Abstract
Lactate is an end product of glycolysis. Owing to the lactate shuttle concept introduced in the early 1980s, increasing researchers indicate lactate as a critical energy source for mitochondrial respiration and as a precursor of gluconeogenesis. Lactate also acts as a multifunctional signaling molecule through receptors expressed in various cells, resulting in diverse biological consequences including decreased lipolysis, immune regulation, and anti-inflammation wound healing, and enhanced exercise performance in association with the gut microbiome. Furthermore, increasing evidence reveals that lactate contributes to epigenetic gene regulation by lactylating lysine residues of histones, which accounts for its key role in immune modulation and maintenance of homeostasis. Here, we summarize the function and mechanism of lactate and lactylation in tumor metabolism and microenvironment.
Keywords: Epigenetic regulation, Lactate, Lactylation, Microenvironment, Tumorigenesis
Introduction
During the last 30 years, growing evidence has demonstrated that lactate has various biological functions, including turning into pyruvate and producing adenosine triphosphate (ATP) through mitochondrial respiration, converting into glucose in gluconeogenesis pathway, and serving as a signal transduction molecule.1 With its receptor, lactate acts as a multifunctional signaling molecule that plays an important role in regulating the tumor microenvironment.2 Numerous studies have reported that chromatin remodeling and posttranslational modification resulted in gene expression pattern alterations.3 Therefore, various types of modifications have been discovered, such as phosphorylation, methylation, acetylation, ubiquitination, glycosylation, butyrylation, glutarylation, malonylation, and succinylation, resulting in particular configurations that shape the post-transcriptional regulation mechanisms.4 Various diseases, including tumors, are characterized by dysregulation of epigenetic modifications and metabolic activities.5 Metabolism and epigenetic modifications may provide novel therapeutic targets for the treatment of tumors. Lactylation, as a new form of modification, has drawn greater attention because of its role in regulating gene expression. In this review, we summarize the function of lactate and lactylation in tumor progression.
Warburg effect and lactate production
During the metabolism of glucose, carbon bonds are oxidized, which produces ATP, supplying energy to mammalian cells and sustaining all mammalian life. In mammals, the end product of glycolysis is lactate, while glucose can be fully oxidized by the mitochondria via respiration, generating CO2. As early as 1926, Otto Warburg found that the glucose uptake dramatically increased in tumor cells, accompanied by producing excessive amounts of lactate, even under aerobic conditions.6 This process, named as Warburg effect, has been studied extensively. Aerobic glycolysis refers to the process of fermenting glucose to create lactate, even when oxygen is present. In addition to cancer, aerobic glycolysis is found in sepsis, infections, inflammatory diseases, and autoimmune disorders.7 Glycolysis produces much less ATP than oxidative phosphorylation, with only two molecules per glucose. However, most cancer cells generate around 60% of their total ATP requirement through glycolysis. Glycolysis has multiple advantages for rapidly proliferating cancer cells. For instance, the glycolytic pathway contributes to cell survival and proliferation by providing raw materials for macromolecular synthesis, and it produces ATP faster than oxidative phosphorylation does.8 Cancer cells’ preference for glycolysis is the result of genetic alterations and overexpression of glycolytic enzymes and metabolite transporters.9 Many genes and proteins are related to the metabolic reprogramming of cancer cells, such as MYC, AKT, epidermal growth factor (EGF), Kirsten rat sarcoma viral oncogene homolog (KRAS), insulin-like growth factor 1 (IGF1), NF-kB, phosphoinositol 3 kinase (PI3K), AMP-activated protein kinase (AMPK), mTOR, and hypoxia-inducible factor 1 (HIF-1).10 Some of these have been found to regulate proteins involved in glycolysis and glutaminolysis. For example, HIF-1 is a key transcription factor that is regulated by cellular oxygen levels and degrades rapidly after synthesis when oxygen concentrations are normal. Even under normoxic conditions, HIF-1 is stabilized by glycolysis products, lactate, and pyruvate, and thus accumulated.11 As a result, the increased expression of glycolytic enzymes regulated by HIF-1 in tumor cells is not merely a substitute for hypoxia.12 HIF-1 can target many genes and regulate their protein expressions, such as the membrane transport proteins glucose transporter 1 (GLUT-1) and monocarboxylate transporter 4 (MCT-4), which transport glucose into the cells and lactate out of the cells.13,14 In addition, an increase in lactate dehydrogenase A (LDHA) expression results in the generation of lactate and NADP, which allows continuous glycolysis and ATP production.15 In addition to glycolysis, lactate can be generated by the tumor-specific isoform of pyruvate kinase (PK) M2, which transforms phosphoenolpyruvate (PEP) into pyruvate and thus increases glycolytic intermediates.16 Numerous studies indicated that PKM2 is highly expressed in various cancers, including non-small-cell lung cancer, melanoma, cervical cancer, etc.17 Lactate accumulation is therefore common in solid tumors. Lactate has long been considered a “metabolic waste product” of aerobic glycolysis, but growing evidence now has firmly demonstrated that lactate can be incorporated into the tricarboxylic acid (TCA) cycle to become a source of energy, and even acts as a multifunctional signaling molecule (Fig. 1).18 Thus, we discuss the function of lactate in tumor progression in this review, including its role in modifying epigenetics.
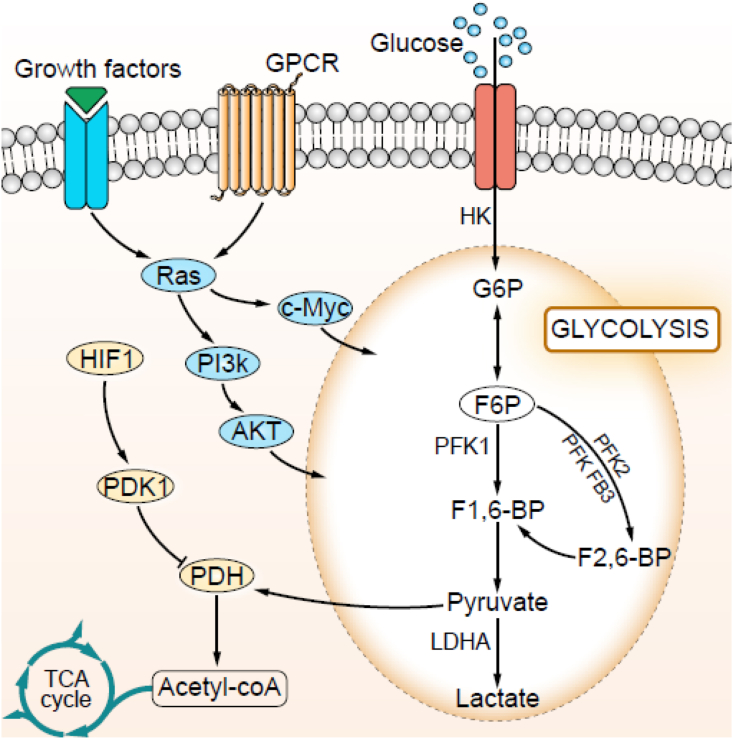
Figure 1.
The molecules regulating lactate production. Tumor cells overexpress HIF-1 and HIF-1 up-regulates enzymes of the glycolytic pathway. Tumor cells overexpress GLUT-1/3 to uptake glucose. Ras-PI3K-Akt and Ras-c-Myc signaling pathways regulate the expression of proteins involved in glycolysis and glutaminolysis.
Lactate acts as an extracellular messenger via lactate receptor GPR81
Since the discovery of the lactate receptor GPR81 in the adipocyte membrane, lactate has been hypothesized to act as a signaling molecule.19 According to Liu's report, lactate suppresses lipolysis in adipocytes via direct activating GPR81 in its physiological concentration range of 1–20 mM.20 In addition to adipose tissue, GPR81 is expressed in many types of cells, including muscle cells, immune cells, tumor cells, and the cells of the central nervous system.21 A 2014 study found that GPR81 was expressed in a variety of cancer cell lines and in the resected tumors from patients with pancreatic cancer.22 Highly glycolytic tumor cells present with high lactate concentration in the tumor microenvironment (TME), hence GPR81, which functions as the receptor for lactate on the cell surface, plays an important role in tumor progression. Lactate is exported into the TME, where extracellular lactate acts on its receptor GPR81 in tumor cells. In addition, it could act on non-cancer cells, where GPR81 is expressed, such as T cells and macrophages in the TME. Both mechanisms would involve facilitating tumor growth and metastasis. Knockdown of GPR81 significantly inhibits the proliferation and metastasis of cancer cells.22,23 Furthermore, the inhibition of GPR81 signaling suppresses pro-angiogenic mediator amphiregulin (AREG) through PI3K/Akt/cAMP-dependent pathway. Surprisingly, GPR81 knockdown resulted in a significant mitochondrial activity reduction and apoptosis increase.23
Lactate-GPR81 signaling also involves in regulating the immune checkpoint pathway. Lactate stimulation induces PD-L1 (programmed death ligand 1) expression in lung cancer cells which is mediated by GPR81.24 Jurkat T cells co-cultured with lactate-treated lung cancer cells significantly reduce IFN-γ production and apoptosis of Jurkat T cells compared with their co-cultured with lactate-untreated lung cancer cells, suggesting that lactate-induced PD-L1 expression presumably resulted in repression of T-cell function and thereby promotion of tumor immune escape. Blockage of the lactate/GPR81 pathway with 3-OBA (3-hydroxy-butyrate) and the PD-1/PD-L1 pathway with cyclic peptide C8 augments CD8+ T cells' function and prominently enhances the activity of metformin in antitumor.25 Deletion of Gpr81 in mice inhibits breast cancer growth in both the constitutive breast cancer model (MMTV-PyMT-Tg) and breast cancer transplant model due to the presence of more tumor-infiltrating T cells and MHCIIhi-immune cells in tumors.26 GPR81 activation contributes to the modulation of cellular DNA repair through the upregulation of DNA repair proteins, such as BRCA1, nibrin, and DNA-PKcs.27 Furthermore, GPR81 enhances doxorubicin resistance through increasing the expression and activity of ABCB1, which acts as the drug-exporting transporter in HeLa cells.28 GPR81 promoting the activity of DNA repair and chemo-resistance both depend on downstream Gi- and PKC-ERK signaling. Interestingly, lactate induces the assembly of Snail/EZH2/STAT3 complex, which enhances STAT3 activity and hence activates GPR81 expression.29
GPR81 also expresses in immune cells and lactate secreted by tumor cells may help confer an immunosuppressive TME. GPR81 is expressed in dendritic cells and macrophages in the colon which play an important role in suppressing immune tolerance and colonic inflammation.30 It could be inferred that similar lactate/GPR81-induced immunosuppression in the TME would be detrimental to the host defense against tumor growth. However, little is known about the role of GPR81 expression in immune cells in the TME. Tumor-derived lactate upregulated GPR81 in MDSCs and activated MDSCs via the GPR81/mTOR/HIF-1α/STAT3 pathway which is necessary for PDAC radio-resistance.31 Ovarian cancer has specific metastatic tropism to the adipose-rich omentum during metastasis, where GPR81 is expressed in adipocytes, which may have a pivotal role in creating a metastatic TME.32 Activation of GPR81 in adipocytes influences hormones and cytokines secretion in adipocytes, which may affect tumor growth, particularly breast and ovarian cancers.21 GPR81 is also expressed in endothelial cells, and lactate induces expression of GPR81 in brain endothelial cells and angiogenesis in glioma (Fig. 2).33
Figure 2.
Autocrine functions of lactate generated by tumor cells via GPR81 expression on tumor cells. Lactate-GPR81 signaling upregulates the expression of PD-L1 involved in immune evasion, BRCA1 and ABCB1 in chemoresistance, and amphiregulin in angiogenesis.
The role of lactate in tumor malignant progression
The released lactate levels of malignancies are remarkably elevated (up to 40 folds) compared to peripheral tissue34 and high concentrations of lactate are positively correlated with nodal or distant metastases and poor survival.35, 36, 37 Accelerated lactate production by aerobic glycolysis continually activated HIF-1α and c-Myc, thereby increasing the expression of pyruvate dehydrogenase kinase 1 (PDK-1) and LDHA, which transforms pyruvate into lactate and contributes to the aerobic glycolysis phenotype. On the other hand, PDK-1 inhibits pyruvate dehydrogenase (PDH) expression and restricts pyruvate entry into the mitochondrial matrix and the Krebs cycle as a result.38 Therefore, high levels of glycolytic flux and lactate production are beneficial for cancer cells' self-sufficiency and sustainability.39 Lactate promotes glioma cell migration by inducing the expression of transforming growth factor-β2 (TGF-β2) and further matrix metalloproteinase-2 (MMP-2).40 The addition of exogenous lactate enhances cell migratory potential in many cancer cell lines in a concentration-dependent manner.41 Although the mechanisms of lactate in promoting cancer metastasis are still not fully understood, high levels of lactate are correlated with a high probability of distant metastasis in the early stage of cancer.42 The incubation of L6 cells with 20 mM lactate increases MCT1 mRNA and protein expression rapidly.43 Therefore, lactate may promote carcinogenesis in susceptible, candidate cancer cells, by stimulating the expression of MCT1. Angiogenesis, the process of new blood vessels formation, is a major step in tumorigenesis. Lactate stimulates the production of VEGF in endothelial cells and increases its angiogenic activity.44 In cancer cells, the release of lactate effectively contributed to the in-vivo development of the tumor vasculature.36,45 Furthermore, lactate can enter into endothelial cells of tumors through MCT1 which stimulates angiogenesis and promotes tumor growth.46 Lactate increases hyaluronan production and therefore promotes the growth and motility of cancer cells,42 while inhibition of lactate production and transportation suppresses tumor angiogenesis. With inhibiting LDHA by oxamate, highly decreased lactate production brings about less angiogenesis. Targeting MCTs and lactate transport across cancer cells effectively inhibits tumor angiogenesis and cell migration.47 Lactate also promotes tumorigenesis independent of MCTs, through binding to its receptor GPR81 on the cell surface.48 Studies have shown that GPR81 is up-regulated in malignant cells. When pancreatic cancer cells with GPR81 silencing were cultured in the medium with a high glycolytic ratio and high lactate concentration, the mitochondrial activity was significantly reduced and cell death was increased.22
The process of tumor cell metastasis involves the degradation of the ECM and invasion of local tissues. Highly proliferative and glycolytic rates in tumors generate a great amount of lactate which creates a low-pH extracellular environment. This promotes the production of de novo actin filament and therefore improves the binding of cancer cell surface integrins to ECM components and enables migration with their assistance.49 Extracellular acidification increases the number and size of tumor cell “invadopodia” which is responsible for amoeboid movement, thereby promoting tumor invasion.50 Furthermore, alkalization of TME directly suppresses tumor invasion, suggesting that acidification of TME is necessary for local invasion. Acidification of TME also activates proteinases MMP-9, cathepsin B, and hyaluronidase-2 released by tumor cells, which degrades the surrounding matrix and promotes tumor cell invasion.51,52 Degrading of ECM by secreted proteinases may also promote the embedding of growth factors such as VEGF, TGFβ, and FGF2 in the matrix, leading to tumor growth and angiogenesis.53 Furthermore, lactate has been demonstrated to promote the expression of CD44, which is commonly up-regulated in tumors and potentiates the adherence of metastatic cancer cells of the breast and prostate to bone marrow endothelium to facilitate bony metastases.54 Acidification of the extracellular milieu in vitro increases lactate production and hence the expression of hyaluronan and CD44 (the predominant receptor for hyaluronan), thereby participating in the process of cancer invasion and metastasis.55 Lactate contributes to the acidification of the local tumor environment which decreases the binding activity of cell integrins to ECM and down-regulates the expression of E-cadherin thereby reducing tumor cell adhesion to neighboring cells and their insult to neighboring vessels.56
Lactate modulates the tumor microenvironment
High levels of aerobic glycolysis and glutaminolysis in tumors release large amounts of lactate and H+ into the extracellular space and thereafter the TME.57 In consequence, these release from tumor cells into TME drives the formation of an acidic TME ranging from 6.3 to 6.9, which promotes tumor growth, angiogenesis, metastasis, drug resistance, and immunosuppression.2 TME includes immune cells, endothelial cells, cancer-associated fibroblasts (CAFs), extracellular matrix (ECM), blood vessels, growth factors, and cellular metabolites related to hypoxia and acidity. Lactate is one of the most significant metabolites in the TME. The concentration of lactate in blood and healthy tissue under physiological condition are in the range of 1.5–3 mM,37 whereas the extracellular lactate concentration of tumor cells reaches 40 mM.35 High levels of lactate also are found in the sera of patients with melanoma, breast cancer, lung cancer, gastrointestinal and urogenital cancers, sarcoma, etc.58
Lactate induces apoptosis of natural killer (NK) and natural killer T (NKT) cells, resulting in the immune escape of tumor cells.59,60 Lactate inhibits interferon (IFN)-γ production and the cytotoxic activity of NK cells.59 Lactate also blocks IFN-γ and interleukin (IL)-4 production of NKT cells in TME through inhibiting mTOR signaling, resulting in preventing the activation of these immune cells.60 Fisher et al. found that high lactate concentration in the tumor environment inhibits T cell proliferation and function.58 Lactate/GPR81-induced immunosuppression also resulted in the inhibition of host defense and breast cancer growth.23 Dendritic cells (DC) are antigen-presenting cells that are an important player in the immune response. Lactate inhibits DC differentiation and makes the cells tolerogenic, which increases IL-10 production; moreover, IL-10 acts as a potent immunosuppressive cytokine that inhibits the maturation of DCs and activation of T cells and suppresses the production of IL-6, IFNγ, TNFα and IL-1β.61,62 Lactate also stimulates the development of myeloid-derived suppressor cells (MDSCs) and exerts broadly immunosuppressive functions by suppressing DC maturation, inhibiting NK cell cytotoxicity, preventing T cell activation, and facilitating regulatory T cell differentiation in TME.63
Macrophages, which are heterogeneous cell populations and function as scavengers, regulate the immune response and maintain tissue homeostasis.64 Macrophage plasticity is partially driven by epigenetic dynamics that can sustain stable phenotypes after activation in the settings of inflammation, autoimmunity, and cancer.65 Typically, activated macrophages can be divided into two phenotypes according to the state of polarization, M1 and M2. M1 macrophages tend to be a pro-inflammatory phenotype and preferentially utilize glycolysis, while M2 macrophages are predominantly present in immune regulation and tissue repair.66 Lactate induces VEGF and metabolizing enzyme arginase-1 (ARG1) expressions in response to HIF1-α, which promotes the polarization of M2 macrophages and hence facilitates tumor growth.67 In the breast cancer model, lactate is a critical metabolite in TME that drives macrophages polarized to M2-type through activating the ERK/STAT3 signaling pathway, which facilitates cell proliferation, migration, and angiogenesis in breast cancer.68 In gastric cancer, lactate contributes macrophage polarization towards an M2-like state through the MCT-HIF1-α signaling.69
Lactate also plays a role in tumor immune escape through binding to its receptor GPR81 or independent of GPR81. Co-culture of Jurkat T cells with GPR81-expressing lung cancer cells exhibits elevated cell proliferation and IFN-γ production compared to co-culturing with GPR81-lacking lung cancer cells.24 When lactate receptor GPR81 on the cell surface is activated in lung cancer, the membrane expression of PD-L1 up-regulates, which causes the tumor immune responses impeded.70 Inversely, inhibition of LDHA enhances the therapy efficacy with anti-programmed cell death-1 (PD1) in melanoma, which may relate to lactate (Fig. 3).71
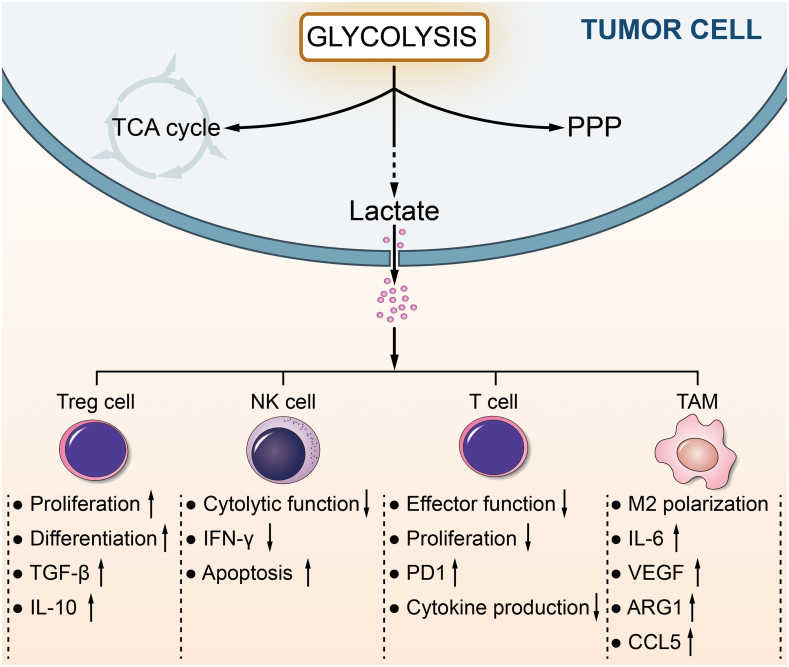
Figure 3.
Lactate in the tumor microenvironment. The tumor microenvironment (TME) is characterized by various cell types, including stromal and immune cells. Tumor cells secrete large amounts of lactate into TME, leading to acidosis, and modulate the metabolism of innate and adaptive immune cells, inhibiting the activation and proliferation of CD8+ T cells and NK cells. Lactate positively affects the immunosuppressive functions of Treg cells. Furthermore, lactate favors the polarization of alternatively activated (M2-like) phenotype macrophages, exerting an anti-inflammatory role, and participates in tumor growth and invasion.
Lactate and lactylation in the epigenetic regulation
Histones are essential components of chromatin, which play a role in the packing of DNA into chromatin and chromosomes and regulate gene expression. The protruding N-terminal and the C-terminal regions of histones often undergo posttranslational modifications. Multiple histone modifications have been discovered, including methylation, acetylation, and ubiquitylation. Those epigenetic modifications to the genome will affect gene expression, and DNA replication and repair.7,72,73 In 2019, Zhang et al. proposed a novel histone modification called lactylation.74 They find that lactylation of histone lysine residues by lactate acts as an epigenetic modification in chromatin that directly stimulates gene transcription. They identify 28 lactylation sites in human and mouse cells by mass spectrometry. The macrophages are stimulated with hypoxia, IFN-γ plus lipopolysaccharide, or bacterial to produce lactate by glycolysis, which increase histone lactylation at their promoters. The increased histone lactylation promotes the expression of M2-like homeostatic genes that are related to wound healing regulation, like Arg1.74 Though the regulatory mechanism of histone lactylation is not clear, many researchers have demonstrated histone lactylation plays a role in tumorigenesis. In ocular melanoma, histone lactylation was elevated and high histone lactylation levels were associated with unfavorable prognosis for the patients.75 Histone lactylation facilitates the expression of YTHDF2, an m6A reader, and causes PER1 and TP53 degradation by the reader’s binding to their respective m6A sites. When non-small cell lung cancer cells were treated with lactate, histone lactylation levels increase, the transcription of glycolytic enzymes (HK1, G6PD, and PKM) was down-regulated, and the transcription of TCA cycle enzymes (SDHA, IDH3G) was up-regulated.76
Mounting evidence has shown that the up-regulation of genes involved in glycolysis in lung fibroblasts, smooth muscle cells, and endothelial cells promotes the progression of lung fibrosis.77,78 In the lungs of humans with idiopathic pulmonary fibrosis or mice models of pulmonary fibrosis induced by bleomycin, histone lactylation increases. When treating macrophages with lactate, the histone lactylation of profibrotic genes, such as platelet-derived growth factor (PDGF), ARG1, thrombospondin 1 (THBS1), and VEGF significantly increase.79 Macrophages can also uptake extracellular lactate by MCT to promote HMGB1 lactylation via a p300/CBP-dependent mechanism, and further secrete lactylated HMGB1 via exosomes to facilitate endothelium permeability.80 During the early phase of somatic cell reprogramming, glycolytic genes were up-regulated by Glis1 and glycolytic flux was raised, resulting in increased acetylation (i.e., H3K27Ac) and lactylation (i.e., H3K18 la) at the loci of pluripotency genes, mainly the SRY-box transcription factor 2 (SOX2), Yamanaka factors POU class 5 homeobox 1 (OCT4), MYC proto-oncogene, and Krüppel-like factor 4 (KLF4), thus facilitating cellular reprogramming.4,81
Conclusions and perspectives
Mounting research has indicated that lactate is a useful metabolic fuel, a precursor of gluconeogenesis, and a signaling molecule for tumor survival and progression. Moreover, lactate plays a role in the regulation of the tumor microenvironment and the epigenetic modification of genes by histone lactylation. The role of lactylation and precise molecular mechanisms of lactylation modifications have not been fully elucidated. Therefore, comprehensive elaboration on how lactate influences epigenetic modifications and gene expression in tumor progression is of great significance. Evaluating histone lactylation levels in tumors and elucidating the mechanism of lactylation in gene expression regulation are anticipated for the cancer therapeutic approach.
Author contributions
Y.Z., Q.P., J.Z., Y.Y., X.Z., A.M. and Y.Q. collected the related papers and drafted the manuscript. Z.Q. and X.Z. revised and finalized the manuscript. All authors read and approved the final version of the manuscript.
Conflict of interests
The authors declare that they have no competing interests.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82060042), Guangxi Natural Science Foundation (China) (No. 2020GXNSFBA297082), Guangxi Science and Technology Program Project (China) (No. AD19245005) and The Basic Ability Enhancement Program for Young and Middle-aged Teachers of Guangxi (China) (No. 2020KY12017).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Zailong Qin, Email: qinzailong@hotmail.com.
Xiang Zheng, Email: xiangzheng12@sina.com.
References
- 1.Certo M., Tsai C.H., Pucino V., et al. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat Rev Immunol. 2021;21(3):151–161. doi: 10.1038/s41577-020-0406-2. [DOI] [PubMed] [Google Scholar]
- 2.Pérez-Tomás R., Pérez-Guillén I. Lactate in the tumor microenvironment: an essential molecule in cancer progression and treatment. Cancers. 2020;12(11):3244. doi: 10.3390/cancers12113244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flavahan W.A., Gaskell E., Bernstein B.E. Epigenetic plasticity and the hallmarks of cancer. Science. 2017;357(6348) doi: 10.1126/science.aal2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen A.N., Luo Y., Yang Y.H., et al. Lactylation, a novel metabolic reprogramming code: current status and prospects. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.688910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egger G., Liang G., Aparicio A., et al. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429(6990):457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 6.Warburg O., Wind F., Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee T.Y. Lactate: a multifunctional signaling molecule. Yeungnam Univ J Med. 2021;38(3):183–193. doi: 10.12701/yujm.2020.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeBerardinis R.J., Chandel N.S. We need to talk about the Warburg effect. Nat Metab. 2020;2(2):127–129. doi: 10.1038/s42255-020-0172-2. [DOI] [PubMed] [Google Scholar]
- 9.Deberardinis R.J. Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet Med. 2008;10(11):767–777. doi: 10.1097/GIM.0b013e31818b0d9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine A.J., Puzio-Kuter A.M. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330(6009):1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 11.Lu H., Forbes R.A., Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277(26):23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 12.Hirschhaeuser F., Sattler U.G.A., Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res. 2011;71(22):6921–6925. doi: 10.1158/0008-5472.CAN-11-1457. [DOI] [PubMed] [Google Scholar]
- 13.Baumeister J., Chatain N., Hubrich A., et al. Hypoxia-inducible factor 1 (HIF-1) is a new therapeutic target in JAK2V617F-positive myeloproliferative neoplasms. Leukemia. 2020;34(4):1062–1074. doi: 10.1038/s41375-019-0629-z. [DOI] [PubMed] [Google Scholar]
- 14.Luo F., Zou Z., Liu X., et al. Enhanced glycolysis, regulated by HIF-1α via MCT-4, promotes inflammation in arsenite-induced carcinogenesis. Carcinogenesis. 2017;38(6):615–626. doi: 10.1093/carcin/bgx034. [DOI] [PubMed] [Google Scholar]
- 15.Cheng L., Qin T., Ma J., et al. Hypoxia-inducible factor-1α mediates hyperglycemia-induced pancreatic cancer glycolysis. Anti Cancer Agents Med Chem. 2019;19(12):1503–1512. doi: 10.2174/1871520619666190626120359. [DOI] [PubMed] [Google Scholar]
- 16.Dayton T.L., Jacks T., Vander Heiden M.G. PKM2, cancer metabolism, and the road ahead. EMBO Rep. 2016;17(12):1721–1730. doi: 10.15252/embr.201643300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu S., Guo Y., Zhang X., et al. Pyruvate kinase M2 (PKM2) in cancer and cancer therapeutics. Cancer Lett. 2021;503:240–248. doi: 10.1016/j.canlet.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 18.de la Cruz-López K.G., Castro-Muñoz L.J., Reyes-Hernández D.O., et al. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front Oncol. 2019;9:1143. doi: 10.3389/fonc.2019.01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge H., Weiszmann J., Reagan J.D., et al. Elucidation of signaling and functional activities of an orphan GPCR, GPR81. J Lipid Res. 2008;49(4):797–803. doi: 10.1194/jlr.M700513-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Liu C., Wu J., Zhu J., et al. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81∗. J Biol Chem. 2009;284(5):2811–2822. doi: 10.1074/jbc.M806409200. [DOI] [PubMed] [Google Scholar]
- 21.Brown T.P., Ganapathy V. Lactate/GPR81 signaling and proton motive force in cancer: role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther. 2020;206 doi: 10.1016/j.pharmthera.2019.107451. [DOI] [PubMed] [Google Scholar]
- 22.Roland C.L., Arumugam T., Deng D., et al. Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Res. 2014;74(18):5301–5310. doi: 10.1158/0008-5472.CAN-14-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee Y.J., Shin K.J., Park S.A., et al. G-protein-coupled receptor 81 promotes a malignant phenotype in breast cancer through angiogenic factor secretion. Oncotarget. 2016;7(43):70898–70911. doi: 10.18632/oncotarget.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng J., Yang H., Zhang Y., et al. Tumor cell-derived lactate induces TAZ-dependent upregulation of PD-L1 through GPR81 in human lung cancer cells. Oncogene. 2017;36(42):5829–5839. doi: 10.1038/onc.2017.188. [DOI] [PubMed] [Google Scholar]
- 25.Chen S., Zhou X., Yang X., et al. Dual blockade of lactate/GPR81 and PD-1/PD-L1 pathways enhances the anti-tumor effects of metformin. Biomolecules. 2021;11(9):1373. doi: 10.3390/biom11091373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown T.P., Bhattacharjee P., Ramachandran S., et al. The lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen-presenting cells in the tumor microenvironment. Oncogene. 2020;39(16):3292–3304. doi: 10.1038/s41388-020-1216-5. [DOI] [PubMed] [Google Scholar]
- 27.Wagner W., Ciszewski W.M., Kania K.D. L- and D-lactate enhance DNA repair and modulate the resistance of cervical carcinoma cells to anticancer drugs via histone deacetylase inhibition and hydroxycarboxylic acid receptor 1 activation. Cell Commun Signal. 2015;13:36. doi: 10.1186/s12964-015-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner W., Kania K.D., Blauz A., et al. The lactate receptor (HCAR1/GPR81) contributes to doxorubicin chemoresistance via ABCB1 transporter up-regulation in human cervical cancer HeLa cells. J Physiol Pharmacol. 2017;68(4):555–564. [PubMed] [Google Scholar]
- 29.Xie Q., Zhu Z., He Y., et al. A lactate-induced Snail/STAT3 pathway drives GPR81 expression in lung cancer cells. Biochim Biophys Acta, Mol Basis Dis. 2020;1866(1) doi: 10.1016/j.bbadis.2019.165576. [DOI] [PubMed] [Google Scholar]
- 30.Ranganathan P., Shanmugam A., Swafford D., et al. GPR81, a cell-surface receptor for lactate, regulates intestinal homeostasis and protects mice from experimental colitis. J Immunol. 2018;200(5):1781–1789. doi: 10.4049/jimmunol.1700604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X., Lu Y., Hang J., et al. Lactate-modulated immunosuppression of myeloid-derived suppressor cells contributes to the radioresistance of pancreatic cancer. Cancer Immunol Res. 2020;8(11):1440–1451. doi: 10.1158/2326-6066.CIR-20-0111. [DOI] [PubMed] [Google Scholar]
- 32.Motohara T., Masuda K., Morotti M., et al. An evolving story of the metastatic voyage of ovarian cancer cells: cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene. 2019;38(16):2885–2898. doi: 10.1038/s41388-018-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miranda-Gonçalves V., Bezerra F., Costa-Almeida R., et al. Monocarboxylate transporter 1 is a key player in glioma-endothelial cell crosstalk. Mol Carcinog. 2017;56(12):2630–2642. doi: 10.1002/mc.22707. [DOI] [PubMed] [Google Scholar]
- 34.Holm E., Hagmüller E., Staedt U., et al. Substrate balances across colonic carcinomas in humans. Cancer Res. 1995;55(6):1373–1378. [PubMed] [Google Scholar]
- 35.Brizel D.M., Schroeder T., Scher R.L., et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51(2):349–353. doi: 10.1016/s0360-3016(01)01630-3. [DOI] [PubMed] [Google Scholar]
- 36.Dhup S., Dadhich R.K., Porporato P.E., et al. Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr Pharmaceut Des. 2012;18(10):1319–1330. doi: 10.2174/138161212799504902. [DOI] [PubMed] [Google Scholar]
- 37.Walenta S., Wetterling M., Lehrke M., et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000;60(4):916–921. [PubMed] [Google Scholar]
- 38.Vaupel P., Schmidberger H., Mayer A. The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol. 2019;95(7):912–919. doi: 10.1080/09553002.2019.1589653. [DOI] [PubMed] [Google Scholar]
- 39.San-Millán I., Brooks G.A. Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis. 2017;38(2):119–133. doi: 10.1093/carcin/bgw127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baumann F., Leukel P., Doerfelt A., et al. Lactate promotes glioma migration by TGF-β2-dependent regulation of matrix metalloproteinase-2. Neuro Oncol. 2009;11(4):368–380. doi: 10.1215/15228517-2008-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goetze K., Walenta S., Ksiazkiewicz M., et al. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol. 2011;39(2):453–463. doi: 10.3892/ijo.2011.1055. [DOI] [PubMed] [Google Scholar]
- 42.Walenta S., Mueller-Klieser W.F. Lactate: mirror and motor of tumor malignancy. Semin Radiat Oncol. 2004;14(3):267–274. doi: 10.1016/j.semradonc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Hashimoto T., Hussien R., Oommen S., et al. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. Faseb J. 2007;21(10):2602–2612. doi: 10.1096/fj.07-8174com. [DOI] [PubMed] [Google Scholar]
- 44.Kumar V.B.S., Viji R.I., Kiran M.S., et al. Endothelial cell response to lactate: implication of PAR modification of VEGF. J Cell Physiol. 2007;211(2):477–485. doi: 10.1002/jcp.20955. [DOI] [PubMed] [Google Scholar]
- 45.Polet F., Feron O. Endothelial cell metabolism and tumour angiogenesis: glucose and glutamine as essential fuels and lactate as the driving force. J Intern Med. 2013;273(2):156–165. doi: 10.1111/joim.12016. [DOI] [PubMed] [Google Scholar]
- 46.Végran F., Boidot R., Michiels C., et al. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71(7):2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- 47.Sonveaux P., Copetti T., De Saedeleer C.J., et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Payen V.L., Mina E., van Hée V.F., et al. Monocarboxylate transporters in cancer. Mol Metabol. 2020;33:48–66. doi: 10.1016/j.molmet.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Webb B.A., Chimenti M., Jacobson M.P., et al. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11(9):671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 50.Busco G., Cardone R.A., Greco M.R., et al. NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. Faseb J. 2010;24(10):3903–3915. doi: 10.1096/fj.09-149518. [DOI] [PubMed] [Google Scholar]
- 51.Putney L.K., Barber D.L. Expression profile of genes regulated by activity of the Na-H exchanger NHE1. BMC Genom. 2004;5(1):46. doi: 10.1186/1471-2164-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bourguignon L.Y.W., Singleton P.A., Diedrich F., et al. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem. 2004;279(26):26991–27007. doi: 10.1074/jbc.M311838200. [DOI] [PubMed] [Google Scholar]
- 53.Shuman Moss L.A., Jensen-Taubman S., Stetler-Stevenson W.G. Matrix metalloproteinases: changing roles in tumor progression and metastasis. Am J Pathol. 2012;181(6):1895–1899. doi: 10.1016/j.ajpath.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Draffin J.E., McFarlane S., Hill A., et al. CD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells. Cancer Res. 2004;64(16):5702–5711. doi: 10.1158/0008-5472.CAN-04-0389. [DOI] [PubMed] [Google Scholar]
- 55.Stern R., Shuster S., Neudecker B.A., et al. Lactate stimulates fibroblast expression of hyaluronan and CD44:the Warburg effect revisited. Exp Cell Res. 2002;276(1):24–31. doi: 10.1006/excr.2002.5508. [DOI] [PubMed] [Google Scholar]
- 56.Paul C.D., Mistriotis P., Konstantopoulos K. Cancer cell motility: lessons from migration in confined spaces. Nat Rev Cancer. 2017;17(2):131–140. doi: 10.1038/nrc.2016.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lyssiotis C.A., Kimmelman A.C. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 2017;27(11):863–875. doi: 10.1016/j.tcb.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fischer K., Hoffmann P., Voelkl S., et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109(9):3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 59.Harmon C., Robinson M.W., Hand F., et al. Lactate-mediated acidification of tumor microenvironment induces apoptosis of liver-resident NK cells in colorectal liver metastasis. Cancer Immunol Res. 2019;7(2):335–346. doi: 10.1158/2326-6066.CIR-18-0481. [DOI] [PubMed] [Google Scholar]
- 60.Kumar A., Pyaram K., Yarosz E.L., et al. Enhanced oxidative phosphorylation in NKT cells is essential for their survival and function. Proc Natl Acad Sci U S A. 2019;116(15):7439–7448. doi: 10.1073/pnas.1901376116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao S., Wu D., Wu P., et al. Serum IL-10 predicts worse outcome in cancer patients: a Meta-analysis. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0139598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nasi A., Fekete T., Krishnamurthy A., et al. Dendritic cell reprogramming by endogenously produced lactic acid. J Immunol. 2013;191(6):3090–3099. doi: 10.4049/jimmunol.1300772. [DOI] [PubMed] [Google Scholar]
- 63.Morrot A., da Fonseca L.M., Salustiano E.J., et al. Metabolic symbiosis and immunomodulation: how tumor cell-derived lactate may disturb innate and adaptive immune responses. Front Oncol. 2018;8:81. doi: 10.3389/fonc.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mehla K., Singh P.K. Metabolic regulation of macrophage polarization in cancer. Trends Cancer. 2019;5(12):822–834. doi: 10.1016/j.trecan.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodriguez R.M., Suarez-Alvarez B., Lopez-Larrea C. Therapeutic epigenetic reprogramming of trained immunity in myeloid cells. Trends Immunol. 2019;40(1):66–80. doi: 10.1016/j.it.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 66.Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Colegio O.R., Chu N.Q., Szabo A.L., et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mu X., Shi W., Xu Y., et al. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle. 2018;17(4):428–438. doi: 10.1080/15384101.2018.1444305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang L., Li S. Lactic acid promotes macrophage polarization through MCT-HIF1α signaling in gastric cancer. Exp Cell Res. 2020;388(2) doi: 10.1016/j.yexcr.2020.111846. [DOI] [PubMed] [Google Scholar]
- 70.San-Millán I., Julian C.G., Matarazzo C., et al. Is lactate an oncometabolite? evidence supporting a role for lactate in the regulation of transcriptional activity of cancer-related genes in MCF7 breast cancer cells. Front Oncol. 2019;9:1536. doi: 10.3389/fonc.2019.01536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Daneshmandi S., Wegiel B., Seth P. Blockade of lactate dehydrogenase-A (LDH-A) improves efficacy of anti-programmed cell death-1 (PD-1) therapy in melanoma. Cancers. 2019;11(4):450. doi: 10.3390/cancers11040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prakash K., Fournier D. Evidence for the implication of the histone code in building the genome structure. Biosystems. 2018;164:49–59. doi: 10.1016/j.biosystems.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 73.Xu H., Wu M., Ma X., et al. Function and mechanism of novel histone posttranslational modifications in health and disease. BioMed Res Int. 2021;2021 doi: 10.1155/2021/6635225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang D., Tang Z., Huang H., et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574(7779):575–580. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu J., Chai P., Xie M., et al. Histone lactylation drives oncogenesis by facilitating m6A reader protein YTHDF2 expression in ocular melanoma. Genome Biol. 2021;22(1):85. doi: 10.1186/s13059-021-02308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang J., Huang D., Jiang Y., et al. Lactate modulates cellular metabolism through histone lactylation-mediated gene expression in non-small cell lung cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.647559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie N., Tan Z., Banerjee S., et al. Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am J Respir Crit Care Med. 2015;192(12):1462–1474. doi: 10.1164/rccm.201504-0780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fernandez I.E., Eickelberg O. New cellular and molecular mechanisms of lung injury and fibrosis in idiopathic pulmonary fibrosis. Lancet. 2012;380(9842):680–688. doi: 10.1016/S0140-6736(12)61144-1. [DOI] [PubMed] [Google Scholar]
- 79.Cui H., Xie N., Banerjee S., et al. Lung myofibroblasts promote macrophage profibrotic activity through lactate-induced histone lactylation. Am J Respir Cell Mol Biol. 2021;64(1):115–125. doi: 10.1165/rcmb.2020-0360OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang K., Fan M., Wang X., et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 2022;29(1):133–146. doi: 10.1038/s41418-021-00841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li L., Chen K., Wang T., et al. Glis1 facilitates induction of pluripotency via an epigenome–metabolome–epigenome signalling cascade. Nat Metab. 2020;2(9):882–892. doi: 10.1038/s42255-020-0267-9. [DOI] [PubMed] [Google Scholar]