Abstract
In recent years, cardiovascular health problems are becoming more and more serious. At the same time, mechanical stimulation closely relates to cardiovascular health. In this context, Piezo1, which is very sensitive to mechanical stimulation, has attracted our attention. Here, we review the critical significance of Piezo1 in mechanical stimulation of endothelial cells, NO production, lipid metabolism, DNA damage protection, the development of new blood vessels and maturation, narrowing of blood vessels, blood pressure regulation, vascular permeability, insulin sensitivity, and maintenance of red blood cell function. Besides, Piezo1 may participate in the occurrence and development of atherosclerosis, diabetes, hypertension, and other cardiovascular diseases. It is worth noting that Piezo1 has dual effects on maintaining cardiovascular health. On the one hand, the function of Piezo1 is necessary to maintain cardiovascular health; on the other hand, under some extreme mechanical stimulation, the overexpression of Piezo1 may bring adverse factors such as inflammation. Therefore, this review discusses the Janus-faced role of Piezo1 in maintaining cardiovascular health and puts forward new ideas to provide references for gene therapy or nanoagents targeting Piezo1.
Keywords: Atherosclerosis, Cardiovascular disease, Endothelial cells (ECs), Mechanical stimulation, Piezo1
Introduction
As the heart beats, it pumps blood through a blood vessel system to the whole body. Along with this process, there are various kinds of mechanical stimulation. These mechanical stimulations include shear stress and stretching, which are closely related to cardiovascular health. In blood vessels, the significant changes in hemodynamics are related to vascular diseases such as atherosclerosis and promote endothelial cells (ECs) proliferation and apoptosis.1 At the same time, in the heart, myocardial cells need to adapt to these mechanical stimuli to ensure the systemic circulation of blood.2 Under the mechanical stimulation of fluid or membrane stretching, the opening of mechanosensitive ion channels on the cytoplasmic membrane is the earliest cellular event. These mechanosensitive ion channels have different structures, ionic selectivity, and gating properties.3, 4, 5, 6, 7, 8, 9 Trimeric Piezo1 cation channel is one of the most important ones. It is non-selective and permeable to Na+, K+ and Ca2+ and their activation in response to pressure stimulation or shear stress induces cell depolarization and Ca2+ influx.6,10, 11, 12 In this paper, we focused on the role of Piezo1 under mechanical stimulation.
In the ordinary physiological environment, Piezo1 can protect the growth and development of blood vessels and bones after mechanical stimulation,12, 13, 14, 15 which indicates that Piezo1 contributes to the maintenance of cardiovascular health. However, under turbulence and high pressure, Piezo1 may cause inflammation,16, 17, 18, 19 endothelial barrier dysfunction,20 thrombosis,21 red blood cell dysfunction22 and other adverse reactions. These pathological environments change the mechanical setting of the cardiovascular system, change the expression of Piezo1, and aggravate the adverse state of the cardiovascular system. Accordingly, the change of Piezo1 expression may affect some chronic cardiovascular diseases, such as diabetes.23
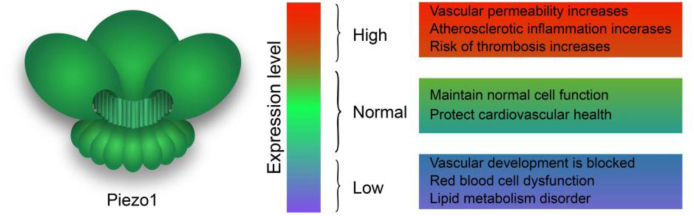
Therefore, we propose a new view on the influence of Piezo1 on cardiovascular health: the existence of Piezo1 is an essential part of maintaining our health, but in special cases, the high expression of Piezo1 will also bring us adverse effects. In other words, the expression level of Piezo1 has a corresponding threshold, when the expression level of Piezo1 is within the safe range of the threshold, it is conducive to our health; on the contrary, when the expression level of Piezo1 is above or below the threshold, Piezo1 may bring disease (Fig. 1). So, what benefits and disadvantages does Piezo1 get to cardiovascular health under mechanical stimulation? We will introduce them one by one below.
Figure 1.
Threshold of Piezo1.
Structure and activation pathway of Piezo1
The structure of molecules determines their functions, properties and affects their distribution in organs, tissues and cells. Piezo1 is expressed in endothelial cells and smooth muscle cells (SMCs), not only in cardiovascular but also in the kidney, skeletal muscle, and pancreas.24 Piezo1 is giant protein (human Piezo1 has 2521 amino acids), each subunit has multiple (>14) predicted transmembrane domains and has no homology with other known proteins.10 The characteristic of the Piezo1 ion channel is that it has a rapid inactivation mechanism. Specifically, while maintaining the mechanical stimulation, the amplitude of Piezo current decreases with time.10 Piezo1 transmits mechanical stimulation between ECs and SMCs and plays the role of a mechanical sensor. Moreover, Piezo1 is responsible for mechanosensitivity in osteoblasts.25
Its structure determines the mechanical sensitivity of Piezo1. When the purified Piezo1 channel was reconstituted into bilayers, the mechanosensitivity was observed, indicating that the channel was a pore-forming subunit,26 which was directly activated by increasing membrane tension.27 The N-terminal domain of Piezo1 is considered to form a mechanical sensor. In contrast, the C-terminal domain constitutes an ion pore composed of the last transmembrane fragment (inner helix), C-terminal extracellular domain, and intracellular C-terminal domain.28, 29, 30, 31 Piezo1 is curved, and its N-terminal domain is similar to the three blades of a propeller and the ion hole on the central axis.32 The N-terminal domain of Piezo1 consists of nine repeats, each of which contains four transmembrane fragments. The conformational changes of the N-terminal domain induced by mechanical stimulation can be transferred to the central pore by reducing the angle between the intracellular helix and the membrane plane outside the channel.33 The main determinant of rapid inactivation of Piezo1 is the hydrophobic gate in the inner pore helix.34 The unstructured region near the beam may play a role in blocking the cytoplasmic fluid, which may restrict permeation by blocking the inner pore of Piezo1.35 In some cell types, including ECs, the native Piezo1 current is slowly inactivated.36,37 A new study shows that sphingophospholipase activity, ceramide, and sphingomyelin are the determinants of natural Piezo gating.38
Therefore, the expression of Piezo1 is related to the sustained activity and rapid inactivation of Piezo1, and its open state may depend on the type of mechanical stimulation and the regulation of its upstream and downstream factors. At present, the reported factors involved in Piezo1 regulation (Table 1) are: sphingomyelin,38 acidification (pk6.9),39 phosphatidylinositol 4-phosphate,40 sphingosine-1-phosphate through Src-dependent phosphorylation,41 fatty acid,42 phosphatidylserine,43 stomatin-like protein-3 (cholesterol and erythrocyte membrane integrin 3),44, 45, 46 β-amyloid peptide,47 polycystin-2,48 trefoil factor family 1,49 CD31 (PECAM-1) and VE-cadherin,50 sarcoplasmic/endoplasmic reticulum calcium ATPase 2,51 spectrin and hyaluronic acid.52
Table 1.
The upstream regulation factors of Piezo1.
| Species | Cell types | In vivo/vitro | |
|---|---|---|---|
| Sphingomyelin38 | Mouse | ECs | In vitro |
| Acidification (pk6.9)39 | Human | HEK293T cells | In vitro |
| Phosphatidylinositol 4-phosphate40 | Mouse | DRG neurons | In vitro |
| Sphingosine-1-phosphate through Src-dependent phosphorylation41 | Mouse | ECs | In vivo |
| Fatty acid42 | Mouse | N2A cells | In vitro |
| Phosphatidylserine43 | Mouse, Human | Myoblasts N2A cells, | In vitro |
| Stomatin-like protein-344−46 | Mouse | DRG neurons | In vitro |
| β-Amyloid peptide47 | Human | HEK293T cells | In vitro |
| Polycystin-248 | Monkey | COS-7 cells | In vitro |
| Trefoil factor family-149 | Human | Gastric cancer cells | In vitro |
| CD31 and VE-cadherin50 sarcoplasmic/endoplasmic | Monkey | COS-7 cells | In vitro |
| Reticulum calcium ATPase-251 | Human | HEK293T cells | In vitro |
| Spectrin and hyaluronic acid52 | Human | ECs | In vitro |
The downstream pathways of Piezo1 (Table 2) include: activation of β1-integrin,53,54 activation of calpain,12,20,53,55,56 phosphorylation of nitric oxide synthase,12, 13, 14 release of ATP,13,22,57 release of adrenomedullin,14 activation of P2Y2 receptor,14 activation of G protein (αq/11 and Gs),13,14 tyrosine phosphorylation of CD31,14 ADAM10 and Notch signaling pathways,58 AKT (protein kinase B) phosphorylation,14 Src phosphorylation,13,55 Ca2+/calmodulin-dependent protein kinase II phosphorylation,59,60 VEGFR2 (vascular endothelial growth factor receptor 2) tyrosine phosphorylation,14 NF-κB (nuclear factor κ-light chain enhancer of activated B cells) phosphorylation and nuclear translocation,61 focal adhesion kinase activation,12,61 VE-cadherin internalization and degradation,20,50,55,62 activation of matrix metalloproteinase-2 and membrane type matrix metalloproteinase-1,41 activation of p38 kinase,63 transcriptional activation of YAP1 (Yes-associated protein 1),64 activation of KLF2 (Krüppel-like factor 2),64 phosphorylation of ERK1/2 (extracellular regulated protein kinases 1/2),65 transcriptional activation of cyclin B,65 enhance the activity of Na + -Ca2+ exchanger 1,66 activate phosphodiesterase 1 and protein kinase A,67 regulate the transcription of AP-1 (activator protein 1),68 mediate the regulation of hypoxia-inducible factor 1α by endothelin-1,68 regulate the static correlation between CD31 and G protein (αq/11),69 regulate F-actin stress tissue62 and nuclear contraction.70 Next, we focus on the role of Piezo1 in response to mechanical stimulation and related pathways.
Table 2.
The downstream pathways of Piezo1.
| Species | Cell types | In vivo/vitro | |
|---|---|---|---|
| β1-integrin53,54 | Hamster, Human | CHO cells, 16HBE cells | In vitro |
| calpain12,20,53,55,56 | Mouse, Hamster, Rat | ECs, CHO cells, PC12 cells | In vitro and in vivo |
| phosphorylation of nitric oxide synthase12, 13, 14 | Mouse, Human | ECs | In vitro and in vivo |
| release of ATP13,22,57 | Mouse, Human | Cs, Urothelial cells, red blood cells | In vitro and in vivo |
| adrenomedullin and P2Y2 receptor | Mouse, Human | ECs | In vitro and in vivo |
| G protein (αq/11 and Gs)13,14 | Mouse, Human | ECs | In vitro and in vivo |
| yrosine phosphorylation of CD3114 | Mouse, Human | ECs | In vitro and in vivo |
| ADAM10 and Notch signaling pathways58 | Human | ECs | In vitro |
| AKT phosphorylation14 | Mouse, Human | ECs | In vitro and in vivo |
| Src phosphorylation13,55 | Mouse | ECs | In vitro and in vivo |
| Ca2+/calmodulin-dependent protein kinase II phosphorylation59,60 | Drosophila, Mouse | Sensory neurons, Osteoblasts | In vitro and in vivo |
| VEGFR2 tyrosine phosphorylation14 | Mouse, Human | ECs | In vitro and in vivo |
| NF-κB phosphorylation and nuclear translocation61 | Mouse, Human | ECs | In vitro and in vivo |
| focal adhesion kinase12,61 | Mouse, Human | ECs | In vitro and in vivo |
| VE-cadherin internalization and degradation20,50,55,62 | Mouse, Monkey | Cs, COS-7 cells | In vitro and in vivo |
| matrix metalloproteinase-2 and membrane type matrix metalloproteinase-141 | Mouse | ECs | In vivo |
| p38 kinase63 | Mouse, Human | Fibroblasts, ECs | In vitro and in vivo |
| YAP1 and KLF264 | Zebrafish | ECs | In vivo |
| phosphorylation of ERK1/2 and cyclin B65 | Dog | MDCK II cells | In vitro |
| Na+-Ca2+ exchanger 166 | Rat | Cajal-like cells | In vivo |
| phosphodiesterase 1 and protein kinase A67 | Hamster, Human | CHO cells, A375-SM cells | In vitro |
| AP-1and mediate the regulation of hypoxia-inducible factor 1α by endothelin-168 | Mouse | Myeloid cells | In vivo |
| egulate the static correlation between CD31 and G protein (αq/11)69 | Human | ECs | In vitro |
| F-actin62 | Mouse | ECs | In vivo |
| nuclear contraction70 | Dog | MDCK cells | In vitro |
The effect of Piezo1 on cardiovascular health under mechanical stimulation
Piezo1 induces mechanical stimulation to promote vascular maturation, which is necessary for embryonic development
Piezo1 is a key endothelial mechanical sensor for early vascular development. In mice, the expression of Piezo1 can be traced back to E9 to 10.5 days of embryonic development. Piezo1 is expressed in the developing cardiovascular system, especially in the endothelium and endocardium. The constitutive, genetic invalidation of Piezo1 is fatal in embryos. The defect of Piezo1 will make the mouse embryo die in the second trimester of pregnancy, but it is worth noting that the cardiovascular generation of mice is normal at this time.11,12 The original picture of these mice dying was that the newly formed vascular plexus did not develop normally. However, in this process, the structure and heartbeat of the heart are not affected, and the loss of Piezo1 leads to the loss of cell perception, which makes the neovascularization unable to mature normally.12 At the same time, the researchers also found that Piezo1 transfection in HEK 293 cells confers sensitivity to shear stress.12 Other studies have found that the opening of Piezo1 and the resulting Ca2+ influx can lead to the activation of MMP-2 (matrix metalloproteinase-2) and MT1MMP (membrane-type matrix metalloproteinase-1) in ECs, thus leading to the germination of ECs.41
In addition to its embryonic expression, Piezo1 also persisted in ECs of adult mice and SMCs of resistant arteries.71 In addition, Piezo1 also exists in the human umbilical vein and fetal placenta ECs.12,72,73 Similarly, in adult animals, conditional deletion of Piezo1 in the endothelium impairs angiogenesis in the event of trauma or hindlimb ischemia.41 The above studies show that Piezo1 has a necessary role in angiogenesis, which is a great contribution of Piezo1 to heart blood health.
Endothelial Piezo1 mediates flow dependent vasodilation and atherosclerosis protection
The endothelium directly contacts blood (including platelets, white blood cells, and various proteins in the blood environment) and represents a barrier to maintain physiological vessel morphology and normal function.74 Therefore, the normal function of ECs is of great significance for the stability of the vascular system.
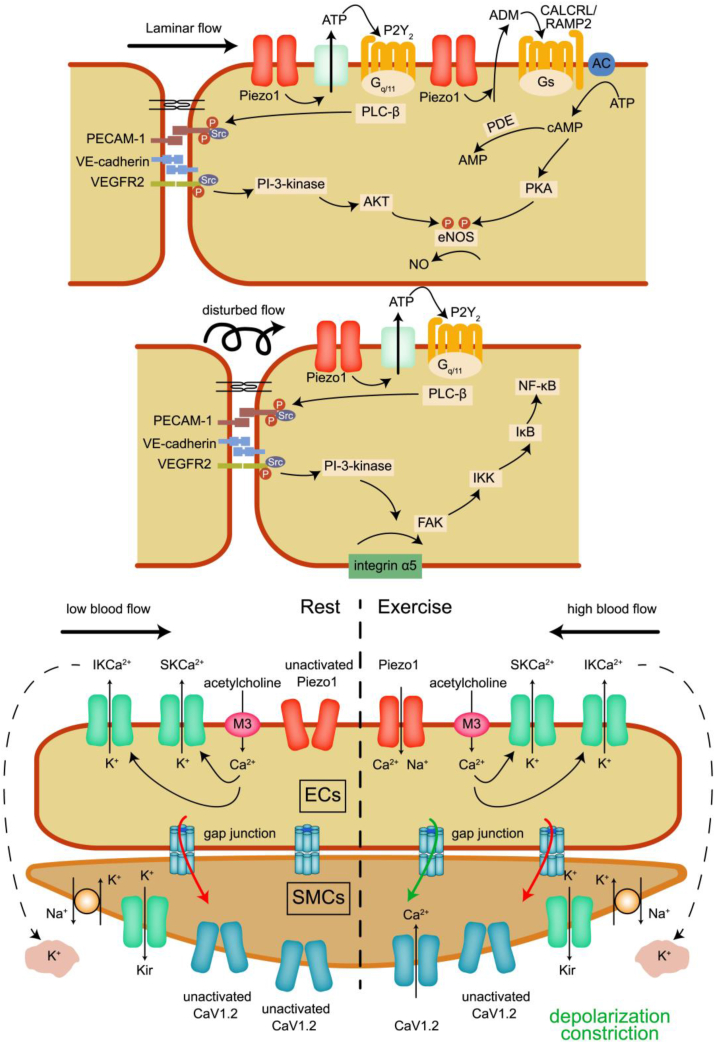
As early as 1982, researchers found NO was an essential endothelium relaxing factor. Recent studies have shown that the expression of Piezo1 plays a vital role in the release of NO from ECs. The shear-activated part of Piezo1 in the mesenteric artery releases ATP from ECs through the pannexin channel. ECs then stimulate Gq/11 mediated activation of mechanosensitive complexes CD31, VE-cadherin, and VEGFR2 downstream of P2Y2 purinergic receptor in an autocrine manner. PI3K (phosphoinositide-3-kinase)/AKT enhances the phosphorylation of eNOS (endothelial nitric oxide synthase) at Ser1176, leading to NO production.13 In the synergistic effect, the increased flow induced the release of Piezo1 dependent adrenomedullin from the endothelium in an undetermined way, resulting in PKA (protein kinase A) downstream Gs-coupled CALCRL (calcitonin receptor like receptor)/RAMP2 (receptor activity modifying protein 2) receptor phosphorylating eNOS at Ser632, thereby further increasing the NO level14). Elevated NO levels, in turn, mediate vasodilation. In normal coronary arteries, blood flow is laminar.75 NO release was boosted by Piezo1 with mechanical stimulation and thereby protecting the blood vessels (Fig. 2A).
Figure 2.
The role of Piezo1 upon mechanical stimulation.14,32,37,61 Piezo1 promotes NO release under laminar flow; Piezo1 regulates the release of NF-κB under disturbed flow; Piezo1 mediates vasoconstriction during exercise.
There are a lot of relevant studies to support this pathway. For example, in adult mice, conditional gene knockout of Piezo1 can prevent flow or Yoda1 induced vasodilation, resulting in decreased phosphorylation of eNOS and reduced NO release.13 The deletion of P2Y2, Gq/11, or pannexin 1/2 in ECs had similar results.13 Besides, mice with endothelium-specific adrenomedullin, adrenomedullin receptor, or Gαs failure also showed that reduced NO release to shear stress, impaired flow-dependent vasodilation, and hypertension.14 In rats, Piezo1 also mediates flow-dependent vasodilation of uterine arteries during pregnancy.76 Finally, miRNA-103a inhibits endothelial Piezo1 and participates in acute myocardial infarction by affecting endothelial function.77
Furthermore, Piezo1 mediated calcium influx is significant for increasing calpain activity and the subsequent rearrangement of local adhesion. Shear stress activates the endothelial Piezo1 channel and downstream Ca2+-dependent calpain, responsible for local adhesion turnover, proteolysis of actin cytoskeleton elements, and arrangement of ECs and blood flow. ECs extracted from Piezo1 knockout mice were not aligned with the flow direction when exposed to atherosclerotic protective flow.26 Whether Piezo1 gene knockout or GsMTx4 inhibition of Piezo1 can inhibit shear stress-induced calcium influx in ECs, resulting in the arrangement of ECs that cannot align with the direction of blood flow.11,12 When the rearrangement of vascular ECs is disordered, the risk of atherosclerosis will increase.
In conclusion, Piezo1 in ECs can be activated under mechanical stimulation, which promotes ECs to produce NO, regulates blood pressure, and prevents atherosclerosis.
Turbulence activated Piezo1 promotes inflammation of atherosclerotic ECs
Blood flow in blood vessels includes laminar flow and turbulent flow. The above-mentioned mechanical stimulation causes Piezo1 to open. It plays a protective role in atherosclerosis through the activation of eNOS and the release of NO, which is in laminar flow condition. However, in the inner curvature of the aortic arch, ECs are affected by turbulence, and the activation of Piezo1 can lead to endothelial inflammation and atherosclerosis.16, 17, 18, 19 At present, researchers have observed that the aggregation of inflammatory ECs reduced after the conditional deletion of endothelial Piezo1. Also, endothelial deletion of Piezo1 or Gq/11 attenuates endothelial inflammation in mice that lack LDL (low-density lipoprotein) receptors and fed a high-fat diet. The activation of integrin α5 and focal adhesion kinase is enhanced by a Piezo1 Gq/11 dependent pathway, leading to the stimulation of the NF-κB path and the expression of Pro atherosclerotic genes. In conclusion, these findings suggest that the activation of Piezo1 by laminar flow is the protective effect of eNOS on atherosclerosis, while turbulence promotes atherosclerosis through the NF-κB pathway (Fig. 2B).61 A new study shows that these pathological phenomena need to be triggered by Piezo1, but with the participation of transient receptor potential vanilloid subfamily 4.78
Here, we summarize the physiological response of Piezo1 in a laminar or turbulent flow. We can conclude: under laminar flow, the expression level of Piezo1 is within the safe range of its threshold, which can play a protective role in atherosclerosis; under turbulent flow, the expression level of Piezo1 will be higher than the threshold, which will cause vascular inflammation reaction and bring adverse effects on cardiovascular health.
Piezo1 mediates exercise-induced vasoconstriction in ECs to maintain blood pressure homeostasis
At rest, the opening of SKCa2+ and IKCa2+ channels in the endothelium induces hyperpolarization of electrically coupled SMCs through gap junctions. At the same time, K+ efflux also activated the Kir channel and/or stimulated Na+/K+ ATPase of SMCs, which also contributed to hyperpolarization and relaxation. The resulting resting hyperpolarization, known as EDH (endothelium-dependent hyperpolarization), can lead to relaxation. Dysfunction of EDH involves various disease states, including hypertension. Various roles acting on EDH are increasingly considered possible therapeutic targets.79
It is generally accepted that life lies in movement. Simultaneously, the research shows that physical activity and other forms of physical exercise provide the primary protection for chronic diseases.80,81 Physical exercise plays a positive role in maintaining cardiovascular health. But in the process of physical exercise, how does blood pressure maintain dynamic stability? High blood flow induced by physical exercise increases the opening of Piezo1, increases the influx of Na+ and Ca2+, and leads to depolarization of mesenteric artery ECs. Depolarization is transmitted to the electrically coupled SMCs through gap junctions and triggers vasoconstriction by opening the Cav1.2 channel.37
The researchers found that in the mesenteric arteries of mice lacking endothelial Piezo1, EDH, which can be prevented by a mixture of K+ channel inhibitor apamin and Chamberlain toxin, was amplified. In addition, when mice lacking endothelial Piezo1 were active on the running wheel, their elevated blood pressure was lower than that of the control group. Therefore, endothelial cell Piezo1 plays a role in increasing hypertension during exercise, and it plays an anti-EDH role through the opening of high blood flow in the endothelium. Endothelial Piezo1 to flow-induced depolarization/contraction seems to be unique to the mesenteric circulation, as the EDH mediated response is not amplified by Piezo1 invalidation in the saphenous or carotid arteries (Fig. 2C).37
In conclusion, the presence of Piezo1 is essential for vasoconstriction during physical exercise, which maintains the dynamic stability of blood pressure.
Basal hardness regulates the expression of Piezo1 and affects myocardial health
Besides shear stress, substrate hardness also affected Piezo1 expression. When culturing human cardiac fibroblasts with Piezo1 siRNA on softer collagen-covered BioFlex plates, the researchers found that the expression of IL-6 (interleukin-6) decreased. However, when the researchers used a conventional rigid plastic tissue culture plate to culture human cardiac fibroblasts with Piezo1 siRNA, the expression of IL-6 did not change. These results suggest that Piezo1 may play a role in detecting the change of substrate hardness or composition and regulate the expression of IL-6 accordingly. Besides, this study also confirmed that Piezo1 could participate in myocardial remodeling.15 In conclusion, the presence of Piezo1 is essential for vasoconstriction during physical exercise, which maintains the dynamic stability of blood pressure.
Cardiac fibroblasts play an essential role in normal physiology and response to injury or stress. The changes in environmental hardness and mechanical stretch of cardiac fibroblasts secondary to fibrosis, cardiac dilatation, or cardiac hemodynamic load induce fibroblasts to differentiate into myofibroblasts and increase the expression of fibrogenic cytokines, ECM (extracellular matrix) 5 protein, and ECM receptor.82, 83, 84 This can protect the integrity and performance of the heart, but the reaction may become inadaptable; excessive deposition of cardiac ECM can lead to fibrosis, thus increasing the hardness of the myocardium and reducing the pumping capacity.85 Due to the role of Piezo1, it is indispensable to maintain myocardial remodeling. Therefore, the normal expression of Piezo1 plays an important role in maintaining heart health.
In another study, researchers used an array of elastic columns as a force sensor to investigate whether the mechanical properties of the substrate can independently modulate piezoelectric activity. They found that Piezo1 was more sensitive to the deflection of the substrate but less sensitive to the change of the hardness of the substrate when the elastic column spacing increased (i.e., the surface roughness decreased). Therefore, the sensitivity of Piezo1 to the substrate depends more on the substrate tension.86
Besides the hardness of the substrate, the extracellular matrix also affects the activity of Piezo1. When analyzing Piezo1 by atomic force microscopy, the researchers found that Piezo1 only responded to higher pushing forces when there was no ECM protein. However, when adding Matrigel to cells, the force intensity required to activate Piezo1 is significantly reduced. Matrigel is a mixture of different ECM proteins, including laminin, collagen, and proteoglycan. These results indicate that the mechanical sensitivity of Piezo1 is closely related to the presence of ECM protein.87
These results indicate that substrate hardness and extracellular matrix are essential factors affecting Piezo1 mechanical sensitivity. Its sensitivity determines the expression level of Piezo1, which has a significant impact on cardiovascular health.
Piezo1 channel mediates heart mechano-chemo transduction
Every beat of the heart experiences dramatic mechanical changes. A new study shows that myocardial cells convert mechanical stretch into Ca2+ and ROS (reactive oxygen species) signals through Piezo1, which determines the mechanical activity of the heart. On the one hand, Piezo1 mediated Ca2+ influx may directly promote cardiac Ca2+ homeostasis through the Ca2+-induced Ca2+ release mechanism. On the other hand, stretch-induced Ca2+ sparks are mediated by a mechano-chemo signaling pathway termed X-ROS signaling, which involves stretch-activation of NADPH (nicotinamide adenine dinucleotide phosphate) oxidase 2 via Rac1-dependent activation of microtubules, leading to the production of ROS that in turn modulates the activity of RyR2. In addition, the researchers found that the loss of cardiac specificity of Piezo1 in mice can damage cardiac function. Notably, heart failure and arrhythmia were observed in mice with cardiac-specific overexpression of Piezo1. Piezo1 is pathologically upregulated in both diseased mouse and human hearts via an autonomic response of cardiomyocytes.88
Through this study, we can conclude. When the expression of Piezo1 is insufficient, the heart function is not complete; but when Piezo1 is overexpressed, the risk of heart disease increased. Piezo1 is essential in the maintenance of cardiac function, and there is a threshold for Piezo1.
The performance of Piezo1's merits and demerits in common cardiovascular diseases
In addition to responding to mechanical stimulation, Piezo1 is also closely related to the development of many cardiovascular diseases. In the process of participating in these lesions, some factors of mechanical stimulation are not clear, so they are introduced separately here.
Piezo1 and hypertension
Piezo1 increases vascular permeability in patients with hypertension
In the case of hypertension, the function of Piezo1 also has two sides. Previous studies have shown that conditional endothelial specific Piezo1 knockout or GsMTx4 inhibition of Piezo1 induced serum leakage can be eliminated when the pulmonary microvascular pressure increases due to left atrial pressure or aortic contraction, which indicates that pressure induced Piezo1 opening in pulmonary microvascular ECs mediates the destruction of endothelial barrier.20 The pharmacological inhibition of calpain mimics the protective effect of Piezo1 failure,20 which indicates that the opening of Piezo1 leads to calpain activation, followed by degradation of the adherent connexin and disintegration of the endothelial barrier. Pulmonary edema usually occurs at high altitudes and also occurs after heart failure and head trauma. It is due to the destruction of the pulmonary capillary endothelial barrier due to the increase of hydrostatic pressure, resulting in plasma leakage to the lung parenchyma.89 These findings suggest that pharmacological inhibition of Piezo1 may be beneficial in the case of pulmonary edema. This also indicates that high level expression of Piezo1 in blood vessels under mechanical stimulation of hypertension may have negative effects on cardiovascular health.
Piezo1 participates in vascular remodeling after hypertensive injury
However, Piezo1 is involved in the increase of vascular permeability and the remodeling of vascular injury caused by hypertension. The study shows that the increased openness of Piezo1 has a nutritional effect on the resistant artery and has an impact on the inner diameter and wall thickness of patients with hypertension. Piezo1 mediates the increase of intracellular calcium and stimulates the activity of transglutaminase, which is a cross-linking enzyme required for arteriolar remodeling. Therefore, Piezo1 plays an essential role in vascular remodeling induced by hypertension.70 This study shows that Piezo1 also made a positive contribution to the pathological environment of hypertension. These phenomena once again suggest that there may be a safety threshold for Piezo1 expression.
Relationship between Piezo1 and diabetes and obesity
Deletion or mutation of Piezo1 may cause diabetes
Diabetes mellitus is a risk factor of susceptibility to vascular diseases.90 Although the hemodynamics of the diabetic artery are still unclear, many reports confirm the relationship between Piezo1 and diabetes. When the researchers studied white adipose tissue (WAT), they found that Piezo1 highly expressed in mature WAT. Therefore, the researchers speculated that Piezo1 mediated mechanical signal transduction is involved in the function and remodelling of adipose tissue, and verified it. The opening of Piezo1 in mature adipocytes resulted in the release of FGF1 (fibroblast growth factor 1), which induced adipocyte precursor differentiation by activating FGF receptor 1. This process is necessary for adipocyte maturation. When mice with Piezo1 deficiency were fed with high-fat diet, the differentiation defect of preadipocytes into mature adipocytes would appear, which would lead to the enlargement of adipocytes, the increase of WAT inflammation and the decrease of insulin sensitivity.23 In addition, in a recently reported case of diabetes mellitus with Piezo1 gene mutation, the level of glycosylated hemoglobin decreased, the level of glycosylated albumin was higher, and the levels of hemoglobin and albumin were normal.91
Deletion of Piezo1 may lead to overeating and obesity
As we all know, obesity is an adverse factor affecting cardiovascular health. Overeating is also one of the important factors leading to obesity. External sensory cues and internal metabolism control the feeding of animals. A recent study has shown that Piezo+ neurons in the PI (pars intercerebralis) are directly activated by the expansion of the Drosophila crop (equivalent to stomach), thus transmitting fast satiety signals along the “brain gut axis” to control food intake. When Piezo1 is knocked out or deleted due to mutation, these flies will over eat. The expression of fly Piezo or mammalian Piezo1 in the neurons of these Piezo1 deficient mutants can inhibit the phenomenon of overeating.92
These results suggest that Piezo1 may be associated with obesity and type 2 diabetes. The normal expression of peizo1 plays a positive role in maintaining the body's lipid metabolism and food intake. But the overexpression of Piezo1 accelerate the maturation of adipocytes, increases the number of adipocytes, and leads to obesity. This once again confirms our view that there is a safety threshold in Piezo1 expression.
Piezo1 maintains normal blood cell function
By measuring the intracellular Ca2+ and pH levels of different blood cell types, the researchers found that yoda1, a mechanosensitive Piezo1 channel activator, significantly increased the intracellular Ca2+ level of red blood cells and decreased the volume of red blood cells, but not in the case of elevated blood glucose. Pathological hyperglycemia may reset Piezo1 mediated mechanical red blood cell volume regulation, leading to microvascular dysfunction and hematopoietic disorders observed in diabetic patients.93 In addition, studies have shown that using GsMTx-4 and GdCl3 as mechanosensitive cation channel inhibitors, targeting Piezo1 and TRPC6 in platelets can reduce the thrombus area. Under normal arterial shear rate, both inhibitors could significantly inhibit thrombus formation on collagen surface, but GdCl3 did not affect platelet morphology or aggregation under static condition. Therefore, it has proved that Piezo1 plays an important role in platelet aggregation and thrombosis.21
Besides, Piezo1 could regulate the mechanical transduction and release of ATP in human erythrocytes by controlling shear induced Ca2+ influx. The researchers found that shear induced ATP release and Ca2+ influx were significantly reduced in human erythrocytes treated with Piezo1 inhibitors or with mutant Piezo1 channels. Moreover, there is a phenomenon worthy of our attention in this study: a critical extracellular Ca2+ concentration is required to trigger significant ATP release, but the ATP pool associated with the membrane in red blood cells also contributes to ATP release. These results indicate that the Piezo1 channel may play a role in maintaining the normal function of red blood cells.22
With the deepening of the research on Piezo1, more and more studies have shown that the allelic mutation of Piezo1 is related to many hereditary erythroid diseases, such as malaria,94 hereditary stem cell disease,95, 96, 97, 98, 99 and so on. Although malaria is caused by parasitism of Plasmodium falciparum, relevant studies have shown that the expression of PfEMP-1 (Plasmodium falciparum erythrocyte surface protein-1) in the 756del heterozygote of Piezo1 is significantly reduced.94 Hereditary stem cell disease is a rare autosomal dominant hereditary disease characterized by hemolytic anemia and erythrocyte dehydration. Its etiology is closely related to the overexpression of Piezo1, which is caused by the GOF (gain-of-function) mutation of Piezo1. Overexpression of Piezo1 will lead to the disruption of intracellular calcium homeostasis and multiple transcription-independent effects in cells, affecting reticulocyte maturation.97
In another study on hereditary stem cell disease, researchers found that Piezo1 can regulate the levels of integrins in and out of red blood cells to regulate the homeostasis of red blood cells.98 In addition, a new study found that the GOF mutation of Piezo1 can affect the iron metabolism of mice and humans and enhance the phagocytosis of macrophages.99
Discussions and prospect
Current studies have shown that Piezo1 plays an essential role in maintaining cardiovascular health under mechanical stimulation. At the same time, Piezo1 is also involved in the occurrence and development of many cardiovascular diseases. Therefore, should we pay more attention to the role of Piezo1 in these lesions?
Piezo1 is crucial to the body in the presence of mechanical stimulation. In the ordinary physiological environment, Piezo1 has a positive protective effect on the growth and development of blood vessels and bones. At the same time, Piezo1 can protect DNA in cells from external mechanical stimulation.100 However, under turbulence and high pressure, Piezo1 may cause adverse reactions such as inflammation, endothelial barrier dysfunction, thrombosis, and red blood cell dysfunction. In addition, Piezo1 is involved in the regulation of adipocyte maturation, which may be an essential target of obesity. But when Piezo1 is silenced, it will lead to a series of pathological conditions, such as increased inflammation of WAT and decreased insulin sensitivity. Here, Piezo1 fully embodies its dual role in maintaining cardiovascular health.
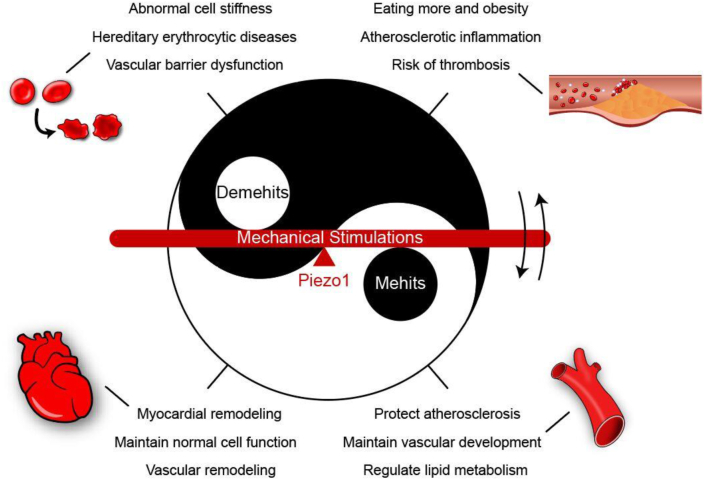
On the one hand, Piezo1 has positive effects on vascular development, myocardial remodelling, blood pressure stability and atherosclerosis; on the other hand, Piezo1 may also aggravate cardiovascular inflammation and cause cell dysfunction. Moreover, its double-sided structure is closely related to its rapid inactivation or sustained activity. This is also the key to its ability to respond to mechanical stimuli and affect cardiovascular health. In addition to the dual nature of Piezo1 in the cardiovascular system, we also found the dual nature of Piezo1 in other studies. The expression of Piezo1 in PDA (pancreatic ductal adenocarcinoma) is related to prolonged survival. About 20% of patients with low Piezo1 expression were 5-year survivors, while no long-term survivors were found in patients with high Piezo1 expression. It is worth noting that although Piezo1 is expressed in PDA tumor cells, the activation and deletion of Piezo1 does not affect the growth of tumor cells in vitro.101 This undoubtedly indicates that the expression level of Piezo1 may be related to the migration of cancer. In a new study on single cell high flux measurement, the researchers found that the stiffness of Piezo1 knockout HEK293T cells was about 80% lower than that of Piezo1 overexpressing HEK293T cells. However, it is worth noting that the cell stiffness of both Piezo1 knockout cells and overexpression cells is lower than normal HEK293T cells.102 The Janus-faced role of Piezo1 are also shown in the immune system. On the one hand, Piezo1 is necessary to maintain the innate immune response.103 On the other hand, specific deletion of Piezo1 can improve some immune diseases (autoimmune neuroinflammation104) and multi-bacterial septicemia.101 Overexpression or underexpression of Piezo1 may affect the function of cells. Only when the expression level of Piezo1 is within a specific normal range can the normal cell function be maintained. The Janus-faced expression of Piezo1 coincides with Yin and Yang in traditional Chinese culture (Fig. 3). The Yin and Yang are both opposite and unified.
Figure 3.
The Yin and Yang of Piezo1 in common cardiovascular diseases.
Therefore, we can conclude that the existence of Piezo1 is an essential part of maintaining our health, but under particular circumstances, the high expression of Piezo1 will also bring us adverse effects. In other words, the expression level of Piezo1 has a corresponding threshold. When the expression level of Piezo1 is in the safe range below the point, it is conducive to our health; on the contrary, when the expression level of Piezo1 exceeds or falls the threshold, Piezo1 may bring disease. Therefore, the activation or inhibition of Piezo1 alone cannot effectively solve these related diseases, which may cause the expression level of Piezo1 to be challenging to maintain in a safe range below the threshold.
But this raises a new question: what is the security threshold of Piezo1? Only through the current research is it challenging to give an accurate answer. However, we can speculate that the safety threshold of Piezo1 is not necessarily the same in different tissues and cells. At the same time, the intensity of mechanical stimulation to maintain Piezo1 expression at a safe threshold was also different. Here, we propose a method to determine the Piezo1 point. Piezo1 in vivo physiological environment threshold was deduced by mechanical measurement and calculation simulation of the physiological environment.
In addition to the threshold of Piezo1, we should also pay attention to the practical application of Piezo1 in dealing with cardiovascular health problems. Percutaneous intervention is the most essential methods for the treatment of cardiovascular diseases. At present, Piezo1 has not been applied in the aspect, but it does not mean that we can ignore Piezo1 as an essential target. Ideal vascular stents need rapid re-endothelialization105 and Piezo1 is a vital protein to maintain the function of ECs. Piezo1 can promote the release of NO from ECs and inhibit the proliferation of vascular SMCs, which is beneficial to increase the re-endothelialization of neointima and prevent restenosis.106 At the same time, stent implantation is accompanied by a lot of mechanical stimulation. Piezo1, as a mechanical sensitive protein, is undoubtedly worthy of attention.
So how Piezo1 could be combined with vascular stents? We believe that using nanotechnology to design the related coatings of Piezo1 should be an effective means. First, we can consider using exosomes. As Piezo1 is a vital cation channel protein distributed in the membrane structure. Therefore, the exosomes should also contain Piezo1 or its related regulatory factors. Exosomes and vascular stent coating related applications have been more mature.107,108 The nano coating with core–shell structure can realize the continuous delivery of drugs in different time periods. Therefore, the nano coating with core–shell structure maybe a better consideration.109,110 The nano coating with core–shell structures can ensure the valid promotion or inhibition of Piezo1 expression at the corresponding stage to ensure that the openness of Piezo1 tends to be more conducive to cardiovascular health. This is the key problem of Piezo1 as a target of gene therapy.
It is a long way to realize the application of Piezo1 in biomaterials or gene therapy. However, this method may be feasible, which is far from the safety threshold of Piezo1. Therefore, we still need to study the relevant regulatory pathways of Piezo1 further, find the threshold of Piezo1, and make the openness of Piezo1 closer to the direction beneficial to our health. It will be closely related to our health on how to regulate Piezo1 efficiently and reasonably.
Author contributions
J. Huang completed data collection and manuscript writing. K. Zhang finished drawing the picture. W. Liu and R. Du participated in data collection and image beautification. H. Zhang, T. Tian and Y. Wang put forward suggestions for revision. G. Wang and T. Yin supervised data collection, the writing and revision of the manuscript.
Conflict of interests
The authors declare no conflict of interest.
Funding
This work was supported by National Natural Science Foundation of China (No. 31971242, and 12032007); the Natural Science Foundation of Chongqing (No. cstc2019jcyj-msxmX0307, cstc2019jcyj-19zdxmX0009, and cstc2019jcyj-zdxmX0028).
Acknowledgements
We thank Research Associate Wang Nan (Cambridge University) for revising the language of the article. The authors also want to thank the support from the Chongqing Engineering Laboratory in Vascular Implants, the Public Experiment Center of State Bioindustrial Base (Chongqing).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Guixue Wang, Email: wanggx@cqu.edu.cn.
Tieying Yin, Email: tieying_yin@cqu.edu.cn.
References
- 1.Sabine A., Bovay E., Demir C.S., et al. FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. J Clin Invest. 2015;125(10):3861–3877. doi: 10.1172/JCI80454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinn T.A., Kohl P. Cardiac mechano-electric coupling: acute effects of mechanical stimulation on heart rate and rhythm. Physiol Rev. 2021;101(1):37–92. doi: 10.1152/physrev.00036.2019. [DOI] [PubMed] [Google Scholar]
- 3.Christensen A.P., Corey D.P. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci. 2007;8(7):510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- 4.Kung C. A possible unifying principle for mechanosensation. Nature. 2005;436(7051):647–654. doi: 10.1038/nature03896. [DOI] [PubMed] [Google Scholar]
- 5.Delmas P., Coste B. Mechano-gated ion channels in sensory systems. Cell. 2013;155(2):278–284. doi: 10.1016/j.cell.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 6.Murthy S.E., Dubin A.E., Patapoutian A. Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat Rev Mol Cell Biol. 2017;18(12):771–783. doi: 10.1038/nrm.2017.92. [DOI] [PubMed] [Google Scholar]
- 7.Hamill O.P., Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81(2):685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 8.Sachs F. Stretch-activated ion channels: what are they? Physiology. 2010;25(1):50–56. doi: 10.1152/physiol.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nilius B., Honoré E. Sensing pressure with ion channels. Trends Neurosci. 2012;35(8):477–486. doi: 10.1016/j.tins.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Coste B., Mathur J., Schmidt M., et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330(6000):55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranade S.S., Qiu Z., Woo S.H., et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci U S A. 2014;111(28):10347–10352. doi: 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J., Hou B., Tumova S., et al. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515(7526):279–282. doi: 10.1038/nature13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S., Chennupati R., Kaur H., Iring A., Wettschureck N., Offermanns S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J Clin Invest. 2016;126(12):4527–4536. doi: 10.1172/JCI87343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iring A., Jin Y.J., Albarrán-Juárez J., et al. Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J Clin Invest. 2019;129(7):2775–2791. doi: 10.1172/JCI123825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blythe N.M., Muraki K., Ludlow M.J., et al. Mechanically activated Piezo1 channels of cardiac fibroblasts stimulate p38 mitogen-activated protein kinase activity and interleukin-6 secretion. J Biol Chem. 2019;294(46):17395–17408. doi: 10.1074/jbc.RA119.009167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn C., Schwartz M.A. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10(1):53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanhoutte P.M., Shimokawa H., Tang E.H., Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009;196(2):193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 18.Chiu J.J., Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gimbrone M.A., García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118(4):620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedrich E.E., Hong Z., Xiong S., et al. Endothelial cell Piezo1 mediates pressure-induced lung vascular hyperpermeability via disruption of adherens junctions. Proc Natl Acad Sci U S A. 2019;116(26):12980–12985. doi: 10.1073/pnas.1902165116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ilkan Z., Wright J.R., Francescut L., Goodall A.H., Mahaut-Smith M.P. Abstract 11593: thrombus formation under flow is inhibited by the mechanosensitive cation channel blockers GsMTx-4 peptide and gadolinium chloride. Circulation. 2015;132(suppl_3):A11593. [Google Scholar]
- 22.Cinar E., Zhou S., DeCourcey J., Wang Y., Waugh R.E., Wan J. Piezo1 regulates mechanotransductive release of ATP from human RBCs. Proc Natl Acad Sci U S A. 2015;112(38):11783–11788. doi: 10.1073/pnas.1507309112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S., Cao S., Arhatte M., et al. Adipocyte Piezo1 mediates obesogenic adipogenesis through the FGF1/FGFR1 signaling pathway in mice. Nat Commun. 2020;11(1):2303. doi: 10.1038/s41467-020-16026-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beech D.J., Kalli A.C. Force sensing by Piezo channels in cardiovascular health and disease. Arterioscler Thromb Vasc Biol. 2019;39(11):2228–2239. doi: 10.1161/ATVBAHA.119.313348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L., You X., Lotinun S., Zhang L., Wu N., Zou W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat Commun. 2020;11(1):282. doi: 10.1038/s41467-019-14146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coste B., Xiao B., Santos J.S., et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483(7388):176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Syeda R., Florendo M.N., Cox C.D., et al. Piezo1 channels are inherently mechanosensitive. Cell Rep. 2016;17(7):1739–1746. doi: 10.1016/j.celrep.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saotome K., Murthy S.E., Kefauver J.M., Whitwam T., Patapoutian A., Ward A.B. Structure of the mechanically activated ion channel Piezo1. Nature. 2018;554(7693):481–486. doi: 10.1038/nature25453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Q., Zhou H., Chi S., et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature. 2018;554(7693):487–492. doi: 10.1038/nature25743. [DOI] [PubMed] [Google Scholar]
- 30.Guo Y.R., MacKinnon R. Structure-based membrane dome mechanism for Piezo mechanosensitivity. Elife. 2017;6 doi: 10.7554/eLife.33660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ge J., Li W., Zhao Q., et al. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature. 2015;527(7576):64–69. doi: 10.1038/nature15247. [DOI] [PubMed] [Google Scholar]
- 32.Douguet D., Patel A., Xu A., Vanhoutte P.M., Honoré E. Piezo ion channels in cardiovascular mechanobiology. Trends Pharmacol Sci. 2019;40(12):956–970. doi: 10.1016/j.tips.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y., Chi S., Guo H., et al. A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel. Nat Commun. 2018;9(1):1300. doi: 10.1038/s41467-018-03570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng W., Gracheva E.O., Bagriantsev S.N. A hydrophobic gate in the inner pore helix is the major determinant of inactivation in mechanosensitive Piezo channels. Elife. 2019;8 doi: 10.7554/eLife.44003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taberner F.J., Prato V., Schaefer I., Schrenk-Siemens K., Heppenstall P.A., Lechner S.G. Structure-guided examination of the mechanogating mechanism of PIEZO2. Proc Natl Acad Sci U S A. 2019;116(28):14260–14269. doi: 10.1073/pnas.1905985116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Del Mármol J.I., Touhara K.K., Croft G., MacKinnon R. Piezo1 forms a slowly-inactivating mechanosensory channel in mouse embryonic stem cells. Elife. 2018;7 doi: 10.7554/eLife.33149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rode B., Shi J., Endesh N., et al. Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nat Commun. 2017;8(1):350. doi: 10.1038/s41467-017-00429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi J., Hyman A.J., De Vecchis D., et al. Sphingomyelinase disables inactivation in endogenous PIEZO1 channels. Cell Rep. 2020;33(1) doi: 10.1016/j.celrep.2020.108225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bae C., Sachs F., Gottlieb P.A. Protonation of the human PIEZO1 ion channel stabilizes inactivation. J Biol Chem. 2015;290(8):5167–5173. doi: 10.1074/jbc.M114.604033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borbiro I., Badheka D., Rohacs T. Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Sci Signal. 2015;8(363):ra15. doi: 10.1126/scisignal.2005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang H., Hong Z., Zhong M., et al. Piezo1 mediates angiogenesis through activation of MT1-MMP signaling. Am J Physiol Cell Physiol. 2019;316(1):C92–C103. doi: 10.1152/ajpcell.00346.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romero L.O., Massey A.E., Mata-Daboin A.D., et al. Dietary fatty acids fine-tune Piezo1 mechanical response. Nat Commun. 2019;10(1):1200. doi: 10.1038/s41467-019-09055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuchiya M., Hara Y., Okuda M., et al. Cell surface flip-flop of phosphatidylserine is critical for PIEZO1-mediated myotube formation. Nat Commun. 2018;9(1):2049. doi: 10.1038/s41467-018-04436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ridone P., Pandzic E., Vassalli M., et al. Disruption of membrane cholesterol organization impairs the activity of PIEZO1 channel clusters. J Gen Physiol. 2020;152(8) doi: 10.1085/jgp.201912515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poole K., Herget R., Lapatsina L., Ngo H.D., Lewin G.R. Tuning Piezo ion channels to detect molecular-scale movements relevant for fine touch. Nat Commun. 2014;5:3520. doi: 10.1038/ncomms4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qi Y., Andolfi L., Frattini F., Mayer F., Lazzarino M., Hu J. Membrane stiffening by STOML3 facilitates mechanosensation in sensory neurons. Nat Commun. 2015;6:8512. doi: 10.1038/ncomms9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maneshi M.M., Ziegler L., Sachs F., Hua S.Z., Gottlieb P.A. Enantiomeric Aβ peptides inhibit the fluid shear stress response of PIEZO1. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-32572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peyronnet R., Martins J.R., Duprat F., et al. Piezo1-dependent stretch-activated channels are inhibited by Polycystin-2 in renal tubular epithelial cells. EMBO Rep. 2013;14(12):1143–1148. doi: 10.1038/embor.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang X.N., Lu Y.P., Liu J.J., et al. Piezo1 is as a novel trefoil factor family 1 binding protein that promotes gastric cancer cell mobility in vitro. Dig Dis Sci. 2014;59(7):1428–1435. doi: 10.1007/s10620-014-3044-3. [DOI] [PubMed] [Google Scholar]
- 50.Chuntharpursat-Bon E., Povstyan O.V., Ludlow M.J., Gaunt H.J., Beech D.J. Cell adhesion molecule interaction with Piezo1 channels is a mechanism for sub cellular regulation of mechanical sensitivity. bioRxiv. 2019 doi: 10.1101/602532. [DOI] [Google Scholar]
- 51.Zhang T., Chi S., Jiang F., Zhao Q., Xiao B. A protein interaction mechanism for suppressing the mechanosensitive Piezo channels. Nat Commun. 2017;8(1):1797. doi: 10.1038/s41467-017-01712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mylvaganam S., Plumb J., Yusuf B., et al. The spectrin cytoskeleton integrates endothelial mechanoresponses. Nat Cell Biol. 2022;24(8):1226–1238. doi: 10.1038/s41556-022-00953-5. [DOI] [PubMed] [Google Scholar]
- 53.Mchugh B.J., Buttery R., Lad Y., Banks S., Haslett C., Sethi T. Integrin activation by Fam38A uses a novel mechanism of R-Ras targeting to the endoplasmic reticulum. J Cell Sci. 2010;123(Pt 1):51–61. doi: 10.1242/jcs.056424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McHugh B.J., Murdoch A., Haslett C., Sethi T. Loss of the integrin-activating transmembrane protein Fam38A (Piezo1) promotes a switch to a reduced integrin-dependent mode of cell migration. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhong M., Wu W., Kang H., et al. Alveolar stretch activation of endothelial Piezo1 protects adherens junctions and lung vascular barrier. Am J Respir Cell Mol Biol. 2020;62(2):168–177. doi: 10.1165/rcmb.2019-0024OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y.Y., Zhang H., Ma T., et al. Piezo1 mediates neuron oxygen-glucose deprivation/reoxygenation injury via Ca2+/calpain signaling. Biochem Biophys Res Commun. 2019;513(1):147–153. doi: 10.1016/j.bbrc.2019.03.163. [DOI] [PubMed] [Google Scholar]
- 57.Miyamoto T., Mochizuki T., Nakagomi H., et al. Functional role for Piezo1 in stretch-evoked Ca2⁺ influx and ATP release in urothelial cell cultures. J Biol Chem. 2014;289(23):16565–16575. doi: 10.1074/jbc.M113.528638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caolo V., Debant M., Endesh N., et al. Piezo1 channel activates ADAM10 sheddase to regulate Notch1 and gene expression. Elife. 2020;9 doi: 10.7554/eLife.50684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song Y., Li D., Farrelly O., et al. The mechanosensitive ion channel piezo inhibits axon regeneration. Neuron. 2019;102(2):373–389. doi: 10.1016/j.neuron.2019.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun W., Chi S., Li Y., et al. The mechanosensitive Piezo1 channel is required for bone formation. Elife. 2019;8 doi: 10.7554/eLife.47454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Albarrán-Juárez J., Iring A., Wang S., et al. Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation. J Exp Med. 2018;215(10):2655–2672. doi: 10.1084/jem.20180483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nonomura K., Lukacs V., Sweet D.T., et al. Mechanically activated ion channel PIEZO1 is required for lymphatic valve formation. Proc Natl Acad Sci U S A. 2018;115(50):12817–12822. doi: 10.1073/pnas.1817070115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blythe N.M., Stylianidis V., Ludlow M.J., et al. Stimulation of cardiac fibroblast Piezo1 channels opposes myofibroblast differentiation and induces IL-6 secretion via Ca2+-mediated p38 MAP kinase activation. bioRxiv. 2019 doi: 10.1101/603456. [DOI] [Google Scholar]
- 64.Duchemin A.L., Vignes H., Vermot J. Mechanically activated Piezo channels control outflow tract valve development through Yap1 and Klf2-Notch signaling axis. Elife. 2019;8 doi: 10.7554/eLife.44706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gudipaty S.A., Lindblom J., Loftus P.D., et al. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature. 2017;543(7643):118–121. doi: 10.1038/nature21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Q., Sun B., Zhao J., et al. Increased Piezo1 channel activity in interstitial Cajal-like cells induces bladder hyperactivity by functionally interacting with NCX1 in rats with cyclophosphamide-induced cystitis. Exp Mol Med. 2018;50(5):1–16. doi: 10.1038/s12276-018-0088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hung W.C., Yang J., Yankaskas C., et al. Confinement-sensing and signal optimization via Piezo1/PKA and myosin II pathways. Cell Rep. 2016;15(7):1430–1441. doi: 10.1016/j.celrep.2016.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Solis A.G., Bielecki P., Steach H.R., et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature. 2019;573(7772):69–74. doi: 10.1038/s41586-019-1485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dela Paz N.G., Frangos J.A. Rapid flow-induced activation of Gαq/11 is independent of Piezo1 activation. Am J Physiol Cell Physiol. 2019;316(5):C741–C752. doi: 10.1152/ajpcell.00215.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jetta D., Gottlieb P.A., Verma D., Sachs F., Hua S.Z. Shear stress-induced nuclear shrinkage through activation of Piezo1 channels in epithelial cells. J Cell Sci. 2019;132(11):jcs226076. doi: 10.1242/jcs.226076. [DOI] [PubMed] [Google Scholar]
- 71.Retailleau K., Duprat F., Arhatte M., et al. Piezo1 in smooth muscle cells is involved in hypertension-dependent arterial remodeling. Cell Rep. 2015;13(6):1161–1171. doi: 10.1016/j.celrep.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 72.Evans E.L., Cuthbertson K., Endesh N., et al. Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation. Br J Pharmacol. 2018;175(10):1744–1759. doi: 10.1111/bph.14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morley L.C., Shi J., Gaunt H.J., et al. Piezo1 channels are mechanosensors in human fetoplacental endothelial cells. Mol Hum Reprod. 2018;24(10):510–520. doi: 10.1093/molehr/gay033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang D., Yan W., Qiu J., et al. Mussel adhesive protein fused with VE-cadherin extracellular domain promotes endothelial-cell tight junctions and in vivo endothelization recovery of vascular stent. J Biomed Mater Res B Appl Biomater. 2020;108(1):94–103. doi: 10.1002/jbm.b.34369. [DOI] [PubMed] [Google Scholar]
- 75.Wang J., Jin X., Huang Y., et al. Endovascular stent-induced alterations in host artery mechanical environments and their roles in stent restenosis and late thrombosis. Regen Biomater. 2018;5(3):177–187. doi: 10.1093/rb/rby006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.John L., Ko N.L., Gokin A., Gokina N., Mandalà M., Osol G. The Piezo1 cation channel mediates uterine artery shear stress mechanotransduction and vasodilation during rat pregnancy. Am J Physiol Heart Circ Physiol. 2018;315(4):H1019–H1026. doi: 10.1152/ajpheart.00103.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang L., Li L., Chen X., Zhang H., Shi Z. miR-103a targeting Piezo1 is involved in acute myocardial infarction through regulating endothelium function. Cardiol J. 2016;23(5):556–562. doi: 10.5603/CJ.a2016.0056. [DOI] [PubMed] [Google Scholar]
- 78.Swain S.M., Liddle R.A. Piezo1 acts upstream of TRPV4 to induce pathological changes in endothelial cells due to shear stress. J Biol Chem. 2021;296 doi: 10.1074/jbc.RA120.015059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Félétou M., Vanhoutte P.M. Endothelium-dependent hyperpolarizations: quo vadis? Acta Physiol. 2017;219(1):100–107. doi: 10.1111/apha.12657. [DOI] [PubMed] [Google Scholar]
- 80.Joyner M.J., Casey D.P. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev. 2015;95(2):549–601. doi: 10.1152/physrev.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lavie C.J., Arena R., Swift D.L., et al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res. 2015;117(2):207–219. doi: 10.1161/CIRCRESAHA.117.305205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deb A., Ubil E. Cardiac fibroblast in development and wound healing. J Mol Cell Cardiol. 2014;70:47–55. doi: 10.1016/j.yjmcc.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turner N.A., Porter K.E. Function and fate of myofibroblasts after myocardial infarction. Fibrogenesis Tissue Repair. 2013;6(1):5. doi: 10.1186/1755-1536-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Herum K.M., Lunde I.G., McCulloch A.D., Christensen G. The soft- and hard-heartedness of cardiac fibroblasts: mechanotransduction signaling pathways in fibrosis of the heart. J Clin Med. 2017;6(5):53. doi: 10.3390/jcm6050053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fan D., Takawale A., Lee J., Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5(1):15. doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bavi N., Richardson J., Heu C., Martinac B., Poole K. PIEZO1-mediated currents are modulated by substrate mechanics. ACS Nano. 2019;13(11):13545–13559. doi: 10.1021/acsnano.9b07499. [DOI] [PubMed] [Google Scholar]
- 87.Hope J.M., Lopez-Cavestany M., Wang W., Reinhart-King C.A., King M.R. Activation of Piezo1 sensitizes cells to TRAIL-mediated apoptosis through mitochondrial outer membrane permeability. Cell Death Dis. 2019;10(11):837. doi: 10.1038/s41419-019-2063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang F., Yin K., Wu K., et al. The mechanosensitive Piezo1 channel mediates heart mechano-chemo transduction. Nat Commun. 2021;12(1):869. doi: 10.1038/s41467-021-21178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.West J.B., Mathieu-Costello O. Vulnerability of pulmonary capillaries in heart disease. Circulation. 1995;92(3):622–631. doi: 10.1161/01.cir.92.3.622. [DOI] [PubMed] [Google Scholar]
- 90.Zhou Y., Tong J., Li X., Li X., Wang G. Numerical simulation of haemodynamics of the descending aorta in the non-diabetic and diabetic rabbits. J Biomech. 2019;91:140–150. doi: 10.1016/j.jbiomech.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 91.Song A., Lu L., Li Y., et al. Low HbA1c with normal hemoglobin in a diabetes patient caused by PIEZO1 gene variant: a case report. Front Endocrinol. 2020;11:356. doi: 10.3389/fendo.2020.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang P., Jia Y., Liu T., Jan Y.N., Zhang W. Visceral mechano-sensing neurons control Drosophila feeding by using Piezo as a sensor. Neuron. 2020;108(4):640–650. doi: 10.1016/j.neuron.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu W., Beerens M., Kiviniemi T.O., Wilson E., Deo R.C., Macrae C.A. Abstract 13908: A novel low-cost cellular functional assay reveals a potential role for Piezo1 in hyperglycemia induced microcirculatory disorders. Circulation. 2019;140(suppl l_1) [Google Scholar]
- 94.Nguetse C.N., Purington N., Ebel E.R., et al. A common polymorphism in the mechanosensitive ion channel PIEZO1 is associated with protection from severe malaria in humans. Proc Natl Acad Sci U S A. 2020;117(16):9074–9081. doi: 10.1073/pnas.1919843117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bae C., Gnanasambandam R., Nicolai C., Sachs F., Gottlieb P.A. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc Natl Acad Sci U S A. 2013;110(12):E1162–E1168. doi: 10.1073/pnas.1219777110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Albuisson J., Murthy S.E., Bandell M., et al. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun. 2013;4:1884. doi: 10.1038/ncomms2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moura P.L., Hawley B.R., Dobbe J.G.G., et al. PIEZO1 gain-of-function mutations delay reticulocyte maturation in hereditary xerocytosis. Haematologica. 2020;105(6):e268–e271. doi: 10.3324/haematol.2019.231159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aglialoro F., Hofsink N., Hofman M., Brandhorst N., van den Akker E. Inside out integrin activation mediated by PIEZO1 signaling in erythroblasts. Front Physiol. 2020;11:958. doi: 10.3389/fphys.2020.00958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ma S., Dubin A.E., Zhang Y., et al. A role of PIEZO1 in iron metabolism in mice and humans. Cell. 2021;184(4):969–982. doi: 10.1016/j.cell.2021.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nava M.M., Miroshnikova Y.A., Biggs L.C., et al. Heterochromatin-driven nuclear softening protects the genome against mechanical stress-induced damage. Cell. 2020;181(4):800–817. doi: 10.1016/j.cell.2020.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aykut B., Chen R., Kim J.I., et al. Targeting Piezo1 unleashes innate immunity against cancer and infectious disease. Sci Immunol. 2020;5(50) doi: 10.1126/sciimmunol.abb5168. [DOI] [PubMed] [Google Scholar]
- 102.Romanov V., Silvani G., Zhu H., Cox C.D., Martinac B. An acoustic platform for single-cell, high-throughput measurements of the viscoelastic properties of cells. Small. 2021;17(3) doi: 10.1002/smll.202005759. [DOI] [PubMed] [Google Scholar]
- 103.Geng J., Shi Y., Zhang J., et al. TLR4 signalling via Piezo1 engages and enhances the macrophage mediated host response during bacterial infection. Nat Commun. 2021;12(1):3519. doi: 10.1038/s41467-021-23683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jairaman A., Othy S., Dynes J.L., et al. Piezo1 channels restrain regulatory T cells but are dispensable for effector CD4+ T cell responses. Sci Adv. 2021;7(28) doi: 10.1126/sciadv.abg5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao Y., Du R., Zhou T., et al. Arsenic trioxide-coated stent is an endothelium-friendly drug eluting stent. Adv Healthc Mater. 2018;7(15) doi: 10.1002/adhm.201800207. [DOI] [PubMed] [Google Scholar]
- 106.Qiu J., Zheng Y., Hu J., et al. Biomechanical regulation of vascular smooth muscle cell functions: from in vitro to in vivo understanding. J R Soc Interface. 2014;11(90) doi: 10.1098/rsif.2013.0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hu S., Li Z., Shen D., et al. Exosome-eluting stents for vascular healing after ischaemic injury. Nat Biomed Eng. 2021;5(10):1174–1188. doi: 10.1038/s41551-021-00705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bunggulawa E.J., Wang W., Yin T., et al. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnol. 2018;16(1):81. doi: 10.1186/s12951-018-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Du R., Wang Y., Huang Y., et al. Design and testing of hydrophobic core/hydrophilic shell nano/micro particles for drug-eluting stent coating. NPG Asia Mater. 2018;10:642–658. [Google Scholar]
- 110.Maruf A., Wang Y., Yin T., et al. Atherosclerosis treatment with stimuli-responsive nanoagents: recent advances and future perspectives. Adv Healthc Mater. 2019;8(11) doi: 10.1002/adhm.201900036. [DOI] [PubMed] [Google Scholar]