Abstract
Purpose
Postmastectomy radiation therapy (PMRT) reduces disease recurrence in appropriately selected patients but may compromise implant-based reconstruction. We investigated whether near-surface dose correlates with radiation-related toxic effects in these patients.
Methods and Materials
Patients receiving PMRT at a single institution from 2016 to 2019 were retrospectively reviewed. Patient demographics and treatment information were collected. Three near-surface structures were retrospectively generated, bound by the chest wall tangent beam as well as the skin surface and the skin-3 mm contour (SR3), skin surface and skin-5 mm contour (SR5), or skin-5 and skin-10 mm contours. Dosimetric analysis of these near-surface contours was performed in 2 Gy intervals. Univariate and multivariate analyses were used to identify predictors of moist desquamation, grade 2+ chest wall pain, use of opiate pain medication, unplanned reconstructive surgery, and implant failure. Logistic regression for each outcome and near-surface contour was performed for receiver-operator area under the curve (AUC) analysis and the Youden J Statistic was used to determine the optimal threshold for each dosimetric parameter.
Results
Of 126 patients reviewed, 109 met the study's eligibility criteria. Median follow-up was 2.3 years. Twenty-five patients (23%) underwent unplanned reconstructive surgery, and 10 (9.2%) experienced implant failure. Among clinical variables, low body mass index and history of smoking predicted unplanned surgery on univariate and multivariate analyses, and moist desquamation predicted grade 2+ chest wall pain. The top dosimetric parameters by AUC for moist desquamation, grade 2+ chest wall pain, use of opiates, unplanned reconstructive surgery, and implant failure were SR5 D10 cc (AUC = 0.701, optimal threshold 57.8 Gy, P < .001), SR3 D10 cc (AUC = 0.600, optimal threshold 56.8 Gy, P = .079), SR5 D10 cc (AUC = 0.642, optimal threshold 57.3 Gy, P = .041), SR3 V44 Gy (AUC = 0.711, optimal threshold 81%, P = .001), and SR3 V44 Gy (AUC = 0.688, optimal threshold 82%, P = .052), respectively.
Conclusions
Near-surface dose correlates with moist desquamation and unplanned reconstructive surgery after PMRT. Further evaluation of prospective optimization of dosimetric parameters related to SR3 and SR5 should be considered.
Introduction
Implant-based breast reconstruction may lead to improved cosmetic outcome, patient satisfaction, and quality of life for women who have undergone a mastectomy as part of their breast cancer treatment.1, 2 Although select women with high-risk features benefit clinically from postmastectomy radiation therapy (PMRT),3, 4, 5, 6, 7 this treatment increases the risk of postreconstruction complications, including infection, capsular contracture, implant malposition and exposure, and implant loss.8 These complications may necessitate additional surgery and compromise the ultimate esthetic outcome of the reconstruction. Counseling patients on the potential interaction between PMRT and implant-based reconstruction is important when discussing radiation therapy.
PMRT plan quality likely plays an important role in the risk of postreconstruction complications. Modern linear accelerators and treatment planning systems allow for improved dose homogeneity and reduced dose to avoidance structures through plan optimization. When reliable dosimetric correlates for adverse outcomes are found, informed planning objectives can be set to reduce the risk of these events. After a mastectomy, the subcutaneous lymphatic plexus and any residual glandular tissue within the chest wall are at risk for harboring residual microscopic disease and are typically included in the clinical target volume.9 The skin and immediately underlying tissue are thus exposed to relatively high radiation doses during PMRT, especially with the use of a tissue-equivalent material (referred to as “bolus”) on the chest wall or a boost dose of radiation to the mastectomy scar. How near-surface dose affects a patient's risk for radiation-related toxic effects, however, is not well characterized. Here we correlate dose to various near-surface chest wall contours with the development of moist desquamation, chest wall pain, the need for additional reconstructive surgery, and implant failure.
Methods and Materials
Patients
Consecutively treated patients who received PMRT at 3 hospitals in a single health care system from 2016 to 2019 were retrospectively analyzed as part of an institutional review board approved study. Women were included if they underwent implant-based reconstruction and had an ipsilateral tissue expander or permanent implant in place at the time of PMRT. Exclusion criteria included pre-PMRT complications, failure to complete PMRT as prescribed, proton therapy, and removal of breast prostheses for reasons definitively unrelated to toxicity or cosmetic concerns. Patients were not excluded based on use of bolus or chest wall mastectomy scar boost. In addition to querying our prospective institutional database, we reviewed patients’ electronic medical records for baseline demographic, disease, and treatment characteristics, as well as treatment outcomes and specific treatment related toxicities, including moist desquamation, chest wall pain, and pain medication use during treatment. Details of patients’ initial breast reconstructions and subsequent surgical interventions related to their breast reconstructions, including reasons for additional surgery, were recorded.
Radiation technique
Chest wall irradiation was typically delivered with 2 deep coplanar tangent photon fields, using inverse-planned, segmented, 3-dimensional (3D) conformal techniques. Regional nodal irradiation was typically delivered with 2 partially wide, coplanar photon fields (typically covering the internal mammary lymph nodes in the first 3 intercostal spaces), also using inverse-planned, segmented, 3D-conformal techniques, with an oblique photon field(s) covering the superior axillary and supraclavicular nodal regions. If dose constraints could not be met with these techniques, volumetric modulated arc therapy (VMAT) was used.
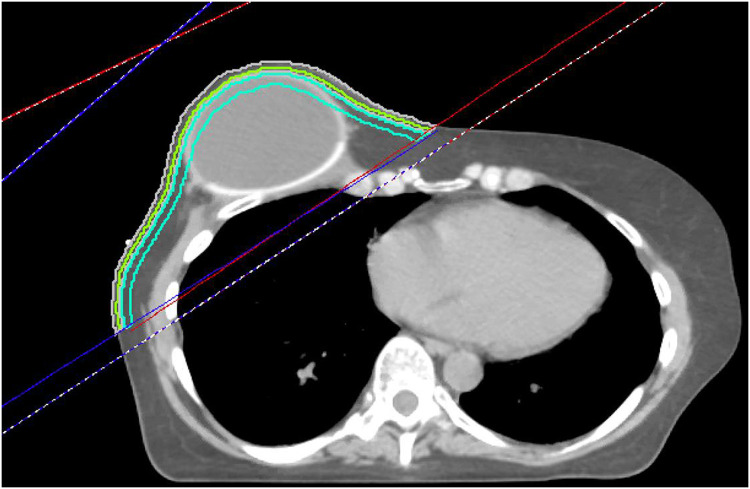
Composite PMRT plans, inclusive of any sequential scar boost administered, were reviewed on Pinnacle Version 16.2.1 (Phillips Medical Systems, Fitchburg, WI) for all patients for whom treatment plan dosimetry was available for review. For these patients, automated contours were created to generate 3 near-surface volumes for analysis of near-surface dose, comprising the volumes bound by the chest wall tangent beam and limited to 0 to 3 mm from skin surface (SR3), 0 to 5 mm from skin surface (SR5), and 5 to 10 mm from skin surface (SR10) (Fig. 1). These structures were confined to the tangent beams covering the chest wall, even when the beam's edge crossed midline to include the skin of the contralateral chest wall. The inframammary fold and skin overlying the axilla were encompassed within the near-surface skin contours. When VMAT was delivered, a similar approach was taken to generate these structures using the unopposed tangent beams from a conventional comparison plan. After generation of SR3, SR5, and SR10, dose-volume histogram data were exported for statistical analysis. During the years that this study cohort was treated, it was our institutional practice to offer daily bolus for patients undergoing PMRT, with potential removal of bolus after observing brisk chest wall erythema. Before export, recalculations were retrospectively performed for treatment plans that had not already accounted for any documented midtreatment bolus prescription modifications to reflect dose with bolus as delivered.
Figure 1.
Representative axial slice of a patient with a right-sided implant postreconstruction.
The red lines represent the medial beam edges, and the blue lines represent the lateral beam edges. The 3 skin rind structures are shown. Lime green represents 0 to 3 mm (SR3), gray represents 0 to 5 mm (SR5), and turquoise represents the 5 to 10 mm rind (SR10). The rinds extend to cover all tissue within the tangent fields, but do not extend into the supraclavicular field.
Endpoints
Our institutional protocol is to perform assessments weekly during the course of radiation and at 2 weeks, 3 months, 6 months, and 12 months after the last dose of radiation therapy and then yearly. Endpoints for analysis included physician- or nurse-reported moist desquamation, grade 2+ chest wall pain, unplanned surgery, and implant failure. Patients were recorded as having an endpoint if any of these were present at any assessment. The presence of moist desquamation was determined by the treating physician and recorded at each on-treatment visit or follow-up, or by a breast-trained radiation oncology nurse at a 2-week post treatment nursing visit. Because SR3, SR5, and SR10 did not extend into the supraclavicular region, moist desquamation was recorded as an event only if it occurred over the chest wall for the purposes of dosimetric correlation. The worst qualitative pain rating and the use of opiates during the acute phase of treatment up to the first follow-up was recorded. Because patient-reported pain was reported either quantitatively on a scale from 1 to 10 or qualitatively using “mild,” “moderate,” or “severe” qualifiers, pain was assessed using a modified brief pain inventory scale and categorized as moderate/severe if any pain severity score was rated 4 or higher.10,11 A pain severity rating of at least 4 out of 10 portends interference in patient function.10 Therefore, grade 2+ chest wall pain was defined as either a pain severity rating of 4 or higher on a 0 to 10 scale or “moderate” or “severe” descriptor. Pain medication use for each patient was reviewed and classified as none, adjunct alone (eg, gabapentin, methocarbamol), NSAID (acetaminophen, ibuprofen), tramadol, or opiates (eg, hydrocodone, oxycodone). Implant failure was defined as the absence of a tissue expander or permanent implant at the time of the last follow-up in a patient who was initially planned for implant-based reconstruction. Unplanned reconstructive surgery was defined as a surgery that was not part of the pretreatment reconstruction plan but performed for alleviate symptoms or for esthetic reasons. In addition to these endpoints, CTCAE (National Cancer Institute Common Terminology Criteria for Adverse Events) version 4.0 toxicities were prospectively recorded at each scheduled follow-up and maintained in our institutional database. These were used to describe both acute and chronic toxicity, Chronic toxicities were recorded beginning at the second follow-up visit, 6 months after completion of radiation, until the most recent follow-up.
Statistical analysis
Descriptive statistics were performed with mean and standard deviation for normally distributed variables, or median and interquartile range otherwise. Baseline characteristics and dosimetric parameters were described. Dose volume histogram parameters extracted included relative volume receiving absolute dose in 2 Gy intervals from 30 Gy to 68 Gy (V30 Gy[%] to V68 Gy[%]) and dose to absolute volumes including D0.1 cc, D0.3 cc, D1 cc, D5 cc, D10 cc, D50 cc, D100 cc.
A Wilcoxon rank-sum test was performed on the continuous values for each dosimetric parameter to determine whether there was a statistical difference in each parameter for those experiencing each endpoint. A P value < .05 was deemed statistically significant. No adjustment for multiple testing was performed.
Dosimetric cut points were determined by receiver-operator curve analysis with the cut point selected by maximizing the Youden J Statistic. The cutpoints with the highest area under the curve (AUC) were assessed to determine which dosimetric parameters provided the best discrimination for each outcome.
A new variable was created for each patient specifying if they met each proposed constraint based on the above AUC analysis. Univariate logistic regression for each outcome was then performed to determine the odds ratio (OR) for each outcome with respect to each proposed constraint. Univariate analysis was also used for clinical parameters to determine their OR for each outcome. Clinical variables included current smoking at the time of surgery, obesity (body mass index [BMI] >30), hypertension, diabetes, menopausal status, histology, receptor status, the type of mastectomy, the type of reconstruction, the type of axillary surgery, final margins, the use of chemotherapy, the use of Herceptin, the use of hormones, and development of moist desquamation in the acute phase was included for all endpoints except moist desquamation itself.
Collinearity of the dosimetric variables was quantified by calculating the variance inflation factor for each group of the top constraints. A score >5 signifies strong collinearity and justifies performing separate multivariable analyses for each collinear variable.12 Due to high collinearity between the dosimetric variables, only the top dosimetric parameter by AUC and any significant clinical variables on univariate analysis for each endpoint were assessed in multivariate analysis. All statistical analysis was performed in R version 4.1.0.
Results
Patient population
Of 126 women receiving PMRT from 2016 to 2019, 116 met clinical criteria for analysis. Of the 10 excluded, 4 patients had no tissue expander or permanent implant in place during treatment, 2 patients experienced pre-PMRT complications, 2 patients received proton therapy, 1 patient did not complete treatment as prescribed, and one patient had clinical evidence of leptomeningeal disease necessitating brain and spine magnetic resonance imaging, which required removal of her tissue expanders. Treatment plans were unavailable for 7 additional patients, leaving 109 patients available for analysis of correlation between near-surface dose and clinical outcomes.
Baseline characteristics are listed in Table 1, with these characteristics broken down by hospital in Table E1. Median follow-up was 2.4 years with a median age of 49. All patients underwent skin-sparing mastectomy, 15 (14%) of whom underwent a nipple-sparing procedure. Axillary lymph node dissection was performed in 70 patients (65%), and the remaining patients underwent a sentinel lymph node procedure alone. Almost all patients (96%) were planned for a 2-stage breast reconstruction with a tissue expander placed at the time of mastectomy; only 4 patients underwent a single-stage reconstruction with a permanent implant placed immediately at the time of mastectomy.
Table 1.
Baseline characteristics
| Characteristic | N = 109* |
|---|---|
| Follow-up (y) | 2.36 (1.54, 3.10) |
| Current smoker | |
| Not smoking | 103 (94%) |
| Smoking | 6 (5.5%) |
| Obese | |
| BMI <30 | 72 (66%) |
| BMI ≥30 | 37 (34%) |
| cT | |
| T0 | 1 (0.9%) |
| T1 | 33 (30%) |
| T2 | 38 (35%) |
| T3 | 27 (25%) |
| T4 | 6 (5.5%) |
| Tis | 4 (3.7%) |
| cN | |
| N0 | 53 (49%) |
| N1 | 49 (45%) |
| N2 | 1 (0.9%) |
| N3 | 6 (5.5%) |
| c Stage | |
| 0 | 4 (3.7%) |
| IA | 25 (23%) |
| IIA | 20 (19%) |
| IIB | 31 (29%) |
| IIIA | 17 (16%) |
| IIIB | 5 (4.6%) |
| IIIC | 6 (5.6%) |
| ypT | |
| T0 | 13 (25%) |
| T1 | 20 (38%) |
| T2 | 12 (23%) |
| T3 | 5 (9.4%) |
| T4 | 1 (1.9%) |
| Tis | 2 (3.8%) |
| ypN | |
| N0 | 21 (40%) |
| N1 | 20 (38%) |
| N1mi | 0 (0%) |
| N2 | 9 (17%) |
| N3 | 3 (5.7%) |
| yp Stage | |
| 0 | 2 (4.8%) |
| IA | 7 (17%) |
| IIA | 11 (26%) |
| IIB | 7 (17%) |
| IIIA | 11 (26%) |
| IIIB | 1 (2.4%) |
| IIIC | 3 (7.1%) |
| pT | |
| T0 | 3 (5.0%) |
| T1 | 20 (33%) |
| T2 | 24 (40%) |
| T3 | 13 (22%) |
| Tis | 0 (0%) |
| pN | |
| N0 | 11 (18%) |
| N1 | 35 (58%) |
| N1mi | 3 (5.0%) |
| N2 | 8 (13%) |
| N3 | 3 (5.0%) |
| p Stage | |
| IA | 3 (5.2%) |
| IB | 3 (5.2%) |
| IIA | 18 (31%) |
| IIB | 17 (29%) |
| IIIA | 14 (24%) |
| IIIC | 3 (5.2%) |
| Age at diagnosis (median, IQR) | 49 (41, 57) |
| Hypertension | 16 (15%) |
| Diabetes | 4 (3.7%) |
| Menopause | |
| Premenopausal | 47 (43%) |
| Perimenopausal | 3 (2.8%) |
| Postmenopausal | 55 (50%) |
| Surgical | 4 (3.7%) |
| Histology | |
| DCIS | 2 (1.8%) |
| IDC | 84 (77%) |
| ILC | 21 (19%) |
| IMC | 2 (1.8%) |
| Receptor status | |
| HR +/HER2– | 82 (75%) |
| HR +/HER2+ | 9 (8.3%) |
| HR +/HER2 unknown | 2 (1.8%) |
| HR–/HER2+ | 8 (7.3%) |
| HR–/HER2– | 8 (7.3%) |
| Type of mastectomy | |
| Nipple sparing | 15 (14%) |
| Skin sparing | 94 (86%) |
| Type of reconstruction | |
| 1 stage | 4 (3.7%) |
| 2 stage | 105 (96%) |
| Axillary surgery type | |
| ALND | 17 (16%) |
| SLN + ALND | 53 (49%) |
| SLN | 38 (35%) |
| Final margin status | |
| Negative | 96 (88%) |
| Positive | 12 (11%) |
| Positive (DCIS) | 1 (0.9%) |
| Sequence of chemotherapy | |
| None | 16 (15%) |
| Adjuvant | 39 (36%) |
| Neoadjuvant | 54 (50%) |
| Herceptin | 18 (17%) |
| Hormones | 86 (80%) |
Abbreviations: ALND = axillary lymph node dissection; BMI = body mass index; DCIS = ductal carcinoma in situ; IDC = invasive ductal carcinoma: ILC = invasive ductal carcinoma; IMC = invasive mammary carcinoma; SLN = sentinel lymph node procedure.
Median (IQR); no. (%).
All patients underwent PMRT with conventional fractionation with 1.8 Gy (40%) or 2 Gy (60%) fractions. Seven patients (6.4%) were treated with VMAT, 2 patients (1.8%) with a dual-isocentric technique, and the remaining 100 patients (92%) with a mono-isocentric technique. Patients received PMRT to the chest wall alone (6.4%) or to the chest wall and regional lymphatics (94%). All patients were treated with a chest wall bolus, although there was variation in the number of fractions of bolus applied (median, 25; interquartile range, 19-30 fractions). One hundred and 4 patients (95%) had 0.5 cm bolus thickness. Eighty-four patients (77%) underwent a sequential mastectomy scar boost with a median dose of 10 Gy (range, 6-12 Gy). The median composite PMRT dose was 60 Gy.
Acute grade 2+ chest wall pain, grade 2+ fatigue, grade 2+ hyperpigmentation, and grade 2+ radiation dermatitis occurred in 58%, 4.6%, 84%, and 56% of patients, respectively. Late grade 2+ arm lymphedema, grade 2+ myositis, and grade 2+ telangiectasia occurred in 2.6%, 1.3%, and 6.5% of patients. 41 patients (38%) experienced moist desquamation within the tangent fields. The location of moist desquamation within the chest wall is shown in Table 2.
Table 2.
Location of moist desquamation
| Location | Count |
|---|---|
| Axilla | 31 |
| Inframammary fold | 10 |
| Chest wall other than axilla or inframammary fold | 6 |
| Unknown | 2 |
Patients counted more than once if >1 location (N = 41).
Twenty-five patients (23%) underwent unplanned reconstructive surgery for infection (n = 8), cosmetic concerns (n = 6), implant exposure (n = 5), contracture (n = 3), necrosis (n = 1), or other reasons (n = 2). Ten patients (11%) ultimately experienced implant failure as of the last follow-up (Table 3).
Table 3.
Reasons for unplanned reconstructive surgery or implant failure
| Cause of unplanned reconstructive surgery or implant failure | Number of patients |
|---|---|
| Infection 12 | 5 |
| Exposure 10 | 2 |
| Wound healing 10 | 2 |
| Cosmetic 7 | 3 |
| Contracture 4 | 0 |
| Other 2 | 2 |
| Necrosis 1 | 1 |
Patients counted more than once if >1 surgery or if reasons multifactorial.
Significant clinical variables on univariate logistic regression are shown in Table 4. Patients who were active smokers at the time of radiation therapy had 7.81 times the odds of undergoing unplanned reconstructive surgery compared with those who were former or never smokers (OR = 7.81, P = .022). Patients with a BMI >30 had decreased odds of undergoing unplanned reconstructive surgery (OR = 0.122, P = .006). Finally, treatment at Hospital 2 (OR = 0.244, P = .017) or 3 (OR = 0.176, P = .007) was associated with a decreased odds of undergoing unplanned reconstructive surgery. The only clinical variable with increased odds of grade 2+ chest wall pain was experiencing moist desquamation (OR = 2.19, P = .049). There were no significant clinical predictors for moist desquamation or implant failure.
Table 4.
Clinical variable univariate logistic regression
| Variable* | OR | 95% CI | P value | |
|---|---|---|---|---|
| Unplanned surgery | ||||
| Current smoker | Smoking | 7.810 | 1.426-59.146 | .022 |
| Obese | BMI ≥30 | 0.122 | 0.019-0.449 | .006 |
| Institution | Hospital 2 | 0.244 | 0.075-0.769 | .017 |
| Hospital 3 | 0.176 | 0.047-0.610 | .007 | |
| Acute grade 2+ pain | ||||
| Moist desquamation | Yes | 2.191 | 1.009-4.841 | .049 |
Abbreviations: BMI = body mass index; OR = odds ratio.
Endpoints implant failure, opiates, and moist desquamation had no significant predictors on univariate logistic regression.
Near-surface dosimetry
The top near-surface dosimetric predictors by AUC for moist desquamation, grade 2+ chest wall pain, use of opiates, unplanned reconstructive surgery, and implant failure were SR5 D10 cc (AUC = 0.701, optimal threshold 57.8 Gy, P < .001), SR3 D10 cc (AUC = 0.600, optimal threshold 56.8 Gy, P = .079), SR5 D10 cc (AUC = 0.642, optimal threshold 57.3 Gy, P = .041), SR3 V44 Gy (AUC = 0.711, optimal threshold 81%, P = .001), and SR3 V44 Gy (AUC = 0.688, optimal threshold 82%, P = .052), respectively. SR10 had no significant dosimetric predictors for any endpoint.
The top 3 dosimetric parameters for SR3 and SR5 that discriminate for each outcome and rind length in univariate analysis by AUC, as well as the mean dose and D10 cc for each endpoint, are displayed in Table E2. The mean dose to SR3 and SR5 are used prospectively at our institution for evaluation and modulation of near-surface dose, as they are not influenced heavily by small changes in dose location, near-surface volume definition, or other dose-volume uncertainties. D10 cc has been shown in prior studies to correlate with acute skin toxicity, and so these parameters were displayed in addition to the top 3 by AUC.13 The variance inflation factors for each of the top 3 dosimetric predictors by AUC with the other 2 factors in the top 3 for each endpoint of SR3 and SR5 are displayed in Table E3.
Multivariate analysis, incorporating each significant clinical variable on univariate analysis and the optimal threshold for the top dosimetric predictor by AUC for each endpoint, is shown in Table 5. The top dosimetric predictors by AUC of unplanned reconstructive surgery, moist desquamation, and use of opiates remained significant on multivariate analysis. Obesity also remained significantly protective for unplanned reconstructive surgery (OR = 0.128, P = .010). Treating hospital and active smoking lost significance in predicting unplanned reconstructive surgery on multivariate analysis. Moist desquamation also lost significance in predicting grade 2+ chest wall pain after controlling for SR5 V54 Gy.
Table 5.
Multivariate analysis with top dosimetric predictor by AUC and significant clinical variables
| Variable | OR | 95% CI | P value | |
|---|---|---|---|---|
| Implant failure | ||||
| SR3 V44 Gy (%) < 82 | 0.123 | 0.007-0.690 | .51 | |
| Unplanned reconstructive surgery | ||||
| Obese | 0.128 | 0.019-0.503 | .10 | |
| SR3 V44 Gy (%) < 81 | 0.229 | 0.050-0.838 | .36 | |
| Institution | Hospital 2 | 0.290 | 0.073-1.082 | .069 |
| Hospital 3 | 0.335 | 0.063-1.645 | .182 | |
| Smoking | 8.682 | 1.127-106.791 | .054 | |
| Moist desquamation | ||||
| SR5 D10 cc (cGy) < 5780 | 0.233 | 0.101-0.517 | <.01 | |
| Acute grade 2+ pain | ||||
| SR5 V54 Gy (%) <10 | 0.423 | 0.174-0.991 | .52 | |
| Moist desquamation | 1.796 | 0.795-4.080 | .159 | |
| Opiates needed | ||||
| SR5 D10 cc (cGy) < 5730 | 0.249 | 0.068-0.733 | .19 | |
Abbreviations: AUC = area under the curve.
Discussion
PMRT increases the risk of complications after mastectomy, with a possible dose-response effect; however, the relationship between dose to the near-surface region and these complications has not been fully elucidated.14, 15, 16, 17 In this cohort of patients undergoing PMRT in the setting of implant-based reconstruction, near-surface dose to SR3 and SR5 were found to predict for moist desquamation, use of opiate pain medication, and unplanned reconstructive surgery. SR10 was found to have no correlations with any endpoint, which is unsurprising given that most dose build-up occurs in the first 5 mm beyond the skin surface. Although there was a strong trend toward SR3 and SR5 predictive ability for grade 2+ chest wall pain, the optimal dosimetric parameters and thresholds did not reach statistical significance. Chest wall pain can be influenced by many factors including surgery, breast reconstruction technique, and patient tolerance, and so it is likely that our study was not powered to detect a component contributed by near-surface dose. Additionally, increased use of stronger pain medications like opiates for these patients, which near-surface dose did predict for, may have masked an increase in chest wall pain. SR3 and SR5 had a similarly strong trend toward predictive ability for implant failure; however, the paucity of events likely made it difficult to detect a significant difference.
Other groups have investigated near-surface dose and found associations with other endpoints of skin toxicity in various patient populations. Muresan et al found that among patients receiving PMRT with implant-based reconstruction, the maximum skin dose and D1 cc to the skin significantly predicted for complications, although the skin structure was not explicitly defined in their publication.18 In another experience of 75 patients in which skin dose was evaluated as a 5 mm strip of skin over the tissue expander, there was an odds ratio of 2.22 for complications for each 5 Gy increase in the D2 cc to the near-surface skin contour.19 Liang et al evaluated the use of proton therapy for 23 patients with breast cancer, 11 of whom received PMRT, and found that the V52.5 CGE and the D10 cc to the near-surface skin contour, defined as 5 mm inward from the skin, predicted for grade 3 radiation dermatitis.13
The clinical utility of these correlations relies on the ability of clinicians to modulate near-surface dose. This can be done with 3D conformal PMRT by adjusting the number of fractions delivered with bolus or by altering mastectomy scar boost use and dose. It can also be done with inverse planned VMAT through prospective contouring of a near-surface volume and using it as an organ at risk. No high-level evidence guides the use of bolus to increase dose to the skin during PMRT in the setting of implant-based reconstruction.9 The use and frequency of chest wall bolus application, as well as the thickness of bolus material, varies significantly in clinical practice. Often, bolus is applied until brisk erythema occurs, at which point radiation is continued without bolus. This practice, however, lacks a priori determination of appropriate near-surface dose that suits a patient's disease characteristics. For example, patients with inadequate surgical margins or primary tumors that are large or involve the skin likely benefit from higher dose to the near-surface region than patients with small primary tumors that were widely excised but were referred for PMRT for lymph node involvement. Recent studies have investigated the de-escalation of near-surface dose. For example, Nichol et al assessed outcomes of patients undergoing PMRT with reconstruction and no bolus and compared with patients undergoing PMRT without reconstruction but with bolus.20 There was no difference in local recurrence between the 2 groups after 10-year follow-up. There is also controversy regarding the use of a sequential mastectomy scar boost.21 Naoum et al found that the addition of a chest wall boost was independently associated with infection, skin necrosis, implant exposure, and implant failure; however, it did not affect locoregional failure.22 In appropriately selected patients, it may be reasonable to reduce or omit use of bolus or sequential mastectomy scar boosts in an effort to reduce near-surface dose and resulting toxicity.
We would caution readers from using rigid near-surface dose constraints from this study without prospective validation. Despite our reporting of the 3 strongest near-surface dose predictors for each endpoint in our patient cohort, this analysis was not powered to detect the optimal dose constraint to the near-surface region. There may also be heterogeneity in the predictive value of different near-surface dose constraints based on body shape, locations of skin folds, and locations of areas of dose heterogeneity on the near-surface volume. This study, however, provides a baseline of parameters for further prospective evaluation. D10 cc to SR3 and SR5 predicted moist desquamation and unplanned reconstructive surgery. Additionally, D10 cc to SR5 predicted for use of opiate pain medication and D10 cc to SR3 had the strongest trend toward predicting clinically significant chest wall pain (AUC = 0.600, optimal threshold 56.8 Gy, P = .079). This would be an ideal dose parameter to evaluate prospectively, as this is the second study to observe its predictive ability for acute skin toxicity.13 One limitation of our study is that, while the general location of moist desquamation was reported, the exact location within the chest wall was unknown. Therefore, we were unable to determine whether focal dose parameters like D10 cc occurred in the area of moist desquamation. For patients who received a sequential mastectomy scar boost, the location of D10 cc invariably was located at the area of the scar boost. The most common reported location of moist desquamation was the axilla, and the second most common location was the inframammary fold. There was a wide variety of locations of boosted mastectomy scars, including scars extending to the axillary fold. Despite this, there may be cases where the location of D10 cc was not the same location as the moist desquamation. Although our study shows near-surface dose predicts for moist desquamation, there are other important contributing factors to moist desquamation like skin fold location and resulting friction with movement and moisture, causing specific areas like the axilla and inframammary fold to be at higher risk. It may be that near-surface dose to these specific high-risk regions of the chest wall may have a larger effect on focal toxicity like moist desquamation, and this may be a future study based on our data.
We found that a lower BMI was associated with a higher incidence of unplanned reconstructive surgery. There have been mixed reports on the association of implant-based reconstruction complications and BMI with some reporting positive correlations of obesity with postmastectomy complications23, 24 and others showing higher incidences of implant failure in patients with lower BMI.25 Although this could be a result of underpowered analysis given the relative rarity of obese patients in our cohort, it may be that the role of BMI in predicting postmastectomy complications is multifactorial. The significantly protective nature of obesity in preventing unplanned reconstructive surgery was unexpected and may also be a result of plastic surgeon patient selection for implant-based reconstruction.
We also found that the hospital being treated at was a significant clinical predictor of unplanned reconstructive surgery on univariate analysis. Although this variable did not remain significant on multivariate analysis, this highlights the potential bias that can occur without controlling for confounding variables. Different plastic surgeons may use different surgical techniques or different patient selection criteria for implant-based reconstruction. Additionally, patient populations may have different baseline risk factors, resulting in variable risk of implant complications with similar near-surface dose. After controlling for clinical factors, near-surface dose remained a significant predictor of unplanned reconstructive surgery.
Given the fact that only women undergoing implant-based reconstruction were included for analysis, it is unclear whether a similar correlation between near-surface dose and complication rate applies to patients undergoing autologous reconstructions without implant reconstruction. It is generally recommended that autologous reconstruction is delayed until after completion of PMRT given the risk for poor cosmetic outcomes or fat necrosis when the flap is irradiated.8 However, some data suggest equivalent outcomes with or without PMRT with autologous reconstruction.16 It should be noted that autologous reconstructions and/or placement of acellular dermal matrix were used in combination with implant-based reconstruction for many patients analyzed. In one analysis of patients undergoing implant-based reconstruction, the strongest predictor of implant failure was absence of coverage of the tissue expander or permanent implant by acellular dermal matrix or a serratus flap at the time of PMRT, with a rate of 32.5% versus 9%.25
Similar caution should be taken in applying these data to patients with prepectoral implant reconstruction. There was a single patient included in this study with prepectoral implant reconstruction, with the remaining patients having subpectoral reconstruction. In a retrospective analysis, Thuman et al found that prepectoral implants resulted in fewer implant failures, regardless of receipt of radiation therapy; however, prepectoral implants resulted in a higher overall complication rate than subpectoral implants in patients who underwent PMRT.26 Specific analysis of near-surface dose and correlation with toxicity in patients undergoing prepectoral implant reconstruction is needed.
Limitations must be acknowledged. This analysis is retrospective and derived from a single health care system. Follow-up is relatively short and may not account for all late PMRT and reconstruction-related complications or additional surgeries. Although we found minimal differences in baseline characteristics of patients between the 3 hospitals, there may be other differences in plastic surgeon patient selection that we did not account for. In general, the threshold for performing additional unplanned reconstructive surgery varies widely between patients and surgeons. In some cases of documented implant failure, patients did not retain breast prostheses, but did convert to autologous reconstruction. The use of the term “failure” may be liberal in such cases because some may have ultimately had an excellent esthetic result. Furthermore, implant failure may have been the result of a patient's priorities and wanting to avoid further surgeries. We also lacked the follow-up time and number of patients to assess how near-surface skin dose relates to local control. Although larger studies with longer follow-up have shown no difference in local control in patients with implant-based reconstruction undergoing PMRT with or without use of bolus, quantifying this relationship would be an ideal future study.20 Risk of skin involvement of disease should always be considered prior offering prospectively reduced near-surface skin dose. Regarding near-surface dose, there are likely discrepancies between treatment plan dosimetry and dosimetry of PMRT as it was delivered given patient setup uncertainties and the difficulties associated with measuring exact skin doses.27 However, using treatment planning systems for optimization of a structure is a practical approach that would be easily translated into clinical practice.
Conclusion
We found that in our study population, near-surface dose correlates with significant acute and chronic toxicities associated with PMRT, including moist desquamation and unplanned reconstructive surgery. Further evaluation of prospective near-surface dose optimization of dosimetric parameters relating to SR3 and SR5 should be considered in select patients to mitigate the toxic effects associated with PMRT. Establishing dose constraints for the near-surface chest wall is critical for minimizing skin-related toxicity.
Disclosures
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Patrick McDermott for useful discussions.
Footnotes
Sources of support: This work had no specific funding.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2023.101283.
Appendix. Supplementary materials
Supplementary Table 1: Variance Inflation Test
Supplementary Table 2: Top Three Parameters by AUC for each Endpoint and Rind Size, including D_mean and D10cc
Supplementary Table 3: Baseline Patient Characteristics by Hospital
References
- 1.McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. 2009;16:2682–2690. doi: 10.1245/s10434-009-0635-x. [DOI] [PubMed] [Google Scholar]
- 2.Galimberti V, Vicini E, Corso G, et al. Nipple-sparing and skin-sparing mastectomy: Review of aims, oncological safety and contraindications. Breast. 2017;34:S82–S84. doi: 10.1016/j.breast.2017.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J National Cancer Inst. 2005;97:116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 4.Overgaard M, Jensen M-B, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 5.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. New Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 6.EBCTCG (Early Breast Cancer Trialists' Collaborative Group) McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.EBCTCG (Early Breast Cancer Trialists' Collaborative Group) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2006;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 8.Network NCC. Breast cancer (version 2.2022). 2022. Available at:https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419. Accessed June 1, 2022.
- 9.Kaidar-Person O, Offersen BV, Hol S, et al. ESTRO consensus guideline for target volume delineation in the setting of postmastectomy radiation therapy after implant-based immediate reconstruction for early-stage breast cancer. Radiother Oncol. 2019;137:159–166. doi: 10.1016/j.radonc.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23:129–138. [PubMed] [Google Scholar]
- 11.Dilworth JT, Griffith KA, Pierce LJ, et al. The impact of chemotherapy on toxic effects and cosmetic outcome in patients receiving whole breast irradiation: An analysis within a statewide quality consortium. Int J Radiat Oncol Biol Phys. 2022;113:266–277. doi: 10.1016/j.ijrobp.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Brown SD, Tauler R, Walczak B, editors. Comprehensive Chemometrics: Chemical and Biochemical Data Analysis. Elsevier; Philadelphia, PA: 2009. [Google Scholar]
- 13.Liang X, Bradley JA, Zheng D, et al. Prognostic factors of radiation dermatitis following passive-scattering proton therapy for breast cancer. Radiat Oncol. 2018;13:72. doi: 10.1186/s13014-018-1004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parekh A, Dholakia AD, Zabranksy DJ, et al. Predictors of radiation-induced acute skin toxicity in breast cancer at a single institution: Role of fractionation and treatment volume. Adv Radiat Oncol. 2017;3:8–15. doi: 10.1016/j.adro.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen M-F, Chen W-C, Lai C-H, et al. Predictive factors of radiation-induced skin toxicity in breast cancer patients. BMC Cancer. 2010;10:508. doi: 10.1186/1471-2407-10-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry T, Brooks S, Sydow N, et al. Complication rates of radiation on tissue expander and autologous tissue breast reconstruction. Ann Surg Oncol. 2010;17:202–210. doi: 10.1245/s10434-010-1261-3. [DOI] [PubMed] [Google Scholar]
- 17.Chung SY, Chang JS, Shin KH, et al. Impact of radiation dose on complications among women with breast cancer who underwent breast reconstruction and post-mastectomy radiotherapy: A multi-institutional validation study. Breast. 2021;56:7–13. doi: 10.1016/j.breast.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muresan H, Lam G, Cooper BT, et al. Impact of evolving radiation therapy techniques on implant-based breast reconstruction. Plast Reconstr Surg. 2017;139:1232e–1239e. doi: 10.1097/PRS.0000000000003341. [DOI] [PubMed] [Google Scholar]
- 19.Chang JS, Song SY, Oh JH, et al. Influence of radiation dose to reconstructed breast following mastectomy on complication in breast cancer patients undergoing two-stage prosthetic breast reconstruction. Frontiers Oncol. 2019;9:243. doi: 10.3389/fonc.2019.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nichol A, Narinesingh D, Raman S, et al. The effect of bolus on local control for patients treated with mastectomy and radiation therapy. Int J Radiat Oncol Biol Phys. 2021;110:1360–1369. doi: 10.1016/j.ijrobp.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy: Clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1539–1569. doi: 10.1200/JCO.2001.19.5.1539. [DOI] [PubMed] [Google Scholar]
- 22.Naoum GE, Salama L, Ho A, et al. The impact of chest wall boost on reconstruction complications and local control in patients treated for breast cancer. Int J Radiat Oncol Biol Phys. 2019;105:155–164. doi: 10.1016/j.ijrobp.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Yoon AP, Qi J, Kim HM, et al. Patient-reported outcomes after irradiation of tissue expander versus permanent implant in breast reconstruction: A multicenter prospective study. Plast Reconstr Surg. 2020;145:917e–926e. doi: 10.1097/PRS.0000000000006724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naoum GE, Ho AY, Shui A, et al. Risk of developing breast reconstruction complications: A machine-learning nomogram for individualized risk estimation with and without postmastectomy radiation therapy. Plast Reconstr Surg. 2022;149:1e–12e. doi: 10.1097/PRS.0000000000008635. [DOI] [PubMed] [Google Scholar]
- 25.Fowble B, Park C, Wang F, et al. Rates of reconstruction failure in patients undergoing immediate reconstruction with tissue expanders and/or implants and postmastectomy radiation therapy. Int J Radiat Oncol Biology Phys. 2015;92:634–641. doi: 10.1016/j.ijrobp.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 26.Thuman JM, Worbowtiz N, Jain A, et al. Impact of radiation on implant-based breast reconstruction in prepectoral versus submuscular planes. Ann Plast Surg. 2021;86(6S Suppl 5):S560–S566. doi: 10.1097/SAP.0000000000002882. [DOI] [PubMed] [Google Scholar]
- 27.McDermott PN. Surface dose and acute skin reactions in external beam breast radiotherapy. Med Dosim. 2020;45:153–158. doi: 10.1016/j.meddos.2019.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Variance Inflation Test
Supplementary Table 2: Top Three Parameters by AUC for each Endpoint and Rind Size, including D_mean and D10cc
Supplementary Table 3: Baseline Patient Characteristics by Hospital