Abstract
Erianthus produces substantial biomass, exhibits a good Brix value, and shows wide environmental adaptability, making it a potential biofuel plant. In contrast to closely related sorghum and sugarcane, Erianthus can grow in degraded soils, thus releasing pressure on agricultural lands used for biofuel production. However, the lack of genomic resources for Erianthus hinders its genetic improvement, thus limiting its potential for biofuel production. In the present study, we generated a chromosome-scale reference genome for Erianthus fulvus Nees. The genome size estimated by flow cytometry was 937 Mb, and the assembled genome size was 902 Mb, covering 96.26% of the estimated genome size. A total of 35 065 protein-coding genes were predicted, and 67.89% of the genome was found to be repetitive. A recent whole-genome duplication occurred approximately 74.10 million years ago in the E. fulvus genome. Phylogenetic analysis showed that E. fulvus is evolutionarily closer to S. spontaneum and diverged after S. bicolor. Three of the 10 chromosomes of E. fulvus formed through rearrangements of ancestral chromosomes. Phylogenetic reconstruction of the Saccharum complex revealed a polyphyletic origin of the complex and a sister relationship of E. fulvus with Saccharum sp., excluding S. arundinaceum. On the basis of the four amino acid residues that provide substrate specificity, the E. fulvus SWEET proteins were classified as mono- and disaccharide sugar transporters. Ortho-QTL genes identified for 10 biofuel-related traits may aid in the rapid screening of E. fulvus populations to enhance breeding programs for improved biofuel production. The results of this study provide valuable insights for breeding programs aimed at improving biofuel production in E. fulvus and enhancing sugarcane introgression programs.
Key words: biofuel, cold stress, Erianthus fulvus, QTLs, reference genome, SWEET family
Erianthus fulvus produces abundant biomass with a high Brix content and can thrive in degraded soils and harsh environments. As a result, it holds promise as a potential biofuel crop. The assembly of a reference genome for E. fulvus, together with exploration of its carbohydrate metabolism at the genomic level, will aid in developing strategies to enhance its use as a biofuel resource.
Introduction
The genus Erianthus from the tribe Andropogoneae of the PACMAD clade in Poaceae is a crucial wild relative of sugarcane (Saccharum spp.) and is of significant interest to sugarcane breeders worldwide. Established by Michaux in 1803, the genus name derives from the Greek words erion, meaning wool, and anthos, meaning flower, in reference to its woolly glumes. It is an important genus in the Saccharum complex and is widely distributed from America (New World species) through the Mediterranean region to India, China, Southeast Asia, and New Guinea (Old World species, Ripidium sect.). Erianthus species (Ripidium sect.) have played a significant role in sugarcane breeding and evolution (Amalraj et al., 2006). Chromosome numbers in Erianthus species are variable, ranging from 2n = 20 to 2n = 60 (Bremer, 1924; Rao and Babu, 1953; Janaki-Ammal, 2008). The genera Saccharum, Erianthus (sect. Ripidium), Sclerostachya, and Narenga form a closely related inter-breeding unit involved in the origin of modern sugarcane cultivars (Mukherjee, 1957). Results of cytological investigations have shown that some chromosomes of Saccharum officinarum L. are similar to those of Miscanthus and Erianthus sect. Ripidium (Daniels and Roach, 1987).
Various species such as Erianthus arundinaceus (Retz.) Jeswiet, Erianthus fulvus, and Erianthus rockii Keng. possess important attributes like higher biomass, vigor, and ratooning ability, as well as resistance to drought, waterlogging, pathogens, and pests (Ram et al., 2001; Cai et al., 2005; Stirling et al., 2011; Wu et al., 2014). Most modern sugarcane cultivars were developed from interspecific crosses between limited progenitor species, presumably S. officinarum, S. spontaneum L., Saccharum robustum E.W.Brandes & Jeswiet ex Grassl, Saccharum barberi Jeswiet, and/or Saccharum sinense Roxb., resulting in a restricted genetic base and limited genetic improvement. Substantial sugarcane breeding gains have been achieved; however, the rate of improvement has remained relatively slow owing to these founder and/or genetic bottleneck effects (Edmé et al., 2006; Singh et al., 2011). Thus, breeders have turned to wild relatives like Miscanthus spp. and Erianthus spp. to introgress desirable traits and broaden the genetic base of cultivated sugarcane (Cai et al., 2005).
Erianthus fulvus can be a beneficial species for introgression programs because of its unique features. It possesses the lowest somatic chromosome count within the Saccharum complex (Amalraj et al., 2006), has a broad geographic distribution and adaptability, and shows strong resistance to drought, cold, and degraded soils (1, 1). The introgression of Erianthus genes into sugarcane varieties is expected to bring about improved multi-ratoon ability and disease and pest resistance (Nair et al., 2017). Furthermore, hybrids between Saccharum and E. arundinaceus or E. fulvus were observed to exhibit superior agronomic characteristics when compared with their parental species (Wang et al., 2010a, 2010b; Fukuhara et al., 2013; Pachakkil et al., 2019). These traits are crucial for sugarcane breeding and can be exploited for the development of cultivars with enhanced adaptability and stress tolerance. Wang et al. (2010a), also found that intergeneric crosses between Saccharum and E. fulvus resulted in the transmission of 7 to 10 E. fulvus chromosomes to the progeny, and they identified 58 true hybrids out of 73 progeny (Wang et al., 2009).
The use of biofuels as an alternative energy source holds significant promise and possesses numerous advantages over fossil fuel-based energy systems. Currently, sugarcane and sorghum are the primary species for biofuel production. However, their utilization for biofuel production not only occupies agricultural lands but also can result in a rapid increase in the market price of sugarcane and sorghum products, particularly table sugar. To address these issues, alternative non-agricultural species with high biomass potential and the ability to grow in degraded non-agricultural soils need to be considered. Erianthus plants exhibit excellent growth under challenging environmental conditions such as drought and acidic, dry, and submerged soils (Matsuo, 2002). In addition, Erianthus spp. have a higher biomass production capacity (40–60 tonnes/hectare/year) than Miscanthus spp. (12–40 tonnes/hectare/year) and switchgrass (7–35 tonnes/hectare/year) (Hattori et al., 2010; (Hattori and Morita, 2010). Erianthus is also a valuable source of feedstock for the biofuel industry because of its high Brix value (Matsuo, 2002). Previous studies have indicated that Erianthus spp. can grow successfully on degraded and agriculturally unsuitable soils, suggesting that land unsuitable for agriculture can be used for biofuel production (Sekiya et al., 2014). The major obstacle to use of E. fulvus in sugarcane breeding programs and biofuel production is a lack of sufficient genomic resources.
The main objective of this study was to produce a reference-quality genome assembly of E. fulvus using a hybrid sequencing method. The newly generated genomic resources were used to clarify phylogenetic ambiguity in the Saccharum complex, which will aid in the selection of suitable parents for future sugarcane breeding programs. In addition, we characterized E. fulvus genes that regulate carbohydrate metabolism, particularly the SWEET sugar transporter gene family, and identified unique mono- and disaccharide transporters. Lastly, we identified ortho-QTL regions associated with traits relevant to the biofuel potential of this species. Our findings hold great potential for future breeding programs to improve biofuel production.
Results and discussion
Genome assembly
Flow cytometry analysis indicated that the genome size of E. fulvus was approximately 937 Mb. A hybrid sequencing strategy that incorporated Illumina, PacBio, and Hi-C reads was used to construct the E. fulvus genome. PacBio sequencing produced approximately 132.3 Gb of long reads, Illumina sequencing 147.4 Gb of short reads, and Hi-C sequencing 189 Gb of high-quality clean reads. A de novo genome assembly was generated from the PacBio long reads and then polished using the Illumina short reads. After two rounds of polishing, a 901.94-Mb draft genome assembly was generated, consisting of 829 scaffolds (Figure 1). The size of this assembly was similar to the estimated genome sizes obtained through flow cytometry (937 Mb) and genome survey analysis (869 Mb) (refer to Figure 3 in Supplemental File 1). Approximately 50.72% of the Hi-C reads had valid contacts with the scaffolds. Using the Hi-C data, 96.38% of the scaffolds were anchored into 10 pseudomolecules that ranged in size from 62 to 115 Mb (Figure 2A). The N50 value of the assembly was 84 Mb, and its GC content was 45.39%. The final assembly covered approximately 96.26% of the estimated genome size (refer to Table 1 in Supplemental File 1). BUSCO assessment (Simão et al., 2015) revealed a high level of gene completeness in the assembled genome: 97.27% of the core genes were complete, and only 1.4% were missing (see Table 1 in Supplemental File 2). This BUSCO value is notably higher than those of other Saccharum complex genome assemblies, such as S. spontaneum (Nascimento et al., 2019), and comparable to those of Miscanthus lutarioriparius L.Liu ex S.L.Chen & Renvoize (Miao et al., 2021) and S. spontaneum (Zhang et al., 2018a). The long terminal repeat (LTR) index (LAI) is a measure of the completeness of repetitive sequences in a genome assembly. Here, the LAI value for E. fulvus was 15.31, which is higher than that of the closely related species M. lutarioriparius (Miao et al., 2021). Almost 99.78% of the Illumina reads were mapped back to the assembled genome, indicating the high quality of the assembly. Assembly statistics of E. fulvus were compared with those of Sorghum bicolor (L.) Moench (NCBIv3), Oryza sativa L. (variety japonica v 4.0), and Arabidopsis thaliana (L.) Heynh. (TAIRv10.1); the N50 value of E. fulvus was comparable to that of O. sativa and higher than those of A. thaliana and S. bicolor (see Figures 1 and 2 in Supplemental File 1).
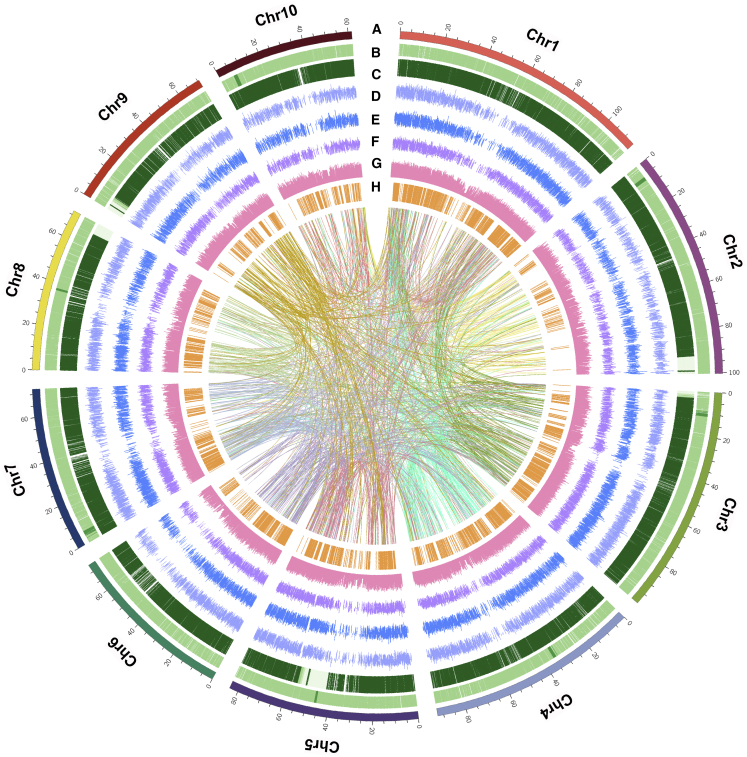
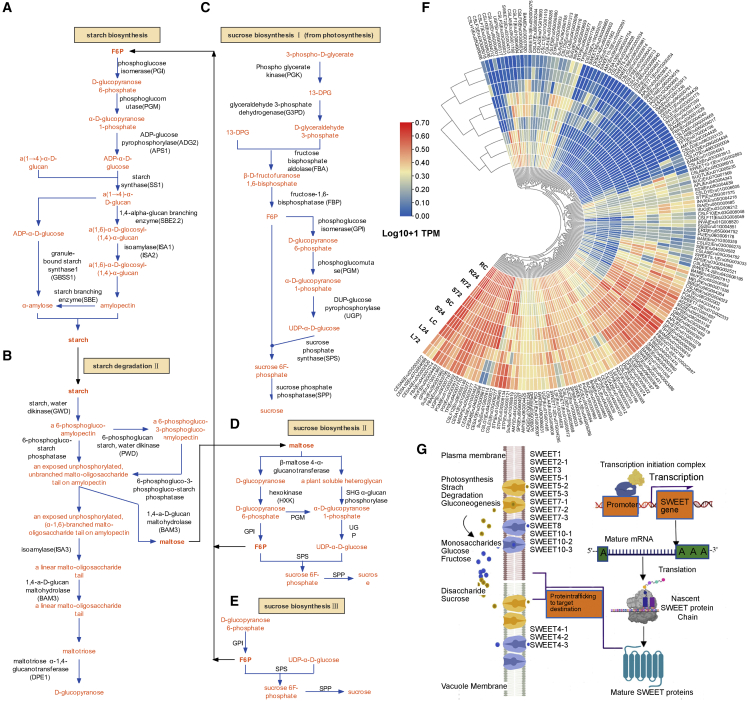
Figure 1.
Circos plot showing the genome characteristics of E. fulvus.
The nine rings from outside to inside are (A) chromosomes, (B)Gypsy elements, (C)copia elements, (D) FPKM values of genes in the stem, (E) FPKM values of genes in the leaf, (F) FPKM values of genes in the root, (G) GC content, (H) ortho-QTL genes, and (I) syntenic relationships of E. fulvus genes.
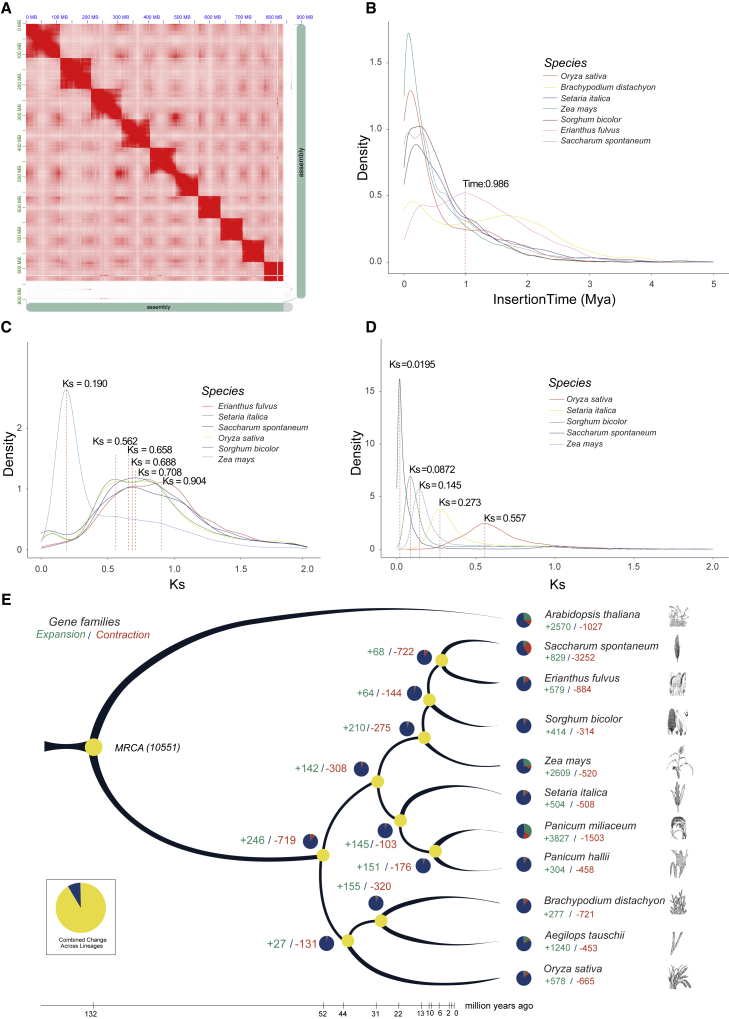
Figure 2.
Evolutionary characteristics of the E. fulvus genome.
(A) Hi-C interaction map.
(B) Depiction of LTR evolution.
(C) Representation of whole-genome duplication (WGD) events during E. fulvus evolution based on synonymous substitution rates (Ks).
(D) Density distribution of Ks values for paired genes calculated within each syntenic block between E. fulvus and other species.
(E) Genome evolution based on single-copy orthologs of 10 species from the PACMAD and BOP clades. A. thaliana was used as an outgroup. Numbers in green indicate expanded gene families, and numbers in red indicate contracted gene families.
Genome annotation
Approximately 67.89% of the E. fulvus genome was repetitive, with LTRs being the most abundant elements at 49.3% (Table 1 of Supplemental File 1, Figure 1). Evaluation of their integration times showed that most intact LTRs in the E. fulvus genome were amplified between 0.2 and 1 million years ago (Mya), with two separate bursts of amplification occurring approximately 1 and 0.2 Mya (Figure 2B). The amplification of copia elements occurred prior to that of gypsy elements (Supplemental File 3). Previous studies have shown that LTR amplifications took place during the Pleistocene epoch, during which the growth of grasslands and other types of vegetation was negatively influenced by low freezing temperatures and limited global atmospheric CO2. Therefore, the expansion and evolution of transposable elements (TEs) may have played a beneficial role in this stressful environment (McClintock, 1984; Grandbastien, 1998; Lisch, 2013). It is possible that LTR amplification in E. fulvus was also an adaptive response to the unfavorable environment during this time. Results indicated significant variation in the proportion of LTRs among species, with higher proportions observed in sorghum (57%), sugarcane (∼52%), and maize (81.4%) than in Brachypodium P.Beauv (21.5%), rice (28.1%), and foxtail millet (31.8%) (Table 3 of Supplemental File 2). The highest gypsy:copia ratio was observed in sorghum (5.4:1). These results suggest that the diversification of grasses may depend on the composition and multiplication of TEs (Kraaijeveld, 2010; Puttick et al., 2015).
The E. fulvus genome was analyzed using a hybrid approach of ab initio prediction and transcriptome mapping, resulting in 35 065 gene models (Table 4 of Supplemental File 2); 90.6% of the predicted genes were complete, and functions were predicted for 79.2%, with 20.8% remaining unannotated (Table 5 of Supplemental File 2). Non-coding RNAs were also identified, including 583 rRNA, 1184 tRNA, 2578 small nuclear RNA (snRNA), and 532 microRNA (miRNA) genes. OrthoMCL was used to identify unique and shared gene families among E. fulvus, Zea mays, S. spontaneum, S. bicolor, and O. sativa. There were 17 563 gene families in E. fulvus, among which 9141 were common to all species and 1073 were unique to E. fulvus (Supplemental File 4). A total of 579 gene families were expanded and 884 contracted in the E. fulvus genome (Figure 2E). There were 4080 single-copy and 6167 multi-copy ortholog groups shared among all Gramineae species. Results from functional enrichment analysis of the expanded and unique gene families are presented in Tables 9 and 10 of Supplemental File 2.
Genome evolution
The genome evolution of E. fulvus was inferred based on single-copy orthologous genes of S. spontaneum, Setaria italica (L.) P.Beauv., Panicum hallii Vasey, Panicum miliaceum L., S. bicolor, E. fulvus, Z. mays (PACMAD clade), O. sativa, Brachypodium distachyon (L.) P.Beauv., and Aegilops tauschii Coss. (BOP clade) (Figure 2E). The results indicated that E. fulvus is evolutionarily closer to S. spontaneum and diverged after S. bicolor. The estimated time of divergence between the PACMAD and BOP clades was approximately 50.4 Mya. To examine whole-genome duplication (WGD) events during E. fulvus evolution, we used synonymous substitution rates (Ks) of inter-chromosomal paralogous gene pairs to determine the age distribution of duplication events. One peak was observed at Ks ∼0.904. The analysis also suggested a WGD event in E. fulvus (Ks ∼0.65) that was shared with other species, except maize, and occurred ∼53.3 Mya (Figure 2C). Data were also analyzed on the basis of pair-wise collinear blocks between the genomes of E. fulvus and other species, and the density distribution of Ks values for paired genes within each syntenic block was calculated (Figure 2D). For orthologous gene pairs, two peaks were observed: Ks = 0.557 between E. fulvus and rice, and Ks = 0.273 between E. fulvus and S. italica. These results suggest that an ancient WGD event occurred prior to the divergence of the BOP and PACMAD clades. Inter-genomic analyses showed highly conserved collinearity among E. fulvus, O. sativa, and S. spontaneum, suggesting a close evolutionary relationship. E. fulvus chromosomes showed explicit synteny with both O. sativa and S. spontaneum chromosomes (Figure 3A and 3C). Specifically, chromosomes 1, 2, and 3 of E. fulvus showed synteny with chromosomes 3/10, 11/12, and 7/9 of rice, respectively. In addition, chromosomes 2 and 10 of E. fulvus demonstrated partial synteny with the telomeric regions of chromosome 12 of rice and chromosomes 5/6 of S. spontaneum. Likewise, chromosome 10 of E. fulvus showed synteny to telomeric regions of chromosome 11 of rice and chromosomes 2/7 of S. spontaneum.
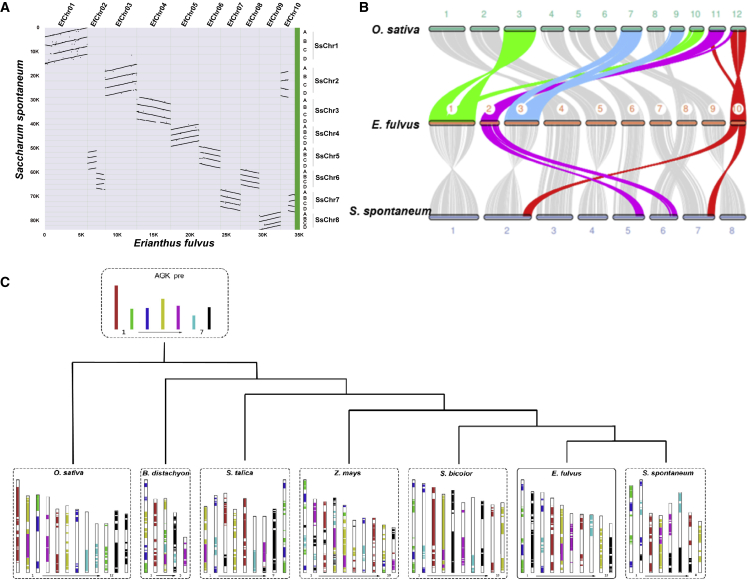
Figure 3.
Inter-genomic analyses.
(A) Correspondence between chromosomes of E. fulvus and S. spontaneum.
(B) Chromosome rearrangements based on AGK gene composition across E. fulvus and other species.
(C) Collinearity and syntenic relationships among E. fulvus, O. sativa, and S. spontaneum.
An evaluation of ancestral grass karyotype (AGK) genes showed that 29.26% of all the genes in E. fulvus corresponded to AGK genes; this proportion was lower than that in other species such as rice (37.19%), S. spontaneum (36.16%), S. italica (42.05%), and S. bicolor (41.79%) (Table 6 of Supplemental File 2). The AGK gene composition was used to examine how the seven ancestral chromosomes have undergone rearrangements to generate the current E. fulvus genome. Seven chromosomes of E. fulvus (chromosomes 2, 4, 6, 7, 8, 9, and 10) were found to have been conserved and not undergone any rearrangements. Notably, ancestral chromosomes 1, 4, 5, 6, and 7 were conserved as E. fulvus chromosomes 4/7, 9, 6, 8, and 2/10, respectively (Figure 3B). Three E. fulvus chromosomes evolved from rearrangements of segments from different ancestral chromosomes. For example, chromosome 1 of E. fulvus evolved as the result of fusion between segments of ancestral chromosomes 2 and 3. Likewise, chromosomes 3 and 5 of E. fulvus evolved from four ancestral chromosomes (3/6 and 4/5, respectively). These results indicate that three pairs of chromosomes have diverged from a common ancestor and undergone separate rearrangements to form three chromosomes in E. fulvus. It was also evident that chromosomes 2 (B. distachyon), 1/5 (O. sativa), 8 (Z. mays), 5 (S. italica), 3 (S. bicolor), 3 (S. spontaneum), and 4/7 (E. fulvus) evolved from ancient chromosome 1, and chromosomes 8 (S. spontaneum), 9 (E. fulvus), 10 (S. bicolor), 4 (S. italica), and 6 (O. sativa) evolved from ancient chromosome 4. Therefore, ancestral chromosomes 1 and 4 are generally evolutionarily conserved in monocots, playing a crucial role in the development of monocot species and protecting their genes from chromosome disturbance during monocot speciation.
Based on the top 0.5% composite likelihood ratio (CLR) values, 4218 selective sweeps were identified in the genome of E. fulvus (Supplemental File 5A). In total, 605 genes were found to have been subjected to selective sweeps. Most sweeps (3241) were located in intergenic regions, and 291 and 426 sweeps were located in exonic and intronic regions, respectively. Approximately 45 sweeps were located in untranslated regions, and 127 showed a downstream location. Gene Ontology (GO) analysis of genes associated with selective sweeps revealed significant enrichment in processes related to cellular regulation, organelle organization, biological regulation, and chromosome organization (Supplemental File 5B). Analysis of nucleotide diversity in E. fulvus from Xizang and Yunnan provinces revealed that strains from Xizang had the highest nucleotide diversity (π = 8.5 × 10−4), and large differences in nucleotide diversity (π ratio) were identified between these two provinces. Based on the top 1% π ratio values, potential selection signals were detected in E. fulvus from Xizang and Yunnan (Supplemental File 5C). GO analysis of candidate genes located in these regions revealed significant enrichment in categories related to lipid and steroid metabolism, suggesting that these regions may have been selected in response to geographic variation.
Phylogenetic reconstruction of Saccharum complex species
We used a combination of whole-genome SNP analysis and genome-wide orthology-based approaches, together with analysis of population structure and differentiation, to determine the true phylogenetic relationships among members of the Saccharum complex. A total of 202 accessions were analyzed using whole-genome SNP-based phylogenetic reconstruction (Table 7 of Supplemental File 2). These accessions belonged to the following groups: (1) the genus Erianthus (97 accessions of E. fulvus, code = ZM); (2) the genus Eulalia (one accession of Eulalia quadrinervis [Hack.] Kuntze, code = SMJM); (3) the genus Miscanthus (17 accessions of Miscanthus floridulus (Labill.) Warb. ex K.Schum. & Lauterb. and Miscanthus sinensis Thunb. Andersson, respective codes = WJM and MANG); (4) the genus Narenga (five accessions of Narenga porphyrocoma [Hance] Bor, code = HBW); (5) the genus Saccharum (56 accessions of S. spontaneum, S. officinarum, S. sinense, S. barberi, and S. robustum, respective codes = GSM, RDZ, ZGZ, YDZ, and DJYS); and (6) an uncertain group composed of 11 accessions of Saccharum arundinaceum (code = BM), 4 accessions of Erianthus bengalense (Retz.) Hubbard. & Vaughn ex Stewart (code = MGZM), 2 accessions of Erianthus munja (code = MGZM), and 7 accessions of Erianthus rockii (code = DZM). The 202 accessions underwent whole-genome resequencing, which generated 2.7 Tb (27.3 billion) paired-end raw reads. The reads were mapped to the Vitis vinifera L. reference genome (Ensemble Plants Release-31) (Kersey et al., 2016) to identify genetic variants. After hard filtering using GATK, 77 726 929 SNPs, 10 278 017 InDels, and 25 000 copy number variants were retained. A core set of 12 549 273 SNPs was generated using a minor allele frequency (MAF) cutoff value ≥0.05. These high-quality SNPs exhibited a transition-to-transversion ratio (Ti/Tv) of 2.88. The genus Saccharum showed the highest genetic diversity (π = 4.7 × 10−3) (Table 8 of Supplemental File 2).
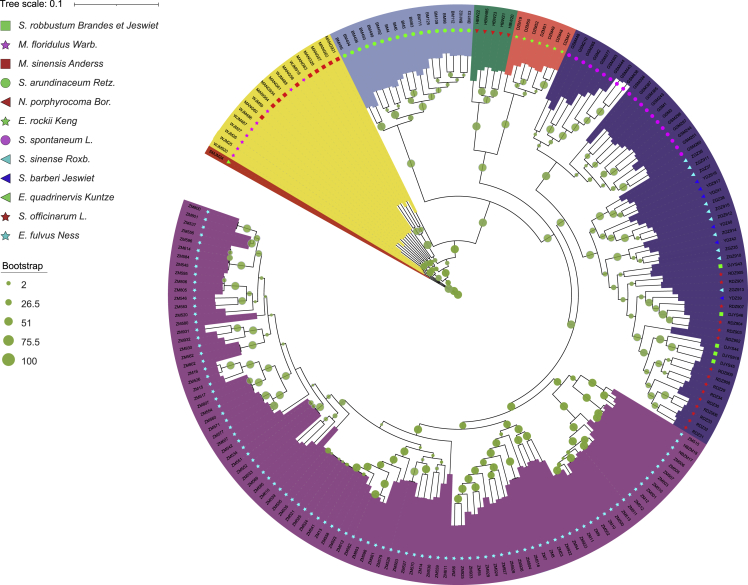
A phylogenetic analysis was performed using the core set of SNPs, with E. quadrinervis Kuntze as an outgroup (Figure 4). A maximum likelihood (ML)-based phylogenetic tree constructed with 100 non-parametric bootstraps revealed six major clusters, with all the E. fulvus accessions forming a separate monophyletic clade (ZMS, magenta). The Saccharum accessions, excluding S. arundinaceum, formed a single admixed group (GZS, blue), suggesting a polyphyletic origin. Notably, S. spontaneum was resolved into a monophyletic sub-cluster, indicating their evolution from a common ancestor, whereas other Saccharum species had a different ancestral origin. E. rockii (DZM, red) accessions formed a sister clade to N. porphyrocoma (HBW, green), and S. arundinaceum (BM, sky blue) and Miscanthus (MANG and WJM, yellow) each formed their own clades. These results suggest that the descendants of a common ancestor diverged to form a polyphyletic lineage of Miscanthus and a larger lineage that, over time, split into sub-lineages of Narenga and E. rockii on one hand and Saccharum and E. fulvus on the other. The genome-wide orthology-based phylogeny had a topology similar to that of the genome-wide SNP-based phylogeny. Detailed orthology-based results, presented in Supplemental File 6, also suggested that E. fulvus is closely related to Saccharum. Both phylogenies showed that S. arundinaceum is distinct from both Erianthus and Saccharum, suggesting that it is a distinct genus. Our orthology results showed that S. arundinaceum is more closely related to Narenga. These phylogenetic reconstructions were corroborated by principal-component analysis (PCA), population structure analysis, and population differentiation results (Supplemental File 6).
Figure 4.
Maximum likelihood (ML) phylogenetic tree based on genome-wide SNPs.
The magenta clade contains E. fulvus (sky-blue star), and the blue clade includes accessions of S. officinarum (red star), S. robustum (green square), S. barberi (blue triangle), S. sinense (sky-blue triangle), and S. spontaneum (magenta circle). The red clade contains accessions of E. rockii (green star), the green clade contains N. porphyrocoma (red triangle), the sky-blue clade contains S. arundinaceum (green circle), and the yellow clade contains Miscanthus (red square and magenta star).
The Saccharum complex is a hypothetical collection of species believed to be responsible for the origin of sugarcane through cross-breeding with each other. This complex includes various genera such as Saccharum, Erianthus sect. Ripidium, Miscanthus sect. Diandra, Sclerostachya, and Narenga (Mukherjee, 1957; Daniels et al., 1975). Several studies have delineated Saccharum from Erianthus (Besse et al., 1998; Nair et al., 2005; Selvi et al., 2006). However, Hodkinson et al. (2002) disputed this delineation and suggested that Saccharum is a polyphyletic group. Our analysis also supports the polyphyletic nature of the Saccharum complex, with E. fulvus exhibiting a sister relationship to other Saccharum species. Furthermore, the taxonomy between Saccharum and Miscanthus is dubious (Clayton W D, Renvoize S A. Genera Graminum: Grasses of the World.[J]. H.M.S.O (Her Majesty's Stationery Office), 1986.; Hodkinson et al., 2002). Previous studies have treated Saccharum as a distinct lineage, phylogenetically distinct from the New World Erianthus and Old World Miscanthus (Grivet et al., 2004; Estep et al., 2014; Evans et al., 2016). Our results suggest that E. fulvus, Saccharum (excluding S. arundinaceum), and Miscanthus are distinct lineages, with Saccharum (excluding S. arundinaceum) more closely related to E. fulvus, and the latter more distinct from Miscanthus. Our results agree with Evans and Joshi (2020), with Erianthus and Miscanthus forming distinct clusters and Miscanthus showing a close relationship to Narenga but divergence from Saccharum. Furthermore, taxonomic relationships among the genera Saccharum, Erianthus, and Tripidium are also unclear. Although E. fulvus is also assigned to the genus Saccharum (refer to NCBI: txid154759), the genus Tripidium is also used interchangeably with Saccharum and Erianthus (refer to NCBI: txid50346). However, our analysis does not support the inclusion of E. fulvus in the genus Saccharum, and it affirms that Tripidium arundinaceum (Retz.) Welker, Voronts. & E.A.Kellogg is distinct from both Saccharum and E. fulvus. This study resolved the ambiguous phylogeny of the Saccharum complex, which may aid in introgression of useful traits into sugarcane from this wild gene pool.
Evaluation of genes related to sucrose and carbohydrate metabolism
Exploration of the carbohydrate metabolism pathway of E. fulvus can provide valuable insights into its potential as a biofuel resource. Carbohydrate metabolism genes of E. fulvus were predicted using a homology-based approach with A. thaliana as a reference (Supplemental File 7). One of the significant determinants of the biofuel potential of a species is its Brix content, which is dependent on the contents of soluble sugars, particularly sucrose, and is regulated by metabolism, transport, and storage of these sugars. Thus, thorough understanding and manipulation of the mechanisms involved in sugar phloem loading and unloading, transport, metabolism, and signaling is essential for enhancing the biofuel potential of E. fulvus. To this end, we evaluated the dynamics of sucrose synthesis and transport. Sucrose can be synthesized in three different ways, and its concentration is determined by a balance between starch synthesis and breakdown (Figure 5). To gain deeper insights into sucrose biosynthesis, we measured the expression levels of related genes under normal growth conditions (28°C) and at low temperature (4°C) for 24 h (T1) and 72 h (T2). Under normal conditions, some key starch biosynthesis genes, such as granule bound starch synthase (GBSS), isoamylase (ISA), and starch branching enzyme (SBE), exhibited the highest TPM values in leaves and the lowest TPM values in roots. A similar pattern was observed for essential starch degradation genes like glucan water dikinase (GWD) and several beta amylases (BAMs) (Figure 5F). Maltose, a byproduct of starch breakdown, is metabolized to sucrose (sucrose biosynthesis II) (Figure 5D). Our analysis revealed tissue-specific variation in the transcript abundance of genes involved in sucrose biosynthesis II, such as UDP-glucose pyrophosphorylase (UGP), phosphoglucomutase (PGM), sucrose phosphate synthase (SPS), and sucrose phosphate phosphatase (SPP). Sucrose is also synthesized from the end products of gluconeogenesis (sucrose biosynthesis III) (Figure 5E). In addition to SPS and SPP, phosphoglucose isomerase (PGI) is also involved in sucrose biosynthesis III. Here, one gene for phosphoglucose isomerase (PGI) exhibited the highest expression in leaves, whereas a different PGI gene showed the highest expression in roots. Sucrose can also be synthesized during photosynthesis (sucrose biosynthesis I) (Figure 5C). In addition to PGI, PGM, UGP, SPS, and SPP, fructose bisphosphate aldolase (FBA) was most highly expressed in roots, whereas fructose-1,6-bisphosphatase (FBP) was most highly expressed in leaves. Two key enzymes, invertase (INV) and sucrose synthase (SuSy), degrade sucrose into fructose and glucose. Among the seven INV genes in E. fulvus, Eru01G008820, Eru05G004218, and Eru05G006584 showed highest expression in leaves, and Eru05G004463, Eru05G000659, and Eru02G002188 showed highest expression in roots under normal conditions. All INV genes showed intermediate expression in stems under normal conditions. We identified five SuSy genes, which showed generally higher expression in roots, followed by stems. Eru01G007814 and Eru09G001960 were the most active SuSy genes in roots, and Eru01G008519 was the most active SuSy in stems under normal conditions (Figure 5F). All SuSy genes showed the lowest expression in leaves under normal conditions. These results indicate that spatial differences in INV and SuSy expression may result in tissue-specific sucrose profiles in E. fulvus.
Figure 5.
Representation of sucrose and starch metabolism.
(A) Pathway depicting starch biosynthesis.
(B) Pathway depicting starch degradation.
(C) Depiction of sucrose biosynthesis during photosynthesis.
(D) Depiction of sucrose biosynthesis from end products of starch degradation.
(E) Depiction of sucrose biosynthesis from end products of gluconeogenesis I.
(F) Heat map showing expression patterns of important genes involved in starch and sucrose metabolism. RC, LC, and SC = root, leaf, and stem control (under normal conditions of 28°C). R24, L24, and S24 = root, leaf, and stem after 24 h at 4°C. R72, L72, and S72 = root, leaf, and stem after 72 h at 4°C.
(G) Diagram representing transcription, translation, and trafficking of EfSWEET proteins to their target destinations. Thirteen EfSWEET proteins were targeted to the plasma membrane and three to the vacuole. These EfSWEET proteins transport either disaccharides like sucrose (orange text, orange transporter in diagram) or monosaccharides like glucose and fructose (purple text and purple transporter in diagram).
Sugar transport in plants is facilitated by various transporter proteins, including sugar transporter proteins (STPs), sucrose transporters (SUCs or SUTs), and members of the SWEET transporter family (Hedrich et al., 2015; Xie et al., 2019). To improve Brix value and, thus, biofuel potential, it is necessary to understand how E. fulvus regulates carbon absorption and sugar transport, particularly of sucrose. Our analysis revealed the presence of eight STPs involved in H+/hexose transport, five of which (Eru03G000121, Eru279G000003, Eru03G008134, Eru02G005933, and Eru06G003577) displayed the highest expression in roots and two of which (Eru01G009940 and Eru05G001575I) displayed the highest expression in leaves under normal conditions. The STPs showed intermediate expression in stems under normal conditions. Among the six SUCs, two (Eru07G001569 and Eru10G000033) displayed the highest expression in stems, and three (Eru05G008064, Eru05G008059, and Eru01G001562) showed the highest expression in roots. STPs and SUCs ensure the transport of various hexoses and the disaccharide sucrose to their respective destinations in E. fulvus. Eru05G002277 and Eru04G006985, which encode tonoplast monosaccharide transporters, and Eru01G008235, which encodes a major facilitator superfamily transmembrane carbohydrate transporter, displayed the highest expression in stems and leaves/roots, respectively.
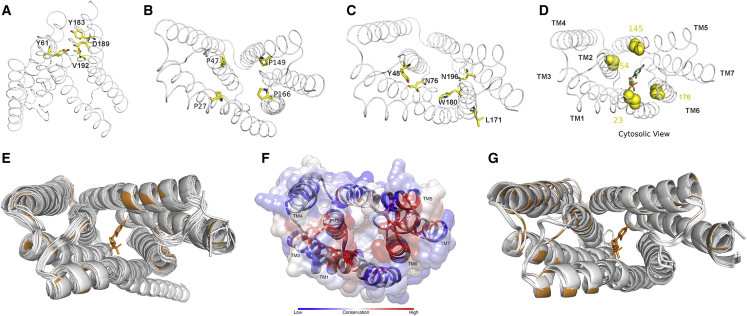
SWEET proteins are a newly discovered class of sugar transporters that play a crucial role in sugar efflux and phloem loading. They can transport various types of sugars, including glucose, galactose, fructose, and sucrose, and they play a critical role in determining the carbon distribution, crop yield, and nutritional and economic value of plants (Singh et al., 2022). Little detailed information is available for the E. fulvus SWEET proteins (EfSWEETs), and it is necessary to study their substrate specificity in order to target specific SWEETs for genetic improvement to increase the Brix value of E. fulvus. We therefore performed structural and functional evaluation of E. fulvus SWEET proteins using the MtN3/saliva SWEET hallmark domain. Sixteen unique EfSWEET proteins were identified (Table 1, Supplemental File 7), clustering into four clades similar to those in A. thaliana (Xie et al., 2019) (Figure 3 of Supplemental File 7). The closest 3D structural analog of EfSWEET proteins was found to be AtSWEET13 (PDB ID 5XPD). The 3D structures of EfSWEET proteins were modeled using Phyre2 (Kelley et al., 2015) and AlphaFold2 (Jumper et al., 2021), revealing seven transmembrane (TM) helices that form a channel similar to the conserved eukaryotic sweet sugar transporter fold (Feng and Frommer, 2015; Tao et al., 2015; Han et al., 2017) (Supplemental File 8). Structural superimpositions with 5XPD revealed a reasonable structural similarity, as shown in Table 1 of Supplemental File 7 and Figure 6E and 6G. A total of 34 amino acid residues were highly conserved across EfSWEETs at an identity threshold of >90%, 13 of which were key functional residues. These key functional residues include Tyr61, Tyr183, Asp189, and Val192, which provide extracellular gating (Tao et al., 2015; Han et al., 2017) (Figure 6A); P27, P47, P149, and P166, which form a proline tetrad essential for transport (Lasko and Brandriss, 1981) (Figure 6B); Tyr48 and Leu169, which provide intracellular gating (Han et al., 2017); and Asn76, Trp180, and Asn196, which surround the binding pocket to facilitate transport (Tao et al., 2015) (Figure 6C). Only Val192 varied across the EfSWEETs and could be an Ile or a Leu. A similar substitution has also been reported across the AtSWEET family (Tao et al., 2015; Han et al., 2017). Four conserved residues (positions 23, 54, 145, and 176) in the central ligand-binding pocket confer functional specificity to the SWEET proteins for either mono- or disaccharides (Han et al., 2017) (Figure 6D and Table 2 of Supplemental File 7). The EfSWEET proteins were classified into mono- and disaccharide transporters based on these conserved residues in the binding pocket (Figure 6G). This classification agreed with estimates of their cavity volumes, which were calculated for the monomeric unit of each EfSWEET protein using the PyVol plugin (Smith et al., 2019) with a monosaccharide transporter protein (AtSWEET13) as a reference (Table 1 of Supplemental File 7). Most EfSWEET monosaccharide transporters have smaller cavity volumes (>900 A3 and <1000 A3) than disaccharide transporters. The cavity volume of EfSWEET monosaccharide transporters is comparable to that of the monosaccharide transporter AtSWEET13. However, there may be no direct correlation between cavity volume and substrate selectivity (mono- or disaccharide transport) because the functional cavity in some sugar transporters may be formed by a homotrimer (Tao et al., 2015) instead of a monomer (Han et al., 2017). Further research is needed to clarify the precise substrate specificity of the EfSWEETs.
Figure 6.
Structural details of EfSWEET proteins.
(A) Tyr61, Tyr183, Asp189, and V192 are vital for extracellular gating.
(B) The proline tetrad (P27, P47, P149, and P166) is essential for the transport route.
(C) Tyr48 and Leu169 are responsible for intracellular gating, and Asn76, Trp180, and Asn196 surround the binding pocket to facilitate transport. Residues are numbered according to 5XPD, and structure figures were created in PyMOL.
(D) The mono- and disaccharide substrate-specific residues highlighted are shown in yellow (23, 54, 145, and 176) and mapped on the 5XPD structure indicated by the gray ribbon, with its substrate analog shown in a stick representation.
(F) Conservation surface mapping of EfSWEET transmembrane domain, colored according to the conservation score derived from the MSA.
(E and G) Structural superimposition of the transmembrane domain of the modeled 3D structure of EfSWEET proteins with AtSWEET13 (PDBID:5XPD). The 5XPD and its disaccharide substrate are shown in orange, whereas all sweet proteins are shown in gray cartoons. (E) Modeled with Alphafold2. (G) Modeled with Phyre2 homology models.
Expression analysis of E. fulvus SWEET genes revealed that five genes (Eru01G008512, Eru04G006195, Eru05G003033, Eru05G003034, and Eru08G000981) exhibited the highest expression in stems under normal conditions. They also demonstrated higher expression levels in stems than in leaves and roots at T1 and T2. On the other hand, six SWEET genes (Eru10G002893, Eru10G002897, Eru10G002891, Eru04G003486, Eru07G004295, and Eru07G002333) exhibited higher expression levels in leaves, and three (Eru02G004313, Eru03G006660, and Eru05G004075) exhibited the highest expression in roots under normal conditions (Figure 5F). These findings provide insights into the spatial dynamics of sucrose metabolism and transport in E. fulvus and offer preliminary information on the type of sugar transported by the EfESWEET proteins. This information may be useful for improving the potential of E. fulvus as a biofuel source through breeding or genetic engineering (Figure 5F).
In addition to soluble sugars, which contribute to Brix content, biomass is also proportionally related to the biofuel potential of a species. Cellulose is the most abundant biopolymer on the planet, and along with other non-cellulosic polysaccharides such as hemicellulose, xyloglucan, galactomannan, and heteromannan, it forms a significant portion of plant biomass. Cellulosic and non-cellulosic structural carbohydrates are synthesized by the cellulose synthase gene superfamily, which includes the CesA (cellulose synthase) gene family and the Csl (cellulose synthase-like) gene family with sub-clades CslA–CslH (Liu et al., 2022). Using protein sequences of the cellulose synthase superfamily from Arabidopsis and rice, a Hidden Markov Model (HMM) was created and used to search the E. fulvus proteome with an e-value threshold of ≤1e−10, resulting in 58 significant hits. These E. fulvus cellulose synthase superfamily proteins were resolved into putative CesA and Csl families based on their evolutionary relationships with homologs from Arabidopsis and rice. Fourteen E. fulvus proteins were identified as CesA members and 44 as Csl members (Figure 4 of Supplemental File 7). CesA proteins are involved in synthesizing cellulose from glucose monomers and are organized into the hexameric rosette complex known as the cellulose synthase complex. CesA proteins function in synthesis of the primary and secondary cell wall. Among the 10 CesA proteins of Arabidopsis, CESA1, CESA3, and CESA6 synthesize the primary cell wall, CESA4, CESA7, and CESA8 synthesize the secondary cell wall, and CESA2, CESA5, CESA9, and CESA10 show tissue-specific activity (Hill et al., 2014). On the basis of their evolutionary relationships with Arabidopsis CesA1, Eru04G008120 and Eru07G004699 may be involved in primary wall synthesis, whereas Eru03G002520, which is more closely related to Arabidopsis CesA8, may be involved in secondary wall synthesis. Further research is required to determine the specific functions of the 14 CesA proteins of E. fulvus identified here. Plant cell walls are complex and are composed of carbohydrates such as cellulose, hemicelluloses, and pectin, which exhibit structural and compositional differences among plants and even among different tissues of the same plant (Liepman and Cavalier., 2012). The nine EfCslA and five EfCslC family members exhibiting 1,4-β-glycan synthase activity may be involved in synthesis of E. fulvus mannan and xyloglucan backbones, respectively. In addition, EfCslA and EfCslC were found to be evolutionarily close, supporting previous findings of a common ancestor and shared structural and physicochemical features (Liepman and Cavalier., 2012). The five identified members of the EfCslD family may be involved in deposition of cellulose in both primary and secondary cell walls (Foreman and Dolan, 2001). All 12 EfCslF members exhibit (1→3; 1→4)-β-D-glucan activity and may be responsible for biosynthesis of (1,3; 1,4)-β-D-glucans, which are characteristic cell wall polysaccharides of grasses and commercially important cereals (Burton et al., 2006; Peng et al., 2019). Ten of the 12 EfCslF genes were located close together within a ∼0.37-Mb region of chromosome 3; eight were clustered into two tandem units of three and five genes each (Figure 5 of Supplemental File 7). Among the different Csl groups, CslA, CslC, and CslD are conserved in all land plants, whereas CslF and CslH are restricted to grasses. CslB and CslG were previously thought to be exclusive to dicots, but a recent study showed that monocots also contain the CslB subfamily (Kaur et al., 2017). Our analysis showed that the E. fulvus genome does not contain any members of the CslB family and has only one member of the CslG family; it contains seven CslH members specific to grasses and five CslE members. The activities of these cellulosic and non-cellulosic carbohydrate biosynthetic genes ensure the proper structure of E. fulvus cell walls, thereby contributing significantly to biomass accumulation.
In a comparative genomics study, we analyzed 303 QTLs of 10 biofuel-related traits from 23 different studies of S. bicolor (Table 3 of Supplemental File 7) and identified orthologous genes in E. fulvus for each trait. E. fulvus had 4920, 1269, 4119, 1304, 539, 2696, 141, 1567, 2940, and 2757 orthologous genes for Brix, cellulose content (CC), fresh biomass (FB), glucose content (GC), hemicellulose content (HC), juice yield (JY), lignin content (LC), sucrose content (SCC), sugar content (SGC), and total dry biomass (TBD), respectively (Supplemental File 9). In total, we found 11 334 SSR markers in 7506 biofuel-related E. fulvus genes. These markers may serve as a valuable resource for quickly screening trait-linked genes and can be used to support breeding programs focused on biofuel development in E. fulvus (Supplemental File 9).
Adaptive response of E. fulvus under cold stress
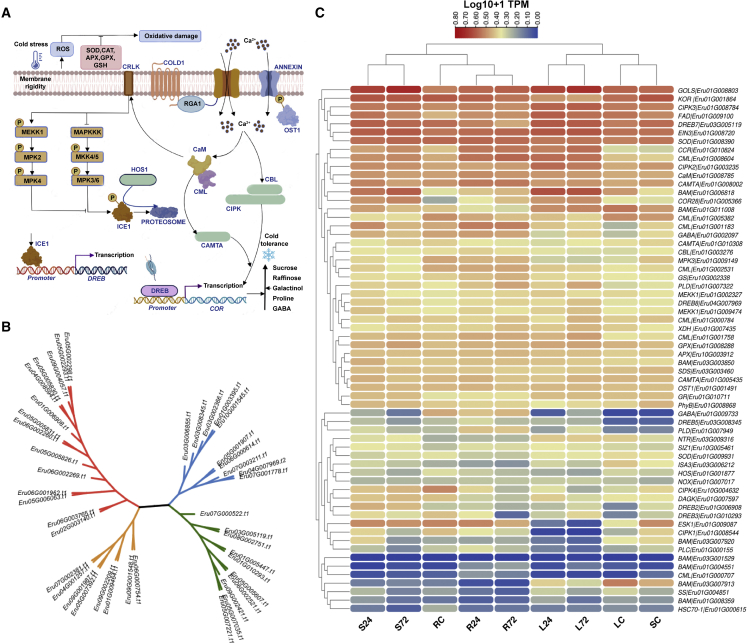
Cold stress affects various aspects of plant growth, including development, yield, and geographic distribution of a species. Plants have evolved intricate mechanisms that help them withstand the effects of cold stress, including the production of soluble sugars, proline, and cold-resistant proteins (Kaplan and Guy, 2004; Kaplan et al., 2007). These mechanisms preserve membrane stability, maintain osmotic potential, inhibit ice crystal formation, and neutralize reactive oxygen species (ROS) (Dong et al., 2009). E. fulvus exhibits broad tolerance to abiotic stresses including cold stress. Based on the expression results under cold stress, we proposed a putative cold tolerance model for E. fulvus (Figure 7A). Our analysis revealed that expression of diacylglycerol kinase (DAGK) was higher in leaves and stems at T1 compared with the control and increased further in stems alone at T2. In roots, DAGK expression decreased with declining temperatures. Expression of fatty acid desaturases (FADs) increased at T1 but began to decline at T2 in all tissues. The activity of DAGK and FADs leads to a reduction in membrane fluidity that is detected by Ca2+ channels, allowing for inflow of Ca2+ into the cytoplasm (Figure 7C). Other Ca2+ transporters such as mechanosensitive MID1-complementing activity 1 (MCA1) and annexin1 (ANN1) also changed in expression under cold stress. The influx of Ca2+ triggered by these transporter activities initiates a cold-regulated gene cascade (Ding et al., 2019). As a result, we observed increased expression of calmodulin (CaM) and several calmodulin-like proteins (CML) at T1 and T2 across all tissues. This calcium signal is converted to a phosphorylation/dephosphorylation signal (Yuan et al., 2018) through activation of Ca2+/CaM-regulated receptor-like kinase 1 (CRLK1), which phosphorylates and activates mitogen-activated protein kinase kinase kinase (MEKK1). Our findings indicate increased expression of MEKK1 at T1 in all tissues, which activates the MEKK1–MKK2–MPK4 pathway and positively regulates cold-responsive genes. Reduced expression of MPK3 suggests deactivation of the MEK4/5–MPK3/6 pathway, which is involved in compromising cold tolerance through degradation of inducer of CBF expression (ICE1) (Zhao et al., 2017).
Figure 7.
Adaptive response of E. fulvus under cold stress.
(A) Putative cold tolerance model of E. fulvus.
(B) Heatmap showing expression of important cold-regulated genes.
(C) Neighbor-joining phylogenetic tree of the functional domains of E. fulvus DREB proteins. L24, S24, and R24 represent the leaf, stem, and root under cold stress at 24 h, and L72, S72, and R72 represent the leaf, stem, and root under cold stress at 72 h.
Although the role of phytochrome B (phyB) in response to cold stress varies (He et al., 2016; Jiang et al., 2020), our analysis showed decreased expression of phyB at T1 and T2 compared with control conditions in all tissues. More research is required to determine the function of phyB in E. fulvus in response to cold stress.
The C-repeat/dehydration response element (CRT/DRE) is a cis-acting DNA element that is sensitive to stress conditions such as drought, cold, and high salt. Proteins that bind to this element are referred to as C-repeat binding factors (CBFs). CBFs bind to the CRT/DRE elements in the promoter regions of multiple COR genes, regulating their expression under various stress conditions, including cold (Gilmour et al., 1998). DREBs, a type of CBF, belong to the APETALA2/ethylene-responsive factor (AP2/ERF) transcription factor superfamily. A genome-wide survey of DREBs in E. fulvus revealed that 42 of 141 AP2/ERF domain-containing proteins were DREBs. These DREBs were dispersed across all chromosomes, with chromosome 5 having the highest number of DREBs (11), followed by chromosome 6 (six). Duplication analysis revealed that three DREB genes (Eru03G002366, Eru01G003395, and Eru10G001545) in E. fulvus had undergone duplication. Expression analysis showed that compared with the controls, the DREB gene Eru03G005119 had higher expression in leaves and stems at two time points (T1 and T2), whereas its expression in roots decreased at low temperatures (Figure 7C). Other DREB genes, such as Eru01G006908, Eru03G008345, and Eru04G007969, showed increased expression in all tissues at T1. These four DREB genes may be potential candidate genes for regulating cold tolerance in E. fulvus. Further research is necessary to confirm the role of the identified DREBs under different stress conditions. Under cold stress, the ICE1 protein binds to and activates the DREBs (Chinnusamy et al., 2003). Although no differential expression pattern was observed for ICE1, a decrease in the expression of high osmotically responsive 1 (HOS1), which stimulates ICE1 breakdown in the nucleus, and an increase in the expression of SIZ1 and OST1, which stabilize ICE1, were observed (Miura et al., 2007; Ding et al., 2015).
Under stress conditions, galactinol, raffinose, and stachyose act as osmoprotectants (Taji et al., 2002; Nishizawa et al., 2008). The gene for galactinol synthase (GolS) was found to have higher expression in all tissues during T1 and T2 compared with control conditions, suggesting that galactinol and/or raffinose may be the primary osmoprotective agents in E. fulvus during cold stress. In addition, expression of beta-amylase genes (Eru01G006818 and Eru03G003850) was enhanced in all tissues during T1 and T2. Glutamate decarboxylase (GAD), which is involved in GABA synthesis, was also upregulated under cold temperatures. Expression of these genes provides osmoprotection to E. fulvus during cold stress. Excessive accumulation of ROS under stress is detrimental to the cell, and ROS must be scavenged to maintain normal cellular function. NADPH oxidases, also known as respiratory burst oxidase homologs (Rbohs), are key players in plant ROS production. Our results showed enhanced expression of an NADPH oxidase family member in all tissues at T1 and T2. Previously, activity of NADPH oxidase was found to peak at 48 h of cold stress and decline thereafter (Zhang et al., 2018b). Our results, however, showed that its transcript abundance continued to increase even after 48 h. Expression of xanthine dehydrogenase (XDH), which performs dual functions of producing and scavenging ROS (Ma et al., 2016), was enhanced under low temperatures. Low temperatures also induced increased expression of antioxidant-related genes such as superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione synthase (GS) (only in leaves), glutathione reductase (GR) (except roots), glutathione peroxidase (GPX), and NADPH dependent thioredoxin reductase (NTR) to counter ROS in E. fulvus (Maxwell et al., 1999; Liu and Lin, 2014; Del Río, 2015; You and Chan, 2015).
Our study provides a high-quality, reference-grade chromosome assembly of E. fulvus, facilitating more in-depth genomic and genetic analysis of this species. This, in turn, has enabled us to resolve the phylogenetic ambiguity of the Saccharum complex, providing guidance for the selection of appropriate parents for sugarcane breeding programs. Our study sheds light on the evolution of the E. fulvus genome through examination of chromosome rearrangements of its ancestral chromosomes. Our other key findings include the spatial expression dynamics of sucrose metabolism, the characterization of EfSWEET proteins as mono- and disaccharide transporters, and the functional dynamics of other sugar transporters. We have identified members of the cellulose synthase gene superfamily involved in the synthesis of cellulosic and non-cellulosic polysaccharides, as well as putative orthologous genes involved in 10 biofuel-related traits. Taken together, these findings will be valuable for targeting specific genes to increase the biofuel potential of E. fulvus. Finally, our putative cold stress model of E. fulvus will contribute to a deeper understanding of its adaptive response.
Methods
Sequencing and genome assembly
E. fulvus plant material was obtained from the Sugarcane Resource Nursery of Yunnan Agricultural University, National Crop Germplasm Resources Platform (Sugarcane), and the National Sugarcane Germplasm Resources Nursery, China. The genome size of E. fulvus was estimated using flow cytometry with the FACSCalibur system (BD Biosciences, San Jose, CA) and internal standards of Zea mays L. B73 (2.3 Gb) and Oryza sativa L. var. Nipponbare (0.43 Gb). DNA was extracted from leaf samples of E. fulvus using a modified CTAB method (Huang et al., 2000), and the quality and quantity of the isolated DNA were assessed by gel electrophoresis and a NanoDrop 2000 spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA). High-quality DNA was used to generate paired-end libraries with insert sizes of 350 bp, 550 bp, 600 bp, and 700 bp for Illumina HiSeq 2000 sequencing (Illumina, San Diego, CA, USA), as well as 20-kb insert-size SMRT libraries for PacBio Sequel sequencing (Pacific Biosciences, Menlo Park, CA, USA). Chromosome conformation capture (Hi-C) libraries were constructed from young leaves cross-linked in formaldehyde. The fixed plant tissue was homogenized into a fine powder in liquid nitrogen, then suspended in extraction buffer for isolation of nuclei. Chromatin from the isolated nuclei was solubilized in SDS, quenched with 1% Triton X-100, and digested with the restriction enzyme DpnII. DNA fragments were labeled with biotin, and their blunt ends were ligated with adaptors (Belton et al., 2012). The Hi-C libraries were sequenced on the Illumina HiSeq X Ten platform at Novogene.
A de novo assembly was generated from PacBio long reads using NextDenovo v2.1-beta.0 (https://github.com/Nextomics/NextDenovo); the process involved self-correction of PacBio raw reads using the NextCorrect module, identification of overlapping regions using Minimap2 (Li, 2018), and construction of a string graph using the NextGraph module. A draft genome assembly was generated after two cycles of polishing with NextPolish v1.0.5 (Hu et al., 2020) using high-quality Illumina short reads. The read pairs generated from Hi-C were then mapped to the draft genome assembly using Juicer v1.6.2 (Durand et al., 2016). Read pairs with valid Hi-C contacts were used as input to the 3D de novo assembly (3D-DNA) pipeline (Dudchenko et al., 2017), followed by assembly of 3D-DNA into pseudomolecules. Finally, the chromosome-scale assembly was refined by correcting mis-joins using Juicebox v1.11.08 (https://github.com/aidenlab/Juicebox). The quality of the assembly was evaluated by mapping paired-end libraries with short insert sizes to the genome assembly using BWA-MEM v0.7.12 (Li and Durbin, 2009). Unigenes assembled with Trinity v2.9.0 (Grabherr et al., 2011) were also mapped to the genome assembly using BLAT (Kent, 2002). Completeness of the assembly was evaluated using BUSCO v4.0.5 (Simão et al., 2015) with the embryophyte lineage as a reference. The LAI was used to evaluate the completeness of repetitive regions of the assembly.
Structural and functional annotation
We used ab initio and evidence-based methods to identify repetitive regions in the E. fulvus genome. We constructed a non-redundant ab initio repeat library using LTR_Finder v1.0.7 (Xu and Wang, 2007) and LTRharvest v1.0 (Ellinghaus et al., 2008) to detect LTR retrotransposons (LTR-RTs). The LTRs were classified using LTR_retriever v2.8 (Ou and Jiang, 2018). We also generated a de novo repeat library using RepeatModeler v2.0 (Price et al., 2005) and used Repeat Masker v4.0.9 (Chen, 2004) to predict ab initio repetitive elements based on the de novo library. Repeat regions were annotated using Repbase v24.06 (Bao et al., 2015) with the aid of RepeatMasker and RepeatProteinMask v4.0.9. Tandem repeats were identified in the assembly using Tandem Repeat Finder (TRF) v4.09 (Benson, 1999). TEs were extracted from the tandem repeats and sorted based on lineage using TEsorter v1.2.1 (Zhang et al., 2022) and HMMs from protein domain databases such as PDD, REXdb (Neumann et al., 2019), and GyDB (Lloréns et al., 2007). We then performed phylogenetic analysis of retrotransposons (RTs) from each classified family based on RT protein domain sequences. Multiple sequence alignment was performed using MAFFT v7.429 (Katoh et al., 2002), and the phylogenetic tree was constructed using FastTree v2.1.11 (Price et al., 2009). We estimated the divergence times between RTs of specific families using PATHd8 (Britton et al., 2007).
Gene models in the E. fulvus genome were predicted using a combination of three methods: de novo, homology-based, and transcriptome-based prediction. The de novo prediction was performed using GeneID v1.4.4 (Parra et al., 2000), GlimmerHMM (Majoros et al., 2004), SNAP (Korf, 2004), and GenScan (Burge and Karlin, 1997) with default parameters. Protein sequences from O. sativa, A. thaliana, B. distachyon, Sesamum indicum, Z. mays, and S. bicolor were obtained from the NCBI database for homology-based prediction, which was performed by aligning these sequences against the E. fulvus genome assembly using TBLASTN v2.2.29+ (Gertz et al., 2006) with a cutoff E-value of 1e−5, followed by assessment with GeneWise v2.4.1 (Birney et al., 2004). The transcriptome-based gene models were generated by assembling RNA sequencing (RNA-seq) reads with Trinity and aligning the transcripts against the genome with PASA v2.4.1 (https://github.com/PASApipeline/). These models were used to train AUGUSTUS v3.2.2 (Stanke et al., 2006), which was used to predict the structure of protein-coding genes. The final gene models were obtained by combining predictions from all methods into a weighted consensus gene structure using EVidenceModeler v1.1.1 (Haas et al., 2008). The final gene models were updated with PASA to include information on untranslated regions and alternative splicing sites.
The predicted genes were functionally annotated using BLASTP (version 2.2.29+) against the SwissProt, TrEMBL, KEGG, and InterPro databases with a cutoff E-value of 1 × 10−5. GO terms for each gene were obtained from the InterPro entries. tRNAscan-SE version 2.0.2 (Lowe and Eddy, 1997) was used to predict and annotate tRNAs, and BLAST version 2.2.29+ with a cutoff E-value of 1 × 10−10 was used to search for homologous rRNA sequences to predict rRNAs. Covariance models from the Rfam database (Kalvari et al., 2018) and INFERNAL version 1.1.2 (Nawrocki et al., 2009) were used to predict miRNAs and snRNAs, respectively.
Genome evolution
The protein sequences of 10 angiosperms (A. thaliana, A. tauschii, B. distachyon, O. sativa, P. hallii, P. miliaceum, S. italica, S. bicolor, Z. mays, and S. spontaneum) were used to infer evolutionary relationships of E. fulvus. OrthoMCL v2.0.9 (Li et al., 2003) was used to identify orthologous genes among these species. Only single-copy orthologous groups were used for phylogenetic analysis; they were aligned with MUSCLE v3.8.31 (Edgar, 2004) and used to construct a phylogenetic tree using MrBayes v3.1.2 (Ronquist and Huelsenbeck, 2003). Sequence divergence times were evaluated using r8s v1.2 (Sanderson, 2003) and confirmed with MCMCTree from the PAML package v4.6 (Yang, 2007). The JC69 nucleotide substitution model and a Markov Chain Monte Carlo (MCMC) burn-in of 10 000 iterations for 100 000 generations were used during the MCMCTree run. The divergence times were calibrated using the TimeTree database (Hedges et al., 2006). We used CAFE v4.0 to detect gene families that had undergone expansion or contraction in the E. fulvus genome (De Bie et al., 2006). GO term enrichment analysis of the significantly contracted and expanded gene families was performed using Ontologizer (http://ontologizer.de/).
SweeD v3.3.2 (Pavlidis et al., 2013) was used to evaluate genome-wide selective signals in E. fulvus through the CLR test, with the grid number set to chromosome length/1000. The top 0.5% CLR values were selected as selective sweeps in the E. fulvus genome. Genome-wide syntenic and collinear blocks were identified among E. fulvus, S. spontaneum, S. italica, S. bicolor, Z. mays, O. sativa, and B. distachyon. Paralogous and orthologous gene pairs between E. fulvus and other species were identified using BLASTP (v2.2.29+) and MCScanX (Wang et al., 2012). MCScanX was used to detect potentially collinear blocks, and syntenic relationships were visualized using the MCscan pipeline (Tang et al., 2008) in the JCVI utility libraries (https://github.com/tanghaibao/jcvi). Paralogous and orthologous gene pairs were aligned with ParaAT (Zhang et al., 2012), and the values of synonymous substitutions per synonymous site (Ks) were estimated using KaKs_Calculator (Wang et al., 2010a, 2010b). To assess the chromosome rearrangements in E. fulvus, the syntenic relationship between pre-γ AGK and E. fulvus was detected using the approach of Murat et al. (2017), and the relative contribution of AGK genes in each E. fulvus chromosome was assessed to determine the rearrangements of the seven ancestral chromosomes that generated the E. fulvus genome during evolution.
Phylogenetic reconstruction and population structure of the Saccharum complex
Whole-genome resequencing data were generated for 202 accessions (Table 7 of Supplemental File 2) of Saccharum complex species and mapped to the V. vinifera reference genome (Ensemble Plants Release-31) (Kersey et al., 2016) using BWA (Li and Durbin, 2009). Variant calling was performed using GATK HaplotypeCaller (Poplin et al., 2017), and joint genotyping was performed across all 202 accessions (Brouard et al., 2019). Hard filtering of the raw variants was carried out with the GATK pipeline, and SNPs with MAF ≤0.05 were filtered to create a core SNP set for phylogenetic analysis. An ML phylogenetic tree was constructed using the core SNP set. The population structure across all 202 accessions was analyzed using PCA of the core SNP data with genome-wide complex trait analysis (GCTA, v1.25.360) and ADMIXTURE v1.361 (Alexander et al., 2009). The number of genetic clusters (K) was predefined between two and nine, and a cross-validation error procedure was performed to determine the best K value and the actual number of genetic stocks in the data.
In addition to the genome-wide SNP-based phylogeny, an ML phylogeny was constructed using genome-wide orthology. Coding sequences of Saccharum complex species with reference genomes available in public databases were retrieved, and paired-end RNA-seq reads were used for species with no reference genomes (Tables 1 and 2 of Supplemental File 6). De novo assemblies of the latter were constructed using Trinity v1.6 (Grabherr et al., 2011). The quality of the assembled transcripts was assessed through read representation using Bowtie 2 (Langmead and Salzberg, 2012) and BUSCO analysis of assembly completeness (Simão et al., 2015). CD-HIT-EST (Li and Godzik, 2006) was used to remove redundant sequences, and TRAPID (Van Bel et al., 2013) was used to identify full-length transcripts with the Plaza 4.5 monocots database (Van Bel et al., 2018) as a reference. The CDSs and ORFs of full-length transcripts were used to infer orthology using Proteinortho (Lechner et al., 2011). MAFFT (Katoh et al., 2013) was used to align single-copy orthologs, AMAS was used for concatenation, and TrimAl (Capella-Gutiérrez et al., 2009) was used to remove spurious sequences and poorly aligned regions. Finally, an ML-based phylogenetic tree was constructed using IQ-TREE and edited using iTOL (Letunic and Bork, 2019).
RNA-seq-based expression analysis
RNA was extracted from leaves, stems, and roots of E. fulvus grown at a normal growth temperature of 28°C (control), 4°C for 24 h (T1), and 4°C for 72 h (T2). Three replicates were used for each tissue under each condition, resulting in a total of 27 paired-end sequencing libraries, which were sequenced on the Illumina HiSeq platform. Trimmomatic (Bolger et al., 2014) was used to remove low-quality reads, and cleaned reads were aligned to the E. fulvus genome using STAR (Dobin et al., 2013). Read count estimation was performed using FeatureCounts (Liao et al., 2014) to generate count data for each gene, and low-count genes were filtered using CPM <1 in at least 25% of the samples. DEBrowser (Kucukural et al., 2019) was used to perform TMM-based normalization of the count data, and differentially expressed genes were identified with edgeR (Robinson et al., 2010) using a fold-change threshold of 2 and an FDR-adjusted p-value cutoff of 0.05. ShinyGO was used to analyze the GO enrichment of the differentially expressed genes (Ge et al., 2020).
Funding
This work was supported by grants from the Major Science and Technology Projects in Yunnan Province (202202AE090021), a special project of Yunnan Key Laboratory of Crop Production and Smart Agriculture (202105AG070007), a sub-project of the National Key Research and Development Program of China (2018YFD1000503), the National Natural Science Foundation of China (31960451, 31560417), a Key Project of Applied Basic Research Program of Yunnan Province (2015FA024), and the ESI Discipline Promotion Program of Yunnan Agricultural University (2019YNAUESIMS01).
Author contributions
Y.D., L.K., and F.L. designed the study. X.W., L.L., R.X., S.G., Q.Y., S.C., H.L., Y.M., L.X., F.X., and Q.S. performed the experiments. L.K., A.M., J.C., Yi.D., X.L., Z.Q., Y.J., G.W., A.S., K.G., Y.P., and T.H. conducted data analyses. L.K., A.M., L.H., and Y.D. wrote the manuscript. A.S., A.M., K.G., X.W., and J.C. edited the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We thank all the individuals who have helped us in this study. No conflict of interest declared.
Published: February 21, 2023
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Supplemental information is available at Plant Communications Online.
Contributor Information
Yang Dong, Email: loyalyang@163.com.
Fusheng Li, Email: lfs810@sina.com.
Accession numbers
The raw sequencing reads, genome assembly, and resequencing data can be accessed from the Erianthus fulvus genome database, EfGD (https://efgenome.ynau.edu.cn/). The data can also be accessed from NCBI using the BioProject ID PRJNA854329.
Supplemental information
OrthoMCL was used to identify orthologous gene groups among the species.
(A) Manhattan plot depicting the genome-wide selective sweep regions in E. fulvus. (B) GO enrichment of genes associated with the selective sweep regions. (C) Manhattan plot depicting the genome-wide nucleotide diversity differences (π ratio) in E. fulvus accessions from Xizang and Yunnan provinces.
References
- Alexander D.H., Novembre J., Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalraj V., Balasundaram N.J. On the taxonomy of the members of ‘Saccharum Complex’. Genet. Resour. Crop. Evol. 2006;53:35–41. [Google Scholar]
- Bao W., Kojima K.K., Kohany O. Repbase Update, a database of repetitive elements in eukaryotic genomes. Mob. DNA. 2015;6:11. doi: 10.1186/s13100-015-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belton J.-M., McCord R.P., Gibcus J.H., Naumova N., Zhan Y., Dekker J. Hi–C: a comprehensive technique to capture the conformation of genomes. Methods. 2012;58:268–276. doi: 10.1016/j.ymeth.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 1999;27:573–580. doi: 10.1093/nar/27.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse P., Taylor G., Carroll B., Berding N., Burner D., McIntyre C.L. Assessing genetic diversity in a sugarcane germplasm collection using an automated AFLP analysis. Genetica. 1998;104:143–153. doi: 10.1023/A:1003436403678. [DOI] [PubMed] [Google Scholar]
- Birney E., Clamp M., Durbin R. GeneWise and genomewise. Genome Res. 2004;14:988–995. doi: 10.1101/gr.1865504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer G. The cyctology of the sugarcane. Genetica. 1924;6:497–525. doi: 10.1007/BF01501148. [DOI] [Google Scholar]
- Britton T., Anderson C.L., Jacquet D., Lundqvist S., Bremer K. Estimating divergence times in large phylogenetic trees. Syst. Biol. 2007;56:741–752. doi: 10.1080/10635150701613783. [DOI] [PubMed] [Google Scholar]
- Brouard J.-S., Schenkel F., Marete A., Bissonnette N. The GATK joint genotyping workflow is appropriate for calling variants in RNA-seq experiments. J. Anim. Sci. Biotechnol. 2019;10:44. doi: 10.1186/s40104-019-0359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge C., Karlin S. Prediction of complete gene structures in human genomic DNA. J. Mol. Biol. 1997;268:78–94. doi: 10.1006/jmbi.1997.0951. [DOI] [PubMed] [Google Scholar]
- Burton R.A., Wilson S.M., Hrmova M., Harvey A.J., Shirley N.J., Medhurst A., Stone B.A., Newbigin E.J., Bacic A., Fincher G.B. Cellulose synthase-like CslF genes mediate the synthesis of cell wall (1, 3; 1, 4)-ß-D-glucans. Science. 2006;311:1940–1942. doi: 10.1126/science.1122975. [DOI] [PubMed] [Google Scholar]
- Cai Q., Aitken K., Fan Y., Piperidis G., Jackson P., McIntyre C. A preliminary assessment of the genetic relationship between Erianthus rockii and the “Saccharum complex” using microsatellite (SSR) and AFLP markers. Plant Sci. 2005;169:976–984. [Google Scholar]
- Capella-Gutiérrez S., Silla-Martínez J.M., Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N. Using RepeatMasker to identify repetitive elements in genomic sequence s. Curr. Protoc. Bioinformatics. 2004;Chapter 4 doi: 10.1002/0471250953.bi0410s05. Unit 4.10. [DOI] [PubMed] [Google Scholar]
- Chinnusamy V., Ohta M., Kanrar S., Lee B.-h., Hong X., Agarwal M., Zhu J.K. ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003;17:1043–1054. doi: 10.1101/gad.1077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton W.D., Renvoize S.A. Her Majesty's Stationery Office; 1986. Genera Graminum: Grasses of the World.[J]. H.M.S.O. [Google Scholar]
- Daniels J., Roach B.T. Developments in Crop Science. Elsevier; 1987. Taxonomy and evolution; pp. 7–84. [Google Scholar]
- Daniels T.E., Silverman S., Jr., Michalski J.P., Greenspan J.S., Sylvester R.A., Talal N. The oral component of Sjögren's syndrome. Oral Surg. Oral Med. Oral Pathol. 1975;39:875–885. doi: 10.1016/0030-4220(75)90108-5. [DOI] [PubMed] [Google Scholar]
- De Bie T., Cristianini N., Demuth J.P., Hahn M.W. CAFE: a computational tool for the study of gene family evolution. Bioinformatics. 2006;22:1269–1271. doi: 10.1093/bioinformatics/btl097. [DOI] [PubMed] [Google Scholar]
- Del Río L.A. ROS and RNS in plant physiology: an overview. J. Exp. Bot. 2015;66:2827–2837. doi: 10.1093/jxb/erv099. [DOI] [PubMed] [Google Scholar]
- Ding Y., Shi Y., Yang S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019;222:1690–1704. doi: 10.1111/nph.15696. [DOI] [PubMed] [Google Scholar]
- Ding Y., Li H., Zhang X., Xie Q., Gong Z., Yang S. OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in Arabidopsis. Dev. Cell. 2015;32:278–289. doi: 10.1016/j.devcel.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C.-H., Zolman B.K., Bartel B., Lee B.-h., Stevenson B., Agarwal M., Zhu J.K. Disruption of Arabidopsis CHY1 reveals an important role of metabolic status in plant cold stress signaling. Mol. Plant. 2009;2:59–72. doi: 10.1093/mp/ssn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko O., Batra S.S., Omer A.D., Nyquist S.K., Hoeger M., Durand N.C., Shamim M.S., Machol I., Lander E.S., Aiden A.P., Aiden E.L. De novo assembly of the Aedes aegypti genome using Hi-C yields chromosome-length scaffolds. Science. 2017;356:92–95. doi: 10.1126/science.aal3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand N.C., Robinson J.T., Shamim M.S., Machol I., Mesirov J.P., Lander E.S., Aiden E.L. Juicebox provides a visualization system for Hi-C contact maps with unlimited zoom. Cell Syst. 2016;3:99–101. doi: 10.1016/j.cels.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmé S.J., Glynn N.G., Comstock J.C. Genetic segregation of microsatellite markers in Saccharum officinarum and S. spontaneum. Heredity. 2006;97:366–375. doi: 10.1038/sj.hdy.6800888. [DOI] [PubMed] [Google Scholar]
- Ellinghaus D., Kurtz S., Willhoeft U. LTRharvest, an efficient and flexible software for de novo detection o f LTR retrotransposons. BMC Bioinformatics. 2008;9:18. doi: 10.1186/1471-2105-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estep M.C., McKain M.R., Vela Diaz D., Zhong J., Hodge J.G., Hodkinson T.R., Layton D.J., Malcomber S.T., Pasquet R., Kellogg E.A. Allopolyploidy, diversification, and the Miocene grassland expansion. Proc. Natl. Acad. Sci. USA. 2014;111:15149–15154. doi: 10.1073/pnas.1404177111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D.L., Joshi S.V. Complete chloroplast genomes of Saccharum spontaneum, Saccharum officinarum and Miscanthus floridulus (Panicoideae: Andropogoneae) reveal the plastid view on sugarcane origins. Syst. Biodivers. 2016;14:548–571. [Google Scholar]
- Evans D.L., Joshi S.V. On the validity of the saccharum complex and the saccharinae subtribe: a re-assesment. BioRxiv. 2020 doi: 10.1101/2020.07.29.226753. Preprint at. [DOI] [Google Scholar]
- Foreman J., Dolan L. Root hairs as a model system for studying plant cell growth. Ann. Bot. 2001;88:1–7. [Google Scholar]
- Feng L., Frommer W.B. Structure and function of SemiSWEET and SWEET sugar transporters. Trends Biochem. Sci. 2015;40:480–486. doi: 10.1016/j.tibs.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Fukuhara S., Terajima Y., Irei S., Sakaigaichi T., Ujihara K., Sugimoto A., Matsuoka M. Identification and characterization of intergeneric hybrid of commercial sugarcane (Saccharum spp. hybrid) and Erianthus arundinaceus (Retz.) Jeswiet. Euphytica. 2013;189:321–327. [Google Scholar]
- Ge S.X., Jung D., Yao R. ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics. 2020;36:2628–2629. doi: 10.1093/bioinformatics/btz931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz E.M., Yu Y.-K., Agarwala R., Schäffer A.A., Altschul S.F. Composition-based statistics and translated nucleotide searches: improving the TBLASTN module of BLAST. BMC Biol. 2006;4:41. doi: 10.1186/1741-7007-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour S.J., Zarka D.G., Stockinger E.J., Salazar M.P., Houghton J.M., Thomashow M.F. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 1998;16:433–442. doi: 10.1046/j.1365-313x.1998.00310.x. [DOI] [PubMed] [Google Scholar]
- Grabherr M.G., Haas B.J., Yassour M., Levin J.Z., Thompson D.A., Amit I., Adiconis X., Fan L., Raychowdhury R., Zeng Q., et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandbastien M. Activation of plant retrotransposons under stress conditions. Trends Plant Sci. 1998;3:181–187. doi: 10.1016/S1360-1385(98)01232-1. [DOI] [Google Scholar]
- Grivet L., Daniels C., Glaszmann J.-C., D'Hont A.J. A review of recent molecular genetics evidence for sugarcane evolution and domestication. Ethnobot. Res. Appl. 2004;2 009-017. [Google Scholar]
- Haas B.J., Salzberg S.L., Zhu W., Pertea M., Allen J.E., Orvis J., White O., Buell C.R., Wortman J.R. Automated eukaryotic gene structure annotation using EVidenceModeler a nd the Program to Assemble Spliced Alignments. Genome Biol. 2008;9:R7. doi: 10.1186/gb-2008-9-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Zhu Y., Liu M., Zhou Y., Lu G., Lan L., Wang X., Zhao Y., Zhang X.C. Molecular mechanism of substrate recognition and transport by the AtSWEET13 sugar transporter. Proc. Natl. Acad. Sci. USA. 2017;114:10089–10094. doi: 10.1073/pnas.1709241114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T., Morita S. Energy crops for sustainable bioethanol production; which, where and how? Plant Prod. Sci. 2010;13:221–234. [Google Scholar]
- Hattori T., Shiotsu F., Doi T., Morita S. Suppression of tillering in Erianthus ravennae (L.) Beauv. due to drought stress at establishment. Plant Prod. Sci. 2010;13:252–255. [Google Scholar]
- He Y., Li Y., Cui L., Xie L., Zheng C., Zhou G., Zhou J., Xie X. Phytochrome B negatively affects cold tolerance by regulating OsDREB1 gene expression through phytochrome interacting factor-like protein OsPIL16 in rice. Front. Plant Sci. 2016;7:1963. doi: 10.3389/fpls.2016.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R., Sauer N., Neuhaus H.E. Sugar transport across the plant vacuolar membrane: nature and regulation of carrier proteins. Curr. Opin. Plant Biol. 2015;25:63–70. doi: 10.1016/j.pbi.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Hedges S.B., Dudley J., Kumar S. TimeTree: a public knowledge-base of divergence times among organisms. Bioinformatics. 2006;22:2971–2972. doi: 10.1093/bioinformatics/btl505. [DOI] [PubMed] [Google Scholar]
- Hill J.L., Jr., Hammudi M.B., Tien M. The Arabidopsis cellulose synthase complex: a proposed hexamer of CESA trimers in an equimolar stoichiometry. Plant Cell. 2014;26:4834–4842. doi: 10.1105/tpc.114.131193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodkinson T.R., Chase M.W., Lledó M.D., Salamin N., Renvoize S.A. Phylogenetics of Miscanthus, Saccharum and related genera (Saccharinae, Andropogoneae, Poaceae) based on DNA sequences from ITS nuclear ribosomal DNA and plastid trnL intron and trnL-F intergenic spacers. J. Plant Res. 2002;115:381–392. doi: 10.1007/s10265-002-0049-3. [DOI] [PubMed] [Google Scholar]
- Hu J., Fan J., Sun Z., Liu S. NextPolish: a fast and efficient genome polishing tool for long-read a ssembly. Bioinformatics. 2020;36:2253–2255. doi: 10.1093/bioinformatics/btz891. [DOI] [PubMed] [Google Scholar]
- Huang J., Ge X., Sun M. Modified CTAB protocol using a silica matrix for isolation of plant genomic DNA. Biotechniques. 2000;28:432–434. doi: 10.2144/00283bm08. [DOI] [PubMed] [Google Scholar]
- Janaki-Ammal E.K. Intergeneric hybrids ofSaccharum. J. Genetics. 2008;41:217–253. [Google Scholar]
- Jiang B., Shi Y., Peng Y., Jia Y., Yan Y., Dong X., Li H., Dong J., Li J., Gong Z., et al. Cold-induced CBF–PIF3 interaction enhances freezing tolerance by stabilizing the phyB thermosensor in Arabidopsis. Mol. Plant. 2020;13:894–906. doi: 10.1016/j.molp.2020.04.006. [DOI] [PubMed] [Google Scholar]
- Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Žídek A., Potapenko A., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalvari I., Nawrocki E.P., Argasinska J., Quinones-Olvera N., Finn R.D., Bateman A., Petrov A.I. Non-coding RNA analysis using the Rfam database. Curr. Protoc. Bioinformatics. 2018;62:e51. doi: 10.1002/cpbi.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan F., Guy C.L. β-Amylase induction and the protective role of maltose during temperature shock. Plant Physiol. 2004;135:1674–1684. doi: 10.1104/pp.104.040808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan F., Kopka J., Sung D.Y., Zhao W., Popp M., Porat R., Guy C.L. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 2007;50:967–981. doi: 10.1111/j.1365-313X.2007.03100.x. [DOI] [PubMed] [Google Scholar]
- Katoh K., Misawa K., Kuma K.i., Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S., Dhugga K.S., Beech R., Singh J. Genome-wide analysis of the cellulose synthase-like (Csl) gene family in bread wheat (Triticum aestivum L.) BMC Plant Biol. 2017;17:193. doi: 10.1186/s12870-017-1142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley L.A., Mezulis S., Yates C.M., Wass M.N., Sternberg M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent W.J. BLAT—the BLAST-like alignment tool. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersey P.J., Allen J.E., Armean I., Boddu S., Bolt B.J., Carvalho-Silva D., Christensen M., Davis P., Falin L.J., Grabmueller C., et al. Ensembl Genomes 2016: more genomes, more complexity. Nucleic Acids Res. 2016;44:D574–D580. doi: 10.1093/nar/gkv1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf I. Gene finding in novel genomes. BMC Bioinf. 2004;5:59. doi: 10.1186/1471-2105-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaijeveld K. Genome size and species diversification. Evol. Biol. 2010;37:227–233. doi: 10.1007/s11692-010-9093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukural A., Yukselen O., Ozata D.M., Moore M.J., Garber M. DEBrowser: interactive differential expression analysis and visualization tool for count data. BMC Genom. 2019;20:1–12. doi: 10.1186/s12864-018-5362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasko P.F., Brandriss M.C. Proline transport in Saccharomyces cerevisiae. J. Bacteriol. 1981;148:241–247. doi: 10.1128/jb.148.1.241-247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner M., Findeiss S., Steiner L., Marz M., Stadler P.F., Prohaska S.J. Proteinortho: detection of (co-) orthologs in large-scale analysis. BMC Bioinf. 2011;12:1–9. doi: 10.1186/1471-2105-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I., Bork P. Interactive Tree of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 2019;47:W256–W259. doi: 10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- Li L., Stoeckert C.J., Jr., Roos D.S. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13:2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34:3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y., Smyth G.K., Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- Liepman A.H., Cavalier D.M. The cellulose synthase-like A and cellulose synthase-like C families: recent advances and future perspectives. Front. Plant Sci. 2012;3:109. doi: 10.3389/fpls.2012.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisch D. How important are transposons for plant evolution? Nat. Rev. Genet. 2013;14:49–61. doi: 10.1038/nrg3374. [DOI] [PubMed] [Google Scholar]
- Liu X., Zhang H., Zhang W., Xu W., Li S., Chen X., Chen H. Genome-wide bioinformatics analysis of Cellulose Synthase gene family in common bean (Phaseolus vulgaris L.) and the expression in the pod development. BMC Genom. Data. 2022;23:1–15. doi: 10.1186/s12863-022-01026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Lin Z. Reactive oxygen species and relative enzyme activities in the development of aerial roots of Chinese Banyan (Ficus microcarpa) J. Plant Growth Regul. 2014;33:160–168. [Google Scholar]
- Lloréns C., Futami R., Bezemer D., Moya A. The Gypsy Database (GyDB) of mobile genetic elements. Nucleic Acids Res. 2007;36:D38–D46. doi: 10.1093/nar/gkm697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe T.M., Eddy S.R. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Wang W., Bittner F., Schmidt N., Berkey R., Zhang L., King H., Zhang Y., Feng J., Wen Y., et al. Dual and opposing roles of xanthine dehydrogenase in defense-associated reactive oxygen species metabolism in Arabidopsis. Plant Cell. 2016;28:1108–1126. doi: 10.1105/tpc.15.00880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoros W.H., Pertea M., Salzberg S.L. TigrScan and GlimmerHMM: two open source ab initio eukaryotic gene-fin ders. Bioinformatics. 2004;20:2878–2879. doi: 10.1093/bioinformatics/bth315. [DOI] [PubMed] [Google Scholar]
- Matsuo K. Ecophysiological characteristics of Erianthus spp. and yielding abilities of three forages under conditions of cattle feces application. JIRCAS Working Rep. 2002;30:187–194. [Google Scholar]
- Maxwell D.P., Wang Y., McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc. Natl. Acad. Sci. USA. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock B. The significance of responses of the genome to challenge. Science. 1984;226:792–801. doi: 10.1126/science.15739260. [DOI] [PubMed] [Google Scholar]
- Miao J., Feng Q., Li Y., Zhao Q., Zhou C., Lu H., Fan D., Yan J., Lu Y., Tian Q., et al. Chromosome-scale assembly and analysis of biomass crop Miscanthus lutarioriparius genome. Nat. Commun. 2021;12:2458–2513. doi: 10.1038/s41467-021-22738-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K., Jin J.B., Lee J., Yoo C.Y., Stirm V., Miura T., Ashworth E.N., Bressan R.A., Yun D.-J., Hasegawa P.M. SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis. Plant Cell. 2007;19:1403–1414. doi: 10.1105/tpc.106.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S.K. Origin and distribution of saccharum. Botanical Gazette. 1957;119:55–61. [Google Scholar]
- Murat F., Armero A., Pont C., Klopp C., Salse J. Reconstructing the genome of the most recent common ancestor of flower ing plants. Nat. Genet. 2017;49:490–496. doi: 10.1038/ng.3813. [DOI] [PubMed] [Google Scholar]
- Nair N.V., Selvi A., Sreenivasan T.V., Pushpalatha K.N., Mary S. Molecular diversity amongSaccharum, Erianthus, Sorghum, Zea and their hybrids. Sugar Tech. 2005;7:55–59. [Google Scholar]
- Nair N.V., Mohanraj K., Sunadaravelpandian K., Suganya A., Selvi A., Appunu C. Characterization of an intergeneric hybrid of Erianthus procerus× Saccharum officinarum and its backcross progenies. Euphytica. 2017;213:267. [Google Scholar]
- Nascimento L.C., Yanagui K., Jose J., Camargo E.L.O., Grassi M.C.B., Cunha C.P., Bressiani J.A., Carvalho G.M.A., Carvalho C.R., Prado P.F., et al. Unraveling the complex genome of Saccharum spontaneum using Polyploid gene assembler. DNA Res. 2019;26:205–216. doi: 10.1093/dnares/dsz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann P., Novák P., Hoštáková N., Macas J. Systematic survey of plant LTR-retrotransposons elucidates phylogenetic relationships of their polyprotein domains and provides a reference for element classification. Mob. DNA. 2019;10:1–17. doi: 10.1186/s13100-018-0144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa A., Yabuta Y., Shigeoka S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008;147:1251–1263. doi: 10.1104/pp.108.122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrocki E.P., Kolbe D.L., Eddy S.R. Infernal 1.0: inference of RNA alignments. Bioinformatics. 2009;25:1335–1337. doi: 10.1093/bioinformatics/btp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou S., Jiang N. LTR_retriever: a highly accurate and sensitive program for identification of long terminal repeat retrotransposons. Plant Physiol. 2018;176:1410–1422. doi: 10.1104/pp.17.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra G., Blanco E., Guigó R. GeneID in Drosophila. Genome Res. 2000;10:511–515. doi: 10.1101/gr.10.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis P., Živkovic D., Stamatakis A., Alachiotis N. SweeD: likelihood-based detection of selective sweeps in thousands of genomes. Mol. Biol. Evol. 2013;30:2224–2234. doi: 10.1093/molbev/mst112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachakkil B., Terajima Y., Ohmido N., Ebina M., Irei S., Hayashi H., Takagi H. Cytogenetic and agronomic characterization of intergeneric hybrids between Saccharum spp. hybrid and Erianthus arundinaceus. Sci. Rep. 2019;9:1748. doi: 10.1038/s41598-018-38316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Pang H., Abbas M., Yan X., Dai X., Li Y., Li Q. Characterization of Cellulose synthase-like D (CSLD) family revealed the involvement of PtrCslD5 in root hair formation in Populus trichocarpa. Sci. Rep. 2019;9:1452–1459. doi: 10.1038/s41598-018-36529-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poplin R., Ruano-Rubio V., DePristo M.A., Fennell T.J., Carneiro M.O., Van der Auwera G.A., Kling D.E., Gauthier L.D., Levy-Moonshine A., Roazen D., et al. Scaling accurate genetic variant discovery to tens of thousands of samples. bioRxiv. 2017 doi: 10.1101/201178. Preprint at. [DOI] [Google Scholar]
- Price A.L., Jones N.C., Pevzner P.A. De novo identification of repeat families in large genomes. Bioinformatics. 2005;21(Suppl 1):i351–i358. doi: 10.1093/bioinformatics/bti1018. [DOI] [PubMed] [Google Scholar]
- Price M.N., Dehal P.S., Arkin A.P. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 2009;26:1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttick M.N., Clark J., Donoghue P.C.J. Size is not everything: rates of genome size evolution, not C-value, correlate with speciation in angiosperms. Proc. Biol. Sci. 2015;282 doi: 10.1098/rspb.2015.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram B., Sreenivasan T., Sahi B., Singh N. Introgression of low temperature tolerance and red rot resistance from Erianthus in sugarcane. Euphytica. 2001;122:145–153. [Google Scholar]
- Rao J., Babu C.J. Chromosome numbers of certain species of Erianthus Michx. Curr. Sci. 1953;22:217. [Google Scholar]
- Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Sanderson M.J. r8s: inferring absolute rates of molecular evolution and divergence ti mes in the absence of a molecular clock. Bioinformatics. 2003;19:301–302. doi: 10.1093/bioinformatics/19.2.301. [DOI] [PubMed] [Google Scholar]
- Sekiya N., Abe J., Shiotsu F., Morita S. Cultivation of erianthus and Napier grass at an abandoned mine in Lampung, Indonesia. Am. J. Plant Sci. 2014;05:1711–1720. [Google Scholar]
- Selvi A., Nair N.V., Noyer J.L., Singh N.K., Balasundaram N., Bansal K.C., Koundal K.R., Mohapatra T. AFLP analysis of the Phenetic organization and genetic diversity in the sugarcane complex, Saccharum and erianthus. Genet. Resour. Crop Evol. 2006;53:831–842. doi: 10.1007/s10722-004-6376-6. [DOI] [Google Scholar]
- Simão F.A., Waterhouse R.M., Ioannidis P., Kriventseva E.V., Zdobnov E.M. BUSCO: assessing genome assembly and annotation completeness with sing le-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- Singh J., Singh A.K., Sharma M.P., Singh P.R., Srivastava A.C. Mechanization of sugarcane cultivation in India. Sugar Tech. 2011;13:310–314. [Google Scholar]
- Singh N., Ujinwal M., Langyan S., Sayyed R.Z., El Enshasy H.A., Kenawy A.A. Genome-wide exploration of sugar transporter (sweet) family proteins in Fabaceae for Sustainable protein and carbon source. PLoS One. 2022;17 doi: 10.1371/journal.pone.0268154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Smith R.H., Dar A.C., Schlessinger A.J.B. PyVOL: A PyMOL plugin for visualization, comparison, and volume calculation of drug-binding sites. bioRxiv. 2019 Preprint at. [Google Scholar]
- Stanke M., Tzvetkova A., Morgenstern B. AUGUSTUS at EGASP: using EST, protein and genomic alignments for improved gene prediction in the human genome. Genome Biol. 2006;7:S11.1–S11.8. doi: 10.1186/gb-2006-7-s1-s11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling G., Cox M., Ogden-Brown J. Proceedings of the 2011 Conference of the Australian Society of Sugar Cane Technologists Held at Mackay, Queensland, Australia, 4-6 May 2011. Australian Society of Sugar Cane Technologists. 2011. Resistance to plant-parasitic nematodes (Pratylenchus zeae and Meloidogyne javanica) in Erianthus and crosses between Erianthus and sugarcane. [Google Scholar]
- Taji T., Ohsumi C., Iuchi S., Seki M., Kasuga M., Kobayashi M., Yamaguchi-Shinozaki K., Shinozaki K. Important roles of drought-and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J. 2002;29:417–426. doi: 10.1046/j.0960-7412.2001.01227.x. [DOI] [PubMed] [Google Scholar]
- Tang H., Wang X., Bowers J.E., Ming R., Alam M., Paterson A.H. Unraveling ancient hexaploidy through multiply-aligned angiosperm gene maps. Genome Res. 2008;18:1944–1954. doi: 10.1101/gr.080978.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Cheung L.S., Li S., Eom J.-S., Chen L.-Q., Xu Y., Perry K., Frommer W.B., Feng L. Structure of a eukaryotic SWEET transporter in a homotrimeric complex. Nature. 2015;527:259–263. doi: 10.1038/nature15391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bel M., Diels T., Vancaester E., Kreft L., Botzki A., Van de Peer Y., Coppens F., Vandepoele K. PLAZA 4.0: an integrative resource for functional, evolutionary and comparative plant genomics. Nucleic Acids Res. 2018;46(D1):D1190–D1196. doi: 10.1093/nar/gkx1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bel M., Proost S., Van Neste C., Deforce D., Van de Peer Y., Vandepoele K. TRAPID: an efficient online tool for the functional and comparative analysis of de novoRNA-Seq transcriptomes. Genome Biol. 2013;14:R134. doi: 10.1186/gb-2013-14-12-r134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.H., Yang Q.H., Li F.S., He L.L., He S.C. Molecular identification of Saccharum spp.× erianthus fulvus hybrids using sequence-characterized amplified region markers. Crop Sci. 2009;49:864–870. [Google Scholar]
- Wang X.H., Yang Q.H., Li F.S., He L.L., He S.C. Characterization of the chromosomal transmission of intergeneric hybrids of Saccharum spp. and erianthus fulvus by genomic in situ Hybridization. Crop Sci. 2010;50:1642–1648. [Google Scholar]
- Wang D., Zhang Y., Zhang Z., Zhu J., Yu J. KaKs_Calculator 2.0: a toolkit incorporating gamma-series methods and sliding window strategies. Dev. Reprod. Biol. 2010;8:77–80. doi: 10.1016/S1672-0229(10)60008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Tang H., DeBarry J.D., Tan X., Li J., Wang X., Lee T.h., Jin H., Marler B., Guo H., et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Huang Y., Lin Y., Fu C., Liu S., Deng Z., Li Q., Huang Z., Chen R., Zhang M. Unexpected inheritance pattern of Erianthus arundinaceus chromosomes in the intergeneric progeny between Saccharum spp. and Erianthus arundinaceus. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H., Wang D., Qin Y., Ma A., Fu J., Qin Y., Hu G., Zhao J. Genome-wide identification and expression analysis of SWEET gene family in Litchi chinensis reveal the involvement of LcSWEET2a/3b in early seed development. BMC Plant Biol. 2019;19:499. doi: 10.1186/s12870-019-2120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Wang H. LTR_FINDER: an efficient tool for the prediction of full-length LTR re trotransposons. Nucleic Acids Res. 2007;35:W265–W268. doi: 10.1093/nar/gkm286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- You J., Chan Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015;6:1092. doi: 10.3389/fpls.2015.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P., Yang T., Poovaiah B.W. Calcium signaling-mediated plant response to cold stress. Int. J. Mol. Sci. 2018;19:3896. doi: 10.3390/ijms19123896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.Zhang H., Li F., Zhong H., Yang Q., He S.J. Identification of sugarcane interspecies hybrids with RAPDs. Afr. J. Biotechnol. 2008;7:1072–10743. [Google Scholar]
- 1.Zhang H., He L., Zhong H., Li F., He S., Yang Q.J. Identification of Intergeneric Hybrids between Saccharum spp. and Erianthus fulvus with ITSs. Afr. J. Biotechnol. 2009;8 1841–18452-4. [Google Scholar]
- Zhang Z., Xiao J., Wu J., Zhang H., Liu G., Wang X., Dai L. ParaAT: a parallel tool for constructing multiple protein-coding DNA alignments. Biochem. Biophys. Res. Commun. 2012;419:779–781. doi: 10.1016/j.bbrc.2012.02.101. [DOI] [PubMed] [Google Scholar]
- Zhang J., Zhang X., Tang H., Zhang Q., Hua X., Ma X., Zhu F., Jones T., Zhu X., Bowers J., et al. Allele-defined genome of the autopolyploid sugarcane Saccharum spontaneum L. Nat. Genet. 2018;50:1565–1573. doi: 10.1038/s41588-018-0237-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li Y., He Y., Hu W., Zhang Y., Wang X., Tang H. Identification of NADPH oxidase family members associated with cold stress in strawberry. FEBS Open Bio. 2018;8:593–605. doi: 10.1002/2211-5463.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R.-G., Li G.-Y., Wang X.-L., Dainat J., Wang Z.-X., Ou S., Ma Y.J. TEsorter: an accurate and fast method to classify LTR-retrotransposons in plant genomes. Hortic. Res. 2022;9:uhac017. doi: 10.1093/hr/uhac017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Wang P., Si T., Hsu C.-C., Wang L., Zayed O., Yu Z., Zhu Y., Dong J., Tao W.A., et al. MAP kinase cascades regulate the cold response by modulating ICE1 protein stability. Dev. Cell. 2017;43:618–629.e5. doi: 10.1016/j.devcel.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
OrthoMCL was used to identify orthologous gene groups among the species.
(A) Manhattan plot depicting the genome-wide selective sweep regions in E. fulvus. (B) GO enrichment of genes associated with the selective sweep regions. (C) Manhattan plot depicting the genome-wide nucleotide diversity differences (π ratio) in E. fulvus accessions from Xizang and Yunnan provinces.