Abstract
Evolutionary perspectives on obesity have aimed to understand how the genetic constitution of individuals has been shaped by selective pressures such as famine, predation or infectious disease. The dual intervention model assumes strong selection on lower and upper limits of adiposity, but negligible fitness implications for intermediate adiposity. These frameworks are agnostic to age, sex and condition. I argue that selection has favoured a ‘crafty genotype’—a genetic basis for accommodating variability in the ‘fitness value’ of fat through phenotypic plasticity, depending on the endogenous and exogenous characteristics of each individual. Hominin evolution occurred in volatile environments. I argue that the polygenetic basis of adiposity stabilizes phenotype in such environments, while also coordinating phenotypic variance across traits. This stability underpins reaction norms through which adiposity can respond sensitively to ecological factors. I consider how the fitness value of fat changes with age, sex and developmental experience. Fat is also differentially distributed between peripheral and abdominal depots, reflecting variable prioritization of survival versus reproduction. Where longevity has been compromised by undernutrition, abdominal fat may promote immediate survival and fitness, while long-term cardiometabolic risks may never materialize. This approach helps understand the sensitivity of adiposity to diverse environmental factors, and why the health impacts of obesity are variable.
This article is part of a discussion meeting issue ‘Causes of obesity: theories, conjectures and evidence (Part I)’.
Keywords: adiposity, evolution, infectious disease, fat distribution, phenotypic plasticity, reaction norm
1. Introduction
Although obesity became a global health epidemic only recently, it has attracted interest from evolutionary biologists for over half a century [1–3]. Body mass index (BMI), the most widely used index of nutritional status, shows substantial variability both within and across populations [4]. Although an inaccurate marker of adiposity at the individual level, BMI trends reflect enormous population variability in body fatness, ranging in adults from below 5% of body weight to over 50% [5]. At a proximate level, the explanation for such variability lies in the interaction between our genes, physiology and environment [6]. At an ultimate level, we need an evolutionary framework to understand why humans are differentially susceptible to obesity [5].
From an evolutionary perspective, our susceptibility to excess weight must broadly derive from the human genome. While fatness is sensitive to environmental factors, obesity has substantial heritability, demonstrated by twin and adoption studies [7]. To date, around 60 genome-wide association studies (GWAS) have linked over 1100 loci with adiposity phenotypes [8], though they explain only a small minority of the heritability. Like height, adiposity appears to be a quintessential polygenetic trait, whereby numerous alleles each contribute a very small magnitude of effect to phenotypic variance [9]. Increasing GWAS sample size will likely allow many more alleles to be identified. Most evolutionary perspectives on obesity have therefore focused on explaining why individuals might differ in alleles associated with BMI and adiposity.
In 1962, Neel published his ‘thrifty genotype’ hypothesis of diabetes susceptibility [1]. This approach assumed that ancestral humans experienced selective pressures from ‘cycles of feast and famine’, through periods of energy-plenty and energy-scarcity. Those more regularly exposed to such conditions were hypothesized to have evolved an enhanced capacity for fat accumulation during times of surplus, which could then be oxidized during famines. In contemporary settings of unlimited food availability, accordingly, thrifty genotypes would drive development of obesity and diabetes. Scientists have searched extensively for thrifty genes, but despite the evidence from GWAS [8,10], the hypothesis remains essentially unsupported [5,11].
An alternative perspective proposes that contemporary genetic variability in BMI emerged in the absence of strong selection. Speakman's ‘drifty genotype’ hypothesis assumes that the emergence of the capacity to use fire and weapons for defence among the genus Homo would have relaxed the selective pressure of predation, weakening upper constraints on body mass and allowing random mutations to accumulate [11].
A challenge for both thrifty and drifty models as originally proposed is that human adiposity is inherently variable within individuals in response to environmental factors; hence genotype has a variable association with phenotype [12]. Attention has therefore shifted to the regulation of body weight and adiposity.
In the short term, both diets and overfeeding interventions have only transient effects, with weight returning to its baseline level [11]. Whether explicitly or implicitly expressed, a common assumption is that weight is regulated around a set point, and that obesity develops when this regulatory system in some way fails. However, adiposity clearly varies over longer periods. One theoretical solution to this dilemma is Speakman's ‘dual intervention point’ model, which assumes that selection has shaped regulatory mechanisms to maintain fat stores above a lower level, necessary to buffer famine, and below an upper level that raises predation risk [11].
While the dual intervention point model clearly represents a conceptual advance, there are several outstanding issues. First, existing evolutionary models are agnostic over age, and yet human adiposity varies profoundly with age in a sex-specific manner, suggesting that fat stores have highly variable ‘fitness value’ through the life-course. Moreover, the sensitivity of adiposity to prevailing ecological conditions and to developmental trajectory indicates that each individual may alter its ‘fitness valuation’ of fat according to its experience. This requires that we pay more attention to the multiple reaction norms, through which a given genotype can give rise to multiple possible phenotypes through the life-course. These reaction norms relate both to developmental plasticity, through which early exposures may leave long-lasting effects on phenotype [13,14], and phenotypic flexibility through adult life, whereby fat stores may increase and decrease in response to successive exposures [15,16].
A second limitation is that most evolutionary models of obesity treat adipose tissue as a ‘petrol tank’, an inert fuel dump where energy is stored at time A for potential use at time B. Such a framework ignores how the metabolic activity of adipose tissue varies by anatomical depot, and offers minimal insight into fat distribution and how it varies with both endogenous and external factors.
Third, characterizing adiposity as a polygenetic trait, where many alleles each have negligible impact on phenotype, begs the question of why its genetic basis should have this profile. Such an ‘infinitesimal’ model helps explain why BMI demonstrates a normal distribution in populations such as hunter–gatherers (though in obesogenic settings, the distribution becomes right-skewed). What are widely termed ‘adiposity' or ‘obesity’ alleles are typically also associated with other physiological traits, and only appear relevant to obesity because they leave a signal in population BMI variability, more so among individuals with high compared with low BMI [17]. Despite their small effects, often equivalent to a few grams of weight [18], selection has clearly acted on adiposity alleles sufficiently strongly to generate the patterns of age and sex variability reviewed below. Paradoxically, minimal attention has been paid to the genetic basis of the reaction norms, through which adiposity can change over time within individuals, both during development and through adult life.
Understanding of this genetic and phenotypic variability in adiposity is currently missing from evolutionary debates on obesity. Optimal strategies for storing fat in unpredictable or changing environments have been explored using mathematical models [19,20], but these address only environmental variability, and do not consider endogenous factors such as age, sex or phenotypic condition. Below, I build on previous frameworks to present a ‘crafty genotype’ hypothesis, aiming to improve understanding both of the polygenic basis of adiposity and its variability with age, sex and life-course experience. First, I revisit the context in which human adiposity evolved.
2. Evolution of human adiposity
Compared with other extant apes, humans have both greater levels of body fatness, especially in females, and a unique life-course profile of adiposity [5,21]. This indicates that adiposity underwent strong selection in the evolution of our lineage; moreover, this evolutionary process connects with the emergence of other ‘quintessential human characteristics', such as our bipedal posture, large brains, sociality, reproductive demography and extended lifespans [5]. All of these traits demonstrate mosaic evolution from the australopithecines onwards [22], and adiposity is likely to have evolved in similar mosaic manner, meaning that different fat depots may have responded to different selective pressures in different periods of time.
The evolution of the genus Homo occurred in the context of the emergence of the savannah niche in Sub-Saharan Africa. While the global climate became overall cooler and more arid in this period, isotopic indices of palaeoclimate also indicate dramatically increasing climatic volatility [23]. Ecological change became so pronounced that it was often experienced across generations and within life-courses, such as transient but extreme El Niño Southern Oscillation (ENSO) events [24]. In other words, the hominin ecological niche was not occasionally disrupted, rather continual disruption was the niche [25–27].
In unpredictable environments, adaptive strategies cannot be elicited directly from the genome, but must instead emerge through plastic responses. Hominin evolution can therefore be considered a sorting process, selecting in favour of phenotypes that could survive and reproduce in increasingly unpredictable environments. Alongside other traits with low plasticity (skeletal anatomy, large brains), selection favoured both larger energy stores and also the capacity to acquire and use them in concert with diverse stimuli and threats [28–32].
Adiposity has a wide range of fitness functions that span the life-history functions of survival and maintenance, growth, reproduction and defence (figure 1). All of these functions can be assumed to have undergone stronger selection in volatile ecological niches. Importantly, humans themselves must have amplified these selective pressures, through their own colonizing behaviour which regularly exposed them to new ecological stresses requiring rapid accommodation [33–35]. The fact that contemporary humans demonstrate remarkably low levels of genetic diversity [36], despite having colonized a huge variety of ecological niches [37], indicates that plastic responses have been the key mode of geographic adaptation. As with other species exposed to volatile environments, human genetic evolution appears to have shifted from local differentiation to the enhancement of plasticity [24,38].
Figure 1.
Schematic diagram illustrating the fitness functions of adipose tissue, and their exposure through human behaviour to the selective pressure of colonizing novel environments. (Online version in colour.)
In this context of persistent ancestral exposure to ecological volatility [23], the evolution of the brain and adiposity in Homo are intricately connected. They act as complementary ‘risk management’ systems, whereby the brain processes acute signals that act on the nervous system, while adipose tissue processes environmental signals that act on energetics and physiological function [28]. Most mammals have specialized in only one of these systems [39], but humans have both, a phenotype consistent with the notion that hominins evolved under persistently high levels of ecological risk. Indeed, evolutionary change in adiposity and metabolism is likely to have preceded the encephalized Homo brain. The brain has obligate fuel requirements, preferentially metabolizing glucose or, during starvation, ketones obtained from fat oxidation [40]. Metabolic adaptations to ecological volatility may have favoured sufficient stabilizing of fuel supply at the level of tissues for substantial expansion of the brain to be viable [24]. As human encephalization is a developmental process, this may further have favoured greater fat stores at birth and in early infancy [41].
Producing encephalized neonates and infants is costly for mothers, especially in colonizing species where conditions at the time of conception may be unrelated to conditions at the time of late gestation and lactation, when reproductive costs are greatest. This selective pressure has favoured high levels of adiposity in females of reproductive age, as well as compensatory reductions in fat-free mass, resulting in major sexual dimorphism in adult human body composition [42]. Humans are quintessential ‘capital breeders’, storing fat in advance so that lactation can be funded regardless of immediate food supply. Colonizing new niches produces boom–bust population dynamics [33], for which fat is highly adaptive: females can use fat stores to ride out lean periods, and then produce offspring rapidly if conditions improve [33]. During the 1974–1975 Bangladesh famine, for example, fertility declined by 34%, but then increased above the long-term background rate by 17% after the famine [43].
However, hominin evolutionary change involved changes not only in adult size and body composition, but also in the tempo and trajectory of maturation, and in longevity [44,45]. Moreover, the Homo genus evolved not only novel developmental trajectories and life stages, but also substantial plasticity therein, so that the age at which developmental milestones are reached is impacted both by genetic constitution and developmental experience [33]. In contemporary humans, fat stores contribute to the regulation of maturation in both sexes, while the timing of menarche is also associated with adult adiposity [14,46]. More fundamentally, these evolutionary trends have left a signal in the association of adiposity with age.
Volatile niches impact every constituent species, and this includes pathogens. Globally, parasites and pathogens are most prevalent in the tropics where Homo evolved [47]; however, the distribution of many diseases also responds sensitively to short-term variation in climate, such as temperature and rainfall. Since infection was the primary cause of human mortality until the recent epidemic of non-communicable disease, immune response was under powerful selective pressure [48,49]. Fat stores play a double role in immune response, not only by supplying energy for costly metabolic processes, but also by secreting pro- and anti-inflammatory cytokines [50,51]. For example, the risk of death in malnourished children is greatest if they have low leptin, a hormone secreted by fat that regulates immune function [31]. Beyond ecological volatility, human sociality may itself have created new niches for infections, initially through larger social groups in ancestral hunter–gatherer populations, and subsequently through the emergence of agriculture. Sedentary farming populations amplified the selective pressure of infection, owing to dense settlements, population growth, pathogens jumping to humans from newly domesticated animals, and unhygienic living conditions, all exacerbated by increased risk of famine [52,53].
Overall, therefore, adiposity appears to represent a defining characteristic of hominin evolution in volatile environments. Enhanced plasticity in energy stores and metabolism contributed to the emergence of inflexible traits such as large brains, and life-history strategies such as sociality and delayed maturation. Fat represents a common energy currency that can fund diverse other physiological processes as required [5], and can therefore allocate energy to any of the four life-history functions, depending on what energy allocation strategy would optimize fitness at any given time. The fitness value of adiposity for contemporary humans in unpredictable environments is demonstrated by an ecological analysis, showing that in both sexes, triceps and subscapular skinfolds are larger in geographical locations with greater inter-annual temperature variability, a marker of ecological volatility [54].
3. The crafty genotype hypothesis
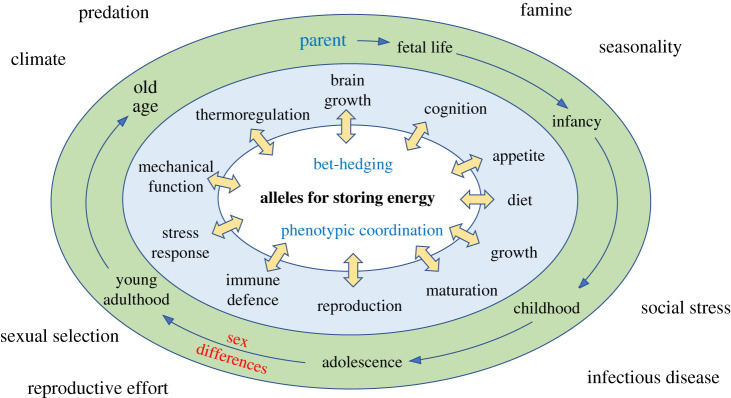
In very rare cases, severe obesity can be attributed to chromosomal deletions or to mutations in specific alleles (monogenic obesity) inherited in Mendelian manner, whose effects are most evident through consanguineous mating [8]. While offering insight into metabolic pathways relevant to weight regulation, monogenic obesity has little relevance to the hypothesis presented here, which focuses on the polygenic basis of adiposity. The miniscule magnitude of effect of a typical adiposity allele may seem to indicate that such alleles are unable to respond to selection. The crafty genotype hypothesis presented here (figure 2) posits a more complex scenario.
Figure 2.
Schematic diagram illustrating how alleles associated with energy stores (adiposity alleles) relate to diverse physiological functions. The relative importance of these functions may vary through the life-course in both systematic ways (age, sex), and individual ways depending on the exposures experienced. (Online version in colour.)
First, it appears that adiposity alleles with large magnitudes of effect have been selected against. This may be an evolved correlate of enhanced plasticity: physiological systems that allow phenotype to respond sensitively to ecological conditions could be impaired if phenotype could be substantially changed by single alleles. A particular example of this is fetal adiposity, where elevated weight at delivery could cause maternal death from obstructed labour. This may explain why fetal fat deposition begins only in the third trimester [55], and why adiposity then increases substantially after birth [56–58].
Second, in stochastic environments, evolution inherently favours genetic bet-hedging—increasing phenotypic variability within a population, thereby increasing the likelihood that some individuals will always survive and reproduce, whatever the environmental state [59]. By distributing the genetic basis of variability in adiposity across numerous alleles, selection is unlikely to systematically eradicate any given allele from the gene pool over a short period, whatever the ecological shocks that occur. Consequently, the overall genetic variability in adiposity and its fitness-enhancing properties are resistant to selection, and are preserved in the population over time.
Third, the role of adiposity alleles in diverse physiological functions (figure 2) suggests that genotype-induced phenotypic variability is subject to coordination. Being fatter may only increase the Darwinian fitness of an organism if multiple other traits shift in a coordinated direction. For example, larger body size will require larger fat stores to survive a given period of negative energy balance, which in turn requires increased appetite [60]. Such associations may be bi-directional, and may also reflect either positive correlations across traits or negative correlations (trade-offs). For example, early reproduction in females may involve a trade-off between linear growth and fat accretion, as reported empirically [46,61] and supported through GWAS [62]. In this scenario, females trade-off early maturation against reduced adult body size (predicted to reduce offspring birth weight), but benefit from an increased capacity to fund lactation and infant growth from their relatively greater adult fat reserves [63].
Fourth, independent of adiposity alleles themselves, selection has also favoured sophisticated mechanisms of plasticity, through which energy stores respond sensitively through reaction norms, both to developmental exposures in early life, and to ongoing ecological stimuli and stresses through adult life [13]. The effect of adiposity alleles may thus interact in complex ways with life-course environmental exposures.
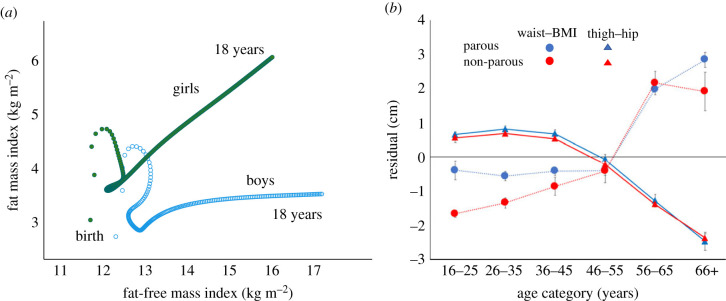
These properties of the crafty genotype relate to its resistance to external selective pressures and its coordination of diverse metabolic traits. This does not mean that adiposity does not evolve, rather the most fundamental factors that interact with these properties are age and sex. That the fitness value of fat varies with age in a sex-specific manner is evident both for total adiposity, adjusted for body size, and its anatomical distribution. Infants rapidly gain fat relative to height, before losing it in early childhood (figure 3a). Boys then show a peak in adiposity before the pubertal spurt in fat-free mass, while girls increase in more linear fashion to adulthood [64–66]. In every human population, young adults show major sexual dimorphism with higher adiposity and lower fat-free mass in females relative to males, reflecting both natural and sexual selective pressures [32,67]. However, the magnitude of these sex differences also varies with ecological conditions [67].
Figure 3.
Age and sex variability in human body composition. (a) Fat mass index plotted against fat-free mass index from birth to 18 years, using the 50th centile of UK reference data [64,65]. Fatness is high in infancy, then declines before increasing again in mid-childhood, though with increasing sexual dimorphism. (b) Variability in fat distribution in Thai women. Residuals from regressing waist girth on BMI, and thigh girth on hip girth, are plotted against age group for parous and non-parous women. In both groups, waist-BMI increases and thigh–hip declines with age, indicating redistribution of body fat, though the data are cross-sectional. Data from the National Sizing Survey of Thailand. Redrawn with permission [78]. (Online version in colour.)
To understand why the distribution of fat as well as its amount varies with age, we must return to the ecological factor that is the strongest selective pressure, namely infectious disease. Mortality from infections is greatest in early life and older age [68], helping understand high levels of adiposity in infancy and the changing distribution of fat by age. The metabolic profile of fat varies markedly by adipose tissue depot. Broadly, abdominal or visceral adipose tissue is more metabolically active than peripheral depots, and a number of studies have linked visceral adiposity with immune function [69–72]. For example, visceral adipose tissue expresses complement genes more than peripheral adipose tissue [70]. However, the anatomical distribution of fat is not fixed, but rather varies in association with various factors.
As adults age, their fat distribution becomes more central and hence more immunogenic [73], a state termed ‘inflammaging’ [74]. In both sexes, abdominal fat accumulates with age while peripheral fat declines, independent of weight change [75–77]. Figure 3b illustrates profound changes in body shape associated with age in Thai women, stratified by whether they have reproduced or not [78]. Young parous women have low waist–BMI ratio and high thigh–hip ratio, indicating a peripheral fat distribution that is used during lactation [79]. Among women in their mid-40s, these traits reverse, indicating substantial redistribution of fat from peripheral to abdominal depots (though the data are cross-sectional). The same patterns are evident in non-parous women, indicating how the young adult body is prepared for reproduction even if it does not occur, and how fat is redistributed to ‘survival’ fat for immune function with older age, when adults can increase their fitness primarily through providing grandparental support [80–82].
That central fat generically funds immune function is indicated by an eco-geographical analysis, where across non-Western populations, a marker of greater pathogen burden was associated with lower subscapular but not triceps skinfold [83]. However, populations also show systematic geographic variability in their fat distribution [84]. Reflecting the importance of infectious disease as a selective pressure, the ‘variable disease selection hypothesis' assumes that the optimal distribution of body fat for immune defence may depend on which specific diseases pose the greatest risk, as individual pathogens create energetic stress in different components of physiology [48]. For example, intra-muscular lipid may be favoured to combat malaria, whereas visceral fat may help defend against gut-borne pathogens [48]. Whether such variability is genetic, or triggered by life-course exposures, is unknown.
The crafty genotype therefore enables fat to be moved around the body depending on age and sex [75–77], in order to maximize the fitness returns from storing energy. Moreover, maternal fat reserves can influence phenotype in the next generation. Energy stored in one generation (liquid capital) can buffer against ecological stresses confronting the offspring, and promote infant growth (somatic capital) [85]. For example, across 12 low- and middle-income countries, high maternal BMI showed protective effects against infant stunting [86], and another multi-country analysis showed similar associations, independent of dietary diversity and maternal wealth and education [87].
4. Varying the fitness value of fat in association with life-course experience
Beyond its overall ‘revaluing’ of the fitness value of fat by age and sex, the ‘crafty genotype’ also allows the value of fat to change according to life-course circumstances and experience, through a range of reaction norms. I focus here on three common exposures, namely low birth weight, child undernutrition, and low social status, while food insecurity is an additional example [88]. All of these initially cause ‘thrifty phenotypes’ [89] with reduced fat-free mass, with implications for survival and longevity. In turn, this may alter the valuation of fat stores. Although fat is an efficient means of storing energy, owing to its high energy density and low ‘access costs’ (energy required to accrete or oxidize fat) per gram, it is more costly to gain fat than fat-free tissue. Investing in fat is therefore a high-risk–high-reward strategy, especially for individuals that are already thin in terms of fat-free mass.
From the 1980s, infants born small were found to be at increased risk of cardiovascular disease, hypertension and type 2 diabetes, and also to have reduced overall survival [90–92]. Subsequent studies linked catch-up growth and adult adiposity as mediating mechanisms [93,94]. In adulthood, the ratio of fat to fat-free mass tends to be elevated following fetal undernutrition [95], but this can be attributed to persistent low fat-free mass, rather than high adiposity per se. The distribution of fat also tends to be more central, but again owing to reduced peripheral fat rather than extreme abdominal fat [96]. Such variability may be considered part of a broader adaptive strategy, whereby following reduced maternal investment in early life, the offspring prioritizes survival and early reproduction over growth and long-term metabolic health [46,96].
Similar patterns of development are evident among survivors of severe child undernutrition. In the short term, both wasting and stunting reduce linear growth, fat-free mass and fat mass, but over time, fatness tends to be preserved whereas deficits in height and fat-free mass persist [97,98]. Again, the distribution of fat appears more central, owing to depletion of peripheral fat [98].
Finally, low socio-economic status is associated with reduced fetal and infant growth [99] and low height and fat-free mass in childhood [100,101], though such associations may be less evident in adult life once fat-free mass is adjusted for the shorter height. In low-income settings, low socio-economic status may also be associated with low adiposity [102]; however, in middle- and high-income countries the relationship reverses, and those of low socio-economic status tend to show greater fat mass and a more central fat distribution [101]. This indicates that poverty drives exposure to both undernutrition in early life and the obesogenic niche at later ages [86]. In social animals in general, increased fat stores may have fitness value for subordinate individuals, compensating for their reduced opportunities to feed [103]. Studies of birds, for example, found that experimentally induced food insecurity increased fat deposition despite no apparent increase in energy intake, owing to compensatory reductions in other metabolic processes [104]. Such mechanisms are certainly plausible in humans, but the magnitude of their effect may only be reliably quantified if diet is controlled, a difficult undertaking in free-living humans.
In each of these cases, all representing forms of thrifty phenotype [89], exposure to nutritional constraint may result in a higher proportion of fat as weight and a more central fat distribution, detrimental to cardiometabolic health. This might be considered a simple life-history trade-off, whereby storing fat generates short-term benefits at a cost to longevity. However, some of these cardiometabolic costs may never be realized, as regardless of whether fat stores are high or low, exposure to undernutrition also shortens life expectancy [105,106]. If long-term costs are subject to ‘future-discounting’, then the short-term benefits of storing fat may be enhanced. Overall, therefore, these examples show how the fitness value of fat can change through shifts in both its metabolic benefits and its costs.
5. Conclusion
Evolutionary models of human obesity to date have been relatively simple, in assuming a constant association between adiposity alleles and selective pressures regardless of age or sex, and ignoring anatomical variability in fat deposition. The polygenetic basis of adiposity has been interpreted as it being non-conducive to selection except at extreme levels, as described in the dual intervention model [11].
The framework presented here offers a novel perspective on the polygenetic basis of adiposity. I argue that selection has favoured a crafty genotype, where the role of numerous alleles helps stabilize the genetic basis of phenotype in order to underpin enhanced plasticity, a strategy that is appropriate for a long-lived organism occupying volatile environments. The crafty genotype allows the fitness value of fat to vary in association with endogenous characteristics and life-course experience. The strategy of storing energy in fat has evolved to a highly sophisticated state, with contrasting fat depots and associated functions whose fitness values vary. This framework, highlighting the plasticity of adipose tissue biology, helps explain why weight gain is sensitive to numerous environmental factors, and why the health impact of obesity itself varies by age, sex, ethnicity and life-course experience. At the population level, the primary cause of trends in obesity is environmental, and has a profound economic basis [5,27].
Acknowledgements
I am grateful for the opportunity to attend the meeting on this topic hosted by the Royal Society.
Data accessibility
This article does not contain any additional data.
Conflict of interest declaration
I declare I have no competing interests.
Author contributions
J.W.: conceptualization, writing—review and editing.
Funding
I received no funding for this study.
References
- 1.Neel V. 1962. Diabetes mellitus: a ‘thrifty’ genotype rendered detrimental by ‘progress’? Am. J. Hum. Genet. 14, 353-362. [PMC free article] [PubMed] [Google Scholar]
- 2.James WP, Trayhurn P. 1976. An integrated view of the metabolic and genetic basis for obesity. Lancet 2, 770-773. ( 10.1016/s0140-6736(76)90602-4) [DOI] [PubMed] [Google Scholar]
- 3.Hoyenga KB, Hoyenga KT. 1982. Gender and energy balance: sex differences in adaptations for feast and famine. Physiol. Behav. 28, 545-563. ( 10.1016/0031-9384(82)90153-6) [DOI] [PubMed] [Google Scholar]
- 4.NCD Risk Factor Collaboration. 2017. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 390, 2627-2642. ( 10.1016/S0140-6736(17)32129-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells JC. 2010. The evolutionary biology of human body fatness: thrift and control. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.Cooper WN, et al. 2012. DNA methylation profiling at imprinted loci after periconceptional micronutrient supplementation in humans: results of a pilot randomized controlled trial. FASEB J. 26, 1782-1790. ( 10.1096/fj.11-192708) [DOI] [PubMed] [Google Scholar]
- 7.Elks CE, Den Hoed M, Zhao JH, Sharp SJ, Wareham NJ, Loos RJF, Ong KK. 2012. Variability in the heritability of body mass index: a systematic review and meta-regression. Front. Endocrinol. 3, 29. ( 10.3389/fendo.2012.00029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loos RJF, Yeo GSH. 2022. The genetics of obesity: from discovery to biology. Nat. Rev. Genet. 23, 120-133. ( 10.1038/s41576-021-00414-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher RA. 1918. The correlation between relatives on the supposition of Mendelian inheritance. Trans. R. Soc. Edinb. 52, 399-433. ( 10.1017/S0080456800012163) [DOI] [Google Scholar]
- 10.Bouchard C. 2007. The biological predisposition to obesity: beyond the thrifty genotype scenario. Int. J. Obes. 31, 1337-1339. ( 10.1038/sj.ijo.0803610) [DOI] [PubMed] [Google Scholar]
- 11.Speakman JR, Elmquist JK. 2022. Obesity: an evolutionary context. Life Metab. 1, 10-24. ( 10.1093/lifemeta/loac002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller MJ, Geisler C, Blundell J, Dulloo A, Schutz Y, Krawczak M, Bosy-Westphal A, Enderle J, Heymsfield SB. 2018. The case of GWAS of obesity: does body weight control play by the rules? Int. J. Obes. 42, 1395-1405. ( 10.1038/s41366-018-0081-6) [DOI] [PubMed] [Google Scholar]
- 13.Orsso CE, Colin-Ramirez E, Field CJ, Madsen KL, Prado CM, Haqq AM. 2020. Adipose tissue development and expansion from the womb to adolescence: an overview. Nutrients 12, 2735. ( 10.3390/nu12092735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierce MB, Leon DA. 2005. Age at menarche and adult BMI in the Aberdeen children of the 1950s cohort study. Am. J. Clin. Nutr. 82, 733-739. ( 10.1093/ajcn/82.4.733) [DOI] [PubMed] [Google Scholar]
- 15.Piersma T, Drent J. 2003. Phenotypic flexibility and the evolution of organismal design. Trends. Ecol. Evol. 18, 228-233. ( 10.1016/S0169-5347(03)00036-3) [DOI] [Google Scholar]
- 16.Sakers A, De Siqueira MK, Seale P, Villanueva CJ. 2022. Adipose-tissue plasticity in health and disease. Cell 185, 419-446. ( 10.1016/j.cell.2021.12.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abadi A, et al. 2017. Penetrance of polygenic obesity susceptibility loci across the body mass index distribution. Am. J. Hum. Genet. 101, 925-938. ( 10.1016/j.ajhg.2017.10.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouchard C. 2021. Genetics of obesity: what we have learned over decades of research. Obesity 29, 802-820. ( 10.1002/oby.23116) [DOI] [PubMed] [Google Scholar]
- 19.Higginson AD, McNamara JM. 2016. An adaptive response to uncertainty can lead to weight gain during dieting attempts. Evol. Med. Public Health 2016, 369-380. ( 10.1093/emph/eow031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mcnamara JM, Houston AI, Higginson AD. 2015. Costs of foraging predispose animals to obesity-related mortality when food is constantly abundant. PLoS ONE 10, e0141811. ( 10.1371/journal.pone.0141811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zihlman AL, Bolter DR. 2015. Body composition in Pan paniscus compared with Homo sapiens has implications for changes during human evolution. Proc. Natl Acad. Sci. USA 112, 7466-7471. ( 10.1073/pnas.1505071112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anton SC, Potts R, Aiello LC. 2014. Human evolution. Evolution of early Homo: an integrated biological perspective. Science 345, 1236828. ( 10.1126/science.1236828) [DOI] [PubMed] [Google Scholar]
- 23.Lisiecki LE, Raymo ME. 2005. A Plio-Pleistocene stack of 57 globally distributed benthic δ18O records. Paleoceanography 20, PA1003. ( 10.1029/2004pa001071) [DOI] [Google Scholar]
- 24.Wells JC. 2012. Ecological volatility and human evolution: a novel perspective on life history and reproductive strategy. Evol. Anthropol. 21, 277-288. ( 10.1002/evan.21334) [DOI] [PubMed] [Google Scholar]
- 25.Bonnefille R, Potts R, Chalié F, Jolly D, Peyron O. 2004. High-resolution vegetation and climate change associated with Pliocene Australopithecus afarensis. Proc. Natl Acad. Sci. USA 101, 12 125-12 129. ( 10.1073/pnas.0401709101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kingston JD. 2007. Shifting adaptive landscapes: progress and challenges in reconstructing early hominid environments. Am. J. Phys. Anthropol. 45, 20-58. ( 10.1002/ajpa.20733) [DOI] [PubMed] [Google Scholar]
- 27.Wells JC. 2016. The metabolic ghetto: an evolutionary perspective on nutrition, power relations and chronic disease. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 28.Wells JC. 2012. The evolution of human adiposity and obesity: where did it all go wrong? Dis. Model Mech. 5, 595-607. ( 10.1242/dmm.009613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henry CJ. 1990. Body mass index and the limits of human survival. Eur. J. Clin. Nutr. 44, 329-335. [PubMed] [Google Scholar]
- 30.Wade GN, Schneider JE. 1992. Metabolic fuels and reproduction in female mammals. Neurosci. Biobehav. Rev 16, 235-272. ( 10.1016/S0149-7634(05)80183-6) [DOI] [PubMed] [Google Scholar]
- 31.Bartz S, et al. 2014. Severe acute malnutrition in childhood: hormonal and metabolic status at presentation, response to treatment, and predictors of mortality. J. Clin. Endocrinol. Metab. 99, 2128-2137. ( 10.1210/jc.2013-4018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jasieńska G, Ziomkiewicz A, Ellison PT, Lipson SF, Thune I. 2004. Large breasts and narrow waists indicate high reproductive potential in women. Proc. R. Soc. Lond. B 271, 1213-1217. ( 10.1098/rspb.2004.2712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wells JC, Stock JT. 2007. The biology of the colonizing ape. Am. J. Phys. Anthropol. 45, 191-222. ( 10.1002/ajpa.20735) [DOI] [PubMed] [Google Scholar]
- 34.Odling-Smee FJ, Laland K, Feldman MW. 2003. Niche construction. Princeton, NJ: Princeton University Press. [Google Scholar]
- 35.Laland KN, Odling-Smee J, Myles S. 2010. How culture shaped the human genome: bringing genetics and the human sciences together. Nat. Rev. Genet. 11, 137-148. ( 10.1038/nrg2734) [DOI] [PubMed] [Google Scholar]
- 36.Gagneux P, et al. 1999. Mitochondrial sequences show diverse evolutionary histories of African hominoids. Proc. Natl Acad. Sci. USA 96, 5077-5082. ( 10.1073/pnas.96.9.5077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groucutt HS, et al. 2015. Rethinking the dispersal of Homo sapiens out of Africa. Evol. Anthropol. 24, 149-164. ( 10.1002/evan.21455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Couper-Johnston R. 2000. El Niño: the weather phenomenon that changed the world. London, UK: Hodder & Stoughton. [Google Scholar]
- 39.Navarrete A, Van Schaik CP, Isler K. 2011. Energetics and the evolution of human brain size. Nature 480, 91-93. ( 10.1038/nature10629) [DOI] [PubMed] [Google Scholar]
- 40.Hasselbalch SG, Madsen PL, Hageman LP, Olsen KS, Justesen N, Holm S, Paulson OB. 1996. Changes in cerebral blood flow and carbohydrate metabolism during acute hyperketonemia. Am. J. Physiol. 270, E746-E751. ( 10.1152/ajpendo.1996.270.5.E746) [DOI] [PubMed] [Google Scholar]
- 41.Kuzawa CW. 1998. Adipose tissue in human infancy and childhood: an evolutionary perspective. Am. J. Phys. Anthropol. 27, 177-209. () [DOI] [PubMed] [Google Scholar]
- 42.Wells JC. 2007. Sexual dimorphism of body composition. Best Pract. Res. Clin. Endocrinol. Metab. 21, 415-430. ( 10.1016/j.beem.2007.04.007) [DOI] [PubMed] [Google Scholar]
- 43.Razzaque A, Alam N, Wai L, Foster A. 1990. Sustained effects of the 1974–5 famine on infant and child mortality in a rural area of Bangladesh. Popul. Stud. 44, 145-154. ( 10.1080/0032472031000144426) [DOI] [PubMed] [Google Scholar]
- 44.Meehan CL, Crittenden AN. 2016. Childhood: origins, evolution, and implications. Albuquerque, NM: University of New Mexico Press. [Google Scholar]
- 45.Caspari R, Lee SH. 2004. Older age becomes common late in human evolution. Proc. Natl Acad. Sci. USA 101, 10 895-10 900. ( 10.1073/pnas.0402857101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wells JCK, Yao P, Williams JE, Gayner R. 2016. Maternal investment, life-history strategy of the offspring and adult chronic disease risk in South Asian women in the UK. Evol. Med. Public Health 2016, 133-145. ( 10.1093/emph/eow011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guernier V, Hochberg ME, Guegan JF. 2004. Ecology drives the worldwide distribution of human diseases. PLoS Biol. 2, e141. ( 10.1371/journal.pbio.0020141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wells JC. 2009. Ethnic variability in adiposity and cardiovascular risk: the variable disease selection hypothesis. Int. J. Epidemiol. 38, 63-71. ( 10.1093/ije/dyn183) [DOI] [PubMed] [Google Scholar]
- 49.Speakman JR. 2018. The evolution of body fatness: trading off disease and predation risk. J. Exp. Biol. 221, jeb167254. ( 10.1242/jeb.167254) [DOI] [PubMed] [Google Scholar]
- 50.Badman MK, Flier JS. 2007. The adipocyte as an active participant in energy balance and metabolism. Gastroenterology 132, 2103-2115. ( 10.1053/j.gastro.2007.03.058) [DOI] [PubMed] [Google Scholar]
- 51.Permana PA, Reardon CL. 2007. What are subcutaneous adipocytes really good for? Viewpoint 2. Exp. Dermatol. 16, 53-55. ( 10.1111/j.1600-0625.2006.00519_4.x) [DOI] [PubMed] [Google Scholar]
- 52.Cohen MN. 1989. Health and the rise of civilization. New Haven, CT: Yale University Press. [Google Scholar]
- 53.Wells JC, Stock JT. 2020. Life history transitions at the origins of agriculture: a model for analysing how niche construction impacts human growth, demography and health. Front. Endocrinol. 11, 325. ( 10.3389/fendo.2020.00325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wells JC, Saunders MA, Lea AS, Cortina-Borja M, Shirley MK. 2019. Beyond Bergmann's rule: global variability in human body composition is associated with annual average precipitation and annual temperature volatility. Am. J. Phys. Anthropol. 170, 75-97. ( 10.1002/ajpa.23890) [DOI] [PubMed] [Google Scholar]
- 55.Ziegler EE, O'Donnell AM, Nelson SE, Fomon SJ. 1976. Body composition of the reference fetus. Growth 40, 329-341. [PubMed] [Google Scholar]
- 56.Andersen GS, Girma T, Wells JCK, Kæstel P, Leventi M, Hother A-L, Michaelsen KF, Friis H. 2013. Body composition from birth to 6 mo of age in Ethiopian infants: reference data obtained by air-displacement plethysmography. Am. J. Clin. Nutr. 98, 885-894. ( 10.3945/ajcn.113.063032) [DOI] [PubMed] [Google Scholar]
- 57.Butte NF, Hopkinson JM, Wong WW, O'Brian Smith E, Ellis KJ. 2000. Body composition during the first 2 years of life: an updated reference. Pediatr. Res. 47, 578-585. ( 10.1203/00006450-200005000-00004) [DOI] [PubMed] [Google Scholar]
- 58.Haas JD, Moreno-Black G, Frongillo EA, Pabon AJ, Pareja LG, Ybarnegaray UJ, Hurtado GL. 1982. Altitude and infant growth in Bolivia: a longitudinal study. Am. J. Phys. Anthropol. 59, 251-262. ( 10.1002/ajpa.1330590304) [DOI] [PubMed] [Google Scholar]
- 59.Philipi T, Seger JH. 1989. Hedging evolutionary bets, revisited. Trends Ecol. Evol. 4, 41-44. [DOI] [PubMed] [Google Scholar]
- 60.Cornelis MC, Rimm EB, Curhan GC, Kraft P, Hunter DJ, Hu FB, Van Dam RM. 2014. Obesity susceptibility loci and uncontrolled eating, emotional eating and cognitive restraint behaviors in men and women. Obesity 22, E135-E141. ( 10.1002/oby.20592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ibáñez L, Ferrer A, Marcos MV, Hierro FR, De Zegher F. 2000. Early puberty: rapid progression and reduced final height in girls with low birth weight. Pediatrics 106, E72. ( 10.1542/peds.106.5.e72) [DOI] [PubMed] [Google Scholar]
- 62.Cousminer DL, et al. 2013. Genome-wide association and longitudinal analyses reveal genetic loci linking pubertal height growth, pubertal timing and childhood adiposity. Hum. Mol. Genet. 22, 2735-2747. ( 10.1093/hmg/ddt104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wells JCK. 2018. Life history trade-offs and the partitioning of maternal investment: implications for health of mothers and offspring. Evol. Med. Public Health 2018, 153-166. ( 10.1093/emph/eoy014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wells JCK, et al. 2012. Body-composition reference data for simple and reference techniques and a 4-component model: a new UK reference child. Am. J. Clin. Nutr. 96, 1316-1326. ( 10.3945/ajcn.112.036970) [DOI] [PubMed] [Google Scholar]
- 65.Wells JCK, Davies PSW, Fewtrell MS, Cole TJ. 2020. Body composition reference charts for UK infants and children aged 6 weeks to 5 years based on measurement of total body water by isotope dilution. Eur. J. Clin. Nutr. 74, 141-148. ( 10.1038/s41430-019-0409-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rolland-Cachera MF, Deheeger M, Guilloud-Bataille M, Avons P, Patois E, Sempé M. 1987. Tracking the development of adiposity from one month of age to adulthood. Ann. Hum. Biol. 14, 219-229. ( 10.1080/03014468700008991) [DOI] [PubMed] [Google Scholar]
- 67.Wells JC. 2012. Sexual dimorphism in body composition across human populations: associations with climate and proxies for short- and long-term energy supply. Am. J. Hum. Biol. 24, 411-419. ( 10.1002/ajhb.22223) [DOI] [PubMed] [Google Scholar]
- 68.Glynn JR, Moss PAH. 2020. Systematic analysis of infectious disease outcomes by age shows lowest severity in school-age children. Sci. Data 7, 329. ( 10.1038/s41597-020-00668-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grant RW, Dixit VD. 2015. Adipose tissue as an immunological organ. Obesity 23, 512-518. ( 10.1002/oby.21003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gabrielsson BG, et al. 2003. High expression of complement components in omental adipose tissue in obese men. Obes. Res. 11, 699-708. ( 10.1038/oby.2003.100) [DOI] [PubMed] [Google Scholar]
- 71.Yang Y-S, Song H-D, Li R-Y, Zhou L-B, Zhu Z-D, Hu R-M, Han Z-G, Chen J-L. 2003. The gene expression profiling of human visceral adipose tissue and its secretory functions. Biochem. Biophys. Res. Commun. 300, 839-846. ( 10.1016/S0006-291X(02)02843-7) [DOI] [PubMed] [Google Scholar]
- 72.Alvehus M, Burén J, Sjöström M, Goedecke J, Olsson T. 2010. The human visceral fat depot has a unique inflammatory profile. Obesity 18, 879-883. ( 10.1038/oby.2010.22) [DOI] [PubMed] [Google Scholar]
- 73.Wells JC, Cole TJ, Treleaven P. 2008. Age-variability in body shape associated with excess weight: the UK National Sizing Survey. Obesity 16, 435-441. ( 10.1038/oby.2007.62) [DOI] [PubMed] [Google Scholar]
- 74.Zamboni M, Nori N, Brunelli A, Zoico E. 2021. How does adipose tissue contribute to inflammageing? Exp. Gerontol. 143, 111162. ( 10.1016/j.exger.2020.111162) [DOI] [PubMed] [Google Scholar]
- 75.Chantler S, Dickie K, Micklesfield LK, Goedecke JH. 2015. Longitudinal changes in body fat and its distribution in relation to cardiometabolic risk in Black South African Women. Metab. Syndr. Relat. Disord. 13, 381-388. ( 10.1089/met.2015.0021) [DOI] [PubMed] [Google Scholar]
- 76.Hughes VA, Roubenoff R, Wood M, Frontera WR, Evans WJ, Fiatarone Singh MA. 2004. Anthropometric assessment of 10-y changes in body composition in the elderly. Am. J. Clin. Nutr. 80, 475-482. ( 10.1093/ajcn/80.2.475) [DOI] [PubMed] [Google Scholar]
- 77.Greendale GA, Han W, Finkelstein JS, Burnett-Bowie S-AM, Huang MH, Martin D, Karlamangla AS. 2021. Changes in regional fat distribution and anthropometric measures across the menopause transition. J. Clin. Endocrinol. Metab. 106, 2520-2534. ( 10.1210/clinem/dgab389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wells JC, Charoensiriwath S, Treleaven P. 2011. Reproduction, aging, and body shape by three-dimensional photonic scanning in Thai men and women. Am. J. Hum. Biol. 23, 291-298. ( 10.1002/ajhb.21151) [DOI] [PubMed] [Google Scholar]
- 79.Rebuffe-Scrive M, Enk L, Crona N, Lönnroth P, Abrahamsson L, Smith U, Björntorp P. 1985. Fat cell metabolism in different regions in women. Effect of menstrual cycle, pregnancy, and lactation. J. Clin. Invest. 75, 1973-1976. ( 10.1172/JCI111914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lieberman DE, Kistner TM, Richard D, Lee I-M, Baggish AL. 2021. The active grandparent hypothesis: physical activity and the evolution of extended human healthspans and lifespans. Proc. Natl Acad. Sci. USA 118, e2107621118. ( 10.1073/pnas.2107621118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gibson MA, Mace R. 2005. Helpful grandmothers in rural Ethiopia: a study of the effect of kin on child survival and growth. Evol. Hum. Behav. 26, 469-482. ( 10.1016/j.evolhumbehav.2005.03.004) [DOI] [Google Scholar]
- 82.Engelhardt SC, Bergeron P, Gagnon A, Dillon L, Pelletier F. 2019. Using geographic distance as a potential proxy for help in the assessment of the grandmother hypothesis. Curr. Biol. 29, 651-656. ( 10.1016/j.cub.2019.01.027) [DOI] [PubMed] [Google Scholar]
- 83.Wells JC, Cortina-Borja M. 2013. Different associations of subscapular and triceps skinfold thicknesses with pathogen load: an ecogeographical analysis. Am. J. Hum. Biol. 25, 594-605. ( 10.1002/ajhb.22418) [DOI] [PubMed] [Google Scholar]
- 84.Wells JC. 2012. Ethnic variability in adiposity, thrifty phenotypes and cardiometabolic risk: addressing the full range of ethnicity, including those of mixed ethnicity. Obes. Rev. 13, 14-29. ( 10.1111/j.1467-789X.2012.01034.x) [DOI] [PubMed] [Google Scholar]
- 85.Wells JC. 2010. Maternal capital and the metabolic ghetto: an evolutionary perspective on the transgenerational basis of health inequalities. Am. J. Hum. Biol. 22, 1-17. ( 10.1002/ajhb.20994) [DOI] [PubMed] [Google Scholar]
- 86.Wells JC, Sawaya AL, Wibaek R, Mwangome M, Poullas MS, Yajnik CS, Demaio A. 2020. The double burden of malnutrition: aetiological pathways and consequences for health. Lancet 395, 75-88. ( 10.1016/S0140-6736(19)32472-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Amugsi DA, Dimbuene ZT, Kimani-Murage EW, Mberu B, Ezeh AC. 2017. Differential effects of dietary diversity and maternal characteristics on linear growth of children aged 6–59 months in sub-Saharan Africa: a multi-country analysis. Public Health Nutr. 20, 1029-1045. ( 10.1017/S1368980016003426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nettle D, Andrews C, Bateson M. 2017. Food insecurity as a driver of obesity in humans: the insurance hypothesis. Behav. Brain Sci. 40, e105. ( 10.1017/S0140525X16000947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hales CN, Barker DJ. 1992. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35, 595-601. ( 10.1007/BF00400248) [DOI] [PubMed] [Google Scholar]
- 90.Baker JL, Olsen LW, Sorensen TI. 2008. Weight at birth and all-cause mortality in adulthood. Epidemiology 19, 197-203. ( 10.1097/EDE.0b013e31816339c6) [DOI] [PubMed] [Google Scholar]
- 91.Barker DJP, Osmond C, Winter PD, Margetts B, Simmonds SJ. 1989. Weight in infancy and death from ischaemic heart disease. Lancet 2, 577-580. ( 10.1016/S0140-6736(89)90710-1) [DOI] [PubMed] [Google Scholar]
- 92.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. 1991. Fetal and infant growth and impaired glucose tolerance at age 64. Br. Med. J. 303, 1019-1022. ( 10.1136/bmj.303.6809.1019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJP. 1999. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. Br. Med. J. 318, 427-431. ( 10.1136/bmj.318.7181.427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Y, et al. 2015. Birth weight and later life adherence to unhealthy lifestyles in predicting type 2 diabetes: prospective cohort study. Br. Med. J. 351, h3672. ( 10.1136/bmj.h3672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kensara OA, Wootton SA, Phillips DI, Patel M, Jackson AA, Elia M. 2005. Fetal programming of body composition: relation between birth weight and body composition measured with dual-energy X-ray absorptiometry and anthropometric methods in older Englishmen. Am. J. Clin. Nutr. 82, 980-987. ( 10.1093/ajcn/82.5.980) [DOI] [PubMed] [Google Scholar]
- 96.Wells JCK, et al. 2019. Low maternal capital predicts life history trade-offs in daughters: why adverse outcomes cluster in individuals. Front. Public Health 7, 206. ( 10.3389/fpubh.2019.00206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wells JCK. 2019. Body composition of children with moderate and severe undernutrition and after treatment: a narrative review. BMC Med. 17, 215. ( 10.1186/s12916-019-1465-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lelijveld N, et al. 2016. Chronic disease outcomes after severe acute malnutrition in Malawian children (ChroSAM): a cohort study. Lancet Glob. Health 4, e654-e662. ( 10.1016/S2214-109X(16)30133-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Victora CG, Barros FC, Vaughan JP, Martines JC, Beria JU. 1987. Birthweight, socio-economic status and growth of Brazilian infants. Ann. Hum. Biol. 14, 49-57. ( 10.1080/03014468700008831) [DOI] [PubMed] [Google Scholar]
- 100.Devakumar D, et al. 2018. Socioeconomic determinants of growth in a longitudinal study in Nepal. Matern. Child Nutr. 14, e12462. ( 10.1111/mcn.12462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bridger Staatz C, Kelly Y, Lacey RE, Blodgett JM, George A, Arnot M, Walker E, Hardy R. 2021. Socioeconomic position and body composition in childhood in high- and middle-income countries: a systematic review and narrative synthesis. Int. J. Obes. 45, 2316-2334. ( 10.1038/s41366-021-00899-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wells JCK, Devakumar D, Manandhar DS, Saville N, Chaube SS, Costello A, Osrin D. 2019. Associations of stunting at 2 years with body composition and blood pressure at 8 years of age: longitudinal cohort analysis from lowland Nepal. Eur. J. Clin. Nutr. 73, 302-310. ( 10.1038/s41430-018-0291-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Witter MS, Swaddle JP. 1995. Dominance, competition, and energetic reserves in the European starling, Sturnus vulgaris. Behav. Ecol. 6, 343-348. ( 10.1093/beheco/6.3.343) [DOI] [Google Scholar]
- 104.Andrews C, Zuidersma E, Verhulst S, Nettle D, Bateson M. 2021. Exposure to food insecurity increases energy storage and reduces somatic maintenance in European starlings (Sturnus vulgaris). R. Soc. Open Sci. 8, 211099. ( 10.1098/rsos.211099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kirkwood TBL, Rose MR. 1991. Evolution of senescence. Phil. Trans. R. Soc. Lond. B 332, 15-24. ( 10.1098/rstb.1991.0028) [DOI] [PubMed] [Google Scholar]
- 106.Kajantie E, Osmond C, Barker DJ, Forsén T, Phillips DI, Eriksson JG. 2005. Size at birth as a predictor of mortality in adulthood: a follow-up of 350 000 person-years. Int. J. Epidemiol. 34, 655-663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article does not contain any additional data.