Abstract
Background:
REFLECT is the first prospective study of Sandoz biosimilar rituximab (SDZ-RTX) in patients with diffuse large B-cell lymphoma (DLBCL).
Objective:
To evaluate the 2-year effectiveness and safety of SDZ-RTX as first-line treatment for DLBCL.
Design:
Real-world, multicenter, open-label, single-arm, non-interventional, post-approval study of SDZ-RTX in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in patients with treatment-naïve CD20‑positive DLBCL.
Methods:
Treatment-naïve, CD20-positive adult patients (⩾18 years) with DLBCL eligible for therapy with R-CHOP were treated with SDZ-RTX-CHOP every 2 or 3 weeks for 6–8 cycles. The effectiveness of SDZ-RTX was measured by the complete response (CR) rate at the end of R-CHOP treatment, as assessed by the treating physician. Progression-free survival (PFS) was assessed at 24 months.
Results:
A total of 169 patients [52.1% female, median (range) age 70 (24−94) years] with DLBCL were included in the full analysis set. At baseline, 19.5% and 24.3% of patients had Ann Arbor disease stage III or IV, respectively, and most patients (80.5%) had Eastern Cooperative Oncology Group Performance Status of 0 or 1. A total of 100 (59.2%) patients completed the 24-month observation period. In total, 110 [65.1%; 95% confidence interval (CI): 57.4–72.3] patients achieved CR as best response and 50 (29.6%; 95% CI: 22.8–37.1) patients achieved partial response. Overall best response rate was 94.7% (95% CI: 90.1–97.5). One-year PFS was 84.9% (95% CI: 78.2–89.6), while 2-year PFS was 78.5% (95% CI: 70.9–84.4); median PFS was not reached within the observational period. A total of 143 (84.6%) patients experienced ⩾1 adverse event, 53 (31.4%) of which were suspected to be related to study drug.
Conclusion:
This real-world, 2-year study reconfirms that first-line treatment of CD20-positive DLBCL with R-CHOP using SDZ-RTX is effective and well tolerated.
Registration:
N/A
Keywords: biosimilar, CHOP, DLBCL, real-world, rituximab
Plain language summary
REFLECT: A study evaluating Sandoz biosimilar rituximab (Rixathon ® ) in combination with CHOP for the treatment of patients with previously untreated diffuse large B-cell lymphoma
Why was this study done?
• Biosimilars are biologic medicines that are highly similar to a reference biologic medicine that is already approved and has been used in patients for several years.
• The REFLECT study was the first study of a biosimilar medicine (Sandoz biosimilar rituximab) in patients with a type of lymphatic cancer called diffuse large B-cell lymphoma (DLBCL).
What did the researchers do?
• Sandoz biosimilar rituximab was given as part of the standard treatment (cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CHOP) in patients with DLBCL who had not received treatment before.
• The researchers aimed to evaluate how well Sandoz biosimilar rituximab worked over a 2-year period.
• The researchers also aimed to look at the safety of Sandoz biosimilar rituximab.
• Patients with DLBCL had to be ⩾18 years of age, in need of treatment, and were classed as suitable for treatment with R-CHOP by their doctor.
• Patients were treated with R-CHOP including Sandoz biosimilar rituximab every 2 or 3 weeks for 6–8 cycles.
What did the researchers find?
• A total of 169 patients with DLBCL were included in the study.
• Just over half (52%) were female and the average age was 67 years.
• Nearly 6 out of 10 (59%) patients completed the 2-year study.
• More than 6 out of 10 (65%) patients achieved complete response and 3 out of 10 (30%) achieved partial response.
• The overall response rate was 95%.
• One-year progression-free survival was 85%, and 2-year progression-free survival was 79%.
• Regarding safety, 85% of patients experienced at least one adverse event; just over 3 out of 10 (31%) of these were suspected to be related to the study drug.
What do the findings mean?
• This 2-year study shows that R-CHOP including Sandoz biosimilar rituximab is effective and well tolerated as the first treatment given to patients with DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most frequent form of non-Hodgkin lymphoma among adults, 1 with an annual incidence of seven to eight cases per 100,000 in western countries.2,3 DLBCL is an aggressive malignancy and median survival has been reported to be less than 1 year in untreated patients.4,5
The chemotherapy regimen, comprising rituximab, three chemotherapy agents (cyclophosphamide, doxorubicin, vincristine), and one steroid (prednisone), is the current standard of care for patients with newly diagnosed DLBCL. 6 The cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) regimen has been used for more than 40 years. With the addition of rituximab to CHOP (R-CHOP) approved by the US Food and Drug Administration in 2006 for use in first-line treatment of patients with DLBCL,6,7 50–60% of patients with DLBCL treated with R-CHOP have been reported to achieve cure.6,8
Sandoz rituximab (SDZ-RTX; Rixathon®) received regulatory approval as a rituximab biosimilar in the EU in 2017. 9 In line with the biosimilar approval process, SDZ-RTX is approved for use in the same indications as reference rituximab (MabThera®, Roche Pharmaceuticals), based on the totality of evidence for biosimilarity. SDZ-RTX is, therefore, approved for the treatment of follicular lymphoma (FL) and DLBCL, chronic lymphocytic leukemia (CLL), severe rheumatoid arthritis (RA), granulomatosis with polyangiitis (GMP, formerly Wegener’s granulomatosis), microscopic polyangiitis (MPA), and pemphigus vulgaris (PV). 9
The SDZ-RTX clinical development program, encompassing studies in patients with FL and RA, demonstrated that SDZ-RTX matched reference rituximab on all clinically relevant attributes, in terms of pharmacokinetics, pharmacodynamics, efficacy, safety, and immunogenicity.10–13 However, no patients with DLBCL were involved in these confirmatory studies; REFLECT is the first prospective study of SDZ-RTX in patients with DLBCL. The prospective, multicenter, open-label, non-interventional study was designed to assess the effectiveness and safety of SDZ-RTX administered in combination with CHOP chemotherapy in treatment-naïve patients with CD20-positive DLBCL under real-world conditions.
Materials and methods
REFLECT was a prospective, observational, multicenter, open-label, single-arm, non-interventional study in treatment-naïve, CD20-positive, adult patients with DLBCL. The study was conducted at 79 sites across Germany, initiated in October 2017 and completed in March 2021. Enrollment was concluded in March 2019.
Patients
In accordance with the Rixathon® Summary of Product Characteristics (SmPC), 9 eligible patients were ⩾18 years old, with a confirmed diagnosis of CD20-positive DLBCL. All patients were selected for therapy with SDZ-RTX in combination with CHOP (R-CHOP), as per the treating physician’s discretion. Patients who had received any prior therapy for DLBCL and/or had any contraindications according to the SmPC for SDZ-RTX, including hypersensitivity to the active substance, active severe infections, immunodeficiency, or severe heart failure, were not eligible for inclusion.
Therapy
Patients received chemotherapy cycles of R-CHOP treatment at visits one to six out of eight (R-CHOP14 infused once every 2 weeks or R-CHOP21 infused once every 3 weeks). In this study, commercially available SDZ-RTX and CHOP were prescribed for DLBCL based on the treating physician’s clinical judgment. The decision to treat the patient with SDZ-RTX was independent from the decision to include the patient in this study. As this was an observational study, only data available from routine clinical practice and local standard of care were recorded. The study did not impose any mandatory treatment regimens, mandate a specific visit schedule, nor require any specific assessments to be carried out by the treating physician; these factors were decided by the investigators according to clinical judgment and clinic routine.
Effectiveness and safety
The primary objective of this study was to evaluate the effectiveness of SDZ-RTX, measured by complete response (CR) rate at the end of R-CHOP treatment, as assessed by the treating physician. For patients who discontinued the study early, the last available assessment was included (last observation carried forward). Secondary objectives were to assess the overall response rate (ORR) at the end of treatment, defined as patients with either a CR or partial response (PR), as well as the progression-free survival (PFS) distribution in these patients at 24 months. Treatment effectiveness for response rate was assessed at the end of treatment and as the best overall response.14–16 PFS was defined as the time from the start of R-CHOP treatment to the first documented progression of disease, or relapse, or death due to any cause within the 24-month observational period. The incidences of adverse events (AEs) and serious AEs (SAEs), including adverse drug reactions, were also reported.
In this non-interventional study, the data outlined in Table 1 were entered into an electronic case report form, but only if these parameters were routinely assessed in daily medical practice and were available in the patients’ medical records. The cut-off date for data collection was 31 March 2021.
Table 1.
Data collected throughout the study.
| Baseline | During therapy and 12-month follow-up |
At end of 12-month study observation | During extended observation (months 18 and 24) |
|---|---|---|---|
| • Patient demographics • Physical examination results, including height and weight • Relevant medical history and comorbidities • ECOG PS/Karnofsky index • DLBCL diagnostic characteristics, including biopsy, staging (Ann Arbor), subtyping, morphology, disease symptoms, immunophenotyping, IPI, target lesions • Details of concomitant medication, including premedication for SDZ-RTX administration • Details of SDZ-RTX treatment • Details of CHOP chemotherapy, and any radiotherapy and/or supportive therapy received • Details of any anti-neoplastic surgery received, including date and location and size of target lesion • QoL assessed by patient-reported outcomes collected using the validated questionnaire EORTC QLQ-C30 • Pregnancy status |
• Physical examination results • ECOG PS/Karnofsky index • Details of concomitant medication • Details of SDZ-RTX treatment • Details of CHOP chemotherapy, and any other radiotherapy and/or supportive therapy received • Details of any anti-neoplastic surgery received, including date and location and size of target lesion • Details of response; CR and PR • Details of any AEs and SAEs experienced • QoL assessed by patient-reported outcomes collected using the validated questionnaire EORTC QLQ-C30 (assessed at months 3, 6, 9, and 12) • Pregnancy status |
• ECOG PS/Karnofsky index • Details of concomitant medication • Details of any anti-neoplastic surgery received, including date and location and size of target lesion • Details of response; CR and PR • Details of any AEs and SAEs experienced • Data on the first subsequent anti-neoplastic therapy received following R-CHOP • Reason for study discontinuation • Pregnancy status |
• Details on patient status, including: ○ Survival ○ Progression or relapse ○ Death (disease-related or not) • Details of any SAEs considered by the investigator to be related to SDZ-RTX (AEs/SAEs that are considered related to disease, therapies other than SDZ-RTX etc. are not required to be reported during this extended observation period) • Details of AESI, including serious AESI • Reason for study discontinuation • Pregnancy status |
AE, adverse event; AESI, adverse event of special interest; CR, complete response; DLBCL, diffuse large B-cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30; IPI, International Prognostic Index; PR, partial response; QoL, quality of life; (R)-CHOP, (rituximab with) cyclophosphamide, doxorubicin, vincristine, and prednisone; SAE, serious adverse event; SDZ-RTX, Sandoz rituximab.
Other assessments included DLBCL subtype analysis (germinal-center B-cell like and activated B-cell like) and hepatitis B virus screening.
Data analysis
All analyses were based on the full analysis set (FAS), which included all patients who received at least one dose of R-CHOP. Treatment response was recorded by the investigators within each participating center. The time of enrollment into the study was defined as the point at which a patient signed the informed consent form at V0 (baseline visit). Patients who dropped out for any reason (for example, lost to follow-up, withdrawal, death) were not replaced. No imputation method was planned for in the effectiveness and safety analysis. The missing values are treated as missing at random. All data analyses were performed by the sponsor.
Continuous variables are summarized by number of patients, mean, standard deviation, minimum, median, and maximum; for selected parameters, 25th and 75th percentiles are also presented. Categorical variables are summarized by number of patients and percentages. Due to the nature of the study, the endpoints are descriptive; hence, no formal statistical testing or sample size calculation based on a formal hypothesis test could be performed. However, the sample size was calculated based on precision of point estimate of CR rate; power evaluation was not applicable. For the expected sample size of approximately 180 eligible patients, with the assumption of a CR rate of 60% and exact binomial distribution, the 95% confidence interval (CI) limits for the point estimate of CR rate were ±7.4%.
This study was designed, implemented, and reported in accordance with the Guidelines for Good Pharmacoepidemiology Practices of the International Society for Pharmacoepidemiology and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. 17
Results
Patient characteristics and treatment
The REFLECT study enrolled 184 treatment-naïve, adult patients with DLBCL; 169 patients received at least one dose of R-CHOP and were included in the FAS (see Table 2). The median (range) age in the FAS was 70 (24−94) years, with 72.2% (n = 122/169) of patients ⩾60 years old. There was a slightly higher proportion of females than males enrolled (52.1% versus 47.9%). The majority of patients (80.5%) reported an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0 or 1 at baseline. In terms of Ann Arbor disease staging, 19.5% (n = 33/169) and 24.3% (n = 41/169) of patients reported a score of III and IV, respectively. One-third of patients (33.2%; n = 56) had an International Prognostic Index (IPI) score of ⩾3 at baseline.
Table 2.
Patient baseline characteristics (FAS population).
| Characteristic | All patients |
|---|---|
| N = 169 | |
| Age at baseline (years) | |
| Median | 70.0 |
| Interquartile range | 58.0–78.0 |
| Min–max | 24–94 |
| Age group (years), n (%) | |
| <60 | 47 (27.8) |
| ⩾60 | 122 (72.2) |
| Gender, n (%) | |
| Female | 88 (52.1) |
| Male | 81 (47.9) |
| BMI at baseline (kg/m²) | |
| Median | 25.00 |
| Interquartile range | 23.10–28.40 |
| Min–max | 17.9–50.8 |
| ECOG PS at baseline, n (%) | |
| 0 | 58 (34.3) |
| 1 | 78 (46.2) |
| 2 | 8 (4.7) |
| 3 | 3 (1.8) |
| Missing | 22 (13.0) |
| IPI score, n (%) | |
| 0 (Low risk) | 11 (6.5) |
| 1 (Low risk) | 38 (22.5) |
| 2 (Low-intermediate) | 36 (21.3) |
| 3 (High-intermediate) | 37 (21.9) |
| 4 (High risk) | 17 (10.1) |
| 5 (High risk) | 2 (1.2) |
| Missing | 28 (16.6) |
| Anti-neoplastic surgery at baseline, n (%) | |
| No | 162 (95.9) |
| Yes | 7 (4.1) |
| DLBCL subtype, n (%) | |
| ABC | 7 (4.1) |
| GCB | 54 (32.0) |
| Not done/available | 108 (64.0) |
| Ann Arbor staging, n (%) | |
| I | 45 (26.6) |
| II1 | 35 (20.7) |
| II2 | 13 (7.7) |
| III | 33 (19.5) |
| IV | 41 (24.3) |
| Not available | 2 (1.2) |
ABC, activated B-cell like; BMI, body mass index; BSA, body surface area; DLBCL, diffuse large B-cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group Performance Status; FAS, full analysis set; GCB, germinal-center B-cell like; IPI, International Prognostic Index.
Age was calculated from date of screening and date of birth. BMI was calculated based on raw data measurements.
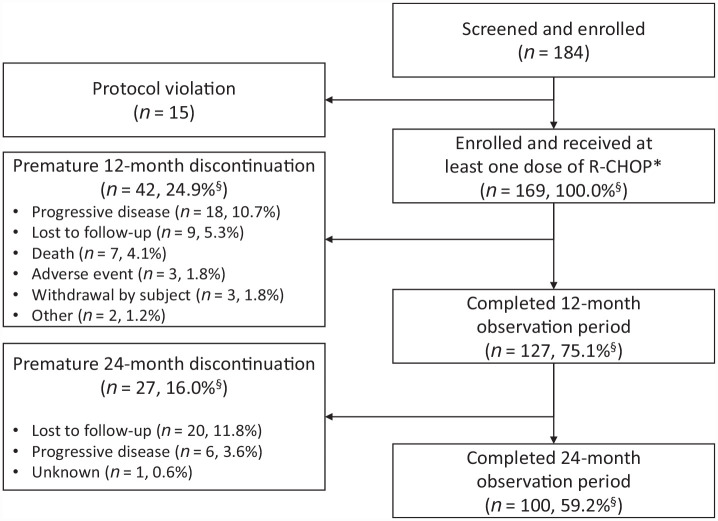
During the study, 75.1% (n = 127) of patients received R-CHOP14 and 24.9% (n = 42) of patients received R-CHOP21. Overall, 24.9% and 4.1% of patients in the FAS discontinued treatment during the follow-up period prior to completion of the 12- and 24-month observation periods, respectively (see Figure 1). The most frequent reason for early discontinuation was progressive disease (10.7% during the 12-month study period and 3.6% during the total 24-month study and extended observation period).
Figure 1.
Patient disposition.
The study was conducted at 79 sites across Germany, initiated in October 2017 and completed in March 2021.
R-CHOP, rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone.
*Full analysis set.
§Percentages are based on N = 169.
Effectiveness
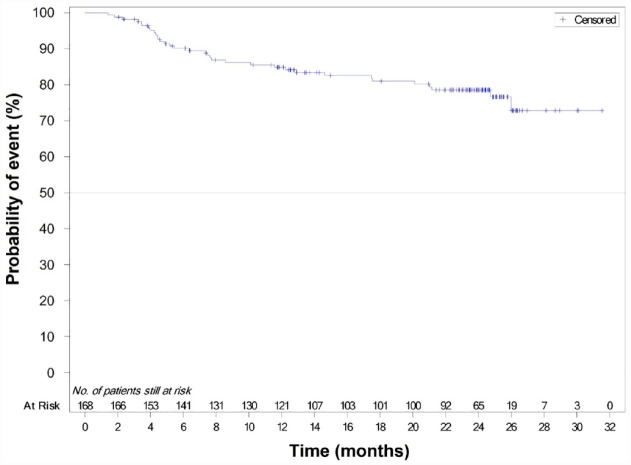
The Kaplan–Meier estimates of 12-, 18-, and 24-month PFS rates were 84.9% (95% CI: 78.2–89.6), 81.0% (95% CI: 73.7–86.4), and 78.5% (95% CI: 70.9–84.4), respectively (see Figure 2). Median PFS was not achieved within the observational period. The evaluation of response at the end of treatment showed an ORR of 89.3% (n = 151/169), with 43.8% (n = 74) of patients achieving CR at the end of treatment and a PR rate of 45.6% (n = 77; see Table 3). According to the best overall response, the ORR was 94.7% (n = 160/169), with 65.1% (n = 110) of patients achieving CR as their best response and 29.6% (n = 50) of patients achieving PR as their best response.
Figure 2.
Kaplan–Meier plot of PFS events (FAS population).
FAS, full analysis set; PFS, progression-free survival.
Table 3.
Summary of response rates (FAS – best overall response and response at end of treatment).
| Type of response to treatment | Best overall response | Response at end of treatment |
|---|---|---|
| All patients N = 169 |
All patients N = 169 |
|
| ORR, n (%) | 160 (94.7) | 151 (89.3) |
| 95% CI for ORR | (90.1–97.5) | (83.7–93.6) |
| CR, n (%) | 110 (65.1) | 74 (43.8) |
| 95% CI for CR | (57.4–72.3) | (36.2–51.6) |
| PR, n (%) | 50 (29.6) | 77 (45.6) |
| 95% CI for PR | (22.8–37.1) | (37.9–53.4) |
| Not available | 0 | 1 (0.6) |
CI, confidence interval; CR, complete response; FAS, full analysis set; ORR, overall response rate; PR, partial response.
ORR = CR + PR.
95% CIs are based on the Clopper–Pearson method.
N is the number of patients in the FAS.
Safety
AEs were reported in 84.6% (n = 143/169) of patients and AEs suspected to be drug-related were reported in 31.4% (n = 53/169; see Table 4). The most common AEs, occurring in >10% of patients, were fatigue (n = 35; 20.7%), anemia (n = 41; 24.3%), polyneuropathy (n = 29; 17.2%), nausea (n = 21; 12.4%), leukopenia (n = 19; 11.2%), and constipation (n = 18; 10.7%). SAEs were reported in 37.3% (n = 63/169) of patients and SAEs suspected to be drug-related were reported in 6.5% (n = 11) of patients. SAEs suspected to be drug-related by Medical Dictionary for Regulatory Activities preferred term were neutropenic sepsis, pneumonia, septic shock, varicella zoster virus infection, hematuria, abdominal pain, erysipelas, tumor lysis syndrome, and peripheral edema. With the exception of pneumonia (three patients) and erysipelas (two patients), any SAEs suspected to be drug-related occurred in only one patient during the study. AEs leading to discontinuation occurred in 13 patients (7.7%) and drug-related AEs leading to discontinuation occurred in three patients (1.8%). AEs requiring dose adjustment or interruption were reported in 24 patients (14.2%). There were eight deaths (4.7%) reported overall, including three deaths (1.8%) during the on-treatment period, defined as within 30 days of the last dose of SDZ-RTX or the last study visit, whichever was later (see Table 4). Seven deaths occurred within the 12-month observation period and one within the 24-month observation period.
Table 4.
Summary of adverse events (FAS population).
| Category, n (%) | All patients |
|---|---|
| N a = 169 | |
| AEs | 143 (84.6) |
| Suspected to be study drug-related | 53 (31.4) |
| SAEs | 63 (37.3) |
| Suspected to be study drug-related | 11 (6.5) |
| AEs leading to discontinuation | 13 (7.7) |
| Suspected to be study drug-related | 3 (1.8) |
| AEs requiring dose interruption and/or change | 24 (14.2) |
| AEs: primary system organ class; preferred term for any events occurring with ⩾10% incidence | |
| Number of subjects with at least one AE | 143 (84.6) |
| General disorders and administration-site conditions | 71 (42.0) |
| Fatigue | 35 (20.7) |
| Mucosal inflammation | 10 (5.9) |
| Pyrexia | 10 (5.9) |
| Blood and lymphatic system disorders | 65 (38.5) |
| Anemia | 41 (24.3) |
| Leukopenia | 19 (11.2) |
| Gastrointestinal disorders | 59 (34.9) |
| Nausea | 21 (12.4) |
| Constipation | 18 (10.7) |
| Nervous system disorders | 57 (33.7) |
| Polyneuropathy | 29 (17.2) |
| Infections and infestations | 50 (29.6) |
| Respiratory, thoracic, and mediastinal disorders | 30 (17.8) |
| Skin and subcutaneous tissue disorders | 25 (14.8) |
| Laboratory investigations | 24 (14.2) |
| All deaths | 8 (4.7 a ) |
| On treatment | 3 (1.8) |
| Septic shock b | 1 (0.6) |
| Pleural effusion | 1 (0.6) |
| Cardiac failure | 1 (0.6) |
| Off treatment | 5 (3.0) |
| Disease progression | 2 (1.2) |
| Pneumonia | 1 (0.6) |
| Enterococcal sepsis | 1 (0.6) |
| Neoplasm progression c | 1 (0.6) |
AE, adverse event; DLBCL, diffuse large B-cell lymphoma; FAS, full analysis set; SAE, serious adverse event.
Categories are not mutually exclusive. Patients with multiple events in the same category are counted only once in that category. Patients with events in more than one category are counted once in each of those categories. On treatment deaths, defined as deaths up to 30 days after the last dose taken of the Sandoz drugs of interest or last visit, whichever is later, are included.
N is the number of patients in the FAS. Percentages are based on N.
This death was reported as treatment-related.
Progression of other malignant neoplasm (non-DLBCL).
Discussion
REFLECT is the first prospective post-approval study to evaluate SDZ-RTX as a curative therapy in treatment-naïve patients with CD20-positive DLBCL. The results reconfirm the expected safety and efficacy profile of SDZ-RTX in combination with CHOP in previously untreated patients with DLBCL treated in a real-world setting. No new safety concerns were observed with SDZ-RTX in this study. In REFLECT, the PFS at 24 months’ extended observation was 78.5%. According to the best overall response, an ORR of 94.7% and CR of 65.1% were observed, and at the end of treatment, an ORR of 89.3% and CR of 43.8% were observed.
The data from REFLECT are comparable with outcomes observed in randomized clinical trials (see Table 5). In a review of trials investigating new frontline therapies for DLBCL management, Mondello and Mian 18 found a 2-year PFS ranging 56–77.6% among patients who received R-CHOP as first-line therapy. Lugtenburg et al. 19 reported a 2-year PFS of 81.5%, with an ORR of 78.0% and a CR/unconfirmed CR of 42% at the end of induction among patients (N = 194) receiving intravenous rituximab plus CHOP, according to Cheson 1999 criteria. 20 A multicenter, randomized R-CHOP dose-intensification study from the United Kingdom (N = 1080; median age 61 years) showed a 2-year PFS of 74.8% in patients receiving R-CHOP21 and 75.4% with R-CHOP14, along with ORRs of 88% and 91% (p = 0.1223), respectively, and CRs/unconfirmed CRs (assessed using the Cheson 1999 criteria) of 63% and 58%. 21 The GOYA study, an international, prospective, open-label, randomized trial of R-CHOP versus CHOP plus obinutuzumab (N = 710; median age 62 years in the R-CHOP group), reported an estimated 5-year PFS of 62.6% for R-CHOP, with an ORR of 77.6% and a CR of 59.1% using positron emission tomography (PET), according to the modified Cheson criteria. 22 In the MabThera International Trial (MInT) Group randomized, unmasked study of R-CHOP-like chemotherapy versus CHOP-like chemotherapy alone (N = 824), patients receiving R-CHOP-like chemotherapy had increased 3-year PFS compared with CHOP-like chemotherapy alone (85% versus 68%; p < 0.0001). A 3-year overall survival benefit was also reported for patients receiving R-CHOP-like chemotherapy versus CHOP-like chemotherapy alone (93% versus 84%; p = 0.0001). Although this study enrolled patients <60 years old, it may be considered relevant for comparison with REFLECT as 27.8% of patients enrolled in REFLECT were also aged <60 years. 23
Table 5.
Summary of studies of rituximab-CHOP in DLBCL.
| Study | Study type | Treatment | Response criteria | N | PFS | ORR | CR/unconfirmed CR |
|---|---|---|---|---|---|---|---|
| Mondello and Mian 18 | Review a | Rituximab plus CHOP (CHOP21 or NR) |
Modified Cheson 2007 criteria or NR | Range 164–370 | Range 56–77.6% at 2 years | NR | NR |
| Lugtenburg et al. 19 | Randomized | Rituximab plus CHOP14 or CHOP21 | Cheson 1999 criteria | 194 | 81.5% at 2 years | 78.0% at end induction | 42% at end induction |
| Cunningham et al. 21 | Randomized | Rituximab plus CHOP14 | Cheson 1999 criteria | 540 | 75.4% at 2 years | 91% at end treatment | 58% at end treatment |
| Rituximab plus CHOP21 | 540 | 74.8% at 2 years | 88% at end treatment | 63% at end treatment | |||
| Sehn et al. 22 | Randomized | Rituximab plus CHOP21 | Modified Cheson 2007 criteria | 710 | 62.6% at 5 years | 77.6% at end treatment | 59.1% at end treatment |
| Pfreundschuh et al. 23 | Randomized | Rituximab plus CHOP-like regimen b | Cheson 1999 criteria | 824 | 85% at 3 years | NR | 86% at day 155 |
| Tilly et al. 24 | Randomized | Rituximab plus CHOP21 | Lugano criteria | 439 | 70.2% at 2 years | 83.8% at end treatment | 74.0% at end treatment |
| Knauf et al. 25 | Observational, prospective | Rituximab plus CHOP14 | NR | 264 | 75.2% at 3 years | 87% c | 59.5% c |
| Rituximab plus CHOP21 | 318 | 79.9% at 3 years | 86% c | 63.5% c | |||
| Lee et al. 26 | Retrospective | Biosimilar rituximab CT-P10 plus CHOP21 | RECIL 2017 | 90 | 74.6% at 1 year | 96.7% c | 86.7% c |
| Rituximab plus CHOP21 | 95 | 79.7% at 1 year | 91.6% c | 84.2% c | |||
| REFLECT | Observational, prospective | Biosimilar rituximab SDZ-RTX plus CHOP14 or CHOP21 | Best overall response |
169 | 78.5% at 2 years | 94.7% d | 65.1% d |
CHOEP, cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete response; DLBCL, diffuse large B-cell lymphoma; MACOP-B, methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin; NR, not reported; ORR, overall response rate; PFS, progression-free survival; PMitCEBO, mitoxantrone, cyclophosphamide, etoposide, bleomycin, vincristine, and prednisone; RECIL, new response evaluation criteria in lymphoma; SDZ-RTX, Sandoz rituximab.
Some studies included had differing patient populations to those in REFLECT.
CHOP21, CHOEP21, MACOP-B, or PMitCEBO.
Timing of assessment not reported.
Best overall response.
The data from REFLECT are also comparable with the outcomes reported in two other real-world studies of patients with DLBCL treated with R-CHOP. The first, a German, prospective study of patients enrolled from 2009 to 2014 (N = 582; median age 68 years), showed a 3-year PFS of 75.2% with R-CHOP14 and 79.9% with R-CHOP21, an ORR of 87% for R-CHOP14 and 86% for R-CHOP21, and unconfirmed CRs of 59.5% and 63.5% for R-CHOP14 and R-CHOP21, respectively; 25 however, this real-world evidence study had no specified criteria for tumor assessment.
Somewhat limited data are available on the use of rituximab biosimilars in DLBCL. The second real-world study includes biosimilar rituximab CT-P10 (Celltrion) in combination with CHOP in patients with DLBCL. 26 In this Korean, single-center, retrospective study, the real-world effectiveness and safety of first-line treatment with the biosimilar rituximab CT-P10 (n = 90) and reference rituximab (n = 95) were compared in patients with DLBCL enrolled from 2016 to 2018. One-year PFS was 74.6% with CT-P10 and 79.7% with reference rituximab; median PFS was not reached in either group. According to the response criteria adopted in this study, ORRs of 96.7% and 91.6%, CRs of 86.7% and 84.2%, and PRs of 10.0% and 7.4% were reported with CT-P10 and reference rituximab, respectively. 26 In addition, a prospective study with limited comparability due to the selection of patients with a good prognosis is reported, 27 and several small studies have been carried out investigating the use of rituximab biosimilars licensed outside of the EU in DLBCL, including China, India, and Turkey.28–31
In REFLECT, R-CHOP therapy achieved similar results for PFS rate (78.5%) and ORR (94.7%, according to best overall response) to those reported in other studies, although CR results differ from study to study as shown in Table 5. This difference in CR rate may be partially explained by variation in study design and patient population. For example, although baseline IPI, ECOG PS, and disease staging were similar between the two biosimilar trial study populations, the population age differed, with a higher median age and wider range for the REFLECT study population compared with that of the Truxima study [70 years (range 24–94) versus 63.5 (range 55–71), respectively]. 26
The REFLECT study has several limitations common to all real-world studies. As a result of the observational nature of this study, clinic visits did not take place at fixed time points for all study participants, and radiologic responses were not available for all visits; this may have introduced variation in how quickly disease progression was identified. In addition, there was no central radiographic assessment as evaluations were performed by the treating physicians at each center. Consequently, computerized tomography and ultrasound imaging were used to assess radiological response in this study, rather than PET scans; this may have impacted the sensitivity and specificity of CR analysis.32,33 Another potential limitation is that the selected sample may not reflect the entire patient population accurately, and selection bias cannot be fully excluded due to the observational study design. Although this study was limited to centers using SDZ-RTX, attempts were made to enroll a variety of centers based on center size and academic affiliation (i.e. academic centers as well as academic-affiliated and non-academic centers). The German clinical research organization appointed by the study sponsor issued a broad invitation to a variety of centers outlining the selection criteria. Despite this effort, the majority of centers involved in this study were medical clinics rather than academic-affiliated hospitals, which may have introduced selection bias as these centers may have identified more patients at an earlier stage in their disease through screening programs. The recruitment of homogeneous patient populations in clinical DLBCL trials represents a significant challenge. Therefore, the differences in results between trials may also be partially attributable to the significant variability in treatment response within a DLBCL population, related mainly to both the pathological and microenvironmental characteristics associated, outlined recently by Wright et al. 34 The quality of therapy alongside supportive medication and care may also be a contributing factor. A final limitation of REFLECT is that – as a result of study design – data can only be interpreted in an observational manner and therefore, no formal statistical analyses can be performed.
In terms of the general safety and tolerability of SDZ-RTX in combination with CHOP, there were no new safety signals shown in the REFLECT study. AEs were reported in 143 patients (84.6%) and AEs suspected to be treatment-related were reported in 53 patients (31.4%). SAEs were reported in 63 patients (37.3%) and SAEs suspected to be treatment-related were reported in 11 patients (6.5%).
The safety findings shown in REFLECT are comparable with rates reported in previous studies. At least one AE occurred in 94.0% of patients (age ⩾18 years) in the R-CHOP group in the recent GOYA study from Sehn et al. 22 and SAEs were reported in 38.4% of patients. Similarly, Lugtenburg et al. 19 reported AEs in 90.4% of patients and SAEs in 33.0% of patients aged 18–80 years, treated with R-CHOP, whereas Delarue et al. 35 reported AEs in 76.0% of patients and SAEs in 49.2% of patients aged 60–80 years. In the Korean, single-center study of CT-P10 in DLBCL, 46.7% of patients experienced AEs of grade ⩾3 receiving CT-P10-CHOP, compared with 42.1% of patients receiving reference rituximab-CHOP. 26
In conclusion, REFLECT is the first prospective post-approval study of Sandoz rituximab (SDZ-RTX; Rixathon®) in combination with CHOP as a curative therapy in treatment-naïve patients with CD20-positive DLBCL. The results reconfirm the expected safety and efficacy profile of R-CHOP in patients with DLBCL treated in a real-world setting. The data support the use of Sandoz rituximab (SDZ-RTX; Rixathon®) as an effective component of R-CHOP in the first-line treatment of DLBCL. Moreover, these results of a new rituximab biosimilar may help to broaden patient access to rituximab-based chemotherapy and support the sustainability of cancer care.
Supplemental Material
Supplemental material, sj-docx-1-tah-10.1177_20406207231183765 for REFLECT: prospective multicenter non-interventional study evaluating the effectiveness and safety of Sandoz rituximab (SDZ-RTX; Rixathon®) in combination with CHOP for the treatment of patients with previously untreated CD20-positive diffuse large B-cell lymphoma by Manfred Welslau, Boris Kubuschok, Julian Topaly, Burkhard Otremba, Thomas Wolff and Galyna Bryn in Therapeutic Advances in Hematology
Acknowledgments
Medical writing and editorial support for all drafts of the article was provided by Sarah Lambert and Caroline McGown of Titan, OPEN Health Communications, supported by Hexal AG, a Sandoz company. The listed authors have authorized the submission of their article via a third party and approved any statements or declarations, for example, conflicting interests, funding, and so on. Factual review and proof-reading were provided by Ines Brueckmann of Hexal AG, a Sandoz company. The authors would like to thank the investigators and patients who participated in the REFLECT study.
Footnotes
ORCID iD: Manfred Welslau  https://orcid.org/0000-0002-3103-0312
https://orcid.org/0000-0002-3103-0312
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Manfred Welslau, Department of Oncology, Klinikum Aschaffenburg, Am Hasenkopf 1, 63739 Aschaffenburg, Germany.
Boris Kubuschok, Department of Hematology and Oncology, Augsburg University Medical Centre, Augsburg, Germany.
Julian Topaly, Klinik für Hämatologie und Onkologie, CaritasKlinikum Saarbrücken, Saarbrücken, Germany.
Burkhard Otremba, Onkologische Praxis Oldenburg, Oldenburg, Germany.
Thomas Wolff, OncoResearch Lerchenfeld GmbH, Hamburg, Germany.
Galyna Bryn, Sandoz, Holzkirchen, Germany.
Declarations
Ethics approval and consent to participate: This study was conducted in accordance with applicable Good Clinical Practice guidelines and with the ethical principles laid down in the Declaration of Helsinki. All patients provided written informed consent prior to entry into the study. Patients who participated in the extended observation period (24 months) re-consented. Ethics approval for the study was obtained from the following ethics committees: Landesärztekammer Baden-Württemberg Ethik-Kommission, Sächsische Landesärztekammer – Ethik-Kommission, Ärztekammer Hamburg – Ethik-Kommission, Ethik-Kommission der Ärztekammer Nordrhein, Carl von Ossietzky Universität Oldenburg, Medizinische Ethik-Kommission, Ethik-Kommission der Ärztekammer Westfalen-Lippe und der Westfälischen Wilhelms-Universität Münster, Ethik-Kommission bei der Ärztekammer Niedersachsen, Ethik-Kommission des Landes Sachsen-Anhalt, Geschäftsstelle Dessau, Ethik-Kommission der Universität zu Lübeck, and Ethik-Kommission bei der Landesärztekammer Rheinland-Pfalz.
Consent for publication: Not applicable.
Author contributions: Manfred Welslau: Conceptualization; Formal analysis; Investigation; Methodology; Writing – review & editing.
Boris Kubuschok: Conceptualization; Formal analysis; Investigation; Methodology; Writing – review & editing.
Julian Topaly: Conceptualization; Formal analysis; Investigation; Methodology; Writing – review & editing.
Burkhard Otremba: Conceptualization; Formal analysis; Investigation; Methodology; Writing – review & editing.
Thomas Wolff: Conceptualization; Formal analysis; Investigation; Methodology; Writing – review & editing.
Galyna Bryn: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The REFLECT study is funded by Hexal AG, a Sandoz company.
Competing interests: BO has received honoraria from Hexal AG, a Sandoz company, for lectures and advisory boards. BK has received honoraria and travel support from Roche and Novartis. GB is an employee of Hexal AG, a Sandoz company. MW, JT, and TW have nothing to disclose.
Availability of data and materials: Not applicable.
References
- 1.Tilly H, Da Silva MG, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015; 26(Suppl. 5): v116–v125. [DOI] [PubMed] [Google Scholar]
- 2.Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood 2006; 107: 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith A, Howell D, Patmore R, et al. Incidence of haematological malignancy by sub-type: a report from the haematological malignancy research network. Br J Cancer 2011; 105: 1684–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koff JL, Flowers CR.Prognostic modeling in DLBCL in the era of immunochemotherapy: where do we go from here? Cancer 2017; 123: 3222–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rovira J, Valera A, Colomo L, et al. Prognosis of patients with diffuse large B cell lymphoma not reaching complete response or relapsing after frontline chemotherapy or immunochemotherapy. Ann Hematol 2015; 94: 803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Li LR, Young KH.New agents and regimens for diffuse large B cell lymphoma. J Hematol Oncol 2020; 13: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration. Drugs@FDA: FDA-approved drugs. Rituxan, https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=103705 (accessed 3 January 2022).
- 8.Liu Y, Barta SK.Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol 2019; 94: 604–616. [DOI] [PubMed] [Google Scholar]
- 9.European Medicines Agency. Rixathon® summary of product characteristics, https://www.ema.europa.eu/en/medicines/human/EPAR/rixathon (2021, accessed 26 May 2022).
- 10.Jurczak W, Zinzani PL, Gaidano G, et al. A phase III efficacy and safety study of the proposed rituximab biosimilar GP2013 versus rituximab in patients with previously untreated advanced follicular lymphoma (abstract no. 1809). In: ASH annual meeting, San Diego, CA, 3–6December2016. [Google Scholar]
- 11.Smolen JS, Cohen SB, Tony HP, et al. Efficacy and safety of Sandoz biosimilar rituximab for active rheumatoid arthritis: 52-week results from the randomized controlled ASSIST-RA trial. Rheumatology 2021; 60: 256–262. [DOI] [PubMed] [Google Scholar]
- 12.Da Silva A, Kronthaler U, Koppenburg V, et al. Target-directed development and preclinical characterization of the proposed biosimilar rituximab GP2013. Leuk Lymphoma 2014; 55: 1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visser J, Feuerstein I, Stangler T, et al. Physicochemical and functional comparability between the proposed biosimilar rituximab GP2013 and originator rituximab. BioDrugs 2013; 27: 495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Younes A, Hilden P, Coiffier B, et al. International working group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann Oncol 2017; 28: 1436–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–586. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline; (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 17.Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–349. [DOI] [PubMed] [Google Scholar]
- 18.Mondello P, Mian M.Frontline treatment of diffuse large B-cell lymphoma: beyond R-CHOP. Hematol Oncol 2019; 37: 333–344. [DOI] [PubMed] [Google Scholar]
- 19.Lugtenburg P, Avivi I, Berenschot H, et al. Efficacy and safety of subcutaneous and intravenous rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in first-line diffuse large B-cell lymphoma: the randomized MabEase study. Haematologica 2017; 102: 1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol 1999; 17: 1244. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet 2013; 381: 1817–1826. [DOI] [PubMed] [Google Scholar]
- 22.Sehn LH, Martelli M, Trneˇný M, et al. A randomized, open-label, phase III study of obinutuzumab or rituximab plus CHOP in patients with previously untreated diffuse large B-cell lymphoma: final analysis of GOYA. J Hematol Oncol 2020; 13: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfreundschuh M, Trümper L, Österborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006; 7: 379–391. [DOI] [PubMed] [Google Scholar]
- 24.Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med 2022; 386: 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knauf W, Abenhardt W, Mohm J, et al. Similar effectiveness of R-CHOP-14 and -21 in diffuse large B-cell lymphoma – data from the prospective German Tumour Registry lymphatic neoplasms. Eur J Haematol 2019; 103: 460–471. [DOI] [PubMed] [Google Scholar]
- 26.Lee K, Ha JL, Jung AR, et al. The clinical outcomes of rituximab biosimilar CTP10 (Truxima®) with CHOP as first-line treatment for patients with diffuse large B-cell lymphoma: real-world experience. Leuk Lymphoma 2020; 61: 1575–1583. [DOI] [PubMed] [Google Scholar]
- 27.Candelaria M, González DE, Delamain MT, et al. Rituximab biosimilar RTXM83 versus reference rituximab in combination with CHOP as first-line treatment for diffuse large B-cell lymphoma: a randomized, double-blind study. Leuk Lymphoma 2019; 60: 3375–3385. [DOI] [PubMed] [Google Scholar]
- 28.Ozbalak M, Mastanzade M, Ozluk O, et al. R-CHOP chemotherapy including biosimilar rituximab (Redditux®) for de-novo diffuse large B-cell lymphoma patients: real-life single center experience. Blood 2021; 138: 4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Y, Song Y, Qin Y, et al. First China approved rituximab biosimilar HLX01: pharmacokinetics, safety and efficacy comparison to reference rituximab in the phase 3 diffuse large B-cell lymphoma study. Blood 2019; 134: 2878. [Google Scholar]
- 30.Visawabandya A, Mukhopadhyay A, Shah S, et al. Comparison of pharmacokinetics and pharmacodynamics of two anti-CD20 monoclonal antibodies (candidate biosimilar DRL-rituximab and innovator reference product rituximab (Mabthera®) in a randomised, multi-centre, double-blind, parallel group study of CHOP with rituximab chemotherapy in patients with CD20-positive diffuse large B-cell lymphoma. Blood 2016; 128: 5391. [Google Scholar]
- 31.Florez A, Matteo TD, Fresnillo G, et al. Clinical pharmacokinetic (PK) and safety (immunogenicity) of rituximab biosimilar RTXM83 in combination with chemotherapy CHOP in patients with diffuse large B-cell lymphoma (DLBCL). Blood 2014; 124: 5472. [Google Scholar]
- 32.Cheson BD, Meignan M.Current role of functional imaging in the management of lymphoma. Curr Oncol Rep 2020; 23: 144. [DOI] [PubMed] [Google Scholar]
- 33.Strati P, Amin Ahmed M, Fowler NH, et al. Pre-treatment maximum standardized uptake value predicts outcome after frontline therapy in patients with advanced stage follicular lymphoma. Haematologica 2020; 105: 1907–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright GW, Huang DW, Phelan JD, et al. A probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer Cell 2020; 37: 551–568.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delarue R, Tilly H, Mounier N, et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomized phase 3 trial. Lancet Oncol 2013; 14: 525–533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tah-10.1177_20406207231183765 for REFLECT: prospective multicenter non-interventional study evaluating the effectiveness and safety of Sandoz rituximab (SDZ-RTX; Rixathon®) in combination with CHOP for the treatment of patients with previously untreated CD20-positive diffuse large B-cell lymphoma by Manfred Welslau, Boris Kubuschok, Julian Topaly, Burkhard Otremba, Thomas Wolff and Galyna Bryn in Therapeutic Advances in Hematology