Abstract
Neutrophils are important immune cells act as the body's first line of defense against infection and respond to diverse inflammatory cues. Many studies have demonstrated that neutrophils display plasticity in inflammatory diseases and cancers. Clarifying the role of neutrophil heterogeneity in inflammatory diseases and cancers will contribute to the development of novel treatment strategies. In this review, we have presented a review on the development of the understanding on neutrophil heterogeneity from the traditional perspective and a high‐resolution viewpoint. A growing body of evidence has confirmed the double‐edged role of neutrophils in inflammatory diseases and tumors. This may be due to a lack of precise understanding of the role of specific neutrophil subsets in the disease. Thus, elucidating specific neutrophil subsets involved in diseases would benefit the development of precision medicine. Thusly, we have summarized the relevance and actions of neutrophil heterogeneity in inflammatory diseases and cancers comprehensively. Meanwhile, we also discussed the potential intervention strategy for neutrophils. This review is intended to deepen our understanding of neutrophil heterogeneity in inflammatory diseases and cancers, while hold promise for precise treatment of neutrophil‐related diseases.
Keywords: cancers, inflammatory diseases, neutrophil extracellular traps, neutrophil heterogeneity, single‐cell sequencing
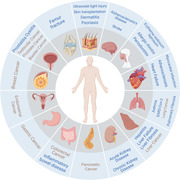
Schematic representation of the neutrophil heterogeneity in inflammatory diseases and cancer (in blue). This figure is created with BioRender.com.
1. INTRODUCTION
Neutrophils are important immune cells, defensing against microbial infections and eliminating pathogenic bacteria. 1 Differentiated and developed in the bone marrow, neutrophils would release and migrate into the blood or tissues, accounting for approximately 50−70% of circulating leukocytes. 2 Various antimicrobial enzymes are found in neutrophils, including myeloperoxidase, acid hydrolytic enzymes, and elastase. 3 As a result of being stimulated by the products of pathogenic bacteria, neutrophils would produce antimicrobial enzymes and display their defensive capabilities. Aside from its important defensive role in combating infections, neutrophils could also trigger inflammatory responses at the site of infection, which in turn lead to immunopathological changes. 4 , 5 Therefore, it is important to clarify the double‐edged role of neutrophils in the body.
Studies in mounting numbers have identified that phenotypically and functionally distinct neutrophil subtypes that exist in circulating or tissue neutrophils under physiological or pathological conditions. 6 In recent years, with the development of single‐cell multiomics sequencing technology and mass cytometry, the accurate and unbiased classification of neutrophils has greatly facilitated our understanding of neutrophil heterogeneity. 7 , 8 A growing number of researchers are finding that the role of different neutrophil subsets in disease may be complex, as some subsets can contribute to the disease process while others would inhibit disease progression. 9 The dual role of neutrophil heterogeneity in inflammatory diseases and cancers has gained increasing attention. 10 , 11 It is hoped that elucidating the role of neutrophil heterogeneity in diseases would lead to a new era of precision medicine in inflammation and cancer‐related diseases.
Therefore, this review aims to highlight the double‐edged role of neutrophils in diseases, systematically summarizing the important roles of neutrophil heterogeneity in inflammatory diseases and cancers. Meanwhile, the potential intervention strategy for neutrophils has been discussed. Overall, this review is intended to deepen our understanding of neutrophil heterogeneity in inflammatory diseases and cancers, holding promise for precise treatment of neutrophil‐related diseases (Figure 1).
FIGURE 1.
Schematic representation of the neutrophil heterogeneity in inflammatory diseases and cancers (in blue). This figure is created with BioRender.com.
2. THE FUNCTIONS AND THE DOUBLE‐EDGED ROLE OF NEUTROPHILS
2.1. Neutrophils in homeostasis
Neutrophils have long been considered as an important part of the innate immune system, playing a pivotal role in controlling infectious diseases. 12 In physiological context, inactivated neutrophils move slowly in the circulation. The origin of neutrophils is derived from hematopoietic cords located at venous sinuses in the bone marrow and derive from a common committed myeloid progenitor cell. 1 The neutrophils development are stimulated by transcription factors, proteins, and receptors such as Egr1, HoxB7, S100A8, S100A9, N‐formyl‐methionyl‐leucyl‐phenylalanine receptor and granulocyte‐macrophage colony stimulating factor receptor. 13 , 14 , 15 , 16 The neutrophil maturation process consists of the following steps: myeloblast→promyelocyte→myelocyte→metamyelocyte→band cell→polymorphonuclear segmented cell. In general, granulocyte colony stimulating factor (G‐CSF) directs the commitment of progenitor cells to the myeloid lineage, promotes proliferation of neutrophil precursors, reduces the transit time through the compartment, and releases of mature neutrophils out of the bone marrow. Once maturation has been completed in the bone marrow and the pool of mature cells is released into the circulation, neutrophils circulate with a set of preformed adhesion and chemotactic receptors and effector proteins to rapidly migrate and respond to multiple microbial and sterile challenges. 17
2.2. Neutrophils in response to stimulation
Upon stimulation by external signals, these neutrophils move rapidly toward the injury site and effect in eliminating pathogenic bacteria and phagocytosis of injured cells. 18 , 19 Neutrophils and their derivatives, such as cytokines, reactive oxygen species (ROS), and neutrophils extracellular traps (NETs), are involved in both innate and adaptive immune responses, influencing the function of other immune cells in various ways and thus participate in the disease process. 18 Neutrophils and NETs could promote macrophage release cytokine while enhancing dendritic cells recruitment and antigen presentation. 20 Furthermore, by regulating cytokine and growth factor production, neutrophils would promote innate immune resolution and repair. In addition, neutrophils might trigger B cell expansion and antibody production. 21 Neutrophils and their derivatives could also affect T cell cytokine production and differentiation, thereby influencing T cell function. 22 During the process of infection or tissue damage, a diverse array of signals could mediate neutrophil activation and forward migration toward the site of damage. Many studies have found that blocked recruitment of neutrophils would lead to the spread of pathogens to the blood and vital organs, resulting in systemic infection and even death. 23 , 24 , 25 Important signals establishing chemoattractant gradients that could take effect in neutrophil recruitment and migration include pathogen‐associated molecular patterns and/or damage‐associated molecular patterns (DAMPs), hydrogen peroxide (H2O2), fMet‐Leu‐Phe (fMLP), lipid mediators, chemokines, and so on. 4 , 5 , 6 As early signals, DAMPs have been reported to be responsible for early neutrophil recruitment. DAMPs could be sensed by pattern recognition receptors (PRRs), including Toll‐like receptors (TLRs) and NOD‐like receptors. 26 , 27 They would bind to and activate G‐protein‐coupled receptors (GPCRs) on neutrophils, thereby inducing resident cells to produce inflammatory mediators and to modulate neutrophil migration, function and behavior. 28 N‐formyl peptides, such as fMLP, is another early signal which could trigger neutrophil chemotaxis and activation. 29 , 30 , 31 Derived from bacterial proteins or released from mitochondria after tissue damage, they would activate human neutrophils by binding to the GPCRs FPR1, FPR2, and FPR3. H2O2 could promote chemotaxis of human and mouse neutrophils. 32 , 33 At present, SRC family kinases (SFKs) have been reported to be a direct sensor to H2O2, required for neutrophils. 32 Interestingly, the peak of H2O2 production occurred around 30 min after injury, and the signal was completely abolished by myeloperoxidase activity of injury‐associated neutrophils 1 h later. 34 Chemokines and lipid mediators usually cause long‐term chemotactic signaling in order to facilitate more sustained recruitment and migration of neutrophils. 35 , 36 , 37 Many studies have reported the involvement of chemokines in neutrophil migration, including CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8. 38 , 39 , 40 These chemokines may be derived from immune and nonimmune cells during tissue infection and injury. 41 , 42 Neutrophils would sense these chemokines through the GPCRs, CXCR1, and CXCR2, 43 , 44 , 45 leading to downstream signaling activation of vasodilator‐stimulated phosphoprotein, phosphoinositide 3‐kinase, and SFKs, contributing to neutrophil‐directed migration. 46 , 47 Lipid mediators, including leukotriene B4 (LTB4), 5‐oxo‐ETE, and 5‐KETE, derived from arachidonic acid, could induce neutrophil chemotaxis. 48 , 49 , 50 During the process of chemotaxis, neutrophils must integrate multiple chemical signals, respond to physical constraints, and prioritize their directional decisions to generate an efficient immune response. A recent study reported that neutrophils were more likely to choose paths with the steepest chemoattractant gradient and the most direct approach angle, and the migration efficiency across planar chambers was inversely correlated with chamber diameter. 51 Many studies have focused on the effect of specific molecules on the migration efficiency of neutrophils. For example, G‐protein‐coupled receptor GPR35 was upregulated in activated neutrophils, and it promoted their migration. 52 Another study reported that CXCL1, derived from endothelial cells, and CXCL2, which was derived from neutrophils, acted in a sequential manner to guide neutrophils through venular walls. 53 In addition, many other molecules, including filamin A, ICAM‐1, IL‐8, G protein signaling 5 (RGS5), Glia maturation factor‐gamma, and MMP2, have also been reported to be involved in affecting the efficiency of neutrophil migrate. 54 , 55 , 56 , 57 , 58 At the site of damaged or infected tissue, neutrophils would fight against pathogens mainly through phagocytosis and production of ROS, bactericidal granulocytes, and NETs. 59 We would focus on the specific effects of neutrophils in the context of different diseases. After the acute inflammatory phase, neutrophil resolution has been shown to be critical in preventing tissue damage and transition to chronic wounds. 60 Neutrophil resolution would occur through neutrophil apoptosis or necrosis, with subsequent clearance by macrophages. 61 Interestingly, some studies have reported that neutrophils would leave the site of damaged or infected tissue in a process termed neutrophil reverse migration. 5 , 61 Wang et al. 62 reported that neutrophils can migrate from the site of inflammation to the blood vessels, and then subsequently return to the bone marrow to promote apoptosis. This process has been known as reverse migration and could also occur through reverse transendothelial migration. 63 Neutrophils could leave the wound in other ways as well, such as metastasis from adjacent tissues and lymphatic metastasis, and eventually spread to other parts of the body. 64 The reverse migration of neutrophils played a crucial role in accelerating the resolution of inflammation. 65 However, another study reported that the return of neutrophils from the wound site to the blood vessel might contribute to the spread of systemic inflammation. 66 This contradiction may be caused by different disease backgrounds. The possible mechanisms of neutrophil reverse migration involved adhesion molecule C (JAM‐C)‐mediated abnormal endothelial cell function, HIF signaling pathway mechanism, lipid mediating mechanism, and CXCL12/CXCR4 signal axis mechanism. 67 , 68 , 69 , 70 In addition, macrophages could also promote neutrophil reverse migration. 71 Moreover, a recent study highlighted the importance of vascular permeability in neutrophil reverse migration. 72 Several studies have described the important role of neutrophil swarming in diseases. 73 , 74 Neutrophil swarming is the formation of a population of neutrophils in infected tissues. The coordination of this population response is essential for the removal of bacteria. 75 As reported, a cell‐intrinsic stop mechanism for the self‐organization of collective behavior suggested the crucial role of GPCR desensitization in attenuating the self‐organized swarming dynamics of neutrophils. In detail, when neutrophils sense high concentrations of swarm‐secreted attractants (LTB4 and CXCL2), the GPCR kinase GRK2 desensitizes the corresponding GPCRs to induce migration arrest. 73 This is a critical step for tissue repair after infection. To date, however, the exact mechanism of neutrophil reverse migration remains unclear. Further research is required to determine the effect of neutrophil reverse migration on different diseases and how to accurately intervene.
2.3. The double‐edged role of neutrophils
Neutrophils play a complex role in diseases, with the ability to both promote and inhibit disease progression. They can promote tumor growth and metastatic progression through the secretion of tumor‐promoting cytokines, enhancement of tumor angiogenesis, modulation of the extracellular matrix, support of tumor cell dissemination, priming of the premetastatic niche, and suppression of antitumor immune responses. 76 , 77 , 78 However, neutrophils can also limit tumor growth and metastatic progression through direct cytotoxicity, antibody‐dependent cell‐mediated cytotoxicity, and inducing adaptive antitumor immunity. 79 , 80 , 81 , 82 The double‐edged role of neutrophils in diseases, both promoting and suppressing diseases, has garnered attention from researchers, with most recent studies highlighting the existence of different subsets of neutrophils with varying functions in blood or tissues. 83 , 84 Neutrophils exhibit heterogeneity in both physiological and pathological conditions. 83 Specific signals at different developmental stages or disease backgrounds would drive neutrophil heterogeneity. 9 We will discuss this in detail in the context of different diseases. Clarification of neutrophil heterogeneity may help explain the dual role of neutrophils in diseases, and understanding the complicated role of neutrophils might benefit the development of treatment for a great many diseases. Therefore, in this review, we focus on how to analyze neutrophil heterogeneity and its double‐sided role in the course of inflammatory diseases and tumors.
3. METHODS OF STUDYING NEUTROPHIL HETEROGENEITY
The advent of high‐resolution methods coupled with investigations of traditional methods in recent years has helped us to reveal the presence of heterogeneity in neutrophils, as presented in various phenotypes, under physiological and pathological contexts 83 , 85 , 86 (Figure 2). Neutrophils from different subpopulations function diversely in inflammatory diseases and cancers, which merits further exploration. 87 , 88 , 89
FIGURE 2.
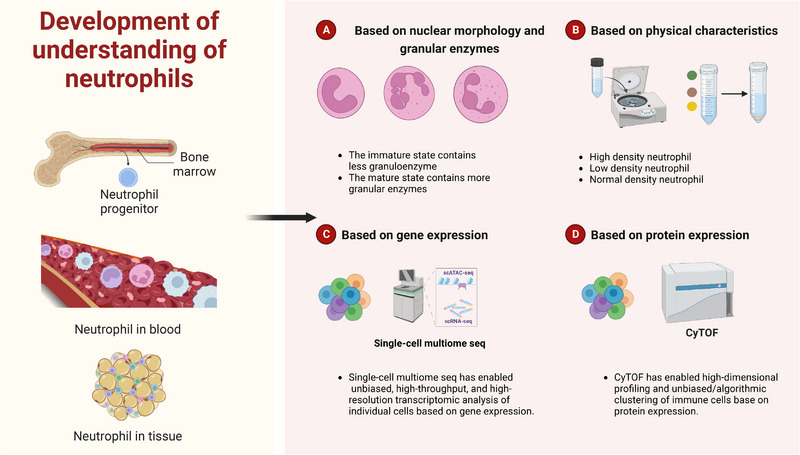
Development of understanding of neutrophils. This figure is created with BioRender.com.
3.1. Studies on neutrophils by the traditional methods
Neutrophil heterogeneity has historically been defined as high‐ and low‐density neutrophils (HDNs and LDNs) through its physical properties. 90 , 91 , 92 , 93 This easy classification of cells has resulted in the retention of this widely accepted definition, despite the lack of information at high resolution. 90 , 93 It is generally accepted that LDNs are immature neutrophils containing few granule enzymes, whereas HDNs are mature neutrophils with higher levels of granule enzymes. 10 , 83 , 94 , 95 , 96 However, complex transitions between these two subpopulations occur in pathological scenarios. 97 , 98 Neutrophils often undergo changes in their nucleus and surface marker genes as they mature and develop. 10 , 99 There are three neutrophil subsets in the mouse bone marrow, including a committed proliferative precursor terming preneutrophils, which would sequentially differentiate into nonproliferating immature and mature neutrophils. Preneutrophils express CD177, whereas differential expression of C‐X‐C chemokine receptor 4 (CXCR4), CXCR2 and CD101 allows for discrimination between immature neutrophils (which are CXCR2−CD101−) and mature neutrophils (which are CXCR2+CD101+). 99 , 100 , 101 As neutrophils mature, surface expression of markers gradually increases, such as CD62L, CD101, or glycosylphosphatidylinositol‐linked receptor Ly6G in mice. 83 , 94 Physiologically, neutrophils would release into the circulation and enter into tissues within a few days, often relying on specific adhesion molecules and regulated by circadian rhythm. 98 , 102 , 103 , 104 , 105 , 106 While under pathological conditions, neutrophils behave in a more complex manner. To be specific, neutrophils die in inflamed or damaged tissues and are even recruited back to the bone marrow by regulating the expression of related receptors. 62 , 107 Attempts have also been made to differentiate the functional differences of neutrophils in cancers according to their density. 96 , 97 , 98 , 108 , 109 Even so, this relies on non‐high‐resolution information, and it often appears to be inaccurate. As an example, some studies have demonstrated that immature LDNs have the ability to promote cancer progression or immunosuppression. 110 , 111 , 112 While some other studies have suggested that mature neutrophils may also possess these same properties. 97 , 98 , 113 Consequently, researches on neutrophil heterogeneity under non‐high‐resolution conditions have been of limited precision and have substantially limited our understanding.
3.2. Studies on neutrophils by high‐resolution methods
Recently, the development of novel technologies, including single‐cell transcriptome sequencing, single‐cell assay for transposase‐accessible chromatin sequencing and mass cytometry, has greatly promoted the accurate and unbiased classification of neutrophils, thus contributing to the advancement of neutrophil heterogeneity. 114 , 115 , 116 , 117 In humans, mice, non‐human primates, and even other mammals, researchers have examined the heterogeneity of neutrophils derived from bone marrow in depth, laying the foundation for understanding how neutrophils develop. 101 , 118 , 119 , 120 , 121 , 122 Studies have revealed that in healthy individuals, 45−65% of circulating neutrophils are CD177+, and about 20−25% of circulating neutrophils express the glycoprotein olfactomedin 4 (OLFM4). 10 , 123 The presence of other circulating neutrophil subpopulations, for example, those expressing the T cell receptor αβ (TCRαβ)+ or proangiogenic CD49d+CXCR4+ vascular endothelial growth factor (VEGFR1)+, is also observed in healthy individuals. 124 , 125 Further evidence has been provided that these circulating neutrophils presented in mice. 126 In addition, the proportion of CXCR4−CD62L+ and CXCR4+CD62L− circulating neutrophils in healthy individuals followed the diurnal regime. The immature CD66b+CD10− neutrophils would generate antibacterial granules, ready to enter into the circulation in response to external signals. 127 These neutrophils, despite their "immature state," are capable of performing innate immune functions and display functional plasticity, as well as immunoregulatory properties. 128 , 129 Physiologically, heterogeneity of circulating neutrophils and neutrophils in tissue is more complex. A more comprehensive discussion would be conducted in the following context relating to multiple diseases. Researchers have developed computational methods to infer cell differentiation trajectories, however, experimental validation has yet to be achieved. 130 , 131 In addition, a further study of the heterogeneity of neutrophils across species is also worthwhile.
4. NEUTROPHIL HETEROGENEITY IN DISEASES
4.1. Neutrophil heterogeneity in inflammation‐related diseases
In both acute and chronic inflammation, the genetic structures of neutrophils appear to undergo a reprogramming process due to the stimulation of external signals, resulting in dynamic transitions between subpopulations. 132 , 133 , 134 In inflammatory diseases, infection or ischemia can induce the production of G‐CSF, GM‐CSF, or other myelopoietic factors, which contribute to different functional neutrophils. 135 , 136 , 137 We will discuss what factors would contribute to neutrophil heterogeneity in the context of diseases. There has been evidence that neutrophil plasticity and heterogeneity are involved in the process of various inflammatory diseases, both physiologically and pathologically. 60 In addition, neutrophils would not only present proinflammation activity via proinflammation neutrophils, neutrophil extracellular traps (NETs), and different neutrophil subsets linked with inflammation process, but also hold anti‐inflammation capacities through various pathways, including anti‐inflammation neutrophils, NETs, and neutrophil subsets that would inhibit inflammation process (Figure 3). Therefore, analyzing neutrophil heterogeneity is of great significance in the field of inflammation‐related diseases, including liver diseases, kidney diseases, respiratory diseases, cardiovascular diseases, and inflammatory bowel diseases (IBDs).
FIGURE 3.
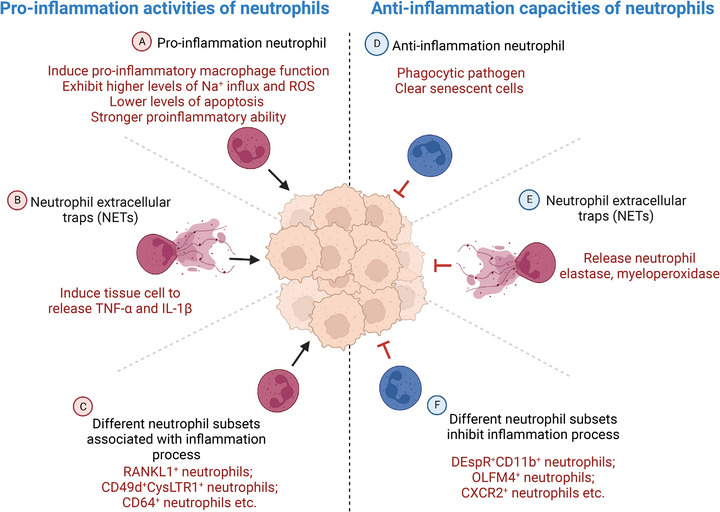
Neutrophils show both proinflammation activity or anti‐inflammation capacities. This figure is created with BioRender.com.
4.1.1. Liver diseases
Liver diseases, including alcoholic liver injury, nonalcoholic fatty liver disease, liver fibrosis, and liver failure, pose serious threats to human life. Neutrophils function vitally in the above‐mentioned diseases. 138 , 139 The accumulation of neutrophils in liver tissue promotes liver injury has been reported by multiple research teams. 140 , 141 , 142 Senger and coworkers 143 found that blocking dipeptidase‐1 on endothelial cells could reduce the recruitment of neutrophils to liver, improving inflammation in liver tissues. Thus, reducing the accumulation of neutrophils in liver tissues could alleviate liver injury, offering new hope for clinical treatment of liver injury. Hwang et al. 144 also reported that the overexpression of C‐X‐C motif chemokine ligand 1 (CXCL1) in mice liver drive steatosis‐to‐nonalcoholic steatohepatitis (NASH) progression through neutrophil‐derived ROS and activation of stress kinases, and this process could be reversed by IL‐22 treatment. Interestingly, another study showed that the absence of neutrophils contributes to resolution of liver inflammation, a process associated with neutrophil‐induced proinflammatory macrophage function. 145 It is likely that neutrophils play different roles for they function distinctively in varied disease stages. HDNs and LDNs exist in liver tissues from clinical samples and alcoholic liver injury mice. Alcohol could induce HDNs to form NETs, thereby leading LDNs to reside in the liver and escape been cleaned‐up by macrophages, causing chronic inflammation. 146 Kolodziejczyk et al. 147 reported the presence of two neutrophil subpopulations in the liver tissue of a mouse model of acute liver failure, among which CXCL2+ neutrophils may be involved in disease progression through their proinflammatory function. In addition, the progression of fibrosis in patients with chronic viral hepatitis has been shown to be associated with IL‐17(+) neutrophils. 148 At present, the liaison between some neutrophil subpopulations and liver‐related diseases has been analyzed. though, the causal relationship between these specific neutrophil subpopulations and diseases remains unclear, warranting further investigation.
4.1.2. Kidney diseases
Glomerulonephritis is a common concomitant of systemic lupus erythematosus (SLE). 149 , 150 The proportion of neutrophils peripheral blood neutrophils and in kidneys is significantly increased in SLE patients and mice. 151 Among them, LDNs exhibited higher activity. 152 Inhibition of infiltrating neutrophils in the kidney ameliorated lupus nephritis progression in mice. 152 Neutrophils also play an important role in other acute and chronic nephritis progression. 153 , 154 Neutrophil subpopulations differ functionally in various kidney diseases. To be specific, the presence of OLFM4+ neutrophils have a negative impact on sepsis‐induced kidney injury, which is associated with renal cell apoptosis and increased plasma creatinine levels. 155 Skopelja‐Gardner et al. 156 reported that acute skin exposure to ultraviolet light drives kidney injury via Ly6G+GFP+ICAM1hiCXCR1lo neutrophils. Another study showed an increase in the proportion of CD14−CD16−CD15+ neutrophils in the peripheral blood of patients with chronic nephritis, and the function of this subset of neutrophils is mainly related to vascular calcification. 157 These findings have further confirmed the significance of neutrophil heterogeneity in nephritis. 89 , 158 Targeting neutrophil elastase has been proven to alleviate chronic nephritis. 159 Nevertheless, the deficiency of efficient drugs that target particular neutrophil subsets exists, requiring additional exploration. There are relatively few studies on neutrophil heterogeneity in renal tissues, and the lack of functional experimental studies on specific neutrophil subsets also warrant further investigation.
4.1.3. Respiratory diseases
The essential role of neutrophils in diseases, including pneumonia, asthma, and chronic obstructive pulmonary disease (COPD), has gradually aroused researchers’ attention. 160 , 161 , 162 During acute lung infection, neutrophils are the first to be recruited in large numbers to the infection site among all cell types, thereby participating in the initial clearance of infected cells. However, a recent study reported that impairing alveoli outnumber alveolar macrophages chemotaxis toward bacteria would induce superfluous neutrophil recruitment, leading to inappropriate inflammation and injury. 163 This illustrates the double‐edged role of neutrophils in respiratory disease. An increasing number of studies in recent years have established the importance of neutrophil heterogeneity in respiratory diseases. 164 , 165 Specifically, the phenotype of neutrophils alters as a disease progresses. 166 The number of CD177+ neutrophils, CD64+ neutrophils and OLFM4+ neutrophils is significantly increased in the peripheral blood of patients with sepsis and asthma, which has been confirmed by several studies. 167 , 168 , 169 , 170 , 171 The CD16high CD62Ldim neutrophils were unveiled to enhance the response to bradykinin in both human isolated small airways and murine tracheae. 172 In hemorrhagic shock and sepsis mouse, inhibition of OLFM4 attenuated lung inflammation in mice. 155 , 173 Acute and chronic respiratory inflammation would mediate the emergence of neutrophil subsets. 174 , 175 Another study revealed the impaired activation and phagocytosis functions of CD123+ neutrophils and PD‐L1+ neutrophils in the peripheral blood of sepsis patients. Subsets that highly express PD‐L1 exerted an immunosuppressive effect, including the inhibition of T cell activation, induction of T cell apoptosis and trans‐differentiation, while the number of CD123+ neutrophils correlated with the severity of the disease. 176 , 177 In a sepsis mouse model, extracellular cold‐inducible RNA‐binding protein (eCIRP) can induce the formation of the proinflammatory phenotype Ly6G+ CD11bhi of LDNs through TLR4. 178 In a mice model of chronic granulomatous pneumonia, G‐CSF mobilized immature CD101neg neutrophil subsets released from the marrow with a proinflammatory phenotype. 179 From the single‐cell scale, researchers have systematically described the heterogeneous population and transcriptome dynamics of neutrophils in the process of maturation, differentiation, and fate determination in both homeostatic and inflammatory states. In the inflammatory state, the heterogenous population of neutrophils did not change, while the proportion, functional characteristics, expression of the transcription factor, and the transformation pathway altered dramatically between populations. This provides basic information for the precise investigation of neutrophil heterogeneity. 180 , 181 It was reported that, compared with normal lung tissue, two distinct neutrophil subsets were generated in Cryptococcus neoformans‐infected lung tissue, and these two subsets highly expressed inflammation‐related genes and oxidative stress‐related genes, respectively. Neutrophils highly expressing inflammation‐related genes can modulate cell‐cell communication with dendritic cells and alveolar macrophages. 182 CD49d+CysLTR1+ neutrophils were present in the nasal lavage fluid from patients with viral respiratory tract infections. 183 A significantly increased amount of CD63+ neutrophils was detected in the blood of cystic fibrosis patients. 184 Both PD‐L1hi and PD‐L1lo neutrophils were present in sputum from cystic fibrosis patients, while in healthy control, only PD‐L1hi neutrophils were present. 185 Moreover, RANKL1+ neutrophils were found specifically present in the peripheral blood of COPD patients. 186 The pivotal role of neutrophil heterogeneity in Coronavirus disease 2019 (COVID‐19) has lately received increasing attention. 187 , 188 , 189 In a recent study, interferon (IFN)‐stimulated gene (ISG) neutrophils were shown absent in the lung tissue of patients with severe COVID‐19. 190 , 191 Immature and PD‐L1+ neutrophils were, in contrast, significantly increased. 192 Other subpopulations, such as CXCR4high/CD62Llow neutrophils and CD16dim/CD62Lbright neutrophils, have also been reported to be involved in the pathogenesis of COVID‐19, which can suppress T cell function. 113 , 193 The beneficial effects of dexamethasone during COVID‐19 were mainly through the downregulation of IFN‐responsive genes and activation of IL‐1R2+ neutrophils. 194 Unlike COVID‐19 infection, the proportion of ICAM‐1+ neutrophils in the peripheral blood of SARS‐CoV‐2‐infected patients varied vastly. 195 This phenotype may be induced by CIRP. 196 Carstensen et al. 197 demonstrated that inhibition of the DEspR+CD11b+ neutrophil subset could alleviate multiple respiratory diseases. 198 While targeting the intervention of CD11b+CD18+ neutrophils would aggravate influenza‐induced lung damage, the specific mechanism of which was related to affecting functions of T cells. 199 Some researchers have speculated that neutrophil differentiation in humans and mice are similar before and after lung inflammation through computational analysis. 200 This, to some extent, indicates that conclusions from mouse‐based studies could provide support for clinical research. The progression of inflammatory diseases usually results from a combination of interactions between neutrophils and other immune cells. At present, the interactions between specific subsets of neutrophils and other immune cells are relatively rare, which is worthy of further investigation.
4.1.4. Cardiovascular diseases
The study of neutrophils, especially neutrophil heterogeneity, is of great momentousness in the field of cardiovascular and cerebrovascular diseases. 201 , 202 , 203 The normal‐density neutrophils (NDNs) and LDN subpopulations in the peripheral blood of patients with hypertension‐associated chronic inflammation exhibited higher levels of Na+ influx and ROS, along with lower levels of apoptosis and stronger proinflammatory ability, thereby promoting the chronic inflammatory response associated with hypertension. 204 Some research teams identified that Ly6G+SiglecF+ (Myc+NFϰB+) neutrophils served as proinflammatory cells in the progression of myocardial infarction (MI). 205 , 206 The CD33highCD16low neutrophils were the main effector neutrophil subset in the peripheral blood of MI patients. 207 Interestingly, compared with young male mice, reduced neutrophil proinflammatory gene expression were found in the infarct region of young females. 208 This suggested that sex differences existed in the function of neutrophils. The proportion of IFN‐responsive/ISG‐expressing neutrophils in the peripheral blood of MI patients was increased. With the help of experiments on mouse models, the researchers indicated that this group of neutrophils originated from the bone marrow and was negatively regulated by Nrf2 activation. 209 In the mouse model of heart failure, two neutrophil subpopulations with different expression patterns were found within the myocardial. Despite the significant increase in the proportion and both having proinflammatory function, the two populations have functional differences in antigen presentation. 210 Patients with ischemic stroke had higher percentages of the overactive senescent (CXCR4bright/CD62Ldim) neutrophil subset and neutrophils with a reverse transendothelial migration (CD54highCXCR1low) phenotype in their peripheral blood than controls, and this was significantly correlated with disease progression. 211 Similarly, the ratio of CXCR4high/CD62Llow neutrophils in the peripheral blood of Parkinson's patients could also reflect the disease process. 212 Neutrophil‐specific deletion of Syk would improve cognitive dysfunction after traumatic injury to the brain. 213 Trivedi et al. 213 reported the existence of a proinflammatory neutrophil subset (antigen‐presenting cell‐like neutrophils) in both hyperlipidemic patients and atherosclerotic mice. This unique phenotype of neutrophils had the ability to activate the adaptive immune response, thereby promoting atherosclerosis progression. Moreover, a positive correlation between the presence of neutrophils and CD3+ T cells was observed. 214 Despite the fact that many studies have confirmed the diversity of neutrophils in peripheral blood in cardiovascular and cerebrovascular diseases, there is insufficient evidence for the heterogeneity of neutrophils in situ in tissues, which may be attributed to the difficulty in obtaining clinical samples.
4.1.5. Inflammatory bowel diseases
IBDs are chronic inflammatory conditions affecting the digestive system. 215 Abnormal function of neutrophils significantly affects the progression of IBDs. 216 Neutrophils and NETs play a pivotal role in IBD. 217 There was upregulation of NETs in the tissues of patients with ulcerative colitis (UC), and NETs could activate the UC lamina propria mononuclear cells to activate extracellular signal‐regulated kinase‐1/2 (ERK1/2), thereby enhancing TNF‐α and interleukin‐1β (IL‐1β) production. 218 Neutrophils exhibit both cytotoxicity and the potential to cause severe tissue damage to the colon tissue. They are also capable of defensive functions. NETs in UC tissues, for instance, have been shown to be advantageous in terms of immune thrombus formation and the prevention of colonic bleeding. 219 The remarkable effect of improving IBD by affecting neutrophil function has gradually attracted researchers’ interest. 220 The artemisinin analogue SM934 or the Annickia polycarpa extract ameliorated UC in mice by inhibiting neutrophil activation or recruitment. 221 , 222 CD177+ neutrophils played a role in protecting intestinal barrier function in IBD by increasing bactericidal activity and IL‐22 production. 223 , 224 Therefore, targeting CD177+ neutrophils may benefit the treatment of IBD. IL‐17+ neutrophils in the colons of mice with dextran sulfate sodium‐induced colitis might contribute to the progression of the disease. 225 Levinsky et al. 226 demonstrated that OLFM4+ neutrophils centrally participated in the pathologic pathway leading to intestinal ischemia/reperfusion injury in mice through experiments involving adoptive transfer of bone marrow. In the realm of IBD drug therapy, the existence of certain neutrophil subsets was also essential. Due to the presence of CXCR2+ neutrophils, patients with UC often appeared insensitive to ustekinumab. 227 Thus, interventions targeting specific neutrophil subsets in colon tissue are expected to pave the way for precise treatment for IBD.
4.1.6. Other inflammatory diseases
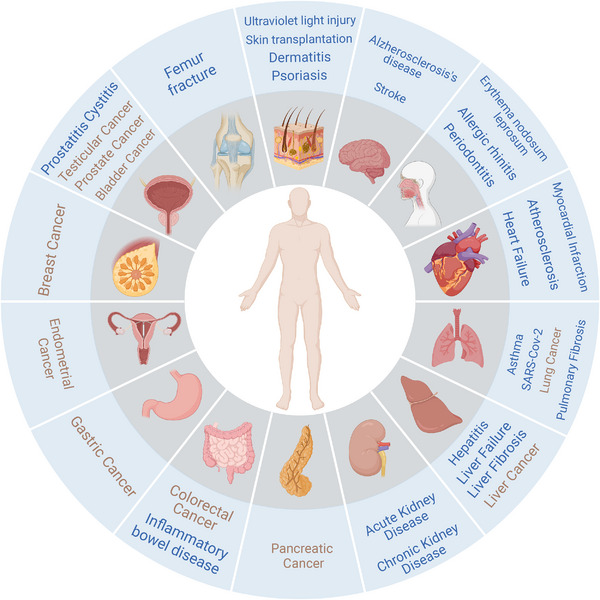
In addition to the above‐mentioned prevalent acute and chronic inflammatory diseases mentioned above, neutrophil heterogeneity is also observed in other inflammatory diseases. Hypodense neutrophils and the ability to form NETs in peripheral blood and gastrointestinal tissues in patients with idiopathic inflammatory myopathy and vasculitis were greatly upregulated. 228 , 229 Neutrophil subpopulations also have fundamental effects on periodontal health regulation, as CD177+ neutrophils were recruited to gingival crevicular fluid in periodontitis preferentially. 230 , 231 , 232 Some researchers have identified specific neutrophil subsets, such as CD14+Ly6Glow neutrophils, that could secrete growth factors NGF and IGF‐1, thereby promoting neuronal survival. 233 , 234 Ly‐6G+SiglecF+ neutrophils specifically exist in the allergic mouse nasal mucosa, exhibiting an activated phenotype. 235 IL‐10R1+ neutrophils were found in the peripheral blood of patients with erythema nodosum leprosum. Senescent CD10negCD16lowCD11blow and immature CD10negCD16negCD11bneg neutrophils in psoriatic skin tissue induced more IL‐17 expression. 236 Induction of CD49d+ neutrophil production favors wound closure after skin transplantation. 237 CD11bhigh/CD11ahigh neutrophils specifically appeared in peripheral blood in a mouse fracture model. 238 In addition, common IFN‐responsive neutrophils played crucial roles in viral infection and other diseases. In summary, numerous studies have elucidated the essential function of neutrophil heterogeneity in both acute and chronic inflammatory diseases, including liver diseases, kidney diseases, respiratory diseases, cardiovascular and cerebrovascular diseases, and colon diseases 4 , 18 , 239 (Figure 4). However, there is currently limited research on the detailed functions of neutrophil subsets in disease development, especially the lack of functional verification of neutrophil subsets in vivo. This represents a potential avenue for future research (Table 1).
FIGURE 4.
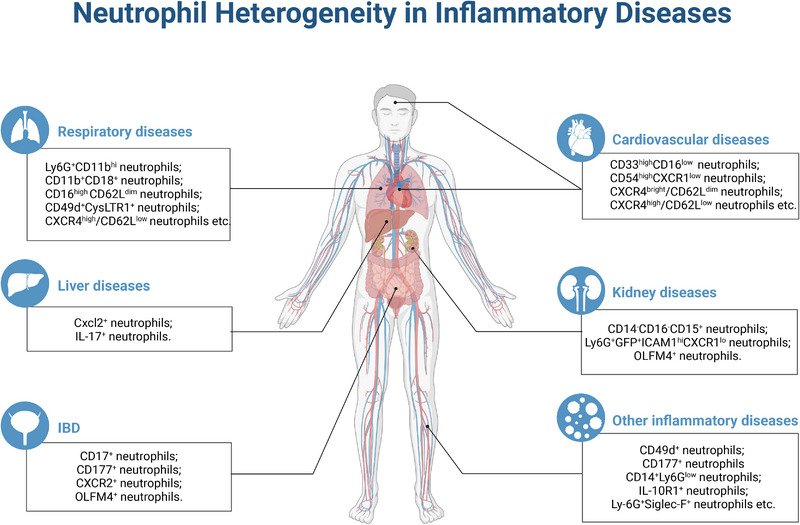
Neutrophil heterogeneity in inflammatory diseases. This figure is created with BioRender.com.
TABLE 1.
Neutrophil heterogeneity in inflammation‐related diseases.
| Diseases | Species | Samples | Neutrophil heterogeneity | Functions | References |
|---|---|---|---|---|---|
| Alcohol‐associated hepatitis | Human/mice | Liver tissues | High‐density neutrophils (HDNs); low‐density neutrophils (LDNs) | HDNs contribute to NETs; LDNs lead to persistent inflammation | 146 |
| Acute liver failure | Mice | Liver tissues | CXCL2+ neutrophils | Regulatory cell infiltration | 147 |
| Chronic viral hepatitis | Human | Liver tissues | IL‐17+ neutrophils | The number of cells increases significantly in the fibrotic stage | 148 |
| Sepsis‐related kidney injury | Human/mice | Blood, kidney tissues | OLFM4+ neutrophils | Juvenile OLFM4‐null mice are protected from sepsis‐related injury | 155 , 173 |
| Kidney inflammation | Mice | Kidney | Ly6G+GFP+ICAM1hiCXCR1lo neutrophils | Promote inflammation | 156 |
| Chronic kidney disease | Human | Blood | CD14−CD16−CD15+ neutrophils | Regulatory vascular calcification | 157 |
| Septic shock | Human | Blood | OLFM4+ neutrophils | The number of cells increases significantly in patients | 167 , 171 |
| Septic shock | Human | Blood | CD177+ neutrophils | The number of cells increases significantly in patients | 168 , 169 |
| Airway hyperreactivity |
Human/mice |
Blood | CD16high CD62Ldim neutrophils | To enhance the response to bradykinin in both human isolated small airways and murine tracheae | 172 |
| Sepsis | Mice | Bone marrow | PD‐L1+ neutrophils | Play an immunosuppressive role in infection | 176 |
| Sepsis | Human | Blood | PD‐L1+ neutrophils, CD123+ neutrophils | The proportion of CD123+ neutrophils correlated with clinical severity | 177 |
| Sepsis | Mice | Blood | Ly6G+CD11bhi neutrophils | Promote inflammation | 178 |
| Chronic granulomatous disease | Mice | Bone marrow | CD101neg neutrophils | Trafficked to the lung and acquired a significantly more proinflammatory transcriptome | 179 |
| Bacterial infection |
Mice; human |
Spleen; blood | Interferon‐stimulated gene (ISG) neutrophils; Ly6Glow CXCR4hi cells; Ly6Ghi CXCR4hi cells | The number of ISG neutrophils increases significantly in spleen of human and mice during infection; the number of Ly6Glow CXCR4hi cells and Ly6Ghi CXCR4hi cells increases significantly in blood and spleen during infection | 180 |
| SARS‐CoV‐2 infection | Non‐human primates | Lung tissue, bronchoalveolar lavage fluid | CD11b+neutrophils | Reflect the severity of the disease | 181 |
| Cryptococcus neoformans (Cn) infection | Mice | Lung | Ox‐PMN; Cyt‐PMN | Ox‐PMNs interact with the fungus and generate ROS; Cyt‐PMNs respond to cytokines to modulate cell‐cell communication with dendritic cells and alveolar macrophages | 182 |
| Acute onset of upper respiratory symptoms | Human | Nasal lavage | CD49d+CysLTR1+ neutrophils | The number of cells increases significantly in patients | 183 |
| Cystic fibrosis | Human | Blood | CD63+ neutrophils | The number of cells increases significantly in patients | 184 |
| Cystic fibrosis | Human | Sputum | PD‐L1high neutrophils | T cell suppression | 185 |
| COPD | Human | Blood | RANKL1+ neutrophils | Correlate with IL‐1β, IL‐6 and IL‐8 in plasma in COPD | 186 |
| SARS‐CoV‐2 infection | Human | Bronchoalveolar lavage fluid | CD16Int neutrophils | Promote inflammation | 187 |
| SARS‐CoV‐2 infection | Human | Blood | DEspR+ neutrophils | Correlate with elevated circulating CCL23, increased NETosis, and the severity of the disease | 189 , 198 |
| SARS‐CoV‐2 infection | Human | Blood | ISG neutrophils | The number of cells decreases significantly in patients with severe disease | 190 |
| SARS‐CoV‐2 infection | Human | Blood | CD16Int low‐density neutrophils | Promote T cell proliferation | 191 |
| SARS‐CoV‐2 infection | Human | Blood | PD‐L1+ neutrophils | Immunosuppressive function | 192 |
| SARS‐CoV‐2 infection | Human | Blood | CXCR4high/CD62Llow neutrophils | Promote inflammation | 193 |
| SARS‐CoV‐2 infection | Human | Blood | IL2+ neutrophils | Anti‐inflammation | 194 |
| SARS‐CoV‐2 infection | Human | Blood | ICAM‐1+ neutrophils | The number of cells increases significantly in patients | 195 |
| Acute lung injury | Human, rhesus macaque, rat | Bronchoalveolar lavage fluid | DEspR+CD11b+/CD66b+ neutrophils | The number of cells increases significantly in disease | 197 |
| Severe influenza virus infection | Mice | Lung | CD11b+CD18+ neutrophils | Regulate T cell function | 199 |
| Hypertension | Human | Blood | Normal‐density neutrophils (NDN), low‐density neutrophils (LDN) | Promote inflammation | 204 |
| Myocardial infarction | Mice | Blood, bone marrow, heart | Ly6G+SiglecF+ neutrophils; ISG neutrophils | Promote inflammation; provide a clinical biomarker | 205 , 206 , 209 |
| Myocardial infarction | Human | Blood | CD33highCD16low neutrophils | An increase in the frequency of hyperactivated | 207 |
| Heart failure | Mice | Heart | CCR2+ neutrophils, CXCR2+ neutrophils | Promote inflammation; antigen presentation | 210 |
| Ischemic stroke | Human | Blood | CXCR4bright/CD62Ldim neutrophils, CD54highCXCR1low neutrophils | Reflect the severity of the disease | 211 |
| Alzheimer's disease | Human | Blood | CXCR4high/CD62Llow neutrophils | Reflect the severity of the disease | 212 |
| Atherosclerosis | Human | Blood | APC‐like neutrophils | Activate the adaptive immune response to promote disease progression | 214 |
| IBD | Human | Blood, colon tissue | CD177+ neutrophils | Play a protective role in IBD through increased bactericidal activity and IL‐22 production | 223 , 224 |
| IBD | Mice | Colon tissue | CD17+ neutrophils | Promote inflammation | 225 |
|
Ischemia/reperfusion injury (IR) |
Mice | Colon tissue | OLFM4+ neutrophils | Lead to intestinal damage and mortality after IR injury | 226 |
| Ulcerative colitis | Human | Colon tissue | CXCR2+ neutrophils | Enrich in nonresponders to ustekinumab therapy | 227 |
| Periodontitis | Human | Blood | CD177+ neutrophils | Preferentially recruited to the gingival crevice of periodontitis patients | 230 |
| Optic nerve and spinal cord injury | Mice | Optic nerve and spinal cord | CD14+Ly6Glow neutrophils | Neuroprotective role by secreting NGF and IGF‐1 | 233 , 234 |
| Allergic rhinitis | Mice | Nasal mucosa | Ly‐6G+SiglecF+ neutrophils | Exhibit an activated phenotype and enhance effector functions | 235 |
| Erythema nodosum leprosum | Human | Blood | IL‐10R1+ neutrophils | Release inflammatory cytokines | 166 |
| Psoriasis | Human | Skin tissue | CD10negCD16lowCD11blow neutrophils; CD10negCD16negCD11bneg neutrophils | Induce IL‐17 and IFN‐γ production by T cells | 236 |
| Wound closure after skin transplantation | Mice |
Blood; Skin tissue |
CD49d+ neutrophils | Play a role in local vessel remodeling during inflammation | 237 |
| Femur fracture | Rat | Blood | CD11bhigh/CD11ahigh neutrophils | The number of cells increases significantly in diseases. | 238 |
The dual role of neutrophils in diseases ought to get sufficient consideration. On the one hand, as innate immune phagocytes, neutrophils function pivotally in immune defense. On the other hand, specific neutrophil subpopulations would promote disease progression. Extensive and comprehensive research of the functional heterogeneity of neutrophils is envisaged to enable precision intervention on neutrophil subpopulations and achieve precise treatment of inflammation‐related diseases.
4.2. Neutrophile heterogeneity in cancer‐related diseases
Over years past, accumulating evidence has demonstrated that neutrophils perform dual roles, exhibiting both pro‐ and antitumor activity. Specific mechanisms are associated with tumor cell proliferation, apoptosis, and the immune microenvironment. 240 , 241 As study advances, researchers have shown that neutrophils undergo phenotype and functional remodulation under the influence of tumors and their microenvironment, playing a complicated role in tumor development and metastasis. 242 , 243 , 244 Besides protumor activity through protumor neutrophils, NETs, and various subsets associated with cancer process, neutrophils also exert antitumor functions via antitumor neutrophils, other derivatives, and subsets inhibiting cancer process (Figure 5).
FIGURE 5.
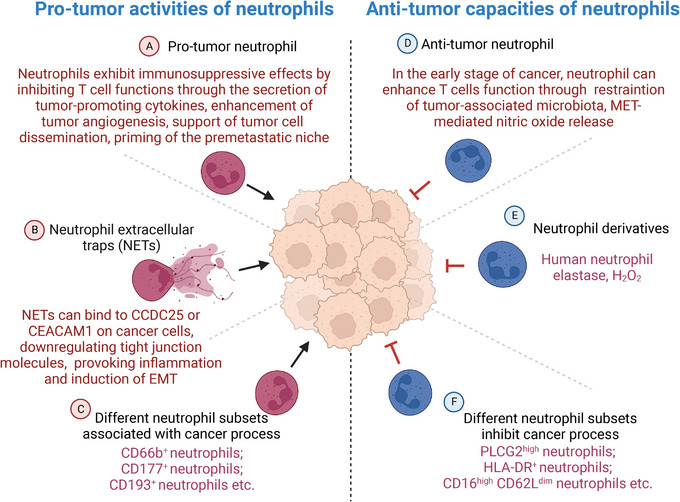
Neutrophils show both protumor activity or antitumor capacities. This figure is created with BioRender.com.
4.2.1. Colorectal cancer
Colorectal cancer (CRC) is one of the most prevalent malignancies globally, accounting for 10% of all cancer cases. The incidence of CRC ranked third among malignant tumors, making it the third leading cause of cancer mortality. 245 , 246 In recent years, several studies have shown that the double role of neutrophils in inflammation‐related diseases, particularly in the development and metastasis of tumors, has been gradually recognized and has garnered growing attention. 247 , 248 Neutrophils and NETs might promote CRC development and metastasis in part by the mediation of tumor metastasis by NETs via binding to CCDC25 or CEACAM1 on cancer cells. 247 , 249 , 250 , 251 , 252 Inhibition of NETs release attenuated colitis as well as colitis‐associated tumorigenesis. 253 , 254 In addition, neutrophils seemed to be protective against the advancement of CRC. Neutrophil elastase has been proven to be inhibitory effective against 35 kinds of cancer cells, including CRC cells. 255 Neutrophils restrained tumor‐associated microbiota and decreased colon tumor development and invasion in an inflammatory‐induced CRC mice model, while neutrophil depletion would in contrast made tumors more aggressive. 256 The role of neutrophils in preventing bacterial invasion may be mediated by IL‐1β. 257 These findings indicated the intricacy of tumor‐associated neutrophil (TAN) involvement in CRC progression. What is more, neutrophils from CRC patients could also exhibit immunosuppressive effects by inhibiting T cell functions through TGFβ. 258 CD177+ neutrophils and CD66b+ neutrophils suppress epithelial cell tumorigenesis in colitis‐associated cancer and became a predictor for a better prognosis in CRC. 259 , 260 In the early stages of CRC, the number of CD66b+ neutrophils were often associated with a favorable prognostic factor. Specific inductions would stimulate the generation of particular neutrophil subsets. 261 For instance, the addition of IL‐21 into bone marrow cell culture increased the amount of CD193+ neutrophils, which migrated readily into the duodenum. 262 Moreover, in a genetic CRC model, suppression of neutrophil‐dependent angiogenesis abrogated resistance to anti‐VEGF antibody. 263 Taken together, these studies suggested that targeting neutrophils is of great significance for CRC treatment.
4.2.2. Hepatocellular carcinoma
The important role of neutrophils in liver cancer has gradually attracted accelerated interest in past few years. 264 , 265 , 266 , 267 According to the present studies, NETs could promote the development from NASH to hepatocellular carcinoma (HCC). 268 Besides, NETs promoted HCC metastasis by downregulating tight junction molecules on adjacent endothelial cells or provoking inflammation. 269 , 270 Neutrophils, especially type‐2 neutrophils (N2), could mediate the formation of an immunosuppressive microenvironment in liver tissue, and neutrophil clearance in mouse models would impede tumor progression. 271 , 272 , 273 The production of neutrophils for specific functions is usually influenced by a variety of factors. For instance, in several models of liver autoimmunity, pharmacologically induced autoantigen‐specific T regulatory type‐1 (TR1) cells and TR1‐cell‐induced B regulatory (Breg) cells used five immunoregulatory cytokines to coordinately recruit neutrophils into the liver and program their transcriptome to generate regulatory neutrophils. 272 Additionally, PD‐L1+ neutrophils have been shown to promote the growth of tumor cells in tissues from HCC patients by inhibiting the proliferation and activation of T cells. 274 The generation of PD‐L1+ neutrophils was likely attributed to the induction of cancer‐associated fibroblasts through the IL6‐STAT3 pathway. 275 Another study also confirmed that, in addition to PD‐L1+ neutrophils, both CCL4+ TANs presented immunosuppressive activity. 276 Neutrophils also have a significant impact on the treatment of HCC. 277 Tumor‐derived lactate would inhibit the efficacy of Lenvatinib in HCC by modulating PD‐L1 expression on neutrophils. 278 The mechanism of action of cabozantinib combined with PD‐1 antibody to enhance antitumor immunity through neutrophil‐based immune responses favored this approach for HCC therapy. 279 Furthermore, in HCC immunotherapy, PD‐L1+ neutrophils would be directly activated by IFN‐γ and subsequently prompting inflammatory response. 280 Therefore, precise targeting of specific neutrophil subsets might reduce the occurrence of immune‐related adverse events to a significant extent. Although the tumor‐killing effect of neutrophils has been reported in other tumors, it has not been reported in liver cancer, which may be a future research direction.
4.2.3. Gastric cancer
With high morbidity and mortality, gastric cancer (GC) is a ubiquitous malignancy worldwide. 281 , 282 Heretofore, surgical treatment remains the first‐line strategy to providing a cure. It is expected that analyzing the molecular events taken place during GC progression would cast new light on GC treatment. 283 Tumor‐derived exosomes could induce neutrophil activation through the HMGB1/TLR4/NF‐κB signaling pathway during GC progression. 284 As well, neutrophils were able to promote GC progression by inducing Th17 cell polarization or inhibiting CD8+ T cell function. 285 , 286 Xia et al. 287 reported that NETs could promote the metastasis of postoperative abdominal infectious complications in GC patients. Induction of epithelial–mesenchymal transition (EMT) has been verified to be another way for NETs to promote GC metastasis. 288 , 289 Five subpopulations of neutrophils were identified in tissues from GC patients. Among them, CXCR4+ neutrophils have proangiogenic and proinvasive properties; while PLCG2high neutrophils can inhibit the invasion and migration of tumor cells. 290 Through single‐cell RNA sequencing, researchers from another team mapped the lymph node metastasis landscape of GC and found that lipocalin 2 (LCN2)+ neutrophils served as a promotor in lymph node metastasis. This suggested that neutrophil subsets might drive metastatic in specific tumor sites. 291 The number of PD‐L1+ neutrophils and CD66b+ neutrophils are associated with the survival of GC patients. 292 , 293 , 294 Interestingly, the number of FasL+PD‐L2+ neutrophils was reported to be associated with poor prognosis of GC in another study. These neutrophils owned immunosuppressive functions on tumor‐specific CD8+ T cells and promoted the growth and progression of human GC tumors both in vitro and in vivo. Targeting the removal of FasL+PD‐L2+ neutrophils could hinder GC progression. 295 The advancement of medical therapy for GC has been quite sluggish thus far. Consequently, the development of targeted drugs for the heterogeneity of neutrophil function is anticipated to yield novel insights for the therapeutic treatment of GC.
4.2.4. Lung cancer
Lung cancer is among the world's most prevalent malignant tumors. The incidence and mortality rate have grown dramatically over the past 50 years, and males were reported to have the highest morbidity and mortality of all malignant tumors. 296 , 297 The proportion of neutrophils in lung cancer patients' tissue or peripheral blood can be utilized to predict the prognosis of lung cancer. 298 , 299 Studies have demonstrated that neutrophils and NETs they generate play a critical role in fostering lung tumor development and metastasis. 300 , 301 , 302 Among these, senescent TANs (CXCR4+CD62Llow) are more likely to form NETs and facilitate metastasis. 303 It has been reported by Tyagi et al. 304 that N2 neutrophils could release LCN2 which would in turn facilitate lung cancer metastasis. The heterogeneity of neutrophils in the peripheral blood of non‐small cell lung cancer patients were greater, and the proportion of LDNs was remarkably upregulated. 305 , 306 Faget et al. 307 reported that Gr1+ neutrophils could favor tumor progression by altering angiogenesis, leading to hypoxia, and sustaining Snail expression. Through MET‐mediated nitric oxide release, neutrophils can also abate tumor growth and metastasis. 79 In recent years, a group of TANs with high expression of SiglecF (sialic acid‐binding immunoglobulin‐like lectin F) has gradually attracted researchers’ attention. SiglecFhigh neutrophils could selectively upregulate the expression of genes associated with tumor‐processes, including angiogenesis, myeloid cell differentiation and recruitment, extracellular matrix remodeling, suppression of T cell responses, and tumor cell proliferation and growth. 308 In the early stages of lung cancer, TANs could enhance the T cell activity to display an antitumor effect. 309 This unique neutrophil population was more mature with a longer lifespan. 98 , 106 , 111 Its function in promoting lung cancer, however, was dependent on the number of osteoblastic cells, suggesting that the function of SiglecFhigh neutrophils was infected by the ratio of cell types. 308 Interestingly, another study on mouse lung cancer showed that, similar to SiglecFhigh neutrophils, neutrophils with Glut1 high expression could live longer in the tumor microenvironment, and could promote the high expression of SiglecF and MMP9, facilitating tumor progression. 310 , 311 However, whether Glut1high neutrophils and SiglecFhigh neutrophils are the same population of neutrophils remains unclear. Another research team identified a group of ISG neutrophils in mouse lung tissue, and the number of ISG neutrophils was significantly reduced in tumor tissue. 312 A subset of tumor‐associated HLA‐DR+ neutrophils was also identified in early‐stage human lung cancer tumor tissue, which can cross‐present antigens to CD8+ T cells, triggering antitumor T cell immune responses. 313 A group of CD66b+/CD10low/CXCR4+/PDL1inter neutrophils were identified in the peripheral blood of advanced lung cancer patients, the existence of which was limited in the advanced stage of lung cancer. 90 At present era, neutrophils have promising potential as effector cells in the treatment of lung cancer. By modifying neutrophil infiltration, for instance, the CXCR2 selective inhibitor SB225002 can boost the therapeutic effect of cisplatin in the treatment of lung cancer. 314 To sum up, neutrophil heterogeneity is extremely important in the prognosis, progression, and treatment of lung cancer. Unfortunately, studies of neutrophil heterogeneity in specific types of lung cancer are limited. For example, it has been reported that neutrophil to lymphocyte ratio predicts the prognosis of extensive small‐cell lung cancer. 315 However, there are few studies on neutrophil heterogeneity in small cell lung cancer.
4.2.5. Breast cancer
Breast cancer is the most common malignancy in women and one of the three most common cancers worldwide, along with lung and colon cancer. 316 Numerous studies have demonstrated that neutrophils could promote the growth and metastasis of breast cancer via the secretion of cytokines and other pathways, 317 , 318 , 319 as well as facilitate breast cancer metastasis by altering other cell functions, such as inhibiting the antimetastasis function of NK cells or mediating the immunosuppressive microenvironment. 320 , 321 Tumor‐derived factors such as G‐CSF and stem cell factor could in turn responsible for activity of immunosuppressive neutrophils, thereby contributing to the advancement of breast cancer. 109 , 322 Interestingly, during the premetastatic stage of breast cancer, tumor‐entrained neutrophils in the lung aggregated and, by generating H2O2, might limit metastatic seeding in the lungs. 80 This partly explains the functional differences of neutrophils at the primary site and the metastatic site. Neutrophils could also play a protumor role by affecting the lipid metabolism of tumor cells. In the context of a low glucose supply in the tumor microenvironment of 4T1 tumor‐bearing mice, neutrophils would engage in fatty acid oxidation, supporting ROS production and amplifying T cell suppression. 109 In addition, a separate study reported that lipids stored within lung neutrophils can be transported to metastatic tumor cells through a macropinocytosis–lysosome pathway, endowing tumor cells with augmented survival and proliferative capacities. 301 Already published is the discovery that neutrophils could exert a mode of destruction of breast cancer cells has already been reported, and the specific mode was related to the regulation function of neutrophils toward CD47‐SIRPα checkpoint through the trogoptosis mechanism. 323 , 324 It was identified by Veillette et al. 324 that C5aR1+ neutrophils would predict worse prognosis of BC patients, which could mechanistically be explained by the ability of C5aR1+ neutrophils in inducing breast cancer glycolysis via increasing ERK1/2‐WTAP‐dependent m6A methylation of ENO1. 325 Moreover, Coffelt et al. 326 reported that in mammary tumor‐bearing K14cre;Cdh1F/F;Trp53F/F (KEP) mice, the tumor microenvironment favors a unique population of metastasis‐inducing neutrophils with upregulation of genes including cKit, Nos2, Prok2, S100a8, and S100a9. Using a panel of 16 distinct genetically engineered mouse models for breast cancer, another analysis also identified the significant enrichment of cKIT+ neutrophils was more likely to potentiates metastatic progression. 327 In previous studies, neutrophil Cathepsin G was found to interact with RAGE (receptor for advanced glycation end products), displaying its antitumor activity. 328 By releasing metastasis‐promoting factors including forming NETs, CXCR4hiCD62Llo aged neutrophils could enhance metastasis of breast cancer cells to the liver or lung. 303 , 329 In summary, neutrophils play a dual role in breast cancer progression. The distinction of which may be attributed to the various phases of the disease and the heterogeneity of neutrophils. which merit additional investigation.
4.2.6. Pancreatic cancer
Pancreatic cancer is one of the most aggressive tumors of the digestive system. The mortality rate is rising annually due to the its stealthy onset, fast development, and ineffective treatment. 330 , 331 Multiple research teams have proven that neutrophils and NETs may greatly enhance the growth and metastasis of pancreatic cancer, and that inhibiting NETs can somewhat delay pancreatic cancer metastasis. 332 , 333 , 334 In an orthotopic pancreatic cancer mouse model, the presence of neutrophils was shown to be associated with functional suppression of the CD8+ T cells. 334 In the pancreatic tumor microenvironment, TAN subsets with various functions exist, among which BHLHE40+ neutrophils have protumor and immunosuppression functions. 335 A recent research revealed that P2RX1neg neutrophils were highly immunosuppressive, hence provoking pancreatic cancer liver metastasis. 335 CD13hi neutrophil‐like myeloid‐derived suppressor cells exert immune suppression in pancreatic ductal adenocarcinoma by influencing the expression of Arginase 1. 336 Targeting neutrophils has also showed encouraging effectiveness in the treatment of pancreatic cancer. 337 Lorlatinib attenuates pancreatic cancer growth by suppressing TAN amount and functions. 338 The therapeutic effect of nivolumab in pancreatic cancer was in association with CD11b+ neutrophil degranulation. 339 Steele et al. 340 revealed that mediating the expression of CXCR2 on neutrophils could improve the efficacy of PD‐1 antibodies in a pancreatic cancer mouse model. This may be due to the fact that high expression of CXCR2 on neutrophils would mediate immunosuppressive microenvironment, effecting T cell entry. 340 , 341 Targeting tumor‐associated CXCR2+ neutrophils and CCR2+ macrophages was reported to improve chemotherapeutic responses in pancreatic ductal adenocarcinoma. 342 In a nutshell, a series of studies have shown that it is necessary to investigate neutrophil heterogeneity in the context of pancreatic cancer treatment. 266 , 267 , 268 , 269 , 270 , 271 , 272 , 273 , 274 , 275 , 276 , 277 , 278 , 279 , 280 , 281 , 282 , 283 , 284 , 285 , 286 , 287 , 288 , 289 , 290 , 291 , 292 , 293 , 294 , 295 , 296 , 297 , 298 , 299 , 300 , 301 , 302 , 303 , 304 , 305 , 306 , 307 , 308 , 309 , 310 , 311 , 312 , 313 , 314 , 315 , 316 , 317 , 318 , 319 , 320 , 321 , 322 , 323 , 324 , 325 , 326 , 327 , 328 , 329 , 330 , 331 , 332 , 333 , 334 , 335 , 336 , 337 , 338 , 339 , 340 , 341 , 342 , 343 For subsequent investigation, the inductive factors of the emergence of diverse neutrophil subsets may be probed, enabling a brand‐new viewpoint for the treatment of pancreatic cancer.
4.2.7. Other cancers
Researchers are also intrigued by the role of neutrophil heterogeneity in other cancers. Neutrophils could regulate the function of CD47‐SIRPα checkpoint through trogoptosis, presenting tumor‐suppressive property in acute promyelocytic leukemia. 344 Meanwhile, combination of SIRPα antibody with CD70 antibody could, to some extent, enhance the antitumor effect of neutrophils. 345 Whether this process is dependent on neutrophils remain obscure. In addition, neutrophils also presented tumoricidal effect in neuroblastoma and endometrial cancer, the mechanism of which might be correlated with the induction of MMP9 expression and ROS generation. 346 , 347 , 348 The ROS‐dependent antitumor effect of neutrophils has also been demonstrated in a mesothelioma mouse model. 349 However, another study showed that CXCR2+CD11b+Ly6Ghi myeloid‐derived suppressor cells (mainly expressing neutrophil marker genes) affected the efficacy of PD‐1 antibodies in rhabdomyosarcoma mice. 350 Three neutrophil subsets were identified in head and neck squamous cell carcinoma (HNSCC) patients: CD16dim CD62Lhigh, CD16high CD62Lhigh and CD16high CD62Ldim neutrophils. Among them, CD16high CD62Ldim neutrophils could inhibit the migration and proliferation of HNSCC cells, as well as inducing cell apoptosis. 351 Another study showed that immunosuppressive neutrophil subsets, such as CD16high CD62Lhigh neutrophils, might be induced by tumor‐produced TGF‐β/IL‐10. 352 Six phenotypically distinct human blood neutrophil populations were identified. The proportion and function of neutrophil subsets were shown to be correlated with melanoma stage, and their changes would favor the differentiation of different melanoma stages. 353 , 354 The profound study of neutrophils has also given hope for the treatment of melanoma. A recent study showed that neutrophils, especially TAN1, were isolated from mice underwent innate immune training by exposure to β‐glucan hold a stronger antitumor effect. 355 The activity of alemtuzumab against B‐cell chronic lymphocytic leukemia was reported to be dependent on neutrophil‐mediated cytotoxicity. 356 Combination of CTLA‐4 and PD‐L1 blockade in patients with chemotherapy‐naive metastatic castration‐resistant prostate cancer was shown to be associated with the modulation of neutrophil subsets in the bone microenvironment. 357 Collectively, neutrophil heterogeneity has been observed in a variety of cancers (Figure 6). The subsets of neutrophils, however, seem to vary from tissue to tissue, requiring researchers to follow up with investigations in specific tumors (Table 2).
FIGURE 6.
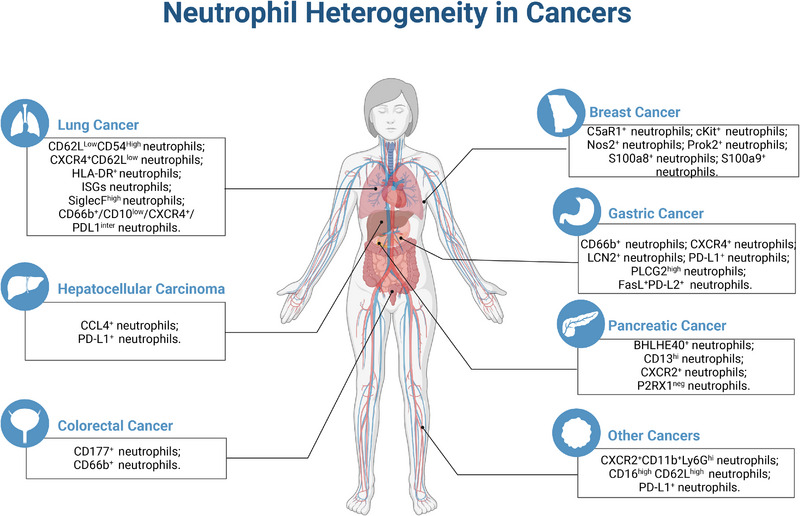
Neutrophil heterogeneity in cancers. This figure is created with BioRender.com.
TABLE 2.
Neutrophil heterogeneity in cancer‐related diseases.
| Diseases | Species | Samples | Neutrophil heterogeneity | Functions | References |
|---|---|---|---|---|---|
| Colorectal cancer | Human | Colon tissues | CD177+ neutrophils | Suppress epithelial cell tumorigenesis | 262 |
| Colorectal cancer | Human | Colon tissues | CD66b+ neutrophils | A favorable prognostic factor in early stages of colon cancer | 263 |
| Hepatocellular carcinoma | Mice | Liver tissues | N2 neutrophils | Immunosuppressive function | 275 |
| Hepatocellular carcinoma | Human, mice | Liver tissues | PD‐L1+ neutrophils | Immunosuppressive function | 277 , 278 |
| Hepatocellular carcinoma | Human, mice | Liver tissues | CCL4+ neutrophils | Recruit macrophages | 279 |
| Hepatocellular carcinoma | Mice | Liver tissues | PD‐L1+ neutrophils | Promote inflammatory response | 283 |
| Gastric cancer | Human | Gastric tissues |
CXCR4+ neutrophils; PLCG2high neutrophils |
Proangiogenesis; inhibit tumor cell invasion and migration | 293 |
| Gastric cancer | Human | Gastric tissues; metastatic lymph nodes | LCN2+ neutrophils | Contribute to lymph node metastasis | 294 |
| Gastric cancer | Human | Gastric tissues | PD‐L1+ neutrophils | Associated with disease progression and reduced GC patient survival | 295 |
| Gastric cancer | Human | Gastric tissues | CD66b+ neutrophils | Predict the poor disease special survival | 296 , 297 |
| Gastric cancer | Human | Gastric tissues | FasL+PD‐L2+ neutrophils | Immunosuppressive function | 298 |
| Breast cancer lung metastasis | Mice; Human | Lung tissues | CXCR4+CD62Llow neutrophils | Contribute to breast cancer lung and liver metastasis | 306 , 331 |
| Breast cancer lung metastasis | Mice; Human | Lung tissues | N2 neutrophils | Contribute to breast cancer lung metastasis | 307 |
| Lung cancer | Human | Blood | Low‐density neutrophils | The number of cells increases significantly in patients | 79 |
| Lung cancer | Mice | Lung tissues | Gr1+ neutrophils | Immunosuppressive function | 309 |
| Lung cancer | Human | Lung tissues | CD62LLowCD54High neutrophils | Release inflammatory cytokines | 312 |
| Lung cancer | Human | Lung tissues | SiglecFhigh neutrophils | Promote tumor growth by inducing Vegfa, Ctsb and Tgfb1 | 311 |
| Lung cancer | Human | Lung tissues | ISG neutrophils | The number of cells decreases significantly in patients. | 317 |
| Lung cancer | Human | Blood | HLA‐DR+ neutrophils | Enhance immune response | 318 |
| Lung cancer | Human | Blood | CD66b+/CD10low/CXCR4+/PDL1inter neutrophils | Relate to advanced lung cancer diagnosis | 90 |
| Breast cancer | Human | Tumor tissues | C5aR1+ neutrophils | Induce breast cancer glycolysis and promote tumor growth | 328 |
| Breast cancer | Human | Tumor tissues | Neutrophils (cKit+, Nos2+, Prok2+, S100a8+, S100a9+) | Promote tumor metastasis | 329 |
| Breast cancer | Mice | Tumor tissues | cKit+ neutrophils | Promote tumor metastasis | 330 |
| Pancreatic ductal adenocarcinoma | Human | Blood, tumor tissues | BHLHE40+ neutrophils | Immunosuppressive function | 338 |
| Pancreatic ductal adenocarcinoma | Human | Tumor tissues | P2RX1neg neutrophils | Immunosuppressive function | 339 |
| Pancreatic ductal adenocarcinoma | Human | Tumor tissues | CD13hi neutrophils | Immunosuppressive function | 340 |
| Pancreatic ductal adenocarcinoma | Human | Tumor tissues | CXCR2+ neutrophils | Immunosuppressive function | 345 , 346 , 347 |
| Rhabdomyosarcoma | Mice | Tumor tissues | CXCR2+CD11b+Ly6Ghi neutrophils | Immunosuppressive function | 354 |
| Neck squamous cell carcinoma | Human | Blood | CD16high CD62Lhigh neutrophils | Correlate with increased survival rate | 355 |
| Melanoma | Human | Blood | PD‐L1+ neutrophils | Correlate with decreased survival rate | 358 |
A comprehensive study of neutrophil heterogeneity would favor us in acquiring a deeper understanding of disease mechanisms and may yield novel insights into cancer treatment.
5. OUTLOOK
In this review, we have provided a comprehensive summary of the significance of neutrophil heterogeneity in inflammatory and tumor‐associated diseases. The presence of neutrophils with different phenotypes in blood, peripheral blood, or tissue has been confirmed by conventional or high‐resolution methods. However, two major challenges regarding neutrophil heterogeneity exist currently. One is to understand the underlying causes of neutrophil heterogeneity, while the other is how neutrophil subsets are defined. There are two potential mechanisms for neutrophil heterogeneity. The first is the intrinsic heterogeneity of neutrophils present in the bone marrow and blood. The second involves exposure to extrinsic factors, either locally or systemically, that modify neutrophil properties. 83 It is noteworthy that the factors inducing neutrophil heterogeneity vary between different diseases, as previously discussed in the context of inflammatory diseases and tumors. It remains difficult to ascertain whether the occurrence of neutrophil heterogeneity is due to a single signal or a combination of multiple factors. This requires more detailed exploration. Additionally, the correlation between neutrophil functions and the phenotypes identified by surface markers is an important issue that warrants discussion. For example, neutrophils with immunosuppressive function and high expression of PD‐L1 would be defined as PD‐L1+ neutrophils. 276 Although some high‐resolution assays such as RNA‐seq have helped in providing functional information of neutrophil subsets and specific expression of maker genes, the relationship between the neutrophil functions and surface makers is still unclear. This represents an important direction of future research.
Despite the fact that extensive evidence has supported the importance of neutrophils as effector cells for disease treatment, many uncertainties remain to be addressed. Currently, studies of neutrophil heterogeneity have been concentrated on the association level. In the upcoming investigation, functions of multiple neutrophil subpopulations should be evaluated based on various transgenic mice. Simultaneously, comprehensive and accurate definition of neutrophil subpopulations along with factors promoting their formation deserve further investigation. Neutrophils exhibit species specificity, and human and mouse neutrophils might function differently in some respects. 312 , 358 Cui et al. 255 reported that human neutrophil elastase selectively killed tumor cells, whereas similar effects did not occur with mouse neutrophil elastase. This indicated that our research of neutrophil heterogeneity in mice did not immediately correlate with clinical cases. In addition, the role of neutrophils also varies widely across different tumors. Neutrophils with high MET expression had a great antitumor effect in the lung cancer model. In the melanoma model, however, the expression of MET would greatly limit the effect of immunotherapy. 79 , 359 Neutrophils function based on the tissue type, the tissue microenvironment, and the stage of disease. Even within the same disease situation, it is possible for neutrophils to perform conflict functions. To be specific, neutrophils showed an antitumor effect by inducing ferroptosis in glioblastoma through MPO; however, the presence of neutrophils in glioblastoma was often seen as a prediction of poor prognosis. 360 The function of neutrophil subsets in peripheral blood from volunteers of different sexes also differs. 361 Differences in neutrophil heterogeneity across species, genders, and disease types have brought great challenges to our study of neutrophil heterogeneity, and will continue to be the focus and difficulty of future research.
From a therapeutic standpoint, investigations targeting specific neutrophil subpopulations are being refined. It is of great significance to develop drugs that target particular neutrophil subpopulation for treatment of inflammation and cancer‐related diseases. Though, approaches that would target particular neutrophil subpopulation remain limited and warrant additional investigation. Presently, some neutrophil‐targeted nanoparticles and nanoformulations targeting specific neutrophil subpopulations have shown favorable alleviative effects. 362 , 363 This may be due to innate ability possessed by cells to sense, integrate and respond to the dynamic environment within the body, rendering them suitable as carriers of therapeutic drugs. In recent years, the design of drug delivery system regarding neutrophils has become a prominent research direction in the field of drug delivery. 364 , 365 There are two types of drug delivery systems: neutrophils as carriers and neutrophil‐membrane‐derived nanovesicles. 364 Several studies have demonstrated that neutrophil‐based drug delivery systems can improve current therapies for inflammatory disorders and cancers. To be specific, Zhang and coworkers 366 prepared a tumor‐penetrating neotype neutrophil cytopharmaceutical (NEs@STING‐Mal‐NP) that conjugated liposomal STING agonists on the surface of neutrophils, inheriting the merits of neutrophils such as proactive tumor vascular extravasation and tissue penetration, significantly boosting the tumor penetration of STING agonists. However, one challenge with neutrophil‐based drug delivery systems is determining the optimal time to deliver nanoparticles for better targeting of infiltrated neutrophils. After all, the time course of neutrophil infiltration is heavily reliant on the pathogenesis of the disease and its stages. The use of effector neutrophil subsets in specific disease context to design drug‐delivery systems may offer a novel approach.
Overall, we have provided information on the fundamental function of neutrophils, the double‐edged role of neutrophils in diseases, and the important function of neutrophilic heterogeneity in inflammatory diseases and tumors. Additionally, potential future research directions for neutrophils have also been discussed. We hope that this review would provide a comprehensive understanding of neutrophils.
AUTHOR CONTRIBUTION
Qu Jiao contributed to the conception and acquisition. Wencheng Zhou and Xinran Cao drafted the manuscript. Qu Jiao and Xinran Cao critically revised the manuscript. Qiang Xu and Yang Sun gave final approval. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
None.
Zhou W, Cao X, Xu Q, Qu J, Sun Y. The double‐edged role of neutrophil heterogeneity in inflammatory diseases and cancers. MedComm. 2023;4:e325. 10.1002/mco2.325
Contributor Information
Jiao Qu, Email: qujiao19920819@163.com.
Yang Sun, Email: yangsun@nju.edu.cn.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Liew PX, Kubes P. The neutrophil's role during health and disease. Physiol Rev. 2019;99(2):1223‐1248. [DOI] [PubMed] [Google Scholar]
- 2. Margraf A, Ley K, Zarbock A. Neutrophil recruitment: from model systems to tissue‐specific patterns. Trends Immunol. 2019;40(7):613‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cassatella MA, Ostberg NK, Tamassia N, Soehnlein O. Biological roles of neutrophil‐derived granule proteins and cytokines. Trends Immunol. 2019;40(7):648‐664. [DOI] [PubMed] [Google Scholar]
- 4. Metzemaekers M, Gouwy M, Proost P. Neutrophil chemoattractant receptors in health and disease: double‐edged swords. Cell Mol Immunol. 2020;17(5):433‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol. 2016;16(6):378‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Filippi MD. Neutrophil transendothelial migration: updates and new perspectives. Blood. 2019;133(20):2149‐2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spitzer MH, Nolan GP. Mass cytometry: single cells, many features. Cell. 2016;165(4):780‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vandereyken K, Sifrim A, Thienpont B, Voet T. Methods and applications for single‐cell and spatial multi‐omics. Nat Rev Genet. 2023:1‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Silvestre‐Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood. 2016;127(18):2173‐2181. [DOI] [PubMed] [Google Scholar]
- 10. Silvestre‐Roig C, Fridlender ZG, Glogauer M, Scapini P. Neutrophil diversity in health and disease. Trends Immunol. 2019;40(7):565‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133(20):2159‐2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosales C. Neutrophils at the crossroads of innate and adaptive immunity. J Leukoc Biol. 2020;108(1):377‐396. [DOI] [PubMed] [Google Scholar]
- 13. Dahl R, Walsh JC, Lancki D, et al. Regulation of macrophage and neutrophil cell fates by the PU.1:c/EBPalpha ratio and granulocyte colony‐stimulating factor. Nat Immunol. 2003;4(10):1029‐1036. [DOI] [PubMed] [Google Scholar]
- 14. Nerlov C, Graf T. PU.1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev. 1998;12(15):2403‐2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosmarin AG, Yang Z, Resendes KK. Transcriptional regulation in myelopoiesis: hematopoietic fate choice, myeloid differentiation, and leukemogenesis. Exp Hematol. 2005;33(2):131‐143. [DOI] [PubMed] [Google Scholar]
- 16. Sasmono RT, Ehrnsperger A, Cronau SL, et al. Mouse neutrophilic granulocytes express mRNA encoding the macrophage colony‐stimulating factor receptor (CSF‐1R) as well as many other macrophage‐specific transcripts and can transdifferentiate into macrophages in vitro in response to CSF‐1. J Leukoc Biol. 2007;82(1):111‐123. [DOI] [PubMed] [Google Scholar]
- 17. Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32(10):452‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. The neutrophil. Immunity. 2021;54(7):1377‐1391. [DOI] [PubMed] [Google Scholar]
- 19. Allen LA. The role of the neutrophil and phagocytosis in infection caused by Helicobacter pylori. Curr Opin Infect Dis. 2001;14(3):273‐277. [DOI] [PubMed] [Google Scholar]
- 20. Phillipson M, Kubes P. The healing power of neutrophils. Trends Immunol. 2019;40(7):635‐647. [DOI] [PubMed] [Google Scholar]
- 21. Dwivedi N, Radic M. Neutrophil activation and B‐cell stimulation in the pathogenesis of Felty's syndrome. Pol Arch Med Wewn. 2012;122(7‐8):374‐379. [PubMed] [Google Scholar]
- 22. Kvedaraite E. Neutrophil‐T cell crosstalk in inflammatory bowel disease. Immunology. 2021;164(4):657‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alves‐Filho JC, Spiller F, Cunha FQ. Neutrophil paralysis in sepsis. Shock. 2010;34(1):15‐21. [DOI] [PubMed] [Google Scholar]
- 24. Sonego F, Castanheira FV, Ferreira RG, et al. Paradoxical roles of the neutrophil in sepsis: protective and deleterious. Front Immunol. 2016;7:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bouma G, Ancliff PJ, Thrasher AJ, Burns SO. Recent advances in the understanding of genetic defects of neutrophil number and function. Br J Haematol. 2010;151(4):312‐326. [DOI] [PubMed] [Google Scholar]
- 26. Gong T, Liu L, Jiang W, Zhou R. DAMP‐sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20(2):95‐112. [DOI] [PubMed] [Google Scholar]
- 27. Zindel J, Kubes P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu Rev Pathol. 2020;15:493‐518. [DOI] [PubMed] [Google Scholar]
- 28. Pittman K, Kubes P. Damage‐associated molecular patterns control neutrophil recruitment. J Innate Immun. 2013;5(4):315‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li L, Chen K, Xiang Y, et al. New development in studies of formyl‐peptide receptors: critical roles in host defense. J Leukoc Biol. 2016;99(3):425‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raoof M, Zhang Q, Itagaki K, Hauser CJ. Mitochondrial peptides are potent immune activators that activate human neutrophils via FPR‐1. J Trauma. 2010;68(6):1332‐1334. 1328‐32; discussion. [DOI] [PubMed] [Google Scholar]
- 31. McDonald B, Pittman K, Menezes GB, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330(6002):362‐366. [DOI] [PubMed] [Google Scholar]
- 32. Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480(7375):109‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klyubin IV, Kirpichnikova KM, Gamaley IA. Hydrogen peroxide‐induced chemotaxis of mouse peritoneal neutrophils. Eur J Cell Biol. 1996;70(4):347‐351. [PubMed] [Google Scholar]
- 34. Pase L, Layton JE, Wittmann C, et al. Neutrophil‐delivered myeloperoxidase dampens the hydrogen peroxide burst after tissue wounding in zebrafish. Curr Biol. 2012;22(19):1818‐1824. [DOI] [PubMed] [Google Scholar]
- 35. Scapini P, Lapinet‐Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195‐203. [DOI] [PubMed] [Google Scholar]
- 36. Norling LV, Perretti M. Proresolving lipid mediators enhance PMN‐mediated bacterial clearance. Proc Natl Acad Sci USA. 2020;117(17):9148‐9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Traidl‐Hoffmann C, Kasche A, Jakob T, et al. Lipid mediators from pollen act as chemoattractants and activators of polymorphonuclear granulocytes. J Allergy Clin Immunol. 2002;109(5):831‐838. [DOI] [PubMed] [Google Scholar]
- 38. Rajarathnam K, Schnoor M, Richardson RM, Rajagopal S. How do chemokines navigate neutrophils to the target site: dissecting the structural mechanisms and signaling pathways. Cell Signal. 2019;54:69‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Matsushima K, Yang D, Oppenheim JJ. Interleukin‐8: an evolving chemokine. Cytokine. 2022;153:155828. [DOI] [PubMed] [Google Scholar]
- 40. Petri B, Sanz MJ. Neutrophil chemotaxis. Cell Tissue Res. 2018;371(3):425‐436. [DOI] [PubMed] [Google Scholar]
- 41. Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36(5):705‐716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354(6):610‐621. [DOI] [PubMed] [Google Scholar]
- 43. Proost P, Struyf S, Van Damme J, Fiten P, Ugarte‐Berzal E, Opdenakker G. Chemokine isoforms and processing in inflammation and immunity. J Autoimmun. 2017;85:45‐57. [DOI] [PubMed] [Google Scholar]
- 44. Russo RC, Garcia CC, Teixeira MM, Amaral FA. The CXCL8/IL‐8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol. 2014;10(5):593‐619. [DOI] [PubMed] [Google Scholar]
- 45. Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400‐2407. [DOI] [PubMed] [Google Scholar]
- 46. Sai J, Raman D, Liu Y, Wikswo J, Richmond A. Parallel phosphatidylinositol 3‐kinase (PI3K)‐dependent and Src‐dependent pathways lead to CXCL8‐mediated Rac2 activation and chemotaxis. J Biol Chem. 2008;283(39):26538‐26547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neel NF, Barzik M, Raman D, et al. VASP is a CXCR2‐interacting protein that regulates CXCR2‐mediated polarization and chemotaxis. J Cell Sci. 2009;122(11):1882‐1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gelfand EW. Importance of the leukotriene B4‐BLT1 and LTB4‐BLT2 pathways in asthma. Semin Immunol. 2017;33:44‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. O'Flaherty JT, Kuroki M, Nixon AB, et al. 5‐Oxo‐eicosanoids and hematopoietic cytokines cooperate in stimulating neutrophil function and the mitogen‐activated protein kinase pathway. J Biol Chem. 1996;271(30):17821‐17828. [DOI] [PubMed] [Google Scholar]
- 50. Konya V, Blattermann S, Jandl K, et al. A biased non‐Galphai OXE‐R antagonist demonstrates that Galphai protein subunit is not directly involved in neutrophil, eosinophil, and monocyte activation by 5‐oxo‐ETE. J Immunol. 2014;192(10):4774‐4782. [DOI] [PubMed] [Google Scholar]
- 51. Ellett F, Marand AL, Irimia D. Multifactorial assessment of neutrophil chemotaxis efficiency from a drop of blood. J Leukoc Biol. 2022;111(6):1175‐1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. De Giovanni M, Tam H, Valet C, Xu Y, Looney MR, Cyster JG. GPR35 promotes neutrophil recruitment in response to serotonin metabolite 5‐HIAA. Cell. 2022;185(5):815‐830. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Girbl T, Lenn T, Perez L, et al. Distinct compartmentalization of the chemokines CXCL1 and CXCL2 and the atypical receptor ACKR1 determine discrete stages of neutrophil diapedesis. Immunity. 2018;49(6):1062‐1076. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roth H, Samereier M, Begandt D, et al. Filamin A promotes efficient migration and phagocytosis of neutrophil‐like HL‐60 cells. Eur J Cell Biol. 2017;96(6):553‐566. [DOI] [PubMed] [Google Scholar]
- 55. Tonetti MS, Imboden MA, Lang NP. Neutrophil migration into the gingival sulcus is associated with transepithelial gradients of interleukin‐8 and ICAM‐1. J Periodontol. 1998;69(10):1139‐1147. [DOI] [PubMed] [Google Scholar]
- 56. Chan EC, Ren C, Xie Z, et al. Regulator of G protein signaling 5 restricts neutrophil chemotaxis and trafficking. J Biol Chem. 2018;293(33):12690‐12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Aerbajinai W, Liu L, Zhu J, Kumkhaek C, Chin K, Rodgers GP. Glia maturation factor‐gamma regulates monocyte migration through modulation of beta1‐integrin. J Biol Chem. 2016;291(16):8549‐8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Monaco S, Sparano V, Gioia M, et al. Enzymatic processing of collagen IV by MMP‐2 (gelatinase A) affects neutrophil migration and it is modulated by extracatalytic domains. Protein Sci. 2006;15(12):2805‐2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173‐182. [DOI] [PubMed] [Google Scholar]
- 60. Herrero‐Cervera A, Soehnlein O, Kenne E. Neutrophils in chronic inflammatory diseases. Cell Mol Immunol. 2022;19(2):177‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hind LE, Huttenlocher A. Neutrophil reverse migration and a chemokinetic resolution. Dev Cell. 2018;47(4):404‐405. [DOI] [PubMed] [Google Scholar]
- 62. Wang J, Hossain M, Thanabalasuriar A, Gunzer M, Meininger C, Kubes P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science. 2017;358(6359):111‐116. [DOI] [PubMed] [Google Scholar]
- 63. Nourshargh S, Renshaw SA, Imhof BA. Reverse migration of neutrophils: where, when, how, and why. Trends Immunol. 2016;37(5):273‐286. [DOI] [PubMed] [Google Scholar]
- 64. de Castro Pinho J, Forster R. Lymph‐derived neutrophils primarily locate to the subcapsular and medullary sinuses in resting and inflamed lymph nodes. Cells. 2021;10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mathias JR, Perrin BJ, Liu TX, Kanki J, Look AT, Huttenlocher A. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol. 2006;80(6):1281‐1288. [DOI] [PubMed] [Google Scholar]
- 66. Wu D, Zeng Y, Fan Y, et al. Reverse‐migrated neutrophils regulated by JAM‐C are involved in acute pancreatitis‐associated lung injury. Sci Rep. 2016;6:20545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Colom B, Bodkin JV, Beyrau M, et al. Leukotriene B4‐neutrophil elastase axis drives neutrophil reverse transendothelial cell migration in vivo. Immunity. 2015;42(6):1075‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Elks PM, van Eeden FJ, Dixon G, et al. Activation of hypoxia‐inducible factor‐1alpha (Hif‐1alpha) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood. 2011;118(3):712‐722. [DOI] [PubMed] [Google Scholar]
- 69. Gewirtz AT, McCormick B, Neish AS, et al. Pathogen‐induced chemokine secretion from model intestinal epithelium is inhibited by lipoxin A4 analogs. J Clin Invest. 1998;101(9):1860‐1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Isles HM, Herman KD, Robertson AL, et al. The CXCL12/CXCR4 signaling axis retains neutrophils at inflammatory sites in zebrafish. Front Immunol. 2019;10:1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tauzin S, Starnes TW, Becker FB, Lam PY, Huttenlocher A. Redox and Src family kinase signaling control leukocyte wound attraction and neutrophil reverse migration. J Cell Biol. 2014;207(5):589‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Marki A, Ley K. Leaking chemokines confuse neutrophils. J Clin Invest. 2020;130(5):2177‐2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kienle K, Lammermann T. Neutrophil swarming: an essential process of the neutrophil tissue response. Immunol Rev. 2016;273(1):76‐93. [DOI] [PubMed] [Google Scholar]
- 74. Hopke A, Scherer A, Kreuzburg S, et al. Neutrophil swarming delays the growth of clusters of pathogenic fungi. Nat Commun. 2020;11(1):2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro‐resolving superfamily of mediators. J Clin Invest. 2018;128(7):2657‐2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Granot Z, Jablonska J. Distinct functions of neutrophil in cancer and its regulation. Mediators Inflamm. 2015;2015:701067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN‐beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120(4):1151‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wu CF, Andzinski L, Kasnitz N, et al. The lack of type I interferon induces neutrophil‐mediated pre‐metastatic niche formation in the mouse lung. Int J Cancer. 2015;137(4):837‐847. [DOI] [PubMed] [Google Scholar]
- 79. Finisguerra V, Di Conza G, Di Matteo M, et al. MET is required for the recruitment of anti‐tumoural neutrophils. Nature. 2015;522(7556):349‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20(3):300‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lopez‐Lago MA, Posner S, Thodima VJ, Molina AM, Motzer RJ, Chaganti RS. Neutrophil chemokines secreted by tumor cells mount a lung antimetastatic response during renal cell carcinoma progression. Oncogene. 2013;32(14):1752‐1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. van Egmond M, Bakema JE. Neutrophils as effector cells for antibody‐based immunotherapy of cancer. Semin Cancer Biol. 2013;23(3):190‐199. [DOI] [PubMed] [Google Scholar]
- 83. Ng LG, Ostuni R, Hidalgo A. Heterogeneity of neutrophils. Nat Rev Immunol. 2019;19(4):255‐265. [DOI] [PubMed] [Google Scholar]
- 84. Deniset JF, Kubes P. Neutrophil heterogeneity: bona fide subsets or polarization states. J Leukoc Biol. 2018;103(5):829‐838. [DOI] [PubMed] [Google Scholar]
- 85. Chatfield SM, Thieblemont N, Witko‐Sarsat V. Expanding neutrophil horizons: new concepts in inflammation. J Innate Immun. 2018;10(5‐6):422‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fukushima K, Nabeshima H, Kida H. Revealing the diversity of neutrophil functions and subsets. Cell Mol Immunol. 2021;18(4):781‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bongers SH, Chen N, van Grinsven E, et al. Kinetics of neutrophil subsets in acute, subacute, and chronic inflammation. Front Immunol. 2021;12:674079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Marzano AV, Borghi A, Wallach D, Cugno M. A comprehensive review of neutrophilic diseases. Clin Rev Allergy Immunol. 2018;54(1):114‐130. [DOI] [PubMed] [Google Scholar]
- 89.Prame Kumar K, Nicholls AJ, Wong CHY. Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018;371(3):551‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shaul ME, Eyal O, Guglietta S, et al. Circulating neutrophil subsets in advanced lung cancer patients exhibit unique immune signature and relate to prognosis. FASEB J. 2020;34(3):4204‐4218. [DOI] [PubMed] [Google Scholar]
- 91. Hacbarth E, Kajdacsy‐Balla A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. 1986;29(11):1334‐1342. [DOI] [PubMed] [Google Scholar]
- 92. Pember SO, Barnes KC, Brandt SJ, Kinkade JM. Density heterogeneity of neutrophilic polymorphonuclear leukocytes: gradient fractionation and relationship to chemotactic stimulation. Blood. 1983;61(6):1105‐1115. [PubMed] [Google Scholar]
- 93. Hassani M, Hellebrekers P, Chen N, et al. On the origin of low‐density neutrophils. J Leukoc Biol. 2020;107(5):809‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ley K, Hoffman HM, Kubes P, et al. Neutrophils: new insights and open questions. Sci Immunol. 2018;3(30). [DOI] [PubMed] [Google Scholar]
- 95. Scapini P, Marini O, Tecchio C, Cassatella MA. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol Rev. 2016;273(1):48‐60. [DOI] [PubMed] [Google Scholar]
- 96. Mackey JBG, Coffelt SB, Carlin LM. Neutrophil maturity in cancer. Front Immunol. 2019;10:1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sagiv JY, Michaeli J, Assi S, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10(4):562‐573. [DOI] [PubMed] [Google Scholar]
- 98. Pfirschke C, Engblom C, Gungabeesoon J, et al. Tumor‐promoting Ly‐6G(+) SiglecF(high) cells are mature and long‐lived neutrophils. Cell Rep. 2020;32(12):108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431‐446. [DOI] [PubMed] [Google Scholar]
- 100. Martin C, Burdon PC, Bridger G, Gutierrez‐Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19(4):583‐593. [DOI] [PubMed] [Google Scholar]
- 101. Evrard M, Kwok IWH, Chong SZ, et al. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity. 2018;48(2):364‐379. e8. [DOI] [PubMed] [Google Scholar]
- 102. Nemeth T, Sperandio M, Mocsai A. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov. 2020;19(4):253‐275. [DOI] [PubMed] [Google Scholar]
- 103. Adrover JM, Del Fresno C, Crainiciuc G, et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity. 2019;50(2):390‐402. e10. [DOI] [PubMed] [Google Scholar]
- 104. Casanova‐Acebes M, Pitaval C, Weiss LA, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153(5):1025‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ballesteros I, Rubio‐Ponce A, Genua M, et al. Co‐option of neutrophil fates by tissue environments. Cell. 2020;183(5):1282‐1297. e18. [DOI] [PubMed] [Google Scholar]
- 106. Boivin G, Ancey PB, Vuillefroy de Silly R, et al. Anti‐Ly6G binding and trafficking mediate positive neutrophil selection to unleash the anti‐tumor efficacy of radiation therapy. Oncoimmunology. 2021;10(1):1876597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Barkaway A, Rolas L, Joulia R, et al. Age‐related changes in the local milieu of inflamed tissues cause aberrant neutrophil trafficking and subsequent remote organ damage. Immunity. 2021;54(7):1494‐1510. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hsu BE, Tabaries S, Johnson RM, et al. Immature low‐density neutrophils exhibit metabolic flexibility that facilitates breast cancer liver metastasis. Cell Rep. 2019;27(13):3902‐3915. e6. [DOI] [PubMed] [Google Scholar]
- 109. Rice CM, Davies LC, Subleski JJ, et al. Tumour‐elicited neutrophils engage mitochondrial metabolism to circumvent nutrient limitations and maintain immune suppression. Nat Commun. 2018;9(1):5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Condamine T, Dominguez GA, Youn JI, et al. Lectin‐type oxidized LDL receptor‐1 distinguishes population of human polymorphonuclear myeloid‐derived suppressor cells in cancer patients. Sci Immunol. 2016;1(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Veglia F, Perego M, Gabrilovich D. Myeloid‐derived suppressor cells coming of age. Nat Immunol. 2018;19(2):108‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wu WC, Sun HW, Chen HT, et al. Circulating hematopoietic stem and progenitor cells are myeloid‐biased in cancer patients. Proc Natl Acad Sci USA. 2014;111(11):4221‐4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Pillay J, Kamp VM, van Hoffen E, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac‐1. J Clin Invest. 2012;122(1):327‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chappell L, Russell AJC, Voet T. Single‐cell (multi)omics technologies. Annu Rev Genomics Hum Genet. 2018;19:15‐41. [DOI] [PubMed] [Google Scholar]
- 115. Han X, Zhou Z, Fei L, et al. Construction of a human cell landscape at single‐cell level. Nature. 2020;581(7808):303‐309. [DOI] [PubMed] [Google Scholar]
- 116. Han X, Wang R, Zhou Y, et al. Mapping the mouse cell atlas by Microwell‐Seq. Cell. 2018;172(5):1091‐1107 e17. [DOI] [PubMed] [Google Scholar]
- 117. Qu J, Yang F, Zhu T, et al. A reference single‐cell regulomic and transcriptomic map of cynomolgus monkeys. Nat Commun. 2022;13(1):4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Dinh HQ, Eggert T, Meyer MA, et al. Coexpression of CD71 and CD117 identifies an early unipotent neutrophil progenitor population in human bone marrow. Immunity. 2020;53(2):319‐334. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Calzetti F, Finotti G, Tamassia N, et al. CD66b(‐)CD64(dim)CD115(‐) cells in the human bone marrow represent neutrophil‐committed progenitors. Nat Immunol. 2022;23(5):679‐691. [DOI] [PubMed] [Google Scholar]
- 120. Weisgrau KL, Vosler LJ, Pomplun NL, et al. Neutrophil progenitor populations of rhesus macaques. J Leukoc Biol. 2019;105(1):113‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Berdnikovs S. The twilight zone: plasticity and mixed ontogeny of neutrophil and eosinophil granulocyte subsets. Semin Immunopathol. 2021;43(3):337‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Rambault M, Doz‐Deblauwe E, Le Vern Y, et al. Neutrophils encompass a regulatory subset suppressing T cells in apparently healthy cattle and mice. Front Immunol. 2021;12:625244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Alder MN, Mallela J, Opoka AM, Lahni P, Hildeman DA, Wong HR. Olfactomedin 4 marks a subset of neutrophils in mice. Innate Immun. 2019;25(1):22‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Puellmann K, Kaminski WE, Vogel M, et al. A variable immunoreceptor in a subpopulation of human neutrophils. Proc Natl Acad Sci USA. 2006;103(39):14441‐14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Massena S, Christoffersson G, Vagesjo E, et al. Identification and characterization of VEGF‐A‐responsive neutrophils expressing CD49d, VEGFR1, and CXCR4 in mice and humans. Blood. 2015;126(17):2016‐2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Christoffersson G, Phillipson M. The neutrophil: one cell on many missions or many cells with different agendas? Cell Tissue Res. 2018;371(3):415‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lawrence SM, Corriden R, Nizet V. The ontogeny of a neutrophil: mechanisms of granulopoiesis and homeostasis. Microbiol Mol Biol Rev. 2018;82(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. van Grinsven E, Textor J, Hustin LSP, Wolf K, Koenderman L, Vrisekoop N. Immature neutrophils released in acute inflammation exhibit efficient migration despite incomplete segmentation of the nucleus. J Immunol. 2019;202(1):207‐217. [DOI] [PubMed] [Google Scholar]
- 129. Leliefeld PHC, Pillay J, Vrisekoop N, et al. Differential antibacterial control by neutrophil subsets. Blood Adv. 2018;2(11):1344‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. La Manno G, Soldatov R, Zeisel A, et al. RNA velocity of single cells. Nature. 2018;560(7719):494‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Qiu X, Mao Q, Tang Y, et al. Reversed graph embedding resolves complex single‐cell trajectories. Nat Methods. 2017;14(10):979‐982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. DeSouza‐Vieira T. Metamorphosis of neutrophil transcriptional programme during Leishmania infection. Parasite Immunol. 2022;44(6):e12922. [DOI] [PubMed] [Google Scholar]
- 133. Chan L, Morovati S, Karimi N, et al. Neutrophil functional heterogeneity and implications for viral infections and treatments. Cells. 2022;11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wigerblad G, Kaplan MJ. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat Rev Immunol. 2022:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Manz MG, Boettcher S. Emergency granulopoiesis. Emergency granulopoiesis. Nat Rev Immunol. 2014;14(5):302‐314. [DOI] [PubMed] [Google Scholar]
- 136. Anzai A, Choi JL, He S, et al. The infarcted myocardium solicits GM‐CSF for the detrimental oversupply of inflammatory leukocytes. J Exp Med. 2017;214(11):3293‐3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Boettcher S, Gerosa RC, Radpour R, et al. Endothelial cells translate pathogen signals into G‐CSF‐driven emergency granulopoiesis. Blood. 2014;124(9):1393‐1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hosseini N, Shor J, Szabo G. Alcoholic hepatitis: a review. Alcohol Alcohol. 2019;54(4):408‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Nati M, Chung KJ, Chavakis T. The role of innate immune cells in nonalcoholic fatty liver disease. J Innate Immun. 2022;14(1):31‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017;127(1):55‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ren R, He Y, Ding D, et al. Aging exaggerates acute‐on‐chronic alcohol‐induced liver injury in mice and humans by inhibiting neutrophilic sirtuin 1‐C/EBPalpha‐miRNA‐223 axis. Hepatology. 2022;75(3):646‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhao X, Yang L, Chang N, et al. Neutrophil recruitment mediated by sphingosine 1‐phosphate (S1P)/S1P receptors during chronic liver injury. Cell Immunol. 2021;359:104243. [DOI] [PubMed] [Google Scholar]
- 143. Choudhury SR, Babes L, Rahn JJ, et al. Dipeptidase‐1 is an adhesion receptor for neutrophil recruitment in lungs and liver. Cell. 2019;178(5):1205‐1221. e17. [DOI] [PubMed] [Google Scholar]
- 144. Hwang S, He Y, Xiang X, et al. Interleukin‐22 ameliorates neutrophil‐driven nonalcoholic steatohepatitis through multiple targets. Hepatology. 2020;72(2):412‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Calvente CJ, Tameda M, Johnson CD, et al. Neutrophils contribute to spontaneous resolution of liver inflammation and fibrosis via microRNA‐223. J Clin Invest. 2019;129(10):4091‐4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Cho Y, Bukong TN, Tornai D, et al. Neutrophil extracellular traps contribute to liver damage and increase defective low‐density neutrophils in alcohol‐associated hepatitis. J Hepatol. 2023;78(1):28‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kolodziejczyk AA, Federici S, Zmora N, et al. Acute liver failure is regulated by MYC‐ and microbiome‐dependent programs. Nat Med. 2020;26(12):1899‐1911. [DOI] [PubMed] [Google Scholar]
- 148. Macek Jilkova Z, Afzal S, Marche H, et al. Progression of fibrosis in patients with chronic viral hepatitis is associated with IL‐17(+) neutrophils. Liver Int. 2016;36(8):1116‐1124. [DOI] [PubMed] [Google Scholar]
- 149. Maria NI, Davidson A. Protecting the kidney in systemic lupus erythematosus: from diagnosis to therapy. Nat Rev Rheumatol. 2020;16(5):255‐267. [DOI] [PubMed] [Google Scholar]
- 150. Rovin BH. Systemic autoimmune diseases and the kidney. Kidney Int. 2020;97(2):222‐225. [DOI] [PubMed] [Google Scholar]
- 151. Saisorn W, Saithong S, Phuengmaung P, et al. Acute kidney injury induced lupus exacerbation through the enhanced neutrophil extracellular traps (and apoptosis) in Fcgr2b deficient lupus mice with renal ischemia reperfusion injury. Front Immunol. 2021;12:669162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Deng Y, Zheng Y, Li D, et al. Expression characteristics of interferon‐stimulated genes and possible regulatory mechanisms in lupus patients using transcriptomics analyses. EBioMedicine. 2021;70:103477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Cahilog Z, Zhao H, Wu L, et al. The role of neutrophil NETosis in organ injury: novel inflammatory cell death mechanisms. Inflammation. 2020;43(6):2021‐2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Nakazawa D, Marschner JA, Platen L, Anders HJ. Extracellular traps in kidney disease. Kidney Int. 2018;94(6):1087‐1098. [DOI] [PubMed] [Google Scholar]
- 155. Stark JE, Opoka AM, Mallela J, et al. Juvenile OLFM4‐null mice are protected from sepsis. Am J Physiol Renal Physiol. 2020;318(3):F809‐F816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Skopelja‐Gardner S, Tai J, Sun X, et al. Acute skin exposure to ultraviolet light triggers neutrophil‐mediated kidney inflammation. Proc Natl Acad Sci USA. 2021;118(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Rodriguez‐Carrio J, Carrillo‐Lopez N, Ulloa C, et al. A subset of low density granulocytes is associated with vascular calcification in chronic kidney disease patients. Sci Rep. 2019;9(1):13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Nishi H, Mayadas TN. Neutrophils in lupus nephritis. Curr Opin Rheumatol. 2019;31(2):193‐200. [DOI] [PubMed] [Google Scholar]
- 159. Bronze‐da‐Rocha E, Santos‐Silva A. Neutrophil elastase inhibitors and chronic kidney disease. Int J Biol Sci. 2018;14(10):1343‐1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Snelgrove RJ, Patel DF, Patel T, Lloyd CM. The enigmatic role of the neutrophil in asthma: friend, foe or indifferent? Clin Exp Allergy. 2018;48(10):1275‐1285. [DOI] [PubMed] [Google Scholar]
- 161. Heeb LEM, Egholm C, Impellizzieri D, Ridder F, Boyman O. Regulation of neutrophils in type 2 immune responses. Curr Opin Immunol. 2018;54:115‐122. [DOI] [PubMed] [Google Scholar]
- 162. Nakamura K, Kageyama S, Kupiec‐Weglinski JW. The evolving role of neutrophils in liver transplant ischemia‐reperfusion injury. Curr Transplant Rep. 2019;6(1):78‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Neupane AS, Willson M, Chojnacki AK, et al. Patrolling alveolar macrophages conceal bacteria from the immune system to maintain homeostasis. Cell. 2020;183(1):110‐125. e11. [DOI] [PubMed] [Google Scholar]
- 164. Zhang Y, Wang Q, Mackay CR, Ng LG, Kwok I. Neutrophil subsets and their differential roles in viral respiratory diseases. J Leukoc Biol. 2022;111(6):1159‐1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Jenkins AR, Holden NS, Jones AW. Inflammatory responses to acute exercise during pulmonary rehabilitation in patients with COPD. Eur J Appl Physiol. 2020;120(10):2301‐2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Laucirica DR, Schofield CJ, McLean SA, et al. Pseudomonas aeruginosa modulates neutrophil granule exocytosis in an in vitro model of airway infection. Immunol Cell Biol. 2022;100(5):352‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Alder MN, Opoka AM, Lahni P, Hildeman DA, Wong HR. Olfactomedin‐4 is a candidate marker for a pathogenic neutrophil subset in septic shock. Crit Care Med. 2017;45(4):e426‐e432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Demaret J, Venet F, Plassais J, et al. Identification of CD177 as the most dysregulated parameter in a microarray study of purified neutrophils from septic shock patients. Immunol Lett. 2016;178:122‐130. [DOI] [PubMed] [Google Scholar]
- 169. Schreiber A, Jannerjahn A, Kettritz R. CD177/NB1 receptor expression is dynamically regulated in sepsis patients. Immunohematology. 2015;31(3):128‐129. [PubMed] [Google Scholar]
- 170. Mortaz E, Alipoor SD, Adcock IM, Mumby S, Koenderman L. Update on neutrophil function in severe inflammation. Front Immunol. 2018;9:2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Kangelaris KN, Clemens R, Fang X, et al. A neutrophil subset defined by intracellular olfactomedin 4 is associated with mortality in sepsis. Am J Physiol Lung Cell Mol Physiol. 2021;320(5):L892‐L902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Ekstedt S, Safholm J, Georen SK, Cardell LO. Dividing neutrophils in subsets reveals a significant role for activated neutrophils in the development of airway hyperreactivity. Clin Exp Allergy. 2019;49(3):285‐291. [DOI] [PubMed] [Google Scholar]
- 173. Kassam AF, Levinsky NC, Mallela JP, et al. Olfactomedin 4‐positive neutrophils are upregulated after hemorrhagic shock. Am J Respir Cell Mol Biol. 2021;64(2):216‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Cortjens B, Ingelse SA, Calis JC, et al. Neutrophil subset responses in infants with severe viral respiratory infection. Clin Immunol. 2017;176:100‐106. [DOI] [PubMed] [Google Scholar]
- 175. Xie Y, Abel PW, Casale TB, Tu Y. T(H)17 cells and corticosteroid insensitivity in severe asthma. J Allergy Clin Immunol. 2022;149(2):467‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Qi X, Yu Y, Sun R, et al. Identification and characterization of neutrophil heterogeneity in sepsis. Crit Care. 2021;25(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Meghraoui‐Kheddar A, Chousterman BG, Guillou N, et al. Two new neutrophil subsets define a discriminating sepsis signature. Am J Respir Crit Care Med. 2022;205(1):46‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Takizawa S, Murao A, Ochani M, Aziz M, Wang P. Frontline Science: extracellular CIRP generates a proinflammatory Ly6G(+) CD11b(hi) subset of low‐density neutrophils in sepsis. J Leukoc Biol. 2021;109(6):1019‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Song Z, Bhattacharya S, Huang G, et al. NADPH oxidase 2 limits amplification of IL‐1beta‐G‐CSF axis and an immature neutrophil subset in murine lung inflammation. Blood Adv. 2023;7(7):1225‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Xie X, Shi Q, Wu P, et al. Single‐cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat Immunol. 2020;21(9):1119‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Fahlberg MD, Blair RV, Doyle‐Meyers LA, et al. Cellular events of acute, resolving or progressive COVID‐19 in SARS‐CoV‐2 infected non‐human primates. Nat Commun. 2020;11(1):6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Deerhake ME, Reyes EY, Xu‐Vanpala S, Shinohara ML. Single‐cell transcriptional heterogeneity of neutrophils during acute pulmonary cryptococcus neoformans infection. Front Immunol. 2021;12:670574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Cheung DS, Sigua JA, Simpson PM, et al. Cysteinyl leukotriene receptor 1 expression identifies a subset of neutrophils during the antiviral response that contributes to postviral atopic airway disease. J Allergy Clin Immunol. 2018;142(4):1206‐1217 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Tirouvanziam R, Gernez Y, Conrad CK, et al. Profound functional and signaling changes in viable inflammatory neutrophils homing to cystic fibrosis airways. Proc Natl Acad Sci USA. 2008;105(11):4335‐4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Ingersoll SA, Laval J, Forrest OA, et al. Mature cystic fibrosis airway neutrophils suppress T cell function: evidence for a role of arginase 1 but not programmed death‐ligand 1. J Immunol. 2015;194(11):5520‐5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Hu X, Sun Y, Xu W, Lin T, Zeng H. Expression of RANKL by peripheral neutrophils and its association with bone mineral density in COPD. Respirology. 2017;22(1):126‐132. [DOI] [PubMed] [Google Scholar]
- 187. Morrissey SM, Geller AE, Hu X, et al. A specific low‐density neutrophil population correlates with hypercoagulation and disease severity in hospitalized COVID‐19 patients. JCI Insight. 2021;6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Hazeldine J, Lord JM. Neutrophils and COVID‐19: active participants and rational therapeutic targets. Front Immunol. 2021;12:680134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. deKay JT, Emery IF, Rud J, et al. DEspR(high) neutrophils are associated with critical illness in COVID‐19. Sci Rep. 2021;11(1):22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Combes AJ, Courau T, Kuhn NF, et al. Global absence and targeting of protective immune states in severe COVID‐19. Nature. 2021;591(7848):124‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. McLeish KR, Shrestha R, Vashishta A, et al. Differential functional responses of neutrophil subsets in severe COVID‐19 patients. Front Immunol. 2022;13:879686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Schulte‐Schrepping J, Reusch N, Paclik D, et al. Severe COVID‐19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182(6):1419‐1440 e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Loyer C, Lapostolle A, Urbina T, et al. Impairment of neutrophil functions and homeostasis in COVID‐19 patients: association with disease severity. Crit Care. 2022;26(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Sinha S, Rosin NL, Arora R, et al. Dexamethasone modulates immature neutrophils and interferon programming in severe COVID‐19. Nat Med. 2022;28(1):201‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Rebillard RM, Charabati M, Grasmuck C, et al. Identification of SARS‐CoV‐2‐specific immune alterations in acutely ill patients. J Clin Invest. 2021;131(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Ode Y, Aziz M, Wang P. CIRP increases ICAM‐1(+) phenotype of neutrophils exhibiting elevated iNOS and NETs in sepsis. J Leukoc Biol. 2018;103(4):693‐707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Carstensen S, Muller M, Tan GLA, et al. Rogue″ neutrophil‐subset [DEspR+CD11b+/CD66b+] immunotype is an actionable therapeutic target for neutrophilic inflammation‐mediated tissue injury ‐ studies in human, macaque and rat LPS‐inflammation models. Front Immunol. 2022;13:1008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Herrera VLM, Walkey AJ, Nguyen MQ, et al. A targetable ‘rogue’ neutrophil‐subset, [CD11b+DEspR+] immunotype, is associated with severity and mortality in acute respiratory distress syndrome (ARDS) and COVID‐19‐ARDS. Sci Rep. 2022;12(1):5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Tak T, Rygiel TP, Karnam G, et al. Neutrophil‐mediated suppression of influenza‐induced pathology requires CD11b/CD18 (MAC‐1). Am J Respir Cell Mol Biol. 2018;58(4):492‐499. [DOI] [PubMed] [Google Scholar]
- 200. Grieshaber‐Bouyer R, Radtke FA, Cunin P, et al. The neutrotime transcriptional signature defines a single continuum of neutrophils across biological compartments. Nat Commun. 2021;12(1):2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Kologrivova I, Shtatolkina M, Suslova T, Ryabov V. Cells of the immune system in cardiac remodeling: main players in resolution of inflammation and repair after myocardial infarction. Front Immunol. 2021;12:664457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Rolfes L, Riek‐Burchardt M, Pawlitzki M, et al. Neutrophil granulocytes promote flow stagnation due to dynamic capillary stalls following experimental stroke. Brain Behav Immun. 2021;93:322‐330. [DOI] [PubMed] [Google Scholar]
- 203. Sim TM, Mak A, Tay SH. Insights into the role of neutrophils in neuropsychiatric systemic lupus erythematosus: current understanding and future directions. Front Immunol. 2022;13:957303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Cerecedo D, Martinez‐Vieyra I, Lopez‐Villegas EO, Hernandez‐Cruz A, Loza‐Huerta ADC. Heterogeneity of neutrophils in arterial hypertension. Exp Cell Res. 2021;402(2):112577. [DOI] [PubMed] [Google Scholar]
- 205. Calcagno DM, Zhang C, Toomu A, et al. SiglecF(HI) marks late‐stage neutrophils of the infarcted heart: a single‐cell transcriptomic analysis of neutrophil diversification. J Am Heart Assoc. 2021;10(4):e019019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Vafadarnejad E, Rizzo G, Krampert L, et al. Dynamics of cardiac neutrophil diversity in murine myocardial infarction. Circ Res. 2020;127(9):e232‐e249. [DOI] [PubMed] [Google Scholar]
- 207. Yiu JYT, Hally KE, Larsen PD, Holley AS. Increased levels of low density neutrophils (LDNs) in myocardial infarction. Acta Cardiol. 2022:1‐8. [DOI] [PubMed] [Google Scholar]
- 208. DeLeon‐Pennell KY, Mouton AJ, Ero OK, et al. LXR/RXR signaling and neutrophil phenotype following myocardial infarction classify sex differences in remodeling. Basic Res Cardiol. 2018;113(5):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Calcagno DM, Ng RP, Toomu A, et al. The myeloid type I interferon response to myocardial infarction begins in bone marrow and is regulated by Nrf2‐activated macrophages. Sci Immunol. 2020;5(51). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Martini E, Kunderfranco P, Peano C, et al. Single‐cell sequencing of mouse heart immune infiltrate in pressure overload‐driven heart failure reveals extent of immune activation. Circulation. 2019;140(25):2089‐2107. [DOI] [PubMed] [Google Scholar]
- 211. Weisenburger‐Lile D, Dong Y, Yger M, et al. Harmful neutrophil subsets in patients with ischemic stroke: association with disease severity. Neurol Neuroimmunol Neuroinflamm. 2019;6(4):e571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Dong Y, Lagarde J, Xicota L, et al. Neutrophil hyperactivation correlates with Alzheimer's disease progression. Ann Neurol. 2018;83(2):387‐405. [DOI] [PubMed] [Google Scholar]
- 213. Trivedi A, Tercovich KG, Casbon AJ, Raber J, Lowell C, Noble‐Haeusslein LJ. Neutrophil‐specific deletion of Syk results in recruitment‐independent stabilization of the barrier and a long‐term improvement in cognitive function after traumatic injury to the developing brain. Neurobiol Dis. 2021;157:105430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Zhao T, Jiang Q, Li W, et al. Antigen‐presenting cell‐like neutrophils foster T cell response in hyperlipidemic patients and atherosclerotic mice. Front Immunol. 2022;13:851713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Chang JT. Pathophysiology of inflammatory bowel diseases. N Engl J Med. 2020;383(27):2652‐2664. [DOI] [PubMed] [Google Scholar]
- 216. Therrien A, Chapuy L, Bsat M, et al. Recruitment of activated neutrophils correlates with disease severity in adult Crohn's disease. Clin Exp Immunol. 2019;195(2):251‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Butin‐Israeli V, Bui TM, Wiesolek HL, et al. Neutrophil‐induced genomic instability impedes resolution of inflammation and wound healing. J Clin Invest. 2019;129(2):712‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Dinallo V, Marafini I, Di Fusco D, et al. Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J Crohns Colitis. 2019;13(6):772‐784. [DOI] [PubMed] [Google Scholar]
- 219. Leppkes M, Lindemann A, Gosswein S, et al. Neutrophils prevent rectal bleeding in ulcerative colitis by peptidyl‐arginine deiminase‐4‐dependent immunothrombosis. Gut. 2022;71(12):2414‐2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Trilleaud C, Gauttier V, Biteau K, et al. Agonist anti‐ChemR23 mAb reduces tissue neutrophil accumulation and triggers chronic inflammation resolution. Sci Adv. 2021;7(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Yan YX, Shao MJ, Qi Q, et al. Artemisinin analogue SM934 ameliorates DSS‐induced mouse ulcerative colitis via suppressing neutrophils and macrophages. Acta Pharmacol Sin. 2018;39(10):1633‐1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Lartey NL, Vargas‐Robles H, Guerrero‐Fonseca IM, et al. Annickia polycarpa extract attenuates inflammation, neutrophil recruitment, and colon damage during colitis. Immunol Lett. 2022;248:99‐108. [DOI] [PubMed] [Google Scholar]
- 223. Zhou G, Yu L, Fang L, et al. CD177(+) neutrophils as functionally activated neutrophils negatively regulate IBD. Gut. 2018;67(6):1052‐1063. [DOI] [PubMed] [Google Scholar]
- 224. Jin M, Zhang H, Wu M, et al. Colonic interleukin‐22 protects intestinal mucosal barrier and microbiota abundance in severe acute pancreatitis. FASEB J. 2022;36(3):e22174. [DOI] [PubMed] [Google Scholar]
- 225. Li Y, Zhu L, Chu Z, et al. Characterization and biological significance of IL‐23‐induced neutrophil polarization. Cell Mol Immunol. 2018;15(5):518‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Levinsky NC, Mallela J, Opoka AM, et al. The olfactomedin‐4 positive neutrophil has a role in murine intestinal ischemia/reperfusion injury. FASEB J. 2019;33(12):13660‐13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Pavlidis P, Tsakmaki A, Pantazi E, et al. Interleukin‐22 regulates neutrophil recruitment in ulcerative colitis and is associated with resistance to ustekinumab therapy. Nat Commun. 2022;13(1):5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Seto N, Torres‐Ruiz JJ, Carmona‐Rivera C, et al. Neutrophil dysregulation is pathogenic in idiopathic inflammatory myopathies. JCI Insight. 2020;5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Carmona‐Rivera C, Khaznadar SS, Shwin KW, et al. Deficiency of adenosine deaminase 2 triggers adenosine‐mediated NETosis and TNF production in patients with DADA2. Blood. 2019;134(4):395‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Dahlstrand Rudin A, Amirbeagi F, Davidsson L, et al. The neutrophil subset defined by CD177 expression is preferentially recruited to gingival crevicular fluid in periodontitis. J Leukoc Biol. 2021;109(2):349‐362. [DOI] [PubMed] [Google Scholar]
- 231. Hirschfeld J. Neutrophil subsets in periodontal health and disease: a mini review. Front Immunol. 2019;10:3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Sansores‐Espana LD, Melgar‐Rodriguez S, Vernal R, Carrillo‐Avila BA, Martinez‐Aguilar VM, Diaz‐Zuniga J. Neutrophil N1 and N2 subsets and their possible association with periodontitis: a scoping review. Int J Mol Sci. 2022;23(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Sas AR, Carbajal KS, Jerome AD, et al. A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat Immunol. 2020;21(12):1496‐1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Jerome AD, Atkinson JR, McVey Moffatt AL, Sepeda JA, Segal BM, Sas AR. Characterization of zymosan‐modulated neutrophils with neuroregenerative properties. Front Immunol. 2022;13:912193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Matsui M, Nagakubo D, Satooka H, Hirata T. A novel Siglec‐F(+) neutrophil subset in the mouse nasal mucosa exhibits an activated phenotype and is increased in an allergic rhinitis model. Biochem Biophys Res Commun. 2020;526(3):599‐606. [DOI] [PubMed] [Google Scholar]
- 236. Rodriguez‐Rosales YA, Langereis JD, Gorris MAJ, et al. Immunomodulatory aged neutrophils are augmented in blood and skin of psoriasis patients. J Allergy Clin Immunol. 2021;148(4):1030‐1040. [DOI] [PubMed] [Google Scholar]
- 237. Turner TC, Sok MCP, Hymel LA, et al. Harnessing lipid signaling pathways to target specialized pro‐angiogenic neutrophil subsets for regenerative immunotherapy. Sci Adv. 2020;6(44). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Teuben MPJ, Hofman M, Greven J, et al. Altered cell surface receptor dynamics and circulatory occurrence of neutrophils in a small animal fracture model. Pathol Res Pract. 2020;216(10):153108. [DOI] [PubMed] [Google Scholar]
- 239. Mouillot P, Witko‐Sarsat V, Wislez M. [Neutrophil plasticity: a new key in the understanding of onco‐immunology] La plasticité des neutrophiles : nouvel élément de compréhension en onco‐immunologie. Rev Mal Respir. 2022;39(7):587‐594. [DOI] [PubMed] [Google Scholar]
- 240. Cerezo‐Wallis D, Ballesteros I. Neutrophils in cancer, a love‐hate affair. FEBS J. 2022;289(13):3692‐3703. [DOI] [PubMed] [Google Scholar]
- 241. Furumaya C, Martinez‐Sanz P, Bouti P, Kuijpers TW, Matlung HL. Plasticity in pro‐ and anti‐tumor activity of neutrophils: shifting the balance. Front Immunol. 2020;11:2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Lecot P, Sarabi M, Pereira Abrantes M, et al. Neutrophil heterogeneity in cancer: from biology to therapies. Front Immunol. 2019;10:2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Rawat K, Syeda S, Shrivastava A. Neutrophil‐derived granule cargoes: paving the way for tumor growth and progression. Cancer Metastasis Rev. 2021;40(1):221‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Lecot P, Ardin M, Dussurgey S, et al. Gene signature of circulating platelet‐bound neutrophils is associated with poor prognosis in cancer patients. Int J Cancer. 2022;151(1):138‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394(10207):1467‐1480. [DOI] [PubMed] [Google Scholar]
- 246. Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325(7):669‐685. [DOI] [PubMed] [Google Scholar]
- 247. Mizuno R, Kawada K, Itatani Y, Ogawa R, Kiyasu Y, Sakai Y. The role of tumor‐associated neutrophils in colorectal cancer. Int J Mol Sci. 2019;20(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Ruland J. Colon cancer: epithelial Notch signaling recruits neutrophils to drive metastasis. Cancer Cell. 2019;36(3):213‐214. [DOI] [PubMed] [Google Scholar]
- 249. Stehr AM, Wang G, Demmler R, et al. Neutrophil extracellular traps drive epithelial‐mesenchymal transition of human colon cancer. J Pathol. 2022;256(4):455‐467. [DOI] [PubMed] [Google Scholar]
- 250. Khan U, Chowdhury S, Billah MM, Islam KMD, Thorlacius H, Rahman M. Neutrophil extracellular traps in colorectal cancer progression and metastasis. Int J Mol Sci. 2021;22(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Yang L, Liu Q, Zhang X, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583(7814):133‐138. [DOI] [PubMed] [Google Scholar]
- 252. Rayes RF, Vourtzoumis P, Bou Rjeily M, et al. Neutrophil extracellular trap‐associated CEACAM1 as a putative therapeutic target to prevent metastatic progression of colon carcinoma. J Immunol. 2020;204(8):2285‐2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Li T, Wang C, Liu Y, et al. Neutrophil extracellular traps induce intestinal damage and thrombotic tendency in inflammatory bowel disease. J Crohns Colitis. 2020;14(2):240‐253. [DOI] [PubMed] [Google Scholar]
- 254. Deng H, Lin C, Garcia‐Gerique L, et al. A novel selective inhibitor JBI‐589 targets PAD4‐mediated neutrophil migration to suppress tumor progression. Cancer Res. 2022;82(19):3561‐3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Cui C, Chakraborty K, Tang XA, et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell. 2021;184(12):3163‐3177. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Triner D, Devenport SN, Ramakrishnan SK, et al. Neutrophils restrict tumor‐associated microbiota to reduce growth and invasion of colon tumors in mice. gastroenterology. 2019;156(5):1467‐1482.[crossref] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Dmitrieva‐Posocco O, Dzutsev A, Posocco DF, et al. Cell‐type‐specific responses to interleukin‐1 control microbial invasion and tumor‐elicited inflammation in colorectal cancer. Immunity. 2019;50(1):166‐180. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Germann M, Zangger N, Sauvain MO, et al. Neutrophils suppress tumor‐infiltrating T cells in colon cancer via matrix metalloproteinase‐mediated activation of TGFbeta. EMBO Mol Med. 2020;12(1):e10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Zhou G, Peng K, Song Y, et al. CD177+ neutrophils suppress epithelial cell tumourigenesis in colitis‐associated cancer and predict good prognosis in colorectal cancer. Carcinogenesis. 2018;39(2):272‐282. [DOI] [PubMed] [Google Scholar]
- 260. Wikberg ML, Ling A, Li X, Oberg A, Edin S, Palmqvist R. Neutrophil infiltration is a favorable prognostic factor in early stages of colon cancer. Hum Pathol. 2017;68:193‐202. [DOI] [PubMed] [Google Scholar]
- 261. Chadwick JW, Fine N, Khoury W, et al. Tissue‐specific murine neutrophil activation states in health and inflammation. J Leukoc Biol. 2021;110(1):187‐195. [DOI] [PubMed] [Google Scholar]
- 262. Takeda Y, Kato T, Nemoto N, et al. Augmentation of the expression of the eotaxin receptor on duodenal neutrophils by IL‐21. Cytokine. 2018;110:194‐203. [DOI] [PubMed] [Google Scholar]
- 263. Itatani Y, Yamamoto T, Zhong C, et al. Suppressing neutrophil‐dependent angiogenesis abrogates resistance to anti‐VEGF antibody in a genetic model of colorectal cancer. Proc Natl Acad Sci USA. 2020;117(35):21598‐21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Chen H, Zhou XH, Li JR, et al. Neutrophils: driving inflammation during the development of hepatocellular carcinoma. Cancer Lett. 2021;522:22‐31. [DOI] [PubMed] [Google Scholar]
- 265. Tsilimigras DI, Brodt P, Clavien PA, et al. Liver metastases. Nat Rev Dis Primers. 2021;7(1):27. [DOI] [PubMed] [Google Scholar]
- 266. Lu C, Rong D, Zhang B, et al. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: challenges and opportunities. Mol Cancer. 2019;18(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Geh D, Leslie J, Rumney R, Reeves HL, Bird TG, Mann DA. Neutrophils as potential therapeutic targets in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2022;19(4):257‐273. [DOI] [PubMed] [Google Scholar]
- 268. van der Windt DJ, Sud V, Zhang H, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68(4):1347‐1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Jiang ZZ, Peng ZP, Liu XC, et al. Neutrophil extracellular traps induce tumor metastasis through dual effects on cancer and endothelial cells. Oncoimmunology. 2022;11(1):2052418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Yang LY, Luo Q, Lu L, et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol. 2020;13(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Xu X, Ye L, Zhang Q, et al. Group‐2 innate lymphoid cells promote HCC progression through CXCL2‐neutrophil‐induced immunosuppression. Hepatology. 2021;74(5):2526‐2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Umeshappa CS, Sole P, Surewaard BGJ, et al. Liver‐specific T regulatory type‐1 cells program local neutrophils to suppress hepatic autoimmunity via CRAMP. Cell Rep. 2021;34(13):108919. [DOI] [PubMed] [Google Scholar]
- 273. Zhou Z, Hu Y, Wu Y, et al. The immunosuppressive tumor microenvironment in hepatocellular carcinoma‐current situation and outlook. Mol Immunol. 2022;151:218‐230. [DOI] [PubMed] [Google Scholar]
- 274. He G, Zhang H, Zhou J, et al. Peritumoural neutrophils negatively regulate adaptive immunity via the PD‐L1/PD‐1 signalling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Cheng Y, Li H, Deng Y, et al. Cancer‐associated fibroblasts induce PDL1+ neutrophils through the IL6‐STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018;9(4):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Xue R, Zhang Q, Cao Q, et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature. 2022;612(7938):141‐147. [DOI] [PubMed] [Google Scholar]
- 277. Wang Y, Zhao Q, Zhao B, et al. Remodeling tumor‐associated neutrophils to enhance dendritic cell‐based HCC neoantigen nano‐vaccine efficiency. Adv Sci (Weinh). 2022;9(11):e2105631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Deng H, Kan A, Lyu N, et al. Tumor‐derived lactate inhibit the efficacy of lenvatinib through regulating PD‐L1 expression on neutrophil in hepatocellular carcinoma. J Immunother Cancer. 2021;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Esteban‐Fabro R, Willoughby CE, Pique‐Gili M, et al. Cabozantinib enhances anti‐PD1 activity and elicits a neutrophil‐based immune response in hepatocellular carcinoma. Clin Cancer Res. 2022;28(11):2449‐2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Siwicki M, Gort‐Freitas NA, Messemaker M, et al. Resident Kupffer cells and neutrophils drive liver toxicity in cancer immunotherapy. Sci Immunol. 2021;6(61). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635‐648. [DOI] [PubMed] [Google Scholar]
- 282. Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. 2020;21(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Tan Z. Recent advances in the surgical treatment of advanced gastric cancer: a review. Med Sci Monit. 2019;25:3537‐3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Zhang X, Shi H, Yuan X, Jiang P, Qian H, Xu W. Tumor‐derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol Cancer. 2018;17(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Shan ZG, Chen J, Liu JS, et al. Activated neutrophils polarize protumorigenic interleukin‐17A‐producing T helper subsets through TNF‐alpha‐B7‐H2‐dependent pathway in human gastric cancer. Clin Transl Med. 2021;11(6):e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Nagaoka K, Shirai M, Taniguchi K, et al. Deep immunophenotyping at the single‐cell level identifies a combination of anti‐IL‐17 and checkpoint blockade as an effective treatment in a preclinical model of data‐guided personalized immunotherapy. J Immunother Cancer. 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Xia X, Zhang Z, Zhu C, et al. Neutrophil extracellular traps promote metastasis in gastric cancer patients with postoperative abdominal infectious complications. Nat Commun. 2022;13(1):1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Zhu T, Zou X, Yang C, et al. Neutrophil extracellular traps promote gastric cancer metastasis by inducing epithelial‑mesenchymal transition. Int J Mol Med. 2021;48(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Li S, Cong X, Gao H, et al. Tumor‐associated neutrophils induce EMT by IL‐17a to promote migration and invasion in gastric cancer cells. J Exp Clin Cancer Res. 2019;38(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Jiang H, Yu D, Yang P, et al. Revealing the transcriptional heterogeneity of organ‐specific metastasis in human gastric cancer using single‐cell RNA Sequencing. Clin Transl Med. 2022;12(2):e730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Qian Y, Zhai E, Chen S, et al. Single‐cell RNA‐seq dissecting heterogeneity of tumor cells and comprehensive dynamics in tumor microenvironment during lymph nodes metastasis in gastric cancer. Int J Cancer. 2022;151(8):1367‐1381. [DOI] [PubMed] [Google Scholar]
- 292. Wang TT, Zhao YL, Peng LS, et al. Tumour‐activated neutrophils in gastric cancer foster immune suppression and disease progression through GM‐CSF‐PD‐L1 pathway. Gut. 2017;66(11):1900‐1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Li S, Sun S, Sun H, et al. A risk signature with inflammatory and immune cells infiltration predicts survival and efficiency of chemotherapy in gastric cancer. Int Immunopharmacol. 2021;96:107589. [DOI] [PubMed] [Google Scholar]
- 294. Cong X, Zhang Y, Zhu Z, et al. CD66b(+) neutrophils and alpha‐SMA(+) fibroblasts predict clinical outcomes and benefits from postoperative chemotherapy in gastric adenocarcinoma. Cancer Med. 2020;9(8):2761‐2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Shan ZG, Zhao YL, Zhang JY, et al. FasL(+) PD‐L2(+) identifies a novel immunosuppressive neutrophil population in human gastric cancer that promotes disease progression. Adv Sci (Weinh). 2022;9(5):e2103543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet. 2021;398(10299):535‐554. [DOI] [PubMed] [Google Scholar]
- 297. Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299‐311. [DOI] [PubMed] [Google Scholar]
- 298. Yang R, Wang X, Ma C, et al. Tumor‐infiltrating neutrophils and peripheral neutrophil‐to‐lymphocyte ratio conversely predicted the prognosis of patients with non‐small cell lung cancer. Cell Immunol. 2022;379:104588. [DOI] [PubMed] [Google Scholar]
- 299. Zhu X, Chen Y, Cui Y. Absolute neutrophil count and mean platelet volume in the blood as biomarkers to detect lung cancer. Dis Markers. 2020;2020:1371964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Xiao Y, Cong M, Li J, et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. 2021;39(3):e7. [DOI] [PubMed] [Google Scholar]
- 301. Li P, Lu M, Shi J, et al. Lung mesenchymal cells elicit lipid storage in neutrophils that fuel breast cancer lung metastasis. Nat Immunol. 2020;21(11):1444‐1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Nolan E, Bridgeman VL, Ombrato L, et al. Radiation exposure elicits a neutrophil‐driven response in healthy lung tissue that enhances metastatic colonization. Nat Cancer. 2022;3(2):173‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Yang C, Wang Z, Li L, et al. Aged neutrophils form mitochondria‐dependent vital NETs to promote breast cancer lung metastasis. J Immunother Cancer. 2021;9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Tyagi A, Sharma S, Wu K, et al. Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating pre‐metastatic niche in lung. Nat Commun. 2021;12(1):474. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 305. Valadez‐Cosmes P, Maitz K, Kindler O, et al. Identification of novel low‐density neutrophil markers through unbiased high‐dimensional flow cytometry screening in non‐small cell lung cancer patients. Front Immunol. 2021;12:703846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Pang J, Yu Q, Chen Y, Yuan H, Sheng M, Tang W. Integrating Single‐cell RNA‐seq to construct a neutrophil prognostic model for predicting immune responses in non‐small cell lung cancer. J Transl Med. 2022;20(1):531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Faget J, Groeneveld S, Boivin G, et al. Neutrophils and snail orchestrate the establishment of a pro‐tumor microenvironment in lung cancer. Cell Rep. 2017;21(11):3190‐3204. [DOI] [PubMed] [Google Scholar]
- 308. Engblom C, Pfirschke C, Zilionis R, et al. Osteoblasts remotely supply lung tumors with cancer‐promoting SiglecF(high) neutrophils. Science. 2017;358(6367). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, et al. Tumor‐associated neutrophils stimulate T cell responses in early‐stage human lung cancer. J Clin Invest. 2014;124(12):5466‐5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Ancey PB, Contat C, Boivin G, et al. GLUT1 expression in tumor‐associated neutrophils promotes lung cancer growth and resistance to radiotherapy. Cancer Res. 2021;81(9):2345‐2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Deryugina EI, Zajac E, Juncker‐Jensen A, Kupriyanova TA, Welter L, Quigley JP. Tissue‐infiltrating neutrophils constitute the major in vivo source of angiogenesis‐inducing MMP‐9 in the tumor microenvironment. Neoplasia. 2014;16(10):771‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Zilionis R, Engblom C, Pfirschke C, et al. Single‐cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity. 2019;50(5):1317‐1334. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Singhal S, Bhojnagarwala PS, O'Brien S, et al. Origin and role of a subset of tumor‐associated neutrophils with antigen‐presenting cell features in early‐stage human lung cancer. Cancer Cell. 2016;30(1):120‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Cheng Y, Mo F, Li Q, et al. Targeting CXCR2 inhibits the progression of lung cancer and promotes therapeutic effect of cisplatin. Mol Cancer. 2021;20(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Drpa G, Sutic M, Baranasic J, et al. Neutrophil‐to‐lymphocyte ratio can predict outcome in extensive‐stage small cell lung cancer. Radiol Oncol. 2020;54(4):437‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389(10074):1134‐1150. [DOI] [PubMed] [Google Scholar]
- 317. Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis‐initiating breast cancer cells. Nature. 2015;528(7582):413‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Szczerba BM, Castro‐Giner F, Vetter M, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566(7745):553‐557. [DOI] [PubMed] [Google Scholar]
- 319. Benevides L, da Fonseca DM, Donate PB, et al. IL17 promotes mammary tumor progression by changing the behavior of tumor cells and eliciting tumorigenic neutrophils recruitment. Cancer Res. 2015;75(18):3788‐3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Spiegel A, Brooks MW, Houshyar S, et al. Neutrophils suppress intraluminal NK cell‐mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016;6(6):630‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Qi M, Xia Y, Wu Y, et al. Lin28B‐high breast cancer cells promote immune suppression in the lung pre‐metastatic niche via exosomes and support cancer progression. Nat Commun. 2022;13(1):897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Casbon AJ, Reynaud D, Park C, et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci USA. 2015;112(6):E566‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Matlung HL, Babes L, Zhao XW, et al. Neutrophils kill antibody‐opsonized cancer cells by trogoptosis. Cell Rep. 2018;23(13):3946‐3959. e6. [DOI] [PubMed] [Google Scholar]
- 324. Veillette A, Chen J. SIRPalpha‐CD47 immune checkpoint blockade in anticancer therapy. Trends Immunol. 2018;39(3):173‐184. [DOI] [PubMed] [Google Scholar]
- 325. Ou B, Liu Y, Yang X, Xu X, Yan Y, Zhang J. C5aR1‐positive neutrophils promote breast cancer glycolysis through WTAP‐dependent m6A methylation of ENO1. Cell Death Dis. 2021;12(8):737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Coffelt SB, Kersten K, Doornebal CW, et al. IL‐17‐producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522(7556):345‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Wellenstein MD, Coffelt SB, Duits DEM, et al. Loss of p53 triggers WNT‐dependent systemic inflammation to drive breast cancer metastasis. Nature. 2019;572(7770):538‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Sionov RV, Fainsod‐Levi T, Zelter T, Polyansky L, Pham CT, Granot Z. Neutrophil cathepsin G and tumor cell RAGE facilitate neutrophil anti‐tumor cytotoxicity. Oncoimmunology. 2019;8(9):e1624129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Peng Z, Liu C, Victor AR, et al. Tumors exploit CXCR4(hi)CD62L(lo) aged neutrophils to facilitate metastatic spread. Oncoimmunology. 2021;10(1):1870811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18(7):493‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Park W, Chawla A, O'Reilly EM. Pancreatic cancer: a review. JAMA. 2021;326(9):851‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Deng J, Kang Y, Cheng CC, et al. DDR1‐induced neutrophil extracellular traps drive pancreatic cancer metastasis. JCI Insight. 2021;6(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Kajioka H, Kagawa S, Ito A, et al. Targeting neutrophil extracellular traps with thrombomodulin prevents pancreatic cancer metastasis. Cancer Lett. 2021;497:1‐13. [DOI] [PubMed] [Google Scholar]
- 334. Zhang Y, Chandra V, Riquelme Sanchez E, et al. Interleukin‐17‐induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J Exp Med. 2020;217(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Wang L, Liu Y, Dai Y, et al. Single‐cell RNA‐seq analysis reveals BHLHE40‐driven pro‐tumour neutrophils with hyperactivated glycolysis in pancreatic tumour microenvironment. Gut. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Zhang J, Xu X, Shi M, et al. CD13(hi) neutrophil‐like myeloid‐derived suppressor cells exert immune suppression through arginase 1 expression in pancreatic ductal adenocarcinoma. Oncoimmunology. 2017;6(2):e1258504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Peng H, Shen J, Long X, et al. Local release of TGF‐beta inhibitor modulates tumor‐associated neutrophils and enhances pancreatic cancer response to combined irreversible electroporation and immunotherapy. Adv Sci (Weinh). 2022;9(10):e2105240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Nielsen SR, Strobech JE, Horton ER, et al. Suppression of tumor‐associated neutrophils by lorlatinib attenuates pancreatic cancer growth and improves treatment with immune checkpoint blockade. Nat Commun. 2021;12(1):3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Li K, Tandurella JA, Gai J, et al. Multi‐omic analyses of changes in the tumor microenvironment of pancreatic adenocarcinoma following neoadjuvant treatment with anti‐PD‐1 therapy. Cancer Cell. 2022;40(11):1374‐1391. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Steele CW, Karim SA, Leach JDG, et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell. 2016;29(6):832‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Chao T, Furth EE, Vonderheide RH. CXCR2‐dependent accumulation of tumor‐associated neutrophils regulates T‐cell immunity in pancreatic ductal adenocarcinoma. Cancer Immunol Res. 2016;4(11):968‐982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Nywening TM, Belt BA, Cullinan DR, et al. Targeting both tumour‐associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut. 2018;67(6):1112‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Tintelnot J, Xu Y, Lesker TR, et al. Microbiota‐derived 3‐IAA influences chemotherapy efficacy in pancreatic cancer. Nature. 2023;615(7950):168‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Bouti P, Zhao XW, Verkuijlen P, et al. Kindlin3‐Dependent CD11b/CD18‐integrin activation is required for potentiation of neutrophil cytotoxicity by CD47‐SIRPalpha checkpoint disruption. Cancer Immunol Res. 2021;9(2):147‐155. [DOI] [PubMed] [Google Scholar]
- 345. Ring NG, Herndler‐Brandstetter D, Weiskopf K, et al. Anti‐SIRPalpha antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci. 2017;114(49):E10578‐E10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Martinez Sanz P, van Rees DJ, van Zogchel LMJ, et al. G‐CSF as a suitable alternative to GM‐CSF to boost dinutuximab‐mediated neutrophil cytotoxicity in neuroblastoma treatment. J Immunother Cancer. 2021;9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Mahiddine K, Blaisdell A, Ma S, Crequer‐Grandhomme A, Lowell CA, Erlebacher A. Relief of tumor hypoxia unleashes the tumoricidal potential of neutrophils. J Clin Invest. 2020;130(1):389‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Blaisdell A, Crequer A, Columbus D, et al. Neutrophils oppose uterine epithelial carcinogenesis via debridement of hypoxic tumor cells. Cancer Cell. 2015;28(6):785‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor‐associated neutrophil phenotype by TGF‐beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Highfill SL, Cui Y, Giles AJ, et al. Disruption of CXCR2‐mediated MDSC tumor trafficking enhances anti‐PD1 efficacy. Sci Transl Med. 2014;6(237). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Millrud CR, Kagedal A, Kumlien Georen S, et al. NET‐producing CD16(high) CD62L(dim) neutrophils migrate to tumor sites and predict improved survival in patients with HNSCC. Int J Cancer. 2017;140(11):2557‐2567. [DOI] [PubMed] [Google Scholar]
- 352. Podaza E, Risnik D, Colado A, et al. Chronic lymphocytic leukemia cells increase neutrophils survival and promote their differentiation into CD16(high) CD62L(dim) immunosuppressive subset. Int J Cancer. 2019;144(5):1128‐1134. [DOI] [PubMed] [Google Scholar]
- 353. Zhu YP, Eggert T, Araujo DJ, Vijayanand P, Ottensmeier CH, Hedrick CC. CyTOF mass cytometry reveals phenotypically distinct human blood neutrophil populations differentially correlated with melanoma stage. J Immunother Cancer. 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Cristinziano L, Modestino L, Capone M, et al. PD‐L1(+) neutrophils as novel biomarkers for stage IV melanoma patients treated with nivolumab. Front Immunol. 2022;13:962669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Kalafati L, Kourtzelis I, Schulte‐Schrepping J, et al. Innate immune training of granulopoiesis promotes anti‐tumor activity. Cell. 2020;183(3):771‐785. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Siders WM, Shields J, Garron C, et al. Involvement of neutrophils and natural killer cells in the anti‐tumor activity of alemtuzumab in xenograft tumor models. Leuk Lymphoma. 2010;51(7):1293‐1304. [DOI] [PubMed] [Google Scholar]
- 357. Subudhi SK, Siddiqui BA, Aparicio AM, et al. Combined CTLA‐4 and PD‐L1 blockade in patients with chemotherapy‐naive metastatic castration‐resistant prostate cancer is associated with increased myeloid and neutrophil immune subsets in the bone microenvironment. J Immunother Cancer. 2021;9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Eruslanov EB, Singhal S, Albelda SM. Mouse versus human neutrophils in cancer: a major knowledge gap. Trends Cancer. 2017;3(2):149‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Peinado H, Aleckovic M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro‐metastatic phenotype through MET. Nat Med. 2012;18(6):883‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Yee PP, Wei Y, Kim SY, et al. Neutrophil‐induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat Commun. 2020;11(1):5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Gupta S, Nakabo S, Blanco LP, et al. Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism. Proc Natl Acad Sci USA. 2020;117(28):16481‐16491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Bachmaier K, Stuart A, Singh A, et al. Albumin nanoparticle endocytosing subset of neutrophils for precision therapeutic targeting of inflammatory tissue injury. ACS Nano. 2022;16(3):4084‐4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Zhao J, Lu H, Xu D, et al. Neutrophil membrane‐coated nanoparticles for enhanced nanosecond pulsed electric field treatment of pancreatic cancer. Int J Hyperthermia. 2022;39(1):1026‐1035. [DOI] [PubMed] [Google Scholar]
- 364. Chu D, Dong X, Shi X, Zhang C, Wang Z. Neutrophil‐based drug delivery systems. Adv Mater. 2018;30(22):e1706245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Han Y, Zhao R, Xu F. Neutrophil‐based delivery systems for nanotherapeutics. Small. 2018;14(42):e1801674. [DOI] [PubMed] [Google Scholar]
- 366. Hao M, Zhu L, Hou S, et al. Sensitizing tumors to immune checkpoint blockage via STING agonists delivered by tumor‐penetrating neutrophil cytopharmaceuticals. ACS Nano. 2023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


