Abstract
Background
Lung cancer, especially non‐small cell lung cancer (NSCLC), is one of the leading causes of cancer‐related deaths worldwide. Vincristine (VCR) is a chemotherapeutic agent for lung cancers; however, its effectiveness is limited by side effects and the development of drug resistance. Patchouli alcohol (PA), from Pogostemon cablin extract, is known to possess anti‐inflammatory and anticancer properties. In this study, we investigated the role of PA in inducing reactive oxygen species (ROS)‐mediated DNA damage in A549 and VCR‐resistant A549/V16 NSCLC cells.
Methods
The anticancer potential of PA was studied using the MTT assay, colony formation, flow cytometry analysis, western blotting, DCFDA staining, immunofluorescence staining, and TUNEL assay techniques.
Results
The intracellular ROS levels were enhanced in PA‐treated cells, activating the CHK1 and CHK2 signaling pathways. PA further inhibited proliferation and colony‐forming abilities and induced cell cycle arrest at the G0/G1 phase by regulating p53/p21 and CDK2/cyclin E1 expression. Moreover, PA increased the percentage of cells in the subG1 phase and induced apoptosis by activating the Bax/caspase‐9/caspase‐3 intrinsic pathway. In addition, drug resistance (p‐glycoprotein) and cancer stem cell (CD44 and CD133) markers were downregulated after PA treatment. Furthermore, combining PA and cisplatin exhibited synergistic inhibitory activity in A549 and A549/V16 cells.
Conclusions
PA induced ROS‐mediated DNA damage, triggered cell cycle arrest and apoptosis, attenuated drug resistance and cancer stem cell phenotypes, and synergistically inhibited proliferation in combination with cisplatin. These findings suggest that PA has the potential to be used for the treatment of NSCLC with or without VCR resistance.
Keywords: apoptosis, non‐small cell lung cancer, patchouli alcohol, ROS, vincristine resistance
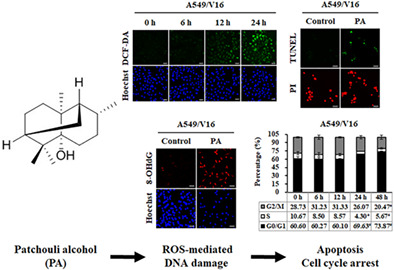
Results indicated that patchouli alcohol (PA) enhanced the expression of intracellular reactive oxygen species (ROS) and oxidative DNA damage maker 8‐OHdG, resulting in induction of cell cycle G0/G1 arresting as well as apoptosis in vincristine (VCR)‐resistant non‐small cell lung cancer (NSCLC) A549/V16 cells. The findings of the present study suggested that PA may be a promising therapeutic agent or adjuvant for the treatment of NSCLC with VCR resistance.
INTRODUCTION
According to the Global Cancer Statistics 2020, from 185 countries for 36 cancers, lung cancer is the leading cause of cancer deaths worldwide, with approximately 2.2 million new cases (11.4% of the total cases) and 1.8 million deaths (18.0% of the total cancer deaths) each year. 1 Lung cancer can be histologically divided into small cell lung cancer (SCLC) and non‐small cell lung cancer (NSCLC). NSCLC accounts for approximately 85% of all cases of lung cancer and is classified as adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. 2 , 3 At the time of diagnosis, NSCLC is often at an advanced stage, with poor prognosis, and the 5‐year survival rate is less than 15%. 4 , 5 Thus, chemotherapy, radiotherapy, immunotherapy, and targeted therapy become the major options of treatment for unresectable advanced NSCLC. 6 , 7 Despite their benefits in patients with NSCLC, these treatments lead to multiple adverse effects and progressive development of drug resistance, 8 , 9 limiting their clinical application. Therefore, there is an urgent need to develop novel and effective therapeutic agents against NSCLC.
Vincristine (VCR), also known as leurocristine, belongs to the vinca alkaloid family and is found in Catharanthus roseus (Madagascar periwinkle). The compound was approved by the US Food and Drug Administration (FDA) in 1963 and is used as a chemotherapeutic drug for the treatment of neuroblastoma, acute lymphocytic and myeloid leukemia, and breast, neck, and lung cancers. 10 , 11 It shows anticancer activity by disrupting the function of microtubules and inhibiting mitosis, thus resulting in cell cycle arrest and even apoptosis. 12 However, the effectiveness of VCR treatment is limited by side effects such as nausea, vomiting, alopecia, and peripheral neuropathy, as well as the development of drug resistance during cancer therapy. 13 There is increasing evidence that ABCB1/p‐glycoprotein (P‐gp), ABCC1/multidrug resistance‐associated protein 1, and ABCG2/breast cancer resistance protein enhance resistance to vinca alkaloids in human cancers. 14 P‐gp is overexpressed in NSCLC cells and efficiently transports VCR from cells, leading to strong resistance to VCR treatment. 15 In addition, several studies have shown that the characteristics of cancer stem cells (CSCs) are related to tumor recurrence, invasion, and drug resistance. 16 Thus, the downregulation of P‐gp expression and CSCs properties may be an effective strategy for the treatment of VCR‐resistant NSCLC.
Many widely used therapeutic agents against cancer are derived from natural sources, such as plants (e.g., vincristine, etoposide, and paclitaxel). Natural compounds have recently been used to overcome drug resistance to VCR in multiple cancers. For example, curcumin induces apoptosis of VCR‐resistant A549 NSCLC cells via reactive oxygen species (ROS)‐regulated p38 MAPK signaling 17 ; pyramidatine sensitizes VCR‐resistant KB oral cancer cells to chemotherapeutic drugs by inhibiting P‐gp expression 18 ; 23‐hydroxybetulinic acid reverses the properties of multidrug resistance in P‐gp‐overexpressing MCF‐7 human breast carcinoma cells. 19 Patchouli alcohol (PA, C15H26O), a natural tricyclic sesquiterpene derived from Pogostemon cablin, has diverse health benefits, including anti‐inflammatory, anti‐influenza virus, antimicrobial, and anticancer properties. 20 , 21 , 22 , 23 , 24 , 25 Previous studies have demonstrated that PA inhibits tumor growth by triggering Akt/mTOR‐mediated autophagy and induces cell cycle arrest and apoptosis by regulating the EGFR‐MAPK pathway in NSCLC. 24 , 25 However, whether PA has inhibitory effects on VCR‐resistant NSCLC cells and the underlying molecular mechanisms remain unclear. Therefore, we investigated the effects of PA on cell proliferation, cell cycle distribution, colony formation, ROS‐mediated DNA damage, apoptosis, drug resistance, and CSCs in a cellular model of VCR‐resistant NSCLC.
METHODS
Chemicals and antibodies
Patchouli alcohol (purity ≥98%) and vincristine (purity ≥98%) were purchased from the Wuhan ChemFaces Biochemical Co., Ltd, and cisplatin (purity ≥95%) was purchased from Cayman Chemical Company. The specific primary antibodies used in this study included those against p‐H2A.X (GeneTex, USA), p‐CHK1 and p‐p53, p‐CHK2 (Biorbyt Ltd.), p21, CDK2, cyclin E1, ERK, p‐ERK, AKT, p‐AKT, Bcl‐2, Bax, caspase‐9, caspase‐3, CD133, CD44, EpCAM, β‐actin (iReal Biotechnology), and P‐gp (Bioss Antibodies Inc.).
Cell culture
The A549 human NSCLC cell line and SVEC mouse vascular endothelial cell line were purchased from the American Type Culture Collection. Cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 1% L‐glutamine, 1% nonessential amino acids, 1% sodium pyruvate, and 1% penicillin and streptomycin mixture, in a humidified 5% CO2 incubator at 37°C. VCR‐resistant A549 cells (A549/V16), obtained from the laboratory of Professor Gwo‐Tarng Sheu with the permission, were established by exposure to increasing concentrations of VCR and maintained in medium with 16 nM VCR. 26 All reagents used in the cell cultures were purchased from Gibco BRL (Life Technologies).
MTT cell viability assay
Cells were cultured in 96‐well plates at a density of 5 × 103 cells/well and treated with a gradient concentration of PA (0–450 μM) for 24, 48, and 72 h. Two hours before the end of incubation, 20 μL of MTT stock solution (5 mg/mL; Amresco) was added directly to each well without removing the medium, and the cells were incubated for 2 h at 37°C in the dark. Subsequently, the medium was replaced with 50 μL dimethyl sulfoxide (DMSO), and cell viability was estimated by calculating the absorbance at 550 nm using a Spectra Max Plus 384 Microplate Reader (Molecular Devices). Experiments were performed at least in triplicate.
Western blotting
After culturing in a 100 mm dish, 2 × 106 cells were incubated with IC60 concentration of PA (112 μM in A549; 135 μM in A549/V16) for 0, 6, 12, 24, and 48 h. Total protein was extracted from the cells using RIPA buffer (Visual Protein) supplemented with protease (Amresco Inc.) and phosphatase inhibitors (Bionovas) on ice for 30 min. After quantification of proteins using the BCA protein assay kit (Pierce; Thermo Scientific), the equivalent amount of protein (20 μg) was separated using 8%–12.5% SDS‐PAGE gel and transferred to PVDF membrane (PALL Corporation), followed by blocking with 5% skim milk. Membranes were washed with 0.5% tween‐20 in TBS (TBS‐T) and immunolabeled with primary antibodies at 4°C overnight. Membranes were washed thrice with TBS‐T and then incubated with biotin‐conjugated secondary antibody (Santa Cruz) for 2 h at 25°C, followed by peroxidase‐conjugated streptavidin incubation (Jackson ImmunoResearch Inc.) for 1 h at 25°C. The bands were visualized using an enhanced chemiluminescence reagent (ECL, T‐Pro Biotechnology) and a GE LAS‐4000 chemiluminescence imaging analyzer (GE Healthcare Life Sciences). Protein blots were quantified by densitometry using ImageJ software (National Institute of Health), and the amounts were expressed relative to the internal reference β‐actin.
Determination of ROS production
Cells were seeded at 2.5 × 105 in a 35 mm dish containing 15 mm coverslips (Assistant) overnight. After treatment with PA for 0–24 h, cells were incubated with 40 μM of 2′,7′‐dichlorodihydrofluorescein diacetate (DCFDA, Sigma‐Aldrich) in 2 mL of medium at 37°C for 30 min and then counterstained with Hoechst 33342 (AAT Bioquest). Fluorescence was immediately detected using a fluorescence microscope, and the intensity was quantified using ImageJ software (National Institute of Health).
Flow cytometry analysis of cell cycle
Cells were cultured in a 100‐mm dish (2 × 106 cells/well) overnight and incubated with IC60 concentration of PA for 0–48 h. The cells were harvested by trypsinization and centrifuged for 10 min at 1500 rpm. After washing thrice with PBS, the cells were resuspended in PBS, added to a propidium iodide (PI, 40 μg/mL) and RNase (100 μg/mL) solution, and incubated in the dark at 4°C overnight. The cell cycle distribution was assessed using FACScan flow cytometry (Beckton Dickinson) and analyzed using FlowJo software (Treestar).
TUNEL staining assay
A terminal deoxynucleotidyl transferase dUTP nick‐end labeling (TUNEL) assay kit (Roche Basel) was used according to the manufacturer's protocol. After incubation with PA (112 μM in A549; 135 μM in A549/V16) for 48 h, A549 and A549/V16 cells were fixed with 10% neutral buffered formalin, inactivated endogenous peroxidase with 3% H2O2 in methanol, and then permeabilized with 0.1% Triton X‐100 in 0.1% sodium citrate on ice. Subsequently, the cells were incubated with a mixed solution containing the enzyme terminal deoxynucleotide transferase and biotinylated dUTP in TdT buffer at 37°C for 1 h, and the nuclei were stained with PI. Finally, apoptotic cells were visualized and counted using a Zeiss Axioskop 2 plus fluorescence microscope (Carl Zeiss).
Immunofluorescence staining assay
A549 and A549/V16 cells (1.5 × 105 cells/well) were plated onto 15 mm coverslips (Assistant) in a 35‐mm dish and exposed to IC60 concentration of PA for 48 h. The cells were fixed with 10% neutral buffered formalin, permeabilized with 1% NP‐40 in PBS, blocked with 5% bovine serum albumin (Sigma‐Aldrich), and then incubated with antibody against P‐gp, CD133, CD44, and EpCAM overnight at 4°C. The cells were incubated with a biotin‐conjugated secondary antibody (Santa Cruz, CA, USA) for 2 h, followed by incubation with Alexa Fluor 488 streptavidin (Jackson ImmunoResearch Inc.) for 1 h. Nuclei were stained with Hoechst 33342 (AAT Bioquest), and images were acquired using a Zeiss Axioskop 2 plus fluorescence microscope (Carl Zeiss).
Colony formation
The cells were incubated in 24‐well plates (5 × 102 cells/well) and allowed to attach, followed by overnight incubation and treatment with PA (0–135 μM). After treatment for 48 h, the drug‐containing medium was replaced with complete culture medium and cultured for 7 days, during which the medium was changed every 3 days. The cells were fixed with cold methanol, stained with 0.1% crystal violet, and washed thoroughly. Finally, the number of colonies (≥50 cells) in each well was counted using a microscope and colony forming efficiency was calculated as the percentage relative to the control (100%). All the samples were done in triplicate and experiments were repeated three times.
Drug combination treatment
Cells were seeded in triplicate in a 96‐well plate and treated with graded concentrations of PA (67, 90, 112, and 135 μM) and cisplatin (3.3, 6.6, 13.3, and 26.6 μM) for 48 h, followed by the detection of cell viability using the MTT assay. Drug–drug interactions were assessed according to the combination index (CI) calculated using CompuSyn software (CompuSyn Inc.) to evaluate the occurrence of synergism (CI < 1), additive effects (CI = 1), and antagonism (CI > 1) using the Chou–Talalay method. 27
Statistical analysis
Data are presented as mean ± standard deviation (SD), representative of at least three independent experiments. One‐way analysis of variance (ANOVA) or unpaired two‐tailed Student's t‐tests were used to determine the statistical significance of differences between groups. All statistical analyses were performed using GraphPad Prism Version 6.01 for Windows (GraphPad Software Inc.), and differences were considered statistically significant when the p‐value was <0.05.
RESULTS
PA inhibited proliferation and cell cycle progression in A549 and A549/V16 cells
First, we investigated the growth‐inhibitory potential of PA in A549 and A549/V16 cells to determine the optimal dose and treatment time for further experiments. The chemical structure of PA, a tricyclic sesquiterpene, is shown in Figure 1a. Cells were treated with different concentrations of PA (45, 67, 90, 112, and 135 μM) or cisplatin (3.3, 6.6, 13.3, 26.6, and 53.1) for 24 and 48 h, and the cell viability of each group was determined using MTT assay. Treatment with PA or cisplatin markedly reduced the viability of A549 (Figure 1b,d) and A549/V16 (Figure 1c,e) cells in a dose‐dependent manner. The IC50 doses of PA and cisplatin at 48 h were 106.18 ± 6.34 and 22.02 ± 3.39 μM in A549 cells, 125.52 ± 2.92 and 11.99 ± 0.03 μM in A549/V16 cells, and 167.31 ± 4.02 and 10.76 ± 0.27 μM in SVEC cells (Table 1), respectively. As shown in Table 2, the higher the value of the selectivity index (SI), the greater the selectivity of the drug for NSCLC cells than for normal cells. PA has higher SI values (1.58, A549; 1.33, A549/V16) than cisplatin (0.49, A549; 0.90, A549/V16) and VCR (1.25, A549; 0.22, A549/V16), suggesting that PA has a high potential for drug development with lower side effects. In this study, we selected the IC60 concentration of PA (112 μM for A549 and 135 μM for A549/V16), inducing a higher percentage of cells with apoptotic morphology, such as cell shrinkage and membrane‐bound apoptotic bodies, than the IC50 concentration, for further examination of various biological activities.
FIGURE 1.

Growth inhibitory effect of patchouli alcohol (PA) in A549 and A549/V16 cells. (a) The chemical structure of PA from the ChemFaces database. (b and c) Cell viabilities of A549 and A549/V16 cells treated with various doses of PA for 0–48 h were measured using 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5 diphenyl tetrazolium bromide (MTT) assay in 96‐well plates. (d and e) The inhibitory effects of cisplatin on cell viabilities were determined using MTT assay. Results are presented as means ± SD from three independent experiments.
TABLE 1.
The IC50 values of PA and cisplatin‐treated A549 and A549/V16 cells.
| Cell line | Time | PA | Cisplatin | VCR |
|---|---|---|---|---|
| A549 | 24 h | 107.84 ± 0.67 | >53.14 | 0.21 ± 0.03 |
| 48 h | 106.18 ± 6.34 | 22.02 ± 3.39 | 0.04 ± 0.01 | |
| A549/V16 | 24 h | 128.94 ± 2.52 | 42.38 ± 1.00 | 0.53 ± 0.03 |
| 48 h | 125.52 ± 2.92 | 11.99 ± 0.03 | 0.23 ± 0.02 | |
| SVEC | 24 h | 158.41 ± 0.48 | 41.65 ± 1.13 | 0.22 ± 0.02 |
| 48 h | 167.31 ± 4.02 | 10.76 ± 0.27 | 0.05 ± 0.01 |
Note: The half‐maximal inhibitory concentration (IC50) values were calculated from dose–response curves of PA or cisplatin at 24 and 48 h and are presented as mean ± standard deviation (μM) from three independent experiments.
Abbreviations: PA, patchouli alcohol; VCR, vincristine.
TABLE 2.
The selective index between non‐small cell lung cancer (NSCLC) and normal cells.
| Cell line | Time | PA | Cisplatin | VCR |
|---|---|---|---|---|
| A549 | 24 h | 1.47 | 0.78 | 1.05 |
| 48 h | 1.58 | 0.49 | 1.25 | |
| A549/V16 | 24 h | 1.23 | 0.98 | 0.42 |
| 48 h | 1.33 | 0.90 | 0.22 |
Note: The selectivity index (SI) was calculated as the IC50 value of the normal cell line divided by the IC50 value of the NSCLC cell line. The higher the SI value, the greater the selectivity of the drug for NSCLC cells.
Abbreviations: PA, patchouli alcohol; VCR, vincristine.
In this study, a colony formation assay was performed to further evaluate the effect of PA on cell proliferation. After cells were treated with IC20, IC40, and IC60 concentration of PA for 48 h and cultured in drug‐free medium for 7 days, the colonies were stained, observed, and counted. The results are illustrated in Figure 2a–d. Colony numbers of A549 and A549/V16 cells were remarkably reduced by PA treatment in a dose‐dependent manner compared with those in the negative control, indicating that PA could suppress the cell proliferation and colony‐forming abilities of A549 and A549/V16 cells. To investigate the mechanism of PA‐induced proliferation inhibition, the cell cycle distributions of PA‐treated A549 and A549/V16 cells were analyzed using flow cytometry. After IC60 concentration of PA treatment for 0, 6, 12, 24, and 48 h, the cell populations in the G0/G1 phase significantly increased in both cell types in a time‐dependent manner (Figure 2e,f). These results suggest that PA could significantly inhibit the growth of NSCLC cells with or without VCR resistance by inducing G0/G1 phase cell cycle arrest.
FIGURE 2.

Effects of patchouli alcohol (PA) on colony formation in A549 and A549/V16 cells. (a and b) Plate colony formation assay was used to evaluate the effect of PA on the colony formation capability of A549 and A549/V16 cells. The cells were treated with PA for 48 h and then incubated in complete culture medium without PA for 7 days. The colonies were visualized using crystal violet staining. (c and d) The number of colonies (>50 cells/colony) in each well were counted and presented as percentage relative to the control (100%). (e and f) Cells were incubated with the IC60 concentration of PA (112 μM in A549; 135 μM in A549/V16) for 0–48 h, the cell cycle distribution was then determined using propidium iodide staining and flow cytometry. The data is presented as mean ± SD from three independent experiments. *p < 0.05, significant decrease compared with control. # p < 0.05, significant increase compared with control.
PA enhanced the levels of intracellular ROS and triggered oxidative DNA damage
Research has shown that ROS plays an important role in regulating metabolic processes in health and disease. However, dysregulation of ROS induces DNA damage and triggers cell death. 28 To determine whether PA induces ROS‐mediated cell damage in A549 and VCR‐resistant A549 cells, intracellular ROS levels and the oxidative DNA damage marker 8‐OHdG were detected using DCFDA and immunofluorescence staining after PA treatment. As shown in Figure 3a–c, the fluorescence intensity in cells was significantly increased to 413.08% ± 40.21% and 380.08% ± 24.28% in PA‐treated A549 and A549/V16 cells at 48 h, respectively, compared with that in the control group. Furthermore, immunofluorescence staining analysis revealed that the protein expression of 8‐OHdG was significantly enhanced in A549 (388.05% ± 27.61%) and A549/V16 (459.39% ± 51.33%) cells after 48 h of PA treatment (Figure 3d–f). Together, these data suggest that PA promotes cell death by inducing ROS production and oxidative DNA damage.
FIGURE 3.
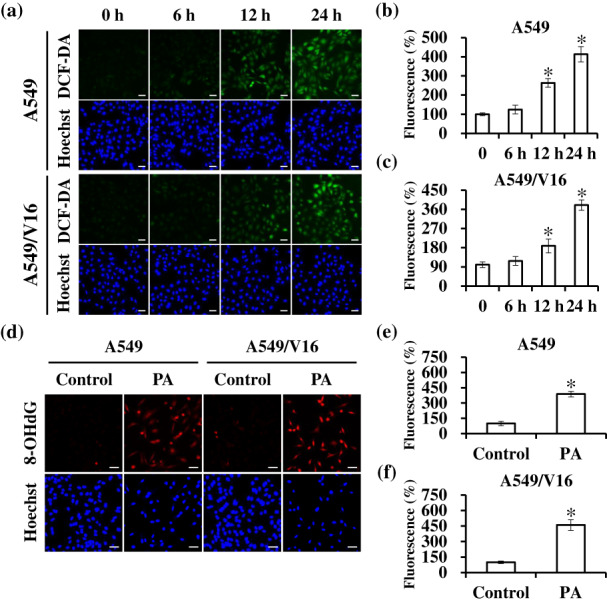
Effects of patchouli alcohol (PA) on reactive oxygen species (ROS) generation and oxidative DNA damage. (a–c) A549 and A549/V16 cells were treated with the IC60 concentration of PA for 0–24 h and then stained with DCFDA and Hoechst 33342. Characteristic fluorescence photographs and quantification indicating intracellular ROS production. Scale bar: 50 μm. (d–f) After PA treatment for 48 h, the levels of oxidative DNA damage maker 8‐OHdG were determined using immunofluorescence staining. Results are shown as means ± SD from three independent experiments. *p < 0.05, compared with control. Scale bar: 50 μm.
PA inhibited cell growth by regulating DNA damage response (DDR)
To confirm the above results, we examined the effect of total cell lysates treated with IC60 concentration of PA on the expression of proteins related to cell growth and DDR using western blotting. As shown in Figure 4, after PA treatment, there was increased expression of DNA damage markers p‐H2A.X, p‐CHK1, and p‐CHK2 mediated by the ROS‐induced DDR, resulting in phosphorylation of the downstream tumor suppressor protein p53. p21 expression, regulated by p53, was upregulated, while CDK2 and cyclin E1 levels, which play an important role in the G1/S transition, were significantly downregulated. Moreover, the expression of proliferation‐related proteins including p‐ERK and p‐AKT was significantly inhibited by PA. These data confirm that PA induced ROS‐mediated DDR to trigger cell cycle arrest and inhibit proliferation of NSCLC cells via the ATM/CHK2 and ATR/CHK1 signaling pathways.
FIGURE 4.
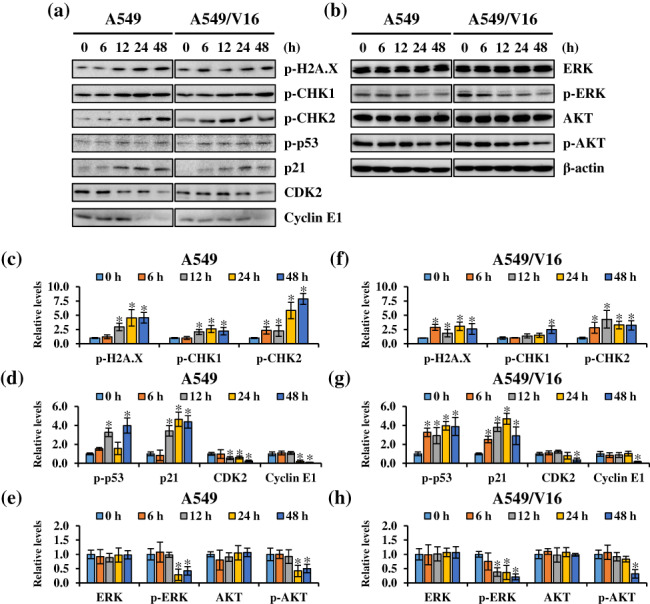
Effects of patchouli alcohol (PA) on protein expression of DNA damage response signaling pathways. (a and b) A549 and A549/V16 cells were treated 112 μM and 135 μM PA for 0–48 h, respectively; the cells were then harvested and prepared for western blot analysis for p‐H2A.X, p‐CHK1, p‐CHK2, p‐p53, p21, CDK2, cyclin E1, ERK, p‐ERK, AKT, and p‐AKT. (c–h) The blots were quantified using ImageJ software, and data are expressed as mean ± SD. β‐actin was the loading control. *p < 0.05, compared with control.
PA induced apoptosis through mitochondria‐mediated caspase activation
The induction of apoptosis, triggered by various stress signals, such as ROS stress, is one of the main mechanisms of most anticancer drugs. 29 As shown by flow cytometry analysis results (Figure 5a,b), the percentage of cells in subG1 phase was significantly increased by approximately 5.14% ± 1.30% to 44.35% ± 5.45% in A549 and 5.88% ± 2.24% to 41.34% ± 4.92% in A549/V16 cells upon PA treatment. A subsequent TUNEL assay was performed to determine the activation of apoptosis and study the possibility of PA‐induced cell death. The results indicated that the percentages of apoptotic cells were significantly increased in PA‐treated A549 (from 4.14% ± 2.48% to 50.37% ± 8.35%) and A549/V16 (from 4.63% ± 1.57% to 43.30% ± 8.87%) cells (Figure 5c,d). Next, the expression levels of apoptotic markers were evaluated using western blotting. Compared with the untreated controls, PA treatment downregulated the expression of antiapoptotic Bcl‐2 and upregulated the expression of proapoptotic Bax and cleaved caspase‐9 and ‐3 (Figure 5e,f). These results demonstrate that PA exerted time‐dependent proapoptotic effects on A549 and A549/V16 cells.
FIGURE 5.
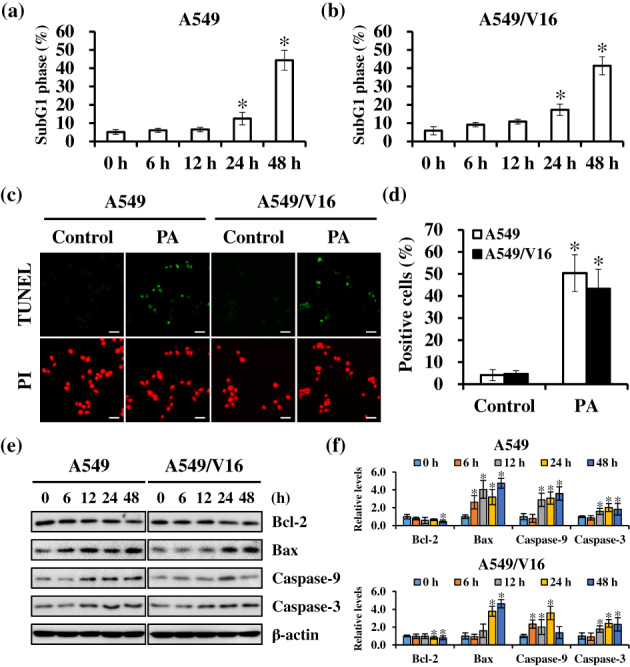
Mitochondrion‐mediated apoptosis of non‐small cell lung cancer (NSCLC) cells were activated by patchouli alcohol (PA) treatment. (a and b) The percentage of cells at subG1 phase increased after PA treatment. (c and d) Cells were exposed to the IC60 concentration of PA for 48 h, and apoptosis was evaluated using TUNEL staining. The nucleus was counterstained with PI and observed under a fluorescence microscopy. Scale bar: 50 μm. (e and f) Expression levels of Bcl‐2, Bax, and cleaved caspase 3/9 in PA‐treated cells were assessed using western blotting. β‐actin was the loading control. Results are shown as means ± SD from three independent experiments. *p < 0.05, compared with control.
PA downregulated the expression of drug resistance and CSCs markers
Studies have shown that P‐gp overexpression and stemness phenotype are related to resistance to chemotherapy drugs against cancer. 15 , 16 To determine the effects of PA on drug resistance, the expression of P‐gp and CSCs markers (CD44, CD133, and EpCAM) in PA‐treated cells was detected using immunofluorescence staining and western blotting. Immunofluorescence staining showed that the expression of P‐gp, CD44, and CD133 was significantly enhanced in A549/V16 cells compared with that in A549 cells; however, the levels of these markers were obviously reduced in both cells after PA treatment (Figure 6a,b). Furthermore, western blotting analysis showed that the protein expression of P‐gp, CD44, and CD133 significantly decreased in a time‐dependent manner after PA treatment (Figure 6c,d). Taken together, these results suggest that PA attenuates VCR drug‐resistance by regulating the efflux pump (P‐gp) and stemness phenotypes (CD44 and CD133) in NSCLC cells.
FIGURE 6.
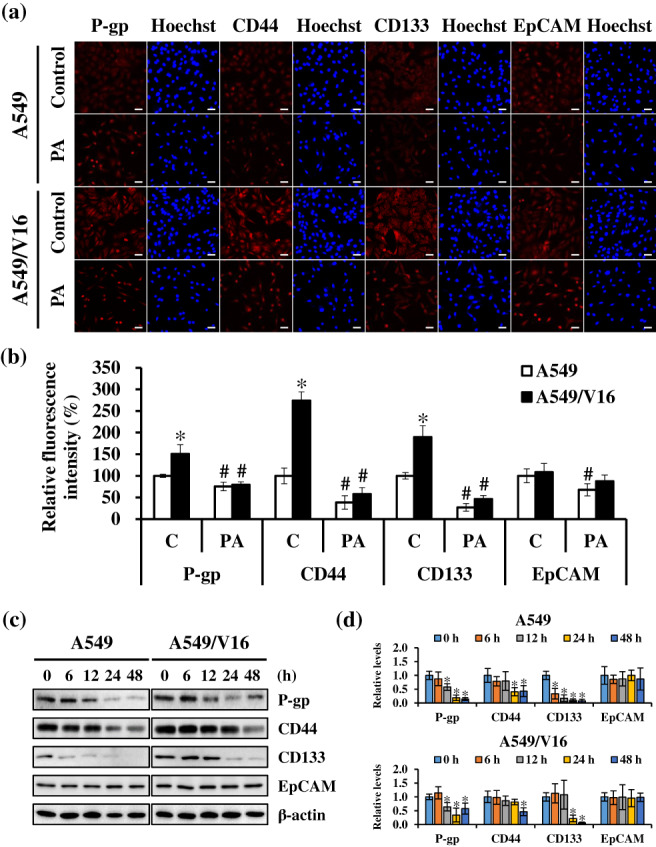
Patchouli alcohol (PA) attenuated the properties of cancer stem cells and drug resistance. (a and b) A549 and A549/V16 cells were treated with the indicated concentrations of PA for 48 h, and protein expressions of P‐gp, CD44, CD133, and EpCAM were detected using immunofluorescence staining. Scale bar: 50 μm. C, control. (c and d) Levels of P‐gp, CD44, CD133, and EpCAM were evaluated using western blotting in cells treated with PA for 6–48 h. β‐actin was an internal control. Data are expressed as the mean ± SD from three independent experiments. *p < 0.05, significant increase compared to A549 cells. # p < 0.05, significant decrease compared with the control.
PA combined with cisplatin synergistically inhibited cell viability
Cisplatin is the first‐line drug for NSCLC and has been recommended in combination with other chemotherapeutic agents for the treatment of advanced lung cancer. Therefore, we explored whether PA works with cisplatin to enhance its growth‐inhibitory activity. Cells were treated with 0–135 μM PA and/or 0–26.6 μM cisplatin for 48 h, and cell viability was determined using the MTT assay. PA treatment promoted antiproliferative effects of cisplatin on NSCLC cells in a concentration‐dependent manner (Figure 7). The drug interactions were determined using the combination index (CI) calculated using CompuSyn software based on the Chou–Talalay method, where CI <1 indicates that the combination has a potential synergistic effect. Most of the CI values for PA combined with cisplatin were less than 1: particularly for 135 μM PA and 26.6 μM cisplatin, CI was 0.68 in A549 and for 135 μM PA and 13.3 μM cisplatin, CI was 0.76 in A549/V16. Collectively, these results suggest that PA/cisplatin cotreatment synergistically suppresses the proliferative ability of NSCLC and VCR‐resistant NSCLC cells.
FIGURE 7.
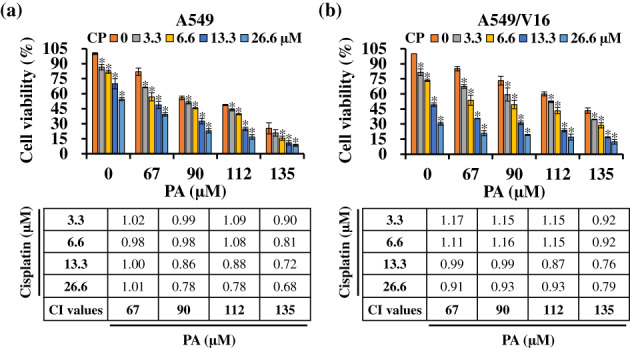
The combination of patchouli alcohol (PA) and cisplatin synergistically inhibited cell growth. A549 (a) and A549/V16 (b) cells were treated with the indicated concentrations of PA and cisplatin for 48 h, and cell viability was measured using 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5 diphenyl tetrazolium bromide (MTT) assay. The combination index (CI) of the data was calculated using CompuSyn software to determine the drug–drug interactions, including synergism (CI < 1), additive effects (CI = 1), and antagonism (CI > 1). Results are expressed as the mean ± SD from three independent experiments. *p < 0.05, compared with the control.
DISCUSSION
Our previous study indicated that PA was the major component (approximately 32.12%) in steam‐distilled extract of Pogostemon cablin, as determined using GC/MS analysis. 23 Evidence has shown that PA exhibits multiple biofunctions, including anti‐inflammatory, antiviral, antimicrobial, and anticancer properties. 20 , 21 , 22 , 23 , 24 , 25 PA has inhibitory activities on the growth of colorectal, prostate, gastric, and lung cancer cells. 24 , 25 , 30 , 31 , 32 In NSCLC, PA treatment suppressed tumor growth in vitro and in vivo by blocking the phosphorylation of EGFR and MER/ERK and activating the JNK pathway. 25 Furthermore, PA arrested the G1/S cycle distribution by affecting p53/p21 and CDK2/cyclin E and activated mitochondrial‐mediated apoptosis by regulating Bcl‐2/Bax/caspase‐9/‐3. Moreover, PA induces autophagy in NSCLC cells by downregulating the Akt/mTOR signaling pathway. 24 VCR has been used in various cancer chemotherapy regimens. 13 However, cancer cells gradually develop resistance to VCR during treatment. Thus, we investigated whether PA had anticancer effects in VCR‐resistant A549/V16 cells. The results showed that PA enhanced ROS‐mediated DNA damage and activated the ATM‐CHK2 and ATR‐CHK1 signaling pathways in both A549 and A549/V16 cells. PA further induced G0/G1 cell cycle arrest and apoptosis by regulating the expression of p53/p21/CKD2/cyclin E1 and Bcl‐2/Bax/caspase‐9/‐3, respectively, consistent with the results in above studies. Moreover, PA reduced drug resistance (P‐gp) and CSCs (CD44, CD133, and EpCAM) markers and synergistically inhibited the growth of NSCLC cells in combination with cisplatin. Based on multifaceted evidence, we propose regulatory pathways for growth inhibition and ROS‐mediated apoptosis induction by PA in NSCLC with or without VCR resistance.
ROS, including singlet oxygen, superoxide, hydroxyl radical, nitric oxide, and hydrogen peroxide, are important secondary messengers in the regulation of cell death or survival when cells are exposed to different stimuli, such as stress conditions, UV radiation, senescence, host defenses, and chemotherapeutic agents. 33 Physiological levels of ROS are responsible for intracellular metabolism, cell signaling for cell growth, differentiation, survival and pathogen defense. 34 However, overexpressed ROS damages cellular components, including carbohydrates, lipids, proteins, and DNA/RNA, resulting in permanent functional changes. 35 Accumulating evidence indicates that ROS‐mediated DNA damage plays a critical role in cancer therapy by triggering DDR mechanisms to promote cell cycle arrest or activate apoptosis. 36 Dysregulation of cell cycle progression and apoptosis are common in cancer cells, and both are often regarded as targets for cancer treatment. In this study, the results showed that PA enhanced the accumulation of intracellular ROS and the expression of the oxidative DNA damage marker 8‐OHdG. Furthermore, PA‐treated cells showed an increasing percentage of cells arrested at G0/G1 or TUNEL‐positive results. These findings suggest that PA induces ROS‐mediated DNA damage, leading to cell cycle arrest and apoptosis in VCR‐resistant NSCLC cells.
ATM and ATR kinases are important sensors of DNA damage in response to oxidative stress and phosphorylate various downstream signaling cascade molecules, including γ‐H2AX, CHK1, and CHK2. 37 , 38 When CHK1 and CHK2 are activated, they phosphorylate the tumor suppressor protein p53, thereby initiating cell cycle checkpoints and DNA repair. 39 The CDK inhibitor p21, regulated by p53, is a cell cycle regulator that triggers cell cycle arrest in the G0/G1 phase by inhibiting the binding of the CDK2/cyclin E1 complex. 40 In contrast, p53 has an important effect on the initiation of apoptosis by regulating proapoptotic (such as Bax and Bad) and antiapoptotic (such as Bcl‐2 and Bcl‐xL) factors. 41 Increasing the ratio of Bax/Bcl‐2 may lead to enhanced permeability of the mitochondrial membrane, release of cytochrome c into the cytoplasm, and activation of the caspase cascade, resulting in the induction of mitochondrial‐mediated apoptosis. 41 In the current study, after the cells were treated with PA, the expressions of p‐H2A.X and the ATM and ATR downstream effector proteins, CHK1, CHK2, and p53, were upregulated. The expression of p21 was increased, while that of CDK2/cyclin E1 was reduced; the ratio of Bax/Bcl‐2 and the expression of cleaved caspase‐3/9 were increased. These results indicate that ROS‐mediated DNA damage by PA triggers DDR, cell cycle arrest, and intrinsic apoptosis via regulation of CHK1/CHK2/p53, CDK2/cyclin E1, and Bax/Bcl‐2/caspase‐9/‐3, respectively.
Researchers have explored chemotherapy resistance in cancer cells and identified several mechanisms underlying multidrug resistance, including (i) increased drug pumping by efflux pumps, such as ABCB1‐encoded P‐glycoprotein (P‐gp), (ii) decreased drug uptake, (iii) enhancement of intracellular drug metabolizing enzymes, (iv) promotion of DNA repair systems, (v) evasion of apoptosis, and (vi) phenotype of CSCs. 42 , 43 The high drug resistance rate of lung cancer may be closely related to the expression of CSCs‐related biomarkers, such as CD44, CD133, and EpCAM, whose mechanisms of action are as follows: (i) CSCs have strong self‐repair ability and low levels of ROS, avoiding the accumulation of DNA damage through the DNA repair system; and (ii) CSCs are in a stagnant state (G0 phase), without effective division and proliferation. 44 , 45 Studies have indicated that targeting P‐gp or CSCs is a strategy for the treatment of drug‐resistant cancer cells. 15 , 43 Our results showed that A549/V16 cells had higher expression of P‐gp, CD44, and CD133 than A549 cells, suggesting that drug pumping out and CSCs characteristics cause the development of VCR resistance in NSCLC cells. However, PA treatment reduced the expression of these proteins in both cell lines, especially in A549/V16 cells. Thus, PA exerts potent anti‐MDR activity in vitro through the attenuation of P‐gp expression and CSCs phenotype in VCR‐resistant NSCLC cells.
Chemotherapy is the classical treatment for lung cancer and includes broad‐spectrum agents such as cisplatin, paclitaxel, and doxorubicin. 46 Cisplatin exerts its anticancer activity by covalently binding to genomic DNA or mitochondrial DNA causing DNA adduct formation and damage, interfering with DNA repair, replication, and transcription, and, finally leading to cell cycle arrest and apoptosis. 47 However, cisplatin has several limitations associated with drug resistance and multiorgan toxicity, including nephrotoxicity, hepatotoxicity, neurotoxicity, and cardiotoxicity. 48 Numerous recent studies have shown that the combination of cisplatin and natural products is a beneficial strategy to attenuate the development of drug resistance and side effects during cisplatin treatment. 48 Therefore, in this study, cisplatin combined with PA was used to treat A549 and A549/V16 cells, and drug–drug interactions were analyzed using the CompuSyn software. The results indicated that the cell viabilities of the drug combination group were lower than those of the cisplatin or PA groups, and most of the CI values were less than 1. This suggests that the combination of cisplatin and PA had synergistic anticancer activity, and PA might be a potentially effective adjuvant in combination therapy for patients with NSCLC.
In conclusion, our results showed that PA suppressed cell proliferation by inducing ROS‐mediated DNA damage, G0/G1 arrest, and apoptosis; attenuated drug resistance by reducing P‐gp expression and CSCs phenotype; and had synergistic effects on growth inhibition in combination with cisplatin in A549 and A549/V16 cells. The findings from the present study indicate that PA may be a promising therapeutic agent or adjuvant for the treatment of NSCLC with or without VCR resistance.
AUTHOR CONTRIBUTIONS
C.‐Y.L., C.‐Y.H., and N.‐M.T. conceived the study and provided the funding; C.‐Y.L., K.‐F.C., Y.‐C.H., and C.‐F.K. performed the experiments and analyzed data; X.‐F.H. and G.‐T.S. conducted statistical analysis; C.‐Y.L. and K.‐F.C. wrote the manuscript. C.‐Y.H. and N.‐M.T. modified the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
ACKNOWLEDGMENTS
Flow cytometry was performed in the Instrument Center of Chung Shan Medical University, which is supported by National Science Council, Ministry of Education and Chung Shan Medical University.
This study was supported by Ditmanson Medical Foundation Chia‐Yi Christian Hospital (grant no. R111‐12); the Ministry of Science and Technology (grant nos. MOST 109‐2320‐B‐040‐012, MOST 110‐2320‐B‐040‐006 and MOST 111‐2320‐B‐040‐022), Taiwan, Republic of China.
Liang C‐Y, Chang K‐F, Huang Y‐C, Huang X‐F, Sheu G‐T, Kuo C‐F, et al. Patchouli alcohol induces G0 /G1 cell cycle arrest and apoptosis in vincristine‐resistant non‐small cell lung cancer through ROS‐mediated DNA damage. Thorac Cancer. 2023;14(21):2007–2017. 10.1111/1759-7714.14982
Chi‐Yen Liang and Kai‐Fu Chang are two first authors contributed equally to this work.
Contributor Information
Chih‐Yen Hsiao, Email: 04504@cych.org.tw.
Nu‐Man Tsai, Email: numan@csmu.edu.tw.
DATA AVAILABILITY STATEMENT
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2. Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol off J Am Soc Clin Oncol. 2013;31(8):992–1001. [DOI] [PubMed] [Google Scholar]
- 3. Gridelli C, Rossi A, Carbone DP, Guarize J, Karachaliou N, Mok T, et al. Non‐small‐cell lung cancer. Nat Rev Dis Primers. 2015;1:15009. [DOI] [PubMed] [Google Scholar]
- 4. Reck M, Rabe KF. Precision diagnosis and treatment for advanced non‐small‐cell lung cancer. N Engl J Med. 2017;377(9):849–61. [DOI] [PubMed] [Google Scholar]
- 5. Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The effect of advances in lung‐cancer treatment on population mortality. N Engl J Med. 2020;383(7):640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Janku F, Stewart DJ, Kurzrock R. Targeted therapy in non‐small‐cell lung cancer—is it becoming a reality? Nat Rev Clin Oncol. 2010;7(7):401–14. [DOI] [PubMed] [Google Scholar]
- 7. Duma N, Santana‐Davila R, Molina JR. Non‐small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94(8):1623–40. [DOI] [PubMed] [Google Scholar]
- 8. Shergold AL, Millar R, Nibbs RJB. Understanding and overcoming the resistance of cancer to PD‐1/PD‐L1 blockade. Pharmacol Res. 2019;145:104258. [DOI] [PubMed] [Google Scholar]
- 9. Terlizzi M, Colarusso C, Pinto A, Sorrentino R. Drug resistance in non‐small cell lung cancer (NSCLC): impact of genetic and non‐genetic alterations on therapeutic regimen and responsiveness. Pharmacol Ther. 2019;202:140–8. [DOI] [PubMed] [Google Scholar]
- 10. Freireich EJ, Wiernik PH, Steensma DP. The leukemias: a half‐century of discovery. J Clin Oncol off J Am Soc Clin Oncol. 2014;32(31):3463–9. [DOI] [PubMed] [Google Scholar]
- 11. Dhyani P, Quispe C, Sharma E, Bahukhandi A, Sati P, Attri DC, et al. Anticancer potential of alkaloids: a key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022;22(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jordan MA. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr Med Chem Anticancer Agents. 2002;2(1):1–17. [DOI] [PubMed] [Google Scholar]
- 13. Skubnik J, Pavlickova VS, Ruml T, Rimpelova S. Vincristine in combination therapy of cancer: emerging trends in clinics. Biology (Basel). 2021;10(9):849–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP‐dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. [DOI] [PubMed] [Google Scholar]
- 15. Syed SB, Lin SY, Arya H, Fu IH, Yeh TK, Charles MRC, et al. Overcoming vincristine resistance in cancer: computational design and discovery of piperine‐inspired P‐glycoprotein inhibitors. Chem Biol Drug des. 2021;97(1):51–66. [DOI] [PubMed] [Google Scholar]
- 16. Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5(4):275–84. [DOI] [PubMed] [Google Scholar]
- 17. Wu MF, Huang YH, Chiu LY, Cherng SH, Sheu GT, Yang TY. Curcumin induces apoptosis of chemoresistant lung cancer cells via ROS‐regulated p38 MAPK phosphorylation. Int J Mol Sci. 2022;23(15):8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Z, Zhu H, Qu S, Tang L, Cao L, Yu W, et al. Pyramidatine (Z88) sensitizes vincristine‐resistant human oral cancer (KB/VCR) cells to chemotherapeutic agents by inhibition of P‐glycoprotein. Anticancer Agents Med Chem. 2018;18(2):286–94. [DOI] [PubMed] [Google Scholar]
- 19. Liu Z, Wen X, Wang G, Zhou Y. Involvement of P‐gp on reversing multidrug resistance effects of 23‐hydroxybetulinic acid on chemotherapeutic agents. Front Pharmacol. 2021;12:796745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeong JB, Shin YK, Lee SH. Anti‐inflammatory activity of patchouli alcohol in RAW264.7 and HT‐29 cells. Food Chem Toxicol. 2013;55:229–33. [DOI] [PubMed] [Google Scholar]
- 21. Yu Y, Zhang Y, Wang S, Liu W, Hao C, Wang W. Inhibition effects of patchouli alcohol against influenza a virus through targeting cellular PI3K/Akt and ERK/MAPK signaling pathways. Virol J. 2019;16(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhong Y, Tang L, Deng Q, Jing L, Zhang J, Zhang Y, et al. Unraveling the novel effect of patchouli alcohol against the antibiotic resistance of helicobacter pylori. Front Microbiol. 2021;12:674560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang XF, Sheu GT, Chang KF, Huang YC, Hung PH, Tsai NM. Pogostemon cablin triggered ROS‐induced DNA damage to arrest cell cycle progression and induce apoptosis on human hepatocellular carcinoma In vitro and In vivo. Molecules. 2020;25(23):5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang L, Chen H, Li R, Li H, Rui X, Zhou L, et al. Mufangji decoction and its active ingredient patchouli alcohol inhibit tumor growth through regulating Akt/mTOR‐mediated autophagy in nonsmall‐cell lung cancer. Evid Based Complement Alternat Med. 2021;2021:2373865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lu X, Yang L, Lu C, Xu Z, Qiu H, Wu J, et al. Molecular role of EGFR‐MAPK pathway in patchouli alcohol‐induced apoptosis and cell cycle arrest on A549 cells in vitro and in vivo. Biomed Res Int. 2016;2016:4567580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chiu LY, Ko JL, Lee YJ, Yang TY, Tee YT, Sheu GT. L‐type calcium channel blockers reverse docetaxel and vincristine‐induced multidrug resistance independent of ABCB1 expression in human lung cancer cell lines. Toxicol Lett. 2010;192(3):408–18. [DOI] [PubMed] [Google Scholar]
- 27. Chou TC. Drug combination studies and their synergy quantification using the Chou‐Talalay method. Cancer Res. 2010;70(2):440–6. [DOI] [PubMed] [Google Scholar]
- 28. Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22(3):377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang Y, Karakhanova S, Hartwig W, D'Haese JG, Philippov PP, Werner J, et al. Mitochondria and mitochondrial ROS in cancer: novel targets for anticancer therapy. J Cell Physiol. 2016;231(12):2570–81. [DOI] [PubMed] [Google Scholar]
- 30. Jeong JB, Choi J, Lou Z, Jiang X, Lee SH. Patchouli alcohol, an essential oil of Pogostemon cablin, exhibits anti‐tumorigenic activity in human colorectal cancer cells. Int Immunopharmacol. 2013;16(2):184–90. [DOI] [PubMed] [Google Scholar]
- 31. Cai J, Zhao J, Gao P, Xia Y. Patchouli alcohol suppresses castration‐resistant prostate cancer progression by inhibiting NF‐kappaB signal pathways. Transl Androl Urol. 2022;11(4):528–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Song Y, Chang L, Wang X, Tan B, Li J, Zhang J, et al. Regulatory mechanism and experimental verification of patchouli alcohol on gastric cancer cell based on network pharmacology. Front Oncol. 2021;11:711984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gupta SC, Hevia D, Patchva S, Park B, Koh W, Aggarwal BB. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal. 2012;16(11):1295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21(7):363–83. [DOI] [PubMed] [Google Scholar]
- 35. Brieger K, Schiavone S, Miller FJ Jr, Krause KH. Reactive oxygen species: from health to disease. Swiss Med Wkly. 2012;142:w13659. [DOI] [PubMed] [Google Scholar]
- 36. Srinivas US, Tan BWQ, Vellayappan BA, Jeyasekharan AD. ROS and the DNA damage response in cancer. Redox Biol. 2019;25:101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marechal A, Zou L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol. 2013;5(9):a012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kastan MB, Bartek J. Cell‐cycle checkpoints and cancer. Nature. 2004;432(7015):316–23. [DOI] [PubMed] [Google Scholar]
- 39. Roos WP, Thomas AD, Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer. 2016;16(1):20–33. [DOI] [PubMed] [Google Scholar]
- 40. Warfel NA, El‐Deiry WS. p21WAF1 and tumourigenesis: 20 years after. Curr Opin Oncol. 2013;25(1):52–8. [DOI] [PubMed] [Google Scholar]
- 41. Reed JC. Mechanisms of apoptosis. Am J Pathol. 2000;157(5):1415–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stavrovskaya AA, Rybalkina EY. Recent advances in the studies of molecular mechanisms regulating multidrug resistance in cancer cells. Biochemistry (Mosc). 2018;83(7):779–86. [DOI] [PubMed] [Google Scholar]
- 43. Cho Y, Kim YK. Cancer stem cells as a potential target to overcome multidrug resistance. Front Oncol. 2020;10:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suresh R, Ali S, Ahmad A, Philip PA, Sarkar FH. The role of cancer stem cells in recurrent and drug‐resistant lung cancer. Adv Exp Med Biol. 2016;890:57–74. [DOI] [PubMed] [Google Scholar]
- 45. Yang B, Wang Y, Chen Z, Feng YM, Shi LL. Effects of apatinib on the "stemness" of non‐small‐cell lung cancer cells In vivo and its related mechanisms. Can Respir J. 2020;2020:2479369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. NSCLC Meta‐Analyses Collaborative Group . Chemotherapy in addition to supportive care improves survival in advanced non‐small‐cell lung cancer: a systematic review and meta‐analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26(28):4617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Achkar IW, Abdulrahman N, Al‐Sulaiti H, Joseph JM, Uddin S, Mraiche F. Cisplatin based therapy: the role of the mitogen activated protein kinase signaling pathway. J Transl Med. 2018;16(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dasari S, Njiki S, Mbemi A, Yedjou CG, Tchounwou PB. Pharmacological effects of cisplatin combination with natural products in cancer chemotherapy. Int J Mol Sci. 2022;23(3):1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.


