Summary
Background
Climate change, in particular the exposure to heat, impacts on human health and can trigger diseases. Pregnant people are considered a vulnerable group given the physiological changes during pregnancy and the potentially long-lasting consequences for the offspring. Evidence published to date on higher risk of pregnancy complications upon heat stress exposure are from geographical areas with high ambient temperatures. Studies from geographic regions with temperate climates are sparse; however, these areas are critical since individuals may be less equipped to adapt to heat stress. This study addresses a significant gap in knowledge due to the temperature increase documented globally.
Methods
Birth data of singleton pregnancies (n = 42,905) from a tertiary care centre in Hamburg, Germany, between 1999 and 2021 were retrospectively obtained and matched with climate data from the warmer season (March to September) provided by the adjacent federal meteorological station of the German National Meteorological Service to calculate the relative risk of heat-associated preterm birth. Heat events were defined by ascending temperature percentiles in combination with humidity over exposure periods of up to 5 days. Further, ultrasound data documented in a longitudinal prospective pregnancy cohort study (n = 612) since 2012 were used to identify pathophysiological causes of heat-induced preterm birth.
Findings
Both extreme heat and prolonged periods of heat exposure increased the relative risk of preterm birth (RR: 1.59; 95% CI: 1.01–2.43; p = 0.045; RR: 1.20; 95% CI: 1.02–1.40; p = 0.025). We identified a critical period of heat exposure during gestational ages 34–37 weeks that resulted in increased risk of late preterm birth (RR: 1.67; 95% CI: 1.14–1.43; p = 0.009). Pregnancies with a female fetus were more prone to heat stress-associated preterm birth. We found heat exposure was associated with altered vascular resistance within the uterine artery.
Interpretation
Heat stress caused by high ambient temperatures increases the risk of preterm birth in a geographical region with temperate climate. Prenatal routine care should be revised in such regions to provide active surveillance for women at risk.
Funding
Found in acknowledgements.
Keywords: Heat stress, Preterm birth, Pregnancy, Climate change, Temperature
Research in context.
Evidence before this study
Pregnancy leaves women especially vulnerable to external stressors. Climate change therefore poses a significant risk of maternal and fetal health. Recently, an increasing number of studies indicate that high temperatures and subsequent heat stress increases the risk of pregnancy adversities such as preterm birth, stillbirth, and low birth weight. Still, the majority of studies were conducted in geographical regions with high average temperatures, leaving a blind spot to the effects of heat periods in regions with temperate climate. Also, even though the harmful effects of excessive heat exposure during pregnancy have been demonstrated, no pathophysiological mechanism has been reported that triggers premature birth following heat stress.
Added value of this study
Although the number of studies addressing the consequences of heat stress in the context of pregnancy is slowly picking up, data originating from regions with temperate climate are still sparse, even though individuals lack acclimatization to high temperatures. We address this gap by investigating the effect of heat exposure on pregnancy outcome in Hamburg, Germany, a northern European city. In order to meet the ambiguity of published heat stress definitions, our study provides the direct comparison of the maximum temperature and apparent temperature, which includes humidity as a factor. Additionally, we depict temperature intensity based on ascending temperature percentiles for varying exposure intervals, which allows to draw conclusion on the effects of both heat intensity and exposure duration. Our study uses data provided by a single clinical centre, which results in a high homogeneity and quality of data acquisition especially relevant in the field of pregnancy care. Finally, by using ultrasound data, our study seeks to provide a starting-point for the identification of pathophysiological causations related to heat-associated pregnancy adversities.
Implications of all the available evidence
Our study highlights the increased risk of late preterm births due to heat stress in a geographical region with temperate climate. This emphasizes the need of health care professionals such as obstetricians to address heat-induced health adversities in regions of the geographical north. Also, we illustrate the importance of an adequate heat stress definition, since the observed effects highly depends on the heat intensity, duration and multifactorial parameters, e.g., humidity.
Introduction
Pregnancy is often compared to a ‘stress test’ given the physiological changes that occur such as increased respiratory rate and cardiac output1,2 and hence, it is not surprising that the course of pregnancy can be easily jeopardized by environmental challenges. Challenges assessed over the last decades mostly focused on maternal infections, distress, disadvantageous socio-economic status and others, which could all be linked to adverse birth outcomes.3,4 In the context of the climate crisis, exposure to high ambient temperatures and consequential heat stress now has to be considered among the challenges that may interfere with pregnancy progression and birth outcomes.5
Strikingly, pregnant women have largely been underrepresented in studies assessing the effect of heat stress carried out to date.6 Yet, it has been proposed that 25,000 infants per year experience a reduction in gestational length in the US as a direct consequence of maternal heat stress.7 Similarly, a recent study concluded that from 2010 to 2020 13,000 preterm births in China were caused by heatwaves.8
Insights emerging from studies focusing on geographical areas world-wide with high average temperatures highlight that heat stress is related to an increased risk of pregnancy complications.9, 10, 11, 12, 13 Clearly, the women assessed in these studies were mostly fully acclimatized to high ambient temperatures,14, 15, 16, 17, 18, 19 whereas studies from geographical regions with temperate climate remain sparse.20, 21, 22, 23 Pregnancy complications that these studies focused on include low fetal weight, stillbirth and preterm birth, the latter defined as delivery of the baby before 37 completed weeks of gestation.11,24,25
Preterm birth affects approximately 11% of all pregnancies worldwide and is one of the most common causes for infant morbidity and mortality. Despite ongoing medical progress, a reduction of preterm birth incidence could not be achieved over the last decade. Therefore, a possible risk perpetuation that may be caused by the ongoing climate crisis must be urgently evaluated.26,27
The aim of the study we here present was to identify the impact of heat exposure on the risk of preterm birth in Hamburg, Germany, a northern European city with temperate climate. We utilized registry data on birth outcomes documented over the last two decades in the University Medical Centre Hamburg-Eppendorf, a tertiary care centre in northern Germany. We evaluated the maximum as well as the apparent temperature, which also considers humidity, to enable a direct comparison to results from studies which solely focused on one of these criteria. Since extreme temperatures can affect the circulatory system during pregnancy due to difficulties in heat dissipation and thermoregulation,28, 29, 30, 31 we leveraged ultrasound data documented in the PRINCE study (Prenatal Identification of Children's Health), a longitudinal prospective pregnancy study performed in Hamburg since the year 2012, to identify heat stress-associated vascular pathogenesis of pregnancy complications.
Methods
Description of study populations
Registry study
Our analysis of heat exposure and preterm birth was based on data on pregnancy outcome retrospectively retrieved from the central electronic patient registry of the Department of Obstetrics and Fetal Health, University Medical Centre Hamburg-Eppendorf; an urban university hospital providing tertiary medical care for more than 497,000 patients per year. We included anonymized data from singleton live births documented between the years 1999 and 2021 (n = 42,905) by the attending physicians. The size of our cohort is determined by the availability of documented patient data in the electronic clinic data base, which was implemented in 1999. The data base query was performed in December 2021 by an authorized clinician. Gestational age (GA) was determined in weeks by the last menstrual period and corrected by first trimester ultrasound, if applicable. We restricted our analyses on birth data obtained during the warmer season of each year, between March and September, as no heat exposure periods occurred outside this seasonal timespan. This restriction resulted in a final study size of n = 25,624.
PRINCE study
The effect of heat stress-associated vascular pathogenesis during pregnancy was determined using data documented in the PRINCE study. This is a prospective longitudinal pregnancy study initiated in 2011 at the Department of Obstetrics and Prenatal Medicine of the University Medical Centre Hamburg-Eppendorf. The PRINCE study included women at an age ≥18 with a viable pregnancy at 12–14 weeks of gestation. Women with chronic infections, known drug or alcohol abuse, multiple pregnancies or pregnancies induced by assisted reproductive technology were excluded. In this study, maternofetal ultrasound was performed at GA of 12–14, 24–26 and 34–36 weeks. At the time point of analysis, PRINCE involved n = 612 study participants, of whom n = 386 gave birth between March and September of the respective year.
Outcome assessment
Preterm and term births were the defined outcomes; whereby preterm birth was defined as GA less than 37 weeks at delivery. Cases of preterm birth were further subdivided into late preterm birth (GA 34+0–36+6), early preterm birth (GA 28+0–33+6) and very early preterm birth (GA 24+0–27+6). Spontaneous delivery was defined by excluding all patients with a code for a Caesarean section, as listed in Table S1.
From the available feto-maternal ultrasound measurements performed at GA 34–36 in the PRINCE study (n = 612), we used the pulsatility index (PI) of the uterine and umbilical arteries for our analyses. For the uterine artery, the arithmetic mean of the PI of the left and right artery was determined. The PI provides information on the peripheral vascular resistance. With regard to the uterine artery, the PI serves as a proxy for uterine blood flow from mother to fetus, whereas the PI of the umbilical artery allows to deduce information on fetal blood flow back to the placenta/mother. Both are of great interest since compromised placental blood flow is known to contribute to preterm birth.28
Exposure assessment
Heat stress, also referred to as heat exposure, was defined as heat events during the week preceding delivery. This exposure window was chosen based on previously published epidemiologic studies on the association of pregnancy outcomes upon heat stress in high ambient temperature environments.16,32,33
In order to standardize the definition of heat stress, we applied two parameters to define heat events: (a) the daily maximum temperature and (b) the daily apparent temperature, which also includes the daily average relative humidity as a factor.11 The apparent temperature was approximated by Thom's Discomfort Index (DI)34 to evaluate heat stress in humans and has been applied since in various studies to monitor thermal discomfort.35, 36, 37 In our study, we used a definition of DI38 adapted for relative humidity as shown in Equation (1):
| (1) |
Data on daily temperature as well as relative humidity were obtained from the federal German National Meteorological Service (Deutscher Wetterdienst) from the meteorological monitoring station Hamburg-Fuhlsbüttel, situated 5 km from the clinic. Temperatures are relatively stable across the city owing to the uniform topography at sea level.
Heat events were identified by applying relative thresholds (the 90th, 95th, 98th and 99th percentile) occurring during the period between March and September for each year. For a heat event, at least 2, 3, 4 or 5 consecutive heat days were taken into consideration. The variation in percentiles representing different heat intensities and exposure duration allows the validation of a possible dose–response relationship between heat and preterm birth and were chosen in accordance to a published study.16
To investigate whether thermoregulation affects the blood flow to the placenta, we focused on heat events defined by the apparent temperature based on the 90th and 95th percentile at 2, 3 or 4 consecutive heat days prior to the ultrasound assessment.
Statistics
Frequencies (%) of maternal and fetal characteristics of the registry study as well as maxima, minima, median and interquartile ranges of PI values from the PRINCE study were described. Further, means, maxima and minima of the environmental conditions for each heat event definition averaged for the entire study period were calculated. For the analysis of the risk of heat-induced preterm birth, the annual number of heat- and non-heat-associated preterm and term births was computed for each heat event definition. Subsequently, we entered the frequency distributions in a contingency table for the four variables “heat-exposed”, “non-heat-exposed”, “term” and “preterm”. In order to test the null hypothesis, postulated as no association between heat exposure and preterm delivery exists, Pearson's chi-squared independence test (Pearson χ2 test) was carried out, with the condition that the underlying assumptions were fulfilled. Relative risks (RR) and 95% confidence intervals (95% CI) for preterm birth after heat exposure for the entire study period were computed. For calculation of 95% CI, the Koopman asymptotic score was used according to published methodologies.39, 40 The risk of heat-induced preterm birth was also independently calculated for the subgroups ‘very early’, ‘early’ and ‘late’ preterm birth, as outlined above. Additionally, we evaluated the influence of fetal sex on heat stress-induced preterm birth. Here, we divided our study population in female and male offspring and calculated the RR for preterm birth after heat exposure for each sex separately. Again, Pearson χ2 test was carried out to test the null hypothesis. Further, we excluded all births which did not match the criteria of spontaneous delivery (specified below) and evaluated the RR for preterm birth upon heat exposure. In a last step, we considered widely accepted risk factors for preterm birth, such as maternal age, and parity, as possible confounders and performed a multiple modified Poisson regression analysis as suggested by Zou et al. 2004.41 Preterm respectively term births were defined as the dependent variable and heat exposure as well as maternal age (dichotomized indicating a pregnancy at risk of preterm birth for women aged under 20 and above 44) were entered in the regression models as binary variables, whereas parity was defined as a continuous covariate. Two-way interaction terms between heat events and age as well as heat events and parity were assessed. Since no significant interactions were identified, we excluded interaction terms from the reported model. We depict the adjusted RR for the effect of heat exposure on the risk of a preterm birth.
To statistically analyse the data obtained from ultrasound examinations, we first performed Shapiro–Wilk test to evaluate if the sampled data follow a Gaussian distribution. To approach Gaussian distribution, we decided to logarithmically (log 10) transform PI values. For the group of non-heat exposed individuals, we follow the assumption of the Central Limit Theorem,42 which postulates normal distribution of data with large sample sizes. Additionally, F-Test was used to evaluate the homogeneity of variance between the two groups (non-heat-exposed vs. heat-exposed). This allowed comparison by unpaired t-test for normally distributed data.
Interactions of outcome and exposure parameters with mediators and confounders are illustrated in a causal directed acyclic graph (Fig. S1).
Details on sample size determination as well as exclusion and inclusion criteria can be found within the method section in the description of study populations. Randomization and blinding of participants was not applicable for this study.
Ethics
The PRINCE study was approved by the ethics committee of the Hamburg Chamber of Physicians under the registration number PV3694, all participants have signed the informed consent forms. Registry data provided by the University Medical Centre Hamburg-Eppendorf were analysed using anonymized data. Anonymization was achieved via the institutional data trust and transfer office. Appropriate safeguards for protecting the confidentiality of registry data were developed and balanced against the risks and benefits of the anticipated research outcomes. The Registry study was filed under the registration number 2023-300330-WF at the Hamburg Chamber of Physicians.
Role of funders
The funders had no role in designing this study, data acquisition, analysis and interpretation, and decision to publish or prepare this manuscript.
Results
Description of study population, ultrasound measurements and exposure data
Among the 25,624 cases of birth included in the registry analysis, 91.49% were classified as term births and 8.51% as preterm birth (Fig. 1a). Among the latter, late preterm births accounted for the majority of preterm birth cases (65.11%), followed by early (27.69%) and very early preterm births (7.20%). These proportions are comparable to other studies investigating the prevalence of preterm birth in Germany.26,43, 44 The frequency of preterm birth was higher in mothers of an age <20 and >45 years. Further characteristics of the study population are described in Table S2. Ambient temperatures from March to September in Hamburg are around 20 °C and can therefore be considered as temperate. However, the recording of the ambient temperatures from 1936 to 2021 highlight a steady increase (Fig. 1b). Study participants live in close proximity to the clinic and the federal meteorological monitoring station. Analysis of residential addresses document that 71.8% of study participants live within a radius of 10 km to the federal meteorological monitoring station and 83.4% live within a radius of 15 km (Fig. 1c). This strongly suggests that the majority of women included in this study were exposed to heat as deduced from the data provided by the federal meteorological monitoring station. The methodological work flow used for the analyses of these study populations is schematically summarized in Fig. 1d.
Fig. 1.
Overview of births and preterm births, course of ambient temperature, the city of Hamburg with the meteorological station,and schematic overview of the experimental design of the analysis, utilizing data from the registry and PRINCE study. (a) Total number of births (grey bars, left Y axis) and percentage of preterm births (solid black line, right Y axis) per year, documented at the University Medical Centre Hamburg-Eppendorf between 1999 and 2021. (b) Average maximum temperature between March and September in Hamburg, Germany during the period 1999–2021 (black line) and heat map depicting the increase in maximum ambient temperatures. Insert: course of average maximum temperature increase (March–September) per year between 1936 and 1998. The colour codes of the heat maps depict the deviation from the mean for each year. (c) The city of Hamburg with the exact location of the University Medical Centre Hamburg-Eppendorf and the federal meteorological monitoring station. Grey circles represent varying distances from the monitoring station. Percentages indicate the residential addresses of registry study participants. (d) Overview of the study design and schematic depiction of the methodological work flow of the two main analyses.
Among the participants of the PRINCE study, available ultrasound examinations conducted between March and September from 2012 to 2021 resulted in a final dataset of n = 386, which is 63.1% of all PRINCE study participants. Characteristics of this study subpopulation are provided in Table S3. The PI, an indicator for peripheral resistance and uterine perfusion, varied from a minimum of 0.56 to a maximum of 1.66 for the umbilical artery with a median of 0.90. The PI of the uterine artery ranged from a minimum of 0.32 to a maximum of 2.00 with a median of 0.65 as listed in Table S4. The absolute values for the environmental conditions, maximum temperature and humidity related to the registry and PRINCE study are summarized in Table S5.
Periods of heat stress increased the relative risk of preterm birth
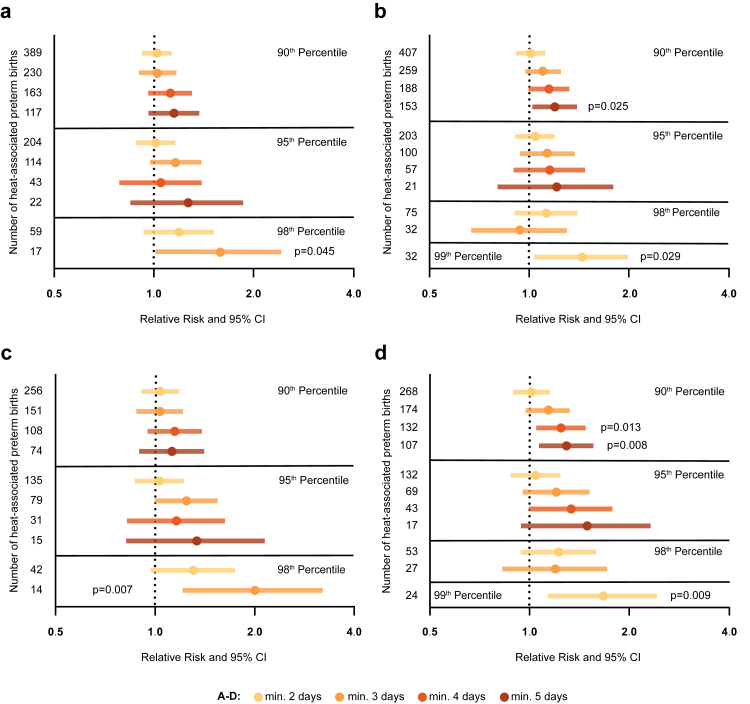
The RR increased with both, the duration and intensity of the heat events. Especially with regard to intense heat events—defined by the 98th percentile and 3 consecutive exposure days—a relevant effect of heat can be assumed, given a RR of 1.59, a 95% CI of 1.01–2.43 and p = 0.045 [Pearson χ2 test] (Fig. 2a; Table S6). When considering the apparent temperature, the RR was generally higher (Fig. 2b; Table S7). Here, the overall increase in the RR, based on the intensity of heat events as well as the exposure period, was reproduced and suggests a dose–response effect. Noteworthy, prolonged heat events of 5 consecutive days in the 90th percentile resulted in an increased RR of 1.20 with positive 95% CI of 1.02–1.40 and p = 0.025 [Pearson χ2 test]. For the apparent temperature, the absolute number of heat-associated preterm births were higher, which enabled the statistical evaluation of the 99th percentile, where exposure for 2 consecutive days was linked to a 1.45 increase in the RR for preterm birth (95% CI: 1.04–2.00; p = 0.029 [Pearson χ2 test]).
Fig. 2.
Periods of heat stress increase the relative risk of preterm birth, especially during gestation weeks 34–36 + 6 (late preterm birth). Relative risk of preterm delivery after heat exposure. Heat events are defined by the daily maximum temperature (a) or the daily apparent temperature (b) for all preterm births. Heat events defined by the daily maximum temperature (c) or the daily apparent temperature (d), stratified for late preterm births (n = 24,863). The number of preterm births per category is shown on the Y-axis; X-axis is displayed in log scale; RR, 95% CI; Pearson χ2.
Women between gestational age 34 and 37 were particularly vulnerable to heat stress-induced preterm birth
Premature onset of birth can be classified in very early, early and late preterm birth. Whilst very early and early preterm births may increase neonatal mortality, children born late preterm still face considerable health inequalities throughout life, including a high risk of infections, allergies and asthma, and neurocognitive impairments.45, 46 Therefore, we performed sensitivity analyses for the distinct subgroups of very early, early and late preterm birth. Strikingly, a higher RR for preterm birth was exclusively detectable in pregnancies exposed to heat events between GA 34+0 and 36+6, accounting for late preterm birth. Here, the most relevant effect of heat can be assumed for heat events defined as 3 consecutive heat days, exceeding the 98th percentile (RR: 2.00; 95% CI: 1.21–3.21; p = 0.007 [Pearson χ2 test]) (Fig. 2c; Table S8). When the apparent temperature was considered as heat stress, the increase of RR in late preterm births was even more pronounced (Fig. 2d; Table S9). Here, prolonged heat exposure exceeding the 90th percentile (min. 4 days: RR: 1.25; 95% CI: 1.05–1.48; p = 0.013 [Pearson χ2 test]; min. 5 days: RR: 1.29; 95% CI: 1.07–1.56; p = 0.008 [Pearson χ2 test]) and heat days exceeding the 99th percentile (RR: 1.67; 95% CI: 1.14–2.43; p = 0.009 [Pearson χ2 test]) were significant. Increased CI observed here likely arise from the decreased study subpopulation (late preterm birth n = 1420). Analyses of early and very early cases of preterm birth allowed to identify a decreased association between heat exposure and preterm birth, suggesting a pathogenesis unrelated to heat stress. However, the sensitivity of these analysis was impaired due to lower numbers (early preterm birth: n = 604; very early preterm birth: n = 157, data not shown).
Heat stress particularly affected pregnancies with a female fetus
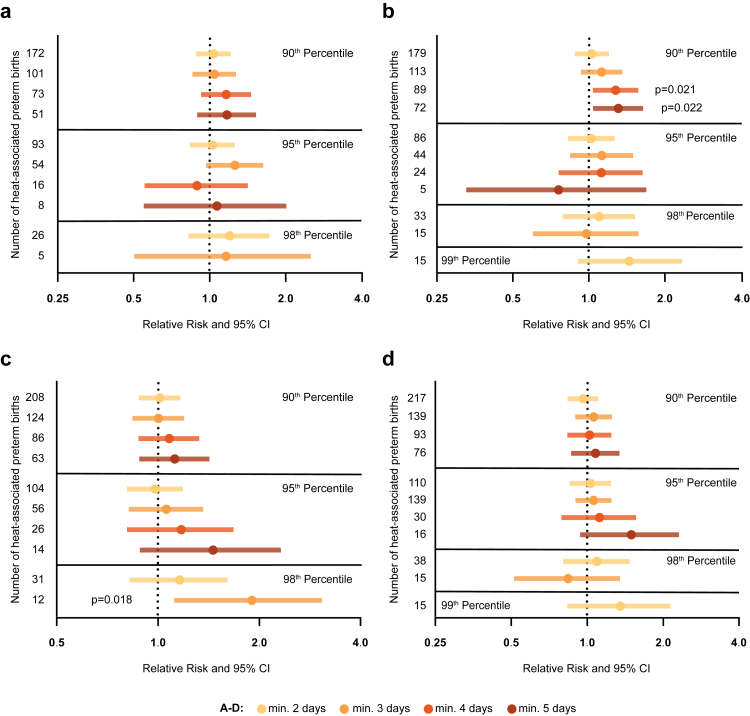
Since sex-specific differences in response to environmental stressors are subject of intense research, we also evaluated the risk of heat stress-associated preterm birth by fetal sex. Our analysis revealed that pregnancies with a female fetus were more vulnerable for preterm birth upon heat stress, defined by the 90th percentile, although statistical significance was exclusively reached when the apparent temperature was taken into account (min. 4 days: RR: 1.28; 95% CI: 1.04–1.57; p = 0.021 [Pearson χ2 test] & min 5 days: RR: 1.31; 95% CI: 1.04–1.64; p = 0.022 [Pearson χ2 test]) (Fig. 3a and b; Tables S10 and S11). Significant heat stress-related effects were not detectable when focusing on male offspring, although slight increases in RR were identified (Fig. 3c and d; Tables S12 and S13). In higher percentiles, we observed a significant heat stress effect on preterm birth rates in pregnancies with a male fetus in the 98th percentile (RR: 1.90; 98% CI: 1.12–3.07; p = 0.018 [Pearson χ2 test]). However, the small cohort size and corresponding low statistical power needs to be taken into consideration with regard to the biological significance of this observation.
Fig. 3.
Heat stress particularly affects pregnancies with a female fetus. Relative risk of preterm delivery after heat exposure stratified for fetal sex. Heat events defined by the daily maximum temperature (a) and the daily apparent temperature (b) in pregnancies with a female offspring, and male offspring (c, d) respectively (female n = 12,552, male n = 12,954). The number of preterm births per category and fetal sex is shown on the Y-axis; X-axis is displayed in log scale; RR, 95% CI; Pearson χ2.
Heat stress increases the risk of a spontaneous onset of birth rather than a medically induced preterm birth
Next, we restricted our analysis of the registry data to cases with spontaneous onset of preterm labour. This was accomplished by excluding all patients with a code for a Cesarian section, as listed in Table S1. These analyses highlight that heat stress indeed increases the risk of a spontaneous onset of preterm birth rather than medically induced preterm birth, e.g., in pregnancies complications requiring immediate, premature delivery via Cesarian section, e.g., in pre-eclampsia (Fig. S2a and b; Tables S14 and S15).
Multiple modified Poisson regression analyses confirmed that the observed heat-associated effects were not confounded by maternal age or parity (Tables S16 and S17).
Heat events and ultrasound measurements; heat stress is associated with an increased placental perfusion and lower peripheral resistance of the uterine artery
We next aimed to identify a pathogenesis through which heat stress may trigger the onset of birth prematurely. Given that exposure to high temperatures is met by an acclimatization response of the cardiovascular system, we focused on circulatory alterations affecting the uterine and fetal blood flow during heat stress. Using the data from ultrasound examinations in the PRINCE study, we evaluated the PI of the uterine artery of the mother as a proxy for uterine blood flow as well as the PI of the umbilical artery for fetal blood flow. Whilst no differences could be identified for the umbilical artery PI between heat-exposed and non-heat-exposed pregnant women (Fig. 4a), the PI of the uterine artery was decreased in pregnancies exposed to heat, reaching levels of significance for 4 consecutive days exceeding the 90th percentile (Fig. 4b). For heat events defined by other percentiles, no significant alterations were detectable (data not shown). Representative ultrasound images of a high and low uterine artery PI taken at GA 34–36 are shown in Fig. 4c and d.
Fig. 4.
Heat stress prior is associated with an increased placental perfusion and lower peripheral resistance of the uterine artery. Logarithmized pulsatility index (logPI) of the umbilical (a) and uterine (b) artery from heat-exposed and non-heat-exposed pregnant women, measured by ultrasound assessment (n = 386). Representative ultrasound images of high (c) and low PI (d), examined at GA 34–36. Scatter-plots represent median + IQR; unpaired t-test.
Discussion
A temperature increase up to 5 °C until the year 2100 has been projected not only for Hamburg, but also other cities and regions with a temperate climate.47 This projection underscores the urgency of recognizing individuals which are highly vulnerable to the impending heat stress. We here provide compelling evidence that heat stress affects the progression of pregnancy by increasing the risk of preterm birth. The uniqueness of our study lies in the geographic region assessed, which is under temperate climate.
We could unearth not only temperature-, but also duration-dependent effects, as durations of a minimum of five consecutive days of heat stress generally had higher effects on the risk of preterm birth than between periods of a minimum of two days on heat. In our analyses, we differentially evaluated two parameters of heat stress on the risk of preterm birth, temperature and humidity. Whilst both parameters gave similar temperature- and duration-dependent effects, the impact of apparent temperature can be considered as higher compared to defining heat stress by the mere temperature alone. Since the definition of apparent temperature also considers humidity, the impeded ability to dissipate heat to the environment by convection or evaporation may account for the higher impact of the apparent temperature compared to temperature alone.48
Interestingly, another study also carried out in Germany focused on seasonality and birth and could not detect an increased risk of preterm birth.49 This suggests that assessment of seasonal patterns does not suffice to identify such risk, as a definition of heat stress must seem to require a clear-cut definition by maximum or apparent temperature, as tested in our study.
Studies conducted in regions where humid and dry-winter subtropical climates are found, such as southern China, California or eastern Australia, also revealed a higher risk of preterm birth.16,18,20,32,50 Despite the temperate climate to which the women in our study were exposed, we here observed—at least in part—a higher effect size. This may either be explained by the lag of northern European populations acclimatizing to increasing heat stress, or the high quality of clinical data. Nonetheless, we did not consider possible cumulative effects of heat stress, atmospheric ozone, and air pollution by particulate matter during pregnancy. Noteworthy, the relationship between temperature, humidity and air pollutants underlies large variations depending on geographical characteristics, the type of air pollutant and seasonality—e.g., concentrations of particulate matter 2.5 and 10 seem to be negatively whereas ozone concentrations positively correlated with temperature.51 When considering air pollution as mediator, regional relationships of these variables must be considered. Although air pollution in Hamburg meets local regulations,52 association of air pollution and heat exposure with adverse pregnancy outcomes should be taken into account in future assessments.53, 54, 55 Additionally, the relationship between indoor and outdoor temperatures as well as physical activity might modulate the risk of heat-associated preterm birth. However, as they are tightly linked to the complex topic of metabolic heat production, they cannot be separately assessed from thermoregulation through nutrition, which is out of the scope of this study.56, 57
We were intrigued by our finding that heat stress particularly affects the risk of late preterm birth (GA 34–36). It can be hypothesized that the threshold for triggering premature delivery by environmental exposures is lowered in late preterm birth compared to women earlier in gestation, since up to 4 weeks before delivery molecular changes can be detected, which induce a shift from pregnancy maintenance to a state of prelabour.58, 59, 60
However, the smaller group sizes affected by early and very early preterm birth must be considered as a limiting factor of the statistical power to detect a heat-related effect.
Strikingly, in our study pregnancies with a female fetus show a higher risk of heat stress-related preterm birth, which is in accordance with results reported in a study conducted in Changsha, China.61 It is well known, that male and female fetuses react differently to intrauterine stress. Pregnancies with a male fetus are per se considered to be at higher risk of complications, e.g., higher rates of preterm birth and stillbirth. This observation is often referred to as ‘male fragility’.62, 63 However, female fetuses have been shown to be more responsive to intrauterine stress and adverse events, which is associated with alterations of fetal growth and placental size.64 However, the underlying pathogenesis of preterm birth in pregnancies with a female fetus is still a matter of speculation. Since a higher heart rate has been observed in female fetuses,65 it is tempting to speculate that this might account for a lower threshold for fetal distress in female fetuses after exposure to heat stress, subsequently triggering the onset of birth prematurely.
In healthy pregnancies, the PI is known to decrease progressively towards term, whereas an abnormal PI can indicate pregnancy adversities such as fetal growth restriction or preeclampsia. In our analyses, we observed that a prolonged exposure to heat stress of 26.6 °C over a period of at least 4 days prior to a fetal ultrasound examination was associated with a lower PI of the uterine artery indicating an increase in placental perfusion. Blood flow to the placenta depends on the systemic blood pressure.66 The systemic blood pressure can be modulated during heat stress as a compensatory mechanism to dissipate heat, e.g., through the skin.48, 67 Our data indicate that heat stress may not only initiate the redistribution of blood to the skin, but also to the fetus. Noteworthy, the maintenance of a constant blood pressure in the context of an increased placental perfusion during heat stress periods burdens the cardiovascular system, as reflected by a higher maternal heart rate (Fig. S3).
The strength of our study lies on the usage of data provided by a single clinical centre, which results in a high homogeneity and quality of data acquisition. Nevertheless, the retrospective character of our study as well as the relatively low number of study participants must be considered as limitation. Our study also benefits from the close proximity between the residence of study participants, the federal meteorological monitoring station and the hospital where all study participants gave birth. Thus, we can deduce that the vast majority of cases included in these analyses were indeed exposed to heat deduced from the data obtained from the federal meteorological station.
The differences in statistical approaches applied in studies assessing the effect of heat stress on health and disease, including the risk of pregnancy complications, limits the options to directly compare findings between studies. The statistical approach we chose, which was based on the Pearson χ2 test and Poisson regression model, provided the RR as a robust and accessible outcome measure. We also considered case crossover approaches, as these provide an additional level of robustness towards individual confounding factors.68, 69 However, these statistical approaches are most appropriate in cohort studies with a larger number of events of interest—in our case women experiencing a heat associated delivery.70, 71 Noteworthy, we refrained from including time series analysis,72 although such statistical approach had been used in studies on pregnancy adversities and heat stress.13, 15, 49 Time series analyses provide important insights on vulnerable exposure windows to heat stress during pregnancy, whilst the scope of our study was based on a specific exposure timeframe, the week preceding delivery.
The ongoing climate crisis highlights the urgent need to address the imminent heat stress-related health risks in the highly vulnerable population of pregnant women and their unborn children. Health care professionals, such as obstetricians must not only be alerted of such risks, but guidelines need to be developed to propose behavioural adaptations and active surveillance tools during heat events.73
Contributors
P.C.A., A.D., E.S., D.Y. and I.G. conceptualized the study. K.H., A.D., P.C.A., A.C.T. and B.H. contributed to clinical data acquisition and provision. P.C.A. and A.D. provided essential resources, substantially contributed to the interpretation of the data and acquired funding to support the study. D.Y., C.W., A.P., and I.G. developed methodology, acquired meteorological data and performed data curation. I.G. performed formal data analysis, D.Y., A.P. and A.D. verified the underlying data. C.W. carried out statistical validation. D.S. validated meteorological aspects. D.Y. and I.G. drafted the first version and developed figures. P.C.A., A.P. and A.D. reviewed the manuscript critically for important intellectual content and conducted editing and rewriting. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Also, all authors have read and approved the final version of the manuscript and were responsible for the decision to submit the manuscript.
Data sharing statement
Meteorological data are publicly available and openly accessible at the online databases of the German Weather Service. Data related to the PRINCE study can—in principle—be made available in anonymized form by the corresponding author after proposal approval. Data from the registry study have been retrieved from the electronic patient files, anonymized by the institutional research platform ‘Datenhotel’ and subsequently made available to the authors for statistical analysis. Data related to the registry study can be made available upon approval of the ethics committee and data protection officer.
Declaration of interests
All authors declare no potential conflicts of interest.
Acknowledgements
I.G. was supported by the Else Kröner-Fresenius-Stiftung iPRIME Scholarship (2021_EKPK.10), UKE, Hamburg and A.P. has been awarded with a Fellowship of Hamburg Institute for Advanced Study. This work made possible by grants provided by the German Research Foundation (Clinical Research Unit 296: AR232/25-2 to P.C.A. and DI2103/2-2 to A.D., Research Unit 5068: AR232/29-1 to P.C.A.) and the Authority for Science, Research and Equality, Hanseatic City of Hamburg, Germany (LFF-FV73) to P.C.A. and A.D. We further acknowledge financial support from the Open Access Publication Fund of UKE - Universitätsklinikum Hamburg-Eppendorf and German Research Foundation.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104651.
Appendix A. Supplementary data
References
- 1.Hegewald M.J., Crapo R.O. Respiratory physiology in pregnancy. Clin Chest Med. 2011;32:1–13. doi: 10.1016/j.ccm.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Tan E.K., Tan E.L. Alterations in physiology and anatomy during pregnancy. Best Pract Res Clin Obstet Gynaecol. 2013;27(6):791–802. doi: 10.1016/j.bpobgyn.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Weck R.H., Paulose T., Flaws J.A. Impact of environmental factors and poverty on pregnancy outcomes. Clin Obstet Gynecol. 2008;51(2):349–359. doi: 10.1097/GRF.0b013e31816f276e. [DOI] [PubMed] [Google Scholar]
- 4.Giudice L.C., Llamas-Clark E.F., DeNicola N., et al. Climate change, women's health, and the role of obstetricians and gynecologists in leadership. Int J Gynaecol Obstet. 2021;155(3):345–356. doi: 10.1002/ijgo.13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perera F., Nadeau K. Climate change, fossil-fuel pollution, and children's health. N Engl J Med. 2022;386(24):2303–2314. doi: 10.1056/NEJMra2117706. [DOI] [PubMed] [Google Scholar]
- 6.Khatana S.A.M., Werner R.M., Groeneveld P.W. Association of extreme heat with all-cause mortality in the contiguous US, 2008-2017. JAMA Netw Open. 2022;5(5) doi: 10.1001/jamanetworkopen.2022.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barreca A., Schaller J. The impact of high ambient temperatures on delivery timing and gestational lengths. Nat Clim Change. 2019;10(1):77–82. [Google Scholar]
- 8.Zhang Y., Hajat S., Zhao L., et al. The burden of heatwave-related preterm births and associated human capital losses in China. Nat Commun. 2022;13(1):7565. doi: 10.1038/s41467-022-35008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chersich M.F., Pham M.D., Areal A., et al. Associations between high temperatures in pregnancy and risk of preterm birth, low birth weight, and stillbirths: systematic review and meta-analysis. BMJ. 2020;371 doi: 10.1136/bmj.m3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carolan-Olah M., Frankowska D. High environmental temperature and preterm birth: a review of the evidence. Midwifery. 2014;30(1):50–59. doi: 10.1016/j.midw.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Kuehn L., McCormick S. Heat exposure and maternal health in the face of climate change. Int J Environ Res Public Health. 2017;14(8):853–865. doi: 10.3390/ijerph14080853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cushing L., Morello-Frosch R., Hubbard A. Extreme heat and its association with social disparities in the risk of spontaneous preterm birth. Paediatr Perinat Epidemiol. 2022;36(1):13–22. doi: 10.1111/ppe.12834. [DOI] [PubMed] [Google Scholar]
- 13.Nyadanu S.D., Tessema G.A., Mullins B., Kumi-Boateng B., Ofosu A.A., Pereira G. Prenatal exposure to long-term heat stress and stillbirth in Ghana: a within-space time-series analysis. Environ Res. 2023;222 doi: 10.1016/j.envres.2023.115385. [DOI] [PubMed] [Google Scholar]
- 14.Schifano P., Asta F., Dadvand P., Davoli M., Basagana X., Michelozzi P. Heat and air pollution exposure as triggers of delivery: a survival analysis of population-based pregnancy cohorts in Rome and Barcelona. Environ Int. 2016;88:153–159. doi: 10.1016/j.envint.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Mohammadi D., Naghshineh E., Sarsangi A., Zare Sakhvidi M.J. Environmental extreme temperature and daily preterm birth in Sabzevar, Iran: a time-series analysis. Environ Health Prev Med. 2019;24(1):5. doi: 10.1186/s12199-018-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ilango S.D., Weaver M., Sheridan P., et al. Extreme heat episodes and risk of preterm birth in California, 2005–2013. Environ Int. 2020;137 doi: 10.1016/j.envint.2020.105541. [DOI] [PubMed] [Google Scholar]
- 17.Vicedo-Cabrera A.M., Olsson D., Forsberg B. Exposure to seasonal temperatures during the last month of gestation and the risk of preterm birth in Stockholm. Int J Environ Res Public Health. 2015;12(4):3962–3978. doi: 10.3390/ijerph120403962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jegasothy E., Randall D.A., Ford J.B., Nippita T.A., Morgan G.G. Maternal factors and risk of spontaneous preterm birth due to high ambient temperatures in New South Wales, Australia. Paediatr Perinat Epidemiol. 2022;36(1):4–12. doi: 10.1111/ppe.12822. [DOI] [PubMed] [Google Scholar]
- 19.Nyadanu S.D., Tessema G.A., Mullins B., Pereira G. Prenatal acute thermophysiological stress and spontaneous preterm birth in Western Australia, 2000-2015: a space-time-stratified case-crossover analysis. Int J Hyg Environ Health. 2022;245 doi: 10.1016/j.ijheh.2022.114029. [DOI] [PubMed] [Google Scholar]
- 20.Dalugoda Y., Kuppa J., Phung H., Rutherford S., Phung D. Effect of elevated ambient temperature on maternal, foetal, and neonatal outcomes: a scoping review. Int J Environ Res Public Health. 2022;19(3) doi: 10.3390/ijerph19031771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox B., Vicedo-Cabrera A.M., Gasparrini A., et al. Ambient temperature as a trigger of preterm delivery in a temperate climate. J Epidemiol Community Health. 2016;70(12):1191–1199. doi: 10.1136/jech-2015-206384. [DOI] [PubMed] [Google Scholar]
- 22.Lee S.J., Hajat S., Steer P.J., Filippi V. A time-series analysis of any short-term effects of meteorological and air pollution factors on preterm births in London, UK. Environ Res. 2008;106(2):185–194. doi: 10.1016/j.envres.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 23.de Bont J., Stafoggia M., Nakstad B., et al. Associations between ambient temperature and risk of preterm birth in Sweden: a comparison of analytical approaches. Environ Res. 2022;213 doi: 10.1016/j.envres.2022.113586. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y., Yu C., Wang L. Temperature exposure during pregnancy and birth outcomes: an updated systematic review of epidemiological evidence. Environ Pollut. 2017;225:700–712. doi: 10.1016/j.envpol.2017.02.066. [DOI] [PubMed] [Google Scholar]
- 25.Dadvand P., Basagaña X., Sartini C., et al. Climate extremes and the length of gestation. Environ Health Perspect. 2011;119(10):1449–1453. doi: 10.1289/ehp.1003241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chawanpaiboon S., Vogel J.P., Moller A.B., et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–e46. doi: 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeitlin J., Saurel-Cubizolles M.J., de Mouzon J., et al. Fetal sex and preterm birth: are males at greater risk? Hum Reprod. 2002;17(10):2762–2768. doi: 10.1093/humrep/17.10.2762. [DOI] [PubMed] [Google Scholar]
- 28.Samuels L., Nakstad B., Roos N., et al. Physiological mechanisms of the impact of heat during pregnancy and the clinical implications: review of the evidence from an expert group meeting. Int J Biometeorol. 2022;8:1505–1513. doi: 10.1007/s00484-022-02301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dreiling C.E., Carman F.S., 3rd, Brown D.E. Maternal endocrine and fetal metabolic responses to heat stress. J Dairy Sci. 1991;74:312–327. doi: 10.3168/jds.S0022-0302(91)78175-7. [DOI] [PubMed] [Google Scholar]
- 30.Andrianakis P., Walker D. Effect of hyperthermia on uterine and umbilical blood flows in pregnant sheep. Exp Physiol. 1994;79(1):1–13. doi: 10.1113/expphysiol.1994.sp003735. [DOI] [PubMed] [Google Scholar]
- 31.Part C., le Roux J., Chersich M., et al. Ambient temperature during pregnancy and risk of maternal hypertensive disorders: a time-to-event study in Johannesburg, South Africa. Environ Res. 2022;212(Pt D) doi: 10.1016/j.envres.2022.113596. [DOI] [PubMed] [Google Scholar]
- 32.Wang J., Williams G., Guo Y., Pan X., Tong S. Maternal exposure to heatwave and preterm birth in Brisbane, Australia. BJOG. 2013;120(13):1631–1641. doi: 10.1111/1471-0528.12397. [DOI] [PubMed] [Google Scholar]
- 33.Ha S., Liu D., Zhu Y., Kim S.S., Sherman S., Mendola P. Ambient temperature and early delivery of singleton pregnancies. Environ Health Perspect. 2017;125(3):453–459. doi: 10.1289/EHP97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thom E. The discomfort index. Weatherwise. 1959;12:57–61. [Google Scholar]
- 35.Gosling S.N., Bryce E.K., Dixon P.G., et al. A glossary for biometeorology. Int J Biometeorol. 2014;58(2):277–308. doi: 10.1007/s00484-013-0729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matzarakis A., Mayer H. The extreme heat wave in Athens in July 1987 from the point of view of human biometeorology. Atmos Environ B Urban Atmos. 1991;25(2):203–211. [Google Scholar]
- 37.de Souza E.S.R., da Silva R.M., de Freitas A.F., Dos Santos J.S., Santos C.A.G., de Lima E.R.V. Thermal comfort conditions at microclimate scale and surface urban heat island in a tropical city: a study on João Pessoa city, Brazil. Int J Biometeorol. 2022;66(6):1079–1093. doi: 10.1007/s00484-022-02260-y. [DOI] [PubMed] [Google Scholar]
- 38.Stathopoulou M.I., Cartalis C., Keramitsoglou I., Santamouris M. Thermal remote sensing of Thom’s discomfort index (DI): comparison with in-situ measurements. Proc SPIE. 2005;5983:131–139. [Google Scholar]
- 39.Koopman P.A.R. Confidence intervals for the ratio of two binomial proportions. Int Biometric Soc. 1984;40(2):513–517. [Google Scholar]
- 40.Fagerland M.W., Lydersen S., Laake P. Recommended confidence intervals for two independent binomial proportions. Stat Methods Med Res. 2015;24(2):224–254. doi: 10.1177/0962280211415469. [DOI] [PubMed] [Google Scholar]
- 41.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X., Astivia O.L.O., Kroc E., Zumbo B.D. How to think clearly about the central limit theorem. Psychol Methods. 2022 doi: 10.1037/met0000448. [DOI] [PubMed] [Google Scholar]
- 43.Jacob J., Lehne M., Mischker A., Klinger N., Zickermann C., Walker J. Cost effects of preterm birth: a comparison of health care costs associated with early preterm, late preterm, and full-term birth in the first 3 years after birth. Eur J Health Econ. 2017;18(8):1041–1046. doi: 10.1007/s10198-016-0850-x. [DOI] [PubMed] [Google Scholar]
- 44.Trotter A., Schreiber R., Sander S., Muche R., Lucke W. Preterm birth rate at 6 centres for perinatal medicine in Baden-Württemberg - potential to reduce prematurity. Z Geburtshilfe Neonatol. 2018;222(5):197–206. doi: 10.1055/a-0721-2232. [DOI] [PubMed] [Google Scholar]
- 45.Natarajan G., Shankaran S. Short- and long-term outcomes of moderate and late preterm infants. Am J Perinatol. 2016;33(3):305–317. doi: 10.1055/s-0035-1571150. [DOI] [PubMed] [Google Scholar]
- 46.Sharma D., Padmavathi I.V., Tabatabaii S.A., Farahbakhsh N. Late preterm: a new high risk group in neonatology. J Matern Fetal Neonatal Med. 2021;34(16):2717–2730. doi: 10.1080/14767058.2019.1670796. [DOI] [PubMed] [Google Scholar]
- 47.von Storch H., Meinke I., Claußen M. Hamburger Klimabericht – Wissen über Klima, Klimawandel und Auswirkungen in Hamburg und Norddeutschland. 1 ed. Springer Spektrum; Berlin, Heidelberg, Germany: 2018. [Google Scholar]
- 48.Cramer M.N., Jay O. Biophysical aspects of human thermoregulation during heat stress. Auton Neurosci. 2016;196:3–13. doi: 10.1016/j.autneu.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Wolf J., Armstrong B. The association of season and temperature with adverse pregnancy outcome in two German states, a time-series analysis. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He J.R., Liu Y., Xia X.Y., et al. Ambient temperature and the risk of preterm birth in Guangzhou, China (2001-2011) Environ Health Perspect. 2016;124(7):1100–1106. doi: 10.1289/ehp.1509778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y., Zhou Y., Lu J. Exploring the relationship between air pollution and meteorological conditions in China under environmental governance. Sci Rep. 2020;10(1):14518. doi: 10.1038/s41598-020-71338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Behörde für Umwelt K., Energie und Agrarwirtschaft . Luftqualität in Hamburg - Jahresbericht 2021. 2022. [Google Scholar]
- 53.Wang L., Luo D., Liu X., et al. Effects of PM(2.5) exposure on reproductive system and its mechanisms. Chemosphere. 2021;264(Pt 1):128436. doi: 10.1016/j.chemosphere.2020.128436. [DOI] [PubMed] [Google Scholar]
- 54.Slama R., Darrow L., Parker J., et al. Meeting report: atmospheric pollution and human reproduction. Environ Health Perspect. 2008;116(6):791–798. doi: 10.1289/ehp.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bekkar B., Pacheco S., Basu R., DeNicola N. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yüzen D., Graf I., Diemert A., Arck P.C. Climate change and pregnancy complications: from hormones to the immune response. Front Endocrinol (Lausanne) 2023;14:1149284. doi: 10.3389/fendo.2023.1149284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kingma B.R., Frijns A.J., Schellen L., van Marken Lichtenbelt W.D. Beyond the classic thermoneutral zone: including thermal comfort. Temperature (Austin) 2014;1(2):142–149. doi: 10.4161/temp.29702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schifano P., Lallo A., Asta F., De Sario M., Davoli M., Michelozzi P. Effect of ambient temperature and air pollutants on the risk of preterm birth, Rome 2001-2010. Environ Int. 2013;61:77–87. doi: 10.1016/j.envint.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 59.Strand L.B., Barnett A.G., Tong S. Maternal exposure to ambient temperature and the risks of preterm birth and stillbirth in Brisbane, Australia. Am J Epidemiol. 2012;175(2):99–107. doi: 10.1093/aje/kwr404. [DOI] [PubMed] [Google Scholar]
- 60.Stelzer I.A., Ghaemi M.S., Han X., et al. Integrated trajectories of the maternal metabolome, proteome, and immunome predict labor onset. Sci Transl Med. 2021;13(592) doi: 10.1126/scitranslmed.abd9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng X., Zhang W., Lu C., Norback D., Deng Q. An epidemiological assessment of the effect of ambient temperature on the incidence of preterm births: identifying windows of susceptibility during pregnancy. J Therm Biol. 2018;74:201–207. doi: 10.1016/j.jtherbio.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 62.Barrett E.S., Lessing J., Wazana A., Székely E., Oberlader T.F. Sex-specific impacts of prenatal stress. 1st ed. Springer; Cham: 2021. [Google Scholar]
- 63.Sandman C.A., Glynn L.M., Davis E.P. Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. J Psychosom Res. 2013;75(4):327–335. doi: 10.1016/j.jpsychores.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sutherland S., Brunwasser S.M. Sex differences in vulnerability to prenatal stress: a review of the recent literature. Curr Psychiatry Rep. 2018;20(11):102. doi: 10.1007/s11920-018-0961-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Widnes C., Flo K., Wilsgaard T., Kiserud T., Acharya G. Sex differences in umbilical artery Doppler indices: a longitudinal study. Biol Sex Differ. 2018;9(1):16. doi: 10.1186/s13293-018-0174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y., Zhao S. Vascular biology of the placenta. Morgan & Claypool Life Sciences; San Rafael (CA): 2010. Integrated systems physiology: from molecules to function to disease. [PubMed] [Google Scholar]
- 67.Lim C.L. Fundamental concepts of human thermoregulation and adaptation to heat: a review in the context of global warming. Int J Environ Res Public Health. 2020;17(21):7795. doi: 10.3390/ijerph17217795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lewer D., Petersen I., Maclure M. The case-crossover design for studying sudden events. BMJ Med. 2022;1(1) doi: 10.1136/bmjmed-2022-000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maclure M., Mittleman M.A. Should we use a case-crossover design? Annu Rev Public Health. 2000;21:193–221. doi: 10.1146/annurev.publhealth.21.1.193. [DOI] [PubMed] [Google Scholar]
- 70.Ha S., Liu D., Zhu Y., Sherman S., Mendola P. Acute associations between outdoor temperature and premature rupture of membranes. Epidemiology. 2018;29(2):175–182. doi: 10.1097/EDE.0000000000000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He S., Kosatsky T., Smargiassi A., Bilodeau-Bertrand M., Auger N. Heat and pregnancy-related emergencies: risk of placental abruption during hot weather. Environ Int. 2018;111:295–300. doi: 10.1016/j.envint.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 72.Fung K.Y., Krewski D., Chen Y., Burnett R., Cakmak S. Comparison of time series and case-crossover analyses of air pollution and hospital admission data. Int J Epidemiol. 2003;32(6):1064–1070. doi: 10.1093/ije/dyg246. [DOI] [PubMed] [Google Scholar]
- 73.Toloo G., FitzGerald G., Aitken P., Verrall K., Tong S. Evaluating the effectiveness of heat warning systems: systematic review of epidemiological evidence. Int J Public Health. 2013;58(5):667–681. doi: 10.1007/s00038-013-0465-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.