Abstract
Verbascoside, which was first discovered in 1963, is a well-known phenylethanoid glycoside (PhG) that exhibits antioxidant, anti-inflammatory, antimicrobial, and neuroprotective activities and contributes to the therapeutic effects of many medicinal plants. However, the biosynthetic pathway of verbascoside remains to be fully elucidated. Here, we report the identification of two missing enzymes in the verbascoside biosynthesis pathway by transcriptome mining and in vitro enzymatic assays. Specifically, a BAHD acyltransferase (hydroxycinnamoyl-CoA:salidroside hydroxycinnamoyltransferase [SHCT]) was shown to catalyze the regioselective acylation of salidroside to form osmanthuside A, and a CYP98 hydroxylase (osmanthuside B 3,3′-hydroxylase [OBH]) was shown to catalyze meta-hydroxylations of the p-coumaroyl and tyrosol moieties of osmanthuside B to complete the biosynthesis of verbascoside. Because SHCTs and OBHs are found in many Lamiales species that produce verbascoside, this pathway may be general. The findings from the study provide novel insights into the formation of caffeoyl and hydroxytyrosol moieties in natural product biosynthetic pathways. In addition, with the newly acquired enzymes, we achieved heterologous production of osmanthuside B, verbascoside, and ligupurpuroside B in Escherichia coli; this work lays a foundation for sustainable production of verbascoside and other PhGs in micro-organisms.
Key words: acyltransferase, cytochrome P450, microbial synthesis, verbascoside, acteoside, phenylpropanoid
This study reports the identification of a BAHD acyltransferase (SHCT) that mediates the regioselective acylation of salidroside to form osmanthuside A and a CYP98 hydroxylase that catalyzes meta-hydroxylations of osmanthuside B to complete the biosynthesis of verbascoside. The heterologous production of verbascoside in Escherichia coli from renewable carbon resources was also achieved.
Introduction
Phenylethanoid glycosides (PhGs) are an important class (>572 members) of natural products found in medicinal herbs and plants; they display multiple biological activities, including neuroprotective, anti-inflammatory, antioxidant, antibacterial, and antiviral activities (Wu et al., 2020). The core structures of PhGs are characterized by a hydroxyphenylethyl moiety that is attached to a β-glucopyranose, which is usually decorated with substituents such as aromatic acids and various sugars through ester or glycosidic linkages. Verbascoside, which contains a hydroxysalidroside residue condensed with caffeoyl and rhamnose moieties, is among the best-known PhGs because of its potential health benefits in humans (Alipieva et al., 2014). Verbascoside is one of the major bioactive ingredients of medicinal plants such as the Echinacea and Plantago species historically used in North America and Europe, as well as “Roucongrong” (Cistanches Herba) and “Dihuang” (Rehmannia glutinosa) used in traditional Chinese medicines (Cheminat et al., 1988; Zubair et al., 2011; Han et al., 2012). Verbascoside is also the major bioactive compound of a dietary supplement popularly consumed in Japan and produced from Sesamum indicum leaves, as well as “Kudingcha” (bitter tea), which is produced from the leaves of Ligustrum robustum in China (He et al., 2003; Fuji et al., 2018). Recent pharmacological studies in vitro or in vivo have demonstrated that verbascoside possesses various biological activities, including anti-inflammatory, antioxidant, neuroprotective, cardiovascular protective, hepatoprotective, and antimicrobial activities (Khan et al., 2022, Xiao et al., 2022).
PhGs such as verbascoside are obtained from plant tissues through direct isolation (Alipieva et al., 2014); thus, the production of PhGs requires farmland and is time consuming. The extraction and purification of natural products from plants are usually laborious processes with low yields (Alipieva et al., 2014; Cravens et al., 2019; Courdavault et al., 2021). Thus, because of the diverse biological activities of PhGs, the total synthesis of PhGs has received significant attention from synthetic chemists. To date, a range of naturally occurring PhGs have been synthesized (Zhou et al., 2006; Das et al., 2007; Khong and Judeh, 2017; Hu et al., 2019). The synthesis of verbascoside has been achieved by several research groups since 1999 (Duynstee et al., 1999; Kawada et al., 1999; Zhang et al., 2021). Overall, however, the chemical synthesis of PhGs involves tedious multistep and low-yield protection–deprotection procedures. Producing natural products in micro-organisms via synthetic biology is a sustainable, environmentally friendly approach and is not limited by season or plant availability (Cravens et al., 2019; Courdavault et al., 2021). To make use of this approach, a complete set of genes encoding the enzymes involved in natural product biosynthesis is needed.
Early biosynthetic studies of PhGs focused mainly on verbascoside. As established by isotope-labeled precursor feeding experiments in cell cultures of Olea europaea and Syringa vulgaris, the caffeoyl moiety is synthesized from phenylalanine through the phenylpropanoid pathway, and the hydroxytyrosol moiety is derived from tyrosine (Ellis, 1983; Saimaru and Orihara, 2010). Potential biosynthetic routes were considered based on verbascoside structure and feeding experiments (Alipieva et al., 2014). Although the transcriptomes of a few plant species that produce verbascoside have been reported, the predicted route of verbascoside biosynthesis beyond the formation of the aglycon tyrosol remains speculative (Torrens-Spence et al., 2018; Zhang et al., 2019; Hou et al., 2022). In a recent study, we reported the biosynthesis of complicated PhGs such as verbascoside, ligupurpuroside B, and ligupurpuroside A based on transcriptome mining of L. robustum and in vitro enzymatic assays (Yang et al., 2021). Specifically, the first glycosylation occurs on tyrosol to form salidroside and is catalyzed by LrUGT85AF8. LrUGT79G7, an osmanthuside A 1,3-rhamnosyltransferase (OART), converts osmanthuside A into osmanthuside B. LrUGT79A19, an osmanthuside B 1,4-rhamnosyltransferase, extends the sugar chain of osmanthuside B to form ligupurpuroside B. Homologs of LrUGT79G7 have also been characterized in several verbascoside-producing species, including S. indicum, O. europaea, R.glutinosa, and C. deserticola. The results indicated that salidroside, osmanthuside A, and osmanthuside B are the first three potential intermediates for the biosynthetic pathways of verbascoside and ligupurpuroside B. However, the enzymes that catalyze the acylation of salidroside to generate osmanthuside A and the hydroxylation of osmanthuside B have remained unknown, and our understanding of the biosynthesis of verbascoside from tyrosol has remained incomplete.
Here, we combined transcriptomes from multiple verbascoside-producing Lamiales species, heterologous expression, and in vitro enzymatic assays to identify missing enzymes in the biosynthesis of verbascoside. Specifically, (1) a hydroxycinnamoyl-co-enzyme A (CoA):salidroside hydroxycinnamoyltransferase (SHCT) catalyzes the p-coumaroyl decoration of salidroside to form osmanthuside A using p-coumaroyl-CoA as the donor substrate. (2) A cytochrome P450 (CYP) enzyme (osmanthuside B 3,3′-hydroxylase [OBH]) catalyzes the meta-hydroxylations of the p-coumaric acid and tyrosol moieties of osmanthuside B to form verbascoside. (3) Homologs of SHCT or OBH were discovered in other verbascoside producers of the Lamiales order. (4) We reconstituted the verbascoside biosynthetic pathway in Escherichia coli and demonstrated the feasibility of microbial synthesis as an alternative approach for producing osmanthuside B and its derivatives (verbascoside and ligupurpuroside B).
Results
Identification of candidate acyltransferases that convert salidroside into osmanthuside A
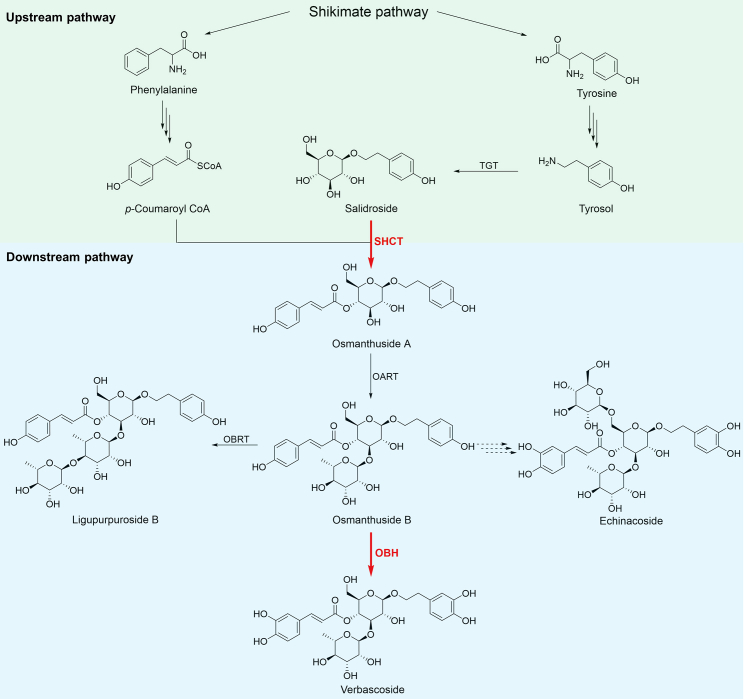
Previous results implied that osmanthuside A is an intermediate in verbascoside biosynthesis (Figure 1) (Yang et al., 2021). In plants, two families of acyltransferases mediate most of the acylation reactions in specialized metabolism. BAHD-acyltransferases (BAHD-ATs), named after the first four biochemically characterized enzymes, use CoA thioesters as the donor substrates, and serine carboxypeptidase-like acyltransferases (SCPL-ATs) use 1-O-β-glucose esters (Bontpart et al., 2015; Petersen, 2016). The putative salidroside hydroxycinnamoyltransferase that converts salidroside into osmanthuside A might be a BAHD-AT or an SCPL-AT (Figure 2A). To identify genes encoding acyltransferases that catalyze the acylation of salidroside, we initially analyzed the transcriptome of L. robustum. Our previous study showed that osmanthuside B and its derivatives accumulate in the leaves of L. robustum and are absent in roots, consistent with the expression level of LrUGT79G7 (Yang et al., 2021). We hypothesized that the target acyltransferases would also be specifically expressed in leaves. Thus, 16 acyltransferase candidates with relatively high expression (fragments per kilobase of transcript per million mapped reads [FPKM] ≥15) in leaves and ≥8-fold higher FPKM in leaves than in roots were selected, comprising BAHD-ATs and SCPL-ATs (Supplemental Table 1).
Figure 1.
Proposed biosynthetic pathway of osmanthuside B and its derivatives, including verbascoside, ligupurpuroside A, and echinacoside.
The proteins labeled in red are reported in this study. TGT, tyrosol glycosyltransferase; SHCT, salidroside hydroxycinnamoyltransferase; OBRT, osmanthuside B 1,4-rhamnosyltransferase; OBH, osmanthuside B hydroxylase.
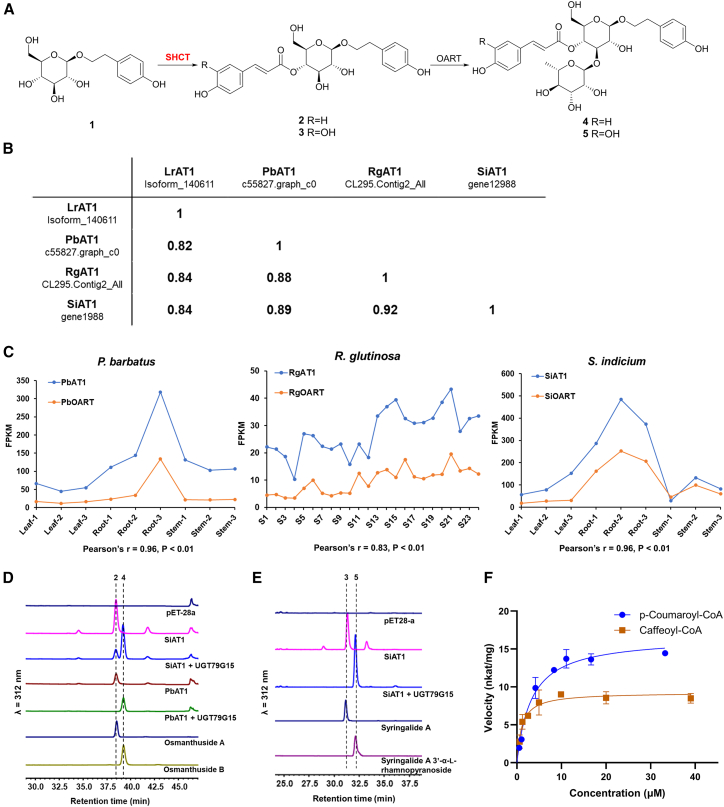
Figure 2.
Identification of SHCTs that convert salidroside into osmanthuside A.
(A) Reactions catalyzed by SHCT and OART. (1) salidroside; (2) osmanthuside A; (3) syringalide A; (4) osmanthuside B; (5) syringalide A 3′-α-L-rhamnopyranoside.
(B) Percentage protein identities of LrAT1 homologs.
(C) Expression profiles of LrAT1 and OART homologs across different samples of P. barbatus, R. glutinosa, and S. indicum. Pearson correlation coefficients of pairs of LrAT1 and OART homologs are shown.
(D) HPLC analysis of reaction mixtures of LrAT1 homologs and RgUGT79G15 using p-coumaroyl-CoA as an acyl donor.
(E) HPLC analysis of reaction mixtures of SiAT1 and RgUGT79G15 using caffeoyl-CoA as an acyl donor.
(F) Michaelis-Menten kinetics of SiAT1 with p-coumaroyl-CoA and caffeoyl-CoA. Means of triplicates and standard deviations are shown.
LrUGT79G7 and its homologs are conserved (∼80% protein identity) in several verbascoside-producing species of the Lamiales order (Yang et al., 2021). Inspired by this information, we assumed that homologs of acyltransferases that acylate salidroside would be found in verbascoside-producing Lamiales plants. To gain more information and narrow the number of acyltransferase candidates, we generated two RNA sequencing (RNA-seq) datasets from different tissues (leaf, stem, and root) of S. indicum and Penstemon barbatus, which are known to produce verbascoside (Xie et al., 2012; Fuji et al., 2018). With the addition of previously reported transcriptomes from R. glutinosa and L. robustum (Zhi et al., 2018; Yang et al., 2021), four RNA-seq datasets were analyzed.
Acyltransferase candidates from L. robustum were used as queries to perform a basic local alignment search tool protein (BLASTP) search against the transcriptomes of R. glutinosa, S. indicum, and P. barbatus. Only isoform_140611, the most highly expressed BAHD acyltransferase in leaf tissue of L. robustum, had a homolog with >80% protein identity in each of the other three species, and these homologs were termed LrAT1, RgAT1, SiAT1, and PbAT1, respectively (Figure 2B). A homolog of LrAT1 with a protein identity of 93.08% (OeAT1, accession number XP_022859703) was also discovered in the verbascoside-producing species O. europaea through a BLASTP search against the nonredundant protein sequence database. However, this homolog was annotated as a “spermidine hydroxycinnamoyl transferase-like enzyme.” Given that biosynthetic genes involved in a particular metabolic pathway are often coordinately regulated at the transcriptional level, we next investigated whether LrAT1 homologs were co-expressed with OART. Pearson correlation coefficients were calculated for each pair of LrAT1 and OART homologs from S. indicum, P. barbatus, and R. glutinosa. As shown in Figure 2C, LrAT1 homologs in all these species showed strong co-expression with OART homologs (Pearson’s correlation coefficient: SiAT1 and SiOART, r = 0.96, P < 0.01; PbAT1 and PbOART, r = 0.96, P < 0.01; RgAT1 and RgOART, r = 0.83, P < 0.01). Therefore, LrAT1 and its homologs were selected as acyltransferase candidates for salidroside acylation and used in further analysis.
Hydroxycinnamoyl-CoA:salidroside hydroxycinnamoyltransferase catalyzes the formation of osmanthuside A
Five acyltransferase candidate genes were individually ligated into the plasmid pET-28a (+) and introduced into E. coli BL21(DE3) for enzyme production. SDS–PAGE analysis showed that the recombinant enzymes were highly expressed, with the exception of LrAT1 (Supplemental Figure 1). The in vitro enzyme assays were initially carried out by incubating crude protein extracts with salidroside and p-coumaroyl-CoA, and the reaction mixtures were analyzed by HPLC. A new product with a retention time of 38.48 min was detected in all five reaction mixtures compared with the control (Figure 2D and Supplemental Figure 2). As determined by liquid chromatography–high-resolution mass spectrometry (LC–HRMS), the peak exhibited a molecular ion with m/z at 445.1528 (Supplemental Figure 3A), which corresponded with the [M – H]– ion of osmanthuside A (calcd for C23H25O9, 445.1493). The product was prepared and purified from a large-scale enzymatic reaction with SiAT1 and confirmed as osmanthuside A by comparing its nuclear magnetic resonance (NMR) spectra with previously reported data (Supplemental Figure 4) (Yang et al., 2021). Two minor products with the same m/z values (Supplemental Figure 5) were also generated in the reaction and may have been formed by the spontaneous migration of the p-coumaroyl moiety of osmanthuside A among the hydroxyl groups of glucose (Zhang et al., 1997). Osmanthuside B is more stable in part because of the substitution of 3-OH with rhamnose, which causes steric hindrance in the migration of the p-coumaroyl group. We then coupled the reaction of SHCTs with UGT79G15 (accession number MZ734613) (Yang et al., 2021), an OART from R. glutinosa with high expression and solubility in E. coli, to check the production of osmanthuside B. When RgUGT79G15, SHCTs, p-coumaroyl-CoA, and UDP-rhamnose were incubated, the reaction mixture contained a major product that was confirmed as osmanthuside B by comparing its retention time (39.20 min) and m/z ([M – H]– calcd for C29H36O13, 591.2072; Found 591.2046) with those of the authentic standard osmanthuside B, as evidenced by LC–HRMS analyses (Figure 2D and Supplemental Figure 3B). For SiAT1, which had a higher catalytic activity, the rearranged products of osmanthuside A were still observed, as RgUGT79G15 could not immediately convert osmanthuside A. However, levels of the side products were much lower than those in the reaction without coupling with RgUGT79G15 (Figure 2D). These results demonstrated that LrAT1 and its homologs are SHCTs that catalyze regioselective acylation of the glucose moiety of salidroside at the C4 position with p-coumaroyl-CoA.
Considering the catalytic promiscuity of BAHD acyltransferases for acyl donors, caffeoyl-CoA was used to test SHCTs, using salidroside as the acceptor. Incubation of purified SiAT1 with caffeoyl-CoA and salidroside resulted in formation of multiple products, and only one new product was detected when coupled with RgUGT79G15 (Figure 2E). This product exhibited a molecular ion of m/z at 607.2037 (Supplemental Figure 6), corresponding to the [M – H]– ion of syringalide A 3′-α-L-rhamnopyranoside isolated from C. deserticola (calcd for C29H36O14, 607.2021) (Han et al., 2012). Because there is no commercially available authentic standard for syringalide A 3′-α-L-rhamnopyranoside, we confirmed the structure by comparing its retention time with that of a standard prepared in an E. coli cell factory. The E. coli strain OB-4 (Supplemental Table 2) performed fermentation in the presence of caffeic acid, and the resulting product was isolated and confirmed as syringalide A 3′-α-L-rhamnopyranoside by comparison of its NMR spectra (Supplemental Figures 7 and 8) with previously reported data (Han et al., 2012). The kinetic parameters for SiAT1 with p-coumaroyl-CoA or caffeoyl-CoA were determined (Figure 2F). The apparent Km and kcat for p-coumaroyl-CoA were 1.66 ± 0.39 μM and 0.779 ± 0.038 s−1, respectively, and the kcat/Km was 469.1 s−1 mM−1. The apparent Km and kcat for caffeoyl-CoA were 1.13 ± 0.42 μM and 0.481 ± 0.039 s−1, respectively, and the kcat/Km was 425.7 s−1 mM−1.
We performed a phylogenetic analysis using SHCTs and other functionally characterized BAHD-ATs that participate in the biosynthesis of phenylpropanoids, flavonoids, terpenoids, and alkaloids (Figure 3 and Supplemental Table 3). The results showed that SHCTs are located in the clade that contains hydroxycinnamoyl-CoA:spermidine hydroxycinnamoyltransferase from Arabidopsis thaliana, hydroxycinnamoyl-CoA:piscidic-acid hydroxycinnamoyltransferase from Actaea racemosa, and hydroxycinnamoyl transferase, which is capable of forming caffeoyl and/or p-coumaroyl esters with malate (Grienenberger et al., 2009; Sullivan, 2009; Werner and Petersen, 2019). SHCTs shared ∼40% sequence identity with these BAHD-ATs at the amino acid level. In addition, SHCTs showed a relatively close phylogenetic relationship with BAHD-ATs involved in the biosynthesis of phenylpropanoid derivatives, such as shikimic or quinic acid ATs and rosmarinic acid synthase.
Figure 3.
Phylogenetic analysis of SHCTs and functionally characterized BAHD-ATs.
Phylogenetic analysis was performed in MEGA7. The maximum likelihood method was used to construct the tree with 1000 bootstrap replicates. The accession numbers of the proteins are listed in Supplemental Table 3.
Identification of candidate enzymes for hydroxylation of osmanthuside B into verbascoside
Previous experiments indicated that meta-hydroxylations of the p-coumaroyl and tyrosol moieties of osmanthuside B are the final two steps in the biosynthesis of verbascoside (Figure 4A) (Yang et al., 2021). The superfamily of CYP enzymes catalyzes diverse oxidative reactions in plant specialized metabolism. To identify putative genes encoding osmanthuside B hydroxylases (OBHs) involved in the biosynthesis of verbascoside, a BLASTP search with known A. thaliana CYP sequences as queries was performed against the transcriptomes of S. indicum, P. barbatus, and R. glutinosa, which produce verbascoside as a secondary metabolite. Initially, the transcriptome of L. robustum was not included in the search, as no verbascoside had been detected in previous work. A total of 166, 44, and 127 CYP candidates were identified from the transcriptomes of S. indicum, P. barbatus, and R. glutinosa, respectively. Encouraged by the strong co-expression of SHCT and OART, we performed hierarchical clustering to identify CYPs that showed expression profiles similar to that of SHCT or OART. As a result, 5, 5, and 8 candidates located in neighboring subclades of SHCT and/or OART were identified in P. barbatus, S. indicum, and R. glutinosa, respectively (Supplemental Figure 9). Moreover, gene9630 of S. indicum, CL4018.Contig2_All of R. glutinosa, and c53863.graph_c0 of P. barbatus shared >80% amino acid sequence identity with each other according to sequence alignments (Figure 4B). As expected, a homolog of gene9630 from O. europaea (accession number XP_022872083.1) was discovered in the nonredundant protein sequence database. Interestingly, a gene9630 homolog with moderate expression (FPKM ∼110) was also identified in the L. robustum transcriptome. These CYPs were designated CYP98A20 (S. indicum), CYP98A167 (O. europaea), CYP98A192 (P. barbatus), CYP98A193 (R. glutinosa), and CYP98A194 (L. robustum) by the Cytochrome P450 Nomenclature Committee.
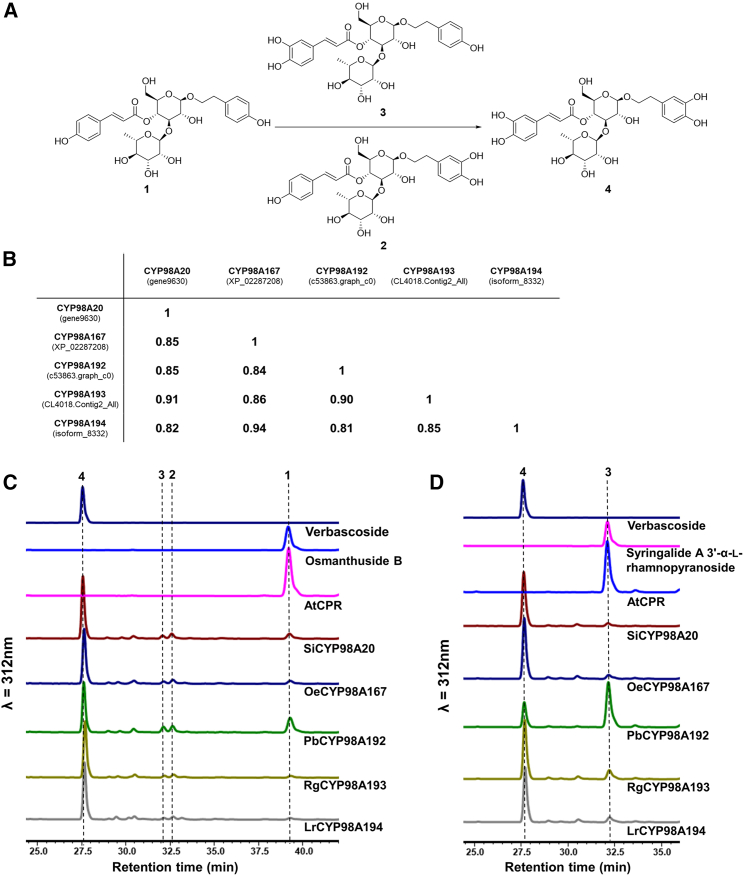
Figure 4.
Identification of OBHs that convert osmanthuside B into verbascoside.
(A) OBH-catalyzed reaction. (1) osmanthuside B; (2) lipedoside A-I; (3) syringalide A 3′-α-L-rhamnopyranoside; (4) verbascoside.
(B) Percentage protein identities of OBH orthologs.
(C) HPLC analysis of microsome assays of OBHs with osmanthuside B as the substrate.
(D) HPLC analysis of microsome assays of OBHs with syringalide A 3′-α-L-rhamnopyranoside as the substrate.
CYP98A20 and its homologs are dual-functional enzymes that catalyze verbascoside formation from osmanthuside B
Five CYP candidate genes were individually ligated into pCf302-AtCPR1 harboring the A. thaliana CYP reductase gene (AtCPR1) (Song et al., 2020) and introduced into Saccharomyces cerevisiae BY4742 for expression. Initially, yeast whole-cell assays were used to functionally characterize P450s, and the yeast strain carrying the empty vector pCf302-AtCPR1 was used as the negative control. Recombinant yeast cultures that expressed CYP homologs and AtCPR1 were supplemented with osmanthuside B. However, no conversion to verbascoside was observed after 24 h, possibly because osmanthuside B had difficulty entering yeast cells. Next, in vitro enzymatic assays with lysed recombinant yeast cells were performed in the presence of NADPH and osmanthuside B, and the reaction mixtures were analyzed by LC–HRMS. All the lysed yeast strains carrying SiCYP98A20 or homologs converted osmanthuside B into a product with the same retention time as verbascoside (Supplemental Figure 10A). The product exhibited a molecular ion of m/z 623.2054 (Supplemental Figure 11A), corresponding to the [M – H]– ion (calcd for C29H36O15, 623.1970). No products were observed in the negative control. In addition, in vitro enzymatic assays were performed by incubating yeast microsome-enriched fractions with NADPH (1 mM) and osmanthuside B (0.1 mM) at 30°C for 6 h. Again, osmanthuside B was converted into verbascoside (Figure 4C). The conversion rates were approximately 88.9% (SiCYP98A20), 93.2% (OeCYP98A167), 73.5% (PbCYP98A192), 96.2% (RgCYP98A193), and 96.4% (LrCYP98A194). The product was further prepared from the large-scale reaction and verified to be verbascoside by comparing its NMR spectra with that of the authentic standard (Supplemental Figure 12). These results convincingly demonstrated that SiCYP98A20 and its homologs convert osmanthuside B into verbascoside by meta-hydroxylations of both the tyrosol and p-coumaroyl moieties of osmanthuside B.
Two minor products were also detected in the reaction mixtures of CYPs, with retention times between those of osmanthuside B and verbascoside in HPLC (Figure 4C). LC–HRMS analysis showed that they exhibited molecular ions of m/z at 607.2037 and 607.2039 (Supplemental Figure 11B and 11C), which corresponded with [M – H]– ions of mono-hydroxylated products of osmanthuside B (calcd for C29H36O14, 607.2021). Peak 3 (Rt = 32.12 min) was confirmed as syringalide A 3′-α-L-rhamnopyranoside by comparing its retention time with that of a standard prepared by fermentation of E. coli strain OB-3 supplemented with caffeic acid. Peak 2 (Rt = 32.64 min) was tentatively assumed to be lipedoside A-I, one of the PhGs isolated from L. pedunculare (He et al., 1994). To confirm that syringalide A 3′-α-L-rhamnopyranoside is the intermediate of verbascoside biosynthesis, microsome-enriched fractions harboring recombinant CYPs were incubated with syringalide A 3′-α-L-rhamnopyranoside (0.1 mM) and NADPH (1 mM) for 6 h. As shown in Figure 4D, syringalide A 3′-α-L-rhamnopyranoside was efficiently converted into verbascoside by all five homologs. The conversion rates were approximately 93.6% (SiCYP98A20), 92.9% (OeCYP98A167), 25.1% (PbCYP98A192), 84.9% (RgCYP98A193), and 90.2% (LrCYP98A194).
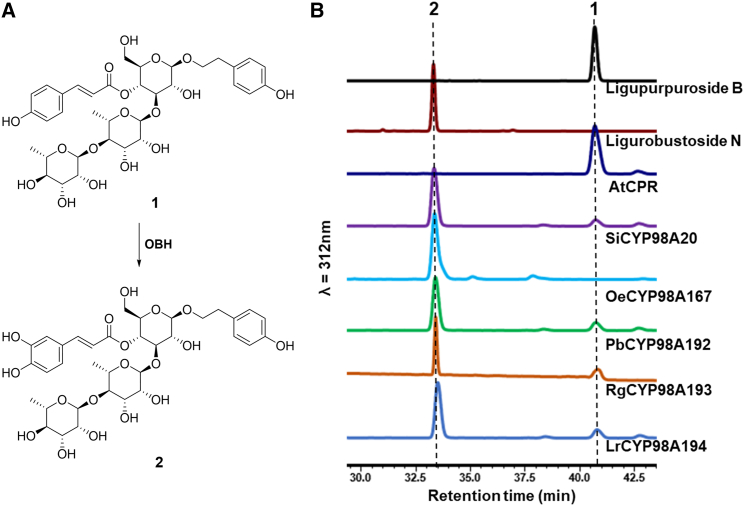
To explore whether the OBHs identified in this study can catalyze the hydroxylation of other intermediates in verbascoside biosynthesis, yeast microsomes harboring OBHs were incubated with osmanthuside A or salidroside. The results showed that these homologs did not catalyze the hydroxylation of osmanthuside A or salidroside. In addition, CYP98 members, such as CYP98A35 from Coffea canephora, are known to catalyze p-coumaroyl shikimate or quinate hydroxylation to form caffeoyl shikimate and chlorogenic acid (Mahesh et al., 2007). Commercially available p-coumaroylquinic acid was tested as a substrate for OBHs. As demonstrated by HPLC and LC–mass spectrometry (MS) analysis, the substrate p-coumaroylquinic acid was converted into chlorogenic acid, but the conversion rates (<5%) were much lower than those of osmanthuside B (Supplemental Figure 13). These results demonstrate that OBHs are dedicated to meta-hydroxylation of osmanthuside B. Because a large amount of ligupurpuroside A accumulated in L. robustum, an enzyme such as LrCYP98A194 may transform ligupurpuroside B into ligupurpuroside A (Figure 5A). We incubated yeast microsomes harboring OBHs with ligupurpuroside B, and a new product was formed. The compound was determined to be ligurobustoside N, a previously reported compound (He et al., 2003) that exhibits meta-hydroxylation on the p-coumaroyl moiety of ligupurpuroside B, by comparison of its retention time (33.49 min) and m/z ([M – H]– calcd for C35H46O18, 753.2600; Found 753.2508) with those of the authentic standard (Figure 5B and Supplemental Figure 11D).
Figure 5.
OBHs convert ligupurpuroside B into ligurobustoside N.
(A) OBH-catalyzed reaction. (1) Ligupurpuroside B; (2) ligurobustoside N; (3) ligupurpuroside A.
(B) HPLC analysis of reaction mixtures of yeast microsome-enriched fractions carrying OBHs with ligupurpuroside B as the substrate.
Protein sequences of the OBHs were compared with those of previously characterized CYP98 family members (Supplemental Table 3). SiCYP98A20 and its homologs showed the highest identities (69%–73%) with CYP98A35 from C. canephora, which hydroxylates p-coumaroylquinic/shikimic acid (Mahesh et al., 2007), and the lowest identities (40%–50%) with CYP98A8 and CYP98A9 from A. thaliana, which hydroxylate tricoumaroylspermidine (Matsuno et al., 2009). Further phylogenetic analysis showed that SiCYP98A20 and its homologs were clustered together in a single subclade (Figure 6). This subclade was phylogenetically close to the subclade composed of CYP98A35 from C. canephora, CYP98A6 from Lithospermum erythrorhizon, and CYP98A112 and CYP98A113 from Phacelia campanularia, the latter three of which hydroxylate 4-coumaroyl-4′-hydroxyphenyllactic acid (Levsh et al., 2019). SiCYP98A20 and its homologs are distantly related to CYP98A8 and CYP98A9 from A. thaliana, which constitute a distinct cluster (Matsuno et al., 2009).
Figure 6.
Phylogenetic analysis of previously characterized CYP98 family members.
Phylogenetic analysis was performed in MEGA7. The maximum likelihood method was used to construct the tree with 1000 bootstrap replicates. The accession numbers of the proteins are listed in Supplemental Table 3.
Functional validation of verbascoside biosynthetic genes in Nicotiana benthamiana
To verify the functions of verbascoside biosynthetic genes, we performed Agrobacterium tumefaciens–mediated transient protein expression in N. benthamiana leaves supplemented with salidroside. As expected, salidroside was transformed into osmanthuside A because of the infiltration of A. tumefaciens harboring SiAT1-carrying plasmids, as detected by HPLC and LC–MS (Supplemental Figure 14). We could not detect the formation of the caffeoyl derivative of salidroside (syringalide A) in tobacco leaves by HPLC. We could only detect a syringalide A peak by MS analysis, indicating that the quantity of syringalide A was low. Subsequently, we transiently co-expressed RgUGT79G15 and SiAT1 in the leaves of N. benthamiana supplemented with salidroside. As a result, osmanthuside B was produced in leaves (Supplemental Figure 14). Similarly, only a trace amount of syringalide A 3′-α-L-rhamnopyranoside was detected by MS analysis. When we further co-expressed CYP98A20 with RgUGT79G15 and SiAT1 in the above experiments, verbascoside was not detected, perhaps because of low access to osmanthuside B as a substrate. To verify the function of OBHs, osmanthuside B was directly infiltrated into the leaves that expressed only SiCYP98A20. As a result, osmanthuside B was converted into verbascoside (Supplemental Figure 14). No product was observed in the empty vector control. These results confirm the roles of SCHT and OBH in the biosynthesis of verbascoside.
De novo biosynthesis of osmanthuside B in E. coli
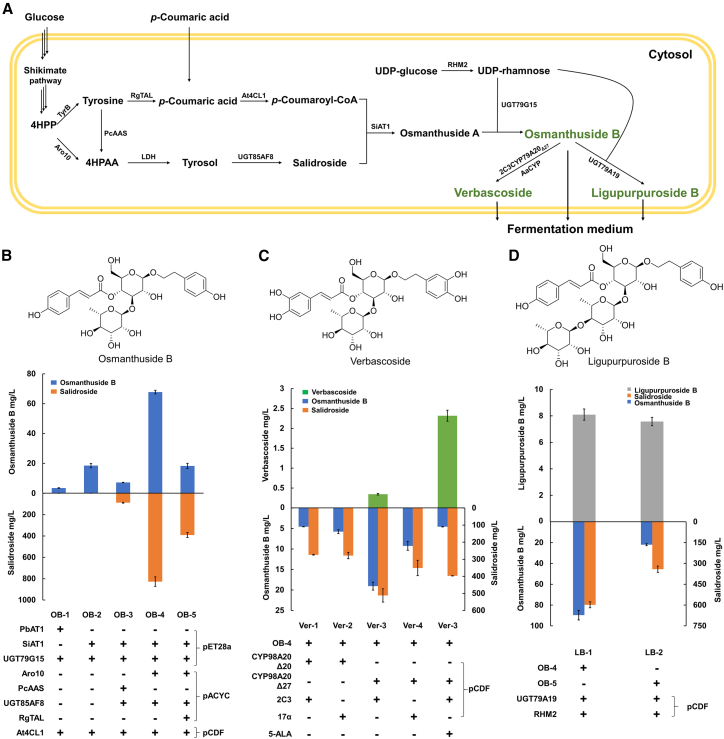
As we now had a complete set of genes for verbascoside biosynthesis, we sought to produce verbascoside and analogs in heterologous hosts. First, we aimed to construct the biosynthetic pathway for osmanthuside B in E. coli(Figure 7A), as osmanthuside B is a common intermediate for many PhGs such as verbascoside, echinacoside, and ligupurpuroside A. The previously engineered E. coli BL21(DE3) strain with enhanced metabolic flux toward tyrosine (BTAL) was used as a chassis strain in this study (Li et al., 2018). Initially, we tested the in vivo cascade reaction of SHCT and OART by feeding salidroside and p-coumaric acid as precursors. PbAT1 and SiAT1 with better catalytic activity and solubility were selected for acylation of salidroside in the following experiments. PbAT1/SiAT1, RgUGT79G15, and a 4-coumarate CoA ligase 1 from A. thaliana (At4CL1) (Ehlting et al., 1999) were introduced into E. coli strain BTAL, resulting in strains OB-1 and OB-2. The strains were cultured by a two-stage cultivation procedure to produce osmanthuside B. Osmanthuside B accumulated in the fermentation media, and its concentration was quantified by HPLC. Strain OB-2 carrying SiAT1 produced 18.48 ± 1.45 mg/L osmanthuside B, and OB-1 harboring PbAT1 produced 3.47 ± 0.23 mg/L osmanthuside B (Figure 7B). Interestingly, production of osmanthuside B was achieved without incorporating the UDP-rhamnose pathway, indicating that RgUGT79G15 can use endogenous dTDP-rhamnose from E. coli as a donor substrate. Strain OB-2 harboring SiAT1, RgUGT79G15, and At4CL1 was selected for biosynthesis of osmanthuside B.
Figure 7.
Biosynthesis of osmanthuside B, verbascoside, and ligupurpuroside B in E. coli.
(A) Engineered biosynthetic pathways for production of osmanthuside B, verbascoside, and ligupurpuroside B. Results of flask culture for osmanthuside B (B), verbascoside (C), and ligupurpuroside B (D). Error bars represent standard error (n = 3).
To avoid adding exogenous salidroside, we incorporated a biosynthetic pathway for salidroside into OB-2. The key precursor 4-hydroxyphenylacetaldehyde can be derived from tyrosine or 4-hydroxyphenylpyruvate. A 4-hydroxyphenylacetaldehyde synthase from Petroselinum crispum (PcAAS) (Torrens-Spence et al., 2012) or a pyruvate decarboxylase from yeast (ARO10) (Bai et al., 2014) and LrUGT85AF8 were introduced into OB-2, yielding strains OB-3 and OB-4. When fed p-coumaric acid in the medium, the OB-4 strain produced 67.76 ± 2.21 mg/L osmanthuside B and 827.23 ± 44.75 mg/L salidroside, and OB-3 produced 7.14 ± 0.19 mg/L osmanthuside B and 88.21 ± 4.11 mg/L salidroside (Figure 7B). To finally achieve production of osmanthuside B from glucose, a tyrosine ammonia-lyase from Rhodotorula glutinis (RgTAL) (Bi et al., 2019), which transforms tyrosine into p-coumaric acid, was introduced into OB-4. Fermentation by the resultant OB-5 strain led to production of 18.23 ± 1.81 mg/L osmanthuside B from 2% glucose in flask cultures, as well as 15.39 ± 0.55 mg/L p-coumaric acid and 390.95 ± 23.17 mg/L salidroside.
Biosynthesis of osmanthuside B derivatives (verbascoside and ligupurpuroside B) in E. coli
We began reconstitution of the verbascoside biosynthetic pathway from the osmanthuside B-producing strain. To achieve production of verbascoside, the OBH sequences must be engineered, as the N-terminal regions of plant CYPs with a transmembrane helix can impede protein folding in E. coli without a correct micro-environment. Their successful expression in E. coli often relies on proper modification of the N terminus, including truncation of the membrane anchor or fusion with hydrophilic peptides (Zhou et al., 2021). Therefore, a series of N-terminally modified variants were designed and generated for SiCYP98A20. Specifically, the first 20 or 27 residues of SiCYP98A20 were truncated according to the transmembrane domains predicted by TMHMM-2.0 (Krogh et al., 2001). A hydrophilic 2C3-tag and 17α-tag were fused to the truncated variants (Barnes et al., 1991; Dimaano et al., 2020), resulting in 2C3-CYP98A20Δ20, 17α-CYP98A20Δ20, 2C3-CYP98A20Δ27, and 17α-CYP98A20Δ27. These CYP98A20 variants and a modified CYP reductase partner (CPR) from Artemisia annua (Chang et al., 2007) were introduced into OB-4 after codon optimization, resulting in strains Ver-1–4. The recombinant strains performed fermentation, supplemented with p-coumaric acid. Verbascoside accumulated in the fermentation media, and its concentration was quantified using HPLC after fermentation. As a result, 0.34 ± 0.02 mg/L verbascoside was measured in the culture medium of the Ver-3 strain that expressed 2C3-CYP98A20Δ27 (Figure 7C). The recombinant strain produced 511.9 ± 39.2 mg/L salidroside and 19.01 ± 0.97 mg/L osmanthuside B, significantly less than that produced by the parental strain OB-4 (Figure 7C). These results indicated that overexpression of CYP98A20 variants disturbed the production of precursors. Given that P450s require heme as a coenzyme, limitation of heme availability can impair P450 function (Park et al., 2022). As the precursor of heme biosynthesis, 5-aminolevulinic acid (5-ALA) was added to the medium to increase the coenzyme heme concentration for CYP activity during fermentation. The production of verbascoside by Ver-3 was thereby increased from 0.34 ± 0.02 mg/L to 2.32 ± 0.14 mg/L. Thus, 5-ALA enhanced the conversion of osmanthuside B to verbascoside catalyzed by heme-requiring CYP98A20. To achieve biosynthesis of verbascoside from glucose in E. coli, 2C3-CYP98A20Δ27 and AaCPR were introduced into E. coli strain OB-5. However, the resulting strain Ver-5 did not produce detectable verbascoside, perhaps because excessive overexpression of the CYP98A20 variant and tyrosine ammonia-lyase RgTAL exerted too much metabolic burden on the engineered strain.
We examined whether LrUGT79A19 could be used to extend the biosynthetic capabilities of the osmanthuside B-producing strains to the production of ligupurpuroside B, a phenylpropanoid trisaccharide in L. robustum (Wong et al., 2001). LrUGT79A19 was introduced into strain OB-4 or OB-5, along with a UDP-rhamnose synthase from A. thaliana (RHM2) (Oka et al., 2007), to further increase the substrate pool for the two rhamnosyltransferases of ligupurpuroside B biosynthesis, yielding strains LB-1 and LB-2. Ligupurpuroside B accumulated in the fermentation medium, and its concentration was quantified by HPLC after fermentation. Strain LB-1 produced 8.09 ± 0.42 mg/L ligupurpuroside B and 89.63 ± 4.58 mg/L osmanthuside B when supplemented with p-coumaric acid. Strain LB-2 directly produced 7.57 ± 0.31 mg/L ligupurpuroside B and 22.01 ± 1.00 mg/L osmanthuside B from 2% glucose (Figure 7D).
Discussion
PhGs are a group of important natural compounds that contribute to the therapeutic effects of many medicinal plants. Their biosynthetic pathways remain largely unknown. Here, by analyzing several species from the Lamiales order, we were able to identify BAHD-ATs (SHCTs) involved in the acylation of salidroside to form osmanthuside A and CYP98 subfamily members (OBHs) that catalyze the hydroxylations of osmanthuside B to produce verbascoside, thus filling the gaps in verbascoside biosynthesis. In addition, OBH and/or SHCT homologs that had not previously been functionally characterized were discovered in 23 Lamiales species, 12 of which have been reported to produce verbascoside (Supplemental Table 4). These homologs might possess the same catalytic functions and participate in verbascoside biosynthesis in these species. Although the biosynthetic pathway of verbascoside appears to be general in Lamiales, further functional characterization of SHCTs, OARTs, and OBHs from other Lamiales species is needed. Other unknown enzymes from close but different biosynthetic pathways could also contribute to the formation of verbascoside because of the complexity of plant phenylethanoid synthesis.
The combination of BAHD-ATs with CYP98 family hydroxylases is frequently observed in formation of the caffeoyl moiety of many phenylpropanoid metabolites and forms a core metabolic module within the phenylpropanoid pathway of land plants (Schoch et al., 2001; Hoffmann et al., 2003). In brief, the CoA-activated p-coumaroyl moiety is esterified with shikimate, quinate, tyramine, spermidine, or phenyllactic acids, and the 3-OH group is then introduced into the aromatic ring of p-coumaric acid by a CYP98 mono-oxygenase.
In this work, SHCTs were shown to catalyze regioselective acylation of the salidroside C4-hydroxy group with a p-coumaroyl/caffeoyl moiety. These BAHD-ATs are more active with a p-coumaroyl moiety than with a caffeoyl moiety, and the catalytic efficiencies are comparable overall. During functional analysis of SHCT in plants, significant quantities of osmanthuside A were detected, and only a negligible amount of the caffeoyl derivative of salidroside was found in tobacco leaves. These results indicate that osmanthuside A is the intermediate for the biosynthesis of osmanthuside B and its derivatives in plants. In addition, SHCTs formed a distinct subclade in the phylogenetic tree, suggesting that these salidroside acyltransferases evolved their catalytic specificity from the same ancestor. SHCTs are more closely phylogenetically related to hydroxycinnamoyl-transferases in the biosynthesis of phenylpropanoids and derivatives, suggesting the role of SHCTs in the formation of osmanthuside A.
Phenolic glycosides with acylated sugar moieties, such as flavonoids and phenylpropanoids, are widely distributed in the plant kingdom. Flavonoid BAHD-ATs have been reported to transfer the malonyl moiety or hydroxycinnamoyl moieties. Most of these BAHD-ATs catalyze acylation of the glucose moiety with strict regioselectivity toward the position of the C6-hydroxy group (Luo et al., 2007). BAHD-ATs also catalyze the acylation of the C4-hydroxyl group or C3-hydroxyl group of the glucose moiety with malonyl-CoA as the donor in the biosynthesis of anthocyanidin derivatives (Suzuki et al., 2004c; Unno et al., 2007). SHCTs discovered in this study catalyze the regiospecific transfer of the p-coumaroyl or caffeoyl group to the C4-hydroxyl group of the glucose moiety of salidroside.
We identified CYP98A20 and its homologs as dual-function meta-hydroxylases that catalyze the final two steps in verbascoside biosynthesis. Two products containing p-coumaroyl or tyrosol moieties of osmanthuside B that were hydroxylated were also detected in the enzymatic assays. This result implied that the enzymatic reactions catalyzed by OBHs in the formation of verbascoside may proceed from either of the two compounds. Several compounds were tested as substrates for OBHs. No conversion was observed for osmanthuside A and salidroside. Intriguingly, OBHs can catalyze hydroxylation of ligupurpuroside B at the C-3 position of the p-coumaroyl moiety to form ligurobustoside N, which has been isolated from leaves of L. robustum (He et al., 2003). Commercially available p-coumaroyl-quinate was tested as a substrate for OBHs. The substrate p-coumaroyl-quinate was converted into chlorogenic acid, but the conversion rates were much lower than that of osmanthuside B (Supplemental Figure 13). These results implied that OBHs are dedicated to meta-hydroxylation of osmanthuside B. The function of OBHs was further confirmed by expression of SiCYP98A20 in tobacco.
In addition to 3′-hydroxylation of the aromatic ring of p-coumaric moieties, other hydroxylation patterns catalyzed by CYP98 members have been reported. For example, CYP98A8 and CYP98A9 from A. thaliana act as tri-coumaroyl-spermidine meta-hydroxylases. AtCYP98A8 also catalyzes 5′-hydroxylation of tri-feruloyl-spermidine to produce di-(hydroxyferuloyl)-sinapoyl-spermidine, a major constituent of the pollen coat (Matsuno et al., 2009). CYP98 members such as CYP98A14 from Coleus blumei have been reported to catalyze the meta-hydroxylation of both p-coumaroyl and phenyllactic acid moieties in the biosynthesis of rosmarinic acid (Eberle et al., 2009). The OBHs discovered in this work, which catalyze the meta-hydroxylations of both tyrosol and p-coumaroyl moieties linked to glucose with an ester bond or a glycosidic linkage, differ from previously characterized CYP98 members. In this process, the CoA-activated p-coumaroyl moiety is first esterified with the glucose moiety of salidroside, catalyzed by SHCTs. Then, a rhamnosyltransferase such as LrUGT79G7 is needed to extend the sugar chain to form a p-coumaroyl disaccharide ester as the substrate for the next CYP98 member, namely, an OBH. This is the first report of enzymes catalyzing meta-hydroxylation of the tyrosol moiety for biosynthesis of hydroxytyrosol-containing natural products.
Overall, the function of the SHCT/OBH pair in verbascoside biosynthesis was supported by gene co-expression analyses, in vitro enzymatic assays, heterologous expression in tobacco, and phylogenetic analysis. We could not rule out alternative pathways in which caffeoyl-CoA was used as the precursor instead of p-coumaroyl-CoA at this stage, and substrate availability is likely to be a critical factor in the control of verbascoside biosynthesis.
The low content of PhGs in most plant species has limited investigation of their activities and industrial applications. We conducted preliminary metabolic engineering efforts in E. coli to achieve the biosynthesis of osmanthuside B and its derivatives. The osmanthuside B produced by the recombinant strain reached ∼90 mg/L when the culture was supplemented with p-coumaric acid under shake-flask conditions without process optimization. De novo production of osmanthuside B from glucose reached ∼22 mg/L. We also demonstrated the E. coli production of osmanthuside B derivatives (verbascoside and ligupurpuroside B) with complicated structures at the mg level. This work expands the use of E. coli as a biosynthetic platform for valuable PhGs. In the future, it should be possible to significantly increase osmanthuside B, verbascoside, and ligupurpuroside B production through a combination of strain engineering and process optimization. For heterologous production of natural products in E. coli, the functional expression of membrane-associated enzymes remains challenging (Zhou et al., 2021). Further improvement of the functional expression of OBHs will be critical for de novo production of verbascoside in E. coli. Computation-aided protein engineering of BAHD-AT and UGTs could be used to enhance the bioconversion of salidroside to osmanthuside B and verbascoside. Because both tyrosol and p-coumaric acid are derived from the shikimate pathway, the metabolic flux between the two precursors needs to be fine tuned.
In conclusion, we have completed the elucidation of the biosynthetic pathway of verbascoside as well as ligupurpuroside B. The discovery and characterization of OBHs and SHCTs expands available knowledge on phenylpropanoid metabolism in the plant kingdom. Our work lays a foundation for elucidating the biosynthetic pathways of PhGs and engineering microbial cell factories for PhG production as an alternative to direct extraction from plants.
Methods
Chemicals and reagents
Salidroside, osmanthuside B, ligupurpuroside B, ligurobustoside N, and verbascoside were purchased from PureChem (Chengdu, China). p-Coumaroyl-CoA was purchased from Weikeqi (Sichuan, China). UDP-rhamnose was purchased from Acmec (Shanghai, China). Syringalide A and syringalide A 3′-α-L-rhamnopyranoside were prepared as detailed in the following section. Osmanthuside A was prepared in our previous study (Yang et al., 2021). Caffeoyl-CoA was prepared as previously reported (Sullivan, 2009). Chromatography-grade acetonitrile and formic acid were purchased from Acmec. Unless otherwise stated, all other chemicals and reagents were purchased from Aladdin (Shanghai, China).
Plant materials and transcriptome sequencing
S. indicum and P. barbatus plants were purchased from local markets in Tianjin (China) and grown under a 16-h light/8-h dark photoperiod at 23°C in a local greenhouse for 30 days. Leaves, stems, and roots from three individuals were frozen with liquid nitrogen and sent to Biomarker Technologies Corporation (Beijing, China) for library construction, RNA-seq on the Illumina NovaSeq 6000 platform in paired-end mode (PE250), and subsequent de novo assembly. Gene expression levels were represented as FPKM values. Transcriptome datasets for R. glutinosa and L. robustum were described previously (Wang et al., 2017; Yang et al., 2021).
Identification of enzyme candidates
For each species, TransDecoder was used to predict putative coding sequences from the transcriptome data using the Pfam database as a reference (Haas et al., 2013). BAHD-AT or CYP candidates were identified by performing local BLASTP searches against predicted proteins using known BAHD-ATs (listed in Supplemental Table 3) or A. thaliana CYP enzymes as baits, with an e-value of 1e−5. SCPL-AT candidates were obtained by HMM searches with PF00450 domain profiles. CD-HIT with a threshold of 0.98 was used to remove redundant sequences (Li and Godzik, 2006), and short sequences (<400 amino acids) were also removed. Hierarchical clustering analysis was performed in TBtools (Chen et al., 2020). FPKM values were scaled across different samples by setting the parameters “Row Scale” and “ZeroToOne.” Cluster parameters were set to “Euclidean” and “Complete.” Pearson correlation analyses were performed using the stats package available in R (version 4.2.2) with FPKM values for genes of interest.
Sequence alignments and phylogenetic analyses
Evolutionary analyses were performed in MEGA7 (Kumar et al., 2016). Multiple sequence alignments were constructed using the ClustalW algorithm. Phylogenetic analysis was carried out using the maximum likelihood method with the JTT matrix-based model and a bootstrap test of 1000 replicates. Initial tree(s) for the heuristic search were obtained automatically by applying the Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the JTT model and then selecting the topology with a superior log likelihood value. BAHD-ATs and CYP98 family enzymes used in the phylogenetic analysis are listed in Supplemental Table 3.
Gene cloning and plasmid construction
Complementary DNAs (cDNAs) were synthesized from total RNA samples using the PrimeScript RT reagent Kit with gDNA Eraser (TransGen, Beijing, China) and the oligo(dT)15 primer. LrAT1, SiAT1, PbAT1, SiCYP98A20, and PbCYP98A192 genes were amplified from cDNA using gene-specific primers (Supplemental Table 5). RgAT1, OeAT1, LrCYP98A194, RgCYP98A193, and OeCYP98A167 genes were synthesized by GENEWIZ (Suzhou, China). For expression in E. coli, BAHD-AT genes were ligated into the pET-28a(+) plasmid between the NheI and SalI restriction sites. For expression in S. cerevisiae, CYP genes were ligated into the yeast expression vector pCf302-AtCPR1 (Song et al., 2020) controlled by the PGK1 promoter. The recombinant plasmids were constructed using the ClonExpress Ultra One Step Cloning Kit (Vazyme, Nanjing, China), followed by transformation into competent DH5α. Positive clones were confirmed by DNA sequencing.
Plasmids pET-28a (+), pCDFDuet-1, and pACYCDuet-1 were used to reconstitute the biosynthetic pathways of PhGs in E. coli. RgUGT79G15, At4CL1, LrUGT79A19, LrUGT85AF8, RgTAL, and ARO10 genes were amplified from plasmids stored in our laboratories (Jiang et al., 2016; Bi et al., 2019; Yang et al., 2021). AaCPR in which the native N-terminal sequence was replaced with the N-terminal leader from C. tropicalis was amplified from the commercial plasmid pCWori-A13AMO-aaCPRct (Addgene) (Chang et al., 2007). RHM2 and PcAAS genes were synthesized without codon optimization. SiCYP98A20 was synthesized with codon optimization for expression in E. coli. The nucleic acid sequences of CYP98A20 mutants (2C3-CYP98A20Δ20, 2C3-CYP98A20Δ27, 17α-CYP98A20Δ20, 17α-CYP98A20Δ27) tested in this study are listed in Supplemental Table 6. Primers used in this section and their use can be found in Supplemental Table 5. To construct a particular plasmid, gene fragments or linearized vectors were amplified by PCR using primers with gene-specific names or “backbones,” followed by digestion with DpnI (Fermentas). Next, DNA fragments were ligated together using the ClonExpress Ultra One Step Cloning Kit (Vazyme). In this way, PT7-RgUGT79G15 was amplified and ligated into pET28a-PbAT1 and pET28a-SiAT1 to generate pET28a-PbAT1-RgUGT79G15 and pET28a-SiAT1-RgUGT79G15, respectively. At4CL1 was amplified and ligated into pCDFDuet1 digested with NdeI and XhoI (Fermentas) to generate pCDFDuet-At4CL1. RHM2 was amplified and ligated into pCDFDuet1 digested with BamHI (Fermentas) to generate pCDFDuet-RHM2. PT7-LrUGT79A19 and PT7-RHM2 were amplified and ligated into pCDFDuet-At4CL1 to generate pCDFDuet-At4CL1-LrUGT79A19-RHM2. CYP98A20 mutants modified by truncation and hydrophilic tags were generated by PCR using specific primers and ligated into pET-28a(+) between the NcoI and SalI restriction sites to generate pET28a-2C3CYP98A20Δ20, pET28a-17αCYP98A20Δ20, pET28a-2C3CYP98A20Δ27, and pET28a-17αCYP98A20Δ27. Next, PT7-CYP98A20 mutants and AaCPR were amplified and ligated into pCDFDuet-At4CL1 to generate four pCDFDuet-At4CL1-mutCYP98A20opt-AaCPR plasmids. ARO10/PcAAS and PT7-LrUGT85AF8 were amplified and ligated into pACYCDuet1 digested with BamHI and SacI (Fermentas) to generate pACYCDuet-Aro10-LrUGT85AF8/pACYCDuet-PcAAS-LrUGT85AF8. RgTAL was amplified and ligated into pACYCDuet-Aro10-LrUGT85AF8 digested with BglII and AatII (Fermentas) to generate pACYCDuet-Aro10-LrUGT85AF8-RgTAL
Culture conditions for recombinant strains
To express candidates in E. coli for functional characterization, E. coli BL21(DE3) strains harboring the BAHD-AT candidates were grown overnight at 37°C. The overnight cultures were diluted 1:50 in 50 mL LB medium containing 50 μg/mL kanamycin. When the OD600 reached 0.6–0.8, protein expression was induced with 0.1 mM IPTG. After incubation at 22°C for 20 h, cells were harvested (4500 rpm, 10 min), resuspended in 5 mL reaction buffer (50 mM Tris-HCl; pH 7.4), and disrupted by sonication. The supernatants were collected after centrifugation (12 000 rpm, 15 min) for enzymatic assays.
To produce PhGs in E. coli, the tyrosine-overproducing strain BTAL was used as the chassis (Li et al., 2018). A two-stage cultivation procedure was used. First, recombinant E. coli strains were cultured overnight in 5 mL LB medium containing appropriate antibiotics (50 μg/mL kanamycin, 50 μg/mL streptomycin, and 25 μg/mL chloramphenicol) at 37°C and inoculated into 50 mL LB medium. When the OD600 reached 0.6–0.8, protein expression was induced with 1 mM IPTG at 25°C for 20–24 h. If needed, 5-ALA was added to a final concentration of 1 mM. Second, cells were harvested by centrifugation (4000 rpm, 10 min) and resuspended in 50 mL modified M9Y medium (5 g/L yeast extract, 3 g/L KH2PO4, 1 g/L NH4Cl, 17.1 g/L Na2HPO4·12H2O, 0.5 g/L NaCl, 14.7 mg/L CaCl2·H2O, 246.5 mg/L MgSO4·7H2O, and 2% glucose) supplemented with 0.2 mM p-coumaric acid, 1 mM salidroside, and 1 mM 5-ALA if needed, followed by 24-h incubation at 30°C. The fermentation media were subjected to HPLC analysis after centrifugation.
To express candidates in S. cerevisiae for functional characterization, pCF302 vectors harboring AtCPR1 and C3H homologs were transformed into S. cerevisiae BY4742 using the LiAC/SS-DNA/PEG protocol (Gietz and Schiestl, 2007). After 3–5 days of incubation at 30°C, positive colonies were selected on a uracil-minus plate (SD-Ura: 6.7 g/L of yeast nitrogen base without amino acids, 0.9 g/L of SD-Ura, 20 g/L of glucose) and verified by colony PCR. A single colony was grown in 5 mL SD-Ura medium at 30°C overnight. The overnight cultures were diluted 1:40 in 200 mL SD-Ura medium and further cultivated at 30°C for 36 h. Cells were harvested by centrifugation (4500 rpm, 10 min).
Purification of recombinant proteins
E. coli cells harvested from 1 L LB medium were resuspended in 100 mL Buffer A (50 mM Tris-HCl, 20 mM imidazole, and 500 mM NaCl; pH 7.4), followed by three passes through the JN-3000PLUS cell crusher (Jnbio, Guangzhou, China), and centrifuged to remove cell debris. The supernatants were collected after centrifugation (15 000 rpm, 30 min, 4°C) and applied to a column loaded with 3 mL Ni-NTA BEADS (Smart-Life Sciences, Changzhou, China). The Ni-NTA beads were washed three times with 15 mL Buffer A and eluted with 15 mL Buffer B (50 mM Tris-HCl, 500 mM imidazole, and 500 mM NaCl; pH 7.4). The elution was centrifuged using Amicon Ultra-15 Centrifugal Filters (Millipore, Burlington, MA) to concentrate enzymes and exchange buffer into Buffer C (50 mM Tris-HCl, 50 mM NaCl, and 10% glycerol; pH 7.4). The protein concentration was determined with a BCA assay kit (Biosharp, Hefei city, China). The purified enzymes were aliquoted and stored at –80°C. All steps were performed on ice with pre-cooled buffers.
Microsome preparation
Yeast cells harvested from 100 mL SD-Ura medium were resuspended in 1 mL lysis buffer (50 mM NaH2PO4, 300 mM NaCl, and 1 mM PMSF; pH 7.4) after washing twice with TEK buffer (50 mM Tris-HCl, 1 mM EDTA, and 100 mM KCl; pH 7.4). We added 0.5 g of glass beads to the cell suspension. The mixtures were oscillated on a vortex oscillator to disrupt cells (vortexed for 1 min and placed on ice for 1 min, repeated five times). Lysate was centrifuged (11 000 g, 20 min) to remove cell debris and intact cells. The supernatant was used as a crude enzyme. To further prepare microsomes, the supernatants were centrifuged at 150 000 g for 1.5 h at 4°C. The harvested microsomes were re-suspended in 1 mL TEG buffer (50 mM Tris-HCl, 1 mM EDTA, and 20% glycerol; pH 7.4), aliquoted, and stored at −80°C. All steps were performed on ice with pre-cooled buffers.
In vitro enzyme assays
The initial enzymatic assays of BAHD-ATs were performed in 100-μL reaction mixtures containing 50 mM Tris-HCl (pH 7.4), 2 mM salidroside, 1 mM p-coumaroyl-CoA, and 90 μL bacterial extract. To couple the reaction with RgUGT79G15, 100 μg purified RgUGT79G15 and 1 mM UDP-rhamnose were added to the reaction mixtures. To test caffeoyl-CoA as an acyl donor, the enzymatic assay was performed in 100-μL reaction mixtures containing 50 mM Tris-HCl (pH 7.4), 100 mM NaCl, 2 mM salidroside, 0.3 mM caffeoyl-CoA, and 10 μg purified SiAT1. The cascade reaction was performed by adding another 20 μg purified RgUGT79G7 or 1 mM UDP-rhamnose. All reaction mixtures were incubated at 30°C for 1 h before adding 100 μL methanol for termination. After centrifugation (12 000 rpm, 10 min), the supernatants were subjected to HPLC or LC–MS analysis. Kinetic characterization of SiAT1 was performed in 100-μL reaction mixtures containing 50 mM Tris-HCl (pH 7.4), 0.5–35 μM p-coumaroyl-CoA or caffeoyl-CoA, and 75 mM salidroside. The mixtures were pre-equilibrated at 30°C for 10 min. Reactions were started by adding 70 ng recombinant enzyme, incubated at 30°C for 30 s, and quenched by addition of 3 μL trifluoroacetic acid (10%). Kinetic constants were calculated by nonlinear Michaelis-Menten regression using GraphPad Prism (version 5). Quantification and analysis of the reaction product were performed by HPLC with syringalide A or syringalide A 3′-α-L-rhamnopyranoside as the authentic standard prepared in the following section.
The enzymatic assays of CYPs were performed in 200-μL reaction mixtures containing 50 mM sodium phosphate buffer (pH 7.4), 1 mM NADPH, 50 μL microsomal proteins or crude enzyme, and 100 μM substrate. After incubation at 30°C for 6 h, the reaction was terminated by adding 200 μL methanol. The mixture was centrifuged at 15 000 g for 10 min. The supernatants were subjected to HPLC or LC–HRMS analysis.
Preparation of osmanthuside A, syringalide A, and syringalide A 3′-α-L-rhamnopyranoside
Osmanthuside A was prepared from the enzymatic assay of SiAT1. The reaction was performed by incubating 40 mL Tris-HCl buffer (50 mM; pH 7.4) containing 20 mg purified SiAT1, 2 mM salidroside, and 0.5 mM p-coumaroyl-CoA at 30°C for 1 h, followed by addition of 1 mL 1 N hydrochloric acid for termination. The product was extracted with ethyl acetate (40 mL × 3). The upper layers were combined and dried with a Rotavapor. The concentrate was re-dissolved in 50% ethanol and subjected to semi-preparative HPLC separation as detailed previously (Yang et al., 2021). Purified osmanthuside A (2.3 mg) was subjected to NMR analysis. p-Coumaroyl-CoA used in this section was prepared as described previously (Sullivan, 2009).
Syringalide A 3′-α-L-rhamnopyranoside and syringalide A were prepared by fermentation performed by the OB-4 and SA-1 strains fed caffeic acid. The OB-4 strain was subjected to a two-stage fermentation as described above and fed with caffeic acid. To produce syringalide A, the SA-1 strain was resuspended in M9Y medium with a lower pH (6.0) at the second stage and incubated at 30°C for 72 h. The supernatants were collected by centrifugation (5000 rpm, 15 min) and concentrated with a Rotavapor. The concentrate was re-dissolved in 50% ethanol and subjected to purification by semi-preparative HPLC separation. Formic acid (0.1%) and acetonitrile were used as the mobile phase in a Shimadzu LC-AD system with an SPD-20A detector and a YMC-Pack ODS-A column (10 × 250 mm, 5 μm). Samples were run at a flow rate of 4 mL/min and an isocratic elution of acetonitrile (18%). Products were detected and quantified by UV absorption at 312 nm. The purified product was freeze dried to obtain syringalide A 3′-α-L-rhamnopyranoside (6.1 mg from 1 L fermentation media) and syringalide A (20.1 mg from 3 L fermentation media), followed by LC–HRMS and NMR analyses. The purified products were used as authentic standard compounds in subsequent experiments.
Transient expression of SiAT1, UGT79G15, and CYP98A12 in N. benthamiana
For transient expression in N. benthamiana, the coding sequences of SiAT1, UGT79G5, and CYP98A12 were ligated into the pEAQ-HT vector between AgeI and XhoI using the ClonExpress Ultra One Step Cloning Kit (Vazyme). After verification by sequencing, recombinant plasmids were transferred into A. tumefaciens LBA4404 by the freeze-thaw method, and the bacteria were grown on LB plates (20 μg/mL rifampicin, 50 μg/mL streptomycin, and 50 μg/mL kanamycin) for 3 days. Colonies were inoculated into 4 mL LB medium (20 μg/mL rifampicin, 50 μg/mL streptomycin, and 50 μg/mL kanamycin) and grown at 30°C overnight. The cultures were pelleted, washed with MMA buffer (10 mM 2-(N-morpholino)ethanesulfonic acid [pH 5.6], 10 mM MgCl2, and 100 μM acetosyringone), and suspended in MMA buffer at room temperature for 2 h. The suspensions were diluted to a concentration equivalent to an OD600 of 0.6 and infiltrated into N. benthamiana leaves (4–6 weeks, with a 16-h light cycle at room temperature) using a 1-mL syringe. For transient co-expression, cultures were prepared separately and resuspended together to give one suspension containing each culture at a concentration equivalent to an OD600 of 0.3 before infiltration. After 4 days, 500 μL 20 mM salidroside or 200 μL 2 mM osmanthuside B were infiltrated into the leaves of N. benthamiana. Leaves were harvested 1 day later, frozen with liquid nitrogen, and ground into powder using mortars and pestles. The powders were extracted with 50% methanol (w:v = 1:5) and centrifuged at 15 000 g for 30 min. The supernatants were filtered through 0.22-μm syringe filters and analyzed by HPLC and LC–HRMS.
HPLC, LC–HRMS, and NMR analyses
HPLC analysis was performed on a Shimadzu LC-20AD HPLC system equipped with an SPD-M40 PDA detector. The analytical column was a SilGreen C18 column (4.6 × 250 mm, 5 μm) (Greenherbs, Beijing, China). The mobile phase contained 0.1% formic acid (A) and acetonitrile (B). The column temperature was set to 40°C. The flow rate was 1 mL/min, and the gradient program was as follows: 0–5 min, 5% B; 5–18 min, 5%–13.2% B; 18–43 min, 18%–21.6% B; 43–50 min, 100% B; and 50–57 min, 5% B. To analyze chlorogenic acid, the mobile phase contained 0.1% formic acid (A) and methanol (B), and the gradient program was as follows: 0–5 min, 5% B; 5–45 min, 5%–100% B; 45–52 min, 100% B; and 52–60 min, 5% B.
LC–HRMS analyses were carried out on an Agilent 1200 HPLC system coupled with a Bruker-MicrOTOF-II mass spectrometer equipped with an electrospray ionization device. Experiments were performed in negative ion mode. The mobile phase contained 5 mM ammonium acetate (A) and acetonitrile (B). The flow rate was 1 mL/min, and the gradient program was as follows: 0–5 min, 18% B; 5–28 min, 18%–20.3% B; 28–35 min, 100% B; and 35–42 min, 18% B. The following MS parameters were used: scan range, 50–1000 Da; scan time, 0.2 s; dry gas flow rate, 6.0 L/min; dry temperature, 180°C; nebulizer pressure, 1 bar; and probe voltage, +4.0 kV. Sodium trifluoroacetate was used for mass calibration. Hystar 3.2 and MicrOTOF control 3.0 were used for data acquisition and processing. DataAnalysis 4.0 (Bruker Daltonics, Bremen, Germany) was used for data analysis.
NMR analyses were performed on a 600 MHz Bruker Advance III spectrometer (Bruker Daltonics) with CD3OD as the solvent.
Funding
This work was supported by the National Key Research and Development Program (2019YFA0905703); the National Natural Science Foundation of China (31970065, U1902214); the Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (TSBICIP-KJGG-002); and the Key Research and Development Plan of Guangdong Province (2022B1111070005).
Author contributions
Y.Y. and T.L. designed the experiments. Y.Y. and D.X. conducted the experiments. Y.Y., D.X., and Y.W. participated in the material preparation. Y.Y. and D.X. performed the data analysis. Y.Y., D.X., and T.L. wrote and reviewed the paper.
Acknowledgments
We thank Dr. Yibin Zhuang and Yi Cai from the Tianjin Institute of Industrial Biotechnology for help with LC–HRMS and NMR analyses. The authors declare the following competing financial interest: Part of the work has been included in a patent application by the Tianjin Institute of Industrial Biotechnology.
Published: March 20, 2023
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Supplemental information is available at Plant Communications Online.
Supplemental information
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplemental information. Additional data related to this paper may be requested from the authors. The sequences functionally characterized in this paper have been deposited in the GenBank database with the following accession numbers: SiCYP98A20, NP_001291339.1; OeCYP98A167, XP_022872083.1; PbCYP98A192, OP595606; RgCYP98A193, QJS39421.1; LrCYP98A194, OP595608; SiAT1, XP_011083883.1; OeAT1, XP_022859703.1; PbAT1, OP595604; RgAT1, OP556356; LrAT1, OP595607.
References
- Alipieva K., Korkina L., Orhan I.E., Georgiev M.I. Verbascoside - a review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014;32:1065–1076. doi: 10.1016/j.biotechadv.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Bai Y., Bi H., Zhuang Y., Liu C., Cai T., Liu X., Zhang X., Liu T., Ma Y. Production of salidroside in metabolically engineered Escherichia coli. Sci. Rep. 2014;4:6640. doi: 10.1038/srep06640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes H.J., Arlotto M.P., Waterman M.R. Expression and enzymatic activity of recombinant cytochrome P450 17-alpha-hydroxylase in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1991;88:5597–5601. doi: 10.1073/pnas.88.13.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi H., Wang S., Zhou W., Zhuang Y., Liu T. Producing gram-scale unnatural rosavin analogues from glucose by engineered Escherichia coli. ACS Synth. Biol. 2019;8:1931–1940. doi: 10.1021/acssynbio.9b00219. [DOI] [PubMed] [Google Scholar]
- Bontpart T., Cheynier V., Ageorges A., Terrier N. BAHD or SCPL acyltransferase? What a dilemma for acylation in the world of plant phenolic compounds. New Phytol. 2015;208:695–707. doi: 10.1111/nph.13498. [DOI] [PubMed] [Google Scholar]
- Chang M.C.Y., Eachus R.A., Trieu W., Ro D.K., Keasling J.D. Engineering Escherichia coli for production of functionalized terpenoids using plant P450s. Nat. Chem. Biol. 2007;3:274–277. doi: 10.1038/nchembio875. [DOI] [PubMed] [Google Scholar]
- Cheminat A., Zawatzky R., Becker H., Brouillard R. Caffeoyl conjugates from Echinacea species: structures and biological activity. Phytochemistry. 1988;27:2787–2794. doi: 10.1016/0031-9422(88)80664-2. [DOI] [Google Scholar]
- Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- Courdavault V., O'Connor S.E., Jensen M.K., Papon N. Metabolic engineering for plant natural products biosynthesis: new procedures, concrete achievements and remaining limits. Nat. Prod. Rep. 2021;38:2145–2153. doi: 10.1039/d0np00092b. [DOI] [PubMed] [Google Scholar]
- Cravens A., Payne J., Smolke C.D. Synthetic biology strategies for microbial biosynthesis of plant natural products. Nat. Commun. 2019;10:2142. doi: 10.1038/s41467-019-09848-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.K., Reddy K.A., Mukkanti K. Total synthesis of phenylpropanoid glycosides, grayanoside A and syringalide B, through a common intermediate. Carbohydr. Res. 2007;342:2309–2315. doi: 10.1016/j.carres.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Dimaano N.G., Yamaguchi T., Fukunishi K., Tominaga T., Iwakami S. Functional characterization of cytochrome P450 CYP81A subfamily to disclose the pattern of cross-resistance in Echinochloa phyllopogon. Plant Mol. Biol. 2020;102:403–416. doi: 10.1007/s11103-019-00954-3. [DOI] [PubMed] [Google Scholar]
- Duynstee H.I., de Koning M.C., Ovaa H., van der Marel G.A., van Boom J.H. Synthesis of verbascoside: a dihydroxyphenylethyl glycoside with diverse bioactivity. Eur. J. Org. Chem. 1999;1999:2623–2632. [Google Scholar]
- Eberle D., Ullmann P., Werck-Reichhart D., Petersen M. cDNA cloning and functional characterisation of CYP98A14 and NADPH:cytochrome P450 reductase from Coleus blumei involved in rosmarinic acid biosynthesis. Plant Mol. Biol. 2009;69:239–253. doi: 10.1007/s11103-008-9420-7. [DOI] [PubMed] [Google Scholar]
- Ehlting J., Büttner D., Wang Q., Douglas C.J., Somssich I.E., Kombrink E. Three 4-coumarate : coenzyme A ligases in Arabidopsis thaliana represent two evolutionarily divergent classes in angiosperms. Plant J. 1999;19:9–20. doi: 10.1046/j.1365-313X.1999.00491.x. [DOI] [PubMed] [Google Scholar]
- Ellis B. Production of hydroxyphenylethanol glycoside in supension cultures of Syringa vulgaris. Phytochemistry. 1983;22:1941–1943. doi: 10.1016/0031-9422(83)80018-1. [DOI] [Google Scholar]
- Fuji Y., Ohtsuki T., Matsufuji H. Accumulation and subcellular localization of acteoside in sesame plants (Sesamum indicum L.) ACS Omega. 2018;3:17287–17294. doi: 10.1021/acsomega.8b02798. [DOI] [Google Scholar]
- Gietz R.D., Schiestl R.H. Frozen competent yeast cells that can be transformed with high efficiency using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2007;2:1–4. doi: 10.1038/nprot.2007.17. [DOI] [PubMed] [Google Scholar]
- Grienenberger E., Besseau S., Geoffroy P., Debayle D., Heintz D., Lapierre C., Pollet B., Heitz T., Legrand M. A BAHD acyltransferase is expressed in the tapetum of Arabidopsis anthers and is involved in the synthesis of hydroxycinnamoyl spermidines. Plant J. 2009;58:246–259. doi: 10.1111/j.1365-313X.2008.03773.x. [DOI] [PubMed] [Google Scholar]
- Haas B.J., Papanicolaou A., Yassour M., Grabherr M., Blood P.D., Bowden J., Couger M.B., Eccles D., Li B., Lieber M., et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013;8:1494–1512. doi: 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Ji L., Boakye-Yiadom M., Li W., Song X., Gao X. Preparative isolation and purification of four compounds from Cistanches deserticola YC Ma by high-speed counter-current chromatography. Molecules. 2012;17:8276–8284. doi: 10.3390/molecules17078276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z.D., Ueda S., Akaji M., Fujita T., Inoue K., Yang C.R. Monoterpenoid and phenylethanoid glycosides from Ligustrum pedunculare. Phytochemistry. 1994;36:709–716. doi: 10.1016/S0031-9422(00)89802-7. [DOI] [PubMed] [Google Scholar]
- He Z.D., Lau K.M., But P.P.H., Jiang R.W., Dong H., Ma S.C., Fung K.P., Ye W.C., Sun H.D. Antioxidative glycosides from the leaves of Ligustrum robustum. J. Nat. Prod. 2003;66:851–854. doi: 10.1021/np020568g. [DOI] [PubMed] [Google Scholar]
- Hoffmann L., Maury S., Martz F., Geoffroy P., Legrand M. Purification, cloning, and properties of an acyltransferase controlling shikimate and quinate ester intermediates in phenylpropanoid metabolism. J. Biol. Chem. 2003;278:95–103. doi: 10.1074/jbc.M209362200. [DOI] [PubMed] [Google Scholar]
- Hou L., Li G., Chen Q., Zhao J., Pan J., Lin R., Zhu X., Wang P., Wang X. De novo full length transcriptome analysis and gene expression profiling to identify genes involved in phenylethanol glycosides biosynthesis in Cistanche tubulosa. BMC Genom. 2022;23:698. doi: 10.1186/s12864-022-08921-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Silipo A., Li W., Molinaro A., Yu B. Synthesis of forsythenethoside A, a neuroprotective macrocyclic phenylethanoid glycoside, and NMR analysis of conformers. J. Org. Chem. 2019;84:13733–13743. doi: 10.1021/acs.joc.9b01956. [DOI] [PubMed] [Google Scholar]
- Jiang J., Bi H., Zhuang Y., Liu S., Liu T., Ma Y. Engineered synthesis of rosmarinic acid in Escherichia coli resulting production of a new intermediate, caffeoyl-phenyllactate. Biotechnol. Lett. 2016;38:81–88. doi: 10.1007/s10529-015-1945-7. [DOI] [PubMed] [Google Scholar]
- Kawada T., Asano R., Hayashida S., Sakuno T. Total synthesis of the phenylpropanoid glycoside, acteoside. J. Org. Chem. 1999;64:9268–9271. doi: 10.1021/jo9906983. [DOI] [Google Scholar]
- Khong D.T., Judeh Z.M.A. Total synthesis of phenylpropanoid glycoside osmanthuside-B6 facilitated by double isomerisation of glucose-rhamnose orthoesters. Org. Biomol. Chem. 2017;15:2638–2646. doi: 10.1039/c7ob00198c. [DOI] [PubMed] [Google Scholar]
- Krogh A., Larsson B., von Heijne G., Sonnhammer E.L. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R.A., Hossain R., Roy P., et al. Anticancer effects of acteoside: Mechanistic insights and therapeutic status. Eur J Pharmacol. 2022;916:174699. doi: 10.1016/j.ejphar.2021.174699. [DOI] [PubMed] [Google Scholar]
- Levsh O., Pluskal T., Carballo V., Mitchell A.J., Weng J.K. Independent evolution of rosmarinic acid biosynthesis in two sister families under the Lamiids clade of flowering plants. J. Biol. Chem. 2019;294:15193–15205. doi: 10.1074/jbc.RA119.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Zhou W., Bi H., Zhuang Y., Zhang T., Liu T. Production of caffeoylmalic acid from glucose in engineered Escherichia coli. Biotechnol. Lett. 2018;40:1057–1065. doi: 10.1007/s10529-018-2580-x. [DOI] [PubMed] [Google Scholar]
- Li W., Godzik A. CD-HIT: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- Luo J., Nishiyama Y., Fuell C., Taguchi G., Elliott K., Hill L., Tanaka Y., Kitayama M., Yamazaki M., Bailey P., et al. Convergent evolution in the BAHD family of acyl transferases: identification and characterization of anthocyanin acyl transferases from Arabidopsis thaliana. Plant J. 2007;50:678–695. doi: 10.1111/j.1365-313X.2007.03079.x. [DOI] [PubMed] [Google Scholar]
- Mahesh V., Million-Rousseau R., Ullmann P., Chabrillange N., Bustamante J., Mondolot L., Morant M., Noirot M., Hamon S., de Kochko A., et al. Functional characterization of two p-coumaroyl ester 3'-hydroxylase genes from coffee tree: evidence of a candidate for chlorogenic acid biosynthesis. Plant Mol. Biol. 2007;64:145–159. doi: 10.1007/s11103-007-9141-3. [DOI] [PubMed] [Google Scholar]
- Matsuno M., Compagnon V., Schoch G.A., Schmitt M., Debayle D., Bassard J.E., Pollet B., Hehn A., Heintz D., Ullmann P., et al. Evolution of a novel phenolic pathway for pollen development. Science. 2009;325:1688–1692. doi: 10.1126/science.1174095. [DOI] [PubMed] [Google Scholar]
- Oka T., Nemoto T., Jigami Y. Functional analysis of Arabidopsis thaliana RHM2/MUM4, a multidomain protein involved in UDP-D-glucose to UDP-L-rhamnose conversion. J. Biol. Chem. 2007;282:5389–5403. doi: 10.1074/jbc.M610196200. [DOI] [PubMed] [Google Scholar]
- Park S.Y., Eun H., Lee M.H., Lee S.Y. Metabolic engineering of Escherichia coli with electron channelling for the production of natural products. Nat. Catal. 2022;5:726–737. doi: 10.1038/s41929-022-00820-4. [DOI] [Google Scholar]
- Petersen M. Hydroxycinnamoyltransferases in plant metabolism. Phytochem. Rev. 2016;15:699–727. doi: 10.1007/s11101-015-9417-1. [DOI] [Google Scholar]
- Saimaru H., Orihara Y. Biosynthesis of acteoside in cultured cells of Olea europaea. J. Nat. Med. 2010;64:139–145. doi: 10.1007/s11418-009-0383-z. [DOI] [PubMed] [Google Scholar]
- Schoch G., Goepfert S., Morant M., Hehn A., Meyer D., Ullmann P., Werck-Reichhart D. CYP98A3 from Arabidopsis thaliana is a 3'-hydroxylase of phenolic esters, a missing link in the phenylpropanoid pathway. J. Biol. Chem. 2001;276:36566–36574. doi: 10.1074/jbc.M104047200. [DOI] [PubMed] [Google Scholar]
- Song W., Zhuang Y., Liu T. Potential role of two cytochrome P450s obtained from Lithospermum erythrorhizon in catalyzing the oxidation of geranylhydroquinone during shikonin biosynthesis. Phytochemistry. 2020;175 doi: 10.1016/j.phytochem.2020.112375. [DOI] [PubMed] [Google Scholar]
- Sullivan M. A novel red clover hydroxycinnamoyl transferase has enzymatic activities consistent with a role in phaselic acid biosynthesis. Plant Physiol. 2009;150:1866–1879. doi: 10.1104/pp.109.136689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Sawada S., Watanabe K., Nagae S., Yamaguchi M.A., Nakayama T., Nishino T. Identification and characterization of a novel anthocyanin malonyltransferase from scarlet sage (Salvia splendens) flowers: an enzyme that is phylogenetically separated from other anthocyanin acyltransferases. Plant J. 2004;38:994–1003. doi: 10.1111/j.1365-313X.2004.02101.x. [DOI] [PubMed] [Google Scholar]
- Torrens-Spence M.P., Pluskal T., Li F.-S., Carballo V., Weng J.-K. Complete pathway elucidation and heterologous reconstitution of Rhodiola salidroside biosynthesis. Mol. Plant. 2018;11:205–217. doi: 10.1016/j.molp.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Torrens-Spence M.P., Gillaspy G., Zhao B., Harich K., White R.H., Li J. Biochemical evaluation of a parsley tyrosine decarboxylase results in a novel 4-hydroxyphenylacetaldehyde synthase enzyme. Biochem. Biophys. Res. Commun. 2012;418:211–216. doi: 10.1016/j.bbrc.2011.12.124. [DOI] [PubMed] [Google Scholar]
- Unno H., Ichimaida F., Suzuki H., Takahashi S., Tanaka Y., Saito A., Nishino T., Kusunoki M., Nakayama T. Structural and mutational studies of anthocyanin malonyltransferases establish the features of BAHD enzyme catalysis. J. Biol. Chem. 2007;282:15812–15822. doi: 10.1074/jbc.M700638200. [DOI] [PubMed] [Google Scholar]
- Wang F., Zhi J., Zhang Z., Wang L., Suo Y., Xie C., Li M., Zhang B., Du J., Gu L., Sun H. Transcriptome analysis of salicylic acid treatment in Rehmannia glutinosa hairy roots using RNA-seq technique for identification of genes involved in acteoside biosynthesis. Front. Plant Sci. 2017;8:787. doi: 10.3389/fpls.2017.00787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner V., Petersen M. A BAHD hydroxycinnamoyltransferase from Actaea racemosa catalyses the formation of fukinolic and cimicifugic acids. Planta. 2019;250:475–485. doi: 10.1007/s00425-019-03181-8. [DOI] [PubMed] [Google Scholar]
- Wong I.Y., He Z.D., Huang Y., Chen Z.Y. Antioxidative activities of phenylethanoid glycosides from Ligustrum purpurascens. J. Agric. Food Chem. 2001;49:3113–3119. doi: 10.1021/jf0100604. [DOI] [PubMed] [Google Scholar]
- Wu L., Georgiev M.I., Cao H., Nahar L., El-Seedi H.R., Sarker S.D., Xiao J., Lu B. Therapeutic potential of phenylethanoid glycosides: a systematic review. Med. Res. Rev. 2020;40:2605–2649. doi: 10.1002/med.21717. [DOI] [PubMed] [Google Scholar]
- Xie J., Tan F., Zhu J., Yue C., Li Q. Separation, purification and quantification of verbascoside from Penstemon barbatus (Cav.) Roth. Food Chem. 2012;135:2536–2541. doi: 10.1016/j.foodchem.2012.07.021. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Ren Q., Wu L. The pharmacokinetic property and pharmacological activity of acteoside: A review. Biomed Pharmacother. 2022;153:113296. doi: 10.1016/j.biopha.2022.113296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wu Y., Zhuang Y., Liu T. Discovery of glycosyltransferases involved in the biosynthesis of ligupurpuroside B. Org. Lett. 2021;23:7851–7854. doi: 10.1021/acs.orglett.1c02873. [DOI] [PubMed] [Google Scholar]
- Zhang S.Q., Li Z.J., Wang A.B., Cai M.S., Feng R. Total synthesis of the phenylpropanoid glycoside, grayanoside A. Carbohydr. Res. 1997;299:281–285. doi: 10.1016/s0008-6215(97)00032-3. [DOI] [Google Scholar]
- Zhang X., Li C., Wang L., Fei Y., Qin W. Analysis of Centranthera grandiflora Benth transcriptome explores genes of catalpol, acteoside and azafrin biosynthesis. Int. J. Mol. Sci. 2019;20:6034. doi: 10.3390/ijms20236034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Yang Y., Wang F., Zhou Z., Zhang H., Zhu Y. An approach to the synthesis of electron-rich and hindered esters and its application to the synthesis of acteoside. Org. Lett. 2021;23:9210–9215. doi: 10.1021/acs.orglett.1c03528. [DOI] [PubMed] [Google Scholar]
- Zhi J., Li Y., Zhang Z., Yang C., Geng X., Zhang M., Li X., Zuo X., Li M., Huang Y., et al. Molecular regulation of catalpol and acteoside accumulation in radial striation and non-radial striation of Rehmannia glutinosa tuberous root. Int. J. Mol. Sci. 2018;19:3751. doi: 10.3390/ijms19123751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A., Zhou K., Li Y. Rational design strategies for functional reconstitution of plant cytochrome P450s in microbial systems. Curr. Opin. Plant Biol. 2021;60 doi: 10.1016/j.pbi.2021.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F.Y., She J., Wang Y.G. Synthesis of a benzyl-protected analog of arenarioside, a trisaccharide phenylpropanoid glycoside. Carbohydr. Res. 2006;341:2469–2477. doi: 10.1016/j.carres.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Zubair M., Nybom H., Lindholm C., Rumpunen K. Major polyphenols in aerial organs of greater plantain (Plantago major L.), and effects of drying temperature on polyphenol contents in the leaves. Sci. Hortic. 2011;128:523–529. doi: 10.1016/j.scienta.2011.03.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplemental information. Additional data related to this paper may be requested from the authors. The sequences functionally characterized in this paper have been deposited in the GenBank database with the following accession numbers: SiCYP98A20, NP_001291339.1; OeCYP98A167, XP_022872083.1; PbCYP98A192, OP595606; RgCYP98A193, QJS39421.1; LrCYP98A194, OP595608; SiAT1, XP_011083883.1; OeAT1, XP_022859703.1; PbAT1, OP595604; RgAT1, OP556356; LrAT1, OP595607.