Abstract
The intellectual history of energy homeostasis, focusing on food intake and energy storage, is briefly reviewed. Physiological energetics was founded by Lavoisier, who in the late eighteenth century invented direct and indirect calorimetry and discovered the role of oxygen in combustion and respiration. Energy was understood well enough by the mid-nineteenth century to realize the physiological energy-balance equation, that energy intake – energy expenditure = energy storage, but this did not greatly influence physiological research for another century. Homeostasis, the concept that many vital physiological variables are actively regulated in narrow envelopes, was developed by Bernard and Cannon between approximately 1870–1940 and remains a central principle of physiology. Kennedy coined the term lipostasis in 1953 to refer to the constancy of fat mass, which Mayer argued was the mechanism regulating body weight. A parameterized control-theory model suggests that a proportional negative-feedback control system incompletely compensates weight loss during persistent negative energy balance, suggesting that Cannon's idea of constancy within a narrow envelope may not fit body-weight regulation well. This modelling encourages further application of control theory to issues in energy homeostasis, including to the development of obesity. It also sets the stage for understanding the underlying neuroendocrine mechanisms.
This article is part of a discussion meeting issue ‘Causes of obesity: theories, conjectures and evidence (Part I)’.
Keywords: regulation, obesity, body weight
1. Introduction
Energy homeostasis refers to the active maintenance, or regulation, of appropriate levels of energy availability (‘active’ in this context refers to a system whose response involves dynamic reacting components [1]). Energy homeostasis is organized hierachically [2]. Cells maintain a constant ATP : ADP ratio of approximately 10, circulating concentrations of energy metabolites are maintained roughly constant, and many data indicate that energy storage is actively regulated [3–7], probably as the result of several interacting influences on natural selection [5–7]. This review presents a brief history of several key concepts related to energy homeostasis: physiological energetics, homeostasis, adipose-tissue regulation and control theory. The last section considers how quantitative modelling of the dynamics of energy-storage regulation might contribute to analyses of three theories of obesity, the dual-intervention-point model (DIPM; [6,7]), the energy-balance model (EBM; [3–5]) and the carbohydrate-insulin model (CIM; [8–11]). The review focuses on energy storage and energy (food) intake; energy expenditure is omitted for brevity.
It is important to recognize that the causal relationships among variables related to energy homeostasis and the mechanisms through which they are regulated remain unclear (see [12] for discussion). Body weight per se seems an unlikely candidate for regulation, although some evidence suggests that there is a gravitostat [13]. Before the obesity epidemic, adult Americans maintained a body-mass index (BMI) that was roughly normally distributed with mean 25 kg m−2 [14], consistent with the operation of an active regulatory system. BMI, however, is a poor measure of energy storage (e.g. [15,16]) and some degree of regulation can be achieved without active regulation (§3.4 in [1]). Adipose tissue seems a more viable candidate. First, it has the highest energy content of any organ, and second, a neuroendocrine negative-feedback mechanism in which leptin links fat mass to eating and energy expenditure has been discovered [5]. Changes in energy stored, however, represent a somewhat variable mix between changes in the mass of adipose tissue and of other tissues that change in concert with adiposity, an issue rarely addressed in obesity research. Huang et al. [17] found that only 48% of obesity-related excess tissue was adipose tissue in men and 67% in women. The triglyceride content of adipose tissue is similarly variable, ranging from 77 to 94% [18]. Finally, it is likely that other regulations related to energy homeostasis affect energy storage indirectly (discussed more in §7).
2. Physiological energetics and energy balance
(a) . Lavoiser
The foundations of physiological energetics were laid by Antoine-Laurent de Lavoisier (1743–1794), together with his wife Marie-Anne Pierrette Paulze Lavoisier (1758–1836), Pierre-Simon Laplace (1749–1827), and others. Lavoisier's careful quantitative analyses led to the ‘law of conservation of mass,’ that the sum of the weights of all products of a chemical reaction equals the sum of the weights of the materials entering the reaction. This enabled Lavoisier to recognize the nature of oxidation for the first time. Although ‘vital’ (i.e. oxygen-containing) air had been isolated previously, its chemistry was obscure. Lavosier [19–21] demonstrated that the chemical products of combustion gained weight by removing oxygen from the air, rather than losing weight as posited by the phlogiston theory. This led him to name oxygen as a novel chemical element.
Lavoisier's investigations of energetics relied on a novel closed calorimeter that enabled the measurement of oxygen consumption, carbon dioxide production, and heat production, which he developed together with Laplace [22]. This apparatus simultaneously began the two main methods to measure energy expenditure, direct calorimetry (measurement of heat production) and indirect calorimetry (measurement of respiratory gas exchange). With it, Lavoisier & Laplace [22] showed that a guinea pig produced nearly the same amount of heat per unit carbon dioxide produced as did oxidation of vegetable material, leading to their famous conclusion ‘la respiration est donc une combustion.’ Further, they discovered that the ratio of oxygen inspired to carbon dioxide expired by guinea pigs was nearly one. This ratio became the respiratory quotient, which, following mid-nineteenth century advances in physiological chemistry, enabled the calculation of relative carbohydrate and lipid oxidation. They also demonstrated that nitrogen played no role in respiration. Finally, Lavoisier and Armand Séguin (1767–1835) extended the work in animals to measure human oxygen consumption for the first time, which they did with subjects at rest and exercising and at different ambient temperatures [23].
Lavoisier [22,24] theorized that heat is a weightless element that is cool when bound to other elements and warm when released. Unlike an element, however, heat is not a conserved quantity, but can be generated by physical work. This was first experimentally verified a few years later [25] and was established quantitatively in the mid-nineteenth century for all the forms of energy then recognized. These and related findings led to the realization that the combination of physical work, heat and other forms of what is now called energy is a conserved quantity. Conservation of energy became part of the first law of thermodynamics.
For further discussion of Lavoisier's contributions, see [26–35].
(b) . Energy balance
The law of conservation of energy implies the energy-balance equation:
where the Δτ represents changes during a time period τ in the potential metabolic energy of chemicals stored in the body, the intake of metabolizable energy in the form of food and the sum of energy expended as physical work, heat and excreta.
The energy balance equation does not indicate causal relationships among the variables. It is consistent both with the possibility that changes in energy stored control food intake and energy expenditure and the possibility that aspects of ingested food other than energy content are critical determinants of energy storage. The relative importance of these possibilities in obesity pathogenesis is currently a controversial research (see §6(b)). The equation is also consistent with the possibility that food intake is controlled mainly by energy expenditure, in the absence of a regulatory mechanism (as described below) and independent of energy storage [36].
3. Homeostatic regulation
(a) . Bernard
Claude Bernard's (1813–1878) importance in physiology rests on several achievements. He made groundbreaking discoveries, including discovery of the role of pancreatic juice in fat digestion [37], of hepatic glycogen [38] and of autonomic controls of circulation, body temperature and glucose metabolism [39]. He contributed a seminal work on the scientific method in physiology and medicine [40,41]; and, most importantly for the present discussion, he enunciated a new fundamental principle of physiology [4,42,43].
Bernard's new principle was that the fluid environment of the organs, tissues and cells of warm-blooded vertebrates must be maintained constant in order for them to maintain normal function. He wrote: ‘It is the fixity of the ‘milieu interieur’ which is the condition of free and independent life … all the vital mechanisms, however varied they may be, have only one object, that of preserving constant the conditions of life in the internal environment’ [42, p. 113]. Bernard based this far-ranging conclusion on his extensive, albeit often crude, measurements of blood glucose, oxygen, carbon dioxide, water content and acidity as well as of body temperature (he correctly surmised that the blood plasma closely mimicked the interstitial fluid that is the immediate environment of most cells). Although many prior investigators had described various physiological compensations [44], Bernard's forceful emphasis of the critical importance of the inner environment together with his many novel investigations of it brought his concept to the centre of physiology, where it remains. Bernard's view, however, was Platonist in that he held that the critical variables of the inner environment are maintained at near absolute constancy. This is discussed more in §3(b).
There is an interesting contrast between Bernard's science and that of another of physiology's foundational figures, Ivan Pavlov (1849–1936). Although surgical anesthesia became available in the middle of his career, Bernard never adopted it. Rather, he depended on acute surgical experiments in unanesthetized animals (which contributed his wife's leaving him and founding an animal-protection society [45,46]). Pavlov found that methods like Bernard's were scientifically, as well as humanely, flawed. Instead, in his work on digestion, for which he won the 1904 Nobel Prize, Pavlov relied on chronic surgical preparations that allowed experiments to be repeated over months in healthy, unstressed animals [47,48]. According to Smith [47, p. R574], ‘this was the platform from which integrative physiology was launched.’ It led to Pavlov's ability to accurately quantify many aspects of digestion and to discover that psychological function affects visceral function; first in ‘psychic secretions’, then in conditioned reflexes; phenomena which occupied him for the last 30 years of his career.
(b) . Cannon
The decades between the careers of Bernard and of Walter B. Cannon (1871–1945) saw numerous improvements in assaying physiological variables as well as discovery of a new modality of physiological signalling, chemical messaging in the form of hormones and neurotransmitters. One of the new methods was x-ray imaging, which Cannon applied to the study of physical digestion beginning while he was a medical student [57–59]. These studies were the cornerstone of the study of gastrointestinal motility. Cannon also noted an association of gastric contractions detected by x-rays and reports of hunger [60], a study that initiated the experimental study of eating [61]. Cannon [62] noted in the course of his work with x-rays that emotional states significantly affected gastrointestinal motility, which led him to his next research direction, the physiological effects of ‘emotional excitement’ [63] (paralleling Pavlov's turn from digestive physiology to conditioned reflexes described in §3(a)). Cannon's investigation of the mechanisms through which emotion had physiological consequences led him to the realization that physiological processes integrate numerous influences to result in both stability and dynamism.
These insights prompted Cannon to revise Bernard's view of the inner environment. Rather than being an unchanging constant, Cannon found that various environmental and physiological situations led to adaptations of the inner environment. Cannon felt that this revision of Bernard's dictum of constancy was worthy of a new name, homeostasis [64,65]. Cannon [64, p. 400] offered the following definition: ‘The coordinated physiological processes which maintain most of the steady states in the organisms are so complex and peculiar to living beings … that I have suggested a special designation for these states, homeostasis. The word does not imply, something set and immobile, a stagnation. It means a condition–a condition which may vary, but is relatively constant.’ This differentiated view of organisms' inner fluid environments has been the core definition of physiological regulation ever since [66].
Cannon [64] theorized that homeostatic regulation was typically achieved by hierarchies of control mechanisms. For example, increases in blood glucose were thought to be countered first by insulin-mediated glucose uptake, then glycogen synthesis, then shunting of plasma glucose into interstitial spaces (Cannon incorrectly believed, that colloidal spaces of connective tissue and skin could absorb excess plasma constituents—‘the analogy implied … is that of a bog or swamp into which water soaks when the supply is bountiful and from which the water seeps back into the distributing system when the supply is meager’ [64, p. 403]), and finally glucose loss via overflow into the urine. Cannon knew of only a single mechanism opposing decreases in blood glucose concentration, a sympathetic-adrenal-medullary response leading to glycogenolysis; identification of glucagon, cortisol and somatostatin lay in the future.
Cannon had little to say about adipose tissue. He believed that fasting blood lipid levels were probably regulated. If so, then adipose tissue was presumably a swamp designed to receive excess lipid. But Cannon [64, p. 415] wrote, ‘what leads to fat storage in some individuals to a greater extent than in others is unknown.’ The concept of regulation of fat mass per se depended on later discoveries (§4.).
(c) . Homeostasis since Cannon
Curt P. Richter (1894–1988) was a third foundational figure in regulatory physiology. Richter's fundamental contribution was recognition of the importance of behaviour in homeostasis. This he showed over decades of research on thermoregulatory behaviour and on specific appetites for various minerals and vitamins that were awakened by deficiencies or by challenges such as pregnancy and lactation. As Richter [69, p. 64] wrote: ‘Both Bernard and Cannon concerned themselves almost entirely with the physiological and chemical regulators of the internal environment. …The results of our own experiments have shown that behaviour or total organism regulators also contribute to the maintenance of a constant internal environment.’ Although their careers overlapped, Cannon did not recognize Richter's importance. For more on Richter, see [70–73].
Richter's emphasis on behavioural homeostatic controls led naturally to analyses of learned behaviours in homeostasis, for example, learned preferences for flavours associated with recovery from vitamin deficiencies and learned aversions of flavours associated with toxins [74]. For further discussions of contemporary evidence for the importance of learning in homeostasis, see [75–77].
Another anticipatory mechanism is allostasis, which refers to a plastic neural network that responds to predictable stressors that might otherwise disrupt homeostasis [78–80]. Importantly, although these responses are adaptive in the short term, they can become maladaptive when repeated frequently. This is referred to as allostatic load, it is thought to contribute to several chronic health problems, including the development of obesity [81,82].
Two further developments since Cannon are the conceptualization of stored energy as a homeostatic variable and the use of control theory to analyse physiological regulation. These are discussed in §§4–6.
4. Regulation of fat mass
(a) . Brobeck
John R. Brobeck (1914–2009) is perhaps best known for his discoveries of the hyperphagia and anorexia syndromes that result from lesion of the ventromedial hypothalamus [83,84] and lateral hypothalamus [85,86], respectively. His own view of his major contribution, however, was different. He wrote, ‘what I introduced is so obvious that now we suppose it was always known’ [87, p. 226]. What he introduced was consideration of all the variables of energy exchange – food intake, physical activity, heat loss and body weight – together in an integrated manner [88].
Importantly, Brobeck did not think that the energy balance equation was relevant to homeostatic regulation. Rather, he thought that the homeostatic regulation of body temperature controlled food intake and physical activity [88,89]. His view of body weight was that ‘regulation of fat storage now appears to be a passive process, subject only indirectly to control’ and that ‘the quantity of fat in the body is determined by mechanisms which regulate food intake, work (or activity) and body temperature and inquiry into the pathogenesis of human obesity must seek to discover abnormalities in the regulation of one or more of these factors' [88, p. 317].
(b) . Kennedy and Mayer
The field's view of energy storage began to change in 1953 with the appearance of a landmark paper by Gordon Kennedy. Based on several lines of evidence, Kennedy [90, p. 578] concluded that ‘the young rat adjusts its food intake so precisely to its energy needs that its fat stores remain almost constant’ and that ‘lipostasis could be achieved [by hypothalamic] sensitivity to the concentrations of circulating metabolites.’ Thus began the concept of regulation of fat mass.
Kennedy's work gained traction when, at a major meeting, Jean Mayer [91] presented it as a complement to his glucostatic theory of meal-to-meal control of eating (which for decades was probably the most researched controller of eating). Mayer expanded Kennedy's idea of mechanism, hypothesizing that ‘long-term regulation of body weight would be based on the fact that animals will mobilize spontaneously, each day, a quantity of fat proportional to, or at least increasing with, the total fat content, [91, p. 39]’ with the quantity also depending on environmental temperature, energy expenditure, etc. Mayer also suggested that there is a ‘privileged’ body weight depending on ‘the type of animals, individual physiologic characteristics, diet, exercise regime, and even individual taste preferences' [91] a remarkably prescient statement (discussed in §5(a)).
5. Control theory
(a) . Introduction
In his history of physiological regulation, Adolph [44] found that early scientists described regulation in terms of adaptation, balancing, compensation, maintenance, self-preservation and the like. Today, theories of homeostatic regulation centre around control theory concepts, in particular negative-feedback control and feed-forward or anticipatory control. Goldstein & Kopin [92, p. 16] state, ‘Negative feedback regulation is a key—if not the key—mechanism for maintaining physiological homeostasis’ (italics original).
This development began with Arturo Rosenblueth, a fellow who worked with both Cannon and the control theorist Norbert Wiener. In 1943, Rosenblueth and Wiener wrote an essay arguing that the much-maligned concept of purpose in behaviour simply referred to behaviour controlled by negative feedback [93], and in 1948 Wiener wrote an influential book describing the varieties of feedback control in machines and animals [94].
Control-theory models have facilitated progress in many branches of physiology. Although negative-feedback control was hypothesized to be the mechanism of Kennedy's lipostasis by Hervey in 1959 [95], mathematical control theory never became prominent in obesity research [1]. This may be beginning to change. Therefore, §5 briefly introduces control theory, and §6 describes some applications to current issues in obesity research.
(b) . Negative-feedback control
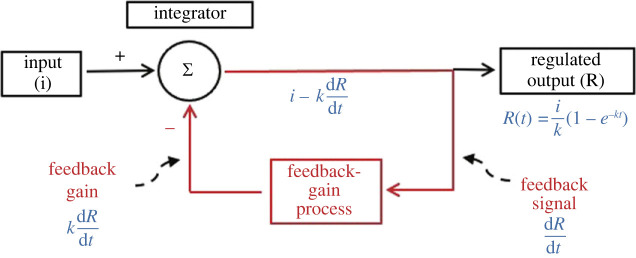
A simple negative-feedback control system involving proportional feedback gain is diagrammed in figure 1 (red and black parts). Each box shown represents a process that transforms the input variable into an output variable. The different variables involved can be transformed to a single variable, enabling the entire system to be mathematically modelled. The system shown is simple in that there is no set point, and proportional because the feedback-gain transformation is a linear function with 0 intercept [1,96]. If the other transforms are also linear, a change in feedback leads to an exponential change in the regulated variable.
Figure 1.
A simple negative-feedback control system (in black and red). The elements of the system are the regulated output that is driven by an input, a feedback signal from the output to a feedback processor that produces feedback-gain signal, and an integrator that subtracts the feedback-gain signal from the input to produce the output signal. Note the closed-loop organization of feedback (red). A mathematical model of the change in output in response to a tonic change in input is shown in blue. Feedback at time t is the change in output from the initial level ; feedback gain is proportional to the feedback (, where k is the constant of proportionality) and is subtracted (Σ) from the input to yield the control signal . Integrating yields the exponential equation: . Note that as time passes, approaches 0 and the final steady-state change in output is simply the ratio of the strengths of the input and feedback-gain signals, Of course, systems with more complex feedback-gain processes and systems responding to dynamic challenges behave in more complex ways.
Figure 1 shows only a single input. This could be comprised multiple components. For example, if the system modelled energy-storage regulation, it might include normal physiological controls of eating, such as basal metabolic rate [35], metabolic fuel availability [2,11], etc., as well as disturbances to normal physiology, such as can be brought about by consumption of large amounts of fat or sugar. Different inputs can activate different control effectors, and different effectors can be recruited at different signal strengths, as in Cannon's model of blood-glucose regulation (§3(b)). Note that negative-feedback control clouds causality, in that there is no clear start or end in a feedback loop [96]. Thus, a defect in any of the processes in a negative-feedback control system can result in dysregulation.
Systems like that in figure 1 return the regulated variable to its initial value following phasic challenges. They fail, however, to fully correct tonic (i.e. prolonged, relatively constant) challenges. Rather, there is always a change in the steady-state. The feedback-gain function is the key to the system's performance. Stronger feedback gain increases response speed and intensity and reduces steady-state changes. As shown in figure 2, feedback-gain processes can also be constructed to include thresholds for activation of control and asymmetric strengths of control for increases and decreases in the regulated variable.
Figure 2.
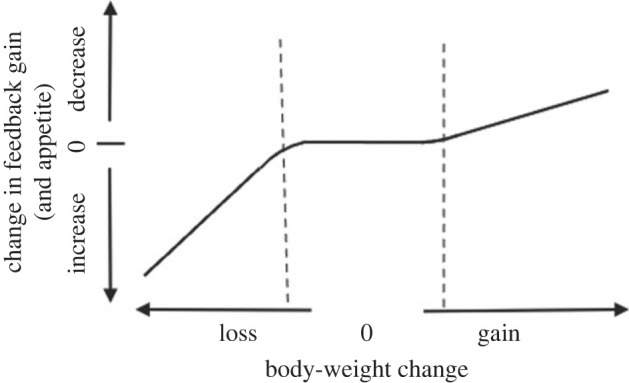
Example of a nonlinear feedback-gain function. The DIPM [6,7] posits that the body-weight regulatory system has thresholds for minimum weight gains and losses required to stimulate weight-regulatory responses and has separate control mechanisms for gains and losses. As the figure shows, a simple negative-feedback system with a curvilinear feedback-gain function and no set-point can result in the same phenomenology. In this example (although not posited by DIPM), the slope of the feedback-gain (and hence, appetite) is greater for weight loss than weight gain. Stronger feedback gain increases response speed and intensity and reduces steady-state changes.
A set-point is an additional input to the feedback loop that provides a target level for the regulated variable. Typically, the feedback signal is subtracted from the set-point signal before entering the feedback-gain process. A common misconception is that the addition of a set-point produces exact regulation. In fact, however, it is the nature of the feedback-gain process that determines the precision of regulation [1,96]. Precise regulation during tonic challenges can be obtained by a strong integral feedback-gain process, in which changes in the feedback signal are accumulated. Integral-feedback gain can be based on a set-point or, equivalently, by accumulating changes from an adaptation level. As there is neither functional nor mechanistic evidence for a set-point in the regulation of energy storage [1,2,76], however, obesity research would be better served if the set-point heuristic were replaced with discussions based on negative-feedback processes [1].
(c) . Feed-forward control
Feed-forward or anticipatory controls refer to situations in which stimuli associated with regulated variables trigger responses prior to any change in the regulated variable [1,76,77]. For example, the cephalic and intestinal (e.g. incretin-mediated) phases of insulin secretion and learned preferences for nutrient-rich foods [75] appear to be anticipatory contributions to the homeostatic regulation of circulating energy metabolites. Feed-forward mechanisms, however, are predictive and therefore often require negative-feedback correction for precise regulation. For example, the direct action of glucose on the pancreatic α-, β- and δ-cells fine tunes the effects of the preceding cephalic and intestinal phases of insulin secretion.
6. Control-theory models of obesity
(a) . Obesity pharmacology
Hall and his colleagues have applied control theory to the dynamics of body-weight loss in individuals with obesity [97–99]. First, in 28 groups of patients with obesity who were chronically treated with one of 14 different pharmacological regimens, dynamic changes in energy expenditure produced by weight loss were modelled using the Hall algorithm [100–102]. Next these data and the patients' weight records were used to estimate energy intake. Finally, exponential functions based on constant pharmacological effects (i.e. a tonic challenge) and a proportional negative-feedback control were fitted to the body weight and energy-intake estimations (as defined in §5(b), proportional means that the feedback-gain signal is proportional to the weight change). No set-point was hypothesized. This yielded good fits for the dynamic courses of body weight and estimated energy intake (mean R2 = 0.87).
Polidori et al. [99] performed a similar analysis using data from patients with type-two diabetes mellitus and overweight or obesity who were treated for one year with the sodium-glucose cotransporter-2 (SGLT2) inhibitor canagliflozin, which produced a urinary-glucose loss of approximately 350 kcal d−1. Measured body weight decreased approximately 3.5 kg, and estimated energy intake increased over approximately 15 weeks to a steady approximately 350 kcal d−1. Again, a proportional negative-feedback model fitted the data well (and addition of an integral control term markedly worsened the fits). If there had been no regulatory response, steady-state weight change would have been approximately 10 kg [102]. The model of energy intake has not yet been validated with measurements of food intake, although Miura et al. [103] found that similar SGLT2-inhibitor treatment was associated with a modest, but significant, increase in subjective hunger.
In summary, these analyses by Hall and his colleagues indicate that simple proportional negative-feedback models fit the dynamic changes in body weight produced by obesity pharmacotherapy remarkably well, suggesting that control-theory models can further understanding of obesity. A critical point is that the fits were achieved without integral control or a set-point, implying that the regulatory system opposes change, but does not have a ‘should-be’ weight to which it returns. This is a conceptual change from the Bernard-Cannon concept of constancy within a relatively narrow envelope (§3(b)). Importantly, whether similar proportional control can predict body-weight dynamics in situations of weight gain remains to be discovered.
(b) . Obesity pathogenesis
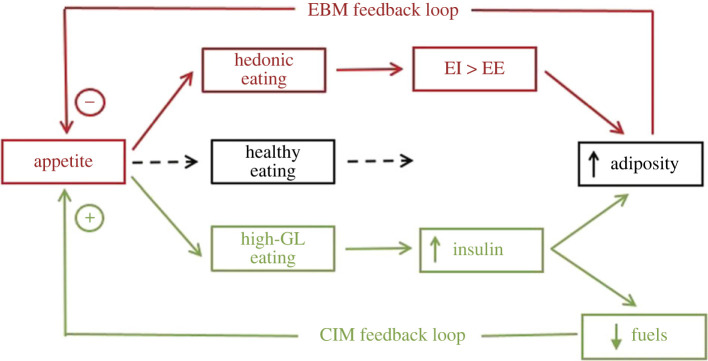
Figure 3 presents a systems diagram amalgamating the feedback loops of two current hypotheses of obesity pathogenesis, the EBM [3–5] and the CIM [8–11]. Both models are hypothetical as neuroendocrine mechanisms mediating the hypothesized causal relations have not been identified. Each model posits that habitual intake of particular diets causes obesity, but the type of diet, the negative feedbacks activated, and their consequences for energy storage all differ. EBM describes the generally held view that excess intake of palatable, energy-dense foods is the most important cause of increased adiposity. Negative feedback from the adipose tissue tends to inhibit eating and reduce the rate of body-weight gain, but usually not strongly enough to prevent the development of obesity. On the basis of a variety of suggestive, but not yet conclusive, evidence, the CIM posits that selection of high glycemic-load (GL; the product of glycemic index and amount of carbohydrate ingested) foods plays a unique role in obesity pathogenesis. By increasing insulin secretion and other anabolic processes, high-GL foods induce postprandial lipogenesis, which directly increases adiposity and, late in the inter-meal interval, decreases the energy available from circulating metabolic-energy substrates. Because the availability and oxidation of metabolic fuels putatively controls appetite [2,11], this decrease stimulates eating and further increase adiposity. In summary, the controls of appetite in EBM and CIM may compete. In EBM, increasing adiposity directly inhibits appetite, whereas in CIM increasing adiposity indirectly stimulates appetite.
Figure 3.
System diagram showing interacting controls of appetite and adiposity hypothesized by EBM (red) and CIM (green). In both models, obesity results from particular habitual dietary choices. In EBM, consumption of palatable, energy-dense foods in excess of energy needs (positive energy balance) leads to obesity, which then inhibits appetite. In CIM, intake of high-GL foods leads to insulin-induced lipogenesis independent of positive energy balance, which decreases circulating metabolic-fuel availability late after meals and stimulates appetite. Note that in EBM, but not CIM, adiposity is in the feedback loop controlling appetite. EI, energy intake; EE, energy expenditure; GL, glycemic load; fuels, circulating metabolic-energy substrates.
Using data to fit control-theory models of the effects of the EBM and CIM could yield insights about the dynamics and potencies of the negative-feedback processes involved and the resultant changes in appetite and adiposity. Consider a hypothetical weight-loss trial in which foods are fed in fixed rations below energy requirements to participants with obesity, and weight change and pre-meal hunger are measured over months. EBM predicts that this regimen should gradually reduce adiposity and thereby activate negative-feedback signals that dynamically increase subjective appetite (intake is fixed) and reduce weight loss owing to metabolic adaptations. CIM predicts that because the low-energy meals of the diet regimen would reduce concentrations of circulating metabolic-energy substrates late in inter-meal intervals, it should activate negative-feedback signals that increase appetite. Importantly, in contrast to EBM, the increase in appetite should occur immediately, as it is not dependent on the development of increased adiposity, and should be relatively constant rather than increasing dynamically.
Next, consider the effect of imposing an isocaloric switch to a higher-GL diet during the trial. EBM predicts that this should not greatly affect the rate of weight loss, so should have little effect on appetite or adiposity. By contrast, CIM predicts that consumption of higher-GL foods should have the two effects described above, increasing both adiposity and appetite.
Finally, consider a trial in which participants with obesity are encouraged to limit their energy intake, but allowed to select the amounts they eat ad libitum, with one group prescribed low-GL foods and the other, high-GL foods. Energy intake and body weight are measured periodically. Assuming the usual initial weight loss and subsequent regain, these data should enable a quantitative comparison of the net negative feedbacks and resultant changes in food intake and body weight posited by EBM and CIM.
7. Summary and conclusion
The review traces the development of the concept of energy homeostasis from Lavoisier's discoveries of oxygen, oxidation and physiological energetics, through Bernard's and Cannon's notions of homeostatic regulation, to the application of homeostatic concepts to energy storage by Brobeck, Kennedy and Mayer, and finally to Hall's recent application of control theory to energy homeostasis. The conceptual challenges faced by scientists throughout this evolution have been emphasized.
Control theory is urged as a potential tool to provide testable models of energy homeostasis, and applications to EBM, CIM and DIPM are discussed. Perhaps the major conceptual insight provided so far is that, at least during weight-loss, body-weight dynamics are accurately fit by simple proportional negative-feedback control, indicating that the concept of constancy and of a body-weight set-point are superfluous. Rather, these data suggest that the body-weight regulatory system opposes change but does not have a ‘ideal’ weight to which it invariably returns. Whether such modelling will facilitate the discovery of the neuroendocrine mechanisms controlling body weight, however, remains to be seen.
Finally, one may ask how the body-weight regulatory system came to be tuned so as to produce the population distribution of adiposity that existed prior to the obesity epidemic [14]. This may have resulted from an optimization process. Such a process would probably involve: first, balancing the opposing evolutionary selection pressures of having sufficient energy stores to survive periods of illness anorexia while maintaining sufficient agility and speed to escape predation [6,7], and, second, balancing potentially competing influences of different physiological regulators, such as the regulation of ATP : ADP ratio and circulating energy substrates mentioned in §1, and other competing regulations, such as thermoregulation. A critical point is that such optimization processes can be mathematically modelled.
Data accessibility
This article has no additional data.
Authors' contributions
N.G.: conceptualization, writing—original draft.
Conflict of interest declaration
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Geary N. 2020. Control-theory models of body-weight regulation and body-weight regulatory appetite. Appetite 144, 104440. ( 10.1016/j.appet.2019.104440) [DOI] [PubMed] [Google Scholar]
- 2.Watts AG, Kanoski SE, Sanchez-Watts G, Langhans W. 2022. The physiological control of eating: signals, neurons, and networks. Physiol. Rev. 102, 689-813. ( 10.1152/physrev.00028.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall KD, et al. 2022. The energy balance model of obesity. Am. J. Clin. Nutr. 115, 1243-1254. ( 10.1093/ajcn/nqac031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LM, Leibel RL. 2017. Obesity pathogenesis. Endocr. Rev. 38, 267-296. ( 10.1210/er.2017-00111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman JM. 2019. Leptin and the endocrine control of energy balance. Nat. Metab. 1, 754-764. ( 10.1038/s42255-019-0095-y) [DOI] [PubMed] [Google Scholar]
- 6.Speakman JR. 2018. The evolution of body fatness: trading off disease and predation risk. J. Exp. Biol. 221, jeb167254. ( 10.1242/jeb.167254) [DOI] [PubMed] [Google Scholar]
- 7.Speakman JR, Elmquist JK. 2022. Obesity: an evolutionary context. Life Metab. 1, 10-24. ( 10.1093/lifemeta/loac002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ludwig DS, et al. 2022. Competing paradigms of obesity pathogenesis. Eur. J. Clin. Nutr. 76, 1209-1221. ( 10.1038/s41430-022-01179-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ludwig DS, et al. 2021. The carbohydrate-insulin model. Am. J. Clin. Nutr. 114, 1873-1885. ( 10.1093/ajcn/nqab270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludwig DS, Sørensen TIA. 2022. An integrated model of obesity pathogenesis that revisits causal direction. Nat. Rev. Endocrinol. 18, 261-262. ( 10.1038/s41574-022-00635-0) [DOI] [PubMed] [Google Scholar]
- 11.Shimy KJ, Feldman HA, Klein GL, Bielak L, Ebbeling CB, Ludwig DS. 2020. Effects of dietary carbohydrate content on circulating metabolic fuel availability in the postprandial state. J. Endocr. Soc. 4, bvaa062. ( 10.1210/jendso/bvaa062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klimentidis YC, et al. 2010. Canaries in the coal mine: a cross-species analysis of the plurality of obesity epidemics. Proc. R. Soc. B 278, 20101890. ( 10.1098/rspb.2010.1890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohlsson C, Jansson JO. 2020. The gravitostat theory. E. Clin. Med. 27, 100530. ( 10.1016/j.eclinm.2020.100530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rony H. 1940. Obesity and leanness. Philadelphia, PA: Lea & Febiger. [Google Scholar]
- 15.Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. 1996. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am. J. Epidemiol. 143, 228-239. ( 10.1093/oxfordjournals.aje.a008733) [DOI] [PubMed] [Google Scholar]
- 16.Nuttal FQ. 2015. Body mass index. Nutr. Today 50, 117-128. ( 10.1097/NT.0000000000000092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwaung P, Bosy-Westphal A, Muller MJ, Geisler C, Heo M, Thomas DM, Kennedy S, Heymsfield SB. 2019. Obesity tissue: composition, energy expenditure, and energy content in adult humans. Obesity (Silver Spring) 27, 1472-1481. ( 10.1002/oby.22557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hübers M, Geisler C, Bosy-Westphal A, Braun W, Pourhassan M, Sørensen TIA, Müller MJ. 2019. Association between fat mass, adipose tissue, fat fraction per adipose tissue, and metabolic risks. Eur. J. Clin. Nutr. 73, 62-71. ( 10.1038/s41430-018-0150-x) [DOI] [PubMed] [Google Scholar]
- 19.Lavoisier AL. 1774. Opuscules phisiques et chymiques. Paris, France: CF Didot. [Google Scholar]
- 20.Lavoisier AL. 1789. Traité Élémentaire de chimie. Paris, France: Cuchet. See https://www.gutenberg.org/cache/epub/52489/pg52489-images.html. [Google Scholar]
- 21.Lavoiser AL. 1790. Elements of chemistry. (Kerr R, translator.) Edinburgh, UK: Creech. See https://www.gutenberg.org/files/30775/30775-h/30775-h.htm.
- 22.Lavoisier AL, Laplace PS. 1780. Mémoire sur la chaleur. Paris, France: Mémoires de l'Académie des sciences. [Google Scholar]
- 23.Séguin AJ, Lavoisier AL. 1789. Premier memoir sur la respiration des animaux. Paris, France: Mémoires de l'Académie des sciences Mem. Acad. Sci. Paris. [Google Scholar]
- 24.Guyton de Morveau LB, Lavoisier AL, Bertholet CL, Fourcroy AF. 1787. Méthode de nomenclature chimique. Paris, France: Cuchet. [Google Scholar]
- 25.Thompson B, Rumford C. 1798. An inquiry concerning the source of the heat which is excited by friction. Phil. Trans. R. Soc. Lond. 88, 80-102. ( 10.1098/rstl.1798.0006) [DOI] [Google Scholar]
- 26.Buchholz AC, Schoeller DA. 2004. Is a calorie a calorie? Am. J. Clin. Nutr. 79, 899S-906S. ( 10.1093/ajcn/79.5.899S) [DOI] [PubMed] [Google Scholar]
- 27.Culotta CA. 1972. Respiration and the Lavoisier tradition: theory and modification, 1777–1850. Trans. Am. Phil. Soc. 62, 3-41. ( 10.2307/1006083) [DOI] [Google Scholar]
- 28.Eagle CT, Sloan J. 1998. Marie Anne Paulze Lavoisier. Chem. Educator 3, 1-18. ( 10.1007/s00897980249a) [DOI] [Google Scholar]
- 29.Guerlac H. 1973. Lavoisier. In Dictionary of scientific biography, vol. 7 (ed. Gillispie C), pp. 87-91. New York, NY: Scribner's. [Google Scholar]
- 30.Hargrove JL. 2006. History of the calorie in nutrition. J. Nutr. 136, 2957-2961. ( 10.1093/jn/136.12.2957) [DOI] [PubMed] [Google Scholar]
- 31.Holmes FL. 1985. Lavoisier and the chemistry of life: an exploration of scientific creativity. Madison, WI: Univ of Wisconsin Press. [Google Scholar]
- 32.Johnson HA. 2008. Revolutionary instruments: Lavoisier's tools as objets d'art. Chem. Heritage 26, 30-35. [Google Scholar]
- 33.Rapaport R. 1963. Antoine-Laurent Lavoisier — a biographical sketch. J. Nutr. 79, 3-8. ( 10.1093/jn/79.1.1) [DOI] [PubMed] [Google Scholar]
- 34.Underwood EA. 1944. Lavoisier and the history of respiration. Proc. R. Soc. Med. 37, 247-262. ( 10.1177/003591574403700603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West JB. 2013. The collaboration of Antoine and Marie-Anne Lavoisier and the first measurements of human oxygen consumption. Am. J. Physiol. Lung Cell. Mol. Physiol. 305, L775-L785. ( 10.1152/ajplung.00228.2013) [DOI] [PubMed] [Google Scholar]
- 36.Blundell JE, Gibbons C, Beaulieu K, Casanova N, Duarte C, Finlayson G, Stubbs RJ, Hopkins M. 2020. The drive to eat in Homo sapiens. Physiol. Behav. 219, 112846. ( 10.1016/j.physbeh.2020.112846) [DOI] [PubMed] [Google Scholar]
- 37.Bernard C. 1849. Du suc pancréatique et de son rôle dans les phénomènes de la digestion. Mémoires du Société de Biologie 1, 99-115. [Google Scholar]
- 38.Bernard C. 1855. Sur le mechanisme de la formation du sucre dans le foie. C. R. Hebd. Acad. Sci. 40, 461-469. [Google Scholar]
- 39.Bernard C. 1876. Leçons sur la chaleur animale, sur les effets de la chaleur, et sur la fièvre. Paris, France: Baillière. [Google Scholar]
- 40.Bernard C. 1865. Introduction à l’étude de la médicine expérimentale. Paris, France: Baillière. See https://www.gutenberg.org/cache/epub/16234/pg16234.html. [Google Scholar]
- 41.Bernard C. 1927. An introduction to the study of experimental medicine. (Greene HC, translator.) New York, NY: Henry Schuman. See http://medlib.bsmu.edu.ua/wp-content/uploads/2019/05/6.pdf. [Google Scholar]
- 42.Bernard C. 1878. Leçons sur les phénomènes de la vie communs aux animaux et aux végétaux. Paris, France: Bailliere. See https://gallica.bnf.fr/ark:/12148/bpt6k62986637.texteImage. [Google Scholar]
- 43.Bernard C. 1974. Phenomena of life common to animals and to plants. (Cook RP, Cook MA, translators.) Dundee UK: Cook. [Google Scholar]
- 44.Adolph EF. 1961. Early concepts of physiological regulations. Physiol. Rev. 41, 737-770. ( 10.1152/physrev.1961.41.4.737) [DOI] [PubMed] [Google Scholar]
- 45.Arunachalam C, Woywodt A. 2010. Turbid urine and beef-eating rabbits: Claude Bernard (1813–78)—a founder of modern physiology. NDT Plus 3, 335-337. ( 10.1093/ndtplus/sfq058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiller J. 1967. Claude Bernard and vivisection. J. History Med. Allied Sci. 22, 246-260. ( 10.1093/jhmas/XXII.3.246) [DOI] [PubMed] [Google Scholar]
- 47.Smith GP. 2000. Pavlov and integrative physiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R743-R755. ( 10.1152/ajpregu.2000.279.3.R743) [DOI] [PubMed] [Google Scholar]
- 48.Smith GP. 2008. Unacknowledged contributions of Pavlov and Barcroft to Cannon's theory of homeostasis. Appetite 51, 428-432. ( 10.1016/j.appet.2008.07.003) [DOI] [PubMed] [Google Scholar]
- 49.Cooper SJ. 2008. From Claude Bernard to Walter Cannon. Appetite 51, 419-427. ( 10.1016/j.appet.2008.06.005) [DOI] [PubMed] [Google Scholar]
- 50.De Luca LA Jr. 2022. A critique on the theory of homeostasis. Physiol. Behav. 247, 113712. 10.1016/j.physbeh.2022.113712. [DOI] [PubMed] [Google Scholar]
- 51.LaFollette H, Shanks N. 1994. Animal experimentation: the legacy of Claude Bernard. Int. Stud. Phil. Sci. 8, 195-210. ( 10.1080/02698599408573495) [DOI] [Google Scholar]
- 52.Noble D. 2008. Claude Bernard, the first systems biologist, and the future of physiology. Exp. Physiol. 93, 16-26. ( 10.1113/expphysiol.2007.038695) [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez de Romo AC. 1989. Tallow and the time capsule: Claude Bernard's discovery of the pancreatic digestion of fat. Hist. Philos. Life Sci. 11, 253-274. [PubMed] [Google Scholar]
- 54.Rodriguez de Romo AC, Borgstein J. 1999. Claude Bernard and pancreatic function revisited after 150 years. Vesalius 5, 18-24. [PubMed] [Google Scholar]
- 55.Sebastian S. 2007. Claude Bernard and an introduction to the study of experimental medicine. J. His. Med. Allied Sci. 62, 495-528. ( 10.1093/jhmas/jrm015) [DOI] [PubMed] [Google Scholar]
- 56.Young FG. 1957. Claude Bernard and the discovery of glycogen. Br. Med. J. 1, 1431-1437. ( 10.1136/bmj.1.5033.1431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cannon WB. 1898. The movements of the stomach studied by means of the Roentgen rays. Am. J. Physiol. 1, 359-382. ( 10.1152/ajplegacy.1898.1.3.359) [DOI] [Google Scholar]
- 58.Cannon WB. 1902. The movements of the intestines studied by means of the Röntgen rays. J. Med. Res. 7, 72-75. [PMC free article] [PubMed] [Google Scholar]
- 59.Cannon WB. 1904. The passage of different foodstuffs from the stomach and through the small intestine. Am. J. Physiol. 12, 387-418. ( 10.1152/ajplegacy.1904.12.4.387) [DOI] [Google Scholar]
- 60.Cannon WB, Washburn AL. 1912. An explanation of hunger. Am. J. Physiol. 29, 441-454. ( 10.1152/ajplegacy.1912.29.5.441) [DOI] [Google Scholar]
- 61.Smith GP. 1997. Eating and the American Zeitgeist. Appetite 29, 191-200. ( 10.1006/appe.1997.0123) [DOI] [PubMed] [Google Scholar]
- 62.Cannon WB. 1945. The way of an investigator. New York, NY: Norton. [Google Scholar]
- 63.Cannon WB. 1915. Bodily changes in pain, hunger, fear, and rage. New York, NY: Applegate. See https://archive.org/details/cu31924022542470/page/n5/mode/2up?ref=ol&view=theater. [Google Scholar]
- 64.Cannon WB. 1929. Organization for physiological homeostasis. Physiol. Rev. 9, 399-431. ( 10.1152/physrev.1929.9.3.399) [DOI] [Google Scholar]
- 65.Cannon WB. 1932. The wisdom of the body. New York, NY: Norton. See https://archive.org/details/wisdomofbody0000walt/page/n5/mode/2up. [Google Scholar]
- 66.Billman GE. 2020. Homeostasis. Front. Physiol. 11, 200. ( 10.3389/fphys.2020.00200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown TH, Fee E. 2002. Walter Bradford Cannon. Am. J. Public Health. 92, 1594-1595. ( 10.2105/AJPH.92.10.1594) [DOI] [Google Scholar]
- 68.Wolfe EL, Barger AC, Benison S. 2000. Walter B. Cannon, science and society. Cambridge, MA: Harvard Univ Press. [Google Scholar]
- 69.Richter CP. 1942–1943 Total self-regulatory functions in animals and human beings. Harvey Lecture Series 38, 63-103. [Google Scholar]
- 70.Geary N. 2013. SSIB Ingestive Classic 4: Curt Richter and the behavioral control of homeostasis. See https://www.ssib.org/web/classic3.php.
- 71.Moran TH, Schulkin J. 2000. Curt Richter and regulatory physiology. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 279, R357-R363. ( 10.1152/ajpregu.2000.279.2.R357) [DOI] [PubMed] [Google Scholar]
- 72.Schulkin J. 2005. Curt Richter. Baltimore, MD: Johns Hopkins University Press. See https://muse.jhu.edu/pub/1/monograph/book/60340. [Google Scholar]
- 73.Smith GP. 2007. Introduction to four papers on Curt Richter and analysis of his scientific practice. Appetite. 49, 347-352. ( 10.1016/j.appet.2007.01.014) [DOI] [PubMed] [Google Scholar]
- 74.Rozin P, Kalat JW. 1971. Specific hungers and poison avoidance as adaptive specializations of learning. Psychol. Rev. 78, 459-486. ( 10.1037/h0031878) [DOI] [PubMed] [Google Scholar]
- 75.de Araujo IE, Schatzker M, Small DM. 2020. Rethinking Food Reward. Annu. Rev. Psychol. 71, 139-164. ( 10.1146/annurev-psych-122216-011643) [DOI] [PubMed] [Google Scholar]
- 76.Booth DA. 2008. Physiological regulation through learnt control of appetites by contingencies among signals from external and internal environments. Appetite 51, 433-441. ( 10.1016/j.appet.2008.06.008) [DOI] [PubMed] [Google Scholar]
- 77.Ramsay DS, Woods SC. 2016. Physiological regulation: how it really works. Cell Metab. 24, 361-364. ( 10.1016/j.cmet.2016.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McEwen BS. 2007. Physiology and neurobiology of stress and adaptation. Physiol. Rev. 87, 873-904. ( 10.1152/physrev.00041.2006) [DOI] [PubMed] [Google Scholar]
- 79.McEwen BS, Gianaros PJ. 2011. Stress- and allostasis-induced brain plasticity. Annu. Rev. Med. 62, 431-445. ( 10.1146/annurev-med-052209-100430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schulkin J, Sterling P. 2019. Allostasis: a brain-centered, predictive mode of physiological regulation. Trends Neurosci. 42, 740-752. ( 10.1016/j.tins.2019.07.010) [DOI] [PubMed] [Google Scholar]
- 81.Sinha R. 2018. Role of addiction and stress neurobiology on food intake and obesity. Biol. Psychol. 131, 5-13. ( 10.1016/j.biopsycho.2017.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tomiyama AJ. 2019. Stress and obesity. Annu. Rev. Psychol. 70, 703-718. ( 10.1146/annurev-psych-010418-102936) [DOI] [PubMed] [Google Scholar]
- 83.Brobeck JR, Tepperman J, Long CNH. 1943. Experimental hypothalamic hyperphagia in the albino rat. Yale J. Biol. Med. 15, 831-853. [PMC free article] [PubMed] [Google Scholar]
- 84.Kissileff HR. 2014. SSIB Ingestive Classic 5: John Brobeck and the hypothalamic control of eating (I). See https://www.ssib.org/web/classic5.php.
- 85.Anand BK, Brobeck JR. 1951. Hypothalamic control of food intake in rats and cats. Yale J. Biol. Med. 24, 123-140. [PMC free article] [PubMed] [Google Scholar]
- 86.Moran TH. 2014. SSIB Ingestive Classic 6: John Brobeck and the hypothalamic control of eating (II). See https://www.ssib.org/web/classic6.php.
- 87.Brobeck JR. 1993. Remembrance of experiments almost forgotten. Appetite 21, 225-231. ( 10.1006/appe.1993.1041) [DOI] [PubMed] [Google Scholar]
- 88.Brobeck JR. 1948. Regulation of energy exchange. Ann. Rev. Physiol. 10, 315-328. ( 10.1146/annurev.ph.10.030148.001531) [DOI] [PubMed] [Google Scholar]
- 89.Strominger JL, Brobeck JR. 1953. A mechanism of regulation of food intake. Yale J. Biol. Med. 25, 383-390. [PMC free article] [PubMed] [Google Scholar]
- 90.Kennedy GC. 1953. The role of depot fat in the hypothalamic control of food intake in the rat. Proc. R. Soc. Lond. B 140, 578-596. ( 10.1098/rspb.1953.0009) [DOI] [PubMed] [Google Scholar]
- 91.Mayer J. 1955. Regulation of energy intake and the body weight. Ann. N Y Acad. Sci. 63, 15-43. ( 10.1111/j.1749-6632.1955.tb36543.x) [DOI] [PubMed] [Google Scholar]
- 92.Goldstein DS, Kopin IJ. 2017 Dec Homeostatic systems, biocybernetics, and autonomic neuroscience. Auton. Neurosci. 208, 15-28. ( 10.1016/j.autneu.2017.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rosenblueth A, Wiener N, Bigelow J. 1943. Behavior, purpose and teleology. Philos Sci. 10, 18-24. ( 10.1086/286788) [DOI] [Google Scholar]
- 94.Wiener N. 1948. Cybernetics or control and communication in the animal and the machine. Cambridge, MA: MIT Press. [Google Scholar]
- 95.Hervey GR. 1959. The effects of lesions in the hypothalamus in parabiotic rats. J. Physiol. I45, 336-352. ( 10.1113/jphysiol.1959.sp006145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Åström KJ, Murray RM. 2016. Feedback systems, 2nd edn. Princeton, NJ: Princeton University Press. [Google Scholar]
- 97.Göbel B, Sanghvi A, Hall KD. 2014. Quantifying energy intake changes during obesity pharmacotherapy. Obesity (Silver Spring) 22, 2105-2108. ( 10.1002/oby.20813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hall KD, Sanghvi A, Göbel B. 2017. Proportional feedback control of energy intake during obesity pharmacotherapy. Obesity 25, 2088-2091. ( 10.1002/oby.21978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Polidori D, Sanghvi A, Seeley RJ, Hall KD. 2016. How strongly does appetite counter weight loss? Quantification of the feedback control of human energy intake. Obesity (Silver Spring) 24, 2289-2295. ( 10.1002/oby.21653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hall KD. 2006. Computational model of in vivo human energy metabolism during semistarvation and refeeding. Am. J. Physiol. 291, E23-E37. ( 10.1152/ajpendo.00523.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hall KD. 2010. Predicting metabolic adaptation, body weight change, and energy intake in humans. Am. J. Physiol. 298, E449-E466. ( 10.1152/ajpendo.00559.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, Swinburn BA. 2011. Quantification of the effect of energy imbalance on bodyweight. Lancet 378, 826-837. ( 10.1016/S0140-6736(11)60812-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miura H, et al. 2019. Effects of ipragliflozin on glycemic control, appetite and its related hormones: a prospective, multicenter, open-label study (SOAR-KOBE study). J. Diabetes Investig. 10, 1254-1261. ( 10.1111/jdi.13015) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.