Abstract
Despite significant improvements in five-year survival rates due to early diagnosis and combination therapy, triple-negative breast cancer (TNBC) treatment remains a major challenge. Finding new and effective targets for diagnosis and drug therapy is urgent for TNBC patients. Jagged-1 (JAG1), one of the canonical ligands of the Notch signaling pathway, is involved in vascular budding and is a poor prognostic factor of TNBC. In this study, combined with quantitative real-time PCR, database analysis, animal experiments, and other means, JAG1 was confirmed to be related to the poor prognosis of TNBC patients. JAG1 was highly expressed in MDA-MB-231 Bone (231B) cells, with stronger invasion and metastasis ability than MDA-MB-231 (231) cells. Treatment of human vascular endothelial cells (HUVEC) with TNBC conditioned medium showed that TNBC JAG1 promoted the angiogenesis of HUVEC. Next, we detected the exosomes extracted from TNBC conditioned medium and found that JAG1 promoted the exosome secretion from 231 cells via ALIX-RAB11A/RAB35. In addition, we also found that the exosomes from JAG1 overexpressed TNBC cells contained more long non-coding RNA (lncRNA) MALAT1, and MALAT1 promoted angiogenesis of HUVEC by targeting miR-140-5p. Finally, the angiogenesis-promoting effect of JAG1 in TNBC was further investigated by matrix gel assay. In conclusion, we reveal that JAG1 has a pro-invasion effect on TNBC and is involved in microenvironment angiogenesis by promoting exosome secretion and the MALAT1-miR-140-5p-JAG1/VEGFA pathway.
Keywords: Angiogenesis, Exosome, JAG1, lncRNAMALAT1, Triple-negative breast cancer
Introduction
Breast cancer has become the world's most common cancer,1 accounting for 11.7% of new cases. Breast cancer still accounts for one in six female cancer deaths, with triple-negative breast cancer (TNBC) leading to a high mortality rate. TNBC does not express progesterone receptor, estrogen receptor, and human epidermal growth factor receptor 2 (HER2), with high vascular density and the tendency of invasion and metastasis.2 Due to lacking hormone receptors and HER2, TNBC patients cannot benefit from endocrine or molecular targeted therapy, and high vascular density reduces the efficacy of antiangiogenic drugs.3 The formation of tumor microvessels guarantees rapid tumor growth and metastasis of carcinoma in situ to distant tissues.4 Additionally, distant metastasis significantly affected the mortality of advanced TNBC patients. Therefore, there is an urgent need to clarify the underlying molecular mechanisms of angiogenesis and distant metastasis in TNBC.
Exosomes are nanoscale vesicles (30–150 nm in diameter) secreted by most living cells and surrounded by a lipid bilayer. They contain a variety of biomolecules,5 playing an important role in distant cell communication. Exosomes from tumor cells contain abundant important contents essential for distant metastasis. It is well known that tumor cells secrete more exosomes than normal cells and carry more pro-metastasis molecules for shaping the pre-metastasis microenvironment.6,7
The Notch signaling pathway plays an important role in the differentiation, proliferation, and apoptosis of tumor cells and promotes the occurrence and development of breast cancer. Notch ligands JAG1 and DLL4 are key ligands that affect the interaction between tumor cells and adjacent cells and participate in angiogenesis. JAG1 can participate in the growth, proliferation, metastasis, and other processes of tumor cells, promote the formation and treatment tolerance of tumor stem cells, and is related to vascular density. DLL4 is involved in the main process of tumor angiogenesis. The latest research found that Notch receptors or ligands can be loaded into exosomes to play a distant role and even affect exosome secretion.8,9 However, it is not clear whether JAG1 is packaged into exosomes to influence microenvironment angiogenesis during distant metastasis of breast cancer.
In this study, human vascular endothelial cells (HUVEC) were treated with conditioned medium from TNBC cells with different expression of JAG1, including MDA-MB-231 (231), 231 cells treated with recombinant protein JAG1 (231-rJAG1), and MDA-MB-231 Bone (231B). Meanwhile, 231B was found to have stronger exosome secretion and angiogenesis promoting ability than 231. Further detection of exosomes in conditioned medium showed that lncRNA MALAT1 in exosomes played an angiogenic role in the TNBC microenvironment. Subsequently, we found that miR-140-5p expression was increased in MALAT1 down-regulated HUVEC, while the expression of JAG1 and vascular endothelial growth factor A (VEGFA) was inhibited. These results suggested that exosomal MALAT1 may promote angiogenesis through the miR-140-5p-JAG1/VEGFA signaling pathway in TNBC.
Materials and methods
Cell culture
Human normal mammary epithelial cells MCF-10A, human breast cancer cells T47D, MCF-7, MDA-MB-231 (231), invasive TNBC cells MDA-MB-231 Bone (231B), and HUVEC were preserved in Chongqing Medical University Key Laboratory of Clinical Laboratory and Diagnostics, Ministry of Education. T47D, MCF-7, 231, 231B and HUVEC cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) high glucose medium (Gibco, USA) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. MCF-10A cells were cultured in a specialized medium (CM-0525, Procell, China). Transcriptome and phenotypic analysis of these cells matched published data.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted with the RNA-Quick Purification Kit (RN001, ES Science, China) according to the manufacturer's instructions. The concentration and purity of RNA were measured by a UV spectrophotometer. A total of 1 μg RNA was reversed into cDNA with RT Master Mix for qPCR Ⅱ (HY–K0511A, MCE, China). qRT-PCR was then performed with SYBR Green qPCR Master Mix (HY–K0523, MCE). The cDNA of miRNA was constructed by using specific reverse transcription primers. GAPDH was used as the internal reference control for mRNA and lncRNA, and U6 was used as the internal reference control for miRNA. The sequences of corresponding primers used are listed in supplementary data.
Database analysis
UALCAN is a database for analyzing and mining transcriptome data from The Cancer Genome Atlas (TCGA). UALCAN was used to detect the relationship between breast cancer subtype and JAG1 mRNA expression. Kaplan–Meier Plotter database collected gene chips and RNA-sequencing datasets from public databases such as the European Genome-Phenotype Archive (EGA), TCGA, and Gene Expression Omnibus (GEO). The progression survival (PPS) of JAG1 in breast cancer was analyzed using the Kaplan–Meier Plotter database. GeneMANIA used extensive genomic and proteomic data to identify genes with similar functions. GeneMANIA was used to identify the relationship among JAG1, VEGFA, and DLL4. GEPIA is a database for gene expression analysis based on genotypic tissue Expression (GTEx) and TCGA datasets. In this study, the correlation between JAG1 and VEGFA in breast cancer tissues was detected using the GEPIA “Correlation” module. Metascape database provides multiple functions such as gene enrichment analysis and protein interaction network. Metascape was used for the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of target Genes for miR-140-5p.
Tumor xenotransplantation experiment
Ten nude mice (females, four weeks of age) were divided into two groups. Suspension of 2 × 106 231 and 231B cells (100 μL) was respectively injected into the mammary pad of nude mice of two groups. After ten days, the tumor size was measured with a vernier caliper every three days and the tumor volume was calculated by the formula: volume = 1/2 × (width)2 × length. At week 4, 100 μL matrigel (356324, Corning, USA) mixed respectively with 231 and 231B cells were injected subcutaneously into the nude mice. After five weeks, the mice were sacrificed, and then the tumor tissues, matrigel plugs, and liver and lung tissues were photographed.
Cell viability experiments
Cell viability was detected by the Cell Counting Kit-8 (CCK-8, HY-K0301, MCE). HUVEC cells were cultured in 96-well plates (90 μL DMEM each well) and treated with conditioned media (CM) from different breast cancer cells (NC, 231-blank-CM, 231-rJAG1-CM, 231B-blank-CM, 231B-DAPT-CM). At 0 h, 24 h, 48 h, and 72 h, 10 μL CCK-8 was added to each well and incubated in the incubator for 1 h before being measured. The absorbance was then measured at 450 nm with a microplate reader.
Vascular formation experiment of matrix glue
To detect the effects of the TNBC microenvironment on angiogenesis, the 96-well plate was added with matrix glue (50 μL/well), and placed in incubator for 45 min at 37 °C. After trypsin digestion, HUVEC cells were suspended in CM of 231 and 231B cells. Then 50 μL suspension containing about 3 × 104 HUVEC cells was added to the 96-well plate above and placed in the incubator at 37 °C. After 4–6 h, the vascular formation was observed under an inverted microscope.
Exosome extraction experiment
Exosomes secreted by 231 and 231B were extracted by ultra-high-speed differential centrifugation. The cell supernatant was centrifuged at 4 °C at 300 g for 10 min, 2,000 g for 20 min, and 10,000 g for 30 min. After each centrifugation, the supernatant was collected and the precipitation was discarded. The final supernatant was filtered by a 0.2 μm pore filter (Millipore, USA) to discard large vesicles. The filtered liquid was centrifuged at 100,000 g for 70 min, and the precipitation was re-suspended with sterile phosphate-buffered saline (PBS). The suspension was then centrifuged again at 100,000 g for 70 min. After each ultra-centrifugation, the supernatant was removed as completely as possible using the pipette. Finally, the external vesicles were dissolved in 50–100 μL sterile PBS and stored at −80 °C for two weeks for subsequent experiments.
Transmission electron microscope (TEM) and nanoparticle tracking analysis (NTA) of exosomes
TEM (HT-7700, Hitachi, Japan) was used to visualize the morphology of exosomes. In brief, 10 μL exosome sample was added to the copper net for precipitation for 1 min. After removing the floating liquid from the filter paper, 10 μL of uranyl acetate was added to the copper net for precipitation for 1 min, and the floating solution was absorbed by filter paper. The net was allowed to dry for several minutes at room temperature. Then imaging results were obtained at 100 kV.
NTA (Malvern Nanosight NS300, UK) analysis was used to identify the particle size of exosome samples. The exosome samples were filtered with PBS, diluted at 1: 300 and then tested on the machine. The sample was analyzed for 60 s and repeated five times. Zeta view processing software was used to process data.
Western blotting
Western blot was used to detect protein expression of RAB11a, and exosome marker proteins CD63 and ALIX. The exosomes and cell proteins were separated by SDS-PAGE and transferred to the PVDF membrane at 200 mA. The membrane was blocked with 5% BSA for 60 min and then incubated with primary antibodies against RAB11a (2413S, CST, USA), CD63 (WL02549, Wanlei), and ALIX (WL03063, Wanlei) at 4 °C overnight. After washing three times, the membrane was incubated with a secondary antibody (ZB-2301, ZSGB-BIO, China) for two hours at room temperature.
Exosome uptake experiment
Exosome samples were dyed with Dil (C1991S, Beyotime, China) according to the instruction. The labeled exosomes were added into HUVEC cells infected with green fluorescent protein (GFP) virus and incubated in the dark for four hours. After fixation with 4% paraformaldehyde, the nuclei were stained with 4,6-diamidino2-phenylindole (DAPI,C02-04002, Bioss, China) for 5 min, and internalized exosomes in HUVEC cells were observed via confocal microscopy.
siRNA transfection experiment
231 cells were transfected with small interfering RNA (siRNA) (GenePharma, China) transfection reagent (siRNA control: Negative control FAM, MALAT1 siRNA: MALAT1-Homo-6131, MALAT1-Homo-7645, and MALAT1-Homo-4108). At least 70% of the cells were fused during transfection, and the concentration of siRNA was 100 nM per transfection. After transfection for 4–6 h, the culture medium was replaced with fresh medium, and cells were collected 24–48 h later for the next experiment. The siRNA sequences are shown in Table 1.
Table 1.
The sequence of siRNA.
| Name | sequence (5′-3′) |
|---|---|
| Negative control FAM | F:UUCUCCGAACGUGUCACGUTT |
| R:ACGUGACACGUUCGGAGAATT | |
| MALAT1-Homo-6131 | F:GCCCUCUAAAUAAGGAAUATT |
| R:UAUUCCUUAUUUAGAGGGCTT | |
| MALAT1-Homo-7645 | F:GGGCUUCUCUUAACAUUUATT |
| R:UAAAUGUUAAGAGAAGCCCTT | |
| MALAT1-Homo-4108 | F:GGGCAAAUAUUGGCAAUUATT |
| R:UAAUUGCCAAUAUUUGCCCTT |
F, forward; R, reverse.
Immunohistochemical test
The tumor tissues and matrigel plugs were embedded in paraffin and sectioned. After dewaxing and antigen retrieval, the sections were blocked with 5% goat serum at room temperature for 1 h. Antibodies against VEGFA (WL0009b, Wanlei, China) and CD31 (WL03674, Wanlei, China) were applied in primary antibody diluent at 1: 200, washed twice with PBS for 3 min, incubated with secondary antibody at 37 °C for 30 min, followed by diaminobenzidine (DAB) for 20 s and hematoxylin for 30 s. The expression of VEGFA and CD31 was observed under an inverted microscope and photographed.
Results
JAG1 promotes malignant metastasis of TNBC
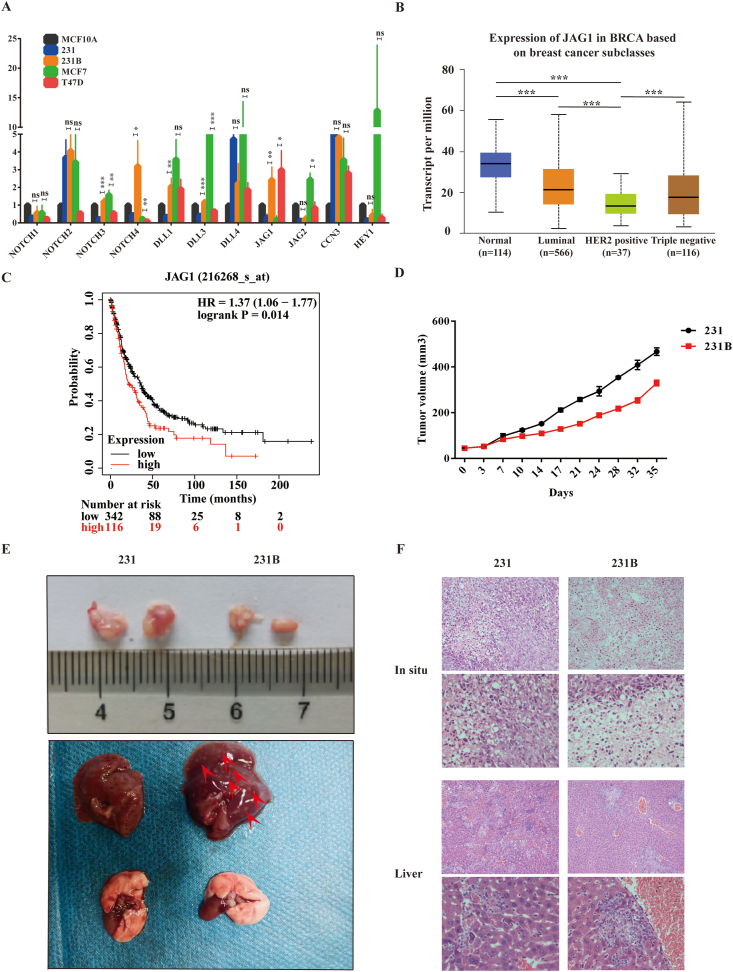
The mRNA expression levels of Notch signaling pathway-related molecules (Notch 1-4, JAG1, JAG2, DLL1, DLL3, DLL4, CCN3, and Hey 1) in TNBC were detected by qRT-PCR, and the results showed that JAG1 expression in 231 cells was lower than that in MCF-10A normal mammary epithelial cells. However, JAG1 expression in 231B cells was higher than in 231 and MCF-10A (Fig. 1A). The mRNA expression of JAG1 in different breast cancer subtypes was analyzed by UALCAN database (Fig. 1B), and the results showed that the average expression of JAG1 in TNBC was 17.566 transcript per million (TPM), while that in normal breast tissue was 33.957 TPM. However, due to the large differences in individual expression of TNBC samples, the difference in JAG1 expression between the normal breast tissue and TNBC was not statistically significant (P > 0.05). In other words, TNBC patients expressed JAG1 at different levels. JAG1 expression levels in the two TNBC cell lines were also highly different, according to our qRT-PCR results. Next, Kaplan–Meier Plotter database analysis (Fig. 1C) found that JAG1 was associated with post-progression survival (PPS) of TNBC (P = 0.014), suggesting a poor prognosis. By injecting 231 and 231B cells into the mammary fat pad of nude mice, hematoxylin and eosin (HE) staining revealed that the nude mice injected with 231B cells showed more liver metastasis and slower tumor growth (Fig. 1D–F). These results indicated that 231B was indeed a more aggressive TNBC cell line, and the expression level of JAG1 was higher than that of 231.
Figure 1.
JAG1 was closely related to the malignant phenotype of TNBC. (A) The expression of the Notch signaling pathway in different breast cancer cell lines was detected by the qRT-PCR assay. (B) The expression of JAG1 in different breast cancer subtypes was analyzed by UALCAN database. (C) The correlation between JAG1 and the survival of breast cancer patients was analyzed by the Kaplan–Meier Plotter database. (D) The tumor volume in nude mice with injection of 231 and 231B into the mammary pad. (E) Tumors in situ and liver metastasis in nude mice. (F) HE staining for tumors in situ and liver metastasis. ns means “Not statistically significant” (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001).
JAG1 regulates angiogenesis in the TNBC microenvironment
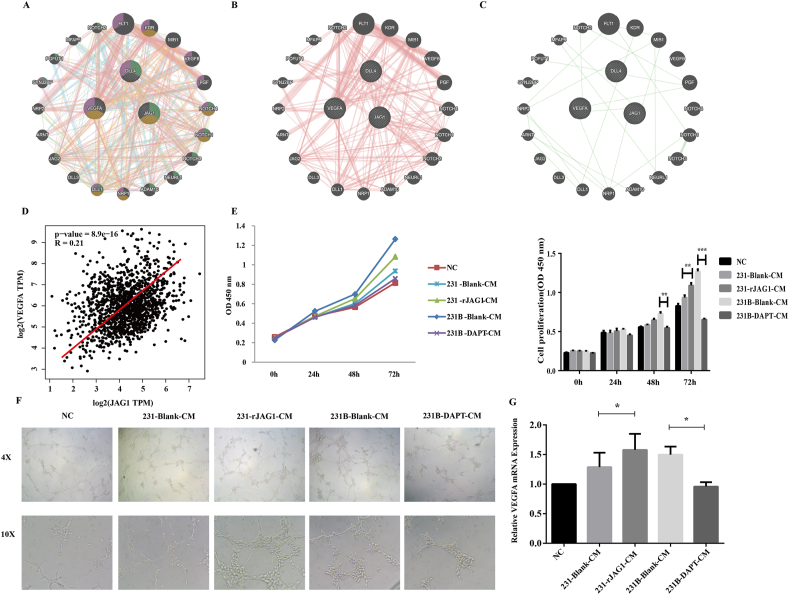
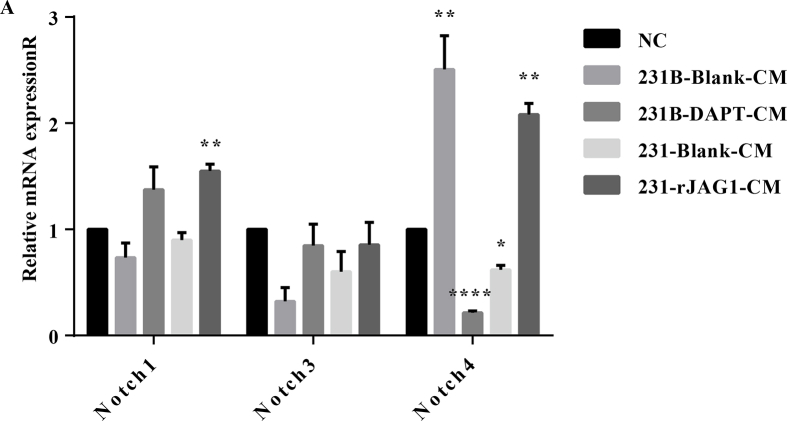
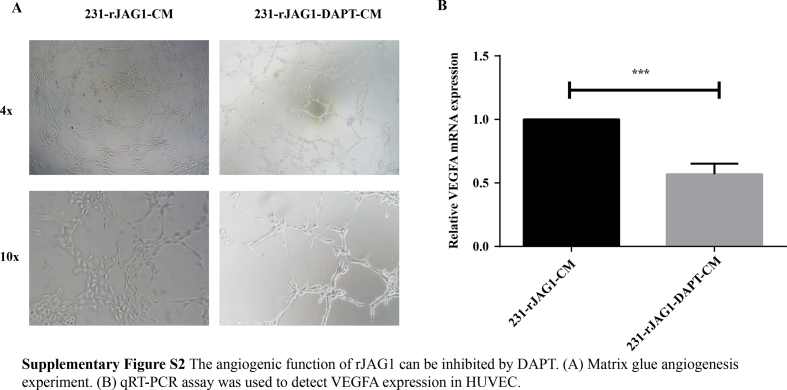
Both DLL4 and JAG1 are transmembrane Notch ligands that regulate angiogenesis by controlling vascular development, endothelial cell (EC) differentiation, and maturation.10,11 In this study, we investigated the relationship among JAG1, VEGFA and DLL4 in the GeneMANIA database (Fig. 2A) and found that JAG1 indeed had complex physical interactions with VEGFA and DLL4 (Fig. 2B). In addition, there were direct Genetic Interactions between JAG1 and VEGFA (Fig. 2C). Previous studies indicated that JAG1 and DLL4 could be packaged on exosomes, bypassing their classic cell–cell contact-dependent signals and affecting angiogenesis at a certain distance. Among them, the relationship between DLL4 and breast cancer angiogenesis has been relatively clear, and the function of JAG1 needs to be further explored. To further explore whether high expression of JAG1 in TNBC can affect angiogenesis remotely through the microenvironment, GEPIA analysis was performed first. The results revealed that JAG1 has a significant positive correlation with VEGFA in breast cancer (Fig. 2D), suggesting that JAG1 may affect VEGFA expression. Then, HUVEC cells were treated with TNBC conditioned medium (CM), and CCK-8 results showed that the rJAG1-231 CM enhanced the proliferation of HUVEC versus 231 CM. Similarly, compared with 231-CM, 231B-CM promoted HUVEC cell proliferation more strongly, and this effect was inhibited by Notch signaling pathway inhibitor DAPT (Fig. 2E). In addition, Notch 4 may be activated as a receptor (Fig. S1). The matrix glue angiogenesis experiment showed that rJAG1 enhanced the angiogenesis promoting ability of 231-CM (Fig. 2F). 231B-CM has stronger angiogenesis promoting ability than 231-CM and can be inhibited by DAPT. The mRNA expression level of VEGFA in HUVEC was detected by qRT-PCR, which further proved that TNBC JAG1 could promote microenvironment angiogenesis (Fig. 2G). The angiogenic activity of rJAG1 was inhibited by DAPT (Fig. S2).
Figure 2.
JAG1 promoted angiogenesis in the TNBC microenvironment. (A–C) GeneMANIA database was used to analyze the relationship of JAG1, VEGFA, and DLL4. (D) Analysis of the correlation of JAG1 and VEGFA via the GEPIA database. (E) CCK-8 assay was performed to detect the effect of TNBC conditioned medium on HUVEC proliferation. (F) Matrigel plug assays to detect the effect of TNBC conditioned medium on HUVEC tubulogenesis ability. (G) qRT-PCR was conducted to detect the expression of VEGFA in HUVEC treated with TNBC conditioned medium (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001).
JAG1 promotes the exosomes secretion of TNBC cells by Alix-RAB11a /RAB35
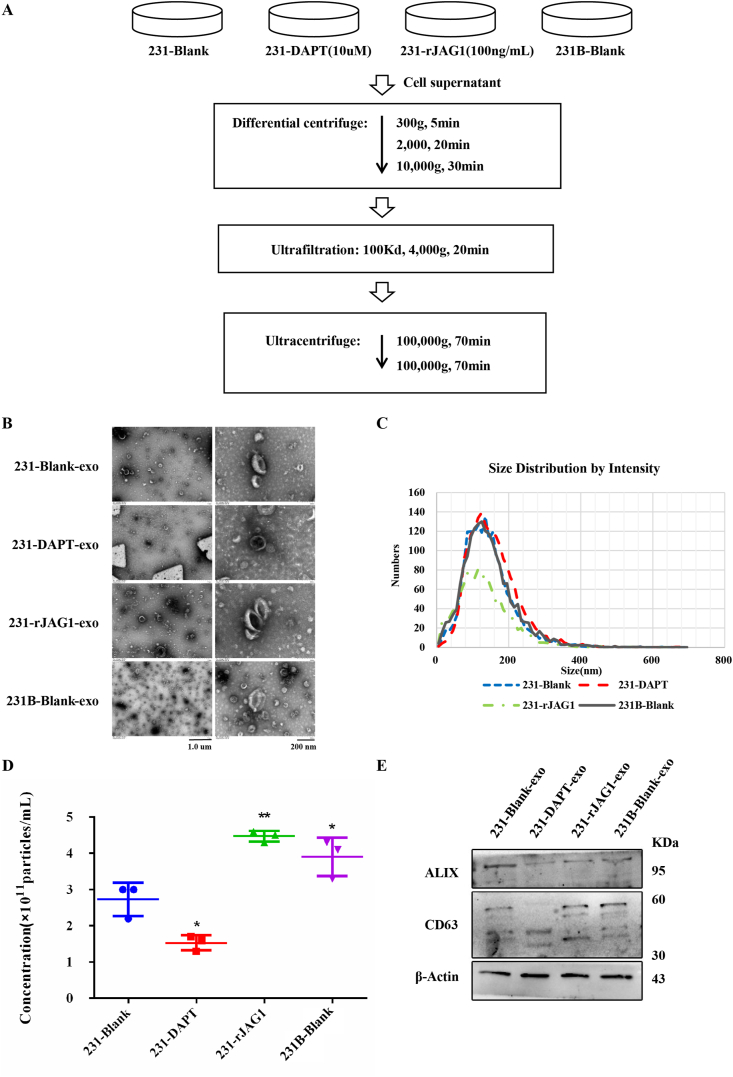
It is believed that exosomes are rich in various bioactive substances, which can promote the formation of tumor niches before metastasis.12 To explore whether the effect of JAG1 on angiogenesis in the TNBC microenvironment is caused by exosomes, the effect of JAG1 on the secretion of TNBC exosomes was analyzed. First, exosomes in breast cancer CM under different treatment conditions were extracted by ultra-high-speed differential centrifugation (Fig. 3A).13 To identify the final centrifuged extract, TEM analysis, NTA analysis, and western blotting were used for characterization. TEM results showed that samples had the typical disc-shaped saucer-like structure of exosomes (Fig. 3B). NTA results showed that the diameter of samples was between 30 and 200 nm (Fig. 3C), which was consistent with the exosome diameter described in existing literature.14 Besides, the concentration of exosome granules in the 231-rJAG1 group and 231B group was significantly higher than those in the 231 group, but significantly decreased after DAPT treatment (Fig. 3D), suggesting that rJAG1 treatment can increase exosome secretion of breast cancer cells. Western blotting results showed the expression of exosome marker proteins ALIX and CD63 in exosome extracts of different groups (Fig. 3E). The above results all confirmed the success of exosome extraction.
Figure 3.
rJAG1 treatment promoted the exosome secretion of 231 cells. (A) Schematic diagram showed the extraction procedure of exosomes from 231-Blank, 231 treated with DAPT (10 μM), 231 treated with rJAG1 (100 ng/mL), and 231B. (B) Representative images of transmission electron microscope (TEM) analysis. (C, D) Particle size analysis to determine the size distribution (C) and particle concentration (D). (E) Western blotting to determine ALIX and CD63 protein levels of exosomes from indicated cells (∗P < 0.05, ∗∗P < 0.01).
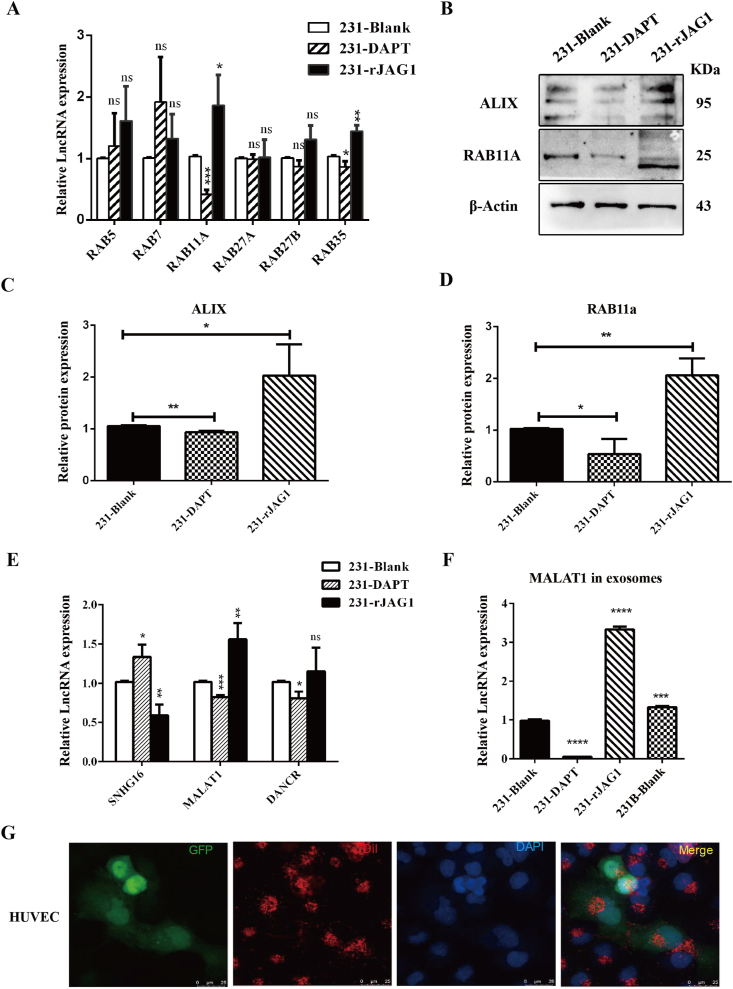
To further clarify the molecular mechanism of JAG1 promoting exosome secretion in TNBC cells, the expression molecules related to exosome formation and release under different treatment conditions were detected in this study. qRT-PCR results showed that mRNA expression levels of exosome release-related molecules RAB11a and RAB35 were significantly up-regulated in the rJAG1 treatment group but down-regulated after DAPT treatment. In contrast, other RAB family molecules showed no significant changes (Fig. 4A). In addition, western blotting results showed that the expression of exosome secret-related protein ALIX in 231 exosomes was up-regulated after rJAG1 treatment, and vice versa after DAPT treatment (Fig. 4B, C). RAB11a protein expression level increased after rJAG1 treatment while DAPT treatment inhibited RAB11a protein expression level (Fig. 4B, D). Therefore, JAG1 can promote exosome secretion through ALIX-RAB11a /RAB35. So, what components of exosomes are at work? It has been reported that JAG1 can be loaded on exosomes to play a long-distance role. However, JAG1 was not packaged on breast cancer exosomes, or the quantity was too small to be detected in this study. Therefore, the focus of this study was shifted to exosomal contents changed by JAG1. Three lncRNAs related to JAG1 that have been experimentally verified were screened out through literature review, including lncRNA SNHG16, MALAT1, and DANCR.15, 16, 17 LncRNA expression in 231 cells and exosomes of different treatment groups were detected by qRT-PCR. The results showed that the MALAT1 expression level in 231 cells was up-regulated after rJAG1 treatment and down-regulated after DAPT treatment (Fig. 4E). Similarly, MALAT1 expression in exosomes was up-regulated in the rJAG1-treated group and 231B group, and down-regulated in the DAPT group (Fig. 4F). Therefore, JAG1 may increase MALAT1 content in TNBC exosomes. Next, through exosome uptake experiments, it was found that TNBC exosomes could be taken in large quantities by HUVEC, indicating that exosomes may affect the angiogenesis of the TNBC microenvironment, and this function may be closely related to MALAT1 in exosomes.
Figure 4.
JAG1 promotes exosome biogenesis and release through RAB11A and ALIX. (A) qRT-PCR to test RAB5, RAB7, RAB11A, RAB27A, RAB27B, and RAB35 expression in 231-Blank, 231 treated with DAPT, and 231 treated with rJAG1. (B) Western blotting was used to test ALIX and RAB11A protein expression in 231 treated by DAPT and rJAG1. Quantification of ALIX (C) and RAB11A (D) protein expression in (B). qRT-PCR to test lncRNA expression of SNHG16, MALAT1, and DANCR in cell (E) and exosomes (F) of 231-Blank, 231-DAPT, 231-rJAG1. (G) The uptake of tumor exosomes (Red) by HUVEC (Green) was detected by immunofluorescence assay (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001).
Exosomal MALAT1 targeting miR-140-5p-JAG1/VEGFA signaling
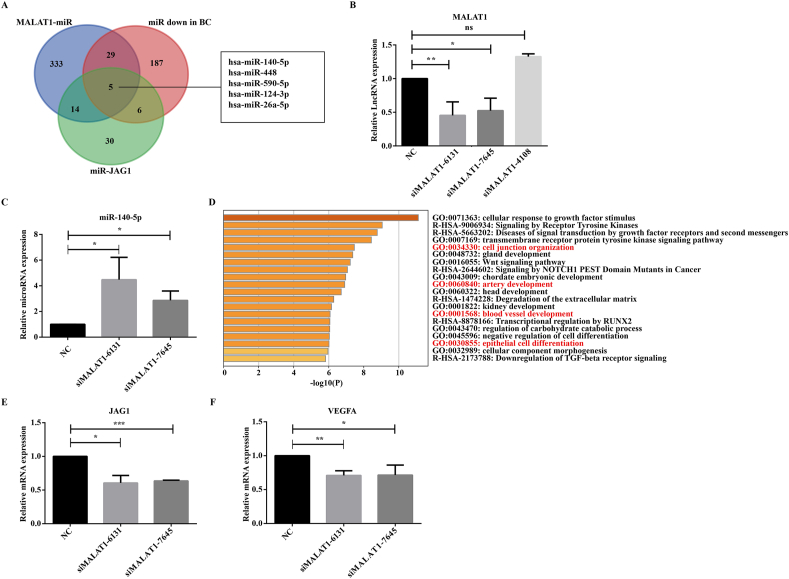
To explore the mechanism of how MALAT1 affects angiogenesis of HUVEC, 381 miRNAs interacting with MALAT1 were found through the ENCORI database and RNAInter database, and 227 miRNAs expressed down-regulated in breast cancer were found through the miRcancer database. A total of 55 miRNAs targeting JAG1 that appeared in three or more miRNA targeting databases were screened out from seven miRNA targeting databases. The intersection of the three was taken, and the results showed that five miRNAs met the requirements, namely miR-140-5p, miR-448, miR-590-5p, miR-124-3p, and miR-26a-5p (Fig. 5A). After interfering HUVEC with siRNAs specific targeting to MALAT1 (siMalat1-6131, siMalat1-7645, siMalat1-4108), we found that siMalat1-6131 and siMalat1-7645 successfully down-regulated MALAT1 expression (Fig. 5B). Studies have experimentally proved that MALAT1 can bind to miR-140-5p.18 Moreover, after MALAT1 interfered, the expression of miR-140-5p in HUVEC was increased significantly (P < 0.05) (Fig. 5C), while the expressions of other miRNAs were not significantly affected. Therefore, MALAT1 may play a role by neutralizing the expression of miR-140-5p. Then KEGG analysis found that miR-140-5p was closely associated with multiple vascular pathways (cell junction blood vessel development, artery development, and epithelial cell differentiation) (Fig. 5D). The mRNA expressions of VEGFA and JAG1 in HUVEC cells were further detected, and it was found that after MALAT1 knockdown, the mRNA expressions of VEGFA and JAG1 in HUVEC cells were decreased (Fig. 5E, F), which further supported the conclusion drawn in Figure 2. Previous studies have shown that JAG1 is a novel target of miR-140-5p in diseases such as glioma and arthritis.19,20 Thus, this study suggested that MALAT1 carried by TNBC exosomes promoted the angiogenesis of HUVEC through the cascade of miR-140-5p-JAG1/VEGFA signaling.
Figure 5.
Exosomal MALAT1 promotes the angiogenesis of HUVEC through a cascade of miR-140-5p/JAG1/VEGFA signals. (A) Screening MALAT1-related miRNAs in breast cancer by Venn diagram. (B) qRT-PCR to verify the knockdown efficiency of the three interference fragments. (C) qRT-PCR to detect the expression of miR-140-5p in HUVEC. (D) KEGG pathway analysis of miR-140-5p. (E, F) qRT-PCR to detect JAG1 (E) and VEGFA (F) expression (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001).
JAG1 promotes angiogenesis in TNBC
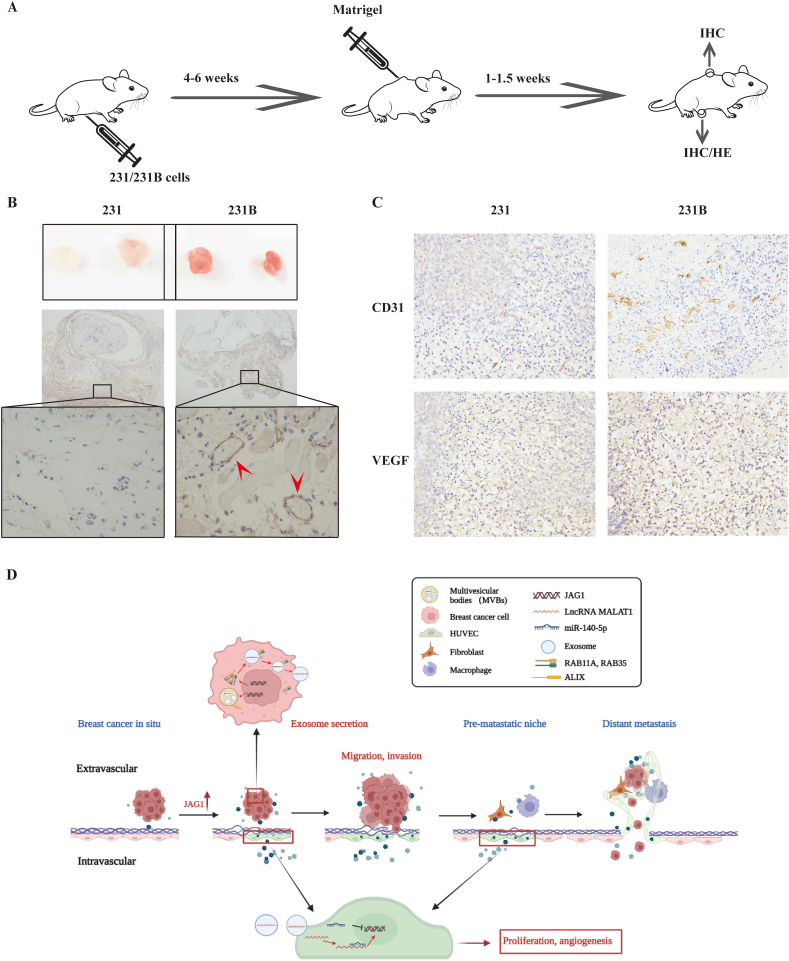
To verify whether JAG1 promotes TNBC angiogenesis in vivo, we conducted a matrigel plugs experiment in nude mice inoculated with 231 and 231B. One week later, the plugs were removed, and immunohistochemical staining was performed to detect the expression of vascular endothelial cell marker CD31 in the matrigel plugs (Fig. 6A, B). It was found that in the nude mice inoculated with 231B, the vascular veins in the plugs were more abundant, and the expression of CD31 was higher. VEGFA and CD31 were also more expressed in 231B tumor tissue than in 231 tumor tissue, suggesting that the degree of TNBC malignancy may be related to angiogenesis ability. Since JAG1 is highly expressed in 231B cells, JAG1 likely plays a role in promoting angiogenesis in the TNBC microenvironment.
Figure 6.
Experiments in vivo showed that JAG1 was correlated with angiogenesis in the TNBC microenvironment. (A) The procedures of animal experiments. (B) Images of plugs and immunohistochemical staining to detect CD31 protein expression level (white arrows showing typical microvessels). (C) Immunohistochemical staining to detect protein expression levels of CD31 and VEGF. (D) JAG1-mediated angiogenesis in the breast cancer microenvironment.
Discussion
TNBC patients benefited from early screening and combination therapy, and the 5-year survival rate was significantly improved.21 However, compared with other types of breast cancer, TNBC is more prone to invasion, metastasis, and recurrence and cannot benefit from endocrine or molecular targeted therapy. The 5-year survival rate of patients with stage IV TNBC is only about 28%.22,23 The high vascular density of TNBC provides abundant nutrients for tumor growth, thus promoting its malignant progression, invasion and metastasis. Therefore, targeting angiogenesis may be one of the breakthroughs in clinical treatment. At present, anti-vascular therapy for TNBC mainly targets VEGF signaling pathway, and representative drugs include bevacizumab and apatinib. Nevertheless, clinical trials have been conducted that the overall survival (OS) of TNBC patients was not significantly improved after treatment.24 Drug resistance has become an important challenge of antiangiogenic therapy due to the upregulation of alternative angiogenic factors. These studies suggest that the mechanism of TNBC tumor angiogenesis is complex and needs to be further elucidated.
Evidence has clearly shown the roles of VEGF and DLL4/Notch 1 signaling pathway in tumor angiogenesis. Notch signaling is expressed in almost all organs, coordinating cell proliferation, differentiation and apoptosis, and determining cell fate.25 Notch signaling has four receptors (Notch1–4) and five ligands (DLL1, DLL3, DLL4, JAG1 and JAG2).26 The relationship between Notch signaling and the occurrence and development of breast cancer has been confirmed, but the effects of different ligands and receptors presented certain heterogeneity. Thus, it is necessary to discuss the function of Notch signaling in breast cancer systematically. So, what is the effect of JAG1 on TNBC tumor angiogenesis? We found a positive correlation between JAG1 and VEGFA through gene co-expression analysis, suggesting that JAG1 and VEGFA may synergistically promote vascular endothelial cell generation. We used the 231B conditioned medium with high JAG1 expression to treat vascular endothelial cells. The results of qRT-PCR and CCK-8 experiments revealed that it could promote the proliferation of vascular endothelial cells. At the same time, the matrix gel tube forming experiment showed that TNBC with high JAG1 expression could significantly promote the tube forming ability of vascular endothelial cells. These results are consistent with the effects of VEGFA on angiogenesis reported by Sung-Hoying et al,27,28 supporting our hypothesis that JAG1 and VEGFA have similar functions in synergistically promoting vascular endothelial cell canalization.
Exosomes are a group of vesicles between 30 and 150 nm in diameter fused with plasma membrane by multivesicular bodies (MVBs).29 This biological process can be divided into three steps: formation of intraluminal vesicles (ILVs), transport of MVBs to lipid membrane, and fusion with the plasma membrane.30 Many molecules are involved in exosome biogenesis and release, such as RAB11a and RAB35, which are members of the Ras superfamily of small G proteins involved in the transport and fusion of MVBs to the plasma membrane. That is, it regulates the exosomal release.31,32 ALIX, also known as PDCD6IP, is involved in MVB biogenesis and affects exosome production.33,34 This study showed that Notch pathway activation by rJAG1 significantly promoted the secretion of M231 exosomes, while Notch pathway inhibition by DAPT inhibited exosome secretion. Mechanistically, we found that the expressions of RAB11a, RAB35, and ALIX, which are involved in the production and release of exosomes, also increased after JAG1 treatment, suggesting that JAG1 may promote the production and release of exosomes through RAB11a, RAB35, and ALIX. It has been reported that the Notch signaling pathway is involved in cell vesicle transport. For example, Notch 4 can target RAB5a and RAB11a and affect exosome secretion containing enzyme NADPH oxidase 2 (NOX2).35
Tumor-related molecules in exosomes are potential early diagnostic markers for tumor metastasis, including proteins, DNA, RNA, etc.36 As the basic molecular unit of intracellular executive function, protein has the disadvantages of large molecular weight, limited content, and inconvenient detection methods. However, lncRNAs are a class of non-coding RNAs whose length is greater than 200 nucleotides.37 On the one hand, lncRNA, as a regulatory factor of gene expression, can participate in various diseases through epigenetic, transcription, and post-transcriptional regulation, with the effect of signal amplification.38 On the other hand, clinical PCR detection of lncRNA is quick, simple, and easy to be quantified. In this study, the level of MALAT1 in 231 exosomes was significantly upregulated after 231B and rJAG1 treatment, suggesting that MALAT1 in TNBC exosomes may play a role in long-distance signaling, thus shaping the pre-metastasis microenvironment.
Exosomes can be ingested by HUVEC, so this study explored downstream targets of MALAT1 in HUVEC cells. Five target genes of MALAT1 were screened out from the database, and miR-140-5p may play an important role after qRT-PCR experiment verification. After MALAT1 knockdown, both JAG1 and VEGFA expressions in HUVEC were inhibited, while JAG1 is one of the target genes of miR-140-5p. Therefore, in the TNBC microenvironment, exosomes delivered MALAT1 may play an angiogenic role through miR-140-5p-JAG1/VEGFA. The matrix thrombus with a denser vascular distribution was observed in nude mice inoculated with 231B, confirming the previous conclusion in vivo.
Conclusions
This study further revealed the effect of JAG1 on the TNBC malignant phenotype through a series of in vitro and in vivo functional experiments. It was preliminarily confirmed that JAG1 could promote the angiogenesis of breast cancer. Mechanically, JAG1 may affect miR-140-5p through exosomal MALAT1 to promote the angiogenesis of HUVEC. JAG1 and VEGFA have a synergistic effect on promoting angiogenesis, suggesting that JAG1 could be a potential target for anti-vascular therapy of TNBC. However, whether JAG1 interacts with DLL4 and other Notch ligands to regulate tumor angiogenesis needs further experimental verification.
Ethics declaration
All experiments involving animals and procedures were approved by the Institutional Animal Care and Use Committee of Chongqing Medical University (2021-023).
Author contributions
Experiment plan and perform: JPL, YTS, and MMW. Figures preparation: YTS; data analysis: JPL and MMW. MQX, FMZ, and ZQH helped to perform experiments. Drafting of the manuscript: MT. All authors agree on the final published version.
Conflict of interests
We declare that this study was conducted without any commercial or financial relationships.
Acknowledgements
We thank for the help of the teachers and students in the Clinical Laboratory of Clinical Laboratory and Diagnostics of Chongqing Medical University.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2022.07.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure s1.tif.
Supplementary figure s2.tif.
References
- 1.World health organization . 2020. Latest Global Cancer Data: Cancer Burden Rises to 19.3 Million New Cases and 10.0 Million Cancer Deaths in 2020.https://www.iarc.who.int/faq/latest-global-cancer-data-2020-qa/ [Google Scholar]
- 2.Lehmann B.D., Bauer J.A., Chen X., et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gluz O., Liedtke C., Gottschalk N., Pusztai L., Nitz U., Harbeck N. Triple-negative breast cancer: current status and future directions. Ann Oncol. 2009;20(12):1913–1927. doi: 10.1093/annonc/mdp492. [DOI] [PubMed] [Google Scholar]
- 4.Li Z., Kang Y. Emerging therapeutic targets in metastatic progression: a focus on breast cancer. Pharmacol Ther. 2016;161:79–96. doi: 10.1016/j.pharmthera.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 6.Nair S., Tang K.D., Kenny L., Punyadeera C. Salivary exosomes as potential biomarkers in cancer. Oral Oncol. 2018;84:31–40. doi: 10.1016/j.oraloncology.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Saber S.H., Ali H.E.A., Gaballa R., et al. Exosomes are the driving force in preparing the soil for the metastatic seeds: lessons from the prostate cancer. Cells. 2020;9(3):564. doi: 10.3390/cells9030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan E., Asada H.H., Ge R. Extracellular vesicle-carried jagged-1 inhibits HUVEC sprouting in a 3D microenvironment. Angiogenesis. 2018;21(3):571–580. doi: 10.1007/s10456-018-9609-6. [DOI] [PubMed] [Google Scholar]
- 9.Boelens M.C., Wu T.J., Nabet B.Y., et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159(3):499–513. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco R., Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. 2013;3(1):a006569. doi: 10.1101/cshperspect.a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kume T. Novel insights into the differential functions of Notch ligands in vascular formation. J Angiogenes Res. 2009;1:8. doi: 10.1186/2040-2384-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wortzel I., Dror S., Kenific C.M., Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. 2019;49(3):347–360. doi: 10.1016/j.devcel.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Lobb R.J., Becker M., Shu W.W., et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031. doi: 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478) doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang T.H., Liang L.Z., Liu X.L., et al. LncRNA UCA1/miR-124 axis modulates TGFβ1-induced epithelial-mesenchymal transition and invasion of tongue cancer cells through JAG1/Notch signaling. J Cell Biochem. 2019;120(6):10495–10504. doi: 10.1002/jcb.28334. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y., Fan B., Ren Z., Liu B., Wang Y. Long noncoding RNA DANCR contributes to docetaxel resistance in prostate cancer through targeting the miR-34a-5p/JAG1 pathway. OncoTargets Ther. 2019;12:5485–5497. doi: 10.2147/OTT.S197009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H., Fan L., Zhang Y., Cao Y., Liu X. SNHG16 aggravates chronic constriction injury-induced neuropathic pain in rats via binding with miR-124-3p and miR-141-3p to upregulate JAG1. Brain Res Bull. 2020;165:228–237. doi: 10.1016/j.brainresbull.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Bao M., Liu G., Song J., Gao Y. Long non-coding RNA MALAT1 promotes odontogenic differentiation of human dental pulp stem cells by impairing microRNA-140-5p-dependent downregulation of GIT2. Cell Tissue Res. 2020;382(3):487–498. doi: 10.1007/s00441-020-03246-1. [DOI] [PubMed] [Google Scholar]
- 19.Yang H.L., Gao Y.M., Zhao J.A. miR-140-5p inhibits human glioma cell growth and invasion by targeting JAG1. Mol Med Rep. 2017;16(3):3634–3640. doi: 10.3892/mmr.2017.6951. [DOI] [PubMed] [Google Scholar]
- 20.Cao F., Chen Y., Wang X., et al. Therapeutic effect and potential mechanisms of intra-articular injections of miR-140-5p on early-stage osteoarthritis in rats. Int Immunopharm. 2021;96:107786. doi: 10.1016/j.intimp.2021.107786. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute (NCI) 2021. Cancer Stat Facts: Female Breast Cancer.https://seer.cancer.gov/statfacts/html/breast.html [Google Scholar]
- 22.Burke E.E., Kodumudi K., Ramamoorthi G., Czerniecki B.J. Vaccine therapies for breast cancer. Surg Oncol Clin. 2019;28(3):353–367. doi: 10.1016/j.soc.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 23.America Cancer Society . 2021. Survival Rates for Breast Cancer.https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html [Google Scholar]
- 24.Robert N.J., Diéras V., Glaspy J., et al. RIBBON-1:randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29(10):1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 25.Ballhause T.M., Jiang S., Baranowsky A., et al. Relevance of Notch signaling for bone metabolism and regeneration. Int J Mol Sci. 2021;22(3):1325. doi: 10.3390/ijms22031325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van de Walle I., Waegemans E., De Medts J., et al. Specific Notch receptor-ligand interactions control human TCR-αβ/γδ development by inducing differential Notch signal strength. J Exp Med. 2013;210(4):683–697. doi: 10.1084/jem.20121798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying T.H., Lin C.L., Chen P.N., Wu P.J., Liu C.J., Hsieh Y.H. Angelol-A exerts anti-metastatic and anti-angiogenic effects on human cervical carcinoma cells by modulating the phosphorylated-ERK/miR-29a-3p that targets the MMP2/VEGFA axis. Life Sci. 2022;296:120317. doi: 10.1016/j.lfs.2022.120317. [DOI] [PubMed] [Google Scholar]
- 28.Wang H., Chen L., Wang S., et al. Tetrandrine promotes angiogenesis via transcriptional regulation of VEGF-A. Vasc Pharmacol. 2021;141:106920. doi: 10.1016/j.vph.2021.106920. [DOI] [PubMed] [Google Scholar]
- 29.Pegtel D.M., Gould S.J. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 30.Hessvik N.P., Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai S., Hou W., Yao Y., et al. Exocyst controls exosome biogenesis via Rab11a. Mol Ther Nucleic Acids. 2021;27:535–546. doi: 10.1016/j.omtn.2021.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klinkert K., Echard A. Rab35 GTPase: a central regulator of phosphoinositides and F-actin in endocytic recycling and beyond. Traffic. 2016;17(10):1063–1077. doi: 10.1111/tra.12422. [DOI] [PubMed] [Google Scholar]
- 33.Larios J., Mercier V., Roux A., Gruenberg J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J Cell Biol. 2020;219(3) doi: 10.1083/jcb.201904113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baietti M.F., Zhang Z., Mortier E., et al. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 35.Jin K., Wen Z., Wu B., et al. NOTCH-induced rerouting of endosomal trafficking disables regulatory T cells in vasculitis. J Clin Invest. 2021;131(1) doi: 10.1172/JCI136042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mashouri L., Yousefi H., Aref A.R., Ahadi A.M., Molaei F., Alahari S.K. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75. doi: 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barile L., Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63–78. doi: 10.1016/j.pharmthera.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 38.Sun Z., Yang S., Zhou Q., et al. Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol Cancer. 2018;17(1):82. doi: 10.1186/s12943-018-0831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]