Abstract
Purpose
Anemia, especially iron-deficiency anemia during pregnancy, significantly impacts maternal health, fetal growth, and development. Moringa leaf is an iron-rich food that can overcome anemia, but there is a lack of evidence on the association between fresh moringa leaf consumption and maternal hemoglobin level during pregnancy. The aim of this study is to test the effect of fresh moringa leaf consumption during pregnancy on maternal hemoglobin levels in southern Ethiopia.
Methods
A community-based comparative cross-sectional study was conducted from May to June 2022 among 230 fresh moringa leaf consumers and 230 non-consumers pregnant women. Data were collected using an interviewer-administered structured questionnaire and hemoglobin level was determined by HemoCue Hb 301. Multivariate multilevel linear regression models were fitted using Statistical Software for Data Science (STATA) version 14.
Results
The overall mean hemoglobin level among pregnant women was 11.76 g/dl ± 1.47 [12.06 g/dl ± 1.22 among fresh moringa leaf consumers and 11.45 g/dl ± 1.64 among non-consumers] with a significant coefficient of association (β) of 0.90 g/dl [β = 0.90 g/dl, 95% CI: 0.54, 1.27]. A number of under-five children, bleeding during the current pregnancy, male-headed household, and current antenatal care visit were the individual-level factors. Distance from the nearest health facility and urban dweller was identified as the community-level factor associated with maternal hemoglobin level during pregnancy.
Conclusion
This study showed that the consumption of fresh moringa leaf during pregnancy increases the level of hemoglobin. So policymakers and maternal and child health program managers need to target moringa tree scale-up and encourage fresh moringa leaf consumption during pregnancy, but its use needs additional rigorous clinical trials. In addition to this, mark the above factors in their efforts to increase maternal hemoglobin levels during pregnancy.
Keywords: fresh moringa leaf consumption, hemoglobin level, pregnant women, Southern Ethiopia
Introduction
Anemia, particularly iron-deficiency anemia during pregnancy, significantly affects maternal health, fetal growth, and development.1,2 During pregnancy, anemia is diagnosed when the hemoglobin (Hgb) level is below 11g/dl. Low hemoglobin levels result in insufficient oxygen and nutrient delivery to the body, placenta, and fetus. It also serves as a proxy for nutritional and health status and is associated with a higher incidence of maternal and infant morbidity and mortality.3–5
Globally, in 2019, over 500 million (29.9%) reproductive-age women were anemic, of which 36.5% were pregnant women.6 Of these pregnant women, 42.7% live in low- and middle-income countries.7 In sub-Saharan Africa, the prevalence of anemia during pregnancy was 35.6%,8 with a pooled prevalence of 26.4% in Ethiopia.9 Between 2011 and 2016, there was a 7% rise in the incidence of anemia among women of reproductive age.10 The magnitude among pregnant women varies from 25.2% to 56.8%, with more than half of it caused by iron deficiency anemia.11–15
Studies have indicated that anemia during pregnancy increases the risk of maternal death,16 preterm birth,17 postpartum complications,18 perinatal asphyxia,19 intra uterine growth retardation,17 small for gestational age,20 childhood under nutrition,21 neonatal mortality,22 reduced cognitive and motor development in later life19,23 and increased risk of developing chronic non-communicable diseases.24 Due to this, it is crucial to concentrate on maternal nutrition during pregnancy in order to reach the global nutrition target of a 50% reduction of anemia in women of reproductive age by 202525 and in directly to achieve SDG 2030.26
Despite different interventions to reduce the incidence of anemia like dietary counseling and iron with folic acid supplementation (IFAS) during pregnancy through antenatal care; but adherence to IFAS was below 50%27–31 which is insufficient to address the problem as needed. As a result, consumption of culturally accepted iron-rich foods source is essential to prevent the incidence of anemia during pregnancy.32
Moringa is a culturally accepted food source rich in iron and abundant in southern Ethiopia, where 100g of locally cooked fresh moringa leaf provides 43.5% of the recommended daily iron intake for adults.33 However, it is underutilized.34,35 Previous studies have shown that consuming moringa reduces malnutrition, pregnancy complications, and micronutrient deficiencies.33,36–39 In addition, a review of the current evidence shows that consumption of moringa leaf during pregnancy increases iron levels and prevents anemia during pregnancy.40 Study conducted in different areas of the world revealed that before and after intervention of moringa capsule or powder among pregnant women, hemoglobin level mean and standard derivation was 11.19 ± 0.8 and 11.93 ± 0.9 in Indonesia,38 10.17 ± 1.0 and 12.12 ± 1.09 in Kanyakumari District,41 10.9 g/dl and >11 g/dl in Waimital Village, Indonesia,42 respectively. The average difference in hemoglobin level of pregnant women before and after moringa administration was 1.82g/dl43 and 0.73±1.29 in Indonesia.38
The majority of moringa research has concentrated on the use of moringa extract (capsules and dry powder). However, our research specifically examines the effect of fresh moringa leaf consumption during pregnancy on hemoglobin level scares in Ethiopia. Hence, this particular study aims to investigate the effects of consuming fresh moringa leaves during pregnancy on hemoglobin level and identify any individual or community-level factors contributing to hemoglobin levels among pregnant women in south Ethiopia.
Materials and Methods
Study Setting, Design and Period
A community-based comparative cross-sectional study was conducted from May to June 2022 in Arba Minch Zuria and Chencha district of Gamo zone. The main towns of Arba Minch Zuria and Chencha district are located 434 kms and 443 kms far to the south of Addis Ababa, the capital city of Ethiopia, respectively, with a total population 353,019 in the year 2021/22 as projected from the 2007 Ethiopian census with 12,214 expected number of pregnancy. There are two hospitals, nine health centers, sixty-three health posts, and different levels of private health facilities providing curative, preventive, and rehabilitative services for the population.
Population of the Study and Inclusion Criteria
The source populations for this study were all pregnant women living in Arba Minch Zuria and Chencha district for at least 6 months. The study population was selected pregnant women with gestational age between 20 and 26 weeks and not unable to communicate at the time of recruitment living in the selected kebeles (the smallest administrative units in Ethiopia) of Arba Minch Zuria and Chencha district.
Sample Size Determination and Sampling Method
The sample size was calculated by comparing two-population means of equal sample size for the two groups using G*Power44 version 3.1.9. Assuming 95% CI, 80% power, 11.494 ± 1.24 expected mean and SD of hemoglobin level among fresh moringa leaf consumers and 9.675 ± 1.28 expected mean and SD of hemoglobin level among non-consumers,43 consumer to non-consumer ratio of 1:1, 10% possible non response, using effect size of 0.345,46 and design effect of 1.5. Based on the above assumptions, the calculated sample size was 460 (230 consumers and 230 non-consumers). The sample was obtained using a multistage sampling method. Initially, 20 kebeles, 10 from Arba Minch Zuria and 10 from Chencha district were selected using lottery methods. Eligible pregnant women were identified and the sample was allocated proportionally based on the number of eligible pregnant women in each kebele. Then, the required sample was designated using computer-generated simple random sampling techniques.
Study Variable
Pregnant woman hemoglobin level was the outcome variable and community-level explanatory variables were consumption of fresh moringa leaf, community wealth status, community source of drinking water, community media exposure, place of residence and distance from the nearest health center; whereas, individual-level variables were socio-demographic, obstetric, health and dietary related factors.
Data Collection Instrument and Techniques
Ten nurses and two public health professionals were used to collect and supervise the data collection, respectively. Two days of training were conducted for data collectors and supervisors. Three laboratory technicians measured the hemoglobin level. Data were collected using a pre-tested, face-to-face interviewer-administered structured questionnaire that address socio-demographic, obstetric, health and dietary related factors.
Data were collected digitally using an open-source toolkit (kobo Collect). A template of questions was prepared in a computer database and deployed to the server then downloaded to the cell phones of data collectors. Training was provided on how to download the template and upload the data. HemoCue Hb 301 machine was used to measure hemoglobin level.
Operational Definition
Moringa Consumption
Women, who consumed fresh moringa leaf in the last one month for at least four days per week, regardless of amount or frequency, we classified as consumer and not consumed ever as non-consumer.
Measurement
Hemoglobin Level
Hemoglobin level of pregnant women was measured using HemoCue Hb 301 machine, safety lancet and microcuvette. The machine was a pre calibrated instrument designed for the measurement of hemoglobin concentration. A blood sample is collected from each participant using a figure-pricking method to collect drops of blood from the left ring finger. The first and second drops of blood were wiped away. A third drop was then used to measure hemoglobin levels. Blood was drawn through microcuvettes and inserted into the HemoCue machine and the results were recorded.47 We used the adjustment factor for altitude correction in order to obtain the comparison of both groups.48
Women nutritional status was assessed using mid upper arm circumference (MUAC) and measured using a flexible non stretchable standard tape measured at the mid-point between the tips of the ulna. Measurements were taken on the right arm with an accuracy of 0.1 cm. Women with MUAC greater than or equal to 23cm are considered well-nourished and less than 23 cm as under nourished.49
Minimum Dietary Diversity- Women (MDD-W)
The data of minimum dietary diversity score was collected by using a 24 hour dietary recall method according to FAO’s 2016 guideline. To each food group that a woman ate in 24 hours of the day before the data collection score of 1 for yes was given otherwise 0 for no. The score was made by counting the number of food groups. Finally, a woman who had got 5 scores or more out of 10 was categorized as adequate otherwise inadequate dietary diversity.50
Data Processing and Analysis
Data were checked online, approved for its completeness and consistency daily and downloaded after completion of data collection, then exported to STATA version 14 for analysis. Descriptive statistics such as frequency, percentages, mean and standard deviations were used to describe the characteristics of the study participants. Exploratory factor analysis using the principal components analysis (PCA) method was employed to determine household wealth status. An Independent t-test was employed to compare the mean hemoglobin level among fresh moringa leaf consumer and non-consumer.
Bivariate analysis was employed on all individual and community level explanatory variables to assess their association with the outcome variable. Variables with a p-value of less than 0.25 in bivariate analyses and plausible variables were included in multivariable multilevel linear regression analysis. First, we estimated an intercept-only model (with only the outcome variable). Intra-class correlation coefficient (Icc) was checked to examine clustering and the extent to which community-level factors explained the unexplained variance of the null model (Icc=0.1212, indicating the preference of multilevel model). Secondly, we included all individual-level variables in the model (model I). In the third stage, we fitted community-level variables only (model II) and the final model was fitted with both individual and community-level variables (model III). Model III, the model with the lowest Akaike’s information criteria (AIC) and Bayesian information criterion (BIC), was selected as the best model for an analysis. ß coefficients with 95% CI in the multivariable multilevel mixed effect linear regression analysis were used to decide whether those individual and community-level variables were statistically significant (p < 0.05) or not. The Breusch-Pagan/Cook-Weisberg and skewness/kurtosis tests were used to check the assumptions of equal variance and normality.
Results
Socio Demographic and Health Related Characteristics of Fresh Moringa Leaf Consumer and Non-Consumer
A total of 460 (230 fresh moringa leaf consumer and 230 non-consumer) participated in the study. There is no significant difference between the two groups (Table 1) in marital status, household head, number of family member (“Supplementary Table 1”), number of pregnancy, history of abortion prior to this pregnancy, pregnancy status, had nausea/vomiting, history of anemia during current pregnancy, history of bleeding during current pregnancy, meal frequency per day and consumption animal source food (ASF) (“Supplementary Table 2”).
Table 1.
Socio-Demographic, Health, and Dietary-Related Characteristics of Fresh Moringa Leaf Consumers and Non-Consumers Pregnant Women in Southern Ethiopia
| Variable | Total No (%) (n=460) | Consumer No (%)(n=230) | Non-Consumer No (%)(n=230) | p-value |
|---|---|---|---|---|
| Marital status | 0.37 | |||
| Married | 458 (99.57) | 229 (99.57) | 229 (99.57) | |
| Other | 2 (0.43) | 1 (0.38) | 1 (0.38) | |
| Household head | 0.38 | |||
| Male | 438 (95.22) | 217 (94.35) | 221 (96.09) | |
| Female | 22 (4.78) | 13 (5.65) | 9 (3.91) | |
| Number of Family members | 0.26 | |||
| Less than or equal to five | 402 (87.39) | 205 (89.13) | 197 (85.65) | |
| Greater than five | 58 (12.61) | 25 (10.87) | 33 (14.35) | |
| Number of gravida | 0.19 | |||
| Primigravida | 113 (24.57) | 58 (25.22) | 55 (23.91) | |
| Multigravida | 306 (66.52) | 157 (68.26) | 149 (64.78) | |
| Grand multigravida | 41 (8.91) | 15 (6.52) | 26 (11.30) | |
| Abortion before this pregnancy | 0.30 | |||
| No | 407 (88.48) | 200 (86.96) | 207 (90.00) | |
| Yes | 53 (11.52) | 30 (13.04) | 23 (10.00) | |
| Pregnancy status | 0.23 | |||
| Unplanned | 154 (33.48) | 83 (36.09) | 71 (30.87) | |
| Planned | 306 (66.52) | 147 (63.91) | 159 (69.13) | |
| Gestational age | 0.29 | |||
| 20 to 23wks | 92 (40.00) | 81 (35.22) | 173 (37.61) | |
| 24 to 26 | 138 (60.00) | 149 (64.78) | 287 (62.39) | |
| Had Nausea/ vomiting | 0.70 | |||
| No | 218 (47.39) | 107 (46.52) | 111 (48.26) | |
| Yes | 242 (52.61) | 123 (53.48) | 119 (51.74) | |
| Anemia during the current pregnancy | 0.79 | |||
| No | 445 (96.74) | 223 (96.96) | 222 (96.52) | |
| Yes | 15 (3.26) | 7 (3.04) | 8 (3.48) | |
| Bleeding during the current pregnancy | 0.31 | |||
| No | 451 (98.04) | 224 (97.39) | 227 (98.70) | |
| Yes | 9 (1.96) | 6 (2.61) | 3 (1.30) | |
| Animal source food (ASF) | 0.06 | |||
| Not consume ASF | 113 (49.13) | 133 (57.83) | 246 (53.48) | |
| Consume ASF | 117 (50.87) | 97 (42.17) | 214 (46.52) | |
| Meal frequency per day | 0.85 | |||
| Three times and less | 106 (46.09) | 108 (46.96) | 214 (46.52) | |
| Four and more times | 124 (53.91) | 122 (53.04) | 246 (53.48) |
Dietary Related Characteristics of Fresh Moringa Leaf Consumer and Non-Consumer
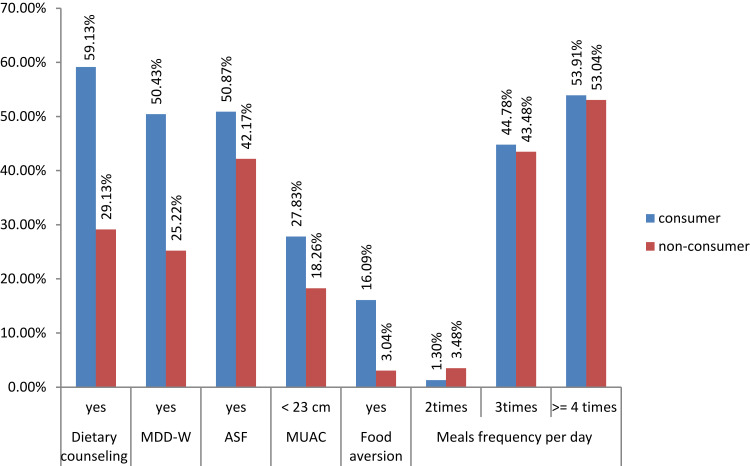
More than three-fourth of the participants, 72.17% of fresh moringa leaf consumers and 81.83% of non-consumers, were well nourished and more than half of the participants consumed at least one additional meal. One hundred and thirty six (59.13%) of fresh moringa leaf consumers and 61 (29.13%) non-consumers received dietary counseling during pregnancy. One hundred and sixteen (50.43%) fresh moringa leaf consumers and 58 (25.22%) non-consumers consumed adequate dietary diversity. One hundred and seventeen (50.87%) fresh moringa leaf consumers and 97 (42.17%) non-consumers consumed animal source food (Figure 1). Our study showed that no significant association between hemoglobin level and animal-based food consumption, intake of 5 or more food groups, and receiving dietary counseling.
Figure 1.
Dietary-related characteristics of fresh moringa leaf consumer and non-consumer pregnant women in Southern Ethiopia.
The Association Between Moringa Leaf Consumption and Maternal Hemoglobin Level
The mean hemoglobin level of the study participants was 11.76 g/dl ± 1.47 (11.45 g/dl ± 1.64 among consumers and 12.06 g/dl ± 1.22 among non-consumers). The mean hemoglobin level was 0.61 g/dl [95% CI 0.34, 0.87] higher among fresh moringa leaf consumer than non-consumer (Table 2).
Table 2.
The Effect of Fresh Moringa Leaf Consumption During Pregnancy on Maternal Hemoglobin Level Among Pregnant Women in Southern Ethiopia
| Fresh Moringa Leaf | Observation | Mean ± SD | [95% C I] | t- test | p-value |
|---|---|---|---|---|---|
| All participant | 460 | 11.76 ± 1.47 | 11.62, 11.89 | ||
| Consumers | 230 | 12.06 ± 1.22 | 11.90, 12.22 | 4.50 | 0.001 |
| Non-consumers | 230 | 11.45 ± 1.64 | 11.24, 11.67 | ||
| Mean difference, 95% CI | 0.61g/dl | 0.34, 0.87 |
Factors Associated with Hemoglobin Level Among Pregnant Women
An intra-cluster correlation coefficient (Icc) of 0.1212 for the null model indicated that 12.12% of the variability in hemoglobin levels among pregnant women was due to intra-cluster variability, also confirming the use of multilevel models for analysis. The between cluster difference decreased from 12.12% in null model to 11.75% in the Model I that had only the individual-level variables. Again, the between cluster differences were slightly decreased from 11.75% in model I to 10.88% in the community level only model (Model II). Finally, Icc decreased to 11.72% in the complete model (Model III) that encompassed both individual and community-level factors. Log likelihood ratio test was established −813.25733, 1 in the null model and −773.98152, 78.55 in the final model (Model III), this shows that increment and confirms model adequacy. The values of AIC were confirmed as 1632.515 in the null model and 1569.963 in the final model (Model III) this expresses a succeeding decrease, which shows that there is a considerable improvement from the empty model to individual and community level-only models and again to the complete model. This confirms the goodness-of-fit of the final model established in the analysis. Hence, model III was chosen for forecasting the change of hemoglobin among pregnant women.
The final model (Model III) shows that hemoglobin levels among pregnant women who consumed fresh moringa leaf were 0.90 g/dl (β = 0.90, 95% CI: 0.54, 1.27) higher than those who did not. The level of hemoglobin was 0.57 g/dl higher among women who lived in urban as compared to those who lived in rural (β = 0.57, 95% CI: 0.22, 0.92). Pregnant women from male-headed households had hemoglobin levels 0.87 g/dl (β = 0.87, 95% CI: 0.40, 1.33) higher than those from female-headed households. Hemoglobin levels were 0.49 g/dl higher among pregnant women with one child than those with two children under 5 years of age (β = 0.49, 95% CI: 0.17, 0.82). The hemoglobin level of women who attend antenatal care in the current pregnancy was 0.45g/dl (β = 0.45, 95% CI: 0.17, 0.74) higher than their counterpart. Hemoglobin level was 1.07 g/dl decreased among women who faced bleeding in the current pregnancy as compared to their counterpart (β = −1.07, 95% CI: −1.91, −0.22). As distance to the nearest health center increased by 1km, the women's hemoglobin level decreased by 0.10g/dl (β = −0.10, 95% CI: −0.15, - 0.06) (Table 3).
Table 3.
Multilevel Mixed Effect Linear Regression Analysis of Individual and Community-Level Factors Associated with Hemoglobin Level Among Pregnant Women in Southern Ethiopia
| Variable | Null Model | Model I | Model II | Model III |
|---|---|---|---|---|
| Hemoglobin level | 11.74 (11.45, 12.02)** | |||
| Health facility distance | −0.08 (−0.12, −0.029)** | −0.10 (−0.15, −0.06)** | ||
| Moringa Consumption | ||||
| No | 1 | 1 | ||
| Yes | 0.99 (0.61, 1.36)** | 0.90 (0.54, 1.27)** | ||
| Place of residence | ||||
| Rural | 1 | 1 | ||
| Urban | 0.63 (0.27, 0.98)* | 0.57 (0.22, 0.92)* | ||
| House hold head | ||||
| Husband | 0.75 (0.26, 1.23)* | 0.87 (0.40, 1.33)** | ||
| Wife | 1 | 1 | ||
| Number of under 5 children | ||||
| No | 0.27 (−0.06, 0.61) | 0.32 (−0.01, 0.64) | ||
| One | 0.47 (0.13, 0.81)* | 0.49 (0.17, 0.82)* | ||
| Two | 1 | 1 | ||
| Bleeding faced in this pregnancy | ||||
| No | 1 | 1 | ||
| Yes | −1.06 (−1.95,0.17)* | −1.07 (−1.91, −0.22)* | ||
| ANC visit | ||||
| No | 1 | 1 | ||
| Yes | 0.45 (0.16, 0.75)* | 0.45 (0.17, 0.74)* | ||
| Variance | ||||
| Between district | 0.26 (0.11, 0.64) | 0.24, (0.09, 0.58) | 0.21(0.09, 0.53) | 0.21 (0.08, 0.52) |
| Between individual | 1.90 (1.67, 2.17) | 1.78 (1.56, 2.03) | 1.74 (1.53, 1.99) | 1.61 (1.41, 1.83) |
| ICC% | 12.12% | 11.75% | 10.88% | 11.72% |
| Model comparison | ||||
| Log likelihood | −813.25733, 1 | −798.01195, 30.49 | −792.06777, 42.38 | −773.98152, 78.55 |
| AIC | 1632.515 | 1612.024 | 1596.136 | 1569.963 |
| BIC | 1644.908 | 1645.074 | 1620.923 | 1615.407 |
Notes: 1: Reference group, *p-value 0.05–0.01, **p-value <0.01.
Discussion
In this study, consumption of fresh moringa leaf had a significant association with the hemoglobin level of pregnant women in the study area. The hemoglobin level was 0.90g/dl higher among fresh moringa leaf consumers than non-consumers. This is in line with previous studies conducted in Indonesia, India, Senegal, Bangladesh and recent reviews37,38,42,51–56 where the intake of moringa in different forms treats anemia. This may be due to the nutrient content of moringa. Moringa is an iron-rich food source with easily digestible proteins, vitamins and minerals.33,57 Also, consumption of fresh moringa leaf increases the level of serum protein, vitamins A and C.36,42 Studies indicated that vitamin C enhances the bioavailability and absorption of iron also serum retinol maintains normal hemoglobin levels and serum protein facilitate iron absorption and transportation.43,58–61 These imply that the consumption of fresh moringa leaf had an effect on hemoglobin level.
In this study, the mean hemoglobin level was decreased by 0.10 g/dl [95% CI: −0.15, −0.06] for each km increase in distance to the nearest health center. This finding is supported by studies conducted in eastern Africa and sub-Saharan Africa.62,63 This may be due to being near to health facilities may easily access health facilities for different services and may increase the chance of dietary counseling and the likelihood of timely diagnosis for infectious disease and contribute to preventing anemia.64–68
Our study shows that the level of hemoglobin in pregnant women was 0.57 g/dl higher among urban dwellers as compared to rural dwellers, which is in line with studies conducted in different parts of Ethiopia and sub-Saharan Africa.9,65,69–71 This might be due to the fact that women living in urban setting had increased intake of iron-rich food, diversified food, adherence to IFAS, accessible for health service, health information and media; this might enhance knowledge and practices that reduce the incidence of anemia during pregnancy. Also, urban pregnant women had higher decision-making power, employment status, and health seeking behaviors, which may reduce the incidence of anemia during pregnancy. Moreover, being a rural dweller increases the likelihood of infection, which might lower the level of hemoglobin.
The hemoglobin level of pregnant women from male-headed households was 0.87 g/dl higher than those from female-headed household. This finding was supported by studies conducted in sub-Saharan Africa and Ethiopia.71,72 This may be due to female household head was relation between low level of education and employment, this makes their livelihood insecure and leads to food insecurity; finally end up with different health problems including iron deficiency. In addition, the low awareness and treatment-seeking behavior of female household heads contribute to anemia.73–75
The level of hemoglobin was 0.49 g/dl higher among pregnant women who had one child as compared to those who had two children below the age of 5 years. This is supported by studies conducted in various regions of Ethiopia.65,72,76 One possible explanation for this is that women experience a depletion of iron due to the frequent pregnancies and resulting loss of blood during delivery and postnatal period. If a woman becomes pregnant too soon after giving birth, she may not have had enough time to replenish the lost blood, which increases her susceptibility to anemia.77,78 Additionally, stress and birth-fear can lead to inadequate food intake in women who give birth more frequently, contributing further to the development of anemia79 and also to this one possible reason for this could be that having more young children under the age of five could result in a greater workload and less time for a woman to take care of herself and provide food for her family.80
Hemoglobin level was 1.07 g/dl decreased among mothers who faced bleeding in the current pregnancy as compared to their counterpart. This finding is comparable with study conducted in north-west and central Ethiopia.76,81 This may be due to the fact that bleeding during the current pregnancy causes depletion of iron which in turn causes anemia.
The hemoglobin level of mothers who attend antenatal care in the current pregnancy was 0.45 g/dl higher than their counterparts. This is supported by recent systematic review and meta-analysis in Ethiopia.9 This possible reason might be that when ANC visits increases the opportunity for getting a sufficient number of iron folate tablets and increases adherence to iron with folic acid.82
It is the fact that consumption of diversified83 and animal source food raises the likelihood of dietary iron intake84 consequently increases the hemoglobin level. Contrary to this fact, in our study and other studies conducted in different parts of the world, we lack significant association between hemoglobin level and consumption of animal-based food studies carried out in Ghana85 and rural Bangladesh.86
Similarly, we found no significant connection between dietary diversity and maternal hemoglobin levels, which agrees with the rural Bangladesh study.86 It may be due to the use of 24 hour recall method to identify consumption of diversified and animal-based food that under estimate the intake because the method does not identify the usual dietary intake.
Regarding counseling, our study found that women who did not receive dietary counseling during ANC visit had no statistically significant association with hemoglobin levels, which is consistent with a study done in Indonesia87 but inconsistent with a study conducted in Ethiopia.88 A plausible explain this discrepancy, it is possible that the quality and content of dietary counseling received may vary. Therefore, further research needs to investigate the type of information provided during ANC encounters and how it is imparted.
Strength and Limitation
The study relied on the participant’s oral responses regarding their history of fresh moringa leaf consumption during the present pregnancy and does not inquire as to how much was consumed daily; therefore, this could have some impact on the results. To minimize recall bias we used consumption history before one month of the study period. Moreover, information on some important confounding variables such as malaria and intestinal parasitic infection were not investigated but to reduce this we used current pregnancy history of malaria and intestinal parasitic infection attack including frequency, bed net use, and intestinal parasitic infection drug use as treatment. In addition to this, we included the key potential confounders in the multivariable regression model. Another limitation is the shortage of similar studies limited us from making further comparative discussions.
Conclusion
Our findings reveal that consumption of fresh moringa leaf during pregnancy can increase hemoglobin level. Hemoglobin level was also found to be affected by place of residence, number of under-five children, bleeding during the current pregnancy, current antenatal care visit, headed of the households, and distance to the nearest health facilities. Thus, policy maker’s program planners, clinical and public health interventions should enhance moringa leaf consumption, birth spacing, maternal health service utilization and accessibility of health facilities.
Acknowledgments
We are grateful to Jimma University for its financial support. We would like to thank Arba Minch university institutional university cooperation (AMU_IUC) project 3 for providing micro cuvettes (Hema Cue Hb 301). We are also indebted to our data collectors, supervisors and study participants.
Funding Statement
This study was funded by Jimma University, Ethiopia. The supporting organization, Jimma University, had no role in study design, data collection analysis, publication decisions, and manuscript preparation.
Abbreviations
ANC, antenatal care; CI, Confidence interval; CSA, Central statistics agency; FAO, Agriculture Organization of the United Nation; GA, gestational age; IRB, Institutional Review Board; MUAC, Mid Upper Arm Circumference; PCA, principal components analysis.
Data Sharing Statement
All related data and its supporting information are within the paper.
Ethics Approval and Informed Consent
All methods of this study were carried out in accordance with the declaration of Helsinki-ethical principle for medical research involving human subjects. An ethical approval and clearance was obtained from the institutional review board (IRB) institute of health, Jimma University with a reference number of (THRPG1/469/2022) before the commencement of data collection. A letter of cooperation was obtained from the Gamo zone health office. Informed consent was obtained from the study participants after informing the purpose of the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The author declared that they have no competing interests concerning the research, authorship, and publication of this article.
References
- 1.Williamson C. Nutrition in pregnancy: latest guidelines and advice. Primary Health Care. 2006;16(7):23. [Google Scholar]
- 2.Gernand AD, Schulze KJ, Stewart CP, West KP, Christian P. Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nat Rev Endocrinol. 2016;12(5):274–289. doi: 10.1038/nrendo.2016.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. Document Reference. World Health Organization; 2011. [Google Scholar]
- 4.DeMaeyer EM, Dallman P, Gurney JM, et al. Preventing and Controlling Iron Deficiency Anaemia Through Primary Health Care: A Guide for Health Administrators and Programme Managers. World Health Organization; 1989. [Google Scholar]
- 5.World Health Organization. Reproductive Health Indicators: Guidelines for Their Generation, Interpretation and Analysis for Global Monitoring. World Health Organization; 2006. [Google Scholar]
- 6.World Health Organization WHO global anaemia estimates, 2021 edition. Anaemia in Women and Children. Available from: https://www.who.int/data/gho/data/themes/topics/anaemia_in_women_and_children. accessed February 18, 2022. 2021.
- 7.Rahman MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low-and middle-income countries: systematic review and meta-analysis, 2. Am J Clin Nutr. 2016;103(2):495–504. doi: 10.3945/ajcn.115.107896 [DOI] [PubMed] [Google Scholar]
- 8.Fite MB, Assefa N, Mengiste B. Prevalence and determinants of anemia among pregnant women in sub-Saharan Africa: a systematic review and meta-analysis. Arch Public Health. 2021;79(1):1–11. doi: 10.1186/s13690-021-00711-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geta TG, Gebremedhin S, Omigbodun AO. Prevalence and predictors of anemia among pregnant women in Ethiopia: systematic review and meta-analysis. PLoS One. 2022;17(7):e0267005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agency CS. Ethiopian Demographic and Health Survey (EDHS); 2016.
- 11.World Health Organization. The Global Prevalence of Anaemia in 2011. World Health Organization; 2015. [Google Scholar]
- 12.Addis Alene K, Mohamed Dohe A. Prevalence of anemia and associated factors among pregnant women in an urban area of Eastern Ethiopia. Anemia. 2014;2014:1–7. doi: 10.1155/2014/561567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mihiretie H, Fufa M, Mitiku A, et al. Magnitude of anemia and associated factors among pregnant women attending antenatal care in Nekemte health center, Nekemte, Ethiopia. J Med Microbiol Diagn. 2015;4(3):1. doi: 10.4172/2161-0703.1000197 [DOI] [Google Scholar]
- 14.Bekele A, Tilahun M, Mekuria A. Prevalence of anemia and its associated factors among pregnant women attending antenatal care in health institutions of Arba Minch Town, Gamo Gofa Zone, Ethiopia: a cross-sectional study. Anemia. 2016;2016:1–9. doi: 10.1155/2016/1073192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asrie F. Prevalence of anemia and its associated factors among pregnant women receiving antenatal care at Aymiba Health Center, northwest Ethiopia. J Blood Med. 2017;8:35. doi: 10.2147/JBM.S134932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray-Kolb LE, Chen L, Chen P, Shapiro M, Caulfield L. CHERG iron report: maternal mortality, child mortality, perinatal mortality, child cognition, and estimates of prevalence of anemia due to iron deficiency. Baltimore, MD; 2012. [Google Scholar]
- 17.Bakhtiar UJ, Khan Y, Nasar R. Relationship between maternal hemoglobin and perinatal outcome. Age. 2007;25:24. [Google Scholar]
- 18.Yilmaz E, Işitan OY, Soysal Ç, Yilmaz ZV, Kara OF, Küçüközkan T. The influence of anemia on maternal and neonatal outcomes in adolescent pregnant. J Surg Med. 2018;2(2):69–73. [Google Scholar]
- 19.Gonzales-Medina C, Arango-Ochante P. Maternal anemia and perinatal outcomes. Rev Peru Ginecol Obstet. 2019;65(4):519–526. doi: 10.31403/rpgo.v65i2221 [DOI] [Google Scholar]
- 20.Nair M, Choudhury MK, Choudhury SS, et al. Association between maternal anaemia and pregnancy outcomes: a cohort study in Assam, India. BMJ Global Health. 2016;1(1):e000026. doi: 10.1136/bmjgh-2015-000026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christian P, Lee SE, Donahue Angel M, et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low-and middle-income countries. Int J Epidemiol. 2013;42(5):1340–1355. doi: 10.1093/ije/dyt109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bililign N, Legesse M, Akibu M. A review of low birth weight in Ethiopia: socio-demographic and obstetric risk factors. Glob J Res Rev. 2018;5(1):4. [Google Scholar]
- 23.Haas JD, Brownlie IVT. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131(2):676S–90S. doi: 10.1093/jn/131.2.676S [DOI] [PubMed] [Google Scholar]
- 24.Jornayvaz FR, Vollenweider P, Bochud M, Mooser V, Waeber G, Marques-Vidal P. Low birth weight leads to obesity, diabetes and increased leptin levels in adults: the CoLaus study. Cardiovasc Diabetol. 2016;15(1):1–10. doi: 10.1186/s12933-016-0389-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Global Nutrition Targets 2025: Policy Brief Series. World Health Organization; 2014. [Google Scholar]
- 26.Assembly G. Resolution Adopted by the General Assembly on 11 September 2015. New York: United Nations; 2015. [Google Scholar]
- 27.Gebremariam AD, Tiruneh SA, Abate BA, Engidaw MT, Asnakew DT, Budhathoki SS. Adherence to iron with folic acid supplementation and its associated factors among pregnant women attending antenatal care follow up at Debre Tabor General Hospital, Ethiopia, 2017. PLoS One. 2019;14(1):e0210086. doi: 10.1371/journal.pone.0210086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demis A, Geda B, Alemayehu T, Abebe H. Iron and folic acid supplementation adherence among pregnant women attending antenatal care in North Wollo Zone northern Ethiopia: institution based cross-sectional study. BMC Res Notes. 2019;12(1):1–7. doi: 10.1186/s13104-019-4142-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Assefa H, Abebe SM, Sisay M. Magnitude and factors associated with adherence to Iron and folic acid supplementation among pregnant women in Aykel town, Northwest Ethiopia. BMC Pregnancy Childbirth. 2019;19(1):1–8. doi: 10.1186/s12884-019-2422-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jikamo B, Samuel M. Non-adherence to iron/folate supplementation and associated factors among pregnant women who attending antenatal care visit in selected public health institutions at Hosanna Town, Southern Ethiopia, 2016. J Nutr Disord Ther. 2018;8(230):2161–0509.1000230. doi: 10.4172/2161-0509.1000230 [DOI] [Google Scholar]
- 31.Gebremichael TG, Haftu H, Gereziher TA. Time to start and adherence to iron-folate supplement for pregnant women in antenatal care follow up; Northern Ethiopia. Patient Prefer Adherence. 2019;13:1057. doi: 10.2147/PPA.S184168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ndegwa SK. Anemia and its associated factors among pregnant women attending antenatal clinic at Mbagathi county hospital, Nairobi county, Kenya. Afr J Health Sci. 2019;32(1):59–73. [Google Scholar]
- 33.Abuye C, Urga K, Knapp H, et al. A compositional study of Moringa stenopetala leaves. East Afr Med J. 2003;80(5):247–252. doi: 10.4314/eamj.v80i5.8695 [DOI] [PubMed] [Google Scholar]
- 34.Gebrezihar TA, Desta Z, Hagos H. Assessing factors affecting moringa production at North-western Zone of Tigray, Ethiopia. Agric Sci. 2020;2(1):17–p17. doi: 10.30560/as.v2n1p17 [DOI] [Google Scholar]
- 35.Terfassa G, Negeyo D. Pre-extension demonstration of moringa preparation and utilization methods in East Shoa Zones of Oromia, Ethiopia. J Biomater. 2020;4(1):17–22. doi: 10.11648/j.jb.20200401.12 [DOI] [Google Scholar]
- 36.Nadimin HV, As’ad S, Buchari A. The extract of moringa leaf has an equivalent effect to iron folic acid in increasing hemoglobin levels of pregnant women: a randomized control study in the coastal area of Makassar. Int J Sci Basic Appl Res. 2015;22(1):287–294. [Google Scholar]
- 37.Idohou-Dossou N, Diouf A, Gueye A, Guiro A, Wade S. Impact of daily consumption of Moringa (Moringa oleifera) dry leaf powder on iron status of Senegalese lactating women. Afr J Food Agric Nutr Dev. 2011;11(4). doi: 10.4314/ajfand.v11i4.69176 [DOI] [Google Scholar]
- 38.Iskandar I, Hadju V, As’ad S, Natsir R. Effect of Moringa oleifera leaf extracts supplementation in preventing maternal anemia and low-birth-weight. Int J Sci Res. 2015;5(2):1–3. [Google Scholar]
- 39.Kumssa DB, Joy EJ, Young SD, Odee DW, Ander EL, Broadley MR. Variation in the mineral element concentration of Moringa oleifera Lam. and M. stenopetala (Bak. f.) Cuf.: role in human nutrition. PLoS One. 2017;12(4):e0175503. doi: 10.1371/journal.pone.0175503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hadju V, Dassir M, Sadapotto A, et al. Effects of Moringa Oleifera leaves and honey supplementation during pregnancy on mothers and newborns: a review of the current evidence. Open Access Maced J Med Sci. 2020;8:208–214. doi: 10.3889/oamjms.2020.4670 [DOI] [Google Scholar]
- 41.Chrysholite Jenisha C, Rajitha S. Effectiveness of drumstick leaves soup on hemoglobin level among antenatal mothers. Int J Med Health Res. 2018;4(11):43–49. [Google Scholar]
- 42.Astuti AD, Rochmaedah S. The effect of consumption of moringa leaves to pregnant women’s hemoglobin levels in the village of Waimital, Kairatu district, West Seram Region, Maluku in 2019. Proceeding International Conference Syedza Saintika; 2020. [Google Scholar]
- 43.Laiskodat J, Kundaryanti R, Novelia S. The effect of Moringa Oleifera on hemoglobin level in pregnancy. Nurs Health Sci J. 2021;1(2):136–141. doi: 10.53713/nhs.v1i2.65 [DOI] [Google Scholar]
- 44.Kang H. Sample size determination and power analysis using the G*Power software. J Educ Eval Health Prof. 2021;18:17. doi: 10.3352/jeehp.2021.18.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen JC Jr. Sample size calculation for two independent groups: a useful rule of thumb. Proc Singapore Healthc. 2011;20(2):138–140. doi: 10.1177/201010581102000213 [DOI] [Google Scholar]
- 46.Van Belle G. Statistical Rules of Thumb. John Wiley & Sons; 2011. [Google Scholar]
- 47.Kuma MN, Tamiru D, Belachew T, Maugeri A. Hemoglobin level and associated factors among pregnant women in rural Southwest Ethiopia. Biomed Res Int. 2021;2021:1–11. doi: 10.1155/2021/9922370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Umar Z, Rasool M, Asif M, et al. Evaluation of hemoglobin concentration in pregnancy and correlation with different altitude: a study from Balochistan plateau of Pakistan. Open Biochem J. 2015;9(7):7–14. doi: 10.2174/1874091X01509010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gelebo DG, Gebremichael MA, Asale GA, Berbada DA. Prevalence of undernutrition and its associated factors among pregnant women in Konso district, southern Ethiopia: a community-based cross-sectional study. BMC Nutr. 2021;7(1):1–13. doi: 10.1186/s40795-021-00437-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.F FAO. Minimum Dietary Diversity for Women: A Guide for Measurement. Vol. 82. Rome: FAO; 2016. [Google Scholar]
- 51.Nur R, Demak IPK, Yane EB. The effect of moringa leaf extract in increasing Hb levels of pregnant women during COVID-19 pandemic in Parigi Regency, Central Sulawesi, Indonesia. Int J Health Sci. 2022;6(1):6019–6028. doi: 10.53730/ijhs.v6nS1.6230 [DOI] [Google Scholar]
- 52.Sitohang P, Candriasih P, Amdani S. Effect of Moringa (Moringa oleifera) biscuit administration on hemoglobin levels of pregnant women. Int J Sci Basic Appl Res. 2018;37:243–252. [Google Scholar]
- 53.Thenmozhi P, Nirmala M, Subalakshmi P. Moringa oleifera leaves soup on hemoglobin among antenatal mothers. Education. 2020;34(40):5. [Google Scholar]
- 54.Khanam M, Sanin KI, Ara G, et al. Effects of Moringa oleifera leaves on hemoglobin and serum retinol levels and underweight status among adolescent girls in rural Bangladesh. Front Nutr. 2022;9. doi: 10.3389/fnut.2022.959890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Talath S, Hemalatha K. A review on significance of Moringa oleifera in the treatment of heme-related disorders and malnutrition. J Pharm Negat. 2022;13(4):473–491. [Google Scholar]
- 56.Perera C, Galappatti D, Thrimavithana A, et al. Effect of Moringa oleiferaon haematological parameters: a systematic review; 2022.
- 57.Yisehak K, Solomon M, Tadelle M. Contribution of Moringa (Moringa stenopetala, Bac.), a highly nutritious vegetable tree, for food security in south Ethiopia: a review. Asian J Appl Sci. 2011;4(5):477–488. doi: 10.3923/ajaps.2011.477.488 [DOI] [Google Scholar]
- 58.Nair KM, Iyengar V. Iron content, bioavailability and factors affecting iron status of Indians. Indian J Med Res. 2009;130(5):634–645. [PubMed] [Google Scholar]
- 59.Pavord S, Myers B, Robinson S, et al. UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol. 2012;156(5):588–600. doi: 10.1111/j.1365-2141.2011.09012.x [DOI] [PubMed] [Google Scholar]
- 60.National RC. Conference on Hemoglobin: 2–3 May 1957. Washington, DC: The National Academies Press; 1958. [PubMed] [Google Scholar]
- 61.Frazer DM, Anderson GJ. The regulation of iron transport. Biofactors. 2014;40(2):206–214. [DOI] [PubMed] [Google Scholar]
- 62.Teshale AB, Tesema GA, Worku MG, Yeshaw Y, Tessema ZT. Anemia and its associated factors among women of reproductive age in Eastern Africa: a multilevel mixed-effects generalized linear model. PLoS One. 2020;15(9):e0238957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seifu BL, Tesema GA. Individual-and community-level factors associated with anemia among children aged 6–23 months in sub-Saharan Africa: evidence from 32 sub-Saharan African countries. Arch Public Health. 2022;80(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tounkara M, Sangho O, Beebe M, et al. Geographic access and maternal health services utilization in Sélingué health district, Mali. Matern Child Health J. 2022;26(3):649–657. doi: 10.1007/s10995-021-03364-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tirore LL, Mulugeta A, Belachew AB, et al. Factors associated with anaemia among women of reproductive age in Ethiopia: multilevel ordinal logistic regression analysis. Matern Child Nutr. 2021;17(1):e13063. doi: 10.1111/mcn.13063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rouamba T, Samadoulougou S, Ouédraogo M, Hien H, Tinto H, Kirakoya-Samadoulougou F. Asymptomatic malaria and anaemia among pregnant women during high and low malaria transmission seasons in Burkina Faso: household-based cross-sectional surveys in Burkina Faso, 2013 and 2017. Malar J. 2021;20(1):1–13. doi: 10.1186/s12936-021-03703-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Starck T, Bulstra CA, Tinto H, et al. The effect of malaria on haemoglobin concentrations: a nationally representative household fixed-effects study of 17,599 children under 5 years of age in Burkina Faso. Malar J. 2021;20(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.James SR, Abasiatti AM, Umoiyoho AJ, Abiona OO. Association between hookworm infection and anaemia among antenatal attendees in a university teaching hospital in southern Nigeria. Trop J Obstet Gynaecol. 2015;32(2):97–104. [Google Scholar]
- 69.Assefa E. Multilevel analysis of anemia levels among reproductive age groups of women in Ethiopia. SAGE Open Med. 2021;9:2050312120987375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abate TW, Getahun B, Birhan MM, et al. The urban–rural differential in the association between household wealth index and anemia among women in reproductive age in Ethiopia, 2016. BMC Womens Health. 2021;21(1):1–8. doi: 10.1186/s12905-021-01461-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Worku MG, Alamneh TS, Teshale AB, et al. Multilevel analysis of determinants of anemia among young women (15–24) in sub-Sahara Africa. PLoS One. 2022;17(5):e0268129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Woldegebriel AG, Gebregziabiher Gebrehiwot G, Aregay Desta A, et al. Determinants of anemia in pregnancy: findings from the Ethiopian health and demographic survey. Anemia. 2020;2020:1–9. doi: 10.1155/2020/2902498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wondimu H, Delelegn W, Dejene K. What do female-headed households’ livelihood strategies in Jimma city, South west Ethiopia look like from the perspective of the sustainable livelihood approach? Cogent Soc Sci. 2022;8(1):2075133. doi: 10.1080/23311886.2022.2075133 [DOI] [Google Scholar]
- 74.Negesse A, Jara D, Temesgen H, et al. The impact of being of the female gender for household head on the prevalence of food insecurity in Ethiopia: a systematic-review and meta-analysis. Public Health Rev. 2020;41(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ali JH. Gender differences in household headship and level of awareness on anaemia among Ethiopian women: evidences from a nationwide cross-sectional survey. Ethiop J Health Dev. 2018;32(2). [Google Scholar]
- 76.Getaneh D, Bayeh A, Belay B, Tsehaye T, Mekonnen Z. Assessment of the prevalence of anemia and its associated factors among pregnant women in Bahir Dar city administration, north-west Ethiopia. J Pregnancy Child Health. 2018;5(367):2. [Google Scholar]
- 77.Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72(1):257S–64S. doi: 10.1093/ajcn/72.1.257S [DOI] [PubMed] [Google Scholar]
- 78.Cai J, Ren T, Lu J, et al. Physiologic requirement for iron in pregnant women, assessed using the stable isotope tracer technique. Nutr Metab. 2020;17(1):1–9. doi: 10.1186/s12986-020-00452-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gelaw T, Ketema TG, Beyene K, Gurara MK, Ukke GG. Fear of childbirth among pregnant women attending antenatal care in Arba Minch town, southern Ethiopia: a cross-sectional study. BMC Pregnancy Childbirth. 2020;20(1):1–7. doi: 10.1186/s12884-020-03367-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elder L, Ransom E. Nutrition of women and adolescent girls: why it matters. Population reference bureau–inform, empower, advance; 2003. Available from: https://www.prb.org/nutritionofwomenandadolescentgirlswhyitmatters. Accessed July 7, 2023.
- 81.Abebaw A, Gudayu TW, Kelkay B. Proportion of immediate postpartum Anaemia and associated factors among postnatal mothers in Northwest Ethiopia: a cross-sectional study. Anemia. 2020;2020:1–10. doi: 10.1155/2020/8979740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Desta M, Kassie B, Chanie H, et al. Adherence of iron and folic acid supplementation and determinants among pregnant women in Ethiopia: a systematic review and meta-analysis. Reprod Health. 2019;16(1):1–14. doi: 10.1186/s12978-019-0848-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deriba BS, Bulto GA, Bala ET, Souza RT. Nutritional-related predictors of anemia among pregnant women attending antenatal care in central Ethiopia: an unmatched case-control study. Biomed Res Int. 2020;2020:1–9. doi: 10.1155/2020/8824291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jackson J, Williams R, McEvoy M, MacDonald-Wicks L, Patterson A. Is higher consumption of animal flesh foods associated with better iron status among adults in developed countries? A systematic review. Nutrients. 2016;8(2):89. doi: 10.3390/nu8020089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lambrecht NJ, Wilson ML, Baylin A, et al. Associations between livestock ownership and lower odds of anaemia among children 6–59 months old are not mediated by animal-source food consumption in Ghana. Matern Child Nutr. 2021;17(3):e13163. doi: 10.1111/mcn.13163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andrews C, Shrestha R, Ghosh S, et al. Consumption of animal source foods, especially fish, is associated with better nutritional status among women of reproductive age in rural Bangladesh. Matern Child Nutr. 2022;18(1):e13287. doi: 10.1111/mcn.13287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suryana S, Fitri Y, Yunianto AE, Bustami B, Lusiana SA. Nutritional education to the nutritional maternal knowledge and iron intake among toddlers with anemia. Open Access Maced J Med Sci. 2022;10(E):1434–1439. doi: 10.3889/oamjms.2022.7017 [DOI] [Google Scholar]
- 88.Taddese E, Alemu DG, Haider MR, Haile ZT. Association between receipt of nutritional counselling during antenatal care visits and anaemia: a cross‐sectional study. J Hum Nutr Diet. 2022. doi: 10.1111/jhn.13089 [DOI] [PubMed] [Google Scholar]