Abstract
Hepatocellular carcinoma (HCC) has a very high incidence and fatality rate, and in most cases, it is already at an advanced stage when diagnosed. Therefore, early prevention and detection of HCC are two of the most effective strategies. However, the methods recommended in the practice guidelines for the detection of HCC cannot guarantee high sensitivity and specificity except for the liver biopsy, which is known as the “gold standard”. In this review, we divided the detection of HCC into pre-treatment diagnosis and post-treatment monitoring, and found that in addition to the traditional imaging detection and liver biopsy, alpha fetoprotein (AFP), lens culinaris-agglutinin-reactive fraction of AFP (AFP-L3), protein induced by vitamin K absence or antagonist-II (PIVKA-II) and other biomarkers are excellent biomarkers for HCC, especially when they are combined together. Most notably, the emerging liquid biopsy shows great promise in detecting HCC. In addition, lactic dehydrogenase (LDH), suppressor of cytokine signaling (SOCS) and other relevant biomarkers may become promising biomarkers for HCC post-treatment monitoring. Through the detailed introduction of the diagnostic technology of HCC, we can have a detailed understanding of its development process and then obtain some enlightenment from the diagnosis, to improve the diagnostic rate of HCC and reduce its mortality.
Keywords: HCC, Invasive diagnosis, Minimally invasive diagnosis, Noninvasive diagnosis, Prognostic monitoring
Introduction
According to the global cancer statistics in 2018,1 HCC ranked 7th in number and the death rate ranked 3rd, as well as the data of 2021 cancer statistics in America2 showed that, HCC ranked 6th in the death rate, indicating that HCC is a serious worldwide problem. HCC has been found to be caused by many risk factors, such as hepatitis B virus (HBV), hepatitis C virus (HCV), aflatoxin and alcohol.3,4 The most optimal treatment for HCC has still not been found. Moreover, HCC is insidious and often diagnosed in the middle or late stage, which leads to the best treatment time is missed and the prognosis is extremely poor. The present feasible prevention mode is only to avoid exposure to risk factors, or to conduct regular physical examinations.
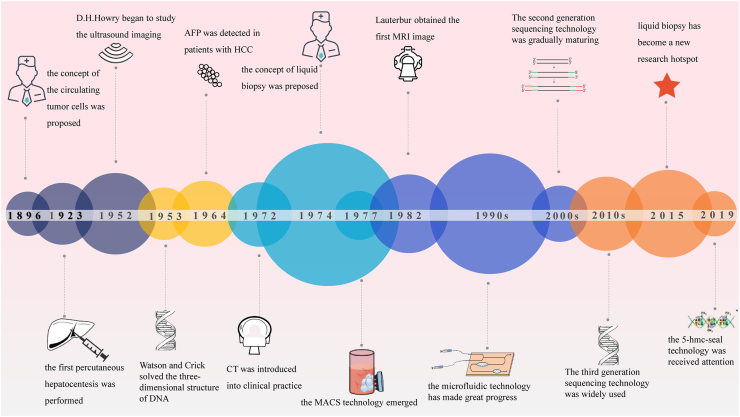
It is undoubtedly important for the diagnosis of HCC. At present, the main clinical diagnostic methods are laboratory examination, imaging examination and liver biopsy. With the development of detection technologies, new methods such as the triple detection of HCC, other tumor markers, combined detection and liquid biopsy have emerged. Although most of them have not been applied in clinical practice, they have broad prospects. In Figure 1, advances in HCC detection are listed in chronological order.
Figure 1.
Timer shaft of the development of HCC detection technologies. Some techniques related to the detection of HCC are listed in chronological order, and the trend of the detection techniques shows that the liquid biopsy has become a hot research direction.
Given the current situation, by consulting many domestic and foreign studies, this review generalized in detail the technologies that can be used for the early screening and prognosis monitoring of HCC, while we analyzed some promising new diagnostic applications to enlighten the strategies to detect HCC.
Invasive diagnosis of HCC
The invasive diagnosis of HCC mainly refers to the living tissue examination of liver puncture, which is called the “gold standard”. In 1923, Bingel performed the first diagnostic percutaneous liver biopsy.5 Its main scope of applications includes the diagnosis of the chronic hepatitis and fatty liver, the guidance of liver disease treatment, and the evaluation of the liver donor's status before and after the liver transplantation, especially for HCC. According to a study,6 41% of suspicious imaging cases were confirmed to be non-HCC by liver biopsy. Imagine that if these patients were not confirmed by liver biopsy, they would receive unnecessary transplants, excisions, and other treatments. Accordingly, liver biopsy plays an important role in the final diagnosis.
For most early HCC, liver nodules can be seen in the imaging examination, so it is particularly important to judge whether the liver nodules are benign or malignant. The guidelines for the management of HCC, published in 2001 by the European Association for the Study of the Liver (EASL),7 showed that 1–2 cm of liver nodules should be confirmed by biopsy. However, Khalili K et al8 found that liver nodules of 1–2 cm are only 14%–23% of malignancy. Liver nodules less than 1 cm are less likely to be HCC, while EASL recommends an ultrasound every four months.9 And those larger than 2 cm can be diagnosed by contrast enhanced, multiphasic computed tomography (CT) or magnetic resonance imaging (MRI).9,10 At present, there is no consensus on the management of liver nodules of 1–2 cm. Pathological diagnosis is more challenging for nodules <2 cm in size, since sampling errors are more likely to occur and these lesions are often well differentiated.
Given the invasive nature of liver biopsy, it is the “gold standard” for the final diagnosis, but not the best option for the early screening.
Noninvasive diagnosis of HCC
With the emergence and development of ultrasound, CT and MRI technologies, a more complete imaging examination model has been formed. The so-called noninvasive diagnosis for HCC mainly refers to imaging examination.
Ultrasound
Mechanical waves with a frequency higher than 20,000 Hz are usually called the ultrasonic waves, which have two vibration modes. It is transmitted in human tissues in the form of a longitudinal wave, which is transmitted and received by an ultrasonic probe. When the ultrasonic wave passes through the different tissues with different acoustic characteristic impedances, the wave can make different changes, including reflection, refraction, diffraction, scattering, attenuation and Doppler effect. The ultrasound machine receives these changes, and transforms these signals into different images to diagnose the human body. With the development of medicine, acoustics and computer technology, there are an increasing number of diagnostic modes in ultrasound (Table 1).
Table 1.
Classification of the ultrasonic diagnostic techniques.
| Name | Introductions | Characteristics | Applications | Ref. |
|---|---|---|---|---|
| A-type ultrasound | The amplitude modulation display method is adopted to display the echo amplitude of the tissue interface | It only reflects the one-dimensional structure information of human tissues, without scanning, the image cannot be called ultrasonic image | Brain, eyeball and pleural effusion examination | 142 |
| M-type ultrasound | The target is propagated with the sound beam and its displacement changes with time | It only reflects the one-dimensional structure information of human tissue, without scanning, the image cannot become ultrasonic image | Cardiovascular system examination | 143 |
| B-type ultrasound | The brightness modulation display method is adopted to obtain the anatomical information of the human body | It can form a section map by one-dimensional scanning | It is the most basic clinical model which can provide the anatomical information of the human organs | 144 |
| Three-dimensional imaging technology | It collects and processes a set of two-dimensional images, and then carries out three-dimensional reconstruction | It can obtain C plane or even F surface which cannot be obtained by the B-type ultrasound | As a supplement of the B-type ultrasound, it can display stereo images of the tissues and organs | 145 |
| Color Doppler ultrasound | It detects the doppler signal from the echo and converts it into an image | Color images can be used to show the direction and relative velocity of the blood flow | It can get the information about the body's blood flow and assess the tumor's blood supply | 146 |
In 1952, D.H.Howry began to study the ultrasound imaging,11 after which ultrasound began to be used in clinical practice, including the HCC detection. For HCC, liver ultrasound is the preferred screening method recommended by all major guidelines. The American Association for the Study of Liver Disease (AASLD) guidelines (2018) recommended that liver ultrasound should be performed every six months in patients with or without alpha fetoprotein (AFP).12 For early HCC, there was no significant change in the morphology and outline under the liver ultrasound images. With the progression of the lesion, the liver will locally bulge outward, thicken, or even irregularly shape. Therefore, the acoustic characteristic impedance in different areas of the liver is different, and the echo intensity of ultrasound images could be used for the pathological classification of HCC.
To enhance the contrast of human tissues under ultrasound images, contrast-enhanced ultrasound (CEUS) has emerged. The first use of CEUS was reported in echocardiography by Gramiak and Shah in 1968.13 This was mainly due to the development of the microbubble contrast agents and corresponding imaging technology. Microbubbles are formed by coating gas with a thickness of tens of nanometers, which can produce strong sound scattering. It can increase the difference in the acoustic scattering signal intensity between the parts with and without contrast agent, thus increasing the image contrast.14 First, a contrast agent with different acoustic characteristic impedances from human tissues is injected into the body cavity or blood vessels. Then, the imaging contrast will be increased, the display of the viscera or lesion will be enhanced, and information on blood perfusion will be obtained. In abdominal organs, CEUS can be used for the qualitative diagnosis of focal liver lesions. In addition, most HCCs are focal liver lesions and are supplied by the hepatic artery system through abnormal unmatched arteries.15 Therefore, CEUS can also be used in the qualitative diagnosis of HCC. The blood supply mode and the time of the contrast agent staying in the HCC can be shown, which can increase the sensitivity of HCC detection compared to the common ultrasound. The sensitivity of CEUS in HCC detection proposed in the clinical practice guidelines for the management of HCC in Asia–Pacific (2017) is similar to that of dynamic CT or dynamic MRI.16 Although the detection effect of CEUS for HCC has been recognized in many aspects, different contrast agents have different degrees of problems in stability, dispersion, cyclic effective survival time and so on,17 which limits the clinical application of CEUS to a certain extent.
Not only the choice of contrast agent, but also the ultrasound itself also has some limitations. For instance, the anatomical range of display is limited, the images are not as clear as the CT and MRI, and the accuracy of examination results sometimes depends on the technical level and the experience of operators.18 With the development of technology, ultrasound will be increasingly closely related to the computer, engineering, digital communication and material science. Based on this trend, ultrasound will be applied to the accurate diagnosis of HCC.
X-ray CT
Generally, CT refers to X-ray computer body layer imaging. The basic principle is that an X-ray harness with high collimation is used to scan the transverse planes of the human body, and the X-ray through the planes is received by the detector, which converts the signal into digital information and image information. Human tissues can be divided into three categories according to density: high density, medium density, and low density. The high-density tissues absorb more X-rays so that white shadows will be shown on the images while fewer X-rays can pass through. At the same time, the thicker the tissues, the fewer X-rays can pass through, resulting in white shadows. That is, CT uses the X-rays to penetrate the tissues of different densities and thickness, then produces different degrees of absorption to achieve diagnosis.
CT was introduced into clinical practice in 1972 and it was used only to examine the traumatic brain in the beginning.19 In 1997, the multi-slice spiral CT emerged.20 The so-called multi-slice meant that the detector had a multi row structure, and with one exposure, the detector could simultaneously receive multiple layers of the human images, making the scanning speed reach the subsecond level. At present, multi-slice spiral CT has become a routine examination method for liver diseases and can make qualitative diagnosis and evaluations of liver-occupying lesions.21
The liver lesions described above include the HCC. Under plain scan CT, most of the HCC lesions are of low density, while a few of them are of high density. When the plain scan does not show abnormality but other auxiliary examinations indicate the possibility of HCC, an enhanced CT should be performed. The water-soluble organic iodine contrast agent was injected intravenously into the patient before scanning. At this time, the density of the lesions can be increased because of the iodine contrast agent, and the most obvious is that the blood vessels also show white shadows because of the increased density. Therefore, enhanced CT has an important diagnostic significance for blood-rich HCC. Ma et al22 performed plain scan CT and enhanced CT in 40 HCC patients. The most obvious differences were observed in the late arterial phase, and the detection rate of HCC was the highest on enhanced CT (78.7%). This study confirmed that dynamic enhanced CT is significant for the diagnosis of HCC compared with plain scan CT.
CT techniques can be widely used in the early screening and prognosis monitoring of HCC. It has excellent performance in density resolution and image overlap, but its radiation dose is high and cannot show the whole organ structures and lesion locations, resulting in some application limitations.
Magnetic resonance imaging (MRI)
There is a large amount of water in the human body, which is rich in hydrogen-1. Hydrogen-1 has spin characteristics, which acts like a small magnet. When the human body is in a strong external magnetic field, hydrogen-1 absorbs energy to produce a magnetic resonance phenomenon. When the magnetic field emission pulse is stopped, hydrogen-1 releases the energy quickly and returns to the original state. This process of the energy release is called the relaxation process. It is collected by the receiver in vitro, and the image is obtained by the computer. What was said above is the principle of nuclear MRI. The black and white grayscale in MRI images is called the signal intensity. The contrast of the black and white grayscale reflects the difference in the inter-tissue relaxation time T1 and T2 (the time required for the relaxation process). There are two basic imaging methods, T1-weighted imaging (T1WI) and T2-weighted imaging (T2WI). All tissues and their lesions in the human body have relatively constant T1 and T2 values, which are inherent parameters.
In 1973, Lauterbur obtained the first MRI image by using the principle of hydrogen atomic nuclear magnetic resonance.23 In 1984, Philips revolutionized the world's first surface coil,24 resulting in images showing very small details, enabling MRI to be better applied clinically. Similarly, HCC can be well detected by MRI. The tumor portion of typical HCC is longer than the surrounding hepatic parenchyma on T1 and T2, showing a low signal on T1WI and a high signal on T2WI.
To further improve the resolution of MRI on human tissues and show some small lesions or diffuse lesions, improving MRI contrast has become an important research direction. The signal strength of MRI depends on the scanning time parameters and organizational characteristic parameters. The former can be controlled by the operator, while the latter includes the relaxation times T1 and T2. Recent decades of research have shown that changing the local magnetic field around the hydrogen protons can change T1 and T2, while some rare earth elements (such as Gd, etc.) had this effect.25,26 The hepatobiliary contrast agents, such as the Gd-BOPTA, Gd-DTPA, Gd-EOB-DTPA, etc., can provide some information on the tumor vascular and hepatocyte function in one examination.27,28 Di Martino et al29 improved the diagnostic accuracy for HCC in the range of 1–2 cm to 90% by using the Gd-EOB-DTPA. At present, Gd-EOB-DTPA is used to help distinguish the early HCC from several benign or borderline nodules.30,31
MRI has been widely used in the diagnosis and prognosis monitoring of HCC. However, it still has limitations, such as technical complexity, high diagnosis cost, long imaging time, higher sensitivity to artifacts, and inconsistency in images quality, especially when it is difficult for the patients to hold their breath and keep still, the image quality is worse.32
Minimally invasive diagnosis of HCC
For the minimally invasive diagnosis of HCC, it mainly refers to the technology of obtaining tumor-related substances from the blood, including serum proteins and circulating metabolites.
AFP combined with ultrasound
AFP was first discovered in 1956 and was reported by Halbrecht et al33 Notably, it was first found in the blood of HCC patients in 1964.34 Since then, it has become the most widely used marker in the diagnosis of HCC.35,36 Normally, the concentration of AFP in serum is lower than 20 ng/mL35, but it can be over 400 ng/mL with the disease, especially in HCC.37 The Chinese Standard for Diagnosis and Treatment of Primary Liver Cancer in China (2019)38 recommended ultrasound combined with serum AFP as a method to screen high-risk groups early. However, the AASLD guidelines and EASL guidelines preferred the use of the ultrasound alone for early screening.12,39,40 A recent study41 found that 20% of patients had insufficient ultrasound quality for the detection of HCC, especially in patients with increased hepatic nodules and liver cirrhosis caused by non-alcoholic steatohepatitis (NASH). The data showed that the number of cirrhotic patients with NASH who then develop HCC is increasing year by year,42 so the quality of ultrasound would face increasingly serious challenges. A retrospective study43 was conducted in 67 patients with non-viral liver cirrhosis over 13 years, and all 67 patients underwent HCC screening with every 6-month AFP measurement and every 6–12-monthly upper abdominal ultrasound. Data showed that about 1/4 of the cirrhotic patients, ultrasound alone was insufficient for early screening.
AFP combined with liver ultrasound has important diagnostic significance for high-risk patients with non-alcoholic cirrhosis and non-viral cirrhosis, which could realize early screening for high-risk HCC patients.
Triple detection of HCC
The triple detection of HCC is a combination of the serum protein markers used in early diagnosis, which are AFP, Lens culinaris-agglutinin-reactive fraction of AFP (AFP-L3) and protein induced by vitamin K absence or antagonist-II (PIVKA-II). AFP-L3 was first detected by Taheta K in the blood of HCC patients in 1998.44 Even in the early stage of HCC, especially when the tumor is supplied by the hepatic artery, malignant liver cells would produce the AFP-L3, which is a highly specific marker of HCC.45 PIVKA-II is also known as DCP, which was first detected in 1984 and considered a serum marker for HCC.46 It is abnormal prothrombin due to the disturbance of vitamin K circulation in liver cells.
AFP, AFP-L3 and PIVKA-II have been included in the National Health Insurance of Japan as clinical serological biomarkers.47, 48, 49 Best et al50 enrolled 285 newly-diagnosed HCC patients and 402 patients with chronic liver diseases for the serum levels of AFP, AFP-L3 and PIVKA-II. The results showed that the specificity and sensitivity of the triple detection group were significantly higher than those of the other groups, with a sensitivity of 85.6% and a specificity of 93.3%. In addition, many studies51, 52, 53 also proved that triple detection of HCC plays an important role in the diagnosis and prognosis monitoring of HCC.
Other tumor markers
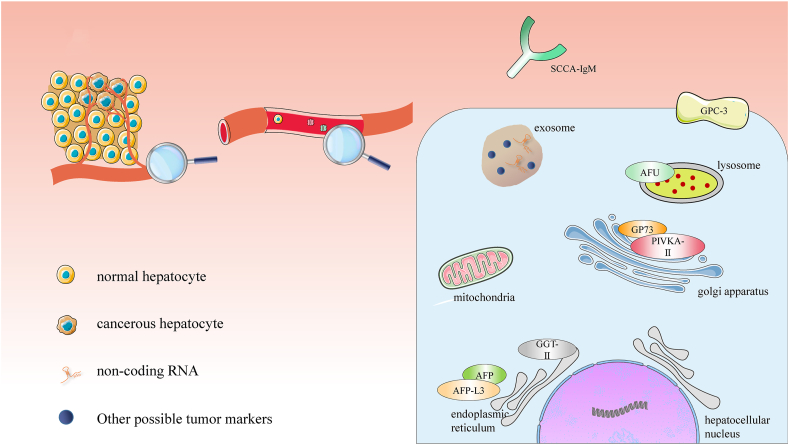
In addition to the markers mentioned above, other tumor markers have also gradually shown special roles in early tumor screening and prognosis monitoring, such as glypican-3 (GPC-3), alpha-L-fucosidase (AFU) and Golgi glycoprotein 73 (GP73). A great number of studies have shown that the diagnostic effect of combined detection of tumor markers is much better than that of AFP alone.
GPC-3 is a member of the family of membrane-bound heparan sulfate proteoglycans, which are anchored on the cell surface by glycosylphosphatidylinositol. It is soluble and can be detected in human serum.54,55 Similar to AFP, GPC-3 is widely expressed in mammalian, but it cannot be detected in normal adult liver tissues.56, 57, 58 In 1997, Hsu et al59 reported for the first time that the expression of GPC-3 in most HCC was higher than that in the normal liver, cholangiocarcinoma and metastatic carcinomas of the liver. Yao et al60 found that GPC-3 expression continued to be upregulated as HCC progressed. Therefore, the detection of the serum GPC-3 is significant for the diagnosis of HCC. Sun B et al61 registered 76 HCC patients to analyze the significance of GPC-3 single or combined with AFP detection in the diagnosis of HCC. The results showed that the detection of GPC-3 combined with AFP can significantly improve the sensitivity and specificity.
AFU is a kind of acid hydrolase, which is widely distributed in various tissues, cells and body fluids of the human body. In 1984, Deugnier et al62 identified the overexpression of AFU in HCC patients for the first time. As a result, some studies on the diagnostic effect of AFU on HCC were carried out.63,64 Mossad et al65 studied 40 HCC patients, 30 cirrhotic patients and 20 healthy controls and examined their serum expression levels of squamous cell carcinoma antigen-immunoglobulin M (SCCA-IgM), AFU and AFP. The results showed that when the SCCA-IgM, AFU and AFP were combined, the sensitivity of the HCC detection was greatly improved.
GP73 is a resident protein of Golgi apparatus. Its apparent molecular weight is approximately 73KD; therefore, it is called GP73.66 It was first found in 2000 by Kladney et al.67 Some studies68, 69, 70 have shown that GP73 is highly expressed in the hepatocytes of HCC patients, regardless of whether there is a viral infection. Moreover, the expression level of GP73 in primary HCC was positively correlated with the degree of the tumor differentiation.71,72 Hou et al73 measured the serum levels of GP73, gamma-glutamyl transferase isoenzyme II (GGT-II) and AFP in 79 HCC patients, 16 cirrhotic patients, 30 chronic hepatitis patients and 28 healthy people. The results showed that when the three were combined, the sensitivity reached 96.3%. Obviously, the combined detection of AFP, GP73 and GGT-II can greatly improve the diagnostic sensitivity of HCC with important implications.
The above detailed results are recorded in Table 2, and the expression of various biomarkers in HCC cells is shown in Figure 2. We found that the sensitivity and specificity of the combined detection of tumor markers were significantly higher than those of AFP alone.
Table 2.
Sensitivity and specificity of different markers for combined diagnosis.
| Tumor Markers | Cutoff value | Unit | Sensitivity/% | Specificity/% | Reference |
|---|---|---|---|---|---|
| AFP | 10 | ng/mL | 68.8 | 88.1 | 50 |
| AFP-L3 | 10 | % | 64.2↓ | 91.5↑ | – |
| PIVKA-Ⅱ | 7.5 | ng/mL | 57.2↓ | 95.0↑ | – |
| AFP + AFP-L3+PIVKA-Ⅱ | – | – | 85.6↑ | 93.3↑ | – |
| AFP | 404.2 | ng/mL | 77.6 | 81.3 | 61 |
| GPC-3 | 272.5 | ng/mL | 75.0↓ | 81.8↑ | – |
| AFP + GPC-3 | – | – | 85.5↑ | 91.5↑ | – |
| AFP | 48 | ng/mL | 70.0 | 53.3 | 65 |
| SCCA-IgM | 233 | AU/mL | 87.5↑ | 66.0↑ | – |
| AFU | 25 | U/L | 87.5↑ | 98.0↑ | – |
| AFP + SCCA-IgM + AFU | – | – | 92.5↑ | 62.1↑ | – |
| AFP | – | – | 55.6 | 86.7 | 73 |
| GP73 | 78.1 | ng/L | 73.4↑ | 80.0↓ | – |
| GGT-Ⅱ | – | – | 68.4↑ | 97.1↑ | – |
| AFP + GP73+GGT-Ⅱ | – | – | 96.3↑ | – | – |
Abbreviations: AFP, Alpha fetoprotein; AFP-L3, Lens culinaris-agglutinin-reactive fraction of AFP; AFU, Alpha-L-fucosidase; GP73, Golgi glycoprotein 73; GPC-3, Glypican-3; GGT-II, Gamma-glutamyl transferase isoenzyme II; PIVKA-II, Protein induced by vitamin K absence or antagonist-II; SCCA-IgM, Squamous cell carcinoma antigen-immunoglobulin M.
Figure 2.
Tumor markers mentioned in the combined detection of HCC. Tumor markers are explained at the cell level and are good biomarkers for HCC.
Of note, around 90% of HCC patients are accompanied by a background of cirrhosis.74 Liver cirrhosis is commonly considered the early lesion for most HCC cases, which developed from progressive fibrosis due to various chronic liver diseases, especially NAFLD (non-alcoholic fatty liver disease).75, 76, 77 There is no doubt that liver biopsy is the gold standard for staging of fibrosis but it has obvious limitations of invasiveness, high-risk and high-cost.78 Therefore, it is essential to develop reliable noninvasive approaches to predict the potential progression of NAFLD to cirrhosis and HCC by assessing liver fibrosis. At present, many NITs (non-invasive tests) have been applied to identify patients with high risk for advanced fibrosis or cirrhosis, including NFS (NAFLD fibrosis score), FIB-4 index (Fibrosis-4), and APRI (aspartate aminotransferase-to-platelet ratio index).79, 80, 81 NFS is a simple scoring system consisting of easily accessible clinical and laboratory data, that accurately distinguished the severity of fibrosis in NAFLD patients.79 Previous study has shown that a high NFS (≥–1.455; adjusted HR 1.87; 95% CI 1.54–2.28; P < 0.001) is strongly associated with developing HCC.82 It gives a hint that patients with a high NFS may be a high-risk group for HCC, which could be distinguished at an early stage through surveillance and further detection to reduce the mortality rate. Similar to NFS, FIB-4 is also a reliable tool for predicting the stage of fibrosis.83,84 Both NFS and FIB-4 are recommended by the EASL-EASD-EASO Clinical Practice Guidelines as part of the diagnostic scheme for preliminary screening of advanced fibrosis.85 Besides, some other tools are also widely used in clinical practice, such as APRI, BARD score and HFS (Hepamet fibrosis score).86,87 All these prediction models are economical and efficient for identifying patients with advanced fibrosis or cirrhosis, which improves the efficacy of HCC surveillance. Meanwhile, successful application of them decreased unnecessary liver biopsies for most patients. What's more, early detection of precancerous conditions is of great significance for patients, who could be referred to hepatologists for further targeted examinations to make a definite diagnosis.
Altogether, it is promising to monitor HCC by NITs and specific tumor markers in clinical practice, for which not only avoid patients from needless biopsy but also provide a cost-efficient method to identify HCC high-risk patients.
Liquid biopsy of HCC
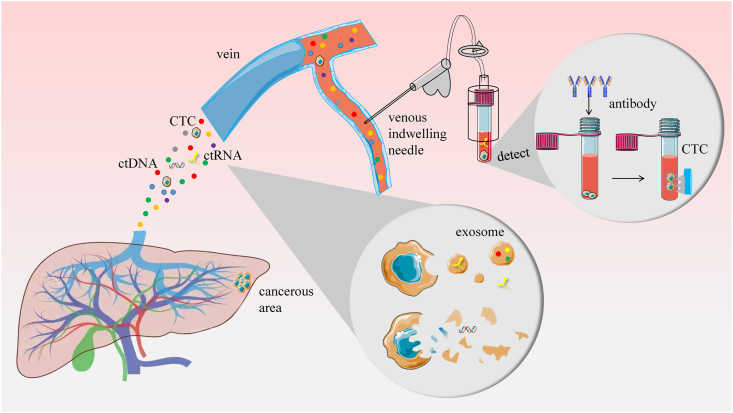
The concept of liquid biopsy was first proposed by Sorrells RB in 1974.88 It is widely regarded as another method of cancer tissue biopsy that will contribute to more sensitive early screening and prognosis monitoring of HCC. At present, liquid biopsy, which obtains tumor-related information from blood, is most valued by the scientific community. The biomarkers related to HCC in the blood are undoubtedly circulating tumor cells, circulating tumor DNA and circulating tumor RNA. In Figure 3, the specific contents and principles of the liquid biopsy are summarized.
Figure 3.
Principles and methods of liquid biopsy. HCC can release circulating tumor cells, circulating tumor DNA, and circulating tumor RNA into the blood. Circulating tumor cells exist in the patient's blood as single cells, and their presence can be detected by drawing a small amount of blood and using magnetic-activated cell sorting technology. Circulating tumor DNA is released into the blood mainly by tumor cell lysis. Circulating tumor RNA is secreted by exosomes. All of them are excellent biomarkers for detecting HCC.
Circulating tumor cells
Australian scholar Ashworth proposed the concept of circulating tumor cells (CTCs) in 1896.89 It refers to the tumor cells that enter the blood circulation system from the primary or the secondary tumors spontaneously or due to the diagnosis or treatment. It exists in the circulatory system in the form of a single cell. CTC detection plays an important role in the diagnosis of most tumors. Recent studies90, 91, 92 have shown that the clinical classification of HCC is positively correlated with the number of CTCs in the blood. However, when cancer cells leave the tumor and enter the bloodstream, most are destroyed by immune cells, and only a few survived.93 Therefore, the number of CTCs in peripheral blood is very small, and thus, a very sensitive identification method is needed. At present, magnetic-activated cell sorting technology (MACS) is the most representative positive enrichment method. In 1977, Molday et al published the use of iron-containing polymeric microspheres conjugated to lectins for the separation of red blood cells and antibody-coated cells.94,95 It is the first and only method approved by the Food and Drug Administration for the diagnosis of CTCs in malignant diseases.96 The principle is to synthesize superparamagnetic immune complexes by using the specific binding principle of cell surface antigens and antibodies,97 to isolate CTCs from peripheral blood. In addition, some studies98,99 conducted the method of combining MACS with a microfluidic chip, which realized the efficient enrichment technology of CTCs. Microfluidic chip was established as an interdisciplinary field of research by combining micro/nano-device technologies, chemical sensor technology, and analytical chemistry in the 1990s.100 Undoubtedly, a microfluidic chip will be used in the diagnosis of HCC in the future.
Li et al101 proposed the “three kinds of antibodies” method to detect CTCs in the serum of HCC patients. These three antibodies were anti-ASGPR antibody, anti-CPS1 antibody and anti-P-CK antibody, respectively. They compared the method with previous methods (using anti-CPS1 or anti-P-CK single antibody detection). The results showed that the “three kinds of antibodies” method detected a higher CTC count, which may confirm that the new strategy could improve the sensitivity of CTC detection in HCC patients.
In general, the enrichment and characterization of circulating HCC cells are the two major steps in the diagnosis of HCC. However, such a detection method is complex and time-consuming. Fortunately, most HCCs are carcinogenic changes caused by the liver cirrhosis. If early CTC screening is only used in cirrhotic patients, it could reduce the workload of diagnosis to a certain extent.
Circulating tumor DNA
Circulating tumor DNA (ctDNA) is a small extracellular fragment of cell-free DNA (cfDNA) released by tumor cells, which is a part of cfDNA and can be detected in the early stage of tumors. In 1953, Watson and Crick solved the three-dimensional structure of DNA.102 In the 2000s, second-generation sequencing technology gradually matured.103 Besides, in the 2010s, third-generation sequencing technology was widely used.104 With the rapid development of gene sequencing technology, it can be detected and counted or even traced dynamically in blood. DNA methyltransferase is one of the most deeply studied mammalian epigenetic modifications. It is responsible for DNA to establish and maintain methylation patterns.105 In normal cells, it ensures the normal gene expression and stable gene silencing. However, numerous studies have shown that the presence of highly methylated regions leads to the inactivation of certain tumor suppressor genes.106, 107, 108 DNA methylation and histone modification lead to the tumor suppressor gene silencing and the chromosome instability, which leads to the occurrence and the development of a variety of cancers.109, 110, 111
Recently, Cai et al112 observed the level of the 5-hmc modification in the cfDNA of 2,554 Chinese individuals (including 1,204 HCC patients) by using the 5-hmc-seal technique. The sensitivity and specificity of detecting HCC were 82.7% and 67.4%, respectively. Compared with serum AFP detection (sensitivity 44.8%, specificity 76.1%), the sensitivity was significantly improved.
In a manner of speaking, with the maturity of comprehensive technology, ctDNA detection technology will be the most mainstream liquid biopsy technology in the future.
Circulating tumor RNA
Circulating tumor RNA (ctRNA) is usually present in exosomes and secreted by tumor cells into the circulatory system. One of the most striking is microRNA (miRNA), which is a small non-coding RNA (17–25 nucleotides) that binds to complementary sequences in target mRNA to induce degradation.113 In cancer, miRNAs may play a role as tumor suppressor genes or carcinogens.114,115 Amr et al116 evaluated the diagnostic potential of miRNA-122 and miRNA-224 in HCC and found that their diagnostic specificities were 98% and 93%, respectively. The most noteworthy was, when miRNA-122 was combined with AFP, the sensitivity was 97.5%, and the specificity reached 100%, which is superior to any other method used alone in the study.
Liquid biopsy, which is noninvasive and can dynamically detect tumor information, has become a research hot spot in recent years. However, an ideal, widely applicable detection technique should have the following characteristics: high sensitivity and high specificity; safe and reliable; efficient and feasible; and reasonable cost. However, at present, liquid biopsy has not been able to meet the conditions of the efficient and feasible and reasonable cost. Therefore, there will be much room for improvement in the future.
Prognostic monitoring of HCC
The above invasive, noninvasive and minimally invasive diagnosis can play an important role in the early screening, diagnosis and prognosis of HCC. There are also some other detection methods, which are mainly aimed at monitoring the prognosis of HCC after treatment.
Lactic dehydrogenase
Lactic dehydrogenase (LDH) is a glycolysis enzyme existing in various tissues and tumors of the human body. It is the key enzyme for the pyruvate conversion to the lactic acid under anaerobic conditions.117,118 The biological relationship between hypoxia and LDH is established by the abnormal activation of hypoxia-inducible factor 1α (HIF-1α).119 There is growing evidence that hypoxia may contribute to the progression of cancers.120, 121, 122 Hypoxia in the tumor microenvironment is sufficient to activate several downstream genes dependent on HIF and promote them expression, including the vascular endothelial growth factor gene, erythropoietin gene and many enzyme genes involved in glucose metabolism.123, 124, 125, 126, 127 These results also explained that hypoxia can promote the progression of cancers. Therefore, it can be inferred that LDH can provide anaerobic conditions for tumor cells, thus promoting the progression of cancers. Some studies128,129 have shown that LDH can be used as a prognostic index for HCC patients. Faloppi et al130 collected the LDH values in 78 HCC patients one month before and after the treatment with Solafinib. The results showed that HCC patients with decreased LDH seemed to have a better prognosis after treatment with Solafinib. Similarly, many other studies131, 132, 133 have shown that lower LDH levels could predict a better prognosis in HCC patients treated by transcatheter arterial embolization (TAE) or radical hepatectomy. Therefore, LDH can be used as a potential indicator to predict the prognosis of HCC patients after treatments. However, there is no denying that more studies are needed to confirm this hypothesis.
Suppressor of cytokine signaling proteins
Cytokines are bioactive small molecular peptides secreted by cells, which transmits biological information by binding to receptors on the surface of cells, thus regulating a variety of biological processes. Suppressor of cytokine signaling (SOCS) was first discovered by Endo et al134 in 1997. Members of the SOCS family are the key regulators of cytokine homeostasis and play an important role in cell proliferation, differentiation, maturation and apoptosis, which are involved in tumorigenesis and the development of cancer.135, 136, 137
Jiang et al138 studied the relationship between the SOCS 3 methylation status and the prognosis of HCC patients after transhepatic arterial chemotherapy and embolization (TACE). The 3-year and 5-year survival rates of patients with SOCS3 methylation were significantly lower than those of patients without the SOCS3 methylation. Some studies139, 140, 141 have also proven that the level of the SOCS and the methylation status of SOCS can be important monitoring factors for the prognosis of HCC patients after treatment. Increased SOCS expression or increased SOCS methylation suggested poor prognosis in HCC.
Simple blood detection can achieve the prognosis of HCC detection, which is significant to HCC patients and can reduce the medical harm caused by the secondary examination. However, the clinical feasibility of these blood detection substances still needs to be proven.
Future prospects for HCC detection
The diagnosis of HCC should not be limited to the imaging tests or blood tests but should be updated with the rapid development of multidisciplinary technology. The MACS and microfluidic chip methods used in liquid biopsy are good examples. With the development of nanotechnology, it has also shown significant advantages in the detection of HCC. Nanochannels are formed by inserting nanomaterials into the lipid bilayer of a cell. When a voltage is applied to the electrodes at both ends of the nanochannel, a constant ion current can be generated. If the nucleic acid sequence passes, the resistance of the nanochannel will be changed due to the physical occupation, which will lead to a change in the ion current, and the purpose of the detection will be achieved by monitoring the change in this current. The above-mentioned miRNA can be used as biomarkers of cancer, and they are also nucleic acid sequences that can be secreted by tumor cells into the circulatory system. Therefore, nanoscale technology will be a good aid for liquid biopsy to detect HCC.
The close link between the liver and the gut gives us another object of study in which changes in the gut microenvironment may indicate changes in the liver microenvironment. The intestinal flora is called a “new virtual metabolic organ”, which can play an “axis” role in the liver. Normally, the tight junctions of intestinal epithelial cells are a natural barrier for bacteria and their metabolites to enter the blood circulation. However, in patients with chronic liver diseases, the detoxification, degradation and clearance of lipopolysaccharide and other bacterial products are inhibited, and intestinal epithelial permeability is increased, which allows lipopolysaccharide and peptidoglycan to enter the portal circulation, thereby increasing the inflammation. When we focus on the culprits that alter the gut microenvironment - the intestinal flora, we may be able to peek into the development of liver disease.
Most HCC develop from hepatitis or cirrhosis, which are different from other cancers. Therefore, we should make early diagnosis and early treatment, actively implement individualized diagnosis, provide a reasonable diagnosis plan for each “pre-cancer patient”, and prevent the progression of HCC in a timely manner, eventually reducing the overall incidence and mortality of HCC.
Conclusion
Most HCC patients have fatty liver, liver cirrhosis and other diseases in the early stage. For these patients, regular ultrasound and minimally invasive diagnosis should be used for early screening. Meanwhile, we should actively search for more suitable serum markers of HCC to improve the sensitivity and specificity of early screening. For CT, MRI and liver biopsy, these detection methods are relatively complex and should be used for diagnosis and even the final diagnosis. The clinical application of the new liquid biopsy technology is temporarily limited. However, it is possible to break this limitation by searching for specific substances secreted from the surface of circulating HCC cells. For the prognosis of HCC patients after treatment, the long-term course and treatment require multiple examinations, and thus, if we continue to apply routine diagnostic technologies, it will undoubtedly increase the burden of the patients and hospitals, so we need to look for newer biomarkers. With the development of diagnostic and therapeutic techniques, we believe that HCC will become a medically overcoming problem in the future.
Author contributions
JD and TX designed the study. QY, YSS and RA were major contributors in writing the manuscript. FL and ZW made substantial contributions to the design of the manuscript and revised it critically for important intellectual content. QF and LJC created all the figures. All authors read and approved the final manuscript.
Conflict of interests
Authors declare no conflict of interests.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81970542, 82072300, 81871674 and 81902084).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Tao Xu, Email: ahmuxutao@163.com.
Lijian Chen, Email: chenlijian77@126.com.
Jian Du, Email: dujane@163.com.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 3.Yu K., Mei Y., Wang Z., Liu B., Deng M. LncRNA LINC00924 upregulates NDRG2 to inhibit epithelial-mesenchymal transition via sponging miR-6755-5p in hepatitis B virus-related hepatocellular carcinoma. J Med Virol. 2022;94(6):2702–2713. doi: 10.1002/jmv.27578. [DOI] [PubMed] [Google Scholar]
- 4.Chu Y.J., Yang H.I., Wu H.C., et al. Aflatoxin B1 exposure increases the risk of hepatocellular carcinoma associated with hepatitis C virus infection or alcohol consumption. Eur J Cancer. 2018;94:37–46. doi: 10.1016/j.ejca.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant A., Neuberger J. Guidelines on the use of liver biopsy in clinical practice. British Society of Gastroenterology. Gut. 1999;45(Suppl 4):IV1–IV11. doi: 10.1136/gut.45.2008.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rastogi A. Changing role of histopathology in the diagnosis and management of hepatocellular carcinoma. World J Gastroenterol. 2018;24(35):4000–4013. doi: 10.3748/wjg.v24.i35.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruix J., Sherman M., Llovet J.M., et al. Clinical management of hepatocellular carcinoma. conclusions of the Barcelona-2000 EASL conference. European association for the study of the liver. J Hepatol. 2001;35(3):421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 8.Kim T.K., Lee K.H., Jang H.J., et al. Analysis of gadobenate dimeglumine-enhanced MR findings for characterizing small (1-2-cm) hepatic nodules in patients at high risk for hepatocellular carcinoma. Radiology. 2011;259(3):730–738. doi: 10.1148/radiol.11101549. [DOI] [PubMed] [Google Scholar]
- 9.Hartke J., Johnson M., Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 2017;34(2):153–159. doi: 10.1053/j.semdp.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 10.European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Howry D.H., Bliss W.R. Ultrasonic visualization of soft tissue structures of the body. J Lab Clin Med. 1952;40(4):579–592. [PubMed] [Google Scholar]
- 12.Heimbach J.K., Kulik L.M., Finn R.S., et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 13.Gramiak R., Shah P.M. Echocardiography of the aortic root. Invest Radiol. 1968;3(5):356–366. doi: 10.1097/00004424-196809000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Spann S., Fetzer D.T. Contrast-enhanced ultrasound in chronic liver diseases. Magn Reson Imag Clin N Am. 2021;29(3):291–304. doi: 10.1016/j.mric.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Choi J.Y., Lee J.M., Sirlin C.B. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014;273(1):30–50. doi: 10.1148/radiol.14132362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omata M., Cheng A.L., Kokudo N., et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindner J.R. Contrast echocardiography: current status and future directions. Heart. 2021;107(1):18–24. doi: 10.1136/heartjnl-2020-316662. [DOI] [PubMed] [Google Scholar]
- 18.Knight A.E., Trutna C.A., Rouze N.C., et al. Full characterization of in vivo muscle as an elastic, incompressible, transversely isotropic material using ultrasonic rotational 3D shear wave elasticity imaging. IEEE Trans Med Imag. 2022;41(1):133–144. doi: 10.1109/TMI.2021.3106278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalender W.A. X-ray computed tomography. Phys Med Biol. 2006;51(13):R29–R43. doi: 10.1088/0031-9155/51/13/R03. [DOI] [PubMed] [Google Scholar]
- 20.Hu H. Multi-slice helical CT: scan and reconstruction. Med Phys. 1999;26(1):5–18. doi: 10.1118/1.598470. [DOI] [PubMed] [Google Scholar]
- 21.Shi C., Xian M., Zhou X., Wang H., Cheng H.D. Multi-slice low-rank tensor decomposition based multi-atlas segmentation: application to automatic pathological liver CT segmentation. Med Image Anal. 2021;73:102152. doi: 10.1016/j.media.2021.102152. [DOI] [PubMed] [Google Scholar]
- 22.Ma A.D., Zhang Y., Xue Z., Li K. Angiogenesis of hepatocellular carcinoma under multislice spiral CT plain scan and enhanced scan. J Biol Regul Homeost Agents. 2015;29(4):895–903. [PubMed] [Google Scholar]
- 23.Menon D.K., Peden C.J., Hall A.S., Sargentoni J., Whitwam J.G. Magnetic resonance for the anaesthetist. Part I: physical principles, applications, safety aspects. Anaesthesia. 1992;47(3):240–255. doi: 10.1111/j.1365-2044.1992.tb02130.x. [DOI] [PubMed] [Google Scholar]
- 24.Phillips C.G., Zeki S., Barlow H.B. Localization of function in the cerebral cortex. Past, present and future. Brain. 1984;107(Pt 1):327–361. [PubMed] [Google Scholar]
- 25.Aouidat F., Boumati S., Khan M., Tielens F., Doan B.T., Spadavecchia J. Design and synthesis of gold-gadolinium-core-shell nanoparticles as contrast agent: a smart way to future nanomaterials for nanomedicine applications. Int J Nanomed. 2019;14:9309–9324. doi: 10.2147/IJN.S224805. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Vuong Q.L., Van Doorslaer S., Bridot J.L., et al. Paramagnetic nanoparticles as potential MRI contrast agents: characterization, NMR relaxation, simulations and theory. MAGMA. 2012;25(6):467–478. doi: 10.1007/s10334-012-0326-7. [DOI] [PubMed] [Google Scholar]
- 27.Ji S., Wang Z., Xia S. Application of ultrasound combined with enhanced MRI by Gd-BOPTA in diagnosing hepatocellular carcinoma. Am J Transl Res. 2021;13(6):7172–7178. [PMC free article] [PubMed] [Google Scholar]
- 28.Van Beers B.E., Pastor C.M., Hussain H.K. Primovist, eovist: what to expect? J Hepatol. 2012;57(2):421–429. doi: 10.1016/j.jhep.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 29.Di Martino M., De Filippis G., De Santis A., et al. Hepatocellular carcinoma in cirrhotic patients: prospective comparison of US, CT and MR imaging. Eur Radiol. 2013;23(4):887–896. doi: 10.1007/s00330-012-2691-z. [DOI] [PubMed] [Google Scholar]
- 30.Murakami T., Sofue K., Hori M. Diagnosis of hepatocellular carcinoma using Gd-EOB-DTPA MR imaging. Magn Reson Med Sci. 2022;21(1):168–181. doi: 10.2463/mrms.rev.2021-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y.C., Chou C.T., Lin C.P., Chen Y.L., Chen Y.F., Chen R.C. The value of Gd-EOB-DTPA-enhanced MR imaging in characterizing cirrhotic nodules with atypical enhancement on Gd-DTPA-enhanced MR images. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0174594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Funk E., Thunberg P., Anderzen-Carlsson A. Patients' experiences in magnetic resonance imaging (MRI) and their experiences of breath holding techniques. J Adv Nurs. 2014;70(8):1880–1890. doi: 10.1111/jan.12351. [DOI] [PubMed] [Google Scholar]
- 33.Halbrecht I., Klibanski C. Identification of a new normal embryonic haemoglobin. Nature. 1956;178(4537):794–795. doi: 10.1038/178794a0. [DOI] [PubMed] [Google Scholar]
- 34.Tatarinov IuS. Detection of embryo-specific alpha-globulin in the blood serum of a patient with primary liver cancer. Vopr Med Khim. 1964;10:90–91. [PubMed] [Google Scholar]
- 35.Hu X., Chen R., Wei Q., Xu X. The landscape of alpha fetoprotein in hepatocellular carcinoma: where are we? Int J Biol Sci. 2022;18(2):536–551. doi: 10.7150/ijbs.64537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang S.L., Liu L.P., Yang S., et al. Preoperative serum alpha-fetoprotein and prognosis after hepatectomy for hepatocellular carcinoma. Br J Surg. 2016;103(6):716–724. doi: 10.1002/bjs.10093. [DOI] [PubMed] [Google Scholar]
- 37.Choucair K., Kamran S., Saeed A. Clinical evaluation of Ramucirumab for the treatment of hepatocellular carcinoma (HCC): place in therapy. Onco Targets Ther. 2021;14:5521–5532. doi: 10.2147/OTT.S268309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Department of Medical Administration National health and health commission of the People's Republic of China. guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition) Zhonghua Gan Zang Bing Za Zhi. 2020;28(2):112–128. doi: 10.3760/cma.j.issn.1007-3418.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 39.European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Tzartzeva K., Obi J., Rich N.E., et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology. 2018;154(6):1706–1718.e1. doi: 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simmons O., Fetzer D.T., Yokoo T., et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45(1):169–177. doi: 10.1111/apt.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe T., Tsuchiya A., Takeuchi S., et al. Development of a non-alcoholic steatohepatitis model with rapid accumulation of fibrosis, and its treatment using mesenchymal stem cells and their small extracellular vesicles. Regen Ther. 2020;14:252–261. doi: 10.1016/j.reth.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Worland T., Harrison B., Delmenico L., Dowling D. Hepatocellular carcinoma screening utilising serum alpha-fetoprotein measurement and abdominal ultrasound is more effective than ultrasound alone in patients with non-viral cirrhosis. J Gastrointest Cancer. 2018;49(4):476–480. doi: 10.1007/s12029-017-0006-y. [DOI] [PubMed] [Google Scholar]
- 44.Taketa K. Characterization of sugar chain structures of human alpha-fetoprotein by lectin affinity electrophoresis. Electrophoresis. 1998;19(15):2595–2602. doi: 10.1002/elps.1150191506. [DOI] [PubMed] [Google Scholar]
- 45.Li D., Mallory T., Satomura S. AFP-L3: a new generation of tumor marker for hepatocellular carcinoma. Clin Chim Acta. 2001;313(1–2):15–19. doi: 10.1016/s0009-8981(01)00644-1. [DOI] [PubMed] [Google Scholar]
- 46.Liebman H.A., Furie B.C., Tong M.J., et al. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310(22):1427–1431. doi: 10.1056/NEJM198405313102204. [DOI] [PubMed] [Google Scholar]
- 47.Kokudo N., Makuuchi M. Evidence-based clinical practice guidelines for hepatocellular carcinoma in Japan: the J-HCC guidelines. J Gastroenterol. 2009;44(Suppl 19):119–121. doi: 10.1007/s00535-008-2244-z. [DOI] [PubMed] [Google Scholar]
- 48.Kokudo N., Takemura N., Hasegawa K., et al. Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49(10):1109–1113. doi: 10.1111/hepr.13411. [DOI] [PubMed] [Google Scholar]
- 49.Park S.J., Jang J.Y., Jeong S.W., et al. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine (Baltim) 2017;96(11) doi: 10.1097/MD.0000000000005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Best J., Bilgi H., Heider D., et al. The GALAD scoring algorithm based on AFP, AFP-L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma. Z Gastroenterol. 2016;54(12):1296–1305. doi: 10.1055/s-0042-119529. [DOI] [PubMed] [Google Scholar]
- 51.Gao J., Song P. Combination of triple biomarkers AFP, AFP-L3, and PIVAKII for early detection of hepatocellular carcinoma in China: expectation. Drug Discov Ther. 2017;11(3):168–169. doi: 10.5582/ddt.2017.01036. [DOI] [PubMed] [Google Scholar]
- 52.Piratvisuth T., Tanwandee T., Thongsawat S., et al. Multimarker panels for detection of early stage hepatocellular carcinoma: a prospective, multicenter, case-control study. Hepatol Commun. 2022;6(4):679–691. doi: 10.1002/hep4.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qi F., Zhou A., Yan L., et al. The diagnostic value of PIVKA-II, AFP, AFP-L3, CEA, and their combinations in primary and metastatic hepatocellular carcinoma. J Clin Lab Anal. 2020;34(5) doi: 10.1002/jcla.23158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Filmus J., Capurro M., Rast J. Glypicans. Genome Biol. 2008;9(5):224. doi: 10.1186/gb-2008-9-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li N., Gao W., Zhang Y.F., Ho M. Glypicans as cancer therapeutic targets. Trends Cancer. 2018;4(11):741–754. doi: 10.1016/j.trecan.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Capurro M.I., Xu P., Shi W., Li F., Jia A., Filmus J. Glypican-3 inhibits hedgehog signaling during development by competing with patched for hedgehog binding. Dev Cell. 2008;14(5):700–711. doi: 10.1016/j.devcel.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 57.Guo M., Zhang H., Zheng J., Liu Y. Glypican-3: a new target for diagnosis and treatment of hepatocellular carcinoma. J Cancer. 2020;11(8):2008–2021. doi: 10.7150/jca.39972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou F., Shang W., Yu X., Tian J. Glypican-3: a promising biomarker for hepatocellular carcinoma diagnosis and treatment. Med Res Rev. 2018;38(2):741–767. doi: 10.1002/med.21455. [DOI] [PubMed] [Google Scholar]
- 59.Hsu H.C., Cheng W., Lai P.L. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: biological significance and temporospatial distribution. Cancer Res. 1997;57(22):5179–5184. [PubMed] [Google Scholar]
- 60.Yao M., Wang L., Fang M., Zheng W., Dong Z., Yao D. Advances in the study of oncofetal antigen glypican-3 expression in HBV-related hepatocellular carcinoma. Biosci Trends. 2016;10(5):337–343. doi: 10.5582/bst.2016.01176. [DOI] [PubMed] [Google Scholar]
- 61.Sun B., Huang Z., Wang B., et al. Significance of glypican-3 (GPC3) expression in hepatocellular cancer diagnosis. Med Sci Mon Int Med J Exp Clin Res. 2017;23:850–855. doi: 10.12659/MSM.899198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deugnier Y., David V., Brissot P., et al. Serum alpha-L-fucosidase: a new marker for the diagnosis of primary hepatic carcinoma? Hepatology. 1984;4(5):889–892. doi: 10.1002/hep.1840040516. [DOI] [PubMed] [Google Scholar]
- 63.Fawzy Montaser M., Amin Sakr M., Omar Khalifa M. Alpha-L-fucosidase as a tumour marker of hepatocellular carcinoma. Arab J Gastroenterol. 2012;13(1):9–13. doi: 10.1016/j.ajg.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 64.Xing H., Qiu H., Ding X., et al. Clinical performance of alpha-L-fucosidase for early detection of hepatocellular carcinoma. Biomarkers Med. 2019;13(7):545–555. doi: 10.2217/bmm-2018-0414. [DOI] [PubMed] [Google Scholar]
- 65.Mossad N.A., Mahmoud E.H., Osman E.A., Mahmoud S.H., Shousha H.I. Evaluation of squamous cell carcinoma antigen-immunoglobulin M complex (SCCA-IGM) and alpha-L-fucosidase (AFU) as novel diagnostic biomarkers for hepatocellular carcinoma. Tumour Biol. 2014;35(11):11559–11564. doi: 10.1007/s13277-014-2467-y. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y., Hu X., Liu S., Zhou S., Chen Z., Jin H. Golgi phosphoprotein 73: the driver of epithelial-mesenchymal transition in cancer. Front Oncol. 2021;11:783860. doi: 10.3389/fonc.2021.783860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kladney R.D., Bulla G.A., Guo L., et al. GP73, a novel Golgi-localized protein upregulated by viral infection. Gene. 2000;249(1–2):53–65. doi: 10.1016/S0378-1119(00)00136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gatselis N.K., Tornai T., Shums Z., et al. Golgi protein-73: a biomarker for assessing cirrhosis and prognosis of liver disease patients. World J Gastroenterol. 2020;26(34):5130–5145. doi: 10.3748/wjg.v26.i34.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiao C., Cui L., Piao J., Qi Y., Yu Z. Clinical significance and expression of serum Golgi protein 73 in primary hepatocellular carcinoma. J Cancer Res Therapeut. 2018;14(6):1239–1244. doi: 10.4103/0973-1482.199784. [DOI] [PubMed] [Google Scholar]
- 70.Kladney R.D., Cui X., Bulla G.A., Brunt E.M., Fimmel C.J. Expression of GP73, a resident golgi membrane protein, in viral and nonviral liver disease. Hepatology. 2002;35(6):1431–1440. doi: 10.1053/jhep.2002.32525. [DOI] [PubMed] [Google Scholar]
- 71.Gao G., Dong F., Xu X., Hu A., Hu Y. Diagnostic value of serum golgi protein 73 for HBV-related primary hepatic carcinoma. Int J Clin Exp Pathol. 2015;8(9):11379–11385. [PMC free article] [PubMed] [Google Scholar]
- 72.Riener M.O., Stenner F., Liewen H., et al. Golgi phosphoprotein 2 (GOLPH2) expression in liver tumors and its value as a serum marker in hepatocellular carcinomas. Hepatology. 2009;49(5):1602–1609. doi: 10.1002/hep.22843. [DOI] [PubMed] [Google Scholar]
- 73.Hou S.C., Xiao M.B., Ni R.Z., et al. Serum GP73 is complementary to AFP and GGT-II for the diagnosis of hepatocellular carcinoma. Oncol Lett. 2013;6(4):1152–1158. doi: 10.3892/ol.2013.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bartolomeo N., Trerotoli P., Serio G. Progression of liver cirrhosis to HCC: an application of hidden Markov model. BMC Med Res Methodol. 2011;11:38. doi: 10.1186/1471-2288-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang D.Q., El-Serag H.B., Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18(4):223–238. doi: 10.1038/s41575-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walker M., El-Serag H.B., Sada Y., et al. Cirrhosis is under-recognised in patients subsequently diagnosed with hepatocellular cancer. Aliment Pharmacol Ther. 2016;43(5):621–630. doi: 10.1111/apt.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu S., Ericson M., Fanjul A., et al. Genome-wide CRISPR screening to identify drivers of TGF-β-induced liver fibrosis in human hepatic stellate cells. ACS Chem Biol. 2022;17(4):918–929. doi: 10.1021/acschembio.2c00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Castera L., Friedrich-Rust M., Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(5):1264–1281. doi: 10.1053/j.gastro.2018.12.036. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Angulo P., Hui J.M., Marchesini G., et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 80.Lin Z.H., Xin Y.N., Dong Q.J., et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53(3):726–736. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 81.Sterling R.K., Lissen E., Clumeck N., et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 82.Kim G.A., Lee H.C., Choe J., et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol. 2017;S0168-S8278(17):32294–32298. doi: 10.1016/j.jhep.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 83.Lee J., Vali Y., Boursier J., et al. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: a systematic review. Liver Int. 2021;41(2):261–270. doi: 10.1111/liv.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Younes R., Caviglia G.P., Govaere O., et al. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with non-alcoholic fatty liver disease. J Hepatol. 2021;75(4):786–794. doi: 10.1016/j.jhep.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 85.European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 86.Ampuero J., Pais R., Aller R., et al. Development and validation of hepamet fibrosis scoring system-A simple, noninvasive test to identify patients with nonalcoholic fatty liver disease with advanced fibrosis. Clin Gastroenterol Hepatol. 2020;18(1):216–225. doi: 10.1016/j.cgh.2019.05.051. e5. [DOI] [PubMed] [Google Scholar]
- 87.Vilar-Gomez E., Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: clinical prediction rules and blood-based biomarkers. J Hepatol. 2018;68(2):305–315. doi: 10.1016/j.jhep.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 88.Sorrells R.B. Synovioanalysis (“liquid biopsy”) J Ark Med Soc. 1974;71(1):59–62. [PubMed] [Google Scholar]
- 89.Pantel K., Alix-Panabières C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med. 2010;16(9):398–406. doi: 10.1016/j.molmed.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 90.Guo L., Xu D., Lu Y., Peng J., Jiang L. Detection of circulating tumor cells by reverse transcription-quantitative polymerase chain reaction and magnetic activated cell sorting in the peripheral blood of patients with hepatocellular carcinoma. Mol Med Rep. 2017;16(5):5894–5900. doi: 10.3892/mmr.2017.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu J., Wang Z., Zhang H., Wang Y., Li D.Q. Survivin-positive circulating tumor cells as a marker for metastasis of hepatocellular carcinoma. World J Gastroenterol. 2021;27(43):7546–7562. doi: 10.3748/wjg.v27.i43.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y., Zhang X., Zhang J., et al. Microfluidic chip for isolation of viable circulating tumor cells of hepatocellular carcinoma for their culture and drug sensitivity assay. Cancer Biol Ther. 2016;17(11):1177–1187. doi: 10.1080/15384047.2016.1235665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Correnti M., Raggi C. Stem-like plasticity and heterogeneity of circulating tumor cells: current status and prospect challenges in liver cancer. Oncotarget. 2017;8(4):7094–7115. doi: 10.18632/oncotarget.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grützkau A., Radbruch A. Small but mighty: how the MACS-technology based on nanosized superparamagnetic particles has helped to analyze the immune system within the last 20 years. Cytometry A. 2010;77(7):643–647. doi: 10.1002/cyto.a.20918. [DOI] [PubMed] [Google Scholar]
- 95.Molday R.S., Yen S.P., Rembaum A. Application of magnetic microspheres in labelling and separation of cells. Nature. 1977;268(5619):437–438. doi: 10.1038/268437a0. [DOI] [PubMed] [Google Scholar]
- 96.Wang X., Sun L., Zhang H., et al. Microfluidic chip combined with magnetic-activated cell sorting technology for tumor antigen-independent sorting of circulating hepatocellular carcinoma cells. PeerJ. 2019;7 doi: 10.7717/peerj.6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mühlberger M., Janko C., Unterweger H., et al. Non-magnetic chromatographic separation of colloidally metastable superparamagnetic iron oxide nanoparticles and suspension cells. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1122–1123:83–89. doi: 10.1016/j.jchromb.2019.05.033. [DOI] [PubMed] [Google Scholar]
- 98.Lin S., Zhi X., Chen D., et al. A flyover style microfluidic chip for highly purified magnetic cell separation. Biosens Bioelectron. 2019;129:175–181. doi: 10.1016/j.bios.2018.12.058. [DOI] [PubMed] [Google Scholar]
- 99.Zhang X., Wei X., Men X., et al. Dual-multivalent-aptamer-conjugated nanoprobes for superefficient discerning of single circulating tumor cells in a microfluidic chip with inductively coupled plasma mass spectrometry detection. ACS Appl Mater Interfaces. 2021;13(36):43668–43675. doi: 10.1021/acsami.1c11953. [DOI] [PubMed] [Google Scholar]
- 100.Kimura H., Sakai Y., Fujii T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metabol Pharmacokinet. 2018;33(1):43–48. doi: 10.1016/j.dmpk.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 101.Li J., Chen L., Zhang X., et al. Detection of circulating tumor cells in hepatocellular carcinoma using antibodies against asialoglycoprotein receptor, carbamoyl phosphate synthetase 1 and pan-cytokeratin. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0096185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Watson J.D., Crick F.H. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953;171(4356):737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- 103.Stein L.D. The case for cloud computing in genome informatics. Genome Biol. 2010;11(5):207. doi: 10.1186/gb-2010-11-5-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Dijk E.L., Auger H., Jaszczyszyn Y., Thermes C. Ten years of next-generation sequencing technology. Trends Genet. 2014;30(9):418–426. doi: 10.1016/j.tig.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y., Wendte J.M., Ji L., Schmitz R.J. Natural variation in DNA methylation homeostasis and the emergence of epialleles. Proc Natl Acad Sci U S A. 2020;117(9):4874–4884. doi: 10.1073/pnas.1918172117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin Q., Chen J.W., Yin H., et al. DNA N6-methyladenine involvement and regulation of hepatocellular carcinoma development. Genomics. 2022;114(2):110265. doi: 10.1016/j.ygeno.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 107.Mehdipour P., Murphy T., De Carvalho D.D. The role of DNA-demethylating agents in cancer therapy. Pharmacol Ther. 2020;205:107416. doi: 10.1016/j.pharmthera.2019.107416. [DOI] [PubMed] [Google Scholar]
- 108.Saghafinia S., Mina M., Riggi N., Hanahan D., Ciriello G. Pan-cancer landscape of aberrant DNA methylation across human tumors. Cell Rep. 2018;25(4):1066–1080. doi: 10.1016/j.celrep.2018.09.082. e8. [DOI] [PubMed] [Google Scholar]
- 109.Huo M., Zhang J., Huang W., Wang Y. Interplay among metabolism, epigenetic modifications, and gene expression in cancer. Front Cell Dev Biol. 2021;9:793428. doi: 10.3389/fcell.2021.793428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Matei D., Nephew K.P. Epigenetic attire in ovarian cancer: the emperor's new clothes. Cancer Res. 2020;80(18):3775–3785. doi: 10.1158/0008-5472.CAN-19-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nowacka-Zawisza M., Wiśnik E. DNA methylation and histone modifications as epigenetic regulation in prostate cancer (Review) Oncol Rep. 2017;38(5):2587–2596. doi: 10.3892/or.2017.5972. [DOI] [PubMed] [Google Scholar]
- 112.Cai J., Chen L., Zhang Z., et al. Genome-wide mapping of 5-hydroxymethylcytosines in circulating cell-free DNA as a non-invasive approach for early detection of hepatocellular carcinoma. Gut. 2019;68(12):2195–2205. doi: 10.1136/gutjnl-2019-318882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.O'Brien J., Hayder H., Zayed Y., Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne) 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ali Syeda Z., Langden S.S.S., Munkhzul C., Lee M., Song S.J. Regulatory mechanism of micro RNA expression in cancer. Int J Mol Sci. 2020;21(5):1723. doi: 10.3390/ijms21051723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Van Roosbroeck K., Calin G.A. Cancer hallmarks and microRNAs: the therapeutic connection. Adv Cancer Res. 2017;135:119–149. doi: 10.1016/bs.acr.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 116.Amr K.S., Elmawgoud Atia H.A., Elazeem Elbnhawy R.A., Ezzat W.M. Early diagnostic evaluation of miR-122 and miR-224 as biomarkers for hepatocellular carcinoma. Genes Dis. 2017;4(4):215–221. doi: 10.1016/j.gendis.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Urbańska K., Orzechowski A. Unappreciated role of LDHA and LDHB to control apoptosis and autophagy in tumor cells. Int J Mol Sci. 2019;20(9):2085. doi: 10.3390/ijms20092085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xiao W., Liu W., Yin L., et al. Serum hydroxybutyrate dehydrogenase as an early predictive marker of the severity of acute pancreatitis: a retrospective study. BMC Gastroenterol. 2020;20(1):393. doi: 10.1186/s12876-020-01521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tan P., Chen J., Xie N., et al. Is preoperative serum lactate dehydrogenase useful in predicting the outcomes of patients with upper tract urothelial carcinoma? Cancer Med. 2018;7(10):5096–5106. doi: 10.1002/cam4.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tao J., Yang G., Zhou W., et al. Targeting hypoxic tumor microenvironment in pancreatic cancer. J Hematol Oncol. 2021;14(1):14. doi: 10.1186/s13045-020-01030-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thienpont B., Steinbacher J., Zhao H., et al. Tumour hypoxia causes DNA hypermethylation by reducing TET activity. Nature. 2016;537(7618):63–68. doi: 10.1038/nature19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang X., Zhao D., Xie H., Hu Y. Interplay of long non-coding RNAs and HIF-1α: a new dimension to understanding hypoxia-regulated tumor growth and metastasis. Cancer Lett. 2021;499:49–59. doi: 10.1016/j.canlet.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 123.Elzakra N., Kim Y. HIF-1α metabolic pathways in human cancer. Adv Exp Med Biol. 2021;1280:243–260. doi: 10.1007/978-3-030-51652-9_17. [DOI] [PubMed] [Google Scholar]
- 124.Hu Q., Qin Y., Ji S., et al. UHRF1 promotes aerobic glycolysis and proliferation via suppression of SIRT4 in pancreatic cancer. Cancer Lett. 2019;452:226–236. doi: 10.1016/j.canlet.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 125.Li T., Mao C., Wang X., Shi Y., Tao Y. Epigenetic crosstalk between hypoxia and tumor driven by HIF regulation. J Exp Clin Cancer Res. 2020;39(1):224. doi: 10.1186/s13046-020-01733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rashid M., Zadeh L.R., Baradaran B., et al. Up-down regulation of HIF-1α in cancer progression. Gene. 2021;798:145796. doi: 10.1016/j.gene.2021.145796. [DOI] [PubMed] [Google Scholar]
- 127.Xiong Q., Liu B., Ding M., Zhou J., Yang C., Chen Y. Hypoxia and cancer related pathology. Cancer Lett. 2020;486:1–7. doi: 10.1016/j.canlet.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 128.Guo L., Ren H., Pu L., Zhu X., Liu Y., Ma X. The prognostic value of inflammation factors in hepatocellular carcinoma patients with hepatic artery interventional treatments: a retrospective study. Cancer Manag Res. 2020;12:7173–7188. doi: 10.2147/CMAR.S257934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kong W., Zuo X., Liang H., et al. Prognostic value of lactate dehydrogenase in patients with hepatocellular carcinoma: a meta-analysis. BioMed Res Int. 2018:1723184. doi: 10.1155/2018/1723184. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Faloppi L., Scartozzi M., Bianconi M., et al. The role of LDH serum levels in predicting global outcome in HCC patients treated with sorafenib: implications for clinical management. BMC Cancer. 2014;14:110. doi: 10.1186/1471-2407-14-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Faloppi L., Bianconi M., Memeo R., et al. Lactate dehydrogenase in hepatocellular carcinoma: something old, something new. BioMed Res Int. 2016;2016:7196280. doi: 10.1155/2016/7196280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Scartozzi M., Faloppi L., Bianconi M., et al. The role of LDH serum levels in predicting global outcome in HCC patients undergoing TACE: implications for clinical management. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang J.P., Wang H.B., Lin Y.H., et al. Lactate dehydrogenase is an important prognostic indicator for hepatocellular carcinoma after partial hepatectomy. Transl Oncol. 2015;8(6):497–503. doi: 10.1016/j.tranon.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Endo T.A., Masuhara M., Yokouchi M., et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387(6636):921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 135.Keewan E., Matlawska-Wasowska K. The emerging role of suppressors of cytokine signaling (SOCS) in the development and progression of leukemia. Cancers. 2021;13(16):4000. doi: 10.3390/cancers13164000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Linossi E.M., Nicholson S.E. Kinase inhibition, competitive binding and proteasomal degradation: resolving the molecular function of the suppressor of cytokine signaling (SOCS) proteins. Immunol Rev. 2015;266(1):123–133. doi: 10.1111/imr.12305. [DOI] [PubMed] [Google Scholar]
- 137.Xie J., Wang M., Cheng A., et al. The role of SOCS proteins in the development of virus- induced hepatocellular carcinoma. Virol J. 2021;18(1):74. doi: 10.1186/s12985-021-01544-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jiang B.G., Wang N., Huang J., et al. Tumor SOCS3 methylation status predicts the treatment response to TACE and prognosis in HCC patients. Oncotarget. 2017;8(17):28621–28627. doi: 10.18632/oncotarget.16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Niwa Y., Kanda H., Shikauchi Y., et al. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene. 2005;24(42):6406–6417. doi: 10.1038/sj.onc.1208788. [DOI] [PubMed] [Google Scholar]
- 140.Pasha H.F., Mohamed R.H., Radwan M.I. RASSF1A and SOCS1 genes methylation status as a non-invasive marker for hepatocellular carcinoma. Cancer Biomarkers. 2019;24(2):241–247. doi: 10.3233/CBM-181638. [DOI] [PubMed] [Google Scholar]
- 141.Zhang M., Liu S., Chua M.S., et al. SOCS5 inhibition induces autophagy to impair metastasis in hepatocellular carcinoma cells via the PI3K/Akt/mTOR pathway. Cell Death Dis. 2019;10(8):612. doi: 10.1038/s41419-019-1856-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Steinberg B.D. Digital beamforming in ultrasound. IEEE Trans Ultrason Ferroelectrics Freq Control. 1992;39(6):716–721. doi: 10.1109/58.165556. [DOI] [PubMed] [Google Scholar]
- 143.Bunce S.M., Hough A.D., Moore A.P. Measurement of abdominal muscle thickness using M-mode ultrasound imaging during functional activities. Man Ther. 2004;9(1):41–44. doi: 10.1016/s1356-689x(03)00069-9. [DOI] [PubMed] [Google Scholar]
- 144.Somer J.C. Electronic sector scanning for ultrasonic diagnosis. Ultrasonics. 1968;6(3):153–159. doi: 10.1016/0041-624x(68)90277-1. [DOI] [PubMed] [Google Scholar]
- 145.Hanna G.B., Shimi S.M., Cuschieri A. Randomised study of influence of two-dimensional versus three-dimensional imaging on performance of laparoscopic cholecystectomy. Lancet. 1998;351(9098):248–251. doi: 10.1016/S0140-6736(97)08005-7. [DOI] [PubMed] [Google Scholar]
- 146.Krejza J., Mariak Z., Walecki J., Szydlik P., Lewko J., Ustymowicz A. Transcranial color Doppler sonography of basal cerebral arteries in 182 healthy subjects: age and sex variability and normal reference values for blood flow parameters. AJR Am J Roentgenol. 1999;172(1):213–218. doi: 10.2214/ajr.172.1.9888770. [DOI] [PubMed] [Google Scholar]