Abstract
Malignant tumor is still a major problem worldwide. During tumorigenesis or tumor development, tumor suppressor p53-binding protein 2 (TP53BP2), also known as apoptosis stimulating protein 2 of p53 (ASPP2), plays a critical role in p53 dependent and independent manner. Expression of TP53BP2 is highly correlated with the prognosis and survival rate of malignant tumor patients. TP53BP2 can interact with p53, NF-κB p65, Bcl-2, HCV core protein, PP1, YAP, CagA, RAS, PAR3, and other proteins to regulate cell function. Moreover, TP53BP2 can also regulate the proliferation, apoptosis, autophagy, migration, EMT and drug resistance of tumor cells through downstream signaling pathways, such as NF-κB, RAS/MAPK, mevalonate, TGF-β1, PI3K/AKT, aPKC-ι/GLI1 and autophagy pathways. As a potential therapeutic target, TP53BP2 has been attracted more attention. We review the role of TP53BP2 in tumorigenesis or tumor development and the signal pathway involved in TP53BP2, which may provide more deep insight and strategies for tumor treatment.
Keywords: Binding proteins, Signaling pathways, TP53, TP53BP2, Tumorigenesis, Tumor development
Introduction
Malignant tumor is still a major problem threatening human health worldwide. According to statistics, among the number of new cases and deaths in 2020, breast cancer, lung cancer and prostate cancer are the three most common cancers reported, while lung cancer, liver cancer and stomach cancer are the three most common causes of cancer death.1 Moreover, Isabelle Soerjomataram et al2 predicted that cancer incidence would double in 2070 compared to 2020. Therefore, it is critical to explore tumor pathogenesis and effective treatment.
Abnormal expression of some tumor suppressor genes is often associated with the pathogenesis of human tumors.3, 4, 5, 6 Among them, the expression of tumor suppressor p53-binding protein 2 (TP53BP2), also known as apoptosis stimulating protein 2 of p53 (ASPP2), is often reduced due to the methylation of promoter CpG island.7 TP53BP2 is the most distinctive member of the ASPP family. TP53BP2 was initially identified as a p53 binding protein, which can regulate p53-mediated apoptosis.8, 9, 10 In recent years, many literatures have reported that TP53BP2 plays an important role in tumorigenesis or tumor development in a p53-dependent and independent manner.11, 12, 13, 14 In addition, clinical studies reported that TP53BP2 expression is significantly downregulated in many tumors, such as lung cancer,15 gastric cancer16 and breast cancer.13 Therefore, we believe that TP53BP2 may be a potential antitumor target. Herein, we systematically review the biological structure and binding proteins of TP53 and TP53BP2, the role of TP53BP2 and its isoforms in tumorigenesis or tumor development and the signaling pathways involved in TP53BP2, which may help to further understand its potential anti-tumor effects and mechanisms.
Biological structure and function of TP53 protein
To better understand TP53BP2, we first comprehensively introduce the structure, function and interacting proteins of TP53.
Biological structure of TP53 protein
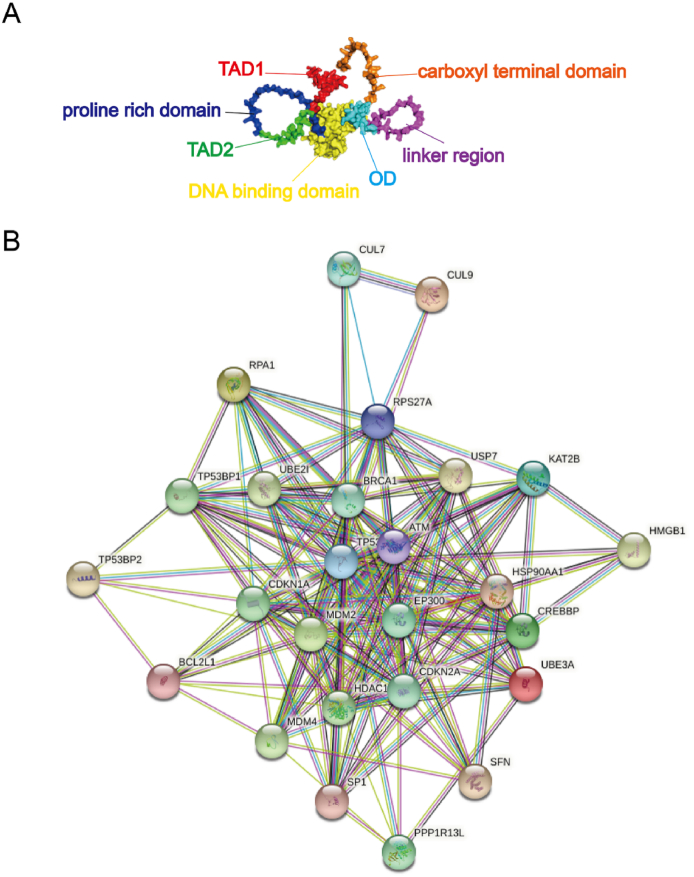
TP53 was initially considered as an oncogene, but increasing research data show that it is a tumor suppressor.17 The tumor suppressor protein p53 encoded by TP53 is composed of 393 amino acids. There are two activation domains (TAD) (AA,1–40 and 41–61) at the N-terminal, which mediate the transcriptional activity of p53. Followed by a proline rich domain (AA, 62–94), this domain has been implicated in p53-mediated cell death and growth suppression. After the N-terminal is the DNA binding domain (AA, 95–292), which can specifically bind to DNA and is the site of about 80% TP53 mutation. The C-terminal includes a linker region (AA, 293–325), oligomerization domain (OD) (AA, 326–356) and carboxyl terminal domain (AA, 357–393). The OD region also includes a nuclear output signal sequence18 (Fig. 1).
Figure 1.
Biological structure and interacting proteins network diagram of TP53. (A) Biological structure of TP53. (B) TP53 interacting proteins network diagram (Quoted from the String database).
Biological function of TP53 protein
Tumor prevention is largely dependent on TP53, which is mutated in approximately 50% of all tumors.17 Under normal conditions, TP53 is maintained in a low-level state in vivo. When activated by stress, TP53 inhibits the cell cycle or promotes apoptosis. There are many interacting proteins of TP53 protein, including MDM2, MDM4, BCL2L1, TP53BP2, etc., which together regulate the cell cycle or apoptosis (Fig. 1). Among them, TP53BP2, discovered at the time of its earliest identification, can interact with TP53 to regulate TP53 by enhancing the DNA binding and transactivation functions of TP53 on the promoters of proapoptotic genes.19
Biological structure and function of TP53BP2 protein
Biological structure of TP53BP2 protein
TP53BP2, the first member of the ASPP family, is a protein encoded by 1128 amino acids.19 Prior to the identification of full-length TP53BP2, Kuniyoshi Iwabuchi and Louie Naumovski identified 53BP2 and BBP, two of the N-terminal spliceosomes of TP53BP2, are composed of 523 and 1005 amino acids, respectively, using yeast two-hybrid system and N-terminal of anti-apoptotic protein Bcl-2 as baits.8,19,20 Structurally, 53BP2/BbP/TP53BP2 have a proline rich region (Pro), four ankyrin repeats (Ank) and an SH3 domain at the C-terminal. BbP and TP53BP2 both have α-helix domain at the N-terminal, while only TP53BP2 has a ubiquitin-like folding structure (ULD)21 (Fig. 2).
Figure 2.
Biological structure of 53BP2 (A), Bbp (B) and TP53BP2 (C).
TP53BP2 binding proteins
How does TP53BP2 perform its biological function? It was reported that TP53BP2 has many binding proteins, which interact with each other to regulate cell function jointly. Among them, more proteins bind to the C-terminal of TP53BP2. The Ank-SH3 domain at the C-terminal of TP53BP2 can interact with the DNA binding domain of p53,8,22 BH4 motif or pro-apoptotic regulator factor of Bcl-2,20,23 and the sites 236–253 and 293–313 of NF-κB p65 to regulate apoptosis.24,25 The Ank-SH3 domain binds to the Hepatitis C virus (HCV) core protein and blocks the interaction between p53 and TP53BP2 to inhibit p53-mediated apoptosis.26 The Ank-SH3 domain binds to the C-terminal of APCL to regulate the localization of TP53BP2 in cells.27 The Ank-SH3 domain binds to the phosphotyrosine binding domain and central domain of insulin receptor substrate 1 (IRS-1) to regulate IRS mediated insulin signaling pathway, growth and development.28,29 The Pro-Ank-SH3 domain interacts with C-terminal Src kinase (Csk) to regulate Src kinase and then regulate epithelial integrity.30 Prior to the Ank domain, there is an RVKF sequence, known as RVxF motif, which binds to the catalytic domain of Protein Phosphatase 1 (PP1) to inhibit PP1 phosphorylase phosphatase activity. SH3 domain also binds to the C-terminal of PP1 to distinguish PP1 subtypes. In addition, the binding of TP53BP2 and PP1 may regulate the tumor suppressive function of TP53BP2.31, 32, 33 The SH3 domain and YPPPPY sequence of TP53BP2 combine with VPMRLR sequence and WW1 domain of Yes-associated protein (YAP) to regulate the phosphorylation of YAP and then regulate the migration of tumor cells.34, 35, 36 The Ank domain binds to a factor inhibiting HIF-1 (FIH-3), thereby regulating the binding of TP53BP2 to protein activated receptor-3 (Par3).37 The Pro domain can bind to the N-terminal of cytotoxic associated gene A (CagA), inhibit the tumor suppressive function of TP53BP2 and lead to the oss of cell polarity38, 39, 40 (Fig. 3; Fig. S1).
Figure 3.
TP53BP2 interacting proteins network diagram and C-terminal binding proteins. (A) TP53BP2 interacting proteins network diagram (Quoted from the String database). (B) DNA binding domain of p53 interact with the ANK-SH3 domain at the C-terminal of TP53BP2. Asn1072 of TP53BP2 binds to Asn247 of p53 (red), and Asp1091 and Glu1092 bind to Arg273 and Arg280(blue). (C) DNA binding domain of Bcl2 interact with the ANK-SH3 domain at the C-terminal of TP53BP2. Asp950 of TP53BP2 binds to Arg164 of p53 (red), Ser1014 binds to Arg26 (blue), Glu1069 binds to Lys17 and His94 (yellow), and Asp991 binds to Arg106 (green). (D) DNA binding domain of NF-κB p65 interact with the ANK-SH3 domain at the C-terminal of TP53BP2. Glu936 of TP53BP2 binds to Lys301, Arg302, Lys303 and Arg304 of NF-κB p65, and Glu1035 binds to Arg236.
There are also some binding proteins at the N-terminal of TP53BP2; for example, the combination of the N-terminal of TP53BP2 and RAS regulates cell aging and autophagy and enhances p53-mediated apoptosis.41 Binding with Par3 maintains the integrity of tight junctions and cell polarity42 (Fig. S2). In addition, some proteins can bind to both the N-terminal and C-terminal of TP53BP2; for example, Amyloid precursor protein-binding protein 1 (APP-BP1) combined with TP53BP2 inhibits apoptosis and ubiquitin pathway.43 DDA3 combined with TP53BP2 inhibited p53-mediated Bax activation.44 Ddx42p (one of the dead box proteins) binds to the N-terminal of TP53BP2 and the Ank-SH3 domain to regulate apoptosis45 (Fig. S3). TP53BP2 also has intramolecular interactions. The Pro domain can be combined with the Ank-SH3 domain to regulate the interaction between TP53BP2 and other proteins.46
Role of TP53BP2 and its isoforms in tumorigenesis or tumor development
Role of TP53BP2 in tumorigenesis or tumor development
TP53BP2 plays an important role in tumorigenesis or tumor development. Numerous in vitro and animal experiments have shown that reduced TP53BP2 expression can inhibit apoptosis of tumor cells, cause malignant proliferation, disrupt cell polarity, promote cell migration, and enhance autophagy and drug resistance to chemotherapy. Clinical trials have also shown that the expression of TP53BP2 is often reduced in human malignancies, and reduced TP53BP2 expression is often associated with tumorigenesis or tumor development, progression, poor prognosis, and low survival. In addition, there are some isoforms of TP53BP2, but these isoforms have opposite functions to TP53BP2 and are likely to be oncogenes. So, how does TP53BP2 play its role? Herein, we elaborate on the role of TP53BP2 and its isoforms in different tumors.
TP53BP2 and liver cancer
Currently, liver cancer is the seventh most common tumor globally, and is the second leading cause of death among cancer patients.1 In terms of autophagy, the decrease of TP53BP2 expression enhances autophagy in hepatocellular carcinoma (HCC) cells, promoting the survival and drug resistance of HCC cells. Moreover, clinical trials also show that the low expression of TP53BP2 is related to the low survival rate of HCC patients.47 In addition, TP53BP2 can also inhibit the autophagy of HepG2 cells by activating the mTOR pathway.48 In terms of tumor growth, the decrease of TP53BP2 expression can enhance the tumor initiation ability and growth by enhancing the mevalonate pathway of HCC cells,11 and the overexpression of TP53BP2 can instantaneously induce the apoptosis of liver cancer cells. However, there is an apoptosis escape mechanism in HCC. The overexpression of TP53BP2 for a long time (more than 48 h) cannot induce apoptosis, because long-term overexpression will make nuclear epidermal growth factor receptors in HCC inhibit the formation of TP53BP2-p53 complex by inducing SOS1 expression.49 Subsequent studies found that bidirectional regulators participated in this escape mechanism as an activator.50 Preclinical studies also showed that diethylnitrosamine (DEN) could activate NF-κB pathway in TP53BP2 deficient mice and promote the formation of mouse liver cancer model.51 Regarding chemotherapy, TP53BP2 can enhance the chemosensitivity of HCC by inhibiting the expression of X-linked inhibitors of apoptosis protein (XIAP).52 The deletion of TP53BP2 disrupts stem-like properties and chemotherapeutic resistance to HCC via Src/FAK/Snail axis.53 A clinical study also found that HCC patients with low expression of TP53BP2 had a higher recurrence rate after transcatheter arterial chemoembolization (TACE) treatment than HCC patients with high expression of TP53BP2.54
TP53BP2 and breast cancer
Global statistics in 2020 showed that breast cancer is the first common malignancy.1 Breast cancer is divided into many subtypes, but the current research on TP53BP2 in breast cancer is limited to lobular and triple-negative breast cancer. In triple-negative breast cancer tissues, the expression of miR-30b-5p is significantly higher than that in para-carcinoma tissues. The expression of miR-30b-5p negatively regulates the expression of TP53BP2, thereby activating the PI3K/AKT pathway and promoting cell proliferation and migration.13,55 There is a truncated TP53BP2 (t-TP53BP2) in lobular carcinoma. t-TP53BP2 promotes tumorigenesis or tumor development by two different mechanisms: The interaction between t-TP53BP2 and PP1 induces actomyosin relaxation, which leads to tumor initiation, while TP53BP2-mediated YAP activation enhances tumor progression.56
TP53BP2 and gastric cancer
Gastric cancer is the third leading cause of cancer death globally, and its occurrence is largely attributed to Helicobacter pylori infection.1,57 CagA is one of the most dangerous factors in Helicobacter pylori.58 In Helicobacter pylori infected cells, CagA can promote the binding of TP53BP2 and p53, but this binding leads to the degradation of p53 and inhibits cell apoptosis.40 Meng et al reported that the infection rate of Helicobacter pylori was high in cancer and precancer tissues, resulting in low expression of TP53BP2.59 A recent study also found that the CagA-TP53BP2 complex can disrupt cell polarity and lead to epithelial–mesenchymal transition (EMT).38 In addition, Gen et al16 demonstrated in vitro and animal experiments that TP53BP2 inhibited invasion of gastric cancer cells and TGF-β-induced EMT by inhibiting E3 ubiquitin ligase-mediated degradation of Smad7.
TP53BP2 and other malignant tumors
In addition to liver cancer, breast cancer and gastric cancer, TP53BP2 is also widely involved in tumorigenesis or tumor development of other malignant tumors. The expression of TP53BP2 decreased significantly in endometrial endometrioid adenocarcinoma,60 oral cancer61 and spinal tumor.62 Inhibition of TP53BP2 expression in endometrial cancer cells promotes invasion by inhibiting LSR and inducing p-YAP expression.36 During the development of esophageal phosphorus cancer, from normal esophageal epithelium to low-grade intraepithelial neoplasia, then to high-grade intraepithelial neoplasia, and finally develop into esophageal cancer, the expression of TP53BP2 gradually decreases, the 5-year overall survival (OS) rate of patients is only 13.2%, and the OS of patients with high expression of TP53BP2 is significantly higher than patients with low expression.63 In colorectal cancer, the mRNA and protein levels of TP53BP2 are significantly lower than those in adjacent tissues.64 In renal cell carcinoma, histone deacetylatlase 1 (HDAC1) induces reduced expression of TP53BP2, promoting cell proliferation and resistance to 5-fluorouracil in vitro and animal experiments. Clinical trials have shown that the down-regulation of TP53BP2 protein is associated with increased TNM stage, and the survival rate and prognosis of patients are also poor.12 Low expression of TP53BP2 is associated with poor prognosis in patients with gallbladder cancer, and down-regulation of TP53BP2 expression promotes invasion and metastasis of gallbladder cancer cells through the aPKC-ι/GLI1 pathway.65 In pancreatic cancer cell lines with TP53BP2 overexpression or knockout, cell proliferation activity was significantly inhibited or promoted, and in vitro and animal experiments also showed that the decrease of TP53BP2 could make cells insensitive to gemcitabine.66
In conclusion, TP53BP2 affects tumorigenesis or tumor development from the perspective of cell proliferation, apoptosis, autophagy, migration, EMT and chemotherapeutic drug resistance (Table 1).
Table 1.
Expression and biological function of TP53BP2 in different tumors.
| Tumor types | TP53BP2 expression in tumor | Biological function | References |
|---|---|---|---|
| Hepatocellular carcinoma | Lower expression | Promote proliferation, autophagy and chemotherapeutic drug resistance; Associated with patient prognosis and survival | 11,47,48,49,50,51,52,53,54 |
| Breast cancer | Lower expression | Promote proliferation and migration | 13,53 |
| Gastric cancer | Lower expression | Promote proliferation, migration, cell polarity and EMT | 16,38,59 |
| Endometrial endometrioid adenocarcinoma | Lower expression | TP53BP2 might become candidate markers for the early diagnosis of EEA | 60 |
| Oral cancer | Lower expression | Associated with advanced and moderately differentiated disease | 61 |
| Spinal tumor | Lower expression | Promote proliferation | 62 |
| Endometrial cancer | Lower expression | Promote cell migration | 36 |
| Esophageal squamous cell carcinoma | Lower expression | Promote ESCC generation and migration | 63 |
| Colorectal cancer | Lower expression | Promote proliferation | 64 |
| Renal cell carcinoma | Lower expression | Promote proliferation; Associated with patient prognosis | 12 |
| Gallbladder cancer | Lower expression | Promote cell migration; Associated with patient prognosis | 65 |
| Pancreatic cancer | Lower expression | Promote proliferation and chemotherapeutic drug resistance | 66 |
Role of TP53BP2 isoforms in tumorigenesis or tumor development
In recent years, some studies have found multiple isoforms of TP53BP2, but these isoforms have lost their tumor inhibition effect, and promote tumorigenesis or tumor development. ΔN-TP53BP2, an isoform of N-terminal deletion of about 254 amino acids, is often expressed in breast cancer. ΔN-TP53BP2 suppresses p53 target gene transactivation, promoter occupancy, and endogenous p53 target gene expression in response to DNA damage. Moreover, ΔN-TP53BP2 promotes progression through the cell cycle. In addition, ΔN-TP53BP2 can also combine with TP53BP2 and halt its function, and may inhibit the expression of TP53BP2.67 TP53BP2, another isoform that lacks most of the C-terminal, results in its inability to interact with p53. TP53BP2 is highly expressed in human colorectal tumors (CRC) and promotes tumorigenesis or tumor development by enhancing cell proliferation, promoting cell migration and endowing chemotherapy-induced apoptosis resistance.68 From these data, the functions of ΔN-TP53BP2 and TP53BP2 are opposite to those of TP53BP2. It is of great significance to get a deep insight into TP53BP2 isoforms for clinical treatment.
Suppression of cancer signaling pathways by TP53BP2
In the above part, we reviewed the role of TP53BP2 in tumorigenesis or development, but this is not the only role of TP53BP2. TP53BP2 also needs to form a cascade through downstream signal pathways to promote or inhibit tumors. Next, we elaborate on the signal pathways involved in TP53BP2 (Fig. 4).
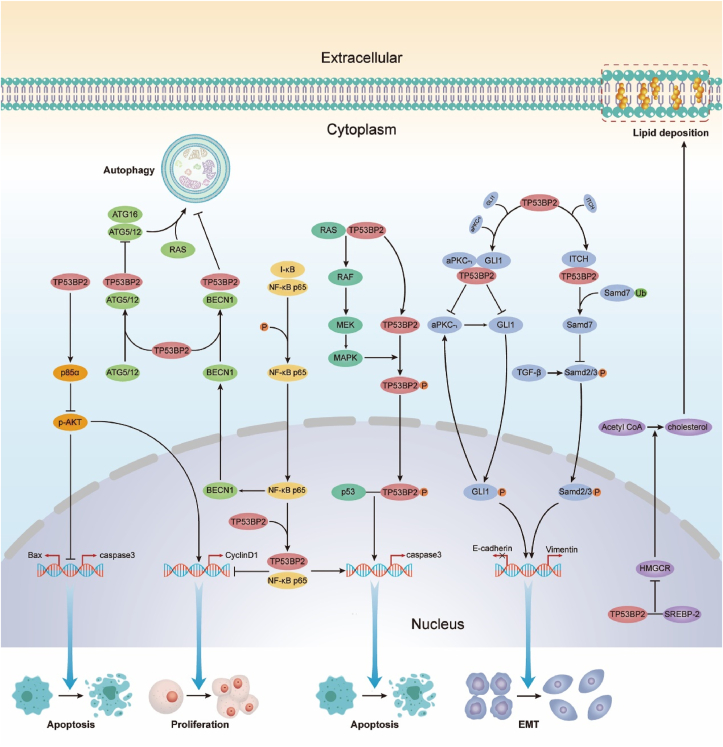
Figure 4.
Suppression of cancer signaling pathways by TP53BP2.
The NF-κB pathway
The NF-κB pathway is an important inflammatory signaling pathway and a key mediator connecting inflammation and tumor.69,70 NF-κB is involved in regulating cell apoptosis, proliferation, tumor migration and invasion, and abnormal activation of NF-κB is often observed in cancer tissues.71,72 The binding pattern of NF-κB to TP53BP2 is similar to that of its natural inhibitor I-κB, suggesting that TP53BP2 may also be an inhibitor of NF-κB.25 Wang et al51 investigated the effects of TP53BP2 on the NF-κB pathway and hepatocellular carcinoma, suggesting that TP53BP2 knockdown can promote hepatic inflammation and hyperplasia by activating the NF-κB pathway, and overexpression of TP53BP2 can inhibit NF-κB pathway and prevent HCC. In TP53BP2 deficient squamous cell carcinoma (SCC) mice, TP53BP2 binds to I-κB and mediates p63 inhibition by inhibiting ΔNp63 (a component of the NF-κB transcription complex) via Rel/A P65, and regulates SCC through inflammatory signaling pathway.73
RAS signaling pathway
RAS is one of the earliest oncogenes, and mutations of RAS are found in about 20% of human tumors.74 Carcinogenic RAS can transform cells and promote tumorigenesis. Previous studies have shown that TP53BP2 binds to RAS-GTP via the N-terminal on the cell membrane to activate the RAS signaling pathway, thereby enhancing p53-mediated apoptosis41 and activate the RAS/RAF/MEK/ERK pathway to promote cell senescence.75 TP53BP2 is also a novel substrate of mitogen-activated protein kinase (MAPK). RAF/MAPK pathway is the most studied downstream RAS signaling pathway. First, TP53BP2 interacts with activated RAS on the cell membrane to activate RAF. Activated RAF activates mitogen-activated and extracellular signal kinase (MEK). MEK phosphorylation further activates MAPK, which in turn phosphorylates Ser827 of TP53BP2. Finally, phosphorylated TP53BP2 interacts with p53 to promote cell apoptosis.76
Mevalonate pathway
The stability and structure of plasma membrane of cells are largely dependent on cholesterol, and a previous study has shown that there is abundant cholesterol in the cell membrane of cancer cells.77 Cholesterol synthesis is mainly achieved through the mevalonate pathway, and some tumors have been shown to depend on this pathway for growth.78 3-hydroxy-3-methylglutaryl CoA reductase (HMGCR) is the rate-limiting enzyme of the mevalonate pathway. Its transcriptional activation is controlled by sterol regulatory element binding protein-2 (SREBP-2). Under the regulation of SREBP-2, acetyl CoA is converted into cholesterol and another non-sterol isoprene for cell needs.79 It was found that the interaction between TP53BP2 and SREBP-2 in the nucleus negatively regulated the mevalonate pathway. In HCC-LM3 cells with decreased TP53BP2 expression, HMGCR mRNA and protein levels were significantly increased, as well as intracellular cholesterol content. Clinical trials have also shown that patients with high levels of TP53BP2 and low levels of HMGCR have a better prognosis.11
The TGF-β1 pathway
Transforming growth factor β1 (TGF-β1) is one of the components of the tumor microenvironment, which is involved in cell proliferation, differentiation, inflammation and other cellular regulatory pathways. TGF-β1 is a typical factor promoting liver fibrosis. A previous study has found that TP53BP2 may reduce the fibrosis of LX-2 cells by inhibiting the autophagy induced by TGF-β1, and also has an anti-TGF -β1 anti-apoptosis effect.80 In addition, TGF-β1 can induce EMT through the Samd2/3 signal.81 Gen et al16 showed that inhibiting the expression of TP53BP2 can significantly inhibit the expression of Samd7. As a negative regulator of Smad2/3, inhibiting Smad7 can promote the phosphorylation and nuclear accumulation of Smad2/3 in GC cells and then promote TGF-β1 induced EMT. It is well established that Smad7 can be ubiquitinated and degraded by ITCH (a HECT type E3 ubiquitin ligase). Subsequently, the immunoprecipitation and ubiquitination experiments show that TP53BP2 reduces the ubiquitination of Smad7 by interacting with ITCH, and Smad7 negatively regulates Smad2/3, thereby inhibiting TGF-β1-Smad2/3 pathway mediated EMT.
PI3K/AKT pathway
Phosphoinositol-3-kinase (PI3K) pathway transmits extracellular signals and activates tyrosine kinase receptor (AKT) and G-protein-coupled receptor, which are required for normal cell growth.82 The PI3K/AKT pathway is a key regulator of survival during cell stress, and abnormal activation of this pathway is often observed in tumors.13,83, 84, 85 A recent study showed that inhibiting TP53BP2 expression promotes the growth and migration of triple negative breast cancer (TNBC) cells through this pathway.13 PI3KR1 (P85 α) negatively regulates the PI3K pathway. After inhibiting the expression of TP53BP2, the expression of p85α decreased and the p-Akt increased. In summary, these studies suggest that down-regulation of TP53BP2 activates the PI3K/AKT pathway in TNBC cells.
aPKC-ι/GLI1 pathway
The transcription factor glioma-associated oncogene homolog 1 (GLI1) is a key factor in the Hedgehog (Hh) pathway, whose abnormal activation often leads to tumorigenesis.86 aPKC-ι can activate the Hh pathway to promote the proliferation of cancer cells.87 Li Tian et al further investigated the role of TP53BP2 in an aPKC-ι pathway. Research shows that TP53BP2 can interact with aPKC-ι and GLI1, while knockdown of TP53BP2 reduced this effect (including the interaction between TP53BP2 and GLI1 in the nucleus) and increased aPKC-ι and GLI1 expression. In addition, as aPKC-ι expression increases, GLI1 is phosphorylated and transferred to the nucleus, where it binds to downstream promoters to release chemokine factors, such as CCL2, CCL5, TNFA, and aPKC-ι, which may form positive feedback to further amplify signals, ultimately leading to EMT in gallbladder carcinoma cells.65
Autophagy pathway
Autophagy is a lysosomal dependent cellular degradation process. The induction of autophagy under stress is very critical for maintaining intracellular homeostasis. Autophagy has beneficial and harmful effects in the early and late stages of hepatocarcinogenesis.88 Many studies have shown that TP53BP2 is involved in the regulation of autophagy. TP53BP2 is structurally similar to ATG12 and LC3 at the N-terminal and can competitively bind ATG5/ATG12 with ATG16 to inhibit RAS-induced autophagy.89 As the expression of TP53BP2 is often reduced in pancreatic cancer, autophagy of pancreatic cancer cells is enhanced, leading to increased resistance to gemcitabine.66 The decrease of TP53BP2 in HCC patients also leads to increased BECN1-dependent autophagy and promotes the survival of HCC cells.47
By expounding the signal pathways involved in TP53BP2, we found that under normal circumstances, most of the downstream signal pathways of TP53BP2 are inactive, but some are active, such as the NF-κB pathway, Mevalonate pathway, TGF-β1 pathway, PI3K/AKT pathway. The aPKC-ι/GLI1 way is off, but RAS signal pathway is active. Under normal circumstances, the combination of TP53BP2 and RAS promotes p53 mediated apoptosis. However, the expression of TP53BP2 is often reduced in tumors, which leads to the failure of Ras signaling pathway to function normally, while the NF-κB pathway, Mevalonate pathway, TGF-β1 pathway, PI3K/AKT pathway, aPKC-ι/GLI1 way activation promotes the proliferation, autophagy, metastasis, and lipid deposition of tumor cells, and inhibits the apoptosis of tumor cells. Therefore, can TP53BP2 be used as a therapeutic target to inhibit tumorigenesis or tumor development through appropriate measures?
Prospects
TP53BP2 has been reported as a tumor therapeutic target in some studies. Recombinant human adenovirus TP53BP2 can inhibit HCC by inhibiting the expression of p-ERK and p-STAT3, cooperating with oxaliplatin to inhibit cell proliferation, autophagy and promote apoptosis.90 In colorectal cancer, oxaliplatin induces phosphorylation of Ser92/Ser361 of TP53BP2, and phosphorylated TP53BP2 promotes apoptosis of HTC116 cells in a p53-dependent manner.91 In addition, in colorectal cancer, TP53BP2 can inhibit autophagy and promote chemotherapy drug resistance in a p53-independent manner, thereby promoting oxaliplatin mediated apoptosis.92 The combination of herpes simplex virus 1 thymidine kinase (HSV-TK), ganciclovir (GCV), recombinant adenovirus p53, and recombinant adenovirus TP53BP2 improves the efficacy of HCC patients lacking functional p53, however, single ASPP2 overexpression cannot induce apoptosis of p53 mutated HCC cells.14 The natural extract luteolin can significantly inhibit the proliferation of HepG2 cells and significantly up-regulate the expression of p53 and TP53BP2.93
Although the current research has found that overexpression of TP53BP2 can effectively improve tumorigenesis or tumor development, it is noteworthy that TP53BP2 also has an important p53-dependent function. TP53BP2 was first discovered through p53, which binds to wild-type p53 but cannot bind to mutant p53. The combination of TP53BP2 and p53 promotes p53-mediated transcriptional activation and cell apoptosis. p53 mutations have been observed in 50% of malignant tumors, making TP53BP2 unable to function normally. Therefore, tumor treatment for TP53BP2 alone may not achieve good results. For example, overexpression of TP53BP2 alone could not induce apoptosis of p53 mutant cells. Wang et al only studied the effect of luteolin on HepG2 with wild type of p53, but did not know what effect it would have in cells with p53 deletion or mutation. Therefore, the path of using TP53BP2 as a gene drug or chemotherapy drug and screening drugs targeting TP53BP2 to treat malignant tumors is still in infancy and needs further exploration.
Further study on the regulation of TP53BP2 is also conducive to the discovery of TP53BP2 targeted drugs. In recent years, the regulation of TP53BP2 has been explored. Current studies have shown that E2F, signal translator and activator of transcription 1 (STAT1) and RAS association domain family 10 (RASSF10) can positively regulate the expression of TP53BP2,94, 95, 96 while HDAC1 inhibits TP53BP2 expression by blocking the binding of E2F to TP53BP2.12 In addition, mir-21 and mir-30B-5p can negatively regulate the expression of TP53BP2.55,97 However, these regulations are limited to the transcriptional level, and the regulation of TP53BP2 in other aspects, such as post-transcriptional level, translation level, and post-translation level, remains unclear and needs to be studied.
The evidence for TP53BP2 as a tumor suppressor gene is gaining increasing interest. Further studies on the role of TP53BP2 in tumor genesis and development and the signaling pathways involved in TP53BP2 may provide more evidence and strategies for tumor treatment.
Author contributions
XNL and YS designed the study. YFH was major contributors in writing the manuscript. KC, BXK, MYC and SSD made substantial contributions to the design of the manuscript and revised it critically for important intellectual content. DXC provided financial support. All authors read and approved the final manuscript.
Conflict of interests
The authors have no conflict of interests to declare.
Funding
This work was supported by Beijing Municipal Natural Science Foundation (China) (No. 7192084); The Capital Health Research and Development of Special (China) (No. 2020-2-1152); Beijing Municipal Institute of Public Medical Research Development and Reform Pilot Project (China) (No. Jingyiyan 2019-6); National Natural Science Foundation of China (No. 81672026); Research and demonstration application of clinical diagnosis and treatment technology in the capital (China) (No. Z191100006619064).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2022.08.014.
Contributor Information
Ying Shi, Email: yingshi@ccmu.edu.cn.
Xiaoni Liu, Email: liuxiaoni888@ccmu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Multimedia component 1
References
- 1.Cao W., Chen H.D., Yu Y.W., Li N., Chen W.Q. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. 2021;134(7):783–791. doi: 10.1097/CM9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soerjomataram I., Bray F. Planning for tomorrow: global cancer incidence and the role of prevention 2020-2070. Nat Rev Clin Oncol. 2021;18(10):663–672. doi: 10.1038/s41571-021-00514-z. [DOI] [PubMed] [Google Scholar]
- 3.Weinberg R.A. The retinoblastoma protein and cell cycle control. Cell. 1995;81(3):323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 4.Powell S.M., Zilz N., Beazer-Barclay Y., et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359(6392):235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 5.Hainaut P., Hollstein M. p53 and human cancer: the first ten thousand mutations. Adv Cancer Res. 2000;77:81–137. doi: 10.1016/s0065-230x(08)60785-x. [DOI] [PubMed] [Google Scholar]
- 6.Ryan K.M., Vousden K.H. Characterization of structural p53 mutants which show selective defects in apoptosis but not cell cycle arrest. Mol Cell Biol. 1998;18(7):3692–3698. doi: 10.1128/mcb.18.7.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Z.J., Lu X., Zhang Y., et al. Downregulated mRNA expression of ASPP and the hypermethylation of the 5'-untranslated region in cancer cell lines retaining wild-type p53. FEBS Lett. 2005;579(7):1587–1590. doi: 10.1016/j.febslet.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 8.Iwabuchi K., Bartel P.L., Li B., Marraccino R., Fields S. Two cellular proteins that bind to wild-type but not mutant p53. Proc Natl Acad Sci U S A. 1994;91(13):6098–6102. doi: 10.1073/pnas.91.13.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joerger A.C., Ang H.C., Veprintsev D.B., Blair C.M., Fersht A.R. Structures of p53 cancer mutants and mechanism of rescue by second-site suppressor mutations. J Biol Chem. 2005;280(16):16030–16037. doi: 10.1074/jbc.M500179200. [DOI] [PubMed] [Google Scholar]
- 10.Gorina S., Pavletich N.P. Structure of the p53 tumor suppressor bound to the ankyrin and SH3 domains of 53BP2. Science. 1996;274(5289):1001–1005. doi: 10.1126/science.274.5289.1001. [DOI] [PubMed] [Google Scholar]
- 11.Liang B., Chen R., Song S., et al. ASPP2 inhibits tumor growth by repressing the mevalonate pathway in hepatocellular carcinoma. Cell Death Dis. 2019;10(11):830. doi: 10.1038/s41419-019-2054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H., Wang X., Zhang C., et al. HDAC1-induced epigenetic silencing of ASPP2 promotes cell motility, tumour growth and drug resistance in renal cell carcinoma. Cancer Lett. 2018;432:121–131. doi: 10.1016/j.canlet.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Wu T., Song H., Xie D., et al. Silencing of ASPP2 promotes the proliferation, migration and invasion of triple-negative breast cancer cells via the PI3K/AKT pathway. Int J Oncol. 2018;52(6):2001–2010. doi: 10.3892/ijo.2018.4331. [DOI] [PubMed] [Google Scholar]
- 14.Liu X., Wang S., Guo X., et al. Exogenous p53 and ASPP2 expression enhances rAdV-TK/GCV-induced death in hepatocellular carcinoma cells lacking functional p53. Oncotarget. 2016;7(14):18896–18905. doi: 10.18632/oncotarget.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S., Shi G., Yuan H., et al. Abnormal expression pattern of the ASPP family of proteins in human non-small cell lung cancer and regulatory functions on apoptosis through p53 by iASPP. Oncol Rep. 2012;28(1):133–140. doi: 10.3892/or.2012.1778. [DOI] [PubMed] [Google Scholar]
- 16.Gen Y., Yasui K., Kitaichi T., et al. ASPP2 suppresses invasion and TGF-β1-induced epithelial-mesenchymal transition by inhibiting Smad7 degradation mediated by E3 ubiquitin ligase ITCH in gastric cancer. Cancer Lett. 2017;398:52–61. doi: 10.1016/j.canlet.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Vogelstein B., Lane D., Levine A.J. Surfing the p53 network. Nature. 2000;408(6810):307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 18.Levine A.J. Targeting therapies for the p53 protein in cancer treatments. Annu Rev Cell Biol. 2019;3:21–34. [Google Scholar]
- 19.Samuels-Lev Y., O'Connor D.J., Bergamaschi D., et al. ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell. 2001;8(4):781–794. doi: 10.1016/s1097-2765(01)00367-7. [DOI] [PubMed] [Google Scholar]
- 20.Naumovski L., Cleary M.L. The p53-binding protein 53BP2 also interacts with Bc12 and impedes cell cycle progression at G2/M. Mol Cell Biol. 1996;16(7):3884–3892. doi: 10.1128/mcb.16.7.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn J., Byeon I.L., Byeon C.H., Gronenborn A.M. Insight into the structural basis of pro- and antiapoptotic p53 modulation by ASPP proteins. J Biol Chem. 2009;284(20):13812–13822. doi: 10.1074/jbc.M808821200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson R.A., Lu X., Jones E.Y., Siebold C. Biochemical and structural studies of ASPP proteins reveal differential binding to p53, p63, and p73. Structure. 2008;16(2):259–268. doi: 10.1016/j.str.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Katz C., Benyamini H., Rotem S., et al. Molecular basis of the interaction between the antiapoptotic Bcl-2 family proteins and the proapoptotic protein ASPP2. Proc Natl Acad Sci U S A. 2008;105(34):12277–12282. doi: 10.1073/pnas.0711269105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J.P., Hori M., Takahashi N., Kawabe T., Kato H., Okamoto T. NF-kappaB subunit p65 binds to 53BP2 and inhibits cell death induced by 53BP2. Oncogene. 1999;18(37):5177–5186. doi: 10.1038/sj.onc.1202904. [DOI] [PubMed] [Google Scholar]
- 25.Benyamini H., Leonov H., Rotem S., Katz C., Arkin I.T., Friedler A. A model for the interaction between NF-kappa-B and ASPP2 suggests an I-kappa-B-like binding mechanism. Proteins. 2009;77(3):602–611. doi: 10.1002/prot.22473. [DOI] [PubMed] [Google Scholar]
- 26.Cao Y., Hamada T., Matsui T., Date T., Iwabuchi K. Hepatitis C virus core protein interacts with p53-binding protein, 53BP2/Bbp/ASPP2, and inhibits p53-mediated apoptosis. Biochem Biophys Res Commun. 2004;315(4):788–795. doi: 10.1016/j.bbrc.2004.01.124. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa H., Koyama K., Murata Y., Morito M., Akiyama T., Nakamura Y. APCL, a central nervous system-specific homologue of adenomatous polyposis coli tumor suppressor, binds to p53-binding protein 2 and translocates it to the perinucleus. Cancer Res. 2000;60(1):101–105. [PubMed] [Google Scholar]
- 28.Hakuno F., Kurihara S., Watson R.T., Pessin J.E., Takahashi S.I. 53BP2S, interacting with insulin receptor substrates, modulates insulin signaling. J Biol Chem. 2007;282(52):37747–37758. doi: 10.1074/jbc.M702472200. [DOI] [PubMed] [Google Scholar]
- 29.Liu C., Luan J., Bai Y., et al. Aspp2 negatively regulates body growth but not developmental timing by modulating IRS signaling in zebrafish embryos. Gen Comp Endocrinol. 2014;197:82–91. doi: 10.1016/j.ygcen.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Langton P.F., Colombani J., Aerne B.L., Tapon N. Drosophila ASPP regulates C-terminal Src kinase activity. Dev Cell. 2007;13(6):773–782. doi: 10.1016/j.devcel.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Helps N.R., Barker H.M., Elledge S.J., Cohen P.T. Protein phosphatase 1 interacts with p53BP2, a protein which binds to the tumour suppressor p53. FEBS Lett. 1995;377(3):295–300. doi: 10.1016/0014-5793(95)01347-4. [DOI] [PubMed] [Google Scholar]
- 32.Bertran M.T., Mouilleron S., Zhou Y., et al. ASPP proteins discriminate between PP1 catalytic subunits through their SH3 domain and the PP1 C-tail. Nat Commun. 2019;10(1):771. doi: 10.1038/s41467-019-08686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Royer C., Sandham E., Slee E., et al. ASPP2 maintains the integrity of mechanically stressed pseudostratified epithelia during morphogenesis. Nat Commun. 2022;13(1):941. doi: 10.1038/s41467-022-28590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Espanel X., Sudol M. Yes-associated protein and p53-binding protein-2 interact through their WW and SH3 domains. J Biol Chem. 2001;276(17):14514–14523. doi: 10.1074/jbc.M008568200. [DOI] [PubMed] [Google Scholar]
- 35.Romero-Pérez L., Garcia-Sanz P., Mota A., et al. A role for the transducer of the Hippo pathway, TAZ, in the development of aggressive types of endometrial cancer. Mod Pathol. 2015;28(11):1492–1503. doi: 10.1038/modpathol.2015.102. [DOI] [PubMed] [Google Scholar]
- 36.Konno T., Kohno T., Okada T., et al. ASPP2 suppression promotes malignancy via LSR and YAP in human endometrial cancer. Histochem Cell Biol. 2020;154(2):197–213. doi: 10.1007/s00418-020-01876-8. [DOI] [PubMed] [Google Scholar]
- 37.Janke K., Brockmeier U., Kuhlmann K., et al. Factor inhibiting HIF-1 (FIH-1) modulates protein interactions of apoptosis-stimulating p53 binding protein 2 (ASPP2) J Cell Sci. 2013;126(Pt 12):2629–2640. doi: 10.1242/jcs.117564. [DOI] [PubMed] [Google Scholar]
- 38.Buti L., Ruiz-Puig C., Sangberg D., et al. CagA-ASPP2 complex mediates loss of cell polarity and favors H. pylori colonization of human gastric organoids. Proc Natl Acad Sci U S A. 2020;117(5):2645–2655. doi: 10.1073/pnas.1908787117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nešić D., Buti L., Lu X., Stebbins C.E. Structure of the Helicobacter pylori CagA oncoprotein bound to the human tumor suppressor ASPP2. Proc Natl Acad Sci U S A. 2014;111(4):1562–1567. doi: 10.1073/pnas.1320631111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buti L., Spooner E., Van der Veen A.G., Rappuoli R., Covacci A., Ploegh H.L. Helicobacter pylori cytotoxin-associated gene A (CagA) subverts the apoptosis-stimulating protein of p53 (ASPP2) tumor suppressor pathway of the host. Proc Natl Acad Sci U S A. 2011;108(22):9238–9243. doi: 10.1073/pnas.1106200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y., Godin-Heymann N., Wang X.D., Bergamaschi D., Llanos S., Lu X. ASPP1 and ASPP2 bind active RAS, potentiate RAS signalling and enhance p53 activity in cancer cells. Cell Death Differ. 2013;20(4):525–534. doi: 10.1038/cdd.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sottocornola R., Royer C., Vives V., et al. ASPP2 binds Par-3 and controls the polarity and proliferation of neural progenitors during CNS development. Dev Cell. 2010;19(1):126–137. doi: 10.1016/j.devcel.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y., Liu W., Naumovski L., Neve R.L. ASPP2 inhibits APP-BP1-mediated NEDD8 conjugation to cullin-1 and decreases APP-BP1-induced cell proliferation and neuronal apoptosis. J Neurochem. 2003;85(3):801–809. doi: 10.1046/j.1471-4159.2003.01727.x. [DOI] [PubMed] [Google Scholar]
- 44.Sun W.T., Hsieh P.C., Chiang M.L., Wang M.C., Wang F.F. p53 target DDA3 binds ASPP2 and inhibits its stimulation on p53-mediated BAX activation. Biochem Biophys Res Commun. 2008;376(2):395–398. doi: 10.1016/j.bbrc.2008.08.168. [DOI] [PubMed] [Google Scholar]
- 45.Uhlmann-Schiffler H., Kiermayer S., Stahl H. The DEAD box protein Ddx42p modulates the function of ASPP2, a stimulator of apoptosis. Oncogene. 2009;28(20):2065–2073. doi: 10.1038/onc.2009.75. [DOI] [PubMed] [Google Scholar]
- 46.Rotem S., Katz C., Benyamini H., et al. The structure and interactions of the proline-rich domain of ASPP2. J Biol Chem. 2008;283(27):18990–18999. doi: 10.1074/jbc.M708717200. [DOI] [PubMed] [Google Scholar]
- 47.Chen R., Wang H., Liang B., et al. Downregulation of ASPP2 improves hepatocellular carcinoma cells survival via promoting BECN1-dependent autophagy initiation. Cell Death Dis. 2016;7(12) doi: 10.1038/cddis.2016.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu P., Shi H., Jiao Y., Chen D., Shi H. [TP53BP2/ASPP2 inhibits autophagy of HepG2 cells by activating mTOR pathway in a p53-independent manner] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2019;35(11):986–991. [PubMed] [Google Scholar]
- 49.Liu K., Jiang T., Ouyang Y., et al. Nuclear EGFR impairs ASPP2-p53 complex-induced apoptosis by inducing SOS1 expression in hepatocellular carcinoma. Oncotarget. 2015;6(18):16507–16516. doi: 10.18632/oncotarget.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu K., Lin D., Ouyang Y., et al. Amphiregulin impairs apoptosis-stimulating protein 2 of p53 overexpression-induced apoptosis in hepatoma cells. Tumour Biol. 2017;39(3) doi: 10.1177/1010428317695026. [DOI] [PubMed] [Google Scholar]
- 51.Wang S., Kou B., Chai M., et al. Knockout of ASPP2 promotes DEN-induced hepatocarcinogenesis via the NF-κB pathway in mice. Cancer Gene Ther. 2022;29(2):202–214. doi: 10.1038/s41417-021-00300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang T., Gao Y., Liu D., et al. ASPP2 enhances chemotherapeutic sensitivity through the down-regulation of XIAP expression in a p53 independent manner in hepatocellular carcinoma. Biochem Biophys Res Commun. 2019;508(3):769–774. doi: 10.1016/j.bbrc.2018.11.181. [DOI] [PubMed] [Google Scholar]
- 53.Xu L., Tong X., Zhang S., et al. ASPP2 suppresses stem cell-like characteristics and chemoresistance by inhibiting the Src/FAK/Snail axis in hepatocellular carcinoma. Tumour Biol. 2016;37(10):13669–13677. doi: 10.1007/s13277-016-5246-0. [DOI] [PubMed] [Google Scholar]
- 54.Mao J., Tan Z., Pan X., Meng F. ASPP2 expression predicts the prognosis of patients with hepatocellular carcinoma after transcatheter arterial chemoembolization. Exp Ther Med. 2021;21(4):397. doi: 10.3892/etm.2021.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu T., Song H., Xie D., et al. miR-30b-5p promotes proliferation, migration, and invasion of breast cancer cells via targeting ASPP2. BioMed Res Int. 2020;2020 doi: 10.1155/2020/7907269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schipper K., Drenth A.P., van der Burg E., et al. Truncated ASPP2 drives initiation and progression of invasive lobular carcinoma via distinct mechanisms. Cancer Res. 2020;80(7):1486–1497. doi: 10.1158/0008-5472.CAN-19-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uemura N., Okamoto S., Yamamoto S., et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345(11):784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 58.Ruggiero P. Helicobacter pylori infection: what's new. Curr Opin Infect Dis. 2012;25(3):337–344. doi: 10.1097/QCO.0b013e3283531f7c. [DOI] [PubMed] [Google Scholar]
- 59.Meng W.D., Chu R.X., Wang B.Z., Wang L.P., Ma L.L., Wang L.X. Helicobacter pylori infection and expressions of apoptosis-related proteins p53, ASPP2 and iASPP in gastric cancer and precancerous lesions. Pathol Biol. 2013;61(5):199–202. doi: 10.1016/j.patbio.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 60.Liu W.K., Jiang X.Y., Ren J.K., Zhang Z.X. Expression pattern of the ASPP family members in endometrial endometrioid adenocarcinoma. Onkologie. 2010;33(10):500–503. doi: 10.1159/000319692. [DOI] [PubMed] [Google Scholar]
- 61.Patel K.D., Barasiya Y.V., Patel J.B., Patel P.S. Apoptosis stimulating protein of p53 (ASPP) 1 and ASPP2 m-RNA expression in oral cancer. Arch Oral Biol. 2020;119 doi: 10.1016/j.archoralbio.2020.104920. [DOI] [PubMed] [Google Scholar]
- 62.Huang W., Li X., Cai L. Effects of ASPP2 on proliferation and apoptosis of malignant spinal tumor cells. Int J Clin Exp Pathol. 2017;10(7):8023–8030. [PMC free article] [PubMed] [Google Scholar]
- 63.Liu B., Yang L., Li X.J., et al. Expression and significance of ASPP2 in squamous carcinoma of esophagus. Kaohsiung J Med Sci. 2018;34(6):321–329. doi: 10.1016/j.kjms.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yin L., Lin Y., Wang X., et al. The family of apoptosis-stimulating proteins of p53 is dysregulated in colorectal cancer patients. Oncol Lett. 2018;15(5):6409–6417. doi: 10.3892/ol.2018.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tian L., Deng Z., Xu L., et al. Downregulation of ASPP2 promotes gallbladder cancer metastasis and macrophage recruitment via aPKC-ι/GLI1 pathway. Cell Death Dis. 2018;9(11):1115. doi: 10.1038/s41419-018-1145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song B., Bian Q., Zhang Y.J., et al. Downregulation of ASPP2 in pancreatic cancer cells contributes to increased resistance to gemcitabine through autophagy activation. Mol Cancer. 2015;14:177. doi: 10.1186/s12943-015-0447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Hook K., Wang Z., Chen D., et al. ΔN-ASPP2, a novel isoform of the ASPP2 tumor suppressor, promotes cellular survival. Biochem Biophys Res Commun. 2017;482(4):1271–1277. doi: 10.1016/j.bbrc.2016.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rieger I., Tsintari V., Overkamp M., et al. ASPP2κ is expressed in human colorectal carcinoma and promotes chemotherapy resistance and tumorigenesis. Front Mol Biosci. 2021;8 doi: 10.3389/fmolb.2021.727203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liang D.Y., Sha S., Yi Q., et al. Hepatitis B X protein upregulates decoy receptor 3 expression via the PI3K/NF-κB pathway. Cell Signal. 2019;62 doi: 10.1016/j.cellsig.2019.109346. [DOI] [PubMed] [Google Scholar]
- 70.DiDonato J.A., Mercurio F., Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246(1):379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 71.He G., Karin M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21(1):159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang S., Pettaway C.A., Uehara H., Bucana C.D., Fidler I.J. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20(31):4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 73.Tordella L., Koch S., Salter V., et al. ASPP2 suppresses squamous cell carcinoma via RelA/p65-mediated repression of p63. Proc Natl Acad Sci U S A. 2013;110(44):17969–17974. doi: 10.1073/pnas.1309362110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3(1):11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 75.Wang Z., Liu Y., Takahashi M., et al. N terminus of ASPP2 binds to Ras and enhances Ras/Raf/MEK/ERK activation to promote oncogene-induced senescence. Proc Natl Acad Sci U S A. 2013;110(1):312–317. doi: 10.1073/pnas.1201514110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Godin-Heymann N., Wang Y., Slee E., Lu X. Phosphorylation of ASPP2 by RAS/MAPK pathway is critical for its full pro-apoptotic function. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0082022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beloribi-Djefaflia S., Vasseur S., Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5(1):e189. doi: 10.1038/oncsis.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moon S.H., Huang C.H., Houlihan S.L., et al. p53 represses the mevalonate pathway to mediate tumor suppression. Cell. 2019;176(3):564–580. doi: 10.1016/j.cell.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dooley K.A., Millinder S., Osborne T.F. Sterol regulation of 3-hydroxy-3-methylglutaryl-coenzyme A synthase gene through a direct interaction between sterol regulatory element binding protein and the trimeric CCAAT-binding factor/nuclear factor Y. J Biol Chem. 1998;273(3):1349–1356. doi: 10.1074/jbc.273.3.1349. [DOI] [PubMed] [Google Scholar]
- 80.Lin M., Chang Y., Xie F., Shi Y., Pang L., Chen D. ASPP2 inhibits the profibrotic effects of transforming growth factor-β1 in hepatic stellate cells by reducing autophagy. Dig Dis Sci. 2018;63(1):146–154. doi: 10.1007/s10620-017-4816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu J., Lamouille S., Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19(2):156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fruman D.A., Chiu H., Hopkins B.D., Bagrodia S., Cantley L.C., Abraham R.T. The PI3K pathway in human disease. Cell. 2017;170(4):605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Datta S.R., Brunet A., Greenberg M.E. Cellular survival: a play in three Akts. Genes Dev. 1999;13(22):2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 84.Zhou L., Li S., Sun J. Ginkgolic acid induces apoptosis and autophagy of endometrial carcinoma cells via inhibiting PI3K/Akt/mTOR pathway in vivo and in vitro. Hum Exp Toxicol. 2021;40(12):2156–2164. doi: 10.1177/09603271211023789. [DOI] [PubMed] [Google Scholar]
- 85.Yu T., An Q., Cao X.L., et al. GOLPH3 inhibition reverses oxaliplatin resistance of colon cancer cells via suppression of PI3K/AKT/mTOR pathway. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118294. [DOI] [PubMed] [Google Scholar]
- 86.Blotta S., Jakubikova J., Calimeri T., et al. Canonical and noncanonical Hedgehog pathway in the pathogenesis of multiple myeloma. Blood. 2012;120(25):5002–5013. doi: 10.1182/blood-2011-07-368142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Justilien V., Walsh M.P., Ali S.A., Thompson E.A., Murray N.R., Fields A.P. The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer Cell. 2014;25(2):139–151. doi: 10.1016/j.ccr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gual P., Gilgenkrantz H., Lotersztajn S. Autophagy in chronic liver diseases: the two faces of Janus. Am J Physiol Cell Physiol. 2017;312(3):C263–C273. doi: 10.1152/ajpcell.00295.2016. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y., Wang X.D., Lapi E., et al. Autophagic activity dictates the cellular response to oncogenic RAS. Proc Natl Acad Sci U S A. 2012;109(33):13325–13330. doi: 10.1073/pnas.1120193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu X., Xu J., Wang S., et al. Synergistic inhibitory effects on hepatocellular carcinoma with recombinant human adenovirus Aspp2 and oxaliplatin via p53-independent pathway in vitro and in vivo. Int J Oncol. 2017;51(4):1291–1299. doi: 10.3892/ijo.2017.4105. [DOI] [PubMed] [Google Scholar]
- 91.Hou Q., Zhao H., Gong W., et al. [Phosphorylation status of ASPP2 modulates p53 apoptotic function in oxaliplatin-induced apoptosis of colorectal cancer HCT116 cells] Zhonghua Zhongliu Zazhi. 2014;36(6):418–423. [PubMed] [Google Scholar]
- 92.Shi Y., Han Y., Xie F., et al. ASPP2 enhances oxaliplatin (L-OHP)-induced colorectal cancer cell apoptosis in a p53-independent manner by inhibiting cell autophagy. J Cell Mol Med. 2015;19(3):535–543. doi: 10.1111/jcmm.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Y.P., Zhou L., Gong X.G. [Pro-apoptotic effects of luteolin on hepatoma HepG2 cells] Zhejiang Da Xue Xue Bao Yi Xue Ban. 2013;42(5):504–510. [PubMed] [Google Scholar]
- 94.Chen D., Padiernos E., Ding F., Lossos I.S., Lopez C.D. Apoptosis-stimulating protein of p53-2 (ASPP2/53BP2L) is an E2F target gene. Cell Death Differ. 2005;12(4):358–368. doi: 10.1038/sj.cdd.4401536. [DOI] [PubMed] [Google Scholar]
- 95.Turnquist C., Wang Y., Severson D.T., et al. STAT1-induced ASPP2 transcription identifies a link between neuroinflammation, cell polarity, and tumor suppression. Proc Natl Acad Sci U S A. 2014;111(27):9834–9839. doi: 10.1073/pnas.1407898111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Richter A.M., Küster M.M., Woods M.L., et al. RASSF10 is a TGFβ-target that regulates ASPP2 and E-cadherin expression and acts as tumor suppressor that is epigenetically downregulated in advanced cancer. Cancers. 2019;11(12):1976. doi: 10.3390/cancers11121976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu L., Wang L., Li X., et al. Effect of miR-21 on apoptosis in hepatoblastoma cell through activating ASPP2/p38 signaling pathway in vitro and in vivo. Artif Cell Nanomed Biotechnol. 2019;47(1):3729–3736. doi: 10.1080/21691401.2019.1664561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1