Abstract
Taxoids such as paclitaxel (Taxol) are an important class of anticancer drugs that bind β-tubulin and stabilize cellular microtubules. To provide new chemical tools for studies of microtubules, we synthesized derivatives of paclitaxel modified at the 7-position with the small coumarin-derived fluorophore Pacific Blue (PB). Three of these Pacific Blue-Taxoids termed PB-Gly-Taxol, PB-β-Ala-Taxol, and PB-GABA-Taxol bind purified crosslinked microtubules with affinities of 34–265 nM, where the affinity can be tuned based on the length of an amino acid linker. When added to living cells in the presence of verapamil or probenecid as inhibitors of efflux, these compounds allow visualization of the microtubule network by confocal microscopy. We describe methods for the synthesis of these probes, determination of their affinities for crosslinked tubulin, and imaging of microtubules in living HeLa cells. We further describe their uptake by Caco-2 cells and two transporter-deficient Caco-2 knockout cell lines in the absence and presence of efflux inhibitors by flow cytometry. These studies revealed that p-glycoprotein (MDR1) and multi-drug resistance protein 2 (MRP2) are major mediators of efflux of these molecular probes. These compounds provide useful tools for studies of microtubules and cellular efflux transporters in living cells.
Keywords: Taxoid, taxane, paclitaxel, taxol, microtubules, tubulin, fluorophore, p-glycoprotein, MDR1, multi-drug resistance protein 2, MRP2, pacific blue, verapamil, probenecid
1. Introduction
Small molecules that stabilize microtubules represent an important class of anticancer therapeutics [1,2]. Paclitaxel (Taxol), a natural product isolated from the Pacific Yew tree [3], is one of the most widely used drugs in this class of compounds. These drugs bind with high affinity to a specific site on the interior of microtubules [4], affect tubulin polymerization dynamics, promote the formation of micronuclei, cause mitotic arrest, and impact signaling pathways [5–7], but their mechanisms of antitumor activity are not fully understood [8–10]. Given the success of this class of drugs, the discovery and development of novel taxoids is ongoing and of substantial interest [11]. To investigate the molecular mechanisms of these anticancer agents, labeled derivatives have been extensively employed. In early studies, radiolabeled [3H]Taxol was used to demonstrate that paclitaxel binds the polymerized αβ dimer of tubulin in vitro with a 1:1 stoichiometry, without appreciable affinity for unassembled tubulin [12]. One of the first fluorescent taxoids used for structural [13] and quantitative binding [14] studies was 3′-N-m-aminobenzamido-3′-N-debenzamidopaclitaxel (N-AB-PT), an analogue of paclitaxel modified on a phenyl ring of a benzoate with a single additional amino group. Competition binding studies with N-AB-PT demonstrated that paclitaxel associates with a high-affinity binding site on GMPcPP-stabilized microtubules from bovine brain with Kd = 19 nM [15]. N-AB-PT itself exhibits a similarly high affinity (Kd = 15 nM) for these microtubules [15,14], but this probe requires UV excitation (e.g. at 320 nm), making it unsuitable for tubulin binding assays in living cells.
A variety of other fluorescent probes of microtubules have been studied in vitro and in cells [16,17]. Flutax-2, comprising paclitaxel linked at the 7-position to Oregon Green via a β-Ala (or L-Ala) linker, has been widely used and is commercially available. This modification at the 7-position of paclitaxel can preserve high affinity for tubulin, and Flutax-2 (L-Ala) binds crosslinked microtubules with Kd = 14 nM as measured by fluorescence anisotropy [18]. Competition experiments by fluorescence anisotropy with this probe were used to measure binding affinities of paclitaxel (Kd = 27 nM) and docetaxel (Kd = 17 nM) for glutaraldehyde-crosslinked microtubles [19]. Other fluorescent taxoids that link fluorophores to the primary amine of the side chain of docetaxel such as the BODIPY 564/570 Taxol (Botax, Kd = 2.2 μM)[20] and silicon rhodamine (SiR)-tubulin (SirTub) [21,9], exhibit substantially lower affinity for microtubules. However, SirTub can be used for superresolution imaging of these structures [21], and it has been used to measure apparent intracellular Kd values of taxoids in competition binding studies in living cells by confocal microscopy [9]. SirTub can be prepared from docetaxel and the SiR-carboxyl fluorophore in three steps [21], but production of the SiR-carboxyl fluorophore requires a nine-step synthesis [22].
In HeLa cells, tubulin represents ca. 4% of total cellular protein [23,24] and is estimated to be present at ~ 20 μM [9]. By promoting the polymerization of tubulin in cells, paclitaxel associates specifically with microtubules, and less than 5% is observed in cellular membranes [25]. Despite its high affinity in vitro, we [26] and others [27] previously showed that the dianionic and highly hydrophilic Oregon Green fluorophore of Flutax-2 (cLogDpH7.4 = 0.5, calculated with ChemAxon MarvinView 20.7) deleteriously impacts its specificity for tubulin in living cells. To more closely mimic the polarity of paclitaxel (cLogP = 3.3) with fluorescent probes, we previously synthesized three novel fluorescent taxoids termed PB-Gly-Taxol, PB-β-Ala-Taxol, and PB-GABA-Taxol [26]. These more hydrophobic probes (cLogDpH7.4 = 2.4–2.8) comprise paclitaxel modified at the 7-position by the amino acids glycine, β-alanine, or gamma amino butyric acid (GABA), linked to the small Pacific Blue (PB) fluorophore (Figure 1). Because this monoanionic coumarin (pKa = 3.7) fluorophore exhibits a high quantum yield (QY=0.75) and can be efficiently excited with 405 nm violet lasers found on many confocal microscopes and flow cytometers (λEx=400 nm, λEm=447 nm, ε=29,500 M−1cm−1), Pacific Blue can generally be readily detected in living cells.
Figure 1.
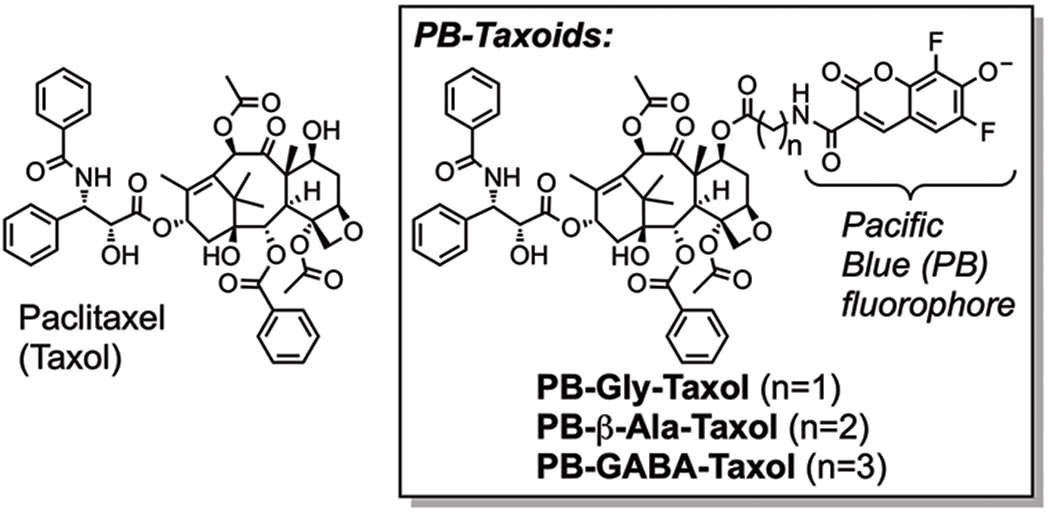
Comparison of the structures of paclitaxel with the fluorescent molecular probes PB-Gly-Taxol, PB-β-Ala-Taxol, and PB-GABA-Taxol.
The affinities of taxoids for microtubules have traditionally been measured with classic biochemical methods using purified crosslinked tubulin [28–30]. Using these methods and a fluorescence enhancement assay, we found that PB-Gly-Taxol binds glutaraldehyde-crosslinked bovine brain microtubules with Kd = 34 ± 6 nM [26]. However, this high affinity is remarkably sensitive to the length of the linker, and when the glycine of PB-Gly-Taxol is systematically replaced with β-alanine (PB-β-Ala-Taxol, Kd = 63 ± 8 nM), or gamma-amino butyric acid (PB-GABA-Taxol, Kd = 265 ± 55 nM), affinity for tubulin is reduced (Figure 2). As shown in Figure 3, molecular docking studies suggest that insertion of the Pacific Blue moiety into a pocket adjacent to the Taxol binding site may impact the affinity of these probes. The higher affinity of PB-Gly-Taxol was additionally correlated with greater cytotoxic potency towards HeLa cells (IC50, 48 h = 60 nM, 25 μM verapamil), whereas PB-β-Ala-Taxol (IC50, 48 h = 330 nM, 25 μM verapamil) and PB-GABA-Taxol (IC50, 48 h = 580 nM, 25 μM verapamil) are less cytotoxic. In contrast, the higher affinity Flutax-2 (IC50, 48 h = 1310 nM, 25 μM verapamil) was found to be the least cytotoxic probe under these conditions, likely due to its greater hydrophilicity and its engagement of alternative targets in cells. Verapamil was added in these assays to inhibit MDR1 and MRP efflux transporters [31], resulting in enhanced binding of these probes to microtubules and increased cytotoxicity. Compared to Flutax-2, these Pacific Blue taxoids exhibit greater specificity for microtubules in living cells, and they are more efficient substrates of efflux transporters that can be inhibited by verapamil.
Figure 2.

Quantification of affinities of the fluorescent probes PB-Gly-Taxol, PB-β-Ala-Taxol, and PB-GABA-Taxol (25 nM) for bovine brain tubulin by enhancement of fluorescence.
Figure 3.
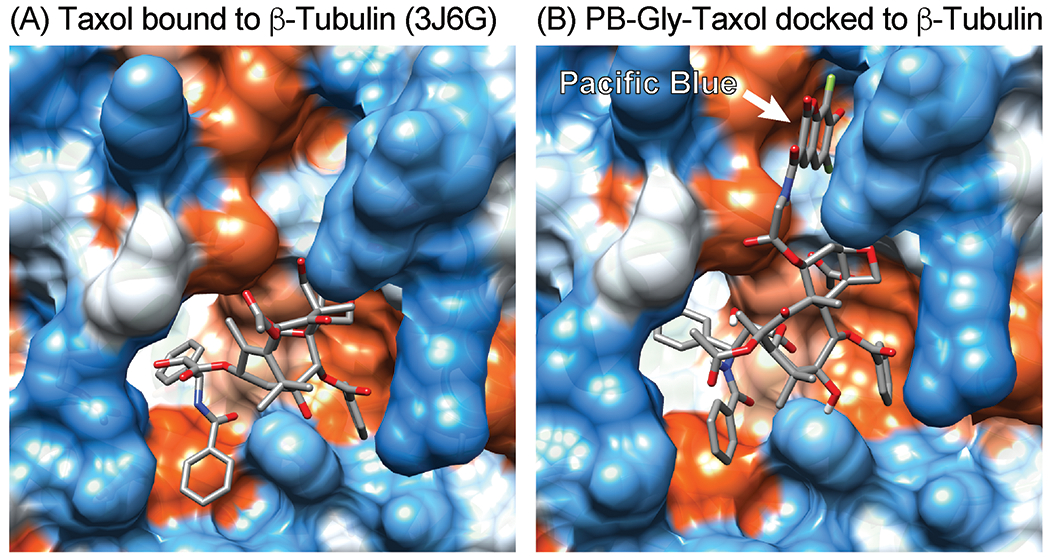
Comparison of the cryo-EM structure of paclitaxel bound to tubulin with a model of PB-Gly-Taxol docked to tubulin. (A) Paclitaxel bound to β-tubulin (PDB ID: 3J6G). (B) A pose of PB-Gly-Taxol docked to the Taxol binding site (AutoDock Vina). Pacific Blue may engage a pocket adjacent to the Taxol binding site to enhance affinity.
In this chapter, we describe methods to synthesize PB-Taxoids (Figure 4), methods to quantify their affinities for purified crosslinked microtubules using fluorescence enhancement, and methods to image cellular microtubules with these compounds by confocal microscopy in HeLa cells (Figure 5). We additionally describe methods to analyze the uptake of both PB-Gly-Taxol and PB-GABA-Taxol in commercially available wild-type and homozygous transporter-knockout Caco-2 cell lines by flow cytometry to further characterize their mechanisms of cellular efflux [32] (Figure 6).
Figure 4.

Synthesis of PB-Gly-Taxol as a representative PB-Taxoid. Substituting Fmoc-Gly-OH with other Fmoc-protected amino acids allows access to other PB-Taxoids.
Figure 5.
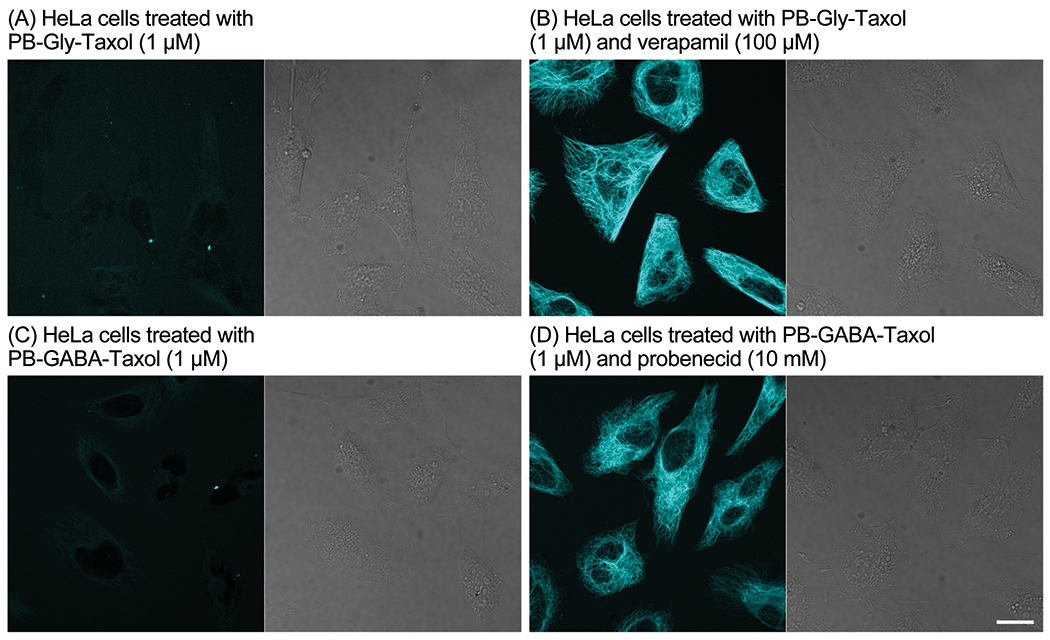
Confocal fluorescence (left) and DIC (right) microscopy of HeLa cells treated for 1 h with PB-Gly-Taxol (A, B) and PB-GABA-Taxol (C, D) without (A, C) and with (B, D) the efflux inhibitors verapamil (B) and probenecid (D). Scale bar: 20 μm.
Figure 6.
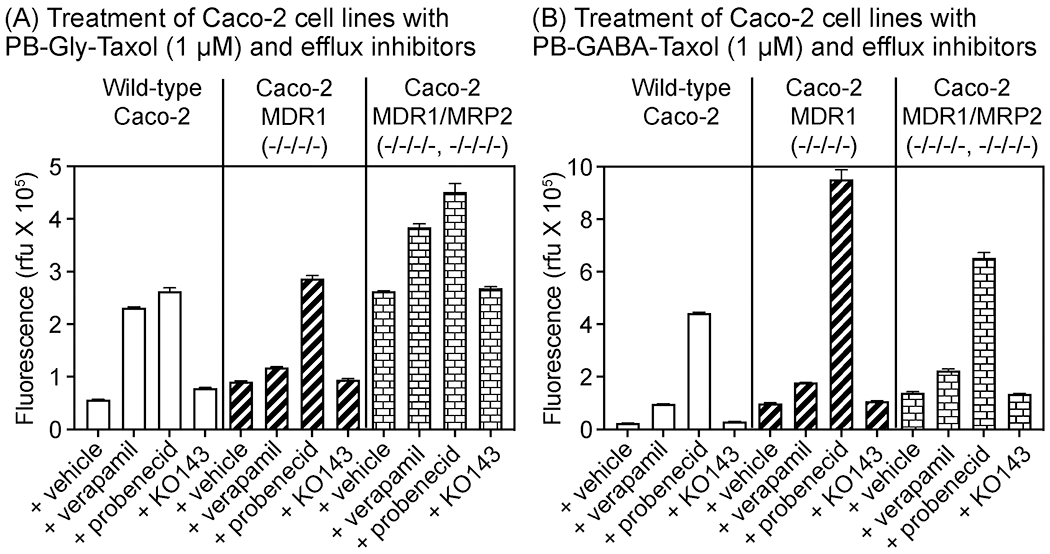
Analysis of uptake of PB-Gly-Taxol and PB-GABA-Taxol by trypsinized Caco-2 wild-type, MDR1 (−/−/−/−), and MDR1/MRP2 (−/−/−/−,−/−/−/−) cell lines by flow cytometry. Cells were treated with PB-Taxoids (1 μM) and DMSO vehicle (0.2%) or efflux inhibitors (verapamil (100 μM), probenecid (10 mM), and KO143 (10 μM)) for 1 h. Error bars = SD, n=3.
We were interested in investigating the efflux properties of PB-Gly-Taxol and PB-GABA-Taxol because the efficacy of paclitaxel in patients can be limited by expression of transporters that expel these drugs from the cellular environment. Rapid efflux of taxoids by cancer cells can be promoted by ATP-binding cassette transporters such as p-glycoprotein (MDR1), transporters of the MRP family (ABCC), and BCRP (ABCG2) [33,34]. To evaluate the substrate activity of PB-Gly-Taxol and PB-GABA-Taxol for these efflux transporters, we measured uptake of these probes in Caco-2 cells, where the expression of efflux transporters has been well-characterized [35–38]. We investigated the uptake of these probes in three commercially available cell lines: Caco-2, MDR1-knockout Caco-2, and MDR1/MRP2 double knockout Caco-2, by flow cytometry. These cells were treated in suspension after trypsinization, avoiding known [35,38] differential effects of cellular polarity on transporter expression.
To inhibit specific transporters, we treated cells with verapamil (100 μM), probenecid (10 mM), and KO143 (10 μM). These small molecules are known to inhibit p-glycoprotein, MRP transporters, and BCRP, respectively, at these concentrations [39,40]. As shown in Figure 6, both PB-Gly-Taxol and PB-GABA-Taxol are sensitive substrates of p-glycoprotein as evidenced by a 4-fold increase in the fluorescence of Caco-2 cells upon inhibition of p-glycoprotein by verapamil. Consistent with this interpretation, verapamil was less effective in the Caco-2 MDR1 and MDR1/MRP2 knockout cells and affected probe uptake by less than 2-fold. In contrast, despite their structural similarities, PB-GABA-Taxol was found to be a substantially more sensitive substrate of MRP transporters than PB-Gly-Taxol when these transporters were inhibited with probenecid. However, MRP transporters contribute to efflux of both of these probes. With PB-GABA-Taxol, probenecid enhanced uptake by > 18-fold in wild-type Caco-2 cells, 9-fold in MDR1 knockout cells, and 4-fold in the MDR1/MRP2 knockout cells. In contrast, when cells were treated under the same conditions with PB-Gly-Taxol, probenecid enhanced uptake by < 5-fold in wild-type Caco-2 cells, 3-fold in the MDR1 knockout cells, and < 2-fold in the MDR1/MRP2 knockout cells. Uptake of these probes was not affected by treatment with KO143, indicating that they are not substrates of BCRP [35,38,39,36]. Overall, these results indicate that these PB-Taxoids recapitulate key aspects of the efflux substrate activity of paclitaxel but exhibit differential sensitivities to efflux by MRP2.
Compared to other fluorescent probes of microtubules, PB-Taxoids exhibit several potential advantages. One is their straightforward synthesis in a four pot, five step process. Another is the ability to readily detect these compounds in living cells by excitation at 405 nm with violet lasers that are found on many confocal microscopes and flow cytometers. Additionally, PB-Gly-Taxol exhibits high affinity for microtubules, within a factor of two of paclitaxel, but the affinity of these probes can be systematically varied by up to 8-fold by selecting the appropriate linker between the fluorophore and the drug. These changes in affinity are correlated with cellular cytotoxicity, which allows selection of probes with higher or lower cytotoxicity profiles. These linker changes additionally affect the selectivity for efflux transporters, which may provide useful tools for profiling of transporter expression. Because these probes mimic aspects of the biological properties of paclitaxel including highly selective binding to microtubules and sensitivity to similar mechanisms of cellular efflux, they may be beneficial for studies of the antitumor mechanisms of action of this class of drugs. Further development of these probes may also offer improved methods for the discovery of novel drugs that engage the taxoid-binding site of tubulin.
2. Materials
2.1. Synthesis of PB-Gly-Taxol as a representative PB-Taxoid.
Paclitaxel.
Tert-butyldimethylchlorosilane.
Imidazole.
2-((2-(9H-Fluoren-9-ylmethoxycarbonylamino)acetyl)amino)acetic acid. Substitute with other Fmoc-protected amino acids to synthesize PB-β-Ala-Taxol or PB-GABA-Taxol.
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride.
4-Dimethyl aminopyridine.
Piperidine.
1 M Tetrabutyl ammonium fluoride in THF.
N,N-Diisopropylethylamine.
Silica gel (Silicycle, #R12030B).
Pacific Blue-NHS ester (see Note 1).
2.2. Quantification of the affinity of PB-Taxoids for purified crosslinked microtubules
Tubulin protein, pre-formed microtubules from bovine brain (Cytoskeleton, # MT001).
Glycerol-EDTA (GAB) buffer (10 mM sodium phosphate, 1 mM EDTA, 1 mM GTP, 3.4 M glycerol, pH 6.5), for taxol binding assay.
GAB buffer containing 20 mM glutaraldehyde.
GAB buffer containing 60 mM sodium borohydride.
100 mM GTP.
Corning 96-well solid black polystyrene microplate.
Microplate shaker.
Fluorescence microplate reader or fluorimeter equipped with filters for excitation and quantification of emission of Pacific Blue (e.g. 390-420 nm band pass excitation filter and 450 nm long pass emission filter).
2.3. Analysis of PB-Taxoids by confocal fluorescence imaging and flow cytometry
Cell line (HeLa, ATCC CCL-2; Caco-2, ATCC HTB-37; Caco-2 MDR1 (−/−/−/−) Knockout, Sigma-Aldrich MTOX1001; Caco-2 MDR1/MRP2 (−/−/−/−,−/−/−/−) Double Knockout, Sigma-Aldrich MTOX1005)
Complete growth medium (DMEM, Sigma-Aldrich #D6429, supplemented with 10% (HeLa) or 20% (Caco-2) Fetal Bovine Serum, Gibco #26140).
Trypsin-EDTA solution.
Phosphate buffered saline (PBS, pH 7.4, 1 mM K2HPO4, 155 mM NaCl, 3 mM Na2HPO4) supplemented with 2% bovine serum albumin (BSA) fraction V. Keep ice-cold for washing of cells before analysis by flow cytometry.
Clear, 96-well untreated microplate.
Slides for imaging of living cells by confocal microscopy.
Rotary hybridization oven or incubating microplate shaker.
Confocal fluorescence microscope.
Flow cytometer equipped with a 405 nm violet laser for excitation of Pacific Blue fluorescence (450 nm emission filter).
3. Methods
3.1. Synthesis of PB-Gly-Taxol as a representative PB-Taxoid.
3.1.1. Synthesis of TBS-Taxol
Dry a single-necked round bottom flask (25 mL) equipped with a magnetic stir bar in an oven (150 °C) for 1 h. Seal this flask with a rubber septum after removing it from the oven. Evacuate the flask under high vacuum (5 min) and flush with dry argon. Repeat at least 3 times, allowing the flask to return to room temperature.
Quickly weigh 500 mg of paclitaxel (0.59 mmol, 1 equiv.) into the dry flask.
Introduce 10 mL of anhydrous dimethylformamide into the flask using a syringe. Stir to effect complete dissolution of the starting material.
Sequentially add 208 mg of imidazole (3.06 equiv.) and 542 mg of tert-butyldimethylchlorosilane (3.61 mmol).
Stir the reaction mixture at room temperature for 16 h under Ar.
Remove and analyze an aliquot of the reaction mixture by TLC (eluent: ethyl acetate/hexane (1:1)). Monitor reaction progress by comparison to the limiting reagent (paclitaxel).
Upon completion, dilute and the transfer the reaction mixture into a 250 mL separatory funnel using 100 mL of dichloromethane.
Sequentially wash the dichloromethane layer in the separatory funnel with saturated solution of 50 mL of aqueous ammonium chloride, 50 mL of water, and 50 mL of saturated aqueous sodium chloride.
Separate organic layer and dry over anhydrous magnesium sulfate.
Remove the magnesium sulfate by filtration and concentrate the filtrate to isolate the crude product.
Purify the TBS-Taxol product by silica gel flash chromatography (100:0 hexane/ethyl acetate to 0:100 hexane/ethyl acetate, > 90% yield).
3.1.2. Synthesis of Fmoc-Gly-TBS-Taxol
Dry a single-necked round bottom flask (10 mL) equipped with a magnetic stir bar in an oven (150 °C) for 1 h. Seal this flask with a rubber septum after removing it from the oven. Evacuate the flask under high vacuum (5 min) and flush with dry argon. Repeat at least 3 times, allowing the flask to return to room temperature.
Quickly weigh 61.4 mg of 2-((2-(9H-fluoren-9-ylmethoxycarbonylamino)acetyl)amino)acetic acid (0.207 mmol, 4 equiv.), 39.6 mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (0.207 mmol, 4 equiv.), and 3.15 mg of 4-dimethyl aminopyridine (0.0258 mmol, 0.5 equiv.) into the dry flask.
Seal the flask using a rubber septum and introduce 1 mL of anhydrous dichloromethane into the flask using a syringe.
Stir the mixture at room temperature under Ar for 30 min.
Dry a 1-dram vial in an oven (150 °C) for 1 hour. Seal the vial with a rubber septum after removing for the oven. Evacuate the vial under vacuum (5 min) and flush with dry argon. Repeat this process 3 times.
Quickly weigh 50 mg of TBS-Taxol (0.516 mmol, 1.0 equiv.) into the dry vial and add 2 mL of anhydrous dichloromethane to dissolve.
Add this solution using a syringe to the previously prepared mixture in the round bottom flask.
Stir the reaction mixture at room temperature for 12 h under Ar.
Remove and analyze an aliquot of the reaction mixture by TLC (eluent: ethyl acetate/hexane (1:1)). Monitor reaction progress by comparison to the limiting reagent (TBS-Taxol).
Upon completion, pipet the crude mixture onto a silica gel column prewetted with hexane. Dilute any residual crude product remaining in the reaction flask with a minimal amount of dichloromethane and transfer to the column.
Purify the product by flash chromatography (100:0 hexane/ethyl acetate to 0:100 hexane/ethyl acetate). After removal of solvents under vacuum, the pure product is obtained as an off white solid (78% yield).
3.1.3. Synthesis of PB-Gly-TBS-Taxol
Dry a 1-dram vial equipped with a magnetic stir bar in an oven (150 °C) for 1 hour. Seal this vial with a rubber septum after removing it from the oven. Evacuate the flask under high vacuum (5 min) and flush with dry argon. Repeat at least 3 times, allowing the flask to return to room temperature.
Quickly weigh 14 mg of Fmoc-Gly-TBS-Taxol (0.011 mmol, 1.0 equiv.) into the dry vial and reseal the vial using a rubber septum.
In a separate flask prepare a 20% (v/v) solution of piperidine in 2 mL of anhydrous dimethyl formamide.
Add 1 mL of this solution to the 1-dram vial using a syringe.
Stir the mixture at room temperature for 30 min.
Remove and analyze an aliquot of the reaction mixture by TLC (eluent: ethyl acetate/hexane (1:1)). Monitor reaction progress by comparison to the limiting reagent (Fmoc-Gly-TBS-Taxol).
Upon conversion of starting material to product, remove the volatile components of the reaction mixture (DMF and piperidine) by drying the vial under high vacuum for several hours.
Redissolve the crude mixture in 0.5 mL of anhydrous dimethyl formamide.
Add 5 mg of Pacific Blue-NHS ester (0.015 mmol, 1.4 equiv.) and 0.006 mL of N,N-diisopropylethylamine (0.034 mmol, 3.1 equiv.) to this vial.
Seal the vial with a rubber septum and stir under Ar for 16 h.
Purify the crude reaction mixture by reverse phase preparative HPLC with a gradient of water and acetonitrile (both containing 0.1% v/v trifluoroacetic acid, see Note 2).
Concentrate the fractions containing the product using a rotary evaporator to remove acetonitrile. Remove the residual water by lyophilization in a foil-wrapped flask to obtain the pure product as an off-white solid (85% yield).
3.1.4. Synthesis of PB-Gly-Taxol
Dry a one-necked round bottom flask equipped with a magnetic stir bar in an oven (150 °C) for 1 hour. Seal this flask with a rubber septum after removing it from the oven. Evacuate the flask under high vacuum (5 min) and flush with dry argon. Repeat at least 3 times, allowing the flask to return to room temperature.
Quickly weigh 10 mg of PB-Gly-TBS-Taxol (0.008 mmol, 1.0 equiv.) into the dry flask and reseal the flask using a rubber septum.
Add 0.5 mL of anhydrous tetrahydrofuran to the flask using a syringe and stir for 5 min.
Add 0.016 mL of 1 M tetrabutyl ammonium fluoride in tetrahydrofuran (0.016 mmol, 2.0 equiv.) to this flask.
Stir the mixture at room temperature under Ar for 90 min.
Remove and analyze an aliquot of the reaction mixture by TLC (eluent: dichloromethane/methanol (9:1)). Monitor reaction progress by comparison to the limiting reagent (PB-Gly-TBS-Taxol).
Upon conversion of starting material to product, concentrate the reaction mixture to dryness using a rotary evaporator.
Redissolve the crude mixture in 1 mL of dimethyl sulfoxide.
Purify the crude reaction mixture by reverse phase preparative HPLC with a gradient of water and acetonitrile (both containing 0.1% v/v trifluoroacetic acid, see Note 2).
Concentrate the fractions containing the product using a rotary evaporator to remove acetonitrile. Remove residual water by lyophilization in a foil-wrapped flask to obtain the pure product as an off-white solid (67% yield).
3.2. Quantification of affinity of PB-Taxoids for purified crosslinked microtubules
3.2.1. Preparation of crosslinked microtubules
Reconstitute 500 μg lyophilized tubulin protein at 50 μM in 182 μL GAB buffer containing 1 mM GTP. This buffer must be prepared fresh before each use by addition of 2 μL of 100 mM GTP to 180 μL complete GAB buffer.
Incubate the reconstituted tubulin in GAB buffer at 37 °C for 30 min to induce microtubule assembly.
Crosslink pre-formed microtubules in 20 mM glutaraldehyde to prevent disassembly by addition of 20 μL GAB buffer containing 200 mM glutaraldehyde followed by incubation at 37 °C for 10 min.
Quench unreacted glutaraldehyde in 60 mM sodium borohydride by addition of 20 μL ice-cold GAB buffer containing 600 mM sodium borohydride. This will reduce labile imine crosslinks and form stable amine crosslinks.
These conditions provide 90% incorporation of tubulin into microtubule polymers. Clarify this solution by centrifugation at 16,800 g for 10 min at room temperature followed by discarding the supernatant to remove free tubulin.
Gently resuspend the pellet in 50 μL GAB buffer to dissolve, and determine the final concentration of crosslinked microtubules using a NanoDrop spectrophotometer or similar instrument based on the molar absorptivity of tubulin heterodimers, ε = 115,000 M−1cm−1 at 280 nm, and the Beer-Lambert equation (concentration = A/εl, where A = Absorbance, ε = molar extinction coefficient, and l = pathlength).
3.2.2. Preparation of an absorbance-normalized concentrated stock solution of PB-Taxoids in DMSO
Weigh ~1 mg of the PB-Taxoid in a microcentrifuge tube (1.5 mL). Add 0.5 mL of molecular biology grade DMSO. Sonicate to ensure complete dissolution and obtain a concentration of ~1.8 mM.
Determine the exact concentration of this DMSO stock solution by obtaining the absorbance spectrum of an aliquot (e.g. 5 μL) in PBS containing 10% DMSO and 0.5% Triton X-100 (in a final volume of 1 mL) with the Beer-Lambert equation (PB-Gly-Taxol ε415nm = 24,300 M−1 cm−1). Dilute the DMSO stock solution to a final concentration of 1 mM by addition of molecular biology-grade DMSO using the dilution equation C1V1 = C2V2, where C = concentration and V = volume.
3.2.3. Quantification of the affinity of PB-Taxoids for purified crosslinked microtubules
Starting with a 3.2 μM solution of microtubules in GAB buffer, use serial dilution to prepare ten 2-fold dilutions in triplicate. This will be used as a 2X solution.
Aliquot 50 μL of these 2X microtubules into 96-well black polystyrene microplates suitable for fluorescence measurements, in triplicate.
Prepare a solution of 50 nM PB-Taxoid in GAB buffer and aliquot 50 μL into each well to afford a final concentration of 25 nM (a value below the Kd) in a final volume of 100 μL (see Note 3).
Cover the plate with an aluminum seal to protect from light and prevent photobleaching, and gently shake the plate for 1 h at 22 °C.
Using a fluorescence microplate reader, measure the fluorescence intensity from Pacific Blue (Isample). Additionally, calculate average fluorescence (Imt) intensities of the background signal of microtubules alone by averaging three sample intensities for each concentration of tubulin in microtubules. Calculate average fluorescence (If) intensities of the free ligand by averaging three sample intensities of the Pacific Blue probe in GAB buffer. Calculate background-subtracted fluorescence (I) signals as I = Isample – Imt. Calculate the change in fluorescence intensity for each sample by subtracting the average (n=6) fluorescence of the free ligand (If). Plot this change in fluorescence intensity (up to 13-fold for PB-Gly-Taxol, up to 4.5-fold for PB-β-Ala-Taxol, and up to 3.4-fold for PB-GABA-Taxol) against the concentration of tubulin and use curve fitting with a one-site specific binding model (GraphPad Prism 9) to calculate the dissociation constant (Kd). Concentrations of crosslinked microtubules that allow 20% to 80% complexation of the PB-Taxoid are optimal for curve-fitting.
As a control, 10 μM paclitaxel can be added as a competitor to block the increase in fluorescence of PB-Taxoids upon binding to confirm the specific interaction with the Taxol-binding site of tubulin.
3.3. Imaging of binding of PB-Taxoids to microtubules by confocal microscopy of living cells
Grow cells in a T-75 flask. Harvest cells by washing twice with 5 mL of PBS followed by immediate trypsinization. For trypsinization, add 5 mL of trypsin-EDTA solution and incubate at 37 °C for 5 min. Once cells are suspended, add 5 mL of complete growth medium to neutralize the trypsin. Transfer cells into a conical 15 mL tube and centrifuge cells for 2 min at 700 g. Discard the media and resuspend the cell pellet in complete growth medium.
Using a cell counter or flow cytometer, measure the concentration of cells. Prepare a cell solution (3 mL) adjusted to a density of 3 × 105 cells/mL in complete medium. Add 300 μL per well to chambers of an Ibidi 8-well μ-Slide. Cover and incubate the cells at 37 °C, 5% CO2 until the cells approach confluency.
Prepare a 1 μM solution of PB-Taxoid in 1 mL of complete growth medium at a final concentration of 0.2% DMSO. Plan to aliquot 200 μL into each well to be imaged (see Note 4). Prepare a negative control lacking the PB-Taxoid in medium containing 0.2% DMSO, a positive control of medium containing 1 μM PB-Taxoid, and a positive control of medium containing 1 μM PB-Taxoid and 100 μM verapamil or 10 mM probenecid (see Note 5).
Remove complete growth medium from each well of the Ibidi 8-well μ-Slide and wash cells with 200 μL of PBS. Immediately aliquot 200 μL of the complete growth medium containing PB-Taxoid into each well.
Incubate the cells for 1 h at 37°C with 5% CO2.
Image immediately with a confocal laser-scanning fluorescence microscope. Excite PB-Taxoids with a 405 nm laser and collect emission from 425 nm to 500 nm.
3.4. Flow cytometry analysis of binding of PB-Taxoids to microtubules in living cells
Grow Caco-2 cells in a T-75 flask. Harvest cells by washing twice with 5 mL of PBS followed by immediate trypsinization. For trypsinization, add 5 mL of trypsin-EDTA solution and incubate at 37 °C for 5 min. Once cells are suspended, add 5 mL of complete growth medium to neutralize the trypsin. Transfer cells into a conical 15 mL tube and centrifuge cells for 2 min at 700 g. Discard the media and resuspend the cell pellet in complete growth medium.
Adjust the cell density to 1 × 106/mL in complete growth medium.
Prepare 1 mL of cell aliquots in Eppendorf tubes and treat with 1 μM PB-Taxoid, with or without efflux inhibitors (100 μM verapamil or 10 mM probenecid or 10 μM KO143 (see Note 5)).
Incubate treated cells in a rotating hybridization oven or similar gently shaking incubator at 37 °C for 1 h.
Wash cells to remove excess unbound PB-Taxoid by centrifuging for 2 min at 700 g, remove media, wash cells with ice-cold PBS, briefly centrifuge again to pellet, and resuspend in ice-cold PBS containing 2% BSA.
Keep cells on ice and analyze immediately, either using single-tubes for sampling or after transferring to an ice-cooled 96-well plate for sampling by a flow cytometer. Collect the fluorescence emission of Pacific Blue (10,000 events) by excitation at 405 nm with a 450 nm emission filter.
Gate 10,000 living cells, measure the median fluorescence of Pacific Blue, and analyze the standard deviation of three replicates.
Acknowledgements
This research was supported by the NIH (R01-CA211720) and the OSU Comprehensive Cancer Center (2P30-CA016058).
4 Notes
Pacific Blue-NHS ester is commercially available from a variety of vendors, but it can also be synthesized in gram quantities from inexpensive starting materials [41].
Purification was performed on an Agilent 1200 HPLC using a Hamilton PRP-1 column (250 mm length, 21.2 mm ID, 7 mm particle size). The gradient was set as 90:10 water/acetonitrile to 0:100 water/acetonitrile over 20 min.
Prepare concentrations of microtubules that enable 20% to 80% complexation of the PB-Taxoid held at a concentration below its Kd to enable equilibrium binding. The highest concentration of microtubules should be orders of magnitude higher than that of the PB-Taxoid tracer to achieve saturation binding.
When using an 8-well Ibidi μ-Slide for imaging, add less than 300 μL of treatment media per well to prevent potential cross-contamination of adjacent wells.
Probenecid has relatively poor aqueous solubility but is soluble in DMSO. Prepare a 10X solution of probenecid in complete growth medium (100 mM, 10% DMSO) by vigorous vortex mixing. Remove any insoluble probenecid using a 0.2 μm syringe filter or high-speed centrifugation with collection of the supernatant.
Decontaminate biohazardous waste. For solids, autoclave. For liquids, treat with 10% bleach for 20 min (Lawrence Berkeley National Laboratory ES&H manual (PUB-3000)).
References
- 1.Kaul R, Risinger AL, Mooberry SL (2019) Microtubule-Targeting Drugs: More than Antimitotics. J Nat Prod 82:680–685 doi: 10.1021/acs.jnatprod.9b00105 [DOI] [PubMed] [Google Scholar]
- 2.Stefano F (2014) Epothilones: From Discovery to Clinical Trials. Curr Topics Med Chem 14:2312–2321 doi: 10.2174/1568026614666141130095855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horwitz SB (1994) Taxol (paclitaxel): mechanisms of action. Ann Oncol 5 Suppl 6:S3–S6 [PubMed] [Google Scholar]
- 4.Prota AE, Bargsten K, Zurwerra D, Field JJ, Diaz JF, Altmann KH, Steinmetz MO (2013) Molecular Mechanism of Action of Microtubule-Stabilizing Anticancer Agents. Science 339:587–590 doi: 10.1126/science.1230582 [DOI] [PubMed] [Google Scholar]
- 5.Zasadil LM, Andersen KA, Yeum D, Rocque GB, Wilke LG, Tevaarwerk AJ, Raines RT, Burkard ME, Weaver BA (2014) Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci Transl Med 6:229ra243 doi: 10.1126/scitranslmed.3007965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L (1996) Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res 56:816–825 [PubMed] [Google Scholar]
- 7.Wang TH, Popp DM, Wang HS, Saitoh M, Mural JG, Henley DC, Ichijo H, Wimalasena J (1999) Microtubule dysfunction induced by paclitaxel initiates apoptosis through both c-Jun N-terminal kinase (JNK)-dependent and -independent pathways in ovarian cancer cells. J Biol Chem 274:8208–8016 doi: 10.1074/jbc.274.12.8208 [DOI] [PubMed] [Google Scholar]
- 8.Komlodi-Pasztor E, Sackett D, Wilkerson J, Fojo T (2011) Mitosis is not a key target of microtubule agents in patient tumors. Nat Rev Clin Oncol 8:244–250 doi: 10.1038/nrclinonc.2010.228 [DOI] [PubMed] [Google Scholar]
- 9.Pineda JJ, Miller MA, Song Y, Kuhn H, Mikula H, Tallapragada N, Weissleder R, Mitchison TJ (2018) Site occupancy calibration of taxane pharmacology in live cells and tissues. Proc Natl Acad Sci U S A 115:E11406–E11414 doi: 10.1073/pnas.1800047115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver BA (2014) How Taxol/paclitaxel kills cancer cells. Mol Biol Cell 25:2677–2681 doi: 10.1091/mbc.E14-04-0916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yared JA, Tkaczuk KH (2012) Update on taxane development: new analogs and new formulations. Drug Des Devel Ther 6:371–384 doi: 10.2147/DDDT.S28997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz JF, Andreu JM (1993) Assembly of purified GDP-tubulin into microtubules induced by taxol and taxotere: reversibility, ligand stoichiometry, and competition. Biochemistry 32:2747–2755 doi: 10.1021/bi00062a003 [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Poliks B, Cegelski L, Poliks M, Gryczynski Z, Piszczek G, Jagtap PG, Studelska DR, Kingston DG, Schaefer J, Bane S (2000) Conformation of microtubule-bound paclitaxel determined by fluorescence spectroscopy and REDOR NMR. Biochemistry 39:281–291 doi: 10.1021/bi991936r [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Edsall R Jr., Jagtap PG, Kingston DG, Bane S (2000) Equilibrium studies of a fluorescent paclitaxel derivative binding to microtubules. Biochemistry 39:616–623 doi: 10.1021/bi992044u [DOI] [PubMed] [Google Scholar]
- 15.Sharma S, Lagisetti C, Poliks B, Coates RM, Kingston DG, Bane S (2013) Dissecting paclitaxel-microtubule association: quantitative assessment of the 2’-OH group. Biochemistry 52:2328–2336 doi: 10.1021/bi400014t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barasoain I, Diaz JF, Andreu JM (2010) Fluorescent taxoid probes for microtubule research. Methods Cell Biol 95:353–372 doi: 10.1016/S0091-679X(10)95019-X [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharyya B, Kapoor S, Panda D (2010) Fluorescence spectroscopic methods to analyze drug-tubulin interactions. Methods Cell Biol 95:301–329 doi: 10.1016/s0091-679x(10)95017-6 [DOI] [PubMed] [Google Scholar]
- 18.Buey RM, Calvo E, Barasoain I, Pineda O, Edler MC, Matesanz R, Cerezo G, Vanderwal CD, Day BW, Sorensen EJ, Lopez JA, Andreu JM, Hamel E, Diaz JF (2007) Cyclostreptin binds covalently to microtubule pores and lumenal taxoid binding sites. Nat Chem Biol 3:117–125 doi: 10.1038/nchembio853 [DOI] [PubMed] [Google Scholar]
- 19.Andreu JM, Barasoain I (2001) The interaction of baccatin III with the taxol binding site of microtubules determined by a homogeneous assay with fluorescent taxoid. Biochemistry 40:11975–11984 [DOI] [PubMed] [Google Scholar]
- 20.Ross JL, Santangelo CD, Makrides V, Fygenson DK (2004) Tau induces cooperative Taxol binding to microtubules. Proc Natl Acad Sci U S A 101:12910–12915 doi: 10.1073/pnas.0402928101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lukinavicius G, Reymond L, D’Este E, Masharina A, Gottfert F, Ta H, Guther A, Fournier M, Rizzo S, Waldmann H, Blaukopf C, Sommer C, Gerlich DW, Arndt H-D, Hell SW, Johnsson K (2014) Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat Methods 11:731–733 doi:10.1038/nmeth.2972 http://www.nature.com/nmeth/journal/v11/n7/abs/nmeth.2972.html#supplementary-information [DOI] [PubMed] [Google Scholar]
- 22.Lukinavicius G, Umezawa K, Olivier N, Honigmann A, Yang G, Plass T, Mueller V, Reymond L, Correa IR Jr., Luo ZG, Schultz C, Lemke EA, Heppenstall P, Eggeling C, Manley S, Johnsson K (2013) A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins. Nat Chem 5:132–139 doi: 10.1038/nchem.1546 [DOI] [PubMed] [Google Scholar]
- 23.Thrower D, Jordan MA, Wilson L (1991) Quantitation of cellular tubulin in microtubules and tubulin pools by a competitive ELISA. J Immunol Methods 136:45–51 doi: 10.1016/0022-1759(91)90248-e [DOI] [PubMed] [Google Scholar]
- 24.Bulinski JC, Morgan JL, Borisy GG, Spooner BS (1980) Comparison of methods for tubulin quantitation in HeLa cell and brain tissue extracts. Anal Biochem 104:432–439. doi: 10.1016/0003-2697(80)90095-0 [DOI] [PubMed] [Google Scholar]
- 25.Kuh HJ, Jang SH, Wientjes MG, Au JL (2000) Computational model of intracellular pharmacokinetics of paclitaxel. J Pharmacol Exp Ther 293:761–770 [PubMed] [Google Scholar]
- 26.Lee MM, Gao Z, Peterson BR (2017) Synthesis of a Fluorescent Analogue of Paclitaxel That Selectively Binds Microtubules and Sensitively Detects Efflux by P-Glycoprotein. Angew Chem Int Ed 56:6927–6931 doi: 10.1002/anie.201703298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duchi S, Dambruoso P, Martella E, Sotgiu G, Guerrini A, Lucarelli E, Pessina A, Coccè V, Bonomi A, Varchi G (2014) Thiophene-based compounds as fluorescent tags to study mesenchymal stem cell uptake and release of taxanes. Bioconjug Chem 25:649–655 doi: 10.1021/bc5000498 [DOI] [PubMed] [Google Scholar]
- 28.Díaz JF, Barasoain I, Andreu JM (2003) Fast kinetics of Taxol binding to microtubules. Effects of solution variables and microtubule-associated proteins. J Biol Chem 278:8407–8419 doi: 10.1074/jbc.M211163200 [DOI] [PubMed] [Google Scholar]
- 29.Diaz JF, Strobe R, Engelborghs Y, Souto AA, Andreu JM (2000) Molecular recognition of taxol by microtubules. Kinetics and thermodynamics of binding of fluorescent taxol derivatives to an exposed site. J Biol Chem 275:26265–26276 doi: 10.1074/jbc.M003120200 [DOI] [PubMed] [Google Scholar]
- 30.Buey RM, Barasoain I, Jackson E, Meyer A, Giannakakou P, Paterson I, Mooberry S, Andreu JM, Díaz JF (2005) Microtubule Interactions with Chemically Diverse Stabilizing Agents: Thermodynamics of Binding to the Paclitaxel Site Predicts Cytotoxicity. Chem Biol 12:1269–1279 doi: 10.1016/j.chembiol.2005.09.010 [DOI] [PubMed] [Google Scholar]
- 31.Römermann K, Wanek T, Bankstahl M, Bankstahl JP, Fedrowitz M, Müller M, Löscher W, Kuntner C, Langer O (2013) (R)-[(11)C]verapamil is selectively transported by murine and human P-glycoprotein at the blood-brain barrier, and not by MRP1 and BCRP. Nucl Med Biol 40:873–878 doi: 10.1016/j.nucmedbio.2013.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin C, Walker J, Rothnie A, Callaghan R (2003) The expression of P-glycoprotein does influence the distribution of novel fluorescent compounds in solid tumour models. Br J Cancer 89:1581–1589 doi: 10.1038/sj.bjc.6601300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Němcová-Fürstová V, Kopperová D, Balušíková K, Ehrlichová M, Brynychová V, Václavíková R, Daniel P, Souček P, Kovář J (2016) Characterization of acquired paclitaxel resistance of breast cancer cells and involvement of ABC transporters. Toxicol Appl Pharmacol 310:215–228 doi: 10.1016/j.taap.2016.09.020 [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y-K, Wang Y-J, Gupta P, Chen Z-S (2015) Multidrug Resistance Proteins (MRPs) and Cancer Therapy. AAPS J 17:802–812 doi: 10.1208/s12248-015-9757-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prime-Chapman HM, Fearn RA, Cooper AE, Moore V, Hirst BH (2004) Differential MRP1-6 isoform expression and function in human intestinal epithelial Caco-2 cells. J Pharmacol Exp Ther 311:476–484 doi: 10.1124/jpet.104.068775 [DOI] [PubMed] [Google Scholar]
- 36.Huisman MT, Chhatta AA, van Tellingen O, Beijnen JH, Schinkel AH (2005) MRP2 (ABCC2) transports taxanes and confers paclitaxel resistance and both processes are stimulated by probenecid. Int J Cancer 116:824–829 doi: 10.1002/ijc.21013 [DOI] [PubMed] [Google Scholar]
- 37.Mease K, Sane R, Podila L, Taub ME (2012) Differential selectivity of efflux transporter inhibitors in Caco-2 and MDCK-MDR1 monolayers: a strategy to assess the interaction of a new chemical entity with P-gp, BCRP, and MRP2. J Pharm Sci 101:1888–1897 doi: 10.1002/jps.23069 [DOI] [PubMed] [Google Scholar]
- 38.Hirohashi T, Suzuki H, Chu XY, Tamai I, Tsuji A, Sugiyama Y (2000) Function and expression of multidrug resistance-associated protein family in human colon adenocarcinoma cells (Caco-2). J Pharmacol Exp Ther 292:265–270 [PubMed] [Google Scholar]
- 39.Potschka H, Baltes S, Löscher W (2004) Inhibition of multidrug transporters by verapamil or probenecid does not alter blood-brain barrier penetration of levetiracetam in rats. Epilepsy Res 58:85–91 doi: 10.1016/j.eplepsyres.2003.12.007 [DOI] [PubMed] [Google Scholar]
- 40.Dei S, Braconi L, Romanelli MN, Teodori E (2019) Recent advances in the search of BCRP- and dual P-gp/BCRP-based multidrug resistance modulators. Cancer Drug Resist 2:710–743 doi: 10.20517/cdr.2019.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee MM, Peterson BR (2016) Quantification of Small Molecule-Protein Interactions using FRET between Tryptophan and the Pacific Blue Fluorophore. ACS Omega 1:1266–1276 doi: 10.1021/acsomega.6b00356 [DOI] [PMC free article] [PubMed] [Google Scholar]


