Abstract
NK2 genes (NKX2 gene cluster in humans) encode for homeodomain-containing transcription factors that are conserved along the phylogeny. According to the most detailed classifications, vertebrate NKX2 genes are classified into two distinct families, NK2.1 and NK2.2. The former is constituted by NKX2-1 and NKX2-4 genes, which are homologous to the Drosophila scro gene; the latter includes NKX2-2 and NKX2-8 genes, which are homologous to the Drosophila vnd gene. Conservation of these genes is not only related to molecular structure and expression, but also to biological functions. In Drosophila and vertebrates, NK2 genes share roles in the development of ventral regions of the central nervous system. In vertebrates, NKX2 genes have a relevant role in the development of several other organs such as the thyroid, lung, and pancreas. Loss-of-function mutations in NKX2-1 and NKX2-2 are the monogenic cause of the brain-lung-thyroid syndrome and neonatal diabetes, respectively. Alterations in NKX2-4 and NKX2-8 genes may play a role in multifactorial diseases, autism spectrum disorder, and neural tube defects, respectively. NKX2-1, NKX2-2, and NKX2-8 are expressed in various cancer types as either oncogenes or tumor suppressor genes. Several data indicate that evaluation of their expression in tumors has diagnostic and/or prognostic value.
Keywords: Drosophila melanogaster, Evolutionary conservation, Homeobox, Homeotic genes, NK2 genes
Introduction
Homeobox-containing genes are defined by the presence of a conserved 180 bp sequence called homeobox, whose name derives from its presence within several homeotic genes in Drosophila melanogaster.1 Homeobox-containing genes encode transcription factors that have a pivotal role during animal development.2,3 Homeobox sequences encode for the homeodomain (HD) which is the DNA-binding domain of these transcription factors. The HD is extremely conserved along the phylogeny and is constituted by three α-helices folding together to generate a relatively rigid structure, in which major sequence-specific DNA contact is established by the third (recognition) helix located on the DNA major groove.4, 5, 6, 7
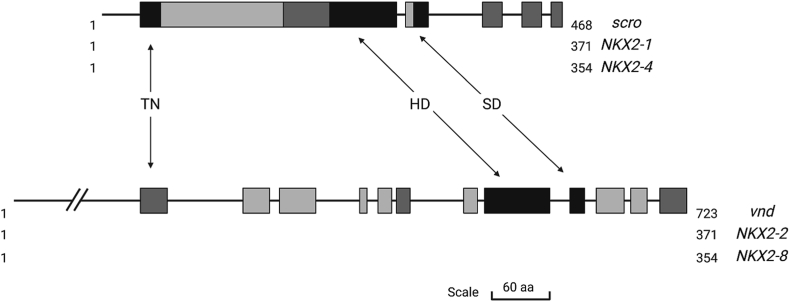
In humans, more than 200 homeobox-containing genes have been identified8 and, for most of them, a specific role during development has been delineated.9 Mutations in several homeobox-containing genes are either causative of monogenic diseases or risk factors for polygenic ones.10,11 Based on sequence conservation, homeobox-containing genes are grouped into different classes, subclasses, and families.8 Referring to the work by Holland and colleagues,8 NK2 genes are part of the Antennapedia (antp) class and include two distinct families, NK2.1 and NK2.2. The former is constituted by NKX2-1 and NKX2-4 genes, which are homologous to the Drosophila scro gene; the latter includes NKX2-2 and NKX2-8 genes, which are homologous to the Drosophila vnd gene. Drosophila-related genes are much bigger than human genes, mostly for differential intron length. The homeobox coding sequence is contained within a single exon in all genes, except for scro. Together with the NK4 family members, NK2.1/2 proteins interact with DNA by contacting sequences having a 5’-CAAT-3’ core.6,12 In addition to the HD, NK2.1/2 proteins share two other domains, the tinman (TN) domain, located near the amino-terminal, and the NK2-specific domain (SD), located at the C-terminal.13, 14, 15 Similarities between NK2 proteins are shown in Figure 1. Both the HD and the SD are extremely conserved in scro and vnd homologous proteins. The TN domain is highly conserved among scro-homologous proteins but less among vnd-homologous ones.
Figure 1.
Structural similarity among NK2.1 and NK2.2 proteins. Schematic representation of the similarity among scro/NKX2-1/NKX2-4 and vnd/NKX2-2/NKX2-8 proteins. Colors represent the percentage of sequence homology: light gray boxes represent sequences with homology <20%; dark gray boxes represent sequences with homology between 20% and 80%; black boxes represent sequences with homology >80%. Black lines represent fragments of Drosophila proteins not present in human NK proteins. Numbers represent protein length. HD: homeodomain; SD: NK2-specific domain; TN: tinman domain. Created with Biorender.com.
Drosophila scro and vnd genes are located in different chromosomes. Instead, chromosomal co-localization and genomic proximity are present for human NKX2-1 and NKX2-8 on chromosome 14 and NKX2-2 and NKX2-4 on chromosome 20. This peculiar localization into clusters suggests that the members of each cluster share the same regulation pattern. Indeed, NKX2-1 and NKX2-8 are co-expressed in the thyroid gland while NKX2-2 and NKX2-4 are co-expressed in the hypothalamus and the pituitary gland. By using the TADKB tool (http://dna.cs.miami.edu/TADKB/browse.php), it is evident that each couple is part of the same topological associating domain (TAD) in different cell lines.
Alterations in the NKX2 family are related to distinct human diseases. Here, after delineating the major biological functions of these genes, their roles in human genetic disorders are reviewed.
Expression and biological functions of NK2.1 and NK2.2 genes
NK2 gene expression and its functions in different species indicate remarkable conservation along the phylogeny. In particular, the identity of the ventral regions of the central nervous system (CNS), from Drosophila to humans, appears to be regulated by these genes. As stated in the introduction, compared to Drosophila, NK2 genes are duplicated in vertebrates.16,17 Gene duplication is a major driver of molecular evolution, being responsible for the origin of new genes.18 Since functional divergence among duplicated genes is required for their maintenance within the genome, “neo-functionalization” occurs when one of the duplicated copies acquires new functions.19 Alternatively, “sub-functionalization” occurs when ancestral functions of the progenitor gene are partitioned between duplicated copies.20 Both mechanisms have been likely involved in NK gene evolution. Indeed, a model including neo- and sub-functionalization together has been proposed.21
To understand the aforementioned concept, the peculiar expressions and functions of Drosophila scro and vnd will be initially summarized, and then the description of human orthologues will be discussed.
Drosophila melanogaster
Scro
During Drosophila embryogenesis, scro is expressed in the pharyngeal primordia and later maintained in the pharynx. This gene is also expressed in bilateral clusters of procephalic neuroblasts and segmental clusters of neuronal precursors in the ventral nerve cord. Then, scro expression is detected in the larval and adult optic lobes.22 Homozygous loss-of-function mutations of scro are lethal during both embryonic and early larval development. Deformed neuroepithelial cells in the developing optic lobe and severely malformed adult optic lobes are present in mutants,23 indicating that scro plays a role in the development and/or function of this tissue. Indeed, one of the scro targets is the pigment-dispersing factor (PDF) encoding gene, which is expressed in ventral lateral neurons (LNvs), acting as a central pacemaker for circadian locomotor activity rhythms. Scro acts as a repressor, binding to a cis-acting element of the pdf gene.24
Vnd
During Drosophila development, vnd is expressed in the ventral column of neuroectoderm and neuroblasts and is maintained in a subset of neurons derived from these neuroblasts.25, 26, 27 Loss of vnd transforms the ventral column into the intermediate column identity, and ectopic vnd transforms their identity the other way around. Therefore, this gene is critical in specifying the dorsoventral axis during Drosophila CNS development.27 The major known molecular function of vnd is transcriptional repression. By performing misexpression experiments in Drosophila larva, it has been shown that vnd represses ind and msh gene expression and that the addition of vnd binding sites to a heterologous enhancer is sufficient to mediate repression. Moreover, the authors show that transcriptional repression occurs by interaction with Groucho protein in the TN domain.28
Homo sapiens
Human orthologues retain some features of Drosophila genes but acquire new and tissue-specific ones. Indeed, studies investigating the expression and functions of NK2 genes have been performed using different animal models. In this section, we will refer to the vertebrate homeobox-containing genes with the Homo sapiens-related name, notwithstanding several of the reviewed data that have been obtained in mouse, zebrafish, or other species.
NKX2-1
NKX2-1 (also named TTF-1, TITF-1, T-EBP) is expressed during the development of the thyroid, lungs, and several regions of the ventral forebrain.29 During thyroid development, NKX2-1 expression initiates at the very beginning of gland morphogenesis in the ventral endoderm of the primitive pharynx (thyroid anlage) and continues up to adulthood.30 In addition to the follicular thyroid cells, NKX2-1 is expressed in the C-cells as well as in parathyroid glands.31 NKX2-1 contributes to the expression of several genes related to the differentiation of thyroid follicular cells such as thyroglobulin (TG), thyroid peroxidase (TPO), and thyrotropin receptor (TSHR).11,32 Indeed, experiments using conditional NKX2-1 knockout mice suggest that this gene is required for the maintenance of the normal architecture and functions of differentiated thyroids.33
As for airway development, NKX2-1 represents the earliest marker of lung fate. It is later confined to the alveolar and bronchial epithelium.34 Indeed, it is first expressed in the prospective lung domain of the developing foregut endoderm at E9 during mouse embryogenesis. Ikonomou and colleagues demonstrated that Nkx2-1-positive lung epithelial primordial progenitors express Wnt and Tgf-β superfamily pathways shaping their cell-fate determination from pre-specified embryonic foregut.35 This transcription factor is also essential for the induction of lung branching morphogenesis.36, 37, 38 NKX2-1 appears essential also in the septation of the tracheoesophageal primordium.38 Kuwahara and colleagues demonstrated by single-cell RNA-sequencing that tracheal commitment is NKX2-1 independent, since Nkx2-1−/− mutant embryos did not experience a transcriptome-wide tracheal-to-esophageal conversion.39 Some NKX2-1 independent mechanisms contribute to tracheal cartilage specification, as some disorganized cartilage still forms in Nkx2-1−/− mutants. Moreover, they highlighted that NKX2-1 is able to bind the regulatory sequences of sonic hedgehog (Shh) and Wnt Family Member 7B (Wnt7b) regulating their expression to control mesenchymal specification to cartilage and smooth muscle, coupling epithelial identity with mesenchymal specification.39
In adulthood, elevated NKX2-1 expression is detected in lung alveolar type II epithelial cells as well as in epithelial cells-based lining bronchi and bronchioles.40 This transcription factor is essential for the expression of distinct surfactant proteins (A, B, C, D), Clara cell secretory proteins, and others.41 By using conditional knockout in mouse, Snyder and co-workers have demonstrated that NKX2-1 deletion induces loss of pulmonary commitment and conversion to gastric lineage; such an effect is mediated by recruitment of transcription factors Foxa1 and Foxa2 to lung-specific promoters, preventing activation of the gastric-specific transcription program.42
To date, several different NKX2-1 interactors have been so far identified in lung-related cells including Pax8, GATA6, STAT3, retinoic acid receptor (RAR) and associated cofactors, nuclear factor-I (NFI–B), AP1 family members, and BR22.43, 44, 45, 46, 47, 48, 49 Moreover, in the murine respiratory epithelial cell within the lungs, NKX2-1 interacts with the surfactant protein C promoter together with the TAZ protein.50 By using immortalized lung epithelial cells, the interaction between NKX2-1, PARP1, and PARP2 proteins has been shown as well as the enhancement in the activity of the promoter of surfactant protein B gene.51
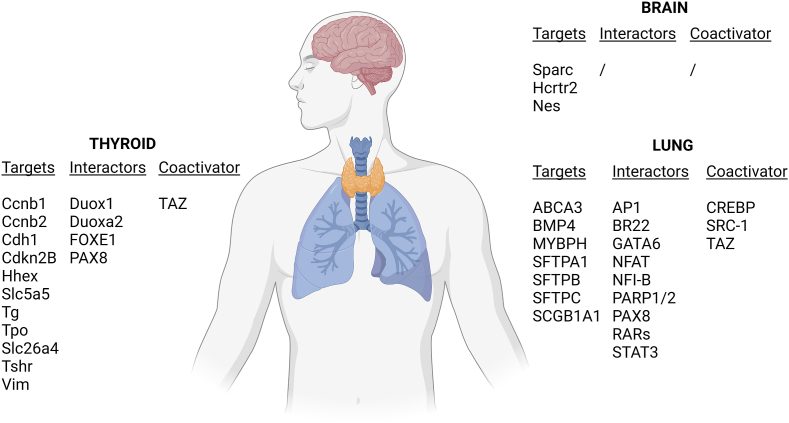
During development, NKX2-1 is expressed in restricted regions of the brain within the diencephalon, in some regions of the hypothalamus and the neurohypophysis, and in the telencephalon.34 In preoptic and hypothalamic areas, NKX2-1 is expressed in the ventral region of the ventricular neuroepithelium. Though this gene is not expressed in Rathke's pouch (derived from the oral epithelium and primordium of the anterior and the intermediate parts of the pituitary), NKX2-1 null mice lack completely the pituitary gland. Indeed, this indicates that NKX2-1 expression in the floor of the diencephalon and in the infundibulum is required for a fully development of the anterior and intermediary pituitary from Rathke's pouch.37 It has been shown that NKX2-1 is expressed in hypothalamic proopiomelanocortin (POMC) neurons from early development to adulthood. Being essential for POMC expression, it is considered a member of the multi-component system contributing to body weight regulation.52 Moreover, by controlling angiotensinogen expression in the subfornical organ, NKX2-1 contributes to the regulation of body fluids homeostasis.53 In the cortex, NKX2-1 has been shown to contribute to the interneuron subtype specification.54 NKX2-1 interactors, targets, and coactivators in the brain, lungs, and thyroid are summarized in Figure 2.
Figure 2.
NKX2-1 transcriptional web. Representation of NKX2-1 targets, interactors, and co-activators in the brain, lungs, and thyroid. Created with Biorender.com.
NKX2-4
NKX2-4 results from the duplication of NKX2-1.16,55 NKX2-4 is indeed expressed in a restricted area of the hypothalamus. Differently from NKX2-1, NKX2-4 is not expressed in the telencephalon. Like NKX2-1, instead, NKX2-4 is expressed in the most ventral region of the hypothalamic area.55 Thus, in this region, NKX2-1 and NKX2-4 appear to have redundant functions. In fact, experiments in zebrafish highlighted that the combined morpholino gene silencing, but not a single one, is required to obtain a significant modification of hypothalamus development.56 By genetic experiments in zebrafish, it has been shown that, in several hypothalamic neurons, NKX2-4 (as well as NKX2-1) controls bsx, a gene encoding a homeodomain-containing protein, which plays a critical role in the differentiation of multiple neuromodulatory cell types in the forebrain.57 NKX2-4 has been detected in the thyroid gland only in the rainbow trout. Experiments in HeLa cells highlighted that NKX2-4 is able to transactivate the thyroglobulin promoter, even if to a lesser extent compared to NKX2-1.58
NKX2-2
During development, NKX2-2 is expressed in the CNS, pancreas, and intestine, in which regulates the cell fate of several cell lineages.59, 60, 61 In the developing pancreas, endocrine progenitors show the NKX2-2 highest expression.62 In adulthood, NKX2-2 pancreatic expression is detected in the alpha, the beta, and the pancreatic polypeptide (PP) cells but not in delta ones.61 NKX2-2 null mice die shortly after birth developing severe hyperglycemia, thus indicating that this transcription factor is essential for the final differentiation of pancreatic beta cells.61,63 The insulin encoding gene has been identified as a direct target of NKX2-2.64 Using rat cells, the interaction between NKX2-2 and calmodulin-binding transcription activator 1 (Camta1) has been demonstrated. Indeed, this interaction controls the promoter activity of the miR-212/miR-132 cluster, whose overexpression increases insulin secretion.65 During both intestinal development and adulthood, NKX2-2 is expressed in epithelial enteroendocrine cells located in the villus and crypt. These cell types are thus able to give rise to all intestinal epithelial cell types, thus having stem cell properties during normal intestinal cell renewal.66
In the early development of the neural tube, NKX2-2 is expressed in most ventral progenitors. In particular, this gene is expressed in the ventral regions of the diencephalon, the hypothalamus, and the thalamus, partially overlapping with NKX2-1-expressing regions. Indeed, both contribute to the brain morphological differentiation.13 In the ventral neural tube, the identity specification of neurons is dependent on the phased activity of the signaling protein Shh. By analyzing neurogenesis in the ventral spinal cord and hindbrain, it has been shown that NKX2-2 plays a pivotal role in ventral neuronal patterning; in these regions, it triggers neuronal identity by interpreting Shh phased signals.59 By using cells derived from primary cultures of rat oligodendrocyte precursors, Wei and co-workers have found that NKX2-2 represses the expression of myelin basic protein by interacting with two in-cis regulatory elements.67 They also showed that the transcription factor Sp1 reversed the repressive effect of NKX2.2 by competing with NKX2-2 for its binding sites.
NKX2-8
NKX2-8 (named Nkx2.9 in mouse) has been identified through the binding of its protein product to the regulatory elements of the α-fetoprotein gene.68 However, it has been shown that NKX2-8 expression is not detectable in the liver at any developmental stage as well as in adulthood, with the absence of morphological anomalies in this organ.69 In contrast, NKX2-8 is expressed in developing thyroid, pharynx, and lung. In the latter, NKX2-8 is detected in tracheobronchial stem cells, in which it downregulates the proliferation activity. In fact, when this gene is inactivated, abnormal expansion of this cell type is observed, inducing bronchial dysplasia.69 It has been proposed that in developing lungs NKX2-8 downregulates NKX2-1 gene and antagonizes the transcriptional activity of the latter on its target genes.69 As NKX2-1 and NKX2-2, NKX2-8 is expressed in the ventral region of the developing neural tube. In particular, its expression is restricted to V3 interneurons of the spinal cord and visceral motoneurons in the hindbrain. However, because of functional redundancy with NKX2-2, inactivation of NKX2-8 has no effects on spinal cord physiology. Instead, its inactivation in the hindbrain affects the development of branchial motoneurons, always determining morphological alteration of the accessory nerve (11th) and, only in some embryos, the abnormalities of the glossopharyngeal (9th) and vagal (10th) nerves.70 Thus, though overlapping expression of the two genes, NKX2-2 is not able to fully substitute NKX2-8 for motoneuron differentiation of the ventral hindbrain. Chromatin immunoprecipitation (ChIP) experiments suggest that NKX2-8 binds to and regulates genes having a role in apoptosis and fatty acid oxidation, including CPT1A and CPT2 genes; moreover, demonstrated that NKX2-8 can interact with Sin3A/HDAC1/SAP18 complex binding with their promoters.71 By performing cell line transfection experiments, a synergic effect between NKX2-8 and serum response factor on a minimal cardiac alpha-actin promoter has been demonstrated.72
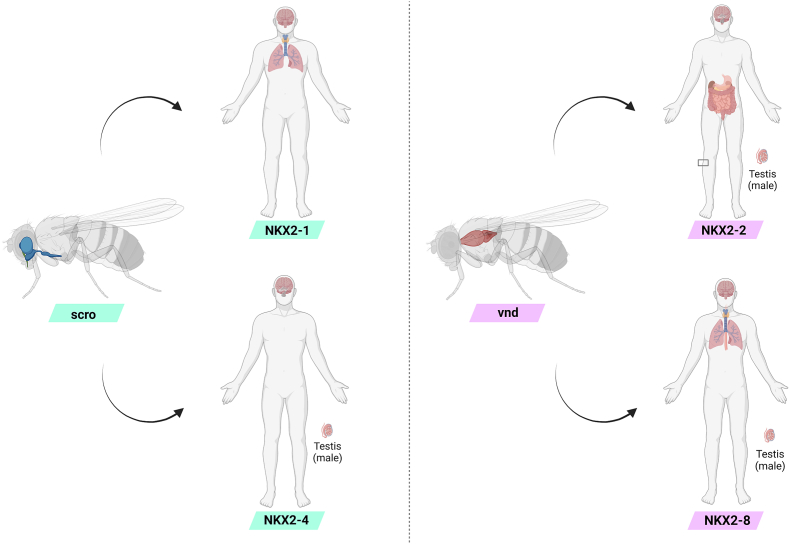
A summary of the tissue expression of Drosophila scro and vnd together with human NK2.1/2 family members is provided in Figure 3. Moreover, the differential localization of human NK2.1/2 genes expression during development and adulthood is summarized in Table 1.
Figure 3.
Tissue expression of Drosophila scro and vdn together with their human orthologues. Left panel: scro is expressed in Drosophila's central nervous system and pharynx, while its human orthologues are expressed in the brain, lung, and thyroid (NKX2-1) and brain and testis (NKX2-4), respectively. Right panel: vnd is expressed in Drosophila's ventral nerve cord, while its human orthologues are expressed in the brain, skin, gastrointestinal system, and testis (NKX2-2), and brain, lungs, thyroid, esophagus, and testis (NKX2-8), respectively. Created with Biorender.com.
Table 1.
Differential localization of human NK2 genes expression during development and adulthood.
| NKX2 gene | Major Tissues Expression during development | Major Tissues Expression during adulthood |
|---|---|---|
| NKX2-1 | lung, tracheoesophageal primordium, thyroid, diencephalon, hypothalamus, neurohypophysis, telencephalon, hypothalamus | lung, thyroid, hypothalamus, pituitary gland |
| NKX2-2 | pancreas, intestine, diencephalon, hypothalamus, thalamus | pancreas, intestine, hypothalamus, pituitary gland |
| NKX2-4 | telencephalon, hypothalamus | hypothalamus, pituitary gland |
| NKX2-8 | thyroid, pharynx, lung, spinal cord, hindbrain | lung, thyroid, esophagus, hypothalamus, pituitary gland |
NKX2 genes in human diseases
NKX2-1
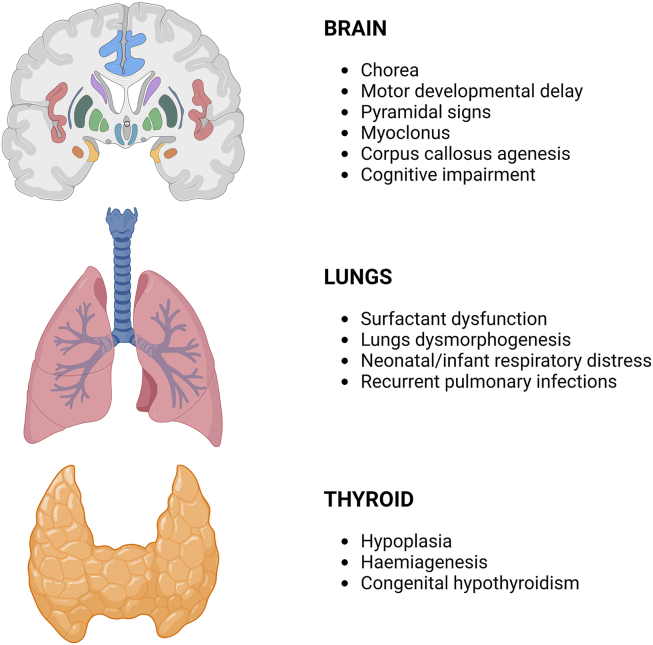
NKX2-1 heterozygous alterations have been identified and associated with a syndromic form initially named brain-lung-thyroid syndrome due to the broad phenotypic spectrum including a variable combination of lungs, thyroid, and neurological defects. Recently, since the varied manifestations of this syndrome, the disorders have been referred to as NKX2-1-related disorders.73 The main characteristics of this disease are represented by benign hereditary chorea, respiratory distress syndrome, and congenital hypothyroidism (Fig. 4). Indeed, the hallmark of brain-lung-thyroid syndrome (BLT) is childhood-onset chorea, commonly beginning in early infancy (most commonly around the first year of life) or, less frequently, in late childhood or adolescence. Progression occurs up to the second decade and then remains static. Lung disease is the second most common manifestation varying from respiratory distress syndrome in newborns to interstitial lung disease in young children. In the elders, pulmonary fibrosis may occur. Hypothyroidism is usually mild, often subclinical, due to various forms of thyroid abnormalities including hypoplasia, haemiagenesis, or athyreosis.32,74
Figure 4.
Main characteristics of the brain-lung-thyroid syndrome (BLTS). Illustration summarizes the main features of BLTS, sorted by organs involved. Created with Biorender.com.
NKX2-1 alterations exhibit autosomal dominant inheritance with variable expressivity and penetrance but frequently occur de novo. So far, about 100 alterations involving the NKX2-1 gene have been reported, the majority of which are point mutations.75 Notwithstanding, whole gene deletions and deletions proximal to NKX2-1 have been found in subjects with BLT syndrome, suggesting the presence of an upstream regulatory region.76
The reported mechanism of disease is haploinsufficiency.77 Indeed, truncating mutations removing, for example, the nuclear localization signal produces a protein that is unable to translocate into the nucleus, while missense variant impaired NXK2-1 binding to its targets, thus corroborating dosage imbalance.78 To date, no genotype–phenotype correlations have been observed, as the manifestations of NKX2-1-related disorders vary among individuals with the same pathogenic variant even within the same family.78 The severity of the phenotype is not consistent with NKX2-1 mutation type (missense or nonsense), deletion size, or location within functional domains (homeobox and transcription regulation). Larger contiguous gene deletions have been associated with a more severe spectrum of the brain–lung–thyroid triad (often with additional clinical characteristics), while point nonsense mutations, affecting the terminal regions of the protein, are reported in those with a milder clinical phenotype.73,74,79 However, this putative genotype–phenotype association is not seen in all cases, as reported by a colleague reporting whole gene deletions triggering a milder disease.80 All these data underpin a high degree of variability, suggesting that there may be other contributory environmental and/or epigenetic factors.
NKX2-1 plays also a role in neoplastic disease, especially in thyroid and lung cancer. In thyroid follicular cells-derived neoplasia, NKX2-1 is highly expressed in differentiated carcinomas (both papillary and follicular subtypes) but not in the undifferentiated (i.e., anaplastic) ones.81,82 Anaplastic carcinoma often arises in the context of differentiated thyroid carcinoma and it has been recently shown that NKX2-1 expression is lost during the transition from differentiated to anaplastic carcinoma.83,84 Moreover, NKX2-1 silencing in the non-tumorigenic FRTL5 rat cell line reduces proliferation.85 Thus, it seems that during thyroid tumorigenesis NKX2-1 could have an “oncogenic” or “tumor suppressor” effect depending on cell context, differentiated or not, respectively. In addition to tumors originating from follicular cells, NKX2-1 is expressed in medullary thyroid carcinomas (originating from parafollicular cells).86 Data suggesting the NKX2-1 bivalent functions (pro or anti-oncogenic) has been gained also for lung cancer.
About 70% of adenocarcinomas express NKX2-1 and retain features of the terminal respiratory unit (TRU) to a certain extent.87, 88, 89 Despite its role as a lineage-survival oncogene in lung adenocarcinomas, NKX2-1 expression is also known to be associated with favorable prognosis in affected patients.90 It has been demonstrated that NKX2-1 regulated genes involved in lung cytoskeletal and cell–cell organization, negatively affecting cell motility, invasion, and metastasis.91 On the other hand, a study by Winslow et al. highlighted that Nkx2-1 downregulation represses Hmga2 expression and acquisition of metastatic ability in a mouse model of lung adenocarcinoma with conditionally activated Kras and loss-of-function p53 mutant alleles.92
Furthermore, NKX2-1 represses TGF-b-induced epithelial-to-mesenchymal transition (EMT) by impairing TGF-b-related induction of Snail and Slug, as well as by reducing TGF-b production.93 All these data points to the notion that NKX2-1 plays a double-edged role in cancer.91,94 NKX2-1 is also expressed in small cell lung cancer (SCLC) where it represents a powerful diagnostic tool.95, 96, 97 According to the knowledge that NKX2-1 represses the gastric-specific gene expression (see above), NKX2-1 negative murine lung tumors express gastrointestinal transcriptome.42
Finally, rearrangements of NKX2-1 with T cell receptor or immunoglobulin heavy chain loci were identified in T-cell acute lymphoblastic leukemia98 and ectopic expression of NKX2-1 has been observed in diffuse large B-cell lymphoma.99,100
NKX2-4
To the best of our knowledge, the only disease in which NKX2-4 appears to be involved is autism spectrum disorder (ASD). Meta-analysis-based studies have shown that variants of NKX2-4 could represent a significant genetic risk factor for ASD.101 Regarding to neoplastic diseases, hyper-methylation in the NKX2-4-containing area has been found in adenoid cystic carcinoma of the salivary gland, although the exact meaning of these data is not fully clear.102 Aberrant activation of NKX2-4 has been found in erythroblastic AML determining the deregulation of genes involved in differentiation events and, therefore, contributing to the generation of specific AML subtypes.103 The partial redundancy between NKX2-1 and NKX2-4 may partially explain the apparently slight effects of NKX2-4 gene variants in human diseases. Accordingly, in the gnomAD database (gnomad.broadinstitute.org) this gene seems not to be intolerant to loss-of-function mutations.
NKX2-2
In agreement with the role of NKX2-2 in pancreas development, mutations of this gene are causative of neonatal diabetes. By studying subjects burdened by this disease and born by consanguineous parents, Flanagan and co-workers have found homozygous loss-of-function mutations of NKX2-2.104 These patients also show several features of developmental delay (affecting intellectual, sensory, and motor functions) consistent with the role of NKX2-2 in the development of several brain regions. In addition to diabetes, NKX2-2 mutations are associated with obesity. Indeed, an infant with a homozygous NKX2-2 loss-of-function mutation suffering severe obesity has been described.105 Interestingly, in this subject, obesity was ascribed to elevated ghrelin levels; in fact, he showed a paradoxical increase of this orexigenic hormone after an oral glucose tolerance test. The absence of NKX2-2 function could increase ghrelin levels by the fate change of pancreatic islet cell progenitors from insulin-to ghrelin-producing cells.
Ewing sarcoma is a malignant tumor with metastatic potential, affecting the bone and soft tissues of children and young adults. Most cases of Ewing sarcoma show the presence of the recurrent chromosomal translocation t(11; 22) (q24; q12), which encodes the aberrant fusion protein EWS/FLI that acts as a transcription factor able to dysregulate the expression of relevant genes involved in the development of this tumor type.106,107 By using a sophisticated experimental approach, it has been demonstrated that NKX2-2 is a fundamental factor in the pathogenesis of Ewing sarcoma.108 In addition to the relevance in the pathogenesis of Ewing sarcoma, the expression of this transcription factor has a relevant role in routine pathological discrimination of round cell sarcomas. In fact, Yoshida and co-workers have found that evaluation of NKX2-2 expression by immunohistochemistry is a marker tool for Ewing sarcoma with a sensitivity of 93% and a specificity of 89%, in the differential diagnosis of small round cell tumors.109 When combined with the expression of CD99, the specificity is increased to 98%.110
Nagel and co-workers found overexpression of NKX2-2 in Hodgkin lymphoma; they demonstrated that the IL17 receptor gene IL17RB activated NKX2-2 transcription. Downstream analysis indicates that NKX2-2 disturbs B-cell differentiation processes inhibiting transcription of homeobox gene MSX1 while activating expression of basic helix-loop-helix factor NEUROD1.100
NKX2-8
The only human non-cancerous diseases with which NKX2-8 has been so far associated are neural tube defects (NTDs), a group of common congenital defects characterized by failure of neural tube closure.111 NTDs are multifactorial in their etiology with genetic factors playing a significant role (heritability up to 70%).112 By analyzing the Weimaraner breed dogs affected by spinal dysraphism, it was shown that these animals harbor a homozygous loss-of-function mutation in NKX2-8.113 In the same study, Safra and co-workers evaluated 149 patients with lumbosacral myelomeningocele for NKX2-8 mutations and found six subjects heterozygous for the p.S62T and one for the p.A94T missense mutations. By comparing this cohort with a control population from the Exome Variant Server (EVS), the authors found a significant difference although substitutions in these genes are quite frequent in the gnomAD database, suggesting a potential low predisposing effect (if any) on NTDs.
The functional redundancy with other NKX genes may explain the lack of data relating to NKX2-8 and non-cancerous human diseases. Instead, this gene is frequently involved in various cancer types. Coactivation of NKX2-1 and NKX2-8 is associated with poor prognosis in non-small-cell lung cancer (NSCLC), and resistance to cisplatin in vitro.114 Indeed, NKX2-8 seems to foster clinical aggressiveness in this disease. However, such an effect could be context-dependent. In fact, data have been produced indicating NKX2-8 as a potential tumor suppressor in squamous cell tumors which are generally NKX2-1 negative.115 Indeed, data from many other neoplasms suggests an onco-suppressor activity for NKX2-8 gene. In fact, downregulation of this gene determines i) the decrease in survival and angiogenesis promotion in oesophageal cancer,116 ii) tumor progression and prognosis in hepatic cell carcinoma,117 iii) the higher recurrence rate and platinum resistance in epithelial ovarian cancer, and iv) the increase in lymph node metastasis and worse prognosis in urothelial carcinoma.118 Conversely, it has been demonstrated that NKX2-8 upregulation inhibits epithelial-to-mesenchymal transition and reduces motility as well as invasiveness in cell lines from urothelial carcinoma.119
Conclusions
The discovery of homeobox-containing genes, which includes the NKX2 family, represents a dramatic leap forward in the understanding of the molecular genetics of embryogenesis. Indeed, NKX2 genes exert essential functions in normal developmental patterning since they regulate tissue maturation in a spatially and temporally defined order. As they regulate a series of vital cellular functions, any alteration (i.e., mutation) dysregulates identifiable tissue-specific pathways.
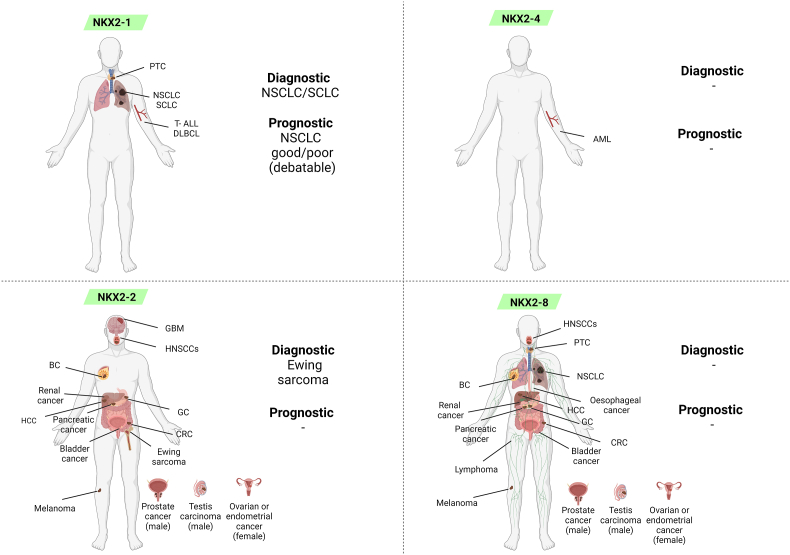
No matter the similarity of gene sequences, NKX2 genes have been associated with diverse human conditions. NKX2-1 haploinsufficiency has a cardinal role in the brain-lung-thyroid syndrome; NKX2-8 is associated with NTDs; NKX2-2 plays a role in the onset of diabetes and obesity; NKX2-4 alteration is related to ASD. NK2.1/2 expression in cancer tissues is summarized in Figure 5.
Figure 5.
NK2.1/2 expression in cancer. Illustration summarizes NK2.1 and NK2.2 involvement in cancer, together with data regarding diagnostic and/or prognostic potential as cancer markers. AML: acute myeloid leukemia; BC: breast cancer; CRC: colorectal cancer; DLBCL: diffuse large B cell lymphoma; GBM: glioblastoma; GC: gastric cancer; HCC: hepatocellular carcinoma; HNSCCs: head and neck squamous cell carcinomas; NSCLC: non-small cell lung cancer; PTC: papillary thyroid cancer; SCLC: small cell lung cancer; T-ALL: T-cell acute lymphoblastic leukemia. Created with Biorender.com.
All these data contribute to fostering the idea that NK genes evolved by both neo- and sub-functionalization. However, notwithstanding the heterogeneity, NKX2 genes represent a valuable biomarker in cancer (such as lung cancer).
Author contributions
Conceptualization: G.D.; Writing – original draft: C.M.; Writing – review and editing: C.M., F.B. and G.D.
Conflict of interests
The authors declare that there is no competing interests.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Gehring W.J., Hiromi Y. Homeotic genes and the homeobox. Annu Rev Genet. 1986;20:147–173. doi: 10.1146/annurev.ge.20.120186.001051. [DOI] [PubMed] [Google Scholar]
- 2.Gehring W.J. Homeo boxes in the study of development. Science. 1987;236(4806):1245–1252. doi: 10.1126/science.2884726. [DOI] [PubMed] [Google Scholar]
- 3.Dressler G.R. An update on the vertebrate homeobox. Trends Genet. 1989;5:129–131. doi: 10.1016/0168-9525(89)90048-6. [DOI] [PubMed] [Google Scholar]
- 4.Desplan C., Theis J., O'Farrell P.H. The sequence specificity of homeodomain-DNA interaction. Cell. 1988;54(7):1081–1090. doi: 10.1016/0092-8674(88)90123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müller M., Affolter M., Leupin W., et al. Isolation and sequence-specific DNA binding of the Antennapedia homeodomain. EMBO J. 1988;7(13):4299–4304. doi: 10.1002/j.1460-2075.1988.tb03328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damante G., Pellizzari L., Esposito G., et al. A molecular code dictates sequence-specific DNA recognition by homeodomains. EMBO J. 1996;15(18):4992–5000. [PMC free article] [PubMed] [Google Scholar]
- 7.Qian Y.Q., Billeter M., Otting G., et al. The structure of the Antennapedia homeodomain determined by NMR spectroscopy in solution: comparison with prokaryotic repressors. Cell. 1989;59(3):573–580. doi: 10.1016/0092-8674(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 8.Holland P.W.H., Booth H.A.F., Bruford E.A. Classification and nomenclature of all human homeobox genes. BMC Biol. 2007;5:47. doi: 10.1186/1741-7007-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright C.V.E. Vertebrate homeobox genes. Curr Opin Cell Biol. 1991;3(6):976–982. doi: 10.1016/0955-0674(91)90116-g. [DOI] [PubMed] [Google Scholar]
- 10.Boncinelli E. Homeobox genes and disease. Curr Opin Genet Dev. 1997;7(3):331–337. doi: 10.1016/s0959-437x(97)80146-3. [DOI] [PubMed] [Google Scholar]
- 11.D’Elia A.V., Tell G., Paron I., et al. Missense mutations of human homeoboxes: a review. Hum Mutat. 2001;18(5):361–374. doi: 10.1002/humu.1207. [DOI] [PubMed] [Google Scholar]
- 12.Damante G., Fabbro D., Pellizzari L., et al. Sequence-specific DNA recognition by the thyroid transcription factor-1 homeodomain. Nucleic Acids Res. 1994;22(15):3075–3083. doi: 10.1093/nar/22.15.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price M., Lazzaro D., Pohl T., et al. Regional expression of the homeobox gene Nkx-2.2 in the developing mammalian forebrain. Neuron. 1992;8(2):241–255. doi: 10.1016/0896-6273(92)90291-k. [DOI] [PubMed] [Google Scholar]
- 14.Bodmer R. Heart development in Drosophila and its relationship to vertebrates. Trends Cardiovasc Med. 1995;5(1):21–28. doi: 10.1016/1050-1738(94)00032-Q. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C., Huang H., Chen Z., et al. The transcription factor NKX2-2 regulates oligodendrocyte differentiation through domain-specific interactions with transcriptional corepressors. J Biol Chem. 2020;295(7):1879–1888. doi: 10.1074/jbc.RA119.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C.C., Brodnicki T., Copeland N.G., et al. Conserved linkage of NK-2 homeobox gene pairs Nkx2-2/2-4 and Nkx2-1/2-9 in mammals. Mamm Genome. 2000;11(6):466–468. doi: 10.1007/s003350010089. [DOI] [PubMed] [Google Scholar]
- 17.Holland P.W.H. Evolution of homeobox genes. Wiley Interdiscip Rev Dev Biol. 2013;2(1):31–45. doi: 10.1002/wdev.78. [DOI] [PubMed] [Google Scholar]
- 18.Ohno S. Duplication of Regulatory Genes and Receptors. Evolution by Gene Duplication. Springer Berlin Heidelberg; Berlin, Heidelberg: 1970. pp. 82–88. [Google Scholar]
- 19.Force A., Lynch M., Pickett F.B., et al. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999;151(4):1531–1545. doi: 10.1093/genetics/151.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoltzfus A. On the possibility of constructive neutral evolution. J Mol Evol. 1999;49(2):169–181. doi: 10.1007/pl00006540. [DOI] [PubMed] [Google Scholar]
- 21.He X., Zhang J. Rapid subfunctionalization accompanied by prolonged and substantial neofunctionalization in duplicate gene evolution. Genetics. 2005;169(2):1157–1164. doi: 10.1534/genetics.104.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaffran S., Das G., Frasch M. The NK-2 homeobox gene scarecrow (scro) is expressed in pharynx, ventral nerve cord and brain of Drosophila embryos. Mech Dev. 2000;94:237–241. doi: 10.1016/s0925-4773(00)00298-7. [DOI] [PubMed] [Google Scholar]
- 23.Yoo S., Nair S., Kim H.J., et al. Knock-in mutations of scarecrow, a Drosophila homolog of mammalian Nkx2.1, reveal a novel function required for development of the optic lobe in Drosophila melanogaster. Dev Biol. 2020;461(2):145–159. doi: 10.1016/j.ydbio.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Nair S., Bahn J.H., Lee G., et al. A homeobox transcription factor scarecrow (SCRO) negatively regulates Pdf neuropeptide expression through binding an identified cis-acting element in Drosophila melanogaster. Mol Neurobiol. 2020;57(4):2115–2130. doi: 10.1007/s12035-020-01874-w. [DOI] [PubMed] [Google Scholar]
- 25.Jiménez F., Martin-Morris L.E., Velasco L., et al. Vnd, a gene required for early neurogenesis of Drosophila, encodes a homeodomain protein. EMBO J. 1995;14(14):3487–3495. doi: 10.1002/j.1460-2075.1995.tb07355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mellerick D.M., Nirenberg M. Dorsal-ventral patterning genes restrict NK-2 homeobox gene expression to the ventral half of the central nervous system of Drosophila embryos. Dev Biol. 1995;171(2):306–316. doi: 10.1006/dbio.1995.1283. [DOI] [PubMed] [Google Scholar]
- 27.Weiss J.B., Von Ohlen T., Mellerick D.M., et al. Dorsoventral patterning in the Drosophila central nervous system: the intermediate neuroblasts defective homeobox gene specifies intermediate column identity. Genes Dev. 1998;12(22):3591–3602. doi: 10.1101/gad.12.22.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cowden J., Levine M. Ventral dominance governs sequential patterns of gene expression across the dorsal-ventral axis of the neuroectoderm in the Drosophila embryo. Dev Biol. 2003;262(2):335–349. doi: 10.1016/s0012-1606(03)00395-6. [DOI] [PubMed] [Google Scholar]
- 29.Guan L., Zhao X., Tang L., et al. Thyroid transcription factor-1: structure, expression, function and its relationship with disease. BioMed Res Int. 2021;2021 doi: 10.1155/2021/9957209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Felice M., di Lauro R. Thyroid development and its disorders: genetics and molecular mechanisms. Endocr Rev. 2004;25(5):722–746. doi: 10.1210/er.2003-0028. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki K., Kobayashi Y., Katoh R., et al. Identification of thyroid transcription factor-1 in C cells and parathyroid cells. Endocrinology. 1998;139(6):3014–3017. doi: 10.1210/endo.139.6.6126. [DOI] [PubMed] [Google Scholar]
- 32.Mio C., Grani G., Durante C., et al. Molecular defects in thyroid dysgenesis. Clin Genet. 2020;97(1):222–231. doi: 10.1111/cge.13627. [DOI] [PubMed] [Google Scholar]
- 33.Kusakabe T., Kawaguchi A., Hoshi N., et al. Thyroid-specific enhancer-binding protein/NKX2.1 is required for the maintenance of ordered architecture and function of the differentiated thyroid. Mol Endocrinol. 2006;20(8):1796–1809. doi: 10.1210/me.2005-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazzaro D., Price M., de Felice M., et al. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113(4):1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- 35.Ikonomou L., Herriges M.J., Lewandowski S.L., et al. The in vivo genetic program of murine primordial lung epithelial progenitors. Nat Commun. 2020;11(1):635. doi: 10.1038/s41467-020-14348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minoo P., Hamdan H., Bu D., et al. TTF-1 regulates lung epithelial morphogenesis. Dev Biol. 1995;172(2):694–698. doi: 10.1006/dbio.1995.8080. [DOI] [PubMed] [Google Scholar]
- 37.Kimura S., Hara Y., Pineau T., et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10(1):60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 38.Minoo P., Su G., Drum H., et al. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(−/−) mouse embryos. Dev Biol. 1999;209(1):60–71. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- 39.Kuwahara A., Lewis A.E., Coombes C., et al. Delineating the early transcriptional specification of the mammalian trachea and esophagus. Elife. 2020;9 doi: 10.7554/eLife.55526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou L., Lim L., Costa R.H., et al. Thyroid transcription factor-1, hepatocyte nuclear factor-3beta, surfactant protein B, C, and Clara cell secretory protein in developing mouse lung. J Histochem Cytochem. 1996;44(10):1183–1193. doi: 10.1177/44.10.8813084. [DOI] [PubMed] [Google Scholar]
- 41.Boggaram V. Thyroid transcription factor-1 (TTF-1/Nkx2.1/TITF1) gene regulation in the lung. Clin Sci (Lond) 2009;116(1):27–35. doi: 10.1042/CS20080068. [DOI] [PubMed] [Google Scholar]
- 42.Snyder E.L., Watanabe H., Magendantz M., et al. Nkx2-1 represses a latent gastric differentiation program in lung adenocarcinoma. Mol Cell. 2013;50(2):185–199. doi: 10.1016/j.molcel.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.di Palma T., Nitsch R., Mascia A., et al. The paired domain-containing factor Pax8 and the homeodomain-containing factor TTF-1 directly interact and synergistically activate transcription. J Biol Chem. 2003;278(5):3395–3402. doi: 10.1074/jbc.M205977200. [DOI] [PubMed] [Google Scholar]
- 44.Liu C., Glasser S.W., Wan H., et al. GATA-6 and thyroid transcription factor-1 directly interact and regulate surfactant protein-C gene expression. J Biol Chem. 2002;277(6):4519–4525. doi: 10.1074/jbc.M107585200. [DOI] [PubMed] [Google Scholar]
- 45.Yan C., Naltner A., Martin M., et al. Transcriptional stimulation of the surfactant protein B gene by STAT3 in respiratory epithelial cells. J Biol Chem. 2002;277(13):10967–10972. doi: 10.1074/jbc.M109986200. [DOI] [PubMed] [Google Scholar]
- 46.Yan C., Naltner A., Conkright J., et al. Protein-protein interaction of retinoic acid receptor alpha and thyroid transcription factor-1 in respiratory epithelial cells. J Biol Chem. 2001;276(24):21686–21691. doi: 10.1074/jbc.M011378200. [DOI] [PubMed] [Google Scholar]
- 47.Bachurski C.J., Yang G.H., Currier T.A., et al. Nuclear factor I/thyroid transcription factor 1 interactions modulate surfactant protein C transcription. Mol Cell Biol. 2003;23(24):9014–9024. doi: 10.1128/MCB.23.24.9014-9024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sever-Chroneos Z., Bachurski C.J., Yan C., et al. Regulation of mouse SP-B gene promoter by AP-1 family members. Am J Physiol. 1999;277(1):L79–L88. doi: 10.1152/ajplung.1999.277.1.L79. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y.S., Yang M.C., Wang B., et al. BR22, a novel protein, interacts with thyroid transcription factor-1 and activates the human surfactant protein B promoter. Am J Respir Cell Mol Biol. 2001;24(1):30–37. doi: 10.1165/ajrcmb.24.1.4050. [DOI] [PubMed] [Google Scholar]
- 50.Park K.S., Whitsett J.A., di Palma T., et al. TAZ interacts with TTF-1 and regulates expression of surfactant protein-C. J Biol Chem. 2004;279(17):17384–17390. doi: 10.1074/jbc.M312569200. [DOI] [PubMed] [Google Scholar]
- 51.Maeda Y., Hunter T.C., Loudy D.E., et al. PARP-2 interacts with TTF-1 and regulates expression of surfactant protein-B. J Biol Chem. 2006;281(14):9600–9606. doi: 10.1074/jbc.M510435200. [DOI] [PubMed] [Google Scholar]
- 52.Orquera D.P., Tavella M.B., de Souza F.S.J., et al. The homeodomain transcription factor NKX2.1 is essential for the early specification of melanocortin neuron identity and activates Pomc expression in the developing hypothalamus. J Neurosci. 2019;39(21):4023–4035. doi: 10.1523/JNEUROSCI.2924-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Son Y.J., Hur M.K., Ryu B.J., et al. TTF-1, a homeodomain-containing transcription factor, participates in the control of body fluid homeostasis by regulating angiotensinogen gene transcription in the rat subfornical organ. J Biol Chem. 2003;278(29):27043–27052. doi: 10.1074/jbc.M303157200. [DOI] [PubMed] [Google Scholar]
- 54.Butt S.J.B., Sousa V.H., Fuccillo M.V., et al. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59(5):722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Small E.M., Vokes S.A., Garriock R.J., et al. Developmental expression of the Xenopus nkx2-1 and nkx2-4 genes. Mech Dev. 2000;96(2):259–262. doi: 10.1016/s0925-4773(00)00400-7. [DOI] [PubMed] [Google Scholar]
- 56.Manoli M., Driever W. nkx2.1 and nkx2.4 genes function partially redundant during development of the zebrafish hypothalamus, preoptic region, and pallidum. Front Neuroanat. 2014;8:145. doi: 10.3389/fnana.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schredelseker T., Veit F., Dorsky R.I., et al. Bsx is essential for differentiation of multiple neuromodulatory cell populations in the secondary prosencephalon. Front Neurosci. 2020;14:525. doi: 10.3389/fnins.2020.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uemae Y., Sakamoto J., Hidaka Y., et al. Gene expression, function, and diversity of Nkx2-4 in the rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol. 2014;206:193–202. doi: 10.1016/j.ygcen.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 59.Briscoe J., Sussel L., Serup P., et al. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398(6728):622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- 60.Desai S., Loomis Z., Pugh-Bernard A., et al. Nkx2.2 regulates cell fate choice in the enteroendocrine cell lineages of the intestine. Dev Biol. 2008;313(1):58–66. doi: 10.1016/j.ydbio.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sussel L., Kalamaras J., Hartigan-O'connor D.J., et al. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125(12):2213–2221. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- 62.Jørgensen M.C., Ahnfelt-Rønne J., Hald J., et al. An illustrated review of early pancreas development in the mouse. Endocr Rev. 2007;28(6):685–705. doi: 10.1210/er.2007-0016. [DOI] [PubMed] [Google Scholar]
- 63.Mastracci T.L., Lin C.S., Sussel L. Generation of mice encoding a conditional allele of Nkx2.2. Transgenic Res. 2013;22(5):965–972. doi: 10.1007/s11248-013-9700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cissell M.A., Zhao L., Sussel L., et al. Transcription factor occupancy of the insulin gene in vivo. Evidence for direct regulation by Nkx2.2. J Biol Chem. 2003;278(2):751–756. doi: 10.1074/jbc.M205905200. [DOI] [PubMed] [Google Scholar]
- 65.Mollet I.G., Malm H.A., Wendt A., et al. Integrator of stress responses calmodulin binding transcription activator 1 (Camta1) regulates miR-212/miR-132 expression and insulin secretion. J Biol Chem. 2016;291(35):18440–18452. doi: 10.1074/jbc.M116.716860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gross S., Balderes D., Liu J., et al. Nkx2.2 is expressed in a subset of enteroendocrine cells with expanded lineage potential. Am J Physiol Gastrointest Liver Physiol. 2015;309(12):G975–G987. doi: 10.1152/ajpgi.00244.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei Q., Miskimins W.K., Miskimins R. Stage-specific expression of myelin basic protein in oligodendrocytes involves Nkx2.2-mediated repression that is relieved by the Sp1 transcription factor. J Biol Chem. 2005;280(16):16284–16294. doi: 10.1074/jbc.M500491200. [DOI] [PubMed] [Google Scholar]
- 68.Wen P., Locker J. A novel hepatocytic transcription factor that binds the alpha-fetoprotein promoter-linked coupling element. Mol Cell Biol. 1994;14(10):6616–6626. doi: 10.1128/mcb.14.10.6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tian J., Mahmood R., Hnasko R., et al. Loss of Nkx2.8 deregulates progenitor cells in the large airways and leads to dysplasia. Cancer Res. 2006;66(21):10399–10407. doi: 10.1158/0008-5472.CAN-06-1564. [DOI] [PubMed] [Google Scholar]
- 70.Pabst O., Rummelies J., Winter B., Arnold H.H. Targeted disruption of the homeobox gene Nkx2.9 reveals a role in development of the spinal accessory nerve. Development. 2003;130(6):1193–1202. doi: 10.1242/dev.00346. [DOI] [PubMed] [Google Scholar]
- 71.Zhu J., Wu G., Song L., et al. NKX2-8 deletion-induced reprogramming of fatty acid metabolism confers chemoresistance in epithelial ovarian cancer. EBioMedicine. 2019;43:238–252. doi: 10.1016/j.ebiom.2019.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reecy J.M., Yamada M., Cummings K., et al. Chicken Nkx-2.8: a novel homeobox gene expressed in early heart progenitor cells and pharyngeal pouch-2 and-3 endoderm. Dev Biol. 1997;188(2):295–311. doi: 10.1006/dbio.1997.8641. [DOI] [PubMed] [Google Scholar]
- 73.Monti S., Nicoletti A., Cantasano A., et al. NKX2.1-Related Disorders: a novel mutation with mild clinical presentation. Ital J Pediatr. 2015;41:45. doi: 10.1186/s13052-015-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peall K.J., Kurian M.A. Benign hereditary chorea: an update. Tremor Other Hyperkinet Mov (N Y) 2015;5:314. doi: 10.7916/D8RJ4HM5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kharbanda M., Hermanns P., Jones J., et al. A further case of brain-lung-thyroid syndrome with deletion proximal to NKX2-1. Eur J Med Genet. 2017;60(5):257–260. doi: 10.1016/j.ejmg.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 76.Thorwarth A., Schnittert-Hübener S., Schrumpf P., et al. Comprehensive genotyping and clinical characterisation reveal 27 novel NKX2-1 mutations and expand the phenotypic spectrum. J Med Genet. 2014;51(6):375–387. doi: 10.1136/jmedgenet-2013-102248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cavaliere E., Gortan A.J., Passon N., et al. NKX2.1 Run-on mutation associated to familial brain-lung-thyroid syndrome. Clin Genet. 2021;100(1):114–116. doi: 10.1111/cge.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferrara A.M., De Michele G., Salvatore E., et al. A novel NKX2.1 mutation in a family with hypothyroidism and benign hereditary chorea. Thyroid. 2008;18(9):1005–1009. doi: 10.1089/thy.2008.0085. [DOI] [PubMed] [Google Scholar]
- 79.Inzelberg R., Weinberger M., Gak E. Benign hereditary chorea: an update. Park Relat Disord. 2011;17(5):301–307. doi: 10.1016/j.parkreldis.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 80.Hamvas A., Deterding R.R., Wert S.E., et al. Heterogeneous pulmonary phenotypes associated with mutations in the thyroid transcription factor gene NKX2-1. Chest. 2013;144(3):794–804. doi: 10.1378/chest.12-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fabbro D., di Loreto C., Beltrami C.A., et al. Expression of thyroid-specific transcription factors TTF-1 and PAX-8 in human thyroid neoplasms. Cancer Res. 1994;54(17):4744–4749. [PubMed] [Google Scholar]
- 82.Miettinen M., Franssila K.O. Variable expression of keratins and nearly uniform lack of thyroid transcription factor 1 in thyroid anaplastic carcinoma. Hum Pathol. 2000;31(9):1139–1145. doi: 10.1053/hupa.2000.16667. [DOI] [PubMed] [Google Scholar]
- 83.Odate T., Oishi N., Kawai M., et al. Progression of papillary thyroid carcinoma to anaplastic carcinoma in metastatic lymph nodes: solid/insular growth and hobnail cell change in lymph nodes are predictors of subsequent anaplastic transformation. Endocr Pathol. 2021;32(3):347–356. doi: 10.1007/s12022-021-09674-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ragazzi M., Torricelli F., Donati B., et al. Coexisting well-differentiated and anaplastic thyroid carcinoma in the same primary resection specimen: immunophenotypic and genetic comparison of the two components in a consecutive series of 13 cases and a review of the literature. Virchows Arch. 2021;478(2):265–281. doi: 10.1007/s00428-020-02891-9. [DOI] [PubMed] [Google Scholar]
- 85.Rossi D.L., Acebrón A., Santisteban P. Function of the homeo and paired domain proteins TTF-1 and Pax-8 in thyroid cell proliferation. J Biol Chem. 1995;270(39):23139–23142. doi: 10.1074/jbc.270.39.23139. [DOI] [PubMed] [Google Scholar]
- 86.Katoh R., Miyagi E., Nakamura N., et al. Expression of thyroid transcription factor-1 (TTF-1) in human C cells and medullary thyroid carcinomas. Hum Pathol. 2000;31(3):386–393. doi: 10.1016/s0046-8177(00)80255-5. [DOI] [PubMed] [Google Scholar]
- 87.Yatabe Y., Mitsudomi T., Takahashi T. TTF-1 expression in pulmonary adenocarcinomas. Am J Surg Pathol. 2002;26(6):767–773. doi: 10.1097/00000478-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 88.Kwei K.A., Kim Y.H., Girard L., et al. Genomic profiling identifies TITF1 as a lineage-specific oncogene amplified in lung cancer. Oncogene. 2008;27(25):3635–3640. doi: 10.1038/sj.onc.1211012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kendall J., Liu Q., Bakleh A., et al. Oncogenic cooperation and coamplification of developmental transcription factor genes in lung cancer. Proc Natl Acad Sci U S A. 2007;104(42):16663–16668. doi: 10.1073/pnas.0708286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anagnostou V.K., Syrigos K.N., Bepler G., et al. Thyroid transcription factor 1 is an independent prognostic factor for patients with stage I lung adenocarcinoma. J Clin Oncol. 2009;27(2):271–278. doi: 10.1200/JCO.2008.17.0043. [DOI] [PubMed] [Google Scholar]
- 91.Yamaguchi T., Hosono Y., Yanagisawa K., et al. NKX2-1/TTF-1:an enigmatic oncogene that functions as a double-edged sword for cancer cell survival and progression. Cancer Cell. 2013;23(6):718–723. doi: 10.1016/j.ccr.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 92.Winslow M.M., Dayton T.L., Verhaak R.G.W., et al. Suppression of lung adenocarcinoma progression by Nkx2-1. Nature. 2011;473(7345):101–104. doi: 10.1038/nature09881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saito R.A., Watabe T., Horiguchi K., et al. Thyroid transcription factor-1 inhibits transforming growth factor-beta-mediated epithelial-to-mesenchymal transition in lung adenocarcinoma cells. Cancer Res. 2009;69(7):2783–2791. doi: 10.1158/0008-5472.CAN-08-3490. [DOI] [PubMed] [Google Scholar]
- 94.Mu D. The complexity of thyroid transcription factor 1 with both pro- and anti-oncogenic activities. J Biol Chem. 2013;288(35):24992–25000. doi: 10.1074/jbc.R113.491647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.di Loreto C., di Lauro V., Puglisi F., et al. Immunocytochemical expression of tissue specific transcription factor-1 in lung carcinoma. J Clin Pathol. 1997;50(1):30–32. doi: 10.1136/jcp.50.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ordóñez N.G. Value of thyroid transcription factor-1 immunostaining in distinguishing small cell lung carcinomas from other small cell carcinomas. Am J Surg Pathol. 2000;24(9):1217–1223. doi: 10.1097/00000478-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 97.Elmas H., Diel R., Önal B., et al. Recommendations for immunocytochemistry in lung cancer typing: an update on a resource-efficient approach with large-scale comparative Bayesian analysis. Cytopathology. 2022;33(1):65–76. doi: 10.1111/cyt.13051. [DOI] [PubMed] [Google Scholar]
- 98.Homminga I., Pieters R., Langerak A.W., et al. Integrated transcript and genome analyses reveal NKX2-1 and MEF2C as potential oncogenes in T cell acute lymphoblastic leukemia. Cancer Cell. 2011;19(4):484–497. doi: 10.1016/j.ccr.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 99.Nagel S., Ehrentraut S., Tomasch J., et al. Ectopic expression of homeobox gene NKX2-1 in diffuse large B-cell lymphoma is mediated by aberrant chromatin modifications. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0061447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nagel S., MacLeod R.A.F., Meyer C., et al. NKL homeobox gene activities in B-cell development and lymphomas. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0205537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alonso-Gonzalez A., Calaza M., Rodriguez-Fontenla C., et al. Novel gene-based analysis of ASD GWAS: insight into the biological role of associated genes. Front Genet. 2019;10:733. doi: 10.3389/fgene.2019.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bell A., Bell D., Weber R.S., et al. CpG island methylation profiling in human salivary gland adenoid cystic carcinoma. Cancer. 2011;117(13):2898–2909. doi: 10.1002/cncr.25818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nagel S., Pommerenke C., Meyer C., et al. NKL homeobox genes NKX2-3 and NKX2-4 deregulate megakaryocytic-erythroid cell differentiation in AML. Int J Mol Sci. 2021;22(21) doi: 10.3390/ijms222111434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Flanagan S.E., de Franco E., Lango Allen H., et al. Analysis of transcription factors key for mouse pancreatic development establishes NKX2-2 and MNX1 mutations as causes of neonatal diabetes in man. Cell Metabol. 2014;19(1):146–154. doi: 10.1016/j.cmet.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Auerbach A., Cohen A., Ofek Shlomai N., et al. NKX2-2 mutation causes congenital diabetes and infantile obesity with paradoxical glucose-induced ghrelin secretion. J Clin Endocrinol Metab. 2020;105(11):3486–3495. doi: 10.1210/clinem/dgaa563. [DOI] [PubMed] [Google Scholar]
- 106.Delattre O., Zucman J., Plougastel B., et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359(6391):162–165. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- 107.Lessnick S.L., Braun B.S., Denny C.T., et al. Multiple domains mediate transformation by the Ewing’s sarcoma EWS/FLI-1 fusion gene. Oncogene. 1995;10(3):423–431. [PubMed] [Google Scholar]
- 108.Smith R., Owen L.A., Trem D.J., et al. Expression profiling of EWS/FLI identifies NKX2.2 as a critical target gene in Ewing's sarcoma. Cancer Cell. 2006;9(5):405–416. doi: 10.1016/j.ccr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 109.Yoshida A., Sekine S., Tsuta K., et al. NKX2.2 is a useful immunohistochemical marker for Ewing sarcoma. Am J Surg Pathol. 2012;36(7):993–999. doi: 10.1097/PAS.0b013e31824ee43c. [DOI] [PubMed] [Google Scholar]
- 110.Shibuya R., Matsuyama A., Nakamoto M., et al. The combination of CD99 and NKX2.2, a transcriptional target of EWSR1-FLI1, is highly specific for the diagnosis of Ewing sarcoma. Virchows Arch. 2014;465(5):599–605. doi: 10.1007/s00428-014-1627-1. [DOI] [PubMed] [Google Scholar]
- 111.Greene N.D., Copp A.J. Neural tube defects. Annu Rev Neurosci. 2014;37:221–242. doi: 10.1146/annurev-neuro-062012-170354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leck I. Causation of neural tube defects: clues from epidemiology. Br Med Bull. 1974;30(2):158–163. doi: 10.1093/oxfordjournals.bmb.a071187. [DOI] [PubMed] [Google Scholar]
- 113.Safra N., Bassuk A.G., Ferguson P.J., et al. Genome-wide association mapping in dogs enables identification of the homeobox gene, NKX2-8, as a genetic component of neural tube defects in humans. PLoS Genet. 2013;9(7) doi: 10.1371/journal.pgen.1003646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hsu D.S., Acharya C.R., Balakumaran B.S., et al. Characterizing the developmental pathways TTF-1, NKX2-8, and PAX9 in lung cancer. Proc Natl Acad Sci U S A. 2009;106(13):5312–5317. doi: 10.1073/pnas.0900827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Harris T., Pan Q., Sironi J., et al. Both gene amplification and allelic loss occur at 14q13.3 in lung cancer. Clin Cancer Res. 2011;17(4):690–699. doi: 10.1158/1078-0432.CCR-10-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin C., Song L., Gong H., et al. Nkx2-8 downregulation promotes angiogenesis and activates NF-κB in esophageal cancer. Cancer Res. 2013;73(12):3638–3648. doi: 10.1158/0008-5472.CAN-12-4028. [DOI] [PubMed] [Google Scholar]
- 117.Qu L., Deng B., Zeng Y., et al. Decreased expression of the Nkx2.8 gene correlates with tumor progression and a poor prognosis in HCC cancer. Cancer Cell Int. 2014;14:28. doi: 10.1186/1475-2867-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu C., Zhang Z., Liao W., et al. The tumor-suppressor gene Nkx2.8 suppresses bladder cancer proliferation through upregulation of FOXO3a and inhibition of the MEK/ERK signaling pathway. Carcinogenesis. 2012;33(3):678–686. doi: 10.1093/carcin/bgr321. [DOI] [PubMed] [Google Scholar]
- 119.Yu C., Liu Z., Chen Q., et al. Nkx2.8 inhibits epithelial-mesenchymal transition in bladder urothelial carcinoma via transcriptional repression of Twist1. Cancer Res. 2018;78(5):1241–1252. doi: 10.1158/0008-5472.CAN-17-1545. [DOI] [PubMed] [Google Scholar]