Abstract
Objectives:
Rechallenge/continuation of clozapine in association with colony-stimulating factors (CSFs) following neutropenia/agranulocytosis has been reported, but many questions remain unanswered about efficacy and safety. This systematic review aims to assess the efficacy and safety of rechallenging/continuing clozapine in patients following neutropenia/agranulocytosis using CSFs.
Methods:
MEDLINE, Embase, PsycInfo, and Web of Science databases were searched from inception date to July 31, 2022. Articles screening and data extraction were realized independently by two reviewers, according to Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 systematic review guidance. Included articles had to report on at least one case where clozapine was rechallenged/continued using CSFs despite previous neutropenia/agranulocytosis.
Results:
Eight hundred forty articles were retrieved; 34 articles met the inclusion criteria, totaling 59 individual cases. Clozapine was successfully rechallenged/continued in 76% of patients for an average follow-up period of 1.9 years. There was a trend toward better efficacy reported in case reports/series, compared with consecutive case series (overall success rates of 84% and 60%, respectively, p-value = 0.065). Two administration strategies were identified, “as-needed” and prophylactic, both yielding similar success rates (81% and 80%, respectively). Only mild and transient adverse events were documented.
Conclusions:
Although limited by the relatively small number of published cases, factors such as time of onset to first neutropenia and severity of the episode did not seem to impact the outcome of a subsequent clozapine rechallenge using CSFs. While the efficacy of this strategy remains to be further adequately evaluated in more rigorous study designs, its long-term innocuity warrants considering its use more proactively in the management of clozapine hematological adverse events as to maintain this treatment for as many individuals as possible.
Keywords: Clozapine, human colony-stimulating factor, neutropenia, agranulocytosis, rechallenge
Introduction
Clozapine (CLZ) is the most efficacious antipsychotic used in treatment-resistant schizophrenia (TRS), including in the early stage of treatment, which affects nearly one-third of all patients with schizophrenia (Leclerc et al., 2021; Meltzer, 1997; Williams et al., 2017). Although it often represents their only opportunity for recovery and reduces suicidality, CLZ use in schizophrenia is as low as 4% in Canada, 2.5%‒5.5% in the United States, and 4.3%‒12.8% in the United Kingdom (Bachmann et al., 2017; Latimer et al., 2013; Siskind et al., 2016; Stroup et al., 2014; Whiskey et al., 2021). These low rates are largely due to the potential occurrence of neutropenia/agranulocytosis, which require a strict hematological surveillance (Gee et al., 2014). Indeed, neutropenia/agranulocytosis, which can be life-threatening, occur in 3.8% and 0.4%‒0.9% of CLZ users, respectively (Li et al., 2020; Myles et al., 2018). Of note, these higher rates of neutropenia, compared to other antipsychotics, could also be explained in part by surveillance bias resulting from stricter hematological monitoring as well as undiagnosed cases of benign ethnic neutropenia (BEN) (Taylor et al., 2022). Neutropenia/agranulocytosis induced by CLZ mostly occur during the first 6–12 months of treatment (Alvir et al., 1993; Schulte, 2006). Other risks factors for developing neutropenia while receiving CLZ include being younger, Afro-American, having low baseline absolute neutrophil count (ANC) values and using drugs also associated with neutropenia (Lally and Flanagan, 2016).
In most countries, ANC values below 1.5 × 109 cells/L during CLZ treatment warrant its discontinuation, but this threshold can be lowered for individuals with BEN. In the United States, since 2015, this threshold has been lowered to 1.0 × 109 cells/L, which can be further reduced to 0.5 × 109 cells/L for people with BEN (Bastiampillai et al., 2016). Unsurprisingly, discontinuation of CLZ following neutropenia can have tremendous consequences as a significant proportion of patients will quickly relapse from their TRS, which will in turn represent a major step back in their quest for recovery (Meltzer et al., 1996). For this reason, strategies allowing CLZ rechallenge or treatment pursuit despite neutropenia are a subject of growing interest, even though it remains on an off-label basis except in the United States (Bastiampillai et al., 2016). So far, both lithium and granulocyte colony-stimulating factors (G-CSFs), or less frequently granulocyte-macrophage colony-stimulating factors (GM-CSFs), have been used off-label in this context.
Lithium has been used to allow CLZ rechallenge for several decades now, perhaps because psychiatrists were already familiar with this drug. Its use is associated with acute and chronic leukocytosis, an effect which could be mediated by a mobilization of peripheral neutrophils and an increased neutrophils production in the bone marrow (Focosi et al., 2009). In a recent review though, despite this strategy being successful in 87% of the published cases (82/94), existing concerns regarding a potential masking effect of lithium could not be eliminated as its discontinuation seemed to be quickly followed by blood dyscrasias (Boazak et al., 2018). Moreover, lithium use isn’t without risks, as it also has its deal of adverse effects, and long-term use can lead to nephropathy, insipidus diabetes, and thyroid disorders (Grandjean and Aubry, 2009). As for colony-stimulating factor (CSF), these recombinant hematopoietic growth factors stimulate the production of neutrophils and are mainly used in oncology for patients receiving myelosuppressive chemotherapy (Kuderer et al., 2007). Their use for patients treated with CLZ was first described for the treatment of agranulocytosis in order to reduce its duration and prevent its associated complications (Lally et al., 2017b). It was only later that these agents were used in adjunct with CLZ to allow its rechallenge/continuation following neutropenia. Despite two reviews published in 2017, both highlighting the potential usefulness of this strategy, several questions remain unanswered before such approach can be more widely used in clinical practice (Lally et al., 2017a; Myles et al., 2017). On one hand, only case reports/series, as well as one retrospective cohort study, had been published at the time these two reviews were undertaken. As these types of studies are prone to important publication bias, the reported success rates of this strategy are less likely to reflect their real clinical efficacy. On the other hand, while two different CSF administration strategies have been identified, that is, “as-required” and “prophylactic,” the former was only reported in nine cases (Lally et al., 2017a; Myles et al., 2017). Thus, the finding that the “as-required” use of CSF might be associated with better efficacy than the “prophylactic” approach still needs to be better documented (Lally et al., 2017a; Myles et al., 2017). Addressing these elements was therefore deemed relevant in order to better support clinical practice and provide people dealing with TRS the best opportunity to a fulfilling life.
Objectives
The main objective of this systematic review was to evaluate the efficacy and safety of CLZ rechallenge/continuation using CSF following a previous episode of neutropenia or agranulocytosis. Secondary objectives were to assess the efficacy of different strategies of CSF administration as well as to identify potential predicting factors of either successful or unsuccessful CLZ rechallenge/continuation.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 guidance (Page et al., 2021). The online application Covidence Systematic Review Software (Veritas Health Innovation, Melbourne, VIC, Australia), specifically designed to facilitate conductance of systematic review by multiple reviewers, was used for the title/abstract and full-text screenings. The protocol was not registered.
Search strategy
Online searches were conducted in MEDLINE, EMBASE, Web of Science, and PsycINFO electronic databases. EF designed the search strategy, which was independently reviewed by two information specialists of Laval University (see Supplemental Material). The date ranges searched were from databases’ inception date until July 31, 2022. There were no language restrictions; for articles written in languages other than English, French or Spanish, translation services were used. Study methodologies included in this review were case reports or series, retrospective or prospective cohort studies, cross-sectional studies, case-control studies or randomized-controlled trials. Included articles’ references lists were manually searched to retrieve other relevant studies. EF performed the electronic searches. EF and OC reviewed the abstracts of all identified studies to identify papers reporting on CLZ, neutropenia/agranulocytosis, and CSF. Potentially relevant papers were independently reviewed in full-text by E.F. and O.C. In instances where insufficient information was provided to assess eligibility, authors were directly contacted to obtain further details. In one case, the journal’s publisher provided an article’s missing supplemental table. Disagreements between the two reviewers about studies’ eligibility were resolved via consensus through discussion; arbitration was not required.
Inclusion and exclusion criteria
Included articles had to report on at least one patient for whom CLZ had been reintroduced or maintained in combination with the use of CSF following a neutropenia or an agranulocytosis developed while they were on CLZ. Reports of patients for whom a rechallenge strategy with CSF was established, but never administered, were excluded. Since the use of CSF in oncologic settings has already been extensively documented, articles that reported on patients who developed blood dyscrasia while undergoing treatment for cancer were excluded.
Definitions
CLZ rechallenge was defined as resuming the use of CLZ after the drug being previously discontinued for more than 7 days following an episode of neutropenia or agranulocytosis. CLZ treatment continuation was defined as maintaining CLZ despite an episode of neutropenia or agranulocytosis. In both situations, two different CSF administration strategies were considered, based on previous reviews (Lally et al., 2017a; Myles et al., 2017).
First, the use of CSF on a regular basis for a fixed duration, irrespective of ANC values, was classified as a “prophylactic strategy.” Second, the use of CSF only when required according to ANC values was classified as an “as-needed strategy.” Neutropenia was defined as ANC values below 1.5–2.0 × 109 cells/L, while an ANC less than 0.5 × 109 cells/L was considered as agranulocytosis. As for CSF, all available formulations of either G-CSF or GM-CSF, including bio-similar agents, and administered subcutaneously, intramuscularly, or intravenously, were included in this review.
Outcomes
For the evaluation of efficacy outcomes, the CSF administration strategy was deemed successful if CLZ could be maintained at the end of follow-up, whether neutropenia or agranulocytosis episodes reoccurred during that span or not. Conversely, the CSF administration strategy was considered unsuccessful if CLZ had to be discontinued due to blood dyscrasia or its consequential complications (e.g., infections). Discontinuation of CLZ for reasons other than hematological did not constitute failures. For the purpose of statistical analyses, two groups of patients were formed on the basis of efficacy outcome, namely those for whom CLZ could be continued at the end of follow-up and those for whom the treatment had to be discontinued as mentioned above. As for the safety of CSF, any adverse events reported from its use were collected.
Data extraction
Data were independently extracted from each included article by two reviewers, OC and EF, and any discrepancies were reviewed by a third reviewer (LB). Patients’ characteristics were collected (i.e., age, sex, ethnic origin, and main psychiatric diagnosis) and all neutropenia episodes occurring during a patient’s follow-up were documented. For each episode, the following variables were collected: delay of onset following CLZ initiation or rechallenge (or delay since last neutropenia episode in cases of CLZ treatment continuation); ANC nadir; CLZ dose at onset of neutropenia; CLZ treatment outcome (continuation or discontinuation); duration of time between neutropenia and CLZ rechallenge, when applicable; use of CSF concomitantly with CLZ; CSF administration strategy (“prophylactic” or “as-needed”); CSF formulation, route of administration, dosage, administration schedule, and ANC thresholds for the “as-needed” administration; concomitant lithium use; length of follow-up (i.e., duration of time between last neutropenia and end of follow-up or CLZ discontinuation); ongoing CLZ use at the end of follow-up; and CSF adverse events. When available, contributing factors or alternative causes of neutropenia/agranulocytosis were collected in order to assess CLZ accountability.
Statistical analyses
Patients were divided into two groups based on their outcomes and comparisons were made using student’s two-sample t-test or chi-squared test. Analyses were carried out using SPSS® version 28 (IBM Analytics, Armonk, NY, USA).
Results
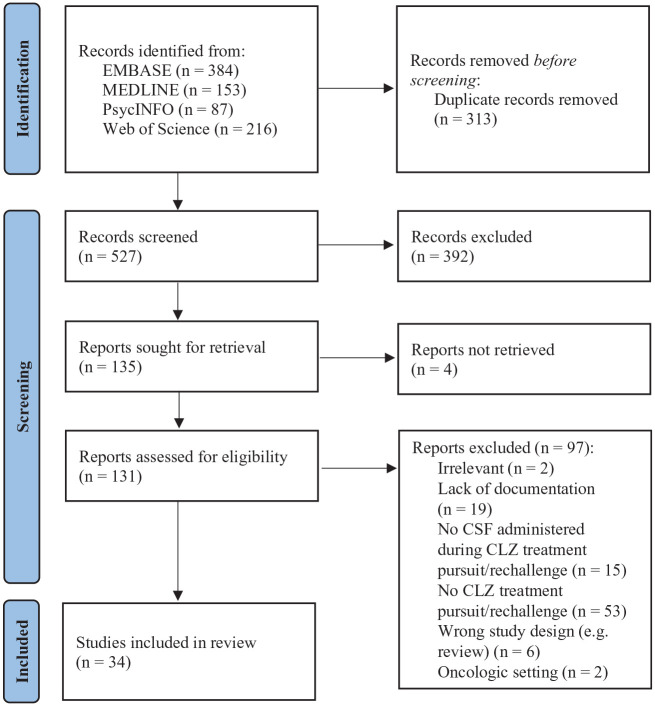
A total of 840 articles were retrieved from electronic searches, from which 313 duplicates were removed as displayed in Figure 1. Review of title and abstract identified 135 potentially relevant articles that were reviewed in their entirety. From these, a total of 34 publications were included in this review; 4 of those reported on cases that were described more than once and were thus excluded from the analysis (Béchard et al., 2020, 2022; Demers et al., 2016, 2020). One case was also reported in two different articles and was therefore only included once in this review (Meyer et al., 2015; Spencer et al., 2012). As detailed in Table 1, 27 articles were case reports or non-consecutive series (total n = 38 cases) (Broughton et al., 2012; Comacchio et al., 2016; Conus et al., 2001; Elmore et al., 2016; Fabre-Bernal et al., 2019; Foster et al., 2017; Freeman et al., 2016; Friedman et al., 2017; Gopalakrishnan et al., 2013; Gugger and Caley, 2007; Hagg et al., 2003; Hazewinkel et al., 2013; Huguet et al., 2013; Joffe et al., 2009; Karst and Lister, 2018; Khan et al., 2013; Majczenko and Stewart, 2008; Martina et al., 2019; Mathewson and Lindenmayer, 2007; Morrow et al., 2020; O’Neill et al., 2021; Rajagopal et al., 2007; Ruiz-Doblado et al., 2020; Sengupta and Harvey, 2010; Shuman et al., 2021; Spencer et al., 2012; Sperner-Unterweger et al., 1998), while there were only 2 consecutive case series (n = 15 cases) (Béchard et al., 2021; Silva et al., 2021) and 1 retrospective cohort study (n = 6 cases) (Meyer et al., 2015), for a total of 59 individual cases.
Figure 1.
Flow chart of reviewed articles.
Source: Adapted from Page et al. (2021).
CLZ: clozapine; CSF: colony-stimulating factor.
Table 1.
CSF success rate according to study methodology.
| Study methodology | Number of studies | Number of cases | CSF strategy success rate | p Value | ||
|---|---|---|---|---|---|---|
| As-needed | Prophylactic | Overall | ||||
| n/N (%) | n/N (%) | n/N (%) | ||||
| Case reports/series | 27 | 38 a | 13/14 (93) | 15/18 (83) | 32/38 (84) | 0.065 |
| Consecutive case series/retrospective cohort study | 3 | 21 b | 8/12 (67) | 5/7 (71) | 13/21 (62) | |
CSF: colony-stimulating factor.
CSF strategy not specified for six cases.
CSF strategy not specified for two cases.
Patients’ characteristics
Among the 59 cases for whom the information was provided, 75% were men with a mean age of 41.6 years old (±14.7, range 17–71) and most were Caucasians (79%). CLZ was most frequently first prescribed for TRS (n = 57) but was also used for bipolar disorder (n = 1) and psychosis associated with Parkinson’s disease (n = 1). Following CLZ initiation, a first neutropenia episode occurred after an average of 4.6 years (±6.9, median = 0.5, range 0.01–25) and 60% arose during the first year of treatment. At the time of occurrence of this neutropenia, the mean CLZ daily dose was 357 mg (±162, range 25–600). During that first neutropenia episode, the average ANC nadir was 0.98 × 109 cells/L (±0.40, median = 1.10, range 0.07–1.60), but the exact value was not specified in 23 cases. There were six cases of agranulocytosis (17%) and 12 patients had ANC values between 0.5 and 1.0 × 109 cells/L (33%). Following this first episode, and after either CLZ rechallenge or pursuit, most patients had one or more subsequent neutropenia episodes (85%). These occurred quicker than for the initial episode in 70% of the cases and the ANC nadir was also lower during the second episode for 74% of the patients.
Efficacy outcomes
Over an average follow-up period of 1.9 years (±2.2, median = 1.0, range 0.04–10.0) following CLZ rechallenge/continuation, CLZ was successfully maintained for 45 out of all 59 patients (76%). As displayed in Table 2, there were no statistically significant differences between patients for whom CLZ was maintained and those for whom it was discontinued. As for CSF strategies, success rates for the “as-needed” and “prophylactic” approaches were similar, when specified (81% and 80%, respectively). There was a trend toward higher success rates in case reports and non-consecutive case series, compared with consecutive case series and the only retrospective cohort study (overall success rates of 84% and 60%, respectively, p-value = 0.065). CSF was used in combination with CLZ after an average of 1.9 neutropenia episodes (±1.1, median = 2.0, range 1–7) and patients further experienced 1.1 episodes following CSF initiation (±1.8, median = 1.0, range 0–9). When reported, G-CSF were more frequently used than GM-CSF, as the latter was used for only two patients, and filgrastim was the most prescribed agent (n = 34), followed by lenograstim (n = 4) and pegfilgrastim (n = 2). Filgrastim was mainly administered subcutaneously (n = 14), but also intramuscularly (n = 2), and doses ranged from 150 (n = 2) to 480 μg (n = 5); the 300-μg dose was most frequently preferred (n = 25). When the “as-needed” strategy was employed, the most common thresholds prompting CSF administration were ANC values below 1.0 (n = 5) and 1.5 × 109 cells/L (n = 3). As for the “prophylactic” strategy, CSF was generally administered once to three times weekly.
Table 2.
Comparison of patients based on CLZ treatment outcome.
| Variables | CLZ discontinued at follow-up (n = 14) | CLZ continued at follow-up (n = 45) | p Value |
|---|---|---|---|
| n (%) | n (%) | ||
| Age, mean ± SD, y | 46.4 ± 14.2 | 39.4 ± 14.7 | 0.200 |
| Gender | |||
| Male | 8 (80) | 28 (74) | 1.000 |
| Female | 2 (20) | 10 (26) | |
| Ethnicity | |||
| Caucasian | 7 (78) | 20 (80) | 1.000 |
| Non-Caucasian | 2 (22) | 5 (20) | |
| Main diagnosis | |||
| Schizophrenia | 13 (93) | 44 (98) | 0.421 |
| Other | 1 (7) | 1 (2) | |
| Time to first neutropenia, mean ± SD, y | 3.0 ± 4.4 | 5.3 ± 7.7 | 0.334 |
| ⩽1 year, n (%) | 7 (58) | 18 (60) | 1.000 |
| >1 year, n (%) | 5 (42) | 12 (40) | |
| ANC nadir at first neutropenia, mean ± SD, ×109 cells/L | 1.12 ± 0.28 | 0.90 ± 0.43 | 0.109 |
| ⩽1.00 × 109 cells/L | 5 (46) | 13 (52) | 0.717 |
| >1.00 × 109 cells/L | 6 (54) | 12 (48) | |
| CLZ dose at first neutropenia, mean ± SD, mg/d | 404 ± 142 | 333 ± 172 | 0.334 |
| Time to second neutropenia, mean ± SD, y | 0.7 ± 1.2 | 0.3 ± 0.5 | 0.357 |
| Delay shorter than first neutropenia | 3 (27) | 6 (32) | 1.000 |
| Delay longer or equal to first neutropenia | 8 (73) | 13 (68) | |
| ANC nadir at second neutropenia, mean ± SD, ×109 cells/L | 0.87 ± 0.47 | 0.71 ± 0.56 | 0.502 |
| ANC nadir lower than at first neutropenia | 3 (43) | 3 (19) | 0.318 |
| ANC nadir higher or equal to first neutropenia | 4 (57) | 13 (81) | |
| Number of neutropenia episodes prior to CSF initiation, mean ± SD | 1.9 ± 0.9 | 2.0 ± 1.2 | 0.933 |
| Number of neutropenia episodes following CSF initiation, mean ± SD | 1.7 ± 1.9 | 0.9 ± 1.8 | 0.234 |
| CSF administration strategy | |||
| As-needed | 5 (50) | 21 (51) | 1.000 |
| Prophylactic | 5 (50) | 20 (49) | |
| Concomitant lithium use | 6 (75) | 9 (41) | 0.215 |
| Follow-up, mean ± SD, y | 2.6 ± 3.3 | 1.7 ± 1.8 | 0.501 |
ANC: absolute neutrophil count; CSF: colony-stimulating factor; d: day; L: litre; mg: milligram; SD: standard deviation; y: year.
Safety outcomes
There were no deaths related to hematological complications nor CSF administration. Adverse events associated to CSF use were only documented for 34 cases out of 59. There were no adverse events noted for most of them (28/34) and the remaining patients experienced only minor events, including rebound leukocytosis (n = 2), mild euphoria (n = 1), flu-like symptoms (n = 1), short-lived back pain (n = 1), and splenomegaly, not clinically significant (n = 1).
Discussion
In this review, the use of CSF to allow CLZ rechallenge/continuation despite neutropenia was found to be successful for 76% of the 59 included cases. This result is in line with two previous reviews, both published in 2017, in which success rates of 75 and 76% were observed based on 32 and 30 patients, respectively (Lally et al., 2017a; Myles et al., 2017). However, these latter findings were based solely on case reports or series, as well as one retrospective cohort study (Meyer et al., 2015), while two consecutive case series have since been published and included in the present review (Béchard et al., 2021; Silva et al., 2021). As could be expected given the reporting bias inherent to case reports/non-consecutive case series, the success rate of 84% reported in these studies was higher than the rate of 60% observed in consecutive case series/retrospective cohort studies. While this lower success rate found in more rigorous study designs may be more reflective of clinical reality, it is nonetheless considerable, especially given the innocuity of CSF. Indeed, only minor and transient adverse events associated with the use of CSF were reported over a mean follow-up period of 1.9 years. Still, in the absence of randomized controlled trials assessing this strategy, it is not possible to conclude on whether or not using CSF is associated with better outcomes than not using it, since patients for whom CSF is used probably differ from those for whom it is not. Indeed, it is likely that CSF use is guided by the degree of certainty about the causality of CLZ in a given case. For instance, CSF was used following a second neutropenia episode for 50% of all patients included in this review, meaning that for these patients, the first rechallenge without CSF had failed; such recurrence increases the likelihood of a causal role of CLZ. Unfortunately, it is impossible to compare the degree of certainty about the causality of the role of CLZ as the information provided in most published cases was not sufficient to properly assess this probability.
Based on available data, it is not yet possible to establish the superiority of any specific CSF administration protocol. Myles et al. (2017) concluded, respectively, that 70% of patients who received prophylactic CSF were still on CLZ at the end of follow-up, compared with 100% of patients who received it on an “as-needed” basis. Meanwhile, Lally et al. (2017a) also reported on a 70% effectiveness rate for CSF prophylactic use, compared with 89% for the “as-needed” strategy. However, these high success rates relied only on a small number of cases, that is, seven and nine cases, respectively. Such a clear-cut distinction between these two approaches was not evidenced in this present review, as CSF prophylactic use was successful in 80% of the cases (n = 20), compared with a success rate of 81% for the “as-needed” strategy (n = 21). This difference is partly driven by a recent consecutive case series in which there were four unsuccessful cases associated with the “as-needed” CSF administration scheme (Béchard et al., 2021). A less ambiguous finding from this present review is that G-CSFs are preferred over GM-CSFs and that the short-acting formulation of filgrastim has been most widely used in this particular setting. Considering that many patients do not require prolonged CSF use, it would seem most adequate to administer single doses of filgrastim 300 μg whenever ANC values drop below a prespecified threshold, such as 1.0 × 109 cells/L, at least during the first few weeks following CLZ rechallenge. This would prevent unnecessary utilization of CSF, since human recombinant CSFs remain costly despite the availability of less expensive biosimilar versions, while not jeopardizing patients’ safety. Indeed, should neutropenia reoccur following rechallenge, it should be caught earlier on provided that blood monitoring is done weekly for at least the first 6 months. In the event of recurrent neutropenia requiring multiple CSF doses, a prophylactic strategy could be initiated with once to thrice weekly administrations of filgrastim. In any case, hematologists should be actively involved whenever a CLZ rechallenge is undertaken following blood dyscrasia.
As for potential predicting factors of either successful or unsuccessful CLZ rechallenge or pursuit, no characteristics were found to differentiate individuals for whom CLZ could be maintained or not at follow-up. Noteworthy, although not statistically significant, patients for whom CLZ rechallenge was successful had lower ANC nadir at first neutropenia episode than those for whom rechallenge was unsuccessful. Furthermore, agranulocytosis was experienced by 6 patients at first blood dyscrasia event, among whom 5 were nevertheless successfully rechallenged with CLZ. These findings suggest that rechallenging a patient who developed CLZ-induced agranulocytosis should not necessarily constitute an absolute contraindication, under strict follow-up conditions.
Results of this review need to be interpreted while taking into account some limitations. First, as discussed previously, non-consecutive case reports/series are prone to publication bias. Although two of the 30 included articles were consecutive case series, representing together 27% (16/59) of all included cases (Béchard et al., 2021; Silva et al., 2021), case reports/non-consecutive case series still account for a majority of the included articles. In order to further limit the impact of publication bias and to get a clearer picture of the efficacy and safety of CSF use to allow CLZ rechallenge/continuation, prospective cohort studies with larger samples of consecutive patients would be particularly relevant. Second, even though the number of included cases is roughly twice those included in Lally et al.’s (2017a) and Myles et al.’s (2017) reviews, the relatively small sample of 59 cases still limited the capacity to detect potential predictive factors to either CLZ rechallenge/continuation success or failure with the adjunction of CSF. The paucity of data provided in numerous of the included records further contributed to this issue. Third, one important element undocumented in almost all cases was the assessment of causality between CLZ treatment and occurrence of neutropenia/agranulocytosis. Indeed, other causes for neutropenia were only rarely explicitly explored, such as the use of other medications associated with hematological toxicity (e.g., anticonvulsants such as carbamazepine and valproate, antibiotics such as trimethoprim-sulfamethoxazole and penicillins) as well as conditions such as infections, metabolic disorders, and BEN (Curtis, 2017). Therefore, a favorable outcome of CLZ rechallenge/continuation while using CSF might result partly from a lesser risk of recurrence of neutropenia due to a dubious involvement of CLZ in the initial one. Additionally, while first episodes of neutropenia typically occur within the first year of CLZ treatment in more than 90% of all patients, the fact that this proportion was only 60% of all cases included in this review could suggest that for a relatively large number of patients, blood dyscrasias may not have been related to CLZ (Alvir et al., 1993; Schulte, 2006). Finally, this present review couldn’t explore the clinical outcome of patients for whom CLZ could successfully be rechallenged/continued nor for those for whom CLZ had to be discontinued since objective measurement of the disease’s severity was detailed in only one article (Béchard et al., 2021).
Conclusion
While statistical power remains limited by the relatively small number of published cases, factors such as time of onset to first neutropenia and severity of the episode do not necessarily impact the outcome of a subsequent CLZ rechallenge using CSF. Thus, although the efficacy of CSF use to allow successful CLZ rechallenge remains to be adequately evaluated using more robust study designs, its long-term innocuity warrants considering its use more proactively in the management of CLZ hematological adverse events as to maintain this treatment for as many individuals as possible. Although there are insufficient data pointing toward any difference in terms of efficacy and safety between administering CSF on a prophylactic basis or “as-needed” only, the latter could improve accessibility to this costly medication. Further research is essential to further identify which patients would be better suited for this strategy. Hopefully, the development of new treatment strategies that ensure the safety of CLZ treatment, even in the occurrence of serious adverse events, will promote its widespread use and offer patients the best chance of recovery.
Supplemental Material
Supplemental material, sj-xlsx-1-jop-10.1177_02698811231154111 for Clozapine rechallenge or continuation despite neutropenia or agranulocytosis using colony-stimulating factor: A systematic review by Olivier Corbeil, Laurent Béchard, Émilien Fournier, Maude Plante, Marc-André Thivierge, Charles-Émile Lafrenière, Maxime Huot-Lavoie, Sébastien Brodeur, Anne-Marie Essiambre, Marc-André Roy and Marie-France Demers in Journal of Psychopharmacology
Footnotes
Availability of data: The complete search strategy used for this review and all data extracted from included studies are available upon reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Olivier Corbeil  https://orcid.org/0000-0002-4192-853X
https://orcid.org/0000-0002-4192-853X
Laurent Béchard  https://orcid.org/0000-0001-6697-1799
https://orcid.org/0000-0001-6697-1799
Émilien Fournier  https://orcid.org/0000-0001-6356-6448
https://orcid.org/0000-0001-6356-6448
Supplemental material: Supplemental material for this article is available online.
References
- Alvir JM, Lieberman JA, Safferman AZ, et al. (1993) Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. The New England Journal of Medicine 329: 162–167. [DOI] [PubMed] [Google Scholar]
- Bachmann CJ, Aagaard L, Bernardo M, et al. (2017) International trends in clozapine use: A study in 17 countries. Acta Psychiatrica Scandinavica 136: 37–51. [DOI] [PubMed] [Google Scholar]
- Bastiampillai T, Gupta A, Chan SK, et al. (2016) Changes for clozapine monitoring in the United States. Molecular Psychiatry 21: 858–860. [DOI] [PubMed] [Google Scholar]
- Béchard L, Corbeil O, Plante M, et al. (2020) Clozapine rechallenge following neutropenia using granulocyte colony-stimulating factor: A Quebec case series. Schizophrenia Bulletin 46: S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béchard L, Corbeil O, Plante M, et al. (2021) Clozapine rechallenge following neutropenia using granulocyte colony-stimulating factor: A Quebec case series. Journal of Psychopharmacology 35: 1152–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béchard L, Morasse-Bégis M, Corbeil O, et al. (2022) Clozapine rechallenge or continuation despite neutropenia, an extended follow-up of a consecutive Quebec case series. Journal of Clinical Psychopharmacology 42: 391–395. [DOI] [PubMed] [Google Scholar]
- Boazak M, Goldsmith DR, Cotes RO. (2018) Mask off? Lithium augmentation for clozapine rechallenge after neutropenia or agranulocytosis: Discontinuation might be risky. The Primary Care Companion for CNS Disorders 20: 18l02282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton T, Millward T, Geelan S. (2012) A case report of clozapine augmentation with granulocyte colony stimulating factor (G-CSF). The Journal of Forensic Psychiatry & Psychology 23: 125–133. [Google Scholar]
- Comacchio C, Dusi N, Lasalvia A. (2016) Successful use of single doses of granulocyte-colony stimulating factor (G-CSF) in the treatment of late-onset agranulocytosis associated with clozapine in a patient with treatment-resistant schizophrenia: A case report. Journal of Clinical Psychopharmacology 36: 173–174. [DOI] [PubMed] [Google Scholar]
- Conus P, Nanzer N, Baumann P. (2001) An alternative to interruption of treatment in recurrent clozapine-induced severe neutropenia. The British Journal of Psychiatry 179: 180. [DOI] [PubMed] [Google Scholar]
- Curtis BR. (2017) Non-chemotherapy drug-induced neutropenia: Key points to manage the challenges. Hematology 2017: 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers MF, Fortier L, Breault AS, et al. (2016) Case-report of filigrastin use in clozapine-related neutropenia. Early Intervention in Psychiatry 10: 139–139. [Google Scholar]
- Demers MF, Fortier L, Corbeil O, et al. (2020) Continuing clozapine despite recurrent neutropenia: A 8 year follow-up case report with granulocyte-colony stimulating factor as-required use. Asian Journal of Psychiatry 48: 101541. [DOI] [PubMed] [Google Scholar]
- Elmore H, Lewin J, Bradley M, et al. (2016) Use of granulocyte colony-stimulating factor in a neutropenic HIV-infected patient on clozapine. Psychosomatics 57: 651–654. [DOI] [PubMed] [Google Scholar]
- Fabre-Bernal C, Gordillo-Montano MJ, Remesal-Cobreros R, et al. (2019) “Great responders and recovery”. Clozapine associated to granulocyte colony stimulating factor. Actas Españolas de Psiquiatría 47: 70–78. [PubMed] [Google Scholar]
- Focosi D, Azzara A, Kast RE, et al. (2009) Lithium and hematology: Established and proposed uses. Journal of Leukocyte Biology 85: 20–28. [DOI] [PubMed] [Google Scholar]
- Foster J, Lally J, Bell V, et al. (2017) Successful clozapine re-challenge in a patient with three previous episodes of clozapine-associated blood dyscrasia. BJPsych Open 3: 22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman GM, Jr, Martin BA, Hu RJ. (2016) G-CSF dosing to prevent recurrent clozapine-induced agranulocytosis. The American Journal of Psychiatry 173: 643. [DOI] [PubMed] [Google Scholar]
- Friedman J, Yeboah E, Hermenau M. (2017) Addition of filgrastim (neupogen) for clozapine rechallenge in the case of Parkinson disease patient. Clinical Neuropharmacology 40: 233–234. [DOI] [PubMed] [Google Scholar]
- Gee S, Vergunst F, Howes O, et al. (2014) Practitioner attitudes to clozapine initiation. Acta Psychiatrica Scandinavica 130: 16–24. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan R, Subhalakshmi TP, Kuruvilla A, et al. (2013) Clozapine re-challenge under the cover of Filgrastim. Journal of Postgraduate Medicine 59: 54–55. [DOI] [PubMed] [Google Scholar]
- Grandjean EM, Aubry JM. (2009) Lithium: Updated human knowledge using an evidence-based approach: Part III: Clinical safety. CNS Drugs 23: 397–418. [DOI] [PubMed] [Google Scholar]
- Gugger J, Caley C. (2007) Clozapine treatment in a patient with a persistently low neutrophil count. Clinical Schizophrenia & Related Psychoses 1: 270–272. [Google Scholar]
- Hagg S, Rosenius S, Spigset O. (2003) Long-term combination treatment with clozapine and filgrastim in patients with clozapine-induced agranulocytosis. International Clinical Psychopharmacology 18: 173–174. [DOI] [PubMed] [Google Scholar]
- Hazewinkel AW, Bogers JP, Giltay EJ. (2013) Add-on filgrastim during clozapine rechallenge unsuccessful in preventing agranulocytosis. General Hospital Psychiatry 35: 576.e511–572. [DOI] [PubMed] [Google Scholar]
- Huguet G, Lillo-Le Louet A, Darnige L, et al. (2013) [Clozapine rechallenge in resistant schizophrenia disorder affecting “super sensitive” patients, after neutropenia under clozapine: A case report]. L'Encéphale 39 Suppl 1: S42–S48. [DOI] [PubMed] [Google Scholar]
- Joffe G, Eskelinen S, Sailas E. (2009) Add-on filgrastim during clozapine rechallenge in patients with a history of clozapine-related granulocytopenia/agranulocytosis. The American Journal of Psychiatry 166: 236. [DOI] [PubMed] [Google Scholar]
- Karst A, Lister J. (2018) Utilization of G-CSF and GM-CSF as an alternative to discontinuation in clozapine-induced neutropenia or leukopenia: A case report and discussion. The Mental Health Clinician 8: 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Harvey J, Sengupta S. (2013) Continuing clozapine with granulocyte colony-stimulating factor in patients with neutropenia. Therapeutic Advances in Psychopharmacology 3: 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuderer NM, Dale DC, Crawford J, et al. (2007) Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: A systematic review. Journal of Clinical Oncology 25: 3158–3167. [DOI] [PubMed] [Google Scholar]
- Lally J, Flanagan RJ. (2016) Severe neutropenia and agranulocytosis. In:Manu, Flanagan, Ronaldson KJ. (eds) Life-Threatening Effects of Antipsychotic Drugs. London: Academic Press, pp.105–148. [Google Scholar]
- Lally J, Malik S, Krivoy A, et al. (2017. a) The use of granulocyte colony-stimulating factor in clozapine rechallenge: A systematic review. Journal of Clinical Psychopharmacology 37: 600–604. [DOI] [PubMed] [Google Scholar]
- Lally J, Malik S, Whiskey E, et al. (2017. b) Clozapine-associated agranulocytosis treatment with granulocyte colony-stimulating factor/granulocyte-macrophage colony-stimulating factor: A systematic review. Journal of Clinical Psychopharmacology 37: 441–446. [DOI] [PubMed] [Google Scholar]
- Latimer E, Wynant W, Clark R, et al. (2013) Underprescribing of clozapine and unexplained variation in use across hospitals and regions in the Canadian province of Quebec. Clinical Schizophrenia & Related Psychoses 7: 33–41. [DOI] [PubMed] [Google Scholar]
- Leclerc LD, Demers MF, Bardell A, et al. (2021) A chart audit study of clozapine utilization in early psychosis. Journal of Clinical Psychopharmacology 41: 275–280. [DOI] [PubMed] [Google Scholar]
- Li XH, Zhong XM, Lu L, et al. (2020) The prevalence of agranulocytosis and related death in clozapine-treated patients: A comprehensive meta-analysis of observational studies. Psychological Medicine 50: 583–594. [DOI] [PubMed] [Google Scholar]
- Majczenko TG, Stewart JT. (2008) Failure of filgrastim to prevent severe clozapine-induced agranulocytosis. Southern Medical Journal 101: 639–640. [DOI] [PubMed] [Google Scholar]
- Martina AC, Ee JS, Lamberti JS. (2019) A case of clozapine induced agranulocytosis 25 years after starting treatment: Effective use of lithium for augmentation in rechallenge. Schizophrenia Research 210: 308–309. [DOI] [PubMed] [Google Scholar]
- Mathewson KA, Lindenmayer JP. (2007) Clozapine and granulocyte colony-stimulating factor: Potential for long-term combination treatment for clozapine-induced neutropenia. Journal of Clinical Psychopharmacology 27: 714–715. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. (1997) Treatment-resistant schizophrenia: The role of clozapine. Current Medical Research and Opinion 14: 1–20. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Lee MA, Ranjan R, et al. (1996) Relapse following clozapine withdrawal: Effect of neuroleptic drugs and cyproheptadine. Psychopharmacology 124: 176–187. [DOI] [PubMed] [Google Scholar]
- Meyer N, Gee S, Whiskey E, et al. (2015) Optimizing outcomes in clozapine rechallenge following neutropenia: A cohort analysis. The Journal of Clinical Psychiatry 76: e1410–1416. [DOI] [PubMed] [Google Scholar]
- Morrow O, Gibson L, Bhamra M, et al. (2020) G-CSF mediated neutrophil augmentation in a unique case of comorbid idiopathic Parkinson’s disease and treatment-resistant schizophrenia on clozapine. Therapeutic Advances in Psychopharmacology 10: 2045125320956414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles N, Myles H, Clark SR, et al. (2017) Use of granulocyte-colony stimulating factor to prevent recurrent clozapine-induced neutropenia on drug rechallenge: A systematic review of the literature and clinical recommendations. The Australian and New Zealand Journal of Psychiatry 51: 980–989. [DOI] [PubMed] [Google Scholar]
- Myles N, Myles H, Xia S, et al. (2018) Meta-analysis examining the epidemiology of clozapine-associated neutropenia. Acta Psychiatrica Scandinavica 138: 101–109. [DOI] [PubMed] [Google Scholar]
- O’Neill E, Carolan D, Kennedy S, et al. (2021) Late-onset neutropenia in long-term clozapine use and its management utilizing prophylactic G-CSF. Case Reports in Psychiatry 2021: 6640681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, et al. (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal G, Graham JG, Haut FF. (2007) Prevention of clozapine-induced granulocytopenia/agranulocytosis with granulocyte-colony stimulating factor (G-CSF) in an intellectually disabled patient with schizophrenia. Journal of Intellectual Disability Research 51: 82–85. [DOI] [PubMed] [Google Scholar]
- Ruiz-Doblado S, Plasencia-Garcia de, Diego B, Perea-Perez R, et al. (2020) Severe clozapine-induced agranulocytosis: Successful treatment with G-CSF and rechallenge of clozapine plus D2 potentiation therapy (amisulpride). Actas Españolas de Psiquiatría 48: 89–91. [PubMed] [Google Scholar]
- Schulte P. (2006) Risk of clozapine-associated agranulocytosis and mandatory white blood cell monitoring. The Annals of Pharmacotherapy 40: 683–688. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Harvey J. (2010) Management of clozapine-associated neutropenia: Combination of G-CSF and continued clozapine treatment. The International Journal of Neuropsychopharmacology 13: 104–105. [Google Scholar]
- Shuman M, Moss L, Dilich A. (2021) Never say never: Successful clozapine rechallenge after multiple episodes of neutropenia. Focus (American Psychiatric Publishing) 19: 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E, Higgins M, Hammer B, et al. (2021) Clozapine re-challenge and initiation following neutropenia: A review and case series of 14 patients in a high-secure forensic hospital. Therapeutic Advances in Psychopharmacology 11: 20451253211015070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind D, McCartney L, Goldschlager R, et al. (2016) Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: Systematic review and meta-analysis. The British Journal of Psychiatry 209: 385–392. [DOI] [PubMed] [Google Scholar]
- Spencer BW, Williams HR, Gee SH, et al. (2012) Granulocyte colony stimulating factor (G-CSF) can allow treatment with clozapine in a patient with severe benign ethnic neutropaenia (BEN): A case report. Journal of Psychopharmacology 26: 1280–1282. [DOI] [PubMed] [Google Scholar]
- Sperner-Unterweger B, Czeipek I, Gaggl S, et al. (1998) Treatment of severe clozapine-induced neutropenia with granulocyte colony-stimulating factor (G-CSF). Remission despite continuous treatment with clozapine. The British Journal of Psychiatry 172: 82–84. [DOI] [PubMed] [Google Scholar]
- Stroup TS, Gerhard T, Crystal S, et al. (2014) Geographic and clinical variation in clozapine use in the United States. Psychiatric Services 65: 186–192. [DOI] [PubMed] [Google Scholar]
- Taylor D, Vallianatou K, Whiskey E, et al. (2022) Distinctive pattern of neutrophil count change in clozapine-associated, life-threatening agranulocytosis. Schizophrenia (Heidelb) 8: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiskey E, Barnard A, Oloyede E, et al. (2021) An evaluation of the variation and underuse of clozapine in the United Kingdom. Acta Psychiatrica Scandinavica 143: 339–347. [DOI] [PubMed] [Google Scholar]
- Williams R, Malla A, Roy M-A, et al. (2017) What is the place of clozapine in the treatment of early psychosis in Canada? The Canadian Journal of Psychiatry 62: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-xlsx-1-jop-10.1177_02698811231154111 for Clozapine rechallenge or continuation despite neutropenia or agranulocytosis using colony-stimulating factor: A systematic review by Olivier Corbeil, Laurent Béchard, Émilien Fournier, Maude Plante, Marc-André Thivierge, Charles-Émile Lafrenière, Maxime Huot-Lavoie, Sébastien Brodeur, Anne-Marie Essiambre, Marc-André Roy and Marie-France Demers in Journal of Psychopharmacology