Abstract
Hispanic/Latino adults are a growing segment of the older U.S. population yet are underrepresented in brain aging research. We aimed to characterize brain aging among diverse Hispanic/Latino individuals. Hispanic/Latino individuals (unweighted n = 2273 ages 35–85 years; 56% female) from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) population-based study underwent magnetic resonance imaging (MRI) as part of the SOL- Investigation of Neurocognitive Aging MRI (SOL-INCA-MRI) ancillary study (2018–2022). We performed linear regressions to calculate age associations with brain volumes for each outcome (total (global) brain, hippocampal, lateral ventricle, total white matter hyperintensity (WMH), individual cortical lobar, and total cortical gray matter) and tested modification by sex. Older age was associated with smaller gray matter volumes and larger lateral ventricle and WMH volumes. Age-related differences in global brain volumes and gray matter volumes in specific regions (i.e., the hippocampus and temporal and occipital lobes) were less pronounced among women. Our findings warrant further investigation into sex-specific mechanisms of brain aging using longitudinal studies.
Keywords: Brain, Aging, Hispanics, Latinos, Brain Volumes, White Matter Hyperintensities
1. Introduction
Hispanic/Latino individuals accounted for over 50% of the United States population growth in the past 10 years (Jones et al., 2021). Despite this growth across all age groups, little is known about the aging Hispanic/Latino brain. Therefore, the Study of Latinos-Investigation of Neurocognitive Aging (SOL-INCA) and SOL-INCA Magnetic Resonance Imaging (SOL-INCA-MRI) were launched to identify biological underpinnings of healthy and neurodegenerative brain aging.
Our current understanding of brain aging is largely based on non-Hispanic/Latino White samples. Existing evidence, albeit mixed, largely points to differences in brain structure between non-Hispanic/Latino White and Hispanic/Latino adults (Brickman et al., 2008; Burke et al., 2018; DeCarli et al., 2008; Minagar et al., 2000; O’Bryant et al., 2021). For example, white matter hyperintensities (WMHs), a measure of cerebrovascular disease, is greater among U.S. Caribbean Hispanic/Latino adults (Brickman et al., 2008; Zahodne et al., 2015) but similar or reduced in predominantly Mexican American samples (DeCarli et al., 2008; Mungas et al., 2009) when compared to non-Hispanic/Latino White individuals. More consistently, middle-aged and older Hispanic/Latino individuals have larger global brain volumes and/or smaller ventricle size compared to their non-Hispanic/Latino White counterparts which may indicate differences in underlying brain aging patterns (Brickman et al., 2008; Burke et al., 2018; DeCarli et al., 2008; Minagar et al., 2000; Stickel et al., 2021). Despite these distinctions, other factors, such as sex differences which tend to favor larger global brain volumes and region-specific morphometrics in women when adjusting for head size (Cowell et al., 1994; Driscoll et al., 2009; Geerlings et al., 2010; Raz et al., 2004a) remain relatively unexplored in the Hispanic/Latino aging brain (McKay et al., 2014; Prabhakaran et al., 2008).
Understanding age-related brain profiles in healthy middle-aged and older Hispanic/Latino adults will lead to increased precision in differential diagnosis of healthy versus neurodegenerative disease. Thus, we conducted a characterization of brain volumes among a diverse Hispanic/Latino U.S. population using data from the SOL-INCA-MRI. We predicted that older age would be associated with smaller global gray matter, lobar, and hippocampal volumes and larger lateral ventricle and white matter hyperintensity volumes. Further, we predicted that age-related differences in global and lobar cortical volumes would be exacerbated in men relative to women.
2. Material and methods
2.1. Data
The Hispanic Community Health Study/Study of Latinos (HCHC/SOL) is an ongoing, population-based prospective cohort study of diverse Hispanic/Latino adults representative of 4 targeted U.S. metropolitan areas (Bronx, NY; Chicago, IL; Miami, FL and San Diego, CA). At the baseline Visit 1 (2008–2011), HCHS/SOL enrolled n = 16,415 individuals 18–74 years of age. Detailed descriptions of the aims, scope, target population, and sampling design of the HCHS/SOL are published elsewhere (LaVange et al., 2010; Sorlie et al., 2010). The SOL-INCA (n = 6377; 2016–2018) is an HCHS/SOL ancillary study that evaluated the cognitive performance of individuals ages 50-years and older at HCHS/SOL Visit 2 who also underwent cognitive assessment at Visit 1 and returned for a second parent study visit. The parent HCHS-SOL study used complex survey sampling designs, so that estimates can be generalizable to the target Hispanic/Latino metropolitan populations. A detailed discussion of the SOL-INCA study and its design have been published elsewhere (González et al., 2019a).
SOL-INCA-MRI leverages the HCHS/SOL cohort and neurocognitive data from the SOL-INCA to examine brain health among the Hispanic/Latino population which is underserved and faces disparities in vascular risk factors. In particular, SOL-INCA-MRI seeks to examine brain health and the role of vascular risk factor burden on cerebrovascular pathology and Alzheimer’s disease risk using state of the science MRI techniques. Data collection is ongoing with an aim to collect brain MRIs on 2,800 participants. Approximately 2,400 participants will be recruited from SOL-INCA with participant selection enriched for individuals with cognitive impairment (González et al., 2019b) and the remaining cognitively healthy subjects randomly sampled with sex and field center matching to the participants with cognitive impairment. Briefly, respondents scoring below 1 SD on any of the cognitive test scores with complaints of decline in memory or thinking and no, or minimal functional impairment were considered impaired. Mild cognitive impairment status was based on National Institute on Aging and Diagnostic and Statistical Manual of Mental Disorders-5 criteria (American Psychiatric Association, 2013; González et al., 2019b; McKhann et al., 2011). Additionally, 400 younger (between 35 and 50 years of age at Visit 2) participants will be randomly selected from the parent HCHS/SOL study to achieve a lifespan perspective on Hispanic/Latino brain health. For the current study, we used all available data, as of April 10, 2022, including unweighted n = 2273 participants (weightings male = 46%, female = 54%), ages 35–85 years, who had completed MRI imaging and relevant image processing; thus, these analyses represent a preliminary report of age and sex associates with brain aging. Supplemental Table 1 shows descriptives for the present SOL-INCA MRI sample 50+ years and the SOL-INCA sample from which participants were recruited.
2.2. MRI acquisition and analysis
Brain images were collected using 3T MRI scanners [GE 3T 750 (3 sites) or Philips 3T Achieva TX (1 site)]. Sequences of interest for the current research included high-resolution T1-weighted structural images (1 mm3 ) and fluid-attenuated inversion recovery (3DFLAIR). All images were processed using analysis pipelines developed in the Imaging of Dementia and Aging (IDeA) laboratory at UC Davis. The process included a number of steps.
Removal of nonbrain tissues: The skull is removed using a convolutional neural net method (Fletcher et al., 2021) followed by human quality control to provide generally minor cleanup if needed. Structural MRI brain images are then nonlinearly registered performed by a cubic B-spline deformation (Rueckert et al., 2006) to a minimal deformation template (MDT) synthetic brain image (Kochunov et al., 2001) optimized for 60 years and older age range.
Image intensity inhomogeneity correction: We utilize a template-based iterative method for correcting field inhomogeneity bias (Fletcher et al., 2012a).
Gray, white and cerebrospinal fluid measurement: Image segmentation is based on an Expectation-Maximization (EM) algorithm that iteratively refines segmentation estimates to produce outputs that are most consistent with the input intensities from the native-space T1 images along with a model of image smoothness (Fletcher et al., 2012b; Rajapakse et al., 1996). Like all EM algorithms, the system must be initialized with a reasonable estimate. The initial estimate is produced from the template-space warps of previously segmented images; because locations of gray matter/white matter/cerebrospinal fluid tissues are known in the template space, transforming these masks back to each image’s native space produces rough estimate 3-tissue segmentations. We then calculate the mean and standard deviation of the image intensities in locations labeled as each tissue type. These values form the initial parameters for a Gaussian model of image intensity for each class. At each iteration, the algorithm uses a Gaussian model of T1-weighted image intensity for each tissue class to produce a segmentation. In the first iteration, these models are estimated as described above. The segmentation yielded by these appearance models alone is then refined using a Markov Random Field (MRF) model, a computational statistical method that efficiently produces a label map consistent with both the input intensities and image smoothness statistics. Inference in the MRF is computed using an adaptive priors model (Fletcher et al., 2012b). This refined segmentation from the MRF is then used to compute new Gaussian intensity models for each tissue class, and the algorithm repeats, iteratively switching between calculating Gaussian appearance models and MRF-based segmentation, until convergence. The MRF-based segmentation at the final iteration is used as the final output segmentation.
White matter hyperintensity (WMH): Assessment of WMH is performed on a combination of FLAIR and 3D T1 images using a modified Bayesian probability structure based on a previously published method of histogram fitting (DeCarli et al., 1999). Prior probability maps for WMH were created from more than 700 individuals with semi-automatic detection of WMH followed by manual editing. Likelihood estimates of the native image are calculated through histogram segmentation and thresholding. All segmentation is initially performed in standard space resulting in probability likelihood values of WMH at each voxel in the white matter. These probabilities are then thresholded at 3.5 standard deviations above the mean to create a binary WMH mask. Further segmentation is based on a modified Bayesian approach that combines image likelihood estimates, spatial priors and tissue class constraints. The segmented WMH masks are then back transformed on to native space for tissue volume calculation. Volumes are log-transformed to normalize population variance.
Automatic hippocampal segmentation: MRI-derived hippocampal volumetry has been a widely used biomarker in Alzheimer’s disease to improve early diagnosis (Frisoni et al., 2013), enrich subject selection (Lorenzi et al., 2010), and monitor treatment efficacy (Hampel et al., 2010; Hampel et al., 2011). To address this need, the EADC-ADNI Working Group established a Delphi panel to determine the optimum protocol (Boccardi et al., 2014a), selected orientation parameters (Boccardi et al., 2014b) and developed the final, rigorously tested protocol along with making publicly available labels from over 100 ADNI subjects (Bocchetta et al., 2014). Our hippocampal segmentation method employs a standard atlas based diffeomorphic approach (Vercauteren et al., 2007) with the minor modification of label refinement. We further modified this approach to include the EADC-ADNI harmonized hippocampal masks to assure standardization across cohorts.
Region of interest-based analysis: We use the Desikan-Killiany-Tourville Atlas (Klein and Tourville, 2012) to parcellate the cerebral cortex. Lobar volumes are created by mask fusion. Regional measures are calculated by back transformation of the atlas into segmented image native space. A voting scheme is used to assure precise labeling of each region after interpolation of the atlas into native space.
All MRI outcomes were residualized to total cranial volume. Standardized (z-scored) residualized values were used across models to facilitate interpretation across outcomes using a common scale. MRI outcomes of interest included total brain, hippocampal, lateral ventricle, total WMH, individual cortical lobar (frontal, parietal, temporal, occipital), and total cortical gray matter volumes. Lateral ventricle and total WMH measures were natural log transformed prior to residualization to normalize variance.
2.3. Primary exposures
Age (in years) at SOL-INCA-MRI and self-identified sex (male, female).
2.4. Covariables
Self-reported Hispanic/Latino heritage (Dominican, Central American, Cuban, Mexican, Puerto Rican, South American, Other/Mixed groups) and height (inches) at Visit 1.
2.5. Analytic strategy
First, we provided descriptive statistics for the SOL-INCA-MRI sample (Table 1). Second, we used linear regression models to separately model associations between age and sex with each brain outcome. In each case, we fit 2 models to determine crude and fully covariables adjusted associations (Tables 2A and B); see Supplemental Table 2 for raw brain volumes by age and stratified by sex). Age was modeled both as a linear (primary) and quadratic (Supplemental Tables 3A and B) exposures. To facilitate interpretation of these associations, we estimated and plotted average marginal estimates (AMEs; means/probabilities) and their 95% confidence intervals across the age range (35–85 years) for men and women. Third, we tested if sex modified the relationships between age and brain structure and visualized resulting significant interactions (Table 3, Fig. 1). Finally, we performed 2 sets of sensitivity analyses. The first set of sensitivity analyses, examined the associations between age and sex with brain outcomes (second step above) specific to participants who were cognitively healthy (i.e., individuals that did not meet criteria for mild cognitive impairment; Supplemental Tables 4, 5A and B) (González et al., 2019b). The second set of sensitivity analyses reproduced the second step (above) while excluding individuals who were identified to have infarct on MRI, given that men were more likely to have infarct than women. All results were weighted to account for the non-probability sampling design and survey regression techniques used to incorporate the stratification and clustering of observations and allow for appropriate inferences to the target population.
Table 1.
Descriptive statistics for Study of Latinos – Investigation of Neurocognitive Aging MRI target population
| Male | Female | Total | |||
|---|---|---|---|---|---|
| Unweighted N | 741 | 1541 | 2282 | ||
| % | 44% | 56% | 100.0 | ||
| Age (%; [SE]) | |||||
| <50 y | 19.9 (2.2) | 10.8 (1.1) | 14.8 (1.2) | p = 0.008 | |
| 50–59 y | 16.2 (l.7) | 18.6 (1.2) | 17.5 (1.0) | ||
| 60–69 y | 30.7 (2.4) | 33.0 (1.6) | 32.0 (1.4) | ||
| 70+ y | 33.2 (3.9) | 37.6 (2.1) | 35.7 (2.0) | ||
| Heritage (%; [SE]) | |||||
| Dominican | 5.7 (1.1) | 10.6 (1.3) | 8.4 (1.0) | p = 0.026 | |
| Central American | 6.7 (1.2) | 8.5 (1.1) | 7.7 (0.8) | ||
| Cuban | 29.3 (3.6) | 22.6 (2.5) | 25.6 (2.3) | ||
| Mexican | 33.0 (2.8) | 31.6 (2.2) | 32.3 (2.0) | ||
| Puerto-Rican | 16.5 (2.1) | 15.8 (1.5) | 16.1 (1.3) | ||
| South American | 4.3 (0.8) | 7.4 (1.2) | 6.0 (0.8) | ||
| Mixed/Other | 4.4 (1.4) | 3.4 (0.8) | 3.8 (0.8) | ||
| Birthplace (%; [SE]) | |||||
| Foreign/Island-born | 84.9 (2.1) | 91.2 (1.1) | 88.4 (1.1) | p = 0.002 | |
| U.S. born | 15.1 (2.1) | 8.8 (1.1) | 11.6 (1.1) | ||
| Education (%; [SE]) | |||||
| Less than High School | 30.1 (2.7) | 37.2 (2.1) | 34.1 (1.7) | p = 0.091 | |
| High School or Equivalent | 22.2 (2.3) | 19.0 (1.3) | 20.5 (1.3) | ||
| More than High School | 47.7 (3.3) | 43.7 (2.0) | 45.5 (1.9) | ||
| Field center (%; [SE]) | |||||
| Bronx | 24.3 (2.5) | 28.8 (2.0) | 26.8 (1.8) | p = 0.359 | |
| Chicago | 13.9 (1.5) | 12.0 (1.1) | 12.9 (1.1) | ||
| Miami | 37.9 (3.7) | 35.1 (2.8) | 36.3 (2.7) | ||
| San Diego | 23.9 (2.5) | 24.0 (2.3) | 24.0 (1.9) | ||
| Infarct on MRI (%; [SE]) | |||||
| No | 89.7 (1.9) | 93.8 (0.9) | 92.0 (1.0) | p = 0.032 | |
| Yes | 10.3 (1.9) | 6.2 (0.9) | 8.0 (1.0) | ||
| Baseline Framingham Risk Score (%; [SE]) | |||||
| Low | 31.8 (2.8) | 60.1 (2.1) | 47.7 (1.9) | p < 0.001 | |
| Medium | 33.3 (2.7) | 24.1 (1.9) | 28.2 (1.6) | ||
| High | 34.8 (3.7) | 15.8 (2.0) | 24.1 (2.1) | ||
| Age (Mean; [SD]) | 62.5 (10.5) | 64.9(11.9) | 63.8(11.5) | p = 0.013 | |
| Height (Mean; [SD]) | 169.6(5.6) | 156.0(6.7) | 162.0(9.2) | p < 0.001 |
Key: MRI, magnetic resonance imaging; SD, standard deviation; SE, standard error.
Table 2A.
Associations with brain volumes. Results are based on regression models. All outcomes are standardized values (z-scored) residualized for total cranial volume. Age treated linearly.
| Total brain volume Estimates [95% CI] |
Hippocampus Estimates [95% CI] |
Log lateral ventricle Estimates [95% CI] |
Log WMH Estimates [95% CI] |
|
|---|---|---|---|---|
| Female | ref | ref | ref | ref |
| Male | −0.186b [−0.326;−0.045] | 0.078 [−0.133;0.289] | 0.251c [0.107;0.395] | 0.159 [−0.004;0.321] |
| Age | −0.062c [−0.068;−0.057] | −0.022c [−0.028;−0.016] | 0.046c [0.040;0.053] | 0.043c [0.038;0.048] |
| Height | −0.004 [−0.012;0.004] | −0.003 [−0.013;0.007] | −0.003 [−0.012;0.007] | −0.006 [−0.014;0.002] |
| Dominican | ref | ref | ref | ref |
| Central American | −0.157 [−0.340;0.027] | 0.052 [−0.141;0.245] | −0.016 [−0.217;0.185] | −0.498c [−0.722;−0.274] |
| Cuban | −0.245b [−0.420;−0.071] | −0.042 [−0.263;0.179] | 0.086 [−0.115;0.287] | −0.434c [−0.623;−0.246] |
| Mexican | −0.193a [−0.350;−0.035] | 0.039 [−0.130;0.208] | −0.123 [−0.285;0.040] | −0.593c [−0.761;−0.425] |
| Puerto-Rican | −0.310b [−0.514;−0.106] | −0.172 [−0.377;0.034] | 0.190 [−0.013;0.393] | 0.118 [−0.072;0.307] |
| South American | −0.110 [−0.348;0.128] | −0.042 [−0.316;0.232] | −0.048 [−0.318;0.221] | −0.475c [−0.692;−0.258] |
| Mixed/Other | −0.105 [−0.581;0.371] | 0.414 [−0.042;0.871] | 0.142 [−0.147;0.431] | −0.074 [−0.352;0.203] |
| Intercept | 4.839c [3.407;6.272] | 1.838a [0.273;3.403] | −2.666b [−4.463;−0.869] | −1.477a [−2.799;−0.154] |
Key: CI, confidence interval; WMH, white matter hyperintensities.
= p < 0.05
= p < 0.01,
= p < 0.001
Table 2B.
Associations with brain volumes. Results are based on regression models. All outcomes are standardized values (z-scored) residualized for total cranial volume. Age treated linearly
| Frontal cortical gray Estimates [95% CI] |
Occipital cortical gray Estimates [95% CI] |
Temporal cortical gray Estimates [95% CI] |
Parietal cortical gray Estimates [95% CI] |
Combined gray Estimates [95% CI] |
|
|---|---|---|---|---|---|
| Female | ref | ref | ref | ref | ref |
| Male | −0.155 [−0.334;0.023] | 0.185a [0.031;0.339] | 0.016 [−0.148;0.181] | −0.084 [−0.250;0.081] | −0.047 [−0.201;0.106] |
| Age | −0.029c [−0.035;−0.022] | −0.021 c [−0.028;−0.014] | −0.043c [−0.048;−0.037] | −0.025c [−0.032;−0.018] | −0.041 c [−0.047;−0.034] |
| Height | −0.003 [−0.012;0.006] | −0.008 [−0.019;0.004] | 0.001 [−0.007;0.008] | −0.008 [−0.018;0.002] | −0.005 [−0.014;0.003] |
| Dominican | ref | ref | ref | ref | ref |
| Central American | −1.062c [−1.322;−0.803] | −0.629c [−0.893;−0.366] | −0.138 [−0.395;0.118] | −0.525c [−0.786;−0.264] | −0.899c [−1.174;−0.625] |
| Cuban | −1.267c [−1.481;−1.053] | −0.634c [−0.876;−0.392] | 0.038 [−0.200;0.276] | −0.777c [−1.011;−0.543] | −1.020c [−1.248;−0.792] |
| Mexican | −1.103c [−1.304;−0.902] | −0.765c [−0.979;−0.552] | −0.136 [−0.359;0.086] | −0.685c [−0.913;−0.457] | −1.002c [−1.222;−0.781] |
| Puerto-Rican | −0.457c [−0.698;−0.217] | −0.332b [−0.580;−0.085] | −0.180 [−0.422;0.062] | −0.336b [−0.578;−0.093] | −0.473c [−0.731;−0.216] |
| South American | −1.003c [−1.250;−0.757] | −0.561c [−0.821;−0.302] | −0.109 [−0.459;0.241] | −0.495c [−0.785;−0.205] | −0.833c [−1.121;−0.545] |
| Mixed/Other | −0.762a [−1.438;−0.085] | −0.537b [−0.923;−0.150] | −0.026 [−0.315;0.264] | −0.273 [−0.678;0.132] | −0.615a [−1.149;−0.081] |
| Intercept | 3.242c [1.802;4.682] | 3.077b [1.064;5.090] | 2.690c [1.446;3.934] | 3.426c [1.626;5.225] | 4.299c [2.803;5.795] |
Key: CI, confidence interval.
= p < 0.05
= p < 0.01,
= p < 0.001
Table 3.
Wald (F)-tests for main effects and sex interactions (with linear age)
| Total brain F (df) |
Hippocampus F (df) |
Log lateral ventricle F (df) |
Log white matter hyperintensities F (df) |
||
|---|---|---|---|---|---|
| Sex | 2.3[1532] | 1.61[1532] | 3.04[1532] | 0.04[1532] | |
| Age | 400.27[1532]d | 17.02[1532]d | 236.19[1532]d | 209.17[1532]d | |
| Height | 0[1532] | 0.05[1532] | 5.49[1532] b | 1.47[1532] | |
| Heritage | 2.12[6527]b | 2.64[6527]b | 2.86[6527]c | 10.65[6527]d | |
| Sexb Age | 11.4[1532] d | 4.48[1532] b | 1.3[1532] | 3.3[1532]a | |
| Sexb Height | 0.57[1532] | 0.47[1532] | 3.02[1532] | 0.13[1532] | |
| Sexb Heritage | 1.25[6527] | 1.9[6527] | 1.05[6527] | 1.15[6527] | |
| Frontal cortical gray F (df) |
Occipital cortical gray F (df) |
Temporal cortical gray F (df) |
Parietal cortical gray F (df) |
Combined gray F (df) |
|
| Sex | 0.39[1532] | 8.17[1532]c | 0.55[1532] | 4.28[1532]b | 5.93[1532]b |
| Age | 87.66[1532]d | 22.36[1532]d | 114.83[1532]d | 22.06[1532]d | 132.69[1532]d |
| Height | 0.04[1532] | 0.76[1532] | 0.23[1532] | 0.07[1532] | 0.16[1532] |
| Heritage | 31.69[6527]d | 7.59[6527]d | 2.01[6527]a | 8.46[6527]d | 20.43[6527d |
| Sexb Age | 0.01[1532] | 4.36[1532] b | 4.39[1532] b | 3.62[1532]a | 3.38[1532] a |
| Sexb Height | 0.18[l532] | 5.73[1532]b | 0[1532] | 3.24[1532] | 3.31[1,532] a |
| 2.23[6527]b | 1.54[6527] | 1.24[6527] | 1.13[6527] | 1.68[6527] |
Bold indicates significant effects of interest (i.e., Sex* Age)
Key: df = degrees of freedom.
= p < 0.07,
= p < 0.05,
= p < 0.01,
= p < 0.001
Fig. 1.
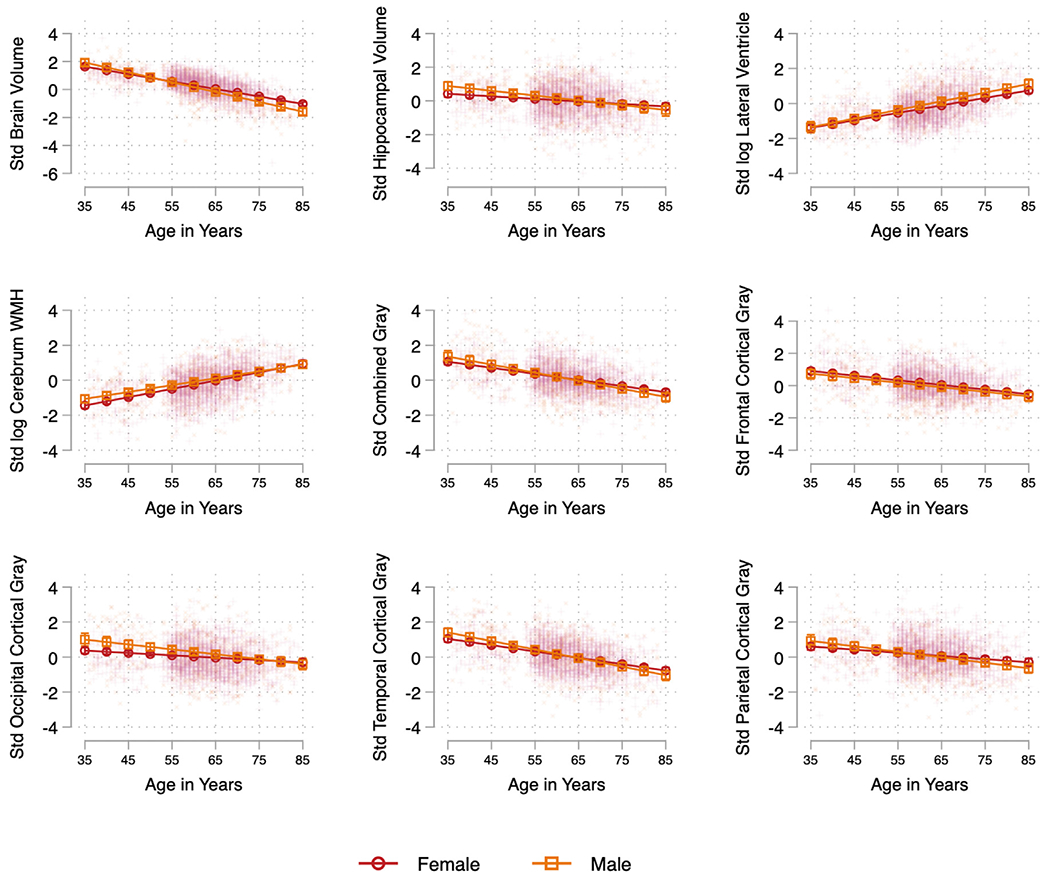
Linear associations between age and brain markers by sex, adjusted for Hispanic/Latino heritage and height. Models include interactions between sex and each of the model included covariates. Note: Covariates included self-reported Hispanic/Latino heritage (Dominican, Central American, Cuban, Mexican, Puerto Rican, South American, Other/Mixed groups) and height (inches) at Visit 1. Abbreviations: Std, standardized; WMH, white matter hyperintensities.
3. Results
3.1. Descriptives of the target population
Average age was 63.8 years ± 11.5 and 56% were women, over two-thirds had achieved high school or equivalent education or higher, 11.6% were U.S. Born, 24.1% met criteria for high risk on the Framingham Cardiovascular Risk Score, (D’agostino et al., 2008) and 8.0% had MRI confirmed infarct(s). Women were slightly older, shorter, and had lower cardiovascular disease risk (based on the Framingham Cardiovascular Risk Score; D’agostino et al., 2008) and lower prevalence of MRI-confirmed infarct(s) compared to males (Table 1).
3.2. Age-related differences
Adjusted for sex, height, and Hispanic/Latino heritage, we found significant and consistent linear associations between age and each of the brain volumes considered with evidence of linear decrements in total brain, hippocampal, and frontal, parietal, temporal, occipital, and combined cortical gray matter volumes with each additional year of age (Table 2A, B). Older age was associated with larger lateral ventricle and WMH volumes.
3.3. Sex differences
Adjusting for age, height, and Hispanic/Latino heritage, women had larger total brain volumes (β = 0.19 [0.33–0.04]; p < 0.01) and smaller lateral ventricle volumes (β = −0.25 [−0.11 to −0.39]; p < 0.001) compared to men. We also found evidence for smaller occipital gray matter volumes among women. Men and women did not differ in hippocampal, frontal, parietal, temporal, total cortical gray matter, or WMH volumes (Table 2A, and B).
3.4. Sex modifications in brain aging outcomes
Tests of interactions between sex and linear age suggested more pronounced age-related differences in brain structure among men relative to women. Sex modification effects were evident for total brain (p < 0.001) and hippocampal volumes (p < 0.05) as well as occipital (p < 0.05), temporal (p < 0.05), with a trend evident for parietal gray matter (p = 0.058) volumes but not frontal, total cortical gray volumes, lateral ventricle, or WMH volumes (Table 3, Fig. 1).
3.5. Quadratic effects of age
We found evidence for a curvilinear decrease in total brain volumes and an increase in lateral ventricle and WMH volumes as a function of age (Supplemental Table 3A and B). The curvilinear age change was particularly notable for total brain volume starting around age 55-years (Supplemental Fig. 1).
3.6. Cognitively healthy sensitivity analysis
In analyses limited to individuals who were cognitively healthy (i.e., did not meet criteria for mild cognitive impairment; Supplemental Table 4; González et al., 2019b), results largely followed the same patterns (Supplemental Table 5A and B). Notably, sex significantly modified the association between age with WMH volumes such that age-related differences in WMH volumes were more pronounced among women compared to men (Supplemental Table 6).
3.7. Infarct exclusion analysis
In analyses limited to individuals who did not have infarct on MRI, results largely followed the same patterns (Supplemental Tables 7–9).
4. Discussion
In this SOL-INCA-MRI preliminary report of 2273 diverse Hispanic/Latino adults ages 35-85 years, we found that older age was largely associated with smaller whole brain and regional volumes and larger lateral ventricle and white matter hyperintensity volumes. Sex differences in brain volumes were detected, even after adjusting for differences in head size, where women tended to have less pronounced age-related differences in some brain measures than men. Our study goes beyond ethnic comparisons of clinic-based samples to provide information from the largest sample of community-dwelling individuals of Hispanic/Latino heritage with neuroimaging data. Thus, our findings set the foundation to study both healthy cognitive aging and ADRD risk among diverse Hispanic/Latino adults. Given the substantial and growing older Hispanic/Latino population (Colby and Ortman, 2015), having detailed information on brain aging can inform normative brain data to compare and contrast to differences associated with vascular and degenerative diseases.
Consistent with existing literature (DeCarli et al., 2005; Fotenos et al., 2005), older age was associated with smaller global and lobar brain volumes among Hispanic/Latino individuals. Age-related atrophy in several brain regions has been confirmed in longitudinal studies of predominantly non-Hispanic/Latino White adults though patterns of volumetric decline vary by brain region (Driscoll et al., 2009; Pfefferbaum et al., 2013). Specifically, several studies have pointed to susceptibility to age-related atrophy in the frontal lobes and relative preservation in posterior regions, especially the occipital lobe (Cowell et al., 1994; DeCarli et al., 2005; Fjell et al., 2009; Jernigan et al., 2001; Pfefferbaum et al., 2013). In the present study, older age was associated with differences across all lobar cortices, but trends toward more pronounced aging differences (i.e., smaller volumes) at older ages were evident only in the parietal lobes. Curvilinear age-related changes in parietal lobe volumes were also detected among non-Hispanic/Latino White adults in the Framingham Heart Study (DeCarli et al., 2005). More pronounced aging differences in older age were evident for global brain volumes in both the present study and Framingham Heart Study, suggesting a cumulative pattern that emerges. Additionally, the patterns observed in the present study were maintained when excluding individuals with mild cognitive impairment, suggesting that these are robust patterns of normal aging.
The hippocampus has been shown to have greater age-related declines than other regions within (Foster et al., 2019; Raz et al., 2004b) and outside of the temporal lobe (Fjell et al., 2013; Jernigan et al., 2001), but other investigations, typically cross-sectional studies, have indicated minimal age-related differences in hippocampal volumes in middle-age (Mu et al., 1999) and even older adulthood (Brickman et al., 2008). Physically healthy samples may demonstrate subtle age-related differences in hippocampal or overall temporal lobe volumes that are then difficult to detect in small samples (DeCarli et al., 1994; Mu et al., 1999). Although Caribbean Hispanic/Latino adults sampled in the Washington Heights–Inwood Columbia Aging Project (Brickman et al., 2008) did not show age-related differences in hippocampal volumes, we observed smaller volumes with older age across our diverse sample of Hispanic/Latino individuals, that may reflect differences in methods of hippocampal measures, with WHICAP being manual traces of the anterior portion, whereas SOL-INCA-MRI analysis was atlas based and included the entire length of the hippocampus. Importantly, in the present study, the age-related differences in hippocampal volumes were observed when controlling for heritage, reducing the likelihood that population heterogeneity could explain these differences.
Sex differences in brain volumes exist among the Hispanic/Latino adults in our sample. Hispanic/Latina women had larger global brain volumes but smaller occipital gray matter volumes compared to men after controlling for head size. Additionally, women demonstrated more stability across the aging spectrum (i.e., less age-related differences) in global brain volumes and hippocampal, temporal, and occipital gray matter volumes compared to men. Thus, consistent with several cross-sectional and longitudinal studies (Brickman et al., 2008; Cowell et al., 1994; DeCarli et al., 2005; Driscoll et al., 2009; Geerlings et al., 2010; Raz et al., 2004a), we observed relative brain structure advantages among women compared to men, although the degree of sex differences was sometimes small (Fotenos et al., 2005). Small differences in brain aging within a cognitively healthy sample, as seen in the present study, could indicate differential risk for ADRD and preclinical stages of Alzheimer’s disease (Fotenos et al., 2005). Despite these seeming advantages, however, women tend to be at increased risk for ADRDs relative to men (Alzheimer’s Association, 2021) particularly among the oldest old (Mayeda et al., 2016). Animal studies suggest differential bioenergetic (metabolic) changes in aging by sex (e.g., in the hippocampus; Zhao et al., 2016), and risk factors for ADRD may differ by sex (Armstrong et al., 2019; Peterson and Tom, 2021). Therefore, our findings warrant longitudinal investigations into sex-specific risk factors for brain aging and ADRD among Hispanic/Latino adults which may manifest as the cohort ages.
In line with previous work, older age (Brickman et al., 2008; Fjell et al., 2009; Pfefferbaum et al., 2013) and male sex (DeCarli et al., 2005) were associated with larger lateral ventricle size, after controlling for head size. The lateral ventricle, and especially the inferior lateral ventricle, show faster increases with age relative to other ventricles (Fjell et al., 2009), suggesting more pronounced age-related atrophy in temporal regions of the brain. In particular, we detected increasingly pronounced associations between age and ventricle size with older age. Also consistent with previous findings (DeCarli et al., 2005), older age was linked to more pronounced WMH volumes possibly related to the accumulation of cardiovascular disease risk factors which are highly prevalent in this population (Daviglus et al., 2012). Although women in our sample had lower cardiovascular disease risk compared to men, we only detected sex differences in WMH volumes among our cognitively healthy subsample (data not shown). Specifically, women had lower WMH volumes compared to men at younger ages but similar WMH load in older ages. Our findings are similar to patterns found in other population-based cohorts (Brickman et al., 2008; DeCarli et al., 2005), suggesting greater susceptibility for WMH burden among women with aging (Sachdev et al., 2009). This may be due, in part, to men and women showing distinct vulnerability to specific risk factors for WMHs (e.g., smoking status, hypertension; Sachdev et al., 2009), and/or heritability for WMHs which may be slightly greater among women (Atwood et al., 2004). However, men have higher prevalence of other cerebrovascular risk markers (e.g., subclinical infarcts, cerebral microbleeds) than women (Graff-Radford et al., 2017; Prabhakaran et al., 2008). These sex-based considerations should be studied further within the diverse Hispanic/Latino community. Importantly, several modifiable risk factors (e.g., diet, physical activity, smoking status) may contribute to cardiovascular disease risk differences in heritage groups (Daviglus et al., 2012) which may then translate to differences in WMH volumes.
4.1. Strengths and limitations
Our results should be interpreted with some limitations in mind. First, despite having a substantial number of MRI analyzed scans from a representative sample of Hispanic/Latino individuals, our results are preliminary and continued data collection will further strengthen inference. Second, the data are cross-sectional and do not reflect atrophy or other brain changes such as incident vascular injury. Third, we used a broad lens to examine brain structure (i.e., focusing on global and lobar volumes) with slightly more specificity for indicators of Alzheimer’s pathology (i.e., hippocampal volumes, lateral ventricular volumes). Examining other specific brain markers (e.g., other region-specific volumes including prefrontal and/or precuneus regions or levels of coincident plasma amyloid and phosphorylated tau biomarkers) (Bayram et al., 2018) may also prove useful in further characterizing the aging brain. Fourth, we did not test the association of brain measures with cognitive outcomes although we did conduct a sensitivity analysis excluding those with mild cognitive impairment (Dong et al., 2015; Fletcher et al., 2018). Fifth, we did not test for modification by social determinants of health (e.g., access to insurance, literacy; Rodriguez et al., 2022; Sadhu et al., 2019) which may point to potential modifiable factors related to brain aging. Further work will more completely address these issues.
5. Conclusions
Based on data from a diverse cohort of Hispanic/Latino adults, we found cross-sectional evidence of age-related differences in brain structure among 2,273 individuals, ages 35-85 years. Older age was associated with smaller global and regional gray matter volumes and larger lateral ventricle and WMH volumes. Women had larger global brain yet smaller occipital gray matter volumes than men, and age-related differences in brain structure (i.e., global, hippocampal, temporal, and occipital volumes) were less pronounced among women. Given the relative paucity of brain aging information on Hispanic/Latino individuals, our work lays a foundation for researchers and clinicians to refer to when working with this population.
Supplementary Material
Acknowledgements
We thank our study staff and participants for their contributions to advancing scientific knowledge. Dr. Stickel and colleagues are supported by R01 AG048642, R56 AG048642 RF1 AG054548 and RF1 AG061022 (National Institute of Aging). Additional support includes K08 AG075351, L30 AG074401, and U54CA267789 to Dr. Stickel, R01AG067568 to Dr. Ramos, P30AG062429 to Dr. González, and P30AG010129 and P30AG072972 to Dr. DeCarli. The Hispanic Community Health Study/Study of Latinos was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, NIH Institution-Office of Dietary Supplements.
Footnotes
Verification
The present findings have not been published before nor is this work being considered for publication in another journal. All authors approved submission of this manuscript. If accepted for publication, this manuscript will not be published elsewhere without the written consent of the copyright holder.
Disclosure statement
The authors have no actual or potential conflicts of interest.
CRediT authorship contribution statement
Ariana M. Stickel: Writing – original draft. Wassim Tarraf: Formal analysis, Supervision, Visualization, Writing – original draft. Kevin A. González: Formal analysis, Visualization, Writing – original draft. Vladamir Ivanovic: Methodology, Writing – review & editing. Alejandra Morlett Paredes: Writing – review & editing. Donglin Zeng: Writing – review & editing. Jianwen Cai: Writing – review & editing. Carmen R. Isasi: Writing – review & editing. Robert Kaplan: Writing – review & editing. Richard B. Lipton: Writing – review & editing. Martha Daviglus: Writing – review & editing. Fernando D. Testai: Writing – review & editing. Melissa Lamar: Writing – review & editing. Linda C. Gallo: Writing – review & editing. Gregory A. Talavera: Writing – review & editing. Marc D. Gellman: Writing – review & editing. Alberto R. Ramos: Writing – review & editing. Hector M. González: Conceptualization, Project administration, Supervision, Funding acquisition, Writing – original draft. Charles DeCarli: Conceptualization, Project administration, Supervision, Funding acquisition, Methodology, Writing – original draft.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neurobiolaging.2023.02.007.
References
- Armstrong NM, An Y, Beason-Held L, Doshi J, Erus G, Ferrucci L, Davatzikos C, Resnick SM, 2019. Sex differences in brain aging and predictors of neurodegeneration in cognitively healthy older adults. Neurobiol. Aging 81, 146–156. doi: 10.1016/j.neurobiolaging.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association, 2021. 2021 Alzheimer’s disease facts and figures. Vol. 17. [DOI] [PubMed] [Google Scholar]
- APA, 2013. Diagnostic and Statistcal Manual of Mental Disorders-5. American Psychiatric Association, Washington, DC. [Google Scholar]
- Atwood LD, Wolf PA, Heard-Costa NL, Massaro JM, Beiser A, D’Agostino RB, DeCarli C, 2004. Genetic variation in white matter hyperintensity volume in the Framingham Study. Stroke. 35 (7), 1609–1613. doi: 10.1161/01.STR.0000129643.77045.10. [DOI] [PubMed] [Google Scholar]
- Bayram E, Caldwell JZK, Banks SJ, 2018. Current understanding of magnetic resonance imaging biomarkers and memory in Alzheimer’s disease. Alzheimers Dement. (N Y) 4, 395–413. doi: 10.1016/j.trci.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardi M, Bocchetta M, Apostolova LG, Barnes J, Bartzokis G, Corbetta G, DeCarli C, deToledo-Morrell L, Firbank M, Ganzola R, Gerritsen L, Henneman W, Killiany RJ, Malykhin N, Pasqualetti P, Pruessner JC, Redolfi A, Robitaille N, Soininen H, Tolomeo D, Wang L, Watson C, Wolf H, Duvernoy H, Duchesne S, Jack CR Jr., Frisoni GBfor the EADC-ADNI Working Group on The Harmonized Protocol for Manual Hippocampal Segmentation and for the Alzheimer’s Disease Neuroimaging Initiative, 2014a. Delphi definition of the EADC-ADNI Harmonized Protocol for hippocampal segmentation on magnetic resonance. Alzheimers Dement 11, 126–138. doi: 10.1016/j.jalz.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardi M, Bocchetta M, Apostolova LG, Preboske G, Robitaille N, Pasqualetti P, Collins LD, Duchesne S, Jack CR Jr., Frisoni GB, 2014b. Establishing magnetic resonance images orientation for the EADC-ADNI manual hippocampal segmentation protocol. J. Neuroimaging 24 (5), 509–514. doi: 10.1111/jon.12065. [DOI] [PubMed] [Google Scholar]
- Bocchetta M, Boccardi M, Ganzola R, Apostolova LG, Preboske G, Wolf D, Ferrari C, Pasqualetti P, Robitaille N, Duchesne S, Jack CR Jr., Frisoni GB Segmentation, ADC-ADNI Working Group on The Harmonized Protocol for Manual Hippocampal Segmentation and for the Alzheimer’s Disease Neuroimaging Initiative, 2015. Harmonized benchmark labels of the hippocampus on magnetic resonance: the EADC-ADNI project. Alzheimers Dement 11. doi: 10.1016/j.jalz.2013.12.019, 151-60.e5. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, Reitz C, Small SA, Mayeux R, DeCarli C, Brown TR, 2008. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch. Neurol 65 (8), 1053–1061. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SL, Rodriguez MJ, Barker W, Greig-Custo MT, Rosselli M, Loewenstein DA, Duara R, 2018. Relationship between cognitive performance and measures of neurodegeneration among Hispanic and White Non-Hispanic individuals with normal cognition, mild cognitive impairment, and dementia. J. Int. Neuropsychol. Soc 24 (2), 176–187. doi: 10.1017/S1355617717000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby SL, Ortman JM, 2015. Projections of the size and composition of the US population: 2014 to 2060. Population Estimates and Projections. US Census Bureau, pp. P25–1143 Current Population Reports. [Google Scholar]
- Cowell PE, Turetsky BI, Gur RC, Grossman RI, Shtasel DL, Gur RE, 1994. Sex differences in aging of the human frontal and temporal lobes. J. Neurosc 14 (8), 4748–4755. doi: 10.1523/jneurosci.14-08-04748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB, 2008. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 117 (6), 743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- Daviglus ML, Talavera GA, Aviles-Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RC, LaVange L, Penedo F, Perreira K, Pirzada A, Schneiderman N, Wassertheil-Smoller S, Sorlie PD, Stamler J, 2012. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA 308 (17), 1775–1784. doi: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA, 2005. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol. Aging 26 (4), 491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, Jack L, Carmelli D, 1999. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 30 (3), 529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Murphy DG, Gillette JA, Haxby JV, Teichberg D, Schapiro MB, Horwitz B, 1994. Lack of age-related differences in temporal lobe volume of very healthy adults. AJNR. Am. J. Neuroradiol 15 (4), 689–696. [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Reed BR, Jagust W, Martinez O, Ortega M, Mungas D, 2008. Brain behavior relationships among African Americans, Whites, and Hispanics. Alzheimer Dis. Assoc. Dis 22 (4), 382–391. doi: 10.1097/WAD.0b013e318185e7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Nabizadeh N, Caunca M, Cheung YK, Rundek T, Elkind MS, DeCarli C, Sacco RL, Stern Y, Wright CB, 2015. Cognitive correlates of white matter lesion load and brain atrophy: the Northern Manhattan Study. Neurology. 85 (5), 441–449. doi: 10.1212/WNL.0000000000001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM, 2009. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 72 (22), 1906–1913. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM, 2009. One-year brain atrophy evident in healthy aging. J. Neurosci 29 (48), 15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Holland D, Dale AM, Walhovd KB, Alzheimer Disease Neuroimaging, I., 2013. Critical ages in the life course of the adult brain: nonlinear subcortical aging. Neurobiol. Aging 34 (10), 2239–2247. doi: 10.1016/j.neurobiolaging.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E, Carmichael O, Decarli C, 2012a. MRI non-uniformity correction through interleaved bias estimation and B-spline deformation with a template. Conf. Proc. IEEE Eng. Med. Biol. Soc 2012, 106–109. doi: 10.1109/EMBC.2012.6347195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E, DeCarli C, Fan AP, Knaack A, 2021. Convolutional neural net learning can achieve production-level brain segmentation in structural magnetic resonance imaging. Front. Neurosci 15, 683426. doi: 10.3389/fnins.2021.683426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E, Gavett B, Harvey D, Farias ST, Olichney J, Beckett L, DeCarli C, Mungas D, 2018. Brain volume change and cognitive trajectories in aging. Neuropsychology. 32 (4), 436. doi: 10.1037/neu0000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E, Singh B, Harvey D, Carmichael O, Decarli C, 2012b. Adaptive image segmentation for robust measurement of longitudinal brain tissue change. Conf. Proc. IEEE Eng. Med. Biol. Soc 2012, 5319–5322. doi: 10.1109/EMBC.2012.6347195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CM, Kennedy KM, Hoagey DA, Rodrigue KM, 2019. The role of hippocampal subfield volume and fornix microstructure in episodic memory across the lifespan. Hippocampus. 29 (12), 1206–1223. doi: 10.1002/hipo.23133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL, 2005. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 64 (6), 1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Bocchetta M, Chetelat G, Rabinovici GD, de Leon MJ, Kaye J, Reiman EM, Scheltens P, Barkhof F, Black SE, Brooks DJ, Carrillo MC, Fox NC, Herholz K, Nordberg A, Jack CR Jr., Jagust WJ, Johnson KA, Rowe CC, Sperling RA, Thies W, Wahlund LO, Weiner MW, Pasqualetti P, Decarli C, 2013. Imaging markers for Alzheimer disease: which vs how. Neurology. 81 (5), 487–500. doi: 10.1212/WNL.0b013e31829d86e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerlings MI, Appelman AP, Vincken KL, Algra A, Witkamp TD, Mali WP, van der Graaf Y, Group SS, 2010. Brain volumes and cerebrovascular lesions on MRI in patients with atherosclerotic disease. The SMART-MR study. Atherosclerosis. 210 (1), 130–136. doi: 10.1016/j.atherosclerosis.2009.10.039. [DOI] [PubMed] [Google Scholar]
- González HM, Tarraf W, Fornage M, González KA, Chai A, Youngblood M, de los Angeles Abreu M, Zeng D, Thomas S, Talavera GA, 2019a. A research framework for cognitive aging and Alzheimer’s disease among diverse US Latinos: design and implementation of the Hispanic Community Health Study/Study of Latinos—Investigation of Neurocognitive Aging (SOL-INcA). Alzheimer’s & Dement. 15 (12), 1624–1632. doi: 10.1016/j.jalz.2019.08.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González HM, Tarraf W, Schneiderman N, Fornage M, Vásquez PM, Zeng D, Youngblood M, Gallo LC, Daviglus ML, Lipton RB, 2019b. Prevalence and correlates of mild cognitive impairment among diverse Hispanics/Latinos: Study of Latinos-Investigation of Neurocognitive Aging results. Alzheimer’s Dement. 15 (12), 1507–1515. doi: 10.1016/j.jalz.2019.08.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford J, Simino J, Kantarci K, Mosley TH Jr., Griswold ME, Windham BG, Sharrett AR, Albert MS, Gottesman RF, Jack CR Jr., Vemuri P, Knopman DS, 2017. Neuroimaging correlates of cerebral microbleeds: the ARIC Study (Atherosclerosis Risk in Communities). Stroke 48 (11), 2964–2972. doi: 10.1161/STROKEAHA.117.018336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Frank R, Broich K, Teipel SJ, Katz RG, Hardy J, Herholz K, Bokde AL, Jessen F, Hoessler YC, Sanhai WR, Zetterberg H, Woodcock J, Blennow K, 2010. Biomarkers for Alzheimer’s disease: academic, industry and regulatory perspectives. Nat. Rev. Drug. Discov 9 (7), 560–574. doi: 10.1038/nrd3115. [DOI] [PubMed] [Google Scholar]
- Hampel H, Wilcock G, Andrieu S, Aisen P, Blennow K, Broich K, Carrillo M, Fox NC, Frisoni GB, Isaac M, Lovestone S, Nordberg A, Prvulovic D, Sampaio C, Scheltens P, Weiner M, Winblad B, Coley N, Vellas B, Oxford Task Force G, 2011. Biomarkers for Alzheimer’s disease therapeutic trials. Prog. Neurobiol 95 (4), 579–593. doi: 10.1016/j.pneurobio.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR, 2001. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol. Aging 22 (4), 581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Jones N, Marks R, Ramirez R, Ríos-Vargas M, 2021. 2020 Census illuminates racial and ethnic composition of the country. Available at: https://www.census.gov/library/stories/2021/08/improved-race-ethnicity-measures-reveal-united-states-population-much-more-multiracial.html. Accessed October 13, 2021.
- Klein A, Tourville J, 2012. 101 labeled brain images and a consistent human cortical labeling protocol. Front. Neurosci 6, 171. doi: 10.3389/fnins.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Lancaster JL, Thompson P, Woods R, Mazziotta J, Hardies J, Fox P, 2001. Regional spatial normalization: toward an optimal target. J. Comput. Assist. Tomogr 25 (5), 805–816. doi: 10.1097/00004728-200109000-00023. [DOI] [PubMed] [Google Scholar]
- LaVange LM, Kalsbeek WD, Sorlie PD, Avilés-Santa LM, Kaplan RC, Barnhart J, Liu K, Giachello A, Lee DJ, Ryan J, Criqui MH, Elder JP, 2010. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann. Epidemiol 20 (8), 642–649. doi: 10.1016/j.annepidem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi M, Donohue M, Paternico D, Scarpazza C, Ostrowitzki S, Blin O, Irving E, Frisoni GB, Alzheimer’s Disease Neuroimaging I, 2010. Enrichment through biomarkers in clinical trials of Alzheimer’s drugs in patients with mild cognitive impairment. Neurobiol. Aging 31 (8), 1443–1451. doi: 10.1016/j.neurobiolaging.2010.04.036, 1451 e1441. [DOI] [PubMed] [Google Scholar]
- Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA, 2016. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 12 (3), 216–224. doi: 10.1016/j.jalz.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay DR, Knowles EE, Winkler AA, Sprooten E, Kochunov P, Olvera RL, Curran JE, Kent JW Jr., Carless MA, Goring HH, Dyer TD, Duggirala R, Almasy L, Fox PT, Blangero J, Glahn DC, 2014. Influence of age, sex and genetic factors on the human brain. Brain Imaging. Behav 8 (2), 143–152. doi: 10.1007/s11682-013-9277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH, 2011. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dement. 7 (3), 263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagar A, Sevush S, Bertran A, 2000. Cerebral ventricles are smaller in Hispanic than non-Hispanic patients with Alzheimer’s disease. Neurology. 55 (3), 446–448. doi: 10.1212/wnl.55.3.446. [DOI] [PubMed] [Google Scholar]
- Mu Q, Xie J, Wen Z, Weng Y, Shuyun Z, 1999. A quantitative MR study of the hippocampal formation, the amygdala, and the temporal horn of the lateral ventricle in healthy subjects 40 to 90 years of age. AJNR Am. J. Neuroradiol 20 (2), 207–211. [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Farias ST, Decarli C, 2009. Age and education effects on relationships of cognitive test scores with brain structure in demographically diverse older persons. Psychol. Aging 24 (1), 116–128. doi: 10.1037/a0013421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryant SE, Johnson LA, Barber RC, Braskie MN, Christian B, Hall JR, Hazra N, King K, Kothapalli D, Large S, Mason D, Matsiyevskiy E, McColl R, Nandy R, Palmer R, Petersen M, Philips N, Rissman RA, Shi Y, Toga AW, Vintimilla R, Vig R, Zhang F, Yaffe K, Team HS, 2021. The health & aging brain among Latino Elders (HABLE) study methods and participant characteristics. Alzheimers Dement. (Amst) 13 (1), e12202. doi: 10.1002/dad2.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A, Tom SE, 2021. A lifecourse perspective on female sex-specific risk factors for later life cognition. Curr Neurol Neurosci Rep 21 (9), 46. doi: 10.1007/s11910-021-01133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV, 2013. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. Neuroimage 65, 176–193. doi: 10.1016/j.neuroimage.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran S, Wright CB, Yoshita M, Delapaz R, Brown T, DeCarli C, Sacco RL, 2008. Prevalence and determinants of subclinical brain infarction: the Northern Manhattan Study. Neurology. 70 (6), 425–430. doi: 10.1212/01.wnl.0000277521.66947.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse JC, Giedd JN, DeCarli C, Snell JW, McLaughlin A, Vauss YC, Krain AL, Hamburger S, Rapoport JL, 1996. A technique for single-channel MR brain tissue segmentation: application to a pediatric sample. Magn. Reson. Imag 14 (9), 1053–1065. doi: 10.1016/s0730-725x(96)00113-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD, 2004a. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol. Aging 25 (3), 377–396. doi: 10.1016/s0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD, 2004b. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 62 (3), 433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Mendoza L, Rodriguez I, Rosselli M, Loewenstein D, Burke S, Orozco A, Duara R, 2022. Cultural factors related to neuropsychological performance and brain atrophy among Hispanic older adults with amnestic Mild Cognitive Impairment (aMCI): A pilot study. Appl. Neuropsychol. Adult 29 (3), 364–372. doi: 10.1080/23279095.2020.1761368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert D, Aljabar P, Heckemann RA, Hajnal JV, Hammers A, 2006. Diffeomorphic registration using B-splines Med. Image Comput. Comput. Assist. Interv 9 (Pt 2), 702–709. [DOI] [PubMed] [Google Scholar]
- Sachdev PS, Parslow R, Wen W, Anstey KJ, Easteal S, 2009. Sex differences in the causes and consequences of white matter hyperintensities. Neurobiol. Aging 30 (6), 946–956. doi: 10.1016/j.neurobiolaging.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Sadhu M, Nicholson TF, Garcia R, Lampley S, Rain M, Fritz A, Jalalizadeh B, Van Enkevort E, Palka J, Brown ES, 2019. Relationship between trust in neighbors and regional brain volumes in a population-based study. Psychiatry. Res. Neuroimaging 286, 11–17. doi: 10.1016/j.pscychresns.2019.03.002. [DOI] [PubMed] [Google Scholar]
- Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Schneiderman N, Raij L, Talavera G, Allison M, LaVange L, Chambless LE, Heiss G, 2010. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann. Epidemiol 20 (8), 629–641. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickel AM, McKinnon AC, Matijevic S, Grilli MD, Ruiz J, Ryan L, 2021. Apolipoprotein E epsilon4 allele-based differences in brain volumes are largely uniform across late middle aged and older Hispanic/Latino- and non-Hispanic/Latino Whites without dementia. Front. Aging. Neurosci. 13, 627322. doi: 10.3389/fnagi.2021.627322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercauteren T, Pennec X, Perchant A, Ayache N, 2007. Non-parametric diffeomorphic image registration with the demons algorithm. Med Image. Comput. Comput. Assist. Interv 10 (Pt 2), 319–326. doi: 10.1007/978-3-540-75759-7_39. [DOI] [PubMed] [Google Scholar]
- Zahodne LB, Manly JJ, Narkhede A, Griffith EY, DeCarli C, Schupf NS, Mayeux R, Brickman AM, 2015. Structural MRI predictors of late-life cognition differ across African Americans, Hispanics, and Whites. Curr. Alzheimer Res 12 (7), 632–639. doi: 10.2174/1567205012666150530203214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Mao Z, Woody SK, Brinton RD, 2016. Sex differences in metabolic aging of the brain: insights into female susceptibility to Alzheimer’s disease. Neurobiol. Aging 42, 69–79. doi: 10.1016/j.neurobiolaging.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


