Abstract
Background
Breech presentation is associated with increased complications. Turning a breech baby to head first presentation using external cephalic version (ECV) attempts to reduce the chances of breech presentation at birth so as to avoid the adverse effects of breech vaginal birth or caesarean section. Interventions such as tocolytic drugs and other methods have been used in an attempt to facilitate ECV.
Objectives
To assess, from the best evidence available, the effects of interventions such as tocolysis, acoustic stimulation for midline spine position, regional analgesia (epidural or spinal), transabdominal amnioinfusion, systemic opioids and hypnosis, or the use of abdominal lubricants, on ECV at term for successful version, presentation at birth, method of birth and perinatal and maternal morbidity and mortality.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 September 2014) and the reference lists of identified studies.
Selection criteria
Randomised and quasi‐randomised trials comparing the above interventions with no intervention or other methods to facilitate ECV at term.
Data collection and analysis
We assessed eligibility and trial quality. Two review authors independently assessed for inclusion all potential studies identified as a result of the search strategy and independently extracted the data using a specially designed data extraction form.
Main results
We included 28 studies, providing data on 2786 women. We used the random‐effects model for pooling data because of clinical heterogeneity between studies. A number of trial reports gave insufficient information to allow clear assessment of risk of bias. We used GradePro software to carry out formal assessments of quality of the evidence for beta stimulants versus placebo and regional analgesia with tocolysis versus tocolysis alone.
Tocolytic parenteral beta stimulants were effective in increasing cephalic presentations in labour (average risk ratio (RR) 1.68, 95% confidence interval (CI) 1.14 to 2.48, five studies, 459 women, low‐quality evidence) and in reducing the number of caesarean sections (average RR 0.77, 95% CI 0.67 to 0.88, six studies, 742 women, moderate‐quality evidence). Failure to achieve a cephalic vaginal birth was less likely for women receiving a parenteral beta stimulant (average RR 0.75, 95% CI 0.60 to 0.92, four studies, 399 women, moderate‐quality evidence). No clear differences in fetal bradycardias were identified, although this was reported for only one study, which was underpowered for assessing this outcome. Failed external cephalic version was reported in nine studies (900 women), and women receiving parenteral beta stimulants were less likely to have failure compared with controls (average RR 0.70, 95% CI 0.60 to 0.82, moderate‐quality evidence). Perinatal mortality and serious morbidity were not reported. Sensitivity analysis by study quality was consistent with overall findings.
For other classes of tocolytic drugs (calcium channel blockers and nitric oxide donors), evidence was insufficient to permit conclusions; outcomes were reported for only one or two studies, which were underpowered to demonstrate differences between treatment and control groups. Little evidence was found regarding adverse effects, although nitric oxide donors were associated with increased risk of headache. Data comparing different tocolytic drugs were insufficient.
Regional analgesia in combination with a tocolytic was more effective than the tocolytic alone for increasing successful versions (assessed by the rate of failed ECVs; average RR 0.61, 95% CI 0.43 to 0.86, five studies, 409 women, moderate‐quality evidence), and no difference was identified in cephalic presentation in labour (average RR 1.44, 95% CI 0.78 to 2.66, three studies, 279 women, very low‐quality evidence), caesarean sections (average RR 0.74, 95% CI 0.40 to 1.37, three studies, 279 women, very low‐quality evidence) nor fetal bradycardia (average RR 1.48, 95% CI 0.62 to 3.57, two studies, 210 women, low‐quality evidence), although studies were underpowered for assessing these outcomes. Studies did not report on failure to achieve a cephalic vaginal birth (breech vaginal deliveries plus caesarean sections) nor on perinatal mortality or serious infant morbidity.
Data were insufficient on the use of regional analgesia without tocolysis, vibroacoustic stimulation, amnioinfusion, systemic opioids and hypnosis, and on the use of talcum powder or gel to assist external cephalic version, to permit conclusions about their effectiveness and safety.
Authors' conclusions
Parenteral beta stimulants were effective in facilitating successful ECV, increasing cephalic presentation in labour and reducing the caesarean section rate, but data on adverse effects were insufficient. Data on calcium channel blockers and nitric acid donors were insufficient to provide good evidence.
The scope for further research is clear. Possible benefits of tocolysis in reducing the force required for successful version and possible risks of side effects need to be addressed further. Further trials are needed to compare the effectiveness of routine versus selective use of tocolysis and the role of regional analgesia, fetal acoustic stimulation, amnioinfusion and abdominal lubricants, and the effects of hypnosis, in facilitating ECV. Although randomised trials of nitric oxide donors are small, the results are sufficiently negative to discourage further trials. Intervention fidelity for ECV can be enhanced by standardisation of the techniques and processes used for clinical manipulation of the fetus in the abdominal cavity and ought to be the subject of further research.
Plain language summary
Ways to help turn a breech baby to head first presentation at the end of pregnancy
Babies born in the breech position (bottom first) are at increased risk of complications at birth because of a delay in birth of the head. Turning a breech baby to head first in late pregnancy may reduce these complications. A procedure called 'external cephalic version (ECV)' describes when practitioners use their hands on the woman's abdomen to gently try to turn the baby from the breech position to head first. A number of treatments may help the success of ECV. These include using tocolytic drugs (drugs like beta stimulants and calcium channel blockers that relax the womb), stimulating the baby with sound through the mother’s abdomen (acoustic stimulation), increasing the fluid surrounding the baby (transabdominal amnioinfusion), injecting pain‐relieving drugs into the mother’s lower back to produce regional analgesia (epidural or spinal analgesia), giving the mother opioid drugs to help her relax, using hypnosis and applying gel or talcum powder to the mother's abdomen.
This review of trials found 28 randomised controlled studies involving 2786 women. Most studies looked at the effects of tocolytic beta stimulant drugs. Results showed that babies are more likely to turn head first during ECV and to remain head first for the start of labour, if women receive beta stimulants. These drugs also reduced the number of caesarean sections, but insufficient data on possible adverse effects were collected. Little information on other types of tocolytic drugs was available, although nitric oxide donors were associated with an increase in headaches. In addition, too little evidence was available to show whether the other ways of trying to help ECV are effective. Further research is needed if we are to increase the success of ECV.
Summary of findings
Summary of findings for the main comparison. Beta stimulant compared with placebo for helping to turn babies with breech presentation when ECV was used.
| Beta stimulant compared with placebo for helping to turn babies with breech presentation when ECV was used | ||||||
| Patient or population: patients with breech presentation Settings: studies in hospital settings Intervention: beta stimulant Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Beta stimulant | |||||
| Cephalic presentation at birth (primary) | Study population | RR 1.68 (1.14 to 2.48) | 459 (5 studies) | ⊕⊕⊝⊝ Low1,2 | ||
| 294 per 1000 | 494 per 1000 (335 to 729) | |||||
| Moderate | ||||||
| 255 per 1000 | 428 per 1000 (291 to 632) | |||||
| Cephalic vaginal birth not achieved (CS + breech vaginal birth) primary outcome | Study population | RR 0.75 (0.6 to 0.92) | 399 (4 studies) | ⊕⊕⊕⊝ Moderate3 | ||
| 727 per 1000 | 545 per 1000 (436 to 669) | |||||
| Moderate | ||||||
| 708 per 1000 | 531 per 1000 (425 to 651) | |||||
| Caesarean section (primary) | Study population | RR 0.77 (0.67 to 0.88) | 742 (6 studies) | ⊕⊕⊕⊝ Moderate1 | ||
| 670 per 1000 | 516 per 1000 (449 to 590) | |||||
| Moderate | ||||||
| 707 per 1000 | 544 per 1000 (474 to 622) | |||||
| Fetal bradycardia (primary) | Study population | RR 2.81 (0.12 to 66.17) | 58 (1 study) | ⊕⊝⊝⊝ Very low4,5 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Failed external cephalic version | Study population | RR 0.7 (0.6 to 0.82) | 900 (9 studies) | ⊕⊕⊕⊝ Moderate1 | ||
| 654 per 1000 | 458 per 1000 (393 to 537) | |||||
| Moderate | ||||||
| 632 per 1000 | 442 per 1000 (379 to 518) | |||||
| Perinatal mortality | See comment | See comment | Not estimable | 0 (0) | See comment | No data reported |
| Perinatal morbidity | See comment | See comment | Not estimable | 0 (0) | See comment | No data reported |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. Most studies contributing data had design limitations. 2. I2 > 60%. Effect size varied considerably. 3. All studies providing data had design limitations. 4. The one study included is of poor quality, as it is an unblinded quasi‐RCT. 5. Wide 95% CI crossing the line of no effect; small sample size and low event rate.
Summary of findings 2. Regional analgesia (with tocolysis) versus no intervention of regional analgesia (with or without tocolysis) for breech presentation.
| Regional analgesia (with tocolysis) versus no intervention of regional analgesia (with or without tocolysis) for breech presentation | ||||||
| Patient or population: patients with breech presentation Settings: studies in hospital settings Intervention: regional analgesia (with tocolysis) versus no intervention of regional analgesia (with or without tocolysis) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Regional analgesia (with tocolysis) vs no intervention of regional analgesia (with or without tocolysis) | |||||
| Cephalic presentation at birth (primary) | Study population | RR 1.63 (0.75 to 3.53) | 279 (3 studies) | ⊕⊝⊝⊝ Very low1,2,3 | ||
| 393 per 1000 | 640 per 1000 (295 to 1000) | |||||
| Moderate | ||||||
| 352 per 1000 | 574 per 1000 (264 to 1000) | |||||
| Cephalic vaginal birth not achieved (CS + breech vaginal birth) (primary) | Study population | RR 0.65 (0.47 to 0.89) | 108 (1 study) | ⊕⊕⊝⊝ Low4,5 | ||
| 741 per 1000 | 481 per 1000 (348 to 659) | |||||
| Moderate | ||||||
| 741 per 1000 | 482 per 1000 (348 to 659) | |||||
| Caesarean section (primary) | Study population | RR 0.74 (0.4 to 1.37) | 279 (3 studies) | ⊕⊝⊝⊝ Very low1,2,3 | ||
| 650 per 1000 | 481 per 1000 (260 to 891) | |||||
| Moderate | ||||||
| 685 per 1000 | 507 per 1000 (274 to 938) | |||||
| Fetal bradycardia (primary) | Study population | RR 1.48 (0.62 to 3.57) | 210 (2 studies) | ⊕⊕⊝⊝ Low1,3 | ||
| 85 per 1000 | 126 per 1000 (53 to 303) | |||||
| Moderate | ||||||
| 86 per 1000 | 127 per 1000 (53 to 307) | |||||
| Failed external cephalic version | Study population | RR 0.61 (0.43 to 0.86) | 409 (5 studies) | ⊕⊕⊕⊝ Moderate1 | ||
| 585 per 1000 | 357 per 1000 (251 to 503) | |||||
| Moderate | ||||||
| 577 per 1000 | 352 per 1000 (248 to 496) | |||||
| Perinatal morbidity | See comment | See comment | Not estimable | 0 (0) | See comment | No data reported |
| Perinatal mortality | See comment | See comment | Not estimable | 0 (0) | See comment | No data reported |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. All studies contributing data had design limitations. 2. I2 > 60%; direction and size of effect inconsistent. 3. Wide 95% CI crossing the line of no effect and small sample size. 4. Study contributing data had design limitations. 5. Estimate based on small sample size.
Background
Description of the condition
Breech presentation occurs when the baby is positioned bottom first. It is more common in early pregnancy, and the incidence decreases with increasing gestational age. The incidence at term is about 3% to 4% (Hickok 1992). Breech presentation may be caused by an underlying fetal or maternal abnormality, it may be an apparently chance occurrence or it may be related to an otherwise benign variant such as cornual placental position (the placenta situated in an upper lateral corner of the uterus). In the latter two instances, breech presentation places a healthy baby and mother at increased risk of a complicated vaginal birth or caesarean section. Therefore, it is understandable that obstetricians, midwives and consumer groups take considerable interest in this topic. Prevention of harm with reduction of risk for mother and baby is a quantifiable outcome of carefully gathering this research evidence. Knowing what works best for whom and in what circumstances is the concern of clinical researchers working in this field.
Considerable disagreement surrounds the management of breech (bottom first) presentation, with respect to both the place of external cephalic version (ECV) and the type of birth. Interpretation of the findings of non‐randomised trials is confounded by the fact that breech presentation per se appears to be a marker for poor perinatal outcome. For example, the incidence of childhood handicap following breech presentation has been found to be high (16%) for both babies born vaginally and those born by caesarean section (Danielian 1996). Randomised trials of planned mode of birth for vaginal breech birth have shown short‐term benefit for the breech presenting baby managed by planned caesarean section compared with planned vaginal birth, although the impact on future pregnancies remains uncertain (Hofmeyr 2003). Two‐year outcomes of one of these randomised trials showed no significant difference in the combined risk of death/neurodevelopmental delay between planned vaginal and planned caesarean groups (Whyte 2004). Despite this, these results have had a profound effect on clinical practice, and in many institutions, caesarean section for breech presentation has become routine. Under these circumstances, the impact of ECV on caesarean section rates would be expected to be greater than was the case in previous trials in institutions in which vaginal breech birth was common. The increased rate of caesarean section for breech presentation has decreased the rate of vaginal breech births, and concern has arisen that practitioners are losing the skill of supporting women who have vaginal breech births.
Breech presentation can be classified as complete, frank or incomplete. A complete breech occurs when the baby’s hips and knees are flexed, with feet near the buttocks. A frank breech presentation is seen when the baby's legs are extended up to its head. Incomplete breech presentations include a footling breech, in which one or both legs are extended below the baby's bottom, and a kneeling breech, whereby the knees are the presenting part of the breech. Although underlying reasons may explain the breech presentation, the baby may have a more difficult vaginal birth because of the delay in birth of the head.
Description of the intervention
External cephalic version
During an ECV, practitioners use their hands on the woman's abdomen to gently try to turn the baby from the breech position to the head‐down position. A video of the procedure can be viewed at https://www.youtube.com/watch?v=fKaNZfUno50.
External cephalic version before term became a part of routine obstetrical practice on the basis of the self‐evident immediate effectiveness of the procedure, as well as reassuring results from several non‐randomised trials, and in spite of the negative results of the only randomised trial reported before 1980 (Brosset 1956). The popularity of ECV before term waned after the mid‐1970s, in part because of reports of an increase in perinatal mortality associated with the procedure (Bradley‐Watson 1975), which, in retrospect, may have been due to application of undue force and the increasing perception of caesarean section as a safer option than ECV or breech birth.
Before the mid‐1970s, ECV was usually attempted before term because of the belief that the procedure would seldom be successful at term. Subsequent studies showed that with the use of tocolysis, ECV could be achieved in a substantial proportion of women with breech presentation at term (37 or more completed weeks of pregnancy). Predictors of unsuccessful version include engaged presenting part, fetal head not easily palpable and tense uterus (Lau 1997).
Initially, successful ECV at a late stage of pregnancy was considered to have become possible only because of the use of tocolytic drugs to relax the uterus. However, later studies showed that ECV at term was frequently possible without tocolysis. The overall success rate was 60% in a systematic review of randomised controlled trials in which some trials included facilitation and others did not (Hofmeyr 1996).
The question, therefore, arose as to whether tocolysis should be used routinely for ECV at term, or only in those cases in which difficulty is anticipated or initial attempts fail.
A number of interventions to try to make ECV easier and more successful have been suggested, including use of tocolytic drugs, vibroacoustic stimulation, regional analgesia, amnioinfusion, maternal hydration, systemic opioid drugs, hypnosis and abdominal lubricants.
How the intervention might work
Tocolysis to facilitate ECV at term
Beta stimulants, such as salbutamol, ritodrine, hexoprenaline or terbutaline, are widely used tocolytics. They are usually given intravenously. Possible side effects for mother and baby include tachycardia (increase in heart rate).
Calcium channel blockers, like nifedipine, can be administered orally (Smith 2000). These drugs can be associated with hypotension (fall in blood pressure).
Nitric oxide donors, such as intravenous nitroglycerine (Belfort 1993) or sublingual glyceryl trinitrate/nitroglycerine spray (Reddick 1997; Yanny 2000), have been suggested as alternative tocolytics.
Vibroacoustic stimulation to facilitate ECV at term
This procedure is performed when the baby is stimulated using sound applied to the mother's abdomen to provoke the baby to move out of the midline position. It has been studied in one small trial, which is included in this review (Johnson 1995).
Regional analgesia to facilitate ECV at term
Regional analgesia includes spinal and epidural anaesthesia. Epidural analgesia is provided when an anaesthetic drug is infused into the epidural space. Spinal analgesia is given when an anaesthetic drug is injected into the cerebrospinal fluid. In a retrospective cohort study, ECV at term was successful in 59% of 32 women with epidural analgesia, and in 24% of 37 women without (Carlan 1994). In an uncontrolled study, ECV under epidural analgesia was successful in nine of 16 women (56%) in whom initial attempts had failed (Neiger 1998a; Neiger 1998b). Common adverse effects of these analgesics include hypotension and headache.
Amnioinfusion to facilitate ECV at term
An amnioinfusion is a procedure whereby saline is infused into the amniotic sac to increase the volume of fluid to enable the baby to turn more easily. Amnioinfusions can be done transabdominally or transvaginally. In an uncontrolled study, six women with failed ECV had a successful repeat attempt following transabdominal amnioinfusion with 700 mL to 900 mL warmed saline (Benifla 1995). To our knowledge, no randomised trials have determined the effectiveness of this intervention.
Systemic opioids to facilitate ECV at term
Systemic opioids may facilitate ECV by relaxing the mother and reducing her sense of discomfort during the procedure.
Hypnosis to facilitate ECV at term
Different types of hypnosis may facilitate ECV by promoting relaxation, thereby potentially reducing the woman's sense of discomfort during the procedure.
Talcum powder and gel to facilitate ECV at term
Powder or gel applied to the woman's abdomen may act as a lubricant, possibly allowing smoother hand movements during attempts to turn the baby.
Why it is important to do this review
It is important to assess whether various interventions do increase the effectiveness of ECV in turning a breech baby to head first presentation and to help guide their use in clinical practice. Many of these interventions are commonly used, and it is important for doctors to be able to apply evidence‐based medicine in this setting to offer the mother the greatest chance of success when undergoing an ECV. It must also be determined whether any of these interventions is associated with possible harm to mother or fetus.
Readers are referred to previous reviews of the topic (Hofmeyr 1989; Hofmeyr 1991; Hofmeyr 1992; Hofmeyr 1993; Hofmeyr 2014; Zhang 1993) ‐ see also related Cochrane reviews: 'Cephalic version by postural management for breech presentation' (Hofmeyr 2012b); 'Cephalic version by moxibustion for breech presentation' (Coyle 2012); 'External cephalic version for breech presentation at term' (Hofmeyr 2012a); and 'External cephalic version for breech presentation before term' (Hutton 2006).
Objectives
To assess, from the best evidence available, the effects of interventions such as tocolysis, acoustic stimulation for midline spine position, regional analgesia (epidural or spinal), transabdominal amnioinfusion, systemic opioids and hypnosis, or the use of abdominal lubricants, on ECV at term for successful version, presentation at birth, method of birth and perinatal and maternal morbidity and mortality.
Methods
Criteria for considering studies for this review
Types of studies
Clinical trials comparing the effects of interventions such as routine tocolysis versus selective or no use of tocolysis, or different tocolytics, epidural or spinal analgesia, amnioinfusion, maternal hydration, systemic opioids and fetal acoustic stimulation in midline fetal spine positions or hypnosis or abdominal lubricants on clinically meaningful outcomes, with random or quasi‐random allocation to treatment and control groups and with violations of allocated management and exclusions after allocation not sufficient to materially affect outcomes.
Types of participants
Women with singleton breech presentations at term and no contraindications to ECV or the intervention being studied, with or without previous failed ECV.
Types of interventions
A. Tocolytic drugs.
B. Vibroacoustic stimulation in midline fetal spine positions.
C. Regional analgesia.
D. Amnioinfusion.
E. Systemic opioids.
To avoid duplication of data, we have listed the interventions under study in order, from A to E. Each intervention will be compared with placebo and with only those interventions above it on the list. Thus, the intervention 'Regional analgesia' (C) will be compared with placebo, then with tocolytic drugs (A), then with vibroacoustic stimulation (B) and finally with other regional analgesia (C). When C is compared with C, different types of regional analgesia are compared with each other, so epidural may be compared with spinal analgesia as an intervention to facilitate ECV. Interventions identified in the future will be added to the end of the list.
In this update we identified trials examining other types of interventions used to facilitate ECV.
F. Hypnosis*.
G. Abdominal lubricants* (talcum powder versus gel).
*We decided to include these interventions, although they were not prespecified in the original protocol.
Types of outcome measures
Primary outcomes
Cephalic presentation at labour and at birth.
Failure to achieve cephalic vaginal birth (composite outcome: caesarean section plus vaginal breech birth)*.
Caesarean section.
Fetal bradycardia or prolonged decelerations as defined by trial authors.
Secondary outcomes
Failed external cephalic version.
Difficult external cephalic version.
Maternal palpitations.
Maternal headaches.
Maternal hypotension.
Operative vaginal birth.
Maternal mortality.
Maternal morbidity.
Perinatal mortality.
Perinatal morbidity.
We have included other outcomes, not specified here, when they were reported in the studies and when we considered them to be clinically important: vaginal breech birth, Apgar less than seven at five minutes, neonatal seizures, admission to neonatal unit, birth trauma, flushing in women, placental abruption, maternal discomfort, pain scores, maternal satisfaction with the procedure and maternal side effects (nausea and vomiting, dizziness and drowsiness).
*In this version of the review, a new primary outcome has been added. The purpose of ECV is to avoid breech presentation, which increases the risk of caesarean section and of breech vaginal delivery. For this reason, and to increase consistency with other related Cochrane reviews, we have added a composite outcome "Failure to achieve cephalic vaginal birth," which represents caesarean section plus vaginal breech births.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (30 September 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For this update, we used the following methods. These methods are based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all potential studies identified as a result of the search strategy. We resolved disagreements through discussion, or, if required, we consulted the other review authors to achieve consensus.
Data extraction and management
We designed a form on which to record extracted data. For eligible studies, two review authors extracted data using the agreed upon form. We resolved discrepancies through discussion, or, if required, we consulted the third review author. We entered the data into Review Manager software (RevMan 2014) and checked them for accuracy.
When information in trial reports was unclear, we planned to contact report authors to request further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion with the other review authors.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions before assignment and assessed whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants; and
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the quantity, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total number of randomly assigned participants), reasons for attrition or exclusion when reported and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported, or could be supplied by the trial authors, we planned to reinclude missing data in the analyses that we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation); or
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed these methods as:
low risk of bias (when it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (when not all of the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported); or
unclear risk of bias
(6) Other bias (checking for bias due to problems not covered by the methods listed above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to the methods listed above, we planned to assess the likely magnitude and direction of bias and whether we considered it likely to impact the findings. When sufficient data were available, we explored the impact of the level of bias by undertaking sensitivity analyses ‐ seeSensitivity analysis.
For this update, we assessed the quality of the evidence using the GRADE approach (Schunemann 2009) to assess the quality of the body of evidence related to the following outcomes.
Cephalic presentation at labour and at birth.
Failure to achieve cephalic vaginal birth.
Caesarean section.
Fetal bradycardia or prolonged decelerations as defined by trial authors.
Failed external cephalic version.
Perinatal mortality.
Perinatal morbidity.
We graded the evidence and included 'Summary of findings' tables for two comparisons.
Tocolytics (parenteral beta stimulants) versus placebo.
Regional analgesia with tocolysis versus tocolysis alone.
The GRADE profiler (Grade 2014) was used to import data from Review Manager 5.3 (RevMan 2014) to create ’Summary of findings’ tables. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes by using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. Evidence can be downgraded from 'high quality' by one level for serious, or by two levels for very serious, limitations depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we have presented results as summary risk ratios with 95% confidence intervals.
Continuous data
We used mean differences if outcomes were measured in the same way between trials. We used standardised mean differences to combine trials that measured the same outcome but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually randomised trials if they were otherwise eligible. For this version of the review, we identified no such trials; if they are included in future updates, we will adjust sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions based on an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and will conduct sensitivity analyses to investigate the effects of variation in the ICC. If we identify both cluster‐randomised trials and individually randomised trials, we plan to synthesise relevant information. We will consider it reasonable to combine the results from both if little heterogeneity is noted between study designs, and if the interaction between effects of the intervention and choice of the randomisation unit is considered unlikely.
We will also acknowledge heterogeneity in the randomisation unit and will perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We did not plan to include trials with a cross‐over design. One of the trials that was otherwise eligible for inclusion randomly assigned women to two groups (parallel design), but if after two attempts the randomised ECV method was not successful, the trial protocol allowed the alternative method to be used (Vallikkannu 2014). We treated this study as a parallel‐group randomised controlled trial and used only data collected before any cross‐over to the alternative method.
Other unit of analysis issues
We excluded trials including multiple pregnancies.
In this version of the review, we did not include trials with multiple treatment arms; if we identify such trials for inclusion in future updates, we will use the methods set out in theCochrane Handbook for Systematic Reviews of Interventions for analysis.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, review authors will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis (i.e. we attempted to include in the analyses all participants randomly assigned to each group). The denominator for each outcome in each trial was the number randomly assigned minus the number of participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero or the P value (< 0.10) in the Chi² test for heterogeneity was low. If we identified substantial heterogeneity (> 30%), we planned to explore this by performing prespecified subgroup analysis.
Assessment of reporting biases
In future updates, if 10 or more studies are included in the meta‐analysis, we plan to investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate this.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used the random‐effects model for pooling data because of clinical heterogeneity in the included studies in various comparisons. The random‐effects summary represents the average range of possible treatment effects, and we have discussed the clinical implications of differing treatment effects between trials. If the average treatment effect was not considered clinically meaningful, we did not combine trials. We have presented results as the average treatment effect with 95% confidence intervals, and when heterogeneity between trials was noted, with estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated this by using subgroup analyses.
When data were available, we planned to carry out the following analysis.
nulliparous versus multiparous women.
We restricted subgroup analysis to the review's primary outcomes.
We assessed subgroup differences by performing interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses by quoting the Chi² statistic and the P value, and results of the interaction test by reporting the I² value.
Sensitivity analysis
We explored heterogeneity by performing sensitivity analysis, looking at primary outcomes only, and by excluding trials with greater risk of bias. We considered studies at low risk of bias when they had low risk of bias in generation of the randomisation sequence, concealment of allocation and loss to follow‐up.
Results
Description of studies
Results of the search
In total the search identified 56 reports corresponding to 36 studies. In the previous published review (Cluver 2012), 25 studies met the inclusion criteria, and in this update, we have included three additional trials (Munoz 2014; Reinhard 2012; Vallikkannu 2014). The 28 included studies involved a total of 2786 women. We have set out information about all of the included trials in the Characteristics of included studies tables.
Included studies
We found 17 studies involving 1876 women that assessed tocolytic drugs (Bujold 2003a; Bujold 2003b; Chung 1996; Collaris 2009; El‐Sayed 2004; Fernandez 1997; Hilton 2009; Impey 2005; Kok 2008; Marquette 1996; Nor Azlin 2005; Nor Azlin 2008; Robertson 1987; Stock 1993; Tan 1989; Vani 2009; Yanny 2000). These drugs included beta stimulants (salbutamol, ritodrine, hexoprenaline and terbutaline), a calcium channel blocker (nifedipine) and a nitric oxide donor (nitroglycerine/glyceryl trinitrate).
We found one study involving 26 women that assessed vibroacoustic stimulation (Johnson 1995).
We found six studies involving 554 women that assessed regional analgesia (Delisle 2001; Dugoff 1999; Mancuso 2000; Schorr 1997; Weiniger 2007; Weiniger 2010). Five of these studies used a tocolytic drug as well in both groups (Dugoff 1999; Mancuso 2000; Schorr 1997; Weiniger 2007; Weiniger 2010), and one study allowed doctors to use a tocolytic at their discretion (Delisle 2001). None of the studies looked at regional analgesia alone.
We found no studies on amnioinfusion.
We found one study involving 95 women that compared regional analgesia with systemic opioids, with both groups also receiving a tocolytic drug (Sullivan 2009).
One study with 60 women examined a systemic opioid (remifentanil) compared with placebo (Munoz 2014).
One study involving 80 women compared two types of hypnosis/relaxation (Reinhard 2012), and one (with 95 women) looked at the application of talcum powder versus gel to assist ECV (Vallikkannu 2014). In this final study after two failed attempts at ECV, cross‐over to the other method occurred, and although analysis was done by intention‐to‐treat (according to original allocation), a proportion of women in both groups received both methods, making interpretation of results difficult; for this reason we have included in the review only data related to the period before the cross‐over.
Three studies are awaiting classification (Andarsio 2000; Hollard 2003; Tan 2008) ‐ seeCharacteristics of studies awaiting classification ‐ and two are ongoing (Burgos 2012; Passerini 2013) ‐ seeCharacteristics of ongoing studies.
Excluded studies
We excluded three studies (Dockeray 1984; Guittier 2013; Wallace 1984) ‐ seeCharacteristics of excluded studies.
Risk of bias in included studies
See the table of Characteristics of included studies and Figure 1.
1.
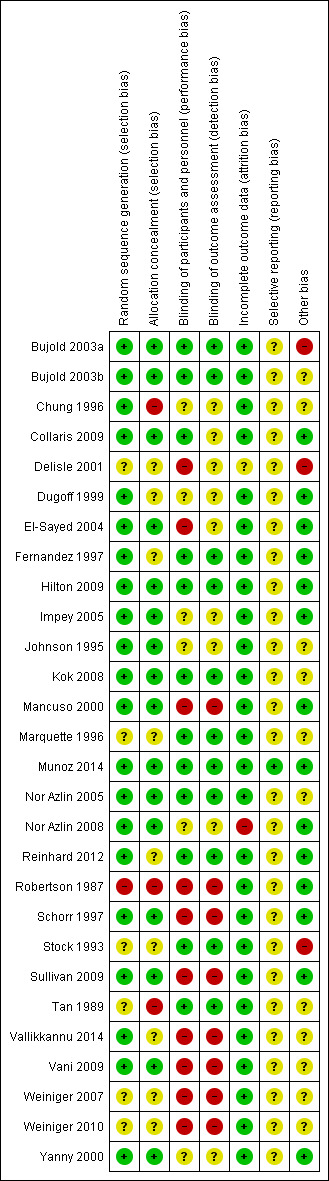
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged 16 studies to be at low risk of bias for both adequate sequence generation and adequate allocation concealment (Bujold 2003a; Bujold 2003b; Collaris 2009; El‐Sayed 2004; Hilton 2009; Impey 2005; Johnson 1995; Kok 2008; Mancuso 2000; Munoz 2014; Nor Azlin 2005; Nor Azlin 2008; Schorr 1997; Sullivan 2009; Vani 2009; Yanny 2000). We considered one study to be at high risk of bias for both sequence generation and allocation concealment (Robertson 1987). The remaining studies were unclear, or we observed a mixture of low, high and unclear risk of bias (Figure 1).
Blinding
We judged 11 studies to be adequately blinded with low risk of bias for both performance bias (women and staff blinded) and detection bias (outcome assessors blinded) (Bujold 2003a; Bujold 2003b; Fernandez 1997; Hilton 2009; Kok 2008; Marquette 1996; Munoz 2014; Nor Azlin 2005; Reinhard 2012; Stock 1993; Tan 1989). Eight studies were at high risk of bias for both performance and detection bias (Mancuso 2000; Robertson 1987; Schorr 1997; Sullivan 2009; Vallikkannu 2014; Vani 2009; Weiniger 2007; Weiniger 2010). For the remaining studies, blinding was not clearly reported or performance or detection bias was noted (Figure 1).
Incomplete outcome data
We considered 26 studies at low risk of bias when considering attrition. In one study, risk of bias was unclear for this domain (Delisle 2001), and in another study, loss to follow‐up meant that for some outcomes the study was at high risk of bias (Nor Azlin 2008) (Figure 1).
Selective reporting
We classified all but one of the studies as unclear because we did not assess the trial protocols (Figure 1).
Other potential sources of bias
We considered 14 studies at low risk of bias in terms of other potential sources of bias, and three at high risk. The remaining studies were unclear on this (Figure 1).
Effects of interventions
We included 28 studies, involving 2786 women (Characteristics of included studies).
Comparison 1. Tocolysis versus placebo for external cephalic version (ECV) at term (13 studies with 15,468 women)
Thirteen studies involving 1548 women looked at this comparison using various tocolytic drugs (Bujold 2003a; Chung 1996; Fernandez 1997; Hilton 2009; Impey 2005; Kok 2008; Marquette 1996; Nor Azlin 2005; Robertson 1987; Stock 1993; Tan 1989; Vani 2009; Yanny 2000).
Beta stimulants: Nine studies looked at beta stimulants. Six studies involving 639 women looked at parenteral ritodrine (Chung 1996; Impey 2005; Marquette 1996; Nor Azlin 2005; Robertson 1987; Stock 1993); two studies involving 174 women looked at oral and parenteral salbutamol (Tan 1989; Vani 2009); and one study involving 103 women looked at parenteral terbutaline (Fernandez 1997).
Calcium channel blockers: One study involving 320 women looked at oral nifedipine (Kok 2008).
Nitric oxide donors: Three studies involving 282 women looked at parenteral or sublingual nitroglycerine/glyceryl nitrate (Bujold 2003a; Hilton 2009; Yanny 2000).
The overall quality of the studies was reasonable. We judged seven studies to have low risk of bias for both sequence generation and allocation concealment (Bujold 2003a; Hilton 2009; Impey 2005; Kok 2008; Nor Azlin 2005; Vani 2009; Yanny 2000) and eight studies to have adequate blinding (Bujold 2003a; Fernandez 1997; Hilton 2009; Kok 2008; Marquette 1996; Nor Azlin 2005; Stock 1993; Tan 1989). SeeFigure 1.
We have used random‐effects models throughout these comparisons because of the clinical heterogeneity observed between studies. We have presented data for different classes of tocolytics together in the same forest plots, but we have not pooled results. Findings for different classes of tocolytic drugs are also reported separately in the text, as different classes of drugs have different mechanisms of action. For most outcomes, evidence mainly relates to beta stimulants.
Primary outcomes
We found a statistically significant increase in cephalic presentation at labour and at birth with the use of parenteral beta stimulants (average risk ratio (RR) 1.68, 95% confidence interval (CI) 1.14 to 2.48, five studies, 459 women, random‐effects Tau² = 0.12, I² = 64%, Chi² P value 0.03, evidence graded as low quality; Analysis 1.1). Relatively little evidence was found for other classes of tocolytic drugs. One study with 310 women examined the use of a calcium channel blocker and did not demonstrate a difference between intervention and control groups for cephalic presentation at birth (RR 1.13, 95% CI 0.87 to 1.48); single studies examining parenteral and sublingual nitric oxide donors also showed no statistically significant differences between intervention and control groups (RR 1.58, 95% CI 0.91 to 2.76, participants = 125, and, RR 0.74, 95% CI 0.52 to 1.05, participants = 99, respectively).
1.1. Analysis.
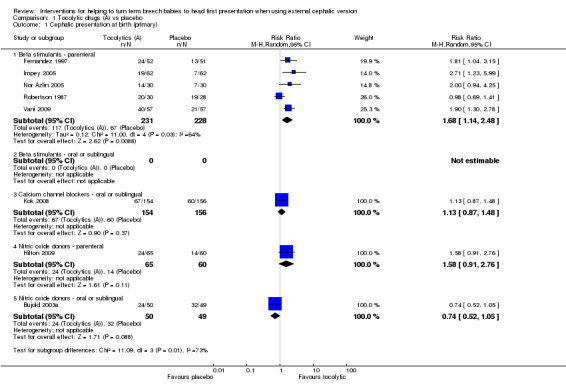
Comparison 1 Tocolytic drugs (A) vs placebo, Outcome 1 Cephalic presentation at birth (primary).
Failure to achieve a cephalic vaginal birth was less likely for women receiving a beta stimulant (average RR 0.75, 95% CI 0.60 to 0.92, four studies, 399 women, evidence graded as moderate quality; Analysis 1.2). One study examined the use of a sublingual nitric oxide donor and reported no clear evidence of differences between groups (RR 1.22, 95% CI 0.86 to 1.72, participants = 99).
1.2. Analysis.
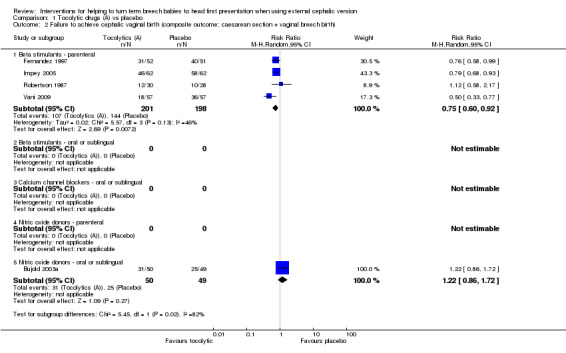
Comparison 1 Tocolytic drugs (A) vs placebo, Outcome 2 Failure to achieve cephalic vaginal birth (composite outcome: caesarean section + vaginal breech birth).
We noted a significant reduction in caesarean sections with the use of beta stimulants to facilitate ECV (average RR 0.77, 95% CI 0.67 to 0.88, participants = 742, six studies, I² = 25%; Analysis 1.3). Evidence on the impact of calcium channel blockers and parenteral nitric oxide donors was limited and showed no clear difference in the rates of caesarean section between treatment and control groups (calcium channel blocker: RR 1.11, 95% CI 0.88 to 1.40, one study, participants = 310; parenteral nitric oxide donor: RR 0.83, 95% CI 0.67 to 1.02, one study, participants = 125).
1.3. Analysis.
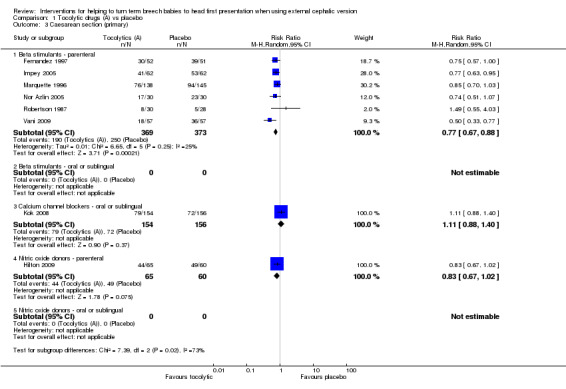
Comparison 1 Tocolytic drugs (A) vs placebo, Outcome 3 Caesarean section (primary).
We identified no significant difference in fetal bradycardia in any of the studies reporting this outcome (beta stimulants: RR 2.81, 95% CI 0.12 to 66.17, participants = 58, one study, evidence graded as very low quality; calcium channel blocker: RR 1.11, 95% CI 0.50 to 2.43, participants = 310, one study; oral nitric oxide donor: RR 0.39, 95% CI 0.08 to 1.93, participants = 99, one study; Analysis 1.4).
1.4. Analysis.
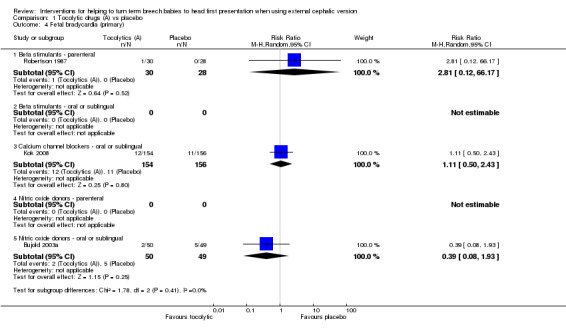
Comparison 1 Tocolytic drugs (A) vs placebo, Outcome 4 Fetal bradycardia (primary).
Only one class of tocolytic drugs, the beta stimulants, had a reasonable number of trials to allow firm conclusions regarding primary outcomes (seeTable 1).
Secondary outcomes
We found a statistically significant reduction in failure of ECV when parenteral beta stimulant drugs were used (average RR 0.70, 95% CI 0.60 to 0.82, participants = 900, nine studies, I² = 34%, evidence graded as moderate quality; Analysis 1.5). For other types of tocolytics (oral beta stimulants, oral calcium channel blockers, parenteral or sublingual nitric oxide donors), evidence was insufficient to demonstrate any differences between groups for failure of ECV (RR 1.00, 95% CI 0.56 to 1.79, participants = 45, one study; RR 0.93, 95% CI 0.78 to 1.11, participants = 310, one study; RR 0.86, 95% CI 0.70 to 1.06, participants = 126, one study; average RR 1.04, 95% CI 0.55 to 1.96, participants = 156, two studies, respectively).
1.5. Analysis.
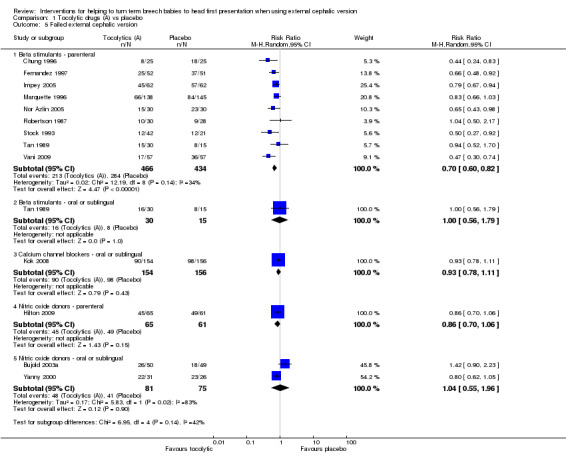
Comparison 1 Tocolytic drugs (A) vs placebo, Outcome 5 Failed external cephalic version.
Too few studies assessed most of our other secondary outcomes to reveal clear differences between groups. No statistically significant differences between groups were identified for difficult ECV (parenteral beta stimulants: RR 0.50, 95% CI 0.16 to 1.54, participants = 63, one study; Analysis 1.6); maternal palpitation (parenteral beta stimulants: RR 5.00, 95% CI 0.25 to 101.89, participants = 114, one study; parenteral nitric oxide donors: RR 0.49, 95% CI 0.05 to 5.27, participants = 117, one study; Analysis 1.7); or maternal hypotension, which was reported for single studies examining parenteral and sublingual nitric oxide donors (RR 1.47, 95% CI 0.26 to 8.50, participants = 117, and, RR 5.88, 95% CI 0.73 to 47.07, participants = 99, respectively; Analysis 1.9).
1.6. Analysis.

Comparison 1 Tocolytic drugs (A) vs placebo, Outcome 6 Difficult external cephalic version.
1.7. Analysis.
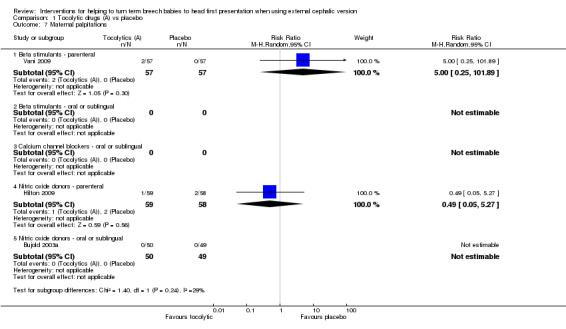
Comparison 1 Tocolytic drugs (A) vs placebo, Outcome 7 Maternal palpitations.
1.9. Analysis.
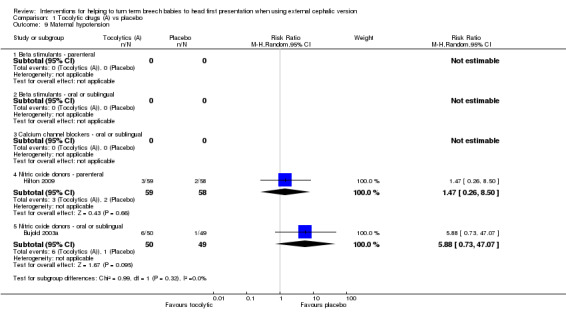
Comparison 1 Tocolytic drugs (A) vs placebo, Outcome 9 Maternal hypotension.
Two studies examining the use of parenteral or sublingual nitric oxide donors reported maternal headaches, and women receiving active treatment were more likely to experience headache compared with those given placebo (RR 18.68, 95% CI 1.11 to 313.77, participants = 117, and, RR 10.29, 95% CI 2.55 to 41.56, participants = 99, respectively; Analysis 1.8).
1.8. Analysis.
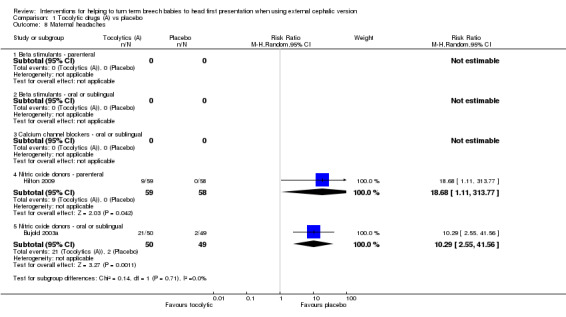
Comparison 1 Tocolytic drugs (A) vs placebo, Outcome 8 Maternal headaches.
Other outcomes were not reported or were reported in single studies, and evidence was insufficient to reveal differences between groups receiving tocolysis versus placebo.
Operative vaginal birth (calcium channel blocker: RR 0.34, 95% CI 0.09 to 1.22, 310 women; Analysis 1.10).
Maternal mortality (not reported).
Maternal morbidity (not reported).
Perinatal mortality (calcium channel blocker: 310 participants, no events; Analysis 1.13).
Perinatal morbidity (not reported).
1.10. Analysis.
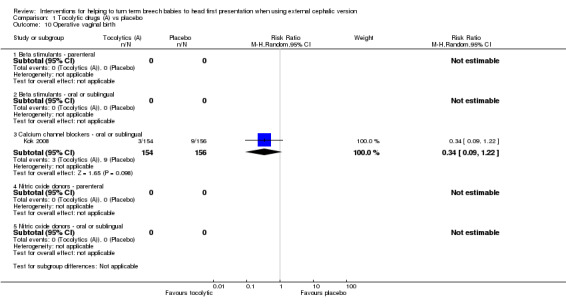
Comparison 1 Tocolytic drugs (A) vs placebo, Outcome 10 Operative vaginal birth.
1.13. Analysis.
Comparison 1 Tocolytic drugs (A) vs placebo, Outcome 13 Perinatal mortality.
In this version of the review, we added the outcome vaginal breech birth, which was reported in one study with no evidence of a difference between groups (RR 1.00, 95% CI 0.30 to 3.28, one study, 124 women; Analysis 1.15).
1.15. Analysis.
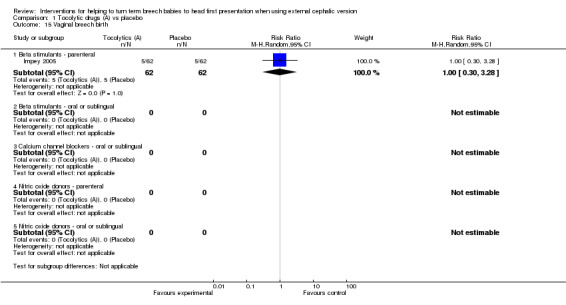
Comparison 1 Tocolytic drugs (A) vs placebo, Outcome 15 Vaginal breech birth.
Non‐prespecified outcomes
An additional six outcomes were reported that we had not specified in the protocol: Apgar less than seven at five minutes (beta stimulants: no events, two studies, 227 infants), neonatal seizures (beta stimulants: no events, one study, 124 infants), admission to neonatal unit (beta stimulants: average RR 1.00, 95% CI 0.30 to 3.36, two studies, 238 infants; Analysis 1.18), birth trauma (beta stimulant: no events, one study, 144 women) and flushing in women (calcium channel blocker: RR 23.30, 95% CI 1.38 to 391.91, one study, 310 women; Analysis 1.20). We found too few data on these outcomes to report findings with confidence.
1.18. Analysis.
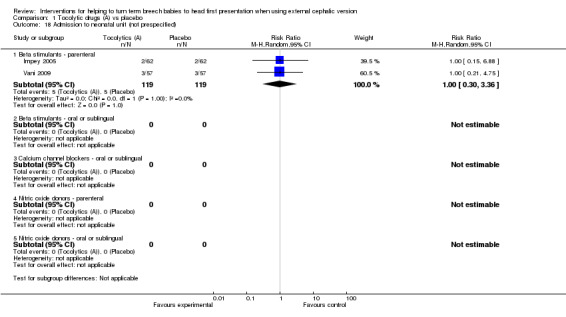
Comparison 1 Tocolytic drugs (A) vs placebo, Outcome 18 Admission to neonatal unit (not prespecified).
1.20. Analysis.
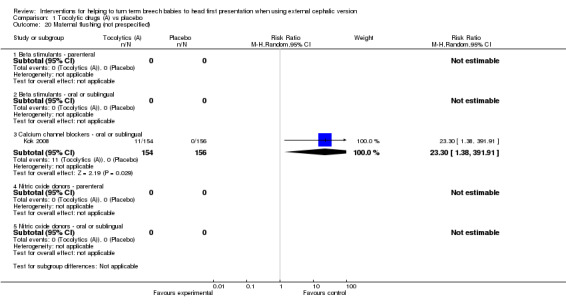
Comparison 1 Tocolytic drugs (A) vs placebo, Outcome 20 Maternal flushing (not prespecified).
Subgroup analysis by parity
Six studies reported the data by parity (Chung 1996; Hilton 2009; Impey 2005; Nor Azlin 2005; Stock 1993; Tan 1989), but only two reported data on our primary outcomes (Hilton 2009; Impey 2005). Interaction tests showed no differences between nulliparous and multiparous women. Cephalic presentation in labour and at birth was not statistically significant, but the numbers of women were small (average RR 1.89, 95% CI 0.98 to 3.62, two studies, 249 women, interaction test Chi² = 0.00, df = 1, P value 0.95; Analysis 21.1). We observed a significant reduction in caesarean sections (average RR 0.84, 95% CI 0.74 to 0.95, two studies, 249 women, interaction test Chi² = 0.68, df = 1, P = 0.41; Analysis 21.2) and in failed ECV (average RR 0.78, 95% CI 0.66 to 0.92, six studies, 513 women, interaction test Chi² = 2.07, df = 1, P = 0.08, Analysis 21.4), with no differences between nulliparous and multiparous women.
21.1. Analysis.
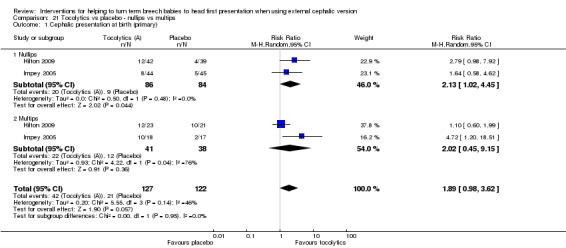
Comparison 21 Tocolytics vs placebo ‐ nullips vs multips, Outcome 1 Cephalic presentation at birth (primary).
21.2. Analysis.
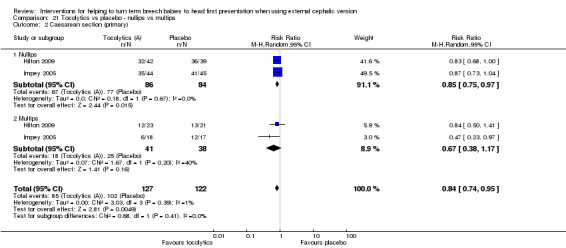
Comparison 21 Tocolytics vs placebo ‐ nullips vs multips, Outcome 2 Caesarean section (primary).
21.4. Analysis.
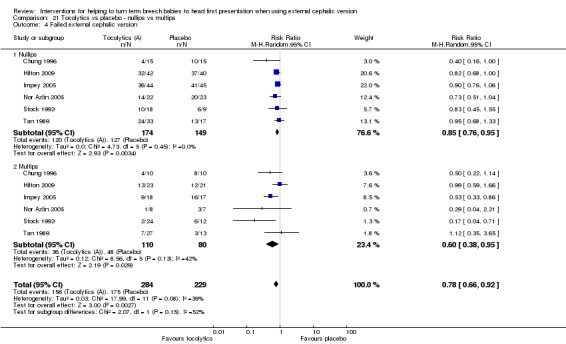
Comparison 21 Tocolytics vs placebo ‐ nullips vs multips, Outcome 4 Failed external cephalic version.
Subgroup analysis by study quality
We considered two studies to be at high risk of bias in terms of randomisation, concealment of allocation or completeness of data (Fernandez 1997; Robertson 1987); both of these studies examined parenteral beta stimulants. Even with these studies temporarily excluded, parenteral beta stimulants still appeared to be effective in achieving cephalic presentation at birth (average RR 2.03, 95% CI 1.49 to 2.77, three studies, 289 women). Findings for the outcome of failure to achieve cephalic vaginal birth remained non‐significant for parenteral beta stimulants (average RR 0.65, 95% CI 0.38 to 1.11, two studies, 238 women). There remained a significant reduction in caesarean sections for parenteral beta stimulants when studies at high risk of bias were temporarily removed (average RR 0.75, 95% CI 0.63 to 0.89, four studies, 581 women). Fetal bradycardia remained as showing no significant difference identified.
Comparison 2. Tocolytics versus other tocolytics (four studies with 344 women)
Four studies, or parts of studies, involving 344 women looked at these comparisons.
Calcium channel blockers (A2) versus beta stimulants (A1): Two studies involving 176 women made this comparison (Collaris 2009; Nor Azlin 2008).
Nitric oxide donors (A3) versus beta stimulants (A1): Two studies involving 168 women made this comparison (Bujold 2003b; El‐Sayed 2004).
Nitric oxide donors (A3) versus calcium channel blockers (A2): No studies looked at this comparison.
One study involving 63 women included three groups and compared two different beta stimulants ‐ hexoprenaline and ritodrine ‐ versus placebo (Stock 1993). We are not comparing different drugs within the same class, so these data are omitted from this section (although the data are included in the section on tocolysis versus placebo).
The overall quality of the studies was reasonable. All four studies were assessed as being at low risk of bias in terms of both sequence generation and allocation concealment (Bujold 2003b; Collaris 2009; El‐Sayed 2004; Nor Azlin 2008). Blinding was considered adequate in one study, in which women, staff and outcome assessors were blinded (Bujold 2003b). (SeeFigure 1.)
Primary outcomes
We obtained too few data on these outcomes to report findings with confidence.
Cephalic presentation at birth
Calcium channel blockers versus beta stimulants (RR 0.62, 95% CI 0.39 to 0.98, one study, 90 women; Analysis 2.1).
Nitric oxide donors versus beta stimulants (RR 0.56, 95% CI 0.29 to 1.09, one study, 74 women; Analysis 2.1.2).
2.1. Analysis.
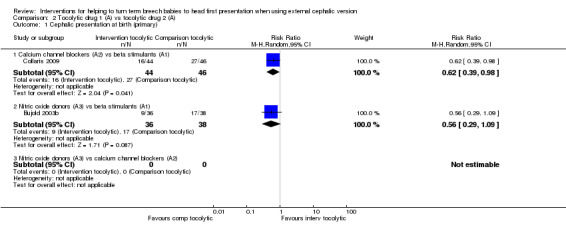
Comparison 2 Tocolytic drug 1 (A) vs tocolytic drug 2 (A), Outcome 1 Cephalic presentation at birth (primary).
Cephalic vaginal birth not achieved
Nitric oxide donors versus beta stimulants (RR 1.13, 95% CI 0.88 to 1.47, one study, 74 women; Analysis 2.2).
2.2. Analysis.
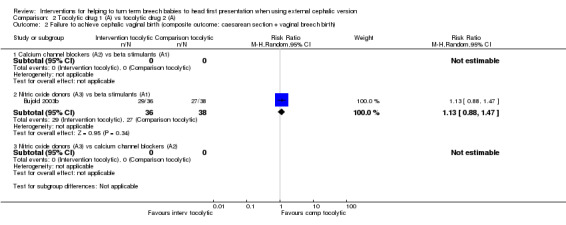
Comparison 2 Tocolytic drug 1 (A) vs tocolytic drug 2 (A), Outcome 2 Failure to achieve cephalic vaginal birth (composite outcome: caesarean section + vaginal breech birth).
Caesarean section
Calcium channel blockers versus beta stimulants (average RR 1.28, 95% CI 1.03 to 1.59, two studies, 170 women; Analysis 2.3).
2.3. Analysis.
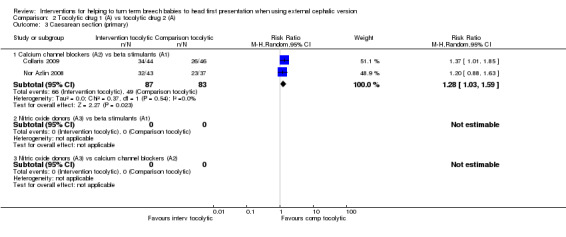
Comparison 2 Tocolytic drug 1 (A) vs tocolytic drug 2 (A), Outcome 3 Caesarean section (primary).
Fetal bradycardia
Calcium channel blockers versus beta stimulants (average RR 1.17, 95% CI 0.46 to 3.03, two studies, 170 infants; Analysis 2.4).
Nitric oxide donors versus beta stimulants (RR 1.06, 95% CI 0.16 to 7.10, one study, 74 infants; Analysis 2.4.1).
2.4. Analysis.
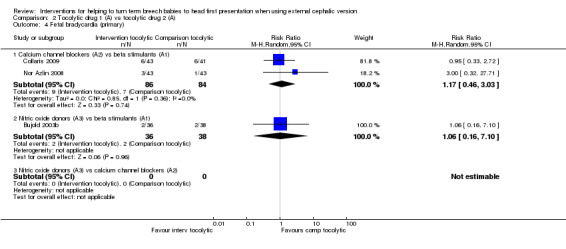
Comparison 2 Tocolytic drug 1 (A) vs tocolytic drug 2 (A), Outcome 4 Fetal bradycardia (primary).
Secondary outcomes
Failed ECV
Calcium channel blockers versus beta stimulants (average RR 1.41, 95% CI 1.06 to 1.86, two studies, 176 women; Analysis 2.5.1).
Nitric oxide donors versus beta stimulants (average RR 1.48, 95% CI 1.13 to 1.94, two studies, 133 women; Analysis 2.5.2).
2.5. Analysis.

Comparison 2 Tocolytic drug 1 (A) vs tocolytic drug 2 (A), Outcome 5 Failed external cephalic version.
Difficult ECV
Calcium channel blockers versus beta stimulants (RR 5.22, 95% CI 0.26 to 105.81, one study, 90 women; Analysis 2.6).
2.6. Analysis.
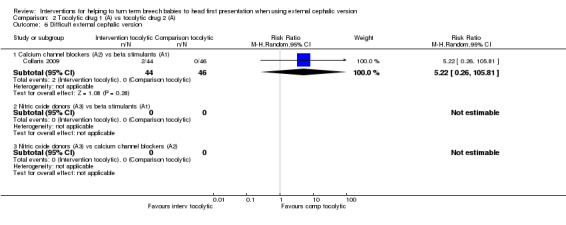
Comparison 2 Tocolytic drug 1 (A) vs tocolytic drug 2 (A), Outcome 6 Difficult external cephalic version.
Non‐prespecified outcomes
Other non‐prespecified outcomes (maternal palpitations, headache, hypotension and infant admission to neonatal intensive care unit) were reported in single studies with small sample sizes, and evidence was insufficient to allow firm conclusions.
Subgroup analysis by parity
One small study involving 86 women compared nifedipine versus terbutaline and reported data by parity (Nor Azlin 2008). Failed ECV, the only outcome reported by parity, did not show a significant difference between the two tocolytic drugs, although the interaction tests showed no differences between nulliparous and multiparous women (RR 1.38, 95% CI 0.90 to 2.13, one study, 86 women interaction test Chi² = 0.35, df = 1, P value 0.55; Analysis 22.4).
22.4. Analysis.
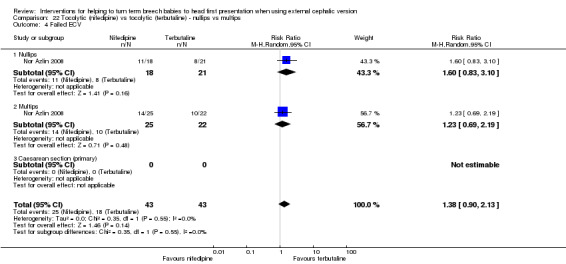
Comparison 22 Tocolytic (nifedipine) vs tocolytic (terbutaline) ‐ nullips vs multips, Outcome 4 Failed ECV.
Sensitivity analyses by study quality
Studies were insufficient for this analysis.
Comparison 3. Vibroacoustic stimulation in midline fetal spine positions versus placebo (one study, 26 women)
One study involving 26 women (of whom 23 provided data) looked at this comparison and reported only on the number of women in whom ECV failed (Johnson 1995). The quality of the study was reasonable, but only 26 women were included. So the finding of a statistically significant reduction in failed ECV cannot be relied upon (RR 0.09, 95% CI 0.01 to 0.60, one study, 23 women; Analysis 3.5).
3.5. Analysis.
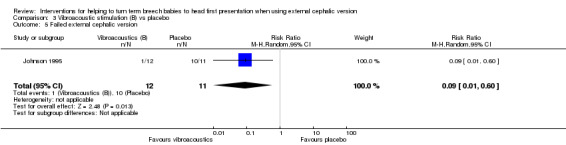
Comparison 3 Vibroacoustic stimulation (B) vs placebo, Outcome 5 Failed external cephalic version.
Other primary and secondary outcomes were not reported.
Comparison 4. Vibroacoustic stimulation versus tocolytics
We found no studies assessing this comparison.
Comparison 5. Comparison of different types of vibroacoustic stimulation
We found no studies assessing this comparison.
Comparison 6. Regional analgesia versus placebo (six studies, 554 women)
Six studies involving 554 women looked at this comparison. Four of these studies addressed the effect of spinal analgesia on ECV (Delisle 2001; Dugoff 1999; Weiniger 2007; Weiniger 2010); two studies assessed the effect of epidural analgesia (Mancuso 2000; Schorr 1997).
All studies except one (Delisle 2001) used a tocolytic drug as well in both arms; the one exception allowed clinicians to choose to use a tocolytic drug if they wished (Delisle 2001). This study reported on very few of our prespecified outcomes. We have analysed separately the use of regional analgesia with or without tocolysis. Findings for primary outcomes for regional analgesia (with tocolysis) are set out in Table 2.
The quality of the studies was generally unclear. Only two studies were considered to have low risk of bias in terms of sequence generation and allocation concealment (Mancuso 2000; Schorr 1997). The remainder of the studies were mostly unclear around risk of bias (Figure 1).
Primary outcomes
For regional analgesia with tocolysis versus tocolysis alone, we found no statistically significant differences identified for the primary outcomes: cephalic presentation at labour and at birth (average RR 1.44, 95% CI 0.78 to 2.66, three studies, 279 women, random‐effects, Tau² = 0.24, I² = 80%, Chi² P value 0.006; Analysis 6.1); caesarean section (average RR 0.74, 95% CI 0.40 to 1.37, three studies, 279 women, random‐effects, Tau² = 0.26, I² = 88%, Chi² P value 0.0003; Analysis 6.3); and fetal bradycardia (average RR 1.48, 95% CI 0.62 to 3.57, two studies, 210 women, random‐effects, Tau² = 0.05, I² = 8%, Chi² P value 0.30; Analysis 6.4). Failure to achieve cephalic vaginal delivery was not reported.
6.1. Analysis.
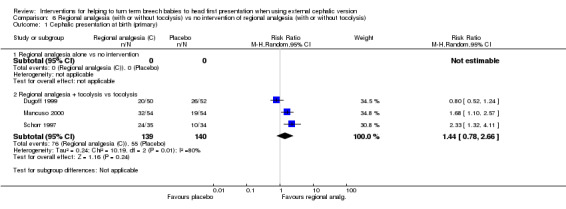
Comparison 6 Regional analgesia (with or without tocolysis) vs no intervention of regional analgesia (with or without tocolysis), Outcome 1 Cephalic presentation at birth (primary).
6.3. Analysis.
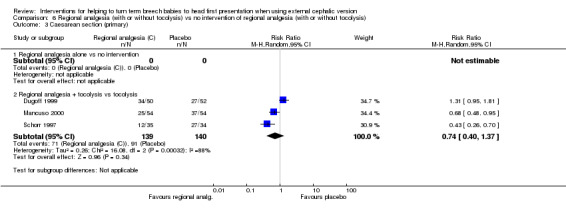
Comparison 6 Regional analgesia (with or without tocolysis) vs no intervention of regional analgesia (with or without tocolysis), Outcome 3 Caesarean section (primary).
6.4. Analysis.
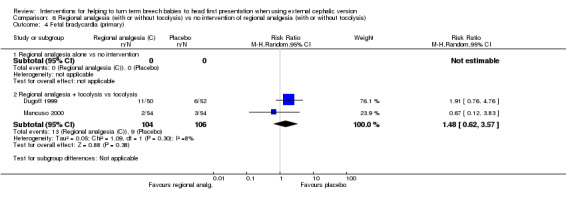
Comparison 6 Regional analgesia (with or without tocolysis) vs no intervention of regional analgesia (with or without tocolysis), Outcome 4 Fetal bradycardia (primary).
Secondary outcomes
We did identify a significant reduction in the number of failures of ECV with regional analgesia with tocolysis (RR 0.61, 95% CI 0.43 to 0.86, participants = 409, five studies, I² = 56%; Analysis 6.5). This outcome was also reported in the single study examining regional analgesia without tocolysis versus no intervention, and no evidence suggested a difference between groups (RR 0.89, 95% CI 0.70 to 1.14, participants = 141). None of our other secondary outcomes were reported (operative vaginal birth, maternal mortality, maternal morbidity, perinatal mortality, perinatal morbidity).
6.5. Analysis.
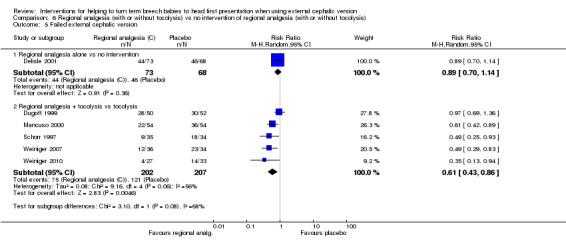
Comparison 6 Regional analgesia (with or without tocolysis) vs no intervention of regional analgesia (with or without tocolysis), Outcome 5 Failed external cephalic version.
Outcomes not prespecified
Some studies assessed placental abruption and maternal discomfort but identified no differences with regional analgesia. Three studies examined maternal hypotension, and regional analgesia with tocolysis was associated with increased risk of hypotension (average RR 11.58, 95% CI 1.53 to 87.50, participants = 280, three studies, I² = 0%; Analysis 6.9).
6.9. Analysis.
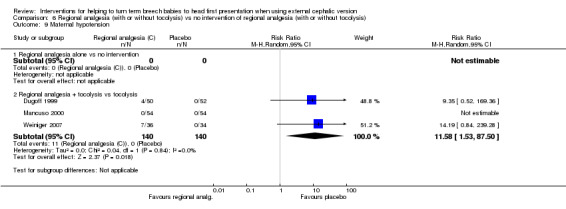
Comparison 6 Regional analgesia (with or without tocolysis) vs no intervention of regional analgesia (with or without tocolysis), Outcome 9 Maternal hypotension.
Subgroup analysis by parity
Six studies reported data by parity, but it was not possible to undertake any subgroup analysis.
Sensitivity analysis by study quality
Good quality data were insufficient for subgroup sensitivity analysis for this comparison, as only two (Mancuso 2000; Schorr 1997) of the six identified were considered to have low risk of bias in terms of randomisation, concealment of allocation or completeness of data.
Comparison 7. Regional analgesia versus tocolytics
We found no studies assessing this comparison.
Comparison 8. Regional analgesia versus vibroacoustic stimulation
We found no studies assessing this comparison.
Comparison 9. Comparison of different types of regional analgesia
We found no studies assessing this comparison.
Comparison 10. Amnioinfusion versus placebo
We found no studies assessing this comparison.
Comparison 11. Amnioinfusion versus tocolytics
We found no studies assessing this comparison.
Comparison 12. Amnioinfusion versus vibroacoustic stimulation
We found no studies assessing this comparison.
Comparison 13. Amnioinfusion versus regional analgesia
We found no studies assessing this comparison.
Comparison 14. Comparison of different types of amnioinfusion
We found no studies assessing this comparison.
Comparison 15. Systemic opioids versus placebo (one study, 60 women)
One study with 60 women was included in this comparison (Munoz 2014); in this trial intravenous patient‐controlled remifentanil was compared with an intravenous placebo.
Primary outcomes
Presentation at birth was not reported. Trialists reported "transient" fetal bradycardia, but the study was underpowered to demonstrate a statistically significant difference between groups (RR 0.31, 95% CI 0.09 to 1.04; Analysis 15.3). The frequency of caesarean section was very similar in the two groups (RR 0.99, 95% CI 0.63 to 1.57) (Analysis 15.4).
15.3. Analysis.

Comparison 15 Systemic opioids (E) vs placebo, Outcome 3 Fetal bradycardia (primary).
15.4. Analysis.

Comparison 15 Systemic opioids (E) vs placebo, Outcome 4 Caesarean section (primary).
Secondary outcomes
No clear evidence was found of a difference between groups in failure of ECV (RR 0.77, 95% CI 0.47 to 1.26; Analysis 15.5) nor in frequency of operative vaginal birth (RR 0.94, 95% CI 0.20 to 4.27; Analysis 15.10).
15.5. Analysis.
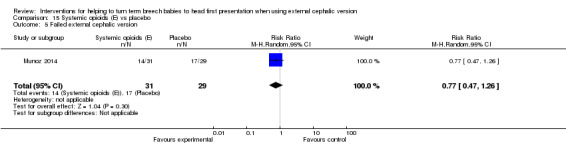
Comparison 15 Systemic opioids (E) vs placebo, Outcome 5 Failed external cephalic version.
15.10. Analysis.
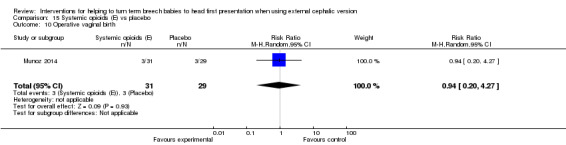
Comparison 15 Systemic opioids (E) vs placebo, Outcome 10 Operative vaginal birth.
Other secondary outcomes were not reported.
Other outcomes
Several non‐prespecified outcomes were reported in this trial. Women receiving the opioid had lower pain scores compared with control participants (mean difference (MD) ‐1.80 (on a 10‐point scale), 95% CI ‐3.04 to ‐0.56; Analysis 15.15), and maternal satisfaction with the procedure was increased in the group receiving remifentanil (MD 2.60, 95% CI 1.25 to 3.95; Analysis 15.16). No significant difference between groups was found in maternal side effects (nausea and vomiting, dizziness and drowsiness), although the study was underpowered to demonstrate differences for most outcomes (Analysis 15.17; Analysis 15.18; Analysis 15.19).
15.15. Analysis.
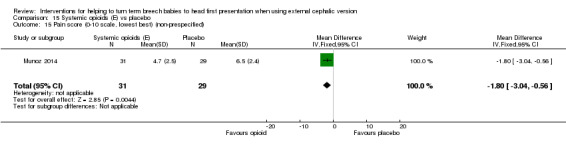
Comparison 15 Systemic opioids (E) vs placebo, Outcome 15 Pain score (0‐10 scale, lowest best) (non‐prespecified).
15.16. Analysis.

Comparison 15 Systemic opioids (E) vs placebo, Outcome 16 Maternal satisfaction score (lower score worst) (non‐prespecified).
15.17. Analysis.
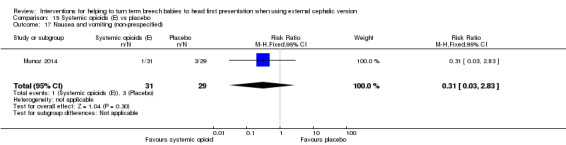
Comparison 15 Systemic opioids (E) vs placebo, Outcome 17 Nausea and vomiting (non‐prespecified).
15.18. Analysis.
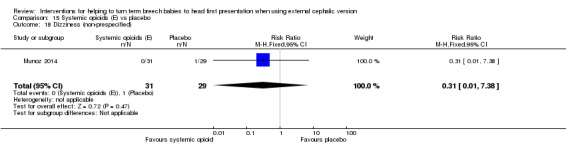
Comparison 15 Systemic opioids (E) vs placebo, Outcome 18 Dizziness (non‐prespecified).
15.19. Analysis.
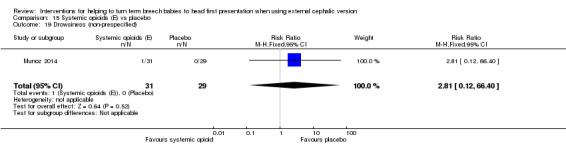
Comparison 15 Systemic opioids (E) vs placebo, Outcome 19 Drowsiness (non‐prespecified).
Comparison 16. Systemic opioids versus tocolytics
We found no studies assessing this comparison.
Comparison 17. Systemic opioids versus vibroacoustic stimulation
We found no studies assessing this comparison.
Comparison 18. Systemic opioids versus regional analgesia
One study, involving 95 women, assessed this comparison (Sullivan 2009). The quality of the study was good; only lack of blinding might contribute to bias. The remaining assessments were consistent with low risk of bias (Figure 1).
Primary outcomes
The rate of cephalic presentation at birth was not reported. No significant differences between groups were reported for the outcome failure to achieve vaginal cephalic birth (RR 1.18, 95% CI 0.90 to 1.54). Also no clear difference between groups was seen in terms of the numbers of women undergoing caesarean section (RR 1.18, 95% CI 0.90 to 1.54, 95 women; Analysis 18.3). A similar rate of fetal bradycardia was observed in the two groups (RR 0.71, 95% CI 0.24 to 2.09, 94 women; Analysis 18.4).
18.3. Analysis.
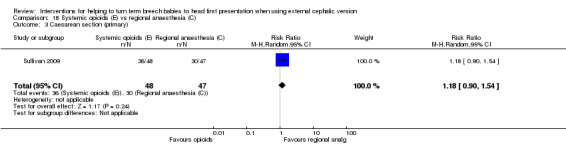
Comparison 18 Systemic opioids (E) vs regional anaesthesia (C), Outcome 3 Caesarean section (primary).
18.4. Analysis.
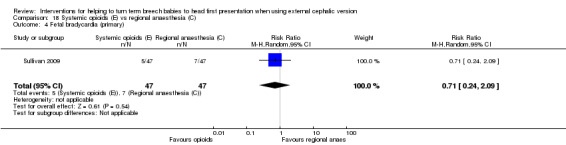
Comparison 18 Systemic opioids (E) vs regional anaesthesia (C), Outcome 4 Fetal bradycardia (primary).
Secondary outcomes
No statistically significant difference was observed in frequency of failure of ECV when a systemic opioid was compared with regional analgesia (RR 1.29, 95% CI 0.93 to 1.80, 95 women; Analysis 18.5).
18.5. Analysis.
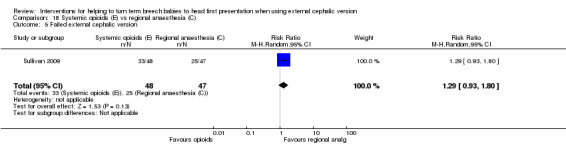
Comparison 18 Systemic opioids (E) vs regional anaesthesia (C), Outcome 5 Failed external cephalic version.
Comparison 19. Systemic opioids versus amnioinfusion
We found no studies assessing this comparison.
Comparison 20. Comparison of different systemic opioids
We found no studies assessing this comparison.
Comparison 23. Hypnosis versus neurolinguistic programming (one study, 80 women)
One study with 80 women compared two types of hypnosis.
Primary outcomes
No primary outcomes were reported.
Secondary outcomes
No significant evidence suggested that one hypnosis technique was more effective than the other (RR 1.08, 95% CI 0.74 to 1.57; Analysis 23.5), and women in both groups reported a similar degree of pain relief during the procedure, as measured on a scale of one to 10 (MD 0.10, 95% CI ‐0.87 to 0.67, one study, 80 women (non‐prespecified outcome); Analysis 23.15).
23.5. Analysis.
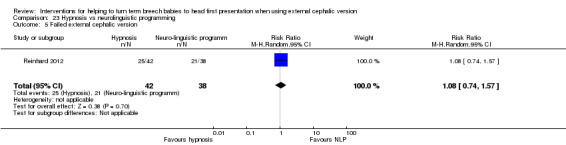
Comparison 23 Hypnosis vs neurolinguistic programming, Outcome 5 Failed external cephalic version.
23.15. Analysis.
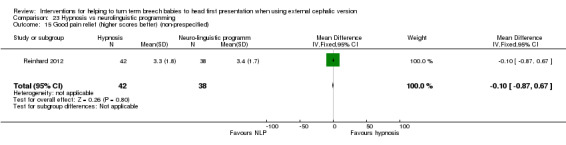
Comparison 23 Hypnosis vs neurolinguistic programming, Outcome 15 Good pain relief (higher scores better) (non‐prespecified).
Comparison 24. Talcum powder versus gel to assist with ECV (one study, 95 women)
One study compared the use of talcum powder versus gel applied to the woman's abdomen to assist with ECV. If after one round of attempts (two attempts) using the allocated method, ECV was not successful, the alternative method could be tried. This meant that many of the women in the trial with initial failed ECV crossed over to the other method; outcome data were very difficult to interpret because although analysis was performed according to original allocation, women may have received both methods. We therefore report here only the secondary outcome related to failure of ECV after the first round of attempts (using the allocated method). There was insufficient evidence to demonstrate whether talcum was more or less effective than gel in assisting version (RR 1.26, 95% CI 0.84 to 1.89, one study, 80 women; Analysis 24.5).
24.5. Analysis.
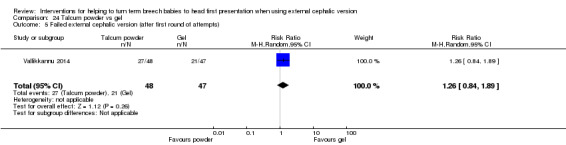
Comparison 24 Talcum powder vs gel, Outcome 5 Failed external cephalic version (after first round of attempts).
Discussion
Summary of main results
Parenteral beta stimulants, used to help external cephalic version of breech babies, were effective in increasing the number of women going into labour with their baby in a cephalic presentation and in reducing the number of women undergoing caesarean section. However, data on possible adverse effects are insufficient. Data derived by comparison of other classes of tocolytic drugs (calcium channel blockers and nitric acid donors) were also insufficient. We identified no difference in response between nulliparous women and multiparous women in terms of successful external cephalic version (ECV), babies in the cephalic presentation during labour and caesarean section.
Use of regional analgesia, in combination with a tocolytic drug, to facilitate ECV was effective in terms of increasing successful versions, but the data show no benefit in terms of babies in the cephalic presentation during labour or reduction in caesarean sections.
Data were insufficient on the use of vibroacoustic stimulation, amnioinfusion, systemic opioids, hypnosis or abdominal lubricants for helping to turn breech babies using ECV techniques.
Overall completeness and applicability of evidence
Available evidence does not describe many of the prespecified outcomes, in particular, possible adverse effects.
Quality of the evidence
The overall quality of the evidence was reasonable, with studies on regional analgesia unable to be blinded. However, several assessments will have yielded insufficient data to provide an answer with any degree of assurance. We carried out formal assessments of quality of the evidence using GRADEpro for parenteral beta stimulants and regional analgesia with tocolysis. For both of these comparisons, the evidence was graded from moderate to very low quality.
Potential biases in the review process
Evidence in this review was derived from studies identified in a detailed search process. Trials comparing interventions to help external cephalic version of breech babies at term that have not been published may not have been identified. We attempted to minimise bias in the review process by having two review authors independently extract data.
Agreements and disagreements with other studies or reviews
We are not aware of any other systematic reviews on this topic. Studies within the review seem to be in reasonable agreement.
Authors' conclusions
Implications for practice.
Beta stimulant tocolytics, given parenterally to facilitate external cephalic version of breech babies, increased the number of babies in the cephalic presentation during labour and birth, and reduced the number of caesarean sections performed. However, insufficient data were collected on possible adverse effects on mother or baby. Other groups of tocolytics, calcium channel blockers and nitric acid donors yielded insufficient data to provide good evidence. Data on other possible facilitators of external cephalic version (ECV) were insufficient to provide useful evidence.
Use of regional analgesia in combination with tocolytic drugs increased the rate of success of ECV, but data were insufficient to indicate whether this was associated with an increase in cephalic presentation at birth or a change in the rate of caesarean section.
Implications for research.
Future research needs to carefully assess any potential adverse effects on both mother and baby.
Routine tocolysis for ECV at term
Further controlled trials of routine tocolysis for ECV at term are needed. In particular, possible benefits of routine tocolysis used to reduce the force required for successful ECV, and possible risks of maternal cardiovascular side effects, need to be addressed further. Additional trials are also needed to compare the effectiveness of routine versus selective use of tocolysis; investigators should include short‐term and long‐term outcome measures that assess morbidity according to type of birth.
Although randomised trials of nitroglycerine have been small, the results are sufficiently negative to discourage further trials.
Fetal acoustic stimulation for ECV at term
The results presented in this review are sufficiently encouraging to justify further trials of this procedure. Short‐term and long‐term outcomes must be assessed.
Regional analgesia for ECV at term
Further trials are needed. The effect of vaginal displacement of the presenting part should be assessed. Fluid received by the regional analgesia group and by the control group should be similar, and whether tocolytic agents should be used adjunctively needs further investigation.
Amnioinfusion for ECV at term
Transabdominal amnioinfusion to increase amniotic fluid volume might facilitate ECV and should be investigated.
Hydration to increase amniotic fluid volume for ECV at term
Intravenous or oral hydration before ECV attempts to increase amniotic fluid volume should be investigated as a separate intervention (Hofmeyr 2002).
Systemic opioids for ECV at term
Given the general adverse effects of opioids (Bricker 2002), research might be better focused on other possible facilitators for ECV of breech babies.
Other interventions
Evidence on other interventions such as hypnosis or abdominal lubricants is insufficient; adequately designed and powered trials may throw light on whether such interventions are worthwhile.
What's new
| Date | Event | Description |
|---|---|---|
| 9 March 2016 | Amended | We have corrected a typographical error in Analysis 6.1 (in relation to Schorr 1997). This edit does not affect the analysis/results or conclusions of this review. |
History
Protocol first published: Issue 3, 1996 Review first published: Issue 3, 1996
| Date | Event | Description |
|---|---|---|
| 30 September 2014 | New search has been performed | We have updated the search and identified 6 new studies. We have included 3 new trials in the review (Munoz 2014; Reinhard 2012; Vallikkannu 2014), excluded 1 study (Guittier 2013) and identified 2 trial registrations for ongoing studies (Burgos 2012; Passerini 2013) |
| 30 September 2014 | New citation required but conclusions have not changed | Overall results are similar to those reported in the previous version of the review. A new author joined the review team to assist with the update. We have incorporated a summary of findings table |
| 30 September 2011 | New search has been performed | Search updated: 10 new trials added to review We have moved Andarsio 2000 from categorisation as an 'Included study' to 'Awaiting classification' because we need to know the route of administration of the drug before we can include data on subgroups in the updated review. We are trying to obtain this information |
| 19 May 2011 | New citation required and conclusions have changed | Beta stimulants are now recommended for facilitating external cephalic version at term, but data on adverse effects were insufficient. Data on calcium channel blockers and nitric acid donors were insufficient to provide good evidence New authors helped update this review |
| 1 October 2009 | Amended | Search updated: 19 reports added to Studies awaiting classification |
| 3 November 2008 | Amended | Converted to new review format |
| 31 March 2004 | New search has been performed | One new trial added to studies awaiting classification (Hollard 2003) |
| 30 September 2003 | New citation required and conclusions have changed | With inclusion of Bujold 2003a and Bujold 2003b, we have changed the recommendation regarding nitroglycerine |
| 30 September 2003 | New search has been performed | Search updated. 2 new trials included (Bujold 2003a; Bujold 2003b) |
Acknowledgements
We thank the Cochrane Pregnancy and Childbirth team for technical support, in particular Steve Milan for assisting with data extraction in the previously published version of this review (Cluver 2012).
We thank the NIHR Programme Grant, which enabled Therese Dowswell (TD) to join our review team for this update. TD is supported by the NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines.
Data and analyses
Comparison 1. Tocolytic drugs (A) vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cephalic presentation at birth (primary) | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Beta stimulants ‐ parenteral | 5 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [1.14, 2.48] |
| 1.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Calcium channel blockers ‐ oral or sublingual | 1 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.87, 1.48] |
| 1.4 Nitric oxide donors ‐ parenteral | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.91, 2.76] |
| 1.5 Nitric oxide donors ‐ oral or sublingual | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.52, 1.05] |
| 2 Failure to achieve cephalic vaginal birth (composite outcome: caesarean section + vaginal breech birth) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Beta stimulants ‐ parenteral | 4 | 399 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.60, 0.92] |
| 2.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Calcium channel blockers ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Nitric oxide donors ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Nitric oxide donors ‐ oral or sublingual | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.86, 1.72] |
| 3 Caesarean section (primary) | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Beta stimulants ‐ parenteral | 6 | 742 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.67, 0.88] |
| 3.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Calcium channel blockers ‐ oral or sublingual | 1 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.88, 1.40] |
| 3.4 Nitric oxide donors ‐ parenteral | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.67, 1.02] |
| 3.5 Nitric oxide donors ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Fetal bradycardia (primary) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Beta stimulants ‐ parenteral | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.12, 66.17] |
| 4.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Calcium channel blockers ‐ oral or sublingual | 1 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.50, 2.43] |
| 4.4 Nitric oxide donors ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Nitric oxide donors ‐ oral or sublingual | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.08, 1.93] |
| 5 Failed external cephalic version | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Beta stimulants ‐ parenteral | 9 | 900 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.60, 0.82] |
| 5.2 Beta stimulants ‐ oral or sublingual | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.56, 1.79] |
| 5.3 Calcium channel blockers ‐ oral or sublingual | 1 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.78, 1.11] |
| 5.4 Nitric oxide donors ‐ parenteral | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.06] |
| 5.5 Nitric oxide donors ‐ oral or sublingual | 2 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.55, 1.96] |
| 6 Difficult external cephalic version | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Beta stimulants ‐ parenteral | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.16, 1.54] |
| 6.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Calcium channel blockers ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 Nitric oxide donors ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 Nitric oxide donors ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal palpitations | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Beta stimulants ‐ parenteral | 1 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 101.89] |
| 7.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Calcium channel blockers ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Nitric oxide donors ‐ parenteral | 1 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.05, 5.27] |
| 7.5 Nitric oxide donors ‐ oral or sublingual | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Maternal headaches | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Beta stimulants ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Calcium channel blockers ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Nitric oxide donors ‐ parenteral | 1 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 18.68 [1.11, 313.77] |
| 8.5 Nitric oxide donors ‐ oral or sublingual | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 10.29 [2.55, 41.56] |
| 9 Maternal hypotension | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Beta stimulants ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Calcium channel blockers ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Nitric oxide donors ‐ parenteral | 1 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.26, 8.50] |
| 9.5 Nitric oxide donors ‐ oral or sublingual | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 5.88 [0.73, 47.07] |
| 10 Operative vaginal birth | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Beta stimulants ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Calcium channel blockers ‐ oral or sublingual | 1 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.22] |
| 10.4 Nitric oxide donors ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Nitric oxide donors ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal mortality | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Beta stimulants ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Calcium channel blockers ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.4 Nitric oxide donors ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.5 Nitric oxide donors ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal morbidity | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Beta stimulants ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Calcium channel blockers ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.4 Nitric oxide donors ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.5 Nitric oxide donors ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Perinatal mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Beta stimulants ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Calcium channel blockers ‐ oral or sublingual | 1 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.4 Nitric oxide donors ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.5 Nitric oxide donors ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Perinatal morbidity | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Beta stimulants ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Calcium channel blockers ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.4 Nitric oxide donors ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.5 Nitric oxide donors ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Vaginal breech birth | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 Beta stimulants ‐ parenteral | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.30, 3.28] |
| 15.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Calcium channel blockers ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.4 Nitric oxide donors ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.5 Nitric oxide donors ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Apgar < 7 at 5 minutes (not prespecified) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 Beta stimulants ‐ parenteral | 2 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Calcium channel blockers ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 Nitric oxide donors ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.5 Nitric oxide donors ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Neonatal seizures (not prespecified) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 Beta stimulants ‐ parenteral | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Calcium channel blockers ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.4 Nitric oxide donors ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.5 Nitric oxide donors ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Admission to neonatal unit (not prespecified) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 Beta stimulants ‐ parenteral | 2 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.30, 3.36] |
| 18.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Calcium channel blockers ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.4 Nitric oxide donors ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.5 Nitric oxide donors ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Birth trauma (not prespecified) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 Beta stimulants ‐ parenteral | 1 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Calcium channel blockers ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.4 Nitric oxide donors ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.5 Nitric oxide donors ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Maternal flushing (not prespecified) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 20.1 Beta stimulants ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Beta stimulants ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Calcium channel blockers ‐ oral or sublingual | 1 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 23.30 [1.38, 391.91] |
| 20.4 Nitric oxide donors ‐ parenteral | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.5 Nitric oxide donors ‐ oral or sublingual | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
1.16. Analysis.
Comparison 1 Tocolytic drugs (A) vs placebo, Outcome 16 Apgar < 7 at 5 minutes (not prespecified).
1.17. Analysis.
Comparison 1 Tocolytic drugs (A) vs placebo, Outcome 17 Neonatal seizures (not prespecified).
1.19. Analysis.
Comparison 1 Tocolytic drugs (A) vs placebo, Outcome 19 Birth trauma (not prespecified).
Comparison 2. Tocolytic drug 1 (A) vs tocolytic drug 2 (A).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cephalic presentation at birth (primary) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.39, 0.98] |
| 1.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 1 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.29, 1.09] |
| 1.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Failure to achieve cephalic vaginal birth (composite outcome: caesarean section + vaginal breech birth) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 1 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.88, 1.47] |
| 2.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section (primary) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.03, 1.59] |
| 3.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Fetal bradycardia (primary) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.46, 3.03] |
| 4.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 1 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.16, 7.10] |
| 4.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Failed external cephalic version | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 2 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.06, 1.86] |
| 5.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.13, 1.94] |
| 5.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Difficult external cephalic version | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 5.22 [0.26, 105.81] |
| 6.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal palpitations | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.23, 2.78] |
| 7.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 1 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.10, 2.71] |
| 7.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Maternal headaches | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 1 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 3.52 [1.05, 11.76] |
| 8.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal hypotension | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 1 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 3.17 [0.35, 29.06] |
| 9.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Operative vaginal birth | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal mortality | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal morbidity | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Perinatal mortality | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Perinatal morbidity | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Vaginal breech birth (not prespecified) | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Apgar < 7 at 5 minutes (not prespecified) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Neonatal seizures (not prespecified) | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Admissions to neonatal unit (not prespecified) | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Birth trauma (not prespecified) | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Maternal flushing (not prespecified) | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 20.1 Calcium channel blockers (A2) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Nitric oxide donors (A3) vs beta stimulants (A1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Nitric oxide donors (A3) vs calcium channel blockers (A2) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
2.7. Analysis.

Comparison 2 Tocolytic drug 1 (A) vs tocolytic drug 2 (A), Outcome 7 Maternal palpitations.
2.8. Analysis.
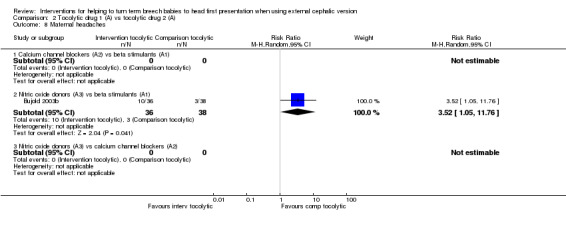
Comparison 2 Tocolytic drug 1 (A) vs tocolytic drug 2 (A), Outcome 8 Maternal headaches.
2.9. Analysis.
Comparison 2 Tocolytic drug 1 (A) vs tocolytic drug 2 (A), Outcome 9 Maternal hypotension.
2.16. Analysis.
Comparison 2 Tocolytic drug 1 (A) vs tocolytic drug 2 (A), Outcome 16 Apgar < 7 at 5 minutes (not prespecified).
2.18. Analysis.
Comparison 2 Tocolytic drug 1 (A) vs tocolytic drug 2 (A), Outcome 18 Admissions to neonatal unit (not prespecified).
Comparison 3. Vibroacoustic stimulation (B) vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cephalic presentation at birth (primary) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Failure to achieve cephalic vaginal birth (composite outcome: caesarean section + vaginal breech birth) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section (primary) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Fetal bradycardia (primary) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Failed external cephalic version | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.60] |
| 6 Difficult external cephalic version | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal palpitations | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Maternal headaches | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal hypotension | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Operative vaginal birth | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal morbidity | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Perinatal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Perinatal morbidity | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Regional analgesia (with or without tocolysis) vs no intervention of regional analgesia (with or without tocolysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cephalic presentation at birth (primary) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Regional analgesia alone vs no intervention | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Regional analgesia + tocolysis vs tocolysis | 3 | 279 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.78, 2.66] |
| 2 Failure to achieve cephalic vaginal birth (composite outcome: caesarean section + vaginal breech birth) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section (primary) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Regional analgesia alone vs no intervention | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Regional analgesia + tocolysis vs tocolysis | 3 | 279 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.40, 1.37] |
| 4 Fetal bradycardia (primary) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Regional analgesia alone vs no intervention | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Regional analgesia + tocolysis vs tocolysis | 2 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.62, 3.57] |
| 5 Failed external cephalic version | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Regional analgesia alone vs no intervention | 1 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.70, 1.14] |
| 5.2 Regional analgesia + tocolysis vs tocolysis | 5 | 409 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.43, 0.86] |
| 6 Difficult external cephalic version | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Regional analgesia alone vs no intervention | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Regional analgesia + tocolysis vs tocolysis | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal palpitations | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Regional analgesia alone vs no intervention | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Regional analgesia + tocolysis vs tocolysis | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Maternal headaches | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Regional analgesia alone vs no intervention | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Regional analgesia + tocolysis vs tocolysis | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal hypotension | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Regional analgesia alone vs no intervention | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Regional analgesia + tocolysis vs tocolysis | 3 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 11.58 [1.53, 87.50] |
| 10 Operative vaginal birth | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Regional analgesia alone vs no intervention | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Regional analgesia + tocolysis vs tocolysis | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal mortality | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Regional analgesia alone vs no intervention | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Regional analgesia + tocolysis vs tocolysis | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal morbidity | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Regional analgesia alone vs no intervention | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Regional analgesia + tocolysis vs tocolysis | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Perinatal morbidity | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Regional analgesia alone vs no intervention | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Regional analgesia + tocolysis vs tocolysis | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Perinatal mortality | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Regional analgesia alone vs no intervention | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Regional analgesia + tocolysis vs tocolysis | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Placental abruption (not prespecified) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 Regional analgesia alone vs no intervention | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Regional analgesia + tocolysis vs tocolysis | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.01, 8.31] |
| 16 Maternal discomfort (not prespecified) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 Regional analgesia alone vs no intervention | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Regional analgesia + tocolysis vs tocolysis | 2 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.03, 1.04] |
6.15. Analysis.
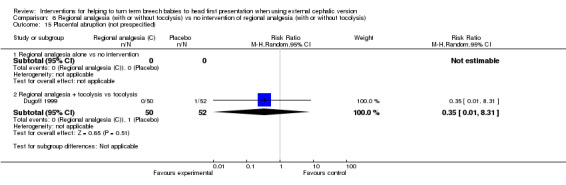
Comparison 6 Regional analgesia (with or without tocolysis) vs no intervention of regional analgesia (with or without tocolysis), Outcome 15 Placental abruption (not prespecified).
6.16. Analysis.

Comparison 6 Regional analgesia (with or without tocolysis) vs no intervention of regional analgesia (with or without tocolysis), Outcome 16 Maternal discomfort (not prespecified).
Comparison 15. Systemic opioids (E) vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cephalic presentation at birth (primary) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Failure to achieve cephalic vaginal birth (composite outcome: caesarean section + vaginal breech birth) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Fetal bradycardia (primary) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.09, 1.04] |
| 4 Caesarean section (primary) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.63, 1.57] |
| 5 Failed external cephalic version | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.47, 1.26] |
| 6 Difficult external cephalic version | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal palpitations | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Maternal headaches | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal hypotension | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Operative vaginal birth | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.20, 4.27] |
| 11 Maternal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal morbidity | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Perinatal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Perinatal morbidity | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Pain score (0‐10 scale, lowest best) (non‐prespecified) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.8 [‐3.04, ‐0.56] |
| 16 Maternal satisfaction score (lower score worst) (non‐prespecified) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [1.25, 3.95] |
| 17 Nausea and vomiting (non‐prespecified) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 2.83] |
| 18 Dizziness (non‐prespecified) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.38] |
| 19 Drowsiness (non‐prespecified) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 66.40] |
Comparison 18. Systemic opioids (E) vs regional anaesthesia (C).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cephalic presentation at birth (primary) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Failure to achieve cephalic vaginal birth (composite outcome: caesarean section + vaginal breech birth) | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.90, 1.54] |
| 3 Caesarean section (primary) | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.90, 1.54] |
| 4 Fetal bradycardia (primary) | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.24, 2.09] |
| 5 Failed external cephalic version | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.93, 1.80] |
| 6 Difficult external cephalic version | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal palpitations | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Maternal headaches | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal hypotension | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Operative vaginal birth | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal morbidity | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Perinatal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Perinatal morbidity | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
18.2. Analysis.
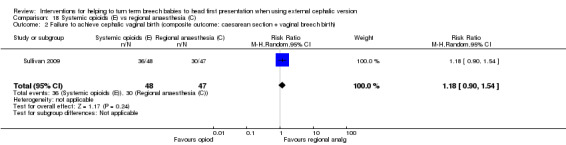
Comparison 18 Systemic opioids (E) vs regional anaesthesia (C), Outcome 2 Failure to achieve cephalic vaginal birth (composite outcome: caesarean section + vaginal breech birth).
Comparison 21. Tocolytics vs placebo ‐ nullips vs multips.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cephalic presentation at birth (primary) | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [0.98, 3.62] |
| 1.1 Nullips | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [1.02, 4.45] |
| 1.2 Multips | 2 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [0.45, 9.15] |
| 2 Caesarean section (primary) | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.74, 0.95] |
| 2.1 Nullips | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.75, 0.97] |
| 2.2 Multips | 2 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.38, 1.17] |
| 3 Fetal bradycardia (primary) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Nullips | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Multips | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Failed external cephalic version | 6 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.66, 0.92] |
| 4.1 Nullips | 6 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.76, 0.95] |
| 4.2 Multips | 6 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.38, 0.95] |
Comparison 22. Tocolytic (nifedipine) vs tocolytic (terbutaline) ‐ nullips vs multips.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cephalic presentation at birth (primary) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Nullips | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Multips | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section (primary) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Nullips | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Multips | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Fetal bradycardia (primary) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Nullips | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Multips | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Failed ECV | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.90, 2.13] |
| 4.1 Nullips | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.83, 3.10] |
| 4.2 Multips | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.69, 2.19] |
| 4.3 Caesarean section (primary) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 23. Hypnosis vs neurolinguistic programming.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cephalic presentation at birth (primary) | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Failure to achieve cephalic vaginal birth (composite outcome: caesarean section + vaginal breech birth) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section (primary) | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4 Fetal bradycardia (primary) | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5 Failed external cephalic version | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.74, 1.57] |
| 6 Difficult external cephalic version | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7 Maternal palpitations | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8 Maternal headaches | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9 Maternal hypotension | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10 Operative vaginal birth | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11 Maternal mortality | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12 Maternal morbidity | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13 Perinatal mortality | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14 Perinatal morbidity | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15 Good pain relief (higher scores better) (non‐prespecified) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.87, 0.67] |
Comparison 24. Talcum powder vs gel.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cephalic presentation at birth (primary) | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Failure to achieve cephalic vaginal birth (composite outcome: caesarean section + vaginal breech birth) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section (primary) | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4 Fetal bradycardia (primary) | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5 Failed external cephalic version (after first round of attempts) | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.84, 1.89] |
| 6 Difficult external cephalic version | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7 Maternal palpitations | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8 Maternal headaches | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9 Maternal hypotension | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10 Operative vaginal birth | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11 Maternal mortality | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12 Maternal morbidity | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13 Perinatal mortality | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14 Perinatal morbidity | 0 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bujold 2003a.
| Methods | RCT. | |
| Participants |
Inclusion criteria: women with singleton breech pregnancy at 36 to 40 weeks' gestation Women also given NST and US evaluation for EFW, fetal morphologic features, AFI and placental location. After NST, if women met criteria, clinician verified breech mobility by abdominal palpation. N = 99. Exclusion criteria: IUGR (defined as an EFW (determined by US examination, < 10th percentile for GA), oligohydramnios (defined as AFI ≤ 5 cm), presence of a placenta previa or an abruptio placenta, a previous uterine scar other than a low transverse caesarean delivery, active labour, rupture of membranes, fetal anomalies incompatible with life, a non‐mobile breech by abdominal palpation, any contraindication to vaginal delivery, a medical/allergic contraindication to nitroglycerine. |
|
| Interventions |
Intervention:tocolysis: nitroglycerine ‐ nitric oxide donor (A3) ‐ sublingual. 2 sublingual sprays of 400 micrograms nitroglycerine given 3 minutes before ECV. N = 50. Comparison:placebo: 2 sublingual sprays of placebo given 3 minutes before ECV. N = 49. |
|
| Outcomes | ECV success (at end of procedure); vertex presentation at labour and at birth; vaginal birth; CS; headache; blood pressure; maternal tachycardia; birthweight. | |
| Notes | Sainte‐Justine Hospital, April 1999 to August 2002. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation table. |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled trial with identical preparations. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, clinician and assessor were blinded. Intravenous ritodrine and placebo were supplied in identical form; sublingual nitroglycerine and placebo were also supplied in identical form by the hospital pharmacy. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, clinician and assessor were blinded. Intravenous ritodrine and placebo were supplied in identical form; sublingual nitroglycerine and placebo were also supplied in identical form by the hospital pharmacy. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No indication of loss of participants. Not mentioned whether the analysis was intention‐to‐treat; appears that a total of 99 women were randomly assigned and all completed. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol, but the trial authors said they would compare the rate of vertex presentation at time of birth and the rate of vertex vaginal birth. They reported only that there was no difference and have not provided data. Other outcomes may also be left out. |
| Other bias | High risk | Halfway through the trial, an interim analysis was performed by the data safety monitoring board. This board decided to stop the trial because of a statistically significant (P value < 0.01) higher rate of side effects and a trend toward a lower rate of successful ECV in 1 group. This decision was based on the likelihood (< 1%) that that group would ultimately show a significant increase in the success rate of ECV, and the likelihood (> 95%) that the subsequently randomly assigned women would be exposed to increased risk of adverse outcomes without potential benefit if the trial was completed. Investigators were informed, and the trial was stopped. No statistically significant differences were observed between the 2 groups with regard to maternal age, GA, EFW, AFI, placental location and type of breech. |
Bujold 2003b.
| Methods | RCT. | |
| Participants |
Inclusion: women with singleton breech pregnancy at 36 to 40 weeks' gestation. N = 74. Exclusion criteria: IUGR, oligohydramnios, placenta praevia, placenta abruptio, uterine scar other than low transverse CS, active labour, ruptured membranes, fetal anomalies incompatible with life, any contraindication to vaginal birth, contraindications to trial medications, non‐reactive CTG. CTG and US performed. |
|
| Interventions |
Intervention:tocolysis ‐ nitroglycerine ‐ nitric oxide donor (A3) ‐ sublingual. Nitroglycerine, 2 sublingual sprays of 400 micrograms nitroglycerine plus IV placebo. N = 38. Comparison:tocolysis ‐ ritodrine ‐ beta stimulant (A1) ‐ parenteral. Ritodrine 15 mg in 1.5 mL plus 20 mL 5% dextrose water by IVI at 111 micrograms per minute, plus placebo sublingual spray. N = 36. Maximum 4 ECV attempts with US control. |
|
| Outcomes | Rate of successful ECV; headaches; blood pressure; maternal heart rate; palpitations, hypotension and prolonged fetal heart rate decelerations (fetal bradycardia). | |
| Notes | Sainte‐Justine Hospital, April 1999 to August 2001. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised table of randomisation. For every 6 women who were entered, 3 women were assigned to the ritodrine group and 3 women were assigned to the nitroglycerine group. |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled trial with identical preparations. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | IV ritodrine and placebo were supplied by the hospital pharmacy in identical form; sublingual nitroglycerine and placebo were also supplied by the hospital pharmacy in identical form. The nurse and the attending physician were blinded to the contents of the infusion or the sublingual spray. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | IV ritodrine and placebo were supplied by the hospital pharmacy in identical form; sublingual nitroglycerine and placebo were also supplied by the hospital pharmacy in identical form. Staff were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No indication suggested that any women were excluded after randomisation or were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Study stopped after 74 women were enrolled because ritodrine was withdrawn from the market in July 2001 ‐ not because of benefit. No statistically significant differences between the 2 groups with regard to maternal age, GA, maternal weight, AFI and Frank breech. However, a significant difference was observed between the 2 groups at admission with respect to mean blood pressure (mm Hg)* at admission for the ritodrine group: 98 (67‐125); mean blood pressure (mm Hg)* at admission for the nitroglycerine group: 90 (75‐106) (P value 0.03). |
Chung 1996.
| Methods | RCT, stratified by parity. | |
| Participants |
Inclusion criteria: women with singleton breech presentation, as confirmed by US, at 36 to 38 weeks' gestation. N = 51 recruited but 50 analysed. Exclusion criteria: contraindication to tocolytic therapy, scarred uterus, antepartum haemorrhage, hypertension, impaired fetal growth, oligohydramnios, vaginal delivery contraindicated, abnormal umbilical artery Doppler flow pattern. |
|
| Interventions |
Intervention:tocolytic: ritodrine ‐ beta stimulant (A1) ‐ parenteral IVI of ritodrine 0.4 mg/mL in 5% dextrose at 1.5 mL/min via an infusion pump, for 15 minutes before and during ECV attempt. If uterine contractions appeared to be preventing successful version, the infusion rate was increased in steps of 0.75 mL/min. Compared with matching 5% dextrose infusion. ECV attempted by 2 investigators, followed by repeat US scan and CTG. N = 25. Comparison:placebo: N = 25. |
|
| Outcomes | Failed ECV attempt. Other data presented according to successful or failed ECV attempt: non‐cephalic presentation at birth (1/24 vs 23/26); CS (5/24 vs 19/26). 1 intrauterine death occurred 4 weeks after successful ECV (group not stated). Subgroup analysis showed that statistically significant benefit was limited to nulliparous women. |
|
| Notes | Paired sequential analysis reached significance after 10 pairs. Trial was continued because of erroneous statistical calculations. Thereafter little benefit was seen from tocolysis. Study authors suggest that tocolysis is helpful only during the learning phase of the technique. A subsequent trial (published earlier) from the same group showed no benefit of tocolysis (Stock 1993). Nulliparous and parous women randomly assigned separately. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers: sequential paired design. |
| Allocation concealment (selection bias) | High risk | Randomisation code was known to 1 of the authors who attended each woman throughout the procedure and for 20 minutes thereafter. He did not take part in version attempts. It is not clear whether allocation in pairs may have enabled the unblinded study author to know the next allocation in some cases, which could introduce selection bias, as could the study author knowing the code even if he did not undertake the procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant was blinded; 2 doctors who attempted the version were blind to randomisation throughout, but the code was known to a third review author, who was in attendance throughout. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participant was blinded; 2 doctors who attempted the version were blind to randomisation throughout, but the code was known to a third review author, who was in attendance throughout. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman was excluded because of absent end‐diastolic flow in the umbilical artery before commencement of the procedure (unclear whether they were excluded before randomisation). Unclear whether the analysis was intention‐to‐treat. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Groups judged by study authors to be similar in age, parity and mean gestation (Table 1, page 721). |
Collaris 2009.
| Methods | RCT. | |
| Participants |
Inclusion criteria: woman not in labour, with singleton pregnancy in breech or transverse lie at 36 to 41 weeks' gestation. N = 90. Exclusion criteria: in keeping with recommendations of the American College of Obstetricians and Gynecologists on ECV. |
|
| Interventions |
Intervention:tocolytic: nifedipine ‐ calcium channel blocker (A2) ‐ oral. Oral nifedipine (10 mg) and SQ saline placebo. N = 44. Comparison:tocolytic: terbutaline ‐ beta stimulant (A1) ‐ parenteral. Subcutaenous terbutaline (250 micrograms) with oral placebo. N = 46. |
|
| Outcomes | Primary outcomes were successful ECV and CS. Secondary outcomes were cephalic fetal presentation at delivery, numerical rating score for satisfaction with ECV, preference for injection or tablet, post ECV. Also, CTG assessment, labour onset, prelabour membrane rupture and various neonatal outcomes. |
|
| Notes | Women who had a failed ECV on first attempt could be re‐randomised. Study authors did a primary analysis on 90 women, but then undertook a secondary analysis by adding in repeat ECV attempts. We will consider only the primary analysis data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, variable blocks of 8 or 12. |
| Allocation concealment (selection bias) | Low risk | No indication that this was an issue; did use sequential opening of sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and providers blinded; unclear whether outcome assessor was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants and providers blinded; unclear whether outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For some outcomes, data were collected only for a subset of participants; CTGs of 7 women (7%) were missing from the files. Analysis of participants at primary enrolment was performed on an intention‐to‐treat basis. All women received treatment as allocated. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No significant differences were noted in baseline criteria; no other biases were apparent. |
Delisle 2001.
| Methods | RCT. | |
| Participants | Inclusion criteria: singleton non‐vertex; age 18 or older; GA 36 weeks or more; intact membranes; reactive CTG. N = 141. | |
| Interventions |
Intervention:regional analgesia (C). Spinal analgesia with bupivacaine 0.25% 1 mL plus 20 mcg fentanyl vs control; 4 ECV attempts. N = 73. Comparison:standard care. N = 68. Nitroglycerine tocolysis was used per operator preference. |
|
| Outcomes | ECV failure; non‐reassuring CTG (1/73 vs 0/68). | |
| Notes | Conference abstract, August 1998 to June 2001. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given. |
| Allocation concealment (selection bias) | Unclear risk | No information given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and clinicians were not blinded; unclear whether outcome assessor was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants and clinicians were not blinded; unclear whether outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Little information given. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | High risk | Baseline data: reported as similar in maternal age, GA at time of ECV, parity and birthweight. However, clinicians, who were not blinded to the intervention, were able to give IV tocolysis by choice; this could lead to an imbalance between groups. No information on this is given in the Conference abstract, but this is a potential source of bias. |
Dugoff 1999.
| Methods | RCT. | |
| Participants |
Inclusion criteria: breech presentation, 36 weeks or more, reactive CTG, intact membranes, minimum 2 × 2 cm pocket of amniotic fluid. N = 102 in the main paper (in abstract, reported as 101 women). Exclusion criteria: gross fetal anomaly, uterine malformation, EFW > 4000 g, fetal growth restriction, placenta praevia, third‐trimester vaginal bleeding, labour, contraindications to spinal analgesia or terbutaline. |
|
| Interventions |
Intervention:regional analgesia (C) + tocolytic. Spinal analgesia with 10 mcg sufentanil and 1 mL 0.25% bupivacaine and 500 mL lactated Ringer's prehydration. N = 50 (49 in abstract; we will use detail from the detailed publication) Comparison:standard care + tocolytic. N = 52. ECV with terbutaline 0.25 mg was attempted usually by 2 operators, and was stopped for fetal bradycardia, maternal discomfort. Up to 4 attempts were allowed. Vaginal elevation of the presenting part not used. |
|
| Outcomes | Successful ECV; breech delivery; CS. | |
| Notes | University of Colorado Health Sciences Centre and Denver Health Medical Centre, USA. October 1993 to August 1997. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, computer‐generated sequence. |
| Allocation concealment (selection bias) | Unclear risk | Allocation by cards in sealed envelopes. Cards designating "spinal" or "no spinal" were placed in sealed opaque envelopes that were opened after women signed informed consent forms. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Likely that the women and the clinician were not blinded to whether or not women received an epidural. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of whether investigators were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Spontaneous version occurred before ECV in 4 women in the spinal group (after the spinal was given) and in 1 woman in the no spinal group. These women were included in the intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No differences in baseline characteristics were noted. |
El‐Sayed 2004.
| Methods | RCT ‐ with cross‐over for some unsuccessful ECVs. | |
| Participants |
Inclusion criteria: term singleton pregnancy with breech presentation. N = 59. Exclusion criteria: Maternal exclusion criteria included chronic hypertension, preeclampsia, placental abruption, placenta praevia, maternal cardiac disease, chorioamnionitis and previous uterine surgery. Fetal exclusion criteria included ruptured membranes, IUGR (EFW < 10th centile for GA by US), decreased AFI or oligohydramnios, fetal anomalies incompatible with life and an extended fetal head. |
|
| Interventions |
Intervention:tocolytic: nitroglycerine ‐ nitric oxide donor (A3) ‐ parenteral. IV nitroglycerin (100 μg IV × 2). N = 30. Comparison:tocolytic: terbutaline ‐ beta stimulant (A1) ‐ parenteral. Terbutaline (0.25 mg SQ). N = 29. After successful ECV, the decision to induce then or wait for spontaneous labour was left to the doctor. After failed ECV, the options were intervention with the other drug in the trial, discharge with appointment for CS or immediate CS; the decision was left to the doctor. |
|
| Outcomes | Successful ECV; difficult ECV; palpitations; headaches; method of delivery; light‐headedness; flushing; reversion (back to breech after ECV). | |
| Notes | We have used only data on initial "Failed ECV" because of the cross‐over element of this study. We are contacting study authors to clarify the other outcome data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation done by a third party not involved in the trial. 30 labels bearing the word 'nitroglycerin' and 30 labels bearing the word 'terbutaline.' Labels were placed on 60 unmarked opaque envelopes, which were sealed, shuffled thoroughly and numbered sequentially. |
| Allocation concealment (selection bias) | Low risk | Labels were placed on 60 unmarked opaque envelopes, which were sealed and numbered sequentially. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participant or doctor. Differing routes of administration of drugs, IV or SQ, meant that people would know which drug was being administered. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether the assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman was assigned to terbutaline before it was confirmed that the baby was breech; excluded as fetus had a cephalic presentation. This was considered insufficient to influence the analysis. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No statistically significant differences in pretreatment maternal or fetal characteristics (see Table 1, on p 2053). These were maternal age, GA at ECV, multiparity, EFW, body mass index, anterior placenta and ECV by maternal‐fetal medicine attending. |
Fernandez 1997.
| Methods | RCT. | |
| Participants |
Inclusion criteria: singleton, non‐cephalic pregnancy; > 36 weeks' gestation. N = 103. Exclusion criteria: younger than 17 years of age, prior uterine surgery, ruptured membranes, placenta praevia, anomalous fetus, multiple gestation, sensitivity to terbutaline, other maternal medical complications. |
|
| Interventions |
Intervention:tocolytic: terbutaline ‐ beta stimulant (A1) ‐ parenteral. Terbutaline 0.25 mg in unlabelled insulin syringe given SQ 15 to 30 minutes before ECV attempts. Forward then backward roll attempted. N = 52. Comparison:placebo. N = 51. |
|
| Outcomes | Successful ECV; CS. | |
| Notes | Parkland Memorial Hospital, Dallas, Texas, USA. January 1994 to June 1995. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised computer tables ‐ randomisation by pharmacy using computer‐generated random sequence. |
| Allocation concealment (selection bias) | Unclear risk | No mention in article. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Terbutaline or placebo obtained from pharmacy in unlabelled syringe. Placebo was an equal volume of normal saline. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled trial with blinding of staff. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusion of women or loss to follow‐up. Appears to be an intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | With exception of maternal age, the 2 groups did not differ at baseline. Mean age for terbutaline group: 23.4. Mean age for placebo group: 25.7. |
Hilton 2009.
| Methods | RCT, stratified by parity and hospital. | |
| Participants |
Inclusion criteria: non‐cephalic singleton pregnancies over 37 weeks with normal AFI. Participants split into nulliparous (N = 82) and multiparous (N = 44). N = 126. Exclusion criteria: labour, ruptured membranes, history of third‐trimester bleeding, any preexisting uterine scar, pregnancy‐induced hypertension or gestational diabetes, oligohydramnios, hydramnios, IUGR, macrosomia, maternal hypotension, inability to comprehend the consent form. |
|
| Interventions |
Intervention:tocolytic: nitroglycerine ‐ nitric oxide donor (A3) parenteral. IV nitroglycerine (10 mL of 100 micrograms/mL). N = 65 (nulliparous = 42, multiparous = 23). Comparison:placebo. N = 61 (nulliparous = 40, multiparous = 21). |
|
| Outcomes | ECV success; cephalic presentation at delivery; CS rate; maternal discomfort; headaches; flushing; hypotension; palpitations; fetal heart rate abnormalities. | |
| Notes | Nulliparous group: 4 women excluded after randomisation. In experimental group, 1 excluded for pregnancy‐induced hypertension, and 1 excluded for decreased AFI. Control group: 1 excluded as woman was in labour, and 1 excluded because of cephalic presentation. 1 woman in placebo group lost to follow‐up. Multiparous group: 3 women excluded after randomisation. In experimental group, 2 excluded as presentation was cephalic at the time of version. In placebo group, 1 woman excluded as presentation was cephalic at time of version. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation tables used. |
| Allocation concealment (selection bias) | Low risk | Using separate randomisation sequences for nulliparous and multiparous women at each hospital site, participants were assigned a study number from sequentially numbered opaque envelopes. The study number was forwarded to the pharmacy, and allocated treatment was provided on the basis of the corresponding study number from randomisation tables kept in the pharmacy. No further details provided on randomisation sequences used. Group of allocation was unknown to obstetrician, nurse, anaesthesiologist and woman. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment was prepared as 10 mL of clear fluid in a 10 mL syringe with 10 mL of 100 micrograms/mL of nitroglycerin for women in the nitroglycerin group, or 10 mL of normal saline for women in the placebo group. Syringes for nitroglycerine and placebo were visually indistinguishable. Group for allocation was unknown to obstetrician, nurse, anaesthesiologist and woman. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Treatment was prepared as 10 mL of clear fluid in a 10 mL syringe with 10 mL of 100 micrograms/mL of nitroglycerin for women in the nitroglycerin group, or 10 mL of normal saline for women in the placebo group. Syringes for nitroglycerine and placebo were visually indistinguishable. Group for allocation was unknown to obstetrician, nurse, anaesthesiologist and woman. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman lost to follow‐up. No apparent exclusion of women after randomisation. 7 women did not undergo ECV, but their outcomes were included in the analysis. Nulliparous group: 2 in nitroglycerine group excluded: 1 had pregnancy‐induced hypertension, 1 had a decreased AFI. Nulliparous group: 2 in placebo group excluded: 1 cephalic, 1 lost to follow‐up. Multiparous group: 2 in nitroglycerine group excluded: had cephalic presentations. Multiparous group: 1 in placebo group excluded: had cephalic presentation. Data on fetal heart rate abnormalities were available for 61 women in nitroglycerine group and 58 in placebo group; for side effects, the numbers were 59 and 58, respectively. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | Baseline data: similar for maternal age, GA and anterior placenta in nulliparous and multiparous trials. |
Impey 2005.
| Methods | RCT. | |
| Participants |
Inclusion criteria: nulliparous women, singleton breech presentation at 36 weeks, or multiparous at 37 or more weeks. Eligible for inclusion if an unsuccessful attempt at ECV (without tocolysis) was reported, with normal CTG. N = 144. Exclusion criteria: preexisting indication for CS, unstable lie, fetal compromise (abdominal circumference below 3rd centile, either umbilical artery resistance index above 97th centile or deepest amniotic fluid pocket 2 cm, rhesus isoimmunisation. |
|
| Interventions |
Intervention:tocolytic: ritodrine ‐ beta stimulant (A1) ‐ parenteral. Tocolysis administered as ritodrine hydrochloride (Yutopar infusion of 50 mg (10 mg/mL)) added to 12 mL dextrose saline (total 17 mL of ritodrine 3 mg/mL). N = 62. Comparison:placebo: 17 mL dextrose saline infusion by the same route at the same rate. N = 62. |
|
| Outcomes | Primary outcome cephalic presentation at birth. Secondary outcomes: incidence of successful ECV, CS, length of hospital inpatient stay, incidence of neonatal Apgar scores < 7 at 5 minutes, neonatal admission, rare neonatal outcomes and mean cord arterial pH. McGill pain scale was used to measure intensity of pain. |
|
| Notes | Setting: Breech Clinic, John Radcliffe Hospital, Oxford: women from community clinics and other local hospitals were referred in at 36 or 37 weeks' gestation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated in a ratio of 1:1 using random block sizes up to 20. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes opened in sequential order. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Specific detail missing, but states same infusion, same timeline, same observation for both control and intervention. In discussion, study authors identified problems with blinding of researcher and medical practitioner as potential threats. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Specific detail missing, but states same infusion, same timeline, same observation for both control and intervention. In discussion, study authors identified problems with blinding of researcher and medical practitioner as potential threats. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Potential sample 505, of whom 284 were deemed eligible for inclusion in the trial. Of these, 13 refused and 47 were not offered. All 124 participants (62 in each arm) completed the trial. Intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No differences in age or gestation seen in baseline data. |
Johnson 1995.
| Methods | RCT cross‐over ‐ using here only data from first part. | |
| Participants | Inclusion criteria: women scheduled for attempted ECV with the fetal spine in the midline (back‐up or back‐down) on US examination. N = 26. All women approached agreed to participate. Exclusion criteria: oligohydramnios (AFI < 5 cm), fetal or uterine anomalies, ruptured amniotic membranes, active labour, engagement of presenting part, fetal heart rate decelerations. | |
| Interventions |
Intervention:vibroacoustic stimulation (B). Fetal acoustic stimulation for 1 to 3 seconds with a Western Electric Division AT&T (Phoenix) model 5C electrolarynx over the fetal head, or over the nurse's upper arm (dummy). Physician blinded by leaving the room during the intervention. N = 12. Comparison:placebo. N = 11. |
|
| Outcomes | Persistent midline spine position on US (stimulation 1/13, control 13/13); failed ECV attempt. Data on method of delivery not included because followed cross‐over treatment. | |
| Notes | 2 hospitals in Arizona, USA, 1 January 1993 to 31 December 1994. After randomisation, 1 from the treatment group and 2 from the control group were excluded because the breech was found to be deeply engaged in the pelvis during the initial ECV attempt. None had changed position to the spine lateral position, and no further attempts at ECV were made. In keeping with the pre‐stated protocol for this review, these women have been included in the outcomes as originally allocated. Those women in whom ECV failed were crossed over to the other intervention arm. This review considers only data from the first intervention, according to the original allocation Results of the 'cross‐over' part of the study are not included. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Selection of sequential envelopes generated by a table of random numbers and handed out by research nurse. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Clinicians were blinded, but the nurse applying the stimulation and the women could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Clinicians reported blinded, but the nurse applying the stimulation and the women could not be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 women (12%) (1 treatment and 2 control) excluded, as breech was deeply engaged. This loss should be insufficient to affect the comparison. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | No baseline data are available for assessment. |
Kok 2008.
| Methods | Multi‐centre RCT. | |
| Participants | Inclusion criteria: term singleton, breech presentation pregnancies. GA of 36 weeks onwards. N = 320. Exclusion criteria: maternal exclusion: any contradiction to labour or vaginal birth, scarred uterus other than transverse in the lower segment, known uterine anomalies, placental abruption in the obstetric history, preeclampsia, maternal cardiac disease, third‐trimester bleeding. Fetal exclusion: IUGR (EFW < 5th percentile for GA assessed by ultrasonography), fetal anomalies or an extended fetal head, oligohydramnios (defined as an AFI ≤ 5 cm) and non‐reassuring signs of fetal well‐being. | |
| Interventions |
Intervention: tocolytic: nifedipine ‐ calcium channel blocker (A2) ‐ oral. Nifedipine (2 doses of 10 mg) orally. N = 160 but 154 analysed. Comparison:placebo: placebo capsules. N = 160 but 156 analysed. |
|
| Outcomes | Primary: successful ECV defined as a fetus in cephalic position 30 minutes after the ECV procedure. Secondary: fetal presentation at birth, mode of birth and adverse maternal (major side effects due to medication, hypotension with fetal consequences, anaphylactic shock due to the medication and any adverse cardiac events due to medication intake) and fetal events (fetal death, emergency delivery, fetal bradycardia, premature rupture of the membranes and placental abruption within 24 hours after the ECV procedure). Minor side effects: nausea, dizziness and flushing and cessation of treatment because of side effects. |
|
| Notes | Nifedipine group ‐ 2 women excluded as they were less than 39 weeks' gestation, 2 excluded as they were repeat versions, 2 women lost to follow‐up. Placebo group ‐ 2 women excluded as they were less than 38 weeks, 2 excluded as they were repeat versions. Study reports no events for fetal death; emergency delivery less than 24 hours; placental abruption less than 24 hours; premature rupture of membranes less than 24 hours; maternal hypotension with fetal consequences; anaphylactic shock. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer blocks of 10, stratified for centre and parity. |
| Allocation concealment (selection bias) | Low risk | Pharmacy prepared sealed opaque containers with study medication and kept an allocation list until completion of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. All participants, nurses and doctors who performed the ECV were blinded to the assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded. All participants, nurses and doctors who performed the ECV were blinded to the assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 women were lost to follow‐up or were excluded in the nifedipine group, and 4 women in the control group. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Baseline characteristics were similar. Baseline characteristics (Table 1) indicate generally good balance, although there appears to be some imbalance in placental anterior localisation (44 vs 55). Some imbalances were seen in some of the ethnicity data (Central African (4 vs 10) and Other (18 vs 9). |
Mancuso 2000.
| Methods | RCT. | |
| Participants |
Inclusion criteria: women undergoing ECV attempt. Age 18 years or greater, singleton pregnancy, 37 weeks or more, breech or transverse presentation, intact membranes, EFW 2000 to 4000 g, reassuring fetal heart rate testing. N = 108. Exclusion criteria: placenta praevia, prior classical CS, third‐trimester bleeding, AFI < 5 or > 25 cm, known uterine malformation, suspected major fetal anomaly, active‐phase labour |
|
| Interventions |
Intervention:regional analgesia (C) + tocolytic. Lumbar epidural analgesia with 3 + 10 mL 2% lidocaine, with epinephrine test dose and fentanyl 100 micrograms. N = 54. Comparison:no regional analgesia + tocolytic. N = 54. All received Ringer's Lactate 1500 mL IV, and terbutaline 0.25 mg SQ. |
|
| Outcomes | Presentation after ECV attempt; presentation at birth; fetal bradycardia causing cessation of ECV attempts; the way women gave birth. | |
| Notes | Tripler Army Medical Centre, Honolulu, Hawaii, December 1994 to June 1998. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers table. |
| Allocation concealment (selection bias) | Low risk | ...with group assignments sealed in sequentially numbered opaque envelopes randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is not possible to blind people to epidurals. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It is not possible to blind people to epidurals. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions or loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | Baseline data were similar between groups; no evidence of other bias. |
Marquette 1996.
| Methods | RCT. | |
| Participants |
Inclusion criteria: women with singleton breech presentation, 36 to 41 weeks' gestation, reactive CTG, breech mobile on abdominal palpation. N = 283. Exclusion criteria: impaired fetal growth (estimated weight < 10th percentile), oligohydramnios (AFI < 5), placenta praevia, placental abruption, uterine scar other than low transverse CS, active labour, ruptured membranes, fetal anomalies incompatible with life, contraindication to vaginal delivery, contraindication to tocolysis. |
|
| Interventions |
Intervention:tocolytic: ritodrine ‐ beta stimulant (A1) ‐ parenteral. IVI, for 20 minutes before and during ECV attempt, of ritodrine 111 micrograms/min or placebo. Maximum of 3 ECV attempts as forward or backward flip. CTG was repeated. N = 138. Comparison:placebo. N = 145 |
|
| Outcomes | Duration of infusion (tocolysis mean 32.1 (SD 1.04) vs control 31.7 (1.12) minutes); unsuccessful ECV; CTG results (all reactive); time from ECV to birth (average 2 weeks); maternal and fetal complications (maternal complications < 4%, similar between groups); mode of birth; birthweight (3370 (39) vs 3382 (44) grams). | |
| Notes | Groups differed in terms of frank breech (tocolysis 59/138 vs control 43/145) and nulliparity (58/138 vs 49/145). Parity (nulliparous 34% vs parous 61%), but not type of breech, affected ECV success rate; therefore results controlled for parity. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random assignment of every 10 patients enrolled: 5 to ritodrine and 5 to control. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo and treatment made up in pharmacy in identical phials and administered IV in the same solution at the same rate. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo and treatment made up in pharmacy in identical phials and administered IV in the same solution at the same rate. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions or loss to follow‐up reported. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Ritodrine group had higher proportion of nulliparous women. It is unclear whether this would impact the comparison of outcomes. |
Munoz 2014.
| Methods | 2‐arm RCT. Individual women randomly assigned. | |
| Participants |
Dates of data collection: April 2010 to March 2011. Setting: tertiary hospital in Spain with more than 3000 births a year. Inclusion criteria: women with non‐cephalic presentation between 36 and 41 weeks' gestation (All non‐labouring pregnant women at 36 to 41 weeks' gestation with a non‐cephalic presentation confirmed by ultrasound scan were invited to participate). N = 60. Exclusion criteria: fetal abnormalities, intrauterine fetal death, suspicion of fetal growth restriction, fetal weight above 3800 g, maternal cardiovascular disease, American Society of Anesthesiologists class > 2, severe hypertension, allergy to any trial medications, amniotic fluid index < 4 cm, Doppler cerebroplacental ratio > 5th percentile, abnormal cardiotocographic recordings, contraindications to vaginal delivery, uterine abnormalities, coagulation disorders, Rhesus incompatibility, multiple gestation, rupture of membranes and/or placental abruption. |
|
| Interventions |
Experimental intervention: remifentanil. Remifentanil at 0.1 lg/kg/min, with rescue boluses on demand of 0.1 lg/kg/min and a lockout period of 4 minutes. Given by patient‐controlled pump.All women given IV infusion of ritodrine 200 lg/min for tocolysis. All women given 1 g paracetamol in 100 mL saline (IV) 5 minutes before ECV. N = 31. Control/Comparison intervention: placebo. Study control solution at 0.1 lg/kg/min, with rescue boluses on demand of 0.1 lg/kg/min and a lockout period of 4 minutes. Given by patient‐controlled pump. All women given IV infusion of ritodrine 200 lg/min for tocolysis. All women given 1 g paracetamol (IV) 5 minutes before ECV. N = 29 |
|
| Outcomes | Pain score (numerical rating scale 0 to 10, no pain to worst pain imaginable); success of ECV; CS; adverse events (nausea, vomiting, dizziness, etc); mode of birth; fetal bradycardia. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “…computer‐generated random sequence…”. |
| Allocation concealment (selection bias) | Low risk | Hospital pharmacy prepared 100 mL infusion bags that contained remifentanil (1 mg) or saline, which were labelled with the patient code and sent to the operative room. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women blind to allocation (placebo‐controlled trial). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Anaesthesiologists, midwives and obstetricians were blinded to allocation group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “After randomisation, three women were excluded: two for spontaneous conversion of the fetus to a cephalic presentation, and one who declined to participate”. Obstetric data for 2 further women were lost, but they were included in the statistical analysis on an intention‐to‐treat basis. |
| Selective reporting (reporting bias) | Low risk | Trial registration form (Valero 2010) lists outcomes, all of which were reported in main study publication (Munoz 2014). |
| Other bias | Low risk | No imbalance in age; BMI; estimated fetal weight; ethnicity; parity; previous CS; presentation; placenta and amniotic fluid volume. |
Nor Azlin 2005.
| Methods | RCT. | |
| Participants | Inclusion criteria: singleton term breech pregnancy at a tertiary hospital. N = 60. Exclusion criteria: previous CS or other uterine scar, uterine malformation, antepartum haemorrhage, hypertension, diabetes mellitus, IUGR, oligohydramnios, fetal anomalies, early or active labour, contraindications to IV ritodrine, contraindication to vaginal delivery | |
| Interventions |
Intervention: tocolytic: ritodrine ‐ beta stimulant (A1) ‐ parenteral. Ritodrine (IV) ‐ 0.4 mg/mL in 5% dextrose infused at 1.5 mL/min. N = 30 (nulliparous 22 and multiparous 8). Comparison:placebo. N = 30 (nulliparous 23 and multiparous 7). |
|
| Outcomes | Successful ECV. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random‐number generator. |
| Allocation concealment (selection bias) | Low risk | Sealed numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Obstetricians and women were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Obstetricians and women were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions or incomplete data. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | More babies in the frank breech position in the ritodrine group. |
Nor Azlin 2008.
| Methods | RCT. | |
| Participants | Inclusion criteria: singleton term pregnancies with a breech presentation. N = 86. Exclusion criteria: oligohydramnios, macrosomia, presence of a contraindication for vaginal delivery, previous caesarean delivery, multiple pregnancy, hypertension in pregnancy, rhesus‐negative mother, previous history of abruptio placenta, lethal fetal anomaly, contraindication against nifedipine or terbutaline. | |
| Interventions |
Intervention:tocolytic: nifedipine ‐ calcium channel blocker (A2) ‐ oral. Oral nifedipine (20 mg). N = 43 (nulliparous 18, multiparous 25). Comparison:tocolytic: terbutaline ‐ beta stimulant (A1) ‐ parenteral. IV terbutaline (50 μg). N = 43 (nulliparous 21, multiparous 22) (6 lost to further follow‐up). |
|
| Outcomes | Successful ECV, difficult ECV, palpitations, hypotension, method of delivery, perinatal morbidity. | |
| Notes | 6 successful ECVs from the terbutaline group were lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐number generator. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, numbered envelopes in sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Clinicians doing ECV were blinded to the tocolytic drug, women were not blinded because 1 group had oral administration and the other IV. Clinicians doing the ECV were in control of the success rate and were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Clinicians doing ECV were blinded to the tocolytic drug, women were not blinded because 1 group had oral administration and the other IV. Clinicians doing the ECV were in control of the success rate and were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Among those who had successful ECV, 6 from terbutaline group were lost to follow‐up, so although all women were included in the assessment of the success of ECV, the outcome of CS is at risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | The study was not stopped early. Baseline characteristics were similar between groups in maternal age, GA, AFI, parity and type of breech presentation. No other biases were identified. |
Reinhard 2012.
| Methods | RCT comparing 2 interventions (clinical hypnosis or NLP) (a control group receiving no intervention was used as a historical comparison – data for this group have not been included in the review). Single‐centre, stratified by parity. | |
| Participants | Control group. From January 1, 2009, to October 31, 2010, a control group were all ECVs, during which time neither hypnosis nor NLP was used. These data will not be included. Setting: a tertiary university hospital in Germany. Johann Wolfgang Goethe University Hospital in Frankfurt am Main. Inclusion criteria: pregnant women with a singleton fetus in a breech position at the scheduled date of the ECV at or after 370/7 (259 days) weeks' gestation, normal amniotic fluid index, with advanced level of German language. N = 80. Exclusion criteria: women in active labour (regular uterine contractions and rupture of membranes), contraindications for a vaginal birth (such as placenta praevia) and planned birth by caesarean section even if the fetus turned to a cephalic position. |
|
| Interventions |
Experimental intervention: hypnosis. 20‐Minute standardized clinical hypnosis intervention via head phones (Bose, QuietComfort 15) before ECV procedure was carried out. Hypnosis intervention was a voice recording of one of the trialists (a certified hypnotherapist who underwent training in the fundamentals of NLP). A relaxation induction was utilised, in which the therapist focused on breathing and concentrated on various parts of the body for trance deepening. N = 42. Control/Comparison intervention: neurolinguistic programming. 20‐Minute standardised NLP intervention via head phones (Bose, QuietComfort 15) before ECV procedure was carried out. Hypnosis intervention was a voice recording of 1 of the trialists (a certified hypnotherapist who underwent training in the fundamentals of NLP). N = 38. |
|
| Outcomes | ECV success; women’s views (results reported as means derived from Likert 6‐point scale). | |
| Notes | We contacted the study author for more information re the NLP intervention and received additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Off‐centre randomisation sequence based on block randomisation was calculated and assigned by the Institute of Biostatistics and Mathematical Modeling. |
| Allocation concealment (selection bias) | Unclear risk | Allocation at the point of randomisation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention was double‐blinded, that is, the participant and the clinician who carried out the ECV procedure did not know the kind of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Intervention was double‐blinded, that is, the participant and the clinician who carried out the ECV procedure did not know the kind of intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up evident after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol. |
| Other bias | Low risk | Other bias not apparent. |
Robertson 1987.
| Methods | Quasi‐RCT | |
| Participants |
Inclusion criteria: breech presentation suitable for ECV at term (37 to 41 weeks). N = 58. Exclusion criteria: oligohydramnios, estimated fetal weight < 2500 g or > 4000 g, non‐reactive NST. |
|
| Interventions |
Intervention:tocolytic: ritodrine ‐ beta stimulant (A1) ‐ parenteral. Use of tocolysis (ritodrine infusion 200 micrograms per minute for 20 minutes) compared with no tocolysis. All women had IV lines. Repeat version attempt with tocolysis was successful in 1/9, with initial failure in the control group (for immediate success rate, this review considered only the initial attempt). N = 30. Comparison:no tocolytic. N = 28. |
|
| Outcomes | Non‐cephalic presentation at birth; CS; immediate ECV success. | |
| Notes | Tacoma, Washington, USA. July 1984 to May 1987. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocated according to last digit of social security number. |
| Allocation concealment (selection bias) | High risk | Allocated according to last digit of social security number. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 women lost to follow‐up in the intervention group and 5 in the control group. This seems unlikely to impact outcomes. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | Groups were similar in age, parity, maternal weight, GA, EFW. No other biases apparent. |
Schorr 1997.
| Methods | RCT. | |
| Participants | Inclusion criteria: breech presentation or transverse lie. N = 69. Exclusion criteria: placenta praevia, fetal compromise, fetal growth restriction, ruptured membranes. | |
| Interventions |
Intervention:regional analgesia (C) + tocolytic. Epidural analgesia with 2% lidocaine with 1:200,000 epinephrine (N = 35); prehydration with 2000 mL lactated Ringer's solution vs no epidural (N = 34). All women received 0.25 mg terbutaline SQ. ECV attempted up to 3 times, with vaginal elevation of the presenting part when necessary. N = 35. Comparison:no regional analgesia + tocolytic. N = 34. 250 mg terbutaline given as adjunct. |
|
| Outcomes | Successful ECV, complications, mode of birth, presentation at delivery. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation cards placed in permuted blocks of 10 by Division of Biostatistics. |
| Allocation concealment (selection bias) | Low risk | Allocation put in sealed opaque envelopes, and all investigators participating in clinical aspects of the study were blinded to the randomisation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is not possible to blind people to the use of epidurals. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It is not possible to blind people to the use of epidurals. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although 5 women declined randomisation, there seemed to be no exclusions and no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No differences in baseline data. No other biases apparent. |
Stock 1993.
| Methods | RCT. | |
| Participants | Inclusion criteria: breech presentation between 36 and 42 weeks with no contraindication to ECV. N = 63. Exclusion criteria: diabetes, heart disease, thyrotoxicosis, ruptured membranes, multiple pregnancy, uterine scar, placenta praevia, oligohydramnios, impaired fetal growth, nuchal cord, placenta praevia. | |
| Interventions |
Intervention 1:tocolytic: ritodrine ‐ beta stimulant (A1) ‐ parenteral. Group B: ritodrine 0.3 mg per minute infusion for 30 minutes and during the procedure, and placebo bolus injection. N = 21. Intervention 2:tocolytic: hexoprenaline ‐ beta stimulant (A1) ‐ parenteral. Group C: placebo infusion and hexoprenaline 10 micrograms bolus injection. N = 21. Comparison:placebo. Group A: placebo infusion and bolus injection. N = 21. For the purposes of this review, which addresses the effectiveness of IV tocolysis for ECV rather than the evaluation of specific tocolytic agents, intervention 1 (group B) and intervention 2 (group C) have been combined. Nulliparous = 18 in tocolytic groups and 9 in placebo group. Multiparous = 24 in tocolytic groups and 12 in placebo group. We have not set up a subgroup comparison of 1 beta stimulant vs another, so the data from this study on failed ECV for ritodrine (7/21) vs hexoprenaline (5/21) are not included as a direct comparison. |
|
| Outcomes | Immediate ECV success; ECV completed < 1 minute; fetal bradycardia during ECV. | |
| Notes | Improved ECV success rate with tocolysis reached statistical significance for hexoprenaline but not for ritodrine. Study authors decided not to continue the ritodrine/placebo arm of the trial to completion. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Each investigator had a separate randomisation sequence. These were in sets of 3 to the 3 groups, stratified for parity.' Method of randomisation not specified. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled trial. Practitioners were blind to group allocation, as were the women. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Practitioners were blind to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of exclusions of loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | High risk | No statistically significant differences between groups in terms of parity, height, age or gestation at time of ECV (see Table 2 on page 266). No differences between groups regarding fetal biparietal diameter, abdominal circumference. Femur length or AFI. Women in ritodrine group were significantly lighter than those in the other 2 groups. Trial stopped early for benefit. |
Sullivan 2009.
| Methods | RCT. | |
| Participants | Inclusion criteria: singleton breech presentations after 36 weeks' gestation. N = 95. Exclusion criteria: patients with contraindications to neuraxial anaesthesia, allergies to study medications. | |
| Interventions |
Intervention:systemic opioids (E) + tocolytic. Systemic opioids (50 µg fentanyl). N = 47. Comparison:regional analgesia (C) + tocolytic. CSE anaesthesia (bupivacaine 2.5 mg and 15 µg fentanyl followed by 45 mg lidocaine and 15 µg epinephrine). N = 48. Both groups received terbutaline. |
|
| Outcomes | Successful ECV, hypotension, decelerations of FHR, persistent decelerations, CS. | |
| Notes | 1 woman excluded after randomisation before ECV because of non‐reassuring CTG. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random‐number table. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman was excluded after randomisation because she underwent an emergency CS. Other possible exclusions are unclear. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | No significant differences between the 2 groups in age, parity, GA, height, weight or obstetrician predicted difficulty of version. Other biases not identified. |
Tan 1989.
| Methods | RCT. | |
| Participants | Inclusion criteria: breech presentation beyond 33 weeks' gestation without contraindication to ECV. N = 90. Exclusion criteria: signs of growth restriction, vaginal bleeding in the third trimester, toxaemia of pregnancy, labour, polyhydramnios, placenta praevia, previous CS scar, contracted pelvis, fetal malformation and uterine malformation. | |
| Interventions |
Intervention 1:tocolytic: salbutamol ‐ beta stimulant (A1) ‐ parenteral. Group 2 received an IVI of salbutamol until maternal heart rate exceeded 100 beats per minute for 30 minutes. N = 30 (nulliparous 17, multiparous 13). Intervention 2:tocolytic: salbutamol ‐ beta stimulant (A1) ‐ oral. Group 1 received salbutamol 4 mg orally 3 times a day for at least 1 day. N = 30 (nulliparous 16, multiparous 14). Comparison:placebo. Group 3 received no salbutamol. N = 30 (nulliparous 17, multiparous 13). Groups 1 and 3 received dummy IV lines |
|
| Outcomes | Immediate ECV success. | |
| Notes | Singapore. This study compared 2 different routes of administration (oral and IV) of a tocolytic drug to facilitate ECV. So it provides data only for tocolysis vs placebo, and the different routes of administration are considered in subgroups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | High risk | 2 stacks of randomised cards divided according to parity with each stack further subdivided by a colour code for gestation A or B. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States that clinicians were blinded to treatment and dummy IVs were inserted. Clinicians did not know parity or gestation. Women's status unclear, but clinicians more likely to be able to influence outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States that clinicians were blinded to treatment and dummy IVs were inserted. Clinicians did not know parity or gestation. Women's status unclear, but clinicians more likely to be able to influence outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions apparent after randomisation and no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | The 3 groups were similar in placental site, abdominal girth, maternal weight, fetal birthweight and stratification of parity and gestation across groups Failed ECV: Time taken was significantly longer (10.5 + 4.9 vs 5.6 + 3.9; P value < 0.001) and onset of labour was significantly earlier (17.6 + 9.8 vs 25.2 + 14.9 days; P value < 0.02), implying that longer manipulation hastened the onset of labour by 70%. |
Vallikkannu 2014.
| Methods | RCT 2‐arm (then cross‐over for second attempt; data following cross‐over have not been included in the review). | |
| Participants |
Dates of data collection: 18 Jan 2011 to 23 Dec 2012. Setting: University Hospital, Kuala Lumpur, Malaysia. 6000 to 7000 births a year. Inclusion criteria: women scheduled for ECV (≥ 36 weeks' gestation). Scheduled ECV, breech presentation or transverse lie, singleton gestation, gestational age ≥ 36 weeks, intact membranes, non‐anomalous fetus, reassuring fetal status on cardiotocogram. N = 95. Exclusion criteria: regular contractions were present, estimated fetal weight < 2 kg, oligohydramnios (amniotic fluid index < 5 cm), severe hypertension, recent antepartum haemorrhage, uterine scar, related allergy and any potential contraindication to vaginal birth. |
|
| Interventions |
Experimental intervention: powder. Commercially available baby talcum powder was applied to the woman’s abdomen by the operator. N = 48. 250 mcg terbutaline was administered subcutaneously 5 to 10 minutes before ECV was attempted. In the first round, a maximum of 2 attempts at ECV were permitted. An attempt comprised a continuous manoeuvre typically lasting not longer than 2 to 3 minutes. Fetal presentation and heart rate were then checked by ultrasound. If ECV was unsuccessful but fetal heart rate was normal and the woman was agreeable, a second attempt was made with the same allocated aid. After completion of the first round of a maximum of 2 attempts, the participant was asked to record her ECV‐related pain score, and the operator was asked to provide a satisfaction score with use of the allocated aid, using a 10 point visual numerical rating scale (VNRS ‐ scored from 1 to 10, marked as higher score more desirable result). Following an unsuccessful first round of ECV, if fetal status was reassuring on cardiotocogram (i.e. until at least 2 fetal heart rate accelerations were observed in the context of a normal baseline, baseline variability and absence of decelerations), and both the provider and the woman were willing, a second round of up to 2 ECV attempts was permitted with cross‐over to the opposing aid, i.e. powder to gel, gel to powder. A further terbutaline dose was given for the second round, which was conducted in similar fashion to the first round. Control/Comparison intervention: gel. Ultrasound aqueous gel was applied to the woman’s abdomen by the operator. N = 47. 250 mcg terbutaline was administered subcutaneously 5 to 10 minutes before ECV was attempted. In the first round, a maximum of 2 attempts at ECV were permitted. An attempt comprised a continuous manoeuvre typically lasting not longer than 2 to 3 minutes. Fetal presentation and heart rate were then checked by ultrasound. If ECV was unsuccessful but fetal heart rate was normal and the woman was agreeable, a second attempt was made with the same allocated aid. After completion of the first round of a maximum of 2 attempts, the participant was asked to record her ECV‐related pain score, and the operator was asked to provide a satisfaction score with use of the allocated aid, using a 10 point visual numerical rating scale (VNRS ‐ scored from 1 to 10, marked as higher score more desirable result). Following an unsuccessful first round of ECV, if fetal status was reassuring on cardiotocogram (i.e. until at least 2 fetal heart rate accelerations were observed in the context of a normal baseline, baseline variability and absence of decelerations), and both the provider and the woman were willing, a second round of up to 2 ECV attempts was permitted with cross‐over to the opposing aid, i.e. powder to gel, gel to powder. A further terbutaline dose was given for the second round, which was conducted in similar fashion to the first round. |
|
| Outcomes | Self‐reported pain; success of ECV; operator’s VNRS satisfaction score (identical scale to the pain VNRS described above) with the agent used; significant post‐ECV cardiotocogram anomaly; cephalic presentation at birth; caesarean (and indication); neonatal outcomes of Apgar score, umbilical cord arterial blood pH and base deficit and neonatal admission; gestational age at birth; blood loss at birth and birthweight; fetal or neonatal death; neonatal hypoxic‐ischaemic encephalopathy and major abruptio placenta. ECV was considered a success if cephalic presentation was demonstrated on ultrasound immediately after an attempt. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “…computer generated randomisation sequence obtained from http://www.random.org...”. |
| Allocation concealment (selection bias) | Unclear risk | “…randomisation envelopes were prepared by an author (NV who was not involved in recruitment) in a single block of 100…sequential opening of the lowest numbered sealed opaque envelope remaining just before the start of ECV”. 5 envelopes were not accounted for. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “Blinding of providers and patients to the intervention was not attempted as it was considered unachievable.” It was not clear whether staff were using their usual or preferred method (it was stated that talcum powder had mainly been used, although some staff had started to use gel for ECV). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding was not attempted, as it was considered unachievable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 48 randomly assigned to powder and 47 to gel. Recruitment ceased when all 100 numbered envelopes were used. 5 numbered envelopes could not be accounted for (2 allocated to powder and 3 allocated to gel). All participants received powder or gel as allocated for their first round of ECV. Primary analysis was per protocol. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | No baseline imbalances in age; gestation; parity; nulliparous; weight; height; BMI; ethnicity; etc. When possible, we have used the data related to the first attempt only, but the assessment of pain seems to be pooled in the published paper. |
Vani 2009.
| Methods | RCT. | |
| Participants |
Inclusion criteria: healthy women, singleton fetus in breech presentation, 37 to 39 weeks with intact membranes, no signs of labour and a clinically EFW 2 to 4 kg. USS performed to confirm breech presentation and to ascertain fetal neck position and location of the placenta. N = 114. Exclusion criteria: AFI outside range of 5 to 25, fetal hyperextended neck, placenta previa, gross fetal anomalies, hypertension, gestational diabetes, antepartum haemorrhage, uterine scar (from CS, myomectomy or perforation), uterine malformation allergy or contraindication to salbutamol or contraindication to a trial of labour even if in cephalic presentation. |
|
| Interventions |
Intervention:tocolytic: salbutamol ‐ beta stimulant (A1) ‐ parenteral. Salbutamol (IV dose of 0.1 mg salbutamol with further boluses every 5 minutes). N = 57. Comparison:placebo. N = 57. |
|
| Outcomes | Successful ECV, palpitations, hypotension, fetal presentation at delivery, method of delivery, perinatal morbidity, Apgar scores. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated by a random‐number generator. |
| Allocation concealment (selection bias) | Low risk | Sealed numbered opaque envelopes prepared in blocks of 4. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label RCT and IV administration of tocolytic was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label RCT and IV administration of tocolytic was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions after randomisation and no loss to follow‐up. All women received their allocated treatment. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | A significant difference was noted in the location of the placenta between the 2 groups. Significantly more women in the tocolysis group had a placenta attached to the fundus. Fewer women in the intervention group had a placenta in the anterior upper segment and more in the posterior upper segment, although statistical significance is not reported. It is unclear whether this would have an impact on outcomes. Post hoc multivariate logistic regression analyses incorporating placental location and allocated treatment as independent co‐variables in the analysis with successful ECV and CS reported separately as dependent outcomes. After control for placental location in both models for successful ECV and CS salbutamol, tocolysis remained significantly associated with increased ECV success (adjusted OR 3.4; 95% CI 1.4 to 8.2; decreased CS adjusted OR 3.4; 95% CI 0.14 to 0.79). |
Weiniger 2007.
| Methods | RCT. | |
| Participants |
Inclusion criteria: All eligible nulliparous women who requested ECV after 37 weeks' gestation during the period from September 2002 to May 2006 were approached for recruitment before the ECV procedure. Inclusion criteria included American Society of Anesthetists status I to II at 37 to 40 weeks' gestation, and no fetal abnormality. N = 70. Exclusion criteria: women with a breech presenting fetus who requested elective caesarean delivery, either after failed ECV at another institution or because they did not wish to try ECV at all, were not included or followed up, and data regarding these women were not collected. Women with any of the following were excluded: previous uterine surgery or uterine anomaly, contraindication for vaginal delivery, contraindications for regional analgesia, woman's refusal of regional analgesia, neuropathy, severe back pain with neurological radiation, poor communication and morbid obesity (body mass index > 40 kg/m2). |
|
| Interventions |
Intervention:regional analgesia (C) + tocolytic. Spinal analgesia (bupivacaine 7.5 mg). N = 36. Comparison: no regional analgesia + tocolytic. N = 34. Both groups received 50 mg ritodrine or 20 mg nifedipine sublingually. |
|
| Outcomes | Successful ECV. | |
| Notes | In spinal group: 1 woman excluded as morbidly obese, 1 women requested to not have spinal after randomisation. In placebo group: 1 woman excluded as morbidly obese, 1 refused ECV after randomisation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation cards randomly inserted into envelopes by a physician not involved in the study. |
| Allocation concealment (selection bias) | Unclear risk | Study allocation was by sequentially numbered sealed envelopes containing a concealed allocation card designating the participant to receive (group S), or not receive (group N), spinal analgesia. Allocation sequence was concealed until after enrolment, and informed consent was obtained before study assignment of the participant was revealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is not possible to blind people to regional analgesia. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It is not possible to blind people to regional analgesia. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women in each group were excluded from the analysis (1 in each group declined ECV or the intervention, and 1 protocol violation was reported in each group). This was considered insufficient to impact the analysis. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Women in the intervention group were significantly younger than those in the control group, but other baseline characteristics were similar between groups (height, weight, weight gain, EFW, GA, amniotic fluid volume, placental position, fetal presentation, position of fetal spine, tocolytic used). |
Weiniger 2010.
| Methods | RCT. | |
| Participants |
Inclusion criteria: Healthy multiparae at term requesting ECV for breech presentation, without fetal or uterine anomaly, were enrolled after written informed consent, and all eligible multiparae requesting ECV after 37 weeks' gestation were approached for recruitment before the ECV. ASA status I to II, 37 to 40 complete weeks' gestation, no fetal abnormality (including IUGR), no contraindication for vaginal delivery or no contraindication for regional analgesia. N = 64. Exclusion criteria: previous CS, previous myomectomy with uterine cavity penetration or uterine anomaly, morbid obesity (body mass index 40 kg/m2), AFI 7 cm, neuropathy, severe back pain with radicular radiation, patient refusal of regional analgesia, poor communication or request for elective CS (after failed ECV at another institution or not wishing to attempt ECV). |
|
| Interventions |
Intervention:regional analgesia (C) + tocolytic. Spinal analgesia (bupivacaine 7.5 mg). N = 31. Comparison: no regional analgesia + tocolytic. N = 33. Ritodrine (50 mg IV) used as muscle relaxant until April 2003, when it was replaced by nifedipine (20 mg orally). |
|
| Outcomes | Successful ECV. | |
| Notes | 1 woman's data not analysed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...concealed cards allocated at random by a physician not involved in study enrolment..." |
| Allocation concealment (selection bias) | Unclear risk | "Women were randomised using numbered sealed envelopes containing concealed cards allocated at random by a physician not involved in study enrolment". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The 2 experienced ECV‐performing obstetricians were not blinded. Women could not be blinded either |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding attempted |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman (in analgesia group) refused ECV after randomisation. 1 woman randomly assigned to receive spinal analgesia did not receive the intended treatment, as the anaesthetist was unable to locate the dura (her ECV was unsuccessful, but she was analysed as intention‐to‐treat in the spinal analgesia group) 2 women with breech presentation in consecutive pregnancies were enrolled twice in the current study. A further analysis without these women was performed to exclude potential bias for the primary outcome. The success of ECV with spinal analgesia excluding the repeat data was 23 of 27 (85.1%) vs 19 of 33 (57.5%) without analgesia (P value < 0.02) None of this would be sufficient to have an impact on the analysis |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Similar maternal age, GA, weight at time of ECV and height. Similar too in terms of breech in past pregnancy, EFW, AFI. Study authors report there was no difference according to parity in the rate of successful ECV within intention‐to‐treat groups. However, it should be noted that for parity 1, 13 were included in the spinal analgesia group and 21 in the no analgesia group. |
Yanny 2000.
| Methods | RCT. | |
| Participants | Inclusion criteria: women with breech presentation choosing ECV, cardiotocograph and US examination acceptable, failed initial ECV attempt without tocolysis. N = 57. | |
| Interventions |
Intervention:tocolytic: glycerol trinitrate/nitroglycerine ‐ nitric oxide donor (A3) ‐ sublingual. Glyceryl trinitrate sublingual spray 800 µg. N = 31. Comparison:placebo. N = 26. Labelled sprays A and B; repeat ECV attempt; if unsuccessful and uterus not relaxed, salbutamol infusion and repeat ECV attempt. |
|
| Outcomes | Side effects: maternal discomfort; blood pressure; pulse, after spray administration; ECV success; uterine relaxation (poor 8/30 nitroglycerine vs 9/25 placebo, reasonable 11/30 vs 8/25, good 7/30 vs 8/25, excellent 4/30 vs 0/25); salbutamol required (13/31 vs 14/26); dose of salbutamol. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Used opaque sealed envelopes, but no information as to whether they were sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions were reported after randomisation, and no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Low risk | Baseline characteristics for the women were similar between the 2 groups in maternal age, parity, extended legs and liquor volume. No other biases were apparent. |
AFI: amniotic fluid index. BMI: body mass index. CI: confidence interval. CS: caesarean section. CSE: combined spinal epidural. CTG: cardiotocography. ECV: external cephalic version. EFW estimated fetal weight. GA: gestational age. IUGR: intrauterine growth restriction. IV: intravenous. IVI: intravenous infusion. min(s): minute(s). NST: non‐stress test. OR: odds ratio. RCT: randomised controlled trial. SD: standard deviation. SQ: subcutaneous. US: ultrasound. vs: versus.
A: Tocolytic drugs: A1 ‐ beta stimulants; A2 ‐ calcium channel blockers; A3 ‐ nitric oxide donors. B: Vibroacoustic stimulation. C: Regional analgesia. D: Amnioinfusion. E: Systemic opioids.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dockeray 1984 | Non‐randomised follow‐up study comparing outcomes of patients who had an ECV vs patients who had a breech vaginal delivery. |
| Guittier 2013 | This was not a randomised trial. 63 women undergoing ECV under hypnosis between 2010 and 2013 were compared with 122 women receiving standard care between 2005 and 2008. |
| Wallace 1984 | Non‐randomised follow‐up study after randomised trial of ECV with tocolysis. |
ECV: external cephalic version.
Characteristics of studies awaiting assessment [ordered by study ID]
Andarsio 2000.
| Methods | RCT. |
| Participants | Inclusion criteria: women undergoing ECV attempt. Unit: individual women. N = 35 women included. |
| Interventions |
Intervention:tocolysis: nitroglycerine ‐ nitric oxide donor (A3) ‐ no route reported. Nitroglycerine: only abstract available, no dose or route of administration given. N = 18. Comparison:tocolysis: terbutaline ‐ beta stimulant (A1) ‐ no route reported. Terbutaline: only abstract available, no dose or route of administration given. N = 17. |
| Outcomes | ECV success. |
| Notes | Preliminary abstract report only reviewed. This study was included in the previous publication (Cluver 2012), but we cannot include in this update until we have information on the routes of administration used. We are writing to study authors to request this information. |
Hollard 2003.
| Methods | RCT. |
| Participants | Inclusion criteria: normal singleton breech pregnancy, gestational age 36 weeks or more, intact membranes, not in labour. N = 36. |
| Interventions |
Intervention:regional analgesia (C). 1000 mL IVI prehydration and intrathecal injection of 6 mg 2% lidocaine with 15 mcg fentanyl. Followed by the same protocol as comparison group. N = 19. Comparison:no regional analgesia + tocolytic. 0.25 mg SQ terbutaline and ECV attempted by a MFM physician. N = 17. |
| Outcomes | Maternal pain (reduced in spinal analgesia group) and satisfaction (no difference) on visual scale; ECV success. |
| Notes | January 1998 to January 2003. It is unclear whether both groups received terbutaline or just the comparison group. We are writing to study authors to clarify this and other details of the study. |
Tan 2008.
| Methods | Double‐blind RCT. |
| Participants | Women with a singleton baby in the breech position. Gestation ≥ 36 weeks (check for early confirmation of gestational age), intact membranes and assuring fetal status on cardiotocograph. N = at least 103 women. |
| Interventions | 250 µg or 500 µg of bolus subcutaneous terbutaline followed by ECV 15 minutes later with a maximum of 2 attempts. |
| Outcomes | Primary: immediate success rate of ECV; caesarean section; cephalic presentation at birth. Secondary: post‐ECV cardiotocograph abnormalities; neonatal nursery admission; Apgar score at 5 minutes; umbilical cord arterial blood, pH; adverse drug events; visual analogue scale satisfaction score with ECV; indication for operative delivery. |
| Notes | Study reported as completed, but no information or data available as yet. |
ECV: external cephalic version. IVI: intravenous infusion. RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Burgos 2012.
| Trial name or title | Open randomised controlled trial to evaluate the efficacy and safety of remifentanil versus nitrous oxide in external cephalic version at term in singleton pregnancy in breech presentation (REMIVER). |
| Methods | Single‐centre randomised parallel‐group controlled trial. Analysis by intention‐to‐treat. |
| Participants | Women 18 to 65 with term pregnancy, singleton pregnancy in breech position (estimated enrolment: 180 women). Setting: hospital in Spain. |
| Interventions | Remifentanil vs inhaled nitrous oxide. |
| Outcomes | Rate of successful ECV, analgesic effect, safety, caesarean rates, acceptability of procedures to the women. |
| Starting date | July 2012 (expected final data collection date: July 2013). |
| Contact information | Jorge Burgos, jburgoss@sego.es |
| Notes |
Passerini 2013.
| Trial name or title | Maternal oral hydration and external cephalic version. |
| Methods | Randomised trial. |
| Participants | 164 pregnant women over 18 years of age with breech presentation at term. |
| Interventions | Women in the intervention will be asked to drink 2 litres of water in 2 hours; the control group will receive no intervention. |
| Outcomes | Successful external cephalic version, amniotic fluid volume, type of birth. |
| Starting date | October 2011 (expected final data collection date: January 2014). |
| Contact information | virna.zobbi@unimib.it |
| Notes |
ECV: external cephalic version.
Differences between protocol and review
We have allocated all outcomes to be primary or secondary outcomes. We have included further interventions examined in recent trials in addition to those originally prespecified in the protocol. We have added an outcome ‐ cephalic vaginal birth not achieved (caesarean section + vaginal breech births) ‐ to enhance consistency with the findings of other related reviews. Additional outcomes are reported that were not specified in the protocol: vaginal breech birth, Apgar less than seven at five minutes, neonatal seizures, admission to neonatal unit, birth trauma, flushing in women, placental abruption, maternal discomfort, pain scores, maternal satisfaction with the procedure and maternal side effects (nausea and vomiting, dizziness and drowsiness).
Contributions of authors
G. Justus Hofmeyr (JH) prepared the original version of the review. Gill Gyte (GG) and JH revised the review in 2004. Cathy Cluver (CC), JH, Marlene Sinclair (MS) and GG revised the review in 2011, and CC and MS undertook data extraction. JH, CC and GG entered and checked data. CC, JH, MS and Therese Dowswell (TD) revised the review in 2014. TD and GG undertook data extraction and checked the data entered. CC, JH and GG are responsible for editing the review and maintaining it.
Sources of support
Internal sources
University of the Witwatersrand, South Africa.
University of Fort Hare, South Africa.
The University of Liverpool, UK.
External sources
South African Medical Research Council, South Africa.
UNDP/UNFPA/WHO/World Bank (HRP), Switzerland.
-
National Institute for Health Research (NIHR), UK.
NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines
Declarations of interest
TD is paid by the UK NHS to work on a range of Cochrane Reviews. The Funders have no influence on the content or conclusions of the reviews I work on. I have received payment from NIHR for my work on this and other reviews.
GJH receives royalties for two chapters authored in UpToDate.
Edited (no change to conclusions)
References
References to studies included in this review
Bujold 2003a {published data only}
- Bujold E, Boucher M, Rinfret D, Berman S, Ferreira E, Marquette GP. Sublingual nitroglycerin versus placebo as a tocolytic for external cephalic version: a randomized controlled trial in parous women. American Journal of Obstetrics and Gynecology 2003;189:1070‐3. [DOI] [PubMed] [Google Scholar]
- Bujold E, Boucher M, Rinfret D, Marquette G. Sublingual nitroglycerin versus placebo as a tocolytic for external cephalic version: a randomized controlled trial in parous women [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S103. [DOI] [PubMed] [Google Scholar]
Bujold 2003b {published data only}
- Bujold E, Marquette GP, Ferreira E, Gauthier RJ, Boucher M. Sublingual nitroglycerin versus intravenous ritodrine as tocolytic for external cephalic version: a double‐blind randomized trial. American Journal of Obstetrics and Gynecology 2003;188(6):1454‐7; discussion 1457‐9. [DOI] [PubMed] [Google Scholar]
Chung 1996 {published data only}
- Chung T, Neale E, Lau TK, Rogers M. A randomized, double blind, controlled trial of tocolysis to assist external cephalic version in late pregnancy. Acta Obstetricia et Gynecologica Scandinavica 1996;75:720‐4. [DOI] [PubMed] [Google Scholar]
- Neale EJ, Lau TK, Chung A, Cohn M, Baldwin S, Rogers M. A randomized double blind controlled trial of tocolysis to assist external cephalic version in late pregnancy. 27th British Congress of Obstetrics and Gynaecology; 1995 July 4‐7; Dublin, Ireland. 1995:76.
Collaris 2009 {published data only}
- Collaris R, Tan PC. Oral nifedipine versus subcutaneous terbutaline tocolysis for external cephalic version: a double‐blind randomised trial. BJOG: an international journal of obstetrics and gynaecology 2009;116(1):74‐81. [DOI] [PubMed] [Google Scholar]
Delisle 2001 {published data only}
- Delisle MF, Kamani A, Douglas J, Bebbington M. Antepartum external cephalic version under spinal anesthesia: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S115. [Google Scholar]
Dugoff 1999 {published data only}
- Dugoff L, Jones OW, Stamm C, Mohling S, Hawkins J. A prospective, randomized trial evaluating the use of spinal anesthesia in external cephalic version. American Journal of Obstetrics and Gynecology 1998;178(1):S71. [DOI] [PubMed] [Google Scholar]
- Dugoff L, Stamm CA, Jones OW, Mohling SI, Hawkins JL. The effect of spinal anesthesia on the success rate of external cephalic version: a randomized trial. Obstetrics & Gynecology 1999;93(3):345‐9. [DOI] [PubMed] [Google Scholar]
El‐Sayed 2004 {published data only}
- El‐Sayed Y, Chitkara U, Reley ET, Cohen SE, Holbrook RH, Druzin M. Nitroglycerin versus terbutaline for external cephalic version. American Journal of Obstetrics and Gynecology 1998;178(1):S71. [DOI] [PubMed] [Google Scholar]
- El‐Sayed YY, Pullen K, Riley ET, Lyell D, Druzin ML, Cohen SE, et al. Randomized comparison of intravenous nitroglycerin and subcutaneous terbutaline for external cephalic version under tocolysis. American Journal of Obstetrics and Gynecology 2004;191:2051‐5. [DOI] [PubMed] [Google Scholar]
Fernandez 1997 {published data only}
- Fernandez CO, Bloom S, Wendel G. A prospective, randomized, blinded comparison of terbutaline versus placebo for singleton, term external cephalic version. American Journal of Obstetrics and Gynecology 1996;174:326. [Google Scholar]
- Fernandez CO, Bloom SL, Smulian JC, Ananth CV, Wendel GD Jr. A randomized placebo‐controlled evaluation of terbutaline for external cephalic version. Obstetrics & Gynecology 1997;90(5):775‐9. [DOI] [PubMed] [Google Scholar]
Hilton 2009 {published data only}
- Hilton J. Intravenous nitroglycerin for external cephalic versions trial (INVERT): a randomized, double‐blinded placebo‐controlled trial. JOGC: Journal of Obstetrics and Gynaecology Canada 2007;29(6 Suppl 1):S16. [Google Scholar]
- Hilton J, Allan B, Swaby C, Wah R, Jarrell J, Wood S, et al. Intravenous nitroglycerin for external cephalic version: a randomized controlled trial. Obstetrics & Gynecology 2009;114(3):560‐7. [DOI] [PubMed] [Google Scholar]
Impey 2005 {published data only}
- Impey L, Pandit M. Tocolysis for repeat external cephalic version in breech presentation at term: a randomised, double‐blinded, placebo‐controlled trial. BJOG: an international journal of obstetrics and gynaecology 2005;112:627‐31. [DOI] [PubMed] [Google Scholar]
Johnson 1995 {published data only}
- Johnson RL, Elliot JP. Fetal acoustic stimulation, an adjunct to external cephalic version: a blinded, randomized crossover study. American Journal of Obstetrics and Gynecology 1995;173:1369‐72. [DOI] [PubMed] [Google Scholar]
Kok 2008 {published data only}
- Kok M. Management of breech presentation: external cephalic version with tocolysis: a multicentre randomised controlled trial, 2005. Netherlands Trial Register (http://www.trialregister.nl ) (accessed 1 November 2005).
- Kok M, Bais JM, Lith JM, Papatsonis DM, Kleiverda G, Hanny D, et al. Nifedipine as a uterine relaxant for external cephalic version: a randomized controlled trial. Obstetrics & Gynecology 2008;112(2 Pt 1):271‐6. [DOI] [PubMed] [Google Scholar]
Mancuso 2000 {published data only}
- Mancuso KM, Yancey MK, Murphy JA, Markenson GR. Epidural analgesia for cephalic version: a randomized trial. Obstetrics & Gynecology 2000;95(5):648‐51. [DOI] [PubMed] [Google Scholar]
Marquette 1996 {published data only}
- Marquette GP, Boucher M, Theriault D, Rifret D. Does the use of a tocolytic agent affect the success rate of external cephalic version?. American Journal of Obstetrics and Gynecology 1996;175:859‐61. [DOI] [PubMed] [Google Scholar]
Munoz 2014 {published data only}
- Munoz H, Guerra S, Perez‐Vaquero P, Valero C, Aizpuru F, Lopez‐Picado A. Remifentanil versus placebo for analgesia during external cephalic version: a randomised clinical trial. International Journal of Obstetric Anesthesia 2014;23(1):52‐7. [DOI] [PubMed] [Google Scholar]
- Valero CA. Remifentanil versus paracetamol for pain treatment external cephalic versions, 2010. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 May 2013).
Nor Azlin 2005 {published data only}
- Nor Azlin MI, Haliza H, Mahdy ZA, Anson I, Fahya MN, Jamil MA. Tocolysis in term breech external cephalic version. International Journal of Gynecology & Obstetrics 2005;88:5‐8. [DOI] [PubMed] [Google Scholar]
Nor Azlin 2008 {published data only}
- Nor Azlin MI, Maryasalwati I, Norzilalwati MN, Zaleha AM, Mohammad AJ, Zainul RMR. Nifedipine versus terbutaline for tocolysis in external cephalic version. International Journal of Gynecology & Obstetrics 2008;102(3):263‐6. [DOI] [PubMed] [Google Scholar]
Reinhard 2012 {published data only}
- Reinhard J, Peiffer S, Reichenbach L, Tottel E, Reitter A, Sinanovic B, et al. The effects of clinical hypnosis versus neuro‐linguistic programming (NLP) before external cephalic version (ECV) ‐ a prospective off‐centre randomised double blind controlled trial. Archives of Gynecology and Obstetrics 2012;286(Suppl 1):S213‐S214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard J, Peiffer S, Reichenbach L, Tottel E, Reitter A, Yuan J, et al. Clinical hypnosis before external cephalic version. Archives of Gynecology and Obstetrics 2012;286(Suppl 1):S215. [Google Scholar]
- Reinhard J, Peiffer S, Snger N, Herrmann E, Yuan J, Louwen F. The effects of clinical hypnosis versus neurolinguistic programming (NLP) before external cephalic version (ECV): a prospective off‐centre randomised, double‐blind, controlled trial. Evidence‐based Complementary and Alternative Medicine 2012;2012:Article ID 626740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard J, Sanger N, Hanker L, Reichenbach L, Yuan J, Herrmann E, et al. Delivery mode and neonatal outcome after a trial of external cephalic version (ECV): a prospective trial of vaginal breech versus cephalic delivery. Archives of Gynecology and Obstetrics 2013;287(4):663‐8. [DOI] [PubMed] [Google Scholar]
Robertson 1987 {published data only}
- Robertson AW, Kopelman JN, Read JA, Duff P, Magelssen DJ, Dashow EE. External cephalic version at term: is a tocolytic necessary?. Obstetrics & Gynecology 1987;70:896‐9. [PubMed] [Google Scholar]
Schorr 1997 {published data only}
- Schorr SJ, Speights SE, Ross EL, Bofill JA, Rust OA, Norman PF, et al. A randomized trial of epidural anesthesia to improve external cephalic version success. American Journal of Obstetrics and Gynecology 1997;177(5):1133‐7. [DOI] [PubMed] [Google Scholar]
Stock 1993 {published data only}
- Stock A, Chung T, Rogers M, Ming WW. Comparison of placebo, ritodrine and hexoprenaline for external cephalic version at term. Proceedings of 2nd International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1993 Sept 7‐10; Hong Kong. 1993:141.
- Stock A, Chung T, Rogers M, Ming WW. Randomized, double blind, placebo controlled comparison of ritodrine and hexoprenaline for tocolysis prior to external cephalic version at term. Australian and New Zealand Journal of Obstetrics and Gynaecology 1993;3:265‐8. [DOI] [PubMed] [Google Scholar]
Sullivan 2009 {published data only}
- Bauchat JR, Grobman WA, Sullivan JT, McCarthy RJ, Fitzgerald PC, Wong CA. Effect of combined spinal‐epidural analgesia versus systemic opioid analgesia on fetal heart rate for external cephalic version. Anesthesiology 2007;106(Suppl 1):17. [Google Scholar]
- Sullivan JT, Bauchat JR, Grobman WA, McCarthy RJ, Wong CA. Impact of CSE versus systemic opioid on fetal heart rate pattern during external cephalic version. Anesthesiology 2007;107:Abstract no: A772. [Google Scholar]
- Sullivan JT, Grobman WA, Bauchat JR, Scavone BM, Grouper S, McCarthy RJ, et al. A randomized controlled trial of the effect of combined spinal‐epidural analgesia on the success of external cephalic version for breech presentation. International Journal of Obstetric Anesthesia 2009;18(4):328‐34. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Patel R, Robles C, Grouper S, Wong CA. The impact of combined spinal‐epidural analgesia on success of external cephalic version. Anesthesiology 2006;105:A917. [Google Scholar]
- Sullivan JT, Scavone BM, Grouper S, Patel R, Robles C, McCarthy RJ, et al. A randomized controlled trial of the impact of combined spinal‐epidural analgesia on the success of external cephalic version for breech presentation. Anesthesiology 2006;104(Suppl 1):10. [DOI] [PubMed] [Google Scholar]
Tan 1989 {published data only}
- Tan GWT, Jen SW, Tan SL, Salmon YM. A prospective randomised controlled trial of external cephalic version comparing two methods of uterine tocolysis with a non‐tocolysis group. Singapore Medical Journal 1989;30:155‐8. [PubMed] [Google Scholar]
Vallikkannu 2014 {published data only}
- Vallikkannu N, Nadzratulaiman WN, Omar SZ, Si Lay K, Tan PC. Talcum powder or aqueous gel to aid external cephalic version: a randomised controlled trial. BMC Pregnancy and Childbirth 2014;14(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vani 2009 {published data only}
- Vani S, Lau SY, Lim BK, Omar SZ, Tan PC. Intravenous salbutamol for external cephalic version. International Journal of Gynecology & Obstetrics 2009;104(1):28‐31. [DOI] [PubMed] [Google Scholar]
Weiniger 2007 {published data only}
- Weiniger CF. Spinal analgesia versus no analgesia: study for external cephalic version (ongoing trial), 2006. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006).
- Weiniger CF, Ginosar Y, Elchalal U, Sharon E, Nokrian M, Ezra Y. External cephalic version for breech presentation with or without spinal analgesia in nulliparous women at term: a randomized controlled trial. Obstetrics & Gynecology 2007;110(6):1343‐50. [DOI] [PubMed] [Google Scholar]
- Weiniger CF, Ginosaur Y, Elchalal U, Einav S, Nucrietin M, Guage P, et al. Prospective randomised study of external cephalic version for breech presentation at term in nulliparous women: spinal analgesia versus no analgesia. International Journal of Obstetric Anesthesia 2007;16(Suppl 1):S21. [Google Scholar]
Weiniger 2010 {published data only}
- Weiniger C, Ginosar Y, Sela H, Elchalal U, Weissman C, Ezra Y. External cephalic version at term in multiparous women with or without spinal analgesia: randomized controlled trial. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S34. [Google Scholar]
- Weiniger CF, Ginosar Y, Elchalal U, Sela HY, Weissman C, Ezra Y. Randomized controlled trial of external cephalic version in term multiparae with or without spinal analgesia. British Journal of Anaesthesia 2010;104(5):613‐8. [DOI] [PubMed] [Google Scholar]
Yanny 2000 {published data only}
- Yanny H, Johanson R, Baldwin K, Lucking L, Fitzpatrick R, Jones P. Double‐blind randomised controlled trial of glyceryl trinitrate spray for external cephalic version. British Journal of Obstetrics and Gynaecology 2000;107:562‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Dockeray 1984 {published data only}
- Dockeray CJ, Gleeson RP. An evaluation of perinatal outcome following external cephalic version under general anaesthesia. Irish Journal of Medical Science 1984; Vol. 153:325.
Guittier 2013 {published data only}
- Guittier MJ, Guillemin F, Farinelli EB, Irion O, Boulvain M, Tejada BM. Hypnosis for the control of pain associated with external cephalic version: a comparative study. Journal of Alternative & Complementary Medicine 2013;19(10):820‐6. [DOI] [PubMed] [Google Scholar]
Wallace 1984 {published data only}
- Wallace RL, Dorsten JP, Eglinton GS. External cephalic version with tocolysis. Observations and continuing experience at the Los Angeles County/University of Southern California Medical Center. Journal of Reproductive Medicine 1984;29:745‐8. [PubMed] [Google Scholar]
References to studies awaiting assessment
Andarsio 2000 {published data only}
- Andarsio F, Feng TI. External cephalic version: nitroglycerin versus terbutaline. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S161. [Google Scholar]
Hollard 2003 {published data only}
- Hollard A, Lyons C, Rumney P, Hunter M, Reed E, Nageotte M. The effect of intrathecal anesthesia on the success of external cephalic version (ECV). American Journal of Obstetrics and Gynecology 2003;189(6):S140. [Google Scholar]
Tan 2008 {published data only}
- Tan PC. A double‐blind randomised trial of 250 µg versus 500 µg bolus dose of terbutaline as a tocolytic agent in external cephalic version, 2008. Current Controlled Trials (www.controlled‐trials.com) (accessed 12 May 2010).
References to ongoing studies
Burgos 2012 {published data only}
- Burgos J. Open randomized controlled trial to evaluate the efficacy and safety of remifentanil versus nitrous oxide in external cephalic version at term in singleton pregnancy in breech presentation (REMIVER), 2012. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 May 2013).
- Del‐Rey L. Clinical trial to evaluate the efficacy and safety of two drugs for pain relieve for pregnant women whose fetus is breech, to attempt to turn the baby, 2011. EU Clinical Trials Register (accessed 31 May 2013).
Passerini 2013 {published data only}
- Passerini CG. Maternal oral hydration and external cephalic version, 2013. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 5 February 2014).
Additional references
Belfort 1993
- Belfort MA. Intravenous nitroglycerin as a tocolytic agent for intrapartum external cephalic version. South African Medical Journal 1993;83:656. [PubMed] [Google Scholar]
Benifla 1995
- Benifla JL, Goffinet F, Bascou V, Darai E, Proust A, Madelenat P. Transabdominal amnio‐infusion facilitates external version maneuver after initial failure. Six successful attempts. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1995;24:319‐22. [PubMed] [Google Scholar]
Bradley‐Watson 1975
- Bradley‐Watson PJ. The decreasing value of external cephalic version in modern obstetric practice. American Journal of Obstetrics and Gynecology 1975;123:237‐40. [DOI] [PubMed] [Google Scholar]
Bricker 2002
- Bricker L, Lavender T. Parenteral opioids for labor pain relief: a systematic review. American Journal of Obstetrics and Gynecology 2002;186(5 Suppl):S94‐S109. [DOI] [PubMed] [Google Scholar]
Brosset 1956
- Brosset A. The value of prophylactic external version in cases of breech presentation. Acta Obstetricia et Gynecologica Scandinavica 1956;35:555‐62. [DOI] [PubMed] [Google Scholar]
Carlan 1994
- Carlan SJ, Dent JM, Huckaby T, Whittington EC, Shaefer D. The effect of epidural anesthesia on safety and success of external cephalic version at term. Anesthesia and Analgesia 1994;79:525‐8. [DOI] [PubMed] [Google Scholar]
Coyle 2012
- Coyle ME, Smith CA, Peat B. Cephalic version by moxibustion for breech presentation. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD003928.pub3] [DOI] [Google Scholar]
Danielian 1996
- Danielian PJ, Wang J, Hall MH. Long term outcome by method of delivery of fetuses in breech presentation at term: population based follow up. BMJ 1996;312:1451‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grade 2014 [Computer program]
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2014. McMaster University, 2014.
Hickok 1992
- Hickok DE, Gordon DC, Milberg JA, Williams MA, Daling JR. The frequency of breech presentation by gestational age at birth: a large population‐based study. American Journal of Obstetrics and Gynecology 1992;166(3):851‐2. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hofmeyr 1989
- Hofmeyr GJ. Breech presentation and abnormal lie in late pregnancy. In: Chalmers I, Enkin MW, Keirse MJNC editor(s). Effective Care in Pregnancy and Childbirth. Oxford: Oxford University Press, 1989:653‐65. [Google Scholar]
Hofmeyr 1991
- Hofmeyr GJ. External cephalic version at term: how high are the stakes?. British Journal of Obstetrics and Gynaecology 1991;98:1‐3. [DOI] [PubMed] [Google Scholar]
Hofmeyr 1992
- Hofmeyr GJ. Breech presentation and shoulder dystocia in childbirth. Current Opinion in Obstetrics and Gynecology 1992;4:807‐12. [PubMed] [Google Scholar]
Hofmeyr 1993
- Hofmeyr GJ. External cephalic version at term. Fetal Maternal Medicine Review 1993;5:213‐22. [Google Scholar]
Hofmeyr 1996
- Hofmeyr GJ, Kulier R. External cephalic version for breech presentation at term. Cochrane Database of Systematic Reviews 1996, Issue 1. [DOI: 10.1002/14651858.CD000083] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2002
- Hofmeyr GJ, Gülmezoglu AM. Maternal hydration for increasing amniotic fluid volume in oligohydramnios and normal amniotic fluid volume. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD000134] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2003
- Hofmeyr GJ, Hannah ME. Planned caesarean section for term breech delivery. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD000166] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2012a
- Hofmeyr GJ, Kulier R. External cephalic version for breech presentation at term. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD000083.pub2] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2012b
- Hofmeyr GJ, Kulier R. Cephalic version by postural management for breech presentation. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD000051.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmeyr 2014
- Hofmeyr GJ. External cephalic version. UpToDate, Waltham, Post TW (Ed), UptoDate, MA (Accessed October 2014).
Hutton 2006
- Hutton EK, Hofmeyr GJ. External cephalic version for breech presentation before term. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD000084.pub2] [DOI] [PubMed] [Google Scholar]
Lau 1997
- Lau TK, Lo KW, Wan D, Rogers MS. Predictors of successful external cephalic version at term: a prospective study. British Journal of Obstetrics and Gynaecology 1997;104(7):798‐802. [DOI] [PubMed] [Google Scholar]
Neiger 1998a
- Neiger R, Hennessy M, Patel M. Reattempting failed external cephalic version under epidural anesthesia. American Journal of Obstetrics and Gynecology 1998;178(1):S71. [DOI] [PubMed] [Google Scholar]
Neiger 1998b
- Neiger R, Hennessy MD, Patel M. Reattempting failed external cephalic version under epidural anesthesia. American Journal of Obstetrics and Gynecology 1998;179(5):1136‐9. [DOI] [PubMed] [Google Scholar]
Reddick 1997
- Reddick LF, Livingston E, Bell E. Sublingual aerosol nitroglycerin for uterine relaxation in attempted external version. American Journal of Obstetrics and Gynecology 1997;176:496‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [PUBMED: 19839216] [DOI] [PubMed] [Google Scholar]
Smith 2000
- Smith P, Anthony J, Johanson R. Nifedipine in pregnancy. British Journal of Obstetrics and Gynaecology 2000;107(3):299‐307. [DOI] [PubMed] [Google Scholar]
Valero 2010
- Valero CA. Remifentanil versus paracetamol for pain treatment external cephalic versions, 2010. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 May 2013).
Whyte 2004
- Whyte H, Hannah ME, Saigal S, Hannah WJ, Hewson S, Amankwah K, et al. Outcomes of children at 2 years after planned cesarean birth versus planned vaginal birth for breech presentation at term: the International Randomized Term Breech Trial. American Journal of Obstetrics and Gynecology 2004;191(3):864‐71. [DOI] [PubMed] [Google Scholar]
Zhang 1993
- Zhang J, Bowes WA, Fortney JA. Efficacy of external cephalic version: a review. Obstetrics and Gynecology 1993;82:306‐12. [PubMed] [Google Scholar]
References to other published versions of this review
Cluver 2012
- Cluver CA, Hofmeyr GJ, Gyte GML, Sinclair M. Interventions for helping to turn term breech babies to head first presentation when using external cephalic version. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD000184.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmeyr 1995
- Hofmeyr GJ. Routine tocolysis for external cephalic version at term. [revised 04 October 1993]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Hofmeyr 2004
- Hofmeyr GJ, Gyte GML. Interventions to help external cephalic version for breech presentation at term. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD000184.pub2] [DOI] [PubMed] [Google Scholar]


