Abstract
Cardiolipin is a unique phospholipid of the inner mitochondrial membrane (IMM) as well as in bacteria. It performs several vital functions such as resisting osmotic rupture and stabilizing the supramolecular structure of large membrane proteins, like ATP synthases and respirasomes. The process of cardiolipin biosynthesis results in the production of immature cardiolipin. A subsequent step is required for its maturation when its acyl groups are replaced with unsaturated acyl chains, primarily linoleic acid. Linoleic acid is the major fatty acid of cardiolipin across all organs and tissues, except for the brain. Linoleic acid is not synthesized by mammalian cells. It has the unique ability to undergo oxidative polymerization at a moderately accelerated rate compared to other unsaturated fatty acids. This property can enable cardiolipin to form covalently bonded net-like structures essential for maintaining the complex geometry of the IMM and gluing the quaternary structure of large IMM protein complexes. Unlike triglycerides, phospholipids possess only two covalently linked acyl chains, which constrain their capacity to develop robust and complicated structures through oxidative polymerization of unsaturated acyl chains. Cardiolipin, on the other hand, has four fatty acids at its disposal to form covalently bonded polymer structures. Despite its significance, the oxidative polymerization of cardiolipin has been overlooked due to the negative perception surrounding biological oxidation and methodological difficulties. Here, we discuss an intriguing hypothesis that oxidative polymerization of cardiolipin is essential for the structure and function of cardiolipin in the IMM in physiological conditions. In addition, we highlight current challenges associated with the identification and characterization of oxidative polymerization of cardiolipin in vivo. Altogether, the study provides a better understanding of the structural and functional role of cardiolipin in mitochondria.
Keywords: Mitochondria, Cardiolipin, Linoleic acid, Oxidative polymerization
Graphical abstract
1. Introduction
Cardiolipin has been the subject of extensive research, with a focus on its crucial role in cellular function and pathology, particularly in mitochondrial function. While many roles of cardiolipin have been investigated, the molecular mechanisms involved in the function of cardiolipin remain poorly understood. To spotlight this problem, we have compiled a set of findings that highlight the significance of four acyl chains in cardiolipin molecules, with a major focus on the role of linoleic acid.
2. Structure of cardiolipin
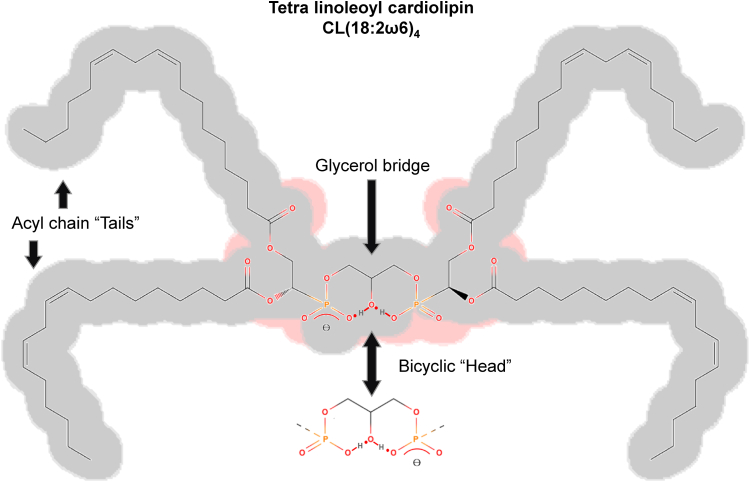
Cardiolipin has a unique structure of dimeric phospholipids connected on the head by glycerol (Fig. 1). It was first isolated from beef hearts in the early 1940s by Mary C. Pangburn [1]. Despite its name, cardiolipin is ubiquitous in all organs/tissues; it is considered the signature phospholipid in the inner mitochondrial membrane (IMM) [2,3] and plasma membrane of bacteria [4]. The amount of cardiolipin in the IMM can vary depending on the organism, tissue type, and physiological state. However, it is estimated that cardiolipin makes up about 20% of the total lipid composition of the IMM in mammals [5]. The presence of cardiolipin in prokaryotes and mitochondria in eukaryotes has been used as an argument for the endosymbiotic hypothesis [6].
Fig. 1.
Structure of cardiolipin. Cardiolipin possesses a distinctive structure consisting of dimeric phospholipids linked by a glycerol moiety at the head group. The predominant acyl chain constituent of mammalian cardiolipin in cells of natural organisms is linoleic acid (18:2ω6). At neutral pH, cardiolipin exhibits a single positive charge, as a single proton becomes sequestered within a bicyclic resonance structure generated by the two phosphates and the central hydroxyl group.
The unique feature of cardiolipin structure, the presence of two phosphate groups can provide two negative charges, a fact that may become important for protein cross-links and protein-protein interactions in general. However, in aqueous dispersions with neutral pH, cardiolipin contains a single charge only, because one proton gets trapped in a bicyclic resonance structure (Fig. 1) formed by the two phosphates and the central hydroxyl group [4,7,8]. It was suggested that cardiolipin as a proton trap may aggregate the oxidative phosphorylation proteins into a patch while it restricts pumped protons within its proximity supplying protons to the ATP synthase with minimal changes in the bulk phase pH [9].
3. Synthesis and maturation of cardiolipin
The biosynthesis of cardiolipin occurs in mitochondria. Cardiolipin synthase joins cytidine diphosphate diacylglycerol (CDP-DAG), a central lipid intermediate for several pathways, and phosphatidylglycerol, a polar membrane lipid, results in unmatured cardiolipin and cytidine monophosphate (CMP) [4]. Mammalian cardiolipin synthesis is reviewed in detail elsewhere [8]. The acyl chain composition of cardiolipin is not controlled by the substrate specificity of their biosynthesis [10], and clear dependency of the cardiolipin composition from nutritionally available lipids is known [11].
Cardiolipin maturation refers to the process by which the acyl chains of cardiolipin molecules are replaced after their initial synthesis. This process is crucial for cardiolipin to be fully functional. First, cardiolipin is deacylated to monolyso-cardiolipin by cardiolipin-specific lipase including Cld1 [12]. Monolysocardiolipin acyltransferase (MLCLAT1) on the matrix-leaflet of the IMM, tafazzin (phospholipid-lysophospholipid transacylase) on the intermembrane-space-facing leaflets of the inner and outer mitochondrial membrane, and acyl-CoA:lysocardiolipin acyltransferase-1 (ALCAT1) on the mitochondria-associated-membranes of endoplasmic reticulum all can re-acylate monolyso-cardiolipins [8]. Among these acyl transfer enzymes, tafazzin is the only member of this enzyme family that is conserved throughout evolution from yeast to higher eukaryotes [8]. Barth's syndrome is characterized by an alteration in the enzyme tafazzin, which is mainly responsible for the maturation of cardiolipin. This alteration results in the buildup of monolyso-cardiolipins that have lost one of their four acyl chains. Tafazzin is a cardiolipin remodeling enzyme, which replaces acyl groups of cardiolipin with mainly linoleic acid (18:2) in animal tissues. Unlike other two remodeling enzymes which transfer acyl chains from acyl-CoA [8], tafazzin transfers fatty acids from phospholipids to lysophospholipids, and transacylation activities were about 10-fold higher for linoleoyl (18:2) groups than for oleoyl (18:1) groups, and they were negligible for arachidonoyl (20:4) groups [13]. Tafazzin deficiency induced a significant decrease in the abundance of cardiolipin-containing linoleoyl groups (72:8, tetralinoleoyl cardiolipin) in the heart of tafazzin knockdown mice [14].
Linoleic acid is a major fatty acid of cardiolipin in animal tissues [15,16]. Initial studies reported that rat liver mitochondria comprise 59% linoleic acid [17], while later studies showed heart and liver cardiolipins almost exclusively contain linoleic acid [18]. The brain was an exception; in mouse and pig brain cardiolipin, oleic acid (18:1) was found to be a major fatty acid, in addition to the longer chain unsaturated fatty acids including arachidonic acid (20:4) and docosahexaenoic acid (DHA, 22:6) [18].
The remodeling (maturation) of cardiolipin is crucial to its function. A high proportion of saturated cardiolipins in mitochondria led to osmotic instability, and polyunsaturated cardiolipin improves the oxidative capacity of mitochondria [19]. Tafazzin knockout shows similar defects as cardiolipin synthase (CLS) knockout in the supramolecular geometry of FoF1 ATP synthase [20]. Remodeled cardiolipin supports the high concentration of proteins in the IMM [21]. Acyl compositional changes in cardiolipin are important for mitochondrial maturation and cardiolipin remodeling during heart development [22]. The progressive loss of cardiac tetralinoleoyl cardiolipin, possibly attributable to decreased remodeling, occurs in response to chronic cardiac overload [23]. Several studies suggest that promoting cardiolipin content (linked with or independently of mitochondrial biogenesis) or restoring cardiolipin acyl chain remodeling, or integrity towards oxidative stress could help counteract the deleterious effects of obesity [24].
In mammalian cells, linoleic acid (18:2) is the major acyl chain constituent of cardiolipin [18]. On the other hand, the fatty acid profile of cultured cells differs from that of natural cells. They have twice the level of monounsaturated fatty acids and half the level of polyunsaturated fatty acids (PUFAs). This difference is not primarily due to cell lines derived from cancers, but rather due to limited access to lipids and the inability to synthesize PUFAs de novo as vertebrate cells [25]. The acyl chain composition of cardiolipin is dependent on the availability of dietary fats [26,27]. In contrast to mammalian cells, mature cardiolipin species in laboratory-grown yeast primarily consist of palmitoleic acid (16:1) and oleic acid (18:1). However, when the yeast culture medium is supplemented with linoleic acid (18:2) and arachidonic acid (20:4), PUFAs are incorporated into yeast cardiolipin [28]. Fatty acids of cardiolipin are responsive to changes in dietary lipids in the liver, heart [29], and skeletal muscle [30]. Interestingly, clinical testing of dietary interventions for Barth syndrome has not been conducted thus far. However, it has been observed that supplemental linoleic acid attenuated, to some extent, the impaired contractile phenotype in vitro in tafazzin knocked-down soleus [31]. Furthermore, the supplementation of linoleic acid in Barth syndrome fibroblasts restored cardiolipin levels [32], and the use of linoleic acid-rich oil altered circulating cardiolipin species and fatty acid composition in adults [33].
4. Role of cardiolipin in the IMM structure
Cardiolipin exists almost exclusively in the IMM [34]. In isolated mitochondria, the outer membrane can be easily ruptured by hypotonic shock revealing a mitoplast, its IMM is exposed but still intact [35]. The IMM is generally tougher and more mechanically resistant than the outer membrane. This is surprising because the IMM contains an unusually high percentage (greater than 70%) of proteins [36]. Cardiolipin can protect IMM from osmotic rupture, as shown in liposomes [37]. By using cryo-electron microscopy, IMM proteins are found to be non-randomly arranged and IMM may be resistant to freeze-fracture [38]. Apparently, non-covalent interactions alone are not sufficient to support a robust and dynamic structure of the IMM. Liposomal membranes have a simple structure and are smaller in size [39], and cannot completely mimic mitochondria which possess a complex morphology and physicochemical characteristics.
In Escherichia coli, cardiolipin in the membrane increase in response to osmotic stress [40]. Cardiolipin in Bacillus subtilis protects its membrane against surfactin-induced permeabilization [41]. Obvious cardiolipin domains were discovered at the poles and septa in bacteria [40,42], and cardiolipins are believed to stabilize highly curved parts of the IMM [43]. Cardiolipins and phosphatidylethanolamines are known to generate negative curvature elastic stress in bilayers [42,[44], [45], [46]]. Cardiolipin directly interacts with Mic27, which is vital for the assembly of itself into the mitochondrial contact site and cristae organizing system (MICOS) complex [47,48]. Isolated heart mitochondria from tafazzin knockdown mice showed increased basal swelling in the absence of external calcium overload [49]. Mitochondrial swelling due to increased IMM permeability to ions through various mechanisms plays an important role in physiological and pathological conditions [50,51]. Accordingly, there is a crosstalk between mitochondrial swelling and IMM structural proteins such as OPA1 [52] which plays an essential role in the structural remodeling of cristae membrane [53]. Cardiolipin participates in MICOS assembly [54] and through specific binding to OPA1 facilitates interactions between OPA1 molecules during mitochondrial fusion [55].
5. Stabilizing quaternary structure of membrane protein complex by cardiolipin
Schlame et al., pointed out the ubiquitous nature of cardiolipin-protein interactions, stating that many structurally unrelated proteins can engage in a strong binding with cardiolipin [56]. The role of cardiolipin in the stability of integral membrane proteins is reviewed elsewhere [57]. Using 2D-DIGE and iTRAQ analyses, we have shown that the expression of mitochondrial proteins, including IMM proteins was significantly affected in the hearts of tafazzin knockdown mice [58]. Cardiolipin stabilizes respiratory chain supercomplexes in yeast [59], as cryo-EM analysis of yeast complex suggested possible cardiolipin density between complex III and IV [60]. Complexes III and IV associate to form respiratory supercomplexes. Both homodimeric supercomplexes contain tightly associated cardiolipin required for function [61] and the removal of tightly bound cardiolipin results in the destabilization of the quaternary structure of bovine heart cytochrome c oxidase [62]. Cardiolipin is critical for the degree of oligomerization and the degree of order in ATP synthase assemblies [20]. Cardiolipin was found to be crucial for the structural stability of respirasomes, which is essential for maintaining the health of cells and tissues [63]. We have shown the mitochondria of mice whose cardiolipin remodeling enzyme tafazzin was knocked down, demonstrated greatly reduced respirasome without changes of individual complexes [49]. Cardiolipin is also essential for mitochondrial calcium uniporter stability and activity [64]. Kumar and coauthors observed Erwinia ligand-gated ion channel (ELIC) in its native environment with detergent-free cryo-EM. They found ELIC-bound cardiolipin found to be in a non-bilayer conformation. One of the two 1,2-acylated glycerol moieties is “buried” well below the line expected to be occupied by the outer leaflet glycerol groups, and two acyl chains nearly reach the opposite side of the membrane, whereas the other two acyl chains “curve away” from an axis perpendicular to the bilayer plane [65]. Interestingly, the cardiolipin remodeling enzyme tafazzin is found to be an integral component of respiratory complex I assembly nucleation [66].
6. Challenges in elucidating the IMM structure in vivo
Elucidation of the IMM structure at the molecular level still remains challenging and high-resolution techniques such as electron microscopy and mass spectrometry have several weaknesses that limit interpretations of structural and functional data. These techniques use several steps including isolation, solubilization, and/or fixation, which can alter and exclude target structures. In favor of this, orderly arranged IMM proteins were observed only when snap freeze rather than fixed tissues were used [38,67]. Notably, freezing per se also disrupts cell structure if the process of freezing is not fast enough [68]. The pitfalls associated with sample preparation for mitochondrial research have been discussed earlier [69,70]. Cryo-electron microscopy is a powerful method to reveal the structure of quaternary structures of membrane proteins, but it requires the solubilization of target proteins with detergents that mimic lipids interacting with the proteins [70]. The structure of a mitochondrial ATP synthase with bound native cardiolipin has been resolved, but acyl tails of lipids were truncated according to map density [71]. McAuley et al., investigated the interaction between cardiolipin and the photoreaction center using X-ray crystallography, the ends of the acyl chains were not resolved in the electron density, presumably because they were mobile and therefore disordered [72]. Crystallography of a membrane protein provides a static view in the context of a detergent micelle rather than in a membrane [73]. Although cardiolipin head is often found interacting with membrane proteins with these methods, their fatty acyl groups were rarely fully resolved, since electron density was present only for the acyl chains close to the headgroup [73].
Analysis of cardiolipin and its oxidation uses LC-MS which requires a liquid phase prior to ionization [74,75]. Polymerized cardiolipins are expected to form high-molecular-weight aggregates that are likely to be excluded by these steps, and the ionized fragments of these polymers would likely result in complicated rather than easily identifiable fragments. Schlame et al. (2001) resolved homologous series of oxidized cardiolipins by mass spectrometry, but only after in vitro oxidation of pure cardiolipin [76]. Imaging mass spectrometry using direct ionization without a liquid phase is available, but the resolution is not enough to resolve mitochondria from other organelles and still needs fixation [77]. Oxidative polymerization is rarely considered in membrane molecular dynamics simulations [46,78,79]. The introduction of acyl chain polymerization into membrane simulations would greatly complicate calculations.
7. Cardiolipin in pathology
The various aspects of cardiolipin deficiency in patients with Barth syndrome and tafazzin knockdown mice have been reviewed elsewhere [80,81]. In addition to Barth syndrome, which develops due to a deficiency of tafazzin, the enzyme responsible for cardiolipin remodeling [82], cardiolipin is also involved in various cellular stress and death pathways in pathological conditions. Anti-cardiolipin antibodies (ACA) are autoantibodies that target cardiolipin in platelets, endothelial cells, and the heart. These antibodies can cause blood clots, leading to a condition called antiphospholipid syndrome (APS), which is associated with thrombotic events such as deep vein thrombosis, pulmonary embolism, stroke, and myocardial infarction [83]. In addition to its thrombotic effects, ACA can also cause pregnancy complications such as recurrent miscarriages, pre-eclampsia, and fetal growth restriction [84]. The exact mechanisms by which ACA contribute to these pathological processes are not fully understood, but they may involve the activation of platelets and the coagulation system, as well as interference with endothelial function [85]. Low cardiolipin content associated with impaired mitochondrial function has been reported in the animal model of Alzheimer's disease [86], as well as other pathological conditions including amyotrophic lateral sclerosis (ALS) [87], traumatic brain injury (TBI) [88], and synaptosomes derived from aged animals [86]. The effects of global ischemia and cardiopulmonary resuscitation resulted in selective oxidation and hydrolysis of cardiolipins, lyso-cardiolipins, and oxygenated free fatty acids accumulation, caspase 3/7 activation in the brain, and resulting motor and cognitive dysfunction [89]. Alterations of cardiolipin played a crucial role in mitochondrial dysfunction and neuronal death following spinal cord injury (SCI), and pharmacological inhibition of cardiolipin alterations showed a potential therapeutic target for ameliorating secondary SCI [90]. Deuterated PUFAs protected against oxidative stress-induced cell injury by inhibiting lipid peroxidation and preserving mitochondrial bioenergetics function, demonstrating their therapeutic potential in preventing disorders associated with PUFA peroxidation [91]. Elamipretide, a cardiolipin-binding peptide, improved structural and functional integrity of mitochondria in the heart subjected to ischemia-reperfusion. The peptide enhanced ETC activity, cristae network integrity, and biomimetic membrane properties [92].
The role of cardiolipin in cell death signaling is reviewed elsewhere [93]. The externalization of cardiolipin to the outer mitochondrial membrane is an important mechanism for identifying damaged or dysfunctional mitochondria and targeting them for elimination via mitophagy in neuronal cells [94]. Drp1, a cytosolic protein involved in stress-induced mitochondrial fission, contains a conserved cardiolipin-binding motif [95]. On the other hand, a cardiolipin-bound form of cytochrome c is thought to initiate apoptosis via a lipid transfer step [96]. Ruan and coauthors identified a positively charged helix that interacts with cardiolipin in gasdermin D, which has a crucial role in pyroptosis as a pore-forming protein [97]. An activated form of gasdermin D moves to the plasma membrane of bacteria where it lyses lipids such as cardiolipin, forming pores during pyroptosis [98]. Cardiolipin was shown to promote the pore-forming activity of alpha-synuclein oligomers in mitochondrial membranes, closely associated with the etiology of Parkinson's disease [99]. Tau protein, a protein responsible for Alzheimer's disease pathology, would interact with their cardiolipin-rich mitochondrial membrane domains, leading to membrane pore formation (independent of mitochondrial permeability transition) and compromised mitochondrial structural integrity [100]. Numerous studies are currently dedicated to investigating cardiolipin integrity as a potential therapeutic target. These efforts aim to develop effective interventions that can mitigate the pathophysiological consequences associated with cardiolipin abnormalities [101].
8. Linoleic acid and oxidative polymerization
Linoleic acid is the major fatty acid of cardiolipin from animal tissues, among other unsaturated fatty acids except in the brain [15,16]. It does not make sense that mitochondria, which is the center of crucial redox reactions, favor oxidation-labile PUFA in its crucial structural component, not to mention the majority of them cannot even be synthesized by themselves. Many authors have suggested that the acyl chains of cardiolipin are highly unsaturated that may play an important role in membrane fluidity and protein interaction. However, Schlame (2008) pointed out that there is no characteristic degree of unsaturation among cardiolipins from different organisms, and considering its minor proportion (20%) in the IMM, it is hard to hypothesize that cardiolipin would determine the overall fluidity of the membrane [4]. Both saturated and unsaturated fatty acids can be oxidized, and the rate of oxidation increases with the degree of unsaturation of acyl chains. For instance, the oxidation rate of linoleic acid (18:2) is 22 times higher than that of oleic acid (18:1) [102]. Notably, mammalian cells are not able to synthesize linoleic acid. Linoleic acid is mostly found in plant oils, and mammals should obtain it as an essential fatty acid from food, although there is no specific information on the amount of linoleic acid required for correction of the symptoms associated with its deficiency [103].
9. Oxidative polymerization of linolenic acid may be vital for the structural function of cardiolipin
What structural, metabolic, and functional features of linoleic acid make it unique? One of the well-characterized linoleic acid pathways is the biosynthesis of prostaglandins via arachidonic acid (20:4) [104,105]. Besides biology, linoleic acid is well known for being a key component of drying oils and frying oils. In paint and varnishes, linoleic acid containing triglycerides underwent oxidative polymerization (Fig. 2) resulting in a covalently bonded 2-D net-like structure [106]. The oxidative polymerization of PUFAs is a chemical process that occurs when they are exposed to oxidative conditions, including oxygen, heat, or metal catalysts. This reaction leads to the formation of polymerized products or degraded byproducts depending on the extent of the reaction [107]. In frying oils, the amount of linoleic acid (C18:2) tends to diminish during the heating time, indicating that the PUFA chains present in triglycerides are primarily responsible for the polymerization reaction of triglycerides [108]. The unique property of linoleic acid may be that its unsaturation falls within an optimal range that allows it to undergo oxidative polymerization rather than excessive oxidative degradation [109], especially considering the high local concentration of acyl chains and antioxidants in the membrane. With oxidation catalysts, most antioxidants and amino acids enhance lipid polymerization [110]. Several studies identified linoleic acid (an omega-6 fatty acid) as a potential pro-inflammatory and cardiovascular health risk factor. This is because the pathways by which it is converted into inflammatory eicosanoids are well-characterized. However, this hypothesis was not supported by randomized controlled feeding studies [111]. Epidemiologic studies provided little if any evidence that linoleic acid contributes to cardiovascular disease, cancer, or inflammation although where the inverse correlations may exist [112,113].
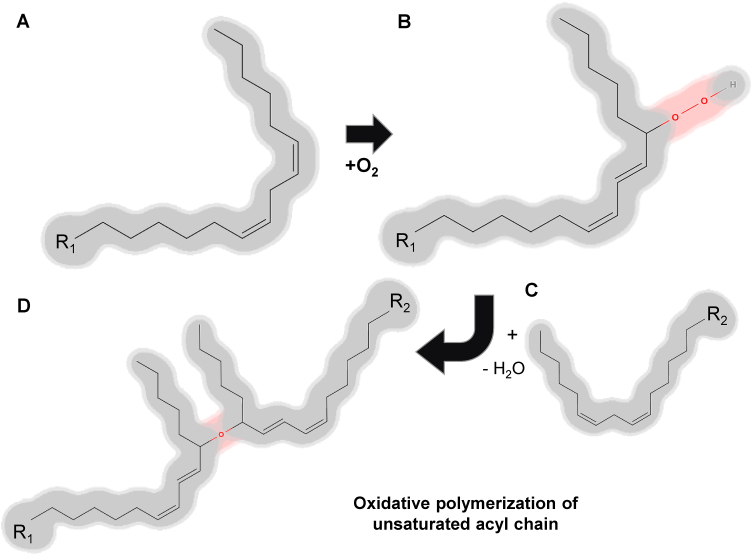
Fig. 2.
Oxidative polymerization of the unsaturated acyl chain. The oxidative polymerization of unsaturated fatty acids (A) is a consequence of autoxidation, which involves the addition of oxygen to the unsaturated fatty acyl chain (B) followed by subsequent crosslinking (C). Initially, an oxygen molecule is incorporated into carbon-hydrogen (C–H) bonds that are adjacent to one of the double bonds present within the unsaturated fatty acid (B). The resultant hydroperoxides are prone to undergo crosslinking reactions, whereby bonds are established between neighboring fatty acyl chains (C), leading to the formation of a polymer network (D).
Oxidative polymerization of the linoleoyl chain may help explain the mechanism of function of cardiolipin in the IMM under physiological conditions. In contrast to triglycerides, phospholipids possess only two covalently linked acyl chains, which constrain their capacity to develop robust and complicated structures through oxidative polymerization. Cardiolipin, on the other hand, has four acyl chains at its disposal (Fig. 3). Polymerized cardiolipin may be able to reinforce the mechanical structure of the IMM thereby, providing covalently bonded net-like structures and gluing membrane proteins into quaternary structures and lipid bilayers which is essential to normal mitochondrial function. We observed high levels (over 70%) of oxidized cardiolipin in untreated hearts by quantitative analysis of cardiolipin using high-resolution mass spectrometry [49] suggesting that the oxidized cardiolipin may play a vital role in healthy mitochondria.
Fig. 3.
The advantage of cardiolipin in oxidative polymerization. Phospholipids (A) have a limitation in forming complex and sturdy structures via oxidative polymerization due to their possession of only two covalently linked acyl chains, in comparison to triglycerides (B). However, cardiolipin (C) contains four acyl chains, thus allowing for the development of a strong and intricate polymer structure. The potential site of crosslinking via unsaturated bonds was highlighted in green. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Human cells generally function in a reduced state, but some degree of localized oxidation is needed [114]. We showed that increased oxidative stress does not stimulate mitochondria swelling [115]. Extensive research on lipid oxidation has traditionally emphasized the toxic degradative pathway of fatty acids, however, given the high local concentration of unsaturated acyl chains and availability of antioxidants in membranes, polymerization is likely to be a favored pathway for linoleoyl chain [106,116] prior to further oxidation into the toxic degradation (Fig. 4), which underscores the need for further investigation into this topic. The “antioxidant paradox” is a term commonly used to describe the discrepancy between the involvement of reactive oxygen species in various human diseases and the lack of significant preventive or therapeutic outcomes observed in most studies involving the administration of high doses of dietary antioxidants to human subjects [114].
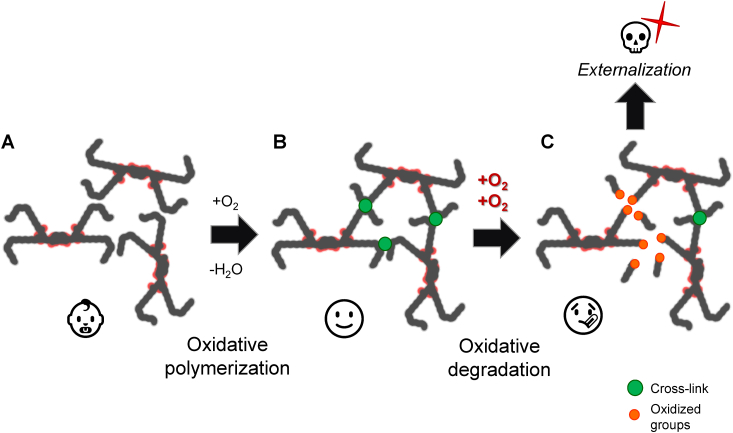
Fig. 4.
Oxidative polymerization of cardiolipin: physiological versus pathological oxidation. Oxidative polymerization of cardiolipin facilitates the creation of a covalently linked, mechanically robust structure (B) than without it (A). However, excessive or pathological oxidation of cardiolipin can result in the breakage of acyl chains, leading to the release of toxic lipid peroxidation products (C). The loss and externalization of cardiolipin can serve as a death signal, contributing to programmed cell death (apoptosis) and other forms of cellular demise. Proper regulation of cardiolipin oxidation may be crucial for maintaining mitochondria integrity. Other membrane components are omitted for simplicity.
10. Conclusions and future perspectives
In conclusion, we develop an intriguing hypothesis that the molecular mechanism underlying the structural function of cardiolipin can be attributed to the oxidative polymerization of unsaturated acyl chains, particularly linoleic acid under physiological conditions. The four acyl chains present in cardiolipin offer a unique advantage over other phospholipids with only two acyl chains, as they can form significantly stronger and intricate covalently bonded structures via oxidative polymerization. Despite the potential significance of this mechanism, it has been largely overlooked in the field of biology due to the methodological challenges associated with studying it and the common negative perception of oxidation. However, more advanced structural studies are required to recognize and investigate the potential role of oxidative polymerization of cardiolipin. The exploration of cardiolipin's biological function and its impact on maintaining mitochondrial function and health is crucial to advancing our understanding of the fundamentals of biochemistry. Additionally, this research could pave the way for the development of more stable liposomes, which can be utilized in a wide range of medical applications.
Author contributions
Conception and draft writing: Se.J, review and revision of the manuscript: Sa.J, administrative, technical, or material support: Sa.J.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was funded by the National Science Foundation grant 2006477 (awarded to SaJ), and the National Institutes of Health grants R16GM145390 (awarded to SaJ), in part by U54MD007600, R25GM061838 (to UPR-MSC). The authors apologize to all colleagues whose important studies were not cited due to space restrictions.
Data availability
No data was used for the research described in the article.
References
- 1.Pangborn M.C. Isolation and purification of a serologically active phospholipid from beef heart. J. Biol. Chem. 1942;143:247–256. [Google Scholar]
- 2.Hatch G.M. Cardiolipin: biosynthesis, remodeling and trafficking in the heart and mammalian cells (Review) Int. J. Mol. Med. 1998;1(1):33–41. doi: 10.3892/ijmm.1.1.33. [DOI] [PubMed] [Google Scholar]
- 3.Baile M.G., Lu Y.W., Claypool S.M. The topology and regulation of cardiolipin biosynthesis and remodeling in yeast. Chem. Phys. Lipids. 2014;179:25–31. doi: 10.1016/j.chemphyslip.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlame M. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J. Lipid Res. 2008;49(8):1607–1620. doi: 10.1194/jlr.R700018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horvath S.E., Daum G. Lipids of mitochondria. Prog. Lipid Res. 2013;52(4):590–614. doi: 10.1016/j.plipres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Martin W., Hoffmeister M., Rotte C., Henze K. An overview of endosymbiotic models for the origins of eukaryotes, their ATP-producing organelles (mitochondria and hydrogenosomes), and their heterotrophic lifestyle. Biol. Chem. 2001;382(11):1521–1539. doi: 10.1515/BC.2001.187. [DOI] [PubMed] [Google Scholar]
- 7.Kates M., Syz J.Y., Gosser D., Haines T.H. pH-dissociation characteristics of cardiolipin and its 2'-deoxy analogue. Lipids. 1993;28(10):877–882. doi: 10.1007/BF02537494. [DOI] [PubMed] [Google Scholar]
- 8.Lu Y.W., Claypool S.M. Disorders of phospholipid metabolism: an emerging class of mitochondrial disease due to defects in nuclear genes. Front. Genet. 2015;6:3. doi: 10.3389/fgene.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haines T.H., Dencher N.A. Cardiolipin: a proton trap for oxidative phosphorylation. FEBS Lett. 2002;528(1–3):35–39. doi: 10.1016/s0014-5793(02)03292-1. [DOI] [PubMed] [Google Scholar]
- 10.Oemer G., Edenhofer M.L., Wohlfarter Y., Lackner K., Leman G., Koch J., Cardoso L.H.D., Lindner H.H., Gnaiger E., Dubrac S., Zschocke J., Keller M.A. Fatty acyl availability modulates cardiolipin composition and alters mitochondrial function in HeLa cells. J. Lipid Res. 2021;62 doi: 10.1016/j.jlr.2021.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGee C.D., Lieberman P., Greenwood C.E. Dietary fatty acid composition induces comparable changes in cardiolipin fatty acid profile of heart and brain mitochondria. Lipids. 1996;31(6):611–616. doi: 10.1007/BF02523831. [DOI] [PubMed] [Google Scholar]
- 12.Beranek A., Rechberger G., Knauer H., Wolinski H., Kohlwein S.D., Leber R. Identification of a cardiolipin-specific phospholipase encoded by the gene CLD1 (YGR110W) in yeast. J. Biol. Chem. 2009;284(17):11572–11578. doi: 10.1074/jbc.M805511200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y., Malhotra A., Ren M., Schlame M. The enzymatic function of tafazzin. J. Biol. Chem. 2006;281(51):39217–39224. doi: 10.1074/jbc.M606100200. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y., Powers C., Moore V., Schafer C., Ren M., Phoon C.K., James J.F., Glukhov A.V., Javadov S., Vaz F.M., Jefferies J.L., Strauss A.W., Khuchua Z. The PPAR pan-agonist bezafibrate ameliorates cardiomyopathy in a mouse model of Barth syndrome. Orphanet J. Rare Dis. 2017;12(1):49. doi: 10.1186/s13023-017-0605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minkler P.E., Hoppel C.L. Separation and characterization of cardiolipin molecular species by reverse-phase ion pair high-performance liquid chromatography-mass spectrometry. J. Lipid Res. 2010;51(4):856–865. doi: 10.1194/jlr.D002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pennington E.R., Funai K., Brown D.A., Shaikh S.R. The role of cardiolipin concentration and acyl chain composition on mitochondrial inner membrane molecular organization and function. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019;1864(7):1039–1052. doi: 10.1016/j.bbalip.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colbeau A., Nachbaur J., Vignais P.M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim. Biophys. Acta. 1971;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- 18.Oemer G., Lackner K., Muigg K., Krumschnabel G., Watschinger K., Sailer S., Lindner H., Gnaiger E., Wortmann S.B., Werner E.R., Zschocke J., Keller M.A. Molecular structural diversity of mitochondrial cardiolipins. Proc. Natl. Acad. Sci. U. S. A. 2018;115(16):4158–4163. doi: 10.1073/pnas.1719407115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luévano-Martínez L.A., Pinto I.F.D., Yoshinaga M.Y., Miyamoto S. In yeast, cardiolipin unsaturation level plays a key role in mitochondrial function and inner membrane integrity. Biochim. Biophys. Acta Bioenerg. 2022;1863(7) doi: 10.1016/j.bbabio.2022.148587. [DOI] [PubMed] [Google Scholar]
- 20.Acehan D., Malhotra A., Xu Y., Ren M., Stokes D.L., Schlame M. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys. J. 2011;100(9):2184–2192. doi: 10.1016/j.bpj.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Y., Erdjument-Bromage H., Phoon C.K.L., Neubert T.A., Ren M., Schlame M. Cardiolipin remodeling enables protein crowding in the inner mitochondrial membrane. EMBO J. 2021;40(23) doi: 10.15252/embj.2021108428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao H., Li B., Wang Z., Mu J., Tian Y., Jiang B., Zhang S., Gong X., Shui G., Lam S.M. Lipidome atlas of the developing heart uncovers dynamic membrane lipid attributes underlying cardiac structural and metabolic maturation. Research. 2022;2022 doi: 10.34133/research.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sparagna G.C., Chicco A.J., Murphy R.C., Bristow M.R., Johnson C.A., Rees M.L., Maxey M.L., McCune S.A., Moore R.L. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J. Lipid Res. 2007;48(7):1559–1570. doi: 10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Prola A., Pilot-Storck F. Cardiolipin alterations during obesity: exploring therapeutic opportunities. Biology (Basel) 2022;11(11) doi: 10.3390/biology11111638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Else P.L. The highly unnatural fatty acid profile of cells in culture. Prog. Lipid Res. 2020;77 doi: 10.1016/j.plipres.2019.101017. [DOI] [PubMed] [Google Scholar]
- 26.Ji J., Greenberg M.L. Cardiolipin function in the yeast S. cerevisiae and the lessons learned for Barth syndrome. J. Inherit. Metab. Dis. 2022;45(1):60–71. doi: 10.1002/jimd.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley R.M., Stark K.D., Duncan R.E. Influence of tissue, diet, and enzymatic remodeling on cardiolipin fatty acyl profile. Mol. Nutr. Food Res. 2016;60(8):1804–1818. doi: 10.1002/mnfr.201500966. [DOI] [PubMed] [Google Scholar]
- 28.Tyurina Y.Y., Lou W., Qu F., Tyurin V.A., Mohammadyani D., Liu J., Hüttemann M., Frasso M.A., Wipf P., Bayir H., Greenberg M.L., Kagan V.E. Lipidomics characterization of biosynthetic and remodeling pathways of cardiolipins in genetically and nutritionally manipulated yeast cells. ACS Chem. Biol. 2017;12(1):265–281. doi: 10.1021/acschembio.6b00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feillet-Coudray C., Fouret G., Casas F., Coudray C. Impact of high dietary lipid intake and related metabolic disorders on the abundance and acyl composition of the unique mitochondrial phospholipid, cardiolipin. J. Bioenerg. Biomembr. 2014;46(5):447–457. doi: 10.1007/s10863-014-9555-y. [DOI] [PubMed] [Google Scholar]
- 30.Fajardo V.A., McMeekin L., Saint C., LeBlanc P.J. Cardiolipin linoleic acid content and mitochondrial cytochrome c oxidase activity are associated in rat skeletal muscle. Chem. Phys. Lipids. 2015;187:50–55. doi: 10.1016/j.chemphyslip.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Elkes M., Andonovski M., Vidal D., Farago M., Modafferi R., Claypool S.M., LeBlanc P.J. The influence of supplemental dietary linoleic acid on skeletal muscle contractile function in a Rodent model of barth syndrome. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.731961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valianpour F., Wanders R.J., Overmars H., Vaz F.M., Barth P.G., van Gennip A.H. Linoleic acid supplementation of Barth syndrome fibroblasts restores cardiolipin levels: implications for treatment. J. Lipid Res. 2003;44(3):560–566. doi: 10.1194/jlr.M200217-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Cole R.M., Angelotti A., Sparagna G.C., Ni A., Belury M.A. Linoleic acid-rich oil alters circulating cardiolipin species and fatty acid composition in adults: a randomized controlled trial. Mol. Nutr. Food Res. 2022;66(15) doi: 10.1002/mnfr.202101132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X.X., Tsoi B., Li Y.F., Kurihara H., He R.R. Cardiolipin and its different properties in mitophagy and apoptosis. J. Histochem. Cytochem. 2015;63(5):301–311. doi: 10.1369/0022155415574818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burnette B., Batra P.P. A combination of methods for the preparation of highly intact mitoplasts from beef heart mitochondria. Anal. Biochem. 1985;145(1):80–86. doi: 10.1016/0003-2697(85)90329-x. [DOI] [PubMed] [Google Scholar]
- 36.Krauss S. 2001. Mitochondria: Structure and Role in Respiration. [Google Scholar]
- 37.Nagamachi E., Hirai Y., Tomochika K., Kanemasa Y. Studies on osmotic stability of liposomes prepared with bacterial membrane lipids by carboxyfluorescein release. Microbiol. Immunol. 1992;36(3):231–234. doi: 10.1111/j.1348-0421.1992.tb01661.x. [DOI] [PubMed] [Google Scholar]
- 38.Allen R.D., Schroeder C.C., Fok A.K. An investigation of mitochondrial inner membranes by rapid-freeze deep-etch techniques. J. Cell Biol. 1989;108(6):2233–2240. doi: 10.1083/jcb.108.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akbarzadeh A., Rezaei-Sadabady R., Davaran S., Joo S.W., Zarghami N., Hanifehpour Y., Samiei M., Kouhi M., Nejati-Koshki K. Liposome: classification, preparation, and applications. Nanoscale Res. Lett. 2013;8(1):102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romantsov T., Guan Z., Wood J.M. Cardiolipin and the osmotic stress responses of bacteria. Biochim. Biophys. Acta. 2009;1788(10):2092–2100. doi: 10.1016/j.bbamem.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinkas D., Fišer R., Kozlík P., Dolejšová T., Hryzáková K., Konopásek I., Mikušová G. Bacillus subtilis cardiolipin protects its own membrane against surfactin-induced permeabilization. Biochim. Biophys. Acta Biomembr. 2020;1862(10) doi: 10.1016/j.bbamem.2020.183405. [DOI] [PubMed] [Google Scholar]
- 42.Renner L.D., Weibel D.B. Cardiolipin microdomains localize to negatively curved regions of Escherichia coli membranes. Proc. Natl. Acad. Sci. U. S. A. 2011;108(15):6264–6269. doi: 10.1073/pnas.1015757108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beltrán-Heredia E., Tsai F.C., Salinas-Almaguer S., Cao F.J., Bassereau P., Monroy F. Membrane curvature induces cardiolipin sorting. Commun. Biol. 2019;2:225. doi: 10.1038/s42003-019-0471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teague W.E., Jr., Soubias O., Petrache H., Fuller N., Hines K.G., Rand R.P., Gawrisch K. Elastic properties of polyunsaturated phosphatidylethanolamines influence rhodopsin function. Faraday Discuss. 2013;161:383–395. doi: 10.1039/c2fd20095c. discussion 419-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikon N., Ryan R.O. Cardiolipin and mitochondrial cristae organization. Biochim. Biophys. Acta Biomembr. 2017;1859(6):1156–1163. doi: 10.1016/j.bbamem.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson B.A., Ramanathan A., Lopez C.F. Cardiolipin-dependent properties of model mitochondrial membranes from molecular simulations. Biophys. J. 2019;117(3):429–444. doi: 10.1016/j.bpj.2019.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong J., Ye F., Lin J., He H., Song Z. The metabolism and function of phospholipids in Mitochondria. Mitochondrial Commun. 2023;1:2–12. [Google Scholar]
- 48.Anand R., Kondadi A.K., Meisterknecht J., Golombek M., Nortmann O., Riedel J., Peifer-Weiß L., Brocke-Ahmadinejad N., Schlütermann D., Stork B. MIC26 and MIC27 cooperate to regulate cardiolipin levels and the landscape of OXPHOS complexes. Life Sci. Alliance. 2020;3(10) doi: 10.26508/lsa.202000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jang S., Lewis T.S., Powers C., Khuchua Z., Baines C.P., Wipf P., Javadov S. Elucidating mitochondrial electron transport chain supercomplexes in the heart during ischemia-reperfusion. Antioxidants Redox Signal. 2017;27(1):57–69. doi: 10.1089/ars.2016.6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szabo I., Zoratti M. Mitochondrial channels: ion fluxes and more. Physiol. Rev. 2014;94(2):519–608. doi: 10.1152/physrev.00021.2013. [DOI] [PubMed] [Google Scholar]
- 51.Javadov S., Chapa-Dubocq X., Makarov V. Different approaches to modeling analysis of mitochondrial swelling. Mitochondrion. 2018;38:58–70. doi: 10.1016/j.mito.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jang S., Javadov S. OPA1 regulates respiratory supercomplexes assembly: the role of mitochondrial swelling. Mitochondrion. 2020;51:30–39. doi: 10.1016/j.mito.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cogliati S., Frezza C., Soriano M.E., Varanita T., Quintana-Cabrera R., Corrado M., Cipolat S., Costa V., Casarin A., Gomes L.C., Perales-Clemente E., Salviati L., Fernandez-Silva P., Enriquez J.A., Scorrano L. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell. 2013;155(1):160–171. doi: 10.1016/j.cell.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedman J.R., Mourier A., Yamada J., McCaffery J.M., Nunnari J. MICOS coordinates with respiratory complexes and lipids to establish mitochondrial inner membrane architecture. Elife. 2015;4 doi: 10.7554/eLife.07739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ban T., Ishihara T., Kohno H., Saita S., Ichimura A., Maenaka K., Oka T., Mihara K., Ishihara N. Molecular basis of selective mitochondrial fusion by heterotypic action between OPA1 and cardiolipin. Nat. Cell Biol. 2017;19(7):856–863. doi: 10.1038/ncb3560. [DOI] [PubMed] [Google Scholar]
- 56.Schlame M., Rua D., Greenberg M.L. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 2000;39(3):257–288. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- 57.Musatov A., Sedlák E. Role of cardiolipin in stability of integral membrane proteins. Biochimie. 2017;142:102–111. doi: 10.1016/j.biochi.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 58.Huang Y., Powers C., Madala S.K., Greis K.D., Haffey W.D., Towbin J.A., Purevjav E., Javadov S., Strauss A.W., Khuchua Z. Cardiac metabolic pathways affected in the mouse model of barth syndrome. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0128561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfeiffer K., Gohil V., Stuart R.A., Hunte C., Brandt U., Greenberg M.L., Schägger H. Cardiolipin stabilizes respiratory chain supercomplexes. J. Biol. Chem. 2003;278(52):52873–52880. doi: 10.1074/jbc.M308366200. [DOI] [PubMed] [Google Scholar]
- 60.Rathore S., Berndtsson J., Marin-Buera L., Conrad J., Carroni M., Brzezinski P., Ott M. Cryo-EM structure of the yeast respiratory supercomplex. Nat. Struct. Mol. Biol. 2019;26(1):50–57. doi: 10.1038/s41594-018-0169-7. [DOI] [PubMed] [Google Scholar]
- 61.Zhang M., Mileykovskaya E., Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J. Biol. Chem. 2002;277(46):43553–43556. doi: 10.1074/jbc.C200551200. [DOI] [PubMed] [Google Scholar]
- 62.Sedlák E., Robinson N.C. Destabilization of the quaternary structure of bovine heart cytochrome c oxidase upon removal of tightly bound cardiolipin. Biochemistry. 2015;54(36):5569–5577. doi: 10.1021/acs.biochem.5b00540. [DOI] [PubMed] [Google Scholar]
- 63.Wittig I., Schägger H. Supramolecular organization of ATP synthase and respiratory chain in mitochondrial membranes. Biochim. Biophys. Acta. 2009;1787(6):672–680. doi: 10.1016/j.bbabio.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 64.Ghosh S., Basu Ball W., Madaris T.R., Srikantan S., Madesh M., Mootha V.K., Gohil V.M. An essential role for cardiolipin in the stability and function of the mitochondrial calcium uniporter. Proc. Natl. Acad. Sci. U. S. A. 2020;117(28):16383–16390. doi: 10.1073/pnas.2000640117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar P., Cymes G.D., Grosman C. Structure and function at the lipid-protein interface of a pentameric ligand-gated ion channel. Proc. Natl. Acad. Sci. U. S. A. 2021;118(23) doi: 10.1073/pnas.2100164118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schiller J., Laube E., Wittig I., Kühlbrandt W., Vonck J., Zickermann V. Insights into complex I assembly: function of NDUFAF1 and a link with cardiolipin remodeling. Sci. Adv. 2022;8(46) doi: 10.1126/sciadv.add3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cieciura L., Rydzyński K., Pieta P., Klimek I. Freeze-fracture studies on mitochondrial membranes of spermatocytes. Cell Tissue Res. 1986;244(2):437–441. doi: 10.1007/BF00219219. [DOI] [PubMed] [Google Scholar]
- 68.Müllers Y., Meiser I., Stracke F., Riemann I., Lautenschläger F., Neubauer J.C., Zimmermann H. Quantitative analysis of F-actin alterations in adherent human mesenchymal stem cells: influence of slow-freezing and vitrification-based cryopreservation. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0211382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jang S., Chapa-Dubocq X.R., Fossati S., Javadov S. Analysis of mitochondrial calcium retention capacity in cultured cells: permeabilized cells versus isolated mitochondria. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.773839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jang S., Javadov S. Current challenges in elucidating respiratory supercomplexes in mitochondria: methodological obstacles. Front. Physiol. 2018;9:238. doi: 10.3389/fphys.2018.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mühleip A., McComas S.E., Amunts A. Structure of a mitochondrial ATP synthase with bound native cardiolipin. Elife. 2019;8 doi: 10.7554/eLife.51179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McAuley K.E., Fyfe P.K., Ridge J.P., Isaacs N.W., Cogdell R.J., Jones M.R. Structural details of an interaction between cardiolipin and an integral membrane protein. Proc. Natl. Acad. Sci. U. S. A. 1999;96(26):14706–14711. doi: 10.1073/pnas.96.26.14706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duncan A.L., Ruprecht J.J., Kunji E.R.S., Robinson A.J. Cardiolipin dynamics and binding to conserved residues in the mitochondrial ADP/ATP carrier. Biochim. Biophys. Acta Biomembr. 2018;1860(5):1035–1045. doi: 10.1016/j.bbamem.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tyurina Y.Y., Domingues R.M., Tyurin V.A., Maciel E., Domingues P., Amoscato A.A., Bayir H., Kagan V.E. Characterization of cardiolipins and their oxidation products by LC-MS analysis. Chem. Phys. Lipids. 2014;179:3–10. doi: 10.1016/j.chemphyslip.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tyurina Y.Y., Tyurin V.A., Anthonymuthu T., Amoscato A.A., Sparvero L.J., Nesterova A.M., Baynard M.L., Sun W., He R., Khaitovich P., Vladimirov Y.A., Gabrilovich D.I., Bayır H., Kagan V.E. Redox lipidomics technology: looking for a needle in a haystack. Chem. Phys. Lipids. 2019;221:93–107. doi: 10.1016/j.chemphyslip.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schlame M., Haller I., Sammaritano L.R., Blanck T.J. Effect of cardiolipin oxidation on solid-phase immunoassay for antiphospholipid antibodies. Thromb. Haemostasis. 2001;86(6):1475–1482. [PubMed] [Google Scholar]
- 77.Sparvero L.J., Tian H., Amoscato A.A., Sun W.Y., Anthonymuthu T.S., Tyurina Y.Y., Kapralov O., Javadov S., He R.R., Watkins S.C., Winograd N., Kagan V.E., Bayır H. Direct Mapping of Phospholipid Ferroptotic Death Signals in Cells and Tissues by Gas Cluster Ion Beam Secondary Ion Mass Spectrometry (GCIB-SIMS) Angew Chem. Int. Ed. Engl. 2021;60(21):11784–11788. doi: 10.1002/anie.202102001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vähäheikkilä M., Peltomaa T., Róg T., Vazdar M., Pöyry S., Vattulainen I. How cardiolipin peroxidation alters the properties of the inner mitochondrial membrane? Chem. Phys. Lipids. 2018;214:15–23. doi: 10.1016/j.chemphyslip.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 79.Dahlberg M., Maliniak A. Molecular dynamics simulations of cardiolipin bilayers. J. Phys. Chem. B. 2008;112(37):11655–11663. doi: 10.1021/jp803414g. [DOI] [PubMed] [Google Scholar]
- 80.Jiang Z., Shen T., Huynh H., Fang X., Han Z., Ouyang K. Cardiolipin regulates mitochondrial ultrastructure and function in mammalian cells. Genes (Basel) 2022;13(10) doi: 10.3390/genes13101889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dudek J., Hartmann M., Rehling P. The role of mitochondrial cardiolipin in heart function and its implication in cardiac disease. Biochim. Biophys. Acta Mol. Basis Dis. 2019;1865(4):810–821. doi: 10.1016/j.bbadis.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 82.Schlame M., Ren M. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 2006;580(23):5450–5455. doi: 10.1016/j.febslet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 83.Petri M. Antiphospholipid syndrome. Transl. Res. 2020;225:70–81. doi: 10.1016/j.trsl.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Linnemann B. Antiphospholipid syndrome - an update. Vasa. 2018;47(6):451–464. doi: 10.1024/0301-1526/a000723. [DOI] [PubMed] [Google Scholar]
- 85.Huang S., Ninivaggi M., Chayoua W., de Laat B. VWF, platelets and the antiphospholipid syndrome. Int. J. Mol. Sci. 2021;22(8) doi: 10.3390/ijms22084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lores-Arnaiz S., Lombardi P., Karadayian A.G., Cutrera R., Bustamante J. Changes in motor function and brain cortex mitochondrial active oxygen species production in aged mice. Exp. Gerontol. 2019;118:88–98. doi: 10.1016/j.exger.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 87.Chaves-Filho A.B., Pinto I.F.D., Dantas L.S., Xavier A.M., Inague A., Faria R.L., Medeiros M.H.G., Glezer I., Yoshinaga M.Y., Miyamoto S. Alterations in lipid metabolism of spinal cord linked to amyotrophic lateral sclerosis. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-48059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anthonymuthu T.S., Kenny E.M., Hier Z.E., Clark R.S.B., Kochanek P.M., Kagan V.E., Bayır H. Detection of brain specific cardiolipins in plasma after experimental pediatric head injury. Exp. Neurol. 2019;316:63–73. doi: 10.1016/j.expneurol.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ji J., Baart S., Vikulina A.S., Clark R.S., Anthonymuthu T.S., Tyurin V.A., Du L., St Croix C.M., Tyurina Y.Y., Lewis J., Skoda E.M., Kline A.E., Kochanek P.M., Wipf P., Kagan V.E., Bayır H. Deciphering of mitochondrial cardiolipin oxidative signaling in cerebral ischemia-reperfusion. J. Cerebr. Blood Flow Metabol. 2015;35(2):319–328. doi: 10.1038/jcbfm.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu N.K., Deng L.X., Wang M., Lu Q.B., Wang C., Wu X., Wu W., Wang Y., Qu W., Han Q., Xia Y., Ravenscraft B., Li J.L., You S.W., Wipf P., Han X., Xu X.M. Restoring mitochondrial cardiolipin homeostasis reduces cell death and promotes recovery after spinal cord injury. Cell Death Dis. 2022;13(12):1058. doi: 10.1038/s41419-022-05369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Andreyev A.Y., Tsui H.S., Milne G.L., Shmanai V.V., Bekish A.V., Fomich M.A., Pham M.N., Nong Y., Murphy A.N., Clarke C.F., Shchepinov M.S. Isotope-reinforced polyunsaturated fatty acids protect mitochondria from oxidative stress. Free Radic. Biol. Med. 2015;82:63–72. doi: 10.1016/j.freeradbiomed.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 92.Allen M.E., Pennington E.R., Perry J.B., Dadoo S., Makrecka-Kuka M., Dambrova M., Moukdar F., Patel H.D., Han X., Kidd G.K., Benson E.K., Raisch T.B., Poelzing S., Brown D.A., Shaikh S.R. The cardiolipin-binding peptide elamipretide mitigates fragmentation of cristae networks following cardiac ischemia reperfusion in rats. Commun. Biol. 2020;3(1):389. doi: 10.1038/s42003-020-1101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pizzuto M., Pelegrin P. Cardiolipin in immune signaling and cell death. Trends Cell Biol. 2020;30(11):892–903. doi: 10.1016/j.tcb.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 94.Chu C.T., Ji J., Dagda R.K., Jiang J.F., Tyurina Y.Y., Kapralov A.A., Tyurin V.A., Yanamala N., Shrivastava I.H., Mohammadyani D., Wang K.Z.Q., Zhu J., Klein-Seetharaman J., Balasubramanian K., Amoscato A.A., Borisenko G., Huang Z., Gusdon A.M., Cheikhi A., Steer E.K., Wang R., Baty C., Watkins S., Bahar I., Bayir H., Kagan V.E. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat. Cell Biol. 2013;15(10):1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mahajan M., Bharambe N., Shang Y., Lu B., Mandal A., Madan Mohan P., Wang R., Boatz J.C., Manuel Martinez Galvez J., Shnyrova A.V., Qi X., Buck M., van der Wel P.C.A., Ramachandran R. NMR identification of a conserved Drp1 cardiolipin-binding motif essential for stress-induced mitochondrial fission. Proc. Natl. Acad. Sci. U. S. A. 2021;118(29) doi: 10.1073/pnas.2023079118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McMillin J.B., Dowhan W. Cardiolipin and apoptosis. Biochim. Biophys. Acta. 2002;1585(2–3):97–107. doi: 10.1016/s1388-1981(02)00329-3. [DOI] [PubMed] [Google Scholar]
- 97.Ruan J., Xia S., Liu X., Lieberman J., Wu H. Cryo-EM structure of the gasdermin A3 membrane pore. Nature. 2018;557(7703):62–67. doi: 10.1038/s41586-018-0058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salami A., Bettadapura S., Wang S. Gasdermin D kills bacteria. Microbiol. Res. 2023;272 doi: 10.1016/j.micres.2023.127383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ghio S., Camilleri A., Caruana M., Ruf V.C., Schmidt F., Leonov A., Ryazanov S., Griesinger C., Cauchi R.J., Kamp F., Giese A., Vassallo N. Cardiolipin promotes pore-forming activity of alpha-synuclein oligomers in mitochondrial membranes. ACS Chem. Neurosci. 2019;10(8):3815–3829. doi: 10.1021/acschemneuro.9b00320. [DOI] [PubMed] [Google Scholar]
- 100.Camilleri A., Ghio S., Caruana M., Weckbecker D., Schmidt F., Kamp F., Leonov A., Ryazanov S., Griesinger C., Giese A., Cauchi R.J., Vassallo N. Tau-induced mitochondrial membrane perturbation is dependent upon cardiolipin. Biochim. Biophys. Acta Biomembr. 2020;1862(2) doi: 10.1016/j.bbamem.2019.183064. [DOI] [PubMed] [Google Scholar]
- 101.Paradies G., Paradies V., Ruggiero F.M., Petrosillo G. Role of cardiolipin in mitochondrial function and dynamics in health and disease: molecular and pharmacological aspects. Cells. 2019;8(7) doi: 10.3390/cells8070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mao X., Chen W., Huyan Z., Sherazi S.T.H., Yu X. Impact of linolenic acid on oxidative stability of rapeseed oils. J. Food Sci. Technol. 2020;57(9):3184–3192. doi: 10.1007/s13197-020-04349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Trumbo P., Schlicker S., Yates A.A., Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet Assoc. 2002;102(11):1621–1630. doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- 104.Wlodawer P., Samuelsson B. On the organization and mechanism of prostaglandin synthetase. J. Biol. Chem. 1973;248(16):5673–5678. [PubMed] [Google Scholar]
- 105.Christie W.W., Harwood J.L. Oxidation of polyunsaturated fatty acids to produce lipid mediators. Essays Biochem. 2020;64(3):401–421. doi: 10.1042/EBC20190082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Poth U. Ullmann's Encyclopedia of Industrial Chemistry; 2001. Drying oils and related products. [Google Scholar]
- 107.Lazzari M., Chiantore O. Drying and oxidative degradation of linseed oil. Polym. Degrad. Stabil. 1999;65(2):303–313. [Google Scholar]
- 108.Olivares-Carrillo P., Ríos A.P.d.l., Quesada-Medina J., Cifre J.G.H., Baños F.G.D. Viscosity as a measure of oil composition changes due to thermal degradation. Appl. Rheol. 2014;24(5):41–46. [Google Scholar]
- 109.Stenberg C., Svensson M., Johansson M. A study of the drying of linseed oils with different fatty acid patterns using RTIR-spectroscopy and chemiluminescence (CL) Ind. Crop. Prod. 2005;21(2):263–272. [Google Scholar]
- 110.Wolman M. Oxidation of lipids and membranes I: in vitro formation of peroxidative lipid polymers. J. Supramol. Struct. 1975;3(1):80–89. doi: 10.1002/jss.400030109. [DOI] [PubMed] [Google Scholar]
- 111.Farvid M.S., Ding M., Pan A., Sun Q., Chiuve S.E., Steffen L.M., Willett W.C., Hu F.B. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation. 2014;130(18):1568–1578. doi: 10.1161/CIRCULATIONAHA.114.010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Whelan J., Fritsche K. Linoleic acid. Adv. Nutr. 2013;4(3):311–312. doi: 10.3945/an.113.003772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Belury M.A. Linoleic acid, an omega-6 fatty acid that reduces risk for cardiometabolic diseases: premise, promise and practical implications. Curr. Opin. Clin. Nutr. Metab. Care. 2023;26(3):288–292. doi: 10.1097/MCO.0000000000000919. [DOI] [PubMed] [Google Scholar]
- 114.Halliwell B. The antioxidant paradox. Lancet. 2000;355(9210):1179–1180. doi: 10.1016/S0140-6736(00)02075-4. [DOI] [PubMed] [Google Scholar]
- 115.Jang S., Javadov S. Association between ROS production, swelling and the respirasome integrity in cardiac mitochondria. Arch. Biochem. Biophys. 2017;630:1–8. doi: 10.1016/j.abb.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Frankel E.N. Lipid oxidation. Prog. Lipid Res. 1980;19(1–2):1–22. doi: 10.1016/0163-7827(80)90006-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.