Abstract
A 52‐year‐old female never‐smoker with an abnormal shadow in the right lung detected on radiography was referred to our institution. Contrast‐enhanced computed tomography revealed an irregular nodule in the upper lobe of the right lung, suggestive of a pulmonary vascular abnormality. Angiography revealed a direct communication between the right internal mammary artery (IMA) and the right upper lobe pulmonary artery branches, with dilated and tortuous vascular proliferation. As multiple branch arteries were seen flowing into the upper lobe from the IMA, transcatheter selective embolization of these vessels and right upper lobectomy by video‐assisted thoracoscopic surgery were performed. Contrary to the clinical diagnosis, the pathological finding was a pulmonary adenocarcinoma of the right upper lobe. Additional lymph node dissection was performed later. We report an extremely rare and unprecedented case of pulmonary adenocarcinoma fed by the right IMA, with a literature review.
Keywords: lung cancer, selective arterial embolization, systemic artery‐to‐pulmonary artery fistulas
In this report, we present an extremely rare and unprecedented case of pulmonary adenocarcinoma receiving its nutritional supply from the right internal mammary artery (IMA). The patient was diagnosed with a systemic arterio‐pulmonary fistula (SAPAF) and underwent a right upper lobectomy due to the lesion's growth over time. However, pathological examination revealed that the lesion was a pulmonary adenocarcinoma associated with SAPAF.
INTRODUCTION
Diseases, such as lung cancer, tuberculosis, and pulmonary arteriovenous malformations (PAVMs) present as nodular shadows on chest radiography. Systemic arterio‐pulmonary artery fistulas (SAPAFs) and systemic arterio‐pulmonary vessel fistulas, which are circulatory shunts from systemic arteries to pulmonary vessels, are rare diseases that appear as nodular shadows on chest radiography. 1 , 2 Bronchoscopy is performed to evaluate for malignancies, pulmonary mycobacteriosis, or other diseases. However, bronchoscopy is contraindicated for vascular malformations due to an increased risk of bleeding. 3 Here, we report a rare surgical case of pulmonary adenocarcinoma, preoperatively diagnosed as SAPAF. This case highlights that malignant tumors can further complicate pulmonary vascular abnormalities.
CASE REPORT
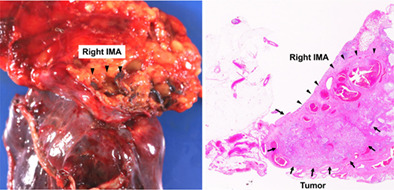
A 52‐year‐old female never‐smoker, with an abnormal shadow in the right lung detected on radiography, was referred to our institution. Computed tomography (CT) revealed an irregular nodule in the upper lobe of the right lung (Figure 1a). Contrast‐enhanced CT revealed a tortuous vascular component inside the nodule (Figure 1b), with high signal intensity on T2‐weighted magnetic resonance imaging (MRI) and gradual contrast on dynamic MRI (Figure S1a,b), suggestive of pulmonary vascular malformation. Though catheter angiography was performed under the suspicion of a pulmonary arteriovenous fistula, no communication between the pulmonary artery and vein was detected (Figure 2a). Selective right internal mammary arteriography revealed communication with the right pulmonary artery (Figure 2b). Because the lesion gradually increased in size, a video‐assisted thoracic surgery (VATS) right upper lobectomy was performed for definitive diagnosis and treatment. Selective embolization of the branch arteries arising from the internal mammary artery (IMA) was preoperatively performed by a radiologist (Figure 2c). Thoracoscopic findings revealed an intrathoracic adhesion between the right upper lobe and anterior mediastinum (Figure 3a) and the mediastinal fatty tissue was resected with the lung. Positron emission tomography (PET) was not performed due to the low malignant risk of the lesion. However, pathological examination revealed a pulmonary adenocarcinoma (invasive adenocarcinoma with micropapillary carcinoma, Figure 3b) associated with a SAPAF. Intraoperatively, no effusion or dissemination was observed. The tumor measured 32 × 22 mm, and invaded the visceral pleura. It was pathologically diagnosed as pT4 due to invasion of the mediastinal fat tissue. The right IMA was confirmed to be the feeding artery of the tumor (Figure 3c, d).
FIGURE 1.
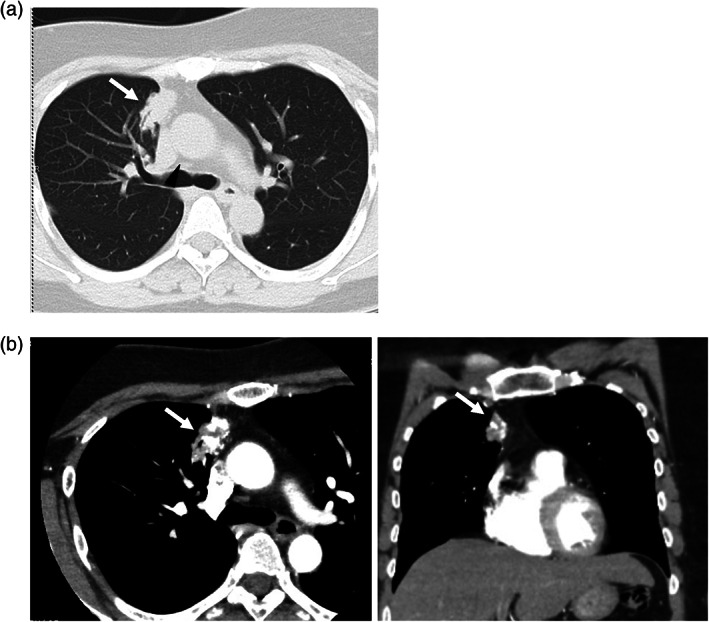
(a) Computed tomography (CT) showing a 31‐mm irregular nodule in the anterior segment (S3) of the upper lobe of the right lung (white arrow). (b) Contrast‐enhanced CT showed a tubular structure (white arrow).
FIGURE 2.

(a) Right pulmonary arteriography showed pulmonary arteries distributed around the lesion, but no arteriovenous shunt was observed. (b) Selective right internal mammary arteriography showed markedly dilated tortuous vascular growth and communication with the right pulmonary artery. IMA, internal mammary artery, PA, pulmonary artery, PV, pulmonary vein. (c) After embolization of the branch of the internal mammary and distal feeders to the fistula, only minimal filling of the network of aberrant blood vessels was identified. No filling of the pulmonary artery was seen.
FIGURE 3.
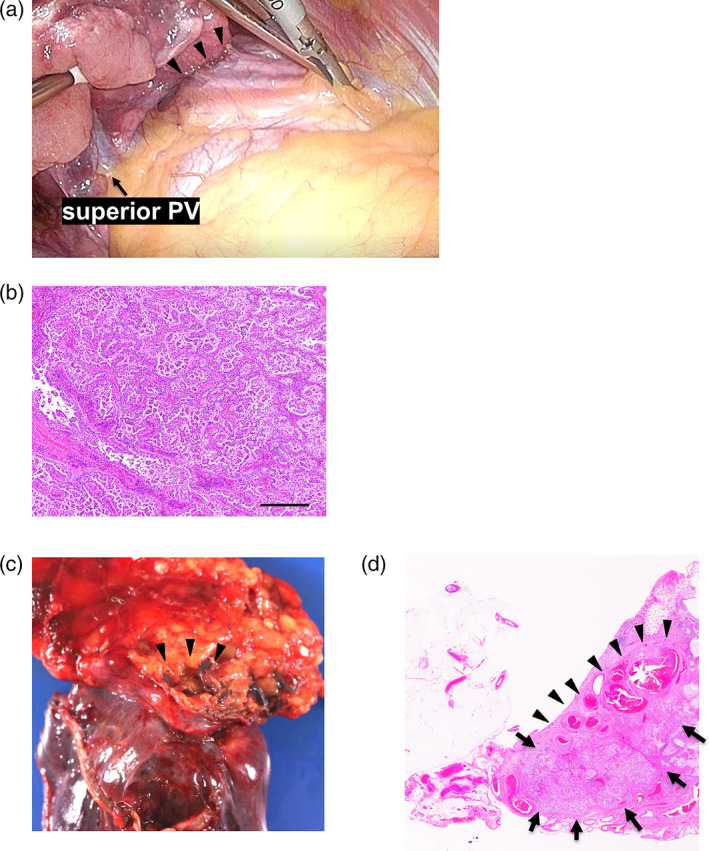
(a) Thoracoscopic findings showed that the right upper lobe was adherent to the anterior mediastinum. PV, pulmonary vein. (b) Microscopic findings of papillary adenocarcinoma. Scale bar, 200 μm. (c) Macroscopic findings in the right upper lobe and anterior mediastinal adipose tissue. A right medial mammary artery fragment can be seen (arrowhead). (d) Pulmonary adenocarcinoma (arrow) and right internal mammary artery inflow (arrowhead) were observed in a loupe view using hematoxylin–eosin staining.
At a later date, additional hilar and upper mediastinal lymph node dissection was performed. The pathological stage was pT4N0M0, stage IIIA. No EGFR mutations, ALK fusion genes, or BRAFV600E were detected, and PD‐L1 expression was low. Her postoperative course was uneventful, and she was discharged on postoperative day 11. She received four cycles of adjuvant chemotherapy with cisplatin and pemetrexed. No tumor recurrence was observed 11 months after the treatment.
DISCUSSION
SAPAF occurs due to pulmonary sequestration, 4 congenital heart disease, 5 tuberculosis, 6 Takayasu's arteritis, 7 trauma, 8 and thoracotomy. 9 The most commonly reported systemic arteries involved are the internal mammalian, intercostal, and subclavian arteries. 10 Abnormal chest shadows, hemoptysis, heart murmurs, and anginal symptoms after coronary artery bypass surgery are known clues for diagnosing SAPAF. 11 , 12 , 13 Unfortunately, these disease‐specific symptoms were absent in the present case.
Catheter angiography of the circulatory system is vital in diagnosing a pulmonary vascular malformation. In our case, pulmonary arteriography ruled out PAVM because no circulatory shunt was detected between the pulmonary artery and pulmonary vein. Instead, communication between the right IMA and right pulmonary artery, through the pleura, was observed. Lung resection or ligation of the inflow vessels is performed for SAPAF treatment. 1 , 11 However, vascular embolization 14 has been increasingly reported. In this case, since the lesion had grown over time, a lobectomy was performed to make a definitive diagnosis. Enucleation or partial resection was not performed to avoid intraoperative hemorrhage.
According to a report of 80 cases of intrapulmonary vascular involvement of the internal thoracic artery, 15 85% of the cases were acquired. In addition to inflammatory disease, chest trauma, and thoracic surgery, malignant lymphoma was reported by Dunn et al. 16 as an underlying disorder. They reported angiographic findings of blood shunting from the left mammalian artery into the left pulmonary artery, via an intrapulmonary Hodgkin's lymphoma infiltrating the anterior mediastinum. However, it was unclear whether the lymphoma drew blood flow from the systemic artery to the pulmonary artery, or whether pre‐existing vascular abnormalities promoted tumorigenesis.
In our case, pathological examination confirmed that the IMA passed through the resected mediastinal tissue into the lung parenchyma, suggesting that the right IMA nourished the tumor and the involvement of the systemic‐pulmonary fistula.
As the patient had an unremarkable medical history, there was no evidence that proved the complete absence of other congenital factors. It was unknown whether the lung cancer was the real cause of SAPAF development. It also remains unclear whether the vascular involvement abnormally promoted tumor formation, as in the case of Hodgkin's lymphoma described above.
This study reported a case of pulmonary adenocarcinoma associated with SAPAF. A lung cancer fed by a systemic artery has not previously been reported. Clinicians should be aware that pulmonary vascular abnormalities might be further complicated. Even if pulmonary vascular abnormalities are suspected preoperatively, intraoperative pathology might be advisable if an elastic hard mass is palpable in the resected lung.
AUTHOR CONTRIBUTIONS
Yuta Ishida and Takuya Fukazawa wrote the manuscript with input from all authors. All authors approved the final version of the manuscript before submission.
CONFLICT OF INTEREST STATEMENT
The authors report no competing interest.
Supporting information
Figure S1.
ACKNOWLEDGMENTS
The authors thank Dr Etsuko Yokota and Dr. Katsuya Kato for the discussions.
Ishida Y, Yukawa T, Nagasaki Y, Minami D, Fujiwara H, Monobe Y, et al. A surgical case of pulmonary adenocarcinoma in the right upper lobe associated with a systemic artery‐to‐pulmonary artery fistula. Thorac Cancer. 2023;14(21):2085–2089. 10.1111/1759-7714.14985
REFERENCES
- 1. Burchell HB, Clagett OT. The clinical syndrome associated with pulmonary arteriovenous fistulas, including a case report of a surgical cure. Am Heart J. 1947;34:151–62. [DOI] [PubMed] [Google Scholar]
- 2. Ito T, Sakamoto T, Norio H, Kaji T, Okada Y. An arteriovenous fistula between the internal mammary artery and the pulmonary vein following blunt chest trauma. Cardiovasc Intervent Radiol. 2005;28:120–3. [DOI] [PubMed] [Google Scholar]
- 3. Muthu V, Ram B, Sehgal IS, Dhooria S, Prasad KT, Bal A, et al. Predictors of severe bleeding during endobronchial biopsy: experience of 537 cases. J Bronchol Interv Pulmonol. 2019;26:273–9. [DOI] [PubMed] [Google Scholar]
- 4. Clements BS, Warner JO. Pulmonary sequestration and related congenital bronchopulmonary‐vascular malformations: nomenclature and classification based on anatomical and embryological considerations. Thorax. 1987;42:401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arvay A, Csakany G, Tomory E. Systemic arteria pulmonary arterial communication in a case of Fallot's pentalogy. Acta Radiol Diagn (Stockh). 1965;3:151–5. [DOI] [PubMed] [Google Scholar]
- 6. Nellens P, Stevens C, Verstraeten J, Heyndrickx GR. Internal mammary to pulmonary artery fistula associated with healed tuberculosis. Acta Cardiol. 1980;35:55–61. [PubMed] [Google Scholar]
- 7. Yamada I, Shibuya H, Matsubara O, Umehara I, Makino T, Numano F, et al. Pulmonary artery disease in Takayasu's arteritis: angiographic findings. AJR Am J Roentgenol. 1992;159:263–9. [DOI] [PubMed] [Google Scholar]
- 8. Saito T, Matsuda M, Yamaguchi T, Matsuzaki T, Nakagawa S. A case of a traumatic systemic‐pulmonary arteriovenous fistula. Jpn Heart J. 1975;16:196–203. [DOI] [PubMed] [Google Scholar]
- 9. Riehl G, Chaffanjon P, Frey G, Sessa C, Brichon PY. Postoperative systemic artery to pulmonary vessel fistula: analysis of three cases. Ann Thorac Surg. 2003;76:1873–7. [DOI] [PubMed] [Google Scholar]
- 10. Thurer RJ. Communication between the pulmonary and systemic circulations. Ann Thorac Surg. 1976;21:114–22. [DOI] [PubMed] [Google Scholar]
- 11. Kuo CL, Lin KH, Ko KH, Huang TW. The aberrant systemic‐pulmonary artery communication: three‐dimensional image simulation. J Cardiothorac Surg. 2019;14:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stafford RW, Kronenberg MW, Dunbar JD, Wooley CF. Continuous precordial murmurs due to internal mammary artery fistulas including internal mammary to pulmonary artery fistulas. Am J Cardiol. 1969;24:414–9. [DOI] [PubMed] [Google Scholar]
- 13. Johnson JA, Schmaltz R, Landreneau RJ, Wright WP, Curtis JJ, Walls JT, et al. Internal mammary artery graft to pulmonary vasculature fistula: a cause of recurrent angina. Ann Thorac Surg. 1990;50:297–8. [DOI] [PubMed] [Google Scholar]
- 14. Lee JK, Park JH, Kim J, Kim SJ, Lee AR, Lee CH, et al. Embolization of multiple systemic artery to pulmonary artery fistula with recurrent hemoptysis. Tuberc Respir Dis (Seoul). 2013;75:120–4. [Epub Sep 30]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abdul Jabbar A, Patel A, Marzlin N, Altabaqchali S, Hasan M, al‐Zubaidi M, et al. Internal mammary artery‐to‐pulmonary vasculature fistula: systematic review of case reports. Vasc Med. 2017;22:426–31. [DOI] [PubMed] [Google Scholar]
- 16. Dunn RP, Wexler L. Systemic‐to‐pulmonary fistula in intrapulmonary Hodgkin's disease. Chest. 1974;66:590–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.


