Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) is an effective treatment for acute myeloid leukemia (AML). Pediatric patients with AML who relapse after HSCT have an extremely poor prognosis. We performed a retrospective study of pediatric patients diagnosed with AML from August 2015 to October 2019 who were treated with HSCT. Kaplan–Meier analyses were used to evaluate overall survival (OS), event-free survival (EFS), and cumulative recurrence rate (CRR). Cox regression analysis was used to determine the association between the baseline characteristics and relapse. A total of 37 pediatric patients met the inclusion criteria. Twenty-eight (75.7%) patients survived, and 9 (24.3%) patients died. The OS rates of AML patients treated with HSCT were 89.2% ± 5.1%, 75.7% ± 7.1%, and 75.7% ± 7.1% at 1, 3, and 5 years, respectively, and the CRRs were 11.4% ± 5.4%, 24.7% ± 7.7%, and 33.1% ± 10.4% at 1, 3, and 5 years after HSCT, respectively; four of nine children who relapsed after transplantation died. Induction with etoposide rather than homoharringtonine and fungal infections could be high-risk factors for recurrence after transplantation. The association between homoharringtonine-based induction therapy and a low recurrence rate persisted after adjusting for age, sex, risk stratification, fusion genes, and fungal infections. This study clarifies the clinical features and poor prognosis of post-transplant relapse in pediatric AML and indicates the urgent need for effective therapy for patients who relapse after HSCT.
Keywords: acute myeloid leukemia, stem cell transplantation, post-transplant relapse, homoharringtonine
Introduction
Leukemia is the most common type of cancer in children (aged 0–14 years). The incidence of leukemia is 42.33 per million in China 1 . Although AML accounts for approximately only one in four of all acute leukemias in children, it is the second most common cancer type in children aged 5 years and older and in adolescents (aged 15–19 years) 1 . AML is characterized by greater than 20% myeloid blasts and is the most aggressive cancer with a variable prognosis depending upon the molecular subtype. In recent years, treatment outcomes for AML have improved due to precision risk stratification and optimized chemotherapy regimens, with overall survival (OS) reaching 70%2,3. The current standard regimen for the treatment of AML is induction chemotherapy with anthracyclines and cytarabine, followed by a choice of chemotherapy consolidation for patients with low to moderate risk or HSCT for patients with high risk, which depends on the patient’s risk stratification 4 . Approximately 80% to 90% of patients with AML achieve complete remission (CR) after induction therapy; however, AML has a high recurrence rate of approximately 40% 5 . After aggressive treatment with strong chemotherapy and HSCT, the prognosis for patients with recurrent AML remains very poor 6 . HSCT is undoubtedly an important treatment for AML, especially for patients with high risk and refractory/relapsed disease 7 . However, the high incidence of transplantation complications, the prognostic impact, the risk stratification of transplantation patients, and the timing of transplantation interventions in the overall management of AML remain controversial in different research centers. This study retrospectively analyzed the clinical outcomes of 37 pediatric patients with AML treated with HSCT after induction of remission with the Chinese Children’s Leukemia Group (CCLG)–AML2015 regimen at the Hematoma Center of Beijing Children’s Hospital, Capital Medical University, and analyzed possible factors affecting prognosis.
Patients and Methods
Patients
The study included 37 patients who underwent HSCT for AML at the Hematology Center of Beijing Children’s Hospital, Capital Medical University. Patients were selected if they met all of the following criteria: (1) have a clear diagnosis and typing of morphology–immunology–cytogenetics–molecular biology (MICM) and risk stratification, (2) high-risk AML treated with the CCLG–AML2015 regimen before HSCT, (3) first allogeneic HSCT, and (4) initial diagnosis between 2015.8 and 2019.10. Patients who met any of the following criteria were not included in this study: (1) diagnosis of acute promyelocytic leukemia (APL) and (2) temporary or palliative treatment without following the standard treatment protocol. The following data were recorded from the database of the hospital: demographics, diagnosis of MICM, induction regimen, disease status before HSCT, transplant characteristics, and complications.
Treatment Protocols
AML-CCLG2015 regime
Induction therapy included two courses. Patients were randomly randomized 1:1 into one of the following two groups: the VP-16 group (DAE + IAE) and the HHT group (DAH + IAH). Consolidation therapies included MA, HA, CLASP, and HAs. However, not every consolidation regimen was used in patients enrolled prior to HSCT, and physicians suggested transplantation for patients after they achieved remission depending on their condition. The specific medications used in the regimen are detailed in Table 1. The CCLG-2015 protocol was a prospective and randomized clinical trial (registered trial number: ChiCTR-IPR-15006816). We used block randomization without stratification to minimize investigator and participant bias. The block size was set to 8, and a random sequence was generated according to a random number table. Allocation was concealed with random envelopes. This clinical trial was approved by the Institutional Ethics Committee of Beijing Children’s Hospital.
Table 1.
AML-CCLG2015 Regime.
| Regime | Drugs | Days | Injection methods | Dose (per day) | |
|---|---|---|---|---|---|
| Induction therapy | DAE/DAH Course I |
DNR | 1, 3, 5 | IV | 40 mg/m2 |
| Ara-C | 1 to 7 | IV | 200 mg/m2 | ||
| HHT (H group) | 1 to 5 | IV | 3 mg/m2 | ||
| VP-16 (E group) | 1 to 5 | IV | 100 mg/m2 | ||
| IAE/IAH Course II |
IDA | 1, 3, 5 | IV | 10 mg/m2 | |
| Ara-C | 1 to 7 | IV | 200 mg/m2 | ||
| HHT (H group) | 1 to 5 | IV | 3 mg/m2 | ||
| VP-16 (E group) | 1 to 5 | IV | 100 mg/m2 | ||
| Consolidation therapy | MA Course III |
Mitozantrone | 1, 2 | IV | 10 mg/m2 |
| Ara-C | 1 to 3 | IV | 4 g/m2 | ||
| HA Course IV |
HHT | 1 to 7 | IV | 3 mg/m2 | |
| Ara-C | 1 to 3 | IV | 2 g/m2 | ||
| CLASP Course V |
L-ASP | 2 | IM | 6000 U/m2 | |
| Ara-C (SR) | 1,2 | IV | 6 g/m2 | ||
| Ara-C (MR, HR) | 1 to 3 | IV | 6 g/m2 | ||
A(Ara-C): cytarabine; AML: acute myeloid leukemia; CCLG: Chinese Children’s Leukemia Group; D(DNR): daunorubicin; E(VP-16): etoposide; H(HHT): homoharringtonine; HR: high risk; I(IDA): idarubicin; IR: medium risk; IV: intravenous injection; L-ASP: L-asparaginasum; M: mitozantrone; SR: standard risk; DAE: DNR+Ara-C+VP-16; DAH: DNR+Ara-C+HHT; IAE: IDA+Ara-C+VP-16; IAH: IDA+Ara-C+HHT; MA: Mitozantrone+Ara-C; HA: HHT+Ara-C; CLASP: Ara-C+L-ASP.
HSCT
(1) Indications for HSCT
If there is an appropriate donor, HSCT should be considered for patients with high-risk AML after course III of consolidation treatment.
(2) Donor and stem cell source
Donor sources include unrelated donors and allogeneic and haploidentical donors. Stem cell sources include bone marrow and peripheral blood stem cells.
Conditioning regime
Patients were pretreated with a modified conditioning regimen before transplantation: Ara-C 4 g/m2.d, D-10 to -9; busulfan (BU) 3.2 mg/kg.d, D-8 to -6; cyclophosphamide (CY) 1.8 g/m2.d, D-5 to -4; semustine (CCNU) 250 mg/m2.d, D-1; total body irradiation (TBI) + fludarabine (FLU) + CY was used as the main conditioning regimen for patients with myeloid sarcoma.
Prevention of graft versus host disease
In this study, mycophenolate mofetil (MMF) was used in combination with cyclosporine (CsA) and short-course methotrexate (MTX) for the prevention of graft versus host disease (GVHD). CsA was given at 2.5 mg/kg.d intravenously from D7, and after stabilization, it was changed to 4–6 mg/kg.d orally. The dose of CsA was adjusted according to the serum drug concentration to control the serum concentration at approximately 200 ng/mL, and the dose was gradually reduced from D60. For related haploidentical grafts, MMF was given orally from D1, and MTX was given 15 mg/m2 intravenously (D1, 3, 6, 9). Some patients transplanted with unrelated grafts were given antithymocyte globulin (ATG) to prevent acute GVHD (aGVHD), and ATG was given 2.5 mg/m2 d, D-5 to -2. The dose and duration of administration were adjusted according to the presence or absence of GVHD.
Implantation standard assay
The chimerism rate was detected by polymerase chain reaction (PCR) for short tandem repeats (STR). Sex chromosome transformation and STR transferred to donor type or blood type transformation were used as evidence of whether the donor graft had implanted. The date of granulocyte implantation was the first day when neutrophils were ≥0.5 × 109/L for three consecutive days. The date of platelet implantation was the first day when platelets were ≥20 × 109/L for seven consecutive days and no platelet transfusion was given.
Donor lymphocyte infusion
Measurable residual disease (MRD) was detected by flow cytometry, 1 month after transplantation. MRD was tested every 3 months thereafter if MRD turned negative and monthly if MRD remained positive. Donor lymphocyte infusion (DLI) was given monthly to AML patients with MRD positivity or MRD elevation greater than 1 log level 6 months after HSCT or with recurrence of extramedullary lesions, with CD3+ cell count starting at 1 × 106/kg.
Definition
High risk at the time of initial diagnosis was defined as having one adverse genetic and molecular abnormality, myeloid sarcoma or transformed AML. CR was defined as the presence of fewer than 5% blasts in the bone marrow, no leukemic blasts in the peripheral blood or extramedullary sites, and recovery of blood counts. Relapse was defined as loss of CR (morphological relapse) or recurrence of leukemic blasts in the extramedullary sites (extramedullary relapse), and MRD turned to >10–2 with CR also belonging to relapse (molecular biology relapse).
Statistical Methods
The study population was analyzed by disease outcomes (relapse or nonrelapse). Patients’ demographic and clinical characteristics are described as the median (interquartile range [IQR]: Q1–Q3) or as percentages, as appropriate. Differences between groups were evaluated using the Mann–Whitney U test for continuous variables and the chi-square test for categorical variables. Kaplan–Meier methods were used to evaluate the incidence rate of outcomes. We selected the variables with P < 0.20 in the analysis of the single factors for Kaplan–Meier survival curve estimation. Variables with P < 0.1 and well-established predictors were selected as confounding variables in the multivariable analyses. Cox regression analysis was used to determine the association between the baseline characteristics and relapse. In this model, we adjusted for age and sex. Mononuclear cell (MNC) counts were transformed into a classification variable based on the tertile and incorporated into survival analysis. The primary objective of this study was to evaluate cumulative recurrence rate (CRR), which was defined as the interval from the date of HSCT to the first relapse post-transplant or the end of the study (death or last follow-up). OS was defined as the interval from the date of AML diagnosis to death or the last follow-up. Event-free survival (EFS) was defined as the interval from the date of HSCT to the first event or last follow-up, and death and relapse were treated as events. The probabilities of CRR, OS, and EFS were estimated by the Kaplan–Meier method. Analyses were performed with SPSS 26.0 (IBM, Chicago, IL, USA) and R version 3.4.0 (R Core Team, Vienna, Austria). All the tests were two-sided, and a P value of <0.05 was considered statistically significant.
Results
Baseline Characteristics of Patients
Among the 37 enrolled patients, the median age was 6.00 (IQR: 3.00–10.50) years, and 26% were male. Baseline characteristics among the nonrelapse and relapse groups are presented in Table 2. In general, the baseline characteristics were well balanced between the two groups; however, patients with relapse had not been induced with the DAH regimen (P = 0.005), had higher MNC counts (P = 0.040), had paternal haploSCT (P = 0.143), had cytomegalovirus (CMV) infection (P = 0.143), had fungal infection (P = 0.025), and had a shorter follow-up time after HSCT (P = 0.009).
Table 2.
Baseline Characteristics According to Control and Relapse.
| Variables | Total, N = 37 | Non-relapse, N = 28 | Relapse, N = 9 | P |
|---|---|---|---|---|
| Age of HSCT, years | 6.00 (3.00–10.50) | 4.50 (2.20–10.75) | 7.00 (4.50–10.00) | 0.320 |
| Gender (male) | 26 (70.27) | 20 (71.43) | 6 (66.67) | 0.786 |
| Initial WBC (>100 × 109/L) | 1 (2.70) | 1 (3.57) | 0 (0.00) | 0.565 |
| MLL rearrangement (+) | 11 (29.73) | 8 (28.57) | 3 (33.33) | 0.786 |
| AML1-ETO (+) | 10 (27.03) | 8 (28.57) | 2 (22.22) | 0.709 |
| Complex karyotype | 6 (16.22) | 4 (14.29) | 2 (22.22) | 0.574 |
| Myeloid sarcoma | 8 (21.62) | 5 (17.86) | 3 (33.33) | 0.963 |
| CNS leukemia | 2 (5.41) | 2 (7.14) | 0 (0.00) | 0.410 |
| Testicular leukemia | 0 (0) | 0 (0) | 0 (0) | - |
| Risk stratification | 0.963 | |||
| Low/middle | 8 (21.62) | 5 (17.86) | 3 (33.33) | |
| High | 29 (78.38) | 23 (82.14) | 6 (66.67) | |
| Induction regime | 0.005 | |||
| DAE | 18 (48.65) | 10 (35.71) | 8 (88.89) | |
| DAH | 19 (51.35) | 18 (64.29) | 1 (11.11) | |
| CR1 | 37 (100) | 28 (100) | 9 (100) | - |
| Donor (father) | 21 (56.8) | 14 (50.00) | 7 (77.8) | 0.143 |
| Donor (relative) | 37 (100) | 28 (100) | 9 (100) | - |
| HLA10/10 | 3 (8.11) | 3 (10.71) | 0 (0.00) | 0.306 |
| Donor gender (male) | 23 (62.16) | 16 (57.14) | 7 (77.78) | 0.267 |
| Same genders between donor and recipient | 22 (59.46) | 16 (57.14) | 6 (66.67) | 0.613 |
| MNC count, 108/L | 8.94 (7.11–12.77) | 8.37 (6.66–12.13) | 12.67 (8.92–16.62) | 0.040 |
| CD34+ cell count, 106/L | 7.98 (6.59–9.94) | 8.12 (6.78–9.95) | 7.95 (6.09–9.36) | 0.735 |
| Time to N implantation | 12.00 (11.00–15.75) | 12.00 (11.00–16.00) | 12.00 (11.00–13.00) | 0.440 |
| Time to PLT implantation | 12.00 (11.00–16.00) | 12.50 (11.00–16.25) | 12.00 (10.50–13.00) | 0.647 |
| DLI | 14 (37.84) | 9 (32.14) | 5 (55.56) | 0.208 |
| aGCHD classification | 0.306 | |||
| 1 | 14 (37.84) | 12 (42.86) | 2 (22.22) | |
| 2 | 8 (21.62) | 5 (17.86) | 3 (33.33) | |
| 3 | 7 (18.92) | 4 (14.29) | 3 (33.33) | |
| 4 | 2 (5.41) | 2 (7.14) | 0 (0.00) | |
| cGVHD | 25 (67.57) | 18 (64.29) | 7 (77.78) | 0.452 |
| CMV infection | 21 (56.76) | 14 (50.00) | 7 (77.78) | 0.143 |
| EBV infection | 6 (16.22) | 5 (17.86) | 1 (11.11) | 0.633 |
| BKV infection | 2 (5.41) | 2 (7.14) | 0 (0.00) | 0.410 |
| HPV B19 infection | 1 (2.70) | 1 (3.57) | 0 (0.00) | 0.565 |
| Bacterial Infection | 14 (37.84) | 10 (35.71) | 4 (44.44) | 0.639 |
| Fungal infection | 7 (18.92) | 3 (10.71) | 4 (44.44) | 0.025 |
| Hemorrhagic cystitis | 10 (27.03) | 8 (28.57) | 2 (22.22) | 0.709 |
| Follow up after HSCT, months | 37.00 (11.0–45.50) | 41.00 (28.25–46.75) | 15.00 (4.00–29.00) | 0.009 |
| Survival time, months | 42.00 (25.5–50.50) | 43.00 (32.00–50.75) | 40.00 (13.50–50.00) | 0.480 |
Normal data are given as medians; qualitative variables are given as numbers (percentages). AML: acute myeloid leukemia; CMV: cytomegalovirus; CNS: central nervous system; DAE: DNR+Ara-C+VP-16; DAH: DNR+Ara-C+HHT; CR1: first complete remission; CD: Cluster of differentiation; DLI: donor lymphocyte infusion; HLA: human leukocyte antigen; MLL rearrangement: excluding MLL-AF9; MNC: mononuclear cell; N: neutrophils; PLT: platelet; risk stratification: risk stratification at the time of initial diagnosis, and the final risk stratification may escalate due to poor treatment outcomes; WBC: white blood cell; EBV: Epstein-Barr virus; BKV: BKpolyomavirus; HPV: human papilloma virus.
Survival Analysis
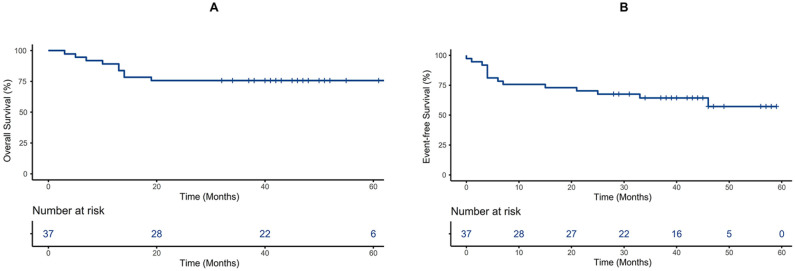
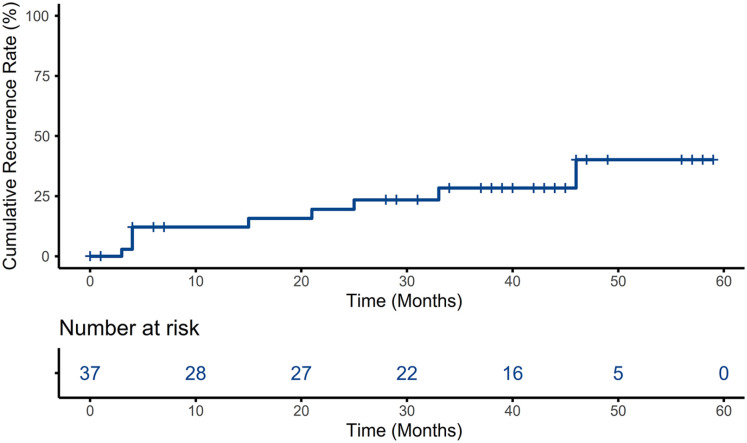
By the follow-up date, 28 (75.7%) patients survived, 9 (24.3%) patients died, and 9 (24.3%) patients relapsed. The OS rates of AML patients treated with HSCT were 89.2% ± 5.1%, 75.7% ± 7.1%, and 75.7% ± 7.1% at 1, 3, and 5 years, respectively; the EFS rates were 75.7% ± 7.1%, 64.4% ± 8.0%, and 57.2% ± 9.8% at 1, 3, and 5 years after HSCT, respectively; and the CRR rates were 11.4% ± 5.4%, 24.7% ± 7.7%, and 33.1% ± 10.4% at 1, 3, and 5 years after HSCT, respectively (Figs. 1 and 2). The causes of death included relapse (n = 4, 44.4%), infection (n = 2, 22.2%), aGVHD (n = 2, 22.2%), and cGVHD (n = 1, 11.1%). The treatment and prognosis of relapsed patients are shown in Table 3. Fourteen patients (37.8%) were treated with DLI for positive MRD conversion or logarithmic grade elevation, and each patient received 4.7 ± 4.15 DLI on average. Only five patients who received DLI progressed to relapse.
Figure 1.
OS estimated by Kaplan–Meier, time from the initial diagnosis of AML (A), EFS estimated by Kaplan–Meier, time from the day of HSCT (B). AML: acute myeloid leukemia; EFS: event-free survival; HSCT: hematopoietic stem cell transplantation; OS: overall survival.
Figure 2.
CRR estimated by Kaplan–Meier, time from the day of HSCT. CRR: cumulative recurrence rate; HSCT: hematopoietic stem cell transplantation.
Table 3.
Treatment and Prognosis of Patients Who Relapsed After HSCT.
| No. | Relapse time | Relapse sites | Main treatment | Effect | Outcome |
|---|---|---|---|---|---|
| P1 | 3 m+ | Bone marrow | Treatment abandoning | – | Death |
| P2 | 4 m+ | Bone marrow, testiclesskin | DLI, Treatment abandoning | – | Death |
| P3 | 4 m+ | Myeloid sarcoma | Secondary HSCT | Second relapse of myeloid sarcoma | Death |
| P4 | 5 m+ | Bone marrow, mesentery, submandibular gland | Treatment abandoning | – | Death |
| P5 | 15 m+ | Bone marrow (molecular biology) | Secondary HSCT | Sustained CR, MRD Fluctuation, given DLI again | Survival |
| P6 | 22 m+ | CNS | Secondary HSCT | CNS (–) | Survival |
| P7 | 26 m+ | Bone marrow (molecular biology) | DLI | Sustained CR, MRD Fluctuation | Survival |
| P8 | 35 m+ | Bone marrow | Secondary HSCT | Sustained CR | Survival |
| P9 | 46 m+ | Bone marrow (molecular biology) | Secondary HSCT | Sustained CR, MRD (–) | Survival |
CNS: central nervous system; CR: complete remission; DLI: donor lymphocyte infusion; HSCT: hematopoietic stem cell transplantation; m: month; MRD: minimal residual disease; Relapse time: from the day of stem cell infusion.
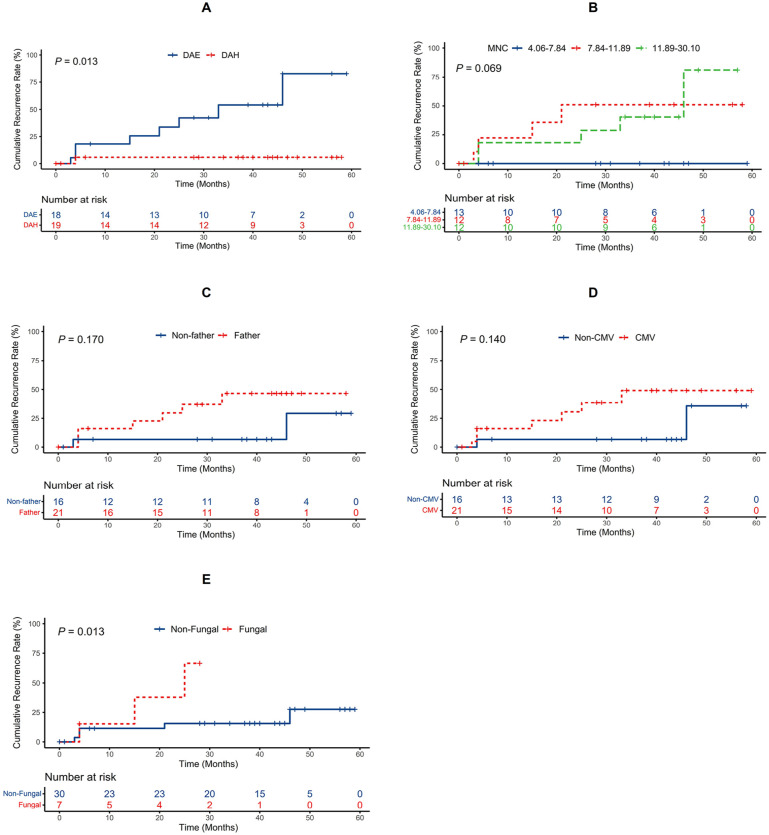
The Kaplan–Meier curve showed that patients treated with the induction regimen of DAE experienced a significantly higher risk of relapse after HSCT than patients induced with the DAH regimen (log-rank test, P = 0.013 < 0.05; Fig. 3A). Patients with fungal infection experienced a higher risk of relapse than patients without fungal infection (log-rank test, P = 0.013 < 0.05; Fig. 3E). The Kaplan–Meier curve also showed that patients who had a paternap donor or who had CMV infection after HSCT experienced a higher risk of relapse than patients in the corresponding groups, but these differences were not statistically significant (log-rank test, P = 0.17 > 0.05 and P = 0.14 > 0.05, respectively; Fig. 3C, D). The Kaplan–Meier curve showed that the relapse risk between patients who had infused different MNC counts was not significantly different (log-rank test, P = 0.069 > 0.05; Fig. 3B). The association between homoharringtonine-based induction therapy and low recurrence rate persisted after adjusting for age, sex, risk stratification, fusion genes, and fungal infections. The adjusted hazard ratios were 0.093 (95% confidence interval (CI) = 0.009–0.920; P = 0.042) for homoharringtonine versus etoposide-based induction therapy (Table 4).
Figure 3.
Kaplan–Meier estimate value and the risk of relapse ranked by induction program (A), infusion counts of MNC (B), whether donor was father (C), CMV infection (D), and fungal infection (E). CMV: cytomegalovirus; MNC: mononuclear cell.
Table 4.
Association Analysis of Clinical Features and Relapse in COX Regression.
| Variables | HR (95% confidence interval) | P |
|---|---|---|
| Age | 0.510 (0.081–3.266) | 0.474 |
| Sex | 0.463 (0.049–4.363) | 0.501 |
| Risk stratification | 2.525 (0.259–24.655) | 0.426 |
| Induction Program | 0.093 (0.009–0.920) | 0.042 |
| MLL-rearrangement | 0.326 (0.057–1.851) | 0.206 |
| fungal infections | 0.325 (0.073–1.447) | 0.140 |
Adjusted age, sex, risk stratification, MLL-rearrangement and fungal infections. HR: hazards ratio.
GVHD
There were 31 patients (83.8%) who had acute GVHD, 22 of whom had I–II GVHD. Twenty-five patients (67.6%) had chronic GVHD, one patient had interstitial lung disease resulting in decreased lung function, and one patient died of chronic intestinal GVHD.
Discussion
Allogeneic HSCT in AML patients in remission is the strongest anti-leukemic therapy available because of the cytoreductive conditioning and the immunologic anti-leukemic graft-versus-leukemia effect. Allogeneic HSCT is more suitable for the subtype of AML where conventional chemotherapy is unlikely to achieve long-lasting remission, especially in refractory/recurrent AML 8 . Previous studies 9 have shown that allogeneic HSCT treatment reduces the relapse rate by 10% in children with newly diagnosed AML, but post-transplant relapse is the leading cause of post-transplant mortality, and once relapsed, treatment options are limited with extremely poor efficacy.
In this study, we focused on analyzing and summarizing the recurrence and death after transplantation in pediatric AML patients and identifying adverse factors of post-transplantation recurrence by analyzing the primary disease characteristics, transplantation characteristics, and post-transplantation complications. Our study showed that the 5-year OS rates of AML patients treated with HSCT were 75.7% ± 7.1% at 5 years, and the 5-year CRR rates were 33.1% ± 10.4% after HSCT, while a study 10 of transplanted AML patients in our hospital during 2005 to 2015 showed that the 5-year OS was 61. 1% ± 8.7%, and the 5-year CRR was 16. 0% ± 6.0%. Data from different time periods showed a significant improvement in the OS rate and an increase in the CRR in transplant patients. The improvement in survival may be due to improvements in HSCT conditioning regimes, diversity in donor selection, infection control and means of pathogen surveillance, advances in comprehensive supportive care, and maturation of transplant teams. The increased recurrence rate and higher survival rates seem to be contradictory phenomena, which we consider to be related to advances in MRD detection technology, allowing physicians to detect recurrence earlier and give patients pre-emptive treatment, such as DLI.
As treatment-related toxicity and mortality continue to improve, relapse has become the most important cause of treatment failure, and new approaches are needed to make the next large leap forward in transplantation for AML. This study showed that post-transplant leukemia relapse was the leading cause of death, with 4 of 9 children who relapsed after transplantation experiencing death. It is worth noting that up to 44.4% of deaths were attributed to leukemia, which highlights how difficult it is to manage AML once patients have developed post-transplant relapse. DLI can enhance the graft-versus-leukemia effect and is an important method to reduce relapse after HSCT 11 . In this study, 14 patients (37.8%) were treated with DLI for positive MRD conversion or logarithmic grade elevation, and only 5 of them progressed to relapse. A retrospective study 12 showed that 46 patients with high-risk AML given DLI treatment 120 days after HSCT had a higher 7-year OS than matched controls (34 patients) (67% vs. 31%, P < 0. 001). The success of therapeutic DLI is strongly correlated with the leukemia load at the time of relapse and the remission status at the time of DLI application 13 . Therefore, it is important to monitor for MRD regularly after transplantation for early detection of relapse. Regrettably, there is a lack of clear guidelines on the indications, timing, and dose of prophylactic DLI.
AML therapy is approaching an era where the promise of targeting small molecule inhibitors and biologics agents has been realized since 2017 14 . Therefore, new directions have emerged for post-transplant maintenance therapy to prevent recurrence. Clinical trials using post-transplant FLT3 inhibitors, such as sorafenib and midostaurin, have shown feasibility, safety, and encouraging post-transplant outcomes 15 Venetoclax has been shown to be effective in combination with hypomethylating agents or low-dose cytarabine in children with AML 16 and should be taken seriously as maintenance therapy after HSCT. In addition, one study 17 showed that donor memory-like natural killer (NK) cells robustly expand and persist with potent anti-leukemic activity in the absence of exogenous cytokines and presented a novel immunotherapy platform for AML that relapsed after allogeneic HSCT in combination with DLI 17 . The role of other cell-based therapies, including chimeric antigen receptor T-cell immunotherapy (CAR-T), in AML is currently being investigated 18 .
In this study, univariate analysis showed that fungal infections after transplantation might be a high-risk factor for recurrence after transplantation. Although multifactor analysis was not statistically significant, it was still evident on the survival curve that patients without fungal infections had a lower recurrence rate. Interestingly, the patients included in this study were treated with two regimens of DHA and DAE for induction therapy, and the Kaplan–Meier curve showed that patients treated with the induction regimen of DAE experienced a significantly higher risk of relapse after HSCT than patients induced with the DAH regimen. The association between homoharringtonine-based induction therapy and low recurrence rate persisted after adjusting for age, sex, risk stratification, fusion genes and fungal infections, providing more favorable evidence for considering homoharringtonine-based induction therapy as a favorable factor in reducing post-transplant recurrence. Although homoharringtonine is a commonly used chemotherapeutic agent that has been used as standard therapy for AML in China 19 , the relationship between homoharringtonine and relapse after transplatation and the reason homoharringtonine reducing relapse have not been reported in previous studies. But it is a great interest, and a larger/prospective study should been considered. Previous studies10,20 have shown that myeloid sarcoma and the number of remissions are important poor prognostic factors. Myeloid sarcoma leads to lower survival rates and shorter survival times. Those with a high number of remissions have a worse prognosis than patients in first remission. A retrospective study 20 showed that the 5-year OS and 5-year disease-free survival (DFS) of 51 patients treated with HSCT because of myeloid sarcomas were 47% and 36%, respectively. All patients were transplanted after their first remission in this study, and no adverse association between myeloid sarcoma and relapse was found, which was considered to be related to the small sample size. However, of the five children who received a second HSCT treatment, one patient who had a recurrence of myeloid sarcoma after the first HSCT had a second recurrence of myeloid sarcoma after the second transplantation and eventually died, while the remaining four children with nonmyeloid sarcoma recurrence had a good prognosis, suggesting to some extent there is a poor prognosis for myeloid sarcoma recurrence.
GVHD is an important transplant-related comorbidity. In this study, due to the short observational follow-up period, the impact of chronic GVHD later on survival is not clear. Attention should be given to chronic GVHD, and its impact on the quality of survival should not be ignored.
This study was influenced by the small sample size due to the prevalence of AML in children, and future multicenter studies should include larger sample sizes and longer follow-up to reduce bias. HSCT is an effective treatment for AML, especially refractory/relapsed AML. Post-transplant relapse is a major cause of death, and secondary transplantation after relapse is an effective means of salvage for such patients. It is believed that with the in-depth understanding of the pathogenesis of AML, the improvement of chemotherapy regimens and supportive therapy, and the advancement of transplantation technology, we can further reduce transplant infection-related mortality, explore post-transplant immunotherapy to reduce AML recurrence rates, and further improve overall AML survival.
Acknowledgments
The authors sincerely thank the patients who participated in this study, their families who actively cooperated, and the healthcare professionals who treated and cared for the patients in the clinical setting.
Footnotes
Authors’ Contributions: Bin Wang and Xiaojia Wen collected data and drafted the manuscript. Xiaojia Wen was responsible for extracting, analyzing data, and interpreting results. Bin Wang, Maoquan Qin, and Huyong Zheng designed treatment protocol. All authors participated in treating the patients. All authors read and approved the final manuscript. Bin Wang and Xiaojia Wen contributed equally to this work.
Availability of Data and Material: The data and material of this study are available from the corresponding author on reasonable request.
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Research Ethics and Patient Consent: This study was approved by the Medical Ethics Committee of Beijing Children’s Hospital, Capital Medical University (ethical approval number: 2015-43). Informed consent was obtained from all patients or legal guardians in accordance with the principles of the Declaration of Helsinki.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (NSFC) (grant no. 82070154), Beijing Natural Science Foundation (grant no. 7222056), and Capital’s Funds for Health Improvement and Research (CFH) (grant no. 2022-1-2091).
ORCID iD: Huyong Zheng  https://orcid.org/0000-0002-1484-9131
https://orcid.org/0000-0002-1484-9131
References
- 1.Ni X, Li Z, Li X, Zhang X, Bai G, Liu Y, Zheng R, Zhang Y, Xu X, Liu Y, Jia C, et al. Socioeconomic inequalities in cancer incidence and access to health services among children and adolescents in China: a cross-sectional study. Lancet. 2022;400(10357):1020–32. [DOI] [PubMed] [Google Scholar]
- 2.Rasche M, Zimmermann M, Borschel L, Bourquin JP, Dworzak M, Klingebiel T, Lehrnbecher T, Creutzig U, Klusmann JH, Reinhardt D. Successes and challenges in the treatment of pediatric acute myeloid leukemia: a retrospective analysis of the AML-BFM trials from 1987 to 2012. Leukemia. 2018;32(10):2167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, Pounds S, Razzouk BI, Lacayo NJ, Cao X, Meshinchi S, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11(6):543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tasian SK, Pollard JA, Aplenc R. Molecular therapeutic approaches for pediatric acute myeloid leukemia. Front Oncol. 2014;4:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorman MF, Ji L, Ko RH, Barnette P, Bostrom B, Hutchinson R, Raetz E, Seibel NL, Twist CJ, Eckroth E, Sposto R, et al. Outcome for children treated for relapsed or refractory acute myelogenous leukemia (rAML):a Therapeutic Advances in Childhood Leukemia (TACL) Consortium study. Pediatr Blood Cancer. 2010;55(3):421–29. [DOI] [PubMed] [Google Scholar]
- 6.Duncan CN, Majhail NS, Brazauskas R, Wang Z, Cahn JY, Frangoul HA, Hayashi RJ, Hsu JW, Kamble RT, Kasow KA, Khera N, et al. Long-term survival and late effects among one-year survivors of second allogeneic hematopoietic cell transplantation for relapsed acute leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2015;21(1):151–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisdorf D, Zhang MJ, Arora M, Horowitz MM, Rizzo JD, Eapen M. Graft-versus-host disease induced graft-versus-leukemia effect: greater impact on relapse and disease-free survival after reduced intensity conditioning. Biol Blood Marrow Transplant. 2012;18(11):1727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez-Arteaga A, Gyurkocza B. Recent advances in allogeneic hematopoietic cell transplantation for acute myeloid leukemia. Curr Opin Hematol. 2020;27(2):115–21. [DOI] [PubMed] [Google Scholar]
- 9.Niewerth D, Creutzig U, Bierings MB, Kaspers GJL. A review on allogeneic stem cell transplantation for newly diagnosed pediatric acute myeloid leukemia. Blood. 2010;116(13):2205–14. [DOI] [PubMed] [Google Scholar]
- 10.Jia C, Zhou X, Wang B, Zhu G, Qin M. Clinical observation of allogeneic hematopoietic stem cell transplantation for the treatment of acute myeloid leukemia in children. Journal of Capital Medical University. 2019;40(2):163–68. [Google Scholar]
- 11.Yan C-H, Wang Y, Wang J-Z, Chen Y-H, Chen Y, Wang F-R, Sun Y-Q, Mo X-D, Han W, Chen H, Zhang X-h, et al. Minimal residual disease- and graft-vs.-host disease-guided multiple consolidation chemotherapy and donor lymphocyte infusion prevent second acute leukemia relapse after allotransplant. J Hematol Oncol. 2016;9(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jedlickova Z, Schmid C, Koenecke C, Hertenstein B, Baurmann H, Schwerdtfeger R, Tischer J, Kolb HJ, Schleuning M. Long-term results of adjuvant donor lymphocyte transfusion in AML after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51(5):663–67. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto T, Fukuda T, Nakashima M, Henzan T, Kusakabe S, Kobayashi N, Sugita J, Mori T, Kurokawa M, Mori SI. Donor lymphocyte infusion for relapsed hematological malignancies after unrelated allogeneic bone marrow transplantation facilitated by the Japan Marrow Donor Program. Biol Blood Marrow Transplant. 2017;23(6):938–44. [DOI] [PubMed] [Google Scholar]
- 14.Newell LF, Cook RJ. Advances in acute myeloid leukemia. BMJ. 2021;375:n2026. [DOI] [PubMed] [Google Scholar]
- 15.Blackmon A, Aldoss I, Ball BJ. FLT3 inhibitors as maintenance therapy after allogeneic stem-cell transplantation. Blood Lymphat Cancer. 2022;12:137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karol SE, Alexander TB, Budhraja A, Pounds SB, Canavera K, Wang L, Wolf J, Klco JM, Mead PE, Das Gupta S, Kim SY, et al. Venetoclax in combination with cytarabine with or without idarubicin in children with relapsed or refractory acute myeloid leukaemia: a phase 1, dose-escalation study. Lancet Oncol. 2020;21(4):551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bednarski JJ, Zimmerman C, Berrien-Elliott MM, Foltz JA, Becker-Hapak M, Neal CC, Foster M, Schappe T, McClain E, Pence PP, Desai S, et al. Donor memory-like NK cells persist and induce remissions in pediatric patients with relapsed AML after transplant. Blood. 2022;139(11):1670–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreidieh F, Abou Dalle I, Moukalled N, El-Cheikh J, Brissot E, Mohty M, Bazarbachi A. Relapse after allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia: an overview of prevention and treatment. Int J Hematol. 2022;116(3):330–40. [DOI] [PubMed] [Google Scholar]
- 19.Jin J, Wang JX, Chen FF, Wu DP, Hu J, Zhou JF, Hu JD, Wang JM, Li JY, Huang XJ, Ma J, et al. Homoharringtonine-based induction regimens for patients with de-novo acute myeloid leukaemia: a multicentre, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2013;14(7):599–608. [DOI] [PubMed] [Google Scholar]
- 20.Chevallier P, Mohty M, Lioure B, Michel G, Contentin N, Deconinck E, Bordigoni P, Vernant J-P, Hunault M, Vigouroux S, Blaise D, et al. Allogeneic hematopoietic stem-cell transplantation for myeloid sarcoma: a retrospective study from the SFGM-TC. J Clin Oncol. 2008;26(30):4940–43. [DOI] [PubMed] [Google Scholar]