To the Editor:
Combination therapy with vascular endothelial growth factor signaling pathway inhibitors (VSPIs) and immune checkpoint inhibitors (ICIs) has led to major improvements in cancer survival. These survival improvements have not been observed to the same degree in people with chronic kidney disease (CKD) and cancer. CKD is highly prevalent in people with cancer1 and is associated with reduced survival in those diagnosed with some types of cancer.2 People with CKD have been poorly represented in cancer trials,3 and the evidence base for the use of ICI and VSPI in people with CKD is important because of their association with adverse kidney events.4 We assessed the extent to which people with markers of kidney disease are represented in clinical trials of combination therapy with ICI and VSPI.
We systematically searched MEDLINE, EMBASE, and Cochrane library databases (PROSPERO CRD42022337942) and followed Preferred Reporting Items for Systematic Reviews and Meta-analyses statement guidelines. The inclusion criteria used the PICO (population, intervention, comparison, and outcome) framework and included adult populations with any solid organ cancer receiving concurrent ICI and VSPI treatment in phase II-IV trials. Two reviewers independently assessed published articles and extracted data. The study did not require ethics approval and used a systematic narrative synthesis with quantitative analysis.
The primary outcomes of interest were: i) exclusion criteria related to kidney disease from trial protocols and ii) information about the representation of people with kidney disease in trials of combination therapy with ICI and VSPI.
Initial search identified 4,893 references, of which 32 trials spanning April 6, 2018 to December 4, 2022 and evaluating 11,066 participants met our pre-specified inclusion criteria. Most participants were assessed in Phase III trials (12 of 32 trials, 87.5% of participants); the remaining participants were assessed in phase II trials. There were 10 different combinations of ICI and VSPI. We could not obtain 1 trial’s full eligibility criteria (Zhang et al 2021, representing 0.3% of participants); this trial was excluded from the analysis (Table 1).
Table 1.
Trials that met eligibility criteria, with trial characteristics and exclusion criterion used
| Study | VSPI | ICI | Trial Population (n) | Tumor Site | Excluded With any Form of Kidney Disease | Exclusion Criterion Definition | Creatinine Clearance Cut-off (mL/min) | Were Patients with Proteinuria Excluded? | Was Baseline Kidney Function Available? | Were Patients on Immunosuppression Excluded? | Were Patients With Solid Organ Transplants Excluded |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase III randomized controlled trials | |||||||||||
| Motzer 2019 | Axitinib | Avelumab | 886 | Renal cell | Yes | Creatinine clearance | 50 | Yes | No | Yes | No |
| Choueiri 2021 | Cabozantinib | Nivolumab | 651 | Renal cell | Yes | Serum Creatinine in relation to upper limit of normal or Creatinine clearance | 40 | Yes | No | Yes | No |
| Colombo 2021 | Bevacizumab | Pembrolizumab | 617 | Gynae | Yes | Serum Creatinine in relation to upper limit of normal or Creatinine clearance | 60 | Unspecified | No | Yes | No |
| Andre 2020 | Bevacizumab | Pembrolizumab | 307 | Colorectal | Yes | Serum Creatinine in relation to upper limit of normal or Creatinine clearance | 60 | Unspecified | No | Yes | No |
| Makker 2022 | Lenvatinib | Pembrolizumab | 827 | Gynae | Yes | Serum Creatinine in relation to upper limit of normal or Creatinine clearance | 30 | Yes | No | Yes | Yes |
| Moore 2021 | Bevacizumab | Atezolizumab | 1301 | Gynae | Yes | Serum Creatinine in relation to upper limit of normal | NA | Yes | No | Yes | Yes |
| Motzer 2021 | Lenvatinib | Pembrolizumab | 1069 | Renal cell | Yes | Creatinine clearance | 30 | Yes | No | Yes | Yes |
| Rini 2019 - keynote | Axitinib | Pembrolizumab | 861 | Renal cell | Yes | Serum Creatinine or Creatinine clearance | 40 | Yes | No | Yes | Yes |
| Finn 2020 | Bevacizumab | Atezolizumab | 501 | Liver | Yes | Serum Creatinine or Creatinine clearance | 50 | Yes | No | Yes | Yes |
| Sugawara 2021 | Bevacizumab | Nivolumab | 550 | Lung | Yes | Serum Creatinine or Creatinine clearance | 50 | Unspecified | No | Yes | Unspecified |
| Socinski 2018 | Bevacizumab | Atezolizumab | 1202 | Lung | Yes | Serum Creatinine in relation to upper limit of normal | NA | Yes | No | Yes | Yes |
| Rini 2019 - Immotion | Bevacizumab | Atezolizumab | 915 | Renal cell | Yes | Measured or Creatinine clearance | 30 | Yes | No | Yes | Yes |
| Phase II randomized controlled trials | |||||||||||
| Lheureux 2022 | Cabozantinib | Nivolumab | 82 | Gynae | Yes | Serum Creatinine in relation to upper limit of normal or Creatinine clearance | 50 | Yes | No | Yes | Yes |
| Mettu 2022 | Bevacizumab | Atezolizumab | 133 | Colorectal | Yes | Creatinine clearance | 50 | Yes | No | Yes | Yes |
| Nayak 2021 | Bevacizumab | Pembrolizumab | 80 | GBM | Yes | Serum Creatinine in relation to upper limit of normal | NA | Yes | No | Yes | No |
| McDermott 2018 | Bevacizumab | Atezolizumab | 305 | Renal cell | Yes | Serum Creatinine in relation to upper limit of normal or Creatinine clearance | 40 | Yes | No | Yes | Yes |
| Redman 2022 | Bevacizumab | Avelumab | 26 | Colorectal | Yes | Creatinine clearance | 30 | Unspecified | No | Yes | Yes |
| Phase II non-randomized multi-arm trials | |||||||||||
| Nayak 2022 | Bevacizumab | Durvalumab | 159 | GBM | Yes | Serum Creatinine in relation to upper limit of normal or Creatinine clearance | 50 | Yes | No | Yes | Yes |
| Awada 2020 | Axitinib | Avelumab | 54 | GBM | Yes | Creatinine clearance | 30 | Yes | No | Yes | No |
| Phase II non-randomized single-arm trials | |||||||||||
| Cousin 2021 | Regorafenib | Avelumab | 46 | Colorectal | Yes | Creatinine clearance | 30 | Yes | No | Yes | Yes |
| Cousin 2022 | Regorafenib | Avelumab | 34 | Other | Yes | Creatinine clearance | 30 | Yes | No | Yes | Yes |
| Kawazoe 2020 | Lenvatinib | Pembrolizumab | 29 | Gastric | Yes | Serum Creatinine in relation to upper limit of normal | NA | Yes | No | Yes | No |
| Lam 2021 | Bevacizumab | Atezolizumab | 40 | Lung | Yes | Serum Creatinine in relation to upper limit of normal or Creatinine clearance | 50 | Yes | No | Yes | No |
| Lee C 2022 | Cabozantinib | Nivolumab | 47 | Renal cell | Yes | Serum Creatinine in relation to upper limit of normal or Creatinine clearance | 30 | Yes | No | Yes | No |
| Lee J 2022 |
Bevacizumab | Atezolizumab | 42 | Lung | Yes | Creatinine clearance | 30 | Yes | No | Yes | No |
| Liu 2019 | Bevacizumab | Nivolumab | 38 | Gynae | Yes | Serum Creatinine in relation to upper limit of normal or Creatinine clearance | 60 | Yes | No | Yes | No |
| Makker 2019 | Lenvatinib | Pembrolizumab | 54 | Gynae | Yes | Serum Creatinine in relation to upper limit of normal or Creatinine clearance | 40 | Yes | No | Yes | Yes |
| McGregor 2019 | Bevacizumab | Atezolizumab | 60 | Renal cell | Yes | Creatinine clearance | 30 | Yes | No | Yes | Yes |
| Seto 2022 | Bevacizumab | Atezolizumab | 39 | Lung | Yes | Serum Creatinine | NA | Yes | No | Yes | Unspecified |
| Wilky 2019 | Axitinib | Pembrolizumab | 33 | Other | Yes | Serum Creatinine in relation to upper limit of normal or Creatinine clearance | 60 | Unspecified | No | Yes | Unspecified |
| Zhang 2021 | Lenvatinib | Pembrolizumab | 38 | Other | Unspecified | Unspecified | NA | Unspecified | No | Unspecified | Unspecified |
| Zsiros 2021 | Bevacizumab | Pembrolizumab | 40 | Gynae | Yes | Creatinine clearance | 60 | Yes | No | Yes | Yes |
Abbreviations: n, number; VSPI, VEGF-signaling pathway inhibitor; ICI, immune checkpoint inhibitor; GBM, Glioblastoma; Gyne, Gynecological cancers; NA, not applicable.
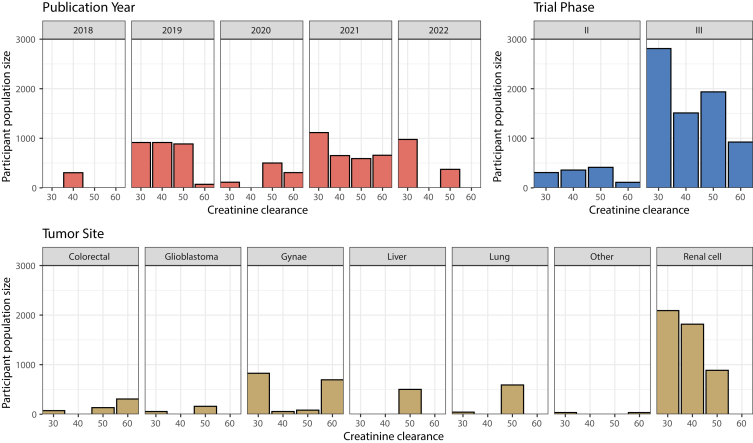
All trials contained at least 1 exclusion criterion pertaining to kidney disease. Creatinine Clearance (CrCl) was the most common exclusion criterion, either alone or in combination with another criterion (26 of 31 trials, 75.7% of participants). No trials using the criterion CrCl included people with CrCl of <30 mL/min. The CrCl cut-off values were inconsistent by trial phase, tumor site, publication year, and agents used in combination (Figure 1, Fig S1-S3). Six trials (6 of 31, 17.2% of participants) accepted alternatives measures of glomerular filtration rate (GFR). Participants with evidence of proteinuria were excluded in 26 of 31 trials (85.9% of participants). Semi-quantitative detection on urinalysis (24 of 31 trials, 84.9% of participants) was the most used exclusion criterion, either alone (3 of 31 trials, 1.1% of participants) or in combination with quantitative methods. All trials excluded people on immunosuppressive therapy. No trial published participants’ baseline kidney function or proteinuria in the primary results article.
Figure 1.
Bar graphs to demonstrate the creatinine clearance values used for exclusion from trials of combination therapy with VEGF-signaling pathway inhibitors and immune checkpoint inhibitors, presented according to participant population size.
We found that all published trials of combination therapy with ICI and VSPI excluded people with evidence of kidney disease. No study included people with advanced CKD and few studies included people with proteinuria. The findings are concerning given that both drugs are associated with adverse kidney effects when used alone, and in combination.4 The under-representation of people with CKD in trials may undermine external validity of the trial and the generalizability of results.
The evidence for administration of VSPI or ICI in advanced CKD is mainly from published case series or retrospective analysis. The paucity of safety data may deny the access of people with CKD to effective anti-cancer therapy or unnecessarily expose them to excess risk of adverse effects. All identified trials excluded people treated with immunosuppressive medications; however, the use of these agents in kidney transplant recipients is increasing. A recent analysis demonstrated high rates of transplant rejection following ICI initiation.5
Concerns have been raised about heterogeneity regarding laboratory measurements used for cancer trial eligibility, including kidney function.6 The accuracy of creatinine-based GFR estimating equations is susceptible to several factors. Moreover, cancer patients may have reduced creatinine generation because of sarcopenia, leading to overestimation of GFR.7 Inaccuracies in GFR estimation could expose patients to potentially toxic doses or, conversely, to inadequate dosing of medications with reduced anti-cancer efficacy.
Renalism, the systematic undertreatment of people with CKD, is not unique to cancer therapies.8,9 Given that CKD is more common among older people, ethnic minorities, and those from socioeconomically deprived backgrounds,10 improving the evidence base of people with CKD is crucial in reducing health care inequalities.
Limitations to this review include its strategy to capture eligibility criteria for original trials, potentially missing post-licensing data or pre-trial safety data. We may not have captured efforts to report the representation of participants with kidney disease in secondary trial publications. We could not find 1 of 32 full trial protocols; however, this trial included only 0.3% of the total number of participants.
In conclusion, no trial included people with advanced CKD or kidney transplant recipients and few included people with proteinuria. Given CKD’s high prevalence in people with cancer and its association with worse cancer outcomes, targeted efforts should improve the representation of people with CKD in cancer trials to enhance external validity. Where exclusions are biologically justified, standardizing the approach using relevant markers of kidney function would improve the clinical application.
Article Information
Authors’ Contributions
Study design: all authors; search design: BMPE, SR, JSL; search performance, extraction and collation of data: BMPE, SR; data analysis: BMPE, JSL; data analysis and figures: BMPE; supervision: PBM, NNL, RJJ. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was in part funded by a Chief Scientist Office Lectureship (number PCL/20/10) to Dr Lees.
Financial Disclosure
Outside the submitted work, Dr Lees reports personal fees from Pfizer, Astra Zeneca, and Bristol-Myers Squibb; Professor Mark reports personal fees and/or non-financial support from Vifor, Napp, Pharmacosmos, Astra Zeneca, Astellas, Novartis, and grants from Boehringer Ingelheim; Dr Lang reports personal fees and non-financial support from Roche, Pfizer, Novartis, Astra Zeneca, Pharmacosmos, Vifor Pharma, and grant support from Roche Diagnostics, Astra Zeneca, and Boehringer (all paid to the University of Glasgow, his employing institution). Professor Jones reports research support from Clovis, Astellas, Exelixis, AstraZeneca, and Roche, honoraria from Clovis, Astellas, AstraZeneca, Roche, Ipsen, Bristol-Myers-Squibb, Pfizer, Merck Serono, Merck Sharp Dohme, Janssen, Bayer, and Novartis. The remaining authors declare that they have no relevant financial interests.
Disclaimer
The views expressed in the submitted article are the authors’ and not an official position of the institution or funder.
Peer Review
Received December 19, 2022. Evaluated by 2 external peer reviewers, with direct editorial input from the Editor-in-Chief. Accepted in revised form March 19, 2023.
Footnotes
Figure S1. PRISMA flowchart
Figure S2. Bar plot of creatinine clearance cut-off values in trials by publication year, trial phase, and tumor site
Figure S3. Heat map of creatinine clearance cut-off values and trial population size
Supplementary Material
Figure S1-S3.
References
- 1.Launay-Vacher V., Oudard S., Janus N., et al. Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer. 2007;110(6):1376–1384. doi: 10.1002/cncr.22904. [DOI] [PubMed] [Google Scholar]
- 2.Lees J.S., Ho F., Parra-Soto S., et al. Kidney function and cancer risk: an analysis using creatinine and cystatin C in a cohort study. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitchlu A., Shapiro J., Amir E., et al. Representation of patients with chronic kidney disease in trials of cancer therapy. JAMA. 2018;319(23):2437–2439. doi: 10.1001/jama.2018.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao L., Yang X., Yi C., Zhu H. Adverse events of concurrent immune checkpoint inhibitors and antiangiogenic agents: a systematic review. Front Pharmacol. 2019;10:1173. doi: 10.3389/fphar.2019.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murakami N., Mulvaney P., Danesh M., et al. A multi-center study on safety and efficacy of immune checkpoint inhibitors in cancer patients with kidney transplant. Kidney Int. 2021;100(1):196–205. doi: 10.1016/j.kint.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sprangers B., Perazella M.A., Lichtman S.M., Rosner M.H., Jhaveri K.D. Improving cancer care for patients with CKD: the need for changes in clinical trials. Kidney Int Rep. 2022;7(9):1939–1950. doi: 10.1016/j.ekir.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chancharoenthana W., Wattanatorn S., Vadcharavivad S., Eiam-Ong S., Leelahavanichkul A. Agreement and precision analyses of various estimated glomerular filtration rate formulae in cancer patients. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-55833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konstantinidis I., Nadkarni G.N., Yacoub R., et al. Representation of patients with kidney disease in trials of cardiovascular interventions. JAMA Intern Med. 2016;176(1):121–124. doi: 10.1001/jamainternmed.2015.6102. [DOI] [PubMed] [Google Scholar]
- 9.Major R., Selvaskandan H., Makkeyah Y.M., Hull K., Kuverji A., Graham-Brown M. The exclusion of patients with CKD in prospectively registered interventional trials for COVID-19—a rapid review of international registry data. J Am Soc Nephrol. 2020;31(10):2250–2252. doi: 10.1681/ASN.2020060877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lees J.S., Hanlon P., Butterly E.W., et al. Effect of age, sex, and morbidity count on trial attrition: meta-analysis of individual participant level data from phase 3/4 industry funded clinical trials. BMJ Med. 2022;1(1) doi: 10.1136/bmjmed-2022-000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1-S3.