Abstract
Diabetic vascular complications can affect both microvascular and macrovascular. Diabetic microvascular complications, such as diabetic nephropathy, diabetic retinopathy, diabetic neuropathy, and diabetic cardiomyopathy, are believed to be caused by oxidative stress. The Nox family of NADPH oxidases is a significant source of reactive oxygen species and plays a crucial role in regulating redox signaling, particularly in response to high glucose and diabetes mellitus. This review aims to provide an overview of the current knowledge about the role of Nox4 and its regulatory mechanisms in diabetic microangiopathies. Especially, the latest novel advances in the upregulation of Nox4 that aggravate various cell types within diabetic kidney disease will be highlighted. Interestingly, this review also presents the mechanisms by which Nox4 regulates diabetic microangiopathy from novel perspectives such as epigenetics. Besides, we emphasize Nox4 as a therapeutic target for treating microvascular complications of diabetes and summarize drugs, inhibitors, and dietary components targeting Nox4 as important therapeutic measures in preventing and treating diabetic microangiopathy. Additionally, this review also sums up the evidence related to Nox4 and diabetic macroangiopathy.
Keywords: Nox4, Diabetic vascular complications, Epigenetic regulation, Nox4 inhibitors, Dietary strategies
1. Introduction
Diabetic microangiopathy is the earliest and most common complication of diabetes, which is caused by micro-arterial, capillary, and micro-venous lesions. It can involve all tissues and organs of the body, in particular the kidney, retina, myocardium, and nerve tissue clinically manifested as diabetic kidney disease (DKD), diabetic retinopathy (DR), diabetic cardiomyopathy (DCM), and diabetic neuropathy, respectively. The role of oxidative stress in promoting diabetic microangiopathy has been acknowledged as critical in recent decades. Reactive oxygen species (ROS) are generated through several pathways, including the mitochondrial electron transport chain, cytochrome P450, xanthine oxidase, and uncoupled nitric oxide synthase [[1], [2], [3], [4]]. Apart from that, nicotinamide adenine dinucleotide phosphate oxidase (Nox) is considered to be a significant contributor to the production of ROS in the organism [5,6]. The Nox family comprises of seven isoforms so far, which are Nox1, Nox2, Nox3, Nox4, Nox5, Doux1, and Doux 2, where Nox4 is the most well-studied one.
Combined with the latest research progress, the objective of this review is to concentrate on the molecular properties and biological functions of No×4 and its role in diabetic microangiopathy, especially the mechanism of Nox4 action on different cell types during the development of DKD, and summarizes therapeutic approaches targeting Nox4 in diabetic microangiopathy. Finally, evidence is drawn for the association of Nox4 with diabetic macroangiopathy, a complication frequently observed in patients with diabetes, which can lead to conditions such as diabetic atherosclerosis, aortic disease, coronary artery disease, peripheral arterial disease, and cerebrovascular disease.
2. Molecular properties and biological functions of Nox4 and other Nox proteins
Nox was first discovered in macrophages, otherwise known as the phagocyte Nox family proteins. The oxidase complex consists of six subunits, namely gp91phox (also called Nox2), p22phox, p47phox, p67phox, p40phox, and Rac. Among them, gp91phox, located on the plasma membrane with p22phox, is the major catalytic subunit, while the other four subunits are distributed in the cytoplasm, also called regulatory subunits. When exposed to an external stimulus, the regulatory subunits could form an enzymatic complex by binding to p22phox, which catalyzes the reaction of NADPH-dependent oxygen molecules that reduces into superoxide anions and thus exert cellular defense functions. Subsequently, a series of homologs of Nox2 (the non-phagocyte Nox family proteins) were identified in multiple cell types, including Nox1, Nox3, Nox4, Nox5, Duox1, and Duox2, and then the scientific community called Nox2 and its homologs collectively as Nox family proteins. The differences in the structure of the Nox family proteins lead to the variance in their activation mechanisms and biological functions. Nox1, Nox3, and Nox4, sharing a similar structure with Nox2, use p22phox as an activator [7]. Notably, to exert biological effects, Nox1-3 need to bind to their respective regulatory subunits to form active Nox complexes, proving that the Nox complexes are mediated by complex protein-protein interactions. Comparatively, Nox4 does not require further activation by other regulatory subunits [8], its expression level is directly regulated by various external stimuli instead. Conversely, Nox5, Duox1, and Duox2 isoforms are not subject to regulation by p22phox or other regulatory subunits [7,9], but rely on intracellular EF-hand calcium-binding domains to bind to enzymes and thus exert their biological functions [9,10].
Nox1 was initially discovered in the colonic epithelium and shares 60% homology with Nox2, whose regulative subunits are the p47phox homolog (Nox Organizing protein 1, NoxO1), the p67phox homolog (Nox Activating protein 1, NoxA1), and Rac. Some reports have also suggested that p47phox and p67phox might be capable of partially replacing NoxO1 and NoxA1, respectively. Similar to Nox2, Nox1 is a superoxide-generating enzyme that mainly produces superoxide anions [9,11]; Nox3 is discovered to be highly expressed in various regions of the inner ear, such as the vestibule, the cochlear sensory epithelium, and the spiral ganglia, thus named as the inner ear NADPH oxidase. Nox3 shares 56% homology with Nox2, whose regulatory subunit is NoxO1. However, the formation mechanism of Nox3 complex is still to be elucidated. Similar to Nox2, the oxidation product of Nox3 is a superoxide anion [12]. Nox4, initially identified from the kidney, is a protein containing 578 amino acids, which shares 39% homology with Nox2 [9]. Its activity is largely dependent on its expression level in tissues or cells [4]. Recently, it was found that both Poldip2 (formerly referred to as NoxR1) and Tks5 (structurally related to p47phox protein) can interact with p22phox and enhance Nox4 activity consequently [13,14], implying that one or more unknown proteins may regulate the formation of the Nox4 complex and affect its activity. Different from other Nox proteins, Nox4 can further convert superoxide anions into hydrogen peroxide (H2O2). Although Nox4 primarily produces H2O2, Nox4-dependent superoxide production has been detected in vascular, cardiac, and renal cells and tissues in some studies [5,15,16]; Nox5, also known as Ca2+-dependent homolog, has been identified in lymphoid tissue and testis sharing 27% homology with Nox2 [17]. However, the functional importance of this isoform remains uncertain as it is only found in the human vasculature but not in rodents, limiting the study of its role in vascular lesions. Similar to Nox2, Nox5 mainly produces superoxide anions. Duox 1 and Duox 2 were initially named as thyroid oxidase owing to their expression largely in the thyroid gland. The proteins show 53% and 47% homology with Nox2, respectively [18]. Current studies have found their association with hormone synthesis, but it is still unknown whether they are involved in the development of vascular lesions. Duox 1 and Duox 2 are similar to Nox4 in that they mainly produce H2O2. In the NADPH oxidase family, the expressions of Nox1, Nox2, Nox4, and Nox5 have been identified in target organs of diabetic complications, including blood vessels, retina, kidney, and peripheral nerves [9]. However, the expression of Nox3, Duox 1, and Duox 2 in tissues involved in diabetic microangiopathy has not yet been reported. Despite significant progress in studying the molecular properties and biological functions of Nox isomers, the specific functions of each Nox remain unclear. Nevertheless, it is undeniable that Nox plays a crucial role in end-organ damage in conditions such as diabetes, kidney disease, hypertension, and atherosclerosis. This review focuses primarily on Nox4 due to its abundantly expressed in various tissues, which has made it the most extensively studied member of the Nox family.
Nox4 was initially thought to be unique in the kidney because it was most abundantly expressed in this organ and was thus termed renal NADPH oxidase (Renox) [19]. However, more and more recent studies have found that Nox4 also expresses and functions in non-renal cells, including cardiomyocytes [20], neurons [21], vascular smooth muscle cells [15], and osteoblasts [22]. Currently, inducers such as hyperglycemia [23,24], insulin [25], transforming growth factor-β (TGF-β) [26,27], angiotensin II (Ang II) [28,29], oxidized low density lipoprotein (Ox-LDL) [30,31], insulin-like growth factor 1 (IGF-1) [32,33], vascular endothelial growth factor (VEGF) [34,35], aldosterone [36,37], and prostacyclin [38]have been reported to inspire the upregulation of Nox4, leading to excessive ROS production. Diabetic microvascular complications can further increase the levels of Nox4-derived ROS in cells and tissues by upregulating the abovementioned inducers, particularly Ang Ⅱ and TGF-β, through hyperglycemia. Unlike phagocyte NADPH oxidases that mainly serves cellular defense, ROS produced by non-phagocyte NADPH oxidases (e.g. Nox4) functions basically as secondary messengers for signaling in various biological processes, including cell proliferation, differentiation, apoptosis, growth factor receptor signaling, senescence, gene expression, oxygen sensing and angiogenesis [4,6,9,39,40], and its excessive generation may induce the emergence and advancement of diseases.
3. Nox4 and diabetic microangiopathy
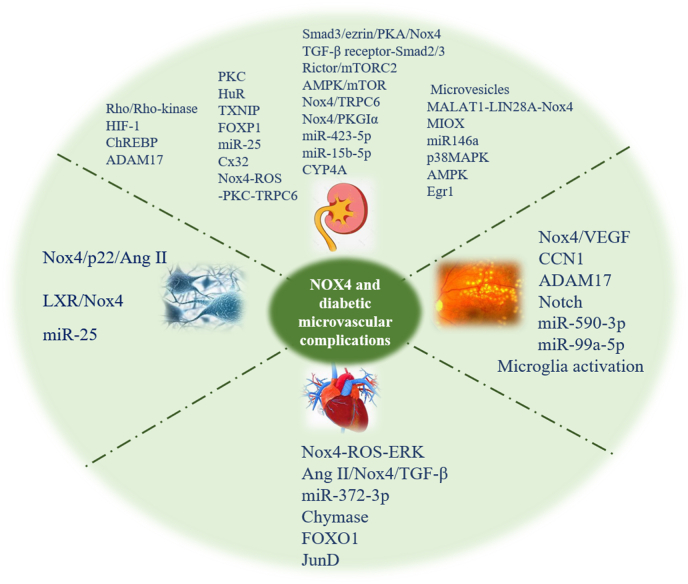
In recent years, Nox4 has been identified to play a crucial part in hyperglycemia-triggered renal damage, meanwhile, its impact on other microangiopathies caused by hyperglycemia has also been recognized gradually. This review article centers on exploring the impact of Nox4 in the development of DKD, DR, DCM, and diabetic neuropathy (Fig. 1), with special attention given to its role in DKD (Fig. 2). Additionally, this paper examines the practicality of targeting Nox4 as a potential therapy for addressing microvascular complications in diabetes.
Fig. 1.
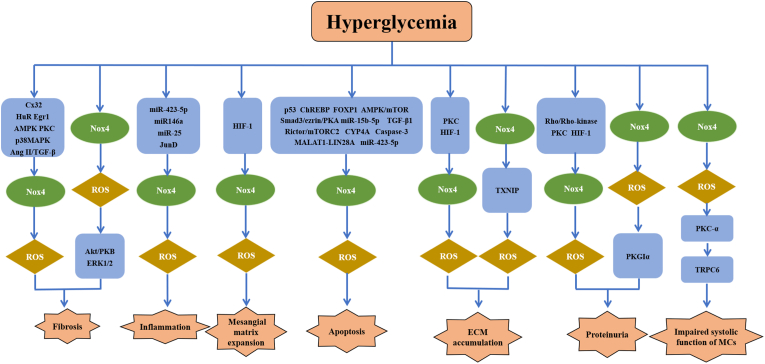
Upstream and downstream effectors of Nox4 involved in diabetic microvascular complications exposed to high glucose and diabetes. Abbreviations: HIF-1: hypoxia inducible factor-1; ChREBP: carbohydrate-responsive element-binding protein; PKC: protein kinase C; TXNIP: thioredoxin interacting protein; FOXP1: forkhead box prote1; Cx32: Connexin 32; TRPC6: transient receptor potential channel 6; AMPK: AMP-activated protein kinase; CYP4A: 4A family cytochrome P450; MIOX: Myo-Inositol Oxygenase; Egr1: early growth response 1; VEGF: vascular endothelial growth factor; Ang II: angiotensin II; TGF-β: transforming growth factor-β; LXR: liver X receptor.
Fig. 2.
Nox4-dependent signaling pathways associated with diabetic kidney disease and their consequences, including renal fibrosis, inflammation, mesangial matrix expansion, apoptosis, ECM accumulation, proteinuria and impaired systolic function of MCs.
3.1. Nox4 and diabetic kidney disease
Diabetic kidney disease, a diabetes-induced microangiopathy of the kidney, is among the most prevalent and severe diabetic microvascular complications. It is reported that 20–40% of diabetic individuals may develop DKD—the main cause of end-stage renal disease (ESRD) [41]. Primary pathological features of DKD are glomerular and tubular hypertrophy, mesangial cell injury, extracellular matrix accumulation, glomerular and tubular basement membrane thickening, and podocyte dysfunction, finally leading to proteinuria, glomerulosclerosis, and tubular interstitial fibrosis [42]. Affected cells in DKD mainly include glomerular mesangial cells, glomerular epithelial cells (also called as podocytes), and tubular epithelial cells. Under pathological conditions, excessive production of tissue or cellular ROS leads to oxidative stress, which is critical in the onset and advancement of DKD. Nox4 is thought to be a primary origin of oxidative stress in the diabetic kidney and a key mediator of redox signaling among glomerular and tubular cells exposed to high glucose environments. Nox4 is widely distributed in a variety of DKD-associated renal cells, whose abnormal activation may induce the development of glomerular mesangial cell hypertrophy, extracellular matrix protein accumulation, podocyte apoptosis, inflammation, and tubulointerstitial fibrosis. Such variation in structure or function may be influenced by cell type, subcellular localization, molecular concentration, disease stage, and other unexplored factors [43].
3.1.1. Nox4 and diabetic kidney
Due to its substantial expression in renal tissue, Nox4 is thought to be critical in the pathogenesis of DKD. In streptozotocin (STZ)-induced diabetic rat kidneys, Etoh et al. detected an elevation in the expression of Nox4, p22phox, and DNA damage marker 8-hydroxydeoxyguanosine (8-OHdG) induced by ROS, however, the expression level could be restored through insulin treatment [44], which suggested that hyperglycemia-induced upregulation of Nox4 may be a significant contributor in the process of oxidative damage in diabetic kidney. Subsequently, more in vivo and in vitro studies on DKD models have shown that the knockdown or silencing of Nox4 greatly slowed down oxidative damage in the kidney [5,23,45].
Currently, the molecular mechanisms by which diabetes and high glucose-regulated Nox4 are intricate and not fully elucidated. Rho/Rho-kinase and protein kinase C (PKC) may be key factors in high glucose-induced upregulation of Nox4 expression. An in vivo study has confirmed that Rho-kinase is contributes to renal vascular endothelial dysfunction in type 1 diabetes (T1DM) by enhancing oxidative stress, and Fasudil, a Rho-kinase inhibitor, could effectively reverse the upregulation of renal cortical Nox4 expression and the elevated excretion of urinary albumin and 8-OHdG in diabetic rats [46,47], suggesting that Rho/Rho-kinase may be an upstream regulator of Nox4. In another study, compared to wild-type diabetic mice, PKC-β−/− diabetic mice showed reduced Nox4 expression in the renal cortex, declined urinary level of oxidative stress indicators such as isoprostane and 8-OHdG, alleviated proteinuria, and significantly improved glomerular structure and function [48]. It indicates that PKC, as a potential upstream regulator of Nox4, is a significant contributor to oxidative damage in diabetic kidney, but additional in vitro researches are necessary to illustrate the specific types of kidney cells where this regulatory mechanism works.
Furthermore, Hu et al. reported that the knockdown of Egr1 in HFD/STZ-induced mice and TGF-β1-treated renal tubular epithelial cells (HK-2) may downregulate the expression of Nox4 and α-SMA, reduce ROS level, and alleviate epithelial-mesenchymal transition (EMT). In contrast, transfecting cells via Egr1 encoded plasmid (pcDNA3-Egr1) could reverse such alterations [49]. It provides evidence that Egr1 functions as a transcriptional activator of Nox4 influencing the oxidative stress in DKD. Recently, there are also reports indicating the critical role of the Connexin 32 (Cx32)-Nox4 signaling axis in DKD, showing a marked downregulation of Cx32 expression and a notable increase of Nox4 expression in the kidneys of STZ-induced diabetic mice. After Cx32 overexpression treatment, diabetic mice witnessed a drop in Nox4 level, accompanied by the normalization of renal function and fibrosis, while Cx32 deficiency led to the opposite result. This study further established that Cx32 mitigates renal fibrosis in diabetic mice by inhibiting Smurf1 expression and thus promoting Nox4 degradation [50]. It is evident that targeting the Cx32-Nox4 signaling pathway appears to be a promising approach for the development of new therapies for DKD. Furthermore, Nayak et al. found that HIF-1 expression increased in the kidney of OVE26 type 1 diabetic mice, resulting in higher Nox4 protein expression and NADPH-dependent ROS generation, glomerular hypertrophy in the whole kidney, mesangial matrix expansion, extracellular matrix accumulation, and urinary albumin excretion. In contrast, HIF-1 inhibitor (YC-1) could reverse the above phenomena, while the blood glucose level remained unchanged [51]. This study suggests that HIF-1 may be implicated in the oxidative damage in DKD by regulating the Nox4 mechanism. Lately, studies have revealed an upregulation of carbohydrate-responsive element-binding protein (ChREBP) and thioredoxin interacting protein (TXNIP) expression in the kidneys of type 2 diabetes mellitus (T2DM) patients and diabetic mice. The knockdown of ChREBP may inhibit the protein expression levels of TXNIP, Nox4, 8-OHdG, and heme oxygenase-1 in the kidney, improving kidney function and apoptosis. This phenomenon has also been confirmed in high glucose-induced HK-2 cell experiments [52], suggesting that ChREBP may function as an upstream regulator of Nox4 for preventiing and treating DKD effectively. Another research showed that ADAM17 and NADPH oxidase activity, Nox4 and fibronectin expression, and cellular collagen content increased in the renal cortex of OVE26 type 1 diabetic mice and the mouse proximal tubular epithelial cells (MCTs) cultured with high glucose, and provoked matrix protein accumulation through Nox4-derived oxidative stress. Comparatively, when cells were pre-exposed with ADAM17 inhibitor (TMI-005) or transfected with ADAM17-siRNA, Nox4 expression and oxidative stress-induced matrix protein accumulation were inhibited [53]. The study suggests that ADAM17 is an upstream regulator of Nox4 and could emerge as a novel therapeutic target for DKD.
Nox4 can also activate multiple signaling pathways as a second messenger and thus get involved in key events of DKD, while renal hypertrophy and extracellular matrix accumulation are among the initial characteristic of the disease. Gorin et al. confirmed that the regulation of renal hypertrophy and fibronectin expression may be facilitated by ROS derived from Nox4 in STZ-induced diabetic rats by activating two protein kinases, Akt/PKB and ERK1/2 [5]. As the disease progresses, inflammatory and fibrotic changes occur gradually in the kidney, which are important pathogeneses for the prolonged development of DKD. Some research has reported that Nox4 is connected to inflammation and fibrosis in the kidney affected by diabetes [54]. In a study conducted on the STZ-induced ApoE−/− diabetic mouse model, Nox4 knockdown or inhibition (GKT137831) was found to be protective against glomerular injury, manifested by reduced proteinuria, structural lesions, glomerular extracellular matrix protein (Collagen IV, Fibronectin) accumulation, glomerular oxidation products, glomerular macrophage infiltration, and declined expression of renal monocyte chemoattractant protein-1 (MCP-1) and nuclear transcription factor kappa B (NF-κB) [45,55]. The research indicates that Nox4 is potentially a prospect for reducing inflammation of diabetic kidney. In addition, two recent studies conducted by Chen et al. further confirmed that Nox4 could mediate the development of diabetic renal fibrosis [50,56]. In summary, Nox4, a key mediator in redox signaling, participates in abnormal renal structural alteration as a second messenger.
3.1.2. Nox4 and glomerular mesangial cells
Glomerular mesangial cells are very active cells within the glomerulus and have the property of secreting cellular matrix. Nox4 is involved in the oxidative injury of glomerular mesangial cells caused by high glucose. As reported, in diabetic rat renal cortex and rat glomerular mesangial cells exposed to high glucose, the expression of Nox4 and the superoxide products derived by NAPDH oxidase were increased, and Nox4 was found to be mainly localized to mitochondria, participating in mitochondrial ROS production. The suppression of Nox4 by siRNA may lead to a significantly reduction in high glucose-induced mitochondrial superoxide production in mesangial cells [57], showing that glomerular mesangial cell injury caused by high glucose is linked to Nox4-derived oxidative damage in mitochondria.
-
1)
Upstream regulatory mechanism of Nox4
Activation of PKC is essential for NADPH oxidase-mediated ROS production in numerous biological processes. It was shown that the levels of gene expression for Nox4 and p22phox were augmented in mesangial cells subjected to high glucose exposure for an extended period (day 7 or 8). However, such changes were completely reversed by the combination with PKC inhibitors on the latter two days, suggesting that high glucose may activate Nox4 through a PKC-dependent mechanism [44]. Despite this, to better elucidate the association between Nox4-generated ROS and PKC, Thallas-Bonke et al. used diabetic mice (C57Bl6/J) with a genetic deletion of Nox4 and observed that the lack of Nox4 not only attenuated glomerulosclerosis, increased mesangial matrix, diabetes-associated proteinuria, and renal oxidative damage, but also inhibited renal PKC activity and the expression of its different isoforms (PKC-α, PKC-β1) [58], showing that Nox4 deletion may attenuate renal structural and functional damage through the PKC pathway, further confirming that Nox4 inhibition is an effective measure to mitigate diabetic kidney injury. The above two studies reveal that the regulatory mechanism between PKC and Nox4 may be bidirectional, and more studies are needed to certify it subsequently.
Furthermore, it was shown that the activation of HuR in high glucose-exposed glomerular mesangial cells (MCs) and STZ-injected diabetic mouse models is a prerequisite for increased Nox4 expression and ROS generated by high glucose, and subsequent fibrotic injury in MCs. When cells were pretreated with HuR siRNAs, Nox4 protein expression was significantly inhibited and fibrotic injury in MCs was thence reduced. Interestingly, after overexpression treatment of HuR, Nox4 expression also increased in MCs even without high glucose exposure [59]. The above results support the involvement of HuR in high glucose-induced fibrotic damage in MCs by regulating Nox4, which provides a new strategy to inhibit the advancement of chronic kidney disease. Additionally, experiments have identified TXNIP as a possible intermediate in the process of ROS production by mitochondria and Nox4 in high glucose-induced MCs. The ROS generated by the whole cell and the mitochondria, Nox4 protein expression, and NAPDH oxidase activity significantly raised in the high glucose-treated MCs of wild-type C3H mice, whereas MCs cultured from TXNIP-deficient Hcb-19 mice manifested no corresponding changes in the high glucose environment. Notably, MCs from TXNIP deficient C3H mice exhibited responses similar to Hcb-19 MCs, while Hcb-19 MCs after TXNIP adenoviral transfection restored mitochondrial ROS production and Nox4 expression [60], which profoundly illustrated that TXNIP is an upstream regulator mediating Nox4-dependent ROS generation stimulated by high glucose in MCs. Meanwhile, Xiang et al. discovered that high glucose stimulus remarkably inhibited FOXP1 expression and enhanced ROS production in MCs. FOXP1 overexpression treatment prevented the abnormal activation of Akt/mammalian target of rapamycin (mTOR) signaling in MCs exposed by high glucose, significantly reduced Nox4 expression and ROS generation, and resisted oxidative stress, proliferation, and extracellular matrix accumulation in MCs [61], implying that FOXP1 regulates abnormal Nox4 activation in high glucose-induced MCs by blocking the Akt/mTOR pathway.
-
2)
Downstream regulatory mechanisms of Nox4
Recent reports have emphasized that in kidneys of diabetic mice and high glucose-induced rat MCs, the expression of Nox4, Intercellular Adhesion Molecule-1 (ICAM-1) and fibronectin (FN) was increased, while the expression of Cx32 and TRPC6 protein was significantly reduced. FN and ICAM-1 were the main factors aggravating diabetic kidney fibrosis, while Cx32 has been proven to be protective against diabetic renal fibrosis. To confirm that the abnormal activation of Nox4 or excessive reduction of Cx32 is connected to the fibrotic process induced by high glucose in MCs, it was found that both Nox4 gene silencing and Cx32 gene overexpression could reverse the profibrotic process induced by high glucose, which further validated the interaction between Cx32 and Nox4 [56]. In conclusion, this study shows that the Cx32-Nox4 signaling pathway is involved in the fibrotic damage process in the diabetic kidney. Excitingly, both cellular and animal experiments have also revealed that Polydatin (PD) can reverse fibrotic changes in DKD models, however, overexpression of Nox4 and knockdown of Cx32 could both abolish the inhibitory effect of PD on ICAM-1 and FN expression exposed by high glucose in rat glomerular mesangial cells [56]. Research has also shown that resveratrol could alleviate renal fibrosis in db/db diabetic mice by activating AMP-activated protein kinase (AMPK) and thereby reducing the overexpression of Nox4 in high glucose-induced fibroblasts [62]. The above results suggest that PD/resveratrol can relieve fibrosis through Cx32-Nox4 or AMPK/Nox4/ROS signaling pathway in diabetic kidney, providing clues to further exploring the molecular mechanism of Nox4-mediated diabetic kidney fibrosis.
In addition, a considerable decrease in TRPC6 protein induced by high glucose may be a key factor in the impaired contractile function of MCs, mainly due to increased Nox4-derived ROS production and the activation of PKC-α. Overexpression of Nox4 in rat MCs infected with adenovirus encoding human Nox4 (Ad-Nox4) or the use of PKC activator (PMA) could both significantly reduce TRPC6 expression. In contrast, knockdown of Nox4 by siRNA-Nox4 or overexpression of TRPC6 significantly increased TRPC6 expression, thereby reversing the contractile function impairment of high glucose-induced MCs. This study suggests that the Nox4-ROS-PKC-TRPC6 pathway may be a potential molecular mechanism for contractile function damage of high glucose-induced MCs, and the molecules are inter-regulative [63].
3.1.3. Nox4 and podocytes
Podocyte damage occurs in the initial stage of DKD and plays a key role in early proteinuria [64]. Meanwhile, Nox4 is a vital contributor to the development of podocyte injury. One study found that the expression of VEGF and renin decreased in male floxedNox4 and podocyte-specific Nox4 deletion (podNox4KO) mice, such was also the case with the accumulation of glomerular collagen fibronectin (Collagen IV, etc.), glomerulosclerosis, glomerular basement membrane thickening, and mesangial expansion. In addition, knockdown of podocyte Nox4 effectively inhibited the overproduction of ROS, glomerular MCP-1, andPKC-α induced by diabetes [65], indicating that Nox4 is engaged in podocyte injury and albuminuria production in DKD.
-
1)
Downstream regulatory mechanisms of Nox4
The role of Nox4 in ROS generation and apoptosis of high glucose-induced podocytes has been confirmed by many studies [1,66]. In glomeruli of OVE26 type 1 diabetic mouse and high glucose-exposed mouse podocytes, Nox4 protein expression was upregulated, along with an increase in cellular ROS, NADPH oxidase-dependent superoxide, and 4A family cytochrome P450 (CYP4A), along with podocyte apoptosis and/or foot process effacement, resulting in proteinuria. However, cellular and animal experiments uncovered that CYP4A inhibitor (HET0016) could effectively block the impact of high glucose on NADPH oxidase activity, Nox4 protein and gene expression, and podocyte apoptosis [1]. This study indicates that the inhibition of downstream molecules of Nox4 through the inhibition of Nox4 and its upstream molecules might be linked with the prevention of high glucose-induced podocyte apoptosis and the reduction of proteinuria. Recently, it has been found that ezrin (Thr567) and phosphorylated Smad3 (Ser423/425) were significantly enhanced in high glucose-treated podocytes, while phosphorylated PKA (Thr197) and cAMP were substantially lowered, accompanied by increased Nox4 expression and apoptosis. To better elucidate the relationship between the abovementioned phenomena and podocyte apoptosis, as well as the mechanisms wherein, Smad3, ezrin, and Nox4 were inhibited, respectively, and results revealed that ROS, Nox4 expression, and apoptosis levels were significantly reduced in high glucose-treated podocytes. Moreover, inhibition of Smad3 also blocked the upregulation of phosphorylated ezrin, and downregulation of PKA activity induced by high glucose. Meanwhile, shRNA-ezrin or ezrin inhibitor (NSC) could prevent the reduction of phosphorylated PKA and cAMP content. While PKA activator (forskolin) notably hindered high glucose-mediated enhancement of Nox4 expression, ROS production, and apoptosis levels [66]. The above evidence strongly suggests that the Smad3/ezrin/PKA/Nox4 pathway may be one of the potential molecular pathways of podocyte apoptosis caused by high glucose, and the molecules are inter-regulative. It has also been demonstrated that Nox4 is involved in TGF-β1-triggered podocyte apoptosis in mice, accompanied by increased ROS production and abnormal activation of caspase-3, while knockdown of Smad2 or Smad3 could block the TGF-β1-induced upregulation in Nox4 expression, ROS production, and abnormal activation of caspase-3 [67]. This study further suggests that the TGF-β receptor-Smad2/3 pathway may serve as a novel pathway for TGF-β1-induced podocyte Nox4 upregulation and podocyte apoptosis.
A rising number of evidence supports the idea that AMPK is an indispensable cellular energy sensor that may have a vital function in the regulation of podocyte apoptosis [68]. Evidence suggests that high glucose may induce podocyte apoptosis by inhibiting AMPK activity, increasing Nox4 expression and NADPH oxidase activity, and activating p53 and PUMA (p53 up-regulated modulator of apoptosis), whereas siRNA-Nox4 and siRNA-p53 significantly inhibited the occurrence of high glucose-triggered podocyte apoptosis, while induction of AMPK activation decidedly repressed Nox4 and p53 expression, oxidative stress, and podocyte damage [69]. The study indicates that AMPK may negatively regulate the Nox4-dependent p53 apoptotic pathway in diabetic glomerular epithelial cells or high glucose-induced podocytes, and it proves that Nox4 could serve as a mediator of podocyte apoptosis exposed in high glucose, shedding new light on Nox4 in diabetic podocyte injury. Based on the fact that rapamycin reduces proteinuria in diabetic patients, the hypothesis that mTOR mediates diabetic podocyte injury was explored further. In OVE26 type 1 diabetic mice and high glucose-induced mouse podocytes, AMPK was significantly downregulated and mTOR was significantly upregulated accompanied by increased Nox4, NADPH oxidase activity, and podocyte apoptosis, while low doses of rapamycin or siRNA-mTOR effectively reversed these changes by inhibiting mTOR, suggesting that mTOR serves as an upstream regulator of podocyte Nox4. Furthermore, in vitro studies showed that AMPK activator (AICAR) attenuated the excessive activation of mTOR stimulated by high glucose [70]. Results have established that high glucose-induced Rictor/mTORC2/Akt activation is associated with podocyte apoptosis, and could upregulate Nox4-derived ROS production. However, in OVE26 type 1 diabetic mice, treatment with antisense oligonucleotides targeting Rictor could effectively inhibit mTORC2, reduce renal Nox4 expression and NADPH oxidase activity, and lower podocyte shedding and apoptosis, GBM thickness, and proteinuria [71]. The above study illustrates that AMPK/mTOR and Rictor/mTORC2 pathways are involved in Nox4-mediated ROS production and podocyte apoptosis in diabetic kidney injury.
It has been found that the activation of Nox4-dependent PKGIα (cGMP-dependent protein kinase Iα) in rat podocytes cultured with high glucose may increase the permeability of podocytes and trigger proteinuria, thereby providing new evidence for the mechanism of podocyte injury. However, the above phenomenon could be reversed by small interfering RNAs of Nox4 and PKGIα [72]. Similarly, knockdown of Nox4 in type 1 diabetic Dahl SS rat model resulted in a drop in Nox4-derived H2O2 production, thereby inhibiting TRPC6 channel activation, reducing calcium influx, and protecting podocytes from damage [73]. These studies reveal a new signaling mechanism targeting Nox4/PKGIα or Nox4/TRPC6, providing a novel approach to prevent and treat podocyte injury in DKD.
In addition, Nox4-derived ROS could facilitate podocyte inflammation and fibrosis. In high glucose-induced human podocytes, Nox4 gene silencing may bring about downregulation of pro-inflammatory and pro-fibrotic markers associated with DKD [45], suggesting that the gene targeting or pharmacological blocking of Nox4 plays a protective part in the pro-inflammatory and pro-fibrotic mechanisms of diabetic glomerular podocytes.
Lately, it has been proposed that the formation of microvesicles (MVs) may be related to podocyte injury in diabetic patients. Experiment proved that high glucose treatment could eminently increase the number of MVs and the expression of Nox4 in mouse podocytes, while Nox4 siRNA may slow down the formation of podocyte-derived MVs induced by high glucose. This study shows that MVs may increase at the trigger of podocyte microangiogenesis through the Nox4-derived ROS pathway, and hence involve in the diabetic podocyte injury pathway [74].
3.1.4. Nox4 and renal tubular epithelial cells
Similar to glomeruli, Nox4 gene and protein expression enhanced in the renal tubules of STZ-induced type 1 diabetic rats. As we all know, statins could reduce cholesterol level, and a mounting volume of evidence is demonstrating its anti-inflammatory and anti-oxidant effects. All this portrays it as a protective agent against DKD [[75], [76], [77]]. A study reported that statins inhibited glomerular and tubular Nox4 expression in db/db diabetic mice, accompanied by reductions in urinary 8-OHdG, 8-isoprostaglandin F2α (8-epi-PGF2a), and proteinuria [75]. This study suggests that statins reduce Nox4-derived oxidative stress and thereby improve DKD. Although there is no direct evidence whether statins relieve renal tubular epithelial cell damage under high glucose conditions by downregulating Nox4, this study indicates that Nox4 may be implicated in the pathogenesis of diabetic renal tubular injury.
-
1)
Upstream regulatory mechanisms of Nox4
Similar to podocytes, metformin exerts its effect mainly by activating AMPK. It has been shown that metformin or AMPK activators could attenuate the high glucose-mediated augment in Nox4 protein expression and ROS in human renal tubular epithelial cells. They could also prevent epithelial-mesenchymal transition mainly by normalizing the boosting of mesenchymal marker protein (α-SMA) and the suppression of epithelial marker protein (E-cadherin) caused by high glucose. AMPK inhibitors or siRNA-AMPK could block the effects of metformin [78]. The above results suggest that AMPK has the potential to modulate the expression of Nox4 and its activation may help attenuate the renal tubular interstitial fibrosis induced by high glucose.
One recent scientific research stated that MALAT1, LIN28A, and Nox4 expression were upregulated in high glucose-triggered HK-2 cells, while the production of ROS and inflammatory factors were also increased, resulting in reduced cell activity and apoptosis. Moreover, it was found that MALAT1 interacted with LIN28A to activate AMPK/mTOR signaling and enhanced the stability of Nox4, thereby aggravating renal tubular epithelial injury. Yet knockdown of MALAT1, LIN28A or Nox4 reversed these phenomena [79]. Above studies confirmed that MALAT1-LIN28A-Nox4 pathway promises a cutting-edge approach for the management of DKD renal tubular epithelial cell injury.
-
2)
Downstream regulatory mechanisms of Nox4
The accumulation of extracellular matrix facilitates the progression of DKD. To confirm the pro-fibrotic effect of Nox4, rat Proximal tubule cells (PTCs) were transduced with adenovirus construct expressing Nox4, and the findings revealed that hyperexpression of Nox4 stimulated NADPH oxidase activity and fibronectin expression [32]. Another study on db/db diabetic mice and mouse proximal tubules exposed to high glucose showed amplification of Nox4 and p22phox protein expression, but no effect on other Nox expressions. Downregulation of Nox4 gene expression by using small interfering RNA and GK-136901 (a Nox1/4 inhibitor) diminished high glucose-induced NADPH oxidase-derived ROS production. Furthermore, in vivo and in vitro models showed a noteworthy increase in p38MAPK phosphorylation, fibronectin, and TGF-β1/2. At the same time, p38MAPK inhibitor (SB-203580) significantly attenuated the accumulation of fibronectin, while GK-136901 inhibited the activation of p38MAPK phosphorylation and attenuated fibronectin and TGF-β expression [23]. It is evident that p38MAPK is a downstream regulator of Nox4 in the signaling pathway connecting high glucose and renal tubular cell injury, and that Nox4 may depend on the p38MAPK redox-sensitive signaling pathway to participate in the molecular mechanism of fibrosis in T2DM nephropathy.
3.1.5. Nox4 and renal tubular interstitial cells
Evidence is still scarce on the role of high glucose-mediated oxidative stress or Nox-derived ROS in mesenchymal cell damage, especially in the conversion of renal fibroblasts into myofibroblasts. Despite glucose has been exhibited to induce extracellular matrix accumulation in renal fibroblasts, there is no proof that ROS derived by Nox4 or any other Nox oxidase can mediate these effects. More research has been centered on the impact and mechanism of Nox4 in TGF-β-induced interstitial cell injury [26].
Currently, angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are the most effective treatments to prevent or alleviate DKD [80,81]. The common mechanism may be correlated with the repression of renal NADPH oxidase in diabetic rats by these two drugs [82,83]. According to the state of the art, Nox4-induced ROS is a major cause that triggers and accelerates the development of DKD. And more and more studies have illustrated that gene targeting or pharmacological inhibition of Nox4 has a renoprotective effect in the pathogenesis of DKD, so Nox4 is a prospective target for pharmacological intervention in the management of DKD. In addition to direct inhibition of Nox4, exploring upstream and downstream regulators of Nox4 and using them as targets is also considered as one of the effective measures for DKD prevention and treatment (Table 1).
Table 1.
Regulatory mechanism of Nox4 in diabetic kidney disease.
| Area of action | Regulating factors/Signal pathways | Main results | Reference |
| Kidney | Rho/Rho-kinase | Upregulated renal cortical Nox4 expression; increased urinary albumin, 8-OHdG excretion; Rho-kinase inhibitor (Fasudil) reversed the above effects. | [46,47] |
| HIF-1 | Upregulated Nox4 protein expression and NADPH-dependent ROS production; Resulted in total renal glomerular hypertrophy, mesangial matrix expansion, extracellular matrix accumulation and urinary albumin excretion. HIF-1 inhibitor (YC-1) could reverse the above phenomena. | [51] | |
| ChREBP | Upregulated the protein expression levels of TXNIP, Nox4, 8-OHdG, and HO-1. The knockdown of ChREBP improved kidney function and apoptosis. | [52] | |
| ADAM17 | Upregulated Nox4 expression and NADPH oxidase activity; increased fibronectin expression and matrix protein accumulation. ADAM17 inhibitor (TMI-005) or transfected with ADAM17-siRNA suppressed Nox4 expression and oxidative stress-induced matrix protein accumulation. | [53] | |
| Mesangial cells (MCs) | PKC | Increased gene expression levels of Nox4 and p22phox; induced renal hypertrophy, glomerular enlargement, and hyperfiltration; Lack of PKC-β can protect against diabetes-induced renal dysfunction, fibrosis, and renal oxidative damage. | [44,58] |
| HuR | Upregulated Nox4 protein and gene expression, increased ROS production and fibrotic injury. HuR siRNAs inhibited the above results. | [59] | |
| TXNIP | Increased overall and mitochondrial ROS, Nox4 protein expression, and NAPDH oxidase activity. TXNIP-deficient showed no corresponding changes in the high glucose environment. | [60] | |
| FOXP1 | FOXP1 overexpression prevented abnormal activation of Akt/mTOR signaling, significantly reduced Nox4 expression and ROS production, and resisted proliferation, oxidative stress and extracellular matrix accumulation. | [61] | |
| Cx32 | Cx32 overexpression inhibited the expression of Smurf1 and promoted the degradation of Nox4; downregulated the expression of FN and ICAM-1 and normalized renal function and fibrosis. | [50,56] | |
| Nox4-ROS-PKC-TRPC6 | Overexpression of TRPC6 reversed the contractile function impairment of high glucose-induced MCs. | [63] | |
| podocyte | AMPK/mTOR | Downregulated AMPK, upregulated mTOR, increased Nox4, NADPH oxidase activity and podocyte apoptosis; inhibition of mTOR effectively reversed the above changes. | [70] |
| Rictor/mTORC2 | Upregulated Nox4 expression, increased podocyte shedding and apoptosis, GBM thickness and proteinuria production. Antisense oligonucleotides targeting Rictor inhibited mTORC2 and normalized the above variations. | [71] | |
| CYP4A | CYP4A inhibitor (HET0016) blocked the impact of high glucose on NADPH oxidase activity, Nox4 protein and gene expression, and podocyte apoptosis. | [1] | |
| Smad3/ezrin/PKA/Nox4 | Increased levels of phosphorylated Smad3 and phosphorylated ezrin, decreased levels of phosphorylated PKA, accompanied by increased levels of Nox4 expression and apoptosis. | [66] | |
| TGF-β receptor-Smad2/3 | Increased the expression of Nox4 and ROS production, abnormal activation of Caspase-3, podocyte apoptosis; knockdown of Smad2/3 blocked the above phenomena caused by TGF-β1. | [67] | |
| Nox4/PKGIα | The activation of Nox4-dependent PKGIα increased the permeability of podocytes, leading to proteinuria; small interfering RNAs of Nox4 and PKGIα reversed the above phenomena. | [72] | |
| Nox4/TRPC6 | Increased Nox4-derived H2O2 production and calcium influx, activated TRPC6 channel, leading to podocyte damage; knockdown of Nox4 reversed the above results. | [73] | |
| Microvesicles (MVs) | Increased Nox4 expression and the number of MVs, stimulated podocyte microangiogenesis; Nox4 siRNA slowed down the formation of podocyte-derived MVs induced by high glucose. | [74] | |
| Renal tubular epithelial cells | Egr1 | Knockdown of Egr1 downregulated Nox4 and α-SMA expression, reduced ROS generation, and alleviated EMT. | [49] |
| AMPK | AMPK activators attenuated the increase of Nox4 protein expression and ROS, restored the upregulation of mesenchymal marker protein (α-SMA) and downregulation of epithelial marker protein (E-cadherin) by high glucose, alleviated the renal tubular interstitial fibrosis. | [78] | |
| MALAT1-LIN28A-Nox4 | Upregulated MALAT1, LIN28A, and Nox4 expression, increased the production of ROS and inflammatory factors, resulted in apoptosis; activated AMPK/mTOR signaling and enhanced Nox4 stability; knockdown of MALAT1, LIN28A or Nox4 reversed these phenomena. | [79] | |
| p38MAPK | Upregulated Nox4 and p22phox protein expression levels, increased p38MAPK phosphorylation, fibronectin, and TGF-β1/2; Nox1/4 inhibitor (GK-136901) inhibited the activation of p38MAPK phosphorylation and attenuated fibronectin and TGF-β expression. | [23] |
3.2. Nox4 and diabetic retinopathy
Diabetic retinopathy is a microangiopathy of the retina caused by diabetes mellitus and is the main cause of blindness in diabetic patients. Patients do not show any symptoms in the early stage, however, when they notice the loss of vision, the lesion may have gone serious indeed, which means prevention is of great importance when it comes to DR in diabetic patients. DR manifests in two stages: a) Retinal microvascular damage and capillary degeneration; b) The onset of an exaggerated compensatory angiogenic reaction. Initial alterations in the retinal microcirculation involve disruptions in blood flow, thickening of the basement membrane, eventual loss of mural cells, and the formation of acellular capillaries. Endothelial apoptosis and capillary dropout result in a hypoxic inner retina, modifications in growth factors, and an elevation of inflammatory mediator. With the progression of the disease, pathological angiogenesis generated abnormal preretinal microvessels [84]. ROS is a signaling factor in the vascular system, and excessive ROS in the retina is associated with retinal angiogenesis, however, the precise mechanism by which this occurs is not fully established.
Nox4 has been connected with the development of DR in many animal models as well as high glucose-cultured retinal endothelial cells [24,[85], [86], [87]]. Similar to DKD, Nox4 is an crucial source of ROS generation in DR [88]. Nox4-derived ROS plays a pivotal role in the pathogenesis of DR [89]. Nox4 is essential for angiogenesis in DR where VEGF serves as a potent angiogenic factor. An experimental study showed increased gene and protein expression of Nox4, NADPH oxidase activity, ROS and VEGF levels in the retina of db/db diabetic mice and high glucose-induced retinal capillary endothelial cells (RCECs). Interestingly, Nox4 inhibition or lovastatin treatment significantly reversed the above changes, suggesting that Nox4 is a vital contributor to ROS generation in the retina and Nox4-mediated VEGF overexpression is a major contributor to diabetic retinal vascular permeability [85,90]. In addition, the role of Nox4-mediated VEGF expression in the neovascularization of insulin-intensive treatment-induced DR has been supported by relevant evidence [91], suggesting that Nox4 may promote angiogenesis in a VEGF-dependent manner. Moreover, it renders Nox4 a potential therapeutic target. DR is associated with excessive VEGF levels in the retina.
Another study found that carotenoids inhibited VEGF-induced increase in Nox4 activity and oxidative stress in human retinal microvascular endothelial cells (HRMECs) [34]. Although the effect of carotenoids on high glucose-induced Nox4 activity in HRMECs has not been reported, carotenoids may have some potential significance in the prevention and treatment of DR.
Similar to DKD, scholars have discovered that knockdown of the ADAM17 gene in STZ-induced diabetic mice endothelial cells could downregulate Nox4 expression and reduce oxidative stress significantly [92], suggesting that ADAM17 is an upstream regulator of Nox4 and has the potential to become a novel therapeutic target for diabetic microangiopathy. Li et al. proposed that CCN1 gene expression is involved in the development of DR. Knockdown of CCN1 (siRNA or CRISPR-Cas9) in HRVECs significantly reduced Nox4 protein expression levels and ROS production, inhibited oxidative stress and thus protected endothelial cell integrity [93]. Therefore, the CCN1/Nox4 axis could be a potential approach for the treatment of DR.
Notch signaling plays a vital part in high glucose-induced retinal cell injury. Activated by high glucose, the signaling pathway could upregulate the expression of Nox4, causing an overproduction of Nox4-derived ROS and increased apoptosis in human retinal endothelial cells (HRECs). In contrast, application of γ-secretase inhibitor (GSI) to suppress Notch activity evidently diminished the mRNA and protein levels of Nox4 and apoptosis induced by high glucose [87]. Besides, Yao et al. discovered that activation of the Notch signaling pathway was also associated with high glucose-induced apoptosis of renal tubular epithelial cells [94]. From the above studies, it is clear that Notch is a potential upstream regulator of Nox4, so the inhibition of Notch signaling or downregulation of Nox4 expression could serve as a viable therapeutic target for DR.
Perivascular apoptosis is a major feature of early diabetic retinopathy, where microglia are associated with diabetic retinal microvascular pericyte apoptosis. It was found that in lipopolysaccharide (LPS)-activated microglia and pericyte culture systems, microglia induced pericyte ROS overproduction mainly by stimulating pericytes to upregulate Nox (especially Nox4) and downregulate UCP2 expression, leading to a decrease in pericyte ΔΨm, an enhancement in nuclear NF-κB-p65 expression, and a heightening of cleaved caspase-3, which promoted pericyte oxidative damage and apoptosis. Notably, we could completely reverse the abovementioned apoptosis via DPI—a Nox inhibitor that suppresses pericyte ROS overproduction induced by microglia. On the other hand, research showed that ROS elevation in diabetes may also trigger microglia, which in turn further stimulates ROS production, forming a vicious cycle [95]. Consequently, regulating microglia activation and function as well as reducing Nox4-derived ROS production may be a novel approach to protect against pericytes apoptosis in DR.
Recent findings have revealed that Nox4-derived ROS may engage in the development of early neurodegeneration or apoptosis in diabetic retinopathy by downregulating BDNF/SIRT1 expression or activating Caspase-3, while NADPH oxidase inhibitor (Apocynin) can effectively reverse the deleterious effects of Nox4-induced ROS production [86], suggesting that the efficient blocking of Nox4 may potentially prevent DR development. A summary of the regulatory mechanisms of Nox4 in DR is provided in Table 2.
Table 2.
Regulatory mechanism of Nox4 in diabetic retinopathy.
| Models | Intervention | Regulating factors/Signal pathways | Main results | Conclusion | Reference | |
|---|---|---|---|---|---|---|
| The retina of db/db diabetic mice and high glucose-induced RCECs | Nox4 inhibition or lovastatin treatment | Nox4/VEGF | Nox4↓, NADPH oxidase activity↓, ROS↓, VEGF↓; Nox4-mediated VEGF overexpression led to diabetic retinal vascular permeability and neovascularization | Nox4 may promote angiogenesis in a VEGF-dependent manner. | [[89], [90], [91]] | |
| STZ-induced diabetic mice endothelial cells | Knockdown of the ADAM17 gene | ADAM17 | Nox4↓, oxidative stress↓ | ADAM17 is an upstream regulator of Nox4 and could be a new therapeutic target for diabetic microangiopathy. | [92] | |
| HRVECs | Knockdown of CCN1 (siRNA or CRISPR-Cas9) | CCN1 | Nox4↓, ROS↓, oxidative stress↓ | CCN1/Nox4 axis could be a potential approach for the treatment of DR. | [93] | |
| High glucose-induced HRECs | γ-secretase inhibitor (GSI) | Notch | Notch activity↓, Nox4↓, ROS↓, apoptosis↓ | Notch is a potential upstream regulator of Nox4. | [87] | |
| LPS-activated microglia and pericyte culture systems | a Nox inhibitor (DPI) | Microglia activation | Nox4↓, UCP2↑, ROS↓, ΔΨm↑, NF-κB-p65↓, cleaved Caspase-3↓, inhibited pericyte oxidative damage and apoptosis | Activated microglia may promote pericyte apoptosis by enhancing ROS production. | [95] | |
3.3. Nox4 and diabetic cardiomyopathy
Diabetic cardiomyopathy is a microangiopathy of the heart caused by diabetes mellitus. It is independent from hypertensive heart disease, coronary atherosclerotic heart disease, heart valve disease, and other cardiac lesions. As an irreversible process, it is mainly diagnosed at a late stage and has been viewed as a major cause of heart failure in diabetic patients. Structural and functional abnormalities in DCM are mainly manifested as left ventricular hypertrophy, fibrosis, apoptosis, increased oxidative stress, and inflammation, prompting cardiac dysfunction [96].
Diabetes or high glucose-induced ROS is an essential mediator of cardiac dysfunction. Nox4 is considered to be one of the major Nox family members that induces cardiac ROS production [97]. It could get expressed in wide range of cardiac cells, including cardiomyocytes, endothelial cells, fibroblasts, and vascular smooth muscle cells [20], and Nox4 overactivation is responsible for the development of cardiac injury in DCM. Numerous studies have shown that Nox4 expression level would significantly increase in both T1DM and T2DM myocardial tissues [[98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108]] and the excessive production of Nox4-derived ROS is interralated with the observed cardiac hypofunction and structural abnormalities [101,108]. Consistent with the results of animal experiments, Nox4 expression was also increased in high glucose-cultured cardiomyocytes and correlated with enhanced oxidative stress or diminished antioxidant levels [103,[108], [109], [110]]. Nox4 antisense oligonucleotide-regulated ROS are essential for attenuating early myocardial structural damage and dysfunction in diabetes. In vitro studies also suggested that significant suppression of Nox4 could prevent the increase in cardiomyocyte NADPH oxidase activity, molecular markers of fibrosis (Fibronectin, Collagen, α-SMA, β-MHC), and the development of myocardial hypertrophy induced by high glucose [108]. This study provides compelling evidence that Nox4 is a crucial source of ROS in diabetic cardiomyopathy, and suggests that Nox4-derived ROS may have a vital impact in the molecular mechanisms of fibrosis in diabetic cardiomyopathy. Matrix metalloproteinases are involved in the remodeling process of myocardial fibers, and the sustained induction and activation of this enzyme in diabetic cardiomyopathy is one of the markers of cardiac fibrosis [111]. In vivo studies revealed an increased expression of collagen (Collagen Ⅰ, Collagen Ⅲ) and matrix metalloproteinases (MMP-2, MMP-9) in myocardial tissue of STZ-induced diabetic rats, indicating the formation of myocardial fibrosis. Besides, in vitro studies further revealed that the expression of Nox4 and phosphorylated ERK1/2 as well as the production of mitochondrial and total cellular ROS were increased in rat cardiac fibroblasts treated with high glucose, while phosphorylated ERK1/2 and MMP-2 expressions significantly reduced after treatment with siRNA-Nox4 [110]. It conveys that the Nox4-ROS-ERK signaling pathway is crucial in the fibrotic process of diabetic cardiomyopathy and Nox4 may be an important target for the prevention and treatment of diabetic cardiomyopathy.
Since hyperglycemia regulates cardiac Ang II and TGF-β levels, both of which are shown to induce cardiac Nox4 expression [107,112,113], it can be inferred that the regulation of Nox4 by Ang II or TGF-β may play a critical role in the pathogenesis of diabetic cardiomyopathy. Reports have unveiled the interaction between Ang II and Nox4 in diabetic myocardial tissues and cells. It has been shown that Ang II receptor blockers could effectively inhibit the overexpression of Ang II type 1 receptor (AT1R), Nox4, and p22phox in the myocardial tissue of STZ-induced diabetic rats, which is accompanied by reduced oxidative stress and pro-fibrotic factor TGF-β expression, and improved cardiac function [99]. However, the evidence on their connection is not yet sufficient. As we all know, Chymase is an important promotor of Ang II. A report showed that the expressions of Chymase, 8-OHdG, and Nox4 are significantly upregulated in myocardial tissue of diabetic hamsters, along with a significant increase in myocardial Ang II levels and fibrosis indexes. While the inhibition of Chymase normalized myocardial Ang II levels and completely reversed Nox4-mediated oxidative stress and myocardial fibrosis in STZ-induced diabetic hamsters [107]. The above studies indicate that Nox4 may mediate the myocardial fibrosis process induced by Ang II in diabetic cardiomyopathy, and the inhibition of Ang II or Nox4 may become an important therapeutic target for diabetic cardiomyopathy alleviation. TGF-β is a cardiac cytokine and a potent pro-fibrotic marker involved in the development of cardiac fibrosis and heart failure. Similar to Ang II, upregulation of Nox4 in animal or cellular models of diabetic cardiomyopathy is usually associated with increased TGF-β expression [98,99,101,105,109,112]. Ang II and TGF-β play a key role in myocardial hypertrophy and fibrosis, and Ang II-induced TGF-β expression in cardiomyocytes and fibroblasts is prerequisite for Ang II-mediated myocardial hypertrophy and fibrosis [114]. Moreover, Nox oxidase is an intracellular signaling molecule necessary for Ang II-induced TGF-β upregulation in cardiomyocytes [115]. All this suggests that Ang II, TGF-β, and Nox oxidase are not individuals in cardiomyocytes, but rather parts of a signaling network that regulate events such as cardiac remodeling. At present, relatively few studies have revealed the interaction between advanced glycation end products (AGEs) and Nox4 in the pathogenesis of DCM, though some have reported that the expression of AGE receptors (RAGE) and Nox4 are both significantly upregulated, and both are regulated by PPARγ in the myocardial tissue of isoproterenol-induced diabetic rats [116]. Apart from that, further studies are needed to elucidate whether the AGE-RAGE pathway triggers diabetic myocardial injury by regulating Nox4.
Lately, scholars have been exploring new mechanisms that regulate the redox status of Nox4 in diabetic cardiomyopathy. Previous studies have shown that Kruppel-like factor 5 (KLF5) is an important regulator of cardiovascular remodeling [117], and then KLF5 was confirmed as a novel gene associated with DCM [118]. Recent studies have indicated that diabetes-mediated activation of FOXO1 increased KLF5 expression in cardiomyocytes, hence stimulating Nox4 expression and ceramide accumulation, causing cardiac dysfunction in diabetic mice [119]. The report further refines the potential mechanism of Nox4 in diabetic cardiomyopathy, and demonstrates that KLF5 is a new therapeutic target besides Nox4 for the prevention and treatment of diabetic cardiomyopathy. Hussain et al. found that the downregulation of JunD is substantial in hyperglycemia and ROS-induced myocardial dysfunction. Interestingly, diabetic mice with cardiac-specific overexpression of JunD were protected from hyperglycemia-induced increases in oxidative stress and inflammatory mediators such as Nox4, NF-κB, aberrant epigenetic regulation, and cardiac dysfunction [104]. The study provides new insights into the molecular mechanisms by which hyperglycemia or diabetes regulates Nox4 in cardiac myocytes or tissues (Table 3) and provides new targets for the prevention and treatment of DCM.
Table 3.
Regulatory Mechanism of Nox4 in Diabetic cardiomyopathy.
| Models | Intervention | Regulating factors/Signal pathways | Main results | Conclusion | Reference |
|---|---|---|---|---|---|
| The myocardial tissue of STZ-induced diabetic rat | – | Nox4-ROS-ERK | Collagen Ⅰ/Ⅲ↑, MMP-2/9↑, Nox4↑, Phosphorylated ERK1/2↑, ROS↑ | Nox4 plays an important role in the fibrotic process of diabetic cardiomyopathy and may be an important target for the prevention and treatment of diabetic cardiomyopathy. | [110] |
| The myocardial tissue of STZ-induced diabetic rat | Ang II receptor blockers | Ang II/Nox4 | AT1R↓, Nox4↓, p22phox↓, Oxidative stress↓, TGF-β↓, improved cardiac function | The regulation of Nox4 by Ang II or TGF-β may play a critical role in the pathogenesis of diabetic cardiomyopathy. | [99,107,112,113] |
| The myocardial tissue of diabetic hamsters | Chymase-specific inhibitor (TEI-F00806/TEI-E00548) | Chymase/Ang II | 8-OHdG↓, Nox4↓, Ang II↓, fibrosis indexes↓ | Chymase inhibition might prevent oxidative stress and diabetic cardiomyopathy at an early stage by reducing local AngII production. | [107] |
| Diabetic mice | – | FOXO1 | KLF5↑, Nox4↑, ceramide accumulation and cardiac dysfunction | KLF5 is a new therapeutic target for the prevention and treatment of diabetic cardiomyopathy. | [119] |
| STZ-induced diabetic mice | Cardiac-specific overexpression of JunD | JunD | Nox4、NF-κB and other mediators of oxidative stress and inflammation↓, aberrant epigenetic regulation and cardiac dysfunction↓ | Pave the way for tissue-specific therapeutic modulation of JunD to prevent diabetic cardiomyopathy. | [104] |
3.4. Nox4 and diabetic neuropathy
Diabetic neuropathy is a neurological microangiopathy caused by diabetes mellitus, which can involve both central and peripheral nerves, with diabetic peripheral neuropathy being the most common in clinical practice. The latest research reported that at least 50% of diabetes patients will progress to diabetic neuropathy [120]. Apart from maintaining blood glucose at a normal level, there are still no other targets or effective therapeutic means, making it urgent to investigate the pathogenesis of diabetic neuropathy and find the therapeutic targets.
Similar to other complications such as DKD, high glucose-mediated ROS production has been proven the most important pathogenic factor associated with the pathogenesis of diabetic neuropathy. Evidence has portrayed NADPH oxidase as a major source of ROS production in various cells and a key factor in various physiological and pathological processes. Notably, Nox4 is widely distributed in the central nervous system including cortical neurons, astrocytes, microglia, hippocampal pyramidal cells, Purkinje cells, and cerebellar granule neurons [121], and is also expressed in the peripheral nervous system including dorsal root ganglion neurons, sympathetic ganglion neurons, and Schwann cells [122,123]. Studies have shown that Nox4-produced ROS are involved in the tumorigenic transformation of glioblastoma and in the pathogenesis of ischemic stroke [124,125], and the main role of Nox4-mediated oxidative stress in the pathogenesis of diabetic neuropathy is under hot debate.
Recently, an in vitro study investigating the mechanism of Schwann cell apoptosis caused by high glucose showed that high glucose increased the gene and protein expressions of Nox4 and pro-apoptotic protein Caspase-3, coupled with increased intracellular ROS levels and Schwann cell apoptosis, and these changes were associated with the development of diabetic peripheral neuropathy. However, through the downregulation of intracellular ROS levels and the gene and protein levels of Caspase-3, the siRNA-Nox4-treated group saw an effective inhibition of high glucose-induced cellular oxidative stress and apoptotic damage [123]. It suggests that the effective inhibition of Nox4 may be a strategy for the treatment of diabetic peripheral neuropathy. Meanwhile, similar results were found by Ji et al. in a HFD/STZ-induced diabetic neuropathy rat model, which showed increased protein expressions of Nox4 and Caspase-3 and higher lipid peroxide and hydroperoxide levels in the model group, together with slowed nerve conduction velocity and reduced axonal area, leading to neurological dysfunction. On contrary, DPI, the NADPH oxidase inhibitor, did not effectively reverse these injuries [126], which may partly result from the insufficient duration of DPI application (only 1 day). It should be noted that the study mainly focused on the effect of DPI on sciatic nerve injury caused by Bupivacaine, hence the result does not mean that DPI has no effect on the prevention and treatment of diabetic neuropathy, instead, it may provide clues on whether the effective inhibition of Nox4 could ameliorate diabetic neuropathy.
Eid et al. recently used a T1DM mouse model, in vitro Schwann cells, and T2DM patient skin biopsies to demonstrate the critical role of liver X receptor (LXR) and Nox4 in diabetic peripheral neuropathy. Specifically, T1DM mice exhibited neurophysiological defects, sensorimotor abnormalities, and defective peripheral myelin gene expression, concomitant with significantly reduced LXR expression and increased Nox4 expression and activity in Schwann cells, peripheral nerves of mice, and skin biopsies from T2DM patients. Furthermore, targeted activation of LXR or specific inhibition of Nox4 in vivo and in vitro attenuated ROS production in high glucose-stimulated Schwann cells and diabetes-induced peripheral nerves, thereby reversing peripheral nervous functional changes and restoring the homeostatic distribution of myelin proteins. This study further proposed novel key mediators in the pathogenesis of diabetic peripheral neuropathy, and showed that targeting the LXR/Nox4 axis is a promising therapeutic approach [127].
Microvascular endothelial cell dysfunction can impair the normal structure and function of peripheral nerves, and one study suggested that a phytoactive drug (DA-9801) for diabetic neuropathy treatment attenuates Ang II-induced oxidative damage in human dermal microvascular endothelial cells by inhibiting the expression of Nox4 and p22 [128]. However, there is still a lack of report on whether DA-9801 could inhibit high glucose-induced activation of Nox4 in microvascular endothelial cells. Hence further deliberation on this study can better explain the pathogenesis of Nox4 in diabetes or high glucose-induced peripheral microangiopathy, and provide a theoretical basis for novel key targets. The regulatory mechanisms of Nox4 in DR are summarized in Table 4.
Table 4.
Regulatory Mechanism of Nox4 in Diabetic neuropathy.
| Models | Intervention | Regulating factors/Signal pathways | Main results | Conclusion | Reference |
|---|---|---|---|---|---|
| The schwann cells of newborn Wistar rats were cultured in vitro | siRNA-Nox4 treatment | Nox4/ROS | ROS↓, Caspase-3↓, Oxidative stress↓, Apoptosis↓ | Effective inhibition of Nox4 can be a protective strategy for developing drugs for diabetic peripheral neuropathy. | [123] |
| T1DM mice, high glucose-stimulated Schwann cells, and T2DM patient skin biopsies | Targeted activation of LXR or specific inhibition of Nox4 | LXR/Nox4 | LXR↑, Nox4↓, ROS↓, neurophysiological defects↓, sensorimotor abnormalities↓, and defective peripheral myelin gene expression↓ | Targeting the LXR/Nox4 axis is a promising therapeutic approach for diabetic peripheral neuropathy. | [127] |
| Human skin microvascular endothelial cells | DA-9801 treatment | Nox4/p22/Ang II | Nox4↓, p22↓, Ang II↓, Oxidative stress↓ | To explore the pathogenesis of Nox4 in diabetic or hyperglycemic peripheral microangiopathy and to provide a theoretical basis for finding key targets. | [128] |
4. Epigenetic regulation for Nox4 and diabetic microvascular complications
4.1. Epigenetic regulation for Nox4 and diabetic kidney disease
Researchers have also explored the mechanism of NADPH oxidase regulation by microRNAs (miRNAs) in DKD models. Among the five miRNAs (miR-25, miR-32, miR-92a, miR-92b, miR-363) presumed capable to bind to Nox4, it was found that miR-25 levels were significantly reduced in kidney of diabetic rats and glomerular mesangial cells of rats treated with high glucose, accompanied by increased expression levels of Nox4. In vitro studies have found that effective inhibition of miR-25 significantly increased the gene and protein expression levels of Nox4 [129]. Similarly, in kidneys of diabetic mice and high glucose-treated mesangial cells, a remarkable increase in the expression of Nox4 was found, while the gene expression levels of miR-25 precursors and mature miR-25 were reduced significantly [130]. The above study justifies the inference that miR-25 acts as an endogenous gene silencing factor regulating the expression of Nox4 in DKD.
Investigation has demonstrated the epigenetic regulation mechanism of Myo-Inositol Oxygenase (MIOX) in diabetic tubulopathy. Upward regulation of MIOX expression in human renal tubular epithelial cells and a robuts binding of specificity protein Sp-1 transcription factor with MIOX promoter were discovered under high glucose conditions. In addition, DNA hypomethylation and histone hyperacetylation of the MIOX promoter may cause the excessive expression of ROS and Nox4, which were associated with mechanisms underlying diabetic tubulopathy [131]. It provides hints that MIOX may regulate Nox4 expression in diabetic tubulopathy through epigenetic regulation.
In recent years, there are increasing reports about microRNAs regulating abnormal activation of Nox4 in diabetic glomerular epithelial cells. miR-423-5p expression was reduced and Nox4 expression was elevated in clinical kidney tissues from individuals with DKD and in high glucose-treated mouse podocytes. Functional analysis confirmed that Nox4 is a direct downstream target of miR-423-5p, and overexpression of miR-423-5p enhanced cell viability while inhibiting ROS production, apoptosis, inflammatory response, and cytoskeletal damage caused by variations in podocyte-specific proteins [132]. This study demonstrates that miR-423-5p overexpression inhibits ROS production by targeting Nox4, thereby protecting against high glucose-induced podocyte injury and providing a prospective therapeutic strategy for DKD. Furthermore, Fu et al. observed that high glucose exposure markedly raised Nox4 protein and gene expression levels in mouse podocytes, and that treatment with miR-15b-5p overexpression eliminated Nox4 overactivation, increased malondialdehyde content, and decreased antioxidant enzyme activity induced by high glucose [133], suggesting that miR-15b-5p may directly or indirectly regulate the expression of Nox4. However, further studies are necessary to confirm whether miR-15b-5p exerts its antioxidant effect by directly targeting Nox4.
Wan et al. explored the function of microRNA146a (miR146a) in a DKD model. It was found that the expression of miR146a was suppressed while Nox4 expression level was signally increased in the kidney of DKD mice. Meanwhile, overexpression of miR146a in HK-2 cells downregulated Nox4 protein level, ROS generation, oxidative stress, and inflammation. At the same time, it decreased protein expression of vascular cell adhesion molecule-1 (VCAM-1) and ICAM-1. The above-mentioned results suggest that miR146a/Nox4 performs a vital function in the regulation of oxidative stress and inflammation in DKD renal tubules [134]. Therefore, miR146a is an upstream regulator of Nox4 and may offer a novel avenue or taget for the treatment of DKD.
4.2. Epigenetic regulation for Nox4 and diabetic retinopathy
Recently, miR-590-3p has been found to be associated with pyroptosis in DR. Downregulation of miR-590-3p and upregulation of NLRP1/Nox4 were observed in high glucose-cultured HRMECs. Inhibition of miR-590-3p could upregulate NLRP1, Nox4/ROS/TXNIP/NLRP3 pathway, and Caspase-1 protein expression. Conversely, overexpression of miR-590-3p could reverse the above effects. Simultaneous transfection of Nox4 and NLRP1 siRNA in HRMECs did not significantly improve cellular activity even in the presence of miR-590-3p mimics, demonstrating that miR-590-3p regulates pyroptosis by targeting Nox4 and NLRP1 [135]. It shows that miR-590-3p may be involved in the pathogenesis of DR as an upstream regulator of Nox4.
And a study has shown that histone deacetylase can reduce the expression level of Nox4 and inhibit retinal angiogenesis, but whether this process applies in diabetes-induced retinopathy needs to be confirmed further [136].
MiR-99a-5p is implicated in regulating diabetes progression. In this study, T2DM patients were divided into three groups: non-DR (NDR), non-proliferative DR (NPDR) and proliferative DR (PDR). The expression of miR-99a-5p was found to decrease gradually with disease progression, which means miR-99a-5p level was negatively correlated with T2DM. Additionally, overexpression of miR-99a-5p mitigated the deleterious effects of high glucose on HRMECs' proliferation. Nox4 was identified as a downstream target of miR-99a-5p, and its upregulation triggered by high glucose was decreased by miR-99a-5p overexpression [137]. In summary, miR-99a-5p improves DR by inhibiting the abnormal proliferation and migration of human retinal microvascular endothelial cells through targeting Nox4.
4.3. Epigenetic regulation for Nox4 and diabetic cardiomyopathy
A study found that STZ-induced mice, when given intracardiac injection of miR-372-3p KD lentivirus, could significantly improve the development of DCM. Ultrasound quantification revealed that in DCM mice, there showed a decline in left ventricular fractional shortening (LVFS) and left ventricular ejection fraction (LVEF) in DCM mice, along with an increase in left ventricular internal diameter at end-diastole (LVIDd) and left ventricular internal diameter at end-systole (LVIDs). However, treatment with miR-372-3p KD significantly reversed these indices, suggesting that miR-372-3p KD can effectively improve cardiac insufficiency and systolic dysfunction. In addition, results showed that miR-372-3p KD treatment raised expression of p-PI3K, p-AKT, p-mTOR, p-P70S6K and HIF-1α, while downregulating Nox2, and Nox4 expression [138], indicating that miR-372-3p is an upstream target of PI3K/AKT/mTOR/HIF-1α signaling pathway, as well as Nox2 and Nox4, and may involve in the angiogenesis of DCM mice.
4.4. Epigenetic regulation for Nox4 and diabetic neuropathy
Similar to DKD, microRNAs may play a facilitative or protective role in the pathogenesis of diabetic neuropathy. It has been reported that the expression of Nox4 and ROS levels in the sciatic nerve of db/db diabetic mice was increased along with a decrease in miR-25 level. The effects were exacerbated in db/db diabetic mice treated with miR-25 inhibitors, yet normalized in the miR-25 precursor treatment group. In addition, miR-25 precursors inhibited PKC activation in db/db diabetic mice, reduced the levels of AGEs and RAGE, and further downregulated inflammatory factors that contribute to peripheral neuropathological processes [139]. These findings validate that miR-25 reduces ROS production in diabetic peripheral nerves by antagonizing the activity of Nox4, but the mechanism in which miR-25 targets Nox4 to exert neuroprotective effects remains to be further explored subsequently.
In conclusion, epigenetic regulation of Nox4 and diabetic microvascular complications are summarized in Table 5.
Table 5.
Epigenetic regulation of Nox4 and diabetic microvascular complications.
| Diabetes complications | Regulating factors/Signal pathways | Main results | Reference |
|---|---|---|---|
| Diabetic Kidney Disease | miR-25 | Effective inhibition of miRNA-25 increased Nox4 gene and protein expression levels. | [129,130] |
| Myo-Inositol Oxygenase | DNA hypomethylation and histone hyperacetylation of the MIOX promoter led to the overexpression of ROS and Nox4. | [131] | |
| miR-423-5p | miR-423-5p overexpression inhibited Nox4-dependent ROS production; enhanced cell viability; inhibited apoptosis, inflammatory response and cytoskeletal damage. | [132] | |
| miR-15b-5p | miR‐15b‐5p overexpression eliminated Nox4 overactivation, increased malondialdehyde content and decreased antioxidant enzyme activity induced by high glucose. | [133] | |
| miR146a | miR146a overexpression downregulated Nox4 protein level, ROS generation, oxidative stress, and inflammation; decreased VCAM-1 and ICAM-1 protein expression. | [134] | |
| Diabetic Retinopathy | miR-590-3p | Inhibition of miR-590-3p upregulated NLRP1, Nox4/ROS/TXNIP/NLRP3 pathway and Caspase-1 protein expression. Conversely, overexpression of miR-590-3p could reverse the above effects. | [135] |
| miR-99a-5p | As DR progressed, miR-99a-5p levels decreased and levels of Nox4, FPG, HOMA-IR and HbA1c increased in diabetic patients. In contrast, overexpression of miR-99a-5p mitigated the deleterious effects of HG. | [137] | |
| Diabetic cardiomyopathy | miR-372-3p | miR-372-3p KD treatment raised expression of p-PI3K, p-AKT, p-mTOR, p-P70S6K and HIF-1α, while downregulating Nox2, and Nox4 expression. In addition, LVFS and LVEF were increased, LVIDd and LVIDs were decreased. | [138] |
| Diabetic neuropathy | miR-25 | Decreased miR-25 levels, accompanied by increased Nox4 expression and ROS levels, as well as inhibition of PKC activation, reduced AGEs and RAGE levels. | [139] |
5. Nox4 inhibitors for diabetic microvascular complications
The complex pathogenesis of diabetic complications has resulted in a lack of fully elucidated specific mechanisms and effective molecular intervention targets and tools. As a result, current treatments for diabetic complications are mainly based on comprehensive symptomatic therapy. However, studies have shown that some existing therapeutic drugs or inhibitors may improve the condition of diabetic complications by regulating the expression of Nox4 (Table 6).
Table 6.
Nox4 inhibitors for diabetic microvascular complications.
| Diabetes complications | drugs/Inhibitors | Models | Main results | Reference |
|---|---|---|---|---|
| Diabetic Kidney Disease | Ginsenoside Rb1 | high glucose-treated mouse podocytes | It significantly inhibited the expression levels of Caspase-9 and Nox4 proteins, and reduced apoptosis and mitochondrial damage. | [140] |
| Sacubitril | Zucker Obese rats | In both the sac/val-treated and val-treated groups, the expression levels of Nox2 and Nox4 were significantly decreased, indicating an improvement in oxidative stress. However, gene expression of podocin and nephrin decreased only after sac/val treatment. | [142] | |
| Fluorofenidone (AKF-PD) | – | Its protection mechanism may involve downregulation of NADPH oxidase, upregulation of GPx and SOD, and modulation of the AGE and PKC pathways to reduce renal oxidative stress. | [143] | |
| Fenofibrate | STZ-induced diabetic rats | There was a boost in CAT and SOD enzyme activity and glutathione content, along with a decrease in mRNA and protein expressions of Nox4, IL-18, and p53. Additionally, fenofibrate improved creatinine clearance and protein excretion. | [144] | |
| Dapagliflozin | db/db mice | It significantly reduced blood glucose and HbA1c levels, and improved albuminuria and urinary TBARS levels. It also downregulated the renal expression of NF-κB p65, MCP-1, Nox4, Nox2 and p47phox, thereby improving renal inflammation and oxidative stress. | [145] | |
| Liraglutide | STZ-induced diabetic rats | Inhibits the expression of oxidative stress markers, renal NADPH oxidase components (Nox4, gp91phox, p22phox, p47phox), TGF-β and fibronectin, as well as significantly improves urinary albumin excretion via the cAMP-PKA pathway. | [146] | |
| Huidouba (HDB) | STZ induced unilateral nephrectomy diabetic rats | Fasting blood glucose, mAlb/Ucr, Scr, BUN and renal MDA levels were significantly decreased, and effectively reducing the expression of renal Nox4 and upregulating the expression of renin and WT1 in diabetic rats, thus alleviating the damage of podocytes. | [147] | |
| Baoshenfang Formula (BSF) | clinical trial/high glucose-cultured podocytes | 24-h urinary protein and renal function were notably improved; high glucose-cultured podocytes were given BSF treatment, MDA, ROS and Bax levels were reduced, while markedly increasing SOD and Bcl-2 levels, it also greatly reduced the expression of Nox4 and phospho-p38, acting as an anti-apoptotic potential to protect the kidney. | [148] | |
| Rehmanniae Radix (RR) and Cornus officinalis (CO) | KK-Ay mice (DKD mice) | It can inhibit the activation of AGEs/Nox4/p65 NF-κB pathway in podocytes and reduce apoptosis. The above drugs act as antioxidants and anti-inflammatory agents to improve DKD. | [149] | |
| Plumbagin | HK-2/STZ-induced diabetic mice | Significantly inhibited TGF-β1-induced overexpression of Nox4, fibronectin and collagen IV; Reversal of renal fibrotic alterations and accumulation of extracellular matrix proteins. | [150] | |
| GKT137831 | STZ-induced ApoE−/− mice/immortalized human podocytes | Effectively inhibits the expression of Nox4, leading to a decrease in renal cortical superoxide production and nitrotyrosine accumulation in glomeruli, reduces macrophage infiltration in the diabetic kidney, attenuates the expression of MCP-1 and NF-κB; Attenuates the gene expression of key proteins involved in renal fibrosis, such as collagen IV, fibronectin, connective tissue growth factor and a-SMA, reduces ROS production and improve proteinuria. | [45,151] | |
| GKT136901 | db/db mice | Inhibits Nox4 expression, reduces oxidative stress and ERK1/2 phosphorylation, attenuates albuminuria and TBARS levels, and protects kidney structures. | [152] | |
| Nacetylcysteine | DKD mice | Inhibition of ROS overproduction, VCAM-1 and ICAM-1 protein expression, and NF-κB, TNF-α, IL-6 and IL-1β activity. | [134] | |
| APX-115 | high glucose-induced podocytes | Completely inhibited the expression of pro-inflammatory molecules (NF-κB, MCP-1, Nox2, Nox4) and pro-fibrotic factors (TGF-β1, PAI-1 and collagen IV), acting as an anti-inflammatory and anti-fibrotic agent. | [153] | |
| Diabetic Retinopathy | lovastatin | high glucose-cultured RCECs/db/db mice | Effectively reduce the mRNA and protein expression of Nox4 and the level of ROS and VEGF, thus improving the permeability of retinal blood vessels, inhibiting retinal oxidative stress and improving the disruption of the blood-retinal barrier. | [85] |
| Fenofibrate | C57BL/6J-Ins2Akita mice | Significant inhibition of Nox4 and Nox2 expression levels and increased phosphorylation of LRP6. And increased expression of antioxidant enzymes (SOD1 and SOD2), improved diabetic vascular leakage. | [154] | |
| Dapagliflozin | lens of fructose-induced T2DM rats | The expression of GLUT5, p47/p67-phox, Nox4 and RAGE was reduced. In addition, dapagliflozin reduced RAGE-induced NADPH oxidase expression in lens epithelial cells (LEC) by inactivating GLUTs and reducing ROS production. | [155] | |
| Conbercept | DR mice | Significantly inhibited the gene expression of Nox4 and p22phox, as well as the accumulation of chronic inflammation in the mouse retina, while reducing retinal endothelial cell proliferation and retinal angiogenesis. | [156] | |
| Gambogic acid (GA) | high glucose-induced HRECs | It decreased the levels of IL-6, IL-8 and TNF-α and significantly increased the level of IL-10, inhibited the activity of the Nox4/NLRP3 pathway, thereby suppressing apoptosis and inflammatory responses in HRECs under high glucose conditions. | [157] | |
| Sulodexide | High glucose incubation of porcine retinal arterioles | Inhibited protein overexpression of Nox4 and Nox5 and suppressed ROS production in a concentration-dependent manner, preventing oxidative damage and endothelial dysfunction to the retinal arterial vasculature. | [158] | |
| Panax notoginseng saponin | high glucose-induced RCECs | Increased activity of antioxidant enzymes (SOD, MnSOD, CAT and GSH-PX) and decreased Nox4 expression, thereby reducing cellular oxidative damage in high glucose-induced RCECs. | [141] | |
| GLX7013114 | diabetic rats | Effectively inhibited nitric/oxidative stress, attenuated apoptosis by reducing Caspase-3 expression, and suppressed micro/giant cell activation. In addition, it attenuated diabetes-induced elevation of VEGF, blood-retinal permeability evaluation index (Evans-Blue leakage) and pro-inflammatory cytokines (TNF-α protein and IL-1β/IL-6) mRNA, and significantly increased the mean PERG amplitude. | [159] | |
| GKT136901 and GKT137831 | dimethyl oxalylglycine-stimulated retinal endothelial cells | Significantly reduces ROS production and VEGF expression, and shows potent anti-inflammatory effects. | [160,161] | |
| Diabetic cardiomyopathy | chrysin | isoproterenol-induced myocardial injury in diabetic rats | It downregulated RAGE expression and inhibited Nox4, 8-OHdG, NF-κBp65/IKK-β, TNF-α, Bax, and Caspase-3, while enhancing Bcl-2 expression and the activity of GSH, CAT, and MnSOD, thereby reducing myocardial oxidative stress, inflammation and apoptosis. | [162] |
| GKT137831 | ventricular myocytes | This Nox4 inhibitor did not significantly inhibit Hi Glu/ThmG-induced ROS. | [163] | |
| Diabetic neuropathy | Liraglutide | STZ-induced diabetic rats | It has a protective effect on peripheral nerves and also inhibits the gene and protein expression of Nox4 in the sciatic nerve. | [164] |
| DA-9801 | HDMECs | Blockade of Ang II-induced NADPH oxidase (Nox4 and p22phox subunits) expression and increased ROS production, attenuated Ang II-induced oxidative stress and reduced the upregulation of p38 MAPK, ERK, ICAM-1, VCAM-1 and E-selectin and caspase 9, 7 and 3 activities. | [128] |
5.1. Nox4 inhibitors for diabetic kidney disease
He et al. discovered that ginsenoside Rb1 (Rb1), which is the major active component of Panax notoginseng, showed therapeutic effects on DKD. In high glucose-treated mouse podocytes, the intervention of Rb1 significantly inhibited Caspase-9 and Nox4 protein expression levels, and reduced podocyte apoptosis and mitochondrial damage [140]. Furthermore, Panax notoginseng saponin was seen to raise the activity of antioxidant enzymes, such as SOD, MnSOD, CAT, and GSH-PX, while decreasing Nox4 expression in high glucose-induced RCECs, which attenuated high glucose-induced cellular oxidative damage in RCECs [141]. The above study provides new evidence to the mechanism of Panax notoginseng against DKD.
Sacubitril (sac) is a neprilysin inhibitor, and valsartan (val) is an Ang II type 1 receptor blocker. Results of a study showed that in Zucker Obese rats, Nox2, Nox4 expressions and nitroso-oxidative stress were increased, and these effects occurred in concert with a decrease in the expression of podocin and nephrin, resulting in glomerular and tubular damage. Notably, in both sac/val-treated and val-treated groups, Nox2 and Nox4 expression levels were significantly decreased, indicating an improvement in oxidative stress. However, only after sac/val treatment did the gene expressions of podocin and nephrin dropped [142]. These findings suggest that the combination of sakubatril and valsartan can be more effective in protecting the kidney.
Fluorofenidone (AKF-PD) is a recently developed drug used to delay the progression of DKD. Some scholars suggest that its protection mechanism may involve downregulation of NADPH oxidase, upregulation of GPx and SOD, and modulation of the AGE and PKC pathways to reduce renal oxidative stress. This makes it a potential therapeutic option for the clinical treatment of DKD [143].
Fenofibrate, a PPAR-α agonist, has been found to improve the development of DKD. In STZ-induced diabetic rats treated with fenofibrate, there was a boost in CAT and SOD enzyme activity and glutathione content, along with a decrease in mRNA and protein expressions of Nox4, IL-18, and p53. Additionally, fenofibrate improved creatinine clearance and protein excretion, thus restored renal function [144]. In summary, fenofibrate curbed oxidative stress, inflammation, and apoptosis, contributing to the improvement of DKD.
Dapagliflozin, a novel hypoglycemic agent, not only lowers blood glucose, but also has anti-inflammatory properties and ameliorates oxidative stress and renal fibrosis. A study found that dapagliflozin treatment in db/db mice significantly reduced blood glucose and HbA1c levels, as well as ameliorated albuminuria and and urine thiobarbituric acid reactive substance (TBARS) levels. Moreover, it downregulated the renal expressions of NF-κB p65, MCP-1, Nox4, Nox2, and p47phox [145], thereby improving diabetes-related renal inflammation and oxidative stress.
Liraglutide is a glucagon-like peptide-1 (GLP-1) analogue, while GLP-1 is considered to be an effective therapeutic agent for T2DM. Studies have shown that oxidative stress markers (renal dihydroethidium staining and urinary 8-hydroxy-2′-deoxyguanosine), expressions of renal NADPH oxidase components (Nox4, gp91phox, p22phox, p47phox), TGF-β and fibronectin, and urinary albumin excretion were significantly elevated in STZ-induced diabetic rats. However, liraglutide intervention significantly ameliorated these changes and improved DKD injury. Previous studies have confirmed that the main effects of GLP-1 are mediated through the activation of adenylate cyclase and cAMP production. This study showed that the PKA inhibitor (H89) and adenylate cyclase inhibitor (SQ22536) were able to reverse the inhibitory effects of liraglutide on NADPH oxidase; nonetheless, Epac2 (another downstream pathway of elevated cAMP levels) inhibition had no reversal effect [146]. These results suggest that liraglutide inhibits the expression of NAPDH oxidase (containing Nox4) via the cAMP-PKA pathway and exerts a renoprotective effect.
Huidouba (HDB), a Tibetan medicine, has been used for thousands of years in the Mount Emei area to nourish kidney yin and maintain blood glucose homeostasis. Yang et al. found that in STZ induced unilateral nephrectomy plus HFD feeding diabetic rats, HDB treatment led to significant reductions in fasting blood glucose, mAlb/Ucr, Scr, BUN, and renal MDA level. Moreover, it efficiently downregulated renal Nox4 expression and upregulated nephrin and WT1 expression in diabetic rats, which relieved podocyte damage [147]. These results suggest that HDB may be used as a potential traditional medicine for the treatment of DKD.
In a clinical trial on Baoshenfang Formula (BSF) for DKD, 72 subjects were divided into normal control, DKD, and BSF treatment groups. The results showed that 24-h urinary protein and renal function were notably improved in patients after BSF treatment compared to the control group. In addition, an in vitro study found that when high glucose-cultured podocytes were given BSF treatment, MDA, ROS and Bax levels were reduced, while markedly increasing SOD and Bcl-2 levels. What's more, BSF treatment greatly decreased Nox4 and phospho-p38 expressions, while high glucose-mediated apoptosis was blocked by p38 MAPK inhibitor and Nox4-siRNA restricted the phosphorylation of p38 [148], showing the anti-apoptotic potential of BSF for kidney protection partly through inhibiting the Nox4/ROS/p38 pathway.
Rehmanniae Radix (RR) and Cornus officinalis (CO) are commonly used herbal combinations for the treatment of DKD. While catalpol (Cat) and loganin (Log) are the main active components of RR and CO, respectively, they can effectively treat DKD by counteracting apoptosis, oxidative stress, and inflammation. Studies have shown that Cat and Log treatment, either alone or in combination, could improve renal function and pathomorphological impairment in KK-Ay mice (DKD model), meanwhile, such treatment was also able to inhibit the activation of AGEs/Nox4/p65 NF-κB pathway in podocytes, especially the combined treatment, which can significantly reduce apoptosis and act as an antioxidant and anti-inflammatory agent to improve DKD [149].
TGFβ1 has been proven to play a role in renal fibrosis and dysfunction in DKD. Co-culturing of HK-2 with TGFβ1±plumbagin, an inhibitor of Nox4, revealed that TGFβ1 increased the mRNA expression of Nox4. However, treatment with Plumbagin and Nox4-siRNA significantly inhibited TGF-β1-induced overexpression of Nox4, fibronectin, and collagen IV in HK-2 cells. Furthermore, plumbagin treatment reversed the renal fibrotic alterations and extracellular matrix protein accumulation in STZ-induced diabetic mice [150]. The results of this study indicate that plumbagin can alleviate the development of DKD by modulating Nox4 signaling pathways.
GKT137831 is a potent and highly selective inhibitor of Nox1/4 that has demonstrated significant therapeutic potential in the treatment of DKD. In STZ-induced ApoE−/− mice, GKT137831 was observed to effectively suppress the expression of Nox4, leading to a reduction in renal cortical superoxide production and nitrotyrosine accumulation in the glomeruli. Consequently, GKT137831 resulted in attenuation of diabetes-induced glomerular injury, including a decrease in albuminuria, ECM accumulation and tubulointerstitial area [45]. Moreover, GKT137831 was found to reduce macrophage infiltration in diabetic kidneys and mitigate expressions of MCP-1 and NF-κB. In vitro experiments using immortalized human podocytes revealed that GKT137831 could alleviate critical proteins involved in renal fibrosis, such as gene expressions of collagen IV, fibronectin, connective tissue growth factor and a-SMA. GKT137831 also reduced ROS production and improved proteinuria [151]. Collectively, these findings highlight the potential of GKT137831 as a therapeutic agent for the treatment of DKD.
DKD is specifically characterized by albuminuria and renal injury. In db/db mice, both plasma and urinary levels of TBARS, which are markers of systemic and renal oxidative stress, were significantly increased. This increase was accompanied by a significant upregulation of Nox4 expression. Treatment with GKT136901 inhibited Nox4 expression, reduced oxidative stress and ERK1/2 phosphorylation, attenuated albuminuria and TBARS levels, and preserved renal structures. Furthermore, renal ERK1/2 activation was found to be elevated in db/db mice, whereas activation of p38MAPK or JNK was not observed to increase, suggesting that increased Nox4 promotes diabetic renal damage through the regulation of renal ERK1/2 activation [152]. Therefore, it can be inferred that diabetes-related Nox4 may contribute to renal injury by modulating ERK1/2 activation.
According to a previous study, miR146a was identified as an upstream regulator of Nox4. In the kidneys of DKD mice, the expression of miR146a was suppressed, while the expression of Nox4 was significantly increased. Moreover, this study also confirmed that overexpression of miR146a in HK-2 cells along with the application of Nox4 inhibitor (Nacetylcysteine) led to further inhibition of ROS overproduction, VCAM-1 and ICAM-1 protein expression, and the activity of NF-κB, TNF-α, IL-6, and IL-1β [134]. These findings suggest that overexpression of miR146a may downregulate the expression of Nox4, and consequently improve oxidative stress and inflammatory response in DKD renal tubules.
APX-115 is a novel pan-Nox inhibitor that has demonstrated promising effects in db/db mice. Specifically, APX-115 intervention caused a containment in Nox1, Nox2, Nox4 protein expression in the diabetic kidney, a significant reduction in plasma 8-isoprostane level, and a lowering in MCP-1, TNFα and IL-6 expression in the diabetic kidney. Notably, APX-115 intervention also brought about significant improvements in insulin resistance, lipid profiles, proteinuria, and creatinine level. In addition, in high glucose-induced podocytes, APX-115 treatment completely suppressed the expression of proinflammatory molecules (NF-κB, MCP-1, Nox2, Nox4), and profibrotic factors (TGF-β1, PAI-1, and collagen IV) [153]. These results indicates that APX-115 boasts anti-inflammatory and anti-fibrotic effects, ultimately leading to renoprotection.
5.2. Nox4 inhibitors for diabetic retinopathy
A study found hypoxia and high glucose-cultured RCECs had significantly higher mRNA and protein expression of Nox4, as well as significantly higher levels of ROS and VEGF. This phenomenon was also observed in the retinas of db/db mice. However, administration of lovastatin treatments almost completely reversed the above changes in vivo and in vitro studies [85]. These findings suggest that lovastatin can improve retinal vascular permeability by inhibiting the expression of Nox4 and VEGF, thereby suppressing retinal oxidative stress and ameliorating blood-retinal barrier breakdown, which implies the potential use of lovastatin as a therapeutic option for diabetic retinopathy.
Fenofibrate and dapagliflozin have therapeutic effects on diabetic retinopathy in addition to their role in alleviating DKD. In the retinas of C57BL/6J-Ins2Akita mice (a genetic T1DM model) and high glucose-treated ARPE-19 cells, the expression levels of Nox4 and Nox2 as well as LRP6 phosphorylation were significantly increased, while the expression of antioxidant enzymes (SOD1 and SOD2) was reduced, resulting in diabetic vascular leakage. Conversely, fenofibrate treatment significantly improved these phenomena. Interestingly, overexpression of Nox4 directly activated high glucose-induced LRP6 phosphorylation in ARPE19, and knockdown of Nox4 suppressed LRP6 phosphorylation [154], suggesting that fenofibrate may improve diabetic retinopathy through the Nox4/LRP6 pathway.
Dagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, is an emerging compound for the treatment of diabetes. A recent study found that SGLT2, GLUT1, GLUT5, p47/p67-phox, Nox4 and RAGE levels were significantly elevated in lens of fructose-induced T2DM rats. However, dapagliflozin treatment reduced GLUT5, p47/p67-phox, Nox4 and RAGE expressions. Additionally, scholars have demonstrated that dapagliflozin downregulated RAGE-induced NADPH oxidase expression in lens epithelial cells (LECs) via inactivation of GLUTs and reduction in ROS production [155]. Based on these results, dapagliflozin may downregulate Nox4 expression via SGLT2 inactivation and RAGE reduction, thereby alleviating diabetic retinopathy.
Conbercept, a newly developed anti-VEGF drug, significantly inhibited the gene expression of Nox4, p22phox, and the accumulation of chronic inflammation in the retina of mice with proliferative DR, while reducing retinal endothelial cell proliferation and retinal angiogenesis. Consequently, Conbercept could be a promising target drug for limiting Nox4-mediated DR angiogenesis [156].
Treating high glucose cultured HRECs with gambogic acid (GA) significantly decreased IL-6, IL-8 and TNF-α levels, while significantly increasing the level of IL-10. GA also inhibited the activity of Nox4/NLRP3 pathway, which suppressed cell apoptosis and inflammatory response of HRECs under high glucose conditions [157]. These findings suggest that GA has a protective effect against diabetic retinopathy.
High glucose incubation of porcine retinal arterioles for 2 h impaired endothelium-dependent vasodilation and elevated ROS levels, as shown in a recent study. However, the application of sulodexide inhibited protein overexpression of Nox4, Nox5 and ROS production in a concentration-dependent manner, preventing oxidative injury and endothelial dysfunction of the retinal arterial vasculature [158].
GLX7013114 is a specific inhibitor of Nox4 that has demonstrated promising effects in the treatment of diabetic retinopathy. When topically administered as eye drops, GLX7013114 was found to effectively inhibit nitric/oxidative stress, alleviate apoptosis by reducing Caspase-3 expression, and restrain micro/macroglia cell activation. Moreover, GLX7013114 attenuated diabetes-induced elevation of VEGF, blood-retinal permeability evaluation index (Evans-Blue leakage), and pro-inflammatory cytokines such as TNF-α protein and IL-1β/IL-6 mRNA. In diabetic rats, the mean pattern electroretinography (PERG) amplitude decreased by more than 50% compared to the control group. However, treatment with GLX7013114 significantly increased the mean PERG amplitude compared to the untreated group [159], suggesting that GLX7013114 preserves retinal ganglion cell function and attenuates diabetic retinopathy.
Interestingly, treatment of dimethyl oxalylglycine-stimulated retinal endothelial cells with Nox1/Nox4 inhibitors (GKT136901 and GKT137831) also pronouncedly lowered ROS production and VEGF expression, and showed potent anti-inflammatory effects [160,161], but whether Nox4 can be a attractive and promising therapeutic target for DR needs to be further studied.
5.3. Nox4 inhibitors for diabetic cardiomyopathy
It has been proven that Chrysin, a PPAR-γ agonist, could inhibit the AGE-RAGE axis and ameliorate isoproterenol-induced myocardial injury in diabetic rats. It downregulated RAGE expression and inhibited Nox4, 8-OHdG, NF-κBp65/IKK-β, TNF-α, Bax, and Caspase-3, while enhancing Bcl-2 expression and the activity of GSH, CAT, and MnSOD. All this could reduce myocardial oxidative stress, inflammation, and apoptosis [162].
A study demonstrated that high extracellular glucose (Hi-Glu) promotes ROS production in ventricular myocytes, allowing CaMKII oxidation to activate, thereby accelerating post-infarction mortality. To explore the source of this novel ROS, scholars used inhibitors of Nox2 (Gp91ds-tat peptide), Nox4 (GKT137831), mitochondrial ROS (MitoTempo), and NOS pathway inhibitors (l-NAME, L-NIO and L-NPA) to investigate their involvement, and found that only inhibition of Nox2 or knockout prevented Hi Glu/ThmG-induced ROS production [163].
5.4. Nox4 inhibitors for diabetic neuropathy
To date, the prevention and slowing of the onset and progression of diabetic neuropathy primarily relies on hypoglycemic agents that keep blood glucose at a normal level. A recent in vivo study found that a glucagon-like peptide-1 receptor (GLP-1R) agonist Liraglutide, also a hypoglycemic agent, had a protective effect on peripheral nerves in STZ-induced diabetic rats, and also inhibited the gene and protein expression of Nox4 in the sciatic nerve of diabetic rats [164]. However, whether GLP-1R agonist alleviates diabetic peripheral nerve injury through the inhibition of Nox4 remains hidden. Therefore, it is meaningful to make endeavors in revealing the mechanism of Nox4 in diabetic neuropathy, which may shed light on targeted treatment of diabetic neuropathy.
DA-9801 is a mixture of extracts from Dioscorea japonica and Dioscorea nipponica, and can be used to treat diabetic neuropathy. Excessive oxidative stress and inflammation have been reported to cause microvascular changes and damage in the vascular endothelium, impairing the normal function and structure of peripheral nerves. In human dermal microvascular endothelial cells (HDMECs), Ang II induced increased expression of NADPH oxidases (Nox4 and p22phox subunits) and ROS production. However, pre-treatment with DA9801 effectively blocked the above processes, thereby attenuating Ang II-induced oxidative stress and reducing the upregulation of p38 MAPK, ERK, ICAM-1, VCAM-1, and E-selectin, as well as caspase 9, 7, and 3 activities [128], thereby ameliorating diabetic neuropathy by inhibiting endothelial cell inflammation and apoptosis.
6. Natural food components that inhibit Nox4 and prevent diabetic microvascular complications
Nox4 is widely expressed in human body, whose activity enhancement could lead to a variety of diabetic microangiopathies. Hence efforts are made to discover natural food components that inhibit Nox4 (Table 7) and limit the use of compounds with toxic side effects, so as to elevate the safety.
Table 7.
Natural food components that inhibit Nox4 and prevent diabetic microvascular complications.
| Diabetes complications | Food components with therapeutic effects | Reference |
|---|---|---|
| Diabetic Nephropathy | 1,25(OH)2D3 | [165] |
| Diarylheptanes and lignans | [167] | |
| Maackiain | [168] | |
| punicalagin | [169] | |
| Diosgenin | [170] | |
| Complanatoside A | [171] | |
| Jujuboside A | [172] | |
| curcumin | [173] | |
| Resveratrol | [54,56] | |
| Diabetic Retinopathy | Folic acid | [174] |
| Malvidin | [175] | |
| Carotenoids | [34] | |
| Diabetic cardiomyopathy | Daidzein | [176] |
| Diabetic neuropathy | Honey (potential) | [177] |
6.1. Natural food components for diabetic kidney disease
Our previous study revealed a potential protective effect of 1,25(OH)2D3 in the prevention and treatment of DKD. As compared with the Zucker lean (ZL) control group, Zucker diabetic fatty (ZDF) rats showed a significant boost in renal MDA content, ROS production, 4-HNE and 8-OHdG protein expression, and a concomitant decrease in GSH content. In contrast, 1,25(OH)2D3 supplementation effectively reversed these variations, and the results further imply that 1,25(OH)2D3 may relieve the oxidative damage in diabetic kidney by inhibiting PARP1, activating SIRT1, and reducing Nox4 protein expression [165]. Similarly, another study found that in 3T3L1 adipocytes subjected to high glucose, supplementation with 1,25(OH)2D3 could significantly reduce Nox4 protein expression, ROS production, and phosphorylation levels of NF-κB and AMPK, hence obviously inhibiting cellular oxidative damage [166]. Moreover, 1,25(OH)2D3 boasts high oral bioavailability and well-proved safety in human studies and clinical trials. That is why we could leverage its antioxidant property in the prevention and treatment of DKD.
Natural food components such as diarylheptanes and lignans, acacia root extracts, and anthocyanin extracts have therapeutic effects on diabetes. Some scholars have compared the activity spectrum of Nox4, Nox2, and Nox5, and found several active chemicals from edible plants that specifically inhibit Nox4, especially diarylheptanes and lignans, whose efficacy is comparable to the best available medication [167]. Moreover, it has been found that Maackiain extracted from the roots of Sophora japonica could significantly reduce the protein expression of renal Nox4 as well as the gene and protein expressions of Bax, Caspase-9, and Caspase-3 in HFD/STZ-induced diabetic rats, thus reducing oxidative stress, inflammation and apoptosis in diabetic kidney [168].
Phytochemicals such as carotenoids [34], punicalagin, diosgenin, complanatoside A, Jujuboside A, Curcumin, and resveratrol have therapeutic effects on DKD. A study found that the major polyphenol (punicalagin) in pomegranate exhibited protective effects against DKD in a HFD/STZ-induced diabetic mice model. The results showed that punicalagin could decrease proteins expression of IL-1β, Caspase-1, GSDMD, and NLRP3 in diabetic mice kidney, and could effectively reduce high glucose-induced high expression of Nox4 protein [169]. This study suggests that administration of punicalagin can significantly relieve oxidative stress, inflammation, and pyroptosis in mice, exhibiting potential therapeutic implications for DKD. Latest studies have found that diosgenin isolated from vegetables and herbs could downregulate renal Nox4 protein expression level and serum inflammatory factors, enhance antioxidant enzyme (SOD, CAT) activity, and reduce the expression of Bax, CytC, Apaf-1, Caspase-9, p-PERK, and Caspase-12. Consequently, it could inhibit oxidative damage, inflammatory response, and apoptosis mediated by mitochondrial and endoplasmic reticulum stress, thereby preventing the development of DKD [170].
It has been found that Complanatoside A (CA) may also be used for the relief of DKD. As was reported, significant downregulation of Nox4 expression, inhibition of epithelial-mesenchymal transition, and reduction in renal tubular epithelial cell fibrosis were observed upon administration of CA in a mouse model of STZ-induced diabetes and in HK-2 cells induced by TGF-β1. Interestingly, overexpression of Nox4 in HK-2 cells abolished the effect of CA, and CA's inhibition of TGF-β1-induced renal fibrosis was even more pronounced once Nox4 was silenced [171].
Additionally, it was found that in diabetic SD rats, the administration of Jujuboside A could significantly reduce the expression of Nox4 and mitochondrial apoptosis proteins (Bax, CytC, Apaf-1, caspase 9), while enhancing SOD, CAT, and GPx activities, thereby inhibiting renal oxidative stress and apoptosis [172]. Research has also demonstrated that oral administration of curcumin in STZ diabetic rats significantly reversed renal dysfunction, including the recovery of creatinine clearance, BUN, and proteinuria indicators towards baseline levels, reduction of Nox4 and p67phox protein expression, and an increase in antioxidant enzyme activity [173]. This study confirms that curcumin is a potential therapy for the prevention and treatment of DKD.
Similarly, it has also been demonstrated that resveratrol can reduce elevated Nox4 expression in the kidney of db/db diabetic mice by activating AMPK, and thus dramatically alleviate diabetic renal fibrosis (i.e. FSP-1, Fibronectin, α-SMA) [62]. Resveratrol and its derivatives have been found to improve renal function in diabetic kidney disease by modulating inflammation and the metabolic memory of hyperglycemia. In non-obese diabetic mice, resveratrol intervention reduced Nox4 expression and led to a return to baseline levels of blood glucose, BUN, SCr, 24h-UMA, and inflammatory factors (RAGE, NF-кB) [54]. In another study, treatment of STZ-induced diabetic mice with polydatin, a resveratrol derivative, increased Cx32 expression and subsequently reduced Nox4 expression. Specifically, overexpression of Nox4 or knockdown of Cx32 abolished the inhibitory effects of polydatin on FN and ICAM-1 expression [56]. These studies suggest that polydatin may decrease renal oxidative damage and renal fibrosis via the Cx32-Nox4 signaling pathway.
6.2. Natural food components for diabetic retinopathy
According to a recent study, folic acid intervention in db/db diabetic mice significantly downregulated protein expression levels of Vav2, Nox4, VEGFR, IL-1β, and NLRP3 in retinal tissues, reduced retinal angiogenesis and oxidative stress, thus showed a protective effect against diabetic retinopathy [174]. It has also been found that blueberry anthocyanin extract (BAE) and its major components Malvidin (Mv), Malvidin-3-glucoside (Mv-3-glc), and Malvidin-3-galactoside (Mv-3-gal) can reduce cellular ROS production, boost cell viability and the activity of CAT and SOD. Mv, in particular, substantially inhibited high glucose-induced Nox4 overexpression in HRCECs, thus protecting it from oxidative damage and providing a new orientation for treating diabetic retinopathy [175].
6.3. Natural food components for diabetic cardiomyopathy
Studies have reported that Daidzein has ameliorative effects on diabetic cardiomyopathy. The latest research found that STZ-induced diabetic rats treated with Daidzein reduced protein expression of Nox4 and RAC-1, increased plasma levels of AMPK and SIRT1, and alleviated necrosis and fibrosis in cardiac tissues [176]. This study suggests that Daidzein treatment may attenuate oxidative stress, necrosis, and fibrotic damage in diabetic myocardial tissue through the inhibition of Nox4.
6.4. Natural food components for diabetic neuropathy
Honey coupled with insulin showed a significant effect on diabetic neuropathy. Three groups of diabetic neuropathic Wistar rats were treated with honey, insulin, and honey + insulin. Compared with the diabetic group, all the treatment groups showed reduced malondialdehyde levels and improved total antioxidant capacity. In particular, the honey + insulin treatment group drastically improved sensory nerve conduction velocity and mitigated dyslipidemia in diabetic rats. In addition, the honey + insulin treatment group showed a greater drop in fasting blood glucose compared with the insulin treatment group, which was comparable to that of the non-diabetic control group [177]. This study suggests that honey has hypoglycemic, hypolipidemic, and antioxidant effects, so it may serve as an adjunct to insulin to manage diabetic neuropathy.
7. Nox4 and diabetic macrovascular complications
7.1. Nox4 and diabetic atherosclerosis
The NADPH oxidase enzyme, Nox4, is the primary producer of ROS in the vascular system; however, its role in atherosclerosis remains a topic of debate. The precise impact of Nox4 on the development and progression of this condition remains uncertain.
Some studies suggest that downregulating Nox4 expression may have positive effects on atherosclerosis. In diabetes, advanced glycation end product-modified low-density lipoproteins (AGE-LDL) are prevalent and can promote the development of atherosclerosis by causing vascular cell dysfunction. Smooth muscle cells (SMCs), which play a key role in the early progression of atherosclerotic plaque, show increased ROS production and NADPH oxidase subunit gene expression (Nox1, Nox4, p22phox, p67phox), as well as increased cell proliferation when cultured under AGE-LDL conditions. This is partly due to the up-regulation of specific receptors such as LRP1, CD36, and RAGE, which enhance NADPH oxidase activity in human vascular smooth muscle cells (VSMCs), leading to a pro-oxidative state [178]. In conclusion, AGE-LDL can activate human VSMCs and up-regulate NADPH oxidase-induced pro-oxidative state through the CD36, LRP1, and RAGE receptors, accelerating the progression of diabetic atherosclerosis.
Neointimal hyperplasia, a pathological feature of atherosclerosis and restenosis, is associated with diabetes mellitus (DM). The combination therapy of linagliptin and metformin significantly inhibited neointimal hyperplasia and improved endothelium-independent contraction in the balloon-injured carotid artery of diabetic rats. Furthermore, high glucose-induced VSMCs remodeling was reversed by the combined use of linagliptin and metformin, which activated AMPK and inhibited Nox4 expression [179]. The study demonstrated the potential protective effect of the combination therapy on VSMC remodeling through the AMPK/Nox4 signal pathway, resulting in the improvement of neointima hyperplasia in diabetic rats. In addition, research indicated that treatment with manganese (Mn) in high glucose-treated human umbilical vein endothelial cells increased adiponectin level, inhibited ROS production and Nox4 expression, and downregulated ICAM-1 expression by upregulating endothelial cell Disulfide bond A-like protein (DsbA-L) [180]. These findings suggest that Mn supplementation may potentially improve diabetes-induced atherosclerosis by modulating the Nox4-dependent ROS production.
Abnormal VSMCs migration is an important pathological process in vascular occlusive diseases such as atherosclerosis and restenosis, which are accelerated by diabetes mellitus. In the obese Zucker rat (ZO), a model of obesity and insulin resistance, ZO aortic SMCs showed a significant increase in Nox4 mRNA and protein levels compared to the control ZL rats. Knockdown of Nox4 inhibited the oxidation of sarco-/endoplasmic reticulum Ca(2+) ATPase (SERCA) and neointima formation after ZO common carotid artery injury [181]. The study suggests that Nox4 plays a critical role in the pathogenesis of vascular occlusive diseases in T2DM, and targeting Nox4 may provide a potential therapeutic strategy for these diseases.
The study showed that Salvianolic acid B (Sal B) improves endothelial function in diabetic rats with blood glucose fluctuations. The mechanism of Sal B involves suppression of endothelial cell apoptosis and stimulation of endothelial nitric oxide synthase (eNOS) phosphorylation (Ser 1177), leading to restoration of NO metabolites and reduction of oxidative stress. Sal B also significantly reduced the protein levels of Nox2 and Nox4, two main isoforms of NADPH oxidase that are major sources of ROS in the vasculature. This reduction in Nox4 protein levels may contribute to the improvement of endothelial function in diabetic rats [182]. Overall, the study indicates that Sal B has a vascular protective effect in diabetes, and the regulation of Nox4 may be one of the underlying mechanisms.
The study investigated the role of ARL15 in high glucose-induced endothelial dysfunction in human umbilical vein endothelial cells (HUVECs). The results showed that up-regulation of ARL15 attenuates HG-induced impairment in HUVECs by increasing NO production and the active phosphorylation of the IR/IRS1/AKT/eNOS pathway, while decreasing ROS and MDA production and increasing SOD level. ARL15 overexpression also reduced the expression of Nox2 and Nox4, two major sources of ROS in the vasculature [183]. These findings suggest that ARL15 may have a protective effect against HG-induced endothelial impairment through the regulation of Nox4 and other pathways involved in oxidative stress. Besides, rosuvastatin treatment reversed impaired endothelium-dependent dilatations and prevented exaggerated contractions to angiotensin II and phenylephrine in renal arteries and aortae from db/db mice. Specifically, rosuvastatin reduced the elevated expression of Nox4, p22(phox), and p67(phox), which are components of NAD(P)H oxidase that produce ROS. Rosuvastatin also inhibited AT1R, Rac1, nitrotyrosine, and phosphorylation of ERK1/2 and p38 MAPK [184]. Overall, this study suggests that rosuvastatin has vasoprotective effects by inhibiting Nox4 and other components of the AT1R-NAD(P)H oxidase cascade.
High glucose induces proliferation and oxidative stress in aortic VSMCs of pigs, accompanied by reduced levels of antioxidant enzymes and increased levels of MDA and ROS. This is associated with increased PKCζ phosphorylation in the aorta of diabetic mice, leading to overexpression of Nox4 and ROS generation [185]. However, nicotinamide treatment has been shown to reverse these effects, indicating its ability to reduce oxidative stress and prevent diabetic atherosclerotic lesions by inhibiting Nox4 expression.
In HFD-induced diabetic metabolic stress (DMS) LDLR−/− mice, increased expression of iPLA2β was found to promote migratory responses, leading to accelerated atherogenesis. This was accompanied by increased Nox4 expression and activity, H2O2 production, and macrophage content in aortic sinus atherosclerotic lesions, which were positively correlated with iPLA2β-specific enzyme activity. In vitro experiments using isolated mouse peritoneal macrophages cultured with high glucose and LDL revealed that inhibition of iPLA2β prevented Nox4 upregulation, H2O2 production, and MCP-1-induced macrophage migration, while restoration of iPLA2β expression reversed these effects. Additionally, the use of Nox4 inhibitor GKT137831 or Nox4 silencing attenuated MCP-1-induced macrophage migration, while Nox4 overexpression restored migration response. In summary, DMS-induced upregulation of iPLA2β leads to increased Nox4 expression and H2O2 production, which enhances MCP-1-induced macrophage migration and accelerates atherosclerosis [186].
Ursolic acid (UA) prevents metabolic priming and hyper-reactivity to MCP-1 by inhibiting Nox4 expression and protecting against Nox4-dependent dysregulation of redox-sensitive processes, including actin turnover and MAPK-signaling. UA also blocks the metabolic stress-induced increase in global protein-S-glutathionylation and restores MKP1 protein expression and phosphatase activity [187]. These findings suggest that dysfunctional blood monocytes may be primary targets of UA and related compounds, providing a novel mechanism for the anti-inflammatory and athero- and renoprotective properties of UA.
In diabetes, the inflammatory response induced by high glucose is a major cause of endothelial dysfunction and an important contributor to the development of atherosclerosis. High glucose has been shown to increase the expression of adhesion molecules, such as ICAM-1 and VCAM-1, in HUVECs through activation of the NADPH oxidase/NF-κB pathway, leading to inflammation. However, high glucose-induced NF-κB binding activity and expression of ICAM-1 and VCAM-1 were inhibited by Nox4 siRNA or NADPH oxidase inhibitors [188]. This study suggests that NADPH oxidase/NF-κB pathway plays a role in high glucose-induced inflammation in endothelial cells.
In high glucose-induced human aortic endothelial cells (HAECs) and THP-1 monocytes, increased expression of Nox4 promotes excessive ROS generation, leading to increased expression of ICAM-1 and VCAM-1 in HAECs, which leads to increased monocyte-endothelial cell adhesion and migration, causing endothelial dysfunction and worsening of diabetic atherosclerotic lesions [189]. Overall, this study suggests that Nox4 plays a crucial role in promoting inflammation and endothelial dysfunction in diabetes.
Another study found that the expression of Nox4, HSF1, and PAI-1 were elevated in hearts of diabetic mice and (glyLDL)-induced mouse embryo fibroblasts (MEFs). However, abovementioned alterations were prevented by Diphenyleneiodonium (a nonspecific Nox inhibitor) or small interfering RNA for p22phox in MEFs [190]. It suggests that Nox4 may be a potential therapeutic target for cardiovascular disease in diabetic patients.
Other research suggest that upregulating Nox4 expression may have positive effects on atherosclerosis. SMCs proliferation and fibrosis are key factors in the development of advanced atherosclerotic lesions. Studies show that in STZ-induced ApoE−/− mice, genes related to SMCs function, such as SM-α-actin and calponin, were reduced, while platelet-derived growth factor (PDGF), osteopontin (OPN), and fibronectin were increased. In Nox4−/− ApoE−/− diabetic mice, genetic deletion of Nox4 further increased PDGF, OPN, collagen I, and the proliferation marker Ki67. Aortic SMCs isolated from Nox4-deficient mice showed reduced expression of contractile genes, increased ECM production, and elevated levels of ROS associated with Nox1. Further research revealed that the loss of Nox4 led to the activation of the PDGF signaling pathway, which resulted in a loss of calponin and increased fibronectin expression. Additionally, upregulation of Nox1 promoted OPN and Ki67 expression [191]. Therefore, Nox4 plays an anti-atherosclerotic role in diabetic models by inhibiting the PDGF signaling pathway and suppressing Nox1 activity.
Similarly, both decreased Nox4 gene expression and increased Nox1 gene expression were observed in the aorta of STZ-treated ApoE-deficient mice. Deletion of the Nox4 isoform led to increased atherosclerotic plaque area, reduced H2O2 production, and increased oxidative capacity and nitrotyrosine levels. This resulted in increased Nox2 staining in intraplaque foam cells and smooth muscle cells, as well as increased MCP-1 concentrations, macrophage accumulation, and proinflammatory factor expression within the aortic wall. These changes ultimately led to increased fibrillar collagen deposition and expression of the pro-fibrotic marker connective tissue growth factor (CTGF), exacerbating atherosclerotic lesions in mice. In contrast, deletion of the Nox1 isoform had the opposite effect as deletion of Nox4 [192]. Therefore, decreased Nox4 gene expression and increased Nox1 gene expression together exacerbated diabetic atherosclerotic lesions.
Atherosclerosis-prone conditions, such as disturbed blood flow, type I diabetes, and a Western diet, downregulate endothelial Nox4 mRNA in arteries. To investigate the role of endothelial Nox4 in atherosclerosis, mice were generated carrying a human Nox4 P437H dominant negative mutation (Nox4DN) on ApoE-deficient mice. Nox4DN led to increased type I diabetes-induced aortic stiffness and atherosclerotic lesions. Besides, significant upregulation of soluble epoxide hydrolase 2 (sEH) was observed in Nox4DN endothelial cells (ECs) and inhibition of sEH activity in Nox4DN EC suppressed inflammation and macrophage adhesion to EC. In contrast, overexpression of endothelial wild-type Nox4 suppressed sEH, ameliorated Western diet-induced atherosclerosis, and decreased aortic stiffness [193]. Therefore, these findings suggest that endothelial Nox4 plays a crucial role in atherosclerosis by regulating sEH expression and inflammation, and overexpression of wild-type Nox4 may have anti-atherosclerotic effects.
Insulin resistance in T2DM causes endothelial cells to produce more superoxide and less vasoprotective NO, leading to impaired glucose disposal and endothelial dysfunction. In mIGFREO mice (IGF-1R EC overexpressing) with specific insulin and IGF-1 resistance in endothelial cells, increased expression of Nox4 was observed due to reduced expression of miR-25, resulting in increased H2O2 production without an increase in superoxide. This increased H2O2 production mediated enhanced whole-body insulin sensitivity, as evidenced by increased glucose disposal and uptake into muscle and fat in response to insulin. Treatment with catalase restored insulin tolerance to wild-type levels, indicating that H2O2 release enhances insulin-mediated glucose reduction in a paracrine manner [194]. Therefore, combined insulin and IGF-1 resistance restricted to the endothelium leads to a potentially favorable adaptation with increased Nox4-derived H2O2 generation mediating enhanced whole-body insulin sensitivity.
Previous studies have demonstrated that high glucose or hyperglycemia can decrease the expression of GATA4 in HUVECs and in the endothelium of diabetic mice. Further investigation showed that GATA4 is a transcription factor of Nox4. Treatment with high glucose inhibited the activity of GATA4, leading to reduced Nox4 expression. In contrast, overexpression of GATA4 increased Nox4 expression. Simvastatin, a drug that preserves endothelial function, increased the expression of both GATA4 and Nox4 in HUVECs exposed to high glucose. This protective effect was reduced when GATA4 was inhibited with siRNA [195]. In summary, GATA4 regulates Nox4 transcription and protects against hyperglycemia-induced endothelial dysfunction, indicating a potential therapeutic target for preventing diabetic complications.
Evidence evaluated the effects of GKT137831, a Nox1/4 inhibitor, on atherosclerosis and end-organ damage in STZ-induced ApoE−/− mice. The results showed that the mice had increased plaque area, decreased Nox4 gene expression, and increased oxidative stress markers. Administration of GKT137831 at a lower dose [30 mg/(kg.day)] reduced plaque area, increased Nox4 gene expression and H2O2 levels, and decreased oxidative stress markers and MCP-1 protein expression. However, a higher dose [60 mg/(kg.day)] decreased Nox4 gene expression and nitrotyrosine accumulation, and up-regulated TNF-α, IL1-β and MIF protein levels. Overall, the effects of GKT137831 were dose-dependent and tissue-specific [55]. Further studies are needed to determine the relative balance of inhibiting Nox4 vs Nox1 in the macrovascular complications.
However, few studies have also shown no significant effect of Nox4 on diabetic atherosclerosis. In high glucose conditions, Nox1 and Nox4 gene expression and ROS production increase in HAECs, leading to enhanced expression of inflammatory markers (MCP-1 and VCAM-1) and fibrosis markers (CTGF). Nox1 knockdown reduces ROS production and decreases the expression of these markers, while Nox4 knockdown does not. Similarly, in STZ-induced ApoE−/− diabetic mice, Nox1 knockdown attenuates the development of aortic plaque formation and treatment with the specific Nox inhibitor GKT137831 prevents the formation of atherosclerotic plaques, but Nox4 knockdown does not affect plaque formation [196]. These findings suggest that Nox1 expression, but not Nox4, plays a crucial role in the development of diabetes-related atherosclerosis.
7.2. Nox4 and diabetic aortic disease
DM is associated with vascular diseases, which may result from NADPH oxidase-derived ROS and inflammation. In db/db mice, a T2DM model, increased Nox4 expression and NADPH oxidase activity were observed, leading to impaired vasodilation. The progression of diabetes from 4 to 12 weeks was associated with increased Nox4 mRNA, matrix remodeling-related cytokines, and superoxide levels [197]. These findings suggest that Nox4 is among the NADPH oxidase subunits involved in ROS production in the vasculature of db/db mice, contributing to DM-associated vascular diseases.
Elevated oxidative stress plays a crucial role in the development of atherosclerosis and endothelial dysfunction in diabetes-related vascular disease. A study found that STZ-induced type 1 diabetic ApoE−/− mice had significantly increased Nox4 and SOD expression, resulting in elevated oxidative stress in both the aorta and mesenteric arteries compared to non-diabetic ApoE−/− mice. The study also revealed that the expression levels of eNOS mRNA and protein were significantly increased in mesenteric arteries. In the advanced stage of diabetes, although Nox4 and SOD expression levels did not increase, eNOS expression levels continued to rise, leading to an increase in oxidative stress [198]. These findings suggest that the increase in oxidative stress in the vasculature of STZ-induced diabetic type 1 ApoE−/− mice is associated with changes in the expression of eNOS, SOD, and Nox4. The sustained increase in eNOS expression levels in the late stages of diabetes may promote hyperglycemia-induced oxidative stress.
Insulin-resistant and diabetic rats show impaired NO-dependent vasodilation. Berberine treatment in diabetic rats reduces glucose and triglyceride levels, increases eNOS mRNA and protein expression, and decreases Nox4 protein expression, resulting in increased NO bioavailability. This protects against aortic vasorelaxation in diabetic rats and restores diabetic endothelial dysfunction [199]. These findings suggest that the decrease in Nox4 expression may be a potential therapeutic target for diabetic vascular complications.
Research indicated that downregulating acid sphingomyelinase (ASM) in rat aortic endothelial cells (RAECs) treated with palmitic acid could improve insulin resistance in T2DM vascular endothelial cells. This downregulation enhanced glucose transporter-4 (Glut4) expression and glucose uptake, reduced ceramide production, and increased eNOS and NO production. It could also inhibit Nox2- and Nox4-dependent O2- production. Furthermore, the ASM selective inhibitor amitriptyline improved acetylcholine-induced vasodilation in rats fed on high-fat diet with STZ injection, while reducing ceramide, Nox2, and Nox4 protein expression levels in aortic endothelial cells. Overall, ASM downregulation appears to ameliorate insulin resistance and vascular dysfunction by suppressing Nox2 and Nox4 expression in RAECs [200].
A diabetic mice model was used to investigate the role of fibronectin containing extra domain A (EDA + FN) in diabetes-induced endothelial dysfunction. It was revealed that STZ-induced diabetes impaired endothelial vasodilation to acetylcholine, regardless of genotype. However, STZ + EDA−/− mice exhibited increased endothelial dysfunction compared with STZ + EDA+/+ and STZ + EDAwt/wt mice. Analysis of the underlying mechanisms showed that STZ + EDA−/− mice exhibited increased oxidative stress, exhibiting enhanced aortic superoxide anion, nitrotyrosine levels, and expression of Nox4 and TGF-β1. In contrast, Nox1 expression and antioxidant potential were similar in aortas from the three genotypes. Furthermore, STZ + EDA+/+ vessels exhibited decreased eNOS expression, this was offset by an increase in eNOS coupling and function [201]. Overall, these findings suggest that while EDA + FN participates in vascular remodeling, it also plays a crucial role in limiting diabetic endothelial dysfunction by preventing vascular oxidative stress and Nox4 expression.
Li et al. investigated the effects of sequoyitol on endothelial dysfunction in type 2 diabetic rats. T2DM was induced by low-dose STZ and a high-fat and high-sugar diet, which resulted in impaired glucose and insulin metabolism, increased insulin resistance, reduced insulin sensitivity index, diminished endothelium-dependent vasodilation, disrupted endothelial integrity, vascular smooth muscle cell hypertrophy and disorder, decreased antioxidant capacity and NO activity, increased lipid peroxidation, and altered eNOS and Nox4 expression in the aorta. sequoyitol treatment for six weeks ameliorated these abnormalities by lowering blood glucose, enhancing insulin sensitivity, improving endothelial function, increasing eNOS and NO levels, decreasing Nox4 expression and lipid peroxidation in the aorta [202]. These findings suggest that sequoyitol may protect the aortic endothelium in diabetic rats by modulating eNOS and Nox4 expression.
Research has explored the effects of protocatechuic acid (PCA), a naturally occurring phenolic acid found in many plants, on aortic oxidative status in type 2 diabetic rats. T2DM was induced by high-fat diet + high-fructose + low-dose STZ, which caused significant reductions in GSH and SOD levels, significant increases in MDA content, RAGE content, Nox4 gene expression, and serum AGEs levels, and significant decrease in eNOS gene expression in the aortic tissue of diabetic rats compared to the control group. Moreover, the aorta of diabetic rats showed mild inflammatory response, focal endothelial damage, and medial elastic lamina smooth muscle proliferation. PCA treatment significantly improved these changes, indicating its ameliorating effect on aortic oxidative status in T2DM rats by modulating the AGE-RAGE-Nox4 pathway [203].
Wang et al. investigated the effects of Oxymatrine (OMT) on diabetes-associated aortic endothelial dysfunction and its underlying mechanisms in diabetic rats. They found that the diabetic rats had reduced body weight, increased plasma glucose levels, and oxidative stress, as well as endothelial injury and dysfunction, including vasodilative and histologic changes. Additionally, the expressions of eNOS protein and mRNA were decreased, while the expression of Nox4 protein was increased in the aortas of the diabetic rats. However, treatment with OMT reversed all these diabetes-induced effects by enhancing NO bioavailability through upregulating eNOS expression and downregulating Nox4 expression [204]. These findings suggest that OMT may have therapeutic potential for treating diabetes-associated aortic endothelial dysfunction, whose mechanism may involve regulating the balance between eNOS and Nox4 expression.
Abdelmageed et al. investigated the potential effects of cinnamaldehyde (CIN) on the insulin signaling pathways in a rat model of T2DM induced by a high-fat diet, fructose, and STZ. The results showed that CIN treatment significantly improved glucose tolerance, insulin sensitivity, and insulin secretion, as well as lipid profile and liver function. CIN also decreased oxidative stress and inflammation, as evidenced by decreased malondialdehyde and increased glutathione and superoxide dismutase levels in both the liver and aorta. Furthermore, CIN treatment upregulated the insulin signaling pathway, as indicated by increased mRNA expression of insulin receptor substrate1, phosphatidylinositol 3-kinase regulatory subunit 1, and AKT serine/threonine kinase 2, increased levels of phosphorylated AKT ser473, and decreased expression of Nox4 in liver tissue. Additionally, CIN treatment decreased serum levels of advanced glycation end products and their receptors in the aorta [205]. These findings suggest that CIN may have potential antidiabetic and antioxidant effects, possibly through upregulating the eNOS and IRS1/PI3K/AKT2 signaling pathway and attenuating the elevation of AGEs, RAGE, and Nox4.
As we all know, the accumulation of AGEs and activation of RAGE lead to sustained oxidative stress in vascular tissue, contributing to diabetic vascular complications. While Nox4 generates ROS in the vasculature and plays a key role in the AGE/RAGE signaling pathway. In this study, glycine administration was found to attenuate oxidative stress by suppressing the AGE/RAGE pathway in the aorta of diabetic rats and HUVECs. This was associated with reduced Nox4 expression, which in turn reduced ROS generation. Additionally, glycine treatment restored the function of the crucial enzyme glyoxalase-1 (Glo1), which suppressed the AGE/RAGE pathway and reduced the formation of AGEs [206]. These findings suggest that glycine may protect against diabetic macrovascular complications by inhibiting the AGE/RAGE pathway and subsequent oxidative stress by improving Glo1 function and suppressing Nox4 expression.
In db/db diabetic mice, the aortas showed higher NADPH oxidase activity and increased superoxide levels, leading to impaired vasodilation. Diabetes progression was associated with increased Nox1, Nox4, and p22phox subunit mRNAs, as well as the induction of matrix-remodeling related cytokines, including CTGF, bone morphogenetic protein 4 (BMP-4), and OPN. Treatment with the superoxide scavenger Tempol reduced superoxide production, plasma glucose, and lipid levels, as well as BMP-4 and OPN protein expression in diabetic mice [197]. This suggests that DM-induced vascular inflammation involves both ROS-sensitive and -insensitive pathways, with Nox4 potentially associated due to its increased mRNA expression in diabetic mice.
Oxidative stress is a key contributor to diabetic vascular dysfunction. In an in vivo study conducted in db/db mice, aortic O(2)(−) production and Nox1, Nox2, and Nox4 mRNA levels were elevated, while Cu/Zn SOD protein and PPARγ mRNA levels were decreased. Treatment with the PPARγ agonist (rosiglitazone) for 1-week significantly reduced aortic O(2)(−) production and Nox1, 2 and 4 expression, but failed to increase Cu/Zn SOD or PPARγ in the aortic tissue of db/db mice [207]. These findings suggest that short-term treatment with rosiglitazone can rapidly inhibit the expression of vascular NADPH oxidase and O(2)(−) production, with Nox4 potentially playing a role, to protect the vasculature.
Research have investigated whether ellagic acid (EA) exerts a vasculo-protective effect under diabetic conditions by Nox4-mediated ROS production. The results demonstrated that EA treatment significantly reduced ROS production, ERK1/2 activation, and Nox4 expression in HAECs and intact rat aortas exposed to high glucose conditions. Furthermore, EA treatment improved endothelium-dependent vasodilation and decreased endothelial dysfunction [208]. These findings suggest that EA exerts vasculo-protective effect under diabetic conditions by inhibiting Nox4-mediated ROS production and ERK1/2 activation, highlighting its potential as a therapeutic target for diabetic vascular complications.
Research showed that Y396, a synthesized analog of rhynchophylline, could directly target the EGFR and inhibit its abnormal phosphorylation induced by high glucose and EGF. This led to the inhibition of downstream translocation to the nucleus of E2F1, its transcriptional activity, and expression of Nox4. Y396 treatment ameliorated diabetes-induced endothelial malfunction by inhibiting EGFR, which reduced downstream oxidative stress in the aortas of T1DM mice and primary RAECs. Y396 also preserved tunicamycin-induced endothelial dysfunction and oxidative stress. Moreover, Y396 protected against diabetes-induced endothelium malfunction in vivo through EGFR inhibition and downstream oxidative stress [209]. Overall, the study highlights the role of Nox4 in diabetes-induced endothelial dysfunction and suggests that Y396 may represent a potential therapeutic target for preventing or treating diabetic vascular complications by inhibiting EGFR-mediated oxidative stress and Nox4 expression.
Zhang et al. explored the protective effects of Astragaloside IV (AS-IV) on STZ-induced endothelial dysfunction and elucidate the underlying mechanisms. It was shown that STZ-injected mice exhibited impaired aortic endothelium-dependent vasorelaxation in response to acetylcholine and increased vasoconstriction, both of which were reversed following AS-IV treatment. The restoration of vascular relaxation was associated with enhanced eNOS phosphorylation levels and reduced ROS formation. Furthermore, AS-IV decreased the expression of various NADPH subunits, including Nox2, Nox4, and Rac-1. This suggests that diabetes-induced vascular injury may stem from eNOS inhibition or activation of Nox-derived ROS generation [210]. Consequently, AS-IV treatment could potentially alleviate oxidative stress in patients with diabetes exhibiting endothelial dysfunction.
Adiponectin (APN) and leptin are adipokines linked to vascular complications in diabetes mellitus. Scholars found that high glucose upregulated leptin expression in VSMCs and caused increased ROS production and upregulation of Nox4 expression. Nox4 mediated APN synthesis in VSMCs under high glucose condition. Furthermore, high glucose activated the RhoA/ROCK pathway and induced the polymerization of globular actin (G-actin) into filamentous actin (F-actin), decreasing the G/F-actin ratio [211]. Overall, these findings suggest that Nox4 plays a crucial role in hyperglycemia-induced oxidative stress and vascular remodeling in T2DM, highlighting its potential as a therapeutic target.
In diabetes, up-regulated Nox generates ROS that contributes to structural-functional alterations of the vascular wall. Histone deacetylase (HDAC)-dependent mechanisms may mediate vascular Nox overexpression in diabetic conditions. HDAC1 and HDAC2 proteins along with Nox1, Nox2, and Nox4 levels were found to be significantly elevated in the aortas of diabetic mice compared to non-diabetic animals. Treatment of diabetic mice with suberoylanilide hydroxamic acid (SAHA), a pan-HDAC inhibitor, mitigated the aortic expression of Nox1, Nox2, and Nox4 subtypes and NADPH-stimulated ROS production. In vitro studies on human aortic SMCs line showed that high concentrations of glucose increased HDAC1 and HDAC2 protein levels, leading to increased Nox1/4/5 expression, ROS production, and the formation of malondialdehyde-protein adducts. SAHA significantly reduced the above-mentioned conditions. Overexpression of HDAC2 up-regulated the Nox1/4/5 gene promoter activities in SMCs, and physical interactions of HDAC1/2 and p300 proteins with Nox1/4/5 promoters were detected at the sites of active transcription. High glucose-induced histone H3K27 acetylation enrichment at the promoters of Nox1/4/5 genes in SMCs. These findings suggest that HDACs mediate vascular Nox up-regulation in diabetes by regulating histone acetylation at Nox gene promoters. HDAC inhibition with SAHA reduces vascular ROS production in experimental diabetes, possibly by a mechanism involving negative regulation of Nox expression [212]. Overall, the study sheds light on the epigenetic regulation of Nox expression in diabetes and highlights the potential therapeutic value of HDAC inhibitors in the treatment of diabetic vascular complications.
Perivascular sympathetic neurons can secrete various trophic factors to regulate vascular function through paracrine signaling, including netrin-1, a diffusible protein that can be secreted outside the cell. Wang et al. investigated the role of netrin-1 in modulating sympathetic neuron paracrine signaling and its impact on vascular adventitial remodeling under hyperglycemic conditions. The findings suggest that netrin-1 deficiency in the supernatant from primary rat superior cervical ganglia (SCG) neurons contributes to adventitial fibroblast (AFs) remodeling, characterized by proliferation, migration, and collagen deposition. Moreover, UNC5b was identified as the netrin-1 specific receptor expressed in AFs. Treatment with netrin-1 inhibited H2O2 production derived from Nox4 through the UNC5b/CAMP/PKA signal pathway, resulting in the amelioration of AFs remodeling. In vivo, downregulation of netrin-1 in the perivascular sympathetic nerve was observed in the adventitia of the aorta in T2DM rats, accompanied by adventitial remodeling. Netrin-1 intervention restored the abnormalities and inhibited Nox4 expression in the aorta adventitia [213]. Overall, the study highlights the role of Nox4 in vascular adventitial remodeling under hyperglycemia and suggests that netrin-1 may represent a potential therapeutic target for preventing or reversing diabetic vascular complications by modulating sympathetic neuron paracrine signaling and Nox4-derived H2O2 production in AFs.
A study investigated the effects of cocoa on oxidative stress and vascular sclerosis and remodeling in diabetes. It compared male ZDF rats fed on standard AIN-93G diet and cocoa diet. It showed that ZDF rats on standard AIN-93G diet had increased aortic pulse pressure, intimal thickness, and elastic fiber damage, as well as elevated levels of Nox2, Nox4, ROS, pERKs, pJNKs, and SIRT1 in the aortic tissue. Cocoa intake ameliorated these alterations by preventing SIRT-1 depletion, downregulating Nox2 and Nox-4 expression, reducing ROS levels, and inhibiting ERKs and JNKs activation. The study also found that ZDF rats on standard AIN-93G diet had decreased nuclear levels of p-Nrf2, metallothionein (MT), and antioxidant enzyme activities such as GPx and glutathione reductase (GR) in the aorta. Cocoa diet restored these levels and enhanced the antioxidant defense system [214]. The study suggested that cocoa may protect the aortic tissue from oxidative stress and vascular damage in diabetes by modulating SIRT1, Nox2, Nox4, Nrf2, and their associated signaling pathways.
In diabetic rats, the mRNA expression of myocardial Nox4 was significantly increased, leading to increased oxidative stress in the heart. However, treatment with telmisartan was found to significantly alleviate the condition. At the same time, it may upregulate the expression of myocardial adiponectin receptor 2 and GLUT4, while downregulating the expression of Nox4, MCP-1, p22phox, and CTGF in the heart. Telmisartan was also found to induce a protective role on the vascular system by upregulating the expression of adipoR1 and downregulating the expression of MCP-1 and NF-κB in the abdominal aorta of diabetic rats [215]. Taken together, these findings suggest that telmisartan may protect against diabetic cardiovascular complications by regulating the expression of Nox4 and other key proteins involved in the pathogenesis of heart and vascular dysfunction in diabetes.
In a study aimed to investigate the protective effect of Salvia miltiorrhiza Bunge-Radix Puerariae (DG) on diabetic vascular injury, DG administration was found to reduce oxidative stress and improve diabetic vascular injury, as evidenced by decreased serum levels of H2O2 and MDA, and increased levels of SOD and CAT. DG also inhibited the expression of ICAM-1, VCAM-1, and Nox4 in the aorta of STZ- induced diabetic rats and endothelial cells [216]. All these suggest that DG has a potential therapeutic effect on diabetic vascular complications by reducing oxidative stress and inhibiting the expression of Nox4 and other adhesion molecules.
Lignophenols (LP) are derivatives of lignin, an abundant polymer in plants. This study investigated whether LP could attenuate vascular oxidative stress and inflammation in diabetic rats induced by STZ. After 5 weeks of treatment, the aorta of diabetic rats treated with LP showed reduced NAD(P)H oxidase subunit Nox4 expression, superoxide production, and inflammatory marker expression compared to untreated diabetic rats [217]. It suggests that Nox4 contributes to the vascular impairment associated with diabetes and that LP could be a potential therapeutic strategy for diabetic vascular complications by inhibiting Nox4.
In diabetes, endothelial inflammation and dysfunction play a crucial role in the development of vascular diseases. An in vitro and in vivo study found that compared to db/+ mice, db/db diabetic mice exhibited elevated vascular inflammation, increased binding of monocytes to the aorta, raised expression of serum chemokines (MCP-1/JE and KC), vascular inflammatory chemokines (MCP-1/JE and KC), and adhesion molecules’ (VCAM-1 and ICAM-1) mRNA, and reduced endothelium-dependent vasodilation. Interestingly, supplementation with blueberries in diabetic mice effectively reversed the vascular inflammatory response and significantly reduced the expression of Nox4 and IκKβ in the aortic vessels and glucose-palmitate-induced ECs [218]. This study suggests that blueberries may improve diabetes-induced endothelial inflammation injury through the Nox4 signaling pathway.
Recent study has found the vascular protection effects of Coreopsis tinctoria Nutt. flower (CTF) against diabetic endothelial dysfunction. It was proved that CTF's active fractions A-2-2 and A-2-3 protected over high glucose-induced HUVEC dysfunction by upregulating IRS-1, Akt, and eNOS expression levels while downregulating Nox4 expression. This led to decreased oxidative stress markers and inflammation. The protective effects were mediated through the JAK2/IRS1/PI3K/Akt/eNOS signaling pathway and were inhibited by intervention with AG490 and LY294002 inhibitors [219]. These findings suggest that CTF may have potential therapeutic benefits for managing vascular complications related to diabetes by targeting Nox4-mediated oxidative stress responses.
Hyperglycemia-induced endothelial dysfunction is a well-known complication of diabetes mellitus and atherosclerosis characterized by enhanced inflammation and endothelial-monocyte adhesion, where Nox4 is involved. A study showed that high glucose levels induced Nox4-dependent ROS generation, which, in turn, induced CIKS, an upstream regulator of NF-κB and AP-1 that plays a crucial role in inflammation and injury. CIKS is physically associated with IKKβ and JNK, and CIKS knockdown could inhibit high glucose-induced inflammatory signaling and endothelial dysfunction. Furthermore, similar to high glucose levels, AGE-HSA, AOPPsHSA, and oxLDL induced CIKS-dependent endothelial dysfunction. Aortas from diabetic mice showed enhanced DPI-inhibitable ROS generation and CIKS expression. Targeting Nox4 may be a potential therapeutic strategy for preventing or treating diabetic vascular complications by inhibiting high glucose-induced ROS generation and subsequent CIKS-mediated inflammation and endothelial dysfunction [189].
In a study investigating the role of Nox4 in atherosclerosis development in diabetic ApoE−/− mice, it showed that deletion of Nox4 attenuated plaque formation and reduced oxidative stress in the aortic sinus. In addition, Nox4 deletion prevented the diabetes-mediated increases in T-cell recruitment and aberrant activation of CD4+ T-cells in the draining lymph nodes. It may also mitigate the pro-inflammatory state in the aortic sinus by down-regulating the expression of pro-inflammatory cytokines and chemotactic molecules. However, Nox4 deletion did not affect systemic levels of RANTES [220]. These findings suggest that Nox4 plays a crucial role in the pathogenesis of atherosclerosis in diabetic mice by regulating oxidative stress, T-cell recruitment and activation, and local inflammation.
In T2DM rats, high glucose can induce the formation and activation of NLRP3 inflammasome, which is associated with endothelial barrier dysfunction. However, treatment with acarbose has been shown to inhibit the formation and activation of NLRP3 inflammasome, as well as the Nox4-dependent superoxide (O2·-) generation that regulates it. These effects of acarbose were observed in RAECs, where it enhanced the expression of junction proteins, such as ZO-1 and VE-Cadherin, and abolished vascular hyperpermeability associated with NLRP3 inflammasome inhibition. In vivo, acarbose intervention was found to relieve vascular leakage and improve vasodilatory response in the aortic endothelium of diabetic rats by restoring the expression of ZO-1, VE-Cadherin, Nox4, and NLRP3 inflammasome [221]. These findings suggest that acarbose may have a potential significance for cardiovascular protection in diabetic patients by inhibiting Nox4 oxidase-dependent O2·- production and subsequently inhibiting NLRP3 inflammasome formation, which leads to the amelioration of endothelial barrier dysfunction.
In a study using IGF-II/LDLR−/−ApoB100/100 mice that exhibited mild hyperglycemia and hyperinsulinemia with complex atherosclerotic lesions, treatment with NFAT blocker A-285222 for 4 weeks significantly increased the expression of the atheroprotective isoform Nox4 in the aorta of male mice, while changes in Nox2 were not significant. In vitro experiments showed that inhibition of NFAT effectively increased expression of Nox4 in VSMCs exposed to H2O2 and of catalase in VSMCs exposed to 3-Morpholinosydnonimine hydrochloride (SIN-1). Additionally, ROS/RNS levels were significantly lower in high glucose-induced VSMCs from NFATc3 knockout mice [222]. These findings suggest that inhibition of NFAT may upregulate Nox4 and catalase expression, leading to reduced oxidative stress and inflammation, and thus may be a promising approach for the treatment of diabetic macrovascular complications.
Zhao et al. found that saskatoon berry (SB) powder reduced monocyte adhesion to the aorta of diabetic db/db mice by inhibiting the expression of various inflammatory, stress, and fibrinolytic regulators, including Nox4, which is involved in the production of ROS – a factor in the pathogenesis of atherosclerosis. The expression of Nox4 was increased in the aorta and heart apex of db/db mice on chow, but treatment with 5% SB powder inhibited this increase [223]. Therefore, it suggests that SB powder may boast cardiovascular protective effects.
NADPH oxidase, a key transcription factor and primary source of ROS, plays a crucial role in regulating adhesion molecule expression and diabetic vascular complications. A study revealed that STZ-induced diabetic rats had significantly increased serum levels of PGE2, ET, H2O2, and NO, along with thickened aortic walls, increased outer membrane fibrosis, smooth muscle rupture, and enlarged nuclei. The aortic tissue showed elevated expression levels of ICAM-1, Lox-1, Nox2, Nox4, E-selectin, and NF-κB p65 genes and proteins, as well as a higher number of cells positively stained for ICAM-1, Nox2, Nox4, and NF-κB p65. Interestingly, these changes were effectively attenuated by puerarin treatment [224]. The study demonstrates that puerarin improves diabetic macrovascular complications and protects diabetic aortas by inhibiting the expression of Nox2, Nox4, and adhesion molecules.
Toll-like receptor 4 (TLR4) plays a crucial role in obesity and diabetes-related endothelial dysfunction by increasing oxidative stress. In the study, type 2 diabetic mice with mutated TLR4 showed protection from hyperglycemia and hypertension. The mechanism involves the downregulation of NADPH oxidase isoforms Nox1 and Nox4 in the arteries of these mice, leading to reduced oxidative stress and improved endothelial function [225]. This suggests that targeting TLR4/NADPH oxidase could be a potential therapeutic approach for managing obesity and diabetes-associated endothelial dysfunction.
Endothelial cell apoptosis is a critical factor in the development of vascular complications in T2DM. Argirein, a NADPH oxidase inhibitor, has been shown to improve cardiac function in T2DM. This study aimed to investigate whether argirein could prevent vascular dysfunction in T2DM by inhibiting endothelial cell apoptosis via Nox4. In vitro, argirein normalized high glucose-induced increases in BAX and Caspase-3 expression and decreased Bcl2 levels in RAECs. Argirein also suppressed annexin V-FITC binding and DNA fragmentation in RAECs, demonstrating its anti-apoptotic properties. Furthermore, argirein blocked ET-1/Nox4-dependent superoxide (O2-.) generation, which regulated endothelial cell apoptosis in RAECs. In vivo, argirein restored Nox4 and BAX expression in the aortic endothelium of high-fat diet-fed rats following streptozocin injection, indicating its potential as a therapeutic agent for preventing T2DM-related vascular complications [226]. In T2DM, it has been found that argirein could increase glucose uptake and Glut4 expression while reversing the phosphorylation of IRS-1-ser307 and AKT-ser473, leading to increased eNOS and NO production in insulin-resistant RAECs induced by palmitic acid (PA). Additionally, argirein blocked Nox4-dependent superoxide generation, which regulated glucose metabolism in RAECs during PA stimulation. In vivo, argirein increased the release of endothelial NO, relieved the vasodilatory response, and restored the expression of Nox4 and pIRS-1-ser307 in aorta endothelium of high-fat diet-fed rats injected with streptozocin [227]. These findings suggest that argirein may provide a novel approach for preventing vascular complications by improving endothelial insulin resistance through inhibiting Nox4-dependent redox signaling in RAECs.
A study reported that calcitonin gene-related peptide (CGRP) alleviated high-glucose-induced cell apoptosis, increased NO production, and enhanced eNOS mRNA expression while reversing the upregulated expression of iNOS and Ang II caused by high glucose. Moreover, CGRP attenuated intracellular ROS production through ERK1/2-Nox4 signaling pathway modulation and reduced translocation of p47phox [228]. These findings suggest that the protective effect of CGRP on RAECs against oxidative injury induced by high glucose may be attributed to its ability to inhibit ERK1/2-Nox4 activity.
Glucose-induced oxidative stress is mediated by the upregulation of Nox4 and its subunits, p47phox and Rac-1, which leads to increased ROS production and apoptosis. The translocation of p47phox to the cell membrane is an indicator of Nox4 activation, which can be inhibited by pretreatment with DPI or GLP-1. GLP-1 treatment suppressed Nox4 activation in HUVECs, leading to reduced oxidative stress and apoptosis. In STZ-induced diabetic rats, GLP-1 treatment attenuated high glucose-induced oxidative stress by inhibiting Nox4, p47phox, and Rac-1 expression and translocation of p47phox. This was accompanied by the amelioration of hyperglycemia and dyslipidemia [229]. These findings suggest that GLP-1 can be useful in the clinical management of diabetic vascular complications by suppressing Nox4-mediated oxidative stress.
A study investigated the role of SENP3 in high glucose-induced aortic endothelial dysfunction and vascular disease in diabetes. It found that high glucose upregulated SENP3 expression in HAECs and in the aorta of type 1 diabetic mice. High glucose also impaired HAECs viability, migration, and apoptosis, and increased the expression of ICAM-1 and VCAM-1, which enhanced the adhesion of THP-1 monocytes to HAECs. These effects were reversed by SENP3 knockdown, suggesting that SENP3 mediated high glucose-induced endothelial damage. The study also revealed that high glucose elevated ROS levels and Nox4 expression in HAECs, which were suppressed by SENP3 knockdown or by N-acetyl-l-cysteine (NAC), a free radical scavenger [230]. The study indicated that SENP3 contributed to high glucose-induced aortic endothelial dysfunction and vascular disease in diabetes via a ROS-dependent mechanism.
Research have investigated the mechanism by which Nox4 mediates sustained activation of the tyrosine kinase Src in response to IGF-I stimulation. The findings suggest that Nox4 recruitment to the plasma membrane scaffold protein SHPS-1 allows localized ROS generation, which mediates sustained Src activation through oxidation. Grb2, a component of the SHPS-1 signaling complex, plays a crucial role in Nox4 recruitment through phosphorylation of Nox4 Tyr-491 and binding to its SH2 domain. Disruption of Nox4 recruitment to SHPS-1 impairs downstream signaling and biological actions, including cell proliferation [231]. These results provide insights into the regulation of signal transduction by localized Nox4-derived ROS and the critical role of Grb2 in Nox4 recruitment and sustained Src activation.
The researchers measured the protein expression of nox1 and Nox4 in aortic vessels of STZ-induced T1DM rats and found a significant increase in nox1 expression but no change in Nox4 expression. The study also observed a decrease in endothelial NO production and sensitivity to acetylcholine and nitroglycerin in diabetic vessels. Taken together, the findings suggest that nox1 and other factors contribute to endothelial dysfunction in diabetes mellitus, with Nox4 playing a minor role [232]. Therefore, nox1 could be a promising therapeutic target for preventing or treating vascular complications in diabetes mellitus.
7.3. Nox4 and diabetic coronary artery disease
Due to the limited number of studies on Nox4 and diabetic coronary artery disease, the precise role of Nox4 in this context remains uncertain and warrants further researches.
Ezetimibe is a potent cholesterol absorption inhibitor. The results showed that ezetimibe treatment reduced vascular superoxide levels and attenuated NADPH oxidase subunit gp91(phox) and Nox4 expression, suggesting a decrease in Nox4-mediated oxidative stress. This attenuation of oxidative stress improved vascular endothelial function through the restoration of phospho-Akt and phospho-eNOS and led to the prevention of cardiac interstitial fibrosis, coronary arterial thickening, and macrophage infiltration [233]. The study provides important insights into the potential use of ezetimibe for the treatment of coronary artery disease associated with T2DM, with Nox4 as a potential target for future research.
Coronary microvascular disease (CMD) is associated with higher cardiac mortality in patients with diabetes, but the molecular mechanisms involved are poorly understood. The findings revealed that diabetic mice and endothelial-specific HuR-KO mice exhibited significant reductions in coronary flow velocity reserve (CFVR) and capillary density in the left ventricle. Analysis of mRNA levels in cardiac endothelial cells (CECs) showed that HuR, Cx40, and Nox4 were decreased in diabetic and HuR-KO mice compared to control mice. Notably, Nox4 protein levels were not altered in CECs of diabetic mice compared with control mice. Although the study suggests that decreased HuR contributes to the development of CMD in diabetes through downregulation of gap junction protein Cx40 in CECs, the role of Nox4 in this pathogenesis is not clear [234]. These findings may have important implications for the development of therapeutic strategies, with further studies needed to determine the specific role of Nox4 in the pathogenesis of CMD in diabetic mice.
7.4. Nox4 and diabetic peripheral vascular disease
Peripheral arterial disease (PAD) is an occlusive arterial disease primarily caused by atherosclerosis that affects the lower extremities. Study has demonstrated elevated Nox4 expression in diabetic patients with high inflammation and identified Nox4 as a critical mediator in the development and complications of lower limb ischemia, especially in diabetic patients. Plasma TNF-α showed a positive correlation with Nox4 expression, particularly in diabetic patients. The study also suggests that the neutrophil-to-lymphocyte ratio (NLR) and TNF-α are suitable markers of mortality in CLTI [235]. These findings shed light on the molecular mechanisms underlying CLTI and highlight Nox4 as a potential therapeutic target for the treatment of PAD. Further research on Nox4 is needed to develop effective treatments for patients with advanced PAD.
A preclinical study aimed to investigate the role of Nox in impaired vascular compensation to arterial occlusion in a diet-induced obese (DIO) mouse model. The study found that increased expression of Nox2, Nox4, p22(phox), and p47(phox) was observed in DIO mice, which was associated with impaired collateral growth and hindlimb perfusion. Treatment with Nox2 or p47(phox) inhibitors or ablation of Nox2 or p47(phox) improved collateral growth and hindlimb perfusion in DIO mice, highlighting the potential of selective Nox component suppression or inhibition as an effective therapy for claudicants. Elevated expression of Nox4 in DIO mice suggests that it may also contribute to the impairment of vascular compensation to arterial occlusion and requires further research [236]. Overall, this study sheds light on the mechanisms underlying impaired vascular compensation in obese mice and provides insights into potential therapeutic strategies for treating claudicants, with NADPH oxidase as a potential target for future research.
7.5. Nox4 and diabetic cerebrovascular disease
Limited research found that liraglutide attenuated the accumulation Nox2 and Nox4 in the cerebrum and cerebellum of STZ-induced diabetic rats. It is suggested that liraglutide may ameliorate DM-induced microvascular oxidative stress, glycation, and inflammation, which contribute to impaired microcirculatory regulation, including vascular dilation and barrier function in the brain in vivo [237].
8. Conclusions
In summary, this review highlights recent progress in the biology of Nox4 and its upstream and downstream regulatory mechanisms in DKD, as well as a variety of other diabetic microangiopathies associated with oxidative stress. Both in vivo and in vitro experimental evidence has emphasized the significance of Nox4 in the pathogenesis of diabetic microangiopathy. Interestingly, this review also summarizes the mechanisms of Nox4 in regulating microvascular complications of diabetes from new perspectives such as epigenetics. This reveals that Nox4 may serve as a therapeutic target for diabetic microvascular complications.
Consequently, developing safe and efficient Nox4 inhibitors or drugs or finding natural nutrients that inhibit Nox4 may prove to be important therapeutic measures in the prevention and treatment of diabetic microangiopathy. The mechanisms of action of existing drugs or inhibitors targeting Nox4 for the treatment of diabetic microangiopathy has been investigated. Furthermore, recent studies have shown that many herbal and natural food components also have hypoglycemic and anti-diabetic microangiopathy effects, which has increased the interest of scientists in the search for safe and effective intervention measures to prevent diabetes and its complications. Of course, this review has its shortcomings. For example, it is only focused on Nox4, but other Nox isoforms have not been summarized.
Given that Nox4 is mainly expressed in the kidney, research on Nox4 and DKD is the most abundant in terms of both mechanisms and treatment measures, which may be the key difference from other Nox family proteins. Among them, it is noteworthy that there is currently no available research exploring the relationship between Nox4 inhibitors and diabetic neuropathy. The epigenetic studies mentioned in this review mainly focus on microRNA, with minimal research on other epigenetic factors such as DNA methylation and histone modification. And there are few studies exploring the multi-omics aspects of Nox4 and diabetic microvascular complications. All of these areas may be a research direction that needs to be strengthened in the future. Furthermore, the available evidence investigating drugs, inhibitors, or natural food components targeting Nox4 has mainly focused on animal and cellular pre-clinical models of diabetic microvascular complications. However, the efficacy and safety of therapeutic agents that inhibit Nox4 require more clinical trials to be conducted for clarification. Ultimately, it is important to note that the role of Nox4 in diabetic macroangiopathy is complex and not yet fully understood. While most studies indicate that Nox4 plays a harmful role in diabetic macroangiopathy, there is also some research suggesting that Nox4 may have a protective effect in diabetic atherosclerosis.
Funding
This study was financially supported by Hubei Key Laboratory of Food Nutrition and Safety (Huazhong University of Science and Technology, Grant number FNS-HBKL2021A04), Hubei Key Laboratory of Kidney Disease Pathogenesis and Intervention (Grant number SB202107), and Science and Technology Project of Hebei Education Department (Grant number QN2022016).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Our gratitude gose to Xiaohao Liu from Beijing Normal University for assisting us in refining the English language.
Contributor Information
Gang Liu, Email: cardio2004@hebmu.edu.cn.
Liping Hao, Email: haolp@mails.tjmu.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Eid A.A., Gorin Y., Fagg B.M., et al. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes. 2009;58(5):1201–1211. doi: 10.2337/db08-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thum T., Fraccarollo D., Schultheiss M., et al. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56(3):666–674. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 3.Shen G.X. Oxidative stress and diabetic cardiovascular disorders: roles of mitochondria and NADPH oxidase. Can. J. Physiol. Pharmacol. 2010;88(3):241–248. doi: 10.1139/Y10-018. [DOI] [PubMed] [Google Scholar]
- 4.Lee S.R., An E.J., Kim J., et al. Function of NADPH oxidases in diabetic nephropathy and development of nox inhibitors. Biomol Ther (Seoul) 2020;28(1):25–33. doi: 10.4062/biomolther.2019.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorin Y., Block K., Hernandez J., et al. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J. Biol. Chem. 2005;280(47):39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 6.Lassegue B., San Martin A., Griendling K.K. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res. 2012;110(10):1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawahara T., Ritsick D., Cheng G., et al. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J. Biol. Chem. 2005;280(36):31859–31869. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- 8.Martyn K.D., Frederick L.M., Von Loehneysen K., et al. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell. Signal. 2006;18(1):69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 10.Banfi B., Tirone F., Durussel I., et al. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5) J. Biol. Chem. 2004;279(18):18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 11.Banfi B., Clark R.A., Steger K., et al. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 2003;278(6):3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 12.Banfi B., Malgrange B., Knisz J., et al. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 2004;279(44):46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 13.Lyle A.N., Deshpande N.N., Taniyama Y., et al. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ. Res. 2009;105(3):249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz B., Shani G., Pass I., et al. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci. Signal. 2009;2(88):ra53. doi: 10.1126/scisignal.2000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clempus R.E., Sorescu D., Dikalova A.E., et al. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler. Thromb. Vasc. Biol. 2007;27(1):42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ago T., Kuroda J., Pain J., et al. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2010;106(7):1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banfi B., Molnar G., Maturana A., et al. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 2001;276(40):37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 18.De Deken X., Wang D., Many M.C., et al. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J. Biol. Chem. 2000;275(30):23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 19.Geiszt M., Kopp J.B., Várnai P., et al. Identification of renox, an NAD(P)H oxidase in kidney. Proc. Natl. Acad. Sci. U. S. A. 2000;97(14):8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cave A.C., Brewer A.C., Narayanapanicker A., et al. NADPH oxidases in cardiovascular health and disease. Antioxidants Redox Signal. 2006;8(5–6):691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 21.Vallet P., Charnay Y., Steger K., et al. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132(2):233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 22.Yang S., Zhang Y., Ries W., et al. Expression of Nox4 in osteoclasts. J. Cell. Biochem. 2004;92(2):238–248. doi: 10.1002/jcb.20048. [DOI] [PubMed] [Google Scholar]
- 23.Sedeek M., Callera G., Montezano A., et al. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2010;299(6):F1348–F1358. doi: 10.1152/ajprenal.00028.2010. [DOI] [PubMed] [Google Scholar]
- 24.Meng W., Shah K.P., Pollack S., et al. A genome-wide association study suggests new evidence for an association of the NADPH Oxidase 4 (NOX4) gene with severe diabetic retinopathy in type 2 diabetes. Acta Ophthalmol. 2018;96(7):e811–e819. doi: 10.1111/aos.13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babelova A., Avaniadi D., Jung O., et al. Role of Nox4 in murine models of kidney disease. Free Radic. Biol. Med. 2012;53(4):842–853. doi: 10.1016/j.freeradbiomed.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 26.Bondi C.D., Manickam N., Lee D.Y., et al. NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J. Am. Soc. Nephrol. 2010;21(1):93–102. doi: 10.1681/ASN.2009020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan F., Wang Y., Wu X., et al. Nox4 and redox signaling mediate TGF-beta-induced endothelial cell apoptosis and phenotypic switch. Cell Death Dis. 2014;5(1) doi: 10.1038/cddis.2013.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Block K., Eid A., Griendling K.K., et al. Nox4 NAD(P)H oxidase mediates Src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II: role in mesangial cell hypertrophy and fibronectin expression. J. Biol. Chem. 2008;283(35):24061–24076. doi: 10.1074/jbc.M803964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang N., Gonzalez-Vicente A., Garvin J.L. Angiotensin II-induced superoxide and decreased glutathione in proximal tubules: effect of dietary fructose. Am. J. Physiol. Ren. Physiol. 2020;318(1):F183–F192. doi: 10.1152/ajprenal.00462.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee C.F., Qiao M., Schroder K., et al. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circ. Res. 2010;106(9):1489–1497. doi: 10.1161/CIRCRESAHA.109.215392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma S., Bai Z., Wu H., et al. The DPP-4 inhibitor saxagliptin ameliorates ox-LDL-induced endothelial dysfunction by regulating AP-1 and NF-kappaB. Eur. J. Pharmacol. 2019;851:186–193. doi: 10.1016/j.ejphar.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 32.New D.D., Block K., Bhandhari B., et al. IGF-I increases the expression of fibronectin by Nox4-dependent Akt phosphorylation in renal tubular epithelial cells. Am. J. Physiol. Cell Physiol. 2012;302(1):C122–C130. doi: 10.1152/ajpcell.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng D., Lv D.D., Fang J. Insulin-like growth factor-I induces reactive oxygen species production and cell migration through Nox4 and Rac1 in vascular smooth muscle cells. Cardiovasc. Res. 2008;80(2):299–308. doi: 10.1093/cvr/cvn173. [DOI] [PubMed] [Google Scholar]
- 34.Keegan G., Pardhan S., Chichger H. Lutein and zeaxanthin attenuates VEGF-induced neovascularisation in human retinal microvascular endothelial cells through a Nox4-dependent pathway. Exp. Eye Res. 2020;197 doi: 10.1016/j.exer.2020.108104. [DOI] [PubMed] [Google Scholar]
- 35.Calabriso N., Massaro M., Scoditti E., et al. Extra virgin olive oil rich in polyphenols modulates VEGF-induced angiogenic responses by preventing NADPH oxidase activity and expression. J. Nutr. Biochem. 2016;28:19–29. doi: 10.1016/j.jnutbio.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 36.Wilkinson-Berka J.L., Tan G., Jaworski K., et al. Identification of a retinal aldosterone system and the protective effects of mineralocorticoid receptor antagonism on retinal vascular pathology. Circ. Res. 2009;104(1):124–133. doi: 10.1161/CIRCRESAHA.108.176008. [DOI] [PubMed] [Google Scholar]
- 37.Rana I., Suphapimol V., Jerome J.R., et al. Angiotensin II and aldosterone activate retinal microglia. Exp. Eye Res. 2020;191 doi: 10.1016/j.exer.2019.107902. [DOI] [PubMed] [Google Scholar]
- 38.Basuroy S., Bhattacharya S., Leffler C.W., et al. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. Am. J. Physiol. Cell Physiol. 2009;296(3):C422–C432. doi: 10.1152/ajpcell.00381.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 40.Leonarduzzi G., Sottero B., Testa G., et al. New insights into redox-modulated cell signaling. Curr. Pharmaceut. Des. 2011;17(36):3994–4006. doi: 10.2174/138161211798764906. [DOI] [PubMed] [Google Scholar]
- 41.Parving H.H., Hovind P. Microalbuminuria in type 1 and type 2 diabetes mellitus: evidence with angiotensin converting enzyme inhibitors and angiotensin II receptor blockers for treating early and preventing clinical nephropathy. Curr. Hypertens. Rep. 2002;4(5):387–393. doi: 10.1007/s11906-002-0069-3. [DOI] [PubMed] [Google Scholar]
- 42.Kanwar Y.S., Wada J., Sun L., et al. Diabetic nephropathy: mechanisms of renal disease progression. Exp. Biol. Med. 2008;233(1):4–11. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 43.Holterman C.E., Read N.C., Kennedy C.R. Nox and renal disease. Clin. Sci. (Lond.) 2015;128(8):465–481. doi: 10.1042/CS20140361. [DOI] [PubMed] [Google Scholar]
- 44.Etoh T., Inoguchi T., Kakimoto M., et al. Increased expression of NAD(P)H oxidase subunits, NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibity by interventive insulin treatment. Diabetologia. 2003;46(10):1428–1437. doi: 10.1007/s00125-003-1205-6. [DOI] [PubMed] [Google Scholar]
- 45.Jha J.C., Gray S.P., Barit D., et al. Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J. Am. Soc. Nephrol. 2014;25(6):1237–1254. doi: 10.1681/ASN.2013070810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gojo A., Utsunomiya K., Taniguchi K., et al. The Rho-kinase inhibitor, fasudil, attenuates diabetic nephropathy in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2007;568(1–3):242–247. doi: 10.1016/j.ejphar.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Yin H., Ru H., Yu L., et al. Targeting of Rho kinase ameliorates impairment of diabetic endothelial function in intrarenal artery. Int. J. Mol. Sci. 2013;14(10):20282–20298. doi: 10.3390/ijms141020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohshiro Y., Ma R.C., Yasuda Y., et al. Reduction of diabetes-induced oxidative stress, fibrotic cytokine expression, and renal dysfunction in protein kinase Cbeta-null mice. Diabetes. 2006;55(11):3112–3120. doi: 10.2337/db06-0895. [DOI] [PubMed] [Google Scholar]
- 49.Hu F., Xue M., Li Y., et al. Early growth response 1 (Egr1) is a transcriptional activator of NOX4 in oxidative stress of diabetic kidney disease. J. Diabetes Res. 2018;2018 doi: 10.1155/2018/3405695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Z., Sun X., Chen Q., et al. Connexin32 ameliorates renal fibrosis in diabetic mice by promoting K48-linked NADPH oxidase 4 polyubiquitination and degradation. Br. J. Pharmacol. 2020;177(1):145–160. doi: 10.1111/bph.14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nayak B.K., Shanmugasundaram K., Friedrichs W.E., et al. HIF-1 mediates renal fibrosis in OVE26 type 1 diabetic mice. Diabetes. 2016;65(5):1387–1397. doi: 10.2337/db15-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen N., Song S., Yang Z., et al. ChREBP deficiency alleviates apoptosis by inhibiting TXNIP/oxidative stress in diabetic nephropathy. J. Diabet. Complicat. 2021;35(12) doi: 10.1016/j.jdiacomp.2021.108050. [DOI] [PubMed] [Google Scholar]
- 53.Ford B.M., Eid A.A., Gooz M., et al. ADAM17 mediates Nox4 expression and NADPH oxidase activity in the kidney cortex of OVE26 mice. Am. J. Physiol. Ren. Physiol. 2013;305(3):F323–F332. doi: 10.1152/ajprenal.00522.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xian Y., Gao Y., Lv W., et al. Resveratrol prevents diabetic nephropathy by reducing chronic inflammation and improving the blood glucose memory effect in non-obese diabetic mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020;393(10):2009–2017. doi: 10.1007/s00210-019-01777-1. [DOI] [PubMed] [Google Scholar]
- 55.Gray S.P., Jha J.C., Kennedy K., et al. Combined NOX1/4 inhibition with GKT137831 in mice provides dose-dependent reno- and atheroprotection even in established micro- and macrovascular disease. Diabetologia. 2017;60(5):927–937. doi: 10.1007/s00125-017-4215-5. [DOI] [PubMed] [Google Scholar]
- 56.Chen Z.Q., Sun X.H., Li X.J., et al. Polydatin attenuates renal fibrosis in diabetic mice through regulating the Cx32-Nox4 signaling pathway. Acta Pharmacol. Sin. 2020;41(12):1587–1596. doi: 10.1038/s41401-020-0475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Block K., Gorin Y., Abboud H.E. Subcellular localization of Nox4 and regulation in diabetes. Proc. Natl. Acad. Sci. U. S. A. 2009;106(34):14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thallas-Bonke V., Jha J.C., Gray S.P., et al. Nox-4 deletion reduces oxidative stress and injury by PKC-alpha-associated mechanisms in diabetic nephropathy. Phys. Rep. 2014;2(11) doi: 10.14814/phy2.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi Q., Lee D.Y., Feliers D., et al. Interplay between RNA-binding protein HuR and Nox4 as a novel therapeutic target in diabetic kidney disease. Mol. Metabol. 2020;36 doi: 10.1016/j.molmet.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shah A., Xia L., Goldberg H., et al. Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species generation by mitochondria and the NADPH oxidase, Nox4, in mesangial cells. J. Biol. Chem. 2013;288(10):6835–6848. doi: 10.1074/jbc.M112.419101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiang H., Xue W., Wu X., et al. FOXP1 inhibits high glucose-induced ECM accumulation and oxidative stress in mesangial cells. Chem. Biol. Interact. 2019;313 doi: 10.1016/j.cbi.2019.108818. [DOI] [PubMed] [Google Scholar]
- 62.He T., Xiong J., Nie L., et al. Resveratrol inhibits renal interstitial fibrosis in diabetic nephropathy by regulating AMPK/NOX4/ROS pathway. J. Mol. Med. (Berl.) 2016;94(12):1359–1371. doi: 10.1007/s00109-016-1451-y. [DOI] [PubMed] [Google Scholar]
- 63.Graham S., Gorin Y., Abboud H.E., et al. Abundance of TRPC6 protein in glomerular mesangial cells is decreased by ROS and PKC in diabetes. Am. J. Physiol. Cell Physiol. 2011;301(2):C304–C315. doi: 10.1152/ajpcell.00014.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanwar Y.S., Sun L., Xie P., et al. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu. Rev. Pathol. 2011;6:395–423. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jha J.C., Thallas-Bonke V., Banal C., et al. Podocyte-specific Nox4 deletion affords renoprotection in a mouse model of diabetic nephropathy. Diabetologia. 2016;59(2):379–389. doi: 10.1007/s00125-015-3796-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo W., Gao H., Pan W., et al. High glucose induces Nox4 expression and podocyte apoptosis through the Smad3/ezrin/PKA pathway. Biol Open. 2020 doi: 10.1242/bio.055012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Das R., Xu S., Quan X., et al. Upregulation of mitochondrial Nox4 mediates TGF-beta-induced apoptosis in cultured mouse podocytes. Am. J. Physiol. Ren. Physiol. 2014;306(2):F155–F167. doi: 10.1152/ajprenal.00438.2013. [DOI] [PubMed] [Google Scholar]
- 68.Rogacka D., Audzeyenka I., Piwkowska A. Regulation of podocytes function by AMP-activated protein kinase. Arch. Biochem. Biophys. 2020;692 doi: 10.1016/j.abb.2020.108541. [DOI] [PubMed] [Google Scholar]
- 69.Eid A.A., Ford B.M., Block K., et al. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J. Biol. Chem. 2010;285(48):37503–37512. doi: 10.1074/jbc.M110.136796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eid A.A., Ford B.M., Bhandary B., et al. Mammalian target of rapamycin regulates Nox4-mediated podocyte depletion in diabetic renal injury. Diabetes. 2013;62(8):2935–2947. doi: 10.2337/db12-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eid S., Boutary S., Braych K., et al. mTORC2 signaling regulates nox4-induced podocyte depletion in diabetes. Antioxidants Redox Signal. 2016;25(13):703–719. doi: 10.1089/ars.2015.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piwkowska A., Rogacka D., Audzeyenka I., et al. High glucose increases glomerular filtration barrier permeability by activating protein kinase G type Ialpha subunits in a Nox4-dependent manner. Exp. Cell Res. 2014;320(1):144–152. doi: 10.1016/j.yexcr.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 73.Ilatovskaya D.V., Blass G., Palygin O., et al. A NOX4/TRPC6 pathway in podocyte calcium regulation and renal damage in diabetic kidney disease. J. Am. Soc. Nephrol. 2018;29(7):1917–1927. doi: 10.1681/ASN.2018030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li M., Zhang T., Wu X., et al. High glucose provokes microvesicles generation from glomerular podocytes via NOX4/ROS pathway. Biosci. Rep. 2019;39(11) doi: 10.1042/BSR20192554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fujii M., Inoguchi T., Maeda Y., et al. Pitavastatin ameliorates albuminuria and renal mesangial expansion by downregulating NOX4 in db/db mice. Kidney Int. 2007;72(4):473–480. doi: 10.1038/sj.ki.5002366. [DOI] [PubMed] [Google Scholar]
- 76.Bruder-Nascimento T., Callera G., Montezano A.C., et al. Renoprotective effects of atorvastatin in diabetic mice: downregulation of RhoA and upregulation of Akt/GSK3. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0162731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zahr R.S., Chappa P., Yin H., et al. Renal protection by atorvastatin in a murine model of sickle cell nephropathy. Br. J. Haematol. 2018;181(1):111–121. doi: 10.1111/bjh.15157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee J.H., Kim J.H., Kim J.S., et al. AMP-activated protein kinase inhibits TGF-beta-, angiotensin II-, aldosterone-, high glucose-, and albumin-induced epithelial-mesenchymal transition. Am. J. Physiol. Ren. Physiol. 2013;304(6):F686–F697. doi: 10.1152/ajprenal.00148.2012. [DOI] [PubMed] [Google Scholar]
- 79.Song P., Chen Y., Liu Z., et al. LncRNA MALAT1 aggravates renal tubular injury via activating LIN28A and the Nox4/AMPK/mTOR signaling Axis in diabetic nephropathy. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.895360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lewis E.J., Hunsicker L.G., Bain R.P., et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 1993;329(20):1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 81.Brenner B.M., Cooper M.E., De Zeeuw D., et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 82.Onozato M.L., Tojo A., Goto A., et al. Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEI and ARB. Kidney Int. 2002;61(1):186–194. doi: 10.1046/j.1523-1755.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 83.Tojo A., Asaba K., Onozato M.L. Suppressing renal NADPH oxidase to treat diabetic nephropathy. Expert Opin. Ther. Targets. 2007;11(8):1011–1018. doi: 10.1517/14728222.11.8.1011. [DOI] [PubMed] [Google Scholar]
- 84.Durham J.T., Herman I.M. Microvascular modifications in diabetic retinopathy. Curr. Diabetes Rep. 2011;11(4):253–264. doi: 10.1007/s11892-011-0204-0. [DOI] [PubMed] [Google Scholar]
- 85.Li J., Wang J.J., Yu Q., et al. Inhibition of reactive oxygen species by Lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: role of NADPH oxidase 4. Diabetes. 2010;59(6):1528–1538. doi: 10.2337/db09-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ahmad A., Nawaz M.I., Siddiquei M.M., et al. Apocynin ameliorates NADPH oxidase 4 (NOX4) induced oxidative damage in the hypoxic human retinal Muller cells and diabetic rat retina. Mol. Cell. Biochem. 2021;476(5):2099–2109. doi: 10.1007/s11010-021-04071-y. [DOI] [PubMed] [Google Scholar]
- 87.Jiao W., Ji J., Li F., et al. Activation of the NotchNox4reactive oxygen species signaling pathway induces cell death in high glucosetreated human retinal endothelial cells. Mol. Med. Rep. 2019;19(1):667–677. doi: 10.3892/mmr.2018.9637. [DOI] [PubMed] [Google Scholar]
- 88.Mustapha N.M., Tarr J.M., Kohner E.M., et al. NADPH oxidase versus mitochondria-derived ROS in glucose-induced apoptosis of pericytes in early diabetic retinopathy. J Ophthalmol. 2010;2010 doi: 10.1155/2010/746978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sedeek M., Montezano A.C., Hebert R.L., et al. Oxidative stress, Nox isoforms and complications of diabetes--potential targets for novel therapies. J Cardiovasc Transl Res. 2012;5(4):509–518. doi: 10.1007/s12265-012-9387-2. [DOI] [PubMed] [Google Scholar]
- 90.Li J., Wang J.J., Zhang S.X. NADPH oxidase 4-derived H2O2 promotes aberrant retinal neovascularization via activation of VEGF receptor 2 pathway in oxygen-induced retinopathy. J. Diabetes Res. 2015;2015 doi: 10.1155/2015/963289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meng D., Mei A., Liu J., et al. NADPH oxidase 4 mediates insulin-stimulated HIF-1alpha and VEGF expression, and angiogenesis in vitro. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0048393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shalaby L., Thounaojam M., Tawfik A., et al. Role of endothelial ADAM17 in early vascular changes associated with diabetic retinopathy. J. Clin. Med. 2020;9(2):400. doi: 10.3390/jcm9020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li H., Li T., Wang H., et al. Diabetes promotes retinal vascular endothelial cell injury by inducing CCN1 expression. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.689318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yao M., Gao F., Wang X., et al. Nox4 is involved in high glucose-induced apoptosis in renal tubular epithelial cells via Notch pathway. Mol. Med. Rep. 2017;15(6):4319–4325. doi: 10.3892/mmr.2017.6516. [DOI] [PubMed] [Google Scholar]
- 95.Ding X., Zhang M., Gu R., et al. Activated microglia induce the production of reactive oxygen species and promote apoptosis of co-cultured retinal microvascular pericytes. Graefes Arch. Clin. Exp. Ophthalmol. 2017;255(4):777–788. doi: 10.1007/s00417-016-3578-5. [DOI] [PubMed] [Google Scholar]
- 96.Boudina S., Abel E.D. Diabetic cardiomyopathy, causes and effects. Rev. Endocr. Metab. Disord. 2010;11(1):31–39. doi: 10.1007/s11154-010-9131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burgoyne J.R., Mongue-Din H., Eaton P., et al. Redox signaling in cardiac physiology and pathology. Circ. Res. 2012;111(8):1091–1106. doi: 10.1161/CIRCRESAHA.111.255216. [DOI] [PubMed] [Google Scholar]
- 98.Thandavarayan R.A., Watanabe K., Ma M., et al. Dominant-negative p38alpha mitogen-activated protein kinase prevents cardiac apoptosis and remodeling after streptozotocin-induced diabetes mellitus. Am. J. Physiol. Heart Circ. Physiol. 2009;297(3):H911–H919. doi: 10.1152/ajpheart.00124.2009. [DOI] [PubMed] [Google Scholar]
- 99.Arozal W., Watanabe K., Veeraveedu P.T., et al. Effects of angiotensin receptor blocker on oxidative stress and cardio-renal function in streptozotocin-induced diabetic rats. Biol. Pharm. Bull. 2009;32(8):1411–1416. doi: 10.1248/bpb.32.1411. [DOI] [PubMed] [Google Scholar]
- 100.Liu C., Hua N., Fu X., et al. Metformin regulates the expression of SK2 and SK3 in the Atria of rats with type 2 diabetes mellitus through the NOX4/p38MAPK signaling pathway. J. Cardiovasc. Pharmacol. 2018;72(5):205–213. doi: 10.1097/FJC.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 101.Alaeddine L.M., Harb F., Hamza M., et al. Pharmacological regulation of cytochrome P450 metabolites of arachidonic acid attenuates cardiac injury in diabetic rats: the role of cytochromes P450 metabolites in diabetic cardiomyopathy. Transl. Res. 2021;235:85–101. doi: 10.1016/j.trsl.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 102.Lu Y., Zhu S., Wang X., et al. ShengMai-san attenuates cardiac remodeling in diabetic rats by inhibiting NOX-mediated oxidative stress. Diabetes Metab Syndr Obes. 2021;14:647–657. doi: 10.2147/DMSO.S287582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tan Y.Y., Chen L.X., Fang L., et al. Cardioprotective effects of polydatin against myocardial injury in diabetic rats via inhibition of NADPH oxidase and NF-kappaB activities. BMC Complement Med Ther. 2020;20(1):378. doi: 10.1186/s12906-020-03177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hussain S., Khan A.W., Akhmedov A., et al. Hyperglycemia induces myocardial dysfunction via epigenetic regulation of JunD. Circ. Res. 2020;127(10):1261–1273. doi: 10.1161/CIRCRESAHA.120.317132. [DOI] [PubMed] [Google Scholar]
- 105.Fan L., Xiao Q., Zhang L., et al. CAPE-pNO2 attenuates diabetic cardiomyopathy through the NOX4/NF-kappaB pathway in STZ-induced diabetic mice. Biomed. Pharmacother. 2018;108:1640–1650. doi: 10.1016/j.biopha.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 106.Novoa U., Arauna D., Moran M., et al. High-intensity exercise reduces cardiac fibrosis and hypertrophy but does not restore the nitroso-redox imbalance in diabetic cardiomyopathy. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/7921363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maeda Y., Inoguchi T., Takei R., et al. Chymase inhibition prevents myocardial fibrosis through the attenuation of NOX4-associated oxidative stress in diabetic hamsters. J Diabetes Investig. 2012;3(4):354–361. doi: 10.1111/j.2040-1124.2012.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maalouf R.M., Eid A.A., Gorin Y.C., et al. Nox4-derived reactive oxygen species mediate cardiomyocyte injury in early type 1 diabetes. Am. J. Physiol. Cell Physiol. 2012;302(3):C597–C604. doi: 10.1152/ajpcell.00331.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.You Q., Wu Z., Wu B., et al. Naringin protects cardiomyocytes against hyperglycemia-induced injuries in vitro and in vivo. J. Endocrinol. 2016;230(2):197–214. doi: 10.1530/JOE-16-0004. [DOI] [PubMed] [Google Scholar]
- 110.Zheng D., Dong S., Li T., et al. Exogenous hydrogen sulfide attenuates cardiac fibrosis through reactive oxygen species signal pathways in experimental diabetes mellitus models. Cell. Physiol. Biochem. 2015;36(3):917–929. doi: 10.1159/000430266. [DOI] [PubMed] [Google Scholar]
- 111.Taye A., Abouzied M.M., Mohafez O.M. Tempol ameliorates cardiac fibrosis in streptozotocin-induced diabetic rats: role of oxidative stress in diabetic cardiomyopathy. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2013;386(12):1071–1080. doi: 10.1007/s00210-013-0904-x. [DOI] [PubMed] [Google Scholar]
- 112.Kakoki M., Bahnson E.M., Hagaman J.R., et al. Engulfment and cell motility protein 1 potentiates diabetic cardiomyopathy via Rac-dependent and Rac-independent ROS production. JCI Insight. 2019;4(12) doi: 10.1172/jci.insight.127660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cucoranu I., Clempus R., Dikalova A., et al. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ. Res. 2005;97(9):900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 114.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc. Res. 2004;63(3):423–432. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 115.Wenzel S., Taimor G., Piper H.M., et al. Redox-sensitive intermediates mediate angiotensin II-induced p38 MAP kinase activation, AP-1 binding activity, and TGF-beta expression in adult ventricular cardiomyocytes. Faseb. J. 2001;15(12):2291–2293. doi: 10.1096/fj.00-0827fje. [DOI] [PubMed] [Google Scholar]
- 116.Rani N., Bharti S., Bhatia J., et al. Chrysin, a PPAR-gamma agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem. Biol. Interact. 2016;250:59–67. doi: 10.1016/j.cbi.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 117.Shindo T., Manabe I., Fukushima Y., et al. Krüppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat. Med. 2002;8(8):856–863. doi: 10.1038/nm738. [DOI] [PubMed] [Google Scholar]
- 118.Di R.M., Yang C.X., Zhao C.M., et al. Identification and functional characterization of KLF5 as a novel disease gene responsible for familial dilated cardiomyopathy. Eur. J. Med. Genet. 2020;63(4) doi: 10.1016/j.ejmg.2019.103827. [DOI] [PubMed] [Google Scholar]
- 119.Kyriazis I.D., Hoffman M., Gaignebet L., et al. KLF5 is induced by FOXO1 and causes oxidative stress and diabetic cardiomyopathy. Circ. Res. 2021;128(3):335–357. doi: 10.1161/CIRCRESAHA.120.316738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Feldman E.L., Callaghan B.C., Pop-Busui R., et al. Diabetic neuropathy. Nat. Rev. Dis. Prim. 2019;5(1):41. doi: 10.1038/s41572-019-0092-1. [DOI] [PubMed] [Google Scholar]
- 121.Sorce S., Krause K.H. NOX enzymes in the central nervous system: from signaling to disease. Antioxidants Redox Signal. 2009;11(10):2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- 122.Wilson C., Munoz-Palma E., Gonzalez-Billault C. From birth to death: a role for reactive oxygen species in neuronal development. Semin. Cell Dev. Biol. 2018;80:43–49. doi: 10.1016/j.semcdb.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 123.Yu T., Xin Q., Xu F., et al. [Research on the mechanism of high glucose affecting the apoptosis of schwann cells by Nox4 NADPH oxidase] Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2019;35(2):130–134. doi: 10.12047/j.cjap.5719.2019.029. [DOI] [PubMed] [Google Scholar]
- 124.Shono T., Yokoyama N., Uesaka T., et al. Enhanced expression of NADPH oxidase Nox4 in human gliomas and its roles in cell proliferation and survival. Int. J. Cancer. 2008;123(4):787–792. doi: 10.1002/ijc.23569. [DOI] [PubMed] [Google Scholar]
- 125.Kleinschnitz C., Grund H., Wingler K., et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8(9) doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ji Z.H., Liu Z.J., Liu Z.T., et al. Diphenyleneiodonium mitigates bupivacaine-induced sciatic nerve damage in a diabetic neuropathy rat model by attenuating oxidative stress. Anesth. Analg. 2017;125(2):653–661. doi: 10.1213/ANE.0000000000002186. [DOI] [PubMed] [Google Scholar]
- 127.Eid S.A., El Massry M., Hichor M., et al. Targeting the NADPH oxidase-4 and liver X receptor pathway preserves schwann cell integrity in diabetic mice. Diabetes. 2020;69(3):448–464. doi: 10.2337/db19-0517. [DOI] [PubMed] [Google Scholar]
- 128.Hong O.K., Lee S.S., Yoo S.J., et al. Effects of DA-9801 on the inflammation and apoptosis induced by angiotensin II in human dermal microvascular endothelial cells. J. Pharmacol. Sci. 2021;145(1):52–59. doi: 10.1016/j.jphs.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 129.Fu Y., Zhang Y., Wang Z., et al. Regulation of NADPH oxidase activity is associated with miRNA-25-mediated NOX4 expression in experimental diabetic nephropathy. Am. J. Nephrol. 2010;32(6):581–589. doi: 10.1159/000322105. [DOI] [PubMed] [Google Scholar]
- 130.Oh H.J., Kato M., Deshpande S., et al. Inhibition of the processing of miR-25 by HIPK2-Phosphorylated-MeCP2 induces NOX4 in early diabetic nephropathy. Sci. Rep. 2016;6 doi: 10.1038/srep38789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sharma I., Dutta R.K., Singh N.K., et al. High glucose-induced hypomethylation promotes binding of Sp-1 to myo-inositol oxygenase: implication in the pathobiology of diabetic tubulopathy. Am. J. Pathol. 2017;187(4):724–739. doi: 10.1016/j.ajpath.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu Y., Zhang J., Fan L., et al. miR-423-5p suppresses high-glucose-induced podocyte injury by targeting Nox4. Biochem. Biophys. Res. Commun. 2018;505(2):339–345. doi: 10.1016/j.bbrc.2018.09.067. [DOI] [PubMed] [Google Scholar]
- 133.Fu Y., Wang C., Zhang D., et al. miR-15b-5p ameliorated high glucose-induced podocyte injury through repressing apoptosis, oxidative stress, and inflammatory responses by targeting Sema3A. J. Cell. Physiol. 2019;234(11):20869–20878. doi: 10.1002/jcp.28691. [DOI] [PubMed] [Google Scholar]
- 134.Wan R.J., Li Y.H. MicroRNA146a/NAPDH oxidase4 decreases reactive oxygen species generation and inflammation in a diabetic nephropathy model. Mol. Med. Rep. 2018;17(3):4759–4766. doi: 10.3892/mmr.2018.8407. [DOI] [PubMed] [Google Scholar]
- 135.Gu C., Draga D., Zhou C., et al. miR-590-3p inhibits pyroptosis in diabetic retinopathy by targeting NLRP1 and inactivating the NOX4 signaling pathway. Invest. Ophthalmol. Vis. Sci. 2019;60(13):4215–4223. doi: 10.1167/iovs.19-27825. [DOI] [PubMed] [Google Scholar]
- 136.Hakami N.Y., Dusting G.J., Peshavariya H.M., Trichostatin A. A histone deacetylase inhibitor suppresses NADPH Oxidase 4-Derived Redox Signalling and Angiogenesis. J. Cell Mol. Med. 2016;20(10):1932–1944. doi: 10.1111/jcmm.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yu H., Zhang X., Wang X., et al. MiR-99a-5p inhibits the proliferation and migration of human retinal microvascular endothelial cells by targeting NOX4. Horm. Metab. Res. 2023;55(2):142–148. doi: 10.1055/a-1982-3926. [DOI] [PubMed] [Google Scholar]
- 138.Han Z., Zhao D., Han M., et al. Knockdown of miR-372-3p inhibits the development of diabetic cardiomyopathy by accelerating angiogenesis via activating the PI3K/AKT/mTOR/HIF-1α signaling pathway and suppressing oxidative stress. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/4342755. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 139.Zhang Y., Song C., Liu J., et al. Inhibition of miR-25 aggravates diabetic peripheral neuropathy. Neuroreport. 2018;29(11):945–953. doi: 10.1097/WNR.0000000000001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.He J.Y., Hong Q., Chen B.X., et al. Ginsenoside Rb1 alleviates diabetic kidney podocyte injury by inhibiting aldose reductase activity. Acta Pharmacol. Sin. 2022;43(2):342–353. doi: 10.1038/s41401-021-00788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fan Y., Qiao Y., Huang J., et al. Protective effects of Panax notoginseng saponins against high glucose-induced oxidative injury in rat retinal capillary endothelial cells. Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/5326382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Habibi J., Aroor A.R., Das N.A., et al. The combination of a neprilysin inhibitor (sacubitril) and angiotensin-II receptor blocker (valsartan) attenuates glomerular and tubular injury in the Zucker Obese rat. Cardiovasc. Diabetol. 2019;18(1):40. doi: 10.1186/s12933-019-0847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qin J., Peng Z., Yuan Q., et al. AKF-PD alleviates diabetic nephropathy via blocking the RAGE/AGEs/NOX and PKC/NOX Pathways. Sci. Rep. 2019;9(1):4407. doi: 10.1038/s41598-018-36344-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yaribeygi H., Mohammadi M.T., Rezaee R., et al. Fenofibrate improves renal function by amelioration of NOX-4, IL-18, and p53 expression in an experimental model of diabetic nephropathy. J. Cell. Biochem. 2018;119(9):7458–7469. doi: 10.1002/jcb.27055. [DOI] [PubMed] [Google Scholar]
- 145.Tang L., Wu Y., Tian M., et al. Dapagliflozin slows the progression of the renal and liver fibrosis associated with type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2017;313(5):E563–e576. doi: 10.1152/ajpendo.00086.2017. [DOI] [PubMed] [Google Scholar]
- 146.Hendarto H., Inoguchi T., Maeda Y., et al. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism. 2012;61(10):1422–1434. doi: 10.1016/j.metabol.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 147.Yang K., Bai Y., Yu N., et al. Huidouba improved podocyte injury by down-regulating Nox4 expression in rats with diabetic nephropathy. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.587995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cui F.Q., Tang L., Gao Y.B., et al. Effect of Baoshenfang Formula on podocyte injury via inhibiting the NOX-4/ROS/p38 pathway in diabetic nephropathy. J. Diabetes Res. 2019;2019 doi: 10.1155/2019/2981705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen Y., Chen J., Jiang M., et al. Loganin and catalpol exert cooperative ameliorating effects on podocyte apoptosis upon diabetic nephropathy by targeting AGEs-RAGE signaling. Life Sci. 2020;25 doi: 10.1016/j.lfs.2020.117653. [DOI] [PubMed] [Google Scholar]
- 150.Yong R., Chen X.M., Shen S., et al. Plumbagin ameliorates diabetic nephropathy via interruption of pathways that include NOX4 signalling. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0073428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gorin Y., Cavaglieri R.C., Khazim K., et al. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am. J. Physiol. Ren. Physiol. 2015;308(11):F1276–F1287. doi: 10.1152/ajprenal.00396.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sedeek M., Gutsol A., Montezano A.C., et al. Renoprotective effects of a novel Nox1/4 inhibitor in a mouse model of Type 2 diabetes. Clin. Sci. (Lond.) 2013;124(3):191–202. doi: 10.1042/CS20120330. [DOI] [PubMed] [Google Scholar]
- 153.Cha J.J., Min H.S., Kim K.T., et al. APX-115, a first-in-class pan-NADPH oxidase (Nox) inhibitor, protects db/db mice from renal injury. Lab. Invest. 2017;97(4):419–431. doi: 10.1038/labinvest.2017.2. [DOI] [PubMed] [Google Scholar]
- 154.Liu Q., Zhang X., Cheng R., et al. Salutary effect of fenofibrate on type 1 diabetic retinopathy via inhibiting oxidative stress-mediated Wnt/β-catenin pathway activation. Cell Tissue Res. 2019;376(2):165–177. doi: 10.1007/s00441-018-2974-z. [DOI] [PubMed] [Google Scholar]
- 155.Chen Y.Y., Wu T.T., Ho C.Y., et al. Blocking of SGLT2 to eliminate NADPH-induced oxidative stress in lenses of animals with fructose-induced diabetes mellitus. Int. J. Mol. Sci. 2022;23(13):7142. doi: 10.3390/ijms23137142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xia J.P., Liu S.Q., Wang S. Intravitreal conbercept improves outcome of proliferative diabetic retinopathy through inhibiting inflammation and oxidative stress. Life Sci. 2021;265 doi: 10.1016/j.lfs.2020.118795. [DOI] [PubMed] [Google Scholar]
- 157.Ye X., Xiang F., Hu Y. Gambogic acid affects high glucose-induced apoptosis and inflammation of retinal endothelial cells through the NOX4/NLRP3 pathway. Ann. Transl. Med. 2023;11(4):168. doi: 10.21037/atm-22-6591. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 158.Dauth A., Bręborowicz A., Ruan Y., et al. Sulodexide prevents hyperglycemia-induced endothelial dysfunction and oxidative stress in porcine retinal arterioles. Antioxidants. 2023;12(2):388. doi: 10.3390/antiox12020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dionysopoulou S., Wikstrom P., Bucolo C., et al. Topically administered NOX4 inhibitor, GLX7013114, is efficacious in treating the early pathological events of diabetic retinopathy. Diabetes. 2023;72(5):638–652. doi: 10.2337/db22-0515. [DOI] [PubMed] [Google Scholar]
- 160.Appukuttan B., Ma Y., Stempel A., et al. Effect of NADPH oxidase 1 and 4 blockade in activated human retinal endothelial cells. Clin. Exp. Ophthalmol. 2018;46(6):652–660. doi: 10.1111/ceo.13155. [DOI] [PubMed] [Google Scholar]
- 161.Deliyanti D., Wilkinson-Berka J.L. Inhibition of NOX1/4 with GKT137831: a potential novel treatment to attenuate neuroglial cell inflammation in the retina. J. Neuroinflammation. 2015;12:136. doi: 10.1186/s12974-015-0363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rani N., Bharti S., Bhatia J., et al. Chrysin, a PPAR-γ agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem. Biol. Interact. 2016;250:59–67. doi: 10.1016/j.cbi.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 163.Lu S., Liao Z., Lu X., et al. Hyperglycemia Acutely increases cytosolic reactive oxygen species via O-linked GlcNAcylation and CaMKII activation in mouse ventricular myocytes. Circ. Res. 2020;126(10):e80–e96. doi: 10.1161/CIRCRESAHA.119.316288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ma J., Shi M., Zhang X., et al. GLP1R agonists ameliorate peripheral nerve dysfunction and inflammation via p38 MAPK/NFkappaB signaling pathways in streptozotocininduced diabetic rats. Int. J. Mol. Med. 2018;41(5):2977–2985. doi: 10.3892/ijmm.2018.3509. [DOI] [PubMed] [Google Scholar]
- 165.Wang D., Li Y., Wang N., et al. 1alpha,25-Dihydroxyvitamin D3 prevents renal oxidative damage via the PARP1/SIRT1/NOX4 pathway in Zucker diabetic fatty rats. Am. J. Physiol. Endocrinol. Metab. 2020;318(3):E343–E356. doi: 10.1152/ajpendo.00270.2019. [DOI] [PubMed] [Google Scholar]
- 166.Manna P., Achari A.E., Jain S.K. Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high fat diet-fed diabetic mice. Arch. Biochem. Biophys. 2017;615:22–34. doi: 10.1016/j.abb.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 167.Kofler P.A., Pircher H., Von Grafenstein S., et al. Characterisation of Nox4 inhibitors from edible plants. Planta Med. 2013;79(3–4):244–252. doi: 10.1055/s-0032-1328129. [DOI] [PubMed] [Google Scholar]
- 168.Guo J., Li J., Wei H., et al. Maackiain protects the kidneys of type 2 diabetic rats via modulating the Nrf2/HO-1 and TLR4/NF-κB/Caspase-3 pathways. Drug Des. Dev. Ther. 2021;15:4339–4358. doi: 10.2147/DDDT.S326975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.An X., Zhang Y., Cao Y., et al. Punicalagin protects diabetic nephropathy by inhibiting pyroptosis based on TXNIP/NLRP3 pathway. Nutrients. 2020;12(5):1516. doi: 10.3390/nu12051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhong Y., Liu J., Sun D., et al. Dioscin relieves diabetic nephropathy via suppressing oxidative stress and apoptosis, and improving mitochondrial quality and quantity control. Food Funct. 2022;13(6):3660–3673. doi: 10.1039/d1fo02733f. [DOI] [PubMed] [Google Scholar]
- 171.Ren C., Bao X., Lu X., et al. Complanatoside A targeting NOX4 blocks renal fibrosis in diabetic mice by suppressing NLRP3 inflammasome activation and autophagy. Phytomedicine. 2022;104 doi: 10.1016/j.phymed.2022.154310. [DOI] [PubMed] [Google Scholar]
- 172.Zhong Y., Luo R., Liu Q., et al. Jujuboside A ameliorates high fat diet and streptozotocin induced diabetic nephropathy via suppressing oxidative stress, apoptosis, and enhancing autophagy. Food Chem. Toxicol. 2022;159 doi: 10.1016/j.fct.2021.112697. [DOI] [PubMed] [Google Scholar]
- 173.Soetikno V., Watanabe K., Sari F.R., et al. Curcumin attenuates diabetic nephropathy by inhibiting PKC-α and PKC-β1 activity in streptozotocin-induced type I diabetic rats. Mol. Nutr. Food Res. 2011;55(11):1655–1665. doi: 10.1002/mnfr.201100080. [DOI] [PubMed] [Google Scholar]
- 174.Lei X.W., Li Q., Zhang J.Z., et al. The protective roles of folic acid in preventing diabetic retinopathy are potentially associated with suppressions on angiogenesis, inflammation, and oxidative stress. Ophthalmic Res. 2019;62(2):80–92. doi: 10.1159/000499020. [DOI] [PubMed] [Google Scholar]
- 175.Huang W., Yan Z., Li D., et al. Antioxidant and anti-inflammatory effects of blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/1862462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Laddha A.P., Kulkarni Y.A. Daidzein mitigates myocardial injury in streptozotocin-induced diabetes in rats. Life Sci. 2021;284 doi: 10.1016/j.lfs.2021.119664. [DOI] [PubMed] [Google Scholar]
- 177.Sirisha A., Gaur G.S., Pal P., et al. Effect of honey and insulin treatment on oxidative stress and nerve conduction in an experimental model of diabetic neuropathy Wistar rats. PLoS One. 2021;16(1) doi: 10.1371/journal.pone.0245395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sima A.V., Botez G.M., Stancu C.S., et al. Effect of irreversibly glycated LDL in human vascular smooth muscle cells: lipid loading, oxidative and inflammatory stress. J. Cell Mol. Med. 2010;14(12):2790–2802. doi: 10.1111/j.1582-4934.2009.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang W.X., Tai G.J., Li X.X., et al. Inhibition of neointima hyperplasia by the combined therapy of linagliptin and metformin via AMPK/Nox4 signaling in diabetic rats. Free Radic. Biol. Med. 2019;143:153–163. doi: 10.1016/j.freeradbiomed.2019.07.030. [DOI] [PubMed] [Google Scholar]
- 180.Burlet E., Jain S.K. Manganese supplementation increases adiponectin and lowers ICAM-1 and creatinine blood levels in Zucker type 2 diabetic rats, and downregulates ICAM-1 by upregulating adiponectin multimerization protein (DsbA-L) in endothelial cells. Mol. Cell. Biochem. 2017;429(1–2):1–10. doi: 10.1007/s11010-016-2931-7. [DOI] [PubMed] [Google Scholar]
- 181.Tong X.Y. Upregulation of nox4 by TGF beta 1 oxidizes SERCA and inhibits NO in arterial smooth muscle of the prediabetic zucker rat (vol 107, pg 975, 2010) Circ. Res. 2012;110(11):E88–E89. doi: 10.1161/CIRCRESAHA.110.221242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ren Y., Tao S., Zheng S., et al. Salvianolic acid B improves vascular endothelial function in diabetic rats with blood glucose fluctuations via suppression of endothelial cell apoptosis. Eur. J. Pharmacol. 2016;79:308–315. doi: 10.1016/j.ejphar.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 183.Shen J., Liu M., Xu J., et al. ARL15 overexpression attenuates high glucose-induced impairment of insulin signaling and oxidative stress in human umbilical vein endothelial cells. Life Sci. 2019;220:127–135. doi: 10.1016/j.lfs.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 184.Tian X.Y., Wong W.T., Xu A., et al. Rosuvastatin improves endothelial function in db/db mice: role of angiotensin II type 1 receptors and oxidative stress. Br. J. Pharmacol. 2011;164(2b):598–606. doi: 10.1111/j.1476-5381.2011.01416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang F., Bao Y., Shen X., et al. Niazirin from Moringa oleifera Lam. attenuates high glucose-induced oxidative stress through PKCζ/Nox4 pathway. Phytomedicine. 2021;86 doi: 10.1016/j.phymed.2019.153066. [DOI] [PubMed] [Google Scholar]
- 186.Tan C., Day R., Bao S., et al. Group VIA phospholipase A2 mediates enhanced macrophage migration in diabetes mellitus by increasing expression of nicotinamide adenine dinucleotide phosphate oxidase 4. Arterioscler. Thromb. Vasc. Biol. 2014;34(4):768–778. doi: 10.1161/ATVBAHA.113.302847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ullevig S.L., Kim H.S., Nguyen H.N., et al. Ursolic acid protects monocytes against metabolic stress-induced priming and dysfunction by preventing the induction of Nox4. Redox Biol. 2014;2:259–266. doi: 10.1016/j.redox.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ning R.B., Zhu J., Chai D.J., et al. RXR agonists inhibit high glucose-induced upregulation of inflammation by suppressing activation of the NADPH oxidase-nuclear factor-kappaB pathway in human endothelial cells. Genet. Mol. Res. 2013;12(4):6692–6707. doi: 10.4238/2013.December.13.3. [DOI] [PubMed] [Google Scholar]
- 189.Venkatesan B., Valente A.J., Das N.A., et al. CIKS (Act1 or TRAF3IP2) mediates high glucose-induced endothelial dysfunction. Cell. Signal. 2013;25(1):359–371. doi: 10.1016/j.cellsig.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhao R., Le K., Moghadasian M.H., et al. Regulatory role of NADPH oxidase in glycated LDL-induced upregulation of plasminogen activator inhibitor-1 and heat shock factor-1 in mouse embryo fibroblasts and diabetic mice. Free Radic. Biol. Med. 2013;61:18–25. doi: 10.1016/j.freeradbiomed.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 191.Di Marco E., Gray S.P., Kennedy K., et al. NOX4-derived reactive oxygen species limit fibrosis and inhibit proliferation of vascular smooth muscle cells in diabetic atherosclerosis. Free Radic. Biol. Med. 2016;97:556–567. doi: 10.1016/j.freeradbiomed.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gray S.P., Di Marco E., Kennedy K., et al. Reactive oxygen species can provide atheroprotection via NOX4-dependent inhibition of inflammation and vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2016;36(2):295–307. doi: 10.1161/ATVBAHA.115.307012. [DOI] [PubMed] [Google Scholar]
- 193.Hu P., Wu X., Khandelwal A.R., et al. Endothelial Nox4-based NADPH oxidase regulates atherosclerosis via soluble epoxide hydrolase. Biochim. Biophys. Acta, Mol. Basis Dis. 2017;1863(6):1382–1391. doi: 10.1016/j.bbadis.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Viswambharan H., Yuldasheva N.Y., Imrie H., et al. Novel paracrine action of endothelium enhances glucose uptake in muscle and fat. Circ. Res. 2021;129(7):720–734. doi: 10.1161/CIRCRESAHA.121.319517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Xu H., Wang Z., Sun Z., et al. GATA4 protects against hyperglycemia-induced endothelial dysfunction by regulating NOX4 transcription. Mol. Med. Rep. 2018;17(1):1485–1492. doi: 10.3892/mmr.2017.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gray S.P., Di Marco E., Okabe J., et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation. 2013;127(18):1888–1902. doi: 10.1161/CIRCULATIONAHA.112.132159. [DOI] [PubMed] [Google Scholar]
- 197.San Martin A., Du P., Dikalova A., et al. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in Type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 2007;292(5):H2073–H2082. doi: 10.1152/ajpheart.00943.2006. [DOI] [PubMed] [Google Scholar]
- 198.Ding H., Hashem M., Triggle C. Increased oxidative stress in the streptozotocin-induced diabetic apoE-deficient mouse: changes in expression of NADPH oxidase subunits and eNOS. Eur. J. Pharmacol. 2007;561(1–3):121–128. doi: 10.1016/j.ejphar.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 199.Wang C., Li J., Lv X., et al. Ameliorative effect of berberine on endothelial dysfunction in diabetic rats induced by high-fat diet and streptozotocin. Eur. J. Pharmacol. 2009;620(1–3):131–137. doi: 10.1016/j.ejphar.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 200.Li X., Jin S.J., Su J., et al. Acid sphingomyelinase down-regulation alleviates vascular endothelial insulin resistance in diabetic rats. Basic Clin. Pharmacol. Toxicol. 2018;123(6):645–659. doi: 10.1111/bcpt.13073. [DOI] [PubMed] [Google Scholar]
- 201.Gortan Cappellari G., Barazzoni R., Cattin L., et al. Lack of fibronectin extra domain A Alternative splicing exacerbates endothelial dysfunction in diabetes. Sci. Rep. 2016;6 doi: 10.1038/srep37965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li X.W., Hao W., Liu Y., et al. [Effect of sequoyitol on expression of NOX4 and eNOS in aortas of type 2 diabetic rats] Yao Xue Xue Bao. 2014;49(3):329–336. [PubMed] [Google Scholar]
- 203.Abdelmageed M.E., Shehatou G.S.G., Suddek G.M., et al. Protocatechuic acid improves hepatic insulin resistance and restores vascular oxidative status in type-2 diabetic rats. Environ. Toxicol. Pharmacol. 2021;83 doi: 10.1016/j.etap.2020.103577. [DOI] [PubMed] [Google Scholar]
- 204.Wang L., Li X., Zhang Y., et al. Oxymatrine ameliorates diabetes-induced aortic endothelial dysfunction via the regulation of eNOS and NOX4. J. Cell. Biochem. 2019;120(5):7323–7332. doi: 10.1002/jcb.28006. [DOI] [PubMed] [Google Scholar]
- 205.Abdelmageed M.E., Shehatou G.S., Abdelsalam R.A., et al. Cinnamaldehyde ameliorates STZ-induced rat diabetes through modulation of IRS1/PI3K/AKT2 pathway and AGEs/RAGE interaction. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019;392(2):243–258. doi: 10.1007/s00210-018-1583-4. [DOI] [PubMed] [Google Scholar]
- 206.Wang Z., Zhang J., Chen L., et al. Glycine suppresses AGE/RAGE signaling pathway and subsequent oxidative stress by restoring Glo1 function in the aorta of diabetic rats and in HUVECs. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/4628962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hwang J., Kleinhenz D.J., Rupnow H.L., et al. The PPARgamma ligand, rosiglitazone, reduces vascular oxidative stress and NADPH oxidase expression in diabetic mice. Vasc. Pharmacol. 2007;46(6):456–462. doi: 10.1016/j.vph.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 208.Rozentsvit A., Vinokur K., Samuel S., et al. Ellagic acid reduces high glucose-induced vascular oxidative stress through ERK1/2/NOX4 signaling pathway. Cell. Physiol. Biochem. 2017;44(3):1174–1187. doi: 10.1159/000485448. [DOI] [PubMed] [Google Scholar]
- 209.Wang Z.J., Chang L.L., Wu J., et al. A novel rhynchophylline analog, Y396, inhibits endothelial dysfunction induced by oxidative stress in diabetes through epidermal growth factor receptor. Antioxidants Redox Signal. 2020;32(11):743–765. doi: 10.1089/ars.2018.7721. [DOI] [PubMed] [Google Scholar]
- 210.Zhang Y., Mao X.D., Cao A.L., et al. Astragaloside IV prevents endothelial dysfunction by improving oxidative stress in streptozotocin-induced diabetic mouse aortas. Exp. Ther. Med. 2021;22(5):1197. doi: 10.3892/etm.2021.10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.El Atab O., Ghantous C.M., El-Zein N., et al. Involvement of caveolae in hyperglycemia-induced changes in adiponectin and leptin expressions in vascular smooth muscle cells. Eur. J. Pharmacol. 2022;919 doi: 10.1016/j.ejphar.2021.174701. [DOI] [PubMed] [Google Scholar]
- 212.Manea S.A., Antonescu M.L., Fenyo I.M., et al. Epigenetic regulation of vascular NADPH oxidase expression and reactive oxygen species production by histone deacetylase-dependent mechanisms in experimental diabetes. Redox Biol. 2018;16:332–343. doi: 10.1016/j.redox.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang H.F., Yu Q.Q., Zheng R.F., et al. Inhibition of vascular adventitial remodeling by netrin-1 in diabetic rats. J. Endocrinol. 2020;244(3):445–458. doi: 10.1530/JOE-19-0258. [DOI] [PubMed] [Google Scholar]
- 214.Alvarez-Cilleros D., Lopez-Oliva M.E., Morales-Cano D., et al. Dietary cocoa prevents aortic remodeling and vascular oxidative stress in diabetic rats. Mol. Nutr. Food Res. 2019;63(18) doi: 10.1002/mnfr.201900044. [DOI] [PubMed] [Google Scholar]
- 215.Guo Z., Zhang R., Li J., et al. Effect of telmisartan on the expression of adiponectin receptors and nicotinamide adenine dinucleotide phosphate oxidase in the heart and aorta in type 2 diabetic rats. Cardiovasc. Diabetol. 2012;11:94. doi: 10.1186/1475-2840-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhao W., Yuan Y., Zhao H., et al. Aqueous extract of Salvia miltiorrhiza Bunge-Radix Puerariae herb pair ameliorates diabetic vascular injury by inhibiting oxidative stress in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2019;129:97–107. doi: 10.1016/j.fct.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 217.Mukai Y., Norikura T., Fujita S., et al. Effect of lignin-derived lignophenols on vascular oxidative stress and inflammation in streptozotocin-induced diabetic rats. Mol. Cell. Biochem. 2011;348(1–2):117–124. doi: 10.1007/s11010-010-0645-9. [DOI] [PubMed] [Google Scholar]
- 218.Petersen C., Bharat D., Wankhade U.D., et al. Dietary blueberry ameliorates vascular complications in diabetic mice possibly through NOX4 and modulates composition and functional diversity of gut microbes. Mol. Nutr. Food Res. 2022;66(8) doi: 10.1002/mnfr.202100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Li Y., Huang C., Fu W., et al. Screening of the active fractions from the Coreopsis tinctoria Nutt. Flower on diabetic endothelial protection and determination of the underlying mechanism. J. Ethnopharmacol. 2020;253 doi: 10.1016/j.jep.2020.112645. [DOI] [PubMed] [Google Scholar]
- 220.Di Marco E., Gray S.P., Chew P., et al. Differential effects of NOX4 and NOX1 on immune cell-mediated inflammation in the aortic sinus of diabetic ApoE-/- mice. Clin. Sci. (Lond.) 2016;130(15):1363–1374. doi: 10.1042/CS20160249. [DOI] [PubMed] [Google Scholar]
- 221.Li X.X., Ling S.K., Hu M.Y., et al. Protective effects of acarbose against vascular endothelial dysfunction through inhibiting Nox4/NLRP3 inflammasome pathway in diabetic rats. Free Radic. Biol. Med. 2019;145:175–186. doi: 10.1016/j.freeradbiomed.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 222.Blanco F., Heinonen S.E., Gurzeler E., et al. In vivo inhibition of nuclear factor of activated T-cells leads to atherosclerotic plaque regression in IGF-II/LDLR(-/-)ApoB(100/100) mice. Diabetes Vasc. Dis. Res. 2018;15(4):302–313. doi: 10.1177/1479164118759220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhao R., Le K., Li W., et al. Effects of Saskatoon berry powder on monocyte adhesion to vascular wall of leptin receptor-deficient diabetic mice. J. Nutr. Biochem. 2014;25(8):851–857. doi: 10.1016/j.jnutbio.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 224.Li W., Zhao W., Wu Q., et al. Puerarin improves diabetic aorta injury by inhibiting NADPH oxidase-derived oxidative stress in STZ-induced diabetic rats. J. Diabetes Res. 2016;2016 doi: 10.1155/2016/8541520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Liang C.F., Liu J.T., Wang Y., et al. Toll-like receptor 4 mutation protects obese mice against endothelial dysfunction by decreasing NADPH oxidase isoforms 1 and 4. Arterioscler. Thromb. Vasc. Biol. 2013;33(4):777–784. doi: 10.1161/ATVBAHA.112.301087. [DOI] [PubMed] [Google Scholar]
- 226.Su J., An X.R., Li Q., et al. Improvement of vascular dysfunction by argirein through inhibiting endothelial cell apoptosis associated with ET-1/Nox4 signal pathway in diabetic rats. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-30386-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Li Q., Su J., Jin S.J., et al. Argirein alleviates vascular endothelial insulin resistance through suppressing the activation of Nox4-dependent O(2)(-) production in diabetic rats. Free Radic. Biol. Med. 2018;121:169–179. doi: 10.1016/j.freeradbiomed.2018.04.573. [DOI] [PubMed] [Google Scholar]
- 228.Guo Y., Zhang Q., Chen H., et al. The protective role of calcitonin gene-related peptide (CGRP) in high-glucose-induced oxidative injury in rat aorta endothelial cells. Peptides. 2019;121 doi: 10.1016/j.peptides.2019.170121. [DOI] [PubMed] [Google Scholar]
- 229.Li Q., Lin Y., Wang S., et al. GLP-1 inhibits high-glucose-induced oxidative injury of vascular endothelial cells. Sci. Rep. 2017;7(1):8008. doi: 10.1038/s41598-017-06712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chen F., Ma D., Li A. SENP3 regulates high glucose-induced endothelial dysfunction via ROS dependent signaling. Diabetes Vasc. Dis. Res. 2020;17(6) doi: 10.1177/1479164120970895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Xi G., Shen X.C., Wai C., et al. Recruitment of Nox4 to a plasma membrane scaffold is required for localized reactive oxygen species generation and sustained Src activation in response to insulin-like growth factor-I. J. Biol. Chem. 2013;288(22):15641–15653. doi: 10.1074/jbc.M113.456046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wendt M.C., Daiber A., Kleschyov A.L., et al. Differential effects of diabetes on the expression of the gp91phox homologues nox1 and nox4. Free Radic. Biol. Med. 2005;39(3):381–391. doi: 10.1016/j.freeradbiomed.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 233.Fukuda M., Nakamura T., Kataoka K., et al. Ezetimibe ameliorates cardiovascular complications and hepatic steatosis in obese and type 2 diabetic db/db mice. J. Pharmacol. Exp. Therapeut. 2010;335(1):70–75. doi: 10.1124/jpet.110.170373. [DOI] [PubMed] [Google Scholar]
- 234.Si R., Cabrera J.T.O., Tsuji-Hosokawa A., et al. HuR/Cx40 downregulation causes coronary microvascular dysfunction in type 2 diabetes. JCI Insight. 2021;6(21) doi: 10.1172/jci.insight.147982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Caicedo D., Alvarez C.V., Perez-Romero S., et al. The inflammatory pattern of chronic limb-threatening ischemia in muscles: the TNF-alpha hypothesis. Biomedicines. 2022;10(2):489. doi: 10.3390/biomedicines10020489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Distasi M.R., Mund J.A., Bohlen H.G., et al. Impaired compensation to femoral artery ligation in diet-induced obese mice is primarily mediated via suppression of collateral growth by Nox2 and p47phox. Am. J. Physiol. Heart Circ. Physiol. 2015;309(7):H1207–H1217. doi: 10.1152/ajpheart.00180.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Baylan U., Korn A., Emmens R.W., et al. Liraglutide treatment attenuates inflammation markers in the cardiac, cerebral and renal microvasculature in streptozotocin-induced diabetic rats. Eur. J. Clin. Invest. 2022;52(9) doi: 10.1111/eci.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.