Abstract
N6-methyladenosine (m6A) RNA modification is widely perceived as the most abundant and common modification in transcripts. This modification is dynamically regulated by specific m6A “writers”, “erasers” and “readers” and is reportedly involved in the occurrence and development of many diseases. Since m6A RNA modification was discovered in the 1970s, with the progress of relevant research technologies, an increasing number of functions of m6A have been reported, and a preliminary understanding of m6A has been obtained. In this review, we summarize the mechanisms through which m6A RNA modification is regulated from the perspectives of expression, posttranslational modification and protein interaction. In addition, we also summarize how external and internal environmental factors affect m6A RNA modification and its functions in tumors. The mechanisms through which m6A methylases, m6A demethylases and m6A-binding proteins are regulated are complicated and have not been fully elucidated. Therefore, we hope to promote further research in this field by summarizing these mechanisms and look forward to the future application of m6A in tumors.
Keywords: N6-methyladenosine (m6A), Regulatory mechanisms, RNA modification, Therapy, Tumor
Introduction
Epigenetics is the study of reversible, heritable changes in genes in the absence of nuclear DNA sequence changes. This field mainly includes DNA modifications (such as methylation) and histone and chromatin modifications. In the 1970s, scientists identified complex base-methyl nucleoside patterns on RNA that were identified as N6-methyladenosine (m6A).1 Although m6A has been known for decades, the significance of its biological properties has become better understood in recent years due to technical breakthroughs. More than 160 different RNA modifications have been identified to date,2,3 and of these, m6A is the most abundant modification among messenger RNAs (mRNAs), transfer RNAs (tRNAs), ribosomal RNAs (rRNAs) and small nuclear RNAs (snRNAs).4, 5, 6 This modification is widely distributed in more than 7000 mRNAs and 300 noncoding RNA (ncRNAs) transcripts in human cells and is enriched in the 3′-untranslated regions (3′ UTRs) of linear RNAs and near the last exon in ncRNAs.7, 8, 9, 10 Studies have shown that m6A plays a critical role in all aspects of RNA metabolism, including stability, splicing, nuclear export and translation.11, 12, 13, 14, 15 In this review, we focus on the mechanisms through which m6A methylation is regulated and the role of m6A in tumor progression.
m6A writers, erasers, and readers
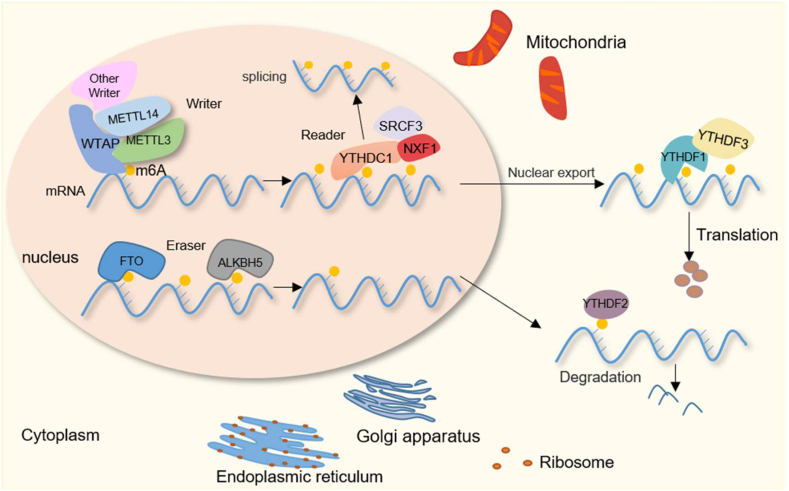
Many studies have confirmed that m6A is a reversible modification. Methylases, demethylases and m6A-binding proteins are the main proteins involved in the regulation of this dynamic process and are also known as the “writers”, “erasers” and “readers” of m6A, respectively (Fig. 1). m6A modification is involved in a series of important biological processes.16
Figure 1.
Mechanisms and molecular functions of m6A modification. METTL3/METTL14/WTAP and other methylases form a “writer” complex, which is responsible for the adding of m6A to mRNA. This modification is removed by the demethylation enzymes FTO and ALKBH5. In the nucleus, YTHDC1 binds to target mRNA with m6A modifications to promote its splicing and also interacts with SRSF3 to promote the binding of target mRNAs to SRSF3 and NXF1, which facilitates the transport of mRNA from the nucleus to the cytoplasm. In the cytoplasm, YTHDF1 promotes mRNA translation with the help of YTHDF3. YTHDF2 recognizes m6A and promotes mRNA degradation.
Methylases, also known as the “writer” complex, have a core of methyltransferase-like protein 3/14 (METTL3/14) and WT1-associated protein (WTAP),17 and RNA-binding motif protein 15/15 B (RBM15/15 B), zinc finger CCCH-type containing 13 (ZC3H13) and vir-like m6A methyltransferase associated (VIRMA/KIAA1429) participate in the formation of subunits of this complex.18, 19, 20 Recently, human CCHC zinc finger-containing protein (ZCCHC4) was identified as a novel m6A writer that mediates the methylation of 28 S rRNAs.21 METTL16 (METTL3 homolog) is considered the “writer” of precursor mRNA and several ncRNAs, including U6 snRNA and long noncoding RNAs (lncRNAs).22,23 More “writers” in this “writer” complex may be discovered in the future.
Human obesity-associated protein (FTO) and ALKB homolog 5 (ALKBH5), known as the “erasers” of m6A, are selective demethylation enzymes that regulate gene expression and cell fate through the oxidative removal of methyl groups from m6A.24,25 In addition, ALKB homolog 1 (ALKBH1) can demethylate the methylcytidine (mC) of mRNA in mammalian cells.26 ALKB homolog 3 (ALKBH3) regulates 1-meA and 3-meC demethylation in endogenous methylated RNA,27 and ALKB homolog 7 (ALKBH7) acts as an RNA demethylase and thereby controls newborn mitochondrial RNA processing and mitochondrial activity.28 These findings add to our understanding of the dynamic modifications of m6A.
m6A “readers”, which constitute a type of m6A-binding protein mainly in the YT521-B homology (YTH) and heterogeneous nuclear ribonucleoprotein (hnRNP) domain families, selectively recognize RNA with m6A modification to mediate its degradation, splicing and translation.29 Several m6A-binding proteins have been identified, and these include YTH family proteins (YTHDC1/2 and YTHDF1/2/3).13,30,31 YTHDC1 promotes the splicing of target mRNA by recruiting the pre-mRNA splicing factor SRSF3 while preventing SRSF10 mRNA binding.32 Moreover, the binding of YTHDC1 to SRSF3 promotes the interaction between SRSF3 and nuclear RNA export factor 1 (NXF1) on target mRNAs and transfers mRNA from the nucleus to the cytoplasm.13 YTHDF1 enhances the translation efficiency of target mRNA with the help of YTHDF3.14 Moreover, YTHDF2 can selectively identify and bind mRNA with m6A modification to promote degradation.11 Additionally, insulin-like growth factor 2 mRNA-binding proteins (IGF2BP1/2/3),33,34 hnRNPA2B1 andeukaryotic translation initiation factor 3 (eIF3) have been identified as m6A “readers”.35,36
m6A-mediated molecular mechanisms
Early studies indicated that m6A modification is involved in pre-mRNA splicing.37 METTL3 significantly affects the p53 mRNA splicing pattern and apoptosis.9 FTO regulates the exon splicing of the lipid-forming regulator RUNX1T1 by regulating the m6A levels near the splicing site.38 Subsequent studies further confirmed the regulatory effect of FTO on mRNA splicing.39 ALKBH5, another demethylase, is also reportedly involved in the regulation of target mRNA splicing.25 The m6A “reader” protein hnRNP regulates the splicing of target mRNAs,40 and hnRNP G interacts synergistically with RNA polymerase II (RNAPII) to regulate alternative splicing within the transcriptome.41 In addition to mammals, m6A modification also affects the splicing of mRNA in Drosophila melanogaster and thus affects the expression of sex-determining core genes.12 Further research on the association between m6A and mRNA splicing is needed to reveal its potential role.
“Writers”, “readers”, and “erasers” reportedly regulate mRNA export. The main complex involved in mRNA export is TREX, and the m6A methylase complex can regulate mRNA export by recruiting TREX.42 METTL3 reportedly regulates the nuclear export of mature mRNA of clock gene Per2.43 ALKBH5 knockout results in accelerated mRNA nuclear export, which suggests that m6A plays an important role in regulating mRNA export.25 YTHDC1 interacts with SRSF3 to mediate the export of methylated mRNA from the nucleus to the cytoplasm.44
The most identified function of m6A on RNA metabolism is its effect on mRNA stability, as demonstrated by metabolic radioisotope labeling studies conducted in the 1970s.45 m6A modification is negatively correlated with mRNA stability and gene expression in mouse embryonic stem cells (mESCs).46 Many subsequent studies have confirmed the effect of m6A modification on mRNA stability.47, 48, 49 The demethylation enzymes FTO and ALKBH5 also play a role by regulating the stability of mRNA.50,51 m6A modifications on target mRNAs are recognized by different RNA-binding proteins and mediate different fates, and of these proteins, proteins belonging to the YTHDF family selectively recognize and accelerate target mRNA degradation.11,52, 53, 54 Unlike the YTHDF family, the IGF2BP family primarily promotes the stability and storage of target mRNAs.34 Therefore, scientists are also considering the possibility of using m6A as a marker for the half-life of mRNA.
A large number of studies have linked m6A to an increased translation rate. METTL3 enhances mRNA translation by recruiting the translation initiation factor eIF3, whereas deletion of METTL3 inhibits translation.55,56 Both FTO and ALKBH5 reportedly regulate downstream mRNA translation.57,58 YTHDF1 actively promotesprotein translation by recognizing m6A modifications on target mRNAs.14 Under heat shock conditions, YTHDF2 recognizes m6A modifications located at the 5′UTR of the transcript and facilitates translation.59 YTHDF3 regulates mRNA translation due to its interaction with translation-related proteins.60 The influence of m6A on mRNA metabolism may affect more than one aspect, and different RNA-binding proteins that simultaneously recognize an m6A modification might mediate different metabolic processes.
Dysfunction of m6A in tumors
Dysregulation of the m6A pathway plays an important role in the occurrence and development of diseases, and many studies have shown that m6A can promote the progression of tumors, such as breast cancer,61 lung cancer,62 glioblastoma,63,64 acute myeloid leukemia,65,66 and liver cancer.67 Many reviews have addressed these diseases in detail, and they will thus not be included in this paper. In this review, we focus on the role of upstream factors in tumor regulation through m6A.
During the period of tumor occurrence and development, many environmental factors, such as cigarette smoke, pollution and heavy metals, play an important or even decisive role. One study found that exposure to particulate matter (PM), sodium arsenite, bisphenol A (BPA), vinclozolin and other environmental toxicants leads to a significant decrease in the global m6A levels.68 In long-term diethylnitrosamine (DEN) exposure-induced hepatocellular carcinoma (HCC), FTO is decreased, which promotes HCC development by regulating energy homeostasis and glucose metabolism.69 Fusaric acid (FA), a food-borne mycotoxin, downregulates p53 by promoting hypermethylation and reducing the m6A level of the p53 promoter, which results in promotion of the occurrence and development of hepatocellular carcinoma.70 FA can also regulate the expression of m6A-related enzymes to affect the overall m6A level in hepatic cells.71 This finding suggests that FA may regulate the expression of p53 in the same way, but the specific mechanism still needs to be further explored. Fumonisin B is a common contaminant of cereal grains, and exposure to FB increases the overall levels of m6 A modification and oxidative stress in human hepatoma (HepG2) cells.72 FTO is inhibited by the ethyl ester form of meclofenamic acid (MA2), which enhances the function of the chemotherapy drug temozolomide in restraining the proliferation of glioma cells.73
In the tumor microenvironment, many factors play a role by regulating m6A modification. m6A is also changed as a result of endogenous stimuli such as heat shock, glucose starvation and oxidative stress.74 Glucose starvation triggers autophagy and activates the nuclear factor kappa B (NF-κB) pathway and thereby FTO. FTO has been instrumental in the development of melanoma and anti-PD-1 resistance therapies.75 The hypoxia-inducible factors HIF-1α and HIF-2α promote the mRNA and protein expression of NANOG, which encodes pluripotency factors, by enhancing ALKBH5 in breast cancer cells, and NANOG increases the percentage of breast cancer stem cells (BCSCs) and promotes their growth and metastases.76 Under the condition of the accumulation of reactive oxygen species (ROS) induced by hypoxia or chemotherapy, the expression of YTHDF1 is downregulated, which in turn promotes the expression of NF-E2 p45-related factor 2 (Nrf2) and its antioxidant genes downstream of aldo-keto reductase 1C1 (AKR1C1) and ultimately induces resistance to cisplatin in non-small cell lung cancer (NSCLC).77 Stress granules (SGs) are dynamic structures that include stalled translation mRNA and can affect the translation and stability of mRNA.78 In human osteosarcoma cells, oxidative stress promotes formation of the METTL3/METTL14/WTAP complex, which mediates m6A modification at the 5′ UTR of mRNA. This modification pattern allows the transport of mRNA from the translatable pool to SGs. Thereafter, the modified mRNA is identified by YTHDF3, and the translation process is blocked.79 Under hypoxic conditions, HIF-1α induces YTHDF1 expression, and subsequently, YTHDF1 promotes the translation of ATG2A and ATG14 (autophagy-related genes) in an m6A-dependent manner, which contributes to the progression of HCC.80 Free radicals, as specific regulatory factors, can directly or indirectly regulate the posttranslational modification of histones81; therefore, these factors may regulate the expression of m6A-related enzymes similarly in response to oxidative stress lesions in the body.
The above discussion notes that many factors, such as environmental factors and oxidative stress, can regulate tumor growth by regulating m6A. Although it is well known how they regulate m6A, the role that m6A plays in this process is less clear. More studies are needed to address this complex question and to uncover the complex functions of m6A in tumorigenesis and development.
Multilevel regulation of m6A in tumors
Most studies on m6A have focused on the regulation of its downstream transcripts, but the potential regulatory mechanism of m6A itself has not been fully revealed. To date, some studies have revealed these mechanisms from different aspects.
Transcription-level regulation of m6A molecules by transcription factors or histone modifications
The expression of m6A can be regulated by changes in transcription factors and alterations in the chromatin state. The initiation of eukaryotic transcription is very complex, and transcription factors play an important role in this process. A previous study found that ALKBH5 could be regulated as a direct transcriptional target of hypoxia-inducible factor-1 (HIF-1) (a core transcription factor that regulates oxygen homeostasis) under hypoxia-induced conditions.82 In a hypoxic microenvironment, HIF-1α and HIF-2α promotes the expression of NANOG by upregulating ALKBH5 in breast cancer cells, which leads to the promotion of tumor growth and metastasis.76 Subsequently, TFEB bind to the conserved element E-box of the ALKBH5 promoter to activate its transcription constitutionally. In contrast, TFEB inhibits METTL3 expression by decreasing the mRNA stability of METTL3.83 In epithelial ovarian cancer (EOC), ALKBH5 mediates the upregulation of HOXA10 by maintaining the stability of HOXA10 mRNA, and upregulated HOXA10 interacts with the TAAA region of the ALKBH5 promoter as a transcription factor. The positive feedback loop increases the proliferation and cisplatin resistance of EOC cells in vivo and in vitro.84 Zfp217 acts as a transcription factor and binds to the promoter of the FTO gene to activate the transcription of FTO and thus modulate m6A mRNA modification.85
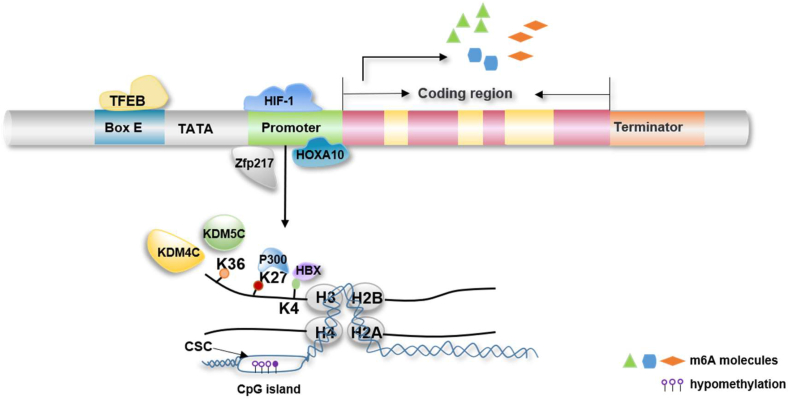
In addition, previous studies have shown that histone H3 trimethylation at lysine 36 (H3K36me3) can be a determinant of m6A and found that most m6A peaks overlap with H3K36me3 modifications. METTL14 writes m6A modifications on new transcripts by directly recognizing and binding H3K36me3.86 Another study showed that lysine-specific histone demethylase 5C (KDM5C) inhibits METTL14 transcription and promotes the metastasis of colorectal cancer (CRC) by demethylating H3K36me3 in the METTL14 promoter.87 The acetylation of histone H3K27 in the METTL3 promoter is mediated byp300 and promotes the transcription of METTL3. Subsequently, increased METTL3 expression promotes tumor angiogenesis and glycolysis in GC by enhancing the stability of HDGF mRNA in an IGF2BP3-dependent manner.88 KDM4C also reportedly regulates the expression of ALKBH5 by increasing the alteration in the chromatin state of ALKBH5. This process is achieved by reducing the H3K9me3 levels and promoting MYB and Pol II recruitment. ALKBH5 subsequently affects the mRNA stability of the receptor tyrosine kinase AXL inan m6A-dependent manner and maintains leukemia stem cell (LSC) function.89 A recent study demonstrated that HBX mediates H3K4me3 modification of the ALKBH5 gene promoter in HBV infection and subsequently promotes the expression of ALKBH5 in a WDR5-dependent manner, and the resulting increased ALKBH5 promotes high expression of HBX in an m6A-dependent manner; this positive feedback pathway ultimately promotes HBV-induced hepatocellular carcinoma.90 Cigarette smoke condensate (CSC) increases the expression of METTL3 by reducing the methylation of its promoter, and subsequently, METTL3 promotes the development and progression of pancreatic cancer by inducing the excessive maturation of miR-25-3p.91 Further studies have shown that CSC also induces ALKBH5 CpG island hypomethylation, which results in reductions in the LINC00278-SORF1 micropeptide levels, downregulation of LINC00278 mRNA, and thereby promotion of the progression of esophageal squamous cell carcinoma (ESCC)92 (Fig. 2).
Figure 2.
Transcription-level regulation of m6A molecules. The transcription level of m6A molecules is mainly regulated by two aspects: 1. Activation of transcription factors. The transcription factor TFEB can bind to the conserved E-box in the promoter to promote ALKBH5 expression. Hypoxia-inducible factor-1 (HIF-1) directly binds to the ALKBH5 promoter and promotes its transcription, HOXA10 promotes ALKBH5 transcription by binding to the ALKBH5 promoter, and Zfp217 binds to the FTO promoter to activate its transcription. 2. Histone modification. The histone demethylase KDM4C regulates ALKBH5 transcription by modifying histone H3K36me3 in the ALKBH5 promoter. HBX mediates the modification of K3K4me3 in the ALKBH5 promoter to promote ALKBH5 transcription, and KDM5C promotes its transcription through demethylated METTL14 histone H3K36me3 modification. P300 regulates METTL3 transcription by mediating the acetylation of METTL3 histone K27. Cigarette smoke condensate (CSC) causes hypomethylation of METTL3 and ALKBH5 CpG islands and regulates their transcription level.
The above discussion notes that the transcriptional activity and chromatin accessibility of m6A can affect its expression. In addition, m6A modification is inversely correlated with chromatin alterations. Specifically, m6A modification sites are located on chromosome-associated regulatory RNAs (carRNAs), and decreased m6A modification levels in carRNAs will increase their expression and promote an open chromatin state and downstream transcription.93 METTL3 also regulates m6A modifications on the histone methyltransferase EZH2, which in turn regulates the H3K27me3 levels.94 These findings link chromatin state dynamics to the regulation of m6A enzyme expression.
Posttranscriptional regulation of m6A molecules by ncRNAs
Previous reports have suggested that m6A regulates ncRNAs, including their splicing, transportation, degradation and expression.95 Interestingly, the level of m6A is also affected by ncRNAs. To date, most studies on the regulatory effect of ncRNAs on m6A have focused on microRNAs (miRNAs), which can regulate m6A expression through sequence pairing, promote the binding of METTL3 to miRNA target genes, and thus change the global m6A modification level.96 In NSCLC, miR-33a directly targets 3′ UTR of METTL3 mRNA and reduces its expression, which eventually leads to attenuation of the proliferation of NSCLC cells.97 In breast cancer, metformin reduces the expression of METTL3 by targeting miR-483-3p, which reduces the m6A levels and ultimately inhibits the proliferation of breast cancer cells. In this process, miR-483-3p also functions by binding to the 3′ UTR of METTL3.98 MiR-320d inhibits the expression of KIF3C by targeting the 3′ UTR of METTL3 and decreasing its expression, which inhibits the progression of prostate cancer.99 Other studies found that HBXIP upregulates the expression of METTL3 in breast cancer cells by inhibiting miRNA let-7g, which downregulates METTL3 expression by binding to its 3′ UTR. In turn, METTL3 promotes HBXIP expression in an m6A-dependent manner and eventually forms the positive feedback loop HBXIP/let-7g/METTL3/HBXIP, which accelerates the proliferation of breast cancer cells.100 Moreover, another research group found that HBXIP silencing in gastric cancer (GC) reduces METTL3 expression, inhibits GC cell proliferation, migration, and invasion, and promotes apoptosis.101 Additionally, in HCC cells, HBXIP promotes the expression of METTL3 at the mRNA and protein levels and then promotes the metabolic reprogramming of HCC cells.102 However, whether HBXIP also regulates METTL3 in a miRNA-dependent manner remains unclear. A study found that miR-4429 inhibits the progression of GC by targeting METTL3 and inhibiting its mRNA and protein expression.103 METTL3 is significantly elevated in hepatoblastoma (HB), suggesting a poor prognosis. Further investigation revealed that METTL3 may serve as a downstream target of miR-186, which inhibits tumor invasion by directly targeting METTL3.104 miR-600 also reportedly inhibits lung cancer progression by decreasing METTL3 expression.105
In addition to METTL3, METTL14 is directly regulated by miR-103-3P, which also functions by targeting its 3′ UTR.106 Similarly, miR-29a decreases the expression of WTAP and ERK by binding to their 3′ UTRs, inhibits the proliferation and metastasis of glioblastoma stem cells (GSCs), and promotes apoptosis.107 However, whether WTAP plays a role in an m6A-dependent manner still needs to be further studied. The one report showed that miR-145 reduces the expression of YTHDF2 by targeting its 3′ UTR, and YTHDF2 increases the total m6A level in HCC and inhibits the occurrence, proliferation, invasion and metastasis of HCC.108 Lysine-specific demethylase 5 (KDM5) is highly expressed in prostate cancer (PCa). This demethylase inhibits miR-495 transcription and expression by binding to its promoter. Subsequently, miR-495 inhibits MOB3B expression by targeting YTHDF2. This miR-495/YTHDF2/m6A-MOB3B axis augments PCa tumor growth.109 In thyroid cancer (TC), IGF2BP2 acts as a direct target of miR-204. MALAT1 competitively binds to IGF2BP2 with miR-204 to promote MYC expression, which promotes TC proliferation, migration, and invasion.110 The miR-96 antagomir promotes cancer progression in CRC by regulating m6A modification in the MYC transcript, and this result is achieved by the regulation of FTO in an AMPKα2-dependent manner.111
It is clear that the above mentioned ncRNAs, particularly miRNAs, appear to preferentially target the 3′ UTR of m6A enzymes to regulate their expression. However, whether this “preference” applies to other ncRNAs is unknown. In addition, as discussed above, miRNAs always appear to negatively regulate m6A modification, but the conclusion drawn is probably one-sided, and it is that additional positive regulators have not yet been detected. In addition, whether ncRNAs bind to m6A enzymes to regulate their expression by promoting their mRNA degradation, inhibiting their translation, or in other manners remains unclear. These questions are equally worth exploring.
In summary, the above-described results indicate that the proper biological function of m6A may require the appropriate regulation of transcription factors and histone modifications. Dysregulation of these processes may lead to changes in m6A and result in tumors or autoimmune diseases. Therefore, additional studies are needed to uncover the mechanisms of m6A-modified transcription, and it is believed that more scholars will reveal its regulatory mechanism in the future, which will improve our understanding and utilization of m6A modification (Fig. 3).
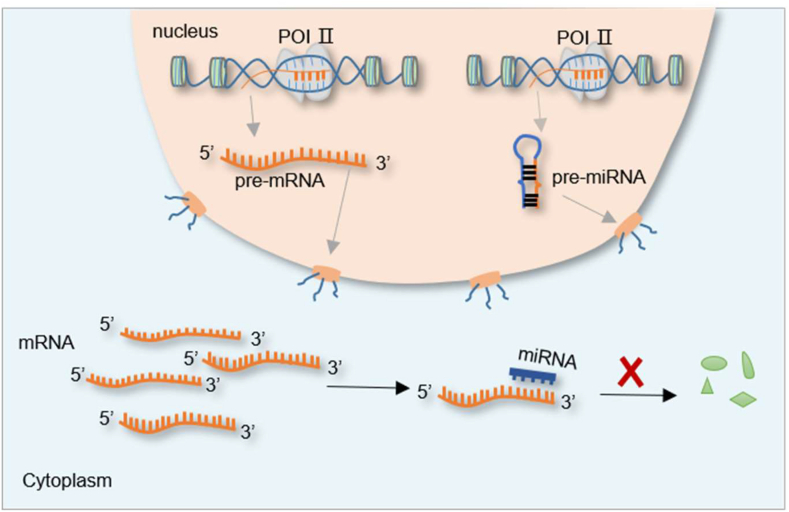
Figure 3.
m6A is regulated by ncRNAs. Mature miRNA binds to the 3′ untranslated region of m6A mRNA in the cytoplasm and inhibits its expression. Specifically, miR-33a can directly target the 3′ UTR of METTL3 mRNA and inhibit its expression. The miRNA let-7g downregulates its expression by targeting the 3′ UTR of METTL3, whereas mir-145 downregulates its expression by targeting the 3′ UTR of YTHDF2. In addition, the expression of METTL14, FTO, YTHDF2 and IGF2BP is also negatively regulated by miRNAs.
Posttranslational modifications of m6A molecules
In terms of posttranslational modifications (PTMs), SUMOylation modifications reportedly regulate m6A-modified molecules. SUMOylation is the process of attaching small ubiquitin-related modifier (SUMO) to protein substrates at specific lysine residues.112,113 This reversible PTM can change the stability, localization, protein–protein interactions and activity of the target protein.114, 115, 116, 117
Du Y et al found that METTL3 could be modified by the SUMO1-specific protease SENP1 on lysine residues K177, K211, K212 and K215. The methyltransferase activity of METTL3 is reduced after SUMOylation. However, the mechanism through which SUMOylation affects the activity of METTL3 remains unclear,118 and SUMOylation of METTL3 does not change its stability, localization, or interaction with METTL14 and WTAP. This type of modification was verified in the NSCLC cell line H1299.118 Mitogen stimulation upregulates UBC9 in liver cancer cells, which modifies METTL3 through the small ubiquitin modifier SUMO1; then, SUMOylated METTL3 regulates EMT progression by controlling Snail mRNA homeostasis.119 ROS can promote the SUMOylation of ALKBH5 by activating ERK/JNK signaling. SUMOylated ALKBH5 cannot bind to its substrate, which significantly increases the m6A level on the entire mRNA and eventually leads to the rapid and effective induction of genes involved in various biological processes, including DNA damage repair.120 An analysis of disease-free survival in patients with lung adenocarcinoma showed that high expression of YTHDF2 and SUMO1 predict poor prognosis. Subsequently, the researchers found that the SUMOylation site of YTHDF2 is on K571 in vivo and in vitro. The SUMOylation modification of YTHDF2 has little effect on its ubiquitination and localization but significantly increases its binding affinity with m6A-modified mRNA, leading to gene expression disorder and cancer progression.62 However, the mechanisms through which SUMOylation affects the activity of m6A molecules remain unclear. Methyl transferase domain (MTD, residues 369–590) and two Cys-Cys-Cys-His (CCCH)-type zinc finger motifs (ZNF, 259–340) are needed for the correct functions of METTL3 and METTL14.121 The researchers found that the SUMOylation modification sites are close to the ZNF motif of METTL3 but far from the MTD domain; therefore, they speculate that SUMOylation may spatially affect the binding of ZNF and MTD to substrate mRNA and ultimately inhibits m6A methyltransferase activity,118 but additional research is needed to confirm this hypothesis.
In addition to SUMOylation, the ubiquitination/autophagy-lysosome pathway of the m6A reader IGF2BP2 could be blocked by LINRIS(upregulated in CRC tissues from patients with poor overall survival (OS), which facilitates Myc-mediated glycolysis and promotes the progression of CR.122 Phosphorylation also reportedly regulates m6A modification. S43, S50 and S525 of METTL3 and S306 and S341 of WTAP could be phosphorylated by ERK, and USP5 subsequently stabilizes METTL3 by deubiquitination,123 which results in maintenance of the stability of the m6A methyltransferase complex to ensure the level of m6A on intracellular mRNA.123 EGFR/SRC/ERK signaling phosphorylates YTHDF2 at serine 39 and threonine 381, which stabilizes YTHDF2. YTHDF2 is needed for GBM cell proliferation, invasion, and tumorigenesis.124
PTMs are essential for protein activity and stability. However, few posttranscriptional modifications have been found on m6A enzymes; thus, future research on PTMs and m6A should first pay attention to whether other PTM modifications exist on m6A enzymes. Another difficulty is the relationship between these posttranslational modifications because whether they function through synergy or antagonism is unclear. This important problem also needs to be resolved in the future (Table 1).
Table 1.
Summary of m6A posttranslational modifications.
| Molecule | Modification | Modification site | Mechanism of modification | Functions of modification |
|---|---|---|---|---|
| METTL3 | SUMOylation | Lysine residues K177, K211, K212 and K215 | m6A methyl transferase activity is significantly inhibited | Regulation of EMT progression118,119 |
| METTLE | Phosphorylation | S43/S50/S525 | Control of m6A methyl transferase activity | Loss of METTTL3/WTAP phosphorylation leaves mouse embryonic stem cells in a pluripotent state123 |
| WTAP | Phosphorylation | S306/S341 | Maintenance of the stability of the m6A methyltransferase complex | Stabilization of the m6A mRNA level in mouse embryonic stem cells123 |
| ALKBH5 | SUMOylation | / | SUMOylated ALKBH5 cannot bind to its substrate | Rapid and efficient induction ofDNA damage repair inside genes120 |
| YTHDF2 | SUMOylation | K571 | Increase the binding affinity to m6A-modified mRNA | Promotion of gene expression disorders and cancer progression62 |
Regulation of the function of m6A molecules by ncRNAs
As detailed in the previous discussion, ncRNAs can regulate the expression of m6A molecules by binding to their 3′ UTR; in addition, ncRNAs can regulate the levels of m6A by recruiting m6A molecules. The lncRNA GAS5-AS1 is the antisense RNA of GAS5. A recent study found that GAS5-AS1 combines with ALKBH5 and that ALKBH5 regulates the m6A modification of GAS5 mRNA to enhance its stability in a YTHDF2-dependent manner. The increased GAS5 ultimately plays an antitumor role by inhibiting the proliferation, invasion, migration and metastasis of cervical cancer cells.125 A novel circRNA, HSA_circ_0008399 (circ 0008399), binds to WTAP and promotes formation of the WTAP/METTL3/METTL14 m6A methyltransferase complex. Subsequently, the mRNA stability of TNF alpha-induced protein 3 (TNFAIP3) is increased in an m6A-dependent manner, and its expression is increased to reduce the chemotherapy sensitivity of metastatic bladder cancer (BC) to cisplatin (CDDP).126 The lncRNA ARHGAP5-AS1 is highly expressed and related to autophagy impairment in gastric cancer. In the nucleus, ARHGAP5-AS1 recruits METTL3 to increase the m6A levels of the target mRNA ARHGAP5 and thus increase its stability. Upregulated ARHGAP5 promotes chemoresistance and suggests poor prognosis in gastric cancer.127 The lncRNA FOXM1 (FOXM1-AS) enhances FOXM1 expression by promoting the binding of ALKBH5 to the nascent transcripts of FOXM1 and reducing its m6A levels and then promotes glioblastoma stem-like cell (GSC) tumorigenesis.128 Similarly, the lncRNA GATA3-As recruits KIAA1429, a “reader” of m6A, to GATA3 pre-mRNA, which results in GATA3 pre-mRNA degradation and RNA-binding protein HuR separation. Promotion of the growth and metastasis of HCC indicates an adverse prognosis in liver cancer.129
A study conducted by Zhu et al revealed another interesting consequence: the lncRNA LINC00266-1 encodes a 71-amino-acid peptide named RNA-binding regulatory peptide (RBRP), which interacts with IGF2BP1 and promotes binding to target transcripts, such as c-Myc mRNA, by increasing the mRNA stability and expression of c-Myc to promote colorectal cancer (CRC) tumorigenesis.130 The m6A reader IGF2BP1 is recruited by the lncRNA KB-1980E6.3 to retain the stability of c-Myc mRNA. These factors form the lncRNA KB-1980E6.3/IGF2BP1/c-Myc signaling axis that maintains the stemness of BCSCs.131
These results show another mechanism through which ncRNAs regulate m6A. Specifically, ncRNAs regulate the expression of m6A modification molecules, and the functions of m6A enzymes can also be regulated through recruitment or interaction. However, whether this regulation is accidental or normal in physiological processes is unclear, and whether the same ncRNAs can not only regulate the production of m6A molecules but also participate in the regulation of their function remains to be determined. Do ncRNAs regulate m6A in other ways? Additional research might be needed to answer these questions.
Regulation of the interaction of m6A molecules with target mRNAs
METTL3 is related to the maintenance of leukemia stages, is located at transcriptional start sites (TSSs) with clear transcription factor and histone modification characteristics, and regulates the expression of mRNAs encoded by its target genes. However, METTL3 mapping to the transcription start site must depend on CEBPZ, a CCAAT box-binding factor that recruits METTL3 to chromatin sites.132 Recent studies showed that the target gene 5′ untranslated nuclear cap-binding subunit 3 (NCBP3) recruits METTL3 in response to hypoxia stress/stress and increases the mRNA m6A levels in cardiomyocytes. The NCBP3/METTL3/eIF4A2 regulatory axis plays an important role in the hypoxia stress of cardiomyocytes.133 In intestinal epithelial cells infected by E. coli K88, the transcription factor FOXO6 recruits METTL3 to form the METTL3/FOXO6 complex. FOXO6 is responsible for the transcriptional activation of GPR161, and METTL3 is responsible for the m6A modification of new GPR161 transcripts, promoting GPR161 transcription and subsequently regulating the expression of β-defensin.134 DDX46 is a member of the RNA helicase dead-box family. DDX46 binds to the CCGGUU conserved motif of mRNA encoding antiviral molecules. When viral infection occurs, DDX46 recruits the m6A demethylase ALKBH5 and increases its binding. The DDX46-binding antiviral molecule mRNA is demethylated to induce nuclear retention, which blocks the protein expression of these antiviral molecules, reduces the production of interferon, and ultimately inhibits the natural antiviral immune response.135 DDX5, another member of the DDX family, interacts with METTL3 after viral infection and regulates the methylation of antiviral mRNA molecules by affecting the formation of the METTL3-METTL14 heterodimer complex, which negatively regulates natural immunity and promotes viral infection.136 ALKBH5 is directly regulated by DDX3, which leads to decreased m6A methylation in FOXM1 and then ascent NANOG transcript and thus contributes to tumor chemoresistance.137
METTL3 interacts with viral RNA-dependent RNA polymerase 3D during EV71 C4 subtype infection, and this interaction induces SUMOylation and ubiquitination of the polymerase and ultimately promotes polymerase stability and viral replication.138,139 During hepatitis B virus (HBV) infection, the viral HBV X (HBx) protein interacts with METTL3 and METTL14, which stimulates their nuclear import and achieves cotranscriptional m6A modification of viral RNAs.140
These reports show another mechanism in which m6A enzymes work by binding to each other and suggest that m6A is not an independent modification pattern. It is more likely one part of a biological process. The mechanism through which m6A collaboratively functions with other molecules may be of interest (Fig. 4).
Figure 4.
Multilevel regulation of m6A. In the nucleus, transcription factors bind to the promoter of m6A molecules and activate their transcription. Histone modifications such as methylation or acetylation also affect the transcription level of m6A molecules. After transcription, ncRNAs, particularly miRNAs, regulate their expression by binding to mRNAs of m6A molecules in the cytoplasm. After translation, the polypeptide chain of m6A molecules is regulated by posttranslational modifications, such as phosphorylation, SUMOylation, and acetylation, and then folds into the correct functional structure. When it plays a regulatory role, the interaction between m6A molecules and target mRNA is regulated by ncRNAs and other molecules, which ultimately affects the m6A modification level on target mRNA, mediatesits splicing, nuclear output, stability, translation and other metabolic processes and affects tumor progression.
Future prospects
In this review, we summarize relevant reports on m6A regulation from the perspective of m6A molecule transcription and expression levels, posttranscriptional modification levels, and other mechanisms. At the transcriptional and expression levels, many ncRNAs and transcription factors reportedly regulate the expression of m6A molecules. Are other molecules involved in this regulation? How are the upstream signaling pathways associated with these molecules regulated? In addition, many studies have shown that a specific miRNA can target multiple genes, and a gene can also be targeted by multiple miRNAs. Is this complex phenomenon involved in the m6A modification process? How can we explain this phenomenon in the future? These questions may complicate the mechanisms involved in m6A modification, and this difficult problem will be faced in future research. Regarding modifications after translation, SUMOylation and phosphorylation reportedly regulate the activity and stability of m6A molecules. More than 300 different posttranscriptional modifications have been identified, and these include phosphorylation, acetylation, ubiquitination, carboxylation, ribosylation and disulfide bond formation. In addition to the several modification methods of m6A molecules found thus far, other posttranscriptional modification methods may be involved in the regulation of m6A molecules, and this possibility is worth further exploration. m6A molecules show regulatory functions by interacting with nucleic acids, such as noncoding RNAs, or ribonucleic acid-binding proteins, such as transcription factors, but the mechanism through which m6A binds to these substances and the regulation of this process remain unanswered questions that can be addressed in future research. In addition to specific regulatory mechanisms, internal and external factors in tumor development can participate in the regulation of m6A modifications and thus play a role in the regulation of tumor progression. It is worth noting that the specific molecular mechanisms of these regulatory processes have not been fully elucidated. Furthermore, whether m6A molecules are regulated by only a single factor and whether m6A molecules are feedback regulated by these influencing factors in tumorigenesis are worthy of future consideration and research efforts.
A growing body of evidence shows that m6A modification plays a dual role in cancer. On the one hand, m6A modification regulates the expression of oncogenes or tumor suppressor genes and thus affects tumor progression, as has been elucidated in many excellent reviews. On the other hand, the m6A levels and the expression and activity of m6A molecules can be regulated to affect the role of m6A in cancer, as summarized in this paper.
An increasing number of m6A inhibitors have been reported, and some inhibitors have been applied to tumor therapy and have achieved good results. However, many molecules are involved in the dynamic regulation of m6A modification, and many of these may change during tumor occurrence and development. Moreover, most “writers” and “erasers” need “readers” to play a role, which makes the targeting of future therapies a major problem. Which m6A molecules play a major role in tumor development? If multiple m6A molecules change, is it necessary to use multiple m6A inhibitors in combination? These problems make it difficult to target m6A molecules in tumor therapy. However, studying more upstream influencing factors of m6A, obtaining a better understanding of m6A and its role in tumors, and making improvements upstream may lead to the avoidance of side effects and treatment difficulties caused by the use of multiple inhibitors. In addition, the simultaneous regulation of m6A upstream factors and application of m6A inhibitors may achieve better results in the treatment of tumors.
Author contributions
LF drafted the manuscript. RD, BC, ML and JT discussed and revised the manuscript. SW designed the study and revised the manuscript. All the authors read and approved the final manuscript.
Conflict of interests
The authors declare that there are no conflicts of interests.
Funding
This work was supported by the Research Project of Jiangsu Commission of Health (China) (No. K2019019).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Desrosiers R., Friderici K., Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71(10):3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He C. Grand challenge commentary: RNA epigenetics? Nat Chem Biol. 2010;6(12):863–865. doi: 10.1038/nchembio.482. [DOI] [PubMed] [Google Scholar]
- 3.Saletore Y., Meyer K., Korlach J., Vilfan I.D., Jaffrey S., Mason C.E. The birth of the Epitranscriptome: deciphering the function of RNA modifications. Genome Biol. 2012;13(10):175. doi: 10.1186/gb-2012-13-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper T.A. Implications of widespread covalent modification of mRNA. Circ Res. 2012;111(12):1491–1493. doi: 10.1161/CIRCRESAHA.112.281071. [DOI] [PubMed] [Google Scholar]
- 5.Saneyoshi M., Harada F., Nishimura S. Isolation and characterization of N6-methyladenosine from Escherichia coli valine transfer RNA. Biochim Biophys Acta. 1969;190(2):264–273. doi: 10.1016/0005-2787(69)90078-1. [DOI] [PubMed] [Google Scholar]
- 6.Iwanami Y., Brown G.M. Methylated bases of ribosomal ribonucleic acid from HeLa cells. Arch Biochem Biophys. 1968;126(1):8–15. doi: 10.1016/0003-9861(68)90553-5. [DOI] [PubMed] [Google Scholar]
- 7.Csepany T., Lin A., Baldick C.J., Jr., Beemon K. Sequence specificity of mRNA N6-adenosine methyltransferase. J Biol Chem. 1990;265(33):20117–20122. [PubMed] [Google Scholar]
- 8.Narayan P., Rottman F.M. An in vitro system for accurate methylation of internal adenosine residues in messenger RNA. Science. 1988;242(4882):1159–1162. doi: 10.1126/science.3187541. [DOI] [PubMed] [Google Scholar]
- 9.Dominissini D., Moshitch-Moshkovitz S., Schwartz S., et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485(7397):201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 10.Meyer K.D., Saletore Y., Zumbo P., Elemento O., Mason C.E., Jaffrey S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149(7):1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X., Lu Z., Gomez A., et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505(7481):117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haussmann I.U., Bodi Z., Sanchez-Moran E., et al. M 6 A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature. 2016;540(7632):301–304. doi: 10.1038/nature20577. [DOI] [PubMed] [Google Scholar]
- 13.Roundtree I.A., Luo G.Z., Zhang Z., et al. YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. Elife. 2017;6 doi: 10.7554/eLife.31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X., Zhao B.S., Roundtree I.A., et al. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161(6):1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M., Zha X., Wang S. The role of N6-methyladenosine mRNA in the tumor microenvironment. Biochim Biophys Acta Rev Cancer. 2021;1875(2) doi: 10.1016/j.bbcan.2021.188522. [DOI] [PubMed] [Google Scholar]
- 16.Pinello N., Sun S., Wong J.J. Aberrant expression of enzymes regulating m 6 A mRNA methylation: implication in cancer. Cancer Biol Med. 2018;15(4):323–334. doi: 10.20892/j.issn.2095-3941.2018.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J., Yue Y., Han D., et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10(2):93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patil D.P., Chen C.K., Pickering B.F., et al. M(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537(7620):369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knuckles P., Lence T., Haussmann I.U., et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m 6 A machinery component Wtap/Fl(2)D. Genes Dev. 2018;32(5–6):415–429. doi: 10.1101/gad.309146.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yue Y., Liu J., Cui X., et al. VIRMA mediates preferential m 6 A mRNA methylation in 3'UTR and near stop Codon and associates with alternative polyadenylation. Cell Discovery. 2018;4:10. doi: 10.1038/s41421-018-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren W., Lu J., Huang M., et al. Structure and regulation of ZCCHC4 in m 6 A-methylation of 28S rRNA. Nat Commun. 2019;10(1):5042. doi: 10.1038/s41467-019-12923-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warda A.S., Kretschmer J., Hackert P., et al. Human METTL16 is a N6-methyladenosine (m 6 A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 2017;18(11):2004–2014. doi: 10.15252/embr.201744940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pendleton K.E., Chen B., Liu K., et al. The U6 snRNA m 6 A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell. 2017;169(5):824–835. doi: 10.1016/j.cell.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia G., Fu Y., Zhao X., et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7(12):885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng G., Dahl J.A., Niu Y., et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49(1):18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma C.J., Ding J.H., Ye T.T., Yuan B.F., Feng Y.Q. AlkB homologue 1 demethylates N3-methylcytidine in mRNA of mammals. ACS Chem Biol. 2019;14(7):1418–1425. doi: 10.1021/acschembio.8b01001. [DOI] [PubMed] [Google Scholar]
- 27.Ueda Y., Ooshio I., Fusamae Y., et al. AlkB homolog 3-mediated tRNA demethylation promotes protein synthesis in cancer cells. Sci Rep. 2017;7 doi: 10.1038/srep42271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L.S., Xiong Q.P., Peña Perez S., et al. ALKBH7-mediated demethylation regulates mitochondrial polycistronic RNA processing. Nat Cell Biol. 2021;23(7):684–691. doi: 10.1038/s41556-021-00709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berlivet S., Scutenaire J., Deragon J.M., Bousquet-Antonelli C. Readers of the m 6 A epitranscriptomic code. Biochim Biophys Acta Gene Regul Mech. 2019;1862(3):329–342. doi: 10.1016/j.bbagrm.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Shi H., Wang X., Lu Z., et al. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 2017;27(3):315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wojtas M.N., Pandey R.R., Mendel M., Homolka D., Sachidanandam R., Pillai R.S. Regulation of m 6 A transcripts by the 3'→5' RNA helicase YTHDC2 is essential for a successful meiotic program in the mammalian germline. Mol Cell. 2017;68(2):374–387. doi: 10.1016/j.molcel.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 32.Xiao W., Adhikari S., Dahal U., et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61(4):507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Müller S., Glaß M., Singh A.K., et al. IGF2BP1 promotes SRF-dependent transcription in cancer in a m6A- and miRNA-dependent manner. Nucleic Acids Res. 2019;47(1):375–390. doi: 10.1093/nar/gky1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang H., Weng H., Sun W., et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20(3):285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alarcón C.R., Goodarzi H., Lee H., Liu X., Tavazoie S., Tavazoie S.F. HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell. 2015;162(6):1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer K.D., Patil D.P., Zhou J., et al. 5' UTR m(6)A promotes cap-independent translation. Cell. 2015;163(4):999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoltzfus C.M., Dane R.W. Accumulation of spliced avian retrovirus mRNA is inhibited in S-adenosylmethionine-depleted chicken embryo fibroblasts. J Virol. 1982;42(3):918–931. doi: 10.1128/jvi.42.3.918-931.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao X., Yang Y., Sun B.F., et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24(12):1403–1419. doi: 10.1038/cr.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartosovic M., Molares H.C., Gregorova P., Hrossova D., Kudla G., Vanacova S. N6-methyladenosine demethylase FTO targets pre-mRNAs and regulates alternative splicing and 3'-end processing. Nucleic Acids Res. 2017;45(19):11356–11370. doi: 10.1093/nar/gkx778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.König J., Zarnack K., Rot G., et al. iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nat Struct Mol Biol. 2010;17(7):909–915. doi: 10.1038/nsmb.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou K.I., Shi H., Lyu R., et al. Regulation of co-transcriptional pre-mRNA splicing by m 6 A through the low-complexity protein hnRNPG. Mol Cell. 2019;76(1):70–81. doi: 10.1016/j.molcel.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lesbirel S., Viphakone N., Parker M., et al. The m 6 A-methylase complex recruits TREX and regulates mRNA export. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-32310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fustin J.M., Doi M., Yamaguchi Y., et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155(4):793–806. doi: 10.1016/j.cell.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 44.Roundtree I.A., Luo G.Z., Zhang Z., et al. YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. Elife. 2017;6 doi: 10.7554/eLife.31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sommer S., Lavi U., Darnell J.E., Jr. The absolute frequency of labeled N-6-methyladenosine in HeLa cell messenger RNA decreases with label time. J Mol Biol. 1978;124(3):487–499. doi: 10.1016/0022-2836(78)90183-3. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Li Y., Toth J.I., Petroski M.D., Zhang Z., Zhao J.C. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol. 2014;16(2):191–198. doi: 10.1038/ncb2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li D., Cai L., Meng R., Feng Z., Xu Q. METTL3 modulates osteoclast differentiation and function by controlling RNA stability and nuclear export. Int J Mol Sci. 2020;21(5):1660. doi: 10.3390/ijms21051660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang W., Zhao Y., Zhang H., Peng Y., Rui Z. METTL3 enhances NSD2 mRNA stability to reduce renal impairment and interstitial fibrosis in mice with diabetic nephropathy. BMC Nephrol. 2022;23(1):124. doi: 10.1186/s12882-022-02753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H., Wang C., Lan L., et al. METTL3 promotes oxaliplatin resistance of gastric cancer CD133+ stem cells by promoting PARP1 mRNA stability. Cell Mol Life Sci. 2022;79(3):135. doi: 10.1007/s00018-022-04129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lv D., Ding S., Zhong L., et al. M 6 A demethylase FTO-mediated downregulation of DACT1 mRNA stability promotes Wnt signaling to facilitate osteosarcoma progression. Oncogene. 2022;41(12):1727–1741. doi: 10.1038/s41388-022-02214-z. [DOI] [PubMed] [Google Scholar]
- 51.Feng L., Fan Y., Zhou J., Li S., Zhang X. The RNA demethylase ALKBH5 promotes osteoblast differentiation by modulating Runx2 mRNA stability. FEBS Lett. 2021;595(15):2007–2014. doi: 10.1002/1873-3468.14145. [DOI] [PubMed] [Google Scholar]
- 52.Li J., Chen K., Dong X., et al. YTHDF1 promotes mRNA degradation via YTHDF1-AGO2 interaction and phase separation. Cell Prolif. 2022;55(1) doi: 10.1111/cpr.13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang C.S., Zhu Y.Q., Xu Q.C., et al. YTHDF2 promotes intrahepatic cholangiocarcinoma progression and desensitises cisplatin treatment by increasing CDKN1B mRNA degradation. Clin Transl Med. 2022;12(6):e848. doi: 10.1002/ctm2.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin Y., Jin X., Nie Q., et al. YTHDF3 facilitates triple-negative breast cancer progression and metastasis by stabilizing ZEB1 mRNA in an m6A-dependent manner. Ann Transl Med. 2022;10(2):83. doi: 10.21037/atm-21-6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin S., Choe J., Du P., Triboulet R., Gregory R.I. The m(6)A methyltransferase METTL3 promotes translation in human cancer cells. Mol Cell. 2016;62(3):335–345. doi: 10.1016/j.molcel.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choe J., Lin S., Zhang W., et al. mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature. 2018;561(7724):556–560. doi: 10.1038/s41586-018-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu J., Chen M., Huang H., et al. Dynamic m6A modification regulates local translation of mRNA in axons. Nucleic Acids Res. 2018;46(3):1412–1423. doi: 10.1093/nar/gkx1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han Z., Wang X., Xu Z., et al. ALKBH5 regulates cardiomyocyte proliferation and heart regeneration by demethylating the mRNA of YTHDF1. Theranostics. 2021;11(6):3000–3016. doi: 10.7150/thno.47354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou J., Wan J., Gao X., Zhang X., Jaffrey S.R., Qian S.B. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature. 2015;526(7574):591–594. doi: 10.1038/nature15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li A., Chen Y.S., Ping X.L., et al. Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27(3):444–447. doi: 10.1038/cr.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu F., Yu X., He G. m6A-mediated tumor invasion and methylation modification in breast cancer microenvironment. JAMA Oncol. 2021;2021 doi: 10.1155/2021/9987376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hou G., Zhao X., Li L., et al. SUMOylation of YTHDF2 promotes mRNA degradation and cancer progression by increasing its binding affinity with m6A-modified mRNAs. Nucleic Acids Res. 2021;49(5):2859–2877. doi: 10.1093/nar/gkab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong F., Qin X., Wang B., et al. ALKBH5 facilitates hypoxia-induced paraspeckle assembly and IL8 secretion to generate an immunosuppressive tumor microenvironment. Cancer Res. 2021;81(23):5876–5888. doi: 10.1158/0008-5472.CAN-21-1456. [DOI] [PubMed] [Google Scholar]
- 64.Qiu Z., Zhao L., Shen J.Z., et al. Transcription elongation machinery is a druggable dependency and potentiates immunotherapy in glioblastoma stem cells. Cancer Discov. 2022;12(2):502–521. doi: 10.1158/2159-8290.CD-20-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sheng Y., Wei J., Yu F., et al. A critical role of nuclear m6A reader YTHDC1 in leukemogenesis by regulating MCM complex-mediated DNA replication. Blood. 2021;138(26):2838–2852. doi: 10.1182/blood.2021011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feng M., Xie X., Han G., et al. YBX1 is required for maintaining myeloid leukemia cell survival by regulating BCL2 stability in an m6A-dependent manner. Blood. 2021;138(1):71–85. doi: 10.1182/blood.2020009676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rong D., Wu F., Lu C., et al. m6A modification of circHPS5 and hepatocellular carcinoma progression through HMGA2 expression. Mol Ther Nucleic Acids. 2021;26:637–648. doi: 10.1016/j.omtn.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cayir A., Barrow T.M., Guo L., Byun H.M. Exposure to environmental toxicants reduces global N6-methyladenosine RNA methylation and alters expression of RNA methylation modulator genes. Environ Res. 2019;175:228–234. doi: 10.1016/j.envres.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 69.Mittenbühler M.J., Saedler K., Nolte H., et al. Hepatic FTO is dispensable for the regulation of metabolism but counteracts HCC development in vivo. Mol Metabol. 2020;42 doi: 10.1016/j.molmet.2020.101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghazi T., Nagiah S., Chuturgoon A.A. Fusaric acid decreases p53 expression by altering promoter methylation and m6A RNA methylation in human hepatocellular carcinoma (HepG2) cells. Epigenetics. 2021;16(1):79–91. doi: 10.1080/15592294.2020.1788324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghazi T., Nagiah S., Chuturgoon A.A. Fusaric acid induces hepatic global m6A RNA methylation and differential expression of m6A regulatory genes in vivo - a pilot study. Epigenetics. 2022;17(6):695–703. doi: 10.1080/15592294.2021.1975937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arumugam T., Ghazi T., Chuturgoon A.A. Fumonisin B1 alters global m6A RNA methylation and epigenetically regulates Keap1-Nrf2 signaling in human hepatoma (HepG2) cells. Arch Toxicol. 2021;95(4):1367–1378. doi: 10.1007/s00204-021-02986-5. [DOI] [PubMed] [Google Scholar]
- 73.Xiao L., Li X., Mu Z., et al. FTO inhibition enhances the antitumor effect of temozolomide by targeting MYC-miR-155/23a cluster-MXI1 feedback circuit in glioma. Cancer Res. 2020;80(18):3945–3958. doi: 10.1158/0008-5472.CAN-20-0132. [DOI] [PubMed] [Google Scholar]
- 74.Tardu M., Jones J.D., Kennedy R.T., Lin Q., Koutmou K.S. Identification and quantification of modified nucleosides in Saccharomyces cerevisiae mRNAs. ACS Chem Biol. 2019;14(7):1403–1409. doi: 10.1021/acschembio.9b00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang S., Wei J., Cui Y.H., et al. M 6 A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nat Commun. 2019;10(1):2782. doi: 10.1038/s41467-019-10669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang C., Samanta D., Lu H., et al. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m⁶A-demethylation of NANOG mRNA. Proc Natl Acad Sci USA. 2016;113(14):E2047–E2056. doi: 10.1073/pnas.1602883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi Y., Fan S., Wu M., et al. YTHDF1 links hypoxia adaptation and non-small cell lung cancer progression. Nat Commun. 2019;10(1):4892. doi: 10.1038/s41467-019-12801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buchan J.R., Parker R. Eukaryotic stress granules: the ins and outs of translation. Mol Cell. 2009;36(6):932–941. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anders M., Chelysheva I., Goebel I., et al. Dynamic m 6 A methylation facilitates mRNA triaging to stress granules. Life Sci Alliance. 2018;1(4) doi: 10.26508/lsa.201800113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Q., Ni Y., Zhang L., et al. HIF-1α-induced expression of m6A reader YTHDF1 drives hypoxia-induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation. Signal Transduct Targeted Ther. 2021;6:76. doi: 10.1038/s41392-020-00453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.García-Giménez J.L., Garcés C., Romá-Mateo C., Pallardó F.V. Oxidative stress-mediated alterations in histone post-translational modifications. Free Radic Biol Med. 2021;170:6–18. doi: 10.1016/j.freeradbiomed.2021.02.027. [DOI] [PubMed] [Google Scholar]
- 82.Thalhammer A., Bencokova Z., Poole R., et al. Human AlkB homologue 5 is a nuclear 2-oxoglutarate dependent oxygenase and a direct target of hypoxia-inducible factor 1α (HIF-1α) PLoS One. 2011;6(1) doi: 10.1371/journal.pone.0016210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song H., Feng X., Zhang H., et al. METTL3 and ALKBH5 oppositely regulate m 6 A modification of TFEB mRNA, which dictates the fate of hypoxia/reoxygenation-treated cardiomyocytes. Autophagy. 2019;15(8):1419–1437. doi: 10.1080/15548627.2019.1586246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nie S., Zhang L., Liu J., et al. ALKBH5-HOXA10 loop-mediated JAK2 m6A demethylation and cisplatin resistance in epithelial ovarian cancer. J Exp Clin Cancer Res. 2021;40(1):284. doi: 10.1186/s13046-021-02088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song T., Yang Y., Wei H., et al. Zfp217 mediates m6A mRNA methylation to orchestrate transcriptional and post-transcriptional regulation to promote adipogenic differentiation. Nucleic Acids Res. 2019;47(12):6130–6144. doi: 10.1093/nar/gkz312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang H., Weng H., Zhou K., et al. Histone H3 trimethylation at lysine 36 guides m 6 A RNA modification co-transcriptionally. Nature. 2019;567(7748):414–419. doi: 10.1038/s41586-019-1016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen X., Xu M., Xu X., et al. METTL14-mediated N6-methyladenosine modification of SOX4 mRNA inhibits tumor metastasis in colorectal cancer. Mol Cancer. 2020;19(1):106. doi: 10.1186/s12943-020-01220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Q., Chen C., Ding Q., et al. METTL3-mediated m 6 A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut. 2020;69(7):1193–1205. doi: 10.1136/gutjnl-2019-319639. [DOI] [PubMed] [Google Scholar]
- 89.Wang J., Li Y., Wang P., et al. Leukemogenic chromatin alterations promote AML leukemia stem cells via a KDM4C-ALKBH5-AXL signaling axis. Cell Stem Cell. 2020;27(1):81–97. doi: 10.1016/j.stem.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 90.Qu S., Jin L., Huang H., Lin J., Gao W., Zeng Z. A positive-feedback loop between HBx and ALKBH5 promotes hepatocellular carcinogenesis. BMC Cancer. 2021;21(1):686. doi: 10.1186/s12885-021-08449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang J., Bai R., Li M., et al. Excessive miR-25-3p maturation via N6-methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression. Nat Commun. 2019;10(1):1858. doi: 10.1038/s41467-019-09712-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu S., Zhang L., Deng J., et al. A novel micropeptide encoded by Y-linked LINC00278 links cigarette smoking and AR signaling in male esophageal squamous cell carcinoma. Cancer Res. 2020;80(13):2790–2803. doi: 10.1158/0008-5472.CAN-19-3440. [DOI] [PubMed] [Google Scholar]
- 93.Liu J., Dou X., Chen C., et al. N 6-methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science. 2020;367(6477):580–586. doi: 10.1126/science.aay6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen J., Zhang Y.C., Huang C., et al. M 6 A regulates neurogenesis and neuronal development by modulating histone methyltransferase Ezh2. Dev Reprod Biol. 2019;17(2):154–168. doi: 10.1016/j.gpb.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martin S.E., Gan H., Toomer G., Sridhar N., Sztuba-Solinska J. The m 6 A landscape of polyadenylated nuclear (PAN) RNA and its related methylome in the context of KSHV replication. RNA. 2021;27(9):1102–1125. doi: 10.1261/rna.078777.121. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Chen T., Hao Y.J., Zhang Y., et al. M(6)A RNA methylation is regulated by microRNAs and promotes reprogramming to pluripotency. Cell Stem Cell. 2015;16(3):289–301. doi: 10.1016/j.stem.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 97.Du M., Zhang Y., Mao Y., et al. miR-33a suppresses proliferation of NSCLC cells via targeting METTL3 mRNA. Biochem Biophys Res Commun. 2017;482(4):582–589. doi: 10.1016/j.bbrc.2016.11.077. [DOI] [PubMed] [Google Scholar]
- 98.Cheng L., Zhang X., Huang Y.Z., et al. Metformin exhibits antiproliferation activity in breast cancer via miR-483-3p/METTL3/m 6 A/p21 pathway. Oncogenesis. 2021;10(1):7. doi: 10.1038/s41389-020-00290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ma H., Zhang F., Zhong Q., Hou J. METTL3-mediated m6A modification of KIF3C-mRNA promotes prostate cancer progression and is negatively regulated by miR-320d. Aging. 2021;13(18):22332–22344. doi: 10.18632/aging.203541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cai X., Wang X., Cao C., et al. HBXIP-elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let-7g. Cancer Lett. 2018;415:11–19. doi: 10.1016/j.canlet.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 101.Yang Z., Jiang X., Li D., Jiang X. HBXIP promotes gastric cancer via METTL3-mediated MYC mRNA m6A modification. Aging. 2020;12(24):24967–24982. doi: 10.18632/aging.103767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang N., Wang T., Li Q., et al. HBXIP drives metabolic reprogramming in hepatocellular carcinoma cells via METTL3-mediated m6A modification of HIF-1α. J Cell Physiol. 2021;236(5):3863–3880. doi: 10.1002/jcp.30128. [DOI] [PubMed] [Google Scholar]
- 103.He H., Wu W., Sun Z., Chai L. miR-4429 prevented gastric cancer progression through targeting METTL3 to inhibit m 6 A-caused stabilization of SEC62. Biochem Biophys Res Commun. 2019;517(4):581–587. doi: 10.1016/j.bbrc.2019.07.058. [DOI] [PubMed] [Google Scholar]
- 104.Cui X., Wang Z., Li J., et al. Cross talk between RNA N6-methyladenosine methyltransferase-like 3 and miR-186 regulates hepatoblastoma progression through Wnt/β-catenin signalling pathway. Cell Prolif. 2020;53(3) doi: 10.1111/cpr.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wei W., Huo B., Shi X. miR-600 inhibits lung cancer via downregulating the expression of METTL3. Cancer Manag Res. 2019;11:1177–1187. doi: 10.2147/CMAR.S181058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun Z., Wang H., Wang Y., et al. miR-103-3p targets the m 6 A methyltransferase METTL14 to inhibit osteoblastic bone formation. Aging Cell. 2021;20(2) doi: 10.1111/acel.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xi Z., Wang P., Xue Y., et al. Overexpression of miR-29a reduces the oncogenic properties of glioblastoma stem cells by downregulating Quaking gene isoform 6. Oncotarget. 2017;8(15):24949–24963. doi: 10.18632/oncotarget.15327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang Z., Li J., Feng G., et al. microRNA-145 modulates N6-methyladenosine levels by targeting the 3'-untranslated mRNA region of the N6-methyladenosine binding YTH domain family 2 protein. J Biol Chem. 2017;292(9):3614–3623. doi: 10.1074/jbc.M116.749689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Du C., Lv C., Feng Y., Yu S. Activation of the KDM5A/miRNA-495/YTHDF2/m6A-MOB3B axis facilitates prostate cancer progression. J Exp Clin Cancer Res. 2020;39(1):223. doi: 10.1186/s13046-020-01735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ye M., Dong S., Hou H., Zhang T., Shen M. Oncogenic role of long noncoding RNAMALAT1 in thyroid cancer progression through regulation of the miR-204/IGF2BP2/m6A-MYC signaling. Mol Ther Nucleic Acids. 2020;23:1–12. doi: 10.1016/j.omtn.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yue C., Chen J., Li Z., Li L., Chen J., Guo Y. microRNA-96 promotes occurrence and progression of colorectal cancer via regulation of the AMPKα2-FTO-m6A/MYC axis. J Exp Clin Cancer Res. 2020;39(1):240. doi: 10.1186/s13046-020-01731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hay R.T. SUMO: a history of modification. Mol Cell. 2005;18(1):1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 113.Gill G. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 2004;18(17):2046–2059. doi: 10.1101/gad.1214604. [DOI] [PubMed] [Google Scholar]
- 114.Geiss-Friedlander R., Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8(12):947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 115.Chen C., Zhu C., Huang J., et al. SUMOylation of TARBP2 regulates miRNA/siRNA efficiency. Nat Commun. 2015;6:8899. doi: 10.1038/ncomms9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu C., Chen C., Huang J., et al. SUMOylation at K707 of DGCR8 controls direct function of primary microRNA. Nucleic Acids Res. 2015;43(16):7945–7960. doi: 10.1093/nar/gkv741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhu C., Chen C., Chen R., et al. K259-SUMOylation of DGCR8 promoted by p14ARF exerts a tumor-suppressive function. J Mol Cell Biol. 2016;8(5):456–458. doi: 10.1093/jmcb/mjw030. [DOI] [PubMed] [Google Scholar]
- 118.Du Y., Hou G., Zhang H., et al. SUMOylation of the m6A-RNA methyltransferase METTL3 modulates its function. Nucleic Acids Res. 2018;46(10):5195–5208. doi: 10.1093/nar/gky156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu H., Wang H., Zhao W., et al. SUMO1 modification of methyltransferase-like 3 promotes tumor progression via regulating Snail mRNA homeostasis in hepatocellular carcinoma. Theranostics. 2020;10(13):5671–5686. doi: 10.7150/thno.42539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu F., Wei J., Cui X., et al. Post-translational modification of RNA m6A demethylase ALKBH5 regulates ROS-induced DNA damage response. Nucleic Acids Res. 2021;49(10):5779–5797. doi: 10.1093/nar/gkab415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang P., Doxtader K.A., Nam Y. Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol Cell. 2016;63(2):306–317. doi: 10.1016/j.molcel.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Y., Lu J.H., Wu Q.N., et al. LncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer. Mol Cancer. 2019;18(1):174. doi: 10.1186/s12943-019-1105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sun H.L., Zhu A.C., Gao Y., et al. Stabilization of ERK-phosphorylated METTL3 by USP5 increases m 6 A methylation. Mol Cell. 2020;80(4):633–647. doi: 10.1016/j.molcel.2020.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fang R., Chen X., Zhang S., et al. EGFR/SRC/ERK-stabilized YTHDF2 promotes cholesterol dysregulation and invasive growth of glioblastoma. Nat Commun. 2021;12(1):177. doi: 10.1038/s41467-020-20379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang X., Zhang J., Wang Y. Long noncoding RNA GAS5-AS1 suppresses growth and metastasis of cervical cancer by increasing GAS5 stability. Am J Transl Res. 2019;11(8):4909–4921. [PMC free article] [PubMed] [Google Scholar]
- 126.Wei W., Sun J., Zhang H., et al. Circ0008399 interaction with WTAP promotes assembly and activity of the m 6 A methyltransferase complex and promotes cisplatin resistance in bladder cancer. Cancer Res. 2021;81(24):6142–6156. doi: 10.1158/0008-5472.CAN-21-1518. [DOI] [PubMed] [Google Scholar]
- 127.Zhu L., Zhu Y., Han S., et al. Impaired autophagic degradation of lncRNA ARHGAP5-AS1 promotes chemoresistance in gastric cancer. Cell Death Dis. 2019;10(6):383. doi: 10.1038/s41419-019-1585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang S., Zhao B.S., Zhou A., et al. M 6 A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31(4):591–606. doi: 10.1016/j.ccell.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lan T., Li H., Zhang D., et al. KIAA1429 contributes to liver cancer progression through N6-methyladenosine-dependent post-transcriptional modification of GATA3. Mol Cancer. 2019;18(1):186. doi: 10.1186/s12943-019-1106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhu S., Wang J.Z., Chen D., et al. An oncopeptide regulates m 6 A recognition by the m 6 A reader IGF2BP1 and tumorigenesis. Nat Commun. 2020;11(1):1685. doi: 10.1038/s41467-020-15403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhu P., He F., Hou Y., et al. A novel hypoxic long noncoding RNA KB-1980E6.3 maintains breast cancer stem cell stemness via interacting with IGF2BP1 to facilitate c-Myc mRNA stability. Oncogene. 2021;40(9):1609–1627. doi: 10.1038/s41388-020-01638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barbieri I., Tzelepis K., Pandolfini L., et al. Promoter-bound METTL3 maintains myeloid leukaemia by m 6 A-dependent translation control. Nature. 2017;552(7683):126–131. doi: 10.1038/nature24678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ye F., Wang X., Tu S., et al. The effects of NCBP3 on METTL3-mediated m6A RNA methylation to enhance translation process in hypoxic cardiomyocytes. J Cell Mol Med. 2021;25(18):8920–8928. doi: 10.1111/jcmm.16852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zong X., Wang H., Xiao X., et al. Enterotoxigenic Escherichia coli infection promotes enteric defensin expression via FOXO6-METTL3-m 6 A-GPR161 signalling axis. RNA Biol. 2021;18(4):576–586. doi: 10.1080/15476286.2020.1820193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zheng Q., Hou J., Zhou Y., Li Z., Cao X. The RNA helicase DDX46 inhibits innate immunity by entrapping m 6 A-demethylated antiviral transcripts in the nucleus. Nat Immunol. 2017;18(10):1094–1103. doi: 10.1038/ni.3830. [DOI] [PubMed] [Google Scholar]
- 136.Xu J., Cai Y., Ma Z., et al. The RNA helicase DDX5 promotes viral infection via regulating N6-methyladenosine levels on the DHX58 and NFκB transcripts to dampen antiviral innate immunity. PLoS Pathog. 2021;17(4) doi: 10.1371/journal.ppat.1009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shriwas O., Priyadarshini M., Samal S.K., et al. DDX3 modulates cisplatin resistance in OSCC through ALKBH5-mediated m 6 A-demethylation of FOXM1 and NANOG. Apoptosis. 2020;25(3–4):233–246. doi: 10.1007/s10495-020-01591-8. [DOI] [PubMed] [Google Scholar]
- 138.Hao H., Hao S., Chen H., et al. N6-methyladenosine modification and METTL3 modulate Enterovirus 71 replication. Nucleic Acids Res. 2019;47(1):362–374. doi: 10.1093/nar/gky1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu Y., Zheng Z., Shu B., et al. SUMO modification stabilizes Enterovirus 71 polymerase 3D to facilitate viral replication. J Virol. 2016;90(23):10472–10485. doi: 10.1128/JVI.01756-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim G.W., Siddiqui A. Hepatitis B virus X protein recruits methyltransferases to affect cotranscriptional N6-methyladenosine modification of viral/host RNAs. Proc Natl Acad Sci U S A. 2021;118(3) doi: 10.1073/pnas.2019455118. [DOI] [PMC free article] [PubMed] [Google Scholar]