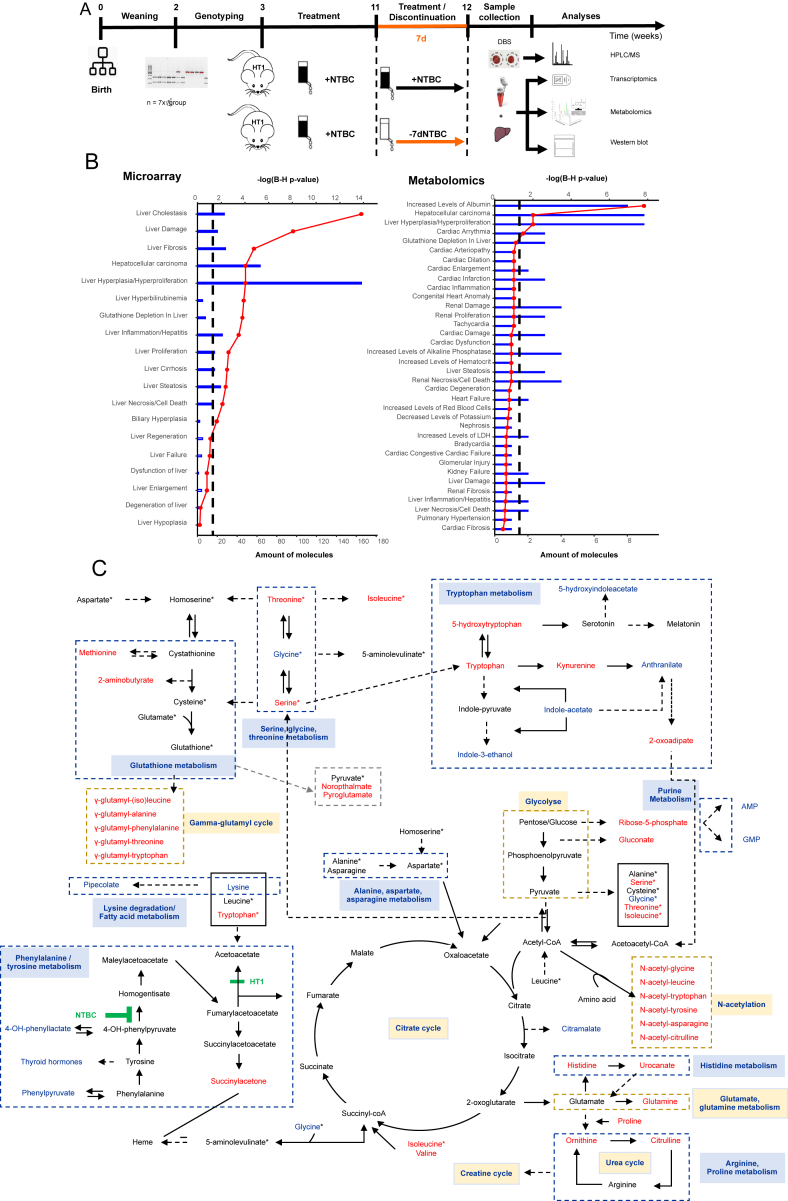
Hereditary tyrosinemia type 1 (HT1) is a life-threatening disease caused by the patient's inability to break down tyrosine due to loss-of-function mutations in the fumarylacetoacetate hydrolase (FAH) enzyme (Fig. S1). Currently, the only available life-saving treatment is nitisinone (NTBC). However, nitisinone therapy comes with debilitating side effects and requires a strict drug regime combined with a tyrosine- and phenylalanine-restricted diet. Consequently, therapy adherence is often experienced as an additional burden. In this study, transcriptional profiling was conducted parallel to high-resolution metabolomics on, respectively, liver tissue and serum samples of Fah-deficient mice. The experimental workflow of our study is shown in Figure 1A. Canonical pathway analyses showed manifest activation of the NRF2-stress response, associated with hepatocellular damage and mainly driven by a markedly increased oxidative burden. Other altered canonical pathways were primarily a downstream consequence of the latter. Furthermore, we observed an overall aminoacidemia and changes related to aminoacyl-tRNA biosynthesis, which postulates that short-term nitisinone discontinuation can already put the patient at risk for hepatocellular carcinoma development. Finally, we did not only identify changes in numerous pathways, directly and indirectly, related to the metabolism of tyrosine and glutathione but also the presence of “side-chain” metabolites, such as y-glutamyl- and N-acetyl-coupled amino acids, indicating that non-directly related pathways like phase II biotransformation reactions are also affected.
Figure 1.
Transcriptional (by microarray) and metabolic (by LC-QTOF-MS) profiling of Fah-deficient mice upon seven-day nitisinone treatment discontinuation compared to continuously treated controls. (A) Summary of the experimental workflow including mouse gender, genotype and age, treatment groups, sample collection, and data analyses. (B) Ingenuity toxicological classification of transcriptional data using liver tissue and metabolic data using serum. The top x-axis and red line charts represent categories ranked by their -log (B–H P-value), the lower x-axis and bar charts represent the total number of differentially expressed molecules, and the black dashed line represents the B–H corrected P-value ≤0.05 threshold. (C) Summary of potentially affected metabolic pathways in serum samples, identified by high-resolution metabolomics. Pathways were visualized based on the KEGG pathway database (http://www.genome.jp/kegg/pathway.html). Identified metabolites that were more than 1.5-fold up- or down-regulated are colored in red and blue, respectively, and the metabolites in black were not identified or altered in this study. Metabolites with an asterisk (∗) are duplicates in this diagram to avoid the complexity of pathways. Solid and dashed arrows indicate respectively the single and multiple steps involved between two metabolites or pathways.
First, in-depth microarray analyses were performed on whole livers using Affymetrix mouse gene 2.0 arrays. The transcriptional response upon seven days of nitisinone deprivation revealed alterations in numerous genes responsible for hepatic diseases, liver damage, liver regeneration, and liver cancer (Fig. 1B), which corresponds with our previous findings in the FRG mouse model.1 Canonical pathway analysis using the Ingenuity Pathway Analysis (IPA) software showed manifest activation of the NRF2-stress response, associated with hepatocellular damage and mainly driven by a markedly increased oxidative burden. Most other observed transcriptional changes in canonical pathways can be considered as a downstream effect of this oxidative stress response, i.e., inhibition of inflammation and acute phase response, depletion of reduced glutathione, and attempted activation of thioredoxin/glutathione peroxidase functions (Fig. S2).
Although transcriptome analysis revealed perturbated pathways, it remains unclear if this translates into metabolic changes. Therefore, to study the association between transcriptome and metabolome, comparative metabolic profiling was conducted by LC-QTOF-MS on mouse serum in the negative (ESI-) and positive (ESI+) polarity. The total data processing flow with all applied cleaning and filter steps and their corresponding results are summarized in Table S1. Data revealed a complex set of changes, but generally highly concordant with the altered gene expression. Most significant changes were observed in metabolites (histidine, glutamine, glycine, methionine, valine, lysine, isoleucine, threonine, tryptophan) that were found to be enriched in “Aminoacyl-tRNA biosynthesis” (Fig. S3 and Table S2). Aminoacyl-tRNA synthases (i) are responsible for the first step of protein synthesis by linking amino acids to their cognate transfer RNAs (tRNAs), (ii) function as regulators in cellular processes to maintain homeostasis, and (iii) are generally regarded as housekeeping molecules without additional functions. However, recent studies suggest that these enzymes are involved in various physiological and pathological processes and that mutations, functional alterations, or dysregulation of these enzymes might be pathologically associated with, among others, tumorigenesis.
When focussing on altered metabolites directly relevant to the clinical manifestations observed in HT1 patients, we found that in the phenylalanine and tyrosine degradation pathway, only phenylpyruvate and 4-hydroxyphenyllactate were significantly decreased in serum samples seven days post nitisinone discontinuation. Remarkably, tyrosine and 4-hydroxyphenylpyruvate, both metabolites proximal to the site of action of nitisinone, were not found to be significantly decreased after seven days of withdrawal compared to continuously treated mice. Instead, a decrease of 4-hydroxyphenyllactate was observed. This indicates that 4-hydroxyphenylpyruvate has been degraded or has been shifted to 4-hydroxyphenyllactate, as there is an equilibrium between both metabolites (Fig. 1C). In addition, the increase of thyroid stimulating hormone could be a consequence of hypertyrosinemia as tyrosine is the precursor for thyroid hormones, melanin, and catecholamines. Both results were also reported in alkaptonuria patients after nitisinone treatment, where tyrosine decrease was much greater compared to our results and where the prevalence of hyperthyroidism was significantly higher (16.0%) than the 3.7% prevalence in the general population.2,3
Interestingly, besides increased levels of amino acids in serum, we observed the presence of “side-chain” metabolites, such as y-glutamyl- and N-acetyl- coupled amino acids (Fig. 1C and Table S3). These metabolites are related to glutathione metabolism, indicating that non-directly related pathways like phase II metabolic biotransformation reactions are also affected. Phase II metabolism involves conjugation reactions with sulphate, glucuronide, glutathione, mercapturic acid, amino acid, methyl, and acetyl. Especially y-glutamyl, glucuronide, and acetyl conjugates were observed as alternative metabolites in the analyzed serum samples. Moreover, gene expression analyses of liver tissue support this observation, where activation of glutathione metabolism and the y-glutamyl-cycle was identified (Fig. S2). Together, this indicates that, besides the conventional pathways, the body eliminates or detoxifies the excess of amino acids by an altered route of metabolism by making conjugated and thus more water-soluble products. These results are consistent with literature where generalized hyperaminoaciduria and small amounts of phase II metabolites are reported in urine.4 Contrary, phase I conjugates of hydroxylation, oxidation, reduction, and hydrolysis were less present.
Associated with the glutathione metabolism, increased levels of 2-aminoisobutyrate, noropthalmate, methionine, and pyroglutamate were observed. These latter two are intermediates of the biosynthesis of glutathione and were reported to be elevated in various experimental models with cardio- and hepatotoxicity due to oxidative stress.5 Together with the observed inhibited ferroptosis signaling pathway and activated vitamin C transport in liver tissue, and a decrease of ascorbate (a compound with anti-oxidative capacities) in serum (Fig. S2), these aberrations could be a secondary response to reverse cell death, oxidative stress, and DNA damage induced by the accumulation of toxic tyrosine-derivates. Thereby, alteration of the ferroptosis signaling pathway was previously described in the literature by Wen-Dai Boa et al who reported that iron overload in HT1 induces liver injury through the Sp1/Tfr2/hepcidin axis.
Other noteworthy changes were associated with the tryptophan pathway (Fig. 1C). Tryptophan, 5-hydroxytryptophan, 2-oxoadipate, kynurenine, N-acetyltryptophan, and y-glutamyl-tryptophan were significantly increased, while 5-hydroxyindoleacetate, anthranilate, indole-3-acetate, and indole-3-ethanol were significantly decreased upon nitisinone deprivation. Also, indoxylsulfate (a metabolite of the indolepyruvate pathway from tryptophan) and 5-hydroxyondoleacetate (an intermediate of the serotonin catabolism) were decreased. Interestingly, indole-derivatives are tryptophan-derived metabolites produced by intestinal microorganisms. Studies suggest that indole and its derivatives play an important role in the pathogenesis of several gastrointestinal disorders, liver diseases, and neurological disorders.
Finally, we observed a decrease in metabolites involved in liver-specific anabolic functions (mevalonic acid and hydroxybutyric) and a depletion of key nucleotides (AMP and GMP), further supporting the oxidative stress response observation at the metabolic level. The remaining unmapped metabolites (Fig. S4E, F and Table S3) did not follow a clear theme and will require further investigation to unravel the reasons behind their alteration.
Author contributions
HCV performed all experiments and data analysis. HCV and JDK designed the experiments, wrote the main manuscript, and prepared figures. BN and AD helped perform LC-MS/MS of serum samples. SVL advised statistical testing and helped write R scripts. LM performed DBS analyses. JDK, TV, DDB, PG, GC, GM, and JG provided financial and infrastructural support. All co-authors revised, improved the language and content of the manuscript, and agreed to the published version of the manuscript.
Conflict of interests
The authors declared no conflict of interests.
Funding
This research was funded by the Research Foundation – Flanders (FWO) (No. 1518619N and G041521N), the Research Council (OZR) of the Vrije Universiteit Brussel (VUB), the Hercules Foundation, Wetenschappelijk Fonds Willy Gepts (WFWG) from the UZ Brussel and a Medical Grant from Swedish Orphan Biovitrum (SOBI) (No. AIIFUND37).
Acknowledgements
The authors thank Claes Paul and Heymans Anja for their technical assistance during the animal experiments and microarray, respectively.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2022.11.013.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Multimedia component 1
References
- 1.Colemonts-Vroninks H., Neuckermans J., Marcelis L., et al. Oxidative stress, glutathione metabolism, and liver regeneration pathways are activated in hereditary tyrosinemia type 1 mice upon short-term nitisinone discontinuation. Genes. 2020;12(1):3. doi: 10.3390/genes12010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davison A.S., Norman B.P., Ross G.A., et al. Evaluation of the serum metabolome of patients with alkaptonuria before and after two years of treatment with nitisinone using LC-QTOF-MS. JIMD Rep. 2019;48(1):67–74. doi: 10.1002/jmd2.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avadhanula S., Introne W.J., Auh S., et al. Assessment of thyroid function in patients with alkaptonuria. JAMA Netw Open. 2020;3(3) doi: 10.1001/jamanetworkopen.2020.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim S.Z., Kupke K.G., Ierardi-Curto L., et al. Hepatocellular carcinoma despite long-term survival in chronic tyrosinaemia I. J Inherit Metab Dis. 2000;23(8):791–804. doi: 10.1023/a:1026756501669. [DOI] [PubMed] [Google Scholar]
- 5.Geenen S., Guallar-Hoyas C., Michopoulos F., et al. HPLC-MS/MS methods for the quantitative analysis of 5-oxoproline (pyroglutamate) in rat plasma and hepatic cell line culture medium. J Pharm Biomed Anal. 2011;56(3):655–663. doi: 10.1016/j.jpba.2011.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1