Lysine-specific demethylase 1 (LSD1), as a histone lysine demethylase, demethylates monomethyl and dimethyl of histone H3 on lysine 4 (H3K4) and lysine 9 (H3K9). Studies have reported that high LSD1 expression promotes cell proliferation, migration, and invasion by regulating chromatin morphology and gene expression, and is closely related to the development of non-small cell lung cancer (NSCLC).1 However, the underlying mechanism of LSD1 on cell proliferation and migration remains unclear.
The programmed-death ligand 1 (PD-L1), one of the key immune inhibitory molecules, plays an important role in tumorigenesis by affecting T cell activation and tumor immune response. Interestingly, it is reported that PD-L1 also promotes NSCLC cell proliferation.2 Some reports have revealed that LSD1 expression is associated with PD-L1 level in clinical TNBC specimens and B16 tumors.3,4 We were thus inspired to explore the non-immune functions of PD-L1 in lung adenocarcinoma (LUAD) cell proliferation and migration.
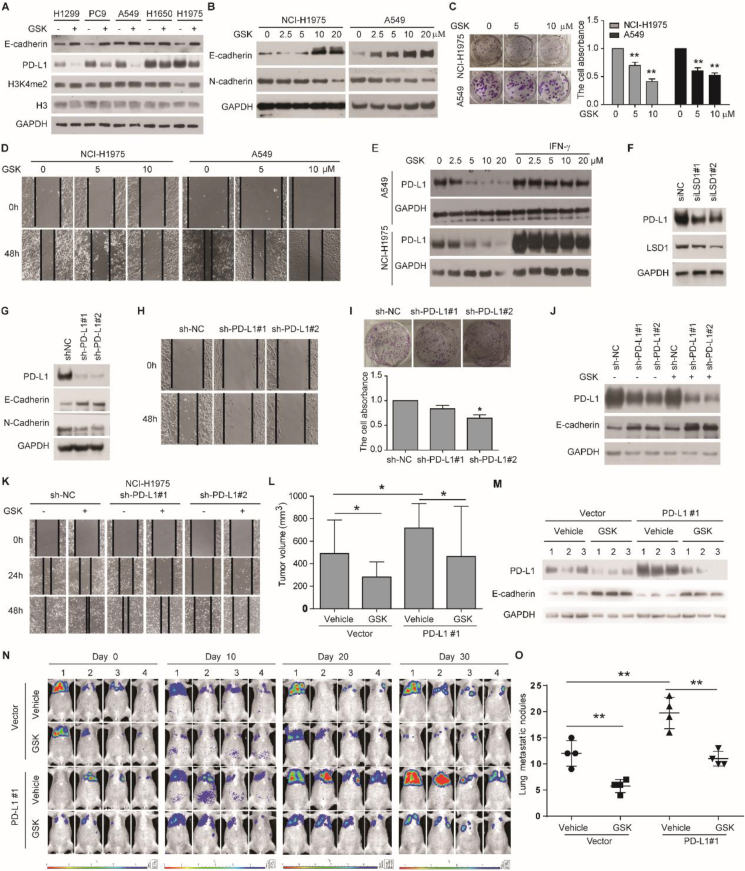
Clinical data from the GEPIA database and the Linkedomics database showed that LSD1 expression in tumor tissues (TCGA-LUAD) was higher than that in normal tissues (Fig. S1A), and LSD1 expression was also higher in T4 stage and M1 stage based on the TNM Classification of Malignant Tumors (Fig. S1B–D). These data suggest that LSD1 is associated with metastasis in LUAD. To investigate the effect of LSD1 on cell proliferation and EMT, specific LSD1 inhibitor GSK-LSD1 2HCL (GSK) was used to treat LUAD cells. The results showed that GSK increased the expression of epithelial protein E-cadherin in all tested LUAD cells (Fig. 1A). Meanwhile, GSK elevated E-cadherin, H3K4me2 and H3K9me2 levels, downregulated the level of mesenchymal protein N-cadherin, and limited clone formation ability and scratch healing ability in a dose-dependent manner in A549 and NCI–H1975 cells (Fig. 1B–D; Fig. S2A, B). Knock-down of LSD1 in NCI–H1975 cells also increased the expression of E-cadherin, and decreased the expression of N-cadherin and scratch healing ability (Fig. S2C, D). Thus, these results support the idea that inhibition of LSD1 prevents cell proliferation and migration in LUAD cell lines.
Figure 1.
Inhibition of LSD1 suppresses tumor growth and metastasis by downregulating PD-L1 expression in lung adenocarcinoma. (A) Expression of E-cadherin, PD-L1, and H3K4me2 in cells treated with 10 μM GSK for 4 days. (B) Expression of E-cadherin and N-cadherin in cells treated with GSK for 4 days. (C) The cellular clone formation ability in cells treated with GSK. (D) The scratch healing ability in cells treated with GSK. (E) Expression of PD-L1 in cells treated with GSK or 10 ng/mL IFN-γ for 4 days. (F) Expression of PD-L1 and LSD1 in NCI–H1975 cells infected with LSD1-specific siRNA and the corresponding negative control. (G) Expression of PD-L1, E-cadherin, and N-cadherin. (H) the scratch healing ability and (I) the cellular clone formation ability in NCI–H1975 cells transfected with sh-NC and sh-PD-L1. (J) Expression of PD-L1 and E-cadherin, and (K) scratch healing ability in NCI–H1975 cells transfected with sh-NC or sh-PD-L1 and treated with 10 μM GSK. (L) Tumor volume. (M) Expression of PD-L1 and E-cadherin in the tumor. (N) Enhanced bioluminescence imaging of mice on day 0, day 10, day 20, and day 30 after GSK injection. (O) Lung metastatic nodules. ∗P < 0.05, ∗∗P < 0.01.
The expression of PD-L1 varied in different cell lines and was significantly higher in A549, NCI–H1650, and NCI–H1975 cells than that in NCI–H1299 and PC-9 cells (Fig. 1A; Fig. S3A). Inconsistent with previous reports,3,4 GSK significantly reduced PD-L1 expression in all LUAD cells (Fig. 1A), in dose- and time-dependent manners in A549 and NCI–H1975 cells (Fig. 1E; Fig. S3B). In addition, inhibition of LSD1 reduced PD-L1 expression stimulated by IFN-γ (Fig. 1E). Knock-down of LSD1 also significantly lowered protein levels of PD-L1 (Fig. 1F; Fig. S3C). Furthermore, cycloheximide alone or in combination with MG132 prevented the PD-L1 inhibited by GSK (Fig. S3D). Meanwhile, we confirmed that GSK significantly decreased PD-L1 mRNA level (Fig. S3E). Although it has been reported that P53 can downregulate PD-L1 expression via miR-34, but GSK did not change P53 expression, regardless of the presence of IFN-γ (Fig. S3F). Taken together, these results strongly suggest that inhibition of LSD1 downregulates PD-L1 expression by reducing the transcription of PD-L1 in LUAD cell lines.
Data from the GEPIA database and the Linkedomics database showed that PD-L1 reduces disease-free survival, over-expresses in the N4 stage, and is negatively correlated with E-cadherin expression in LUAD (Fig. S4A–C). Importantly, knock-down of PD-L1 increased E-cadherin expression, and decreased N-cadherin expression, cell migration ability, scratch healing ability, and clone formation ability (Fig. 1G–I; Fig. S4D, E). Overexpression of PD-L1 had the opposite results (Fig. S4F–H). Thus, PD-L1 is involved in LUAD progression by promoting cell proliferation and migration. To investigate whether PD-L1 participates in the regulation of LSD1 on cell proliferation and migration, we evaluated the effects of GSK on LUAD cancer cells with knock-down or overexpression of PD-L1. Effects of GSK on E-cadherin expression and scratch healing ability were further enhanced by PD-L1 knock-down but prevented by ectopic PD-L1 overexpression (Fig. 1J, K; Fig. S5A–D). After the expression of PD-L1, the inhibition of LSD1 still has a certain effect on tumor growth and migration, indicating that the catalytic activity of LSD1 not only works through PD-L1 but also has other unknown mechanisms, which need to be studied. Therefore, these data suggest that down-regulation of PD-L1 is important for LSD1 inhibitors to inhibit the proliferation and migration of LUAD cells.
It has been reported that IFN-γ regulates PD-L1 expression through the JAK-STAT pathway.5 Here, we found that genetic or pharmacological inhibition of LSD1 significantly reduced the protein levels of IFNGR1, IFNGR2, p-JAK1, and p-JAK2, regardless of the presence of IFN-γ (Fig. S6A–C). Moreover, GSK also downregulated the mRNA level of IFNGR1 and IFNGR2 (Fig. S6D). JAK1 and JAK2 pan inhibitor Ruxolitinib (RUX) decreased the protein levels of p-STAT3 and PD-L1 and increased E-cadherin expression (Fig. S6E). Importantly, the combination of GSK and RUX further decreased the expression of p-STAT3 and PD-L1, and elevated E-cadherin expression (Fig. S6E). Collectively, these results implicate that inhibiting LSD1 downregulates PD-L1 expression and upregulates E-cadherin expression by influencing the JAK pathway.
In the subcutaneous xenograft model, A549 cells transfected with the control vector or PD-L1 vector were inoculated into BALB/C athymic mice, followed by intraperitoneal injection of GSK. The results showed that overexpression of PD-L1 promoted tumor growth and GSK significantly inhibited tumor growth (Fig. 1L; Fig. S7A, B). In addition, PD-L1 overexpression decreased E-cadherin expression, while GSK significantly downregulated PD-L1 expression and upregulated E-cadherin expression in tumor samples (Fig. 1M). Thus, the data demonstrate that PD-L1 expression promotes tumor growth, while GSK inhibited PD-L1 expression and tumor growth in vivo. We also established A549 tumor-metastasis models. The results showed that PD-L1 promoted tumor metastasis, indicated by enhanced bioluminescence imaging and tumor nodules on the lung, while GSK inhibited tumor metastasis (Fig. 1N, O; Fig. S7C). Therefore, PD-L1 promotes lung metastasis, and inhibition of LSD1 has an anti-metastasis effect in vivo.
In conclusion, the major findings of this study are as follows: (i) PD-L1 has non-immune regulatory functions on LUAD cells, including promoting proliferation and migration; (ii) inhibition of LSD1 prevents the LUAD cell proliferation, migration, tumor growth, and metastasis through the downregulation of PD-L1 expression; (iii) inhibition of LSD1 downregulates PD-L1 expression through the JAK pathway. Taken together, LSD1 is first reported to regulate cell proliferation and migration through PD-L1, which provides insights into PD-L1's non-immune functions in LUAD cells.
Author contributions
Pengxing He and Yichao Xu designed the research project. Pengxing He, Linna Du, Pan Hao, Han Yang, Yufei Ren, Huiqin Kang, and Yanli Ding performed the experiments. Pengxing He, Yichao Xu, and Wen Zhao analyzed the data. Pengxing He and Yichao Xu wrote the manuscript; Pengxing He, Yichao Xu, and Hongmin Liu provided critical discussion and revised the paper.
Conflict of interests
The authors declare no conflict of interests.
Funding
This work was supported by the National Natural Science Foundation of China (No. U2004101 for Peng-xing He; No. U21A20416 for Hong-min Liu).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2022.11.002.
Contributor Information
Yichao Xu, Email: xuyc2017@zzu.edu.cn.
Hongmin Liu, Email: liuhm@zzu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Stewart C.A., Byers L.A. Altering the course of small cell lung cancer: targeting cancer stem cells via LSD1 inhibition. Cancer Cell. 2015;28(1):4–6. doi: 10.1016/j.ccell.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du W., Zhu J., Zeng Y., et al. KPNB1-mediated nuclear translocation of PD-L1 promotes non-small cell lung cancer cell proliferation via the Gas6/MerTK signaling pathway. Cell Death Differ. 2021;28(4):1284–1300. doi: 10.1038/s41418-020-00651-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheng W., LaFleur M.W., Nguyen T.H., et al. LSD1 ablation stimulates anti-tumor immunity and enables checkpoint blockade. Cell. 2018;174(3):549–563.e19. doi: 10.1016/j.cell.2018.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin Y., Vasilatos S.N., Chen L., et al. Inhibition of histone lysine-specific demethylase 1 elicits breast tumor immunity and enhances antitumor efficacy of immune checkpoint blockade. Oncogene. 2019;38(3):390–405. doi: 10.1038/s41388-018-0451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song T.L., Nairismägi M.L., Laurensia Y., et al. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood. 2018;132(11):1146–1158. doi: 10.1182/blood-2018-01-829424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.