Abstract
In accordance with Article 43 of Regulation (EC) 396/2005, EFSA received a request from the European Commission to review the existing maximum residue levels (MRLs) for the non‐approved active substance fenarimol in view of the possible lowering of the MRLs. EFSA investigated the origin of the current EU MRLs. For existing EU MRLs that reflect previously authorised uses in the EU, or that are based on obsolete Codex maximum residue limits, or import tolerances that are not required any longer, EFSA proposed the lowering to the limit of quantification. EFSA performed a chronic and acute dietary risk assessment for the revised list of MRLs to allow risk managers to take the appropriate decisions. For some commodities, further risk management discussions are required to decide which of the risk management options proposed by EFSA should be implemented in the EU MRL legislation.
Keywords: consumer risk assessment, toxicological evaluation, residue definitions, MRL setting, fenarimol, non‐approved active substance
Summary
The European Commission submitted a request to EFSA for a targeted review of maximum residue limits (MRLs) for 10 active substances no longer approved in the EU, but for which MRLs greater than the limit of quantification (LOQ) are still in place and for which Member States have identified potential consumer health risks. Separate reasoned opinions should be provided in accordance with Article 43 of Regulation (EC) 396/2005, for each of the substances included in this mandate, one of them being fenarimol.
In accordance with the terms of reference, EFSA investigated the origin of the current EU MRLs for fenarimol, and whether they are sufficiently substantiated. An EU MRL is considered substantiated if it is sufficiently supported by data and established for uses still authorised or based on Codex maximum residue limit (CXL) or import tolerance that are still in place and relevant. Accordingly, MRLs that were derived for previously authorised EU uses are obsolete and should be lowered to the LOQ. For those commodities for which the existing EU MRLs are based on a CXL, EFSA investigated whether the CXLs are still in place and whether they are sufficiently supported by data. Obsolete or insufficiently supported Codex MRLs are also candidates for being lowered to the LOQ. To identify possible import tolerances, EFSA consulted Member States and the United Kingdom on good agricultural practices authorised in third countries that were evaluated at national level which might justify maintaining certain MRLs as import tolerances. Following this Member State consultation, the United Kingdom notified a total of 11 import tolerances still in place. An evaluation report was not provided as the GAPs submitted were already assessed in a previous MRL application. EFSA also screened the quality of the toxicological reference values (TRVs) derived at EU level and by the Joint Meeting on Pesticide residues (JMPR). As EFSA identified critical issues related to the available toxicological database, EFSA organised an expert consultation (Pesticides Peer Review Teleconference 98) to discuss the toxicological profile and the TRVs for fenarimol.
EFSA prepared a draft reasoned opinion that was shared with Member States and the European Reference Laboratories (EURLs) for consultation via a written procedure. Comments received were considered during the finalisation of this reasoned opinion. The following conclusions are derived.
The metabolism of fenarimol in plants and animals was previously investigated in the framework of the EU evaluation for inclusion in Annex I to Directive 91/414/EEC, previous MRL applications, as well as by the JMPR. According to the results of the metabolism studies assessed, the residue definition for enforcement and risk assessment, both for plant and animal products, is fenarimol.
Analytical methods are available for the enforcement of the proposed residue definition in high water content and high acid content commodities with an LOQ of 0.01 mg/kg. Fenarimol can be enforced in food of animal origin with an LOQ of 0.01 mg/kg in muscle and fat, milk, eggs, kidney, liver and fish. According to the EURLs, a QuEChERS (or QuOil) multiresidue and a single‐residue analytical method are available with an LOQ of 0.01 mg/kg for the routine analysis of fenarimol in the four main matrix groups of plant origin, and a default LOQ of 0.01 mg/kg is also deemed achievable to monitor fenarimol in all commodities of animal origin.
The origin of all current MRLs set for fenarimol (based on formerly approved uses or on CXLs) was investigated, and a list of MRLs was identified as not sufficiently substantiated: CXLs or import tolerances for cherries, peaches, table and wine grapes, bananas and hops; EU MRLs for apricots, strawberries, raspberries (red and yellow), currants (black, red and white), gooseberries (green, red and yellow) gherkins, courgettes, other cucurbits with edible peel, watermelons and other cucurbits with inedible peel. No fall‐back MRLs were identified for any of these crops. Moreover, further risk management discussions are required to decide whether the existing EU MRL for pome fruits, cucumbers, melons and pumpkins should be maintained or lowered to the LOQ.
A screening of the quality of the TRVs set at EU level and of those established by the JMPR was performed, and the set of toxicological studies used to derive these TRVs was assessed according to the current standards. As critical issues were identified, a Member States experts' consultation took place. Experts concluded that the TRVs cannot be confirmed for fenarimol since the available data do not provide sufficient evidence to exclude the genotoxicity potential of fenarimol, the data available were insufficient compared to current standards and uncertainty factors could not be established. Accordingly, the EU acceptable daily intake (ADI) and acute reference dose (ARfD) derived in 1996 and 2001, respectively, do not comply with the current scientific standards. Therefore, EFSA recommends that risk managers discuss whether these TRVs should be withdrawn. The following data would be required to finalise the toxicological assessment which is a prerequisite to derive robust TRVs:
an assessment of the validity of analytical methods used in feed, body fluids and tissues, air and any additional matrices used in support of the toxicological studies was not conducted;
if possible, an assessment of the toxicological relevance of unknown impurities potentially present in the technical specification and present in fenarimol‐treated commodities;
an interspecies comparative in vitro metabolism study on animal species used in pivotal studies and on human material;
additional studies to conclude on the genotoxic potential of fenarimol;
investigations on the neurotoxic and immunotoxic potential of fenarimol;
additional toxicological data to complete the ED assessment;
an up‐to‐date literature search;
full re‐evaluation of the toxicological data package and reporting relevant details on the studies and the results in accordance with the current standards.
The same limitations regarding the (geno)toxicity data package are applicable to JMPR values.
Chronic and acute exposure calculations were performed using revision 3.1 of PRIMo, considering commodities for which CXLs and EU MRLs were found to be sufficiently substantiated, while all CXLs/MRLs that were revoked or are no longer substantiated were proposed to be lowered to the appropriate LOQ, as well as all other commodities for which no GAP was reported under this review. Comparing to the current EU TRVs, no exceedances were observed, and the highest chronic exposure represented 14% of the ADI (Dutch toddler). The highest acute exposure amounted to 76% of the ARfD (melons). Nevertheless, EFSA emphasises that as the toxicological assessment revealed deficiencies and concerns regarding the toxicological studies available for fenarimol and considering that EU TRVs do not meet the current scientific standards, the risk assessment cannot be finalised, and the results presented under the current review are indicative only.
Due to the deficiencies identified regarding the toxicological studies available for fenarimol, none of the existing EU MRLs/CXLs listed in the summary table below are recommended for inclusion in Annex II to the Regulation. If a decision to withdraw the TRVs is taken, EFSA recommends that risk managers discuss whether all MRLs currently implemented in EU Regulation should be lowered to the respective LOQs.
Summary table
| Code(a) | Commodity | Existing MRL(b) (mg/kg) | Outcome of the review | |
|---|---|---|---|---|
| MRL proposal (mg/kg) | Comment | |||
| Residue definition for enforcement (plants and animal products): fenarimol | ||||
| 0130000 | Pome fruits | 0.1 |
0.1 or LOQ Further consideration by risk managers needed |
Risk management discussion is needed to decide whether the existing MRL needs to be lowered as it is not confirmed if the GAP behind this import tolerance is still authorised in the country of origin. In addition, it should be discussed whether the existing MRL needs to be lowered as the risk assessment could not be finalised, lacking robust TRVs for fenarimol. |
| 0140010 | Apricots | 0.5 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
| 0140020 | Cherries (sweet) | 1.5 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
| 0140030 | Peaches | 0.5 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
|
0151010 0151020 |
Table and wine grapes | 0.3 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
| 0152000 | Strawberries | 0.3 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
| 0153030 | Raspberries (red and yellow) | 0.1 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
| 0154030 | Currants (black, red and white) Gooseberries (green, red and yellow) | 1 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
| 0163020 | Bananas | 0.2 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
| 0232010 | Cucumbers | 0.2 |
0.2 or LOQ Further consideration by risk managers needed |
Risk management discussion is needed to decide whether the existing MRL needs to be lowered as it is not confirmed if the GAP behind this import tolerance is still authorised in the country of origin. In addition, it should be discussed whether the existing MRL needs to be lowered as the risk assessment could not be finalised, lacking robust TRVs for fenarimol. |
|
0232020 0232030 0232990 |
Gherkins, Courgettes, Other cucurbit with edible peel | 0.2 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
|
0233010 0233020 |
Melons Pumpkins | 0.2 |
0.2 or LOQ Further consideration by risk managers needed |
Risk management discussion is needed to decide whether the existing MRL needs to be lowered as it is not confirmed if the GAP behind this import tolerance is still authorised in the country of origin. In addition, it should be discussed whether the existing MRL needs to be lowered as the risk assessment could not be finalised, lacking robust TRVs for fenarimol. |
|
0233030 0233990 |
Watermelons Other cucurbits with inedible peel | 0.05 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
| 0700000 | Hops | 5 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
MRL: maximum residue limit; CXL: Codex residue limit; LOQ: limit of quantification; TRV: toxicological reference value; ARfD: acute reference dose; GAP: good agricultural practice.
(a): Commodity code number according to Annex I of Regulation (EC) No 396/2005.
(b): MRL currently set under Regulation (EC) No 318/2014.
Background
In March 2021, a Member State submitted to the European Commission the results of a screening performed on all maximum residue levels (MRLs) of active substances used in plant protection products that are not approved in the EU. The list contained 904 substances; for 297 of them, at least one MRL was set at a level above the limit of quantification (LOQ).
For 219 of these substances, the MRLs are not related to the uses of the substances in plant protection products (e.g. MRLs reflect the use of biocides or veterinary medical product, or MRLs are set to account for their occurrence in certain food due to environmental persistence, or their natural occurrence). For the other 78 substances, the MRLs were established either based on formerly approved uses in the EU, on import tolerance requests, or on Codex maximum residue limits (CXLs).
Some of these substances were never approved in the EU, or their approval was withdrawn before 2008, and therefore, they did not fall within the scope of the systematic review of all existing MRLs under Article 12 of Regulation (EC) No 396/2005 1 .
A second Member State conducted additional analysis, identifying potential consumer risk for some of the MRLs set for these active substances.
Based on these analyses, the European Commission conducted a prioritisation exercise to identify substances for which existing MRLs should be reviewed with high priority. The prioritisation was also discussed and agreed with Member States during several meetings of the Standing Committee on Plants, Animals, Food and Feed (SCoPAFF), section Phytopharmaceuticals – Pesticides residues (September 2021, 2 November 2021 3 and February 2022 4 ). The SCoPAFF agreed that 10 active substances, for which potential consumer risks were identified, should be assessed by EFSA as a priority. One of the substances identified for being assessed with high priority is fenarimol.
The European Commission proposed to mandate EFSA to provide a targeted review of MRLs for the substances concerned without delay. Due to the urgency of the subject, EFSA was invited to consider, if appropriate, delivering a separate reasoned opinion for each of the substances included in this mandate, as to be able to start providing outcomes to the Commission as soon as possible and successively. In this reasoned opinion, EFSA covered the targeted review of the MRLs for fenarimol.
Terms of reference (as provided by the requestor)
EFSA was requested by the European Commission, according to Article 43 of Regulation (EC) No 396/2005, to prepare a reasoned opinion on fenarimol. In particular, the following tasks should be performed:
to investigate the origin of the current EU MRLs (e.g. MRL based on formerly approved uses in the EU, on import tolerance requests or on CXLs). This analysis should allow to verify if the CXLs/import tolerances are still justified 5 and to identify MRLs that do not correspond to import tolerances or currently established CXLs (non‐verified CXL/import tolerances);
to consult Member States on information about good agricultural practices authorised in third countries and already evaluated at MS level, which might support maintaining the existing import tolerances or setting of new (lowered) import tolerances, if this is necessary in view of consumer protection;
to identify fall‐back MRLs for MRLs that do not correspond to a verified CXLs/import tolerance; these fall‐back MRLs could be either a lower import tolerance or a lower CXL established more recently. If no fall‐back MRL can be identified, the MRL should be considered for lowering to the appropriate LOQ;
to consult the EU Reference Laboratories (EURLs) on the LOQs achievable during routine analyses for all commodities;
- to perform an indicative screening of the chronic and acute consumer exposure related to the existing EU MRLs reflecting the verified CXLs/import tolerances, fall‐back MRLs and/or proposed revised LOQ MRLs, using the newest version of the Pesticide Residues Intake Model (PRIMo) based on the available residue definitions for risk assessment and, if not available, residue definitions for enforcement derived at EU level or by JMPR. The following scenarios should be calculated:
- Scenario 1:
- Values at the appropriate LOQ: All MRLs that are based on former EU uses and all CXLs that were revoked by the Codex Committee on Pesticide Residues (CCPR) should be lowered to the appropriate LOQ;
- Non‐LOQ values to be considered: CXLs that were previously taken over in EU legislation, CXLs that were covered by still existing (higher) EU MRLs to be considered at the value of the CXL, MRLs based on existing import tolerances;
to derive the input values for commodities of animal origin for the consumer exposure calculation from the relevant assessment where the MRLs for animal products were derived. However, if the respective risk assessment values (HR/STMR) cannot be retrieved from the available sources, the exposure shall be calculated with the existing MRL. If the existing MRL is no longer justified and no fall‐back MRL can be retrieved, the existing MRL should be considered for being lowered to the LOQ; in this case, the risk assessment screening should be performed with the LOQ;
to examine the available information in order to screen the quality of the toxicological reference values (TRVs) set at EU level and of those established by JMPR. This screening should also consider the completeness of the set of toxicological studies used to derive the TRVs, as to assess if it would be acceptable according to the current standards. In case deficiencies are identified, these should be highlighted along with the resulting uncertainties;
to examine the available information in order to screen the quality of the residue definitions for risk assessment set at EU level and of those established by JMPR. In case deficiencies are identified, these should be highlighted along with the resulting uncertainties;
to compare the indicative chronic and acute dietary exposure to the toxicological reference values derived at EU level or, if not available, to the toxicological reference values derived by JMPR;
to report information on the classification of the substance under the CLP Regulation 8 and whether the active substance meets the criteria for endocrine disruptors;
to assess, in all cases, the contribution of MRLs at the LOQ to the exposure in all exposure scenarios;
to recommend MRLs that do not pose an unacceptable risk to consumers, where possible, and advise risk managers on alternative options. Where relevant, EFSA should indicate whether the achievable LOQs are sufficiently protective for consumers;
to share its draft reasoned opinion for consultation with Member States (MSs) and EURLs before finalising it.
EFSA accepted the mandate and to deliver its assessment by finalising separate reasoned opinions for each of the substances included in this mandate, including fenarimol, by 22 May 2023. Subsequently, an extension of the deadline to 31 October 2023 was agreed with the European Commission.
Assessment
To address the complex terms of reference (ToR), EFSA used the following approach:
In Section 1 (Regulatory background information on fenarimol), information on classification of the active substance under CLP regulation and on endocrine properties is reported (addressing ToR 10).
In Section 2.1 (Nature of residues and residue definitions), a screening of the quality of residue definitions is reported (addressing ToR 8).
In Section 2.2 (analytical methods for MRLs enforcement), information on analytical methods for MRLs enforcement provided by the EURLs on the LOQs achievable during routine residues analysis is reported (ToR 4). In addition, EFSA summarised the information on the analytical methods assessed previously by EFSA and the JMPR.
In Section 2.3 (existing MRLs), information on the origin of the current MRLs is reported in tabular format (ToR 1). In the same section, information provided by MSs on good agricultural practices (GAPs) authorised in third countries and previously evaluated in view of setting import tolerances can be found (ToR 2). This information, together with information on existing CXLs, is used to derive possible fall‐back MRLs (ToR 3) that are also reported in the table if available.
In Section 3 (toxicological reference values), the quality of the TRVs set in the EU and by JMPR are assessed (ToR 7).
In Section 4 (consumer risk assessment), an indicative screening of the chronic and acute consumer exposure is presented (ToR 5 and 6). The dietary exposure assessment Scenario 1 is performed as requested in ToR 5 (a). Scenario 2 is not relevant for the assessment of fenarimol, as all CXLs were revoked and IT set in EU Regulation were implemented and evaluated by EFSA after 2009. This section also addresses ToR 11 (contribution of MRLs at the LOQ to the total exposure) and ToR 9 (comparison of the dietary exposure with the TRVs derived at EU and JMPR level); however, noting that following the experts' meeting on mammalian toxicology, it was concluded that the TRVs do not comply with the current scientific standards.
In the Conclusions and Recommendations section, EFSA presents the MRL proposals that are unlikely to pose an unacceptable risk to consumers, where possible, and the ones for which further consideration is required (ToR 12).
EFSA has based its assessment on the following documents:
the EU evaluation (UK, 1996, 2000) for inclusion in Annex I to Directive 91/414/EEC;
the review report on fenarimol (European Commission, 2007);
the Reports and Evaluations of the JMPR (FAO and WHO, 1995, 1996);
the reports of the Codex Committee on Pesticide Residues (CCPR, 1997, 1998, 2021);
the previous reasoned opinions on fenarimol (EFSA, 2008, 2011).
As requested by the terms of reference (ToR 2), Member States were invited to submit by 18 October 2022 the good agricultural practices (GAPs) that are authorised in third countries and already evaluated at national level, in the format of specific GAP forms, as well as the supporting residue data, in the format of an evaluation report. In the framework of this consultation, seven Member States (CZ, DE, ES, FR, IT, NL and SE) and UK 9 provided feedback regarding fenarimol. The United Kingdom notified a total of 11 import tolerances still in place. The EU reference laboratories (EURLs) were also consulted (ToR 4) to provide an evaluation report on the availability of analytical methods for enforcement and the LOQs achievable during routine analysis in plants and animal commodities. The EURLs report on analytical methods (EURLs, 2022) submitted during the collection of data is considered as main supporting document to this reasoned opinion and, thus, made publicly available. In addition, an expert consultation in the area of mammalian toxicology was conducted in March 2023; the peer review meeting report TC 98 (EFSA, 2023a) is also considered as main supporting document.
On the basis of the data submitted by the MSs, the EURLs, the data available in the Joint Meeting on Pesticide residues (JMPR) Evaluation reports and taking into account the conclusions derived by EFSA in previous opinions and the screening of the available toxicological data with regard to their completeness and quality according to current standards, EFSA prepared a draft reasoned opinion, which was circulated to Member States and EURLs for consultation via a written procedure in May 2023. Comments received by 26 May 2023 were considered during the finalisation of this reasoned opinion (ToR 13).
Further supporting document to this reasoned opinion is the Member States consultation report (EFSA, 2023b). The exposure calculations for all crops reported in the framework of this review performed using the EFSA Pesticide Residues Intake Model (PRIMo) are also key supporting documents made publicly available as background document to this reasoned opinion.
1. Regulatory background information on fenarimol
The key events concerning the regulatory history of fenarimol, the background information, together with the relevant published documents are summarised in Table 1.
Table 1.
Background information
| Process | Status | Comments, references |
|---|---|---|
| Approval status | Not approved |
Decision on inclusion of fenarimol for a limited period of time in Annex I of Council Directive 91/414/EEC by Decision 2006/134/EC (a) . Considering the several areas of concern that were identified during the peer review the inclusion of the active substance in Annex I already expired on 30 June 2008. |
| EFSA conclusion available | No | – |
| MRL review performed | No | – |
| EU MRL applications or other EU assessments | Yes, see comments |
The values of the CXL voted (CAC, 1997, 1999) are in line with the values of EU MRL in Reg (EC) 149/2008 (b) . However, the CXLs were revoked by CAC 2021 following discussion in CCPR 52 (2021). Review of MRLs of concern (Art. 43): Review of MRLs for apples, pears, peaches, bananas, tomatoes and peppers (EFSA, 2008). Legally implemented by Regulation (EC) 1097/2009 (c) . MRL application (Art. 10): Import tolerances in apples, bananas, cherries, cucumbers, melons, pumpkins, peaches, strawberries, table grapes and wine grapes, tomatoes and watermelons (EFSA, 2011). Legally implemented by Regulation (EU) No 318/2014 (d) . |
| Classification under CLP Regulation | See comments |
Lact. H362 ‘may cause harm to breast‐fed children’ Repr. 2, H361fd ‘suspected of damaging fertility. Suspected of damaging the unborn child’. (CLP00 (e) ). Cut‐off criteria regarding classification are not met. |
| Endocrine effects of a.s. | Yes, see comments | Although an ED assessment has not been performed fully according to ECHA and EFSA guidance (ECHA and EFSA, 2018), with the data available and taking into account the uncertainties, it can be concluded that endocrine disruptors criteria are met for fenarimol according to current standards. |
a.s: active substance; MRL: maximum residue limit; CXL: Codex maximum residue limit; CCPR: Codex Committee on Pesticide Residues; CAC: Codex Alimentarius Commission; CLP: classification, labelling and packaging; ED: endocrine disruptor; ECHA: European chemicals agency.
Commission Directive 2006/134/EC of 11 December 2006 amending Council Directive 91/414/EEC to include fenarimol as active substance OJ L 349, 12.12.2006, pp. 32–36. OJ L 314M, 1.12.2007, pp. 463–467.
Commission Regulation (EC) No 149/2008 of 29 January 2008 amending Regulation (EC) No 396/2005 of the European Parliament and of the Council by establishing Annexes II, III and IV setting maximum residue levels for products covered by Annex I. OJ L 58, 1.3.2008, pp. 1–398.
Commission Regulation (EC) No 1097/2009 of 16 November 2009 amending Annex II to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for dimethoate, ethephon, fenamiphos, fenarimol, methamidophos, methomyl, omethoate, oxydemeton‐methyl, procymidone, thiodicarb and vinclozolin in or on certain products. OJ L 301, 17.11.2009, pp. 6–22.
Commission Regulation (EU) No 318/2014 of 27 March 2014 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for fenarimol, metaflumizone and teflubenzuron in or on certain products OJ L 93, 28.3.2014, p. 28–57.
Annex VI of Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, pp. 1–1355.
2. Residue definitions and existing EU MRLS
2.1. Nature of residues and residue definitions
As requested in point 8 of the terms of reference, EFSA summarised in this section the information used to derive the residue definitions for plant and animal products. Table 2 covers the studies submitted in the framework of the EU evaluation for inclusion in Annex I to Directive 91/414/EEC, in the framework of an MRL application as well as in the framework of the JMPR evaluation for the setting of CXLs.
Table 2.
Available metabolism studies
| Primary crops | Crop groups | Crop(s) | Application(s) | Sampling (DAT) | Comment/source |
|---|---|---|---|---|---|
| Fruit crops | Apple | Foliar (spray appl.), Total seasonal rate of 80–200 g a.s./ha in 11 (a) appl., 1–2 weeks intervals between treatments | 6 h, 29, 49 DALT | [14C‐carbinol]‐fenarimol (FAO and WHO, 1995, 1996; UK, 1996; EFSA, 2011) | |
| Post‐harvest (mist spray appl.), 1 mL of formulated product/apple eq. to 8.93 g a.s./kg of apples | 14 | 2 studies: [14C‐carbinol]‐fenarimol and mixture of [14C‐carbinol], [14C‐4‐chlorophenyl] and [14C‐2‐chlorophenyl]‐fenarimol (FAO and WHO, 1995, 1996; UK, 1996; EFSA, 2011) | |||
| Grapes | Foliar (spray appl.), total seasonal rate of 166 g a.s./ha in 4 appl. (2 weeks intervals between treatments) or 1 × 500 mg/L formulation (dose unspecified) | 0, 15, 30, 45, 60 | Mixture of [14C‐carbinol], [14C‐4‐chlorophenyl] and [14C‐2‐chlorophenyl]‐fenarimol (FAO and WHO, 1995, 1996; UK, 1996; EFSA, 2011) | ||
| Cucumber | Foliar (spray appl.), 1 × 24.7 g a.s./ha | 4 | [14C‐carbinol]‐fenarimol (FAO and WHO, 1995, 1996; UK, 1996; EFSA, 2011) | ||
| Root crops | – | – | – | No study available | |
| Leafy crops | – | – | – | No study available | |
| Cereals/grass | – | – | – | No study available | |
| Pulses/oilseeds | – | – | – | No study available |
| Livestock | Animal | Dose (mg/kg DM/day) | Duration (day) | Comment/source |
|---|---|---|---|---|
| Chicken | 0.7 or 7 | 5 | [14C‐carabinol] (FAO and WHO, 1995, 1996; UK, 1996) | |
| Laying hens | 0.6 | 7 days, then 23 days of epuration |
[14C‐carabinol] (FAO and WHO, 1995, 1996; UK, 1996) Only eggs were analysed |
|
| Ruminant, goat | 2 × 10 | 5 | [14C‐carbinol] (FAO and WHO, 1995, 1996; UK, 1996) | |
| Pigs | 2 × 1 | 5 | 1 pig with [14C‐carbinol], 1 with [14C‐2‐chlorophenyl] and 1 with [14C‐4‐chlorophenol] (FAO and WHO, 1995, 1996; UK, 1996) |
a.s.: active substance; DAT: days after treatment; DALT: days after last treatment; DM: dry matter.
The first application out of 11 is conducted with non‐radiolabelled active substance.
Metabolism studies on apple, grapes and cucumber were assessed in the framework of the EU evaluation (UK, 1996) for inclusion in Annex I to Directive 91/414/EEC, in the framework of the JMPR evaluation (FAO and WHO, 1995, 1996) for the setting of CXLs and in the framework of an MRL application (EFSA, 2011). Despite the deficiencies in the metabolism studies, given the three studies available on fruits and fruiting vegetables and considering the chemical structure of the molecule, as unchanged parent fenarimol was the predominant component of the residue, the residue definition for monitoring and risk assessment in plant commodities was proposed as fenarimol.
The nature of fenarimol residues in livestock was investigated and assessed in the framework of EU evaluation (UK, 1996) and in the framework of the JMPR evaluation (FAO and WHO, 1995, 1996) for the setting of CXLs. No metabolism studies on livestock were reviewed in the MRL application (EFSA, 2011), because the calculated dietary burden did not exceed the trigger value of 0.1 mg/kg DM. In the metabolism study with pigs, fenarimol was identified in liver and kidney samples at low levels. In the metabolism study with goats, a number of metabolites were formed (e.g. o‐chlorobenzoic acid and the methyl sulfone derivative of fenarimol neither of which were identified in the rat study), but they occurred at very low levels and are not expected to exceed 0.01 mg/kg following the feeding of crops which had been treated according to the GAPs presented in JMPR report (FAO and WHO, 1996). In the metabolism study with chicken, the highest total residue occurred in liver and kidneys, but no identification was attempted given that dietary burden for chickens were not expected to exceed 0.1 mg/kg DM. In the metabolism study with laying hen where only eggs were sampled, the highest total residue was detected on day 7, but with residues below 0.01 mg/kg (expressed as fenarimol equivalent) and therefore no identification of the residue was attempted. Thus, a residue definition as fenarimol was proposed for enforcement and risk assessment (FAO and WHO, 1995, 1996).
Table 3 below summarises the residue definitions derived at EU level and by the JMPR. The EU residue definitions for enforcement are the ones set in Regulation (EC) No 396/2005. EU residue definitions for risk assessment were proposed in the framework of the EU evaluation (UK, 1996) and in the framework of an MRL application (EFSA, 2011). The same residue definitions for enforcement and risk assessment were derived by the JMPR (FAO and WHO, 1995, 1996).
Table 3.
Residue definitions derived at EU level and by JMPR
| Type of residue definition (RD) | Commodity group | EU residue definition | JMPR residue definitions |
|---|---|---|---|
| RD for enforcement | Plant products | Fenarimol | Fenarimol |
| Animal products | Fenarimol | Fenarimol | |
| RD for risk assessment | Plant products |
Fenarimol (fruit crops only) |
Fenarimol (fruit crops only) |
| Animal products |
Fenarimol (UK, 1996) |
Fenarimol |
|
| Comments: The residue definitions are fully comparable. | |||
2.2. Analytical methods for MRLs enforcement
Analytical methods for the determination of fenarimol residues were assessed in the framework of the EU evaluation (UK, 1996) for inclusion in Annex I to Directive 91/414/EEC, in the framework of the JMPR evaluation (FAO and WHO, 1995, 1996) for the setting of CXLs and in the framework of an MRL application (EFSA, 2011). Analytical methods are available to enforce residues of fenarimol in high water content and high acid content commodities with an LOQ of 0.01 mg/kg. These methods are not stereoselective and therefore determine the sum of the two isomers of fenarimol (EFSA, 2011).
Fenarimol can be enforced in food of animal origin with an LOQ of 0.01 mg/kg in muscle, kidney, liver, fat milk and eggs (FAO and WHO, 1995, 1996; UK, 1996).
During the data collection, the EURLs provided information on a QuEChERS (or QuOil) analytical method using GC–MS/MS technique (also LC–MS/MS for dry commodities), with an LOQ of 0.01 mg/kg for the routine analysis of fenarimol in high water content, high acid content, high oil content and dry commodities. In the four main matrix groups of plant origin, even lower LOQs were successfully validated (for high water and high acid content as well as dry commodities down to 0.002 mg/kg and for high fat content commodities down to 0.005 mg/kg).
According to the EURLs, in commodities of animal origin (muscle, liver, eggs and fish), fenarimol can be monitored with a default LOQ of 0.01 mg/kg. Even lower levels down to 0.001 mg/kg were successfully validated for these three commodities. Based on the experience gained with these matrices, an LOQ of 0.01 mg/kg for animal fat, kidney and milk is deemed achievable (EURLs, 2022). It is concluded that analytical methods are available for all commodities under assessment, except for hops. The EURLs reported that the analytical standard for fenarimol is commercially available (EURLs, 2022).
Table 4 provides an overview of the analytical methods available and their respective LOQs. It is concluded that analytical methods are available for all commodities under assessment, except for hops. The EURLs reported that the analytical standard for fenarimol is commercially available (EURLs, 2022).
Table 4.
Analytical methods available
| Commodity group | Analytical method available | LOQ (mg/kg) | Source | |
|---|---|---|---|---|
| Plant commodities | High water content | Yes (methanol or another hydrophilic solvent system for extraction; GC‐ECD) | 0.01 |
UK (1996) EFSA (2011) |
| Yes (QuEChERS method with LC‐MS/MS) | 0.01 | EFSA (2011) | ||
|
Yes (QuEChERS method with GC‐MS/MS) |
0.01 | EURLs (2022) | ||
| High oil content | Yes (QuEChERS and QuOil method with GC‐MS/MS) | 0.01 | EURLs (2022) | |
| High acid content | Yes (methanol or another hydrophilic solvent system for extraction; GC‐ECD) | 0.01 |
UK (1996) EFSA (2011) |
|
| Yes (QuEChERS method with LC‐MS/MS) | 0.01 | EFSA (2011) | ||
|
Yes (QuEChERS method with GC‐MS/MS) |
0.01 | EURLs (2022) | ||
| Dry |
Yes (QuEChERS method with GC‐MS/MS and LC‐MS/MS) |
0.01 | EURLs (2022) | |
| Animal commodities | Muscle | Yes (methanol/acetonitrile or methanol/methylene chloride extraction; extracts cleaned up on a Florisil column; analysis with GC‐ECD) | 0.01 |
UK (1996) |
| Yes (Q‐EMR method with GC‐MS/MS) | 0.01 | EURLs (2022) | ||
| Kidney | Yes (GC‐ECD) | 0.01 |
UK (1996) |
|
| – | 0.01 (a) | EURLs (2022) | ||
| Liver | Yes (GC‐ECD) | 0.01 |
UK (1996) |
|
| Yes (Q‐EMR method with GC‐MS/MS) | 0.01 | EURLs (2022) | ||
| Fat | Yes (hexane/1‐chlorobutane extraction; extracts cleaned up on a Florisil column; analysis with GC‐ECD) | 0.01 |
UK (1996) |
|
| – | 0.01 (a) | EURLs (2022) | ||
| Milk | Yes (acetonitrile extraction then washed with hexane and partitioned with methylene chloride; extracts cleaned up on a Florisil column; analysis with GC‐ECD) | 0.01 |
UK (1996) |
|
| – | 0.01 (a) | EURLs (2022) | ||
| Eggs | Yes (methanol/acetonitrile or methanol/methylene chloride extraction; extracts cleaned up on a Florisil column; analysis with GC‐ECD) | 0.01 |
UK (1996) |
|
| Yes (Q‐EMR method with GC‐MS/MS or GC‐Orbitrap) | 0.01 | EURLs (2022) | ||
| Fish | Yes (Q‐EMR method with GC‐MS/MS) | 0.01 | EURLs (2022) | |
LOQ: limit of quantification; GC‐ECD: gas chromatography with electron capture detector; GC‐MS/MS: gas chromatography with tandem mass spectrometry; LC‐MS/MS: liquid chromatography with tandem mass spectrometry; QuEChERS: Quick, Easy, Cheap, Effective, Rugged and Safe (analytical method); Q‐EMR: QuEChERS with Enhanced Matrix Removal.
Although no validation data are available for this specific commodity within the EURLs, it is assumed that the reported LOQ would be achievable based on the experience gained with fenarimol in other matrices.
2.3. Existing MRLs
The EU MRLs for fenarimol are established in Annex II of Regulation (EC) No 396/2005. For a number of food products, Codex maximum residue limits (CXLs) have been taken over in the EU legislation. It should be noted that in the framework of the current review, UK notified uses of fenarimol authorised in third countries, detailed in Appendix A.
EFSA reported in Table 5, the existing EU MRLs set above the LOQ for the respective crops, including information on the source of the MRLs together with the relevant GAPs and the references to the assessment where the MRL proposal was derived. In response to ToR 1 which requests to provide an analysis whether the existing EU MRL, the CXL or the import tolerance established for a crop is sufficiently substantiated, EFSA applied the following criteria:
Table 5.
Background information on current MRLs for fenarimol established at a level above the LOQ (CXLs/import tolerances) and verification whether these values are sufficiently substantiated
| Commodity | Existing MRL (mg/kg) | Source of existing MRL | cGAP for existing MRL | Existing MRL substantiated? (Y/N) | Fall‐back GAP | Fall‐back MRL (mg/kg) | Comment |
|---|---|---|---|---|---|---|---|
| Pome fruits | 0.1 | EU MRL (Reg. (EU) 318/2014) derived from Import Tolerance (EFSA, 2011) | USA and Indonesia on apples: foliar application, 10 × 105 g a.s./ha, PHI 30 days (EFSA, 2011) | tbd | No fall‐back GAP identified | – |
The MRL is derived from an import tolerance on apples based on USA and Indonesia uses (EFSA, 2011). UK reported a reference to these GAPs in the framework of the current review, without specifying their authorisation status. The MRL derived for apples was implemented in Regulation (EU) No 318/2014 for the whole group of pome fruits. The USA use is no longer authorised (CFR, 2019) and no information is available on the use in Indonesia. All CXLs were revoked. As no information is available whether the use still exists in Indonesia, risk managers should further discuss whether the MRL is substantiated. |
| Apricots | 0.5 | Reg (EC) 149/2008 | – | N | No fall‐back GAP identified | – |
No uses in third countries were reported. All CXLs were revoked (CAC, 2021) and no import tolerance GAP identified. Therefore, the MRL is not substantiated. |
| Cherries (sweet) | 1.5 | EU MRL (Reg. (EU) 318/2014) derived from Import Tolerance (EFSA, 2011) | USA: foliar application, 5 × 105 g a.s./ha, PHI 0 day (EFSA, 2011) | N | No fall‐back GAP identified | – | The MRL is derived from an import tolerance based on a USA use (EFSA, 2011). UK reported a reference to this GAP in the framework of the current review, without specifying its authorisation status. The USA use is no longer authorised (CFR, 2019). All CXLs were revoked. In the absence of known third country use, the MRL is not substantiated. |
| Peaches | 0.5 | CXL (CAC, 1999) | Spain: foliar application, 1 × 48 g a.s./ha, PHI 7 days (FAO and WHO, 1995) | N | No fall‐back GAP identified | – |
Although notified by UK, the import tolerance assessed previously by EFSA was not supported by sufficient data (EFSA, 2011). All CXLs were revoked (CAC, 2021). Therefore, the MRL is not substantiated. |
| Table and wine grapes | 0.3 | CXL (CAC, 1999) | UK: foliar application, 400 g a.s./ha, PHI 14 days (FAO and WHO, 1995, 1996) | N | No fall‐back GAP identified | – |
Although notified by UK, the import tolerance assessed previously by EFSA was not supported by sufficient data (EFSA, 2011). All CXLs were revoked (CAC, 2021). Therefore, the MRL is not substantiated. |
| Strawberries | 0.3 | Reg (EC) 149/2008 | – | N | No fall‐back GAP identified | – |
No uses in third countries were reported The import tolerance assessed previously by EFSA was not supported by sufficient data (EFSA, 2011). All CXLs were revoked (CAC, 2021). Therefore, the MRL is not substantiated. |
| Raspberries (red and yellow) | 0.1 | Reg (EC) 149/2008 | – | N | No fall‐back GAP identified | – |
No uses in third countries were reported. All CXLs were revoked (CAC, 2021) and no import tolerance GAP identified. Therefore, the MRL is not substantiated. |
| Currants (black, red and white) Gooseberries (green, red and yellow) | 1 | Reg (EC) 149/2008 | – | N | No fall‐back GAP identified | – |
No uses in third countries were reported. All CXLs were revoked (CAC, 2021) and no import tolerance GAP identified. Therefore, the MRL is not substantiated. |
| Bananas | 0.2 | EU MRL (Reg. (EC) 1097/2009) derived from CXL (EFSA, 2008) | 6 × 200 g a.s./ha, PHI 7 days (EFSA, 2011) | N | No fall‐back GAP identified | – |
Although notified by UK, the import tolerance application to support a use of unknown origin was not supported by sufficient data (EFSA, 2011). All CXLs were revoked (CAC, 2021). Therefore, the MRL is not substantiated. |
| Cucumbers | 0.2 | Reg (EU) 149/2008 | Mexico: foliar application, 2 × 108 g a.s./ha, PHI 1 day (EFSA, 2011) | tbd | No fall‐back GAP identified | – | An import tolerance application to support a use in Mexico was supported by data (EFSA, 2011). UK reported a reference to this GAP in the framework of the current review, without specifying its status. However, as no information is available whether the use still exists in Mexico, risk managers should further discuss whether the MRL is substantiated. |
| Gherkins, Courgettes, Other cucurbit with edible peel | 0.2 | Reg (EC) 149/2008 | – | N | No fall‐back GAP identified | – |
No uses in third countries were reported. All CXLs were revoked (CAC, 2021) and no import tolerance GAP identified. Therefore, the MRL is not substantiated. |
| Melons | 0.2 | EU MRL (Reg. (EU) 318/2014) derived from Import Tolerance (EFSA, 2011) | Mexico: foliar application, 2 × 108 g a.s./ha, PHI 1 day (EFSA, 2011) | tbd | No fall‐back GAP identified | – |
An import tolerance application to support a use in Mexico was supported by data (EFSA, 2011). UK reported a reference to this GAP in the framework of the current review, without specifying its status All CXLs were revoked. As no information is available whether the use still exists in Mexico, risk managers should further discuss whether the MRL is substantiated. |
| Pumpkins | 0.2 | EU MRL (Reg. (EU) 318/2014) derived from Import Tolerance (EFSA, 2011) | Mexico: foliar application, 2 × 108 g a.s./ha, PHI 1 day (EFSA, 2011) | tbd | No fall‐back GAP identified | – |
The MRL is derived from an import tolerance based on a Mexico use (EFSA, 2011). UK reported a reference to this GAP in the framework of the current review, without specifying its status. All CXLs were revoked. As no information is available whether the use still exists in Mexico, risk managers should further discuss whether the MRL is substantiated. |
| Watermelons and other cucurbits with inedible peel | 0.05 | Reg (EC) 149/2008 | – | N | No fall‐back GAP identified | – |
An import tolerance application to support a use in Mexico was not supported by sufficient data (EFSA, 2011). All CXLs were revoked (CAC, 2021). Therefore, the MRL is not substantiated. |
| Hops | 5 | CXL (CAC, 1999) | DE: foliar application, 4 × 60 g a.s./ha, PHI 10 days (FAO and WHO, 1995, 1996) | N | No fall‐back GAP identified | – |
All CXLs were revoked (CAC, 2021) and no import tolerance GAP identified. Therefore, the MRL is not substantiated. |
MRL: maximum residue limit; CXL: Codex maximum residue limit; IT: import tolerance; CAC: Codex Alimentarius Commission; CCPR: Codex committee on pesticide Residues: GAP: good agricultural practice; cGAP: critical good agricultural practice; a.s.: active substance; PHI: preharvest interval; tbd: to be discussed.
A CXL is considered substantiated if:
it is still in place (CXL has not been withdrawn from the Codex system);
the CXL is sufficiently supported by data;
the enforcement residue definition is identical with the EU residue definition.
An import tolerance is considered substantiated if:
the GAP in the country of origin is still authorised;
the import tolerance is sufficiently supported by data;
the MRL in the country of origin is established at a level corresponding to the EU MRL (taking into account the potential difference in the RDs);
in case the residue definition in the country of origin is different, the import tolerance is substantiated if sufficient information is available to derive an MRL for the EU RD.
An existing EU MRLs is not substantiated if:
it is based on a previously authorised EU use;
it is based on a previous CXL that has been revoked/withdrawn;
it is based on an import tolerance that is no longer relevant as the use in the country of origin is not confirmed.
In order to address ToR 3, 5 and 6, in cases where the current CXLs or import tolerances are not sufficiently substantiated, Table 5 includes information on possible fall‐back GAPs and the associated fall‐back MRLs. In the last column of this table, additional considerations relevant for taking risk management decisions are also reported.
In 2021, CCPR52 proposed the removal of all Codex MRLs for fenarimol on the basis of public health concerns and/or lack of support. The EU did not express any reservation in CAC in 2021. Therefore, all MRLs originating from the adoption of CXLs are considered not substantiated and should be lowered to the LOQ.
Although all CXLs were revoked in 2021, it cannot be excluded that there are still authorisations for fenarimol worldwide. Some MRLs are based on import tolerances established following the EFSA opinion (EFSA, 2011) based on an assessment conducted by UK as Evaluating Member State (EMS). The GAPs notified by the UK in the framework of the current review are the same as the one previously assessed by EFSA. However, no information was provided by UK on the status of the GAPs in the countries of origin. According to the register of tolerances and exemptions for pesticide chemical residues (Code of Federal Regulation – CFR, 2019), in the USA, the tolerances for fenarimol residues in concerned foods (apples and cherries) expired in 2016. No information was found on whether the uses in other third countries for the other concerned foods (apples in Indonesia, cucumbers, melons, pumpkins and watermelons in Mexico) are still in place. Therefore, the substantiation of the corresponding MRLs is to be discussed, as risk managers might want to confirm whether the GAPs supporting the import tolerances are still authorised.
3. Toxicological reference values
EFSA was mandated to examine the available information in order to screen the quality of the toxicological reference values (TRVs) set at EU level and of those established by the JMPR and to assess the completeness of the set of toxicological studies used to derive the TRVs according to the current standards. In case deficiencies are identified, these should be highlighted along with the resulting uncertainties (ToR 7).
The TRVs for fenarimol reported in Table 6 were proposed by the rapporteur Member State (RMS) in 1996 (ADI) and in 2000 in an addendum to the DAR (ARfD); the TRVs were formally adopted by the European Commission with the approval of fenarimol by Commission Directive 2006/134/EC 10 in force until 13 June 2011. In 1995, the JMPR derived an ADI which can be found in Table 7. An ARfD was not established by the JMPR, as not considered at the time of the assessment.
Table 6.
Toxicological reference values (TRVs) set at EU level
| TRV | Value | Reference | Comments |
|---|---|---|---|
| ADI | 0.01 mg/kg bw per day | European Commission (2007) | Based on the NOAEL of 1.3 mg/kg bw per day for reduced body weight gain, increase in fatty liver and hyperplastic liver nodules observed at 2.47 mg/kg bw per day in a 2‐year study in rats and applying an UF of 100 |
| ARfD | 0.02 mg/kg bw | European Commission (2007) | Based on a NOAEL for fertility of 2 mg/kg bw per day and applying and UF of 100 |
ADI: acceptable daily intake; ARfD: acute reference dose; bw: body weight; NOAEL: no observed adverse effect level; UF: uncertainty factor.
Table 7.
Toxicological reference values (TRVs) set by the JMPR
| TRV | Value | Reference | Comments |
|---|---|---|---|
| ADI | 0.01 mg/kg bw per day | FAO and WHO (1995) | Based on the overall NOAEL of 1.2 mg/kg bw per day seen in several carcinogenicity studies in rats and applying an UF of 100 |
| ARfD | – | – | Not considered at the time of the assessment |
ADI: acceptable daily intake; ARfD: acute reference dose; bw: body weight; NOAEL: no observed adverse effect level; UF: uncertainty factor.
EFSA screened the completeness and the quality of the toxicological studies that were used to derive the EU and the JMPR TRVs, focussing on the question whether the studies meet current scientific standards. EFSA did not undertake a full review of the original studies; the basis of the TRV derivation was scrutinised based on the available data reported mainly in the original DAR and addenda (UK, 1996, 2000).
During this scrutiny, EFSA identified critical issues related to the available toxicological database which were discussed with Member State experts in mammalian toxicology in the Pesticides Peer Review Teleconference 98 in March 2023 (EFSA, 2023a).
The discussions with the Member State experts focussed on the following three critical points:
the genotoxicity data set;
the robustness of the available data to derive toxicological reference values, i.e. the ADI, the ARfD and respective UF;
the endocrine disrupting potential of fenarimol.
The genotoxicity data package available for fenarimol contains studies assessing partially the three endpoints, i.e. gene mutation in bacterial and mammalian cells (in vitro), clastogenicity (in vitro and in vivo) and aneugenicity (in vivo). In addition, considering the difficulty to evaluate the studies with any certainty given the short summary presented at the time of the assessment, one expert provided a quantitative structure–activity relationship (QSAR) analysis. 11
The studies for gene mutation showed negative results but presented limitations such as lack of repeated experiments in all tests. One of the test guidelines was deleted in the meantime (in vitro unscheduled DNA synthesis assay (TG 482)) that was considered relevant and reliable at the time of the assessment to clarify the gene mutation potential of the test substance but is not considered reliable anymore. Regarding clastogenicity and aneugenicity, one mouse in vivo micronucleus test showed slight positive effects, whereas another negative study has limitations, tabulated results are not provided, and the equivocal results cannot be independently assessed. The studies were conducted in the 80s according to the OECD test guidelines in place at the time or previously to their publication and thus present significant deviations compared to current test guidelines, such as low number of cells analysed, too low concentrations or too short exposure time. The lack of a search for public literature was also noted as an additional uncertainty. The QSAR analysis provided was also considered insufficient to clarify the uncertainties, as presenting itself medium to low confidence in the results. Overall, the experts considered that the data package is not reliable, there are too many uncertainties in the available data set and the genotoxicity potential of fenarimol cannot be concluded for the three endpoints, gene mutation, clastogenicity and aneugenicity.
The endocrine disrupting potential (ED) of fenarimol was discussed as the active substance was identified as a potential ED in rodents, but the human relevance of its mode of action was questioned. 12 In single/multigeneration studies, EATS‐mediated and/or sensitive adverse effects have been seen on the reproduction in rats and mice, but not in guinea pigs or rabbits. Reduced fertility, dystocia, reduced liveborn litter size and reduced post‐partum survival occurred in rats; some of these effects have also been seen in mice, but at higher dose levels. Reduce fertility was observed in males, the other effects were observed in females and a consequence to treatment‐related effects on parturition.
In these studies, the NOAEL for systemic (parental) toxicity was 4 mg/kg bw per day (EFSA noted 2.9 mg/kg bw per day adjusting to the lowest value of dose intake) and the NOAEL for reproductive effects was approximately 1 mg/kg bw per day (EFSA noted 0.66 mg/kg bw per day adjusting to the lowest value of dose intake). Investigations in rats indicate that the adverse effects of fenarimol on fertility and parturition are hormonally mediated and a result of fenarimol inhibiting aromatase activity. It is noted that critical endpoints for an ED assessment were not investigated (in vitro and in vivo) and the database is considered incomplete according to the current guidance (ECHA and EFSA, 2018) (i.e. lack of EATS‐mediated and sensitive endpoints measured in vivo); the studies presented shortcomings (due to limitations in the conduct and reporting of the studies); however, there was direct evidence in vitro that fenarimol inhibits the enzyme aromatase responsible for the metabolism of androgens to oestrogens, notably testosterone to oestradiol, this is considered the basis for the adverse effects on fertility and parturition seen in rats and mice. Accordingly, the ED criteria are met for fenarimol as the adverse effects are likely a consequence of the ED mode of action. This is supported by established Adverse Outcome Pathways (AOP), such as the AOP n° 7 and AOP n° 153. It was concluded that human relevance of these effects and mode of action cannot be discarded.
In addition, considering the quality of the studies and of the reporting that does not allow an independent assessment of the data in the dossier, lack of some of the ED‐mediated and sensitive endpoints measured in vivo, lack of in vivo and in vitro mechanistic studies in the dossier, and the lack of a systematic literature review, a potential threshold for the ED effects of fenarimol in humans cannot be defined with any certainty. It is noted that the RMS disregarded the reproductive toxicity NOAELs to derive TRVs, considering these effects in rats not relevant to human health.
With regard to the toxicological data package needed to derive an ADI and ARfD for fenarimol according to the current data requirements, 13 the experts identified major limitations and missing data. Due to the deficiencies listed below, the experts concluded that the derivation of toxicological reference values according to current scientific standards is not possible 14 :
according to current standards, the genotoxic potential of fenarimol was found to be inconclusive;
an assessment of the validity of analytical methods used in feed, body fluids and tissues, air and any additional matrices used in support of the toxicological studies was not conducted;
toxicological relevance of unknown impurities potentially present in the technical specification and present in fenarimol‐treated commodities cannot be assessed;
an interspecies comparative in vitro metabolism study performed on animal species used in pivotal studies and on human material is not available to determine the relevance of the toxicological animal data to humans and whether additional testing of potential unique human metabolites would be required;
an up‐to‐date search for published literature is missing;
investigations on the neurotoxic and immunotoxic potential of fenarimol have not been performed;
fenarimol should be considered an endocrine disruptor according to current standards. Additional investigations of the endocrine disruptive potential of fenarimol according to the current ECHA/EFSA Guidance (ECHA and EFSA, 2018) would be needed to complete the respective assessment and derive a reliable threshold;
the summary of the toxicological studies reported in the DAR (UK, 1996) is quite short, it is not reported as would be expected according to current standards (e.g. with tabulated results) and an independent review of the findings cannot be undertaken. The RMS does not report the good laboratory practices compliance and acceptability of the studies that were mostly performed during the 70s‐80s, and not performed according to OECD TG.
Considering the high uncertainties identified, in particular the inconclusive genotoxicity potential of fenarimol, the lack of neurotoxicity investigations, the incomplete ED assessment, the old studies that did not measure relevant parameters to this active substance and the incomplete reporting of the data, a critical NOAEL (point of departure) cannot be identified and adding an UF to the previously established TRVs was not considered feasible. Accordingly, the ADI and ARfD derived in 1996 and 2000, respectively, in the EU do not comply with the current scientific standards. The JMPR values suffer from the same limitations as it appears to be based on the same data package.
4. Consumer risk assessment
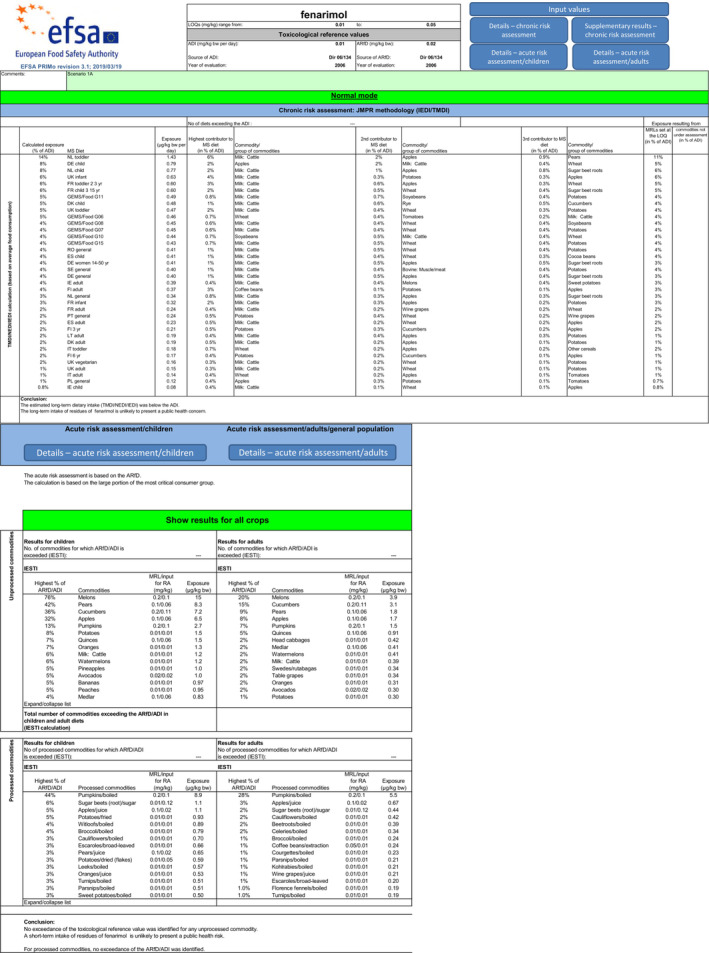
In order to address ToR 5 (a) (Scenario 1), ToR 6 and ToR 11, EFSA calculated the chronic and acute dietary exposure, based on the current residue definition for risk assessment, i.e. fenarimol. Chronic and acute exposure calculations for all crops reported in the framework of this review were performed using revision 3.1 of the EFSA PRIMo (EFSA, 2018, 2019). All input values included in the exposure calculations are summarised in Appendix C.
As for some commodities, EFSA suggested two risk management options (i.e. for pome fruits, cucumbers, melons and pumpkins; see Table 5; Appendix C), the following two subscenarios were calculated:
- Scenario 1A:
- ○ All CXLs and EU MRLs that were recommended for further risk management discussion (labelled as ‘to be discussed’ in Table 5) were considered for the exposure assessment, using the relevant risk assessment value for the current MRL. For the chronic exposure assessment, the calculation is based on the supervised trials median residue levels (STMR) derived for raw agricultural commodities. For the acute exposure assessment, the calculation is based on the highest residue levels (HR) expected in raw agricultural commodities.
- ○ For commodities for which the CXLs/MRLs were revoked or are no longer substantiated, the appropriate LOQ was used as input value for the exposure calculation.
- ○ All other commodities where no GAP was reported in the framework of the MRL review were included in the calculation with the appropriate LOQ.
Scenario 1B:
○ Same input values as in scenario 1A, except for the CXL/MRLs labelled as ‘to be discussed’ in Table 5, for which the appropriate LOQ was used, assuming that a risk management decision on the lowering of these MRLs would be taken.
The risk assessment scenario as described in ToR 5 (b) (Scenario 2) is not relevant for the assessment of fenarimol, as all CXLs were revoked and IT set in EU Regulation were implemented and evaluated by EFSA after 2009. In addition, Scenario 2 would be identical to Scenario 1B.
The acute and chronic exposure calculations were compared to current EU TRVs (European Commission, 2007), noting that during the experts' meeting on mammalian toxicology held in March 2023, the experts concluded that these TRVs do not comply with the current scientific standards (see Section 3).
Screenshots of the report sheet of the indicative PRIMo calculations for scenario 1A and 1B are presented in Appendix B.
In scenarios 1A, the highest chronic exposure was calculated for Dutch toddler, representing 14% of the ADI. The contribution of the MRLs set at the LOQ to the exposure represents 11% of the ADI. The highest acute exposure was calculated for melons, representing 76% of the ARfD.
In scenarios 1B, the highest chronic exposure was calculated for Dutch toddler, representing 13% of the ADI. The contribution of the MRLs set at the LOQ to the exposure represents 13% of the ADI. The highest acute exposure was calculated for potatoes, representing 8% of the ARfD.
EFSA highlights that the toxicological assessment revealed deficiencies regarding the toxicological studies available for fenarimol (EFSA, 2023a). Therefore, considering the high level of uncertainty affecting the TRVs currently set in EU Regulation, the risk assessment requested in ToR 5 cannot be finalised and the results presented in this review are indicative only.
Conclusions and recommendations
The metabolism of fenarimol in plants and animals was previously investigated in the framework of the EU evaluation for inclusion in Annex I to Directive 91/414/EEC, previous MRL applications, as well as by the JMPR. According to the results of the metabolism studies assessed, the residue definition for enforcement and risk assessment, both for plant and animal products, is fenarimol.
Analytical methods are available for the enforcement of the proposed residue definition in high water content and high acid content commodities with an LOQ of 0.01 mg/kg. Fenarimol can be enforced in food of animal origin with an LOQ of 0.01 mg/kg in muscle and fat, milk, eggs, kidney, liver and fish. According to the EURLs, a QuEChERS (or QuOil) multi‐residue and a single‐residue analytical method are available with an LOQ of 0.01 mg/kg for the routine analysis of fenarimol in the four main matrix groups of plant origin, and a default LOQ of 0.01 mg/kg is also deemed achievable to monitor fenarimol in all commodities of animal origin.
The origin of all current MRLs set for fenarimol (based on formerly approved uses or on CXLs) was investigated, and a list of MRLs was identified as not sufficiently substantiated: CXLs or import tolerances for cherries, peaches, table and wine grapes, bananas and hops; EU MRLs for apricots, strawberries, raspberries (red and yellow), currants (black, red and white), gooseberries (green, red and yellow) gherkins, courgettes, other cucurbits with edible peel, watermelons and other cucurbits with inedible peel. No fall‐back MRLs were identified for any of these crops. Moreover, further risk management discussions are required to decide whether the existing EU MRL for pome fruits, cucumbers, melons and pumpkins should be maintained or lowered to the LOQ.
A screening of the quality of the TRVs set at EU level and of those established by the JMPR was performed, and the set of toxicological studies used to derive these TRVs was assessed according to the current standards. As critical issues were identified, a Member States experts' consultation took place. Experts concluded that the TRVs cannot be confirmed for fenarimol since the available data do not provide sufficient evidence to exclude the genotoxicity potential of fenarimol, the data available were insufficient compared to current standards, and uncertainty factors could not be established. Accordingly, the EU ADI and ARfD derived in 1996 and 2001, respectively, do not comply with the current scientific standards. Therefore, EFSA recommends that risk managers discuss whether these TRVs should be withdrawn. The following data would be required to finalise the toxicological assessment which is a prerequisite to derive robust TRVs:
an assessment of the validity of analytical methods used in feed, body fluids and tissues, air and any additional matrices used in support of the toxicological studies;
if possible, an assessment of the toxicological relevance of unknown impurities potentially present in the technical specification and present in fenarimol‐treated commodities;
an interspecies comparative in vitro metabolism study on animal species used in pivotal studies and on human material;
additional studies to conclude on the genotoxic potential of fenarimol;
investigations on the neurotoxic and immunotoxic potential of fenarimol;
additional toxicological data to complete the ED assessment;
an up‐to‐date search for public literature;
full re‐evaluation of the toxicological data package and reporting relevant details on the studies and the results in accordance with the current standards.
The same limitations regarding the (geno)toxicity data package are applicable to JMPR values.
Chronic and acute exposure calculations were performed using revision 3.1 of PRIMo, considering commodities for which CXLs and EU MRLs were found to be sufficiently substantiated, while all CXLs/MRLs that were revoked or are no longer substantiated were proposed to be lowered to the appropriate LOQ, as well as all other commodities for which no GAP was reported under this review. Comparing to the current EU TRVs, no exceedances were observed, and the highest chronic exposure represented 14% of the ADI (Dutch toddler). The highest acute exposure amounted to 76% of the ARfD (melons). Nevertheless, EFSA emphasises that as the toxicological assessment revealed deficiencies and concerns regarding the toxicological studies available for fenarimol and considering that EU TRVs do not meet the current scientific standards, the risk assessment cannot be finalised, and the results presented under the current review are indicative only.
Due to the deficiencies identified regarding the toxicological studies available for fenarimol, none of the existing EU MRLs/CXLs listed in the table below (Table 8) are recommended for inclusion in Annex II to the Regulation. If a decision to withdraw the TRVs is taken, EFSA recommends that risk managers discuss whether all MRLs currently implemented in EU Regulation should be lowered to the respective LOQs.
Table 8.
Summary table
| Code (a) | Commodity | Existing MRL (b) (mg/kg) | Outcome of the review | |
|---|---|---|---|---|
| MRL proposal (mg/kg) | Comment | |||
| Residue definition for enforcement (plants and animal products): fenarimol | ||||
| 0130000 | Pome fruits | 0.1 |
0.1 or LOQ Further consideration by risk managers needed |
Risk management discussion is needed to decide whether the existing MRL needs to be lowered as it is not confirmed if the GAP behind this import tolerance is still authorised in the country of origin. In addition, it should be discussed whether the existing MRL needs to be lowered as the risk assessment could not be finalised, lacking robust TRVs for fenarimol. |
| 0140010 | Apricots | 0.5 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
| 0140020 | Cherries (sweet) | 1.5 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
| 0140030 | Peaches | 0.5 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
|
0151010 0151020 |
Table and wine grapes | 0.3 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
| 0152000 | Strawberries | 0.3 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
| 0153030 | Raspberries (red and yellow) | 0.1 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
| 0154030 |
Currants (black, red and white) Gooseberries (green, red and yellow) |
1 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
| 0163020 | Bananas | 0.2 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
| 0232010 | Cucumbers | 0.2 |
0.2 or LOQ Further consideration by risk managers needed |
Risk management discussion is needed to decide whether the existing MRL needs to be lowered as it is not confirmed if the GAP behind this import tolerance is still authorised in the country of origin. In addition, it should be discussed whether the existing MRL needs to be lowered as the risk assessment could not be finalised, lacking robust TRVs for fenarimol. |
|
0232020 0232030 0232990 |
Gherkins, Courgettes, Other cucurbit with edible peel | 0.2 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
|
0233010 0233020 |
Melons Pumpkins | 0.2 |
0.2 or LOQ Further consideration by risk managers needed |
Risk management discussion is needed to decide whether the existing MRL needs to be lowered as it is not confirmed if the GAP behind this import tolerance is still authorised in the country of origin. In addition, it should be discussed whether the existing MRL needs to be lowered as the risk assessment could not be finalised, lacking robust TRVs for fenarimol. |
|
0233030 0233990 |
Watermelons Other cucurbits with inedible peel | 0.05 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
| 0700000 | Hops | 5 | LOQ | The existing MRL is not substantiated. Hence, the MRL should be lowered to the LOQ. |
MRL: maximum residue limit; CXL: Codex residue limit; LOQ: limit of quantification; TRV: toxicological reference value; ARfD: acute reference dose; GAP: good agricultural practice.
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
MRL currently set under Regulation (EC) No 318/2014.
Abbreviations
- a.s.
active substance
- ADI
acceptable daily intake
- ARfD
acute reference dose
- bw
body weight
- CAC
Codex Alimentarius Commission
- CCPR
Codex Committee on Pesticide Residues
- cGAP
critical good agricultural practice
- CLP
classification, labelling and packaging
- CXL
Codex maximum residue limit
- ECHA
European chemicals agency
- ED
endocrine disruptor
- EURLs
European Reference Laboratories
- GAP
good agricultural practice
- HR
highest residue
- IT
import tolerance
- JMPR
Joint Meeting on Pesticide residues
- LOQ
limit of quantification
- MRL
maximum residue limit
- NOAEL
no observed adverse effect level
- PHI
preharvest interval
- STMR
median residue value
- TRV
toxicological reference value
- UF
uncertainty factor
Appendix A – Summary of the fall‐back GAPs collected from Member States
1.
| Crop and/or situation | MS or country | F G or I (a) | Pests or group of pests controlled | Preparation | Application | Application rate per treatment | PHI (days) (d) | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type (b) | Conc. a.s. | Method kind | Range of growth stages & season (c) | Number min–max | Interval between application (min) | g a.s./hL min–max | Water L/ha min–max | Rate and unit | ||||||
| Apples | USA and Indonesia | F | Fungi | EC | 120 g/L | Foliar treatment | – | 10 | 7 | 3–4 | – | 70–105 g a.s./ha | 30 | |
| Bananas | – | F | Fungi | EC | – | Foliar treatment | – | 6 | – | – | – | 200 g a.s./ha | 7 |
Notified by UK, but not sufficiently supported in EFSA (2011) The country was not indicated by the EMS |
| Cherries | USA | F | Fungi | EC | 120 g/L | Foliar treatment | – | 5 | – | 105 g a.s./ha | 0 | |||
| Grapes | USA | F | Fungi | EC | 120 g/L | Foliar treatment | – | 4 | 14 | – | – | 44–61 g a.s./ha | 21 | Notified by UK, but not sufficiently supported in EFSA (2011) |
| Peaches | Argentina and Uruguay | F | Fungi | EC | – | Foliar treatment | – | 2 | 2.4 | – | 48–72 g a.s./ha | 20 | Notified by UK, but not sufficiently supported in EFSA (2011) | |
| Strawberries | Japan | G | Fungi | WP | 12% | Foliar treatment | – | 3 | 7 | – | 1,500–2,000 | 45–60 g a.s./ha | 1 | Notified by UK, but not sufficiently supported in EFSA (2011) |
| Morocco | F | Fungi | EC | – | Foliar treatment | – | – | – | 3 | – | – | 1 | Notified by UK, but not sufficiently supported in EFSA (2011) | |
| Tomatoes | Egypt | F | Fungi | SC | 120 g/L | Foliar treatment | – | 2 | 4 | 3 | 960 | 29 g a.s./ha | 4 | Notified by UK, but not sufficiently supported in EFSA (2011) |
| Watermelon | Japan | F | Fungi | SC | 12% | Foliar treatment | – | 4 | – | 1.2 | – | – | 3 | Notified by UK, but not sufficiently supported in EFSA (2011) |
| Cucumber, Pumpkin and Melon | Mexico | F | Fungi | EC | 120 g/L | Foliar treatment | – | 2 | 7 | – | 200–800 | 108 g a.s./ha | 1 | |
MS: Member State.
(a): Outdoor or field use (F), greenhouse application (G) or indoor application (I).
(b): CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide.
(c): Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
(d): PHI – minimum preharvest interval.
Appendix B – Pesticide Residue Intake Model (PRIMo)
1.
PRIMo_EU_(Sc. 1A)
PRIMo_EU_(Sc. 1B)

Appendix C – Input values for the exposure calculations
1.
| Commodity | Existing MRL (mg/kg) | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | ||
| Risk assessment residue definition: Fenarimol | |||||
| Pome fruits | 0.1 |
Scenario 1A: 0.02 Scenario 1B: 0.01* |
Scenario 1A: STMR (EFSA, 2011) Scenario 1B: LOQ |
Scenario 1A: 0.06 Scenario 1B: 0.01* |
Scenario 1A: HR (EFSA, 2011) Scenario 1B: LOQ |
| Apricots | 0.5 | 0.01* | LOQ | 0.01* | LOQ |
| Cherries (sweet) | 1.5 | 0.01* | LOQ | 0.01* | LOQ |
| Peaches | 0.5 | 0.01* | LOQ | 0.01* | LOQ |
| Wine and table grapes | 0.3 | 0.01* | LOQ | 0.01* | LOQ |
| Strawberries | 0.3 | 0.01* | LOQ | 0.01* | LOQ |
| Raspberries (red and yellow) | 0.1 | 0.01* | LOQ | 0.01* | LOQ |
| Currants (red, black and white) and Gooseberries (green, red and yellow) | 1 | 0.01* | LOQ | 0.01* | LOQ |
| Bananas | 0.2 | 0.01* | LOQ | 0.01* | LOQ |
| Cucumbers | 0.2 |
Scenario 1A: 0.03 Scenario 1B: 0.01* |
Scenario 1A: STMR (EFSA, 2011) Scenario 1B: LOQ |
Scenario 1A: 0.11 Scenario 1B: 0.01* |
Scenario 1A: HR (EFSA, 2011) Scenario 1B: LOQ |
| Gherkins, courgettes and other cucurbits with inedible peel | 0.2 | 0.01* | LOQ | 0.01* | LOQ |
| Melons and pumpkins | 0.2 |
Scenario 1A: 0.05 Scenario 1B: 0.01* |
Scenario 1A: STMR (EFSA, 2011) Scenario 1B: LOQ |
Scenario 1A: 0.1 Scenario 1B: 0.01* |
Scenario 1A: HR (EFSA, 2011) Scenario 1B: LOQ |
| Watermelons and other cucurbits with inedible peel | 0.05 | 0.01* | LOQ | 0.01* | LOQ |
| Hops | 5 | 0.05* | LOQ | 0.05* | LOQ |
| Other crops/commodities | See Reg. (EU) 318/2014 | LOQ (a) | |||
STMR: median residue value; HR: highest residue; LOQ: limit of quantification.
*: Indicates that the input value is set at the limit of quantification.
(a): A LOQ of 0.02 mg/kg was applied to tree nuts, oilseeds, oil fruits and herbs and edible flowers, and of 0.05 mg/kg to tea, coffee beans, herbal infusions, cocoa beans, carobs, hops, spices and honey. A default LOQ of 0.01 mg/kg for all other commodities was applied.
Suggested citation: EFSA (European Food Safety Authority) , Bellisai, G ., Bernasconi, G ., Binaglia, M ., Carrasco Cabrera, L ., Castellan, I ., Castoldi, A. F ., Chiusolo, A ., Crivellente, F ., Del Aguila, M ., Ferreira, L ., Giner Santonja, G ., Greco, L ., Istace, F ., Jarrah, S ., Lanzoni, A ., Leuschner, R ., Mangas, I ., Miron, I ., Nave, S ., … Verani, A . (2023). Reasoned Opinon on the targeted review of maximum residue levels (MRLs) for fenarimol. EFSA Journal, 21(7), 1–32. 10.2903/j.efsa.2023.8113
Requestor European Commission
Question number EFSA‐Q‐2022‐00449
Acknowledgements EFSA wishes to thank the following experts from ANSES (French Agency for Food, Environmental and Occupational Health & Safety) for the support provided to this reasoned opinion: Nicolas Breysse, Pierre L'Yvonnet, Tomin‐Gwenn Robin and Xavier Sarda.
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Approved: 27 June 2023
Notes
Regulation (EC) No 396/2005 of the Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.03.2005, pp. 1–16.
Standing Committee on Plants, Animals, Food and Feed Section Phytopharmaceuticals – Pesticide Residues 23–24 September 2021 (https://food.ec.europa.eu/system/files/2021-10/sc_phyto_20210923_ppr_sum.pdf).
Standing Committee on Plants, Animals, Food and Feed Section Phytopharmaceuticals – Pesticide Residues 22–23 November 2021 (https://food.ec.europa.eu/system/files/2021-12/sc_phyto_20211122_ppr_sum_0.pdf).
Standing Committee on Plants, Animals, Food and Feed Section Phytopharmaceuticals – Pesticide Residues 22–23 February 2022 (https://food.ec.europa.eu/system/files/2022-08/sc_phyto_20220222_ppr_sum.pdf).
A CXL is considered justified if it is still in place (i.e. if it has not been withdrawn). An import tolerance is to be considered justified if the GAP in the country of origin is still authorised and the MRL in the country of origin is established at a level corresponding to the EU MRL (taking into account the potential difference in the RDs).
The first EFSA scientific report in preparation of CCPR was prepared in 2010.
The first evaluations of import tolerances under Regulation (EC) No 396/2005 which fully entered into force on 1.9.2008.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006OJ L 353, 31.12.2008, p. 1.
The United Kingdom withdrew from EU on 1 February 2020. In accordance with the Agreement on the Withdrawal of the United Kingdom from the EU, and in particular with the Protocol on IE/NI, the EU requirements on data reporting are also applicable to NI.
Commission Directive 2006/134/EC of 11 December 2006 amending Council Directive 91/414/EEC to include fenarimol as active substance. OJ L 349, 12.12.2006, pp. 32–36.
See experts’ consultation point 2.1 at the Pesticide Peer Review Teleconference 92 (EFSA, 2023a).
See experts’ consultation point 2.3 at the Pesticide Peer Review Teleconference 92 (EFSA, 2023a).
Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the marketOJ L 93, 3.4.2013, pp. 1–84.
See experts’ consultation point 2.2 at the Pesticide Peer Review Teleconference 92 (EFSA, 2023a).
References
- CAC (Codex Alimentarius Commission) , 1997. Report on the Joint FAO/WHO food standards programme. Codex Alimentarius Commission, twenty‐second session, Geneva, 23–28 June 1997.
- CAC (Codex Alimentarius Commission) , 1999. Report on the Joint FAO/WHO food standards programme. Codex Alimentarius Commission, twenty‐third session, Rome, 28 June‐3 July 1999.
- CAC (Codex Alimentarius Commission) , 2021. Report on the Joint FAO/WHO food standards programme. Codex Alimentarius Commission, forty‐fourth session, Virtual, 8–15, 17–18 November and 14 December 2021.
- CCPR (Codex Committee on Pesticide Residues) , 1997. Report of the 29th session of the Codex Committee on Pesticide Residues, The Hague, Netherlands, 7–12 April 1997.
- CCPR (Codex Committee on Pesticide Residues) , 1998. Report of the 30th session of the Codex Committee on Pesticide Residues, The Hague, Netherlands, 20–25 April 1998.
- CCPR (Codex Committee on Pesticide Residues) , 2021. Report of the 52nd session of the Codex Committee on Pesticide Residues, Virtual, 26–30 July and 3 August 2021.
- CFR (Code of Federal Regulations) , 2019. Part 180 – Tolerances and exemptions for pesticide chemical residues in food. Title 40 – Protection of Environment. Chapter I – Environmental protection agency. Subchapter E – Pesticide programs. Vol 26. 01 July 2019.
- ECHA (European Chemicals Agency) and EFSA (European Food Safety Authority) with the technical support of the Joint Research Centre (JRC), Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, Pellizzato F, Tarazona J, Terron A and Van der Linden S, 2018. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal 2018;16(6):5311, 135 pp. 10.2903/j.efsa.2018.5311.ECHA-18-G-01-EN [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2008. MRLs of concern for the active substance fenarimol. EFSA Scientific Report, 161, 1–28. [Google Scholar]
- EFSA (European Food Safety Authority) , 2011. Modification of the existing maximum residue levels for fenarimol in various crops. EFSA Journal 2011;9(9):2350, 32 pp. 10.2903/j.efsa.2011.2350 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato A, Brocca D, Ferreira L, Greco L, Jarrah S, Leuschner R, Medina P, Miron I, Nougadere A, Pedersen R, Reich H, Santos M, Stanek A, Tarazona J, Theobald A and Villamar‐Bouza L, 2018. Guidance on use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA Journal 2018;16(1):5147, 43 pp. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Anastassiadou M and Brancato A, Carrasco Cabrera L, Ferreira L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Tarazona J and Theobald A, Verani A, 2019. Pesticide Residue Intake Model- EFSA PRIMo revision 3.1 (update of EFSA PRIMo revision 3). EFSA supporting publication 2019:EN-1605, 15 pp. 10.2903/sp.efsa.2019.EN-1605 [DOI]
- EFSA (European Food Safety Authority) , 2023a. Report on the pesticide peer review TC 98 – fenarimol. Available online: www.efsa.europa.eu
- EFSA (European Food Safety Authority) , 2023b. Member States consultation report on the targeted review of maximum residue levels (MRLs) for fenarimol prepared by EFSA in the framework of Article 43 of Regulation (EC) No 396/2005, July 2023. Available online: www.efsa.europa.eu
- EURLs (EU Reference Laboratories for Pesticide Residues) , 2022. Evaluation Report prepared under Article 43 of Regulation (EC) No 396/2005. Analytical validations by the EURLs and capability of official laboratories to be considered for the targeted review of the MRLs for fenarimol. Available online: www.efsa.europa.eu
- European Commission , 2007. Review report for the active substance fenarimol finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 3 March 2006 in view of the inclusion of fenarimol in Annex I of Directive 91/414/EEC. 6847/VI/97‐final, 5 January 2007.
- FAO (Food and Agriculture Organization of the United Nations) and WHO (World Health Organization) , 1995. Fenarimol. In: Pesticide residues in food – 1995. Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Geneva, Swiss, 18–27 September 1995. Evaluations, Part I, Residues. FAO Plant Production and Protection Paper 137.
- FAO (Food and Agriculture Organization of the United Nations) and WHO (World Health Organization) , 1996. Fenarimol. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Geneva, Swiss, 18–27 September 1995. FAO Plant Production and Protection Paper 140.
- UK (United Kingdom) , 1996. Draft assessment report on the active substance fenarimol prepared by the rapporteur Member State United Kingdom in the framework of Council Directive 91/414/EEC, March 1996.
- UK (United Kingdom) , 2000. Addendum to the draft assessment report on the active substance fenarimol prepared by the rapporteur Member State United Kingdom in the framework of Council Directive 91/414/EEC, August 2000.


