Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) is the most prevalent neurological complication of chemotherapy for cancer, and has limited effective treatment options. Autologous conditioned serum (ACS) is an effective biologic therapy used by intra-articular injection for patients with osteoarthritis. However, ACS has not been systematically tested in the treatment of peripheral neuropathies such as CIPN. It has been generally assumed that the analgesic effect of this biologic therapy results from augmented concentrations of anti-inflammatory cytokines and growth factors. Here we report that a single intrathecal injection of human conditioned serum (hCS) produced long-lasting inhibition of paclitaxel chemotherapy-induced neuropathic pain (mechanical allodynia) in mice, without causing motor impairment. Strikingly, the analgesic effect of hCS in our experiments was maintained even 8 weeks after the treatment, compared with non-conditioned human serum (hNCS). Furthermore, the hCS transfer-induced pain relief in mice was fully recapitulated by rat or mouse CS transfer to mice of both sexes, indicating cross-species and cross-sex effectiveness. Mechanistically, CS treatment blocked the chemotherapy-induced glial reaction in the spinal cord and improved nerve conduction. Compared to NCS, CS contained significantly higher concentrations of anti-inflammatory and pro-resolving mediators, including IL-1Ra, TIMP-1, TGF-β1, and resolvins D1/D2. Intrathecal injection of anti-TGF-β1 and anti-Il-1Ra antibody transiently reversed the analgesic action of CS. Nanoparticle tracking analysis revealed that rat conditioned serum contained a significantly greater number of exosomes than NCS. Importantly, the removal of exosomes by high-speed centrifugation largely diminished the ACS-produced pain relief, suggesting a critical involvement of small vesicles (exosomes) in ACS’ beneficial effects. Together, our findings demonstrate that intrathecal CS produces a remarkable resolution of neuropathic pain mediated through a combination of small vesicles/exosomes and neuroimmune/neuroglial modulation.
Keywords: ACS (autologous conditioned serum), CIPN (chemotherapy-induced peripheral neuropathy), conditioned serum (CS), exosomes, glial cells, IL-1Ra (interleukin-1 receptor antagonist), non-conditioned serum (NCS), neuroinflammation, spinal cord
1. Introduction
Peripheral neuropathies are common, resulting from metabolic diseases such as diabetes, and exposure to toxins such as chemotherapy. Chemotherapy-induced polyneuropathy (CIPN) is particularly problematic, affecting greater than 50% of individuals treated with paclitaxel (Grisold et al., 2012; Seretny et al., 2014; Sisignano et al., 2014), potentially limiting therapeutic doses of cancer treatments. Chemotherapy causes a loss of epidermal innervation, infiltration of inflammatory cells, neuroinflammation, and neuropathic pain (Chang et al., 2018; Krukowski et al., 2016; Li et al., 2018; Luo et al., 2019; Pachman et al., 2011; Robinson et al., 2014; Sisignano et al., 2014; Zhang et al., 2016). CIPN also produces robust activation of glial cells (e.g., astrocytes and microglia) in the spinal cord and satellite glial cells in the dorsal root ganglion (DRG), leading to upregulation of proinflammatory cytokines and chemokines that promote neuropathic pain (Hu et al., 2018; Ji et al., 2013; Luo et al., 2021; Robinson et al., 2014). There are several commonly used medications for neuropathic pain, such as gabapentenoids, tricyclic antidepressants, and serotonin-norepinephrine reuptake inhibitors. Unfortunately, these pharmacologic options commonly produce side effects and demonstrate limited effectiveness in the treatment of CIPN (Hammack et al., 2002; Kautio et al., 2009; Price et al., 2022; Rao et al., 2007). These limitations have led to the search for novel analgesic agents for the treatment of chronic neuropathic pain.
Biologically based regenerative therapies such as platelet-rich plasma (PRP), autologous conditioned serum (ACS), and mesenchymal stem cells (MSCs) are used with increasing frequency in the treatment of osteoarthritis and musculoskeletal disorders (Buchheit et al., 2020). PRP is one of the more commonly applied biologic therapies; it can be produced relatively easily from blood samples. However, this therapy remains controversial because of variable processing techniques and inconsistent outcomes (Bennell et al., 2021; Buchheit et al., 2022). MSC treatments are also commonly performed in the US, especially from bone marrow aspiration. Although mechanisms of action of MSC therapies were initially thought to be secondary to cellular replacement of cartilage and other damaged tissues, recent research supports that analgesia from MSC therapies is primarily driven by growth factors, anti-inflammatory cytokines, and neuroimmune modulation (Boukelmoune et al., 2021; Buchheit et al., 2020; Chen et al., 2015; Evans et al., 2016; Guo et al., 2011; Huh et al., 2017; Liu et al., 2017). We have shown that the intrathecal administration of bone marrow stromal/stem cells (MSCs) produces long-term analgesia via TGF-β1-mediated neuro-immune modulation (Chen et al., 2015). However, these MSC techniques require multiple steps, including tissue harvest, cell culture, and the potential safety concerns of systemic or intrathecal cellular injection. For these reasons, our lab has been studying a blood-derived, cell-free biologic therapy, ACS, with the goal of an easily deployable, and effective treatment for nerve injury pain.
ACS is currently in clinical use in the US and Europe and has been shown to be effective in the treatment of osteoarthritis and degenerative musculoskeletal conditions (Baltzer et al., 2009; Damjanov et al., 2018; Meijer et al., 2003a). ACS has also been used to successfully treat neuropathic spine disease (lumbar and cervical radiculopathy) (Becker et al., 2007; Goni et al., 2015) and trigeminal neuralgia (Aghamohammadi et al., 2022). The analgesic effect of this biologic therapy has been presumed secondary to the augmented concentrations of IL-1Ra, TGF-β, HGF, IL-10, and other anabolic cytokines (Evans et al., 2016). In particular, IL-1Ra is dramatically increased in the generation of ACS and was thought to be one of the predominant analgesic cytokines. However, when recombinant IL-1Ra is injected in a similar manner for the treatment of OA, it does not provide significant analgesia (Chevalier et al., 2009).
Although effective in the treatment of radicular and trigeminal nerves, trials using ACS for polyneuropathies such as CIPN are still lacking. To investigate the mechanisms by which ACS generates benefits in this neuropathic pain condition, we conducted a series of experiments to assess cross-species behavioral effects (human-mouse, rat-mouse, mouse-mouse, Fig. 1), changes in neurologic function with treatment, and the analgesic impacts of ACS-contained neuroimmune factors and small vesicles such as exosomes. Since we conducted cross-species comparison in this study, we used the term “conditioned serum (CS)”, instead of “autologous conditioned serum (ACS)”. We also included non-conditioned serum (NCS) as control for the CS treatment.
Figure 1.
(A) Schematic of blood collection and blood incubation in human, rat, and mouse samples. (B) Schematic of serum correction for conditioned serum (CS) and non-conditioned serum (NCS) from humans (hCS and hNCS), rats (rCS and rNCS), and mice (mCS and mNCS). (C) Schematic of a mouse model of chemotherapy-induced peripheral neuropathy (CIPN) by four injections of paclitaxel on day 1, 3, 5, and 7 in CD1 mice. (C) Schematic of behavioral tests for mechanical pain (von Frey tests) and cold pain (acetone test). (D) Schematic of a single intrathecal injection of NCS and CS (from human, mouse, and rat) to CD1 mice of both sexes.
2. Materials and methods
2.1. CS incubation from human, rat, and mouse blood
Human CS was generated after collecting blood samples from 3 volunteers (IRB protocol Pro00077946). Blood was collected under aseptic conditions and subsequently incubated for 24 hours at 37°C (Fig. 1A). The incubated product was then transferred under strict aseptic conditions in a biological safety cabinet to a centrifuge tube and spun at 3000g for 10 min. The supernatant was then removed, filtered with a 0.22μ filter, and aliquoted into cryo tubes for storage. The incubated serum was stored in a −20° freezer until ready for use. Rat CS was generated after collecting blood samples via transcardial puncture. Rat CS (from up to 5mL of serum per rat) or mouse CS (from up to 1mL of serum per mouse) was prepared in a parallel manner to human CS preparation, with volume-reduced equipment. Exosomes were removed from rat ACS samples by high-speed centrifuge (100,000 rpm, 2 x 90 min).
2.2. Reagents.
We ordered paclitaxel from Sigma-Aldrich (T7191-25MG), which comes as a 25 mg bottle of powder. Paclitaxel was dissolved by adding 0.7 ml DMSO directly to the powder. Dissolved paclitaxel was then mixed in 100% ethanol and Cremophor EL (Millipore/Sigma) at 1:1 ratio. Anti-TGF-β1 antibody was obtained from R&D Systems (Chen et al., 2015), and anti-IL-ra neutralizing antibody was ordered from R&D Systems (AF-480-SP).
2.3. Animals, neuropathic and postoperative pain models, and drug administration.
Adult CD1 mice (8-10 weeks old, males and females) and Sprague Dawley Rats (200-250 g, males) were purchased from Charles River Laboratories. Animals were randomly assigned to each group. All animals were maintained by the Division of Laboratory Animal Resources (DLAR) at the Duke University Animal Facility. All animal experiments were approved by the Institutional Animal Care and Use Committees (IACUC) of Duke University. Peripheral neuropathy by chemotherapy was induced in mice by four intraperitoneal injections of paclitaxel at a dose of 2 mg/kg on days 0, 2, 4, and 6. Neuropathic pain was also induced in rats and mice by spared nerve injury (SNI) surgery (Decosterd and Woolf, 2000). Mice and rats were anesthetized with isoflurane, and a 5.0 silk tight ligation of the tibial and common peroneal nerves was performed, followed by transection and removal of a 3- to 5-mm portion of the nerve. Notably, the sural nerve, the third peripheral branch of the sciatic nerve, was left intact, and any contact with or stretching of the sural nerve was carefully avoided. Postoperative pain was induced by plantar incision, wherein a 1-cm longitudinal incision of the left plantar aspect of the hind paw, beginning 0.5 cm from the end of the heel, was made with a number 11 surgical blade through the skin, fascia, and plantaris muscle (Brennan et al., 1996; Matsuda et al., 2017). For intrathecal (i.t.) or intraplantar injections, mice were briefly anesthetized with isoflurane (2%), and 10 μl of reagents were injected using a 30G needle. For intrathecal injection, a lumbar puncture was performed between the L5 and L6 spinal levels, and a successful injection was validated by a brisk tail-flick.
2.4. Behavioral analysis.
Hind-paw sensitivity was tested with the use of von Frey filament. These analyses were performed after animals were habituated for at least 2 days in boxes on an elevated metal mesh floor under stable room temperature and humidity. Logarithmically-graded von Frey fibers (0.02-2.56 gram, Stoelting) were applied to the plantar surface of the hind paw. Paw withdrawal threshold was calculated following the previously reported protocol using the up-down method (Luo et al., 2018). Paw withdrawal frequency was measured by applying a 0.16 or 0.4 gram von Frey filament to the plantar surface of the hind paw ten times, with the number of paw withdrawals divided by the ten applications and reported as the withdrawal frequency. Motor function was evaluated by the rotarod test using a rotarod system (IITC Life Science). Mice underwent a two-day training period (2 trials per mouse, 5 rpm rotarod for 10 minutes per trial) before the test. During rotarod testing, the speed of rotation was accelerated from 5 to 15 rpm over 60 seconds with a testing period cut-off of 300 seconds for each trial and 2 total trials performed. The fall latency of each mouse was recorded and averaged. The experimenter was blinded to the treatments given to each mouse. Cold sensitivity was assessed by acetone test. Through the mesh floor two acetone applications (20 μl/application) were gently applied to the bottom of a hind paw using a pipette. Responses to acetone were calculated as duration (seconds) of pain-like behaviors (lifting/licking). Mechanical pain in rats was assessed by an electronic von Frey Anesthesiometer (IITC Life Science Inc.). A hind paw was stimulated with increasing force (0–50 g) of a von Frey filament, presented perpendicularly to the plantar surface (Tao et al., 2021).
2.5. Immunohistochemistry.
Mice were anesthetized with isoflurane and perfused through the ascending aorta with PBS followed by 4% paraformaldehyde. After perfusion, spinal cord tissues were post-fixed, dehydrated with 30% sucrose solution, embedded, and sliced into 30 μm free-floating sections with a cryostat. The sections were blocked with 5% goat serum for 1 h at room temperature and incubated overnight at 4 °C with primary antibody against IBA1 (rabbit, 1:800, Wako, 019-19741) and GFAP (mouse, 1:400, Millipore Sigma, G3893). After washing, the sections were incubated with secondary anti-rabbit Alexa Fluor 488 and anti-Mouse Alexa Fluor 555 antibody (1:400, Invitrogen) for 1 h at room temperature. Stained tissue sections were examined using a Nikon fluorescence microscope. For high-resolution imaging, sections were also captured by a Zeiss LCM 880 confocal microscope. To quantify immunofluorescence, integrated density was measured within laminae I-III of the spinal cord dorsal horn in each optic field by Image J (Version: k1.53). Three to four spinal cord sections were analyzed per mouse and five mice were included per group.
2.6. ELISA
ELISA kits for human IL-1Ra (Catlog#DRA00B) and TIMP-1 (Catlog#DY970-05), Human/Mouse/Rat/Porcine/Canine TGF-β (Catlog#DB100B) were purchased from R&D system. ELISA kits for RvD1 (Catlog#500380) and RvD2 (Catlog#501120) were purchased from Cayman Chemical. Rat IL-10 (Catlog#EK0418) and TIMP-1 (CalLog#EK0583) ELISA kits were purchased from Boster Bio (Pleasanton, CA). ELISA tests were performed with human and rat serum samples, according to the manufacturer’s instructions. For each ELISA assay, 10 μl of serum (CS and NCS) were used. The standard curve was included in each experiment. Cytokines were quantified by using a sandwich ELISA method and measuring OD450 absorbance. RvD1 or RvD2 levels were quantified by using a competitive ELISA method and measuring OD420 absorbance.
2.7. Nerve conduction velocity measurement
In vivo electrophysiology was performed to evaluate sensory nerve conduction velocity (SNCV). Mice were anesthetized with urethane (500 mg/kg, i.p.) to record afferent fibers. Two stimulating electrodes were placed on the fifth toe and ankle (distance was measured to be 1 cm) and a recording cuff electrode was placed on the sciatic nerve. The delay time was measured (ms/cm) based on the difference between the peak times of the spike waveforms by electrical stimulation at the first site (toe) and the second site (ankle). The amplitude (μV) was measured from the first positive spike to the last negative spike. The filter was set to 20 Hz to 5 kHz, and electrical stimulation of 10 V was applied every 60 seconds for 1 ms intervals. We obtained 15 spikes for each mouse and averaged them. Electrical signals were recorded by an 1800 AC amplifier (A-M system) and converted to a distal signal by a Disitata 1440A (Molecular Device). Raw data were analyzed using pClamp10 (Molecular Device).
2.8. Nanoparticle tracking analysis (NTA) for exosomes
NTA was developed to define the physical characteristics of vesicles and determine their concentrations (McNicholas and Michael, 2017). NTA uses light scattering to track Brownian particle motion and calculates particle size and concentration. We used NTA with NanoSight (Fig. 8A) to quantify exosome concentrations in rat serum. The concentrations and errors were averaged from 5 measurements for each sample, and each measurement takes 60 s. The auto analyses were performed in NanoSight NTA Software 3.2 and the detection threshold was setting to 3 for all experiments.
Figure 8.
Nanoparticle tracking analysis showing increased exosome concentration in male rat CS (rCS). (A) Paradigm of nanoparticle tracking analysis. (B) Size distribution and concentrations of extracellular vesicles in rNCS, rCS, and U-rCS in each of five rats and all rats combined. (C) Particle density per mL serum. (D) Normalized extracellular vesicle (EV) concentrations as percentage of rNCS. **p<0.01, ***p<0.001, n=5 rats, one-way ANOVA, followed by Bonferroni posthoc test. rUCS, rat CS after ultracentrifugation. Note that EV is deleted in rUCS.
2.9. Statistics
All data in this study are expressed as mean ± SEM. All sample sizes are included in figure legends. The sample sizes were based on our previous study for paw withdrawal threshold, with a minimum sample size of n=5 for each sex (Luo et al., 2021). This is also based on the assumption for an RM ANOVA analysis for alpha = 0.05 and power = 0.8. The Sample sizes are increased to 8-10 when males and females are combined in Supplementary Figs. 1-6. Animals were randomly assigned to each experimental group. The results of the behavioral tests were analyzed with two-way ANOVA, or one-way ANOVA, followed by Bonferroni’s post-hoc test, or by unpaired Student’s t-test. Differences between groups were deemed statistically significant if the p-value was less than 0.05. Statistical significance was labeled as *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
3. Results
3.1. Intrathecal transfer of human CS to mice produces long-term pain relief and improves nerve conduction in a mouse model of CIPN
We prepared human CS (hCS) and human non-conditioned serum (hNCS), i.e. non-incubated serum (control serum) from two male healthy donors and one female donor (Fig. 1). We then tested the analgesic actions of hCS and hNCS in a murine model of CIPN. To determine the specific effects of hCS, we compared the differences of hCS and hNCS from the same donor. We measured mechanical allodynia, a cardinal feature of neuropathic pain, using von Frey filaments for both paw withdrawal threshold (PWT) and paw withdrawal frequency (PWF, % response to a subthreshold 0.4g filament). Paclitaxel (PTX) induced rapid (<7 days) and sustained (>6 weeks) mechanical allodynia, as shown by a reduction of PWT (Fig. 2A) and an increase in PWF (Fig. 2B). One week after the first PTX treatment, we administered either hCS via intrathecal injection (i.t., 10 μl), as the intrathecal route can target both dorsal root ganglia (DRG) and spinal cord in the pain pathway (Kawasaki et al., 2008). A single i.t. injection of hCS produced significant reduction of mechanical allodynia for >6 weeks, as shown by increased PWT (Fig. 2A) and reduced PWF (Fig. 2B), compared to hNCS. PTX also produced cold hypersensitivity in the acetone test, but this cold pain was not affected by the hCS treatment (Fig. 2C), suggesting a selective effect of hCS on mechanical pain. This result also indicates that paw movement for cold response was unaltered after the hCS treatment. Furthermore, open-field test showed comparable traveling distance of mice treated by hCS or hNCS, indicating no alterations of motor function or activity in the hCS treated animals (Fig. 2D). Because recent studies have shown sex dimorphism in glial and immune modulation of pain (Sorge et al., 2015; Taves et al., 2016), we also generated the CIPN model in female mice. Notably, i.t. injection of hCS was also effective in reducing mechanical allodynia pain in female mice (Supplementary Fig. 1A) and males and females combined (Supplementary Fig. 1B).
Figure 2.
Intrathecal injection of human CS (hCS, from a male doner) alleviates neuropathic pain symptom following PTX-induced CIPN in male mice. (A, B) Von Frey tests for mechanical pain showing paw withdrawal threshold (PWT, A) and paw withdrawal frequency (PWF, B). (C) Acetone test showing cold pain. (D) Open-field test in hCS and hNCS treated animals. hCS produces long-term reduction of PTX-induced mechanical allodynia by increasing PWT (A) and decreasing PWF (B), without effects on cold pain (C) and motor function (D). n=5 male mice per group. *p<0.05, **p<0.01, ***p<0.001, two-way ANOVA, followed by Bonferroni posthoc test. hCS, human conditioned serum; hNCS, human non-conditioned serum.
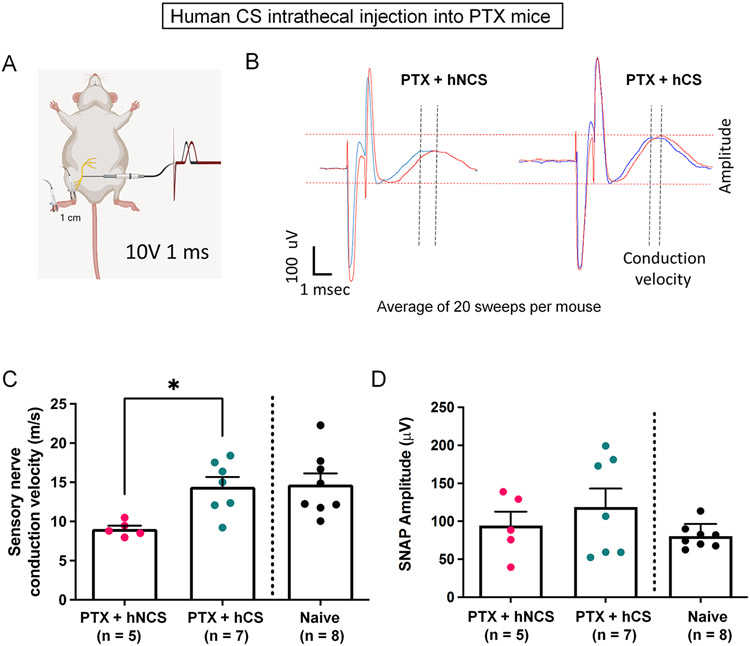
CIPN is additionally characterized by impairment of nerve conduction (Sisignano et al., 2014). We therefore conducted in vivo electrophysiology to measure the sensory nerve conduction velocity (NCV) of the sciatic nerve in naïve and PTX-treated animals (Fig. 3A-D). Compared to naïve control mice, the paclitaxel dose used in this study resulted in a mild reduction in NCS (Fig. 3C). Intrathecal hCS treatment significantly increased NCV (p<0.01, unpaired t-test, Fig. 3B,C), without changing the amplitude of NCV (Fig. 3B,D). This finding indicates that hCS not only relieves pain symptom (mechanical allodynia) but also improves peripheral neurologic function.
Figure 3.
Intrathecal human CS (hCS, from a male donor) treatment significantly improves nerve conduction velocity in male and female mice with CIPN. (A) Schematic of sensory conductance measurement. Two stimulation electrodes were inserted into a hind paw with 1 cm between them and the recording cuff electrode was inserted to the sciatic nerve (1 ms stimulation 10V). (B) Representative nerve conductance trace. left: control mice. right: hCS-treated mice. (C,D) Quantification of nerve conductance velocity (C) and amplitude (D). n = 7 mice for naïve control, n = 5 mice for hNCS with PTX, n = 7 mice for hCS with PTX. *p<0.05, Unpaired t-test.
3.2. Intrathecal administration of rat CS resolves mechanical pain and inhibits spinal cord glial reaction in CIPN mice.
Next, we tested the hypothesis that CS prepared from rats is also effective in suppressing pain in mice. We prepared rat CS (rCS) and rNCS in a parallel manner to the hCS/hNCS processing with the use of volume-reduced processing equipment. We then intrathecally injected 10 μl of rCS to paclitaxel-treated mice. Similar to the effects of hCS, we found that rCS injection resulted in long-lasting pain inhibition. rCS significantly increased PWT for the entire duration of our testing, from 3 hours to 3 weeks (p<0.05, Two-Way ANOVA, Fig. 4A). rCS also significantly decreased PWF from 1 hour to 3 weeks (p<0.05, Two-Way ANOVA, Fig. 4B). Furthermore, i.t. injection of rCS effectively reduced mechanical allodynia in female mice (Supplementary Fig. 2A,B) and males and female combined (Supplementary Fig. 2C,D). Spared nerve injury (SNI), a commonly used animal model to study neuropathic pain, is characterized by the rapid development of mechanical and cold allodynia (Decosterd and Woolf, 2000). We found that rCS was also effective in reducing SNI-induced mechanical allodynia in male rats (Supplementary Fig. 3A), female rats (Supplementary Fig. 3B), and males and females combined (Supplementary Fig. 3C) compared to control serum (rNCS).
Figure 4.
Intrathecal injection of rat CS (rCS) alleviates neuropathic pain symptom in mice. (A, B) Von Frey tests for mechanical pain showing PWT (A) and PWF (B). Note that rCS with 24h incubation, but not 12h incubation, produces long-term reduction of PTX-induced mechanical allodynia by increasing threshold (A) and decreasing frequency (B). n=5 male mice per group. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, two-way ANOVA, followed by Bonferroni posthoc test. Male donors and receivers were tested.
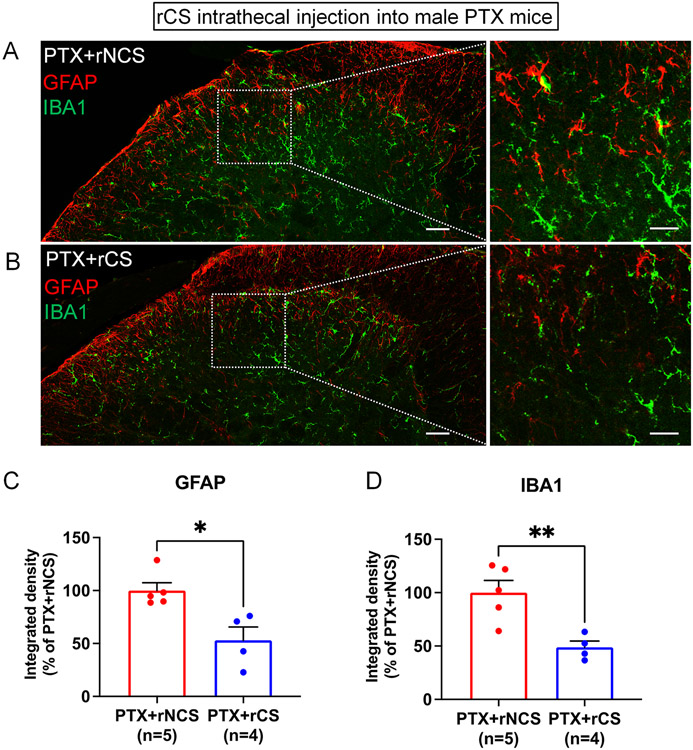
CIPN is known to produce reactive changes in glial cells, such as astrocytes or/and microglia in the spinal cord dorsal horn (Hu et al., 2018; Ji et al., 2013; Luo et al., 2021; Robinson et al., 2014). Mounting evidence supports a critical role of glial cells in different types of pathological pain conditions (Chiang et al., 2012; Milligan and Watkins, 2009; Ren and Dubner, 2010; Salter and Stevens, 2017). We conducted immunohistochemistry of the astroglial marker GFAP and the microglial marker IBA1 in the spinal cord sections of rCS and rNCS-treated mice with CIPN. We measured the intensity of immunofluorescence in the superficial dorsal horn (laminae I-III) where the majority of GFAP staining was observed (Fig. 5A, B). Compared to rNCS, the rCS treatment resulted in significant decreases in GFAP immunostaining (p<0.05, t-test, Fig. 5C) and IBA1 immunostaining (p<0.01, t-test, Fig. 5D). Thus, CS may resolve neuropathic pain via inhibition of chemotherapy-evoked microglial and astroglial reaction in the spinal cord.
Figure 5.
Intrathecal rat ACS (rCS) treatment inhibits glial reaction in the spinal cord dorsal horn of male mice with CIPN. (A,B) Double immunofluorescence staining of the lumbar spinal dorsal horn sections showing GFAP and IBA1 immunostaining following rNCS (A) and rCS treatment (B) in PTX mice. (C-D) Quantification of the integrated density of GFAP (C) and IBA1 (D) in the lumbar spinal dorsal horn of PTX mice treatment with rNCS and rCS (n = 5/group). *p< 0.05, **p< 0.01 vs. control serum (rNCS). Unpaired two-tailed Student’s t test. Spinal cord tissues were harvested one week after the rCS treatment. Male donors and receivers were tested.
3.3. Anti-inflammatory and pro-resolving mediators are enriched in hCS and rCS.
Cytokines play a critical role in the pathogenesis of pain via peripheral and central modulation (Sommer and Kress, 2004; Watkins et al., 2001). Previous studies have revealed that after incubation, human ACS contains higher levels of anti-inflammatory cytokines such as IL-1Ra (Meijer et al., 2003b). We first used ELISA to measure serum levels of IL-1Ra in 3 hCS samples, collected from 3 healthy donors (2 males and 1 female). We found profound increases in IL-1Ra levels in all three hCS samples (p<0.001, vs. hNCS, paired t-test, Fig. 6A). Our earlier report also showed that tissue inhibitor of matrix metalloprotease 1 (TIMP-1) is an endogenous inhibitor of MMP-9 and potently inhibited neuropathic pain in mice and rats (Kawasaki et al., 2008). Notably, TIMP-1 levels increased in all 3 hCS samples (p<0.05, vs. hNCS, paired t-test, Fig. 6B). Resolvins, including D-series resolvins RvD1 and RvD2, belong to a superfamily of specialized pro-resolving mediators (SPMs) and possess potent analgesic actions (Ji, 2022; Serhan, 2014). We used commercial ELISA kits to measure RvD1 and RvD2 levels in hCS and hNCS (Fig. 6C-F). We observed a tendency of increased RvD1 levels in all samples (Fig. 6D, p = 0.2218 vs. hNCS, n=3, paired two-tailed t-test) and found a significant increase of RvD2 levels (Fig. 6E, p = 0.0249, vs. hNCS, n=3, paired two-tailed t-test).
Figure 6.
ELISA analyses of human and rat CS (hCS, rCS) and NCS (hNCS, rNCS). (A-F) Human concentrations of cytokines and resolvins in hCS and control serum (hNCS). (A) IL-1Ra levels in hCS and hNCS. (B) TIMP-1 levels in hCS and hNCS. (C,E) Standard curves of RvD1 and RvD2 ELISA. (D,F) RvD1 and RvD2 levels in hCS and hNCS. *p<0.05, ***p<0.001; paired student’s t-est. n=3 samples from two healthy male volunteers (blue) and one healthy female donor (red). (G, H) Rat serum concentrations of TGF-β1 (G) and TIMP-1 (H) in rCS and control serum (rNCS). *p<0.05, ****p<0.0001; n=8 (rNCS), n=8 (rCS), unpaired student’s t-test.
We also used commercial ELISA kits to measure TGF-β1 and TIMP1 levels in rCS and rNCS samples and found significant increases in levels of both TGF-β1 (p<0.0001, rCS vs. rNCS, n=5, Fig. 6G) and TIMP (p<0.05, rCS vs. rNCS, n=5, Fig. 6H). Collectively, these results suggest that an increase in anti-inflammatory and pro-resolving mediators in hCS and rCS may be responsible for the rapid analgesic effects of the intrathecal CS treatment.
Because our earlier research showed that MSC-induced relief of neuropathic pain is mediated by TGF-β1 (Chen et al., 2015), we tested the effect of intrathecal injection of anti-TGFβ1 neutralizing antibody, given 7 days after the rCS treatment. Compared to control IgG, TGF-β1 neutralizing antibody significantly but transiently decreased PWT at 1 hour (p<0.05, (Fig. 7A). TGFβ1 antibody also significantly increased PWF at 1 hour (p<0.01, Fig. 7B). Furthermore, we found IL-Ira neutralizing antibody transiently reversed the anti-allodynic effect of the rCS in CIPN mice (Fig. 7C, D). These data suggest that CS-induced acute pain relief is mediated by TGF-β1 and IL-Ira.
Figure 7.
Anti-TGF-β1 and anti-IL1ra neutralizing antibodies transiently reverses the anti-allodynic effects of rCS in mice. (A, B) Von Frey tests for PWT (A) and PWF (B) showing a temporary reversal of hCS-induced analgesia at 1 hour post-injection with 4 μg of anti-TGF-β1 antibody. (C, D) Von Frey tests for PWT (C) and PWF (D) showing a temporary reversal of rCS-induced analgesia at 1-3 h post-injection with 4 μg of anti-IL-1ra antibody. Intrathecally-injected IgG was included as control. *p<0.05, **p<0.01, ***, p<0.001, n=5 per group, two-way ANOVA, followed by Bonferroni posthoc test. Male donors and receivers were tested.
3.4. Intrathecal or intraplantar administration of mouse CS inhibits neuropathic and postoperative pain in mice of both sexes
We prepared mouse CS (mCS) and mNCS in a parallel manner to the hCS processing with the use of volume-reduced processing equipment (approximately 1.5 ml of mCS and mNCS were acquired from 5 naive mice respectively). We first conducted mCS treatment one week after SNI in both male and female mice. We found that i.t. mCS injection (10 μl) significantly reduced SNI-induced mechanical allodynia (increasing PWT and decreasing PWF) and cold allodynia in male mice, female mice, and males and females combined (Supplementary Fig. 4A-I). In addition to neuropathic pain models, we also tested the analgesic effect of mCS in a mouse model of postoperative pain by plantar incision. mCS (10 μl) was administrated to the incision site immediately after paw incision. Intraplantar mCS treatment prevented the development of postoperative pain by increasing PWT and decreasing PWF in male mice, female mice, and males and females combined (Supplementary Fig. 5A-F). Finally, we prepared mCS and mNCS from female mice and found that intrathecal injection of female mCS significantly inhibited paclitaxel-induced mechanical allodynia by increasing PWT and decreasing PWF in males, females, and males and females combined (Supplementary Fig. 6A-F). Together, these results suggest that CS is an effective pain therapy for cross-sex treatment.
3.5. Secreted small vesicles/exosomes are increased in CS
To investigate the mechanism underlying the ACS’ long-term pain relief, we measured small vesicles/exosomes in rCS and rNCS. Exosomes have historically been difficult to quantify due to their small sizes. Recently, however, nanoparticle tracking analysis (NTA) has been successfully employed to define the physical characteristics and concentrations of these small vesicles (McNicholas and Michael, 2017). We used NTA with NanoSight (Fig. 8A) to quantify exosome concentrations in rCS and rNCS (control serum) from each of five rats. We observed similar particle sizes of rCS and rNCS, ranging from 80-200 nm (Fig. 8B). This range of small vesicles is indicative of exosomes, as 30-150 nm extracellular vesicles (Jean-Toussaint et al., 2020). Intriguingly, the concentration of particles was increased in all the rCS samples (Fig. 8B). We found ~2-fold increase in particle concentration in 24-hour rCS compared to rNCS (p<0.001, One-Way ANOVA, Fig. 8C, D).
3.6. Secreted small vesicles/exosomes are essential for the analgesic actions of CS
To determine the role of small vesicles/exosomes in CS for the resolution of pain, we depleted serum exosomes by ultracentrifugation at 100,000 rpm for 2 x 90 min (Fig. 9A). This method effectively removed 95% of small vesicles in ACS (Fig. 9C, D). Next, we compared the analgesic effects of rCS, ultracentrifuged rCS (U-rCS), and ultracentrifuged rNCS (U-rNCS). We found that the analgesic actions of rCS were partially lost in animals treated with U-CS (Fig. 9B,C). PWT testing showed significant differences between rCS and U-CS groups at all the time points we tested, including 1, 3, 5, 14, and 28 days (p<0.0001) after the treatment (Fig. 9B). PWF testing also showed significant differences between U-CS and U-NCS groups in these time points (Fig. 9C), including 1, 3, 5, 14, and 28 days (p<0.0001) after the rCS treatment. Exosome-depleted rCS was still able to produce mild and transient pain relief, but its analgesic efficacy was greatly diminished (Fig. 9B,C). Collectively, these findings suggest that exosomes are critically required for sustained pain relief by CS.
Figure 9.
Effects of ultracentrifugation on rCS-induced analgesia in CIPN mice. (A) Schematic of ultracentrifugation (100,000 x g, 120 min, 4°C). (B, C) Von Frey tests for paw withdrawal threshold (B) and withdrawal frequency (C). Ultracentrifuged rCS (U-rCS) showed significantly decreased analgesic effects versus ACS for all measured timepoints up to 28 days following intrathecal injection in CIPN mice. Intrathecally injected ultracentrifuged rNCS (U-rNCS) was used as control. *p<0.05, **p<0.01, ****p<0.001, **p<0.0001, n=5 per group, two-way ANOVA, followed by Bonferroni posthoc test. Male donors and receivers were tested.
4. Discussion
To our knowledge, this is the first pre-clinical study to investigate the analgesic and neurologic response after the cross-species and cross-sex application of conditioned serum. We reported several interesting findings in this study. First, cross-species transfer of hCS, rCS, mCS via a single intrathecal injection resulted in profound inhibition of chemotherapy-induced mechanical allodynia in mice, without impairing motor function. Intrathecal rCS or mCS treatment was also effective in reducing SNI-induced neuropathic pain in rats and mice. By contrast, the same intrathecal injection of human or rodent non-conditioned serum (NCS) had no effects on mechanical pain in the rodent CIPN and SNI models, implying that the analgesic effects of the serum are acquired in the conditioning process. We did not see noticeable sex differences in CS treatment; both sexes showed significant responses to hCS, rCS, and mCS. Strikingly, the analgesic effects of hCS and rCS could sustain for several weeks. Second, rCS treatment significantly inhibited the chemotherapy-induced glial reaction (GFAP and IBA1 upregulations) in the spinal cord dorsal horn and improved the chemotherapy-evoked slowdown of nerve conduction velocity in mice, suggesting that CS may ameliorate neuroinflammatory changes and improve neurologic function in CIPN. Third, rCS contains not only greater concentrations of anti-inflammatory and pro-resolving mediators, but it also holds significantly larger numbers of exosomes than NCS. Finally, the depletion of exosomes from rCS by high-speed centrifuge largely abolished the analgesic actions of rCS in mice, indicating a significant functional contribution of the exosomes in CS.
It was generally believed that ACS relieves pain via augmented concentrations of anti-inflammatory cytokines (e.g., IL-1Ra, IL-4, IL-10, TGFβ1) and growth factors (e.g., HGF) (Evans et al., 2016). Consistent with prior work, we demonstrated significant increases in the concentrations of IL-1Ra in in hCS samples. Functionally, intrathecal administration of anti-TGF-β1 neutralizing antibody transiently reversed CS-induced analgesia. We also observed that hCS and rCS had higher concentrations of TIMP1. As an endogenous inhibitor of MMP-9, TIMP1 has been shown to potently suppress nerve injury-induced neuropathic pain in rodents (Kawasaki et al., 2008). Furthermore, the CS coagulation process produced higher concentrations of SPMs, including resolvins D1 and D2 (Norris et al., 2017). Mounting evidence suggests that RvD1 and RvD2 potently inhibit inflammatory and neuropathic pain via modulation of inflammation, glial activation, and ion channels such as TRPA1 and TRPV1 (Ji et al., 2022). We also found that rCS had significantly higher TGFβ1 levels than rNCS and that the intrathecal administration of anti-TGF-β1 neutralizing antibody transiently reversed CS-induced analgesia. Thus, TGF-β1 signaling, which is involved in the analgesic benefits of MSCs (Chen et al., 2005), also appears to play a role in ACS analgesia. Collectively, it appears that these anti-inflammatory and pro-resolving mediators play a role in ACS-mediated analgesia in animal models of neuropathy.
However, it is important to point out that a single administration of these anti-inflammatory and pro-resolving mediators may only produce short-term pain relief for hours to days (Chen et al., 2015). The actions of these mediators alone are insufficient to account for the long-lasting analgesic effectiveness of CS in animals with neuropathic pain. Our animal data is consistent with human clinical studies where CS has demonstrated significant improvements in pain and longer-term superiority to corticosteroid injection when tested in the treatment of lumbar and cervical radiculopathy (Becker et al., 2007; Goni et al., 2015). The potential therapeutic role of CS-derived exosomes may explain some of the prolonged analgesic responses in the treatment of neuropathy. Our results show that ultracentrifugation depletes small vesicles and exosomes while leaving soluble growth factors and cytokines, allowing us to distinguish the responses of these various factors in the treatment of CIPN. The drastic reduction in analgesic response with the use of ultracentrifuged ACS implies a significant therapeutic effect from exosomes and small particles.
Secretome-based therapies such as ACS remain an attractive option for the treatment of degenerative orthopedic and neurologic conditions. Secretomes contain exosomes, growth factors, cytokines, lipids, nucleic acids, antioxidants, and are largely devoid of immunogenic MHC antigens found in cell-based therapies (Harrell et al., 2019). The CS secretome, likely through multiple mechanisms, has been shown to significantly reduce biomarkers of lipid peroxidation and joint inflammation when compared with PRP for the treatment of osteoarthritis (Shirokova et al., 2020). This reduction in reactive oxygen species may also play a role in the improvement of neuropathy pain in our animal models. Exosomes, nanovesicles with a diameter ranging from approximately 30-150 nm, are part of the secretome of several biologic therapies and are known to play a role in the analgesic effects of MSCs (Shiue et al., 2019). Secretomes have been shown to reduce neuropathic pain in animal models of diabetic neuropathy (Brini et al., 2017) and to promote nerve regeneration after injury (Sumarwoto et al., 2021). Their cell-free nature allows for sterile filtration, potentially reducing contamination risks (Laggner et al., 2020). Our results support the important role of the exosomal component of the CS secretome in the reversal of neuropathic pain.
Exosome release is modulated quantitatively and qualitatively by cellular stress, like the one that occurs during CS processing (Yang and Robbins, 2012). These small vesicles contain various miRNAs such as miR-21, miRNA-186, miR-146a, miR-146b, miR-146a-5p, miR-124, miR-124a, miR-let-7, and miR-30c-5p, and miR-544-3p, known to regulate the expression of ion channels and cytokines and contribute to neural and glial responses to injury (Gallo et al., 2012; Kalluri and LeBleu, 2020; Park et al., 2014; Recchiuti et al., 2011; Simeoli et al., 2017; Tramullas et al., 2018). Exosomal miRNA is able to suppress cartilage degradation by inhibiting MMP activity and promote cartilage growth through increased production of collagen proteins (Mao et al., 2018). In the treatment of diabetic neuropathy, exosome-based treatments have been shown to increase myelin and axonal thickness and improve nerve conduction velocity in mice (Fan et al., 2020). Our data support an important exosomal role in the prolonged analgesic effect of CS. It will be of great interest to investigate exosome profiles in ACS and NIS and further define the molecular pathways involved in analgesic and anabolic tissue responses.
In summary, our research provides evidence that CS, a whole blood secretome, potently attenuates neuropathic pain in animal models of CIPN. Further, the prolonged analgesic effectiveness of CS is largely abolished by the ultracentrifugation of non-soluble factors such as exosomes, supporting the critical role of these small vesicles in the long-term benefits of this therapy. It is also notable that the improvements in animal pain behavior were associated with cellular changes (inhibition of glial reactivity) and functional recovery (increase in conduction velocity). These findings demonstrate that CS treatment may not only resolve chronic pain but also improve neuroinflammation and neural function in CIPN. Future studies are required to further define the exosomal, epigenetic, and analgesic impacts in different animal models, administration routes, and tissue types.
Supplementary Material
Acknowledgments:
This work was partially supported by Duke University Anesthesiology Research Fund, NIH grant R01 grant DE17794, and DoD grants W81XWH2110756 and W81XWH2110885.
Footnotes
Conflict of interest statement: Authors TB, YH, AB, SB, JX, YM, RG, TH, and RRJ have no competing financial interest in this study. Authors JR and PW are employed by Orthogen AG.
References
- Aghamohammadi D, Sharifi S, Shakouri SK, Eslampour Y, Dolatkhah N, 2022. Autologous conditioned serum (Orthokine) injection for treatment of classical trigeminal neuralgia: results of a single-center case series. J Med Case Rep 16, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltzer AWA, Moser C, Jansen SA, Krauspe R, 2009. Autologous conditioned serum (Orthokine) is an effective treatment for knee osteoarthritis. Osteoarthritis Cartilage 17, 152-160–160. [DOI] [PubMed] [Google Scholar]
- Becker C, Heidersdorf S, Drewlo S, Rodriguez S.Z.d., Krämer J, Willburger RE, 2007. Efficacy of Epidural Perineural Injections With Autologous Conditioned Serum for Lumbar Radicular Compression. Spine (Phila Pa 1976) 32, 1803-1808–1808. [DOI] [PubMed] [Google Scholar]
- Bennell KL, Paterson KL, Metcalf BR, Duong V, Eyles J, Kasza J, Wang Y, Cicuttini F, Buchbinder R, Forbes A, Harris A, Yu SP, Connell D, Linklater J, Wang BH, Oo WM, Hunter DJ, 2021. Effect of Intra-articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis: The RESTORE Randomized Clinical Trial. JAMA 326, 2021–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukelmoune N, Laumet G, Tang Y, Ma J, Mahant I, Singh SK, Nijboer C, Benders M, Kavelaars A, Heijnen CJ, 2021. Nasal administration of mesenchymal stem cells reverses chemotherapy-induced peripheral neuropathy in mice. Brain Behav Immun 93, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan TJ, Vandermeulen EP, Gebhart GF, 1996. Characterization of a rat model of incisional pain. Pain 64, 493–501. [DOI] [PubMed] [Google Scholar]
- Brini AT, Amodeo G, Ferreira LM, Milani A, Niada S, Moschetti G, Franchi S, Borsani E, Rodella LF, Panerai AE, Sacerdote P, 2017. Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain. Sci. Rep 7, 9904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchheit T, Huh Y, Maixner W, Cheng J, Ji RR, 2020. Neuroimmune modulation of pain and regenerative pain medicine. J Clin Invest 130, 2164–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchheit T, Hunt C, Eldrige J, Eshraghi Y, Souza D, 2022. Product characteristics should be reported in all biological therapy publications. Reg. Anesth. Pain Med [DOI] [PubMed] [Google Scholar]
- Chang W, Berta T, Kim YH, Lee S, Lee SY, Ji RR, 2018. Expression and Role of Voltage-Gated Sodium Channels in Human Dorsal Root Ganglion Neurons with Special Focus on Nav1.7, Species Differences, and Regulation by Paclitaxel. Neurosci Bull 34, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Park CK, Xie RG, Ji RR, 2015. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-beta secretion. J Clin.Invest 125, 3226–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, Loeuille D, Kivitz AJ, Silver D, Appleton BE, 2009. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 61, 344–352. [DOI] [PubMed] [Google Scholar]
- Chiang CY, Sessle BJ, Dostrovsky JO, 2012. Role of Astrocytes in Pain. Neurochem. Res [DOI] [PubMed] [Google Scholar]
- Damjanov N, Barac B, Colic J, Stevanovic V, Zekovic A, Tulic G, 2018. The efficacy and safety of autologous conditioned serum (ACS) injections compared with betamethasone and placebo injections in the treatment of chronic shoulder joint pain due to supraspinatus tendinopathy: a prospective, randomized, double-blind, controlled study. Med. Ultrason 20, 335–341. [DOI] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ, 2000. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87, 149–158. [DOI] [PubMed] [Google Scholar]
- Evans CH, Chevalier X, Wehling P, 2016. Autologous Conditioned Serum. Phys. Med. Rehabil. Clin. N. Am 27, 893-908–908. [DOI] [PubMed] [Google Scholar]
- Fan B, Li C, Szalad A, Wang L, Pan W, Zhang R, Chopp M, Zhang ZG, Liu XS, 2020. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia 63, 431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo A, Tandon M, Alevizos I, Illei GG, 2012. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One 7, e30679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni VG, Singh Jhala S, Gopinathan NR, Behera P, Batra YK, R HHA, Guled U, Vardhan H, 2015. Efficacy of Epidural Perineural Injection of Autologous Conditioned Serum in Unilateral Cervical Radiculopathy: A Pilot Study. Spine (Phila Pa 1976) 40, E915–921. [DOI] [PubMed] [Google Scholar]
- Grisold W, Cavaletti G, Windebank AJ, 2012. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol. 14 Suppl 4, iv45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wang H, Zou S, Gu M, Watanabe M, Wei F, Dubner R, Huang GT, Ren K, 2011. Bone marrow stromal cells produce long-term pain relief in rat models of persistent pain. Stem Cells 29, 1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack JE, Michalak JC, Loprinzi CL, Sloan JA, Novotny PJ, Soori GS, Tirona MT, Rowland KM Jr., Stella PJ, Johnson JA, 2002. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain 98, 195–203. [DOI] [PubMed] [Google Scholar]
- Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V, 2019. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LY, Zhou Y, Cui WQ, Hu XM, Du LX, Mi WL, Chu YX, Wu GC, Wang YQ, Mao-Ying QL, 2018. Triggering receptor expressed on myeloid cells 2 (TREM2) dependent microglial activation promotes cisplatin-induced peripheral neuropathy in mice. Brain Behav Immun 68, 132–145. [DOI] [PubMed] [Google Scholar]
- Huh Y, Ji R-R, Chen G, 2017. Neuroinflammation, Bone Marrow Stem Cells, and Chronic Pain. Front. Immunol 8, 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Toussaint R, Tian Y, Chaudhuri AD, Haughey NJ, Sacan A, Ajit SK, 2020. Proteome characterization of small extracellular vesicles from spared nerve injury model of neuropathic pain. J Proteomics 211, 103540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, 202. Specialized Pro-Resolving Mediators as Resolution Pharmacology for the Control of Pain and Itch Annual Review of Pharmacology and Toxicology In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Berta T, Nedergaard M, 2013. Glia and pain: Is chronic pain a gliopathy? Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, LeBleu VS, 2020. The biology, function, and biomedical applications of exosomes. Science 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautio AL, Haanpää M, Leminen A, Kalso E, Kautiainen H, Saarto T, 2009. Amitriptyline in the prevention of chemotherapy-induced neuropathic symptoms. Anticancer Res. 29, 2601–2606. [PubMed] [Google Scholar]
- Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR, 2008. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat.Med 14, 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukowski K, Eijkelkamp N, Laumet G, Hack CE, Li Y, Dougherty PM, Heijnen CJ, Kavelaars A, 2016. CD8+ T Cells and Endogenous IL-10 Are Required for Resolution of Chemotherapy-Induced Neuropathic Pain. J Neurosci 36, 11074–11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laggner M, Gugerell A, Bachmann C, Hofbauer H, Vorstandlechner V, Seibold M, Gouya Lechner G, Peterbauer A, Madlener S, Demyanets S, Sorgenfrey D, Ostler T, Erb M, Mildner M, Ankersmit HJ, 2020. Reproducibility of GMP-compliant production of therapeutic stressed peripheral blood mononuclear cell-derived secretomes, a novel class of biological medicinal products. Stem Cell. Res. Ther 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, North RY, Rhines LD, Tatsui CE, Rao G, Edwards DD, Cassidy RM, Harrison DS, Johansson CA, Zhang H, Dougherty PM, 2018. DRG Voltage-Gated Sodium Channel 1.7 Is Upregulated in Paclitaxel-Induced Neuropathy in Rats and in Humans with Neuropathic Pain. J Neurosci 38, 1124–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Hua Z, Shen J, Yin Y, Yang J, Cheng K, Liu A, Wang L, Cheng J, 2017. Comparative Efficacy of Multiple Variables of Mesenchymal Stem Cell Transplantation for the Treatment of Neuropathic Pain in Rats. Mil Med 182, 175–184. [DOI] [PubMed] [Google Scholar]
- Luo X, Chen O, Wang Z, Bang S, Ji J, Lee SH, Huh Y, Furutani K, He Q, Tao X, Ko MC, Bortsov A, Donnelly CR, Chen Y, Nackley A, Berta T, Ji RR, 2021. IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron 109, 2691–2706 e2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Fitzsimmons B, Mohan A, Zhang L, Terrando N, Kordasiewicz H, Ji RR, 2018. Intrathecal administration of antisense oligonucleotide against p38alpha but not p38beta MAP kinase isoform reduces neuropathic and postoperative pain and TLR4-induced pain in male mice. Brain Behav Immun 72, 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Huh Y, Bang S, He Q, Zhang L, Matsuda M, Ji RR, 2019. Macrophage Toll-like Receptor 9 Contributes to Chemotherapy-Induced Neuropathic Pain in Male Mice. J Neurosci 39, 6848–6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G, Zhang Z, Hu S, Zhang Z, Chang Z, Huang Z, Liao W, Kang Y, 2018. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell. Res. Ther 9, 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Oh-Hashi K, Yokota I, Sawa T, Amaya F, 2017. Acquired Exchange Protein Directly Activated by Cyclic Adenosine Monophosphate Activity Induced by p38 Mitogen-activated Protein Kinase in Primary Afferent Neurons Contributes to Sustaining Postincisional Nociception. Anesthesiology 126, 150–162. [DOI] [PubMed] [Google Scholar]
- McNicholas K, Michael MZ, 2017. Immuno-characterization of Exosomes Using Nanoparticle Tracking Analysis. Methods Mol Biol 1545, 35–42. [DOI] [PubMed] [Google Scholar]
- Meijer H, Reinecke J, Becker C, Tholen G, Wehling P, 2003a. The production of anti-inflammatory cytokines in whole blood by physico-chemical induction. Inflamm. Res 52, 404-407–407. [DOI] [PubMed] [Google Scholar]
- Meijer H, Reinecke J, Becker C, Tholen G, Wehling P, 2003b. The production of anti-inflammatory cytokines in whole blood by physico-chemical induction. Inflamm Res 52, 404–407. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR, 2009. Pathological and protective roles of glia in chronic pain. Nat.Rev.Neurosci 10, 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris PC, Libreros S, Chiang N, Serhan CN, 2017. A cluster of immunoresolvents links coagulation to innate host defense in human blood. Sci Signal 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachman DR, Barton DL, Watson JC, Loprinzi CL, 2011. Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clin Pharmacol Ther 90, 377–387. [DOI] [PubMed] [Google Scholar]
- Park CK, Xu ZZ, Berta T, Han Q, Chen G, Liu XJ, Ji RR, 2014. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 82, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R, Smith D, Franklin G, Gronseth G, Pignone M, David WS, Armon C, Perkins BA, Bril V, Rae-Grant A, Halperin J, Licking N, O'Brien MD, Wessels SR, MacGregor LC, Fink K, Harkless LB, Colbert L, Callaghan BC, 2022. Oral and Topical Treatment of Painful Diabetic Polyneuropathy: Practice Guideline Update Summary: Report of the AAN Guideline Subcommittee. Neurology 98, 31–43. [DOI] [PubMed] [Google Scholar]
- Rao RD, Michalak JC, Sloan JA, Loprinzi CL, Soori GS, Nikcevich DA, Warner DO, Novotny P, Kutteh LA, Wong GY, 2007. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3). Cancer 110, 2110–2118. [DOI] [PubMed] [Google Scholar]
- Recchiuti A, Krishnamoorthy S, Fredman G, Chiang N, Serhan CN, 2011. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J. 25, 544–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Dubner R, 2010. Interactions between the immune and nervous systems in pain. Nat.Med 16, 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CR, Zhang H, Dougherty PM, 2014. Astrocytes, but not microglia, are activated in oxaliplatin and bortezomib-induced peripheral neuropathy in the rat. Neuroscience 274, 308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Stevens B, 2017. Microglia emerge as central players in brain disease. Nat Med 23, 1018–1027. [DOI] [PubMed] [Google Scholar]
- Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M, 2014. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 155, 2461–2470. [DOI] [PubMed] [Google Scholar]
- Serhan CN, 2014. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokova L, Noskov S, Gorokhova V, Reinecke J, Shirokova K, 2020. Intra-Articular Injections of a Whole Blood Clot Secretome, Autologous Conditioned Serum, Have Superior Clinical and Biochemical Efficacy Over Platelet-Rich Plasma and Induce Rejuvenation-Associated Changes of Joint Metabolism: A Prospective, Controlled Open-Label Clinical Study in Chronic Knee Osteoarthritis. Rejuvenation Res 23, 401–410. [DOI] [PubMed] [Google Scholar]
- Shiue SJ, Rau RH, Shiue HS, Hung YW, Li ZX, Yang KD, Cheng JK, 2019. Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury-induced pain in rats. Pain 160, 210–223. [DOI] [PubMed] [Google Scholar]
- Simeoli R, Montague K, Jones HR, Castaldi L, Chambers D, Kelleher JH, Vacca V, Pitcher T, Grist J, Al-Ahdal H, Wong LF, Perretti M, Lai J, Mouritzen P, Heppenstall P, Malcangio M, 2017. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat Commun 8, 1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisignano M, Baron R, Scholich K, Geisslinger G, 2014. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol 10, 694–707. [DOI] [PubMed] [Google Scholar]
- Sommer C, Kress M, 2004. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci.Lett 361, 184–187. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS, 2015. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat.Neurosci 18, 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumarwoto T, Suroto H, Mahyudin F, Utomo DN, Romaniyanto, Tinduh D, Notobroto HB, Sigit Prakoeswa CR, Rantam FA, Rhatomy S, 2021. Role of adipose mesenchymal stem cells and secretome in peripheral nerve regeneration. Ann Med Surg (Lond) 67, 102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Luo X, Zhang T, Hershey B, Esteller R, Ji RR, 2021. Spinal Cord Stimulation Attenuates Mechanical Allodynia and Increases Central Resolvin D1 Levels in Rats With Spared Nerve Injury. Front Physiol 12, 687046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taves S, Berta T, Liu DL, Gan S, Chen G, Kim YH, Van de Ven T, Laufer S, Ji RR, 2016. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav Immun 55, 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramullas M, Frances R, de la Fuente R, Velategui S, Carcelen M, Garcia R, Llorca J, Hurle MA, 2018. MicroRNA-30c-5p modulates neuropathic pain in rodents. Sci Transl Med 10. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF, 2001. Glial activation: a driving force for pathological pain. Trends Neurosci. 24, 450–455. [DOI] [PubMed] [Google Scholar]
- Yang C, Robbins PD, 2012. Immunosuppressive exosomes: a new approach for treating arthritis. Int. J. Rheumatol 2012, 573528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li Y, de Carvalho-Barbosa M, Kavelaars A, Heijnen CJ, Albrecht PJ, Dougherty PM, 2016. Dorsal Root Ganglion Infiltration by Macrophages Contributes to Paclitaxel Chemotherapy-Induced Peripheral Neuropathy. J Pain 17, 775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.