Abstract
A better understanding of wheat functional genomics can improve targeted breeding for better agronomic traits and environmental adaptation. However, the lack of gene-indexed mutants and the low transformation efficiency of wheat limit in-depth gene functional studies and genetic manipulation for breeding. In this study, we created a library for KN9204, a popular wheat variety in northern China, with a reference genome, transcriptome, and epigenome of different tissues, using ethyl methyl sulfonate (EMS) mutagenesis. This library contains a vast developmental diversity of critical tissues and transition stages. Exome capture sequencing of 2090 mutant lines using KN9204 genome–designed probes revealed that 98.79% of coding genes had mutations, and each line had an average of 1383 EMS-type SNPs. We identified new allelic variations for crucial agronomic trait-related genes such as Rht-D1, Q, TaTB1, and WFZP. We tested 100 lines with severe mutations in 80 NAC transcription factors (TFs) under drought and salinity stress and identified 13 lines with altered sensitivity. Further analysis of three lines using transcriptome and chromatin accessibility data revealed hundreds of direct NAC targets with altered transcription patterns under salt or drought stress, including SNAC1, DREB2B, CML16, and ZFP182, factors known to respond to abiotic stress. Thus, we have generated and indexed a KN9204 EMS mutant library that can facilitate functional genomics research and offer resources for genetic manipulation of wheat.
Key words: wheat, exome capture sequencing, EMS mutagenesis, functional genomics
The lack of gene-indexed mutants limits functional genomics and molecular breeding in wheat. This study reports the generation and characterization of an EMS-mutagenized library by whole-exome capture in the winter wheat variety KN9204. The library contains 2090 lines with mutations covering 98.79% of coding genes and shows various developmental phenotypes; it can facilitate studies of gene function and provide novel resources for genetic manipulation.
Introduction
Common wheat has been a vital staple crop since the dawn of civilization, and wheat production must be significantly increased to feed the ever-growing population (Hickey et al., 2019). Nonetheless, our understanding of the genetic basis and molecular regulation of wheat productivity, end-use quality, and environmental adaptability has lagged far behind that of other cereal crops (Xiao et al., 2022). One of the bottlenecks is the lack of genetic resources to enable robust and efficient gene characterization and validation for functional genomics. In addition, the breeding process has narrowed the available genetic diversity of wheat germplasm resources (Liang et al., 2021; Pour-Aboughadareh et al., 2021). Over the past decades, induced mutagenesis has played a vital role in gene discovery, functional characterization, and breeding in plants. For example, genome-wide transfer DNA insertion mutagenesis pools have made a significant contribution to functional genomics in Arabidopsis and rice (Krysan et al., 1999; Jeong et al., 2002). Artificial mutagenesis has accelerated breeding, producing trait-improved wheat varieties worldwide. For instance, the world’s first Ug99-resistant wheat variety ‘Eldo Ngano 1’ and the salt-tolerant cultivar H6756 were bred from γ-ray mutants (Forster, 2014), and Luyuan 502, a high-yielding, sprouting- and lodging-resistant wheat cultivar bred from cosmic ray–induced mutants, had a total cultivation area of over 3.6 million hectares by 2017 (Liu et al., 2021). Thus, introduction of novel sequence variation into crop genomes by artificial mutagenesis can widen genetic diversity for functional genomics and crop breeding.
Among the sources of physical and chemical mutagenesis, ethyl methyl sulfonate (EMS) has been widely used in plants. EMS produces random point mutations, mainly G/C to A/T transition (Henry et al., 2014). Compared with physical rays, Activator/Dissociator, and transfer DNA insertion mutagenesis, EMS produces a range of mutation strengths, with strong mutations necessary for studies of gene function and weak mutations suitable for breeding improvement. Wheat can accumulate a higher mutation load without negative effects on survival, owing to its hexaploid nature. This makes it easy to generate saturating mutations covering the whole coding genome. In the past few years, genome editing tools, particularly CRISPR-Cas9, have been used to introduce a mutation at a specific position, opening up an era of functional genomics and precision crop breeding (Gao, 2021). However, genome editing is limited to a few wheat varieties because of the low efficiency of genetic transformation (Wang et al., 2017a). The extensive commercialization of genetically modified crops has been restricted by current regulatory hurdles in many countries (Palan et al., 2021). Therefore, as a non-transgenic approach with no variety limitations, EMS-induced mutagenesis is expected to coexist with CRISPR-Cas9 for crop improvement and contribute to functional genomics in wheat.
Numerous EMS-mutagenized populations have been created in wheat cultivars to expand breeding germplasm, generate novel allelic variations, and identify critical regulators. For instance, herbicide-resistant wheat lines have been isolated by screening mutant pools of ‘Ningchun 4,’ ‘Aikang 58,’ ‘Lunxuan 987,’ ‘Zhoumai 16,’ and ‘Jing RS801’ (Chen et al., 2021b). Allelic variations in the genes ADP-glucose pyrophosphorylase large subunit (AGPL) (Guo et al., 2017), Starch branching enzyme IIa (SBEIIa) (Botticella et al., 2011; Rawat et al., 2019), Waxy (Rawat et al., 2019), Sucrose transporter (SUT1) (Rawat et al., 2019), and wheat GA 20-oxidase (TaGA20ox1) (King et al., 2015) have been identified and used for functional study and breeding. Nevertheless, few genes have been cloned and characterized from EMS-mutagenized populations. This is mainly due to the difficulty of obtaining gene-indexed mutations at a genome-wide scale in wheat, which has a large genome (Uauy et al., 2017; IWGSC et al., 2018). Targeted capture and sequencing of coding regions, with less than 2% of high-value genomic regions enriched for functional variants and low levels of repetitive regions, has been a viable strategy for identifying mutations in wheat (Uauy et al., 2017). Indeed, whole-exome capture followed by sequencing (WES) has been widely used in crops with large genomes owing to its robust, rapid, cost-effective, and high-throughput nature (Kaur and Gaikwad, 2017). Krasileva and colleagues sequenced the coding regions of 1535 Kronos (tetraploid) and 1200 Cadenza (hexaploid, spring wheat) mutant lines using an 84-Mb whole-exome capture assay and established in silico functional genomic resources for wheat (Krasileva et al., 2017). However, the Cadenza mutations did not cover all the annotated genes, and new sequence-indexed mutants are still needed. Furthermore, more mutagenesis libraries with diverse genetic backgrounds are in high demand for functional genomics and breeding, especially in winter wheat, which accounts for 70% of the total wheat acreage (Kadar et al., 2018).
In this study, we generated an EMS-mutagenized library for the elite winter wheat variety KN9204 and identified 2.89 million EMS-type SNPs covering 98.79% of high-confidence genes through WES. The mutant library with abundant morphological diversity and the novel mutations of agronomically related genes provide valuable genetic resources for gene identification and breeding applications. Integrating previously generated multi-omics data from KN9204, we use NAC transcription factors (TFs) as an example to demonstrate the robustness of the mutant pool for functional genomics.
Results
Generation of an EMS-mutagenized library in the wheat cultivar KN9204
Kenong 9204 (KN9204) is a representative winter wheat cultivar widely grown in the north China plains and characterized by semi-dwarf stature (∼74 cm), compact plant architecture (∼6 180 000 fertile spikes ha−1), high yield (∼7500 kg ha−1), and high nitrogen use efficiency (nitrogen uptake efficiency ≈ 65%, nitrogen utilization efficiency ≈ 35%) (Jia et al., 2006; Cui et al., 2016; Shi et al., 2022). Dozens of cultivars derived from KN9204 with improved grain yield, overwintering ability, heat resistance, and milling quality have been authorized (Figure 1A) (Zhao et al., 2015; Li et al., 2021b). With moderate regeneration efficiency, Kenong 199 is generally used as a typical winter variety for genetic transformation in gene functional studies (Wang et al., 2017a). Numerous quantitative trait loci (QTLs) for various traits, including flag leaf size, spike-related traits, root morphology, grain number per spike, and nitrogen use efficiency, have been mapped using biparental genetic populations developed from KN9204 (Fan et al., 2018, 2019; Liu et al., 2020b; Yu et al., 2022; Zhao et al., 2022). A reference genome, transcriptome, and epigenome have been generated for KN9204, including information on chromatin accessibility and core histone modifications and variants from multiple tissues, developmental stages, and environmental treatments (Figure 1B, Supplemental Figure 1) (Li et al., 2018; Liu et al., 2020b; Lin et al., 2022; Shi et al., 2022). Thus, its elite agronomic traits, plain and productive genealogies, multi-dimensional omics data, and preliminarily mapped QTLs make KN9204 an ideal choice for functional genomics and genetic improvement via mutagenesis.
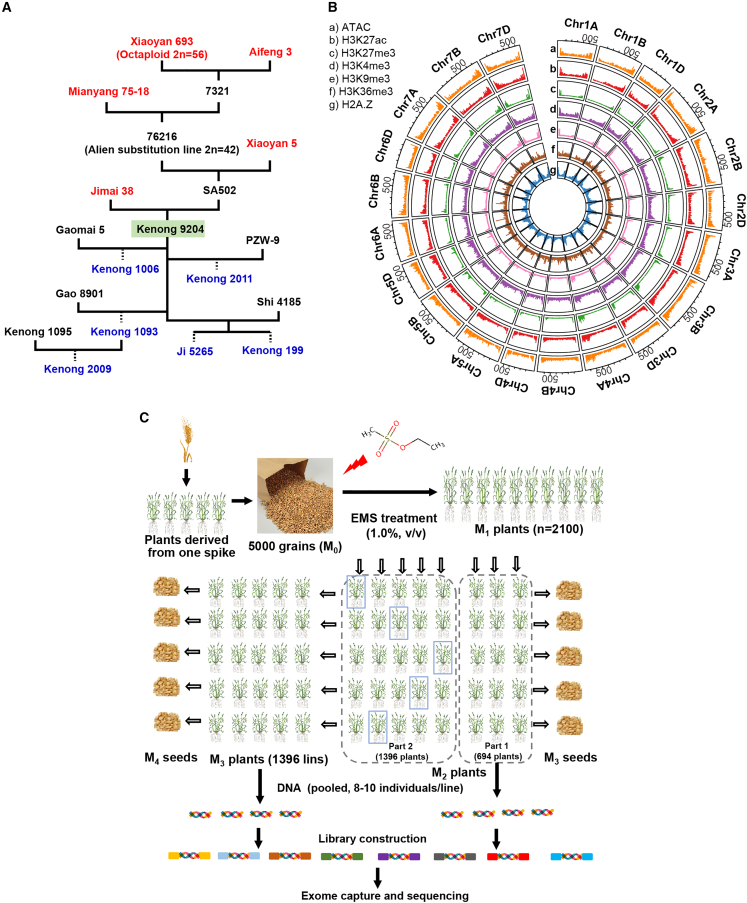
Figure 1.
Construction of an EMS mutant library in the winter wheat variety KN9204.
(A) A simplified pedigree showing the original parents (in red) and offspring (in blue) of Kenong 9204 (KN9204). The indirect parents and other cultivars are in black.
(B) Circos plot summarizing the chromosomal distribution of multi-epigenomic data. From the outer to the inner circle: (a) Assay for Transposase Accessible Chromatin using sequencing (ATAC-seq), (b) H3K27ac, (c) H3K27me3, (d) H3K4me3, (e) H3K9me3, (f) H3K36me3, and (g) H2A.Z. Bar plots in each circle show the density of epigenetic mark intensity.
(C) Overview of construction and whole-exome capture sequencing of the EMS-mutagenized population of KN9204.
Accordingly, we generated a mutant library of KN9204 by treating mature seeds with 1.0% v/v EMS. Approximately 5000 seeds (M0) were used for mutagenesis, and 2134 germinated, resulting in M1 plants. In total, 2090 M1 plants survived and generated the M2 individuals. M2 plants were successively advanced to the M3 generation through single-seed descent with selfing (Figure 1C). Six hundred ninety-four lines from the M2 generation were used to form the mutant population, together with the remaining 1396 lines from the M3 generation. Sixteen progenies of each mutant individual were planted in a row in the field for phenotypic investigation, and 8–10 individuals were pooled for DNA extraction and subsequent exome-capture sequencing (Figure 1C). Seeds were harvested from each mutant line and bulked for later distribution and functional study (Figure 1C).
Diverse developmental variations in the KN9204 mutant library
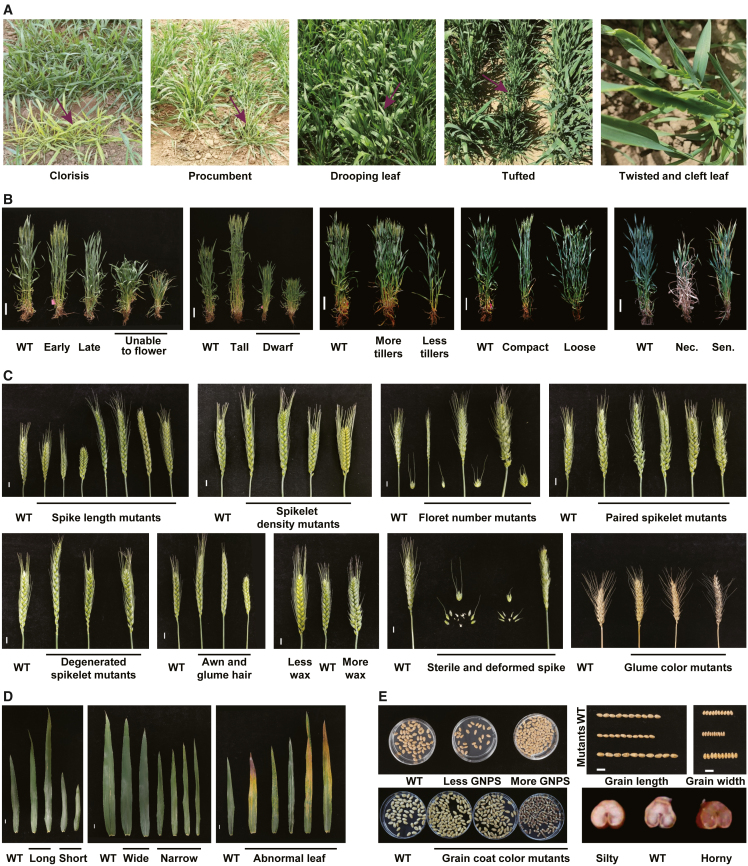
Next, we evaluated the genetic diversity of developmental traits in the KN9204 mutant library throughout the wheat life cycle. Two independent field surveys were performed during the 2021–2022 cropping season (October 2021 to June 2022) in Beijing (Northern Winter Wheat Zone, 39° 55′ N, 116° 23′ E) and in Shijiazhuang (Yellow and Huai Valley Winter Wheat Zone, 38° 04′ N, 114° 28′ E) (Zhou et al., 2018). Anomalous developmental defects were observed and catalogued in both fields, including chlorosis, procumbent growth, twisted leaves, and tufted plants at the seedling stage (Figure 2A); alterations in aspects of plant architecture such as plant height (PH), tiller number, and tiller angle during the heading stage (Figure 2B); changes in spike-related traits such as spike length, spikelet density, and floret number, as well as paired spikelets, degenerated spikelets, and altered glume hairs and awns (Figure 2C); changes in leaf morphology (Figure 2D); alterations in fertility during the grain-filling stage; and changes in grain-related traits such as grain number, grain size, grain plumpness, seed coat color, and endosperm hardness (Figure 2E) at the mature stage. In total, 286 lines (13.68%) exhibited noticeable alterations in qualitative indicators in both fields, and these were categorized into four classes: plant architecture, spike and affiliated organs, leaf shape and color, and other terms. Plant architecture was the largest category, with 123 lines, most of which were PH-related mutants (76.42%, 94 of 123 lines). Spike-related traits were the next largest category, with 114 lines; paired spikelets were the most common alteration (81.57%, 93 of 114 lines), followed by altered auxiliary organs such as awns and glumes (Supplemental Table 1). Premature senescence, wax leaf, and early or late heading were also commonly observed and were present in more than 10 lines each in the KN9204 mutant library (Supplemental Table 1).
Figure 2.
Developmental diversity in various categories within the KN9204 mutant library.
(A) Anomalous developmental defects observed at the seedling stage. Arrows indicate the lines with defects.
(B) Mutants with altered plant architecture at the heading stage. WT, KN9204 wild type; Nec., premature necrosis; Sen., premature senescence. Scale bars, 10 cm.
(C) Pictures of spike and affiliated organ mutants at the grain filling stage. Scale bars, 1 cm.
(D) Leaf shape mutants and abnormal leaves, including lesion mimics, striped leaves, and premature senescence. All photos were taken of the flag leaves. Scale bars, 1 cm.
(E) Grain-related mutant phenotypes. Grains from a single main spike of each mutant were compared with those of wild-type KN9204. GNPS, grain number per spike. Scale bars, 1 cm.
To estimate the phenotypic distribution of important agronomically related traits in the KN9204 mutant library, we investigated 14 quantitative indicators in 200 randomly chosen mutant lines (Supplemental Table 2). Among these indicators, spike length, spikelet number per spike (SPS), grain number per spike, fertile florets per spikelet, and thousand-grain weight fit an approximately normal distribution (Supplemental Figure 2A–2F). For example, the mutant population had a slightly reduced SPS compared with KN9204 on average (mutants versus KN9204, 23.67 versus 25.54) (Supplemental Figure 2B), with a minimum and maximum SPS of 17.25 and 34.62 (Supplemental Table 2). However, PH tended to fit a positively skewed distribution, as 46 lines were taller than 80 cm and only 6 lines were shorter than 60 cm (Supplemental Figure 2G). Grain width and grain roundness showed the opposite pattern, fitting a negatively skewed distribution (Supplemental Figure 2H and 2I).
The high frequency of visible morphological alterations and the wide range of phenotypic variation observed in the KN9204 mutant library indicated that it would be a valuable resource for screening mutant lines that would aid gene functional studies and breeding applications.
Characterizing mutagenesis in the KN9204 mutant library by WES
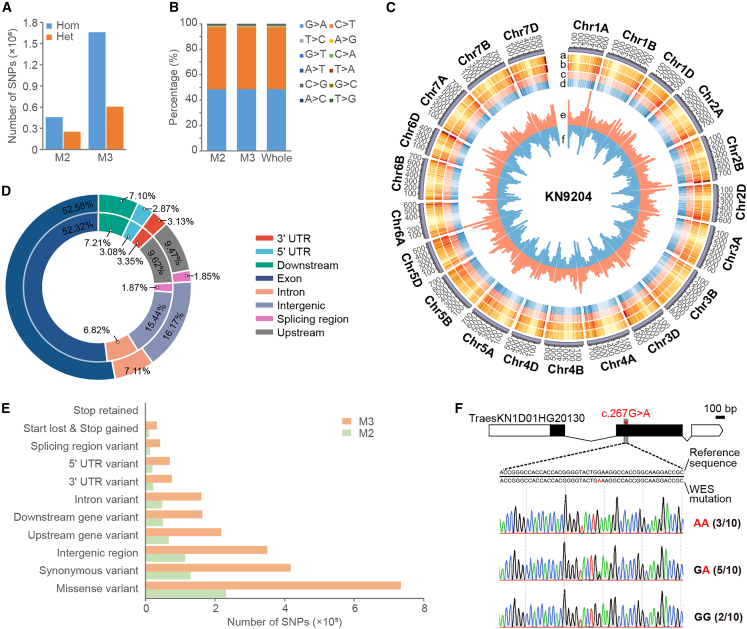
To fill the gap between the genome and the “gene-ome” in hexaploid wheat, we performed WES for the EMS-mutagenized library and the KN9204 wild type using probes that were designed according to the recently assembled KN9204 reference genome (Shi et al., 2022) (see methods for details). On average, 91.62 million high-quality clean reads (paired-end 150 bp, Q30 > 0.85) per sample were generated by WES, covering 99.93% of exome targets with an average depth of 11.63-fold as aligned to the KN9204 reference genome (Supplemental Table 3). Based on the alignment, approximately 57.59% of the target region was sequenced at least five times, whereas 41.57% had a greater than 10-fold sequencing depth (Supplemental Figure 3A). Minimum coverages of 10 mutated reads for heterozygous mutations and 6 for homozygous mutations were used to differentiate real mutations from sequencing errors (Supplemental Figure 3B). Using a modified Genome Analysis Toolkit (GATK) bioinformatics pipeline followed by strict quality filtering (see methods), we identified a total of 2.97 million SNPs, with a homozygous/heterozygous ratio of 1.83 and 2.74 for the M2 and M3 populations, respectively (Figure 3A). The percentage of SNPs that were G → A or C → T transitions was 97.26% (Figure 3B), as EMS preferentially alkylates guanine to O6-ethylguanine, leading to the mispairing with T in place of C (Henry et al., 2014). Consequently, 2.89 million EMS-type SNPs were identified, with an estimated average of 1383 EMS-type mutations per mutant line and 19.94 mutations per kilobase across the mutagenesis population (Supplemental Table 3, Supplemental Figure 3C). No significant differences in mutation frequency were found among the three subgenomes (Supplemental Figure 3D). The distribution and density of mutations correlated well with the genes on each chromosome, suggesting a uniform distribution of mutations (Figure 3C).
Figure 3.
Characterization of mutations in the KN9204 EMS-mutagenized library.
(A) The numbers of homozygous and heterozygous single-nucleotide polymorphism (SNP) mutations in the M2 and M3 populations. Hom, homozygous; Het, heterozygous.
(B) The proportions of nucleotide mutations in M2, M3, and the whole mutant population. EMS-type mutations (C → T and G → A) accounted for more than 97% of the SNPs.
(C) Circos plot of important mutation indicators in the EMS-mutagenized population. From the outer to the inner circle: (a) probe density, (b) mutation density in exons, (c) density of heterozygous mutations (light red), (d) density of homozygous mutations (light blue), (e) numbers of severe mutations (orange), and (f) numbers of SNPs on the chromosome (light blue).
(D) The distribution of SNPs in gene structures, with mutations in the M2 population plotted on the inner circle and those in M3 on the outer one. More than half of the SNPs were located in exons in both populations.
(E) Functional annotation of SNP-caused mutations in genes. Mutations in the M2 population are marked in green and those in M3 in orange.
(F) Validation of WES-identified EMS mutations via Sanger sequencing. Numbers indicate the frequency of specific nucleotides from multiple sequencing results.
To further understand the variation patterns within structural genes, SNPs were categorized and associated with gene structure using SnpEff (Cingolani et al., 2012). Most variations were located in genic regions (Supplemental Figure 3E), and approximately 52.58%, 6.82%, 6.00%, and 1.85% of SNPs in the M3 population were annotated in exons, introns, UTRs, and splicing regions, respectively (Figure 3D). Functional annotation of SNPs in the gene-coding sequence suggested that 33 367 SNPs affected translation termination (stop gained, start lost, or stop retained), 42 257 caused mis-splicing, and 725 491 were non-synonymous protein-coding mutations in the M3 population (Figure 3E). To assess the validity of mutation sites detected by WES and the efficiency of transmission between generations, we randomly selected 22 mutations and performed genotyping by Sanger sequencing (Figure 3F). Eight out of ten homozygous mutations (80.00%) were confirmed to be positive, 10 of 12 heterozygous mutations (83.33%) were detected as homozygous or heterozygous, and two mutations were not detected (Supplemental Data 1). Thus, abundant SNPs were captured in WES, most of which (97.26%) were reliable EMS-type mutations and retained in progeny seeds.
Identification of gene-index mutations in the KN9204 mutant library
Next, we asked how many genome-wide mutations were present in the KN9204 mutant library. We found that 2 383 763 SNPs covered 108 990 genes, representing 98.79% of the annotated high-confidence protein-coding genes and averaging 27.26 SNPs per gene model (Supplemental Table 3). Among these genes, 106 277 contained at least one missense mutation, and 41 266 had at least one high-impact mutation (stop gained, start lost, splice donor/acceptor variant, and stop retained) (Supplemental Table 3). An average of 1168 genes were mutated per line, 28.90 of which were severely mutated (with at least one high-impact mutation) (Supplemental Figure 3F–3H, Supplemental Table 3).
Additional attention was paid to typical lines with visible phenotypic variations. Similar numbers of SNPs were observed in these lines (Figure 4A), but fewer genes were mutated in typical lines than in residual lines (Figure 4B). A markedly reduced number of severely mutated genes per line was observed (Figure 4C), suggesting that these striking mutated phenotypes do not appear to be the cumulative effect of mutated SNPs but are instead due to the mutation of key genes. Notably, 42 non-synonymous novel mutations, including three stop-gained mutations (p.G247∗, p.G386∗, and p.G527∗), were detected in the “Green Revolution” gene Rht-D1 (Li et al., 2013) (Figure 4D). Four mutant lines for these three mutations exhibited marked PH changes, with significantly increased PH in homozygous lines and separation of PH for heterozygous lines (Figure 4E and 4F). Other genes, such as TaTB1, TaQ, WFZP, Ms1, TaNAC019, TaCol-B5, and TaGW2, also contained novel mutations (Dobrovolskaya et al., 2015; Wang et al., 2017b; Dixon et al., 2018; Zhang et al., 2018, 2022; Li et al., 2019; Liu et al., 2020a, 2020c), and many of the mutation sites have not been reported previously (Supplemental Data 2). Thus, the KN9204 EMS-mutagenized library contains novel allelic variations of crucial genes related to yield and agronomic traits and is potentially useful for future breeding applications.
Figure 4.
Novel allelic variations identified in the KN9204 mutant library.
(A–C) SNPs per line (A), mutated genes per line (B), and severely mutated genes per line (C) in typical lines with visible phenotypic variations and residual lines. Wilcoxon’s test was used to determine significant differences.
(D) Non-synonymous novel mutations detected in Rht-D1 (TraesKN4D01HG04200). The positions of previously reported (in blue) and novel non-synonymous mutations (in black) and stop-gained mutations (in red) are shown on a schematic diagram of Rht-D1.
(E and F) The plant height of typical mutant lines for Rht-D1. A comparison of four mutant lines with wild-type KN9204 (E) and photographs of two mutant lines (F) are shown. Note: G386∗ is heterozygous, and the mutant line containing it showed plant height trait segregation. The bar plot shows the plant heights of four mutant lines and wild-type KN9204. Dunnett’s tests were used to determine the differences between each line and the wild type. ∗P < 0.05; ∗∗P < 0.01.
(G) Large numbers of mutations were detected on the rye-derived 1BS region of KN9204. The density of SNPs (in red), annotated genes (gray), mutated genes (green), and severely mutated genes (blue) is displayed using a 2-Mb sliding window. The blue dashed box indicates the rye-derived 1BS region (Chr1B:0–283 Mb).
(H) The number of SNPs, percentage of mutated genes, and percentage of severely mutated genes in the Chr1B:0–283 Mb region in the EMS population. Numbers in the bars indicate the SNP numbers, mutated gene numbers, and severely mutated gene numbers in the Chr1B:0–283 Mb region.
Common wheat is an allohexaploid species with three subgenomes (Ramírez-González et al., 2018; Shi et al., 2022). Most synteny-pair homeologous genes are functionally redundant, although some have differentiated in expression pattern and function (Ramírez-González et al., 2018). The distribution and frequency of EMS-type mutations were further evaluated using syntenic homeologous triads as a unit. The 12 988 triads with at least one mutated homeolog in the population and the triads in which all three homeologs were mutated accounted for 99.41% of all triads (Supplemental Figure 3I). Among the triads, 73.09% had high-impact mutations in at least one homeologous gene, and 12.23% were severely mutated in all three homeologs (Supplemental Table 3). For a given triad, an average of 69.16 lines in the population had at least one mutated homeologous gene, and 1.88 lines had at least one severely mutated homeolog (Supplemental Table 3). Together, these mutations lay a foundation for future functional genomics research.
Chromosome 1BS of KN9204 was substituted by 1RS from rye in breeding and originated from two KN9204 parents (Jimai 38 and Mianyang 75-18) (Figure 1A). We further explored mutagenesis-induced variation on the rye-derived chromosome 1BS in KN9204. There were fewer SNPs, mutated genes, and severely mutated genes on 1BS compared with 1BL, especially in Chr1B:180–280 Mb near the centromere region (Figure 4G). However, this is caused by the low gene density in this region. A total of 36 023 SNPs were detected in the rye-derived 1BS region (Chr1B:0–283 Mb) (Shi et al., 2022), covering 991 genes (98.41% of annotated genes), and 417 of these genes (41.41%) were severely mutated (Figure 4H). Therefore, mutants for many genes on 1BS are still available in the KN9204 mutant library. For instance, 57 SNPs were detected in the nitrate transporter gene TraesKN1B01HG09350, including a stop-gained mutation (p.Gln386∗) (Supplemental Data 2). The ω-secalin proteins, a primary causative factor for the poor quality of 1BL/1RS translocation lines, are encoded by the Sec-1 locus on 1BS (Li et al., 2021a). We obtained several mutants of TraesKN1B01HG01000 (Supplemental Data 2), a secalin gene at the Sec-1 locus, which can potentially improve the end-use quality of KN9204.
To summarize, we have built a nearly complete genome-wide gene-indexed mutation library that can provide a valuable resource for gene functional analysis and may contain desirable allelic variants for breeding.
Exploring KN9204 mutant lines of NAC transcription factors for abiotic stress response
TFs are central regulators of plant development and response to environmental stimuli (Licausi et al., 2013; Phukan et al., 2016; Kaufmann and Airoldi, 2018; Leng and Zhao, 2020), modulating broad patterns of gene expression by binding to cis-elements of their targets. By integrating PlantTFDB, HMMsearch, and BLASTP, we systematically identified 11 005 TFs (Supplemental Figure 4A, Supplemental Data 3), accounting for 10.20% of the annotated high-confidence genes, and categorized them into 57 families (Figure 5A). WRKY, NAC, and B3 were the most prominent TF families, with more than 1000 TFs each (Figure 5A). Similar to previous reports (Evans et al., 2022), many TFs were retained in the two steps of polyploidization during wheat speciation (Supplemental Figure 4B). More than half of the TFs were gained from whole-genome duplication or duplication of large genome segments (Supplemental Figure 4C).
Figure 5.
Evaluation of abiotic stress tolerance of NAC transcription factor mutant lines.
(A) The number of identified TFs and the responsiveness of each TF family to abiotic stress. Only TF families with more than 100 genes are shown. The size of the circle indicates the number of genes that were differentially expressed in roots or leaves of KN9204 under drought/salt stress, with a higher percentage indicated by a darker color.
(B) Summary of the KN9204 EMS mutant lines that contained NAC TF mutations.
(C) The maximum root length (MRL) distribution of 100 mutant lines under different conditions.
(D) The mutant lines exhibited differences in drought and salt stress sensitivity. Each mutant line is plotted as a point, and their MRLs under different conditions are linked with a dashed line.
(E) The ranking of 100 mutant lines using MRLSalt/CK, SLSalt/CK, MRLDrought/CK, and SLDrought/CK. In each box, mutants in the upper quarter, middle half, and lower quarter were defined as tolerant, less sensitive, and sensitive, respectively. Tukey’s multiple comparisons test was used to determine the significance of differences between MRLSalt/CK, SLSalt/CK, MRLDrought/CK, and SLDrought/CK. Different letters indicate significant differences at P < 0.05.
(F) Overview of mutant lines with different responses to drought and salt stress.
(G) The number of NAC TFs that responded to different stresses in each DEG group. TFs for which at least one mutant line exhibited a sensitive or tolerant phenotype under drought and salt stress were counted. TFs that were differentially expressed under drought stress, salt stress, or both conditions were assigned to the drought DEGs, the salt DEGs, and the drought and salt DEGs, respectively.
Abiotic stresses such as drought, salinity, and heat threaten wheat production and food security (Minhas et al., 2017; Shahzad et al., 2021). TFs play a critical regulatory role in response and adaptation to different environmental stresses (Kaufmann and Airoldi, 2018; Leng and Zhao, 2020). Transcriptome analysis of KN9204 seedlings under drought and salt stress indicated that the WRKY, NAC, and bHLH TF families were abundantly represented within the differentially expressed genes (DEGs) (Figure 5A). In particular, all three homeologs of TaNAC071, which contributes to drought tolerance in wheat, were differentially expressed under control and stress conditions in KN9204 (Mao et al., 2021) (Supplemental Figure 4D). Orthologs of some rice NAC TFs reported to participate in drought, salt, heat, and cold tolerance were also identified as DEGs (Supplemental Figure 4D), indicating the potential roles of NAC TFs in response to drought and salt stress in wheat. Lines from the KN9204 mutant library that contained mutations in NAC TFs were therefore surveyed to explore the role of NACs in abiotic stress resistance.
A total of 1138 NAC TFs were identified in wheat and were clustered into three branches on the basis of a phylogenetic tree that included sequences from wheat, Arabidopsis thaliana, and rice (Supplemental Figure 4E). Within the KN9204 mutant library, 857 NAC TFs had mutations, and 57.60% of these had at least one high-impact mutation (Figure 5B). A total of 240 TFs had a homozygous high-impact mutation, with a majority of TFs randomly mutated in a few individuals (≤2) in the mutant library (Figure 5B). One hundred homozygous mutant lines for 80 randomly selected severely mutated NAC TFs were subjected to salt or drought treatment (Supplemental Figure 5A). Based on a pre-test with KN9204 (Supplemental Figure 5B), 250 mM NaCl was used for the salt treatment, and 8% w/v PEG-6000 was used for the drought treatment. Among nine quantitative indicators, seedling length (SL) and maximum root length (MRL) showed good repeatability and moderate responses to salt and drought stress (Supplemental Figure 5C–5E), as reported previously (Lin et al., 2019; Ahmed et al., 2019). The effect of salt stress on SL and MRL was greater than that of drought stress, with a more significant reduction in SL than in MRL (Figure 5C, Supplemental Figure 5F, Supplemental Data 4). However, a distinct sensitivity to drought and salt stress was observed among the 100 mutant lines (Figure 5D, Supplemental Figure 5G).
We sorted the relative MRL values under salt conditions (MRLSalt/CK) and drought conditions (MRLDrought/CK) and the relative SL values under salt conditions (SLSalt/CK) and drought conditions (SLDrought/CK) from smallest to largest, then assigned lines from the upper quarter, middle, and lower quarter to the tolerant, less sensitive, and sensitive groups, respectively (Figure 5E). By integrating these indexes, we assigned the 100 NAC mutant lines to different groups according to their drought and salt stress responses (Figure 5F). Among the 80 NAC TFs, 20 genes were DEGs in KN9204 under drought and salt conditions, corresponding to 34 mutant lines. Notably, 15 of 17 DEGs (21 of 26 mutant lines) under drought conditions were shown to be drought sensitive or drought tolerant. Seven of 10 drought DEGs and all 6 DEGs in both drought and salt conditions had the drought-related phenotype (Figure 5G). Similarly, 6 of 10 salt DEGs had salt-sensitive or salt-tolerant phenotypes (Figure 5G).
Therefore, by integrating the transcriptome dynamics of KN9204 under stress treatments with the KN9204 mutant library, we systematically uncovered 13 mutant lines with 11 interfering NAC TF mutations that showed altered responses to drought and salt stress.
Uncovering the transcriptional regulation of NAC TFs in response to drought and salt stresses
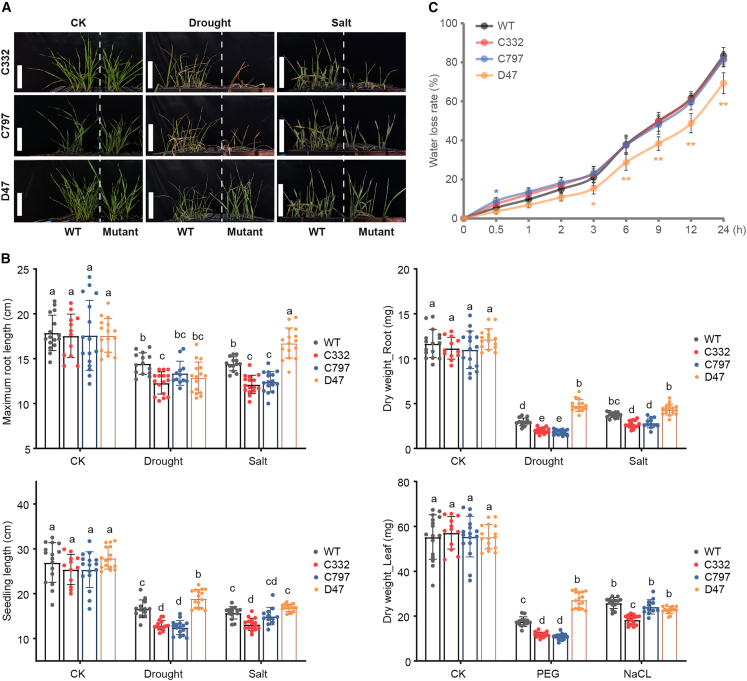
Detailed phenotypic observations were obtained for three lines that contained NAC TF mutations and showed changes in drought and salt sensitivity: C332 (stop-gained mutant of TraesCS5A02G241900, p.Trp142∗), D47 (start-lost mutant of TraesCS5A02G242600), and C797 (stop-gained mutant of TraesCS2D02G576400, p.Trp289∗) (Figure 6A). No noticeable differences were observed between KN9204 and the mutant lines under control conditions (Figure 6A and 6B). The C332 line was sensitive to both drought and salt stress (Figure 6A), with significantly reduced MRL, SL, and dry weight of roots (DW_Root) and leaves (DW_Leaf) compared with KN9204 (Figure 6B). Like the C332 line, the C797 line was sensitive to drought in both roots and leaves. However, it showed salt sensitivity only in the roots and exhibited no changes in leaf morphology, such as seedling length and DW_Leaf (Figure 6A and 6B), suggesting that the C797 mutation has different effects on root and leaf tissues. By contrast, the D47 line was generally resistant to drought and salt stress compared with KN9204 (Figure 6A and 6B). Detached leaves of D47 exhibited a lower water loss rate, suggesting that a greater water-holding capacity was beneficial to its drought and salt tolerance (Figure 6C). The C332 and C797 lines did not differ from KN9204 in water loss rate, suggesting that their sensitivity to drought and salt may not be due to a change in water-holding capacity (Figure 6C).
Figure 6.
Changes in drought and salt stress response in three NAC TF mutant lines.
(A) Assessment of drought and salt tolerance of the NAC TF mutant lines C332, C797, and D47. Photographs of seedlings were taken after 2 weeks of hydroponic growth under normal conditions (CK), drought treatment, and salt treatment.
(B) Quantitative analysis of maximum root length (MRL), seedling length (SL), root dry weight (DW_Root), and leaf dry weight (DW_leaf) in three NAC TF mutant lines under different conditions. The error bars denote ±SD, n = 15. Tukey’s multiple comparisons test was used to determine the significance of trait differences among the four lines (three mutant lines and wild-type KN9204). Different letters indicate a significant difference at P < 0.05.
(C) Water loss rate of isolated leaves under normal conditions. Fresh leaves were isolated from seedlings after hydroponic growth for 2 weeks under normal conditions. The error bars denote ±SD, n = 10. Dunnett’s test was used to determine the differences in water loss rate of three mutant lines compared with the wild type, KN9204. Only differences that reached the significance threshold are labeled (with the color corresponding to the line). ∗P < 0.05; ∗∗P < 0.01.
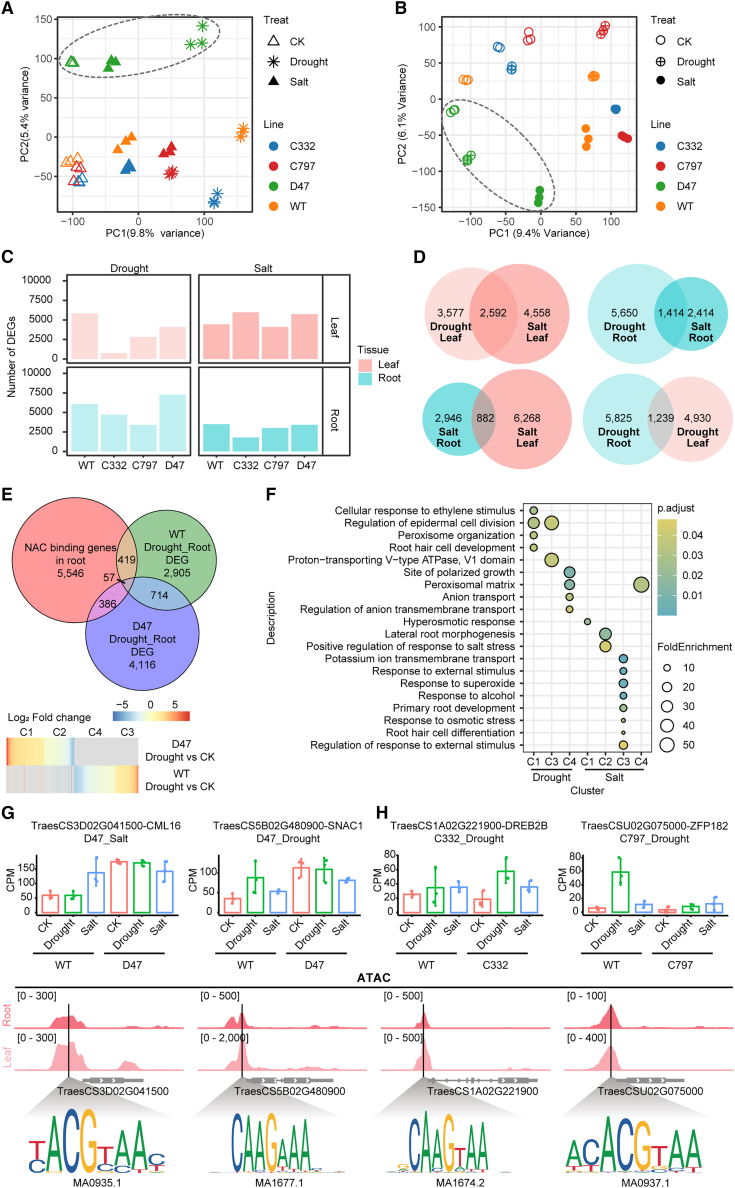
To understand how NAC TFs regulate abiotic stress response, we performed RNA-sequencing (RNA-seq) of root and leaf tissues from KN9204 and the three mutant lines (C332, C797, and D47) under control, salt, and drought treatments (Supplemental Figure 6A). Root and leaf samples were clearly separated by principal component analysis (Supplemental Figure 6A). Within the same tissue, samples could generally be further divided by different treatments (Figure 7A and 7B); the drought treatment was more diverse than the salt treatment compared with the control for roots (Figure 7A), whereas the salt treatment was more diverse for leaves (Figure 7B). The number of DEGs between control and stress samples confirmed these trends (Figure 7C). Interestingly, the D47 line was separated from the other two mutant lines, C332 and C797 (grouped by dashed ovals), and KN9204 fell between these two groups (Figure 7A and 7B). This transcriptome pattern fits well with the resistance of D47 to salt and drought stress, whereas C332 and C797 are more sensitive to both stresses compared with KN9204 (Figure 6). We compared the DEGs induced by drought or salt stress in KN9204 with those in NAC TF mutant lines, defining the altered DEGs as potential genes regulated by NAC TFs (Supplemental Figure 6B, Supplemental Data 5). In all three mutant lines, there were generally more shared DEGs in the same tissue with different treatments than in different tissues with the same treatment (Figure 7D, Supplemental Figure 6C).
Figure 7.
Transcriptional regulation of NAC TF targets in response to drought and salt stresses.
(A and B) Principal component analysis (PCA) of transcriptome data for root samples (A) and leaf samples (B) under different conditions. Each symbol represents one sample. As indicated, samples of different mutant lines, tissues, and treatments are displayed in different shapes and colors. Dashed ovals separate the samples of the D47 line from others.
(C) Numbers of DEGs in the roots and leaves of each mutant line under different conditions.
(D) Venn diagrams show the overlap of salt or drought stress–responsive DEGs in different tissues of the mutant line C332 compared with the control.
(E) Schematic of the strategy used to identify target candidates of TraesCS5A02G242600 (D47). The KN9204 (WT) and D47 mutant line-specific drought-responsive genes and genes with different response levels (log2(FCKN9204/FCmutant) >1) are summed as TraesCS5A02G242600 (D47)-affected drought-responsive genes. Among them, genes with NAC TF binding sites in the open promoter region are considered to be targets of NAC TFs during drought treatment. The heatmap shows the mRNA fold change of 862 identified drought-responsive TraesCS5A02G242600 target candidates in the roots of the WT or the D47 mutant line.
(F) Enriched GO terms in the identified TraesCS5A02G242600 (D47) target candidates under drought or salt stress.
(G and H) Expression patterns of the NAC TF target candidates TaSNAC1-D, OsCML16(G), OsDREB-2B, and OsZFP182(H) under CK, drought, or salt-stress conditions in the WT and various mutant lines (top). These candidates harbor different NAC TF binding motifs under the ATAC-seq peak in their promoter regions in root or leaf tissue, as indicated (bottom).
By integrating chromatin accessibility data generated from KN9204 roots and leaves (Supplemental Figure 1) with the potential binding motifs of NAC TFs (Supplemental Figure 6D) (Castro-Mondragon et al., 2022), we identified potential direct targets of NAC TFs in roots and leaves (Supplemental Figure 6E, Supplemental Data 6). Most potential NAC binding motifs were located less than 1 kb from the transcription start site (Supplemental Figure 6F), and more NAC TF binding motifs were detected in potential NAC targets in the roots (Supplemental Figure 6G). By overlapping the potential NAC targets with NAC-regulated genes from the RNA-seq analysis, we identified 862 drought-responsive and 774 salt-responsive target candidates for TraesCS5A02G242600 (D47-line mutation) in roots and leaves, respectively (Figure 7E, Supplemental Data 6). Gene ontology (GO) analysis showed that genes related to “cellular response to ethylene stimulus,” “root hair cell development,” and antioxidation were enriched, consistent with the morphological changes in the D47 mutant line (Figure 7F). In the drought-responsive TraesCS5A02G242600 target candidates, we found wheat orthologs of SNAC1, OsLG3, OsTIFY3, OsGRAS23, and OsXXT1, and orthologs of OsCML16, SNAC2, and DSM2 were identified among the salt-responsive TraesCS5A02G242600 target candidates (Supplemental Data 6). Wheat orthologs of SNAC1 and OsCML16 contain NAC binding motifs in their accessible promoter regions and show differences in induction between D47 and KN9204 under salt or drought stress (Figure 7G). SNAC1 has been reported to alter root architecture and enhance drought and salt tolerance in rice (Hu et al., 2006), and several hundred target candidates for TraesCS5A02G241900 (C332) and TraesCS2D02G576400 (C797) were identified for salt and drought stress (Supplemental Figure 6H). Among them, DREB2B and ZFP182 are potential targets for further characterization (Figure 7H).
Thus, by taking advantage of the KN9204 mutant library and multi-dimensional omics data generated in the KN9204 background, we can efficiently study gene function to identify NAC TF targets that are involved in regulating drought and salt stress response/adaptation.
Discussion
Indexed KN9204 EMS mutant library enables gene identification and genetic manipulation in winter wheat
Bread wheat is a major crop that feeds 40% of the world’s population. Winter wheat accounts for about 70% of the total wheat acreage, with a higher grain yield, better quality, and broader adaptability than spring wheat (Entz and Fowler, 1991; Kadar et al., 2018). Gene functional studies and genetic manipulation of wheat are continually ongoing, especially for winter varieties. Artificial mutagenesis increases genetic diversity and provides tools for the study of gene function (Krysan et al., 1999; Jeong et al., 2002). Several EMS-mutagenized populations are available in winter wheat (Chen et al., 2012, 2021b; Li et al., 2022). However, the lack of genome-wide gene-indexed information makes it difficult to associate mutant phenotypes with causal DNA variations.
Here, we described whole-exome capture sequencing and characterization of an EMS-mutagenized population of the winter wheat variety KN9204 (Figures 1 and 3). Recently, we assembled a high-quality reference genome for KN9204 (Shi et al., 2022) and designed whole-exome capture probes accordingly. A pseudogenome has been assembled for the spring wheat variety Cadenza (Krasileva et al., 2017), but other varieties such as Lunxuan 987 and Jinmai 47 have not been sequenced (Chen et al., 2021b; Li et al., 2022). The lack of a high-quality reference genome limits the utilization of mutations, including retrieval and selection of desired mutants, primer design, and authentication of variants. The KN9204 mutant pool has advantages in mutation frequency and pool size, important factors that affect the variability of a mutagenized population (Henry et al., 2014). The KN9204 population has a high mutation density of at least one mutation per 50.2 bp, lower than that of Kronos (∼28.7 bp) and Cadenza (∼25.3 bp) (Krasileva et al., 2017) but much higher than that of NN-Gandum-1 (∼47.8 kb) (Hussain et al., 2018), Jinmai 47 (∼47 kb) (Chen et al., 2012), and the T. monococcum accession TA4342-96 (∼92 kb) (Rawat et al., 2012). The KN9204 mutants also have a smaller number of EMS-type SNPs per line compared with Kronos and Cadenza, and the pool has a larger population size and contains variations covering nearly the entire set of coding genes. Furthermore, the KN9204 population contains severe mutations in over 41 000 high-confidence genes, making it a well-balanced option for functional genomics and breeding.
The 1RS chromosome segment from rye has been widely used in breeding programs for agronomic trait improvement (Feuillet et al., 2008; Moskal et al., 2021). Although a chromosome-scale assembly of a 1BL/1RS translocation line has been generated (Ru et al., 2020), no whole-genome reference sequence carrying the 1BL/1RS chromosome has been published other than KN9204 (Shi et al., 2022). In the rye-derived 1BS chromosome region, we detected mutations in 98.41% of the annotated genes and novel variation in ω-secalin protein genes (Figure 4) that may be helpful for understanding the genetic basis of elite trait formation and breeding improvement.
We performed a field survey for visible developmental changes and observed diverse morphological variations in the KN9204 mutant population, covering important agronomic traits (heading date, PH, and leaf morphology), yield traits (SPS, thousand-grain weight, and grain number per spike), and quality trait (grain hardness) (Figure 2). More importantly, we identified novel allelic variations via WES in agronomic- and yield-related genes such as Rht-D1, WFZP, and GW2. In the Green Revolution gene Rht-D1, we detected three novel alleles with truncation in multiple lines, four of which showed altered PH (Figure 4, Supplemental Data 2). Thus, the genetic variation in the KN9204 mutant library has potential for use in the breeding process.
All-in-one KN9204 toolbox for gene functional studies in wheat
In recent years, numerous genomic studies and high-throughput multi-omics sequencing data have enabled wheat research to enter the “big data era” (Xiao et al., 2022). Integrating different layers of omics data can accelerate our understanding of wheat productivity, quality, and environmental adaptation. Because of the elite agronomic traits and high-quality reference genome of KN9204 (Jia et al., 2006; Cui et al., 2016; Shi et al., 2022), we generated transcriptome and epigenomic datasets for this variety, including chromatin accessibility, core histone modifications, and variants from multiple tissues, developmental stages, and environmental treatments (Supplemental Figure 1) (Li et al., 2018; Lin et al., 2022; Shi et al., 2022; Zhang et al., 2022; Zhao et al., 2022). Combined with the KN9204 gene-indexed mutant population established here, these data can now enable wheat functional genomics at different research levels.
NAC TFs are known to regulate abiotic stress responses, especially drought resistance in wheat and other species (Jeong et al., 2010; Mao et al., 2015, 2020, 2021; Chen et al., 2020). In this study, we integrated the transcriptome dynamics of KN9204 under stress treatments with the KN9204 mutant library to systematically survey 100 mutant lines with mutations in 80 NAC TFs. We identified 13 lines with NAC TF mutations that showed altered responses to drought and salt stress (Figure 5). We also used medium-scale screening and verification strategies for developing wheat spikes (Lin et al., 2022). We screened 85 potential TFs, which were identified by integrating a transcriptional regulation network with a genome-wide association study, and found that 44 TFs containing KN9204 mutant lines had altered spike architecture (Lin et al., 2022). For the various agronomic trait QTLs mapped in the KN9204-Jing 411 RIL population (Fan et al., 2018, 2019; Liu et al., 2020b; Yu et al., 2022; Zhao et al., 2022), we expect that verification and genetic effect evaluation of candidate genes can be easily performed using the KN9204 mutants. Thus, the KN9204 EMS population assists with mining, identifying, and verifying genes that underlie essential traits.
Combining multi-omics data generated in KN9204 (Figure 1) with the indexed KN9204 mutant lines can also accelerate the interpretation of molecular regulatory mechanisms for specific genes, especially TFs. Here we provided a snapshot of the transcriptional regulatory analysis of three NAC TFs that participate in drought and salt response/adaptation (Figures 6 and 7) by integrating transcriptome, chromatin accessibility, and TF binding site data previously generated in KN9204 (Shi et al., 2022; Zhang et al., 2023). These multi-dimensional omics data generated in KN9204 are useful for studying the effects of DNA variation on gene expression, transcriptional regulatory networks, and potential interacting proteins.
In summary, we built a mutant library for KN9204 and provided an “all-in-one” toolkit for the wheat research community. Compared with existing wheat mutant libraries, KN9204 is the first published gene-indexed mutant pool for winter wheat. It carries the 1BL/1RS wheat–rye translocated chromosome and shows a balance between gene coverage and background mutation interference. We hope that it will promote wheat functional genomics and the success of wheat mutant breeding.
Methods
Plant material and EMS mutagenesis
The winter wheat variety KN9204 was used to generate the mutant populations. To ensure the purity and uniformity of seeds, we sowed 30 individual plants of KN9204 derived from seeds of one spike and took the grains from well-grown plants for mutagenesis. In brief, approximately 5000 seeds (M0) were pre-soaked in ddH2O for 6 h and then soaked and gently shaken for 12 h in a 1.0% (v/v) solution of EMS (liquid, 99% purity; Coolaber, China) at a ratio of 200 grains/100 ml at room temperature (25°C). The treatment was terminated by washing the seeds for 10 min in 200 mM sodium thiosulfate solution and then thoroughly washing with running water for 1 h. The EMS-treated seeds were sown in the field, and 2090 M1 individuals survived and produced M2 seeds for harvest. Of these, 1396 M2 plants were successively advanced to the M3 generation using a single spike in the greenhouse. Sixteen progenies of the 694 M2 and 1396 M3 individuals were planted in a row in the field for phenotypic investigation and DNA extraction. All individuals in each row were harvested and bulked for later distribution. For each row, the young leaves of 8–10 seedlings were sampled and pooled for genomic DNA extraction using the Plant Genomic DNA Extraction Kit (DP205, TianGen, China) following the instructions provided by the supplier.
Growth phenotyping in the field
Sixteen progenies of the M2 and M3 individuals were planted in the field for phenotypic investigation. Each mutant line was planted in a 1.5-m single-row plot with 10 cm between plants and 25 cm between rows and managed according to local practices. Two independent field surveys were conducted during the 2021–2022 cropping season in Beijing (39° 55′ N, 116° 23′ E) and Shijiazhuang (38° 04′ N, 114° 28′ E). Anomalous developmental defects observed in at least three individuals in both fields were considered to be credible phenotypic variations. Thirteen quantitative indicators were investigated in both fields using 200 randomly chosen mutant lines, and the mean value of each trait was used for analysis. Flowering time was calculated as the days from sowing to half of the spikes in a row flowering. Flag leaf length and width were measured 10 days after flowering on the main shoots of five plants in the center of each row using a leaf area–measuring instrument (Yaxin-1242, Beijing Yaxin Science Instrument Technology, Beijing, China). PH, spike number per plant, and tiller angle were measured 15 days after flowering on 10 uniform plants from the center of each row. PH was measured from the soil to the tip of the uppermost spike (excluding awns). Spikes with at least three grains were counted to measure spike number per plant. Tiller angles were measured with an angle-measuring instrument (TPZW-J-1, TOP, China). Spike length and SPS were measured on 10 prominent tiller spikes. Spike length was measured from the base of the spike to the tip (excluding the awns), and total SPS was counted. These spikes were harvested and used to measure grain number per spike, thousand-grain weight, grain length, grain width, and grain roundness with a Crop Grain Appearance Quality Scanning Machine (SC-G, Wanshen Technology, China).
Construction of exome sequencing libraries
Genomic DNA was quantified using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Approximately 2 μg of genomic DNA was fragmented into 200–300 bp using the Bioruptor UCD-200 sonicator (Diagenode, Denville, NJ). Libraries were constructed using the DNA library prep kit for the BGI platform (BGI, Shenzhen, China). Fragmented DNA was end-repaired with an end-repair enzyme and deoxyadenosine. According to the manufacturer’s instructions, the adapter-ligated libraries were selected for an average insert size of ∼350 bp using magnetic beads (Twist Bioscience, CA, USA). The pre-capture library amplification was performed with four or five PCR cycles. Equal amounts of products from eight libraries were pooled to obtain 4 μg DNA for hybridization.
Hybridization of sample libraries was performed using the customer exome panel (Tcuni, Chengdu, China). The exome panel was designed specifically for the coding sequences of the KN9204 genome and included 1 232 636 probes covering 127 Mb of the genome (98.11% of the coding sequences of high-confidence genes). Hybrids were captured using the M1 capture beads kit (Thermo Fisher, MA, USA), washed, and amplified by ligation-mediated PCR. The quality of the captured libraries was assessed using the Agilent 4200 Tape Station (Agilent, CA, USA). The final captured libraries were sequenced with the BGI T7 platform.
Read mapping and SNP calling
After quality control of raw reads with fastp (v.0.19.5), BWA (v.0.7.17-r1188) was used with default settings to align the reads to the KN9204 reference genome shared by Shi et al. prior to publication (including 110 326 genes; Shi et al., 2022). Reads with MQ < 30 were removed. BAM files were sorted, and PCR duplicates were removed using SAMtools (v.1.9). The GATK best practice workflow was used for SNP calling. A GVCF file was generated for each sample using “HaplotypeCaller.” Then, “GenotypeGVCFs” was used to transform the GVCF file for each sample into a raw VCF file. Finally, BCFtools (v.1.9) was used to filter each VCF file by '%QUAL<100 || INFO/DP < 5'. In total, we obtained 34 156 989 SNPs from all sequenced samples.
Identification of EMS mutations
To reduce the false-positive rate of EMS mutations among the SNP datasets, we used a strict filtering pipeline with three steps: (1) 614 725 SNPs were discovered among three wild-type plants of KN9204. Errors in sequencing or computational processing caused these SNPs, and they were therefore removed from our dataset. (2) EMS mutations in a large genome are rare events. A genomic site is unlikely to be mutated in more than one sample. Any SNP detected in more than one sample was therefore considered an error, and 13 862 902 SNPs remained in our dataset. (3) We removed heterozygous SNPs supported by fewer than 10 reads and homozygous SNPs supported by fewer than 6 reads. As a result, 2 971 607 SNPs were defined as the final EMS mutations and used in our analysis. Because users may want to retrieve all possible mutations, a larger SNP dataset is used on our website, in which one mutation was allowed to be present in no more than four samples.
Validation of EMS mutations
A total of 22 mutations were randomly chosen for validation through direct Sanger sequencing. The main objectives were to confirm the presence of the mutation and assess the mutation transmission efficiency in progeny seeds. For each mutation, at least four plants were used for validation, and the gene-specific PCR primers used to clone genomic regions flanking the target mutation are shown in Supplemental Data 1.
Annotation and phylogeny of NAC TFs
We annotated the TFs by taking the union of ortholog-based results, an HMMsearch approach, and a BLAST-based approach. HMMsearch was performed using Pfam conserved domains of TF families (provided by PlantTFDB) to identify wheat TFs in the wheat genome. Protein sequences of TFs annotated in rice and Arabidopsis were used for ortholog alignment with wheat genes, using the BLASTP method with a cutoff e value ≤ 1e−5. We aligned the NAC protein sequences of wheat, rice, and Arabidopsis using ClustalW and constructed maximum-likelihood phylogenetic trees using the auto setting to detect the best protein model, 100 maximum-likelihood searchers, and 100 rapid bootstraps.
Stress treatments
Seeds of KN9204 and the mutant lines were surface sterilized in 1% sodium hypochlorite for 15 min, washed in distilled water several times, and placed on vermiculite for 5 days. Uniform seedlings were wrapped with a sponge and fixed on a seedling-raising plate through a 1-cm-diameter hole. Before stress treatments, the seedlings were grown hydroponically in half-strength Hoagland solution in the greenhouse for 24 h. Then the seedlings were subjected to drought stress (8% PEG-6000, w/v) or salt stress (250 mM NaCl) for 14 days in a growth chamber at 22°C/18°C (day/night), 16 h/8 h (light/dark), and 50% humidity. All experiments were performed in parallel, and seedlings in normal growth conditions were used as controls. Leaves and roots were collected separately 6 days after stress treatment, frozen immediately in liquid nitrogen, and stored at −80°C.
Transcriptome analyses
Total RNA was isolated from leaves and roots under normal and stress conditions (three biological replicates per treatment) using an RNAprep Pure Plant Kit (TianGen, Beijing, China). A NanoDrop 1000 spectrophotometer and an Agilent Bio-analyzer 2100 system (Agilent Technologies, Beijing, China) were used to determine the quantity and quality of the RNA. Equal amounts of total RNA from 20 samples were pooled for library construction and transcriptome sequencing using a modified strand-specific 3′-end RNA-seq protocol. Raw reads were filtered with fastp v.0.20.1 for adapter removal, low-quality base trimming, and read filtering (Chen et al., 2018). FastQC v.0.11.8 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) was also performed to ensure the high quality of the reads. Clean reads were aligned using HISAT2 v.2.1.0 with default parameters (Kim et al., 2019). The number of paired reads mapped to each gene was counted using featureCounts v.2.0.1 with default parameters (Liao et al., 2014). The count files were then used as input for DEG analysis with DESeq2 v.1.26.0 using the threshold “absolute value of Log2 Fold Change ≥ 1 and FDR ≤ 0.05” (Love et al., 2014). CPM values generated from the count matrix were used to characterize gene expression and for principal component analysis. We obtained mean counts for subsequent clustering and visualization by merging three biological replicates. Functional enrichment was performed using the clusterProfiler v.3.18.1 R package (Yu et al., 2012), and GO annotation files were generated from IWGSC Annotation v.1.1.
Statistical analysis
R (https://cran.r-project.org/; v.4.0.2) was used to compute statistics and generate plots unless otherwise specified. The data statistics and drawings in Figures 4E and 6B were produced using GraphPad Prism 8.0. Wilcoxon’s test was used for two-group comparisons of data (Figures 4A–4C, Supplemental Figures 3D and 6F). Dunnett’s test was used to determine the differences between multiple experimental groups and the controls (Figures 4E and 6C). Tukey’s multiple comparisons test was used to compare the significance of differences between more than three groups (Figures 5E and 6B).
Material distribution
We are promoting the global distribution of this mutant library, for example, by depositing the entire collection in international gene banks. However, owing to the regulatory policy of the funding agency, we need time to communicate and proceed with this process, which usually takes months to years. To meet the immediate needs of users outside China, we have cooperated with MolBreeding Biotech Ltd. to build a mutation query and ordering platform with an English interface (http://kn9204.molbreeding.com/). Through this website, the EMS-type mutations detected in the KN9204 populations at different stringency levels can be visualized using a JBrowse graphic interface. The mutations are colored based on the severity of their effects (red, stop gained/frameshift; violet, missense; green, synonymous; and blue, non-coding regions). Additional mutation information is available by clicking on a particular mutation in the browser. “Blast,” “Get sequence,” and “PrimerServer” tools based on the KN9204 genome and genes are integrated to enable users to easily obtain gene IDs and design genotyping primers. Once the desired mutations are identified, users can order on the “Query” page, and the system will automatically send the order information to both the user and the manager. Seeds will be sent out after payment. In addition, the platform will help to prepare relevant customs clearance documents, enabling orders from abroad to proceed smoothly.
DNA from 8–10 plants was pooled for sequencing of each mutant line, which means that the indexed mutation results may represent a mixture of different seeds. The user should verify the received seeds to confirm particular mutations.
Data availability
The raw RNA-seq and ATAC-seq data reported in this study have been deposited in the Genome Sequence Archive (Chen et al., 2021a) under accession no. CRA009130 and in the National Genomics Data Center (CNCB-NGDC Members and Partners, 2022), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences, where they are publicly accessible at https://ngdc.cncb.ac.cn/gsa. The published RNA-seq and epigenomic data from KN9204 used for analysis were downloaded from CNCB-NGDC under accession nos. CRA008877 (spike-related data, Lin et al., 2022) and PRJCA004416 (leaf- and root-related data, Shi et al., 2022; Zhang et al., 2023).
Funding
This research is supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA24010204) to J.X., the Hebei Natural Science Foundation (C2021205013) and “Full-time introduction of high-end talent research project” (2020HBQZYC004) to X.-g.L., the National Natural Science Foundation of China (U22A6009) to J.-m.L., the Research Program for Network Security and Information of the Chinese Academy of Sciences (CAS-WX2021SF-0109) to F.H. and J.X., the National Key Research and Developmental Program of China (2021YFD1201500) to J.X., and a China Postdoctoral Science Foundation-funded project (2020M680742) to D.-z.W.
Author contributions
J.X. and X.-g.L. designed and supervised the research; J.X. and D.-z.W. wrote the manuscript with the help of H.-j.W., F.H., Y.-h.H., Y.-l.J., X.-g.L., J.-m.L., Y.-m.Y., Y.-q.Z., Y.G., C.-j.Z., W.-q.T., L.W., X.-l.G., S.-z.Z., Y.-j.Z., and X.-l.L.; J.X., Y.-m.Y., D.-z.W., H.-j.W., F.H., and Y.-x.X. prepared all the figures; Y.-p.L., F.C., X.-g.L., and J.-m.L. generated the EMS mutant library; Y.-p.L. and D.-z.W. performed mutant library growth in the field and phenotyping; Y.-x.Z., Z.-x.C., and L.-x.G. performed genomic DNA extraction for WES and Sanger sequencing for verification; H.-j.W. and F.H. performed WES analysis; Y.-x.X. performed the transcriptome and epigenome analysis; and D.-z.W. performed all the rest of the experiments.
Acknowledgments
No conflict of interest is declared.
Published: March 21, 2023
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Supplemental information is available at Plant Communications Online.
Contributor Information
Junming Li, Email: ljm@ms.sjziam.ac.cn.
Fei He, Email: fhe@genetics.ac.cn.
Xigang Liu, Email: xgliu@hebtu.edu.cn.
Jun Xiao, Email: jxiao@genetics.ac.cn.
Supplemental information
References
- Ahmed H.G.M.-D., Sajjad M., Li M., Azmat M.A., Rizwan M., Maqsood R.H., Khan S.H. Selection criteria for drought-tolerant bread wheat genotypes at seedling stage. Sustainability. 2019;11:2584. doi: 10.3390/su11092584. [DOI] [Google Scholar]
- Botticella E., Sestili F., Hernandez-Lopez A., Phillips A., Lafiandra D. High resolution melting analysis for the detection of EMS induced mutations in wheat Sbella genes. BMC Plant Biol. 2011;11:156. doi: 10.1186/1471-2229-11-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Mondragon J.A., Riudavets-Puig R., Rauluseviciute I., Berhanu Lemma R., Turchi L., Blanc-Mathieu R., Lucas J., Boddie P., Khan A., Manosalva Pérez N., et al. JASPAR 2022: the 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2022;50:D165–D173. doi: 10.1093/nar/gkab1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Huang L., Min D., Phillips A., Wang S., Madgwick P.J., Parry M.A.J., Hu Y.-G. Development and characterization of a new TILLING population of common bread wheat (Triticum aestivum L.) PLoS One. 2012;7:e41570. doi: 10.1371/journal.pone.0041570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Zhou Y., Chen Y., Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Gong Y., Gao Y., Zhou Y., Chen M., Xu Z., Guo C., Ma Y. TaNAC48 positively regulates drought tolerance and ABA responses in wheat (Triticum aestivum L.) Crop J. 2020;9:785–793. [Google Scholar]
- Chen T., Chen X., Zhang S., Zhu J., Tang B., Wang A., Dong L., Zhang Z., Yu C., Sun Y., et al. The genome sequence archive family: toward Explosive data growth and diverse data types. Dev. Reprod. Biol. 2021;19:578–583. doi: 10.1016/j.gpb.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Wang Z., Heng Y., Li J., Pei J., Cao Y., Deng X.W., Ma L. Generation of a series of mutant lines resistant to imidazolinone by screening an EMS-based mutant library in common wheat. Crop J. 2021;9:1030–1038. [Google Scholar]
- Cingolani P., Platts A., Wang L.L., Coon M., Nguyen T., Wang L., Land S.J., Lu X., Ruden D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff. Fly. 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CNCB-NGDC Members and Partners Database resources of the National Genomics Data Center, China National Center for Bioinformation in 2022. Nucleic Acids Res. 2022;50:D27–D38. doi: 10.1093/nar/gkab951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui F., Fan X., Chen M., Zhang N., Zhao C., Zhang W., Han J., Ji J., Zhao X., Yang L., et al. QTL detection for wheat kernel size and quality and the responses of these traits to low nitrogen stress. Theor. Appl. Genet. 2016;129:469–484. doi: 10.1007/s00122-015-2641-7. [DOI] [PubMed] [Google Scholar]
- Dixon L.E., Greenwood J.R., Bencivenga S., Zhang P., Cockram J., Mellers G., Ramm K., Cavanagh C., Swain S.M., Boden S.A. TEOSINTE BRANCHED1 regulates inflorescence architecture and development in bread wheat (Triticum aestivum) Plant Cell. 2018;30:563–581. doi: 10.1105/tpc.17.00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaya O., Pont C., Sibout R., Martinek P., Badaeva E., Murat F., Chosson A., Watanabe N., Prat E., Gautier N., et al. FRIZZY PANICLE drives supernumerary spikelets in bread wheat. Plant Physiol. 2015;167:189–199. doi: 10.1104/pp.114.250043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entz M.H., Fowler D.B. Agronomic performance of winter versus spring wheat. Agron. J. 1991;83:527–532. [Google Scholar]
- Evans C.E.B., Arunkumar R., Borrill P. Transcription factor retention through multiple polyploidization steps in wheat. G3. 2022;12:jkac147. doi: 10.1093/g3journal/jkac147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Zhang W., Zhang N., Chen M., Zheng S., Zhao C., Han J., Liu J., Zhang X., Song L., et al. Identification of QTL regions for seedling root traits and their effect on nitrogen use efficiency in wheat (Triticum aestivum L.) Theor. Appl. Genet. 2018;131:2677–2698. doi: 10.1007/s00122-018-3183-6. [DOI] [PubMed] [Google Scholar]
- Fan X., Cui F., Ji J., Zhang W., Zhao X., Liu J., Meng D., Tong Y., Wang T., Li J. Dissection of pleiotropic QTL regions controlling wheat spike characteristics under different nitrogen treatments using traditional and conditional QTL mapping. Front. Plant Sci. 2019;10:187. doi: 10.3389/fpls.2019.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuillet C., Langridge P., Waugh R. Cereal breeding takes a walk on the wild side. Trends Genet. 2008;24:24–32. doi: 10.1016/j.tig.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Forster B.P. The development of “Eldo Ngano 1”: the world’s world’s first Ug99 resistant mutant wheat variety. IAEA Bull. 2014;55:18–19. [Google Scholar]
- Gao C. Genome engineering for crop improvement and future agriculture. Cell. 2021;184:1621–1635. doi: 10.1016/j.cell.2021.01.005. [DOI] [PubMed] [Google Scholar]
- Guo H., Yan Z., Li X., Xie Y., Xiong H., Liu Y., Zhao L., Gu J., Zhao S., Liu L. Development of a high-efficient mutation resource with phenotypic variation in hexaploid winter wheat and identification of novel alleles in the TaAGP.L-B1 gene. Front. Plant Sci. 2017;8:1404. doi: 10.3389/fpls.2017.01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry I.M., Nagalakshmi U., Lieberman M.C., Ngo K.J., Krasileva K.V., Vasquez-Gross H., Akhunova A., Akhunov E., Dubcovsky J., Tai T.H., et al. Efficient genome-wide detection and cataloging of EMS-induced mutations using exome capture and next-generation sequencing. Plant Cell. 2014;26:1382–1397. doi: 10.1105/tpc.113.121590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey L.T., N Hafeez A., Robinson H., Jackson S.A., Leal-Bertioli S.C.M., Tester M., Gao C., Godwin I.D., Hayes B.J., Wulff B.B.H., et al. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019;37:744–754. doi: 10.1038/s41587-019-0152-9. [DOI] [PubMed] [Google Scholar]
- Hu H., Dai M., Yao J., Xiao B., Li X., Zhang Q., Xiong L. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc. Natl. Acad. Sci. USA. 2006;103:12987–12992. doi: 10.1073/pnas.0604882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M., Iqbal M.A., Till B.J., Rahman M.U. Identification of induced mutations in hexaploid wheat genome using exome capture assay. PLoS One. 2018;13:e0201918. doi: 10.1371/journal.pone.0201918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IWGSC. Appels R., Eversole K., Stein N., Feuillet C., Keller B., Rogers J., Pozniak C.J., Choulet F., Distelfeld A., et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science. 2018;361:eaar7191. doi: 10.1126/science.aar7191. [DOI] [PubMed] [Google Scholar]
- Jeong D.-H., An S., Kang H.-G., Moon S., Han J.-J., Park S., Lee H.S., An K., An G. T-DNA insertional mutagenesis for activation tagging in rice. Plant Physiol. 2002;130:1636–1644. doi: 10.1104/pp.014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J.S., Kim Y.S., Baek K.H., Jung H., Ha S.-H., Do Choi Y., Kim M., Reuzeau C., Kim J.-K. Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol. 2010;153:185–197. doi: 10.1104/pp.110.154773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y., An D., Li J., Tong Y., An Z. Study on the effects of root traits on nitrogen uptake by different wheat genotypes at booting stage. Acta Agric. Boreali-Sin. 2006;21:37–40. [Google Scholar]
- Kadar R., Muntean L., Racz I., Ona A., Ceclan A., Hirişcău D. The effect of genotype, climatic conditions and nitrogen fertilization on yield and grain protein content of spring wheat (Triticum aestivum L.) Not. Bot. Horti Agrobot. Cluj-Napoca. 2018;47:515–521. [Google Scholar]
- Kaufmann K., Airoldi C.A. In: Plant Transcription Factors: Methods and Protocols. Yamaguchi N., editor. Springer; 2018. Master regulatory transcription factors in plant development: a blooming perspective. (3–22). [DOI] [PubMed] [Google Scholar]
- Kaur P., Gaikwad K. From genomes to GENE-omes: exome sequencing concept and applications in crop improvement. Front. Plant Sci. 2017;8:2164. doi: 10.3389/fpls.2017.02164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Paggi J.M., Park C., Bennett C., Salzberg S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019;37:907–915. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R., Bird N., Ramirez-Gonzalez R., Coghill J.A., Patil A., Hassani-Pak K., Uauy C., Phillips A.L. Mutation scanning in wheat by exon capture and next-generation sequencing. PLoS One. 2015;10:e0137549. doi: 10.1371/journal.pone.0137549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasileva K.V., Vasquez-Gross H.A., Howell T., Bailey P., Paraiso F., Clissold L., Simmonds J., Ramirez-Gonzalez R.H., Wang X., Borrill P., et al. Uncovering hidden variation in polyploid wheat. Proc. Natl. Acad. Sci. USA. 2017;114:E913–E921. doi: 10.1073/pnas.1619268114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan P.J., Young J.C., Sussman M.R. T-DNA as an insertional mutagen in Arabidopsis. Plant Cell. 1999;11:2283–2290. doi: 10.1105/tpc.11.12.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng P., Zhao J. Transcription factors as molecular switches to regulate drought adaptation in maize. Theor. Appl. Genet. 2020;133:1455–1465. doi: 10.1007/s00122-019-03494-y. [DOI] [PubMed] [Google Scholar]
- Li A., Yang W., Lou X., Liu D., Sun J., Guo X., Wang J., Li Y., Zhan K., Ling H.-Q., et al. Novel natural allelic variations at the Rht-1 loci in wheat. J. Integr. Plant Biol. 2013;55:1026–1037. doi: 10.1111/jipb.12103. [DOI] [PubMed] [Google Scholar]
- Li Y., Fu X., Zhao M., Zhang W., Li B., An D., Li J., Zhang A., Liu R., Liu X. A genome-wide view of transcriptome dynamics during early spike development in bread wheat. Sci. Rep. 2018;8:15338. doi: 10.1038/s41598-018-33718-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Zhou J., Jia H., Gao Z., Fan M., Luo Y., Zhao P., Xue S., Li N., Yuan Y., et al. Mutation of a histidine-rich calcium-binding-protein gene in wheat confers resistance to Fusarium head blight. Nat. Genet. 2019;51:1106–1112. doi: 10.1038/s41588-019-0426-7. [DOI] [PubMed] [Google Scholar]
- Li G., Wang L., Yang J., He H., Jin H., Li X., Ren T., Ren Z., Li F., Han X., et al. A high-quality genome assembly highlights rye genomic characteristics and agronomically important genes. Nat. Genet. 2021;53:574–584. doi: 10.1038/s41588-021-00808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Meng X., Li D., Zhao M., Sun S., Li Y., Lu G., Qiao W. A method for quantification of heat resistance of quality in different wheat cultivars. IOP Conf. Ser. Earth Environ. Sci. 2021;792:012044. [Google Scholar]
- Li Y., Xiong H., Zhang J., Guo H., Zhou C., Xie Y., Zhao L., Gu J., Zhao S., Ding Y., et al. Genome-wide and exome-capturing sequencing of a gamma-ray-induced mutant reveals biased variations in common wheat. Front. Plant Sci. 2022;12:793496. doi: 10.3389/fpls.2021.793496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Liu H.-J., Yan J., Tian F. Natural variation in crops: realized understanding, continuing promise. Annu. Rev. Plant Biol. 2021;72:357–385. doi: 10.1146/annurev-arplant-080720-090632. [DOI] [PubMed] [Google Scholar]
- Liao Y., Smyth G.K., Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- Licausi F., Ohme-Takagi M., Perata P. APETALA2/Ethylene Responsive Factor (AP2/ERF) transcription factors: mediators of stress responses and developmental programs. New Phytol. 2013;199:639–649. doi: 10.1111/nph.12291. [DOI] [PubMed] [Google Scholar]
- Lin Y., Yi X., Tang S., Chen W., Wu F., Yang X., Jiang X., Shi H., Ma J., Chen G., et al. Dissection of phenotypic and genetic variation of drought-related traits in diverse Chinese wheat landraces. Plant Genome. 2019;12:190025. doi: 10.3835/plantgenome2019.03.0025. [DOI] [PubMed] [Google Scholar]
- Lin X., Xu Y., Wang D., Yang Y., Zhang X., Bie X., Wang H., Jiang J., Ding Y., Lu F., et al. Systematic mining and genetic characterization of regulatory factors for wheat spike development. bioRxiv. 2022 doi: 10.1101/2022.11.11.516122. Preprint at. [DOI] [Google Scholar]
- Liu H., Wang K., Tang H., Gong Q., Du L., Pei X., Ye X. CRISPR/Cas9 editing of wheat TaQ genes alters spike morphogenesis and grain threshability. J. Genet. Genomics. 2020;47:563–575. doi: 10.1016/j.jgg.2020.08.004. [DOI] [PubMed] [Google Scholar]
- Liu J., Li H., Zhang N., Meng D., Zhi L., Ren X., Ji J., Su Z., Li J., Fan X., et al. Transcriptomic analysis reveals the contribution of QMrl-7B to wheat root growth and development. Front. Plant Sci. 2020;4549 doi: 10.3389/fpls.2022.1062575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Xie Y., Guo H., Zhao L., Xiong H., Gu J., Zhao S. In: Mutation Breeding, Genetic Diversity and Crop Adaptation to Climate Change. Sivasankar S., Ellis N., Jankuloski L., Ingelbrecht I., editors. CABI; 2021. New mutation techniques for crop improvement in China; pp. 47–52. [Google Scholar]
- Liu Y., Hou J., Wang X., Li T., Majeed U., Hao C., Zhang X. The NAC transcription factor NAC019-A1 is a negative regulator of starch synthesis in wheat developing endosperm. J. Exp. Bot. 2020;71:5794–5807. doi: 10.1093/jxb/eraa333. [DOI] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H., Wang H., Liu S., Li Z., Yang X., Yan J., Li J., Tran L.-S.P., Qin F. A transposable element in a NAC gene is associated with drought tolerance in maize seedlings. Nat. Commun. 2015;6:8326. doi: 10.1038/ncomms9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H., Li S., Wang Z., Cheng X., Li F., Mei F., Chen N., Kang Z. Regulatory changes in TaSNAC8-6A are associated with drought tolerance in wheat seedlings. Plant Biotechnol. J. 2020;18:1078–1092. doi: 10.1111/pbi.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H., Li S., Chen B., Jian C., Mei F., Zhang Y., Li F., Chen N., Li T., Du L., et al. Variation in cis-regulation of a NAC transcription factor contributes to drought tolerance in wheat. Mol. Plant. 2021;15:276–292. doi: 10.1016/j.molp.2021.11.007. [DOI] [PubMed] [Google Scholar]
- Minhas P.S., Rane J., Pasala R.K. In: Abiotic Stress Management for Resilient Agriculture. Minhas P.S., Rane J., Pasala R.K., editors. Springer; 2017. Abiotic stresses in agriculture: an overview; p. 3. (8). [Google Scholar]
- Moskal K., Kowalik S., Podyma W., Łapiński B., Boczkowska M. The pros and cons of rye chromatin introgression into wheat genome. Agronomy. 2021;11:456. [Google Scholar]
- Palan B., Bhattacharya A., Char B. TILLING in the era of precise genome editing. Indian J. Biotechnol. 2021;20:9–16. [Google Scholar]
- Phukan U.J., Jeena G.S., Shukla R.K. WRKY transcription factors: molecular regulation and stress responses in plants. Front. Plant Sci. 2016;7:760. doi: 10.3389/fpls.2016.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pour-Aboughadareh A., Kianersi F., Poczai P., Moradkhani H. Potential of wild relatives of wheat: ideal genetic resources for future breeding programs. Agronomy. 2021;11:1656. [Google Scholar]
- Ramírez-González R.H., Borrill P., Lang D., Harrington S.A., Brinton J., Venturini L., Davey M., Jacobs J., van Ex F., Pasha A., et al. The transcriptional landscape of polyploid wheat. Science. 2018;361:eaar6089. doi: 10.1126/science.aar6089. [DOI] [PubMed] [Google Scholar]
- Rawat N., Sehgal S.K., Joshi A., Rothe N., Wilson D.L., McGraw N., Vadlani P.V., Li W., Gill B.S. A diploid wheat TILLING resource for wheat functional genomics. BMC Plant Biol. 2012;12:205. doi: 10.1186/1471-2229-12-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat N., Joshi A., Pumphrey M., Singh L., Mahlandt A., Chhabra B., Wilson D., Gill B., Poland J., Tiwari V. A TILLING resource for hard red winter wheat variety jagger. Crop Sci. 2019;59:1666–1671. [Google Scholar]
- Ru Z., Juhasz A., Li D., Deng P., Zhao J., Gao L., Wang K., Keeble-Gagnere G., Yang Z., Li G., et al. 1RS.1BL molecular resolution provides novel contributions to wheat improvement. bioRxiv. 2020 doi: 10.1101/2020.09.14.295733. Preprint at. [DOI] [Google Scholar]
- Shahzad A., Ullah S., Dar A.A., Sardar M.F., Mehmood T., Tufail M.A., Shakoor A., Haris M. Nexus on climate change: agriculture and possible solution to cope future climate change stresses. Environ. Sci. Pollut. Res. Int. 2021;28:14211–14232. doi: 10.1007/s11356-021-12649-8. [DOI] [PubMed] [Google Scholar]
- Shi X., Cui F., Han X., He Y., Zhao L., Zhang N., Zhang H., Zhu H., Liu Z., Ma B., et al. Comparative genomic and transcriptomic analyses uncover the molecular basis of high nitrogen-use efficiency in the wheat cultivar Kenong 9204. Mol. Plant. 2022;15:1440–1456. doi: 10.1016/j.molp.2022.07.008. [DOI] [PubMed] [Google Scholar]
- Uauy C., Wulff B.B.H., Dubcovsky J. Combining traditional mutagenesis with new high-throughput sequencing and genome editing to reveal hidden variation in polyploid wheat. Annu. Rev. Genet. 2017;51:435–454. doi: 10.1146/annurev-genet-120116-024533. [DOI] [PubMed] [Google Scholar]
- Wang K., Liu H., Du L., Ye X. Generation of marker-free transgenic hexaploid wheat via an Agrobacterium-mediated co-transformation strategy in commercial Chinese wheat varieties. Plant Biotechnol. J. 2017;15:614–623. doi: 10.1111/pbi.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Li J., Chen S., Heng Y., Chen Z., Yang J., Zhou K., Pei J., He H., Deng X.W., Ma L. Poaceae-specific MS1 encodes a phospholipid-binding protein for male fertility in bread wheat. Proc. Natl. Acad. Sci. USA. 2017;114:12614–12619. doi: 10.1073/pnas.1715570114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., Liu B., Yao Y., Guo Z., Jia H., Kong L., Zhang A., Ma W., Ni Z., Xu S., et al. Wheat genomic study for genetic improvement of traits in China. Sci. China Life Sci. 2022;65:1718–1775. doi: 10.1007/s11427-022-2178-7. [DOI] [PubMed] [Google Scholar]
- Yu G., Wang L.-G., Han Y., He Q.-Y. clusterProfiler: an R Package for comparing biological themes among gene clusters. OMICS A J. Integr. Biol. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Liu Y., Zou Z., Sun X., Zhang J., Song S., Wang L., Qin R., Sun H., Cui F., et al. QTL detection for internode diameter and its association with yield-related traits in wheat. Cereal Res. Commun. 2022;51:101–113. doi: 10.1007/s42976-022-00283-0. [DOI] [Google Scholar]
- Zhang H., Cui F., Zhao L., Zhang X., Chen J., Zhang J., Li Y., Niu Y., Wang L., Gao C., et al. Epigenetic modifications mediate cultivar-specific root development and metabolic adaptation to nitrogen availability in wheat. bioRxiv. 2023 doi: 10.1101/2023.02.24.529862. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Jia H., Li T., Wu J., Nagarajan R., Lei L., Powers C., Kan C.-C., Hua W., Liu Z., et al. TaCol-B5 modifies spike architecture and enhances grain yield in wheat. Science. 2022;376:180–183. doi: 10.1126/science.abm0717. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li D., Zhang D., Zhao X., Cao X., Dong L., Liu J., Chen K., Zhang H., Gao C., Wang D. Analysis of the functions of TaGW2 homoeologs in wheat grain weight and protein content traits. Plant J. 2018;94:857–866. doi: 10.1111/tpj.13903. [DOI] [PubMed] [Google Scholar]
- Zhao C.-H., Fan X.-L., Wang W.-L., Zhang W., Han J., Chen M., Ji J., Cui F., Li J.-M. Genetic composition and its transmissibility analysis of wheat candidate backbone parent Kenong 9204. Acta Agron. Sin. 2015;41:574. [Google Scholar]
- Zhao C., Liu X., Liu H., Kong W., Zhao Z., Zhang S., Wang S., Chen Y., Wu Y., Sun H., et al. Fine mapping of QFlw-5B, a major QTL for flag leaf width in common wheat (Triticum aestivum L.) Theor. Appl. Genet. 2022;135:2531–2541. doi: 10.1007/s00122-022-04135-7. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Chen Z., Cheng M., Chen J., Zhu T., Wang R., Liu Y., Qi P., Chen G., Jiang Q., et al. Uncovering the dispersion history, adaptive evolution and selection of wheat in China. Plant Biotechnol. J. 2018;16:280–291. doi: 10.1111/pbi.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw RNA-seq and ATAC-seq data reported in this study have been deposited in the Genome Sequence Archive (Chen et al., 2021a) under accession no. CRA009130 and in the National Genomics Data Center (CNCB-NGDC Members and Partners, 2022), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences, where they are publicly accessible at https://ngdc.cncb.ac.cn/gsa. The published RNA-seq and epigenomic data from KN9204 used for analysis were downloaded from CNCB-NGDC under accession nos. CRA008877 (spike-related data, Lin et al., 2022) and PRJCA004416 (leaf- and root-related data, Shi et al., 2022; Zhang et al., 2023).