Abstract
Glucosinolates (GSLs), found mainly in species of the Brassicaceae family, are one of the most well-studied classes of secondary metabolites. Produced by the action of myrosinase on GSLs, GSL-derived hydrolysis products (GHPs) primarily defend against biotic stress in planta. They also significantly affect the quality of crop products, with a subset of GHPs contributing unique food flavors and multiple therapeutic benefits or causing disagreeable food odors and health risks. Here, we explore the potential of these bioactive functions, which could be exploited for future sustainable agriculture. We first summarize our accumulated understanding of GSL diversity and distribution across representative Brassicaceae species. We then systematically discuss and evaluate the potential of exploited and unutilized genes involved in GSL biosynthesis, transport, and hydrolysis as candidate GSL engineering targets. Benefiting from available information on GSL and GHP functions, we explore options for multifunctional Brassicaceae crop ideotypes to meet future demand for food diversification and sustainable crop production. An integrated roadmap is subsequently proposed to guide ideotype development, in which maximization of beneficial effects and minimization of detrimental effects of GHPs could be combined and associated with various end uses. Based on several use-case examples, we discuss advantages and limitations of available biotechnological approaches that may contribute to effective deployment and could provide novel insights for optimization of future GSL engineering.
Key words: glucosinolate, glucosinolate hydrolysis product, metabolic engineering, Brassicaceae, multifunctional crop ideotype
Options for multifunctional Brassicaceae crop ideotypes with customized glucosinolate profiles to meet future demand for food diversification and sustainable crop production are explored in this review. An integrated roadmap of glucosinolate engineering is then proposed based on an overview of glucosinolate diversity, molecular mechanisms, and functions, highlighting the phenotypic consequences of gene modification and the importance of maximizing the advantages of different biotechnologies.
Introduction
The prevalence and versatility of glucosinolates (GSLs) and GSL-derived hydrolysis products (GHPs) have underpinned the success and wide diversity of crops in the plant family Brassicaceae, as well as species in 16 other Brassicales families (Barco and Clay, 2019). Although GSLs have evolved in plants by providing strategies to manage challenging biotic and abiotic environments, they are mostly compatible with the human gut system and, with some exceptions, confer beneficial properties to crops and derived by-products (Liu et al., 2021; Sikorska-Zimny and Beneduce, 2021). The ability of humans to digest tissues from every part of Brassicaceae plant anatomy has led to domestication-related selection of diverse morphotypes distinguished by the proliferation of different organs and tissues (Cheng et al., 2016). The extent to which this has been achieved in the past few thousand years is almost unique among flowering plants (Mabry et al., 2018; Gallagher et al., 2019; Bryce et al., 2021; McAlvay et al., 2021) and is likely due to the absence of more toxic plant secondary metabolites such as cyanogenic glycosides (Moreira et al., 2018).
This review aims to provide an overview of current knowledge and opportunities for future GSL engineering. The availability of annotated genomes, first for Arabidopsis and then for each of the Brassica crops and other Brassicaceae species, has provided a rich source of information that complements the increased resolution of chemical analysis and functional mode-of-action studies for GSLs, precursors, and GHPs (Wang et al., 2013; Guo et al., 2016; Bell et al., 2020; Harun et al., 2020; Song et al., 2021; Zhao et al., 2021). Understanding the detailed evolution of distinct GSL subclasses within different taxa, along with relevant biosynthetic and regulatory networks, now enables the development of predictive strategies for metabolic engineering to define specifications or ideotypes. In particular, there is scope to recombine subnetworks and pathways to deliver desirable GSLs/GHPs and reduce antinutritional counterparts in a wider range of crops, which could provide both unique flavors for the human diet and specific resistance to crop pests and pathogens (Miao et al., 2021). Based on our review, we propose an integrated roadmap for generating multifunctional Brassicaceae crops to meet future demand for food diversification and sustainable crop production. Use-case examples are outlined to explore the possibilities of crop ideotype design and the availability of current biotechnological approaches, while also considering how beneficial effects of GHPs could be maximized and detrimental effects minimized in target crops.
Diversity of GSL structures and taxonomic distribution
Glucosinolates (GSLs) are a large group of sulfur-rich, amino acid-derived secondary metabolites with similar chemical structures that include a β-D-thioglucose group, a sulfonated oxime group, and an amino acid-derived R group (or side chain). A series of analytical methods have been developed to identify and isolate diverse GSL structures and quantify GSL content (Blažević et al., 2020). By mid-2021, at least 90 distinct GSL structures had been satisfactorily characterized in plants, with a further ∼49 structures remaining to be examined (Blažević et al., 2020; Montaut et al., 2020; Trabelcy et al., 2021). GSLs are mostly reported and studied in species of the Brassicaceae family, which includes a wide range of globally important vegetable, fodder, forage, condiment mustard, and oilseed crops and accounts for most (84 out of 90) well-characterized GSL structures. Using the comprehensive GSL number system for enumeration, these GSLs are 5, 11, 12, 15, 19, 22, 23, 24R, 24S, 28–31, 38S, 40R, 40S, 43, 45–48, 50R, 51, 54, 56–58, 61–69, 72, 73, 77, 79, 80, 82–84, 87, 88, 92, 94, 95, 101, 105, 107, 111, 112, 114, 117, 121R, 121S, 122R, 122S, 125–127, 129–143, 148, 149, 151, and 152 (Fahey et al., 2001; Blažević et al., 2020; Montaut et al., 2020). Most GSLs in Brassicaceae crops are derived biosynthetically from either methionine (Met), phenylalanine (Phe), tyrosine (Tyr), or tryptophan (Trp) (Wu et al., 2021a). Met-derived aliphatic GSLs appear to predominate in most species, both in content and number of structures (Table 1; Supplemental Table 1). Indeed, Met-derived aliphatic GSLs constitute the majority of all known GSLs (Blažević et al., 2020). Much of this diversity derives from chain elongation of the Met with consecutive CH2 groups early in GSL biosynthesis (Figure 1A). Accordingly, the Met-derived aliphatic GSLs are often assigned to different subgroups according to the number of C atoms between the side-chain sulfur and the oxime carbon (Table 1; Supplemental Table 1). For some Met-derived aliphatic GSLs, the terminal methyl and sulfur are eliminated, and in those cases the subgroups simply reflect the number of carbon atoms in the side chain (Blažević et al., 2020). The usual GSLs in common Brassicaceae species belong to the C3–C5 subgroups, corresponding to one to three added CH2 groups, but in some crops such as watercress (Nasturtium officinale) and wasabi (Eutrema japonicum), as well as non-domesticated species, longer side chains are found, up to the C11 subgroup (Blažević et al., 2020) (Table 1; Supplemental Table 1). Trp-derived GSLs are usually known as indole GSLs because the indole moiety from Trp contained in these GSLs is structurally conserved in general. Only four indole GSLs are commonly encountered in crops and typically all occur (Table 1). Only three GSLs derived from Phe occur at significant levels in crops. Glucotropaeolin is predominant in garden cress (Lepidium sativum) seeds and contains a benzyl side chain that is directly derived from the precursor amino acid (Table 1). Gluconasturtiin is predominant in several organs of watercress and results from a single-chain elongation of Phe (Figure 1B; Table 1; Supplemental Table 1). Further hydroxylation of gluconasturtiin results in glucobarbarins that occur at high levels in some weeds, at moderate levels in watercress, and at very low levels in rapeseed (Brassica napus) (Blažević et al., 2020) (Table 1; Supplemental Table 1). Only one GSL derived from Tyr, sinalbin, is common in crops; it carries the same phenol group as the precursor amino acid and for this reason has numerous chemical similarities to the indole GSLs (Blažević et al., 2020). The full structural diversity of GSLs is richer than we can fully capture here and includes GSLs derived from alanine, valine (Val), leucine (Leu), isoleucine (Ile), and glutamate (Blažević et al., 2020). We solely list one Ile-derived GSL, glucocochlearin (Table 1), to represent branched-chain GSLs (derived from branched-chain amino acids Val, Leu, Ile, and their homologs). Although glucocochlearin occurs rarely in Brassicaceae crops (Agerbirk et al., 2021), it has been detected in several species: at relatively high levels in seeds of Chinese cabbage (Brassica rapa spp. pekinensis), kale (Brassica oleracea var. acephala), mustard (Brassica juncea), and radish (Raphanus sativus) and at lower levels in other organs of Chinese cabbage, kale, rapeseed, pak choi (B. rapa spp. chinensis), wasabi, and horseradish (Armoracia rusticana) (Table 1; Supplemental Table 1).
Table 1.
The classification of major GSLs and their taxonomic distribution among important Brassicaceae species.
| Precursor amino acid | Group | Trivial name | Numberc | Semisystemic name |
Arabidopsis thalianab |
Brassica carinata |
Brassica juncea |
Brassica napus |
Brassica nigra |
Brassica oleraceab |
Brassica rapab |
Eruca sativa |
Lepidium sativum |
Nasturtium officinale |
Raphanus sativus |
Sinapis alba |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seeda | Seed | Seed | Seed | Seed | Leaf | Leaf | Leaf | Seed | Leaf | Root | Seed | |||||
| Met | C3 | sinigrin | 107 | 2-propenyl GSL | 0 | +++ | + | tr,† | +++ | + | 0 | 0 | 0 | 0 | 0 | tr |
| glucoiberverin | 95 | 3-(methylthio)propyl GSL | tr | 0 | tr | 0 | 0 | tr | 0 | 0 | 0 | 0 | 0 | 0 | ||
| glucoiberin | 73 | 3-(methylsulfinyl)propyl GSL | tr | 0 | tr | 0 | 0 | + | 0 | 0 | 0 | tr | 0 | 0 | ||
| C4 | glucoerucin | 84 | 4-(methylthio)butyl GSL | +++ | 0 | + | tr | 0 | tr | tr,† | + | 0 | 0 | +,† | 0 | |
| glucoraphasatin | 83 | 4-(methylthio)but-3-enyl GSL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | +++ | 0 | ||
| glucoraphanin | 64 | 4-(methylsulfinyl)butyl GSL | + | 0 | + | + | 0 | ++ | tr,† | ++ | 0 | 0 | tr | 0 | ||
| glucoraphenin | 63 | 4-(methylsulfinyl)but-3-enyl GSL | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 | ++,† | 0 | ||
| gluconapin | 12 | 3-butenyl GSL | 0 | tr | +++ | ++ | 0 | tr | +++ | 0 | 0 | 0 | 0 | tr | ||
| progoitrin | 24R | (R)-2-hydroxybut-3-enyl GSL | 0 | + | + | +++ | 0 | + | ++ | + | 0 | 0 | 0 | + | ||
| epiprogoitrin | 24S | (S)-2-hydroxybut-3-enyl GSL | 0 | 0 | 0 | tr | 0 | ++ | 0 | +,† | 0 | 0 | 0 | 0 | ||
| C5 | glucoberteroin | 94 | 5-(methylthio)pentyl GSL | + | 0 | tr | tr | 0 | 0 | 0 | tr,† | 0 | 0 | 0 | 0 | |
| glucoalyssin | 72 | 5-(methylsulfinyl)pentyl GSL | tr | 0 | tr | + | 0 | tr,† | + | tr | 0 | 0 | 0 | 0 | ||
| glucobrassicanapin | 101 | 4-pentenyl GSL | 0 | 0 | tr | + | 0 | tr | ++ | 0 | 0 | 0 | +,† | 0 | ||
| gluconapoleiferin | 38S | 2-hydroxypent-4-enyl GSL | 0 | 0 | tr,† | tr | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | ||
| C6–C10d | +++ | 0 | 0 | tr,† | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 | ||||
| Phe/Tyr | glucotropaeolin | 11 | benzyl GSL | 0 | + | tr,† | 0 | 0 | 0 | 0 | tr,† | +++ | tr | 0 | tr | |
| sinalbin | 23 | 4-hydroxybenzyl GSL | 0 | 0 | 0 | tr,† | 0 | 0 | 0 | tr,† | 0 | 0 | 0 | +++ | ||
| gluconasturtiin | 105 | 2-phenylethyl GSL | tr | 0 | tr | tr | tr | + | tr,† | tr | 0 | +++ | +,† | 0 | ||
| glucobarbarins | 40 | 2-hydroxy-2-phenylethyl GSLs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 | ||
| Trp | glucobrassicin | 43 | 3-indolylmethyl GSL | + | tr | tr | tr | tr | ++ | + | tr | 0 | + | tr | tr | |
| 4-hydroxyglucobrassicin | 28 | 4-hydroxyindol-3-ylmethyl GSL | tr | tr | tr,† | + | tr | 0 | tr,† | + | 0 | tr | tr,† | 0 | ||
| neoglucobrassicin | 47 | 1-methoxyindol-3-ylmethyl GSL | 0 | 0 | tr | tr | 0 | tr,† | + | tr,† | 0 | 0 | 0 | tr | ||
| 4-methoxyglucobrassicin | 48 | 4-methoxyindol-3-ylmethyl GSL | 0 | 0 | tr | tr | tr | tr | tr | + | 0 | tr | tr | 0 | ||
| Ile | glucocochlearin | 61 | 1-methylpropyl GSL | 0 | 0 | ++ | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 |
+++, the predominant GSL, which accounts for ≥40% of the total GSL content in an organ. ++, GSL that accounts for ≥20% and <40% of the total GSL content in an organ. +, GSL that accounts for ≥1% and <20% of the total GSL content in an organ. tr, GSL remains in trace amounts, accounting for <1% of the total GSL content in an organ. 0, GSL that was not detected in an organ. †, GSL that was detected in only a few cultivars but was absent in other cultivars of a species. All the GSL content provided here are dry weight data.
The representative organ is selected to illustrate GSL profiles of each species.
GSL profiles of Col-0 ecotype, pak choi, and white cabbage are selected to represent A. thaliana, B. rapa, and B. oleracea, respectively.
The bold GSL numbers proposed by Fahey et al. (2001) are listed here for reference in Supplemental Table 1. In particular, the number 40 is used to represent glucobarbarins, which include two epimers, glucobarbarin (40S) and epiglucobarbarin (40R).
Long-chain GSLs are included in the group C6–C10 and specified in Supplemental Table 1, which includes (1) 6-(methylthio)hexyl GSL (88), also glucolesquerellin; (2) 6-(methylsulfinyl)hexyl GSL (67), also glucohesperin; (3) 7-(methylthio)heptyl GSL (87); (4) 7-(methylsulfinyl)heptyl GSL (66), also glucoibarin; (5) 8-(methylthio)octyl GSL (92); (6) 8-(methylsulfinyl)octyl GSL (69), also glucohirsutin; (7) 8-(methylsulfonyl)octyl GSL (80); (8) 9-(methylsulfonyl)nonyl GSL (79); and (9) 10-(methylsulfinyl)decyl GSL (65), also glucocamelinin.
Data sources: Matthäus and Luftmann (2000); Brown et al. (2003); Boyd et al. (2006); Bellostas et al. (2007); Gill et al. (2007); Velasco et al. (2008); Pasini et al. (2012); Augustine et al. (2013b); Wiesner et al. (2013b); Chun et al. (2013); Zhu el . (2013); Agerbirk et al. (2014); Bhandari et al. (2015); Zhang et al. (2015c); Yi et al. (2015, 2016); Giallourou et al. (2016); Taranto et al. (2016); Jeon et al. (2017, 2022); Park et al. (2017); Klopsch et al. (2018); Andini et al. (2019); Bell et al. (2021); Li et al. (2021b); Gohain et al. (2021); Wang et al. (2022b); Missinou et al. (2022).
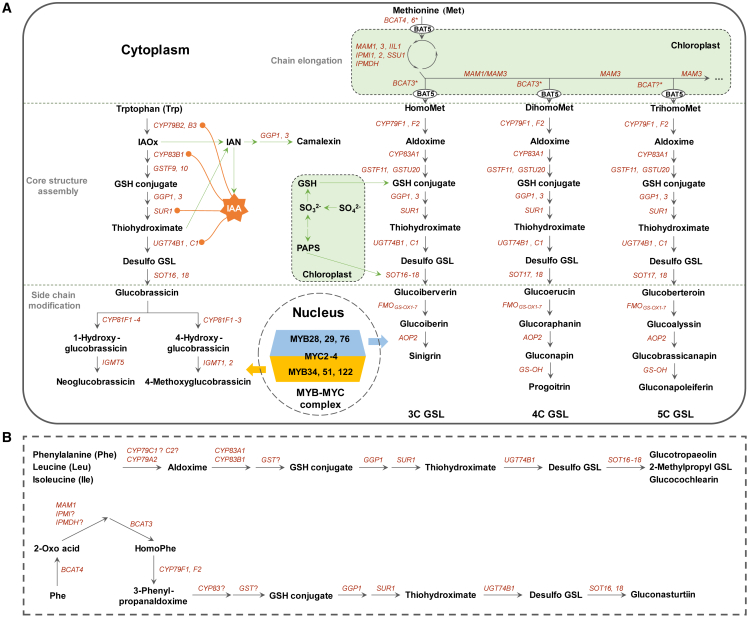
Figure 1.
De novo biosynthetic pathways of GSLs in A. thaliana.
(A)De novo biosynthesis of Met-derived aliphatic GSLs and Trp-derived indole GSLs. BCAT3 is a chloroplast enzyme that has a specific role in the production of homoMet and dihomoMet. BCAT4 and BCAT6 are both cytosolic enzymes. Catalyzed by MAMs, chain-elongated 2-keto acids can be transaminated either in chloroplasts by BCAT3 or by BCAT4/BCAT6 following export by BAT5 from the chloroplasts to the cytoplasm. This transport process is not shown in Figure 1. The negative feedback loops between IAA and GSL biosynthetic genes are indicated by orange lines. The camalexin biosynthetic pathway that intersects with GSL biosynthesis intermediates is indicated by green arrows. GSH, glutathione; PAPS, 3′-phosphoadenosine-5′-phosphosulfate; IAOx, indole-3-acetaldoxime; IAN, indole-3-acetonitrile.
(B) The proposed biosynthetic pathways of Phe-derived glucotropaeolin, Leu-derived 2-methylpropyl GSL, and Ile-derived glucocochlearin (upper), as well as homoPhe-derived gluconasturtiin (lower). The involvement and function of genes that are followed by a question mark (“?”) remain to be investigated in A. thaliana.
Information sources: Hull et al. (2000); Mikkelsen et al. (2000, 2004); Bak et al. (2001); Bak and Feyereisen (2001); Reintanz et al. (2001); Chen et al. (2003); Naur et al. (2003); Grubb et al. (2004, 2014); Schuster et al. (2006); Knill et al. (2008); Sønderby et al. (2010b); Geu-Flores et al. (2011); Lächler et al. (2015); Liu et al. (2016b); Petersen et al. (2019); Harun et al. (2020); Wang et al. (2020); Shang et al. (2022).
Beyond this structural diversity, the content, concentration, and dynamic changes of GSLs vary among plant growth stages, organs, and tissues (Bell and Wagstaff, 2017). Comparison of available GSL information between tissues and organs of several Brassicaceae species clearly shows that these variations in GSL profiles are associated with different taxa (Table 1; Supplemental Table 1). In B. napus, for example, progoitrin and gluconapin are the major GSLs in seeds, whereas glucobrassicanapin is predominant in leaves. Among B. napus oilseed cultivars, the difference between traditional high-GSL and modern selected low-GSL (canola) types is primarily due to a significant reduction in progoitrin and gluconapin, as well as a moderate reduction in 4-hydroxyglucobrassicin (March et al., 1989; Fieldsend and Milford, 1994; Li et al., 1999; Andini et al., 2019; Missinou et al., 2022). Nevertheless, progoitrin remains the most abundant GSL in the seeds of low-GSL cultivars (Velasco et al., 2008). The Phe-derived gluconasturtiin is frequently most abundant in roots, particularly in Brassica species (Kirkegaard and Sarwar, 1998; Bhandari et al., 2015) (Supplemental Table 1).
Over time, the ancestors of most Brassicales families have undergone β (∼85 million years ago) and α (∼31.8 million years ago) whole-genome duplication (WGD) events, with a series of recent gene duplications and rearrangements (Edger et al., 2015, 2018). This has provided the opportunity for divergence and specialization of genes involved in GSL biosynthesis, transport, and hydrolysis (Barco and Clay, 2019). Subsequently, the evolution of diverse GSL profiles has been accelerated through domestication and artificial selection, with the accumulation of morphologically distinct subspecies and subsequent radiation into locally adapted landraces and numerous modern cultivars (Chalhoub et al., 2014; Kim et al., 2016; Branca et al., 2018; Chopra et al., 2020; Arias et al., 2021). This provides us with a broad and flexible platform that contains abundant species, genetic loci, and GSL resources and could be exploited for GSL engineering, as we discuss in detail below.
Candidate targets for current and future GSL metabolic engineering
Manipulating genes involved in biosynthesis to alter GSL diversity
Over the past few decades, a number of genes involved in GSL biosynthesis have been identified and characterized in the model plant Arabidopsis thaliana (Harun et al., 2020) and subsequently in other Brassicaceae crop species, particularly in the genus Brassica (Zhu et al., 2012; Augustine et al., 2013a; Kim et al., 2013; Zhang et al., 2015a; Seo et al., 2016; Sharma et al., 2016; Yin et al., 2017; Liu et al., 2020; Tandayu et al., 2022). Apart from biosynthesis of indole GSLs, de novo biosynthetic pathways of intact GSL molecules involve elongation of side chains, assembly of core structures, and secondary modification of side chains (Figure 1A). Previous attempts have been made to extend GSL diversity in various Brassicaceae species through different molecular approaches (Miao et al., 2021). However, it should be recognized that metabolic engineering is a complex undertaking, given the innate interactions among GSLs, phytohormones (Malka and Cheng, 2017), glutathione (Sønderby et al., 2010b), phytoalexins (Geu-Flores et al., 2011), and primary metabolic pathways (Borpatragohain et al., 2016) that also affect plant growth and development. We therefore considered those genes that may affect GSL profiles and also have pleiotropic effects on normal plant growth (Tables 2 and 3; Supplemental Table 2). Although several genes have already been exploited as engineering targets in different species (Miao et al., 2021), a few that appear capable of modifying GSL profiles are yet to be explored. However, the potential for negative effects on normal plant growth and agronomic traits after molecular engineering may reduce the total number of available genes. We further collated a subset of biosynthetic genes, for some of which targeted mutagenesis has led to perturbations in normal plant growth and important agronomic traits, as well as in GSL profiles (Tables 2 and 3; Supplemental Table 2). This enables us to explore the feasibility and potential of exploiting these genes as future GSL engineering targets.
Table 2.
Phenotypic alterations following mutagenesis of genes involved in the GSL chain elongation step.
| Locus | Strategy | Species | Plant growth and agronomic traitsa |
GSL content |
Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| Height | Fertility | Yield | Survival | Met-derived | Indole | ||||
| MAM1 | KO | A. thaliana | Nc | +c |
Kroymann et al., (2001) Textor et al. (2007) |
||||
| OE | B. rapa | N | N | N | N | +b | +b | Zang et al. (2008) | |
| MAM3 | OE | A. thaliana | N | N | N | N | Nc |
Field et al. (2004); Textor et al. (2007) |
|
| KO | A. thaliana | +c | Nc | Textor et al. (2007) | |||||
| GS-ELONG (MAMs) | KD | B. napus | N | N | N | N | –b | Nb | Liu et al. (2011) |
| BCAT4 | KO | A. thaliana | – | + |
Schuster et al. (2006); Sawada et al. (2009) |
||||
| KO | A. thaliana | N | N | N | –/N | + | Lächler et al. (2015) | ||
| BCAT6 | KO | A. thaliana | N | N | N | N | N | Lächler et al. (2015) | |
| BCAT3 | KO | A. thaliana | +/N | –/+ | Knill et al. (2009) | ||||
| KO | A. thaliana | N | N | N | N/+ | + | Lächler et al. (2015) | ||
| BCAT3BCAT4 | KO | A. thaliana | – | + | Knill et al. (2009) | ||||
| BCAT4BCAT6 | KO | A. thaliana | N | N | N | – | + | Lächler et al. (2015) | |
| BCAT3BCAT4BCAT6 | KO | A. thaliana | – | – | – | + | Lächler et al. (2015) | ||
| IPMI1 | KO | A. thaliana | N | N | N | N | –/N | Knill et al. (2009) | |
| KO and KD | A. thaliana | N | N | N | N | N | N |
He et al. (2010); Imhof et al. (2014) |
|
| IPMI2 | KO | A. thaliana | N | N | N | N/+ | N |
Knill et al. (2009); Imhof et al. (2014) |
|
| KO | A. thaliana | N | N | He et al. (2010) | |||||
| IPMI1IMPI2 | KO and KD | A. thaliana | –c | Nc | He et al. (2010) | ||||
| IPMDH | KO | A. thaliana | N | N | N | N | –c | +c |
He et al. (2009); Sawada et al. (2009); He et al. (2011) |
KO, knockout; KD, knockdown; OE, overexpression.
Several representative plant growth and agronomic traits are selected to show phenotypic alterations following gene mutagenesis. Plant height refers to the height of plants at full maturity or to putative height because dwarfism and subsequent death may occur at early stages. Fertility refers to plant male fertility rate at the flowering stage. Yield refers to per-plant seed yield at full maturity. Survival refers to the observed plant survival rate under normal growth conditions. Because the source data represent a mixture of descriptive and quantitative information, N (no changes), + (increase), and – (decrease) are used to indicate, although not precisely quantify, observed alterations in plant growth, agronomic traits, and GSL content.
The available GSL data are only from plant leaves. Other available GSL data are derived from the combination of leaves and seeds. A single N, +, or – is used to indicate the overall alteration in content in leaves and seeds. A virgule (“/”) is used to show distinct alterations in leaves/seeds.
GSL alterations are not supported by specific data but are speculated from the relevant description or calculated by indirect data evidence described in the source literature.
Table 3.
Phenotypic alterations following mutagenesis of genes involved in the GSL core structure assembly step.
| Locus | Strategy | Species | Plant growth and agronomic traitsa |
GSL contenta |
Tissue/organ | Benefit | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Height | Fertility | Yield | Survival | Met-derived | Indole | ||||||
| CYP79F1 | KO | A. thaliana | – | – | – | –/+/– | +/N/+ | leaf/root/seed | Reintanz et al. (2001) | ||
| OE | B. rapa | – | + | N | leaf | Zang et al. (2008) | |||||
| CYP79F2 | KO | A. thaliana | N | N | N | N | +/N/N | leaf/root/seed | Chen et al. (2003) | ||
| KO | A. thaliana | N | N | N | N | –/+/–/– | N/+/+/+ | leaf/stem/root/flower | Tantikanjana et al. (2004) | ||
| OE | A. thaliana | – | N | leaf/root/seed | Chen et al. (2003) | ||||||
| CYP79F1CYP79F2 | KO | A. thaliana | – | – | – | – | +/+/–/+ | leaf/stem/root/flower | Tantikanjana et al. (2004) | ||
| CYP79B2CYP79B2 | OE | A. thaliana | – | – | N | + | leaf | resistance to Pseudomonas syringae pv. maculicola |
Hull et al. (2000); Mikkelsen et al. (2000) |
||
| KO | A. thaliana | N | N | N | N | Zhao et al. (2002) | |||||
| CYP79B3CYP79B3 | KO | A. thaliana | N | N | N | N | Zhao et al. (2002) | ||||
| OE | B. rapa | N | root | Zang et al. (2009) | |||||||
| CYP79B2CYP79B3 | KO | A. thaliana | – | – | Zhao et al. (2002) | ||||||
| CYP83A1 | KO | A. thaliana | N | N | N | N | +/N | leaf/seed | resistance to Erysiphe cruciferarum, Glovinomyces orontii, and G. cichoracearum |
Hemm et al. (2003); Weis et al. (2013), 2014; Liu et al. (2016a) |
|
| OE | A. thaliana | N | N | N | N | N | N | seedling/leaf | Naur et al. (2003) | ||
| OE | B. napus | + | N | leaf/seed | Zhang et al. (2015b) | ||||||
| CYP83B1 | KO | A. thaliana | – | – | – | seedling |
Bak et al. (2001); Bak and Feyereisen (2001) |
||||
| OE | A. thaliana | – | – | + | seedling | Bak and Feyereisen (2001) | |||||
| OE | A. thaliana | – | + | N | leaf | Naur et al. (2003) | |||||
| CYP79B2CYP79B3CYP83B1 | OE | B. rapa | N | root | Zang et al. (2009) | ||||||
| GSTF11 | KO | A. thaliana | N | N | N | N | – | N | leaf/seed | Zhang et al. (2022) | |
| GSTU20 | KO | A. thaliana | N | N | N | N | – | N | leaf/seed | Zhang et al. (2022) | |
| GSTF11GSTU20 | KO | A. thaliana | – | N | leaf/seed | Zhang et al. (2022) | |||||
| GGP1GGP3 | KD | A. thaliana | – | + | leaf | Geu-Flores et al. (2011) | |||||
| SUR1 | KO | A. thaliana | – | – | – | – | – | shoot | Mikkelsen et al. (2004); Grubb et al. (2014) | ||
| OE | A. thaliana | N | N | N | N | N | N | Mikkelsen et al. (2004) | |||
| UGT74B1 | KO | A. thaliana | – | – | – | – | – | leaf/shoot |
Grubb et al. (2004); Grubb et al. (2014) |
||
| OE | B. oleracea | N | N | + | leaf | Zheng et al. (2021) | |||||
| OE | B. napus | + | + | leaf/seed | resistance to Sclerotinia sclerotiorum and Botrytis cinerea | Zhang et al. (2015b) | |||||
| UGT74C1 | KO | A. thaliana | N | N | N | N | N | N | shoot | Grubb et al. (2014) | |
| UGT74B1UGT74C1 | KO | A. thaliana | – | – | – | – | – | – | shoot | Grubb et al. (2014) | |
| SOT16 | KO | A. thaliana | N | N | N | N | Nb | Nb | Klein and Papenbrock (2009) | ||
| OE | A. thaliana | +b | Klein and Papenbrock (2009) | ||||||||
The abbreviations of mutagenesis strategies and phenotypic alterations (N, +, –) follow Table 2. A single N, +, or – is used both for GSL alteration of a single tissue/organ and a similar alteration among multiple tissues/organs. A virgule (“/”) is used to show distinct alterations in GSL content among different tissues/organs based on the order in the column "Tissue/organ.”
GSL alterations were not supported by specific data but are speculated from the relevant description or calculated by indirect data evidence described in the source literature.
Enzymes encoded by GSL biosynthetic genes have been engineered in different species; these include methylthioalkylmalate synthases (MAMs), cytochrome P450 enzymes (CYP79F1, CYP79Bs, CYP83B1, and CYP83A1), UDP-glycosyltransferase 74B1 (UGT74B1), flavin-containing monooxygenases (FMOGS-OXs), and 2-oxoglutarate-dependent dioxygenase 2 (AOP2) (Tables 2 and 3; Supplemental Table 2). In the chain-elongation step, MAMs have been ideal engineering targets because of their dominant roles in determining the structures of Met-derived aliphatic GSLs by facilitating the chain elongation reaction of Met derivatives (Figure 1A). Apart from its role in biosynthesis of Met-derived aliphatic GSLs, MAM1 is also responsible for the elongation of Phe to generate homoPhe, the precursor of gluconasturtiin (Shang et al., 2022) (Figure 1B). Other chain-elongation genes also exhibit the ability to modify GSL profiles (Table 2), including three genes (BCAT3, BCAT4, and BCAT6) encoding branched-chain-amino-acid aminotransferases that are potential candidate engineering targets. Single- and double-knockout mutants of these genes exhibit similar alterations in GSL profiles, with elevated indole GSLs and decreased Met-derived aliphatic GSLs (Table 2). Only the triple mutant showed abnormal morphological phenotypes (Table 2). Another important GSL biosynthesis enzyme, 3-isopropylmalate dehydratase (IPMI), comprises a large subunit (IPMI LSU1) and three small subunits (IPMI SSU1–3), which are encoded by IIL1, IPMI SSU1, IPMI1, and IPMI2, respectively (Lächler et al., 2020). The phenotypic defects exhibited by IIL1 and IPMI SSU1 knockout mutants are associated with the important roles of these enzymes in plant primary metabolism and developmental processes, making them unsuitable for molecular engineering (Knill et al., 2009; Sureshkumar et al., 2009; Imhof et al., 2014) (Table 2). However, unlike IIL1 and IPMI SSU1, a combination of IPMI1 and IPMI2 could represent a target for GSL engineering, although single IPMI1 or IPMI2 knockout/knockdown mutants showed limited alterations in GSL profiles (Table 2). Finally, the gene encoding 3-isopropylmalate dehydrogenase 1 (IPMDH) also has the potential to be engineered in more species owing to its ability to alter Met-derived aliphatic GSLs; more importantly, the knockout mutant showed an agronomic phenotype indistinguishable from that of wild-type plants (Table 2).
In the core structure assembly step, indole-3-acetaldoxime (IAOx) is a Trp-derived intermediate involved in indole GSL biosynthesis that also represents a key intersection point connecting primary and secondary metabolism as well as auxin biosynthetic pathways (Figure 1A). As an important naturally synthesized auxin, indole-3-acetic acid (IAA) dynamically regulates plant growth and development (Zhao, 2010). It is believed that numerous plant species can use aldoximes as precursors for IAA biosynthesis (Perez et al., 2021), and for A. thaliana, functional mutation of several GSL biosynthetic genes (CYP79B2, CYP79B3, CYP83B1, SUR1, and UGT74B1) has shown pleiotropic indirect effects on IAA homeostasis via effects on IAOx (Figure 1A). Functional mutation of these genes not only alters GSL profiles but also leads to abnormal IAA-related phenotypes (Table 3). Among these genes, SUPERROOT1 (SUR1) may not be suitable as a potential GSL engineering target. This is because functional loss of SUR1 results in the elimination of almost all GSLs, a lack of seed development, and plant death, whereas overexpression of SUR1 has no effect on GSL profiles or plant morphology (Table 3; Supplemental Table 2). Apart from these IAA-related genes, CYP79F1 and CYP79F2 have shown correlations with cytokinin levels, and their functional mutagenesis in A. thaliana and B. rapa leads to exaggerated plant morphology (Table 3). CYP79A2 solely controls the conversion of Phe to phenylacetaldoxime, which is the precursor of glucotropaeolin and a naturally occurring auxin phenylacetic acid (Wittstock and Halkier, 2000). Overexpression of CYP79A2 leads to high-auxin phenotypes with overaccumulation of glucotropaeolin and phenylacetic acid (Perez et al., 2021). If such genes are to be appropriate targets for GSL engineering, these negative phytohormone-related phenotypes will need to be overcome in the future. Given the wide range of Brassicaceae species with complex polyploid genomes that harbor multiple homologs of a high proportion of genes, there is considerable scope for selection of appropriate paralogs for mutagenesis that may reduce or even eliminate undesirable pleiotropic changes, expanding the range of GSL engineering targets.
In addition to phytohormone-related GSL biosynthetic genes, other genes within the core structure assembly step can alter GSL profiles without affecting normal plant growth. CYP83A1 has been an ideal engineering target because knockout mutants exhibit positive effects in controlling several biotrophic fungi (Table 3; Supplemental Table 2). This suggests potential for CYP83A1 orthologs in GSL engineering of a wider range of Brassicaceae crops as a strategy to improve specific disease resistance. GSTF11 and GSTU20 are two newly verified genes encoding glutathione-S-transferases. Knockout mutants did not show morphological alterations and had a significantly reduced Met-derived aliphatic GSL content, making them appropriate targets for GSL engineering (Table 3). GGP1 and GGP3, which encode γ-glutamyl peptidases, connect glutathione sulfur-donating pathways with GSL biosynthetic pathways and are also involved in indole-sulfur phytoalexin biosynthetic pathways in A. thaliana (Geu-Flores et al., 2011; Klein and Sattely, 2017) (Figure 1A). Trp-derived phytoalexins, of which camalexin is a representative, are similar to GSLs and contribute to plant resistance against various pathogens (Pedras et al., 2011; Ahuja et al., 2012; Plaszkó et al., 2022). In the A. thaliana double mutant ggp1-1ggp3-1, indole GSL content was enhanced and camalexin content was reduced (Geu-Flores et al., 2011) (Table 3), but the effects on morphological phenotypes and plant resistance to specific pathogens remain unknown. However, accumulation of camalexin is confined to the tribe Camelineae (Bednarek et al., 2011). In Brassica species, different indole-sulfur phytoalexins such as brassinin occur and can undergo further reactions controlled by a series of downstream genes to form numerous derived phytoalexins (Pedras et al., 2011; Plaszkó et al., 2022). The metabolic connection between indole GSLs and brassinin biosynthesis has been established in cabbage, indicating the likely involvement of SUR1 and GGPs in the relevant pathways (Klein and Sattely, 2017). Therefore, if GGPs and SUR1 were selected for engineering in more Brassicaceae species, future studies should carefully examine both the possible negative effects on plant growth and agronomic traits and also the resulting changes in plant resistance to specific pathogens. At the end of the core structure assembly step, three genes encoding cytosolic sulfotransferases (SOT16–18) are responsible for the sulfonation of desulfo GSLs. Although the substrate specificities of SOT enzymes for different desulfo GSLs have been elucidated in A. thaliana and B. napus, it remains unclear whether functional mutation of SOTs can significantly alter GSL profiles (Klein and Papenbrock, 2009; Hirschmann and Papenbrock, 2015). Located downstream of GSL biosynthesis, their engineering potential is probably limited, although this remains to be verified in future work.
In the side-chain secondary modification step, different gene families (FMOGS-OXs, AOP2, GS-OH, CYP81Fs, and IGMTs) ultimately determine GSL side-chain structures, and there is no evidence that mutagenesis of these downstream genes has negative effects on normal plant growth and agronomic traits (Supplemental Table 2). This underlies their potential to be engineered in a wide range of species. As mentioned above, FMOGS-OXs and AOP2 have been used to modify GSLs in species besides A. thaliana (e.g., B. rapa), but other secondary modification genes have not (Supplemental Table 2). For Met-derived aliphatic GSLs, GS-OH encodes a 2-oxoacid-dependent dioxygenase that is solely responsible for the conversion from gluconapin to progoitrin (Figure 1A). Transgenic research in A. thaliana and B. oleracea has revealed multiple potential functions of GS-OH in GSL engineering: knockout or silencing may eliminate goitrogenic progoitrin, whereas overexpression may promote progoitrin accumulation to control specific generalist insects such as Trichoplusia ni (Hansen et al., 2008; Wu et al., 2022). For indole GSLs, four CYP81F genes (CYP81F1–4) and three genes encoding indole glucosinolate O-methyltransferases (IGMT1, IGMT2, and IGMT5) fine-tune the conversion among different indole GSL structures (Figure 1A). Among these genes, CYP81F2 controls the conversion from glucobrassicin to 4-methoxyglucobrassicin (Figure 1A). The hydrolysis of 4-methoxyglucobrassicin confers plant resistance against green peach aphid (Myzus persicae) by post-ingestive breakdown, independent of classical myrosinases (Pfalz et al., 2009), and against fungi through the action of the atypical myrosinase PEN2 to activate the innate immune response in A. thaliana (Bednarek et al., 2009; Clay et al., 2009). These results indicate the great potential for CYP81F2 (and likewise IGMT1 and IGMT2) to be overexpressed in more species that overaccumulate 4-methoxyglucobrassicin to control fungal diseases, although the resulting effects on normal plant growth remain to be investigated. Finally, IGMT5, which is solely responsible for the conversion from 1-hydroxyglucobrassicin to neoglucobrassicin, also represents an engineering target (Figure 1A). Functional loss of IGMT5 has been shown to enhance resistance to the root-knot nematode Meloidogyne javanica, indicating its potential role in Arabidopsis belowground defense (Pfalz et al., 2016) and inspiring us to make similar engineering efforts in other species.
Detailed understanding of GSL biosynthetic gene functions in the model plant A. thaliana has facilitated recent progress in the biosynthetic pathways of Phe-derived GSLs and corresponding engineering attempts in GSL-free species. Through the use of comparative genomic tools and transcriptomic data, a relatively clear de novo pathway for biosynthesis of gluconasturtiin from Phe and its further hydroxylation was proposed in the wild species Barbarea vulgaris, in which oxidized phenylethyl GSLs predominate (Agerbirk and Olsen, 2015; Liu et al., 2016b). Subsequently, the genetic mechanisms of Phe-derived GSL biosynthesis were investigated in a genome-wide association study on B. rapa (Shang et al., 2022). Based on the collective results, we provide the potential biosynthetic pathways of two Phe-derived GSLs in A. thaliana (Figure 1B), glucotropaeolin and gluconasturtiin, whose potent health-promoting effects have been widely reported (see section “generating multifunctional crops with defined GSL profiles and novel end uses”; Table 4). Attempts to heterologously produce Phe-derived glucotropaeolin and gluconasturtiin by expressing biosynthetic genes from A. thaliana or co-expressing biosynthetic genes from A. thaliana and B. vulgaris have been successful in potato (Solanum tuberosum) and tobacco (Nicotiana benthamiana) (Møldrup et al., 2012; González-Romero et al., 2021; Wang et al., 2021). Although the substrate specificity of the involved enzymes was clearly revealed in the engineering studies, their in planta functions in Phe-derived GSL biosynthesis, especially for gluconasturtiin, remain to be clarified in A. thaliana (Figure 1B). The broad specificity of newly characterized CYP79C enzymes, which accept both Leu and to some extent Ile in addition to Phe (Figure 1B) and the altered specificity of CYP79C1 in the presence of chain elongating enzymes were surprising (Wang et al., 2020). Therefore, further studies are also required to fill gaps in the proposed biosynthetic pathways of GSLs derived from Leu, Ile, and additional amino acids in A. thaliana (Figure 1B). This would help us understand how activities of specific enzymes are orchestrated to catalyze the formation of rare and unexploited GSLs, beyond the context currently dominated by Met-derived aliphatic GSLs in A. thaliana and most Brassicaceae crop species. Such insights could be further exploited and applied in a wide range of engineering contexts.
Table 4.
Odor descriptions and health effects of major GHPs found in important Brassicaceae species.
| GHP | GSLa | Odor description | Beneficial/detrimental effects on human healthb |
|---|---|---|---|
| Benzyl ITC | glucotropaeolin (11) | chemical, pungent, green, rotten grass, cooked, watercress-like (Masuda et al., 1996; Bell et al., 2021) | anticarcinogenic, anti-inflammatory, antidiabetic, anti-obesity, and neuroprotective activity and prevention of multiple sclerosis (Wu et al., 2021b; Sundaram et al., 2022) |
| 3-Butenyl ITC | gluconapin (12) | green, bitter, pungent, aromatic, wasabi-like, vegetable-like, cabbage-like (Masuda et al., 1996; Bell et al., 2018, 2021) | anticarcinogenic activity (Sundaram et al., 2022) antimicrobial activity (Jang et al., 2010) prevention of postprandial hypertriglyceridemia (Washida et al., 2010) |
| 4-Hydroxybenzyl ITC | sinalbin (23) | pungent (Bell et al., 2018) | anticarcinogenic activity (Monu et al., 2014; Jurkowska et al., 2018) |
| 5-vinyloxazolidine-2-thiones (goitrins)c | progoitrin (24R) and epiprogoitrin (24S) | strongly bitter (Bell et al., 2018) | goitrogenic effect and inhibition of thyroid hormone production∗ (Stoewsand, 1995; Felker et al., 2016) antiviral activity (Niexing et al., 2020) |
| Indole-3-carbinol | glucobrassicin (43) | unpleasant (Bell et al., 2018) | cardioprotective and neuroprotective activity (Kamal et al., 2022) anticarcinogenic, antioxidant, antimicrobial, antiviral (including COVID-19) and anti-inflammatory, anti-obesity, antidiabetic, cardioprotective activity (Singh et al., 2021; Centofanti et al., 2022) |
| 4-(Methylsulfinyl)butyl ITC (sulforaphane) |
glucoraphanin (64) | no taste or flavor? (Bell et al., 2021) | anticarcinogenic activity (at least 17 types of cancer) (Kaiser et al., 2021) antimicrobial, antiangiogenic, antioxidant and cardioprotective activity (Mahn and Castillo, 2021; Mangla et al., 2021; Kamal et al., 2022) antiviral activity (including COVID-19) (Mahn and Castillo, 2021; Ordonez et al., 2022) anti-inflammatory activity (Wei et al., 2022) antidiabetic activity (Mangla et al., 2021; Wang et al., 2022a) Recovery and prevention of muscle atrophy (Vargas-Mendoza et al., 2022) Prevention of alcohol intolerance (Mahn and Castillo, 2021) Neuroprotective activity (Uddin et al., 2020) |
| 7-(Methylsulfinyl)heptyl ITC | glucoibarin (66) | unknown | anticarcinogenic activity (DeVito et al., 2000; Rose et al., 2005a) |
| 6-(Methylsulfinyl)hexyl ITC (hesperin) |
glucohesperin (67) | unknown | anticarcinogenic activity (Trio et al., 2017) anti-inflammatory and neuroprotective activity (Jaafaru et al., 2018) antiallergic activity (Yamada-Kato et al., 2012) |
| 8-(Methylsulfinyl)octyl ITC (hirsutin) |
glucohirsutin (69) | unknown | anti-inflammatory activity (DeVito et al., 2000; Rose et al., 2005b) |
| 5-(Methylsulfinyl)pentyl ITC (alyssin) |
glucoalyssin (72) | unknown | anticarcinogenic activity (Milczarek et al., 2018; Pocasap et al., 2019) antimicrobial activity (Nowicki et al., 2021) |
| 3-(Methylsulfinyl)propyl ITC (iberin) |
glucoiberin (73) | unknown | anticarcinogenic activity (Kim and Singh, 2009; Wang et al., 2016; Pocasap et al., 2019) antioxidant activity (Ernst et al., 2013) antimicrobial activity (Nowicki et al., 2021) |
| 4-(Methylthio)but-3-enyl ITC (raphasatin) |
glucoraphasatin (83) | pungent (Bell et al., 2018) | anticarcinogenic and antioxidant activity (Sundaram et al., 2022) |
| 4-(Methylthio)butyl ITC (erucin) |
glucoerucin (84) | radish-like, cabbage-like, mushroom-like (Kroener and Buettner, 2017; Wei et al., 2021) | anticarcinogenic activity (Singh et al., 2020) antioxidant activity (Cedrowski et al., 2021) anti-inflammatory, neuroprotective and cardioprotective activity (Jaafaru et al., 2018; Kamal et al., 2022) anti-obesity activity (Chae et al., 2015) antihypertensive activity (Martelli et al., 2020) |
| 7-(Methylthio)heptyl ITC | 7-(methylthio)heptyl GSL (87) | sweet, fatty, flowery, plastic, radish-like, pickle-like (Masuda et al., 1996; Kroener and Buettner, 2017) | antimicrobial activity (Shin et al., 2004) antiplatelet activity (Kumagai et al., 1994) |
| 6-(Methylthio)hexyl ITC (lesquerellin) |
glucolesquerellin (88) | sweet, fatty, acidic, radish-like (Masuda et al., 1996; Kroener and Buettner, 2017) | anticarcinogenic activity (Yano et al., 2000; Korenori et al., 2013) antiallergic activity (Yamada-Kato et al., 2012) antimicrobial activity (Shin et al., 2004) antiplatelet activity (Kumagai et al., 1994) |
| 5-(Methylthio)pentyl ITC (berteroin) |
glucoberteroin (94) | radish-like, pickle-like, mushroom-like (Masuda et al., 1996; Kroener and Buettner, 2017) | Prevention of non-alcoholic fatty liver disease (Kim et al., 2021) anti-inflammatory activity (Jung et al., 2014) anticarcinogenic activity (Kim and Singh, 2009) |
| 3-(Methylthio)propyl ITC (iberverin) |
glucoiberverin (95) | strongly radish-like, pungent, sulfurous, vegetative, horseradish-like, mushroom-like, gooseberry-like (Masuda et al., 1996; Kroener and Buettner, 2017; Bell et al., 2021; Wei et al., 2021) | anticarcinogenic activity (Kim and Singh, 2009; Wang et al., 2016) antioxidant activity (Ernst et al., 2013) antimicrobial activity (Nowicki et al., 2021) |
| 4-Pentenyl ITC | glucobrassicanapin (101) | green, acrid, pungent, peppery, sulfurous, musty, fragrant, mustard-like, horseradish-like (Masuda et al., 1996; Bell et al., 2018, 2021) | antimicrobial activity (Jang et al., 2010) |
| 2-Phenylethyl ITC | gluconasturtiin (105) | strongly radish-like, strong watercress aroma, pungent, green, horseradish-like, gooseberry-like, tingling sensation (Masuda et al., 1996; Kroener and Buettner, 2017; Bell et al., 2018, 2021; Wei et al., 2021) | anticarcinogenic activity (Sundaram et al., 2022) antioxidant, anti-inflammatory and antimicrobial activity (Coscueta et al., 2022) cardioprotective and neuroprotective activity (Kamal et al., 2022) |
| Allyl ITC | sinigrin (107) | strongly pungent, spicy, bitter, sulfurous, lachrymatory, mustard-like, garlic-like, onion-like, horseradish-like (Masuda et al., 1996; Kroener and Buettner, 2017; Bell et al., 2018, 2021) | anticarcinogenic, anti-inflammatory, antimicrobial, antioxidant, and wound-healing activity (Mazumder et al., 2016) antiviral activity (including COVID-19) (Guijarro-Real et al., 2021) antidiabetic and antihyperglycemic activity (Abbas et al., 2017; Zhang and Wang, 2022) potential goitrogenic effect (Lee and Kwon, 2015) |
| Allyl thiocyanate | sinigrin (107) | peppery, pungent, musty, sulfurous, mustard-like, horseradish-like (Bell et al., 2018, 2021) | potential goitrogenic effect (Lee and Kwon, 2015) |
The bold GSL numbers are placed in brackets after GSL names, and the order of GHPs is arranged by GSL numbers.
The detrimental effects of GHPs on human health are marked with an asterisk.
Goitrins include two enantiomers: (1) (S)-goitrin, also (S)-5-vinyloxazolidine-2-thione, which is formed from the hydrolysis of progoitrin (24R); and (2) (R)-goitrin, also (R)-5-vinyloxazolidine-2-thione, which is formed from the hydrolysis of epiprogoitrin (24S).
In addition to biosynthetic enzymes, MYB transcription factors have a global impact on biosynthetic gene expression and total GSL content (Figure 1A), endowing them with considerable engineering potential. MYB28, MYB29, and MYB76 regulate the expression of genes related to Met-derived aliphatic GSL biosynthesis (Sønderby et al., 2010a). Owing to their relative functional conservation among species and prominent roles in determining overall Met-derived aliphatic GSL content, MYB28 and MYB29 have been widely used for GSL engineering of different Brassicaceae species (Miao et al., 2021; Hölzl et al., 2023; Zhou et al., 2023). MYB76 mainly plays an auxiliary role in regulating secondary modification genes (Sønderby et al., 2010a). As an engineering target, it could be combined with MYB28, MYB29, or biosynthetic genes to make a more significant impact on GSL profiles in practical applications. For indole GSLs, MYB34, MYB51, and MYB122 directly regulate constitutive and pathogen-induced de novo biosynthesis of glucobrassicin and indirectly control accumulation of IAOx derivatives in A. thaliana (Frerigmann and Gigolashvili, 2014; Frerigmann et al., 2016). MYB34 and MYB51 are crucial for GSL biosynthesis in roots and shoots separately, whereas MYB122 plays an accessory role but becomes particularly significant in the context of environmental stress (Frerigmann and Gigolashvili, 2014). Overexpression of MYB34 and MYB122 gives rise to exaggerated IAA phenotypes, whereas that of MYB51 does not, mainly because of its role in activating core structure assembly genes both upstream and downstream of IAOx (Gigolashvili et al., 2007). Considering the importance of minimizing negative effects of target genes after mutagenesis, MYB51 appears to be a primary choice for GSL engineering. To obtain engineering targets for a wider range of species, there is a need to identify and characterize MYB34 and MYB122 orthologs that lack negative effects on normal plant growth or agronomic traits.
Simultaneous manipulation of genes involved in transport and biosynthesis to modify GSL spatiotemporal distribution
GSL biosynthesis and accumulation have distinct spatiotemporal patterns (Jørgensen et al., 2015). Once assembly of intact GSL molecules is complete, they are transported into neighboring specialized sulfur-rich cells (S-cells) for storage (Schuster et al., 2006), as well as accumulating in specific tissues and organs remote from their site of synthesis (Jørgensen et al., 2015). In recent years, identification and investigation of GSL transporters (GTRs) in A. thaliana have provided new information on how GSLs are retained, mobilized, and allocated throughout plant growth and development (Burow and Halkier, 2017; Xu et al., 2019; Hunziker et al., 2021).
GTR proteins belong to the nitrate transporter 1/peptide transporter (NRT1/PTR) family (also known as the NPF family), which is present in a wide range of plant taxa (Nour-Eldin et al., 2012). GTR1 (AtNPF2.10) and GTR2 (AtNPF2.11) were first identified and characterized in A. thaliana (Nour-Eldin et al., 2012), and their roles as GSL importers have been demonstrated in inter-organ transport (e.g., the seed loading process), as well as intra-organ/tissue retention and allocation of Met-derived aliphatic and indole GSLs based on symplastic or apoplastic pathways (Andersen et al., 2013; Andersen and Halkier, 2014; Madsen et al., 2014; Xu et al., 2016, 2019; Nintemann et al., 2018; Hunziker et al., 2019, 2021). Distinct from GTR1 and GTR2, the newly discovered GTR3 (AtNPF2.9) exhibits a strong preference for indole GSLs in A. thaliana (Jørgensen et al., 2017). It is capable of retaining indole GSLs in roots and, along with GTR1 and GTR2, participates in seed loading (Nour-Eldin et al., 2012), root exudation (Xu et al., 2016), intra-leaf distribution (Madsen et al., 2014), translocation for storage in S-cells of the stem (Xu et al., 2019), and transport of indole GSLs between roots, shoots, leaves, and flower stalks (Andersen et al., 2013; Andersen and Halkier, 2014; Jørgensen et al., 2017; Hunziker et al., 2021).
The specific ability of GTRs to alter GSL spatiotemporal distribution, especially in the seed GSL loading process, has aroused considerable interest in creating novel Brassicaceae crop cultivars that possess high GSL concentrations in vegetative parts and corresponding low concentrations in seeds (Nour-Eldin et al., 2017; Nambiar et al., 2021; He et al., 2022). Such germplasm has been generated by editing a GTR2 homolog on chromosome A06 in B. napus and, most importantly, it exhibited no impairment of normal plant growth (He et al., 2022). This result reflects the specific function of GTR orthologs in retaining GSLs in specific organs. In addition to the effects of GTRs, the effects of biosynthetic gene expression on GSL spatiotemporal distribution should not be neglected. It has been widely observed that the expression patterns of several biosynthetic genes make partial contributions to GSL phenotypes (Mikkelsen et al., 2000; Reintanz et al., 2001; Chen et al., 2003; Grubb et al., 2004, 2014; Redovniković et al., 2012; Kong et al., 2016). For example, MAM1 and MAM3 exhibited similar expression patterns during A. thaliana development, with distinct expression levels between tissues, although MAM1 exhibited significantly higher expression levels that corresponded to a higher concentration of short-chain GSLs compared with long-chain GSLs (Redovniković et al., 2012). A similar phenomenon was also observed in a study of FMOGS-OX6 and FMOGS-OX7 (Li et al., 2016). Transcription factors also influence the spatiotemporal distribution of GSLs; for example, MYB28 orthologs have been shown to maintain high levels of GSLs in leaves and low levels in seeds of A. thaliana and B. napus (Sønderby et al., 2010a; Liu et al., 2020). Therefore, observed GSL phenotypes appear at the very least to be co-determined by biosynthetic gene expression and GTRs (Tan et al., 2022), and their synergistic effects on GSL spatiotemporal distribution should be considered for engineering. It may be feasible to achieve a desired spatiotemporal GSL distribution in selected tissues and organs by molecular modification of GTRs, biosynthetic genes, and transcription factors, as well as by exploitation of unidentified GSL exporters in the future.
Selecting targets from unexploited hydrolysis genes for modification of GHPs
The well-known glucosinolate–myrosinase system, also known as the “mustard oil bomb,” plays an essential role in Brassicaceae plant defense. Myrosinases are stored in myrosin cells that are isolated from S-cells (Lüthy and Matile, 1984). When plant tissues are disrupted, myrosinases are released to interact with biologically inert GSLs and catalyze their degradation into bioactive isothiocyanates (ITCs) associated with multiple biological functions (Wittstock et al., 2016a). ITC is the major GHP type at neutral cell pH, and a hydroxy moiety at the C2 position leads to spontaneous cyclization to form oxazolidine-2-thiones (Wittstock and Halkier, 2002) (Figure 2A). The oxazolidine-2-thiones have received considerable scientific attention, as the dominant GSL (progoitrin) in classical varieties of B. napus exhibits a structural feature that results in an oxazolidine-2-thione product, (S)-goitrin (see section “generating multifunctional crops with defined GSL profiles and novel end uses”; Table 4). In A. thaliana, six genes of the β-thioglucoside glucohydrolase family (TGGs) that encode myrosinases have been identified (Figure 2A). TGG1 and TGG2 function in aerial plant parts, whereas TGG4 and TGG5 function in roots. However, TGG3 and TGG6 have been demonstrated to be pseudogenes and do not encode functional myrosinases (Zhang et al., 2002; Meijer et al., 2009). In addition, PEN2 encodes an atypical myrosinase beta-glucosidase 26 that is mainly involved in hydrolysis of glucobrassicin and 4-methoxyglucobrassicin and plays an essential role in the innate immune response (Bednarek et al., 2009).
Figure 2.
The production pathways of various GHPs catalyzed by myrosinases and governed by different specifier proteins.
The unstable intermediate aglycones produced by myrosinases are enclosed in square brackets.(A) Catalyzed by TGG-encoding myrosinases, an intermediate aglycone is produced, which can spontaneously convert into an isothiocyanate or further cyclize to form an oxazolidine-2-thione at typical cell pH.(B) The pathways of simple nitrile production in the presence of ESPs or NSPs.(C) The production of an epithionitrile from an alkenyl aglucone in the presence of ESPs.
(D) The production of a thiocyanate from an allyl, a 4-methylthiobutyl, or a benzyl aglycone in the presence of TFPs.
Information sources: Lüthy and Benn (1977); Bones and Rossiter (1996); Burow et al. (2006, 2008); Kuchernig et al. (2011); Agerbirk and Olsen (2012); Gumz et al. (2015); Wittstock et al. (2016a, 2016b).
Non-ITC GHPs can also be produced in the presence of specifier proteins at the expense of ITC formation in A. thaliana (Figures 2B and 2C). Nitrile-specifier proteins (NSPs) are solely responsible for the formation of simple nitriles, regardless of GSL side-chain structures (Figure 2B). Epithiospecifier proteins (ESPs) can promote formation of epithionitrile from alkenyl GSLs with double bonds at the end of side chains (Figure 2C). Apart from epithionitriles, simple nitriles can also be formed in the presence of ESPs, a process that is confined to the conversion of non-alkenyl and indole GSLs (Lambrix et al., 2001) (Figure 2B). In general, there are one ESP- and five NSP-encoding genes involved in GSL hydrolysis in A. thaliana. ESP1 can be expressed in aerial tissues and organs, although its expression patterns differ among various A. thaliana ecotypes, some of which lack ESPs (Burow et al., 2009). NSPs showed distinct organ-specific expression patterns, with NSP1 mainly functioning in seedlings and roots, NSP2 in seeds, and NSP3 in roots. The functions of NSP4 and NSP5 remain unclear (Wittstock and Burow, 2010; Wittstock et al., 2016b). Organic thiocyanates are possible GHPs formed in the presence of thiocyanate-forming proteins (TFPs), which have been found in a small subset of Brassicaceae species such as Thlaspi arvense (TaTFPs) and L. sativum (LsTFPs) (Burow et al., 2007; Kuchernig et al., 2011). To date, only three GSLs, sinigrin (allyl GSL), glucotropaeolin (benzyl GSL), and glucoerucin (4-(methylthio)butyl GSL), have been observed that could be stably converted into relevant thiocyanates (Figure 2D). LsTFP only converts glucotropaeolin into benzyl thiocyanate, and TaTFP only converts allyl GSL into allyl thiocyanate (Burow et al., 2007; Kuchernig et al., 2011; Gumz et al., 2015). In addition to thiocyanates, simple nitriles and epithionitriles can also be produced by TFPs upon myrosinase-catalyzed GSL hydrolysis (Burow et al., 2007; Kuchernig et al., 2011; Gumz et al., 2015).
Although specifier proteins are crucial for the formation of non-ITC GHPs, external variables such as pH and the presence of metal ions (especially Fe2+) can significantly affect GHP composition. Without specifier proteins, low pH (pH < 5) or addition of Fe2+ can result in the production of simple nitriles (Bones and Rossiter, 2006; Burow et al., 2006). When present, specifier protein activity can be enhanced by addition of Fe2+ or Fe3+ (ESPs), and changes in pH can also affect specifier protein activity, changing the final GHP composition (Burow et al., 2006, 2007; Kuchernig et al., 2011).
To the best of our knowledge, there have been few if any attempts to produce desired GHPs by engineering myrosinase- and specifier protein-encoded genes in different species. Although extensive exploration and manipulation of genes involved in GSL biosynthesis and transport have created desired GSL phenotypes in crop species, these alterations manifest themselves prior to the final biological functions and desired end uses. Hence, we suggest that manipulations of genes involved in GSL hydrolysis are likely to be more profitable because they are directly associated with desired end products and biological functions. Diverse GHPs could be overaccumulated in specific tissues and organs or mass-produced in vitro through gene or enzyme modification or under controlled conditions such as specific pHs and Fe2+/Fe3+ concentrations. These GHPs may provide substrates for final products that meet multiple future demands, either directly or after additional processing.
Generating multifunctional crops with defined GSL profiles and novel end uses
Prior to GSL engineering, it is critical to consider future demand for the development of multifunctional crops corresponding to available information on GSL and GHP functions. This may determine the selection of target crops and the roadmap for design of crop ideotypes (Figure 3A). The demand may be considered from two perspectives: food diversification and/or sustainable crop production. The former requires multifunctional crop ideotypes whose accumulated GSLs contribute pleasing flavors to the daily diet while exhibiting health-promoting or harmless properties for humans and livestock. The latter requires multifunctional crop ideotypes whose accumulated GSLs enhance specific plant resistance to endemic pests and pathogens, thus reducing pesticide use and assisting in the development of sustainable agriculture. Regardless of any possible technical challenges in practical application, the corresponding crop ideotypes are expected to be proposed first according to specific requirements (Figure 3A).
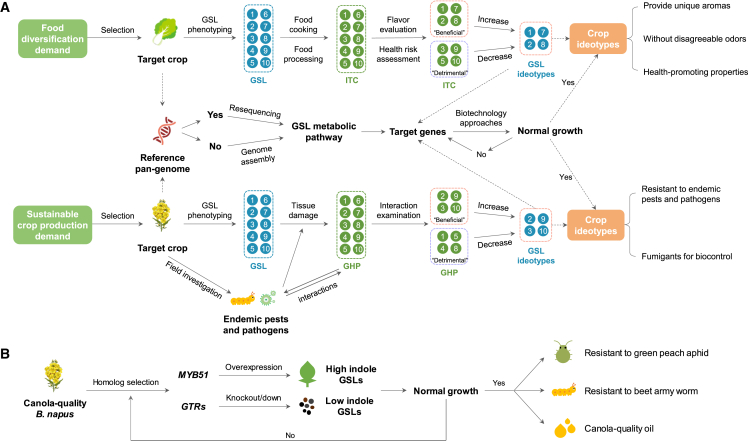
Figure 3.
Roadmap for generating Brassicaceae crop ideotypes with defined GSL profiles.
(A) Two routes for developing multifunctional crop ideotypes based on expected future demand for food diversification and sustainable crop production.
(B) A feasible molecular route for development of a canola-quality B. napus ideotype that is resistant to endemic green peach aphids.
Optional crop ideotypes based on the demand for food diversification
Food flavor is an important factor that contributes to consumer preference. Although the overall flavor of a Brassicaceae plant can derive from hundreds of odorants, it is widely believed that ITCs, the main type of GHP produced during food processing (Figure 2A), are the major distinguishing factors (Wieczorek et al., 2018; Bell et al., 2021) (Table 4). For example, considerable breeding effort has already been successful in modifying GSL profiles to reduce bitterness in Brussels sprouts (B. oleracea var. gemmifera) and ensure that they are more palatable (Fenwick et al., 1983; Van Doorn et al., 1998). More generally, there is scope for modifying GSL profiles in edible plant parts to create various crop ideotypes with distinct flavors using available biotechnological approaches. In the daily diet, pungent and spicy tastes of condiments made from sinigrin-rich species, such as wasabi, horseradish, and mustard, are sought after by large numbers of consumers (Table 4). Although the flavor of these three components is similar (contributed mainly by allyl ITC), wasabi tastes more moderate, fresher, and sweeter, partially owing to less gluconasturtiin (with a strongly radish-like and pungent odor) and the presence of ω-alkenyl ITCs, long-chain ω-methylthioalkyl, and ω-methylsulfinylalkyl ITCs (Uchida et al., 2012) (Table 4; Supplemental Table 1). Nevertheless, the extremely strict cultivation conditions and long growth period of wasabi make it scarce and costly for consumers (Depree et al., 1998). This leads to the prevalence of cheaper horseradish, mustard, and their by-products in the market because they are much easier to grow, manage, and process (Agneta et al., 2013). Given that the GSL profiles of wasabi and horseradish are already established (Supplemental Table 1), horseradish could be a target species for increasing the concentrations of GSLs associated with wasabi aroma through biotechnological approaches to attract interest from consumers. Other sinigrin-rich species, such as the less frequently cultivated black mustard and Ethiopian mustard (Table 1), also have the potential for genetic improvement to deliver wasabi-like flavors in their derived products.
For vegetable and oil species, the balance of flavor and health is more important (Bell and Wagstaff, 2017). To maximize the flavor of vegetables and minimize possible health risks from oil crops, there have been concerted efforts to reduce the concentrations of bitter, pungent, and health-threatening GSLs in specific organs and tissues. For example, until the 1970s, the levels (100–180 μmol/g dry weight) of GSLs in rapeseed (Wathelet et al., 1993; Chen et al., 2018; Wang et al., 2018; Tan et al., 2022), particularly progoitrin, were recognized as having a goitrogenic effect on livestock fed protein-rich seed meal, as well as a bitter and spicy taste following extraction of oil for human consumption (Table 4). This has driven the development and extensive cultivation of canola-quality B. napus as a major global temperate oil crop after major modifications of seed composition that reduced levels of GSL (10–30 μmol/g dry weight) and erucic acid (Stenfansson and Kondra, 1975; Wathelet et al., 1993; Chen et al., 2018). Increasing evidence indicates that numerous naturally occurring GHPs detected in important Brassicaceae vegetables (sulforaphane, allyl ITC, phenethyl ITC, benzyl ITC, products of indole-3-methyl ITC such as indole-3-carbinol, etc.) have health-promoting effects (e.g., anticarcinogenic, anti-inflammatory, neuroprotective, anti-obesity, and antiviral activity) and desirable aromas, such as sweet and flowery odors (Table 4). For this reason, there is scope for developing crop ideotypes with further overaccumulation of health-promoting GSLs while maintaining or enhancing overall desirable flavor. For example, it appears that sulforaphane exhibits no significant taste or flavor (Table 4), and it has been widely proven to be one of the most potent anticarcinogenic ITCs (Garcia-Oliveira et al., 2021). This underpins the very successful commercial release of the broccoli cultivar Beneforté, with highly enriched glucoraphanin concentrations (Sivapalan et al., 2018). Similar efforts have also been made—and could be further extended—in species such as rapeseed (Liu et al., 2012), kale (Araki et al., 2013; Qian et al., 2015), mustard (Augustine and Bisht, 2015a), and several subspecies of B. rapa (Liu et al., 2017), as well as underutilized rocket salad (Eruca sativa) (Table 1). Enriching GSLs in a species can provide health-promoting effects but may also result in disagreeable odors. For instance, gluconasturtiin is predominant in watercress edible organs (Table 1) and contributes anticarcinogenic, antimicrobial, and many other health-promoting effects, but it can also generate a high-intensity pungent odor and “tingling” taste sensation (Bell et al., 2021) (Table 4). Although the C6–C8 GSLs present in watercress contribute sweetish and flowery aromas (Tables 1 and 4; Supplemental Table 1), these may be masked because of their lower concentrations and the predominance of gluconasturtiin. Therefore, it may be feasible to enhance the overall aroma of watercress and attract more consumers by further enriching long-chain GSLs and reducing gluconasturtiin in edible organs. Alongside the intake of raw and fresh vegetables, a wider range of cooking methods may be explored to reduce undesirable odors prior to or during ingestion, or indeed following digestion (Nugrahedi et al., 2015; Marcinkowska et al., 2021; Wieczorek et al., 2021). Furthermore, our increasing understanding of aroma-active compounds in vegetable species is likely to promote development of additional aroma-associated GSLs and reduction of GSLs associated with negative odors (Engel et al., 2006; Guijarro-Real et al., 2020; Bell et al., 2021; Marcinkowska et al., 2021; Wei et al., 2021; Wieczorek et al., 2021). It should be noted that, for a few double-high oil landraces of B. napus, B. juncea, and B. rapa that are still grown in India and southwest China (Kang et al., 2021), the unique aroma of the derived oil is still preferred by local consumers (Yao et al., 2020; Liang et al., 2023). This inspires us to further dissect the unique aroma compounds that could be valuable for flavor enhancement of canola oil.
Optional crop ideotypes based on the demand for sustainable crop production
In addition to affecting food flavor, GSLs can also confer resistance in Brassicaceae species. Through the glucosinolate–myrosinase system, diverse GHPs are released to play dual roles in complex plant–herbivore and plant–pathogen interactions (Figure 2A–2D). In plant–herbivore studies, insect herbivores are generally classified as generalists or specialists, depending on their feeding habits on host plants. Some generalists tend to be repelled or poisoned by GHPs, whereas several GSL specialists tend to be stimulated to oviposit or feed on Brassicaceae plant tissues (Winde and Wittstock, 2011). Over the past decade, numerous studies have demonstrated these kinds of relationships between specific GSLs/GHPs and insect herbivores (Ahuja et al., 2009; Liu et al., 2021). Likewise, similar relationships have been observed between specific GSLs/GHPs and plant pathogens (Plaszkó et al., 2021, 2022). These observations have motivated ongoing biocontrol attempts to alter concentrations of GSLs that increase resistance to specific pests and pathogens (Table 3; Supplemental Table 2). Moreover, the use of chemical pesticides may have inadvertently increased because of diminished resistance to specific pests, pathogens, and even birds in double-low oil crop production. Although there is a lack of systematic data to support this possibility, bird damage has become severe in recent years, at least in China’s canola fields, and there is a significant negative correlation between Met-derived GSLs and levels of bird damage (Zhao et al., 2016). Comprehensive field investigations will be required to verify this relationship in more regions, where yield losses will hopefully be offset by use of GSL-enriched ideotype cultivars.
The release of volatile ITCs can contribute to exogenous biocontrol in agricultural production, and the advantages of biofumigation for control of soil-borne pests and pathogens and reduction of greenhouse gas emissions have been widely demonstrated (Sarwar et al., 1998; Lazzeri et al., 2010; Peng et al., 2021; Plaszkó et al., 2021). This has been achieved through Brassicaceae crop rotation or soil incorporation of GSL-rich green tissues or seed meal (Sarwar et al., 1998). More extensive adoption of biofumigation is expected to benefit from further enrichment of specific GSLs in specific crop tissues and organs, and subsequently in their by-products. In addition to exogenous application, enhancing endogenous resistance via overaccumulation of specific GSLs could be easily achieved for oil crops because the transport of increased GSLs from vegetative organs to seeds could be disturbed by knocking out GTR proteins, thereby reducing negative effects on oil quality (Nour-Eldin et al., 2017; Nambiar et al., 2021; He et al., 2022; Tan et al., 2022). However, for vegetable species whose aerial parts are usually edible, flavor may be negatively affected if resistance is obtained by enhancing endogenous GSL concentrations (Wieczorek et al., 2018). An alternative solution is to knock out/down TGG genes to reduce the production of bitter ITCs, an approach that may be useful for vegetables usually consumed raw, although it could result in higher susceptibility of target plants to specific pests or pathogens (e.g., green peach aphid) (Borgen et al., 2012).
A roadmap for crop ideotype design to address future demand
Based on the reviewed advances and discussion of genetic mechanisms that regulate GSL biosynthesis, transport, and hydrolysis, as well as functional associations with insect herbivores, pathogens, and human and livestock health, we propose an integrated roadmap for specifying crop ideotype options that may be delivered through sophisticated metabolic engineering using combined biotechnological approaches (Figure 3A). Our expectation is that future multifunctional Brassicaceae crops and their by-products will accumulate desirable GSLs in specific organs and tissues to more precisely meet future demands for food diversification and sustainable crop production.
According to the proposed roadmap (Figure 3A), once the target crop is selected, GSL phenotyping with existing analytical strategies is required for target organs or tissues. GHP profiles are expected to be deduced based on GSL profiles that correspond to different tissue exposures, such as food processing and cooking, or tissue disruption resulting from pathogen or pest damage. For food diversification, characterization of ITCs that contribute to flavor and assessments of associated health risks for humans and livestock are necessary to ascertain which ITCs primarily contribute beneficial effects (pleasing flavors, harmless or health-promoting effects) and which contribute detrimental effects (disagreeable flavors or harmful health effects). For sustainable crop production contexts, field investigation is required to establish an inventory of endemic pathogens and pests. This may be based on reviewing available information or on contextual examination of plant–herbivore, tritrophic, or plant–pathogen interactions, which would help to ascertain which GHPs primarily contribute beneficial effects (resistance to specific pests and pathogens) and which contribute detrimental effects (making plants susceptible or attractive to specific pests and pathogens). In addition to phenotypic evaluations, investigation of GSL metabolic pathways is also necessary. According to recent statistics, 58 Brassicaceae genomes have been sequenced, assembled, and published as of December 2022 (https://plabipd.de/plant_genomes_pa.ep), and pan-genome sequences of a few important species (e.g., B. rapa and B. napus) are also available (Song et al., 2020; Cai et al., 2021). With the published reference pan-genome sequences, detection of candidate GSL genes with existing high-throughput resequencing and bioinformatic tools is relatively straightforward. For species that lack reference genomes and pan-genome sequences, de novo genome assembly is now routine and is expected to be performed as a preliminary step. The complete GSL metabolic pathway could subsequently be generated via comparative genomic and pathway modelling tools. Such data frameworks, which combine information on GSL phenotypes and genotypes, will enable predictions of which GSLs can be increased and, correspondingly, which can be reduced. Finally, novel crop chemotypes can be defined and used as a template to design engineering approaches for development of target crop cultivars close to the designed ideotypes.
Biotechnological approaches for future GSL engineering
Although many ideotypes can be enumerated dependent on specific demands, the likelihood of achieving a specific GSL engineering goal will depend on the feasibility and complexity of applying available biotechnological approaches. Gene editing or transgenic approaches are available for GSL engineering, which have been demonstrated for most Brassica species (Li et al., 2021a; Neequaye et al., 2021; He et al., 2022; Tan et al., 2022) (Tables 2 and 3; Supplemental Table 2), and are expected to become available for a wider range of Brassicaceae species (Miao et al., 2021; Neequaye et al., 2021; Kim et al., 2022). In future GSL engineering work, selection of an appropriate combination of target genes involved in GSL biosynthesis, transport, and hydrolysis is an important preliminary step in the design of crop ideotypes. Based on this step and the proposed roadmap (Figure 3A), the most feasible route may be chosen for practical breeding. For example, green peach aphid is one of the most common generalist herbivores and usually feeds on Brassica species (Desneux et al., 2006). To inhibit aphid infestation in canola fields, MYB51 and GTRs could be engineering targets for development of a more resistant crop ideotype (Figure 3B). Overexpression of MYB51 homologs enhances indole GSL concentrations without affecting normal plant growth (Gigolashvili et al., 2007) and is expected to increase resistance to green peach aphids and another generalist herbivore, the beet armyworm (Spodoptera exigua) (Kim et al., 2008; Borgen et al., 2012). However, enrichment of indole GSLs in seeds may negatively affect the flavor of canola oil (Wieczorek et al., 2018). The knockout/knockdown of GTR homologs is expected to resolve this issue by disturbing GSL loading in the seed (Nour-Eldin et al., 2017; Nambiar et al., 2021; He et al., 2022; Tan et al., 2022; Hölzl et al., 2023). Weakened performance of green peach aphids on GTR knockout plants has also been observed in A. thaliana (Madsen et al., 2015). The final canola ideotype is expected to exhibit normal growth and enhanced resistance to green peach aphids in vegetative organs and low GSL levels in seeds, thus maintaining the quality of canola oil (Figure 3B). If normal plant growth is negatively affected after gene mutation, especially for GTR genes, other appropriate orthologs could be selected to evaluate plant growth effects (Figure 3B). In this route, MYB51 could be replaced by other indole GSL biosynthesis genes such as CYP79B2, CYP83A1, UGT74B1, SOT16, CYP81F2, MYB34, MYB122, and even the newly characterized WRKY33, whose overexpression may also drive accumulation of indole GSLs and potentially increase resistance to green peach aphids, other pests, and/or pathogens (Frerigmann and Gigolashvili, 2014; Tao et al., 2022) (Table 3; Supplemental Table 2). Beyond manipulation of endogenous GSL genes, introgression of exogenous genes into target species by transformation can also promote overaccumulation of novel desirable GSLs in Brassicaceae species. For example, ectopic expression of sorghum (Sorghum bicolor) CYP79A1 and CYP71E1 caused overaccumulation of the novel GSL sinalbin in transgenic A. thaliana lines (Bak et al., 1999, 2000; Petersen et al., 2001), and plant morphological phenotypes and contents of other GSLs remained unaffected in CYP79A1 transgenic lines (Kristensen et al., 2005). This approach relies on the broad substrate specificity of CYP79 enzymes in numerous species; these enzymes can catalyze the conversion of amino acids into corresponding oximes and are expected to be used in GSL engineering of more Brassicaceae species (Bak et al., 2006).
There remain several limitations and obstacles to be surmounted in the practical application of molecular engineering approaches. First, current market restrictions for genetically modified crops are strict, hindering their adoption, although such barriers may be reduced for gene editing (Pixley et al., 2022). Second, the extent to which GSL and GHP profiles can be altered by current transgenic or gene-editing approaches is still limited. This could be improved by protein engineering, which is a combination of rational design, directed evolution, and many other methods that optimize the properties of enzymes in a more controlled, targeted, and accurate manner (Kapoor et al., 2017). Proteomic engineering has been used to alter the substrate specificity of MAM synthases toward different GSLs through in planta and in vivo site-directed mutagenesis of key active residues, an effort made possible by the recent biophysical dissection of MAM synthase crystal structures in A. thaliana and B. juncea (Kumar et al., 2019; Petersen et al., 2019). A similar mutagenesis study was performed on GTR1, assisted by a bioinformatics protocol designed to identify the potential binding site of GSLs more efficiently than with biophysical methods (Peña-Varas et al., 2022). Therefore, as a potentially powerful method, protein engineering is expected to be applied in more GSL metabolic contexts, together with integration of further developed multi-omics techniques. Third, possible negative effects of gene modification may affect the selection of target genes and may be difficult to overcome. Fourth, genes usually exist as multiple copies within complex Brassicaceae genomes, and multiple evaluations of specific paralogs are likely to be required to determine which are functional and less likely to perturb normal plant growth. Moreover, interactions among GSL genes may differ in complex genomes. In A. thaliana, for example, AtMYB28 has been shown to be a positive regulator of AtAOP2 (Sønderby et al., 2010a), whereas three homologs encoding BrMYB28 in B. rapa act as negative regulators of BrAOP2 (Seo et al., 2016). Even in allotetraploid B. napus, RNAi silencing of MAM genes surprisingly induced biosynthesis of C3 Met-derived aliphatic GSLs (Liu et al., 2011). We expect that this problem will be progressively overcome as GSL genetic mechanisms in complex genomes are more fully elucidated in the future.
Apart from molecular approaches, traditional intra- and interspecific hybridization is still a powerful and universal approach for generating diverse crop ideotypes. Especially for field selection, GSL diversity can be increased in target crops by gene introgression, which also enables the co-selection of GSL traits and other important agronomic traits. For example, GSL diversity was enriched in resynthesized allotetraploid B. napus using different genome resynthesis approaches in various combinations (Kräling et al., 1990; Hill et al., 2003; Cleemput and Becker, 2012). In a resynthesized B. napus (AACC) germplasm pool developed by interspecific hybridization between B. rapa (AA) and Brassica carinata (BBCC), B-genome-related sinigrin is introgressed in both leaves and seeds of most accessions (Table 1; Hu et al., 2019; Qin et al., 2020). Where health-promoting GSLs exist in one species, such as glucoraphanin in broccoli, these could be further enriched to achieve higher content via recombination. Beneforté is an F1 hybrid developed by crossing broccoli with the related wild species B. villosa (Traka et al., 2013). The introgressed B. villosa MYB28 allele leads to a significant increase in glucoraphanin in Beneforté varieties (Sivapalan et al., 2018). Health-promoting GSLs absent in one species may also be significantly enriched to detectable levels by recombination of alleles within or between homoeologous chromosomes. In the A-genome diploid species B. rapa, for example, glucoraphanin is almost undetectable owing to the presence of three functional BrAOP2 genes (Zhang et al., 2015a). Through a marker-assisted backcrossing process, two BrAOP genes (BrAOP2.2 and BrAOP2.3) were replaced with non-functional counterparts, leading to a significant increase in glucoraphanin content (Liu et al., 2017). Following hybridization, it is crucial to determine an appropriate method of artificial selection that ensures the retention of specific alleles. This can be accelerated using a traditional marker-assisted approach or by using genomic selection and other bioinformatic analysis techniques that can detect more subtle connections between genotypes and phenotypes (Spindel and McCouch, 2016).
Overall, we hope that the collective knowledge and discussion presented in this review will stimulate development of a comprehensive toolbox and a positive outlook for sophisticated engineering of GSL profiles in a wider range of Brassicaceae species. As additional genes are waiting to be identified and their roles resolved, novel engineering targets will continue to emerge (Harun et al., 2021). This will increase the options available for modifying GSL profiles by gene-editing or transgenic approaches. There are several more potential directions for future GSL engineering that we cannot fully capture here, such as control of greenhouse growth conditions (Bell and Wagstaff, 2017) and postharvest storage, cooking, and processing protocols to obtain desired GSLs and GHPs (Wu et al., 2021a); optimization of sulfur fertilizer supply to enrich GSL accumulation (Borpatragohain et al., 2019); production of GSLs by in vitro plant cell cultures (Petersen et al., 2018); and induction of GSL production by exogenous phytohormones (Wiesner et al., 2013a; Augustine and Bisht, 2015b; Wang et al., 2015) or radiation (Banerjee et al., 2016). We believe that combining such explorations will facilitate the accessibility of GSL engineering in crop breeding, ultimately enabling development of multifunctional Brassicaceae crops to meet sustainable demand in the future.
Prospects
Since the initial discovery of GSLs in the early nineteenth century, our understanding of GSLs and GHPs has advanced with available chemical, genetic, and genomic analysis technologies. This has led to the thorough elucidation of variations in GSL structure, distribution, biosynthesis, hydrolysis, and transport, providing great potential for knowledge-based manipulation of GSLs and GHPs in different Brassicaceae species. However, the discussion in this review is still limited to a small number of species that have been intensively selected and domesticated. In fact, the genetic diversity harbored within the entire Brassicaceae family is far beyond our current understanding (Hu et al., 2021). Wild species are waiting to be exploited and their abundant GSL variations and other desirable traits introgressed into current major crops. They may even be used directly as novel crops through the establishment of an efficient de novo domestication strategy (Yu et al., 2021). At least some wild edible species, such as wall rocket (Diplotaxis erucoides), are expected to be developed as new crops that contain desirable GSLs (Clemente-Villalba et al., 2020; Guijarro-Real et al., 2020). Apart from exploitation of novel germplasm, advances in technology will expand the reach of GSL engineering. In particular, it is necessary to establish the feasibility of combined tissue-specific targeting of GSLs that contribute separately to human and livestock nutrition, health, and plant defense. This approach need not be applied solely to Brassicaceae species, because systems for the de novo production of desirable GSLs have been established in GSL-free species (e.g., potato and tobacco) (González-Romero et al., 2021; Wang et al., 2021). The underlying driver for accumulating all the relevant knowledge is the demand to produce more nutritious and healthier food for a growing human population in a sustainable manner with a reduced reliance on synthetic pesticides.
Funding
This work was supported by the National Natural Science Foundation of China (32171982 and 31970564).
Author contributions
J.Z. conceptualized the manuscript. H.Q. drafted the manuscript and prepared all the tables, figures, and supplemental tables. J.Z. and G.J.K. drafted part of the manuscript and revised the manuscript, tables, figures, and supplemental tables. P.B. revised the manuscript, tables, figures, and supplemental tables. All authors approved the final manuscript for submission.
Acknowledgments
We acknowledge Dr. Jinling Meng and Dr. Matthew N. Nelson for giving valuable suggestions on the manuscript. Some of the icons used in Figure 3 were purchased and cited from the website https://www.51yuansu.com/. No conflict of interest is declared.
Published: February 23, 2023
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Supplemental information is available at Plant Communications Online.
Supplemental information
References
- Abbas Q., Hassan M., Raza H., Kim S.J., Chung K.W., Kim G.H., Seo S.Y. In vitro, in vivo and in silico anti-hyperglycemic inhibition by sinigrin. Asian Pac. J. Trop. Med. 2017;10:372–379. doi: 10.1016/j.apjtm.2017.03.019. [DOI] [PubMed] [Google Scholar]
- Agerbirk N., Olsen C.E. Glucosinolate structures in evolution. Phytochemistry. 2012;77:16–45. doi: 10.1016/j.phytochem.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Agerbirk N., Olsen C.E. Glucosinolate hydrolysis products in the crucifer Barbarea vulgaris include a thiazolidine-2-one from a specific phenolic isomer as well as oxazolidine-2-thiones. Phytochemistry. 2015;115:143–151. doi: 10.1016/j.phytochem.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Agerbirk N., Hansen C.C., Kiefer C., Hauser T.P., Ørgaard M., Asmussen Lange C.B., Cipollini D., Koch M.A. Comparison of glucosinolate diversity in the crucifer tribe Cardamineae and the remaining order Brassicales highlights repetitive evolutionary loss and gain of biosynthetic steps. Phytochemistry. 2021;185:112668. doi: 10.1016/j.phytochem.2021.112668. [DOI] [PubMed] [Google Scholar]
- Agerbirk N., Olsen C.E., Cipollini D., Ørgaard M., Linde-Laursen I., Chew F.S. Specific glucosinolate analysis reveals variable levels of epimeric glucobarbarins, dietary precursors of 5-phenyloxazolidine-2-thiones, in watercress types with contrasting chromosome numbers. J. Agric. Food Chem. 2014;62:9586–9596. doi: 10.1021/jf5032795. [DOI] [PubMed] [Google Scholar]
- Agneta R., Möllers C., Rivelli A.R. Horseradish (Armoracia rusticana), a neglected medical and condiment species with a relevant glucosinolate profile: a review. Genet. Resour. Crop Evol. 2013;60:1923–1943. [Google Scholar]
- Ahuja I., Kissen R., Bones A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012;17:73–90. doi: 10.1016/j.tplants.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Ahuja I., Rohloff J., Bones A.M. Defence mechanisms of Brassicaceae: implications for plant-insect interactions and potential for integrated pest management. Sustain. Agric. 2009;2:623–670. [Google Scholar]
- Andersen T.G., Halkier B.A. Upon bolting the GTR1 and GTR2 transporters mediate transport of glucosinolates to the inflorescence rather than roots. Plant Signal. Behav. 2014;9:277400-10. doi: 10.4161/psb.27740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen T.G., Nour-Eldin H.H., Fuller V.L., Olsen C.E., Burow M., Halkier B.A. Integration of biosynthesis and long-distance transport establish organ-specific glucosinolate profiles in vegetative Arabidopsis. Plant Cell. 2013;25:3133–3145. doi: 10.1105/tpc.113.110890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andini S., Dekker P., Gruppen H., Araya-Cloutier C., Vincken J.P. Modulation of glucosinolate composition in Brassicaceae seeds by germination and fungal elicitation. J. Agric. Food Chem. 2019;67:12770–12779. doi: 10.1021/acs.jafc.9b05771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki R., Hasumi A., Nishizawa O.I., Sasaki K., Kuwahara A., Sawada Y., Totoki Y., Toyoda A., Sakaki Y., Li Y., et al. Novel bioresources for studies of Brassica oleracea: identification of a kale MYB transcription factor responsible for glucosinolate production. Plant Biotechnol. J. 2013;11:1017–1027. doi: 10.1111/pbi.12095. [DOI] [PubMed] [Google Scholar]
- Arias T., Niederhuth C.E., McSteen P., Pires J.C. The molecular basis of kale domestication: transcriptional profiling of developing leaves provides new insights into the evolution of a Brassica oleracea vegetative morphotype. Front. Plant Sci. 2021;12:637115. doi: 10.3389/fpls.2021.637115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine R., Bisht N.C. Biofortification of oilseed Brassica juncea with the anti-cancer compound glucoraphanin by suppressing GSL-ALK gene family. Sci. Rep. 2015;5:18005. doi: 10.1038/srep18005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine R., Bisht N.C. Biotic elicitors and mechanical damage modulate glucosinolate accumulation by co-ordinated interplay of glucosinolate biosynthesis regulators in polyploid Brassica juncea. Phytochemistry. 2015;117:43–50. doi: 10.1016/j.phytochem.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Augustine R., Majee M., Gershenzon J., Bisht N.C. Four genes encoding MYB28, a major transcriptional regulator of the aliphatic glucosinolate pathway, are differentially expressed in the allopolyploid Brassica juncea. J. Exp. Bot. 2013;64:4907–4921. doi: 10.1093/jxb/ert280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine R., Mukhopadhyay A., Bisht N.C. Targeted silencing of BjMYB28 transcription factor gene directs development of low glucosinolate lines in oilseed Brassica juncea. Plant Biotechnol. J. 2013;11:855–866. doi: 10.1111/pbi.12078. [DOI] [PubMed] [Google Scholar]
- Bak S., Feyereisen R. The involvement of two P450 enzymes, CYP83B1 and CYP83A1, in auxin homeostasis and glucosinolate biosynthesis. Plant Physiol. 2001;127:108–118. doi: 10.1104/pp.127.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak S., Olsen C.E., Halkier B.A., Møller B.L. Transgenic tobacco and Arabidopsis plants expressing the two multifunctional sorghum cytochrome P450 enzymes, CYP79A1 and CYP71E1, are cyanogenic and accumulate metabolites derived from intermediates in dhurrin biosynthesis. Plant Physiol. 2000;123:1437–1448. doi: 10.1104/pp.123.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak S., Olsen C.E., Petersen B.L., Møller B.L., Halkier B.A. Metabolic engineering of p-hydroxybenzylglucosinolate in Arabidopsis by expression of the cyanogenic CYP79A1 from Sorghum bicolor. Plant J. 1999;20:663–671. doi: 10.1046/j.1365-313x.1999.00642.x. [DOI] [PubMed] [Google Scholar]
- Bak S., Paquette S.M., Morant M., Morant A.V., Saito S., Bjarnholt N., Zagrobelny M., Jørgensen K., Osmani S., Simonsen H.T., et al. Cyanogenic glycosides: a case study for evolution and application of cytochromes P450. Phytochemistry Rev. 2006;5:309–329. [Google Scholar]
- Bak S., Tax F.E., Feldmann K.A., Galbraith D.W., Feyereisen R. CYP83B1, a cytochrome P450 at the metabolic branch point in auxin and indole glucosinolate biosynthesis in Arabidopsis. Plant Cell. 2001;13:101–111. doi: 10.1105/tpc.13.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A., Rai A.N., Penna S., Variyar P.S. Aliphatic glucosinolate synthesis and gene expression changes in gamma-irradiated cabbage. Food Chem. 2016;209:99–103. doi: 10.1016/j.foodchem.2016.04.022. [DOI] [PubMed] [Google Scholar]
- Barco B., Clay N.K. Evolution of glucosinolate diversity via whole-genome duplications, gene rearrangements, and substrate promiscuity. Annu. Rev. Plant Biol. 2019;70:585–604. doi: 10.1146/annurev-arplant-050718-100152. [DOI] [PubMed] [Google Scholar]
- Bednarek P., Piślewska-Bednarek M., Svatoš A., Schneider B., Doubský J., Mansurova M., Humphry M., Consonni C., Panstruga R., Sanchez-Vallet A., et al. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science. 2009;323:101–106. doi: 10.1126/science.1163732. [DOI] [PubMed] [Google Scholar]
- Bednarek P., Piślewska-Bednarek M., Ver Loren van Themaat E., Maddula R.K., Svatoš A., Schulze-Lefert P. Conservation and clade-specific diversification of pathogen-inducible tryptophan and indole glucosinolate metabolism in Arabidopsis thaliana relatives. New Phytol. 2011;192:713–726. doi: 10.1111/j.1469-8137.2011.03824.x. [DOI] [PubMed] [Google Scholar]
- Bell L., Wagstaff C. Enhancement of glucosinolate and isothiocyanate profiles in Brassicaceae crops: addressing challenges in breeding for cultivation, storage, and consumer-related traits. J. Agric. Food Chem. 2017;65:9379–9403. doi: 10.1021/acs.jafc.7b03628. [DOI] [PubMed] [Google Scholar]
- Bell L., Chadwick M., Puranik M., Tudor R., Methven L., Kennedy S., Wagstaff C. The Eruca sativa genome and transcriptome: a targeted analysis of sulfur metabolism and glucosinolate biosynthesis pre and postharvest. Front. Plant Sci. 2020;11:525102. doi: 10.3389/fpls.2020.525102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell L., Kitsopanou E., Oloyede O.O., Lignou S. Important odorants of four Brassicaceae species, and discrepancies between glucosinolate profiles and observed hydrolysis products. Foods. 2021;10:1055. doi: 10.3390/foods10051055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell L., Oloyede O.O., Lignou S., Wagstaff C., Methven L. Taste and flavor perceptions of glucosinolates, isothiocyanates, and related compounds. Mol. Nutr. Food Res. 2018;62:1700990. doi: 10.1002/mnfr.201700990. [DOI] [PubMed] [Google Scholar]
- Bellostas N., Sørensen J.C., Sørensen H. Profiling glucosinolates in vegetative and reproductive tissues of four Brassica species of the U-triangle for their biofumigation potential. J. Sci. Food Agric. 2007;87:1586–1594. [Google Scholar]
- Bhandari S.R., Jo J.S., Lee J.G. Comparison of glucosinolate profiles in different tissues of nine Brassica crops. Molecules. 2015;20:15827–15841. doi: 10.3390/molecules200915827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blažević I., Montaut S., Burčul F., Olsen C.E., Burow M., Rollin P., Agerbirk N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry. 2020;169:112100. doi: 10.1016/j.phytochem.2019.112100. [DOI] [PubMed] [Google Scholar]
- Bones A.M., Rossiter J.T. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol. Plantarum. 1996;97:194–208. [Google Scholar]
- Bones A.M., Rossiter J.T. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry. 2006;67:1053–1067. doi: 10.1016/j.phytochem.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Borgen B.H., Ahuja I., Thangstad O.P., Honne B.I., Rohloff J., Rossiter J.T., Bones A.M. “Myrosin cells” are not a prerequisite for aphid feeding on oilseed rape (Brassica napus) but affect host plant preferences. Plant Biol. 2012;14:894–904. doi: 10.1111/j.1438-8677.2012.00578.x. [DOI] [PubMed] [Google Scholar]
- Borpatragohain P., Rose T.J., King G.J. Fire and brimstone: molecular interactions between sulfur and glucosinolate biosynthesis in model and crop Brassicaceae. Front. Plant Sci. 2016;7:1735. doi: 10.3389/fpls.2016.01735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borpatragohain P., Rose T.J., Liu L., Raymond C.A., Barkla B.J., King G.J. Seed glucosinolate yield is maximized by higher rates of sulfur nutrition than required for seed yield in condiment mustard (Brassica juncea L.) PLoS One. 2019;14:e0213429. doi: 10.1371/journal.pone.0213429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd L.A., McCann M.J., Hashim Y., Bennett R.N., Gill C.I.R., Rowland I.R. Assessment of the anti-genotoxic, anti-proliferative, and anti-metastatic potential of crude watercress extract in human colon cancer cells. Nutr. Cancer. 2006;55:232–241. doi: 10.1207/s15327914nc5502_15. [DOI] [PubMed] [Google Scholar]
- Branca F., Chiarenza G.L., Cavallaro C., Gu H., Zhao Z., Tribulato A. Diversity of Sicilian broccoli (Brassica oleracea var. italica) and cauliflower (Brassica oleracea var. botrytis) landraces and their distinctive bio-morphological, antioxidant, and genetic traits. Genet. Resour. Crop Evol. 2018;65:485–502. [Google Scholar]
- Brown P.D., Tokuhisa J.G., Reichelt M., Gershenzon J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry. 2003;62:471–481. doi: 10.1016/s0031-9422(02)00549-6. [DOI] [PubMed] [Google Scholar]
- Bryce C.M., Davis M.S., Gompper M.E., Hurt A., Koster J.M., Larson G., Ostrander E.A., Udell M.A.R., Urfer S., Wirsing A.J., et al. Biology’s best friend: bridging disciplinary gaps to advance canine science. Integr. Comp. Biol. 2021;61:76–92. [Google Scholar]
- Burow M., Halkier B.A. How does a plant orchestrate defense in time and space? Using glucosinolates in Arabidopsis as case study. Curr. Opin. Plant Biol. 2017;38:142–147. doi: 10.1016/j.pbi.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Burow M., Bergner A., Gershenzon J., Wittstock U. Glucosinolate hydrolysis in Lepidium sativum - identification of the thiocyanate-forming protein. Plant Mol. Biol. 2007;63:49–61. doi: 10.1007/s11103-006-9071-5. [DOI] [PubMed] [Google Scholar]
- Burow M., Markert J., Gershenzon J., Wittstock U. Comparative biochemical characterization of nitrile-forming proteins from plants and insects that alter myrosinase-catalysed hydrolysis of glucosinolates. FEBS J. 2006;273:2432–2446. doi: 10.1111/j.1742-4658.2006.05252.x. [DOI] [PubMed] [Google Scholar]
- Burow M., Losansky A., Müller R., Plock A., Kliebenstein D.J., Wittstock U. The genetic basis of constitutive and herbivore-induced ESP-independent nitrile formation in Arabidopsis. Plant Physiol. 2009;149:561–574. doi: 10.1104/pp.108.130732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burow M., Zhang Z.Y., Ober J.A., Lambrix V.M., Wittstock U., Gershenzon J., Kliebenstein D.J. ESP and ESM1 mediate indol-3-acetonitrile production from indol-3-ylmethyl glucosinolate in Arabidopsis. Phytochemistry. 2008;69:663–671. doi: 10.1016/j.phytochem.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Cai X., Chang L., Zhang T., Chen H., Zhang L., Lin R., Liang J., Wu J., Freeling M., Wang X. Impacts of allopolyploidization and structural variation on intraspecific diversification in Brassica rapa. Genome Biol. 2021;22:166. doi: 10.1186/s13059-021-02383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedrowski J., Dąbrowa K., Przybylski P., Krogul-Sobczak A., Litwinienko G. Antioxidant activity of two edible isothiocyanates: sulforaphane and erucin is due to their thermal decomposition to sulfenic acids and methylsulfinyl radicals. Food Chem. 2021;353:129213. doi: 10.1016/j.foodchem.2021.129213. [DOI] [PubMed] [Google Scholar]
- Centofanti F., Alonzi T., Latini A., Spitalieri P., Murdocca M., Chen X., Cui W., Shang Q., Goletti D., Shi Y., et al. Indole-3-carbinol in vitro antiviral activity against SARS-Cov-2 virus and in vivo toxicity. Cell Death Dis. 2022;8:491. doi: 10.1038/s41420-022-01280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae S.Y., Seo S.G., Yang H., Yu J.G., Suk S.J., Jung E.S., Ji H., Kwon J.Y., Lee H.J., Lee K.W. Anti-adipogenic effect of erucin in early stage of adipogenesis by regulating Ras activity in 3T3-L1 preadipocytes. J. Funct.Foods. 2015;19:700–709. [Google Scholar]
- Chalhoub B., Denoeud F., Liu S., Parkin I.A.P., Tang H., Wang X., Chiquet J., Belcram H., Tong C., Samans B., et al. Early allopolyploid evolution in the post-neolithic Brassica napus oilseed genome. Science. 2014;345:950–953. doi: 10.1126/science.1253435. [DOI] [PubMed] [Google Scholar]
- Chen B., Xu K., Li H., Gao G., Yan G., Qiao J., Wu X. Evaluation of quality traits and their genetic variation in global collections of Brassica napus L. Plant Genet. Resour. 2018;16:146–155. [Google Scholar]
- Chen S., Glawischnig E., Jørgensen K., Naur P., Jørgensen B., Olsen C.E., Hansen C.H., Rasmussen H., Pickett J.A., Halkier B.A. CYP79F1 and CYP79F2 have distinct functions in the biosynthesis of aliphatic glucosinolates in Arabidopsis. Plant J. 2003;33:923–937. doi: 10.1046/j.1365-313x.2003.01679.x. [DOI] [PubMed] [Google Scholar]
- Cheng F., Sun R., Hou X., Zheng H., Zhang F., Zhang Y., Liu B., Liang J., Zhuang M., Liu Y., et al. Subgenome parallel selection is associated with morphotype diversification and convergent crop domestication in Brassica rapa and Brassica oleracea. Nat. Genet. 2016;48:1218–1224. doi: 10.1038/ng.3634. [DOI] [PubMed] [Google Scholar]
- Chopra R., Johnson E.B., Emenecker R., Cahoon E.B., Lyons J., Kliebenstein D.J., Daniels E., Dorn K.M., Esfahanian M., Folstad N., et al. Identification and stacking of crucial traits required for the domestication of pennycress. Nat. Food. 2020;1:84–91. [Google Scholar]
- Chun J.H., Arasu M.V., Lim Y.P., Kim S.J. Variation of major glucosinolates in different varieties and lines of rocket salad. Hortic. Environ. Biotechnol. 2013;54:206–213. [Google Scholar]
- Clay N.K., Adio A.M., Denoux C., Jander G., Ausubel F.M. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science. 2009;323:95–101. doi: 10.1126/science.1164627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleemput S., Becker H.C. Genetic variation in leaf and stem glucosinolates in resynthesized lines of winter rapeseed (Brassica napus L.) Genet. Resour. Crop Evol. 2012;59:539–546. [Google Scholar]
- Clemente-Villalba J., Ariza D., García-Garví J.M., Sánchez-Bravo P., Noguera-Artiaga L., Issa-Issa H., Hernández F., Carbonell-Barrachina Á.A. Characterization and potential use of Diplotaxis erucoides as food ingredient for a sustainable modern cuisine and comparison with commercial mustards and wasabis. Eur. Food Res. Technol. 2020;246:1429–1438. [Google Scholar]
- Coscueta E.R., Sousa A.S., Reis C.A., Pintado M.M. Phenylethyl isothiocyanate: a bioactive agent for gastrointestinal health. Molecules. 2022;27:794. doi: 10.3390/molecules27030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depree J.A., Howard T.M., Savage G.P. Flavour and pharmaceutical properties of the volatile sulphur compounds of wasabi (Wasabia japonica) Food Res. Int. 1998;31:329–337. [Google Scholar]
- Desneux N., Rabasse J.M., Ballanger Y., Kaiser L. Parasitism of canola aphids in France in autumn. J. Pest Sci. 2006;79:95–102. [Google Scholar]
- DeVito W.J., Stone S., Mori K., Shamgochian M. Ethanol inhibits prolactin- and tumor necrosis factor-α-but not gamma interferon-induced expression of intercellular adhesion molecule-1 in human astrocytoma cells. J. Cell. Biochem. 2000;77:455–464. [PubMed] [Google Scholar]
- Edger P.P., Hall J.C., Harkess A., Tang M., Coombs J., Mohammadin S., Schranz M.E., Xiong Z., Leebens-Mack J., Meyers B.C., et al. Brassicales phylogeny inferred from 72 plastid genes: a reanalysis of the phylogenetic localization of two paleopolyploid events and origin of novel chemical defenses. Am. J. Bot. 2018;105:463–469. doi: 10.1002/ajb2.1040. [DOI] [PubMed] [Google Scholar]
- Edger P.P., Heidel-Fischer H.M., Bekaert M., Rota J., Glöckner G., Platts A.E., Heckel D.G., Der J.P., Wafula E.K., Tang M., et al. The butterfly plant arms-race escalated by gene and genome duplications. Proc. Natl. Acad. Sci. USA. 2015;112:8362–8366. doi: 10.1073/pnas.1503926112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel E., Martin N., Issanchou S. Sensitivity to allyl isothiocyanate, dimethyl trisulfide, sinigrin, and cooked cauliflower consumption. Appetite. 2006;46:263–269. doi: 10.1016/j.appet.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Ernst I.M.A., Palani K., Esatbeyoglu T., Schwarz K., Rimbach G. Synthesis and Nrf2-inducing activity of the isothiocyanates iberverin, iberin and cheirolin. Pharmacol. Res. 2013;70:155–162. doi: 10.1016/j.phrs.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Fahey J.W., Zalcmann A.T., Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- Felker P., Bunch R., Leung A.M. Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in Brassica vegetables, and associated potential risk for hypothyroidism. Nutr. Rev. 2016;74:248–258. doi: 10.1093/nutrit/nuv110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick G.R., Griffiths N.M., Heaney R.K. Bitterness in brussels sprouts (Brassica oleracea L. var. gemmifera): the role of glucosinolates and their breakdown products. J. Sci. Food Agric. 1983;34:73–80. [Google Scholar]
- Field B., Cardon G., Traka M., Botterman J., Vancanneyt G., Mithen R. Glucosinolate and amino acid biosynthesis in Arabidopsis. Plant Physiol. 2004;135:828–839. doi: 10.1104/pp.104.039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieldsend J., Milford G.F.J. Changes in glucosinolates during crop development in single- and double- low genotypes of winter oilseed rape (Brassica napus): I. Production and distribution in vegetative tissues and developing pods during development and potential role in the recycling. Ann. Appl. Biol. 1994;124:531–542. [Google Scholar]
- Frerigmann H., Gigolashvili T. MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Mol. Plant. 2014;7:814–828. doi: 10.1093/mp/ssu004. [DOI] [PubMed] [Google Scholar]
- Frerigmann H., Piślewska-Bednarek M., Sánchez-Vallet A., Molina A., Glawischnig E., Gigolashvili T., Bednarek P. Regulation of pathogen-triggered tryptophan metabolism in Arabidopsis thaliana by MYB transcription factors and indole glucosinolate conversion products. Mol. Plant. 2016;9:682–695. doi: 10.1016/j.molp.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Gallagher E., Yuxiang Y., Mabry M.E., Pires C.J. Breeding the dog of the plant world: development of doubled haploid Brassica oleracea. Poster Presented at Botany 2019 Annual Meeting. July 27-31, 2019. Tucson, AZ. 2019 [Google Scholar]
- Garcia-Oliveira P., Otero P., Pereira A.G., Chamorro F., Carpena M., Echave J., Fraga-Corral M., Simal-Gandara J., Prieto M.A. Status and challenges of plant-anticancer compounds in cancer treatment. Pharmaceuticals. 2021;14:157. doi: 10.3390/ph14020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geu-Flores F., Møldrup M.E., Böttcher C., Olsen C.E., Scheel D., Halkier B.A. Cytosolic γ-glutamyl peptidases process glutathione conjugates in the biosynthesis of glucosinolates and camalexin in Arabidopsis. Plant Cell. 2011;23:2456–2469. doi: 10.1105/tpc.111.083998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giallourou N., Oruna-Concha M.J., Harbourne N. Effects of domestic processing methods on the phytochemical content of watercress (Nasturtium officinale) Food Chem. 2016;212:411–419. doi: 10.1016/j.foodchem.2016.05.190. [DOI] [PubMed] [Google Scholar]
- Gigolashvili T., Berger B., Mock H.P., Müller C., Weisshaar B., Flügge U.I. The transcription factor HIG1/MYB51 regulates indolic glucosinolate biosynthesis in Arabidopsis thaliana. Plant J. 2007;50:886–901. doi: 10.1111/j.1365-313X.2007.03099.x. [DOI] [PubMed] [Google Scholar]
- Gill C.I.R., Haldar S., Boyd L.A., Bennett R., Whiteford J., Butler M., Pearson J.R., Bradbury I., Rowland I.R. Watercress supplementation in diet reduces lymphocyte DNA damage and alters blood antioxidant status in healthy adults. Am. J. Clin. Nutr. 2007;85:504–510. doi: 10.1093/ajcn/85.2.504. [DOI] [PubMed] [Google Scholar]
- Gohain B., Kumar P., Malhotra B., Augustine R., Pradhan A.K., Bisht N.C. A comprehensive Vis-NIRS equation for rapid quantification of seed glucosinolate content and composition across diverse Brassica oilseed chemotypes. Food Chem. 2021;354:129527. doi: 10.1016/j.foodchem.2021.129527. [DOI] [PubMed] [Google Scholar]
- González-Romero M.E., Rivera C., Cancino K., Geu-Flores F., Cosio E.G., Ghislain M., Halkier B.A. Bioengineering potato plants to produce benzylglucosinolate for improved broad-spectrum pest and disease resistance. Transgenic Res. 2021;30:649–660. doi: 10.1007/s11248-021-00255-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb C.D., Zipp B.J., Kopycki J., Schubert M., Quint M., Lim E.-K., Bowles D.J., Pedras M.S.C., Abel S. Comparative analysis of Arabidopsis UGT74 glucosyltransferases reveals a special role of UGT74C1 in glucosinolate biosynthesis. Plant J. 2014;79:92–105. doi: 10.1111/tpj.12541. [DOI] [PubMed] [Google Scholar]
- Grubb C.D., Zipp B.J., Ludwig-Müller J., Masuno M.N., Molinski T.F., Abel S. Arabidopsis glucosyltransferase UGT74B1 functions in glucosinolate biosynthesis and auxin homeostasis. Plant J. 2004;40:893–908. doi: 10.1111/j.1365-313X.2004.02261.x. [DOI] [PubMed] [Google Scholar]
- Guijarro-Real C., Plazas M., Rodríguez-Burruezo A., Prohens J., Fita A. Potential in vitro inhibition of selected plant extracts against SARS-CoV-2 chymotripsin-like protease (3CLPro) activity. Foods. 2021;10:1503. doi: 10.3390/foods10071503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guijarro-Real C., Prohens J., Rodríguez-Burruezo A., Fita A. Consumers acceptance and volatile profile of wall rocket (Diplotaxis erucoides) Food Res. Int. 2020;132:109008. doi: 10.1016/j.foodres.2020.109008. [DOI] [PubMed] [Google Scholar]
- Gumz F., Krausze J., Eisenschmidt D., Backenköhler A., Barleben L., Brandt W., Wittstock U. The crystal structure of the thiocyanate-forming protein from Thlaspi arvense, a kelch protein involved in glucosinolate breakdown. Plant Mol. Biol. 2015;89:67–81. doi: 10.1007/s11103-015-0351-9. [DOI] [PubMed] [Google Scholar]
- Guo R., Huang Z., Deng Y., Chen X., XuHan X., Lai Z. Comparative transcriptome analyses reveal a special glucosinolate metabolism mechanism in Brassica alboglabra sprouts. Front. Plant Sci. 2016;7:1497. doi: 10.3389/fpls.2016.01497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen B.G., Kerwin R.E., Ober J.A., Lambrix V.M., Mitchell-Olds T., Gershenzon J., Halkier B.A., Kliebenstein D.J. A novel 2-oxoacid-dependent dioxygenase involved in the formation of the goiterogenic 2-hydroxybut-3-enyl glucosinolate and generalist insect resistance in Arabidopsis. Plant Physiol. 2008;148:2096–2108. doi: 10.1104/pp.108.129981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harun S., Abdullah-Zawawi M.R., Goh H.H., Mohamed-Hussein Z.A. A comprehensive gene inventory for glucosinolate biosynthetic pathway in Arabidopsis thaliana. J. Agric. Food Chem. 2020;68:7281–7297. doi: 10.1021/acs.jafc.0c01916. [DOI] [PubMed] [Google Scholar]
- Harun S., Afiqah-Aleng N., Karim M.B., Altaf Ul Amin M., Kanaya S., Mohamed-Hussein Z.-A. Potential Arabidopsis thaliana glucosinolate genes identified from the co-expression modules using graph clustering approach. PeerJ. 2021;9:e11876. doi: 10.7717/peerj.11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Chen B., Pang Q., Strul J.M., Chen S. Functional specification of Arabidopsis isopropylmalate isomerases in glucosinolate and leucine biosynthesis. Plant Cell Physiol. 2010;51:1480–1487. doi: 10.1093/pcp/pcq113. [DOI] [PubMed] [Google Scholar]
- He Y., Chen L., Zhou Y., Mawhinney T.P., Chen B., Kang B.H., Hauser B.A., Chen S. Functional characterization of Arabidopsis thaliana isopropylmalate dehydrogenases reveals their important roles in gametophyte development. New Phytol. 2011;189:160–175. doi: 10.1111/j.1469-8137.2010.03460.x. [DOI] [PubMed] [Google Scholar]
- He Y., Mawhinney T.P., Preuss M.L., Schroeder A.C., Chen B., Abraham L., Jez J.M., Chen S. A redox-active isopropylmalate dehydrogenase functions in the biosynthesis of glucosinolates and leucine in Arabidopsis. Plant J. 2009;60:679–690. doi: 10.1111/j.1365-313X.2009.03990.x. [DOI] [PubMed] [Google Scholar]
- He Y., Yang Z., Tang M., Yang Q.-Y., Zhang Y., Liu S. Enhancing canola breeding by editing a glucosinolate transporter gene lacking natural variation. Plant Physiol. 2022;188:1848–1851. doi: 10.1093/plphys/kiac021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemm M.R., Ruegger M.O., Chapple C. The Arabidopsis ref2 mutant is defective in the gene encoding CYP83A1 and shows both phenylpropanoid and glucosinolate phenotypes. Plant Cell. 2003;15:179–194. doi: 10.1105/tpc.006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J., Lethenborg P., Li P.W., Rahman M.H., Sørensen H., Sørensen J. Inheritance of progoitrin and total aliphatic glucosinolates in oilseed rape (Brassica napus L) Euphytica. 2003;134:179–187. [Google Scholar]
- Hirschmann F., Papenbrock J. The fusion of genomes leads to more options: a comparative investigation on the desulfo-glucosinolate sulfotransferases of Brassica napus and homologous proteins of Arabidopsis thaliana. Plant Physiol. Biochem. 2015;91:10–19. doi: 10.1016/j.plaphy.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Hölzl G., Rezaeva B.R., Kumlehn J., Dörmann P. Ablation of glucosinolate accumulation in the oil crop Camelina sativa by targeted mutagenesis of genes encoding the transporters GTR1 and GTR2 and regulators of biosynthesis MYB28 and MYB29. Plant Biotechnol. J. 2023;21:189–201. doi: 10.1111/pbi.13936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D., Jing J., Snowdon R.J., Mason A.S., Shen J., Meng J., Zou J. Exploring the gene pool of Brassica napus by genomics-based approaches. Plant Biotechnol. J. 2021;19:1693–1712. doi: 10.1111/pbi.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D., Zhang W., Zhang Y., Chang S., Chen L., Chen Y., Shi Y., Shen J., Meng J., Zou J. Reconstituting the genome of a young allopolyploid crop, Brassica napus, with its related species. Plant Biotechnol. J. 2019;17:1106–1118. doi: 10.1111/pbi.13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull A.K., Vij R., Celenza J.L. Arabidopsis cytochrome P450s that catalyze the first step of tryptophan-dependent indole-3-acetic acid biosynthesis. Proc. Natl. Acad. Sci. USA. 2000;97:2379–2384. doi: 10.1073/pnas.040569997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker P., Halkier B.A., Schulz A., Turner S. Arabidopsis glucosinolate storage cells transform into phloem fibres at late stages of development. J. Exp. Bot. 2019;70:4305–4317. doi: 10.1093/jxb/erz176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker P., Lambertz S.K., Weber K., Crocoll C., Halkier B.A., Schulz A. Herbivore feeding preference corroborates optimal defense theory for specialized metabolites within plants. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2111977118. e2111977118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof J., Huber F., Reichelt M., Gershenzon J., Wiegreffe C., Lächler K., Binder S. The small subunit 1 of the Arabidopsis isopropylmalate isomerase is required for normal growth and development and the early stages of glucosinolate formation. PLoS One. 2014;9:e91071. doi: 10.1371/journal.pone.0091071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaafaru M.S., Abd Karim N.A., Enas M.E., Rollin P., Mazzon E., Abdull Razis A.F. Protective effect of glucosinolates hydrolytic products in neurodegenerative diseases (NDDs) Nutrients. 2018;10:580. doi: 10.3390/nu10050580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M., Hong E., Kim G.H. Evaluation of antibacterial activity of 3-butenyl, 4-pentenyl, 2-phenylethyl, and benzyl isothiocyanate in Brassica vegetables. J. Food Sci. 2010;75:412–416. doi: 10.1111/j.1750-3841.2010.01725.x. [DOI] [PubMed] [Google Scholar]
- Jeon B.W., Oh M.-H., Kim H.S., Kim E.O., Chae W.B. Glucosinolate variation among organs, growth stages and seasons suggests its dominant accumulation in sexual over asexual-reproductive organs in white radish. Sci. Hortic. 2022;291:110617. [Google Scholar]
- Jeon J., Bong S.J., Park J.S., Park Y.K., Arasu M.V., Al-Dhabi N.A., Park S.U. De novo transcriptome analysis and glucosinolate profiling in watercress (Nasturtium officinale R. Br.) BMC Genomics. 2017;18:401. doi: 10.1186/s12864-017-3792-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen M.E., Nour-Eldin H.H., Halkier B.A. Transport of defense compounds from source to sink: lessons learned from glucosinolates. Trends Plant Sci. 2015;20:508–514. doi: 10.1016/j.tplants.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Jørgensen M.E., Xu D., Crocoll C., Ernst H.A., Ramírez D., Motawia M.S., Olsen C.E., Mirza O., Nour-Eldin H.H., Halkier B.A. Origin and evolution of transporter substrate specificity within the NPF family. Elife. 2017;6:e19466. doi: 10.7554/eLife.19466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y.J., Jung J.I., Cho H.J., Choi M.S., Sung M.K., Yu R., Kang Y.H., Park J.H.Y. Berteroin present in cruciferous vegetables exerts potent anti-inflammatory properties in murine macrophages and mouse skin. Int. J. Mol. Sci. 2014;15:20686–20705. doi: 10.3390/ijms151120686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkowska H., Wróbel M., Szlęzak D., Jasek-Gajda E. New aspects of antiproliferative activity of 4-hydroxybenzyl isothiocyanate, a natural H2S-donor. Amino Acids. 2018;50:699–709. doi: 10.1007/s00726-018-2546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser A.E., Baniasadi M., Giansiracusa D., Giansiracusa M., Garcia M., Fryda Z., Wong T.L., Bishayee A. Sulforaphane: a broccoli bioactive phytocompound with cancer preventive potential. Cancers. 2021;13:4796. doi: 10.3390/cancers13194796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal R.M., Abdull Razis A.F., Mohd Sukri N.S., Perimal E.K., Ahmad H., Patrick R., Djedaini-Pilard F., Mazzon E., Rigaud S. Beneficial health effects of glucosinolates-derived isothiocyanates on cardiovascular and neurodegenerative diseases. Molecules. 2022;27:624. doi: 10.3390/molecules27030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L., Qian L., Zheng M., Chen L., Chen H., Yang L., You L., Yang B., Yan M., Gu Y., et al. Genomic insights into the origin, domestication and diversification of Brassica juncea. Nat. Genet. 2021;53:1392–1402. doi: 10.1038/s41588-021-00922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor S., Rafiq A., Sharma S. Protein engineering and its applications in food industry. Crit. Rev. Food Sci. Nutr. 2017;57:2321–2329. doi: 10.1080/10408398.2014.1000481. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Lee B.W., Schroeder F.C., Jander G. Identification of indole glucosinolate breakdown products with antifeedant effects on Myzus persicae (green peach aphid) Plant J. 2008;54:1015–1026. doi: 10.1111/j.1365-313X.2008.03476.x. [DOI] [PubMed] [Google Scholar]
- Kim N., Jeong Y.M., Jeong S., Kim G.B., Baek S., Kwon Y.E., Cho A., Choi S.B., Kim J., Lim W.J., et al. Identification of candidate domestication regions in the radish genome based on high-depth resequencing analysis of 17 genotypes. Theor. Appl. Genet. 2016;129:1797–1814. doi: 10.1007/s00122-016-2741-z. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Singh S.V. D,L-Sulforaphane causes transcriptional repression of androgen receptor in human prostate cancer cells. Mol. Cancer Ther. 2009;8:1946–1954. doi: 10.1158/1535-7163.MCT-09-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.B., Li X., Kim S.J., Kim H.H., Lee J., Kim H., Park S.U. MYB transcription factors regulate glucosinolate biosynthesis in different organs of Chinese cabbage (Brassica rapa ssp. pekinensis) Molecules. 2013;18:8682–8695. doi: 10.3390/molecules18078682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.C., Ahn W.S., Cha A., Jie E.Y., Kim S.W., Hwang B.H., Lee S., Hwang H. Development of glucoraphanin-rich broccoli (Brassica oleracea var. italica ) by CRISPR/Cas9-mediated DNA-free BolMYB28 editing. Plant Biotechnol. Rep. 2022;16:123–132. [Google Scholar]
- Kim Y.J., Park S.Y., Lee J.H. Berteroin ameliorates lipid accumulation through AMPK-mediated regulation of hepatic lipid metabolism and inhibition of adipocyte differentiation. Life Sci. 2021;282:119668. doi: 10.1016/j.lfs.2021.119668. [DOI] [PubMed] [Google Scholar]
- Kirkegaard J.A., Sarwar M. Biofumigation potential of brassicas: I. Variation in glucosinolate profiles of diverse field-grown brassicas. Plant Soil. 1998;201:71–89. [Google Scholar]
- Klein A.P., Sattely E.S. Biosynthesis of cabbage phytoalexins from indole glucosinolate. Proc. Natl. Acad. Sci. USA. 2017;114:1910–1915. doi: 10.1073/pnas.1615625114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Papenbrock J. Kinetics and substrate specificities of desulfo-glucosinolate sulfotransferases in Arabidopsis thaliana. Physiol. Plant. 2009;135:140–149. doi: 10.1111/j.1399-3054.2008.01182.x. [DOI] [PubMed] [Google Scholar]
- Klopsch R., Witzel K., Artemyeva A., Ruppel S., Hanschen F.S. Genotypic variation of glucosinolates and their breakdown products in leaves of Brassica rapa. J. Agric. Food Chem. 2018;66:5481–5490. doi: 10.1021/acs.jafc.8b01038. [DOI] [PubMed] [Google Scholar]
- Knill T., Reichelt M., Paetz C., Gershenzon J., Binder S. Arabidopsis thaliana encodes a bacterial-type heterodimeric isopropylmalate isomerase involved in both Leu biosynthesis and the Met chain elongation pathway of glucosinolate formation. Plant Mol. Biol. 2009;71:227–239. doi: 10.1007/s11103-009-9519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knill T., Schuster J., Reichelt M., Gershenzon J., Binder S. Arabidopsis branched-chain aminotransferase 3 functions in both amino acid and glucosinolate biosynthesis. Plant Physiol. 2008;146:1028–1039. doi: 10.1104/pp.107.111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W., Li J., Yu Q., Cang W., Xu R., Wang Y., Ji W., Wang Y., Xu R., Kong W., et al. Two novel flavin-containing monooxygenases involved in biosynthesis of aliphatic glucosinolates. Front. Plant Sci. 2016;7:1292. doi: 10.3389/fpls.2016.01292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenori Y., Tanigawa S., Kumamoto T., Qin S., Daikoku Y., Miyamori K., Nagai M., Hou D.X. Modulation of Nrf2/Keap1 system by wasabi 6-methylthiohexyl isothiocyanate in ARE-mediated NQO1 expression. Mol. Nutr. Food Res. 2013;57:854–864. doi: 10.1002/mnfr.201200689. [DOI] [PubMed] [Google Scholar]
- Kräling K., Röbbelen G., Thies W., Herrmann M., Ahmadi M.R. Variation of seed glucosinolates in lines of Brassica napus. Plant Breeding. 1990;105:33–39. [Google Scholar]
- Kristensen C., Morant M., Olsen C.E., Ekstrøm C.T., Galbraith D.W., Møller B.L., Bak S. Metabolic engineering of dhurrin in transgenic Arabidopsis plants with marginal inadvertent effects on the metabolome and transcriptome. Proc. Natl. Acad. Sci. USA. 2005;102:1779–1784. doi: 10.1073/pnas.0409233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener E.-M., Buettner A. Unravelling important odorants in horseradish (Armoracia rusticana) Food Chem. 2017;232:455–465. doi: 10.1016/j.foodchem.2017.04.042. [DOI] [PubMed] [Google Scholar]
- Kroymann J., Textor S., Tokuhisa J.G., Falk K.L., Bartram S., Gershenzon J., Mitchell-Olds T. A gene controlling variation in Arabidopsis glucosinolate composition is part of the methionine chain elongation pathway. Plant Physiol. 2001;127:1077–1088. [PMC free article] [PubMed] [Google Scholar]
- Kuchernig J.C., Backenköhler A., Lübbecke M., Burow M., Wittstock U. A thiocyanate-forming protein generates multiple products upon allylglucosinolate breakdown in Thlaspi arvense. Phytochemistry. 2011;72:1699–1709. doi: 10.1016/j.phytochem.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Kumagai H., Kashima N., Seki T., Sakurai H., Ishii K., Ariga T. Analysis of volatile components in essential oil of upland wasabi and their inhibitory effects on platelet aggregation. Biosci. Biotechnol. Biochem. 1994;58:2131–2135. [Google Scholar]
- Kumar R., Lee S.G., Augustine R., Reichelt M., Vassão D.G., Palavalli M.H., Allen A., Gershenzon J., Jez J.M., Bisht N.C. Molecular basis of the evolution of methylthioalkylmalate synthase and the diversity of methionine-derived glucosinolates. Plant Cell. 2019;31:1633–1647. doi: 10.1105/tpc.19.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lächler K., Clauss K., Imhof J., Crocoll C., Schulz A., Halkier B.A., Binder S. In Arabidopsis thaliana substrate recognition and tissue- as well as plastid type-specific expression define the roles of distinct small subunits of isopropylmalate isomerase. Front. Plant Sci. 2020;11:808. doi: 10.3389/fpls.2020.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lächler K., Imhof J., Reichelt M., Gershenzon J., Binder S. The cytosolic branched-chain aminotransferases of Arabidopsis thaliana influence methionine supply, salvage and glucosinolate metabolism. Plant Mol. Biol. 2015;88:119–131. doi: 10.1007/s11103-015-0312-3. [DOI] [PubMed] [Google Scholar]
- Lambrix V., Reichelt M., Mitchell-Olds T., Kliebenstein D.J., Gershenzon J. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. Plant Cell. 2001;13:2793–2807. doi: 10.1105/tpc.010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzeri L., D’Avino L., Gies D. Additional benefits of the efficacy in containing soilborne pest and pathogens with biofumigant plants and materials. Acta Hortic. 2010;883:323–329. [Google Scholar]
- Lee J., Kwon H. In vitro metabolic conversion of the organic breakdown products of glucosinolate to goitrogenic thiocyanate anion. J. Sci. Food Agric. 2015;95:2244–2251. doi: 10.1002/jsfa.6943. [DOI] [PubMed] [Google Scholar]
- Li X., Sandgrind S., Moss O., Guan R., Ivarson E., Wang E.S., Kanagarajan S., Zhu L.-H. Efficient protoplast regeneration protocol and CRISPR/Cas9-mediated editing of glucosinolate transporter (GTR) genes in rapeseed (Brassica napus L.) Front. Plant Sci. 2021;12:680859. doi: 10.3389/fpls.2021.680859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Kiddle G., Bennett R., Doughty K., Wallsgrove R. Variation in the glucosinolate content of vegetative tissues of Chinese lines of Brassica napus L. Ann. Appl. Biol. 1999;134:131–136. [Google Scholar]
- Li Y., Yu Y., Xu L., Guo E., Zang Y., He Y., Zhu Z. Transcriptome profiling reveals candidate key genes involved in sinigrin biosynthesis in Brassica nigra. Horticulturae. 2021;7:173. [Google Scholar]
- Liang Q., Xiong W., Zhou Q., Cui C., Xu X., Zhao L., Xuan P., Yao Y. Glucosinolates or erucic acid, which one contributes more to volatile flavor of fragrant rapeseed oil? Food Chem. 2023;412:135594. doi: 10.1016/j.foodchem.2023.135594. [DOI] [PubMed] [Google Scholar]
- Liu S., Bartnikas L.M., Volko S.M., Ausubel F.M., Tang D. Mutation of the glucosinolate biosynthesis enzyme cytochrome P450 83A1 monooxygenase increases camalexin accumulation and powdery mildew resistance. Front. Plant Sci. 2016;7:227. doi: 10.3389/fpls.2016.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Huang H., Yi X., Zhang Y., Yang Q., Zhang C., Fan C., Zhou Y. Dissection of genetic architecture for glucosinolate accumulations in leaves and seeds of Brassica napus by genome-wide association study. Plant Biotechnol. J. 2020;18:1472–1484. doi: 10.1111/pbi.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Zhang X., Yang H., Agerbirk N., Qiu Y., Wang H., Shen D., Song J., Li X. Aromatic glucosinolate biosynthesis pathway in Barbarea vulgaris and its response to Plutella xylostella infestation. Front. Plant Sci. 2016;7:83. doi: 10.3389/fpls.2016.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Hammerlindl J., Keller W., McVetty P.B.E., Daayf F., Quiros C.F., Li G. MAM gene silencing leads to the induction of C3 and reduction of C4 and C5 side-chain aliphatic glucosinolates in Brassica napus. Mol. Breeding. 2011;27:467–478. [Google Scholar]
- Liu Z., Hirani A.H., McVetty P.B.E., Daayf F., Quiros C.F., Li G. Reducing progoitrin and enriching glucoraphanin in Braasica napus seeds through silencing of the GSL-ALK gene family. Plant Mol. Biol. 2012;79:179–189. doi: 10.1007/s11103-012-9905-2. [DOI] [PubMed] [Google Scholar]
- Liu Z., Liang J., Zheng S., Zhang J., Wu J., Cheng F., Yang W., Wang X. Enriching glucoraphanin in Brassica rapa through replacement of BrAOP2.2/BrAOP2.3 with non-functional genes. Front. Plant Sci. 2017;8:1329. doi: 10.3389/fpls.2017.01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Wang H., Xie J., Lv J., Zhang G., Hu L., Luo S., Li L., Yu J. The roles of cruciferae glucosinolates in disease and pest resistance. Plants. 2021;10:1097. doi: 10.3390/plants10061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthy B., Matile P. The mustard oil bomb: rectified analysis of the subcellular organisation of the myrosinase system. Biochem. Physiol. Pflanz. 1984;179:5–12. [Google Scholar]
- Lüthy J., Benn M.H. Thiocyanate formation from glucosinolates: a study of the autolysis of allylglucosinolate in Thlaspi arvense L. seed flour extracts. Can. J. Biochem. 1977;55:1028–1031. doi: 10.1139/o77-153. [DOI] [PubMed] [Google Scholar]
- Mabry M.E., Gallagher E., Gore M.A., Labate J.A., Turner S.D., Baxter I.R., Kliebenstein D.J., Pires C.J. Poster Presented at Botany 2018 Annual Meeting. July 21-25, 2019. Rochester, MN. 2018. Brassica oleracea: the dog of the plant world; pp. 2–3. [Google Scholar]
- Madsen S.R., Kunert G., Reichelt M., Gershenzon J., Halkier B.A. Feeding on leaves of the glucosinolate transporter mutant gtr1gtr2 reduces fitness of Myzus persicae. J. Chem. Ecol. 2015;41:975–984. doi: 10.1007/s10886-015-0641-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen S.R., Olsen C.E., Nour-Eldin H.H., Halkier B.A. Elucidating the role of transport processes in leaf glucosinolate distribution. Plant Physiol. 2014;166:1450–1462. doi: 10.1104/pp.114.246249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahn A., Castillo A. Potential of sulforaphane as a natural immune system enhancer: a review. Molecules. 2021;26:752. doi: 10.3390/molecules26030752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malka S.K., Cheng Y. Possible interactions between the biosynthetic pathways of indole glucosinolate and auxin. Front. Plant Sci. 2017;8:2131. doi: 10.3389/fpls.2017.02131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangla B., Javed S., Sultan M.H., Kumar P., Kohli K., Najmi A., Alhazmi H.A., Al Bratty M., Ahsan W. Sulforaphane: a review of its therapeutic potentials, advances in its nanodelivery, recent patents, and clinical trials. Phytother. Res. 2021;35:5440–5458. doi: 10.1002/ptr.7176. [DOI] [PubMed] [Google Scholar]
- March G. de, Mcgregor D.I., Séguin-Shwartz G. Glucosinolate content of maturing pods and seeds of high and low glucosinolate summer rape. Can. J. Plant Sci. 1989;69:929–932. [Google Scholar]
- Marcinkowska M., Frank S., Steinhaus M., Jeleń H.H. Key odorants of raw and cooked green kohlrabi (Brassica oleracea var. gongylodes L.) J. Agric. Food Chem. 2021;69:12270–12277. doi: 10.1021/acs.jafc.1c04339. [DOI] [PubMed] [Google Scholar]
- Martelli A., Piragine E., Citi V., Testai L., Pagnotta E., Ugolini L., Lazzeri L., Di Cesare Mannelli L., Manzo O.L., Bucci M., et al. Erucin exhibits vasorelaxing effects and antihypertensive activity by H2S-releasing properties. Br. J. Pharmacol. 2020;177:824–835. doi: 10.1111/bph.14645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Harada Y., Tanaka K., Nakajima M., Tabeta H. ACS Symposium Series. 1996. Characteristic odorants of wasabi (Wasabia japonica matum), Japanese horseradish, in comparison with those of horseradish (Armoracia rusticana) pp. 67–78. [Google Scholar]
- Matthäus B., Luftmann H. Glucosinolates in members of the family brassicaceae: separation and identification by LC/ESI-MS-MS. J. Agric. Food Chem. 2000;48:2234–2239. doi: 10.1021/jf991306w. [DOI] [PubMed] [Google Scholar]
- Mazumder A., Dwivedi A., du Plessis J. Sinigrin and its therapeutic benefits. Molecules. 2016;21:416. doi: 10.3390/molecules21040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlvay A.C., Ragsdale A.P., Mabry M.E., Qi X., Bird K.A., Velasco P., An H., Pires J.C., Emshwiller E. Brassica rapa domestication: untangling wild and feral forms and convergence of crop morphotypes. Mol. Biol. Evol. 2021;38:3358–3372. doi: 10.1093/molbev/msab108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Sun X., Tan D., Gong S., Meijer J., Zhang J. The two non-functional myrosinase genes TGG3 and TGG6 in Arabidopsis are expressed predominantly in pollen. Plant Science. 2009;177:371–375. [Google Scholar]
- Miao H., Zeng W., Wang J., Zhang F., Sun B., Wang Q. Improvement of glucosinolates by metabolic engineering in Brassica crops. aBIOTECH. 2021;2:314–329. doi: 10.1007/s42994-021-00057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen M.D., Hansen C.H., Wittstock U., Halkier B.A. Cytochrome P450 CYP79B2 from Arabidopsis catalyzes the conversion of tryptophan to indole-3-acetaldoxime, a precursor of indole glucosinolates and indole-3-acetic acid. J. Biol. Chem. 2000;275:33712–33717. doi: 10.1074/jbc.M001667200. [DOI] [PubMed] [Google Scholar]
- Mikkelsen M.D., Naur P., Halkier B.A. Arabidopsis mutants in the C-S lyase of glucosinolate biosynthesis establish a critical role for indole-3-acetaldoxime in auxin homeostasis. Plant J. 2004;37:770–777. doi: 10.1111/j.1365-313x.2004.02002.x. [DOI] [PubMed] [Google Scholar]
- Milczarek M., Mielczarek L., Lubelska K., Dąbrowska A., Chilmonczyk Z., Matosiuk D., Wiktorska K. In vitro evaluation of sulforaphane and a natural analog as potent inducers of 5-fluorouracil anticancer activity. Molecules. 2018;23:3040. doi: 10.3390/molecules23113040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missinou A.A., Ferreira De Carvalho J., Marnet N., Delhaye T., Hamzaoui O., Abdel Sayed D., Guitton Y., Lebreton L., Langrume C., Laperche A., et al. Identification and quantification of glucosinolates and phenolics in a large panel of Brassica napus highlight valuable genetic resources for chemical ecology and breeding. J. Agric. Food Chem. 2022;70:5245–5261. doi: 10.1021/acs.jafc.1c08118. [DOI] [PubMed] [Google Scholar]
- Møldrup M.E., Geu-Flores F., de Vos M., Olsen C.E., Sun J., Jander G., Halkier B.A. Engineering of benzylglucosinolate in tobacco provides proof-of-concept for dead-end trap crops genetically modified to attract Plutella xylostella (Diamondback moth) Plant Biotechnol. J. 2012;10:435–442. doi: 10.1111/j.1467-7652.2011.00680.x. [DOI] [PubMed] [Google Scholar]
- Montaut S., Read S., Blažević I., Nuzillard J.M., Roje M., Harakat D., Rollin P. Investigation of the glucosinolates in Hesperis matronalis L. And Hesperis laciniata all.: unveiling 4′-O-β-D-apiofuranosylglucomatronalin. Carbohydr. Res. 2020;488:107898. doi: 10.1016/j.carres.2019.107898. [DOI] [PubMed] [Google Scholar]
- Monu E.A., David J.R.D., Schmidt M., Davidson P.M. Effect of white mustard essential oil on the growth of foodborne pathogens and spoilage microorganisms and the effect of food components on its efficacy. J. Food Prot. 2014;77:2062–2068. doi: 10.4315/0362-028X.JFP-14-257. [DOI] [PubMed] [Google Scholar]
- Moreira X., Abdala-Roberts L., Gols R., Francisco M. Plant domestication decreases both constitutive and induced chemical defences by direct selection against defensive traits. Sci. Rep. 2018;8:12678. doi: 10.1038/s41598-018-31041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambiar D.M., Kumari J., Augustine R., Kumar P., Bajpai P.K., Bisht N.C. GTR1 and GTR2 transporters differentially regulate tissue-specific glucosinolate contents and defence responses in the oilseed crop Brassica juncea. Plant Cell Environ. 2021;44:2729–2743. doi: 10.1111/pce.14072. [DOI] [PubMed] [Google Scholar]
- Naur P., Petersen B.L., Mikkelsen M.D., Bak S., Rasmussen H., Olsen C.E., Halkier B.A. CYP83A1 and CYP83B1, two nonredundant cytochrome P450 enzymes metabolizing oximes in the biosynthesis of glucosinolates in Arabidopsis. Plant Physiol. 2003;133:63–72. doi: 10.1104/pp.102.019240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neequaye M., Stavnstrup S., Harwood W., Lawrenson T., Hundleby P., Irwin J., Troncoso-Rey P., Saha S., Traka M.H., Mithen R., Østergaard L. CRISPR-Cas9-mediated gene editing of MYB28 genes impair glucoraphanin accumulation of Brassica oleracea in the field. Cris. J. 2021;4:416–426. doi: 10.1089/crispr.2021.0007. [DOI] [PubMed] [Google Scholar]
- Nie L.X., Wu Y.L., Dai Z., Ma S.C. Antiviral activity of Isatidis radix derived glucosinolate isomers and their breakdown products against influenza A in vitro/ovo and mechanism of action. J. Ethnopharmacol. 2020;251:112550. doi: 10.1016/j.jep.2020.112550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nintemann S.J., Hunziker P., Andersen T.G., Schulz A., Burow M., Halkier B.A. Localization of the glucosinolate biosynthetic enzymes reveals distinct spatial patterns for the biosynthesis of indole and aliphatic glucosinolates. Physiol. Plant. 2018;163:138–154. doi: 10.1111/ppl.12672. [DOI] [PubMed] [Google Scholar]
- Nour-Eldin H.H., Andersen T.G., Burow M., Madsen S.R., Jørgensen M.E., Olsen C.E., Dreyer I., Hedrich R., Geiger D., Halkier B.A. NRT/PTR transporters are essential for translocation of glucosinolate defence compounds to seeds. Nature. 2012;488:531–534. doi: 10.1038/nature11285. [DOI] [PubMed] [Google Scholar]
- Nour-Eldin H.H., Madsen S.R., Engelen S., Jørgensen M.E., Olsen C.E., Andersen J.S., Seynnaeve D., Verhoye T., Fulawka R., Denolf P., Halkier B.A. Reduction of antinutritional glucosinolates in Brassica oilseeds by mutation of genes encoding transporters. Nat. Biotechnol. 2017;35:377–382. doi: 10.1038/nbt.3823. [DOI] [PubMed] [Google Scholar]
- Nowicki D., Krause K., Szamborska P., Żukowska A., Cech G.M., Szalewska-Pałasz A. Induction of the stringent response underlies the antimicrobial action of aliphatic isothiocyanates. Front. Microbiol. 2021;11:591802. doi: 10.3389/fmicb.2020.591802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugrahedi P.Y., Verkerk R., Widianarko B., Dekker M. A mechanistic perspective on process-induced changes in glucosinolate content in Brassica vegetables: a review. Crit. Rev. Food Sci. Nutr. 2015;55:823–838. doi: 10.1080/10408398.2012.688076. [DOI] [PubMed] [Google Scholar]
- Ordonez A.A., Bullen C.K., Villabona-Rueda A.F., Thompson E.A., Turner M.L., Merino V.F., Yan Y., Kim J., Davis S.L., Komm O., et al. Sulforaphane exhibits antiviral activity against pandemic SARS-CoV-2 and seasonal HCoV-OC43 coronaviruses in vitro and in mice. Commun. Biol. 2022;5:242. doi: 10.1038/s42003-022-03189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W., Kim K.S., Jang Y.S., Lee K., Kim S.J., Ahn S.J., Hong S.W., Lee Y.H. Variation in glucosinolate contents of cruciferous plants. Rec. Nat. Prod. 2017;11:185–192. [Google Scholar]
- Pasini F., Verardo V., Caboni M.F., D’Antuono L.F. Determination of glucosinolates and phenolic compounds in rocket salad by HPLC-DAD-MS: evaluation of Eruca sativa Mill. and Diplotaxis tenuifolia L. genetic resources. Food Chem. X. 2012;133:1025–1033. [Google Scholar]
- Pedras M.S.C., Yaya E.E., Glawischnig E. The phytoalexins from cultivated and wild crucifers: chemistry and biology. Nat. Prod. Rep. 2011;28:1381–1405. doi: 10.1039/c1np00020a. [DOI] [PubMed] [Google Scholar]
- Peña-Varas C., Kanstrup C., Vergara-Jaque A., González-Avendaño M., Crocoll C., Mirza O., Dreyer I., Nour-Eldin H., Ramírez D. Structural insights into the substrate transport mechanisms in GTR transporters through ensemble docking. Int. J. Mol. Sci. 2022;23:1595. doi: 10.3390/ijms23031595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Liu H., Shen M., Chen R., Li J., Dong Y. The inhibitory effects of different types of Brassica seed meals on the virulence of Ralstonia solanacearum. Pest Manag. Sci. 2021;77:5129–5138. doi: 10.1002/ps.6552. [DOI] [PubMed] [Google Scholar]
- Perez V.C., Dai R., Bai B., Tomiczek B., Askey B.C., Zhang Y., Rubin G.M., Ding Y., Grenning A., Block A.K., Kim J. Aldoximes are precursors of auxins in Arabidopsis and maize. New Phytol. 2021;231:1449–1461. doi: 10.1111/nph.17447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A., Hansen L.G., Mirza N., Crocoll C., Mirza O., Halkier B.A. Changing substrate specificity and iteration of amino acid chain elongation in glucosinolate biosynthesis through targeted mutagenesis of Arabidopsis methylthioalkylmalate synthase 1. Biosci. Rep. 2019;39 doi: 10.1042/BSR20190446. BSR20190446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A., Wang C., Crocoll C., Halkier B.A. Biotechnological approaches in glucosinolate production. J. Integr. Plant Biol. 2018;60:1231–1248. doi: 10.1111/jipb.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B.L., Andréasson E., Bak S., Agerbirk N., Halkier B.A. Characterization of transgenic Arabidopsis thaliana with metabolically engineered high levels of p-hydroxybenzylglucosinolate. Planta. 2001;212:612–618. doi: 10.1007/s004250000429. [DOI] [PubMed] [Google Scholar]
- Pfalz M., Mukhaimar M., Perreau F., Kirk J., Hansen C.I.C., Olsen C.E., Agerbirk N., Kroymann J. Methyl transfer in glucosinolate biosynthesis mediated by indole glucosinolate O-methyltransferase 5. Plant Physiol. 2016;172:2190–2203. doi: 10.1104/pp.16.01402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalz M., Vogel H., Kroymann J. The gene controlling the indole glucosinolate modifier1 quantitative trait locus alters indole glucosinolate structures and aphid resistance in Arabidopsis. Plant Cell. 2009;21:985–999. doi: 10.1105/tpc.108.063115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pixley K.V., Falck-Zepeda J.B., Paarlberg R.L., Phillips P.W.B., Slamet-Loedin I.H., Dhugga K.S., Campos H., Gutterson N. Genome-edited crops for improved food security of smallholder farmers. Nat. Genet. 2022;54:364–367. doi: 10.1038/s41588-022-01046-7. [DOI] [PubMed] [Google Scholar]
- Plaszkó T., Szűcs Z., Vasas G., Gonda S. Effects of glucosinolate-derived isothiocyanates on fungi: a comprehensive review on direct effects, mechanisms, structure-activity relationship data and possible agricultural applications. J. Fungi. 2021;7:539. doi: 10.3390/jof7070539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaszkó T., Szűcs Z., Vasas G., Gonda S. Interactions of fungi with non-isothiocyanate products of the plant glucosinolate pathway: a review on product formation, antifungal activity, mode of action and biotransformation. Phytochemistry. 2022;200:113245. doi: 10.1016/j.phytochem.2022.113245. [DOI] [PubMed] [Google Scholar]
- Pocasap P., Weerapreeyakul N., Thumanu K. Alyssin and iberin in cruciferous vegetables exert anticancer activity in HepG2 by increasing intracellular reactive oxygen species and tubulin depolymerization. Biomol. Ther. 2019;27:540–552. doi: 10.4062/biomolther.2019.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., Sun B., Miao H., Cai C., Xu C., Wang Q. Variation of glucosinolates and quinone reductase activity among different varieties of Chinese kale and improvement of glucoraphanin by metabolic engineering. Food Chem. 2015;168:321–326. doi: 10.1016/j.foodchem.2014.07.073. [DOI] [PubMed] [Google Scholar]
- Qin H., Zhang W., Wang M., Xiong S., Hu D., Sun X., Hu L., Meng J., Zou J. Characterize glucosinolates of four Brassica species and interspecific tranferring of specific glucosinoaltes. Journal of Plant Genetic Resources. 2020;21:94–104. (in Chinese) [Google Scholar]
- Redovniković I.R., Textor S., Lisnić B., Gershenzon J. Expression pattern of the glucosinolate side chain biosynthetic genes MAM1 and MAM3 of Arabidopsis thaliana in different organs and developmental stages. Plant Physiol. Biochem. 2012;53:77–83. doi: 10.1016/j.plaphy.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Reintanz B., Lehnen M., Reichelt M., Gershenzon J., Kowalczyk M., Sandberg G., Godde M., Uhl R., Palme K. bus, a bushy Arabidopsis CYP79F1 knockout mutant with abolished synthesis of short-chain aliphatic glucosinolates. Plant Cell. 2001;13:351–367. doi: 10.1105/tpc.13.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose P., Huang Q., Ong C.N., Whiteman M. Broccoli and watercress suppress matrix metalloproteinase-9 activity and invasiveness of human MDA-MB-231 breast cancer cells. Toxicol. Appl. Pharmacol. 2005;209:105–113. doi: 10.1016/j.taap.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Rose P., Won Y.K., Ong C.N., Whiteman M. β-Phenylethyl and 8-methylsulphinyloctyl isothiocyanates, constituents of watercress, suppress LPS induced production of nitric oxide and prostaglandin E2 in RAW 264.7 macrophages. Nitric Oxide. 2005;12:237–243. doi: 10.1016/j.niox.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Sarwar M., Kirkegaard J.A., Wong P.T.W., Desmarchelier J.M. Biofumigation potential of brassicas. Plant Soil. 1998;201:103–112. [Google Scholar]
- Sawada Y., Kuwahara A., Nagano M., Narisawa T., Sakata A., Saito K., Hirai M.Y. Omics-based approaches to methionine side chain elongation in arabidopsis: characterization of the genes encoding methylthioalkylmalate isomerase and methylthioalkylmalate dehydrogenase. Plant Cell Physiol. 2009;50:1181–1190. doi: 10.1093/pcp/pcp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster J., Knill T., Reichelt M., Gershenzon J., Binder S. BRANCHED-CHAIN AMINOTRANSFERASE4 is part of the chain elongation pathway in the biosynthesis of methionine-derived glucosinolates in Arabidopsis. Plant Cell. 2006;18:2664–2679. doi: 10.1105/tpc.105.039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M.S., Jin M., Chun J.H., Kim S.J., Park B.S., Shon S.H., Kim J.S. Functional analysis of three BrMYB28 transcription factors controlling the biosynthesis of glucosinolates in Brassica rapa. Plant Mol. Biol. 2016;90:503–516. doi: 10.1007/s11103-016-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang G., Zhao H., Tong L., Yin N., Hu R., Jiang H., Kamal F., Zhao Z., Xu L., Lu K., et al. Genome-wide association study of phenylalanine derived glucosinolates in Brassica rapa. Plants. 2022;11:1274. doi: 10.3390/plants11091274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M., Mukhopadhyay A., Gupta V., Pental D., Pradhan A.K. BjuB.CYP79F1 regulates synthesis of propyl fraction of aliphatic glucosinolates in oilseed mustard Brassica juncea: functional validation through genetic and transgenic approaches. PLoS One. 2016;11:e0150060. doi: 10.1371/journal.pone.0150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin I.S., Masuda H., Naohide K. Bactericidal activity of wasabi (Wasabia japonica) against Helicobacter pylori. Int. J. Food Microbiol. 2004;94:255–261. doi: 10.1016/S0168-1605(03)00297-6. [DOI] [PubMed] [Google Scholar]
- Sikorska-Zimny K., Beneduce L. The glucosinolates and their bioactive derivatives in Brassica: a review on classification, biosynthesis and content in plant tissues, fate during and after processing, effect on the human organism and interaction with the gut microbiota. Crit. Rev. Food Sci. Nutr. 2021;61:2544–2571. doi: 10.1080/10408398.2020.1780193. [DOI] [PubMed] [Google Scholar]
- Singh A.A., Patil M.P., Kang M.J., Niyonizigiye I., Kim G.D. Biomedical application of indole-3-carbinol: a mini-review. Phytochem. Lett. 2021;41:49–54. [Google Scholar]
- Singh D., Arora R., Bhatia A., Singh H., Singh B., Arora S. Molecular targets in cancer prevention by 4-(methylthio)butyl isothiocyanate - a comprehensive review. Life Sci. 2020;241:117061. doi: 10.1016/j.lfs.2019.117061. [DOI] [PubMed] [Google Scholar]
- Sivapalan T., Melchini A., Saha S., Needs P.W., Traka M.H., Tapp H., Dainty J.R., Mithen R.F. Bioavailability of glucoraphanin and sulforaphane from high-glucoraphanin broccoli. Mol. Nutr. Food Res. 2018;62:1700911. doi: 10.1002/mnfr.201700911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sønderby I.E., Burow M., Rowe H.C., Kliebenstein D.J., Halkier B.A. A complex interplay of three R2R3 MYB transcription factors determines the profile of aliphatic glucosinolates in Arabidopsis. Plant Physiol. 2010;153:348–363. doi: 10.1104/pp.109.149286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sønderby I.E., Geu-Flores F., Halkier B.A. Biosynthesis of glucosinolates - gene discovery and beyond. Trends Plant Sci. 2010;15:283–290. doi: 10.1016/j.tplants.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Song J.M., Guan Z., Hu J., Guo C., Yang Z., Wang S., Liu D., Wang B., Lu S., Zhou R., et al. Eight high-quality genomes reveal pan-genome architecture and ecotype differentiation of Brassica napus. Nat. Plants. 2020;6:34–45. doi: 10.1038/s41477-019-0577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Wei Y., Xiao D., Gong K., Sun P., Ren Y., Yuan J., Wu T., Yang Q., Li X., et al. Brassica carinata genome characterization clarifies U’s triangle model of evolution and polyploidy in Brassica. Plant Physiol. 2021;186:388–406. doi: 10.1093/plphys/kiab048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindel J.E., McCouch S.R. When more is better: how data sharing would accelerate genomic selection of crop plants. New Phytol. 2016;212:814–826. doi: 10.1111/nph.14174. [DOI] [PubMed] [Google Scholar]
- Stefansson B.R., Kondra Z.P. Tower summer rape. Can. J. Plant Sci. 1975;55:343–344. [Google Scholar]
- Stoewsand G.S. Bioactive organosulfur phytochemicals in Brassica oleracea vegetables-A review. Food Chem. Toxicol. 1995;33:537–543. doi: 10.1016/0278-6915(95)00017-v. [DOI] [PubMed] [Google Scholar]
- Sundaram M.K., R P., Haque S., Akhter N., Khan S., Ahmad S., Hussain A. Dietary isothiocyanates inhibit cancer progression by modulation of epigenome. Semin. Cancer Biol. 2022;83:353–376. doi: 10.1016/j.semcancer.2020.12.021. [DOI] [PubMed] [Google Scholar]
- Sureshkumar S., Todesco M., Schneeberger K., Harilal R., Balasubramanian S., Weigel D. A genetic defect caused by a triplet repeat expansion in Arabidopsis thaliana. Science. 2009;323:1060–1063. doi: 10.1126/science.1164014. [DOI] [PubMed] [Google Scholar]
- Tan Z., Xie Z., Dai L., Zhang Y., Zhao H., Tang S., Wan L., Yao X., Guo L., Hong D. Genome- and transcriptome-wide association studies reveal the genetic basis and the breeding history of seed glucosinolate content in Brassica napus. Plant Biotechnol. J. 2022;20:211–225. doi: 10.1111/pbi.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandayu E., Borpatragohain P., Mauleon R., Kretzschmar T. Genome-wide association reveals trait loci for seed glucosinolate accumulation in Indian mustard (Brassica juncea L.) Plants. 2022;11:364. doi: 10.3390/plants11030364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantikanjana T., Mikkelsen M.D., Hussain M., Halkier B.A., Sundaresan V. Functional analysis of the tandem-duplicated P450 genes SPS/BUS/CYP79F1 and CYP79F2 in glucosinolate biosynthesis and plant development by ds transposition-generated double mutants. Plant Physiol. 2004;135:840–848. doi: 10.1104/pp.104.040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H., Miao H., Chen L., Wang M., Xia C., Zeng W., Sun B., Zhang F., Zhang S., Li C., Wang Q. WRKY33-mediated indolic glucosinolate metabolic pathway confers resistance against Alternaria brassicicola in Arabidopsis and Brassica crops. J. Integr. Plant Biol. 2022;64:1007–1019. doi: 10.1111/jipb.13245. [DOI] [PubMed] [Google Scholar]
- Taranto F., Francese G., Di Dato F., D’Alessandro A., Greco B., Onofaro Sanajà V., Pentangelo A., Mennella G., Tripodi P. Leaf metabolic, genetic, and morphophysiological profiles of cultivated and wild rocket salad (Eruca and Diplotaxis Spp.) J. Agric. Food Chem. 2016;64:5824–5836. doi: 10.1021/acs.jafc.6b01737. [DOI] [PubMed] [Google Scholar]
- Textor S., De Kraker J.W., Hause B., Gershenzon J., Tokuhisa J.G. MAM3 catalyzes the formation of all aliphatic glucosinolate chain lengths in Arabidopsis. Plant Physiol. 2007;144:60–71. doi: 10.1104/pp.106.091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabelcy B., Chinkov N., Samuni-Blank M., Merav M., Izhaki I., Carmeli S., Gerchman Y. Investigation of glucosinolates in the desert plant Ochradenus baccatus (Brassicales: resedaceae). Unveiling glucoochradenin, a new arabinosylated glucosinolate. Phytochemistry. 2021;187:112760. doi: 10.1016/j.phytochem.2021.112760. [DOI] [PubMed] [Google Scholar]
- Traka M.H., Saha S., Huseby S., Kopriva S., Walley P.G., Barker G.C., Moore J., Mero G., van den Bosch F., Constant H., et al. Genetic regulation of glucoraphanin accumulation in Beneforté® broccoli. New Phytol. 2013;198:1085–1095. doi: 10.1111/nph.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trio P.Z., Kawahara A., Tanigawa S., Sakao K., Hou D.X. DNA microarray profiling highlights Nrf2-mediated chemoprevention targeted by wasabi-derived isothiocyanates in HepG2 cells. Nutr. Cancer. 2017;69:105–116. doi: 10.1080/01635581.2017.1248296. [DOI] [PubMed] [Google Scholar]
- Uchida K., Miura Y., Nagai M., Tominaga M. Isothiocyanates from Wasabia japonica activate transient receptor potential ankyrin 1 channel. Chem. Senses. 2012;37:809–818. doi: 10.1093/chemse/bjs065. [DOI] [PubMed] [Google Scholar]
- Uddin M.S., Mamun A.A., Jakaria M., Thangapandiyan S., Ahmad J., Rahman M.A., Mathew B., Abdel-Daim M.M., Aleya L. Emerging promise of sulforaphane-mediated Nrf2 signaling cascade against neurological disorders. Sci. Total Environ. 2020;707:135624. doi: 10.1016/j.scitotenv.2019.135624. [DOI] [PubMed] [Google Scholar]
- Van Doorn H.E., Van Der Kruk G.C., Van Holst G.J., Raaijmakers-Ruijs N.C.M.E., Postma E., Groeneweg B., Jongen W.H.F. The glucosinolates sinigrin and progoitrin are important determinants for taste preference and bitterness of brussels sprouts. J. Sci. Food Agric. 1998;78:30–38. doi: 10.1021/jf970523z. [DOI] [PubMed] [Google Scholar]
- Vargas-Mendoza N., Madrigal-Santillán E., Álvarez-González I., Madrigal-Bujaidar E., Anguiano-Robledo L., Aguilar-Faisal J.L., Morales-Martínez M., Delgado-Olivares L., Rodríguez-Negrete E.V., Morales-González Á., Morales-González J.A. Phytochemicals in skeletal muscle health: effects of curcumin (from Curcuma longa Linn) and sulforaphane (from Brassicaceae) on muscle function, recovery and therapy of muscle atrophy. Plants. 2022;11:2517. doi: 10.3390/plants11192517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco P., Soengas P., Vilar M., Cartea M.E., Del Rio M. Comparison of glucosinolate profiles in leaf and seed tissues of different Brassica napus crops. J. Am. Soc. Hortic. Sci. 2008;133:551–558. [Google Scholar]
- Wang B., Wu Z., Li Z., Zhang Q., Hu J., Xiao Y., Cai D., Wu J., King G.J., Li H., Liu K. Dissection of the genetic architecture of three seed-quality traits and consequences for breeding in Brassica napus. Plant Biotechnol. J. 2018;16:1336–1348. doi: 10.1111/pbi.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Crocoll C., Agerbirk N., Halkier B.A. Engineering and optimization of the 2-phenylethylglucosinolate production in Nicotiana benthamiana by combining biosynthetic genes from Barbarea vulgaris and Arabidopsis thaliana. Plant J. 2021;106:978–992. doi: 10.1111/tpj.15212. [DOI] [PubMed] [Google Scholar]
- Wang C., Dissing M.M., Agerbirk N., Crocoll C., Halkier B.A. Characterization of Arabidopsis CYP79C1 and CYP79C2 by glucosinolate pathway engineering in Nicotiana benthamiana shows substrate specificity toward a range of aliphatic and aromatic amino acids. Front. Plant Sci. 2020;11:57. doi: 10.3389/fpls.2020.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Chen M., Guo R., Ding Y., Zhang H., He Y. The improvement of sulforaphane in type 2 diabetes mellitus (T2DM) and related complications: a review. Trends Food Sci. Technol. 2022;129:397–407. [Google Scholar]
- Wang N., Wang W., Liu C., Jin J., Shao B., Shen L. Inhibition of growth and induction of apoptosis in A549 cells by compounds from oxheart cabbage extract. J. Sci. Food Agric. 2016;96:3813–3820. doi: 10.1002/jsfa.7575. [DOI] [PubMed] [Google Scholar]
- Wang Y., Pan Y., Liu Z., Zhu X., Zhai L., Xu L., Yu R., Gong Y., Liu L. De novo transcriptome sequencing of radish (Raphanus sativus L.) and analysis of major genes involved in glucosinolate metabolism. BMC Genomics. 2013;14:836. doi: 10.1186/1471-2164-14-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang Q., Sun H., Zhang Z., Qian H., Zhao X., He H., Zhang L. Glucosinolate profiles in different organs of 111 radish accessions and candidate genes involved in converting glucobrassicin to 4-hydroxyglucobrassicin. J. Agric. Food Chem. 2022;70:488–497. doi: 10.1021/acs.jafc.1c05107. [DOI] [PubMed] [Google Scholar]
- Wang Z., Yang R., Guo L., Fang M., Zhou Y., Gu Z. Effects of abscisic acid on glucosinolate content, isothiocyanate formation and myrosinase activity in cabbage sprouts. Int. J. Food Sci. Technol. 2015;50:1839–1846. [Google Scholar]
- Washida K., Miyata M., Koyama T., Yazawa K., Nomoto K. Suppressive effect of Yamato-mana (Brassica rapa L. oleifera group) constituent 3-butenyl glucosinolate (gluconapin) on postprandial hypertriglyceridemia in mice. Biosci. Biotechnol. Biochem. 2010;74:1286–1289. doi: 10.1271/bbb.100018. [DOI] [PubMed] [Google Scholar]
- Wathelet J.P., Wagstaffe P.J., Boenke A., Marlier M., Severin M. The certified total glucosinolate content in three rapeseeds (CRMs 190, 366 and 367) Fresenius J. Anal. Chem. 1993;347-347:396–399. [Google Scholar]
- Wei L.Y., Zhang J.K., Zheng L., Chen Y. The functional role of sulforaphane in intestinal inflammation: a review. Food Funct. 2022;13:514–529. doi: 10.1039/d1fo03398k. [DOI] [PubMed] [Google Scholar]
- Wei S., Xiao X., Wei L., Li L., Li G., Liu F., Xie J., Yu J., Zhong Y. Development and comprehensive HS-SPME/GC–MS analysis optimization, comparison, and evaluation of different cabbage cultivars (Brassica oleracea L. var. capitata L.) volatile components. Food Chem. 2021;340:128166. doi: 10.1016/j.foodchem.2020.128166. [DOI] [PubMed] [Google Scholar]
- Weis C., Hildebrandt U., Hoffmann T., Hemetsberger C., Pfeilmeier S., König C., Schwab W., Eichmann R., Hückelhoven R. CYP83A1 is required for metabolic compatibility of Arabidopsis with the adapted powdery mildew fungus Erysiphe cruciferarum. New Phytol. 2014;202:1310–1319. doi: 10.1111/nph.12759. [DOI] [PubMed] [Google Scholar]
- Weis C., Pfeilmeier S., Glawischnig E., Isono E., Pachl F., Hahne H., Kuster B., Eichmann R., Hückelhoven R. Co-immunoprecipitation-based identification of putative BAX INHIBITOR-1-interacting proteins involved in cell death regulation and plant-powdery mildew interactions. Mol. Plant Pathol. 2013;14:791–802. doi: 10.1111/mpp.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek M.N., Majcher M.A., Jeleń H.H. Identification of aroma compounds in raw and cooked broccoli. Flavour Fragr. J. 2021;36:576–583. [Google Scholar]
- Wieczorek M.N., Walczak M., Skrzypczak-Zielińska M., Jeleń H.H. Bitter taste of Brassica vegetables: the role of genetic factors, receptors, isothiocyanates, glucosinolates, and flavor context. Crit. Rev. Food Sci. Nutr. 2018;58:3130–3140. doi: 10.1080/10408398.2017.1353478. [DOI] [PubMed] [Google Scholar]
- Wiesner M., Hanschen F.S., Schreiner M., Glatt H., Zrenner R. Induced production of 1-methoxy-indol-3-ylmethyl glucosinolate by jasmonic acid and methyl jasmonate in sprouts and leaves of pak choi (Brassica rapa ssp. chinensis) Int. J. Mol. Sci. 2013;14:14996–15016. doi: 10.3390/ijms140714996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner M., Zrenner R., Krumbein A., Glatt H., Schreiner M. Genotypic variation of the glucosinolate profile in pak choi (Brassica rapa ssp. chinensis) J. Agric. Food Chem. 2013;61:1943–1953. doi: 10.1021/jf303970k. [DOI] [PubMed] [Google Scholar]
- Winde I., Wittstock U. Insect herbivore counteradaptations to the plant glucosinolate-myrosinase system. Phytochemistry. 2011;72:1566–1575. doi: 10.1016/j.phytochem.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Wittstock U., Burow M. Glucosinolate breakdown in Arabidopsis: mechanism, regulation and biological significance. Arabidopsis Book. 2010;8:e0134. doi: 10.1199/tab.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittstock U., Halkier B.A. Cytochrome P450 CYP79A2 from Arabidopsis thaliana L. catalyzes the conversion of L-phenylalanine to phenylacetaldoxime in the biosynthesis of benzylglucosinolate. J. Biol. Chem. 2000;275:14659–14666. doi: 10.1074/jbc.275.19.14659. [DOI] [PubMed] [Google Scholar]
- Wittstock U., Halkier B.A. Glucosinolate research in the Arabidopsis era. Trends Plant Sci. 2002;7:263–270. doi: 10.1016/s1360-1385(02)02273-2. [DOI] [PubMed] [Google Scholar]
- Wittstock U., Kurzbach E., Herfurth A.M., Stauber E.J. Glucosinolate breakdown. Adv. Bot. Res. 2016;80:125–169. [Google Scholar]
- Wittstock U., Meier K., Dörr F., Ravindran B.M. NSP-Dependent simple nitrile formation dominates upon breakdown of major aliphatic glucosinolates in roots, seeds, and seedlings of Arabidopsis thaliana Columbia-0. Front. Plant Sci. 2016;7:1821. doi: 10.3389/fpls.2016.01821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Zhang T., Wang Y., Chen M., Yang J., Li F., Deng Y., Zhu Z., Lei J., Chen G., et al. BocODD1 and BocODD2 regulate the biosynthesis of progoitrin glucosinolate in Chinese kale. Int. J. Mol. Sci. 2022;23:14781. doi: 10.3390/ijms232314781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Huang H., Childs H., Wu Y., Yu L., Pehrsson P.R. Glucosinolates in Brassica vegetables: characterization and factors that influence distribution, content, and intake. Annu. Rev. Food Sci. Technol. 2021;12:485–511. doi: 10.1146/annurev-food-070620-025744. [DOI] [PubMed] [Google Scholar]
- Wu Y.-Y., Xu Y.-M., Lau A.T.Y. Anti-cancer and medicinal potentials of moringa isothiocyanate. Molecules. 2021;26:7512. doi: 10.3390/molecules26247512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Hanschen F.S., Witzel K., Nintemann S.J., Nour-Eldin H.H., Schreiner M., Halkier B.A. Rhizosecretion of stele-synthesized glucosinolates and their catabolites requires GTR-mediated import in Arabidopsis. J. Exp. Bot. 2016;68:3205–3214. doi: 10.1093/jxb/erw355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Hunziker P., Koroleva O., Blennow A., Crocoll C., Schulz A., Nour-Eldin H.H., Halkier B.A. GTR-mediated radial import directs accumulation of defensive glucosinolates to sulfur-rich cells in the phloem cap of Arabidopsis inflorescence Stem. Mol. Plant. 2019;12:1474–1484. doi: 10.1016/j.molp.2019.06.008. [DOI] [PubMed] [Google Scholar]
- Yamada-Kato T., Nagai M., Ohnishi M., Yoshida K. Inhibitory effects of wasabi isothiocyanates on chemical mediator release in RBL-2H3 rat basophilic leukemia cells. J. Nutr. Sci. Vitaminol. 2012;58:303–307. doi: 10.3177/jnsv.58.303. [DOI] [PubMed] [Google Scholar]
- Yano T., Yajima S., Virgona N., Yano Y., Otani S., Kumagai H., Sakurai H., Kishimoto M., Ichikawa T. The effect of 6-methylthiohexyl isothiocyanate isolated from Wasabia japonica (wasabi) on 4-(methylnitrosamino)-1-(3-pyridyl)-1-buatnone-induced lung tumorigenesis in mice. Cancer Lett. 2000;155:115–120. doi: 10.1016/s0304-3835(00)00364-5. [DOI] [PubMed] [Google Scholar]
- Yao Y., Liu C., Xiong W., Liang Q., Xuan P., Zeng X., Zeng S., Zhou Q., Huang F. Silicon dioxide as an efficient adsorbent in the degumming of rapeseed oil. J. Clean. Prod. 2020;268:122344. [Google Scholar]
- Yi G., Lim S., Chae W.B., Park J.E., Park H.R., Lee E.J., Huh J.H. Root glucosinolate profiles for screening of radish (Raphanus sativus L.) genetic resources. J. Agric. Food Chem. 2016;64:61–70. doi: 10.1021/acs.jafc.5b04575. [DOI] [PubMed] [Google Scholar]
- Yi G.-E., Robin A.H.K., Yang K., Park J.-I., Kang J.-G., Yang T.-J., Nou I.-S. Identification and expression analysis of glucosinolate biosynthetic genes and estimation of glucosinolate contents in edible organs of Brassica oleracea subspecies. Molecules. 2015;20:13089–13111. doi: 10.3390/molecules200713089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L., Chen H., Cao B., Lei J., Chen G. Molecular characterization of MYB28 involved in aliphatic glucosinolate biosynthesis in Chinese kale (Brassica oleracea var. alboglabra Bailey) Front. Plant Sci. 2017;8:1083. doi: 10.3389/fpls.2017.01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Lin T., Meng X., Du H., Zhang J., Liu G., Chen M., Jing Y., Kou L., Li X., et al. A route to de novo domestication of wild allotetraploid rice. Cell. 2021;184:1156–1170.e14. doi: 10.1016/j.cell.2021.01.013. [DOI] [PubMed] [Google Scholar]
- Zang Y.X., Kim D.H., Park B.S., Hong S.B. Metabolic engineering of indole glucosinolates in Chinese cabbage hairy roots expressing Arabidopsis CYP79B2, CYP79B3, and CYP83B1. Biotechnol. Bioprocess Eng. 2009;14:467–473. [Google Scholar]
- Zang Y.X., Kim J.H., Park Y.D., Kim D.H., Hong S.B. Metabolic engineering of aliphatic glucosinolates in Chinese cabbage plants expressing Arabidopsis MAM1, CYP79F1, and CYP83A1. BMB Rep. 2008;41:472–478. doi: 10.5483/bmbrep.2008.41.6.472. [DOI] [PubMed] [Google Scholar]
- Zhang A., Luo R., Li J., Miao R., An H., Yan X., Pang Q. Arabidopsis glutathione-S-transferases GSTF11 and GSTU20 function in aliphatic glucosinolate biosynthesis. Front. Plant Sci. 2022;12:816233. doi: 10.3389/fpls.2021.816233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang S. Antidiabetic potential of sinigrin against streptozotocin-induced diabetes via modulating inflammation and oxidative stress. Appl. Biochem. Biotechnol. 2022;194:4279–4291. doi: 10.1007/s12010-021-03739-x. [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu Z., Liang J., Wu J., Cheng F., Wang X. Three genes encoding AOP2, a protein involved in aliphatic glucosinolate biosynthesis, are differentially expressed in Brassica rapa. J. Exp. Bot. 2015;66:6205–6218. doi: 10.1093/jxb/erv331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Pontoppidan B., Xue J., Rask L., Meijer J. The third myrosinase gene TGG3 in Arabidopsis thaliana is a pseudogene specifically expressed in stamen and petal. Physiol. Plant. 2002;115:25–34. doi: 10.1034/j.1399-3054.2002.1150103.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Huai D., Yang Q., Cheng Y., Ma M., Kliebenstein D.J., Zhou Y. Overexpression of three glucosinolate biosynthesis genes in Brassica napus identifies enhanced resistance to Sclerotinia sclerotiorum and Botrytis cinerea. PLoS One. 2015;10:e0140491. doi: 10.1371/journal.pone.0140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li B., Huai D., Zhou Y., Kliebenstein D.J. The conserved transcription factors, MYB115 and MYB118, control expression of the newly evolved benzoyloxy glucosinolate pathway in Arabidopsis thaliana. Front. Plant Sci. 2015;6:343. doi: 10.3389/fpls.2015.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010;61:49–64. doi: 10.1146/annurev-arplant-042809-112308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Chen Z., Chen J., Chen B., Tang W., Chen X., Lai Z., Guo R. Comparative transcriptomic analyses of glucosinolate metabolic genes during the formation of Chinese kale seeds. BMC Plant Biol. 2021;21:394. doi: 10.1186/s12870-021-03168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Hull A.K., Gupta N.R., Goss K.A., Alonso J., Ecker J.R., Normanly J., Chory J., Celenza J.L. Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev. 2002;16:3100–3112. doi: 10.1101/gad.1035402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Jiang M., Zhang W., Yang J., Lu G., Cheng Y., Fu G., Li P., Zhang X. Bird damage and glucosinolates of canola (Brassica napus L.) Chinese Journal of Oil Crop Sciences. 2016;38:111–115. (in Chinese) [Google Scholar]
- Zheng H., Zhang C., Wang Y., Zhou W., Chen J., Yan X., Li Z., Huang S., Li M., Tang Y., et al. Overexpression of the glucosyltransferase gene BoaUGT74B1 enhances the accumulation of indole glucosinolates in Chinese kale. Sci. Hortic. (Amsterdam) 2021;288:110302. [Google Scholar]
- Zhou X., Zhang H., Xie Z., Liu Y., Wang P., Dai L., Zhang X., Wang Z., Wang Z., Wan L., et al. Natural variation and artificial selection at the BnaC2.MYB28 locus modulate Brassica napus seed glucosinolate. Plant Physiol. 2023;191:352–368. doi: 10.1093/plphys/kiac463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B., Wang Z., Yang J., Zhu Z., Wang H. Isolation and expression of glucosinolate synthesis genes CYP83A1 and CYP83B1 in pak choi (Brassica rapa L. ssp. chinensis var. communis (N. Tsen & S.H. Lee) hanelt) Int. J. Mol. Sci. 2012;13:5832–5843. doi: 10.3390/ijms13055832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B., Yang J., Zhu Z. Variation in glucosinolates in pak choi cultivars and various organs at different stages of vegetative growth during the harvest period. J. Zhejiang Univ. Sci. B. 2013;14:309–317. doi: 10.1631/jzus.B1200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.