Abstract
Plants being sessile in nature, are exposed to unwarranted threats as a result of constantly changing environmental conditions. These adverse factors can have negative impacts on their growth, development, and yield. Hormones are key signaling molecules enabling cells to respond rapidly to different external and internal stimuli. In plants, melatonin (MT) plays a critical role in the integration of various environmental signals and activation of stress-response networks to develop defense mechanisms and plant resilience. Additionally, melatonin can tackle the stress-induced alteration of cellular redox equilibrium by regulating the expression of redox hemostasis-related genes and proteins. The purpose of this article is to compile and summarize the scientific research pertaining to MT's effects on plants' resilience to biotic and abiotic stresses. Here, we have summarized that MT exerts a synergistic effect with other phytohormones, for instance, ethylene, jasmonic acid, and salicylic acid, and activates plant defense-related genes against phytopathogens. Furthermore, MT interacts with secondary messengers like Ca2+, nitric oxide, and reactive oxygen species to regulate the redox network. This interaction triggers different transcription factors to alleviate stress-related responses in plants. Hence, the critical synergic role of MT with diverse plant hormones and secondary messengers demonstrates phytomelatonin's importance in influencing multiple mechanisms to contribute to plant resilience against harsh environmental factors.
Keywords: Melatonin, Redox, Nitric oxide, Hydrogen sulfide, Phytohormones, Plant immunity
Graphical abstract
Highlights
-
•
Melatonin acts as a redox network regulator in plants via regulating secondary messengers signaling.
-
•
Melatonin regulates the activity of redox-sensitive proteins and transcription factors.
-
•
Melatonin influences gene expression and physiological processes in response to stresses.
-
•
Melatonin synergically work with other hormones to confer plant resistance and stress adaptability.
1. Introduction
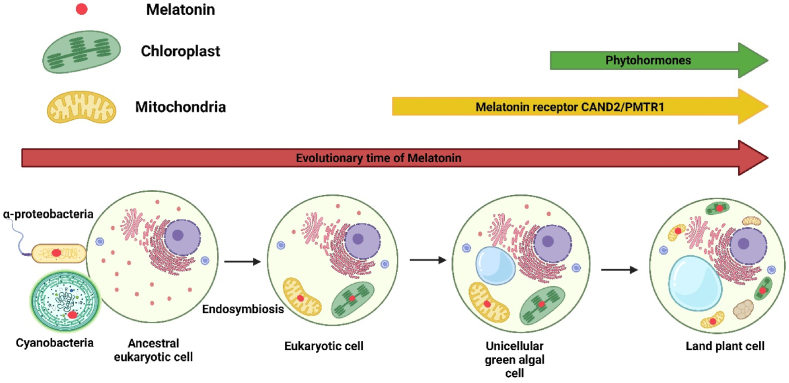
Phytohormones are a class of signaling molecules usually produced in low concentration under control conditions; however, their production level gradually increases in response to stress stimuli [1]. Among various phytohormones, phytomelatonin (N-acetyl-5-methoxytryptamine) has emerged as a novel and universal signaling molecule that influences plant growth and stimulates defense responses against bacterial, viral, and fungal diseases, as well as abiotic stresses. The first report on melatonin's (MT) role in plants was published in 2000 [2]. This study demonstrated that MT is present in certain plant tissues. There are two primary sites for MT synthesis in plants: mitochondria and chloroplasts [3]. According to the evolutionary history of MT, mitochondria probably evolved from a α-proteobacteria, and chloroplasts are possibly related to cyanobacteria (Fig. 1) [3,4]. Based on the evolutionary perspective, it seems that MT kept its primary role as an antioxidant and also acquired various essential signaling transduction functions during plant evolution [5,6]. In fact, MT and its precursors/derivatives could scavenge free radicals through various processes and considered as a potent antioxidant compared with vitamins C and E [7,8].
Fig. 1.
Evolution of phytomelatonin (MT). At the beginning of life on Earth, it is believed that MT was first produced by prokaryotes. During the early stages of evolution, the progenitor cells engulfed cyanobacteria and α-proteobacteria, evolving into mitochondrial and chloroplast-containing plant cells, which were able to produce MT. The MT receptors CAND2/PMTR1 were first originated in single-celled green algae, Coccomyxa subellipsoidea and Botryococcus braunii. Following plant acclimatization, the phytohormone pathways initiated and increased the ability of the plant to cope with biotic stresses with the aid of MT. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
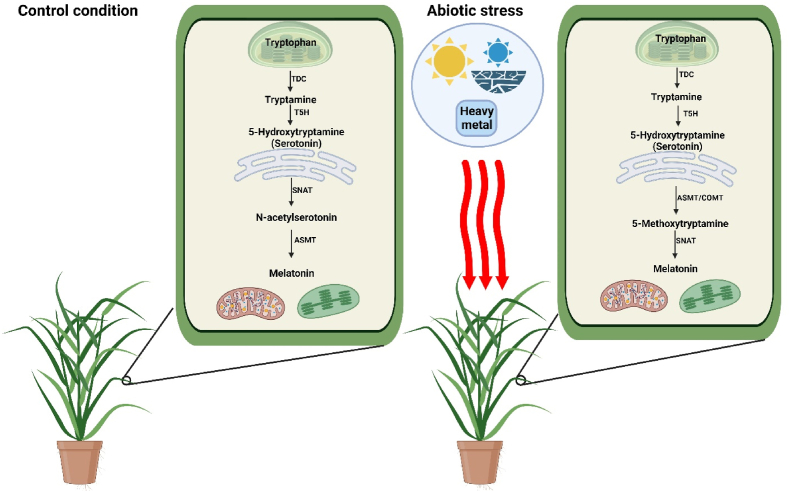
The biosynthetic pathway of this ancient molecule (melatonin) initiates from tryptophan. In order to fully comprehend how plants produce MT under varying conditions, it is imperative to examine the profound regulation of enzymes involved in its biosynthesis. MT is synthesized by six enzymes in rice (Oryza sativa) which include (1) cytoplasm localized tryptophan decarboxylation (TDC), (2) tryptamine-5-hydroxylase (T5H), an enzyme present in the endoplasmic reticulum, (3) chloroplast localized serotonin N-acetyltransferase (SNAT), cytoplasm residing (4) N-acetylserotonin O-methylthransferase (ASMT) (5) caffeic acid 3-O-methyltransferase (COMT) and (6) tryptophan hydroxylase (TPH) [9]. In contrast to rice, Arabidopsis and Nicotiana tabacum do not possess TDC due to no functional TDC genes in their genome [7]. The alternative of TDC in Arabidopsis is probably tyrosine decarboxylase (TYDC) which decarboxylates aromatic amino acids in a pyridoxal-5-phosphate-dependent manner [10]. Arabidopsis contains TYDC gene (At4g28680), which may show low-level TDC-like activity in serotonin (SER) production, the key precursor of MT biosynthesis [7]. In cereals like wheat and rice, internal and environmental factors play an important role in phytomelatonin biosynthesis. For example, tryptophan is converted into MT in plants by two pathways depending upon normal/stress growth conditions. The primary carboxylation of tryptophan occurs in the cytoplasm of plants by TDC during normal plant growth. In rice, TDC has a relatively small gene family that comprises three genes, and overexpression of TDC promotes the elevation of SER and tryptamine (TRYP) [11,12], while lower content of tryptamine, serotonin, and MT was observed in down-regulated TDC [13]. Subsequently, T5H breaks down TRYP into SER and is dependent on acetyl-coenzyme A (Ac-CoA) in the endoplasmic reticulum. In the third step, serotonin is transformed to N-acetylserotonin by SNAT in the chloroplast. N-acetylserotonin is further altered into MT in the cytoplasm by ASMT [1]. When plants are under stress conditions, then MT is formed by methylating serotonin to produce 5-methyltryptamine, and subsequently acetylating 5-methyltryptamine to form MT (Fig. 2) [14]. Hence, the existence of two MT metabolic pathways helps plants to switch between battle or rest mode depending upon the stress or normal growing conditions, respectively.
Fig. 2.
Biosynthesis pathway of melatonin in rice plants subjected to normal and adverse growth conditions. TDC, tryptophan decarboxylase; T5H, tryptamine-5-hydroxylase; ASMT, N-acetylserotonin methyltransferase; COMT, acetylserotonin O-methyltransferase; SNAT, serotonin N-acetyltransferase.
MT degradation in cells is carried out via enzymatic and non-enzymatic pathways. Melatonin is also transformed enzymatically in plants, where 2-hydroxyl and 3-hydroxyl melatonin are produced by the enzymes melatonin-2-hydroxylase (M2H) and melatonin-3-hydroxylase (M3H), respectively [15,16]. The non-enzymatic breakdown of melatonin, which happens in both plants and animals, is associated with its reaction with free radicals or other ROS or photocatalytic degradation in the presence of UV light, which confirms its antioxidant capacity [8,17]. In plants, redox network is the combination of genes, enzymes, and metabolites that maintain cellular homeostasis. The environmental stresses often affect plant's normal physiological activity and productivity by generating excessive reactive oxygen species (ROS) such as superoxide anion (O2·-), hydrogen peroxide (H2O2), or hydroxyl radical (·OH) and reactive nitrogen species (RNS) like nitric oxide (NO), nitrogen dioxide (NO2), non-radical species peroxynitrite (ONOO−) and S-nitrosoglutathione (GSNO) [1]. The cellular redox system becomes unbalanced because of the higher accumulation of these ROS and RNS, whereas lower/moderate production of these chemical species participates as cellular messengers in the cells to regulate essential cellular processes [18]. Plants have evolved a sophisticated antioxidant mechanism to regulate ROS homeostasis in response to oxidative stress. There is a wide variety of enzymatic and non-enzymatic antioxidants found in the plants, for example, ascorbate peroxidase (APX), thioredoxin (TRX), guaiacol peroxidase (POD), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione reductase (GR) peroxiredoxins (PRX), proline, and ascorbic acid, which are regarded as a significant part of redox hub [19]. Plants have also been shown to interact with ROS/RNS through other signaling pathways, such as nitric oxide, calcium, and hormones [20]. In conjunction with other plant hormones, MT has emerged as a new warrior to protect plants from the damaging effects of abiotic and biotic threats [21]. Several studies have demonstrated that MT protects against abiotic stress by stimulating ROS scavenging system to mitigate heat [22], cold [23], salinity [24], heavy metals [25] and drought stress [26,27]. Although the mechanism by which phytomelatonin confers abiotic tolerance is well-documented, the genetic mechanism involved in MT coordination with secondary messengers is still the least understood. Furthermore, little research has been conducted about its effects on biotic tolerance, and they have not been extensively reviewed. The objective of the present review is to comprehensively discuss the recent studies that (1) reveal the significance of the emerging features of MT in mitigating biotic and abiotic stresses, (2) to explore the molecular mechanisms of plant stress regulation, and (3) evaluating the potential of this powerful molecule in improving plants' defense. Additionally, MT cross-talk with different plant hormones/secondary messengers was also discussed, along with the identification of future opportunities for research to address existing gaps in the literature.
2. Coordination of MT and secondary messengers under abiotic stress in plants
2.1. MT and Ca2+ function synergistically to improve plant tolerance
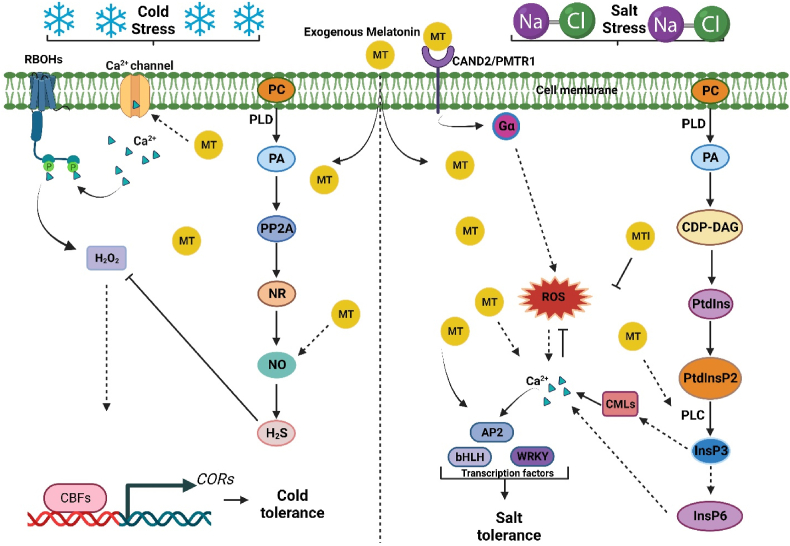
In plants, Calcium (Ca2+) is a versatile intracellular signaling molecule that can regulate multiple cellular processes under normal and stressful conditions. Among the most important Ca2+ receptors, calmodulin (CaM) and CaM-like proteins (CMLs) act as the main Ca2+ sensing proteins in stress-mediated signaling pathways in plants [28]. Several studies have indicated that CML genes are associated with plants’ growth and development, the metabolism of cells, the innate immune system, and the resistance to abiotic and biological stresses [[29], [30], [31]]. For instance, CML44 overexpression can enhance tomato tolerance to salt, drought, and cold [32]. In Arabidopsis, the higher CML8 expression level could trigger salicylic acid (SA) [33], and over-expressed CML20 negatively regulates the ABA signaling to reduce drought tolerance [34]. The anti-stress phytohormones MT corporates with micro-nutrients like Ca2+ to alleviate plant stress [35]. There are several pathways through which MT triggers the production of Ca2+. In animal cells, MT binds with CaM to regulate the Ca2+-dependent cellular mechanism [36]. A recent study suggests that the MT receptor in plants CAND2/PMTR1 is present in the outer membrane of mitochondria because it has a slight amino acid similarity with MT1, a melatonin receptor in humans, which also resides in mitochondria [6]. However, the subcellular localization of CAND2/PMTR1 in Arabidopsis [37] and Phaseolus vulgaris [38] shows its presence in the plasma membrane. The CAND2/PMTR1 perceives the MT signaling and stimulates the G-protein G , which further triggers the production of H2O2 and Ca2+ [37]. In P. vulgaris, the addition of MT up-regulated the CML genes and promoted the induction of Ca2+ [38]. When exposed to salt stress, the membrane-bounded phospholipids undergo phosphorylation to produce phosphatidylinositol 4,5-bisphosphate (PtdInsP2). The addition of MT up-regulates the phospholipase C (PLC) biosynthesis gene, enhancing the degradation of PtdInsP2 to generate lipid-binding diacylglycerol (DAG) and soluble inositol 1,4,5-triphosphate (InsP3), which is subsequently phosphorylated into phytic acid (InsP6) (Fig. 3) [[39], [40], [41]]. The CDPKs are Ca2+ sensing proteins that detect fluctuation in intracellular Ca2+ levels and initiate downstream signaling processes to mitigate stress responses. In stress signal transduction, CDPK could be activated by ROS, which can phosphorylate and activate the NADPH-mediated respiratory burst oxidase homologs (RBOHs), to trigger downstream defense response for better adaptability under unfavorable conditions [42]. It has been reported that MT also activates the calcium-dependent protein kinase (CDPK) via Ca2+ signaling [43]. In response to higher H2O2 accumulation in tomatoes leaves; the foliar application of MT resulted in higher expression of CDPK biosynthesis genes (CDPK1 and CDPK2), which gradually increased the Ca2+ signaling to regulate diverse physiological processes to improve redox balance and stress adaptation [44].
Fig. 3.
A regulatory model of interaction between melatonin (MT) and secondary messengers under abiotic stress. During cold stress, the membrane-bounded phospholipid phosphatidylcholine (PC) is degraded by phospholipase D (PLD) to produce phosphatidic acid (PA), which subsequently modulates the protein phosphatase 2A (PP2A), nitrate reductase (NR), nitric oxide (NO), and H2S. The exogenous application of MT triggers the Ca2+ channel to release Ca2+, which binds directly to the N-terminus of respiratory burst oxidase homologs (RBOHs), cold-induced Ca2+ activates respiratory burst oxidase activity to produce H2O2, thereby provoking Ca2+ transients in plant cells and causing downstream responses involving CBF-dependent pathways that promote cold tolerance. In case of salt stress, the PA is phosphorylated into cytidine diphosphate-diacylglycerol (CDP-DG), phosphatidylinositol (PtdIns), phosphatidylinositol bisphosphate (Ptd InsP2). The addition of MT up-regulates the phospholipase C (PLC), which hydrolysis the Ptd InsP2 into inositol 1,4,5 triphosphate (InsP3) and phosphorylated in subsequent steps to form phytic acid (InsP6), which triggers the release of Ca2+ in the cytoplasm. The MT receptor CAND2/PMTR1 also involves in the accumulation of Ca2+ by triggering G-protein G , which promotes ROS and Ca2+ production. Increased MT and Ca2+ influx up-regulates the transcription factors (AP2, bHLH, WRKY) to mitigate salt stress.
The synergy between MT and Ca2+ was investigated by inhibiting the MT synthesis gene GhCOMT (Gh_D12G2680) in upland cotton through virus-induced gene silencing technology (VIGS). Consequently, the mutant plants became more susceptible to salt stress [45]. The strong chelating properties of InsP6 store the Ca2+ in the storage vacuole and releases Ca2+ into the cytosol upon needed; however, the putative InsP6-Ca2+ channel is still undetermined [45,46]. In GhCOMT mutant plants, the PtdInsP2 degrading enzyme encoding gene PLC was down-regulated along with lower Ca2+ content. The MT foliar application to the GhCOMT silenced plants significantly upregulated the transcriptional levels of genes associated with InsP3 and InsP6 biosynthesis [45], leading to higher accumulation of InsP3 and InsP6 and further enhancement of Ca2+ production. As a result, the plants exhibited reduced salt-stress sensitivity (Fig. 3) [45]. Another study reported that the MT application induced the expression of calmodulin-related genes and the influx of Ca2+ in barley seedlings under salt stress, leading to modulating different antioxidant enzyme processes via ROS scavenging system consisting of enzymatic antioxidants and enhancing plant tolerance [47].
Different stress responses trigger different Ca2+ signaling pathways to counter the stress in plants. Cold stress primarily affects plant cell membranes, detecting stress signals and activating various cellular responses [48,49]. During low-temperature stress, the Ca2+ channels are activated, resulting in rapid Ca2+ signal induction that confers cold tolerance by activating downstream phosphorylation pathways, including C-repeat binding factors (CBFs) [23]. The overexpression level of CBF in plants enhances cold tolerance, while mutations diminish it [50]. Due to the amphipathic properties of MT readily crosses the plasma membrane into the cytosol [51] and stimulates the Ca+2 channels to induce the influx of Ca2+ into the cytoplasm (Fig. 3); subsequently, Ca2+ attaches to the N-terminus RBOHs to generate H2O2 [23]. Thus, Ca2+ transients are induced in plant cells, which trigger downstream responses involving CBF-dependent pathways. However, blocking of Ca2+ flux inhibited the melatonin-induced CBF pathway leading to plant susceptibility to cold stress [23]. The interplay between melatonin and Ca2+ was further studied in Dalbergia odorifera [52] and broad bean [53]. These studies provided evidence of coordinated actions between MT and Ca2+ in mitigating drought and arsenic-induced damage by reducing lipid peroxidation, respectively. Hence, the result from these studies explains that Ca2+ and melatonin work together to mitigate plant stress responses. However, the genetic mechanism involved in the synergic relationship between Ca2+ and melatonin is still unclear and needs further investigation to explore it.
2.2. Interaction between MT and nitric oxide signaling
A key signaling molecule known as nitric oxide (NO) regulates plants' developmental and stress responses. In plants, NO regulates cellular redox homeostasis through post-translational modifications (PTMs) by interacting with reduced glutathione (GSH) to produce S-nitrosoglutathione in a reversible redox modification process known as S-nitrosylation. The stress-induced NO triggers the S-nitrosylation of histone deacetylase (HDA19) protein (Fig. 4), which may repress the transcriptional level of genes responsible for plant tolerance or susceptibility [54,55]. Although NO and melatonin exhibit independent interaction through multiple signaling pathways, there is a certain degree of companionship [56]. According to Ref. [57]; rapeseed seedlings exposed to NaCl stress produced NO-mediated S-nitrosylation, which was subsequently elevated by the exogenous melatonin and sodium nitroprusside (SNP) treatment. Nonetheless, PTIO-dependent depletion of NO led to lower S-nitrosylation of target proteins, indicating that NO promotes salinity tolerance by acting downstream of melatonin [57]. During cold conditions, the external application of MT elevates the transcriptional activity of ZAT6/10/12 transcriptional factors (TFs) in Arabidopsis [58,59] and ZAT2/6/12 in tomatoes [60] and cucumbers [61]. These TFs govern the transcriptional expression of CBF and antioxidant genes. The ZATs attach to the promoter site (TACAAT) of CBFs genes and over-expresses the CBF1/2/3 to minimize low-temperature sensitivity in plants [62]. MT stimulates the expression of CBF genes, which triggers the production of l-arginine by promoting the expression of arginine decarboxylase 1/2 (ADC1 and ADC2), ornithine decarboxylase (ODC) and ornithine aminotransferase (OAT) in arginine pathway [56,60]. Finally, Citrulline was formed from l-arginine, and NO was produced due to the activity of the nitric oxide synthase (NOS) enzyme [63]. MT has been reported to elevate the NOS activity by increasing the expression level of genes related to NOS (Fig. 4) [64]. Similarly, MT also contributes to NO signaling by regulating genes related to S-nitrosoglutathione reductase (GSNOR) and Nitrate reductase (NR). In tomatoes, the exogenous treatment of MT increased the endogenous NO production by up-regulating NR activity and inhibiting the GSNOR activity [65].
Fig. 4.
A systematic model illustrating the cross-talk between melatonin (MT) and nitric oxide (NO) in plant cells. During abiotic stresses, NO is produced and induces the tryptophan decarboxylase (TDC), and tryptamine (Trm) is further hydroxylated by tryptamine 5- hydroxylase (T5H) to serotonin. In a subsequent step, serotonin is converted to 5-methyltryptamine, and serotonin N-acetyltransferase (SNAT) acetylate 5-methyltryptamine to synthesize MT under stress conditions. The compound sodium nitroprusside (SNP), acts as a NO source and protects against abiotic stresses. The addition of SNP inhibited the synthesis of hydroxyindole-O-methyltransferase (HIOMT; a MT synthase; however, the expression of HIOMT was up-regulated in the presence of exogenous MT or abiotic stress. The exogenous MT also up-regulates ZATs, which bind with CBFs and trigger the synthesis of l-arginine from the arginine pathway. NOS converts l-arginine to NO, further converted into S-nitrosoglutathione (GSNO) by reduced glutathione (GSH). GSNO subsequently breakdown into NO and releases S-nitrosylation (SNO) by the action of S-nitrosoglutathione reductase. In response to stress, Nitrate reductase (NR) also activate to produce NO. The combined effect of MT and NO regulates the MAPK cascade, further activating the metal-responsive transcription factor-1 (MTF-1) and alleviating heavy metal toxicity in plants. On the other hand, SNO promotes histone deacetylase (HDA19) to inhibit the acetylation of histone, leading to CBF gene suppression.
Environmental stresses promote the interaction between MT and NO in order to regulate a variety of physiological and biochemical pathways in plants. Previous studies have examined the impact of MT on plants under the stress of cadmium (Cd) [66]. During Cd stress, MTbiosynthesis genes are transcriptionally activated, thus resulting in melatonin production [67]. The Cd-induced MT production relies on various factors such as H2O2, NO, and light intensity. The MAPK cascade reaction is activated in rice cells exposed to Cd by increasing levels of H2O2 and NO, thereby inducing MT biosynthetic genes (TDC and T5H) (Fig. 4) [68]. Another report described the synergistic role of MT and NO signaling in mitigating Cd and Pb-stressed soybean. The conjunction treatment of MT and SNP significantly reduced the intracellular levels of jasmonic and abscisic acid by elevating catabolic genes (CYP707A1 and CYP707A2) and down-regulation of NCED3 [43]. Furthermore, MT and NO mitigate the mobilization of Cd and Pb by augmenting Ca2+ and K+ through up-regulation of metal translocation transcription factors, MTF-1 and WRKY27 [43]. A similar study reported that maize plants exposed to Pb exhibited reduced Pb-toxicity due to a higher accumulation of endogenous NO content when supplemented with MT (0.05 or 0.10 mM) [69]. The tolerance to Pb toxicity was diminished by cPTIO, an NO scavenger, by lowering the endogenous NO. According to these findings, MT-induced antioxidant defense is mediated by NO and MT/NO interplay, which might play a significant role in the ability of maize to tolerate Pb stress [69].
2.3. Interplay of MT-H2S signaling
In plant cells, hydrogen sulfide (H2S) was formerly considered to be a harmful gaseous molecule; however, this concept has changed as plants utilize H2S in various developmental processes [[70], [71], [72]]. When present in low concentrations, it affects several growth and development characteristics, including germination rates [73], stomatal regulation [74], and the development of adventitious roots [75]. Moreover, H2S can cause post-translational modifications to proteins through persulfidation (S-sulfhydration), resulting in altered protein localizations and different functional outcomes. For instance, persulfidating ATG8 (an autophagy-related ubiquitin-like protein) inhibits the accumulation of autophagy in Arabidopsis roots [76].
H2S interacts with phytohormones (Auxin, MT, ETH, ABA) in a significant manner to modulate stress, growth, and development [[77], [78], [79]]. MT contributes a vital role in maintaining H2S homeostasis in plants; a recent study on Solanum lycopersicum L. var. cherry under salinity stress demonstrated that the combined effects of MT and H2S mitigated the consequences of NaCl-induced growth abnormalities in tomato seedlings [80]. The activation of L/D-cysteine desulfhydrase (LCD or L-DES/DCD) is a salient enzymatic process for producing H2S. The L/D cysteine undergoes hydrolysis to generate H2S, pyruvate, and NH4+ [78,81]. According to Ref. [80]; melatonin contributes to maintaining H2S homeostasis in tomatoes' cells in response to salt stress by modulating the expression of cytosolic L-DES isoforms. The pre-treatment of MT in cucumbers under salt stress stimulates the generation of H2S by L/D-cysteine desulfhydrase, influencing photosynthetic efficiency and ROS burst [82]. When MT is used to mitigate salt stress, H2S also functions as a downstream target of MT via interacting with NO and MAPK cascades. Furthermore, there has been evidence of downstream interaction between NO and H2S in pepper plants (Capsicum annuum L) that were affected by salinity or iron deficiency [77]. MT foliar application induced an elevated level of endogenous H2S and NO under salinity and iron deficiency stress. However, the effects were reversed in response to H2S and NO inhibitors (HT and cPTIO, respectively). Additionally, the MT application to tomato seedlings increases the l-cysteine desulfhydrase's activity [80]. As a result of the MT treatment, the plants can effectively achieve a greater K+/Na+ ratio, maintaining a higher level of antioxidant activity and reducing ROS generation [83]. Excess ROS were substantially scavenged by supplementing chromium-stressed tomato seedlings with MT, maintaining ROS homeostasis in the plants [84]. A significant increase in H2S was observed following the application of 30 mM MT to tomato seedlings under chromium stress. In contrast, 1 mM hypotaurine (H2S scavenger) inhibited the effect of MT in reducing Cr-toxicity [84]. Consequently, it was concluded that MT requires intracellular H2S to ameliorate the negative effects of Cr on tomato seedlings. A recent study also demonstrated the importance of H2S and the MT interaction in regulating the stomatal movement due to drought stress [85]. It was found that exogenous MT enhanced the production of H2S and elevated the transcriptional level of LCD and DES1 under normal or adverse growth conditions. During drought stress, H2S generated after MT treatment stimulated stoma closure through the regulation of genes associated with K+ channel such as AKT1, KC1, KCO1, and KAT1 in Arabidopsis to improve drought resistance [85]. The mutation of lcd and des1, inhibited the MT's ability to improve drought tolerance, suggesting that MT is partially dependent upon H2S to increase drought stress tolerance [85].
2.4. MT and redox network interactions
An intracellular redox network is a complex system of reactions involving oxidants (such as ROS) and antioxidants (such as glutathione, etc.). These reactions are important for maintaining cellular homeostasis and protecting cells from oxidative damage [86]. In response to stressful conditions, ROS are used by the cell as signaling agents to cross-talk with phytohormones and regulate post-transcriptional modification through SUMOylation, a process by which target proteins are covalently bonded with small ubiquitin-like modifier (SUMO). Previously, it has been demonstrated that ROS interacts with SA and SUMO to attenuate herbicide stress in Arabidopsis [87,88]. Similarly, MT also interacts with ROS/RNS and helps to recover redox homeostasis within plant cells by generating intracellular antioxidants, such as ascorbic acid (ASA), CAT, GPX, PRX, SOD, NR, NOS, and GSH (Fig. 5) In addition to its influence on gene expression, MT can also affect signal transduction pathway-related signaling cascades [51]. During the low-temperature condition, ROS burst rapidly oxidizes membrane-bounded fatty acids, resulting in the deposition of malondialdehyde (MDA) and enhanced electrolyte leakage in plant cells [48,89]. The antioxidant activity of MT plays a central role in the scavenging of ROS by increasing the antioxidant enzymes activity under stress response. In response to salt stress, the crimson seedless grapevine plants pre-treated with MT exhibited salinity tolerance by lowering the ROS accumulation via promoting the TF MYB108A to bind with the promoter region of ACS1 (1-aminocyclopropane-1-carboxylic acid) gene and triggering the biosynthesis of ethylene (ETH) [90]. In addition, MT enhances stress tolerance by NADPH oxidase-modulated ROS signaling. Besides NADPH oxidase, the cell membrane-based enzyme RBOH is the major generator of apoplastic ROS by producing O2·- dismutated to H2O2 [1]. The plant RBOHs comprises a multigene family with nine to ten RBOH genes in rice and Arabidopsis, respectively [91]. The knockout mutants of rbohF in Arabidopsis showed salt sensitivity and altered K+/Na+ homeostasis [92]. Under salinity stress, ROS over-accumulation leads to oxidative damage in the cell. The MT promotes salt tolerance by elevating OsRBOHF-dependent ROS signaling to activate stress-responsive genes as well as, increasing K+ retention in roots by enhancing the key ion transporters like OsAKT1, OsHAK1, and OsHAK5 [91].
Fig. 5.
Systematic model explaining the interaction of reactive oxygen species (ROS) and reactive nitrogen species (NOS) with melatonin (MT). (1) The environmental stresses induce alternation in the redox balance. (2) Increasing ROS/RNS levels causes oxidative/nitrosative stress. (3)The redox imbalance triggers the MT biosynthetic genes to produce (4) MT. (5) The MT triggers its receptor (CAND/PMTR1) to induce changes in (6) NOS-like enzyme and NR, which elevates the NO levels and antioxidant (CAT, SOD, APX, GPX, PRX) levels. (7) Consequently, these enzymes degrade the ROS species to stabilize cellular homeostasis. (8) The direct action of MT (and its byproducts) also inhibits the increased level of ROS/RNS. (9) The regulation of NO via interaction of its biosynthesis enzymes with MT and with O2·- to form ONOO− triggers the signaling cascade response. (10) The MT triggers other phytohormones to interact with NO signaling to activate plant defense response. ASA: ascorbic acid; GSH: glutathione; RbOH: respiratory burst oxidase homolog; GPX: glutathione peroxidase; SOD: superoxide dismutase; NR: nitrate reductase; NOS: nitric oxide synthase; CAT: catalase; PRX: peroxiredoxin.
MT also affects the transcription of genes related to redox homeostasis. Plants that have been treated with MT exhibit increased expression of enzymes related to redox metabolisms. For instance, ROS are generated in chloroplast and mitochondria under heat stress due to the increasing rate of electron leakage from the chloroplastic/mitochondrial electron transport chain. The leaked electrons react with oxygen to form superoxide radicals and are later converted into H2O2 and hydroxyl radicals via the action of NADPH dehydrogenase, alternative oxidase, aconitase, and MnSOD in mitochondria [93]. In chloroplast, toxic superoxide radicals are converted into less toxic H2O2 via membrane-bound Cu/ZnSOD, and finally, thylakoid-bound POX decomposes H2O2 into the water [94]. It was observed that the exogenous application of MT alleviates oxidative stress in celery seedlings via promoting the expression of pyruvate metabolizing enzymes and peroxidases genes in the phenylpropanoid biosynthesis pathway under heat stress [95]. The expression of PODs and glutathione metabolism-related genes at the transcriptional level may provide molecular support for melatonin-mediated attenuation of oxidative stress in stressed plants [96]. The root priming with MT promotes root growth via suppressing LOX-mediated JA synthesis and oxidative stress under copper exposure. In plants, LOX genes catalyze the oxygenation of polyunsaturated fatty acids, linoleic acid and also generate toxic superoxide anion, peroxide, and free radicals that can damage DNA, proteins, and membranes [97]. The MT pre-treatment suppressed the level of linoleic acid and the expression of LOX, and stimulated the activities of many redox-related genes such as peroxidases, glutamate dehydrogenase, and glutathione that were suppressed in Cu2+-stressed melon roots, which showed that MT regulates root development via multiple mechanisms [97]. The detoxification of superoxide anions and H2O2 is also carried out by free polyamines, while conjugated polyamines scavenge other ROS [98,99]. The MT application promoted the polyamine biosynthesis by upregulating the expression level of N-carbamoylputrescine amidase (NCA), spermidine synthase (SPDS), ADC, and ODC. The higher protein level of ADC and ODC consequently increased the GABA contents in stressed plants. These findings revealed that MT could regulate polyamine metabolism and subsequently strengthen cell tolerance against oxidative damage [99].
MT is thought to mitigate H2O2 bursts under unfavorable conditions by promoting the absorption of ROS by anti-oxidative enzymes or by increasing the efficiency of ascorbate/glutathione synthesis (Fig. 5). Recently, the antioxidant potential of MT was investigated against combined stress of high-temperature (40 °C or higher) and drought in tomato seedlings in field conditions [100]. The foliar application of MT enhanced the SOD, POD, CAT, APX and GR activity over accumulated ROS, thereby restoring the redox homeostasis [100]. The pre-treatment of MT in Bermuda grass may reduce the production of H2O2 and modulate plant growth, and alleviate ROS accumulation by modulating ROS synthesis and accumulation [101]. Furthermore, MT mitigates paraquat-induced oxidative stress in Zea mays [102], Arabidopsis [51], and Pisum sativum [103]. In Arabidopsis, MT inhibits cadmium accumulation by modulating various heavy metal transporter genes, such as ABC transporters and PLANT CADMIUM RESISTANCE 2 (PCR2), and downregulating the miR398; a micro RNA that negatively regulates the Cu/Zn Superoxide Dismutase genes (CSD1 and CSD2). As a result, the melatonin-mediated over-expression of CSD1 and CSD2 helps decrease the ROS and attenuates the oxidative damage in Arabidopsis [104]. The application of MT treatment on Malus hupehensis seedlings grown in AlCl3 contaminated soil [105], revealed that roots treated with MT showed induction of TF gene SENSITIVE TO PROTON RHIZOTOXICITY 1 (STOP1), which improves the Al tolerance in plants by interacting with other TF NAC2. The STOP1 directly binds with the promoter of ALUMINUM SENSITIVE 3 (ALS3) and SODIUM HYDROGEN EXCHANGER 2 (NHX3) to modulate their transcription and promote H+ and Al3+ exchange in vacuole in AlCl3 stress. Furthermore, the MT induced STOP1 transcription elevated the SOD, POD and CAT enzymes to mitigate the cellular oxidative damage under AlCl3 stress [105]. These findings indicate the crucial role of MT in facilitating plant adaptation to adverse environmental conditions by maintaining multiple mechanisms, including redox network. However, more research is needed to determine the exact nature of MT and redox homeostasis intricate connections in the context of biotic/abiotic stresses.
3. MT, ETH, JA, and SA: the cooperation against phytopathogens
Plants have evolved a unique dual-tiered immune system capable of defending themselves against diverse pathogenic microbes. The Bacterial flagellin, fungal chitin, etc. are some of the known pathogen-associated molecular patterns (PAMPs), which are recognized by receptors to trigger the first immune response in plants, generally known as PAMP-triggered immunity (PTI) [78,[106], [107], [108]]. The second layer of immune response is triggered by plant resistance (R) proteins that detect pathogens' specific effectors (Avr proteins). This process refers to effector-triggered immunity (ETI).
The plant immune signaling network is regulated by a variety of plant hormones acting downstream of PTI and ETI activation. These hormones contribute significantly to resistance against pests and phytopathogens. SA, ETH, jasmonic acid (JA), and MT are the key phytohormones involved in plant resistance [109,110]. These phytohormones interplay with each other to confer plant resistance. Following is the cross-talk between MT, SA, ETH, and JA to trigger defense responses against phytopathogens.
3.1. The conquest against viral attack
Plant disease caused by viruses results in substantially reduced yield and economic losses because viruses are difficult to control as compared to fungi or bacteria in infected plants [111]. Similar to other phytohormones, the exogenous MT treatment confers resistance to viral invasion in Nicotiana glutinosa and Solanum lycopersicum [112]. The MT treatment increased the SA production and elevated the expression level of pathogenesis-related protein 1 (PR1) and PR5 in S. lycopersicum infected with tobacco mosaic virus (TMV) [112]. In cereal crops, the rice stripe virus (RSV) causes yellow and green stripes and necrotic lesions. Laodelphax striatellus, a small plant hopper, is generally responsible for the transmission of RSV [113]. MT and NO applied exogenously to rice can promote resistance to RSV [113]. Furthermore, both compounds induced the transcription of two plant defense-related genes (OsPR1b and OsWRKY45), indicating that MT and NO cooperate to enhance resilience against RSV in rice plants [113]. Previously [114], investigated the effects of MT on apples infected with the Apple Stem Grooving Virus (ASGV) [114]. In general, apples are grown vegetatively through grafting. Hence, it's crucial that the apple planting material is free from viruses to prevent their transmission from one generation to the next. The MT treatment of virus-infected shoots significantly exterminates ASGV, based on the MT concentration used and the culture duration on the MT-containing shoot proliferation medium [114]. In another recent study, MT and SA were evaluated for possible anti-viral activity against Alfalfa Mosaic Virus (AMV) on eggplants [115]. The foliar application of MT or SA effectively attenuated the AMV infection via stimulating the activities of antioxidant enzymes and upregulating the PR3 and MPK1 defense gene regulation [115].
3.2. The defenders against bacterial invasion
MT interacts with other phytohormones by mediating the transcription of genes related to ETH, SA, and JA pathways [116]. Environmental stress responses strongly influence ETH and its signaling components. A recent finding indicated that Xanthomonas axonopodis pv. manihotis (Xam) infestation increases levels of intracellular ETH, which modulates disease resistance in plants by inducing the production of MT in cassava roots [117]. Moreover, the ethylene-responsive transcription factor ETHYLENE INSENSITIVE LIKE 5 (EIL5), known to promote disease resistance, is crucial for the ethylene-induced accumulation of MT in cassava. Overexpression of ERF94 up-regulated the PR2, PR3, PR9 genes in potatoes [118]. Heat stress transcription factor 20 (Hsf20) physically interacts with EIL5 and led to the activation of the MT biosynthetic gene ASMT2 (Fig. 6), suggesting a link between ETH and MT in plant disease resistance [117]. Furthermore, increasing of EIL5 protein promotes Hsf20-specific interaction with PR3, thereby contributing to the anti-bacterial properties of PR3. Similarly, another report also suggested that exogenous application of MT inhibited food-borne Bacillus subtilis pathogen in cherry tomatoes and up-regulated the ACO1, a gene encoding an ACC oxidase via converting 1-aminocyclopropane-l-carboxylic acid (ACC) into ethylene [119]. The MT interaction with ETH and salicylic acid-mediated defense mechanism was also discussed by Ref. [120]. The pre-treatment of Arabidopsis leaves with 10 μm MT significantly inhibited the growth of Pst DC3000 and showed high induction levels of defense-related genes such as ACS6, ICS1(ISOCHORISMATE SYNTHASE 1), PDF1.2 (PLANT DEFENCIN 1.2), and PR1, a well-known marker for the SA-mediated immunity in plants. Among these genes, PR1 showed a significant transcriptional expression level. Additionally, the PR1 gene was also induced by treatment with MT, suggesting that MT has similar signaling properties to SA [120]. Except for SA, the exogenous treatment of ET or JA induces PDF1.2 [121]. Similarly, exogenous application of MT on Xanthomonas oryzae pv. oryzae infected rice plants induces PDF1.2 via stimulating the JA biosynthetic genes [122]. Foliar application of MT to Citrus sinensis also suppressed the pathogenicity of gram-negative bacteria Candidatus Liberibacter asiaticus by positively mediating the SA and JA contents [123], indicating that MT cooperates with other phytohormones to fight against phytopathogens.
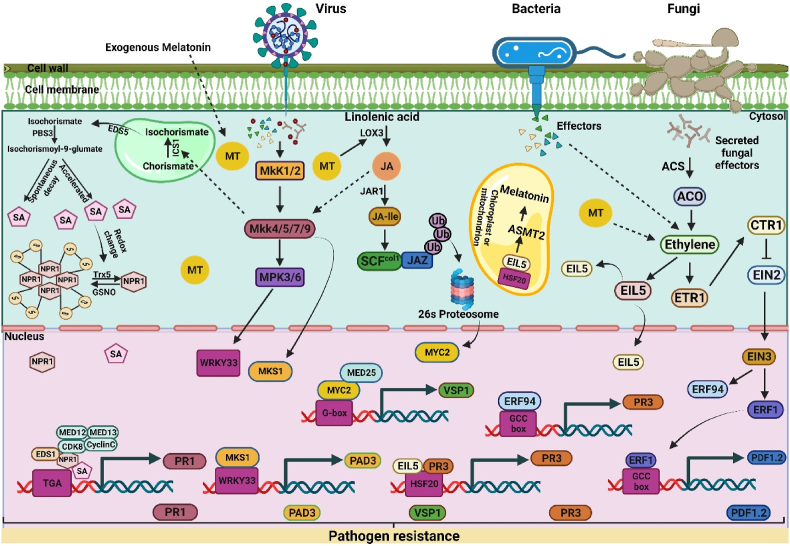
Fig. 6.
Schematic representation of melatonin (MT) interplay with salicylic acid (SA), jasmonic acid (JA), and ethylene (ETH) under phyto-pathogen invasion. (1) MT-SA interaction: A biotrophic pathogen invade plants by releasing effectors into the host cells. The exogenous application of MT up-regulates the ICS gene through mitogen-activated protein kinase (Mkk4/5/7/9). ICS is involved in the catalyzation and conversion of chorismite into isochorismate, and Enhanced Disease Susceptibility 5 (EDS5) exports this protein to the cytoplasm. As a result of PBS3's cytosolic activity, l-glutamate, and isochorismate are conjugated to synthesis isochorismate-9-glutamate. As a result of spontaneous decay, SA is produced from isochorismate-9-glutamate. In addition to acting as a Pyruvoyl glutamine lyase, the EPS1 may also be capable of breaking down N-pyruvoyl-l-glutamate into SA. As a result of the interaction between WRKY transcription factors and NPR1, CDK8 is recruited to the W-box sequence in the promoter of NPR1 gene. The MT-mediated over-expression of ICS and pathogen-induced defense signaling promote SA accumulation. Moreover, SA stimulates redox reactions through which NPR1 oligomers are reduced to monomers. NPR1 monomers migrate from the cytosol to the nucleus, forming an association with transcription factors (TGA), CDK8, EDS1, and SA, which results in the transcription of PR genes. (2) MT-JA interaction: Exo-/endogenous MT increases the endogenous level of JA via up-regulating the LOX genes. Pathogen-secreted effectors are recognized by pattern recognition receptors (PRR), which stimulate the MEK1/2 cascades. MT triggers the activation of MKK4/5/7/9, and MKK4 induces the activation of MPK3/MPK6 and MKS1 (MPK4's substrate). Camalexin (a phytoalexin) is biosynthesized by the WRKY33 transcription factor. WRKY33 is phosphorylated by MPK3/MPK6, which enhances its transactivation activity. As a result of the complex formed between WRKY33 and MKS1, Phytoalexin Deficient 3 (PAD3) is transcribed, activating the camalexin synthesis. In elicited cells, SCFcol1 is formed by associating Col1 with SKP1, Cullin, and JA-Ile protein. The SCFcol1 ubiquitinates the JAZ family of repressor proteins and degrades these by the 26S proteasome. Degradation of JAZ stimulates downstream JA responses due to the release of MYC2 from inhibition. MEDIATOR25 (MED25) stimulates wound-responsive gene VSP1 transcription by bonding with MYC2. (3) Ethylene stimulates MT production: During invasion on plants, the necrotrophic pathogen releases its effectors into the plant's cell, resulting in the activation of genes encoding ethylene production, such as 1-aminocyclopropane-1-carboxylic acid synthase (ACS) and 1-aminocyclopropane-l-carboxylic acid oxidase (ACO) that can produce ethylene. The addition of MT also stimulates the production of ethylene and up-regulation of ETHYLENE INSENSITIVE LIKE 5 (EIL5). The EIL5 directly binds with HEAT STRESS TRANSCRIPTION FACTOR 20 (HSF20) and leads to the activation of N-acetylserotonin O-methyltransferase 2 (ASMT2) gene, which produces the MT. The ethylene levels are low under control/normal conditions, leading to the activation of Ethylene receptor 1 (ERT1), which binds with CTR1 and suppresses the downstream signaling pathways. However, a higher level of ethylene inactivates Raf-like protein CTR1 by binding to its receptors and activating ethylene insensitive 2 (EIN2) and ethylene insensitive 3 (EIN3) transcription factors. EIN3 is responsible for regulating ERF1 and ERF94 expression. The ERF1 and ERF94 interact with the GCC box and activate PDF1.2 and PR3 genes, respectively. The higher protein concentration of EIL5 facilities the binding of the Hsf20 and PR3 and further promotes the synthesis of PR3, which triggers the pathogen resistance genes in the plant.
Several genes related to plant defense are activated by MT, which increases resistance to pathogens. In Pst DC3000 infected Arabidopsis, the exogenous MT application activates MPK3 and MPK6 through MKK4/5/7/9 cascades (Fig. 6), suggesting that MT-mediated innate immunity is activated by MAPK signaling through these cascades [124]. Serotonin N-acetyltransferase (SNAT) serves as a penultimate enzyme in the metabolism of MT. Arabidopsis snat mutants infected with Pst avrRpt2 exhibited 50% lower MT contents as compared to the wild-type. Furthermore, the reduced levels of MT in snat mutants resulted in diminished SA levels because MT acts upstream of SA synthesis. Thus, decreased SA levels may have contributed to higher disease susceptibility in snat mutants, attenuating defense gene expression [125]. In order to stimulate disease resistance, SA acquisition is necessary. The exogenous MT treatment triggers the activation of the SA receptor NPR1 and SA-activated defense genes PRs, EDS1, and PAD4 to function critically in plant immunity [122,126,127]. The MAPK cascade has been studied for its potential role in activating SA-dependent immune response [128]. A significant increase in MPK3 and MPK6 expression was observed in Arabidopsis when exogenous MT was added; however, this induction is abolished in MT receptor mutants pmtr1/cand2 [129]. This indicates that MT sends signals through PMTR1/CAND2 to MAPK cascade and induces plant immunity through an SA-mediated mechanism.
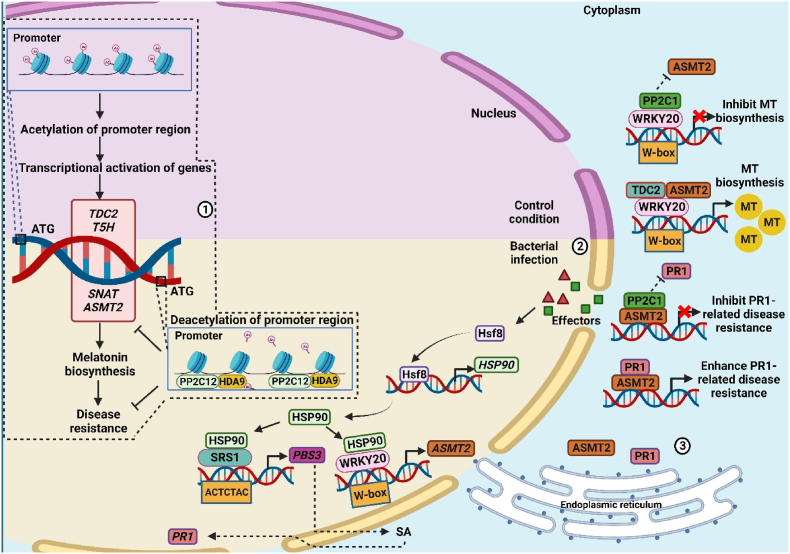
During environmental stress signaling in plants, the role of histone deacetylation (HDA) proteins cannot be neglected as these govern the expression level of target genes via altering the transcriptional activity of genes. HDA9 is a histone deacetylase that repressively modulates plant resistance against cassava bacterial blight (CBB) by reducing the lysine 5 of histone 4 (H4K5) at the promoter sites of MT biosynthetic gene, hence, inhibiting MT production (Fig. 7) [130]. The MT spray ameliorated the sensitivity to CBB disease in HDA9 overexpressing cassava plants [130]. Furthermore, protein phosphatase (PP2C1/12), an inhibitor of ASMT2 gene, forms complexes with WRKY20 [131] and HDA9 [130], respectively, and limits the transcriptional regulation of MT biosynthesis genes (Fig. 7). In cassava plants, the bacterial effectors stimulate the transcription of Hsf8 in the nucleus; the Hsf8 attaches to the promoter region of HSP90 and promotes the expression of HSP90 [132]. At the promoter of ASMT2, the HSP90 interact with WRKY20 and induces a higher expression level of ASMT2. Similarly, HSP90 also binds with SHI-related sequence (SRS1), at the avrPphB susceptible 3 (PBS3) promoter region (ACTCTAC) to activate the PBS3, a SA biosynthetic gene localized in cytoplasm and nucleus, leading to elevated PR1 expression level [132]. The MT biosynthetic ASMT2 protein physically forms a complex with PR1 protein in the cytoplasm and enhances the plant defense mechanism (Fig. 7) [133] However, PP2C1 interferes with the interaction of ASMT2 and PR1 by binding itself with the ASMT2 and inhibits the PR1-mediated immune responses (Fig. 7) [133]. Hence, PP2C1/12 and HAD9 illustrate an antagonist relationship with MT-mediated plant immune response and fine-tune the MT biosynthesis in plant cells.
Fig. 7.
Systematic model showing (1) epigenetic regulation of MT biosynthesis, (2) transcriptional regulation of MT and SA through heat shock factors (HSFs), and (3) fine-tuning of ASMT2/PR1-related activity. HDA9: HISTONE DEACETYLATION 9; HSP 90: Heat Shock Protein 90; PBS3: AVRPPHB SUSCEPTIBLE3; PP2C1/12: protein phosphatase; MT: melatonin; SRS1: SHI-related sequence; TDC2: tryptophan decarboxylation 2; T5H: tryptamine-5-hydroxylase; ASMT2: N-acetylserotonin O-methylthransferase 2, PR1: pathogenesis-related protein 1.
3.3. The antifungal activity by MT, ETH, JA, and SA
Among the most severe plant diseases, fungal diseases jeopardize crop production [134]. According to various studies, MT enhances plant immunity [117,119,120,124,135]. The accumulation of MT by the overexpression of SNAT1 and SNAT2 genes in watermelon and cucurbits contributed to resistance against powdery mildew and soil-borne oomycetes by altering the expression of genes associated with the defense mechanisms mediated by ETI and PAMP [136]. According to Ref. [134]; the MT treatment alleviated potato blight by inhibiting mycelial growth and causing Phytophthora infestans to undergo structural changes. Based on the transcriptome analysis, MT application resulted in the down-regulation of genes associated with pathogen virulence, resulting in decreased fungal infection in plants [134]. Similarly, when Capsicum annum was treated with 100 μM MT, the mycelial growth of colletotrichum gloeosporioides and colletotrichum acutatum was reduced to 76% and 71%, respectively, due to the MT-induced over-expression of chitinase (CaChilll2) gene, which induces degradation of the fungal cell wall [137]. Verticillium dahlia, a soil-borne fungus, severely reduces crop production and fiber quality in cotton [138]. The exogenous MT application on V. dahlia-infected cotton elevated the expression of gossypol, mevalonate (MVA), and phenylpropanoid biosynthesis genes, resulting in a higher accumulation of lignin and gossypol. On the other hand, the cotton plants became vulnerable to V. dahliae by suppressing intracellular MT with VIGS technology. Prior to inoculating VIGS plants with V. dahliae, a pre-treatment with MT restored the compromised resistance [138]. MT also promotes the growth of soil-borne beneficial fungus, arbuscular mycorrhizal fungi (AMF), which forms a symbiotic relationship with plant roots and improves plant resistance to stressful conditions [[139], [140], [141]]. Ahammed and colleagues demonstrated that MT stimulated the proliferation of AMF to confer resistance in Cucumis sativus roots against Fusarium oxysporum. The MT application decreased the F. oxysporum-induced photosynthesis diminution and reversed the effects of F. oxysporum infection on AMF colonization in C. sativus roots [142].
During the fungal attack, MT cooperates with other phytohormones to mitigate the fungal infection in plants. In bananas, Fusarium oxysporum infects the xylem and causes wilting. The exogenous MT treatment on infected bananas causes up-regulation of HSP90s, and causes the reprogramming of phytohormones like SA, JA, and ETH in order to promote fungal resistance [135]. According to numerous studies, the JA signaling network also participates in MT-mediated plant immunity; however, the effects of MT on the JA pathway vary significantly among species [143,144]. Recently transcriptome sequencing also revealed the significance of MT-mediated resistance against Botrytis elliptica in Lilium by modulating phenylpropanoid, JA, and SA signaling pathways [145]. MT induces alternation in the gene expression level through DNA methylation, which is primarily carried out by methyltransferases (MET1) at CG and CHH sites of the plant's DNA [146,147]. The MT-treated grape berries significantly reduced DNA methylation by down-regulating MET genes, resulting in accelerated transcriptional activity of EDS1 and other plant defense-related genes [146]. Similarly, the expression level of SA signaling genes (PR1, PR5, and NPR1), WRKY22 transcripts (WRKY22-1, WRKY22-6), and MAPK cascade-related gene transcripts (MPK3-1, MPK3-2) were elevated in MT-treated ginseng leaves and enhanced plant immunity against gray mold [148]. In tomatoes, the addition of MT elevated the methyl jasmonate contents by upregulating allene oxide cyclase (AOC) and lipoxygenase (LOX) genes present upstream of JA biosynthesis. Furthermore, MT treatment substantially diminished the expression levels of JA signaling inhibitor gene JASMONATE ZIM DOMAIN 1 (JAZ1) (Fig. 6), resulting in the up-regulation of defense-related genes against B. cinerea by enhancing the JA signaling network [149]. Another recent investigation also demonstrated that MT mediates the jasmonic acid and phenyl-propane signaling pathways in blueberry fruits to enhance their resistance to postharvest disease caused by B. cinerea [150]. These results suggest that MT cooperates with other hormones to trigger the plant's innate immune mechanism against fungi.
4. MT homologs: enhances plant immunity against biotic stress
In plants and animals, the MT biosynthesis pathway is differently regulated. Plants possess a more complex biosynthetic pathway for MT compared to vertebrates. It's a well-known fact that MT reduces the severity of biotic stresses in plants; however, its production is costly, thus limiting its use in agriculture [151]. Contrary to MT biosynthesis in plants, synthetic MT is produced in laboratories for therapeutic purposes in humans. 5-methoxyindole is used as the main starting material in the four-step reaction of commercial synthesis of MT. Recently, the use of MT homologs as new arsenals against phyto-pathogens to enhance plant immunity is becoming popular. It's been reported that two homologs of MT, namely 5-methoxytryptamine and 5-methoxyindole were used to study their biological activity in stimulating the defense mechanism in Nicotina benthamiana against Phytophthora nicotianae [151]. Invasion of pathogens occurs primarily through the stomata. Upon pathogen attack, the plant produces ABA to close the stomata to avoid disease infection. A recent study found that MT mediates ABA metabolism and stomatal movement under heat stress in tomatoes [22] and under drought stress in Malus hupehensis and Malus prunifolia [152]. Thus, MT prevents pathogen invasion by promoting stomatal closure by modulating ABA signaling. Similar to MT, the homologs of MT also induce stomatal closure to inhibit pathogen entry into the plant cells [151]. Previous studies have exhibited that the MT receptor (CAND2/PMTR1) regulates stomatal closure induced by MT in Arabidopsis thaliana [37]. Based on the similar amino acid sequence of CAND2/PMTR1 in Arabidopsis, the in silico analysis revealed the four receptors for trP47363, trP13076, trP49122, and trP40966 in N. benthamiana leaves, among which trP47363 and trP13076 transmembrane receptors were identified as essential components in the regulation of stomata by MT homologs; 5-methoxytryptamine and 5-methoxyindole [151]. These homologs also significantly enhanced the SA biosynthetic genes, causing SA accumulation and up-regulation of the PR1 gene to confer resistance against pathogens. The MT homolog, 5-methoxyindole has also been reported to inhibit the Fusarium graminearum growth and conidia germination by inducing ROS accumulation and deformation of F. graminearum hyphae [153].
5. Conclusion and future perspectives
MT regulates diverse developmental phases and defense mechanisms by acting independently and in conjunction with other phytohormones. Based on recent findings, the generation of ROS and RNS in response to pathogen infection implicates in activation of MT biosynthesis [144]. The elevated MT triggers the activation of other phytohormones and augments the callose deposits, mevalonate, phenylpropanoid, linin, gossypol, antioxidant enzymes, Ca2+, NO, and H2S, all of which play a role in improving plant immunity. In this review, we also highlighted the synergic role of MT with secondary signaling molecules. Secondary messengers such as Ca2+, NO, and H2S are essential mediators of the plant stress response, and these coordinate with phytohormones to initiate a defense response. However, the genetic mechanism underlying the synergetic relationship between MT and secondary messengers like Ca2+ is still ambiguous.
Even though MT is globally gaining attention as a new plant hormone, several issues still need to be resolved. For instance, MT is biosynthesized in mitochondria and chloroplast. The accumulation of MT exerts activation of ICS gene, which is also present in chloroplast and responsible for the biosynthesis of SA. The question is how MT activates the ICS gene. Similarly, ETH and JA are also influenced by the exogenous application of MT by upregulating their biosynthetic genes. Nevertheless, how does MT affect the regulatory mechanism of the biosynthesis genes in phytohormones production? What transcription factors are involved in the process? The MT interaction with other hormones could be better comprehended by using MT homologs, as these activate the MT-mediated signaling pathway in the host plants against pathogens by regulating the production of other phytohormones. This indicates that almost all phytohormones are well connected with each other to fight phyto-pathogens and maintain internal homeostasis stable in adverse environmental conditions. Based on the information summarized here, it is apparent that despite numerous studies of MT-mediated plant defense mechanisms against bacterial and fungal diseases, the molecular and genetic mechanism involved in plant immunity against viral infection is the least studied and warrants further investigation.
In the future, comprehensive fundamental research is required to evaluate the regulation of endogenous MT generation and its interplay with several defense-associated processes in laboratory and field environments. In plants, significant research progress has been made in cassava and rice regarding the genetic mechanism of MT. However, the genetic mechanism of MT in other plant species, such as Arabidopsis and tobacco, has not been thoroughly studied due to the lack of some functional MT biosynthetic genes. Despite this, MT is still produced in these plants; however, the exact genetic and metabolic pathway remains unclear. The role of PMTR1 in the perception of MT in plant growth and development and in stress resistance has been demonstrated in several studies; however, the specific signal-transduction pathways are still unclear. Is it possible that plants contain other MT receptors? What is the mechanism by which PMTR1 regulates MAPK cascades? The structure of MT is similar to that of auxin, which requires different types of transporters for its distribution [9]. Studies have shown that MT is transported across plasma and mitochondrial membranes by glucose transporters GLUT1 and PEPT1/2 in mammals [154,155]. Although, in plants, the MT transporters are unknown, further research on MT transporters may provide insight into the mechanisms by which this new phytohormone operates.
Authors contributions
K.M.S.S. prepared figures. All authors contributed substantially to this article's writing and agreed with the submitted version.
Declaration of competing interest
All authors declare no conflict of interests.
Acknowledgments
We are sincerely thankful for the funding by National Natural Science Foundation of China (32250410283, 32250410314, 31800386 and 32000201), Jiangsu Outstanding Postdoctoral Program (2022ZB676, 2022ZB677, 2022ZB678, 2023ZB863), Natural Science Foundation of Jiangsu Province (BK20211319), and Natural Science Foundation of the Jiangsu Higher Education institutions of China (21KJB210004). We also would like to thank State Key Laboratory of Lake Science and Environment (No. 2022SKL013) and the Science and Technology Planning Social Development Project of Zhenjiang City (No. SH2021005). Finally, we would like to acknowledge BioRender, as all the figures were created on this platform.
Contributor Information
Biying Zhao, Email: zhaoby@ujs.edu.cn.
Faisal Islam, Email: faysal224@yahoo.com.
Jian Chen, Email: jianchen@ujs.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Arnao M.B., Hernández-Ruiz J. Melatonin and reactive oxygen and nitrogen species: a model for the plant redox network. Melatonin Res. 2019;2:152–168. [Google Scholar]
- 2.Murch S., KrishnaRaj S., Saxena P. Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John's wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep. 2000;19:698–704. doi: 10.1007/s002990000206. [DOI] [PubMed] [Google Scholar]
- 3.Li X., Rengel Z., Chen Q. Phytomelatonin prevents bacterial invasion during nighttime. Trends Plant Sci. 2022;4:331–334. doi: 10.1016/j.tplants.2021.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Tan D.X., Manchester L.C., Liu X., Rosales‐Corral S.A., Acuna‐Castroviejo D., Reiter R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin's primary function and evolution in eukaryotes. J. Pineal Res. 2013;54:127–138. doi: 10.1111/jpi.12026. [DOI] [PubMed] [Google Scholar]
- 5.Tan D.-X. Melatonin and plants. J. Exp. Bot. 2015;66:625–626. [Google Scholar]
- 6.Zhao D., Wang H., Chen S., Yu D., Reiter R.J. Phytomelatonin: an emerging regulator of plant biotic stress resistance. Trends Plant Sci. 2021;26:70–82. doi: 10.1016/j.tplants.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Back K. Melatonin metabolism, signaling and possible roles in plants. Plant J. 2021;105:376–391. doi: 10.1111/tpj.14915. [DOI] [PubMed] [Google Scholar]
- 8.Tan D.X., Manchester L.C., Terron M.P., Flores L.J., Reiter R.J. One molecule, many derivatives: a never‐ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q., Arnao M.B. vol. 73. Oxford University Press UK; 2022. pp. 5773–5778. (Phytomelatonin: an Emerging New Hormone in Plants). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutensohn M., Klempien A., Kaminaga Y., Nagegowda D.A., Negre‐Zakharov F., Huh J.H., Luo H., Weizbauer R., Mengiste T., Tholl D. Role of aromatic aldehyde synthase in wounding/herbivory response and flower scent production in different Arabidopsis ecotypes. Plant J. 2011;66:591–602. doi: 10.1111/j.1365-313X.2011.04515.x. [DOI] [PubMed] [Google Scholar]
- 11.Byeon Y., Park S., Lee H.Y., Kim Y.S., Back K. Elevated production of melatonin in transgenic rice seeds expressing rice tryptophan decarboxylase. J. Pineal Res. 2014;56:275–282. doi: 10.1111/jpi.12120. [DOI] [PubMed] [Google Scholar]
- 12.Kang S., Kang K., Lee K., Back K. Characterization of rice tryptophan decarboxylases and their direct involvement in serotonin biosynthesis in transgenic rice. Planta. 2007;227:263–272. doi: 10.1007/s00425-007-0614-z. [DOI] [PubMed] [Google Scholar]
- 13.Lee K., Back K. Melatonin‐deficient rice plants show a common semidwarf phenotype either dependent or independent of brassinosteroid biosynthesis. J. Pineal Res. 2019;66 doi: 10.1111/jpi.12537. [DOI] [PubMed] [Google Scholar]
- 14.Back K., Tan D.X., Reiter R.J. Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016;61:426–437. doi: 10.1111/jpi.12364. [DOI] [PubMed] [Google Scholar]
- 15.Byeon Y., Back K. Molecular cloning of melatonin 2‐hydroxylase responsible for 2‐hydroxymelatonin production in rice (O ryza sativa) J. Pineal Res. 2015;58:343–351. doi: 10.1111/jpi.12220. [DOI] [PubMed] [Google Scholar]
- 16.Lee K., Zawadzka A., Czarnocki Z., Reiter R.J., Back K. Molecular cloning of melatonin 3‐hydroxylase and its production of cyclic 3‐hydroxymelatonin in rice (Oryza sativa) J. Pineal Res. 2016;61:470–478. doi: 10.1111/jpi.12361. [DOI] [PubMed] [Google Scholar]
- 17.Hardeland R., Tan D.X., Reiter R.J. Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J. Pineal Res. 2009;47:109–126. doi: 10.1111/j.1600-079X.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 18.Gu Q., Xiao Q., Chen Z., Han Y. Crosstalk between melatonin and reactive oxygen species in plant abiotic stress responses: an update. Int. J. Mol. Sci. 2022;23:5666. doi: 10.3390/ijms23105666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foyer C.H., Noctor G. Oxidant and antioxidant signalling in plants: a re‐evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005;28:1056–1071. [Google Scholar]
- 20.Chokshi K., Pancha I., Ghosh A., Mishra S. Nitrogen starvation-induced cellular crosstalk of ROS-scavenging antioxidants and phytohormone enhanced the biofuel potential of green microalga Acutodesmus dimorphus. Biotechnol. Biofuels. 2017;10:1–12. doi: 10.1186/s13068-017-0747-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Y., Lv Y., Shi Y., Li T., Chen Y., Zhao D., Zhao Z. The role of phyto-melatonin and related metabolites in response to stress. Molecules. 2018;23:1887. doi: 10.3390/molecules23081887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jahan M.S., Shu S., Wang Y., Hasan M.M., El-Yazied A.A., Alabdallah N.M., Hajjar D., Altaf M.A., Sun J., Guo S. Melatonin pretreatment confers heat tolerance and repression of heat-induced senescence in tomato through the modulation of ABA-and GA-mediated pathways. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.650955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang J., Guo Y., Li J., Su Z., Wang C., Zhang R., Wei C., Ma J., Zhang X., Li H. Positive interaction between H2O2 and Ca2+ mediates melatonin-induced CBF pathway and cold tolerance in watermelon (Citrullus lanatus L.) Antioxidants. 2021;10:1457. doi: 10.3390/antiox10091457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhan H., Nie X., Zhang T., Li S., Wang X., Du X., Tong W., Song W. Melatonin: a small molecule but important for salt stress tolerance in plants. Int. J. Mol. Sci. 2019;20:709. doi: 10.3390/ijms20030709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Awan S.A., Khan I., Rizwan M., Irshad M.A., Xiaosan W., Zhang X., Huang L. Reduction in the Cadmium (Cd) Accumulation and Toxicity in Pearl Millet (Pennisetum Glaucum L.) by Regulating Physio-Biochemical and Antioxidant Defense System via Soil and Foliar Application of Melatonin. Environ. Pollut. 2023 doi: 10.1016/j.envpol.2023.121658. [DOI] [PubMed] [Google Scholar]
- 26.EL-Bauome H.A., Abdeldaym E.A., Abd El-Hady M.A., Darwish D.B.E., Alsubeie M.S., El-Mogy M.M., Basahi M.A., Al-Qahtani S.M., Al-Harbi N.A., Alzuaibr F.M. Exogenous proline, methionine, and melatonin stimulate growth, quality, and drought tolerance in cauliflower plants. Agriculture. 2022;12:1301. [Google Scholar]
- 27.Zhao C., Yang M., Wu X., Wang Y., Zhang R. Physiological and transcriptomic analyses of the effects of exogenous melatonin on drought tolerance in maize (Zea mays L.) Plant Physiol. Biochem. 2021;168:128–142. doi: 10.1016/j.plaphy.2021.09.044. [DOI] [PubMed] [Google Scholar]
- 28.Xu L., Zhang F., Tang M., Wang Y., Dong J., Ying J., Chen Y., Hu B., Li C., Liu L. Melatonin confers cadmium tolerance by modulating critical heavy metal chelators and transporters in radish plants. J. Pineal Res. 2020;69 doi: 10.1111/jpi.12659. [DOI] [PubMed] [Google Scholar]
- 29.Delk N.A., Johnson K.A., Chowdhury N.I., Braam J. CML24, regulated in expression by diverse stimuli, encodes a potential Ca2+ sensor that functions in responses to abscisic acid, daylength, and ion stress. Plant Physiol. 2005;139:240–253. doi: 10.1104/pp.105.062612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnan F., Ranty B., Charpenteau M., Sotta B., Galaud J.P., Aldon D. Mutations in AtCML9, a calmodulin‐like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant J. 2008;56:575–589. doi: 10.1111/j.1365-313X.2008.03622.x. [DOI] [PubMed] [Google Scholar]
- 31.McCormack E., Tsai Y.-C., Braam J. Handling calcium signaling: arabidopsis CaMs and CMLs. Trends Plant Sci. 2005;10:383–389. doi: 10.1016/j.tplants.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Munir S., Liu H., Xing Y., Hussain S., Ouyang B., Zhang Y., Li H., Ye Z. Overexpression of calmodulin-like (ShCML44) stress-responsive gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses. Sci. Rep. 2016;6:1–20. doi: 10.1038/srep31772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park H.C., Park C.Y., Koo S.C., Cheong M.S., Kim K.E., Kim M.C., Lim C.O., Lee S.Y., Yun D.-J., Chung W.S. AtCML8, a calmodulin-like protein, differentially activating CaM-dependent enzymes in Arabidopsis thaliana. Plant Cell Rep. 2010;29:1297–1304. doi: 10.1007/s00299-010-0916-7. [DOI] [PubMed] [Google Scholar]
- 34.Wu X., Qiao Z., Liu H., Acharya B.R., Li C., Zhang W. CML20, an Arabidopsis calmodulin-like protein, negatively regulates guard cell ABA signaling and drought stress tolerance. Front. Plant Sci. 2017;8:824. doi: 10.3389/fpls.2017.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma A., Wang J., Xu D., Tao S., Chong S., Yan D., Li Z., Yuan H., Zheng B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020;713 doi: 10.1016/j.scitotenv.2020.136675. [DOI] [PubMed] [Google Scholar]
- 36.Argueta J., Solís-Chagoyán H., Estrada-Reyes R., Constantino-Jonapa L.A., Oikawa-Sala J., Velázquez-Moctezuma J., Benítez-King G. Further evidence of the melatonin calmodulin interaction: effect on CaMKII activity. Int. J. Mol. Sci. 2022;23:2479. doi: 10.3390/ijms23052479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei J., Li D.X., Zhang J.R., Shan C., Rengel Z., Song Z.B., Chen Q. Phytomelatonin receptor PMTR 1‐mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018;65 doi: 10.1111/jpi.12500. [DOI] [PubMed] [Google Scholar]
- 38.Zhao H., Gao Y., Du Y., Du J., Han Y. Genome-wide analysis of the CML gene family and its response to melatonin in common bean (Phaseolus vulgaris L.) Sci. Rep. 2023;13:1–12. doi: 10.1038/s41598-023-28445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia Q., Kong D., Li Q., Sun S., Song J., Zhu Y., Liang K., Ke Q., Lin W., Huang J. The function of inositol phosphatases in plant tolerance to abiotic stress. Int. J. Mol. Sci. 2019;20:3999. doi: 10.3390/ijms20163999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan M.S.S., Basnet R., Ahmed S., Bao J., Shu Q. Mutations of OsPLDa1 increase lysophospholipid content and enhance cooking and eating quality in rice. Plants. 2020;9:390. doi: 10.3390/plants9030390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan M.S.S., Basnet R., Islam S.A., Shu Q. Mutational analysis of OsPLDα1 reveals its involvement in phytic acid biosynthesis in rice grains. J. Agric. Food Chem. 2019;67:11436–11443. doi: 10.1021/acs.jafc.9b05052. [DOI] [PubMed] [Google Scholar]
- 42.Seybold H., Trempel F., Ranf S., Scheel D., Romeis T., Lee J. Ca2+ signalling in plant immune response: from pattern recognition receptors to Ca2+ decoding mechanisms. New Phytol. 2014;204:782–790. doi: 10.1111/nph.13031. [DOI] [PubMed] [Google Scholar]
- 43.Imran M., Khan A.L., Mun B.-G., Bilal S., Shaffique S., Kwon E.-H., Kang S.-M., Yun B.-W., Lee I.-J. Melatonin and nitric oxide: dual players inhibiting hazardous metal toxicity in soybean plants via molecular and antioxidant signaling cascades. Chemosphere. 2022;308 doi: 10.1016/j.chemosphere.2022.136575. [DOI] [PubMed] [Google Scholar]
- 44.Gong B., Yan Y., Wen D., Shi Q. Hydrogen peroxide produced by NADPH oxidase: a novel downstream signaling pathway in melatonin‐induced stress tolerance in Solanum lycopersicum. Physiol. Plantarum. 2017;160:396–409. doi: 10.1111/ppl.12581. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y., Fan Y., Rui C., Zhang H., Xu N., Dai M., Chen X., Lu X., Wang D., Wang J. Melatonin improves cotton salt tolerance by regulating ROS scavenging system and Ca 2+ signal transduction. Front. Plant Sci. 2021;12:1239. doi: 10.3389/fpls.2021.693690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munnik T., Vermeer J.E. Osmotic stress‐induced phosphoinositide and inositol phosphate signalling in plants. Plant Cell Environ. 2010;33:655–669. doi: 10.1111/j.1365-3040.2009.02097.x. [DOI] [PubMed] [Google Scholar]
- 47.Tian X., He X., Xu J., Yang Z., Fang W., Yin Y. Mechanism of calcium in melatonin enhancement of functional substance-phenolic acid in germinated hulless barley. RSC Adv. 2022;12:29214–29222. doi: 10.1039/d2ra05289j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Islam F., Khan M.S.S., Ahmed S., Abdullah M., Hannan F., Chen J. OsLPXC negatively regulates tolerance to cold stress via modulating oxidative stress, antioxidant defense and JA accumulation in rice. Free Radic. Biol. Med. 2023;199:2–16. doi: 10.1016/j.freeradbiomed.2023.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Khan M.S.S., Islam F., Chen H., Chang M., Wang D., Liu F., Fu Z.Q., Chen J. Transcriptional coactivators: driving force of plant immunity. Front. Plant Sci. 2022;80 doi: 10.3389/fpls.2022.823937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jia Y., Ding Y., Shi Y., Zhang X., Gong Z., Yang S. The cbfs triple mutants reveal the essential functions of CBF s in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 2016;212:345–353. doi: 10.1111/nph.14088. [DOI] [PubMed] [Google Scholar]
- 51.Weeda S., Zhang N., Zhao X., Ndip G., Guo Y., Buck G.A., Fu C., Ren S. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cisse E.-H.M., Zhang L.-J., Pu Y.-J., Miao L.-F., Li D.-D., Zhang J., Yang F. Exogenous Ca 2+ associated with melatonin alleviates drought-induced damage in the woody tree Dalbergia odorifera. J. Plant Growth Regul. 2021:1–16. [Google Scholar]
- 53.Siddiqui M.H., Alamri S., Khan M.N., Corpas F.J., Al-Amri A.A., Alsubaie Q.D., Ali H.M., Kalaji H.M., Ahmad P. Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J. Hazard Mater. 2020;398 doi: 10.1016/j.jhazmat.2020.122882. [DOI] [PubMed] [Google Scholar]
- 54.Kimura Y., Goto Y.-I., Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxidants Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 55.Zheng Y., Li Z., Cui X., Yang Z., Bao C., Pan L., Liu X., Chatel‐Innocenti G., Vanacker H., Noctor G. S‐Nitrosylation of the histone deacetylase HDA19 stimulates its activity to enhance plant stress tolerance in Arabidopsis. Plant J. 2023;4:836–854. doi: 10.1111/tpj.16174. [DOI] [PubMed] [Google Scholar]
- 56.Zhu Y., Gao H., Lu M., Hao C., Pu Z., Guo M., Hou D., Chen L.-Y., Huang X. Melatonin-nitric oxide crosstalk and their roles in the redox network in plants. Int. J. Mol. Sci. 2019;20:6200. doi: 10.3390/ijms20246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao G., Zhao Y., Yu X., Kiprotich F., Han H., Guan R., Wang R., Shen W. Nitric oxide is required for melatonin-enhanced tolerance against salinity stress in rapeseed (Brassica napus L.) seedlings. Int. J. Mol. Sci. 2018;19:1912. doi: 10.3390/ijms19071912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khanna K., Bhardwaj R., Alam P., Reiter R.J., Ahmad P. Phytomelatonin: a master regulator for plant oxidative stress management. Plant Physiol. Biochem. 2023;196:260–269. doi: 10.1016/j.plaphy.2023.01.035. [DOI] [PubMed] [Google Scholar]
- 59.Song L., Tan Z., Zhang W., Li Q., Jiang Z., Shen S., Luo S., Chen X. Exogenous melatonin improves the chilling tolerance and preharvest fruit shelf life in eggplant by affecting ROS-and senescence-related processes. Horticult. Plant J. 2022;3:523–540. [Google Scholar]
- 60.Aghdam M.S., Luo Z., Jannatizadeh A., Sheikh-Assadi M., Sharafi Y., Farmani B., Fard J.R., Razavi F. Employing exogenous melatonin applying confers chilling tolerance in tomato fruits by upregulating ZAT2/6/12 giving rise to promoting endogenous polyamines, proline, and nitric oxide accumulation by triggering arginine pathway activity. Food Chem. 2019;275:549–556. doi: 10.1016/j.foodchem.2018.09.157. [DOI] [PubMed] [Google Scholar]
- 61.Zhao H., Zhang K., Zhou X., Xi L., Wang Y., Xu H., Pan T., Zou Z. Melatonin alleviates chilling stress in cucumber seedlings by up-regulation of CsZat12 and modulation of polyamine and abscisic acid metabolism. Sci. Rep. 2017;7:4998. doi: 10.1038/s41598-017-05267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi H., Chan Z. The cysteine2/histidine2‐type transcription factor ZINC FINGER OF ARABIDOPSIS THALIANA 6‐activated C‐REPEAT‐BINDING FACTOR pathway is essential for melatonin‐mediated freezing stress resistance in Arabidopsis. J. Pineal Res. 2014;57:185–191. doi: 10.1111/jpi.12155. [DOI] [PubMed] [Google Scholar]
- 63.Corpas F.J., González-Gordo S., Palma J.M. NO source in higher plants: present and future of an unresolved question. Trends Plant Sci. 2022;27:116–119. doi: 10.1016/j.tplants.2021.11.016. [DOI] [PubMed] [Google Scholar]
- 64.Liu J., Yang J., Zhang H., Cong L., Zhai R., Yang C., Wang Z., Ma F., Xu L. Melatonin inhibits ethylene synthesis via nitric oxide regulation to delay postharvest senescence in pears. J. Agric. Food Chem. 2019;67:2279–2288. doi: 10.1021/acs.jafc.8b06580. [DOI] [PubMed] [Google Scholar]
- 65.Wen D., Gong B., Sun S., Liu S., Wang X., Wei M., Yang F., Li Y., Shi Q. Promoting roles of melatonin in adventitious root development of Solanum lycopersicum L. by regulating auxin and nitric oxide signaling. Front. Plant Sci. 2016;7:718. doi: 10.3389/fpls.2016.00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cai S.Y., Zhang Y., Xu Y.P., Qi Z.Y., Li M.Q., Ahammed G.J., Xia X.J., Shi K., Zhou Y.H., Reiter R.J. HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal Res. 2017;62 doi: 10.1111/jpi.12387. [DOI] [PubMed] [Google Scholar]
- 67.Byeon Y., Lee H.Y., Hwang O.J., Lee H.J., Lee K., Back K. Coordinated regulation of melatonin synthesis and degradation genes in rice leaves in response to cadmium treatment. J. Pineal Res. 2015;58:470–478. doi: 10.1111/jpi.12232. [DOI] [PubMed] [Google Scholar]
- 68.Lee K., Choi G.H., Back K. Cadmium‐induced melatonin synthesis in rice requires light, hydrogen peroxide, and nitric oxide: key regulatory roles for tryptophan decarboxylase and caffeic acid O‐methyltransferase. J. Pineal Res. 2017;63 doi: 10.1111/jpi.12441. [DOI] [PubMed] [Google Scholar]
- 69.Okant M., Kaya C. The role of endogenous nitric oxide in melatonin-improved tolerance to lead toxicity in maize plants. Environ. Sci. Pollut. Control Ser. 2019;26:11864–11874. doi: 10.1007/s11356-019-04517-3. [DOI] [PubMed] [Google Scholar]
- 70.Chen J., Zhou H., Xie Y. SnRK2.6 phosphorylation/persulfidation: where ABA and H2S signaling meet. Trends Plant Sci. 2021;26:1207–1209. doi: 10.1016/j.tplants.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 71.de Bont L., Mu X., Wei B., Han Y. Abiotic stress-triggered oxidative challenges: where does H2S act? J. Genet. Genom. 2022;49:748–755. doi: 10.1016/j.jgg.2022.02.019. [DOI] [PubMed] [Google Scholar]
- 72.Liu H., Wang J., Liu J., Liu T., Xue S. Hydrogen sulfide (H2S) signaling in plant development and stress responses. aBIOTECH. 2021;2:32–63. doi: 10.1007/s42994-021-00035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma P., Meyyazhagan A., Easwaran M., Sharma M.M.M., Mehta S., Pandey V., Liu W.-C., Kamyab H., Balasubramanian B., Baskaran R. Hydrogen Sulfide: a new warrior in assisting seed germination during adverse environmental conditions. Plant Growth Regul. 2022;98:401–420. [Google Scholar]
- 74.Zhang W., Wang L., Zhang L., Kong X., Zhang J., Wang X., Pei Y., Jin Z. H2S-mediated balance regulation of stomatal and non-stomatal factors responding to drought stress in Chinese cabbage. Horticult. Res. 2023;10:uhac284. doi: 10.1093/hr/uhac284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y., Wei L., Feng L., Zhang M., Hu D., Tie J., Liao W. Hydrogen sulfide promotes adventitious root development in cucumber under salt stress by enhancing antioxidant ability. Plants. 2022;11:935. doi: 10.3390/plants11070935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aroca A., Yruela I., Gotor C., Bassham D.C. Persulfidation of ATG18a regulates autophagy under ER stress in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2023604118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaya C., Higgs D., Ashraf M., Alyemeni M.N., Ahmad P. Integrative roles of nitric oxide and hydrogen sulfide in melatonin‐induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol. Plantarum. 2020;168:256–277. doi: 10.1111/ppl.12976. [DOI] [PubMed] [Google Scholar]
- 78.Khan M.S.S., Islam F., Ye Y., Ashline M., Wang D., Zhao B., Fu Z.Q., Chen J. The interplay between hydrogen sulfide and phytohormone signaling pathways under challenging environments. Int. J. Mol. Sci. 2022;23:4272. doi: 10.3390/ijms23084272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiang Z.X., Li W., Lu Y.T., Yuan T.T. Hydrogen sulfide alleviates osmotic stress‐induced root growth inhibition by promoting auxin homeostasis. Plant J. 2023;114:1369–1384. doi: 10.1111/tpj.16198. [DOI] [PubMed] [Google Scholar]
- 80.Mukherjee S., Bhatla S.C. Exogenous melatonin modulates endogenous H 2 S homeostasis and L-cysteine desulfhydrase activity in salt-stressed tomato (Solanum lycopersicum L. var. cherry) seedling cotyledons. J. Plant Growth Regulat. 2021;40:2502–2514. [Google Scholar]
- 81.Chen T., Tian M., Han Y. Hydrogen sulfide: a multi-tasking signal molecule in the regulation of oxidative stress responses. J. Exp. Bot. 2020;71:2862–2869. doi: 10.1093/jxb/eraa093. [DOI] [PubMed] [Google Scholar]
- 82.Sun Y., Ma C., Kang X., Zhang L., Wang J., Zheng S., Zhang T. Hydrogen sulfide and nitric oxide are involved in melatonin-induced salt tolerance in cucumber. Plant Physiol. Biochem. 2021;167:101–112. doi: 10.1016/j.plaphy.2021.07.023. [DOI] [PubMed] [Google Scholar]
- 83.Srivastava V., Chowdhary A.A., Verma P.K., Mehrotra S., Mishra S. Hydrogen sulfide‐mediated mitigation and its integrated signaling crosstalk during salinity stress. Physiol. Plantarum. 2022;174 doi: 10.1111/ppl.13633. [DOI] [PubMed] [Google Scholar]
- 84.Khan M.N., Siddiqui M.H., Mukherjee S., AlSolami M.A., Alhussaen K.M., AlZuaibr F.M., Siddiqui Z.H., Al-Amri A.A., Alsubaie Q.D. Melatonin involves hydrogen sulfide in the regulation of H+-ATPase activity, nitrogen metabolism, and ascorbate-glutathione system under chromium toxicity. Environ. Pollut. 2023;323 doi: 10.1016/j.envpol.2023.121173. [DOI] [PubMed] [Google Scholar]
- 85.Wang Z., Mu Y., Zhang L., Liu Z., Liu D., Jin Z., Pei Y. Hydrogen sulfide mediated the melatonin induced stoma closure by regulating the K+ channel in Arabidopsis thaliana. Environ. Exp. Bot. 2023;205 [Google Scholar]
- 86.Ding H., Wang B., Han Y., Li S. The pivotal function of dehydroascorbate reductase in glutathione homeostasis in plants. J. Exp. Bot. 2020;71:3405–3416. doi: 10.1093/jxb/eraa107. [DOI] [PubMed] [Google Scholar]
- 87.Castro P.H., Couto D., Santos M.Â., Freitas S., Lourenço T., Dias E., Huguet S., Marques da Silva J., Tavares R.M., Bejarano E.R. SUMO E3 ligase SIZ1 connects sumoylation and reactive oxygen species homeostasis processes in Arabidopsis. Plant Physiol. 2022;189:934–954. doi: 10.1093/plphys/kiac085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khan M.S.S., Chen J. Regulation of the Interplay between Reactive Oxygen Species and SUMOylation Pathways: an Essential Role for SIZ1. Plant Physiol. 2022;189:454–456. doi: 10.1093/plphys/kiac110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hayat F., Sun Z., Ni Z., Iqbal S., Xu W., Gao Z., Qiao Y., Tufail M.A., Jahan M.S., Khan U. Exogenous melatonin improves cold tolerance of strawberry (Fragaria× ananassa Duch.) through modulation of DREB/CBF-COR pathway and antioxidant defense system. Horticulturae. 2022;8:194. [Google Scholar]
- 90.Xu L., Xiang G., Sun Q., Ni Y., Jin Z., Gao S., Yao Y. Melatonin enhances salt tolerance by promoting MYB108A-mediated ethylene biosynthesis in grapevines. Horticult. Res. 2019;6 doi: 10.1038/s41438-019-0197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu J., Shabala S., Zhang J., Ma G., Chen D., Shabala L., Zeng F., Chen Z.H., Zhou M., Venkataraman G. Melatonin improves rice salinity stress tolerance by NADPH oxidase‐dependent control of the plasma membrane K+ transporters and K+ homeostasis. Plant Cell Environ. 2020;43:2591–2605. doi: 10.1111/pce.13759. [DOI] [PubMed] [Google Scholar]
- 92.Ma L., Zhang H., Sun L., Jiao Y., Zhang G., Miao C., Hao F. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress. J. Exp. Bot. 2012;63:305–317. doi: 10.1093/jxb/err280. [DOI] [PubMed] [Google Scholar]
- 93.Sharma P., Jha A.B., Dubey R.S., Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Botany. 2012;2012 [Google Scholar]
- 94.Miller G., Suzuki N., Ciftci‐Yilmaz S., Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- 95.Li M., Zhou J., Du J., Li X., Sun Y., Wang Z., Lin Y., Zhang Y., Wang Y., He W. Comparative physiological and transcriptomic analyses of improved heat stress tolerance in celery (Apium graveolens L.) caused by exogenous melatonin. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms231911382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu Q., Feng Z., Xu W., Vetukuri R.R., Xu X. Exogenous melatonin-stimulated transcriptomic alterations of Davidia involucrata seedlings under drought stress. Trees (Berl.) 2021;35:1025–1038. [Google Scholar]
- 97.Hu Z., Fu Q., Zheng J., Zhang A., Wang H. Transcriptomic and metabolomic analyses reveal that melatonin promotes melon root development under copper stress by inhibiting jasmonic acid biosynthesis. Horticult. Res. 2020;7 doi: 10.1038/s41438-020-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saha J., Brauer E.K., Sengupta A., Popescu S.C., Gupta K., Gupta B. Polyamines as redox homeostasis regulators during salt stress in plants. Front. Environ. Sci. 2015;3:21. [Google Scholar]
- 99.Wang L., Yang M., Dong Y., Reiter R.J., Xu Y., Lin X., Luo Z., Li L. Melatonin confers enhanced polyamine metabolism and cell tolerance in Vitis vinifera against oxidative damage: quantitative proteomic evidence. Postharvest Biol. Technol. 2022;184 [Google Scholar]
- 100.Annadurai M.K.K., Alagarsamy S., Karuppasami K.M., Ramakrishnan S., Subramanian M., Venugopal P.R., Muthurajan R., Vellingiri G., Dhashnamurthi V., Veerasamy R. Melatonin decreases negative effects of combined drought and high temperature stresses through enhanced antioxidant defense system in tomato leaves. Horticulturae. 2023;9:673. [Google Scholar]
- 101.Xie C., Xiong X., Huang Z., Sun L., Ma J., Cai S., Yu F., Zhong W., Chen S., Li X. Exogenous melatonin improves lead tolerance of bermudagrass through modulation of the antioxidant defense system. Int. J. Phytoremediat. 2018;20:1408–1417. doi: 10.1080/15226514.2018.1488813. [DOI] [PubMed] [Google Scholar]
- 102.Fathi N., Kazemeini S.A., Alinia M., Mastinu A. The effect of seed priming with melatonin on improving the tolerance of Zea mays L. var saccharata to paraquat-induced oxidative stress through photosynthetic systems and enzymatic antioxidant activities. Physiol. Mol. Plant Pathol. 2023;124 [Google Scholar]
- 103.Szafrańska K., Reiter R.J., Posmyk M.M. Melatonin application to Pisum sativum L. seeds positively influences the function of the photosynthetic apparatus in growing seedlings during paraquat-induced oxidative stress. Front. Plant Sci. 2016;7:1663. doi: 10.3389/fpls.2016.01663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gu Q., Chen Z., Yu X., Cui W., Pan J., Zhao G., Xu S., Wang R., Shen W. Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant Sci. 2017;261:28–37. doi: 10.1016/j.plantsci.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 105.C. Wang, C. Bian, J. Li, L. Han, D. Guo, T. Wang, Z. Sun, C. Ma, X. Liu, Y. Tian, X. Zheng, Melatonin promotes Al3+ compartmentalization via H+ transport and ion gradients in Malus hupehensis, Plant Physiol. 2023 Jun 13:kiad339. doi: 10.1093/plphys/kiad339. [DOI] [PubMed]
- 106.Arnaud D., Deeks M.J., Smirnoff N. Organelle-targeted biosensors reveal distinct oxidative events during pattern-triggered immune responses. Plant Physiol. 2023;191:2551–2569. doi: 10.1093/plphys/kiac603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen J., Zhang J., Kong M., Freeman A., Chen H., Liu F. More stories to tell: NONEXPRESSOR OF PATHOGENESIS‐RELATED GENES1, a salicylic acid receptor. Plant Cell Environ. 2021;44:1716–1727. doi: 10.1111/pce.14003. [DOI] [PubMed] [Google Scholar]
- 108.Garcia A.G., Steinbrenner A.D. Bringing plant immunity to light: a genetically encoded, bioluminescent reporter of pattern-triggered immunity in Nicotiana benthamiana. Mol. Plant Microbe Interact. 2023;36:139–149. doi: 10.1094/MPMI-07-22-0160-TA. [DOI] [PubMed] [Google Scholar]
- 109.Hernández-Ruiz J., Giraldo-Acosta M., El Mihyaoui A., Cano A., Arnao M.B. Melatonin as a possible natural anti-viral compound in plant biocontrol. Plants. 2023;12:781. doi: 10.3390/plants12040781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khan M., Ali S., Manghwar H., Saqib S., Ullah F., Ayaz A., Zaman W. Melatonin function and crosstalk with other phytohormones under normal and stressful conditions. Genes. 2022;13:1699. doi: 10.3390/genes13101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hadidi A., Barba M., Candresse T., Jelkmann W. Virus and Virus-like Diseases of Pome and Stone Fruits. Am. Phytopath. Soc. 2011;96:461. [Google Scholar]
- 112.Zhao L., Chen L., Gu P., Zhan X., Zhang Y., Hou C., Wu Z., Wu Y.F., Wang Q.C. Exogenous application of melatonin improves plant resistance to virus infection. Plant Pathol. 2019;68:1287–1295. [Google Scholar]
- 113.Lu R., Liu Z., Shao Y., Sun F., Zhang Y., Cui J., Zhou Y., Shen W., Zhou T. Melatonin is responsible for rice resistance to rice stripe virus infection through a nitric oxide-dependent pathway. Virol. J. 2019;16:1–8. doi: 10.1186/s12985-019-1228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen L., Wang M.R., Li J.W., Feng C.H., Cui Z.H., Zhao L., Wang Q.C. Exogenous application of melatonin improves eradication of apple stem grooving virus from the infected in vitro shoots by shoot tip culture. Plant Pathol. 2019;68:997–1006. [Google Scholar]
- 115.Sofy A.R., Sofy M.R., Hmed A.A., Dawoud R.A., Refaey E.E., Mohamed H.I., El-Dougdoug N.K. Molecular characterization of the Alfalfa mosaic virus infecting Solanum melongena in Egypt and the control of its deleterious effects with melatonin and salicylic acid. Plants. 2021;10:459. doi: 10.3390/plants10030459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ayyaz A., Shahzadi A.K., Fatima S., Yasin G., Zafar Z.U., Athar H-u-R, Farooq M.A. Uncovering the role of melatonin in plant stress tolerance. Theor. Exp. Plant Physiol. 2022;34:335–346. [Google Scholar]
- 117.Wei Y., Zhu B., Ma G., Shao X., Xie H., Cheng X., Zeng H., Shi H. The coordination of melatonin and anti‐bacterial activity by EIL5 underlies ethylene‐induced disease resistance in cassava. Plant J. 2022;111:683–697. doi: 10.1111/tpj.15843. [DOI] [PubMed] [Google Scholar]
- 118.Charfeddine M., Samet M., Charfeddine S., Bouaziz D., Gargouri Bouzid R. Ectopic expression of StERF94 transcription factor in potato plants improved resistance to Fusarium solani infection. Plant Mol. Biol. Rep. 2019;37:450–463. [Google Scholar]
- 119.Zhu G.-Y., Sha P.-F., Zhu X.-X., Shi X.-C., Shahriar M., Zhou Y.-D., Wang S.-Y., Laborda P. Application of melatonin for the control of food-borne Bacillus species in cherry tomatoes. Postharvest Biol. Technol. 2021;181 [Google Scholar]
- 120.Lee H.Y., Byeon Y., Back K. Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J. Pineal Res. 2014;57:262–268. doi: 10.1111/jpi.12165. [DOI] [PubMed] [Google Scholar]
- 121.Reymond P., Farmer E.E. Jasmonate and salicylate as global signals for defense gene expression. Curr. Opin. Plant Biol. 1998;1:404–411. doi: 10.1016/s1369-5266(98)80264-1. [DOI] [PubMed] [Google Scholar]
- 122.Chen X., Laborda P., Liu F. Exogenous melatonin enhances rice plant resistance against Xanthomonas oryzae pv. oryzae. Plant Dis. 2020;104:1701–1708. doi: 10.1094/PDIS-11-19-2361-RE. [DOI] [PubMed] [Google Scholar]
- 123.Nehela Y., Killiny N. Melatonin is involved in citrus response to the pathogen huanglongbing via modulation of phytohormonal biosynthesis. Plant Physiol. 2020;184:2216–2239. doi: 10.1104/pp.20.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee H.Y., Back K. Mitogen‐activated protein kinase pathways are required for melatonin‐mediated defense responses in plants. J. Pineal Res. 2016;60:327–335. doi: 10.1111/jpi.12314. [DOI] [PubMed] [Google Scholar]
- 125.Lee H.Y., Byeon Y., Tan D.X., Reiter R.J., Back K. Arabidopsis serotonin N‐acetyltransferase knockout mutant plants exhibit decreased melatonin and salicylic acid levels resulting in susceptibility to an avirulent pathogen. J. Pineal Res. 2015;58:291–299. doi: 10.1111/jpi.12214. [DOI] [PubMed] [Google Scholar]
- 126.Shi H., Chen Y., Tan D.X., Reiter R.J., Chan Z., He C. Melatonin induces nitric oxide and the potential mechanisms relate to innate immunity against bacterial pathogen infection in Arabidopsis. J. Pineal Res. 2015;59:102–108. doi: 10.1111/jpi.12244. [DOI] [PubMed] [Google Scholar]
- 127.Sun Y., Liu Z., Lan G., Jiao C., Sun Y. Effect ofexogenous melatonin on resistance of cucumber to downy mildew. Sci. Hortic. 2019;255:231–241. [Google Scholar]
- 128.Koo S.C., Moon B.C., Kim J.K., Kim C.Y., Sung S.J., Kim M.C., Cho M.J., Cheong Y.H. OsBWMK1 mediates SA-dependent defense responses by activating the transcription factor OsWRKY33. Biochem. Biophys. Res. Commun. 2009;387:365–370. doi: 10.1016/j.bbrc.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 129.Yang Q., Peng Z., Ma W., Zhang S., Hou S., Wei J., Dong S., Yu X., Song Y., Gao W. Melatonin functions in priming of stomatal immunity in Panax notoginseng and Arabidopsis thaliana. Plant Physiol. 2021;187:2837–2851. doi: 10.1093/plphys/kiab419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao H., Ge Z., Zhou M., Zeng H., Wei Y., Liu G., Yan Y., Reiter R.J., He C., Shi H. Histone deacetylase 9 regulates disease resistance through fine‐tuning histone deacetylation of melatonin biosynthetic genes and melatonin accumulation in cassava. J. Pineal Res. 2023;74 doi: 10.1111/jpi.12861. [DOI] [PubMed] [Google Scholar]
- 131.Bai Y., Wei Y., Yin H., Hu W., Cheng X., Guo J., Dong Y., Zheng L., Xie H., Zeng H. PP2C1 fine‐tunes melatonin biosynthesis and phytomelatonin receptor PMTR1 binding to melatonin in cassava. J. Pineal Res. 2022;73 doi: 10.1111/jpi.12804. [DOI] [PubMed] [Google Scholar]
- 132.Wei Y., Zhu B., Liu W., Cheng X., Lin D., He C., Shi H. Heat shock protein 90 co-chaperone modules fine-tune the antagonistic interaction between salicylic acid and auxin biosynthesis in cassava. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2021.108717. [DOI] [PubMed] [Google Scholar]
- 133.Guo J., Bai Y., Wei Y., Dong Y., Zeng H., Reiter R.J., Shi H. Fine‐tuning of pathogenesis‐related protein 1 (PR1) activity by the melatonin biosynthetic enzyme ASMT2 in defense response to cassava bacterial blight. J. Pineal Res. 2022;72 doi: 10.1111/jpi.12784. [DOI] [PubMed] [Google Scholar]
- 134.Zhang S., Zheng X., Reiter R.J., Feng S., Wang Y., Liu S., Jin L., Li Z., Datla R., Ren M. Melatonin attenuates potato late blight by disrupting cell growth, stress tolerance, fungicide susceptibility and homeostasis of gene expression in Phytophthora infestans. Front. Plant Sci. 2017;8:1993. doi: 10.3389/fpls.2017.01993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wei Y., Hu W., Wang Q., Zeng H., Li X., Yan Y., Reiter R.J., He C., Shi H. Identification, transcriptional and functional analysis of heat‐shock protein 90s in banana (M USA acuminata L.) highlight their novel role in melatonin‐mediated plant response to F usarium wilt. J. Pineal Res. 2017;62 doi: 10.1111/jpi.12367. [DOI] [PubMed] [Google Scholar]
- 136.Mandal M.K., Suren H., Ward B., Boroujerdi A., Kousik C. Differential roles of melatonin in plant‐host resistance and pathogen suppression in cucurbits. J. Pineal Res. 2018;65 doi: 10.1111/jpi.12505. [DOI] [PubMed] [Google Scholar]
- 137.Ali M., Tumbeh Lamin-Samu A., Muhammad I., Farghal M., Khattak A.M., Jan I., ul Haq S., Khan A., Gong Z.-H., Lu G. Melatonin mitigates the infection of Colletotrichum gloeosporioides via modulation of the chitinase gene and antioxidant activity in Capsicum annuum L. Antioxidants. 2020;10:7. doi: 10.3390/antiox10010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li C., He Q., Zhang F., Yu J., Li C., Zhao T., Zhang Y., Xie Q., Su B., Mei L. Melatonin enhances cotton immunity to Verticillium wilt via manipulating lignin and gossypol biosynthesis. Plant J. 2019;100:784–800. doi: 10.1111/tpj.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aseel D.G., Rashad Y.M., Hammad S.M. Arbuscular mycorrhizal fungi trigger transcriptional expression of flavonoid and chlorogenic acid biosynthetic pathways genes in tomato against Tomato Mosaic Virus. Sci. Rep. 2019;9:9692. doi: 10.1038/s41598-019-46281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Miozzi L., Vaira A.M., Catoni M., Fiorilli V., Accotto G.P., Lanfranco L. Arbuscular mycorrhizal symbiosis: plant friend or foe in the fight against viruses? Front. Microbiol. 2019;10:1238. doi: 10.3389/fmicb.2019.01238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Oyewole B., Olawuyi O., Odebode A., Abiala M. Influence of Arbuscular mycorrhiza fungi (AMF) on drought tolerance and charcoal rot disease of cowpea. Biotechnol. Rep. 2017;14:8–15. doi: 10.1016/j.btre.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ahammed G.J., Mao Q., Yan Y., Wu M., Wang Y., Ren J., Guo P., Liu A., Chen S. Role of melatonin in arbuscular mycorrhizal fungi-induced resistance to Fusarium wilt in cucumber. Phytopathology. 2020;110:999–1009. doi: 10.1094/PHYTO-11-19-0435-R. [DOI] [PubMed] [Google Scholar]
- 143.Betsuyaku S., Katou S., Takebayashi Y., Sakakibara H., Nomura N., Fukuda H. Salicylic acid and jasmonic acid pathways are activated in spatially different domains around the infection site during effector-triggered immunity in Arabidopsis thaliana. Plant Cell Physiol. 2018;59:8–16. doi: 10.1093/pcp/pcx181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zeng H., Bai Y., Wei Y., Reiter R.J., Shi H. Phytomelatonin as a central molecule in plant disease resistance. J. Exp. Bot. 2022;73:5874–5885. doi: 10.1093/jxb/erac111. [DOI] [PubMed] [Google Scholar]
- 145.Xie X., Han Y., Yuan X., Zhang M., Li P., Ding A., Wang J., Cheng T., Zhang Q. Transcriptome analysis reveals that exogenous melatonin confers Lilium disease resistance to Botrytis elliptica. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.892674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gao S., Ma W., Lyu X., Cao X., Yao Y. Melatonin may increase disease resistance and flavonoid biosynthesis through effects on DNA methylation and gene expression in grape berries. BMC Plant Biol. 2020;20:1–15. doi: 10.1186/s12870-020-02445-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim M., Ohr H., Lee J.W., Hyun Y., Fischer R.L., Choi Y. Temporal and spatial downregulation of Arabidopsis MET1 activity results in global DNA hypomethylation and developmental defects. Mol. Cell. 2008;26:611. [PMC free article] [PubMed] [Google Scholar]
- 148.Yang Q., Li J., Ma W., Zhang S., Hou S., Wang Z., Li X., Gao W., Rengel Z., Chen Q. Melatonin increases leaf disease resistance and saponin biosynthesis in Panax notogiseng. J. Plant Physiol. 2021;263 doi: 10.1016/j.jplph.2021.153466. [DOI] [PubMed] [Google Scholar]
- 149.Liu C., Chen L., Zhao R., Li R., Zhang S., Yu W., Sheng J., Shen L. Melatonin induces disease resistance to Botrytis cinerea in tomato fruit by activating jasmonic acid signaling pathway. J. Agric. Food Chem. 2019;67:6116–6124. doi: 10.1021/acs.jafc.9b00058. [DOI] [PubMed] [Google Scholar]
- 150.Qu G., Wu W., Ba L., Ma C., Ji N., Cao S. Melatonin enhances the postharvest disease resistance of blueberries fruit by modulating the jasmonic acid signaling pathway and phenylpropanoid metabolites. Front. Chem. 2022;10 doi: 10.3389/fchem.2022.957581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kong M., Sheng T., Liang J., Ali Q., Gu Q., Wu H., Chen J., Liu J., Gao X. Melatonin and its homologs induce immune responses via receptors trP47363-trP13076 in Nicotiana benthamiana. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.691835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li C., Liang B., Chang C., Wei Z., Zhou S., Ma F. Exogenous melatonin improved potassium content in Malus under different stress conditions. J. Pineal Res. 2016;61:218–229. doi: 10.1111/jpi.12342. [DOI] [PubMed] [Google Scholar]
- 153.Kong M., Liang J., Ali Q., Wen W., Wu H., Gao X., Gu Q. 5-methoxyindole, a chemical homolog of melatonin, adversely affects the phytopathogenic fungus Fusarium graminearum. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222010991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huo X., Wang C., Yu Z., Peng Y., Wang S., Feng S., Zhang S., Tian X., Sun C., Liu K. Human transporters, PEPT 1/2, facilitate melatonin transportation into mitochondria of cancer cells: an implication of the therapeutic potential. J. Pineal Res. 2017;62 doi: 10.1111/jpi.12390. [DOI] [PubMed] [Google Scholar]
- 155.Mayo J.C., Sainz R.M., González-Menéndez P., Hevia D., Cernuda-Cernuda R. Melatonin transport into mitochondria. Cell. Mol. Life Sci. 2017;74:3927–3940. doi: 10.1007/s00018-017-2616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.