Abstract
Hydrogen sulfide (H2S) is one of the three known gas signal transducers, and since its potential physiological role was reported, the literature on H2S has been increasing. H2S is involved in processes such as vasodilation, neurotransmission, angiogenesis, inflammation, and the prevention of ischemia-reperfusion injury, and its mechanism remains to be further studied. At present, the role of post-translational processing of proteins has been considered as a possible mechanism for the involvement of H2S in a variety of physiological processes. Current studies have shown that H2S is involved in S-sulfhydration, phosphorylation, and S-nitrosylation of proteins, etc. This paper focuses on the effects of protein modification involving H2S on physiological and pathological processes, looking forward to providing guidance for subsequent research.
Keywords: Hydrogen sulfide, Modification, Phosphorylation, S-nitrosylation, S-sulfhydration
Introduction
Hydrogen sulfide (H2S) is the third gas signaling molecule synthesized in mammalian cells after carbon monoxide and nitric oxide. In the past 30 years, the physiological and pathological processes of H2S in the human body have been studied extensively.1 H2S has long been considered a toxic gas with a rotten egg odor, but it has now been shown to be an important gas signaling molecule in both prokaryotes and eukaryotes.2,3 H2S is widely present in mammals and acts as a signaling molecule in the central and peripheral nervous system, cardiovascular, immune, endocrine, reproductive, and digestive systems,4, 5, 6, 7, 8 mediating diastolic and vasculogenesis, cell death, inflammation, and anticancer or carcinogenic effects.9, 10, 11, 12, 13
Post-translational modification (PTM) refers to the chemical modification of proteins after translation. For most proteins, this is a later step in protein biosynthesis. Precursor proteins are inactive and often undergo a series of post-translational processes before they become functional mature proteins. The types of processing are varied and generally fall into the following categories: phosphorylation, methylation, acetylation, sumoylation, and ubiquitylation.14, 15, 16, 17, 18 When proteins are made, 20 different amino acids are combined to make them. After protein translation, other biochemical functional groups of the protein (e.g., acetate, phosphate, different lipids, and carbohydrates) can attach to the protein to alter its chemistry, or cause structural changes (e.g., the establishment of disulfide bonds) to broaden its function.17
This paper aims to explore the PTM process in which H2S participates and its role in organisms, hoping to inspire researchers.
Anabolism of H2S in the body
Enzymes that synthesize H2S
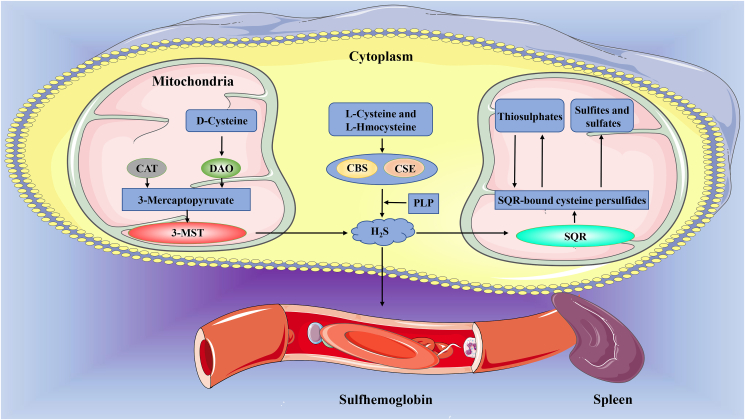
H2S synthesis in vivo is affected by a variety of enzymes, three of which are best understood: cystathionine β-synthase (CBS), cystathionine gamma-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST).19 Naturally, CBS and CSE are mainly located in the cytoplasm,20 while 3-MST is mainly present in mitochondria and cytoplasm.21,22 However, under the condition of hypoxia, the mitochondrial-targeted sequence at the c-terminal of CBS can be recognized by Hsp70 and transported to the mitochondria, while CSE enters the mitochondrial cavity mediated by Translocase of the Outer Membrane 2020,23. The optimal pH of CBS and CSE was 8.5–9.0, and that of 3-MST was 7.4,24, 25, 26 but the pH of cytosol was 7–7.4, and the pH of the mitochondrial cavity was 8.0, which was weakly alkaline.27 Under hypoxic conditions, CBS and CSE are transported to the mitochondrial cavity, which is favorable for H2S production under this pH condition, this may be an autoregulation mechanism of cells under hypoxia. The substrates catalyzed by these three enzymes are l-cysteine, l-homocysteine, and 3-mercaptopyruvate, and then little amounts of l-cystine and d-cysteine. Among them, l-cysteine and l-homocysteine are the main substrates of CBS and CSE, its catalytic reaction requires the participation of cofactor pyridoxal-5-phosphate,28 3-mercaptopyruvate is the important substrate of cysteine aminotransferase: 3-MST Axis.29,30 Meanwhile, d-amino acid oxidase in mitochondria and peroxisome catalyzed H2S formation from d-cysteine with the assistance of 3-MST,31,32 while selenium-binding protein 1, recently discovered in adipocytes, catalyzes the production of H2S, formaldehyde, and H2O2 from methanethiol.33 The specific reactions catalyzed by these enzymes have been discussed in several review.34,35 But these enzymes are expressed differently in different tissues (cells) and produce H2S with different efficiency, how do these enzymes work together to regulate the production/distribution of H2S is unclear.
H2S metabolism
H2S is discharged from the body mainly by excretion, and respiration accounts for only a very small part of the excretion.36 Before excretion, H2S needs to undergo enzyme-induced catabolism and non-enzyme-induced catabolism, and enzyme-induced catabolism is the main pathway.35 In the mitochondria, H2S is catabolized by the sulfide quinone oxidoreductase system (SQR) to form SQR-bound cysteine persulfides, which are then broken down into thiosulphates (a reversible process) and also into sulfites and sulfates, which are excreted in urine.37,38 H2S can also be decomposed into sulfites by ethylmalonic encephalopathy 1 in the mitochondrial matrix.39 In the non-enzymatic way, the spleen excretes the sulfhemoglobin formed by the combination of H2S in red blood cells with methemoglobin.40
The rate of H2S production in living organisms is very fast, such as in the liver, kidney, and brain of mice, but high concentrations of H2S are harmful to mammals, and the level of H2S in tissues must be strictly controlled, suggesting that the production and metabolism of H2S is a tightly regulated and relatively balanced process41, 42, 43 (Fig. 1).
Figure 1.
The main way of synthesis and metabolism of H2S. Endogenous H2S is mainly produced by three enzyme-catalyzed substrates of CBS, CSE, and 3-MST, and its path mainly includes enzymatic and non-enzymatic degradation. CAT: cysteine aminotransferase; CBS: cystathionine β-synthase; CSE: cystathionine gamma-lyase; DAO: d-amino acid oxidase; 3-MST: 3-mercaptopyruvate sulfurtransferase; PLP: pyridoxal-5-phosphate; SQR: sulfide quinone oxidoreductase system.
H2S and S-sulfhydration
S-sulfhydration, also known as S-sulfuration or S-persulfidation, is the recently discovered H2S or polysulfide-induced PTM, which forms persulfides by chemically modifying specific cysteine residues of the target protein.44 Polysulfides can transfer sulfur atoms to cysteine residues with low pKa,45 convert cysteine –SH groups to –SSH, leading to protein S-sulfhydration and cell signal transduction.46,47 There is growing evidence showing that this PTM contributes to the signal transduction mediated by H2S in mammals and participates in the physiological and pathological processes of the cardiovascular system, urinary system, nervous system, and reproductive system (Table 1).
Table 1.
Sites and functions of protein S-sulfhydration in different systems.
| Location | Protein | Site(s) | Functions | Refs. |
|---|---|---|---|---|
| Cardiovascular system | PPARγ | Cys139 | Adipogenesis | 53 |
| PTP1B | Cys215 | Restore ER stress homeostasis | 56 | |
| MuRF1 | Cys44 | Protect cardiac structural protein | 59 | |
| Keap-1 | Cys151 | Anti-oxidative stress | 60,61 | |
| PDI | Cys53/Cys57 and Cys397/Cys400 | Reduce ER stress | 65 | |
| HuR | Cys13 | Preserve endothelial cell function and delay atherogenesis | 66 | |
| TRPV4 | Unknown | Vasodilatation | 71 | |
| TRPV1 | Unknown | Vasodilatation | 72 | |
| CaMKII | Cys6 | Improve mitochondrial function | 75 | |
| MEK1 | Cys341 | DNA damage repair | 76 | |
| SP-1 | Cys68 and Cys755 | Maintain the stability of metabolites and protect endothelial cells | 78 | |
| Cys664 | Inhibit cardiac hypertrophy | 79 | ||
| IGF1R | Unknown | Inhibit the proliferation of vascular smooth muscle cells | 80 | |
| Kir6.1 | Cys43 | Vasodilatation | 81 | |
| Nervous system | Parkin | Unknown | Neuroprotective effect | 87,88 |
| CTSS | Cys25 | Inhibit ATP-induced neuroinflammation and Aβ1–42 synthesis | 97 | |
| GSK3β | Cys218 | Neuroprotective effect | 98 | |
| GAPDH | Cys150 | Cognitive dysfunction | 106 | |
| SR | Unknown | Improve synaptic function | 113 | |
| PKA, PKC, CAMKII | Unknown | Mediate rapid excitatory synaptic transmission | 117 | |
| Kidney | Keap-1 | Cys151 | Promote cells from oxidative stress | 118,119 |
| GAPDH | Cys150 | Increase ATP production | 121 | |
| Cys156 or Cys152 | Decrease ATP production | 115,120 | ||
| EGFR | C797/C798 | Urination and excrete sodium, and lower blood pressure | 123 | |
| Sirt1 | Unknown | Regulation epigenetic function | 125 | |
| Liver | PGC-1α, Glucose-6-phosphatase, Fructose-1, 6-bisphosphatase | Unknown | Enhance the liver glucose produce | 129 |
| Testis | PDE | Unknown | Restore testosterone synthesis | 130 |
| Myoblasts | metallothionein-1 | Unknown | Protects myoblasts from oxidative stress induced by cadmium | 131 |
| Melanoma cells | Human serum albumin | Cys34 | Exert anti-oxidative stress, inhibit tyrosinase and melanin accumulation | 132 |
CaMKII: Ca2+/calmodulin-dependent protein kinase II; CTSS: Cathepsin S; EGFR: endothelial growth factor receptor; ER: endoplasmic reticulum; GSK3β: glycogen synthase kinase 3β; HuR: human antigen R; IGF1R: Insulin-like growth factor-1 receptor; Keap-1: Kelch-like ECH-associated protein1; Kir6.1: An ATP-sensitive potassium channel; MEK1: mitogen-activated extracellular signal-regulated kinase 1; MuRF1: muscle RING finger-1; Parkin: An E3 ubiquitin ligase; PDE: phosphodiesterase; PDI: protein disulphide isomerase; PGC-1α: peroxisome proliferator-activated receptor-γ coactivator-1α; PPARγ: peroxisome proliferator-activated receptor γ, PTP1B: protein tyrosine phosphatase 1B; Sirt1: silent mating-type information regulator 2 homolog 1; SP1: specific protein-1, SR: serine racemase; TRPV4 and TRPV1: transient receptor potential family member.
S-sulfhydration in the cardiovascular system
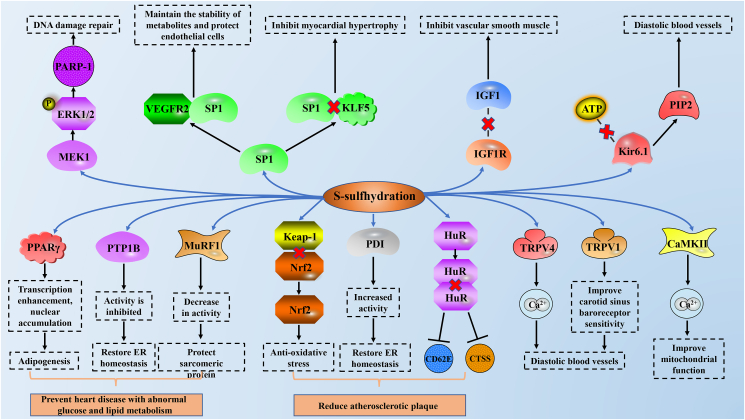
The presence of endogenous H2S has been detected in the arteries and hearts of many animals, including humans,48,49 and has been proven to be widely involved in physiological and pathological processes, such as vasodilation, arterial contraction, cardioprotection, and plaque formation.50, 51, 52 Peroxisome proliferator-activated receptor γ (PPARγ) is the leading factor of blood glucose and lipid metabolism. H2S can directly S-sulfhydrate PPARγ at the Cys139 site, increasing nuclear accumulation of PPARγ. It enhanced the DNA binding activity of PPARγ response element promoter and promoted the expression of adipogenic gene, and the mutation of PPARγ at Cys139 site blocked the S-sulfhydration of H2S.53 Protein tyrosine phosphatase 1B (PTP1B) plays an important role in endoplasmic reticulum (ER) stress and is thought to play an important role in obesity-induced cardiomyopathy and septic shock-induced cardiovascular dysfunction.54,55 H2S induces inhibition of PTP1B activity by S-sulfhydration at Cys215, promoting phosphorylation and activation of protease-like ER kinases, and restoring ER homeostasis, all of which are absent in CSE knockout HeLa cells.56 Muscle RING finger-1 (MuRF1) is a ubiquitin-linked enzyme, which was first found to play a role in skeletal muscle atrophy and cardiomyopathy.57 It is specifically expressed in the M-line and cytoplasm of cardiomyocytes and can degrade sarcomere including troponin I, troponin T, and titin.58 Knockdown MuRF1, mutation of MuRF1 at Cys44 in neonatal rat cardiomyocytes, or S-sulfhydration of MuRF1 by NaHS at Cys44 can reduce the protection of diabetic patients from MuRF1 induced degradation of cardiac structural proteins.59 These results suggest that S-sulfhydration may play an important role in cardiovascular disease induced by abnormal glucose and lipid metabolism.
It has been proposed that H2S -induced protein S-sulfhydration may be a new therapeutic target for preventing diabetes from accelerating atherosclerosis.60 Kelch-like ECH-associated protein1 (Keap-1) acts as a negative receptor for nuclear factor E2-related factor 2 (Nrf2) to protect cells from oxidative stress. In human gastric epithelial cells, NaHS S-sulfhydrates Keap-1, promotes Keap-1/Nrf2 dissociation, and increases Nrf2 transcriptional activity, thereby reducing ischemia-reperfusion induced oxidative stress and cell damage.61 Keap-1 is S-sulfhydrated in embryonic fibroblasts of wild-type (WT) mice, but not in mice with CSE knockdown. NaHS S-sulfhydrates Keap-1 at Cys151 regulates the localization, activity, and target gene expression of Nrf2 in mouse embryonic fibroblasts. The Cys151 mutation weakened the S-sulfhydration of Keap-1, failed to promote the nuclear translocation of Nrf2, and prevented cell senescence. Defects in a functional domain of Keap-1 (Tramtrack and Bric-á-Brac 2 dimerization domain) eliminated NaHS-induced S-sulfhydration. GYY4137 (an H2S donor) reduced arteriosclerotic plaque formation and reactive oxygen species (ROS) levels in LDL-receptor knockout (LDLr−/−) mice, but not in LDLr−/− and Nrf2−/− knockout mice. Similarly, foam cells and oxidative stress levels in peritoneal macrophages isolated from WT mice were reduced by GYY4137, whereas those isolated from Nrf2−/− were not.62 In endothelial cells stimulated by oxidized LDL and high glucose, the S-sulfhydration of GYY4137 on Keap-1 at Cys151 and Cys273 promotes the dissociation of Keap-1/Nrf2, while mutations at Cys151A eliminate GYY4137-induced Keap-1/Nrf2 dissociation, Nrf2 nuclear translocation, and ROS clearance.60 Protein disulphide isomerase (PDI) activity is closely related to endothelial function and is up-regulated in plaques. ROS manages cytoplasmic PDI activity, ROS-mediated specific oxidase regulates ER PDI activity, and H2S has an anti-oxidative stress effect and can directly remove hyperhomocysteinaemia (HHcy) induced ROS.63,64 S-sulfhydration of PDI by NaHS and GYY4137 at Cys53/Cys57 and Cys397/Cys400 inhibits HHcy-induced PDI dysfunction and ER stress, and reduces atherosclerotic plaque formation.65 Both CD62E and cathepsin S (CTSS) are associated with endothelial cell activation and atherosclerosis. The constitutive S-sulfhydration of CSE-derived H2S at Cys13 reduces the dimer and activity of human antigen R (HuR), thereby preventing its binding to target mRNA and reducing the expression of target protein (for example CD62E and CTSS). However, due to vascular inflammation, Ser377 of CSE is phosphorylated, its activity is inhibited, and CD62E expression is increased, accelerating the formation of endothelial dysfunction and atherosclerosis. Similarly, atherosclerotic plaques increased and lumen area decreased in carotid arteries in mice with CSE knockdown, this can be reversed by SG1002 (a slow-releasing polysulfide donor).66
H2S regulates voltage-activated calcium channels in the cardiovascular system.67,68 The concentration of NaHS dependently inhibits L-type calcium currents in cardiomyocytes, which can be eliminated by DTT (dithiothreitol, a small molecule organic reducing agent), and NaHS reduces functional free sulfhydryl groups in L-type calcium channels, providing indirect evidence for H2S-mediated S-sulfhydration in voltage-activated Ca2+ channels.69 Transient receptor potential (TRP) channels are Ca2+ osmotic ion channels with angiogenic effect and are also regulated by H2S.70 Inhibition of TRPV4 (TRP family member) inhibited the vasodilation of H2S-induced Ca2+ and K+ influx, while Na2S treatment of aortic endothelial cells enhanced the S-sulfhydration of TRPV4. H2S-mediated vasodilation required the activation of TRPV4-dependent Ca2+ influx in endothelial cells. In addition, TRPV4 causes vasodilation through Ca2+ and is enhanced by S-sulfhydration.71 In the carotid sinus of spontaneously hypertensive rats, endogenous H2S and CBS regulate blood pressure through TRPV1 (another TRP family member), and NaHS supplementation increases the expression of mRNA, protein, and S-sulfhydrated TRPV1 to enhance the sensitivity of carotid baroreceptors and thus dilate blood vessels.72 Ca2+/calmodulin-dependent protein kinase II (CaMKII) mediates the mitochondrial damage in patients with heart failure by regulating cardiac excitation-contraction coupling, apoptosis, Ca2+ homeostasis, and ROS metabolism.73,74 CaMKII activity affects cardiomyocyte Ca2+ homeostasis, intracellular Ca2+ flux, and mitochondrial Ca2+ processing. The S-sulfhydration of CaMKII by H2S at Cys6 inhibits the activity of CaMKII, thus improving mitochondrial function, inhibiting mitochondrial apoptosis, and protecting myocardial cells.75
Moreover, in human endothelial cells and fibroblasts, H2S induces S-sulfhydration of mitogen-activated extracellular signal-regulated kinase 1 (MEK1) at Cys341 to promote phosphorylation and nuclear transfer of extracellular regulated protein kinases (ERK1/2), thereby activating poly (ADP-ribose) polymerase-1 (PARP-1) and mediating DNA damage repair.76 Specific protein-1 (SP-1) is an important transcription factor with multiple functions in the cardiovascular system.77 The S-sulfhydration of SP-1 by NaHS at Cys68 and Cys755 can stabilize and enhance the binding of SP-1 to the vascular endothelial growth factor receptor 2 promoter, maintain the stability of metabolites and protect endothelial cells.78 H2S reduces the promoter activity of Krüppel-like factor 5 (KLF5) and the expression of mRNA, and the S-sulfhydration of SP-1 by H2S at Cys664 weaken the activity of SP-1 and KLF5 promoter binding and inhibits cardiac hypertrophy.79 H2S inhibits the expression of insulin-like growth factor-1 receptor (IGF1R), and the binding ability of S-sulfhydrated IGF1R and IGF1 is weakened, thereby inhibiting the proliferation of vascular smooth muscle cells.80 The S-sulfhydration of H2S to Kir6.1 (an ATP-sensitive potassium channel) at Cys43 reduces the binding of Kir6.1-ATP and enhances the binding of Kir6.1-phosphatidylinositol (4,5)-bisphosphate to activate Kir6.1, increase the activity of KATP channels and relax blood vessels.81 All of these S-sulfhydrations play an important role in the physiological and pathological processes of the cardiovascular system, but the interaction between them needs to be further explored (Fig. 2).
Figure 2.
The role of different proteins in the cardiovascular system after S-sulfhydration. After protein S-sulfhydration, it exerts anti-oxidative stress in the cardiovascular system, restores ER homeostasis, regulates ion channels, and regulates glucose and lipid metabolism. It plays a role in blood pressure regulation, atherosclerotic plaque formation, and cardiac hypertrophy. CaMKII: Ca2+/calmodulin-dependent protein kinase II; CTSS: cathepsin S; ER: endoplasmic reticulum; ERK1/2: extracellular regulated protein kinase 1/2; HuR: human antigen R; IGF1: insulin-like growth factor-1; IGF1R: insulin-like growth factor-1 receptor; Keap-1: Kelch-like ECH-associated protein1; Kir6.1: ATP-sensitive potassium channel 6.1; KLF5: Krüppel-like factor 5; MEK1: mitogen-activated extracellular signal-regulated kinase 1; MuRF1: muscle RING finger-1; Nrf2: nuclear factor E2-related factor 2; PARP-1: poly (ADP-ribose) polymerase-1; PDI: protein disulphide isomerase; PIP2: phosphatidylinositol (4,5)-bisphosphate; PPARγ:peroxisome proliferator-activated receptor γ; PTP1B: protein tyrosine phosphatase 1B; SP1: specific protein-1; TRPV4 and TRPV1: transient receptor potential family member; VEGFR2: vascular endothelial growth factor receptor 2.
S-sulfhydration in the nervous system
The study of H2S in the central nervous system began with the discovery of sulfide in the brain, and then the synthesis of endogenous H2S was detected in the brain.82,83 Importantly, the metabolic disorder of H2S is involved in the development of various neurological diseases. Such as Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD).84 Parkin, as an E3 ubiquitin ligase, can ubiquitinate the target protein of the proteasome. The mutation of parkin leads to the death of dopaminergic cells in the development of PD.85,86 The S-sulfhydration of parkin is related to its activity, and the S-nitrosylation weakens its activity. Studies have shown that the parkin of S-sulfhydration in PD patients is significantly reduced, which means that the activity of Parkin is closely related to PD.87,88 The S-sulfhydration of H2S on parkin enhances its activity, thereby exerting a neuroprotective effect in PD. AD is a progressive neurodegenerative disease with insidious onset. Clinically, it is characterized by general dementia such as memory impairment, aphasia, apraxia, agnosia, impairment of visual-spatial skills, executive dysfunction, and personality and behavior changes.89,90 Although the cause of AD is unknown so far, H2S is believed to be involved in the development of AD.91,92 Neuroinflammation and excessive Aβ deposition synergistically promote the development of AD,93 signal transducer and activator of transcription 3 (STAT3) mediates neuroinflammation and Aβ deposition in the process of AD, and the inhibition of CTSS in microglia can produce neuroprotective effects in AD.94, 95, 96 In mouse glial cells BV2, H2S reduces ATP-induced ROS production, inflammatory response, and Aβ1-42 deposition by eliminating phosphorylation of STAT3 and S-sulfhydration of CTSS at Cys25.97 The S-sulfhydration of glycogen synthase kinase 3β (GSK3β) by H2S at Ctys218 inhibits the phosphorylation of Tau (a major component of the neurofibrillary tangles) beyond Ser396, while the hyperphosphorylation of Tau reduces its affinity for microtubules and causes its aggregation.98 In addition, it was found in the autopsy of AD patients and mice that the expression of CSE and the effect of S-sulfhydration decreased, indicating that the increase in the content of H2S and the degree of S-sulfhydration can inhibit the progression of AD.98,99
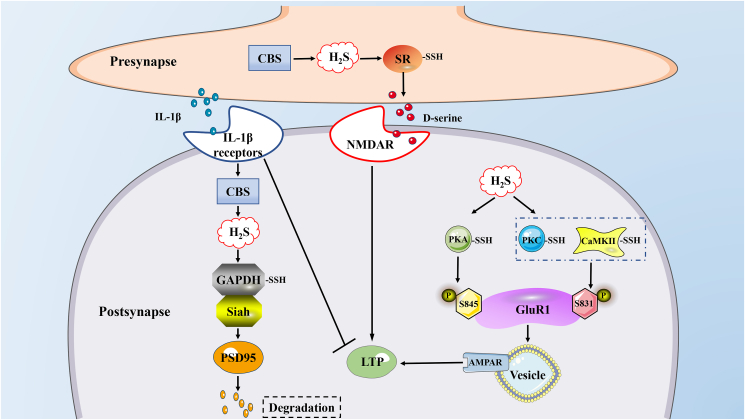
Memory impairment exists in several neurodegenerative diseases and is caused by a variety of pathological and physiological mechanisms, including neuroinflammation and aging.100, 101, 102 Interleukin-1β (IL-1β) is a pro-inflammatory factor widely found in the brain,103 postsynaptic density 95 (PSD95) is the core scaffold protein of the postsynaptic protein network, which is involved in the regulation of synaptic stability, strength, and plasticity.104,105 IL-1β plays a crucial role in learning and memory by regulating PSD95. IL-1β mediates the increase of H2S and leads to the decrease of neuronal PSD95. This effect is due to the S-sulfhydration of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) at Cys150 by H2S, which enhances the binding of GAPDH and Siah (an E3 ligase protein), and enhances IL-1β-induced ubiquitination mediation. The induced PSD95 degrades, which in turn leads to cognitive dysfunction. When CBS is knockout, IL-1β-induced synapse reduction and memory impairment are significantly alleviated. This study provides a new explanation for inflammation-induced memory impairment: IL-1β through the S-sulfhydration of GAPDH by H2S leads to the combination of GAPDH and Siah and increases the stability of Siah. Stable Siah promotes the ubiquitination of PSD95.106 Long-term potentiation (LTP), a cellular model for memory, is bidirectionally regulated by various redox signals.107,108 The increase of LTP is closely related to the improvement of synaptic function related to aging.109,110 Studies have shown that LTP damage in elderly animals is related to the decrease in N-methyl-d-aspartate subtype glutamate receptor (NMDAR) activity induced by endogenous d-serine.111,112 H2S increases the availability of d-serine through the S-sulfhydration of serine racemase (SR) to enhance LTP, and the increase of SR S-sulfhydration reduces the S-nitrosylated SR and enhances the activity of SR. CBS knockout and drug inhibition both damage hippocampal LTP, and this effect can be reversed by supplementation of exogenous H2S and d-serine. In addition, after knocking out the SR in the hippocampus, the effect of H2S on SR was eliminated, indicating that H2S regulates d-serine through S-sulfhydrates SR and plays an important role in hippocampal LTP. In summary, this study shows that S-sulfhydration of SR improves NMDAR-dependent synaptic function. There are pieces of literature that LTP and memory in the brain are also related to the concentration of IL-1β, that is, high concentrations of IL-1β inhibit synaptic strength and LTP,113 while physiological levels of IL-1β promote LTP and the formation of memory,114 and the increase in IL-1β causes an increase in the production of H2S.106 Therefore, whether the concentration of IL-1β affects LTP remains to be verified. In addition, GAPDH, a key regulator of astrocyte SR, is S-sulfhydrated in the kidney and synapse, but its effect on the astrocyte needs to be further clarified.106,115 α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) mediates rapid excitatory synaptic transmission in the central nervous system, and its dynamic expression in the postsynaptic membrane is related to the induction and maintenance of LTP and long-term depression (LTD), and participates in the regulation of learning and memory activities. Phosphorylation of AMPAR subunits in neurons is an important regulatory mechanism that controls their functions.116 A variety of protein kinases (such as PKA, PKC, CAMKII) and protein phosphatase type 2A are key regulators of AMPAR phosphorylation. H2S can enhance the activity of PKA, PKC, and CAMKII through S-sulfhydration, thereby phosphorylating GluR1 (AMPAR subunit) at Ser845 and Ser841, consequently increasing the surface activity of GluR1, and these effects can be reversed by DTT.117 To sum up, H2S affects the formation of memory in many ways in the nervous system, and the generation of H2S in different parts may have the opposite effect. This may be related to the bell-shaped pharmacological model of H2S.13 In short, H2S impacts on memory needs further study (Fig. 3).
Figure 3.
S-sulfhydration plays a role in synapses and participates in memory formation. H2S participates in memory formation through S-sulfhydration of GAPDH, SR, PKA, PKC, and CaMKII, which may all be related to LTP. AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; CaMKII: Ca2+/calmodulin-dependent protein kinase II; CBS: cystathionine β-synthase; GluR1: AMPAR subunit; LTP: long-term potentiation; NMDAR: N-methyl-d-aspartate subtype glutamate receptor; PSD95: postsynaptic density 95; Siah: E3 ubiquitin protein ligase; SR: serine racemase.
S-sulfhydration in kidney
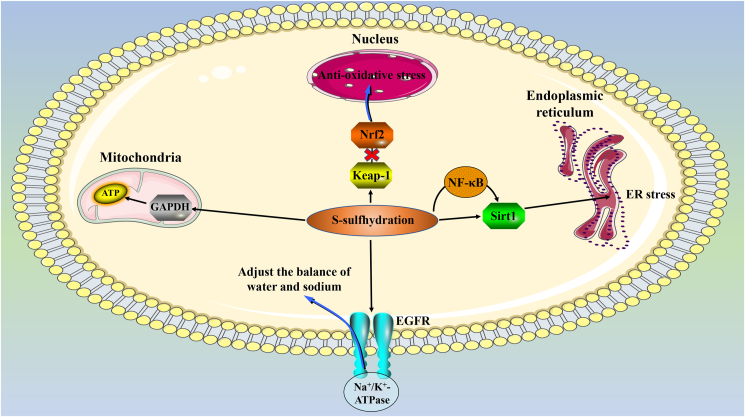
H2S plays an important role in the physiological and pathological processes of the kidneys by regulating the blood flow, endocrine, and metabolic functions of the kidneys. Its S-sulfhydration to proteins acts on the kidneys mainly through the following channels: 1) Keap-1, which plays a role in the cardiovascular system, also plays a role in the kidney. The S-sulfhydration of Keap-1 at Cys151 by H2S dissociates the transcription factor Nrf-2, promotes the transfer of Nrf-2 to the nucleus, and promotes cells from oxidative stress.60 And Nrf-2 is thought to play a role in artificially induced kidney damage after ischemia-reperfusion.118,119 2) The S-sulfhydration of GAPDH not only affects its activity but also protects GAPDH from harmful PTM. The S-sulfhydration of GAPDH at Cys150 enhances its activity, and the S-sulfhydration at Cys156 or Cys152 weakens its activity.115,120 At the same time, the S-sulfhydration of Cys150 also competitively weakens the S-nitrosylation at Cys150, and the S-nitrosylation will weaken the activity of GAPDH.121 GAPDH activity is positively correlated with ATP production. In acute kidney injury, the production of ATP is essential for the recovery of renal function.122 3) S-sulfhydration of proteins can also adjust the balance of water and sodium. S-sulfhydration at the C797/C798 site of endothelial growth factor receptor (EGFR) induces the endocytosis of Na+/K+-ATPase, which leads to the loss of renal tubular Na+/K+-ATPase function, induces mice to urination and excrete sodium, and lower blood pressure.123 4) Silent mating-type information regulator 2 homolog 1 (Sirt1) participates in aging, metabolism, and tolerance to oxidative stress by deacetylating its various substrates (including histones, transcription factors, and co-regulators).124 H2S can not only directly S-sulfhydrate Sirt1 to increase its activity, but also increase the expression of Sirt1 through S-sulfhydration of its upstream transcription factor NF-κβ, and the S-sulfhydration of Sirt1 also reduces its deacetylation ability, which plays an important role in the regulation of its epigenetic function.125, 126, 127 In addition, H2S also S-sulfhydrates certain proteins in the PI3K/Akt, ERK1/2, and ATF4 pathways, thereby reducing ER stress76,128 (Fig. 4).
Figure 4.
The role of S-sulfhydration in the kidney. S-sulfhydration regulates ATP production, anti-oxidative stress, regulation of ER stress and water-sodium balance in the kidney. EGFR: endothelial growth factor receptor; ER: endoplasmic reticulum; Keap-1: Kelch-like ECH-associated protein1; Nrf2: nuclear factor E2-related factor 2; Sirt1: silent mating-type information regulator 2 homolog 1.
S-sulfhydration in other systems
In addition, in hepatocytes, H2S can regulate the expression of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) through glucocorticoid receptor and cAMP/PKA pathways, can also directly S-sulfhydrate PGC-1a to enhance its activity, and can also S-sulfhydrate glucose-6-phosphatase and fructose-1,6-bisphosphatase to enhance the liver glucose produce.129 In the testis, CBS-catalyzed H2S inhibits phosphodiesterase (PDE) expression through S-sulfhydrated PDE, activates the cAMP/PKA pathway, and restores testosterone synthesis.130 In myoblasts, CSE-induced H2S protects myoblasts from oxidative stress induced by cadmium by S-sulfhydrating metallothionein-1.131 In melanoma cells, H2S S-sulfhydrates human serum albumin at Cys34 to exert anti-oxidative stress and inhibit tyrosinase and melanin accumulation.132
S-sulfhydration and other PTMs
Except for S-sulfhydration, no other PTMs directly induced by H2S have been found for the time being. But proteins S-sulfhydration can indirectly regulate other types of PTM, such as inducing protein phosphorylation, competitive inhibition of S-nitrosylation, methylation, etc.56,121 It is unclear whether H2S can induce other types of PTM to produce biological effects.
The detection method and principle of S-sulfhydration
The current methods for detecting S-sulfhydration mainly include modified biotin-switch technique, N-ethyl maleimide blocking method, tag-switch assay. The modified biotin-switch technique utilizes the property of the thiol blocking agent iodoacetic acid (IAA) to react with free thiols and protein persulfides. IAA reacts with S-sulfhydrated proteins to form alkylated S-sulfhydrated compounds, which are then cleaved with dithiothreitol (DTT) to modify the compounds. Specific cysteine was then labeled with iodoacetamide-linked biotin. However, in this method, DTT will also cleave S-nitrosothiols, resulting in errors.56 Maleimide selectively interacts with the sulfhydryl group of cysteine and labels sulfhydrated and non-sulfhydrated cysteines. The use of fluorescently labeled maleimide forms adducted disulfides with persulfides in the sample, which are decomposed by DTT resulting in a decrease in fluorescence. Because of the availability of commercial reagents, this method is relatively simple and can be quantitatively analyzed. This method cannot be used to label S-sulfhydrated proteins because persulfides are already involved in the reaction.127 The tag-switch assay uses two reagents to label protein persulfides in two steps. The first step uses a thiol blocker (methylsulfonyl benzothiazole) to label-free thiols and persulfides. When properly labeled, disulfide bonds in persulfide adducts can exhibit higher reactivity with certain nucleophiles than those commonly found in proteins. Therefore, only persulfide adducts can be labeled with available nucleophile-containing tag-switching reagent. In the second step, the persulfide adduct can be labeled with a labeled cyanoacetic acid derivative (nucleophile). The advantage of this method is that the intracellular S-sulfhydration can be observed under a fluorescence microscope.
These methods have their advantages and disadvantages,133, 134, 135 therefore, more effective methods need to be developed to identify the effects and sites of S-sulfhydration.
H2S donors and their possible challenges as a drug
Since the discovery of the physiological function of H2S, more and more H2S donors have also been discovered or synthesized. Among the natural compounds, there are not only inorganic substances such as NaHS, Na2S, but also Allicin, Ajoene, Diallyl sulfide, Diallyl disulfide from garlic and sulforaphane from cruciferous plants. The anti-inflammatory and anticancer effects of garlic and cruciferous plants may be related to H2S donors.136, 137, 138, 139, 140, 141, 142, 143 Synthetic compounds such as GYY4137, 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione, S-propargyl-cysteine are pure H2S donors.144,145 In recent years, the number of compounds developed in conjunction with other drugs or donors has gradually increased, such as the non-steroidal anti-inflammatory drug H2S-NSAIDs containing H2S,146 and there are NOSH-aspirin and NOSH-sulindac containing H2S and NO donors developed using the synergy of NO and H2S.147,148
Since the concentration of H2S in living cells is difficult to measure, the concentration of the donor is now used as the drug concentration, but high concentrations of H2S are toxic to the organism, and different tissues have different tolerance to H2S. Therefore, how to target H2S donors to target tissues and maintain appropriate concentrations is a challenge for drug development. Even so, individual differences are also issues that need to be considered.
Concluding remarks and prospects
The cellular and biological effects of H2S are mainly mediated through the following ways: (1) interaction with ion channels, (2) mutual effect with second messengers, (3) direct or indirect PTM, (4) regulation Redox balance, and (5) oxygen sensing and mitochondrial bioenergetics.149, 150, 151 However, as the research progresses, S-sulfhydration, this kind of PTM directly induced by H2S, has attracted more and more attention. S-sulfhydration has been shown to be involved in all the biological effects of H2S.
Protein S-sulfhydration is a post-translational modification directly induced by H2S, which may be the molecular mechanism of H2S action. However, not all S-sulfhydration can change the spatial structure and activity of a protein. Even the S-sulfhydration of the same protein can exhibit different effects, which may be determined by the position of S-sulfhydration cysteine residues. If the S-sulfhydrated cysteine locates in a key structural domain, it is essential to maintain the activity and function of the protein. However, the exact nature of the crosstalk between S-sulfhydration and other PTMs is still unclear and needs to be further elucidated.
Although there are many problems to be solved, the importance of S-sulfhydration is self-evident. More information about S-sulfhydration will help us understand the role of S-sulfhydration in the mechanism of H2S. In addition, protein S-sulfhydration (induction or blocking) may become a potential new target for drug design, this may provide a new direction for the development and application of H2S-based drugs.
Author contributions
DW, HC, and XJ conceived the study and drafted the manuscript. HC, LQ, KL, YQ, and JZ prepared the figures. All authors read and approved the final manuscript.
Conflict of interests
The authors declare that they have no competing interests.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81802718 and 81670088), the Training Program for Young Backbone Teachers of Institutions of Higher Learning in Henan Province, China (No. 2020GGJS038), and the Foundation of Science & Technology Department of Henan Province, China (No. 202102310480, 222102310490, and 222102310495).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Xin-Ying Ji, Email: 10190096@vip.henu.edu.cn.
Dong-Dong Wu, Email: ddwubiomed2010@163.com.
References
- 1.Farrugia G., Szurszewski J.H. Carbon monoxide, hydrogen sulfide, and nitric oxide as signaling molecules in the gastrointestinal tract. Gastroenterology. 2014;147(2):303–313. doi: 10.1053/j.gastro.2014.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouillaud F., Blachier F. Mitochondria and sulfide: a very old story of poisoning, feeding, and signaling? Antioxidants Redox Signal. 2011;15(2):379–391. doi: 10.1089/ars.2010.3678. [DOI] [PubMed] [Google Scholar]
- 3.Olson K.R. Mitochondrial adaptations to utilize hydrogen sulfide for energy and signaling. J Comp Physiol B. 2012;182(7):881–897. doi: 10.1007/s00360-012-0654-y. [DOI] [PubMed] [Google Scholar]
- 4.Martin G.R., McKnight G.W., Dicay M.S., Coffin C.S., Ferraz J.G., Wallace J.L. Hydrogen sulphide synthesis in the rat and mouse gastrointestinal tract. Dig Liver Dis. 2010;42(2):103–109. doi: 10.1016/j.dld.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Mani S., Li H., Untereiner A., et al. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127(25):2523–2534. doi: 10.1161/CIRCULATIONAHA.113.002208. [DOI] [PubMed] [Google Scholar]
- 6.Linden D.R., Levitt M.D., Farrugia G., Szurszewski J.H. Endogenous production of H2S in the gastrointestinal tract: still in search of a physiologic function. Antioxidants Redox Signal. 2010;12(9):1135–1146. doi: 10.1089/ars.2009.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackstone E., Morrison M., Roth M.B. H2S induces a suspended animation-like state in mice. Science. 2005;308(5721):518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 8.Blackstone E., Roth M.B. Suspended animation-like state protects mice from lethal hypoxia. Shock. 2007;27(4):370–372. doi: 10.1097/SHK.0b013e31802e27a0. [DOI] [PubMed] [Google Scholar]
- 9.Zhao W., Zhang J., Lu Y., Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20(21):6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai W.J., Wang M.J., Moore P.K., Jin H.M., Yao T., Zhu Y.C. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res. 2007;76(1):29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Kolluru G.K., Shen X., Bir S.C., Kevil C.G. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zanardo R.C., Brancaleone V., Distrutti E., Fiorucci S., Cirino G., Wallace J.L. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20(12):2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 13.Hellmich M.R., Szabo C. Hydrogen sulfide and cancer. Handb Exp Pharmacol. 2015;230:233–241. doi: 10.1007/978-3-319-18144-8_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphrey S.J., James D.E., Mann M. Protein phosphorylation: a major switch mechanism for metabolic regulation. Trends Endocrinol Metabol. 2015;26(12):676–687. doi: 10.1016/j.tem.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y., Ren B., Yang J., et al. The role of histone methylation in the development of digestive cancers: a potential direction for cancer management. Signal Transduct Targeted Ther. 2020;5(1):143. doi: 10.1038/s41392-020-00252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng S., Marmorstein R. Protein N-terminal acetylation: structural basis, mechanism, versatility, and regulation. Trends Biochem Sci. 2021;46(1):15–27. doi: 10.1016/j.tibs.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vu L.D., Gevaert K., De Smet I. Protein language: post-translational modifications talking to each other. Trends Plant Sci. 2018;23(12):1068–1080. doi: 10.1016/j.tplants.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Krajewski W.A. Ubiquitylation: how nucleosomes use histones to evict histones. Trends Cell Biol. 2019;29(9):689–694. doi: 10.1016/j.tcb.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Li Z., Polhemus D.J., Lefer D.J. Evolution of hydrogen sulfide therapeutics to treat cardiovascular disease. Circ Res. 2018;123(5):590–600. doi: 10.1161/CIRCRESAHA.118.311134. [DOI] [PubMed] [Google Scholar]
- 20.Fu M., Zhang W., Wu L., Yang G., Li H., Wang R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc Natl Acad Sci U S A. 2012;109(8):2943–2948. doi: 10.1073/pnas.1115634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Módis K., Coletta C., Erdélyi K., Papapetropoulos A., Szabo C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J. 2013;27(2):601–611. doi: 10.1096/fj.12-216507. [DOI] [PubMed] [Google Scholar]
- 22.Nagahara N., Ito T., Kitamura H., Nishino T. Tissue and subcellular distribution of mercaptopyruvate sulfurtransferase in the rat: confocal laser fluorescence and immunoelectron microscopic studies combined with biochemical analysis. Histochem Cell Biol. 1998;110(3):243–250. doi: 10.1007/s004180050286. [DOI] [PubMed] [Google Scholar]
- 23.Teng H., Wu B., Zhao K., Yang G., Wu L., Wang R. Oxygen-sensitive mitochondrial accumulation of cystathionine β-synthase mediated by Lon protease. Proc Natl Acad Sci U S A. 2013;110(31):12679–12684. doi: 10.1073/pnas.1308487110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadav P.K., Martinov M., Vitvitsky V., et al. Biosynthesis and reactivity of cysteine persulfides in signaling. J Am Chem Soc. 2016;138(1):289–299. doi: 10.1021/jacs.5b10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pazicni S., Lukat-Rodgers G.S., Oliveriusová J., et al. The redox behavior of the heme in cystathionine beta-synthase is sensitive to pH. Biochemistry. 2004;43(46):14684–14695. doi: 10.1021/bi0488496. [DOI] [PubMed] [Google Scholar]
- 26.Yadav P.K., Yamada K., Chiku T., Koutmos M., Banerjee R. Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J Biol Chem. 2013;288(27):20002–20013. doi: 10.1074/jbc.M113.466177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boron W.F. Regulation of intracellular pH. Adv Physiol Educ. 2004;28(1–4):160–179. doi: 10.1152/advan.00045.2004. [DOI] [PubMed] [Google Scholar]
- 28.Zaichko N.V., Melnik A.V., Yoltukhivskyy M.M., Olhovskiy A.S., Palamarchuk I.V. Hydrogen sulfide: metabolism, biological and medical role. Ukrainian Biochem J. 2014;86(5):5–25. [PubMed] [Google Scholar]
- 29.Pedre B., Dick T.P. 3-Mercaptopyruvate sulfurtransferase: an enzyme at the crossroads of sulfane sulfur trafficking. Biol Chem. 2021;402(3):223–237. doi: 10.1515/hsz-2020-0249. [DOI] [PubMed] [Google Scholar]
- 30.Hipólito A., Nunes S.C., Vicente J.B., Serpa J. Cysteine aminotransferase (CAT): a pivotal sponsor in metabolic remodeling and an ally of 3-mercaptopyruvate sulfurtransferase (MST) in cancer. Molecules. 2020;25(17):3984. doi: 10.3390/molecules25173984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shibuya N., Koike S., Tanaka M., et al. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat Commun. 2013;4:1366. doi: 10.1038/ncomms2371. [DOI] [PubMed] [Google Scholar]
- 32.Yoboue E.D., Sitia R., Simmen T. Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages. Cell Death Dis. 2018;9(3):331. doi: 10.1038/s41419-017-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Randi E.B., Casili G., Jacquemai S., Szabo C. Selenium-binding protein 1 (SELENBP1) supports hydrogen sulfide biosynthesis and adipogenesis. Antioxidants (Basel) 2021;10(3):361. doi: 10.3390/antiox10030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C.W., Moore P.K. H2S synthesizing enzymes: biochemistry and molecular aspects. Handb Exp Pharmacol. 2015;230:3–25. doi: 10.1007/978-3-319-18144-8_1. [DOI] [PubMed] [Google Scholar]
- 35.Kabil O., Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxidants Redox Signal. 2014;20(5):770–782. doi: 10.1089/ars.2013.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toombs C.F., Insko M.A., Wintner E.A., et al. Detection of exhaled hydrogen sulphide gas in healthy human volunteers during intravenous administration of sodium sulphide. Br J Clin Pharmacol. 2010;69(6):626–636. doi: 10.1111/j.1365-2125.2010.03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein A., Bailey S.M. Redox biology of hydrogen sulfide: implications for physiology, pathophysiology, and pharmacology. Redox Biol. 2013;1(1):32–39. doi: 10.1016/j.redox.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabil O., Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285(29):21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiranti V., Viscomi C., Hildebrandt T., et al. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat Med. 2009;15(2):200–205. doi: 10.1038/nm.1907. [DOI] [PubMed] [Google Scholar]
- 40.Chenuel B., Sonobe T., Haouzi P. Effects of infusion of human methemoglobin solution following hydrogen sulfide poisoning. Clin Toxicol (Phila). 2015;53(2):93–101. doi: 10.3109/15563650.2014.996570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olson K.R. A practical look at the chemistry and biology of hydrogen sulfide. Antioxidants Redox Signal. 2012;17(1):32–44. doi: 10.1089/ars.2011.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kabil O., Motl N., Banerjee R. H2S and its role in redox signaling. Biochim Biophys Acta. 2014;1844(8):1355–1366. doi: 10.1016/j.bbapap.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vitvitsky V., Kabil O., Banerjee R. High turnover rates for hydrogen sulfide allow for rapid regulation of its tissue concentrations. Antioxidants Redox Signal. 2012;17(1):22–31. doi: 10.1089/ars.2011.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toohey J.I. Sulfur signaling: is the agent sulfide or sulfane? Anal Biochem. 2011;413(1):1–7. doi: 10.1016/j.ab.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 45.Greiner R., Pálinkás Z., Bäsell K., et al. Polysulfides link H2S to protein thiol oxidation. Antioxidants Redox Signal. 2013;19(15):1749–1765. doi: 10.1089/ars.2012.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kimura H. Signaling molecules: hydrogen sulfide and polysulfide. Antioxidants Redox Signal. 2015;22(5):362–376. doi: 10.1089/ars.2014.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimura H. Hydrogen sulfide: from brain to gut. Antioxidants Redox Signal. 2010;12(9):1111–1123. doi: 10.1089/ars.2009.2919. [DOI] [PubMed] [Google Scholar]
- 48.Webb G.D., Lim L.H., Oh V.M., et al. Contractile and vasorelaxant effects of hydrogen sulfide and its biosynthesis in the human internal mammary artery. J Pharmacol Exp Therapeut. 2008;324(2):876–882. doi: 10.1124/jpet.107.133538. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y.H., Lu M., Hu L.F., Wong P.T., Webb G.D., Bian J.S. Hydrogen sulfide in the mammalian cardiovascular system. Antioxidants Redox Signal. 2012;17(1):141–185. doi: 10.1089/ars.2011.4005. [DOI] [PubMed] [Google Scholar]
- 50.Yang G., Wu L., Jiang B., et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322(5901):587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kiss L., Deitch E.A., Szabó C. Hydrogen sulfide decreases adenosine triphosphate levels in aortic rings and leads to vasorelaxation via metabolic inhibition. Life Sci. 2008;83(17–18):589–594. doi: 10.1016/j.lfs.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johansen D., Ytrehus K., Baxter G.F. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury: evidence for a role of K ATP channels. Basic Res Cardiol. 2006;101(1):53–60. doi: 10.1007/s00395-005-0569-9. [DOI] [PubMed] [Google Scholar]
- 53.Cai J., Shi X., Wang H., et al. Cystathionine γ lyase-hydrogen sulfide increases peroxisome proliferator-activated receptor γ activity by sulfhydration at C139 site thereby promoting glucose uptake and lipid storage in adipocytes. Biochim Biophys Acta. 2016;1861(5):419–429. doi: 10.1016/j.bbalip.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Kandadi M.R., Panzhinskiy E., Roe N.D., Nair S., Hu D., Sun A. Deletion of protein tyrosine phosphatase 1B rescues against myocardial anomalies in high fat diet-induced obesity: role of AMPK-dependent autophagy. Biochim Biophys Acta. 2015;1852(2):299–309. doi: 10.1016/j.bbadis.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 55.Coquerel D., Neviere R., Delile E., et al. Gene deletion of protein tyrosine phosphatase 1B protects against sepsis-induced cardiovascular dysfunction and mortality. Arterioscler Thromb Vasc Biol. 2014;34(5):1032–1044. doi: 10.1161/ATVBAHA.114.303450. [DOI] [PubMed] [Google Scholar]
- 56.Krishnan N., Fu C., Pappin D.J., Tonks N.K. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal. 2011;4(203):ra86. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bodine S.C., Latres E., Baumhueter S., et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294(5547):1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 58.Centner T., Yano J., Kimura E., et al. Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J Mol Biol. 2001;306(4):717–726. doi: 10.1006/jmbi.2001.4448. [DOI] [PubMed] [Google Scholar]
- 59.Sun X., Zhao D., Lu F., et al. Hydrogen sulfide regulates muscle RING finger-1 protein S-sulfhydration at Cys(44) to prevent cardiac structural damage in diabetic cardiomyopathy. Br J Pharmacol. 2020;177(4):836–856. doi: 10.1111/bph.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie L., Gu Y., Wen M., et al. Hydrogen sulfide induces Keap1 S-sulfhydration and suppresses diabetes-accelerated atherosclerosis via Nrf2 activation. Diabetes. 2016;65(10):3171–3184. doi: 10.2337/db16-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo C., Liang F., Shah Masood W., Yan X. Hydrogen sulfide protected gastric epithelial cell from ischemia/reperfusion injury by Keap1 s-sulfhydration, MAPK dependent anti-apoptosis and NF-κB dependent anti-inflammation pathway. Eur J Pharmacol. 2014;725:70–78. doi: 10.1016/j.ejphar.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 62.Yang G., Zhao K., Ju Y., et al. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxidants Redox Signal. 2013;18(15):1906–1919. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- 63.Okumura M., Kadokura H., Inaba K. Structures and functions of protein disulfide isomerase family members involved in proteostasis in the endoplasmic reticulum. Free Radic Biol Med. 2015;83:314–322. doi: 10.1016/j.freeradbiomed.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 64.Chang L., Geng B., Yu F., et al. Hydrogen sulfide inhibits myocardial injury induced by homocysteine in rats. Amino Acids. 2008;34(4):573–585. doi: 10.1007/s00726-007-0011-8. [DOI] [PubMed] [Google Scholar]
- 65.Jiang S., Xu W., Chen Z., et al. Hydrogen sulphide reduces hyperhomocysteinaemia-induced endothelial ER stress by sulfhydrating protein disulphide isomerase to attenuate atherosclerosis. J Cell Mol Med. 2021;25(7):3437–3448. doi: 10.1111/jcmm.16423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bibli S.I., Hu J., Sigala F., et al. Cystathionine γ lyase sulfhydrates the RNA binding protein human antigen R to preserve endothelial cell function and delay atherogenesis. Circulation. 2019;139(1):101–114. doi: 10.1161/CIRCULATIONAHA.118.034757. [DOI] [PubMed] [Google Scholar]
- 67.Ping N.N., Li S., Mi Y.N., Cao L., Cao Y.X. Hydrogen sulphide induces vasoconstriction of rat coronary artery via activation of Ca(2+) influx. Acta Physiol (Oxf) 2015;214(1):88–96. doi: 10.1111/apha.12475. [DOI] [PubMed] [Google Scholar]
- 68.Zhang W., Xu C., Yang G., Wu L., Wang R. Interaction of H2S with calcium permeable channels and transporters. Oxid Med Cell Longev. 2015;2015:323269. doi: 10.1155/2015/323269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang R., Sun Y., Tsai H., Tang C., Jin H., Du J. Hydrogen sulfide inhibits L-type calcium currents depending upon the protein sulfhydryl state in rat cardiomyocytes. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Munaron L., Avanzato D., Moccia F., Mancardi D. Hydrogen sulfide as a regulator of calcium channels. Cell Calcium. 2013;53(2):77–84. doi: 10.1016/j.ceca.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 71.Naik J.S., Osmond J.M., Walker B.R., Kanagy N.L. Hydrogen sulfide-induced vasodilation mediated by endothelial TRPV4 channels. Am J Physiol Heart Circ Physiol. 2016;311(6):H1437–H1444. doi: 10.1152/ajpheart.00465.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu W., Liao Y., Huang Y., et al. Endogenous hydrogen sulfide enhances carotid sinus baroreceptor sensitivity by activating the transient receptor potential cation channel subfamily V member 1 (TRPV1) channel. J Am Heart Assoc. 2017;6(5) doi: 10.1161/JAHA.116.004971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rokita A.G., Anderson M.E. New therapeutic targets in cardiology: arrhythmias and Ca2+/calmodulin-dependent kinase II (CaMKII) Circulation. 2012;126(17):2125–2139. doi: 10.1161/CIRCULATIONAHA.112.124990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Toko H., Takahashi H., Kayama Y., et al. Ca2+/calmodulin-dependent kinase IIdelta causes heart failure by accumulation of p53 in dilated cardiomyopathy. Circulation. 2010;122(9):891–899. doi: 10.1161/CIRCULATIONAHA.109.935296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu D., Hu Q., Tan B., Rose P., Zhu D., Zhu Y.Z. Amelioration of mitochondrial dysfunction in heart failure through S-sulfhydration of Ca(2+)/calmodulin-dependent protein kinase II. Redox Biol. 2018;19:250–262. doi: 10.1016/j.redox.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao K., Ju Y., Li S., Altaany Z., Wang R., Yang G. S-sulfhydration of MEK1 leads to PARP-1 activation and DNA damage repair. EMBO Rep. 2014;15(7):792–800. doi: 10.1002/embr.201338213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang P., Zhang Y., Pang J., et al. Loss of Jak2 impairs endothelial function by attenuating Raf-1/MEK1/Sp-1 signaling along with altered eNOS activities. Am J Pathol. 2013;183(2):617–625. doi: 10.1016/j.ajpath.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 78.Saha S., Chakraborty P.K., Xiong X., et al. Cystathionine β-synthase regulates endothelial function via protein S-sulfhydration. FASEB J. 2016;30(1):441–456. doi: 10.1096/fj.15-278648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meng G., Xiao Y., Ma Y., et al. Hydrogen sulfide regulates krüppel-like factor 5 transcription activity via specificity protein 1 S-sulfhydration at Cys664 to prevent myocardial hypertrophy. J Am Heart Assoc. 2016;5(9) doi: 10.1161/JAHA.116.004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shuang T., Fu M., Yang G., Wu L., Wang R. The interaction of IGF-1/IGF-1R and hydrogen sulfide on the proliferation of mouse primary vascular smooth muscle cells. Biochem Pharmacol. 2018;149:143–152. doi: 10.1016/j.bcp.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 81.Mustafa A.K., Sikka G., Gazi S.K., et al. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res. 2011;109(11):1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Warenycia M.W., Goodwin L.R., Benishin C.G., et al. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem Pharmacol. 1989;38(6):973–981. doi: 10.1016/0006-2952(89)90288-8. [DOI] [PubMed] [Google Scholar]
- 83.Abe K., Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16(3):1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang X., Bian J.S. Hydrogen sulfide: a neuromodulator and neuroprotectant in the central nervous system. ACS Chem Neurosci. 2014;5(10):876–883. doi: 10.1021/cn500185g. [DOI] [PubMed] [Google Scholar]
- 85.Chia S.J., Tan E.K., Chao Y.X. Historical perspective: models of Parkinson's disease. Int J Mol Sci. 2020;21(7):2464. doi: 10.3390/ijms21072464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kunath T., Natalwala A., Chan C., et al. Are PARKIN patients ideal candidates for dopaminergic cell replacement therapies? Eur J Neurosci. 2019;49(4):453–462. doi: 10.1111/ejn.14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vandiver M.S., Paul B.D., Xu R., et al. Sulfhydration mediates neuroprotective actions of parkin. Nat Commun. 2013;4:1626. doi: 10.1038/ncomms2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chung K.K., Thomas B., Li X., et al. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304(5675):1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- 89.Fessel J. Alzheimer's disease combination treatment. Neurobiol Aging. 2018;63:165. doi: 10.1016/j.neurobiolaging.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 90.Krantic S. Editorial: from current diagnostic tools and therapeutics for Alzheimer's disease towards earlier diagnostic markers and treatment targets. Curr Alzheimer Res. 2017;14(1):2–5. doi: 10.2174/156720501401161201104858. [DOI] [PubMed] [Google Scholar]
- 91.Vandini E., Ottani A., Zaffe D., et al. Mechanisms of hydrogen sulfide against the progression of severe Alzheimer's disease in transgenic mice at different ages. Pharmacology. 2019;103(1–2):50–60. doi: 10.1159/000494113. [DOI] [PubMed] [Google Scholar]
- 92.Kumar M., Sandhir R. Hydrogen sulfide in physiological and pathological mechanisms in brain. CNS Neurol Disord - Drug Targets. 2018;17(9):654–670. doi: 10.2174/1871527317666180605072018. [DOI] [PubMed] [Google Scholar]
- 93.Cai Z., Hussain M.D., Yan L.J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer's disease. Int J Neurosci. 2014;124(5):307–321. doi: 10.3109/00207454.2013.833510. [DOI] [PubMed] [Google Scholar]
- 94.Reichenbach N., Delekate A., Plescher M., et al. Inhibition of Stat3-mediated astrogliosis ameliorates pathology in an Alzheimer's disease model. EMBO Mol Med. 2019;11(2) doi: 10.15252/emmm.201809665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lowry J.R., Klegeris A. Emerging roles of microglial cathepsins in neurodegenerative disease. Brain Res Bull. 2018;139:144–156. doi: 10.1016/j.brainresbull.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 96.Chandra S.R. Alzheimer's disease: an alternative approach. Indian J Med Res. 2017;145(6):723–729. doi: 10.4103/ijmr.IJMR_74_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cao L., Cao X., Zhou Y., et al. Hydrogen sulfide inhibits ATP-induced neuroinflammation and Aβ(1-42) synthesis by suppressing the activation of STAT3 and cathepsin S. Brain Behav Immun. 2018;73:603–614. doi: 10.1016/j.bbi.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 98.Hanger D.P., Anderton B.H., Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med. 2009;15(3):112–119. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 99.Giovinazzo D., Bursac B., Sbodio J.I., et al. Hydrogen sulfide is neuroprotective in Alzheimer's disease by sulfhydrating GSK3β and inhibiting Tau hyperphosphorylation. Proc Natl Acad Sci U S A. 2021;118(4) doi: 10.1073/pnas.2017225118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cotman C.W., Berchtold N.C., Christie L.A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 101.Nyberg L. Neuroimaging in aging: brain maintenance. F1000Res. 2017;6:1215. doi: 10.12688/f1000research.11419.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nyberg L., Pudas S. Successful memory aging. Annu Rev Psychol. 2019;70:219–243. doi: 10.1146/annurev-psych-010418-103052. [DOI] [PubMed] [Google Scholar]
- 103.Coleman L.G., Jr., Zou J., Qin L., Crews F.T. HMGB1/IL-1β complexes regulate neuroimmune responses in alcoholism. Brain Behav Immun. 2018;72:61–77. doi: 10.1016/j.bbi.2017.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han Q., Lin Q., Huang P., et al. Microglia-derived IL-1β contributes to axon development disorders and synaptic deficit through p38-MAPK signal pathway in septic neonatal rats. J Neuroinflammation. 2017;14(1):52. doi: 10.1186/s12974-017-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bustos F.J., Ampuero E., Jury N., et al. Epigenetic editing of the Dlg4/PSD95 gene improves cognition in aged and Alzheimer's disease mice. Brain. 2017;140(12):3252–3268. doi: 10.1093/brain/awx272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mir S., Sen T., Sen N. Cytokine-induced GAPDH sulfhydration affects PSD95 degradation and memory. Mol Cell. 2014;56(6):786–795. doi: 10.1016/j.molcel.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 107.Cai F., Wang F., Lin F.K., et al. Redox modulation of long-term potentiation in the hippocampus via regulation of the glycogen synthase kinase-3beta pathway. Free Radic Biol Med. 2008;45(7):964–970. doi: 10.1016/j.freeradbiomed.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 108.Kamsler A., Segal M. Hydrogen peroxide modulation of synaptic plasticity. J Neurosci. 2003;23(1):269–276. doi: 10.1523/JNEUROSCI.23-01-00269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bodhinathan K., Kumar A., Foster T.C. Intracellular redox state alters NMDA receptor response during aging through Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2010;30(5):1914–1924. doi: 10.1523/JNEUROSCI.5485-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang Y.J., Wu P.F., Long L.H., et al. Reversal of aging-associated hippocampal synaptic plasticity deficits by reductants via regulation of thiol redox and NMDA receptor function. Aging Cell. 2010;9(5):709–721. doi: 10.1111/j.1474-9726.2010.00595.x. [DOI] [PubMed] [Google Scholar]
- 111.Mothet J.P., Rouaud E., Sinet P.M., et al. A critical role for the glial-derived neuromodulator D-serine in the age-related deficits of cellular mechanisms of learning and memory. Aging Cell. 2006;5(3):267–274. doi: 10.1111/j.1474-9726.2006.00216.x. [DOI] [PubMed] [Google Scholar]
- 112.Turpin F.R., Potier B., Dulong J.R., et al. Reduced serine racemase expression contributes to age-related deficits in hippocampal cognitive function. Neurobiol Aging. 2011;32(8):1495–1504. doi: 10.1016/j.neurobiolaging.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 113.Williamson L.L., Bilbo S.D. Chemokines and the hippocampus: a new perspective on hippocampal plasticity and vulnerability. Brain Behav Immun. 2013;30:186–194. doi: 10.1016/j.bbi.2013.01.077. [DOI] [PubMed] [Google Scholar]
- 114.McAfoose J., Baune B.T. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33(3):355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 115.Jarosz A.P., Wei W., Gauld J.W., et al. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is inactivated by S-sulfuration in vitro. Free Radic Biol Med. 2015;89:512–521. doi: 10.1016/j.freeradbiomed.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 116.Diering G.H., Huganir R.L. The AMPA receptor code of synaptic plasticity. Neuron. 2018;100(2):314–329. doi: 10.1016/j.neuron.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li Y.L., Zhou J., Zhang H., et al. Hydrogen sulfide promotes surface insertion of hippocampal AMPA receptor GluR1 subunit via phosphorylating at serine-831/serine-845 sites through a sulfhydration-dependent mechanism. CNS Neurosci Ther. 2016;22(9):789–798. doi: 10.1111/cns.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu M., Reddy N.M., Higbee E.M., et al. The Nrf2 triterpenoid activator, CDDO-imidazolide, protects kidneys from ischemia-reperfusion injury in mice. Kidney Int. 2014;85(1):134–141. doi: 10.1038/ki.2013.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu Q.Q., Wang Y., Senitko M., et al. Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPARγ, and HO-1. Am J Physiol Ren Physiol. 2011;300(5):F1180–F1192. doi: 10.1152/ajprenal.00353.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mustafa A.K., Gadalla M.M., Sen N., et al. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2(96):ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hara M.R., Agrawal N., Kim S.F., et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7(7):665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 122.Basile D.P., Anderson M.D., Sutton T.A. Pathophysiology of acute kidney injury. Compr Physiol. 2012;2(2):1303–1353. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ge S.N., Zhao M.M., Wu D.D., et al. Hydrogen sulfide targets EGFR Cys797/Cys798 residues to induce Na(+)/K(+)-ATPase endocytosis and inhibition in renal tubular epithelial cells and increase sodium excretion in chronic salt-loaded rats. Antioxidants Redox Signal. 2014;21(15):2061–2082. doi: 10.1089/ars.2013.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Godoy J.A., Zolezzi J.M., Braidy N., Inestrosa N.C. Role of Sirt1 during the ageing process: relevance to protection of synapses in the brain. Mol Neurobiol. 2014;50(3):744–756. doi: 10.1007/s12035-014-8645-5. [DOI] [PubMed] [Google Scholar]
- 125.Du C., Lin X., Xu W., et al. Sulfhydrated sirtuin-1 increasing its deacetylation activity is an essential epigenetics mechanism of anti-atherogenesis by hydrogen sulfide. Antioxidants Redox Signal. 2019;30(2):184–197. doi: 10.1089/ars.2017.7195. [DOI] [PubMed] [Google Scholar]
- 126.Katto J., Engel N., Abbas W., Herbein G., Mahlknecht U. Transcription factor NFκB regulates the expression of the histone deacetylase SIRT1. Clin Epigenet. 2013;5(1):11. doi: 10.1186/1868-7083-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sen N., Paul B.D., Gadalla M.M., et al. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol Cell. 2012;45(1):13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mazza R., Pasqua T., Cerra M.C., Angelone T., Gattuso A. Akt/eNOS signaling and PLN S-sulfhydration are involved in H₂S-dependent cardiac effects in frog and rat. Am J Physiol Regul Integr Comp Physiol. 2013;305(4):R443–R451. doi: 10.1152/ajpregu.00088.2013. [DOI] [PubMed] [Google Scholar]
- 129.Untereiner A.A., Wang R., Ju Y., Wu L. Decreased gluconeogenesis in the absence of cystathionine gamma-lyase and the underlying mechanisms. Antioxidants Redox Signal. 2016;24(3):129–140. doi: 10.1089/ars.2015.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang J., Wang J., Shen T., Hong R., Tang S., Zhao X.H. (2) S catalysed by CBS regulates testosterone synthesis through affecting the sulfhydrylation of PDE. J Cell Mol Med. 2021;25(7):3460–3468. doi: 10.1111/jcmm.16428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang Y., Ali A., Jin Z., Pei Y., Yang G. Induction of cystathionine gamma-lyase expression and metallothionein-1 S-sulfhydration alleviate cadmium-induced cell death in myoblast cells. Ecotoxicol Environ Saf. 2019;179:222–231. doi: 10.1016/j.ecoenv.2019.04.063. [DOI] [PubMed] [Google Scholar]
- 132.Ikeda M., Ishima Y., Kinoshita R., et al. A novel S-sulfhydrated human serum albumin preparation suppresses melanin synthesis. Redox Biol. 2018;14:354–360. doi: 10.1016/j.redox.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jaffrey S.R., Snyder S.H. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE. 2001;2001(86):pl1. doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- 134.Mustafa A.K., Gadalla M.M., Snyder S.H. Signaling by gasotransmitters. Sci Signal. 2009;2(68):re2. doi: 10.1126/scisignal.268re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang D., Macinkovic I., Devarie-Baez N.O., et al. Detection of protein S-sulfhydration by a tag-switch technique. Angew Chem Int Ed Engl. 2014;53(2):575–581. doi: 10.1002/anie.201305876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Arditti F.D., Rabinkov A., Miron T., et al. Apoptotic killing of B-chronic lymphocytic leukemia tumor cells by allicin generated in situ using a rituximab-alliinase conjugate. Mol Cancer Therapeut. 2005;4(2):325–331. [PubMed] [Google Scholar]
- 137.Padilla-Camberos E., Zaitseva G., Padilla C., Puebla A.M. Antitumoral activity of allicin in murine lymphoma L5178Y. Asian Pac J Cancer Prev APJCP. 2010;11(5):1241–1244. [PubMed] [Google Scholar]
- 138.Hitchcock J.K., Mkwanazi N., Barnett C., et al. The garlic compound Z-ajoene, S-thiolates COX2 and STAT3 and dampens the inflammatory response in RAW264.7 macrophages. Mol Nutr Food Res. 2021;65(3) doi: 10.1002/mnfr.202000854. [DOI] [PubMed] [Google Scholar]
- 139.Ye Y., Yang H.Y., Wu J., Li M., Min J.M., Cui J.R. Z-ajoene causes cell cycle arrest at G2/M and decrease of telomerase activity in HL-60 cells. Zhonghua Zhongliu Zazhi. 2005;27(9):516–520. [PubMed] [Google Scholar]
- 140.Shukla Y., Arora A., Singh A. Antitumorigenic potential of diallyl sulfide in Ehrlich ascites tumor bearing mice. Biomed Environ Sci. 2002;15(1):41–47. [PubMed] [Google Scholar]
- 141.Thejass P., Kuttan G. Antiangiogenic activity of diallyl sulfide (DAS) Int Immunopharm. 2007;7(3):295–305. doi: 10.1016/j.intimp.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 142.Jo H.J., Song J.D., Kim K.M., Cho Y.H., Kim K.H., Park Y.C. Diallyl disulfide induces reversible G2/M phase arrest on a p53-independent mechanism in human colon cancer HCT-116 cells. Oncol Rep. 2008;19(1):275–280. [PubMed] [Google Scholar]
- 143.Abbaoui B., Riedl K.M., Ralston R.A., et al. Inhibition of bladder cancer by broccoli isothiocyanates sulforaphane and erucin: characterization, metabolism, and interconversion. Mol Nutr Food Res. 2012;56(11):1675–1687. doi: 10.1002/mnfr.201200276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee Z.W., Teo X.Y., Tay E.Y., et al. Utilizing hydrogen sulfide as a novel anti-cancer agent by targeting cancer glycolysis and pH imbalance. Br J Pharmacol. 2014;171(18):4322–4336. doi: 10.1111/bph.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hasegawa U., Tateishi N., Uyama H., van der Vlies A.J. Hydrolysis-sensitive dithiolethione prodrug micelles. Macromol Biosci. 2015;15(11):1512–1522. doi: 10.1002/mabi.201500156. [DOI] [PubMed] [Google Scholar]
- 146.Elsheikh W., Blackler R.W., Flannigan K.L., Wallace J.L. Enhanced chemopreventive effects of a hydrogen sulfide-releasing anti-inflammatory drug (ATB-346) in experimental colorectal cancer. Nitric Oxide. 2014;41:131–137. doi: 10.1016/j.niox.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 147.Kodela R., Chattopadhyay M., Velázquez-Martínez C.A., Kashfi K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid has enhanced chemo-preventive properties compared to aspirin, is gastrointestinal safe with all the classic therapeutic indications. Biochem Pharmacol. 2015;98(4):564–572. doi: 10.1016/j.bcp.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kodela R., Chattopadhyay M., Kashfi K. Synthesis and biological activity of NOSH-naproxen (AVT-219) and NOSH-sulindac (AVT-18A) as potent anti-inflammatory agents with chemotherapeutic potential. Medchemcomm. 2013;4(11) doi: 10.1039/C3MD00185G. 10.1039/C3MD00185G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jiang B., Tang G., Cao K., Wu L., Wang R. Molecular mechanism for H(2)S-induced activation of K(ATP) channels. Antioxidants Redox Signal. 2010;12(10):1167–1178. doi: 10.1089/ars.2009.2894. [DOI] [PubMed] [Google Scholar]
- 150.Wallace J.L., Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov. 2015;14(5):329–345. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 151.Filipovic M.R. Persulfidation (S-sulfhydration) and H2S. Handb Exp Pharmacol. 2015;230:29–59. doi: 10.1007/978-3-319-18144-8_2. [DOI] [PubMed] [Google Scholar]