Abstract
Rationale & Objective
The benefit–risk profile of rivaroxaban versus warfarin for atrial fibrillation (AF) in patients with chronic kidney disease is uncertain. We compared rivaroxaban with warfarin across the range of kidney function in adults with AF.
Study Design
Multicenter retrospective cohort.
Setting & Participants
Adults with AF and a measure of estimated glomerular filtration rate (eGFR); using administrative data from 5 jurisdictions across Australia and Canada (2011-2018). Kidney function was categorized as eGFR ≥60, 45-59, 30-44, and <30 mL/min/1.73 m2. Patients receiving dialysis and kidney transplant recipients were excluded.
Exposures
New dispensation of either rivaroxaban or warfarin.
Outcomes
Composite (1) effectiveness outcome (all-cause death, ischemic stroke, or transient ischemic attack) and (2) major bleeding events (intracranial, gastrointestinal, or other) at 1 year.
Analytical Approach
Cox proportional hazards models accounting for propensity score matching were performed independently in each jurisdiction and then pooled using random-effects meta-analysis.
Results
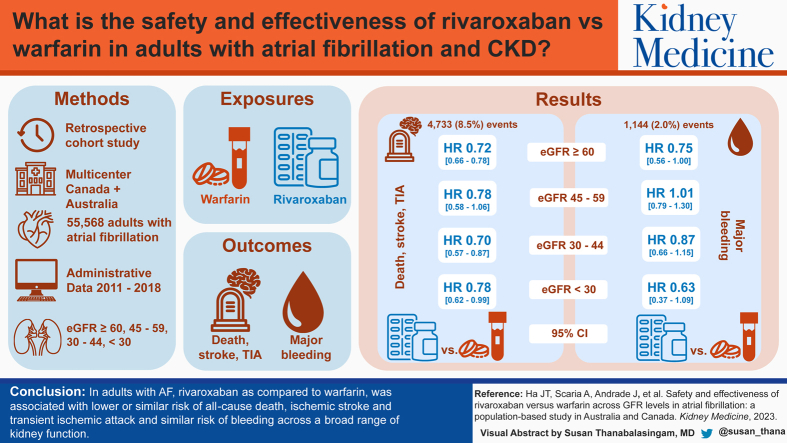
55,568 patients (27,784 rivaroxaban–warfarin user matched pairs; mean age 74 years, 46% female, 33.5% with eGFR <60 mL/min/1.73 m2) experienced a total of 4,733 (8.5%) effectiveness and 1,144 (2.0%) bleeding events. Compared to warfarin, rivaroxaban was associated with greater or similar effectiveness across a broad range of kidney function (pooled HRs of 0.72 [95% CI, 0.66-0.78], 0.78 [95% CI, 0.58-1.06], 0.70 [95% CI, 0.57-0.87], and 0.78 [95% CI, 0.62-0.99]) for eGFR ≥60, 45-59, 30-44, and <30 mL/min/1.73 m2, respectively). Rivaroxaban was also associated with similar risk of major bleeding across all eGFR categories (pooled HRs of 0.75 [95% CI, 0.56-1.00], 1.01 [95% CI, 0.79-1.30], 0.87 [95% CI, 0.66-1.15], and 0.63 [95% CI, 0.37-1.09], respectively).
Limitations
Unmeasured treatment selection bias and residual confounding.
Conclusions
In adults with AF, rivaroxaban compared with warfarin was associated with lower or similar risk of all-cause death, ischemic stroke and transient ischemic attack and similar risk of bleeding across a broad range of kidney function.
Plain-Language Summary
This real-world study involved a large cohort of 55,568 adults with atrial fibrillation from 5 jurisdictions across Australia and Canada. It showed that the favorable safety (bleeding) and effectiveness (stroke or death) profile of rivaroxaban compared with warfarin was consistent across different levels of kidney function. This study adds important safety data on the use of rivaroxaban in patients with reduced kidney function, including those with estimated glomerular filtration rate <30 mL/min/1.73 m2 in whom the risks and benefits of rivaroxaban use is most uncertain. Overall, the study supports the use of rivaroxaban as a safe and effective alternative to warfarin for atrial fibrillation across differing levels of kidney function.
Index Words: Atrial fibrillation, bleeding, chronic kidney disease, death, direct oral anticoagulant, eGFR, rivaroxaban, stroke, warfarin
Graphical abstract
Editorial, ●●●
Editorial, 100686
Chronic kidney disease (CKD) and atrial fibrillation (AF) are common interrelated major public health concerns. Both conditions are strong risk factors for stroke or systemic embolism, congestive heart failure, myocardial infarction, and all-cause death.1, 2, 3, 4 Direct oral anticoagulants (DOACs) are now a mainstay of anticoagulant therapy for the prevention of cardiovascular thrombotic events. Rivaroxaban is commonly prescribed for people with AF and early-stage CKD.5 This is supported by CKD subgroup data from randomized trials indicating superior benefit–risk profiles compared to vitamin K antagonists in this group.6 Whether similarly favorable benefit–risk profiles exist for rivaroxaban across the spectrum of CKD remains uncertain.
Although rivaroxaban is approved for patients with greatly reduced kidney function (to the lowest limit of CrCl 15 mL/min),7 this is based on limited pharmacokinetic data without comprehensive assessment of cardiovascular thrombotic events and bleeding outcomes, comparing rivaroxaban with warfarin, in high-risk patients with AF and CrCl <30 mL/min.8 It remains uncertain whether the clinical benefit of rivaroxaban seen in patients with AF and mild-moderate CKD9 extends to those with advanced CKD who have been excluded from most randomized trials. Several population-based studies have compared rivaroxaban with warfarin, focused on assessing the risks of stroke and bleeding, but have demonstrated conflicting findings: with both higher5 and lower10,11 risk of bleeding reported along with variability in the risk of ischemic stroke.5,10,12, 13, 14, 15 They have also had a number of limitations including single-center design, lack of organ-specific bleeding data, and limited sample sizes for the high-risk eGFR <30 mL/min/1.73 m2 subgroup where clinical data is most lacking,5,10,12, 13, 14, 15 limiting the generalizability of their findings across the range of kidney function.
We conducted a large, multicenter cohort study of adults with AF to comprehensively assess the effectiveness (risk of all-cause death, ischemic stroke, or transient ischemic attack [TIA]), and safety (risk of hospitalization for major bleeding: either intracranial, upper or lower gastrointestinal, or other) of rivaroxaban compared with warfarin across the range of kidney function.
Methods
Study Design and Source Population
We conducted a retrospective, propensity score-matched parallel cohort study using a common protocol for health care data from 5 jurisdictions across Australia and Canada. In Australia, we used linked data from the EXamining ouTcomEs in chronic Disease in the 45 and Up [EXTEND45] Study,16 a population-based cohort study assembled on the Sax Institute’s 45 and Up Study,17 with participants randomly sampled from the Services Australia Medicare enrollment database, which provides near complete coverage of the population. In Canada, we used administrative and registry health care data from the provinces of Alberta, British Columbia, Manitoba, and Ontario (see Table S1 for details). The study design, source population, and analytical methods have been described previously.18
Cohort Definition
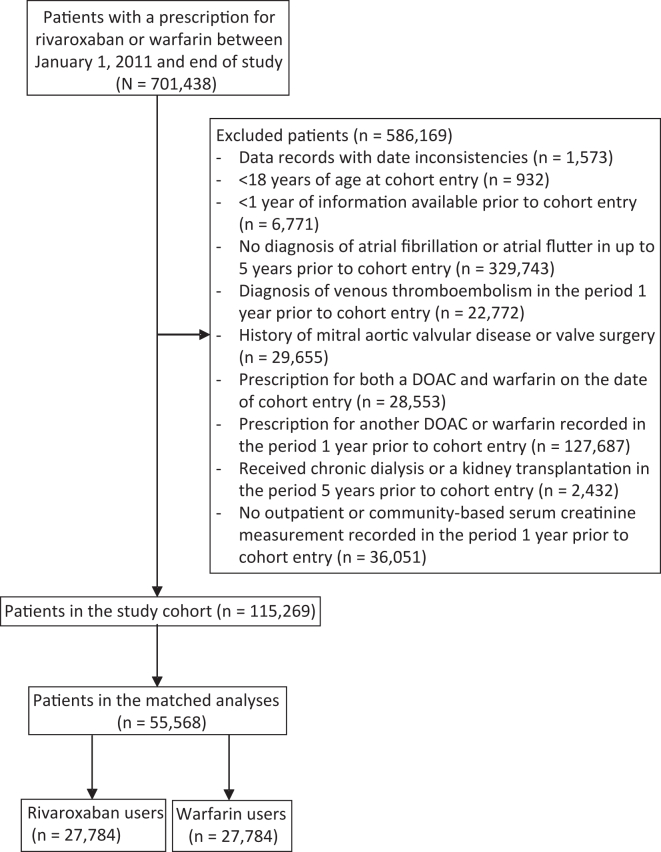
The study cohort included adults with at least one outpatient serum creatinine measurement within 1 year before the cohort entry date who were newly dispensed either rivaroxaban or warfarin between January 1, 2011 and the end date of the available data in each jurisdiction (latest end date: December 31, 2018). Cohort entry (ie, index date) was defined as the date of the first dispensed rivaroxaban or warfarin prescription during the study period. Patients were eligible for inclusion if they had a diagnosis of AF or atrial flutter (International Classification of Diseases [ICD], Ninth Revision code 427.31/2; Tenth Revision code I4819,20) in up to 5 years before and including the date of cohort entry. We excluded patients who had (1) less than 1 year of information in the database before cohort entry; (2) a diagnosis of venous thromboembolism within 1 year before cohort entry; (3) a history of mitral aortic valvular disease or valve surgery in the period 5 years before cohort entry; (4) a prescription for another DOAC (dabigatran or apixaban) or warfarin within 1 year before cohort entry; or (5) both a DOAC and warfarin dispensed on the date of their first prescription (Fig 1). We excluded patients who had received maintenance dialysis or kidney transplantation in the 5-year period before the cohort entry date.
Figure 1.
Identification of study cohort. DOAC, direct oral anticoagulant.
New rivaroxaban and warfarin users were matched at the time of the first dispensed prescription. Matching was based on propensity scores (the propensity to receive rivaroxaban) calculated at the time of cohort entry (detailed below). In the primary intention-to-treat analysis, exposure to either rivaroxaban or warfarin was considered a time-fixed variable throughout the study follow-up.
Assessment of Kidney Function
We estimated baseline glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.21 Participants were grouped into the following eGFR categories: ≥60, 45-59, 30-44, and <30 mL/min/1.73 m2.
Covariates
Demographic characteristics and comorbid conditions were assessed using health care data files of the participating sites. Comorbid conditions were defined by ICD-9 and ICD-10 diagnosis codes based on data available for the 365 days preceding cohort entry (Table S2). Comorbid conditions included diabetes, hypertension, congestive heart failure, cerebrovascular disease (including TIA and stroke [ischemic or hemorrhagic]), and history of bleeding requiring hospitalization. We assessed baseline use of prescription antiplatelet agents, nonsteroidal anti-inflammatory drugs, and proton pump inhibitors, defined as ≥1 dispensed prescription of these agents within 90 days before cohort entry. For descriptive purposes, we calculated the CHA2DS2-VASc22 score, a risk score that estimates the risk of stroke in patients with AF, and a modified HAS-BLED23 score, a risk score that estimates the risk of major bleeding in patients with AF.
Outcomes
We assessed the 1-year composite outcomes of (1) all-cause death, first hospitalization for ischemic stroke, or TIA (hereafter referred to as the effectiveness outcome); and (2) first hospitalization for major bleeding, defined as intracranial, upper or lower gastrointestinal, or other bleeding (identified using validated ICD-9 and ICD-10 codes24,25; Table S2). Individual components of the effectiveness and bleeding outcomes were also assessed separately. Participants were followed from their date of cohort entry until the earliest of the following events: date of the outcome, death, end of the 1-year follow-up period, or study end (December 31, 2018).
Statistical Analyses
A multivariable logistic regression model that included demographic information, year of cohort entry, comorbid conditions, and prescription medication use (listed above) was developed to estimate the conditional probability of being prescribed rivaroxaban within each eGFR category. We used 1-to-1 matching without replacement and a caliper width of 0.2 of the standard deviation of the logit of the propensity score.26 Matching was conducted within each eGFR category to ensure similar distribution of measured baseline covariates between rivaroxaban and warfarin users by eGFR category. Balance in baseline covariates between rivaroxaban and warfarin users before and after matching was determined using the standardized difference. Meaningful imbalance was defined as an absolute standardized difference >10%.27
Poisson regression was used to summarize study outcome event rates (expressed as per 100 person-years) by oral anticoagulant type (rivaroxaban or warfarin use) and eGFR category. Rates of the effectiveness and safety outcomes were used to estimate the absolute risk difference of study outcomes between rivaroxaban users and warfarin users by eGFR category. Cox proportional hazards models (accounting for clustering within matched pairs using robust sandwich estimator of variance and adjusted for baseline eGFR category) were constructed to estimate the association between rivaroxaban/warfarin use and outcomes within 1 year from the date of cohort entry by eGFR category. Within each eGFR category, warfarin users were considered as the reference category in estimating the hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) for each of the study outcomes. Separate models were constructed for the effectiveness and bleeding outcomes (overall and individually). Analyses were performed independently in each jurisdiction according to a common protocol using SAS, version 9.4 (SAS Institute Inc). A two-sided P value <0.05 was considered statistically significant.
Meta-analysis
Summary estimates of HRs and corresponding 95% CIs were obtained within each eGFR category by pooling site-level data using the DerSimonian and Laird random-effects model. The percentage of variability across sites attributable to heterogeneity beyond chance were estimated using the I2 statistic28 where values of ≤25%, >25 to 75%, and >75% corresponded to low, moderate, and high levels of heterogeneity, respectively. Meta-regression assessed for modification of the comparative effectiveness and safety of rivaroxaban versus warfarin by eGFR category. Meta-analysis was performed with Stata software, version 16.1 (Stata Corp).
Sensitivity Analyses
Additional prespecified sensitivity analyses were performed: (1) all analyses were repeated using an as-treated approach (in which patients were censored on oral anticoagulant treatment switches or discontinuation); (2) adjusted Cox models for baseline eGFR as well as variables observed to be meaningfully imbalanced when covariate balance was assessed in each eGFR category (Table S3); (3) tested ascertainment of study outcome events using both hospital and emergency department data (in jurisdictions where emergency department data were available); and (4) we assessed the comparative effectiveness of rivaroxaban and warfarin initiation by eGFR category on the outcome of myocardial infarction (both as part of the composite outcome and individually).
Ethics Approval
In New South Wales, Australia, ethical approval for the EXTEND45 Study was obtained from the New South Wales Population and Health Services Research Ethics Committee (HREC/13/CIPHS/69). The 45 and Up Study received ethics approval from the University of NSW Human Research Ethics Committee. In the Canadian provinces of Alberta, British Columbia, and Manitoba, the study was approved by the institutional review boards of the University of Calgary (REB18-0471_REN2), University of British Columbia Providence Health Care Research Institute (H18-02319), and University of Manitoba (HS22072), respectively. In Ontario, Canada, the use of administrative health data was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board.
Results
Patient Characteristics
We identified 115,269 eligible adults with a diagnosis of AF and a new prescription for rivaroxaban (n=63,108) or warfarin (n=52,161) who had at least 1 outpatient baseline serum creatinine measurement (Fig 1). There were differences in baseline characteristics between the rivaroxaban and warfarin groups of the unmatched cohort. Rivaroxaban users, compared with warfarin users, had higher mean eGFR, and comorbid conditions such as diabetes and cerebrovascular disease were less prevalent (Table 1). Patterns in rivaroxaban and warfarin prescribing during the study period (2011-2019) are shown in Fig S1.
Table 1.
Baseline Characteristics of Patients With Atrial Fibrillation, by Rivaroxaban and Warfarin Use, Before and After Propensity Score Matching
| Unmatched Cohort |
Standardized Differencea | Matched Cohort |
Standardized Differencea | |||
|---|---|---|---|---|---|---|
| Rivaroxaban | Warfarin | Rivaroxaban | Warfarin | |||
| Number of patients | 63,108 | 52,161 | 27,784 | 27,784 | ||
| Age (y); mean (SD)a | 74.1 (9.4) | 75.5 (10.2) | 0.180 | 74.2 (9.8) | 74.5 (10.3) | 0.030 |
| Female | 29,578 (46.9%) | 24,094 (46.2%) | 0.041 | 12,711 (45.7%) | 12,773 (46.0%) | 0.015 |
| eGFR (mL/min/1.73 m2); mean (SD)a | 70.8 (17.5) | 62.7 (22.2) | 0.399 | 68.5 (19.1) | 68.0 (19.5) | 0.031 |
| ≥60 | 45,809 (72.6%) | 29,668 (56.9%) | 18,475 (66.5%) | 18,475 (66.5%) | ||
| 45-59 | 12,060 (19.1%) | 10,111 (19.4%) | 5,498 (19.8%) | 5,498 (19.8%) | ||
| 30-44 | 4,554 (7.2%) | 7,344 (14.1%) | 3,137 (11.3%) | 3,137 (11.3%) | ||
| <30 | 685 (1.1%) | 5,038 (9.7%) | 674 (2.4%) | 674 (2.4%) | ||
| Diabetes | 19,489 (30.9%) | 17,797 (34.1%) | 0.157 | 8,980 (32.3%) | 8,971 (32.3%) | 0.015 |
| Hypertension | 46,673 (74.0%) | 40,029 (76.7%) | 0.135 | 20,513 (73.8%) | 20,751 (74.7%) | 0.020 |
| Myocardial infarction | 4,543 (7.2%) | 7,538 (14.5%) | 0.196 | 2,885 (10.4%) | 3,052 (11.0%) | 0.021 |
| Congestive heart failure | 13,005 (20.6%) | 18,960 (36.3%) | 0.353 | 7,928 (28.5%) | 7,986 (28.7%) | 0.021 |
| Cerebrovascular disease | 8,882 (14.1%) | 11,004 (21.1%) | 0.194 | 5,025 (18.1%) | 5,056 (18.2%) | 0.018 |
| Peripheral vascular disease | 1,433 (2.3%) | 3,114 (6.0%) | 0.130 | 1,069 (3.8%) | 1,178 (4.2%) | 0.021 |
| Chronic obstructive pulmonary disease | 5,774 (9.1%) | 8,759 (16.8%) | 0.110 | 3,683 (13.3%) | 3,816 (13.7%) | 0.010 |
| Liver disease | 2,675 (4.2%) | 2,182 (4.2%) | 0.014 | 1,208 (4.3%) | 1,185 (4.3%) | 0.010 |
| Cancer | 18,848 (30.0%) | 15,077 (28.9%) | 0.018 | 7,881 (28.4%) | 7,899 (28.4%) | 0.005 |
| Prior major bleeding requiring hospital admissionb | 729 (1.2%) | 949 (1.8%) | 0.056 | 378 (1.4%) | 378 (1.4%) | 0.010 |
| Prescription drug use | ||||||
| Antiplatelet agents | 4,079 (6.5%) | 4,866 (9.3%) | 0.108 | 2,116 (7.6%) | 2,119 (7.6%) | 0.018 |
| Nonsteroidal anti-inflammatory drugs | 11,627 (18.4%) | 8,296 (15.9%) | 0.018 | 4,514 (16.2%) | 4,506 (16.2%) | 0.006 |
| Proton pump inhibitors | 15,809 (25.1%) | 15,009 (28.8%) | 0.050 | 7,163 (25.8%) | 7,283 (26.2%) | 0.011 |
| CHA2DS2-VASc score | ||||||
| 0-1 | 5,068 (8.0%) | 2,979 (5.7%) | 0.131 | 2,261 (8.1%) | 1,799 (6.5%) | 0.039 |
| ≥2 | 58,040 (92.0%) | 49,182 (94.3%) | 0.122 | 25,523 (91.9%) | 25,985 (93.5%) | 0.024 |
| Modified HAS-BLED score | ||||||
| 0-2 | 18,778 (29.8%) | 18,993 (36.4%) | 0.112 | 19,094 (68.7%) | 19,106 (68.8%) | 0.023 |
| ≥3 | 44,330 (70.2%) | 33,168 (63.6%) | 0.127 | 8,690 (31.3%) | 8,678 (31.2%) | 0.015 |
Note: Data are presented as the number of patients and the corresponding percentage of the cohort unless otherwise indicated. Cells of tables with patient counts <5 were suppressed by participating centers due to privacy restrictions. The sum of count data may thus differ slightly from the presented total. CHA2DS2-VASc and modified HAS-BLED scores estimate the risk of stroke and bleeding, respectively, in patients with atrial fibrillation.
Abbreviations: eGFR, estimated glomerular filtration rate; SD, standard deviation.
sample size weighted mean or standardized difference.
bleeding defined as first hospitalization for intracranial, gastrointestinal, or other bleeding.
A total of 55,568 patients (27,784 rivaroxaban–warfarin user matched pairs) were included in the propensity score-matched analyses (EXTEND45 = 530; Alberta = 10,774; British Columbia = 110; Manitoba = 3,004; Ontario = 41,150). Overall, balance was achieved across all covariates after matching (Table 1). The mean age of the matched cohort was 74 years, 33.5% (n=18,618) had eGFR <60 mL/min/1.73 m2 (2.4% [n=1,348] had eGFR <30 mL/min/1.73 m2, with the majority 2.2% [n=1,200] having eGFR 15-29 mL/min/1.73 m2). Of the matched cohort, 51,508 (92.7%) had CHA2DS2-VASc scores ≥2, and 17,368 (31.3%) had HAS-BLED scores ≥3. Median number of days (range across the 5 jurisdictions from which data was pooled) from last AF/atrial flutter diagnosis to cohort entry date was 12 (3-274) days (Table S4).
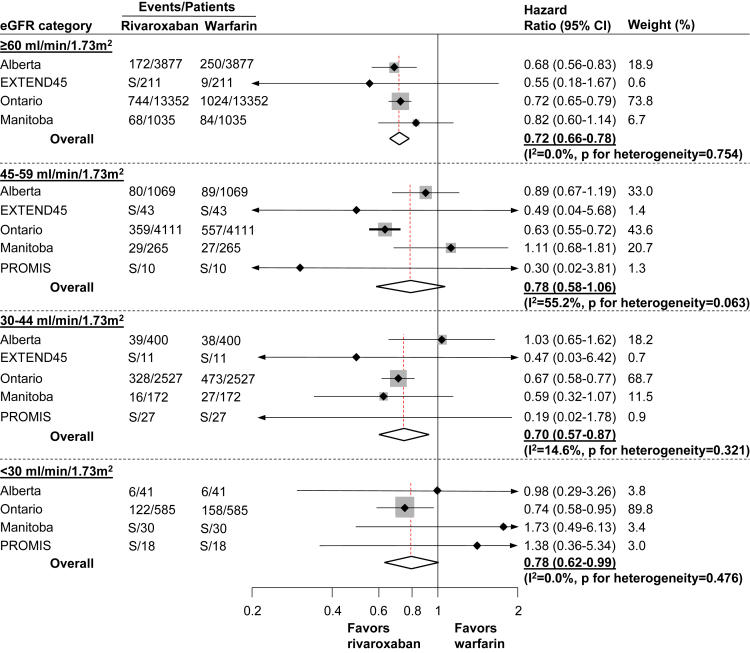
Effectiveness Outcome
A total of 4,733 patients experienced the effectiveness outcome (8.5%) over the 1-year follow-up period. The number of ischemic stroke or TIA events was 577 (1.0%), and there were 4,287 deaths (7.7%). Compared to warfarin, rivaroxaban initiation was associated with similar or greater effectiveness (HRs of 0.72 [95% CI, 0.66-0.78], 0.78 [95% CI, 0.58-1.06], 0.70 [95% CI, 0.57-0.87], and 0.78 [95% CI, 0.62-0.99]), across the strata of kidney function, for eGFR ≥60, 45-59, 30-44, and <30 mL/min/1.73 m2, respectively (Fig 2). There was no evidence of heterogeneity across jurisdictions within eGFR groups, except for eGFR 45-59 mL/min/1.73 m2 (I2 = 55.2%; P for heterogeneity = 0.06). Meta-regression showed that the association was consistent across eGFR categories and across all jurisdictions (P = 0.75). Compared with warfarin, rivaroxaban initiation was associated with similar or lower risk of all-cause death (HRs of 0.70 [95% CI, 0.65-0.77], 0.78 [95% CI, 0.57-1.07], 0.71 [95% CI, 0.63-0.82], and 0.76 [95% CI, 0.59-0.97]), across the strata of kidney function, for eGFR ≥60, 45-59, 30-44, and <30 mL/min/1.73 m2, respectively (Table S5). Further, compared with warfarin, rivaroxaban initiation was associated with similar risk of ischemic stroke or TIA (HRs of 0.80 [95% CI, 0.54-1.19], 0.75 [95% CI, 0.53-1.04], 0.71 [95% CI, 0.23-2.20] and 0.99 [95% CI, 0.45-2.17]), across the strata of kidney function, for eGFR ≥60, 45-59, 30-44, and <30 mL/min/1.73 m2, respectively (Table S5).
Figure 2.
Hazard ratios (95% CIs) for the effectiveness outcomea by eGFR category; within each eGFR category, warfarin initiation was considered as the reference category in estimating the hazard ratios and their 95% CI.
Meta-regression showed that the association between rivaroxaban initiation (compared with warfarin initiation) and the effectiveness outcome was not modified by eGFR category (P = 0.75).
aDefined as the composite of all-cause death, ischemic stroke, or transient ischemic attack.
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; EXTEND45, Examining Outcomes in Chronic Disease in the 45 and Up Study (the data source used in the state of New South Wales, Australia. In EXTEND45, there were no rivaroxaban–warfarin matched pairs included for eGFR <30 mL/min/1.73 m2); PROMIS, Patient Records and Outcome Management Information System (data source used in the province of British Columbia, Canada; PROMIS is an integrated registry and clinical information system for chronic kidney disease [eGFR <60 mL/min/1.73 m2] and therefore was excluded from eGFR category ≥60 mL/min/1.73m2); S, number of outcome events were <5 and cells were suppressed to meet privacy restrictions.
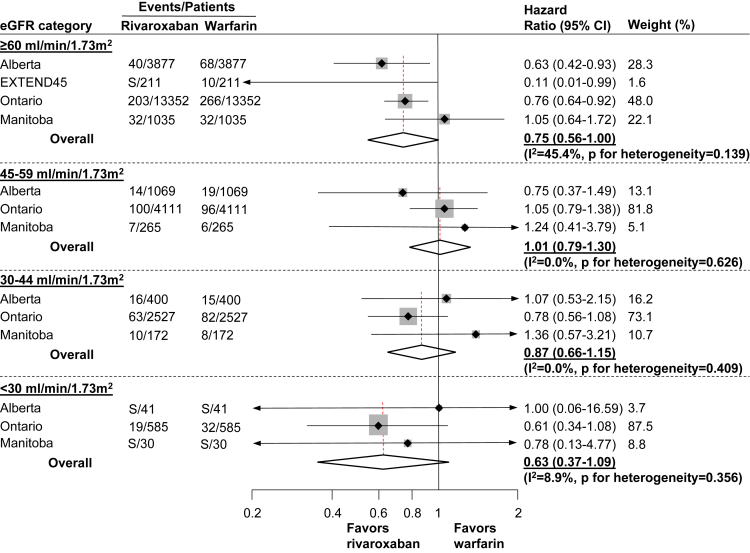
Major Bleeding Outcomes
A total of 1,144 (2.0%) experienced a major bleeding event. The numbers of intracranial, upper or lower gastrointestinal, and other bleeding events were 138 (0.2%), 731 (1.3%), and 267 (0.5%), respectively. Overall, incidence rates of the major bleeding outcomes increased with progressively lower eGFR in both rivaroxaban and warfarin users (Table S6).
Rivaroxaban was associated with similar risk of major bleeding across all categories of eGFR (HRs for eGFR categories ≥60, 45-59, 30-44, and <30 mL/min/1.73 m2: 0.75 [95% CI, 0.56-1.00], 1.01 [95% CI, 0.79-1.30], 0.87 [95% CI, 0.66-1.15], and 0.63 [95% CI, 0.37-1.09], respectively; Fig 3). There was no evidence of heterogeneity across jurisdictions within eGFR groups, except for eGFR ≥60 mL/min/1.73 m2 for major bleeding (I2 = 45.4%). Meta-regression showed that the comparative safety of rivaroxaban versus warfarin was consistent across eGFR categories and the 5 jurisdictions (P = 0.76). Similar results were observed when components of the major bleeding outcome were assessed individually (Table S5).
Figure 3.
Hazard ratios (95% CIs) for the major bleeding outcomea by eGFR category; within each eGFR category, warfarin initiation was considered as the reference category in estimating the hazard ratios and their 95% CIs.
Meta-regression showed that the association between rivaroxaban initiation (compared with warfarin initiation) and the safety outcome was not modified by eGFR category (P = 0.76).
aDefined as the first hospitalization for major bleeding (intracranial, upper or lower gastrointestinal, or other bleeding).
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; EXTEND45, Examining Outcomes in Chronic Disease in the 45 and Up Study (data source used in the state of New South Wales, Australia; due to 0 cells [ie, no outcome events] recorded for eGFR categories 45-59 mL/min/1.73 m2 and eGFR 30-44 mL/min/1.73 m2, data from EXTEND45 were excluded from these eGFR categories; there were no rivaroxaban–warfarin matched pairs included for eGFR <30 mL/min/1.73 m2); PROMIS, Patient Records and Outcome Management Information System (the data source used in the province of British Columbia, Canada; PROMIS is an integrated registry and clinical information system for chronic kidney disease [eGFR <60 mL/min/1.73 m2] and therefore was excluded from eGFR category ≥60 mL/min/1.73 m2 group); S, number of outcome events were <5 and cells were suppressed to meet privacy restrictions.
Patterns of Reduced Rivaroxaban Dosing According to eGFR Category
Forty-six percent of rivaroxaban users were prescribed a reduced dose. Among rivaroxaban users with eGFR ≥60, 45-59, 30-44, and <30 mL/min/1.73 m2, the proportion of patients prescribed reduced doses were 36%, 53%, 77%, and 86%, respectively (Fig S2).
Sensitivity Analysis
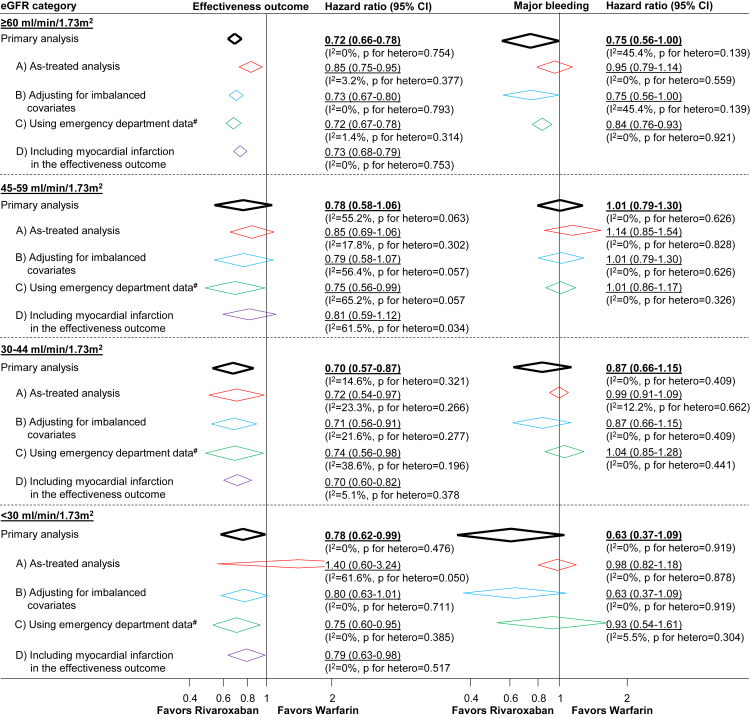
Results generally remained unchanged (Fig 4) in all sensitivity analyses including (1) as-treated analysis; (2) models adjusted for covariates identified as imbalanced when covariate balance was evaluated according to eGFR category; (3) analyses using both hospitalization and emergency department data to assess the occurrence of study outcome events in jurisdictions where data were available; and (4) the outcome of nonfatal myocardial infarction (individually and as part of the effectiveness outcome) by eGFR category.
Figure 4.
Sensitivity analyses assessing the comparative effectiveness and safety of rivaroxaban and warfarin according to eGFR category.a (A) As-treated analysis in which patients were censored on oral anticoagulant treatment switches or discontinuation; (B) Analysis adjusted for baseline eGFR category and variables observed to be meaningfully imbalanced when covariate balance was assessed in each eGFR category; (C) Analysis in which the occurrence of study outcome events were identified using both hospital and emergency department data (in participating sites where emergency department data were available) and (D) Analysis in which the comparative effectiveness outcome additionally included myocardial infarction.
aThe percentage of variability across sites attributable to heterogeneity beyond chance were estimated using the I2 statistic where values of ≤25%, >25% to 75%, and >75% correspond to low, moderate, and high levels of heterogeneity, respectively.
Discussion
In this large multicenter study of adults with AF, rivaroxaban versus warfarin was associated with similar or better effectiveness and similar safety across a broad range of kidney function. Findings were consistent across the jurisdictions and a series of sensitivity analyses. Despite its limitations as an observational study, it has important clinical implications given the lack of trials including patients with AF and CKD, especially advanced CKD.
DOAC therapy is a key component of the prevention of thromboembolic events in people with AF and is widely used in this patient population. However, these agents are prescribed less frequently in patients with reduced kidney function than those with normal kidney function,29 attributable to uncertainty regarding the risk of bleeding in individuals with reduced kidney function. Our results have important implications in this regard. First, our findings on the favorable benefit–risk profile of rivaroxaban in patients with AF and moderately reduced kidney function support its ongoing use in this patient group. This was initially demonstrated in the ROCKET-AF and J-ROCKET-AF trials, which included patients with CrCl 30 to 50 mL/min.9,30,31 Our study substantially adds to this studied population, including over 8,600 rivaroxaban users with eGFR 30-59 mL/min/1.73 m2. Second, our study adds important safety data. It raises no obvious concerns regarding the comparative safety of rivaroxaban versus warfarin across a broad range of kidney function, showing a similar risk of organ-specific major bleeding, including gastrointestinal bleeding.
There remains insufficient evidence to establish benefits or harms of DOACs or warfarin in patients with advanced CKD, who have been largely excluded from randomized trials.6 These patients may be preferentially prescribed warfarin over DOACs due to reduced drug elimination of DOACs at lower creatinine clearances, lack of widely accessible reversal agents, and lack of an assay to reliably measure the anticoagulant effect of any DOAC in CKD.9 This study contributes important data suggesting no safety signal or lack of effectiveness for rivaroxaban compared to warfarin in over 1,300 participants with eGFR <30 mL/min/1.73 m2, providing some reassurance for its use as an alternative to warfarin.
Our findings are also largely consistent with previous cohort studies examining the influence of kidney function on ischemic stroke and major bleeding in patients with AF treated with rivaroxaban or warfarin, with a few exceptions.12, 13, 14 A major study by Shin et al5 demonstrated that patients with AF and eGFR <60 mL/min/1.73 m2 derived similar benefits from DOACs for prevention of ischemic stroke compared with warfarin but surprisingly had a higher risk of bleeding. In comparison, our study suggests that rivaroxaban is at least a safe alternative to warfarin in patients with moderately reduced kidney function.
The strengths of our study include assessment of the effectiveness and safety of rivaroxaban in patients with AF across a broad range of kidney function in a large binational cohort. In addition, our study involved a large and diverse patient population, including individuals typically excluded from randomized trials, with around a third of the matched cohort at high cardiovascular risk, with diabetes, congestive heart failure, and/or eGFR <60 mL/min/1.73 m2. Previous cohort studies have focused on stroke and bleeding outcomes without attention to other cardiovascular thrombotic events including nonfatal myocardial infarction in a high-risk population with reduced kidney function. When comparing rivaroxaban with warfarin, we have demonstrated no clear difference in the risk of myocardial infarction in patients with AF across all categories of eGFR. We acknowledge the sensitivity analysis including myocardial infarction could have been strengthened by more comprehensive data on antiplatelet use. However, our linked data was not able to capture concurrent use of aspirin, an over-the-counter medication in the included jurisdictions.
Our study also has limitations. Data on appropriate dosing of rivaroxaban in patients with reduced kidney function and time in the therapeutic range of warfarinized patients were not available. We assessed bleeding events requiring hospitalization (due to differences in data availability across jurisdictions), which may have underestimated the full extent of bleeding rates in the study, although our findings were consistent when including emergency department data where available. Considered together, these may reflect the lower major bleeding rate (2.0%) in the matched cohorts compared to previous cohorts and trials. However, our study provides real-world evidence for the prescription of oral anticoagulants in patients with moderately reduced kidney function where international normalized ratios can be labile.12,30 Although patients with eGFR <30 mL/min/1.73 m2 were included, patients with poor kidney function (eGFR <15 mL/min/1.73 m2) were still underrepresented in our cohort, which limited our ability to further assess the safety and effectiveness of rivaroxaban compared with warfarin in this patient group. Hence, the possibility of excess bleeding risk with rivaroxaban in this high-risk population cannot be excluded. We assessed study outcomes within 1 year from rivaroxaban or warfarin initiation, and therefore, the risk–benefit ratio of rivaroxaban in patients with AF and CKD over longer periods was not studied. Finally, as an observational study, the potential for unmeasured residual confounding remains.
In this large cohort of adults with AF, rivaroxaban compared with warfarin was associated with greater or similar effectiveness and similar safety across a broad range of kidney function. Our results suggest rivaroxaban may have a favorable benefit–risk ratio in patients with AF independent of kidney function, including those with advanced nondialysis CKD.
Article Information
Authors’ Full Names and Academic Degrees
Jeffrey T. Ha, MBBS, Anish Scaria, BSc(Hons), MSc, Jason Andrade, MD, Sunil V. Badve, MBBS, PhD, Peter Birks, MD, Sarah E. Bota, MSc, Anna Campaign, PhD, Ognjenka Djurdjev, MSc, Amit X. Garg, MD, PhD, Ziv Harel, MD, MSc, Brenda Hemmelgarn, MD, PhD, Carinna Hockham, MSc, DPhil, Matthew T. James, MD, PhD, Meg J. Jardine, MBBS, PhD, Dickson Lam, MBBS, Adeera Levin, MD, Eric McArthur, MSc, Pietro Ravani, MD, PhD, Selena Shao, MSc, Manish M. Sood, MD, MSc, Zhi Tan, MSc, Navdeep Tangri, MD, PhD, Reid Whitlock, MSc, Martin Gallagher, MBBS, PhD, and Min Jun, MScMed(ClinEpi), PhD
Authors’ Contributions
Study conception: MJun, AS, AG, BRH, MTJ, MJJ, AL, MS, NT, and MG; study design and conduct: all authors; study protocol and design of study analytical plan: MJun and AS; study data acquisition: MJun, PB, SEB, OD, AG, BH, CH, MTJ, MJJ, AL, MS, NT, and RW; statistical analysis: AS, AC, EM, SS, ZT, and RW; supervision: MJun. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This study was funded by a Project Grant (1148060) from the National Health and Medical Research Council of Australia (NHMRC). The funding organizations had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. JTH is supported by an Australian Government Research Training Program Scholarship and a University Postgraduate Award from the University of New South Wales, Sydney, Australia. AXG was supported by the Dr. Adam Linton Chair in Kidney Health Analytics and a Clinician Investigator Award from the Canadian Institutes of Health Research (CIHR). MTJ was supported by a CIHR New Investigator Award. MMS is supported by the Jindal Research Chair in the Prevention of Kidney Disease, Alberta, Canada. MJun is supported by a Scientia Fellowship from the University of New South Wales, Sydney, Australia.
Financial Disclosure
MJun reports receiving unrestricted grant support from VentureWise (a wholly owned commercial subsidiary of NPS MedicineWise) to conduct a commissioned project funded by AstraZeneca, outside the submitted work. MTJ was the principal investigator of an investigator-initiated research grant from Amgen Canada, outside the submitted work. MMS received speakers’ fees from AstraZeneca. The remaining authors declare that they have no relevant financial interests.
Acknowledgments
This study was made possible through data sharing agreements (de-identified, aggregated data) between the George Institute for Global Health, Australia and participating Canadian research centers in the provinces of Alberta, British Columbia, Manitoba, and Ontario. The study was completed using administrative and health care data from 5 jurisdictions across Australia (in the state of New South Wales) and Canada (Alberta, British Columbia, Manitoba, and Ontario).
New South Wales (NSW), Australia: Data from the EXTEND45 study (a population-based cohort study assembled on the Sax Institute’s 45 and Up Study,17 a large prospective study of a cohort of NSW residents aged ≥45 years originally recruited between 2005 and 2009) were used. Of the 267,357 participants originally recruited to the 45 and Up Study, patient data on 530 participants were included in the current study. This research was completed using data collected through the 45 and Up Study (www.saxinstitute.org.au). The 45 and Up Study is managed by the Sax Institute in collaboration with major partner Cancer Council NSW; and partners: the Heart Foundation; NSW Ministry of Health; NSW Department of Communities and Justice; and Australian Red Cross Lifeblood. We thank the many thousands of people participating in the 45 and Up Study. Record linkage of the 45 and Up Study baseline questionnaire responses with corresponding information from the NSW Admitted Patient Data Collection, the Registry of Births, Deaths and Marriages, and community laboratory testing services was performed by the NSW Centre for Health Record Linkage (CHeReL) covering the period 2005-2014. CHeReL uses a probabilistic procedure to link records, in which records with an uncertain probability of being true matches are checked by hand. Its current estimated false positive rate is 0.5% (CHeReL; https://www.cherel.org.au). The Medicare Benefits Schedule (MBS; recorded claims for subsidized medical and diagnostic services in Australia) and Pharmaceutical Benefits Scheme (PBS; recorded claims for subsidized pharmaceutical products in Australia) data were provided to the Sax Institute by Services Australia. Record linkage of the 45 and Up Study data to the MBS and PBS data was facilitated by the Sax Institute using a unique identifier provided by Services Australia and based on deterministic matching. All linked data available in the EXTEND45 study were accessed through the Secure Unified Research Environment (SURE).
Alberta, Canada: This study is based in part by data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions contained herein are those of the researchers and do not represent the views of the Government of Alberta or Alberta Health Services.
British Columbia, Canada: This study is based on data obtained from BC Renal and PopData BC, and the analysis supported by BC Kidney Research Unit, which is supported by both BC Renal and University of British Columbia.
Manitoba, Canada: The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Manitoba Population Research Data Repository under project #2019-009_(HIPC# 2018/2019-41). The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, or other data providers is intended or should be inferred. Data used in this study are from the Manitoba Population Research Data Repository housed at the Manitoba Centre for Health Policy, University of Manitoba and were derived from data provided by Manitoba Health, Winnipeg Regional Health Authority, Vital Statistics, and Shared Health Diagnostic Services.
Ontario, Canada: The analysis performed in Ontario using datasets linked by unique encoded identifiers and was supported by ICES Kidney, Dialysis and Transplantation Program at the ICES Western site. ICES is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). Core funding for ICES is provided by the Academic Medical Organization of Southwestern Ontario (AMOSO), the Schulich School of Medicine and Dentistry (SSMD), Western University, and the Lawson Health Research Institute (LHRI). This study also received funding from the National Health and Medical Research Council (NHMRC). Parts of this material are based on data and/or information compiled and provided by CIHI and the Ontario Ministry of Health. However, the analyses, conclusions, opinions, and statements expressed in the material are those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. We thank IQVIA Solutions Canada Inc. for use of their Drug Information Database.
Disclaimer
The results, opinions, conclusions, and statements expressed herein are those of the authors and are independent from the funding sources. No endorsement by any of the funding sources or governments of the participating research centers’ jurisdictions is intended or should be inferred.
Peer Review
Received October 9, 2022. Evaluated by 3 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form April 4, 2023.
Footnotes
Complete author and article information provided before references.
Figure S1: Patterns in rivaroxaban and warfarin prescribing during the study period (2011-2019).
Figure S2: Rivaroxaban dosing patterns in the study cohort according to eGFR category.
Table S1: Data Sources Used in the Study Across the Participating Centers
Table S2: ICD-9-CM and ICD-10-CA Diagnosis Codes for the Identification of Atrial Fibrillation, Covariates, and Study Outcomes
Table S3: Baseline Characteristics of Patients With Atrial Fibrillation by eGFR Category, Before and After Propensity Score Matching
Table S4: Distribution of Time Between AF/Atrial Flutter Diagnosis and First Prescription for Rivaroxaban or Warfarin (Cohort Entry Date)
Table S5: Pooled Hazard Ratios (95% Confidence Intervals) for the Individual Components of the Effectiveness and Major Bleeding Outcomes by eGFR Category
Table S6: Incidence Rates (95% CIs) of the Effectiveness and Major Bleeding Outcomes by Rivaroxaban and Warfarin Use and eGFR Category
Supplementary Material
Fig S1-S2, Table S1-S6.
References
- 1.Go A.S., Fang M.C., Udaltsova N., et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2009;119(10):1363–1369. doi: 10.1161/CIRCULATIONAHA.108.816082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massicotte-Azarniouch D., Kuwornu J.P., Carrero J.J., et al. Incident atrial fibrillation and the risk of congestive heart failure, myocardial infarction, end-stage kidney disease, and mortality among patients with a decreased estimated GFR. Am J Kidney Dis. 2018;71(2):191–199. doi: 10.1053/j.ajkd.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Masson P., Webster A.C., Hong M., Turner R., Lindley R.I., Craig J.C. Chronic kidney disease and the risk of stroke: a systematic review and meta-analysis. Nephrol Dial Transplant. 2015;30(7):1162–1169. doi: 10.1093/ndt/gfv009. [DOI] [PubMed] [Google Scholar]
- 4.Bansal N., Fan D., Hsu C.Y., Ordonez J.D., Go A.S. Incident atrial fibrillation and risk of death in adults with chronic kidney disease. J Am Heart Assoc. 2014;3(5) doi: 10.1161/JAHA.114.001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin J.I., Secora A., Alexander G.C., et al. Risks and benefits of direct oral anticoagulants across the spectrum of GFR among incident and prevalent patients with atrial fibrillation. Clin J Am Soc Nephrol. 2018;13(8):1144–1152. doi: 10.2215/cjn.13811217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ha J.T., Neuen B.L., Cheng L.P., et al. Benefits and harms of oral anticoagulant therapy in chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2019;171(3):181–189. doi: 10.7326/m19-0087. [DOI] [PubMed] [Google Scholar]
- 7.Bayer Australia Ltd. Rivaroxaban (Xarelto) product information. Accessed February 27, 2022.
- 8.Kubitza D., Becka M., Mueck W., et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct Factor Xa inhibitor. Br J Clin Pharmacol. 2010;70(5):703–712. doi: 10.1111/j.1365-2125.2010.03753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox K.A., Piccini J.P., Wojdyla D., et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011;32(19):2387–2894. doi: 10.1093/eurheartj/ehr342. [DOI] [PubMed] [Google Scholar]
- 10.Coleman C.I., Kreutz R., Sood N.A., et al. Rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and severe kidney disease or undergoing hemodialysis. Am J Med. 2019;132(9):1078–1083. doi: 10.1016/j.amjmed.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Yao X., Tangri N., Gersh B.J., et al. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2017;70(21):2621–2632. doi: 10.1016/j.jacc.2017.09.1087. [DOI] [PubMed] [Google Scholar]
- 12.Yao X., Inselman J.W., Ross J.S., et al. Comparative effectiveness and safety of oral anticoagulants across kidney function in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2020;13(10) doi: 10.1161/CIRCOUTCOMES.120.006515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weir M.R., Berger J.S., Ashton V., et al. Impact of renal function on ischemic stroke and major bleeding rates in nonvalvular atrial fibrillation patients treated with warfarin or rivaroxaban: a retrospective cohort study using real-world evidence. Curr Med Res Opin. 2017;33(10):1891–1900. doi: 10.1080/03007995.2017.1339674. [DOI] [PubMed] [Google Scholar]
- 14.Wetmore J.B., Roetker N.S., Yan H., Reyes J.L., Herzog C.A. Direct-acting oral anticoagulants versus warfarin in medicare patients with chronic kidney disease and atrial fibrillation. Stroke. 2020;51(8):2364–2373. doi: 10.1161/strokeaha.120.028934. [DOI] [PubMed] [Google Scholar]
- 15.Loo S.Y., Coulombe J., Dell’Aniello S., Brophy J.M., Suissa S., Renoux C. Comparative effectiveness of novel oral anticoagulants in UK patients with non-valvular atrial fibrillation and chronic kidney disease: a matched cohort study. BMJ Open. 2018;8(1) doi: 10.1136/bmjopen-2017-019638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foote C., Hockham C., Sukkar L., et al. EXamining ouTcomEs in chroNic Disease in the 45 and Up Study (the EXTEND45 Study): protocol for an Australian linked cohort study. JMIR Res Protoc. 2020;9(4) doi: 10.2196/15646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bleicher K., Summerhayes R., Baynes S., et al. Cohort profile update: the 45 and Up study. Int J Epidemiol. 2023;52(1):e92–e101. doi: 10.1093/ije/dyac104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jun M, Scaria A, Andrade J, et al. Kidney function and the comparative effectiveness and safety of direct oral anticoagulants versus warfarin in adults with atrial fibrillation: a multicenter observational study. Eur Heart J Qual Care Clin Outcomes. Published online October 27, 2022. https://doi.org/10.1093/ehjqcco/qcac069 [DOI] [PubMed]
- 19.Jensen P.N., Johnson K., Floyd J., Heckbert S.R., Carnahan R., Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(0 1 suppl 1):141–147. doi: 10.1002/pds.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao R.J.R., Andrade J.G., Deyell M.W., Jackson H., McAlister F.A., Hawkins N.M. Sensitivity, specificity, positive and negative predictive values of identifying atrial fibrillation using administrative data: a systematic review and meta-analysis. Clin Epidemiol. 2019;11:753–767. doi: 10.2147/CLEP.S206267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lip G.Y.H., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J.G.M. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 23.Pisters R., Lane D.A., Nieuwlaat R., de Vos C.B., Crijns H.J.G.M., Lip G.Y.H. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 24.Kokotailo R.A., Hill M.D. Coding of stroke and stroke risk factors using International Classification of Diseases, revisions 9 and 10. Stroke. 2005;36(8):1776–1781. doi: 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]
- 25.Arnason T., Wells P.S., van Walraven C., Forster A.J. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118(2):253–262. doi: 10.1016/j.thromres.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Austin P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin P.C., Mamdani M.M. A comparison of propensity score methods: a case-study estimating the effectiveness of post-AMI statin use. Stat Med. 2006;25(12):2084–2106. doi: 10.1002/sim.2328. [DOI] [PubMed] [Google Scholar]
- 28.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinecke H., Nabauer M., Gerth A., et al. Morbidity and treatment in patients with atrial fibrillation and chronic kidney disease. Kidney Int. 2015;87(1):200–209. doi: 10.1038/ki.2014.195. [DOI] [PubMed] [Google Scholar]
- 30.Patel M.R., Mahaffey K.W., Garg J., et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 31.Hori M., Matsumoto M., Tanahashi N., et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation – the J-ROCKET AF study. Circ J. 2012;76(9):2104–2111. doi: 10.1253/circj.cj-12-0454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1-S2, Table S1-S6.