Glycosylation is a ubiquitous molecular modification in the process of cellular metabolism regulation, which plays a vital role in maintaining and regulating cellular functions. Deficiency in glycosylation enzymes may jeopardize the glycosylation process and eventually lead to the metabolic disease, manifesting with either a neurologic or multisystem phenotype, which is also known as congenital disorder of glycosylation (CDG).1 It could be classified into type I CDG and type II CDG, and the latter was referred to as the abnormal formation of nascent glycoprotein. Phosphomannomutase 2 CDG (PMM2-CDG), the most prevalent CDG with an incidence rate of 1 in 20,000 individuals, is mainly due to the metabolic disorder of phosphomannomutase 2 (PMM2).2 PMM2 participates in the conversion of mannose-6-phosphate to mannose-1-phosphate. The cysteine residues, as one of the least abundant amino acids, have unique attributes to maintain the stability of protein structure, especially for catalytic activity and protein folding.3 Therefore, cysteine mutations have been identified in various diseases including PMM2-CDG. Suggested by the crystal structure of PMM2 (PDB ID: 7O5Z),4 four cysteine mutations in the hPMM2, C9Y, C103F, C192G, and C241S, have been found in PMM2-CDG. After bioinformatic analysis by PROVEAN, the four cysteine mutations were predicted with “Deleterious”, ranking with the best two were C103F and C192G (C9Y: −5.493, C103F: −6.339, C192G: −11.176, C241S: −2.986). However, the underlying pathological mechanism is still unclear. Among them, C103F and C192G have only been reported in clinical study without a detailed explanation, so they were selected for our research. Two mutants were located near the linker region 1 and 2; C103F was located on the No. 4 α helix and C192G was located on the No. 7 α helix (Fig. S1A). The purpose of this study was to explore the structure and function roles of PMM2 clinical mutation and investigate the role of cysteine in PMM2 which might provide new insights into preventive and therapeutic strategies for PMM2-CDG.
The protein of PMM2 wild-type (WT), C103F, and C192G were purified from the Escherichia coli system, then spectroscopic experiments were conducted under physiological and stress conditions. From our studies, we obtained the properties of the structural and biological functions of WT and cysteine mutants of PMM2, including solubility, structural stability, thermal stability, oxidative stress sensitivity, cell apoptosis, and molecular simulations.
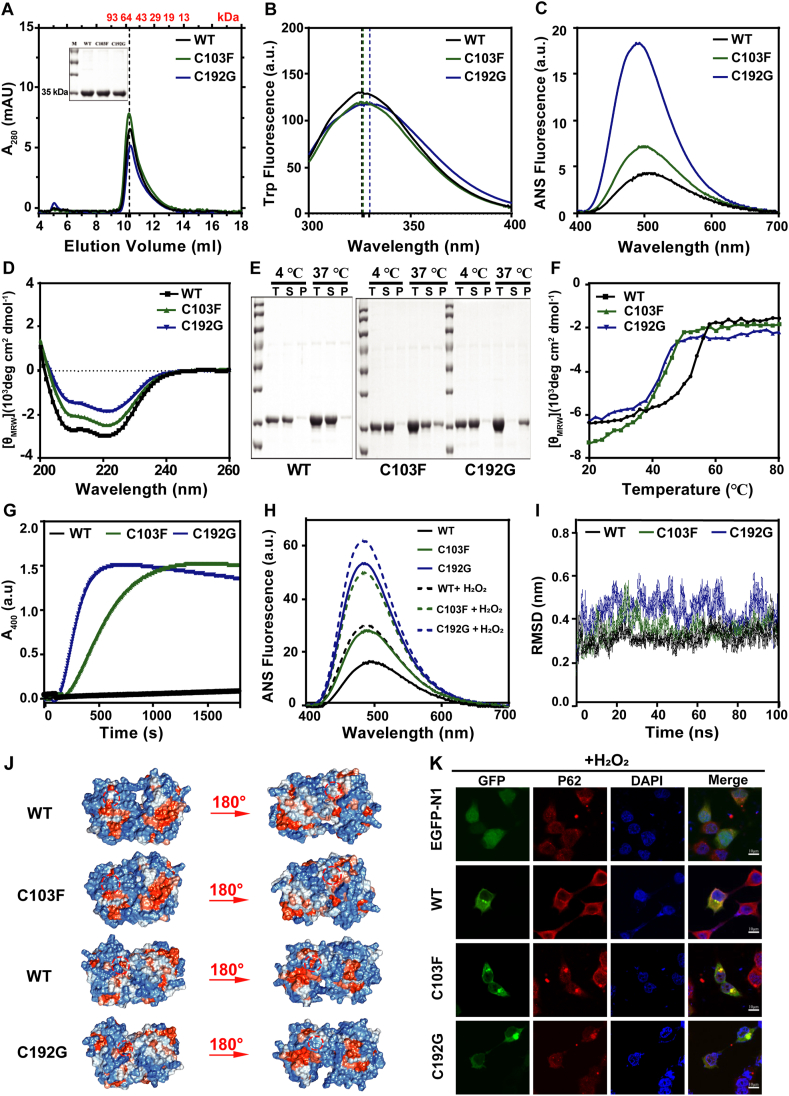
According to the results of size exclusion chromatography (SEC) analysis and SDS-PAGE, a single main peak and band indicated a high homogeneity of the purified proteins (Fig. 1A). Basic physicochemical properties of mutants and WT were demonstrated by a series of spectra experiments. The intrinsic tryptophan fluorescence (Trp Fluorescence) showed that the C192G was accompanied by an about 2 nm redshift of the maximum emission wavelength (Emax), suggesting that the structure of C192G variants became loose (Fig. 1B). The ANS fluorescence data provided clear evidence that the cysteine substitution induced a large change in the non-polarity of the ANS-binding site, and the C192G variant caused a larger change than the C103F variant did (Fig. 1C). Far-CD spectra presented that the ratio of α-helix and β-sheet of C103F and C192G was significantly lower than that in WT (Fig. 1D). Those findings collectively indicated that the structure of the mutations became looser, which may lead to the low solubility of the mutants, especially the C192G (Fig. S1B).
Figure 1.
The pathogenic cysteine mutants (C103F and C192G) disrupted the structure of PMM2. (A) SEC profiles of 100 μL 10 μM purified PMM2-WT, C103F, and C192G proteins. The inset shows the SDS-PAGE analysis of the purified proteins. (B) Intrinsic Trp fluorescence spectra, (C) ANS fluorescence spectra, and (D) Far-UV CD spectra of the 10 μM WT, C103F, and C192G. (E) SDS-PAGE analysis of the soluble and insoluble fractions of protein solutions incubated at 37 °C for 4 h, T: total, S: supernatant, P: precipitate. (F) The ellipticity at 222 nm of Far-UV CD upon temperature-series changes. (G) Thermal aggregation kinetics of the proteins obtained by heating the protein solutions at 50 °C. (H) ANS fluorescence spectra of the 10 μM WT, C103F, and C192G after 1 mM H2O2 treatment for 12 h at 4 °C. (I) Cα root mean square deviation (RMSD) of the dimeric WT and Cys mutants from the initial structure time for all simulations. (J) Surface electrostatic potentials of the dimeric WT and Cys mutants. The Cys mutants altered the distribution of charged residues and the representative areas were indicated by dotted circles. (K) Representative confocal images of HEK293T cells with exogenous expression of the WT, C103F, and C192G fused by GFP at the N-terminus with 2 mM H2O2 treatment. Exogenously expressed PMM2 proteins were visualized by GFP (green), the aggresomes were marked by P62 (red), and the nucleus was stained by DAPI (blue). Scale bars = 10 μm.
To assess the effect of cysteine substitution on protein stability at physiological temperature (37 °C), we monitored the aggregation of samples by measuring the turbidity at 400 nm (A400) at consecutive time points. As our results showed, the A400 value of C103F and C192G reached to a plateau at 4 h and was significantly higher than that of WT (Fig. S2A). During treatment at 37 °C for 4 h, the samples were further subjected to SDS-PAGE analysis. Compared to the native state, the two mutants went into the pellet fraction not in the soluble fraction, especially for the C192G mutant, almost all of the protein was found in precipitation (Fig. 1E; Fig. S2D). These findings revealed that the cysteine substitution made the protein more unstable at physiological temperature (37 °C). Meanwhile, the cysteine mutants also brought about drastic structural changes at physiological temperature, confirmed by the alterations of Trp fluorescence and Far-CD spectra (Fig. S2B, C).
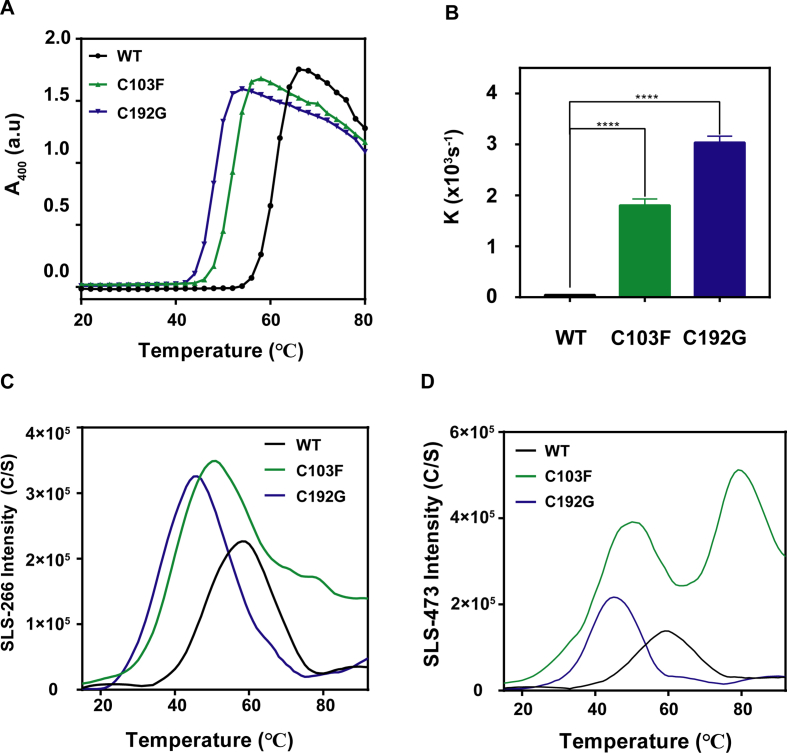
In order to investigate the thermal stability of PMM2 WT and cysteine mutants, we applied circular dichroism (CD) spectroscopy, A400 and UNcle System. The results showed that cysteine mutants had significantly decreased starting and midpoint temperatures of thermal denaturation (Tm) when measured by the transition curves that were obtained from the changes of E222 in Far-UV CD (Fig. 1F) and A400 (Fig. S3A). C103F and C192G mutants lose their CD signal at a lower temperature than WT. The C103F and C192G mutants began to form aggregates at 42 °C and 44 °C respectively, whereas the WT kept soluble till 56 °C (Fig. S3A). The thermal aggregation kinetics experiments (at 50 °C) implied that the mutants aggregated with a shorter lag time and a faster aggregation rate (Fig. 1G; Fig. S3B). The UNcle results also demonstrated that the mutants were more susceptible to temperature (Fig. S3C, D). Notably, the mutants had a higher peak and lower temperature for the formation of small (E266) and large (E473) aggregates.
Oxidative damage is major stress faced by many kinds of cells.5 To compare the stability of WT and cysteine mutants during the redox environment, we treated the purified proteins and cells with hydrogen peroxide (H2O2) to induce oxidative stress. The results for purified proteins indicated that WT and mutants were all sensitive to oxidative stress (Fig. S4A–D). Non-reducing SDS-PAGE results indicated that cysteine mutant might exist as a higher multimeric form, and the degradation happened as well (Fig. S4E, F). The oxidative damage from H2O2 will lead to protein misfolding caused by the disruption of the interaction between atoms, e.g., hydrogen bonding, and disulfide bond. Thus, the misfolded proteins eventually exhibit protein aggregation or degradation.
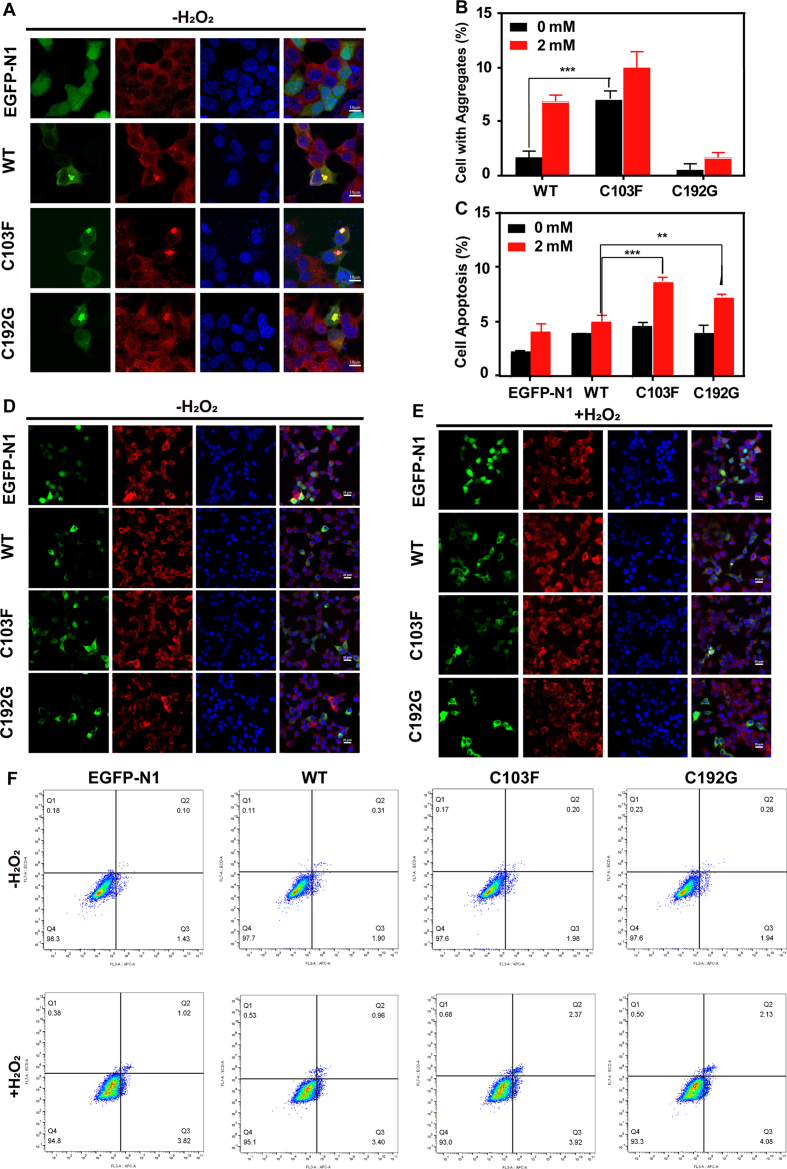
In HEK 293T cell lines, the location of overexpressed WT and cysteine mutants were mostly distributed in the cytoplasm. Whereas a small fraction of cells formed aggregates, which were co-localized with aggresome marker protein (P62) (Fig. 1K; Fig. S5A). The ratio of cells with aggregates was significantly increased after 2 mM H2O2 treatment, meanwhile, cysteine mutants formed more aggregates both under normal and oxidative stress conditions (Fig. S5B, D, E), and a larger percentage of cell apoptosis (Fig. S5C, F), suggesting that the cysteine mutants were more sensitive to oxidative condition. We speculated that the lower aggregates ratio within C192G-overexpressed cells may be attributed to the presumable presence of degraded C129G mutant in cells and the cumulative effect of cell apoptosis. These results were consistent with those for the purified proteins in a tube. To our knowledge, this is the first study to demonstrate that PMM2 forms aggregates in cells.
For the purpose of monitoring the aggregation process caused by Cys mutation, molecular dynamic (MD) simulation of the dimer was performed. Alignment of the dimeric structures accomplished by simulation of PMM2 and cysteine mutants indicated that the mutations apparently altered the overall folding of PMM2 (Fig. S6A, B). The Cα RMSF and RMSD values of C192G was highly fluctuated (Fig. 1I; Fig. S6C, D). However, in the cap domain (aa 87–184), different waveforms existed between the 2 mutants. The alignment of well-balanced simulated structures indicated that the cystenine mutants exhibited a looser structure and exposed a larger hydrophobic surface than WT (Fig. 1J). Moreover, C103F significantly decreased the subunit binding energy, especially in the electronic part (Fig. S6E–G).
Taken together, MD structural analysis presented the changes in structure in detail. Combined MD structural analysis and the above results from spectroscopic experiments could provide precise insights into the pathological mechanism by which cysteine mutations altered the molecular structure of PMM2.
In this project, we have documented for the first time that PMM2 mutant formed aggregates and elevated cell apoptosis. Furthermore, we proposed that cysteine mutation may disrupt the formation of disulfide bonds, as verified by Free thiol measurement (Fig. S1C). This suggested that potential disulfide bonds might have significant effects on the conformation and thermal stability of PMM2. These results provided proof of concept for the clinical treatment of PMM-CDG. Except for pharmacological partners, combinations with antioxidant agents as therapeutic approaches are also worthy of investigation.
Author contributions
L. Hu and J. Mao conceived, designed, and supervised the research. Y. Pan, L. Lin, and F. Yu performed the experiments. X. He, J. Sun, and G. Bai identified pathogenic variants and bioinformation analysis. L. Hu, J. Mao, Y. Pan, F. Yu, and L. Lin performed data analyses. Y. Pan and L. Hu wrote the manuscript. All authors read and approved the final version of the manuscript.
Conflict of interests
The authors declare no competing interests.
Funding
This study was financially supported by the Natural Science Foundation of Zhejiang Province (China) (No. LQ22C070004).
Acknowledgements
We are extremely grateful to all members of the Hu Lab and Mao Lab, past and present. We thank Dr. Xiangjun Chen for his helpful suggestions on this manuscript. We thank the National Clinical Research Center for Child Health (Hangzhou, China) for the great support.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2022.10.002.
Contributor Information
Jianhua Mao, Email: maojh88@zju.edu.cn.
Lidan Hu, Email: hulidan@zju.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
figs6.
References
- 1.Reily C., Stewart T.J., Renfrow M.B., et al. Glycosylation in health and disease. Nat Rev Nephrol. 2019;15(6):346–366. doi: 10.1038/s41581-019-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam C., Krasnewich D.M. In: GeneReviews® [Internet] Adam M.P., Everman D.B., Mirzaa G.M., et al., editors. University of Washington, Seattle; Seattle (WA): 2005. PMM2-CDG; pp. 1993–2022. [updated 2021 May 20] [Google Scholar]
- 3.Poole L.B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic Biol Med. 2015;80:148–157. doi: 10.1016/j.freeradbiomed.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briso-Montiano A., Del Caño-Ochoa F., Vilas A., et al. Insight on molecular pathogenesis and pharmacochaperoning potential in phosphomannomutase 2 deficiency, provided by novel human phosphomannomutase 2 structures. J Inherit Metab Dis. 2022;45(2):318–333. doi: 10.1002/jimd.12461. [DOI] [PubMed] [Google Scholar]
- 5.Held J.M. Redox systems biology: harnessing the sentinels of the cysteine redoxome. Antioxidants Redox Signal. 2020;32(10):659–676. doi: 10.1089/ars.2019.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.