Summary
Ubc13 is required for Lys63‐linked polyubiquitination and innate immune responses in mammals, but its functions in plant immunity still remain largely unknown. Here, we used molecular biological, pathological, biochemical, and genetic approaches to evaluate the roles of rice OsUbc13 in response to pathogens. The OsUbc13‐RNA interference (RNAi) lines with lesion mimic phenotypes displayed a significant increase in the accumulation of flg22‐ and chitin‐induced reactive oxygen species, and in defence‐related genes expression or hormones as well as resistance to Magnaporthe oryzae and Xanthomonas oryzae pv oryzae. Strikingly, OsUbc13 directly interacts with OsSnRK1a, which is the α catalytic subunit of SnRK1 (sucrose non‐fermenting‐1‐related protein kinase‐1) and acts as a positive regulator of broad‐spectrum disease resistance in rice. In the OsUbc13‐RNAi plants, although the protein level of OsSnRK1a did not change, its activity and ABA sensitivity were obviously enhanced, and the K63‐linked polyubiquitination was weaker than that of wild‐type Dongjin (DJ). Overexpression of the deubiquitinase‐encoding gene OsOTUB1.1 produced similar effects with inhibition of OsUbc13 in affecting immunity responses, M. oryzae resistance, OsSnRK1a ubiquitination, and OsSnRK1a activity. Furthermore, re‐interfering with OsSnRK1a in one OsUbc13‐RNAi line (Ri‐3) partially restored its M. oryzae resistance to a level between those of Ri‐3 and DJ. Our data demonstrate OsUbc13 negatively regulates immunity against pathogens by enhancing the activity of OsSnRK1a.
Keywords: rice, OsUbc13, K63‐linked polyubiquitination, OsSnRK1a, pathogen resistance
Short abstract
Inhibition of OsUbc13 activates autoimmune responses, and enhances the activity of OsSnRK1a, one OsUbc13's direct interaction partners, through attenuating its K63‐linked polyubiquitination, thereby increasing pathogen resistance in rice.
Introduction
Plants have evolved a two‐layered immune system to prevent the attack of pathogen: PAMP‐triggered immunity (PTI) governed by pattern recognition receptors and effector‐triggered immunity (ETI) conferred by highly polymorphic resistance (R) proteins (Faulkner and Robatzek, 2012; Jones and Dangl, 2006; Schwessinger and Zipfel, 2008; Spoel and Dong, 2012). One typical feature of ETI is the hypersensitive response (HR), which is an important defence mechanism employed by plants to protect themselves from biotic stress. In the infection site, plants rapidly undergo programmed cell death (PCD) that arrests the growth and spread of the pathogen (Wu et al., 2014; Zebell and Dong, 2015). HR is also usually accompanied by the generation of reactive oxygen species (ROS), expression of pathogenesis‐related (PR) proteins, accumulation of callose and thickening of cell walls at the infection sites (Jones and Dangl, 1996). Given its severe impact on cell fate and its high efficiency in fighting pathogen damage, HR must be tightly monitored in the absence of pathogen attack to balance plant growth and defence (Mukhtar et al., 2016).
Since plants lack adaptive immunity, their immune system is largely dependent on two types of innate immunity, PTI and ETI. Mammalian innate immunity is closely regulated by a cascade of ubiquitination systems (Jiang and Chen, 2011). Ubiquitination is a post‐translational protein modification that regulates numerous important cellular processes in all eukaryotes. Ubiquitin (Ub), a highly conserved 76‐amino acid polypeptide, is first activated by forming a thioester complex with the Ub‐activating enzyme (Uba or E1). Next, Ub‐conjugating enzyme (Ubc or E2) catalyses the transfer of activated Ub to Ub ligase (E3) depending on the active‐site cysteine. Finally, E3 mediates ubiquitination of the target proteins (Ye and Rape, 2009). The final fates of target proteins after ubiquitination usually depend on whether the protein is polyubiquitinated or monoubiquitinated. During polyubiquitination, the C‐terminal of the next Ub is connected to one of the seven surface lysine residues (Lys6, 11, 27, 29, 33, 48 or 63) on the previous Ub (Komander and Rape, 2012). Different multi‐Ub chains display different chemical and topological properties. For instance, the Lys48(K48)‐linked chain causes degradation of target proteins (Hershko and Ciechanover, 1998; Komander and Rape, 2012; Wickliffe et al., 2011), while the Lys63(K63)‐linked chain is usually involved in changing the activity of the target protein (Chen and Sun, 2009). Formation of the K63‐linked poly‐Ub chain requires a unique heterodimeric complex formed by the canonical E2, Ubc13, and another Ubc‐like protein named Ubc‐E2 variant (UEV) (Hofmann and Pickart, 1999; Romero‐Barrios and Vert, 2018).
In mammals, Ubc13‐mediated polyubiquitination of K63 plays an important role in the regulation of innate and adaptive immune responses, such as signal transduction and activating nuclear factor kappa enhancer‐binding protein (NF‐κB), which is a key immune modulator (Jiang and Chen, 2011; Wu and Karin, 2015). After signal recognition, a complex formed by Ubc13, Uev1A, and an E3 ubiquitin ligase (TRAF6) is responsible for catalysing the K63‐linked polyubiquitination of two kinase complexes, including TGFβ‐activated kinase 1 (TAK1) and IκB kinase (IKK). This modification promotes the phosphorylation of the IKK β subunit by TAK1, which in turn activates IKK. Knockout of Ubc13 in experimental mice has been reported to result in autoimmunity (Chang et al., 2012), embryonic lethality (Fukushima et al., 2007; Yamamoto et al., 2006a) and defects in cell proliferation (Yamamoto et al., 2006b).
Different researchers have identified and cloned Ubc13 and UEV family genes from several different plant species (Guo et al., 2015, 2016; Liu et al., 2021; Mural et al., 2013; Wang et al., 2017a; Wen et al., 2006, 2008; Zang et al., 2012), indicating that their functions should be conserved. In Arabidopsis, two Ubc13s (AtUbc13A/B) interact with all four UEVs (designated AtUEV1A‐D) and promote K63‐linked polyubiquitination in vitro (Wen et al., 2008). And these two Ubc13 genes have been reported to be involved in the regulation of DNA damage repair (Wen et al., 2006), apical dominance (Yin et al., 2007), iron metabolism (Li and Schmidt, 2010) and auxin signalling (Wen et al., 2014). Recently, Wang et al. (2019) revealed that Arabidopsis Ubc13 differentially regulates two PCD pathways in responses to pathogen and low‐temperature stress. The ubc13 double mutant exhibited hypothermia‐induced and salicylic acid (SA)‐dependent lesion‐mimicking phenotypes. But unlike typical lesion mimic mutants, ubc13 did not show enhanced resistance to virulent bacterial and fungal pathogens (Wang et al., 2019). In addition, the tomato Ubc13 (Fni3/Sl‐Ubc13‐2) and UEV (Suv) have been found to positively regulate plant immunity through interaction with Fen, one protein kinase playing a pivotal role in ETI. The Ub‐conjugating activity of tomato Ubc13 and its interaction with Fen appear to be required for cell death triggered by Fen overexpression (OE) in Nicotiana benthamiana and by several NLR/effector pairs (Mural et al., 2013). In the rice genome, only one hypothetical gene (Os01g0673600) named OsUbc13 showed high sequence identity with a pair of highly conserved Arabidopsis Ubc13. OsUbc13 has been identified to form stable heterodimers with all highly conserved UEVs (OsUEV1A–D) for mediating Ub chain assembly linked by K63 and also be required for tolerance to DNA damage (Wang et al., 2017a; Zang et al., 2012). Human ovarian tumour domain‐containing Ub aldehyde‐binding protein 1 (OTUB1) is a deubiquitinating enzyme, which can directly interact with Ubc13 and strongly suppress Ubc13‐dependent K63‐linked ubiquitination by preventing Ub transfer (Nakada et al., 2010; Wiener et al., 2012). Similarly, the interactions were detected in vitro and in vivo between the rice OsOTUB1.1 and OsUbc13 proteins (Wang et al., 2017b). However, whether OsUbc13 functions in rice innate immunity and disease resistance is unclear.
The sucrose non‐fermenting‐1 (SNF1) protein kinase family comprises SNF1 in Saccharomyces cerevisiae, the AMP‐activated protein kinase (AMPK) in mammals, and the SNF1‐related protein kinases (SnRKs) in higher plants. The conserved SNF1 family serves as the cellular energy sensor and regulator (Halford and Hey, 2009; Hardie, 2007; Hardie et al., 1998; Wang et al., 2021). In plants, SnRKs emerge as promising candidates to balance growth and defence (Cho et al., 2012; Kim et al., 2015; Nukarinen et al., 2016; Seo et al., 2011). These kinases form heterotrimeric complexes with a catalytic α‐subunit and regulatory β‐ and γ‐subunits. The α‐subunit consists of a highly conserved N‐terminal Ser/Thr kinase domain and a large C‐terminal regulatory domain that mediates its interaction with the β‐ and γ‐subunits (Broeckx et al., 2016; Hardie et al., 2012; Hedbacker and Carlson, 2008). The SnRK family of plants is subdivided into three subfamilies, SnRK1, SnRK2, and SnRK3, of which SnRK1 is most similar to SNF1 and AMPK (Coello et al., 2011; Ding et al., 2009; Hrabak et al., 2003). Increasing evidence has shown that SnRK1s play an important role in plants' immune responses. Plants overexpressing SnRK1 are more resistant to geminivirus infection, while SnRK1‐silenced plants are more susceptible (Hao et al., 2003; Shen et al., 2011; Shen and Hanley‐Bowdoin, 2021). OE of TaSnRK1α in wheat plants increases the resistance against F. graminearum and silencing of TaSnRK1α has the opposite effect (Jiang et al., 2020). SnRK1 phosphorylates and destabilizes WRKY3 to enhance barley immunity to powdery mildew (Han et al., 2020). Rice has three genes encoding the α‐subunit of the SnRK1 complex, which can be subdivided into two subgroups, OsSnRK1a (OSK1) and OsSnRK1b (OSK24/35) (Takano et al., 1998). Among them, OSK35 positively regulates defence against Magnaporthe oryzae and Xanthomonas oryzae pv. oryzae (Xoo) (Kim et al., 2015). OsSnRK1a functions in the sugar signalling cascade (Lu et al., 2007) and energy homeostasis (Wang et al., 2021). Furthermore, OsSnRK1a has been reported to confer broad‐spectrum disease resistance and act as a master switch that regulates growth‐immunity trade‐offs in rice (Filipe et al., 2018), but its protein turnover and the mechanism by which OsSnRK1a‐mediated immunity is regulated remains largely unknown.
In this research, we investigated the molecular function of OsUbc13 in rice immunity. The OsUbc13‐RNA interference (RNAi) lines displayed lesion mimic phenotypes, accompanied by a significant increase in the accumulation of flg22‐ and chitin‐induced ROS, and in defence‐related gene expression or hormones. Unexpectedly, in contrast to the susceptibility caused by the Arabidopsis Ubc13 mutation, knockdown of OsUbc13 significantly increased rice resistance to M. oryzae and Xoo. Notably, OsUbc13 directly interacts with OsSnRK1a, which is the α catalytic subunit of SnRK1 (sucrose nonfermentation 1‐related protein kinase 1) and positively regulates broad‐spectrum disease resistance in rice. In the OsUbc13‐RNAi plants, although the protein abundance of OsSnRK1a did not change, its activity and sensitivity to ABA were obviously enhanced, and K63‐linked polyubiquitination was weaker than that of wild‐type Dongjin (DJ). OE of the deubiquitinase‐encoding gene OsOTUB1.1 produced similar effects to the inhibition of OsUbc13 in affecting immunity responses, M. oryzae resistance, OsSnRK1a ubiquitination, and OsSnRK1a activity. Furthermore, re‐interfering with OsSnRK1a in one OsUbc13‐RNAi line (Ri‐3) partially restored its M. oryzae resistance to a level between those of Ri‐3 and DJ. Our data demonstrate OsUbc13 negatively regulates immunity against pathogens by enhancing the activity of OsSnRK1a.
Results
Phenotypic characterization of the OsUbc13‐RNAi lines
To survey the potential function of OsUbc13 in rice innate immunity and disease resistance, 15 independent RNA interference (RNAi) lines of OsUbc13 were successfully produced. Two lines (Ri‐1 and Ri‐3), in which the relative expression level of OsUbc13 was approximately one‐tenth of that in wild‐type DongJin (DJ, Figure 1a), were selected for follow‐up studies. Under field conditions in summer (Fuzhou, China), the tips of lower (older) leaves of the two OsUbc13‐RNAi lines exhibited irregular brown necrotic lesions approximately 30 days post‐sowing. The lesions then spread gradually, eventually extending to whole leaves, leading to severe leaf withering and premature senescence (Figure 1b,c). Cell death lesions eventually appeared on the top of OsUbc13‐RNAi plants, and by the heading and grain‐filling stage, these gene‐silenced plants exhibited a pronounced senescence phenotype (Figure 1b). When OsUbc13‐RNAi lines were grown in the greenhouse, less severe lesions were visible about 40 days after planting in soil, and longer in the nutrient solution. These variations in both the timing of lesion appearance and in lesion severity suggest that cell death in OsUbc13‐RNAi plants is influenced by growth conditions. In addition to cell death and premature senescence, two key agronomic traits including tiller number and grain number per panicle were adversely affected in OsUbc13‐RNAi plants (Figure S1), which is consistent with perturbation of the OsUbc13 gene in the Zhonghua11 (ZH11) background by another research group (Wang et al., 2017b). To determine whether the development of lesions in the OsUbc13‐RNAi lines involves altered hydrogen peroxide (H2O2) accumulation, we performed 3,3′‐diaminobenzidine (DAB) staining. As shown in Figure 1c, strong brown staining is prominent around leaf lesions in OsUbc13‐RNAi plants, as opposed to in the leaves of wild‐type DJ, indicating that a significant amount of H2O2 was accumulated when OsUbc13 was silenced. We further quantified that the concentration of H2O2 in OsUbc13‐RNAi is significantly higher than that in wild‐type DJ plants (Figure 1d), and so is the production of malondialdehyde (MDA, Figure S2a), another biomarker related to cell death and lipid peroxidation (Ayala et al., 2014; Ruan et al., 2019). Photosynthetic pigment content was measured to check the effect of lesions on OsUbc13‐RNAi plants. The levels of chlorophyll a, chlorophyll b and carotenoids were significantly reduced in Ri‐1 and Ri‐3 compared with DJ (Figure S2b), suggesting that the formation of necrotic lesions had a negative effect on photosynthetic pigment content, which might be the cause of poor agronomic performance of the OsUbc13‐RNAi lines.
Figure 1.

The OsUbc13‐RNAi lines exhibit lesion mimic phenotype accompanied with reactive oxygen species burst and accelerated leaf senescence. (a) qRT‐PCR analysis of OsUbc13 expression in two OsUbc13‐RNAi (Ri‐1 and Ri‐3) lines. OsActin1 gene was used as an internal control. Data are shown as means ±SE; n = 3 (***P < 0.001; Student's t‐test). (b) Whole plants of the wild‐type DJ and two OsUbc13‐RNAi lines at the heading and grain filling stage in the paddy field. Scale bar = 15 cm. (c) Lesion mimic phenotypes and DAB staining on leaves of the OsUbc13‐RNAi plants at 30‐day post‐sowing in soil, compared with that of DJ. Scale bar = 1 cm. (d) Detection of the H2O2 content of DJ and the OsUbc13‐RNAi plants at 30‐day post‐sowing in soil. Data are shown as means ±SE; n = 3 (**P < 0.01, ***P < 0.001; Student's t‐test). (e) ROS accumulation dynamics in DJ and OsUbc13‐RNAi plants after flg22 and water (mock) treatments. Data are shown as means ±SE; n = 3. (f) ROS accumulation dynamics in DJ and OsUbc13‐RNAi plants after chitin and water (mock) treatments. Data are shown as means ±SE; n = 3.
To further investigate whether the ROS signalling pathway is impaired in OsUbc13‐RNAi plants, we compared the dynamics of flg22‐ or chitin‐induced ROS generation using a chemical luminescence assay. The ROS production rate was faster in OsUbc13‐RNAi than in DJ in response to both flg22 and chitin treatments (Figure 1e,f). ROS levels peaked ~9 min after flg22 treatment, and these levels were nearly 2.40 or 2.99 times higher in Ri‐1 or Ri‐3 than in DJ, respectively (Figure 1e). With chitin treatment, the ROS levels peaked ~23 min, and the levels were nearly 2.30 or 2.60 times higher in Ri‐1 or Ri‐3 than in DJ, respectively (Figure 1f). Even in the control treated with water, the basal ROS level was also higher in Ri‐1 and Ri‐3 than in DJ (Figure 1e,f). Taken together, these results indicate that the ROS signalling pathway is enhanced in OsUbc13‐RNAi plants.
Enhanced resistance of OsUbc13‐RNAi plants against Magnaporthe oryzae and Xanthomonas oryzae pv. oryzae
Given that OsUbc13‐RNAi had hypersensitivity‐like lesions and more ROS accumulation after treatment with PAMPs (flg22 and chitin), we speculated that these transgenic plants may exhibit enhanced resistance to rice pathogens. Before testing this hypothesis, we first examined the response of the OsUbc13 gene to M. oryzae infection through quantitative reverse transcription PCR (qRT‐PCR). As illustrated in Figure 2a, the expression level of OsUbc13 in wild‐type DJ seedlings was induced by inoculation with GUY11, a virulent isolate of M. oryzae; the induced expression reached a peak at 3‐ and 6‐day post‐inoculation, indicating that OsUbc13 may participate in rice responses to M. oryzae infection. To evaluate the blast resistance, detached leaves of 1‐month‐old seedlings of DJ and OsUbc13‐RNAi were inoculated with GUY11 using the punch method in a controlled environment. Although the OsUbc13‐RNAi plants growing in greenhouse did not display lesion mimic phenotypes when challenged with GUY11, both relative lesion area and fungal biomass in the lesions of which were significantly lower than in DJ (Figure 2bd). The disease resistance test was repeated with a spraying inoculation method using conidia. The two OsUbc13‐RNAi lines develop only small, scattered lesions compared with DJ which shows typical blast lesions (Figure 2e,f). In another test, fully expanded flag leaves of DJ and OsUbc13‐RNAi were inoculated with one bacterial blight pathogen Xoo strain PXO99. At 15‐day post‐inoculation, the lesion lengths were much shorter on OsUbc13‐RNAi plants than on DJ plants (Figure 2g,h). These results demonstrate that the OsUbc13‐RNAi plants display significantly enhanced resistance to both M. oryzae and Xoo. In addition to RNAi lines, we also tried to generate knockout lines using the CRISPR/Cas9 method (Ma et al., 2015) to investigate OsUbc13's function in disease resistance. However, we were unable to obtain successful transgenic plants, probably due to the lethality of knocking out OsUbc13.
Figure 2.

The OsUbc13‐RNAi lines display enhanced resistance to both M. oryzae and Xoo. (a) qRT‐PCR analysis of OsUbc13 expression level at different time after inoculation with the compatible isolate GUY11 of M. oryzae. Two‐week‐old wild‐type DJ seedlings were used for inoculation. The seedlings sprayed only with 0.025% Tween 20 were used as negative control (Mock). OsActin1 gene was used as an internal control. Data are shown as means ±SE; n = 3. (b) The lesions on DJ and OsUbc13‐RNAi leaves at 8 days after punch inoculation with the compatible M. oryzae isolate GUY11. Scale bar = 1 cm. (c) Relative lesion area (%) in leaves of (b) indicates significant differences between DJ and OsUbc13‐RNAi. Data are shown as means ±SE; n = 15 (***P < 0.001; Student's t‐test). (d) Relative fungal biomass, measured as MoPot2 by qRT‐PCR, in leaves of (b) was normalized to OsUbq DNA (Park et al., 2012). Data are shown as means ±SE; n = 3 (***P < 0.001; Student's t‐test). (e) The lesions on DJ and OsUbc13‐RNAi leaves at 8 days after spraying inoculation with the compatible M. oryzae isolate GUY11. Scale bar = 1 cm. (f) Relative lesion area (%) in leaves of (e) indicates significant differences between DJ and OsUbc13‐RNAi. Data are shown as means ±SE; n = 5 (***P < 0.001; Student's t‐test). (g) The lesions on DJ and OsUbc13‐RNAi leaves at 18 days after inoculation with the compatible Xoo isolate PXO99. (h) Lesion lengths in leaves of (g) indicate significant differences between DJ and OsUbc13‐RNAi. Data are shown as means ±SE; n = 6 (***P < 0.001; Student's t‐test).
OE lines of the OsUbc13 gene were successfully constructed (Figure S3a). In contrast to the RNAi lines, the two OE lines (OE17‐2 and OE18‐3) did not exhibit obvious reduced or enhanced resistance to M. oryzae compared with DJ (Figure S3b,c). These results demonstrate that the disruption of OsUbc13 is responsible for enhanced disease resistance.
To ascertain whether enhanced disease resistance response stemmed from elevated expression of defence‐related genes, we examined their expression by qRT‐PCR. These defence genes mainly consisted of those involved in jasmonic acid (JA) and SA signalling and/or biosynthesis. As components of the JA signalling, JAMyb, PBZ1, and JAZ8 genes (Sathe et al., 2019) were highly expressed in Ri‐1 and Ri‐3 compared with that in DJ. PAL3, PR1a, PR1b, PR3, PR4, and PR10, which are known to be involved in the SA signalling pathway (Park et al., 2012), were all also significantly up‐regulated in Ri‐1 and Ri‐3 (Figure 3a). Notably, in Ri‐1 and Ri‐3, the expression of AOS2, which encodes allene oxide synthase (a key enzyme in the JA biosynthetic pathway), was increased to about 25‐ and 70‐fold higher than that in DJ, respectively; the expression of ICS1, which encodes isochorismate synthase (a key enzyme in the SA biosynthetic pathway), was elevated to about threefold (Figure 3a). Furthermore, the abundance of various forms of SA and JA, including free SA, SA 2‐O‐β‐Glucoside (SAG), free JA, methyl jasmonate (MEJA), N‐[(−)‐jasmonoyl]‐(L)‐valine (JA‐Val), and N‐[(−)‐jasmonoyl]‐(S)‐isoleucine (JA‐Ile, the active form of JA) was stronger in OsUbc13‐RNAi than that in DJ (Figure 3b). Collectively, these data suggest that OsUbc13‐RNAi lines confer enhanced disease resistance by enhancing flg22‐ and chitin‐triggered ROS accumulation and possibly by activating the SA and JA signalling pathways in rice.
Figure 3.

Constitutive expression of several defence‐related genes and contents of defence phytohormones in OsUbc13‐RNAi and DJ. (a) qRT‐PCR was used to analyse the expression of defence‐related genes involved in JA or SA signalling/synthetic pathway. Total RNA was extracted from the leaves of OsUbc13‐RNAi and DJ plants at 30‐day post‐sowing in soil. OsActin1 gene was used as an internal control. Data are shown as means ±SE; n = 3 (**P < 0.01, ***P < 0.001; Student's t‐test). (b) Contents of free salicylic acid (SA), salicylic acid 2‐O‐β‐glucoside (SAG), free jasmonic acid (JA), methyl jasmonate (MEJA), N‐[(−)‐Jasmonoyl]‐(L)‐valine (JA‐Val), and jasmonoyl‐L‐isoleucine (JA‐Ile) in OsUbc13‐RNAi and DJ. The leaves of OsUbc13‐RNAi and DJ plants at 30‐day post‐sowing in soil were used to extract SA, SAG, JA, MEJA, JA‐Val, and JA‐Ile. Data are shown as means ±SE; n = 3 (*P < 0.05, **P < 0.01, ***P < 0.001; Student's t‐test).
OsUbc13 interacts with OsSnRK1a
The identification and in‐depth study of OsUbc13‐interacting proteins are crucial to revealing its molecular mechanisms regulating innate immunity and disease resistance in rice. Coincidentally, in our previous study, 20 potential OsUbc13‐interacting partners have been found through yeast two‐hybrid (Y2H) assay (Wang et al., 2014). Based on predicted protein function and existing literature reports, among these candidate proteins, Os10g0445500 (hypersensitive response‐like lesion‐inducer family protein, named by us as OsHRLI), Os05g0409300 (similar to cysteine protease inhibitor, named by us as OsCPI) and Os08g0199300 (similar to YyaF/YCHF TRANSFAC/OBG family small GTpase plus RNA binding domain TGS, known and named as OsYchF1) may be related to the disease resistance of rice (Cheung et al., 2016; Wang et al., 2014; Zhang et al., 2020a). Os05g053050 (Oryza sativa S‐phase kinase‐associated protein 1‐like protein 1, known and named as OsSnRK1a) has been confirmed to have the function of improving broad‐spectrum disease resistance in rice (Filipe et al., 2018; Wang et al., 2014).
To further confirm the interactions of these four proteins with OsUbc13, we performed point‐to‐point Y2H analysis. The full‐length coding sequence (CDS) of OsUbc13 gene was ligated with the Gal4 DNA‐binding domain of the bait vector pGBKT7 (BK‐OsUbc13); the full‐length CDS of OsHRLI, OsCPI, OsYchF1, or OsSnRK1a gene was fused to the Gal4 activation domain (AD) of the prey vector pGADT7 (AD‐OsHRLI, AD‐OsCPI, AD‐ OsYchF1, and AD‐OsSnRK1a). As shown in Figure 4a, OsUbc13 is only physically associated with OsSnRK1a in the Y2H system, but not with the other three proteins. Next, a firefly split‐luciferase complementation imaging (LCI) assay was carried out to consolidate the evidence for the interaction between the two. The N‐terminal of firefly luciferase (nLuc) was fused with OsSnRK1a, whereas the C‐terminal (cLuc) was connected to OsUbc13. OsSnRK1a‐nLuc and cLuc‐OsUbc13 were transiently co‐expressed in wild‐type tobacco (N. benthamiana) leaves. OsSnRK1a‐nLuc/cLuc and cLuc‐OsUbc13/nLuc were used as negative controls. A strong fluorescence signal was observed in leaves co‐expressing OsSnRK1a‐nLuc and cLuc‐OsUbc13, but not in the leaves expressing plasmids for negative controls (Figure 4b). Furthermore, co‐immunoprecipitation (Co‐IP) assays in tobacco leaves also confirmed that OsUbc13 interacts with OsSnRK1a in vivo, because we found that OsSnRK1a co‐precipitated with OsUbc13‐eGFP but not with the control eGFP (Figure 4c). Bimolecular fluorescence complementation (BiFC) experiments were performed to determine where OsUbc13 and OsSnRK1a interact in plant cells. The N‐ and C‐terminal of yellow fluorescent protein (YFP) were coupled to OsSnRK1a or OsUbc13 (OsSnRK1a‐nYFP/cYFP and OsUbc13‐nYFP/cYFP), respectively. And then, these recombinant vectors were transiently expressed in leaf cells of wild‐type tobacco (N. benthamiana). The strong YFP signal was visualized mainly from the OsSnRK1a‐nYFP/OsUbc13‐cYFP and OsSnRK1a‐cYFP/OsUbc13‐nYFP complementation assay in the cytoplasm and nucleus of the transformed cells, but not in the negative controls (Figure 4d). At the same time, we found that OsSnRK1a was mainly localized to the cytoplasm and nucleus (Figure 4e), which is consistent with previous findings and OsUbc13's localization (Cho et al., 2012; Liu et al., 2021; Wang et al., 2014, 2021). Interaction of OsSnRK1a and OsUbc13 in the yeast or plant cells could be either direct or indirect, possibly involving an E3 ligase. To distinguish between these possibilities, we used a pull‐down assay to examine the binding of OsUbc13 to OsSnRK1a in vitro, in which the 3 × Flag‐OsSnRK1a‐GFP and 8 × His‐OsUbc13‐GFP recombinant proteins were obtained from a cell‐free protein synthesis system (Zhang et al., 2020b). The results showed that the interaction between OsUbc13 and OsSnRK1a does not require other proteins and is direct (Figure 4f). Taken overall, we conclude that OsUbc13 directly interacts with OsSnRK1a both in vivo and in vitro.
Figure 4.

OsUbc13 interacts with OsSnRK1a. (a) Y2H assay. The pGBKT7 plasmid containing the OsUbc13 coding sequence (BK‐OsUbc13) and the pGADT7 plasmid containing the OsHRLI, OsYchF1, OsCPI, and OsSnRK1a coding sequence (AD‐OsHRLI, ‐OsYchF1, ‐OsCPI, ‐OsSnRK1a) were co‐transformed into yeast cells (AH109). Yeast cells co‐transformed with AD‐T/BK‐53 or AD‐T/BK‐Lam vectors were used as the positive or negative control, respectively. Interaction of OsUbc13 with OsSnRK1a was indicated by the ability of yeast cells to grow on dropout medium lacking Leu, Trp, His, and Ade for 5 days after plating. (b) LCI assay. Agrobacterial strains containing different combinations of plasmids were co‐infiltrated into tobacco leaves. A cooled charge‐coupled imaging apparatus was used to capture the images. No signal was obtained for the negative controls in which OsSnRK1a‐nLuc was co‐expressed with cLuc, and cLuc‐OsUbc13 was co‐expressed with nLuc. The pseudocolor bar indicates the range of luminescence intensity. (c) Co‐IP assay. OsUbc‐eGFP and OsSnRK1a were co‐expressed in tobacco leaves to detect the interaction between OsUbc13 and OsSnRK1a. An anti‐GFP affinity matrix was used for immunoprecipitation and anti‐OsSnRK1a was used for immunoblot analysis. Red asterisks indicate nonspecific bands. Experiments were repeated two times with similar results. (d) BiFC assay. Fluorescence was observed in the nuclear compartment of transformed tobacco (N. benthamiana) cells, resulting from the complementation of OsSnRK1a‐nYFP+OsUbc13‐cYFP or OsSnRK1a‐cYFP+OsUbc13‐nYFP. No signal was obtained for the negative controls in which OsHRLI‐nYFP was co‐expressed with OsUbc13‐cYFP, and OsHRLI‐cYFP was co‐expressed with OsUbc13‐nYFP. YFP signal was detected by confocal microscopy. Scale bars = 25 μM. (e) Subcellular localization of OsSnRK1a in leaves of tobacco (N. benthamiana). EGFP, enhanced green fluorescent protein; RFP‐H2B, a nuclear marker. Scale bars = 25 μM. (f) Pull‐down assay. 3 × Flag‐OsSnRK1a‐GFP and 8 × His‐OsUbc13‐GFP recombinant proteins expressed by CFPS (cell‐free protein synthesis) reactions were used in the pull‐down assay. Equal amounts of 3 × Flag‐OsSnRK1a‐GFP and 8 × His‐OsUbc13‐GFP were incubated with His‐tag magnetic beads. Pull‐down products were detected using anti‐His and anti‐Flag antibodies. Experiments were repeated two times with similar results.
The C‐terminal fragment of OsSnRK1a and the 89th cysteine residue of OsUbc13 are responsible for their interaction
As reported previously (Emanuelle et al., 2016; Filipe et al., 2018), the OsSnRK1a protein, consisting of 505 amino acid residues, comprises an amino (N)‐terminal serine/treonine kinase catalytic domain (S_TKc) followed by a ubiquitin‐associated domain (UBA) and a kinase‐associated 1 (KA1) domain at its carboxyl (C)‐terminal (Figure 5a, top). To identify the region of OsSnRK1a essential for the interaction with OsUbc13, we fused six truncated OsSnRK1a variants to the Gal4 AD of the prey vector and examined the interactions of these variants with OsUbc13 by Y2H analysis. As shown in Figure 5a, deletion of the C‐terminal amino acid residues 456–505, 328–505, or 287–505 of OsSnRK1a (AD‐OsSnRK1a1–455, AD‐OsSnRK1a1–327, and AD‐OsSnRK1a1–286) completely abolished the OsUbc13‐OsSnRK1a interaction, whereas only retaining the 219 or 178 residues at the C‐terminus of OsSnRK1a that harbour the KA1 domain (AD‐OsSnRK1a287–505 and AD‐OsSnRK1a328–505) did not affect the OsUbc13‐OsSnRK1a interaction. This result showed that the C‐terminal region of OsSnRK1a is required for its interaction with OsUbc13. Further mapping revealed that 178 amino acids spanning the C‐terminal KA1 domain (OsSnRK1a328–505), rather than just the KA1 domain, are exclusively involved in the OsUbc13‐OsSnRK1a interaction because an OsSnRK1a variant in which the N‐terminal amino acids 1–455 were deleted and only the KA1 domain was retained (AD‐OsSnRK1a456–505) could not physically associate with OsUbc13 (Figure 5a).
Figure 5.

Y2H screening assay to identify necessary sites and regions required for the interaction between OsUbc13 and OsSnRK1a. (a) Interaction test between OsUbc13 and different truncated OsSnRK1a proteins with specific deletion. Top, the schematic diagram of OsSnRK1a protein. S_TKc, serine/treonine kinase catalytic domain; UBA, ubiquitin associated domain (UBA); KA1, kinase‐associated 1 domain. Low, the interaction was indicated by the ability of yeast cells to grow on dropout medium lacking Leu, Trp, His, and Ade for 5 days after plating. Yeast cells co‐transformed with AD‐T/BK‐53 or AD‐T/BK‐Lam vectors were used as the positive or negative control, respectively. (b) Interaction test between OsUbc13(C89G) and OsSnRK1a, or between OsUbc13 and OsSnRK1a(K43M)/(K139R). Interaction was indicated by the ability of yeast cells to grow on dropout medium lacking Leu, Trp, His, and Ade for 5 days after plating. Yeast cells co‐transformed with AD‐T/BK‐53 or AD‐T/BK‐Lam vectors were used as the positive or negative control, respectively. (c) OsUEV1B interacts with OsSnRK1a. Interaction was indicated by the ability of yeast cells to grow on dropout medium lacking Leu, Trp, His, and Ade for 5 days after plating. Yeast cells co‐transformed with AD‐T/BK‐53 or AD‐T/BK‐Lam vectors were used as the positive or negative control, respectively.
Zang et al. (2012) demonstrated that OsUbc13 shows a high degree of conservation with Ubc13s from other eukaryotic organisms. As might be expected with this overall high level of amino acid sequence similarity between OsUbc13 and other Ubc13 homologues, it has all of the functionally important amino acids for biochemical activities of Ubc13, namely, Cys‐89 located in the putative active site for ubiquitin thioester formation, Met‐66, which is involved in the interaction with an E3 ligase, and three pocket residues (Glu‐57, Phe‐59, and Arg‐72) that determine binding specificity for Uev protein (VanDemark et al., 2001; Wooff et al., 2004). The positions of these amino acids are strictly identical to those in Fni3 (Figure S4), a homologue of the Ubc13‐type ubiquitin‐conjugating enzyme in tomato that catalyses exclusively K63‐linked ubiquitination (Mural et al., 2013). Substitution of Cys‐89 in the active site of Fni3 with Gly (Fni3C89G) essentially abolishes the Fen‐Fni3 interaction (Mural et al., 2013). As expected, the substitution of Cys‐89 of OsUbc13 (OsUbc13C89G) almost completely eliminated the interaction between OsUbc13 and OsSnRK1a in the Y2H system (Figure 5b). To validate the protein kinase (PK) function of OsSnRK1a in the OsSnRK1a‐OsUbc13 interaction, inactive forms of OsSnRK1a were prepared as ATP binding site‐mutated PK (OsSnRK1aK43M, substitution of Lys‐43 with Met) and as catalytically inactive PK (OsSnRK1aK139R, substitution of Lys‐139 with Arg) (Cho et al., 2012). As shown in Figure 5b, mutation of Lys‐43 or Lys‐139 in OsSnRK1a had no difference on the interaction between OsSnRK1a and OsUbc13 in the Y2H system. These results suggest that the OsUbc13‐OsSnRK1a interaction may mainly depend on the ubiquitin‐conjugating enzyme activity of OsUbc13 rather than the kinase activity of OsSnRK1a.
Formation of the K63‐linked poly‐Ub chain requires Ubc13 and another Ubc‐like protein named UEV (Hofmann and Pickart, 1999; Romero‐Barrios and Vert, 2018). In our recent study, OsUEV1B has been reported to interact with OsUbc13 and be required for phosphate homeostasis in rice (Liu et al., 2021). According to this, we speculated that OsUEV1B can also associate with OsSnRK1a to assist OsUb13 in the K63‐linked ubiquitination of OsSnRK1a. As shown in Figure 5c, the Y2H assay proved our hypothesis that OsUEV1B does interact with OsSnRK1a. However, unlike the OsUbc13‐RNAi lines, the osuev1b mutant did not show significantly improved resistance to rice blast (Figure S5), which may be related to the functional redundancy of the OsUEV1 protein family. As a potential target of OsUbc13‐OsUEV1B complex, OsVDAC1, a voltage‐gated anion channel protein, functions in maintaining phosphorus homeostasis in rice (Liu et al., 2021), but the osvdac1 mutant also displayed similar blast sensitivity with wild‐type DJ (Figure S5). These findings drove us to focus our next studies on OsSnRK1a.
Silencing OsUbc13 attenuates K63‐linked polyubiquitination of OsSnRK1a and improves SnRK1 activity
Although in a different background, silencing of OsUbc13 by RNAi resulted in phenotypes similar to those of plants overexpressing OsSnRK1a (Filipe et al., 2018), at least with respect to the resistance to M. oryzae and Xoo and the number of tillers per plant (Figures 2b–h and S1b). Given the interaction between OsUbc13 and OsSnRK1a, we speculated that there were at least two possibilities for the emergence of these similar phenotypes. First, the protein level of OsSnRK1a was increased in the OsUbc13‐RNAi line; second, the activity of OsSnRK1a was enhanced. Both will help to increase the activity of the SnRK1 complex. Growing evidence suggests that OE of the α catalytic subunit of the SnRK1 complex can enhance total SnRK1 activity (Jossier et al., 2009; Wang et al., 2020; Yu et al., 2018). In our study, transient expression of the OsSnRK1a‐EGFP fusion protein in tobacco leaves increased the total SnRK1 activity by about 34.05% compared with the expression of empty EGFP alone (Figure S6). To test the first possibility, we detected the protein level of OsSnRK1a using anti‐OsSnRK1a antibody. OsSnRK1a protein level was similar to that of wild‐type DJ in both the interference and OE lines of OsUbc13, and there was no obvious change (Figure 6a). Therefore, the first possibility might be ruled out. Likewise, silencing or OE of OsUbc13 had no significant effect on the transcription of OsSnRK1a (Figure S7).
Figure 6.

Detection of OsSnRK1a protein content, SnRK1 activity, and polyubiquitination degree. (a) The protein level of OsSnRK1a in leaves of DJ, OsUbc13‐RNAi and ‐OE plants at 30‐day post‐sowing in soil was detected using anti‐OsSnRK1a. Coomassie brilliant blue (CBB) staining was used as a loading control. Band intensity was calculated by ImageJ software. Experiments were repeated three times with similar results. (b) The SnRK1 kinase activity in leaves of DJ and OsUbc13‐RNAi plants at 30‐day post‐sowing in soil. Data are shown as means ±SE; n = 6 (*P < 0.05, **P < 0.01; Student's t‐test). (c) In vivo polyubiquitination level of OsSnRK1a in DJ, OsUbc13‐OE line (OE17‐2), and OsUbc13‐RNAi line (Ri‐3). Crude protein extracted from seedlings at 30‐day post‐sowing in soil was immunoprecipitated by anti‐OsSnRK1a antibody and detected using antibodies that specifically recognize K48/K63‐polyubiquitin conjugates (anti‐K48 and K63) and anti‐OsSnRK1a antibody. Experiments were repeated two times with similar results.
To test another possibility, we directly measured SnRK1 activity in seedlings of OsUbc13‐RNAi lines. As shown in Figure 6b, the activity of SnRK1 in Ri‐1 and Ri‐3 was approximately 1.25‐ and 1.37‐fold higher than that of wild‐type DJ, respectively. It has been reported that OE of Arabidopsis SnRK1.1 confers an ABA‐hypersensitive phenotype (Jossier et al., 2009). To assess the ABA sensitivity of OsUbc13‐RNAi lines, we treated Ri‐1 and Ri‐3 seeds on half‐strength Murashige and Skoog (1/2 MS) medium supplemented with or without 3 μM ABA for 8 d. After ABA treatment, the OsUbc13‐RNAi lines had significantly shorter roots and shoots than those of wild‐type DJ, exhibiting hypersensitivity to ABA (Figure S8a,b). Without ABA treatment, the root and shoot lengths of OsUbc13‐RNAi lines were similar to DJ, and the roots of Ri‐1 were even longer than DJ (Figure S8a,c). These results demonstrate that silencing OsUbc13 may increase the activity of OsSnRK1a rather than changing its protein content.
As the two most frequently detected ubiquitination modifications to date, K48‐linked polyubiquitination often leads to the degradation of target protein (Hershko and Ciechanover, 1998; Komander and Rape, 2012; Wickliffe et al., 2011), while K63‐linked polyubiquitination is involved in altering the activity of target protein (Chen and Sun, 2009; Li et al., 2013; Tang et al., 2019; Zhang et al., 2019). Ubc13 and its cofactor UEV are unique among E2 ubiquitin‐conjugating enzymes in that they catalyse exclusively the formation of K63‐linked polyubiquitin chains (Lim and Lim, 2011). In tomatoes, Fni3(Ubc13) and Suv (UEV) are reported to together catalyse K63‐specific polyubiquitination (Mural et al., 2013). Based on these findings, we investigated whether the ubiquitination of OsSnRK1a is mediated by OsUbc13. We purified OsSnRK1a from the leaves of DJ, OsUbc13‐OE (OE17‐2), and OsUbc13‐RNAi (Ri‐3) seedlings using anti‐OsSnRK1a polyclonal antibodies. And then, the immunoprecipitated proteins were immunoblotted with two specific ubiquitin antibodies, K48‐ and K63‐Ub chain antibodies (anti‐K48 and anti‐K63). As shown in Figure 6c, the degree of K63‐linked polyubiquitination of immunoprecipitated OsSnRK1a was indistinguishable in OE17‐2 but was visibly attenuated in Ri‐3, relative to those in DJ. The levels of K48‐linked polyubiquitin chains in all materials were very low and did not differ (Figure 6c), suggesting that down‐regulated expression of OsUbc13 has reduced the specific binding of K63‐linked ubiquitin chains on OsSnRK1a. Taken together, these data show that inhibition of OsUbc13 expression attenuates K63‐linked polyubiquitination on OsSnRK1a, resulting in the enhanced activity of SnRK1, which in turn improves disease resistance.
Deubiquitinating enzyme OsOTUB1.1 also physically interacts with OsSnRK1a
In humans, OTUB1, a deubiquitinating enzyme, forms a complex in vivo with Ubc13 and prevents ubiquitin transfer (Nakada et al., 2010; Wiener et al., 2012). OsOTUB1.1 (Os08g0537800), a homologue of TUB1 in rice, can interact with OsUbc13 and cleave both K48‐ and K63‐linked polyubiquitin (Wang et al., 2017b). More importantly, rice plants overexpressing OsOTUB1.1 exhibit leaf necrosis like OsUbc13‐RNAi lines (Wang et al., 2017b), which prompted us to examine the possibility that OsOTUB1.1 can also interact with OsSnRK1a. As expected, we found an interaction between OsOTUB1.1 and OsSnRK1a in the Y2H system (Figure 7a). To identify the region of OsSnRK1a essential for the interaction with OsOTUB1.1, we fused six truncated OsSnRK1a variants to the prey vector and examined the interaction between these variants and OsOTUB1.1. As shown in Figure 7a, deletion of the C‐terminal amino acid residues 456–505, 328–505, or 287–505 of OsSnRK1a (AD‐OsSnRK1a1–455, AD‐OsSnRK1a1–327, and AD‐OsSnRK1a1–286) completely abolished the OsOTUB1.1‐OsSnRK1a interaction, whereas only retaining the 219 or 178 residues at the C‐terminus of OsSnRK1a that harbour the KA1 domain (AD‐OsSnRK1a287–505 and AD‐OsSnRK1a328–505) did not affect the OsOTUB1.1‐OsSnRK1a interaction. This result showed that the C‐terminal region of OsSnRK1a is also required for its interaction with OsOTUB1.1. Further mapping revealed that 178 amino acids spanning the C‐terminal KA1 domain (OsSnRK1a328–505), rather than just the KA1 domain, are exclusively involved in the OsOTUB1.1‐OsSnRK1a interaction because an OsSnRK1a variant in which the N‐terminal amino acids 1–455 were deleted and only the KA1 domain was retained (AD‐OsSnRK1a456–505) could not physically associate with OsOTUB1.1 (Figure 7a). These findings in Y2H assay indicate that the key region of interaction between OsOTUB1.1 and OsSnRK1a is consistent with that of the OsUbc13‐OsSnRK1a interaction.
Figure 7.

OsOTUB1.1 interacts with OsSnRK1a. (a) Interaction test between OsOTUB1.1 and different truncated OsSnRK1a proteins with specific deletion. Top, the schematic diagram of OsSnRK1a protein. S_TKc, serine/treonine kinase catalytic domain; UBA, ubiquitin‐associated domain; KA1, kinase‐associated 1 domain. Low, the interaction was indicated by the ability of yeast cells to grow on dropout medium lacking Leu, Trp, His, and Ade for 5 days after plating. Yeast cells co‐transformed with AD‐T/BK‐53 or AD‐T/BK‐Lam vectors were used as the positive or negative control, respectively. (b) BiFC assay. Fluorescence was observed in the nuclear compartment of transformed tobacco (N. benthamiana) cells, resulting from the complementation of OsSnRK1a‐nYFP+OsOTUB1.1‐cYFP or OsSnRK1a‐cYFP+OsOTUB1.1‐nYFP. No signal was obtained for the negative controls in which OsSnRK1a1–455‐nYFP was co‐expressed with OsOTUB1.1‐cYFP, and OsSnRK1a1–455‐cYFP was co‐expressed with OsOTUB1.1‐nYFP. YFP signal was detected by confocal microscopy. Scale bars = 25 μM. (c) LCI assay. Agrobacterial strains containing different combinations of plasmids were co‐infiltrated into tobacco leaves. A cooled charge‐coupled imaging apparatus was used to capture the images. No signal was obtained for the negative controls in which OsSnRK1a‐nLuc was co‐expressed with cLuc, and cLuc‐OsOTUB1.1 was co‐expressed with nLuc. The pseudocolor bar indicates the range of luminescence intensity.
BiFC assay was performed to determine whether and where OsOTUB1.1 and OsSnRK1a interact in plant cells. The N‐ or C‐terminal of YFP was coupled to OsOTUB1.1 (OsOTUB1.1‐nYFP/cYFP), respectively. And then, these newly constructed recombinant vectors were transiently expressed with OsSnRK1a‐n/cYFP in tobacco leaves. The strong YFP signal was visualized mainly in the OsSnRK1a‐nYFP/OsOTUB1.1‐cYFP and OsSnRK1a‐cYFP/OsOTUB1.1‐nYFP complementation assay in the cytoplasm and nucleus of the transformed cells, but not in the negative controls (Figure 7b). Furthermore, LCI assay was carried out to further confirm the interaction in plants. The cLuc was connected to OsOTUB1.1. OsSnRK1a‐nLuc and cLuc‐OsOTUB1.1 were transiently co‐expressed in tobacco leaves. A strong fluorescence signal was observed in leaves co‐expressing OsSnRK1a‐nLuc and cLuc‐OsOTUB1.1, but not in the leaves expressing plasmids for negative controls (Figure 7c). Together, these results demonstrate that OsOTUB1.1 also interacts with OsSnRK1a in planta.
OsOTUB1. 1‐OE lines confer enhanced resistance to Magnaporthe oryzae, increased SnRK1 activity, and attenuated K63 ubiquitination of OsSnRK1a
Considering that OsOTUB1.1 can also interact with OsSnRK1a, we wondered whether OsOTUB1.1 is involved in rice disease resistance. First, we obtained two independent OsOTUB1.1‐ OE lines (OsOTUB1.1‐OE‐1 and OsOTUB1.1‐OE‐2, Figure S9a) in the background of Zhonghua 11 (ZH11, Wang et al., 2017b). Then, we inoculated them with M. oryzae isolate GUY11 by the punch method, and we found that the two OsOTUB1.1‐OE lines both increased disease resistance to M. oryzae, which showed a smaller lesion area and less fungal biomass than the wild‐type ZH11 (Figure 8a–c). To ascertain whether enhanced disease resistance response stemmed from elevated expression of PR genes, we examined their expression by qRT‐PCR. As components of JA signalling or biosynthesis, WRKY45, JAMyb, JAZ8, PBZ1, and AOS2 genes (Sathe et al., 2019) were significantly up‐regulated in the two OsOTUB1.1‐OE lines compared with ZH11 (Figure S9b–f). Most of the SA‐signalling genes, PAL3, PR1a, PR3, PR4, PR10, PR4, and PR10 were highly expressed in OsOTUB1.1‐OE lines compared with ZH11 (Figure S9g–m). Collectively, these data suggest that, similar to the OsUbc13‐RNAi lines, the OsOTUB1.1‐OE lines are likely to elevate disease resistance by activating SA and JA signalling pathways in rice.
Figure 8.

The OsOTUB1.1‐OE lines display enhanced resistance to M. oryzae. (a) The lesions on ZH11 and OsOTUB1.1‐OE leaves at 8 days after punch inoculation with the compatible M. oryzae isolate GUY11. Scale bar = 1 cm. (b) Relative lesion area (%) in leaves of (a) indicates significant differences between ZH11 and OsOTUB1.1‐OE. Data are shown as means ±SE; n = 12 (***P < 0.001; Student's t‐test). (c) Relative fungal biomass, measured as MoPot2 by qRT‐PCR, in leaves of (a) was normalized to OsUbq DNA (Park et al., 2012). Data are shown as means ± SE; n = 3 (**P < 0.01; Student's t‐test). (d) The protein level of OsSnRK1a in leaves of ZH11 and OsOTUB1.1‐OE plants at 30‐day post‐sowing in soil was detected using anti‐OsSnRK1a. Coomassie brilliant blue (CBB) staining was used as a loading control. Band intensity was calculated by ImageJ software. Experiments were repeated three times with similar results. (e) The SnRK1 kinase activity in leaves of ZH11 and OsOTUB1.1‐OE plants at 30‐day post‐sowing in soil. Data are shown as means ±SE; n = 3 (*P < 0.05; Student's t‐test). (f) In vivo polyubiquitination level of OsSnRK1a in ZH11 and OsOTUB1.1‐OE lines. Crude protein extracted from seedlings at 30‐day post‐sowing in soil was immunoprecipitated by anti‐OsSnRK1a antibody and detected using antibodies that specifically recognize K48/K63‐polyubiquitin conjugates (anti‐K48 and K63) and anti‐OsSnRK1a antibody. Experiments were repeated three times with similar results.
Although in a different background, OE of OsOTUB1.1 also showed identical phenotypes to those of plants overexpressing OsSnRK1a, in terms of the resistance to M. oryzae, plant height, and the number of tillers per plant (Filipe et al., 2018; Wang et al., 2017b). We speculated that there are two possibilities for the appearance of the above phenotypes, the protein level or the activity of OsSnRK1a was increased in the OsOTUB1.1‐OE line. Because there was no difference in the abundance of the OsSnRK1a transcript between ZH11 and OsOTUB1.1‐OE lines (Figure S9n), so we assayed the protein contents of OsSnRK1a using anti‐OsSnRK1a antibody. As shown in Figure 8d, the level of OsSnRK1a protein was not significantly increased in the OsOTUB1.1‐OE line compared with wild‐type ZH11, ruling out one of these possibilities. The other possibility was investigated by measuring SnRK1 activity in seedlings of OsOTUB1.1‐RNAi lines. As shown in Figure 8e, the SnRK1activity of OsOTUB1.1‐OE‐1/2 was approximately 1.45‐fold higher than that of wild‐type ZH11. And then, the immunoprecipitated OsSnRK1a proteins from ZH11 and the two OsOTUB1.1‐OE seedlings were immunoblotted using anti‐K48 and anti‐K63 antibodies. The degree of K63‐linked polyubiquitination of OsSnRK1a was visibly attenuated in OsOTUB1.1‐OE lines. The levels of K48‐linked polyubiquitin chains in all materials were very low and did not differ (Figure 8f). Collectively, the above results prove that, like inhibiting OsUbc13, overexpressing OsOTUB1.1 reduces the K63‐linked ubiquitin chains on OsSnRK1a, enhances SnRK1 activity, and in turn improves rice blast resistance.
OsUbc13 affects rice blast resistance partially dependent on OsSnRK1a
To provide genetic evidence for the regulation of OsSnRK1a by OsUbc13, we obtained double RNAi lines by transforming the OsSnRK1a‐RNAi plasmids into one OsUbc13‐RNAi line (Ri‐3), namely US‐dRNAi. Two independent transgenic lines, US‐dRNAi‐5 and US‐dRNAi‐7, were identified. The qRT‐PCR confirmed the decrease in target transcript abundance in the US‐dRNAi lines (Figure 9a). OsSnRK1a protein levels in the US‐dRNAi lines were also significantly lower than that in DJ (Figure 9b). Then, we inoculated them with M. oryzae isolate GUY11 by the punch method, and we found that down‐regulation of OsSnRK1a in US‐dRNAi plants resulted in decreased disease resistance to M. oryzae to a level intermediate to those of OsUbc13‐RNAi lines and wild‐type DJ (Figure 9c). Statistically, the two US‐dRNAi lines displayed smaller lesion areas and less fungal biomass compared with wild‐type DJ, but these did not reach the levels of OsUbc13‐RNAi (Figure 9d,e). Thus, genetically decreasing OsSnRK1a expression partially suppressed rice blast resistance phenotypes of OsUbc13‐RNAi lines in rice.
Figure 9.
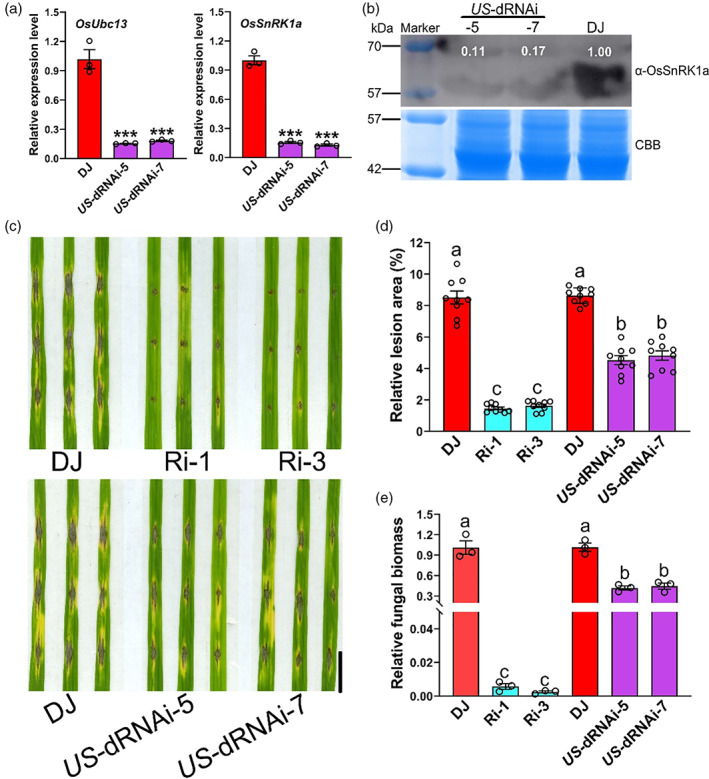
Repression of OsSnRK1a partially reduces the higher resistance to M. oryzae in OsUbc13‐RNAi line (Ri‐3). (a) Expression levels of OsUbc13 and OsSnRK1a in DJ and US‐dRNAi plants. The double RNA interference materials named US‐dRNAi were obtained from the re‐interference of OsSnRK1a in the OsUbc13‐RNAi homozygous line (Ri‐3). OsActin1 gene was used as an internal control. Data are shown as means ±SE; n = 3 (***P < 0.001; Student's t‐test). (b) The protein level of OsSnRK1a in leaves of DJ and US‐dRNAi plants at 30‐day post‐sowing in soil was detected using anti‐OsSnRK1a. Coomassie brilliant blue (CBB) staining was used as a loading control. Experiments were repeated two times with similar results. (c) The lesions on DJ, OsUbc13‐RNAi, and US‐dRNAi leaves at 8 days after punch inoculation with the compatible M. oryzae isolate GUY11. Scale bar = 1 cm. (d) Relative lesion area (%) in leaves of (b) indicates significant differences between DJ, OsUbc13‐RNAi, and US‐dRNAi. Data are shown as means ±SE; n = 9. Significant difference was determined by ANOVA; values with different letters indicate a significant difference from each other (P < 0.05). (e) Relative fungal biomass, measured as MoPot2 by qRT‐PCR, in leaves of (b) was normalized to OsUbq DNA (reference). Data are shown as means ±SE; n = 3 (**P < 0.01; Student's t‐test).
Discussion
Ubiquitin‐conjugating enzyme (Ubc) 13 plays a key role for the regulation of both innate and adaptive immune responses in mammalian immune systems (Jiang and Chen, 2011). Consequently, the Ubc13 protein has become a target of pathogens in suppressing host immunity (Sanada et al., 2012). Compared with its well‐studied mammalian ortholog, the function of Ubc13 in plants is less explored. Fni3, the homologue of mammalian Ubc13 in tomato, is solely responsible for catalysing Lys63(K63)‐linked ubiquitination and has been reported to regulate HR‐related PCD and plays a role in ETI (Mural et al., 2013). Arabidopsis Ubc13 differentially regulates two PCD pathways in responses to pathogen and low‐temperature stress (Wang et al., 2019), suggesting that Ubc13 has a conserved function to regulate immune responses in different plants. In addition to immunity, plant Ubc13 (Li and Schmidt, 2010; Wen et al., 2006, 2014; Yin et al., 2007) and Ubc13‐mediated K63 polyubiquitination (Johnson and Vert, 2016; Martins et al., 2015; Romero‐Barrios and Vert, 2018; Tomanov et al., 2014) have been implicated in numerous biological processes.
The rice genome only encodes one Ubc13 protein named OsUbc13. However, its function in rice immunity and disease resistance has not been fully understood. In the present study, we first found that silencing the OsUbc13 gene in the DongJin (DJ) background triggers a series of immune responses, including typical necrotic lesions, cell death, and ROS burst (Figure 1). Additionally, two key agronomic traits including tiller number and grain number per panicle were adversely affected in OsUbc13‐RNAi plants (Figure S1b,c), which is consistent with perturbation of the OsUbc13 gene in the Zhonghua11 (ZH11) background by another research group (Wang et al., 2017b). Next, through personal communication with the first author of this article (Wang et al., 2017b), we learned that disrupting the OsUbc13 gene in ZH11 also leads to a lesion‐mimic phenotype, further consolidating our results. We also tried to knockout OsUbc13 using the CRISPR/Cas9 method, but all the transgenic lines of T0 generation died at the seedling stage. Moreover, it has been reported that OsUbc13 is a candidate housekeeping gene (Zang et al., 2012), so it is possible that the knockout of OsUbc13 is lethal and the normal growth of rice must depend on a functional OsUbc13.
In plants, for example, enhanced defence is often accompanied by a ‘syndrome’ of compromised morphology such as growth retardation, male sterility, and reduced plant size. In other words, such compromised morphology often results in immunity activation as observed in transgenic rice overexpressing OsWRKY45 and Ideal Plant Architecture1 , knockdown of miR156, and knockout of OsDOF11 or OsALDH2B1, which is often referred to as trade‐offs between growth and defence (Goto et al., 2015; Ke et al., 2020; Li et al., 2016; Wu et al., 2018; Yang et al., 2012). So, the reduction of tiller numbers and grain numbers per panicle in the OsUbc13‐RNAi lines was probably caused by autoactivation of immune responses. Arabidopsis ubc13 double mutant has similar immune responses with the OsUbc13‐RNAi lines. However, these responses of ubc13 are induced by low temperature, which only appears when grown at 16 °C, not at 22 °C (Wang et al., 2019). In addition to ROS bursts and cell death, many lesion‐mimic mutants exhibit enhanced disease resistance (Ma et al., 2019; Qiao et al., 2010; Qiu et al., 2021; Yamanouchi et al., 2002). Unlike typical lesion‐mimic mutants, Arabidopsis ubc13 double mutant did not enhance disease resistance against virulent bacterial and fungal pathogens, but diminished HR and compromised ETI against avirulent bacterial pathogens (Wang et al., 2019). Unexpectedly, in our study, the OsUbc13‐RNAi lines were found to show enhanced resistance to both fungal and bacterial pathogens, accompanied by up‐regulated expression of defence‐related genes and elevated levels of defence hormones (Figures 2 and 3). Although both homologous Ubc13s are involved in autoimmune responses, their contributions to disease resistance in Arabidopsis (dicot) and rice (monocot) are diametrically opposed, demonstrating the complexity of Ubc13's regulatory mechanisms on immune responses. Similarly, there are differences in the NPR1‐mediated PCD and resistance in Arabidopsis and rice. In Arabidopsis, AtNPR1 is a negative regulator of PCD (Fu et al., 2012; Furniss and Spoel, 2015). By contrast, OsNPR1 positively regulates PCD in rice (Bai et al., 2011; Chern et al., 2005; Liu et al., 2017; Yuan et al., 2007).
Sucrose non‐fermenting 1 (SNF1)‐related kinase 1 (SnRK1) is a central energy sensor kinase in plants that is functionally and evolutionarily conserved with SNF1 in yeast and AMP‐activated kinase (AMPK) in animals (Baena‐González and Lunn, 2020; Broeckx et al., 2016; Crepin and Rolland, 2019; Crozet et al., 2014). The eukaryotic AMPK/SNF1/SnRK1 protein kinases typically function as heterotrimeric complexes composed of one α‐catalytic subunit and two regulatory subunits, β and γ (Broeckx et al., 2016; Crozet et al., 2014). Several recent studies have shown that SnRK1 in plants is involved in various metabolic pathways (Cai, 2020; Hu et al., 2022; Liang et al., 2021; Wang et al., 2020, 2021; Zhang et al., 2020c). Notably, increasing evidence indicates that SnRK1 also plays an important role in plant biotic interactions (Han et al., 2020; Hao et al., 2003; Jiang et al., 2020; Shen et al., 2011; Shen and Hanley‐Bowdoin, 2021). In rice, the three genes encoding α‐catalytic subunit of SnRK1 are classified into two subfamilies, namely OsSnRK1a (OSK1) and OsSnRK1b (OSK24 and OSK35). Among them, OSK35 positively regulates defence against M. oryzae and Xoo (Kim et al., 2015). Recently, OsSnRK1a has been reported to confer broad‐spectrum disease resistance and act as a master switch that regulates growth‐immunity trade‐offs in rice (Filipe et al., 2018), but the mechanisms of OsSnRK1a‐mediated immune regulation remain unknown.
Wang et al., (2014) identified that OsSnRK1a may be a potential interacting protein of OsUbc13. Based on Y2H, LCI, Co‐IP, and BiFC analysis, we confirmed that OsUbc13 associates with OsSnRK1a in vivo (Figure 4a–d). Conventionally, ubiquitin‐conjugating enzymes (Ubcs or E2s) are thought to interact primarily with ubiquitin ligases (E3s). Nevertheless, E2 enzymes have been shown in several cases to interact with substrate proteins of ubiquitination (Laine et al., 2006; Mural et al., 2013; Shembade et al., 2007). In vitro pull‐down assay showed that the interaction between OsUbc13 and OsSnRK1a is direct (Figure 4e). Coincidentally, a previous literature also reported that Fni3, an ortholog of OsUbc13 in tomato (Figure S4), directly binds a protein kinase Fen and catalyses exclusively K63‐linked ubiquitination (Mural et al., 2013). Further analysis revealed that 178 amino acids containing the C‐terminal KA1 domain of OsSnRK1a (OsSnRK1a328–505), rather than just the KA1 domain, are essential for the OsUbc13‐OsSnRK1a interaction (Figure 5a). Substitution of Cys‐89 (the active site for ubiquitin thioester formation) in Fni3 and OsUbc13 with Gly essentially abolishes their interactions with Fen or OsSnRK1a (Mural et al., 2013; Figure 5b). However, as a protein kinase (PK), substitution of Lys‐43 (ATP binding site for PK) or Lys‐139 (catalytic site for PK) in OsSnRK1a (Cho et al., 2012) does not affect its interaction with OsUbc13 (Figure 5b), indicating that their binding may be more dependent on the ubiquitin‐conjugating enzyme activity of OsUbc13 rather than the kinase activity of OsSnRK1a.
Formation of K63‐linked polyubiquitination requires specific dimeric complex formed by Ubc13 and another Ubc‐E2 variant (UEV) protein because Ubc13 lacks active cysteine residues found in other E2s (Broomfield et al., 1998; Hofmann and Pickart, 1999; Romero‐Barrios and Vert, 2018). In our previous study, it was found that OsUEV1B, an Ubc enzyme variant protein, interacts with OsUbc13 and is required for phosphate homeostasis in rice (Liu et al., 2021). As expected, OsUEV1B was also able to bind to OsSnRK1a in the Y2H system (Figure 5c). However, unlike the OsUbc13‐RNAi lines, the osuev1b mutant did not exhibit stronger blast resistance than wild‐type DJ (Figure S5), probably due to functional redundancy among the four members of the OsUEV1 family (named OsUEV1A‐D) in rice, whereas OsUbc13 is unique (Wang et al., 2017a; Zang et al., 2012). These findings imply that the enhanced disease resistance after interfering with OsUbc13 is likely related to the altered ubiquitination level of OsSnRK1a.
Considering that the effects of down‐expression of OsUbc13 or over‐expression of OsSnRK1a on rice disease resistance are consistent, we speculate that the protein level or activity of OsSnRK1a will be elevated in OsUbc13‐RNAi lines. By western blot analysis, we found that the protein content of OsSnRK1a did not change significantly in both the OsUbc13 interference and OE lines, and the same was true at the transcriptional level (Figures 6a and S7). OsSnRK1a encodes the α catalytic subunit of SnRK1, and it has been reported that OE of Arabidopsis SnRK1.1 confers higher SnRK1 activity an ABA‐hypersensitive phenotype (Jossier et al., 2009). Excitingly, in the present study, SnRK1 activity (as indicated by OsSnRK1a activity), was significantly improved in the OsUbc13‐RNAi lines, and it was accompanied by increased ABA sensitivity (Figures 6b and S8). Unlike conventional K48‐linked ubiquitination that mainly serves as a signal for 26S proteasome‐mediated degradation of substrate proteins, K63‐linked polyubiquitination has been shown to play nonproteolytic, regulatory roles in various cellular processes (Bhoj and Chen, 2009; Duncan et al., 2006; Fisk and Yaffe, 1999; Lauwers et al., 2009; Martinez‐Forero et al., 2009). So far, Ubc13 is the only known E2 that mediates the polyubiquitination linked by K63, and K63‐linked polyubiquitination, similar to phosphorylation or sumoylation, changes the activities of target proteins (Pickart, 2001). In recent years, there have been such reports. For example, HECTD3, a new E3 ubiquitin ligase, interacts with caspase‐8 death effector domains and ubiquitinates caspase‐8 with K63‐linked polyubiquitin chains that do not target caspase‐8 for degradation but decrease the caspase‐8 activation (Li et al., 2013). K63‐linked ubiquitination suppresses RIPK1 kinase activity to regulate cell death during embryogenesis and inflammation (Tang et al., 2019; Zhang et al., 2019). Using two specific ubiquitin antibodies, K48‐ and K63‐Ub chain antibodies (anti‐K48 and anti‐K63), we found the degree of K63‐linked polyubiquitination for OsSnRK1a in Ri‐3 (one OsUbc13‐RNAi line) was visibly attenuated, relative to those in DJ. The levels of K48‐linked polyubiquitin chains did not differ (Figure 6c). Taken together, these data support our hypothesis that down‐regulation of OsUbc13 enhances the kinase activity of OsSnRK1a, most likely by attenuating K63‐linked polyubiquitination of OsSnRK1a. Substitution of the active ubiquitination site (K276R) in AtPIP2;1 significantly reduces its ubiquitination (Chen et al., 2021). According to previous research on rice ubiquitome (Xie et al., 2015), there are two predicted ubiquitinated lysine motifs (EKub) in the KA1 domain of OsSnRK1a, at positions 458 and 476. Further LC–MS/MS and point mutation analysis would be worthwhile to determine whether these two lysine residues (K458 and K476) are active ubiquitination sites and their effects on ubiquitination and activity of OsSnRK1a.
Surprisingly, our further study revealed that OsOTUB1.1, a deubiquitinase known to interact with OsUbc13 for cleaving both K48‐ and K63‐linked polyubiquitin, also binds to OsSnRK1a, and even the key regions required for their bindings are consistent (Figure 7). Rice plants overexpressing OsOTUB1.1 exhibit similar phenotypes with OsUbc13‐RNAi lines, including leaf necrosis (Wang et al., 2017b). Phenotypic identification and qRT‐PCR results showed that OE of OsOTUB1.1 (Figure S9a) also significantly increased rice resistance to M. oryzae and the expression of defence‐related genes (Figures 8a–c and S9b–m). Likewise, OE of OsOTUB1.1 increased SnRK1 activity and reduced the K63‐linked polyubiquitination of OsSnRK1a, also having little effect on OsSnRK1a at the transcriptional and translational levels (Figures 8d–f and S9n). These results further suggest that K63‐linked polyubiquitination inhibits the activity of OsSnRK1a, which can be alleviated by down‐regulating OsUbc13 or up‐regulating OsOTUB1.1. Further investigation is warranted to determine whether OsUbc13 and OsOTUB1.1 competitively interact with OsSnRK1a and their relationship in rice immunity.
After re‐interfering with OsSnRK1a in one OsUbc13‐RNAi line (Ri‐3), the M. oryzae resistance of the double‐interfering materials (name US‐dRNAi) was reduced to an intermediate level between the wild‐type DJ and OsUbc13‐RNAi (Figure 9), providing genetic evidence that OsUbc13 negatively regulates blast resistance partially via OsSnRK1a. We present a possible working model for OsUbc13‐OsSnRK1a module based on our results and those of previous reports (Figure 10). In wild‐type DJ plants, K63‐linked polyubiquitination of OsSnRK1a is maintained at high levels by interacting with OsUbc13, resulting in inhibition of its activity and, thus, becoming susceptible to M. oryzae. However, in the OsUbc13‐RNAi plants, K63‐linked polyubiquitination of OsSnRK1a is attenuated, increasing its activity, which in turn enhances resistance to M. oryzae. A major task in understanding Ubc13‐mediated K63 polyubiquitination in plants and its biological relevance is to identify its cognate E3s. So far, in Arabidopsis, only one RING domain‐containing protein, RGLG2, and another F‐box protein, CPR1, have been identified to interact with Ubc13 and function in apical dominance and immunity (Wang et al., 2019; Yin et al., 2007). Yeast two‐hybrid analyses using the U‐box‐domain regions of armadillo (ARM)‐U‐box E3 Ub‐ligases and the Ub‐conjugating (UBC) domains of E2s showed that, among 40 rice E2s, 11 E2s accounted for 70% of the interactions with 17 ARM‐U‐box E3s. But none of the ARM‐U‐box E3 had been identified to interact with OsUbc13 (Bae and Kim, 2014). In this study, we have identified OsUbc13 capable of direct interaction with OsSnRK1a in vitro, indicating that ubiquitination of the latter by the former may be independent of additional E3, which needs to be confirmed by in vitro ubiquitination assay. Silencing of OsSnRK1a in the Ri‐3 only partially rescued the phenotype of blast resistance. Identifying other proteins that interact with OsUbc13 will help us to understand OsUbc13‐mediated immunity responses and disease resistance in rice more comprehensively.
Figure 10.

A proposed model for OsUbc13‐mediated blast resistance. In wild‐type DJ plants, OsUbc13 interacts with OsSnRK1a, resulting in high levels of K63‐linked polyubiquitination on OsSnRK1a, inhibition of its activity, and becoming susceptible to M. oryzae. In the OsUbc13‐RNAi plants, a small amount of OsUbc13 does not allow more K63‐linked polyubiquitination on OsSnRK1a, leading to increased activity of OsSnRK1a and enhanced resistance to M. oryzae.
Materials and methods
Plant materials and growth conditions
The OsUbc13‐RNAi and OsUbc13‐OE transgenic plants, as well as previously obtained osuev1b and osvdac1 mutants (Liu et al., 2021) were generated in the background of Oryza sativa ssp. japonica cv. DongJin (DJ). The OsOTUB1.1‐OE lines in the background of Oryza sativa ssp. japonica cv. Zhonghua11 (ZH11) were kindly provided by Prof. Xiangdong Fu and Dr. Shuansuo Wang (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences). The double RNA interference materials named US‐dRNAi were obtained from the re‐interference of OsSnRK1a in the OsUbc13‐RNAi homozygous line (Ri‐3). For disease and other phenotype characterizations, the sterilized rice seeds were germinated on 1/2 Murashige & Skoog (MS) medium. Seedlings were grown in a greenhouse under 12‐h/12‐h days (30 °C)/nights (25 °C) photoperiod at a photon density of approximately 200 mmol/m2/s and approximately 60% humidity, or in the experimental field of Fujian Agriculture and Forestry University (Sanming, China).
Expression constructs and their transformation in plants
The RNAi constructs were obtained by subcloning a fragment (~200 bp) from the coding sequence (CDS) of OsUbc13 or OsSnRK1a into the pTCK303 vector (Wang et al., 2004) in the sense and antisense orientations, respectively. The full‐length CDS of OsUbc13 (462 bp) was cloned into the modified pCAMBIA1300 vector (target gene driven by the maize Ub gene promoter) to generate OE constructs. The resulting constructs were transformed into DJ or Ri‐3 by Agrobacterium tumefaciens‐mediated transformation. The transformants were screened by PCR amplification using primers specific for HYG or G148. The primers used are listed in Table S1.
RNA extraction and quantitative reverse transcription PCR analysis
RNA extraction and quantitative real‐time PCR analysis were performed as previously described (Liu et al., 2016). Briefly, fresh plant tissues were harvested and immediately ground into a fine powder in liquid nitrogen. Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. The DNase‐treated RNA was reverse transcribed using SuperScript III reverse transcriptase (Invitrogen) according to the manufacturer's instructions. Quantitative reverse transcription PCR (qRT‐PCR) was performed in an optical 96‐well plate using SYBR Premix Ex Taq (TaKaRa) and the CFX96 Real‐Time PCR Detection System (Bio‐Rad). The PCR thermal cycling protocol was 95 °C for 10 s, followed by 40 cycles at 95 °C for 5 s and 60 °C for 30 s. OsActin1 gene was used as the internal reference, and data analyses were performed using the 2−ΔΔCt method as described previously (Livak and Schmittgen, 2001).
DAB staining for H2O2 in leaves
3,3′‐Diaminobenzidine (DAB, Sigma‐Aldrich) staining was performed following a published method with some modifications (Liu et al., 2018). Briefly, leaves (second from the top) at 30‐day post‐sowing in soil were detached and immersed in 1% DAB solution in HCl‐acidified (pH 3.8). After 30 min under vacuum, the samples were incubated at room temperature for 24 h in the dark. The samples were then bleached by boiling in ethanol to remove the chlorophyll and reveal the brown spots, which are indicative of the reaction of DAB with H2O2. The samples were observed and imaged under a scanner.
Measurement of H2O2 contents
H2O2 contents were measured as previously described with some modifications (Liu et al., 2018). Briefly, leaves (second from the top) at 30‐day post‐sowing in soil were used to measure H2O2 contents. The contents were measured spectrophotometrically after reaction with potassium iodide (KI). The reaction mixture consisted of 0.5 mL of 0.1% trichloroacetic acid (TCA), leaf extract supernatant, 0.5 mL of 100 mM potassium phosphate buffer (pH 7.8) and 1 mL reagent (1 M KI, w/v in fresh double‐distilled water). The blank control consisted of 1 mL 0.1% TCA and 1 mL KI in the absence of leaf extract. After 1 h of reaction in darkness, the absorbance was measured at 390 nm. The amount of H2O2 was calculated using a standard curve prepared with known concentrations of H2O2.
Measurement of reactive oxygen species burst
ROS burst was measured following PAMP treatment (flg22 and chitin) as previously described (Liu et al., 2017; Park et al., 2012; Schwacke and Hager, 1992). Briefly, leaves (second from the top) at 12‐day post‐sowing in nutrient solution were punched into disks (0.25 cm2), which were submerged in distilled water overnight. Three disks per sample were then placed in a 1.5‐mL microcentrifuge tube containing 100 mL luminol (Sigma‐Aldrich), 1 mL horseradish peroxidase (Sigma‐Aldrich), and 100 nM flg22 (Phytotech) or 8 nM hexa‐N‐acetyl‐chitohexaose (APExBIO); distilled water was used as a control. The luminescence was immediately measured at 10‐s intervals over a period of 20 min or 41 min in a Glomax 20/20 luminometer (Promega). Three biological replications (three disks for each replication) were performed for each sample of the three treatments.
Measurement of malondialdehyde and photosynthetic pigment
The seedlings at 30‐day post‐sowing in soil were used to measure MDA and photosynthetic pigment. The contents of chlorophyll a, chlorophyll b, and carotenoids were determined according to He et al. (2018). The contents of malonaldehyde (MDA) were measured following the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Pathogen infection
To evaluate rice blast disease resistance, the clipped leaves (second from the top) or the whole seedlings at 20‐day post‐sowing in nutrient solution were inoculated with M. oryzae strain GUY11 (Li et al., 2017; Liu et al., 2017). GUY11 was gown on complete agar medium for 2 weeks before producing spores. Spores were collected via flooding of the fungal agar cultures with sterile water, and the spore concentration in the suspension was adjusted to 1 × 105 conidia/mL before inoculation. For the punch method, we dip 5 μL spore suspension for each drop using a pipette at 3 spots on each leaf which was wounded with a mouse ear punch and then put them in a culture dish that contains 0.1% 6‐benzylaminopurine (6‐BA) sterile water to keep moist (Ono et al., 2001; Park et al., 2012). For spraying method, the whole seedlings were sprayed with spore suspension. The inoculated leaves or seedlings were transferred to a growth chamber with a photoperiod of 12 h light and 12 h dark, 28 °C, and 80% relative humidity, and lesion areas were measured using a scanner and ImageJ software after inoculation for 8 days. Relative fungal DNA amount was calculated using the threshold cycle value (Ct) of M. oryzae Pot2 DNA against the Ct of rice genomic ubiquitin DNA (Park et al., 2012).
To evaluate bacterial blight disease, rice plants were inoculated with Xoo by the leaf‐clipping method as previously described (Liu et al., 2017). The PXO99 strain was separately suspended in distilled water and adjusted to 109 viable cells/mL (OD600 = 1). Scissors were dipped into the bacterial suspensions and then used to remove the distal tips (5 cm) of flag leaves. At least three individual plants and three tillers of each plant were inoculated. The infected plants were grown in a greenhouse under 12‐h/12‐h days (30 °C)/nights (25 °C) photoperiod at a photon density of approximately 200 mmol/m2/s and approximately 60% humidity. Disease was scored by measuring the lesion length at 18 days post‐inoculation.
Plant hormone analysis
The quantification of plant hormones was performed as previously described (Dobrev and Vankova, 2012; Liu et al., 2020). In brief, 3 g of plant leaves at 30 days after sowing in soil were rapidly frozen in liquid nitrogen and homogenized into a powder, and the powder was extracted with 1 mL methanol containing 20% water at 4 °C for 12 h. The extract was centrifuged at 12000 g under 4 °C for 15 min. The supernatant was collected and evaporated to dryness under nitrogen gas stream and reconstituted in 100 mL of acetonitrile containing 5% water. The solution was centrifuged, and the supernatant was collected for analysis using an LC‐ESI‐MS/MS system (HPLC, Shim‐pack UFLC SHIMADZU CBM30A system; MS, Applied Biosystems 4500 Q TRAP). The experiments were performed by Wuhan Metware Biotechnology Co., Ltd (Wuhan, China).
Y2H assay
The full‐length CDS of OsUbc13, OsUbc13 C89G and OsOTUB1.1 was fused to pGBKT7 (Clontech) to generate bait vectors (BK‐OsUbc13 and BK‐OsOTUB1.1) that contain the Gal4 DNA‐BD. Full‐length CDS of OsHRLI, OsCPI, OsYchF1, OsSnRK1a, OsSnRK1a K43M , and OsSnRK1a K139R was inserted into pGADT7 (Clontech) to produce prey vectors (AD‐OsHRLI, AD‐OsCPI, AD‐OsYchF1, AD‐OsSnRK1a, AD‐OsSnRK1aK43M, and OsSnRK1aK139R) with the Gal4 DNA‐AD. To identify specific regions critical for the interactions, multiple truncated OsSnRK1a sequences were ligated with pGADT7. Yeast two‐hybrid assays were performed as described previously (Liu et al., 2021). The bait and prey vectors were co‐transformed into the yeast strain AH109 and physical interactions were indicated by the ability of cells to grow on a dropout medium lacking Leu, Trp, His, and Ade for 5 days after plating.
Luciferase complementation imaging assay
LCI assay was performed as previously described (Chen et al., 2008; Liu et al., 2021). The pCAMBIA1300‐nLuc and pCAMBIA1300‐cLuc vectors were used for this analysis. The cLuc‐OsUbc13, cLuc‐OsOTUB1.1, and OsSnRK1a‐nLuc constructs were obtained by enzyme digestion and ligation or by seamless cloning. Different pairs of constructs were co‐transformed into wild‐type tobacco (N. benthamiana, 4 weeks old) leaves by Agrobacterium infiltration. After infection for 2 days, 1 mM precooled luciferin was sprayed on to the leaves, and then the samples were incubated in the dark for 5–10 min. The images were captured using a cooled charge‐coupled device imaging apparatus.
Co‐IP assay
Co‐IP assay was performed as previously described with some modifications (Hu et al., 2019). Briefly, total protein was extracted from infiltrated tobacco (N. benthamiana, 4 weeks old) leaves (co‐expressing OsUbc13‐EGFP and OsSnRK1a) with protein extraction buffer (50 mM Tris–HCl (pH 7.5), 100 mM NaCl, 1 mM EDTA, 10 mM NaF, 5 mM Na3VO4, 0.25% Triton X‐100, 0.25% NP‐40, 1 mM PMSF, 1× protease inhibitor cocktail), and then incubated with 20 μL anti‐GFP agarose beads (Chromotek, gta‐20) for 2 h at 4 °C. The beads were washed five times with wash buffer (50 mM Tris–HCl (pH 7.5), 100 mM NaCl, 1 mM EDTA, 1 mM PMSF and 1× protease inhibitor cocktail), and the precipitated proteins were eluted with 2× SDS loading buffer at 95 °C for 3 min. The samples were subjected to immunoblot analysis using anti‐GFP (TransGene Biotech) and anti‐OsSnRK1a (PhytoAB) antibodies. For all immunoblot analyses for OsUbc13‐EGFP, the protein samples were not boiled.
Bimolecular fluorescence complementation assay
The BiFC assay was performed as previously described (Bracha‐Drori et al., 2004; Liu et al., 2021). To produce a fusion with either the N‐ or the C‐terminal fragment of YFP, OsUbc13, OsSnRK1a, OsHRLI, OsSnRK1a1–455 and OsOTUB1.1 were subcloned into the pCAMBIA1300‐nYFP or pCAMBIA1300‐cYFP vectors, respectively. Corresponding BiFC plasmids and negative controls were co‐expressed in wild‐type tobacco (N. benthamiana, 4 weeks old) leaves by Agrobacterium infiltration. After infection for 2 days, the YFP fluorescence was detected with a 514 nm laser (Leica TCS SP5).
Subcellular localization analysis
Subcellular localization assay was performed as described previously (Liu et al., 2021). Briefly, the full‐length CDS of OsSnRK1a was amplified by PCR and directionally inserted into pCAMBIA1300‐35S:EGFP. Cultures of the A. tumefaciens strain EHA105 harbouring the pCAMBIA1300‐35S:OsSnRK1a‐EGFP constructs were used to infect the healthy leaves of tobacco (N. benthamiana, 4 weeks old) expressing 35S:RFP‐histone 2B (nuclear marker, Martin et al., 2009). After infection for 2 days, the fluorescent signals were observed using a confocal microscope (Leica TCS SP5).
Cell‐free protein synthesis and pull‐down assay
Coupled cell‐free transcription and translation reactions were performed as previously described (Zhang et al., 2020b) with some modifications. To generate plasmids for CFPS reactions, the full‐length CDSs of OsUbc13 and OsSnRK1a were inserted into pD2P‐8 × His‐GFP and pD2P‐3 × Flag‐GFP vectors by seamless cloning, respectively. Standard CFPS reactions were carried out in 1.5‐mL microcentrifuge tubes. Each reaction (15 μL) contains the following components: 16.7 μg/mL recombinant plasmids (8 × His‐OsUbc13‐GFP or 3 × Flag‐OsSnRK1a‐GFP), 25 mM HEPES‐KOH (pH 7.4), 120 mM potassium glutamate, 6 mM magnesium glutamate, 1.5 mM of each ATP, GTP, CTP, and UTP, 0.1 mM of each of 20 amino acids, 25 mM creatine phosphate, 1.7 mM DTT, 1 mM putrescine, 0.5 mM spermidine, 0.27 mg/mL creatine phosphokinase (from rabbit muscle; Sigma–Aldrich), 60 U T7 RNA polymerase (Thermo Fisher Scientific), and 50% (v/v) cell extract. All reactions were mixed using the above conditions and incubated at 23 °C for 5 h unless otherwise noted. The recombinant protein was purified with magnetic beads containing His and Flag tags, respectively.
Cell‐free expressed and purified OsUbc13 and OsSnRK1a fusion proteins (100 μL each) were mixed into the same 1.5‐mL centrifuge tube and incubated at 4 °C with rotation for 4 h. After that, 70 μL His‐tag magnetic beads were added and mixed. After incubation at 4 °C for 1 h, the mixture was placed on a magnetic stand, and the supernatant was discarded. 1 mL of washing buffer was added to the above 1.5‐mL centrifuge tube, mixed by inversion, centrifuged briefly and placed on a magnetic stand. When the solution was clear, the supernatant was discarded, and the process was repeated six times. Finally, the remaining magnetic beads in the centrifuge tube were mixed with 50 μL of loading buffer and heated at 100 °C for 5–10 min for immunoblotting analysis using anti‐His and anti‐Flag antibodies (TransGene Biotech).
Detection of the protein levels of OsSnRK1a
The seedlings at 30‐day post‐sowing in soil were used to detect the protein levels of OsSnRK1a. Total protein was extracted with extraction buffer (50 mM Tris–HCl (pH 7.5), 100 mM NaCl, 1 mM EDTA, 10 mM NaF, 5 mM Na3VO4, 0.25% Triton X‐100, 0.25% NP‐40, 1 mM PMSF, 1× protease inhibitor cocktail) and adjusted to equal concentrations. OsSnRK1a abundance was detected by western blotting using the anti‐OsSnRK1a antibody (PhytoAB).
SnRK1 activity assay
The rice seedlings at 30‐day post‐sowing in soil, or the tobacco leaves after transient expression of EGFP and OsSnRK1a‐EGFP for 2 days, were used to detect SnRK1 activity as previously described (Liang et al., 2021). Fresh plant samples (1 g) were ground in 1 mL of cold extraction buffer containing 100 mM Tricine‐NaOH (pH 8.0), 25 mM NaF, 5 mM dithiothreitol, 2 mM tetrasodium pyrophosphate, 0.5 mM ethylene diamine tetraacetic acid, 0.5 mM ethylene glycol tetraacetic acid, 1 mM benzamidine, 1 mM phenylmethylsulfonyl fluoride, 1 mM protease inhibitor cocktail (Sigma P9599), phosphatase inhibitors (PhosStop; Roche), and insoluble polyvinylpyrrolidone with a final concentration of 2% (w/v). The homogenate was transferred to two cold microfuge tubes and centrifuged at 12000 g for 5 min at 4 °C. A pre‐equilibrated 2.5‐mL centrifuge column was used to desalt the supernatant (750 μL). AMARA polypeptide was used as the substrate. SnRKl activity was measured using the Universal Kinase Activity Kit (R&D Systems, Zhang et al., 2009).
ABA sensitivity analysis
To test ABA sensitivity, the wild‐type DJ and OsUbc13‐RNAi lines were germinated on 1/2 MS medium for 3 d. After germination, the seedlings with similar shoot and root length were transplanted to transparent plastic plates with 1/2 MS medium containing 3 μM of ABA or water as a control. After 1 week of growth, the phenotypes were recorded, and the shoot length of these seedlings was measured.
Detection of the ubiquitination levels of OsSnRK1a
The rice seedlings at 30‐day post‐sowing in soil were used to detect the ubiquitination levels of OsSnRK1a as previously described (Chen et al., 2021). Total protein was extracted with extraction buffer (50 mM Tris–HCl (pH 7.5), 100 mM NaCl, 1 mM EDTA, 10 mM NaF, 5 mM Na3VO4, 0.25% Triton X‐100, 0.25% NP‐40, 1 mM PMSF, 1× protease inhibitor cocktail). Anti‐OsSnRK1a antibodies coupled to IgG agarose were added to the crude protein, incubated at 4 °C for 2 h, and washed at least five times with PBS. The obtained immunoprecipitates were suspended in protein loading buffer and boiled at 95 °C for 5 min before being analysed by SDS‐PAGE, and the separated proteins were transferred to a nitrocellulose membrane (GE Healthcare). The OsSnRK1a protein was then detected by probing the membrane with an anti‐OsSnRK1a antibody (PhytoAB), and polyubiquitinated forms were detected by probing with antibodies that recognize total ubiquitin conjugates, antibodies that specifically recognize K48‐polyubiquitin conjugates, or antibodies that specifically recognize K63‐polyubiquitin conjugates (Abcam).
Funding
This study was supported by the National Natural Science Foundation of China (grant nos. 32171932 and 31601232), the Natural Science Foundation of Fujian Province (grant no. 2021 J01088), the Joint Funds of Science and Technology R&D Program of Henan Province (grant no. 222301420101), Fujian Agriculture and Forestry University Program for Distinguished Young Scholar (grant no. xjq201706), and China Postdoctoral Science Foundation (grant no. 2017 M612108).
Author contributions
J.L. and W.X. designed the project and wrote the manuscript. J.L., B.N., and B.Y. conducted the experiments, organized, and analysed the data. F.X., Q.Z., and Y.W. contributed to the experimental design and manuscript revision. All authors have read and approved the final manuscript.
Conflict of interest statement
None declared.
Supporting information
Figure S1 Several agronomic traits of DJ and OsUbc13‐RNAi plants. (a) Plant height. (b) Tiller number per plant. (c) Grain number per panicle. (D) 1000‐grain weight. Data are shown as means ±SE; (a) to (c), n = 17; (d), n = 13 (***P < 0.001; Student's t‐test). All phenotypic data were measured in paddy‐grown rice plants under normal cultivation conditions.
Figure S2 MDA and Photosynthetic pigment content in DJ and OsUbc13‐RNAi leaves. The rice leaves at 30‐day post sowing in soil were used to measure MDA (a) and photosynthetic pigment (b). Data are shown as means ±SE; n = 3 (*P < 0.05, ***P < 0.01, ***P < 0.001; Student's t‐test).
Figure S3 Overexpression of OsUbc13 did not affect the resistance to M. oryzae. (a) qRT‐PCR analysis of OsUbc13 expression in OsUbc13‐OE lines (OE17‐2 and OE18‐3). OsActin1 gene was used as an internal control. Data are shown as means ±SE; n = 3 (***P < 0.001; Student's t‐test). (b) The lesions on DJ and OsUbc13‐OE leaves at 8‐day after punch inoculation with the compatible M. Oryzae isolate GUY11. Scale bar = 1 cm. (c) Relative lesion area (%) in leaves of (b) indicates no significant differences between DJ and OsUbc13‐OE. Data are shown as means ±SE; n = 6.
Figure S4 Protein sequences of rice OsUbc13 and tomato Fni3. Red letters indicate functionally important amino acid residues for biochemical activities of Ubc13. Cys‐89, the active site for ubiquitin thioester formation; Met‐66, which is involved in the interaction with an E3 ligase; Glu‐57, Phe‐59, and Arg‐72, three pocket residues, determine binding specificity for Uev protein. Purple letters indicate the only 5 different amino acid residues between OsUbc13 and Fni3.
Figure S5 Mutation of OsUEV1B or OsVDAC1 did not affect the resistance to M. oryzae. (A) The lesions on DJ, osuev1b, and osvdac1 leaves at 8‐day after punch inoculation with the compatible M. Oryzae isolate GUY11. Scale bar = 1 cm. (B) Relative lesion area (%) in leaves of (A) indicates no significant differences between DJ and osuev1b or osvdac1. Data are shown as means ±SE; n = 6.
Figure S6 SnRK1 kinase activity in tobacco leaves after transient expression of empty EGFP or OsSnRK1a‐EGFP fusion protein. (a) qRT‐PCR analysis of OsSnRK1a expression in tobacco leaves after infection for 2 days. NtEF‐1α gene was used as an internal control. Data are shown as means ±SE; n = 3 (***P < 0.001; Student's t‐test). (b) SnRK1 activity in tobacco leaves after infection for 2 days. Data are shown as means ±SE; n = 3 (***P < 0.001; Student's t‐test).
Figure S7 qRT‐PCR analysis of OsSnRK1a expression level in transgenic lines of OsUbc13. (a) OsUbc13‐RNAi lines. (b) OsUbc13‐OE lines. Data are shown as means ±SE; n = 3.
Figure S8 The OsUbc13‐RNAi lines exhibited increased ABA sensitivity. (a) Phenotypes of DJ and OsUbc13‐RNAi seeds after 8 days of growth on 1/2 MS medium with or without 3 μM ABA. Scale bar = 2 or 5 cm. (b) Shoot and root lengths with ABA treatment. Data are shown as means ±SE; n = 10 (**P < 0.01, ***P < 0.001; Student's t‐test). (c) Shoot and root lengths without ABA treatment. Data are shown as means ±SE; n = 10 (**P < 0.01; Student's t‐test).
Figure S9 Constitutive expression of several defense‐related genes in OsOTUB1.1‐OE and wild‐type ZH11. Total RNA was extracted from the leaves of OsOTUB1.1‐OE and ZH11 plants at 30‐day post sowing in soil. qRT‐PCR was used to analyze the genes expression. (a) OsOTUB1.1 expression. (b) to (f) The expression of defense‐related genes involved in JA signaling/synthetic pathway. (g) to (m) The expression of defense‐related genes involved in SA signaling pathway. (n) OsSnRK1a expression. Data are shown as means ±SE; n = 3 (**P < 0.01, ***P < 0.001; Student's t‐test).
Table S1 Primers used in this study
Acknowledgements
We thank Prfo. Xiangdong Fu and Dr. Shuansuo Wang (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) for providing the seeds of OsOTUB1.1‐OE lines. We thank Dr. Huakun Zheng (College of Life Sciences, Fujian Agriculture and Forestry University) for guiding the experiments on rice blast resistance identification. We thank Dr. Huimei Wang (China Rice Research Institute) for providing the Xoo strain PXO99.
References
- Ayala, A. , Munoz, M.F. and Arguelles, S. (2014) Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4‐hydroxy‐2‐nonenal. Oxid. Med. Cell. Longev. 2014, 360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae, H. and Kim, W.T. (2014) Classification and interaction modes of 40 rice E2 ubiquitin‐conjugating enzymes with 17 rice ARM‐U‐box E3 ubiquitin ligases. Biochem. Biophys. Res. Commun. 444, 575–580. [DOI] [PubMed] [Google Scholar]
- Baena‐González, E. and Lunn, J.E. (2020) SnRK1 and trehalose 6‐phosphate‐two ancient pathways converge to regulate plant metabolism and growth. Curr. Opin. Plant Biol. 55, 52–59. [DOI] [PubMed] [Google Scholar]
- Bai, W. , Chern, M. , Ruan, D. , Canlas, P.E. , Sze‐To, W.H and Ronald, P.C. (2011) Enhanced disease resistance and hypersensitivity to BTH by introduction of an NH1/OsNPR1 paralog. Plant Biotechnol. J. 9, 205–215. [DOI] [PubMed] [Google Scholar]
- Bhoj, V.G. and Chen, Z.J. (2009) Ubiquitylation in innate and adaptive immunity. Nature 458, 430–437. [DOI] [PubMed] [Google Scholar]
- Bracha‐Drori, K. , Shichrur, K. , Katz, A. , Oliva, M. , Angelovici, R. , Yalovsky, S. and Ohad, N. (2004) Detection of protein‐protein interactions in plants using bimolecular fluorescence complementation. Plant J. 40, 419–427. [DOI] [PubMed] [Google Scholar]
- Broeckx, T. , Hulsmans, S. and Rolland, F. (2016) The plant energy sensor: Evolutionary conservation and divergence of SnRK1 structure, regulation, and function. J. Exp. Bot. 67, 6215–6252. [DOI] [PubMed] [Google Scholar]
- Broomfield, S. , Chow, B.L. and Xiao, W. (1998) MMS2, encoding a ubiquitin‐conjugating‐enzyme‐like protein, is a member of the yeast error‐free postreplication repair pathway. Proc. Natl. Acad. Sci. U. S. A. 95, 5678–5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Y. (2020) SnRK1‐ZmRFWD3‐Opaque2: A nexus of seed nutrient accumulation and diurnal cycles. Plant Cell 32, 2671–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, J.H. , Xiao, Y. , Hu, H. , Jin, J. , Yu, J. , Zhou, X. , Wu, X. et al. (2012) Ubc13 maintains the suppressive function of regulatory T cells and prevents their conversion into effector‐like T cells. Nat. Immunol. 13, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z.J. and Sun, L.J. (2009) Nonproteolytic functions of ubiquitin in cell signaling. Mol. Cell 33, 275–286. [DOI] [PubMed] [Google Scholar]
- Chen, H. , Zou, Y. , Shang, Y. , Lin, H. , Wang, Y. , Cai, R. , Tang, X. et al. (2008) Firefly luciferase complementation imaging assay for protein‐protein interactions in plants. Plant Physiol. 146, 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Liu, R. , Wu, Y. , Wei, S. , Wang, Q. , Zheng, Y. , Xia, R. et al. (2021) ERAD‐related E2 and E3 enzymes modulate the drought response by regulating the stability of PIP2 aquaporins. Plant Cell 33, 2883–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chern, M. , Fitzgerald, H.A. , Canlas, P.E. , Navarre, D.A. and Ronald, P.C. (2005) Overexpression of a rice NPR1 homolog leads to constitutive activation of defense response and hypersensitivity to light. Mol. Plant Microbe Interact. 18, 511–520. [DOI] [PubMed] [Google Scholar]
- Cheung, M.Y. , Li, X. , Miao, R. , Fong, Y.H. , Li, K.P. , Yung, Y.L. , Yu, M.H. et al. (2016) ATP binding by the P‐loop NTPase OsYchF1 (an unconventional G protein) contributes to biotic but not abiotic stress responses. Proc. Natl. Acad. Sci. U. S. A. 113, 2648–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, Y.H. , Hong, J.W. , Kim, E.C. and Yoo, S.D. (2012) Regulatory functions of SnRK1 in stress‐responsive gene expression and in plant growth and development. Plant Physiol. 158, 1955–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coello, P. , Hey, S.J. and Halford, N.G. (2011) The sucrose non‐fermenting‐1‐related (SnRK) family of protein kinases: potential for manipulation to improve stress tolerance and increase yield. J. Exp. Bot. 62, 883–893. [DOI] [PubMed] [Google Scholar]
- Crepin, N. and Rolland, F. (2019) SnRK1 activation, signaling, and networking for energy homeostasis. Curr. Opin. Plant Biol. 51, 29–36. [DOI] [PubMed] [Google Scholar]
- Crozet, P. , Margalha, L. , Confraria, A. , Rodrigues, A. , Martinho, C. , Adamo, M. , Elias, C.A. et al. (2014) Mechanisms of regulation of SNF1/AMPK/SnRK1 protein kinases. Front. Plant Sci. 5, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, X. , Richter, T. , Chen, M. , Fujii, H. , Seo, Y.S. , Xie, M. , Zheng, X. et al. (2009) A rice kinase‐protein interaction map. Plant Physiol. 149, 1478–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrev, P.I. and Vankova, R. (2012) Quantification of abscisic Acid, cytokinin, and auxin content in salt‐stressed plant tissues. Methods Mol. Biol. 913, 251–261. [DOI] [PubMed] [Google Scholar]
- Duncan, L.M. , Piper, S. , Dodd, R.B. , Saville, M.K. , Sanderson, C.M. , Luzio, J.P. and Lehner, P.J. (2006) Lysine‐63‐linked ubiquitination is required for endolysosomal degradation of class I molecules. EMBO J. 25, 1635–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelle, S. , Doblin, M.S. , Stapleton, D.I. , Bacic, A. and Gooley, P.R. (2016) Molecular Insights into the Enigmatic Metabolic Regulator, SnRK1. Trends Plant Sci. 21, 341–353. [DOI] [PubMed] [Google Scholar]
- Faulkner, C. and Robatzek, S. (2012) Plants and pathogens: putting infection strategies and defence mechanisms on the map. Curr. Opin. Plant Biol. 15, 699–707. [DOI] [PubMed] [Google Scholar]
- Filipe, O. , De Vleesschauwer, D. , Haeck, A. , Demeestere, K. and Hofte, M. (2018) The energy sensor OsSnRK1a confers broad‐spectrum disease resistance in rice. Sci. Rep. 8, 3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk, H.A. and Yaffe, M.P. (1999) A role for ubiquitination in mitochondrial inheritance in Saccharomyces cerevisiae . J. Cell Biol. 145, 1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Z.Q. , Yan, S. , Saleh, A. , Wang, W. , Ruble, J. , Oka, N. , Mohan, R. et al. (2012) NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486, 228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima, T. , Matsuzawa, S. , Kress, C.L. , Bruey, J.M. , Krajewska, M. , Lefebvre, S. , Zapata, J.M. et al. (2007) Ubiquitin‐conjugating enzyme Ubc13 is a critical component of TNF receptor‐associated factor (TRAF)‐mediated inflammatory responses. Proc. Natl. Acad. Sci. U. S. A. 104, 6371–6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furniss, J.J. and Spoel, S.H. (2015) Cullin‐RING ubiquitin ligases in salicylic acid‐mediated plant immune signaling. Front. Plant Sci. 6, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, S. , Sasakura‐Shimoda, F. , Suetsugu, M. , Selvaraj, M.G. , Hayashi, N. , Yamazaki, M. , Ishitani, M. et al. (2015) Development of disease‐resistant rice by optimized expression of WRKY45 . Plant Biotechnol. J. 13, 753–765. [DOI] [PubMed] [Google Scholar]
- Guo, H. , Wen, R. , Liu, Z. , Datla, R. and Xiao, W. (2015) Molecular cloning and functional characterization of two brachypodium distachyon UBC13 genes whose products promote k63‐linked polyubiquitination. Front. Plant Sci. 6, 1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, H. , Wen, R. , Wang, Q. , Datla, R. and Xiao, W. (2016) Three Brachypodium distachyon uev1s promote Ubc13‐mediated Lys63‐linked polyubiquitination and confer different functions. Front. Plant Sci. 7, 1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford, N.G. and Hey, S.J. (2009) Snf1‐related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signalling in plants. Biochem. J. 419, 247–259. [DOI] [PubMed] [Google Scholar]
- Han, X. , Zhang, L. , Zhao, L. , Xue, P. , Qi, T. , Zhang, C. , Yuan, H. et al. (2020) SnRK1 phosphorylates and destabilizes WRKY3 to enhance barley immunity to powdery mildew. Plant Commun. 1, 100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao, L. , Wang, H. , Sunter, G. and Bisaro, D.M. (2003) Geminivirus AL2 and L2 proteins interact with and inactivate SNF1 kinase. Plant Cell 15, 1034–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie, D.G. (2007) AMP‐activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 8, 774–785. [DOI] [PubMed] [Google Scholar]
- Hardie, D.G. , Carling, D. and Carlson, M. (1998) The AMP‐activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 67, 821–855. [DOI] [PubMed] [Google Scholar]
- Hardie, D.G. , Ross, F.A. and Hawley, S.A. (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 13, 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y. , Zhang, Z. , Li, L. , Tang, S. and Wu, J.L. (2018) Genetic and physio‐biochemical characterization of a novel premature senescence leaf mutant in rice (Oryza sativa L.). Int. J. Mol. Sci. 19, 2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedbacker, K. and Carlson, M. (2008) SNF1/AMPK pathways in yeast. Front. Biosci. 13, 2408–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko, A. and Ciechanover, A. (1998) The ubiquitin system. Annu. Rev. Biochem. 67, 425–479. [DOI] [PubMed] [Google Scholar]
- Hofmann, R.M. and Pickart, C.M. (1999) Noncanonical MMS2‐encoded ubiquitin‐conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell 96, 645–653. [DOI] [PubMed] [Google Scholar]
- Hrabak, E.M. , Chan, C.W. , Gribskov, M. , Harper, J.F. , Choi, J.H. , Halford, N. , Kudla, J. et al. (2003) The Arabidopsis CDPK‐SnRK superfamily of protein kinases. Plant Physiol. 132, 666–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Y. , Han, X. , Yang, M. , Zhang, M. , Pan, J. and Yu, D. (2019) The Transcription Factor INDUCER OF CBF EXPRESSION1 Interacts with ABSCISIC ACID INSENSITIVE5 and DELLA proteins to fine‐tune abscisic acid signaling during seed germination in arabidopsis. Plant Cell 31, 1520–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Y. , Liu, J. , Lin, Y. , Xu, X. , Xia, Y. , Bai, J. , Yu, Y. et al. (2022) Sucrose non‐fermenting‐1‐related protein kinase 1 regulates sheath‐to‐panicle transport of non‐structural carbohydrates during rice grain filling. Plant Physiol. 189, 1694–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X. and Chen, Z.J. (2011) The role of ubiquitylation in immune defence and pathogen evasion. Nat. Rev. Immunol. 12, 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, C. , Hei, R. , Yang, Y. , Zhang, S. , Wang, Q. , Wang, W. , Zhang, Q. et al. (2020) An orphan protein of Fusarium graminearum modulates host immunity by mediating proteasomal degradation of TaSnRK1α. Nat. Commun. 11, 4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, A. and Vert, G. (2016) Unraveling K63 polyubiquitination networks by sensor‐based proteomics. Plant Physiol. 171, 1808–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, A.M. and Dangl, J.L. (1996) Logjam at the Styx: programmed cell death in plants. Trends Plant Sci. 1, 114–119. [Google Scholar]
- Jones, J.D. and Dangl, J.L. (2006) The plant immune system. Nature 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Jossier, M. , Bouly, J.P. , Meimoun, P. , Arjmand, A. , Lessard, P. , Hawley, S. , Grahame Hardie, D. et al. (2009) SnRK1 (SNF1‐related kinase 1) has a central role in sugar and ABA signalling in Arabidopsis thaliana. Plant J. 59, 316–328. [DOI] [PubMed] [Google Scholar]
- Ke, Y. , Yuan, M. , Liu, H. , Hui, S. , Qin, X. , Chen, J. , Zhang, Q. et al. (2020) The versatile functions of OsALDH2B1 provide a genic basis for growth‐defense trade‐offs in rice. Proc. Natl. Acad. Sci. U. S. A. 117, 3867–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, C.Y. , Vo, K.T.X. , An, G.H. and Jeon, J.S. (2015) A rice sucrose non‐fermenting‐1 related protein kinase 1, OSK35, plays an important role in fungal and bacterial disease resistance. J. Korean Soc. Appl. Biol. Chem. 58, 669–675. [Google Scholar]
- Komander, D. and Rape, M. (2012) The ubiquitin code. Annu. Rev. Biochem. 81, 203–229. [DOI] [PubMed] [Google Scholar]
- Laine, A. , Topisirovic, I. , Zhai, D. , Reed, J.C. , Borden, K.L. and Ronai, Z. (2006) Regulation of p53 localization and activity by Ubc13. Mol. Cell. Biol. 26, 8901–8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwers, E. , Jacob, C. and Andre, B. (2009) K63‐linked ubiquitin chains as a specific signal for protein sorting into the multivesicular body pathway. J. Cell Biol. 185, 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. and Schmidt, W. (2010) A lysine‐63‐linked ubiquitin chain‐forming conjugase, UBC13, promotes the developmental responses to iron deficiency in Arabidopsis roots. Plant J. 62, 330–343. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Kong, Y. , Zhou, Z. , Chen, H. , Wang, Z. , Hsieh, Y.C. , Zhao, D. et al. (2013) The HECTD3 E3 ubiquitin ligase facilitates cancer cell survival by promoting K63‐linked polyubiquitination of caspase‐8. Cell Death Dis. 4, e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Yang, D.L. , Sun, L. , Li, Q. , Mao, B. and He, Z. (2016) The systemic acquired resistance regulator OsNPR1 attenuates growth by repressing auxin signaling through promoting IAA‐amido synthase expression. Plant Physiol. 172, 546–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Zhu, Z. , Chern, M. , Yin, J. , Yang, C. , Ran, L. , Cheng, M. et al. (2017) A natural allele of a transcription factor in rice confers broad‐spectrum blast resistance. Cell 170, e115. [DOI] [PubMed] [Google Scholar]
- Liang, J. , Zhang, S. , Yu, W. , Wu, X. , Wang, W. , Peng, F. and Xiao, Y. (2021) PpSnRK1α overexpression alters the response to light and affects photosynthesis and carbon metabolism in tomato. Physiol. Plant. 173, 1808–1823. [DOI] [PubMed] [Google Scholar]
- Lim, K.L. and Lim, G.G. (2011) K63‐linked ubiquitination and neurodegeneration. Neurobiol. Dis. 43, 9–16. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Zhang, C. , Wei, C. , Liu, X. , Wang, M. , Yu, F. , Xie, Q. et al. (2016) The RING finger ubiquitin E3 ligase OsHTAS enhances heat tolerance by promoting H2O2‐induced stomatal closure in rice. Plant Physiol. 170, 429–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q. , Ning, Y. , Zhang, Y. , Yu, N. , Zhao, C. , Zhan, X. , Wu, W. et al. (2017) OsCUL3a negatively regulates cell death and immunity by degrading OsNPR1 in rice. Plant Cell 29, 345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Sun, X. , Xu, F. , Zhang, Y. , Zhang, Q. , Miao, R. , Zhang, J. et al. (2018) Suppression of OsMDHAR4 enhances heat tolerance by mediating H2O2‐induced stomatal closure in rice plants. Rice 11, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Liu, H. , He, J. , Zhang, S. , Han, H. , Wang, Z. , Liu, W.C. et al. (2020) RIN13‐mediated disease resistance depends on the SNC1‐EDS1/PAD4 signaling pathway in Arabidopsis. J. Exp. Bot. 71, 7393–7404. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Liao, W. , Nie, B. , Zhang, J. and Xu, W. (2021) OsUEV1B, an Ubc enzyme variant protein, is required for phosphate homeostasis in rice. Plant J. 106, 706–719. [DOI] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2−ΔΔCt Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lu, C.A. , Lin, C.C. , Lee, K.W. , Chen, J.L. , Huang, L.F. , Ho, S.L. , Liu, H.J. et al. (2007) The SnRK1A protein kinase plays a key role in sugar signaling during germination and seedling growth of rice. Plant Cell 19, 2484–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X. , Zhang, Q. , Zhu, Q. , Liu, W. , Chen, Y. , Qiu, R. , Wang, B. et al. (2015) A robust CRISPR/Cas9 system for convenient, high‐efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 8, 1274–1284. [DOI] [PubMed] [Google Scholar]
- Ma, J. , Wang, Y. , Ma, X. , Meng, L. , Jing, R. , Wang, F. , Wang, S. et al. (2019) Disruption of gene SPL35, encoding a novel CUE domain‐containing protein, leads to cell death and enhanced disease response in rice. Plant Biotechnol. J. 17, 1679–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, K. , Kopperud, K. , Chakrabarty, R. , Banerjee, R. , Brooks, R. and Goodin, M.M. (2009) Transient expression in Nicotiana benthamiana fluorescent marker lines provides enhanced definition of protein localization, movement and interactions in planta. Plant J. 59, 150–162. [DOI] [PubMed] [Google Scholar]
- Martinez‐Forero, I. , Rouzaut, A. , Palazon, A. , Dubrot, J. and Melero, I. (2009) Lysine 63 polyubiquitination in immunotherapy and in cancer‐promoting inflammation. Clin. Cancer Res. 15, 6751–6757. [DOI] [PubMed] [Google Scholar]
- Martins, S. , Dohmann, E.M. , Cayrel, A. , Johnson, A. , Fischer, W. , Pojer, F. , Satiat‐Jeunemaitre, B. et al. (2015) Internalization and vacuolar targeting of the brassinosteroid hormone receptor BRI1 are regulated by ubiquitination. Nat. Commun. 6, 6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar, M.S. , McCormack, M.E. , Argueso, C.T. and Pajerowska‐Mukhtar, K.M. (2016) Pathogen tactics to manipulate plant cell death. Curr. Biol. 26, R608–R619. [DOI] [PubMed] [Google Scholar]
- Mural, R.V. , Liu, Y. , Rosebrock, T.R. , Brady, J.J. , Hamera, S. , Connor, R.A. , Martin, G.B. et al. (2013) The tomato Fni3 lysine‐63‐specific ubiquitin‐conjugating enzyme and suv ubiquitin E2 variant positively regulate plant immunity. Plant Cell 25, 3615–3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada, S. , Tai, I. , Panier, S. , Al‐Hakim, A. , Iemura, S. , Juang, Y.C. , O'Donnell, L. et al. (2010) Non‐canonical inhibition of DNA damage‐dependent ubiquitination by OTUB1. Nature 466, 941–946. [DOI] [PubMed] [Google Scholar]
- Nukarinen, E. , Nagele, T. , Pedrotti, L. , Wurzinger, B. , Mair, A. , Landgraf, R. , Bornke, F. et al. (2016) Quantitative phosphoproteomics reveals the role of the AMPK plant ortholog SnRK1 as a metabolic master regulator under energy deprivation. Sci. Rep. 6, 31697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono, E. , Wong, H.L. , Kawasaki, T. , Hasegawa, M. , Kodama, O. and Shimamoto, K. (2001) Essential role of the small GTPase Rac in disease resistance of rice. Proc. Natl. Acad. Sci. U. S. A. 98, 759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, C.H. , Chen, S. , Shirsekar, G. , Zhou, B. , Khang, C.H. , Songkumarn, P. , Afzal, A.J. et al. (2012) The Magnaporthe oryzae effector AvrPiz‐t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen‐associated molecular pattern‐triggered immunity in rice. Plant Cell 24, 4748–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart, C.M. (2001) Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70, 503–533. [DOI] [PubMed] [Google Scholar]
- Qiao, Y. , Jiang, W. , Lee, J. , Park, B. , Choi, M.S. , Piao, R. , Woo, M.O. et al. (2010) SPL28 encodes a clathrin‐associated adaptor protein complex 1, medium subunit micro 1 (AP1M1) and is responsible for spotted leaf and early senescence in rice (Oryza sativa). New Phytol. 185, 258–274. [DOI] [PubMed] [Google Scholar]
- Qiu, T. , Zhao, X. , Feng, H. , Qi, L. , Yang, J. , Peng, Y.L. and Zhao, W. (2021) OsNBL3, a mitochondrion‐localized pentatricopeptide repeat protein, is involved in splicing nad5 intron 4 and its disruption causes lesion mimic phenotype with enhanced resistance to biotic and abiotic stresses. Plant Biotechnol. J. 19, 2277–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero‐Barrios, N. and Vert, G. (2018) Proteasome‐independent functions of lysine‐63 polyubiquitination in plants. New Phytol. 217, 995–1011. [DOI] [PubMed] [Google Scholar]
- Ruan, B. , Hua, Z. , Zhao, J. , Zhang, B. , Ren, D. , Liu, C. , Yang, S. et al. (2019) OsACL‐A2 negatively regulates cell death and disease resistance in rice. Plant Biotechnol. J. 17, 1344–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada, T. , Kim, M. , Mimuro, H. , Suzuki, M. , Ogawa, M. , Oyama, A. , Ashida, H. et al. (2012) The Shigella flexneri effector OspI deamidates UBC13 to dampen the inflammatory response. Nature 483, 623–626. [DOI] [PubMed] [Google Scholar]
- Sathe, A.P. , Su, X. , Chen, Z. , Chen, T. , Wei, X. , Tang, S. , Zhang, X.B. et al. (2019) Identification and characterization of a spotted‐leaf mutant spl40 with enhanced bacterial blight resistance in rice. Rice 12, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacke, R. and Hager, A. (1992) Fungal elicitors induce a transient release of active oxygen species from cultured spruce cells that is dependent on Ca2+ and protein‐kinase activity. Planta 187, 136–141. [DOI] [PubMed] [Google Scholar]
- Schwessinger, B. and Zipfel, C. (2008) News from the frontline: recent insights into PAMP‐triggered immunity in plants. Curr. Opin. Plant Biol. 11, 389–395. [DOI] [PubMed] [Google Scholar]
- Seo, Y.S. , Chern, M. , Bartley, L.E. , Han, M. , Jung, K.H. , Lee, I. , Walia, H. et al. (2011) Towards establishment of a rice stress response interactome. PLoS Genet. 7, e1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shembade, N. , Harhaj, N.S. , Yamamoto, M. , Akira, S. and Harhaj, E.W. (2007) The human T‐cell leukemia virus type 1 Tax oncoprotein requires the ubiquitin‐conjugating enzyme Ubc13 for NF‐κB activation. J. Virol. 81, 13735–13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, W. and Hanley‐Bowdoin, L. (2021) SnRK1: a versatile plant protein kinase that limits geminivirus infection. Curr. Opin. Virol. 47, 18–24. [DOI] [PubMed] [Google Scholar]
- Shen, Q. , Liu, Z. , Song, F. , Xie, Q. , Hanley‐Bowdoin, L. and Zhou, X. (2011) Tomato SlSnRK1 protein interacts with and phosphorylates betaC1, a pathogenesis protein encoded by a geminivirus beta‐satellite. Plant Physiol. 157, 1394–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel, S.H. and Dong, X. (2012) How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 12, 89–100. [DOI] [PubMed] [Google Scholar]
- Takano, M. , Kajiya‐Kanegae, H. , Funatsuki, H. and Kikuchi, S. (1998) Rice has two distinct classes of protein kinase genes related to SNF1 of Saccharomyces cerevisiae, which are differently regulated in early seed development. Mol. Gen. Genet. 260, 388–394. [DOI] [PubMed] [Google Scholar]
- Tang, Y. , Tu, H. , Zhang, J. , Zhao, X. , Wang, Y. , Qin, J. and Lin, X. (2019) K63‐linked ubiquitination regulates RIPK1 kinase activity to prevent cell death during embryogenesis and inflammation. Nat. Commun. 10, 4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomanov, K. , Luschnig, C. and Bachmair, A. (2014) Ubiquitin Lys 63 chains‐ second‐most abundant, but poorly understood in plants. Front. Plant Sci. 5, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDemark, A.P. , Hofmann, R.M. , Tsui, C. , Pickart, C.M. and Wolberger, C. (2001) Molecular insights into polyubiquitin chain assembly: Crystal structure of the Mms2/Ubc13 heterodimer. Cell 105, 711–720. [DOI] [PubMed] [Google Scholar]
- Wang, M. , Chen, C. , Xu, Y. , Jiang, R. , Han, Y. , Xu, Z. and Chong, K. (2004) A practical vector for efficient knockdown of gene expression in rice (Oryza sativa L.). Plant Mol. Biol. Rep. 22, 409–417. [Google Scholar]
- Wang, Y. , Xu, M.Y. , Liu, J.P. , Wang, M.G. , Yin, H.Q. and Tu, J.M. (2014) Molecular identification and interaction assay of the gene (OsUbc13) encoding a ubiquitin‐conjugating enzyme in rice. J. Zhejiang Univ. Sci. B 15, 624–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Zang, Y. , Zhou, X. and Xiao, W. (2017a) Characterization of four rice UEV1 genes required for Lys63‐linked polyubiquitination and distinct functions. BMC Plant Biol. 17, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. , Wu, K. , Qian, Q. , Liu, Q. , Li, Q. , Pan, Y. , Ye, Y. et al. (2017b) Non‐canonical regulation of SPL transcription factors by a human OTUB1‐like deubiquitinase defines a new plant type rice associated with higher grain yield. Cell Res. 27, 1142–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Wen, R. , Wang, J. , Xiang, D. , Wang, Q. , Zang, Y. , Wang, Z. et al. (2019) Arabidopsis UBC13 differentially regulates two programmed cell death pathways in responses to pathogen and low‐temperature stress. New Phytol. 221, 919–934. [DOI] [PubMed] [Google Scholar]
- Wang, W.R. , Liang, J.H. , Wang, G.F. , Sun, M.X. , Peng, F.T. and Xiao, Y.S. (2020) Overexpression of PpSnRK1α in tomato enhanced salt tolerance by regulating ABA signaling pathway and reactive oxygen metabolism. BMC Plant Biol. 20, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Lu, Y. , Li, J. , Zhang, X. , Hu, F. , Zhao, Y. and Zhou, D.X. (2021) SnRK1 stimulates the histone H3K27me3 demethylase JMJ705 to regulate a transcriptional switch to control energy homeostasis. Plant Cell 33, 3721–3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, R. , Newton, L. , Li, G. , Wang, H. and Xiao, W. (2006) Arabidopsis thaliana UBC13: Implication of error‐free DNA damage tolerance and Lys63‐linked polyubiquitylation in plants. Plant Mol. Biol. 61, 241–253. [DOI] [PubMed] [Google Scholar]
- Wen, R. , Torres‐Acosta, J.A. , Pastushok, L. , Lai, X. , Pelzer, L. , Wang, H. and Xiao, W. (2008) Arabidopsis UEV1D promotes Lysine‐63‐linked polyubiquitination and is involved in DNA damage response. Plant Cell 20, 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, R. , Wang, S. , Xiang, D. , Venglat, P. , Shi, X. , Zang, Y. , Datla, R. et al. (2014) UBC13, an E2 enzyme for Lys63‐linked ubiquitination, functions in root development by affecting auxin signaling and Aux/IAA protein stability. Plant J. 80, 424–436. [DOI] [PubMed] [Google Scholar]
- Wickliffe, K.E. , Williamson, A. , Meyer, H.J. , Kelly, A. and Rape, M. (2011) K11‐linked ubiquitin chains as novel regulators of cell division. Trends Cell Biol. 21, 656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener, R. , Zhang, X. , Wang, T. and Wolberger, C. (2012) The mechanism of OTUB1‐mediated inhibition of ubiquitination. Nature 483, 618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooff, J. , Pastushok, L. , Hanna, M. , Fu, Y. and Xiao, W. (2004) The TRAF6 RING finger domain mediates physical interaction with Ubc13. FEBS Lett. 566, 229–233. [DOI] [PubMed] [Google Scholar]
- Wu, X. and Karin, M. (2015) Emerging roles of Lys63‐linked polyubiquitylation in immune responses. Immunol. Rev. 266, 161–174. [DOI] [PubMed] [Google Scholar]
- Wu, L. , Chen, H. , Curtis, C. and Fu, Z.Q. (2014) Go in for the kill: How plants deploy effector‐triggered immunity to combat pathogens. Virulence 5, 710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Lee, S.K. , Yoo, Y. , Wei, J. , Kwon, S.Y. , Lee, S.W. , Jeon, J.S. et al. (2018) Rice transcription factor OsDOF11 modulates sugar transport by promoting expression of sucrose transporter and SWEET genes. Mol. Plant 11, 833–845. [DOI] [PubMed] [Google Scholar]
- Xie, X. , Kang, H. , Liu, W. and Wang, G.L. (2015) Comprehensive profiling of the rice ubiquitome reveals the significance of lysine ubiquitination in young leaves. J. Proteome Res. 14, 2017–2025. [DOI] [PubMed] [Google Scholar]
- Yamamoto, M. , Sato, S. , Saitoh, T. , Sakurai, H. , Uematsu, S. , Kawai, T. , Ishii, K.J. et al. (2006a) Cutting Edge: Pivotal function of Ubc13 in thymocyte TCR signaling. J. Immunol. 177, 7520–7524. [DOI] [PubMed] [Google Scholar]
- Yamamoto, M. , Okamoto, T. , Takeda, K. , Sato, S. , Sanjo, H. , Uematsu, S. , Saitoh, T. et al. (2006b) Key function for the Ubc13 E2 ubiquitin‐conjugating enzyme in immune receptor signaling. Nat. Immunol. 7, 962–970. [DOI] [PubMed] [Google Scholar]
- Yamanouchi, U. , Yano, M. , Lin, H. , Ashikari, M. and Yamada, K. (2002) A rice spotted leaf gene, Spl7, encodes a heat stress transcription factor protein. Proc. Natl. Acad. Sci. U. S. A. 99, 7530–7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, D.L. , Yao, J. , Mei, C.S. , Tong, X.H. , Zeng, L.J. , Li, Q. , Xiao, L.T. et al. (2012) Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. U. S. A. 109, E1192–E1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, Y. and Rape, M. (2009) Building ubiquitin chains: E2 enzymes at work. Nat. Rev. Mol. Cell Biol. 10, 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, X.J. , Volk, S. , Ljung, K. , Mehlmer, N. , Dolezal, K. , Ditengou, F. , Hanano, S. et al. (2007) Ubiquitin lysine 63 chain forming ligases regulate apical dominance in Arabidopsis. Plant Cell 19, 1898–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, W. , Peng, F. , Xiao, Y. , Wang, G. and Luo, J. (2018) Overexpression of PpSnRK1α in tomato promotes fruit ripening by enhancing RIPENING INHIBITOR regulation pathway. Front. Plant Sci. 9, 1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, Y. , Zhong, S. , Li, Q. , Zhu, Z. , Lou, Y. , Wang, L. , Wang, J. et al. (2007) Functional analysis of rice NPR1‐like genes reveals that OsNPR1/NH1 is the rice orthologue conferring disease resistance with enhanced herbivore susceptibility. Plant Biotechnol. J. 5, 313–324. [DOI] [PubMed] [Google Scholar]
- Zang, Y. , Wang, Q. , Xue, C. , Li, M. , Wen, R. and Xiao, W. (2012) Rice UBC13, a candidate housekeeping gene, is required for K63‐linked polyubiquitination and tolerance to DNA damage. Rice 5, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebell, S.G. and Dong, X. (2015) Cell‐cycle regulators and cell death in immunity. Cell Host Microbe. 18, 402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Primavesi, L.F. , Jhurreea, D. , Andralojc, P.J. , Mitchell, R.A. , Powers, S.J. , Schluepmann, H. et al. (2009) Inhibition of SNF1‐related protein kinase1 activity and regulation of metabolic pathways by trehalose‐6‐phosphate. Plant Physiol. 149, 1860–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Zhang, H. , Xu, C. , Li, X. , Li, M. , Wu, X. , Pu, W. et al. (2019) Ubiquitination of RIPK1 suppresses programmed cell death by regulating RIPK1 kinase activation during embryogenesis. Nat. Commun. 10, 4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. , Fang, H. , Shi, X. , He, F. , Wang, R. , Fan, J. , Bai, P. et al. (2020a) A fungal effector and a rice NLR protein have antagonistic effects on a Bowman‐Birk trypsin inhibitor. Plant Biotechnol. J. 18, 2354–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Liu, W.Q. and Li, J. (2020b) Establishing a eukaryotic pichia pastoris cell‐free protein synthesis system. Front. Bioeng. Biotechnol. 8, 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. , Peng, F. , Xiao, Y. , Wang, W. and Wu, X. (2020c) Peach PpSnRK1 participates in sucrose‐mediated root growth through auxin signaling. Front. Plant Sci. 11, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Several agronomic traits of DJ and OsUbc13‐RNAi plants. (a) Plant height. (b) Tiller number per plant. (c) Grain number per panicle. (D) 1000‐grain weight. Data are shown as means ±SE; (a) to (c), n = 17; (d), n = 13 (***P < 0.001; Student's t‐test). All phenotypic data were measured in paddy‐grown rice plants under normal cultivation conditions.
Figure S2 MDA and Photosynthetic pigment content in DJ and OsUbc13‐RNAi leaves. The rice leaves at 30‐day post sowing in soil were used to measure MDA (a) and photosynthetic pigment (b). Data are shown as means ±SE; n = 3 (*P < 0.05, ***P < 0.01, ***P < 0.001; Student's t‐test).
Figure S3 Overexpression of OsUbc13 did not affect the resistance to M. oryzae. (a) qRT‐PCR analysis of OsUbc13 expression in OsUbc13‐OE lines (OE17‐2 and OE18‐3). OsActin1 gene was used as an internal control. Data are shown as means ±SE; n = 3 (***P < 0.001; Student's t‐test). (b) The lesions on DJ and OsUbc13‐OE leaves at 8‐day after punch inoculation with the compatible M. Oryzae isolate GUY11. Scale bar = 1 cm. (c) Relative lesion area (%) in leaves of (b) indicates no significant differences between DJ and OsUbc13‐OE. Data are shown as means ±SE; n = 6.
Figure S4 Protein sequences of rice OsUbc13 and tomato Fni3. Red letters indicate functionally important amino acid residues for biochemical activities of Ubc13. Cys‐89, the active site for ubiquitin thioester formation; Met‐66, which is involved in the interaction with an E3 ligase; Glu‐57, Phe‐59, and Arg‐72, three pocket residues, determine binding specificity for Uev protein. Purple letters indicate the only 5 different amino acid residues between OsUbc13 and Fni3.
Figure S5 Mutation of OsUEV1B or OsVDAC1 did not affect the resistance to M. oryzae. (A) The lesions on DJ, osuev1b, and osvdac1 leaves at 8‐day after punch inoculation with the compatible M. Oryzae isolate GUY11. Scale bar = 1 cm. (B) Relative lesion area (%) in leaves of (A) indicates no significant differences between DJ and osuev1b or osvdac1. Data are shown as means ±SE; n = 6.
Figure S6 SnRK1 kinase activity in tobacco leaves after transient expression of empty EGFP or OsSnRK1a‐EGFP fusion protein. (a) qRT‐PCR analysis of OsSnRK1a expression in tobacco leaves after infection for 2 days. NtEF‐1α gene was used as an internal control. Data are shown as means ±SE; n = 3 (***P < 0.001; Student's t‐test). (b) SnRK1 activity in tobacco leaves after infection for 2 days. Data are shown as means ±SE; n = 3 (***P < 0.001; Student's t‐test).
Figure S7 qRT‐PCR analysis of OsSnRK1a expression level in transgenic lines of OsUbc13. (a) OsUbc13‐RNAi lines. (b) OsUbc13‐OE lines. Data are shown as means ±SE; n = 3.
Figure S8 The OsUbc13‐RNAi lines exhibited increased ABA sensitivity. (a) Phenotypes of DJ and OsUbc13‐RNAi seeds after 8 days of growth on 1/2 MS medium with or without 3 μM ABA. Scale bar = 2 or 5 cm. (b) Shoot and root lengths with ABA treatment. Data are shown as means ±SE; n = 10 (**P < 0.01, ***P < 0.001; Student's t‐test). (c) Shoot and root lengths without ABA treatment. Data are shown as means ±SE; n = 10 (**P < 0.01; Student's t‐test).
Figure S9 Constitutive expression of several defense‐related genes in OsOTUB1.1‐OE and wild‐type ZH11. Total RNA was extracted from the leaves of OsOTUB1.1‐OE and ZH11 plants at 30‐day post sowing in soil. qRT‐PCR was used to analyze the genes expression. (a) OsOTUB1.1 expression. (b) to (f) The expression of defense‐related genes involved in JA signaling/synthetic pathway. (g) to (m) The expression of defense‐related genes involved in SA signaling pathway. (n) OsSnRK1a expression. Data are shown as means ±SE; n = 3 (**P < 0.01, ***P < 0.001; Student's t‐test).
Table S1 Primers used in this study


