Summary
Viral respiratory infections (VRIs) cause seasonal epidemics and pandemics, with their transmission influenced by climate conditions. Despite the risks posed by novel VRIs, the relationships between climate change and VRIs remain poorly understood. In this review, we synthesized existing literature to explore the connections between changes in meteorological conditions, extreme weather events, long-term climate warming, and seasonal outbreaks, epidemics, and pandemics of VRIs from an interdisciplinary perspective. We proposed a comprehensive conceptual framework highlighting the potential biological, socioeconomic, and ecological mechanisms underlying the impact of climate change on VRIs. Our findings suggested that climate change increases the risk of VRI emergence and transmission by affecting the biology of viruses, host susceptibility, human behavior, and environmental conditions of both society and ecosystems. Further interdisciplinary research is needed to address the dual challenge of climate change and pandemics.
Keywords: Climate change, Extreme weather event, Meteorological factor, Seasonality, Viral respiratory infection
Introduction
Viral respiratory infections (VRIs) are the most common category of infectious diseases and one of the leading causes of morbidity and mortality worldwide.1 They can give rise to both repetitive seasonal epidemic outbreaks and catastrophic pandemics. The 21st century has witnessed several large-scale outbreaks and pandemics of VRIs, such as SARS in 2003, influenza H1N1 in 2009, Middle East Respiratory Syndrome (MERS) in 2012, and COVID-19 in 2019, each of which has resulted in severe excess human mortality or morbidity, as well as significant economic disruption. The public health risks of both occurrence and wide-scale transmission caused by VRIs are greater now than ever before. Growing populations and urbanization, the rapid expansion of global travel, civil conflict and migration, climate change, and other human-induced environmental degradations have all contributed to the risk of pandemics.2
Climate change is considered the biggest health threat facing humanity in the 21st century.3 The unprecedented rises in global temperatures and increases in the frequency and amplitude of extreme weather events have occurred as a consequence of human activities, mainly relating to anthropogenic emissions of greenhouse gases (GHG).4,5 Climate change endangers human health and well-being in a variety of ways, with the most prominent and direct being heat-related deaths. Nonetheless, the health impacts can extend beyond the direct effects of high temperatures on deaths and include indirect effects on vector organisms, environmental pollution as well as food and water supply, leading to increases in both climate-sensitive communicable and non-communicable diseases.6 Mounting evidence shows the increase in extreme weather events leads to vector-borne disease outbreaks at higher frequencies and greater scales by affecting pathogens, vectors, and hosts.7 Climate change also creates a cascade of consequences for socioeconomic determinants of health such as economic livelihoods and healthcare access, which in turn disproportionately burden the most vulnerable populations and exacerbate health inequity.8
Concerns have long been raised regarding the relationships between climate and VRIs. The incidence of VRIs (e.g., seasonal influenza) is higher in winter, which is believed to be related to cooler temperatures.9 In recent years, peaks of certain VRIs have seen shifted toward warmer seasons.10,11 VRIs are referred to as climate-sensitive diseases and most of them can be influenced by at least one climatic driver12; however, unanswered questions remain about the relationships between climate change and VRIs. Whether global warming is affecting the epidemiological patterns of VRIs, and how the future epidemic dynamics of VRIs will shift in a rapidly changing climate remain poorly understood. To date, studies have largely focused on the links between VRIs and certain climatic drivers in confined regions and have drawn inconclusive results. Meanwhile, the mechanisms behind the disagreement in those relationships remain unclear. There is a lack of systematic and comprehensive understanding on whether and how the full range of meteorological conditions, extreme weather events and long-term warming affects epidemic dynamics of VRIs.
The primary aim of this review is to fill a gap in the existing literature by synthesizing the recent epidemiological, biological, and ecological evidence on whether and how climate change, manifested in meteorological fluctuations, extreme weather events, and long-term global warming, influences the epidemic dynamics of VRIs, including spatiotemporal distribution of seasonal epidemic, disease outbreaks, and pandemics. It is hoped this synthesis can explain underlying mechanistic pathways grounded on an interdisciplinary perspective, contribute to forming a common consensus, and identify key areas for further research to provide a scientific basis for future pandemic preparation in the context of climate change.
Etiologic and epidemiological characteristics of VRIs
Globally, VRIs are the most common category of infectious diseases and one of the primary causes of morbidity and mortality.13 Hundreds of types of viruses are known causative agents of VRIs, with a spectrum from established, endemic human-adapted viruses to emerging, highly pathogenic viruses from animal reservoirs with pandemic threat.13 Influenza viruses, respiratory syncytial viruses (RSV), parainfluenza viruses (PIV), human metapneumoviruses (HMPV), human coronaviruses (HCoV), rhinoviruses (RV), adenoviruses (ADV), human bocaviruses (HBoV), and enteroviruses (EV) are most frequently detected viral pathogens associated with respiratory infections circulated in populations almost every year.14 Table 1 summarizes the etiologic and epidemiological characteristics of VRIs that commonly circulate in humans.
Table 1.
Characteristics of etiology and seasonality of viral respiratory infections (VRIs).
| Respiratory virus | Family | Structure and genome | Subtypes | Primary routes of transmission | Seasonal patterns |
|---|---|---|---|---|---|
| Influenza virus15 | Orthomyxoviridae | Enveloped ss − RNA viruses | A, B, C | Droplets and aerosols, contact | Winter peaks in temperate regions |
| Respiratory syncytial virus (RSV)10 | Paramyxoviridae | Enveloped ss − RNA viruses | A, B | Direct and indirect contact, droplets, and aerosols | Winter and early spring peaks in temperate regions |
| Human Metapneumovirus (HMPV)14 | Paramyxoviridae | Enveloped ss − RNA viruses | A, B | Droplets and contact | Late winter and spring peaks in temperate regions |
| Parainfluenza viruses (PIV)14 | Paramyxoviridae | Enveloped ss − RNA viruses | 1, 2, 3, 4 | Droplets and contact | PIV-1 and PIV-2 peak in the fall and winter in temperate regions, PIV-3 peaks in warm seasons, PIV-4 sporadically all-year |
| Human coronavirus (HCoV)13,16 | Coronaviridae | Enveloped ss + RNA viruses | OC43, 229E, NL63, HKU1a | Droplet spray and/or aerosol, contact | Winter peaks in most temperate regions |
| Rhinoviruses (RV)17 | Picornaviridae | Enveloped ss + RNA viruses | Species A, B, and C, with >100 serotypes | Contact, droplet spray, and/or aerosol | All-year, with peaks in the autumn and spring in temperate regions |
| Human bocavirus (HBoV)15 | Parvoviridae | Non-enveloped ss + DNA viruses | 1, 2, 3 | Contact, droplet spray, and/or aerosol | All-year, with a possible peak during the summer |
| Adenoviruses (ADV)13,18 | Adenoviridae | Non-enveloped dsDNA viruses | 53 | Contact, possibly droplet spray and/or aerosol | All-year |
| Enterovirus (EV)14 | Picornaviridae | Non-enveloped ss + RNA viruses | D68 | Contact | Summer and autumn |
Other human coronaviruses include MERS-CoV, SARS-CoV and SARS-CoV-2.
Respiratory viruses affect both the upper and lower respiratory tract, causing symptoms ranging from common colds to severe pneumonia requiring hospitalization.14 The severity of infections varies widely, modulated by physiological characteristics, nutrition, personal hygiene, and environmental circumstances that affect the integrity of the respiratory mucosa, respiratory function, and immunological state.13 Children (<5 years), older adults (≥65 years), pregnant women, immunocompromised individuals, and those with underlying cardiopulmonary conditions are most vulnerable.19 VRIs can be spread through virus-laden droplets or aerosols released from the respiratory tract when an infected individual speaks, coughs, and sneezes. In general, the transmission of respiratory viruses can be mostly categorized as occurring through three major routes: (1) direct and/or indirect contact with contaminated surfaces or objects (fomites), (2) short-range droplet-borne transmission, and (3) long-range aerosol transmission.20 Important determinants of the likelihood that droplet-borne viruses reach previously uninfected individuals are droplet sedimentation and evaporation, which are largely influenced by climatic and other environmental conditions.21
Seasonality of respiratory viruses is most pronounced in temperate regions, with winter or early spring peaks in influenza viruses, RSV, HMPV, and HCoV activity.16,22,23 Summer and autumn are typically the prime seasons for EV activity.24 Latitude gradients in the time of transmission peaks, duration, and interannual variability of seasonal cycles of RSV and influenza have been described,25,26 revealing earlier peak timing, shorter duration, and weaker seasonality in low latitude areas. Characterization of PIV seasonality is complicated by complex interactions between subtypes.15 ADV, RV, and HBoV can occur sporadically year-round.27 An understanding of the spatiotemporal dynamics such as seasonality and the underlying causes that determine the occurrence of VRIs outbreaks may provide us with insights into developing more effective prevention strategies.
The determinants of the seasonality of VRIs have been debated for many years. Previous work has revealed that there are distinct seasonal preferences for viral characteristics between enveloped and non-enveloped viruses.28 It has also been presumed that the observed seasonality of VRIs reflects seasonal fluctuations in the physiological state of the host due to varying weather conditions, such as changes in immune response and nutrients. Additionally, seasonal changes in behavioral patterns may also contribute to seasonality,29 and climatic conditions are considered to be one of the most strongly associated external drivers of the seasonality of VRIs.
Effects of meteorological factors on seasonal epidemics of VRIs
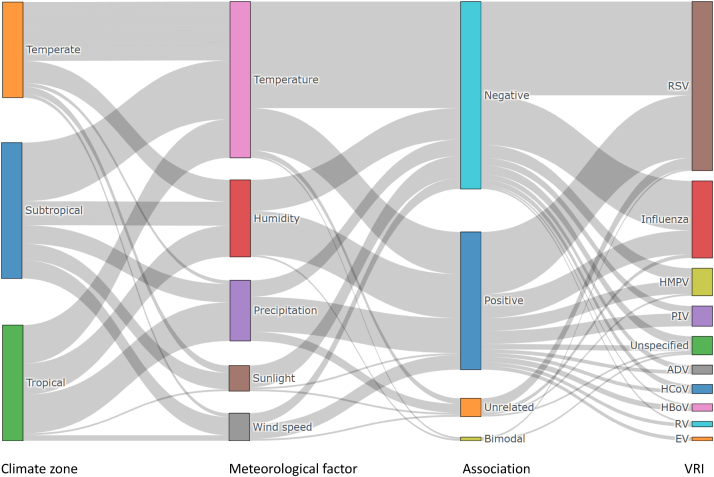
Seasonal epidemics of VRIs are usually considered to be related to seasonal variations of meteorological factors. Many studies have examined the relationship between VRIs and these factors, with temperature, humidity, precipitation, solar radiation, and wind speed most notably proposed as potential driving factors influencing the incidence of seasonal epidemics of VRIs (Fig. 1, see Supplementary Information for references). Generally, low temperature, low humidity, and low solar radiation are associated with seasonal epidemics in northern hemisphere winters, while increased precipitation is associated with tropical and subtropical VRI peaks.30,31 Both negative and positive relationships between wind speed and VRIs have been reported,32, 33, 34 but such associations between wind and VRIs are likely not independent of other meteorological conditions.35 By far, a large body of research investigating the relationship between meteorological factors and VRIs has focused on RSV and influenza, probably due to the high infection rates and relatively well-developed surveillance systems for these two pathogens.36
Fig. 1.
Associations between meteorological factors and viral respiratory infections (VRIs). Sankey diagram displaying the associations of five meteorological factors with VRIs and regional differentiations across temperate, subtropical, and tropical climates as demonstrated in previous epidemiological studies. The width of each gray belt represents the quantity of literature showing a given association (Data and references see Supplementary Information).
Ambient temperature and humidity
Temperature and humidity are the most significant meteorological contributors to the seasonal epidemics of VRIs.37 Earlier laboratory studies have revealed that low temperature and low humidity enhance viral viability and transmission, especially influenza, and can compromise host airway antiviral defense.38, 39, 40 Epidemiological research in temperate countries, such as Canada,41 China,42 Greece,43 and the US,44 has likewise found temperature and humidity negatively correlated with the incidence of VRIs. However, multi-country studies have offered contradictory findings that “cold-dry” and “warm-humid” conditions are related to peaks of VRIs in temperate and tropical climates, respectively.9 The reasons for the observed spatial heterogeneity have been subject to an ongoing discussion. One theory is that outdoor weather conditions may impact people’s behaviors and virus transmission in unique ways in temperate and tropical climates. This suggests that the effects of temperature and humidity on VRIs are far more intricate when analyzed at the population level compared to laboratory studies.
Recent epidemiological studies have explored the synergistic effects of temperature and humidity on VRIs, showing nonlinear relationships with or without thresholds under different temperature-humidity combinations,11,27 suggesting that seasonal epidemics of VRIs are not only shaped by single meteorological factors but likely the composition of multiple meteorological conditions. More quantitative, albeit complex (i.e., J-shaped, U-shaped, and bimodal exposure-response curves), type- or subtype-specific relationships between temperature and VRIs with lagged effects have also been revealed, partly due to the adoption of updated statistical approaches and more precise viral detection and diagnostic methods.45, 46, 47
However, epidemiological studies conducted within and between climatic zones have yielded substantially heterogeneous results regarding the relationship between relative humidity (RH) and VRIs.42,48,49 For example, a recent study conducted in mainland China and Hong Kong found that absolute humidity (AH) is a better indicator than RH when it comes to explaining the seasonal epidemic and transmission of VRIs. Additionally, the study identified a U-shaped relationship between AH and influenza transmissibility.50 Although various relationships have been reported, it is still difficult to quantify the extent to which the incidence of VRI seasonal epidemics can be attributed to these meteorological factors.51
Indoor microclimate
While many studies have provided evidence linking meteorological conditions to VRIs, it is worth noting that most of these studies used outdoor meteorological parameters for correlation and/or regression analysis, which may differ significantly from the actual indoor environment. An essential consideration is that indoor heating and air conditioning can artificially modify the indoor climate, disconnecting individuals from outdoor weather variations, and maintaining a consistent thermal comfort level indoors.15 Indoor heating changes the temperature and RH, while air conditioning lowers the AH of the indoor air.52 Evidence shows that outdoor AH may serve as a proxy for indoor RH and that AH is more important than RH in influencing seasonal patterns of VRIs.53
Studies show that individuals living in homes with indoor heaters that elevate indoor RH levels have lower rates of infections due to decreased virus viability.29 Opposite of what might be expected, virus transmission is more prevalent in indoor environments that are well-air-conditioned but have poor ventilation. The indoor climate and ventilation rates, regulated by outdoor weather conditions, are suggested to play a vital role in moderating the seasonal patterns of VRIs.15 Caution should therefore be taken when interpreting results of previous studies relying on outdoor meteorological data and ecological study designs.
Extreme weather events, climate anomalies, and outbreaks of VRIs
Over the last few decades, extreme weather events have become more frequent, intense, prolonged, and erratic due to climate change. This situation worsening as the world has experienced acute impacts of this phenomenon, with China and Europe experiencing prolonged heatwaves in 2022, East Asia suffering from heavy rainfall and flooding, and widespread wildfires in Siberia and the Eastern Mediterranean. Unfortunately, such occurrences are only a glimpse of what the future may hold, as the likelihood of concurrent extreme events—such as simultaneous heatwaves and wildfires or intensified flooding caused by sea-level rise and heavy rainfall—increases in many regions. These events may have cascading effects on hydrological conditions, air quality, and health determinants, modifying disease exposure, while increasing susceptibility and ultimately leading to an increased risk of infections and outbreaks.54,55
Heatwaves and compound events
Extreme weather events including heatwaves and wildfires are related to a variety of adverse respiratory health impacts, which are associated with an increase in air pollution.56, 57, 58 High-pressure atmospheric conditions, sunlight and low wind speeds during heatwaves contribute to high levels of air pollution. Heatwaves and droughts exacerbate the spread of wildfires, resulting in widespread elevations in levels of particulate matter (PM), which can exacerbate damage to lung function and/or pre-existing respiratory illnesses like COPD and asthma.59,60 A time-stratified case-crossover study in China showed that the risk of acute upper respiratory infections increased by 30% during heatwaves.56 Other studies reported exacerbation of pre-existing respiratory illnesses and increased host susceptibility to VRIs resulting from synergistic effects of compound exposures to aggravated air pollution, heatwaves, wildfire, and droughts.56, 57, 58
Heavy rainfall and consequent floods
The frequency and intensity of heavy rainfall will very likely increase over most regions globally during the 21st century, with more rain-generated flooding.5 In resource-limited tropical low- and middle-income countries (LMICs), which have been proven more vulnerable to the impacts of climate change,5 heavy rainfalls are found to be more likely associated with VRI peaks.61, 62, 63 Previous studies have found that extreme rainfall increases emergency visits and hospital admissions for acute respiratory infections.64, 65, 66 Haynes et al.67 have documented higher RSV incidences peaking in tropical/subtropical countries with high precipitation during wet months (including Bangladesh, Guatemala, and Thailand). It has been hypothesized that VRI epidemics in tropical rain belt LMICs may be related to overcrowding and poor sanitation, although this has yet to be fully proven.68 Flooding as a result of extreme rainfall increases the risk of infections and disease outbreaks both directly and indirectly. A cross-sectional study reported that direct exposure to floodwater increased the risk of influenza-like illnesses (OR = 2.75).69 Disruption of housing, health access, and emergency response infrastructure due to flooding can compromise health system resilience and increase both exposure and vulnerability to VRI risks.70
El Niño and abnormal seasonal fluctuations
El Nino is a climate pattern that signals a greater likelihood of continued abnormally warm sea surface temperatures in the tropical eastern Pacific Ocean, leading to abnormal temperature fluctuations in wintertime.71 A time-series study based on the WHO Global Influenza Programme database found that large-scale outbreaks of influenza increased during El Niño, suggesting that El Niño may have potential explanatory power in predicting influenza outbreaks as a climatic precursor.52 Evidence has shown that abnormal temperature fluctuations in winter result in earlier onset and greater severity of influenza epidemics in the subsequent year.71 According to the Centers for Disease Control and Prevention, all six large-scale outbreaks of influenza in the Northern Hemisphere between 1957 and 2009 occurred during spring to summer,72 which contradicts the assumption that influenza is endemic in winter months in northern latitudes and temperate climates. As climate change intensifies, abnormal temperature fluctuations may increasingly contribute to widespread outbreaks of influenza.
Long-term climate warming and future dynamics of VRIs
Human-induced climate change has already led to approximately a 1.2 °C increase in global average temperature above pre-industrial levels.4 In the long term, climate change, characterized by rising global average temperatures, has led to, and will continue to lead to, a multitude of challenges in the atmosphere, hydrosphere, and biosphere. These include the changes in climate belts, alternations in vegetation coverage, melting of glacial permafrost, and loss of biodiversity.5 If this warming continues at current rates, the rising of global temperatures is projected to overshoot the threshold of 1.5 °C by mid-century, leading to significant, catastrophic, and irreversible consequences for both human societies and natural ecosystems.73
Shift in spatiotemporal dynamics of VRI epidemics
Recent epidemiological surveillance in temperate climates has revealed a shift in the peak timing of RSV seasonal activity towards warmer seasons over the past few decades, along with a shortened epidemic duration.10,74 These trends are hypothesized to be associated with rising global temperatures leading to an expansion of the tropical belt.75 However, confirming this hypothesis through empirical research is currently difficult due to the scarcity of long-term continuous surveillance data.
Despite limited empirical studies, two modeling studies76,77 have explored the potential implications of long-term warming on the spatiotemporal patterns of VRI epidemics (i.e., RSV and influenza). Their findings indicated that climate change could result in a shift in the geographic distribution, duration, and severity of VRIs. Baker et al.76 projected that future RSV epidemics would have a northward shift in regional ranges as epidemic durations became more persistent. Increases in temperature-driven humidity would result in less intense disease outbreaks of RSV, although epidemics became more severe in locations that experienced extreme rainfall, usually in the tropics. Liu et al.77 projected future spatiotemporal trends in intra-season temperature variability and consequent influenza epidemics in northern mid-latitude regions under RCP (i.e., Representative Concentration Pathways) scenarios and found that densely populated areas would undergo the greatest rise in rapid intra-season temperature variability by the end of this century, thereby increasing the risk of influenza morbidity by up to 50%.
Emerging VRIs with pandemic potentials
Respiratory viruses exist in a spectrum from established, endemic human-adapted ones to emerging and zoonotic ones with pandemic potential. Of all the viruses mentioned above, three families of RNA viruses associated with respiratory infections appear to frequently jump species boundaries, including Orthomyxoviridae, Coronaviridae, and Paramyxoviridae (corresponding to five types of virus: influenza virus, HCoV, RSV, PIV, HMPV).78 For those viruses with zoonotic features, diseases can emerge via animal-human transmission, but only in certain cases does their emergence lead to disease outbreaks or even pandemics. According to the model proposed by Morse et al. the process of emergence can be broadly divided into three stages.79 Stage 1 involves the pathogen remaining in its natural reservoir. Large-scale ecological and societal changes can alter the likelihood of cross-species transmission, which can lead to progression into stage 2. Stage 2 is characterized by localized emergence, where spillovers from natural reservoirs result in human-to-human transmission. Finally, stage 3 is marked by the acquisition of sustained human-to-human transmission with low or no human immunity. Increased global connectivity can aid the transition to stage 3, leading to actual pandemics.
It is important to note that each stage of disease emergence is complex and multifactorial, with a variety of biological, ecological, and socioeconomic factors contributing to the emergence and spread of infectious diseases. Our emphasis is on larger-scale climate change modifications that increase the likelihood of spillover from preceding pathogen stages.79 Long-term climate change is expected to facilitate shifts in animal species’ ranges and cross-species viral transmission. A recent modeling study projected that cross-species viral transmission will increase as temperatures rise by 2070, with changing precipitation patterns and vegetation cover changes identified as additional factors that create new opportunities for the spillover of viruses. Climate change-related habitat destruction forces animals to enter new geographic areas where they may come into contact with new hosts and viruses, and increased interaction between species can also create new opportunities for the emergence and transmission of viruses.80 Another recent study that examined samples from the Arctic landscape has quantified the likelihood of climate change boosting virus spillover risk. Their findings show that spillover risk increases with runoff from glacier melt, which is a proxy for climate change.81
Potential mechanistic pathways linking climate change to VRIs
Despite numerous attempts to describe the relationships between climate and VRIs, including meteorological conditions, extreme weather events, and long-term warming, many inconsistencies and contradictions remain. These relationships, primarily derived from epidemiological surveys or ecological studies, require further investigation to understand the underlying mechanisms by which climate change impacts VRIs. This necessitates a dissection of the possible pathways involving biological, social, and environmental factors that mediate or moderate the effects of climate change on VRIs.
Virus viability, transmission, and replication
Meteorological factors affect virus viability and hence transmission, which has been revealed by both in vitro and in vivo studies. In laboratory settings, low temperatures enhance virus viability, especially in lipid-enveloped viruses like influenza and RSV.21 Exposure to intense ultraviolet solar radiation inactivates viruses in vitro. RH affects the physical fate of virus-laden droplets by influencing the sedimentation and evaporation process, so the virus transmission mode could hypothetically be shifted in different humidity conditions.82 Animal transmission models supported a U-shaped curve of influenza virus survival in droplets with RH, with reduced viral transmission efficiency at moderate RH.18,21,39,83 These mechanisms linking meteorological factors, especially temperature and RH, to virus viability and transmission provide a basic biological explanation for the seasonal epidemic of VRIs during temperate winter seasons and could in part mirror the “cold-dry” and “warm-humid” weather conditions potentially favoring virus transmission by different modes.40,53
Virus replication in upper respiratory airways can be enhanced in low-temperature conditions, as shown by in vitro studies.40 A recently proposed hypothesis, known as temperature-dependent viral tropism (TDVT), suggests that endemic respiratory viruses can self-regulate their pathogenicity, thereby maintaining mobility inside hosts, by developing thermal sensitivity within a range that supports organ-specific viral tropism within the human body. Specifically, they replicate most rapidly at temperatures below body temperature.84
Host susceptibility
Meteorological fluctuations, particularly in temperature and humidity, regulate airway antiviral function, affecting the innate and adaptive immunity of the host to VRIs. Inhaled dry air impairs epithelial integrity, mucociliary clearance, and tissue repair function in the airway tract. Additionally, recent research has revealed a previously undiscovered innate immunity mechanism within nasal tissues against viruses responsible for upper respiratory infection is dampened when inhaling cold air.85, 86, 87, 88 There is also plausibility of links between seasonal fluctuations of host immunity and the seasonal epidemic of VRIs, where seasonal meteorological conditions (such as sunlight) play a crucial role in regulating host immunity (such as vitamin D level, which relates to immune clearance against respiratory infections).89 These immunity mechanisms may explain the high incidence of VRI in winter at high latitudes but do not appear to explain the onset of outbreaks during summer heatwaves.
Recent experimental studies provide a plausible biological explanation for increased VRI incidences during heatwaves from a molecular perspective. Researchers simulated the high-temperature environment of a summer heatwave and found that prolonged exposure to extremely high temperatures (≥36 °C) in mice impairs virus-specific CD8+ T cell responses and antibody production following intranasal influenza virus infection.15 In addition, heatwaves, along with wildfires, increase compound exposure to air pollution as well as allergens like pollens, which causes airway irritation and respiratory tract inflammation. This reduces airway responsiveness to harmful stimuli and weakened clearance, exacerbating the damage to lung function.59,60
Human behavior
Human behavior can influence contact rates between infected and susceptible individuals, which can promote virus transmission through close host proximity. Crowding in particular plays a key role in this regard. A study conducted by Murray et al. empirically demonstrated that household crowding moderates the relationship between rainfall and increased risks of VRIs.64 Outdoor climate can also affect human behavior, such as gathering indoors during rainy days and using indoor heating on cold days or air conditioning on hot days. This seems appropriate in explaining the different seasonal patterns of VRI epidemics for both cold temperate and hot subtropical or tropical regions, where such indoor crowding influenced by seasonal weather conditions facilitates human-to-human transmission.53 Seasonal behavioral patterns are a critical mechanism in the transmission of seasonal VRIs, and they can be significantly influenced by the weather.
Population movement
Extreme weather events, including heavy rainfall and flooding, can disrupt housing, healthcare access, and emergency infrastructure, resulting in mass displacement and worsening living conditions.65 This, in turn, can lead to the clustering of people in temporary accommodations with little or no ventilation and increase human-to-human contact, which can elevate the risk of viral transmissions. Moreover, long-term impacts due to climate change can exacerbate these implications. With the increasing intensity and frequency of extreme weather events, rising sea levels and cyclones, and environmental degradation, people are forced to migrate across rural-urban areas and even cross borders.5 This migration further increases the risk of cross-regional and cross-population transmission, leading to infectious disease epidemics or even pandemics.
Agricultural adaptation
Modifications in agricultural practices to climate change impacts and adaptation cannot be overlooked. Climate change has led to prolonged droughts, desertification, and land degradation, which have had slow-onset but significant impacts on agriculture, especially in tropical regions in the developing world where soil quality is already poor. Increases in the frequency and severity of droughts exacerbate food insecurity in those areas currently vulnerable to undernutrition, increasing susceptibility to infectious diseases by compromising host immunity.90,91 Additionally, the intensification and industrialization of agriculture to meet the growing demand for animal-source protein have contributed to the emergence of zoonotic diseases.92 Deforestation and the geographic expansion of farming have also led to the increase in wildlife-livestock-human interfaces as this has pushed agricultural landscapes and wildlife habitats into overlapping environments, facilitating the transmission of novel pathogens between susceptible species.93
Urban environments
Urban areas, including their supporting systems and infrastructure for providing key services, are susceptible to the impacts of climate change, usually in the forms of cascading or compound exposures that result from a combination of extreme events and slowly emerging and ongoing impacts.94 For instance, rising sea levels combined with increased flooding from heavy rainfalls put a strain on water systems and housing. People living in older, poorly heated, and ventilated houses that are already vulnerable due to socioeconomic marginalization are at a greater risk of respiratory illness from dampness.95 Moreover, climate change stressors can hinder disease prevention and control efforts from public health systems by disrupting transportation networks and supply chains, reducing the mobility of healthcare personnel and resources, and restricting access to humanitarian aid.70
Natural environments
Natural environments are experiencing changes in the atmosphere, hydrosphere, and biosphere. These include modifications in average conditions and variability of climates and concurrent factors like amplified ocean acidification and carbon dioxide concentrations in the atmosphere. These changes can also result in ecosystem degradation and biodiversity loss.96 As global temperatures rise, the tropical belt expands, and vegetation cover shifts, causing animal species to undergo geographic range shifts as their habitats shrink or move.75,78 By understanding the impacts of climate change on ecological dynamics, hotspots of vulnerability, particularly in relation to emerging disease risks, can be identified. A previous study by Allen et al. demonstrated that disease emergence risk is elevated in hotspots located in tropical forest regions with high mammal biodiversity and undergoing anthropogenic land use changes associated with agricultural practices.97
Discussion
In this review, we synthesized recent multidisciplinary evidence to summarize the associations of viral respiratory infections (VRIs) with climate change from three dimensions: meteorological factors, extreme weather events, and long-term warming. We also explored potential mechanistic pathways explaining how climate affects VRIs from three aspects, namely biological, social, and environmental mechanisms, intending to foster interdisciplinary knowledge about the interaction between climate change and VRIs. The review covered a total of nine viral pathogens causing VRIs, with emphasis on those that have the potential to cause both repetitive seasonal epidemic outbreaks and catastrophic pandemics. Extensive epidemiological studies have explored the relationships between seasonal epidemics of VRIs and meteorological factors, although significant spatial heterogeneity has been shown at the regional level. However, there is limited research on extreme weather events and their impact on VRIs, with most research consisting of case studies. Even fewer studies have considered the effect of long-term climate warming on the dynamics of VRIs in human populations.
Despite the scattering of previous studies, we have found through systematically integrating interdisciplinary evidence that climate change has direct or indirect effects on the occurrence and transmission of VRIs. As suggested by both biological and epidemiological evidence, temperature and humidity are the most significant meteorological drivers of VRI seasonality. Due to climate change, seasonal epidemics of VRIs may shift spatially and temporally, with rising temperatures and abnormal rainfall patterns being contributing factors. Extreme weather events have the potential to exacerbate the risks of VRI transmission and increase outbreak risks by affecting disease exposure, leading to greater proximity between susceptible hosts. In addition, extreme weather events can increase the vulnerability of people, especially those who reside in resource-limited regions, to multiple stressors related to both climate hazards and infectious diseases, such as displacement and food insecurity. Climate change over the longer term will shift the spatiotemporal dynamic of VRIs, increasing the likelihood of VRIs occurring in locations and seasons previously unaffected. The increasing concern of spillover of emerging zoonotic pathogens and the potential for pandemics is primarily a result of modifications in both natural and social environments as a result of climate change and human-animal-environment interconnectedness.
Our comprehensive review of the three main mechanisms (biological, social, and environmental) that affect the dynamics of VRI epidemics suggests that climate change has significant implications for the different stages of expansion of VRI transmission, from seasonal epidemics to pandemics. Although not all mechanisms affecting the dynamics of VRI epidemics are fully understood, some provide intriguing insights. For example, if the seasonal VRIs occur as an intrinsic oscillation cycle regulated by weather-dependent behavioral and physiological factors, as argued by some researchers,29 climate change could make such oscillations of seasonality increasingly unpredictable. Such instability and unpredictability in the weather-climate system may, in turn, further exacerbate the impact of climate change on the seasonal patterns of VRIs.
VRIs with pandemic potential emerging from animal-human interfaces can be influenced by climate change through multiple processes related to ecological, social, and environmental mechanisms. These mechanisms interact with one another in complex ways as anthropogenic activities reshape the environment.78 The contact patterns between humans and wildlife reservoirs have changed due to urbanization and the intrusion of humans into previously unoccupied regions.12 In addition, increased global connectivity despite the hiatus following the emergence of COVID-19, which includes travel, trade, conflicts, and population movement or displacement, has increased human-to-human contact, has increased the risk of infectious disease transmission in both densely populated and remote areas, and potential for VRIs to more rapidly across borders than ever before.
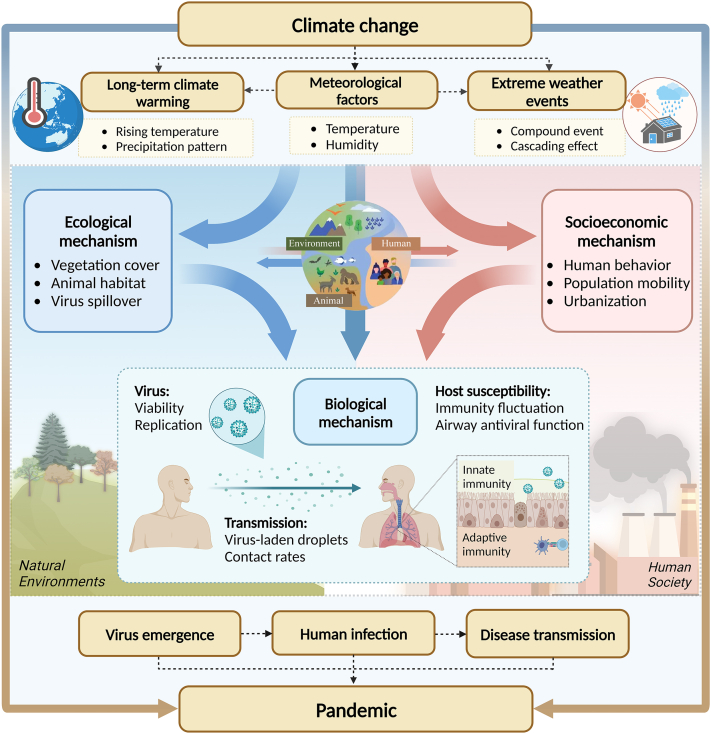
To gain a comprehensive understanding of the complex interaction between climate change and VRIs, we present a conceptual framework of possible mechanistic pathways through which climate change influences VRIs (Fig. 2). The influence of climate change on VRIs can be multi-dimensional, affecting the natural environments, and human society. These have dynamic interactions with underlying factors, such as biological, socioeconomic, and ecological factors, which contribute to the vulnerability of both individuals and communities. Anthropogenic factors, such as rapid increases in urban populations, crowded living environments, intensified global connectivity, land use and cover changes, and deteriorating ecological environments, may lead to more frequent human-animal-environment interactions and amplify the risks of emerging and re-emerging VRIs in the context of climate change.
Fig. 2.
Mechanistic pathways through which climate change influences viral respiratory infections (VRIs). (Created with BioRender.com).
The COVID-19 pandemic may be a harbinger of an upcoming new era, defined by outbreaks of emerging and re-emerging diseases that spread quickly and internationally.2 The interplay of biological, socioeconomic, and ecological factors, together with novel aspects of human-animal-environment interfaces, pose additional challenges concerning the emergence of infectious diseases. Therefore, adopting the One Health approach, which systematically considers the interconnected interaction between humans, animals, and the environment we share, as well as the interconnection of climate change, human health, environmental sanitation, and biodiversity as a whole,98 is instrumental for understanding the regional and global health threats associated with climate change. This perspective can enhance interdisciplinary collaboration in strengthening surveillance and early warning of viral diseases to inform preparation for future pandemics.
Outstanding questions
The findings of existing studies often conflict and have yet to point to a consensus on mechanisms underlying the disagreement in the relationships between climatic drivers and VRIs. Some of the apparent disagreement can be attributed, partly, to study design, statistical approaches, data comparability between study locations and periods, differences in virus types or subtypes/lineages, and diagnostic techniques.51 However, other factors including ecological and socioeconomic determinants with possible mediating or moderating effects on population susceptibility have received less attention. Although previous in vitro experiments and animal models have partly elucidated biological mechanisms of how meteorological factors affect the dynamics of respiratory viruses, these are not always borne out by epidemiological studies due to the complexity of the associations involved and interactions between environmental and social mechanisms. Further research on the mechanisms underlying these complex associations is warranted.
Global warming, changes in precipitation patterns, and increases in the frequency and severity of extreme weather events often result from a combination of interacting processes across multiple spatial and temporal dimensions.99 The combination of climate drivers and hazards is referred to as compound events, which are receiving significant research attention in the context of climate change.4 Moreover, the interaction between changes in multiple climatic conditions, and compound exposure to both climatic hazards and environmental pollution may lead to more complex and severe health effects and risks.5 Previously, most assessments have only considered a single driver or hazard at a time, and have failed to reflect the complex environment that people experience in the real world, potentially leading to underestimation of the compound risks. Further research is required with particular attention to the risks from compound events such as compound exposures to certain ranges of temperature and humidity that result in higher incidence of VRIs.
Early warning systems can be an effective tool for informing the development of disease prevention and control measures, as well as for resource allocation. The integration of multi-source data and techniques to offer proper predictive information is crucial for disease early warning. Traditional epidemiological models for disease prediction and early warning, however, have limited application in time and area scales, and their generalizability is affected by a diversity of climatic conditions across regions. Prior studies have noted the important role of meteorological and climatic factors in predicting VRI onsets. With more effects of climate on VRIs gradually being demonstrated, further work is needed to improve infectious disease prediction and early warning models by incorporating climate data and climate forecasting techniques. Such climate-based models can be instrumental in improving overall predictive power, capturing earlier leading signals of climate change-induced epidemics of certain viruses, assessing risks, and targeting prevention strategies. This requires the disciplinary divide between epidemiologists and atmospheric scientists to be effectively bridged.
Search strategy and selection criteria.
We searched PubMed, MEDLINE, and references from relevant articles using the search terms “climate change”, “meteorological”, “respiratory infections”, and “virus” ((climat∗[Title/Abstract] OR meteorolog∗[Title/Abstract]) AND respiratory [Title/Abstract] AND (virus [Title/Abstract] OR viral [Title/Abstract]) AND English [language] “Climate Change” [Mesh] AND “Respiratory Tract Infections” [Mesh] AND (“Viruses” [Mesh] OR viral [Title/Abstract]) AND English [language]) for papers published in English before March 2023. We performed our literature searches before developing the manuscript, and again prior to submission of the final, revised version. Some reviewers provided references that they deemed of particular importance. Most publications included in the review were from the past five years, but earlier commonly cited publications were not excluded.
Since 2020, studies on climatic conditions and COVID-19 have made up a significant proportion of the literature. However, there is substantial uncertainty about the relationship between climate and COVID-19 due to the rapid variation of SARS-CoV-2 itself, the presence of NPIs, and data availability in different countries. Studies concerning the relationships between climatic variables and COVID-19 were therefore excluded from the literature search.
Conclusions
The dynamics of VRIs are shifting in the face of rapid environmental change. Climate change, as evidenced by alterations in meteorological conditions, an increase in extreme weather events, and long-term climate warming, is likely to increase the risk of the emergence, transmission, and epidemic of VRIs, with changes in seasonality or geographic range potentially leading to elevated transmission and emergence by altering the biology of viruses, host susceptibility, human social behavior, and environmental conditions. Furthermore, the interplay between biological, socioeconomic, and ecological factors, coupled with evolving human-animal-environmental interfaces, pose additional challenges in the emergence of infectious diseases with pandemic potential in the context of climate change. To address these challenges, cross-disciplinary collaborative research should is urgently needed to elucidate the underlying mechanisms, compound exposures, and implications for outbreak early warning systems. Such research demands a concerted effort from epidemiologists, biologists, and atmospheric scientists, and should be given the highest research priority.
Contributors
C.H. initiated and designed the study. Y.H. carried out the literature search, compiled all data, drew the figures, and drafted the manuscript. C.H., Y.H., S.R., W.J.L., and N.J. contributed to the interpretation of results and revised the manuscript. All authors read the final version of the manuscript and approved its submission.
Declaration of interests
All authors declare no relevant conflicts of interest.
Acknowledgements
This work was supported by the grants from the National Key Research and Development Program of China (No. 2018YFA0606200) and Sanming Project of Medicine in Shenzhen, China (No. SZSM202111001). The funder had no role in study design, data collection, data analysis and interpretation, or drafting the manuscript.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104593.
Appendix A. Supplementary data
References
- 1.Troeger C., Blacker B., Khalil I.A., et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–1210. doi: 10.1016/S1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker R.E., Mahmud A.S., Miller I.F., et al. Infectious disease in an era of global change. Nat Rev Microbiol. 2022;20(4):193–205. doi: 10.1038/s41579-021-00639-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . 2021. COP26 special report on climate change and health: the health argument for climate action. [Google Scholar]
- 4.Masson-Delmotte V., Zhai P., Pirani A., et al. Vol. 2. 2021. (Climate change 2021: the physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change). [Google Scholar]
- 5.Pörtner H.-O., Roberts D.C., Adams H., et al. 2022. Climate change 2022: impacts, adaptation and vulnerability. IPCC sixth assessment report. [Google Scholar]
- 6.Zhao Q., Guo Y., Ye T., et al. Global, regional, and national burden of mortality associated with non-optimal ambient temperatures from 2000 to 2019: a three-stage modelling study. Lancet Planet Health. 2021;5(7):e415–e425. doi: 10.1016/S2542-5196(21)00081-4. [DOI] [PubMed] [Google Scholar]
- 7.Rocklöv J., Dubrow R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nat Immunol. 2020;21(5):479–483. doi: 10.1038/s41590-020-0648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romanello M., McGushin A., Di Napoli C., et al. The 2021 report of the lancet countdown on health and climate change: code red for a healthy future. Lancet. 2021;398(10311):1619–1662. doi: 10.1016/S0140-6736(21)01787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamerius J.D., Shaman J., Alonso W.J., et al. Environmental predictors of seasonal influenza epidemics across temperate and tropical climates. PLoS Pathog. 2013;9(3) doi: 10.1371/journal.ppat.1003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozeki S., Oshiro M., Fukumi D., Takeuchi T., Mii S., Nishikado Y. Change over time in seasonality and severity of children hospitalized with respiratory syncytial virus infection in Japan. Pediatr Infect Dis J. 2022;41(8):614–619. doi: 10.1097/INF.0000000000003568. [DOI] [PubMed] [Google Scholar]
- 11.Shobugawa Y., Takeuchi T., Hibino A., et al. Occurrence of human respiratory syncytial virus in summer in Japan. Epidemiol Infect. 2017;145(2):272–284. doi: 10.1017/S095026881600220X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mora C., McKenzie T., Gaw I.M., et al. Over half of known human pathogenic diseases can be aggravated by climate change. Nat Clim Change. 2022;12(9):869–875. doi: 10.1038/s41558-022-01426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Doorn H.R., Yu H. Elsevier; 2020. Viral respiratory infections. Hunter's tropical medicine and emerging infectious diseases; pp. 284–288. [Google Scholar]
- 14.García-Arroyo L., Prim N., Del Cuerpo M., et al. Prevalence and seasonality of viral respiratory infections in a temperate climate region: a 24-year study (1997-2020) Influenza Other Respir Viruses. 2022;16(4):756–766. doi: 10.1111/irv.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moriyama M., Hugentobler W.J., Iwasaki A. Seasonality of respiratory viral infections. Annu Rev Virol. 2020;7(1):83–101. doi: 10.1146/annurev-virology-012420-022445. [DOI] [PubMed] [Google Scholar]
- 16.Li P., Wang Y., Lamers M.M., et al. Recapitulating infection, thermal sensitivity and antiviral treatment of seasonal coronaviruses in human airway organoids. eBioMedicine. 2022;81 doi: 10.1016/j.ebiom.2022.104132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopes G.P., Amorim Í.P.S., Melo B.O., et al. Identification and seasonality of rhinovirus and respiratory syncytial virus in asthmatic children in tropical climate. Biosci Rep. 2020;40(9) doi: 10.1042/BSR20200634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price R.H.M., Graham C., Ramalingam S. Association between viral seasonality and meteorological factors. Sci Rep. 2019;9(1):929. doi: 10.1038/s41598-018-37481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chong K.C., Chen Y., Chan E.Y.Y., et al. Association of weather, air pollutants, and seasonal influenza with chronic obstructive pulmonary disease hospitalization risks. Environ Pollut. 2022;293 doi: 10.1016/j.envpol.2021.118480. [DOI] [PubMed] [Google Scholar]
- 20.Wang C.C., Prather K.A., Sznitman J., et al. Airborne transmission of respiratory viruses. Science. 2021;373(6558) doi: 10.1126/science.abd9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezaei M., Netz R.R. Airborne virus transmission via respiratory droplets: effects of droplet evaporation and sedimentation Majid Rezaei and Roland R. Netz. Curr Opin Colloid Interface Sci. 2021;55 doi: 10.1016/j.cocis.2021.101471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park S., Lee Y., Michelow I.C., Choe Y.J. Global seasonality of human coronaviruses: a systematic review. Open Forum Infect Dis. 2020;7(11):ofaa443. doi: 10.1093/ofid/ofaa443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Wang X., Broberg E.K., Campbell H., Nair H. Seasonality of respiratory syncytial virus and its association with meteorological factors in 13 European countries, week 40 2010 to week 39 2019. Euro Surveill. 2022;27(16) doi: 10.2807/1560-7917.ES.2022.27.16.2100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui B., Zhang D., Pan H., et al. Viral aetiology of acute respiratory infections among children and associated meteorological factors in southern China. BMC Infect Dis. 2015;15:124. doi: 10.1186/s12879-015-0863-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y., Reeves R.M., Wang X., et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Global Health. 2019;7(8):e1031–e1045. doi: 10.1016/S2214-109X(19)30264-5. [DOI] [PubMed] [Google Scholar]
- 26.Heraud J.M., Razanajatovo N.H., Viboud C. Global circulation of respiratory viruses: from local observations to global predictions. Lancet Global Health. 2019;7(8):e982–e983. doi: 10.1016/S2214-109X(19)30277-3. [DOI] [PubMed] [Google Scholar]
- 27.Xu B., Wang J.F., Li Z.J., et al. Seasonal association between viral causes of hospitalised acute lower respiratory infections and meteorological factors in China: a retrospective study. Lancet Planet Health. 2021;5(3):E154–E163. doi: 10.1016/S2542-5196(20)30297-7. [DOI] [PubMed] [Google Scholar]
- 28.Annan A., Ebach F., Corman V.M., et al. Similar virus spectra and seasonality in paediatric patients with acute respiratory disease, Ghana and Germany. Clin Microbiol Infect. 2016;22(4):340–346. doi: 10.1016/j.cmi.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang J.W.-T., Loh T.P. Influenza seasonality. Curr Treat Options Infect Dis. 2016;8(4):343–367. [Google Scholar]
- 30.Ianevski A., Zusinaite E., Shtaida N., et al. Low temperature and low UV indexes correlated with peaks of influenza virus activity in Northern Europe during 2010–2018. Viruses. 2019;11(3):207. doi: 10.3390/v11030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gamba-Sanchez N., Rodriguez-Martinez C.E., Sossa-Briceño M.P. Epidemic activity of respiratory syncytial virus is related to temperature and rainfall in equatorial tropical countries. Epidemiol Infect. 2016;144(10):2057–2063. doi: 10.1017/S0950268816000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao X., Chen Z., Dong H., Zhu C., Yan Y. Epidemiology of influenza in hospitalized children with respiratory tract infection in Suzhou area from 2016 to 2019. J Med Virol. 2020;92(12):3038–3046. doi: 10.1002/jmv.26015. [DOI] [PubMed] [Google Scholar]
- 33.Lim Y.K., Kweon O.J., Kim H.R., Kim T.H., Lee M.K. Clinical features, epidemiology, and climatic impact of genotype-specific human metapneumovirus infections: long-term surveillance of hospitalized patients in South Korea. Clin Infect Dis. 2020;70(12):2683–2694. doi: 10.1093/cid/ciz697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H., Wen S., Zheng J., Chen X., Lv F., Liu L. Meteorological factors affecting respiratory syncytial virus infection: a time-series analysis. Pediatr Pulmonol. 2020;55(3):713–718. doi: 10.1002/ppul.24629. [DOI] [PubMed] [Google Scholar]
- 35.Sundell N., Andersson L.M., Brittain-Long R., Lindh M., Westin J. A four year seasonal survey of the relationship between outdoor climate and epidemiology of viral respiratory tract infections in a temperate climate. J Clin Virol. 2016;84:59–63. doi: 10.1016/j.jcv.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WHO . World Health Organization; Washington DC, USA: 2019. WHO meeting of mid-term review of the RSV surveillance pilot based on the global influenza surveillance and response system: 18–20 December 2017 PAHO. [Google Scholar]
- 37.Lowen A.C., Steel J. Roles of humidity and temperature in shaping influenza seasonality. J Virol. 2014;88(14):7692–7695. doi: 10.1128/JVI.03544-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren S.-Y., Wang W.-B., Hao Y.-G., et al. Stability and infectivity of coronaviruses in inanimate environments. World J Clin Cases. 2020;8(8):1391. doi: 10.12998/wjcc.v8.i8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gustin K.M., Belser J.A., Veguilla V., et al. Environmental conditions affect exhalation of H3N2 seasonal and variant influenza viruses and respiratory droplet transmission in ferrets. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0125874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foxman E.F., Storer J.A., Fitzgerald M.E., et al. Temperature-dependent innate defense against the common cold virus limits viral replication at warm temperature in mouse airway cells. Proc Natl Acad Sci U S A. 2015;112(3):827–832. doi: 10.1073/pnas.1411030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radhakrishnan D., Ouedraogo A., Shariff S.Z., McNally J.D., Benchimol E.I., Clemens K.K. The association between climate, geography and respiratory syncitial virus hospitalizations among children in Ontario, Canada: a population-based study. BMC Infect Dis. 2020;20(1):157. doi: 10.1186/s12879-020-4882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Z.R., Zhu Y., Wang Y.Q., et al. Association of meteorological factors with childhood viral acute respiratory infections in subtropical China: an analysis over 11 years. Arch Virol. 2014;159(4):631–639. doi: 10.1007/s00705-013-1863-8. [DOI] [PubMed] [Google Scholar]
- 43.Sirimi N., Miligkos M., Koutouzi F., Petridou E., Siahanidou T., Michos A. Respiratory syncytial virus activity and climate parameters during a 12-year period. J Med Virol. 2016;88(6):931–937. doi: 10.1002/jmv.24430. [DOI] [PubMed] [Google Scholar]
- 44.Paynter S., Sly P.D., Ware R.S., Williams G., Weinstein P. The importance of the local environment in the transmission of respiratory syncytial virus. Sci Total Environ. 2014;493:521–525. doi: 10.1016/j.scitotenv.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 45.Lei C., Lou C.T., Io K., et al. Viral etiology among children hospitalized for acute respiratory tract infections and its association with meteorological factors and air pollutants: a time-series study (2014-2017) in Macao. BMC Infect Dis. 2022;22(1):588. doi: 10.1186/s12879-022-07585-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qi L., Liu T., Gao Y., et al. Effect of meteorological factors on the activity of influenza in Chongqing, China, 2012-2019. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0246023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma P., Tang X., Zhang L., et al. Influenza A and B outbreaks differed in their associations with climate conditions in Shenzhen, China. Int J Biometeorol. 2022;66(1):163–173. doi: 10.1007/s00484-021-02204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu W.K., Chen D.H., Tan W.P., et al. Paramyxoviruses respiratory syncytial virus, parainfluenza virus, and human metapneumovirus infection in pediatric hospitalized patients and climate correlation in a subtropical region of southern China: a 7-year survey. Eur J Clin Microbiol Infect Dis. 2019;38(12):2355–2364. doi: 10.1007/s10096-019-03693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loh T.P., Lai F.Y., Tan E.S., et al. Correlations between clinical illness, respiratory virus infections and climate factors in a tropical paediatric population. Epidemiol Infect. 2011;139(12):1884–1894. doi: 10.1017/S0950268810002955. [DOI] [PubMed] [Google Scholar]
- 50.Ali S.T., Cowling B.J., Wong J.Y., et al. Influenza seasonality and its environmental driving factors in mainland China and Hong Kong. Sci Total Environ. 2022;818 doi: 10.1016/j.scitotenv.2021.151724. [DOI] [PubMed] [Google Scholar]
- 51.Lam E.K.S., Morris D.H., Hurt A.C., Barr I.G., Russell C.A. The impact of climate and antigenic evolution on seasonal influenza virus epidemics in Australia. Nat Commun. 2020;11(1):2741. doi: 10.1038/s41467-020-16545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oluwole O.S.A. Dynamic regimes of El Niño southern oscillation and influenza pandemic timing. Front Public Health. 2017;5:301. doi: 10.3389/fpubh.2017.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marr L.C., Tang J.W., Van Mullekom J., Lakdawala S.S. Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. J R Soc Interface. 2019;16(150) doi: 10.1098/rsif.2018.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McMichael A.J. Extreme weather events and infectious disease outbreaks. Virulence. 2015;6(6):543–547. doi: 10.4161/21505594.2014.975022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suk J.E., Vaughan E.C., Cook R.G., Semenza J.C. Natural disasters and infectious disease in Europe: a literature review to identify cascading risk pathways. Eur J Public Health. 2020;30(5):928–935. doi: 10.1093/eurpub/ckz111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang A., Hu W., Li J., Wei R., Lin J., Ma W. Impact of heatwaves on daily outpatient visits of respiratory disease: a time-stratified case-crossover study. Environ Res. 2019;169:196–205. doi: 10.1016/j.envres.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 57.Grigorieva E., Lukyanets A. Combined effect of hot weather and outdoor air pollution on respiratory health: literature review. Atmosphere. 2021;12(6):790. [Google Scholar]
- 58.Joshi M., Goraya H., Joshi A., Bartter T. Climate change and respiratory diseases: a 2020 perspective. Curr Opin Pulm Med. 2020;26(2):119–127. doi: 10.1097/MCP.0000000000000656. [DOI] [PubMed] [Google Scholar]
- 59.Allouche J., Cremoni M., Brglez V., et al. Air pollution exposure induces a decrease in type II interferon response: a paired cohort study. eBioMedicine. 2022;85 doi: 10.1016/j.ebiom.2022.104291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guarnieri M., Balmes J.R. Outdoor air pollution and asthma. Lancet. 2014;383(9928):1581–1592. doi: 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gomez-Barroso D., León-Gómez I., Delgado-Sanz C., Larrauri A. Climatic factors and influenza transmission, Spain, 2010–2015. Int J Environ Res Public Health. 2017;14(12):1469. doi: 10.3390/ijerph14121469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Umuhoza T., Oyugi J., Mancuso J.D., Ahmed A., Bulimo W.D. Morbidity burden, seasonality and factors associated with the human respiratory syncytial virus, human parainfluenza virus, and human adenovirus infections in Kenya. IJID Reg. 2021;1:72–78. doi: 10.1016/j.ijregi.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lim K.J., John J.L., Rahim S., et al. A 1-year cross-sectional study on the predominance of influenza among hospitalized children in a tropical area, Kota Kinabalu, Sabah. J Physiol Anthropol. 2022;41(1):11. doi: 10.1186/s40101-022-00285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murray E., Klein M., Brondi L., et al. Rainfall, household crowding, and acute respiratory infections in the tropics. Epidemiol Infect. 2012;140(1):78–86. doi: 10.1017/S0950268811000252. [DOI] [PubMed] [Google Scholar]
- 65.Phung D., Huang C., Rutherford S., Chu C., Wang X., Nguyen M. Association between annual river flood pulse and paediatric hospital admissions in the Mekong Delta area. Environ Res. 2014;135:212–220. doi: 10.1016/j.envres.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 66.Smith G.S., Messier K.P., Crooks J.L., Wade T.J., Lin C.J., Hilborn E.D. Extreme precipitation and emergency room visits for influenza in Massachusetts: a case-crossover analysis. Environ Health Glob. 2017;16(1):1–8. doi: 10.1186/s12940-017-0312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haynes A.K., Manangan A.P., Iwane M.K., et al. Respiratory syncytial virus circulation in seven countries with global disease detection regional centers. J Infect Dis. 2013;208(Suppl 3):S246–S254. doi: 10.1093/infdis/jit515. [DOI] [PubMed] [Google Scholar]
- 68.Chowdhury F.R., Ibrahim Q.S.U., Bari M.S., et al. The association between temperature, rainfall and humidity with common climate-sensitive infectious diseases in Bangladesh. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0199579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Man H., Mughini Gras L., Schimmer B., Friesema I.H.M., de Roda Husman A.M., van Pelt W. Gastrointestinal, influenza-like illness and dermatological complaints following exposure to floodwater: a cross-sectional survey in the Netherlands. Epidemiol Infect. 2016;144(7):1445–1454. doi: 10.1017/S0950268815002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paterson D.L., Wright H., Harris P.N.A. Health risks of flood disasters. Clin Infect Dis. 2018;67(9):1450–1454. doi: 10.1093/cid/ciy227. [DOI] [PubMed] [Google Scholar]
- 71.Ballester J., Rodó X., Robine J.-M., Herrmann F.R. European seasonal mortality and influenza incidence due to winter temperature variability. Nat Clim Change. 2016;6(10):927–930. [Google Scholar]
- 72.Fox S.J., Miller J.C., Meyers L.A. Seasonality in risk of pandemic influenza emergence. PLoS Comput Biol. 2017;13(10) doi: 10.1371/journal.pcbi.1005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Masson-Delmotte V., Zhai P., Pörtner H.O., Roberts D., Skea J., Shukla P.R. Global Warming of 1.5° C: IPCC Special Report on Impacts of Global Warming of 1.5° C above Pre-industrial Levels in Context of Strengthening Response to Climate Change, Sustainable Development, and Efforts to Eradicate Poverty. Cambridge University Press; 2022. [Google Scholar]
- 74.Ferrero F., Torres F., Abrutzky R., et al. Seasonality of respiratory syncytial virus in Buenos Aires. Relationship with global climate change. Arch Argent Pediatr. 2016;114(1):52–55. doi: 10.5546/aap.2016.eng.52. [DOI] [PubMed] [Google Scholar]
- 75.Mamalakis A., Randerson J.T., Yu J.-Y., et al. Zonally contrasting shifts of the tropical rain belt in response to climate change. Nat Clim Change. 2021;11(2):143–151. doi: 10.1038/s41558-020-00963-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baker R.E., Mahmud A.S., Wagner C.E., et al. Epidemic dynamics of respiratory syncytial virus in current and future climates. Nat Commun. 2019;10(1):5512. doi: 10.1038/s41467-019-13562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Q., Tan Z.-M., Sun J., Hou Y., Fu C., Wu Z. Changing rapid weather variability increases influenza epidemic risk in a warming climate. Environ Res Lett. 2020;15(4) [Google Scholar]
- 78.Holmes E.C. COVID-19—lessons for zoonotic disease. Science. 2022;375(6585):1114–1115. doi: 10.1126/science.abn2222. [DOI] [PubMed] [Google Scholar]
- 79.Morse S.S., Mazet J.A.K., Woolhouse M., et al. Prediction and prevention of the next pandemic zoonosis. Lancet. 2012;380(9857):1956–1965. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carlson C.J., Albery G.F., Merow C., et al. Climate change increases cross-species viral transmission risk. Nature. 2022;607(7919):555–562. doi: 10.1038/s41586-022-04788-w. [DOI] [PubMed] [Google Scholar]
- 81.Lemieux A., Colby G.A., Poulain A.J., Aris-Brosou S. Viral spillover risk increases with climate change in High Arctic lake sediments. Proc R Soc B. 2022;289(1985) doi: 10.1098/rspb.2022.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Memarzadeh F. Literature review of the effect of temperature and humidity on viruses. ASHRAE Trans. 2021;118(1):1049–1060. [Google Scholar]
- 83.Pica N., Chou Y.Y., Bouvier N.M., Palese P. Transmission of influenza B viruses in the guinea pig. J Virol. 2012;86(8):4279–4287. doi: 10.1128/JVI.06645-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shaw Stewart P.D., Bach J.L. Temperature dependent viral tropism: understanding viral seasonality and pathogenicity as applied to the avoidance and treatment of endemic viral respiratory illnesses. Rev Med Virol. 2022;32(1):e2241. doi: 10.1002/rmv.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kudo E., Song E., Yockey L.J., et al. Low ambient humidity impairs barrier function and innate resistance against influenza infection. Proc Natl Acad Sci U S A. 2019;116(22):10905–10910. doi: 10.1073/pnas.1902840116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adivitiya, Kaushik M.S., Chakraborty S., Veleri S., Kateriya S. Mucociliary respiratory epithelium integrity in molecular defense and susceptibility to pulmonary viral infections. Biology (Basel) 2021;10(2):95. doi: 10.3390/biology10020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang D., Taha M.S., Nocera A.L., Workman A.D., Amiji M.M., Bleier B.S. Cold exposure impairs extracellular vesicle swarm-mediated nasal antiviral immunity. J Allergy Clin Immunol. 2023;151(2):509–525.e8. doi: 10.1016/j.jaci.2022.09.037. [DOI] [PubMed] [Google Scholar]
- 88.Sacks D., Baxter B., Campbell B.C.V., et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612–632. doi: 10.1177/1747493018778713. [DOI] [PubMed] [Google Scholar]
- 89.Damoiseaux J., Smolders J. The engagement between vitamin D and the immune system: is consolidation by a marriage to be expected? eBioMedicine. 2018;31:9–10. doi: 10.1016/j.ebiom.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wheeler T., von Braun J. Climate change impacts on global food security. Science. 2013;341(6145):508–513. doi: 10.1126/science.1239402. [DOI] [PubMed] [Google Scholar]
- 91.Gwela A., Mupere E., Berkley J.A., Lancioni C. Undernutrition, host immunity and vulnerability to infection among young children. Pediatr Infect Dis J. 2019;38(8):e175–e177. doi: 10.1097/INF.0000000000002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rohr J.R., Barrett C.B., Civitello D.J., et al. Emerging human infectious diseases and the links to global food production. Nat Sustain. 2019;2(6):445–456. doi: 10.1038/s41893-019-0293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Randolph D.G., Refisch J., MacMillan S., et al. Preventing the next pandemic: zoonotic diseases and how to break the chain of transmission. The United Nations Environment Programme (UNEP); 2020. [Google Scholar]
- 94.Zscheischler J., Martius O., Westra S., et al. A typology of compound weather and climate events. Nat Rev Earth Environ. 2020;1(7):333–347. [Google Scholar]
- 95.Lawrence J., Blackett P., Cradock-Henry N.A. Cascading climate change impacts and implications. Climate Risk Manage. 2020;29 doi: 10.1016/j.mex.2020.100893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Malhi Y., Franklin J., Seddon N., et al. Climate change and ecosystems: threats, opportunities and solutions. Philos Trans R Soc Lond B Biol Sci. 2020;375(1794) doi: 10.1098/rstb.2019.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allen T., Murray K.A., Zambrana-Torrelio C., et al. Global hotspots and correlates of emerging zoonotic diseases. Nat Commun. 2017;8(1):1124. doi: 10.1038/s41467-017-00923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amuasi J.H., Lucas T., Horton R., Winkler A.S. Reconnecting for our future: the lancet one health commission. Lancet. 2020;395(10235):1469–1471. doi: 10.1016/S0140-6736(20)31027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zscheischler J., Westra S., van den Hurk B.J.J.M., et al. Future climate risk from compound events. Nat Clim Change. 2018;8(6):469–477. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.