Abstract
Background:
Percutaneous endoscopic gastronomy (PEG) tubes are commonly used to administer enteral nutrition during head and neck cancer (HNC) treatment. However, the benefits of placing a prophylactic feeding tube (PFT; prior to radiotherapy [RT]) or reactive feeding tube (RFT, after RT initiation) are unclear. We sought to compare survival, body mass trends, and hospitalization rates between strategies.
Methods:
We conducted a retrospective cohort study of 11,473 Veterans with stages III–IVC HNC treated with chemoradiotherapy. Patients with PEG tube placement within 30 days prior to treatment initiation (PFT) were compared to all other patients (non-PFT) or patients with PEG tube placement within 3 months after treatment initiation placement (RFT). We compared survival, longitudinal body mass changes, and hospitalization rates for PFT versus non-PFT or RFT patients in propensity score (PS)-matched Cox regression models.
Results:
3,186 (28%) patients received PFT and 8,287 (72%) were non-PFT, of which 1,874 (23%) received RFT. After PS-matching, there were no significant differences in overall survival (HR 0.97, 95% CI 0.92–1.02), HNC-specific survival (HR 0.98, 95% CI 0.92–1.09), change in BMI (p=0.24), or hospitalization rates between PFT and non-PFT groups. Significant differences in hospitalization rates between PFT and RFT groups persisted after PS-matching (−0.11 hospitalizations/month), but no differences were found for other outcomes.
Conclusion:
Timing of PEG tube placement in Veterans with HNC was not associated with any significant survival or body mass advantage. However, patients who received PFT had a lower hospitalization rate than those who received RFT.
Keywords: enteral nutrition, squamous cell carcinoma of head and neck, chemoradiotherapy, survival, veterans, retrospective studies
Introduction
Incident head and neck cancers affect over 65,000 Americans annually.1 The majority of these tumors are squamous cell histology and many are related to human papillomavirus (HPV) infection. Nearly half of these cases are advanced stage at diagnosis.2 Radiotherapy (RT) is a mainstay treatment of head and neck cancers, especially squamous cell carcinomas, with an estimated 75% of head and neck squamous cell carcinomas (HNSCC) requiring RT as primary or adjuvant treatment.3
Maintaining optimal nutritional status is critical for patients being treated for head and neck cancer as these patients are at a unique risk for malnourishment. Tumors in this region can cause dysphagia, poor oral intake, changes in metabolism, and often invade structures critical for chewing and swallowing, leading to cachexia. Furthermore, treatment options, which include surgery, RT, chemotherapy, and their combinations, have several side effects which can impact nutrition, such as altered anatomy, mucositis, xerostomia, fatigue, nausea and vomiting, and intestinal malabsorption.4 Previous studies estimate that over 40% of patients being treated for head and neck cancers are malnourished, leading to poor immune function, impaired quality of life, limited treatment tolerability, and poorer survival.5-8
To minimize the impact of malnutrition and dysphagia for head and neck patients under treatment, enteral nutrition administered by gastrostomy is often implemented and has been shown to increase quality of life, reduce treatment interruptions, and decrease nutrition-related emergency department visits and hospitalization.9,10 Percutaneous endoscopic gastrostomy tubes (PEG) have commonly been placed after symptoms arise or when it becomes medically necessary, a practice termed reactive feeding tube (RFT) placement. However, an alternative treatment pathway has been to place PEG tubes before RT treatment. This strategy, termed prophylactic feeding tube (PFT) placement, is implemented with the rationale that patients who may encounter nutritional problems during treatment will have more rapid access to enteral supplementation. The comparative benefits of PFT over reactive strategies are unclear however, with prior studies demonstrating mixed results regarding both body weight maintenance and survival outcomes.11-18
Most prior studies evaluating the timing of feeding tube placement have been limited by very small sample sizes and lack of appropriate methods to limit confounding. Furthermore, only a few examined the effect of PFT versus RFT on overall survival and cancer-specific survival. Our study aims to fill this gap in knowledge by using national data from the Veterans Affairs (VA) health system to determine the impact of PFT placement on both oncologic and patient-centered outcomes.
Methods
Patient Selection
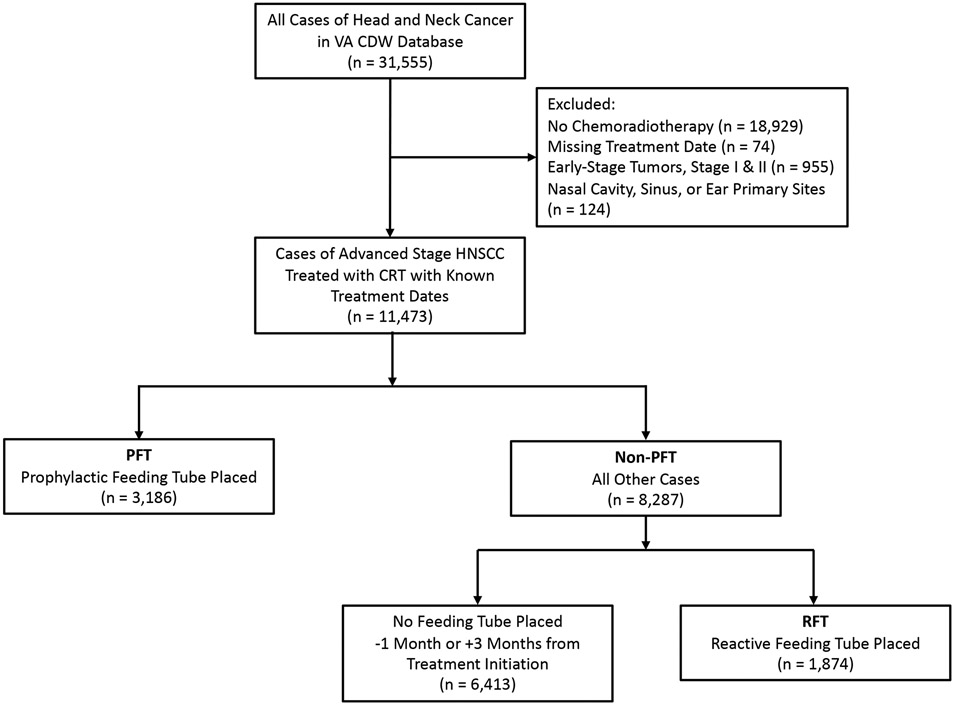
Using the VA Corporate Datawarehouse (CDW), we identified patients with unresected stage III–IVC (American Joint Committee on Cancer, 7th edition) HNSCC treated with chemoradiotherapy (CRT) for curative intent with known treatment dates, a total of 11,473 cases. We included oropharynx, laryngeal, and oral cavity tumors. Nasopharyngeal, sinus, and other less common head and neck subsites for which swallow function is not as prominently affected were excluded. Of these Veterans, 3,186 patients had a PEG tube placed within 30 days prior to treatment initiation (PFT) and 8,287 patients did not have a PEG tube placed prior to treatment initiation (non-PFT). Of the non-PFT patients, 1,874 patients had a PEG tube placed within 3 months after treatment initiation (RFT), and 6,413 patients had no PEG tube placed within this timeframe (Figure 1).
Figure 1.
Consort diagram.
Study Variables
Baseline sociodemographic variables were identified from VA databases. Cancer data, including year of diagnosis, clinical stage, tumor anatomic site, as well as alcohol use data, baseline hearing loss and neuropathy were collected from VA oncology files. Smoking status was classified using VA Health Factors data, augmented with cancer registry data. Estimated glomerular filtration rates (eGFR) were calculated from VA laboratory data. Charlson comorbidity scores were determined by comorbid illnesses identified by diagnostic codes in outpatient and inpatient VA claims databases within 12 months before cancer diagnosis.19 The primary exposure of interest, feeding tube placement and placement date, was ascertained from inpatient and outpatient procedure files using common procedural terminology version 4 (CPT-4) codes 43246 and 49440 or international classification of disease version 9 (ICD-9) 43.11 procedure code. Radiation and chemotherapy administration and initiation dates were identified from registry files and augmented by intravenous medication files.20 Body mass index (BMI) was calculated from weight and height data from biometric data associated with vital sign collection closest to date of diagnosis within six months prior (median interval: 6 days). Hospitalizations were determined from inpatient records.
Our study’s primary outcomes were overall and head and neck cancer-specific survival determined from linked vital status data as well as information on cancer-specific death from cancer registry data. Secondary outcomes included body mass trends and hospitalization rates. Survival times for primary analyses were calculated from the date of cancer diagnosis to the date of death or censoring on January 1, 2018. Head and neck cancer-specific death was determined using cancer registry information regarding cause of death, although some cases were missing cause of death and were excluded from HNCSS analyses.
Statistical Analysis
We tested for differences in baseline and clinical characteristics between PFT and non-PFT groups and between PFT and RFT groups with the t test for normal continuous variables and the chi-square test for categorical variables. To minimize ascertainment bias, we propensity score (PS)-matched the two study arms in both sets of analyses (PFT vs. non-PFT and PFT vs. RFT). We calculated PSs for the study cohort by determining the probability of PFT placement by fitting a logistic regression model. Variables included in the logistic model were as follows: sociodemographic characteristics (age, race, and sex), date of diagnosis, smoking status, alcohol use, primary site of tumor, tumor characteristics (overall stage, T stage, and N stage), comorbidity score, eGFR, baseline neuropathy and hearing loss, BMI, and oncologic surgical procedures. Race, radiation dose, chemotherapy regimen, smoking status, alcohol use, T stage, N stage, and eGFR all had missing values (0.6%, 13.5%, 6.7%, 1.5%, 7.1%, 3.1%, 2.4%, and 7.1%, respectively). BMI at baseline and at 6 months had missing values as well (1.6% and 19.0%, respectively). 76.2% of cases with missing values for BMI at 6 months had died prior to that date. Multiple imputation methods were used to estimate missing values for PS calculations. PS-matched cohorts were then created, and the distribution of patient characteristics by study group were compared before and after matching, using appropriate parametric and non-parametric tests. PFT and non-PFT patients were matched 1:2 for a total of 9,396 subjects for overall survival (OS) and 8,037 subjects for head and neck cancer-specific survival (HNCSS) using a PS caliper of 0.013. PFT and RFT patients were matched 1:1 for a total of 3,736 subjects for OS and 3,076 subjects for HNCSS with PS calipers of 0.015 and 0.017 respectively. We fitted Cox regression models comparing primary outcomes (overall and cancer-specific survival) between intervention groups in the matched cohort with robust standard errors to account for loss of independence due to matching. Based on the number of deaths observed among patients in the cohort, we calculated that the study had an 80% power to detect at least an 11% reduction in the hazard ratio (HR) of death associated with PFT versus RFT using an alpha of 0.05. We then used t tests to compare body mass outcomes between groups. Hospitalization rates were also determined and compared by calculating confidence intervals for the number of hospitalizations per month for the 6-month period following treatment initiation. Three separate sensitivity analyses were performed on all outcomes, first excluding cases with feeding tube placement within one week of treatment initiation, then limiting observations to cases with a baseline BMI < 18.5 kg/m2, and finally excluding stage IVC cases. All analyses were performed in STATA Version 13 (STATA Corp., College Station, TX). This study was approved by the James J Peters VA Medical Center Institutional Review Board.
Results
Baseline Characteristics
We identified 11,473 cases of unresected stage III–IVC (American Joint Committee on Cancer, 7th edition) HNSCC treated with CRT for curative intent with known treatment dates. 3,186 cases received PFT placement (27.8%, Table 1). In comparing PFT and non-PFT groups, patients receiving PFT were more likely to be white (p=0.008), diagnosed with cancer in more recent years (p<0.001), receive a single-agent chemotherapy regimen (p<0.001), have alcohol use history (p=0.02), have oropharynx primary site (p=0.002), have a later stage at diagnosis (p=0.04), and have a higher comorbidity score (p<0.001, Table 1). In comparing PFT and RFT groups, RFT cases were more likely to have been diagnosed with cancer in earlier years (p<0.001), receive a multiagent chemotherapy regimen (p<0.001), have a lower eGFR (p<0.001), have a higher comorbidity score (p=0.01), and less likely to have alcohol use history (p=0.01, results not otherwise shown).
Table 1.
Baseline cohort characteristics by treatment group for PFT vs non-PFT
| Overall Cohort | 1:2 Propensity-matched Cohort | |||||
|---|---|---|---|---|---|---|
| Characteristic | PFT N=3,186 |
Non-PFT N=8,287 |
p-value | PFT N=3,132 |
Non-PFT N=6,264 |
p-value |
| Age, mean (SD) | 61.8 (8.0) | 61.8 (8.2) | 0.99 | 61.8 (8.0) | 61.7 (8.1) | 0.83 |
| Men, N (%) | 3,158 (99.1) | 8,203 (99.0) | 0.63 | 3,104 (99.1) | 6,209 (99.1) | 1.00 |
| Race/Ethnicity, N (%) | 0.008 | 0.41 | ||||
| Caucasian | 2,612 (82.0) | 6,566 (79.2) | 2,563 (81.8) | 5,044 (80.5) | ||
| African American | 449 (14.1) | 1,345 (16.2) | 444 (14.2) | 969 (15.5) | ||
| Hispanic | 76 (2.4) | 261 (3.2) | 76 (2.4) | 157 (2.5) | ||
| Other | 29 (0.9) | 69 (0.8) | 49 (1.6) | 94 (1.5) | ||
| Missing | 20 (0.6) | 46 (0.6) | 0 (0.0) | 0 (0.0) | ||
| Year of diagnosis, N (%) | <0.001 | 0.24 | ||||
| 2000-2005 | 627 (19.7) | 1,833 (22.1) | 625 (20.0) | 1,277 (20.4) | ||
| 2006-2010 | 1,272 (39.9) | 2,791 (33.7) | 1,226 (39.1) | 2,341 (37.4) | ||
| 2011-2014 | 1,287 (40.4) | 3,663 (44.2) | 1,281 (40.9) | 2,646 (42.2) | ||
| Radiation Dose, cGy, mean (SD) | 6,383 (4,822) | 6,594 (6,406) | 0.11 | 6,393 (4,856) | 6,614 (6,408) | 0.11 |
| Chemotherapy Regimen, N (%) | <0.001 | <0.001 | ||||
| Single-agent | 2,021 (63.4) | 4,830 (58.3) | 1,986 (63.4) | 3,678 (58.7) | ||
| Multiagent | 1,023 (32.1) | 2,829 (34.1) | 1,005 (32.1) | 2,119 (33.8) | ||
| NOS | 142 (4.5) | 628 (7.6) | 141 (4.5) | 467 (7.5) | ||
| Smoking Status, N (%) | 0.48 | 0.57 | ||||
| Current cigarette | 1,969 (61.8) | 5,030 (60.7) | 1,930 (61.6) | 3,820 (61.0) | ||
| Current cigar/pipe/smokeless | 63 (2.0) | 160 (1.9) | 62 (2.0) | 126 (2.0) | ||
| Former | 879 (27.6) | 2,319 (28.0) | 866 (27.7) | 1,755 (28.0) | ||
| Never | 236 (7.4) | 643 (7.8) | 236 (7.5) | 460 (7.3) | ||
| Missing | 39 (1.2) | 135 (1.6) | 38 (1.2) | 103 (1.6) | ||
| Alcohol Use, N (%) | ||||||
| Yes | 2,538 (79.7) | 6,358 (76.7) | 0.02 | 2,489 (79.5) | 4,884 (78.0) | 0.44 |
| Missing | 193 (6.1) | 616 (7.4) | 190 (6.1) | 447 (7.1) | ||
| Anatomic Site, N (%) | 0.002 | 0.46 | ||||
| Oral Cavity | 234 (7.3) | 702 (8.5) | 234 (7.5) | 509 (8.1) | ||
| Oropharynx | 1,915 (60.1) | 4,689 (56.6) | 1,865 (59.5) | 3,668 (58.6) | ||
| Hypopharynx/Larynx | 1,037 (32.6) | 2,896 (34.9) | 1,033 (33.0) | 2,087 (33.3) | ||
| Stage at Diagnosis, N (%) | 0.04 | 0.85 | ||||
| III | 631 (19.8) | 1,750 (21.1) | 629 (20.1) | 1,293 (20.6) | ||
| IVA | 2,156 (67.7) | 5,478 (66.1) | 2,118 (67.6) | 4,185 (66.8) | ||
| IVB | 306 (9.6) | 748 (9.0) | 292 (9.3) | 588 (9.4) | ||
| IVC | 93 (2.9) | 311 (3.8) | 93 (3.0) | 198 (3.2) | ||
| Clinical T, N (%) | <0.001 | 0.56 | ||||
| 1 | 322 (10.1) | 972 (11.7) | 321 (10.2) | 699 (11.2) | ||
| 2 | 921 (28.9) | 2,309 (27.9) | 907 (29.0) | 1,792 (28.6) | ||
| 3 | 914 (28.7) | 2,407 (29.0) | 906 (28.9) | 1,807 (28.8) | ||
| 4 | 973 (30.5) | 2,300 (27.8) | 942 (30.1) | 1,836 (29.3) | ||
| Missing | 56 (1.8) | 299 (3.6) | 56 (1.8) | 130 (2.1) | ||
| Clinical N, N (%) | <0.001 | 0.76 | ||||
| 0 | 498 (15.6) | 1,294 (15.6) | 491 (15.7) | 980 (15.6) | ||
| 1 | 527 (16.5) | 1,307 (15.8) | 515 (16.4) | 1,016 (16.2) | ||
| 2 | 1,945 (61.1) | 4,925 (59.4) | 1,910 (61.0) | 3,790 (60.5) | ||
| 3 | 177 (5.6) | 522 (6.3) | 177 (5.6) | 385 (6.2) | ||
| Missing | 39 (1.2) | 239 (2.9) | 39 (1.3) | 93 (1.5) | ||
| eGFR, mg/dl, median (IQR) | 86 (72-104) | 86 (70-101) | 0.02 | 86 (72-103) | 86 (71-102) | 0.50 |
| Baseline Hearing Loss, N (%) | 425 (13.3) | 1,085 (13.1) | 0.73 | 421 (13.4) | 821 (13.1) | 0.65 |
| Baseline Neuropathy | 226 (7.1) | 553 (6.7) | 0.42 | 223 (7.1) | 419 (6.7) | 0.44 |
| Comorbidity Score, N (%) | <0.001 | 0.09 | ||||
| 0 | 1,355 (42.5) | 4,114 (49.6) | 1,350 (43.1) | 2,849 (45.5) | ||
| 1 | 848 (26.6) | 1,915 (23.1) | 826 (26.4) | 1,579 (25.2) | ||
| >1 | 983 (30.9) | 2,258 (27.3) | 956 (30.5) | 1,836 (29.3) | ||
Abbreviations: PFT, prophylactic feeding tube; cGy, centigray; eGFR, estimated glomerular filtration rate; ΔBMI, change in BMI
BMI Results
In the overall, unmatched cohort, PFT cases had a statistically significant lower BMI at baseline than non-PFT cases (p=0.01, Table 3), although the magnitude of this difference was small (0.3 kg/m2). Six months after cancer treatment initiation, mean BMI did not differ in the two groups, nor did the mean change in BMI (both p>0.1; Table 3). There were no differences seen in BMI at baseline, at 6 months, or change over 6 months between the PS-matched PFT and non-PFT cohorts. In a sensitivity analysis excluding cases with feeding tube placement within one week of treatment initiation, baseline BMI was lower for PFT patients (p=0.04), but again there was no difference in BMI or change in BMI at six months by PFT status. No differences in baseline BMI, BMI at 6 months, or change in BMI were seen in sensitivity analyses including only cases with BMI < 18.5 kg/m2 at baseline or excluding stage IVC cases between PFT and non-PFT cohorts. A similar trend was seen in the PFT and RFT cohorts. In the overall, unmatched cohort, PFT cases had statistically significant lower BMI at baseline and change in BMI over 6 months (both p<0.001, Table 3). However, these differences were not seen after PS-matching. In sensitivity analyses excluding cases with feeding tube placement within one week of treatment initiation, differences in BMI at baseline and change over 6 months were once again significant (p=0.02, p=0.004, respectively, Table 3). When comparing cases with BMI < 18.5 kg/m2 at baseline, BMI at baseline did not differ significantly, but BMI at 6 months and change in BMI did (p=0.03, p=0.02, respectively). No differences were seen when excluding stage IVC cases.
Table 3.
Body mass index results and hospitalization rates by treatment group
| Overall Cohort | 1:2 Propensity-matched Cohort | |||||
|---|---|---|---|---|---|---|
| PFT | Non-PFT | p-value | PFT | Non-PFT | p-value | |
| Baseline BMIa, mean (SD) | 25.7 (5.9) | 26.0 (6.0) | 0.01 | 25.7 (5.9) | 25.8 (6.0) | 0.47 |
| BMIa at 6 months, mean (SD) | 22.8 (4.6) | 23.0 (4.8) | 0.11 | 22.9 (4.6) | 22.9 (4.8) | 0.99 |
| ΔBMIa after 6 months, mean (SD) | −3.2 (2.8) | −3.1 (2.9) | 0.53 | −3.2 (2.8) | −3.1 (2.8) | 0.24 |
| Hospitalizations, rateb (95% CI) | 0.23 (0.22-0.24) | 0.23 (0.22-0.23) | 0.38 | 0.23 (0.22-0.24) | 0.23 (0.23-0.24) | 0.44 |
| Overall Cohort | 1:1 Propensity-matched Cohort | |||||
| PFT | RFT | p-value | PFT | RFT | p-value | |
| Baseline BMIa, mean (SD) | 25.7 (5.9) | 26.4 (5.8) | <0.001 | 26.2 (6.1) | 26.4 (5.8) | 0.25 |
| BMIa at 6 months, mean (SD) | 22.8 (4.6) | 23.1 (4.7) | 0.10 | 23.1 (4.7) | 23.0 (4.6) | 0.58 |
| ΔBMIa after 6 months, mean (SD) | −3.2 (2.8) | −3.5 (2.8) | <0.001 | −3.3 (2.8) | −3.5 (2.8) | 0.06 |
| Hospitalizations, rateb (95% CI) | 0.23 (0.22-0.24) | 0.35 (0.34-0.36) | <0.001 | 0.24 (0.23-0.25) | 0.35 (0.34-0.36) | <0.001 |
Abbreviations: PFT, prophylactic feeding tube; BMI, body mass index; SD, standard deviation; ΔBMI, change in BMI; CI, confidence interval
kg/m2
hospitalizations per month
Survival Outcomes
There were no significant differences in overall survival (OS) or head and neck cancer-specific survival (HNCSS) between the PFT and non-PFT groups in crude or PS-matched analyses (Table 4). Median OS for PFT was 43.5 months, while median OS for non-PFT cases was 44.0 months (HR 1.00, 95% CI 0.95–1.05; Table 4). In propensity-score matched groups, PFT median OS was 44.6 months while the matched non-PFT comparator group median OS was 41.8 months. Median HNCSS for PS-matched PFT and non-PFT cases were 52.7 and 50.4 months, respectively.
Table 4.
Cox proportional hazard models for overall survival (OS) and head and neck cancer-specific survival (HNCSS) for PFT vs non-PFT
| PFT Median Survival, mo |
Non-PFT Median Survival, mo |
Hazard Ratio (95% CI) |
|
|---|---|---|---|
| OS | |||
| Comprehensive | 43.5 | 44.0 | 1.00 (0.95-1.05) |
| Matched | 44.6 | 41.8 | 0.97 (0.92-1.02) |
| HNCSS | |||
| Matched | 52.7 | 50.4 | 0.98 (0.92-1.03) |
| Sensitivity Analyses | |||
| Excluding FT placement <1 week from trt initiation | 39.8 | 42.2 | 1.03 (0.97-1.09) |
| Including only BMI < 18.5 kg/m2 | 14.4 | 15.9 | 0.98 (0.84-1.14) |
| Excluding stage IVC | 47.4 | 45.0 | 0.97 (0.92-1.03) |
Abbreviations: PFT, prophylactic feeding tube; mo, months; CI, confidence interval; FT, feeding tube; trt, treatment; BMI, body mass index
Survival outcomes were similar in analyses comparing PFT to RFT patients. The median OS for PFT cases was 43.5 months, compared to 47.2 months for RFT cases (HR 1.02, 95% CI 0.95–1.09; Table 5). In PS-matched cases, PFT median OS was 48.2 months while RFT median OS remained 47.2 months; no significant difference in the hazard of death was noted (HR 0.97, 95% CI 0.89–1.04; Table 5). PFT treated patients also did not differ from RFT patients in cancer-specific survival (HR 1.03, 95% CI 0.94–1.12; Table 5).
Table 5.
Cox proportional hazard models for overall survival (OS) and head and neck cancer-specific survival (HNCSS) for PFT vs RFT
| PFT Median Survival, mo |
RFT Median Survival, mo |
Hazard Ratio (95% CI) |
|
|---|---|---|---|
| OS | |||
| Comprehensive | 43.5 | 47.2 | 1.02 (0.95-1.09) |
| Matched | 48.2 | 47.2 | 0.97 (0.89-1.04) |
| HNCSS | |||
| Matched | 52.2 | 54.2 | 1.03 (0.94-1.12) |
| Sensitivity Analyses | |||
| Excluding FT placement <1 week from trt initiation | 43.3 | 49.3 | 1.05 (0.95-1.15) |
| Including only BMI < 18.5 kg/m2 | 14.8 | 14.5 | 0.96 (0.74-1.24) |
| Excluding stage IVC | 51.4 | 49.2 | 0.96 (0.89-1.04) |
Abbreviations: PFT, prophylactic feeding tube; mo, months; CI, confidence interval; FT, feeding tube; trt, treatment; BMI, body mass index
In sensitivity analyses excluding cases with feeding tube placement within one week of treatment initiation, we found no significant difference in OS for PS-matched cohorts when comparing PFT versus non-PFT (HR 1.03; 95% CI 0.97–1.09; Table 4) or PFT versus RFT patients (HR 1.05; 95% CI 0.95–1.15; Table 5). We further analyzed cases with baseline BMI < 18.5 kg/m2 and similarly found no difference in OS for PS-matched cohorts for PFT versus non-PFT (HR 0.98, 95% CI 0.84–1.14; Table 4) or PFT versus RFT patients (HR 0.96, 95% CI 0.74–1.24; Table 5). Excluding cases with tumor stage IVC likewise did not show any survival differences between the groups.
Hospitalization Rates
Hospitalization rates did not differ between PFT and non-PFT cases (Table 3). In the overall cohort, PFT cases had 0.23 (95% CI 0.22–0.24; Table 3) hospitalizations per month, compared to 0.23 (95% CI 0.22–0.23; Table 3) per month for non-PFT cases. No differences were seen in the PS-matched cohort or the sensitivity analyses (excluding cases with feeding tube placement within one week of treatment initiation, analyzing cases with baseline BMI < 18.5 kg/m2, or excluding stage IVC cases). Hospitalization rates differed significantly between all PFT and RFT cohorts (Table 3). In the overall cohort, RFT cases had a hospitalization rate of 0.35 (95% CI 0.34–0.36), significantly higher than that of PFT cases. Significant differences were also seen in PS- matched cohort (PFT 0.24 95% CI 0.23–0.25 vs RFT 0.35 95% CI 0.34–0.36; Table 3). Statistical significance was retained while excluding cases with feeding tube placement within one week of treatment initiation (PFT 0.24 95% CI 0.23–0.25 vs RFT 0.35 95% CI 0.34–0.36), among cases with BMI < 18.5 kg/m2 (PFT 0.29 95% CI 0.25–0.33 vs RFT 0.40 95% CI 0.35–0.45), and excluding stage IVC cases (PFT 0.24 95% CI 0.23–0.25 vs RFT 0.34 95% CI 0.33–0.35).
Discussion
In a national cohort of Veterans with locally advanced head and neck cancers we found that there was no difference in body mass changes, overall survival, or cancer-specific survival associated with PFT placement when compared to all other advanced head and neck cancer patients or to RFT patients alone, accounting for possible treatment allocation bias. This suggests that the benefits of prophylactic feeding tube placement for survival or body mass-related outcomes may be limited. However, patients had lower rates of hospitalization with PFT placement compared to RFT placement, but not when compared to all other advanced head and neck cancer patients (non-PFT). Other key patient-centered outcomes, such as cancer treatment tolerability, quality of life, comfort, or long-term swallowing outcomes were not assessed in this study and would need to be considered before making definitive guidance regarding this procedure. Nonetheless, PFT placement is a procedure involving potential patient harms and medical costs and the results of our analysis suggest that the role of PFT may deserve further large-scale randomized assessment.
In a recent systematic review of 3 randomized controlled trials, no significant short-term or long-term survival or body mass changes were observed between PFT and RFT outcomes.21 Our study found similar results. However, one study included in the systematic review found that in patients that did experience weight loss, the PFT group lost significantly less weight than the RFT group.14 The same study found some improvements in short-term quality of life outcomes.14 Certainty of evidence was rated as low to moderate for all outcomes and no differences were seen in most outcomes, including overall and disease-free survival, long-term weight changes, BMI changes, nutritional status, treatment interruptions, and long-term quality of life.
Maintaining adequate nutrition and resultant body mass during head and neck cancer therapy has been a primary indication for PFT. Two prior studies have found limited beneficial effects of PFT on BMI outcomes. Salas et al. conducted a small randomized study of PFT vs RFT in head and neck cancer patients and found no difference in BMI trajectory for either 21 PFT versus 18 RFT patients from baseline to end of RT and post-RT.11 Silander et al. also reported a randomized study of PFT (n=64) vs nutritional counseling and enteral supplementation with nasogastric tube (NGT; n=70), if indicated.14 As with the previous trial, that study found no difference in BMI outcomes. Our study provides validation of the body mass outcomes found in these two small, randomized trials in a large, real-world cohort.
Current guidelines suggest PFT for patients with severe weight loss prior to treatment, ongoing dehydration or dysphagia, anorexia, or pain interfering with the ability to eat/drink adequately, significant comorbidities that may be aggravated by poor oral intake, mild to severe aspiration risk, and patients for whom long-term swallowing disorders are likely.22 PFT use has been hypothesized to reduce treatment interruptions, minimize hospitalizations, and therefore improve cancer outcomes and survival. Despite this, studies have consistently failed to show an impact of PFT use on survival. The randomized trial conducted by Silander et al (n=134) found no survival difference associated with PFT.14 Several small comparative retrospective studies have reported similar results.13,15,18 The largest previous retrospective study compared outcomes at two medical centers that differed in regards to standard practices for feeding tube placement for advanced HNSCC patients, finding no difference in 5-year survival according to feeding tube practices.16 These studies are all consistent with our findings of similar OS and HNCSS for patients with and without PFT; furthermore, our study provides results from a national cohort with the largest sample size to date. Although our study is retrospective, we use propensity-score matching to minimize the effect of covariates that may influence interventional group placement.
Our study found that PFT placement significantly reduced the rate of hospitalization within 6 months of treatment initiation compared to RFT placement. Several other studies have also examined hospitalization rates with PFT placement with mixed results. In a small, randomized controlled trial, Brown et al found no differences in number of unplanned hospital admissions between PFT and standard of care.9 A prospective cohort study by the same group, however, found a reduction in nutrition-related hospitalizations associated with PFT.10 Similarly, several other studies have similarly noted increases in hospitalizations associated with RFT placement.16,17,23 In our large, retrospective study, hospitalization rates for all causes were not decreased for PFT compared to the larger group of similar patients but were comparatively increased for RFT patients. We speculate that the reason for the increase in hospitalizations in the RFT group is that RFT placement may signify serious nutritional difficulties, thereby indicating patients at higher risk of future hospitalizations for associated care.
Differences in other patient-centered outcomes in other comparative studies have found both benefits and potential harms associated with PFT strategies. Two studies have found that PFT compared to reactive strategies were associated with better quality of life measures.11,14 In addition, the impact of these strategies on cancer treatment outcomes has been mixed as one17 out of four studies12,15-17 found that PFT reduced treatment interruptions. Finally some potential harms have been identified, with one study finding that PFT increased FT dependence13 and another study finding more FT complications in PFT.16 Our study did not have relevant data to assess these outcomes, but consideration of these factors, along with patient-centered discussions, may be needed to determine the overall appropriateness of PFT.
The strengths of this study include that this is the first large, nationally represented study to evaluate the effect of PFT use on BMI change, survival, and hospitalization rate. Our study utilized detailed clinical data, including longitudinal body mass assessments, high quality vital status data, and extensive documentation of comorbidities, giving us a broader picture of each patient’s health. Some limitations include a non-randomized comparison, although statistical methods to minimize allocation bias were used. Our study lacked data on the exact indications for feeding tube placement, RT dose to the neck, or reasons for hospital admissions. Our study cohort was also overwhelmingly male, limiting generalizability. We also did not have information on quality of life or detailed data on nutritional status beyond body mass measurements and therefore could not assess a full range of important patient-oriented outcomes. Nonetheless, this study provides validation of previous smaller randomized studies as well as additional information that could be used to assess the potential benefits of prophylactic feeding tube placement prior to treatment of advanced HNSCC.
Conclusion
In a cohort of 11,473 Veterans with advanced HNSCC with planned or ongoing chemoradiotherapy, the timing of PEG tube placement had no effect on BMI changes, OS, and HNCSS. However, PFT placement decreased hospitalization rates compared to RFT placement. The decision to use PFT must be weighed against possible improvement in quality-of-life measures and other functional outcomes. Further randomized evaluation is needed to clarify the optimal indications for PFT placement.
Table 2.
Propensity score-matched cohort characteristics by treatment group for PFT vs RFT
| Characteristic | PFT N=1,868 |
RFT N=1,868 |
p-value |
|---|---|---|---|
| Age, mean (SD) | 61.6 (7.9) | 61.7 (8.2) | 0.57 |
| Men, N (%) | 1,853 (99.2) | 1,854 (99.3) | 0.72 |
| Race/Ethnicity, N (%) | 0.92 | ||
| Caucasian | 1,512 (80.9) | 1,516 (81.2) | |
| African American | 282 (15.1) | 272 (14.6) | |
| Hispanic | 47 (2.5) | 49 (2.6) | |
| Other* | 27 (1.5) | 31 (1.7) | |
| Year of diagnosis, N (%) | 0.45 | ||
| 2000-2005 | 429 (23.0) | 462 (24.7) | |
| 2006-2010 | 699 (37.4) | 686 (36.7) | |
| 2011-2014 | 740 (39.6) | 720 (38.5) | |
| Radiation Dose, cGy, mean (SD) | 6,352 (4,202) | 6,647 (6,223) | 0.11 |
| Chemotherapy Regiment, N (%) | 0.002 | ||
| Single-agent | 1,170 (62.6) | 1,066 (57.1) | |
| Multiagent | 610 (32.7) | 713 (38.1) | |
| NOS | 88 (4.7) | 89 (4.8) | |
| Smoking Status, N (%) | 0.95 | ||
| Current cigarette | 1,120 (60.0) | 1,100 (58.9) | |
| Current cigar/pipe/smokeless | 45 (2.4) | 44 (2.4) | |
| Former | 529 (28.3) | 552 (29.6) | |
| Never | 151 (8.1) | 148 (7.9) | |
| Missing | 23 (1.2) | 24 (1.3) | |
| Alcohol Use, N (%) | |||
| Yes | 1,474 (78.9) | 1,431 (76.6) | 0.16 |
| Missing | 110 (5.9) | 124 (6.6) | |
| Anatomic Site, N (%) | 0.73 | ||
| Oral Cavity | 150 (8.0) | 142 (7.6) | |
| Oropharynx | 1,143 (61.2) | 1,131 (60.6) | |
| Hypopharynx/Larynx | 575 (30.8) | 595 (31.9) | |
| Stage at Diagnosis, N (%) | 0.68 | ||
| III | 391 (20.9) | 421 (22.5) | |
| IVA | 1,238 (66.3) | 1,213 (64.9) | |
| IVB | 183 (9.8) | 182 (9.7) | |
| IVC | 56 (3.0) | 52 (2.8) | |
| Clinical T, N (%) | 0.53 | ||
| 1 | 223 (11.9) | 229 (12.3) | |
| 2 | 578 (30.9) | 576 (30.8) | |
| 3 | 534 (28.6) | 550 (29.4) | |
| 4 | 495 (26.5) | 471 (25.2) | |
| Missing | 38 (2.0) | 42 (2.3) | |
| Clinical N, N (%) | 0.90 | ||
| 0 | 258 (13.8) | 271 (14.5) | |
| 1 | 291 (15.6) | 301 (16.1) | |
| 2 | 1,161 (62.2) | 1,131 (60.5) | |
| 3 | 126 (6.7) | 130 (7.0) | |
| Missing | 32 (1.7) | 35 (1.9) | |
| eGFR, mg/dl, median (IQR) | 85 (69-100) | 84 (69-99) | 0.11 |
| Baseline Hearing Loss, N (%) | 251 (13.4) | 257 (13.8) | 0.78 |
| Baseline Neuropathy | 138 (7.4) | 135 (7.2) | 0.85 |
| Comorbidity Score, N (%) | 0.96 | ||
| 0 | 771 (41.3) | 774 (41.4) | |
| 1 | 454 (24.3) | 447 (23.9) | |
| >1 | 643 (34.4) | 647 (34.6) |
Abbreviations: PFT, prophylactic feeding tube; cGy, centigray; eGFR, estimated glomerular filtration rate; ΔBMI, change in BMI
Abbreviations:
- PFT
prophylactic feeding tube
- RFT
reactive feeding tube
Footnotes
Level of Evidence: Level 4
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. Jan 2019;69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Cooper JS, Porter K, Mallin K, et al. National Cancer Database report on cancer of the head and neck: 10-year update. Head & neck. Jun 2009;31(6):748–58. doi: 10.1002/hed.21022 [DOI] [PubMed] [Google Scholar]
- 3.Barton MB, Jacob S, Shafiq J, et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. Jul 2014;112(1):140–4. doi: 10.1016/j.radonc.2014.03.024 [DOI] [PubMed] [Google Scholar]
- 4.Bossola M. Nutritional interventions in head and neck cancer patients undergoing chemoradiotherapy: a narrative review. Nutrients. Jan 5 2015;7(1):265–76. doi: 10.3390/nu7010265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandra RK. Nutrition and the immune system: an introduction. Am J Clin Nutr. Aug 1997;66(2):460S–463S. doi: 10.1093/ajcn/66.2.460S [DOI] [PubMed] [Google Scholar]
- 6.Langius JA, van Dijk AM, Doornaert P, et al. More than 10% weight loss in head and neck cancer patients during radiotherapy is independently associated with deterioration in quality of life. Nutr Cancer. 2013;65(1):76–83. doi: 10.1080/01635581.2013.741749 [DOI] [PubMed] [Google Scholar]
- 7.Paccagnella A, Morello M, Da Mosto MC, et al. Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support Care Cancer. Jul 2010;18(7):837–45. doi: 10.1007/s00520-009-0717-0 [DOI] [PubMed] [Google Scholar]
- 8.Gorenc M, Kozjek NR, Strojan P. Malnutrition and cachexia in patients with head and neck cancer treated with (chemo)radiotherapy. Rep Pract Oncol Radiother. Jul-Aug 2015;20(4):249–58. doi: 10.1016/j.rpor.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown TE, Banks MD, Hughes BGM, Lin CY, Kenny LM, Bauer JD. Randomised controlled trial of early prophylactic feeding vs standard care in patients with head and neck cancer. Br J Cancer. Jun 27 2017;117(1):15–24. doi: 10.1038/bjc.2017.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown TE, Banks MD, Hughes BGM, Lin CY, Kenny LM, Bauer JD. Comparison of Nutritional and Clinical Outcomes in Patients with Head and Neck Cancer Undergoing Chemoradiotherapy Utilizing Prophylactic versus Reactive Nutrition Support Approaches. J Acad Nutr Diet. Apr 2018;118(4):627–636. doi: 10.1016/j.jand.2016.10.013 [DOI] [PubMed] [Google Scholar]
- 11.Salas S, Baumstarck-Barrau K, Alfonsi M, et al. Impact of the prophylactic gastrostomy for unresectable squamous cell head and neck carcinomas treated with radio-chemotherapy on quality of life: Prospective randomized trial. Radiother Oncol. Dec 2009;93(3):503–9. doi: 10.1016/j.radonc.2009.05.016 [DOI] [PubMed] [Google Scholar]
- 12.Nugent B, Parker MJ, McIntyre IA. Nasogastric tube feeding and percutaneous endoscopic gastrostomy tube feeding in patients with head and neck cancer. J Hum Nutr Diet. Jun 2010;23(3):277–84. doi: 10.1111/j.1365-277X.2010.01047.x [DOI] [PubMed] [Google Scholar]
- 13.Chen AM, Li BQ, Lau DH, et al. Evaluating the role of prophylactic gastrostomy tube placement prior to definitive chemoradiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. Nov 15 2010;78(4):1026–32. doi: 10.1016/j.ijrobp.2009.09.036 [DOI] [PubMed] [Google Scholar]
- 14.Silander E, Nyman J, Bove M, Johansson L, Larsson S, Hammerlid E. Impact of prophylactic percutaneous endoscopic gastrostomy on malnutrition and quality of life in patients with head and neck cancer: a randomized study. Head & neck. Jan 2012;34(1):1–9. doi: 10.1002/hed.21700 [DOI] [PubMed] [Google Scholar]
- 15.Williams GF, Teo MT, Sen M, Dyker KE, Coyle C, Prestwich RJ. Enteral feeding outcomes after chemoradiotherapy for oropharynx cancer: a role for a prophylactic gastrostomy? Oral Oncol. May 2012;48(5):434–40. doi: 10.1016/j.oraloncology.2011.11.022 [DOI] [PubMed] [Google Scholar]
- 16.Olson R, Karam I, Wilson G, Bowman A, Lee C, Wong F. Population-based comparison of two feeding tube approaches for head and neck cancer patients receiving concurrent systemic-radiation therapy: is a prophylactic feeding tube approach harmful or helpful? Support Care Cancer. Dec 2013;21(12):3433–9. doi: 10.1007/s00520-013-1936-y [DOI] [PubMed] [Google Scholar]
- 17.Lewis SL, Brody R, Touger-Decker R, Parrott JS, Epstein J. Feeding tube use in patients with head and neck cancer. Head & neck. Dec 2014;36(12):1789–95. doi: 10.1002/hed.23538 [DOI] [PubMed] [Google Scholar]
- 18.Kramer S, Newcomb M, Hessler J, Siddiqui F. Prophylactic versus reactive PEG tube placement in head and neck cancer. Otolaryngol Head Neck Surg. Mar 2014;150(3):407–12. doi: 10.1177/0194599813517081 [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 20.Bauml JM, Vinnakota R, Anna Park YH, et al. Cisplatin Every 3 Weeks Versus Weekly With Definitive Concurrent Radiotherapy for Squamous Cell Carcinoma of the Head and Neck. J Natl Cancer Inst. Sep 18 2018;doi: 10.1093/jnci/djy133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mellors K, Ye X, Van Den Brande J, et al. Comparison of prophylactic percutaneous endoscopic gastrostomy with reactive enteral nutrition in patients with head and neck cancer undergoing radiotherapy or chemoradiotherapy: A systematic review. Clin Nutr ESPEN. Dec 2021;46:87–98. doi: 10.1016/j.clnesp.2021.09.724 [DOI] [PubMed] [Google Scholar]
- 22.National Comprehensive Cancer Network. Head and Neck Cancer (Version 2.2020). Accessed June 16, 2020, https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- 23.Lee JH, Machtay M, Unger LD, et al. Prophylactic gastrostomy tubes in patients undergoing intensive irradiation for cancer of the head and neck. Arch Otolaryngol Head Neck Surg. Aug 1998;124(8):871–5. doi: 10.1001/archotol.124.8.871 [DOI] [PubMed] [Google Scholar]