Abstract
Background:
Tyrosine-kinase inhibitors (TKIs) and immunotherapy represent the backbone treatment for metastatic renal cell carcinoma (mRCC) patients. The aim of the present study was to describe mean corpuscular volume (MCV) and red cell distribution width (RDW) in mRCC patients treated with pazopanib or cabozantinib, and to explore their potential impact on oncological outcomes.
Materials and methods:
We conducted a multicenter retrospective observational study in mRCC patients treated with pazopanib or cabozantinib between January 2012 and December 2020 in nine Italian centers. Descriptive statistics, univariate, and multivariate analyses were performed.
Objectives:
The primary endpoints were the incidence and trend over time of anemia, macrocytosis (elevated MCV), and anisocytosis (elevated RDW). The secondary endpoints were the correlations of MCV and RDW with objective response rate (ORR), progression-free survival (PFS), and overall survival (OS).
Results:
A total of 301 patients were enrolled; mean Hb value was 12.5 g/dl, a mean increase of 1 g/dl was observed at day 15 and maintained at 3 months. Most patients had baseline macrocytosis (MCV levels > 87 fl), with a significant mean increase after 3 months of treatment. At univariate analysis patients with macrocytosis had better ORR, longer PFS, and OS. About one third of patients had baseline anisocytosis (RDW > 16%), with a significant mean increase after 3 months of treatment. At univariate analysis, patients with RDW values ⩽ 16% had higher ORR, longer PFS, and OS. At multivariate analysis, baseline macrocytosis was significantly associated with better PFS in patients treated with pazopanib and baseline anisocytosis with shorter OS in all patients.
Conclusions:
mRCC patients treated with pazopanib or cabozantinib may have baseline macrocytosis and anisocytosis. A significant increase of Hb, MCV, and RDW after TKIs start was observed. Baseline macrocytosis is positively correlated with PFS in patients treated with pazopanib and baseline anisocytosis affects survival of patients treated with TKIs.
Keywords: anisocytosis, cabozantinib, macrocytosis, metastatic renal cell carcinoma, pazopanib, prognostic
Introduction
The treatment of metastatic renal cell carcinoma (mRCC) has undergone deep changes in recent years with the introduction into the clinical practice of immunotherapy and antiangiogenetic tyrosine-kinase inhibitors (TKIs). 1
The first TKI, that showed an advantage in progression-free survival (PFS) and overall survival (OS) compared with cytokines such as interferon-alfa (IFN-α), was sunitinib. 2 The increasing evidence about the efficacy of TKIs in the treatment of mRCC had brought to the introduction and approval of other molecules in this setting. Pazopanib is a multitarget TKI that inhibits vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), fibroblastic growth factor receptor (FGFR), clusted of differentiation 117 (c-KIT) and REarranged during Trasfectin (RET) gene. 3 In a phase III trial, pazopanib demonstrated a significant improvement in PFS as first-line treatment in patients with advanced mRCC. 4 Cabozantinib is a potent inhibitor of VEGFR2, mesenchymal epithelial transition factor (MET) and tyrosine-protein kinase receptor UFO (AXL), 5 and has demonstrated a longer PFS compared with sunitinib in patients with mRCC at intermediate-high risk in first-line setting. 6 Moreover, cabozantinib is approved for the treatment of mRCC patients pre-treated with a VEGFR-TKI, having demonstrated to be superior to the mTOR inhibitor everolimus in this setting. 7
In Italy, both pazopanib and cabozantinib are approved as treatment options for mRCC patients: pazopanib as first-line option and cabozantinib both as first line (in intermediate-poor risk patients) and as further line treatment. 8
TKIs can cause hematologic toxicity such as neutropenia, leukopenia, thrombocytopenia, and anemia.4,6,9 Patients treated with sunitinib suffered an increase in incidence of macrocytosis and macrocytic anemia, despite showing normal levels of B12 vitamin and folic acid. This phenomenon reverses when the treatment is interrupted. 10 Among other etiopathogenetic hypotheses, there seems to be a drug-related inhibition of the erythroid progenitors proliferative pathways, in particular the c-KIT pathway. 11 In addition, the increase in mean corpuscular volume (MCV) during treatment with sunitinib has been proven to have a positive correlation with the outcome: patients who develop macrocytosis have, in fact, a longer PFS. 12
Red cell distribution width (RDW) is another hematological parameter correlated with cancer-related survival in patients with localized RCC: a higher RDW is directly related to the grading and the stage of disease; 13 in addition, the increased RDW is related to the cancer-specific mortality in patients with mRCC who are underwent to partial or total nephrectomy. 14
In clinical practice, patients treated with pazopanib and cabozantinib frequently present baseline macrocytosis (i.e. elevation on MCV) and anisocytosis (i.e. elevation on RDW) or develop these laboratory alterations after the treatment start. However, the incidence of these phenomena and the correlation with the oncological outcomes are underreported.
The aim of the present study was to describe the blood parameters MCV and RDW in patients with mRCC treated with pazopanib and cabozantinib, and to explore their potential impact on oncological outcomes.
Materials and methods
We conducted a multicenter retrospective observational study of patients with mRCC treated with pazopanib or cabozantinib between January 2012 and December 2020 in nine Italian centers. The primary endpoints of the study were the incidence and trend over time of anemia, macrocytosis, and anisocytosis (i.e. elevation of RDW) in patients with mRCC treated with pazopanib and cabozantinib. The secondary endpoints were the correlations of MCV and RDW with objective response rate (ORR), PFS, and OS.
Patients’ characteristics
Patients with unresectable or metastatic RCC, histologically confirmed were included in the study. They received pazopanib or cabozantinib in the advanced setting at any line of treatment.
We collected information about baseline prognostic group using the International mRCC Database Consortium (IMDC) criteria, 15 Eastern Cooperative Oncology Group Performance Status (ECOG PS), pathological characteristics and metastatic sites; biochemical parameters were also required and collected from baseline to the first 3 months from treatment start. Exclusion criteria were patients not treated with cabozantinib or pazopanib, lacking of most medical records.
Patients underwent to disease evaluation about every 3 months by imaging with CT scan and magnetic resonance according to the local practice, using Response Evaluation Criteria in Solid Tumours (RECIST).
Statistics
We used descriptive statistics to report patients’ characteristics. We defined anemia as hemoglobin (Hb) value lower than 12 g/dl; we determined the cut-offs of MCV and RDW to define macrocytosis and anisocytosis, respectively, as the optimal values to maximize the log-rank test.
To determine sample size, we analyzed data from literature. The incidence of macrocytosis found in 120 patients treated with sunitinib was about 67% of cases. 10 We considered plausible to detect a not dissimilar incidence rate for pazopanib and cabozantinib; considering the observation time window and the number of patients treated with these drugs, we expected a sample size of approximately 300 patients.
Paired samples t-test was performed to compare the Hb, MCV, and RDW variations from basal levels and at 15, 29, and 85 days from the beginning of the treatment while the association between clinical-pathological factors and MCV and RDW was evaluated by a generalized linear model, considering each single factor in the univariate setting and all the factors together without any selection in the multivariable approach; in this way, regression coefficients are adjusted for each variable considered in the analysis.
Patients with complete response (CR) and partial response (PR) as best response were defined as ‘responders’: they were used to evaluate the ORR; patients with stable disease (SD), progressive disease (PD) or with not evaluable response (NE) due to clinically PD were defined as ‘non-responders’. Moreover, we considered patients with clinical benefit those that obtained CR or PR or SD as best response, and patient with no clinical benefit those that obtained PD or NE as best response. For the comparison of the MCV and RDW distribution between groups (responders compared with non-responders, clinical benefit compared with no clinical benefit), we used the Mann–Whitney test.
The PFS was defined as the time from the start of therapy with TKI to the disease progression or death, whichever occurred first. The OS was calculated from the start of treatment to death for any cause. We considered as censored patients without progression or death at the last follow-up.
PFS and OS were estimated using the Kaplan–Meier method and compared using the log-rank test; median values were reported with 95% confidence intervals (95% CIs). The median follow-up was calculated with the reverse Kaplan–Meier method. Univariate and multivariate survival analyses were performed by using Cox proportional hazard models, multivariable analysis was performed considering all the factors together without any selection. The chi-square test was used to compare categorical endpoints. Significance levels were set at a value of 0.05, and all p values were two-sided.
We planned the analyses for the overall patient population and within each treatment group (pazopanib or cabozantinib).
The IBM SPSS Statistics for Windows, Version 27.0, IBM Corp., Armonk, New York, USA) and R v.4.1.2 were used for the analyses.
Results
Patient characteristics
We enrolled 301 patients: 179 patients (59%) were treated with pazopanib, while 122 patients (41%) with cabozantinib. Baseline clinical and pathological characteristics of the overall population and TKIs use are shown in Table 1. The median age was 68 years, with a male:female ratio of 2:1. Fifty-three percent of patients had an intermediate prognostic score following the IMDC criteria both in the pazopanib and in the cabozantinib group, while 61% of patients had an Eastern Cooperative Oncology Group Performance Status (ECOC PS) of 0. Most patients underwent a nephrectomy (85%). Patients received pazopanib as first-line treatment in 97% of cases, while cabozantinib was mainly used beyond the first line (42% of patients received it as second-line treatment and 44% after second-line therapy).
Table 1.
Clinical and pathological characteristics of patients.
| Number of patients (%) | Overall
301 (100%) |
Pazopanib group
179 (59%) |
Cabozantinib group
122 (41%) |
|---|---|---|---|
| Median age (range) | 68 (36–89) | 70 (42–89) | 65 (36–85) |
| Sex (%) | |||
| Male | 206 (68.4) | 126 (70.4) | 80 (65.6) |
| Female | 95 (31.6) | 52 (29.4) | 42 (34.4) |
| Histology (%) | |||
| Clear cell | 250 (83.1) | 152 (84.9) | 98 (80.3) |
| Papillary | 24 (8.0) | 11 (6.1) | 13 (10.7) |
| Chromophobe | 8 (2.7) | 5 (2.8) | 3 (2.5) |
| Other | 19 (6.3) | 11 (6.1) | 8 (6.6) |
| IMDC score (%) | |||
| Good | 103 (34.2) | 65 (36.3) | 38 (31.1) |
| Intermediate | 159 (52.8) | 92 (51.4) | 67 (54.9) |
| Poor | 39 (13.0) | 22 (12.3) | 17 (13.9) |
| ECOG PS (%) | |||
| 0 | 183 (60.8) | 10 (61.5) | 73 (59.8) |
| 1 | 102 (33.9) | 61 (34.1) | 41 (33.6) |
| 2–3 | 16 (5.4) | 8 (4.5) | 8 (6.5) |
| NLR (%) | |||
| <3 | 183 (60.8) | 80 (44.7) | 43 (35.2) |
| >3 | 102 (33.9) | 75 (41.9) | 65 (53.3) |
| NA | 38 (12.6) | 24 (13.4) | 14 (11.5) |
| Nephrectomy (%) | |||
| Yes | 256 (85) | 149 (83.2) | 107 (87.7) |
| No | 45 (15) | 30 (16.8) | 15 (12.3) |
| Median number of metastatic sites (range) | 2 (1–8) | 2 (1–6) | 3 (1–8) |
| Sites of metastasis (%) | |||
| Lung | 194 (64.5) | 116 (64.8) | 78 (63.9) |
| Liver | 58 (19.3) | 29 (16.2) | 29 (23.8) |
| Nodes | 126 (41.9) | 58 (32.4) | 68 (55.7) |
| Bone | 112 (37.2) | 53 (29.6) | 59 (48.4) |
| Glands | 58 (19.3) | 30 (33.5) | 28 (23.0) |
| Other | 114 (37.9) | 60 (33.5) | 54 (44.3) |
| Use of PPI (%) | |||
| Yes | 132 (43.9) | 69 (38.5) | 63 (51.6) |
| No | 169 (56.1) | 110 (61.5) | 59 (48.4) |
| Line of treatment (%) | |||
| 1st | 192 (63.8) | 175 (97.8) | 17 (13.9) |
| 2nd | 54 (17.9) | 3 (1.0) | 51 (41.8) |
| ⩾3rd | 55 (18.3) | 1 (0.2) | 54 (44.2) |
ECOG PS, Eastern Cooperative Oncology Group Performance Status; IMDC, International mRCC Database Consortium; NA, not applicable; NLR, neutrophils-lymphocytes ratio; PPI, proton-pump inhibitors.
Hb, MCV, and RDW incidence and trend over time
In the observed period of 3 months, Hb levels showed a significant increase either in all patients and in the pazopanib and cabozantinib groups, increasing with a mean of 1 g/dl as early as at the day 15 (p < 0.0001 in all instances, Supplemental Tables S1, S2, and S3; Supplemental Figures S1 and S2).
We determined a cut-off of 87 fl to define macrocytosis as the optimal values to maximize the log-rank test. MCV values displayed a significant decrease between basal value and 15 and 29 days (median values 88.8 fl, 87.6 fl, and 87.9 fl, respectively; p < 0.0001) and a significant increase between basal and 90 days (median values 89.0 fl and 92.0 fl, respectively; p < 0.0001) in the overall population (Supplemental Table S1, Supplemental Figure S3). This trend was maintained both in the pazopanib and in the cabozantinib group. In the pazopanib group, MCV values showed a significant decrease between basal and day 15 (p = 0.003) and a significant increase between basal and day 85 (p < 0.0001) (Supplemental Table S2, Supplemental Figure S4). In the cabozantinib group, we observed the decreases between basal and day 15 and day 29 as significant variations (p < 0.0001) (Supplemental Table S3, Supplemental Figure S4).
We determined a cut-off of 16% to define anisocytosis as the optimal values to maximize the log-rank test. RDW values showed a constant and significant increase in the overall population during the observed 3-month period, reaching the peak at day 85, with a mean increase of 2.6% in the overall population, of 2.5% in the pazopanib group and of 2.8% in the cabozantinib group (p < 0.0001 in all instances) (Supplemental Table S1, S2, and S3 respectively; Supplemental Figure S5 and S6).
Multivariate analyses
Tables 2 and 3 summarize the correlation by generalized linear model, between baseline MCV and RDW and clinical-pathological characteristics of the population. In the univariate analysis, high levels of MCV are positively correlated with lines of treatment beyond the first (p < 0.0001), treatment with cabozantinib (p < 0.0001), nephrectomy (p = 0.003) and Hb (p < 0.0001). A significative inverse correlation was instead found between MCV and a ECOG PS > 0 (p < 0.0001), an intermediate (p = 0.002) or poor (p < 0.0001) prognostic score according to IMDC criteria and liver metastasis (p = 0.03). At the multivariate analysis, only Hb values remained significant (p < 0.0001).
Table 2.
Correlation between basal MCV and clinical-pathological factors in the overall population: univariate and multivariate analyses.
| Univariate | Multivariate | |
|---|---|---|
| Age (in years) |
p = 0.91 0.005 (0.045) |
p = 0.06 0.081 (0.043) |
| Gender | p = 0.82 | p = 0.60 |
| M | –0.245 (1.070) | 0.519 (1.003) |
| F | 0 | 0 |
| ECOG PS | p < 0.0001 | p = 0.11 |
| 0 | 0 | 0 |
| 1-2-3 | –3.566 (0.998) | –1.706 (1.061) |
| Histology | p = 0.85 | p = 0.39 |
| CC | 0.260 (1.330) | 1.035 (1.213) |
| Not CC | 0 | 0 |
| PPI | p = 0.70 | p = 0.35 |
| Yes | 0.383 (1.004) | 0.897 (0.964) |
| No | 0 | 0 |
| IMDC | p < 0.0001 | p = 0.13 |
| Good | 0 | 0 |
| Intermediate | –3.264 (1.042) p = 0.002 | –0.985 (1.063) p = 0.35 |
| Poor | –8.183 (1.556) p < 0.0001 | –3.473 (1.735) p = 0.045 |
| LINE | p < 0.0001 | p = 0.21 |
| 1st | 0 | 0 |
| ⩾2nd | 3.722 (1.013) | 2.328 (1.841) |
| NLR | p = 0.080 | p = 0.28 |
| <3 | 0 | 0 |
| ⩾3 | –1.786 (1.021) | –1.046 (0.961) |
| Liver mets | p = 0.03 | p = 0.07 |
| Yes | –2.649 (1.246) | –2.191 (1.223) |
| No | 0 | 0 |
| Bone mets | p = 0.47 | p = 0.60 |
| Yes | 0.742 (1.029) | 0.539 (1.016) |
| No | 0 | 0 |
| Nephrectomy | p = 0.003 | p = 0.97 |
| Yes | 4.092 (1.395) | –0.047 (1.422) |
| No | 0 | 0 |
| Number of metastatic site | p = 0.058 | p = 0.63 |
| 1-2 | 0 | 0 |
| ⩾3 | –1.929 (1.018) | –0.526 (1.109) |
| Treatment | p < 0.0001 | p = 0.061 |
| Pazopanib | 0 | 0 |
| Cabozantinib | 3.528 (0.993) | 3.344 (1.784) |
| Hb | p < 0.0001 | p < 0.0001 |
| <12 g/dl | 0 | 0 |
| >12 g/dl | 6.027 (0.954) | 4.473 (1.059) |
Bold values express statistically significant values from our analysis.
ECOG PS, Eastern Cooperative Oncology Group Performance Status; Hb, hemoglobin; IMDC, International mRCC Database Consortium criteria; MCV, mean corpuscular volume; NLR, neutrophil-to-lymphocyte ratio; PPI, proton-pump inhibitors.
Table 3.
Correlation between basal RDW and clinical-pathological factors in the overall population: univariate and multivariate analyses.
| Univariate | Multivariate | |
|---|---|---|
| Age (in years) | 0.001 (0.013) p = 0.98 | 0.010 (0.011) p = 0.52 |
| Gender | p = 0.66 | p = 0.71 |
| M | 0.137 (0.313) | –0.100 (0.266) |
| F | 0 | 0 |
| ECOG PS | p < 0.0001 | p = 0.01 |
| 0 | 0 | 0 |
| 1-2-3 | 1.370 (0.287) | 0.712 (0.281) |
| Histology | p = 0.72 | p = 0.88 |
| CC | 0.142 (0.389) | –0.046 (0.322) |
| Not CC | 0 | 0 |
| PPI | p = 0.012 | p = 0.37 |
| Yes | 0.733 (0.291) | 0.227 (0.256) |
| No | 0 | 0 |
| IMDC | p < 0.0001 | p = 0.67 |
| Good | 0 | 0 |
| Intermediate | 1.029 (0.308) p = 0.001 | 0.119 (0.282) p = 0.63 |
| Poor | 2.033 (0.459) p < 0.0001 | 0.411 (0.460) p = 0.37 |
| LINE | p = 0.006 | p = 0.26 |
| 1st | 0 | 0 |
| ⩾2nd | –0.797 (0.288) | 0.553 (0.488) |
| NLR | p = 0.16 | p = 0.15 |
| <3 | 0 | 0 |
| ⩾3 | 0.396 (0.282) | –0.363 (0.255) |
| Liver mets | p = 0.39 | p = 0.71 |
| Yes | 0.318 (0.367) | 0.085 (0.324) |
| No | 0 | 0 |
| Bone mets | p = 0.20 | p = 0.68 |
| Yes | 0.385 (0.300) | 0.111 (0.269) |
| No | 0 | 0 |
| Nephrectomy | p < 0.0001 | p = 0.01 |
| Yes | –1.698 (0.402) | –0.946 (0.377) |
| No | 0 | 0 |
| No. of metastatic site | p < 0.0001 | p = 0.87 |
| 1–2 | 0 | 0 |
| ⩾3 | 1.044 (0.293) | 0.047 (0.294) |
| Treatment | p = 0.006 | p = 0.42 |
| Pazopanib | 0 | 0 |
| Cabozantinib | 0.798 (0.293) | 0.378 (0.473) |
| Hb | p < 0.0001 | p < 0.0001 |
| <12 g/dl | 0 | 0 |
| >12 g/dl | –2.106 (0.271) | –1.428 (0.281) |
Bold values express statistically significant values from our analysis.
ECOG PS, Eastern Cooperative Oncology Group Performance Status; Hb, hemoglobin; IMDC, International mRCC Database Consortium criteria; NLR, neutrophil-to-lymphocyte ratio; PPI, proton-pump inhibitors; RDW, red cell distribution width.
RDW values were found to have a significant positive correlation in univariate analysis with an ECOG PS > 0 (p < 0.0001), concomitant PPI treatment (p = 0.012), IMDC score intermediate (p = 0.001) or poor (p < 0.0001), three or more metastatic sites (p < 0.0001) and cabozantinib treatment (p = 0.006). However, significant inverse correlations were found between RDW and lines of treatment beyond the first (p = 0.006), nephrectomy (p < 0.0001) and Hb (p < 0.0001).
In the multivariate analysis, a significant inverse correlation was maintained between RDW and nephrectomy (p = 0.01) and Hb values (p < 0.0001), while a significant positive correlation was maintained between RDW and ECOG PS > 0 (p = 0.01).
Oncological outcome
The median follow-up was 47 months (IQR 25–74) in the overall population, 65 months (IQR 36–84) in the pazopanib group and 27 months (IQR 16–31) in the cabozantinib group.
Objective response
The ORR (PR + CR) was 44.5% (95% CI 38.8–50.1) in the overall population. The response rate in the pazopanib group was 46.6% (95% CI 39.3–54.0) and 41.3% (95% CI 32.5–50.1) in the cabozantinib group.
Patients with MCV levels > 87 fl had an ORR of 48.8%, whereas patients with a MCV ⩽ 87 had an ORR of 37.7 % (p = 0.057). The patients that obtained a response had MCV values higher than patients that did not respond (p = 0.06, Supplemental Figure S7).
Patients with RDW values ⩽ 16% had a higher ORR: 50.0% compared with 32.4% in patients with RDW values > 16% (p = 0.004). The patients that obtained a response had RDW values significantly lower than patients that did not respond (p = 0.02, Supplemental Figure S8).
Disease control rate
Patients with MCV levels > 87 fl obtained a clinical benefit as best response in 83.5% of cases, while patients with a MCV ⩽ 87 in 70.8% of cases (p = 0.009). The patients that obtained a clinical benefit had MCV values significantly higher than patients that did not (p = 0.003, Supplemental Figure S9).
Patients with RDW values ⩽ 16% obtained a higher disease control rate (83.3%) compared with those with anisocytosis who had a disease control rate of 67.6% (p = 0.002). The patients that obtained a clinical benefit had RDW values significantly lower than patients that did not (p = 0.001, Supplemental Figure S10).
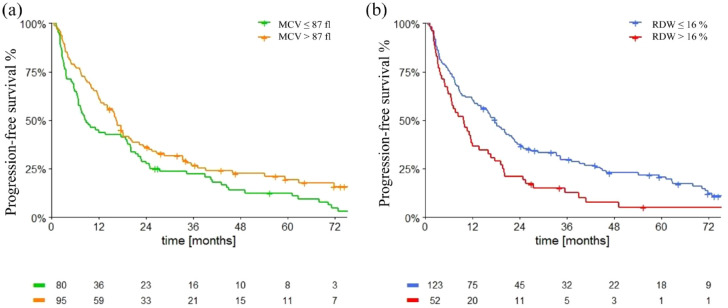
Progression-free survival
Median PFS (mPFS) was 12.0 months (95% CI: 9.5–14.6). MCV and RDW showed to correlate with PFS. Patients with MCV levels > 87 fl had significantly higher mPFS compared with patients with MCV levels ⩽ 87 fl [14.1 months (95% CI 11.0–17.3) versus 8.6 months (95% CI 5.9–11.3), p = 0.031, Figure 1(a)]. However, patients with a higher RDW had a significantly lower mPFS: 9.5 months (95% CI 7.2–11.8) in patients with RDW > 16% versus 16.0 months (95% CI 12.3–20.0) in patients with levels ⩽ 16% (p < 0.0001) (Figure 1(b)).
Figure 1.
Progression-free survival in overall population according to macrocytosis (a) and anisocytosis (b).
MCV, mean corpuscular volume; RDW, red cell distribution width.
The factors associated with PFS at the univariate analyses are shown in Table 4: baseline MCV levels > 87 fl were significantly associated with longer PFS (hazard ratio (HR) 0.76, 95% CI 0.59–0.98, p = 0.03). Baseline RDW levels > 16% were significantly associated with poorer PFS (HR 1.74, 95% CI 1.34–2.26, p < 0.0001). Moreover, ECOG PS > 0, PPI treatment, intermediate-poor IMDC score, NLR ⩾ 3, liver and bone metastasis and number of metastatic sites were significantly related to a shorter PFS, while nephrectomy was significantly related to a longer PFS.
Table 4.
Univariate analyses of PFS in overall population, pazopanib, and cabozantinib groups.
| Overall population | Pazopanib group | Cabozantinib group | |
|---|---|---|---|
| Age (in years) |
p = 0.11 1.01 (1.00–1.02) |
p = 0.09 1.01 (1.00–1.03) |
p = 0.26 1.01 (0.99–1.03) |
| Gender | p = 0.07 | p = 0.10 | p = 0.41 |
| M | 1.00 | 1.00 | 1.00 |
| F | 0.78 (0.59–1.02) | 0.75 (0.53–1.06) | 0.83 (0.53–1.29) |
| ECOG PS | p = 0.002 | p = 0.03 | p = 0.03 |
| 0 | 1.00 | 1.00 | 1.00 |
| 1-2-3 | 1.48 (1.15–1.91) | 1.42 (1.03–1.96) | 1.57 (1.04–2.38) |
| Histology | p = 0.42 | p = 0.62 | p = 0.63 |
| CC | 0.87 (0.62–1.22) | 0.89 (0.57–1.40) | 0.88 (0.52–1.48) |
| Not CC | 1.00 | 1.00 | 1.00 |
| PPI | p < 0.0001 | p = 0.038 | p = 0.003 |
| Yes | 1.62 (1.26–2.08) | 1.40 (1.02–1.94) | 1.90 (1.25–2.90) |
| No | 1.00 | 1.00 | 1.00 |
| IMDC | p < 0.0001 | p < 0.0001 | p = 0.003 |
| Good | 1.00 | 1.00 | 1.00 |
| Intermediate | 1.13 (0.86–1.50) | 1.14 (0.81–1.61) | 1.06 (0.66–1.71) |
| Poor | 2.96 (2.00–4.38) | 3.08 (1.86–5.09) | 2.69 (1.43–5.07) |
| NLR | p = 0.001 | p = 0.025 | p = 0.02 |
| <3 | 1.00 | 1.00 | 1.00 |
| ⩾3 | 1.59 (1.21–2.08) | 1.48 (1.05–2.10) | 1.71 (1.09–2.67) |
| Liver mets | p = 0.03 | p = 0.005 | p = 0.90 |
| Yes | 1.41 (1.03–1.94) | 1.80 (1.19–2.72) | 0.97 (0.59–1.59) |
| No | 1.00 | 1.00 | 1.00 |
| Bone mets | p = 0.01 | p = 0.22 | p = 0.048 |
| Yes | 1.40 (1.08–1.81) | 1.24 (0.88–1.75) | 1.52 (1.01–2.29) |
| No | 1.00 | 1.00 | 1.00 |
| Nephrectomy | p = 0.001 | p = 0.02 | p < 0.0001 |
| Yes | 0.57 (0.41–0.80) | 0.62 (0.41–0.94) | 0.31 (0.17–0.57) |
| No | 1.00 | 1.00 | 1.00 |
| Number of metastatic site | p < 0.0001 | p = 0.002 | p = 0.18 |
| 1–2 | 1.00 | 1.00 | 1.00 |
| ⩾3 | 1.61 (1.24–2.08) | 1.71 (1.22–2.41) | 1.32 (0.88–1.99) |
| Baseline MCV | p = 0.03 | p = 0.03 | p = 0.46 |
| ⩽87 fl | 1.00 | 1.00 | 1.00 |
| >87 fl | 0.76 (0.59–0.98) | 0.70 (0.51–0.97) | 0.86 (0.57–1.29) |
| Baseline RDW | p < 0.0001 | p = 0.004 | p = 0.01 |
| ⩽16% | 1.00 | 1.00 | 1.00 |
| >16% | 1.74 (1.34–2.26) | 1.67 (1.18–2.36) | 1.70 (1.12–2.56) |
Bold values express statistically significant values from our analysis.
ECOG PS, Eastern Cooperative Oncology Group Performance Status; IMDC, International mRCC Database Consortium criteria; MCV, mean corpuscular volume; NLR, neutrophil-to-lymphocyte ratio; PFS, progression-free survival; PPI, proton-pump inhibitors; RDW, red cell distribution width.
At the multivariate analysis, PPI treatment, an intermediate-poor IMDC score and NLR ⩾ 3 remained significantly related to a shorter PFS (Table 5).
Table 5.
Multivariate analyses of PFS in overall population, pazopanib, and cabozantinib groups.
| Overall population | Pazopanib group | Cabozantinib group | |
|---|---|---|---|
| Age (in years) |
p = 0.19 1.01 (1.00–1.02) |
p = 0.08 1.02 (1.00–1.04) |
p = 0.95 1.00 (0.98–1.02) |
| Gender | p = 0.07 | p = 0.18 | p = 0.85 |
| M | 1.00 | 1.00 | 1.00 |
| F | 0.88 (0.64–1.19) | 0.75 (0.49–1.14) | 1.06 (0.61–1.83) |
| ECOG PS | p = 0.86 | p = 0.43 | p = 0.76 |
| 0 | 1.00 | 1.00 | 1.00 |
| 1-2-3 | 1.03 (0.74–1.42) | 1.19 (0.77–1.85) | 1.09 (0.63–1.90) |
| Histology | p = 0.12 | p = 0.07 | p = 0.71 |
| CC | 0.74 (0.51–1.08) | 0.59 (0.34–1.04) | 0.89 (0.49–1.62) |
| Not CC | 1.00 | 1.00 | 1.00 |
| PPI | p = 0.01 | p = 0.047 | p = 0.14 |
| Yes | 1.46 (1.08–1.96) | 1.47 (1.01–2.15) | 1.52 (0.87–2.65) |
| No | 1.00 | 1.00 | 1.00 |
| IMDC | p = 0.001 | p = 0.036 | p = 0.021 |
| Good | 1.00 | 1.00 | 1.00 |
| Intermediate | 1.00 (0.73–1.37) | 1.08 (0.70–1.65) | 0.69 (0.40–1.20) |
| Poor | 2.34 (1.42–3.84) | 2.53 (1.16–5.48) | 1.75 (0.79–3.85) |
| NLR | p = 0.024 | p = 0.052 | p = 0.044 |
| <3 | 1.00 | 1.00 | 1.00 |
| ⩾3 | 1.40 (1.05–1.87) | 1.49 (1.00–2.24) | 1.68 (1.02–2.77) |
| Liver mets | p = 0.08 | p = 0.10 | p = 0.89 |
| Yes | 1.38 (0.96–2.00) | 1.56 (0.92–2.62) | 1.04 (0.59–1.84) |
| No | 1.00 | 1.00 | 1.00 |
| Bone mets | p = 0.12 | p = 0 .39 | p = 0.49 |
| Yes | 1.26 (0.94–1.70) | 1.20 (0.80–1.80) | 1.20 (0.72–2.01) |
| No | 1.00 | 1.00 | 1.00 |
| Nephrectomy | p = 0.86 | p = 0.51 | p = 0.08 |
| Yes | 1.04 (0.69–1.57) | 1.21 (0.69–2.13) | 0.53 (0.26–1.08) |
| No | 1.00 | 1.00 | 1.00 |
| Number of metastatic site | p = 0.74 | p = 0.80 | p = 0.61 |
| 1–2 | 1.00 | 1.00 | 1.00 |
| ⩾3 | 1.06 (0.76–1.47) | 1.06 (0.65–1.74) | 0.86 (0.49–1.52) |
| Baseline MCV | p = 0.41 | p = 0.043 | p = 0.51 |
| ⩽87 fl | 1.00 | 1.00 | 1.00 |
| >87 fl | 0.88 (0.66–1.19) | 0.67 (0.46–0.99) | 1.19 (0.71–2.01) |
| Baseline RDW | p = 0.27 | p = 0.68 | p = 0.10 |
| ⩽16% | 1.00 | 1.00 | 1.00 |
| >16% | 1.20 (0.86–1.67) | 0.90 (0.56–1.46) | 1.59 (0.91–2.78) |
Bold values express statistically significant values from our analysis.
ECOG PS, Eastern Cooperative Oncology Group Performance Status; IMDC, International mRCC Database Consortium criteria; MCV, mean corpuscular volume; NLR, neutrophil-to-lymphocyte ratio; PFS, progression-free survival; PPI, proton-pump inhibitors; RDW, red cell distribution width.
In the pazopanib population, the mPFS was 14.1 months (95% CI 10.6–17.7). Patients with macrocytosis had a mPFS of 16.7 months (95% CI 14.5–18.9), while patients with MCV levels ⩽ 87 fl had a mPFS of 8.1 months (95% CI 5.1–11.1), p = 0.03 (Figure 2(a)). Patients with anisocytosis had a mPFS of 11.2 months (95% CI 8.6–13.7), while patients with RDW levels ⩽ 16% had a mPFS of 16.7 months (95% CI 12.1–21.3), p = 0.004 (Figure 2(b)).
Figure 2.
Progression-free survival in pazopanib group according to macrocytosis (a) and anisocytosis (b).
MCV, mean corpuscular volume; RDW, red cell distribution width.
Baseline macrocytosis was significantly related to longer PFS (HR 0.70, 95% CI 0.51–0.97, p = 0.03), while baseline anisocytosis was significantly related to shorter PFS (HR 1.67, 95% CI 1.18–2.36, p = 0.004). Moreover, ECOG PS > 0, PPI treatment, an intermediate-poor IMDC score, NLR ⩾ 3, liver metastasis and number of metastatic sites were significantly related to a shorter PFS, while nephrectomy was significantly related to a longer PFS (Table 4).
At the multivariate analysis, baseline MCV levels > 87 fl were significantly related to longer PFS (HR 0.67, 95% CI 0.46–0.99, p = 0.043). Moreover, PPI treatment and an intermediate-poor IMDC score remained significantly related to a shorter PFS (Table 5).
In the cabozantinib group, the mPFS was 10.7 months (95% CI 8.9–12.5). Patients with macrocytosis had an mPFS of 11.2 months (95% CI 7.6–14.8), while patients with MCV levels ⩽ 87 fl had a mPFS of 8.6 months (95% CI 6.0–11.2), p = 0.46 (Figure 3(a)). Patients with anisocytosis had a mPFS of 8.2 months (95% CI 5.0–11.4), while patients with RDW levels ⩽ 16% had a mPFS of 14.0 months (95% CI 9.5–18.5), p = 0.01 (Figure 3(b)).
Figure 3.
Progression-free survival in cabozantinib group according to macrocytosis (a) and anisocytosis (b).
MCV, mean corpuscular volume; RDW, red cell distribution width.
Baseline anisocytosis was significantly related to poorer PFS (HR 1.70, 95% CI 1.12–2.56, p = 0.01). Moreover, ECOG PS > 0, PPI treatment, an intermediate-poor IMDC score, NLR ⩾ 3 and bone metastasis were significantly related to a shorter PFS, while nephrectomy was significantly related to a longer PFS (Table 4). At the multivariate analysis, an intermediate-poor IMDC score and NLR ⩾ 3 remained significantly related to a shorter PFS (Table 5).
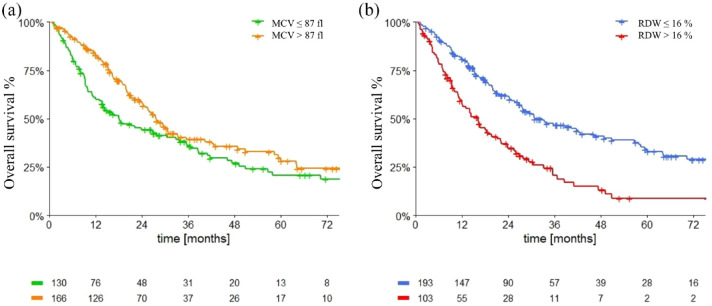
Overall survival
Median OS (mOS) in the overall population was 25.8 months (95% CI 21.3–30.2).
Patients with macrocytosis had mOS of 27.7 months (95% CI 23.4–31.9), while patients with lower MCV had mOS of 18.1 (95% CI 10.2–25.9; p = 0.015 (Figure 4(a)).
Figure 4.
Overall survival in overall population according to macrocytosis (a) and anisocytosis (b).
MCV, mean corpuscular volume; RDW, red cell distribution width.
Patients with RDW levels ⩽ 16% had mOS of 30.9 months (95% CI 21.2–40.5), while patients with RDW levels > 16% had mOS of 16.1 months (95% CI 11.6–20.6; p < 0.0001) (Figure 4(b)).
The results of univariate analysis of OS are summarized in Table 6, baseline macrocytosis was significantly related to longer OS (HR 0.70, 95% CI 0.53–0.94, p = 0.03). Baseline anisocytosis was significantly associated with shorter OS (HR 2.15, 95% CI 1.60–2.90, p < 0.0001). Moreover, ECOG PS > 0, PPI treatment, intermediate-poor IMDC score, NLR ⩾ 3, bone metastasis and number of metastatic sites were significantly related to a shorter OS, while nephrectomy was significantly related to a longer OS.
Table 6.
Univariate analyses of OS in overall population, pazopanib, and cabozantinib group.
| Overall population | Pazopanib group | Cabozantinib group | |
|---|---|---|---|
| Age (in years) |
p = 0.11 1.01 (1.00–1.03) |
p = 0.025 1.02 (1.00–1.04) |
p = 0.17 1.02 (0.99–1.04) |
| Gender | p = 0.06 | p = 0.04 | p = 0.76 |
| M | 1.00 | 1.00 | 1.00 |
| F | 0.74 (0.54–1.02) | 0.64 (0.42–0.98) | 0.92 (0.56–1.53) |
| ECOG PS | p < 0.0001 | p = 0.03 | p = 0.004 |
| 0 | 1.00 | 1.00 | 1.00 |
| 1-2-3 | 1.68 (1.27–2.24) | 1.50 (1.04–2.16) | 1.97 (1.24–3.13) |
| Histology | p = 0.30 | p = 0.86 | p = 0.11 |
| CC | 0.82 (0.57–1.19) | 0.96 (0.58–1.58) | 0.63 (0.36–1.12) |
| Not CC | 1.00 | 1.00 | 1.00 |
| PPI | p < 0.0001 | p = 0.19 | p = 0.001 |
| Yes | 1.67 (1.26–2.22) | 1.28 (0.88–1.86) | 2.30 (1.41–3.75) |
| No | 1.00 | 1.00 | 1.00 |
| IMDC | p < 0.0001 | p < 0.0001 | p < 0.0001 |
| Good | 1.00 | 1.00 | 1.00 |
| Intermediate | 1.61 (1.14–2.25) | 1.42 (0.94–2.16) | 1.88 (1.05–3.38) |
| Poor | 5.80 (3.73–9.01) | 6.18 (3.55–10.76) | 4.95 (2.35–10.42) |
| NLR | p = 0.001 | p = 0.023 | p = 0.02 |
| <3 | 1.00 | 1.00 | 1.00 |
| ⩾3 | 1.73 (1.27–2.36) | 1.59 (1.06–2.36) | 1.80 (1.08–3.00) |
| Liver mets | p = 0.15 | p = 0.18 | p = 0.98 |
| Yes | 1.30 (0.91–1.86) | 1.38 (0.85–2.25) | 1.01 (0.58–1.74) |
| No | 1.00 | 1.00 | 1.00 |
| Bone mets | p = 0.015 | p = 0.11 | p = 0.60 |
| Yes | 1.44 (1.07–1.93) | 1.38 (0.93–2.05) | 1.13 (0.71–1.79) |
| No | 1.00 | 1.00 | 1.00 |
| Nephrectomy | p < 0.0001 | p = 0.001 | p < 0.0001 |
| Yes | 0.46 (0.32–0.65) | 0.47 (0.30–0.73) | 0.28 (0.15–0.53) |
| No | 1.00 | 1.00 | 1.00 |
| Number of metastatic site | p < 0.0001 | p = 0.001 | p = 0.18 |
| 1–2 | 1.00 | 1.00 | 1.00 |
| ⩾3 | 1.88 (1.41–2.51) | 1.92 (1.31–2.80) | 1.37 (0.86–2.19) |
| Baseline MCV | p = 0.03 | p = 0.06 | p = 0.08 |
| ⩽87 fl | 1.00 | 1.00 | 1.00 |
| >87 fl | 0.70 (0.53–0.94) | 0.70 (0.49–1.02) | 0.66 (0.42–1.05) |
| Baseline RDW | p < 0.0001 | p < 0.0001 | p = 0.008 |
| ⩽16% | 1.00 | 1.00 | 1.00 |
| >16% | 2.15 (1.60–2.90) | 2.09 (1.41–3.11) | 1.87 (1.18–2.97) |
Bold values express statistically significant values from our analysis.
ECOG PS, Eastern Cooperative Oncology Group Performance Status; IMDC, International mRCC Database Consortium criteria; MCV, mean corpuscular volume; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PPI, proton-pump inhibitors; RDW, red cell distribution width.
At the multivariate analysis, higher RDW levels were significantly associated with poorer OS (HR 1.53, 95% CI 1.05–2.23, p = 0.028), like PPI treatment, an intermediate-poor IMDC score and NLR ⩾ 3 (Table 7).
Table 7.
Multivariate analyses of OS in overall population, Pazopanib, and Cabozantinib groups.
| Overall population | Pazopanib group | Cabozantinib group | |
|---|---|---|---|
| Age (in years) |
p = 0.055 1.02 (1.00–1.03) |
p = 0.004
1.04 (1.01–1.06) |
p = 0.81 1.00 (0.98–1.03) |
| Gender | p = 0.59 | p = 0.20 | p = 0.55 |
| M | 1.00 | 1.00 | 1.00 |
| F | 0.91 (0.63–1.30) | 0.72 (0.44–1.19) | 1.23 (0.62–2.46) |
| ECOG PS | p = 0.76 | p = 0.47 | p = 0.35 |
| 0 | 1.00 | 1.00 | 1.00 |
| 1-2-3 | 0.94 (0.65–1.37) | 0.83 (0.50–1.38) | 1.34 (0.73–2.47) |
| Histology | p = 0.058 | p = 0.52 | p = 0.15 |
| CC | 0.67 (0.44–1.01) | 0.82 (0.44–1.51) | 0.62 (0.32–1.20) |
| Not CC | 1.00 | 1.00 | 1.00 |
| PPI | p = 0.027 | p = 0.26 | p = 0.058 |
| Yes | 1.47 (1.04–2.06) | 1.28 (0.83–1.99) | 1.97 (0.98–3.97) |
| No | 1.00 | 1.00 | 1.00 |
| IMDC | p < 0.0001 | p < 0.0001 | p = 0.045 |
| Good | 1.00 | 1.00 | 1.00 |
| Intermediate | 1.52 (1.04–2.24) | 1.54 (0.93–2.55) | 1.31 (0.66–2.59) |
| Poor | 4.94 (2.78–8.76) | 6.58 (2.87–15.07) | 3.02 (1.20–7.57) |
| NLR | p = 0.027 | p = 0.26 | p = 0.11 |
| <3 | 1.00 | 1.00 | 1.00 |
| ⩾3 | 1.47 (1.05–2.06) | 1.31 (0.82–2.11) | 1.58 (0.90–2.76) |
| Liver mets | p = 0.28 | p = 0.80 | p = 0.66 |
| Yes | 1.26 (0.83–1.92) | 1.08 (0.59–1.99) | 1.15 (0.62–2.14) |
| No | 1.00 | 1.00 | 1.00 |
| Bone mets | p = 0.24 | p = 0.24 | p = 0.68 |
| Yes | 1.23 (0.87–1.74) | 1.33 (0.83–2.14) | 0.88 (0.47–1.63) |
| No | 1.00 | 1.00 | 1.00 |
| Nephrectomy | p = 0.83 | p = 0.57 | p = 0.06 |
| Yes | 1.05 (0.66–1.67) | 1.21 (0.63–2.36) | 0.50 (0.24–1.04) |
| No | 1.00 | 1.00 | 1.00 |
| Number of metastatic site | p = 0.21 | p = 0.48 | p = 0.96 |
| 1-2 | 1.00 | 1.00 | 1.00 |
| ⩾3 | 1.26 (0.88–1.81) | 1.21 (0.72–2.02) | 1.02 (0.54–1.91) |
| Baseline MCV | p = 0.53 | p = 0.11 | p = 0.63 |
| ⩽87 fl | 1.00 | 1.00 | 1.00 |
| >87 fl | 0.90 (0.63–1.26) | 0.69 (0.44–1.09) | 1.16 (0.64–2.10) |
| Baseline RDW | p = 0.028 | p = 0.38 | p = 0.10 |
| ⩽16% | 1.00 | 1.00 | 1.00 |
| >16% | 1.53 (1.05–2.23) | 1.29 (0.73–2.30) | 1.69 (0.90–3.17) |
Bold values express statistically significant values from our analysis.
ECOG PS, Eastern Cooperative Oncology Group Performance Status; IMDC, International mRCC Database Consortium criteria; MCV, mean corpuscular volume; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PPI, proton-pump inhibitors; RDW, red cell distribution width.
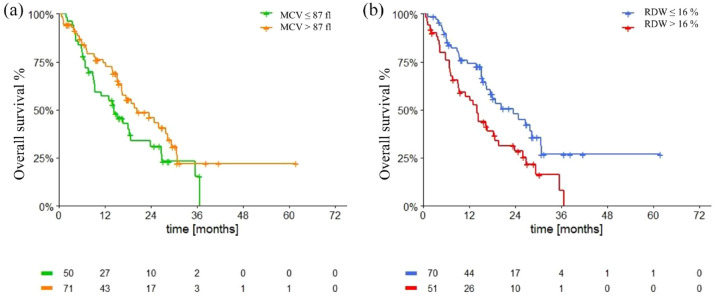
Median OS in the pazopanib group was 30.6 months (95% CI 22.1–39.1). Patients with macrocytosis had a mOS of 35.6 months (95% CI 23.9–47.3), while patients with MCV levels ⩽ 87 fl had a mOS of 22.2 months [95% CI 2.2–42.2; p = 0.06, Figure 5(a)]. Patients with anisocytosis had a mOS of 21.2 months (95% CI 15.1–27.3), while patients with RDW levels ⩽ 16% had a mOS of 46.2 months [95% CI 25.0–67.3; p < 0.0001, Figure 5(b)].
Figure 5.
Overall survival in pazopanib group according to macrocytosis (a) and anisocytosis (b).
MCV, mean corpuscular volume; RDW, red cell distribution width.
Baseline anisocytosis was significantly associated with shorter OS in the pazopanib group (HR 2.09, 95% CI 1.41–3.11, p < 0.0001). Moreover, ECOG PS > 0, age, male sex, an intermediate-poor IMDC score, NLR ⩾ 3 and number of metastatic sites were significantly related to a shorter OS, while nephrectomy was significantly related to a longer OS (Table 6). At the multivariate analysis, only age and an intermediate-poor IMDC score remained significantly related to a poorer OS (Table 7).
Median OS was 18.1 months in the cabozantinib group (95% CI 15.0–21.1).
Patients with macrocytosis had a mOS of 18.8 months (95% CI 12.5–25.1), while patients with MCV levels ⩽ 87 fl had a mOS of 13.1 months [95% CI 6.6–19.6; p = 0.08, Figure 6(a)]. Patients with anisocytosis had a mOS of 14 months (95% CI 8.8–19.1), while patients with RDW levels ⩽ 16% had a mOS of 23.6 months [95% CI 15.4–31.8; p = 0.008, Figure 6(b)].
Figure 6.
Overall survival in cabozantinib group according to macrocytosis (a) and anisocytosis (b).
MCV, mean corpuscular volume; RDW, red cell distribution width.
At univariate analyses, baseline anisocytosis was significantly associated with poorer OS (HR 1.87, 95% CI 1.18–2.97, p = 0.008). Moreover, ECOG PS > 0, PPI treatment, intermediate-poor IMDC score and NLR ⩾ 3 were significantly related to a shorter OS, while nephrectomy was significantly related to a longer OS (Table 6). At the multivariate analysis, only an intermediate-poor IMDC score remained significantly related to a poorer OS (Table 7).
Discussion
The primary endpoint of the study was to describe the incidence and the trend over time of macrocytosis and anisocytosis in patients with mRCC treated with pazopanib or cabozantinib. Different studies have analyzed the correlation between TKI treatment and the development of macrocytosis. Rini et al. 10 found that MCV increased throughout sunitinib therapy and that sunitinib-induced macrocytosis was reversible with drug discontinuation. However, to the best of our knowledge, the incidence of macrocytosis and its correlation to the outcome of patients with mRCC treated with pazopanib or cabozantinib has never been thoroughly assessed.
We observed that 55.8% patients presented macrocytosis before starting treatment, with a significant mean increase after 3 months of treatment of 3 fl in the overall population and of 5 fl in the pazopanib group. Kloth et al. reported retrospective data from patients with solid tumors, treated with different TKIs (i.e. imatinib, pazopanib, sorafenib, sunitinib, or vemurafenib). In patients treated with pazopanib, the rise in MCV levels generally occurred roughly after 3 months of treatment, without a decrease in the first 3 months. Patients with mRCC treated with sunitinib who developed macrocytosis had a significantly longer OS; however, for other tumor-TKI settings, there was no statistically significant difference in survival between patients with and without macrocytosis or substantial increase in MCV levels from baseline. 16 Their conclusions differ from our findings: in fact, our patients treated with pazopanib showed a significant early decrease in MCV values after 2 weeks of treatment, with a later significant increase after 3 months. Moreover, in the pazopanib group patients with MCV > 87 fl had significantly longer PFS. These findings align with those of Kucharz et al., 17 who studied the correlation between macrocytosis during sunitinib treatment and PFS and found that patients who developed macrocytosis after 3 sunitinib treatment cycles had longer PFS times than those without macrocytosis.
In patients treated with cabozantinib, MCV values have never been investigated before. We found a significant decrease in MCV values after 14 and 28 days of treatment and a non-significant increase in values after 3 months. Baseline macrocytosis in the cabozantinib group did not correlate with neither PFS nor OS at multivariate analyses: this could be related with the use of cabozantinib as second line treatment and, in this way, baseline MCV could be influenced by prior treatments.
MCV values also had a significant impact on the ORR: patients with MCV > 87 fl had a higher ORR than those with lower MCV in the overall population and in both the TKI subgroups.
Many studies have demonstrated the association between cancer and elevated inflammatory markers,18–20 therefore different inflammation-related hematologic markers, such as NLR and RDW, are investigated as biomarkers for several solid tumors.21–24 Several studies have demonstrated the prognostic relevance of NLR pre-treatment values for both localized and metastatic clear cell RCC.25–28
More recent studies have analyzed the correlation between RDW and outcome of patients with RCC. Zyczkowski et al. 14 were the first that explored the correlation between RDW values and cancer-specific survival (CSS): a key finding of their study was that CSS was significantly lower in patients with high RDW (>13.9%) compared with patients with lower RDW (<13.9%) and that RDW was an independent prognostic factor of CSS in RCC patients treated with partial or radical nephrectomy. However, Aktepe et al. were the firsts to publish a study regarding the prognostic value of RDW for patients with mRCC treated with targeted therapy. They considered 104 patients treated with either sunitinib or pazopanib and identified 15.4% RDW level as the optimal cut-off value for OS prediction. Their study revealed that patients with high RDW level had inferior PFS and OS than those with low RDW. 29
Our study evaluated a larger (301) cohort of patients with mRCC, treated with pazopanib (59%) or cabozantinib (41%). We determined a baseline RDW cut-off of 16% as the optimal value to maximize the log-rank test. We collected data at the beginning of the treatment and during the first 3 month of treatment. A constant increase in the RDW levels, with a peak after 3 months of therapy was observed. Like Aktepe et al. 29 we found that patients with higher RDW values have poorer PFS and OS than those with lower RDW. At the multivariate analysis, the RDW value was an independent factor significantly associated with poorer OS in the overall population, but not in the groups separated by type of TKI, probably due to the limited number of patients in each group. We also evaluated the correlation between RDW levels and the ORR, revealing that patients with RDW values ⩽ 16% have a significantly higher response to therapy (50.0%) than those with RDW > 16% (32.4%).
An additional finding of our study was the increase in the hemoglobin values after treatment start. In fact, we observed a significant mean increase of 1 g/dl after 2 weeks of treatment with both pazopanib and cabozantinib. These values were then maintained during the first 3 months. The earliest findings about this phenomenon are those of Alexandrescu et al. who described erythrocytosis in five patients treated either with sunitinib or sorafenib for various metastatic cancers (RCC, melanoma, and hepatocellular carcinoma). Erythrocytosis developed with a relatively rapid onset over 1 to 2 weeks, reaching a peak at 4 to 9 weeks after the beginning of TKI treatment: 30 similar occurrences were described in different case reports.31–34
At multivariate analysis, the only factor significantly and positively related to MCV basal levels was Hb ⩾ 12 g/dl. Conversely, the only factor significantly and positively correlated to RDW basal levels at multivariate analysis was ECOG PS ⩾ 1, while nephrectomy and Hb ⩾ 12 g/dl were significantly and inversely correlated with RDW. These findings are in accordance with the fact that anemia (a well-known negative prognostic factor for patients with mRCC) is associated with anisocytosis and lack of macrocytosis; interestingly these two latter factors are in turn associated with poor outcome in our study. In other words, it seems that patients (treated with VEGFR-TKI) without anemia, higher MCV and without anisocytosis belong to a favorable prognostic condition.
We hypothesized that this laboratory pattern (elevated Hb, elevated MCV, low RDW) can be sustained by the erythropoietin (EPO) stimulation as consequence of HIF-α pathway activation: Von-Hippel Lindau protein dysfunction represents the main activation mechanism of HIF-α pathway in RCC. Thus, the laboratory pattern above mentioned could be considered a ‘phenotype’ of the HIF-α pathway activation in these patients. This hypothesis could be corroborated by other studies that found that erythrocytosis secondary to anti-VEGF treatment occurred only in RCC patients, not in patients with other malignancies treated with the same agents. This phenomenon suggests that anti-VEGF-induced elevated EPO levels in serum are more likely to have arisen directly from RCC itself. 32 In addition, some clinical cases reported an elevation of EPO levels in the serum of patients treated with pazopanib despite high levels of Hb, confirming the hypothesis that the erythrocytosis induces by VEGFR inhibitors could be due to an overproduction of EPO.31,33 Moreover, red blood cells (RBCs) with high MCV and low RDW may reflect the presence of erythroblasts in peripheral blood, as a response of increased levels of EPO. The increase in Hb levels over time described in our results is consistent with possible positive feedback on HIF-α pathway induced by the inhibition of the function of downstream proteins (i.e. VEGFR, PDGFR). The increasing levels over time of MCV and RDW could reflect the balance between erythrocytosis stimulation and c-KIT inhibition 30 (Figure 7). As further evidence of what has been hypothesized, it is relevant to note that the drug belzutifan (an HIF-α inhibitor) among the main adverse events causes anemia, acting upstream of the HIF pathway and blocking not only VEGF production but also EPO production. 35
Figure 7.
Hypothetic mechanisms to justify the development of macrocytosis and reduction of anisocytosis in patients treated with pazopanib and cabozantinib. The inhibition of VEGF by TKIs acts on neo-angiogenesis and brings a paradoxal hyperactivation of HIF-α pathway with an over-expression of EPO which acts on bone marrow where the production of immature high-volume RBCs (erythroblasts) are released in the bloodstream.
EPO, erythropoietin; HIF-α, Hypoxia-inducible factor; PDGF-ß, platelet-derived growth factor-ß; RBCs, red blood cells; RCC, renal cancer cell; TGF-ß, tumor growth factor-ß; VEGF, vascular endothelial growth factor; VHL, Von-Hipple Lindau gene.
Another speculative explanation to justify the better outcome of patients with macrocytosis is that, tumoral blood vessels created during pathological neo-angiogenesis have an altered structure: 36 high volume RBCs with a uniform dimension, together with the inhibition of neoangiogenesis through the VEGF-pathway inhibition, reduce the nutritional intake for tumor with a better response to therapy.
Furthermore, the lack of clinical and laboratory prognostic biomarker is an unmet need in mRCC: the laboratory-based biomarkers we identify in the present study are inexpensive, easy to look and could give to the clinicians more information regarding patients’ probability to treatment benefit. In addition, this study confirms previous literature data emerged in patients treated with sunitinib.
The main limitation of the present study is its retrospective nature and the relative limited number of patients enrolled. Moreover, factors that could influence macrocytosis and anisocytosis such as nutritional (i.e. vitamin B12, folate, and iron levels) and endocrine parameters (i.e. preexisting or drug-related alteration of thyroids), were not analyzed due to a lack of data from most of the centers involved in the study. Another limitation of this retrospective study is based on the response evaluation at the treatment: notwithstanding it is performed based on RECIST criteria by treating physicians, it may lack the typical rigor of prospective trials. On the other hand, this makes results to be closer to real-life clinical practice.
Conclusion
To the best of our knowledge, our study is the first retrospective observational study that assessed hemoglobin levels over the time and that evaluate the impact of macrocytosis and anisocytosis in patients treated with TKIs. Strengths of our study are represented by the multicenter involvement, the adequate median follow-up, and the bivalence concerning the TKI type (cabozantinib or pazopanib) and treatment line.
Our results showed that a not negligible proportion of patient with mRCC treated with pazopanib or cabozantinib had baseline macrocytosis or anisocytosis. Moreover, we showed a significant increase of Hb, MCV and RDW after the beginning of these TKIs. Baseline macrocytosis is positively correlated with PFS in patients treated with pazopanib and baseline anisocytosis is a prognostic factor for all patients treated with pazopanib or cabozantinib. The evidence provided by the present study suggest that some laboratory parameters (i.e. Hb, MCV, and RDW) may indirectly reflect the activation of HIF-alfa pathway in patients with mRCC.
We are planning the development of a laboratory-based score including Hb, MCV, and RDW with the aim of tailoring the treatment options for mRCC patients both with TKI-based and immunotherapy only combinations. At the same time, we are designing a prospective randomized trial to validate this biomarker in patients treated with TKIs and immunotherapy for mRCC; in addition we plan to design a prospective study to understand the mechanistic basis of the observed phenomenon in MARECAP trial.
Supplemental Material
Supplemental material, sj-docx-1-tau-10.1177_17562872231187216 for The role of mean corpuscular volume and red cell distribution width in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors: the MARECAP retrospective study by Chiara Tommasi, Giulia Scartabellati, Diana Giannarelli, Ugo De Giorgi, Nicole Brighi, Giuseppe Fornarini, Sara Elena Rebuzzi, Silvia Puglisi, Orazio Caffo, Stefania Kinspergher, Alessia Mennitto, Carlo Cattrini, Matteo Santoni, Elena Verzoni, Alessandro Rametta, Marco Stellato, Andrea Malgeri, Giandomenico Roviello, Matteo Brunelli and Sebastiano Buti in Therapeutic Advances in Urology
Supplemental material, sj-pptx-2-tau-10.1177_17562872231187216 for The role of mean corpuscular volume and red cell distribution width in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors: the MARECAP retrospective study by Chiara Tommasi, Giulia Scartabellati, Diana Giannarelli, Ugo De Giorgi, Nicole Brighi, Giuseppe Fornarini, Sara Elena Rebuzzi, Silvia Puglisi, Orazio Caffo, Stefania Kinspergher, Alessia Mennitto, Carlo Cattrini, Matteo Santoni, Elena Verzoni, Alessandro Rametta, Marco Stellato, Andrea Malgeri, Giandomenico Roviello, Matteo Brunelli and Sebastiano Buti in Therapeutic Advances in Urology
Acknowledgments
None.
Footnotes
ORCID iDs: Chiara Tommasi  https://orcid.org/0000-0002-9230-9288
https://orcid.org/0000-0002-9230-9288
Carlo Cattrini  https://orcid.org/0000-0003-4785-9480
https://orcid.org/0000-0003-4785-9480
Marco Stellato  https://orcid.org/0000-0002-0993-7540
https://orcid.org/0000-0002-0993-7540
Sebastiano Buti  https://orcid.org/0000-0003-0876-0226
https://orcid.org/0000-0003-0876-0226
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Chiara Tommasi, Medical Oncology Unit, University Hospital of Parma, Via Gramsci 14, Parma 43126, Italy; Department of Medicine and Surgery, University of Parma, Parma, Italy; Gruppo Oncologico Italiano di Ricerca Clinica (GOIRC), Parma, Italy.
Giulia Scartabellati, Department of Medicine and Surgery, University of Parma, Parma, Italy.
Diana Giannarelli, Facility Epidemiology and Biostatistic, G-Step, Fondazione Policlinico Universitario A. Gemelli, Scientific Direction, IRCCS, Rome, Italy.
Ugo De Giorgi, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy.
Nicole Brighi, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy.
Giuseppe Fornarini, Medical Oncology Unit, IRCCS Ospedale Policlinico San Martino, Genova, Italy.
Sara Elena Rebuzzi, Medical Oncology Unit, Ospedale San Paolo, Savona, Italy; Department of Internal Medicine and Medical Specialties (Di.M.I.), University of Genova, Genova, Italy.
Silvia Puglisi, Medical Oncology Unit, IRCCS Ospedale Policlinico San Martino, Genova, Italy.
Orazio Caffo, Department of Medical Oncology, Santa Chiara Hospital, Trento, Italy.
Stefania Kinspergher, Department of Medical Oncology, Santa Chiara Hospital, Trento, Italy.
Alessia Mennitto, Division of Oncology, University Hospital ‘Maggiore della Carità’, Novara, Italy.
Carlo Cattrini, Division of Oncology, University Hospital ‘Maggiore della Carità’, Novara, Italy.
Matteo Santoni, Oncology Unit, Macerata Hospital, Macerata, Italy.
Elena Verzoni, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy.
Alessandro Rametta, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy.
Marco Stellato, Department of Medical Oncology, Università Campus Bio-Medico di Roma, Rome, Italy.
Andrea Malgeri, Department of Medical Oncology, Università Campus Bio-Medico di Roma, Rome, Italy.
Giandomenico Roviello, Department of Health Sciences, University of Florence, Florence, Italy.
Matteo Brunelli, Department of Diagnostic and Public Health, Section of Pathology, University of Verona, Verona, Italy.
Sebastiano Buti, Medical Oncology Unit, University Hospital of Parma, Parma, Italy; Department of Medicine and Surgery, University of Parma, Parma, Italy; Gruppo Oncologico Italiano di Ricerca Clinica (GOIRC), Parma, Italy.
Declarations
Ethics approval and consent to participate: We obtained approval from the ethics committee of the coordinating center (Comitato Etico dell’Area Vasta Emilia Nord, protocol number 208/2021/OSS/AOUPR MA.RE.CA.P). For living patients, we collected written informed consent during a follow up visit . For unreachable or deceased patients, we collected data following the indication of our Ethical Committee and following our Privacy Law, guaranteeing the anonymity of data.
Consent for publication: Not applicable.
Author contributions: Chiara Tommasi: Conceptualization; Data curation; Investigation; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Giulia Scartabellati: Data curation; Investigation; Supervision; Writing – original draft.
Diana Giannarelli: Formal analysis; Methodology; Resources; Software; Validation; Writing – original draft; Writing – review & editing.
Ugo De Giorgi: Resources; Writing – review & editing.
Nicole Brighi: Resources; Writing – review & editing.
Giuseppe Fornarini: Resources; Writing – review & editing.
Sara Elena Rebuzzi: Resources; Writing – review & editing.
Silvia Puglisi: Resources; Writing – review & editing.
Orazio Caffo: Resources; Writing – review & editing.
Stefania Kinspergher: Resources; Writing – review & editing.
Alessia Mennitto: Resources; Writing – review & editing.
Carlo Cattrini: Resources; Writing – review & editing.
Matteo Santoni: Resources; Writing – review & editing.
Elena Verzoni: Resources; Writing – review & editing.
Alessandro Rametta: Resources; Writing – review & editing.
Marco Stellato: Resources; Writing – review & editing.
Andrea Malgeri: Resources; Writing – review & editing.
Giandomenico Roviello: Resources; Writing – review & editing.
Matteo Brunelli: Resources; Writing – review & editing.
Sebastiano Buti: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.B. received honoraria as a speaker at scientific events and advisory role by Bristol-Myers Squibb (BMS), Pfizer; MSD, Ipsen, AstraZeneca, and Novartis; he also received research funding from Novartis. For the remaining authors, there are no conflicts of interest. The other authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1.Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019; 30: 706–720. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009; 27: 3584–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyamoto S, Kakutani S, Sato Y, et al. Drug review: pazopanib. Jpn J Clin Oncol 2018; 48: 503–513. [DOI] [PubMed] [Google Scholar]
- 4.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010; 28: 1061–1068. [DOI] [PubMed] [Google Scholar]
- 5.Al-Salama ZT, Keating GM.Cabozantinib: a review in advanced renal cell carcinoma. Drugs 2016; 76: 1771–1778. [DOI] [PubMed] [Google Scholar]
- 6.Choueiri TK, Hessel C, Halabi S, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): progression-free survival by independent review and overall survival update. Eur J Cancer 2018; 94: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373: 1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linee Guida TUMORI DEL RENE. AIOM, https://www.aiom.it/linee-guida-aiom-2020-tumori-del-rene/ (2020, accessed 7 July 2022).
- 9.Cerbone L, Combarel D, Geraud A, et al. Association of cabozantinib pharmacokinetics, progression and toxicity in metastatic renal cell carcinoma patients: results from a pharmacokinetics/pharmacodynamics study. ESMO Open 2021; 6: 100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rini BI, Choueiri TK, Elson P, et al. Sunitinib-induced macrocytosis in patients with metastatic renal cell carcinoma. Cancer 2008; 113: 1309–1314. [DOI] [PubMed] [Google Scholar]
- 11.Schallier D, Trullemans F, Fontaine C, et al. Tyrosine kinase inhibitor-induced macrocytosis. Anticancer Res 2009; 29: 5225–5228. [PubMed] [Google Scholar]
- 12.Bourlon MT, Gao D, Trigero S, et al. Clinical significance of sunitinib-associated macrocytosis in metastatic renal cell carcinoma. Cancer Med 2016; 5: 3386–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang FM, Xu G, Zhang Y, et al. Red cell distribution width is associated with presence, stage, and grade in patients with renal cell carcinoma. Dis Markers 2014; 2014: 860419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Życzkowski M, Rajwa P, Gabrys E, et al. The relationship between red cell distribution width and cancer-specific survival in patients with renal cell carcinoma treated with partial and radical nephrectomy. Clin Genitourin Cancer 2018; 16: e677–e683. [DOI] [PubMed] [Google Scholar]
- 15.Heng DYC, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009; 27: 5794–5799. [DOI] [PubMed] [Google Scholar]
- 16.Kloth JSL, Hamberg P, Mendelaar PAJ, et al. Macrocytosis as a potential parameter associated with survival after tyrosine kinase inhibitor treatment. Eur J Cancer 2016; 56: 101–106. [DOI] [PubMed] [Google Scholar]
- 17.Kucharz J, Giza A, Dumnicka P, et al. Macrocytosis during sunitinib treatment predicts progression-free survival in patients with metastatic renal cell carcinoma. Med Oncol 2016; 33: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014; 106: dju124. [DOI] [PubMed] [Google Scholar]
- 19.Grivennikov SI, Greten FR, Karin M.Immunity, inflammation, and cancer. Cell 2010; 140: 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeuchi H, Kawanaka H, Fukuyama S, et al. Comparison of the prognostic values of preoperative inflammation-based parameters in patients with breast cancer. PLoS ONE 2017; 12: e0177137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng YC, Huang CN, Wu WJ, et al. The prognostic significance of inflammation-associated blood cell markers in patients with upper tract urothelial carcinoma. Ann Surg Oncol 2016; 23: 343–351. [DOI] [PubMed] [Google Scholar]
- 22.Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015; 52: 86–105. [DOI] [PubMed] [Google Scholar]
- 23.Hu L, Li M, Ding Y, et al. Prognostic value of RDW in cancers: a systematic review and meta-analysis. Oncotarget 2017; 8: 16027–16035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albayrak S, Zengin K, Tanik S, et al. Red cell distribution width as a predictor of prostate cancer progression. Asian Pac J Cancer Prev 2014; 15: 7781–7784. [DOI] [PubMed] [Google Scholar]
- 25.de Martino M, Pantuck AJ, Hofbauer S, et al. Prognostic impact of preoperative neutrophil-to-lymphocyte ratio in localized nonclear cell renal cell carcinoma. J Urol 2013; 190: 1999–2004. [DOI] [PubMed] [Google Scholar]
- 26.Hu K, Lou L, Ye J, et al. Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: a meta-analysis. BMJ Open 2015; 5: e006404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semeniuk-Wojtaś A, Lubas A, Stec R, et al. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and C-reactive protein as new and simple prognostic factors in patients with metastatic renal cell cancer treated with tyrosine kinase inhibitors: a systemic review and meta-analysis. Clin Genitourin Cancer 2018; 16: e685–e693. [DOI] [PubMed] [Google Scholar]
- 28.Ohno Y, Nakashima J, Ohori M, et al. Pretreatment neutrophil-to-lymphocyte ratio as an independent predictor of recurrence in patients with nonmetastatic renal cell carcinoma. J Urol 2010; 184: 873–878. [DOI] [PubMed] [Google Scholar]
- 29.Aktepe OH, Guven DC, Sahin TK, et al. The predictive value of red blood cell distribution width for survival outcomes of metastatic renal cell carcinoma patients treated with targeted therapy. Nutr Cancer 2021; 73: 1957–1963. [DOI] [PubMed] [Google Scholar]
- 30.Alexandrescu DT, McClure R, Farzanmehr H, et al. Secondary erythrocytosis produced by the tyrosine kinase inhibitors sunitinib and sorafenib. J Clin Oncol 2008; 26: 4047–4048. [DOI] [PubMed] [Google Scholar]
- 31.Bukhari N, Winquist E.Case: secondary polycythemia due to pazopanib in patients with metastatic renal cell carcinoma. Can Urol Assoc J 2017; 11: E449–E450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W, Cheng J, Mallon C, et al. Symptomatic secondary polycythemia induced by anti-VEGF therapy for the treatment of metastatic renal cell carcinoma: a case series and review. Clin Genitourin Cancer 2015; 13: e391–e395. [DOI] [PubMed] [Google Scholar]
- 33.Fanelli M, Caputo F, Cerma K, et al. Pazopanib-related secondary polycythemia in metastatic myxofibrosarcoma: a case report and review of the literature. J Oncol Pharm Pract 2021; 27: 766–770. [DOI] [PubMed] [Google Scholar]
- 34.Kodaira S, Ehara J, Takamizawa S, et al. Acute lower extremity arterial thrombosis associated with osimertinib-induced erythrocytosis. Am J Case Rep 2021; 22: e932252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonasch E, Donskov F, Iliopoulos O, et al. Belzutifan for renal cell carcinoma in Von Hippel-Lindau disease. N Engl J Med 2021; 385: 2036–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lugano R, Ramachandran M, Dimberg A.Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci 2020; 77: 1745–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tau-10.1177_17562872231187216 for The role of mean corpuscular volume and red cell distribution width in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors: the MARECAP retrospective study by Chiara Tommasi, Giulia Scartabellati, Diana Giannarelli, Ugo De Giorgi, Nicole Brighi, Giuseppe Fornarini, Sara Elena Rebuzzi, Silvia Puglisi, Orazio Caffo, Stefania Kinspergher, Alessia Mennitto, Carlo Cattrini, Matteo Santoni, Elena Verzoni, Alessandro Rametta, Marco Stellato, Andrea Malgeri, Giandomenico Roviello, Matteo Brunelli and Sebastiano Buti in Therapeutic Advances in Urology
Supplemental material, sj-pptx-2-tau-10.1177_17562872231187216 for The role of mean corpuscular volume and red cell distribution width in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors: the MARECAP retrospective study by Chiara Tommasi, Giulia Scartabellati, Diana Giannarelli, Ugo De Giorgi, Nicole Brighi, Giuseppe Fornarini, Sara Elena Rebuzzi, Silvia Puglisi, Orazio Caffo, Stefania Kinspergher, Alessia Mennitto, Carlo Cattrini, Matteo Santoni, Elena Verzoni, Alessandro Rametta, Marco Stellato, Andrea Malgeri, Giandomenico Roviello, Matteo Brunelli and Sebastiano Buti in Therapeutic Advances in Urology