Abstract
Exosomes carry and transmit signaling molecules used for intercellular communication. The generation and secretion of exosomes is a multistep interlocking process that allows simultaneous control of multiple regulatory sites. Protein molecules, mainly RAB GTPases, cytoskeletal proteins and soluble N-ethylmaleimide-sensitive fusion attachment protein receptor (SNARE), are specifically regulated in response to pathological conditions such as altered cellular microenvironment, stimulation by pathogenic factors, or gene mutation. This interferes with the smooth functioning of endocytosis, translocation, degradation, docking and fusion processes, leading to changes in the secretion of exosomes. Large numbers of secreted exosomes are disseminated by the flow of body fluids and absorbed by the recipient cells. By transmitting characteristic functional proteins and genetic information produced under disease conditions, exosomes can change the physiological state of the recipient cells and their microenvironment. The microenvironment, in turn, affects the occurrence and development of disease. Therefore, this review will discuss the mechanism by which exosome secretion is regulated in cells following the formation of mature secretory multivesicular bodies (MVBs). The overall aim is to find ways to eliminate disease-derived exosomes at their source, thereby providing an important new basis for the clinical treatment of disease.
Keywords: Cellular microenvironment, Cytoskeleton, Exosome secretion, RAB GTPase, SNARE
Introduction
Exosomes are extracellular vesicles (EVs) enclosed by a lipid bilayer and characterized as cup-shaped structures with a diameter of 40–160 nm. The lipid membrane of exosomes is derived from the cytoplasmic membrane of parental cells. Similar to the carriage of a truck, it protects the enriched contents loaded within them from degradation by enzymes in the body fluids. These contents include specific proteins,1 lipids2 and nucleic acids.3 Exosome secretion was initially thought to be a means of removing cellular waste resulting from cell damage or disruption of cell homeostasis, but with no significant effect on surrounding cells.4 However, later work showed that exosomes functioned as both garbage trucks and delivery trucks.
Although almost all types of cells can secrete exosomes, the contents of the exosomes vary depending upon the cell type and physiological state through a rigorous sorting mechanism that reflects the composition of the parental cells. Exosomes are widespread in various body fluids and carry important biological information. They can act as signaling complexes to directly stimulate target cells. Moreover, exosomes can also function as bridges to transfer receptors and functional proteins, or to transmit genetic information that alters the phenotype of the receiving cell, thereby representing a new pattern of information transmission systems between cells.5 Exosomes significantly alter cellular behavior and are closely linked to the occurrence and development of a variety of diseases.
Exosomes derived from different cell types are potentially involved in the immune response, antigen presentation, cell migration, cell differentiation, construction of the tumor microenvironment, tumor invasion and a number of other processes.6 These functions of exosomes indicate that they could have great potential value for clinical applications. Using tumors as an example, exosomes derived from cancer cells can be used as biomarkers to assist with diagnosis,7, 8, 9 as therapeutic targets for intervention,10, 11, 12 and potentially as a cancer vaccine to induce active immunity in the body.13,14 Exosomes derived from immune cells can be used directly as drugs for cancer treatment.15,16 In addition, following specific modification, engineered exosomes can also be used as drug delivery carriers for individualized targeted therapy.17,18 Although many research findings have been published in the field of exosomes over the years, the molecular mechanisms that regulate exosome secretion are still poorly understood. A better understanding of the mechanism of exosome biogenesis and secretion and of their role in tumor progression will therefore help to open a new era in cancer diagnosis and treatment.
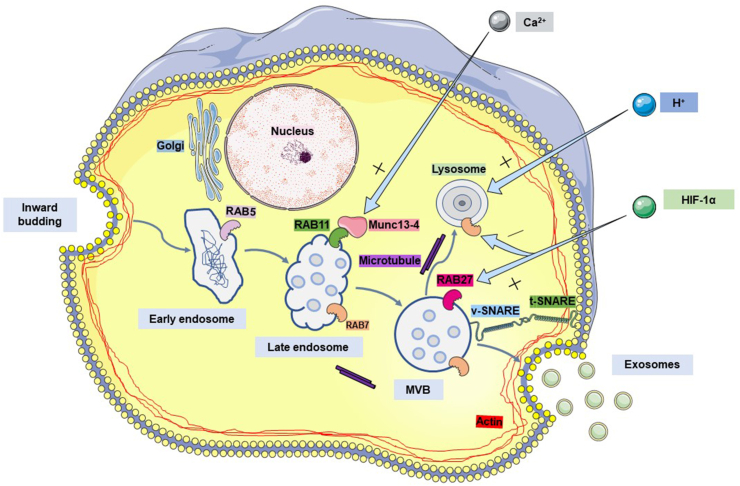
Accumulating evidence shows that exosomes are generated from invaginating buds on the surface of cell membranes. Extracellular proteins, lipids, metabolites and cell membrane proteins are then endocytosed into the cell (internalized) to form primary endocytic vesicles. They fuse with each other to form early endosomes (EEs),19 which subsequently mature and transform into late endosomes (LEs). At this stage, LEs have multiple membrane invaginations that encapsulate specific sorted proteins and nucleic acids to form intraluminal vesicles (ILVs) before these in turn develop into multivesicular bodies (MVBs). The fate of MVBs varies according to the proteins that are expressed on their surface. Some function as “delivery trucks” and are transported to the plasma membrane under the traction of intracellular molecular motors where they fuse with the plasma membrane and release ILVs to the cell exterior. The released ILVs are termed exosomes.20 Other MVBs function as “garbage trucks” by guiding their contents to the lysosomes for degradation and removal (Fig. 1).6,21
Figure 1.
The process of exosome generation and release by cells. As the cell membrane invaginates, some extracellular lipids and proteins are internalized into the cell to form early endosomes. The early endosomes further mature and transform into late endosomes. At this step, multiple membrane invaginations occur in late endosomes to package specifically sorted proteins, nucleic acids and other cargo, resulting in the formation of intraluminal vesicles (ILVs) which later become multivesicular bodies (MVBs). Most MVBs fuse with the plasma membrane and release ILVs to the cell exterior, where they are termed exosomes. A small proportion of MVBs are transported to the lysosome and fuse with it for degradation. RAB family proteins, SNARE tethering proteins and cytoskeletal proteins are all involved in the transport, docking and fusion of MVBs during this process. Conditions in the cellular microenvironment such as the Ca2+ concentration, pH and oxygen concentration are also intimately involved in MVB production and secretion.
The secretion of MVBs outside the cell to form exosomes has three key steps: targeted transport of MVBs, docking of MVBs to the plasma membrane, and fusion of the MVB restriction membrane with the plasma membrane. The correct operation of this process depends on the MVB surface proteins. These proteins are specifically recognized and bound by the receptor on the target membrane, with the whole process functioning as a conveyor belt to deliver the MVB to its correct destination. Many studies have revealed that the RAB GTPase protein family, tethering factors, soluble N-ethylmaleimide-sensitive fusion attachment protein receptor (SNARE) protein family and cytoskeletal proteins are all involved in these regulatory processes. Cytoskeletal proteins provide dynamic support during transport, while SNARE proteins mediate the docking and fusion of MNBs with the plasma membrane. Different members of the RAB family of proteins have different subcellular localizations in the cell. They not only mediate transport to the plasma membrane but also coordinate all of the steps listed above to ensure the accuracy of MVB membrane transport and membrane fusion.
RAB GTPase coordinates the transport of MVBs and their docking and fusion with the plasma membrane
According to previous studies, members of the RAB GTPase family each have their own unique subcellular localizations.22 Following activation, RAB proteins are associated with the membranes of specific organelles. This allows them to have roles at different steps of the membrane transport process, including vesicle formation, vesicle movement and membrane fusion,23 as well as to ensure the correct direction of vesicle transportation. The main RAB GTPases that have been shown to be involved in the production and secretion of exosomes are RAB7, RAB11, RAB27, and RAB35. In addition, although RAB2B, RAB5, and RAB9A also play important roles in the generation of exosomes, but will not be discussed here as they are involved in the early formation of MVBs.
The RAB family of proteins is comprised of small GTPases that belong to the RAS superfamily. They play a key role in various membrane transport events as molecular switches. The RAB protein is in an inactive state when it binds GDP in the cytoplasm, and in an activated state when it binds GTP on the cell membrane and vesicle membrane.24 The RAB protein effectively cycles between these two conformations through a process involving the coordination and cooperation of several functional proteins.
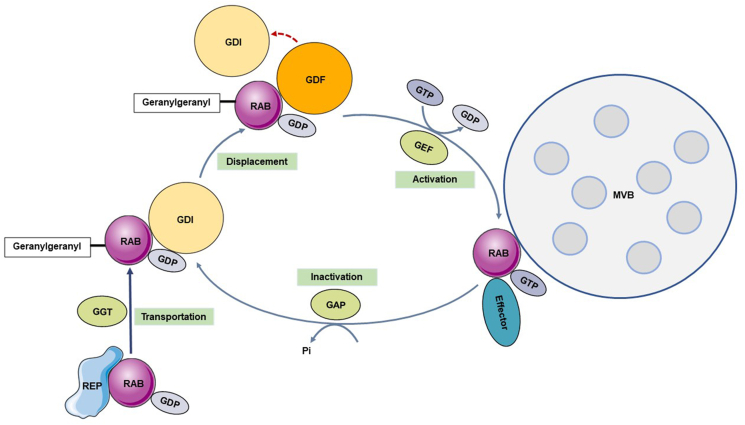
RAB escort proteins (REPs) capture and transport newly synthesized RAB to RAB geranylgeranyl transferase (RGGTase) in this cycle to add hydrophobic geranylgeranyl groups that enable reversible binding with the target membrane.25 Guanine nucleotide exchange factors (GEFs) exchange GDP bound to RAB with GTP,26 activate RAB attached to the target membrane, and enable RAB to further selectively recruit specific effector proteins to complete their functions. GTPase-activating proteins (GAPs) catalyze the activation of GTPase, hydrolyze the GTP bound to RAB, and convert it into an inactive form bound to GDP.27 At this time, GDP dissociation inhibitor (GDI) transports inactivated RAB from the target membrane28 and stores it in the cytoplasm in an inactive form. The next time it is needed, GDI presents it to the target membrane again. Because GDI has a relatively higher affinity for RAB, the latter must be dissociated from GDI with the help of GDI displacement factors (GDFs) so that it can bind to the target membrane (Fig. 2).23,29
Figure 2.
The RAB switch and its dynamic cycling model. The newly synthesized RAB in the cytoplasm is captured by RAB escort protein (REP) in a GDP-bound inactive form and transported to RAB geranylgeranyl transferase (RGGTase). Here, hydrophobic geranylgeranyl groups are added, allowing RAB to reversibly bind to the target membrane. GDP dissociation inhibitor (GDI) recognizes and binds to the inactivated form of RAB protein to stably store RAB in the cytoplasm. Upon activation, GDI displacement factors (GDFs) help RAB dissociate from GDI so that it can bind to the target membrane. Meanwhile, GDP is replaced with GTP by guanine nucleotide exchange factors (GEFs), which then activate RAB protein attached to the target membrane. After this step is completed, RAB recruits GTPase-activating proteins (GAPs) to hydrolyze GTP and convert the protein to an inactive form.
RAB27, mainly RAB27A, mediates the docking, tethering and fusion of MVBs with the plasma membrane
The central role of RAB27 in exosomal secretion has been widely confirmed30, 31, 32 and hence this RAB protein is currently the subject of intense investigation. RAB27 includes RAB27A and RAB27B encoded by two different genes. Their amino acid sequences share 71% homology,33 and they use the same effector proteins.34 The RAB27A and RAB27B subtypes sometimes have repetitive and redundant functions in their regulation of the secretion pathway. Surprisingly, however, RAB27A and RAB27B play different roles in exosome secretion even in the same cell type, which may be due to their interactions with different effector proteins.
The effectors of RAB27 discovered so far are divided into three categories35,36: synaptotagmin-like protein (Slp),37 Slp homolog lacking C2 domains (Slac2)37, 38, 39 and Munc13–4.40 A previous study found that silencing of RAB27A and RAB27B in the cervical cancer cell line HeLa significantly inhibited exosome secretion.41 The difference is that RAB27A is mainly located in CD63-positive MVBs where it mediates docking, tethering, and fusion between MVBs and the plasma membrane by interacting with the effector protein Slp4 instead of mediating docking and fusion between small vesicles to form an enlarged MVB. At the same time, RAB27B is mainly distributed around the nucleus in the trans-Golgi network (TGN) region, with a small amount of it is located in the CD63-positive compartments. Binding of RAB27B to the Slac2b effector protein likely mediates the directed transfer of MVBs along microtubules to the actin-rich cortex and its docking at the periphery of the cell. However, the same experiment revealed that the inhibition of RAB7 or RAB11A had no effect on the secretion of exosomes from HeLa cells.41
In a mouse model, the role of RAB27A in the secretion of exosomes from breast cancer cells was similar to that observed in HeLa cells. In contrast to HeLa cells, the secretion of exosomes by breast cancer cells does not always require RAB27B.42 In addition, the function of RAB27A in promoting the release of exosomes has also been successively verified in melanoma cells,30 bladder cancer cells,43 lung adenocarcinoma cells44 and cortical neuronal cells.45 RAB27B is downregulated by FOXO1 when diabetes occurs, thereby reducing the exosomal secretion from mouse proximal tubule (BUMPT) cells.46 LINC00511, which is highly expressed in hepatocellular carcinoma (HCC) and upregulates the expression of RAB27B, also induces the colocalization of RAB27B and CD63+ MVBs, thereby significantly increasing the secretion of exosomes by HCC cells.47 It has been speculated that different types of cells have different physiological processes, thus leading to different mechanisms of exosome secretion.
RAB11 transports recycling endosomes or MVBs to the peripheral plasma membrane
RAB11 is mainly located on recycling endosomes and promotes their formation and subsequent fusion with the plasma membrane.48,49 Rip11 binds to RAB11 and recruits Myo V to assemble a ternary complex that is transported along polarized actin filaments of the subcortical terminal net to target the peripheral plasma membrane of the cell.50 Munc13–4 (calcium ion-dependent RAB binding protein, encoded by UNC13D) is a calcium ion-dependent RAB effector protein that interacts with RAB27,51,52 RAB11 53 and other RAB proteins. The tethering or docking of vesicles in the extracellular active region is regulated by bridging RAB11 on the vesicle membranes with SNAREs on the plasma membrane, thus reducing their transport speed and mediating their participation in plasma membrane fusion.53 In MDA-MB-231 breast cancer cells, RAB11A participates in the intracellular transport of MVBs by interacting with Munc13–4. It also plays a role in the release of exosomes after stimulation by calcium ions. Similar results have been observed in human lung cancer A549 cells and human pancreatic cancer PANC-1 cells.54
RAB35 regulates the docking or tethering of MVBs to the plasma membrane
RAB35 was the first RAB protein to be discovered. TBC1D10A–C is the GAP for RAB35, which inhibits the activity of RAB35 by hydrolyzing GTP. Inhibition of RAB35 bound to the surface of Oli-neu oligodendrocytes causes endosomes to accumulate in the cell and reduces the secretion of exosomes, suggesting that active RAB35 may promote the docking and tethering of MVBs to the plasma membrane.55 It was later confirmed that RAB35 is specifically involved in exosome secretion in primary oligodendrocytes56 and brain cells.57 Furthermore, the highly expressed lncRNA HOTAIR regulates the transport and docking of MVBs to the plasma membrane of liver cancer cells by regulating RAB35 expression.58
To date, many interesting findings have been made that help to explain how RAB proteins are involved in exosome secretion. However, more comprehensive and in-depth research work is needed to fully understand the exact contributions of RAB protein family members in the biogenesis of exosomes.
RAB7 guides late endosomes to fuse with lysosomes for degradation
RAB7 is mainly responsible for coordinating traffic between late endosomes and lysosomes.59 It relies on a more complicated mechanism to direct MVBs carrying some cargo considered as garbage to lysosomes for degradation, thus avoiding unwanted side effects. Lysosomes are the main sites for degradation in cells and they are rich in hydrolytic enzymes that hydrolyze biomolecules into metabolites.60 Following the transport of MVBs to a certain location, their fate must be decided as either storage, to play a specific role, or to be metabolized by degradation pathways. Lysosomes are important organelles that regulate the secretion of exosomes. The number of exosomes released into the extracellular space is inversely correlated with the number of late endosomes degraded by the lysosomal pathway. In other words, more degradation of late endosomes by lysosomes results in fewer exosomes being released from cells.
Two lysosome degradation pathways have been identified. MVBs are sometimes not directly transported to the lysosome but instead fuse with the autophagosomes before being transported to the lysosome for degradation. Autophagosomes are organelles that phagocytose cytoplasmic proteins, transport them from autophagosomes to lysosomes, and then fuse with the lysosomes to form autophagolysosomes that degrade the contents they encapsulate. This was confirmed by the observation that the enhanced autophagy induced by starvation reduces the release of exosomes.61 In addition, RAB2 has been shown to play a key regulatory role in the fusion of late endosomes with lysosomes,62 as well as the fusion of autophagosomes with lysosomes in Drosophila.63 However, the regulatory mechanism responsible for balancing the secretion and degradation of MVBs remains unclear.
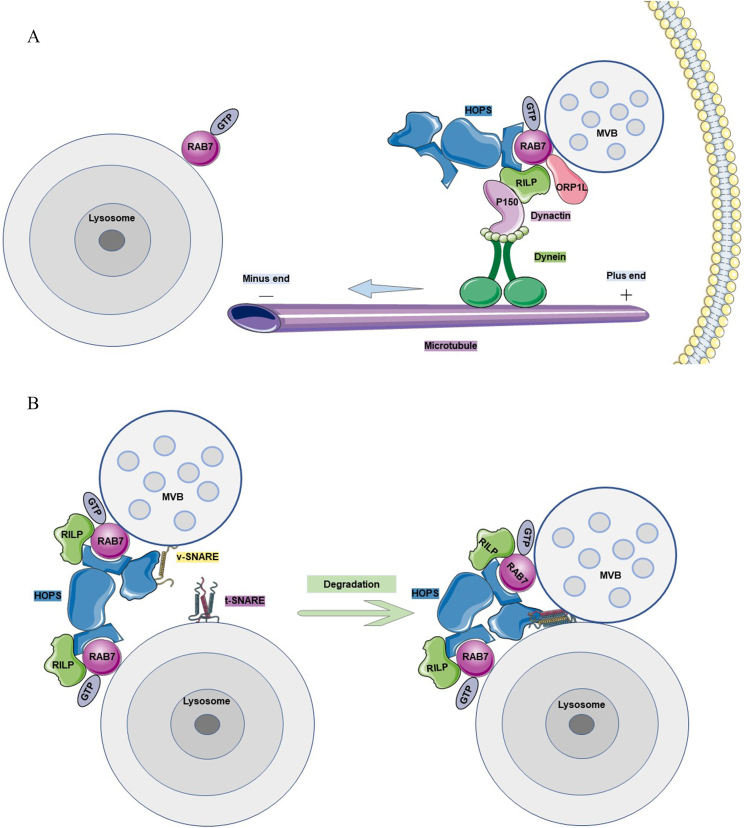
The tethered complex HOPS (homotypic fusion and protein sorting complex) is recruited by the RAB7 GEF RILP (RAB7-interacting lysosomal protein)64 and interacts with RAB7 to activate it to RAB7-GTP. Through the synergistic effect of RAB7 expressed on the late endosome and lysosomal membranes, the v-SNAREs and t-SNAREs with fusion functions are bridged and assembled by the HOPS-RAB7-RILP complex between the adjacent membranes. The “zipper” complex that triggers membrane fusion creates a double membrane opening that mediates the fusion of late endosomes with lysosomes.65,66 For example, the v-SNARE VAMP7 mediates the fusion of the MVB restriction membrane with the plasma membrane. VAMP7 has also been suggested to play a key role in the fusion between late endosomes and lysosomes. Overexpression of the Longin domain in VAMP7 prevents this fusion event from occurring (Fig. 3B).67
Figure 3.
The lysosomal degradation pathway of MVBs. (A) The microtubule system transports MVBs to the lysosome. The motor protein dynein at the plus end of the microtubule interacts with its regulatory factor, dynactin. At the same time, the p150Glued subunit of dynactin forms a complex with RILP, RAB7 and cholesterol-sensitive protein ORP1L in order to transport MVBs to the lysosomes located at the minus end of the microtubule. (B) The HOPS complex participates in the fusion of MVBs with lysosomes. The HOPS complex includes two RAB binding regions and a SNARE binding region. With the assistance of RILP, it can bind to RAB7 on both the MVBs and lysosomes and bring v-SNARE on the MVBs close to t-SNARE on the lysosomes. Once the two SNAREs are tightly tethered, MVBs fuse with the lysosome and are subsequently degraded.
The EPG5 protein is a RAB7 effector involved in the autophagy-lysosomal degradation pathway. The specific tethering function of EPG5 suggests that it functions as a fishing net to combine autophagosomes with RAB7-expressing late endosomes/lysosomes. EPG5 recognizes and captures autophagosomes and late endosomes/lysosomes by simultaneously binding the specific proteins LC3/LGG-1 and RAB7, respectively. Then, the R-SNARE VAMP7/8 on late endosomes/lysosomes and the STX17-SNAP29-Qabc-SNARE complex assembled on autophagosomes are connected in series by EPG5. This promotes the assembly and stabilization of the trans-SNARE complex STX17-SNAP29-VAMP7/8, ultimately causing the three membranes to fuse with each other and promote degradation.68
Cytoskeletal proteins drive MVB movement and exocytosis
If the RAB proteins control the direction of MVB movement like a steering wheel, then the cytoskeletal proteins are responsible for providing the power for MVB movement like an engine. The movement of intracellular vesicles is mediated by cytoskeletal structures such as actin or microtubules and their associated molecular motors to ensure the direction and effectiveness of vesicle transport. The cytoskeleton is the protein fiber network system in eukaryotic cells responsible for support, movement, and transportation. RAB GTPases adjust the short- and long-distance movements of vesicles by recruiting motion adapters or by directly interacting with motors along actin filaments or microtubules, respectively.69,70 However, although scholars have obtained some knowledge, the specific mechanisms used by cytoskeletal proteins in membrane transport are not yet well understood.
Actin coordinates the directional transport of vesicles and the occurrence of membrane fusion events
Actin filaments polymerize beneath the plasma membrane to form a dense network structure, with cortical actin blocking MVBs at the edge of the cell. Therefore, polymerized actin must be dynamically reshaped or cut to allow the MVBs that reach the inner wall of the plasma membrane to fuse and be released from the cell.71 On the other hand, the actin network structure is also thought to promote vesicle docking.72 Therefore, the occurrence of a complete membrane fusion event is thought to require the dynamic disassembly and reassembly of cortical actin.
Rho GTPase family proteins, including key regulators of the actin cytoskeleton, play an important role in the rapid remodeling and contraction of actin.73,74 ERM (Ezrin, Radixin, and Moesin) protein acts as a scaffold protein. Through indirect regulation by the Rho protein, the carboxyl terminus of ERM interacts with F-actin (filamentous actin) to anchor monomeric actin filaments to the plasma membrane in preparation for the polymerization and rearrangement of actin.75 WASP (Wiskott-Aldrich syndrome protein) can be activated as a downstream molecule of Rho GTPases such as Rac and Cdc42. Arp2/3 (actin-related protein 2/3) complexes and actin monomers then directly regulate actin polymerization by binding to the common domain of the carboxyl terminus of the activated WASP family, respectively.76 Interestingly, Cdc42 appears to participate only in the extracellular shedding of larger diameter microvesicles (MVs) (>200 nm) and does not play a role in the release of smaller exosomes.77 The Arp2/3 complex is the core of actin assembly and helps to synthesizes and assembles actin monomers into actin filaments, thereby promoting the formation of filopodia78 and lamellipodia.79 In addition, a special actin-rich subcellular structure, invadopodia, mediates the hydrolysis of matrix proteins and is the specific and key docking and secretion site for CD63-and RAB27A-positive MVBs. Inhibiting or inducing the formation of invadopodia reduces or increases, respectively, the concentration of exosomes released into the medium.80 Branched actin proteins assembled on the cytoplasmic side of the cell membrane serve as docking sites for receiving MVBs transported from a distance. Their quantity and quality directly affects the secretion of exosomes without changing the exosomal loading of cargo. The actin-binding protein cortactin binds to the branched actin located in the cortex and stabilizes its structure, thereby increasing the number of docking sites for MVBs and the stability of exosome secretion.81
Actin also acts in another way that is closely related to its molecular motor function. The myosin V family is a recognized actin motor that specifically attaches to intracellular vesicles in a RAB-dependent manner and drives the vesicles to use actin cables as tracks for directional transport.82,83 A classic and important model for this is RAB27A binding to melanosomes, thereby recruiting melanophilin to the melanosome membrane. Melanophilin then binds to myosin Va and forms a complex that directs melanosomes to the plasma membrane at the periphery of the cell where exocytosis occurs.84
Microtubules drive endosomes for long-distance bidirectional movement
Microtubules are similar to well-built two-way highways, while endosomes enclosed in a lipid bilayer membrane are similar to speeding cars. Endosomes move along the microtubules through the traction of two molecular motors, kinesins and dyneins. Kinesins move toward the positive end of the microtubule that extends to the periphery of the cell,85 while cytoplasmic dyneins move toward the negative end anchored in the center of the microtubule tissue.86 RAB GTPases can adjust movement in any direction along the microtubule by interacting with these two types of microtubule motors.87 The activation of dynein-related movements depends upon their interaction with microtubule-positive end proteins (+TIP proteins), including EB1, EB3, and CLIP-170.
RILP and oxysterol-binding protein-related protein 1L (ORP1L) interact with active RAB7 located in late endosomes and lysosomes to form a ternary complex.88,89 The CAP-Gly region of p150Glued, a subunit of dynactin, interacts with CLIP-17090 or directly with microtubules91 to recruit dynactin to the positive end of microtubules, thereby recruiting and activating dynein.92 Subsequently, cholesterol-sensitive ORP1L transports the RAB7-RILP complex to βIII spectrin (a dynein receptor) on the endosome in the presence of high cholesterol concentrations and binds to dynein to induce the dynein-dynactin complex. This initiates the movements of the late endosome toward the negative end of the microtubule, eventually transporting these late endosomes to the centrosome and lysosome (Fig. 3A).88 ORP1L changes its conformation in the presence of low cholesterol concentrations, thereby exposing the FFAT motif and interacting with the ER protein VAP-A. VAP-A removes the dynein motor from the RAB7-RILP complex, at which time the endosome relocates to the cell periphery.93
SNARE mediates the fusion of MVBs with the plasma membrane in the final step of secretion
The final and critical step for the release of MVBs into the extracellular space to form exosomes is the fusion of the MVB restriction membrane with the cell membrane. This process has been proven by multiple scientists to be dependent on the SNARE protein family. SNARE proteins include the SNAP receptor protein located on the vesicle membrane (vesicle-SNARE, v-SNARE) and the SNAP receptor protein located on the target membrane (target-SNARE, t-SNARE). These mediate specific recognition and fusion of the transport vesicle membrane with the target membrane. v-SNAREs determine the direction of transport and specifically recognize and interact with t-SNAREs to form a complex so that the vesicles can attach to the cell membrane, thus ensuring the accuracy of vesicle transport.
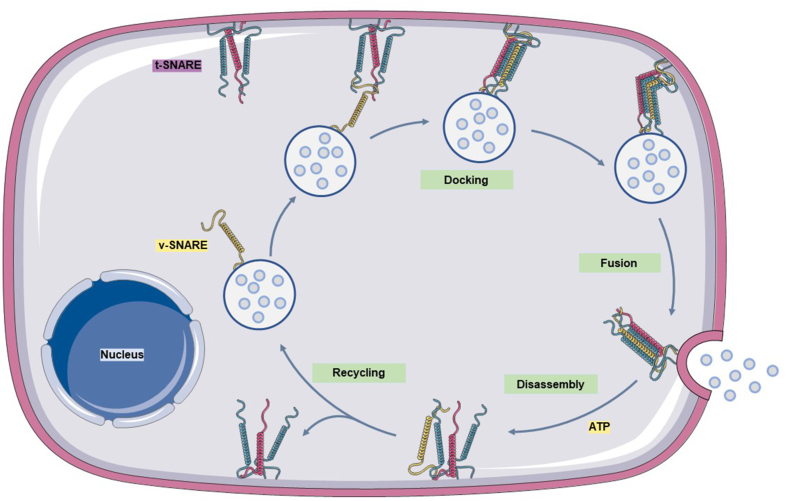
A credible SNARE membrane fusion mechanism has been proposed whereby a v-SNARE from the vesicle membrane and three t-SNAREs from the target membrane are brought into close proximity through traction of the tethering factor.94,95 Cys amino acid residues interact and assemble into a very stable, four-stranded, helical bundle transmembrane complex. The two membranes in contact with each other and gradually fuse based on the bridge provided by the SNARE complex,96,97 opening a path for the release of the vesicle contents. Once this process is complete, the SNARE complex is disassembled in an energy-consuming manner and with the participation of ATPase. Notably, the v-SNAREs that were originally exist on the vesicle have now been transferred to the target membrane through membrane fusion. If these proteins are to function again, they must first be recycled to the vesicle membrane in readiness for the next fusion event (Fig. 4).
Figure 4.
SNARE assembly and disassembly mediate the fusion of MVBs with the cell membrane. One v-SNARE located on the vesicle membrane and three t-SNAREs located on the target membrane are tightly tethered to each other as they approach and assemble into a stable, four-stranded helical bundle transmembrane complex. This further brings the two membranes into contact and promotes fusion, thus releasing the particles in the MVBs from the cells. After this step is complete, the SNARE complex remains on the fused target membrane and is disassembled into monomeric proteins. In addition, the v-SNARE only continues to function after it is recycled to the vesicle membrane.
Many studies have been conducted on the membrane fusion function of SNARE family members during exosome secretion. Vesicle-associated membrane protein (VAMP) is a typical v-SNARE, with VAMP2, VAMP3, VAMP7, and VAMP8 all involved in cell exocytosis. Their roles in exosome secretion are currently under investigation. VAMP2 interacts with syntaxin4 to form a fusion complex that promotes oligodendrocytes to secrete microvesicles similar in size to exosomes.98 In neuronal cells with high-frequency electrophysiological activity, the abundance of VAMP3 (cellubrevin) is upregulated by basic fibroblast growth factor (bFGF), which indirectly increases the frequency of fusion events between MVBs and the plasma membrane and demonstrates that exosome secretion is induced by electrical activity.99 The v-SNARE protein VAMP7 is present in late endosomes and is required for MVBs to fuse with the plasma membrane and release exosomes in leukemia K562 cells.100 YKT6 is also a v-SNARE protein and has been shown to promote the secretion of exosomes specifically loaded with Wnt signaling proteins in HEK293 cells.101 Similarly, both YKT6 gene knockdown and the use of YKT6 inhibitors significantly reduced the number of exosomes secreted by A549 lung cancer cells.102 YKT6 also mediates the fusion of MVBs with the plasma membrane in pancreatic cancer cells through its own palmitoylation and colocalization with VAMP3. This event may be regulated by the lncRNA PVT1.103
On the other hand, t-SNARE proteins include the syntaxin family and the SNAP25 (synaptosome-related protein with a relative molecular mass of 25 kDa) family. In Drosophila S2 cells, Syntaxin1A was shown to be necessary for the secretion of evenness interrupted (Evi) - exosomes.104 pH-sensitive optical reporters connected to tetraspanin have been designed to intuitively observe the process of docking and fusion of MVBs with the plasma membrane (PM) under live total internal reflection fluorescence (TIRF) and dynamic correlative light-electron microscopy. Depletion of Syntaxin4 in the cells was observed to reduce the frequency of MVB-PM fusion.105 Experiments with Caenorhabditis elegans showed that Syntaxin5 is activated or recruited by the GTPase RAL-1 located on the MVB membrane and directs MVBs to dock and fuse with the plasma membrane.106 Syntaxin6 and CD63 are colocalized in prostate cancer cells, with knockdown of the Syntaxin6 gene observed to significantly reduce the number of exosomes secreted by cells, which is related to the loss in ability of Syntaxin6 to form SNARE fusion complexes.107 Another study showed that SNAP23 is phosphorylated by pyruvate kinase type M2 (PKM2)108 or histamine H1 receptor (H1HR)105 and then mediates MVB membrane fusion and exosome release. A similar event was observed after knocking down Syntaxin19 and SNAP29 in HeLa cells, i.e., the number of vesicles that fused with the plasma membrane was significantly reduced. In addition, the loss of SNAP29 resulted in the accumulation of vesicles docked under the cell membrane, providing further evidence that membrane fusion was blocked.109 Together, these findings indicate that SNARE proteins are the main contributors to membrane fusion events. However, more in-depth studies are needed to gain a better understanding of the mechanism of SNARE's action.
The extracellular microenvironment induces exosome secretion
The above discussion relates to the internal cellular changes that affect the secretion of exosomes. The external factors that affect exosome secretion will now be reviewed below. Exosomes secreted by cells carry and transmit signaling molecules to change the cellular microenvironment.110 Conversely, changes in the cellular microenvironment also induce exosome secretion to protect cells from stress responses caused by various endogenous and exogenous stimuli.111 Abnormal stress responses are the basis for various pathological conditions, including cancer. The occurrence and development of cancer are closely related to the state of the tumor microenvironment. The components of the tumor microenvironment are complex and comprise a small ecological niche that includes tumor cells, fibroblasts, endothelial cells, immune cells and mesenchymal stem cells, as well as cytokines, chemokines, and various other proteins.112 As tumors progress, the tumor cells compete with normal cells for nutrients, oxygen, and growth factors. Under this pressure, the stress response mechanism of tumor cells is activated to promote further tumor progression. Exosomes represent a new form of intercellular communication that is activated under stress. The final destination of MVBs for either degradation or secretion depends on the dynamic balance of cells. Tumor cells protect themselves from stress by secreting exosomes.113, 114, 115, 116
The hypoxic state of diseases can stimulate exosome secretion
Hypoxia is an important feature of solid tumors and is considered to be one of the factors that triggers the secretion of exosomes.117 The number of exosomes secreted by breast cancer cells increases in a hypoxic environment. This process is mediated by hypoxia-inducible factor-1α (HIF-1α) activation of hypoxia signaling through the oxygen-sensing pathway. Related miRNAs, especially miR-210, are loaded into exosomes and promote the survival and invasive ability of breast cancer cells.118 Dorayappan et al. found that when exposed to a hypoxic environment, ovarian cancer cells increased the release of exosomes by upregulating RAB27A and downregulating RAB7, LAMP1/2, and neu-1, thus inducing a secretory lysosomal phenotype. However, knockout of the oncogene STAT3 (signal transducer and activator of transcription factor 3) under hypoxic conditions increases the degradation of MVBs within lysosomes and reduces the docking of late endosomes to the plasma membrane. This subsequently reduces the release of exosomes from ovarian cancer cells through increased levels of RAB7, a key protein in lysosome fusion, and decreased levels of RAB27A, a regulatory factor that mediates the docking of late endosomes to the plasma membrane.119
Recently, the hypoxic stress of oral squamous cell carcinoma (OSCC) caused by the tumor microenvironment was shown to affect the anti- and pro-tumor properties of γδT cells by altering the number of exosomes released by cancer cells, which in turn regulated the function of marrow-derived suppressor cells (MDSCs) through the miR-21/PTEN/PD-L1 axis.120 Hypoxia has also been found to stimulate the release of exosomes from hypoxic breast cancer-associated fibroblasts (CAFs) to promote cancer cell invasion.121 Based on these results, a hypoxic microenvironment can increase the secretion of tumor cell-derived exosomes and change the tumor microenvironment.
Exosomes can biogenerate in a calcium-dependent manner
Tumor progression and metastasis are often accompanied by an increased level of intracellular calcium ions (Ca2+), which then acts as an intracellular signal to induce regulated secretion from most cell types. Monensin (MON) is a Na+/H+ exchanger that increases the intracellular Ca2+ concentration through the Na+/Ca2+ reverse transport protein. Savina et al. found that treatment of K562 cells with MON stimulates exosome secretion, and that Ca2+ chelating agents completely eliminated the MON-induced exosome secretion. Similar results were obtained with chloroquine and bafilomycin. Transferrin (TF) was also found to be involved in the secretion of exosomes from K562 cells in a calcium-dependent manner and as a physiological stimulus.122 According to recent studies, the calcium-dependent SNAP receptor and RAB-binding protein Munc13–4 is expressed at high levels in various invasive carcinomas, including lung cancer, breast cancer and pancreatic cancer, which promotes the maturation of MVBs. Meanwhile, Munc13–4 binds to RAB11 in a Ca2+-dependent manner, leading to increased secretion of exosomes by cancer cells.123 Knockout of Munc13–4 reduces the secretion of exosomes carrying the MT1-MMP enzyme by breast cancer cells, thereby reducing their ability to destroy the extracellular matrix.54
Increased glycolysis under hypoxia promotes the secretion of exosomes
Numerous studies have shown that changes in cell metabolism are a core feature of tumors. Compared with normal cells, tumor cells secrete more exosomes. Moreover, the quantity of exosomes secreted by liver cancer and lung cancer cells are positively correlated with aerobic glycolysis. As mentioned above, the level of PKM2 and its phosphorylation levels are increased in tumor cells, thus promoting glucose uptake and lactic acid production. Phosphorylated PKM2 is a protein kinase that phosphorylates the Ser95 residue of SNAP23, a component of the SNARE complex, thereby positively regulating exosome secretion from tumor cells.108 The inhibitory effect of shikonin on glycolysis reduces the release of exosomes, while tumor necrosis factor alpha (TNF-α)-induced glycolysis increases exosome secretion.108 The latest research shows that after the uptake by cancer cells of exosomes secreted by CAFs, their metabolism is reprogrammed to simulate the occurrence of a hypoxic environment that regulates exosome secretion, as evidenced by hyperactive glycolysis and decreased mitochondrial oxidative phosphorylation.124 This result indicates a close relationship between cell metabolism and exosome secretion.
Low intracellular pH promotes the acidification and degradation of MVBs
The accumulation of lactic acid in the extracellular environment is mainly caused by glycolysis and hypoxia, which induce and maintain the typical acidic microenvironment of tumor cells. This acidification is due to the activation of multiple ion channels on the restriction membrane, and the local pH stepwise reduction in pH is a necessary condition for the normal operation of the entire endosomal transport pathway.125 In melanoma cells, an acidic pH increases exosome secretion,126 while alkaline conditions significantly decrease exosome secretion.127 Although an acidic environment has been confirmed to increase the release of exosomes, studies have also shown that autophagy-related genes (Atg), including Atg5 and Atg16L1, destroy the active structure of V-ATPase (vacuolar-type ATPase). This inhibits its proton pump activity and prevents the acidification of MVBs that produce exosomes, thereby reducing their degradation due to fusion with autophagolysosomes and ultimately increasing exosome secretion.128 Similar results were also reported in another study in which storage in an acidic solution (pH = 4.0) reduced the concentration of exosomes. This may be related to the degradation of exosome proteins and to the reduced long-term stability of exosomes in acidic environments, which may subsequently affect their physiological functions.129
In addition to the aforementioned factors that affect the cellular microenvironment, the absence of nutrient and growth factors may also cause cells to secrete more exosomes.130 Tumor cells with DNA damage due to chemotherapy131,132 or radiation exposure133 also secrete more exosomes, thereby promoting the metastasis of cancer cells.
Pathogenic effects of exosomes
The role of exosomes can often be seen everywhere in the occurrence and development of many human diseases. As “delivery trucks”, exosomes are loaded with a large number of cell type-specific bioactive components. Exosomes from different cell sources are spread throughout the body via circulation of body fluids and are taken up by target cells to produce intercellular crosstalk, causing different biological effects.
In general, there are two pathogenic modes of exosomes. The first is that the increased secretion of exosomes enhances the delivery of pathogenic or stimulating biologically active substances to healthy cells. Increased secretion of exosomes with the same contents can maintain the overstimulation of recipient cells. For example, a high-fat diet can trigger the secretion of more exosomes from white adipose tissue to enhance communication with liver tissue. The enrichment of CD36 in these exosomes increases hepatic fat uptake, ultimately leading to fat deposits in the liver and the occurrence of nonalcoholic fatty liver disease.134 Tubular epithelial cells (NRK-52E) stimulated by TGF-β1 show a marked increase in exosome secretion whereby encapsulated miR-21 is transported to fibroblasts and leads to their activation via the PTEN/Akt pathway, thereby aggravating renal fibrosis.135 Similarly, the release of exosomes from HIV-infected cells is increased following stimulation by the HIV accessory protein negative factor (Nef), which rides on these exosomes to target CD4+ T cells, causing their apoptosis and ultimately triggering immunodeficiency in the body.136 Furthermore, when lysosomal dysfunction occurs, α-synuclein-enriched MVBs in neuronal and glial cells cannot be degraded through the normal lysosomal pathway. Instead, they spread to nearby neurons via the secretion of exosomes, resulting in the neurodegenerative disorder of Parkinson's disease (PD).137 Tumor cells are known to have a higher capacity for exosome secretion than any other cell type. The exosomes derived from tumor cells are loaded with a large number of bioactive factors that are advantageous for tumor development. Upon interaction with suitable target cells, they can produce effects such as pro-angiogenesis,138,139 pro-invasion and metastasis,140,141 and pro-immune suppression,142,143 thus increasing the malignant potential.
The second pathogenic mode for exosomes is that reduced secretion can weaken the delivery of protective, biologically active substances to healthy cells. CD8+ T regulatory (Treg) cells actively perform immunosuppressive functions under physiological conditions. When exosome secretion from CD8+ Treg cells is blocked under pathological conditions, NADPH oxidase 2 (NOX2) secreted out of the cell through the exosome endocytosis pathway is blocked in the intercellular compartment. Hence, the CD8+ Treg cells lose their ability to inhibit the activation of surrounding CD4+ T cells. As a consequence, they cannot effectively defend against the inflammatory attack of CD4+ T cells on the aorta and large conductive arteries, resulting in autoimmune giant cell arteritis (GCA).49
Conclusions and significance
In summary, exosomes clearly play important roles in both physiological and pathological conditions. The future development of methods to block the release of exosomes and/or interfere with exosome-mediated communication between cells will therefore be very important. These strategies may be promoted as extremely effective and less traumatic treatments for various diseases. Over the past thirty years, a large number of studies on exosomes have provided more profound, more detailed and more realistic information on the multiple regulatory mechanisms of exosome secretion. However, although some of the relevant regulatory mechanisms are increasingly well understood, the exact mechanisms of action of many regulatory molecules are still not entirely clear. Moreover, it is not fully understood how different MVB subtypes produced by different types of cells can shunt to different secretory pathways. Much more research is needed to explore the mechanism of exosome secretion, to gain a better understanding of the potential use of exosomes, and to translate the results of experimental research into improved capabilities of clinical treatments.
Author contributions
Minxue Xu and Mingbing Xiao: Conceptualization. Mingbing Xiao, Mei Li and Weisong Xu: Supervision and Project administration. Minxue Xu: Writing - Original Draft. Minxue Xu, Jie Ji and Dandan Jin: Visualization. Jie Ji, Dandan Jin, Yue Wu and Yifei Ji: Investigation and Writing- Reviewing and Editing. Tong Wu, Renjie Lin, Shengze Zhu, Feng Jiang and Baijun Bao: Writing- Reviewing and Editing.
All appropriate contributors are listed as authors and all authors have agreed to the manuscript's content and its submission to Genes and Diseases.
Conflict of interests
The authors declare that they have no competing interests.
Funding
This study was supported by grants from Natural Science Foundation of Jiangsu Province, China (No. BK20211105), the Key Research and Development Plan of Jiangsu Province, China (No. BE2019692), the Health Project of Jiangsu Province, China (No. H2019072), the Social Development Foundation of Nantong City, China (No. MS22020005, JCZ21061, MSZ20076 and JCZ20065), the Postgraduate Research & Practice Innovation Program of Jiangsu Province, China (No. KYCX20_2673, KYCX20_2681 and KYCX21_3112).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Mei Li, Email: 13862901258@163.com.
Weisong Xu, Email: xws71@sina.com.
Mingbing Xiao, Email: xmb73@163.com.
References
- 1.Choi D.S., Kim D.K., Kim Y.K., Gho Y.S. Proteomics of extracellular vesicles: exosomes and ectosomes. Mass Spectrom Rev. 2015;34(4):474–490. doi: 10.1002/mas.21420. [DOI] [PubMed] [Google Scholar]
- 2.Skotland T., Sandvig K., Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res. 2017;66:30–41. doi: 10.1016/j.plipres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 4.Harding C., Heuser J., Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 6.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478) doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen I.H., Xue L., Hsu C.C., et al. Phosphoproteins in extracellular vesicles as candidate markers for breast cancer. Proc Natl Acad Sci U S A. 2017;114(12):3175–3180. doi: 10.1073/pnas.1618088114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu W., Hurley J., Roberts D., et al. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann Oncol. 2021;32(4):466–477. doi: 10.1016/j.annonc.2021.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong H., Huang Z., Yang Z., et al. Recent progress in detection and profiling of cancer cell-derived exosomes. Small. 2021;17(35) doi: 10.1002/smll.202007971. [DOI] [PubMed] [Google Scholar]
- 10.Marleau A.M., Chen C.S., Joyce J.A., Tullis R.H. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. 2012;10:134. doi: 10.1186/1479-5876-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poggio M., Hu T., Pai C.C., et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177(2):414–427. doi: 10.1016/j.cell.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie X., Nie H., Zhou Y., et al. Eliminating blood oncogenic exosomes into the small intestine with aptamer-functionalized nanoparticles. Nat Commun. 2019;10(1):5476. doi: 10.1038/s41467-019-13316-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolfers J., Lozier A., Raposo G., et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7(3):297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 14.Hu S., Ma J., Su C., et al. Engineered exosome-like nanovesicles suppress tumor growth by reprogramming tumor microenvironment and promoting tumor ferroptosis. Acta Biomater. 2021;135:567–581. doi: 10.1016/j.actbio.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Samuel M., Gabrielsson S. Personalized medicine and back-allogeneic exosomes for cancer immunotherapy. J Intern Med. 2021;289(2):138–146. doi: 10.1111/joim.12963. [DOI] [PubMed] [Google Scholar]
- 16.Kang Y.T., Niu Z., Hadlock T., et al. On-chip biogenesis of circulating NK cell-derived exosomes in non-small cell lung cancer exhibits antitumoral activity. Adv Sci. 2021;8(6):2003747. doi: 10.1002/advs.202003747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J., Chen D., Ho E.A. Challenges in the development and establishment of exosome-based drug delivery systems. J Contr Release. 2021;329:894–906. doi: 10.1016/j.jconrel.2020.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Liang Y., Duan L., Lu J., Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huotari J., Helenius A. Endosome maturation. EMBO J. 2011;30(17):3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hessvik N.P., Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chavrier P., Parton R.G., Hauri H.P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62(2):317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- 23.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 24.Homma Y., Hiragi S., Fukuda M. Rab family of small GTPases: an updated view on their regulation and functions. FEBS J. 2021;288(1):36–55. doi: 10.1111/febs.15453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seabra M.C. Nucleotide dependence of Rab geranylgeranylation. Rab escort protein interacts preferentially with GDP-bound Rab. J Biol Chem. 1996;271(24):14398–14404. doi: 10.1074/jbc.271.24.14398. [DOI] [PubMed] [Google Scholar]
- 26.Langemeyer L., Perz A., Kümmel D., Ungermann C. A guanine nucleotide exchange factor (GEF) limits Rab GTPase-driven membrane fusion. J Biol Chem. 2018;293(2):731–739. doi: 10.1074/jbc.M117.812941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan X., Eathiraj S., Munson M., Lambright D.G. TBC-domain GAPs for Rab GTPases accelerate GTP hydrolysis by a dual-finger mechanism. Nature. 2006;442(7100):303–306. doi: 10.1038/nature04847. [DOI] [PubMed] [Google Scholar]
- 28.Ullrich O., Horiuchi H., Bucci C., Zerial M. Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature. 1994;368(6467):157–160. doi: 10.1038/368157a0. [DOI] [PubMed] [Google Scholar]
- 29.Sivars U., Aivazian D., Pfeffer S.R. Yip3 catalyses the dissociation of endosomal Rab-GDI complexes. Nature. 2003;425(6960):856–859. doi: 10.1038/nature02057. [DOI] [PubMed] [Google Scholar]
- 30.Peinado H., Alečković M., Lavotshkin S., et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webber J.P., Spary L.K., Sanders A.J., et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34(3):290–302. doi: 10.1038/onc.2013.560. [DOI] [PubMed] [Google Scholar]
- 32.Song L., Tang S., Han X., et al. KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nat Commun. 2019;10(1):1639. doi: 10.1038/s41467-019-09720-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen D., Guo J., Miki T., Tachibana M., Gahl W.A. Molecular cloning and characterization of rab27a and rab27b, novel human rab proteins shared by melanocytes and platelets. Biochem Mol Med. 1997;60(1):27–37. doi: 10.1006/bmme.1996.2559. [DOI] [PubMed] [Google Scholar]
- 34.Kariya Y., Honma M., Hanamura A., et al. Rab27a and Rab27b are involved in stimulation-dependent RANKL release from secretory lysosomes in osteoblastic cells. J Bone Miner Res. 2011;26(4):689–703. doi: 10.1002/jbmr.268. [DOI] [PubMed] [Google Scholar]
- 35.Fukuda M. Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic. 2013;14(9):949–963. doi: 10.1111/tra.12083. [DOI] [PubMed] [Google Scholar]
- 36.Catz S.D. Regulation of vesicular trafficking and leukocyte function by Rab27 GTPases and their effectors. J Leukoc Biol. 2013;94(4):613–622. doi: 10.1189/jlb.1112600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuroda T.S., Fukuda M., Ariga H., Mikoshiba K. The Slp homology domain of synaptotagmin-like proteins 1-4 and Slac2 functions as a novel Rab27A binding domain. J Biol Chem. 2002;277(11):9212–9218. doi: 10.1074/jbc.M112414200. [DOI] [PubMed] [Google Scholar]
- 38.Fukuda M., Kuroda T.S. Slac2-c (synaptotagmin-like protein homologue lacking C2 domains-c), a novel linker protein that interacts with Rab27, myosin Va/VIIa, and actin. J Biol Chem. 2002;277(45):43096–43103. doi: 10.1074/jbc.M203862200. [DOI] [PubMed] [Google Scholar]
- 39.Bare Y., Chan G.K., Hayday T., McGrath J.A., Parsons M. Slac2-b coordinates extracellular vesicle secretion to regulate keratinocyte adhesion and migration. J Invest Dermatol. 2021;141(3):523–532. doi: 10.1016/j.jid.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Shirakawa R., Higashi T., Tabuchi A., et al. Munc13-4 is a GTP-Rab27-binding protein regulating dense core granule secretion in platelets. J Biol Chem. 2004;279(11):10730–10737. doi: 10.1074/jbc.M309426200. [DOI] [PubMed] [Google Scholar]
- 41.Ostrowski M., Carmo N.B., Krumeich S., et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 42.Bobrie A., Krumeich S., Reyal F., et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72(19):4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 43.Ostenfeld M.S., Jeppesen D.K., Laurberg J.R., et al. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res. 2014;74(20):5758–5771. doi: 10.1158/0008-5472.CAN-13-3512. [DOI] [PubMed] [Google Scholar]
- 44.Li W., Hu Y., Jiang T., et al. Rab27A regulates exosome secretion from lung adenocarcinoma cells A549: involvement of EPI64. APMIS. 2014;122(11):1080–1087. doi: 10.1111/apm.12261. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L., Zhang X., Hsieh L.S., Lin T.V., Bordey A. Rab27a-dependent paracrine communication controls dendritic spine formation and sensory responses in the barrel cortex. Cells. 2021;10(3):622. doi: 10.3390/cells10030622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng M., Wen J., Ma Z., et al. FOXO1-mediated downregulation of RAB27B leads to decreased exosome secretion in diabetic kidneys. Diabetes. 2021;70(7):1536–1548. doi: 10.2337/db20-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng X., Li X., Yang S., et al. LINC00511 drives invasive behavior in hepatocellular carcinoma by regulating exosome secretion and invadopodia formation. J Exp Clin Cancer Res. 2021;40(1):183. doi: 10.1186/s13046-021-01990-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grant B.D., Donaldson J.G. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10(9):597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin K., Wen Z., Wu B., et al. NOTCH-induced rerouting of endosomal trafficking disables regulatory T cells in vasculitis. J Clin Invest. 2021;131(1) doi: 10.1172/JCI136042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li B.X., Satoh A.K., Ready D.F. Myosin V, Rab11, and dRip11 direct apical secretion and cellular morphogenesis in developing Drosophila photoreceptors. J Cell Biol. 2007;177(4):659–669. doi: 10.1083/jcb.200610157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neeft M., Wieffer M., de Jong A.S., et al. Munc13-4 is an effector of rab27a and controls secretion of lysosomes in hematopoietic cells. Mol Biol Cell. 2005;16(2):731–741. doi: 10.1091/mbc.E04-10-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh R.K., Mizuno K., Wasmeier C., et al. Distinct and opposing roles for Rab27a/Mlph/MyoVa and Rab27b/Munc13-4 in mast cell secretion. FEBS J. 2013;280(3):892–903. doi: 10.1111/febs.12081. [DOI] [PubMed] [Google Scholar]
- 53.Johnson J.L., He J., Ramadass M., et al. Munc13-4 is a Rab11-binding protein that regulates Rab11-positive vesicle trafficking and docking at the plasma membrane. J Biol Chem. 2016;291(7):3423–3438. doi: 10.1074/jbc.M115.705871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Messenger S.W., Woo S.S., Sun Z., Martin T.F.J. A Ca(2+)-stimulated exosome release pathway in cancer cells is regulated by Munc13-4. J Cell Biol. 2018;217(8):2877–2890. doi: 10.1083/jcb.201710132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsu C., Morohashi Y., Yoshimura S.I., et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189(2):223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frühbeis C., Fröhlich D., Kuo W.P., et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11(7) doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peng K.Y., Pérez-González R., Alldred M.J., et al. Apolipoprotein E4 genotype compromises brain exosome production. Brain. 2019;142(1):163–175. doi: 10.1093/brain/awy289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang L., Peng X., Li Y., et al. Long non-coding RNA HOTAIR promotes exosome secretion by regulating RAB35 and SNAP23 in hepatocellular carcinoma. Mol Cancer. 2019;18(1):78. doi: 10.1186/s12943-019-0990-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langemeyer L., Fröhlich F., Ungermann C. Rab GTPase function in endosome and lysosome biogenesis. Trends Cell Biol. 2018;28(11):957–970. doi: 10.1016/j.tcb.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 60.Saftig P., Puertollano R. How lysosomes sense, integrate, and cope with stress. Trends Biochem Sci. 2021;46(2):97–112. doi: 10.1016/j.tibs.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fader C.M., Sánchez D., Furlán M., Colombo M.I. Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in k562 cells. Traffic. 2008;9(2):230–250. doi: 10.1111/j.1600-0854.2007.00677.x. [DOI] [PubMed] [Google Scholar]
- 62.Lund V.K., Lycas M.D., Schack A., Andersen R.C., Gether U., Kjaerulff O. Rab2 drives axonal transport of dense core vesicles and lysosomal organelles. Cell Rep. 2021;35(2):108973. doi: 10.1016/j.celrep.2021.108973. [DOI] [PubMed] [Google Scholar]
- 63.Lund V.K., Madsen K.L., Kjaerulff O. Drosophila Rab2 controls endosome-lysosome fusion and LAMP delivery to late endosomes. Autophagy. 2018;14(9):1520–1542. doi: 10.1080/15548627.2018.1458170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Kant R., Fish A., Janssen L., et al. Late endosomal transport and tethering are coupled processes controlled by RILP and the cholesterol sensor ORP1L. J Cell Sci. 2013;126(Pt 15):3462–3474. doi: 10.1242/jcs.129270. [DOI] [PubMed] [Google Scholar]
- 65.Furuta N., Fujita N., Noda T., Yoshimori T., Amano A. Combinational soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins VAMP8 and Vti1b mediate fusion of antimicrobial and canonical autophagosomes with lysosomes. Mol Biol Cell. 2010;21(6):1001–1010. doi: 10.1091/mbc.E09-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balderhaar H.J., Ungermann C. CORVET and HOPS tethering complexes - coordinators of endosome and lysosome fusion. J Cell Sci. 2013;126(Pt 6):1307–1316. doi: 10.1242/jcs.107805. [DOI] [PubMed] [Google Scholar]
- 67.Pryor P.R., Mullock B.M., Bright N.A., et al. Combinatorial SNARE complexes with VAMP7 or VAMP8 define different late endocytic fusion events. EMBO Rep. 2004;5(6):590–595. doi: 10.1038/sj.embor.7400150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Z., Miao G., Xue X., et al. The Vici syndrome protein EPG5 is a Rab7 effector that determines the fusion specificity of autophagosomes with late endosomes/lysosomes. Mol Cell. 2016;63(5):781–795. doi: 10.1016/j.molcel.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 69.Granger E., McNee G., Allan V., Woodman P. The role of the cytoskeleton and molecular motors in endosomal dynamics. Semin Cell Dev Biol. 2014;31(100):20–29. doi: 10.1016/j.semcdb.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Titus M.A. Myosin-driven intracellular transport. Cold Spring Harbor Perspect Biol. 2018;10(3):a021972. doi: 10.1101/cshperspect.a021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li P., Bademosi A.T., Luo J., Meunier F.A. Actin remodeling in regulated exocytosis: toward a mesoscopic view. Trends Cell Biol. 2018;28(9):685–697. doi: 10.1016/j.tcb.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 72.Porat-Shliom N., Milberg O., Masedunskas A., Weigert R. Multiple roles for the actin cytoskeleton during regulated exocytosis. Cell Mol Life Sci. 2013;70(12):2099–2121. doi: 10.1007/s00018-012-1156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279(5350):509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 74.Ridley A.J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 75.Matsui T., Maeda M., Doi Y., et al. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140(3):647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gautreau A.M., Fregoso F.E., Simanov G., Dominguez R. Nucleation, stabilization, and disassembly of branched actin networks. Trends Cell Biol. 2022;32(5):421–432. doi: 10.1016/j.tcb.2021.10.006. 00208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang J., Zhuang X., Greene K.S., et al. Cdc42 functions as a regulatory node for tumour-derived microvesicle biogenesis. J Extracell Vesicles. 2021;10(3) doi: 10.1002/jev2.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aloisio F.M. A WICB 50th Favorite: Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells. Mol Biol Cell. 2021;32(21):fe4. doi: 10.1091/mbc.E21-06-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Higgs H.N., Pollard T.D. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu Rev Biochem. 2001;70:649–676. doi: 10.1146/annurev.biochem.70.1.649. [DOI] [PubMed] [Google Scholar]
- 80.Hoshino D., Kirkbride K.C., Costello K., et al. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5(5):1159–1168. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sinha S., Hoshino D., Hong N.H., et al. Cortactin promotes exosome secretion by controlling branched actin dynamics. J Cell Biol. 2016;214(2):197–213. doi: 10.1083/jcb.201601025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fagarasanu A., Rachubinski R.A. Orchestrating organelle inheritance in Saccharomyces cerevisiae. Curr Opin Microbiol. 2007;10(6):528–538. doi: 10.1016/j.mib.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 83.Arai S., Noda Y., Kainuma S., Wada I., Yoda K. Ypt11 functions in bud-directed transport of the Golgi by linking Myo2 to the coatomer subunit Ret2. Curr Biol. 2008;18(13):987–991. doi: 10.1016/j.cub.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 84.Wu X.S., Rao K., Zhang H., et al. Identification of an organelle receptor for myosin-Va. Nat Cell Biol. 2002;4(4):271–278. doi: 10.1038/ncb760. [DOI] [PubMed] [Google Scholar]
- 85.Drummond D.R. Regulation of microtubule dynamics by kinesins. Semin Cell Dev Biol. 2011;22(9):927–934. doi: 10.1016/j.semcdb.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 86.Allan V.J. Cytoplasmic dynein. Biochem Soc Trans. 2011;39(5):1169–1178. doi: 10.1042/BST0391169. [DOI] [PubMed] [Google Scholar]
- 87.Horgan C.P., McCaffrey M.W. Rab GTPases and microtubule motors. Biochem Soc Trans. 2011;39(5):1202–1206. doi: 10.1042/BST0391202. [DOI] [PubMed] [Google Scholar]
- 88.Johansson M., Rocha N., Zwart W., et al. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol. 2007;176(4):459–471. doi: 10.1083/jcb.200606077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma X., Liu K., Li J., et al. A non-canonical GTPase interaction enables ORP1L-Rab7-RILP complex formation and late endosome positioning. J Biol Chem. 2018;293(36):14155–14164. doi: 10.1074/jbc.RA118.001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Valetti C., Wetzel D.M., Schrader M., et al. Role of dynactin in endocytic traffic: effects of dynamitin overexpression and colocalization with CLIP-170. Mol Biol Cell. 1999;10(12):4107–4120. doi: 10.1091/mbc.10.12.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Culver-Hanlon T.L., Lex S.A., Stephens A.D., Quintyne N.J., King S.J. A microtubule-binding domain in dynactin increases dynein processivity by skating along microtubules. Nat Cell Biol. 2006;8(3):264–270. doi: 10.1038/ncb1370. [DOI] [PubMed] [Google Scholar]
- 92.Kesisova I.A., Robinson B.P., Spiliotis E.T. A septin GTPase scaffold of dynein-dynactin motors triggers retrograde lysosome transport. J Cell Biol. 2021;220(2) doi: 10.1083/jcb.202005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rocha N., Kuijl C., van der Kant R., et al. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J Cell Biol. 2009;185(7):1209–1225. doi: 10.1083/jcb.200811005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jahn R., Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490(7419):201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baker R.W., Hughson F.M. Chaperoning SNARE assembly and disassembly. Nat Rev Mol Cell Biol. 2016;17(8):465–479. doi: 10.1038/nrm.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sutton R.B., Fasshauer D., Jahn R., Brunger A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395(6700):347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 97.Antonin W., Fasshauer D., Becker S., Jahn R., Schneider T.R. Crystal structure of the endosomal SNARE complex reveals common structural principles of all SNAREs. Nat Struct Biol. 2002;9(2):107–111. doi: 10.1038/nsb746. [DOI] [PubMed] [Google Scholar]
- 98.Yu Z., Shi M., Stewart T., et al. Reduced oligodendrocyte exosome secretion in multiple system atrophy involves SNARE dysfunction. Brain. 2020;143(6):1780–1797. doi: 10.1093/brain/awaa110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kumar R., Tang Q., Müller S.A., et al. Fibroblast growth factor 2-mediated regulation of neuronal exosome release depends on VAMP3/cellubrevin in hippocampal neurons. Adv Sci. 2020;7(6):1902372. doi: 10.1002/advs.201902372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fader C.M., Sánchez D.G., Mestre M.B., Colombo M.I. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta. 2009;1793(12):1901–1916. doi: 10.1016/j.bbamcr.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 101.Gross J.C., Chaudhary V., Bartscherer K., Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14(10):1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 102.Ruiz-Martinez M., Navarro A., Marrades R.M., et al. YKT6 expression, exosome release, and survival in non-small cell lung cancer. Oncotarget. 2016;7(32):51515–51524. doi: 10.18632/oncotarget.9862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun C., Wang P., Dong W., Liu H., Sun J., Zhao L. LncRNA PVT1 promotes exosome secretion through YKT6, RAB7, and VAMP3 in pancreatic cancer. Aging. 2020;12(11):10427–10440. doi: 10.18632/aging.103268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koles K., Nunnari J., Korkut C., et al. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J Biol Chem. 2012;287(20):16820–16834. doi: 10.1074/jbc.M112.342667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Verweij F.J., Bebelman M.P., Jimenez C.R., et al. Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. J Cell Biol. 2018;217(3):1129–1142. doi: 10.1083/jcb.201703206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hyenne V., Apaydin A., Rodriguez D., et al. RAL-1 controls multivesicular body biogenesis and exosome secretion. J Cell Biol. 2015;211(1):27–37. doi: 10.1083/jcb.201504136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peak T.C., Panigrahi G.K., Praharaj P.P., et al. Syntaxin 6-mediated exosome secretion regulates enzalutamide resistance in prostate cancer. Mol Carcinog. 2020;59(1):62–72. doi: 10.1002/mc.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wei Y., Wang D., Jin F., et al. Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nat Commun. 2017;8:14041. doi: 10.1038/ncomms14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gordon D.E., Bond L.M., Sahlender D.A., Peden A.A. A targeted siRNA screen to identify SNAREs required for constitutive secretion in mammalian cells. Traffic. 2010;11(9):1191–1204. doi: 10.1111/j.1600-0854.2010.01087.x. [DOI] [PubMed] [Google Scholar]
- 110.Yang E., Wang X., Gong Z., Yu M., Wu H., Zhang D. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Targeted Ther. 2020;5(1):242. doi: 10.1038/s41392-020-00359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hou P.P., Chen H.Z. Extracellular vesicles in the tumor immune microenvironment. Cancer Lett. 2021;516:48–56. doi: 10.1016/j.canlet.2021.05.032. [DOI] [PubMed] [Google Scholar]
- 112.Sounni N.E., Noel A. Targeting the tumor microenvironment for cancer therapy. Clin Chem. 2013;59(1):85–93. doi: 10.1373/clinchem.2012.185363. [DOI] [PubMed] [Google Scholar]
- 113.Camussi G., Deregibus M.C., Bruno S., Grange C., Fonsato V., Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res. 2011;1(1):98–110. [PMC free article] [PubMed] [Google Scholar]
- 114.De Maio A. Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: a form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa. Cell Stress Chaperones. 2011;16(3):235–249. doi: 10.1007/s12192-010-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Desdín-Micó G., Mittelbrunn M. Role of exosomes in the protection of cellular homeostasis. Cell Adhes Migrat. 2017;11(2):127–134. doi: 10.1080/19336918.2016.1251000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baixauli F., López-Otín C., Mittelbrunn M. Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front Immunol. 2014;5:403. doi: 10.3389/fimmu.2014.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kumar A., Deep G. Hypoxia in tumor microenvironment regulates exosome biogenesis: molecular mechanisms and translational opportunities. Cancer Lett. 2020;479:23–30. doi: 10.1016/j.canlet.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 118.King H.W., Michael M.Z., Gleadle J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dorayappan K.D.P., Wanner R., Wallbillich J.J., et al. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: a novel mechanism linking STAT3/Rab proteins. Oncogene. 2018;37(28):3806–3821. doi: 10.1038/s41388-018-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li L., Cao B., Liang X., et al. Microenvironmental oxygen pressure orchestrates an anti- and pro-tumoral γδ T cell equilibrium via tumor-derived exosomes. Oncogene. 2019;38(15):2830–2843. doi: 10.1038/s41388-018-0627-z. [DOI] [PubMed] [Google Scholar]
- 121.Xi L., Peng M., Liu S., et al. Hypoxia-stimulated ATM activation regulates autophagy-associated exosome release from cancer-associated fibroblasts to promote cancer cell invasion. J Extracell Vesicles. 2021;10(11) doi: 10.1002/jev2.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Savina A., Furlán M., Vidal M., Colombo M.I. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278(22):20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 123.Woo S.S., James D.J., Martin T.F. Munc13-4 functions as a Ca(2+) sensor for homotypic secretory granule fusion to generate endosomal exocytic vacuoles. Mol Biol Cell. 2017;28(6):792–808. doi: 10.1091/mbc.E16-08-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao H., Yang L., Baddour J., et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 2016;5 doi: 10.7554/eLife.10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Scott C.C., Gruenberg J. Ion flux and the function of endosomes and lysosomes: pH is just the start: the flux of ions across endosomal membranes influences endosome function not only through regulation of the luminal pH. Bioessays. 2011;33(2):103–110. doi: 10.1002/bies.201000108. [DOI] [PubMed] [Google Scholar]
- 126.Parolini I., Federici C., Raggi C., et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284(49):34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ban J.J., Lee M., Im W., Kim M. Low pH increases the yield of exosome isolation. Biochem Biophys Res Commun. 2015;461(1):76–79. doi: 10.1016/j.bbrc.2015.03.172. [DOI] [PubMed] [Google Scholar]
- 128.Guo H., Chitiprolu M., Roncevic L., et al. Atg5 disassociates the V1 V0-ATPase to promote exosome production and tumor metastasis independent of canonical macroautophagy. Dev Cell. 2017;43(6):716–730. doi: 10.1016/j.devcel.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 129.Cheng Y., Zeng Q., Han Q., Xia W. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell. 2019;10(4):295–299. doi: 10.1007/s13238-018-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zou W., Lai M., Zhang Y., et al. Exosome release is regulated by mTORC1. Adv Sci. 2019;6(3):1801313. doi: 10.1002/advs.201801313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aubertin K., Silva A.K., Luciani N., et al. Massive release of extracellular vesicles from cancer cells after photodynamic treatment or chemotherapy. Sci Rep. 2016;6:35376. doi: 10.1038/srep35376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Keklikoglou I., Cianciaruso C., Güç E., et al. Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat Cell Biol. 2019;21(2):190–202. doi: 10.1038/s41556-018-0256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lehmann B.D., Paine M.S., Brooks A.M., et al. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008;68(19):7864–7871. doi: 10.1158/0008-5472.CAN-07-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yan C., Tian X., Li J., et al. A high-fat diet attenuates AMPK α1 in adipocytes to induce exosome shedding and nonalcoholic fatty liver development in vivo. Diabetes. 2021;70(2):577–588. doi: 10.2337/db20-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao S., Li W., Yu W., et al. Exosomal miR-21 from tubular cells contributes to renal fibrosis by activating fibroblasts via targeting PTEN in obstructed kidneys. Theranostics. 2021;11(18):8660–8673. doi: 10.7150/thno.62820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lenassi M., Cagney G., Liao M., et al. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11(1):110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Currim F., Singh J., Shinde A., et al. Exosome release is modulated by the mitochondrial-lysosomal crosstalk in Parkinson's disease stress conditions. Mol Neurobiol. 2021;58(4):1819–1833. doi: 10.1007/s12035-020-02243-3. [DOI] [PubMed] [Google Scholar]
- 138.Zeng Z., Li Y., Pan Y., et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9(1):5395. doi: 10.1038/s41467-018-07810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Song Y., Wang M., Tong H., et al. Plasma exosomes from endometrial cancer patients contain LGALS3BP to promote endometrial cancer progression. Oncogene. 2021;40(3):633–646. doi: 10.1038/s41388-020-01555-x. [DOI] [PubMed] [Google Scholar]
- 140.Yuan X., Qian N., Ling S., et al. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics. 2021;11(3):1429–1445. doi: 10.7150/thno.45351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nie H., Liao Z., Wang Y., Zhou J., He X., Ou C. Exosomal long non-coding RNAs: emerging players in cancer metastasis and potential diagnostic biomarkers for personalized oncology. Genes Dis. 2021;8(6):769–780. doi: 10.1016/j.gendis.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou C., Wei W., Ma J., et al. Cancer-secreted exosomal miR-1468-5p promotes tumor immune escape via the immunosuppressive reprogramming of lymphatic vessels. Mol Ther. 2021;29(4):1512–1528. doi: 10.1016/j.ymthe.2020.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jiang X., Liu G., Li Y., Pan Y. Immune checkpoint: the novel target for antitumor therapy. Genes Dis. 2021;8(1):25–37. doi: 10.1016/j.gendis.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]