Abstract
Lithium (Li) induces severe polyuria and polydipsia in up to 40% of patients undergoing Li treatment. In rats, Li treatment induces a reversible cellular remodeling of the collecting duct (CD), decreasing the fraction of principal-to-intercalated cells. To investigate the potential role of adherens junction proteins, we performed immunohistochemistry on kidney cross-sections from rats treated with Li as well as rats undergoing recovery on a normal diet following 4 weeks of Li-treatment. We performed immunoelectron microscopy on cryosections to determine the ultrastructural localizations. Immunohistochemistry showed that E-cadherin and β-catenin were present in both the lateral and basal plasma membrane domains of CD cells. Immunoelectron microscopy confirmed that β-catenin was localized both to the lateral and the basal plasma membrane. The basal localization of both proteins was absent from a fraction of mainly principal cells after 10 and 15 days of Li-treatment. After 4 weeks of Li-treatment few to no cells were absent of E-cadherin and β-catenin at the basal plasma membrane. After 12 and 19 days of recovery some cells exhibited an absence of basal localization of both proteins. Thus, the observed localizational changes of E-cadherin and β-catenin appear before the cellular remodeling during both development and recovery from Li-NDI:
Keywords: adherens junction, cellular remodeling, collecting duct, diabetes insipidus, lithium
Introduction
Nephrogenic diabetes insipidus (NDI) is a disorder characterized by the inability of the kidney to regulate and maintain the internal fluid homeostasis, causing polyuria and subsequently polydipsia by disrupting the hormone-regulated water reabsorption occurring in the kidney collecting duct (CD). Lithium (Li) enters the CD principal cells through the epithelial sodium channel (ENaC). 1 Li cations are taken up by the principal cells through a concentration gradient and accumulate intracellularly, due to a higher permeability of ENaC for Li than for sodium in addition to a lower affinity of Li than that of sodium to the Na,K-ATPase.2–5 However, it is believed that some cellular efflux of Li occurs through the Na/H exchanger-1 (NHE1).6,7 Li-NDI in rats is characterized by a cellular remodeling of the CD. The fraction of intercalated cells is markedly increased, whereas the fraction of principal cells is decreased. 8 This occurs gradually during NDI development starting at 10 days of treatment in parallel with increasing polyuria and decreased AQP2 levels. 9 After 4 weeks of lithium treatment, long rows of intercalated cells appear. 8 The cellular remodeling has been shown to be reversible after 19 days of recovery on a Li-free diet. 10 Previous proteomic studies have indicated that cell-contact proteins in CD cells are affected by Li treatment in rats, which is consistent with a role of cell contacts in the cellular remodeling.11,12 Potentially, principal cells may be extruded by loss of their attachment to the basement membrane. A similar phenomenon has been observed in the removal of intercalated cells in the developing kidney. 13 Moreover, dilated extracellular spaces between the basal plasma membrane of principal cells and the basement membrane have been documented in Li treated rats. 14 Two proteins associated with adherens junctions are E-cadherin and β-catenin and the abundance of both proteins is increased in the inner medullary CD (IMCD) in response to 2 weeks of Li treatment in rats. 12
Adherens junctions are cell–cell contact complexes necessary for intercellular adhesion and the structural integrity of the epithelium. The adherens junction complex is located just beneath the tight junction complex. It is typically composed of classical cadherins (type I), anchoring the actin filaments between cells. Classical cadherins are calcium-dependent cell adhesion molecules. 15 E-cadherin is a transmembrane protein with its extracellular domain being composed of five repetitive subdomains called cadherin repeats with each repeat exhibiting binding sites for calcium cations. 15 The binding of Ca2+ induces a conformational change between the subdomains rendering the molecule capable of interaction with the extracellular domain of E-cadherin of an adjacent cell. 16 On its cytoplasmic domain, E-cadherin binds to β-catenin and p120 catenin. 17 β-catenin in turn binds α-catenin as the linkage to the actin filaments. In addition to functioning as a linker molecule between cadherins and the actin cytoskeleton, β-catenin has an essential role in the canonical Wnt-signaling pathway. The Wnt-signaling pathway is important in embryogenesis and regeneration in adult tissue, through its influence on cellular proliferation, cell migration, differentiation, and the epithelial-mesenchymal transition (EMT).18,19 When β-catenin is not a constituent of the adherens junction its stability is highly dependent of the Wnt-signaling pathway. Activation of the pathway recruits constituents of a proteasomal degradational complex to the Wnt receptor,20,21 ensuring cytoplasmic stability of β-catenin and its safe translocation to the nucleus and subsequent transcriptional activation of Wnt target genes. 22 However, in the absence of active Wnt-signaling cytoplasmic β-catenin is phosphorylated,23,24 leading to its ubiquitinylation and subsequent degradation.25,26 In addition, the Notch-signaling pathway has been shown to be important for maintaining a principal cell differentiation profile of cells in the CD27,28 and to regulate the Wnt/β-catenin signaling pathway.29,30 Decreased or compromised Notch-signaling has been demonstrated to induce changes in cellular composition of the murine CD during development, 31 in hypokalemic mice, and in ADAM10 KO mice.32,33 ADAM10 is a metalloproteinase important for the initiation of Notch-signaling by extracellular cleavage of the Notch receptor and has been documented to cleave E-cadherin extracellularly as well. 34
As cell–cell contact constituents, E-cadherin and β-catenin have been described to be expressed in the basolateral membrane domains of renal distal tubules and the collecting duct.35–37 However, often no distinction are made between the lateral and basal domains. Importantly, the composition of the cellular adhesion complexes in these domains is distinct. The cellular adhesion between the cell and the basal lamina is ensured by the focal adhesions and hemidesmosomes constituted by proteins of the integrin superfamily. At this localization, the function of E-cadherin and β-catenin is to our knowledge still unknown. In this study, we investigated the cellular localization of E-cadherin and β-catenin in rat kidney during NDI development and recovery.
Materials and Methods
Experimental Animals
In this study, we have used paraffin-embedded kidney blocks from two previously published animal studies (Fig. 19,10). The previously published animal protocols were approved by the Animal Experiments Inspectorate, Denmark. In the first study, rats were fed 40 mmol LiCl/kg dryfood for the first 7 days of Li-treatment followed by a diet containing 60 mmol LiCl/kg dryfood for the remaining treatment period. 9 Rats were fed Li for 4 days (n=3); 10 days (n=3); and 15 days (n=3). In the second study (recovery), rats were fed LiCl for 28 days (40 mmol LiCl/kg dryfood for the first 7 days and 60 mmol LiCl/kg dryfood for the last 21 days) followed by 0 (n=5); 6 (n=4); 12 (n=4); 19 (n=5); or 28 days (n=5) on a normal diet (Fig. 1 10 ). For electron microscopy, ultrathin cryosections from the proximal part of the inner medulla (IM-1) were cut from three control rats perfusion-fixed with 4% paraformaldehyde (PFA) in 0.1 M cacodylate.
Figure 1.
Experimental setup. Paraffin-embedded sections from previously published Li and recovery studies were used.9,10 Short-term Li protocols; control rats and rats treated with Li for 4, 10, and 15 days. Recovery protocols; control rats and rats treated with Li for 28 days followed by recovery on a Li-free diet for 0, 6, 12, 19, and 28 days.
Immunohistochemistry
For single labeling, paraffin-embedded sections (2 µm) were blocked with 0.3% H2O2 in methanol for 30 min followed by antigen retrieval in TEG-buffer. The sections were treated with 50 mM NH4Cl in phosphate-buffered saline (PBS) for 30 min followed by washing with 1% bovine serum albumin (BSA), 0.2% gelatin, 0.05% saponin in PBS. The sections were incubated with either anti-E-cadherin antibody (610181, BD Biosciences, Franklin Lakes, NJ, dilution 1:3000, 1:12,000 or 1:24,000, the higher dilutions was due to increased efficacy of newly bought antibody), anti-Notch1 (sc-376403, Santa Cruz Biotechnology, Dallas, TX, dilution 1:75), anti-β-catenin (sc-7199, Santa Cruz Biotechnology, dilution 1:500), or anti-β-catenin (sc-7963, Santa Cruz Biotechnology, dilution 1:400) at 4°C overnight. For cell counting with the anti-E-cadherin antibody, the dilution of 1:12,000 was used in the recovery protocol, and 1:3000 was used in the Li induction protocol. Next day, sections were rinsed in 0.1% BSA, 0.2% gelatin, 0.05% saponin in PBS, and incubated with corresponding horseradish peroxidase-conjugated secondary antibodies (P0448 or P0447 at dilution 1:200, DAKO, Glostrup, Denmark) for 1 hr. Labeling was detected with 3,3′-Diaminobenzidine tetrahydrochloride (DAB) and sections were counterstained with Mayers Hematoxylin. Negative controls for E-cadherin and β-catenin were performed using isotype-specific mouse immunoglobulins in identical IgG concentrations; IgG1 (X0931, Agilent-Dako A/S, Glostrup, Denmark, control for anti-β-catenin, sc-7963) and IgG2a (X943, Agilent-Dako A/S, control for anti-E-cadherin [610181] [Appendix Fig. A1]). To further confirm the specificity of the E-cadherin (610181) antibody, labeling with two other E-cadherin antibodies (PA5-85088, Invitrogen, Waltham, MA, dilution 1:500 and LS-B12414, Lifespan Biosciences, Lynnwood, WA, dilution 1:1800) was performed (Appendix Fig. A2).
For immunoflourescent labeling, sections were incubated with anti-AQP4 (249-323, Alomone Labs, Jerusalem, Israel, dilution 1:1000), biotinylated anti-H+-ATPase (H7659, 9 dilution 1:100) and either anti-E-cadherin (610181, dilution 1:6000) or anti-β-catenin (sc-7963, dilution 1:100) overnight for triple labeling. For double labeling sections were incubated overnight with anti-β-catenin (sc-7199, dilution 1:150) and anti-E-cadherin (610181, dilution 1:3000). Labeling was detected by Alexa flour-conjugated secondary antibodies (donkey anti-mouse 488 [A21202] and donkey anti-rabbit 555 [A31572], Invitrogen, dilution 1:1000) and (Streptavidin-633 [S21375], Invitrogen, dilution 1:600) for triple labeling and (donkey anti-mouse 555 [A31570] and donkey anti-rabbit 488 [A21206], Invitrogen, dilution 1:1000) for double labeling. In addition, double staining with anti-E-cadherin (610181) and anti-β-catenin (sc-7199) of the proximal tubules in the renal cortex, was also used as a negative control for E-cadherin labeling in accordance with previous studies in rats and humans36,38 (Appendix Fig. A3). The immunoflourescence stainings were analyzed using a Leica TCS SP2 confocal laser scanning system with a Leica DMIRE2 inverted microscope. Images were generated at a 400-Hz scanning speed with an 63× oil objective. In general, qualitative analysis was performed on whole kidney sections. Collecting ducts representative of the IM-1 were selected and imaged.
Immunoelectron Microscopy
Ultrathin cryosections of kidney inner medulla (60 nm) from rats perfusion fixed with 4% PFA in 0.1 M cacodylate were incubated with anti-β-catenin (sc-7963, dilution 1:200) in 0.01 M PBS with 0.1% BSA overnight at 4°C. Next day, sections were incubated with 10-nm gold-conjugated goat anti-mouse (GaM10, BBI Solutions, Crumlin, United Kingdom) secondary antibody at dilution 1:50 for 2 hrs at 4°C. The sections were counterstained with 0.4% uranyl acetate in 1.8% methocel for 10 min. The analysis of the stained cryosections was performed using a JEM-1400 Plus Electron Microscope (JEOL Ltd., Tokyo, Japan).
Cell Culture and Immunoblotting
To further assess antibody specificity, the antibodies were tested using a stable mouse cortical collecting duct cell line (mCCDcl1) 39 Cells were washed and scraped in cold PBS with protease- (Mini cOmplete, Hoffmann-La Roche, Basel, Switzerland) and phosphatase inhibitors (phosSTOP, Hoffmann-La Roche). Homogenization was performed with a Ultrasonic Homogenizer (BioLogics Inc., Cary, North Carolina) and 4× Laemmli’s Sample Buffer with 0.39 M Dithiothreitol (DTT) was added to the cell lysates. SDS-PAGE was performed on Mini-Protean 4–15% TGX precast gels (Bio-Rad Laboratories, Hercules, California) and proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories). Membranes were probed with anti-E-cadherin (610181, dilution 1:4000), β-catenin (sc-7199, dilution 1:1000), and Notch-1 (sc-376403, dilution 1:100) (Appendix Fig. A4).
Cell Counting
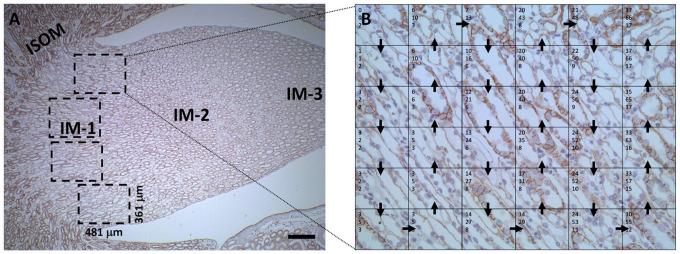
Manual cell counting was performed as described by Bologna-Molina et al. 40 Four images were taken per animal at IM-1 using a 25× objective as indicated in Appendix Fig. A5.
Each image covered an area of 481 µm × 361 µm. For blinding purposes a third-party unaware of the research imported all images to Microsoft Office PowerPoint in a random sequence. A 6 × 6 grid table was placed on top of each image and cell counting proceeded from the uppermost left grid-frame to the uppermost right frame (Appendix Fig. A5). Only CDs with an open lumen and CD cells with visible nuclei were counted. The counted cells were categorized as either positive (definite labeling of E-cadherin at the basal plasma membrane domain), negative (no labeling present at the basal plasma membrane domain), or inconclusive (not possible to either confirm or deny labeling at the basal plasma membrane domain). The data were calculated as the fraction of CD cells in IM-1 with or without basal E-cadherin labeling. The average number of IM-1 CD cells counted per animal ± SD was as following: 4 days Li: experimental (exp.) 358 ± 37 (n=3) and control (con.) 362 ± 84 (n=3); 10 days Li: exp. 357 ± 44 (n=3) and con. 263 ± 40 (n=3); 15 days Li: exp. 397 ± 101 (n=3) and con. 344 ± 113 (n=3); 0 days recovery: exp. 442 ± 125 (n=5) and con. 264 ± 38 (n=4); 6 days recovery: exp. 504 ± 101 (n=5) and con. 363 ± 151 (n=2); 12 days recovery: exp. 669 ± 153 (n=5) and con. 352 ± 87 (n=5); 19 days recovery: exp. 504 ± 95 (n=5) and con. 329 ± 54 (n=5); and 28 days recovery: exp. 371 ± 112 (n=5) and con. 278 ± 62 (n=5).
Results
Localization of E-Cadherin and β-Catenin in the Lateral and Basal Plasma Membrane of Collecting Duct Cells
Immunohistochemistry on kidney sections from control rats showed that E-cadherin and β-catenin were localized in both lateral and basal plasma membrane domains of CD cells in IM-1 (Fig. 2A and B). To confirm whether this localization was at the plasma membrane, immuno electron microscopy was performed for β-catenin. Labeling was observed in the lateral plasma membrane corresponding to adherens junctions and in more distal part of the lateral membrane as well as in the basal plasma membrane of CD cells (Fig. 2C, arrows).
Figure 2.

E-cadherin and β-catenin are present in both the lateral and basal plasma membrane of inner medullary CD cells. Immunohistochemistry of paraffin-embedded kidney sections from control rats labeled for E-cadherin or β-catenin in the proximal part of inner medulla (IM-1, A and B). Both proteins are predominantly localized to the basolateral plasma membrane domain of the IM-1 CD cells (A and B). Scale bar: 50 µm. Kidney cryosections for transmission electron microscopy were incubated with anti-β-catenin, and 10-nm gold-conjugated secondary antibody. β-catenin are localized to the basal (C, arrows, scale bar: 200 nm) and lateral plasma membranes (C, inset, arrows, scale bar: 100 nm). Abbreviations: AJ, adherens junction; BM, basement membrane; CD, collecting duct; TJ, tight junction.
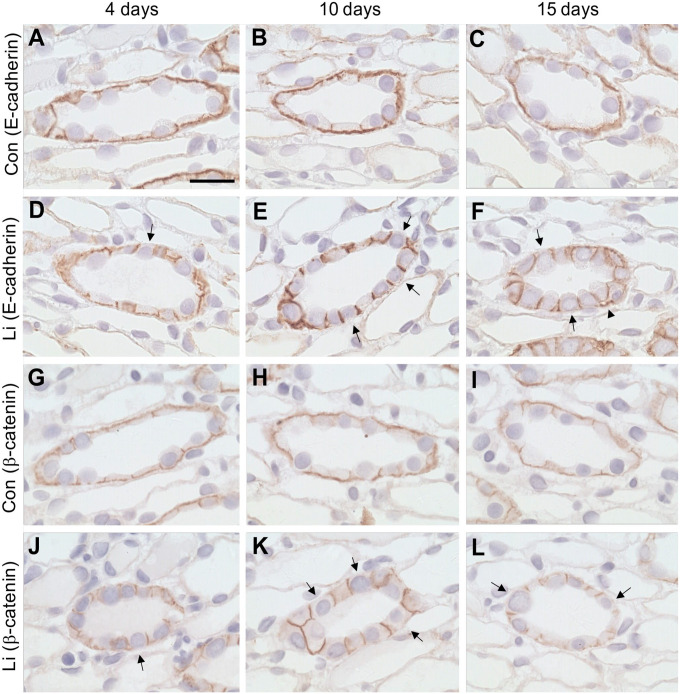
E-cadherin and β-catenin Labeling in the Basal Membrane Is Absent From Many CD Cells During Development of Li-NDI
In control rats, E-cadherin and β-catenin labeling was observed in the lateral and basal plasma membrane domains of CD cells as described above (Fig. 3A-C and G-I). Cells with no or very weak basal labeling were occasionally seen after 4 days Li (Fig. 3D and J, arrows). Cellular counting on E-cadherin labeled sections confirmed the results (Table 1). After 10 and 15 days of Li treatment, the amount of cells with no basal labeling was increased compared with the controls (Fig. 3E-F and K-L, arrows, Table 1). Many IM-1 CD cells were taller with the labeling at the lateral plasma membrane domain being more noticeable. In addition, dilated extracellular spaces at the basal plasma membrane domains were observed after 15 days of Li treatment (Fig. 3F, arrowheads).
Figure 3.
Cells without basal E-cadherin and β-catenin labeling appears at 10 days Li. Immunohistochemistry of paraffin-embedded kidney sections from control rats and rats treated with Li for 4, 10, and 15 days. Sections were incubated with anti-E-cadherin (A-F) or anti-β-catenin (G-I). In the proximal part of IM, no apparent difference in the amount of cells absent of basal E-cadherin or β-catenin labeling was observed after 4 days of Li treatment (A, D, G, J). In contrast, after 10 and 15 days of Li treatment, a noticeable increase in cells absent of the two proteins in the basal plasma membrane domains were observed, when compared with controls (B-C, E-F, H-I, K-L, arrows). In addition, dilated extracellular spaces at the basal plasma membrane domains were observed after 15 days of Li treatment (F, arrowheads). Scale bar: 20 µm.
Table 1.
E-Cadherin Labeling at the Basal Plasma Membrane of CD Cells in IM-1 by Manual Cell Counting.
| Animal (n) | Positive fraction | Negative fraction | Inconclusive fraction |
|---|---|---|---|
| 4 days of Li diet | |||
| Con (3) | 0.86 ± 0.04 | 0.05 ± 0.02 | 0.09 ± 0.03 |
| Li (3) | 0.79 ± 0.04 | 0.09 ± 0.03 | 0.12 ± 0.01 |
| 10 days of Li diet | |||
| Con (3) | 0.91 ± 0.04 | 0.03 ± 0.01 | 0.06 ± 0.04 |
| Li (3) | 0.65 ± 0.04** | 0.24 ± 0.06** | 0.11 ± 0.02 |
| 15 days of Li diet | |||
| Con (3) | 0.92 ± 0.05 | 0.03 ± 0.01 | 0.05 ± 0.03 |
| Li (3) | 0.81 ± 0.07 | 0.07 ± 0.03* | 0.12 ± 0.05 |
| 4 weeks of Li diet | |||
| Con (4) | 0.92 ± 0.06 | 0.03 ± 0.03 | 0.05 ± 0.04 |
| Li (5) | 0.95 ± 0.06 | 0.02 ± 0.02 | 0.04 ± 0.04 |
| 6 days recovery a | |||
| Con (2) | 0.92 ± 0.03 | 0.01 ± 0.02 | 0.07 ± 0.02 |
| Li (5) | 0.91 ± 0.06 | 0.03 ± 0.02 | 0.06 ± 0.04 |
| 12 days recovery | |||
| Con (5) | 0.89 ± 0.02 | 0.03 ± 0.01 | 0.08 ± 0.03 |
| Li (5) | 0.85 ± 0.08 | 0.05 ± 0.04 | 0.10 ± 0.04 |
| 19 days recovery | |||
| Con (5) | 0.89 ± 0.05 | 0.05 ± 0.02 | 0.06 ± 0.02 |
| Li (5) | 0.85 ± 0.04 | 0.07 ± 0.03 | 0.08 ± 0.02 |
| 28 days recovery | |||
| Con (5) | 0.91 ± 0.03 | 0.04 ± 0.02 | 0.05 ± 0.01 |
| Li (5) | 0.90 ± 0.03 | 0.05 ± 0.03 | 0.05 ± 0.01 |
Fraction values are given as means ± SD. Abbreviations: CD, collecting duct; IM-1, inner medulla-1; Negative fraction, no labeling present at the basal plasma membrane domain; Inconclusive fraction, not possible to either confirm or deny labeling at the basal plasma membrane domain; Positive fraction, definite labeling of E-cadherin at the basal plasma membrane domain.
No statistical test was performed, as control group only included two animals.
p<0.05, **p<0.01.
E-cadherin and β-catenin Are Absent From the Basal Membrane of Some CD Cells During Recovery From Li-NDI
After 28 days of Li (0 days recovery), few-to-no CD cells exhibited absence of E-cadherin and β-catenin at the basal plasma membrane domain (Figs. 4A, B and 5A, B). Dilated extracellular spaces were observed (Fig. 4B, arrowheads). After 12 and 19 days of recovery, some cells with no labeling of β-catenin and E-cadherin in the basal plasma membrane domain were observed again (Figs. 4E, H and 5E, H, arrows) although not statistically significant and not to the same extent as during Li treatment (Table 1). After 28 days of recovery, occasionally, cells were identified without basal labeling of both proteins (Figs. 4J and 5J, arrows).
Figure 4.
Cells without basal E-cadherin labeling are not observed after 0 days recovery, but are observable to some extent after 12 days of recovery. Immunohistochemistry of kidney sections from control rats and rats treated with Li for 28 days followed by recovery for 0, 6, 12, 19, and 28 days. Sections were incubated with anti-E-cadherin. After 0 days of recovery, no change in the amount of cells absent of basal E-cadherin was observed in the proximal part of IM (A and B). After 12 and 19 days of recovery, absence of E-cadherin at the basal plasma membrane domains was seen in some cells (E-F, G-H, arrows). After 28 days of recovery, cells absent of basal E-cadherin were occasionally observed (I-J, arrows). Dilated extracellular spaces were observed after 0 days of recovery (B, arrowheads). Scale bar: 20 µm. Abbreviation: IM-1, inner medulla-1.
Figure 5.
Cells without basal β-catenin labeling are not observed after 0 days recovery, but are observable to some extent after 12 days of recovery. Immunohistochemistry of kidney sections from control rats and rats treated with Li for 28 days followed by recovery for 0, 6, 12, 19, and 28 days. Sections were incubated with anti-β-catenin. After 0 days recovery, no change in amount of cells absent of basal β-catenin was observed in IM-1 (A and B). After 12 and 19 days of recovery, absence of β-catenin at the basal plasma membrane was seen in some cells (E-H, arrows). After 28 days of recovery, cells absent of basal β-catenin were occasionally observed (I-J, arrows). Scale bar: 20 µm. Abbreviation: IM-1, inner medulla-1.
β-Catenin and E-Cadherin Are Absent From the Basal Membrane of the Same Principal Cells
To determine if the absence of E-cadherin and β-catenin in the basal plasma membrane domain was celltype specific, we performed a triple immunofluorescent labeling using anti-E-cadherin or anti-β-catenin with anti-AQP4 and anti-H+-ATPase, as markers for principal cells or intercalated cells, respectively. After 10 days of Li treatment, almost all cells exhibiting the absence of E-cadherin labeling at the basal plasma membrane were positive for AQP4 and negative for H+-ATPase (Fig. 6A-F, arrowheads). Cells positive for the H+-ATPase rarely exhibited a weak-to-no labeling of E-cadherin (Fig. 6F, asterisk). After 12 days of recovery, cells absent of E-cadherin were positive for AQP4 expression (Fig. 6G-L, arrowheads). Similarly, cells absent of β-catenin labeling at the basal plasma membrane after 10 days of Li treatment and during recovery were positive for AQP4 (Fig. 7A-L, arrowheads). As for E-cadherin labeling, only very few cells positive for the H+-ATPase exhibited the absence of basal β-catenin labeling (not shown). In accordance with the previous peroxidase stainings, few CD cells exhibited the absence of E-cadherin and β-catenin in the controls (Figs. 6B and 7B and H, arrows).
Figure 6.
E-cadherin is absent primarily in AQP4-positive cells. Triple immunofluorescent labeling of E-cadherin (green), AQP4 (red) and H+-ATPase (blue) on kidney sections from control rats and rats after 10 days Li and after 12 days recovery. 10 days Li induced many cells absent of basal E-cadherin labeling compared with controls (B, E, arrows). Almost all of these cells were AQP4-positive (C, F, arrowheads). Only very few H+-ATPase-positive cells were absent of basal E-cadherin (F, asterisk). After 12 days of recovery, an increase in cells absent of basal E-cadherin was observed when compared with controls (H, K, arrows). These cells were also AQP4-positive (I, L, arrowheads). Scale bar: 30 µm.
Figure 7.
Mainly AQP4-positive cells are absent for β-catenin. Triple immunofluorescent labeling of or β-catenin (green), AQP4 (red) and H+-ATPase (blue) on kidney sections from control rats and rats treated with Li for 10 days and rats under recovery for 12 days. Li treatment for 10 days resulted in more cells absent of β-catenin when compared with controls (B, E, arrows). Almost all of these cells were AQP4-positive (C, F, arrowheads). After 12 days of recovery, cells without basal β-catenin was increased compared with controls (H, K, arrows). These cells also exhibited labeling of AQP4 (I, L, arrowheads). Scale bar: 30 µm.
As E-cadherin and β-catenin localization seemed to be affected in a similar manner by Li treatment, a double immunofluorescent staining with anti-E-cadherin and anti-β-catenin antibodies were performed. E-cadherin and β-catenin colocalized to the plasma membrane domains of the same cells in both controls and Li-treated animals (Fig. 8A and B). After 10 days of Li treatment, the CD cells of the IM-1 with the absence of E-cadherin at the basal plasma membrane domain were concurrently absent of β-catenin and cells absent of β-catenin were also absent of E-cadherin (Fig. 8B, arrows). This was also the case for the few CD cells absent of basal labeling in the controls (Fig. 8A).
Figure 8.
E-cadherin and β-catenin are absent from the basal plasma membrane of the same cells. Double immunofluorescent labeling of β-catenin (green) and E-cadherin (red) on sections from control rats and rats treated with Li for 10 days. β-catenin and E-cadherin labeling colocalizes at basal and lateral plasma membrane domain of CD cells in the proximal part of IM (yellow). CD cells absent of E-cadherin are simultaneously absent of β-catenin and vice versa in both controls (A, arrows) and in rats treated with Li for 10 days (B, arrows). Scale bar: 30 µm. Abbreviations: CD, collecting duct; IM, inner medulla.
Notch-1 Labeling Is Decreased During NDI Development
To further elucidate a potential causal mechanism of the cellular remodeling, we examined whether Notch-signaling is affected during Li-treatment. Immunohistochemical labeling using antibodies against the intracellular domain of Notch-1 revealed a predominantly cytoplasmic and apical labeling in CD cells of control rats (Fig. 9A, C and D). We observed no difference in localization or apparent expression levels in IM-1 of rats treated with Li for 4 days when compared with controls (Fig. 9A and B). In contrast, after 10 days of Li treatment, CD cells showed a decrease in cytoplasmic and apical labeling intensity when compared with controls (Fig. 9C and D). After 15 days of Li treatment, no apparent difference in overall labeling intensity was observed (Fig. 9E and F). However, some CD cells showed mainly apical labeling compared with cytoplasmic labeling (Fig. 9F).
Figure 9.
A decrease in Notch-1 was observed after 10 days of Li-treatment. Immunohistochemistry of paraffin-embedded kidney sections from control rats and rats treated with Li for 4, 10, and 15 days. Sections were incubated with anti-Notch-1. Labeling was observed in the collecting duct cells of the IM, predominantly localized in the cytoplasm, but was also observed at the apical membrane domains. No changes were observed in the localization, nor the amount of labeling after 4 days of Li treatment (A and B). After 10 days of Li treatment, a decrease in labeling intensity was observed in many CDs of the IM-1 when compared with controls (C and D). At 15 days of Li treatment, no apparent changes in labeling intensity of the CDs were observed (E and F). However, in some CD cells the labeling was mainly observed in the apical plasma membrane domains (Fig. 9F). Scale bar: 20 µm. Abbreviations: CD, collecting duct; IM-1, inner medulla-1.
Discussion
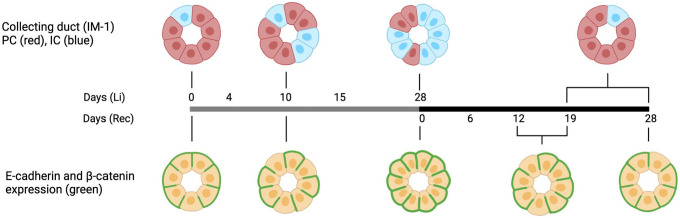
Li induces a cellular remodeling of the rat kidney CD. After 10 and 15 days of Li treatment, an increased intercalated cell density and cellular proliferation were observed, 9 and after 4 weeks of Li treatment, the total amount of cells of the IMCD was increased while long rows of ICs appeared and the fraction of principal cells was decreased 10 (Fig. 10, upper panel). After 19 and 28 days of recovery following 4 weeks of Li treatment, the intercalated cell density was reduced toward control levels, and this was not associated with cellular proliferation 10 (Fig. 10, upper panel). In this study, we have demonstrated that many cells exhibit absence of E-cadherin and β-catenin labeling at the basal plasma membrane at 10 days of Li treatment (Fig. 10, lower panel). Interestingly, almost all IM-1 CD cells exhibited basal labeling of E-cadherin and β-catenin at the same time as an evident remodeling had occurred after 4 weeks of Li-treatment. Thus, the disappearance of E-cadherin and β-catenin in the basal plasma membrane during the development of NDI may trigger the loss of cell contact with the extracellular matrix leading to hyperplasia and cellular remodeling. During the recovery period, there was a slight increase although to a lesser extent than during Li treatment in cells without basal labeling of the two adherens junction proteins (Fig. 10).
Figure 10.
Basal expression of E-cadherin and β-catenin is absent from CD cells before the cellular remodeling. Illustrative timepoint representation of the cellular remodeling and absence of basal expression of E-cadherin and β-catenin. The cellular remodeling with an increase in intercalated cell density is observed to occur gradually from 10 days of Li treatment until 28 days of Li treatment (left upper panel8,9). In addition, the cellular remodeling is reversed toward control levels after 19–28 days of recovery (right upper panel 10 ). Our observations in this study suggest that a fraction of CD cells (primarily principal cells) exhibit absence of the adherens junction proteins, E-cadherin and β-catenin at the basal plasma membrane at 10 days, which is just before the major changes in Li induced cellular remodeling (lower panel, green labeling). In contrast, at 28 days of Li, basal labeling was present in the majority of cells. Furthermore, a small fraction of cells exhibiting absence of basal E-cadherin and β-catenin are observed after 12–19 days of recovery just before reversal of the cellular composition to control levels. Illustration was created with BioRender.com. Abbreviations: CD, collecting duct; IC, intercalated cell; PC, principal cell.
E-cadherin and β-catenin Are Both Absent in Primarily Principal Cells During Development of Li-NDI and During Recovery
We show that mainly AQP4-positive cells exhibit the absence of basal E-cadherin and β-catenin after 10 days of Li. This may support the hypothesis that Li has an effect on cellular adhesion between the principal cells and the basement membrane.9,11 Dilated extracellular spaces in the CD has been observed in this and a previous study. 14 Disruption of cell-adhesions may lead to cellular detachment of principal cells during the development of NDI. A similar event has been seen in the developing kidney, where intercalated cells are removed due to the extrusion of the cells by loss of their cellular attachment to the basement membrane. 13 Thus, there may be an increased turnover of the principal cells as these cells are in a proliferating state followed by extrusion and loss in the urine. However, the potential cellular detachment of cells via loss of E-cadherin and β-catenin, may be contradicted by the still evident lateral labeling observed after Li. Furthermore, the role of E-cadherin and β-catenin in the basal plasma membrane is still unknown. Despite the reduced fraction of principal cells, Li increased the amount of principal cells positive for a proliferation marker Proliferating Cell Nuclear Antigen (PCNA). 9 PCNA is abundant in the S phase of the cell cycle. This can also be explained partly by an arrest of principal cells in the G2 phase of the cell cycle not allowing the cells to enter mitosis. 41
The pattern with absence of basal E-cadherin and β-catenin primarily in principal cells was observed to a lesser extent after 12 days of recovery suggesting that this phenomenon may play a minor role in the recovery phase compared with the induction phase. During recovery, the fraction of intercalated cells decreases toward control levels again. If cellular detachment of intercalated cells through E-cadherin and β-catenin loss is part of the pathophysiology, we would expect a larger fraction of intercalated cells loosing basal presence of E-cadherin and β-catenin during recovery. An additional hypothesis of the cellular remodeling has been proposed to be due to cellular interconversion. 9 This is supported by observations of novel cell types in the CD displaying both intercalated and principal cell markers after Li-treatment and recovery.10,42 In addition, both Type A and Type B intercalated cells have previously been demonstrated to be derived from AQP2-positive cells in the developing kidney. 43 The loss of basal labeling and cellular detachment could potentially occur during a transition from one cell type to another. The transition may also occur via the Wnt-signaling pathway where β-catenin translocation is an essential step. Both the Wnt- and Notch-signaling pathways are potential determinants of cell fates. Downregulation or inactivation of Notch has been associated with a cellular remodeling of the outer medullary CD under hypokalemic nephropathy and of the medullary CD in nephrogenic diabetes insipidus via Hes1.31,32 In addition, in Hes1 knock-out animals, an increased density of intercalated cells of the outer medullary CD was shown to derive from principal cells. 27 In this study, we observed a decrease in labeling of Notch-1 in the CDs of IM-1 after 10 days of Li-treatment, which may suggest a decrease in Notch-signaling and could be indicative of the inability of the CD cells to maintain a principal cell profile, as previously described to be the case with loss of Notch-signaling in the kidney.27,28 The high fraction of PCs normally found in the CD is believed to be achieved by Notch-signaling transduced through lateral inhibition, where activation of Notch-signaling in neighboring cells inhibit IC differentiation and maintain a primarily PC differentiation profile of the CD. 31 Interestingly, the decreased Notch-1 labeling was observed in whole CD tubules and not only in a subset of CD cells within a tubule, proposing the loss of lateral inhibition as having a potential causative role in the cellular remodeling. In addition, Notch-signaling is known to have the potential to limit Wnt-signaling and promote a Notch-ON/Wnt-OFF signaling state by limiting transcriptional activity of β-catenin, through its degradation by an endocytic pathway or by nuclear interactions between the intracellular domain, Notch Intracellular Domain (NICD), and β-catenin. 29 The decreased Notch-1 labeling correlates at a timepoint of Li-treatment that exhibits a high fraction of cells with absence of basal membrane domain labeling of β-catenin. However, we have not yet observed any changes in cytoplasmic or nuclear levels of β-catenin during Li-treatment indicating influence on the Wnt-pathway. Importantly, Notch-signaling in the CD can also be effectuated through the Notch-2 receptor, and further investigation into Notch-2 expression and expressional patterns of Notch ligands, that is, Jagged-1 and Delta-like-1, is necessary to conclude upon the potential role of Notch-signaling in the cellular remodeling of the CD during Li-treatment.
The Role of E-Cadherin and β-Catenin at the Basal Plasma Membrane
The ultrastructural localization of β-catenin was observed at the lateral plasma membranes just beneath the tight junctions corresponding to the adherens junctions and also at the basal plasma membrane. We were not able to perform immunoelectron microscopy for E-cadherin. However, since E-cadherin and β-catenin colocalize at the basolateral plasma membrane domains at light microscopic level, it is very likely that they also colocalize at the ultrastructural level. Previous studies have shown E-cadherin and β-catenin localized to basolateral plasma membrane domains although the function in the basal domain is not known.12,36 The localization is consistent with previous observations of the presence of E-cadherin in the basal plasma membrane of CD cells. 37
We demonstrate that E-cadherin and β-catenin proteins colocalize at the basolateral plasma membrane domains of the same cells in the IMCD, and that the cells absent of E-cadherin labeling at their basal plasma membrane are simultaneously absent of β-catenin and vice versa. These results suggest that E-cadherin and β-catenin are interacting in a complex at this subcellular localization. This is consistent with previous studies, demonstrating that β-catenin translocate to the cytoplasm, when E-cadherin is either degraded following endocytosis or cleaved either extracellularly or intracellularly.34,44 Cell-Extracellular Matrix (ECM) contacts at the basal plasma membrane are hemidesmosomes and focal adhesions. E-cadherin and β-catenin are not known to be part of these complexes. However, it cannot be ruled out to be the case. Neither, can it be ruled out that the complex is part of yet unknown cellular-ECM contact. A protein of the cadherin family has previously been shown to interact with integrins at the basal plasma membrane. 45
The basal membrane may also act as a reservoir for E-cadherin and β-catenin. A previous study show an elevated E-cadherin and β-catenin expression after 1 and 2 weeks of Li. 12 In addition, the CD cells become taller, thus increasing the visibility of their lateral membranes after Li treatment. The increased number of cells and their taller morphology could explain the elevated expression levels of both proteins, as more cell–cell contacts would be expected to be necessary for epithelial integrity. The taller morphology while loss of basal labeling could be indicative of transport, for example, by transcytosis or via the plasma membrane of proteins between the lateral and basal plasma membrane with the basal membrane acting as a reservoir for E-cadherin and β-catenin complexes ready for adherens junction formation. Interestingly, we see no apparent accumulation of β-catenin at the cytoplasm or nuclei of IM-1 CD cells, when absence of basal labeling of both proteins is observed. After 4 weeks of Li treatment, we see that the two proteins are present again at both the basal and lateral membranes. This is regardless of taller cell morphology, which would be consistent with the increased expression of the two proteins. 12 Thus, there may be a refilling of the basal reservoir to compensate for the translocation of the proteins to the adherens junctions in the lateral membrane during Li treatment.
The understanding of how the cellular remodeling occurs is not only of importance for revealing the underlying pathological pathway of Li-NDI but also could potentially reveal mechanisms of cellular plasticity that could be occurring in other pathological pathways. Cellular remodeling usually occurs in wound healing, allergic reactions, infections, and cancer—including tumor invasiveness. In addition, investigating what role the cell–cell contact proteins E-cadherin and β-catenin have in the basal plasma membrane domains in single cell layer epithelia like that of the CD could have implications on the basic cell biological understanding of these adherens junction complexes and their relationship with cell-ECM complexes.
Acknowledgments
We thank for technical assistance and sharing of expertise by Inger Merete S. Paulsen on immunohistochemistry for light and confocal microscopy, Inger Kristoffersen for helping with the handling of the electron microscope and Hanne Sidelmann for immunohistochemistry on cryosections for TEM, Golsha Ayoubi for technical assistance with immunoblotting, and Tina Drejer for helping with cell cultures.
Appendix
Figure A1.
Isotype specific IgG controls. Immunohistochemistry using anti-E-cadherin (610181, IgG2, A) antibodies and corresponding IgG2 control, (B). In the lower panels show labeling with anti-β-catenin (sc-7963, IgG1, C) and corresponding IgG1 controls. Both control sections (B and D) are negative supporting the specificity of the antibodies. Scale bar: 50 µm.
Figure A2.

Double immunofluorescent labeling of β-catenin (sc-7199, green) and E-cadherin (610181, red) on kidney cortex sections from control rats. The two proteins colocalize in the cortical collecting duct (CCD), but not in the proximal tubule (PT). β-catenin is observed (arrowheads) in the proximal tubule, whereas E-cadherin is not. This in accordance with previous studies (Prozialeck et al, 36 Tsuchiya et al 38 ). This supports the specificity of the antibodies. Scale bar: 20 µm.
Figure A3.
Immunohistochemistry using three different antibodies for E-cadherin. The antibodies (610181, panel A; PA5-85088, panel B; and LS-B12414, panel C) were tested on control kidney sections. All three antibodies show labeling in the basolateral plasma membrane. This supports the specificity of the E-cadherin antibody. Scale bar: 20 µm.
Figure A4.

Immunoblotting showing the specificity of (A) E-cadherin (610181), (B) β-catenin (c-7199), and (C) Notch-1 (sc376403) using a mouse cortical collecting duct cell line (mCCDcl1). The E-cadherin antibody recognizes a full-length band at 120 kDa and a cleaved band at 35 kDa (Marambaud et al 44 ). The β-catenin antibody recognizes a band at ~85–90 kDa as expected (Uniprot.org). The Notch-1 antibody recognizes a band at ~110–120 kDa corresponding to a cleaved transmembrane intracellular fragment and a full length ~250–300 kDa (Logeat et al 46 ).
Figure A5.
Manual cell counting procedure. The fraction of cells with and without basal E-cadherin labeling were calculated in the upper part of inner medulla (IM-1). Four images were taken per animal using a 25× objective. Each image covered an area of 481 µm × 361 µm. For blinding purposes a third-party unaware of the research imported all images to Microsoft Office PowerPoint in a random sequence. A 6 × 6 grid table was placed on top of each image and cell counting proceeded from the uppermost left grid-frame to the uppermost right frame. Scale bar: 250 µm. Abbreviation: ISOM, Inner Stripe of Outer Medulla.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: XS contributed to study design; performed immunohistochemistry; light, confocal, and electron microscopic analysis and imaging; figure creation; and manuscript writing. RN carried out the staining for TEM and helped with the electron microscopic analysis. JP contributed with confocal microscopy and image analysis. BMC carried out study design; contributed to microscopic analysis for light, confocal and electron microscopy; contributed to manuscript writing. All authors have read and approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Helen and Ejnar Bjørnows Foundation and the A.P. Moeller Foundation.
ORCID iDs: Jeppe Praetorius  https://orcid.org/0000-0001-6109-247X
https://orcid.org/0000-0001-6109-247X
Birgitte M. Christensen  https://orcid.org/0000-0002-6140-4629
https://orcid.org/0000-0002-6140-4629
Contributor Information
Xabier Sørtvedt, Department of Biomedicine, Aarhus University, Aarhus, Denmark.
Rikke Nielsen, Department of Biomedicine, Aarhus University, Aarhus, Denmark.
Jeppe Praetorius, Department of Biomedicine, Aarhus University, Aarhus, Denmark.
Birgitte M. Christensen, Department of Biomedicine, Aarhus University, Aarhus, Denmark.
Literature Cited
- 1.Christensen BM, Zuber AM, Loffing J, Stehle JC, Deen PM, Rossier BC, Hummler E.AlphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol. 2011;22(2):253–61. doi: 10.1681/ASN.2010070734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kellenberger S, Hoffmann-Pochon N, Gautschi I, Schneeberger E, Schild L.On the molecular basis of ion permeation in the epithelial Na+ channel. J Gen Physiol. 1999;114(1):13–30. doi: 10.1085/jgp.114.1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer LG, Frindt G.Conductance and gating of epithelial Na channels from rat cortical collecting tubule. Effects of luminal Na and Li. J Gen Physiol. 1988;92(1):121–38. doi: 10.1085/jgp.92.1.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunham PB, Senyk O.Lithium efflux through the Na/K pump in human erythrocytes. Proc Natl Acad Sci U S A. 1977;74(7):3099–103. doi: 10.1073/pnas.74.7.3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kortenoeven ML, Li Y, Shaw S, Gaeggeler HP, Rossier BC, Wetzels JF, Deen PM.Amiloride blocks lithium entry through the sodium channel thereby attenuating the resultant nephrogenic diabetes insipidus. Kidney Int. 2009;76(1):44–53. doi: 10.1038/ki.2009.91 [DOI] [PubMed] [Google Scholar]
- 6.Thomsen K, Shirley DG.A hypothesis linking sodium and lithium reabsorption in the distal nephron. Nephrol Dial Transplant. 2006;21(4):869–80. doi: 10.1093/ndt/gfk029 [DOI] [PubMed] [Google Scholar]
- 7.Alsady M, Baumgarten R, Deen PM, de Groot T.Lithium in the kidney: friend and foe? J Am Soc Nephrol. 2016;27(6):1587–95. doi: 10.1681/ASN.2015080907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen BM, Marples D, Kim YH, Wang W, Frøkiaer J, Nielsen S.Changes in cellular composition of kidney collecting duct cells in rats with lithium-induced NDI. Am J Physiol Cell Physiol. 2004;286(4):C952–64. doi: 10.1152/ajpcell.00266.2003 [DOI] [PubMed] [Google Scholar]
- 9.Christensen BM, Kim YH, Kwon TH, Nielsen S.Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol. 2006;291(1):F39–48. doi: 10.1152/ajprenal.00383.2005 [DOI] [PubMed] [Google Scholar]
- 10.Trepiccione F, Capasso G, Nielsen S, Christensen BM.Evaluation of cellular plasticity in the collecting duct during recovery from lithium-induced nephrogenic diabetes insipidus. Am J Physiol Renal Physiol. 2013;305(6):F919–29. doi: 10.1152/ajprenal.00152.2012 [DOI] [PubMed] [Google Scholar]
- 11.Trepiccione F, Pisitkun T, Hoffert JD, Poulsen SB, Capasso G, Nielsen S, Knepper MA, Fenton RA, Christensen BM.Early targets of lithium in rat kidney inner medullary collecting duct include p38 and ERK1/2. Kidney Int. 2014;86(4):757–67. doi: 10.1038/ki.2014.107 [DOI] [PubMed] [Google Scholar]
- 12.Nielsen J, Hoffert JD, Knepper MA, Agre P, Nielsen S, Fenton RA.Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci U S A. 2008;105(9):3634–9. doi: 10.1073/pnas.0800001105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Cha JH, Tisher CC, Madsen KM.Role of apoptotic and nonapoptotic cell death in removal of intercalated cells from developing rat kidney. Am J Physiol. 1996;270(4 Pt 2):F575–92. doi: 10.1152/ajprenal.1996.270.4.F575 [DOI] [PubMed] [Google Scholar]
- 14.Laursen UH, Pihakaski-Maunsbach K, Kwon TH, Østergaard Jensen E, Nielsen S, Maunsbach AB.Changes of rat kidney AQP2 and Na, K-ATPase mRNA expression in lithium-induced nephrogenic diabetes insipidus. Nephron Exp Nephrol. 2004;97(1):e1–16. doi: 10.1159/000077593 [DOI] [PubMed] [Google Scholar]
- 15.Meng W, Takeichi M.Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol. 2009;1(6):a002899. doi: 10.1101/cshperspect.a002899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pokutta S, Herrenknecht K, Kemler R, Engel J.Conformational changes of the recombinant extracellular domain of E-cadherin upon calcium binding. Eur J Biochem. 1994;223(3):1019–26. doi: 10.1111/j.1432-1033.1994.tb19080.x [DOI] [PubMed] [Google Scholar]
- 17.Ozawa M, Baribault H, Kemler R.The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989;8(6):1711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heuberger J, Birchmeier W.Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2(2):a002915. doi: 10.1101/cshperspect.a002915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clevers H.Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–80. doi: 10.1016/j.cell.2006.10.018 [DOI] [PubMed] [Google Scholar]
- 20.Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X.A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438(7069):873–7. doi: 10.1038/nature04185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D.Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7(4):801–9. doi: 10.1016/s1097-2765(01)00224-6 [DOI] [PubMed] [Google Scholar]
- 22.Eastman Q, Grosschedl R.Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr Opin Cell Biol. 1999;11(2):233–40. doi: 10.1016/s0955-0674(99)80031-3 [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X.Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108(6):837–47. doi: 10.1016/s0092-8674(02)00685-2 [DOI] [PubMed] [Google Scholar]
- 24.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A.Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17(5):1371–84. doi: 10.1093/emboj/17.5.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latres E, Chiaur DS, Pagano M.The human F box protein beta-Trcp associates with the Cul1/Skp1 complex and regulates the stability of beta-catenin. Oncogene. 1999;18(4):849–54. doi: 10.1038/sj.onc.1202653 [DOI] [PubMed] [Google Scholar]
- 26.Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, Polakis P.The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol. 1999;9(4):207–10. doi: 10.1016/s0960-9822(99)80091-8 [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee M, deRiso J, Otterpohl K, Ratnayake I, Kota D, Ahrenkiel P, Chandrasekar I, Surendran K.Endogenous notch signaling in adult kidneys maintains segment-specific epithelial cell types of the distal tubules and collecting ducts to ensure water homeostasis. J Am Soc Nephrol. 2019;30(1):110–26. doi: 10.1681/ASN.2018040440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grassmeyer J, Mukherjee M, deRiso J, Hettinger C, Bailey M, Sinha S, Visvader JE, Zhao H, Fogarty E, Surendran K.Elf5 is a principal cell lineage specific transcription factor in the kidney that contributes to Aqp2 and Avpr2 gene expression. Dev Biol. 2017;424(1):77–89. doi: 10.1016/j.ydbio.2017.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acar A, Hidalgo- Sastre A, Leverentz MK, Mills CG, Woodcock S, Baron M, Collu GM, Brennan K.Inhibition of Wnt signalling by Notch via two distinct mechanisms. Sci Rep. 2021;11(1):9096. doi: 10.1038/s41598-021-88618-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishiguro H, Okubo T, Kuwabara Y, Kimura M, Mitsui A, Sugito N, Ogawa R, Katada T, Tanaka T, Shiozaki M, Mizoguchi K, Samoto Y, Matsuo Y, Takahashi H, Takiguchi S.NOTCH1 activates the Wnt/beta-catenin signaling pathway in colon cancer. Oncotarget. 2017;8(36):60378–89. doi: 10.18632/oncotarget.19534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeong HW, Jeon US, Koo BK, Kim WY, Im SK, Shin J, Cho Y, Kim J, Kong YY.Inactivation of Notch signaling in the renal collecting duct causes nephrogenic diabetes insipidus in mice. J Clin Invest. 2009;119(11):3290–300. doi: 10.1172/JCI38416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iervolino A, Prosperi F, De La Motte LR, Petrillo F, Spagnuolo M, D’Acierno M, Siccardi S, Perna AF, Christensen BM, Frische S, Capasso G, Trepiccione F.Potassium depletion induces cellular conversion in the outer medullary collecting duct altering Notch signaling pathway. Sci Rep. 2020;10(1):5708. doi: 10.1038/s41598-020-61882-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo Q, Wang Y, Tripathi P, Manda KR, Mukherjee M, Chaklader M, Austin PF, Surendran K, Chen F.Adam10 mediates the choice between principal cells and intercalated cells in the kidney. J Am Soc Nephrol. 2015;26(1):149–59. doi: 10.1681/ASN.2013070764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, de Strooper B, Hartmann D, Saftig P.ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci U S A. 2005;102(26):9182–7. doi: 10.1073/pnas.0500918102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terada N, Karim MR, Izawa T, Kuwamura M, Yamate J.Immunolocalization of beta-catenin, E-cadherin and N-cadherin in neonate and adult rat kidney. J Vet Med Sci. 2017;79(11):1785–90. doi: 10.1292/jvms.17-0439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prozialeck WC, Lamar PC, Appelt DM.Differential expression of E-cadherin, N-cadherin and beta-catenin in proximal and distal segments of the rat nephron. BMC Physiol. 2004;4:10. doi: 10.1186/1472-6793-4-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SY, Han SM, Kim JE, Chung KY, Han KH.Expression of E-cadherin in pig kidney. J Vet Sci. 2013;14(4):381–6. doi: 10.4142/jvs.2013.14.4.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuchiya B, Sato Y, Kameya T, Okayasu I, Mukai K.Differential expression of N-cadherin and E-cadherin in normal human tissues. Arch Histol Cytol. 2006;69(2):135–45. doi: 10.1679/aohc.69.135 [DOI] [PubMed] [Google Scholar]
- 39.Thomsen ML, Gronkjaer C, Iervolino A, Rej S, Trepiccione F, Christensen BM.Atorvastatin does not ameliorate nephrogenic diabetes insipidus induced by lithium or potassium depletion in mice. Physiol Rep. 2021;9(21):e15111. doi: 10.14814/phy2.15111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bologna-Molina R, Damian-Matsumura P, Molina-Frechero N.An easy cell counting method for immunohistochemistry that does not use an image analysis program. Histopathology. 2011;59(4):801–3. doi: 10.1111/j.1365-2559.2011.03954.x [DOI] [PubMed] [Google Scholar]
- 41.de Groot T, Alsady M, Jaklofsky M, Otte-Höller I, Baumgarten R, Giles RH, Deen PM.Lithium causes G2 arrest of renal principal cells. J Am Soc Nephrol. 2014;25(3):501–10. doi: 10.1681/ASN.2013090988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Himmel NJ, Wang Y, Rodriguez DA, Sun MA, Blount MA.Chronic lithium treatment induces novel patterns of pendrin localization and expression. Am J Physiol Renal Physiol. 2018;315(2):F313–22. doi: 10.1152/ajprenal.00065.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu H, Chen L, Zhou Q, Zhang X, Berger S, Bi J, Lewis DE, Xia Y, Zhang W.Aqp2-expressing cells give rise to renal intercalated cells. J Am Soc Nephrol. 2013;24(2):243–52. doi: 10.1681/ASN.2012080866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis NK.A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002;21(8):1948–56. doi: 10.1093/emboj/21.8.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langhe RP, Gudzenko T, Bachmann M, Becker SF, Gonnermann C, Winter C, Abbruzzese G, Alfandari D, Kratzer MC, Franz CM, Kashef J.Cadherin-11 localizes to focal adhesions and promotes cell-substrate adhesion. Nat Commun. 2016;7:10909. doi: 10.1038/ncomms10909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah NG, Israel A.The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci U S A. 1998;95(14):8108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]