Abstract
Objectives:
To investigate the responsiveness of the motor function measure (MFM) and determine the minimal clinically important difference (MCID) in individuals with 2 common types of congenital muscular dystrophy (CMD).
Design:
Observational, prospective, single center, cohort study.
Setting:
National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health (NIH).
Participants:
Individuals (N=44) with collagen VI-related dystrophies (COL6-RD, n=23) and 21 individuals laminin alpha2-related muscular dystrophy (LAMA2-RD, n=21) enrolled in a 4-year longitudinal natural history study.
Interventions:
Not applicable.
Main Outcome Measures:
Responsiveness of the MFM-32 and the Rasch-scaled MFM-25 and the MCID of the MFM-32 determined from a patient-reported anchor with 2 different methods, within-patient and between-patient.
Results:
The original MFM-32 and Rasch-scaled MFM-25 performed similarly overall in both the COL6-RD and LAMA2-RD populations, with all subscores (D1, standing and transfers; D2, axial and proximal; D3, distal) showing a significant decrease over time, except MFM D1 and D3 for LAMA2-RD. The MFM D1 subscore was the most sensitive to change for ambulant individuals, whereas the MFM D2 subscore was the most sensitive to change for nonambulant individuals. The MCID for the MFM-32 total score was calculated as 2.5 and 3.9 percentage points according to 2 different methods.
Conclusions:
The MFM showed strong responsiveness in individuals with LAMA2-RD and COL6-RD. Because a floor effect was identified more prominently with the Rasch-Scaled MFM-25, the use of the original MFM-32 as a quantitative variable with the assumption of scale linearity appears to be a good compromise. When designing clinical trials in congenital muscular dystrophies, the use of MCID for MFM should be considered to determine if a given intervention effects show not only a statistically significant change but also a clinically meaningful change.
Keywords: Disability evaluation, Muscular dystrophies, Rehabilitation
Congenital muscular dystrophies (CMDs) form a heterogeneous group of congenital and early-onset muscle disorders. This group covers a large spectrum of phenotypic severity from severe and often early fatal disorders to relatively mild conditions compatible with survival into adult life.1 Collagen VI-related dystrophies (COL6-RDs) and laminin alpha2-related muscular dystrophy (LAMA2-RD) together are the most common forms of CMD.
Although the phase 1 study of the antiapoptotic compound omigapil in COL6-RD and LAMA2-RD (ClinicalTrials.gov no.:NCT01805024) marked the completion of the first clinical trial for these 2 types of CMD, more interventional trials are in the planning stages, highlighting the importance of establishing outcome measures and natural history data in these populations. In CMDs, the primary driver of functional impairment, and therefore also the target for rational treatments, is the skeletal muscle. Thus, the reliable assessment of motor function becomes a highly relevant outcome measure. The presence of resulting joint contractures in addition to muscle weakness has the potential to lead to inaccurate motor function assessment and thus presents a challenge to motor outcome scales.2 The Motor Function Measure (MFM)-32, a 32-item motor function scale designed for patients with neuromuscular diseases (NMDs),3 has shown encouraging preliminary results regarding internal consistency, reproducibility, and criterion validity in patients with CMDs.2,4 To investigate additional metrological properties of the MFM-32 using item response theory models, in the specific context of congenital disorders of muscle, we also applied Rasch analysis to a larger dataset and provided a derived scale: the Rasch-scaled MFM-25 for individuals with CMD.4 The Rasch-scaled MFM-25 should be retested in another cohort of individuals with CMD to compare its sensitivity to change versus the original MFM-32.
In addition, the recently published National Institutes of Health (NIH) 4-year CMDs natural history study reported the MFM-32 as the most suitable outcome measure to detect change over a period of 1 year across the spectrum of functional severity in LAMA2-RD and COL6-RD.5 When designing a clinical trial, responsiveness (ie, the ability of an instrument to be responsive to change or to be unresponsive in absence of change) is of prime importance to consider during the choice of the primary outcome measure in addition to its reliability, especially for sample size calculation.6,7
To ensure that statistically significant changes in outcome measures also reflect clinically meaningful implications for the affected individuals in real life, it is also crucial to determine the minimally clinically important difference (MCID), which is to define the clinical importance of a given change in outcome scores separate from their statistical significance. Different approaches have been used to define MCID, including distribution-based methods (eg, effect size, standard error [SE] of the measurement) and anchor-based methods, which use an external “anchor” scale to obtain the perceived change by the patient or caregiver, compared with the scale of interest.8–10 To fully evaluate the benefit of a therapeutic intervention such as would be done in clinical trials in CMD, the target change in the MFM-32 score effects not only has to be statistically significant but should also reflect clinically meaningful improvements for affected individuals with CMD.
Therefore, our main objectives in this analysis were to investigate responsiveness of the MFMs, both for the original MFM-32 and Rasch-scaled MFM-25, in individuals with a diagnosis of COL6-RD or LAMA2-RD, and to establish the MCID of the MFM in CMD, using an anchor-based approach.
Methods
Ethics
Study approval was obtained from the National Institute of Neurological Disorders and Stroke Institutional Review Board (protocol no. 12-N-0095). Individuals or parents who agreed to participate provided informed consent and assent for participants under the age of 18 years.
Individuals
Individuals included in our study were all originally enrolled in an NIH outpatient pediatric clinic between 2010 and 2014 during a longitudinal 4-year natural history study (“Clinical and Molecular Manifestations of Neuromuscular and Neurogenetic Disorders of Childhood”; ClinicalTrials.gov no.: NCT01568658). Additional inclusion criteria applied to our specific study were (1) to be diagnosed with LAMA2-RD or COL6-RD, and (2) to have completed at least 2 MFM-32 assessments 1 year apart during the 4-year natural history study.
Outcome measures
The original MFM-32 consists of 32 items that evaluate 3 functional domains: D1, standing and transfers; D2, axial and proximal motor function; and D3, distal motor function. For each item, the scoring system uses a 4-point Likert scale (0–3) based on the individual’s increasing ability to perform each task without assistance. Raw scores were calculated for each D1, D2, and D3 domain, and a total score (TS) was calculated by adding the scores for each item.3
We previously performed Rasch analysis on a sample of 289 patients with congenital disorders of the muscle and established a conversion of the raw MFM-32 (D1, D2, D3) score into a Rasch-scaled MFM-25 (D1, D2, D3), applying a transformation of MFM-32 ordinal-level scores into linearized measurements via a 2-step procedure.4 Briefly, during the first step, 14 items demonstrating threshold disorders were rescored to 3 response categories, and 7 items that did not fit the Rasch model were deleted to end on a shorter MFM-25 scale. During the second step, the original MFM-32 D1, D2, and D3 scores were converted to a linear level provided by the Rasch analysis as a “person location estimates measures11 (supplemental table S1, available online only at http://www.archives-pmr.org/) to end on the Raschscaled MFM-25 scores. The original MFM-32 total score was not converted into Rasch Measurement, because unidimensionality is one of the most important assumption of the Rasch Model.12
All physical therapists performing the MFM-32 during the study were trained and certified on the scale with established interrater reliability.13 During the medical history, participants or caregivers were asked for an overall assessment of disease evolution: “Have you noticed any change in strength since your last study visit?” Their perceived change was expressed in 3 outcomes: deterioration, stability, or improvement.
Data collection
The participants’ demographic characteristics (sex, age), disease group (COL6-RD or LAMA2-RD), and ambulatory status (defined by the ability to walk 10m indoors without assistance or orthosis) were collected during the baseline visit (defined as the day on which the first MFM-32 was performed for each individual). Scores of the 32 items of the MFM were collected at the baseline visit and at each yearly visit of the longitudinal natural history study. The individuals’ or caregivers’ perceived changes in term of disease evolution were collected each year during follow-up visits.
Data analysis
An analysis of prospectively collected data was performed. Cohort characteristics were described by disease subtype. Continuous variables were quantified using medians and interquartile ranges and were tested for differences between groups using the Wilcoxon rank-sum test. Categorical variables were quantified using number and percent and were tested for differences between groups using Pearson’s chi-square test. The proportion of individuals having the lower limit function score at baseline was used to determine whether a floor effect was present.14,15
To describe the longitudinal behavior of both the original MFM-32 (D1, D2, D3, TS) and the Rasch-scaled MFM-25 (D1, D2, D3), linear mixed effects models with random intercepts were fit for each outcome. Models investigated change in the outcome over continuous time in each CMD subtype (or CMD subtype and severity combination), adjusted for sex, and time-varying covariates of weight, height, and age. Time was defined as time from baseline for an individual on a specific outcome measure.
For MCID, the group average changes in the MFM-32 TS for each self-reported change was consecutively calculated, adjusting for multiple observations per person with a separate linear mixed effects model for each domain and TS. The outcome measure was the change between timepoints for that individual and the predictor variable was the self-report rating.
Using the above group-level estimates for each self-report level, 2 MCID values were consecutively calculated: one by the within-patient method, which is the mean change in score in the patients who improved, and the other by the between-patient method, which is the difference in mean change in score between patients who improved and patients who were stable.
The threshold for significance was set at α of 0.05. All statistical analyses were performed using R, version 3.3.1.a
Results
Of the 48 participants enrolled in the NIH 4-year longitudinal natural history study, 23 with COL6-RD and 21 with LAMA2-RD (age range, 5–21y) were included in our study (fig 1). Table 1 shows the characteristics of the population at baseline. At baseline, differences between percentages of nonambulant participants, distribution of MFM-32 score, and age between each disease group suggested that the disease severity was higher in participants with LAMA2-RD than in participants with COL6-RD. Participants had 2 to 5 MFM-32 measurements during follow-up. No patient had more than 1 consecutive missing measurement between visits and no individual with only 2 visits had a gap between those 2 visits.
Fig 1.
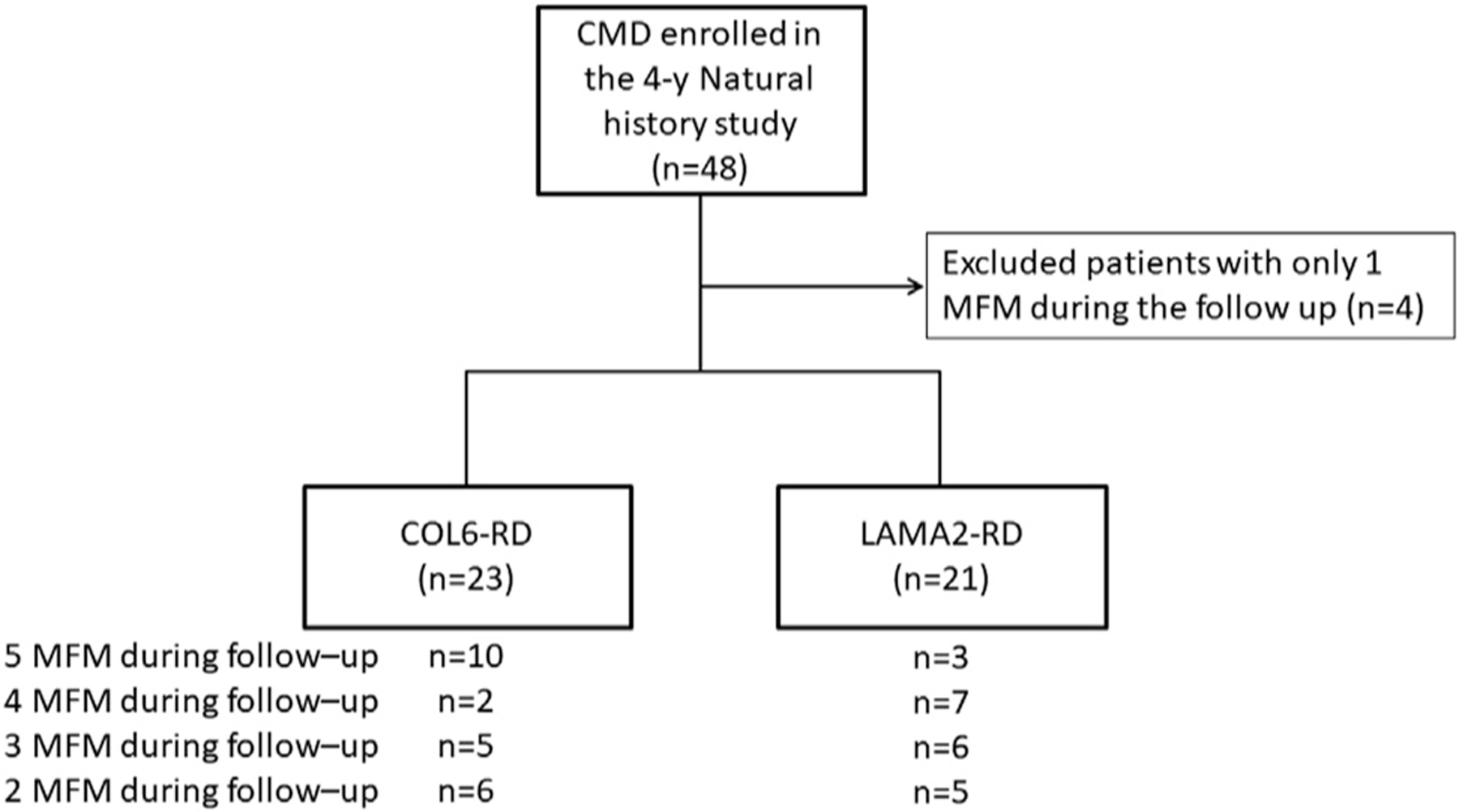
Flow chart of the study.
Table 1.
Cohort characteristics at inclusion
| Characteristic | COL6-RD (n=23) | LAMA2-RD (n=21) | P Value |
|---|---|---|---|
| Age, y, median (IQR) | 9 (7.0–13.5) | 7 (5.0–9.0) | .03 |
| Sex, male, n (%) | 12 (52) | 10 (48) | >.99 |
| Ambulant, n (%) | 16 (70) | 4 (19) | .002 |
| MFM-32 raw scores, median (IQR) | |||
| MFM D1 | 16 (7.0–24.0) | 1 (1.0–4.0) | .006 |
| MFM D2 | 32 (29.5–34.0) | 29 (15.0–31.0) | .01 |
| MFM D3 | 20 (18.5–20.0) | 14 (13.0–16.0) | <.001 |
| MFM TS | 69 (59.5–77.0) | 44 (27.0–52.0) | .002 |
Abbreviation: IQR, interquartile range.
Floor effect
No individuals had a minimal score for D2 and D3 (ie, a score of 0), whether in the original MFM-32 or the Rasch-scaled MFM-25. Regarding the D1 subscore, 3 out of the 23 individuals with COL6-RD had a score of 0 for the original MFM-32 and 6 for the Rasch-scaled MFM-25. For LAMA2-RD, 5 out of the 21 individuals had a score of 0 in D1 for the original MFM-32 and 16 for the Rasch-scaled MFM-25. Regarding nonambulant participants in the sample (both COL6-RD and LAMA2-RD), 8 individuals had a score of 0 in D1 for the original MFM-32 whereas 22 for the Rasch-scaled MFM-25.
MFM responsiveness
Whereas in individuals with COL6-RD, all MFM subscores (D1, D2, D3 and TS) showed a significant decrease over time, MFM subscores D1 and D3 showed a nonsignificant decrease in individuals with LAMA2-RD. These findings were similar for both the MFM-32 and the Rasch-scales MFM-25 (table 2).
Table 2.
Longitudinal estimates of average change per year in COL6-RD (n=23) and LAMA2-RD (n=21) for the MFM-32 and the Raschscaled MFM-25
| COL6-RD |
LAMA2-RD |
|||
|---|---|---|---|---|
| MFM-32 | Rasch-scaled MFM-25 | MFM-32 | Rasch-scaled MFM-25 | |
| MFM D1 | −1.99 (0.44)* | −0.37 (0.10)* | −0.72 (0.47) | −0.10 (0.11) |
| MFM D2 | −1.40 (0.50)† | −0.34 (0.10)* | −1.59 (0.56)† | −0.34 (0.11)† |
| MFM D3 | −0.74 (0.21)* | −0.29 (0.10)† | −0.27 (0.24) | −0.08 (0.11) |
| MFM TS | −4.05 (0.92)* | NA | −2.62 (1.00)† | NA |
NOTE. All values are mean (SE).
Abbreviation: NA, not applicable.
P<.001.
P<.01.
Considering the essentially similar behavior of each scale, from this point only original MFM-32 subscores are provided in the text and for the calculation of the MCID. After categorizing participant function by ambulation status, the MFM D1 subscore appeared to be the most sensitive to change for ambulant participants, whereas the MFM D2 subscore appeared to be the most sensitive to change for nonambulant participants (table 3). Significant change over time was identified for the MFM D3 subscore in nonambulant COL6-RD participants (e1.43; SE, 0.28; P<.001), but not in nonambulant LAMA2-RD participants (e0.28; SE, 0.25; PZ.26). The MFM TS showed a highly significant change over time in ambulant and nonambulant COL6-RD participants, but only in nonambulant LAMA2-RD participants (see table 3).
Table 3.
Longitudinal estimates of average change per year in MFM-32 and Rasch-scaled MFM-25 by disease group and ambulation status
| Ambulant |
Nonambulant |
|||||||
|---|---|---|---|---|---|---|---|---|
| COL6-RD (n=16) |
LAMA2-RD (n=4) |
COL6-RD (n=7) |
LAMA2-RD (n=17) |
|||||
| Subscore or TS | MFM-32 | Rasch-scaled MFM-25 | MFM-32 | Rasch-scaled MFM-25 | MFM-32 | Rasch-scaled MFM-25 | MFM-32 | Rasch-scaled MFM-25 |
| MFM D1 | −2.63 (0.35)* | −0.53 (0.08)* | −2.01 (0.54)* | −0.34 (0.13) | −0.76 (0.42) | −0.14 (0.10) | −0.32 (0.38) | −0.07 (0.09) |
| MFM D2 | −0.44 (0.50) | −0.21 (0.10)† | −0.04 (0.81) | −0.12 (0.17) | −3.17 (0.61)* | −0.58 (0.13)* | −2.01 (0.54)* | −0.40 (0.11)* |
| MFM D3 | −0.33 (0.23) | −0.12 (0.11) | 0.06 (0.39) | 0.06 (0.18) | −1.43 (−0.28)* | −0.56 (0.14)* | −0.28 (0.25) | −0.09 (0.12) |
| MFM TS | −3.38 (0.79)* | NA | −2.02 (1.25) | NA | −5.28 (0.96)* | NA | −2.69 (0.87)† | NA |
NOTE. All values are mean (SE).
Abbreviation: NA, not applicable.
P<.001.
P<.01.
MCID calculation
Sixty-two self-reported impressions of change were available for a total of 35 individuals, with 26 individuals having multiple observations (22 of 62 emphasized worsening, 28 of 62 indicated stabilization, and 12 of 62 demonstrated improvement). The average MFM changes (D1, D2 and D3, and TS) in each group of participant-reported evolution (improved, stable, worsened) are reported in figure 2.
Fig 2.
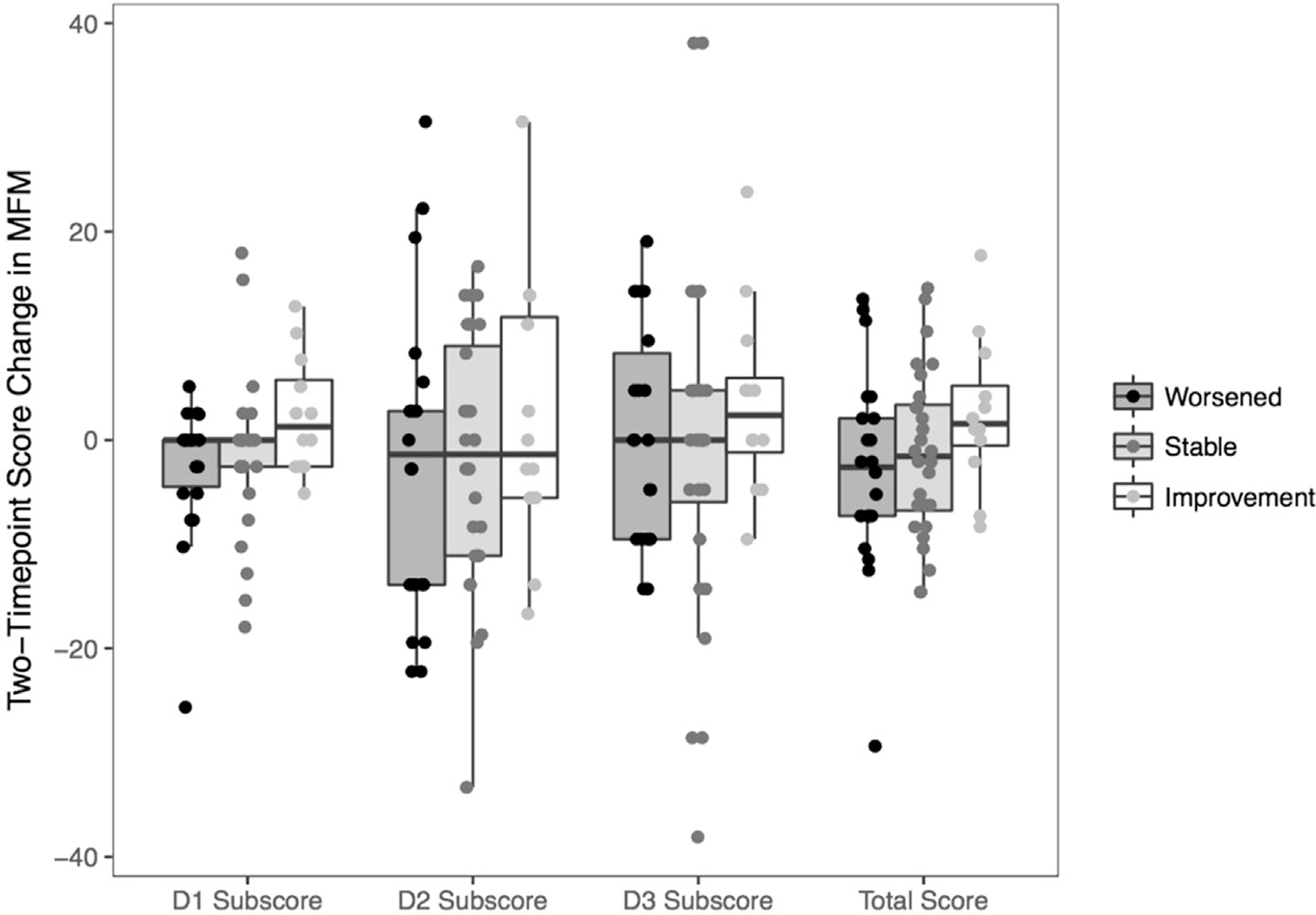
Two-Timepoint score change in MFM (D1, D2, D3 and TS) in each group of participant-reported function (worsened, stable and improvement). Only the first set of timepoints for each individual is shown.
The mean scores changes for D1, D2, D3, and TS improved for participants reporting improvement (2.32 [SE, 1.96]; 2.08 [SE, 3.84]; 3.57 [SE, 3.98]; and 2.52 [SE, 2.44], respectively) and declined for participants reporting stability (e1.82 [SE, 1.38]; e 1.96 [SE, 2.52]; e0.51 [SE, 2.61]; and e1.38 [SE, 1.59]) or deterioration (e1.75 [SE, 1.56]; e2.65 [SE, 2.84]; 0 [SE, 2.94]; and e2.85 [SE, 1.8]). Using the within-patient method (ie, the estimated mean change for patients who improved), the MCID for the MFM-32 TS was 2.5. With the between-patient method (ie, deducting the mean change score of stable patients from the first MCID), the MCID was 3.9.
Discussion
Responsiveness is a key quality required of an outcome measure instrument to be used in a clinical trial. It should not be viewed as only a property of the instrument itself, as responsiveness is also directly related to the specific disease behavior of the condition under study and thus must be determined expressively for each such condition.6,16,17 Using high quality longitudinal data from a 4-year natural history study, we demonstrated the responsiveness of the MFM in participants with 2 essential forms of CMD, LAMA2-RD and COL6-RD. We found a similar trend regarding slopes of change and responsiveness regardless of the type of scale used (original MFM-32 vs the Rasch-scaled MFM-25). Significant differences were found between change over time in different disease and severity groups (LAMA2-RD vs COL6-RD and ambulant vs nonambulant participants), confirming the construct validity of the original MFM-32.
Our results suggest that the responsive domain of interest is D1 for ambulant patients and D2 for nonambulant patients, reflecting a similar pattern to that observed in the spinal muscular atrophy (SMA) population.18 For the D1 domain, specifically in non-ambulant individuals, there was a considerable floor effect in the Rasch-scaled MFM-25, which was less prominent in the original MFM-32, supporting the ability of D1 from the MFM-32 to monitor standing position and transfers in nonambulant patients with CMD. These results confirm our clinical experience suggesting that, in contrast to most SMA and Duchenne muscular dystrophy (DMD) patients, even if CMD patients lose the ability to ambulate, some patients may preserve some abilities pertaining to standing position and transfer functions. The D3 domain was able to capture a change in only the nonambulant COL6-RD individuals, likely because of the progressive course of distal joint contractures and weakness after loss of ambulation with consequences for distal motor function. Within our cohort, LAMA2-RD individuals could be described as having the most severely impaired motor function, including distally, and were likely less subject to change in the D3 domain even though the D3 domain was identified by Barthels et al as very discriminant in the later and most severe stage of DMD.19 For the CMD patient population studied here, the MFM TS appeared particularly valuable because it was able to capture significant changes over time for each disease and severity group. This was observed in all cases except in ambulant LAMA2-RD individuals (only 4 patients), in whom D1 was found to be most responsive to monitor disease progression. The greater floor effect for D1 in the Rasch-scaled MFM-25, the complexity of the score calculation transformation that must be performed for this scale, and the interest of the MFM TS, which is available only for the original MFM-32 support the use of the original MFM-32 rather than the Rasch-scaled MFM-25.
There are many approaches to assess responsiveness of an outcome measure, with the most frequently used being a distribution-based approach like effect size or standardized response mean.20 However, these methods largely do not address consideration for the MCID, which involves defining the clinical importance of a given change in outcome scores separately from their pure statistical significance. Tying changes in an outcome measure to clinical meaningfulness for the patient is of great importance to regulators involved in the drug approval processes. Various methods have been described to determine MCID, with a high amount of variability depending on the method chosen. Several studies have recommended anchor-based approaches instead of distribution-based methods, which assess minimal detectable change rather than important changes21,22 and do not involve participants or caregivers in any way.23 Among anchor-based approaches, both within-patient and between-patient methods can be used with a longitudinal data structure. Because there is no evidence of superiority among these methods, we chose to present both approaches.
In a previous study, a change of 5 points in MFM scores was considered clinically meaningful in a population of 152 individuals affected with various NMDs,24 whereas the MCID in this study was 2.5 or 3.9 depending on the method used. This slight difference may be caused by the mixed population characteristics of the previous study, whereas the present study is more homogeneous with an overall phenotype of more significantly impaired motor function. Other studies have reported an MCID for different scales used in NMDs: the North Star Ambulatory Assessment and the 6MWT in DMD,25,26 the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders in SMA type 1,27 and the modified Hammersmith functional motor scale in SMA types 2 and 3.28 These studies used mainly a distribution-based method. One study used a patient-reported outcome measure, the Pediatric Outcomes Data Collection Instrument questionnaire,29 and found variable MCID values for the 6MWT in the DMD population depending on their functional abilities. This supports the idea that MCID values can differ not only according to the pathology but also based on the severity of the functional impairment.
The present study also highlights that, in the natural history of a degenerative disease of muscle, the functionality can be perceived as improved from one year to the next.
Furthermore, optimal sample size estimation ensures appropriate power to detect significant differences in clinical research studies. Based on expected score change induced by treatment, an estimation of adequate sample size for a clinical trial using the MFM as the primary outcome is allowed by the data of the present study.
Study limitations
In this study, the high quality of the prospectively collected data set and optimal data analysis compensated for the relatively small number of participants, which is related to the rare nature of these diseases. The data point of the perceived-participant and caregiver change used to calculate the MCID was necessarily retrospective because it was based on the participants’ memory of their condition 1 year earlier. Another limitation may be the use of a 3-point Likert scale to express the perceived change of disease evolution, because the use of a larger number of response alternatives could have increased the reliability of test statistics.30 The ability to walk and stand (MFM D1 domain) is usually the most important driving progression of the disease for patients and family members until it bottoms out. In future studies, it could be of interest to try to determine an MCID individually for each of the 3 MFM domains. However, in the current study, a 3-item self-assessment would have further complicated the task for the participant and required assurance of adequate participant understanding of the 3 functional domains at the study outset. To record the perceived change of disease evolution, patients and family members were asked about the change of strength since the last assessment that could be considered as an approximation of the evolution of motor function. A question related to the evolution of the functional abilities could have been more accurate.
Conclusions
We demonstrated robust responsiveness of the MFM in patients with LAMA2-RD and COL6-RD. The use of the original MFM-32 as a quantitative variable with the assumption of scale linearity appears to be a good compromise. The MCID of the MFM-32 will permit the determination of a minimum target efficiency anchored to clinical relevance for the patient. This in turn will help power clinical trials by choosing the most appropriate MFM scores change relative to sample characteristics and to calculate the sample size needed to capture meaningful clinical changes.
Supplementary Material
List of abbreviations:
- 6WMT
6-minute walk test
- CMD
congenital muscular dystrophy
- COL6-RD
collagen VI-related dystrophy
- D1
motor function measure D1 subscore
- D2
motor function measure D2 subscore
- D3
motor function measure D3 subscore
- DMD
Duchenne muscular dystrophy
- LAMA2-RD
laminin, alpha2-related muscular dystrophy
- MCID
minimal clinically important difference
- MFM
motor function measure
- NIH
National Institutes of Health
- NMD
neuromuscular disease
- SMA
spinal muscular atrophy
- TS
motor function measure total score
Footnotes
Work in the National Institute of Neurological Disorders and Stroke, the National Institute of Nursing Research, and the National Institutes of Health (NIH) Mark O. Hatfield Clinical Research Center is supported by intramural funds of the NIH.
Disclosures: Dr Le Goff reports nonfinancial support from Biogen, outside the submitted work. Dr Vuillerot reports grants and personal fees from Biogen, personal fees from Roche, and personal fees from Avexis, outside the submitted work. The other authors have nothing to disclose.
Supplier
R, version 3.3.1; The R Project for Statistical Computing.
References
- 1.Muntoni F, Voit T. The congenital muscular dystrophies in 2004: a century of exciting progress. Neuromuscul Disord 2004;14:635–49. [DOI] [PubMed] [Google Scholar]
- 2.Bönnemann CG, Rutkowski A, Mercuri E, Muntoni F. CMD Outcomes Consortium. 173rd ENMC International Workshop: congenital muscular dystrophy outcome measures 5–7 March 2010, Naarden, The Netherlands. Neuromuscul Disord 2011;21:513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bérard C, Payan C, Hodgkinson I, Fermanian J; MFM Collaborative Study Group. A motor function measure for neuromuscular diseases. Construction and validation study. Neuromuscul Disord 2005;15:463–70. [DOI] [PubMed] [Google Scholar]
- 4.Vuillerot C, Rippert P, Kinet V, et al. Rasch analysis of the motor function measure in patients with congenital muscle dystrophy and congenital myopathy. Arch Phys Med Rehabil 2014;95:2086–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain MS, Meilleur K, Kim E, et al. Longitudinal changes in clinical outcome measures in COL6-related dystrophies and LAMA2-related dystrophies. Neurology 2019;93:e1932–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norman GR, Stratford P, Regehr G. Methodological problems in the retrospective computation of responsiveness to change: the lesson of Cronbach. J Clin Epidemiol 1997;50:869–79. [DOI] [PubMed] [Google Scholar]
- 7.Guyatt GH, Deyo RA, Charlson M, Levine MN, Mitchell A. Responsiveness and validity in health status measurement: a clarification. J Clin Epidemiol 1989;42:403–8. [DOI] [PubMed] [Google Scholar]
- 8.Sloan J, Symonds T, Vargas-Chanes D, Fridley B. Practical guidelines for assessing the clinical significance of health-related quality of life changes within clinical trials. Ther Innov Regul Sci 2003;37:23–31. [Google Scholar]
- 9.de Vet HC, Terwee CB, Ostelo RW, Beckerman H, Knol DL, Bouter LM. Minimal changes in health status questionnaires: distinction between minimally detectable change and minimally important change. Health Qual Life Outcomes 2006;4:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rai SK, Yazdany J, Fortin PR, Aviña-Zubieta JA. Approaches for estimating minimal clinically important differences in systemic lupus erythematosus. Arthritis Res Ther 2015;17:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright BD, Linacre JM. Observations are always ordinal; measurements, however, must be interval. Arch Phys Med Rehabil 1989;70:857–60. [PubMed] [Google Scholar]
- 12.Brentani E, Golia S. Unidimensionality in the Rasch model: how to detect and interpret. Statistica 2007;67:253–61. [Google Scholar]
- 13.Meilleur KG, Jain MS, Hynan LS, et al. Results of a two-year pilot study of clinical outcome measures in collagen VI- and laminin alpha2-related congenital muscular dystrophies. Neuromuscul Disord 2015;25:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McHorney CA, Tarlov AR. Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res 1995;4:293–307. [DOI] [PubMed] [Google Scholar]
- 15.Terwee CB, Bot SDM, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 2007;60:34–42. [DOI] [PubMed] [Google Scholar]
- 16.Kirshner B, Guyatt G. A methodological framework for assessing health indices. J Chronic Dis 1985;38:27–36. [DOI] [PubMed] [Google Scholar]
- 17.Oeffinger D, Bagley A, Rogers S, et al. Outcome tools used for ambulatory children with cerebral palsy: responsiveness and minimum clinically important differences. Dev Med Child Neurol 2008;50:918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vuillerot C, Payan C, Iwaz J, Ecochard R, Bérard C; MFM Spinal Muscular Atrophy Study Group. Responsiveness of the motor function measure in patients with spinal muscular atrophy. Arch Phys Med Rehabil 2013;94:1555–61. [DOI] [PubMed] [Google Scholar]
- 19.Bartels B, Pangalila RF, Bergen MP, Cobben NAM, Stam HJ, Roebroeck ME. Upper limb function in adults with Duchenne muscular dystrophy. J Rehabil Med 2011;43:770–5. [DOI] [PubMed] [Google Scholar]
- 20.Middel B, van Sonderen E. Statistical significant change versus relevant or important change in (quasi) experimental design: some conceptual and methodological problems in estimating magnitude of intervention-related change in health services research. Int J Integr Care 2002;2:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terwee CB, Roorda LD, Dekker J, et al. Mind the MIC: large variation among populations and methods. J Clin Epidemiol 2010;63:524–34. [DOI] [PubMed] [Google Scholar]
- 22.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 2008;61:102–9. [DOI] [PubMed] [Google Scholar]
- 23.King MT. A point of minimal important difference (MID): a critique of terminology and methods. Expert Rev Pharmacoecon Outcomes Res 2011;11:171–84. [DOI] [PubMed] [Google Scholar]
- 24.Vuillerot C, Payan C, Girardot FC, et al. Responsiveness of the motor function measure in neuromuscular diseases. Arch Phys Med Rehabil 2012;93. 2251–6.e1. [DOI] [PubMed] [Google Scholar]
- 25.Mayhew AG, Cano SJ, Scott E, et al. Detecting meaningful change using the North Star Ambulatory Assessment in Duchenne muscular dystrophy. Dev Med Child Neurol 2013;55:1046–52. [DOI] [PubMed] [Google Scholar]
- 26.McDonald CM, Henricson EK, Abresch RT, et al. The 6-minute walk test and other clinical endpoints in Duchenne muscular dystrophy: reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle Nerve 2013;48:357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med 2017;377:1723–32. [DOI] [PubMed] [Google Scholar]
- 28.Swoboda KJ, Scott CB, Crawford TO, et al. SMA CARNI-VAL trial part I: double-blind, randomized, placebo-controlled trial of l-carnitine and valproic acid in spinal muscular atrophy. PLoS One 2010;5:e12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henricson E, Abresch R, Han JJ, et al. The 6-minute walk test and person-reported outcomes in boys with Duchenne muscular dystrophy and typically developing controls: longitudinal comparisons and clinically-meaningful changes over one year. PLoS Curr 2013;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maydeu-Olivares A, Fairchild AJ, Hall AG. Goodness of fit in item factor analysis: effect of the number of response alternatives. Struct Equ Modeling 2017;24:495–505. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


