Abstract
Background
Analysis of the glutamine metabolic pathway has taken a special place in metabolomics research in recent years, given its important role in cell biosynthesis and bioenergetics across several disorders, especially in cancer cell survival. The science of metabolomics addresses the intricate intracellular metabolic network by exploring and understanding how cells function and respond to external or internal perturbations to identify potential therapeutic targets. However, despite recent advances in metabolomics, monitoring the kinetics of a metabolic pathway in a living cell in situ, real-time and holistically remains a significant challenge.
Aim
This review paper explores the range of analytical approaches for monitoring metabolic pathways, as well as physicochemical modeling techniques, with a focus on glutamine metabolism. We discuss the advantages and disadvantages of each method and explore the potential of label-free Raman microspectroscopy, in conjunction with kinetic modeling, to enable real-time and in situ monitoring of the cellular kinetics of the glutamine metabolic pathway.
Key scientific concepts
Given its important role in cell metabolism, the ability to monitor and model the glutamine metabolic pathways are highlighted. Novel, label free approaches have the potential to revolutionise metabolic biosensing, laying the foundation for a new paradigm in metabolomics research and addressing the challenges in monitoring metabolic pathways in living cells.
Supplementary Information
The online version of this article (10.1007/s11306-023-02031-9) contains supplementary material, which is available to authorized users.
Keywords: Metabolic pathways analysis, Glutaminolysis, Flux analysis, Computational modelling, Vibrational spectroscopy, Chemometrics
Introduction
Central carbon metabolism is critical for energy production and includes the glycolysis pathway, the pentose phosphate pathway and the tricarboxylic acid (TCA) cycle. Increased understanding has revealed that the roles of these metabolic pathways are not limited to cellular energetics, but they are also involved in the assembly of the most important cellular building blocks, including nucleotides and amino acids (Zhang et al., 2008). Concomitant with the advances in knowledge and understanding of this metabolic system, a different view of glutamine metabolism has emerged (Reitzer et al., 1979), one that elevates glutamine from a non-essential amino acid to one which plays a fundamental role in cell biosynthesis and bioenergetics, including redox homeostasis, synthesis of metabolites that drive the TCA cycle, generation of antioxidants to eliminate reactive oxygen species (ROS), synthesis of nonessential amino acids, pyrimidines, purines, and macromolecules such as fatty acids for cell replication and activation of cell signaling (Deberardinis & Cheng, 2009; Newsholme et al., 2003). Moreover, glutamine has been shown to be a perfect substrate for oxidative metabolism in glutamine-addicted tumour cells (Wise & Thompson, 2010). These properties make glutamine metabolism an attractive target for therapeutic interventions designed to effectively disrupt cancer cell metabolism (Altman et al., 2016; Matés et al., 2020). Therefore, monitoring and elucidating intracellular glutamine metabolism as a potential therapeutic target, especially in cancer, has attracted the interest of researchers in recent decades (Zhu et al., 2017).
The science of metabolomics is a rapidly growing field of research that focuses on the identification and quantification of metabolites present in a biological system, such as cells or tissues. This field aims to identify and elucidate active metabolic pathways in a given system and to understand how these pathways are affected by different conditions or stimuli. Although there are several approaches to metabolomics research, the primary focus has historically been on the intracellular metabolome, mainly aimed at exploring cellular metabolism to elucidate its functional mechanisms and responses, which is beneficial not only to disease diagnosis but also to many aspects of toxicology and therapeutics (Doroghazi et al., 2014; Guma et al., 2016; Wishart, 2016). In the context of glutamine metabolism, metabolomics can be used to identify the metabolites that are produced or consumed during the breakdown and synthesis of glutamine, and to study the effects of various genetic or environmental factors on this process.
While metabolomics focuses on identifying and quantifying the metabolites, fluxomics has emerged as the process of studying metabolic fluxes, or the rates of biochemical reactions, in a biological system, which is a more dynamic and quantitative approach to understanding metabolism (Emwas et al., 2022). Metabolic fluxes are altered in many diseases, such as cancer, and therefore elucidating how the rate of metabolic processes changes can be of great benefit for the understanding of risk factors, disease development and progression, and development of therapies (Antoniewicz, 2018). Fluxomics approaches such as metabolic flux analysis (MFA) and flux balance analysis (FBA) have advanced various steady-state and kinetic approaches to monitor and analyse metabolic fluxes, which have accelerated the understanding of metabolism (Yasemi & Jolicoeur, 2021). Physicochemical models can be developed to provide insights into how the properties of biological systems come about and how their behaviour can be predicted and regulated (Bordbar et al., 2014; Khodayari et al., 2014). Metabolic network analysis (data-driven approach) based on pseudo steady state (PSS) and dynamic assumptions have been combined with physicochemical models to capture the dynamic behaviour of biological samples (Antoniewicz, 2013a).
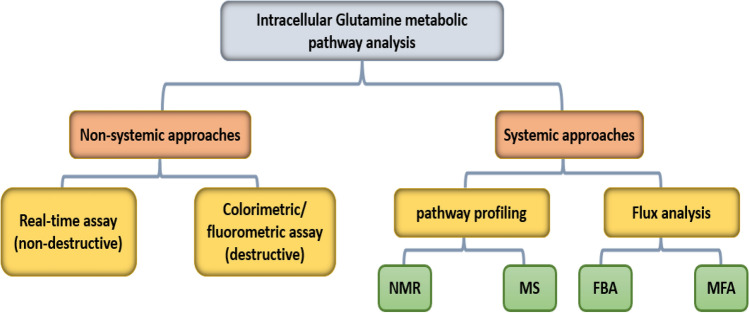
This review paper (as summarised in Fig. 1) explores various analytical approaches used to investigate intracellular metabolism, including metabolic profiling methods [typically using mass spectrometry (MS) and nuclear magnetic resonance (NMR)] and flux analysis techniques (FBA and MFA). Additionally, we discuss two types of physicochemical modeling techniques commonly used in metabolomics and how they have helped to consolidate and expand our understanding of metabolic processes. Using glutamine metabolisation as an exemplar, the limitations and challenges associated with these techniques, as well as their advantages and insights, are explored.
Fig. 1.
Combining analytical approaches, including fluxomics techniques and profiling tools, with physicochemical modelling approaches helps to elucidate a metabolic pathway such as glutamine and provide a deep understanding of cellular function and responses
The lack of a technique that can monitor the kinetics of a metabolic pathway in a living cell in situ is identified as a challenge. To address this limitation, we investigate the potential of label-free vibrational spectroscopy techniques, such as Raman microspectroscopy, which can enable real-time and in situ monitoring of the cellular kinetics of metabolic pathways with subcellular resolution.
In the “Future Perspectives” section, we suggest a combinatorial approach to address the current limitations, which involves utilizing label-free Raman microspectroscopy along with kinetic models trained by the biochemical assays, to enable real-time and in situ monitoring of the cellular kinetics of the glutamine metabolic pathway. While vibrational microspectroscopy is not expected to replace metabolomics, it can potentially complement it by operating in the niche areas where metabolomics falls short. Vibrational spectroscopy can provide the spectral signatures of the cellular complexities, but it cannot distinguish between different metabolites, which demands innovative data analysis tools to extract the dynamics of cellular metabolism, which will be discussed in more detail in the last section.
Glutamine metabolism
Extracellular glutamine is predominantly internalised by cells via the alanine-serine-cysteine transporter 2 (ASCT2), which has itself been the target of many studies, because it is overexpressed in various types of cancer cells (Liu et al., 2018a), including breast cancer, lung cancer and liver cancer (Liu et al., 2018b). Glutamine plays diverse roles in cells, depending on the pathway by which it is metabolised. Figure 2 summarises the main contributions of glutamine to cellular metabolism. After entering the mitochondria, glutamine is converted to glutamate by the enzyme glutaminase (GLS). Subsequently, glutamate undergoes either oxidative deamination by glutamate dehydrogenase (GDH) in the mitochondria, leading to the generation of α-ketoglutarate (α-KG), or transamination in the cytosol, resulting in the production of non-essential amino acids (Li & Le, 2018; Newsholme et al., 2003). α-KG can follow two pathways. In the first, it undergoes oxidation within the TCA cycle [often called the Szent-Györgyi–Krebs cycle, acknowledging the Nobel Prize (1937) winning contributions of Albert Szent-Györgyi in identifying the fumarate, malate and oxaloacetate forming reactions towards the end of the cycle, and those of Hans Adolf Krebs, Nobel Prize (1953), for elucidating the details of the overall cycle], ultimately being converted to pyruvate and lactate in the cytosol through a metabolic process known as glutaminolysis (represented by blue arrows) (‘Albert Szent-Györgyi – Facts—NobelPrize.org’ n.d.; Altman et al., 2016; Krebs & Johnson, 1980; Wang et al., 2010). This process not only drives the TCA cycle and fuels the electron transport chain (ETC), a major energy source, but also participates in supporting redox homeostasis by supplying carbon to the malic enzyme, certain isoforms of which generate NADPH required for anabolic processes such as fatty acid synthesis (Lyssiotis et al., 2013). In the second pathway (represented by red arrows), α-KG undergoes reductive carboxylation, contributing to the synthesis of citrate in the TCA cycle (Yang et al., 2017a), which then contributes in lipid biosynthesis while maintaining the redox balance within the mitochondria (Mullen et al., 2014).
Fig. 2.
Schematic summary of intracellular glutamine metabolism. Glutamine is involved in the synthesis of four main macromolecules in the cytosol: proteins, nucleotides, lipids and fatty acids. In mitochondria, alpha-ketoglutarate derived from glutamine can be further metabolised either by oxidation involving the TCA cycle, termed as the glutaminolysis pathway (indicated by blue arrows), leading to lactate production, or by the reductive carboxylation pathway (indicated by red arrows), leading to lipid synthesis. Gln glutamine, Glc glucose, Glu glutamate, α-KG α-ketoglutarate, Mal malate, OAA oxaloacetate, Cit citrate, GSH glutathione, aa acid amine, Gly glycine, Ser serine, Cys cysteine (Color figure online)
Glutamine also plays a role in promoting the synthesis of macromolecules such as fatty acids, lipids, proteins, and nucleotides by providing carbon and nitrogen for their precursors (DeBerardinis et al., 2007). Additionally, it affects the activity of cytokines such as Tumour Necrosis Factor-alpha (TNF-a) and Interleukin-6 (IL-6) and other factors that boost the immune system (Cruzat et al., 2018). Furthermore, glutamine participates in the production of the antioxidant glutathione (GSH), inhibiting ROS and supporting various biosynthetic pathways crucial for cellular integrity and function (Amores-Sánchez & Medina, 1999).
Abnormalities in glutamate/glutamine metabolism have been linked to disorders such as hyperinsulinism/hyperammonaemia syndrome (Kelly & Stanley, 2001) and mental health disorders like bipolar disorder (Moore et al., 2007) and depression (Abdallah et al., 2014). Moreover, glutamine can be targeted therapeutically, as demonstrated by its ability to repolarize macrophages from an M1 to an M2 phenotype, which can prevent obesity- or diabetes-associated pathology (Ren et al., 2019). Moreover, glutamine metabolism is a crucial topic in cancer research. The Warburg effect, observed in tumor cells, involves the conversion of glycolytic pyruvate to lactate instead of further catabolism through the TCA cycle, leading to reduced energy production per mole of glucose (Manoj et al., 2022; Warburg, 1956). Glutaminolysis serves as an alternative energy source in tumor cells, and its acceleration has been observed in glutamine-addicted cancers such as highly invasive ovarian and mesothelioma cancers (Adhikary et al., 2022; Medina, 2001; Li & Le, 2018; Yang et al., 2017a). However, the role of glutamine in continuous cell proliferation as an anabolic substrate is controversial, as its limited availability in tissues and circulation restricts its biological role in this process (Brosnan, 2003; Lacey & Wilmore, 1990). Glutamine is not replenished upon exhaustion, nor is it produced in sufficient amounts during stress or uncontrolled cancer growth. Therefore, the body must obtain glutamine from the diet during high demands, making it a conditionally essential amino acid (Lacey & Wilmore, 1990). As a result, glutamine can only be considered a conditionally indispensable substrate and/or precursor in the human circulation with limited direct roles as a carbon source in the proliferation and growth of transformed cells.
Apart from its supportive role as a survival factor in the proliferation of cancer cells, glutamine is also involved in cancer suppression through several mechanisms. These include stimulating the activation of the tumour suppressor p53, which causes apoptosis and tumour regression (Suzuki et al., 2010). The expression levels of oncogenes and tumour suppressors have been found to be the most important factors determining the function of glutamine in cancer development or suppression (Iurlaro et al., 2014; Yoo et al., 2020). Therefore, monitoring the glutamine metabolism in a dynamic sense can be valuable for understanding cancer mechanisms and developing novel personalised therapies.
Monitoring the glutamine metabolic pathway
The cellular metabolome accurately reflects the cellular phenotype and function (Beger, 2013), and in vitro metabolomic analysis has played a crucial role in advancing our fundamental understanding of cellular function. However, it is crucial to confirm the results in vivo, as some studies have indicated a significant mismatch between in vivo and in vitro measurements. For example, microglia immunometabolism exhibits a distinct phenotype in vivo compared to primary microglia cultures due to microglia's ability to adapt their energy metabolism to fluctuating nutrient availability (Bernier et al., 2020). Also, while glutamine serves as an anaplerotic substrate in the TCA cycle in glioma cells (DeBerardinis et al., 2007), tracking of 13C-Gln in brain tumor patients has shown minimal contribution of glutamine in the TCA cycle (Marin-Valencia et al., 2012; Mashimo et al., 2014). In this context, monitoring the glutamine metabolic pathway requires both in vitro and in vivo assessment to obtain reliable results. Advanced metabolic imaging techniques such as magnetic resonance spectroscopy (MRS), positron emission tomography (PET), single photon emission computed tomography (SPECT), MS imaging (MSI), and fluorescence imaging enable in vivo evaluation of glutamine metabolism for clinical diagnostics (Ekici et al., 2022). These techniques have already been described and explored in detail in the review by Ekici et al. (2022).
This paper focusses on evaluating the available tools and techniques for monitoring the intracellular glutamine metabolic pathway in in vitro studies. To organize the techniques, they have been grouped into two categories, namely systemic and non-systemic approaches, as illustrated in Fig. 3.
Fig. 3.
Simplified summary of methods for monitoring the glutamine metabolic pathway
Systemic monitoring of the glutamine metabolism by metabolomics
Metabolomics is considered a powerful strategy to gain a systems-level understanding of metabolism and its changes in response to a specific perturbation in biological systems (Ortmayr et al., 2016a, b). This approach works in both a targeted and non-targeted mode, depending on the objective of the study. Metabolomics is treated as a relative quantification technique primarily aimed at obtaining accurate metabolic fingerprints for the maximum number of metabolites detected and subsequently identified in cells (Ortmayr et al., 2016a, b). This systemic metabolomic approach not only measures representative metabolites originating from different metabolic pathways, but can also reveal unknown metabolic pathways and their potential interactions. It thus avoids the limitation of hypothesis-driven methods (in systemic and non-systemic studies), which can lead researchers to overlook the effect of therapy on the entire cellular metabolism (Di Minno et al., 2019; Ribbenstedt et al., 2018; Zhang et al., 2016). Strategies used to holistically study glutamine metabolism include (1) metabolic profiling and, (2) analysis of metabolic activity (fluxes) using stable isotope labelling methods, mostly based on mass spectroscopy (MS) and NMR.
Metabolic profiling
Metabolic profiling approaches allow quantification of hundreds of metabolites in a biological cell, tissue, or organism (Kopka et al., 2004). They provide a more holistic picture of the state of cellular metabolism by providing large-scale detection and information on the structural composition and abundance of a broad range of metabolites, although it must be noted it is not possible to detect every metabolite in a system (Amantonico et al., 2010; Kopka et al., 2004). Untargeted profiling of the metabolome is performed using label-free analytical techniques, MS and NMR-based approaches being the most popular (Kuehnbaum & Britz-Mckibbin, 2013).
Mass spectrometry
The mass spectrometer ionises the chemical components and sorts the ions based on their mass-to-charge ratio (Han & Gross, 2005). The application of MS-based analytical approaches to the field of metabolomics studies has been motivated primarily by the recognised advantages in terms of specificity, sensitivity and a broad detection range that ensure powerful identification and quantification (Theodoridis et al., 2012). An example of its application in the context of glutamine metabolism is the work of Zhou et al. (2012), who elucidated a previously unknown mechanism of glutamine metabolism through a comparative MS analysis of the pancreatic ductal adenocarcinoma cell line. This revealed significant consumption of glutamine as a nitrogen donor in amino acid and nucleotide synthesis (shown by green arrows in Fig. 2), based on the observation of a strong overexpression of the enzyme cytidine triphosphate (CTP) synthase, which catalyses glutamine for the biosynthesis of cytidine, and down-regulation of TCA cycle's enzymes (Zhou et al., 2012).
A serious problem of MS in the identification of metabolites is ion suppression, which is a kind of matrix effect and is mainly caused by the different volatility of metabolites, due to their different nature and structure, which in turn affects the output of the number of ions in the gas phase reaching the detector and eventually leads to errors in the quantification of the analysis (Annesley, 2003; Antignac et al., 2005; Bamba et al., 2012). To address the issue of ion suppression and improve the identification of metabolites in MS analysis, chromatographic separation techniques such as gas chromatography (GC) and liquid chromatography (LC) have been employed (Sandra & Sandra, 2006). These techniques can effectively separate metabolites from interfering matrix compounds in the sample, based on their relative mobilities in the stationary phase which results in better resolution and more accurate quantification of metabolites (Kole et al., 2011). In the flux analysis approach, which will be explored Sect. 3.1.2, the measurement of different isotopomers requires the measurement of different masses, but when it comes to measuring different metabolites, chromatographic separation is preferred to ensure accurate identification and quantification. Moreover, the integration of GC–MS and LC–MS techniques offers a broader coverage of metabolites and facilitates a more comprehensive understanding of complex biological systems in metabolomics studies. Zeki et al. (2020), conducted a comprehensive review highlighting the simultaneous utilisation of GC–MS and LC–MS in untargeted metabolomics investigations, highlighting crucial aspects such as sample preparation, data acquisition, and data processing. Also, advancements in technology have led to the development of new techniques such as supercritical fluid chromatography (SFC), which shows high sensitivity comparable to or even better than LC–MS (Losacco et al., 2019) and technologies like matrix-assisted laser desorption ionization (MALDI) and desorption electrospray ionization (DESI) MS that allow for the spatial identification of metabolites (Matés et al., 2020). These advancements offer further opportunities for improved metabolite analysis.
Another major challenge is the reliability in identifying the detected metabolites. This problem has been solved with Molecular Networking, a visualisation method for tandem MS data (Hollywood et al., 2018). This method assumes that similar molecules have similar MS fragmentation patterns, meaning that they tend to group closely together in a network, and is able to detect the spectral sets of related molecules (Hollywood et al., 2018). Despite all the progress made in MS approaches, such as improving the detection limit to the sub-attomole to zeptomole range, there are still some limitations in using it at the single cell level (Liu et al., 2019).
Nuclear magnetic resonance spectroscopy
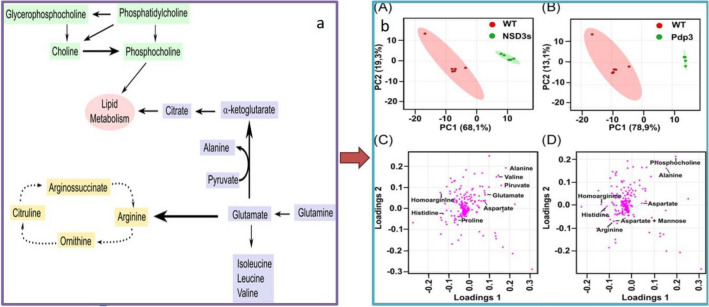
NMR is another high-throughput metabolomics technology that has been used in many studies to analyse metabolomics profiles (Reo, 2002). Recently, Lee et al. used 1H NMR spectroscopy to identify metabolic pathways that are disrupted in gliomas, including lactate, glutamine and alanine, to determine the metabolic characteristics of gliomas that are candidates for targeted therapies (Lee et al., 2019). Another recent study related to glutamine metabolism using NMR revealed a link between the human oncoprotein NSD3s and the rewiring of cancer metabolism by taking the glutaminolysis pathway (Fig. 4a). This study analysed the metabolic response to overexpression of NSD3s in Saccharomyces cerevisiae using 1H NMR and reported that overexpression leads to a simultaneous increase in aspartate and alanine levels and a decrease in arginine levels, which in turn leads to an increase in the rate of glutaminolysis (Fig. 4b) (Rona et al., 2019). Some other applications of NMR as a metabolic profiling tool have been discussed in the work of Larive et al. (2015).
Fig. 4.
a Metabolic pathways of the major metabolites detected on NSD3s and Pdp3 overexpression. The main metabolites detected belong to glutaminolysis (blue), metabolism of cholines (green), and arginine synthesis (yellow). b NMR metabolomics shows similar metabolic profile on NSD3s or Pdp3 overexpression. PCA score plots show a strong class discrimination between control (WT) replicates (red) and NSD3s overexpression (green) (A), and between control (WT) replicates (red) and Pdp3 overexpression (green) (B). PCA loading plots highlight important metabolites for class discrimination between NSD3s+ and WT, such as glutamate, aspartate, and alanine (C), and between Pdp3+ and WT, such as phosphocholine, aspartate, and alanine (D). NMR nuclear magnetic resonance, WT wild-type.
Reproduced from Rona et al. (2019) with permission
On the other hand, analytical instruments for metabolic studies on living cells must not only provide high-resolution data, but also be rapid, since the physiological state of the living cell can change for instance, if it experiences oxygen deprivation for more than a few minutes (Serber et al., 2007). Monitoring the metabolism of living cells by 1D and 2D NMR spectroscopy is limited by the low resolution of the spectra and the long time required, respectively (Motta et al., 2003). Some efforts have been made to solve this issue, such as the application of band-selective optimised flip-angle short-transient (SOFAST) HMQC techniques to 15N-labelled cells, which provide high-resolution spectra of small metabolites directly in living cells in a few seconds (Motta et al., 2003). Although NMR using fluorescent biosensors or optical reporter tags can provide a valuable perspective for identifying the biochemistry of living cells, it, like the MS based approach, is of limited use for in situ tracking of metabolic processes (Lerche et al., 2015), due to the inherent physicochemical properties of small and hydrophilic metabolites, such as low ionisation efficiency and high detection limits (Chalcraft et al., 2009; Kuehnbaum & Britz-Mckibbin, 2013). Furthermore, the datasets generated by NMR and MS are complicated and difficult to model and interpret, although progress continues to be made in statistical approaches to data mining (Coen et al., 2008).
Flux analysis of cellular metabolism
Flux analysis (fluxomics) techniques are used to study the rates of metabolic transitions that are critical for identifying key regulatory nodes in metabolic pathways, based on changes in rates under intra/extracellular perturbations. Understanding the metabolic dynamics and fluxes through these techniques serves as the foundation for comprehending disease mechanisms/phenotypes and developing novel therapies (Metallo & Vander Heiden, 2013; Wishart, 2016). Intracellular metabolic fluxes can be estimated through both MFAs and FBA (Toya et al., 2011). MFA is a technique used to quantify reaction fluxes in metabolic pathways over time, utilising the stable isotope labeling method. In this method, isotopic isomers, or isotopomers, are introduced into the biological system, and based on prior knowledge of carbon transitions through the metabolic pathways, fluxes through different nodes are calculated (Allen & Young, 2020; Yang et al., 2002). MFA estimates intracellular fluxes empirically from labelling patterns in metabolic networks to represent the in vivo metabolic state of the cell (Iwatani et al., 2008). The choice of tracer determines the accuracy of the estimation of metabolic fluxes, as it enables more targeted analysis to investigate specific reactions within the network (Metallo et al., 2009). For example, 13C-labelled glutamine and 15N-labelled glutamine can be used to track the fate of carbons and nitrogen, respectively, in glutamine metabolism (Yang et al., 2017b). There are two main experimental procedures for the estimation of metabolic fluxes by isotope tracers: dynamic (kinetic) and steady-state tracing (Emwas et al., 2022). In kinetic isotope labeling experiments, an unlabelled carbon source like glucose or glutamine is replaced by a labeled one, and the labelling patterns of intermediates and products are measured at different time points (Wang et al., 2020). Conversely, in steady-state experiments, the labelled material is distributed through metabolic pathways until a steady state of label accumulation is achieved in each intracellular metabolite pool (Buescher et al., 2015). Wang et al. provide a review of the development of equations used to determine reaction fluxes derived from labelling patterns applied to biological systems (Wang et al., 2020).
In the context of glutamine metabolic pathways, tracking the fate of labelled metabolites has established the contribution of glutamine carbon atoms to central carbon metabolism pathways, including glutaminolysis, the TCA cycle, and the pentose phosphate pathway (Leippe et al., 2017). Studies using 13C-Gln as an isotopic tracer have revealed that glutamine drives the TCA cycle, and its contribution increases significantly under hypoxia conditions, highlighting the key role of glutaminolysis metabolism in cancer cells (Zhang et al., 2014). Additionally, 13C-labelling studies have provided new insights into the reverse flux or reductive carboxylation of glutamine-derived α-KG through the TCA cycle (Kalyanaraman et al., 2018). In one example, Le et al. (2012) used the tracer [U-13C, 15N]-Gln to investigate the production rate of TCA cycle intermediates from glutamine in human lymphoma B cells (P493) under glucose depletion. The researchers found that P493 cells rely on a glutamine-driven TCA cycle independent of glucose and that inhibiting GLS curbs tumour cell proliferation both in vitro and in vivo. However, intervention or inhibition of the glutamine pathway does not always result in a therapeutically effective outcome such as apoptosis. Alternative metabolic pathways can be activated to compensate for the required energy deficiency (Lopez & Banerji, 2017; Thompson et al., 2017). Numerous studies have reported this, including that of Halama et al. (2018), which investigated the impact of inhibiting GLS C (GAC) in mouse embryonic fibroblasts and found that using the allosteric inhibitor C.968 had no effect on the expected decrease in glutamate and TCA cycle intermediates. These findings suggest that cancer cells may depend on other sources of energy, such as lipid catabolism and autophagy, which are known to accelerate in response to glutamate deficiency.
Isotopic labelling has become a popular approach for studying metabolic pathways, such as glutamine metabolism, and has provided valuable insights into metabolomics science. Nevertheless, there are some drawbacks that must be acknowledged. The primary limitation of isotopic labelling is related to the techniques used to measure isotopic labelling of molecules, such as NMR and MS. NMR spectroscopy is a powerful tool for understanding metabolic fluxes, including reaction rates and metabolite exchange between cell compartments, as well as providing positional labelling information (Tang et al., 2009; Yu et al., 1995). However, there are limitations such as time-consuming sample purification, lower sensitivity and resolution compared to MS, and expensive instrumentation (Lim et al., 2007). The recent review by Giraudeau highlights the ongoing efforts to improve NMR's sensitivity and resolution while maintaining its structure elucidation capabilities (Giraudeau, 2020). Additionally, the high complementarity between MS and NMR has been demonstrated in various studies, and the development of advanced statistical approaches for integrating data from multiple platforms shows promise (Giraudeau, 2020).
Compared to NMR, MS offers high analytical power, allowing accurate and rapid quantification of labelling patterns, making it more suitable for flux measurements due to its widespread availability, lower cost, and sensitive detection of molecular enrichment (Antoniewicz, 2013b; Goudar et al., 2010; Kleijn et al., 2007; Tang et al., 2009). However, MS analysis provides only partial information on the distribution of isotopomers, and it is challenging to extract information about the position of the label from MS data, even when multiple fragments of the same compound are detected (Kohlstedt & Wittmann, 2019). To address this limitation, Tandem-MS (MS/MS) can potentially increase the amount of labelling data available for MFA (Choi & Antoniewicz, 2011). Nonetheless, it remains a destructive technique that cannot monitor cellular kinetics in situ and can only provide a snapshot of the metabolic state at the time of analysis, which may not accurately reflect the dynamic nature of cells and their rapidly changing metabolic state (Wang et al., 2016).
Another limitation of isotopic labelling is that it can introduce potential biases in the metabolism due to the labeled carbon source being processed differently from the unlabelled carbon source, leading to misleading results (Bianco & Perrotta, 2015; Chaube, 2014; Hernandez-Saavedra et al., 2020). These limitations have prompted researchers to explore alternative, non-destructive methods, such as “label-free” techniques, which offer advantages such as low damage to cells, high sensitivity, and small working volumes (Cunningham & Laing, 2008; Krafft et al., 2017).
Non-systemic simple biochemical assays
Biochemical assays adopt different strategies for measuring different aspects of a single node of the metabolism. To generalise, the biochemical assays either target a specific metabolite and quantify it or try to decode the enzyme kinetics, one enzyme at a time. The assays targeting the metabolites measure the changes associated with viable (real-time quantification) or non-viable cells (steady-state quantification). Figure 5 shows the points that can be measured by biochemical assays, a list of which is provided in the Supplementary Information (Table S1). These approaches may better reflect changes in metabolite concentrations (Cajka & Fiehn, 2016).
Fig. 5.
Depiction of the metabolites/enzymes involved in glutamine metabolism that can be measured by biochemical assays. CoA coenzyme-A, Cit citrate, ICT isocitrate, IDH isocitrate dehydrogenase, α-KG α-ketoglutarate, KGDHC α-ketoglutarate dehydrogenase complex, SuccCoA succinyl-CoA, SCS succinyl-CoA synthesize, Succ succinate, SDH succinate dehydrogenase, Fum fumarate, Mal malate, MDH malate dehydrogenase, OAA oxaloacetate, CS citrate synthesize, Glu glutamate, GLS glutaminase, GDH glutamate dehydrogenase
Monitoring glutamine metabolism by enzyme kinetics or metabolite quantification assays in a snapshot
Biochemical assays are commonly used due to their affordability, reproducibility, and simplicity (Majdinasab et al., 2021). Glutamine metabolism is closely linked to mitochondrial GLS, which converts glutamine to glutamate through a deamination reaction. This allows glutamate to enter the TCA cycle, generate ATP and NADPH, regulate GSH homeostasis, and control ROS, as shown in Fig. 2. Measuring intracellular levels of substrates such as glutamate, α-KG, ATP, and GLS activity at different time points can provide insight into glutamine metabolism (Leippe et al., 2017). Various colorimetric and fluorometric detection assays are available to measure changes in the aforementioned enzymes or metabolites in the cell (Fu et al., 2017). However, changes in metabolite concentrations are not a reliable indicator of metabolic rates or changes in directionality since an increase in metabolite concentration could signify an increase in precursor enzyme activity and/or a decrease in successor enzyme activity (Bennett et al., 2009; Fendt et al., 2010).
Additionally, biochemical assays are destructive, requiring cells to be lysed at each time point to quantify intracellular metabolites. Therefore, these approaches do not provide continuous kinetic monitoring of glutamine metabolism. However, steady-state approaches based on mathematical modeling are valid during constant exponential growth phase in batch cultures and steady-state in continuous cultures (Buescher et al., 2015; Yasemi & Jolicoeur, 2021). Combining steady-state kinetic monitoring with computational modeling is widely used to study metabolic changes and identify potential therapeutic targets. For instance, a recent study investigated the effect of Bcl-2-associated athanogene 3 (BAG3) on GLS stabilization and reported increased glutaminolysis, as indicated by quantifying glutamate and α-KG levels in cells using appropriate assays (Zhao et al., 2019). The study also suggested BAG3 as an optional therapeutic target against cancer by demonstrating its involvement in the regulation of glutaminolysis (Zhao et al., 2019).
Dynamic monitoring of glutamine metabolism by extracellular metabolite quantification assays
To monitor the metabolic activity of a pathway, the real-time viability assay is one of the most widely used tools, which measures the rate of uptake/excretion of metabolites such as glucose, glutamine, lactate, and ammonia, providing an overview of overall cellular metabolism (Antoniewicz, 2013a; Stoll et al., 1996). For example, inhibition of glutamine uptake by l-γ-glutamyl-p-nitroanilide (GPNA) (measuring the rate of glutamine degradation from the medium provides information about the rate of glutamine uptake by cells in a culture system) has a dampening effect on the metabolism of gastric cancer cells (Ma et al., 2021).
Furthermore, there are commercially available assays designed to monitor glycolysis flux, which can be used to kinetically monitor glutaminolysis flux as well, because the glutaminolysis pathway leads to lactate production and is hyperactive in proliferating cells. Such assays measure the extracellular acidification rate (ECAR), which is used to quantify proton production and serves as a substitute marker for lactate production (‘Glycolytic rate measurement, glycolysis assay, pH Xtra | Agilent’ n.d.) For instance, Rupprecht et al. determined both ECAR and oxygen consumption rate (OCR) to analyse the influence of glucose and glutamine deficiency on the metabolic activity of N18TG2 cells (Rupprecht et al., 2019). The results showed that glutamine deficiency strongly affected both the OCR and ECAR, leading to their reversal. Glucose deficiency also led to increased glutamine uptake in the cells, indicating that the cells switched to the oxidative metabolism under glucose shortage (Rupprecht et al., 2019). Similarly, Trilla-Fuertes et al. used cell viability assays in combination with computational models to investigate the link between glutaminolysis and breast cancer (Trilla-Fuertes et al., 2020).
Although these assays provided valuable insights, the data alone were not sufficient to fully describe the intracellular fluxes in the metabolic pathway (Junot et al., 2014). Therefore, it is necessary to combine data from these assays, such as the rate of extracellular lactate production, which represents the fluxes of glycolysis and glutaminolysis, with intracellular spectroscopic approaches and physicochemical models to obtain a computational estimate of metabolic fluxes and gain a deeper understanding of cellular metabolism (Buescher et al., 2015; Leippe et al., 2017; Liao et al., 2019).
Metabolic modelling of glutamine metabolism
The physicochemical modelling approach provides a valuable framework for simulating cellular metabolic kinetics. It helps predict cellular phenotypic responses to external factors such as drugs or changing environmental conditions by providing a quantitative understanding of metabolism and its regulation (Becker et al., 2007), which in turn can generate hypotheses to improve overall process performance by optimising and regulating process conditions and enable potentially useful therapeutic interventions. In addition, experimental validation of these predictions can provide new experimental data that can further improve the models (McGillen et al., 2014; Moulin et al., 2021; Vasilakou et al., 2016)..Because the biological experiments provide the flux information needed to construct the models, accurate identification of metabolites and determination of flux changes is critical to the accuracy of subsequent predictions of network properties made using the models (Acosta et al., 2007). In general, there are different types of mathematical models to describe intracellular reaction processes and metabolism, such as constraint-based models, spatial models, dynamical model components, qualitative and logical models, and kinetic models. For the description of metabolism, most studies have been drawn from these two groups: constraint-based models (or stoichiometric models) and kinetic models (Gombert & Nielsen, 2000; Keating et al., 2020; Nielsen, 2017).
Constraint based models
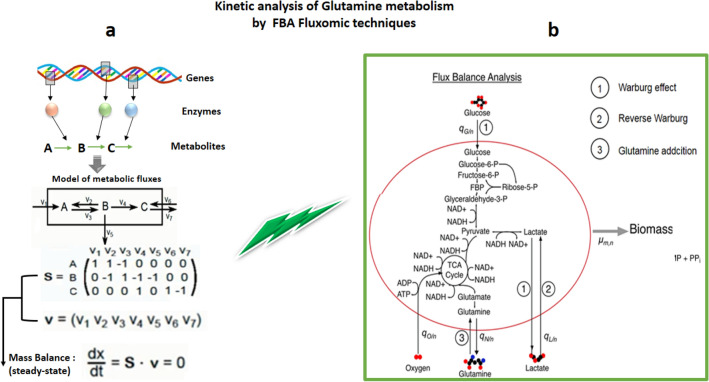
Constraint-based modelling, also known as stoichiometry-based or FBA, is a mathematical modelling technique for rebuilding a metabolic network model of microorganisms in order to simulate and predict metabolic reaction fluxes, on the basis of which the cellular function can be interpreted (Covert et al., 2001; O’Brien et al., 2015). This technique has been developed based on the knowledge of whole genome sequencing (WGS) of microorganisms and the development of tools for annotating gene functions (Covert et al., 2001; O’Brien et al., 2015). FBA predicts reaction fluxes within defined boundaries of a metabolic network reconstruction based on mass balance or steady-state assumptions, meaning that the metabolite concentration cannot change within the system, e.g. dATP/dt = dNADH/dt = 0, upon which a set of FBA equations can be constructed (Maarleveld et al., 2013; Orth et al., 2010). A summarised form of the FBA process is shown in Fig. 6a. FBA models use the principles of reaction stoichiometry and physicochemical constraints that may include thermodynamics or kinetics such as energy balance, mass balance and flux constraints (Cotten & Reed, 2013). These models use Boolean expressions to represent the relationship between genes, proteins and metabolic reactions (Machado et al., 2016). A popular example of this modelling approach is Genome-scale metabolic models that simulate whole cell metabolic reactions based on genome sequences (using the Human Metabolism Map; Recon2) using the COBRA Toolbox v2.0 library (Ryu et al., 2015). Although, it is hardly possible to build a comprehensive mathematical model for a living cell that can include all cellular processes as many aspects of cellular function are still unknown (Nielsen, 2017), several whole-cell models have been developed, such as a model developed by Markus Covert which represents the most comprehensive mathematical model for a whole cell of Mycoplasma genitalium (Karr et al., 2012).
Fig. 6.
a In FBA method, the metabolic reaction fluxes within the boundary can be calculated by solving the equation SV = 0 (S stands for matrix and V stands for reaction rate) using linear programming, optimising or minimising an objective function, such as maximising cell growth, under given constraints. b FBA model for central carbon metabolism. The arrows indicate the flux of metabolites in a simplified representation of cell metabolism and growth. The uptake and production rates of the ith metabolite and nth metabolic phenotype are denoted as qi/n [g/g − DW−hr]. The FBA model is optimised by imposing the maximum growth rate, μm,n [hr−1], as an objective function for each cell type and metabolic phenotype. b Has been adapted from Shan et al. (2018), under the terms of the Creative Commons Attribution License
The quality of flux predictions in this technique is significantly affected by the choice of cellular objective. In cancer cells, lactate production as a cellular objective might show the ability to correctly predict high flux through glycolysis pathway (Schwartz et al., 2015). Zhang and Boley (2022) constructed a non-linear multi-objective FBA model in which an objective function contains three important objective terms, namely: ATP production rate, ATP yield and lactate production rate. Using this model, they were able to show the inducing effect of glutaminolysis on the Warburg effect in cancer cells (Zhang & Boley, 2022). A good example of a FBA model created by Shan et al. for central carbon metabolism is shown in Fig. 6b. The model includes labels for the primary steps of the three hypotheses, which are: (1) the Warburg effect, (2) the Reverse Warburg effect, and (3) Glutamine addiction (Shan et al., 2018). Constraint-based models have provided the fundamental biological insights into metabolic pathways (Xu et al., 2013). For example, Damiani et al. (2017) employed the model of the glucose and glutamine metabolism and reported that, when oxygen conditions are insufficient to fully oxidise the available glucose and glutamine carbons (as in the environment of proliferating cells), redox homeostasis can be maintained through switching on alternative metabolic pathways, like utilising glutamine through reductive carboxylation which results in lipid production. Nevertheless, such models do not provide the level of kinetic details of the metabolic pathway that is required to show how changes in the dynamic concentration of cellular components affect functional behaviour (Srinivasan et al., 2015). In recent years, efforts have been made to apply dynamic control of metabolism on the constraint-based approaches by taking extracellular by-products into account, leading to dynamic FBA (dFBA), through which dynamic models to study a bio-system under transient conditions can be developed to capture the dynamic changes in metabolic fluxes through time series of concentration measurements (Llaneras et al., 2012; Yasemi & Jolicoeur, 2021). Dynamic flux analysis approaches are now increasingly applied to investigate and optimise microbial and mammalian bioprocesses (Antoniewicz, 2013b).
Kinetic models
Kinetic models that express how changes in metabolite concentrations affect local reaction rates are able to dynamically analyse the metabolic state of biological systems and also provide dynamic simulation of metabolism by implementing reaction kinetics (Smallbone & Mendes, 2013). These models require the determination of macroscopic data to obtain a general formulation of the dynamics of the metabolic process (Richelle & Bogaerts, 2015). However, the selection of appropriate model structures is often based on a trial-and-error approach (Richelle & Bogaerts, 2015).
In 1988, Savageau proposed a general kinetic formalism to represent any non-linear function using power equations (S and Volterra Systems, generalised mass actions) (Savageau, 1988). However, these forms were developed to describe the overall reaction rates of a biological network and are not able to describe a double component effect (Richelle & Bogaerts, 2015). Furthermore, parameter estimation in the context of kinetic structure identification is a rather difficult task, as these functions often involve a large number of highly correlated parameters (Richelle & Bogaerts, 2015). Against this background, Haag et al. have proposed an extension of Michaelis–Menten kinetics using the classical extended Monod law to circumvent the problems of identifiability associated with possible over parameterisation (Haag et al., 2003). This general formalism allows for either activation or inhibition effects with a reduced number of parameters. Furthermore, enzyme kinetics are very well illustrated by the Michaelis–Menten equation, which essentially describes the kinetics of an enzyme with two key parameters: the limiting rate (V) and the Michaelis–Menten constant (KM) (Schnell, 2014). Notably, Karry et al. have recently proposed the “inverse Michaelis–Menten equation”, which is intended for kinetic analyses of enzyme reactions at interfaces, especially for enzyme kinetics in steady state, in which the enzyme concentration far exceeds the molar concentration of the available surface sites (Kari et al., 2017).
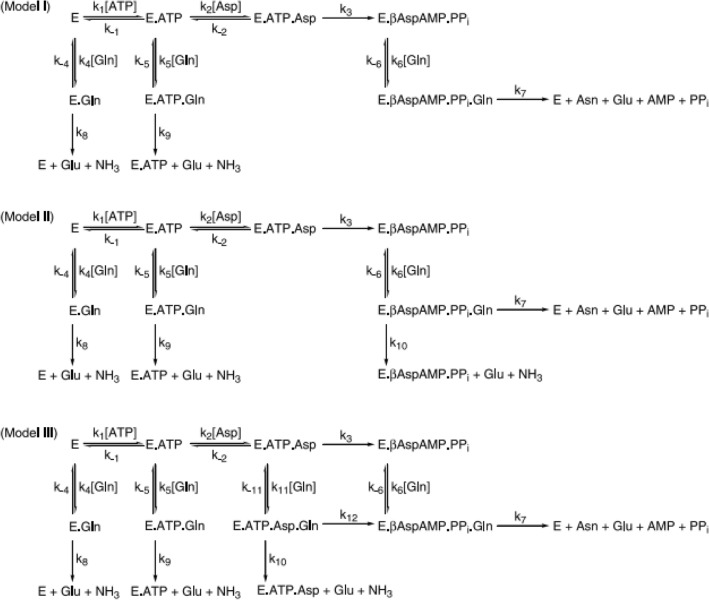
There have been various proposed kinetic models for the metabolic pathway of glutamine (Schuster et al., 2021; Wang et al., 2022). The example shown in Scheme 1 examined the kinetic behaviour of glutamine-dependent asparagine synthetases (AS-B) (Tesson et al., 2003). This model was able to demonstrate that the coordination of the two active sites in AS-B occurs only after the formation of a b-aspartyl AMP intermediate in the synthetase site of the enzyme by incorporating a quaternary E.ATP.Asp.Gln complex. This complex can either proceed to the formation of asparagine or release ammonia by unproductive glutamine hydrolysis (Tesson et al., 2003). Another recent study revealed a new role for glutamine as a major source of acetyl-CoA to revive TCA cycle metabolism, which the authors hypothesised could occur under the condition of mitochondrial dysfunction, such as mutations in the ETC or TCA cycle, by which α-KG (from glutamine) is carboxylated by NADPH-dependent isoforms of isocitrate dehydrogenase to produce isocitrate and then acetyl-CoA (Hensley et al., 2013).
Scheme 1.
Models for the kinetic mechanism of Escherichia coli asparagine synthetase B. (Top) Proposed kinetic mechanism based on product inhibition and isotope trapping experiments (model I) (Middle). Model I modified to include the leakage of ammonia from the intramolecular tunnel (model II). (Bottom) Modified proposal for the AS-B kinetic mechanism that assumes catalytic site coordination after βAspAMP formation and no leakage of ammonia from the intramolecular tunnel (model III). The models are reproduced from Tesson et al. (2003) with permission
The development of the standardised platforms of Systems Biology Markup Language (SBML; Hucka et al., 2003) and Systems Biology Graphical Notation (SBGN; Mi et al., 2009) has led to the emergence of a growing number of open-source computational modelling tools such as Cell Designer (Funahashi et al., 2008), which allows users to easily draw and kinetically model biochemical pathways (Le Novère et al., 2006), and provides an interface to many other metabolic pathway enrichment databases (Xia et al., 2010). Many metabolic models are available in SMBL, along with a MATLAB (The MathWorks, Inc.) script for extracting annotation information (reaction equations) from the corresponding author (Quek et al., 2010). These advances have made kinetic modelling of complex cellular mechanisms and pathways significantly more accessible in recent years (Strutz et al., 2019).
Future perspectives
Cells constantly adjust their metabolic fluxes pathway preferences to respond to any change in the environment in vivo, and probably the same mechanisms control the behaviour of cells in in vitro culture. Therefore, monitoring the kinetic evolution of metabolic processes in living cells is of great value in the quest to understand physiological responses to modulators (like drugs and signalling molecules)and the early onset of disease (Klein et al., 2022). However, intracellular metabolism is a very complicated network, so it is not possible to assess the overall metabolic behaviour based on the properties of the individual components alone (Volkova et al., 2020). Therefore, to understand cellular metabolic behaviour, it must be analysed holistically by taking advantage of multi omics approaches. Furthermore, in situ monitoring and analysis of cellular metabolism should be both systematic and non-destructive in order to gain a deeper understanding of the kinetics and regulatory mechanisms underlying the metabolic network.
Among the techniques recently developed for real-time monitoring of metabolism, vibrational spectroscopies, including IR and Raman spectroscopy, have attracted much attention because they provide rapid and simultaneous data on multiple variables in a non-destructive manner (Moros et al., 2010). A molecular compound consisting of n atoms has 3n − 6 vibrational modes (3n − 5 for linear molecules). Each vibrational mode of a molecule has a specific band pattern in the spectrum of IR absorption or Raman scattering, and occurs at a specific wavenumber related to its transition energy. Therefore, molecular labelling is not required in vibrational spectroscopic techniques because all sample molecules have specific band patterns, or a “fingerprint”. In complex multicomponent samples, the combined chemical fingerprint can be used to characterise the state of the sample (Sato et al., 2018). This advantage makes it unnecessary to lyse, stain or fix the living sample (Sato et al., 2018). Also, the direct immersion of microscope objectives or fibre-optic probes in the culture medium enables the use of these light-based spectroscopic techniques for in situ or in-line monitoring of important parameters (Lourenço et al., 2012). Both techniques, IR absorption and Raman scattering, have been explored as an integral part of Process Analytical Technologies (PAT), in which, according to the Quality by Design paradigm (Food and Drug Administration, 2004), in situ monitoring of a bioprocess, such as metabolism, can minimise the risk of contamination during sampling and the delays (between sampling and analysis time) associated with off-line methods (Bhatia et al., 2018; Graf et al., 2022), and both techniques have been demonstrated to provide very accurate prediction of the concentrations of various compounds in the cell culture medium (Bhatia et al., 2018; Sellick et al., 2010). IR absorption spectroscopy analyses the amount of absorbed infrared light according to the interaction with a molecule that occurs at a certain wavenumber. It is a popular technique, because it affords rapid sampling and operational simplicity (Beć et al., 2020). The fundamental vibrational frequencies appear in the mid-IR (~ 500–3500 cm−1), while the near-IR region (~ 780 nm to 2500 nm) can be used to measure vibrational overtone and combination modes, which are intrinsically orders of magnitude weaker. The weaker absorption in this region gives rise to greater sampling depths, which makes the technique of near-IR spectroscopy popular in, for example, food science (‘Near-Infrared Spectroscopy in Food Science and Technology—Google Books’ n.d.). Mid-IR absorption spectroscopy is well established in analytical chemistry, as a first port of call for materials characterisation and identification and has increasingly found applications in, amongst others, the pharmaceutical sector (Bunaciu et al., 2010). Water is a notoriously strong absorber in the mid-IR, however, and so applications of analysis in aqueous environments are limited, although there have been extensive explorations of biomedical applications of IR based spectroscopic techniques for label free analysis and classification of, for example, (dried) biofluid (Theakstone et al., 2021) and histopathological biopsies (Paraskevaidi et al., 2021). Many studies have used near-NIR and mid-IR spectroscopy to monitor various intracellular metabolic processes, a list of these studies is included in the review paper by Landgrebe et al. (2010). One example is the use of near-IR spectroscopy by Rhiel et al. (2004) for the off-line and real-time monitoring of PC-3 human prostate cancer cells to quantify the consumption rate of glucose and glutamine, which were determined to be 6.8 and 1.8 × 10−17 mol/cell s, respectively (Rhiel et al., 2004). This, and similar works, demonstrate the potential of the technique for online, in situ, real time process monitoring, although there remain some challenges related to these instruments, such as the interpretation of the spectral data, which is difficult due to the overlap of the detected peaks (Abu-Absi et al., 2011; Arnold et al., 2003; Scarff et al., 2008). The technique is also limited in the quantification of intracellular glutamine, as the accurate detection range is above the glutamine level in a mammalian cell culture medium (Foley et al., 2012). Compared to near-IR, mid-IR is known to be a more sensitive technique, and its ability to effectively monitor multiple analytes in a bioprocess in real-time has been confirmed by many studies (Roychoudhury et al., 2006), one of which is the work of Rhiel et al., who successfully used Fourier transform mid-IR spectroscopy for real-time and in situ monitoring of glucose and lactate analytes in bioreactor cultures of Chinese hamster ovary for long-term monitoring at industrial production scale (Rhiel et al., 2002). Although the mid-IR region provides more distinct spectral peaks compared to near-IR, the peaks detected from water in aqueous systems such as biological cells (since most part of the cells are composed of water) interfere greatly by overlapping or masking the peaks of other organic molecules and make the analysis complicated (Abu-Absi et al., 2011).
Often considered a complementary technique to IR absorption (‘Biomedical Applications of Synchrotron Infrared Microspectroscopy: A practical approach—Google Books’ n.d.), Raman spectroscopy quantifies the intensity of inelastically scattered monochromatic light by a molecule at varying frequencies. The frequency shift is due to the change in energy of the photon (which corresponds to the energy of a molecular vibration) as it couples to the vibrations of the molecule (Locke et al., 2019). Raman spectroscopy is particularly sensitive to symmetric, polarisable vibrations (e.g. C=C bonds), whereas asymmetric, polar vibrations (e.g. OH) are very strong IR absorbers. Raman can thus be more readily applied to aqueous systems, and is considered a strong candidate for in vivo biomedical applications (Baker et al., 2018). It should be noted that, according to the report by Li et al., Raman spectroscopy performs better than near-IR spectroscopy in terms of accuracy, selectivity, sensitivity and detection range (Li et al., 2018). Furthermore, because Raman spectroscopy can be performed at source wavelengths in the visible or infrared wavelengths, operation in a confocal microscopic mode has led to increasing application for characterisation and monitoring processes at a cellular and subcellular level, with the prospects of high content spectroscopic analysis (Efeoglu et al., 2018; Farhane et al., 2018; McIntyre et al., 2018).
The potential of Raman spectroscopy for real-time in situ monitoring of key metabolic parameters such as glucose, glutamine and lactate in mammalian cell cultures has been demonstrated (Abu-Absi et al., 2011; Santos et al., 2018). Konorov et al. (2012) investigated the autophagic response to starvation stimuli (glutamine deficiency) in living cells using Raman spectroscopy. Raman spectroscopy was able to non-invasively monitor the dynamics of relevant changes through the starvation state and recovery pathway, and they used fluorescence microscopy with GFP-LC3 labelled cells to confirm the obtained results about generated response in the cells. The results show that glutamine deficiency in both cell lines, MCF-7 (human breast cancer cells) and LMD (mouse metastatic prostate cancer cells), leads to a 2.9-fold and 2.1-fold increase in the autophagosomal compartments, respectively (Konorov et al., 2012). The study also identified the 718 cm−1 Raman band, related to phospholipids, as a spectral marker associated with the response, as it has a much higher intensity in cells lacking glutamine (Konorov et al., 2012).
Although the study of Konorov et al. was innovative in its use of Raman microspectrocopy to monitor the metabolic processes in live cell, the spectral analysis was largely qualitative, or univariate, based on the analysis of individual peaks or peak ratios (Konorov et al., 2012). As biological cells are made up of complex molecules such as proteins and DNA, the Raman spectrum becomes very complex, limiting the ability to identify or analyse a particular molecule due to the overlap of bands (Li et al., 2018). Label-free techniques inherently detect all species present within the sampled area, and identification of specific responses requires multivariate analytical methods to extract the differential signatures that are indicative of cellular injury events or response pathways. These signatures can be influenced by changes in concentration or conformation of any or all constituent biomolecules, as well as environmental factors such as pH. It is becoming increasingly clear that approaches focused on identifying differential spectroscopic signatures, rather than specific bands associated with individual biomolecules, may be more effective. As a result, chemometric or multivariate data analysis tools are necessary for extracting the desired information. These techniques help to extract the quantitative concentration information to facilitate the interpretation of the spectral data by solving problems such as background fluctuations, baseline drift or fluorescence (Bos et al., 2020; O’Connell et al., 2010). In other words, the precision of Raman performance depends on chemometric models or multivariate analyses to process the large dataset consisting of thousands of variables and to identify the relationship between the variables (Yousefi-Darani et al., 2022). In unsupervised analyses, a Principal Components Analysis approach is commonly employed to reduce the dimensionality of the data, and in doing so identify the main spectral features which give rise to variances or between datasets. Systematic and/or evolving sources of variance are better explored, or data mined, using regression or correlation analyses (Byrne et al., 2020). The study by Baradez et al. explored Raman spectroscopy as a tool for in-line monitoring of an autologous T-cell immunotherapy model produced in a stirred tank bioreactor system (Baradez et al., 2018). Chemometric models were constructed based on multivariate regression (Projection to Latent Structures) of the Raman spectral data generated by continuous in line monitoring of the bioreactor against off-line reference data for glucose, lactate, glutamine, glutamate, and ammonia concentrations, with reference also to Raman spectra of the pure components. Figure 7 demonstrates that the multivariate regression approach was able to generate models for real time, in situ monitoring of the constituent components.
Fig. 7.
The correlation between chemometric models for glucose, lactate, glutamine, glutamate, and ammonia with reference bioanalyser measurements collected every 24 h, as well as continuous Raman chemometric analysis. The information pertains to a single bioprocess run involving all four donors, and closed circles in the graph represent the reference bioanalyser measurements.
Reproduced from Baradez et al. (2018) under the Creative Commons Attribution License
The ability of Raman microspectroscopy to monitor the uptake of chemotherapeutic agents such as Doxorubicin (DOX) in in A549 and Calu-1 lung cancer cells has been demonstrated (Farhane et al., 2015a, 2017a). Partial Least Squares Regression (PLSR) was furthermore explored to differentiate between the spectral signatures of the initial intracellular chemical binding of cisplatin (Nawaz et al., 2010, 2011), vincristine (Nawaz et al., 2013) and more recently DOX (Farhane et al., 2015b, 2017a, b; Szafraniec et al., 2016) and Actinomycin (Farhane et al., 2018a) from the subsequent cellular response pathway. 2D Spectral Cross-Correlation has been employed to similarly differentiate between drug binding events and cellular response pathways (Farhane et al., 2018b), and Spectral Cross-Correlation in cellular maps has been demonstrated as a sensitive tool to profile the subcellular distribution of specific spectral signatures (Keating et al., 2012). Another flexible chemometric approach is Multivariate Curve Resolution-Alternating Least Squares (MCR-ALS) analysis, which can be used to data mine spectra which are representative of the time course of a bioprocess and interpret changes in the evolution of the chemical components of the process (Jaumot et al., 2005; Pérez-Guaita et al., 2022). Koch et al. have demonstrated the use of MCR-ALS for extracting data on changes in multiple analytes in a bioprocess measured by FT-IR spectroscopy (Koch et al., 2014). However, chemometric techniques require the isolation of information about the system to avoid abstruseness (Pérez-Guaita et al., 2022). The performance of the MCR-ALS approach is guided by constraints, which ensure, for example, non-negativity of the component spectra (de Juan & Tauler, 2021), and were extended to include kinetic constraints, in a Hard–Soft modelling approach, such that the analysis of the evolution of a series of measurements can constrained to fit a kinetic model (De Juan et al., 2000). Such an approach has been employed, for example, by Perez-Guaita et al. to data mine the Raman spectroscopic signatures of the cellular uptake of and response to DOX (Perez-Guaita et al., 2020, 2022).
The studies to date illustrate that the continued development of protocols for harnessing and interpreting the label free, high content spectral information which Raman microspectroscopic analysis can yield is imperative, and this is further driven by the demands of emerging technologies. Although full spectral coherent Raman microspectroscopic imaging is currently only available in limited frequency ranges, it has already been demonstrated (Camp et al., 2014), and this imaging technique holds great promise for label-free high-content spectroscopic analysis of sub-cellular processes in real-time, as well as unparalleled visualization of cellular processes and function.. In this context, a recent live cell imaging study proposed a new platform for combining stimulated Raman (SRS) with deuterium-labelled glutamine (Gln-d5) to analyse the nature of polyglutamine (polyQ) aggregates in Huntington's disease (Miao & Wei, 2020). They achieved high sensitivity and specific Raman imaging of aggregated Gln-d5 in living cells (Fig. 8), which could lead to the conclusion that (1) these aggregates are 1.8 times denser compared to those with GFP labelling used in fluorescence microscopy, (2) the composition of the proteins depends on the size of the aggregates and (3) the inhibition of aggregate formation reveals the possibility of an intermediate folding state caused by local hyperhydration of the aggregates (the associated resulting data indicate higher C–H/C–D binding ratios favouring the possibility of interaction with heat shock protein (HSP) 40/70) (Miao & Wei, 2020).
Fig. 8.
Live-cell SRS imaging of mHtt-97Q-GFP aggregates with Gln-d5 labelling. a SRS imaging of mHtt aggregates (arrowheaded, 2167 cm−1, C–D on), validated by fluorescence imaging through GFP (Fluorescence). Off-resonance image at 2035 cm−1 shows no signal. b Live-cell SRS images for an mHtt-97Q-GFP aggregate (arrowheaded) at Gln-d5 (2167 cm−1), CH3 (2940 cm−1), and amide I (1664 cm−1) channels on the same set of HeLa cells. c SRS imaging of an mHtt-97Q-GFP aggregate at 2143 cm−1 by leucine-d10 (Leu-d10) labelling. d Average Sagg/Bcell from SRS images of C–D with Gln-d5 labelling (5.75 ± 1.03, n = 13); amide I (2.15 ± 0.34, n = 4); CH3 (1.66 ± 0.14, n = 10); and C–D with Leu-d10 labelling (2.45 ± 0.33, n = 10). Error bar SD.
Reproduced from Miao and Wei (2020) under the terms of the Creative Commons Attribution License
Reviewing the methods used to monitor glutamine metabolism, it can be concluded that Raman spectroscopy has more desirable properties as a quantitative analytical approach compared to conventional analytical methods such as MS or NMR. Label-free monitoring using Raman microspectroscopy not only avoids bias in biological response, but is also capable of non-invasively monitoring the metabolic dynamics of living organisms to develop more effective metabolic intervention strategies for therapeutic approaches (Konorov et al., 2012; Lussier et al., 2019). Moreover, extracellular metabolites can be simultaneously analysed with Raman (Shalabaeva et al., 2017). Therefore, for real-time and in situ monitoring the cellular kinetics of metabolic pathway of glutaminolysis, it is conceivable to employ kinetic models, informed by time series, conventional biochemical models such as the pH extracellular assay (measures the lactate production rate), as the basis on which multivariate data mining techniques such as MCR-ALS can reliably extract the signatures of interest from label-free, Raman microspectroscopic analysis of live cells, and interpret them in terms of their biochemical origin. (Fig. 9).
Fig. 9.
Schematic illustration of proposed paradigm of use of conventional biochemical assays on which to develop metabolic flus models, which can in turn be used to guide the multivariate analysis of label free, live cell Raman microspectroscopic data
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
ZM: Conceptualization, Writing—Original Draft, NP: Writing—Original Draft, OH: Writing—Original Draft, PC: Writing—Original Draft, HJB: Conceptualization, Writing—Original Draft, Supervision, Funding Acquisition.
Funding
Open Access funding provided by the IReL Consortium. ZM and NP are funded by Science Foundation Ireland Frontiers for the Future Award 20/FFPP/8517.
Data Availability
For more detailed data, please refer to the supplementary material of the referenced paper.
Declarations
Conflict of interest
The Authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdallah CG, Jiang L, De Feyter HM, Fasula M, Krystal JH, Rothman DL, et al. Glutamate metabolism in major depressive disorder. American Journal of Psychiatry. 2014;171(12):1320–1327. doi: 10.1176/APPI.AJP.2014.14010067/ASSET/IMAGES/LARGE/APPI.AJP.2014.14010067F2.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Absi NR, Kenty BM, Cuellar ME, Borys MC, Sakhamuri S, Strachan DJ, et al. Real time monitoring of multiple parameters in mammalian cell culture bioreactors using an in-line Raman spectroscopy probe. Biotechnology and Bioengineering. 2011;108(5):1215–1221. doi: 10.1002/BIT.23023. [DOI] [PubMed] [Google Scholar]
- Acosta ML, Sánchez A, García F, Contreras A, Molina E. Analysis of kinetic, stoichiometry and regulation of glucose and glutamine metabolism in hybridoma batch cultures using logistic equations. Cytotechnology. 2007;54(3):189–200. doi: 10.1007/S10616-007-9089-9/FIGURES/8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikary G, Shrestha S, Naselsky W, Newland JJ, Chen X, Xu W, et al. Mesothelioma cancer cells are glutamine addicted and glutamine restriction reduces YAP1 signaling to attenuate tumor formation. Molecular Carcinogenesis. 2022 doi: 10.1002/MC.23497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DK, Young JD. Tracing metabolic flux through time and space with isotope labeling experiments. Current Opinion in Biotechnology. 2020;64:92–100. doi: 10.1016/J.COPBIO.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nature Reviews Cancer. 2016;16(10):619–634. doi: 10.1038/nrc.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amantonico A, Urban PL, Zenobi R. Analytical techniques for single-cell metabolomics: State of the art and trends. Analytical and Bioanalytical Chemistry. 2010;398(6):2493–2504. doi: 10.1007/S00216-010-3850-1. [DOI] [PubMed] [Google Scholar]
- Amores-Sánchez MI, Medina MÁ. Glutamine, as a precursor of glutathione, and oxidative stress. Molecular Genetics and Metabolism. 1999;67(2):100–105. doi: 10.1006/MGME.1999.2857. [DOI] [PubMed] [Google Scholar]
- Annesley TM. Ion suppression in mass spectrometry. Clinical Chemistry. 2003;49(7):1041–1044. doi: 10.1373/49.7.1041. [DOI] [PubMed] [Google Scholar]
- Antignac JP, De Wasch K, Monteau F, De Brabander H, Andre F, Le Bizec B. The ion suppression phenomenon in liquid chromatography–mass spectrometry and its consequences in the field of residue analysis. Analytica Chimica Acta. 2005;529(1–2):129–136. doi: 10.1016/J.ACA.2004.08.055. [DOI] [Google Scholar]
- Antoniewicz MR. Dynamic metabolic flux analysis—Tools for probing transient states of metabolic networks. Current Opinion in Biotechnology. 2013;24(6):973–978. doi: 10.1016/J.COPBIO.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Antoniewicz MR. 13C metabolic flux analysis: Optimal design of isotopic labeling experiments. Current Opinion in Biotechnology. 2013;24(6):1116–1121. doi: 10.1016/J.COPBIO.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Antoniewicz MR. A guide to 13C metabolic flux analysis for the cancer biologist. Experimental and Molecular Medicine. 2018;50(4):1–13. doi: 10.1038/s12276-018-0060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SA, Crowley J, Woods N, Harvey LM, McNeil B. In situ near infrared spectroscopy to monitor key analytes in mammalian cell cultivation. Biotechnology and Bioengineering. 2003;84(1):13–19. doi: 10.1002/BIT.10738. [DOI] [PubMed] [Google Scholar]
- Baker MJ, Byrne HJ, Chalmers J, Gardner P, Goodacre R, Henderson A, et al. Clinical applications of infrared and Raman spectroscopy: State of play and future challenges. The Analyst. 2018;143(8):1735–1757. doi: 10.1039/C7AN01871A. [DOI] [PubMed] [Google Scholar]
- Bamba T, Lee JW, Matsubara A, Fukusaki E. Metabolic profiling of lipids by supercritical fluid chromatography/mass spectrometry. Journal of Chromatography A. 2012;1250:212–219. doi: 10.1016/J.CHROMA.2012.05.068. [DOI] [PubMed] [Google Scholar]
- Baradez MO, Biziato D, Hassan E, Marshall D. Application of Raman spectroscopy and univariate modelling as a process analytical technology for cell therapy bioprocessing. Frontiers in Medicine. 2018 doi: 10.3389/FMED.2018.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beć KB, Grabska J, Huck CW. Biomolecular and bioanalytical applications of infrared spectroscopy—A review. Analytica Chimica Acta. 2020;1133:150–177. doi: 10.1016/J.ACA.2020.04.015. [DOI] [PubMed] [Google Scholar]
- Becker SA, Feist AM, Mo ML, Hannum G, Palsson B, Herrgard MJ. Quantitative prediction of cellular metabolism with constraint-based models: The COBRA Toolbox. Nature Protocols. 2007;2(3):727–738. doi: 10.1038/nprot.2007.99. [DOI] [PubMed] [Google Scholar]
- Beger RD. A review of applications of metabolomics in cancer. Metabolites. 2013;3(3):552–574. doi: 10.3390/METABO3030552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Kimball EH, Gao M, Osterhout R, Van Dien SJ, Rabinowitz JD. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nature Chemical Biology. 2009;5(8):593–599. doi: 10.1038/nchembio.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier LP, York EM, MacVicar BA. Immunometabolism in the brain: How metabolism shapes microglial function. Trends in Neurosciences. 2020;43(11):854–869. doi: 10.1016/J.TINS.2020.08.008. [DOI] [PubMed] [Google Scholar]
- Bhatia H, Mehdizadeh H, Drapeau D, Yoon S. In-line monitoring of amino acids in mammalian cell cultures using Raman spectroscopy and multivariate chemometrics models. Engineering in Life Sciences. 2018;18(1):55–61. doi: 10.1002/ELSC.201700084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco L, Perrotta G. Methodologies and perspectives of proteomics applied to filamentous fungi: From sample preparation to secretome analysis. Open Access International Journal of Molecular Science. 2015;16:16. doi: 10.3390/ijms16035803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biomedical applications of synchrotron infrared microspectroscopy: A practical approach (n.d.). Google Books. Retrieved January 27, 2023, from https://books.google.ie/books?hl=en&lr=&id=R3YoDwAAQBAJ&oi=fnd&pg=PA105&dq=Raman+Microscopy:+Complement+or+Competitor%3F%E2%80%9D,++Hugh+J.+Byrne,+Ganesh+D.+Sockalingum+and+Nick+Stone&ots=-lT7iYLN-K&sig=Z9QtWifUlN1QAjEOQerXbuxCJ4Q&redir_esc=y#v=onepage&q=Raman%20Microscopy%3A%20Complement%20or%20Competitor%3F%E2%80%9D%2C%20%20Hugh%20J.%20Byrne%2C%20Ganesh%20D.%20Sockalingum%20and%20Nick%20Stone&f=false
- Bordbar A, Monk JM, King ZA, Palsson BO. Constraint-based models predict metabolic and associated cellular functions. Nature Reviews Genetics. 2014;15(2):107–120. doi: 10.1038/nrg3643. [DOI] [PubMed] [Google Scholar]
- Bos TS, Knol WC, Molenaar SRA, Niezen LE, Schoenmakers PJ, Somsen GW, Pirok BWJ. Recent applications of chemometrics in one- and two-dimensional chromatography. Journal of Separation Science. 2020;43(9–10):1678–1727. doi: 10.1002/JSSC.202000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan JT. Interorgan amino acid transport and its regulation. The Journal of Nutrition. 2003;133(6):2068S–2072S. doi: 10.1093/JN/133.6.2068S. [DOI] [PubMed] [Google Scholar]
- Buescher JM, Antoniewicz MR, Boros LG, Burgess SC, Brunengraber H, Clish CB, et al. A roadmap for interpreting 13C metabolite labeling patterns from cells. Current Opinion in Biotechnology. 2015;34:189–201. doi: 10.1016/J.COPBIO.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunaciu AA, Aboul-Enein HY, Fleschin S. Application of Fourier transform infrared spectrophotometry in pharmaceutical drugs analysis. Applied Spectroscopy Reviews. 2010;45(3):206–219. doi: 10.1080/00387011003601044. [DOI] [Google Scholar]
- Byrne HJ, Bonnier F, McIntyre J, Parachalil DR. Quantitative analysis of human blood serum using vibrational spectroscopy. Clinical Spectroscopy. 2020;2:100004. doi: 10.1016/J.CLISPE.2020.100004. [DOI] [Google Scholar]
- Cajka T, Fiehn O. Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics. Analytical Chemistry. 2016;88(1):524–545. doi: 10.1021/ACS.ANALCHEM.5B04491/ASSET/IMAGES/LARGE/AC-2015-04491V_0008.JPEG. [DOI] [PubMed] [Google Scholar]
- Camp CH, Lee YJ, Heddleston JM, Hartshorn CM, Walker ARH, Rich JN, et al. High-speed coherent Raman fingerprint imaging of biological tissues. Nature Photonics. 2014;8(8):627–634. doi: 10.1038/nphoton.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalcraft KR, Lee R, Mills C, Britz-McKibbin P. Virtual quantification of metabolites by capillary electrophoresis–electrospray ionization–mass spectrometry: Predicting ionization efficiency without chemical standards. Analytical Chemistry. 2009;81(7):2506–2515. doi: 10.1021/AC802272U/SUPPL_FILE/AC802272U_SI_001.PDF. [DOI] [PubMed] [Google Scholar]
- Chaube R. Absolute quantitation of post-translational modifications. Frontiers in Chemistry. 2014 doi: 10.3389/fchem.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Antoniewicz MR. Tandem mass spectrometry: A novel approach for metabolic flux analysis. Metabolic Engineering. 2011;13(2):225–233. doi: 10.1016/J.YMBEN.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Coen M, Holmes E, Lindon JC, Nicholson JK. NMR-based metabolic profiling and metabonomic approaches to problems in molecular toxicology. Chemical Research in Toxicology. 2008;21(1):9–27. doi: 10.1021/TX700335D/ASSET/IMAGES/LARGE/TX-2007-00335D_0005.JPEG. [DOI] [PubMed] [Google Scholar]
- Cotten C, Reed JL. Mechanistic analysis of multi-omics datasets to generate kinetic parameters for constraint-based metabolic models. BMC Bioinformatics. 2013;14(1):1–13. doi: 10.1186/1471-2105-14-32/FIGURES/6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covert MW, Schilling CH, Palsson B. Regulation of gene expression in flux balance models of metabolism. Journal of Theoretical Biology. 2001;213(1):73–88. doi: 10.1006/JTBI.2001.2405. [DOI] [PubMed] [Google Scholar]
- Cruzat V, Rogero MM, Keane KN, Curi R, Newsholme P. Glutamine: Metabolism and immune function, supplementation and clinical translation. Nutrients. 2018;10(11):1564. doi: 10.3390/NU10111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham BT, Laing LG. Advantages and application of label-free detection assays in drug screening. Expert Opinion on Drug Discovery. 2008;3(8):891–901. doi: 10.1517/17460441.3.8.891. [DOI] [PubMed] [Google Scholar]
- Damiani C, Colombo R, Gaglio D, Mastroianni F, Pescini D, Westerhoff HV, et al. A metabolic core model elucidates how enhanced utilization of glucose and glutamine, with enhanced glutamine-dependent lactate production, promotes cancer cell growth: The Warburg effect. PLoS Computational Biology. 2017;13(9):e1005758. doi: 10.1371/JOURNAL.PCBI.1005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Juan A, Maeder M, Martínez M, Tauler R. Combining hard- and soft-modelling to solve kinetic problems. Chemometrics and Intelligent Laboratory Systems. 2000;54(2):123–141. doi: 10.1016/S0169-7439(00)00112-X. [DOI] [Google Scholar]
- de Juan A, Tauler R. Multivariate Curve Resolution: 50 Years addressing the mixture analysis problem—A review. Analytica Chimica Acta. 2021;1145:59–78. doi: 10.1016/J.ACA.2020.10.051. [DOI] [PubMed] [Google Scholar]
- Deberardinis RJ, Cheng T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2009;29(3):313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(49):19345–19350. doi: 10.1073/PNAS.0709747104/SUPPL_FILE/09747FIG6.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Minno A, Porro B, Turnu L, Manega CM, Eligini S, Barbieri S, et al. Untargeted metabolomics to go beyond the canonical effect of acetylsalicylic acid. Journal of Clinical Medicine. 2019;9(1):51. doi: 10.3390/JCM9010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroghazi JR, Albright JC, Goering AW, Ju KS, Haines RR, Tchalukov KA, et al. A roadmap for natural product discovery based on large-scale genomics and metabolomics. Nature Chemical Biology. 2014;10(11):963–968. doi: 10.1038/nchembio.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeoglu E, Maher MA, Casey A, Byrne HJ. Toxicological assessment of nanomaterials: The role of in vitro Raman microspectroscopic analysis. Analytical and Bioanalytical Chemistry. 2018;410(6):1631–1646. doi: 10.1007/S00216-017-0812-X/FIGURES/1. [DOI] [PubMed] [Google Scholar]
- Ekici S, Nye JA, Neill SG, Allen JW, Shu HK, Fleischer C. Glutamine imaging: A new avenue for glioma management. American Journal of Neuroradiology. 2022;43(1):11–18. doi: 10.3174/AJNR.A7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emwas AH, Szczepski K, Al-Younis I, Lachowicz JI, Jaremko M. Fluxomics—New metabolomics approaches to monitor metabolic pathways. Frontiers in Pharmacology. 2022;13:299. doi: 10.3389/FPHAR.2022.805782/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhane Z, Bonnier F, Byrne HJ. Monitoring doxorubicin cellular uptake and trafficking using in vitro Raman microspectroscopy: Short and long time exposure effects on lung cancer cell lines. Analytical and Bioanalytical Chemistry. 2017;409(5):1333–1346. doi: 10.1007/S00216-016-0065-0/FIGURES/8. [DOI] [PubMed] [Google Scholar]
- Farhane Z, Bonnier F, Byrne HJ. An in vitro study of the interaction of the chemotherapeutic drug Actinomycin D with lung cancer cell lines using Raman micro-spectroscopy. Journal of Biophotonics. 2018;11(1):e201700112. doi: 10.1002/JBIO.201700112. [DOI] [PubMed] [Google Scholar]
- Farhane Z, Bonnier F, Byrne HJ. An in vitro study of the interaction of the chemotherapeutic drug Actinomycin D with lung cancer cell lines using Raman micro-spectroscopy. Journal of Biophotonics. 2018 doi: 10.1002/JBIO.201700112. [DOI] [PubMed] [Google Scholar]
- Farhane Z, Bonnier F, Casey A, Byrne HJ. Raman micro spectroscopy for in vitro drug screening: Subcellular localisation and interactions of doxorubicin. The Analyst. 2015;140(12):4212–4223. doi: 10.1039/C5AN00256G. [DOI] [PubMed] [Google Scholar]
- Farhane Z, Bonnier F, Maher MA, Bryant J, Casey A, Byrne HJ. Differentiating responses of lung cancer cell lines to Doxorubicin exposure: In vitro Raman micro spectroscopy, oxidative stress and bcl-2 protein expression. Journal of Biophotonics. 2017;10(1):151–165. doi: 10.1002/JBIO.201600019. [DOI] [PubMed] [Google Scholar]
- Farhane Z, Nawaz H, Bonnier F, Byrne HJ. In vitro label-free screening of chemotherapeutic drugs using Raman microspectroscopy: Towards a new paradigm of spectralomics. Journal of Biophotonics. 2018;11(3):e201700258. doi: 10.1002/JBIO.201700258. [DOI] [PubMed] [Google Scholar]
- Fendt SM, Buescher JM, Rudroff F, Picotti P, Zamboni N, Sauer U. Tradeoff between enzyme and metabolite efficiency maintains metabolic homeostasis upon perturbations in enzyme capacity. Molecular Systems Biology. 2010;6(1):356. doi: 10.1038/MSB.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley R, Hennessy S, Marison IW. Potential of mid-infrared spectroscopy for on-line monitoring of mammalian cell culture medium components. Applied Spectroscopy. 2012;66(1):33–39. doi: 10.1366/11-06395. [DOI] [Google Scholar]
- Food and Drug Administration. (2004). Guidance for industry, PAT-A framework for innovative pharmaceutical development, manufacturing and quality assurance. FDA. Retrieved February 3, 2023, from http://www.fda.gov/cder/guidance/published.html, https://cir.nii.ac.jp/crid/1570854175899248000
- Fu X, Hu X, Li N, Zheng F, Dong X, Duan J, et al. Glutamine and glutaminolysis are required for efficient replication of infectious spleen and kidney necrosis virus in Chinese perch brain cells. Oncotarget. 2017;8(2):2400. doi: 10.18632/ONCOTARGET.13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi A, Matsuoka Y, Jouraku A, Morohashi M, Kikuchi N, Kitano H. Cell Designer 3.5: A versatile modeling tool for biochemical networks. Proceedings of the IEEE. 2008;96(8):1254–1265. doi: 10.1109/JPROC.2008.925458. [DOI] [Google Scholar]
- Giraudeau P. NMR-based metabolomics and fluxomics: Developments and future prospects. The Analyst. 2020;145(7):2457–2472. doi: 10.1039/D0AN00142B. [DOI] [PubMed] [Google Scholar]
- Glycolytic rate measurement, glycolysis assay, pH Xtra | Agilent. (n.d.). Retrieved April 1, 2023, from https://www.agilent.com/en/product/cell-analysis/real-time-cell-metabolic-analysis/plate-reader-metabolic-assays/ph-xtra-glycolysis-assay-740895
- Gombert AK, Nielsen J. Mathematical modelling of metabolism. Current Opinion in Biotechnology. 2000;11(2):180–186. doi: 10.1016/S0958-1669(00)00079-3. [DOI] [PubMed] [Google Scholar]
- Goudar C, Biener R, Boisart C, Heidemann R, Piret J, de Graaf A, Konstantinov K. Metabolic flux analysis of CHO cells in perfusion culture by metabolite balancing and 2D [13C, 1H] COSY NMR spectroscopy. Metabolic Engineering. 2010;12(2):138–149. doi: 10.1016/J.YMBEN.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Graf A, Woodhams A, Nelson M, Richardson DD, Short SM, Brower M, Hoehse M. Automated data generation for Raman spectroscopy calibrations in multi-parallel mini bioreactors. Sensors. 2022 doi: 10.3390/S22093397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guma M, Tiziani S, Firestein GS. Metabolomics in rheumatic diseases: Desperately seeking biomarkers. Nature Reviews Rheumatology. 2016;12(5):269–281. doi: 10.1038/nrrheum.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag, J. E., Vande Wouwer, A., & Remy, M. (2003). A general model of reaction kinetics in biological systems. In 2003 European control conference (ECC), 2003 (pp. 2929–2934). 10.23919/ECC.2003.7086485
- Halama A, Kulinski M, Dib SS, Zaghlool SB, Siveen KS, Iskandarani A, et al. Accelerated lipid catabolism and autophagy are cancer survival mechanisms under inhibited glutaminolysis. Cancer Letters. 2018;430:133–147. doi: 10.1016/J.CANLET.2018.05.017. [DOI] [PubMed] [Google Scholar]
- Han X, Gross RW. Shotgun lipidomics: Electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrometry Reviews. 2005;24(3):367–412. doi: 10.1002/MAS.20023. [DOI] [PubMed] [Google Scholar]
- Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: Cell biology, physiology, and clinical opportunities. The Journal of Clinical Investigation. 2013;123(9):3678–3684. doi: 10.1172/JCI69600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Saavedra D, Sanders L, Freeman S, Reisz JA, Lee MH, Mickael C, et al. Stable isotope metabolomics of pulmonary artery smooth muscle and endothelial cells in pulmonary hypertension and with TGF-beta treatment. Scientific Reports. 2020 doi: 10.1038/s41598-019-57200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollywood KA, Schmidt K, Takano E, Breitling R. Metabolomics tools for the synthetic biology of natural products. Current Opinion in Biotechnology. 2018;54:114–120. doi: 10.1016/J.COPBIO.2018.02.015. [DOI] [PubMed] [Google Scholar]
- Hucka M, Finney A, Sauro HM, et al. The systems biology markup language (SBML): A medium for representation and exchange of biochemical network models. Bioinformatics. 2003;19(4):524–531. doi: 10.1093/BIOINFORMATICS/BTG015. [DOI] [PubMed] [Google Scholar]
- Iurlaro R, León-Annicchiarico CL, Muñoz-Pinedo C. Regulation of cancer metabolism by oncogenes and tumor suppressors. Methods in Enzymology. 2014;542:59–80. doi: 10.1016/B978-0-12-416618-9.00003-0. [DOI] [PubMed] [Google Scholar]
- Iwatani S, Yamada Y, Usuda Y. Metabolic flux analysis in biotechnology processes. Biotechnology Letters. 2008;30(5):791–799. doi: 10.1007/S10529-008-9633-5/TABLES/1. [DOI] [PubMed] [Google Scholar]
- Jaumot J, Gargallo R, De Juan A, Tauler R. A graphical user-friendly interface for MCR-ALS: A new tool for multivariate curve resolution in MATLAB. Chemometrics and Intelligent Laboratory Systems. 2005;76(1):101–110. doi: 10.1016/J.CHEMOLAB.2004.12.007. [DOI] [Google Scholar]
- Junot C, Fenaille F, Colsch B, Bécher F. High resolution mass spectrometry based techniques at the crossroads of metabolic pathways. Mass Spectrometry Reviews. 2014;33(6):471–500. doi: 10.1002/MAS.21401. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B, Cheng G, Hardy M, Ouari O, Lopez M, Joseph J, et al. A review of the basics of mitochondrial bioenergetics, metabolism, and related signaling pathways in cancer cells: Therapeutic targeting of tumor mitochondria with lipophilic cationic compounds. Redox Biology. 2018;14:316–327. doi: 10.1016/J.REDOX.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kari J, Andersen M, Borch K, Westh P. An inverse Michaelis-Menten approach for interfacial enzyme kinetics. ACS Catalysis. 2017;7(7):4904–4914. doi: 10.1021/ACSCATAL.7B00838/ASSET/IMAGES/LARGE/CS-2017-00838N_0006.JPEG. [DOI] [Google Scholar]
- Karr JR, Sanghvi JC, MacKlin DN, Gutschow MV, Jacobs JM, Bolival B, et al. A whole-cell computational model predicts phenotype from genotype. Cell. 2012;150(2):389–401. doi: 10.1016/J.CELL.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating ME, Bonnier F, Byrne HJ. Spectral cross-correlation as a supervised approach for the analysis of complex Raman datasets: The case of nanoparticles in biological cells. The Analyst. 2012;137(24):5792–5802. doi: 10.1039/C2AN36169H. [DOI] [PubMed] [Google Scholar]
- Keating SM, Waltemath D, König M, Zhang F, Dräger A, Chaouiya C, et al. SBML Level 3: An extensible format for the exchange and reuse of biological models. Molecular Systems Biology. 2020;16(8):e9110. doi: 10.15252/MSB.20199110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A, Stanley CA. Disorders of glutamate metabolism. Mental Retardation and Developmental Disabilities Research Reviews. 2001;7(4):287–295. doi: 10.1002/MRDD.1040. [DOI] [PubMed] [Google Scholar]
- Khodayari A, Zomorrodi AR, Liao JC, Maranas CD. A kinetic model of Escherichia coli core metabolism satisfying multiple sets of mutant flux data. Metabolic Engineering. 2014;25:50–62. doi: 10.1016/J.YMBEN.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Kleijn RJ, Geertman JMA, Nfor BK, Ras C, Schipper D, Pronk JT, et al. Metabolic flux analysis of a glycerol-overproducing Saccharomyces cerevisiae strain based on GC–MS, LC–MS and NMR-derived 13C-labelling data. FEMS Yeast Research. 2007;7(2):216–231. doi: 10.1111/J.1567-1364.2006.00180.X. [DOI] [PubMed] [Google Scholar]
- Klein SG, Alsolami SM, Arossa S, Ramos-Mandujano G, Parry AJ, Steckbauer A, et al. In situ monitoring reveals cellular environmental instabilities in human pluripotent stem cell culture. Communications Biology. 2022;5(1):1–10. doi: 10.1038/s42003-022-03065-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C, Posch AE, Goicoechea HC, Herwig C, Lendl B. Multi-analyte quantification in bioprocesses by Fourier-transform-infrared spectroscopy by partial least squares regression and multivariate curve resolution. Analytica Chimica Acta. 2014;807:103–110. doi: 10.1016/J.ACA.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlstedt M, Wittmann C. GC–MS-based 13C metabolic flux analysis resolves the parallel and cyclic glucose metabolism of Pseudomonas putida KT2440 and Pseudomonas aeruginosa PAO1. Metabolic Engineering. 2019;54:35–53. doi: 10.1016/J.YMBEN.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Kole PL, Venkatesh G, Kotecha J, Sheshala R. Recent advances in sample preparation techniques for effective bioanalytical methods. Biomedical Chromatography. 2011;25(1–2):199–217. doi: 10.1002/BMC.1560. [DOI] [PubMed] [Google Scholar]
- Konorov SO, Jardon MA, Piret JM, Blades MW, Turner RFB. Raman microspectroscopy of live cells under autophagy-inducing conditions. The Analyst. 2012;137(20):4662–4668. doi: 10.1039/C2AN35477B. [DOI] [PubMed] [Google Scholar]
- Kopka J, Fernie A, Weckwerth W, Gibon Y, Stitt M. Metabolite profiling in plant biology: Platforms and destinations. Genome Biology. 2004;5(6):1–9. doi: 10.1186/GB-2004-5-6-109/FIGURES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafft C, Schmitt M, Schie IW, Cialla-May D, Matthäus C, Bocklitz T, Popp J. Label-free molecular imaging of biological cells and tissues by linear and nonlinear Raman spectroscopic approaches. Angewandte Chemie International Edition. 2017;56(16):4392–4430. doi: 10.1002/ANIE.201607604. [DOI] [PubMed] [Google Scholar]
- Krebs HA, Johnson WA. The role of citric acid in intermediate metabolism in animal tissues. FEBS Letters. 1980;117(S1):K2–K10. doi: 10.1016/0014-5793(80)80564-3. [DOI] [PubMed] [Google Scholar]
- Kuehnbaum NL, Britz-Mckibbin P. New advances in separation science for metabolomics: Resolving chemical diversity in a post-genomic era. Chemical Reviews. 2013;113(4):2437–2468. doi: 10.1021/CR300484S/ASSET/IMAGES/MEDIUM/CR-2012-00484S_0017.GIF. [DOI] [PubMed] [Google Scholar]
- Lacey JM, Wilmore DW. Is glutamine a conditionally essential amino acid? Nutrition Reviews. 1990;48(8):297–309. doi: 10.1111/J.1753-4887.1990.TB02967.X. [DOI] [PubMed] [Google Scholar]
- Landgrebe D, Haake C, Höpfner T, Beutel S, Hitzmann B, Scheper T, et al. On-line infrared spectroscopy for bioprocess monitoring. Applied Microbiology and Biotechnology. 2010;88(1):11–22. doi: 10.1007/S00253-010-2743-8/FIGURES/5. [DOI] [PubMed] [Google Scholar]
- Larive CK, Barding GA, Dinges MM. NMR spectroscopy for metabolomics and metabolic profiling. Analytical Chemistry. 2015;87(1):133–146. doi: 10.1021/AC504075G/ASSET/IMAGES/LARGE/AC-2014-04075G_0011.JPEG. [DOI] [PubMed] [Google Scholar]
- Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metabolism. 2012;15(1):110–121. doi: 10.1016/J.CMET.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Jeun SS, Kim SH, Yoo CY, Baek HM, Yang SH. Metabolic profiling of human gliomas assessed with NMR. Journal of Clinical Neuroscience. 2019;68:275–280. doi: 10.1016/J.JOCN.2019.07.078. [DOI] [PubMed] [Google Scholar]
- Leippe D, Sobol M, Vidugiris G, Cali JJ, Vidugiriene J. Bioluminescent assays for glucose and glutamine metabolism: High-throughput screening for changes in extracellular and intracellular metabolites. SLAS Discovery: Advancing Life Sciences R&D. 2017;22(4):366–377. doi: 10.1177/1087057116675612. [DOI] [PubMed] [Google Scholar]
- Le Novère N, Bornstein B, Broicher A, Courtot M, Donizelli M, Dharuri H, et al. BioModels Database: A free, centralized database of curated, published, quantitative kinetic models of biochemical and cellular systems. Nucleic Acids Research. 2006;34(Suppl 1):D689–D691. doi: 10.1093/NAR/GKJ092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerche MH, Jensen PR, Karlsson M, Meier S. NMR insights into the inner workings of living cells. Analytical Chemistry. 2015;87(1):119–132. doi: 10.1021/AC501467X/ASSET/IMAGES/LARGE/AC-2014-01467X_0008.JPEG. [DOI] [PubMed] [Google Scholar]
- Li M, Ebel B, Chauchard F, Guédon E, Marc A. Parallel comparison of in situ Raman and NIR spectroscopies to simultaneously measure multiple variables toward real-time monitoring of CHO cell bioreactor cultures. Biochemical Engineering Journal. 2018;137:205–213. doi: 10.1016/J.BEJ.2018.06.005. [DOI] [Google Scholar]
- Li T, Le A. Glutamine metabolism in cancer. Advances in Experimental Medicine and Biology. 2018;1063:13–32. doi: 10.1007/978-3-319-77736-8_2/COVER. [DOI] [PubMed] [Google Scholar]
- Liao M, Liao W, Xu N, Li B, Liu F, Zhang S, et al. LncRNA EPB41L4A-AS1 regulates glycolysis and glutaminolysis by mediating nucleolar translocation of HDAC2. eBioMedicine. 2019;41:200–213. doi: 10.1016/J.EBIOM.2019.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M, Ye H, Panoskaltsis N, Drakakis EM, Yue X, Cass AEG, et al. Intelligent bioprocessing for haemotopoietic cell cultures using monitoring and design of experiments. Biotechnology Advances. 2007 doi: 10.1016/j.biotechadv.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen X, Zhang Y, Liu J. Advancing single-cell proteomics and metabolomics with microfluidic technologies. The Analyst. 2019;144(3):846–858. doi: 10.1039/C8AN01503A. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhao T, Li Z, Wang L, Yuan S, Sun L. The role of ASCT2 in cancer: A review. European Journal of Pharmacology. 2018;837:81–87. doi: 10.1016/J.EJPHAR.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Llaneras F, Sala A, Picó J. Dynamic estimations of metabolic fluxes with constraint-based models and possibility theory. Journal of Process Control. 2012;22(10):1946–1955. doi: 10.1016/J.JPROCONT.2012.09.001. [DOI] [Google Scholar]
- Locke A, Belsare S, Deutz N, Coté G. Aptamer-switching optical bioassay for citrulline detection at the point-of-care. Journal of Biomedical Optics. 2019;24(12):1. doi: 10.1117/1.JBO.24.12.127002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JS, Banerji U. Combine and conquer: Challenges for targeted therapy combinations in early phase trials. Nature Reviews Clinical Oncology. 2017 doi: 10.1038/nrclinonc.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losacco GL, Veuthey JL, Guillarme D. Supercritical fluid chromatography–mass spectrometry: Recent evolution and current trends. Trends in Analytical Chemistry. 2019;118:731–738. doi: 10.1016/J.TRAC.2019.07.005. [DOI] [Google Scholar]
- Lourenço ND, Lopes JA, Almeida CF, Sarraguça MC, Pinheiro HM. Bioreactor monitoring with spectroscopy and chemometrics: A review. Analytical and Bioanalytical Chemistry. 2012;404(4):1211–1237. doi: 10.1007/S00216-012-6073-9/TABLES/6. [DOI] [PubMed] [Google Scholar]
- Lussier F, Missirlis D, Spatz JP, Masson JF. Machine-learning-driven surface-enhanced Raman scattering optophysiology reveals multiplexed metabolite gradients near cells. ACS Nano. 2019 doi: 10.1021/ACSNANO.8B07024. [DOI] [PubMed] [Google Scholar]
- Lyssiotis CA, Son J, Cantley LC, Kimmelman AC. Pancreatic cancers rely on a novel glutamine metabolism pathway to maintain redox balance. Cell Cycle. 2013;12(13):1987–1988. doi: 10.4161/CC.25307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Wu J, Zhou M, Wu J, Wu Z, Lin L, et al. Inhibition of glutamine uptake improves the efficacy of cetuximab on gastric cancer. Integrative Cancer Therapies. 2021 doi: 10.1177/15347354211045349/ASSET/IMAGES/LARGE/10.1177_15347354211045349-FIG2.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maarleveld TR, Khandelwal RA, Olivier BG, Teusink B, Bruggeman FJ. Basic concepts and principles of stoichiometric modeling of metabolic networks. Biotechnology Journal. 2013;8(9):997–1008. doi: 10.1002/BIOT.201200291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado D, Herrgård MJ, Rocha I. Stoichiometric representation of gene–protein–reaction associations leverages constraint-based analysis from reaction to gene-level phenotype prediction. PLoS Computational Biology. 2016;12(10):e1005140. doi: 10.1371/JOURNAL.PCBI.1005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdinasab M, Daneshi M, Louis Marty J. Recent developments in non-enzymatic (bio)sensors for detection of pesticide residues: Focusing on antibody, aptamer and molecularly imprinted polymer. Talanta. 2021;232:122397. doi: 10.1016/J.TALANTA.2021.122397. [DOI] [PubMed] [Google Scholar]
- Manoj KM, Nirusimhan V, Parashar A, Edward J, Gideon DA. Murburn precepts for lactic-acidosis, Cori cycle, and Warburg effect: Interactive dynamics of dehydrogenases, protons, and oxygen. Journal of Cellular Physiology. 2022;237(3):1902–1922. doi: 10.1002/JCP.30661. [DOI] [PubMed] [Google Scholar]
- Marin-Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang XL, et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metabolism. 2012;15(6):827–837. doi: 10.1016/J.CMET.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagandla S, et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014;159(7):1603–1614. doi: 10.1016/J.CELL.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matés JM, Di Paola FJ, Campos-Sandoval JA, Mazurek S, Márquez J. Therapeutic targeting of glutaminolysis as an essential strategy to combat cancer. Seminars in Cell and Developmental Biology. 2020;98:34–43. doi: 10.1016/J.SEMCDB.2019.05.012. [DOI] [PubMed] [Google Scholar]
- McGillen JB, Kelly CJ, Martínez-González A, Martin NK, Gaffney EA, Maini PK, Pérez-García VM. Glucose-lactate metabolic cooperation in cancer: Insights from a spatial mathematical model and implications for targeted therapy. Journal of Theoretical Biology. 2014;361:190–203. doi: 10.1016/J.JTBI.2014.09.018. [DOI] [PubMed] [Google Scholar]
- McIntyre J, Farhane Z, Efeoglu E, Casey A, Maher M, Byrne HJ, Bonnier F. Advancing Raman microspectroscopy for cellular and subcellular analysis: Towards in vitro high-content spectralomic analysis. Applied Optics. 2018;57(22):E11–E19. doi: 10.1364/AO.57.000E11. [DOI] [PubMed] [Google Scholar]
- Medina MA. Glutamine and cancer. The Journal of Nutrition. 2001;131(9):2539S–2542S. doi: 10.1093/JN/131.9.2539S. [DOI] [PubMed] [Google Scholar]
- Metallo CM, Vander Heiden MG. Understanding metabolic regulation and its influence on cell physiology. Molecular Cell. 2013;49(3):388–398. doi: 10.1016/J.MOLCEL.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metallo CM, Walther JL, Stephanopoulos G. Evaluation of 13C isotopic tracers for metabolic flux analysis in mammalian cells. Journal of Biotechnology. 2009;144(3):167–174. doi: 10.1016/J.JBIOTEC.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Schreiber F, Le Novére N, Moodie S, Sorokin A. Systems biology graphical notation: Activity flow language level 1. Nature Proceedings. 2009;2009:1. doi: 10.1038/npre.2009.3724.1. [DOI] [PubMed] [Google Scholar]
- Miao K, Wei L. Live-cell imaging and quantification of PolyQ aggregates by stimulated Raman scattering of selective deuterium labeling. ACS Central Science. 2020;6(4):478–486. doi: 10.1021/ACSCENTSCI.9B01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CM, Frazier JA, Glod CA, Breeze JL, Dieterich M, Finn CT, et al. Glutamine and glutamate levels in children and adolescents with bipolar disorder: A 4.0-T proton magnetic resonance spectroscopy study of the anterior cingulate cortex. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(4):524–534. doi: 10.1097/CHI.0B013E31802F5F2C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moros J, Garrigues S, de la Guardia M. Vibrational spectroscopy provides a green tool for multi-component analysis. Trends in Analytical Chemistry. 2010;29(7):578–591. doi: 10.1016/J.TRAC.2009.12.012. [DOI] [Google Scholar]
- Motta A, Paris D, Melck D. Principles of nuclear magnetic resonance in one and two dimensions. Analytical Chemistry. 2003;27(3):2405–2411. doi: 10.1021/ac9026934. [DOI] [Google Scholar]
- Moulin C, Tournier L, Peres S. Combining kinetic and constraint-based modelling to better understand metabolism dynamics. Processes. 2021 doi: 10.3390/PR9101701. [DOI] [Google Scholar]
- Mullen AR, Hu Z, Shi X, Jiang L, Boroughs LK, Kovacs Z, et al. Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Reports. 2014;7(5):1679–1690. doi: 10.1016/J.CELREP.2014.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz H, Bonnier F, Knief P, Howe O, Lyng FM, Meade AD, Byrne HJ. Evaluation of the potential of Raman microspectroscopy for prediction of chemotherapeutic response to cisplatin in lung adenocarcinoma. The Analyst. 2010;135(12):3070–3076. doi: 10.1039/C0AN00541J. [DOI] [PubMed] [Google Scholar]
- Nawaz H, Bonnier F, Meade AD, Lyng FM, Byrne HJ. Comparison of subcellular responses for the evaluation and prediction of the chemotherapeutic response to cisplatin in lung adenocarcinoma using Raman spectroscopy. The Analyst. 2011;136(12):2450–2463. doi: 10.1039/C1AN15104E. [DOI] [PubMed] [Google Scholar]
- Nawaz H, Garcia A, Meade AD, Lyng FM, Byrne HJ. Raman micro spectroscopy study of the interaction of vincristine with A549 cells supported by expression analysis of bcl-2 protein. The Analyst. 2013;138(20):6177–6184. doi: 10.1039/C3AN00975K. [DOI] [PubMed] [Google Scholar]
- Near-infrared spectroscopy in food science and technology. Google Books. (n.d.). Retrieved January 27, 2023, from https://books.google.ie/books?hl=en&lr=&id=wsk7SPMOuJAC&oi=fnd&pg=PR5&dq=Near-infrared+spectroscopy+in+food+science+and+technology&ots=Zn2d95YSEh&sig=EIUjegKhZx39ikcbpqP4wABtHLg&redir_esc=y#v=onepage&q=Near-infrared%20spectroscopy%20in%20food%20science%20and%20technology&f=false
- Newsholme P, Procopio J, Ramos Lima MM, Pithon-Curi TC, Curi R. Glutamine and glutamate—Their central role in cell metabolism and function. Cell Biochemistry and Function. 2003;21(1):1–9. doi: 10.1002/CBF.1003. [DOI] [PubMed] [Google Scholar]
- Nielsen J. Systems biology of metabolism. Annual Review of Biochemistry. 2017 doi: 10.1146/annurev-biochem. [DOI] [PubMed] [Google Scholar]
- O’Brien EJ, Monk JM, Palsson BO. Using genome-scale models to predict biological capabilities. Cell. 2015;161(5):971–987. doi: 10.1016/J.CELL.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell, M.-L., Howley, T., Ryder, A. G., & Leger, M. N. (2010). Qualitative analysis using Raman spectroscopy and chemometrics: A comprehensive model system for narcotics analysis. Applied Spectroscopy, 64(10), 1109–1121. Retrieved January 5, 2023, from https://opg.optica.org/abstract.cfm?uri=as-64-10-1109 [DOI] [PubMed]
- Ortmayr K, Causon TJ, Hann S, Koellensperger G. Increasing selectivity and coverage in LC–MS based metabolome analysis. Trends in Analytical Chemistry. 2016;82:358–366. doi: 10.1016/J.TRAC.2016.06.011. [DOI] [Google Scholar]
- Ortmayr K, Charwat V, Kasper C, Hann S, Koellensperger G. Uncertainty budgeting in fold change determination and implications for non-targeted metabolomics studies in model systems. The Analyst. 2016;142(1):80–90. doi: 10.1039/C6AN01342B. [DOI] [PubMed] [Google Scholar]
- Orth JD, Thiele I, Palsson BO. What is flux balance analysis? Nature Biotechnology. 2010;28(3):245–248. doi: 10.1038/nbt.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevaidi M, Matthew BJ, Holly BJ, Hugh BJ, Thulya CPV, Loren C, et al. Clinical applications of infrared and Raman spectroscopy in the fields of cancer and infectious diseases. Applied Spectroscopy Reviews. 2021;56(8–10):804–868. doi: 10.1080/05704928.2021.1946076. [DOI] [Google Scholar]
- Perez-Guaita D, Quintas G, Farhane Z, Tauler R, Byrne HJ. Data mining Raman microspectroscopic responses of cells to drugs in vitro using multivariate curve resolution-alternating least squares. Talanta. 2020;208:120386. doi: 10.1016/J.TALANTA.2019.120386. [DOI] [PubMed] [Google Scholar]
- Pérez-Guaita D, Quintás G, Farhane Z, Tauler R, Byrne HJ. Combining pharmacokinetics and vibrational spectroscopy: MCR-ALS hard-and-soft modelling of drug uptake in vitro using tailored kinetic constraints. Cells. 2022;11(9):1555. doi: 10.3390/CELLS11091555/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quek LE, Dietmair S, Krömer JO, Nielsen LK. Metabolic flux analysis in mammalian cell culture. Metabolic Engineering. 2010;12(2):161–171. doi: 10.1016/J.YMBEN.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Reitzer, L. J., Wice, B. M., & Kennel1, D. (1979). Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. 254(8), 26–35. Retrieved August 18, 2022, from http://www.jbc.org/ [PubMed]
- Ren W, Xia Y, Chen S, Wu G, Bazer FW, Zhou B, et al. Glutamine metabolism in macrophages: A novel target for obesity/type 2 diabetes. Advances in Nutrition. 2019;10(2):221–230. doi: 10.1093/ADVANCES/NMY084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reo NV. NMR-based metabolomics. Drug and Chemical Toxicology. 2002;25(4):375–382. doi: 10.1081/DCT-120014789. [DOI] [PubMed] [Google Scholar]
- Rhiel MH, Cohen MB, Arnold MA, Murhammer DW. On-line monitoring of human prostate cancer cells in a perfusion rotating wall vessel by near-infrared spectroscopy. Biotechnology and Bioengineering. 2004;86(7):852–861. doi: 10.1002/BIT.10834. [DOI] [PubMed] [Google Scholar]
- Rhiel MH, Ducommun P, Bolzonella I, Marison I, Von Stockar U. Real-time in situ monitoring of freely suspended and immobilized cell cultures based on mid-infrared spectroscopic measurements. Biotechnology and Bioengineering. 2002;77(2):174–185. doi: 10.1002/BIT.10134. [DOI] [PubMed] [Google Scholar]
- Ribbenstedt A, Ziarrusta H, Benskin JP. Development, characterization and comparisons of targeted and non-targeted metabolomics methods. PLoS ONE. 2018;13(11):e0207082. doi: 10.1371/JOURNAL.PONE.0207082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richelle A, Bogaerts P. Systematic methodology for bioprocess model identification based on generalized kinetic functions. Biochemical Engineering Journal. 2015;100:41–49. doi: 10.1016/J.BEJ.2015.04.003. [DOI] [Google Scholar]
- Rona GB, Almeida NP, Santos GC, Fidalgo TKS, Almeida FCL, Eleutherio ECA, Pinheiro AS. 1H NMR metabolomics reveals increased glutaminolysis upon overexpression of NSD3s or Pdp3 in Saccharomyces cerevisiae. Journal of Cellular Biochemistry. 2019;120(4):5377–5385. doi: 10.1002/JCB.27816. [DOI] [PubMed] [Google Scholar]
- Roychoudhury P, Harvey LM, McNeil B. The potential of mid infrared spectroscopy (MIRS) for real time bioprocess monitoring. Analytica Chimica Acta. 2006;571(2):159–166. doi: 10.1016/J.ACA.2006.04.086. [DOI] [PubMed] [Google Scholar]
- Rupprecht A, Moldzio R, Mödl B, Pohl EE. Glutamine regulates mitochondrial uncoupling protein 2 to promote glutaminolysis in neuroblastoma cells. Biochimica et Biophysica Acta-Bioenergetics. 2019;1860(5):391–401. doi: 10.1016/J.BBABIO.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JY, Kim HU, Lee SY. Reconstruction of genome-scale human metabolic models using omics data. Integrative Biology. 2015;7(8):859–868. doi: 10.1039/C5IB00002E. [DOI] [PubMed] [Google Scholar]
- Sandra K, Sandra P. Ion suppression: A major concern in mass spectrometry. LCGC North America. 2006;24(5):498–510. doi: 10.56530/LCGC.EU.JI1165R4. [DOI] [Google Scholar]
- Santos RM, Kessler JM, Salou P, Menezes JC, Peinado A. Monitoring mAb cultivations with in situ Raman spectroscopy: The influence of spectral selectivity on calibration models and industrial use as reliable PAT tool. Biotechnology Progress. 2018;34(3):659–670. doi: 10.1002/BTPR.2635. [DOI] [PubMed] [Google Scholar]
- Sato H, Ishigaki M, Taketani A, Andriana BB. Raman spectroscopy and its use for live cell and tissue analysis. Biomedical Spectroscopy and Imaging. 2018;7(3–4):97–104. doi: 10.3233/BSI-180184. [DOI] [Google Scholar]
- Savageau MA. Introduction to S-systems and the underlying power-law formalism. Mathematical and Computer Modelling. 1988;11(C):546–551. doi: 10.1016/0895-7177(88)90553-5. [DOI] [Google Scholar]
- Scarff M, Arnold SA, Harvey LM, McNeil B. Near infrared spectroscopy for bioprocess monitoring and control: current status and future trends. Critical Reviews in Biotechnology. 2008;26(1):17–39. doi: 10.1080/07388550500513677. [DOI] [PubMed] [Google Scholar]
- Schnell S. Validity of the Michaelis-Menten equation—Steady-state or reactant stationary assumption: That is the question. The FEBS Journal. 2014;281(2):464–472. doi: 10.1111/FEBS.12564. [DOI] [PubMed] [Google Scholar]
- Schuster S, Ewald J, Kaleta C. Modeling the energy metabolism in immune cells. Current Opinion in Biotechnology. 2021;68:282–291. doi: 10.1016/J.COPBIO.2021.03.003. [DOI] [PubMed] [Google Scholar]
- Schwartz JM, Barber M, Soons Z. Metabolic flux prediction in cancer cells with altered substrate uptake. Biochemical Society Transactions. 2015;43(6):1177–1181. doi: 10.1042/BST20150149. [DOI] [PubMed] [Google Scholar]
- Sellick CA, Hansen R, Jarvis RM, Maqsood AR, Stephens GM, Dickson AJ, Goodacre R. Rapid monitoring of recombinant antibody production by mammalian cell cultures using Fourier transform infrared spectroscopy and chemometrics. Biotechnology and Bioengineering. 2010;106(3):432–442. doi: 10.1002/BIT.22707. [DOI] [PubMed] [Google Scholar]
- Serber Z, Selenko P, Hänsel R, Reckel S, Löhr F, Ferrell JE, et al. Investigating macromolecules inside cultured and injected cells by in-cell NMR spectroscopy. Nature Protocols. 2007;1(6):2701–2709. doi: 10.1038/nprot.2006.181. [DOI] [PubMed] [Google Scholar]
- Shalabaeva V, Lovato L, La Rocca R, Messina GC, Dipalo M, Miele E, et al. Time resolved and label free monitoring of extracellular metabolites by surface enhanced Raman spectroscopy. PLoS ONE. 2017;12(4):e0175581. doi: 10.1371/JOURNAL.PONE.0175581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan M, Dai D, Vudem A, Varner JD, Stroock AD. Multi-scale computational study of the Warburg effect, reverse Warburg effect and glutamine addiction in solid tumors. PLoS Computational Biology. 2018;14(12):e1006584. doi: 10.1371/JOURNAL.PCBI.1006584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallbone K, Mendes P. Large-scale metabolic models: From reconstruction to differential equations. Industrial Biotechnology. 2013;9(4):179–184. doi: 10.1089/IND.2013.0003/ASSET/IMAGES/LARGE/FIGURE4.JPEG. [DOI] [Google Scholar]
- Srinivasan S, Cluett WR, Mahadevan R. Constructing kinetic models of metabolism at genome-scales: A review. Biotechnology Journal. 2015;10(9):1345–1359. doi: 10.1002/BIOT.201400522. [DOI] [PubMed] [Google Scholar]
- Stoll TS, Ruffieux PA, Schneider M, Von Stockar U, Marison IW. On-line simultaneous monitoring of ammonia and glutamine in a hollow-fiber reactor using flow injection analysis. Journal of Biotechnology. 1996;51(1):27–35. doi: 10.1016/0168-1656(96)01558-1. [DOI] [PubMed] [Google Scholar]
- Strutz J, Martin J, Greene J, Broadbelt L, Tyo K. Metabolic kinetic modeling provides insight into complex biological questions, but hurdles remain. Current Opinion in Biotechnology. 2019;59:24. doi: 10.1016/J.COPBIO.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Tanaka T, Poyurovsky MV, Nagano H, Mayama T, Ohkubo S, et al. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(16):7461–7466. doi: 10.1073/PNAS.1002459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szafraniec E, Majzner K, Farhane Z, Byrne HJ, Lukawska M, Oszczapowicz I, et al. Spectroscopic studies of anthracyclines: Structural characterization and in vitro tracking. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2016;169:152–160. doi: 10.1016/J.SAA.2016.06.035. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi, A. (n.d.). Facts. NobelPrize.org. Retrieved June 5, 2023, from https://www.nobelprize.org/prizes/medicine/1937/szent-gyorgyi/facts/
- Tang YJ, Martin HG, Myers S, Rodriguez S, Baidoo EEK, Keasling JD. Advances in analysis of microbial metabolic fluxes via 13C isotopic labeling. Mass Spectrometry Reviews. 2009;28(2):362–375. doi: 10.1002/MAS.20191. [DOI] [PubMed] [Google Scholar]
- Tesson AR, Soper TS, Ciustea M, Richards NGJ. Revisiting the steady state kinetic mechanism of glutamine-dependent asparagine synthetase from Escherichia coli. Archives of Biochemistry and Biophysics. 2003;413(1):23–31. doi: 10.1016/S0003-9861(03)00118-8. [DOI] [PubMed] [Google Scholar]
- Theakstone AG, Rinaldi C, Butler HJ, Cameron JM, Rose Confield L, Rutherford SH, et al. Fourier-transform infrared spectroscopy of biofluids: A practical approach. Translational Biophotonics. 2021;3(2):e202000025. doi: 10.1002/TBIO.202000025. [DOI] [Google Scholar]
- Theodoridis GA, Gika HG, Want EJ, Wilson ID. Liquid chromatography–mass spectrometry based global metabolite profiling: A review. Analytica Chimica Acta. 2012;711:7–16. doi: 10.1016/J.ACA.2011.09.042. [DOI] [PubMed] [Google Scholar]
- Thompson RM, Dytfeld D, Reyes L, Robinson RM, Smith B, Manevich Y, et al. Glutaminase inhibitor CB-839 synergizes with carfilzomib in resistant multiple myeloma cells. Oncotarget. 2017 doi: 10.18632/oncotarget.16262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toya Y, Kono N, Arakawa K, Tomita M. Metabolic flux analysis and visualization. Journal of Proteome Research. 2011;10(8):3313–3323. doi: 10.1021/PR2002885/SUPPL_FILE/PR2002885_SI_001.ZIP. [DOI] [PubMed] [Google Scholar]
- Trilla-Fuertes L, Gámez-Pozo A, López-Camacho E, Prado-Vázquez G, Zapater-Moros A, López-Vacas R, et al. Computational models applied to metabolomics data hints at the relevance of glutamine metabolism in breast cancer. BMC Cancer. 2020 doi: 10.1186/s12885-020-06764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilakou E, Machado D, Theorell A, Rocha I, Nöh K, Oldiges M, Wahl SA. Current state and challenges for dynamic metabolic modeling. Current Opinion in Microbiology. 2016;33:97–104. doi: 10.1016/J.MIB.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Volkova S, Matos MRA, Mattanovich M, de Mas IM. Metabolic modelling as a framework for metabolomics data integration and analysis. Metabolites. 2020;10(8):303. doi: 10.3390/METABO10080303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18(3):207–219. doi: 10.1016/J.CCR.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huang WE, Cui L, Wagner M. Single cell stable isotope probing in microbiology using Raman microspectroscopy. Current Opinion in Biotechnology. 2016;41:34–42. doi: 10.1016/J.COPBIO.2016.04.018. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wondisford FE, Song C, Zhang T, Su X. Metabolic flux analysis—Linking isotope labeling and metabolic fluxes. Metabolites. 2020;10(11):447. doi: 10.3390/METABO10110447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang C, Chen G. Kinetic modeling: A tool for temperature shift and feeding optimization in cell culture process development. Protein Expression and Purification. 2022;198:106130. doi: 10.1016/J.PEP.2022.106130. [DOI] [PubMed] [Google Scholar]
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–270. doi: 10.1126/SCIENCE.124.3215.269. [DOI] [PubMed] [Google Scholar]
- Wise DR, Thompson CB. Glutamine addiction: A new therapeutic target in cancer. Trends in Biochemical Sciences. 2010;35(8):427–433. doi: 10.1016/J.TIBS.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS. Emerging applications of metabolomics in drug discovery and precision medicine. Nature Reviews Drug Discovery. 2016;15(7):473–484. doi: 10.1038/nrd.2016.32. [DOI] [PubMed] [Google Scholar]
- Xia J, Wishart DS, Valencia A. MetPA: A web-based metabolomics tool for pathway analysis and visualization. Bioinformatics. 2010;26(18):2342–2344. doi: 10.1093/BIOINFORMATICS/BTQ418. [DOI] [PubMed] [Google Scholar]
- Xu Z, Zheng P, Sun J, Ma Y. ReacKnock: Identifying reaction deletion strategies for microbial strain optimization based on genome-scale metabolic network. PLoS ONE. 2013;8(12):e72150. doi: 10.1371/JOURNAL.PONE.0072150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Hua Q, Shimizu K. Metabolic flux analysis in Synechocystis using isotope distribution from 13C-labeled glucose. Metabolic Engineering. 2002;4(3):202–216. doi: 10.1006/MBEN.2002.0226. [DOI] [PubMed] [Google Scholar]
- Yang L, Venneti S, Nagrath D. Glutaminolysis: A hallmark of cancer metabolism. Annual Review of Biomedical Engineering. 2017;19:163–194. doi: 10.1146/ANNUREV-BIOENG-071516-044546. [DOI] [PubMed] [Google Scholar]
- Yasemi M, Jolicoeur M. Modelling cell metabolism: A review on constraint-based steady-state and kinetic approaches. Processes. 2021;9(2):322. doi: 10.3390/PR9020322. [DOI] [Google Scholar]
- Yoo HC, Yu YC, Sung Y, Han JM. Glutamine reliance in cell metabolism. Experimental and Molecular Medicine. 2020 doi: 10.1038/s12276-020-00504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi-Darani A, Paquet-Durand O, von Wrochem A, Classen J, Tränkle J, Mertens M, et al. Generic chemometric models for metabolite concentration prediction based on Raman spectra. Sensors. 2022 doi: 10.3390/S22155581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, White LT, Doumen C, Damico LA, LaNoue KF, Alpert NM, Lewandowski ED. Kinetic analysis of dynamic 13C NMR spectra: Metabolic flux, regulation, and compartmentation in hearts. Biophysical Journal. 1995;69(5):2090–2102. doi: 10.1016/S0006-3495(95)80080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki ÖC, Eylem CC, Reçber T, Kır S, Nemutlu E. Integration of GC–MS and LC–MS for untargeted metabolomics profiling. Journal of Pharmaceutical and Biomedical Analysis. 2020;190:113509. doi: 10.1016/J.JPBA.2020.113509. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ahn WS, Gameiro PA, Keibler MA, Zhang Z, Stephanopoulos G. 13C isotope-assisted methods for quantifying glutamine metabolism in cancer cells. Methods in Enzymology. 2014;542:369–389. doi: 10.1016/B978-0-12-416618-9.00019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhu X, Wang C, Zhang H, Cai Z. Non-targeted and targeted metabolomics approaches to diagnosing lung cancer and predicting patient prognosis. Oncotarget. 2016;7(39):63437. doi: 10.18632/ONCOTARGET.11521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Boley D. Nonlinear multi-objective flux balance analysis of the Warburg Effect. Journal of Theoretical Biology. 2022;550:111223. doi: 10.1016/J.JTBI.2022.111223. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Cheng X, Yang Y, Zhao Y. Lighting up live-cell and in vivo central carbon metabolism with genetically encoded fluorescent sensors optogenetics modules and circuits view project cell metabolism in autophagy view project. Annual Review of Analytical Chemistry. 2008 doi: 10.1146/annurev-anchem-091619-091306. [DOI] [PubMed] [Google Scholar]
- Zhao S, Wang JM, Yan J, Zhang DL, Liu BQ, Jiang JY, et al. BAG3 promotes autophagy and glutaminolysis via stabilizing glutaminase. Cell Death and Disease. 2019;10(4):1–12. doi: 10.1038/s41419-019-1504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Capello M, Fredolini C, Racanicchi L, Piemonti L, Liotta LA, et al. Proteomic analysis reveals Warburg effect and anomalous metabolism of glutamine in pancreatic cancer cells. Journal of Proteome Research. 2012;11(2):554–563. doi: 10.1021/PR2009274/SUPPL_FILE/PR2009274_SI_007.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Ploessl K, Zhou R, Mankoff D, Kung HF. Metabolic imaging of glutamine in cancer. Journal of Nuclear Medicine. 2017;58(4):533–537. doi: 10.2967/JNUMED.116.182345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For more detailed data, please refer to the supplementary material of the referenced paper.