Abstract
A core component of metacognition is cognitive awareness, insight into how one’s cognitive abilities compare to others. Previous studies of cognitive awareness have focused on basic aspects of perception, memory, and learning. Further, studies of the awareness of one’s social cognitive abilities have been limited to examining awareness of others’ thinking (i.e., theory of mind). The current study characterizes awareness of one’s own social cognitive abilities, specifically face recognition awareness, and examines its change across the lifespan. We used a large, web-based sample (N=4,143) with a broad age range (ages 10-70), administering well-validated measures of objective (Cambridge Face Memory Test 3) and self-reported (Cambridge Face Memory Questionnaire) face recognition. We found a robust overall association between objective and self-reported face recognition (r=0.42 in females, r=.36 in males). While we found that face recognition ability peaked in the early-to-mid 30s, face recognition awareness peaked in the early-to-mid 20s, was relatively stable throughout the 20s-40s, and declined in the 50s-60s. Relative subjective vs. objective face recognition bias measures demonstrated that 10-18- and 51–70-year-olds overestimated their self-reported face recognition abilities in comparison to 19-50-year-olds. Finally, compared to males, females had greater face recognition awareness and a bias to relatively underestimate their face recognition abilities.
Keywords: face recognition, metacognition, cognitive awareness, development, aging
Metacognition is the capacity to understand one’s own thinking (Palmer et al., 2014; Frith, 2012) and one core component is the awareness of how one’s cognitive abilities compare to others—cognitive awareness (also referred to as metacognitive knowledge or cognitive insight, for a review, see Norman et al., 2019; and for a metasynthesis, see Zell and Krisan, 2014). Cognitive awareness predicts success in school, from primary education through college (Zhao et al., 2014). In adults, poorer cognitive awareness has been associated with psychiatric disorders (e.g., David et al., 2012; Medalia & Thysen, 2008) and impacts real-world, performance-based tasks such as driving (Ross et al., 2012). Further, previous studies have shown that many domains of cognitive awareness decline with age (e.g., Weil et al, 2013; Palmer et al., 2014) and poor awareness of cognitive decline may be an early indicator of the Alzheimer’s Disease (Cacciamani et al., 2017). Previous studies of cognitive awareness have mainly focused on examining basic aspects of perception, memory, and learning (e.g., Palmer et al., 2014; Soto & Silvanto, 2014). However, another aspect of cognitive awareness that is critical in daily life is awareness of one’s social cognitive abilities, since social relationships are a key contributor to happiness and life satisfaction (Diener & Seligman, 2002; Taylor et al., 2001). Prior studies of social cognitive awareness have been limited to examining awareness of others’ feelings, thoughts, and intentions, i.e., theory of mind (for a review, see Moran, 2013). The goal of the current study was to characterize awareness of one’s own social cognitive abilities and to examine how this awareness changes across the lifespan.
One particularly remarkable social cognitive ability is our proficiency in recognizing a large number of people by their face, with the average person being able to readily identify approximately 5,000 faces (Jenkins et al., 2018). Deficient face recognition ability (e.g., prosopagnosia) can lead to pronounced person-recognition difficulties with significant consequences for daily life functioning, such as increased social anxiety and more limited employment opportunities (Yardley et al., 2008). Having accurate awareness of one’s face recognition abilities can help mitigate issues associated with poor face recognition, such as using compensatory mechanisms or setting up situations where you do not need to rely solely on face recognition. Importantly, it can also lead to more accurate attributions of social behavior and improve social functioning. For example, if one is aware that they have excellent face recognition, they could be less likely to interpret another’s failed recognition of them as a personal slight. Further, prosopagnosics that become aware of the extent of their face recognition deficits through research participation typically report considerable relief and often more accurately reinterpret previous life experiences (e.g., they realize that growing up they were not inattentive or uncaring about others but rather had severe face recognition deficits, Dingfelder, 2019).
Some studies have found that awareness of one’s face recognition ability is rather limited, reporting small to non-significant associations (e.g., Bowles et al., 2009; see review by Palermo et al., 2017). However, more recent studies have reported robust associations between validated measures of objective face recognition (e.g., Cambridge Face Memory Test-CFMT) and self-report measures (e.g., Cambridge Face Memory Questionnaire-CFMQ vs. CFMT, r=.44, Arizpe et al., 2019; Prosopagnosia Index 20-PI-20 vs. CFMT, r=−.39, Gray et al., 2017; Portuguese PI-20 vs. CFMT, r=−.40, Ventura et al., 2018). This suggests that individuals have substantial insight into their face recognition abilities, despite infrequently receiving specific feedback on their face recognition performance compared to skills with more frequent, explicit feedback, e.g., mathematics or sports performance. Fewer studies have examined individual differences or age-related changes in face recognition awareness. Murray et al. (2018) reported that the majority of their developmental prosopagnosics (21 out of 33) did not gain insight into their face recognition deficits until after age 19, suggesting children and adolescents may have reduced awareness of face recognition deficits. With regards to age effects, Bowles et al. (2009) found that whereas adults 18-32 years old showed some modest degree of face recognition awareness (objective/subjective r=.22, p<.05), older adults 55-88 years old showed little-to-no association between subjective and objective face recognition (r=−.05). Interestingly, younger and older adults in Bowles et al. reported very similar levels of subjective face recognition abilities despite younger adults’ better objective face recognition abilities compared to older adults. A recent paper by Murray and Bate (2019) also found that males with developmental prosopagnosia reported significantly fewer subjective face recognition deficits than females with the same level of objective face recognition impairments, perhaps indicating a bias for males to minimize their subjective face recognition deficits.
Despite this handful of studies examining age and gender differences in face recognition awareness, the lifespan trajectory of this metacognitive ability and of cognitive awareness in general remains incompletely characterized. One important question is whether the awareness of an ability develops, peaks, and declines concurrently with the ability itself or rather if they have different lifespan trajectories. The prediction of ability and awareness having similar trajectories comes from a series of studies which have demonstrated that decreased ability is often accompanied by decreased cognitive awareness as well (e.g., Kruger & Dunning, 1999; Ehrlinger et al., 2008). Consistent with these results, studies have found that during a visual contrast-sensitivity task where participants made a judgment followed immediately by a confidence rating, perceptual awareness increased from early to late adolescence (11-18 years old, Weil et al., 2013) and subsequently declined in middle and older age (Palmer et al., 2014), a very similar trajectory to raw contrast sensitivity ability (McKendrick et al., 2013). Weil et al. (2013) also reported that females had greater overall perceptual awareness than males. One drawback to these studies is that they investigated cognitive abilities that peak around the same time when general metacognitive processes have shown to peak (e.g., in the early 20s, Weil et al., 2013). Face recognition ability is unusual—compared to more basic perception abilities, short-term memory, and executive functions that peak in the late-teens and early 20s (Hartshorne & Germine, 2015), the lifetime peak of face recognition is considerably later, in the early-to-mid 30s (Germine et al., 2011). Thus, face recognition ability is a good test case to determine whether cognitive ability and awareness necessarily coincide or rather if face recognition awareness is similar to other meta-cognitive functions that peak in the early 20s (e.g., Reese et al., 2011). Further, previous studies examining lifespan changes in cognitive awareness have used trial-by-trial judgments of awareness (e.g., Weil et al., 2013). These paradigms provide an indication of whether an individual was aware of their performance on a particular trial (i.e., local metacognition, see Soew et al., 2021) and have shown to correlate with metacognitive abilities in general (e.g., Mazancieux et al., 2020). However, examining the relationship between self-report questionnaires about one’s general ability vs. objective ability (i.e., global metacognition, Soew et al., 2021) may capture additional daily life cognitive awareness information, complementing trial-by-trial paradigms. Finally, task-specific self-report measures may be subject to biases (e.g., in easy tasks people are more likely to rate themselves as above average compared to difficult tasks), which can influence how individuals report their abilities in comparison to others (Kruger, 1999) and global metacognition measures may be less susceptible to these effects.
To further characterize the lifespan changes in face recognition awareness, we examined a large, web-based sample (N=4,143) with a broad age range (ages 10-70) on well-validated measures of objective (CFMT3) and self-reported face recognition (CFMQ, Arizpe et al., 2019). Our measure of interest was the strength of association between self-reported and objective face recognition ability, similar to other investigations of cognitive awareness (e.g., Ackerman & Wolman, 2007; Serra-Blasco et al., 2019; Vardy et al., 2006), and how this association differs with age. We also examined age-related changes in subjective vs. objective biases as well as slopes of the best fit objective-predicting-subjective regression line for each age group, i.e., how many units of self-reported ability are traded off for a unit of objective ability. To examine these measures, we divided our sample into seven bins: Ages 10-18, 19-22, 23-26, 27-31, 32-38, 39-50, and 51-70. Each bin had at least 200 participants per gender to ensure that correlations could be reliably calculated for each age bin and across genders (Guadagnoli & Velicer, 1988). Finally, we sought to compare face recognition awareness across genders, considering that females have previously shown greater cognitive awareness on perceptual tasks (e.g., Weil et al., 2013) and males may minimize their face recognition deficits compared to females (Murray & Bate, 2019).
Methods
Participants
Participants performed the study on www.TestMyBrain.org, a cognitive testing website accessed through search engines, social media and news sites, where participants receive feedback on their cognitive performance compared to population norms (Fortenbaugh et al., 2015; Germine et al., 2011; Germine et al., 2012; and Riley et al., 2016). The participants in this study were 4,143 (2,610 female) unpaid volunteers who visited TestMyBrain.org between January 2015 and March 2015. All participants gave informed consent in accordance with guidelines set forth by the Committee on the Use of Human Subjects at Harvard University (protocol # F15795–122) and the Wellesley College Institutional Review Board. Additionally, participants completed a voluntary demographic survey which included questions related to age, gender, location, native language, education, and ethnicity. All participants received feedback on their performance relative to others at the end of the tasks. Notably, data from www.TestMyBrain.org has been shown to be of comparable high quality and reliability when compared with data gathered in a laboratory setting (Germine et al., 2012) and has been extensively used to study population dynamics across various cognitive, perceptual, and neuropsychological tests and experiments (Arizpe et al., 2019; Fortenbaugh et al., 2015; Riley et al., 2016; Rothlein et al., 2018), including face recognition (Germine et al. 2011; Susilo et al., 2013).
Tasks and Procedure
Each participant performed the following tasks in a fixed order: (1) the Cambridge Face Memory Questionnaire, (2) the Cambridge Face Memory Test version 3, and (3) a Famous Faces Memory Test.
The Cambridge Face Memory Questionnaire (CFMQ) is a valid and reliable 18-item self-report questionnaire designed to measure one’s daily face recognition abilities (see Arizpe et al., 2019 and Supplemental Table S1). The CFMQ includes questions assessing the frequency of both positive and negative face recognition occurrences in everyday life and includes one question assessing one’s face recognition skills compared to others. For all but one question, the following five frequency options were given: 1) Never or Almost Never, 2) Not Usually, 3) Sometimes, 4) Frequently, 5) Always or Almost Always. It should be noted that questions 3, 4, 5, 6, 9, 13, 14, 15, 16, and 18 were reverse scored. The first question “Compared to my peers, I think my face recognition skills are…” gave the following options: 1) Far Below Average, 2) Somewhat Below Average, 3) Average, 4) Somewhat Above Average, and 5) Far Above Average. The minimum score on the CFMQ is 18, indicating worse self-reported face recognition, and the maximum is 90.
The Cambridge Face Memory Test (CFMT; Duchaine & Nakayama, 2006) is a widely used test of unfamiliar face recognition in which participants are required to learn and recognize six target faces in conditions of varying difficulty. We used the internet-based version of this test, CFMT3 (Germine et al., 2012). Faces were bald and presented in grayscale with no distinguishing non-facial features. The first part of the test introduced six target faces to participants. Each target face was presented at three different angles for 3 s each. Next, participants performed a 3-alternative forced-choice task with the learned target face and two non-target distractors presented in the same angle and lighting. After the learning phase, all six target faces were shown simultaneously for 20 s and participants were instructed to memorize these faces. Next, participants were then tested on 30 forced-choice trials, where each trial included one of the six target faces and two non-target faces shown in novel views and lighting conditions. After another 20 s study period with the same six target faces, in the last 24 trials visual noise was added to stimuli to make the tasks more difficult.
The CFMT3 is identical to the original version of the CFMT developed by Duchaine and Nakayama (2006), except that instead of photographs of faces, the CFMT3 uses novel artificial faces that were generated with FaceGen software (Singular Inversions, Toronto, ON). As our experiment was widely and publicly available online, we refrained from using the original CFMT in our study to maintain the integrity of the original CFMT for clinical or in-lab use, for example, as part of a diagnosis of prosopagnosia. In terms of the validity of using artificial faces in the CFMT3, there is an ongoing debate and some studies have found that artificial faces are more difficult to remember than real faces (Balas et al., 2015). However, others have found similar overall recognition performance and robust face inversion effects (e.g., Kätsyri, 2018), suggesting very similar processing to real faces. Notably, Wilmer et al. (2010) employed very similar FaceGen facial stimuli in the CFMT format and found a strong correlation with the original CFMT using real faces (r = .76). This is close to internal consistency values reported for the original CFMT (ranging from .75 to .90), suggesting a high correspondence. Further, we previously found a high correlation between the CFMT3 and Famous Face Memory Test (FFMT) scores, r(1516) = 0.47, p < .001 (Arizpe et al, 2019), suggesting that the FaceGen faces in the CFMT3 provide a valid measure of individual differences in face recognition ability.
After the CFMT3, participants also completed one of three versions of a Famous Face Memory Test (FFMT, see Mishra et al., 2019 for an in-depth description). We chose to focus our analyses on the CFMT rather than the FFMT because 1) CFMT is a more widely used measure of individual differences in face recognition ability than the FFMT, and 2) FFMT performance may depend more on one’s previous experience with the famous faces (e.g., degree of interest in and exposure to American media).
Analyses
Our analyses focused on continuous measures of objective (CFMT3) and subjective (CFMQ) face recognition ability as well as metacognitive measures—correlation, slope, and subjective/objective bias, measured for each of the seven age bins: 10-18, 19-22, 23-26, 27-31, 32-38, 39-50, and 51-70. We also analyzed the entire sample and each age bin separately for male and female participants.
To determine the age associated with best performance on the CFMT3, we used a peak analysis method adapted from Germine et al. (2011). The data was fitted to a quadratic function with score plotted linearly and age plotted logarithmically, as this more closely fit a quadratic function as compared to age plotted linearly (see Supplemental Figure 3A). A bootstrap resampling procedure with 1000 samples was used to generate an estimate of the mean of the peak, as well as a standard error and confidence interval, from the maximums of the best-fit quadratic functions of the samples (Efron & Tibshirani, 1993). The standard deviation of these values was used as the estimate of standard error (DiCiccio & Efron, 1996; Germine et al., 2011). A 95% confidence interval was generated using the percentile method (Efron & Tibshirani, 1993).
To measure the strength of the relationship between subjective and objective face recognition ability, i.e., group metacognitive sensitivity, we calculated the Pearson correlation coefficients between CFMT3 and CFMQ for each bin. For converging evidence, within each bin we also calculated the slope and intercept of the best-fit regression line of CFMT3 predicting CFMQ. Although correlations and slopes are often highly associated, correlations represent a scaleless indicator of the linear association between two measures (subjective vs. objective ability) whereas slopes indicate how many units of one measure are traded off for a unit of another, providing some advantages when comparing slopes between groups. As a final measure, we calculated a measure of subjective vs. objective performance bias. To do this, we first z-transformed each participant’s CFMQ and CFMT3 score based on the total sample M and SD, and then calculated a z-score difference of CFMQ minus CFMT3. One drawback to collapsing across all participants on the CFMQ is that one CFMQ item reads, “Compared to my peers, I think my face recognition skills are…”. However, the rest of the items are not relative to one’s peer group and the results were very similar when removing this item.
To compare correlation coefficients in different age bins and across gender, we used the Fisher r-to-z transformation to normalize the sampling distributions of the correlations (Fisher, 1915). We performed a test of equality on the Fisher z transformations and evaluated the results using Chi-square tests, while z-tests were used to compare chronological pairs of age bins (Howell, 2011). Regression slopes in chronologically adjacent age bins were compared using a t-test for testing the difference between two independent regression coefficients, as described in Cohen et al., 2003. False Discovery Rate (FDR) corrected p-values were used to correct for multiple comparisons (Benjamini et al., 1995).
As a robustness check of the data and to ensure our binning method did not influence the findings, we also plotted the results as a continuous moving window of 600 participants, approximately the average bin size of our categorical analyses (see Supplementary Figure S4). We also performed an exploratory multiple regression analysis predicting CFMQ from CFMT3, age, age2, CFMT3 x age, and CFMT3 x age2 (see Supplementary Tables S2).
Sample Size Justification
Our sample size was calculated to examine at least seven age bins across the lifespan. Our main measure of interest was metacognitive accuracy, the correlation between subjective and objective face recognition. To ensure that differences in correlations of .1 or greater between age bins could be reliably detected, we sought to obtain at least 400 participants per age bin (minimum of 200 males and 200 females).
Transparency and Openness
Materials and analysis code for this study are available by emailing the corresponding author. We report how we determined our sample size. This study was not pre-registered.
Results
Participants
There were 4,143 participants with ages ranging from 10 to 70 years1 (M = 32.41, SD = 15.06). As shown in Table 1, there were significantly more female participants than males in the sample (overall female: 63%, overall male: 37%; X2(6, N=4143) = 36.687, p < 0.001), similar to other studies from www.TestMyBrain.org (Germine et al., 2011). With regards to education, 2.4% of the participants attended middle school, 17.2% went to high school/secondary school, 24.6% attended some college/university, 23.3% held a bachelor’s degree, 24.7% received a graduate degree, and 3.1% did not indicate their level of education. For the analyses, the participants’ data were divided into the following seven age bins where every bin included at least 200 individuals from each gender: Age 10-18, Age 19-22, Age 23-26, Age 27-31, Age 32-38, Age 39-50, and Age 51-70. Including at least 200 male and female participants in each age bin ensured that correlations could be reliably calculated for each age bin and across genders (Guadagnoli & Velicer, 1988).
Table 1.
Participant Demographics (N = 4,313)
| Group | Age Range | Average Age | n | Males | Females |
|---|---|---|---|---|---|
| 1 | 10-18 | 16.0 | 777 | 238 | 539 |
| 2 | 19-22 | 20.4 | 600 | 219 | 381 |
| 3 | 23-26 | 24.4 | 537 | 208 | 329 |
| 4 | 27-31 | 28.9 | 504 | 219 | 285 |
| 5 | 32-38 | 34.8 | 485 | 214 | 271 |
| 6 | 39-50 | 44.4 | 573 | 209 | 364 |
| 7 | 51-70 | 59.5 | 667 | 226 | 441 |
Objective and Subjective Face Recognition Performance Across the Lifespan
The goal of the current study was to characterize the change in face recognition awareness across the lifespan. Before quantifying face recognition awareness, we first sought to examine its constituent measures: lifespan changes in objective and subjective (i.e., self-reported) face recognition ability.
Cambridge Face Memory Test
We examined the CFMT3 to characterize lifespan changes in learning and recognizing novel faces. We generally replicated previous CFMT findings (Germine et al., 2011) showing a rapid increase in childhood, peak in the early to mid 30s (M = 33.374, SE = 1.025, 95% CI [31.862, 35.111]) and slow decline in later adulthood (see Figure 1A and Supplemental Figure S1). A two-way ANOVA of gender (male/female) and age (7 age bins) on CFMT scores revealed a main effect of age (F(6, 4142) = 1.665, p < 0.001), but no significant effect of gender nor age x gender interaction (all p’s > .13).
Figure 1. Self-reported (CFMQ) and objective face recognition (CFMT3) and bias (CFMQ - CFMT3) across the lifespan.

Note. CFMQ (Cambridge Face Memory Questionnaire), CFMT3 (Cambridge Face Memory Test version 3), and Bias (CFMQ – CFMT3). Note that the dots represent the average of consecutive 2-year age bins and the blue area represents the 95% confidence interval around the best-fit quadratic function. Positive bias scores indicate better self-report rating compared to objective face recognition ability in comparison to the entire population (over-confidence) whereas negative bias scores indicate lower self-report compared to objective face recognition ability (under-confidence).
Comparing chronological age bins (i.e., Age bins 10-18 vs 19-22; Age 19-22 vs Age 23-26; Age 23-26 vs Age 27-31; Age 27-31 vs Age 32-38; Age 32-38 vs Age 39-50; and Age 39-50 vs Age 51-70) revealed a significant increase in performance between ages 10-18 and 19-22 (t (1375) = −3.94; p < 0.001; False Discovery Rate (FDR) corrected probability: q = 0.000369) as well as significant decrease in performance between ages 39-50 and 51-70 (t(1238) = 3.85; p < 0.001; FDR corrected probability: q = 0.00036).
Cambridge Face Memory Questionnaire
We next examined self-reported face recognition performance across the lifespan using the CFMQ, which revealed a markedly different pattern (see Figure 1B). Scores were relatively flat across the lifespan and increased in later adulthood, indicating higher ratings of self-reported face recognition with age. Further, we found that males rated themselves as better at face recognition than females (see Supplemental Figure S2), even though there were no differences between males and females in objective face recognition performance. Change across the lifespan was confirmed by a two-way ANOVA with gender and age (7 age bins) on CFMQ score, showing a main effect of age (F(6, 4142) = 5.176, p < 0.001) and gender (F(6, 4142) = 12.436, p < 0.001), but no significant age x gender interaction. Comparing chronological pairs of age bins across gender only revealed a significant increase in self-reported recognition between the bins ranges of Age 39-50 and Age 51-70 (t(1238) = −4.54, p < 0.001; FDR corrected probability: q = 0.00003). For all other adjacent age pairs, the differences were not significant (all p’s>.30).
Measuring Face Recognition Awareness Across the Lifespan
The main objective of this study was to characterize age-related changes in individuals’ awareness of their face recognition ability. We first examined our primary measure of face recognition awareness, the correlation between objective and subjective face recognition performance (i.e., group metacognitive sensitivity). As secondary measures, we examined the best-fit regression line of objective measures predicting subjective measures and the subjective minus objective performance bias score.
When examining the associations between objective measures and subjective measures of face recognition for each age group, we found that the associations increased rapidly from 10-18 to 19-22 years old, peaked in the mid 20s, were relatively stable until the mid 40s, and declined in later adulthood (51-70 years). We next evaluated whether this peak matched the peak of objective face recognition ability on the CFMT3. The mid 20s peak of metacognitive accuracy (objective-subjective associations) did not fall within the peak of CFMT3 accuracy, 95 % CI [31.862, 35.111], suggesting that peak objective and subjective awareness do not temporally coincide.
To test whether these lifespan changes were significant, we performed a test of equality on the Fisher z transformations of the 7 age bin correlations and evaluated the results using the Chi-square test (see Methods). We clearly found that the null hypothesis, that all correlation coefficients are equal, should be rejected (c2-statistic = 41.60, p < 0.001). We then tested for differences in adjacent chronological pairs of Pearson correlation coefficients. We found significant correlation differences between 10-18 vs. 19-22 age bins (Z = −3.46; p < 0.001; FDR corrected probability: q =0.003) and 39-50 vs. 51-70 age bins (Z = 2.53, p = 0.011; FDR corrected probability: q = 0.03), while the others were not significant (all p’s > 0.3; see Figure 2).
Figure 2. Group Metacognitive Sensitivity (CFMT3 x CFMQ correlations) across the lifespan.
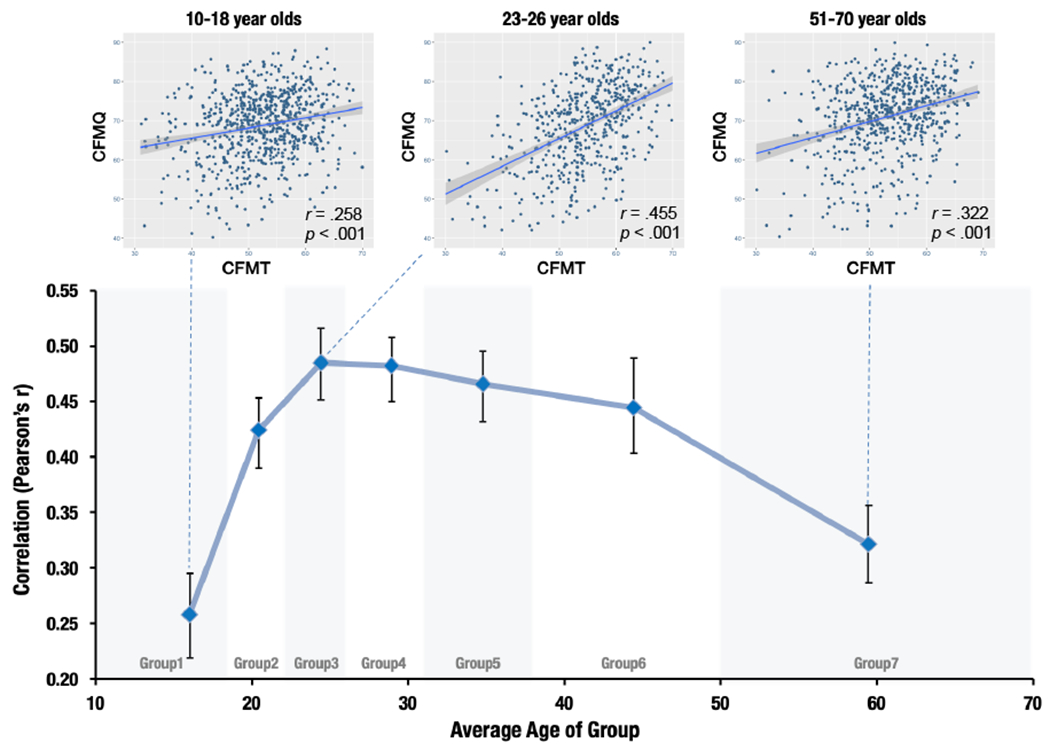
Note. Each age bin data point represents the Pearson correlation between CFMT3 (Cambridge Face Memory Test version 3) and CFMQ (Cambridge Face Memory Questionnaire) for participants in that bin, with error bars indicating 95% confidence intervals. To illustrate the lifespan changes in the association between CFMT3 and CFMQ, individual subject data are shown for 10-18, 23-26, and 51-70 year old age bins.
To further characterize the nature of these age-related differences, we next examined the change in the best-fit regression lines of objective measures predicting subjective face recognition performance across the age bins. These slope measures, compared to the correlation coefficients above which are scaleless indicators of the subjective vs. objective ability relationship, indicate how many units of subjective ability are traded off for a unit of objective ability. That said, it should be noted that as the subjective/objective correlation comes closer to zero, so does the regression slope. As can be seen in Supplemental Figure S3, for CFMT predicting CFMQ, we found that 10-18 year-olds and 51-70 year-olds had shallower slopes than individuals in the mid-years of life. In particular, this was driven by 10-18 and 51-70 year-olds with worse objective face recognition tending to rate themselves as having better face recognition abilities. We confirmed these differences by comparing regression slopes in chronologically adjacent age bins. The results indicated that the slope of the regression lines for CFMT predicting CFMQ significantly differed between 10-18 and 19-22 year-olds (t(1373) = 4.10, p < 0.001; FDR corrected probability: q = 0.0003) as well as between 39-50 vs. 51-70 year-olds (t(1236) = 3.432, p < 0.001; FDR corrected probability: 0.0018), but not for any other adjacent age bins (all p’s > .05). An examination of the y-intercepts showed a very similar pattern to the slopes (see Supplementary Materials).
Lastly, we investigated age-related differences in individuals’ awareness of their face recognition abilities using the subjective minus objective bias scores. We calculated z-score differences between subjective ratings and objective ratings. Positive bias indicates higher self-report ratings compared to objective scores (i.e., over-confidence relative to the population mean) whereas negative bias implies higher objective scores than self-report ratings (i.e., under-confidence relative to the population mean). As can be seen in Figure 1C, we found that 10-18 and 51-70 year-olds had the highest positive bias scores across all age bins, meaning higher subjective than objective ratings (i.e., over-confidence relative to the mean), consistent with the regression slope analysis. In contrast, 19-50 year-olds had slightly negative bias scores, suggesting higher objective than subjective ratings (i.e., under-confidence). These bias differences between age groups were confirmed to be significant by one-way ANOVA (F(6, 4142) = 20.655, p < 0.001). Comparing bias scores for chronological pairs of age bins via unpaired t-tests revealed similar findings to the CFMT vs. CFMQ correlations. For the subjective-objective bias scores, there were significant bias differences between Age 10-18 and Age 19-22 (t(1375) = 4.37, p < 0.001; FDR corrected probability: q = 0.000039) as well as between Age 39-50 and Age 51-70 (t(1238) = −7.58, p < 0.001; FDR corrected probability: q = 0.0000000000004). These results demonstrate that reduced face recognition awareness earlier (Age 10-18) and later (Age 51-70) is also accompanied by overconfidence in one’s face recognition abilities.
Gender Differences in Awareness of Face Recognition Ability
Because studies have shown a female advantage in several aspects of social cognition (e.g., Reading the Mind in the Eyes, see Vellante et al., 2013; face recognition, Herlitz & Loven, 2013; McKelvie et al., 1993; though see Bowles et al., 2009), we next sought to compare face recognition awareness between males and females. We found a relatively similar lifespan pattern in both males and females (see Figure 3A), but interestingly, compared to males, female participants showed a significantly greater overall association between CFMT and CFMQ (females: r=0.419 vs. males: r=0.358, Z = 3.46, p = 0.0257). We then tested for correlation differences in pairwise comparisons for each age bin across gender (e.g., correlation for Female Age 10-18 vs. Male Age 10-18) for CFMT vs. CFMQ. None of the pairwise comparisons were significantly different (all p’s > 0.1).
Figure 3. Group Metacognitive Sensitivity (CFMT x CFMQ correlations) and Bias (CFMQ – CFMT) across the Lifespan Separated by Gender.
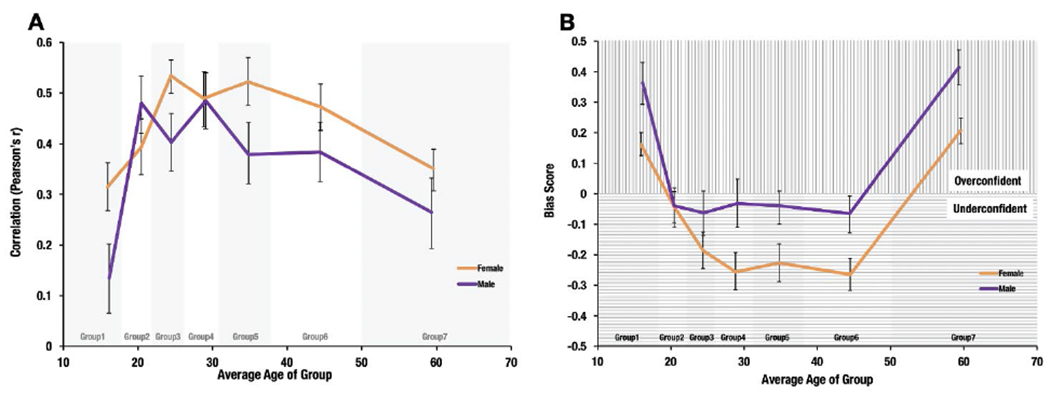
Note. A) Each age bin data point represents the Pearson correlation between CFMT3 (Cambridge Face Memory Test version 3) and CFMQ (Cambridge Face Memory Questionnaire) for participants in that bin, with error bars indicating 95% confidence intervals. For bias scores (B), positive values indicate better self-report rating compared to objective face recognition ability in comparison to the entire population (over-confidence) whereas negative bias scores indicate lower self-report compared to objective face recognition ability (under-confidence). Error bars indicate 95% confidence intervals.
We next compared the regression slopes of objective predicting subjective performance between males and females (see Supplemental Figure 3B). Again, we found an overall difference between male and female regression slopes for CFMT predicting CFMQ (females: 0.637 vs. males: 0.482, t(4139) = 3.70, p < 0.001). In addition, a series of FDR-corrected pairwise comparisons for each age bin revealed significant gender differences for CFMT predicting CFMQ (Age 10-18 (t(773) = 2.987, p = 0.003; FDR corrected probability: q = 0.02), Age 23-26 (t(533) = 2.698, p = 0.007; FDR corrected probability: q = 0.025), and Age 32-38 (t(481) = 2.681, p = 0.008; FDR corrected probability: q = 0.018). Y-intercepts showed a similar pattern (see Supplementary Materials).
Finally, we ran a series of unpaired t-tests to compare subjective minus objective bias scores between males and females. As demonstrated by Figure 3B, the results showed that males had more of a bias than females to report higher self-reported vs. objective face recognition (t(4141) = 3.804, p < 0.001). In fact, the average bias scores for males across all age bins were either positive or near zero. For females, on the other hand, most bias scores were negative except for Age 10-18 and Age 51-70. Pairwise comparisons of age bins revealed several trends that failed to reach significance after correcting for multiple comparisons: Age 10-18 (t(775) = −2.3, p = 0.0217; FDR corrected probability: q = 0.0506), Age 32-38 (t(483) = −1.896, p = 0.0586; FDR corrected probability: q = 0.08204), Age 39-50 (t(571) = −2.121, p = 0.0343; FDR corrected probability: q = 0. 0.060025), and Age 51-70 (t(665) = −2.324, p = 0.0204; FDR corrected probability: q = 0.0714).
Do the Results Observed Depend on Binning the Data?
The purpose of using age bins in the current study was to obtain several stable estimates of correlations, our primary measure of cognitive awareness. To ensure that the results observed did not depend on the particular participant bins that were selected, we similarly examined correlations and slopes using a continuous sliding window approach of 600 participants, approximately the average bin size used in the main analyses in the manuscript. Importantly, these visualizations show highly similar results to the main analyses (see Supplementary Figure S4). Additionally, we performed multiple regressions predicting subjective face recognition from CFMT3, age, age2, CFMT3 x age, and CFMT x age2. Importantly, we found that both CFMT3 x age and CFMT3 x age2 uniquely predicted CFMQ, suggesting that the CFMT/CFMQ relationship varies with age when age is used as a continuous measure (see Supplementary Table S2).
Discussion
The current study is the first to characterize how face recognition awareness changes across the lifespan. We found a robust overall association between objective performance on the CFMT3 and subjective report (r=0.42 in females, r=.36 in males). Notably, the lifespan pattern of face recognition awareness had a different pattern from that of objective and subjective face recognition ability. In particular, face recognition awareness increased between ages 10-18 and the mid-20s (peaking at r=.48), plateaued until around age 50, and decreased in 51–70-year-olds. These findings were complemented by bias measures of subjective vs. objective face recognition, demonstrating that 10-18- and 51–70-year-olds tended to overestimate their self-reported face recognition abilities, particularly those with the lowest face recognition abilities, in comparison to individuals in the mid-years of life. Finally, we found a small but consistent effect of gender on face recognition ability awareness, with females having overall greater face recognition awareness compared to males while at the same time showing a bias to relatively underestimate their face recognition abilities compared to males. Together, this suggests that face recognition awareness may be a distinct process from face recognition ability. We discuss how face recognition awareness may both depend on the amount of feedback that one receives about their face recognition ability in everyday life as well as issues related to self-report, such as forgetting face recognition mistakes.
The current findings provide important insights into social cognition. Studies of social cognitive awareness have primarily focused on the individual’s awareness of others’ feelings, thoughts, and intentions (i.e., theory of mind, Moran, 2013), and its importance for social competence (Hoglund et al., 2008). By using the association between subjective and objective face recognition to measure face recognition awareness, the current study was able to characterize awareness of one’s own social cognitive abilities. Interestingly, our observed peak of face recognition awareness in the mid-20s is earlier than the mid-40s peak performance for a popular theory of mind task, Reading the Mind in the Eyes (Hartshorne & Germine, 2015). Though it would be beneficial for future studies to directly compare these tasks, this provides preliminary support that face recognition self-awareness and social awareness of others may be at least partially separable processes (e.g., see Nichols & Stich, 2003). For example, self-monitoring mechanisms that provide self-knowledge may develop independently, or before, mechanisms that provide awareness of others’ mental states (Nichols & Stich, 2003; though see Goldman, 2006). Though the everyday life implications of greater face recognition awareness remain to be characterized, one likely possibility is that it helps the individual to determine how to behave appropriately or better interpret social situations (e.g., avoiding faux pas) and could provide greater social confidence. For example, if one is aware of their below-average face recognition abilities, when greeted at a social gathering by a potentially familiar person, one could compensate by smiling and saying “good to see you” while asking vague questions to try and determine who the person is without offending them. Poor awareness of one’s face recognition abilities, on the other hand, could have significant negative social consequences such as being seen as uncaring by others or rather erroneously perceiving that others do not care about you.
To our knowledge, this is the first study to use a large sample of participants to characterize finer-grained age-related changes in global metacognition, allowing for a better understanding of cognitive awareness across the lifespan. Other studies have either examined age-related or group differences in local (i.e., within participant) metacognitive abilities (e.g., between adolescents and adults, Weil et al., 2013; between younger and older adults, Palmer et al., 2014). One strength of the current study’s examination of global metacognition is that participants reported about their general ability rather than specifically how well they think they would perform on a particular task or how confident they are in their performance on a single trial. Though local, task-specific metacognitive performance has been shown to correlate with metacognitive abilities in general (Mazancieux et al., 2020), global metacognition may provide important complementary information and likely better generalizes to metacognition in daily life. Additionally, task-specific self-reports, compared to self-reported general ability, typically show greater correlations with the objective task performance (e.g., Hertzog et al., 2000). Thus, our demonstration of strong associations between general self-reported face recognition ability and task performance as well as changes with age suggest these findings are robust and may be even more pronounced when examining task-specific self-report paradigms.
It is notable that the highest level of face recognition awareness, both in terms of strongest subjective/objective relationship and least amount of bias, was reached in the early to mid 20s, preceding the highest levels of objective face recognition ability by approximately 10 years. One explanation for this earlier development of face recognition awareness is increased social feedback. In particular, studies suggest that the size of one’s global social network size is at its greatest level in the early to mid 20s and is followed by a plateau between late 20s and early 30s (Wrzus et al., 2013). This increased social network size during the early to mid 20s is likely related to increased emotional and behavioral autonomy (Hofer & Pikowski, 2002; Kreppner, 1993) as well as entering new school or work environments, exposing individuals in this age range to a much larger number of relevant individuals/to-be-remembered faces. This larger social network could give individuals many more opportunities to try and learn and remember faces as well as feedback on their performance, increasing their self-knowledge. Another factor that could account for the peak of face recognition awareness in the mid 20s is that this is the time when episodic memory is most accurate (Shing et al., 2010). It could be that individuals in their mid 20s are better able to remember their daily face recognition successes and failures than younger and older adults, increasing the accuracy of their self-report.
Similar mechanisms may explain the decline of face recognition awareness amongst 51-70 year-olds. Our finding of poorer face recognition awareness in older adults is consistent with Bowles et al. (2009), who showed that while adults 18-32 years old had a modest degree of face recognition awareness, older adults 55-88 years old showed little-to-no association between subjective and objective face recognition (r=−.05). One possibility is that, compared to younger adults, older adults receive less feedback about their face recognition abilities. Older adults tend to have smaller, more stable social circles than younger adults (Carstensen, 1992; Wrzus et al., 2013). Older adults’ social circles are often composed of individuals whom they have known for extended periods of time such as their spouse, children, relatives, and a decreasing group of close friends (van Tilburg, 1998). These more intimate social circles could result in relatively fewer situations where older adults receive feedback (or fewer situations where they receive negative feedback) on their ability to learn and remember faces, providing them with less insight into their face recognition abilities. An alternative explanation is that as people get older, they forget or underreport their everyday face recognition failures and, thus, rate their subjective face recognition to be better than it is. This phenomenon is consistent with studies that demonstrate fewer self-reported cognitive failures in older adults while they are experiencing objective cognitive decline (for a review, see De Winter, Dodou, & Hancock, 2015). Older adults’ increase in self-reported face recognition ability while at the same time their objective face recognition is declining is consistent with this more general effect. An additional factor could be the age-related positivity effect, in which older adults are motivated to be biased towards positive over negative stimuli and memories as an emotional regulation strategy (Mather & Carstensen, 2005). This positivity effect can result in more emotionally gratifying memory distortions in older adults (Mather & Carstensen, 2005), and thus could also lead to greater forgetting of experiences that reflect negatively on their face recognition ability.
In addition to these lifespan changes, the current study also revealed significant gender differences in face recognition awareness. Compared to males, females had better face recognition awareness overall and were less biased to positively rate their subjective face recognition abilities. This female advantage was not observed on the CFMT, consistent with a recent report using both male and female-face versions of the CFMT (Elbich et al., 2017, though see other studies showing a female advantage in recognition of new faces, Herlitz & Loven, 2013; Mishra et al., 2019). This suggests that the female advantage may be more pronounced for social cognitive awareness and goes along with other studies showing better social cognitive awareness in females (e.g., theory of mind, Kirkland et al., 2013). One explanation is that females tend to have larger and more diverse social networks than males (Antonucci & Akiyama, 1987; Antonucci et al., 1998; Ross & Mirowsky, 1989; Turner & Marino, 1994), which could provide additional interactions and feedback, enhancing their face recognition awareness. This enhanced insight may be specific to social cognitive abilities, as meta-analyses have failed to find consistent gender differences in other domains of self-insight (e.g., Freund & Kasten, 2012). In terms of females’ less positive bias in their subjective report than males, this is consistent with a recent report finding that, in comparison to females, male developmental prosopagnosics reported fewer everyday face recognition failures despite having similar objective face recognition abilities (Murray & Bate, 2019). This difference could be due to females’ better recollection of their daily life face recognition failures, consistent with reports of superior autobiographical memory in women (Davis, 1999; Fuentes & Desrocher, 2013; Pillemer et al., 2003). Alternatively, these biases could reflect the general effect that males are more likely to have more positive self-reports than females (Barber & Odean, 2001; Deaux & Farris, 1977; Lundeberg et al., 1994).
Another important implication of the current findings is in diagnosing developmental prosopagnosics and super face recognizers, those with extraordinary face recognition abilities (Russell et al., 2009). First, the current results demonstrate a generally robust relationship between self-reported and objective face recognition abilities, supporting the diagnostic utility of self-report face recognition questionnaires (e.g., PI-20, Gray et al., 2017; Ventura et al., 2018). Also consistent with prior findings (Arizpe et al., 2019), the current results highlight some of the limits of self-report, such as its susceptibility to bias, and suggest that self-report alone is inadequate to diagnose prosopagnosics and super recognizers. The current findings extend previous work by demonstrating that self-reported face recognition in 10-18 year olds and 51-70 year olds are generally the least accurate and are biased towards overconfidence in their face recognition abilities. In contrast, self-reported face recognition of individuals in their early-to-mid-20s and females in general are the most accurate. Taking into account these age- and gender-related differences in group metacognitive sensitivity and bias when using self-report questionnaires to diagnose prosopagnosia (and potentially super recognizers) would be an important future endeavor.
Though the current study provides insights into social cognitive awareness across the lifespan, it is limited by the cross-sectional nature of the study. Cohort effects, such as younger generations being more adept with social media where they may receive exposure to many faces and new acquaintances, could partially account for the findings. A longitudinal study would allow a better characterization of lifespan changes and help determine if greater face recognition awareness leads to greater objective face recognition ability. Further, the current study does not address whether the effects are specific to face recognition awareness or reflect metacognition/cognitive awareness more generally. Future studies comparing the current paradigm with other facets of social cognitive awareness (e.g., theory of mind), and metacognition/cognitive awareness (e.g., memory) would be useful to better tease this question apart. Another limitation of the current study is that our objective face recognition measure was composed of artificial FaceGen faces. Though studies have found that artificial faces generally behave similar to real faces (e.g., face-inversion effects, Kätsyri, 2018), it would be good to replicate these results using real faces. Finally, though the current study examines global metacognition, it does not speak to local metacognitive efficiency, the ability to distinguish between one’s own correct and incorrect responses given a specific level of task performance (Fleming & Lau, 2014). Future studies would be useful to measure lifespan changes in face recognition metacognitive efficiency and how this corresponds with the current findings.
In sum, the present study provides novel insights into how one aspect of social cognitive awareness, face recognition awareness, changes across the lifespan. Our results demonstrate that face recognition awareness peaks in the early-to-mid 20s, is relatively stable throughout the 20s-40s, and declines in the 50s-60s. They also show that females have consistently greater face recognition awareness than males. Future studies will be useful to better characterize the real-world consequences of poor vs. good face recognition awareness and the benefits of feedback ‘training.’ Finally, it would be useful to determine if these results generalize to awareness of other aspects of social cognition such as facial emotion recognition ability.
Supplementary Material
Public Significance Statement:
This study suggests that people generally have insight into their face recognition ability, particularly from the mid-20s to 40s. We found that both those under 20 and over 50 tend to overestimate their face recognition ability, and that compared to females, males displayed a tendency to overestimate their face recognition ability. We also found that face recognition ability peaks about ten years later than awareness of this ability, which is temporally aligned with the lifetime peak of other more general metacognitive processes. This suggests that, at least in the case of face recognition, ability and metacognitive awareness of that ability do not share a developmental trajectory.
Acknowledgments
This study was funded by R01 from the National Eye Institute grant #RO1EY026057 awarded to JD.
Footnotes
It should be noted that no personal data was collected from minors (participants < 18 years old) and the protocol was in adherence with the Children’s Online Privacy and Protection Act.
References
- Ackerman PL, & Wolman SD (2007). Determinants and validity of self-estimates of abilities and self-concept measures. Journal of Experimental Psychology: Applied, 13(2), 57–78. [DOI] [PubMed] [Google Scholar]
- Antonucci TC, Akiyama H (1987). An examination of sex differences in social support among older men and women. Sex Roles, 11/12, 737–749. [Google Scholar]
- Antonucci TC, Akiyama H, Lansford JE (1998). Negative effects of close social relations. Journal of Applied Family Studies, 47, 379–384. [Google Scholar]
- Arizpe JM, Saad E, Douglas AO, Germine L, Wilmer JB, & DeGutis JM (2019). Self-reported face recognition is highly valid, but alone is not highly discriminative of prosopagnosia-level performance on objective assessments. Behavior research methods, 51(3), 1102–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balas B, & Pacella J (2015). Artificial faces are harder to remember. Computers in human behavior, 52, 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber BM, Odean T, 2001. Boys will be boys: Gender, overconfidence, and common stock investment. Quarterly Journal of Economics, 116, 261–292. [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300. http://www.jstor.org/stable/2346101 [Google Scholar]
- Bowles DC, McKone E, Dawel A, Duchaine B, Palermo R, Schmalzl L, … & Yovel G (2009). Diagnosing prosopagnosia: Effects of ageing, sex, and participant–stimulus ethnic match on the Cambridge Face Memory Test and Cambridge Face Perception Test. Cognitive Neuropsychology, 26(5), 423–455. [DOI] [PubMed] [Google Scholar]
- Cacciamani F, Tandetnik C, Gagliardi G, Bertin H, Habert MO, Hampel H, Boukadida L, Révillon M, Epelbaum S, Dubois B, & INSIGHT-PreAD study group (2017). Low Cognitive Awareness, but Not Complaint, is a Good Marker of Preclinical Alzheimer’s Disease. Journal of Alzheimer’s Disease: JAD, 59(2), 753–762. 10.3233/JAD-170399 [DOI] [PubMed] [Google Scholar]
- Carstensen LL (1992). Social and emotional patterns in adulthood: Support for socioemotional selectivity theory. Psychology and Aging, 7, 331–338. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, and Aiken LS (2003). Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences (3rd edition). Mahwah, NJ: Lawrence Earlbaum Associates. [Google Scholar]
- David AS, Bedford N, Wiffen B, & Gilleen J (2012). Failures of metacognition and lack of insight in neuropsychiatric disorders. Philosophical Transactions of the Royal Society B: Biological Sciences, 367(1594), 1379–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PJ (1999). Gender differences in autobiographical memory for childhood emotional experiences. Journal of Personality and Social Psychology, 76, 498–510. [DOI] [PubMed] [Google Scholar]
- De Winter JCF, Dodou D, & Hancock PA (2015). On the paradoxical decrease of self-reported cognitive failures with age. Ergonomics, 58(9), 1471–1486. [DOI] [PubMed] [Google Scholar]
- Deaux K, & Farris E (1977). Attributing causes for one’s own performance: The effects of sex, norms, and outcome. Journal of Research in Personality, 11, 59–72. doi: 10.1016/0092-6566(77)90029-0. [DOI] [Google Scholar]
- DiCiccio TJ, & Efron B (1996). Bootstrap Confidence Intervals. Statistical Science, 11(3), 189–212. [Google Scholar]
- Diener E, & Seligman ME (2002). Very happy people. Psychological Science, 13(1), 81–84. [DOI] [PubMed] [Google Scholar]
- Dingfelder S (2019, August 21). My Life With Face Blindness. Washington Post. https://www.washingtonpost.com/news/magazine/wp/2019/08/21/feature/my-life-with-face-blindness/ [Google Scholar]
- Duchaine B, & Nakayama K (2006). The Cambridge Face Memory Test: Results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia, 44(4), 576–585. [DOI] [PubMed] [Google Scholar]
- Efron B, & Tibshirani RJ (1994). An introduction to the bootstrap. Chapman & Hall/CRC. [Google Scholar]
- Elbich D, Motta-Mena N, & Scherf S (2017). Similar Neural Network Topology for Men and Women During Face Recognition. Journal of Vision, 17(10), 844–844. [Google Scholar]
- Ehrlinger J, Johnson K, Banner M, Dunning D, & Kruger J (2008). Why the unskilled are unaware: Further explorations of (absent) self-insight among the incompetent. Organizational behavior and human decision processes, 105(1), 98–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA (1915). Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika, 10(4), 507–521. [Google Scholar]
- Fleming SM, & Lau HC (2014). How to measure metacognition. Frontiers in human neuroscience, 8, 443. 10.3389/fnhum.2014.00443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenbaugh FC, DeGutis J, Germine L, Wilmer JB, Grosso M, Russo K, & Esterman M (2015). Sustained attention across the life span in a sample of 10,000: Dissociating ability and strategy. Psychological science, 26(9), 1497–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund PA, & Kasten N (2012). How smart do you think you are? A meta-analysis on the validity of self-estimates of cognitive ability. Psychological bulletin, 138(2), 296. [DOI] [PubMed] [Google Scholar]
- Frith CD (2012). The role of metacognition in human social interactions. Philosophical Transactions of the Royal Society of London Series B Biological Sciences, 367(1599), 2213–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes A, & Desrocher M (2013). The effects of gender on the retrieval of episodic and semantic components of autobiographical memory. Memory, 21(6), 619–632. [DOI] [PubMed] [Google Scholar]
- Germine LT, Duchaine B, & Nakayama K (2011). Where cognitive development and aging meet: Face learning ability peaks after age 30. Cognition, 118(2), 201–210. 10.1016/j.cognition.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Germine L, Nakayama K, Duchaine BC, Chabris CF, Chatterjee G, & Wilmer JB (2012). Is the Web as good as the lab? Comparable performance from Web and lab in cognitive/perceptual experiments. Psychonomic Bulletin & Review, 19(5), 847–857. 10.3758/s13423-012-0296-9. [DOI] [PubMed] [Google Scholar]
- Goldman AI (2006). Simulating Minds. Oxford, Oxford University Press. [Google Scholar]
- Gray KL, Bird G, & Cook R (2017). Robust associations between the 20-item prosopagnosia index and the Cambridge Face Memory Test in the general population. Royal Society Open Science, 4(3), 160923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagnoli E, & Velicer WF (1988). Relation of sample size to the stability of component patterns. Psychological bulletin, 103(2), 265. [DOI] [PubMed] [Google Scholar]
- Hartshorne JK, & Germine LT (2015). When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychological science, 26(4), 433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitz A, & Lovén J (2013). Sex differences and the own-gender bias in face recognition: A meta-analytic review. Visual Cognition, 21(9-10), 1306–1336. [Google Scholar]
- Hertzog C, Park DC, Morrell RW, & Martin M (2000). Ask and ye shall receive: Behavioral specificity in the accuracy of subjective memory complaints. Applied Cognitive Psychology, 14, 257–275. [Google Scholar]
- Hofer M, & Pikowski B (2002). Familien mit Jugendlichen [Families with adolescents]. In Hofer M, Wild E, & Noack P (Eds.), Lehrbuch Familienbeziehungen: Eltern und Kinder in der Entwicklung (pp. 241–264). Göttingen, Germany: Hogrefe. [Google Scholar]
- Hoglund WL, Lalonde CE, & Leadbeater BJ (2008). Social-cognitive competence, peer rejection and neglect, and behavioral and emotional problems in middle childhood. Social Development, 17(3), 528–553. [Google Scholar]
- Howell DC (2011). Statistical methods for psychology. Cengage Learning. [Google Scholar]
- Jenkins R, Dowsett AJ, & Burton AM (2018). How many faces do people know? Proceedings of the Royal Society B: Biological Sciences, 285(1888), 20181319. 10.1098/rspb.2018.1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kätsyri J (2018). Those virtual people all look the same to me: Computer-rendered faces elicit a higher false alarm rate than real human faces in a recognition memory task. Frontiers in psychology, 9, 1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland RA, Peterson E, Baker CA, Miller S, & Pulos S (2013). Meta-analysis Reveals Adult Female Superiority in” Reading the Mind in the Eyes Test”. North American Journal of Psychology, 15(1). [Google Scholar]
- Kreppner K (1993). Eltern-Kind Beziehungen: Kindes und Jugendalter [Parent–child relationships: Childhood and adolescence]. In Auhagen AE & von Salisch M (Ed.), Zwischenmenschliche Beziehungen (pp. 81–104). Göttingen, Germany: Hogrefe [Google Scholar]
- Kruger J (1999). Lake wobegon be gone! the “below-average effect” and the egocentric nature of comparative ability judgments. Journal of Personality and Social Psychology, 77(2), 221–232. doi: 10.1037/0022-3514.77.2.221 [DOI] [PubMed] [Google Scholar]
- Kruger J, & Dunning D (1999). Unskilled and unaware of it: how difficulties in recognizing one’s own incompetence lead to inflated self-assessments. Journal of personality and social psychology, 77(6), 1121. [DOI] [PubMed] [Google Scholar]
- Lundeberg MA, Fox PW, & Punccohar J (1994). Highly confident but wrong: gender differences and similarities in confidence judgments. Journal of Educational Psychology, 86, 114–121. [Google Scholar]
- Mather M, & Carstensen LL (2005). Aging and motivated cognition: the positivity effect in attention and memory. Trends in cognitive sciences, 9(10), 496–502. 10.1016/j.tics.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Mazancieux A, Dinze C, Souchay C, & Moulin CJA (2020). Metacognitive domain specificity in feeling-of-knowing but not retrospective confidence. Neuroscience of Consciousness, 2020(1), Article niaa001. 10.1093/nc/niaa001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvie SJ, Standing L, Jean DS, & Law J (1993). Gender differences in recognition memory for faces and cars: Evidence for the interest hypothesis. Bulletin of the Psychonomic Society, 31(5), 447–448. [Google Scholar]
- McKendrick AM, Weymouth AE, & Battista J (2013). Visual form perception from age 20 through 80 years. Investigative ophthalmology & visual science, 54(3), 1730–1739. 10.1167/iovs.12-10974 [DOI] [PubMed] [Google Scholar]
- Medalia A, & Thysen J (2008). Insight into neurocognitive dysfunction in schizophrenia. Schizophrenia Bulletin, 34(6), 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra MV, Likitlersuang J, Wilmer JB, Cohan S, Germine L, & DeGutis JM (2019). Gender Differences in Familiar Face Recognition and the Influence of Sociocultural Gender Inequality. Scientific Reports, 9(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM (2013). Lifespan development: The effects of typical aging on theory of mind. Behavioural brain research, 237, 32–40. [DOI] [PubMed] [Google Scholar]
- Murray E, Hills PJ, Bennetts RJ, & Bate S (2018). Identifying Hallmark Symptoms of Developmental Prosopagnosia for Non-Experts. Sci Rep, 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E, & Bate S (2019). Self-ratings of face recognition ability are influenced by gender but not prosopagnosia severity. Psychological assessment. [DOI] [PubMed] [Google Scholar]
- Nichols S, & Stich SP (2003). Oxford cognitive science series. Mindreading: An integrated account of pretence, self-awareness, and understanding other minds. New York, NY, US: Clarendon Press/Oxford University Press. [Google Scholar]
- Palermo R, Rossion B, Rhodes G, Laguesse R, Tez T, Hall B, … & Al-Janabi S (2017). Do people have insight into their face recognition abilities?. The Quarterly Journal of Experimental Psychology, 70(2), 218–233. [DOI] [PubMed] [Google Scholar]
- Palmer EC, David AS, & Fleming SM (2014). Effects of age on metacognitive efficiency. Consciousness and Cognition, 28, 151–160. 10.1016/J.CONCOG.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillemer D, Wink P, DiDonato T, & Sanborn R (2003). Gender differences in autobiographical memory styles of older adults. Memory, 11(6), 525–532. [DOI] [PubMed] [Google Scholar]
- Reese E, Haden CA, Baker-Ward L, Bauer P, Fivush R, & Ornstein PA (2011). Coherence of Personal Narratives across the Lifespan: A Multidimensional Model and Coding Method. Journal of cognition and development : official journal of the Cognitive Development Society, 12(4), 424–462. 10.1080/15248372.2011.587854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley E, Okabe H, Germine L, Wilmer J, Esterman M, & DeGutis J (2016). Gender differences in sustained attentional control relate to gender inequality across countries. PloS one, 11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LA, Dodson J, Edwards JD, Ackerman ML, Ball K (2012). Self-rated driving is not related to driving safety in older adults. Accident Analysis and Prevention, 48, 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CE, Mirowsky J (1989). Explaining the social patterns of depression: Control and problem solving—or support and talking. Journal of Health and Social Behavior, 30, 206–219. [PubMed] [Google Scholar]
- Rothlein D, DeGutis J, & Esterman M (2018). Attentional fluctuations influence the neural fidelity and connectivity of stimulus representations. Journal of cognitive neuroscience, 30(9), 1209–1228. [DOI] [PubMed] [Google Scholar]
- Russell R, Duchaine B, & Nakayama K (2009). Super-recognizers: People with extraordinary face recognition ability. Psychonomic bulletin & review, 16(2), 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Elbich DB, & Motta-Mena NV (2017). Investigating the influence of biological sex on the behavioral and neural basis of face recognition. eNeuro, 4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Blasco M, Torres IJ, Vicent-Gil M, Goldberg X, Navarra-Ventura G, Aguilar E, … & Cardoner N (2019). Discrepancy between objective and subjective cognition in major depressive disorder. European Neuropsychopharmacology, 29(1), 46–56. [DOI] [PubMed] [Google Scholar]
- Sheehan MJ, & Nachman MW (2014). Morphological and population genomic evidence that human faces have evolved to signal individual identity. Nature communications, 5(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing YL, Werkie-Bergner M, Brehmer Y, Muller V, Li S, Lindenberger U (2010). Episodic memory across the lifespan: the contributions of associative and strategic components. Neuroscience and Biobehavioral Reviews 34, 1080–1091 [DOI] [PubMed] [Google Scholar]
- Soto D, & Silvanto J (2014). Reappraising the relationship between working memory and conscious awareness. Trends in Cognitive Sciences, 18(10), 520–525. [DOI] [PubMed] [Google Scholar]
- Susilo Tirta & Germine Laura & Duchaine Brad. (2013). Face Recognition Ability Matures Late: Evidence From Individual Differences in Young Adults. Journal of experimental psychology. Human perception and performance, 39, 10.1037/a0033469. [DOI] [PubMed] [Google Scholar]
- Taylor RJ, Chatters LM, Hardison CB, & Riley A (2001). Informal social support networks and subjective well-being among African Americans. Journal of Black Psychology, 27(4), 439–463. [Google Scholar]
- Turner RJ, Marino F (1994). Social support and social structure: A descriptive analysis. Journal of Health and Social Behavior, 35, 193–212. [PubMed] [Google Scholar]
- van Tilburg T (1998). Losing and gaining in old age: Changes in personal network size and social support in a four-year longitudinal study. Journal of Gerontology: Social Sciences, 53B, S313–S323. [DOI] [PubMed] [Google Scholar]
- Vardy J, Wong K, Yi Ql. et al. Assessing cognitive function in cancer patients. Support Care Cancer 14, 1111–1118 (2006). [DOI] [PubMed] [Google Scholar]
- Vellante M, Baron-Cohen S, Melis M, Marrone M, Petretto DR, Masala C, & Preti A (2013). The “Reading the Mind in the Eyes” test: systematic review of psychometric properties and a validation study in Italy. Cognitive Neuropsychiatry, 18(4), 326–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura P, Livingston LA, & Shah P (2018). Adults have moderate-to-good insight into their face recognition ability: Further validation of the 20-item Prosopagnosia Index in a Portuguese sample. Quarterly Journal of Experimental Psychology, 71(12), 2677–2679. 10.1177/1747021818765652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil LG, Fleming SM, Dumontheil I, Kilford EJ, Weil RS, Rees G, … & Blakemore SJ (2013). The development of metacognitive ability in adolescence. Consciousness and cognition, 22(1), 264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmer JB, Germine L, Chabris CF, Chatterjee G, Williams M, Loken E, … & Duchaine B (2010). Human face recognition ability is specific and highly heritable. Proceedings of the National Academy of sciences, 107(11), 5238–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrzus C, Hänel M, Wagner J, & Neyer FJ (2012). Social Network Changes and Life Events Across the Life Span: A Meta-Analysis. Psychological Bulletin. Advance online publication. 10.1037/a0028601. [DOI] [PubMed] [Google Scholar]
- Yardley L, McDermott L, Pisarski S, Duchaine B, & Nakayama K (2008). Psychosocial consequences of developmental prosopagnosia: A problem of recognition. Journal of Psychosomatic Research, 65(5), 445–451. 10.1016/J.JPSYCHORES.2008.03.013 [DOI] [PubMed] [Google Scholar]
- Zell E, & Krizan Z (2014). Do people have insight into their abilities? A metasynthesis. Perspectives on Psychological Science, 9(2), 111–125. [DOI] [PubMed] [Google Scholar]
- Zhao N, Wardeska JG, McGuire SY, & Cook E (2014). Metacognition: An effective tool to promote success in college science learning. Journal of College Science Teaching, 43(4), 48–54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


