Abstract
Endothelial dysfunction results in chronic vascular inflammation, which is critical for the development of atherosclerotic diseases. Transcription factor Gata6 has been reported to regulate vascular endothelial cell activation and inflammation in vitro. Here, we aimed to explore the roles and mechanisms of endothelial Gata6 in atherogenesis.
Endothelial cell (EC) specific Gata6 deletion was generated in the ApoeKO hyperlipidemic atherosclerosis mouse model. Atherosclerotic lesion formation, endothelial inflammatory signaling, and endothelial-macrophage interaction were examined in vivo and in vitro by using cellular and molecular biological approaches. EC-GATA6 deletion mice exhibited a significant decrease in monocyte infiltration and atherosclerotic lesion compared to littermate control mice. Cytosine monophosphate kinase 2 (Cmpk2) was identified as a direct target gene of GATA6 and EC-GATA6 deletion decreased monocyte adherence, migration and pro-inflammatory macrophage foam cell formation through regulation of the CMPK2-Nlrp3 pathway. Endothelial target delivery of Cmpk2-shRNA by intercellular adhesion molecule 2 (Icam-2) promoter-driven AAV9 carrying the shRNA reversed the Gata6 upregulation mediated elevated Cmpk2 expression and further Nlrp3 activation and thus attenuated atherosclerosis. In addition, C–C motif chemokine ligand 5 (Ccl5) was also identified as a direct target gene of Gata6 to regulate monocyte adherence and migration influencing atherogenesis.
This study provides direct in vivo evidence of EC-GATA6 involvement in the regulation of Cmpk2-Nlrp3, as well as Ccl5, on monocyte adherence and migration in atherosclerosis development and advances our understanding of the in vivo mechanisms of atherosclerotic lesion development, and meanwhile provides opportunities for future therapeutic interventions.
Keywords: Atherosclerosis, Gata6 transcription factors, Endothelial cells, Cytosine monophosphate kinase 2 (Cmpk2), Nlrp3 inflammasome
Highlights
-
•
Elevated Gata6 expression in ECs of atherosclerotic lesions, and EC-Gata6 regulates the development of atherosclerosis.
-
•
EC-Gata6 regulates Cmpk2-Nlrp3 pathways resulting in monocyte recruitment and proinflammatory macrophage formation.
-
•
Icam-2 promoter driven AAV9 endothelial target delivery of Cmpk2-shRNA suppresses atherosclerotic lesion formation.
1. Introduction
Atherosclerosis has been recognized as a chronic inflammatory disease resulting in ischemia of the heart and brain and is the leading cause of death worldwide [1,2]. Activated dysfunctional endothelial cells (ECs) recruit leukocytes to the arterial wall leading to vascular wall inflammation which is responsible for the initiation and development of atherosclerosis.
EC dysfunction results in the earliest atherosclerotic lesion, such as the focal permeation, trapping and physicochemical modification of circulating lipoprotein particles in the sub-endothelial space [3,4]. This sets into motion a complex pathogenic sequence [1,3,4], initially involving the selective recruitment of circulating monocytes from the blood into the intima where they differentiate into macrophages and internalize modified lipoproteins to become foam cells, the hallmark of early fatty streak lesions. Thereafter, multiple chemokines and growth factors elaborated by activated endothelium and macrophages act on neighboring smooth muscle cells or their precursors [5] to induce their proliferation and synthesis of extracellular matrix components within the intimal compartment, thus generating a fibromuscular plaque. The final progressive structural vascular remodeling develops advanced atherosclerotic lesions with fibrous caps overlying lipid-rich, necrotic cores consisting of oxidized lipoproteins, cholesterol crystals and cellular debris accompanied by varying degrees of matrix remodeling and calcification. Given the importance of EC dysfunction in the initiation and development of atherosclerosis, a more thorough understanding of the molecular pathways that regulate this process is warranted.
Gata proteins are members of a zinc finger protein family of transcription factors that recognize the consensus (A/T)GATA(A/G) and related sequences [6]. Gata6 is highly expressed in the cardiovascular system and has been implicated in various developmental processes, including cell proliferation and differentiation [[6], [7], [8], [9], [10]]. Previous studies in vitro cultured ECs showed that EC activation was associated with Gata6 nuclear translocation, chromatin binding, and enhanced Gata6-dependent transcriptional activation of vascular cell adhesion molecule-1 (VCAM-1) [11,12], which recruit leukocytes to the arterial wall that is believed to be of primary importance in the pathogenesis of atherosclerosis. However, the direct effect of EC-Gata6 on atherogenesis in vivo has not been clarified.
To study in vivo function of Gata6 in atherosclerosis, endothelial-specific loss of Gata6 in the ApoeKO hyperlipidemic atherosclerosis mouse model was generated and the mutant mice displayed attenuated atherosclerotic lesion formation compared to the corresponding littermate control mice. Mechanism studies showed that EC-Gata6 deletion reduced monocyte adherence, migration and recruitment into the vascular wall. Cytosine (or deoxycytosine) monophosphate kinase 2 (Cmpk2), a family member of nucleotide kinases targeting mitochondria where it converts dCMP to dCDP and then converts to dCTP, was recently reported to be closely associated with NLRP3 inflammasome activation [13]. The NLRP3 inflammasome is implicated in several severe chronic inflammatory disorders such as atherosclerosis [[14], [15], [16]]. We identified Cmpk2, as well as chemokine Ccl5, as direct downstream targets of Gata6, and Cmpk2 acting on the Nlrp3 inflammasome and IL-1β production, together affecting monocyte adherence and migration for atherogenesis. Moreover, we constructed Icam-2 promoter-driven AAV9 target delivery of Cmpk2-shRNA into ECs to reverse the elevated expression of Cmpk2 and in turn Nlrp3 inflammasome activation mediated by EC-Gata6 upregulation in ApoeKO hyperlipidemic mice, and thus reduced atherosclerosis [17]. These data demonstrate an important role of EC-Gata6 in in vivo atherogenesis and uncover a Gata6-Cmpk2-Nlrp3 inflammasome activation and Gata6-Ccl5 as novel regulators of endothelial dysfunction in atherogenesis.
2. Material and methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
2.1. Generation of vascular EC-specific Gata6 loss-of-function in ApoeKO hyperlipidemic atherosclerosis mouse model
The conditional Gata6 knockout mice [18] were crossed with the EC-specific Cdh5CreERT2 line [19] to generate endothelial-specific loss of Gata6 in ApoeKO background, Gata6flox/flox;Cdh5CreERT2;ApoeKO abbreviated as Gata6ECKO;ApoeKO, and fed with a high-fat diet for 8 weeks for the hyperlipidemia atherosclerosis mouse model. Gata6wt/wt; Cdh5CreERT2;ApoeKO mice were used as control and abbreviated as WT; ApoeKO. All animal experiments were approved by the Tongji University Institutional Animal Care and Use Committee and conformed to the guidelines from Directive2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. Mice were sacrificed by an overdose of anesthesia with 1% pentobarbital sodium (100 mg/kg, i.p.) at the indicated time point. The primer sequences of mRNA expression in vivo/in vitro are listed in Online Table I, and the detailed protocols are described in Methods of the online-only Data Supplement. The data, methods used in the analysis, and materials used to conduct the research are available from the corresponding author on reasonable request.
2.2. Construct of Icam-2 promoter-driven AAV9 for target delivery of Cmpk2-shRNA specifically into ECs to evaluate the effects of EC-Gata6-Cmpk2 signal in atherogenesis of ApoeKO hyperlipidemia mice
An Icam-2 promoter-driven Cmpk2-shRNA fusion with GFP AAV9 was constructed for EC-targeted delivery of Cmpk2-shRNA to evaluate the effect of Cmpk2 inhibition on Gata6-dependent atherogenesis [17].
2.3. Statistical analysis
Data are presented as mean ± SEM for at least 3 or 5 independent assays unless otherwise noted. All data were first assessed for normality and equal variance. The D'Agostino-Pearson omnibus normality test was used to check for normality of data distribution. For data passed normality and equal variance, the student t-test was used for 2-sample comparisons; 1-way ANOVA with Tukey post hoc tests was used for comparisons between multiple groups. Nonparametric statistical test with Kruskal-Wallis with Dunn post hoc test or 2-tailed Mann-Whitney U test was performed for sample size less than 5 per group and data failed normality or equal variance test. Values of P < 0.05 were considered as statistically significant.
3. Results
3.1. Elevated Gata6 expression in human coronary endothelium with atherosclerotic plaques or in ApoeKO hyperlipidemic mouse artery and atherosclerotic prone endothelium
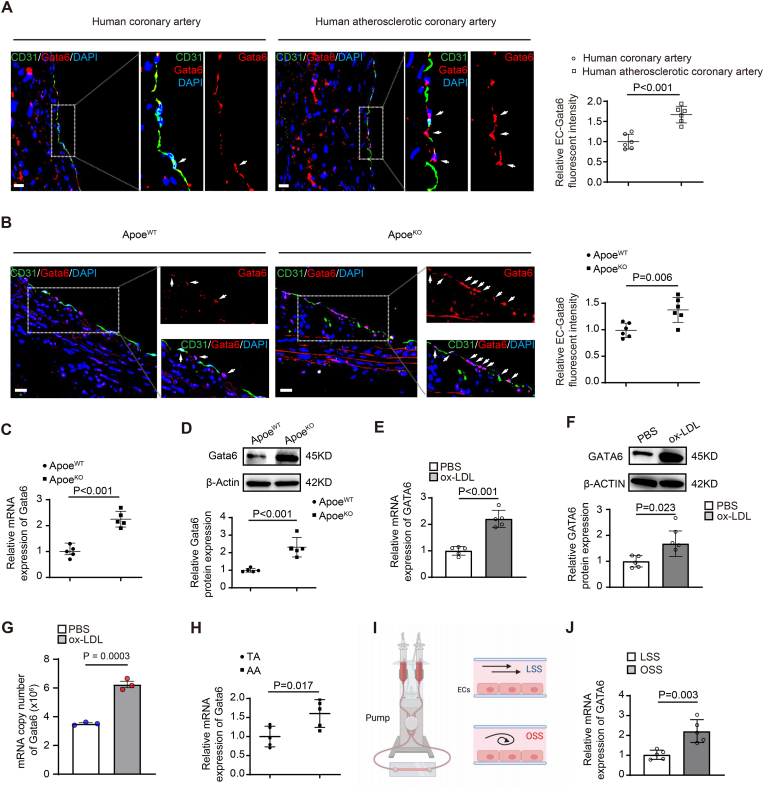
Gata6 is highly expressed in ECs of normal artery [10] and significantly elevated expression was seen in atherosclerotic endothelium of human coronary artery with atherosclerotic plaques (Fig. 1A) and by Co-immunostaining of Gata6 and endothelial marker CD31. Co-immunostaining of Gata6 and endothelial marker CD31 showed Gata6 expression in normal aortic ECs from ApoeWT mice, and the increased expression in the ECs from ApoeKO hyperlipidemic mice (Fig. 1B), which was confirmed by RT-qPCR (Fig. 1C) and Western blot (Fig. 1D). The elevated Gata6 expression in the mouse model was confirmed in human aortic ECs (HAECs) treated with oxidized low-density lipoprotein (oxLDL) by RT-qPCR (Fig. 1E), Western blot (Fig. 1F) and digital PCR (Fig. 1G).
Fig. 1.
Elevated Gata6 expression in the endothelium of atherosclerotic lesions of human coronary artery and Apoe knockout hyperlipidemic mouse artery. (A) The representative images of immunostaining of GATA6 in the endothelium of human coronary artery with or without atherosclerotic lesions by co-staining with EC marker CD31 (n = 6), with quantification data on the right and the arrows indicating Gata6 positive endothelial cells (ECs) (n = 6). (B–D) Gata6 expression in aortic ECs of Apoe knockout (ApoeKO) hyperlipidemic and ApoeWT mice by co-staining with EC marker CD31 (B) (n = 6), quantitative reverse transcription PCR (RT-qPCR) (C) and Western blot (D) (n = 5). (E–G) GATA6 expression in human aortic ECs (HAECs) treated with oxidized low density lipoprotein (oxLDL, 50 μg/ml for 48hr) or vehicle control by RT-qPCR (E), Western blot (F) with quantification data on the right (n = 5) and digital PCR (G) (n = 3). (H) Gata6 expression in aortic arch or thoracic arch ECs by RT-qPCR (n = 5). (I) Schematic diagrams on design of in vitro shear stress flow system and cross sections of cell chamber. (J) GATA6 expression in HAECs treated with oscillatory shear stress (OSS) or laminar shear stress (LSS) by RT-qPCR (n = 5). Quantification of Western blot was carried out with Image J and normalized to loading control β-actin. Unpaired 2-tailed student t-test for A-F, H, J and nonparametric statistical test for G. Scale bars: A, B, 100 μm.
Endothelial cells are critical sensors of shear stress and regulate vascular homeostasis. Branches and bends of arteries are exposed to low oscillatory shear stress (OSS), an environment that promotes vascular dysfunction and atherosclerosis, while physiologically high laminar shear stress (LSS) is protective [20]. Our further study showed elevated Gata6 expression in aortic arch ECs, compared with that in thoracic arch ECs (Fig. 1H), and the same elevated expression was confirmed in HAECs treated with oscillatory shear stress (Fig. 1I and J). These data suggest a possible role of endothelial Gata6 (EC-Gata6) in atherogenesis.
3.2. Loss of endothelial Gata6 attenuates atherosclerotic lesion formation with decreased macrophages in atherosclerotic lesion due to reduced monocyte adhesion and migration
To explore the role of EC-Gata6 in atherosclerotic lesion formation, we generated Gata6ECKO;ApoeKO mice, the specific EC-Gata6 deletion mice on an ApoeKO hyperlipidemic background. The significantly reduced Gata6 expression was confirmed in ECs of EC-Gata6 deletion mice by RT-qPCR (Fig. 2A) and Western blot (Fig. 2B). No significant alteration of body weight, plasma cholesterol were observed between Gata6ECKO;ApoeKO and littermate WT;ApoeKO mice after 8 weeks of high-fat western diet (Online Table IIA). Specific deletion of Gata6 in ECs caused significantly decreased atherosclerotic lesion percentages from 11.0 ± 1.0% (mean ± SEM) to 6.9 ± 0.6% (P < 0.05) as measured by Sudan IV-positive stained area of enface aorta compared with those of WT;ApoeKO mice (Fig. 2C). Similarly, Gata6ECKO;ApoeKO mutant mice exhibited decreased lesion area from 2.0 ± 0.2 × 105 μm [2] to 1.0 ± 0.1 × 105 μm [2] (P < 0.01) and percentage of lesion from 18.3 ± 1.6% to 10.7 ± 1.3% (P < 0.01) (Fig. 2D). There was no significant difference of atherosclerotic lesions in aorta enface and aortic root between male and female mice.
Fig. 2.
Endothelial Gata6 deletion reduces atherosclerotic lesions and lesional macrophages due to decreased macrophage adhesion, migration and reduced pro-inflammatory macrophage foam cell formation. (A–B) Gata6 expression of Gata6fl/fl;Cdh5-CreERT2;ApoeKO(Gata6ECKO;ApoeKO) mutant and Gata6WT/WT;Cdh5-CreERT2;ApoeKO (WT;ApoeKO) littermate wild-type mice by RT-qPCR (A) and Western blot (B) following tamoxifen treatment (100 mg/kg, i.p. every other day for total 4 times) (n = 5). (C–E) The representative images of Sudan IV staining of en face aorta (C), aortic root (D)and F4/80 immunostaining aortic root sections (E) in Gata6ECKO;ApoeKO and littermate wild-type mice following 8 weeks high-fat western diet with quantification data on the right (n = 12, 6 each for male and female mice per group). (F–G) GATA6 expression by RT-qPCR (F) and Western blot (G) with quantification data on the right in HAECs of GATA6-siRNA knockdown and scramble siRNA control(n = 5). (H–J) Adhesion of monocytes to GATA6-siRNA or scramble-siRNA ECs (H), monocyte migration in Boyden chamber assay (I) and macrophages engulf of Dil-labeled oxidized low-density lipoprotein (oxLDL) (J) after treatment of condition medium from GATA6-siRNA or scramble-siRNA ECs, with representative images on the left and quantitative data left (n = 5). (K) The expression of oxidized low density lipoprotein receptor 1 (OLR1), interleukin-1β (IL-1β) and monocyte chemoattractant protein-1 (MCP-1) in macrophages following treatment by oxLDL and condition medium from GATA6-siRNA or scramble-siRNA ECs. Quantification of Western blot was carried out with Image J and normalized to loading control β-actin. Unpaired 2-tailed student t-test for A, B and F–K and two-way ANOVA followed by Turkey post hoc test for C-E. Scale bars: C, 2 mm; D, 500 μm; E, H, I, 100 μm; J, 50 μm.
Next, we characterized the cellular composition of the atherosclerotic lesions by immunostaining of the aortic root, and the number of F4/80+-macrophages within the lesions was reduced from 11.9 ± 0.9% in littermate WT; ApoeKO mice to 7.7 ± 0.7% (P < 0.01) in Gata6ECKO;ApoeKO mutant mice at (Fig. 2E).
To explore the cellular mechanism for the decreased macrophages within the lesion, we constructed GATA6 knockdown HAECs and the knockdown efficiency was confirmed by RT-qPCR (Fig. 2F) and Western blot (Fig. 2G). And, we observed a significantly reduced adherence of THP-1, a human leukemia monocytic cell line labeled with fluorescent RFP-dye, to confluent HAECs with GATA6 was knockdown by siRNA (Fig. 2H, Online Fig. 1A). Furthermore, the adherence of THP-1 to HAECs was increased under oscillating shear stress condition, which was decreased in GATA6 knockdown HAECs (Online Fig. 1B).
The conditional medium from GATA6-siRNA knockdown HAECs significantly decreased monocyte/macrophage migration in the Boyden chamber assay (Fig. 2I). These results suggest that EC-Gata6 is important in the regulation of monocyte adhesion, migration and infiltration into the vessel wall during atherosclerotic lesion development.
3.3. Loss of endothelial GATA6 impedes macrophage phagocytosis and pro-inflammatory macrophage foam cell formation
Phagocytosis initiated by the atherogenic modified LDL through receptors such as scavenger receptors and oxidized low density lipoprotein receptor 1 (OLR1) on macrophages, which triggers a pro-inflammatory immune response and intracellular lipid accumulation, is an important step in foam cell formation and atherosclerosis development. To investigate the roles and mechanisms of EC-GATA6 in macrophage phagocytosis, we stimulated macrophages with EC-conditioned medium, and observed a significant decrease of proinflammatory M1 macrophage markers, interleukin-1β (IL-1β) and TNF-α (tumor necrosis factor-α) in macrophage, and no significant change of M2 macrophage markers (CD115, CD163) by RT-qPCR (Online Fig. 2). Moreover, the EC-conditioned medium from GATA6-knockdown HAECs impeded macrophage phagocytosis of oxLDL to form pro-inflammatory macrophages as demonstrated by reduced numbers of Dil-label oxLDL + macrophage (Fig. 2J), and also reduced expression of oxidized low density lipoprotein receptor 1 (OLR1), monocyte chemoattractant protein-1 (MCP1) (Fig. 2K), which are known to promote atherosclerosis.
3.4. Cmpk2 and Ccl5 are identified as possible Gata6 direct downstream target genes responsible for the EC-Gata6 upregulation-mediated atherosclerotic lesion development through regulation of monocyte/macrophage infiltration and proinflammatory macrophage formation
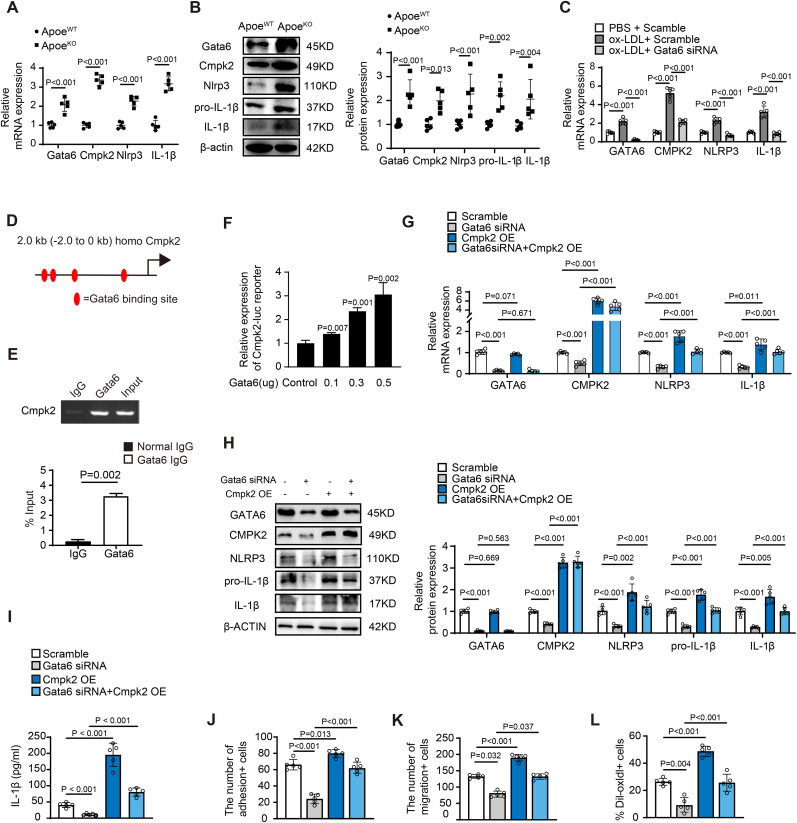
We performed RNA-sequencing using ECs of Gata6 mutant fluorescent reporter mice GATA6ECKO;R26RmT/mG and wild-type control littermates GATA6WT;Cdh5CreERT2;R26RmT/mG in our recent paper [21]. To investigate the potential target genes for EC-Gata6 upregulation-mediated atherogenesis, we re-analyzed the RNA-sequencing data and found a dramatically reduced expression of Cmpk2 in Gata6ECKO mutant mice.
Cmpk2, a family member of nucleotide kinases targeting to mitochondria where it converts dCMP to dCDP, and then to dCTP, was recently reported to be closely associated with NLRP3 inflammasome activation [13], while NLRP3 inflammasome activation and IL-1β production is implicated in atherogenesis [15,16]. Our data showed a significantly elevated expression of Gata6 in aortic ECs of ApoeKO hyperlipidemic mice and that similar increased expression of Cmpk2, and also Nlrp3 and IL-1β was observed by RT-qPCR (Fig. 3A) and Western blot (Fig. 3B) compared with that of littermate wild-type mice. Moreover, GATA6 expression levels varied in parallel with Cmpk2 when they were upregulated after treatment with oxLDL, and GATA6-siRNA knockdown reversed the oxLDL-induced upregulation of GATA6, then Cmpk2, Nlrp3 and IL-1β in HAECs (Fig. 3C), suggesting that Gata6 might regulate Cmpk2, then Nlrp3 and IL-1β expression and thereby influence atherogenesis.
Fig. 3.
Cmpk2 is identified as Gata6 direct downstream target gene in ECs for the regulation of monocyte adhesion, migration and pro-inflammatory macrophage formation. (A–C) The expression of Cytidine/uridine monophosphate kinase 2 (Cmpk2) by RT-qPCR (A) and Western blot (B) with quantification data on the right in aortic ECs of Gata6ECKO;ApoeKO hyperlipidemic and ApoeWT mice (n = 5), and in oxLDL stimulated HAECs of GATA6-siRNA knockdown and scramble siRNA control by RT-qPCR (C) (n = 5). (D) Sequence analysis of GATA6 binding sites (red oval) in the proximal promoter region of CMPK2 gene. (E) Chromatin immunoprecipitation (ChIP) assay of GATA6 and the promoter of CMPK2 gene with gel images on the top. (F) The luciferase activity of proximal-2kb Cmpk2 promoter with Gata6 binding sites by Gata6 expression vector in 293T cells (n = 3). (G–H) The expression of NLRP3 inflammasome components, NLRP3 and IL-1β, in oxLDL-treated ECs by RT-qPCR (G) and Western blot (H) with quantification data on the right, and the effects of GATA6-siRNA, CMPK2 vector for overexpression or the combination on NLRP3 inflammasome activation. (I) Secreted IL-1β level in conditioned medium of GATA6-siRNA or scramble-siRNA ECs by ELISA (n = 5). (J–L) The quantitative data of monocyte adhesion to GATA6-siRNA or scramble-siRNA ECs (J), monocyte migration in Boyden chamber assay (K) and macrophages engulf of Dil-labeled oxLDL (L) after treatment of condition medium from GATA6-siRNA or scramble-siRNA ECs (n = 5). Quantification of Western blot was carried out with Image J and normalized to loading control β-actin. Unpaired 2-tailed student t-test for A-C, G-L and nonparametric statistical test for F.
In order to validate whether Cmpk2 is a direct Gata6 target gene, we performed promoter sequence analysis and found 4 GATA6 binding sites in the proximal promoter region CMPK2 gene (Fig. 3D). Chromatin immunoprecipitation (ChIP) assay showed a direct association of GATA-6 with the CMPK2 promoter (Fig. 3E). To determine whether GATA-6 could directly activate Cmpk2 transcription, we cloned the Cmpk2 promoter sequence of -2kb–0 kb containing 4 Gata6 conserved binding sites into pGL3 basic luciferase reporter vector (Online Fig. 3) and the luciferase reporter assay showed that Gata6 activated the luciferase activity in a dose-dependent manner (Fig. 3F). Further RT-qPCR and Western blot study showed that GATA6-siRNA knockdown significantly reduced the oxLDL-induced elevated expression of GATA6 and further CMPK2, and in turn NLRP3 and IL-1β, while CMPK2 overexpression reversed the GATA6-siRNA knockdown mediated reduced expression of CMPK2, NLRP3 and IL-1β signal (Fig. 3G and H). We also observed a significant decrease in IL-1β secretion in condition medium from Gata6 knockdown ECs by Elisa, which was rescued by overexpression of Cmpk2 in HAECs (Fig. 3I). Moreover, we found a significant reversal of CMPK2 overexpression for GATA6-siRNA mediated reduction of monocyte adhesion to HAECs (Fig. 3J) and MOAECs (Online Fig. 4), monocyte migration (Fig. 3K) and pro-inflammatory foam cell formation (Fig. 3L). These results suggest that Cmpk2 is the Gata6 novel direct downstream target gene and elevated Cmpk2 expression by Gata6 upregulation activates the Nlrp3 inflammasome and increases IL-1β production contributing to atherosclerotic lesion development through regulation of monocyte adhesion, monocyte/macrophage migration and proinflammatory macrophage formation in ApoeKO hyperlipidemic mice.
Fig. 4.
Ccl5 is identified as Gata6 direct downstream target gene in ECs for the regulation of monocyte adhesion and migration. (A–C) The expression of chemokine Ccl5 in ECs of Gata6ECKO;ApoeKO hyperlipidemic and ApoeWT mice (A), in HAECs treated with oxLDL or vehicle (B) and HAECs with GATA6-siRNA and scramble siRNA (C) (n = 5). (D) The sequence analysis of GATA6 binding sites (red oval) in the proximal promoter region of CCL5 gene. (E) Chromatin immunoprecipitation (ChIP) assay of GATA6 and the promoter of CCL5 gene with gel images on the top. (F) The luciferase activity of proximal-2kb Ccl5 promoter with Gata6 binding sites by Gata6 expression vector (n = 3). (G–H) Adhesion of monocytes to GATA6-siRNA and scramble siRNA ECs (G) and monocyte migration in Boyden chamber assay after treatment with condition medium from GATA6-siRNA and scramble siRNA ECs (H), with representative image on the left and quantitative data right (n = 5). (I) Secreted CCL5 level in conditioned medium of GATA6-siRNA or scramble-siRNA ECs by ELISA (n = 5). Unpaired 2-tailed student t-test for A-C, E and G-I and nonparametric statistical test for F. Scale bars: G-H,100 μm
In addition to Gata6 mediated regulation of Cmpk2 expression associated with NLRP3 inflammasome activation leading to atherosclerosis, the C–C motif chemokine ligand 5 (Ccl5) has been widely established to be involved in the regulation of monocyte adhesion and migration contributing to the inflammatory recruitment of classical monocytes and vascular inflammation during the development of atherosclerosis [22,23]. There was a significantly elevated expression of Gata6 and Ccl5 in aortic ECs of ApoeKO mice compared with littermate wild-type mice (Fig. 4A), and also in HAECs treated with oxLDL compared with the vehicle control (Fig. 4B). GATA6-siRNA knockdown significantly reduced the GATA6 upregulation and further CCL5 expression (Fig. 4C). Sequence analysis showed GATA6 binding sites in the promoter region of CCL5 (Fig. 4D) and ChIP assay showed an association of GATA6 with the promoter of CCL5 (Fig. 4E). Luciferase assay confirmed that Gata6 expression vector dose-dependently activated the promoter of Ccl5 containing Gata6 binding sites (Fig. 4F). Moreover, CCL5 overexpression significantly reversed the GATA6-siRNA mediated reduction of monocyte adhesion to HAECs (Fig. 4G) and monocyte migration (Fig. 4H). We also observed a significant decrease in CCL5 secretion in condition medium from Gata6 knockdown ECs by Elisa, which was rescued by overexpression of CCL5 in HAECs (Fig. 4I). These results provide evidence that Ccl5 might also be a novel direct target gene of Gata6 contributing to atherogenesis through regulation of monocyte adhesion, monocyte/macrophage migration.
3.5. Icam-2 promoter-driven AAV9 target delivery of Cmpk2-shRNA into ECs for inhibiting EC-Gata6 upregulation mediated elevation of Cmpk2 expression to protect monocyte adherence and migration for atherogenesis in ApoeKO hyperlipidemic mice
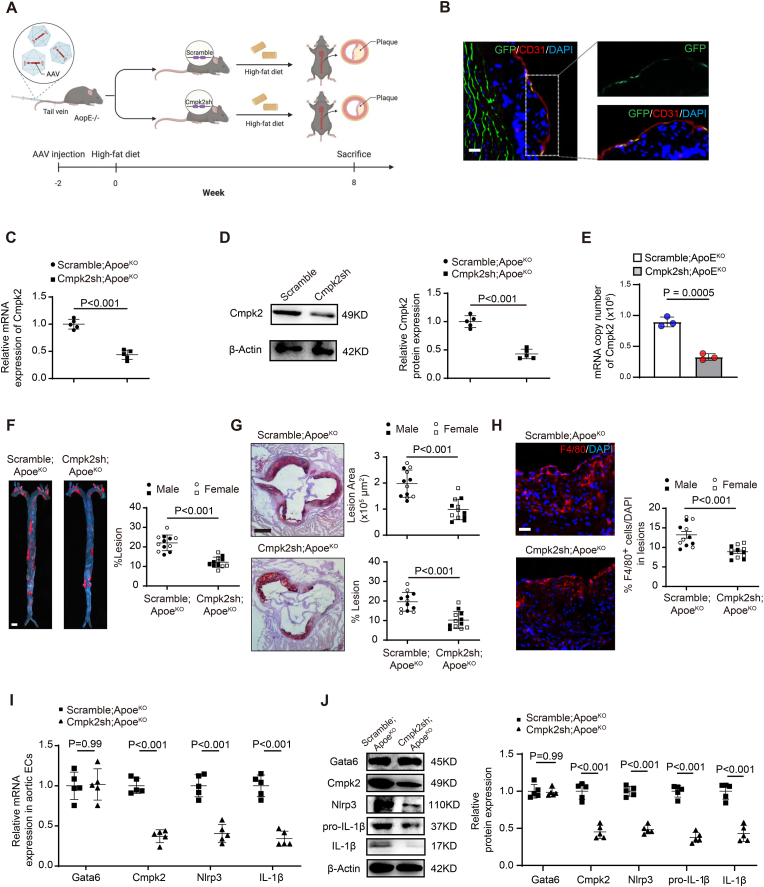
To clarify whether the EC-Gata6 mediated Cmpk2-Nlrp3 signal is required for atherosclerotic lesion development in ApoeKO hyperlipidemic mice, we constructed an intercellular adhesion molecule 2 (Icam-2)-promoter-driven and GFP fusion AAV9 carrying Cmpk2-shRNA to target deliver the shRNA specifically into ECs [17] (Fig. 5A). A significant GFP expression was observed specifically in the CD31-positive staining ECs of mouse aortas following intravenous injection of Icam-2 promoter-driven AAV9 (Fig. 5B) indicating that this Icam-2-AAV9 specifically entered into vascular ECs. Significant inhibition of Cmpk2 expression was confirmed by RT-PCR and Western blot in mouse ECs following administration of Icam-2 AAV9 carrying Cmpk2-shRNA compared with those carrying scramble-shRNA (Fig. 5C and D). Copy numbers of Cmpk2 mRNA in ECs was further quantified by digital PCR, and result demonstrated a significant decrease in mRNA copy number of EC-Cmpk2 in mice with administration of Icam-2 AAV9 carrying Cmpk2-shRNA (Fig. 5E). No significant change of body weight and plasma cholesterol content were obseverd in AAV-Cmpk2 shRNA or scramble shRNA control mice (Online Table IIB). This specific inhibition of the Gata6 upregulation-mediated elevation of Cmpk2 expression in ECs of ApoeKO hyperlipidemic mice by Icam-2 promoter-driven AAV9 carrying Cmpk2-shRNA significantly diminished the enhanced atherosclerotic lesion formation, with the percentage of lesion reduction from 22.1 ± 1.2% (mean ± SEM) to 12.1 ± 0.8% (P < 0.01) in aorta en face (Fig. 5F), and average lesion reduction from 2.0 ± 0.2 × 105 μm [2] to 1.0 ± 0.1 × 105 μm [2] (P < 0.01), as well as percentage lesion reduction from 19.6 ± 1.4% to 10.3 ± 1.3% (P < 0.01) in the aortic root (Fig. 5G), as measured by Sudan IV-positive stained area compared with those by the AAV9 carrying scramble-shRNA. Also, the number of F4/80+-macrophages within the lesions was reduced from 13.2% of ApoeKO mice to 8.9% of ApoeKO mice following administration of Icam-2 promoter-driven AAV9 carrying Cmpk2-shRNA (Fig. 5H). Moreover, Cmpk2-shRNA knockdown in ECs decreased the expression of Nlrp3 and IL-1β (Fig. 5I–J). These data suggest that EC-Cmpk2 knockdown inhibits Nlrp3 inflammasome activation and IL-1β production to reduce monocyte/macrophage infiltration and further alleviate atherosclerotic lesion formation.
Fig. 5.
Icam-2 promoter-driven adeno-associated virus 9 target delivery of Cmpk2-shRNA into ECs inhibits the elevated Gata6-Cmpk2 mediated Nlpr3 inflammasome activation and reduces atherosclerosis. (A) The schematic figure of administration of Icam-2 promoter-driven adeno-associated virus 9 (AAV9) carrying Cmpk2-shRNA or scramble-shRNA by tail vein injection for viral EC target delivery affecting atherosclerotic lesion formation in 8-week high-fat diet ApoeKO mice. (B–E) Viral GFP fluorescence in aortic ECs co-staining with EC marker CD31 (B) and Cmpk2 expression in ECs by RT-qPCR (C), Western blot (D) with quantification data on the right and digital PCR (E) following tail vein injection of Icam-2 promoter-driven AAV9 carrying Cmpk2-shRNA or scramble-shRNA. (F–H) The representative images of Sudan IV staining of en face aorta (F), aortic root (G) and F4/80 immunostaining aortic root sections (H) following administration of Icam-2 AAV9 carrying Cmpk2-shRNA or scramble shRNA, with quantification on the right (n = 12, 6 each for male and female mice per group). (I–J) Gata6, Cmpk2 and in turn Nlrp3 and IL-1β expression by RT-qPCR (I) and Western blot (J) with quantification data on the right in ECs of ApoEKO hyperlipidemic and wild-type mice following tail vein injection of Icam-2 AAV9 carrying Cmpk2-shRNA or scramble-shRNA. Quantification of Western blot was carried out with Image J and normalized to loading control β-actin. Unpaired 2-tailed student t-test for C-D and I-J, two-way ANOVA followed by Turkey post hoc test for F–H. Scale bars: B, 500 μm, 100 μm; F, 2 mm; G, 500 μm; H, 100 μm.
Consistent with these in vivo findings, CMPK2 knockdown in HAECs by CMPK2-siRNA reduced the expression of Nlrp3 and IL-1β (Online Fig. 6A and B), and oxLDL-induced adherence of THP-1 to HAECs (Online Fig. 6C). The conditional medium from CMPK2-siRNA HAECS significantly reduced monocyte/macrophage migration (Online Fig. 6D) and pro-inflammatory macrophage formation (Online Fig. 6E).
Fig. 6.
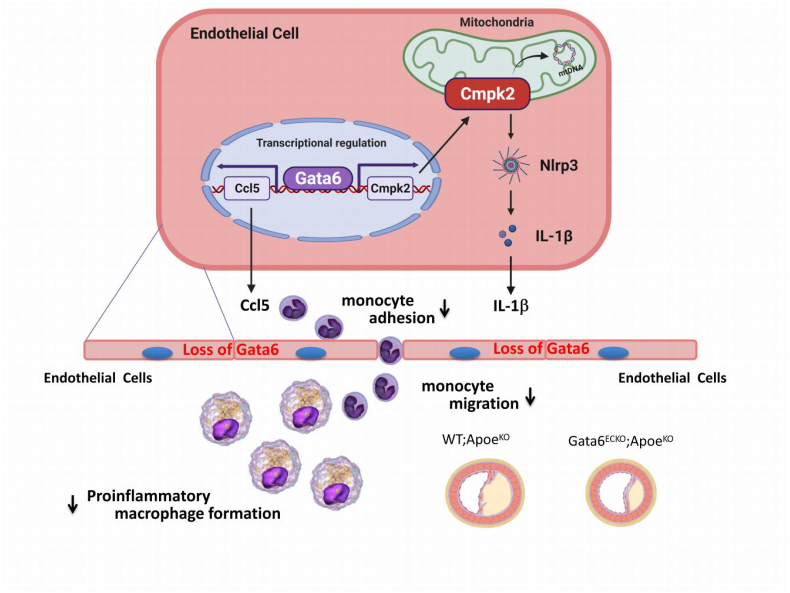
Schematic figure of working model. Model of endothelial Gata6 exerts its atherogenic effects through regulation of vascular inflammation by Cmpk2-Nlrp3 inflammasome activation and chemokine Ccl5 signals that affect the monocyte adherence, migration and recruitment into the vessel wall, as well as the pro-inflammatory macrophage formation.
Taken together, these data indicate that EC-Gata6 exerts its atherogenic effects through regulation of vessel wall inflammation by Cmpk2-Nlrp3 inflammasome activation and the chemokine Ccl5 signal that affects monocyte adherence, migration and recruitment into the vascular wall, as well as pro-inflammatory macrophage formation (Fig. 6).
4. Discussion
Atherosclerosis is a chronic inflammatory disease in which the recruitment and trapping of immune cells, especially monocytes, into the vessel wall results in vascular chronic inflammation for initiation and development of atherosclerotic plaques [[1], [2], [3], [4], [5]]. Endothelial cells, as the innermost layer in all vessels, play an essential role in regulating tissue homeostasis by controlling infiltration of circulating immune cells into the vessel wall, and EC injury or dysfunction is known to promote excessive vascular leukocytes infiltration by alteration of its production of cytokines, chemokines and adhesion molecules contributing to atherosclerosis [2,16,24].
Endothelial mitochondrial redox dysfunction is an important factor causing abnormal function of the endothelium, which plays a central role during atherosclerosis development [25]. Our study showed that EC-Gata6 regulates its downstream target gene Cmpk2, a family member of nucleotide kinases in mitochondria where it converts dCMP to dCDP and then to dCTP, to supply deoxyribonucleotides for mtDNA synthesis necessary for the production of oxidized mtDNA fragments, that is important in Nlrp3 activation for inflammatory response [13]. Endothelial target gene delivery by intercellular adhesion molecule 2 (Icam-2) promoter-driven AAV9 carrying the Cmpk2-shRNAreduced endothelial inflammation and alleviated atherosclerotic lesion formation.
Gata6 is a highly expressed GATA factor in the vessel wall and Gata6 in vascular smooth muscle cells plays an important role in phenotypic modulation contributing to vessel remodeling [21,26,27], while endothelial Gata6 regulation of adhesion molecules such as VACM-1 has been implicated in controlling monocytic cell adhesion crucial for atherosclerosis [11,12]. Further study showed high GATA6/VCAM-1 expression levels in the vessel inner curvature where oscillatory shear stress prevails to facilitate susceptibility to atherosclerosis, and epigenetic factors HDACs and miRNAs, and hormone receptors RARα and RXRα, regulate the proinflammatory GATA6/VCAM-1 signaling in ECs with subsequent inflammatory cell infiltration to control atherosclerotic lesion development [28,29]. Our recent data demonstrated that endothelial Gata6 regulates neointimal formation via targeting PDGF-B in a paracrine manner [21]. Gata6 in ECs is also reported to directly target VCAM1 [11], Tgfβ110. Gata6 in lung ECs was reported to associate with multiple targets, like RhoB, MMP10, MMP1 et al. [30], while no detailed functional experiments are performed. Therefore, the direct evidence of vascular EC restricted Gata transcription factors in the regulation of cytokines and chemokines for excessive infiltration of leukocytes for in vivo atherogenesis needs further investigate.
The NLRP3 inflammasome has been implicated in atherosclerosis recently and the CANTOS (Canakinumab Antiinflammatory Thrombosis Outcome Study) trial highlighted the importance of inflammation specifically, inflammasome-derived inflammation in atherosclerotic disease [31]. CMPK2, a family member of nucleotide kinases targeting mitochondria where it converts dCMP to dCDP and then converts to dCTP, is a rate-limiting enzyme for mtDNA synthesis and production of oxidized mtDNA fragments, and the cytosolic oxidized mtDNA association with the NLRP3 inflammasome complex is required for its activation [14]. Myeloid-specific ablation of LPS-induced CMPK2 abrogates NLRP3 inflammasome activation and reduces pulmonary inflammation and acute respiratory distress syndrome severity without a direct effect on IL-6 [32]. In this study, we identified Cmpk2 as a Gata6 direct downstream target gene, and in vivo and in vitro evidence clarified endothelial Gata6 involvement in the regulation of Cmpk2-dependent Nlrp3 inflammasome activation and production of IL-1β in a paracrine manner that promotes monocyte recruitment to the vessel wall forming proinflammatory macrophages responsible for atherosclerotic lesion development. Therefore, target delivery of Cmpk2-shRNA restricts atherosclerotic lesion development and might provide future opportunities for therapeutic interventions for atherosclerotic diseases.
In addition to regulating Cmpk2 and subsequent Nlrp3 inflammasome activation, Gata6-deficient ECs were associated with chemokine Ccl5 which was also demonstrated to be a direct target of Gata6. Thus, part of the effect of Gata6 deletion could potentially be independent of Cmpk2 dependent Nlrp3 inflammasome activation and might be driven by increased chemokine Ccl5 expression. Ccl5 is a well-known chemokine for monocyte recruitment contributing to increasing macrophage abundance in the vessel wall during atherosclerotic lesion development [22,33,34]. Therefore, Gata6 regulates either the Cmpk2 -Nlrp3 or Ccl5 signal, regulating EC behavior to control circulating monocyte recruitment and atherogenesis.
Funding
This study was supported by funds from the National Natural Science Foundation of China (82270260, 82270432), National Key Research and Development Program (2022YFA1104503), Sino-German Center Mobility Program (M-0680), Shanghai Rising-Star Program (20QA1408100, 21QA1401400), Young Medical Talents Training Program of Pudong Health Bureau of Shanghai (PWRq2021-04), Young Health Talents of Shanghai Municipal Health Commission (2022YQ069, 2022YQ035), Innovative research team of high-level local universities in Shanghai and a key laboratory program of the Education Commission of Shanghai Municipality (ZDSYS14005), Research program of Shenzhen Science and Technology Innovation Committee (JCYJ20190807102605515, JCYJ20220530163601003).
Author contribution statement
Wenrun Wu and Wenzhen Bao conducted most of the in vivo/in vitro experiments, immunofluorescence staining, qPCR and Western blot assay, data interpretation, analysis. Xiaoli Chen assisted in mouse colony maintenance, Cmpk2 AAV experiment, and contributed to manuscript revisions. Yushi Lu, Ji Fang and Jiwen Liu performed RAW migration assay, THP-1 adhesion assay, and in vitro shear stress assay. Sheng Peng and Jingjiang Pi were involved in chip and luciferase experiment and immunofluorescence staining. Brain Tomlinson, Paul Chan revised the manuscript. Qi Zhang, Lin Zhang and Zhongmin Liu provided advice throughout the project. Jie Liu and Yuzhen Zhang provided reagents, resources, advice throughout the project, and wrote the manuscript. Tao Zhuang supervised the entire project and had a major role in experimental design, data analysis, and interpretation, generated all experimental mice.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102775.
Contributor Information
Jie Liu, Email: kenliujie@126.com.
Yuzhen Zhang, Email: yzzhang-tj@tongji.edu.cn.
Tao Zhuang, Email: zhuangtao5217@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Ross R. Atherosclerosis — an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Gimbrone M.A., Jr., García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stary H.C. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler. Thromb. Vasc. Biol. 2000;20:1177–1178. doi: 10.1161/01.atv.20.5.1177. [DOI] [PubMed] [Google Scholar]
- 4.Simionescu N., Vasile E., Lupu F., Popescu G., Simionescu M. Prelesional events in atherogenesis. Accumulation of extracellular cholesterol-rich liposomes in the arterial intima and cardiac valves of the hyperlipidemic rabbit. Am. J. Pathol. 1986;123:109–125. [PMC free article] [PubMed] [Google Scholar]
- 5.Tabas I., García-Cardeña G., Owens G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015;209:13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko L.J., Engel J.D. DNA-binding specificities of the gata transcription factor family. Mol. Cell Biol. 1993;13:4011–4022. doi: 10.1128/mcb.13.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molkentin J.D. The zinc finger-containing transcription factors gata-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J. Biol. Chem. 2000;275:38949–38952. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- 8.Morrisey E.E. Gata-6: the proliferation stops here: cell proliferation in glomerular mesangial and vascular smooth muscle cells. Circ. Res. 2000;87:638–640. doi: 10.1161/01.res.87.8.638. [DOI] [PubMed] [Google Scholar]
- 9.Rosas M., Davies L.C., Giles P.J., Liao C.T., Kharfan B., Stone T.C., et al. The transcription factor gata6 links tissue macrophage phenotype and proliferative renewal. Science. 2014;344:645–648. doi: 10.1126/science.1251414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Froese N., Kattih B., Breitbart A., Grund A., Geffers R., Molkentin J.D., et al. Gata6 promotes angiogenic function and survival in endothelial cells by suppression of autocrine transforming growth factor beta/activin receptor-like kinase 5 signaling. J. Biol. Chem. 2011;286:5680–5690. doi: 10.1074/jbc.M110.176925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umetani M., Mataki C., Minegishi N., Yamamoto M., Hamakubo T., Kodama T. Function of gata transcription factors in induction of endothelial vascular cell adhesion molecule-1 by tumor necrosis factor-alpha. Arterioscler. Thromb. Vasc. Biol. 2001;21:917–922. doi: 10.1161/01.atv.21.6.917. [DOI] [PubMed] [Google Scholar]
- 12.Fan X., Chen X., Feng Q., Peng K., Wu Q., Passerini A.G., et al. Downregulation of gata6 in mtor-inhibited human aortic endothelial cells: effects on tnf-α-induced vcam-1 expression and monocytic cell adhesion. Am. J. Physiol. Heart Circ. Physiol. 2019;316:H408–h420. doi: 10.1152/ajpheart.00411.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong Z., Liang S., Sanchez-Lopez E., He F., Shalapour S., Lin X.J., et al. New mitochondrial DNA synthesis enables nlrp3 inflammasome activation. Nature. 2018;560:198–203. doi: 10.1038/s41586-018-0372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen M.B., Gregersen I., Ø Sandanger, Yang K., Sokolova M., Halvorsen B.E., et al. Targeting the inflammasome in cardiovascular disease. JACC Basic Transl Sci. 2022;7:84–98. doi: 10.1016/j.jacbts.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grebe A., Hoss F., Latz E. Nlrp3 inflammasome and the il-1 pathway in atherosclerosis. Circ. Res. 2018;122:1722–1740. doi: 10.1161/CIRCRESAHA.118.311362. [DOI] [PubMed] [Google Scholar]
- 16.Zhuang T., Liu J., Chen X., Zhang L., Pi J., Sun H., et al. Endothelial foxp1 suppresses atherosclerosis via modulation of nlrp3 inflammasome activation. Circ. Res. 2019;125:590–605. doi: 10.1161/CIRCRESAHA.118.314402. [DOI] [PubMed] [Google Scholar]
- 17.Wang L., Luo J.Y., Li B., Tian X.Y., Chen L.J., Huang Y., et al. Integrin-yap/taz-jnk cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016;540:579–582. doi: 10.1038/nature20602. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y., Goss A.M., Cohen E.D., Kadzik R., Lepore J.J., Muthukumaraswamy K., et al. A gata6-wnt pathway required for epithelial stem cell development and airway regeneration. Nat. Genet. 2008;40:862–870. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., Nakayama M., Pitulescu M.E., Schmidt T.S., Bochenek M.L., Sakakibara A., et al. Ephrin-b2 controls vegf-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 20.Souilhol C., Serbanovic-Canic J., Fragiadaki M., Chico T.J., Ridger V., Roddie H., et al. Endothelial responses to shear stress in atherosclerosis: a novel role for developmental genes. Nat. Rev. Cardiol. 2020;17:52–63. doi: 10.1038/s41569-019-0239-5. [DOI] [PubMed] [Google Scholar]
- 21.Zhuang T., Liu J., Chen X., Pi J., Kuang Y., Wang Y., et al. Cell-specific effects of gata (gata zinc finger transcription factor family)-6 in vascular smooth muscle and endothelial cells on vascular injury neointimal formation. Arterioscler. Thromb. Vasc. Biol. 2019;39:888–901. doi: 10.1161/ATVBAHA.118.312263. [DOI] [PubMed] [Google Scholar]
- 22.Noels H., Weber C., Koenen R.R. Chemokines as therapeutic targets in cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2019;39:583–592. doi: 10.1161/ATVBAHA.118.312037. [DOI] [PubMed] [Google Scholar]
- 23.Zernecke A., Weber C. Chemokines in the vascular inflammatory response of atherosclerosis. Cardiovasc. Res. 2010;86:192–201. doi: 10.1093/cvr/cvp391. [DOI] [PubMed] [Google Scholar]
- 24.Gerhardt T., Ley K. Monocyte trafficking across the vessel wall. Cardiovasc. Res. 2015;107:321–330. doi: 10.1093/cvr/cvv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowak W.N., Deng J., Ruan X.Z., Xu Q. Reactive oxygen species generation and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017;37:e41–e52. doi: 10.1161/ATVBAHA.117.309228. [DOI] [PubMed] [Google Scholar]
- 26.Mano T., Luo Z., Malendowicz S.L., Evans T., Walsh K. Reversal of gata-6 downregulation promotes smooth muscle differentiation and inhibits intimal hyperplasia in balloon-injured rat carotid artery. Circ. Res. 1999;84:647–654. doi: 10.1161/01.res.84.6.647. [DOI] [PubMed] [Google Scholar]
- 27.Fan Y., Gu X., Zhang J., Sinn K., Klepetko W., Wu N., et al. Twist1 drives smooth muscle cell proliferation in pulmonary hypertension via loss of gata-6 and bmpr2. Am. J. Respir. Crit. Care Med. 2020;202:1283–1296. doi: 10.1164/rccm.201909-1884OC. [DOI] [PubMed] [Google Scholar]
- 28.Lee D.Y., Lin T.E., Lee C.I., Zhou J., Huang Y.H., Lee P.L., et al. Microrna-10a is crucial for endothelial response to different flow patterns via interaction of retinoid acid receptors and histone deacetylases. Proc. Natl. Acad. Sci. U. S. A. 2017;114:2072–2077. doi: 10.1073/pnas.1621425114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee D.Y., Yang T.L., Huang Y.H., Lee C.I., Chen L.J., Shih Y.T., et al. Induction of microrna-10a using retinoic acid receptor-α and retinoid x receptor-α agonists inhibits atherosclerotic lesion formation. Atherosclerosis. 2018;271:36–44. doi: 10.1016/j.atherosclerosis.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Ghatnekar A., Chrobak I., Reese C., Stawski L., Seta F., Wirrig E., et al. Endothelial gata-6 deficiency promotes pulmonary arterial hypertension. Am. J. Pathol. 2013;182:2391–2406. doi: 10.1016/j.ajpath.2013.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C., et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 32.Xian H., Liu Y., Rundberg Nilsson A., Gatchalian R., Crother T.R., Tourtellotte W.G., et al. Metformin inhibition of mitochondrial atp and DNA synthesis abrogates nlrp3 inflammasome activation and pulmonary inflammation. Immunity. 2021;54:1463–1477.e1411. doi: 10.1016/j.immuni.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soehnlein O., Libby P. Targeting inflammation in atherosclerosis - from experimental insights to the clinic. Nat. Rev. Drug Discov. 2021;20:589–610. doi: 10.1038/s41573-021-00198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jongstra-Bilen J., Tai K., Althagafi M.G., Siu A., Scipione C.A., Karim S., et al. Role of myeloid-derived chemokine ccl5/rantes at an early stage of atherosclerosis. J. Mol. Cell. Cardiol. 2021;156:69–78. doi: 10.1016/j.yjmcc.2021.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.