Abstract
With the development of high-throughput biology techniques and artificial intelligence, it has become increasingly feasible to design and construct artificial biological parts, modules, circuits, and even whole systems. To overcome the limitations of native promoters in controlling gene expression, artificial promoter design aims to synthesize short, inducible, and conditionally controlled promoters to coordinate the expression of multiple genes in diverse plant metabolic and signaling pathways. Synthetic promoters are versatile and can drive gene expression accurately with smart responses; they show potential for enhancing desirable traits in crops, thereby improving crop yield, nutritional quality, and food security. This review first illustrates the importance of synthetic promoters, then introduces promoter architecture and thoroughly summarizes advances in synthetic promoter construction. Restrictions to the development of synthetic promoters and future applications of such promoters in synthetic plant biology and crop improvement are also discussed.
Key words: plants, synthetic promoter, cis element, gene expression
This review outlines current advances in synthetic promoter design for plants, including analysis of promoter structure, prediction of gene expression, and methods of generating synthetic promoters. Applications and promising trends in synthetic promoter development are highlighted.
Introduction
In recent decades, single or multiple genes have been successfully manipulated to improve crop yield, biotic/abiotic stress tolerance, and the production of secondary metabolites for sustainable agriculture (International Service for the Acquisition of Agri-biotech Applications [ISAAA] database, https://www.isaaa.org/gmapprovaldatabase/). Genetically modified crop varieties have been generated by engineering a single gene (e.g., Cry genes from the bacterium Bacillus thuringiensis, Bts, and glyphosate-tolerant 5-enolpyruvylshikimate-3-phosphate synthases, EPSPs) or multiple genes simultaneously (e.g., phytoene synthase, PSY, and bacterial carotene desaturase, CRTI), resulting in 541 transgenic events in 32 crops for global commercial cultivation (Barry et al., 1997; Beyer et al., 2002; Kumar et al., 2020; ISAAA database 2022). Because of the fitness cost of enhanced resistance gene expression driven by a constitutive promoter (CaMV35S or Ubiquitin), the resistance levels of transgenic crops may be limited (van Hulten et al., 2006; Xu et al., 2017; Matsunaga et al., 2019; Sun et al., 2021). Moreover, unintended pleiotropic effects or toxicity of ectopic gene expression may result in epigenetic silencing (Vaucheret and Fagard, 2001; Abdeen and Miki, 2009). To increase crop yield and quality or produce desired plant metabolites, there is a need for innovative technologies that control the expression of multiple genes at the genomic level (Shah et al., 2015; Kumar et al., 2020). Accordingly, synthetic biology enables the modification, deletion, or insertion of DNA sequences at target regions to precisely control gene expression and cellular metabolic processes, thereby facilitating the restoration of extant traits in crop cultivars via evolutionary and artificial selection breeding, and hopefully redesigning and manipulating whole crop systems (Dey et al., 2015; Yu et al., 2021).
The accurate regulation of gene expression depends mainly on promoters, which establish transcription capacity by recruiting corresponding transcription factors (TFs) (Porto et al., 2014). Alteration of the cis elements in a promoter region can regulate the activities of downstream gene expression (Rushton, 2016). Synthetic promoters are manually built to control target gene transcription using diverse cis elements to regulate the binding activities of TFs. Thus, synthetic promoters allow downstream gene expression under specific environmental conditions or at specific developmental stages, producing spatiotemporal and developmental expression patterns. Synthetic promoters can overcome the limitations of native promoters in terms of transcription efficiency, strength, and induction conditions; they can abolish unnecessary negative feedback and reduce metabolic burden and energy loss (Rushton et al., 2002; Mehrotra et al., 2011; Liu et al., 2013; Liu and Stewart., 2016; Rushton, 2016). Synthetic promoters developed in planta show potential for broad application in metabolic engineering, molecular pharming, and crop breeding (Mithra et al., 2017; Wurtzel et al., 2019).
In this review, we first describe general promoter architecture in plants and the limitations of native promoters in controlling gene expression. Next, we summarize current strategies and techniques for synthetic promoter design. Finally, we highlight alternative uses of synthetic promoters for tuning gene expression
Promoter architecture in plants
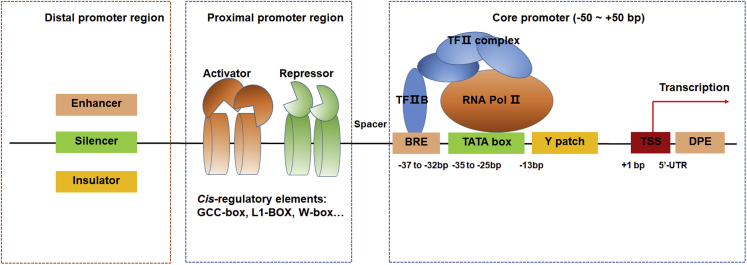
Plant promoters consist primarily of a core promoter region, proximal promoter elements, and distal regions, which are defined by their functions and nucleotide distances from the transcription start site (TSS) (Figure 1; Mithra et al., 2017). The core promoter region typically contains a TATA box (TATA(A/T)A(A/T)), an initiator element (Inr), the TFIIB binding site, and/or downstream promoter elements (Bernard et al., 2010; Murray et al., 2022). The core promoter region encompasses approximately 100 base pairs (bp), from −50 to +50 bp around the TSS (+1) and directly interacts with RNA polymerase Ⅱ to form a preinitiation complex by recruiting TF ⅡD and other basal TF Ⅱs (Porto et al., 2014). Pyrimidine-based Y patches or TC motifs located 13 bp upstream of the TSS have recently been identified as potential cis elements involved in stable expression activity (Yamamoto and Obokata, 2008; Bernard et al., 2010). These elements in the core promoter region have highly conserved positions with respect to the TSS; however, this position conservation does not exist in all genes (Brázda et al., 2021). In addition, the activities of the same core promoter are not fully conserved when analyzed in different genomes, as demonstrated by a comparison between the tobacco epidermis and maize protoplasts (Jores et al., 2021). Core promoters cannot generally drive gene expression at high levels and promote only low basal transcription activities. Although alterations in the architecture and structure of the core promoter lead to changes in gene expression (Jores et al., 2021), core promoters with stable, low expression, such as the 35S mini promoter, are preferred for the assembly of basic transcriptional machinery in synthetic promoters.
Figure 1.
Diagram of promoter architecture in plants.
Plant promoters consist of a core promoter, a proximal promoter region, and distal promoter regions. Distal promoter regions contain enhancer, silencer, and insulator cis elements. The proximal promoter region contains cis elements bound by activator and repressor proteins. The core promoter generally spans from −50 bp to +50 bp and enables assembly of the transcription initiation complex. It contains a TATA box, TF ⅡB recognition elements, a Y patch, and downstream promoter elements (DPEs). There is a spacer sequence of no less than 50 bp between the core promoter and the proximal promoter region. TSS, transcription start site. BRE, TF ⅡB recognition element.
The proximal promoter region spans hundreds of base pairs and includes, among other components, a CAAT box, a GC box, and cis elements that bind various regulators (activators or repressors) (Yamamoto et al., 2011; Rasskazov et al., 2020). In addition to the TATA box, the CAAT box also contributes to the transcription initiation machinery. Sequence analysis and expression-based evaluation of promoter context revealed that proximal promoters in Arabidopsis and rice are mainly located from −200 to +51 bp relative to the TSS (Hiratsuka et al., 2022). Transcriptomics and genomics analyses also showed that TF binding motifs have a bell-shaped distribution from −500 to the TSS and are enriched around −250 bp in several plant species (Weirauch et al., 2014; Ksouri et al., 2021; Rozière et al., 2022). Deep learning revealed that the promoter region spanning from −214 bp to the TSS has a significant effect on gene expression in seven model organisms (Zrimec et al., 2020). These results indicate that the regions from −500 bp to the TSS and from −250 bp to the TATA box constitute the major regulatory region of the proximal promoter and the proximal promoter region enriched in TF binding sites, respectively (Weirauch et al., 2014; Ksouri et al., 2021; Hiratsuka et al., 2022; Rozière et al., 2022). The cis elements of the proximal promoter region show diverse and variable arrangements, and such variations in type and copy number are generally reconstructed to create synthetic promoters.
Enhancers, silencers, insulators, and locus control regions are important distal cis-regulatory elements (CREs) that regulate gene expression in a position- and orientation-independent manner (Schmitz et al., 2022). These regulatory elements, in conjunction with proximal promoter cis elements, mediate gene regulation in an orchestrated manner (Zrimec et al., 2020; Zrimec et al., 2022a, 2022b). Given the uncertainty surrounding the positions of distal CREs, we will consider synthetic promoters to be composed of core and proximal promoter regions, irrespective of distal elements (Schmitz et al., 2022). Thus, the synthetic promoters described here are artificial modules that do not naturally occur in organisms. These promoters are manually created using parts of non-plant, foreign plant, or artificial DNA to control target gene expression in entirely new patterns (Aysha et al., 2018; Belcher et al., 2020).
Strategy of synthetic promoter design
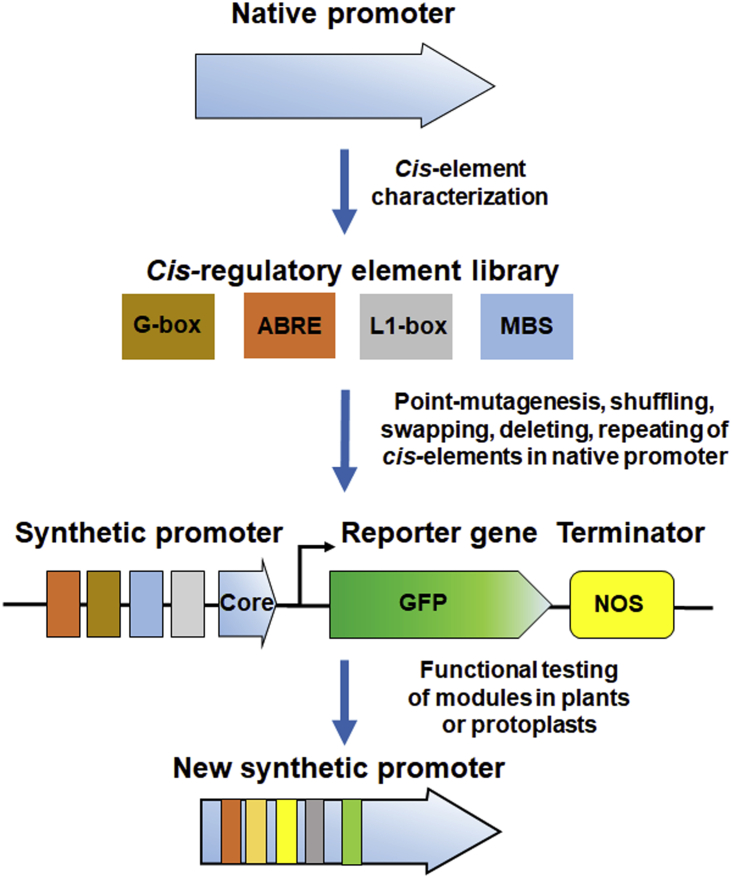
Artificial promoters were initially constructed by manipulating the position, spacing, and number of key cis elements by incorporating them individually or in combination into existing native/core promoters (Cazzonelli, and Velten, 2008; Kumar et al., 2012; Acharya et al., 2014a, 2014b; Deb et al., 2018). Traditionally, the cis elements in native promoters were point mutated, shuffled, repeated, or replaced with novel motifs to generate synthetic promoters with desirable functions (Figure 2). For example, a site-directed mutated cis element was used to generate the DR5 promoter, which is more sensitive to auxin than the original GH3 promoter (Ulmasov et al., 1997; Chen et al., 2013). In addition, different copies of CREs have been spaced and arranged upstream of the minimal promoter to generate novel synthetic promoters (Wu et al., 2018). The spacing between CREs and their distances to the minimal promoter strongly affect the activities of synthetic promoters. CREs or motifs placed extremely close to the core promoter (i.e., <50 bp) may lose their activities owing to steric hindrance because TF Ⅱs can compete with regulatory TFs at the binding sites. Furthermore, spacing differences between two identical CREs may result from functional differences in plant development (Smaczniak et al., 2017). To date, suitable spacing between CREs in synthetic promoters has been determined by functional testing.
Figure 2.
Traditional approach for generating new synthetic promoters.
The cis elements of native promoters are first characterized and then diversified by techniques such as point mutation, shuffling, and repeating of cis elements. The mutated promoters are functionally tested in plants or protoplasts to guide subsequent generation of artificial promoters. ABRE, ABA-responsive element; MBS, MYB transcription factor binding site; Core, core promoter; NOS: nopaline synthase gene terminator.
In addition to the spacing between CREs, the numbers of CREs including a TATA box can be manipulated to improve the associated signal-to-noise ratio and change gene expression (Mogno et al., 2010; Murphy et al., 2010). The inducible strength of a synthetic promoter can be modulated by using different numbers of cis elements. For example, the synthetic pathogen/drought-inducible promoter was generated by combining different copies of regulatory elements (Jameel et al., 2022). These mutation strategies have successfully diversified CREs in native promoters, producing numerous synthetic promoters (Table 1).
Table 1.
Synthetic promoters generated in plants from 2019 to the present.
| Promoter name | cis-element resource | Method used | Expression pattern | Function | Tested plant | Reference |
|---|---|---|---|---|---|---|
| FUASCsV8CP | cassava vein mosaic virus | artificially synthesized | salicylic acid inducibility | enhanced antifungal activity | tobacco | Deb and Dey (2019) |
|
pOp4 pOp6 |
Lac operator CaMV35S mini promoter |
manually synthesized | shoot/root apical meristem, the cambium in Arabidopsis | steroid, dexamethasone inducible | Arabidopsis | |
| Pmec | spacer element, TAGC repeat | manually synthesized | not sure | salt, ABA inducible | tobacco | Dhatterwall et al. (2019); (López-Salmerón et al., 2019) |
| GAL1 | Yeast | manually synthesized | not shown | IPTG inducible | yeast | Naseri et al. (2019) |
| SP-DD; SP-FF; SP-FFDD | CREs from Arabidopsis | hybrid promoter | not confirmed | Ascochyta rabiei, jasmonate, salicylic acid inducible | tobacco | Shokouhifar et al. (2019) |
| BiGSSP2; BiGSSP3; BiGSSP6; BiGSSP7 | Act1, rbcs1 promoter from rice | promoter synthesized | green tissue specific | Bi-directional expression in green tissues | rice | Bai et al. (2020) |
| At2S3 | minimal promoter | artificially synthesized | inducible expression | phosphate responsive | Arabidopsis | Belcher et al. (2020) |
| MiniSyn | CREs from plants | artificially synthesized | constitutive expression | tobacco, oilseed rape, Hordeum vulgare | Cai et al. (2020) | |
|
pro-SmAMP1 pro-SmAMP2 |
Stellaria media | point mutation | proline stress | tobacco | Efremova et al. (2020) | |
| VGWSF; GWVSF; GWSFV | virus inducible cis-element GWSF | insertion of v-box | inducible | pathogen inducible | Arabidopsis | Huang and Li (2020) |
| pCaD | EAS and EAH from hot pepper | CRE deletion and combination | induced by fungal and bacterial pathogens | pathogen-inducible expression | tobacco | In et al. (2020) |
| SynP14-SynP19 | CREs from soybean, CaMV35S | synthetic assemblies | root specific, drought inducible | root specific, drought inducible | soybean hairy roots, Arabidopsis | Jameel et al. (2020) |
|
pKANADI-MotifSYNTHETIC pKANADI-MotifSYNTHETIC pWRKY-MotifSYNTHETIC |
CREs from Arabidopsis | synthetic assemblies | root specific | cell type specific | Arabidopsis | Rich-Griffin et al. (2020) |
| CaMV35S with riboswitch | CaMV35S | module aTheoAz | constitutive expression | theophylline responsive | Arabidopsis | Shanidze et al. (2020) |
| TCSn | bacterial two component signaling | artificially synthesized | cytokinin responsive | cytokinin responsive | rice protoplasts | Ga et al. (2021) |
| CBS:minDFR | yeast operator copper binding site; tomato DFR minimal promoter | manually synthesized | not confirmed | copper inducible | tobacco | |
|
RTBV MMV |
rice tungro bacilliform virus, mirabilis mosaic virus | domain swapping and hybridization | roots and leaves | constitutive expression | tobacco, petunia, rice, pearl millet, bacterium | Gupta et al. (2021) |
| MiniSyn | CREs from plants | artificially synthesized | constitutive expression | tobacco, oilseed rape | Jores et al. (2021) | |
|
S100 D100 |
strawberry vein banding virus, dahlia mosaic virus | element shuffling | abscisic acid, salicylic acid induced | antifungal, antibacterial activity | tobacco | Khadanga et al. (2021) |
|
BL1 BL2 |
ERF53, RD29A minimal promoter | manually synthesized | stress inducible | abiotic stress | Arabidopsis | Kim et al. (2021) |
|
MBR3 FBR3 |
blueberry red ringspot virus | motif deletion, chimeric promoter | constitutive expression in rice | antibacterial antifungal activities | tobacco, rice | Sethi et al. (2021) |
| FvAAT2; FvDOF2 | strawberry | gene editing | fruit expressed | strawberry | Súnico et al. (2021) | |
| SD18-1; SD9-2; SS16-1; SS16-2; SS16-3 | CRES from poplar | SD and SS subdomains shuffled | induced in whole plants | osmotic stress inducible | poplar, tobacco, Arabidopsis | Yang et al. (2021) |
| AtPLGG1; AtBASS6 | combined with 5′ UTR | manually synthesized | chloroplast | induced by temperature, low CO2, and high irradiance | Arabidopsis | |
| STAPs | 35S promoter, effector-binding elements | small synthetic TALE-activated promoter | tunable and tissue-specific expression | dTALE protein inducible | rice | Danila et al. (2022) |
| ZmDRO1B73 | cis elements from maize | manually synthesized | mainly in roots | ABA/drought inducible | maize | Feng et al. (2022) |
| pATFs | gRNA and 35S minimal promoter | synthetic assemblies | gRNA recognized | inducible by gRNAs | tobacco, Arabidopsis | Kar et al. (2022) |
| SP2 | manual combination of cis elements | not tissue specific | drought inducible | Jameel et al. (2022) | ||
| SlDFR | CaMV35S promoters | manual combination of cis elements | dCasEV2.1 responsive | promoted by fungal mPAF A3 part, etc. | tobacco | Moreno-Giménez et al. (2022) |
Synthetic assemblies, manually synthesized: the promoters were synthesized by a DNA synthesis machine or the method was not directly presented.
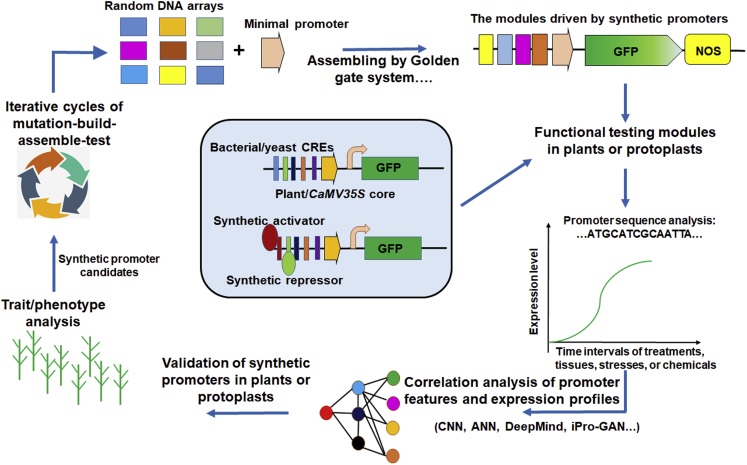
Recent developments in high-throughput molecular biology technologies have facilitated the design of synthetic promoters (Alipanahi et al., 2015; Brückner et al., 2015). Since omics-based methods have been used to explore gene expression profiles, it has become possible to assess multi-dimensional features of promoters and determine the effects of gene expression on biological traits at the genomic level (Contreras-Moreira and Sebastian, 2016; Zrimec et al., 2022a, 2022b; Hong and Cohen, 2022). Computed tomography (CT) technology and crop phenomics provide a basis for rapid and real-time analysis of physiological traits under different conditions throughout plant development (Mochida et al., 2020; Wu et al., 2021). Moreover, machine learning (ML), synthetic promoter libraries (SPLs), and large-scale analysis of promoter expression profiles have enabled the development of mathematical models that describe the relationship between promoter properties and transcriptional strength (Chen et al., 2008; Miyashita et al., 2022; Zhao et al., 2022). At present, synthetic promoter design typically consists of four steps: cis-element characterization, promoter design (or synthesis of random promoter arrays), construction of reporter modules driven by synthetic promoters, and functional testing of the synthetic promoters. After iterative cycles of mutation, construction, screening, and characterization, ideal artificial promoters are obtained that are short, intelligently responsive, and controllable, without notable side effects (Figure 3).
Figure 3.
The minimal promoter approach for synthetic promoter creation.
DNA arrays are synthesized and ligated with a minimal or core promoter to generate SPLs. The synthetic promoters are recombined with a reporter gene such as GFP to generate reporter MSPs. The middle panel shows MSPs in which cis-regulatory elements (CREs) originate from bacteria or yeast. The reporter module is composed of a synthetic promoter, a reporter gene, and a terminator; the synthetic promoter contains cis elements bound by synthetic activators or repressors to conditionally control downstream gene expression. The modules are transiently expressed in plants or protoplasts. After analyzing expression profiles of the reporter gene and DNA sequences of the promoters, promoter datasets are created and used by machine learning (ML) for expression profile prediction. ML models predict promoter sequences and corresponding expression strengths in silico. New synthetic promoters are validated and undergo further iterative rounds if needed. CNN, ANN, DeepMind, and iPro-GAN are deep learning methods.
High-throughput screening of cis elements
The crucial step in the design of efficient synthetic promoters is the identification of cis elements and their functions. The main goals are to identify conserved CREs recognized by TFs and to extract the properties of native promoters, including CRE structures and positions. The functions of cis elements were originally elucidated by functional analysis of deleted or mutated native promoters (Bhullar et al., 2003; Jameel et al., 2022). A number of methods for demonstrating DNA–protein interactions have been widely used to confirm the binding proteins of cis elements. These methods include electrophoretic mobility shift assays (EMSAs) (Gadgil et al., 2001; Hellman and Fried, 2007; Stormo and Zhao, 2010), DNA–protein interaction enzyme-linked immuno-sorbent assays (DPI-ELISA) (Brand et al., 2010), and quantitative DPI-ELISA (qDPI-ELISA) (Fischer et al., 2016). More recently, EMSA sequencing (EMSA-seq), high-throughput sequencing-fluorescent ligand interaction profiling (HiTS-FLIP), ATAC-seq with nuclei sorting, DNA affinity purification sequencing (DAP-seq), chromatin immunoprecipitation sequencing (ChIP-seq), and crosslinking immunoprecipitation sequencing (CLIP-seq) have all been used for rapid discovery of DNA motifs or cis elements at the genomic level (Liu et al., 2002; Chen et al., 2008; Nutiu et al., 2011; Bartlett et al., 2017; Lu et al., 2017). These methods can not only identify genome-wide binding sites for single TFs but also screen candidate TFs binding to multiple CREs.
cis elements can also be identified by co-expression analysis combined with in vivo confirmation assays (Haberer et al., 2006; Liu et al., 2014; Lieberman-Lazarovich et al., 2019). Moreover, multi-omics analyses have been used to predict co-expression modules and networks that connect various developmental pathways and stress responses (Shim et al., 2021). These modules and networks contain large numbers of transcriptional regulatory pairs that demonstrate CRE–TF interactions. Such methods have been used to identify numerous pairs of cis elements and correlated TFs in plants (Zykovich et al., 2009). Currently, data on these CREs and DNA–protein binding pairs are available from a number of databases, including iRegNet, ReMap2022, PLACE, PlantCARE, PlantTFDB, TRANSFAC, and JASPAR (Higo et al., 1999; Hehl and Wingender, 2001; Lescot et al., 2002; Che et al., 2005; Matys et al., 2006; Mahony and Benos, 2007; Guo et al., 2008; Yamamoto and Obokata, 2008; Fornes et al., 2020; Hammal et al., 2022). These databases not only provide experimentally confirmed DNA–protein interactions but also can be used to predict TF candidates that bind to synthetic promoters (Jensen and Liu, 2004; Guo et al., 2008; Bailey et al., 2009; Shahmuradov et al., 2017; Rasskazov et al., 2020). Intrinsic DNA–TF pairs and their DNA shape structures can also be extracted by ML methods to explain dynamic gene expression patterns (Sielemann et al., 2021; Zrimec et al., 2022a, 2022b).
Lieberman-Lazarovich et al. (2019) found that the sequences and positions of CRE variants (such as auxRE motifs) are highly conserved in the promoters of hormone-responsive genes across 45 angiosperms. High salt–responsive CREs were identified using Arabidopsis single-cell RNA sequencing (RNA-seq) data and CRE databases (Uygun et al., 2019), indicating that it is possible to predict gene expression based on the positions and numbers of CREs in a particular gene and to decode the expression profiles of a synthetic promoter during whole-plant growth stages (de Boer et al., 2020). Thus, future studies should identify CRE sequences, positions, and associations with responses to different environmental and developmental signals across plant species (Gasch et al., 2016; Lieberman-Lazarovich et al., 2019; Moore et al., 2022).
Assembling modules driven by synthetic promoters for functional testing
Functional testing of modules driven by synthetic promoters (MSPs) generally involves two steps: MSP assembly and expression pattern analysis. Numerous popular tools (e.g., Gibson, Golden Gate, COMPASS, MoClo, GoldenBraid, MODAL, and PaperClip) have been developed for assembly of reusable DNA parts into working modules, thereby accelerating MSP assembly (Sarrion-Perdigones et al., 2013; Coll et al., 2016; Naseri et al., 2019; Nogueira-López et al., 2019; Lukan et al., 2022). Multiple synthetic parts with different universal adaptors are ligated simultaneously to generate anticipated modules, facilitating assembly work flow (Sarrion-Perdigones et al., 2013; Engler et al., 2014). These methods overcome the limitations of the restriction enzyme digestion and ligation method, enabling efficient and rapid construction of multigene circuits and SPLs.
During SPL construction, various CREs are first synthesized or converted into standard parts with general adaptors. SPLs are then generated by ligating or recombining these CREs with core promoters or a minimal promoter to drive reporter genes (Belcher et al., 2020; Jores et al., 2021; Rao et al., 2021). One example of successful SPL construction is that of Brückner and colleagues, who developed a pathogen-responsive circuit with a designer transcription activator-like effector (dTALE)-activated synthetic promoter (STAP) library recognized by TALE TFs (Brückner et al., 2015). The SPL was composed of a dTALE-DNA binding site and a TATA box flanked by degenerate sequences. The final synthetic promoter drove stable expression of the GUS gene in tobacco plants at a level 10-fold higher than that driven by the CaMV35S promoter (Brückner et al., 2015), demonstrating that SPLs can significantly aid in the construction of new signaling or metabolic pathways in plants while fine-tuning gene expression.
Large-scale confirmation of MSPs in plants remains restricted by transformation efficiency and the lack of a platform for rapid analysis of synthetic promoter expression profiles (Linshiz et al., 2016; Wu et al., 2021; Cao et al., 2022; Maren et al., 2022; Rössner et al., 2022). To circumvent limitations related to transformation efficiency and avoid the cumbersome transformation process, plant protoplasts, tobacco leaves, and model plants such as Marchantia polymorpha have been widely used as hosts for transient expression of MSPs (Jores et al., 2020, 2021; Sauret-Güeto et al., 2020). For example, the expression strengths of 1000 minimal synthetic elements were computationally tested and analyzed in oilseed rape and tobacco protoplasts using a quantitative experimental system. To decrease genotype dependence during generation, the recently developed cut-dip-budding delivery system enabled efficient transformation without the need for tissue culture (Cao et al., 2022). Together, these methods will likely generate large numbers of transgenic cells/plants for functional testing of synthetic promoters.
In addition, with the aid of ML, Self-transcribing active regulatory region sequencing (STARR) sequencing successfully predicted the strengths and activities of synthetic promoters in Arabidopsis, maize, and sorghum (Jores et al., 2021). A fluorescence-inducing laser projector (FLIP) platform equipped with an ultra-low-noise camera enabled researchers to rapidly screen for fluorescent MSP signatures under different stress conditions (Rigoulot et al., 2021). FLIP and CT technology, combined with single-cell omics, may offer a promising approach for precisely exploring the activities of cell-type-specific regulatory elements in plants and may partially overcome the limited accuracy and slow throughput of synthetic promoter testing (Li et al., 2020a, 2020b, 2020c; Dorrity et al., 2021; Marand et al., 2021; Wu et al., 2021; Zhang et al., 2021; Goto-Yamada et al., 2022).
Artificial intelligence for predicting the expression patterns of synthetic promoters
Model-based design of synthetic promoters is an ideal way to construct synthetic promoters in plants (Bhandari et al., 2021; Jores et al., 2021; Zrimec et al., 2022a, 2022b). ML models are generally trained to predict the relationships between synthetic promoter structures and functions. The inputs for these models comprise the promoter sequences and their distribution properties, and the outputs generally include the expression patterns of corresponding synthetic promoters and digitalized traits (Jores et al., 2021; Moore et al., 2022). Establishment of an ML model typically has the following prerequisites: (1) ample random or saturated nucleotide variations in the synthetic promoters; (2) rapid and precise platform to enable large-scale detection of gene expression profiles (Li et al., 2020a, 2020b, 2020c; Wu et al., 2021); (3) massive datasets of DNA–protein interactions (Yamamoto and Obokata, 2008; Alipanahi et al., 2015); and (4) quantitative models or a regression equation to summarize the universal rules of promoter features and expression profiles.
Classical ML methods mainly establish correlations between classified promoter features and expression patterns to interpret the underlying mechanisms of gene regulation (Bhandari et al., 2021). These algorithms, which include linear regression and random forests, use DNA structural properties (such as CREs, the TATA box, K-mer frequencies, and position weight) as explanatory variables to predict gene transcription levels. They are workable when used to estimate the effects of individual CREs on gene expression strength and analyze conserved CREs based on ample transcriptomic data. Accordingly, the TSSPlant tool is trained by a backpropagation algorithm and uses an artificial neural network model to predict TSSs and TATA boxes in plants based on 18 significant compositional and signal features of plant promoter sequences (Shahmuradov et al., 2017).
Gene expression driven by synthetic promoters in plants is affected by their insertion sites in the host genome and the corresponding chromosome conformational structures. Recent reports indicate that nearby enhancers, methylation levels, 3′ UTRs, and even terminators can modulate absolute gene expression levels (Washburn et al., 2019; Zrimec et al., 2020; Li et al., 2021). These factors must be considered when various deep learning methods are developed and used for genomic structure identification and expression analysis (Sartor et al., 2019; Li et al., 2021; Zrimec et al., 2022a, 2022b). Model performance and the accuracy of expression prediction are consistently increased when greater promoter lengths and larger amounts of transcription and/or ATAC-seq data are used in the analysis. For example, Jores et al. (2021) predicted and improved the efficiency and strength of a designed synthetic core promoter through the use of ample mutations and transcriptomic data. Zhao et al. developed the XgBoost model to precisely predict the strength of artificially designed promoter sequences based on SPLs, which enabled the predictable tuning of synthetic promoters to achieve optimal transcriptional strength in bacteria (Zhao et al., 2022). The Zrimec group applied deep learning to large-scale expression datasets to reveal the genetic regulatory code controlling mRNA abundance and directly predict gene expression levels from native DNA sequences (Zrimec et al., 2020).
Recently, Zrimec et al. used generative adversarial networks (GANs) to directly extract properties of genomic and transcriptomic data and increase expression predictions in yeast (Zrimec et al., 2022a; 2022b), demonstrating substantial improvement of deep ML algorithms that use self-adjusting filters to identify sequence properties in gene expression. Advances and comparisons among different algorithms have been well summarized, but the use of efficient deep learning methods based on promoter structure to predict transcription levels in plants is lacking (Zrimec et al., 2021; Yaschenko et al., 2022). Deep ML methods improve prediction accuracy by considering whole-gene regulatory structure, including the structure of the promoter and adjacent regions. They have shown that widespread genetic mutations in large segregated populations can increase resolution and accuracy when evaluating the effects of sequence variants (Chien et al., 2021; Zrimec et al., 2022a, 2022b). Overcoming current limitations—including the lack of broad dynamic expression ranges, spatial and conditionally inducible transcriptomics, and abundant promoter variants for model training—is expected to make the prediction of plant gene expression more accessible (de Boer et al., 2020; Song et al., 2020). Groups of synthetic promoters with expected expression levels generated by ML models can be used in directed evolution and eventually optimized.
Synthetic directed evolution to optimize the structures of synthetic promoters
Recently, synthetic directed evolution (SDE) has emerged as a technique for discovering and modifying plant traits. SDE uses localized sequence diversification (LSD) and selection pressure to evolve promoters with enhanced suitability. SDE is used not only to choose the optimum promoter after testing a large number of random synthetic promoters but also to generate all possible mutations of a particular promoter. After several rounds of mutation, testing, and selection, the newly evolved promoters may produce beneficial quantitative changes in the expression of tissue-specific target genes or in expression strength under specific stress conditions (Oliva et al., 2019; Rao et al., 2021). Because genetic variations that significantly influence crop traits are enriched in regulatory regions (Römer et al., 2010; Zhao et al., 2021), the CREs of native promoters in plants have become hotspots for SDE (Zhang et al., 2017; Li et al., 2020a, 2020b, 2020c, 2022; Zeng et al., 2020).
To decrease the disease susceptibility of rice, random mutations were introduced into the promoters of three host sucrose transporter genes (SWEET11, SWEET13, and SWEET14) by CRISPR-Cas9-mediated gene editing. Inoculation with 63 Xoo strains revealed that these mutations endowed the susceptible varieties IR64 and Ciherang-Sub1 with broad-spectrum resistance to bacterial blight (Oliva et al., 2019). Similarly, mutations were incorporated into targeted promoter regions of maize ZmCLE7 and ZmFCP1, leading to upregulated expression of ZmCLE7 in developing ear primordia and thus to improved yield (Liu et al., 2021). Application of this approach, known as targeting-induced local lesions in genomes (TILLING), to native promoters may provide a practical way to reduce gene pleiotropy and trade-off effects during plant breeding. To overcome time-consuming genetic transformation, the wild type is crossed with transgenic plants carrying multiple guide RNAs (gRNAs) and the Cas9 gene to generate F1 populations. After self-crossing of F1 plants, dozens of tomato plants were efficiently generated in which novel cis-regulatory alleles of CLV3 produced tomatoes with differences in fruit size, inflorescence architecture, and plant growth (Rodriguez-Leal, et al., 2019). This method generates the largest number of potential mutations for the selection of desired phenotypes without repeated genetic transformation and therefore shows promise for the rapid incorporation of mutated promoters into plants.
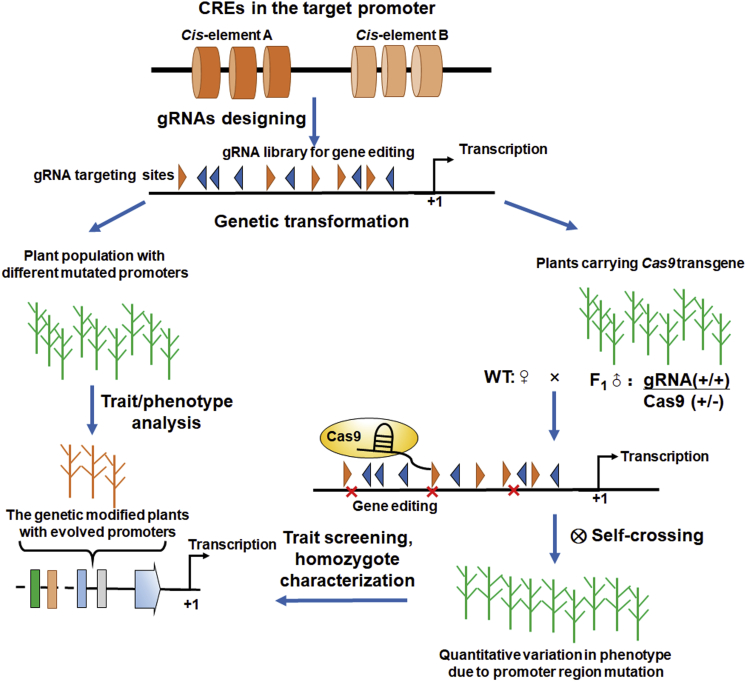
In summary, at least two approaches with different advantages have been developed for synthetic promoter design in plants: the minimal/core promoter approach and the SDE approach (Figure 4; Oliva et al., 2019; Jores et al., 2021). In the minimal promoter approach, random DNA arrays are synthesized and ligated with a core promoter or a 35S minimal promoter to generate synthetic promoters with all possible mutations. The synthetic promoters are then used to drive reporter gene expression for testing in chassis cells such as tobacco epidermal cells or protoplasts (Cai et al., 2020). The detected transcription patterns and sequenced promoter features are then used for ML or deep learning to establish their complex correlations for promoter structure design ahead of synthetic promoter construction. This approach produces evolved promoters and models for use in synthetic promoter design. However, the evolved promoters must be tested in native plants when selecting suitable synthetic promoters for plant biotechnology or breeding.
Figure 4.
The directed evolution approach for synthetic promoter generation in vivo.
Based on the cis elements of native plant promoters, gRNA libraries for gene editing are synthesized and ligated into a CRISPR-Cas9 vector. The positive plasmids are co-transformed into Agrobacterium to create a library with multiple editing sites for use in Agrobacterium infection. Large-scale Agrobacterium-mediated transformation is performed to generate plant populations with directed mutated promoters for trait assessment in the field. To reduce the number of genetic transformations, F1 plants carrying Cas9 and gRNAs are selected and crossed with the wild type (WT) to generate larger plant populations with direct-mutated promoters. After field testing and trait assessment, the genetically modified plants with evolved promoters are chosen as germplasms for plant biotechnology or breeding. Triangles show the binding sites of gRNAs. +1 indicates the TSS.
By contrast, the SDE approach is used to obtain functional synthetic promoters in situ after mutation of pivotal regions rather than arbitrary regions of the target promoter(s) (Hendelman et al., 2021) (Figure 4). The target promoters are mutated by gene editing to generate a number of allele variations, thereby producing plants with evolved promoters for phenotype screening. Because phenotype analysis is completed in the field or greenhouse, plants with the evolved promoters can be used directly in plant breeding (Liu et al., 2021; Wang et al., 2021; Song et al., 2022). In addition, the SDE approach shows potential for evolving the native promoters of multiple co-expressed genes by combinatorial gene editing owing to the conservation of CREs (Lieberman-Lazarovich et al., 2019).
Limitations overcome by synthetic promoters in the control of gene expression
Synthetic promoters were first constructed in the 1990s. Since then, significant advances have been made in the rational design of synthetic promoters for specific goals. In the following sections, we summarize the characteristics of synthetic promoters that are distinct from those of native promoters in the control of gene expression.
Minimal and short synthetic promoters
Most native plant promoters span hundreds of nucleotides, are bound by endogenous or orthologous TFs, and show complex patterns of regulation in response to stress or developmental signals (Liu et al., 2013). Synthetic promoter design uses a divide-and-conquer approach for removal of non-essential elements to construct a minimal promoter for optimum metabolism and energy (Bhullar et al., 2003; Mithra et al., 2017; Aysha et al., 2018; Cai et al., 2020). Because of their low complexity, ideal minimal synthetic promoters are capable of overcoming the restrictions of native promoters and minimizing background noise and unwanted cross-talk between synergetic circuits and endogenous networks. Wang et al. truncated non-essential sequences and incorporated green tissue–specific cis elements into the synthetic promoters of GSSP1, GSSP3, and GSSP5-7, resulting in stronger transcriptional activities (Wang et al., 2015). Minimal synthetic promoters may have varying expression strengths when activated by various TFs and can produce expected expression patterns in transient systems and stable transgenic Arabidopsis plants (Cai et al., 2020). To reduce gene silencing and background disturbance, minimal promoters from bacteria and viruses, such as the CaMV35S minimal promoter, are often selected for synthesis of synthetic promoters. Synthetic promoters with short sequences can decrease the length of expression cassettes and consequently improve transformation efficiency in plants.
Tissue-specific and inducible synthetic promoters
Native tissue-specific promoters can drive gene expression in different tissues or organs; however, few of these promoters have been characterized. To control tissue- and condition-specific gene expression, leaf- (Wang et al., 2015), vascular- (Lv et al., 2009), and root-specific (Mohan et al., 2017) synthetic promoters have been generated. For example, the DR5 promoter, a well-known auxin-responsive synthetic promoter, is widely used to visually quantify the level of indole-3-acetic acid response (0–100 μM) in meristematic tissues (Ulmasov et al., 1997; Chen et al., 2013). Furthermore, Kumar et al. (2012) observed that transcriptional activity was affected by the spacing and number of TGACG motifs. The promoter FS-(TGACG)2, in which an additional TGACG motif was inserted upstream of the original one, exhibited enhanced expression in roots and salicylic acid inducibility (Kumar et al., 2012; Tomaž et al., 2022). Synthetic promoters can also be created by reordering and changing the number of CREs in the promoter, thereby increasing the strength of the endogenous promoter. For example, three hybrid synthetic promoters were constructed with stress-inducible characteristics by rearranging and adding the Dof-1 element (Ranjan and Dey, 2012). Similarly, the pCL1b promoter with alterations in the CRT/DRE element facilitates subtle regulation of cold responses to prevent cold-induced sweetening (Li et al., 2015). SynP15, SynP16, and SynP18 are root-specific and drought-inducible synthetic promoters that were developed by concatenating 11 cis motifs (Jameel et al., 2020). Recently, inducible promoters were designed to generate an inducible circuit that responded to several abiotic stresses without compromising plant growth (Pierre-Jerome et al., 2016). These synthetic promoters can drive tissue-specific and/or stress-inducible gene expression in modified plants, improving biotic/abiotic stress tolerance and productivity.
Synthetic promoters can mitigate gene silencing
To decrease transgene silencing that results from repetitive use of a single promoter, synthetic promoters can combine functionally equivalent cis elements from diverse non-plant organisms, thus maximizing sequence diversity (Kumar et al., 2012, 2015; Gupta et al., 2021; Khadanga et al., 2021; Sethi et al., 2021). Hybrid promoters using the components of two viruses had 2.5 times higher activity than the CaMV35S promoter in prokaryotes and plants (Gupta et al., 2021). Recently, Belcher et al. incorporated CREs from yeast into the upstream region of a plant minimal promoter, generating an artificial promoter that functions in planta (Belcher et al., 2020). Synthetic promoters containing CREs from other organisms avoid homologous recombination and transgene silencing when the same native promoters are used to express multiple transgenes (Shah et al., 2015; Belcher et al., 2020).
Bi-directional synthetic promoters enable gene stacking
Bi-directional promoters (BDPs), which are typically located between adjacent genes in a head-to-head manner, coordinate gene transcription in opposite directions (Shin et al., 2003; Dhadi et al., 2009; Zheng et al., 2011; Araceli et al., 2017; In et al., 2020). As opposed to unidirectional promoters, BDPs can aggregate multiple genes to generate protein products in stoichiometric quantities (Dhadi et al., 2009). BDPs were first made by fusing unidirectional minimal promoters at their 5′ ends in opposite orientations to facilitate gene stacking at a single locus (Chaturvedi et al., 2006; Zhang et al., 2008). In turn, the inducible BDPs P1301A and P1301B were synthesized after integrating the PCEC promoter with different cis motifs (Chaturvedi et al., 2006). Similarly, Li et al. developed a bi-directional duplex promoter (BDDP) by placing two similar core promoters in opposite orientations upstream and downstream of duplicated enhancer elements. The artificial BDDP increased transgene expression by 206- to ∼300-fold (Li et al., 2004). Patro et al. developed a BDP by adding two heterogeneous core promoters in opposite orientations upstream and downstream of three enhancers, thereby improving promoter activity (Patro et al., 2013). These synthetic BDPs overcome the limitation of low expression strength associated with natural BDPs.
BDPs can also be designed to control tissue-specific and/or inducible gene expression (Mehrotra et al., 2011). Araceli et al. synthesized a Pi-induced promoter that responds to low levels of phosphate as well as abiotic stresses (Araceli et al., 2017), and the modified pCaD promoter serves as a good example of a pathogen-inducible BDP (In et al., 2020). A series of BDPs, termed BiGSSPs, were developed by assembling different cis elements to drive reporter genes with highly bi-directional green tissue–specific expression efficiencies (Bai et al., 2020). This work confirmed that the same core promoter can be used to generate BDPs when introducing different introns (OsTub6I and OsAct1) in opposite directions. Recently, BDPs have been used for co-expression of genes in the same metabolic pathways and as multiple gRNAs in the CRISPR-Cas system, thereby enabling multiplex genome editing (Vogl et al., 2018; Ren et al., 2019; Hsieh-Feng and Yang, 2020). BDPs thus provide more flexibility for inducible and spatiotemporal gene editing.
Synthetic promoters as parts of intelligent biosensor modules
Synthetic promoters can be obtained by incorporating heterologous regulatory elements that recognize physical or chemical signals and dynamically adjust gene expression (Samalova et al., 2005; Zürcher et al., 2013). To sense exogenous signals, synthetic promoters containing inducible elements have been fused with a reporter and utilized as a module of in vivo phytosensors (Liu et al., 2011). The signals are recognized by the synthetic promoters, which then drive the expression of the reporter module to amplify signals for rapid digital detection. For example, a synthetic signal transduction module was constructed using an artificial PlantPho promoter to monitor environmental pollutants, explosives, and chemical agents (Antunes et al., 2011). The transcriptional output module from the cis elements of hypoxia-responsive ERF1 and ERF2 acts as an oxygen-sensing machinery and inhibits cysteine oxidase enzymes in plants under hypoxic conditions (Gasch et al., 2016). Phosphate-starvation-driven BDPs based on the M-EZ2 enhancer could respond sensitively to changes in Pi concentration (Araceli et al., 2017). Recently, a pathogen-specific synthetic phytosensor has been developed in potato plants using a synthetic 4×S-box promoter and the Q-system TF as an amplified reporter and is able to detect extracellular signals at the early stages of bacterial infection (Persad-Russell et al., 2022). Varied cis elements (QUAS elements) in the synthetic promoter amplified previously undetectable signals at different levels. Overall, these results show the significant capacity of synthetic promoters in plants as delicate indicators that respond to environmental changes.
Concluding remarks and future perspectives
Synthetic promoters have been successfully used for multiple gene editing, crop improvement, and biosensor design through in situ gene editing (Oliva et al., 2019; Persad-Russell et al., 2020; Hendelman et al., 2021; Kar et al., 2022; Moreno-Giménez et al., 2022). Such promoters have significant potential to fine-tune gene expression and consequently develop superior crop varieties with tailored morphologies and resilience to biotic and abiotic stresses. However, several limitations must be overcome prior to their successful commercial application (Righetti et al., 2021): (1) we lack sufficient information about the features of CREs in promoters, including their spacing, order, and potential regulators. Although great advances have been made in plant genomics, it remains difficult to establish direct correlation between promoter architectures and gene expression compared with advances achieved in bacteria and humans. This limitation necessitates the use of artificial intelligence to predict the effects of numerous environmental and developmental signals involved in gene regulation at the genomic level (Yaschenko et al., 2022; Zrimec et al., 2022a, 2022b). Future studies should consider investigating the overall epigenetic features of CREs at specific times, environmental and developmental stages, and tissues and/or cells (Moore et al., 2022). (2) Highly efficient transformation technology is required to create transgenic plants with synthetic promoters. Large-scale testing of the expression profiles of synthetic promoters typically uses plant protoplasts as the host cells, an approach that provides limited insight into gene expression driven by the synthetic promoters in whole plants under different conditions. Future investigations should aim to develop a gamete (pollen) transformation method that can generate ample transgenic plants for immediate screening of gene expression. (3) A novel method that integrates real-time sequencing with various screening methods, such as the FLIP platform, is required to monitor gene expression and immediately manipulate translational changes (Hagenbeek and Rock, 2001; Rigoulot et al., 2021). Crop phenomics is another method for analyzing plant physiological responses to environmental changes throughout their lifespans, revealing how promoter mutations influence complex plant traits (Mochida et al., 2020).
To overcome these difficulties, three trends have emerged in the design of synthetic plant promoters: (1) the use of synthetic promoters to drive single-cell or tissue-specific gene expression, such as in roots to control single-cell/tissue development, has become feasible. Single-cell sequencing allows for a deeper understanding of the regulatory code of plant promoters and the manner in which it shapes transcription and translation levels, thereby enabling its manipulation to control gene expression in a restricted space (Rich-Griffin et al., 2020; Dorrity, et al., 2021; Brophy et al., 2022). (2) Synthetic promoters can serve as switches to conditionally regulate target gene expression. In such systems, synthetic promoters can sense external signals to turn on/off the expression of synthetic regulators (TFs or synthetic dCas proteins) (Marchisio and Huang, 2017; Li et al., 2019; Garcia-Perez et al., 2022). A module is generally composed of a synthetic promoter and synthetic regulators (e.g., activator, dcas9/12-VP64; repressor, dcas9/12-KARB). Because the proximal promoter regions of target genes contain single or multiple copies of the binding sequence for the gRNA, the module progressively regulates endogenous gene expression in the plant (Papikian et al., 2019; Selma et al., 2019). These modules have potential applications in gene editing and plant metabolic engineering by finely regulating bottleneck gene expression in a pathway (Papikian et al., 2019; Selma et al., 2019; Moreno-Giménez et al., 2022). (3) It is now possible to establish a closed circuit in plants that is completely controlled by synthetic parts independent from the native parts. The closed circuit is composed of only about two or three modules that control gene expression by performing logic operations. Notably, the modified plant traits are predictable (Brophy et al., 2022).
Synthetic promoters have been developed based on modern biotechnologies whose products overlap somewhat with genetically modified organism (GMO) products. To alleviate increasing concerns about such research projects, particularly with respect to biosafety, the governments of different countries have produced legislation and regulations to confine application scope and decrease potential ecological threats. If synthetic promoters become more “intelligent,” we believe that such promoters will develop rapidly and may soon become deployable to improve crop yield and quality.
Author contributions
E.Y., J.W., and M.R. collected the references and wrote the manuscript draft. L.Z. revised the manuscript. K.Z. wrote and revised the manuscript.
Funding
This work was funded by Key Research and Development Projects (nos. 2018YFA0901000 and 2018YFA0901003) and the BIO-Agri. project of SJTU.
Acknowledgments
We thank many colleagues who have contributed to research on promoter analysis and synthetic promoter design in plants and apologize to those whose works were not cited owing to space limitations.
Published: February 9, 2023
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
References
- Abdeen A., Miki B. The pleiotropic effects of the bar gene and glufosinate on the Arabidopsis transcriptome. Plant Biotechnol. J. 2009;7:266–282. doi: 10.1111/j.1467-7652.2009.00398.x. [DOI] [PubMed] [Google Scholar]
- Acharya S., Ranjan R., Pattanaik S., Maiti I.B., Dey N. Efficient chimeric plant promoters derived from plant infecting viral promoter sequences. Planta. 2014;239:381–396. doi: 10.1007/s00425-013-1973-2. [DOI] [PubMed] [Google Scholar]
- Acharya S., Sengupta S., Patro S., Purohit S., Samal S.K., Maiti I.B., Dey N. Development of an intra-molecularly shuffled efficient chimeric plant promoter from plant infecting Mirabilis mosaic virus promoter sequence. J. Biotechnol. 2014;169:103–111. doi: 10.1016/j.jbiotec.2013.08.022. [DOI] [PubMed] [Google Scholar]
- Alipanahi B., Delong A., Weirauch M.T., Frey B.J. Predicting the sequence specificities of DNA-and RNA-binding proteins by deep learning. Nat. Biotechnol. 2015;33:831–838. doi: 10.1038/nbt.3300. [DOI] [PubMed] [Google Scholar]
- Antunes M.S., Morey K.J., Smith J.J., Albrecht K.D., Bowen T.A., Zdunek J.K., Troupe J.F., Cuneo M.J., Webb C.T., Hellinga H.W., et al. Programmable ligand detection system in plants through a synthetic signal transduction pathway. PLoS One. 2011;6:e16292. doi: 10.1371/journal.pone.0016292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araceli O.A., Alfredo C.R., Javier M.M., Luis H.E. A phosphate starvation-driven bidirectional promoter as a potential tool for crop improvement and in vitro plant biotechnology. Plant Biotechnol. J. 2017;15:558–567. doi: 10.1111/pbi.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aysha J., Noman M., Wang F., Liu W., Zhou Y., Li H., Li X. Synthetic promoters: designing the cis regulatory modules for controlled gene expression. Mol. Biotechnol. 2018;60:608–620. doi: 10.1007/s12033-018-0089-0. [DOI] [PubMed] [Google Scholar]
- Bai J., Wang X., Wu H., Ling F., Zhao Y., Lin Y., Wang R. Comprehensive construction strategy of bidirectional green tissue-specific synthetic promoters. Plant Biotechnol. J. 2020;18:668–678. doi: 10.1111/pbi.13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey T.L., Boden M., Buske F.A., Frith M., Grant C.E., Clementi L., Ren J., Li W.W., Noble W.S. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry G.F., Kishore G.M., Padgette S.R., Stallings W.C. Glyphosatetolerant 5-enolpyruvylshikimate-3-phosphate synthases. US Patent 5633435. 1997 [Google Scholar]
- Bartlett A., O'Malley R.C., Huang S.S.C., Galli M., Nery J.R., Gallavotti A., Ecker J.R. Mapping genome-wide transcription-factor binding sites using DAP-seq. Nat. Protoc. 2017;12:1659–1672. doi: 10.1038/nprot.2017.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher M.S., Vuu K.M., Zhou A., Mansoori N., Agosto-Ramos A., Thompson M.G., Scheller H.V., Loqué D., Shih P.M. Design of orthogonal regulatory systems for modulating gene expression in plants. Nat. Chem. Biol. 2020;16:857–865. doi: 10.1038/s41589-020-0547-4. [DOI] [PubMed] [Google Scholar]
- Bernard V., Brunaud V., Lecharny A. TC-motifs at the TATA-box expected position in plant genes: a novel class of motifs involved in the transcription regulation. BMC Genom. 2010;11:166. doi: 10.1186/1471-2164-11-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer P., Al-Babili S., Ye X., Lucca P., Schaub P., Welsch R., Potrykus I. Golden Rice: introducing the beta-carotene biosynthesis pathway into rice endosperm by genetic engineering to defeat vitamin A deficiency. J. Nutr. 2002;132:506S–510S. doi: 10.1093/jn/132.3.506S. [DOI] [PubMed] [Google Scholar]
- Bhandari N., Khare S., Walambe R., Kotecha K. Comparison of machine learning and deep learning techniques in promoter prediction across diverse species. PeerJ. Comput. Sci. 2021;7:e365. doi: 10.7717/peerj-cs.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhullar S., Chakravarthy S., Advani S., Datta S., Pental D., Burma P.K. Strategies for development of functionally equivalent promoters with minimum sequence homology for transgene expression in plants: cis-elements in a novel DNA context versus domain swapping. Plant Physiol. 2003;132:988–998. doi: 10.1104/pp.103.020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand L.H., Kirchler T., Hummel S., Chaban C., Wanke D. DPI-ELISA: a fast and versatile method to specify the binding of plant transcription factors to DNA in vitro. Plant Methods. 2010;6:25. doi: 10.1186/1746-4811-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brázda V., Bartas M., Bowater R.P. Evolution of diverse strategies for promoter regulation. Trends Genet. 2021;37:730–744. doi: 10.1016/j.tig.2021.04.003. [DOI] [PubMed] [Google Scholar]
- Brophy J.A.N., Magallon K.J., Duan L., Zhong V., Ramachandran P., Kniazev K., Dinneny J.R. Synthetic genetic circuits as a means of reprogramming plant roots. Science. 2022;377:747–751. doi: 10.1126/science.abo4326. [DOI] [PubMed] [Google Scholar]
- Brückner K., Schäfer P., Weber E., Grützner R., Marillonnet S., Tissier A. A library of synthetic transcription activator-like effector-activated promoters for coordinated orthogonal gene expression in plants. Plant J. 2015;82:707–716. doi: 10.1111/tpj.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y.M., Kallam K., Tidd H., Gendarini G., Salzman A., Patron N.J. Rational design of minimal synthetic promoters for plants. Nucleic Acids Res. 2020;48:11845–11856. doi: 10.1093/nar/gkaa682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Xie H., Song M., Lu J., Ma P., Huang B., Wang M., Tian Y., Chen F., Peng J., et al. Cut-dip-budding delivery system enables genetic modifications in plants without tissue culture. Innovation. 2022;4:100345. doi: 10.1016/j.xinn.2022.100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzonelli C.I., Velten J. In vivo characterization of plant promoter element interaction using synthetic promoters. Transgenic Res. 2008;17:437–457. doi: 10.1007/s11248-007-9117-8. [DOI] [PubMed] [Google Scholar]
- Chaturvedi C.P., Sawant S.V., Kiran K., Mehrotra R., Lodhi N., Ansari S.A., Tuli R. Analysis of polarity in the expression from a multifactorial bidirectional promoter designed for high-level expression of transgenes in plants. J. Biotechnol. 2006;123:1–12. doi: 10.1016/j.jbiotec.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Che D., Jensen S., Cai L., Liu J.S. BEST: binding-site estimation suite of tools. Bioinformatics. 2005;21:2909–2911. doi: 10.1093/bioinformatics/bti425. [DOI] [PubMed] [Google Scholar]
- Chen X., Guo L., Fan Z., Jiang T. W-AlignACE: an improved Gibbs sampling algorithm based on more accurate position weight matrices learned from sequence and gene expression/ChIP-chip data. Bioinformatics. 2008;24:1121–1128. doi: 10.1093/bioinformatics/btn088. [DOI] [PubMed] [Google Scholar]
- Chen Y., Yordanov Y.S., Ma C., Strauss S., Busov V.B. DR5 as a reporter system to study auxin response in Populus. Plant Cell Rep. 2013;32:453–463. doi: 10.1007/s00299-012-1378-x. [DOI] [PubMed] [Google Scholar]
- Chien C.H., Huang L.Y., Lo S.F., Chen L.J., Liao C.C., Chen J.J., Chu Y.W. Using machine learning approaches to predict target gene expression in rice T-DNA insertional mutants. Front. Genet. 2021;12:798107. doi: 10.3389/fgene.2021.798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll A., Wilson M.L., Gruden K., Peccoud J. GenoCAD Plant grammar to design plant expression vectors for promoter analysis. Methods Mol. Biol. 2016;1482:219–232. doi: 10.1007/978-1-4939-6396-6_14. [DOI] [PubMed] [Google Scholar]
- Contreras-Moreira B., Sebastian A. FootprintDB: analysis of plant cis-regulatory elements, transcription factors, and binding interfaces. Methods Mol. Biol. 2016;1482:259–277. doi: 10.1007/978-1-4939-6396-6_17. [DOI] [PubMed] [Google Scholar]
- Danila F., Schreiber T., Ermakova M., Hua L., Vlad D., Lo S.F., Chen Y.S., Lambret-Frotte J., Hermanns A.S., Athmer B., et al. A single promoter-TALE system for tissue-specific and tuneable expression of multiple genes in rice. Plant Biotechnol. J. 2022;20:1786–1806. doi: 10.1111/pbi.13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer C.G., Vaishnav E.D., Sadeh R., Abeyta E.L., Friedman N., Regev A. Deciphering eukaryotic gene regulatory logic with 100 million random promoters. Nat. Biotechnol. 2020;38:56–65. doi: 10.1038/s41587-019-0315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb D., Dey N. Synthetic Salicylic acid inducible recombinant promoter for translational research. J. Biotechnol. 2019;297:9–18. doi: 10.1016/j.jbiotec.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Deb D., Shrestha A., Maiti I.B., Dey N. Recombinant promoter (MUASCsV8CP) driven totiviral killer protein 4 (KP4) imparts resistance against fungal pathogens in transgenic tobacco. Front. Plant Sci. 2018;9:278. doi: 10.3389/fpls.2018.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey N., Sarkar S., Acharya S., Maiti I.B. Synthetic promoters in planta. Planta. 2015;242:1077–1094. doi: 10.1007/s00425-015-2377-2. [DOI] [PubMed] [Google Scholar]
- Dhadi S.R., Krom N., Ramakrishna W. Genome-wide comparative analysis of putative bidirectional promoters from rice, Arabidopsis and Populus. Gene. 2009;429:65–73. doi: 10.1016/j.gene.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Dhatterwal P., Basu S., Mehrotra S., Mehrotra R. Genome wide analysis of W-box element in Arabidopsis thaliana reveals TGAC motif with genes down regulated by heat and salinity. Sci. Rep. 2019;9:1681. doi: 10.1038/s41598-019-38757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrity M.W., Alexandre C.M., Hamm M.O., Vigil A.L., Fields S., Queitsch C., Cuperus J.T. The regulatory landscape of Arabidopsis thaliana roots at single-cell resolution. Nat. Commun. 2021;12:3334. doi: 10.1038/s41467-021-23675-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efremova L.N., Strelnikova S.R., Gazizova G.R., Minkina E.A., Komakhin R.A. A synthetic strong and constitutive promoter derived from the Stellaria media pro-SmAMP1 and pro-SmAMP2 promoters for effective transgene expression in plants. Genes. 2020;11:1407. doi: 10.3390/genes11121407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler C., Youles M., Gruetzner R., Ehnert T.M., Werner S., Jones J.D.G., Patron N.J., Marillonnet S. A golden gate modular cloning toolbox for plants. ACS Synth. Biol. 2014;3:839–843. doi: 10.1021/sb4001504. [DOI] [PubMed] [Google Scholar]
- Feng X., Jia L., Cai Y., Guan H., Zheng D., Zhang W., Xiong H., Zhou H., Wen Y., Hu Y., et al. ABA-inducible DEEPER ROOTING 1 improves adaptation of maize to water deficiency. Plant Biotechnol. J. 2022;20:2077–2088. doi: 10.1111/pbi.13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S.M., Böser A., Hirsch J.P., Wanke D. Quantitative analysis of protein-DNA Interaction by qDPI-ELISA. Methods Mol. Biol. 2016;1482:49–66. doi: 10.1007/978-1-4939-6396-6_4. [DOI] [PubMed] [Google Scholar]
- Fornes O., Castro-Mondragon J.A., Khan A., van der Lee R., Zhang X., Richmond P.A., Modi B.P., Correard S., Gheorghe M., Baranašić D., et al. JASPAR 2020: update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2020;48:D87–D92. doi: 10.1093/nar/gkz1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ga E., Song J., Min M.K., Ha J., Park S., Lee S.B., Lee J.Y., Kim B.G. Reconstitution of cytokinin signaling in rice protoplasts. Int. J. Mol. Sci. 2021;22:3647. doi: 10.3390/ijms22073647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadgil H., Oak S.A., Jarrett H.W. Affinity purification of DNA-binding proteins. J. Biochem. Biophys. Methods. 2001;49:607–624. doi: 10.1016/s0165-022x(01)00223-8. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez E., Diego-Martin B., Quijano-Rubio A., Moreno-Giménez E., Selma S., Orzaez D., Vazquez-Vilar M. A copper switch for inducing CRISPR/Cas9-based transcriptional activation tightly regulates gene expression in Nicotiana benthamiana. BMC Biotechnol. 2022;22:12. doi: 10.1186/s12896-022-00741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch P., Fundinger M., Müller J.T., Lee T., Bailey-Serres J., Mustroph A. Redundant ERF-VII transcription factors bind to an evolutionarily conserved cis-motif to regulate hypoxia-responsive gene expression in Arabidopsis. Plant Cell. 2016;28:160–180. doi: 10.1105/tpc.15.00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto-Yamada S., Oikawa K., Yamato K.T., Kanai M., Hikino K., Nishimura M., Mano S. Image-based analysis revealing the molecular mechanism of peroxisome dynamics in plants. Front. Cell Dev. Biol. 2022;10:883491. doi: 10.3389/fcell.2022.883491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A.Y., Chen X., Gao G., Zhang H., Zhu Q.H., Liu X.C., Zhong Y.F., Gu X., He K., Luo J. PlantTFDB: a comprehensive plant transcription factor database. Nucleic Acids Res. 2008;36:D966–D969. doi: 10.1093/nar/gkm841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D., Dey N., Leelavathi S., Ranjan R. Development of efficient synthetic promoters derived from pararetrovirus suitable for translational research. Planta. 2021;253:42. doi: 10.1007/s00425-021-03565-9. [DOI] [PubMed] [Google Scholar]
- Haberer G., Mader M.T., Kosarev P., Spannagl M., Yang L., Mayer K.F.X. Large-scale cis-element detection by analysis of correlated expression and sequence conservation between Arabidopsis and Brassica oleracea. Plant Physiol. 2006;142:1589–1602. doi: 10.1104/pp.106.085639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbeek D., Rock C.D. Quantitative analysis by flow cytometry of abscisic acid-inducible gene expression in transiently transformed rice protoplasts. Cytometry. 2001;45:170–179. doi: 10.1002/1097-0320(20011101)45:3<170::aid-cyto1160>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hammal F., de-Langen P., Bergon A., Lopez F., Ballester B. ReMap 2022: a database of Human, Mouse, Drosophila and Arabidopsis regulatory regions from an integrative analysis of DNA-binding sequencing experiments. Nucleic Acids Res. 2022;50:D316–D325. doi: 10.1093/nar/gkab996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehl R., Wingender E. Database-assisted promoter analysis. Trends Plant Sci. 2001;6:251–255. doi: 10.1016/s1360-1385(01)01954-9. [DOI] [PubMed] [Google Scholar]
- Hellman L.M., Fried M.G. Electrophoretic mobility shift assay (EMSA) for detecting protein-nucleic acid interactions. Nat. Protoc. 2007;2:1849–1861. doi: 10.1038/nprot.2007.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendelman A., Zebell S., Rodriguez-Leal D., Dukler N., Robitaille G., Wu X., Kostyun J., Tal L., Wang P., Bartlett M.E., et al. Conserved pleiotropy of an ancient plant homeobox gene uncovered by cis-regulatory dissection. Cell. 2021;184:1724–1739.e16. doi: 10.1016/j.cell.2021.02.001. [DOI] [PubMed] [Google Scholar]
- Higo K., Ugawa Y., Iwamoto M., Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka T., Makita Y., Yamamoto Y.Y. Sequence-based evaluation of promoter context for prediction of transcription start sites in Arabidopsis and rice. Sci. Rep. 2022;12:6976. doi: 10.1038/s41598-022-11169-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C.K.Y., Cohen B.A. Genomic environments scale the activities of diverse core promoters. Genome Res. 2022;32:85–96. doi: 10.1101/gr.276025.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh-Feng V., Yang Y. Efficient expression of multiple guide RNAs for CRISPR/Cas genome editing. Abiotech. 2020;1:123–134. doi: 10.1007/s42994-019-00014-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Li H. Effect of virus inducible cis-element insertion on transcription properties of improved GWSF promoter in Arabidopsis thaliana. Biol. Plant. 2020;64:320–323. [Google Scholar]
- In S., Lee H.A., Woo J., Park E., Choi D. Molecular characterization of a pathogen-inducible bidirectional promoter from hot pepper (Capsicum annuum) Mol. Plant Microbe Interact. 2020;33:1330–1339. doi: 10.1094/MPMI-07-20-0183-R. [DOI] [PubMed] [Google Scholar]
- ISAAA database GM approval database retrieved on sept. 2022. https://www.isaaa.org/gmapproval database/default.asp
- Jameel A., Ketehouli T., Wang Y., Wang F., Li X., Li H. Detection and validation of cis-regulatory motifs in osmotic stress-inducible synthetic gene switches via computational and experimental approaches. Funct. Plant Biol. 2022;49:1043–1054. doi: 10.1071/FP21314. [DOI] [PubMed] [Google Scholar]
- Jameel A., Noman M., Liu W., Ahmad N., Wang F., Li X., Li H. Tinkering cis motifs jigsaw puzzle led to root-specific drought-inducible novel synthetic promoters. Int. J. Mol. Sci. 2020;21:1357. doi: 10.3390/ijms21041357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S.T., Liu J.S. BioOptimizer: a Bayesian scoring function approach to motif discovery. Bioinformatics. 2004;20:1557–1564. doi: 10.1093/bioinformatics/bth127. [DOI] [PubMed] [Google Scholar]
- Jores T., Tonnies J., Dorrity M.W., Cuperus J.T., Fields S., Queitsch C. Identification of plant enhancers and their constituent elements by STARR-seq in tobacco leaves. Plant Cell. 2020;32:2120–2131. doi: 10.1105/tpc.20.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jores T., Tonnies J., Wrightsman T., Buckler E.S., Cuperus J.T., Fields S., Queitsch C. Synthetic promoter designs enabled by a comprehensive analysis of plant core promoters. Nat. Plants. 2021;7:842–855. doi: 10.1038/s41477-021-00932-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar S., Bordiya Y., Rodriguez N., Kim J., Gardner E.C., Gollihar J.D., Sung S., Ellington A.D. Orthogonal control of gene expression in plants using synthetic promoters and CRISPR-based transcription factors. Plant Methods. 2022;18:42. doi: 10.1186/s13007-022-00867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadanga B., Chanwala J., Sandeep I.S., Dey N. Synthetic promoters from strawberry vein banding virus (SVBV) and Dahlia Mosaic Virus (DaMV) Mol. Biotechnol. 2021;63:792–806. doi: 10.1007/s12033-021-00344-5. [DOI] [PubMed] [Google Scholar]
- Kim K., Kim B., Shin J., Kim W.C. Construction of novel promoters based on the characteristics of drought stress specific cis-regulatory element. J. Appl. Biol. Chem. 2021;64:39–48. [Google Scholar]
- Ksouri N., Castro-Mondragón J.A., Montardit-Tarda F., van Helden J., Contreras-Moreira B., Gogorcena Y. Tuning promoter boundaries improves regulatory motif discovery in nonmodel plants: the peach example. Plant Physiol. 2021;185:1242–1258. doi: 10.1093/plphys/kiaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D., Patro S., Ghosh J., Das A., Maiti I.B., Dey N. Development of a salicylic acid inducible minimal sub-genomic transcript promoter from Figwort mosaic virus with enhanced root-and leaf-activity using TGACG motif rearrangement. Gene. 2012;503:36–47. doi: 10.1016/j.gene.2012.04.053. [DOI] [PubMed] [Google Scholar]
- Kumar K., Gambhir G., Dass A., Tripathi A.K., Singh A., Jha A.K., Yadava P., Choudhary M., Rakshit S. Genetically modified crops: current status and future prospects. Planta. 2020;251:91. doi: 10.1007/s00425-020-03372-8. [DOI] [PubMed] [Google Scholar]
- Kumar S., AlAbed D., Whitteck J.T., Chen W., Bennett S., Asberry A., Wang X., DeSloover D., Rangasamy M., Wright T.R., et al. A combinatorial bidirectional and bicistronic approach for coordinated multi-gene expression in corn. Plant Mol. Biol. 2015;87:341–353. doi: 10.1007/s11103-015-0281-6. [DOI] [PubMed] [Google Scholar]
- Lescot M., Déhais P., Thijs G., Marchal K., Moreau Y., Van-de Peer Y., Rouzé P., Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Li W., Zhou Z., Chen H., Xie C., Lin Y. A new rice breeding method: CRISPR/Cas9 system editing of the Xa13 promoter to cultivate transgene-free bacterial blight-resistant rice. Plant Biotechnol. J. 2020;18:313–315. doi: 10.1111/pbi.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Zhang R., Meng X., Chen S., Zong Y., Lu C., Qiu J.L., Chen Y.H., Li J., Gao C. Targeted, random mutagenesis of plant genes with dual cytosine and adenine base editors. Nat. Biotechnol. 2020;38:875–882. doi: 10.1038/s41587-019-0393-7. [DOI] [PubMed] [Google Scholar]
- Li J., Hsu A., Hua Y., Wang G., Cheng L., Ochiai H., Yamamoto T., Pertsinidis A. Single-gene imaging links genome topology, promoter-enhancer communication and transcription control. Nat. Struct. Mol. Biol. 2020;27:1032–1040. doi: 10.1038/s41594-020-0493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Xie C., Song B., Ou Y., Lin Y., Liu X., Zhang H., Liu J. Construction of efficient, tuber-specific, and cold-inducible promoters in potato. Plant Sci. 2015;235:14–24. doi: 10.1016/j.plantsci.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Li Q., Feng Q., Snouffer A., Zhang B., Rodríguez G.R., van-der, Knaap E. Increasing fruit weight by editing a cis-regulatory element in tomato KLUH promoter using CRISPR/Cas9. Front. Plant Sci. 2022;13:879642. doi: 10.3389/fpls.2022.879642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Jiang H., Kong L., Chen Y., Lang K., Fan X., Zhang L., Pian C. Deep6mA: a deep learning framework for exploring similar patterns in DNA N6-methyladenine sites across different species. PLoS Comput. Biol. 2021;17:e1008767. doi: 10.1371/journal.pcbi.1008767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Xiong X., Li J.F. The working dead: repurposing inactive CRISPR-associated nucleases as programmable transcriptional regulators in plants. Abiotech. 2019;1:32–40. doi: 10.1007/s42994-019-00003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.T., Jayasankar S., Gray D.J. Bi-directional duplex promoters with duplicated enhancers significantly increase transgene expression in grape and tobacco. Transgenic Res. 2004;13:143–154. doi: 10.1023/b:trag.0000026074.11859.77. [DOI] [PubMed] [Google Scholar]
- Lieberman-Lazarovich M., Yahav C., Israeli A., Efroni I. Deep conservation of cis-element variants regulating plant hormonal responses. Plant Cell. 2019;31:2559–2572. doi: 10.1105/tpc.19.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linshiz G., Jensen E., Stawski N., Bi C., Elsbree N., Jiao H., Kim J., Mathies R., Keasling J.D., Hillson N.J. End-to-end automated microfluidic platform for synthetic biology: from design to functional analysis. J. Biol. Eng. 2016;10:3–15. doi: 10.1186/s13036-016-0024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Gallagher J., Arevalo E.D., Chen R., Skopelitis T., Wu Q., Bartlett M., Jackson D. Enhancing grain-yield-related traits by CRISPR-Cas9 promoter editing of maize CLE genes. Nat. Plants. 2021;7:287–294. doi: 10.1038/s41477-021-00858-5. [DOI] [PubMed] [Google Scholar]
- Liu W., Mazarei M., Peng Y., Fethe M.H., Rudis M.R., Lin J., Millwood R.J., Arelli P.R., Stewart C.N., Jr. Computational discovery of soybean promoter cis-regulatory elements for the construction of soybean cyst nematode-inducible synthetic promoters. Plant Biotechnol. J. 2014;12:1015–1026. doi: 10.1111/pbi.12206. [DOI] [PubMed] [Google Scholar]
- Liu W., Mazarei M., Rudis M.R., Fethe M.H., Stewart C.N. Rapid in vivo analysis of synthetic promoters for plant pathogen phytosensing. BMC Biotechnol. 2011;11:108. doi: 10.1186/1472-6750-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Stewart C.N., Jr. Plant synthetic promoters and transcription factors. Curr. Opin. Biotechnol. 2016;37:36–44. doi: 10.1016/j.copbio.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Liu W., Yuan J.S., Stewart C.N., Jr. Advanced genetic tools for plant biotechnology. Nat. Rev. Genet. 2013;14:781–793. doi: 10.1038/nrg3583. [DOI] [PubMed] [Google Scholar]
- Liu X.S., Brutlag D.L., Liu J.S. An algorithm for finding protein-DNA binding sites with applications to chromatin-immunoprecipitation microarray experiments. Nat. Biotechnol. 2002;20:835–839. doi: 10.1038/nbt717. [DOI] [PubMed] [Google Scholar]
- López-Salmerón V., Schürholz A.K., Li Z., Schlamp T., Wenzl C., Lohmann J.U., Greb T., Wolf S. Inducible, cell type-specific expression in Arabidopsis thaliana through LhGR-mediated trans-activation. J Vis Exp. 2019 doi: 10.3791/59394. [DOI] [PubMed] [Google Scholar]
- Lu Z., Hofmeister B.T., Vollmers C., DuBois R.M., Schmitz R.J. Combining ATAC-seq with nuclei sorting for discovery of cis-regulatory regions in plant genomes. Nucleic Acids Res. 2017;45:e41. doi: 10.1093/nar/gkw1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukan T., Gruden K., Coll A. Plant X-tender toolbox for the assembly and expression of multiple transcriptional units in plants. Methods Mol. Biol. 2022;2379:81–97. doi: 10.1007/978-1-0716-1791-5_5. [DOI] [PubMed] [Google Scholar]
- Lv X., Song X., Rao G., Pan X., Guan L., Jiang X., Lu H. Construction vascular-specific expression bi-directional promoters in plants. J. Biotechnol. 2009;141:104–108. doi: 10.1016/j.jbiotec.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Mahony S., Benos P.V. STAMP: a web tool for exploring DNA-binding motif similarities. Nucleic Acids Res. 2007;35:W253–W258. doi: 10.1093/nar/gkm272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marand A.P., Chen Z., Gallavotti A., Schmitz R.J. A cis-regulatory atlas in maize at single-cell resolution. Cell. 2021;184:3041–3055.e21. doi: 10.1016/j.cell.2021.04.014. [DOI] [PubMed] [Google Scholar]
- Marchisio M.A., Huang Z. CRISPR-Cas type II-based synthetic biology applications in eukaryotic cells. RNA Biol. 2017;14:1286–1293. doi: 10.1080/15476286.2017.1282024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren N.A., Duan H., Da K., Yencho G.C., Ranney T.G., Liu W. Genotype-independent plant transformation. Hortic. Res. 2022;9:uhac047. doi: 10.1093/hr/uhac047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga W., Shimura H., Shirakawa S., Isoda R., Inukai T., Matsumura T., Masuta C. Transcriptional silencing of 35S driven-transgene is differentially determined depending on promoter methylation heterogeneity at specific cytosines in both plus- and minus-sense strands. BMC Plant Biol. 2019;19:24. doi: 10.1186/s12870-019-1628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matys V., Kel-Margoulis O.V., Fricke E., Liebich I., Land S., Barre-Dirrie A., Reuter I., Chekmenev D., Krull M., Hornischer K., et al. TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res. 2006;34:D108–D110. doi: 10.1093/nar/gkj143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra R., Gupta G., Sethi R., Bhalothia P., Kumar N., Mehrotra S. Designer promoter: an artwork of cis engineering. Plant Mol. Biol. 2011;75:527–536. doi: 10.1007/s11103-011-9755-3. [DOI] [PubMed] [Google Scholar]
- Mithra S.A., Kulkarni K., Srinivasan R. In: Plant Biotechnology: Principles and Applications. Abdin M., Kiran U., Kamaluddin, Ali A., editors. Springer; 2017. Plant promoters: characterization and applications in transgenic technology; pp. 117–172. [Google Scholar]
- Miyashita E., Komatsu K.R., Saito H. Large-scale analysis of RNA-protein interactions for functional RNA motif discovery using FOREST. Methods Mol. Biol. 2022;2509:279–290. doi: 10.1007/978-1-0716-2380-0_17. [DOI] [PubMed] [Google Scholar]
- Mochida K., Nishii R., Hirayama T. Decoding plant-environment interactions that influence crop agronomic traits. Plant Cell Physiol. 2020;61:1408–1418. doi: 10.1093/pcp/pcaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogno I., Vallania F., Mitra R.D., Cohen B.A. TATA is a modular component of synthetic promoters. Genome Res. 2010;20:1391–1397. doi: 10.1101/gr.106732.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan C., Jayanarayanan A.N., Narayanan S. Construction of a novel synthetic root-specific promoter and its characterization in transgenic tobacco plants. 3 Biotech. 2017;7:234. doi: 10.1007/s13205-017-0872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B.M., Lee Y.S., Wang P., Azodi C., Grotewold E., Shiu S.H. Modeling temporal and hormonal regulation of plant transcriptional response to wounding. Plant Cell. 2022;34:867–888. doi: 10.1093/plcell/koab287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Giménez E., Selma S., Calvache C., Orzáez D. GB_SynP: a Modular dCas9-regulated synthetic promoter collection for fine-tuned recombinant gene expression in plants. ACS Synth. Biol. 2022;11:3037–3048. doi: 10.1021/acssynbio.2c00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K.F., Adams R.M., Wang X., Balázsi G., Collins J.J. Tuning and controlling gene expression noise in synthetic gene networks. Nucleic Acids Res. 2010;38:2712–2726. doi: 10.1093/nar/gkq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A., Mendieta J.P., Vollmers C., Schmitz R.J. Simple and accurate transcriptional start site identification using Smar2C2 and examination of conserved promoter features. Plant J. 2022;112:583–596. doi: 10.1111/tpj.15957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseri G., Behrend J., Rieper L., Mueller-Roeber B. COMPASS for rapid combinatorial optimization of biochemical pathways based on artificial transcription factors. Nat. Commun. 2019;10:2615. doi: 10.1038/s41467-019-10224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira-López G., Padilla-Arizmendi F., Inwood S., Lyne S., Steyaert J.M., Nieto-Jacobo M.F., Stewart A., Mendoza-Mendoza A. TrichoGate: an improved vector system for a large scale of functional analysis of Trichoderma genes. Front. Microbiol. 2019;10:2794. doi: 10.3389/fmicb.2019.02794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutiu R., Friedman R.C., Luo S., Khrebtukova I., Silva D., Li R., Zhang L., Schroth G.P., Burge C.B. Direct measurement of DNA affinity landscapes on a high-throughput sequencing instrument. Nat. Biotechnol. 2011;29:659–664. doi: 10.1038/nbt.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva R., Ji C., Atienza-Grande G., Huguet-Tapia J.C., Perez-Quintero A., Li T., Eom J.S., Li C., Nguyen H., Liu B., et al. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 2019;37:1344–1350. doi: 10.1038/s41587-019-0267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papikian A., Liu W., Gallego-Bartolomé J., Jacobsen S.E. Site-specific manipulation of Arabidopsis loci using CRISPR-Cas9 SunTag systems. Nat. Commun. 2019;10:729. doi: 10.1038/s41467-019-08736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro S., Maiti I.B., Dey N. Development of an efficient bi-directional promoter with tripartite enhancer employing three viral promoters. J. Biotechnol. 2013;163:311–317. doi: 10.1016/j.jbiotec.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Persad-Russell R., Mazarei M., Schimel T.M., Howe L., Schmid M.J., Kakeshpour T., Barnes C.N., Brabazon H., Seaberry E.M., Reuter D.N., et al. Specific bacterial pathogen phytosensing is enabled by a synthetic promoter-transcription factor system in potato. Front. Plant Sci. 2022;13:873480. doi: 10.3389/fpls.2022.873480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre-Jerome E., Moss B.L., Lanctot A., Hageman A., Nemhauser J.L. Functional analysis of molecular interactions in synthetic auxin response circuits. Proc. Natl. Acad. Sci. USA. 2016;113:11354–11359. doi: 10.1073/pnas.1604379113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto M.S., Pinheiro M.P.N., Batista V.G.L., dos-Santos R.C., Filho P.d.A.M., de- Lima L.M. Plant promoters: an approach of structure and function. Mol. Biotechnol. 2014;56:38–49. doi: 10.1007/s12033-013-9713-1. [DOI] [PubMed] [Google Scholar]
- Ranjan R., Dey N. Development of vascular tissue and stress inducible hybrid-synthetic promoters through DOF-1 motifs rearrangement. Cell Biochem. Biophys. 2012;63:235–245. doi: 10.1007/s12013-012-9359-9. [DOI] [PubMed] [Google Scholar]
- Rao G.S., Jiang W., Mahfouz M. Synthetic directed evolution in plants: unlocking trait engineering and improvement. Synth. Biol. 2021;6:ysab025. doi: 10.1093/synbio/ysab025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasskazov D., Chadaeva I., Sharypova E., Zolotareva K., Khandaev B., Ponomarenko P., Podkolodnyy N., Tverdokhleb N., Vishnevsky O., Bogomolov A., et al. Plant_SNP_TATA_Z-Tester: a web service that unequivocally estimates the impact of proximal promoter mutations on plant gene expression. Int. J. Mol. Sci. 2020;23:8684. doi: 10.3390/ijms23158684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q., Zhong Z., Wang Y., You Q., Li Q., Yuan M., He Y., Qi C., Tang X., Zheng X., et al. Bidirectional promoter-based CRISPR-Cas9 systems for plant genome editing. Front. Plant Sci. 2019;10:1173. doi: 10.3389/fpls.2019.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Griffin C., Eichmann R., Reitz M.U., Hermann S., Woolley-Allen K., Brown P.E., Wiwatdirekkul K., Esteban E., Pasha A., Kogel K.H., et al. Regulation of cell type-specific immunity networks in Arabidopsis roots. Plant Cell. 2020;32:2742–2762. doi: 10.1105/tpc.20.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righetti E., Uluşeker C., Kahramanoğulları O. Stochastic simulations as a tool for assessing signal fidelity in gene expression in synthetic promoter design. Biology. 2021;10:724. doi: 10.3390/biology10080724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigoulot S.B., Schimel T.M., Lee J.H., Sears R.G., Brabazon H., Layton J.S., Li L., Meier K.A., Poindexter M.R., Schmid M.J., et al. Imaging of multiple fluorescent proteins in canopies enables synthetic biology in plants. Plant Biotechnol. J. 2021;19:830–843. doi: 10.1111/pbi.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Leal D., Xu C., Kwon C.T., Soyars C., Demesa-Arevalo E., Man J., Liu L., Lemmon Z.H., Jones D.S., Van Eck J., et al. Evolution of buffering in a genetic circuit controlling plant stem cell proliferation. Nat. Genet. 2019;51:786–792. doi: 10.1038/s41588-019-0389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer P., Recht S., Strauß T., Elsaesser J., Schornack S., Boch J., Wang S., Lahaye T. Promoter elements of rice susceptibility genes are bound and activated by specific TAL effectors from the bacterial blight pathogen, Xanthomonas oryzae pv. oryzae. New Phytol. 2010;187:1048–1057. doi: 10.1111/j.1469-8137.2010.03217.x. [DOI] [PubMed] [Google Scholar]
- Rössner C., Lotz D., Becker A. VIGS Goes Viral: how VIGS transforms our understanding of plant science. Annu. Rev. Plant Biol. 2022;73:703–728. doi: 10.1146/annurev-arplant-102820-020542. [DOI] [PubMed] [Google Scholar]
- Rozière J., Guichard C., Brunaud V., Martin M.L., Coursol S. A comprehensive map of preferentially located motifs reveals distinct proximal cis-regulatory sequences in plants. Front. Plant Sci. 2022;13:976371. doi: 10.3389/fpls.2022.976371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton P.J. What have we learned about synthetic promoter construction? Methods Mol. Biol. 2016;1482:1–13. doi: 10.1007/978-1-4939-6396-6_1. [DOI] [PubMed] [Google Scholar]
- Rushton P.J., Reinstädler A., Lipka V., Lippok B., Somssich I.E. Synthetic plant promoters containing defined regulatory elements provide novel insights into pathogen- and wound-induced signaling. Plant Cell. 2002;14:749–762. doi: 10.1105/tpc.010412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samalova M., Brzobohaty B., Moore I. pOp6/LhGR: a stringently regulated and highly responsive dexamethasone-inducible gene expression system for tobacco. Plant J. 2005;41:919–935. doi: 10.1111/j.1365-313X.2005.02341.x. [DOI] [PubMed] [Google Scholar]
- Sarrion-Perdigones A., Vazquez-Vilar M., Palací J., Castelijns B., Forment J., Ziarsolo P., Blanca J., Granell A., Orzaez D. GoldenBraid 2.0: a comprehensive DNA assembly framework for plant synthetic biology. Plant Physiol. 2013;162:1618–1631. doi: 10.1104/pp.113.217661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor R.C., Noshay J., Springer N.M., Briggs S.P. Identification of the expressome by machine learning on omics data. Proc. Natl. Acad. Sci. USA. 2019;116:18119–18125. doi: 10.1073/pnas.1813645116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauret-Güeto S., Frangedakis E., Silvestri L., Rebmann M., Tomaselli M., Markel K., Delmans M., West A., Patron N.J., Haseloff J. Systematic tools for reprogramming plant gene expression in a simple model, Marchantia polymorpha. ACS Synth. Biol. 2020;9:864–882. doi: 10.1021/acssynbio.9b00511. [DOI] [PubMed] [Google Scholar]
- Schmitz R.J., Grotewold E., Stam M. Cis-regulatory sequences in plants: their importance, discovery, and future challenges. Plant Cell. 2022;34:718–741. doi: 10.1093/plcell/koab281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selma S., Bernabé-Orts J.M., Vazquez-Vilar M., Diego-Martin B., Ajenjo M., Garcia-Carpintero V., Granell A., Orzaez D. Strong gene activation in plants with genome-wide specificity using a new orthogonal CRISPR/Cas9-based programmable transcriptional activator. Plant Biotechnol. J. 2019;17:1703–1705. doi: 10.1111/pbi.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi L., Deb D., Khadanga B., Dey N. Synthetic promoters from blueberry red ringspot virus (BRRV) Planta. 2021;253:121. doi: 10.1007/s00425-021-03624-1. [DOI] [PubMed] [Google Scholar]
- Shah S.H., Jan S.A., Ahmad N., Khan S., Kumar T., Iqbal A., Nasir F., Noman M., Ali U. Use of different promoters in transgenic plant development: current challenges and future perspectives. Am.-Eurasian J. Agric. Environ. Sci. 2015;15:664–675. [Google Scholar]
- Shahmuradov I.A., Umarov R.K., Solovyev V.V. TSSPlant: a new tool for prediction of plant Pol II promoters. Nucleic Acids Res. 2017;45:e65. doi: 10.1093/nar/gkw1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanidze N., Lenkeit F., Hartig J.S., Funck D. A theophylline-responsive riboswitch regulates expression of nuclear-encoded genes. Plant Physiol. 2020;182:123–135. doi: 10.1104/pp.19.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim S., Park C.M., Seo P.J. iRegNet: an integrative Regulatory Network analysis tool for Arabidopsis thaliana. Plant Physiol. 2021;187:1292–1309. doi: 10.1093/plphys/kiab389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R., Kim M.J., Paek K.H. The CaTin1 (Capsicum annuum TMV-induced clone 1). and CaTin1-2 genes are linked head-to-head and share a bidirectional promoter. Plant Cell Physiol. 2003;44:549–554. doi: 10.1093/pcp/pcg069. [DOI] [PubMed] [Google Scholar]
- Shokouhifar F., Bahrabadi M., Bagheri A., Mamarabadi M. Transient expression analysis of synthetic promoters containing F and D cis-acting elements in response to Ascochyta rabiei and two plant defense hormones. Amb. Express. 2019;9:195. doi: 10.1186/s13568-019-0919-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sielemann J., Wulf D., Schmidt R., Bräutigam A. Local DNA shape is a general principle of transcription factor binding specificity in Arabidopsis thaliana. Nat. Commun. 2021;12:6549. doi: 10.1038/s41467-021-26819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaczniak C., Muiño J.M., Chen D., Angenent G.C., Kaufmann K. Differences in DNA binding specificity of floral homeotic protein complexes predict organ-specific target genes. Plant Cell. 2017;29:1822–1835. doi: 10.1105/tpc.17.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q., Lee J., Akter S., Rogers M., Grene R., Li S. Prediction of condition-specific regulatory genes using machine learning. Nucleic Acids Res. 2020;48:e62. doi: 10.1093/nar/gkaa264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Meng X., Guo H., Cheng Q., Jing Y., Chen M., Liu G., Wang B., Wang Y., Li J., et al. Targeting a gene regulatory element enhances rice grain yield by decoupling panicle number and size. Nat. Biotechnol. 2022;40:1403–1411. doi: 10.1038/s41587-022-01281-7. [DOI] [PubMed] [Google Scholar]
- Stormo G.D., Zhao Y. Determining the specificity of protein-DNA interactions. Nat. Rev. Genet. 2010;11:751–760. doi: 10.1038/nrg2845. [DOI] [PubMed] [Google Scholar]
- Sun D., Li Y., Ma Z., Yan X., Li N., Shang B., Hu X., Cui K., Koiwa H., Zhang X. The epigenetic factor FVE orchestrates cytoplasmic SGS3-DRB4-DCL4 activities to promote transgene silencing in Arabidopsis. Sci. Adv. 2021;7:eabf3898. doi: 10.1126/sciadv.abf3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Súnico V., Higuera J.J., Molina-Hidalgo F.J., Blanco-Portales R., Moyano E., Rodríguez-Franco A., Muñoz-Blanco J., Caballero J.L. The intragenesis and synthetic biology approach towards accelerating genetic gains on strawberry: development of new tools to improve fruit quality and resistance to pathogens. Plants. 2021;11:57. doi: 10.3390/plants11010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaž Š., Gruden K., Coll A. TGA transcription factors-Structural characteristics as basis for functional variability. Front. Plant Sci. 2022;13:935819. doi: 10.3389/fpls.2022.935819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T., Murfett J., Hagen G., Guilfoyle T.J. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uygun S., Azodi C.B., Shiu S.H. Cis-regulatory code for predicting plant cell-type transcriptional response to high salinity. Plant Physiol. 2019;181:1739–1751. doi: 10.1104/pp.19.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van-Hulten M., Pelser M., van-Loon L.C., Pieterse C.M.J., Ton J. Costs and benefits of priming for defense in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2006;103:5602–5607. doi: 10.1073/pnas.0510213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaucheret H., Fagard M. Transcriptional gene silencing in plants: targets, inducers and regulators. Trends Genet. 2001;17:29–35. doi: 10.1016/s0168-9525(00)02166-1. [DOI] [PubMed] [Google Scholar]
- Vogl T., Kickenweiz T., Pitzer J., Sturmberger L., Weninger A., Biggs B.W., Köhler E.M., Baumschlager A., Fischer J.E., Hyden P., et al. Engineered bidirectional promoters enable rapid multi-gene co-expression optimization. Nat. Commun. 2018;9:3589. doi: 10.1038/s41467-018-05915-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Zhu M., Ye R., Liu Z., Zhou F., Chen H., Lin Y. Novel green tissue-specific synthetic promoters and cis-regulatory elements in rice. Sci. Rep. 2015;5:18256. doi: 10.1038/srep18256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Aguirre L., Rodríguez-Leal D., Hendelman A., Benoit M., Lippman Z.B. Dissecting cis-regulatory control of quantitative trait variation in a plant stem cell circuit. Nat. Plants. 2021;7:419–427. doi: 10.1038/s41477-021-00898-x. [DOI] [PubMed] [Google Scholar]
- Washburn J.D., Mejia-Guerra M.K., Ramstein G., Kremling K.A., Valluru R., Buckler E.S., Wang H. Evolutionarily informed deep learning methods for predicting relative transcript abundance from DNA sequence. Proc. Natl. Acad. Sci. USA. 2019;116:5542–5549. doi: 10.1073/pnas.1814551116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirauch M.T., Yang A., Albu M., Cote A.G., Montenegro-Montero A., Drewe P., Najafabadi H.S., Lambert S.A., Mann I., Cook K., et al. Determination and inference of eukaryotic transcription factor sequence specificity. Cell. 2014;158:1431–1443. doi: 10.1016/j.cell.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Wu D., Feng H., Duan L., Dai G., Liu X., Wang K., Yang P., Chen G., Gay A.P., et al. A deep learning-integrated micro-CT image analysis pipeline for quantifying rice lodging resistance related traits. Plant Commun. 2021;2:100165. doi: 10.1016/j.xplc.2021.100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Duan L., Pruneda-Paz J.L., Oh D.H., Pound M., Kay S., Dinneny J.R. The 6×ABRE synthetic promoter enables the spatiotemporal analysis of ABA-mediated transcriptional regulation. Plant Physiol. 2018;177:1650–1665. doi: 10.1104/pp.18.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzel E.T., Vickers C.E., Hanson A.D., Millar A.H., Cooper M., Voss-Fels K.P., Nikel P.I., Erb T.J. Revolutionizing agriculture with synthetic biology. Nat. Plants. 2019;5:1207–1210. doi: 10.1038/s41477-019-0539-0. [DOI] [PubMed] [Google Scholar]
- Xu G., Greene G.H., Yoo H., Liu L., Marqués J., Motley J., Dong X. Global translational reprogramming is a fundamental layer of immune regulation in plants. Nature. 2017;545:487–490. doi: 10.1038/nature22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y.Y., Obokata J. PPDB: a plant promoter database. Nucleic Acids Res. 2008;36:D977–D981. doi: 10.1093/nar/gkm785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y.Y., Yoshioka Y., Hyakumachi M., Obokata J. Characteristics of core promoter types with respect to gene structure and expression in Arabidopsis thaliana. DNA Res. 2011;18:333–342. doi: 10.1093/dnares/dsr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Lee J.H., Poindexter M.R., Shao Y., Liu W., Lenaghan S.C., Ahkami A.H., Blumwald E., Stewart C.N., Jr. Rational design and testing of abiotic stress-inducible synthetic promoters from poplar cis-regulatory elements. Plant Biotechnol. J. 2021;19:1354–1369. doi: 10.1111/pbi.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaschenko A.E., Fenech M., Mazzoni-Putman S., Alonso J.M., Stepanova A.N. Deciphering the molecular basis of tissue-specific gene expression in plants: can synthetic biology help? Curr. Opin. Plant Biol. 2022;68:102241. doi: 10.1016/j.pbi.2022.102241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Lin T., Meng X., Du H., Zhang J., Liu G., Chen M., Jing Y., Kou L., Li X., et al. A route to de novo domestication of wild allotetraploid rice. Cell. 2021;184:1156–1170.e14. doi: 10.1016/j.cell.2021.01.013. [DOI] [PubMed] [Google Scholar]
- Zeng D., Liu T., Ma X., Wang B., Zheng Z., Zhang Y., Xie X., Yang B., Zhao Z., Zhu Q., et al. Quantitative regulation of waxy expression by CRISPR/Cas9-based promoter and 5'UTR-intron editing improves grain quality in rice. Plant Biotechnol. J. 2020;18:2385–2387. doi: 10.1111/pbi.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Gai Y., Wang W., Zhu Y., Chen X., Jiang X. Construction and analysis of a plant transformation binary vector pBDGG harboring a bi-directional promoter fusing dual visible reporter genes. J. Genet. Genomics. 2008;35:245–249. doi: 10.1016/S1673-8527(08)60034-X. [DOI] [PubMed] [Google Scholar]
- Zhang L., Yu H., Ma B., Liu G., Wang J., Wang J., Gao R., Li J., Liu J., Xu J., et al. A natural tandem array alleviates epigenetic repression of IPA1 and leads to superior yielding rice. Nat. Commun. 2017;8:14789. doi: 10.1038/ncomms14789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.Q., Chen Y., Liu Y., Lin W.H., Wang J.W. Single-cell transcriptome atlas and chromatin accessibility landscape reveal differentiation trajectories in the rice root. Nat. Commun. 2021;12:2053. doi: 10.1038/s41467-021-22352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Li J., Yang L., Qin G., Xia C., Xu X., Su Y., Liu Y., Ming L., Chen L.L., et al. An inferred functional impact map of genetic variants in rice. Mol. Plant. 2021;14:1584–1599. doi: 10.1016/j.molp.2021.06.025. [DOI] [PubMed] [Google Scholar]
- Zhao M., Yuan Z., Wu L., Zhou S., Deng Y. Precise prediction of promoter strength based on a de novo synthetic promoter library coupled with machine learning. ACS Synth. Biol. 2022;11:92–102. doi: 10.1021/acssynbio.1c00117. [DOI] [PubMed] [Google Scholar]
- Zheng H., Lei Y., Lin S., Zhang Q., Zhang Z. Bidirectionalization of a methyl jasmonate-inducible plant promoter. Biotechnol. Lett. 2011;33:387–393. doi: 10.1007/s10529-010-0431-5. [DOI] [PubMed] [Google Scholar]
- Zrimec J., Börlin C.S., Buric F., Muhammad A.S., Chen R., Siewers V., Verendel V., Nielsen J., Töpel M., Zelezniak A. Deep learning suggests that gene expression is encoded in all parts of a co-evolving interacting gene regulatory structure. Nat. Commun. 2020;11:6141. doi: 10.1038/s41467-020-19921-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrimec J., Buric F., Kokina M., Garcia V., Zelezniak A. Learning the regulatory code of gene expression. Front. Mol. Biosci. 2021;8:673363. doi: 10.3389/fmolb.2021.673363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrimec J., Fu X., Muhammad A.S., Skrekas C., Jauniskis V., Speicher N.K., Börlin C.S., Verendel V., Chehreghani M.H., Dubhashi D., et al. Controlling gene expression with deep generative design of regulatory DNA. Nat. Commun. 2022;13:5099. doi: 10.1038/s41467-022-32818-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrimec J., Zelezniak A., Gruden K. Toward learning the principles of plant gene regulation. Trends Plant Sci. 2022;27:1206–1208. doi: 10.1016/j.tplants.2022.08.010. [DOI] [PubMed] [Google Scholar]
- Zürcher E., Tavor-Deslex D., Lituiev D., Enkerli K., Tarr P.T., Müller B. A robust and sensitive synthetic sensor to monitor the transcriptional output of the cytokinin signaling network in planta. Plant Physiol. 2013;161:1066–1075. doi: 10.1104/pp.112.211763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zykovich A., Korf I., Segal D.J. Bind-n-Seq: high-throughput analysis of in vitro protein-DNA interactions using massively parallel sequencing. Nucleic Acids Res. 2009;37:e151. doi: 10.1093/nar/gkp802. [DOI] [PMC free article] [PubMed] [Google Scholar]