Abstract
Severe insulin resistance has been linked to some of the most globally prevalent disorders, such as diabetes mellitus, nonalcoholic fatty liver disease, polycystic ovarian syndrome, and hypertension. Hereditary severe insulin resistance syndrome (H-SIRS) is a rare disorder classified into four principal categories: primary insulin receptor defects, lipodystrophies, complex syndromes, and obesity-related H-SIRS. Genes such as INSR, AKT2, TBC1D4, AGPAT2, BSCL2, CAV1, PTRF, LMNA, PPARG, PLIN1, CIDEC, LIPE, PCYT1A, MC4R, LEP, POMC, SH2B1, RECQL2, RECQL3, ALMS1, PCNT, ZMPSTE24, PIK3R1, and POLD1 have been linked to H-SIRS. Its clinical features include insulin resistance, hyperglycemia, hyperandrogenism, severe dyslipidemia, fatty liver, abnormal topography of adipose tissue, and low serum leptin and adiponectin levels. Diagnosis of H-SIRS is based on the presence of typical clinical features associated with the various H-SIRS forms and the identification of mutations in H-SIRS-linked genes by genetic testing. Diet therapy, insulin sensitization, exogenous insulin therapy, and leptin replacement therapy have widely been adopted to manage H-SIRS. The rarity of H-SIRS, its highly variable clinical presentation, refusal to be tested for genetic mutations by patients’ family members who are not severely sick, unavailability of genetic testing, and testing expenses contribute to the delayed or underdiagnoses of H-SIRS. Early diagnosis facilitates early management of the condition, which results in improved glycemic control and delayed onset of diabetes and other complications related to severe insulin resistance. The use of updated genetic sequencing technologies is recommended, and long-term studies are required for genotype–phenotype differentiation and formulation of diagnostic and treatment protocols.
Keywords: Diabetes, Genetics, Insulin resistance, Lipodystrophy, Pathophysiology, Therapy
General overview of severe insulin resistance syndrome
Insulin resistance is a physiological abnormality that increases the likelihood of developing impaired fasting glucose and glucose tolerance, dyslipidemia, and endothelial dysfunction. Insulin resistance is one of the most common endocrine dysfunctions associated with type 2 diabetes, cardiovascular disease, essential hypertension, polycystic ovarian syndrome, nonalcoholic fatty liver disease, certain forms of cancers, and sleep apnea.1 Severe insulin resistance, the extreme form of insulin resistance, has been linked to genetic defects. With the development of gene sequencing technologies, more and more mutations related to insulin resistance have been identified.2
According to the pathogenesis, genetic variation, and clinical presentation, hereditary severe insulin resistance syndrome (H-SIRS) can be classified into (1) primary insulin signaling defects, (2) lipodystrophies, including congenital generalized lipodystrophy (CGL) and familial partial lipodystrophy (FPLD), (3) severe obesity, and (4) complex syndromes.3, 4, 5 The prevalence of H-SIRS varies among the subtypes. A clinical study suggested that patients with H-SIRS account for approximately 0.1%–0.5% of in-hospital patients with diabetes.3 Donohue's syndrome (DS) is a rare (1 in 4 million) but severe H-SIRS of primary insulin receptor defect, and children with this syndrome die in the first year of their life.6 Rabson–Mendenhall syndrome (RMS) is not as severe as DS and the patients easily survive childhood, but their life expectancy is significantly reduced after adolescence.7 The prevalence of CGL is approximately one in 10 million worldwide.8
This review explores the pathogenesis of H-SIRS at the gene level, especially the network of glycometabolism, lipid metabolism, and other signaling pathway disruptions, summarizes the current diagnostic and treatment practices, and highlights the benefits of early optimal management.
Pathophysiology of H-SIRS
Insulin signaling defects
H-SIRS caused by INSR mutations
In 1985, scientists deciphered the gene sequences of insulin receptors from DNA cloning, which proved that they belong to the tyrosine kinase family.9 After insulin binds to the receptor, the tyrosine kinase is activated and subsequently initiates a signaling cascade.
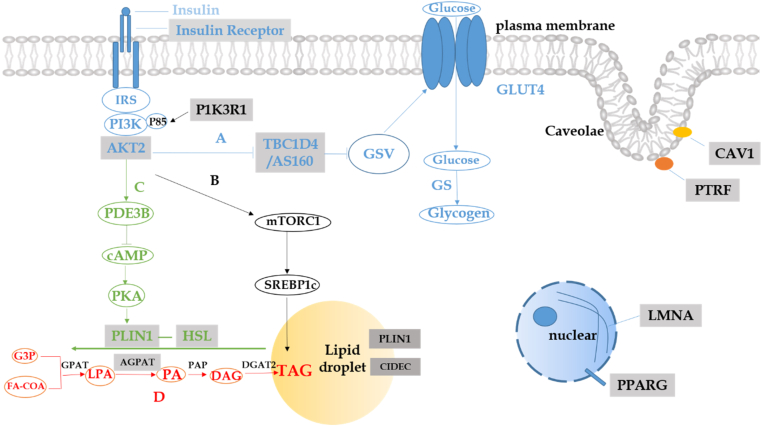
DS, or leprechaunism, and RMS are caused by heterogeneous, homogenous, or compound heterogeneous mutations in the INSR. The severity of the conditions is related to the location of the variants and the number of alleles. In most cases, the patients with homogenous or compound heterogeneous mutations in the α subunit have more severe diseases than the ones with heterogeneous mutations in the β subunit.10,11 However, a patient with the variant leprechaun/Minn-1 and carrying a heterogeneous nonsense mutation and a synonymous mutation has been shown to have severe symptoms and decreased insulin receptor mRNA and protein levels on the cell surface. A synonymous mutation in leprechaun/Minn-1 played a cis-acting role in influencing the transcription rate and the stability of the mRNA.12 The insulin receptor is the first site of action for insulin so that the destruction can disrupt subsequent pathways (Fig. 1).
Figure 1.
H-SIRS related genes and pathogenesis. (A) Insulin binds to insulin receptor and activates receptor tyrosine kinases, and then followed by IRS/PI3K/AKT act as a complex. GLUT4 moving from the inside to the surface of the cell by GSV, due to the inhabitation of TBCID4 phosphorylation, this is the process of insulin-mediated glucose uptake. (B) The expression of SREBP1c is activated by insulin, induced by AKT and mTORC1, mediating the de novo lipogenesis. (C) Activation of PI3K and PDE3B decreased the concentration of cAMP, and decreased phosphorylation of perilipin (encoded by PLIN1) and recruitment of HSL (encoded by LIPE) to inhibit lipolysis. (D) The process of de novo lipogenesis and the enzymes involved. CIDEC is co-localized with the lipid droplet protein perilipin. Caveolae is the flask-like invaginations of the plasma membrane, Caveolin (encoded by CAV1) is the major protein of Caveolae, Cavin (encoded by PTRF) is peripheral membrane protein of Caveolae. Lamins (Lamin A and Lamin C are encoded by LMNA) are intermediate filaments structural protein underlying the inner nuclear membrane. H-SIRS related genes are embedded with gray boxes. AGPAT: 1-acyl-glycerol-3-phosphate acyltransferase; AKT2: AKT serine/threonine kinase 2; cAMP: cyclic adenosine monophosphate; CAV1: Caveolin 1; CIDEC: cell death-inducing DFFA like effector c; DAG: diacylglycerol; DGAT2: DAG-O-acyltransferase; FA-COA: fat acyl coenzyme A; GLUT4: glucose transporter 4; GPAT: glycerol-3-phosphate acyltransferase; GSV: GLUT4-containing storage vesicles; G3P: glycerol-3-phosphate; HSL: hormone-sensitive lipase; INSR: insulin receptor; IRS: insulin receptor substrate; LIPE: lipase E, hormone-sensitive type; LMNA: lamin A/C; LPA: lysophosphatidic acid; mTORC1: mammalian target of rapamycin complex 1; PA: phosphatidic acid; PAP: phosphatidate phosphatase; PDE3B: phospho-diesterase 3B; PI3K: phosphatidylinositol 3-kinase; PLIN1: Perilipin 1; PKA: protein kinase; TAG: triglyceride; PPARG: peroxisome proliferator-activated receptor Gamma; PTRF: polymerase I and transcript release factor; SREBP1c: sterol regulatory element binding transcription factor 1; TBC1D4: TBC1 domain family member 4.
H-SIRS caused by gene mutations related to the post-receptor insulin signaling pathway
Gene mutations other than those related to the insulin receptor can also disrupt the insulin signaling pathway, causing H-SIRS. AKT serine/threonine kinase 2 (AKT2), also called protein kinase B (PKB)-β, is an important intermediate regulator of insulin signaling, which is involved in the metabolic functions of the target tissues of insulin action.13 AKT2 encodes the protein serine/threonine kinase.14 In response to insulin, AKT2 phosphorylates the transcriptional coactivator, peroxisome proliferator-activated receptor-coactivator 1 α (PGC-1 α), and reduces the recruitment of homologous promoters, thereby lowering liver gluconeogenesis and lipid oxidation.15 AKT2 deficiency in mice causes severe diabetes and lipodystrophy,16 which proves that it plays a pertinent role in glucose and lipid metabolism and is involved in both pathways (Fig. 1).
The phosphorylation of TBC1 domain family member 4 (TBC1D4), also called AS160, could inhibit glucose uptake by glucose transporter 4 (GLUT4). GLUT4 is the specific glucose transporter protein present in fat and muscle tissues, moving from the inside to the cell surface, and is inhibited by the phosphorylation of TBC1D4. The activation of IRS/PI3K/AKT inhibits phosphorylation of TBC1D417, 18, 19 (Fig. 1). For example, in Greenlandic patients with homozygous mutations of TBC1D4p.Arg684Ter, GLUT4 protein was decreased in the skeletal muscle and led to diabetes.20
Lipodystrophies
Congenital generalized lipodystrophy
Hereditary lipodystrophy syndromes are classified into CGL and FPLD. CGL is an autosomal recessive disorder. To date, mutations in four genes are associated with congenital lipodystrophy syndrome, known as congenital generalized lipodystrophy type 1 (CGL1), type 2 (CGL2), type 3 (CGL3), and type 4 (CGL4).21,22 1-Acylglycerol-3-phosphate O-acyltransferase 2 (APGAT2) catalyzes the acylation of lysophosphatidic acid to form phosphatidic acid (Fig. 1). Loss-of-function mutations in APGAT2 result in decreased synthesis of triacylglycerol in adipose tissues23 and abnormal differentiation and proliferation of adipocytes, which causes CGL1.24 BSCL2 lipid droplet biogenesis associated, seipin (BSCL2) gene encodes seipin, an endoplasmic reticulum protein,25 interacts with the scaffold protein 14-3-3-β and then binds to the actin-severing protein Cofilin-1, which remodels the actin cytoskeleton to promote adipocyte differentiation and makes the preadipocytes become mature adipocytes. BSCL2 mutation results in the most severe type of lipodystrophy, CGL2.26 Caveolin 1 (CAV1), which is the candidate gene of CGL3, encodes Caveolin, the major membrane protein, to form Caveolae, the flask-like invagination in the plasma membrane. Furthermore, the peripheral membrane protein Cavin, encoded by polymerase I and transcript release factor (PTRF), is essential for the formation and stabilization of Caveolae27,28 and causes CGL4.29 Adipocytes lacking Caveolae result in impaired triglyceride storage ability in mice.30 In patients with CGL3, homozygous loss-of-function mutations have been found in CAV1.31 Meanwhile, heterozygous mutations in CAV1 have also been observed in FPLD7.32,33
Familial partial lipodystrophy
Familial partial lipodystrophy is a disorder caused by mutations in different genes and includes FPLD2, FPLD3, FPLD4, FPLD5, FPLD6, and FPLD7. FPLD1, the Köbberling syndrome, may be caused by several genetic as well as environmental factors.34,35 Lamin A/C (LMNA) encodes Lamin A and Lamin C, the structural proteins of the nuclear membrane, which determine the shape and size of the nucleus. Mutations in the LMNA gene have been linked to a variety of human diseases, such as Dunnigan FPLD2.36,37 In an in vitro experiment, the overexpression of wild-type and mutant gain-of-function laminin A inhibited the maturation of preadipocytes to adipocytes and the de novo synthesis of triglycerides.38 Peroxisome proliferator-activated receptors (PPARs) encompass three subtypes: PPAR-α, PPAR-δ, and PPAR-γ. PPAR-γ is mainly found in adipose tissues and is involved in adipocyte differentiation.39 PPARG encodes PPAR-γ. Mutations in PPARG impair the function of transcription factors and lower the transcriptional activity,40 which result in impaired insulin action and FPLD3.41 Perilipin 1 (PLIN1) encodes perilipin, which is the phosphoprotein located on the surface of intracellular lipid droplets.42 In the feeding state, Perilipin forms a barrier on the surface of the lipid droplets, which restricts lipase entry and promotes triacylglycerol storage. When stimulated by catecholamines, PKA-phosphorylated perilipin recruits hormone-sensitive lipase (HSL) to enhance lipolysis.43 FPLD4 is caused by heterozygous loss-of-function mutations in PLIN1. The mutant protein is associated with smaller lipid droplets, decreased triglyceride storage, and increased basal lipolysis in the preadipocytes.44 Cell death-inducing DFFA like effector C (CIDEC) is a lipid droplet protein also known as FSP27, which is colocalized with perilipin. CIDEC can augment lipid accumulation and regulate lipolysis.45 Dominant-negative PPARG mutations decrease the expression of FSP27, and the lack of CIDEC causes large monocular lipid droplets to separate into multiple small droplets in white adipocytes by increasing lipolysis and decreasing triacylglycerol storage, which leads to FPLD5.46 The LIPE gene encodes HSL,47 which is an intracellular neutral lipase that can hydrolyze several lipids.48 HSL plays a key role in the functioning of adipocytes,49 especially promoting the lipolysis of the stored triglycerides. Homozygous LIPE mutations inhibit lipolysis and may lead to FPLD6 in adults.50 FPLD6 is a progressive condition.51 The zinc metallopeptidase STE24 (ZMPSTE24) is necessary for the processing and maturation of Lamin A. The mutations in ZMPSTE24 may cause mandibuloacral dysplasia (MAD) because of the impaired processing of prelamin A.52,53
Severe obesity
Severe obesity-related H-SIRS is mainly caused by pelanocortin 4 receptor (MC4R), leptin (LEP), proopiomelanocortin (POMC), and SH2B adaptor protein 1 (SH2B1). MC4R mutations impair the colocalization of MC4R and adenylate cyclase 3 (ADCY3) in the primary cilia of hypothalamic neurons and lead to obesity.54 α-melanocyte-stimulating hormone (α-MSH) binds to MC4R55 and regulates food intake. α-MSH deficiency accounts for severe early-onset obesity in patients with POMC mutation.56 Leptin activates PI3K in the hypothalamus to reduce food intake and regulate body fat57,58 via the PI3K-PDE3B-cAMP pathway.59
Complex syndromes
H-SIRS has also been linked to several hereditary complex syndromes, such as Werner syndrome, Bloom syndrome, Alstrom syndrome, microcephalic osteodysplastic primordial dwarfism type 2 (MOPDII), MAD type A (MADA), MAD type B (MADB), SHORT syndrome, and mandibular hypoplasia, progeroid features, deafness, and lipodystrophy (MPDL) syndrome.
The phosphoinositide-3-kinase regulatory subunit 1 (PIK3R1) gene encodes the regulatory subunits p85, p55, and p50 of PI3K.60,61 The mutations in this gene, which often appears in the C-terminal Src homology 2 (SH2) domain of PIK3, lead to the SHORT syndrome. Impaired PI3K activation is associated with the downregulation of the PI3K-AKT-mTOR pathway62 (Fig. 1).
Diagnosis
Clinical presentation
Acanthosis nigricans, ovarian dysfunction, and abnormal glucose homeostasis are the generic clinical features of SIRS. Acanthosis nigricans, a dark velvety thickening of the skin in folds and creases, can be seen in almost all known forms of H-SIRS.3 In young women, oligomenorrhea and severe hyperandrogenism are the earliest signs of H-SIRS that gain clinical attention; however, the underlying hyperinsulinemia goes unrecognized in most cases. Ovarian hyperandrogenism becomes clinically apparent during infancy and/or post-pubertal life. Congenital hyperplasia or an androgen-secreting tumor is the principal differential diagnosis of H-SIRS-related severe hyperandrogenism. However, in H-SIRS, along with severe hyperandrogenism, acanthosis nigricans is also prominent. Puberty is often accelerated in H-SIRS. Abnormal glucose homeostasis is common, but it is not the earliest manifestation. Hypoglycemia precedes hyperglycemia, while postprandial hypoglycemia is a characteristic manifestation of H-SIRS-related hypoglycemia. In H-SIRS, fasting glucose test is inadequate for diagnosing diabetes, and hyperglycemia suggestive of diabetes becomes evident only after oral glucose challenge,.3,5
Dyslipidemia, hepatic steatosis, and abnormal development and topography of the adipose tissues are the characteristic features of the lipodystrophic subtype of H-SIRS, which differentiate them from the insulin signaling defects subtype of H-SIRS. More than a thousand allelic variants of insulin receptor defects have been described, and the most severe syndromes are DS and RMS.4
DS is a rare autosomal recessive syndrome of severe insulin resistance, which is marked by low birth weight, postnatal severe failure to thrive because of malnutrition despite regular feeding, characteristic facial features (microcephaly, pointed chin, low set prominent ear, broad nose, and thick lips), acanthosis nigricans, hirsutism, excessively thick skin, decreased subcutaneous adipose tissues, muscles wasting, relatively large hands and feet, and significant hypertrophic cardiomyopathy that manifests after the first few months of life.6 The classical features of Rabson–Mendenhall syndrome consist of hyperinsulinemia, coarse facial features (globular nose, full lips, and furrowed tongue), acanthosis nigricans, hyperkeratosis, hypertrichosis, dental abnormalities, and growth retardation.7
Lipodystrophies is a group of heterogeneous disorders characterized by generalized or partial loss of adipose tissue, severe dyslipidemia, hepatic steatosis, and low serum leptin and/or adiponectin levels. CGL is an autosomal recessive disorder diagnosed in most patients with total or subtotal absence of adipose tissue along with metabolic abnormalities at birth or early childhood. Nevertheless, a differential diagnosis of CGL should be considered in older patients who have a massive loss of subcutaneous fat because CGL has been diagnosed with genetic testing in some older patients.63 Patients with CGL1 present with generalized loss of subcutaneous fat, muscular hypertrophy, and acromegalic features at birth or early infancy. Subsequently, in early childhood, acanthosis nigricans, hypertriglyceridemia, hirsutism, hepatomegaly with steatosis, micro-albuminemia, myocardial hypertrophy, and insulin resistance appear. Pituitary adenoma, umbilical hernia, and polycystic ovarian syndrome have been reported in 20% of the cases.63,64 Most of the patients are diagnosed with type-2 diabetes mellitus in their second decade of life. CGL2 has similar but relatively severe features when compared with CGL1. The presence of polyneuropathies and severe cognitive disabilities differentiates CGL2 from CGL1. However, these differentiating features have been observed in only one patient with AGPAT2 mutation.65 Apart from the features of CGL1, psycho-behavioral issues, penile hypertrophy, splenomegaly, renal hypertrophy, progressive myoclonic epilepsy, recurrent pancreatitis secondary to severe triglyceridemia, and thyroid carcinoma have been reported in patients with CGL2.66, 67, 68 CAV1 mutations give rise to a highly variable phenotype of lipodystrophic syndromes. The patients with p.F160X mutation in CAV1 have the neonatal progeroid appearance (triangular face, a large anterior fontanelle), lipodystrophy, pulmonary hypertension, cutis marmorata, feeding disorders, and cerebral atrophy.69,70 CAV1 p.Glu38X mutation leads to the classical features of CGL and severe insulin resistance along with recurrent episodes of pneumonia, chronic diarrhea, and hypocalcemia.31 The I134fsdelA-X137 mutation in CAV1 is associated with atypical partial lipodystrophy, with subcutaneous fat loss affecting the upper part of the body and face but sparing the legs, gluteal region, and visceral fat stores. Other manifestations include type 5 hyperlipoproteinemia, recurrent pancreatitis, congenital cataracts, and neurological findings. On the other hand, the -88delC mutation is linked to partial lipodystrophy, with subcutaneous fat loss affecting the arms, legs, and gluteal region but sparing the face, neck, and visceral fat stores. Severe type 5 hyperlipoproteinemia and recurrent pancreatitis are also seen.32 The c.424C>T (p. Q142∗) mutation results in a generalized loss of subcutaneous fat, except for the abdomen and buttocks. A poor musculature is also noted, with clearly visible dermal vessels suggestive of cutis marmorata.33 How mutations in different locations of CAV1 lead to different diseases is still unclear.70 In addition to the generalized loss of subcutaneous fat and the classical features of insulin resistance, muscular dystrophies, decreased range of motion in multiple joints, mild mental retardation, developmental delay, bone abnormalities, and elevated creatine kinase are the typical features of CGL4, which is a progressive disorder caused by a mutation of the PTRF gene.71, 72, 73, 74 Cardiac arrhythmias, mild hypothyroidism, pyloric stenosis, scoliosis, and lordosis have also been reported in several patients.29,75,76 Two different phenotypes have been linked to the mutation in PCYT1A; one is characterized by severe insulin resistance and congenital lipodystrophy with short stature, and the other is related to spondylo-metaphyseal dysplasia with cone-red dystrophy.77,78
As mentioned previously, FPLD is classified into six types. The mutations in the PPARG gene lead to partial lipodystrophy, which is characterized by subcutaneous fat loss and hypertrophy of the muscles in the arms (below the surgical head of the humerus), legs, and gluteal regions. Accumulation of adipose tissues is seen in the face, neck, suprascapular, and abdominal regions. Additionally, type 2 diabetes, severe insulin resistance, and polycystic ovarian syndrome are noted in FPLD3.40,79,80 However, end-stage renal disease with hyperparathyroidism and severe cardiac abnormalities are seen in very few patients. A biallelic mutation in PPARG can lead to congenital generalized lipodystrophy similar to the CGL syndrome.81 Features that differentiate FPLD2 caused by the LMNA mutation from FPDL3 are the loss of truncal fat and subcutaneous fat over the entire arm, with marked muscularity, phlebectasia, presence of cardiomyopathies, and a relatively severe lipodystrophy phenotype. In FPLD4 caused by a mutation in PLIN1, lipoatrophy and muscular appearance limited to the lower limbs and the gluteofemoral region along with centripetal obesity are the differentiating features.82,83 Proteinuria, hypertension, and recurrent strokes are also rarely seen in patients with FPLD4 syndrome.44,84 To the best of our knowledge, one case of CIDEC mutation has been reported, which presented with characteristic features of FPLD syndrome. The patient exhibited lipoatrophic and muscular lower limbs and white adipocytes with multiloculated lipid droplets, which can be considered as the differentiating features of this form of FPLD.85 Mutation in the LIPE gene causes multiple symmetric lipomatosis and insulin resistance with partial lipodystrophy. Partial lipodystrophy associated with the LIPE mutation, known as FPLD6, is marked by subcutaneous fat loss in the lower limbs, accumulation of fat in the face, neck, axilla, and trunk, and neuromuscular manifestations50,86 (Table 1).
Table 1.
Hereditary severe insulin resistance syndromes.
| Gene | Clinical disorder | Inheritance | Clinical feature |
|---|---|---|---|
| INSULIN SIGNALING DEFECTS | |||
| GENERALIZED | |||
| INSR | AD/AR | Extreme hyperinsulinemia, normal lipid profile, no fatty liver, normal or elevated adiponectin, and IGFBP1 | |
| Partial | |||
| ATK2 | FPLD6 | AD | Hepatic steatosis, dyslipidemia, low adiponectin, severe insulin resistance |
| TBCID4 | AD | Postprandial insulin resistance | |
| LIPODYSTROPHIES (Congenitally absent subcutaneous fat, or partial deficiency of adipose tissue, severe dyslipidemia, fatty liver, low adiponectin/leptin) | |||
| Congenital Generalized Lipodystrophies (CGL) | |||
| AGPAT2 | CGL1 | AR | Steatosis, hypertriglyceridemia |
| BSCL2 | CGL2 | AR | Polyneuropathies and cognitive disabilities |
| CAV1 | CGL3 | AR | Short stature, pulmonary arterial hypertension |
| PTRF | CGL4 | AR | Muscular dystrophies, elevated serum CK |
| Familial Partial Lipodystrophies (FPLD) | |||
| LMNA | FPLD2 | AD | Lipoatrophy and remarkable muscular hypertrophy of limbs, gluteofemoral, and truncal region, cardiomyopathy |
| PPARG | FPLD3 | AD | Lipoatrophy limited to Limbs with centripetal obesity, hypertriglyceridemia |
| PLIN1 | FPLD4 | AD | Lipoatrophy limited to limbs and gluteofemoral region |
| CIDEC | FPLD5 | AR | Multilocular lipid droplets fat loss of lower limbs and abdomen |
| LIPE | FPLD6 | AR | Multiple symmetric lipomatosis, lipoatrophy of lower limbs |
| CAV1 | FPLD7 | AD | Early-onset cataracts |
| PCYT1A | Low adiponectin, short stature | ||
| SEVERE OBESITY (Early onset severe hyperphagic obesity, dyslipidemia, hepatic steatosis, normal/high leptin, low/normal adiponectin) | |||
| MC4R | AD/AR | Tall stature, fasting hyperinsulinemia | |
| LEP | AR | Hypogonadotropic hypogonadism, hyperphagia, immune dysfunction | |
| POMC | AR | Red hair and Adrenocorticotropic hormone (ACTH) deficiency | |
| SH2B1 | AD | Insulin resistance, Leptin resistance | |
| COMPLEX SYNDROMES (severe dyslipidemia disproportionate to total body fat mass, severe hepatic steatosis, adipose tissue failure) | |||
| RECQL2 LMNA | Werner syndrome | AR | Cataracts, immunodeficiency limb contractures, premature aging, |
| RECQL3 | Bloom syndrome | AR | Telangiectasia, short stature, increased susceptibility to cancer |
| ALMS1 | Alstrom syndrome | AR | Red-cone dystrophy, deafness, pulmonary or hepatic or renal abnormalities |
| PCNT | MOPDII | AR | Short stature, vascular anomalies |
| LMNA | MADA | AR | Neuropathies, premature aging |
| ZMPST-24 | MADB | AR | Facial and skeletal abnormalities acro-osteolysis of the distal phalanges |
| PIK3R1 | SHORT syndrome | AD/sporadic | Short stature, neurological abnormalities |
| POLD1 | MDPL syndrome | AD/sporadic | Mandibular hypoplasia, joint contractures, crowded teeth, deafness |
Diagnostic approaches and criteria
H-SIRS can be diagnosed based on clinical presentation and characteristic biochemical features, as described in the preceding section. Genetic testing is required to confirm the diagnosis. Definite diagnostic criteria are yet to be formulated; however, some arbitrary criteria proposed earlier can help diagnose the condition. In individuals with normal glucose tolerance and a BMI of <30 kg/m2, fasting insulin of >150 pmol/L is suggestive of H-SIRS, and in individuals with absolute insulin deficiency and a BMI of <30 kg/m2, an exogenous insulin requirement of >1,500 pmol/L is a useful indicator.3 As insulin levels can be influenced by the onset of puberty, decompensation of beta cells, and accumulation of adipose tissue, the diagnosis should be made in light of accompanying clinical features of severe insulin resistance. These features include acanthosis nigricans, hyperandrogenism, and generalized or partial loss of subcutaneous fat.5 DS can be diagnosed based on the presence of a biochemical triad (severe hyperinsulinemia, fasting hypoglycemia, and postprandial hyperglycemia) combined with the characteristic clinical features of DS.6 The criteria to diagnose the CGL forms of H-SIRS comprise the major and minor criteria87, 88, 89 (Table 2). Confirmation of mutations in genes associated with H-SIRS using genetic sequencing techniques, such as whole-exome sequencing and Sanger's sequencing, is required to establish H-SIRS diagnosis.
Table 2.
Congenital Generalized Lipodystrophy diagnosis criteria.
| Major criteria |
| Insulin resistance |
| Loss of subcutaneous fat affecting both trunk and limbs |
| Acromegaloid features |
| Hepatomegaly |
| Hypertriglyceridemia |
| Minor criteria |
| Early onset of puberty in females |
| Mental retardation |
| Hypertrophic cardiomyopathy |
| Hypertrichosis |
| Prominent veins |
| Bone cysts |
| Diagnosis |
| Presence of 3 major criteria OR 2 Major with ≥2 Minor criteria AND/OR Identification of mutation on CGL linked genes |
H-SIRS shows a wide and variable spectrum of presentations, which necessitates multiple additional investigations for thoroughly evaluating the underlying complications. Blood glucose and insulin levels are fundamental investigations that help clinicians to confirm the presence of insulin resistance and provide a basis for differentiating multiple forms of H-SIRS. For example, severe hyperinsulinemia, hypoglycemia in accelerated fasting states, and postprandial hyperglycemia are considered hallmarks of DS,6 and postprandial insulin resistance is associated with TBC1D4 mutation. Hyperinsulinemic euglycemic clamp, homeostasis model assessment of insulin resistance (HOMA-IR), and oral glucose tolerance test (OGTT) can be used for the early diagnosis of insulin resistance. Computer tomography, magnetic resonance imaging, and ultrasonography are useful in determining hepatosplenomegaly and the onset of puberty. Extended ovaries and multiple mature follicles in girls are the signs of early onset of puberty.90 H-SIRS attributed to primary inulin receptor defects presents a normal lipid profile with normal or elevated adiponectin and leptin levels. In contrast, severe dyslipidemia and low adiponectin and leptin levels are sensitive but non-specific indicators of lipodystrophic H-SIRS.3 Therefore, obtaining a complete lipid profile and testing the serum leptin and adiponectin levels can help in the etiological differentiation of H-SIRS. Sex hormone-binding globulin (SHBG) and insulin growth factor binding protein-1 (IGFBP-1) are also elevated in primary insulin receptor defects. Dual-energy X-ray absorptiometry (DEXA) and skin-fold thickness measurements can be used to determine total body fat loss in comparison with predicted fat composition and peripheral lipoatrophy, respectively. Whole-exome sequencing and Sanger sequencing are commonly performed on DNA extracted from peripheral blood samples to detect mutations in candidate genes.
Barriers to the early diagnosis of H-SIRS
The extreme rarity of H-SIRS is one of the key factors that lead to its underdiagnosis in clinical practice. Many clinicians may not face a single patient with H-SIRS in their entire medical practice,91 and some may lack knowledge about the diagnosis and management of the syndrome. Consequently, patients with mild symptoms are underdiagnosed.63,92,93 The immense variability in the clinical presentations of the same form of H-SIRS makes it difficult to be diagnosed in the first clinical encounter. Despite receiving almost the same management, two siblings with the same genetic defect have been reported to show some variations in the clinical course of the syndrome.94 Some patients with a mild phenotype of H-SIRS or presently asymptomatic family members of the identified patients may refuse to undergo genetic testing. Unavailability of genetic testing facilities and the associated expenses are construed to be the other barriers that prevent the early diagnosis of H-SIRS, especially in underdeveloped countries. Hence, the number of cases of H-SIRS reported in developing and underdeveloped countries is relatively low.95
Treatment and surveillance
Treatment
H-SIRS treatment is extremely challenging because of the immense variability in the presentation and the barriers to early diagnosis. Multiple treatment options are available, including dietary modifications, insulin sensitization, insulin therapy, metreleptin therapy, and recombinant human insulin-like growth factor-1 (rhIGF-1) administration. Dietary management by adhering to a low-fat diet, practicing calorie restriction, and prescribing milk powder containing medium-chain fatty acids for infants and acarbose diet for adults constitutes the mainstay of treatment and has been shown to effectively improve the biochemical profile of patients with CGL.94,96 After 4–14 months of treatment with a low-fat and lactose-free diet supplemented with a formula rich in medium-chain fatty acids and lipid-lowering agents, patients with CGL1 and CGL2 showed improvements in glycemic control and liver function, reduction in serum triglycerides and body height, and return to normal weight.91 Neonates presenting with failure to thrive and characteristic features of CGL should be managed with parenteral nutrition containing the minimum amount of essential fatty acids.97,98
Insulin sensitization with metformin or a combination of sensitizing agents with extraneous insulin is the common therapy for all forms of H-SIRS. Although metformin is widely used in treating H-SIRS, it is not always effective. Metformin effectively controlled the progression of the syndrome by improving glycemic control in some patients. Recent studies have suggested that metformin can be used safely, unless the estimated glomerular filtration rate (eGFR) falls below 30 mL/min, with a dose reduction advised at 45 mL/min. Metformin should be used with caution in patients with congestive heart failure requiring pharmacologic treatment, renal impairment with elevated creatinine levels, and advanced age.40,99 Pioglitazone, one of the thiazolidinediones (TZDs), via the activation of PPAR-γ to improve insulin sensitivity, has been shown to increase the expression of genes involved in adipogenesis, including LPL, LEP, and SLC2A4 in fibroblasts. The expressions of these genes were markedly reduced in the fibroblasts of patients with lipodystrophies. The patients treated with pioglitazone demonstrated improvements in insulin sensitivity, glycemic control, plasma leptin, and body fat composition. Commonly used dose regimes of pioglitazone are 4–45 mg per day.100,101 Although pioglitazone treatment did not result in obvious improvements in fat distribution, it effectively improved metabolic control in a patient with FPLD, and HbA1c dropped from 68.3 to 35.5 mmol/mol without any dietary or lifestyle modifications.102 Multidrug therapy with insulin-sensitizing agents, dipeptidyl peptidase-4 inhibitor, and extraneous insulin can sometimes be beneficial if single-drug therapy fails to achieve glycemic control. Two insulin-sensitizing agents, when combined with dipeptidyl peptidase-4 inhibitor, showed promising results in improving glycemic control in a patient with RMS.103
RhIGF-1 improves glycemic control by stimulating peripheral glucose utilization and suppressing glucose production in the liver.104 Studies have established that rhIGF-1 enhances survival in infants with severe receptropathies; moreover, rhIGF-1 tends to improve beta-cell function in adults. Nevertheless, the definite mode of its action and the precise dosing are yet to be established; therefore, it is recommended that rhIGF-1 therapy be initiated only after consultation with experienced physicians. This therapy should be started at a low dose, with a strict follow-up of clinical and biochemical parameters. In case of deterioration or lack of improvement in the clinical and biochemical parameters, the therapy should be immediately discontinued.98
Metreleptin is recombinant human leptin that has recently been approved by the FDA to treat congenital generalized lipodystrophies, and it improves glycemic control and hepatic steatosis.90 Metreleptin therapy alleviates hypertriglyceridemia and reduces the daily requirement of inulin.93 When compared with the use of metreleptin alone, two siblings showed better metabolic control with combined metreleptin and metformin therapy.94 Symptomatic treatment of the accompanying abnormalities, such as cardiac and renal abnormalities and hypertension, is equally important in managing H-SIRS.
Benefits of early diagnosis-led early treatment and surveillance
Early diagnosis of H-SIRS leads to early management, which facilitates improved glycemic control and a significant delay in the onset of diabetes and other complications related to severe insulin resistance.79,93 Furthermore, early diagnosis permits surveillance of the patients for the early identification of complications and prompt modification of the treatment. For example, the early diagnosis-led early treatment of RMS improved glycemic control for a relatively long period and delayed the complications of severe insulin resistance in a patient when compared with his elder sister, who was initially misdiagnosed to have Costello syndrome.7 Early commencement of rhIGF-1 treatment in DS boosts linear growth and prolongs longevity.104 The patients with lipodystrophy syndrome who started early treatment with optimal dietary management and early addition of metreleptin therapy had good glycemic control for a prolonged period, with a marked reduction in the complications associated with severe insulin resistance and lipodystrophies.
Perspectives
According to the complexities, rarity, and variations in H-SIRS, the use of updated genetic sequencing technologies is recommended to make an accurate diagnosis and offer informed genetic counseling to the parents for future pregnancies. Furthermore, amniotic fluid or chorionic villus samples should be prenatally tested to detect mutations. Family members of patients with confirmed H-SIRS should also be genetically tested for the mutations. Long-term studies are required for genotype–phenotype differentiation and formulation of diagnostic and treatment protocols to improve the diagnosis rate, explore more remedies for the different forms of H-SIRS, and determine the actual effectiveness and associated risks. Sodium-glucose co-transporter 2 inhibitor (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP-1 Ras) are the new classes of hypoglycemic agents and hold promise in controlling insulin resistance in H-SIRS. However, further studies are required to confirm the clinical effectiveness of these agents in H-SIRS.
Conclusions
Literature on the various genetic forms of SIRS was reviewed, and based on available data, the pathogenesis and clinical management of H-SIRS were described. Identification of insulin resistance via HOMA-IR or hyperinsulinemic-euglycemic clamp and the presence of three or more classical features of severe insulin resistance, including acanthosis nigricans, hyperandrogenism, hyperglycemia, dyslipidemia, hepatic steatosis, and generalized or partial lipoatrophy, in the absence of autoantibodies associated with insulin resistance or diabetes, are highly suggestive of H-SIRS. To confirm the diagnosis, mutations in H-SIRS-associated genes should be identified by using whole-exome sequencing or Sanger sequencing. Multiple treatment options are currently available for H-SIRS management, which have been proven to be effective in clinical short-term studies. RhIGF-1 therapy improves survival in infants with DS. Two insulin-sensitizing agents, when combined with dipeptidyl peptidase-4 inhibitor, have shown promising results in improving glycemic control in patients with RMS. Optimal diet therapy and early metreleptin therapy are recommended for lipodystrophic H-SIRS. Early diagnosis allows surveillance of patients for the early identification of complications and the prompt modification of treatment accordingly. SGLT2i and GLP-1 Ras are the new hypoglycemic agents, and more studies are required to confirm their effectiveness in treating H-SIRS.
Author contributions
Iqbal J and Jiang HL contributed equally to the review. Iqbal J, Jiang HL, Wu HX and Zhou HD designed the study protocol. Iqbal J, Jiang HL, and Wu HX did the literature review and wrote the review. Li L, Zhou HD, Zhou YH, Hu N, Xiao F, Wang T, and Xu SN contributed to revise the manuscript.
Conflict of interests
Authors declare no conflict of interests.
Funding
This work was supported by the National Natural Science Foundation of China (No. 8217033609, 81770880, 81800788, and 81970762); the Science & Technology Department of Hunan Province (China) (No. 2020SK2080, and 2015JC3012); and Changsha Science & Technology (China) (No. k1906019, and kq1901118).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Reaven G.M. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr. 2005;25:391–406. doi: 10.1146/annurev.nutr.24.012003.132155. [DOI] [PubMed] [Google Scholar]
- 2.Muniyappa R., Lee S., Chen H., Quon M.J. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 3.Semple R.K., Savage D.B., Cochran E.K., Gorden P., O'Rahilly S. Genetic syndromes of severe insulin resistance. Endocr Rev. 2011;32(4):498–514. doi: 10.1210/er.2010-0020. [DOI] [PubMed] [Google Scholar]
- 4.Melvin A., O'Rahilly S., Savage D.B. Genetic syndromes of severe insulin resistance. Curr Opin Genet Dev. 2018;50:60–67. doi: 10.1016/j.gde.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Parker V.E., Semple R.K. Genetics in endocrinology: genetic forms of severe insulin resistance: what endocrinologists should know. Eur J Endocrinol. 2013;169(4):R71–R80. doi: 10.1530/EJE-13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkwood A., Stuart G., Harding L. Donohue syndrome: a review of literature, case series, and anesthetic considerations. Paediatr Anaesth. 2018;28(1):23–27. doi: 10.1111/pan.13273. [DOI] [PubMed] [Google Scholar]
- 7.Al-Kandari H., Al-Abdulrazzaq D., Al-Jaser F., Al-Mulla F., Davidsson L. Rabson-Mendenhall Syndrome in a brother-sister pair in Kuwait: diagnosis and 5 year follow up. Prim Care Diabetes. 2021;15(1):175–177. doi: 10.1016/j.pcd.2020.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350(12):1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 9.Ullrich A., Bell J.R., Chen E.Y., et al. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature. 1985;313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- 10.Whitehead J.P., Soos M.A., Jackson R., Tasic V., Kocova M., O'Rahilly S. Multiple molecular mechanisms of insulin receptor dysfunction in a patient with Donohue syndrome. Diabetes. 1998;47(8):1362–1364. doi: 10.2337/diab.47.8.1362. [DOI] [PubMed] [Google Scholar]
- 11.Hosoe J., Kadowaki H., Miya F., et al. Structural basis and genotype-phenotype correlations of INSR mutations causing severe insulin resistance. Diabetes. 2017;66(10):2713–2723. doi: 10.2337/db17-0301. [DOI] [PubMed] [Google Scholar]
- 12.Kadowaki T., Kadowaki H., Taylor S.I. A nonsense mutation causing decreased levels of insulin receptor mRNA: detection by a simplified technique for direct sequencing of genomic DNA amplified by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990;87(2):658–662. doi: 10.1073/pnas.87.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiteman E.L., Cho H., Birnbaum M.J. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metabol. 2002;13(10):444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 14.Cheng J.Q., Godwin A.K., Bellacosa A., et al. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci U S A. 1992;89(19):9267–9271. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X., Monks B., Ge Q., Birnbaum M.J. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature. 2007;447(7147):1012–1016. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- 16.Garofalo R.S., Orena S.J., Rafidi K., et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112(2):197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roden M., Shulman G.I. The integrative biology of type 2 diabetes. Nature. 2019;576(7785):51–60. doi: 10.1038/s41586-019-1797-8. [DOI] [PubMed] [Google Scholar]
- 18.James D.E., Brown R., Navarro J., Pilch P.F. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988;333(6169):183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- 19.Bouzakri K., Ribaux P., Tomas A., Parnaud G., Rickenbach K., Halban P.A. Rab GTPase-activating protein AS160 is a major downstream effector of protein kinase B/Akt signaling in pancreatic beta-cells. Diabetes. 2008;57(5):1195–1204. doi: 10.2337/db07-1469. [DOI] [PubMed] [Google Scholar]
- 20.Moltke I., Grarup N., Jørgensen M.E., et al. A common Greenlandic TBC1D4 variant confers muscle insulin resistance and type 2 diabetes. Nature. 2014;512(7513):190–193. doi: 10.1038/nature13425. [DOI] [PubMed] [Google Scholar]
- 21.Alcantara D., Elmslie F., Tetreault M., et al. SHORT syndrome due to a novel de novo mutation in PRKCE (Protein Kinase Cɛ) impairing TORC2-dependent AKT activation. Hum Mol Genet. 2017;26(19):3713–3721. doi: 10.1093/hmg/ddx256. [DOI] [PubMed] [Google Scholar]
- 22.Raygada M., Rennert O. Congenital generalized lipodystrophy: profile of the disease and gender differences in two siblings. Clin Genet. 2005;67(1):98–101. doi: 10.1111/j.1399-0004.2004.00372.x. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal A.K., Arioglu E., De Almeida S., et al. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet. 2002;31(1):21–23. doi: 10.1038/ng880. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal A.K., Garg A. Congenital generalized lipodystrophy: significance of triglyceride biosynthetic pathways. Trends Endocrinol Metabol. 2003;14(5):214–221. doi: 10.1016/s1043-2760(03)00078-x. [DOI] [PubMed] [Google Scholar]
- 25.Cui X., Wang Y., Tang Y., et al. Seipin ablation in mice results in severe generalized lipodystrophy. Hum Mol Genet. 2011;20(15):3022–3030. doi: 10.1093/hmg/ddr205. [DOI] [PubMed] [Google Scholar]
- 26.Yang W., Thein S., Wang X., et al. BSCL2/seipin regulates adipogenesis through actin cytoskeleton remodelling. Hum Mol Genet. 2014;23(2):502–513. doi: 10.1093/hmg/ddt444. [DOI] [PubMed] [Google Scholar]
- 27.Ariotti N., Parton R.G. SnapShot: caveolae, caveolins, and cavins. Cell. 2013;154(3):704. doi: 10.1016/j.cell.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Hill M.M., Bastiani M., Luetterforst R., et al. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132(1):113–124. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shastry S., Delgado M.R., Dirik E., Turkmen M., Agarwal A.K., Garg A. Congenital generalized lipodystrophy, type 4 (CGL4) associated with myopathy due to novel PTRF mutations. Am J Med Genet. 2010;152A(9):2245–2253. doi: 10.1002/ajmg.a.33578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L., Brown D., McKee M., et al. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metabol. 2008;8(4):310–317. doi: 10.1016/j.cmet.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim C.A., Delépine M., Boutet E., et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab. 2008;93(4):1129–1134. doi: 10.1210/jc.2007-1328. [DOI] [PubMed] [Google Scholar]
- 32.Cao H., Alston L., Ruschman J., Hegele R.A. Heterozygous CAV1 frameshift mutations (MIM 601047) in patients with atypical partial lipodystrophy and hypertriglyceridemia. Lipids Health Dis. 2008;7:3. doi: 10.1186/1476-511X-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garg A., Kircher M., Del Campo M., Amato R.S., Agarwal A.K., University of Washington Center for Mendelian Genomics Whole exome sequencing identifies de novo heterozygous CAV1 mutations associated with a novel neonatal onset lipodystrophy syndrome. Am J Med Genet. 2015;167A(8):1796–1806. doi: 10.1002/ajmg.a.37115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herbst K.L., Tannock L.R., Deeb S.S., Purnell J.Q., Brunzell J.D., Chait A. Köbberling type of familial partial lipodystrophy: an underrecognized syndrome. Diabetes Care. 2003;26(6):1819–1824. doi: 10.2337/diacare.26.6.1819. [DOI] [PubMed] [Google Scholar]
- 35.Guillín-Amarelle C., Sánchez-Iglesias S., Castro-Pais A., et al. Type 1 familial partial lipodystrophy: understanding the Köbberling syndrome. Endocrine. 2016;54(2):411–421. doi: 10.1007/s12020-016-1002-x. [DOI] [PubMed] [Google Scholar]
- 36.Worman H.J., Bonne G. "Laminopathies": a wide spectrum of human diseases. Exp Cell Res. 2007;313(10):2121–2133. doi: 10.1016/j.yexcr.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Speckman R.A., Garg A., Du F., et al. Mutational and haplotype analyses of families with familial partial lipodystrophy (Dunnigan variety) reveal recurrent missense mutations in the globular C-terminal domain of lamin A/C. Am J Hum Genet. 2000;66(4):1192–1198. doi: 10.1086/302836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boguslavsky R.L., Stewart C.L., Worman H.J. Nuclear lamin A inhibits adipocyte differentiation: implications for Dunnigan-type familial partial lipodystrophy. Hum Mol Genet. 2006;15(4):653–663. doi: 10.1093/hmg/ddi480. [DOI] [PubMed] [Google Scholar]
- 39.Evans R.M., Barish G.D., Wang Y.X. PPARs and the complex journey to obesity. Nat Med. 2004;10(4):355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 40.Hegele R.A., Cao H., Frankowski C., Mathews S.T., Leff T. PPARG F388L, a transactivation-deficient mutant, in familial partial lipodystrophy. Diabetes. 2002;51(12):3586–3590. doi: 10.2337/diabetes.51.12.3586. [DOI] [PubMed] [Google Scholar]
- 41.Barroso I., Gurnell M., Crowley V.E., et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402(6764):880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 42.Blanchette-Mackie E.J., Dwyer N.K., Barber T., et al. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res. 1995;36(6):1211–1226. [PubMed] [Google Scholar]
- 43.Brasaemle D.L., Subramanian V., Garcia A., Marcinkiewicz A., Rothenberg A. Perilipin A and the control of triacylglycerol metabolism. Mol Cell Biochem. 2009;326(1–2):15–21. doi: 10.1007/s11010-008-9998-8. [DOI] [PubMed] [Google Scholar]
- 44.Gandotra S., Le Dour C., Bottomley W., et al. Perilipin deficiency and autosomal dominant partial lipodystrophy. N Engl J Med. 2011;364(8):740–748. doi: 10.1056/NEJMoa1007487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puri V., Konda S., Ranjit S., et al. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem. 2007;282(47):34213–34218. doi: 10.1074/jbc.M707404200. [DOI] [PubMed] [Google Scholar]
- 46.Nishino N., Tamori Y., Tateya S., et al. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J Clin Invest. 2008;118(8):2808–2821. doi: 10.1172/JCI34090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holm C., Kirchgessner T.G., Svenson K.L., et al. Hormone-sensitive lipase: sequence, expression, and chromosomal localization to 19 cent-q13.3. Science. 1988;241(4872):1503–1506. doi: 10.1126/science.3420405. [DOI] [PubMed] [Google Scholar]
- 48.Kraemer F.B., Shen W.J. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res. 2002;43(10):1585–1594. doi: 10.1194/jlr.r200009-jlr200. [DOI] [PubMed] [Google Scholar]
- 49.Albert J.S., Yerges-Armstrong L.M., Horenstein R.B., et al. Null mutation in hormone-sensitive lipase gene and risk of type 2 diabetes. N Engl J Med. 2014;370(24):2307–2315. doi: 10.1056/NEJMoa1315496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zolotov S., Xing C., Mahamid R., Shalata A., Sheikh-Ahmad M., Garg A. Homozygous LIPE mutation in siblings with multiple symmetric lipomatosis, partial lipodystrophy, and myopathy. Am J Med Genet. 2017;173(1):190–194. doi: 10.1002/ajmg.a.37880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xia B., Cai G.H., Yang H., Wang S.P., Mitchell G.A., Wu J.W. Adipose tissue deficiency of hormone-sensitive lipase causes fatty liver in mice. PLoS Genet. 2017;13(12):e1007110. doi: 10.1371/journal.pgen.1007110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moulson C.L., Go G., Gardner J.M., et al. Homozygous and compound heterozygous mutations in ZMPSTE24 cause the laminopathy restrictive dermopathy. J Invest Dermatol. 2005;125(5):913–919. doi: 10.1111/j.0022-202X.2005.23846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agarwal A.K., Fryns J.P., Auchus R.J., Garg A. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum Mol Genet. 2003;12(16):1995–2001. doi: 10.1093/hmg/ddg213. [DOI] [PubMed] [Google Scholar]
- 54.Siljee J.E., Wang Y., Bernard A.A., et al. Subcellular localization of MC4R with ADCY3 at neuronal primary cilia underlies a common pathway for genetic predisposition to obesity. Nat Genet. 2018;50(2):180–185. doi: 10.1038/s41588-017-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu D., Willard D., Patel I.R., et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371(6500):799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 56.Krude H., Biebermann H., Luck W., Horn R., Brabant G., Grüters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19(2):155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 57.Kennedy A., Gettys T.W., Watson P., et al. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab. 1997;82(4):1293–1300. doi: 10.1210/jcem.82.4.3859. [DOI] [PubMed] [Google Scholar]
- 58.Niswender K.D., Morton G.J., Stearns W.H., Rhodes C.J., Myers M.G., Jr., Schwartz M.W. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413(6858):794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 59.Zhao A.Z., Huan J.N., Gupta S., Pal R., Sahu A. A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat Neurosci. 2002;5(8):727–728. doi: 10.1038/nn885. [DOI] [PubMed] [Google Scholar]
- 60.Hoyle J., Yulug I.G., Egan S.E., Fisher E.M. The gene that encodes the phosphatidylinositol-3 kinase regulatory subunit (p85 alpha) maps to chromosome 13 in the mouse. Genomics. 1994;24(2):400–402. doi: 10.1006/geno.1994.1638. [DOI] [PubMed] [Google Scholar]
- 61.Conley M.E., Dobbs A.K., Quintana A.M., et al. Agammaglobulinemia and absent B lineage cells in a patient lacking the p85α subunit of PI3K. J Exp Med. 2012;209(3):463–470. doi: 10.1084/jem.20112533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dyment D.A., Smith A.C., Alcantara D., et al. Mutations in PIK3R1 cause SHORT syndrome. Am J Hum Genet. 2013;93(1):158–166. doi: 10.1016/j.ajhg.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guerreiro V., Bernardes I., Pereira J., et al. Acromegaly with congenital generalized lipodystrophy - two rare insulin resistance conditions in one patient: a case report. J Med Case Rep. 2020;14(1):34. doi: 10.1186/s13256-020-2352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferraria N., Pedrosa C., Amaral D., Lopes L. Berardinelli-Seip syndrome: highlight of treatment challenge. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2012-007734. bcr2012007734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oswiecimska J., Dawidziuk M., Gambin T., et al. A patient with Berardinelli-Seip syndrome, novel AGPAT2 splicesite mutation and concomitant development of non-diabetic polyneuropathy. J Clin Res Pediatr Endocrinol. 2019;11(3):319–326. doi: 10.4274/jcrpe.galenos.2018.2018.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin J., Cao L., Zhao Z., et al. Novel BSCL2 gene mutation E189X in Chinese congenital generalized lipodystrophy child with early onset diabetes mellitus. Eur J Endocrinol. 2007;157(6):783–787. doi: 10.1530/EJE-07-0393. [DOI] [PubMed] [Google Scholar]
- 67.Opri R., Fabrizi G.M., Cantalupo G., et al. Progressive myoclonus epilepsy in congenital generalized lipodystrophy type 2: report of 3 cases and literature review. Seizure. 2016;42:1–6. doi: 10.1016/j.seizure.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 68.Chen R., Yuan X., Wang J., Zhang Y. Clinical and molecular characterization of two Chinese patients with Type 2 congenital generalized lipodystrophy. Gene. 2017;637:57–62. doi: 10.1016/j.gene.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 69.Schrauwen I., Szelinger S., Siniard A.L., et al. A frame-shift mutation in CAV1 is associated with a severe neonatal progeroid and lipodystrophy syndrome. PLoS One. 2015;10(7):e0131797. doi: 10.1371/journal.pone.0131797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han B., Copeland C.A., Kawano Y., et al. Characterization of a caveolin-1 mutation associated with both pulmonary arterial hypertension and congenital generalized lipodystrophy. Traffic. 2016;17(12):1297–1312. doi: 10.1111/tra.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jelani M., Ahmed S., Almramhi M.M., et al. Novel nonsense mutation in the PTRF gene underlies congenital generalized lipodystrophy in a consanguineous Saudi family. Eur J Med Genet. 2015;58(4):216–221. doi: 10.1016/j.ejmg.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 72.Murakami N., Hayashi Y.K., Oto Y., et al. Congenital generalized lipodystrophy type 4 with muscular dystrophy: clinical and pathological manifestations in early childhood. Neuromuscul Disord. 2013;23(5):441–444. doi: 10.1016/j.nmd.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 73.Salle-Teyssières L., Auclair M., Terro F., et al. Maladaptative autophagy impairs adipose function in congenital generalized lipodystrophy due to Cavin-1 deficiency. J Clin Endocrinol Metab. 2016;101(7):2892–2904. doi: 10.1210/jc.2016-1086. [DOI] [PubMed] [Google Scholar]
- 74.Ardissone A., Bragato C., Caffi L., et al. Novel PTRF mutation in a child with mild myopathy and very mild congenital lipodystrophy. BMC Med Genet. 2013;14:89. doi: 10.1186/1471-2350-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rajab A., Straub V., McCann L.J., et al. Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations. PLoS Genet. 2010;6(3):e1000874. doi: 10.1371/journal.pgen.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akinci G., Topaloglu H., Akinci B., et al. Spectrum of clinical manifestations in two young Turkish patients with congenital generalized lipodystrophy type 4. Eur J Med Genet. 2016;59(6–7):320–324. doi: 10.1016/j.ejmg.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Payne F., Lim K., Girousse A., et al. Mutations disrupting the Kennedy phosphatidylcholine pathway in humans with congenital lipodystrophy and fatty liver disease. Proc Natl Acad Sci U S A. 2014;111(24):8901–8906. doi: 10.1073/pnas.1408523111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Testa F., Filippelli M., Brunetti-Pierri R., et al. Mutations in the PCYT1A gene are responsible for isolated forms of retinal dystrophy. Eur J Hum Genet. 2017;25(5):651–655. doi: 10.1038/ejhg.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Francis G.A., Li G., Casey R., et al. Peroxisomal proliferator activated receptor-gamma deficiency in a Canadian kindred with familial partial lipodystrophy type 3 (FPLD3) BMC Med Genet. 2006;7:3. doi: 10.1186/1471-2350-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miehle K., Porrmann J., Mitter D., et al. Novel peroxisome proliferator-activated receptor gamma mutation in a family with familial partial lipodystrophy type 3. Clin Endocrinol (Oxf) 2016;84(1):141–148. doi: 10.1111/cen.12837. [DOI] [PubMed] [Google Scholar]
- 81.Dyment D.A., Gibson W.T., Huang L., Bassyouni H., Hegele R.A., Innes A.M. Biallelic mutations at PPARG cause a congenital, generalized lipodystrophy similar to the Berardinelli-Seip syndrome. Eur J Med Genet. 2014;57(9):524–526. doi: 10.1016/j.ejmg.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 82.Kozusko K., Tsang V., Bottomley W., et al. Clinical and molecular characterization of a novel PLIN1 frameshift mutation identified in patients with familial partial lipodystrophy. Diabetes. 2015;64(1):299–310. doi: 10.2337/db14-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jéru I., Vantyghem M.C., Bismuth E., et al. Diagnostic challenge in PLIN1-associated familial partial lipodystrophy. J Clin Endocrinol Metab. 2019;104(12):6025–6032. doi: 10.1210/jc.2019-00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen R.X., Zhang L., Ye W., et al. The renal manifestations of type 4 familial partial lipodystrophy: a case report and review of literature. BMC Nephrol. 2018;19(1):111. doi: 10.1186/s12882-018-0913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rubio-Cabezas O., Puri V., Murano I., et al. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med. 2009;1(5):280–287. doi: 10.1002/emmm.200900037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sollier C., Capel E., Aguilhon C., et al. LIPE-related lipodystrophic syndrome: clinical features and disease modeling using adipose stem cells. Eur J Endocrinol. 2021;184(1):155–168. doi: 10.1530/EJE-20-1013. [DOI] [PubMed] [Google Scholar]
- 87.Chaudhary H., Panigrihi I., Bhatia P. Oil Red-O Positive lipid blobs on peripheral blood film examination in a muscular infant with the diagnosis of Berardinelli-Seip syndrome. Oxf Med Case Reports. 2019;2019(7):omz062. doi: 10.1093/omcr/omz062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gomes K.B., Pardini V.C., Fernandes A.P. Clinical and molecular aspects of Berardinelli-Seip congenital lipodystrophy (BSCL) Clin Chim Acta. 2009;402(1–2):1–6. doi: 10.1016/j.cca.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 89.Van Maldergem L. In: GeneReviews(®) Adam M.P., Ardinger H.H., Pagon R.A., et al., editors. University of Washington, Seattle,Copyright © 1993-2020; Seattle (WA): 1993. Berardinelli-seip congenital lipodystrophy. [PubMed] [Google Scholar]
- 90.Rostami P., Nakhaeimoghadam M., Bijani F.M., et al. AGPAT2 gene mutation in a child with Berardinelli-Seip congenital lipodystrophy syndrome. Ann Endocrinol (Paris) 2013;74(1):59–61. doi: 10.1016/j.ando.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 91.Liu Y., Li D., Ding Y., et al. Further delineation of AGPAT2 and BSCL2 related congenital generalized lipodystrophy in young infants. Eur J Med Genet. 2019;62(9):103542. doi: 10.1016/j.ejmg.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 92.Roth T., Nair S., Kumar A. Monogenic diabetes secondary to congenital lipodystrophy in a 14-year-old Yemeni girl. J Clin Res Pediatr Endocrinol. 2010;2(4):176–179. doi: 10.4274/jcrpe.v2i4.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vatier C., Vantyghem M.C., Storey C., et al. Monogenic forms of lipodystrophic syndromes: diagnosis, detection, and practical management considerations from clinical cases. Curr Med Res Opin. 2019;35(3):543–552. doi: 10.1080/03007995.2018.1533459. [DOI] [PubMed] [Google Scholar]
- 94.Maeda M., Maeda T., Ebihara K., Ihara K. The long-term management of congenital generalized lipodystrophy (Berardinelli-Seip syndrome): the clinical manifestations of Japanese siblings for approximately 20 years. Clin Pediatr Endocrinol. 2019;28(4):139–145. doi: 10.1297/cpe.28.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schuster J., Khan T.N., Tariq M., et al. Exome sequencing circumvents missing clinical data and identifies a BSCL2 mutation in congenital lipodystrophy. BMC Med Genet. 2014;15:71. doi: 10.1186/1471-2350-15-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sassi R., Bond R.R., Cairns A., et al. PDF-ECG in clinical practice: a model for long-term preservation of digital 12-lead ECG data. J Electrocardiol. 2017;50(6):776–780. doi: 10.1016/j.jelectrocard.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 97.Friguls B., Coroleu W., del Alcazar R., Hilbert P., Van Maldergem L., Pintos-Morell G. Severe cardiac phenotype of Berardinelli-Seip congenital lipodystrophy in an infant with homozygous E189X BSCL2 mutation. Eur J Med Genet. 2009;52(1):14–16. doi: 10.1016/j.ejmg.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 98.Semple R.K., Williams R.M., Dunger D.B. What is the best management strategy for patients with severe insulin resistance? Clin Endocrinol (Oxf) 2010;73(3):286–290. doi: 10.1111/j.1365-2265.2010.03810.x. [DOI] [PubMed] [Google Scholar]
- 99.Madhra M., Noh R.M., Zammitt N.N., Patrick A.W., Love C.D. A complicated pregnancy in a patient with lipodystrophic diabetes attributable to a peroxisome proliferator-activated receptor gamma (PPARG) mutation. Diabet Med. 2012;29(10):e398–e401. doi: 10.1111/j.1464-5491.2012.03742.x. [DOI] [PubMed] [Google Scholar]
- 100.Victoria B., Cabezas-Agrícola J.M., González-Méndez B., et al. Reduced adipogenic gene expression in fibroblasts from a patient with type 2 congenital generalized lipodystrophy. Diabet Med. 2010;27(10):1178–1187. doi: 10.1111/j.1464-5491.2010.03052.x. [DOI] [PubMed] [Google Scholar]
- 101.Mughal A., Kumar D., Vikram A. Effects of Thiazolidinediones on metabolism and cancer: relative influence of PPARγ and IGF-1 signaling. Eur J Pharmacol. 2015;768:217–225. doi: 10.1016/j.ejphar.2015.10.057. [DOI] [PubMed] [Google Scholar]
- 102.Demir T., Onay H., Savage D.B., et al. Familial partial lipodystrophy linked to a novel peroxisome proliferator activator receptor -γ (PPARG) mutation, H449L: a comparison of people with this mutation and those with classic codon 482 Lamin A/C (LMNA) mutations. Diabet Med. 2016;33(10):1445–1450. doi: 10.1111/dme.13061. [DOI] [PubMed] [Google Scholar]
- 103.Moreira R.O., Zagury R.L., Nascimento T.S., Zagury L. Multidrug therapy in a patient with Rabson-Mendenhall syndrome. Diabetologia. 2010;53(11):2454–2455. doi: 10.1007/s00125-010-1879-5. [DOI] [PubMed] [Google Scholar]
- 104.McDonald A., Williams R.M., Regan F.M., Semple R.K., Dunger D.B. IGF-I treatment of insulin resistance. Eur J Endocrinol. 2007;157(Suppl 1):S51–S56. doi: 10.1530/EJE-07-0271. [DOI] [PubMed] [Google Scholar]