Triple-negative breast cancer (TNBC) is highly malignant and refractory to immunotherapy through impeding the immune cell infiltration and inflammation in the tumor microenvironment (TME).1 DNA-dependent protein kinase catalytic subunit (DNA-PKcs) is a member of the phosphatidylinositol 3-kinase-related kinase family, which is required for the non-homologous end joining repair.2 The effect of DNA-PKcs on the generation of cytosolic DNA and inflammation response in tumor immuno-environment is not defined. We found a specific DNA-PKcs inhibitor, NU7441, induced cytosolic DNA, stimulator of interferon genes (STING), and retinoic acid-inducible gene I (RIG-I) signals in vitro. In Balb/c immune-competent mice bearing 4T1 TNBC cells, NU7441 impaired the tumor growth and metastasis, and increased the CD45+ leukocytes, CD4+ T cells, CD8+ T cells, and CD1a+ antigen-presenting cells, as well as MHC-I and interferon alpha receptor (IFNAR) in TME. However, in Balb/c athymic nude mice without IFNAR and CD8+ T cells in TME, NU7741 did not influence tumor growth. These results show that inhibition of DNA-PKcs triggers cytosolic DNA sensing and induces an inflamed TME to promote anti-tumoral immunity, which provides a strategy to alter the inflammation and lymphocyte infiltration in TME to increase the efficacy of immunotherapy in TNBC and other cancers with an immune-suppressive TME.
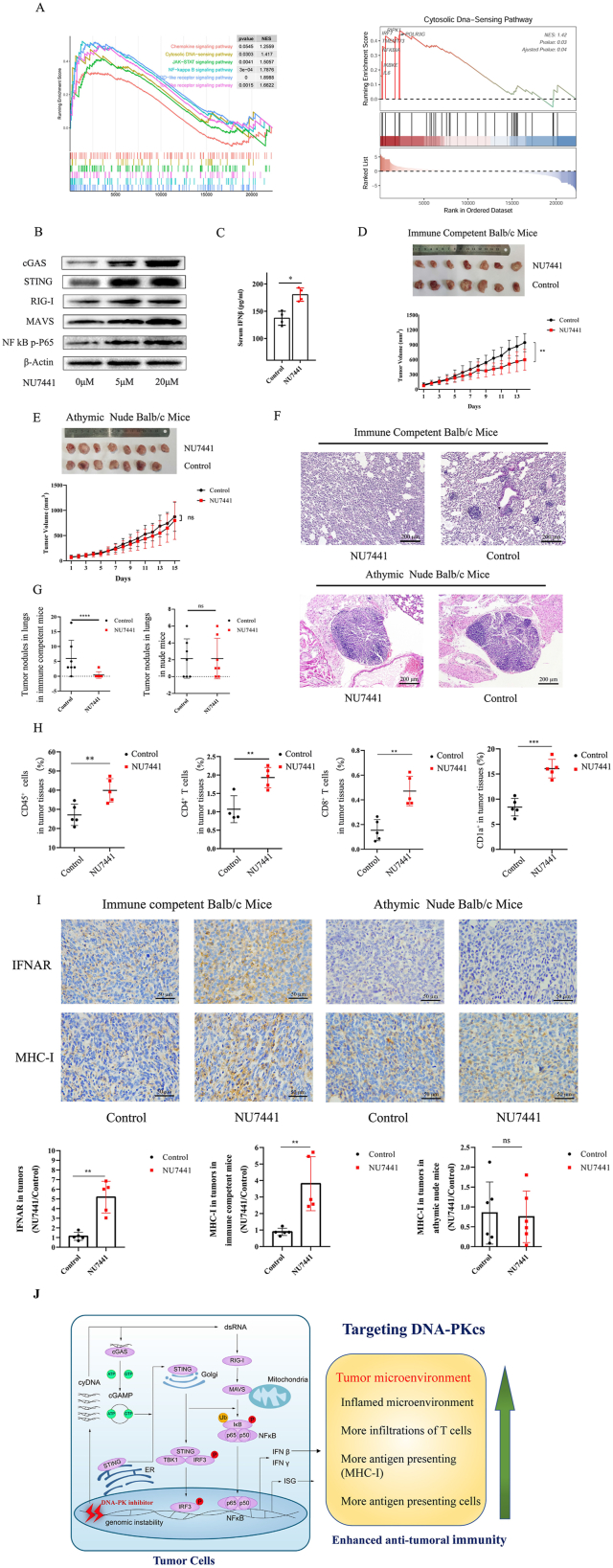
TNBC, characterized by the lack of expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), accounts for about 15% of all breast cancers.3 Chemotherapy is an available treatment opinion for TNBC; however, such tumors rapidly develop resistance to chemotherapy causing a median overall survival of only 8–13 months in metastatic TNBC patients, which is not an acceptable therapy outcome.4 The immune checkpoint inhibitors (ICI), programmed cell death protein 1(PD-1), and programmed death-ligand 1 (PD-L1) would be a promising strategy for TNBC treatment and have been approved in the therapy of metastatic TNBC patients; however, the response rate of metastatic TNBC patients to PD-1/L1 treatment is only about 5%.5 Major impediments of ICI responses in metastatic TNBC are the non-inflamed TME, low antigen presentation, and lymphocyte infiltrations, all of which harshly restrain the efficacy of immunotherapy. Here, we investigated the effects of NU7441 on inflammation in TME and anti-tumoral immunity in TNBC. NU7441 inhibited the growth of human BT549 TNBC cells in a dose-dependent manner (Fig. S1A). Gene ontology analysis of RNA-seq showed NU7441 promoted the leukocyte chemotaxis, migration, and T cell activation (Fig. S1B). Gene Set Enrichment Analysis (GSEA) showed Toll-like receptor signaling, NOD-like signaling, and cytosolic DNA-sensing were up-regulated by NU7441 (Fig. 1A). The factors in cytosolic DNA sensing, STING, IRF7, NFkB, and PolR3G were up-regulated (Fig. 1B), indicating that NU7441 triggers the cytosolic DNA sensing. ELISA results showed NU7441 increased both cell-free dsDNA and ssDNA in the cell culture (Fig. S1C). Western blot showed NU7441 up-regulated cGAS, STING, RIG-I, MAVS, and NFkB P–P65, all of which are involved in cytosolic DNA/RNA sensing and interferon production (Fig. 1C). RT-qPCR showed IFN-I and ISG (interferon-stimulated gene) transcriptions were up-regulated by NU7441 (Fig. S1D, E). To confirm the finding that NU7441 triggers cytosolic DNA sensing and IFN-β, we checked the roles of NU7441 in human MDA-MB-231 TNBC cells and murine CH12F3 lymphoma cells, showing that NU7441 induced cytosolic DNA sensing in MDA-MB-231 cells (Fig. S1F–J) and CH12F3 cells (Fig. S2A–D).
Figure 1.
NU7441 promotes anti-tumoral immunity via triggering cytosolic DNA sensing and inducing an inflamed TME in metastatic triple-negative breast cancer (TNBC). (A) Gene Set Enrichment Analysis (GSEA) showed that Toll-like receptor signaling, NOD-like signaling, and cytosolic DNA-sensing were up-regulated by NU7441. In GESA cytosolic DNA sensing, STING, RIG-I, IRF7, NFkB, and PolR3G were up-regulated. (B) Western blot showed cGAS, STING, RIG-I, MAVS, and P–P65 levels were up-regulated by NU7441 in BT549 cells. (C) In Balb/c mice subcutaneously injected with NU7441(70 mg/kg total dose), ELISA results showed the serum IFN-β was increased by NU7441. (D) NU7441 was intraperitoneally injected into the immune-competent xenograft Balb/c mice bearing 4T1 TNBC cells (100 mg/kg total dose). NU7441 suppressed the tumor growth in immune-competent xenograft Balb/c mice through tumor volume calculation. (E) NU7441 was intraperitoneally injected into athymic nude xenograft Balb/c mice (100 mg/kg total dose). NU7441 did not suppress the tumor growth in athymic nude xenograft mice through tumor volume calculation. (F) The HE staining results showed NU7441 suppressed the TNBC metastasis to the lungs in immune-competent xenograft Balb/c mice but not in athymic nude xenograft mice. (G) NU7441 inhibited tumor metastasis to lungs in immune-competent xenograft mice but had no effects on tumor metastasis in athymic nude xenograft mice through counting tumor nodules. (H) NU7441 increased the infiltrations of CD45+ cells, CD4+ and CD8+ T cells, and CD1a+ antigen-presenting cells in TME in immune-competent Balb/c xenograft mice bearing 4T1 cells through flow cytometry analysis. (I) Immunohistochemistry results showed that NU7441 increased the IFNAR and MHC-I levels in TME in immune-competent xenograft mice. In the TME of athymic nude mice, there is no observable IFNAR expression shown up in immunohistochemistry staining, and MHC-I levels were not altered by NU7441. (J) A diagram exhibiting the roles of DNA-PKcs in cytosolic DNA sensing, inflamed TME, and anti-tumoral immunity. Targeting DNA-PKcs promotes anti-tumoral immunity via triggering cytosolic DNA sensing, inducing an inflammation TME, and recruiting lymphocytes in metastatic TNBC.
After confirming the positive regulation of NU7441 on cytosolic DNA sensing and IFN-β in vitro, we detected the roles of NU7441 in immune-competent Balb/c mice. The immune-competent Balb/c mice were subcutaneously injected with NU7441 (70 mg/kg total dose). The weights of mice were not altered by NU7441 (Fig. S3A). ELISA results showed the serum IFN-β was slightly increased by NU7441 (Fig. 1C). The results of flow cytometry showed the splenic CD4+ T cells, CD8+ T cells, CD11c+ dendritic cells (DC) and CD1a+ cells were not altered by NU7441 (Fig. S3B–D). These results demonstrated NU7441 increased serum IFN-β but did not influence the differentiation of splenic CD4+ T cells, CD8+ T cells, and CD11c+ DC, CD1a+ antigen-presenting cells in vivo.
We tested the effects of NU7441 on anti-tumoral immunity in the xenograft immune competent and athymic nude Balb/c mice bearing 4T1 TNBC cells. The weights of immune-competent mice were not altered during the NU7441 treatment (100 mg/kg total dose; Fig. S4A). The tumor volumes were calculated every day and the removed tumors were weighted, which showed NU7441 suppressed the tumor growth (Fig. 1D; Fig. S4B). The weights of nude xenograft mice were not altered during the NU7441 treatment (Fig. S4C). NU7441 did not suppress the tumor growth in athymic nude xenograft mice (Fig. 1E; Fig. S4D). The lung tissues were collected for HE staining to detect the tumor metastasis, showing NU7441 obviously suppressed the TNBC metastasis to lungs in immune-competent Balb/c mice, but NU7441 did not suppress the TNBC metastasis in athymic nude mice (Fig. 1F, G; Fig. S4E). These results demonstrated that NU7441 promoted anti-tumoral immunity to inhibit TNBC growth and metastasis.
We next analyzed the effects of NU7441 on lymphocyte infiltration and inflammation in TME. In immune-competent xenograft Balb/c mice, NU7441 increased the ratio of CD45+ leukocytes in tumor tissues from about 27% to 40%, also increased CD4+ and CD8+ T cells, and CD1a+ antigen-presenting cells in TME (Fig. 1H; Fig. S5A–C). The athymic nude xenograft Balb/c mice are deficient with CD4+ and CD8+ T cells in TME, and the CD1a+ antigen-presenting cells in TME were not altered by NU7441 (Fig. S5D, E). IFNAR, the receptor of IFN-I, is a pro-inflammatory cytokine, which improved cytosolic T cell survival in the TME to promote anti-tumoral immunity. Immunohistochemistry results showed that NU7441 obviously increased the IFNAR levels in TME in immune-competent xenograft mice, while IFNAR expressions were not shown up in athymic nude mice (Fig. 1I), and NU7441 increased the MHC-I in TME in immune-competent xenograft mice, but not in nude xenograft mice (Fig. 1I). These results demonstrated that NU7441 increased the antigen presentation and IFN mediated inflammation in TME.
The RT-qPCR results showed NU7441 did not influence PD-1 and CTLA-4 transcriptions, but increased the PD-L1 transcription in tumors, lungs, and spleens (Fig. S6A–I). In immune-competent xenograft mice, NU7441 increased the serum IFN-β (Fig. S7A) but did not influence the splenic CD4+ and CD8+ T cells, and CD1a+ antigen-presenting cells (Fig. S7B–D), showing that NU7441 did not cause severe inflammation in the normal immune organ of immune-competent xenograft mice.
We analyzed the infiltration of immune cells in TME using the clinical TCGA data of breast cancer patients with varied DNA-PKcs mutations from TIMER (https://cistrome.shinyapps.io/timer/), which showed the high amplification of DNA-PKcs decreased the infiltration of CD4, CD8 T cells and DC cells in TME compared with the diploid controls (Fig. S8). These bioinformation analyses from the clinical data convinced our experimental results that the inhibition of DNA-PKcs promotes the infiltration of immune cells in TME.
Taken together, our studies demonstrated that NU7441 triggers cytosolic DNA sensing, induces inflammation, and recruits more lymphocytes in TME to increase anti-tumoral immunity in metastatic TNBC (Fig. 1J). Notably, NU7441 has low effects on inflammation and activation of splenic immune cells in immune-competent Balb/c mice and immune-competent xenograft Balb/c mice. These findings can contribute to understanding the mechanism of DNA-PKcs in the control of cytosolic DNA sensing and inflammation in TEM, and also provide a strategy to increase the efficacy of immunotherapy through targeting DNA-PKcs.
Author contributions
C.M. and Y.W. performed the experiments. Q.Y. performed some animal experiments. Z.C., X.G., Z.P., Z.S., J.W., and J.L. aided in the in vitro and animal experiments. Y.G. performed data analysis. W.M. supervised all experiments and wrote the manuscript. All authors approved the final version of the manuscript.
Conflict of interests
The authors have no conflict of interests in this paper.
Funding
This work is supported by Dalian Medical University Yingcai Project (China) to W.M. and Liaoning Provincial Program for Top Discipline of Basic Medical Sciences (China).
Acknowledgements
We would like to express gratitude to Dr. Pixu Liu, Institute Cancer Stem Cell, Dalian Medical University, for providing reagents and helpful suggestions.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2023.01.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Voorwerk L., Slagter M., Horlings H.M., et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25(6):920–928. doi: 10.1038/s41591-019-0432-4. [DOI] [PubMed] [Google Scholar]
- 2.Pannunzio N.R., Watanabe G., Lieber M.R. Nonhomologous DNA end-joining for repair of DNA double-strand breaks. J Biol Chem. 2018;293(27):10512–10523. doi: 10.1074/jbc.TM117.000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gluz O., Liedtke C., Gottschalk N., et al. Triple-negative breast cancer—current status and future directions. Ann Oncol. 2009;20(12):1913–1927. doi: 10.1093/annonc/mdp492. [DOI] [PubMed] [Google Scholar]
- 4.den Brok W.D., Speers C.H., Gondara L., et al. Survival with metastatic breast cancer based on initial presentation, de novo versus relapsed. Breast Cancer Res Treat. 2017;161(3):549–556. doi: 10.1007/s10549-016-4080-9. [DOI] [PubMed] [Google Scholar]
- 5.Adams S., Schmid P., Rugo H.S., et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):397–404. doi: 10.1093/annonc/mdy517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.