Abstract
Introduction
Neuroprotective drugs such as citicoline could improve cognitive performance and quality of life. We studied the effect of citicoline treatment and its association with Vascular Risk Factors (VRF) and APOE on cognition in patients with Subjective Cognitive Complaints (SCC) and Mild Cognitive Impairment (MCI).
Methods
This is an observational and prospective study with citicoline during 12 months follow-up. Eighty-one subjects who met criteria for SCC/MCI, aged 50–75 years with VRF were included and prescribed citicoline 1g/day. Subjects with previous cognitive impairment and any other central nervous system affection were excluded. Wilcoxon Signed Ranks test and paired samples t-test were used to analyze the change in neuropsychological performance.
Results
Mean age of the sample was 68.2 (SD 6.8) years and 26 (32.09%) were females. Fifteen subjects (24.6%) were APOE-ε4 carriers, fifty-six (76.7%) had hypertension, fifty-eight (79.5%) had dyslipidemia, twenty-one (28.8%) had diabetes mellitus and twenty-six (35.6%) had cardiopathy. Thirty-two (43.8%) subjects were diagnosed as SCC and forty-one (56.16%) as MCI. During the follow-up, Tweny-six patients (81.25%) in the group of SCC remained stable, six subjects (18.8%) converted to MCI. Twelve patients (29.9%) with MCI reverted to SCC and twenty-nine patients (70.7%) remained stable. At follow-up, SCC subjects had an improvement in the global language domain (p=0.03), naming (p<0.001), attention (p=0.01) and visuospatial abilities (p<0.01). MCI group showed an improvement in the screening test (p=0.03), delayed memory (p<0.01), global cognition (p=0.04) and in cognitive flexibility (p=0.03). Presence of APOE-ε4 had no impact on the above findings.
Discussion
SCC subjects showed an improvement in language and attention domains, while those with MCI performed better after 12 months in total scores of MoCA and RBANS domains, some converting back to SCC. This supports the idea that citicoline may prevent cognitive decline in patients with cognitive deficits.
Keywords: cerebrovascular disease, cognitive decline, citicoline
Introduction
Stroke is a growing global health-care problem with a substantial burden, resulting in physical or cognitive impairment and disability or even death.1 Almost half of stroke survivors have cognitive disturbances2 and cognitive deficits following stroke are even more common than stroke recurrence.3 Ganguli et al4 observed that previous history of stroke was associated with poor cognitive performance across all the cognitive domains. Benedictus et al5 studied the impact of cerebrovascular lesions in cognitive decline. 344 patients from the Amsterdam Dementia Cohort with subjective cognitive decline were evaluated at 1 year follow-up, demonstrating that 16% of subjects progressed from subjective cognitive complaints (SCC) to mild cognitive impairment (MCI) or dementia, favoring the loss of independence for activities of daily living and accelerating the need for institutionalization.6,7 Other studies pointed out a rate of conversion of 20% in subjects with SCC to MCI.8 In the recent review by Craig et al9 estimated that the prevalence of vascular dementia in the first year after stroke ranges from 1.1% to 39.2%. Based on the 16 studies included in the meta-analysis the pooled prevalence was 18.4% (95% CI 7.4 to 38.7).
Cerebrovascular risk factors (VRF) have traditionally been associated with stroke. Hypertension, diabetes, and hypercholesterolemia increase the risk of vascular cognitive impairment and Alzheimer’s type dementia.10–12 Patients with atherosclerosis, peripheral vascular disease, or diabetes in combination with APOE-ε4 carriers had a higher risk of cognitive impairment than those patients without an e4 allele or VRF.13
Safe and effective neuroprotective drugs could improve the outcome for millions of acute stroke patients through cognitive decline prevention. Citicoline has been proposed to provide neuroprotective effects through multiple mechanisms of action and participates in the biosynthesis of acetylcholine and increases metabolism and levels of norepinephrine and dopamine in the central nervous system.14,15 For its pleiotropic effects it has been proposed as a treatment for acquired brain damage, Parkinson disease, cognitive decline and stroke.14,16,17 Several studies demonstrated beneficial effects in both cognitive vascular decline and neurodegenerative diseases.3,15,18,19
IDEALE was an open-label, multicenter study, to assess the efficacy and safety of treatment with citicoline (1g/day) in patients with MCI of vascular origin.20 A total of 265 subjects ≥ 65 years old with a Mini Mental State Examination (MMSE) ≥ 21 and vascular lesions in neuroimaging tests were assessed. MMSE score in the treatment group remained stable at 3 and 9 months, while the no-treatment group had a decline at 9 months.
One of the critical points about the effectiveness of citicoline is related to the duration of clinical studies, mostly between 3 and 9 months. Therefore, it is difficult to appreciate the real effects of the treatment in the longer term.21
Alvarez-Sabin et al22 evaluated in an open-label randomized study subjects treated with citicoline 1g/day. Of 347 subjects, 199 were evaluated by means of a neuropsychological study at 12 months, showing an improvement in all cognitive domains and a significant improvement in executive functions-attention and temporal orientation compared to the group without treatment. Treatment with citicoline in patients with a first episode of ischemic stroke was safe and may be effective in improving cognitive decline. A second study23 included patients 6 weeks after having suffered a first ischemic stroke and showed that treatment with citicoline 1g/day improved cognition and quality of life in these patients at 2 years. Citicoline has shown a consistent improvement in cognitive function in patients with MCI, especially of vascular origin.24
The studies carried out to date show that the administration of citicoline 1g/day is safe and effective in vascular and post-stroke cognitive impairment, requiring chronic administration (≥ 9 months up to 2 years) to demonstrate its effectiveness.21
A limitation in the vast majority of the studies is the use of screening tests to assess the cognitive status, mainly the MMSE. Other studies pointed that the Montreal Cognitive Assessment (MoCA) is better to assess patients with cerebrovascular pathology.
The study by Sikaroodi et al25 compared performance in MMSE vs MoCA in patients with VRF. Total values in the MoCA were significantly lower in patients with two or more VRF, not showing differences in the MMSE.
Little is known about the effect of citicoline in patients with VRF and SCC. Our objective was to study the effect of citicoline treatment and its association with VRF and APOE in cognitive performance in patients with cognitive complaints and mild cognitive impairment during 12 months follow-up.
Methods
Study Design
This is a prospective interventional study without a control group of the efficacy of citicoline during 12 months follow-up. Patients with cerebrovascular disease who met criteria for SCC or MCI were evaluated at Hospital Universitari MútuaTerrassa due to memory complaints. Subjects with previous cognitive impairment and any other CNS affection were excluded. The study was approved by the local ethic committee and all subjects signed the informed consent. All participants included in the study were prescribed citicoline 1g/day.
Criteria
Demographic data, underlying comorbidities, blood tests included APOE and previous cognitive impairment were collected and evaluated in order to assess subjects’ concordance to criteria. Subjects were required to have a stroke-associated cognitive disorder meeting the following characteristics: a) meet at least 3 or more SCC plus criteria:26 i) memory-focused complaint, ii) onset of complaint in the last 5 years, iii) confirmation of cognitive change by an informant, iv) perception of performing worse than people of the same age, v) APOE-ε4 carriers. Subjects also had to meet criteria for vascular risk factors according to the Framingham Stroke Risk Profile: having had a previous transient ischemic attack or minor stroke in the last year or evidence of small vessel pathology assessed with Fazekas ≥ 1. Exclusion criteria included previous diagnosis of other neurocognitive disorders, history of affective disorder or psychosis, taking psychotropic medications or others that affect cognition (except stable hypnotic medication) in the 4 weeks prior to inclusion, history of cerebrovascular accident or traumatic brain injury that make difficult to conduct neuropsychological assessment and any condition that, in the opinion of the investigator, interferes with compliance with the study procedures.
Procedure
First study visit included the explanation of the protocol and signing the informed consent. Basal neuropsychological assessment was performed and citicoline was prescribed at 1g/day. If possible adverse events occurred, patients attended unscheduled clinical visit. Follow-up clinical visit was performed at 6 and 12 months. Treatment was discontinued or changed to an oral solution in case of adverse events. After 12 months subjects were re-evaluated with the same neuropsychological battery as at baseline, using parallel versions for the RBANS.
Cognitive Measures
A set of subtests were selected to create a Neuropsychological battery specific for this population. All tests are validated in our population and are used internationally. The battery included the MoCA test for screening, Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), Digits Forward and Backward, Corsi Forward and Backward, Trail Making Test A and B (TMT), Symbol Digit Modalities Test (SDMT), Stroop, Phonemic and Semantic fluency and Boston Naming Test from the NEURONORMA project (NN). Hospital Anxiety and Depression Scale (HAD) was administered to assess symptoms of anxiety and depression. A parallel version for the RBANS battery was used in order to avoid learning effect (version A for basal visit and version B for 12 month follow-up). Categorization of SCC or MCI was done with Delayed Memory Index of RBANS <85, considering that 1.5 SD below the mean has been frequently cited in the literature as identifying impairment in MCI.27
Statistical Analysis
Two groups were created considering the cognitive improvement after follow-up, plus 4 subgroups regarding initial SCC or MCI status and the cognitive improvement. Kolmogorov–Smirnov tests were performed to evaluate normal distribution of all quantitative variables in the study.
Descriptives
Demographic and clinical characteristics of subjects were compared across cognitive status, including comparisons regarding the longitudinal cognitive improvement, using chi-square, fisher exact, Mann–Whitney U or Kruskal–Wallis tests as appropriate.
Longitudinal Comparisons of Neuropsychological Scores
Wilcoxon Signed Ranks test and paired samples t-test were used to analyze the change in neuropsychological performance at baseline and 12 months follow-up. The neuropsychological scores of the different cognitive groups, separately at baseline and at follow-up, were compared as appropriate with Mann–Whitney U or Student’s t-test, or with Kruskal–Wallis or ANOVA with Bonferroni post-hoc correction.
Differences Among Groups and Effect of Independent Factors
Additionally, we calculated the magnitude of pre-post raw differences of RBANS sub-domains and of the total scores of the rest of the tests. These differences were compared among cognitive groups following the same method as in the previous analysis. Finally, linear regression models were created to evaluate the effect of all VRF plus APOE-ε4 carrier status over the calculated pre-post cognitive differences.
Statistical analyses were run with SPSS Statistics for Windows, Version 25 (IBM Corp., Armonk, NY). The level of significance for all analyses was set to 0.05.
Results
A total of 81 patients who had VRF, subjective cognitive complaints and/or mild cognitive impairment were included in the study and administered 1g/day of citicoline.
In our cohort, 8 patients discontinued treatment. Of the total sample, 10 (12.34%) patients had minor side-effect reactions after initiating treatment, including headache, dizziness, nausea, and vomiting. Of these patients, 5 (6.17%) improved and continued treatment switching pills for oral solution.
Descriptives
The mean age of the sample was 68.2 (SD 6.8) years and 26 (32.09%) were females. Frequency of cerebrovascular risk factors and APOE-ε4 carriers is shown in Table 1. Thirty-two (43.8%) subjects were diagnosed as SCC and 41 (56.16%) with MCI. No statistical differences were found in clinical characteristics. Subjects in SCC group were significantly younger (p=0.030) and with higher education (p=0.058). Comparing patients who were cognitively improved at 12 months as compared to those who were not, presence of hypertension was significantly higher in the group with no improvement (p=0.005).
Table 1.
Clinical and Demographic Data of the Studied Population
| Characteristics | SCC (N=32) | MCI (N=41) | Sig. |
|---|---|---|---|
| Age, y | 68 (61–72) | 72 (67–73) | 0.030 |
| Sex | |||
| Females | 11 (34.37%) | 15 (36.58%) | 0.854 |
| Males | 21 (65.62%) | 26 (63.41%) | |
| Education, y | 10 (8–15) | 8 (4–10) | 0.058 |
| APOE-ε4 | 6 (23.07%) | 9 (25.71%) | 0.813 |
| Fazekas 1 | 19 (67.85%) | 20 (55.55%) | 0.664 |
| Fazekas 2 | 6 (21.42%) | 11 (30.55%) | |
| Fazekas 3 | 3 (10.71%) | 5 (13.88%) | |
| Hypertension | 25 (78.12%) | 31 (75.06%) | 0.801 |
| Dyslipidemia | 23 (71.87%) | 35 (85.36%) | 0.157 |
| DM | 8 (25%) | 13 (31.07%) | 0.530 |
Note: Data is presented as N (%) or Median (IQR).
Abbreviations: SCC, Subjective Cognitive Complaints; MCI, Mild Cognitive Impairment; Sig., statistical significance; Y, years; DM, Diabetes Mellitus.
Longitudinal Cognitive Status
During the follow-up period, 26 patients (81.25%) in the group of SCC remained stable as SCC, 6 subjects (18.8%) converted to MCI. 12 patients (29.9%) with MCI reverted to SCC and 29 patients (70.7%) remained stable. Patients that had a worsening in cognition were similar for age and education (p>0.05). Differences in baseline performance between SCC and MCI were found in all cognitive tests except for Corsi Backward test.
Neuropsychological performance of all subjects at 12 months follow-up is described in Table 2. When analyzing the follow-up period between groups (Table 3), SCC subjects show an improvement of the RBANS indexes of language (p=0.03), attention (p=0.01) and visuospatial (p<0.01), plus in the denomination of BNT (p<0.001). Diversely, the MCI group showed an improvement in total MoCA (p=0.03), delayed memory index of the RBANS (p<0.01), in total RBANS (p=0.04) and in TMT-B (p=0.03).
Table 2.
Neuropsychological Performance of All Subjects at 12 Months Follow-Up
| Neuropsychological Test/Assessment | Score Mean | Score Mean | Sig. |
|---|---|---|---|
| T0 (Basal) | T1 (12 Months) | ||
| MoCA Total | 23 (18.5–25.5) | 24 (19–26) | 0.009 |
| RBANS IMI | 80.42 ±17.67 | 81.49 ±19.08 | 0.463 |
| RBANS VSPI | 102 (96–112) | 105 (92–109) | 0.167 |
| RBANS LNGI | 92 (87–99) | 96 (88–102) | 0.013 |
| RBANS ATI | 68 (60–85) | 75 (56–88) | 0.019 |
| RBANS DMI | 84.22 ± 17.61 | 86.97 ± 19.03 | 0.051 |
| RBANS Total | 81.58 ± 15.97 | 83.77 ± 17 | 0.039 |
| Digits forward RBANS | 8 (6–9) | 8 (7–10) | 0.023 |
| Digits Backward | 4 (3–5) | 4 (3–5) | 0.748 |
| Corsi Forward | 6 (5–8) | 6 (5–7) | 0.267 |
| Corsi Backward | 5 (4–7) | 5 (4–6) | 0.367 |
| TMT-A | 61 (48.5–93.5) | 60 (45–81) | 0.950 |
| TMT-B | 156.5 (109–217) | 138.5 (99–200) | 0.503 |
| Semantic fluency | 16.84 ± 6.05 | 17.88 ± 6.76 | 0.034 |
| Phonetic fluency | 11 (7.5–14) | 11 (8–15) | 0.765 |
| Stroop Lecture | 78.5 (59–92) | 76.5 (62–86) | 0.859 |
| Stroop Color | 48.16 ± 12.29 | 49.82 ± 12.37 | 0.577 |
| Stroop Interference | 24.78 ± 9.46 | 26.51 ± 10.96 | 0.232 |
| BNT | 49 (42–53) | 50 (42–55) | 0.006 |
| HAD A | 6.42 ± 4.13 | 6.85 ± 4.28 | 0.487 |
| HAD D | 5 (2–8) | 5 (3–8) | 0.110 |
Notes: Data is presented as Mean ± SD or Median (IQR). Statistically significant differences are marked in bold.
Abbreviations: MoCA, Montreal Cognitive Assessment; RBANS IMI, Repeatable Battery for the Assessment of Neuropsychological Status Immediate Memory Index; RBANS VSPI, Repeatable Battery for the Assessment of Neuropsychological Status Visuospatial Index; RBANS LNGI, Repeatable Battery for the Assessment of Neuropsychological Status Language Index; RBANS ATI, Repeatable Battery for the Assessment of Neuropsychological Status Attention Index; RBANS DMI, Repeatable Battery for the Assessment of Neuropsychological Status Delayed Memory Index; TMT, Trail Making Test; BNT, Boston Naming Test.
Table 3.
Neuropsychological Performance at 12 Months Follow-Up Regarding Basal Diagnosis
| Neuropsychological Test/Assessment | SCC | Sig. | MCI | Sig. | ||
|---|---|---|---|---|---|---|
| T0 (Basal)/T1 (12 Months) | T0 (Basal)/T1 (12 Months) | |||||
| MoCA Total | 24 (22–28) | 25 (23–28) | 0.16 | 20 (16–23) | 22 (19–25) | 0.03 |
| RBANS IMI | 90.63 (14.55) | 91.84 (13.21) | 0.54 | 72.46 (15.81) | 73.41 (19.81) | 0.65 |
| RBANS VSPI | 108.50 (102–121) | 105 (98–109) | 0.01 | 102 (84–109) | 100 (84–109) | 0.54 |
| RBANS LNGI | 97.50 (92–104) | 100 (93–105) | 0.03 | 88 (82–96) | 90 (85–98) | 0.14 |
| RBANS ATI | 83.5 (60–88) | 85 (77–91) | 0.01 | 60 (56–75) | 60 (53–82) | 0.62 |
| RBANS DMI | 100.78 (8.16) | 100.59 (11.15) | 0.93 | 71.29 (10.78) | 76.34 (17.07) | 0.01 |
| RBANS Total | 92.97 (11.19) | 94 (9.94) | 0.50 | 72.66 (13.30) | 75.78 (17.17) | 0.04 |
| Digits forward | 8 (7–10) | 9 (8–10) | 0.09 | 7 (6–8) | 7 (6–8) | 0.14 |
| Digits Backward | 4 (3–5) | 4 (3–5) | 0.73 | 3 (3–4) | 3 (3–4) | 0.89 |
| Corsi Forward | 7 (6–8) | 6 (6–8) | 0.38 | 6 (5–7) | 6 (5–7) | 0.46 |
| Corsi Backward | 6 (5–7) | 6 (4–6) | 0.10 | 4 (4–6) | 5 (4–6) | 0.85 |
| TMT-A | 50 (40–68) | 54.5 (36–73.5) | 0.27 | 72 (58–120) | 64.5 (52–118) | 0.36 |
| TMT-B | 127.5 (97–168.5) | 115.5 (94–173.5) | 0.50 | 201.5 (137–272) | 143.5 (115–240) | 0.03 |
| Semantic fluency | 19.81 (5.67) | 21.03 (6.15) | 0.15 | 14.51 (5.32) | 15.41 (6.23) | 0.13 |
| Phonetic fluency | 13 (9–16) | 12.71 (3.51) | 0.60 | 9.5 (5–11) | 9.76 (4.99) | 0.37 |
| Stroop Lecture | 88 (71.5–95.5) | 78.28 (21.52) | 0.33 | 71.5 (48–82) | 71.12 (20.81) | 0.21 |
| Stroop Color | 54.16 (11.13) | 53.87 (10) | 0.87 | 43.26 (11.07) | 46.12 (13.28) | 0.33 |
| Stroop Interference | 28.45 (8.27) | 30 (9.8) | 0.23 | 21.79 (9.40) | 23.32 (11.12) | 0.67 |
| BNT | 51 (48–54) | 53.5 (50–56) | 0.00 | 44 (39–52) | 46 (40–51) | 0.42 |
| HAD A | 7.31 (4.83) | 7.03 (4.06) | 0.62 | 20 (16–23) | 6.7 (4.5) | 0.18 |
| HAD D | 3.5 (1–7.5) | 5 (2.5–7.5) | 0.06 | 72.46 (15.81) | 6 (3–9.5) | 0.52 |
Notes: Data is presented as Mean ± SD or Median (IQR). Statistically significant differences are marked in bold.
Abbreviations: SCC, Subjective Cognitive Complaints; MCI, Mild Cognitive Impairment; Sig., Significance; MoCA, Montreal Cognitive Assessment; RBANS IMI, Repeatable Battery for the Assessment of Neuropsychological Status Immediate Memory Index; RBANS VSPI, Repeatable Battery for the Assessment of Neuropsychological Status Visuospatial Index; RBANS LNGI, Repeatable Battery for the Assessment of Neuropsychological Status Language Index; RBANS ATI, Repeatable Battery for the Assessment of Neuropsychological Status Attention Index; RBANS DMI, Repeatable Battery for the Assessment of Neuropsychological Status Delayed Memory Index; TMT, Trail Making Test; BNT, Boston Naming Test.
The SCC patients that remained stable over 12 months showed a significant mean improvement of 4.5 points in the language index between both assessments, 6.5 points in the attention index, 3 points in delayed memory index, 8.32 points in TMT-B and 2.69 in total RBANS. The MCI patients that reverted to SCC showed a significant mean improvement of 3.5 points in language index, 2 points in attention index, 2 points in denomination BNT and 1 point in MoCA.
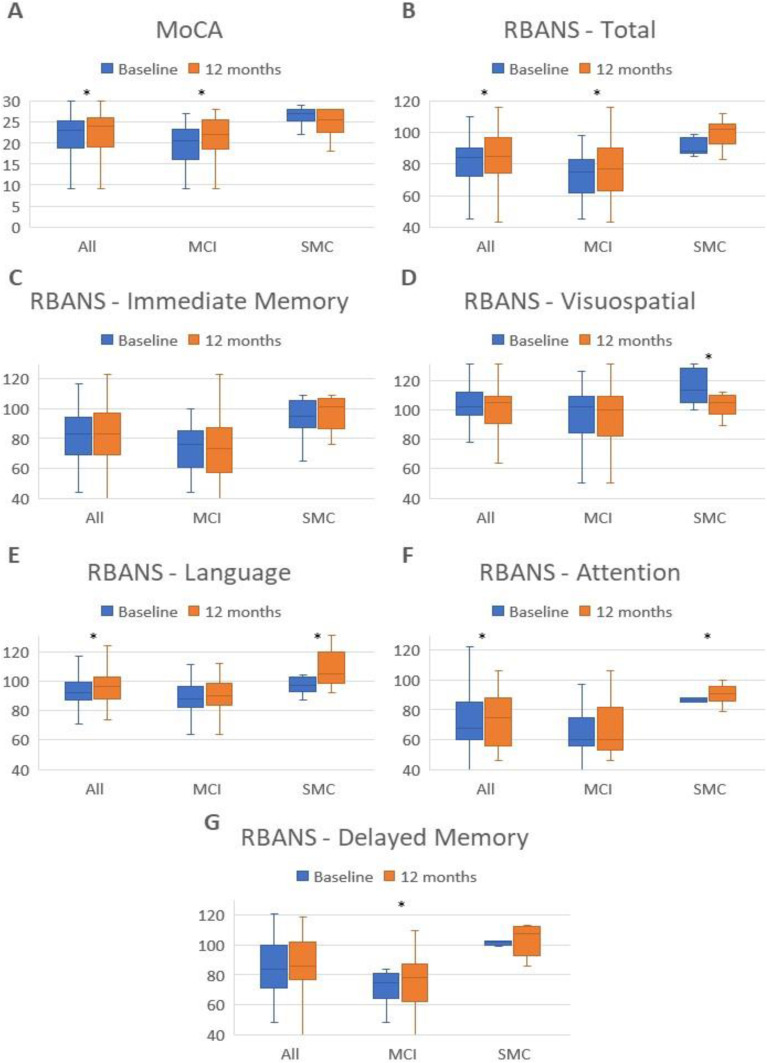
Figure 1 shows the neuropsychological performance changes of the RBANS indexes and total MoCA along follow-up for all groups.
Figure 1.
Neuropsychological performance changes of the RBANS indexes and total MoCA for baseline and 12 months in all groups.
Notes: Statistically significant differences (p < 0.05) are indicated with an asterisk. (A) represents neuropsychological performance for the total MoCA scale. (B) represents neuropsychological performance for the total RBANS scale. (C) shows neuropsychological performance for the Immediate Memory Index of the RBANS. (D) shows neuropsychological performance for the Visuospatial Index of the RBANS. (E) shows neuropsychological performance for the Language Index of the RBANS. (F) shows neuropsychological performance for the Attention Index of the RBANS. (G) shows neuropsychological performance for the Delayed Memory Index of the RBANS.
Abbreviations: MoCA, Montreal Cognitive Assessment; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status.
Regression models including VRF and APOE-ε4 status of all subjects showed no influence over longitudinal change in most cognitive tests. However, an association of hypertension with less change in RBANS attention index (p=0.015), delayed memory index (p=0.020) and total RBANS (p=0.025) was found. Having dyslipidemia correlated with smaller differences along follow-up in RBANS attention index (p=0.005).
Discussion
We aimed to evaluate the possible role of citicoline as an effective and safe treatment in a population with mild vascular cognitive impairment or cognitive complaints.
Both groups (SCC and MCI) were equivalent in terms of sociodemographic characteristics except for age (SCC were younger) and education (SCC had higher education). These differences were not found in participants that had worse scores on follow up. The later had the same sociodemographic characteristics and the groups were homogeneous in terms of APOE and VRF.
Minor adverse events were reported such as headache, dizziness, nausea and vomiting in 10 participants. These results are in line with results reported by Gareri et al14 were only 2.3% of the subjects presented mild adverse events related to citicoline. Similarly, Grieb et al18 revealed a favorable safety profile with only a few adverse events mostly related to digestive symptoms. The later remitted when treatment was interrupted or changed to an oral solution.
Neuropsychological performance at baseline showed significant differences in all cognitive domains between subjects with SCC and MCI. Although these could be expected in the memory domain, a better performance was also observed in the non-memory domains in the SCC group. This is in line with the reported evolutive pattern of vascular dementia.4,5
Regarding the longitudinal changes of the whole sample, an improvement was observed in the screening MoCA total scores, in attention, language, processing speed and memory domains as described previously.22 As described by Sikaroodi et al,24 the MoCA test can be considered as a better screening tool, compared to MMSE, for the detection of cognitive changes in patients with VRF.
Focusing on the subgroups’ evolution, the 81.25% of SCC subjects were stable one year later, and just the 18.8% converted to MCI, according to the previously reported slow progression of vascular-related cognitive deficits.5,8,9 The new finding is that 29.9% of the sample with a baseline diagnosis of MCI reverted to SCC after one year. The subjects presented an improvement in delayed memory, in total RBANS and in a processing speed. Koepsell et al28 reported a rate of conversion from MCI to normal or near-normal cognition of 16% of the subjects at one year follow-up. The citicoline may play a role in stabilizing cognitive decline in MCI subjects with VRF.
We observed cognitive changes even in those subjects who remained in the same stage during the follow-up. SCC subjects showed an improvement in language and attention domains, while those with MCI performed better after 12 months in total scores of MoCA and RBANS, delayed and working memory. These results showing improvement in some domains one year after treatment are consistent with previously described.20–23 The above supports the idea that not only the progression of vascular cognitive deficits can be slowed down, but also partially reverted to some extent. A prolonged intake of citicoline may be related with this improvement by controlling the damage caused by VRFs.
The participants clinical characteristics showed no consistent effect on cognitive changes. Indeed, only the presence of hypertension had a significant effect on the progression or minor improvement in attention, delayed memory and overall cognition. We found that being an APOE-ε4 carrier had no effect on the progression of cognitive deficits. Other studies reported the association among APOE, VRF and cognitive decline.13
Our study has some limitations, as is the lack of a control group. The degree of cognitive decline of our subjects was compared with similar studies and populations. However, cognitive improvement could be partly attributable to patients’ expectation bias, so placebo-controlled studies are needed to better elucidate the efficacy of citicoline in those patients. This is a preliminary observational study that gives room to a possible strengthening by enlarging the size sample and include a control group in future studies. The sample was made up of a larger number of men, as is expected in patients with vascular risk factors in the normal population. Criteria used to define MCI was based on delayed memory recall index of the RBANS, future studies should consider the non-memory domains in the definition of MCI especially in patients with vascular impairment. In our cohort, the learning effect was controlled by using parallel versions of the neuropsychological tests for the principal neuropsychological scale (RBANS).
In conclusion, our study longitudinally analyzed the effect of citicoline over a 1-year period. Overall, our data suggest that a daily intake of citicoline 1g may have a beneficial impact on different cognitive domains, and that may be recommended for its use in patients with cognitive impairment or cognitive complaints related to vascular risk factors. Citicoline neuroprotective effects are likely to occur with prolonged use, so longer longitudinal and placebo-controlled studies are needed.
Acknowledgment
The authors thank the patients and all the physicians and health personnel of HUMT involved in the care of these patients. EPIGENESIS Project. Instituto de Salud Carlos III. Pl17/02089 (J. Krupinski).
Funding Statement
Study was partially supported by unrestricted grant from Ferrer.
Data Sharing Statement
All study data, including raw and analyzed data and materials are available from the corresponding author on reasonable request.
Statement of Ethics
This study protocol was reviewed and approved by Hospital Universitari MútuaTerrassa committee. All participants signed the inform consent to participate in the study. The study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
M.A. fellowship was in part funded from Ferrer unrestricted grant. The authors report no other conflicts of interest in this work.
References
- 1.Alvarez-Sabín J, Román GC. The role of citicoline in neuroprotection and neurorepair in ischemic stroke. Brain Sci. 2013;3(3):1395–1414. doi: 10.3390/brainsci3031395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker Collo S, Feigin VL, Parag V, Lawes CM, Senior H. Auckland stroke outcomes study. Part 2: cognition and functional outcomes 5 years poststroke. Neurology. 2010;75(18):1608–1616. doi: 10.1212/WNL.0b013e3181fb44c8 [DOI] [PubMed] [Google Scholar]
- 3.Alvarez Sabín J, Román GC. Citicoline in vascular cognitive impairment and vascular dementia after stroke. Stroke. 2011;42(Suppl. S1):S40–S43. doi: 10.1161/STROKEAHA.110.606509 [DOI] [PubMed] [Google Scholar]
- 4.Ganguli M, Fu B, Snitz BE, et al. Vascular risk factors and cognitive decline in a population sample. Alzheimer Dis Assoc Disord. 2014;28(1):9–15. doi: 10.1097/WAD.0000000000000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benedictus MR, Van Harten AC, Leeuwis AE, et al. White matter hyperintensities relate to clinical progression in subjective cognitive decline. Stroke. 2015;46(9):2661–2664. doi: 10.1161/STROKEAHA.115.009475 [DOI] [PubMed] [Google Scholar]
- 6.Royall DR, Lauterbach EC, Kaufer D, Malloy P, Coburn KL, Black KJ. The cognitive correlates of functional status: a review from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatr Clin Neurosci. 2007;19(3):249–265. doi: 10.1176/jnp.2007.19.3.249 [DOI] [PubMed] [Google Scholar]
- 7.Gaugler JE, Kane RL, Kane RA, Clay T, Newcomer R. Caregiving and institutionalization of cognitively impaired older people: utilizing dynamic predictors of change. Gerontologist. 2003;43(2):219–229. doi: 10.1093/geront/43.2.219 [DOI] [PubMed] [Google Scholar]
- 8.Fernández-Blázquez MA, Ávila-Villanueva M, Maestú F, Medina M. Specific features of subjective cognitive decline predict faster conversion to mild cognitive impairment. J Alzheimer’s Dis. 2016;52(1):271–281. doi: 10.3233/JAD-150956 [DOI] [PubMed] [Google Scholar]
- 9.Craig L, Hoo ZL, Yan TZ, Wardlaw J, Quinn TJ. Prevalence of dementia in ischemic or mixed stroke populations: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2022;93(2):180–187. doi: 10.1136/jnnp-2020-325796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unverzagt FW, McClure LA, Wadley VG, et al. Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology. 2011;77(19):1729–1736. doi: 10.1212/WNL.0b013e318236ef23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seshadri S, Wolf PA, Beiser A, et al. Stroke risk profile, brain volume, and cognitive function. The Framingham Offspring Study. Neurology. 2004;63(9):1591–1599. doi: 10.1212/01.WNL.0000142968.22691.70 [DOI] [PubMed] [Google Scholar]
- 12.Gorelick PB, Scuteri A, Black SE, et al. AHA/ASA Scientific statement: vascular contributions to cognitive impairment and dementia. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haan MN, Shemanski L, Jagust WJ, Manolino TA, Kuller L. The role of APOE E4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA. 1999;282(1):40–46. doi: 10.1001/jama.282.1.40 [DOI] [PubMed] [Google Scholar]
- 14.Gareri P, Castagna A, Cotroneo AM, et al. The role of citicoline in cognitive impairment: pharmacological characteristics, possible advantages and doubts for an old drug with new perspectives. Clin Interv Aging. 2015;10:1421–1429. doi: 10.2147/CIA.S87886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Secades JJ, Lorenzo JL. Citicoline: pharmacological and clinical review, 2022 update. Rev Neurol. 2022;75(Suppl 5):S1–S89. doi: 10.33588/rn.75s05.2022311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blount PJ, Nguyen CD, McDeavitt JT. Clinical use of cholinomimetic agents: a review. J Head Trauma Rehabil. 2002;17(4):314–321. doi: 10.1097/00001199-200208000-00005 [DOI] [PubMed] [Google Scholar]
- 17.Hurtado O, Lizasoain I, Moro MA. Neuroprotection and recovery: recent data at the bench on citicoline. Stroke. 2011;42(Suppl 1):S33–S35. doi: 10.1161/STROKEAHA.110.597435 [DOI] [PubMed] [Google Scholar]
- 18.Grieb P. Neuroprotective properties of citicoline: facts, doubts and unresolved issues. CNS Drugs. 2014;28(3):185–193. doi: 10.1007/s40263-014-0144-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Cobos R, Frank-Garcia A, Gutierrez-Fernández M, Díez-Tejedor E. Citicoline, use in cognitive decline: vascular and degenerative. J Neurol Sci. 2010;299(1–2):188–192. doi: 10.1016/j.jns.2010.08.027 [DOI] [PubMed] [Google Scholar]
- 20.Cotroneo AM, Castagna A, Putignano S, et al. Effectiveness and safety of citicoline in mild vascular cognitive impairment: the IDEALE study. Clin Interv Aging. 2013;8:131. doi: 10.2147/CIA.S38420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gareri P, Castagna A. Citicoline in vascular cognitive impairment. Some latest evidences. Ann Alzheimers Dement Care. 2017;2(1):18–19. doi: 10.17352/aadc.000004 [DOI] [Google Scholar]
- 22.Alvarez-Sabin J, Ortega G, Jacas C, et al. Long-term treatment with citicoline may improve poststroke vascular cognitive impairment. Cerebrovasc Dis. 2013;35(2):146–154. doi: 10.1159/000346602 [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Sabin J, Santamarina E, Maisterra O, et al. Long-term treatment with citicoline prevents cognitive decline and predicts a better quality of life after a first ischemic stroke. Int J Mol Sci. 2016;17(3):390. doi: 10.3390/ijms17030390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bermejo PE, Dorado R, Zea-Sevilla MA. Role of citicoline in patients with mild cognitive impairment. Neurosci Insights. 2023;18:26331055231152496. doi: 10.1177/26331055231152496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sikaroodi H, Yadegari S, Miri SR. Cognitive impairments in patients with cerebrovascular risk factors: a comparison of Mini Mental Status Exam and Montreal Cognitive Assessment. Clin Neurol Neurosurg. 2013;115(8):1276–1280. doi: 10.1016/j.clineuro.2012.11.026 [DOI] [PubMed] [Google Scholar]
- 26.Sánchez-Benavides G, Grau-Rivera O, Suárez-Calvet M, et al. Brain and cognitive correlates of subjective cognitive decline-plus features in a population-based cohort. Alzheimers Res Ther. 2018;10(1):123. doi: 10.1186/s13195-018-0449-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985 [DOI] [PubMed] [Google Scholar]
- 28.Koepsell TD, Monsell SE. Reversion from mild cognitive impairment to normal or near-normal cognition: risk factors and prognosis. Neurology. 2012;79(15):1591–1598. doi: 10.1212/WNL.0b013e31826e26b7 [DOI] [PMC free article] [PubMed] [Google Scholar]